A kind of monoclonal antibody specifically binding foot-and-mouth disease non-structural protein and its application
A technology of monoclonal antibody and foot-and-mouth disease, applied in the direction of immunoglobulin, anti-virus immunoglobulin, antibody medical components, etc., can solve the problems of identification of immunized animals and naturally infected animals, interference, incomplete purification, etc., to reduce non-specific reactions , high sensitivity, and the effect of widening the linear range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0020] As an embodiment of the present invention, the antibody is monoclonal antibody 7D7, the amino acid sequence of its heavy chain variable region is the amino acid sequence shown in SEQ ID No.2, and its light chain variable region amino acid sequence is SEQ ID No. . The amino acid sequence shown in 4.
[0021] The monoclonal antibody 7D7 is the monoclonal antibody 7D7 of the non-structural protein 3ABC of the foot-and-mouth disease virus, is an IgG antibody, has a relative affinity greater than 5.0 ng / ml, and has good reactivity with the non-structural protein of the foot-and-mouth disease virus.
[0022] The term "foot-and-mouth disease virus antigen" refers to an antigen prepared from the non-structural protein of foot-and-mouth disease virus.
[0023] The term "luminol chemiluminescent system" includes luminescent substrate 1: containing 1.0~6.8×10 -3 mol / L Tris-HCl buffer solution of hydrogen peroxide (0.05~0.5mol / L, pH 8.5), and luminescent substrate 2 (protected fro...
Embodiment 1
[0053] Preparation, identification and inspection of embodiment 1 foot-and-mouth disease virus nonstructural protein monoclonal antibody
[0054] 1.1 Preparation and identification of FMDV nonstructural protein
[0055] According to NCBI (http: / / www.ncbi.nlm.nih.gov) reported in the foot-and-mouth disease virus (accession number is DQ478936.1) in the gene sequence design primer pair 3ABC protein upstream primer 1: 5-AGCAGATCTCAATTCCTTCCCAAAAATCTG-3 ', Downstream primer 1: 5'-AATCACTCGTGGTGCGGTTCGGGGTC-3'. According to the method described by Cao Yimei (Cao Yimei, Liu Zaixin, Lu Zengjun, etc. The expression of non-structural protein gene 3ABC of foot-and-mouth disease virus in E. The 3ABC gene was added, and the cDNA reverse-transcribed from the viral RNA was amplified by PCR with the designed primers. It was detected by 1% agarose gel electrophoresis that a band of about 1.3 Kb was produced, which was in line with the expectation.
[0056]The cloned sequence of the FMDV 3ABC...
Embodiment 2
[0079] Preparation and application of embodiment 2 foot-and-mouth disease virus nonstructural protein antibody detection kit
[0080] 2.1 Preparation of the kit
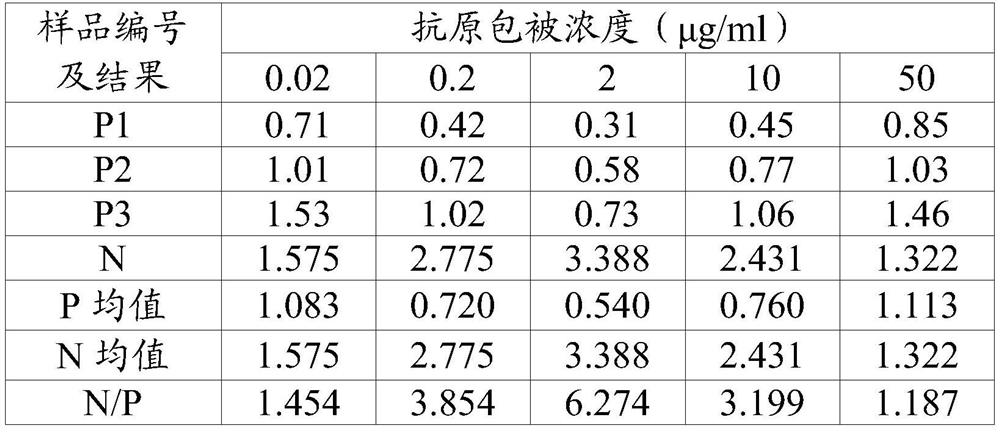
[0081] Coated plate: Dilute the FMDV 3ABC prepared in Example 1.1 with CB buffer (0.02mol / L, pH 9.6) to 2.0μg / ml, coat at 2-8°C overnight, discard the coating solution and press 200μl / well Add PB solution (0.02mol / L, pH 7.4) containing 20% calf serum and block at 37°C for 2 hours, discard the blocking solution, dry and package.
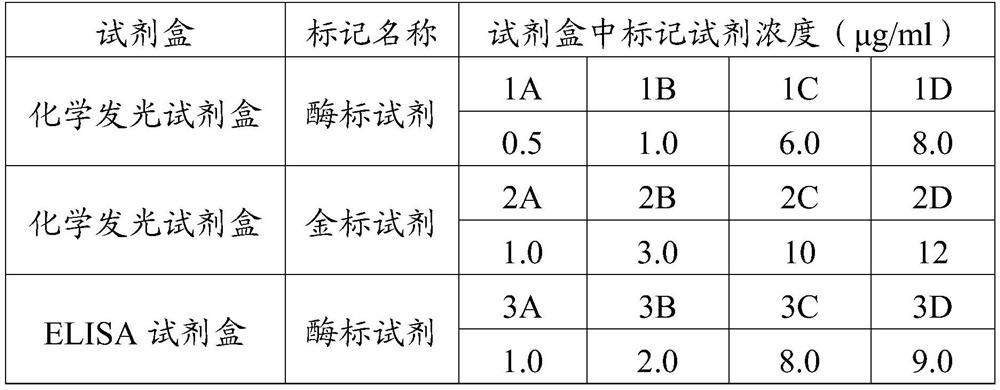
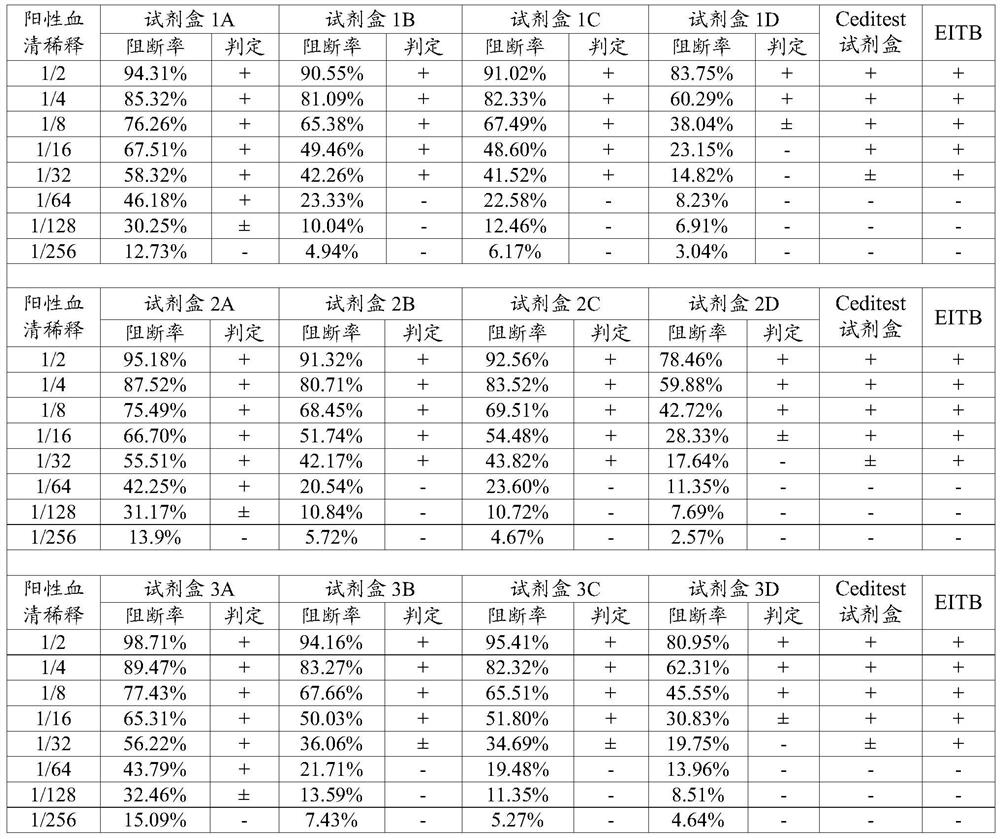
[0082] Enzyme-labeled reagent: Dilute the enzyme-labeled monoclonal antibody 7D7 prepared in Example 1.6 with sample diluent, select a representative concentration to describe the concentration range of the enzyme-labeled reagent in the kit, see Table 1.
[0083] Gold-labeled reagent: Dilute the gold-labeled monoclonal antibody 7D7 prepared in Example 1.7 with sample diluent, select a representative concentration to describe the concentration range of the enzyme-labeled reagent in the kit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com