Patents
Literature
505 results about "Herpes simplex virus DNA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Herpes virus may refer to: the large family of DNA viruses known as Herpesviridae (or herpesviruses) Herpes simplex virus 1 and 2, the two types of herpesvirus responsible for herpes simplex infections
Sugar modified oligonucleotides that detect and modulate gene expression
Compositions and methods are provided for the treatment and diagnosis of diseases amenable to modulation of the production of selected proteins. In accordance with preferred embodiments, oligonucleotides and oligonucleotide analogs are provided which are specifically hybridizable with a selected sequence of RNA or DNA wherein at least one of the 2'-deoxyfuranosyl moieties of the nucleoside unit is modified. Treatment of HIV, herpes virus, papillomavirus and other infections is provided.
Owner:IONIS PHARMA INC
Use of laggera plant abstract in inhibiting herpes simplex virus and hepatitis B virus
InactiveCN1989989AReduced expression functionDigestive systemPharmaceutical delivery mechanismDiseaseCaffeoylquinic acid
The invention involves novel drug use of six-rowed chrysanthemum plant extracts which is used to treating herpes simplex virus (type 1 and / or type 2) and various disease caused by hepatitis B virus infection. The six-rowed chrysanthemum plant extracts is prepared by six-rowed chrysanthemum plant fresh or dry goods through the refining of alcohol-water extraction, column chromatography, alcohol solvent elution, the amount of caffeoyl guinic acid chemical compound is below 30%. The six-rowed chrysanthemum plant extracts prepared in the invention has significant function of inhibiting herpes simplex virus with type 1 (HSV-1), herpes simplex virus type 2 (HSV-2) and hepatitis B virus (HBV) replication, and can reduce effectiveness of HBV e antigen (HBeAg) in the HepG 2.2.15 cell lines, it can be used for treatment various disease caused by said correlate virus infection.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Virulent oncolytic herpes simplex virus strains engineered to counter the innate host response
ActiveUS7731952B2Preserving abilityContinual and sustained deliveryBiocideGenetic material ingredientsAutoimmune responsesHost response
Owner:NEW YORK UNIV
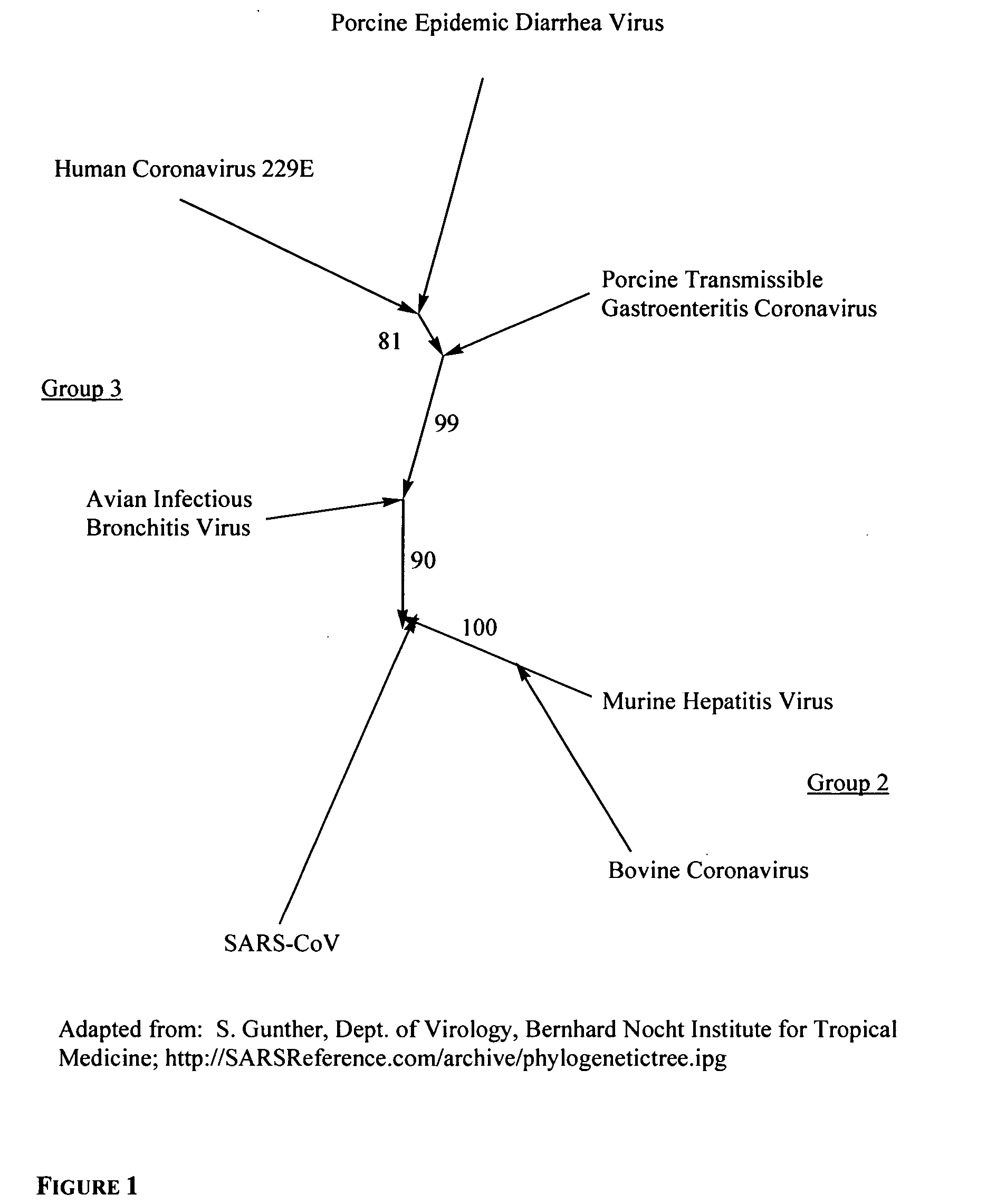
Phospholipids for the treatment of infection by togaviruses, herpes viruses and coronaviruses
InactiveUS20050187192A1BiocidePhosphorous compound active ingredientsHerpes simplex virus DNACompound (substance)
Provided are compounds, methods and pharmaceutical compositions for treating a host, especially a human, infected with a togavirus, herpes virus and / or coronavirus, and in particular SARS-CoV, cytomegalovirus or varicella-zoster virus. The method in one embodiment comprises administering to that host an effective amount of an anti-togavirus, anti-herpes virus and / or anti-coronavirus phospholipid or a pharmaceutically acceptable salt or prodrug thereof. The phospholipid compound is, e.g., a 3-alkylamido-2-alkoxypropylphosphocholine compound or salt thereof. The compound may be administered alone or in combination and / or alternation with one or more other anti-viral agents.
Owner:KUCERA PHARMA
Antigen delivery platforms
InactiveUS20140030292A1Improve stabilityAvoid reduce stimulationSsRNA viruses positive-senseFusion with post-translational modification motifAntigen deliveryHerpes simplex virus DNA
This disclosure provides platforms for delivery of herpes virus proteins to cells, particularly proteins that form complexes in vivo. In some embodiments these proteins and the complexes they form elicit potent neutralizing antibodies. Thus, presentation of herpes virus proteins using the disclosed platforms permits the generation of broad and potent immune responses useful for vaccine development.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Virus coat protein/receptor chimeras and methods of use
InactiveUS7311920B1Sufficient amountMethod can be usedCompound screeningApoptosis detectionCXCR4Immunodeficiency virus
The invention relates to chimeric molecules comprising a virus coat sequence and a receptor sequence that can interact with each other to form a complex that is capable of binding a co-receptor. Such chimeric molecules therefore exhibit functional properties characteristic of a receptor-coat protein complex and are useful as agents that inhibit virus infection of cells due to occupancy of co-receptor present on the cell, for example. In particular aspects, the chimeric polypeptide includes an immunodeficiency virus envelope polypeptide, such as that of HIV, SIV, FIV, FeLV, FPV and herpes virus. Receptor sequences suitable for use in a chimeric polypeptide include, for example, CCR5 and CXCR4 sequences.
Owner:MARYLAND BIOTECH INST UNIV OF
Use of Mutant Herpes Simplex Virus-2 for Cancer Therapy
ActiveUS20090215147A1Strong anti-tumor immune responseUseful in therapyPolypeptide with localisation/targeting motifVectorsNucleotideHerpes simplex virus DNA
Owner:UNIV HOUSTON SYST
Pyrimidine nucleosides and their monophosphate prodrugs for treatment of viral infections and cancer
The present invention is directed to compounds, compositions and methods for treating or preventing cancer and viral infections, in particular, HIV, HCV, Norovirus, Saporovirus, cytomegalovirus (CMV), herpes viruses (HSV-1, HSV-2), Dengue virus, Yellow fever, or HBV in human patients or other animal hosts. The compounds are certain N4-hydroxycytidine nucleosides derivatives, modified monophosphate and phosphonates prodrugs analogs, and pharmaceutically acceptable, salts, prodrugs, and other derivatives thereof. In particular, the compounds show potent antiviral activity against HIV-1, HIV-2, HCV, Norovirus, Saporovirus, cytomegalovirus (CMV), herpes viruses (HSV-1, HSV-2), Dengue virus, Yellow fever, and HBV.
Owner:EMORY UNIVERSITY
Mutant herpes simplex viruses and uses thereof
InactiveUS20040063094A1Overcome reduction in stabilityReduce in quantityMicrobiological testing/measurementFermentationNervous systemMammal
A herpes simplex virus (HSV) comprising (i) an HSV LAT sequence inserted into an essential gene of the HSV; and (ii) a deletion in the endogenous LAT region of the HSV strain. The HSV of the invention can be used in the treatment of disorders of, or injuries to, the nervous system of a mammal.
Owner:BIOVEX LTD
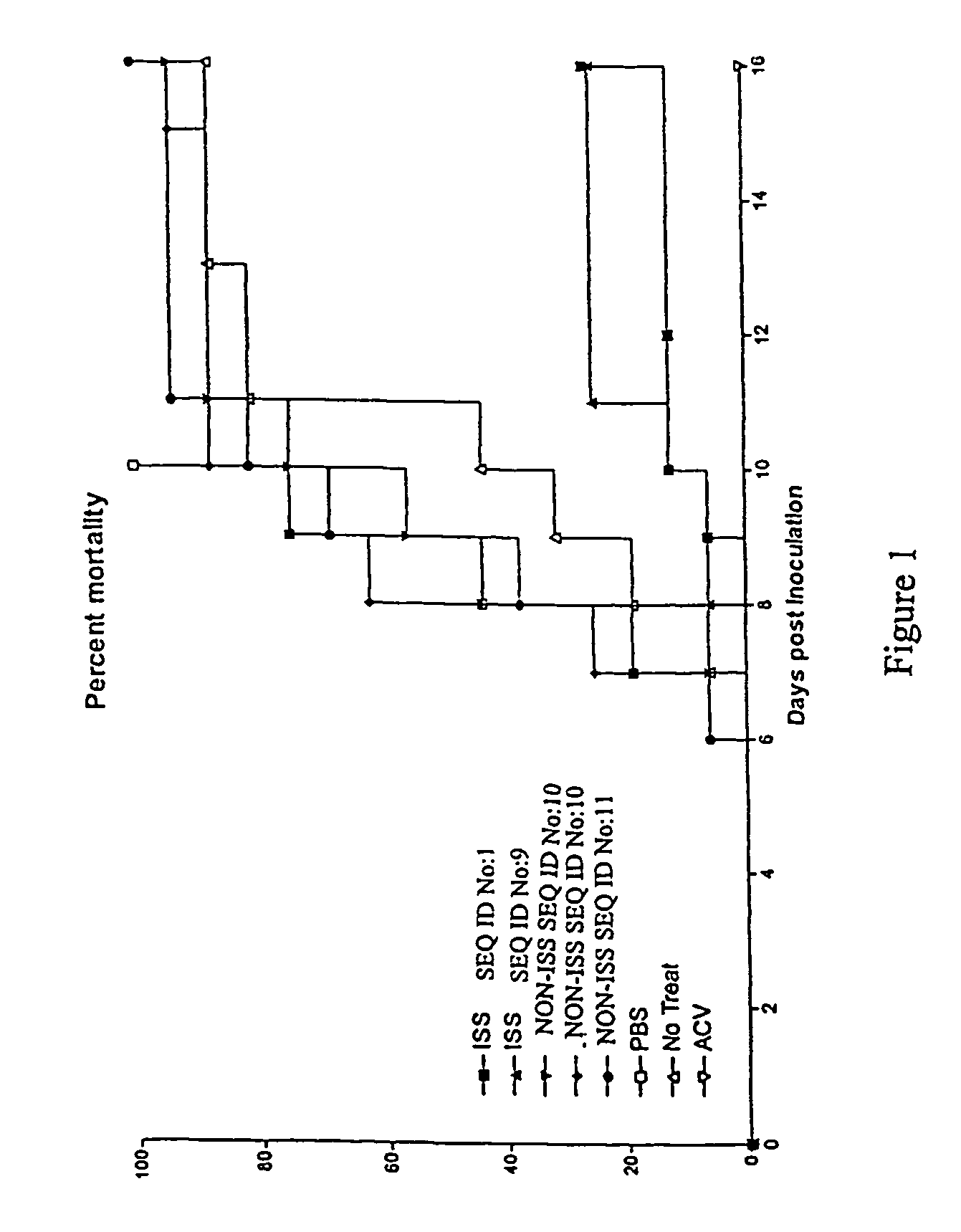
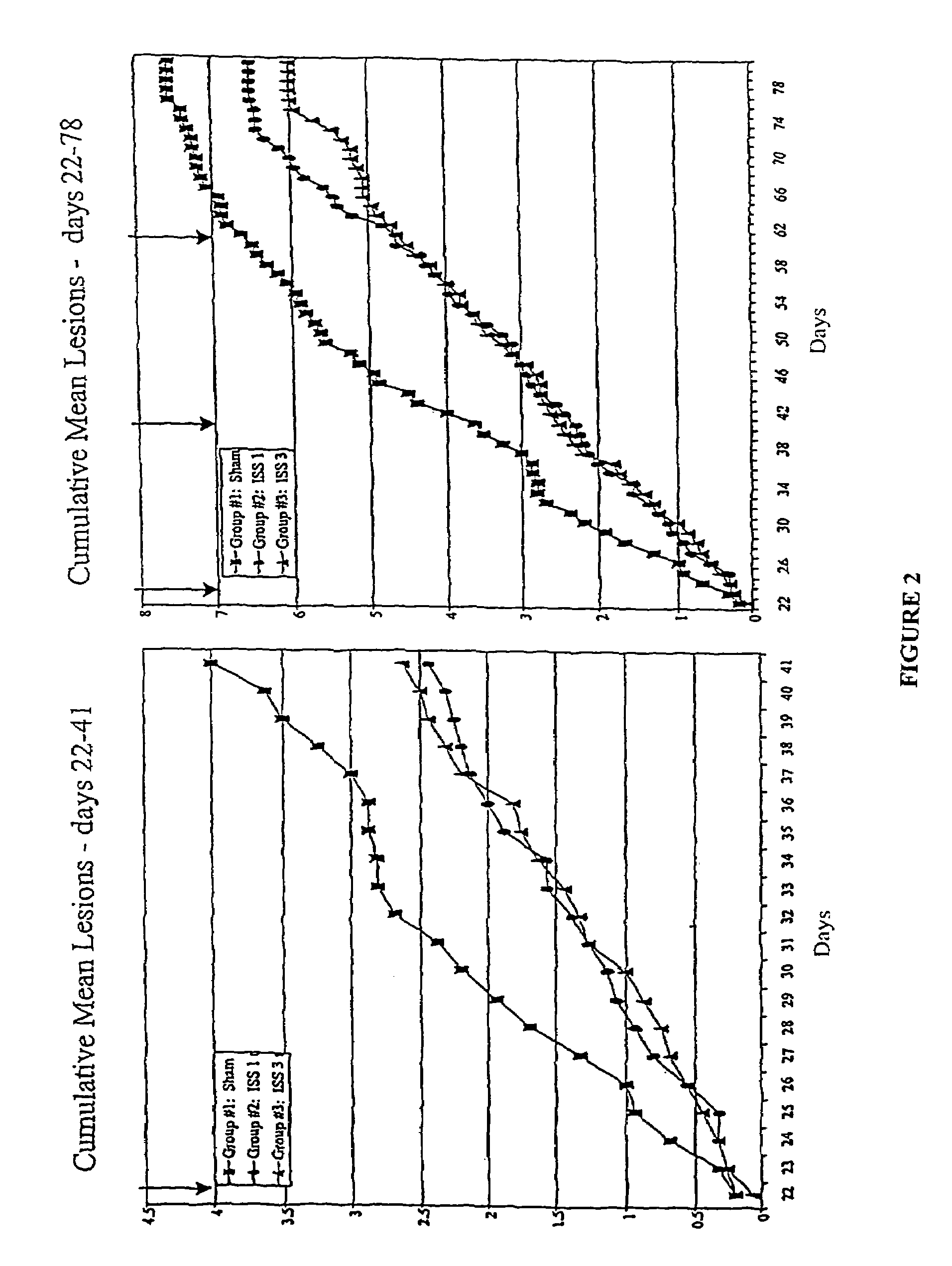
Methods of ameliorating symptoms of herpes infection using immunomodulatory polynucleotide sequences
InactiveUS7157437B2Many symptomReduce incidenceOrganic active ingredientsSugar derivativesAntigenHerpes simplex virus DNA
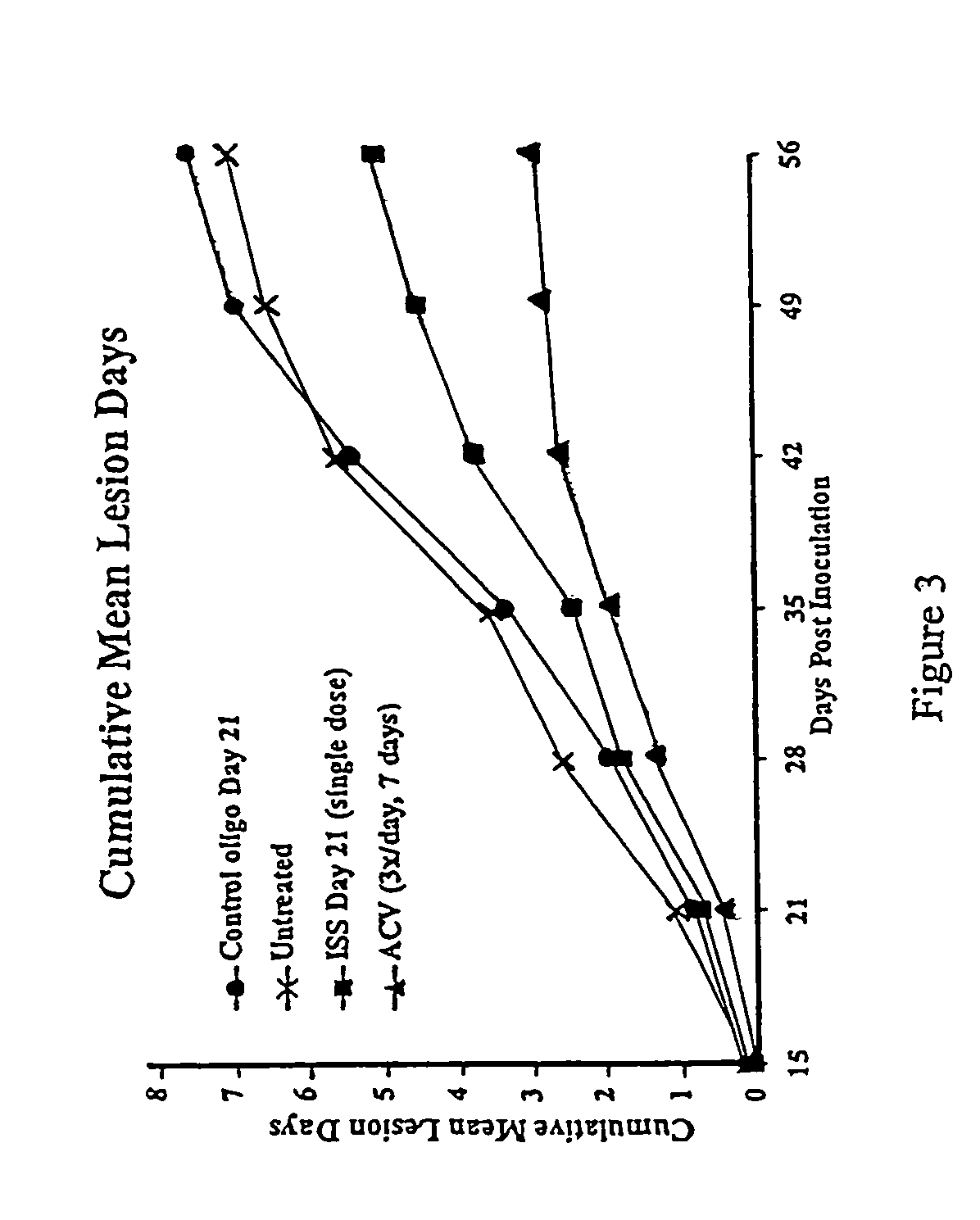
The invention provides new methods of preventing and / or treating herpes virus infections, particularly reducing infection, one or more symptoms and recurrence of one or more symptoms of herpes simplex virus infection. A polynucleotide comprising an immunostimulatory sequence (an “ISS”) is administered to an individual which is at risk of being posed to alphaherpesvirinae, has been exposed to alphaherpesvirinae or is infected with alphaherpesvirinae. The ISS is administered without any alphaherpesvirinae antigens. Administration of the ISS results in reduced incidence, recurrence, and severity of one or more symptoms of alphaherpesvirinae infection.
Owner:DYNAVAX TECH CORP
Equine herpesvirus glycoproteins
InactiveUS6193983B1Improve identityPeptide/protein ingredientsGenetic material ingredientsHerpes simplex virus DNAEquine herpesvirus
A vaccine for the selective immunization of horses against EHV4 and / or EHV1 is provided comprising at least one of (i) EHV4 virus wherein a portion of the gG gene of the EHV4 virus that elicits a type-specific response to EHV4 has been deleted and (ii) EHV1 virus wherein a portion of the gG gene of the EHV1 virus that elicits a type-specific response to EHV1 has been deleted. Antibodies which specifically bind to a epitopes of EHV4 gG or EHV1 also are provided.
Owner:UNIVERSITY OF MELBOURNE
Unit dosage forms for the treatment of herpes simplex
InactiveUS7351715B2Increase successSafe and effective and inexpensiveBiocidePeptide/protein ingredientsDiseaseCell membrane
The components of this invention are chosen because of their complementarity for the prevention or treatment of diseases caused by the herpes simplex virus. L-Lysine favorably increases the physiologic immunomodulation necessary for defense against this virus. Zinc improves and maintains a normal immune response. 2-Deoxy-2-D-glucose and heparin sodium alter the surface interaction between the herpes virus and the cell, preventing fusion and infectivity. N-Acetyl-L-cysteine increases glutathione levels thereby creating a thiol redox barrier to the virus at the cell membrane. Quercetin reduces intraoellular replication of the herpes virus and viral infectivity. Ascorbate, in concert with copper and D-α-tocopherol, provides an antioxidant defense against the herpes virus, which tends to lose latency during period of oxidative, free radical excess. Selenium and quercetin also participate in reducing various oxidative stresses. Together the components of this invention provide the potential for improved resistance to, improved recovery from, and a decreased frequency of recurrence of herpes simplex virus infection.
Owner:CHRONORX
High titer recombinant AAV production
InactiveUS7091029B2Increase final yieldEliminate stepsBiocideGenetic material ingredientsHerpes simplex virus DNAViral vector
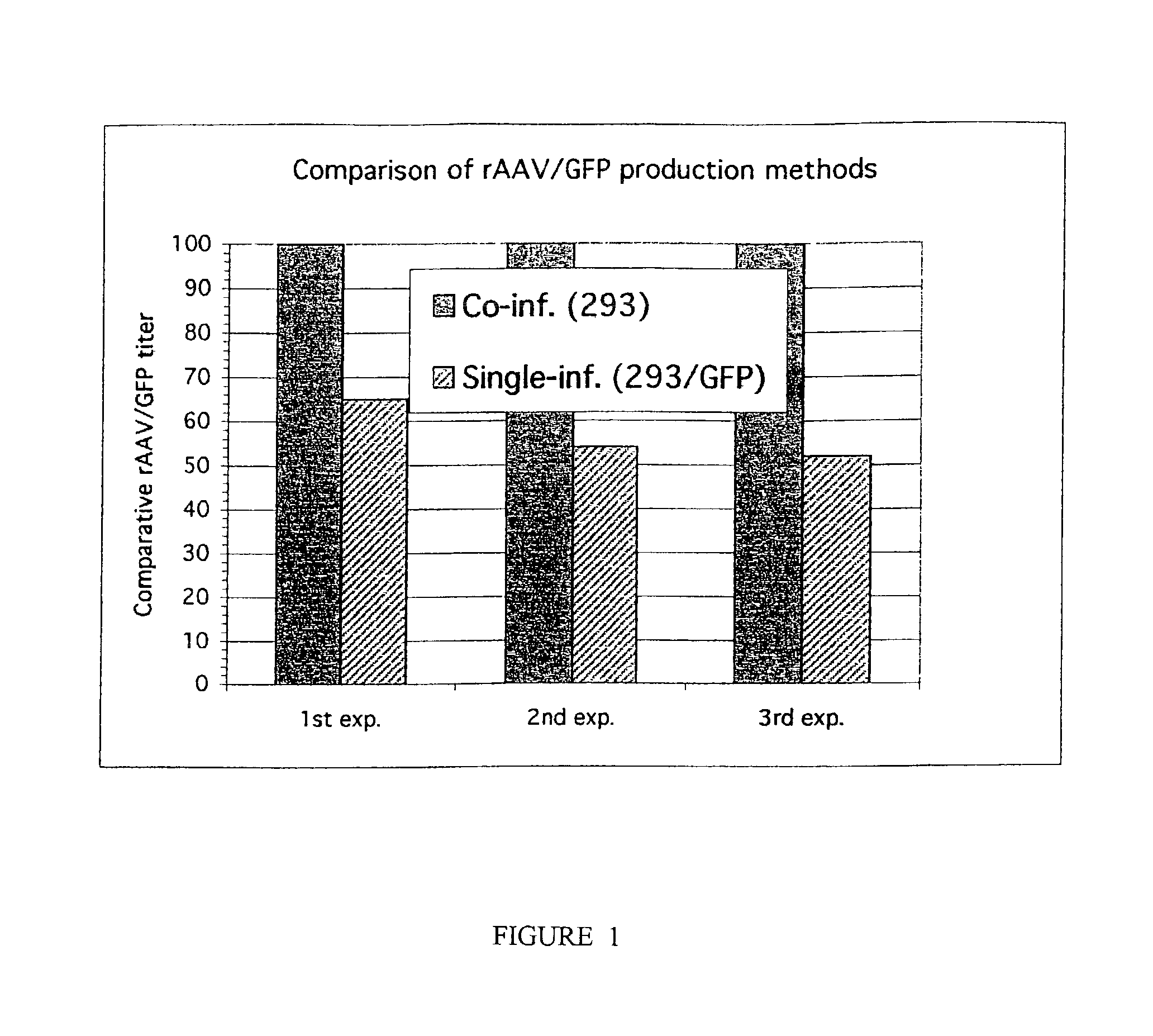
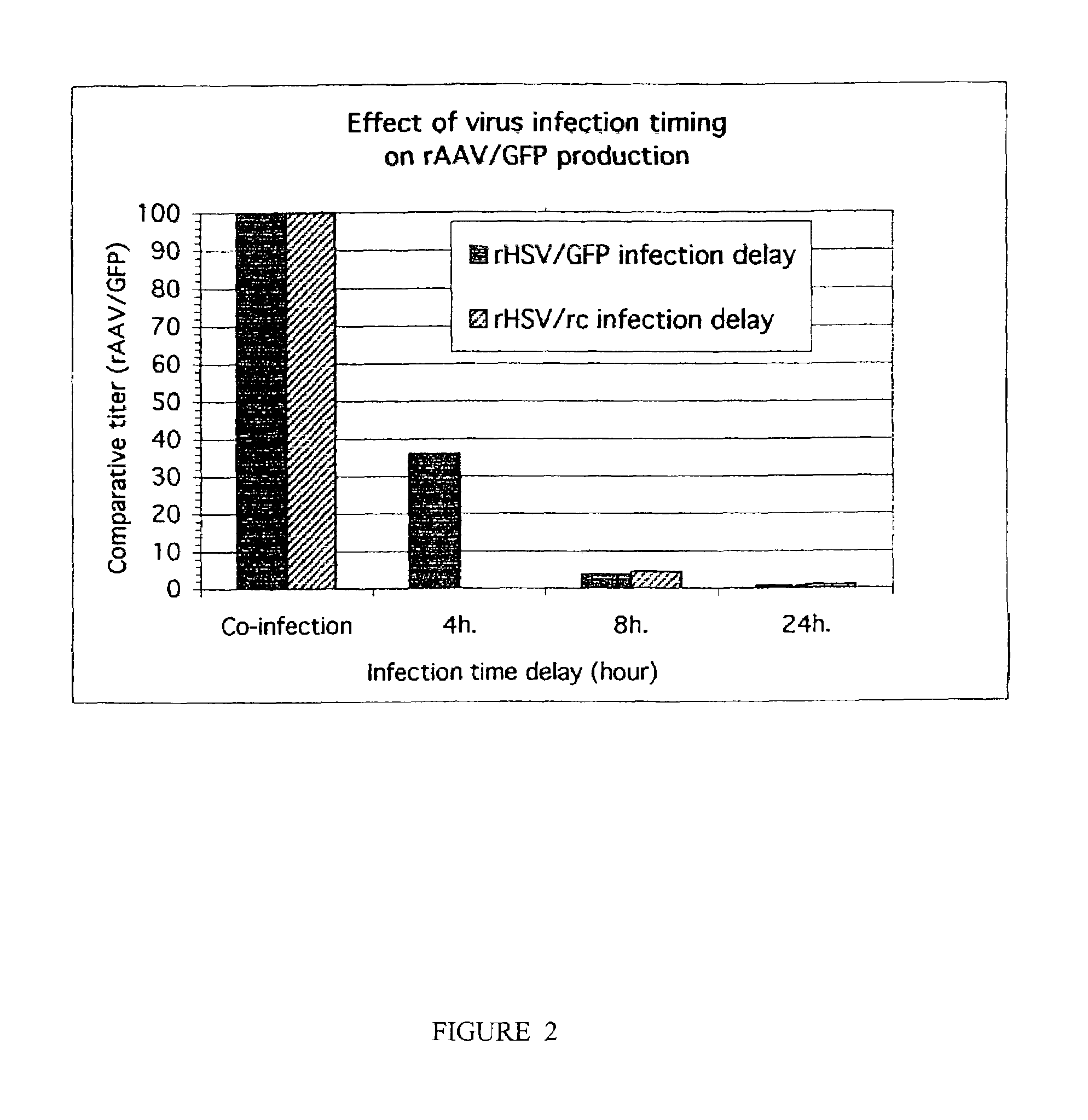
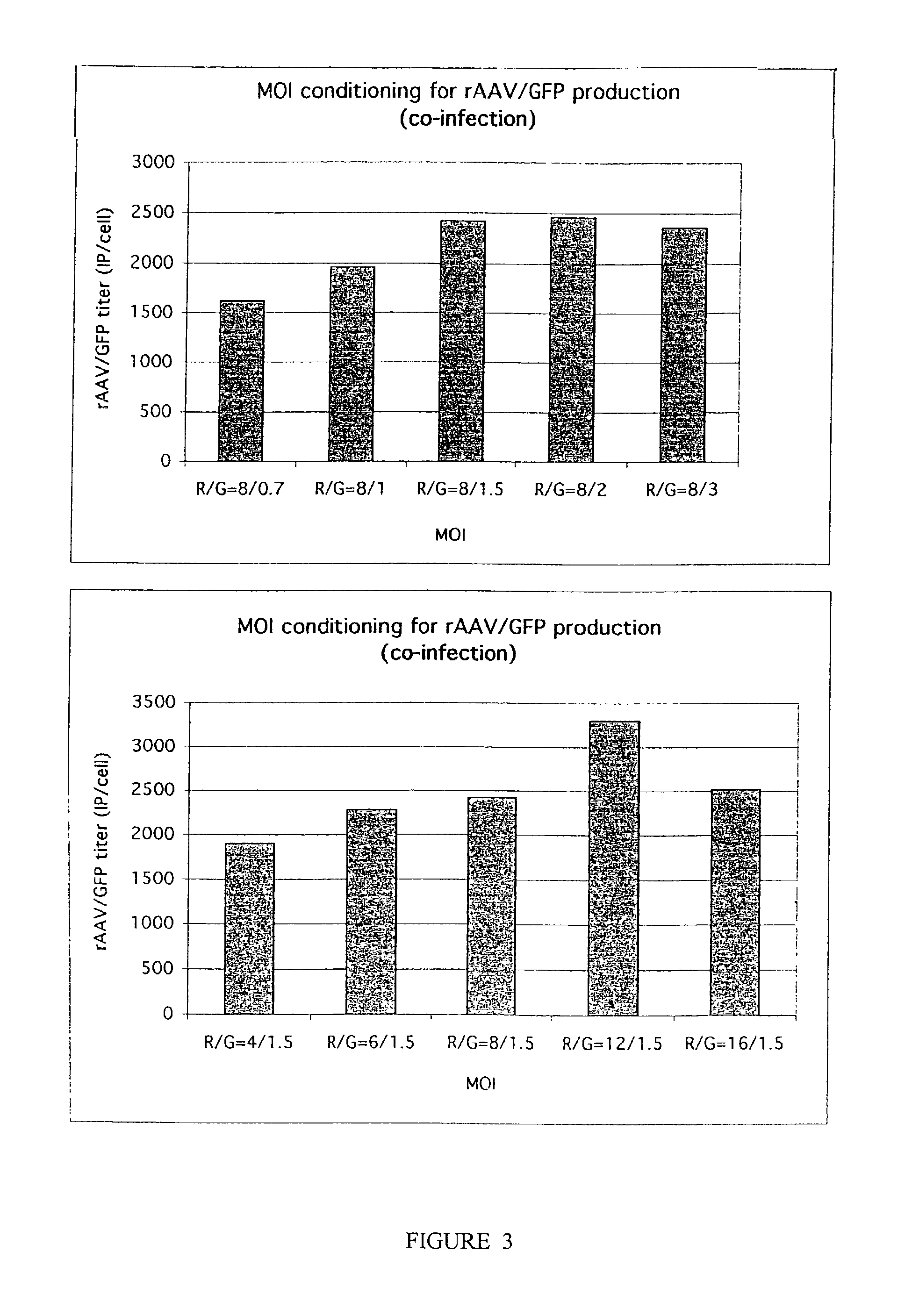
The invention includes methods and compositions for the production of high titer recombinant adeno-associated virus (rAAV). The disclosed rAAV are useful in gene therapy applications. Methods are based on the use of recombinant herpes virus vectors and result in highly efficient production of rAAV.
Owner:APPL GENETIC TECH CORP +2
Methods for treating herpes virus infections
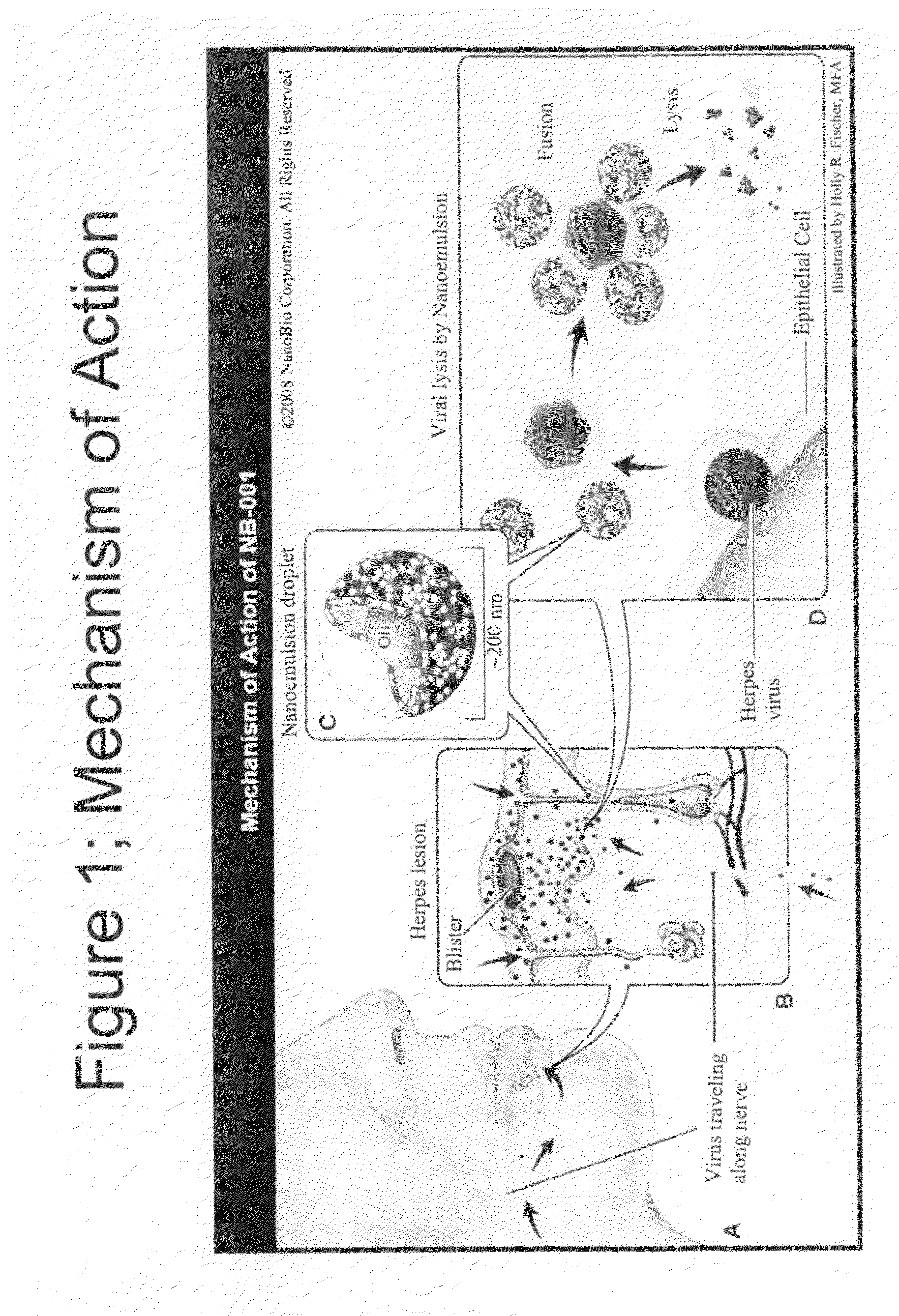
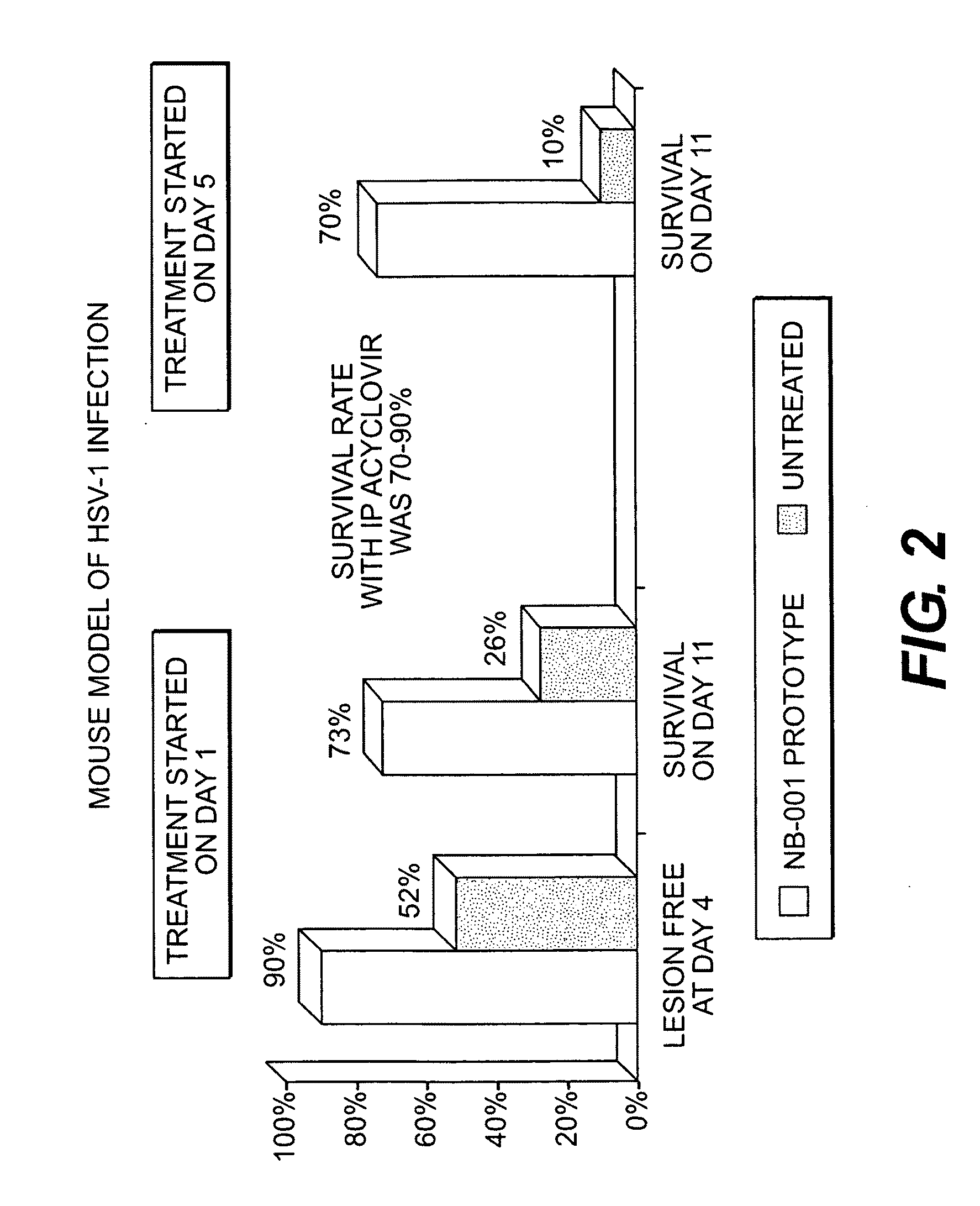
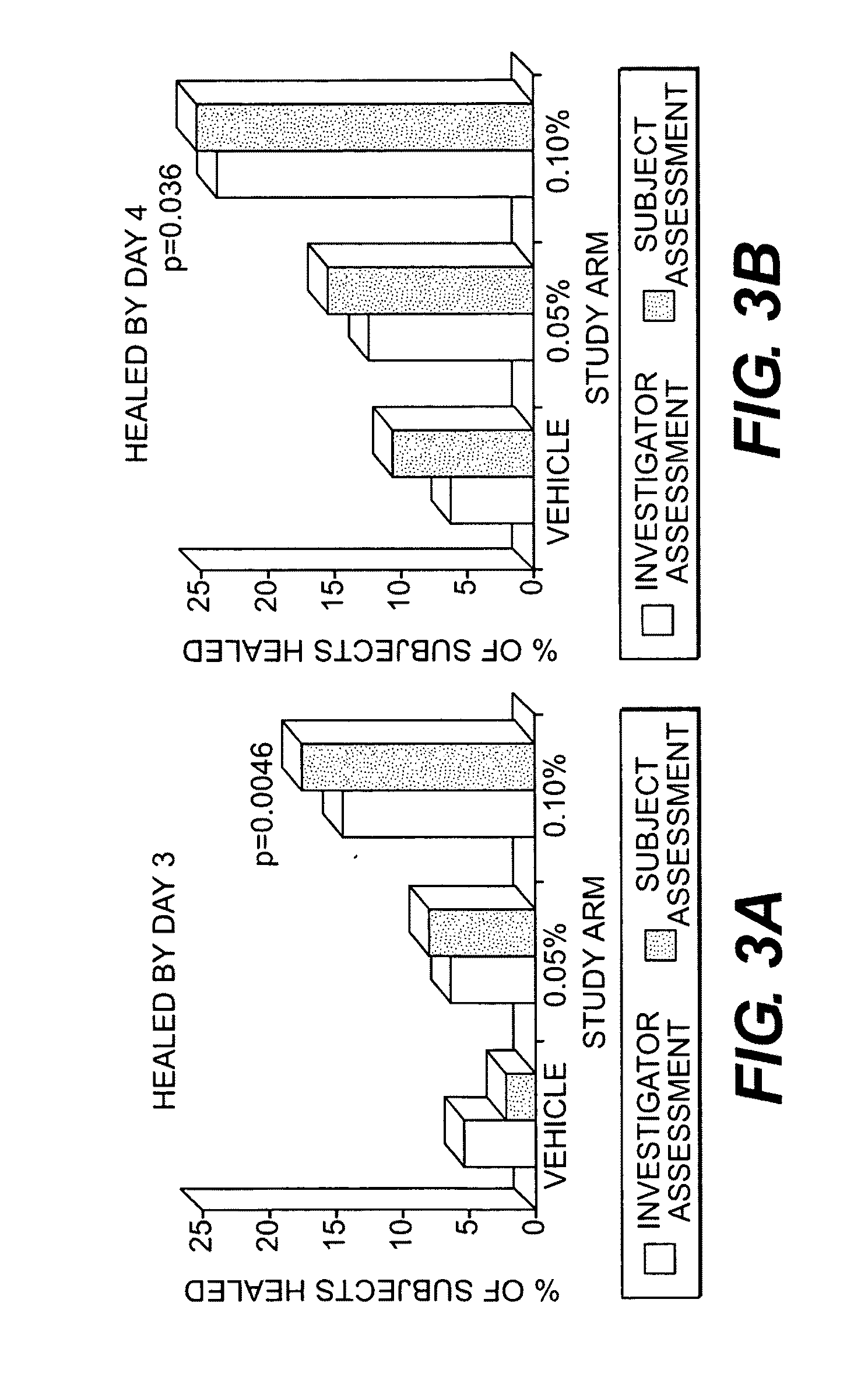
InactiveUS20100075914A1Minimizing reactivationAvoid spreadingBiocideAntiviralsHerpes simplex virus DNAHerpesviridae Infections
Owner:NANOBIO CORP
Genetically engineered herpes virus for the treatment of cardiovascular disease
InactiveUS6846670B2Organic active ingredientsPeptide/protein ingredientsCombined Modality TherapyGenetically engineered
The present invention provides methods of expressing a nucleic acid or producing a proteinaceous composition encoded by a nucleic acid in vascular and cardiovascular cells by administration of a herpesvirus vector. The present invention provides methods of producing a therapeutic benefit in vascular and cardiovascular tissue by administration of a herpesvirus vector. In additional aspects, the invention concerns combination therapies for vascular and cardiovascular diseases comprising administration of a herpesvirus vector and treatment with at least one addition pharmacological agent or surgical procedure.
Owner:UNIV OF CHICAGO AN ILLINOIS CORP THE
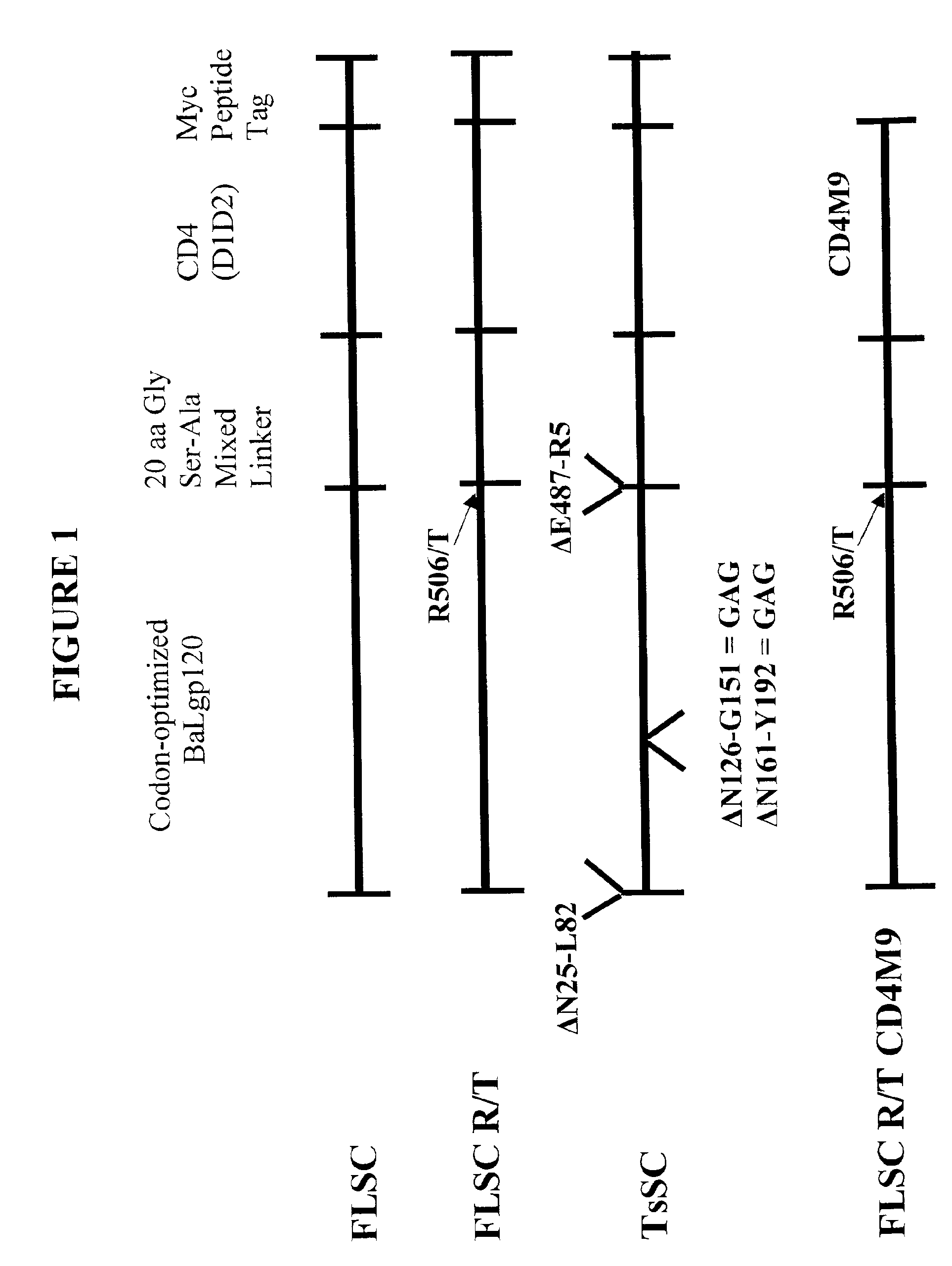
Virus coat protein/receptor chimeras and methods of use
InactiveUS6908612B2Raise the potentialAvoid virus infectionOrganic active ingredientsFungiImmunodeficiency virusHerpes simplex virus DNA
The invention relates to chimeric molecules comprising a virus coat sequence and a receptor sequence that can inter-act with each other to form a complex that is capable of binding a co-receptor. Such chimeric molecules therefore exhibit functional properties characteristic of a receptor-coat protein complex and are useful as agents that inhibit virus infection of cells due to occupancy of a co-receptor present on the cell. In particular aspects, the chimeric polypeptide includes an immunodeficiency virus envelope polypeptide, such as that of HIV, SIV, FIV, FeLV, FPV and herpes virus. Receptor sequences suitable for use in a chimeric polypeptide include, for example, CD4 D1D2 and CD4M9 sequences.
Owner:MARYLAND UNIV OF BIOTECH INST
Herpes viruses for immune modulation
A method of stimulating an immune response in a human or animal subject, which method comprises administering to a subject in need thereof an effective amount of an attenuated herpes virus which:(i) lacks a functional vhs gene, or a functional equivalent thereof;(ii) lacks a functional ICP47 gene, or a functional equivalent thereof; and(iii) is incapable of expressing a substantial amount of functional ICP22, or a functional equivalent thereof, in mammalian dendritic cells.
Owner:BIOVEX LTD
Herpes virus vectors for dendritic cells
InactiveUS6641817B1Efficient infectionLow toxicityBiocideGenetic material ingredientsDiseaseInfected cell
An attenuated herpes virus capable of efficiently infecting a dendritic cell without preventing antigen processing occurring within the infected cell. The attenuated herpes virus and dentrictic cells infected with the virus are useful in immunotherapeutic methods of treating disease.
Owner:BIOVEX LTD
Anti-light antibody therapy for inflammatory bowel disease
InactiveUS20130315913A1Increase currentCompounds screening/testingImmunoglobulins against cytokines/lymphokines/interferonsHerpes simplex virus DNAT lymphocyte
The present invention provides safe therapeutic doses of an antagonist of human LIGHT (lymphotoxin-like, exhibits inducible expression and competes with Herpes Virus Glycoprotein D for Herpes Virus Entry Mediator (HVEM), a receptor expressed by T lymphocytes), as well as methods of monitoring whether a therapeutic dose of an anti-LIGHT antagonist is safe.
Owner:SANOFI SA
Herpes viruses for immune modulation
InactiveUS6713067B2Minimize ToxicityMinimise immune response-inhibitory activityBiocideGenetic material ingredientsDendritic cellHerpes simplex virus DNA
An attenuated herpes virus which lacks a functional vhs gene or a functional equivalent thereof, but which has a functional UL43 gene or functional equivalent thereof, stimulates an immune response when dendritic cells are infected with the virus.
Owner:BIOVEX LTD
Synthetic peptide, inhibitor to DNA viruses
The present invention relates to the identification of the active domain of Herpoxin, a DNA virus-inhibiting-protein which was isolated from cobra venom in U.S. Pat. No. 5,648,339 and has a molecular weight of 13.5 kDa We have isolated a fragment of Herpoxin which contains the active domain and which we have named Herp. Herp mimics the activity of Herpoxin in inhibiting the replication of DNA viruses. A synthetic version of the active fragment was produced having the amino acid sequence Asn-Leu-Tyr-Gln-Phe-Lys-Asn-Met-Ile-Gln. The synthetic version of Herp consisting of ten amino acids inhibits the replication of DNA viruses such as herpes viruses types 1 and 2, cytomegalovirus and varicella zoster virus as well as Tubercle bacilli.
Owner:LIPPS BINIE V +1
Folium isatidis cellulose fiber having antiviral, antibacterial and skin-care functions and preparation method thereof
InactiveCN105603556AEasy to takeGood textile processabilityArtificial filaments from viscoseWet spinning methodsSodium CaseinateEngineering
The invention discloses a folium isatidis cellulose fiber having antiviral, antibacterial and skin-care functions and a preparation method thereof. The folium isatidis cellulose fiber contains, by weight, 2.0-8.0 parts of folium isatidis extract, 2.0-8.0 parts of sodium caseinate, 2-30 parts of porous starch and 2-50 parts of protein. The preparation method includes the following steps that 1, preparation of folium isatidis extract-sodium caseinate compound microcapsules, 2 preparation of a blended spinning stock solution and 3 spinning and post-processing. Compared with conventional viscose fibers, the influenza A virus inactivation rate of the fiber is 82.0% or above, the herpes virus inactivation rate is 84.0% or above, a bacteriostatic activity value is greater than or equal to 2.0, a bactericidal activity value is greater than or equal to 0.2, a variety of amino acids and trace elements are contained in the fiber, the fiber has good skin-care and health-care functions, the physical index of the product can meet the requirements of GB / T14463-2008 viscose staple fiber first-grade products, and the fiber has good wearability and textile processing performance.
Owner:单大伟
Recombinant AAV production in mammalian cells
The present invention includes methods and compositions for the production of high titer recombinant Adeno-Associated Virus (rAAV) in a variety of mammalian cells. The disclosed rAAV are useful in gene therapy applications. Disclosed methods based on co-infection of cells with two or more replication-defective recombinant herpes virus (rHSV) vectors are suitable for high-titer, large-scale production of infectious rAAV.
Owner:APPL GENETIC TECH CORP
Nucleic acid molecules encoding novel herpes antigens, vaccine comprising the same, and methods of use thereof
ActiveUS20140023673A1Effective vaccineGenetic material ingredientsVirus peptidesAntigenHerpes simplex virus DNA
Provided herein are nucleic acid sequences that encode novel consensus amino acid sequences of herpes virus antigens, as well as genetic constructs / vectors and vaccines expressing the sequences. Also provided herein are methods for generating an immune response against herpes virus using the vaccines that are provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Mutant herpes simplex viruses and uses thereof
InactiveUS6719982B1Reduce synthesisLow toxicityNervous disorderPeptide/protein ingredientsNervous systemMammal
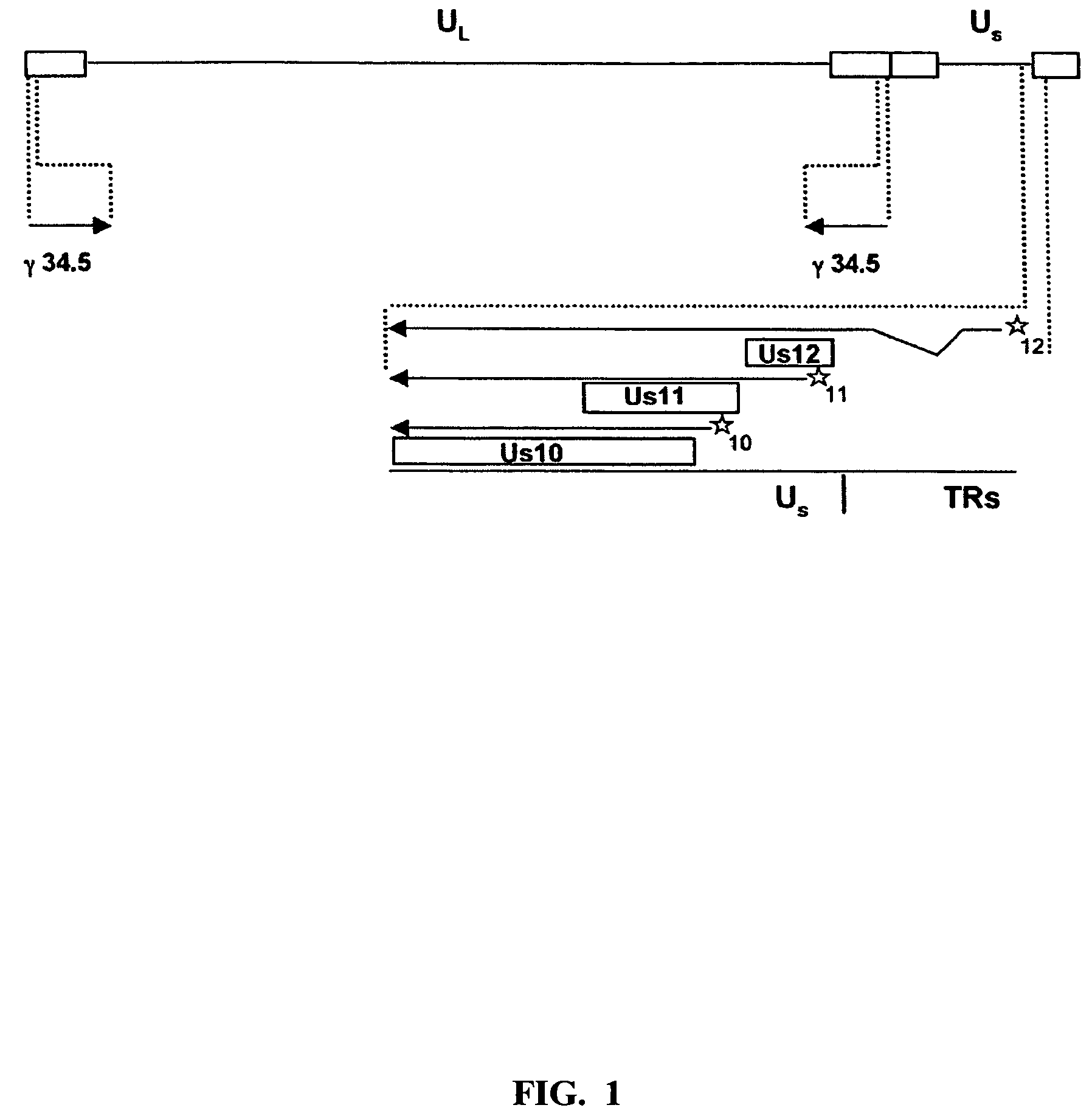
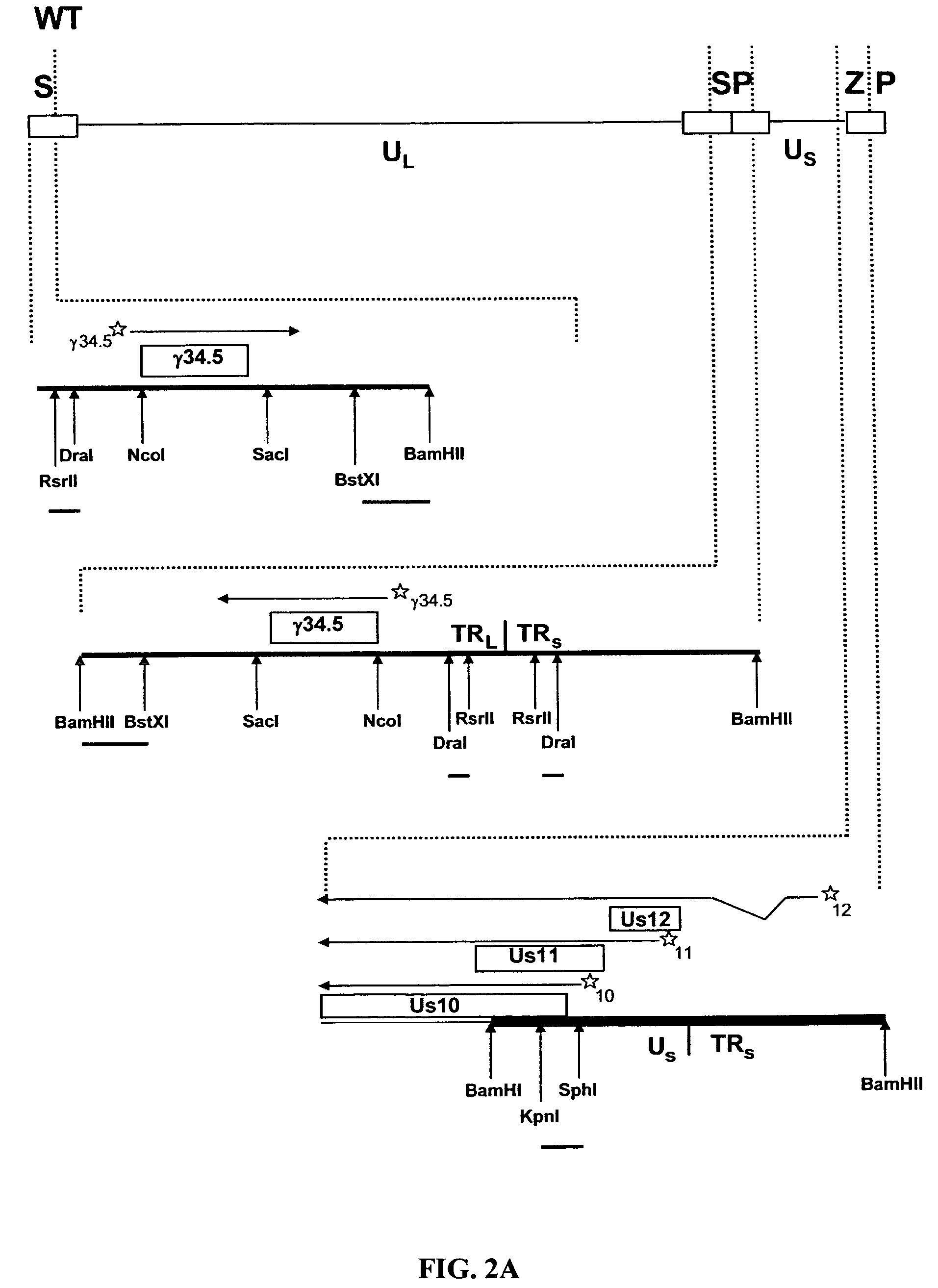
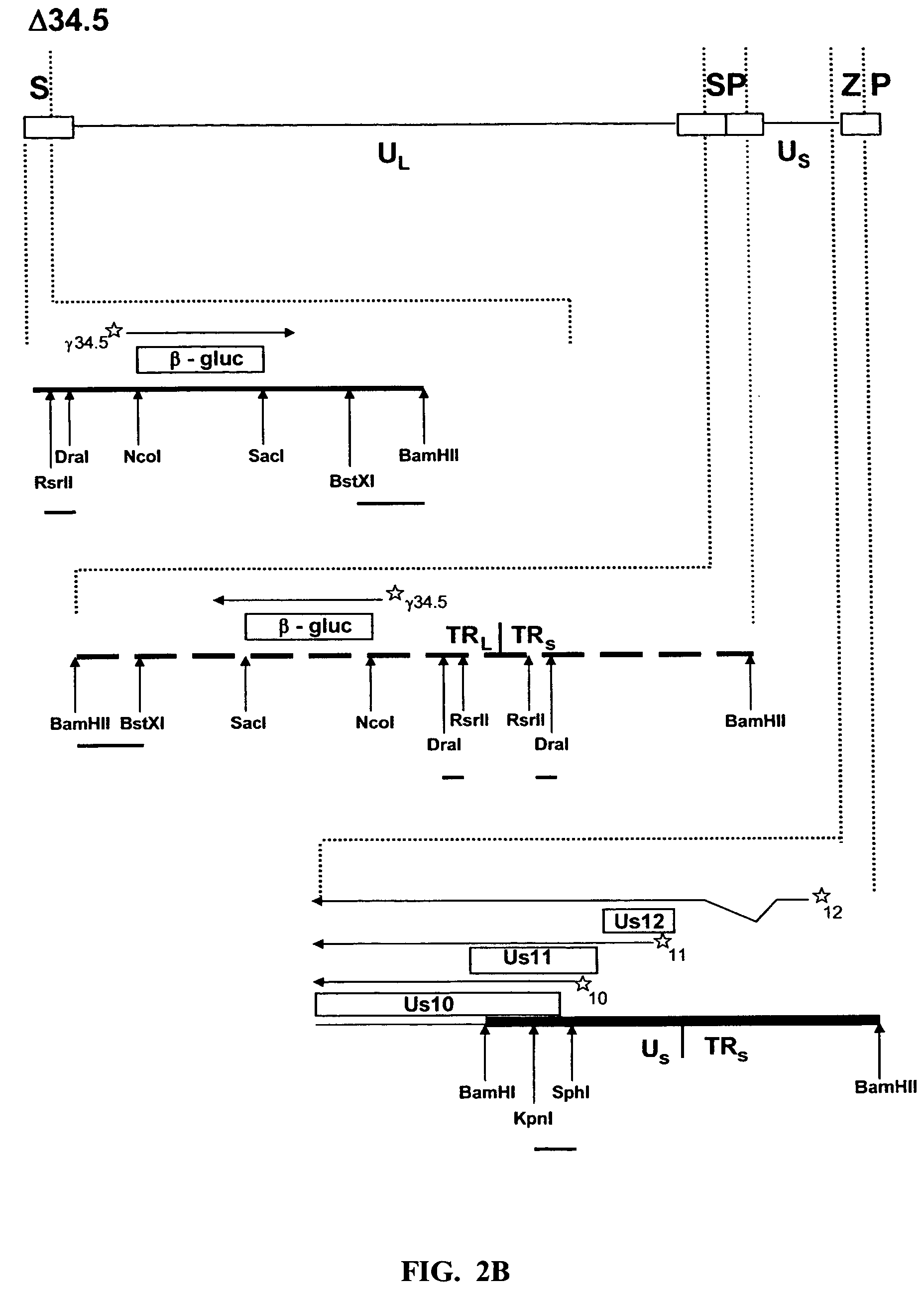
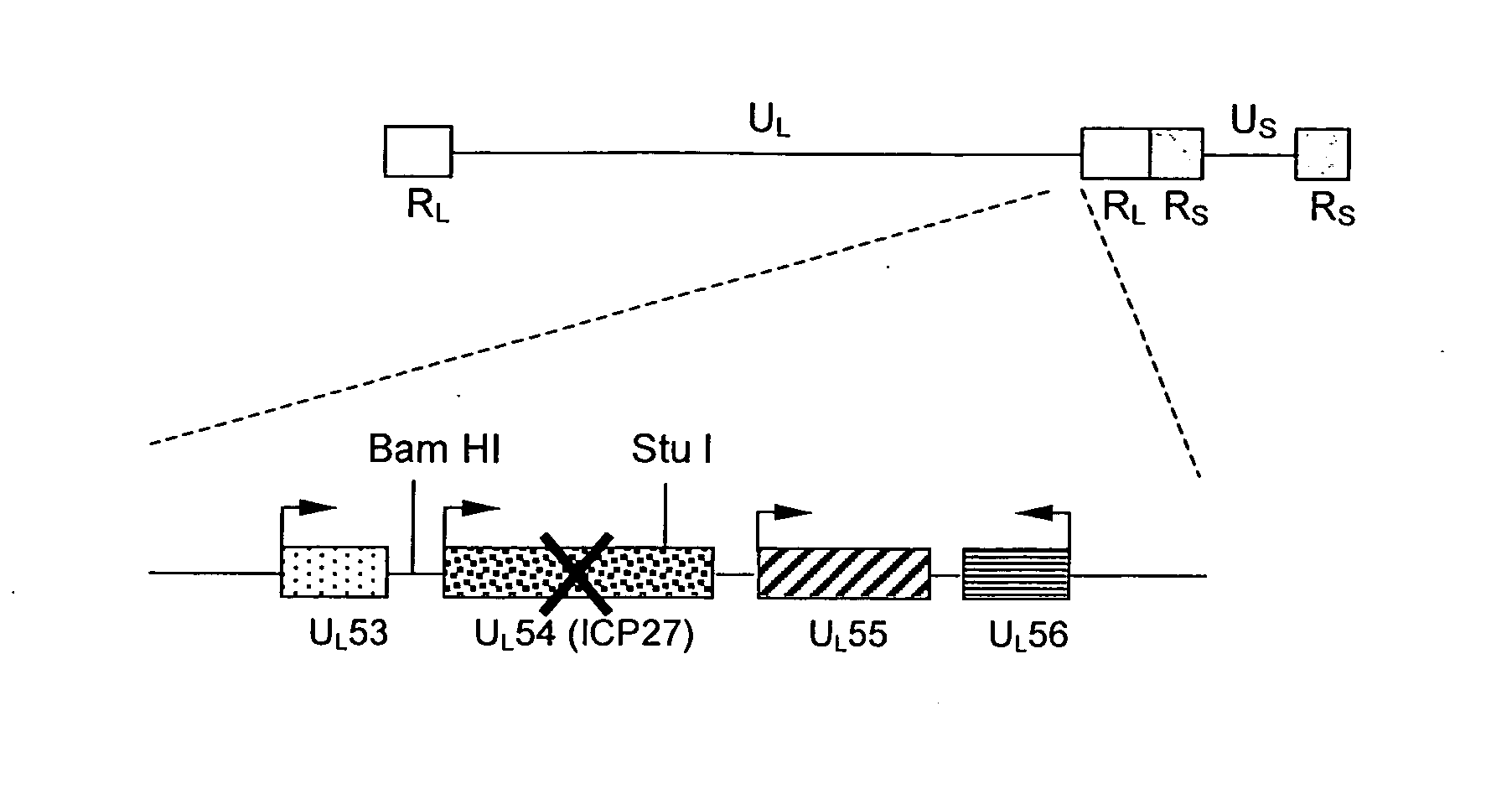
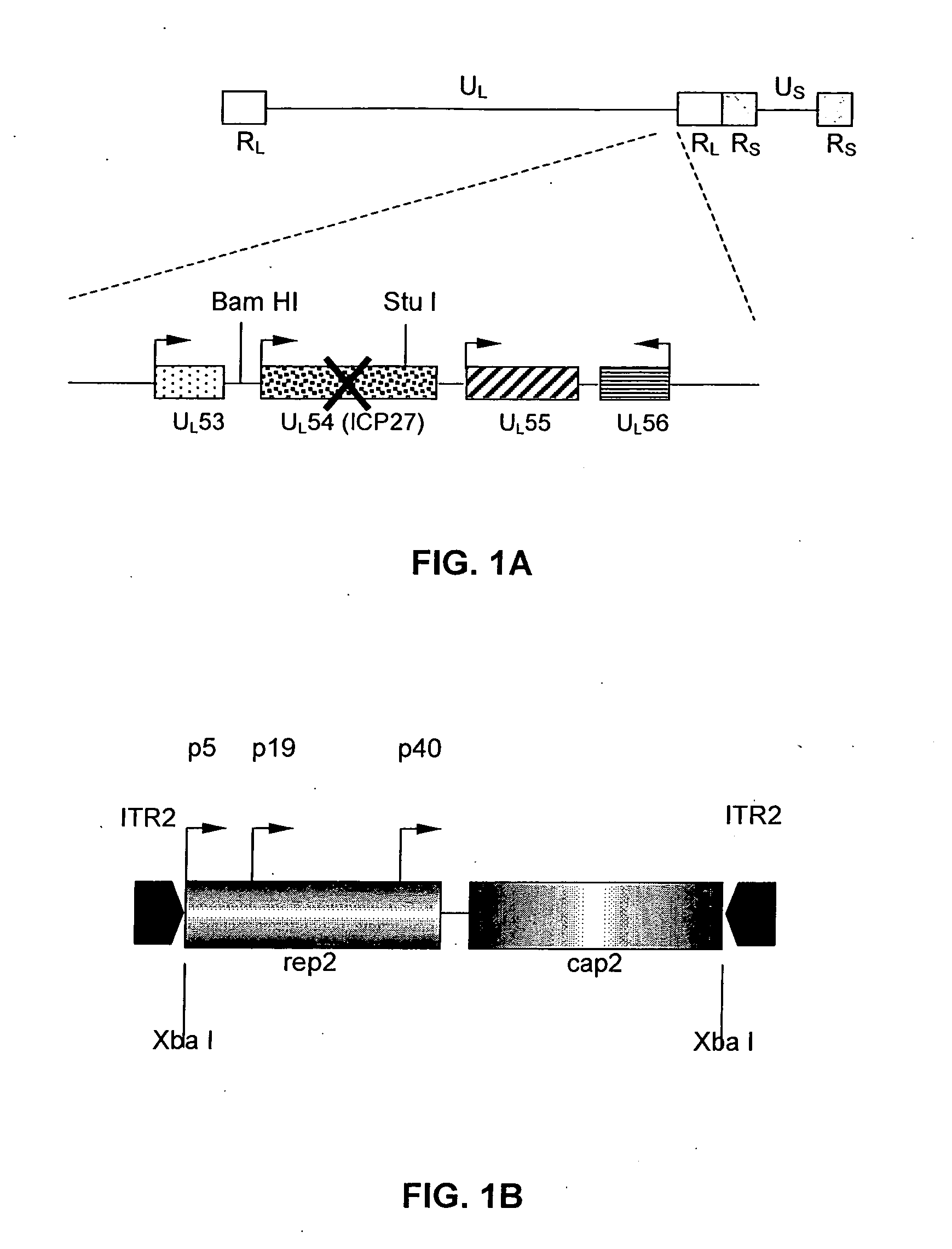
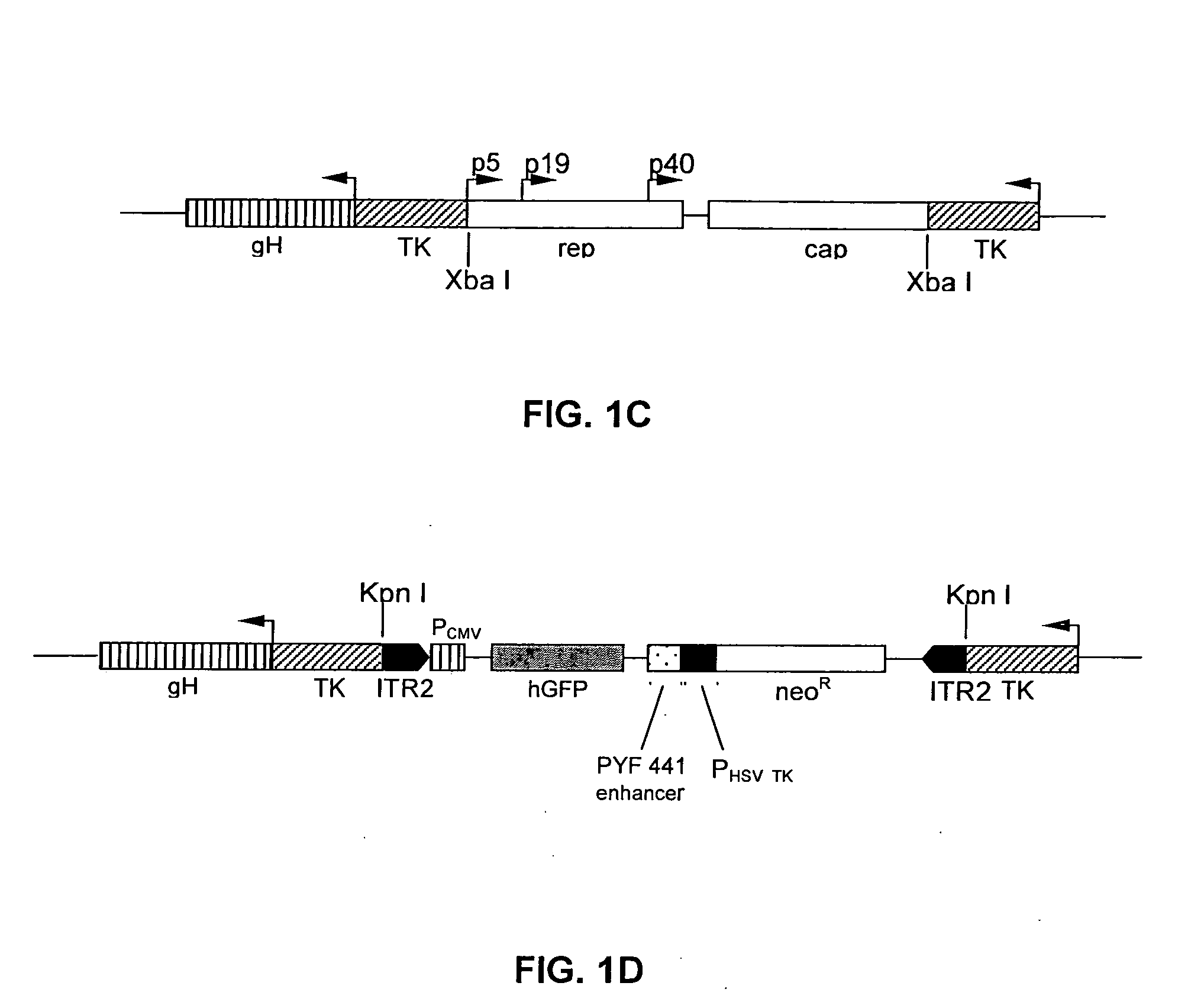
The present invention provides a herpes simplex virus strain having a functional ICP27 gene and lacking at lease a functional ICP4 gene and a functional ICP34.5 gene. It also provides the use of a herpes simplex virus strain which lacks at least a functional ICP4 gene and a functional ICP34.5 gene in the treatment of disorders of, or injuries to, the nervous system of a mammal.
Owner:BIOVEX LTD
Vaccine for treatment and prevention of herpes simplex virus infection
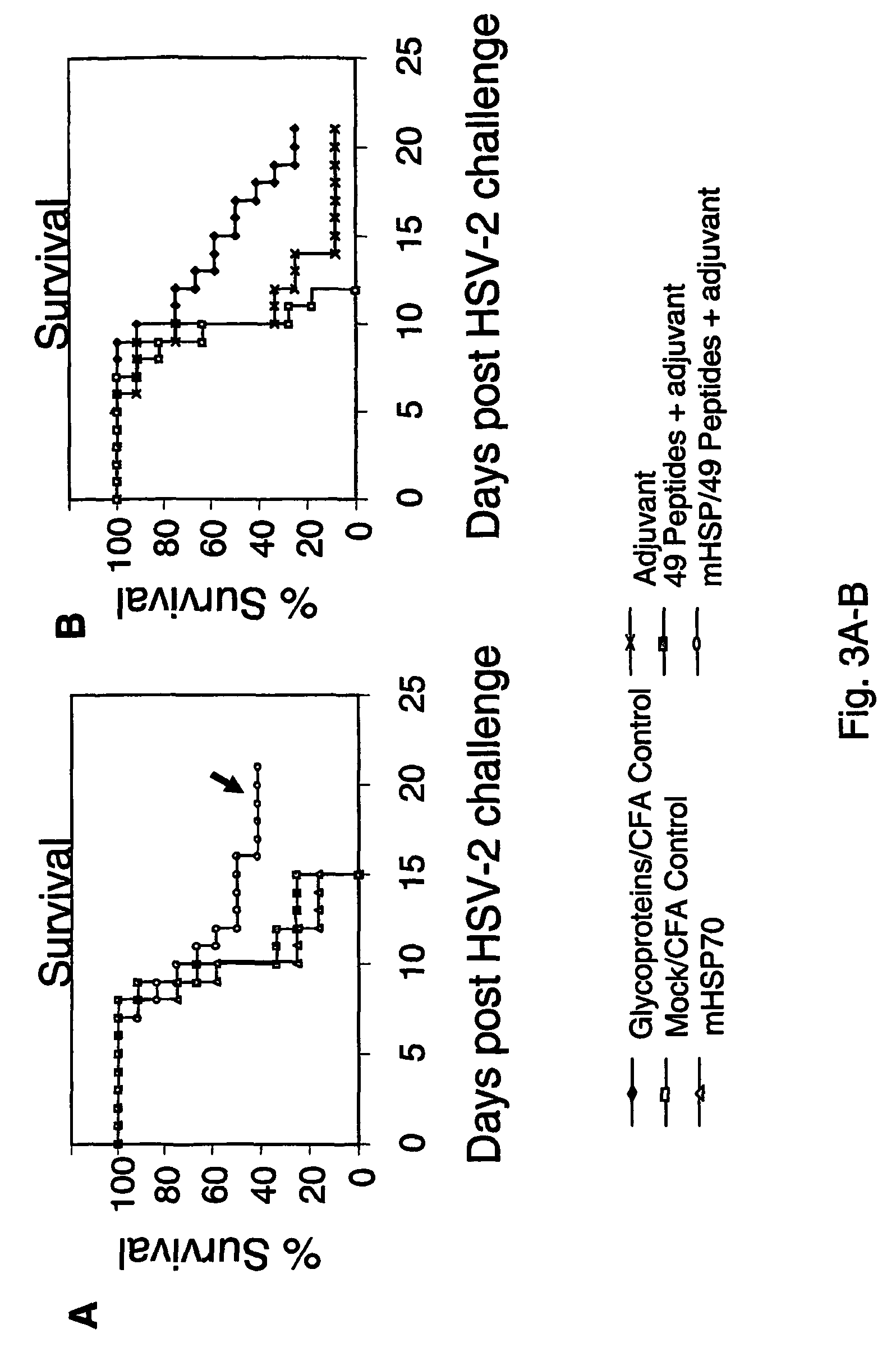
The present invention relates to methods and compositions for the prevention and treatment of herpes virus infections. The invention provides antigenic peptides, and pharmaceutical compositions comprising complexes of antigenic peptides and adjuvants that can activate an immune response against herpes viruses. The invention also provides methods of making the antigenic peptides and complexes of antigenic peptides and adjuvants. Methods of use of the pharmaceutical compositions are also provided.
Owner:AGENUS INC
Recombinant HVT vectors expressing antigens of avian pathogens and uses thereof
ActiveUS9114108B2Effective protectionSsRNA viruses negative-senseViral antigen ingredientsAntigenFowl
The present invention provides recombinant herpesvirus of turkeys (HVT) vectors that contain and express antigens of avian pathogens, compositions comprising the recombinant HVT vectors, polyvalent vaccines comprising the recombinant HVT vectors and one or more wild type viruses or recombinant vectors. The present invention further provides methods of vaccination against a variety of avian pathogens and method of producing the recombinant HVT vectors.
Owner:MERIAL INC
Acyclovir-peptide analogs
InactiveUS20050043246A1Sufficient hydrophilicityEffectively transported into ocular tissueBiocideSenses disorderMedicineHerpes simplex virus DNA
Dipeptide and tripeptide ester derivatives of acyclovir and its analogs are disclosed which are useful to treat herpes virus infections. Also disclosed is a method for preparing a therapeutic agent for targeted delivery to ocular tissue comprising linking the therapeutic agent to one or more groups of the formula —X—Y-Z(n)-R; wherein each X, Y and Z is independently Met, Val, Thr, Tyr, Trp, Ser, Ala or Gly; each R is independently H or an amino-protecting group; and each n is independently 0 or 1.
Owner:UNIVERSITY OF MISSOURI
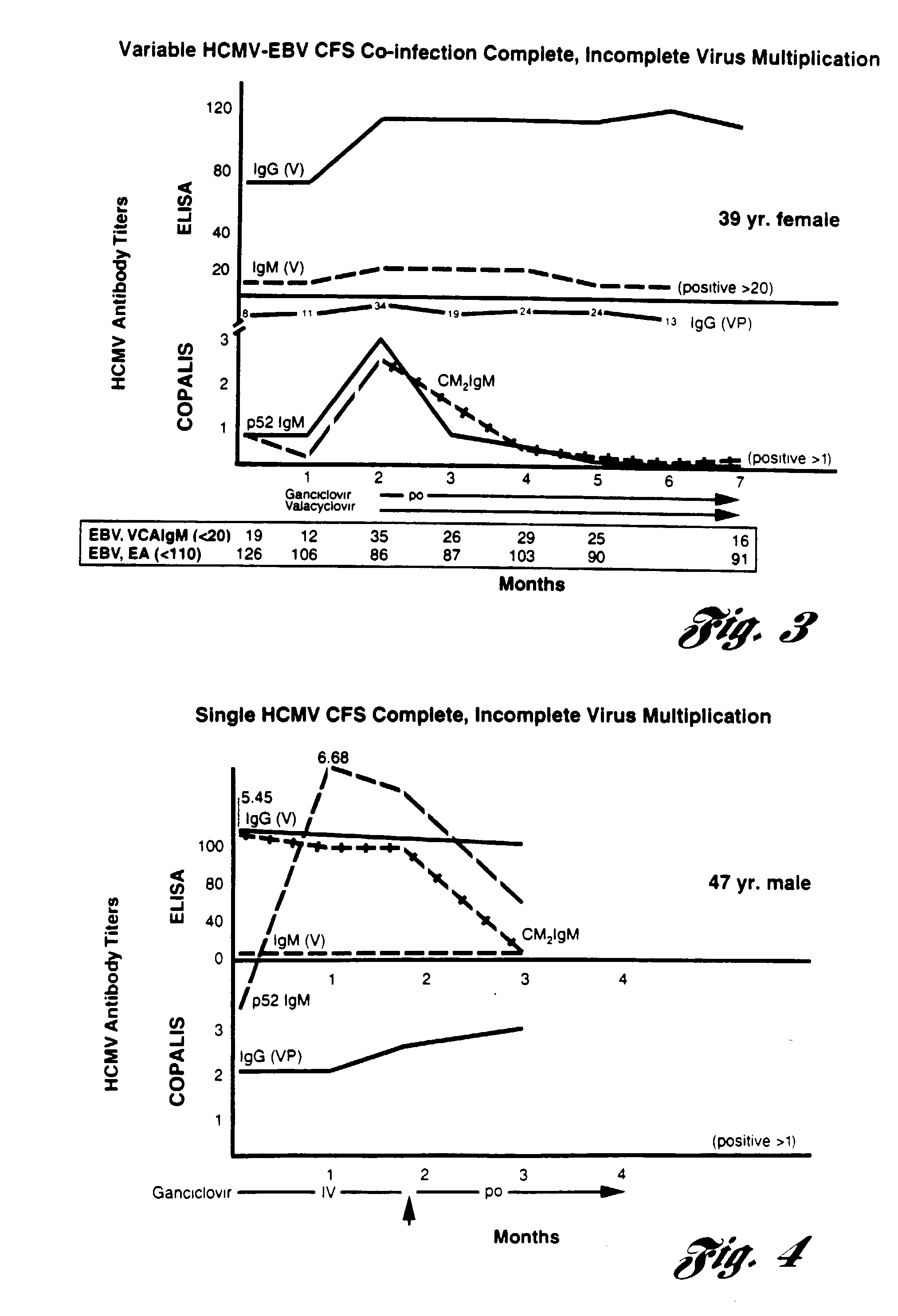
Method for diagnosing and alleviating the symptoms of chronic fatigue syndrome
InactiveUS20040072144A1Relieve symptomsEfficient multiplicationBiocideMicrobiological testing/measurementVirus multiplicationClinical trial
A method for alleviating chronic fatigue syndrome with the administration of antiviral agents. Based on clinical tests, chronic fatigue syndrome is a persistent herpes virus infection including incomplete virus multiplication and thus administration of antiviral agents are shown to alleviate the symptoms associated with the disorder. Based on therapeutic trials, patients receiving the recommended antiviral treatment, have experienced significant reduction or elimination of the symptoms associated with chronic fatigue syndrome. A method of diagnosis of the chronic fatigue syndrome is further disclosed.
Owner:LERNER A MARTIN
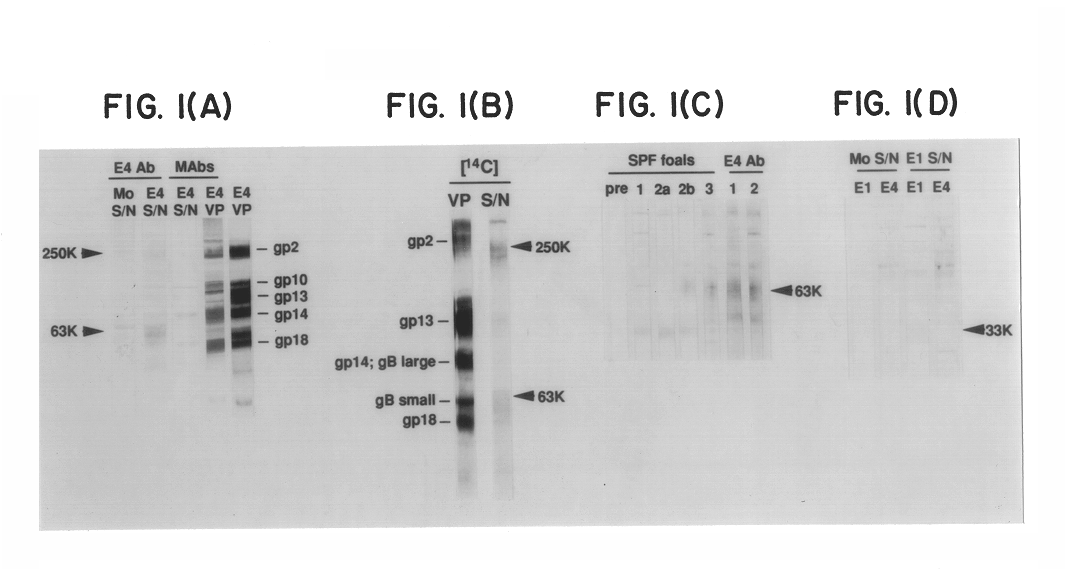
Equine herpesvirus glycoproteins
InactiveUS6544526B1Peptide/protein ingredientsViral antigen ingredientsHerpes simplex virus DNAEquine herpesvirus
A vaccine for the selective immunization of horses against EHV4 and / or EHV1 is provided comprising at least one of (i) EHV4 virus wherein a portion of the gG gene of the EHV4 virus that elicits a type-specific response to EHV4 has been deleted and (ii) EHV1 virus wherein a portion of the gG gene of the EHV1 virus that elicits a type-specific response to EHV1 has been deleted. Antibodies which specifically bind to a epitopes of EHV4 gG or EHV1 also are provided.
Owner:UNIVERSITY OF MELBOURNE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com