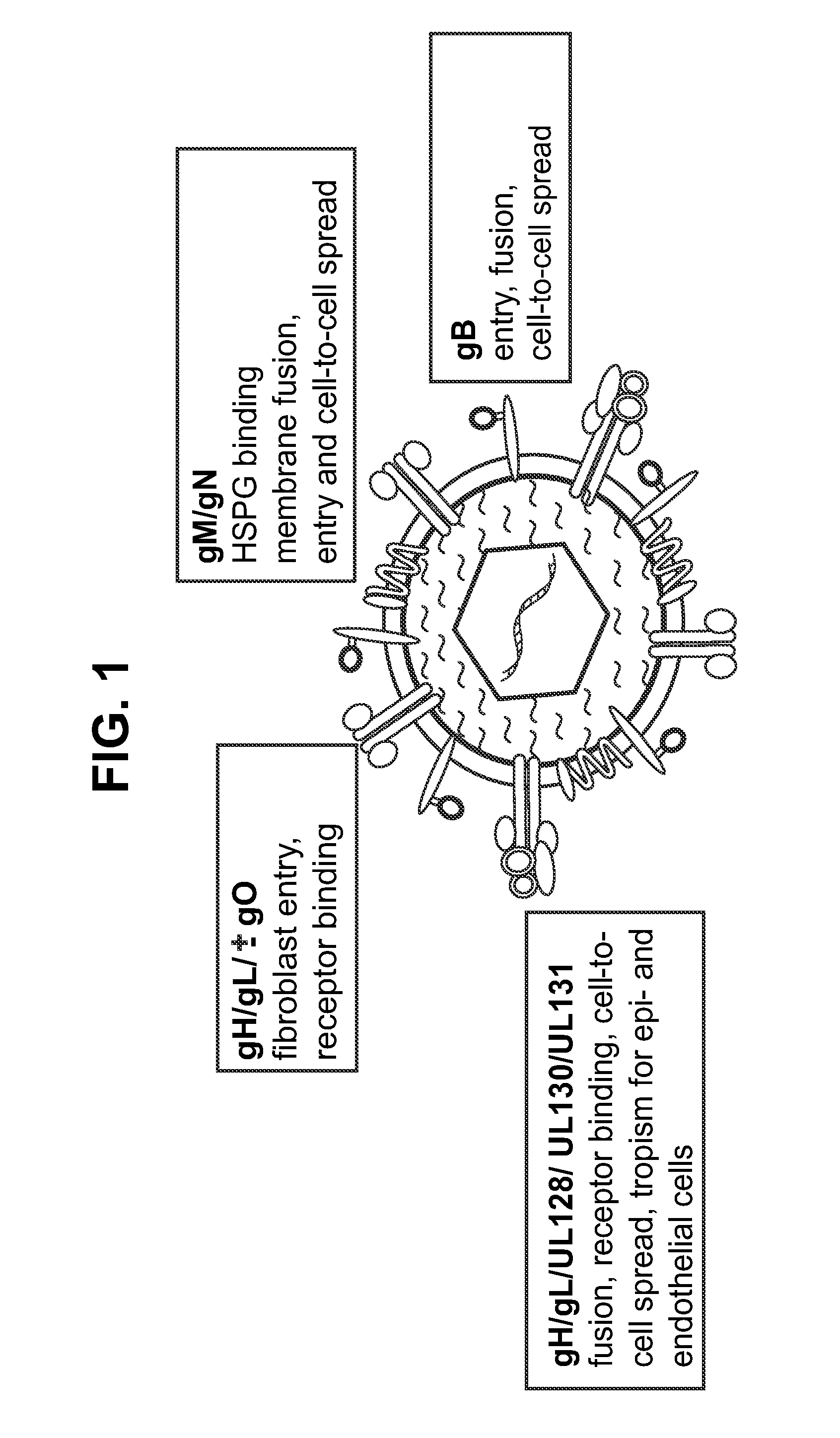
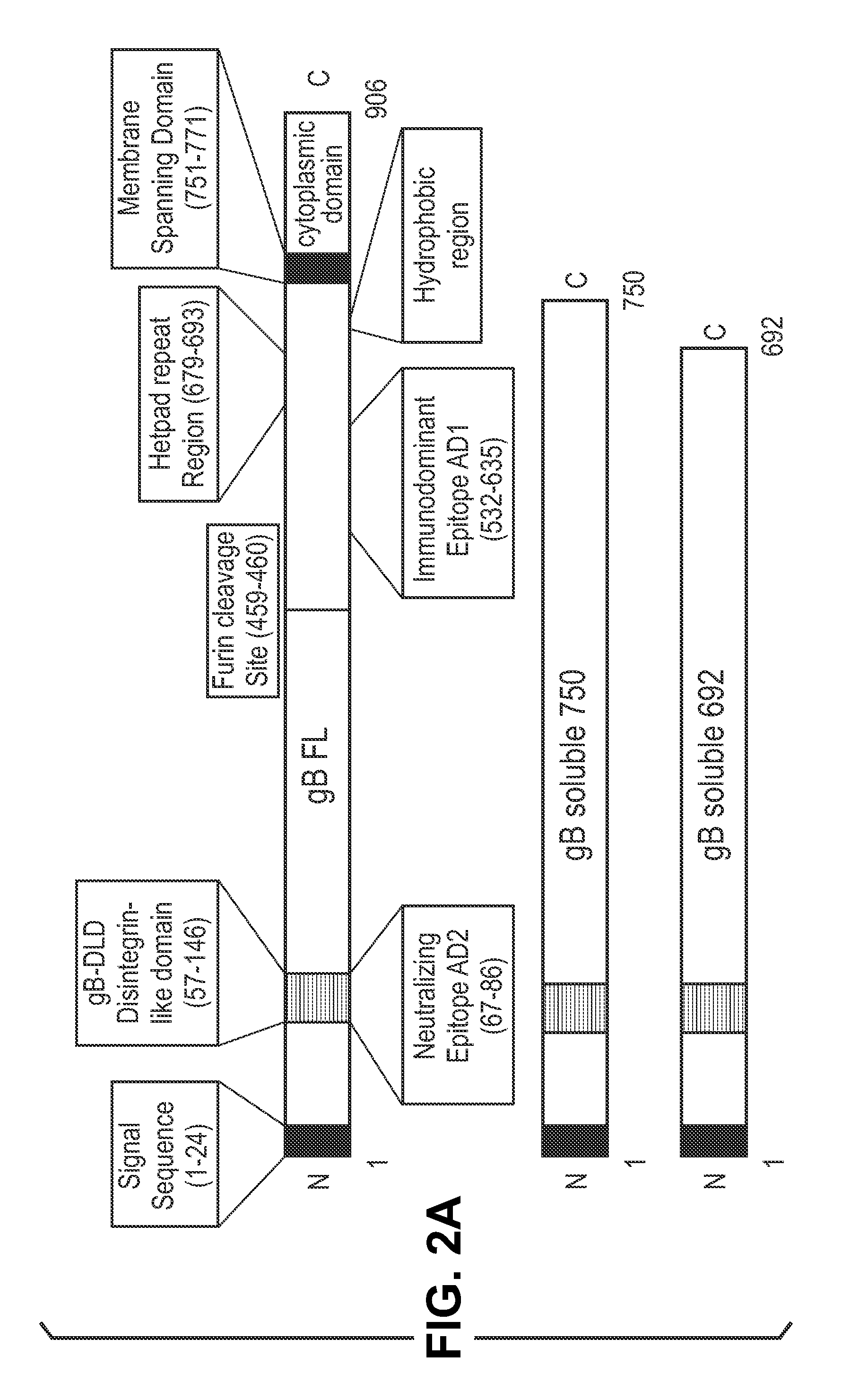
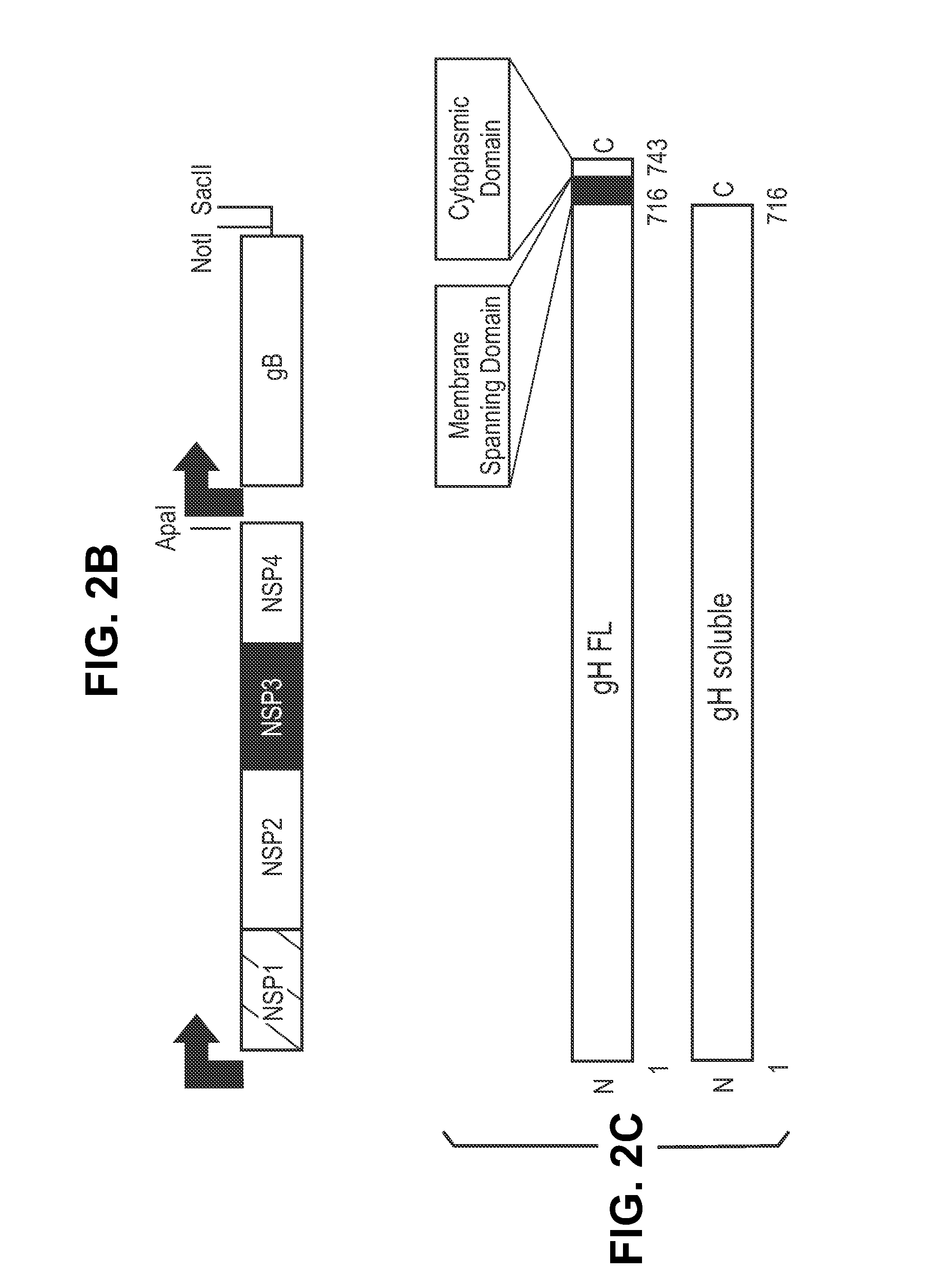
Antigen delivery platforms
a technology of antigens and platforms, applied in the direction of viruses, drug compositions, immunological disorders, etc., can solve the problems of devastating defects in neurological development in neonates, substantial morbidity and mortality, and substantial morbidity and mortality, and achieve the effect of inhibiting the entry of cmv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Delivery of Individual CMV Antigens Using a VRP Platform
[0211]Each of CMV glycoproteins gB and gH induce neutralizing responses, and gB is the dominant antigen among antibodies in human sera that neutralize infection of fibroblasts (Britt et al. (1990) J. Virol. 64(3):1079-85). The following experiments demonstrate in mice a neutralizing response against these antigens delivered using a VRP platform.
[0212]Each CMV antigen was cloned into a pcDNA-6H is vector (Invitrogen) and tested for protein expression before cloning into an alphavirus replicon vector, pVCR 2.1 SalI / XbaI derived from the plasmid described by Perri et al. (J. Virol 77(19)10394-10403 (2003)) producing the constructs shown in FIG. 2. pVCR 2.1 SalI / XbaI is a self-replicating RNA vector that, when electroporated with defective helper capsid and glycoprotein RNA, forms an infectious alphavirus particle.
[0213]pVCR vectors were used to make RNA which was electroporated into baby hamster kidney (BHKV) cells in the presence...
example 2
Construction of Polycistronic Alphavirus Vectors
[0219]CMV produces several multi-protein complexes during infection. To determine whether a single replicon expressing all components of a desired complex can be used to produce the CMV complex in a subject, or whether components of the complex could be co-delivered from multiple replicon vectors, we designed a platform that allows controlled expression of multiple CMV proteins.
[0220]An alphavirus vector (pVCR 2.1 SalI / XbaI) was modified to allow assembly of multiple subgenomic promoters (SGP) and genes of interest (GOI). pVCR 2.1SalI / XbaI ApaI site at 11026-31 bp was changed from GGGCCC (SEQ ID NO:______) to GGCGCC (SEQ ID NO:______). ClaI and PmlI restriction sites added in the region immediately downstream of the first subgenomic promoter and SalI-XbaI insert sites. The sequence at 7727-7754 bp was changed from ctcgatgtacttccgaggaactgatgtg (SEQ ID NO:______) to ATCGATGTACTTCCGAGGAACTCACGTG (SEQ ID NO:______).
[0221]A shuttling vector...
example 3
Production of CMV Complexes
[0232]This example demonstrates that CMV protein complexes can be formed in a cell after delivery of the complex components from a polycistronic alphavirus replicon vector.
[0233]gH / gL and gH / gL / gO Complexes
[0234]Polycistronic gH / gL and gH / gL / gO alphavirus replicons were constructed as described above (shown schematically in FIG. 5A). VRPs containing gH, gL, gO, gH / gL and gH / gL / gO encoding replicons were produced in BHKV cells as described above and used to infect BHKV cells to demonstrate complex formation in vitro. VRP infected ARPE-19 cells produced disulfide linked complexes of gH / gL. gO did not detectably alter gH / gL association (FIG. 5B).
[0235]Immunofluorescence studies were conducted to evaluate the localization of gH and gL delivered alone and when delivered using a polycistronic alphavirus to look at relocalization of the proteins when co-expressed. gH localization did not appear to change in the presence or absence of gL, or gL / gO. gL localization...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com