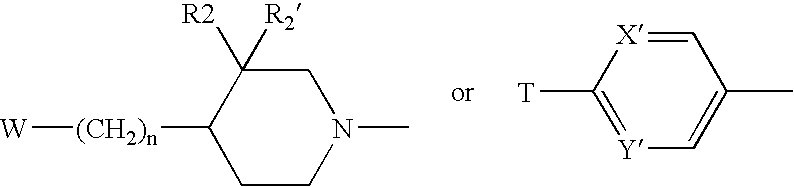Patents
Literature
154 results about "Alphavirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
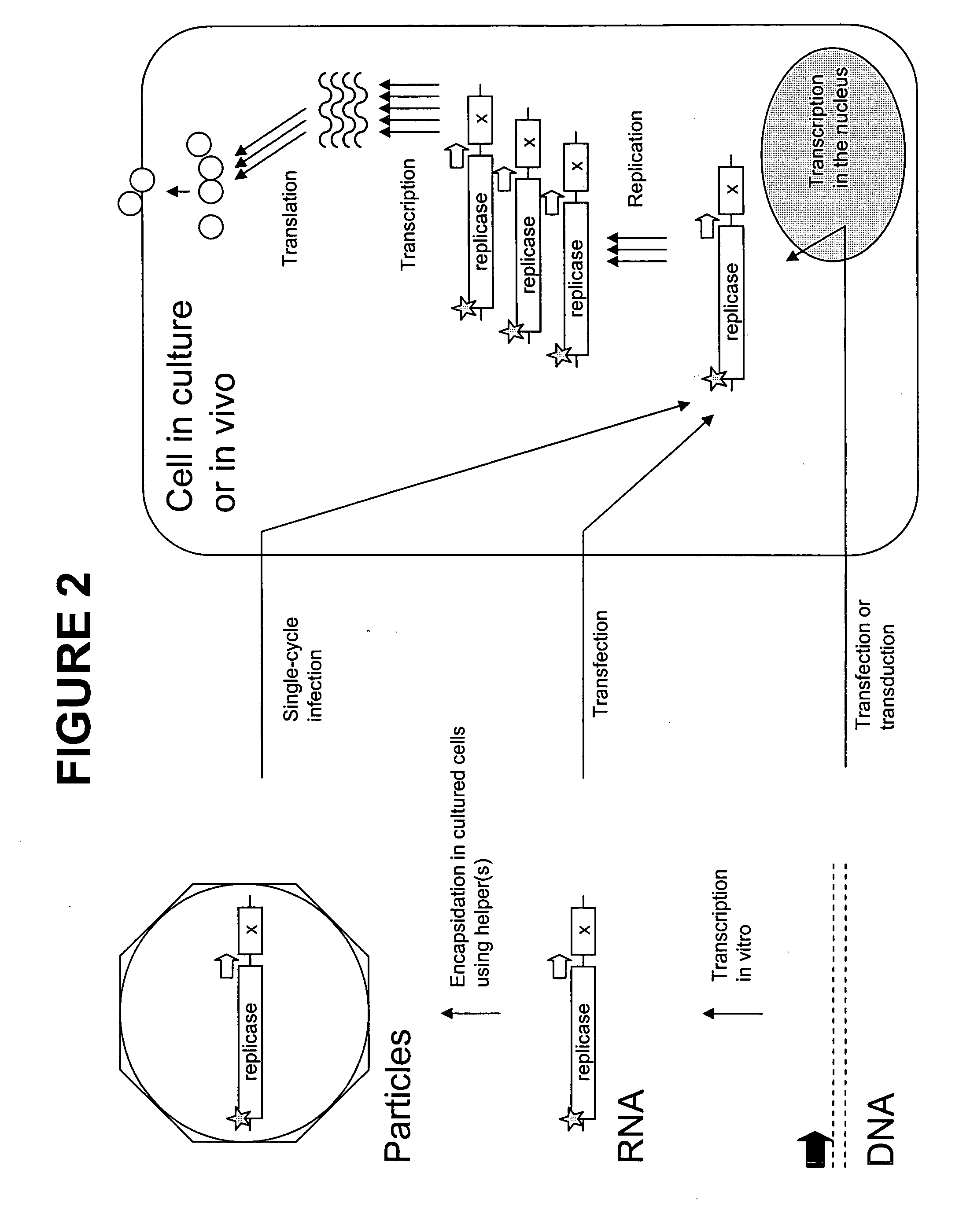
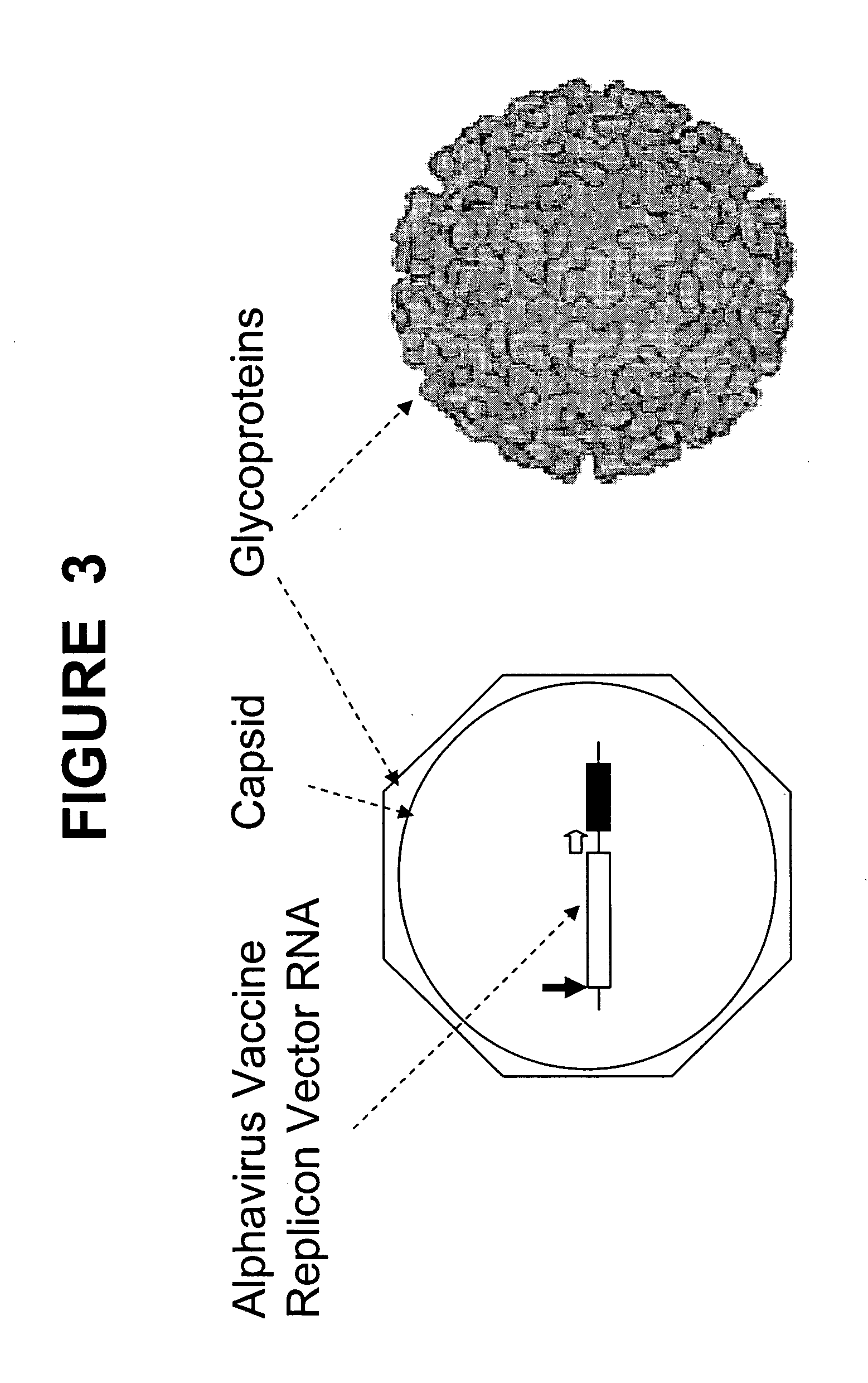
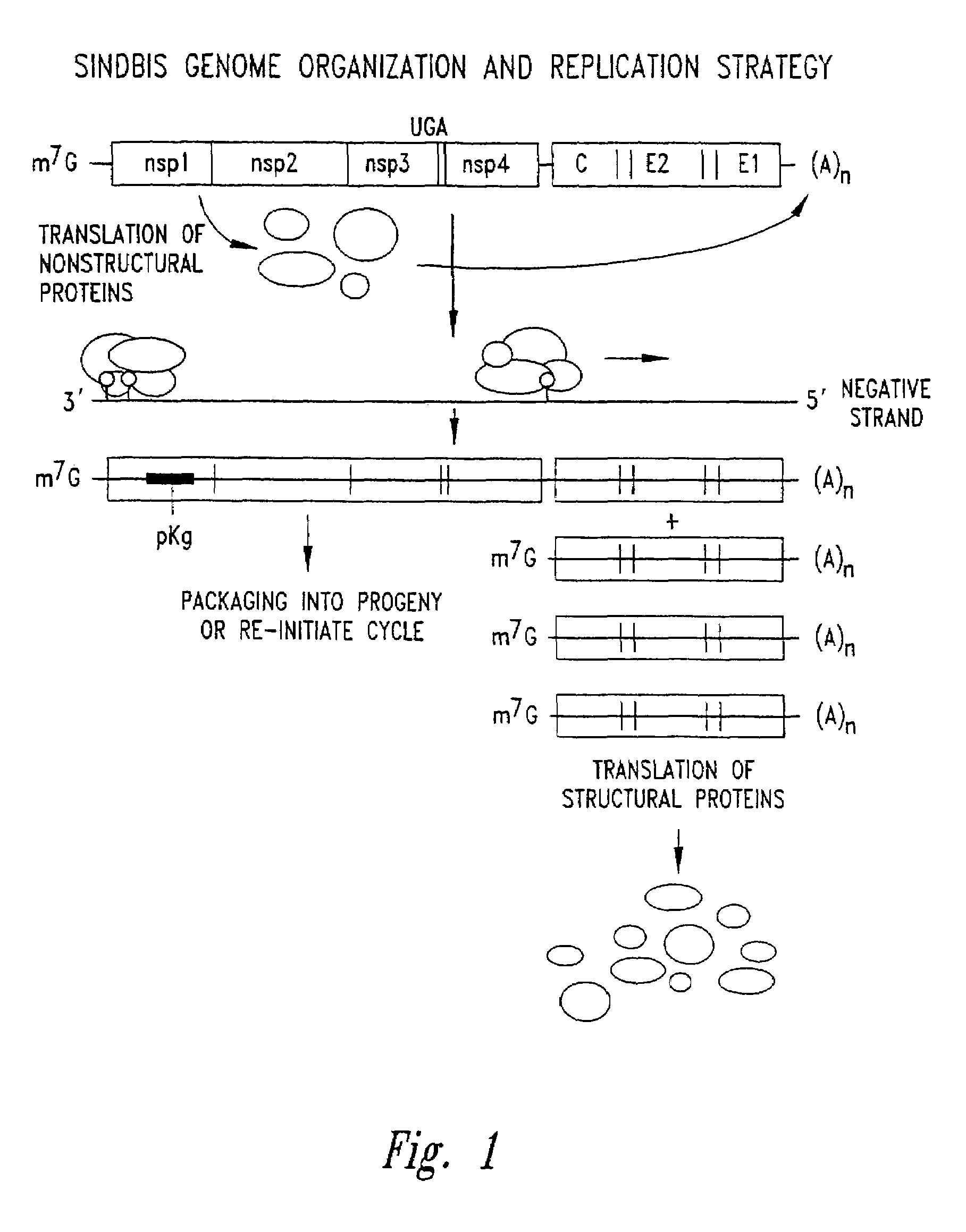
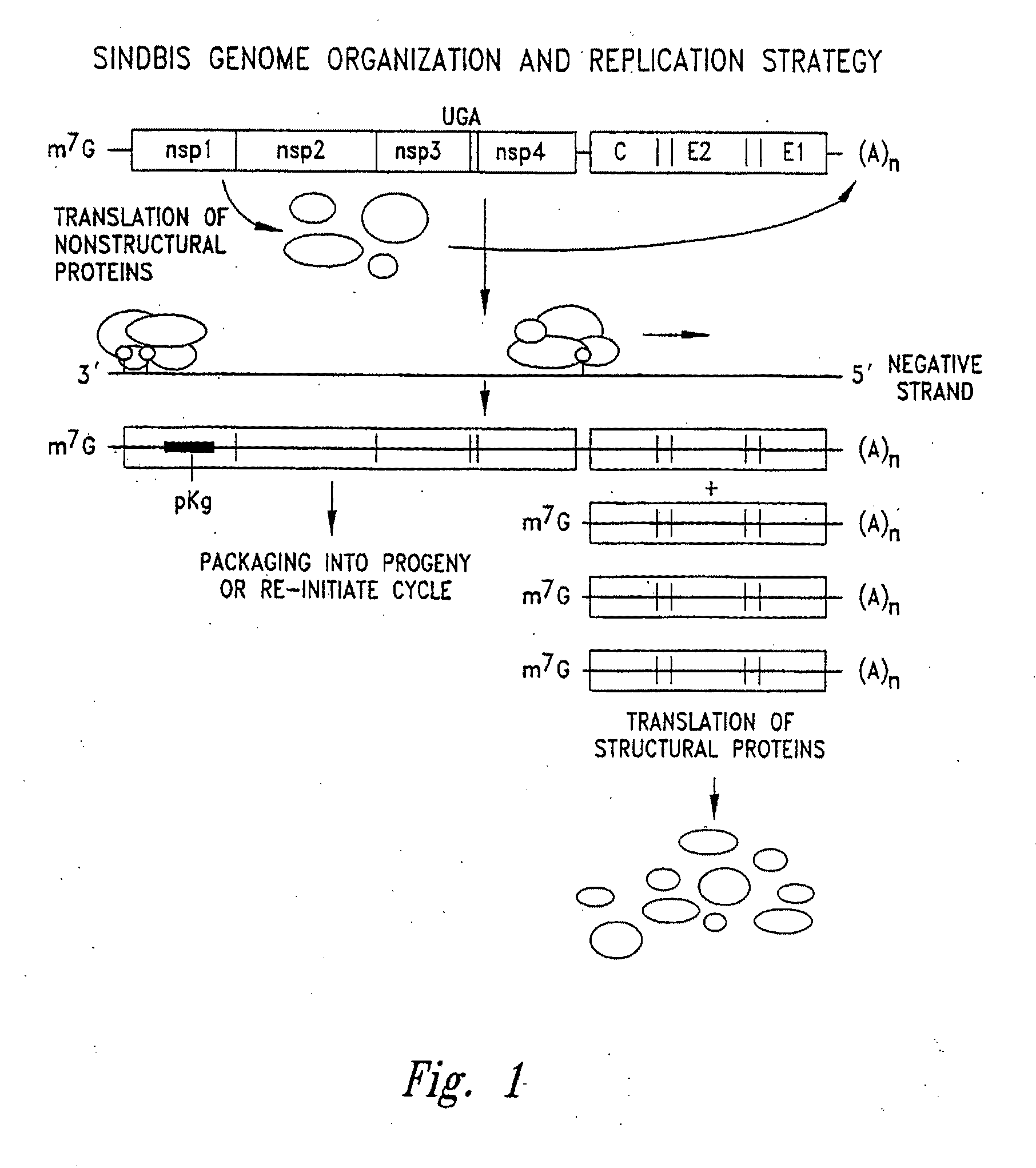
In biology and immunology, an Alphavirus belongs to group IV of the Baltimore classification of the Togaviridae family of viruses, according to the system of classification based on viral genome composition introduced by David Baltimore in 1971. Alphaviruses, like all other group IV viruses, have a positive sense, single-stranded RNA genome. There are thirty alphaviruses able to infect various vertebrates such as humans, rodents, fish, birds, and larger mammals such as horses as well as invertebrates. Transmission between species and individuals occurs mainly via mosquitoes, making the alphaviruses a member of the collection of arboviruses – or arthropod-borne viruses. Alphavirus particles are enveloped, have a 70 nm diameter, tend to be spherical (although slightly pleomorphic), and have a 40 nm isometric nucleocapsid.
Alphavirus particles and methods for preparation
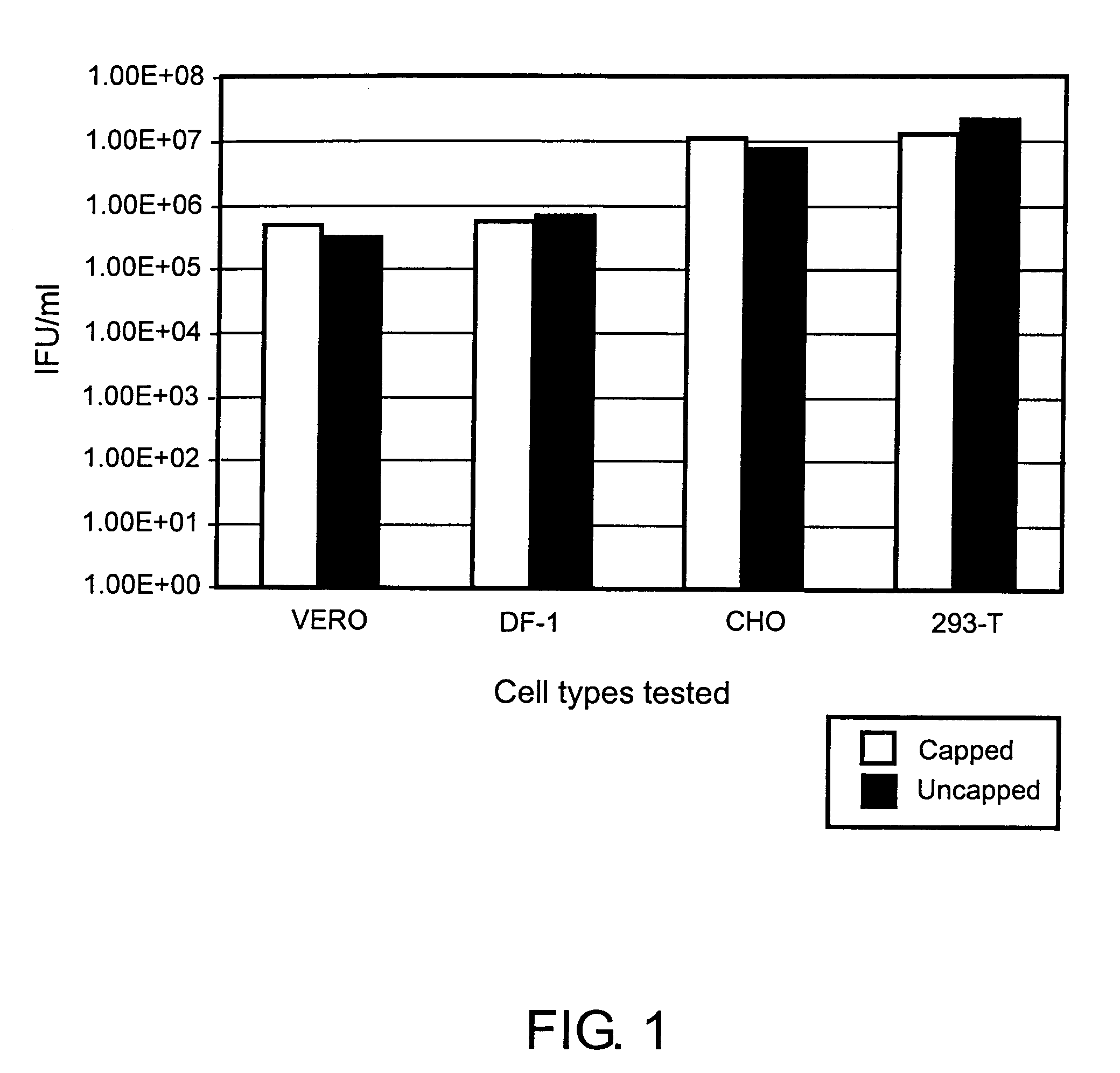
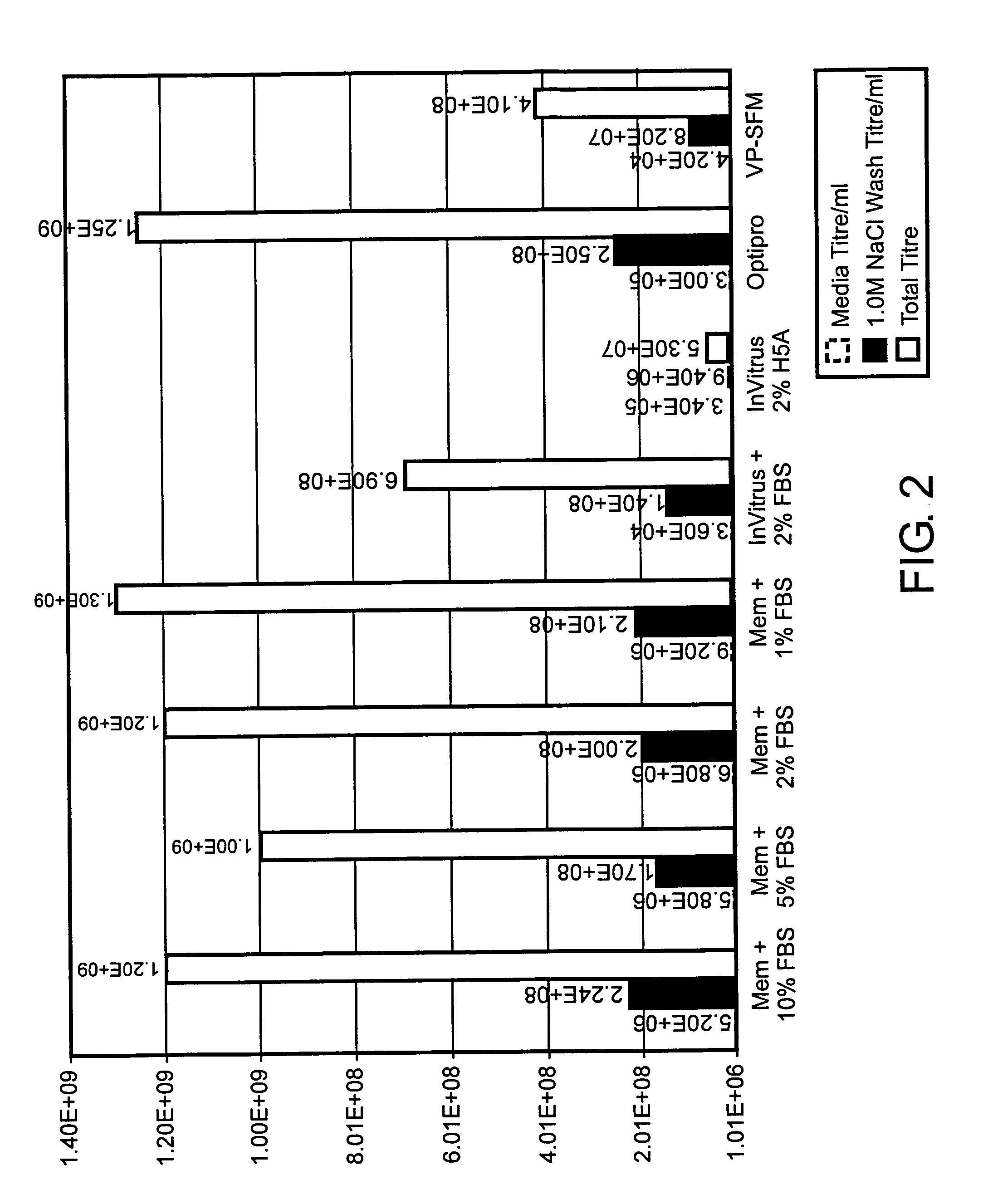
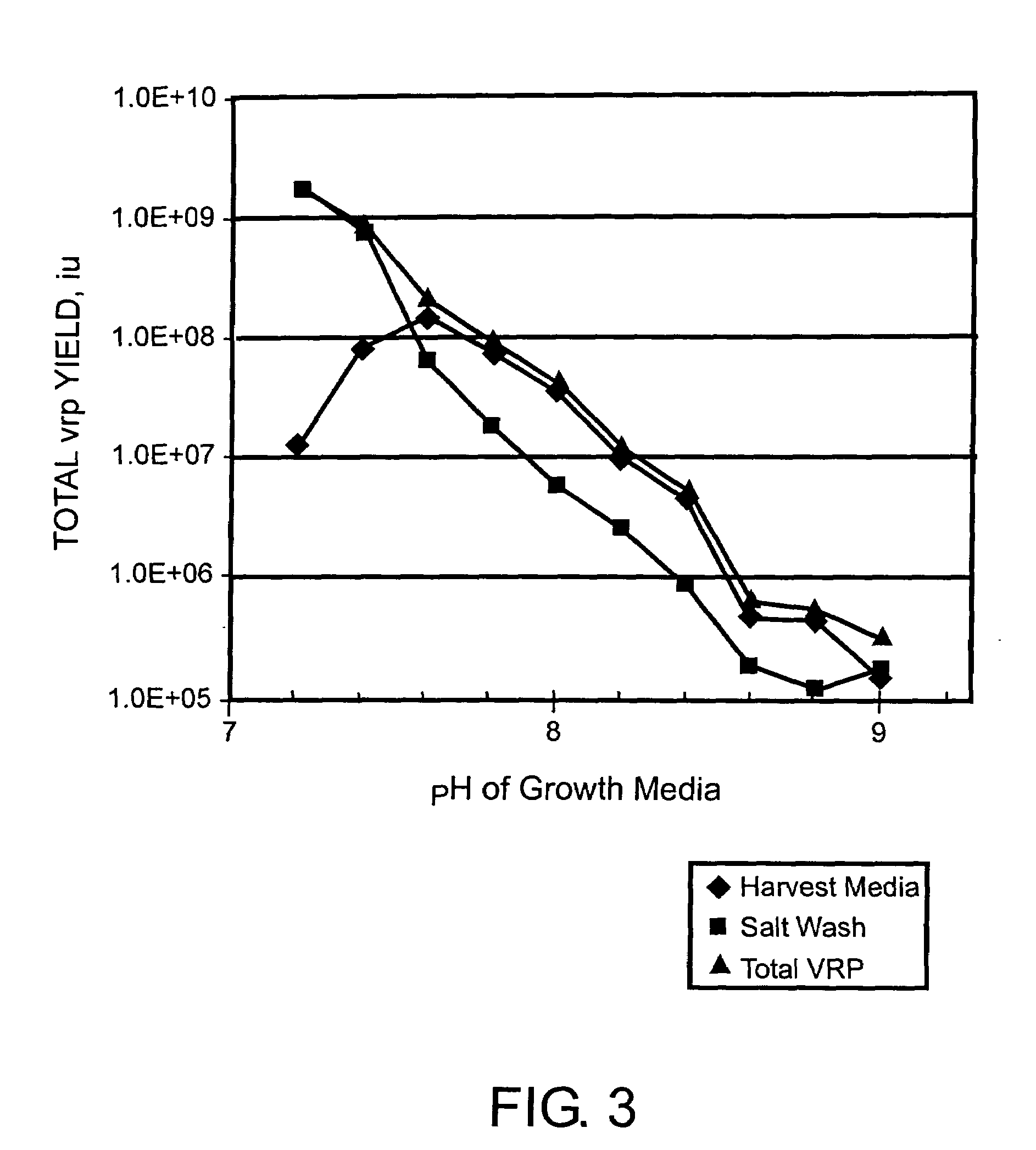
ActiveUS7078218B2Improve salt wash recoveryIncrease ionic strengthSsRNA viruses positive-senseGenetic material ingredientsHigh densityPotassium
Provided herein are methods for producing alphavirus replicon particles in high yield; replicon RNAs are electroporated into permissive cells, where the cells are at a relatively high density, together with at least one helper nucleic acid providing the necessary functions for packaging. After a growth period in appropriate medium, alphavirus replicon particles are harvested from the surfaces of the cells in which they were produced using a salt wash in which the salt concentration is from about 0.2 to about 5 M sodium chloride, calcium chloride, magnesium chloride, potassium chloride, ammonium acetate, ammonium bicarbonate, among others. After dilution, if necessary, the particles can be purified by a suitable chromatographic technique.
Owner:ALPHAVAX INC
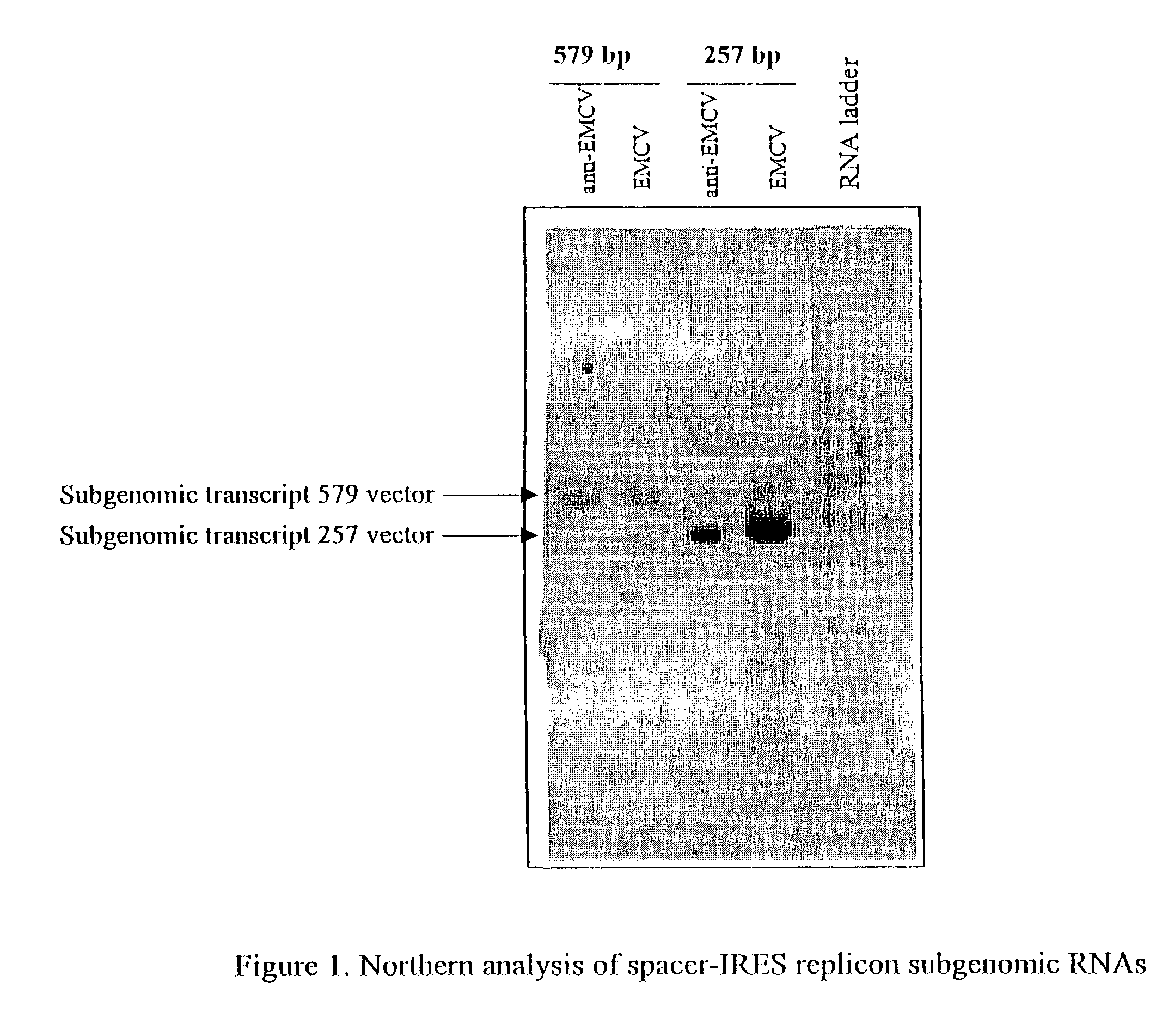
Alphavirus replicons and helper constructs
Owner:ALPHAVAX INC
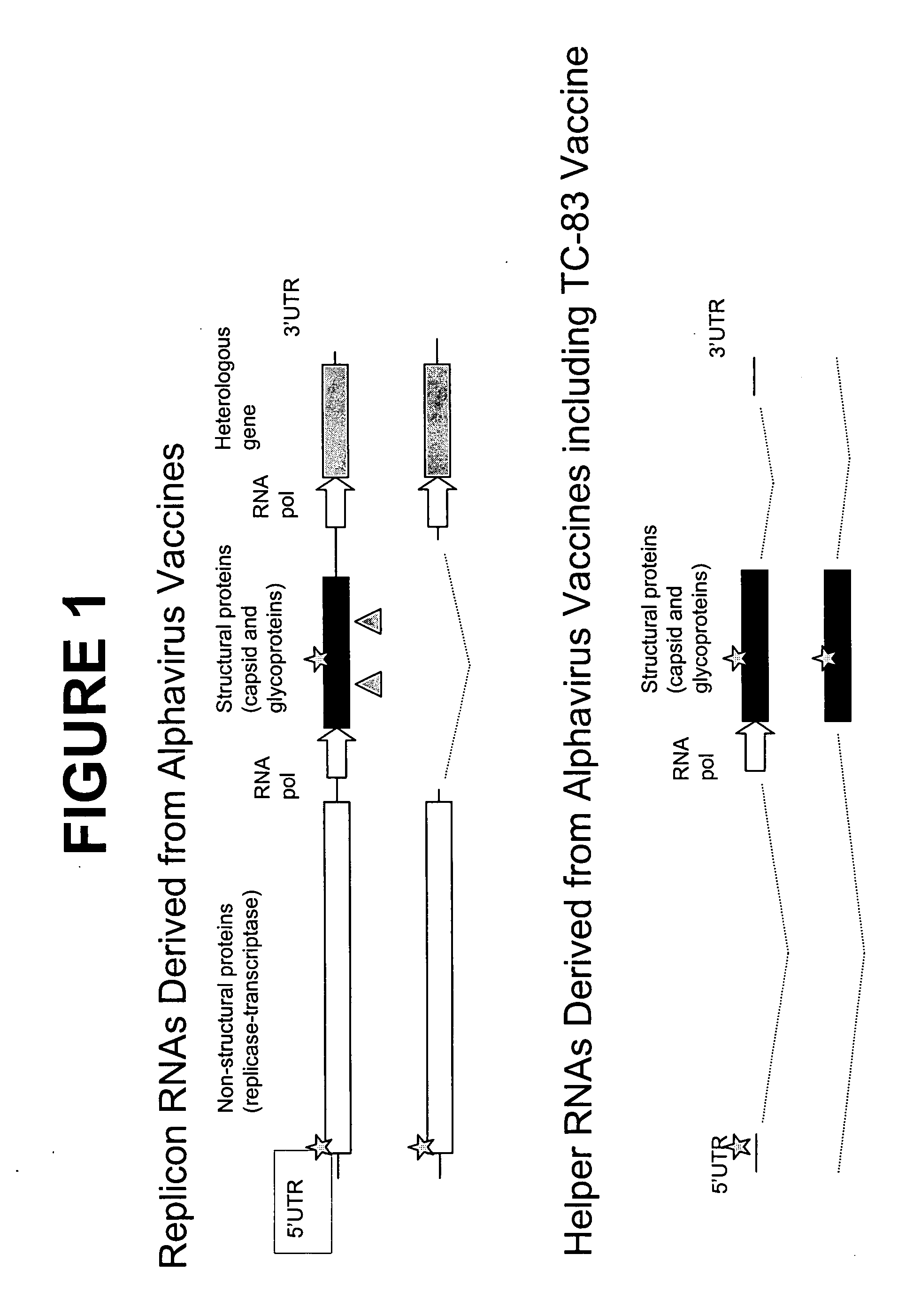
TC-83-derived alphavirus vectors, particles and methods
InactiveUS20050266550A1Reduce expressionMaximizing expressionSsRNA viruses positive-senseGenetic material ingredientsViral replicationImmunogenicity
The present disclosure provides TC-83 VEE-derived replicons, alphaviral replicon particles and immunogenic compositions containing TC-83 alphaviral replicon particles which direct the expression of at least one antigen when introduced into a suitable host cell. The TC-83 VEE-derived ARPs described herein are improved in that they are subject to a lower vector-specific immune response than prior art ARPs.
Owner:ALPHAVAX INC
RNA respiratory syncytial virus vaccines
InactiveUS6060308AFaster replicationImprove efficiencySsRNA viruses negative-senseBiocideF proteinViral Vaccine
A vector comprising a first DNA sequence which is complementary to at least part of an alphavirus RNA genome and having the complement of complete alphavirus DNA genome replication regions, a second DNA sequence encoding a paramyxovirus protein, particularly a respiratory syncytial virus fusion (RSV F) protein or a RSV F protein fragment that generates antibodies that specifically react with RSV F protein, the first and second DNA sequences being under the transcriptional control of a promoter is described. Such vector may be used to produce an RNA transcript which may be used to immunize a host, including a human host, to protect the host against disease caused by paramyxovirus, particularly respiratory syncytial virus, by administration to the host.
Owner:CONNAUGHT LAB
Virus like particle compositions and methods of use
ActiveUS20120003266A1Improve purification effectAvoid infectionFungiSsRNA viruses positive-senseVirus-like particleChikungunya fever
The invention features compositions and methods for the prevention or treatment of one or more strains of Chikungunya virus, as well as other alphavirus-mediated diseases.
Owner:UNITED STATES OF AMERICA
Alphavirus replicons and helper constructs
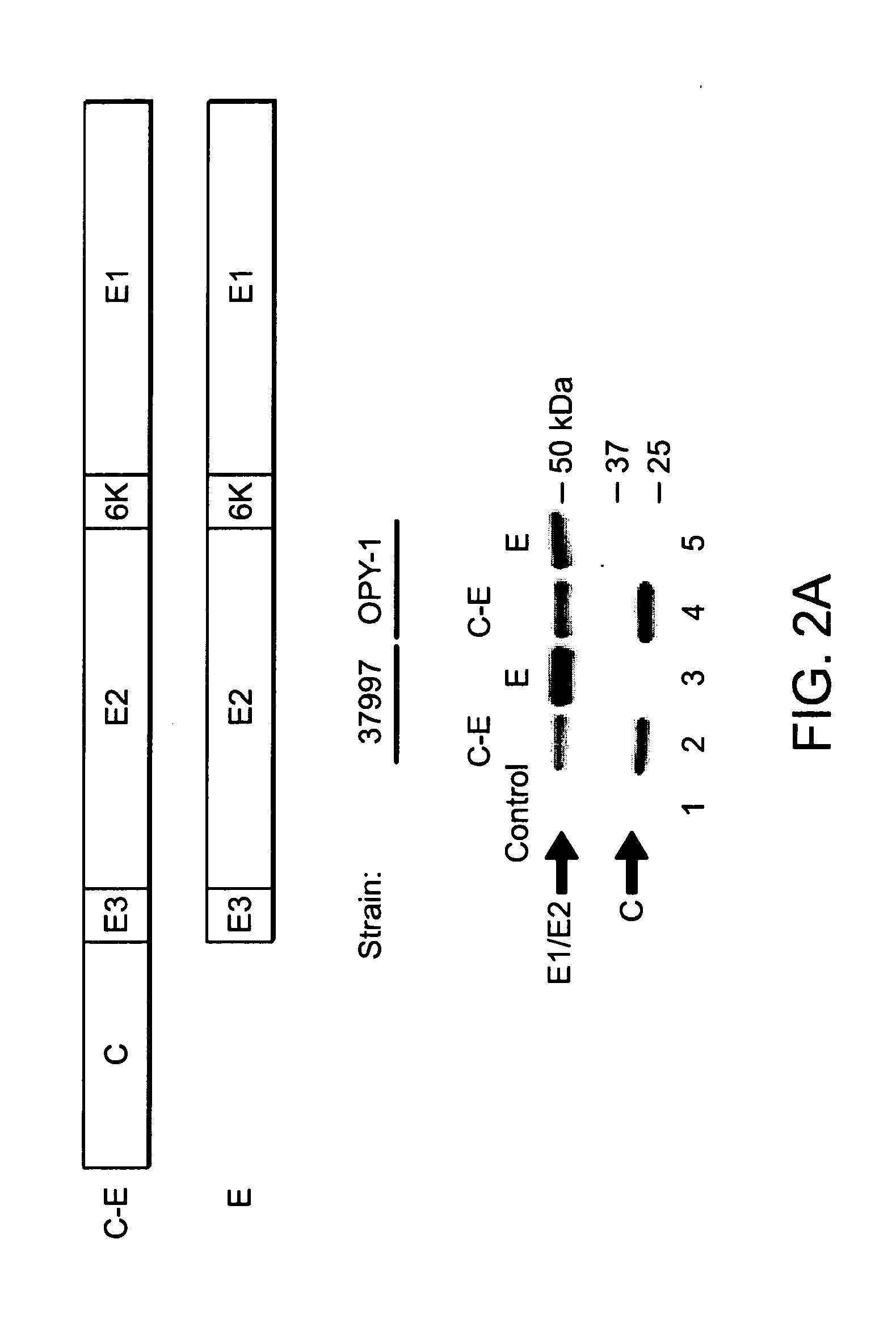
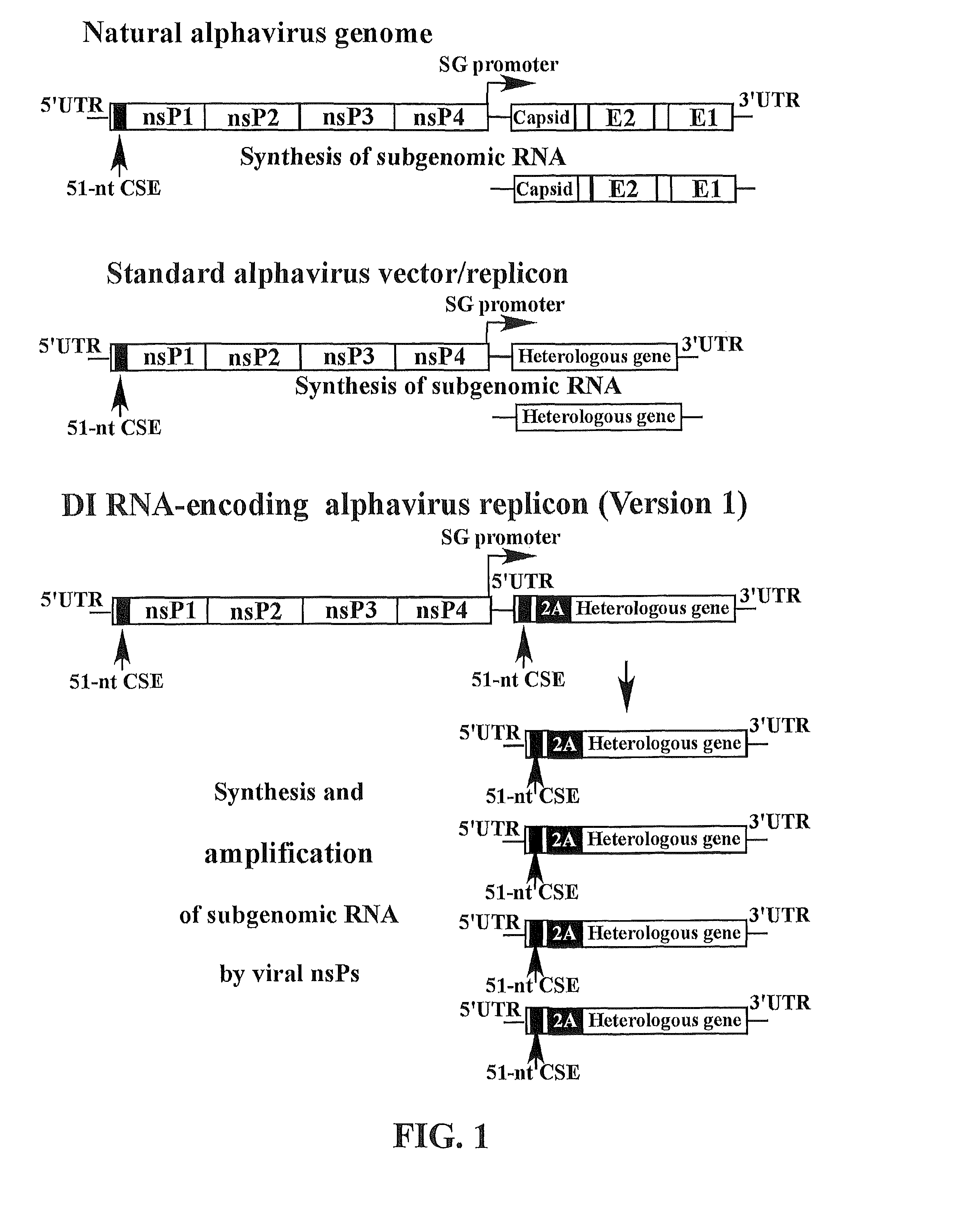
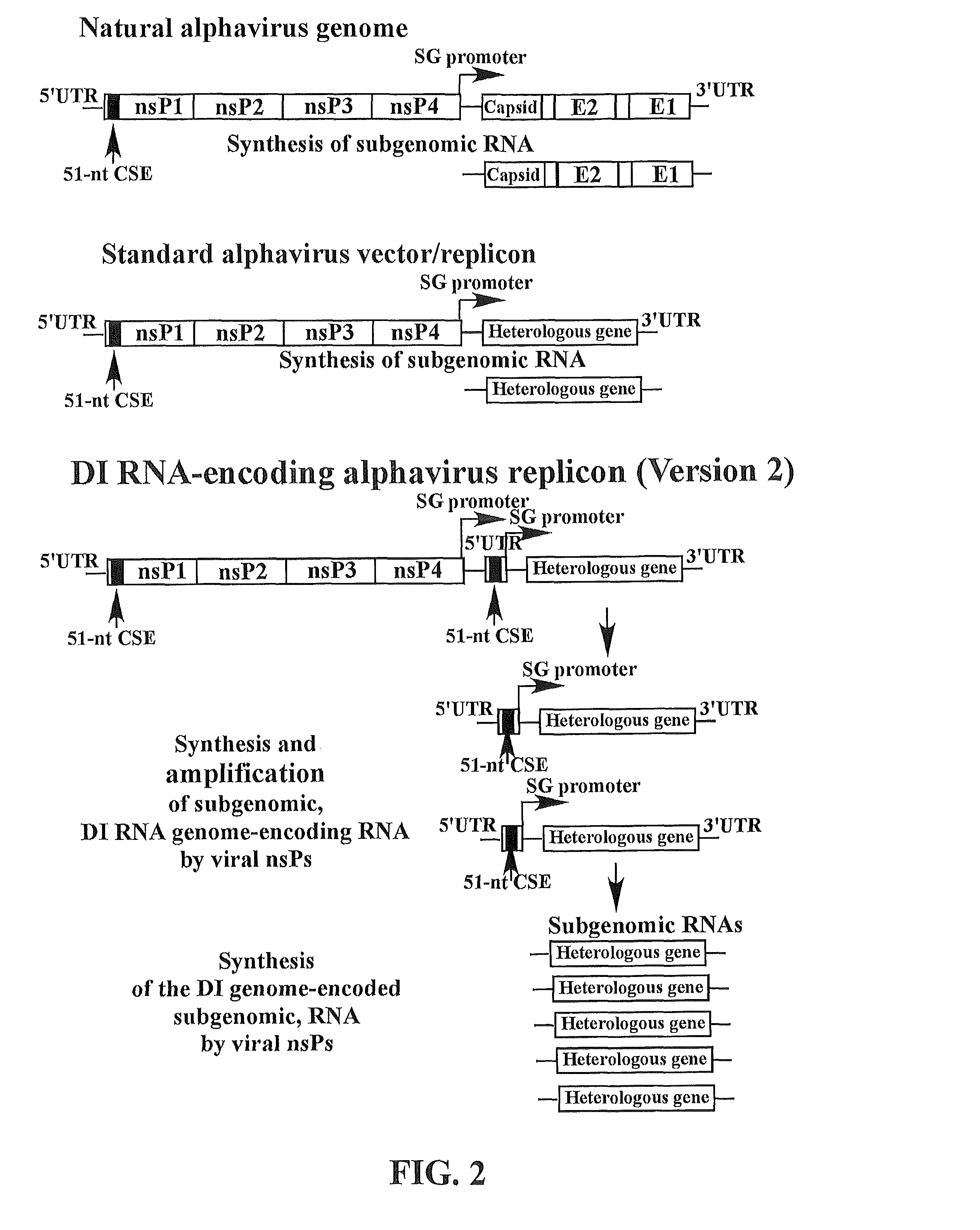
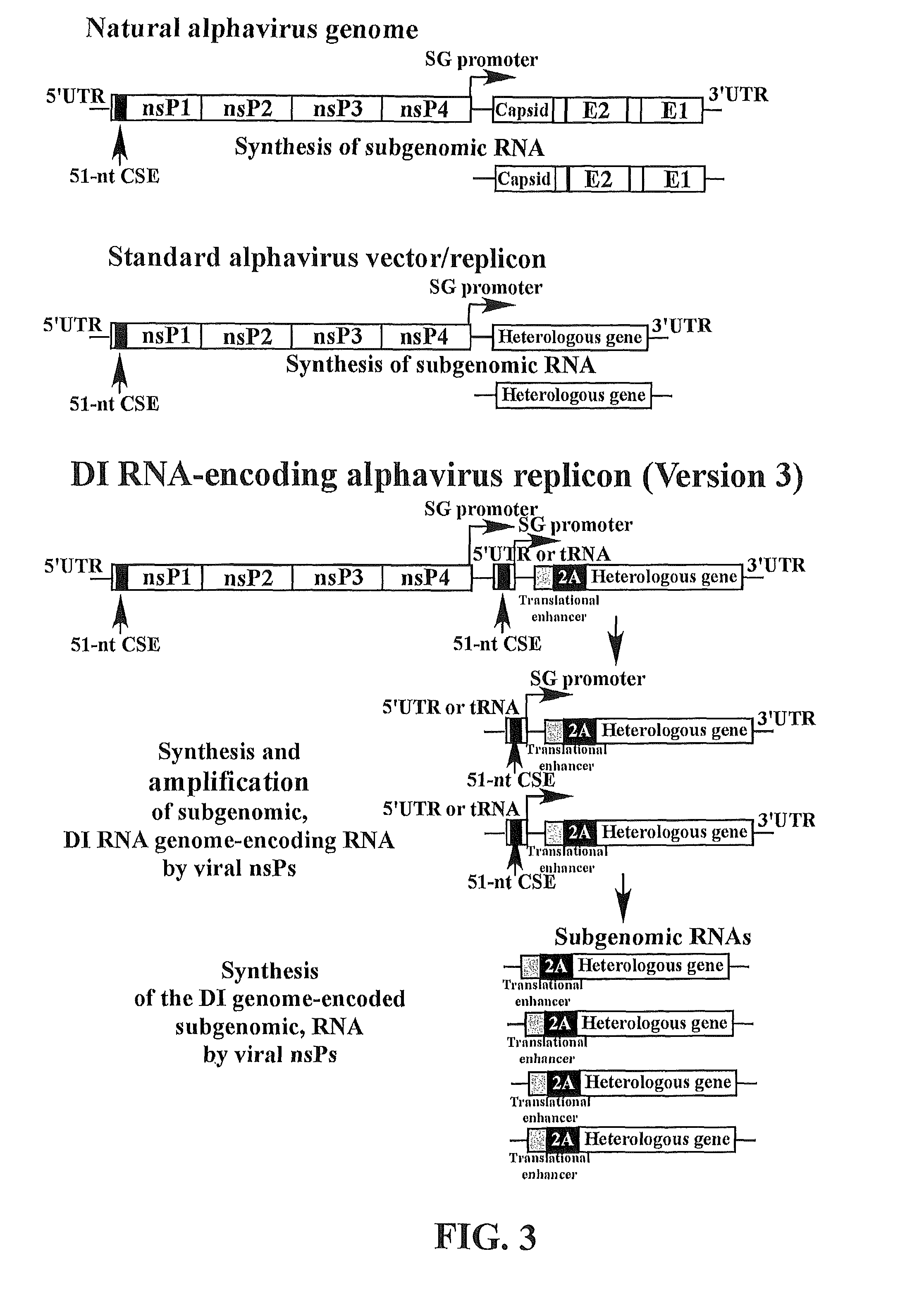
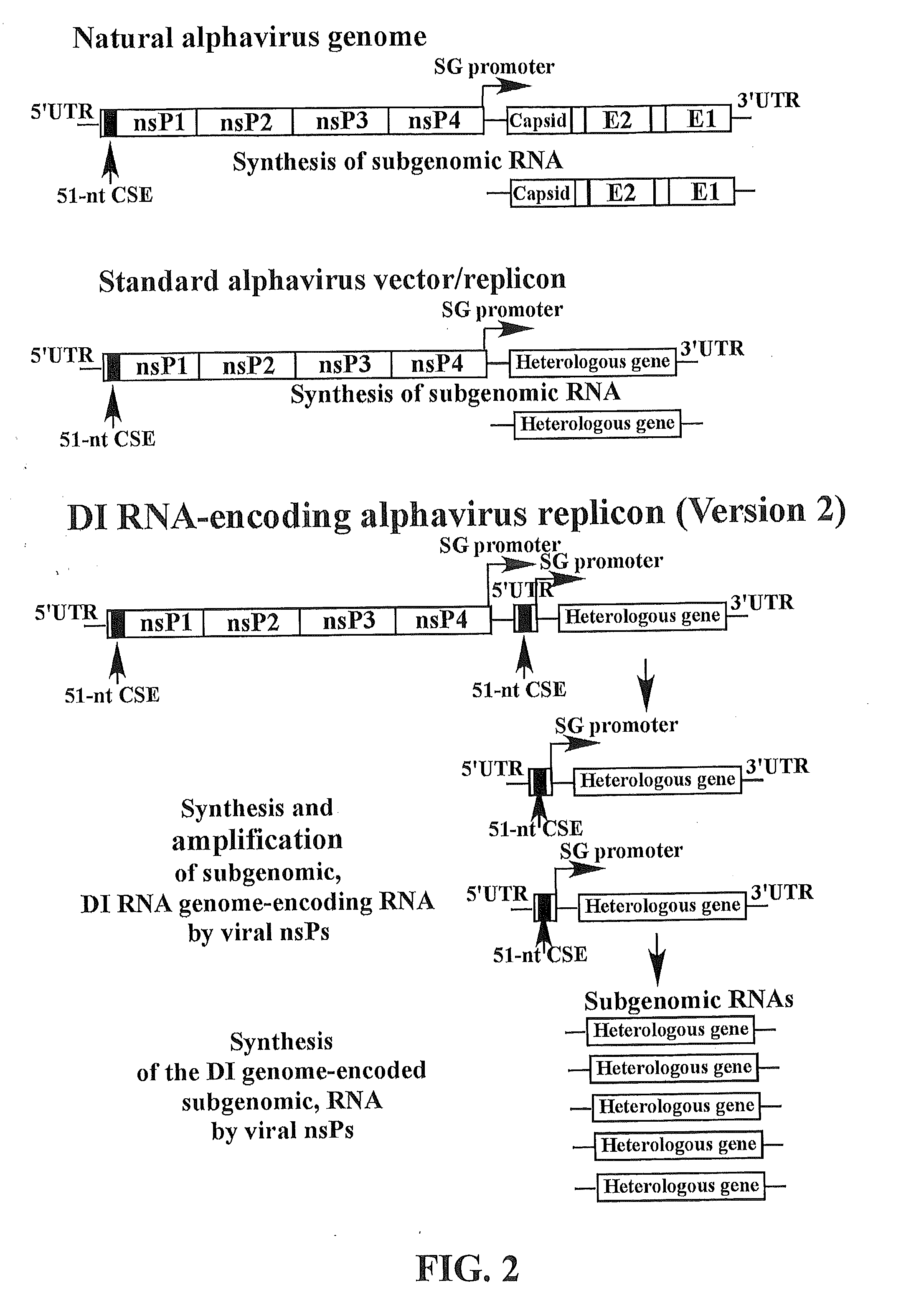
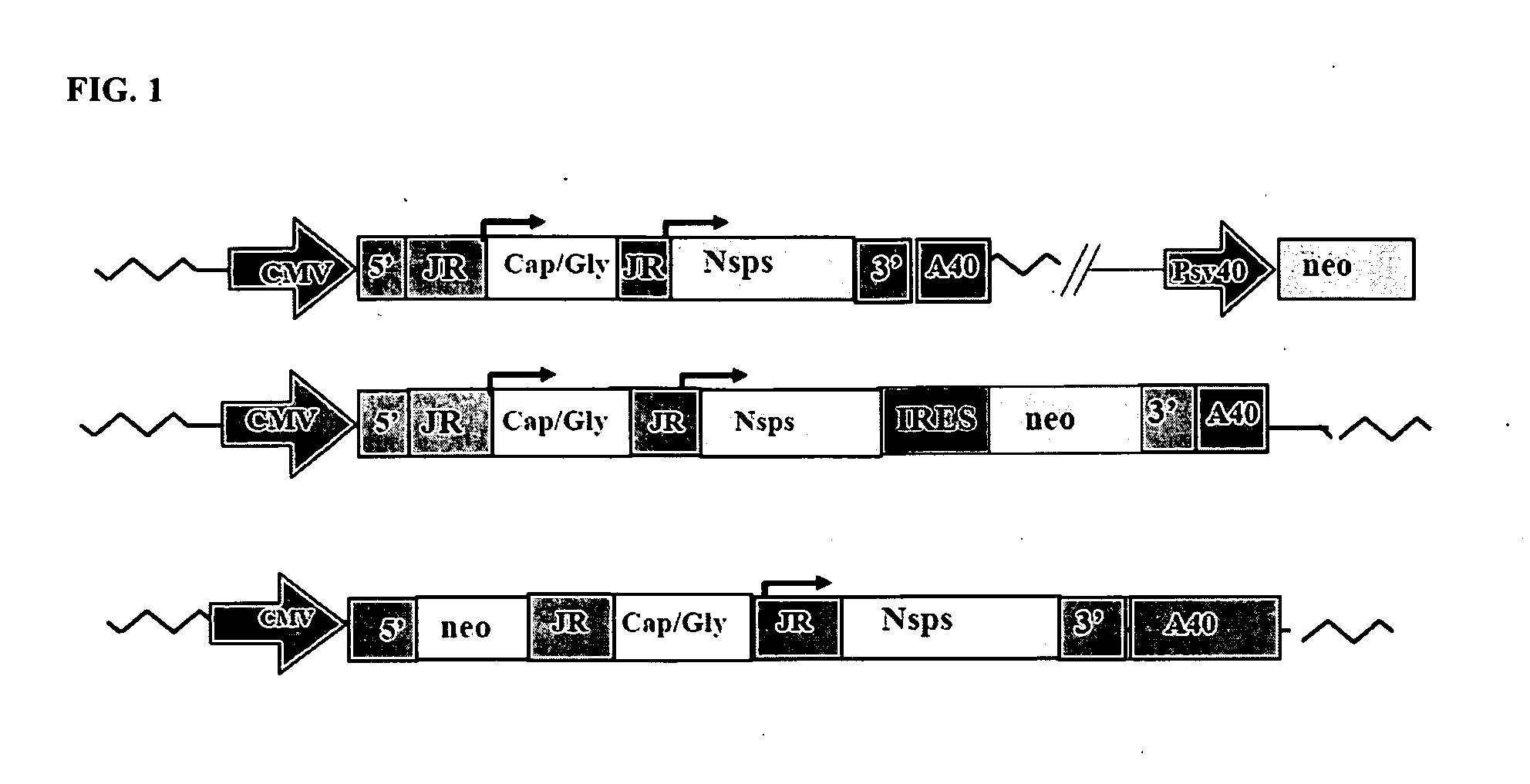
The present invention provides a recombinant nucleic acid comprising: a first nucleic acid sequence encoding a 5′ alphavirus replication recognition sequence; at least one second nucleic acid sequence encoding an alphavirus nonstructural protein; at least one alphavirus subgenomic promoter; at least one IRES element; at least one heterologous nucleic acid; and a third nucleic acid encoding a 3′ alphavirus replication recognition sequence. Further provided are methods of making alphavirus particles comprising a recombinant nucleic acid of this invention and methods of using the compositions of this invention. Also provided is a recombinant helper nucleic acid comprising: a first nucleic acid sequence encoding a 5′ alphavirus replication recognition sequence; an alphavirus subgenomic promoter; an IRES element; a second nucleic acid encoding an alphavirus structural protein; and a third nucleic acid encoding a 3′ alphavirus replication recognition sequence.
Owner:ALPHAVAX INC
Compositions and methods for generating an immune response utilizing alphavirus-based vector systems
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Veterinary pharmaceutical formulacion that comprises an RNA recombinant particle that encodes for a cu/zn superoxide dismutase protein of ruminant pathogenic bacteria and at least one RNA alphavirus belonging to the semliki forest virus family
InactiveUS20110200667A1Improve efficacyProtective efficacyAntibacterial agentsOrganic active ingredientsBrucella abortusZn superoxide dismutase
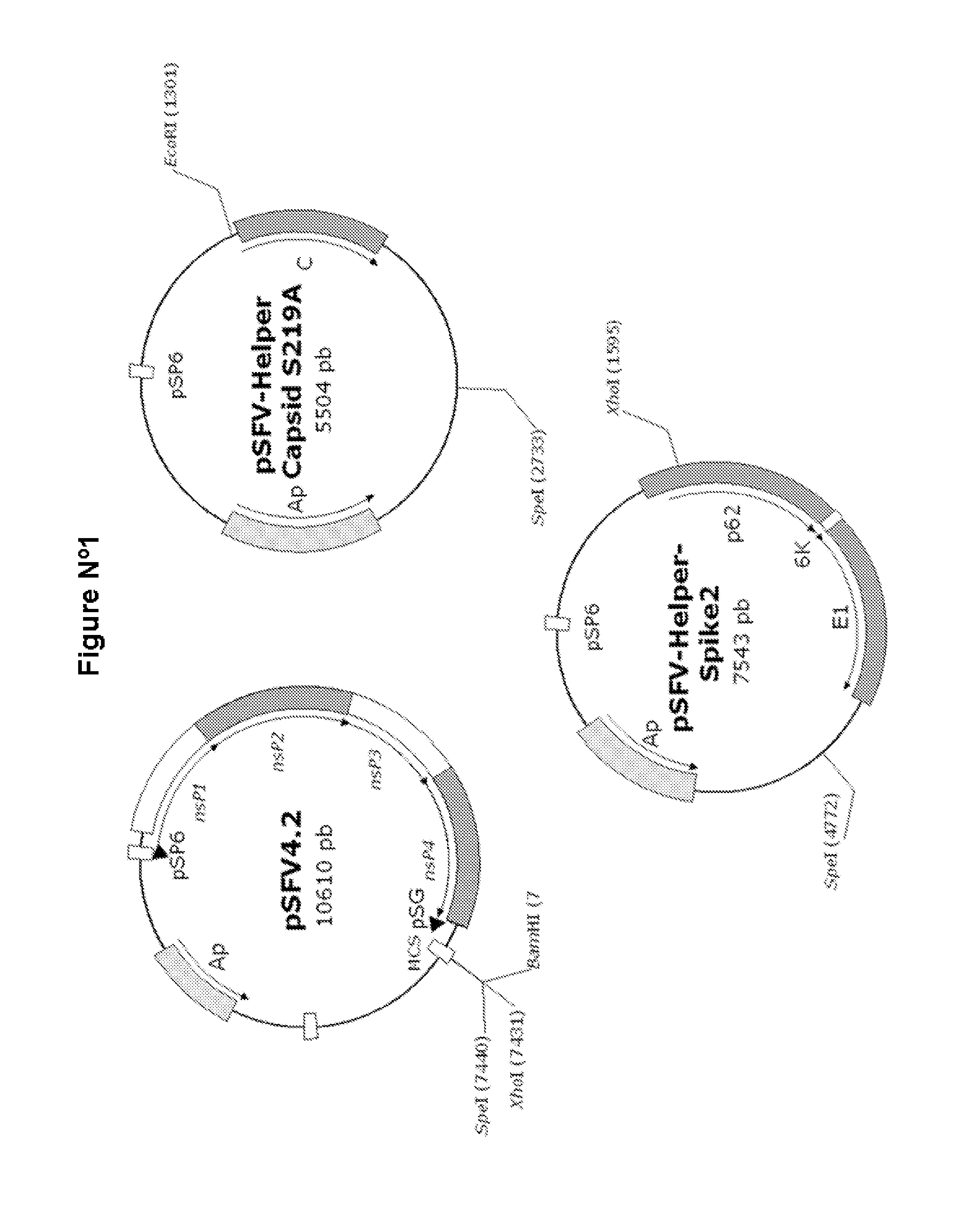
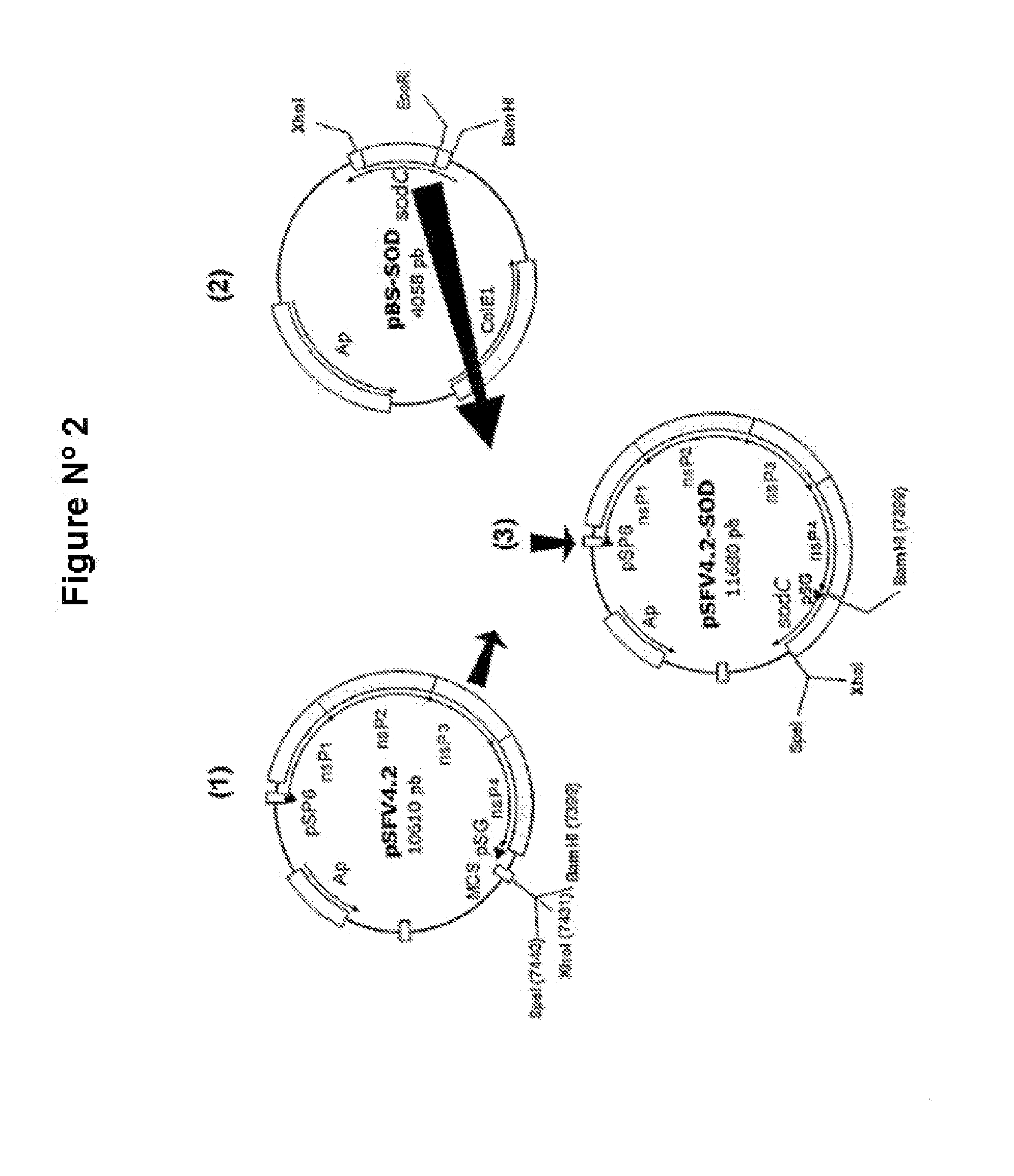
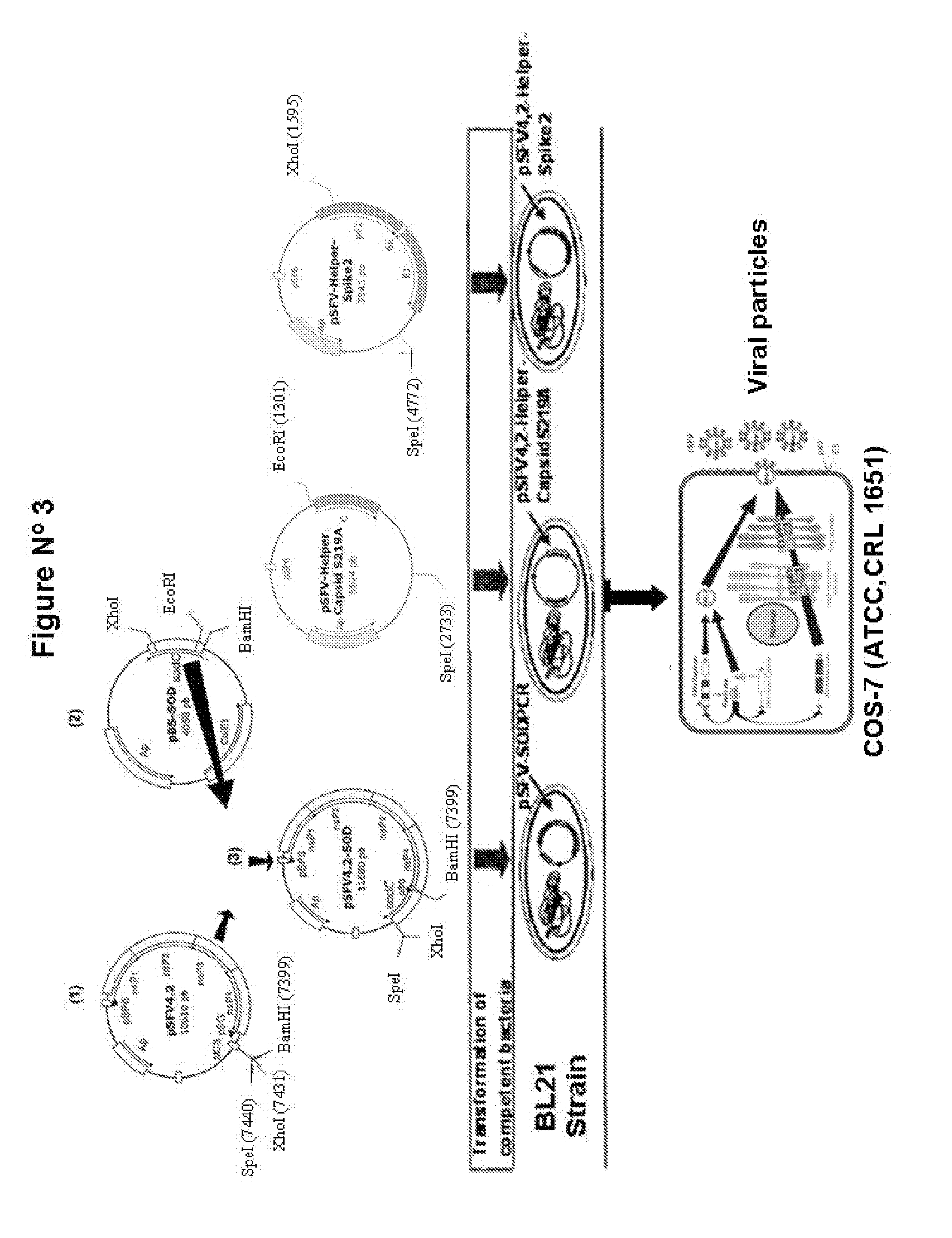
The technology is a veterinary pharmaceutical formulation of two vaccines, one from an RNA viral vector system constituted by an RNA recombinant particle that codifies for a Cu / Zn superoxide dismutase protein of Brucella abortus, and the other based on naked RNA constituted by a recombinant molecule of naked RNA that carries a sequence for the synthesis of at least one recombinant Cu / Zn superoxide dismutase protein of Brucella abortus and some Semliki Forest virus genes. An expression system based on the Semliki Forest virus and a use of this system, in addition to a method for the preparation of the pharmaceutical formulations.
Owner:UNIV DE CONCEPCION
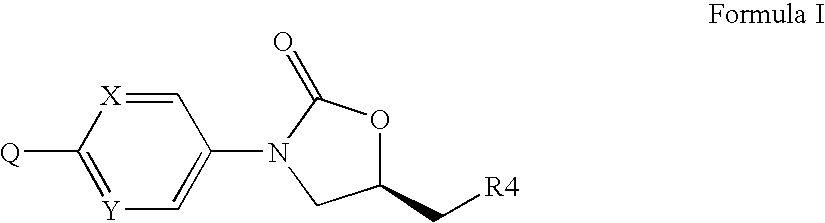
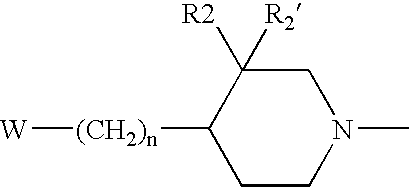
Oxazolidinones Bearing Antimicrobial Activity Composition and Methods of Preparation
The present invention concerns recombinant DNA's comprising cDNA of genomic RNA of a Salmonidae alphavirus preceded by a spacer sequence, under the control of a suitable promoter. Said recombinant DNA's are useful for obtaining expression vectors, producing recombinant Salmonidae alphavirus, and for obtaining vaccines.
Owner:SINDKHEDKAR MILIND D +4
Chimeric alphavirus replicon particles
Chimeric alphaviruses and alphavirus replicon particles are provided including methods of making and using same. Specifically, alphavirus particles are provided having nucleic acid molecules derived from one or more alphaviruses and structural proteins (capsid and / or envelope) from at least two or more alphaviruses. Methods of making, using, and therapeutic preparations containing the chimeric alphavirus particle, are disclosed.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Vectors derived from South African Arbovirus No. 86
InactiveUS6982087B2High expressionImprove sequenceBiocideSsRNA viruses positive-senseConnective tissueNucleotide sequencing
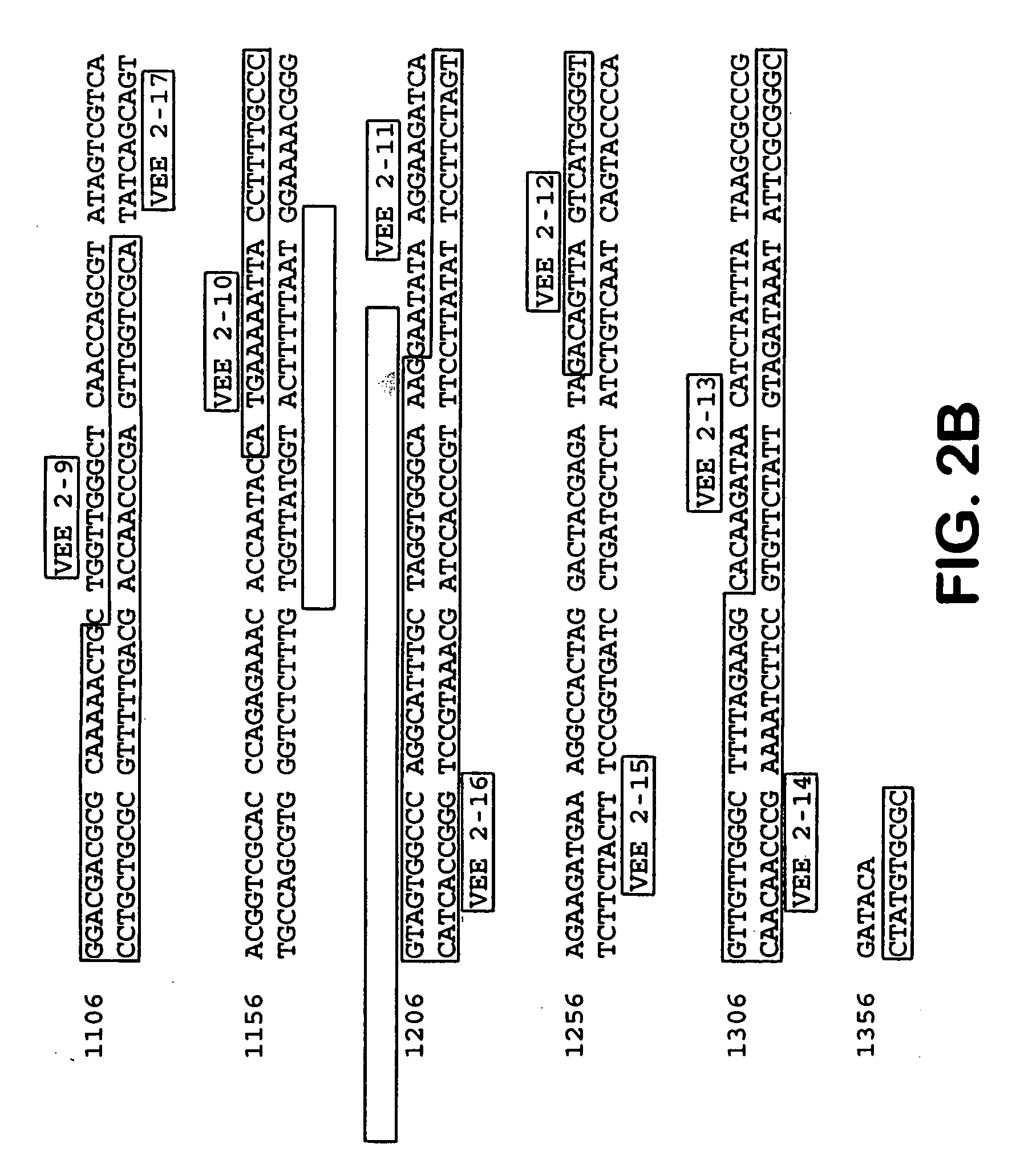
Provided herein are alphavirus vectors derived from South African Arbovirus No. 86 (S.A.AR86) comprising attenuating mutations and methods of making the same. Also provided are improved viral vectors and helper constructs comprising a S.A.AR86 capsid enhancer sequence. The present invention also provides S.A.AR86 replicon and helper constructs comprising an alphavirus capsid enhancer sequence. Further provided are methods of administering an alphavirus vector comprising a heterologous nucleotide sequence (preferably encoding an immunogen or a therapeutic polypeptide) according to the invention to a cell or subject. In preferred embodiments, the alphavirus vector delivers the heterologous nucleotide sequence to the cells of the bone, bone marrow, and / or bone-associated connective tissue.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
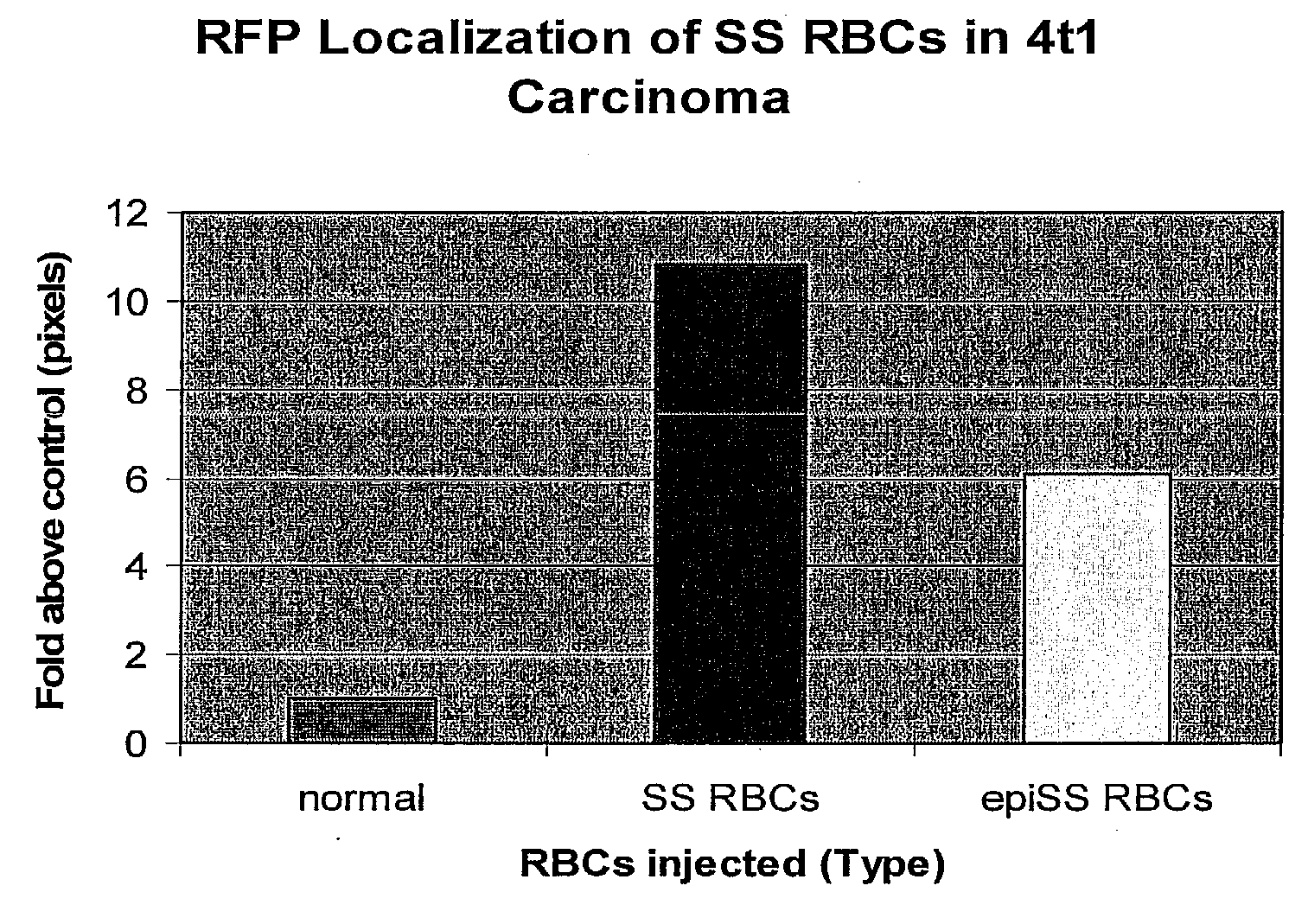
Sickled Erythrocytes, Nucleated Precursors & Erythroleukemia Cells for Targeted Delivery of Oncolytic Viruses, Anti-tumor Proteins, Plasmids, Toxins, Hemolysins & Chemotherapy
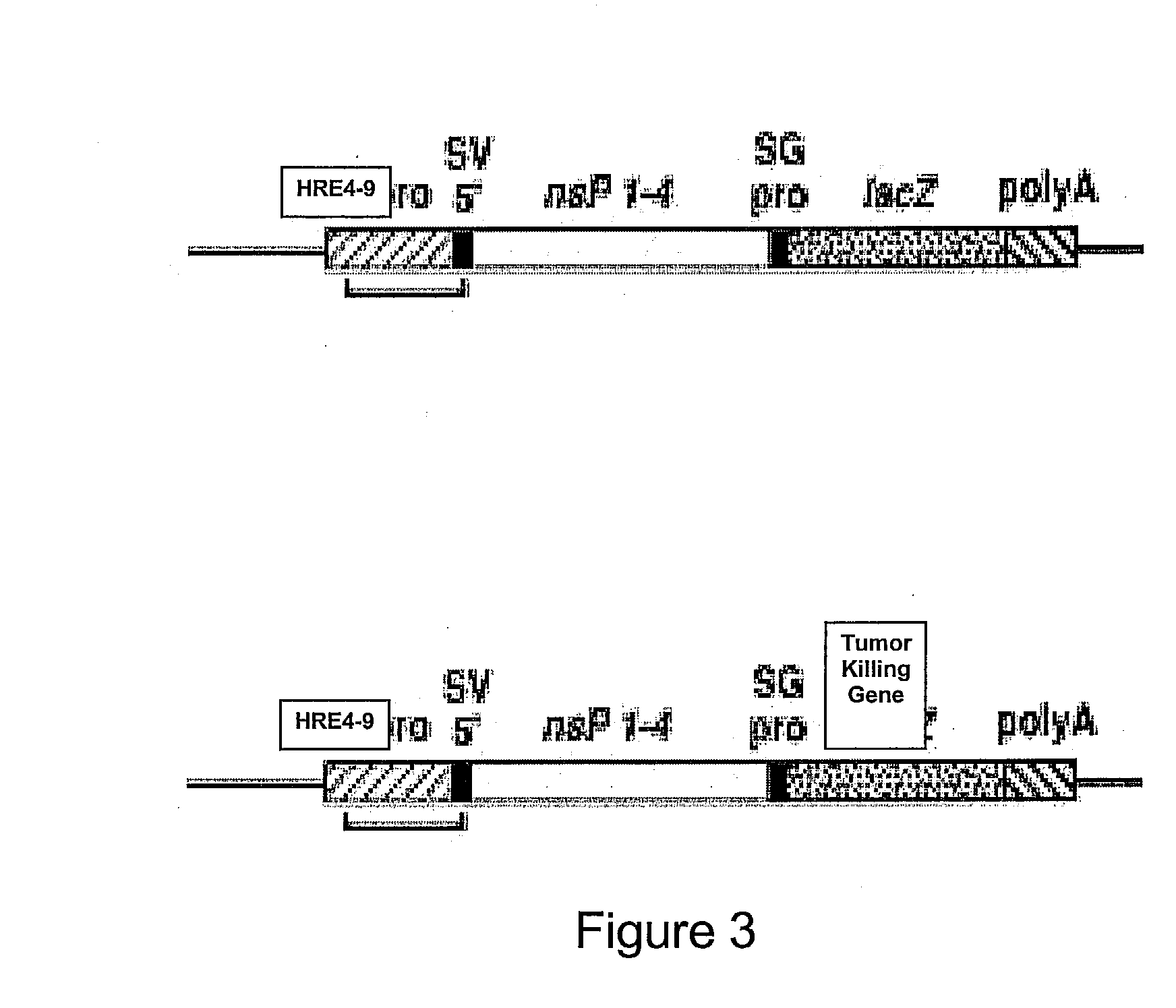
The present invention provides erythrocytes or nucleated erythrocyte precursors from animals or patients with SS or SA hemoglobin or erythroleukemia cells stably transfected with BCAM / Lu which are capable of selectively localizing in tumor vasculature promoting ischemia and occlusion and carrying oncolytic viruses, antitumor proteins, plasmids, toxins and chemotherapy into the tumor milieu. Nucleated erythroid precursors containing SS or SA hemoglobin and transfected with nucleic acids encoding a hypoxia-responsive element and containing nucleic acids encoding expression of oncolytic viruses, superantigens, toxins, viruses, antitumor proteins and chemotherapy are also useful in inducing a potent and specific tumoricidal response. An especially favored carrier is an SS nucleated erythroid precursor transfected with a replication competent oncolytic adenovirus or self-replicating alphavirus expressing a fusogenic membrane glycoprotein or a tumoricidal polypeptide.
Owner:TERMAN DAVID S +1
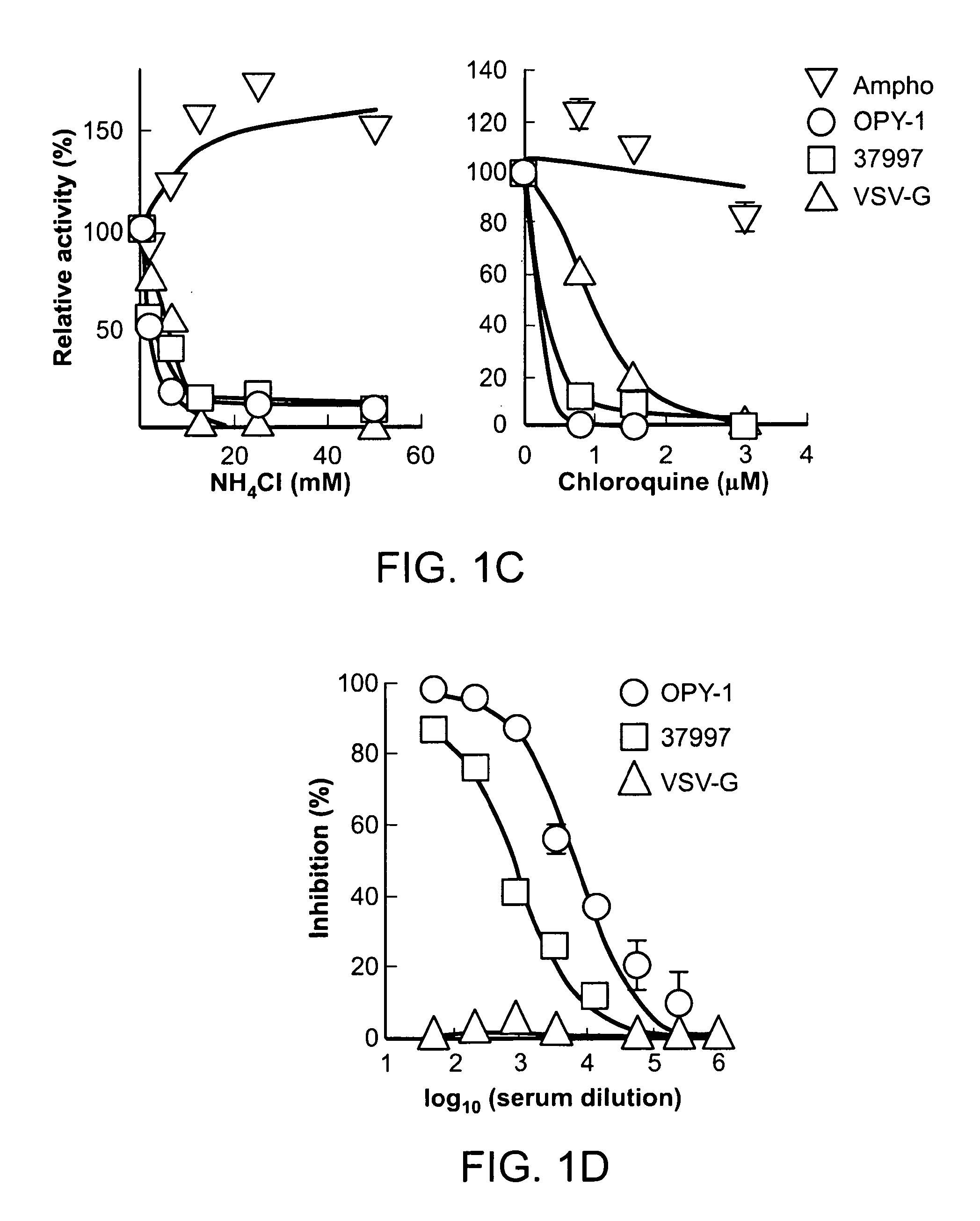
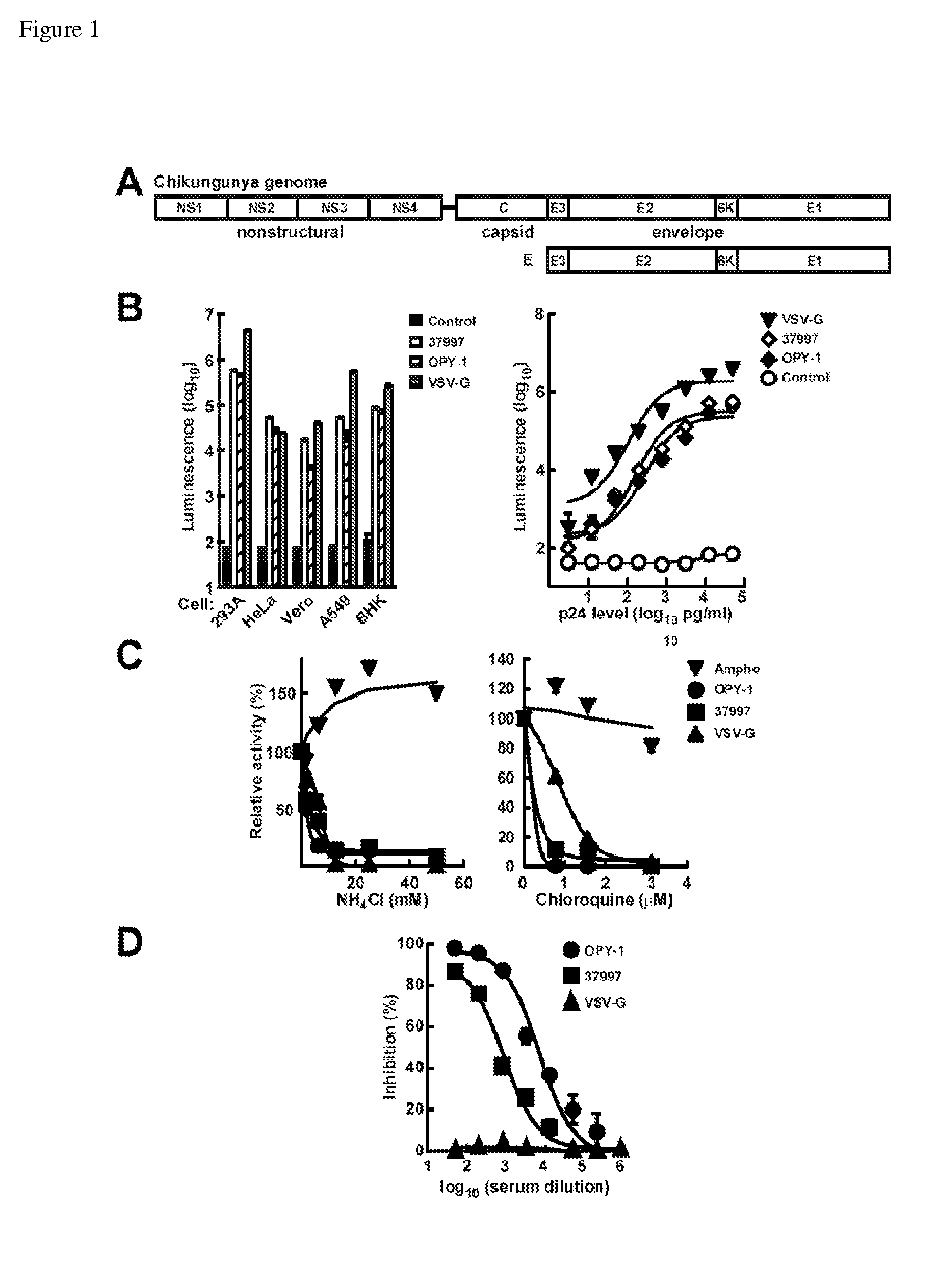
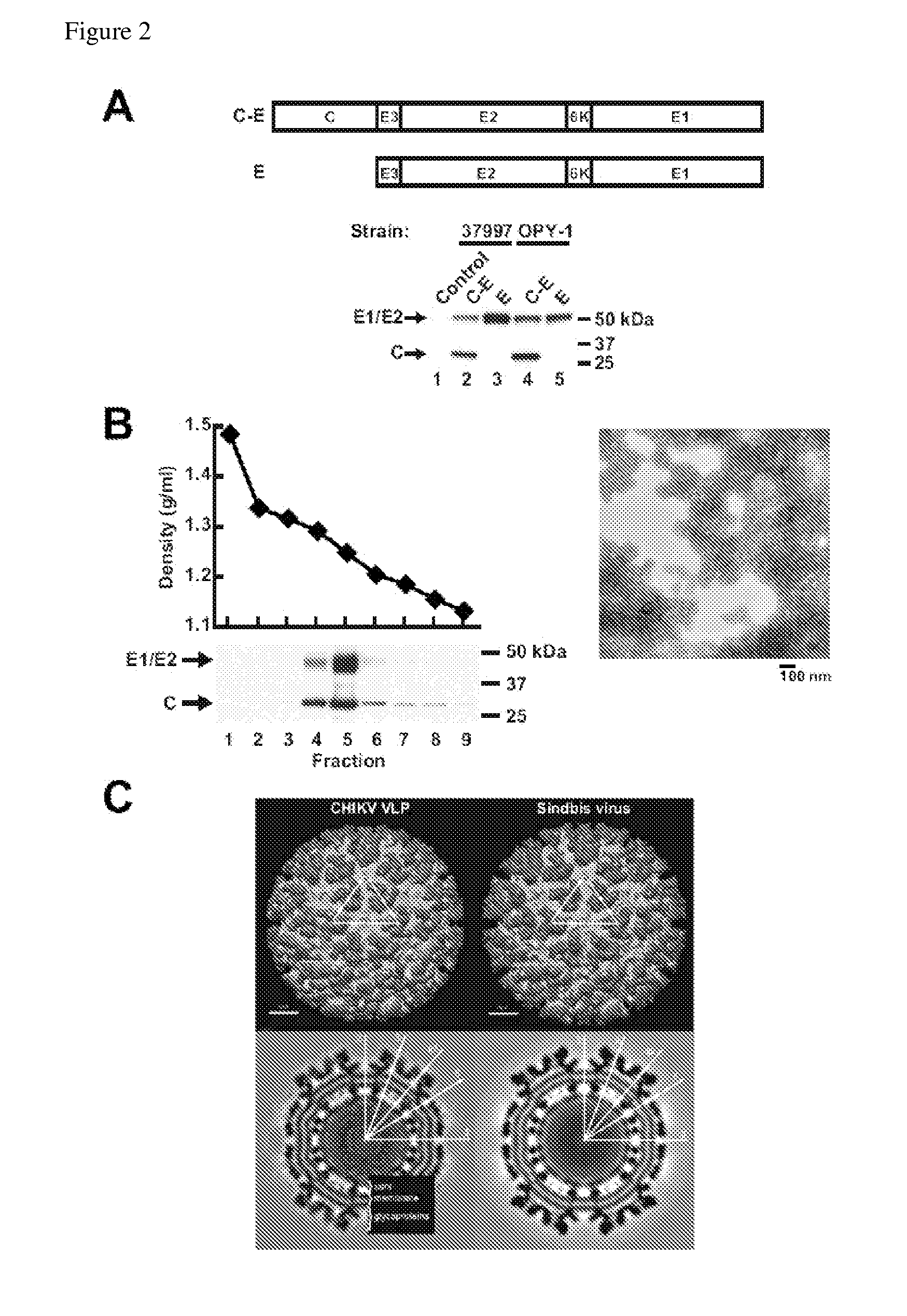
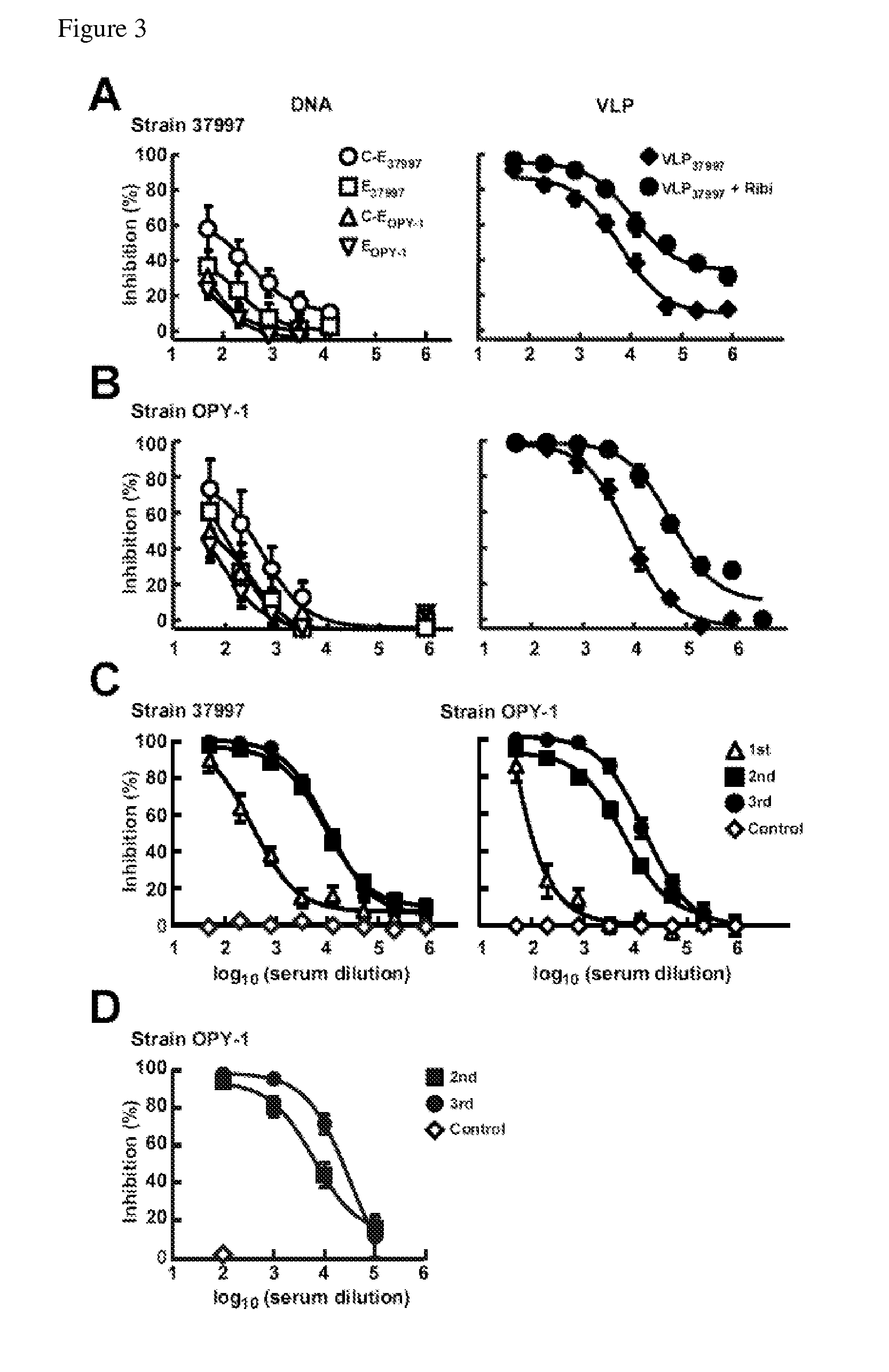
Virus-like particles (VLPs) prepared from chikungunya virus structural proteins
ActiveUS9353353B2Avoid infectionRelieve symptomsSsRNA viruses positive-senseAntipyreticDiseaseVirus-like particle
The invention features compositions and methods for the prevention or treatment of one or more strains of Chikungunya virus, as well as other alphavirus-mediated diseases.
Owner:UNITED STATES OF AMERICA
Virus-like particles and methods of use
ActiveUS20140170186A1Improve purification effectImproving immunogenicitySsRNA viruses positive-senseSugar derivativesVirus-like particleFlavivirus
The invention features modified alphavirus or flavivirus virus-like particles (VLPs). The invention provides methods, compositions, and kits featuring the modified VLPs. The invention also features methods for enhancing production of modified VLPs for use in the prevention or treatment of alphavirus and flavivirus-mediated diseases. The invention also provides methods for delivering agents to a cell using the modified VLPs.
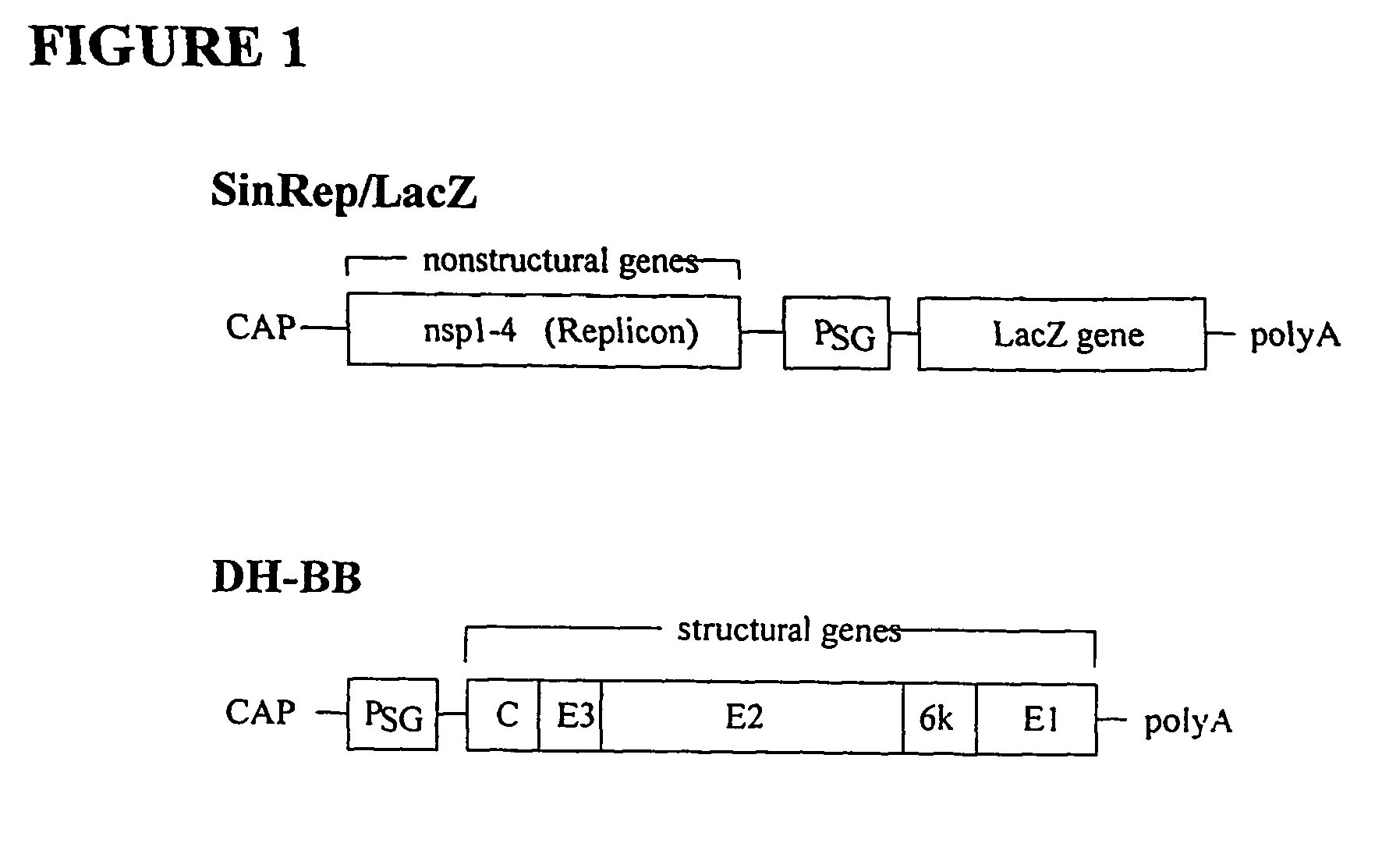
Methods and compositions for alphavirus replicons
The present invention provides alphavirus replicons and methods of their use in producing heterologous protein.
Owner:UAB RES FOUND
Tumor therapy with alphavirus-based and high affinity laminin receptor-targeted vectors
The present invention relates to methods and compositions for treating tumors using vectors that preferentially target tumor cells. In particular, the invention relates to Sindbis virus vectors which have a preferential affinity for high affinity laminin receptors (HALR). These vectors are efficiently targeted to tumors and have the ability to cause tumor necrosis.
Owner:NEW YORK UNIV
Alphavirus vectors having attentuated virion structural proteins
InactiveUS20060099587A1Improving immunogenicityReduce manufacturing costSsRNA viruses positive-senseMicrobiological testing/measurementStructural proteinMutant
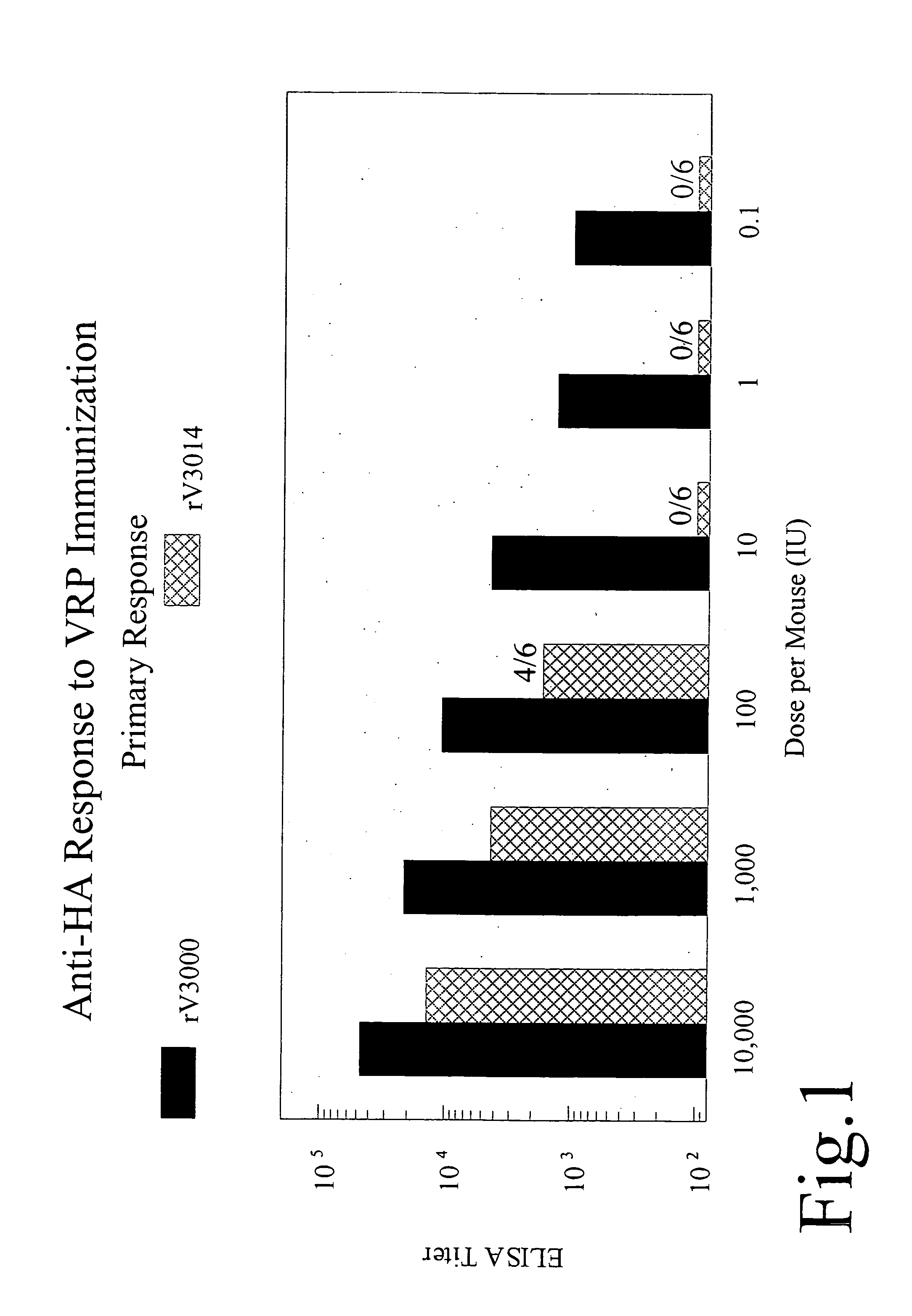
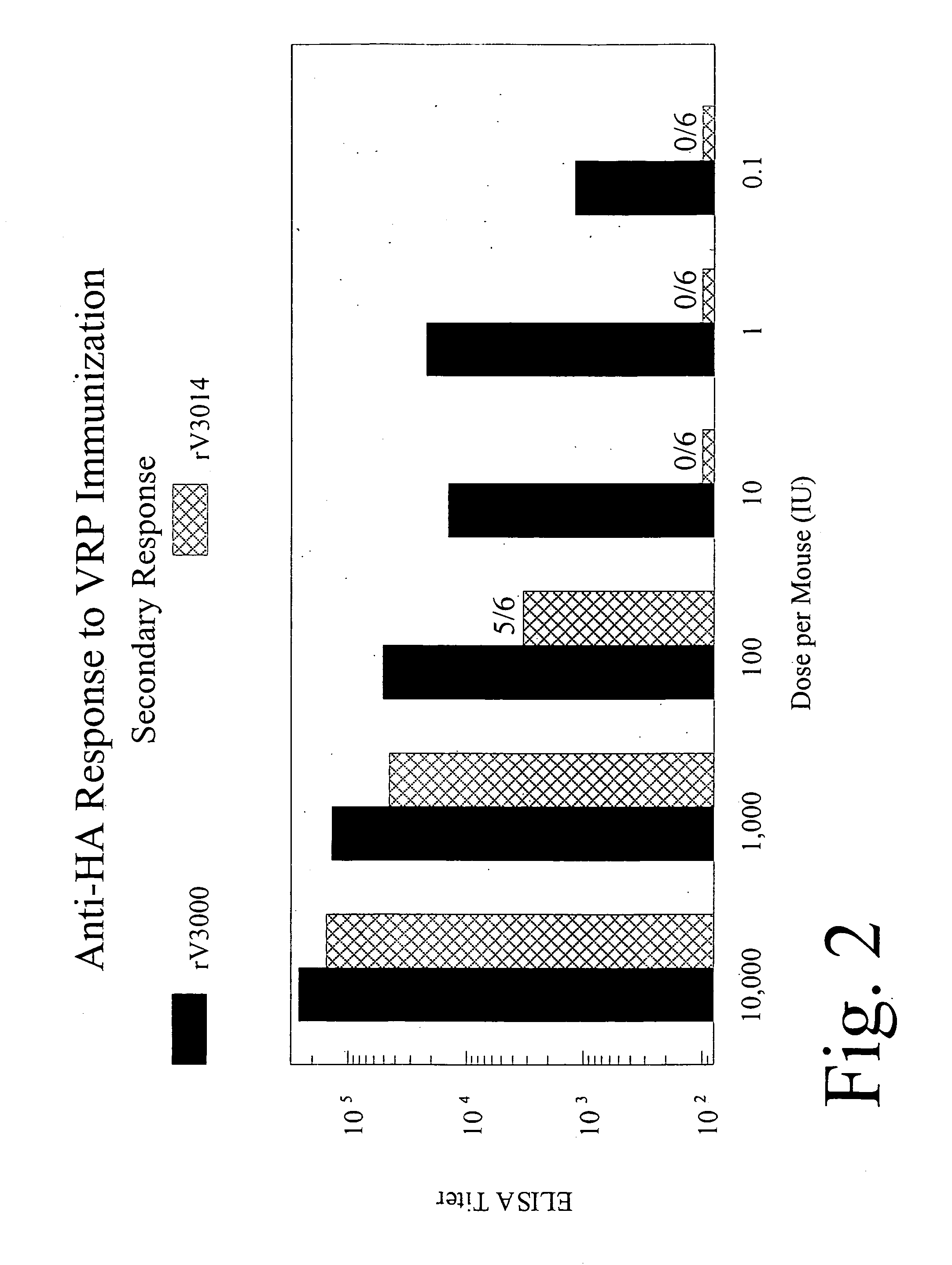
The present invention provides immunogenic compositions and methods that may be used to administer safer (i.e., attenuated) alphavirus vectors (such as alphavirus vectors comprising a VEE virion shell) that retain improved immunogenicity as compared with other attenuated alphaviruses (e.g., the VEE 3014 mutant, described below). In particular embodiments of the invention, the alphavirus vector comprises VEE structural proteins comprising an attenuating mutation in the E1 glycoprotein. In other particular embodiments, the attenuating mutation is in the fusogenic region of the E1 glycoprotein. The present invention enables administration of lower dosages of a safer (i.e., attenuated) virus and, thus, can further reduce manufacturing costs. The present inventors have found that immunogenicity of alphavirus vectors may be influenced by a number of factors including species, site and route of administration.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL +1
Chimeric vectors
ActiveUS20060073594A1Less cytopathicSsRNA viruses positive-senseNucleic acid vectorHeterologousViral vector
The present invention relates to chimeric vectors. More specifically, the invention relates to recombinant poxvirus vectors and viruses that are capable of expressing an alphaviral RNA replicon expressing a heterologous sequence of interest.
Owner:MERIAL INC
Methods and Compositions for Alphavirus Replicons
The present invention provides alphavirus replicons and methods of their use in producing heterologous protein.
Owner:UAB RES FOUND
Cell Line for Propagation of Highly Attenuated AlphaViruses
InactiveUS20110229969A1Reduce productionHigh potencyAnimal cellsSsRNA viruses positive-senseDNACell nucleus
The present invention provides an avian cell that is derived from an avian host cell and stably carries at least one DNA sequence in the cell nucleus encoding an alphavirus polypeptide, a method for preparing such an avian cell, and its use in preparing an alphavirus replican particle.
Owner:PROBIOGEN AG
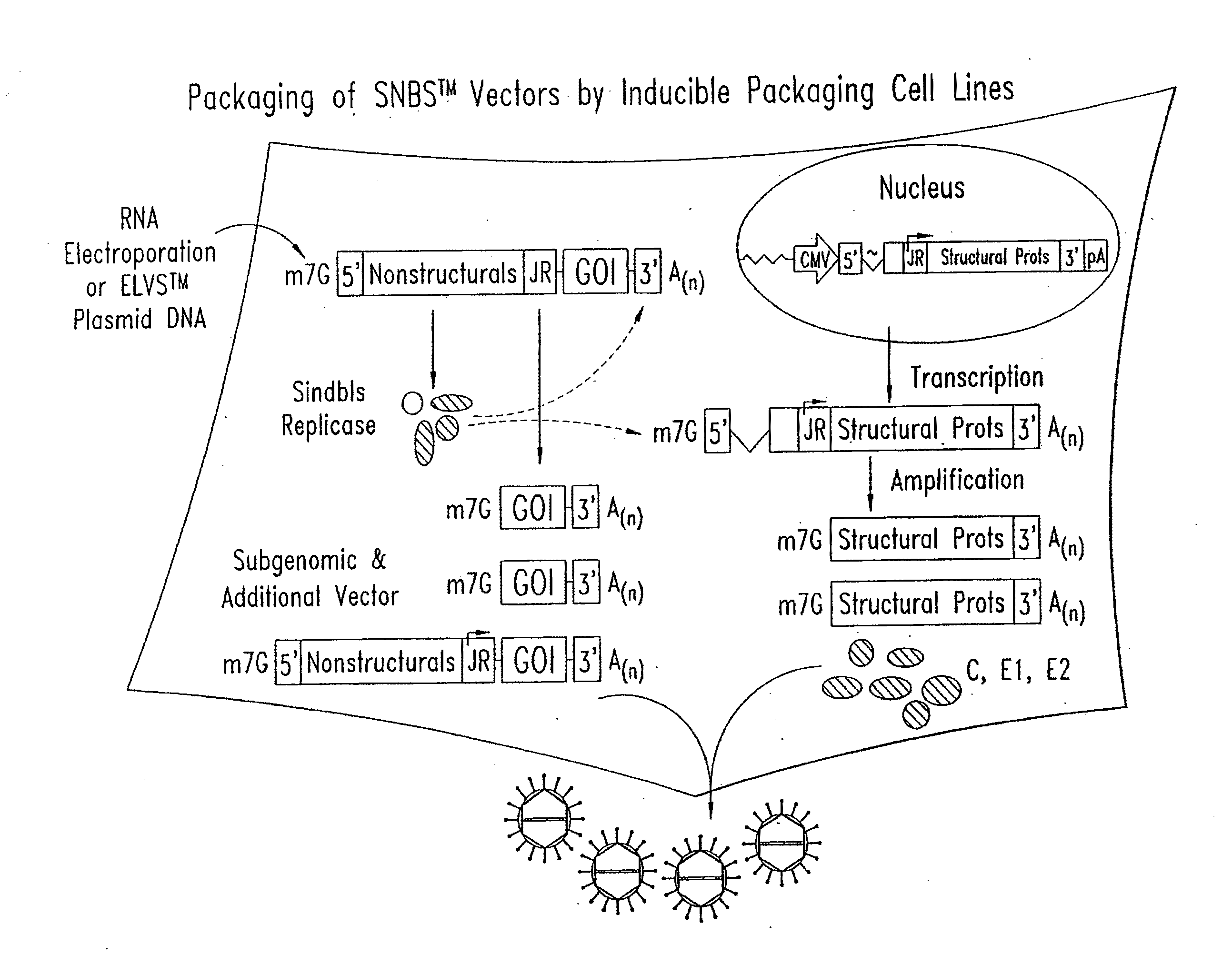
Methods of generating alphavirus particles
Strategies for increasing the productivity of alphavirus packaging cell lines and of reducing the possibility that replication competent virus may be generated during large scale production of recombinant alphavirus particles.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
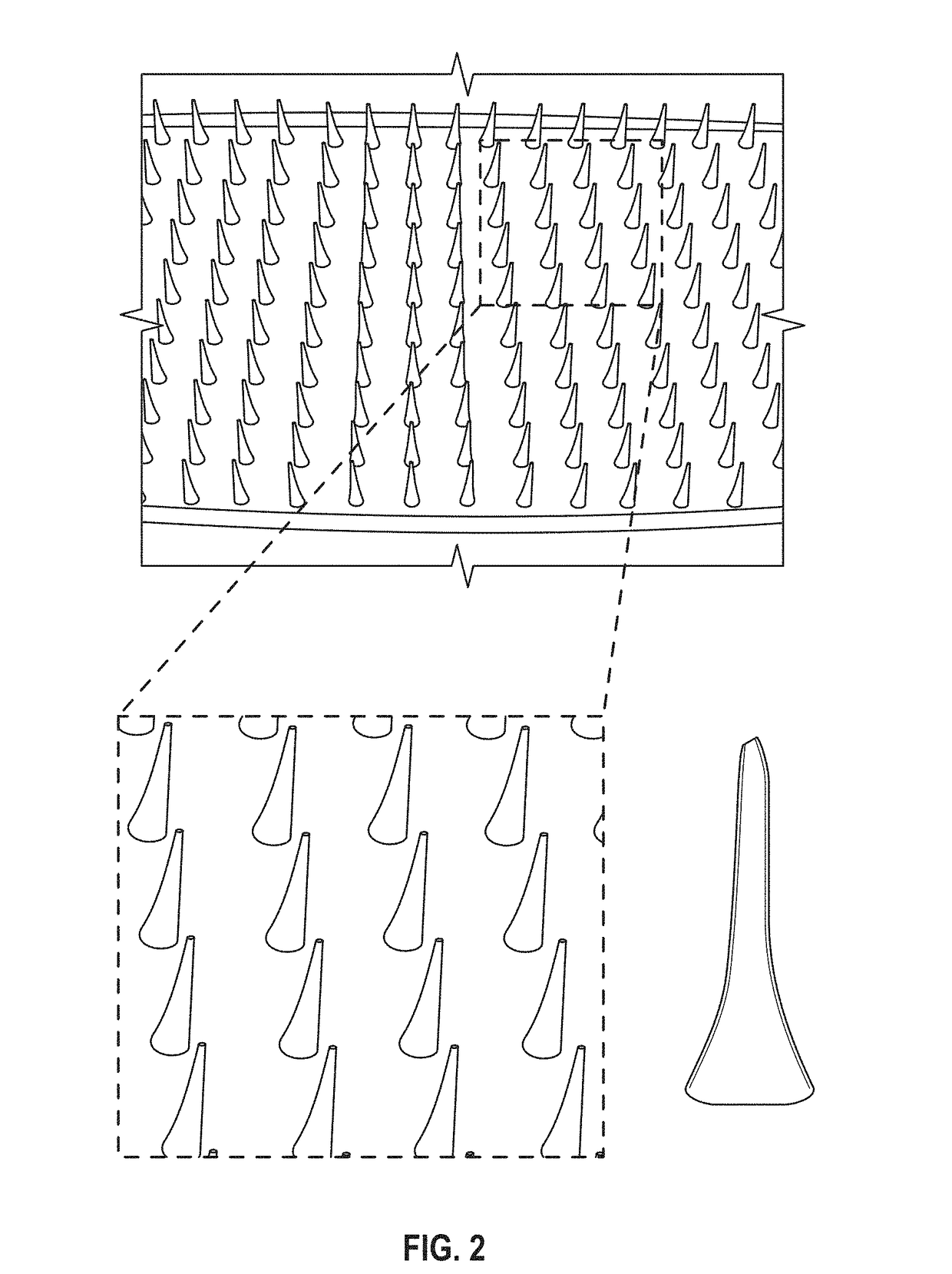
Microneedle compositions and methods of using same
ActiveUS20170196966A1Enhance immune responseEffective immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseViral replicationVirology
Described herein, are microneedle devices comprising a recombinant alphavirus replicon encoding an exogenous polypeptide, wherein the recombinant alphavirus replicon is coated onto or embedded into a plurality of microneedles. Also described herein are methods of preparing a microneedle device comprising a recombinant alphavirus replicon encoding an exogenous polypeptide. Also disclosed herein are methods of inducing an immune response in an individual comprising contacting the individual with a microneedle device comprising a recombinant alphavirus replicon encoding an exogenous polypeptide.
Owner:VERNDARI INC
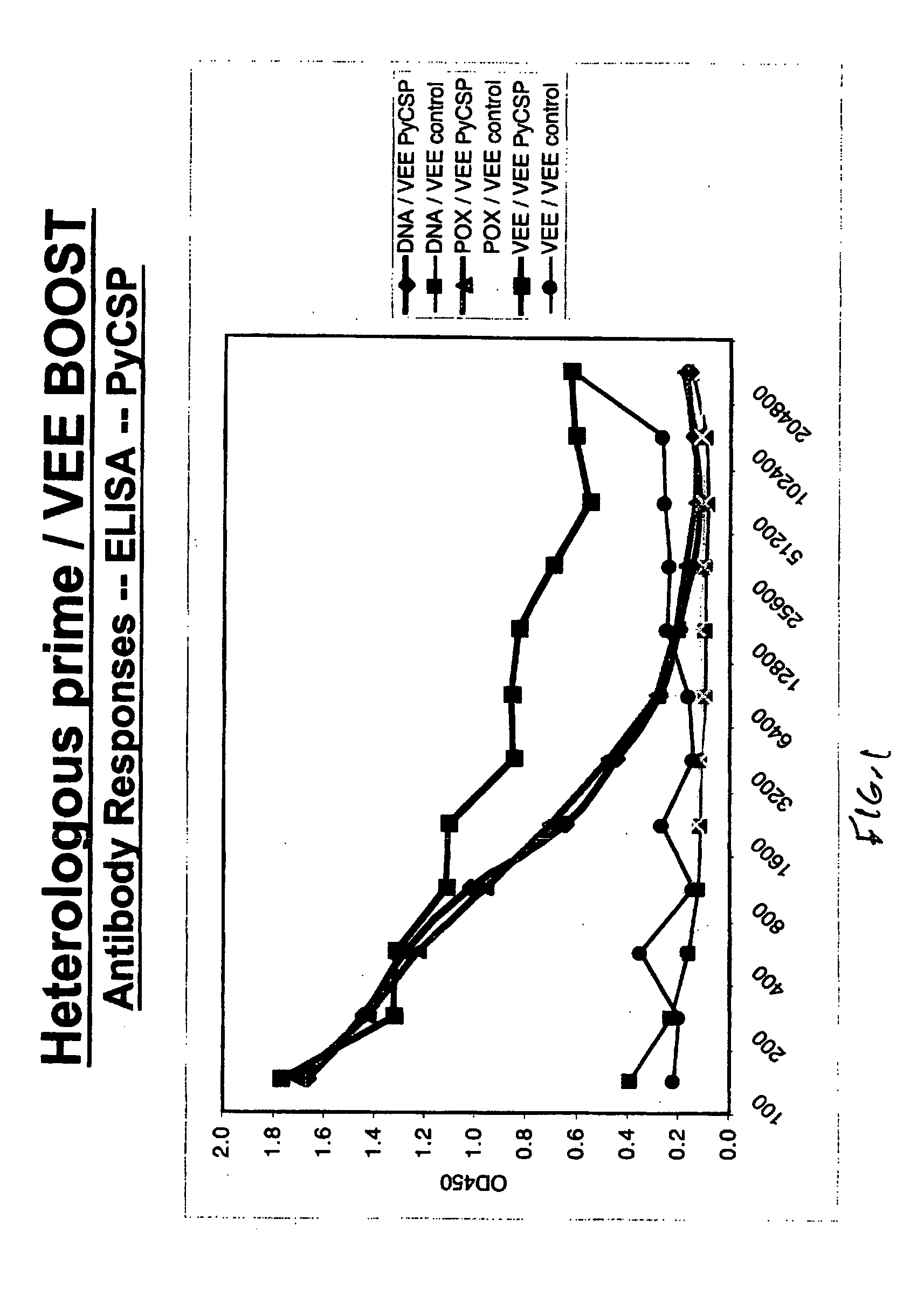
Enhancement of vaccine-induced immune responses and protection by heterologous boosting with alphavirus replicon vaccines
InactiveUS20050208020A1Enhancing and broadening immunogenicityEnhancing and broadening and protective immunityBiocideSsRNA viruses positive-senseHeterologousWhole Organism
The inventive subject matter relates to an immunogenic composition and method of enhancing immunogenicity and protective immunity induced by any subunit or whole organism vaccine or combination of vaccines comprising administering to the subject a priming immunization preparation containing an antigen or fragment thereof, said preparation being selected from the group consisting of: a recombinant virus expression system; a recombinant protein antigen or a recombinant polypeptide; a synthetic peptide; a polynucleotide vector; a whole organism or extract and combinations thereof; and a boosting immunization of at least one alphavirus replicon containing an antigen or fragment thereof. The inventive subject matter further relates to an immunogenic composition and method of inducing an immune response that activates both cellular and humoral arms of the immune system.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE NAVY NAVAL RES LAB WASHINGTON
Recombinant alphavirus-based vectors with reduced inhibition of cellular macromolecular synthesis
InactiveUS7811812B2Reduced and delayed and no inhibitionReduced and delayed and developmentFungiSsRNA viruses positive-senseWild typeInhibitory effect
Isolated nucleic acid molecules are disclosed, comprising an alphavirus nonstructural protein gene which, when operably incorporated into a recombinant alphavirus particle, eukaryotic layered vector initiation system, or RNA vector replicon, has a reduced level of vector-specific RNA synthesis, as compared to wild-type, and the same or greater level of proteins encoded by RNA transcribed from the viral junction region promoter, as compared to a wild-type recombinant alphavirus particle. Also disclosed are RNA vector replicons, alphavirus vector constructs, and eukaryotic layered vector initiation systems which contain the above-identified nucleic acid molecules.
Owner:CARDIOVENTION
Genetic immunization against cervical carcinoma
InactiveUS7198792B2Promote growthDevoid of the capacity to suppress the retinoblastomaVectorsViral antigen ingredientsVector systemCervical carcinoma
An alphavirus vector system comprising nucleic acid of a human papilloma virus origin is disclosed. A method of treating or preventing cervical cancer is also disclosed. The method includes administering the alphavirus vector system and / or a cell comprising nucleic acid derived from human papilloma virus (HPV) to a subject. The alphavirus vector system or the cell may be administered as a vaccine.
Owner:UNIVERSITY OF GRONINGEN
Recombinant alphavirus-based vectors with reduced inhibition of cellular macro-molecular synthesis
InactiveUS20100330121A1Reduced and delayed and no inhibitionReduced delayed no developmentFungiSsRNA viruses positive-senseWild typeInhibitory effect
Isolated nucleic acid molecules are disclosed, comprising an alphavirus nonstructural protein gene which, when operably incorporated into a recombinant alphavirus particle, eukaryotic layered vector initiation system, or RNA vector replicon, has a reduced level of vector-specific RNA synthesis, as compared to wild-type, and the same or greater level of proteins encoded by RNA transcribed from the viral junction region promoter, as compared to a wild-type recombinant alphavirus particle. Also disclosed are RNA vector replicons, alphavirus vector constructs, and eukaryotic layered vector initiation systems which contain the above-identified nucleic acid molecules.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC +1
Alphavirus and Alphavirus Replicon Particle Formulations and Methods
Disclosed are methods for preparing dried (preferably lyophilized) preparations comprising a population of alphaviruses or alphavirus replicon particles, a sugar or polyol, a surfactant and a salt and preparations made by said methods, both in the dried form but also as liquids prior to drying or after reconstituting dried preparations. These preparations may further comprise a plasticizer and / or a bulking agent. These preparations are readily reconstituted, with little or no loss in infectivity of the viruses or replicon particles.
Owner:ALPHAVAX INC
Chimeric alphavirus replicon particles
Chimeric alphaviruses and alphavirus replicon particles are provided including methods of making and using same. Specifically, alphavirus particles are provided having nucleic acid molecules derived from one or more alphaviruses and structural proteins (capsid and / or envelope) from at least two or more alphaviruses. Methods of making, using, and therapeutic preparations containing the chimeric alphavirus particle, are disclosed.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
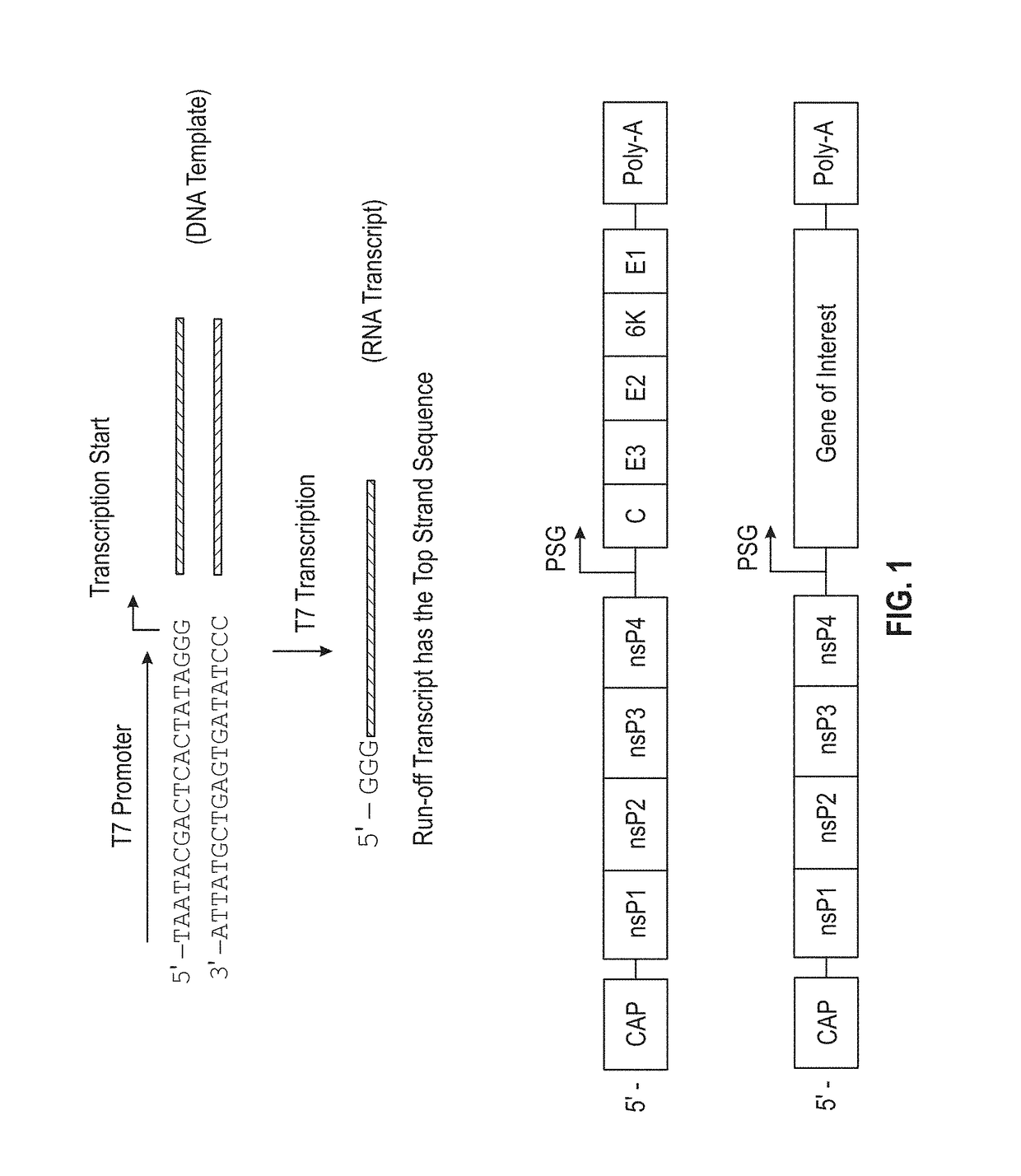
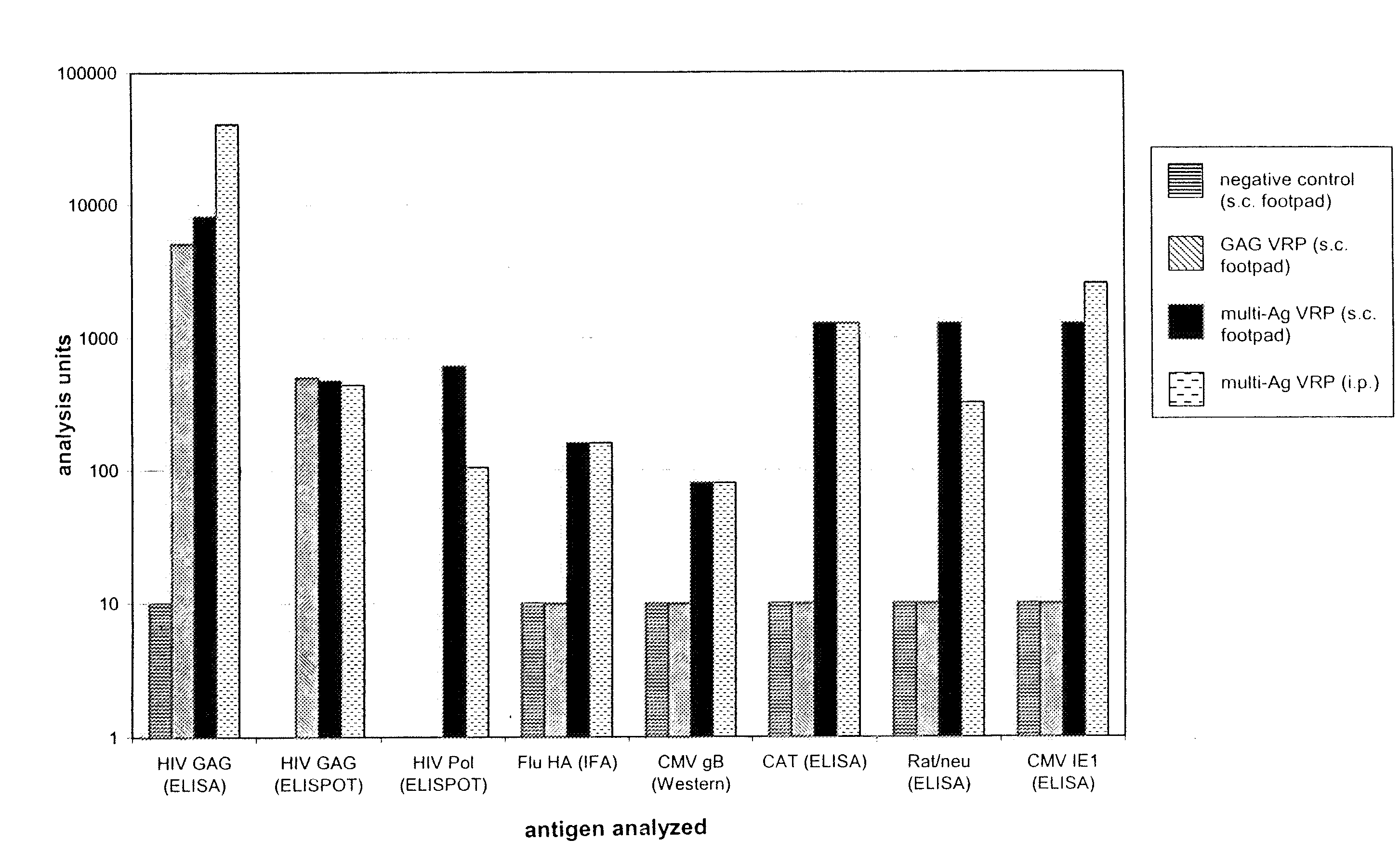
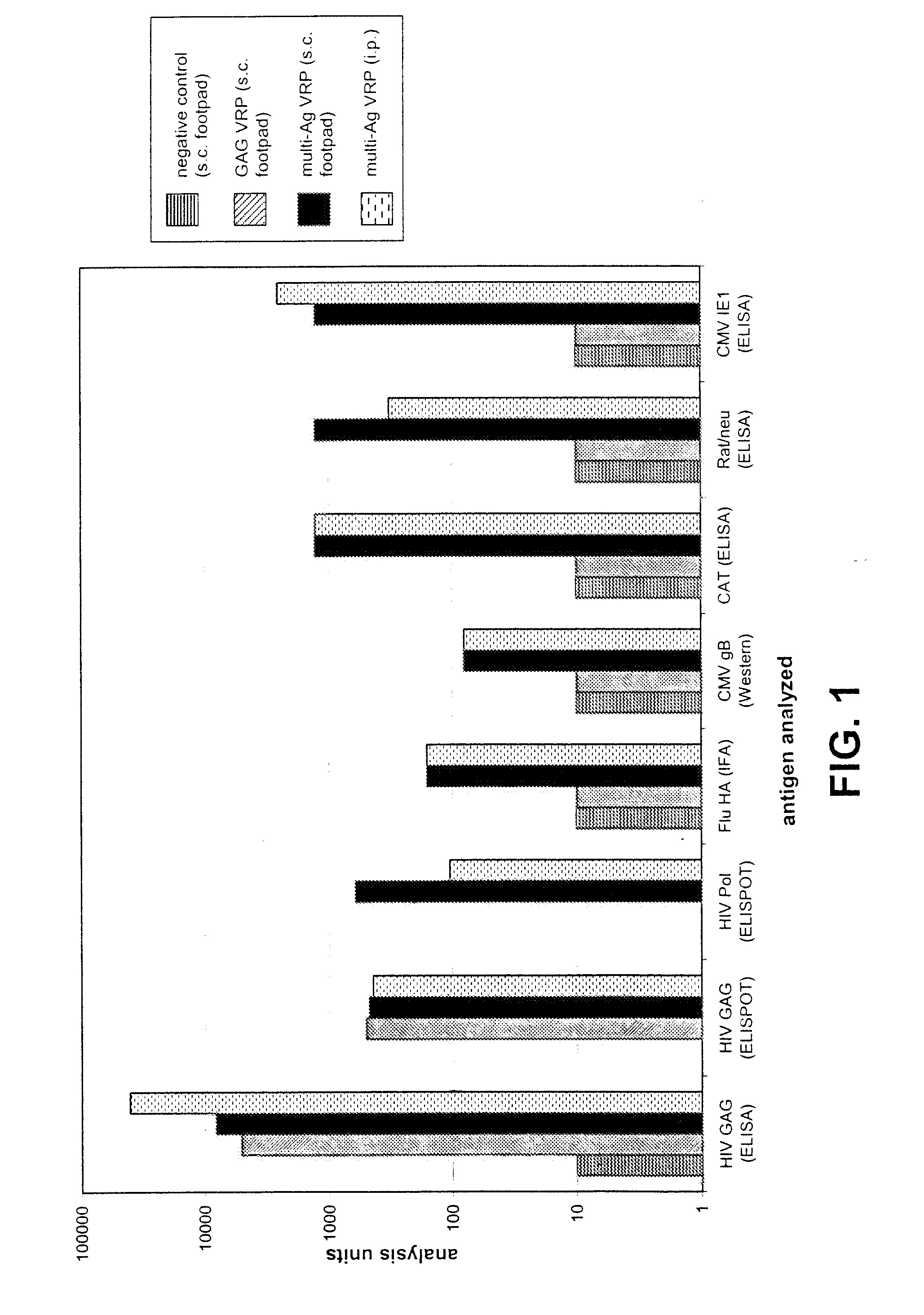
Multi-antigenic alphavirus replicon particles and methods
ActiveUS20080213309A1Easy maintenancePrevent recurrenceBiocideSsRNA viruses positive-senseExpression LibraryViral replication
Viral replicon selected nucleic acid expression libraries are useful for analyzing multiple antigens associated with a parasite, pathogen or neoplasia or for preparing immunogenic compositions for generating immune responses specific for the parasite, pathogen or neoplasia. Alphavirus replicon particles representative of the nucleic acid expression library are preferred. The nucleic acid library can be a random library, or it can be prepared after a selection step, for example, by differential hybridization prior to cloning into the replicon vector.
Owner:ALPHAVAX INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com