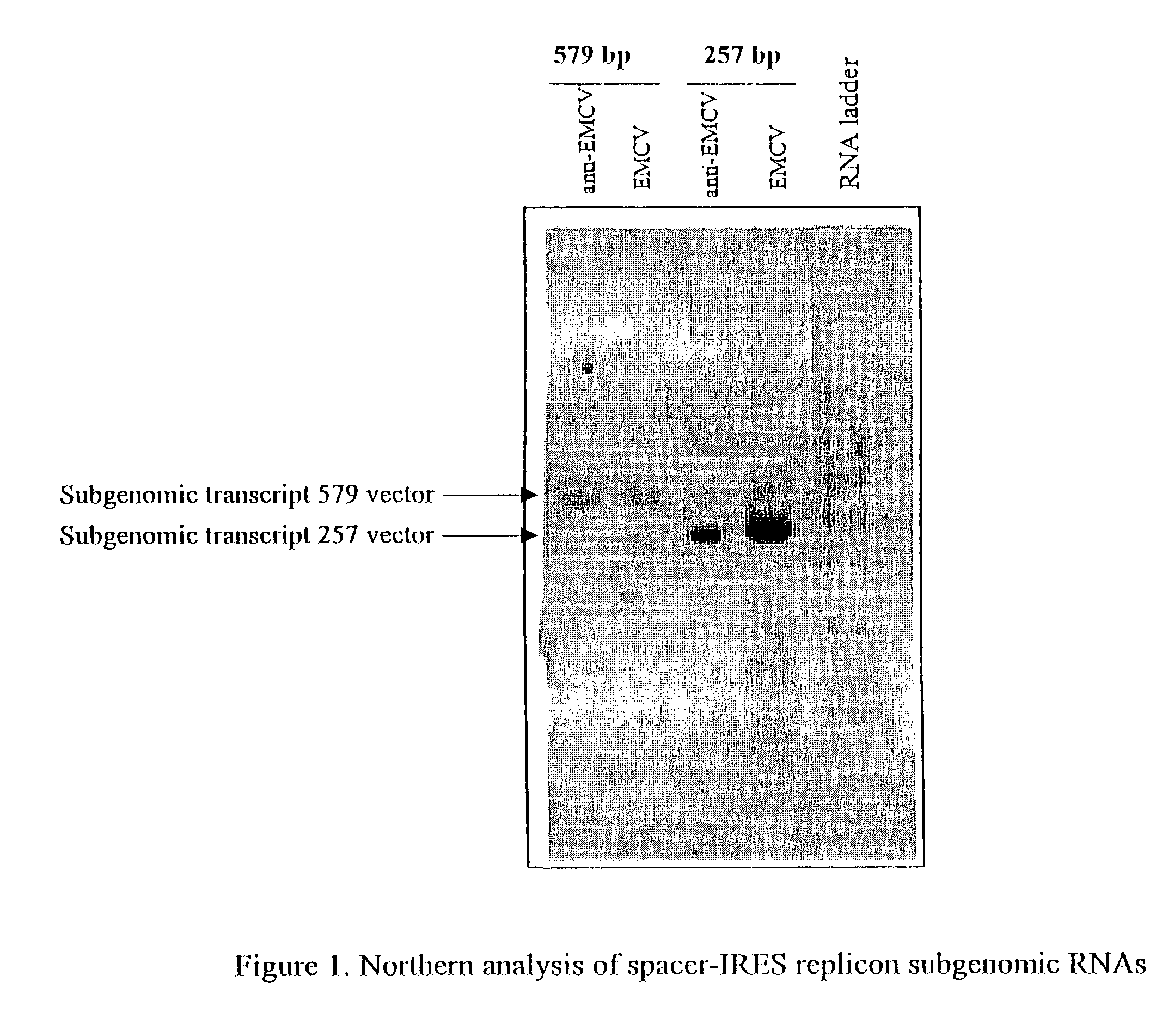
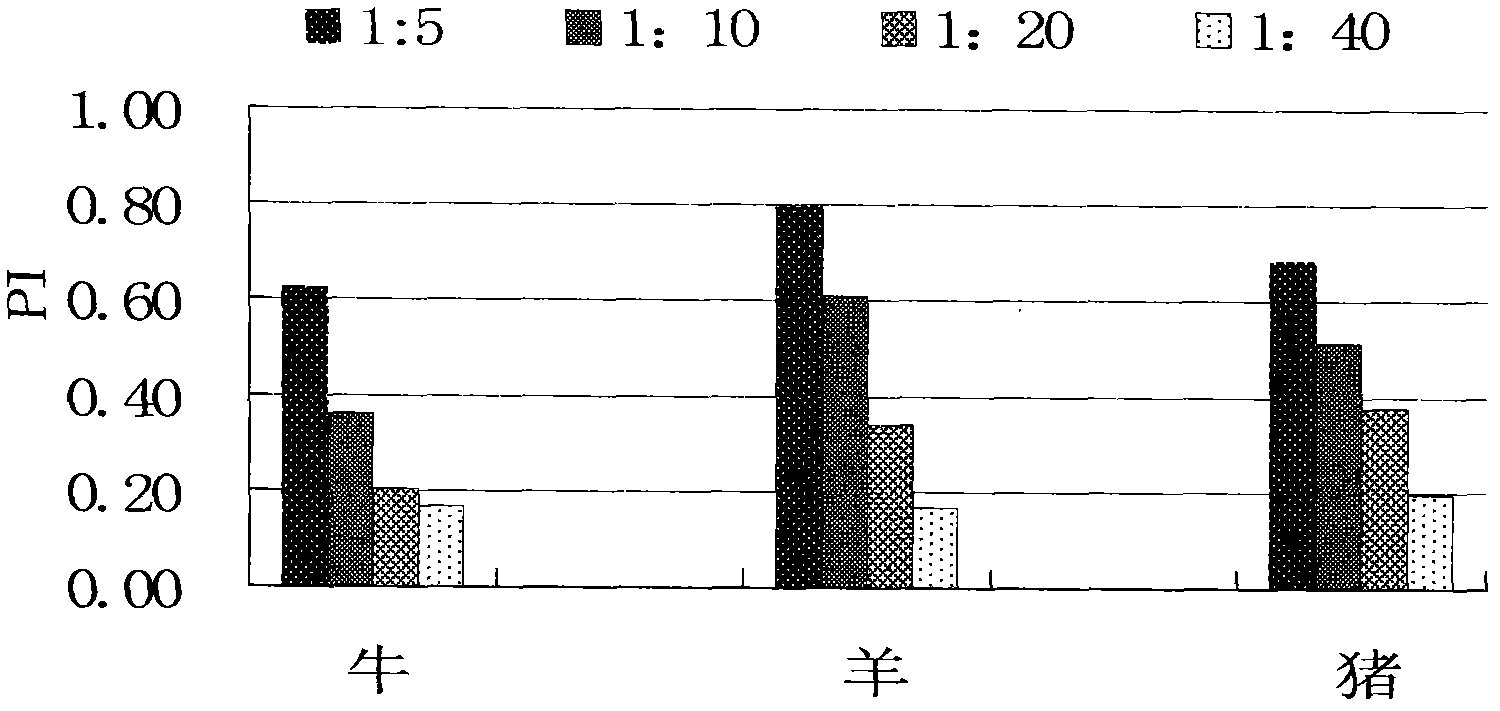
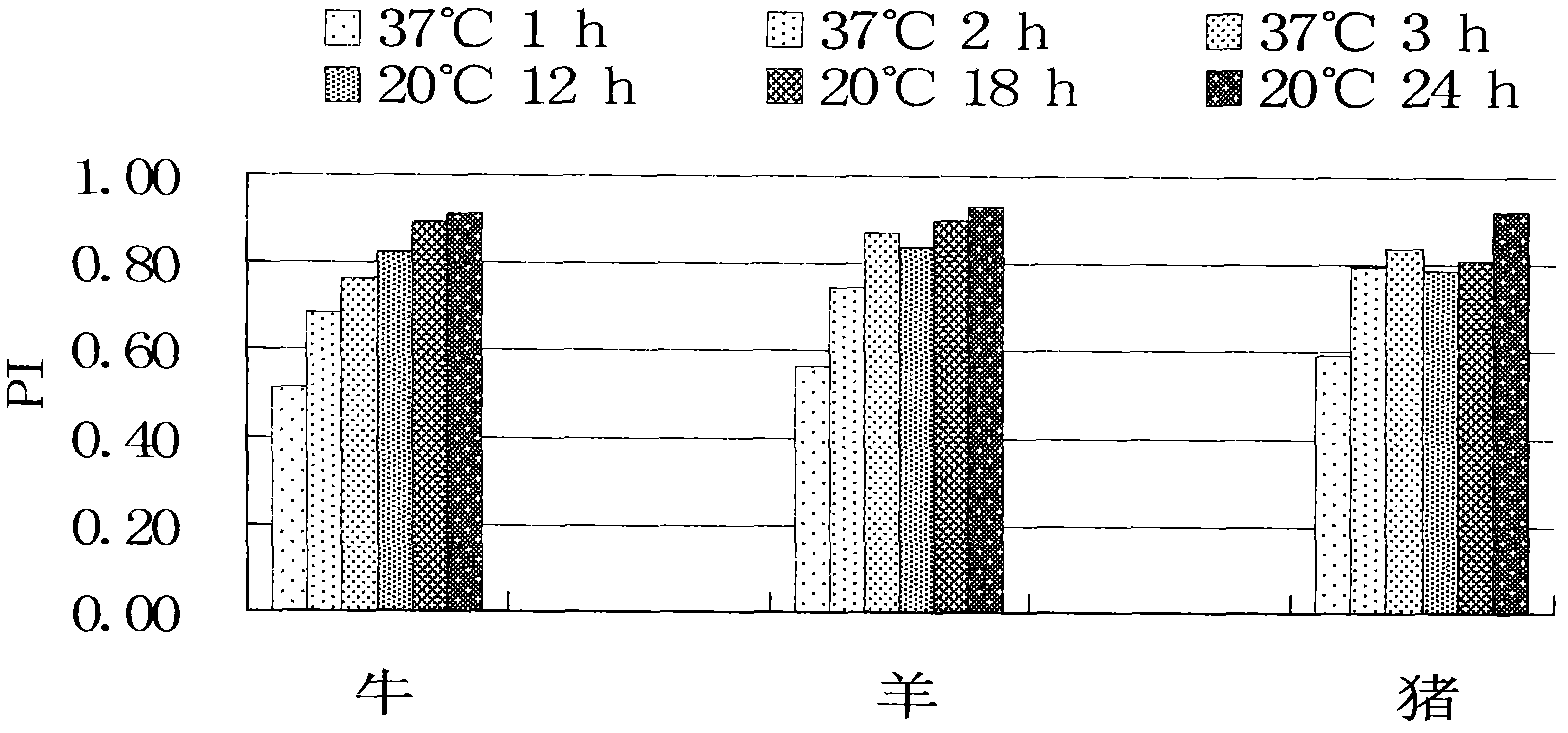
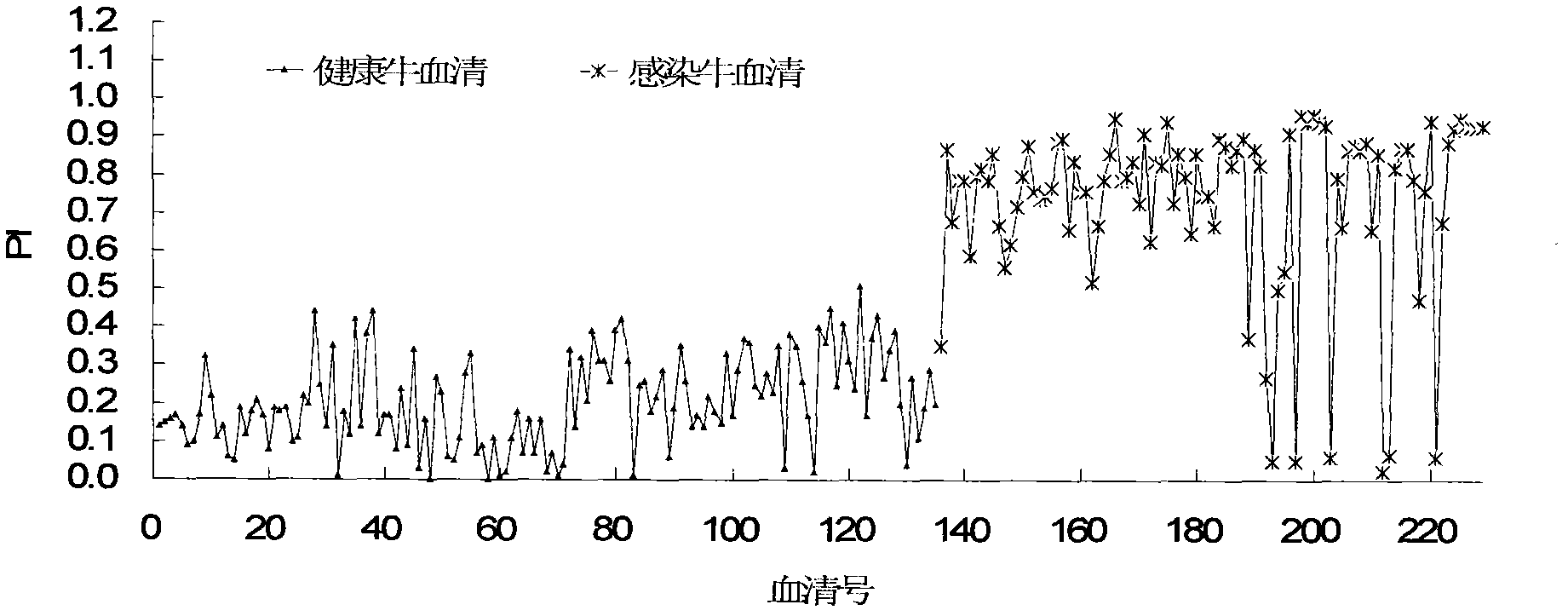
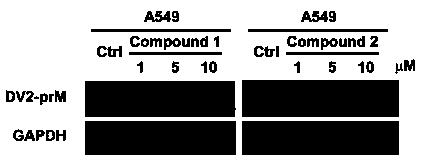
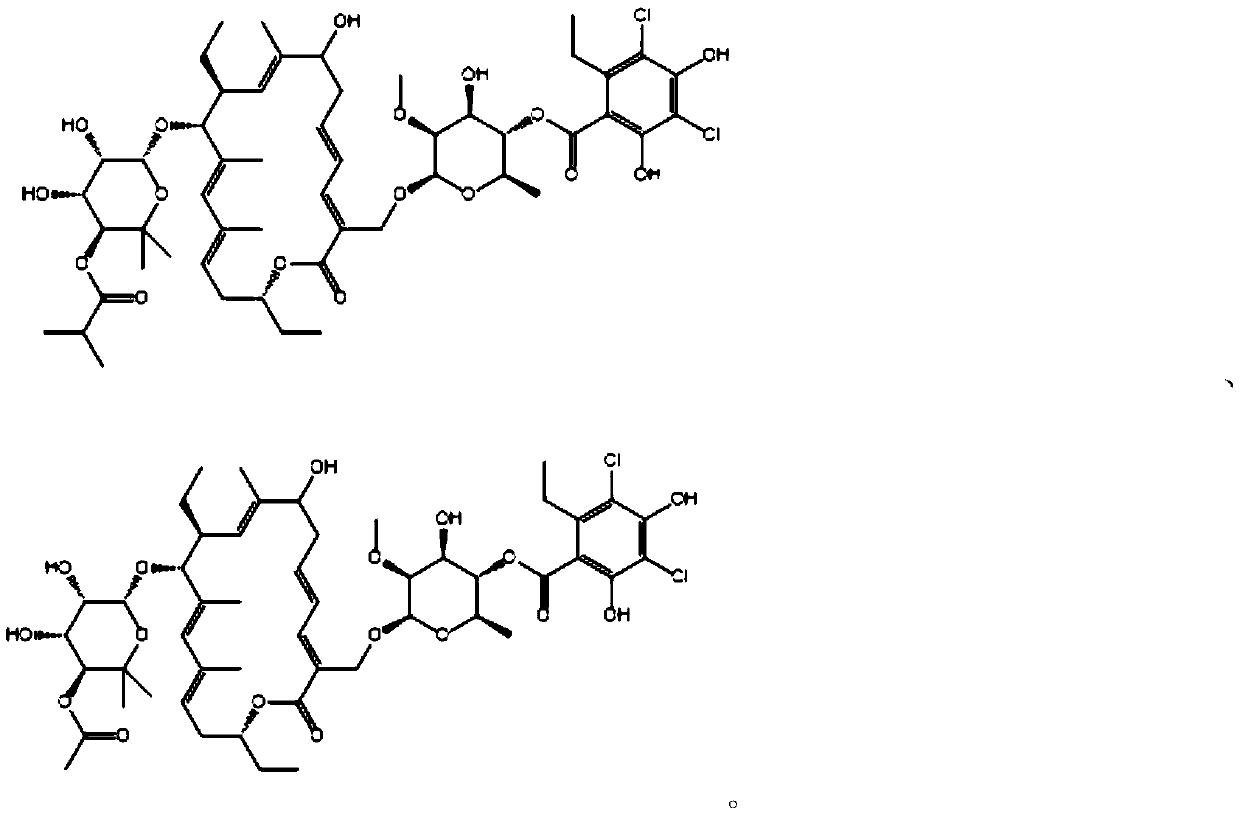
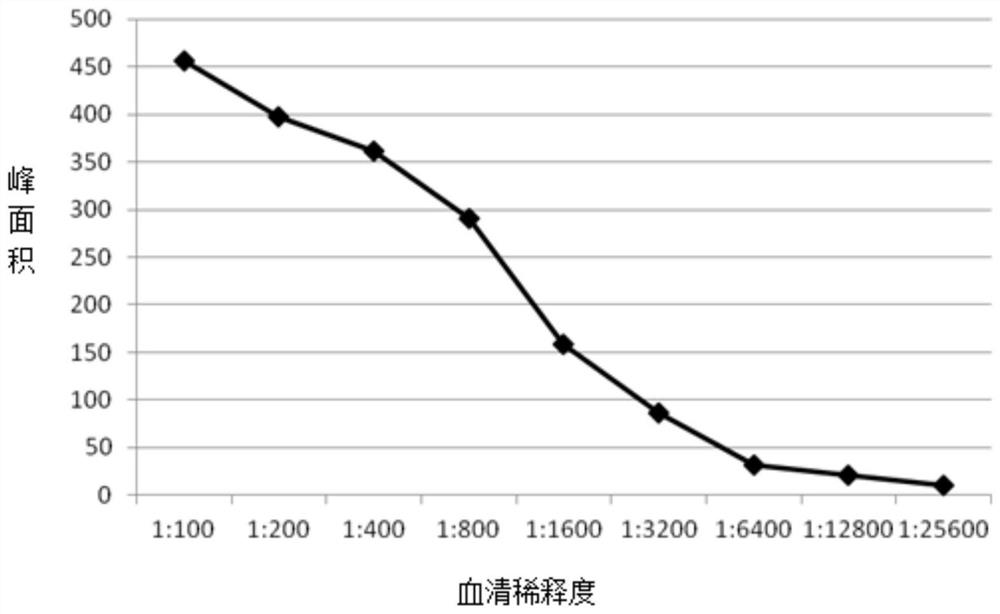
Patents
Literature
37 results about "Viral nonstructural protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
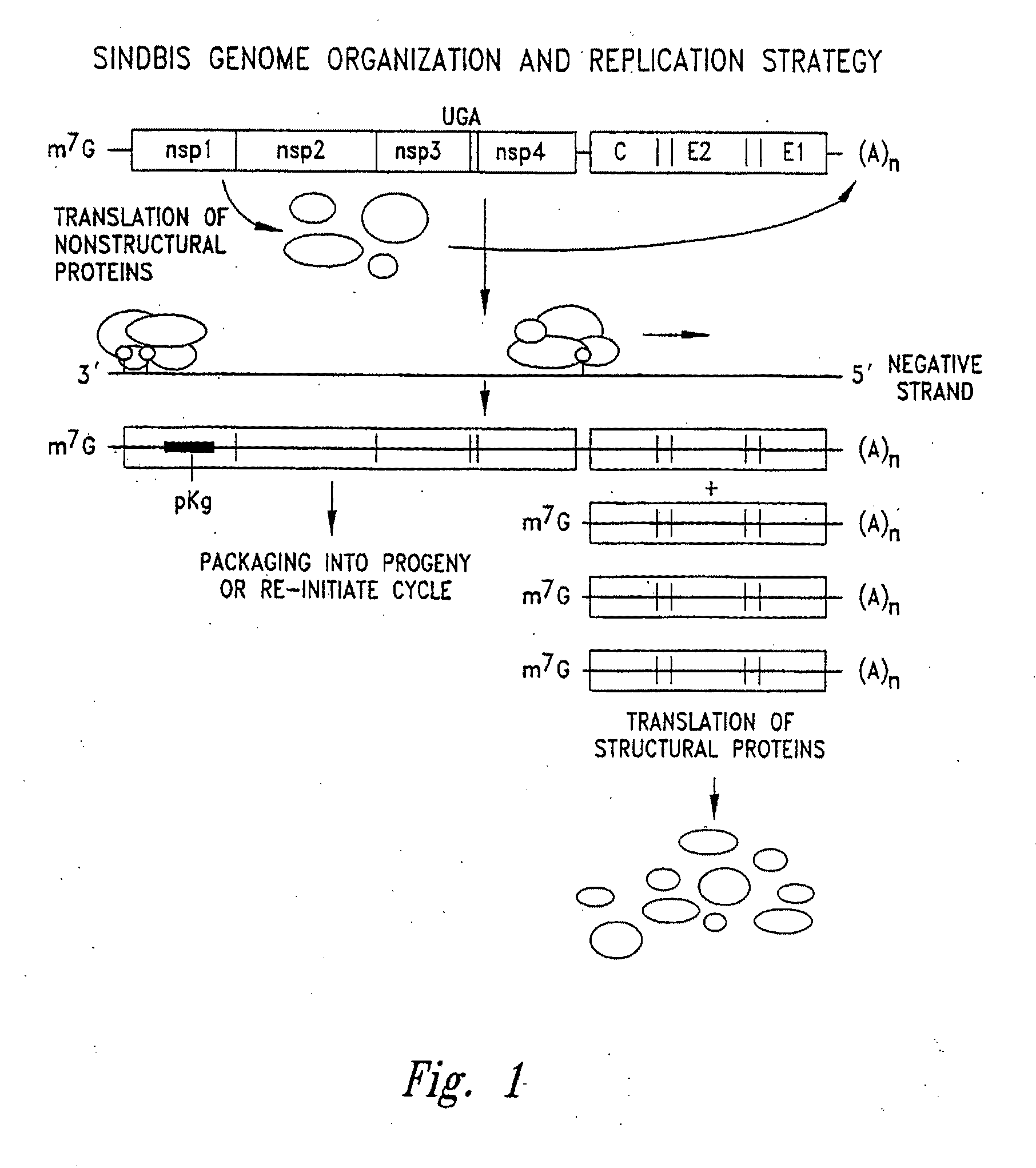
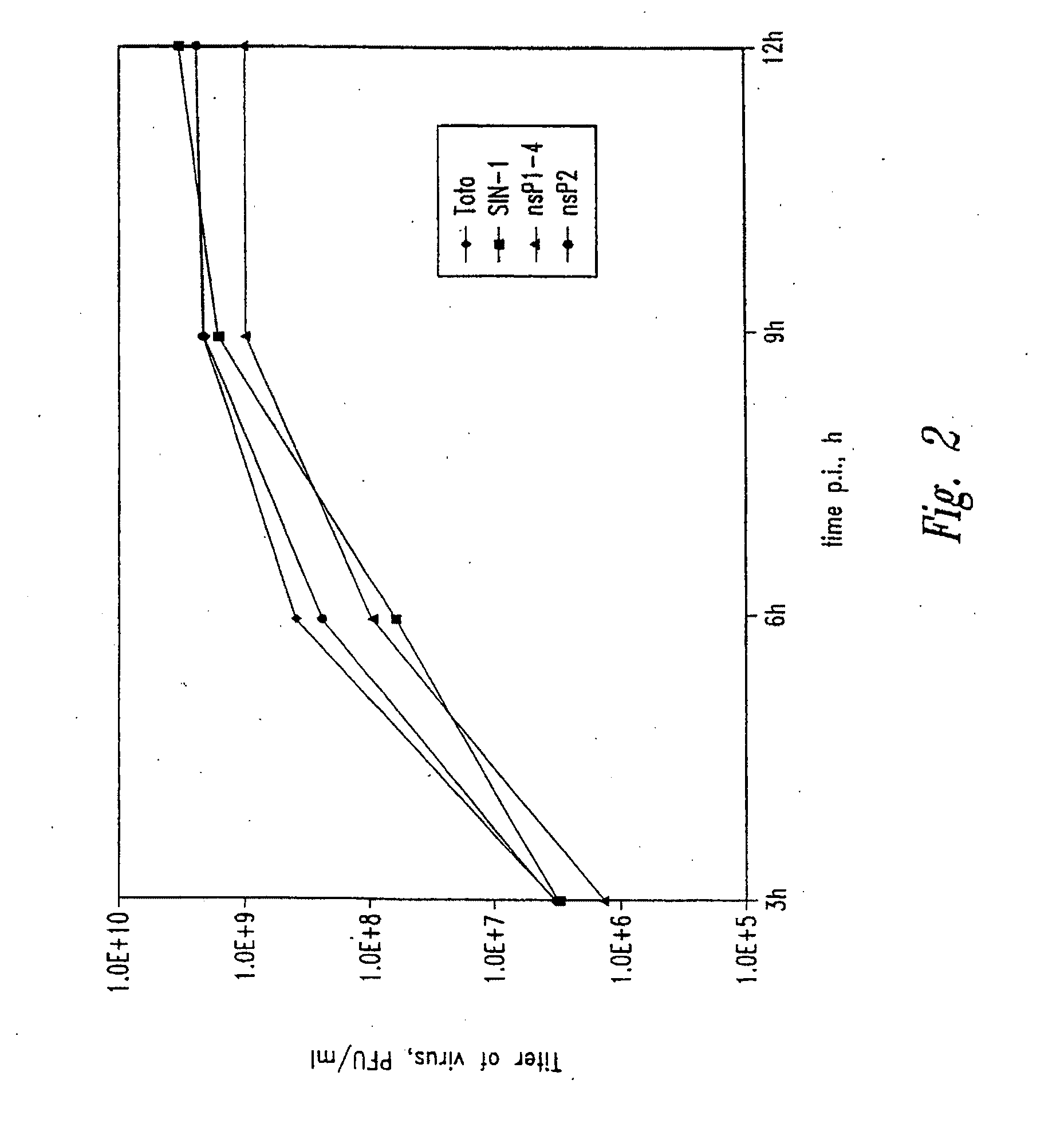
In virology, a nonstructural protein is a protein encoded by a virus but that is not part of the viral particle.
Alphavirus replicons and helper constructs
Owner:ALPHAVAX INC
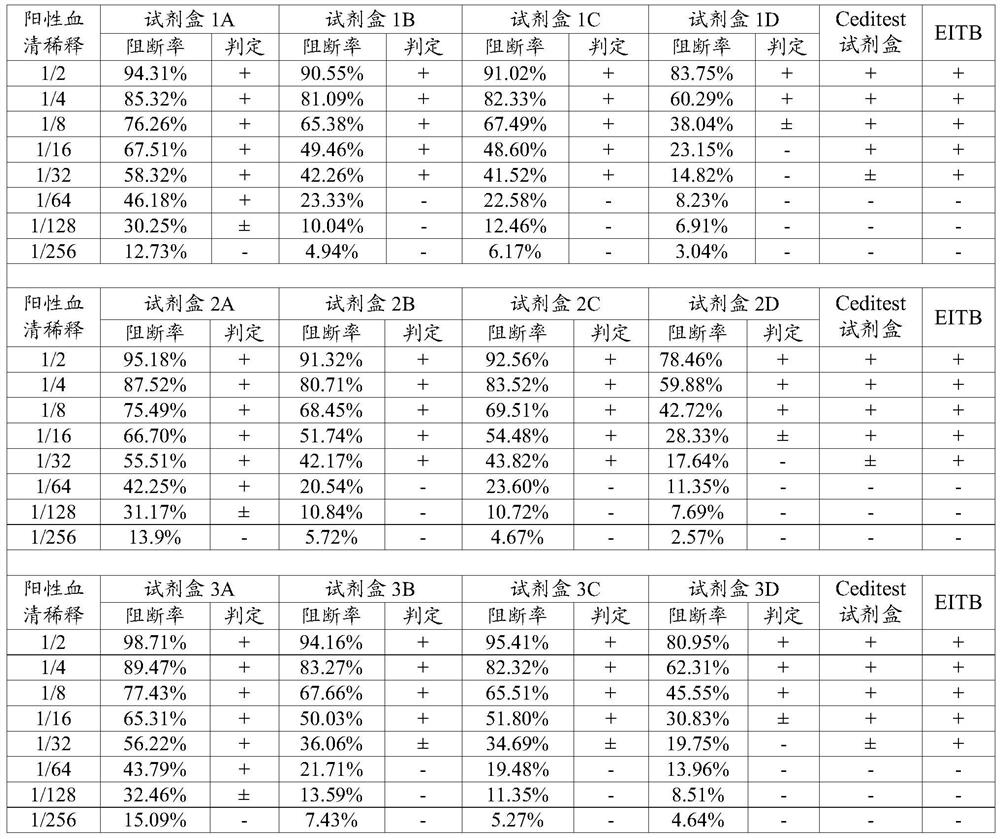
Monoclonal antibody blocking enzyme-linked immunosorbent assay (ELISA) kit and method for detecting nonstructural protein (NSP) antibody of foot-and-mouth disease virus (FMDV)
The invention discloses a monoclonal antibody blocking enzyme-linked immunosorbent assay (ELISA) kit and a method for detecting the nonstructural protein (NSP) antibody of a foot-and-mouth disease virus (FMDV) (FMD NSP B-ELISA); the kit comprises ELISA reaction plates, serum diluent, 25 times concentrated detergent, substrate solution, 100* concentrated ELISA detecting antibody, stop buffer, positive control serum and negative control serum; the ELISA reaction plates are two 96-pore high-affinity ELISA reaction plates, firstly 6* groups of amino acid monoclonal antibody or NSP 2C polyclonal antibody, and then FMDV 3ABC or 2C3AB NSP which is expressed by pronucleus and is provided with 6* groups of amino acid labels is captured through the monoclonal antibody or the polyclonal antibody; and compared with other similar kits, the method has higher coincidence rate and higher positive serum detection rate, and is applicable to detecting the serum of cattle, sheep, pigs and other susceptible animals.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Recombinant alphavirus-based vectors with reduced inhibition of cellular macromolecular synthesis
InactiveUS7811812B2Reduced and delayed and no inhibitionReduced and delayed and developmentFungiSsRNA viruses positive-senseWild typeInhibitory effect
Isolated nucleic acid molecules are disclosed, comprising an alphavirus nonstructural protein gene which, when operably incorporated into a recombinant alphavirus particle, eukaryotic layered vector initiation system, or RNA vector replicon, has a reduced level of vector-specific RNA synthesis, as compared to wild-type, and the same or greater level of proteins encoded by RNA transcribed from the viral junction region promoter, as compared to a wild-type recombinant alphavirus particle. Also disclosed are RNA vector replicons, alphavirus vector constructs, and eukaryotic layered vector initiation systems which contain the above-identified nucleic acid molecules.
Owner:CARDIOVENTION
Recombinant alphavirus-based vectors with reduced inhibition of cellular macro-molecular synthesis
InactiveUS20100330121A1Reduced and delayed and no inhibitionReduced delayed no developmentFungiSsRNA viruses positive-senseWild typeInhibitory effect
Isolated nucleic acid molecules are disclosed, comprising an alphavirus nonstructural protein gene which, when operably incorporated into a recombinant alphavirus particle, eukaryotic layered vector initiation system, or RNA vector replicon, has a reduced level of vector-specific RNA synthesis, as compared to wild-type, and the same or greater level of proteins encoded by RNA transcribed from the viral junction region promoter, as compared to a wild-type recombinant alphavirus particle. Also disclosed are RNA vector replicons, alphavirus vector constructs, and eukaryotic layered vector initiation systems which contain the above-identified nucleic acid molecules.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC +1
Virus-like particles for treatment of viral infections
The invention provides virus-like particles for treatment of viral infections based on the virus causing the infection. The virus-like particles comprise the virus recombinant proteins that form a capsid, recombinant virus membrane proteins attached to the capsid and vRNA packaged within said capsid. The vRNA is generated from a DNA sequence encoding a polypeptide capable of specifically binding to a constant region of a nonstructural protein of the virus that is essential for propagation of the virus.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Non-virus non-structural protein foot-and-mouth disease vaccine and preparation thereof
ActiveCN101322844ASimple methodEfficient methodImmunoglobulins against virusesAntiviralsDiseaseFoot mouth disease virus
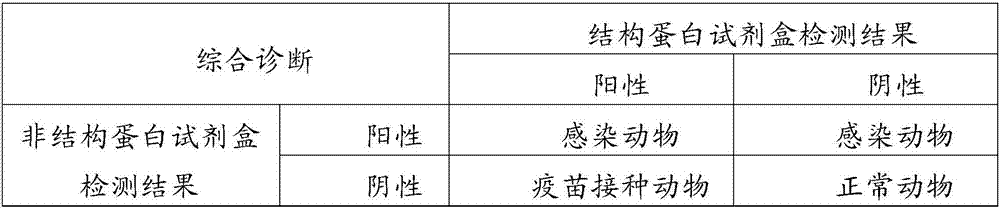
The invention discloses a method for preparing a foot-and-mouth disease vaccine. The preparation method of the invention comprises the steps that monoclonal antibodies of non-structure protein of foot and mouth disease virus is used for removing non-structure protein of foot and mouth disease virus in a target object which is then prepared into the foot-and-mouth disease vaccine, wherein, the target object is a foot-and-mouth disease virus culture or a gene engineering expression product comprising the non-structure protein of foot and mouth disease virus and structure protein of foot and mouth disease virus. The reliability is greatly improved when infected animals are distinguished from immune animals in vaccine immunization animals using the foot-and-mouth disease vaccine of the invention; in addition, the method for preparing the foot-and-mouth disease vaccine of virus-free non-structure protein is simple, convenient and effective without extra expensive equipment and can be applied on a large scale. Therefore, the foot-and-mouth disease vaccine of the virus-free non-structure protein and the preparation method thereof have great significance in the quarantine of the foot-and-mouth disease vaccine and are about to play important roles in the prevention and control of the foot-and-mouth disease.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Nonstructural protein antibody ELISA (enzyme-linked immunosorbent assay) Kit for porcine foot-and-mouth disease virus
ActiveCN107513101AIncreased sensitivityStrong specificitySsRNA viruses positive-senseVirus peptidesChemical synthesisEpitope
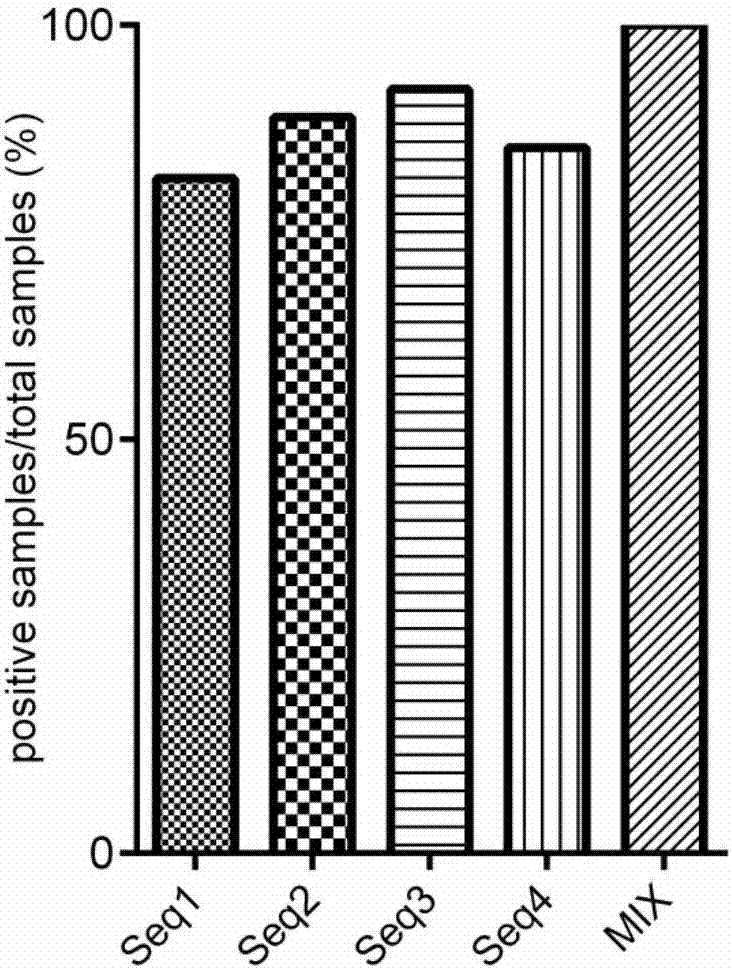
The invention discloses a nonstructural protein antibody ELISA (enzyme-linked immunosorbent assay) kit for a porcine foot-and-mouth disease virus. The kit comprises a foot-and-mouth disease virus nonstructural protein epitope polypeptide coated enzyme-linked reaction plate and an enzyme labeled antibody; foot-and-mouth disease virus nonstructural protein epitope polypeptides are a polypeptide shown as sequence 1, a polypeptide shown as sequence 2, a polypeptide shown as sequence 3 and a polypeptide shown as sequence 4 in a sequence table. According to the kit, a chemically synthesized nonstructural protein antigen peptide is used for coating the reaction plate, antigen dosage is small, sensitivity and specificity are high, and the presence of foot-and-mouth disease virus infection can be effectively detected. The kit provided by the invention has good specificity, sensitivity and high efficiency, and has good market prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Promoterless cassettes for expression of alphavirus structural proteins
ActiveCN101802199ASsRNA viruses positive-senseCell receptors/surface-antigens/surface-determinantsStart codonNucleotide
The present invention provides an isolated RNA molecule comprising: a) an alphavirus 5' replication recognition sequence, wherein at least one initiation codon has been removed from the 5' replication recognition sequence; b) a nucleotide sequence encoding an alphavirus structural protein; and c) an alphavirus 3' replication recognition sequence, with the proviso that the RNA molecule does not contain a promoter that directs transcription of the nucleotide sequence of (b), and wherein the alphavirus 5' and 3' replication recognition sequences of (a) and (c) direct replication of the RNA molecule in the presence of alphavirus nonstructural proteins.
Owner:ALPHAVAX INC
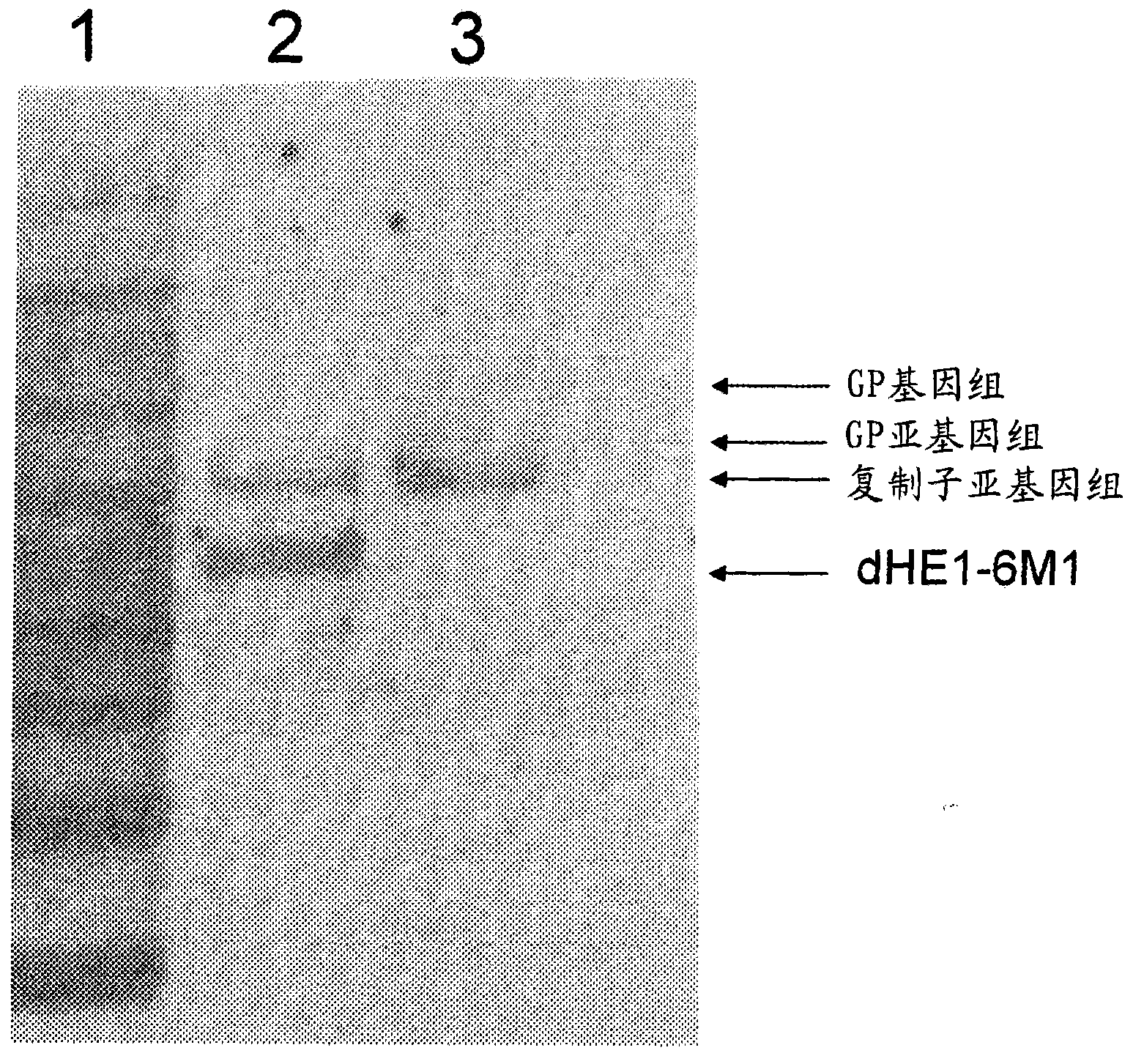
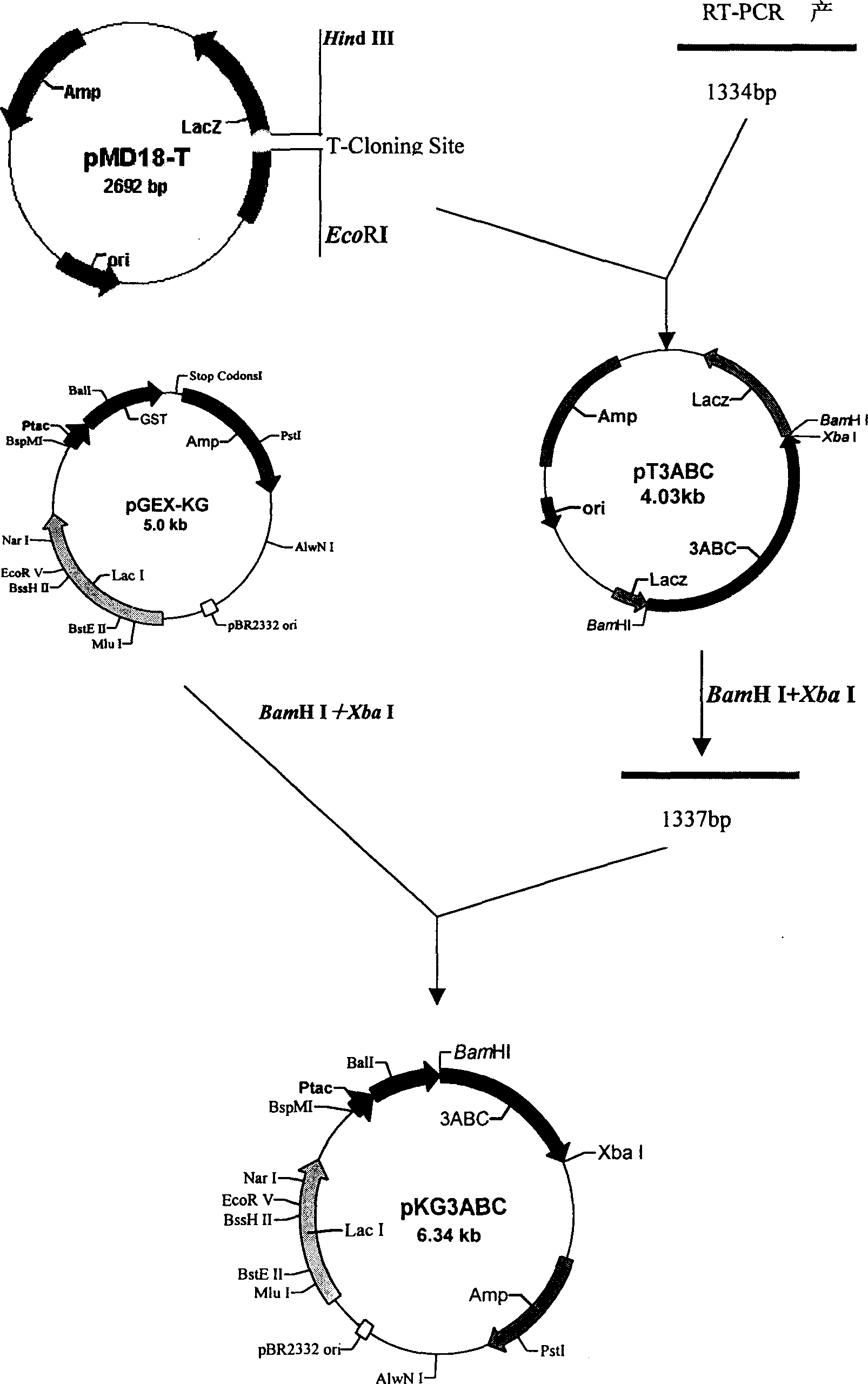
Non-structural protein gene 3ABC of foot-and-mouth disease virus and its preparation and use
The present invention relates to non-structural protein gene 3ABC of foot-and-mouth disease virus, prokaryotic expression vector constituted with the gene, recombinant expression strain Escherichia coli BL21 / pKG3ABC obtained through transforming the vector to colibacillus BL21 and screening, method of expressing non-structural protein gene 3ABC of foot-and-mouth disease virus with the expression strain and purifying the expression protein, and the ELISA discrimination and diagnosis method established with the expression protein. The recombinant expression strain Escherichia coli BL21 / pKG3ABC is preserved in CCTCC and in the preservation number of CCTCC M203068.
Owner:HUAZHONG AGRI UNIV
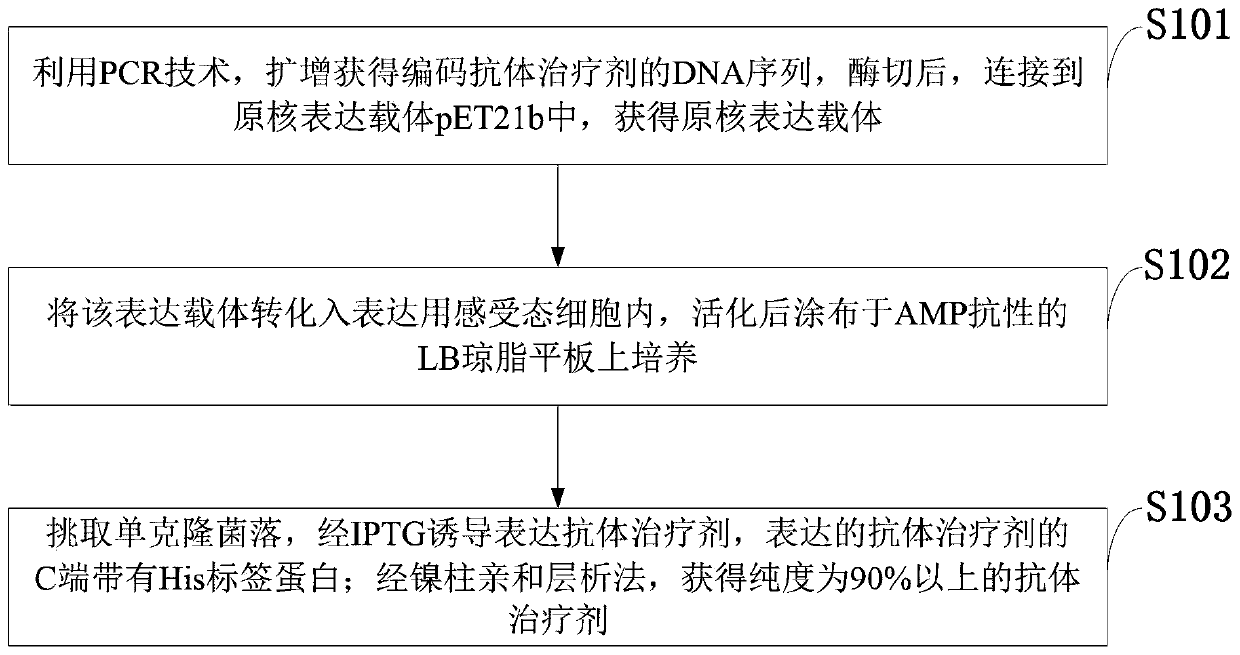
Antibody for inhibiting replication of PRRS virus, expression vector and therapeutic agent
InactiveCN109705212AInhibition of replicationRealize the effect of anti-PRRS virus proliferationImmunoglobulins against virusesAntiviralsNucleotideStructural protein
The present invention belongs to the technical field of genetic engineering, and discloses an antibody for inhibiting replication of a PRRS virus, an expression vector and a therapeutic agent. A nucleotide sequence of the antibody for inhibiting replication of the PRRS virus is SEQ ID NO:1. The expression vector of the present invention is capable of expressing an antibody therapeutic agent capable of entering cells, specifically binding to a PRRS virus non-structural protein Nsp9, and inhibiting proliferation of the PRRS virus in PAMs cells. The antibody therapeutic agent can be used to develop a drug for treating PRRS. The interaction of the antibody therapeutic agent with the PRRS virus Nsp9 is demonstrated by enzyme-linked immunoassay. The cells are incubated with the antibody therapeutic agent, and indirect immunofluorescence experiments are performed to demonstrate that the antibody therapeutic agent is able to enter the cells. At the same time, the antibody therapeutic agent isused for virus inhibition test to prove that the antibody therapeutic agent can inhibit the replication of the PRRS virus.
Owner:NORTHWEST A & F UNIV
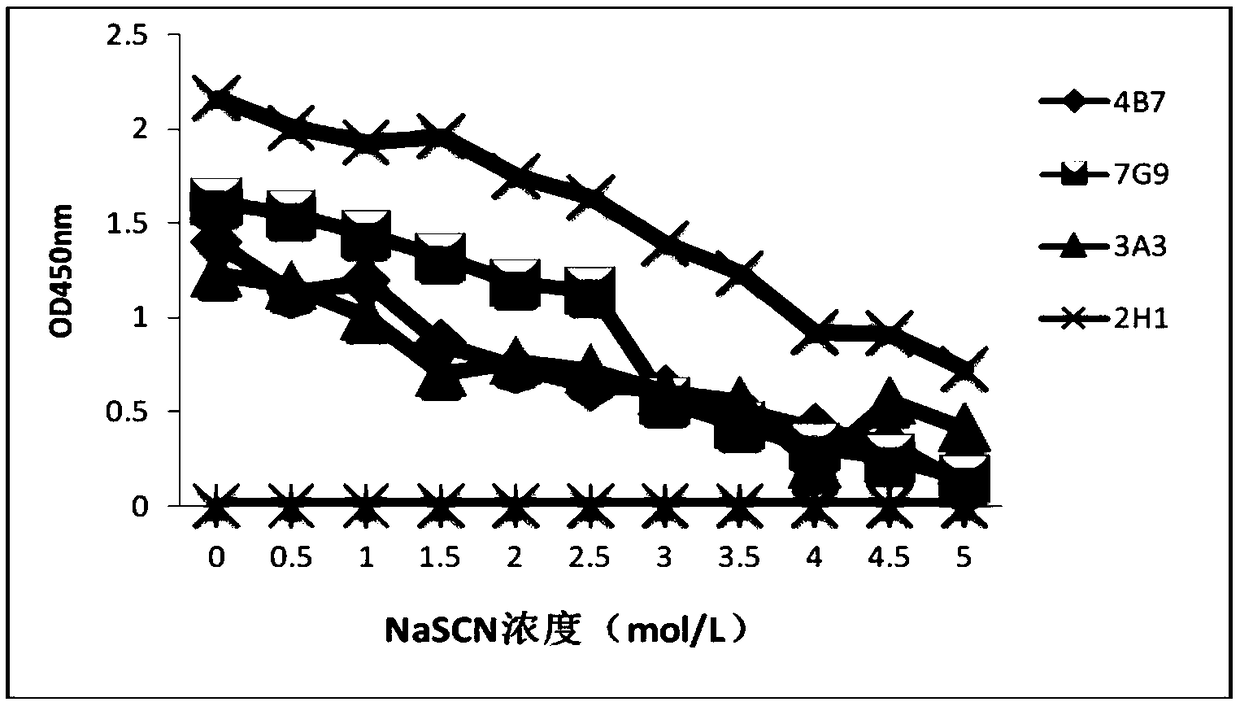
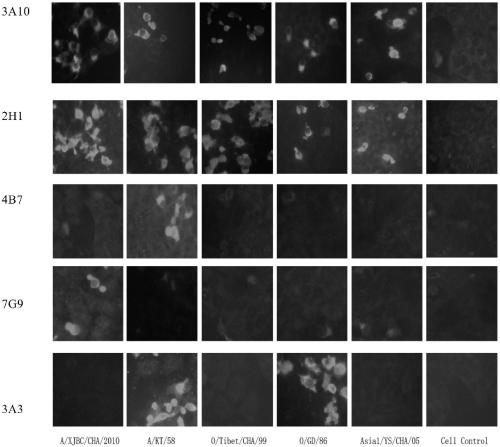
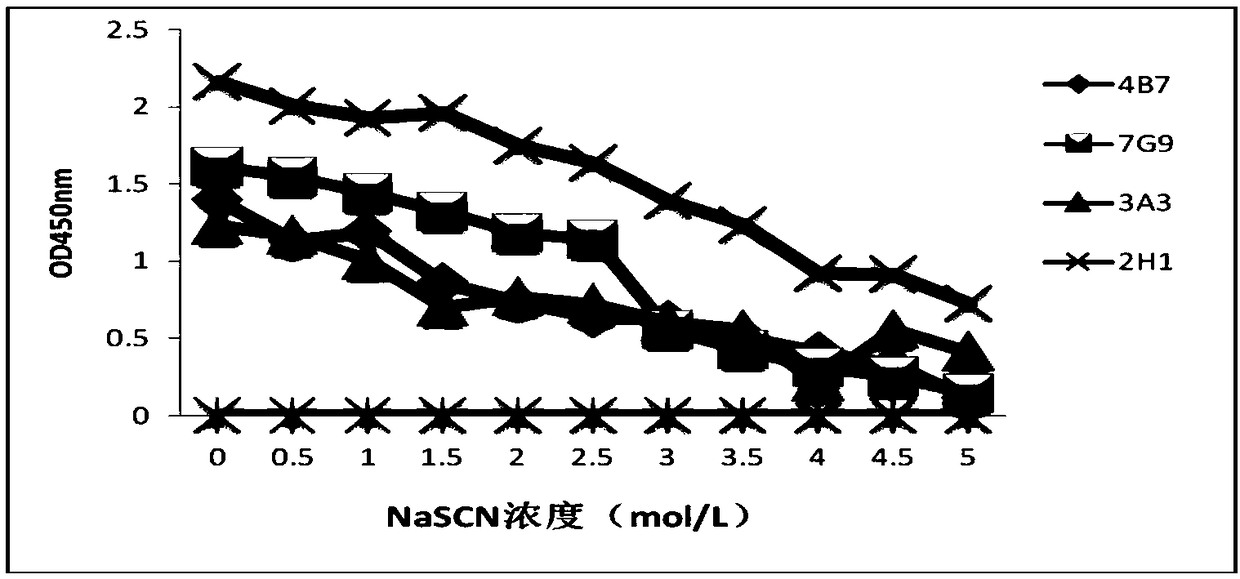
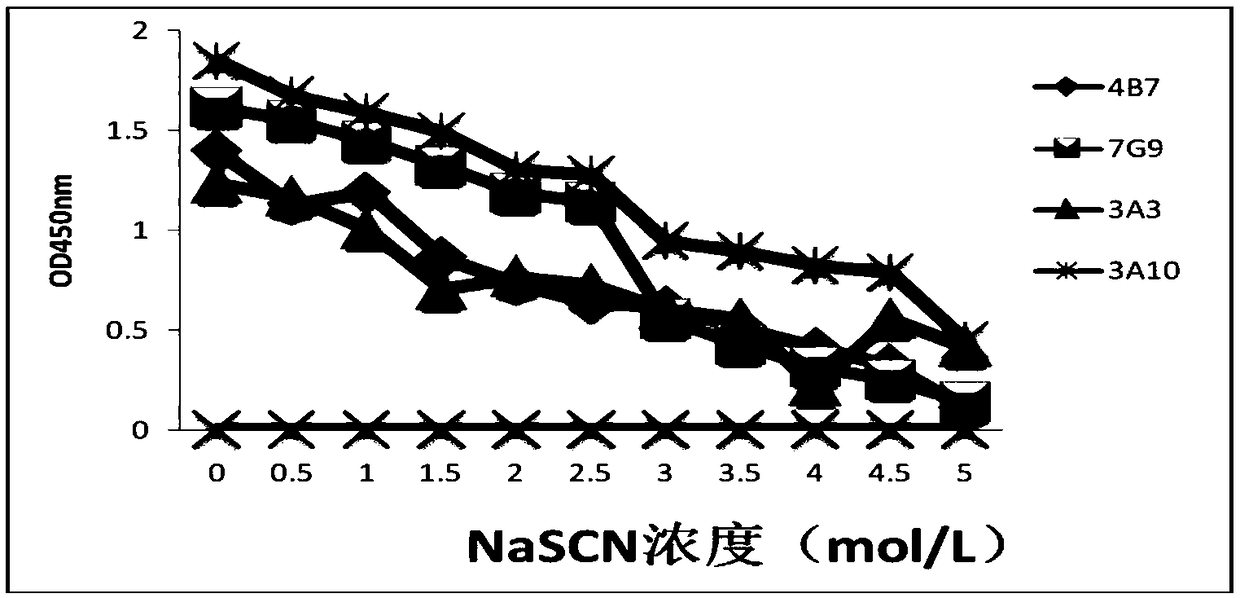
Hybridoma cell line secreting foot-and-mouth disease virus non-structural protein monoclonal antibody 3A10, and application thereof
ActiveCN109295006AGood broad spectrumHighly conservativeBiological material analysisImmunoglobulins against virusesStructural proteinViral nonstructural protein
The invention discloses a hybridoma cell line secreting a foot-and-mouth disease virus non-structural protein monoclonal antibody 3A10, and an application thereof. The hybridoma cell line stably secreting the foot-and-mouth disease virus non-structural protein monoclonal antibody, with the microbial preservation number of CGMCC NO.16213, is finally obtained through screening using a cell fusion technology by immunizing mice with the foot-and-mouth disease non-structural protein. The monoclonal antibody secreted by the hybridoma cell line has the advantages of good broad spectrum, high conservatism, strong affinity and strong competitiveness with serum, and can be used as a detection antibody in a blocking ELISA detection method to accurately distinguish naturally infected animals with thefoot-and-mouth disease and immunized animals. The invention further provides a blocking ELISA detection kit established by using the monoclonal antibody as a detection antibody. The detection kit canbe adopted to detect the serum of any animal species, spans the inter-species barrier, and has the advantages of high reliability, wide application range, good broad spectrum, strong competitiveness,and quickness and convenience in detection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Recombinant alphavirus vectors and methods of using same
InactiveUS20080096274A1SsRNA viruses positive-senseSugar derivativesTherapeutic proteinSindbis virus
The present invention describes nucleic acid segments, recombinant alphavirus vectors, and recombinant alphavirus particles that include a Sindbis viral vector. The Sindbis viral vector includes a nucleic acid segment encoding a fusion protein comprising a Sindbis virus nonstructural protein and a protein or peptide of interest, wherein the production of the fusion protein does not affect viral replication or infection. The protein or peptide of interest may be a marker, diagnostic, or therapeutic protein or peptide. Methods of using such recombinant alphavirus particles to kill tumor cells is also described herein.
Owner:OKLAHOMA THE BOARD OF RGT UNIV OF
Preparation method and application of double-effect vaccine for dengue fever
ActiveCN105601721AStop transmissionReduce bleedingSsRNA viruses positive-senseViral antigen ingredientsWAS PROTEINStructural protein
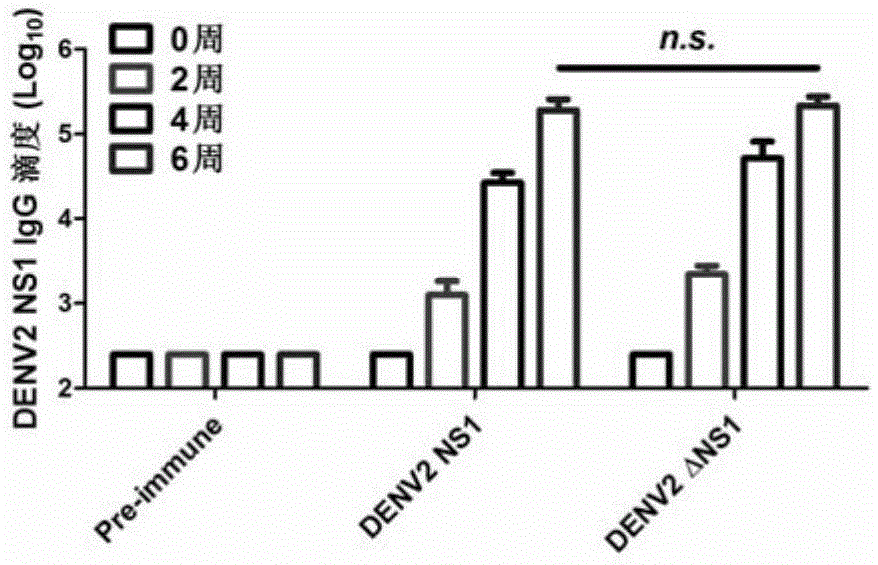
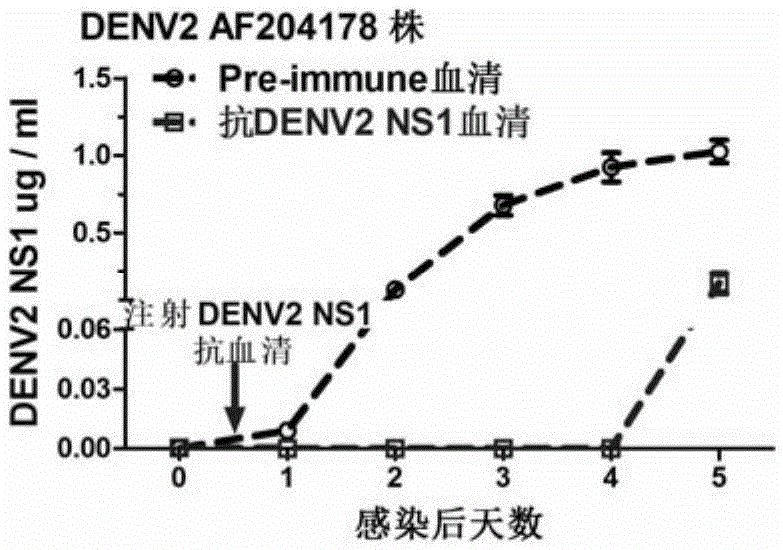
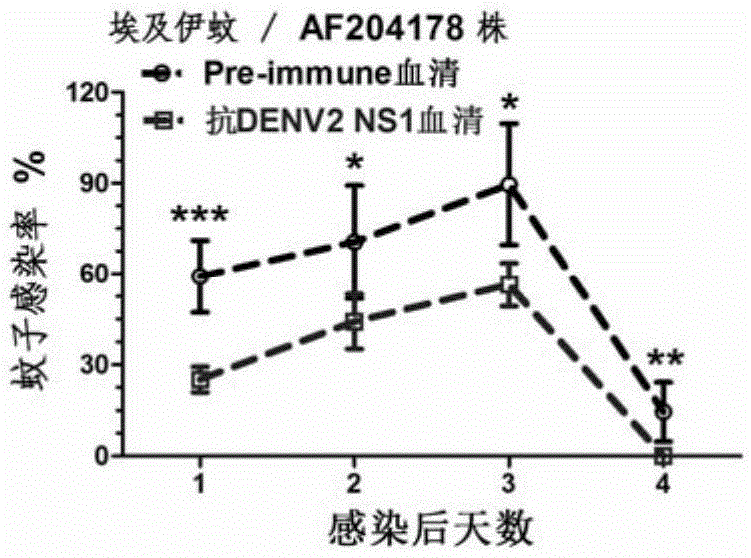
The invention discloses a preparation method and application of a double-effect vaccine for the dengue fever. The invention provides a protein which is protein a) or protein b, wherein protein a) is a protein composed of an amino acid sequence as shown in a sequence 4 in a sequence table, and protein b) is a protein which has same functions as protein a) and is derived from the protein a) by subjecting the sequence 4 in the sequence table to substitution and / or deletion and / or addition of one or more amino acid residues. Experiments in the invention prove that reconstructed dengue virus non-structural protein 1 (DENV delta NS1) can be used as the vaccine; and the double-effect vaccine can protect mankind or mammals from dengue haemorrhagic fever and block propagation of the dengue virus in nature via mosquitoes.
Owner:TSINGHUA UNIV
Detection card for rapidly detecting nonstructural protein antibodies of FMDV (foot-and-mouth disease virus) in sera
InactiveCN107870243AQuick checkHigh sensitivitySsRNA viruses positive-senseVirus peptidesAntigenMicrosphere
The invention discloses a detection card for rapidly detecting nonstructural protein antibodies of an FMDV (foot-and-mouth disease virus) in sera. The detection card comprises a detection card housingand a test strip assembled in a detection card housing, wherein the test strip comprises a plastic base plate with a pressure-sensitive adhesive, and a sample pad, a marker pad, a nitrocellulose membrane and absorbent paper are adhered to the base plate in sequence; the marker pad comprises a carrier substrate and a marker, and is a membrane formed by spraying lanthanide fluorescence detection microspheres and lanthanide fluorescence quality control microspheres on the carrier substrate; the nitrocellulose membrane is coated with recombinant protein of FMDV nonstructural protein as a detection line and coated with rabbit anti-chicken lgY antibodies as a control line, and the markers are the fluorescence detection microspheres labeled with FMDV nonstructural protein recombinant antigens and the fluorescence quality control microspheres labeled with chicken lgY antibodies. The detection card can realize rapid detection of the nonstructural protein antibodies of the FMDV on site and in the field and is used for distinguishing virus infection animals from vaccinated animals.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
Hybridoma cell line secreting foot-and-mouth disease virus non-structural protein monoclonal antibody 2H1, and application thereof
ActiveCN109295005AAccurate distinctionHighly conservativeBiological material analysisMicroorganism based processesStructural proteinViral nonstructural protein
The invention discloses a hybridoma cell line secreting a foot-and-mouth disease virus non-structural protein monoclonal antibody 2H1, and an application thereof. The hybridoma cell line stably secreting the foot-and-mouth disease virus non-structural protein monoclonal antibody, with the microbial preservation number of CGMCC NO.16212, is finally obtained through screening using a cell fusion technology by immunizing mice with the foot-and-mouth disease non-structural protein. The monoclonal antibody secreted by the hybridoma cell line has the advantages of good broad spectrum, high conservatism, strong affinity and strong competitiveness with serum, and can be used as a detection antibody in a blocking ELISA detection method to accurately distinguish naturally infected animals with the foot-and-mouth disease and immunized animals. The invention further provides a blocking ELISA detection kit established by using the monoclonal antibody as a detection antibody. The detection kit can be adopted to detect the serum of any animal species, spans the inter-species barrier, and has the advantages of high reliability, wide application range, good broad spectrum, strong competitiveness, and quickness and convenience in detection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
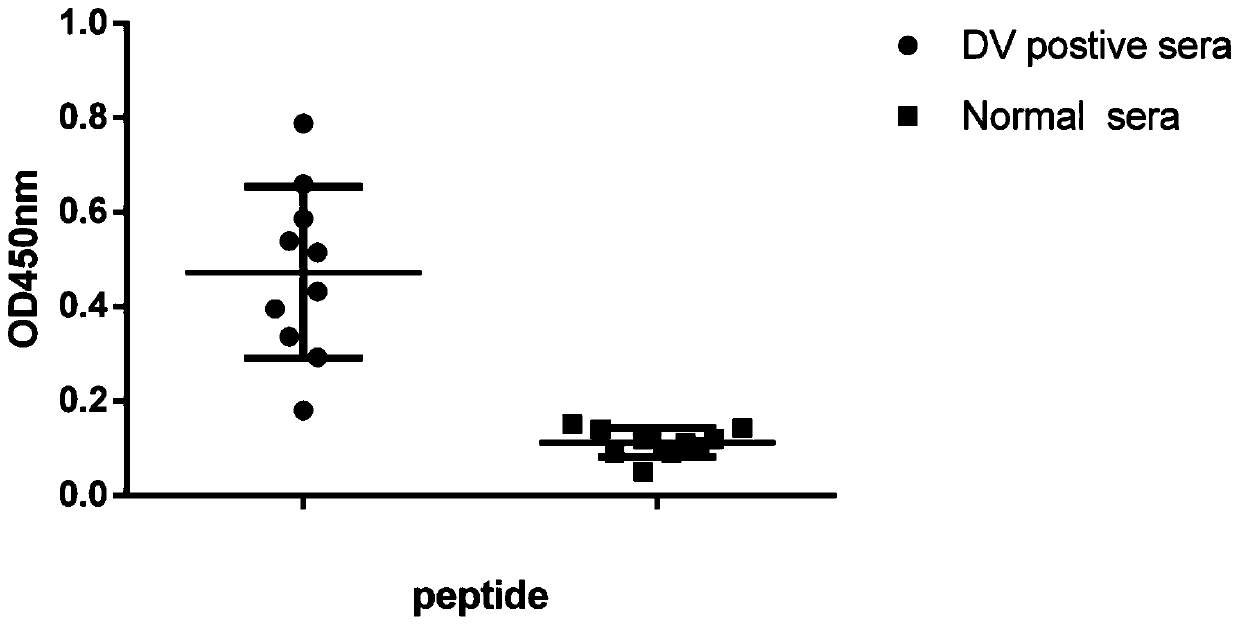
Antigen epitope polypeptide located on nostructal protein 1 of dengue virus and application of antigen epitope polypeptide
ActiveCN111378019AImprove responseSsRNA viruses positive-senseAntibody mimetics/scaffoldsAntigen epitopeAntigen
The invention discloses an antigen epitope polypeptide located on a nostructal protein 1 of a dengue virus and application of the antigen epitope polypeptide. An antigen polypeptide with high reactivity is screened out from a sequence of the nostructal protein 1 of the dengue virus and is subjected to recombinant expression. Verified by tests, the polypeptide can have relatively high reactivity toIgM in blood serum of a dengue virus infected patient. The antigen epitope polypeptide disclosed by the invention has a huge potential value in early diagnosis on DENV infections and epidemiologicalinvestigations.
Owner:中国人民解放军南部战区疾病预防控制中心
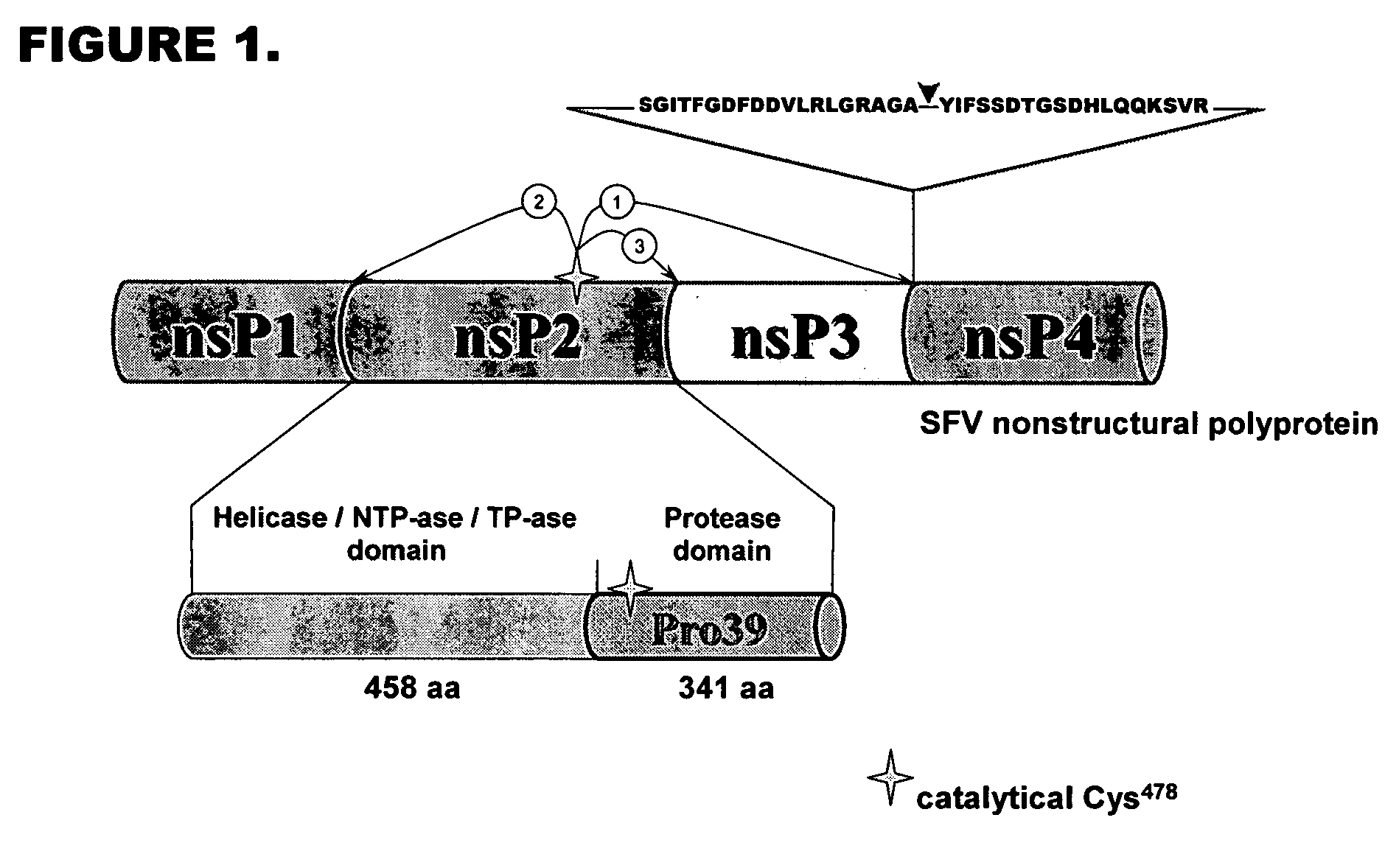
Optimized recognition site of the alphavirus non-structural protease for tag removal and specific processing of recombinant proteins
InactiveUS20050282254A1Easy to useImprove efficiencySsRNA viruses positive-senseBacteriaProteinase activityProtein purification
The present invention discloses highly efficient novel recognition sites for Pro39 protease. The invention further provides a wide range of conditions for protein purification and modification with the novel recognition sites. The invention even further provides expression vectors for expression of fusion proteins in cells and method to purify fusion proteins.
Owner:QUATTROMED
Virus-like particles for treatment of viral infections
The invention provides virus-like particles for treatment of viral infections based on the virus causing the infection. The virus-like particles comprise the virus recombinant proteins that form a capsid, recombinant virus membrane proteins attached to the capsid and vRNA packaged within said capsid. The vRNA is generated from a DNA sequence encoding a polypeptide capable of specifically binding to a constant region of a nonstructural protein of the virus that is essential for propagation of the virus.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Method of elevating prediction accuracy of grouping subjects with severe dengue infection
PendingUS20220268774A1Improve forecast accuracyReduce rateDisease diagnosisBiological testingStructural proteinSevere dengue
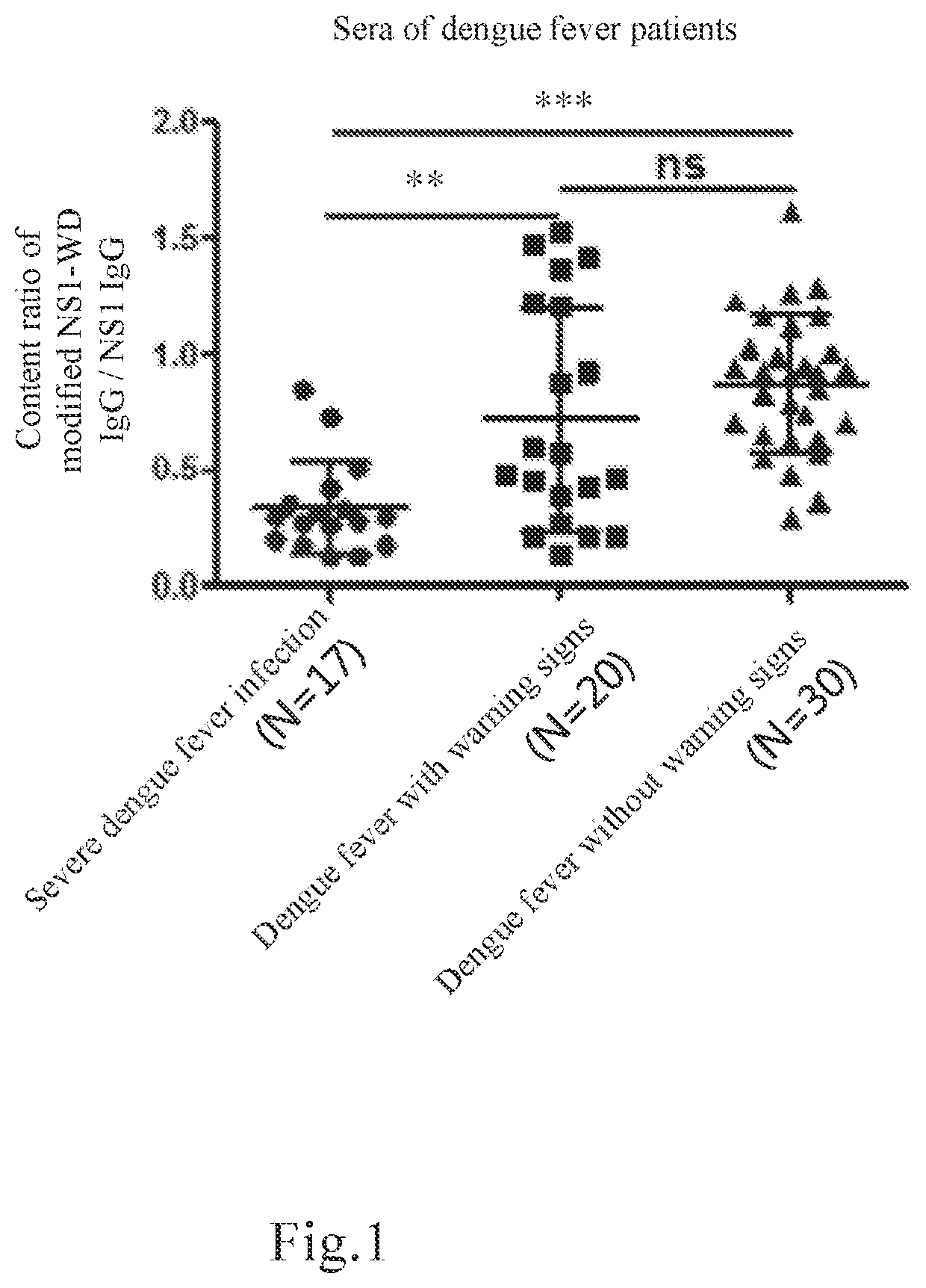
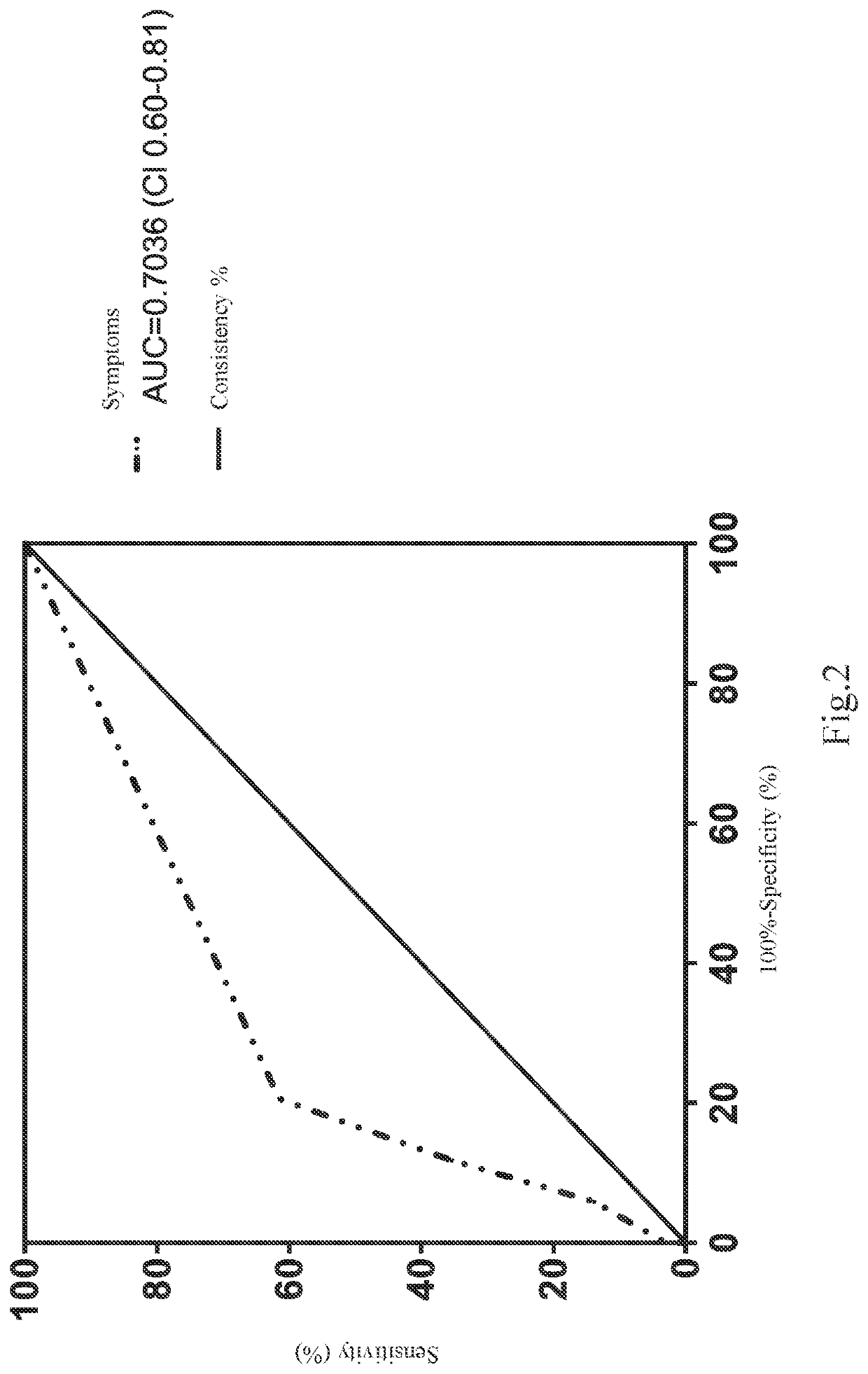
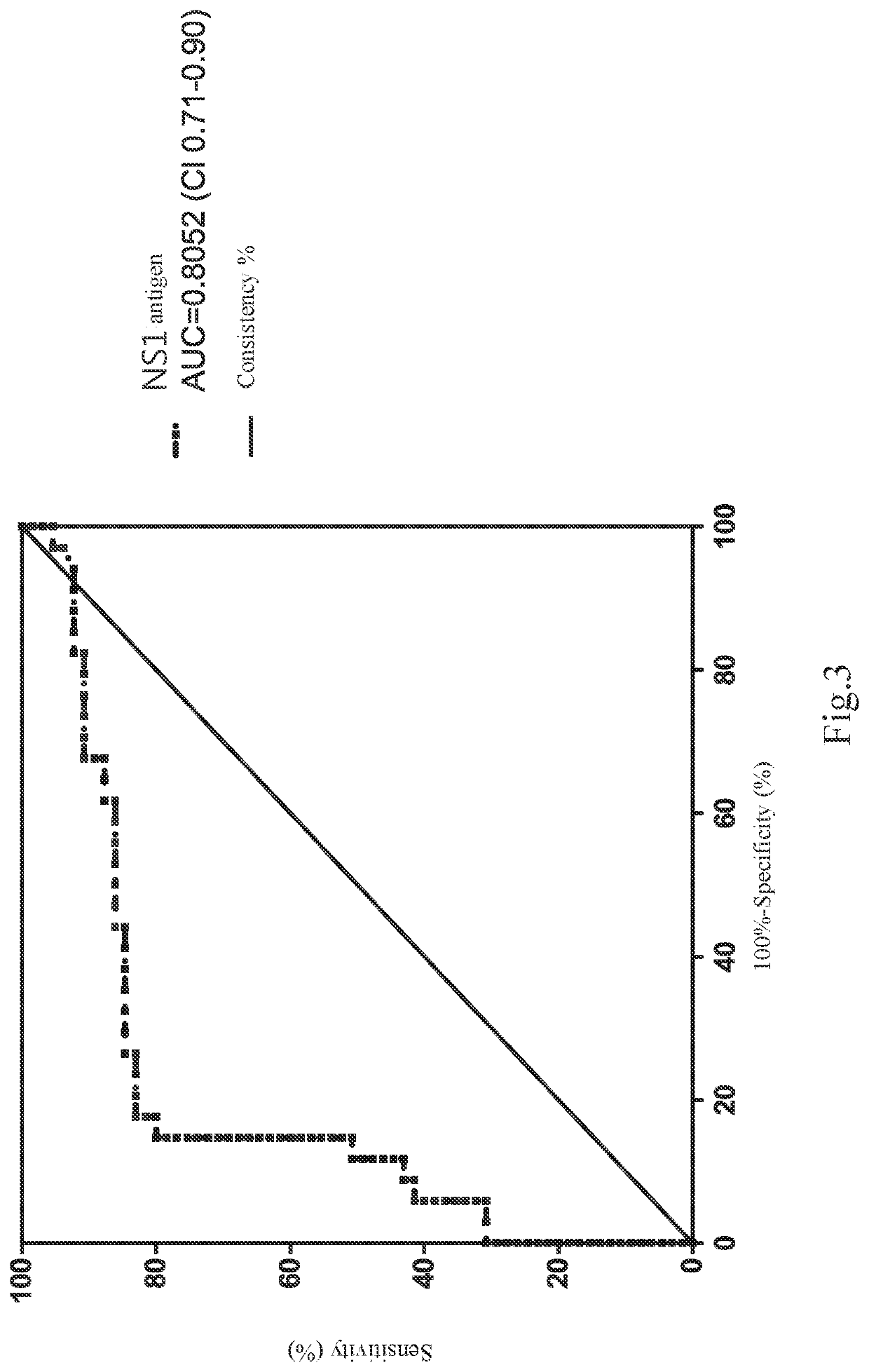
The present invention relates to a method of elevating prediction accuracy of grouping subjects with severe dengue infection. In the method, a non-structural protein 1 (NS1) and an endogenous anti-NS1 antibody of dengue virus in an ex vivo biological specimen are detected and crossly compared, leading in reduce of false negative rates of testing results, as well as elevating grouping accuracy of patients with severe dengue infection.
Owner:NAT CHENG KUNG UNIV
Expression and purification of amino terminal region of SARS coronavirus non-structural protein nsp2, and crystalline structure of amino terminal region
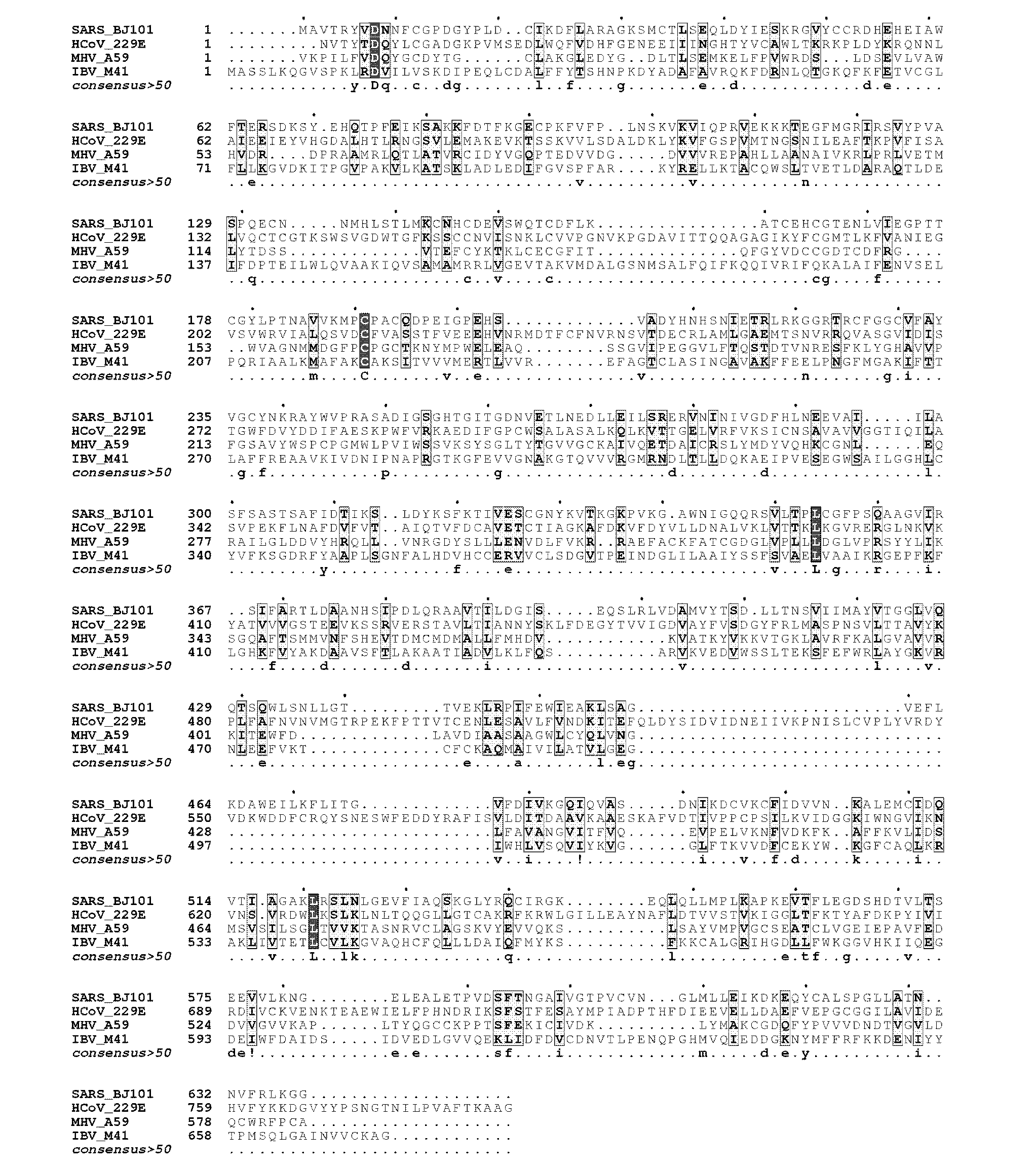
The invention provides a crystalline three-dimensional structure of an amino terminal region of an SARS (severe acute respiratory syndrome) coronavirus non-structural protein nsp2. The amino terminal region of the SARS coronavirus non-structural protein nsp2 comprises amino acids at positions in a range of about 1-50 to about 150-300 of the SARS coronavirus non-structural protein nsp2; and atoms in the crystalline three-dimensional structure have at least 40% of atomic coordinates in a table 1, or the average root mean square deviation of the main-chain carbon-skeleton atomic structure coordinates of the at least 40% amino acids of the crystalline three-dimensional structure of the amino terminal region of the SARS coronavirus non-structural protein nsp2 and the coordinates in the table 1 is not greater than 1.5 angstroms. The invention also provides an expression, purification and crystallization method of the SARS coronavirus non-structural protein nsp2, the crystalline structure of the SARS coronavirus non-structural protein nsp2, and theoretic guiding meanings and applications of the crystalline structure in functional researches, latent drug screening and drug designs.
Owner:TIANJIN INT JOINT ACADEMY OF BIOTECH & MEDICINE
High-throughput Zika virus nonstructural protease activity assay buffer and activity assay method
ActiveCN106434849BHigh activityThe result is accurateMicrobiological testing/measurementStructural proteinNaCl - Sodium chloride
Owner:TIANJIN INT JOINT ACADEMY OF BIOTECH & MEDICINE
Application of fidaxomicin in the preparation of medicines for treating related diseases and/or symptoms caused by dengue virus infection
ActiveCN107929300BImprove securityHigh activityOrganic active ingredientsAntiviralsDiseaseClostridium difficile infections
The invention discloses an application of fidaxomicin in preparing medicines for treating related diseases and / or symptoms caused by dengue virus infection. The fidaxomicin, as a clinically approved drug for resisting clostridium difficile infection, is high in safety; and in addition, the fidaxomicin, which is high in activity of inhibiting the dengue virus infection and relatively strong in binding capacity with dengue virus non-structural protein NS5, is expected to function as a novel drug for resisting the dengue virus infection.
Owner:SUN YAT SEN UNIV
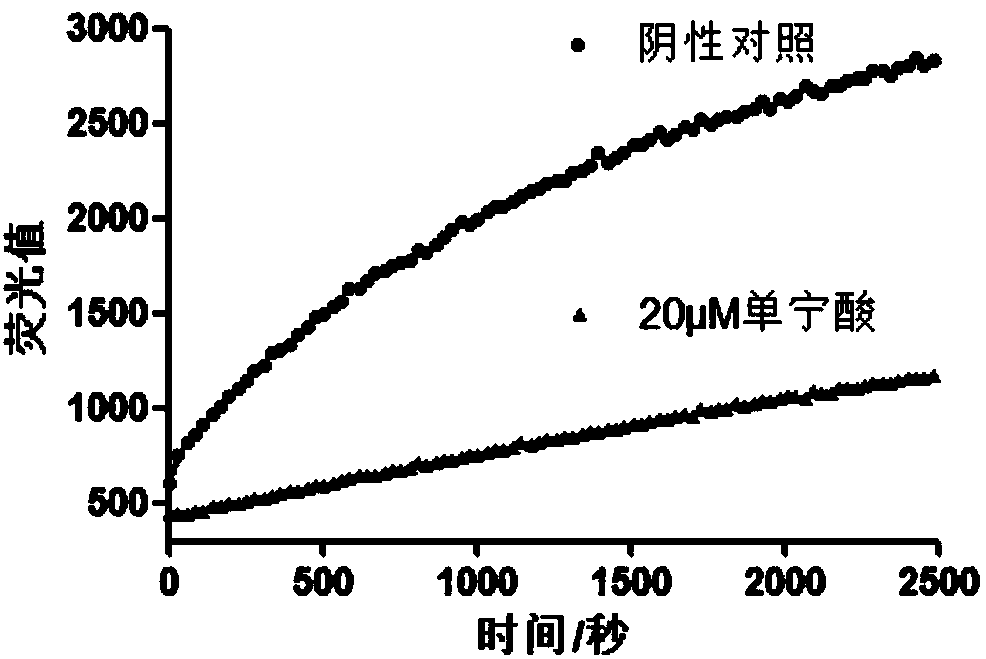
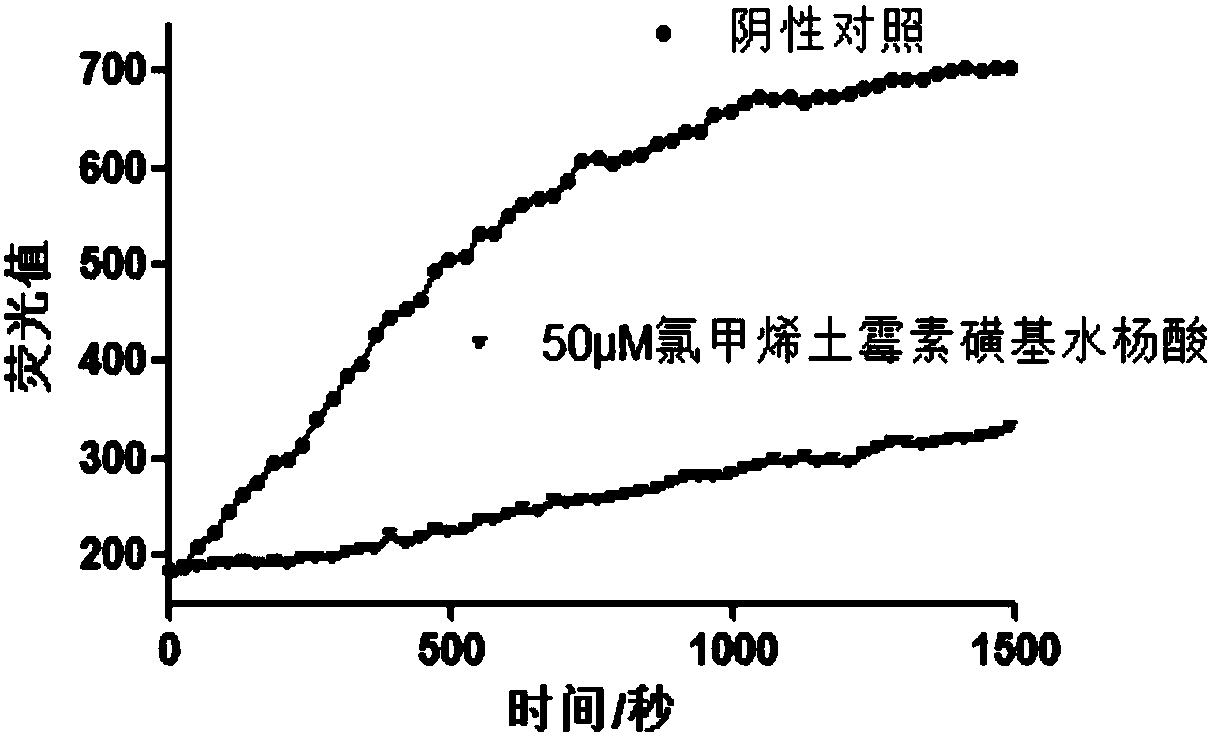
Application of a series of compounds with tannic acid as representation in resisting infection of chikungunya and other alphaviruses
InactiveCN107823629AEnhanced inhibitory effectTetracycline active ingredientsAntiviralsProteaseBiology
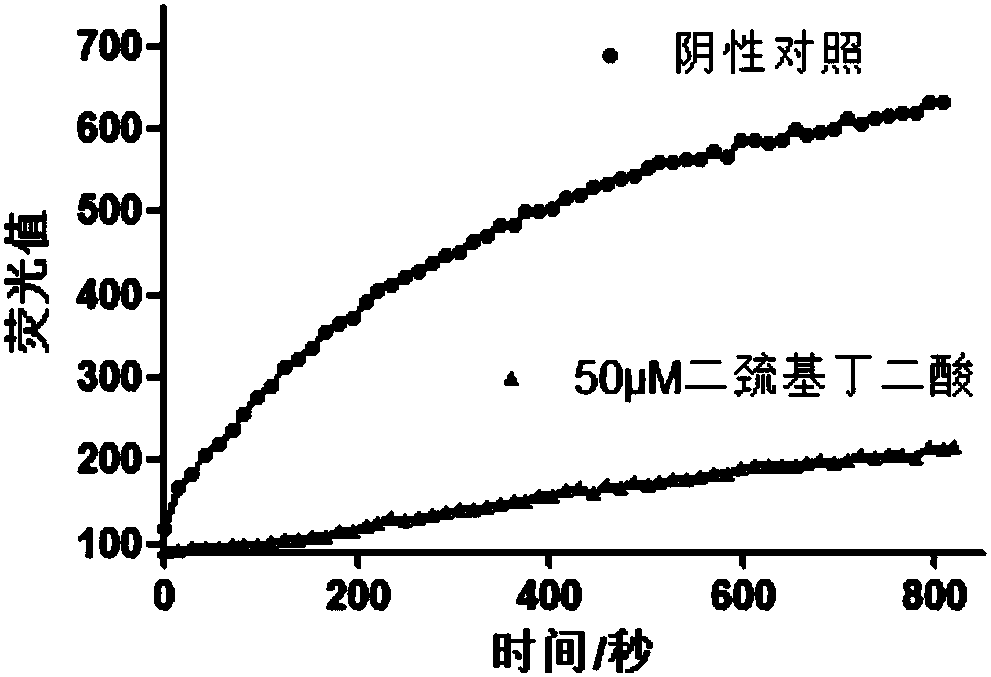
The invention provides an application of a series of compounds with tannic acid as representation in resisting infection of chikungunya and other alphaviruses, and specifically relates to the application of tannic acid, meclocycline sulfosalicylate, 2,3-dimercaptosuccinic acid and oritavancin diphosphate in resisting infection of chikungunya and other alphaviruses; the compounds can be used for inhibition of the activity of chikungunya virus nsP2 protease (protease of a nonstructural protein 2), and are expected to become potential drugs for resisting infection of CHIKV and other alphaviruses.
Owner:TIANJIN INT JOINT ACADEMY OF BIOTECH & MEDICINE
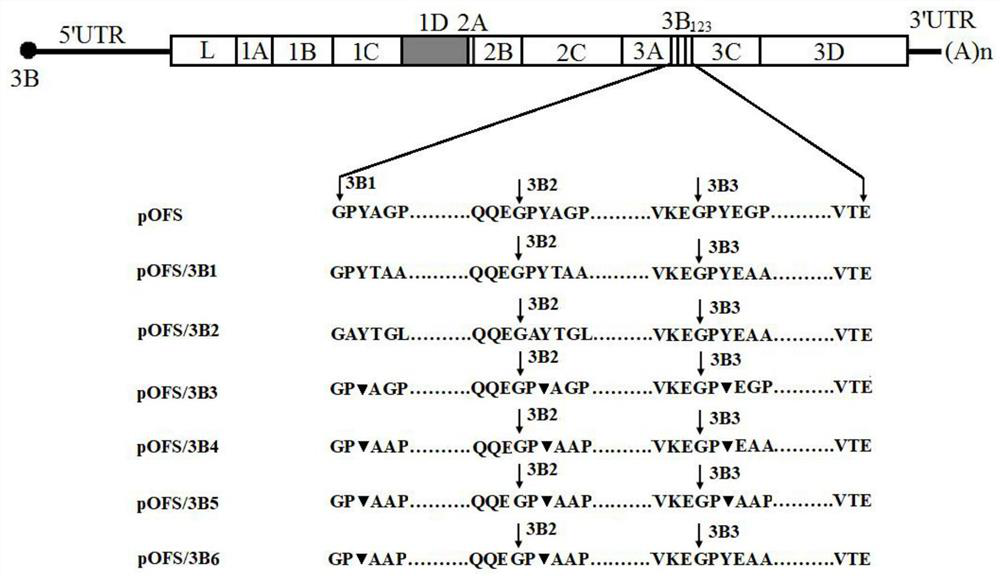
A foot-and-mouth disease virus non-structural protein 3b dominant epitope deletion-marked strain and its preparation method and application
ActiveCN112029735BEffective controlEfficient purificationSsRNA viruses positive-senseViral antigen ingredientsDiseaseStructural protein
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Application of tiacumicin derivative to preparation of medicine for treating related diseases and/or symptoms caused by dengue virus infection
ActiveCN107714713AImprove securityHigh activityOrganic active ingredientsAntiviralsDiseaseClostridium difficile infections
The invention discloses an application of a tiacumicin derivative to preparation of a medicine for treating related diseases and / or symptoms caused by dengue virus infection. Tiacumicin serving as a clostridium difficile infection resisting medicine clinically approved for use is high in safety, and the tiacumicin derivative can remarkably inhibit dengue viruses, is high in activity, has strong binding capacity with dengue virus non-structural protein NS5 and can be expected to be a novel effective dengue virus infection resisting medicine.
Owner:SUN YAT SEN UNIV +1
Application of tiacumicin derivatives in preparation of medicines for treating related diseases and/or symptoms caused by dengue virus infection
ActiveCN107714713BImprove securityHigh activityOrganic active ingredientsAntiviralsDiseaseClostridium difficile infections
The invention discloses an application of a tiacumicin derivative to preparation of a medicine for treating related diseases and / or symptoms caused by dengue virus infection. Tiacumicin serving as a clostridium difficile infection resisting medicine clinically approved for use is high in safety, and the tiacumicin derivative can remarkably inhibit dengue viruses, is high in activity, has strong binding capacity with dengue virus non-structural protein NS5 and can be expected to be a novel effective dengue virus infection resisting medicine.
Owner:SUN YAT SEN UNIV +1
A test strip for the differential diagnosis of foot-and-mouth disease virus-infected animals and vaccine-immunized animals
The invention discloses a test paper strip for differential diagnosis of foot-and-mouth disease virus-infected animals and vaccine-immunized animals. Three epitope polypeptides on viral non-structural proteins 2B, 2C, and 3B. The artificial antigen prepared by the present invention has the advantages of easy acquisition, stable structure, purity up to 99%, low cost, rapid mass production, etc., which solves the problem of low expression of traditionally expressed proteins, difficulty in ensuring natural spatial structure, difficulty in renaturation, difficulty in The removal of bacterial proteins affects the specificity of the test results and other shortcomings, and also solves the risk of incomplete virus inactivation when using inactivated viruses. The invention also pre-treats the gold-labeled pad, which is more conducive to the gold-labeled pad’s water absorption and the release of gold-labeled protein, effectively solving the slow and incomplete release of gold-labeled protein in the existing test paper, which affects the detection accuracy, sensitivity and issues such as shelf life.
Owner:HENAN ACAD OF AGRI SCI
Virus-like particles for treatment of viral infections
The invention provides virus-like particles for treatment of viral infections based on the virus causing the infection. The virus-like particles comprise the virus recombinant proteins that form a capsid, recombinant virus membrane proteins attached to the capsid and vRNA packaged within said capsid. The vRNA is generated from a DNA sequence encoding a polypeptide capable of specifically binding to a constant region of a nonstructural protein of the virus that is essential for propagation of the virus.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
A kind of monoclonal antibody specifically binding foot-and-mouth disease non-structural protein and its application
ActiveCN109320606BHigh sensitivityImprove linear rangeSsRNA viruses positive-senseViral antigen ingredientsDiseaseStructural protein
The invention discloses a foot-and-mouth disease virus non-structural protein monoclonal antibody 7D7, a foot-and-mouth disease virus non-structural protein antibody detection kit containing the monoclonal antibody and an application thereof. The antibody detection kit provided by the invention has high sensitivity and can obviously widen the linear range of detection; the detection is not limited by animal species, and is suitable for large-scale screening of foot-and-mouth disease diseases of artiodactyl animals. The vaccine prepared by the conjugate containing the monoclonal antibody 7D7 can remove non-structural proteins, and can avoid the interference of FMDV non-structural protein antibodies after immunizing animals, thereby accurately distinguishing infected animals from immunized animals.
Owner:LUOYANG PULIKE WANTAI BIOTECH
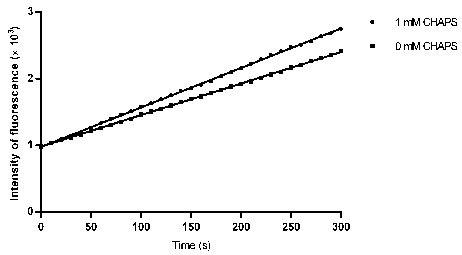
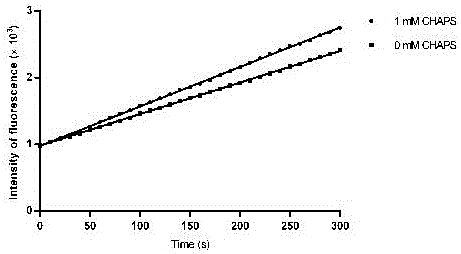
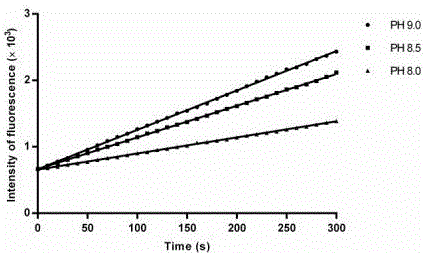
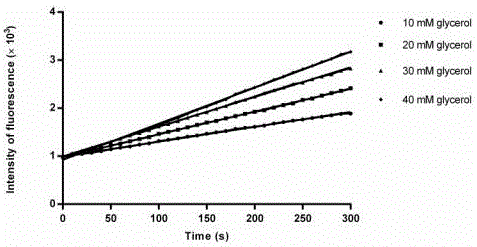
Activity determination buffer solution and activity determination method of high-throughput Zika virus non-structural protease
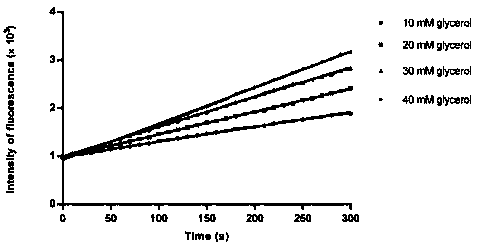
ActiveCN106434849AHigh activityThe result is accurateMicrobiological testing/measurementAdditive ingredientGlycerol
The invention discloses an activity determination buffer solution and an activity determination method of high-throughput Zika virus non-structural protease, wherein the buffer solution consists of the following buffer ingredients: 10-200mM of trihydroxymethyl aminomethane, 0-20mM of sodium chloride, 0-1mM of CHAPS, 0-20mM of galactose and glycerol which is 10-40% of in volume percentage, and the pH value of the buffer solution is at 8.0-9.0. The buffer solution provided by the invention can improve the activity of the protease in a process of detecting the enzymatic activity of Zika viruses, so that the high-throughput Zika virus non-structural protease activity determination method, which makes use of the buffer solution, is more accurate in result, and subsequently an effect of guiding the screening of Zika virus protease activity inhibitors is achieved and the research and development progress of medicines targeted to the Zika viruses is promoted.
Owner:TIANJIN INT JOINT ACADEMY OF BIOTECH & MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com