Recombinant alphavirus-based vectors with reduced inhibition of cellular macro-molecular synthesis
a technology of recombinant alphavirus and macromolecular synthesis, which is applied in the direction of fungi, drug compositions, immunological disorders, etc., can solve the problems of permanent sequelae, death, and general self-limiting infection, and achieve the effect of reducing, delayed, or no inhibition of cellular synthesis, reducing, or no inhibition of cp
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Isolation and Characterization of SIN1
[0323]Below, the identification and molecular characterization of a positive strand RNA virus which exhibits reduced inhibition of host macromolecular synthesis and is capable of establishing persistent infection in vertebrate cells, as compared to lytic, cytopathogenic wild type strains of the same virus, is described. For example, Sindbis virus is used as a prototype representative of the Alphavirus genus.
A. Isolation, Plaque Purification, and Characterization of SIN-1 from a Wild-Type Sindbis Virus Stock
[0324]The isolation, molecular cloning, and characterization of a Sindbis virus variant strain is described. This strain is able to establish productive persistent infection in the absence of cytopathicity, but produce levels of virus equivalent to that of wild-type virus.
[0325]A high-titered (>108 PFU / ml) wild-type stock obtained by infection of BHK cells (ATCC No. CCL-10) with Sindbis virus (CMCC #4639) at low MOI (≦0.1). To facilitate infec...
example 2
Isolation and Characterization of Positive Strand RNA Viruses which Exhibit Reduced Inhibition of Host Macromolecular Synthesis
[0352]The derivation of virus variants exhibiting the desired phenotypes of reduced, delayed or no inhibition of host cell macromolecular synthesis is dependent on the generation, characterization, and isolation of sequences which differ from that of wild-type virus. However, in addition to example 1, there are no obvious or previously disclosed methods to select for or identify coding or non-coding viral sequence changes that result in alteration of this virus-based inhibition of macromolecular synthesis, or the generation of viruses that lead to persistent, rather than lytic, infection. The present invention provides specific methods, using alphaviruses as an example, that enable one to overcome these obstacles.
A. Biological Selection of Virus Variants
[0353]The biological derivation of virus variants which result in reduced, delayed, or no inhibition of ho...
example 3
Preparation of SIN1-Based RNA Vector Replicons
A. Construction of the SIN-1 Basic Vector
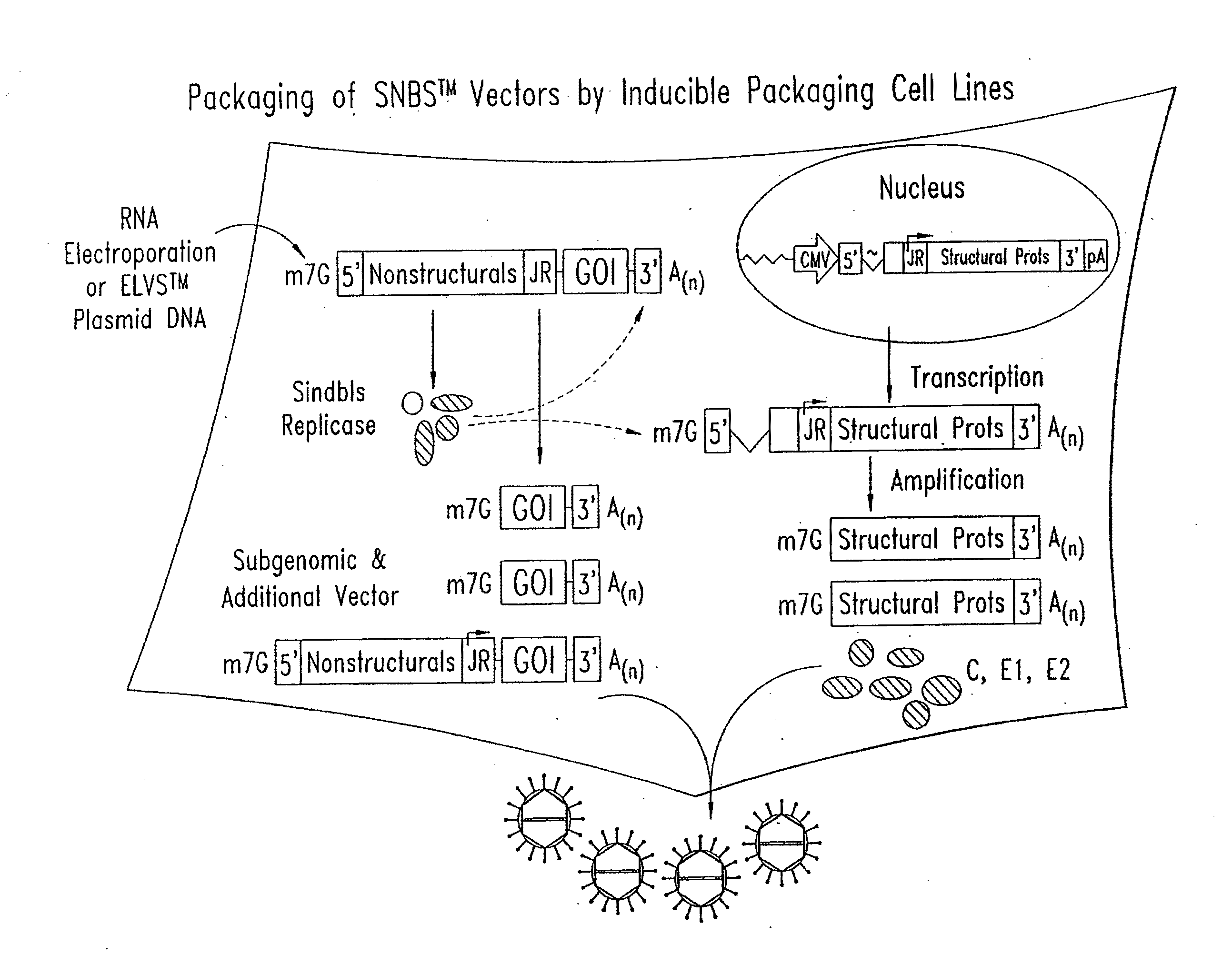
[0370]SIN-1 derived vector backbones were constructed and inserted into a plasmid DNA containing a bacteriophage RNA polymerase promoter, such that transcription in vitro produced an RNA molecule that acts as a self-replicating molecule (replicon) upon introduction into susceptible cells. The basic SIN-1 RNA vector replicon was comprised of the following ordered elements: SIN-1 nsPs genes, subgenomic RNA promoter region, a polylinker sequence, which may contain heterologous sequence insertions, the SIN-1 3′ non translated region (NTR), and a poly adenylate sequence. In addition, nsP genes of the desired phenotype, derived using methods such as those of Example 2, also may be substituted. Following transfection into susceptible cells, autonomous replication of the RNA vector replicon occurs as for virus, and the heterologous sequences are synthesized as highly abundant subgenomic mRNA molecules, wh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com