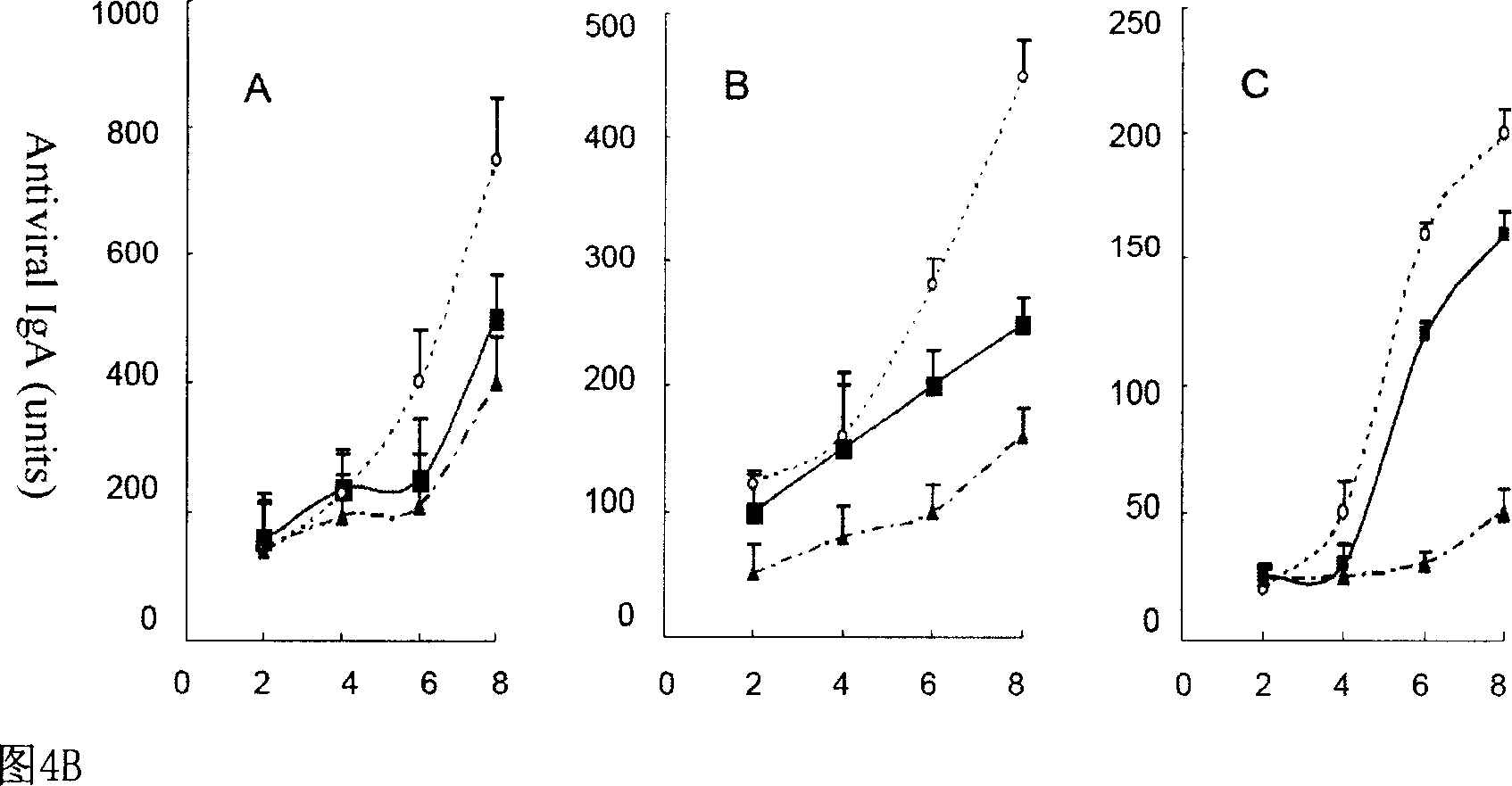
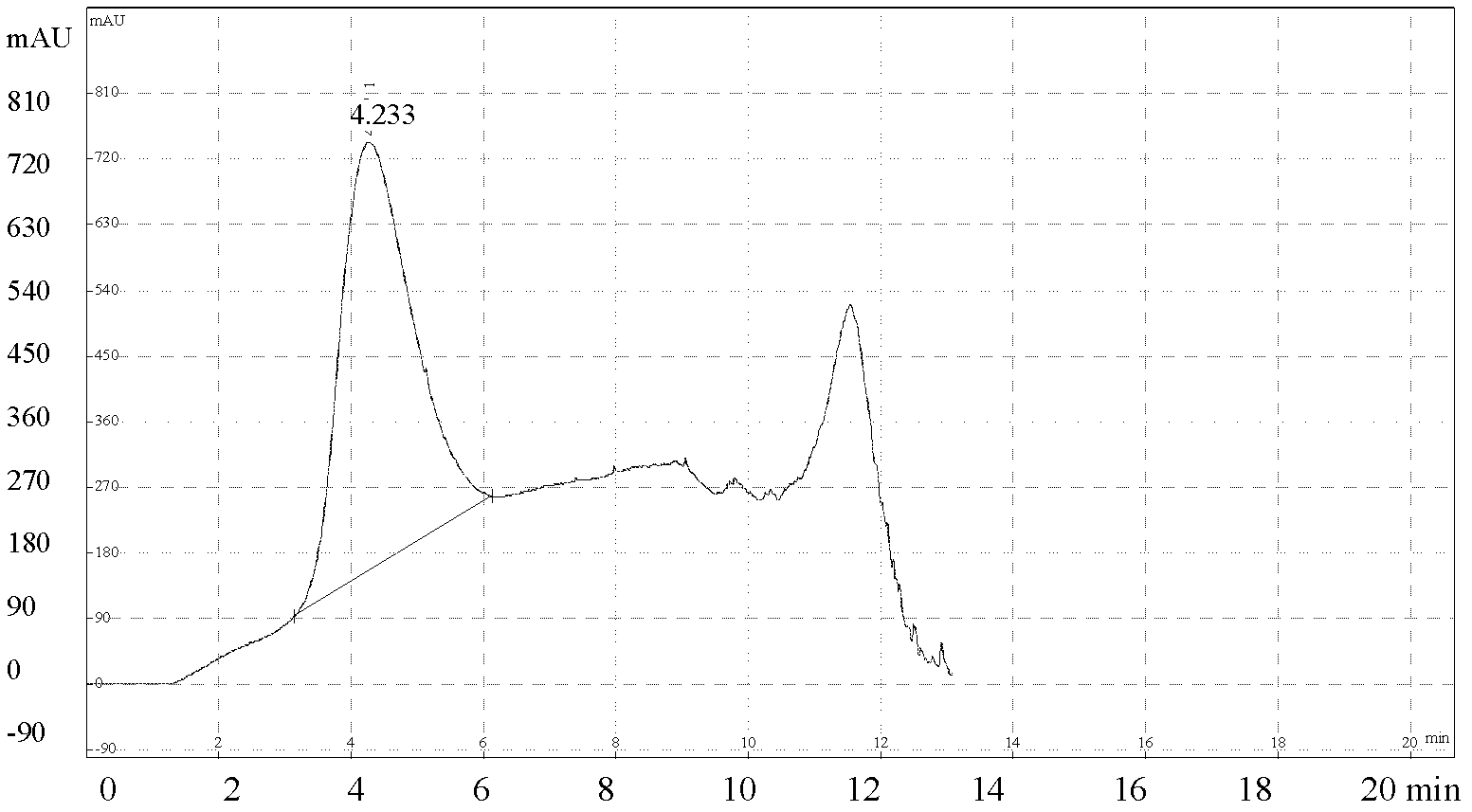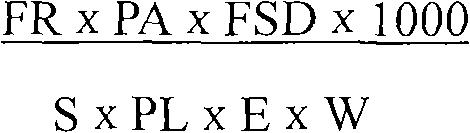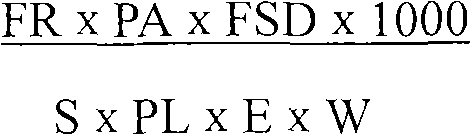Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
661 results about "Foot-and-mouth disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Foot-and-mouth disease (FMD) or hoof-and-mouth disease (HMD) is an infectious and sometimes fatal viral disease that affects cloven-hoofed animals, including domestic and wild bovids. The virus causes a high fever for between two and six days, followed by blisters inside the mouth and on the feet that may rupture and cause lameness.
Method for identifying animal-based components in meat and meat products by utilizing SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) method
The invention relates to a method for identifying the animal-based components in meat and meat products by utilizing an SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) method, and belongs to the field of food sanitation inspection. The method provided by the invention is utilized to identify the pig, cattle, sheep, chicken and fish-based components in the meat products; the SDS-PAGE is carried out on the soluble proteins which are extracted from the processed sample by carrying out different temperature thermal treatments on fresh animal muscle tissues; and the pig, cattle, sheep, chicken and fish-based components in the meat products are judged according to an electrophoresis pattern. The method provided by the invention has the characteristics of strong specificity and sensitivity, and visual and reliable result judgment, and is simple to operate and has the extremely important significances on preventing animal disease pathogens such as foot-and-mouth disease, avian influenza and the like from spreading into China, preventing illegal retailers from concealing commodity components and guaranteeing the health of people.
Owner:QINGDAO AGRI UNIV +1
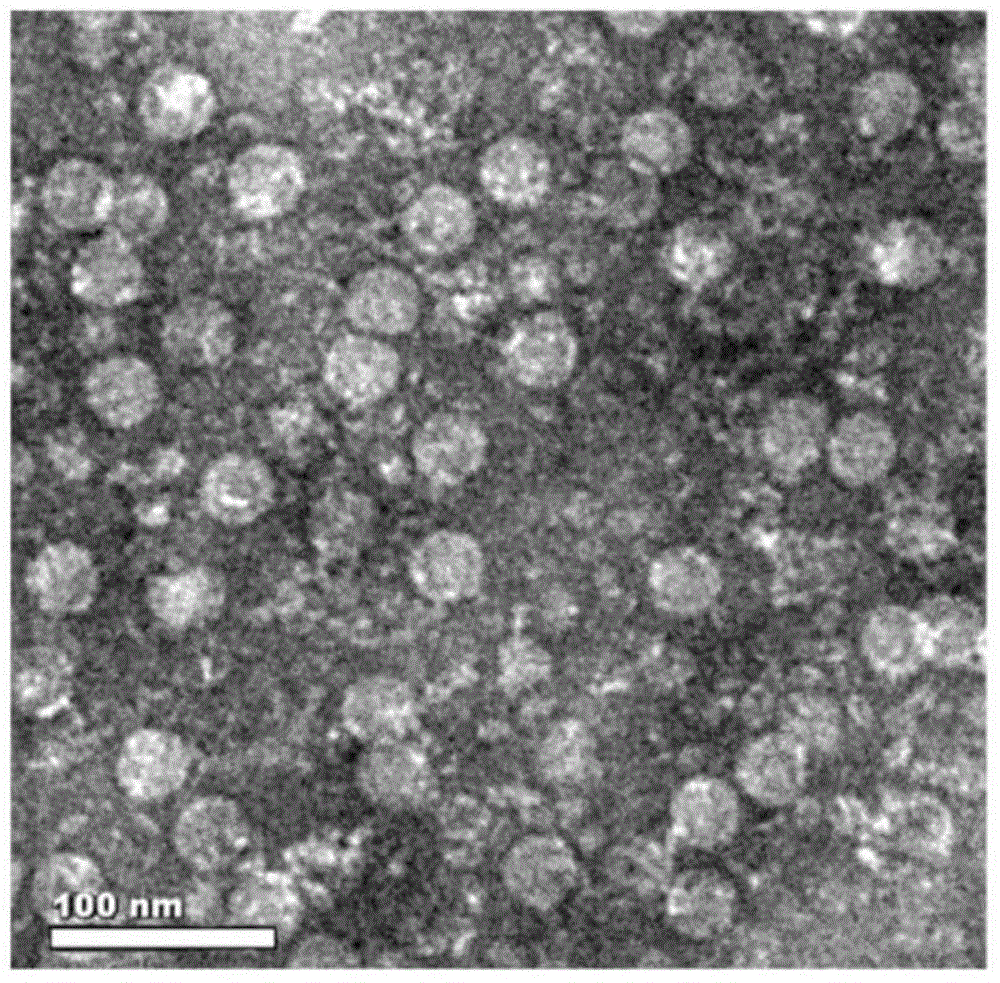
Foot-and-mouth disease virus capsid protein tandem coexpressions and virus-like particle preparation method
ActiveCN104404074AHigh activityNatural binding activityBacteriaInactivation/attenuationEscherichia coliVirus-like particle
The invention relates to escherichia coli-derived single-plasmid-tandem soluble coexpression foot-and-mouth disease virus capsid proteins VP0 (which is a VP4 and VP2 fusion gene), VP1 and VP3, and a foot-and-mouth disease virus capsid protein virus-like particle preparation method. Foot-and-mouth disease virus capsid protein virus-like particles can be used for preparation of a foot-and-mouth disease vaccine. According to the method, a plurality of aspects of escherichia coli-derived soluble coexpression foot-and-mouth disease virus capsid protein are studied, by comprehensive use of tandem coexpression and SUMO(suggested upper merged ontology) technology with a tag for soluble coexpression of the foot-and-mouth disease virus capsid proteins VP0 (which is the VP4 and VP2 fusion gene), VP1 and VP3, the ultimate objective protein accounts for about 20% of total bacterial protein, and the foot-and-mouth disease virus capsid proteins obtained by purification can be successfully assembled into the virus like particles.
Owner:SA BIOTECH (SUZHOU) PTE LTD
Development of a Marker Foot and Mouth Disease Virus Vaccine Candidate That is Attenuated in the Natural Host
ActiveUS20120315295A1Easy to replaceQuick changeSsRNA viruses positive-senseSugar derivativesSerotypeViral Vaccine
We have generated novel molecularly marked FMDV A24LL3DYR and A24LL3BPVKV3DYR vaccine candidates. The mutant viruses contain a deletion of the leader coding region (LL) rendering the virus attenuated in vivo and negative antigenic markers introduced in one or both of the viral non-structural 3Dpol and 3B proteins. The vaccine platform includes unique restriction endonuclease sites for easy swapping of capsid proteins for different FMDV subtypes and serotypes. The mutant viruses produced no signs of FMD and no shedding of virulent virus in cattle. No clinical signs of disease or fever were observed and no transmission to in-contact animals was detected in pigs inoculated with live A24LL3DYR. Cattle immunized with chemically inactivated vaccine candidates showed an efficacy comparable to a polyvalent commercial FMDV vaccine. These vaccine candidates used in conjunction with a cELISA provide a suitable target for DIVA companion tests.
Owner:US SEC AGRI
Method for rapidly, accurately and repeatedly determining foot-and-mouth disease vaccine antigen 146S
InactiveCN104634891AGood repeatabilityReduce mistakesComponent separationChromatographic separationPhosphate
The invention discloses a method for rapidly, accurately and repeatedly determining foot-and-mouth disease vaccine antigen 146S. A size exclusion high-efficiency liquid-phase chromatographic column in a molecular weight separation range of 2*10<4> to 1*10<7>Da is adopted to carry out the chromatographic separation on a detected sample on a high-efficiency liquid-phase chromatography. The operation pressure of the chromatography is 1.0MPa to 2.5MPa, the flow rate in the chromatographic column is 0.5 to 1.0 ml / min, a flow phase is phosphate buffer (pH 7.0 to 7.5) containing 0.1M sodium sulfate, and the column temperature is 15 to 25 DEG C. An ultraviolet and laser detector is used for detecting an optical signal of effluent at an outlet of the size exclusion high-efficiency liquid-phase chromatographic column, and a peak area of a sample can be analyzed by virtue of a computer software system of the high-efficiency liquid-phase chromatography. A standard curve of the absorption peak area and 146S concentration is established by virtue of a relation between the ultraviolet absorption peak and the concentration of different 146S standard products of different concentrations. Chromatograph is carried out on the detected sample through the size exclusion high-efficiency liquid-phase chromatographic column. The ultraviolet absorption peak area is measured, and the concentration of 146S in the detected sample can be acquired according to the standard curve.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
O-type foot-and-mouth disease virus multi-epitope mucous membrane immunization vaccine and use
This invention relates to a fusion protein used for preventing aftosa, its preparation method and application. This fusion protein contains O type foot-and-mouth disease virus main cytomembrane protein VP1 epitope, colibacillus thermolability toxin B subunit, thymus derived cell epitope and purification label.
Owner:GUANGZHOU PUTAI BIOTECH
O type foot and mouth disease virus-like particle and preparation method thereof and application
ActiveCN106479986AHigh expressionImprove assembly efficiencySsRNA viruses positive-senseViral antigen ingredientsAnimal testingStructural protein
The invention discloses an O type foot and mouth disease virus-like particle and a preparation method of the O type foot and mouth disease virus-like particle and an application. The O type foot and mouth disease virus-like particle is formed by assembling structural protein VP0, VP1 and optimized OP3 structural proteins of O type foot and mouth disease virus, wherein the gene sequence of the VP1 is shown as SEQ ID NO.1; the gene sequence of the VP0 is shown as SEQ ID NO.2; the gene sequence of the optimized VP3 is shown as SEQ ID NO.3. The invention tries to perform artificially missing on a part of section of the structural protein VP3 of O type foot and mouth disease virus; the result shows that the protein expression amount of the VP3 gene after the artificial missing is improved by 20% in comparison to that before mutation; the assembling efficiency of the virus-like particle is also improved by 15%; moreover, the animal test result shows that the immunogenicity thereof is good and free from significant difference with VLPs obtained through unmissed VP3 assembling. The invention provides a new technical manner for the research of the foot and mouth disease vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Formulations for alteration of biophysical properties of mucosal lining
InactiveUS20070053844A1Altered propertyReduce transmissionAntibacterial agentsPowder deliveryActive agentContamination
Conductive formulations containing conductive agents, such as salts, ionic surfactants, or other substances that are in an ionized state or easily ionized in an aqueous or organic solvent environment, and methods of use, have been developed. One or more active agents, such as antivirals, antimicrobials, anti-inflammatories, proteins or peptides, may optionally be included with the formulation. The active agent may be administered with or incorporated into the formulation, or may be administered after the conductive formulation is administered. When applied to mucosal lining fluids, the formulation alters the physical properties such as the surface tension, surface elasticity, and bulk viscosity of the mucosal lining. The formulation is administered in an amount sufficient to alter biophysical properties in the mucosal linings of the body. The formulations may be administered for several different purposes: reducing the spreading of infectious diseases, both viral and bacterial, such as SARS, influenza, tuberculosis, and RSV in humans and hoof and mouth disease in cloven-tooted animals; minimizing ambient contamination due to particle formation during breathing, coughing, sneezing, or talking which is particularly important in the clean room applications; decreasing or preventing the occurrence of obstructive sleep apnea and some cases of irritable bowel syndrome; and controlling the uptake kinetics of drug molecules and pathogens.
Owner:PULMATRIX
Formulations decreasing particle exhalation
Formulations have been developed for pulmonary delivery to treat or reduce the infectivity of diseases such as viral infections, especially tuberculosis, SARS, influenza and respiratory synticial virus in humans and hoof and mouth disease in animals, or to reduce the symptoms of allergy or other pulmonary disease. Formulations for pulmonary administration include a material that significantly alters physical properties such as surface tension and surface elasticity of lung mucus lining fluid, which may be isotonic saline and, optionally, a carrier. The formulation may be administered as a liquid solution, suspension, aerosol, or powder where the particles consist basically of an osmotically active solute. Drugs, especially antivirals or antibiotics, may optionally be included with the formulation. These may be administered with or incorporated into the formulation.
Owner:PULMATRIX
Method for expanding antigen spectrum of foot-and-mouth disease vaccine strain by reverse genetic operation and preparation method of vaccine
ActiveCN101948811AHigh protection rateBroad antigen spectrumVirus peptidesMicroorganism based processesImmune effectsSoutheast asia
The invention relates to a method for expanding the antigen spectrum of a foot-and-mouth disease vaccine strain by reverse genetic operation and a preparation method of a vaccine. The amino acid sequence of the VP3 and VP1 structural proteins of the foot-and-mouth disease virus strain of the invention is represented by the amino acid residues from a position 304 to a position 736 in SEQ ID No.4. Experiments show that the vaccine prepared from the mutant virus strain obtained by the invention can resist porcine epidemic viruses of China O / TL / Taiwan / 97 lineage, Pan-Asia O / China / 99 lineage and Southeast Asia Myanmar O / GS / 2010 / 98 lineage, has a characteristic of wide antigen spectrum, can immunize pigs and obviously improve the rate of protection against foot-and-mouth disease viruses which are of the same type and have antigenicity difference, achieves an immune effect of cross protection, and is expected to play an important role in the prevention and control of foot-and-mouth disease.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Large-scale preparation method for foot-and-mouth disease totivirus marked vaccine with high yield, high purity and high safety and product thereof
ActiveCN104491855AMark stableEnsure safetyMicroorganism based processesAntiviralsSucroseUltrafiltration
The invention discloses a large-scale preparation method for foot-and-mouth disease totivirus marked vaccine with high yield, high purity and high safety and a product thereof. The method comprises the following steps: a)collecting a virus solution; b)performing deep filtration on a membrane, performing ultrafiltration and performing enzymolysis on nuclease; c)purifying through a strong anion exchange adsorption bed or an adsorption film; d)depositing by PEG, extracting by chloroform-isoamyl aleohl; e)inactivating; F)performing density gradient centrifugation on an inactivation liquid through cane sugar and purifying; g)performing ultrafiltration dialysis and aseptic filtration; and h)reserving a stock solution or emulsifying. The provided foot-and-mouth disease totivirus marked vaccine antigen is uniform and complete foot-and-mouth virus particle, The vaccine is injected into body, so animal infection and immunization can be completely distinguished, does not contain foot-and-mouth disease virus non-structural protein and other virus particle, and does not contain animal-based foreign protein, polypeptide and oligopeptides, animal latent anaphylactic reaction, carcinogenesis and latent risk such as mad cow disease for causing animal infectious diseases due to vaccine injection can be effectively reduced, and the vaccine has no influence on animal food safety and trade.
Owner:吕宏亮 +2
Kit for quantitative detection on O type foot-and-mouth disease virus antibody through fluorescence immunoassay magnetic particles
InactiveCN108107220AAdequate responseIncrease binding areaBiological testingBiotin-streptavidin complexSorbent
The invention discloses a kit for quantitative detection on an O type foot-and-mouth disease virus antibody through fluorescence immunoassay magnetic particles. The kit consists of O type foot-and-mouth disease virus antibody negative serum, O type foot-and-mouth disease virus antibody positive serum, VP1 coating magnetic beads, a biotinylation goat-anti-pig antibody, a streptavidin marking fluorescent substance, a cleaning solution and an enhancing solution. The magnetic beads used in the kit have relatively large binding areas, so that the detection range is greatly increased, the reaction time is shortened, and the sensitivity is improved. The kit has a relatively wide stimulation spectrum and a relatively narrow emitting spectrum, the cost can be reduced, and the sensitivity can be improved; compared with a conventional fluorescent substance, the kit is relatively wide in detection range and relatively good in specificity. Due to adoption of a streptavidin-biotin signal amplification system, the detection sensitivity is further improved, and the kit is relatively high in sensitivity when being compared with ELISA (Enzyme-Linked Immuno Sorbent Assay) and chemiluminiscence. Together with a full-automatic detector, on-site automatic operation can be achieved, one or more samples can be simultaneously detected, and the kit is simple, convenient and rapid to operate and low in price.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
Dual real-time fluorescence quantitative PCR detection kit for foot-and-mouth disease and Seneca valley virus
InactiveCN107326100ARapid differential testImprove throughputMicrobiological testing/measurementMicroorganism based processesFluorescenceRapid identification
The invention provides a combination of a primer and a probe used for dual real-time fluorescence quantitative PCR detection of the foot-and-mouth disease virus and the Seneca valley virus and a detection kit. The sequences of the combination of the primer and the probe are respectively shown in the SEQ ID NO:1-7. The invention further provides a non-diagnostic-purpose dual real-time fluorescence quantitative PCR detection method for the foot-and-mouth disease virus and the Seneca valley virus. The three serotype foot-and-mouth disease viruses of O, A and AsiaI and the Seneca valley virus are rapidly identified and detected at the same time in the same reaction, the detection is finished within two hours, and the primer, the probe and the detection method have the advantages of rapidness, specificity, sensitivity and high throughput, and meet the requirements on large-batch rapid identification and detection of the three serotype foot-and-mouth disease viruses of O, A and AsiaI and the Seneca valley virus.
Owner:HENAN CENT FOR ANIMAL DISEASE CONTROL & PREVENTION
Method for quantification of 146S content in foot-and-mouth disease antigen by using liquid chromatography detection system
The present invention discloses a method for quantification of 146s content in foot-and-mouth disease antigen by using a liquid chromatography detection system. The method comprises: adopting protamine sulfate to purify virus, adopting PEG6000 to concentrate the virus, carrying out sucrose density gradient ultracentrifugation on the concentrated virus, adopting a liquid chromatography detection system to detect an absorption peak of the centrifugated sample at a wavelength of 259 nm, and calculating 146S content according to the following formula: C=FRS / 76Wb. The method can be used for determination of the 146S content in various types of foot-and-mouth disease antigens. With quantification of the 146S content in the foot-and-mouth disease antigen, foot-and-mouth disease vaccine preparation can be guided, vaccine efficacy can be indirectly evaluated, and major guiding significances are provided for enhancing quality of the foot-and-mouth disease vaccine in our country.
Owner:内蒙古必威安泰生物科技有限公司
Method for culturing baby hamster kidney (BHK) 21 cell in serum-free way, and vaccine preparation method
InactiveCN102115729AIncrease culture densityIncrease productivityMicroorganism based processesAntiviralsBiotechnologyHamster
The invention provides a method for culturing baby hamster kidney (BHK) 21 cell in a serum-free way, which leads the BHK 21 cell to be inoculated into a cell culture medium for culturing, wherein the cell culture medium comprises a basic culture medium and further comprises 100-200g / 100L of soy protein, 100-200g / 100L of pea protein, 100-200g / 100L of broad bean protein, 0-100g / 100L of potato protein, 0-200g / 100L of wheat gluten protein and 50-100g / 100L of rice protein by taking the volume of solvent of the culture medium as reference. In addition, the invention also provides a vaccine (such asrabies vaccine and foot-and-mouth disease vaccine) preparation method comprising the method for culturing the BHK 21 cell. The BHK 21 cell cultured by the culture medium containing the vegetable protein is high in culture density, beneficial to separating down-stream products of the cells, low in cost, small in batch difference, good in safety and suitable for producing virus host, expression vector and the like of biological products such as vaccine and the like.
Owner:BEIJING SKYWING TECH CO LTD
Method for determining components and estimating anti-gen content of foot-and-mouth disease vaccine
ActiveCN105467138AEasy to operateStrong specificityBiological material analysisBiological testingSerum igeAgricultural science
The invention provides a method for determining components and estimating the anti-gen content of a foot-and-mouth disease vaccine. The method comprises the following steps: (1) performing emulsion resolving on a foot-and-mouth disease vaccine to separate out an antigen phase; (2) concentrating the antigen phase; (3) using purified foot-and-mouth disease virus Asia 1-type, O-type and A-type 146S antigens to immunize animals and prepare detection serums; (4) diluting the concentrated antigens by different multiples and respectively performing immunodiffusion tests on the detection serums; (5) judging the components of the foot-and-mouth disease vaccine according to precipitation lines, that is, whether the vaccine is an O-type univalent vaccine, an O-type + A-type bivalent vaccine or an O-type + Asia 1-type + A-type trivalent vaccine; (6) estimating the content of the antigen in a detected vaccine sample according to the highest antigen dilution multiple at which the precipitation lines appear. The method can not only identify the components of the foot-and-mouth disease vaccine but also estimate the contents of all the components, and is simple to operate, strong in specificity, good in stability and suitable for basic-level application.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Foot-and-mouth disease genetic engineering mixed epitope vaccine and preparation method thereof
ActiveCN103007273AGood immune protectionUniform response levelAntiviralsAntibody medical ingredientsGenetic engineeringPolyinosinic Acids
The invention discloses a foot-and-mouth disease genetic engineering mixed epitope vaccine and a preparation method thereof. The vaccine consists of the following four parts: a serial B cell epitope recombinant protein BI consisting of main neutralizing epitops of O-type foot-and-mouth disease viruses in Cathay, Transasia and Mya 98 pedigrees with a gene sequence of SEQ ID NO:1 and an amino acid sequence of SEQ ID NO:2, a T-cell epitope recombinant protein TI consisting of serial connection of universal T-cell epitope and a plurality of foot-and-mouth disease virus specific T-cell epitopes with a gene sequence of SEQ ID NO:3 and an amino acid sequence of SEQ ID NO:4, Toll-like receptor 3 agonist-polyinosinic acid-polycytidysic acid and / or Toll-like receptor7 / 8 agonist-R848 serving as immunopotentiator, and 201 oil adjuvant. When being used for immunizing a pig, the BI and TI mixed epitope vaccine prepared by utilizing the method can produce a protective immunization effect the same as or better than that of an inactivated influenza virus Vaccines, and has a cross protection effect to viruses of the three pedigrees, so that the vaccine is a novel immune-enhanced O-type foot-and-mouth genetic engineering mixed epitope vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Method for preparing foot-and-mouth disease antigen
ActiveCN101121938APromote safe productionReduce consumptionSsRNA viruses positive-senseVirus peptidesAntigenTransfer vector
The invention provides a method for expressing foot-and-mouth disease antigens in insects using recombinant baculoviruses, which includes: cloning different gene combinations of foot-and-mouth disease into baculovirus delivery vectors to construct transfer vectors; using the constructed transfer vectors to transfer Infect the baculovirus and perform DNA recombination to obtain the recombinant baculovirus; infect the insect host with the recombinant baculovirus; culture the infected insect host to express the foot-and-mouth disease antigen; collect and purify the expressed foot-and-mouth disease antigen. The method of the present invention uses a baculovirus expression system to safely and efficiently produce foot-and-mouth disease antigens in a silkworm bioreactor. The prepared antigens are extremely safe and can directly produce vaccines to immunize animals. The method of preparing foot-and-mouth disease antigen of the present invention does not require investment in building a factory, has no three wastes, consumes very little energy such as electricity and water resources, and its production cost is also significantly lower than the traditional method of preparing foot-and-mouth disease antigen. It is safe, efficient, has low energy consumption and low cost. Low and many advantages.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
Formulations for alteration of biophysical properties of mucosal lining
InactiveCN101237853AReduce disseminationReduce surrounding pollutionAntibacterial agentsOrganic active ingredientsActive agentIn vivo
The inventors have developed conductive formulations and methods of use thereof containing conductive agents that are ionic or readily ionizable in aqueous or organic solvent environments, such as salts, ionic surfactants, or other substances. The formulation may optionally contain one or more active agents, eg, antiviral, antimicrobial, anti-inflammatory proteins or peptides. The active agent can be co-administered or incorporated into the formulation, or can be administered after administration of the conductive formulation. When applied to a mucosal lining fluid, the formulation alters the physical properties of the mucosal lining, such as surface tension, surface elasticity, and bulk viscosity. The formulations are administered in amounts sufficient to alter the biophysical properties of the mucosal lining in vivo. The formulation can be administered for several different purposes: to reduce the spread of viral and bacterial infectious diseases such as SARS, influenza, tuberculosis, and RSV in humans, and foot-and-mouth disease in artiodactyls; Ambient pollution caused by particle formation when sneezing or talking is particularly important in the use of clean rooms; reducing or preventing the occurrence of obstructive sleep apnea and some cases of irritable bowel syndrome; and controlling the intake of drug molecules and pathogens into the dynamics.
Owner:PULMATRIX
Multiplex PCR kit for simultaneously detecting four viruses carried by ruminants
ActiveCN104450966AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesDNA ContaminationMultiplex pcrs
The invention discloses a multiplex PCR kit for simultaneously detecting four viruses carried by ruminants. The kit comprises four pairs of specific primers. The experiment proves that bluetongue, foot-and-mouth disease, peste des petits ruminants and vesicular stomatitis virus nucleic acid in clinical samples of ruminants can be simultaneously detected. The kit has the characteristics of high sensitivity, high specificity and simplicity in operation, the detection time can be saved, and the condition that DNA contamination occurs in detection is reduced.
Owner:艾军 +2
Method for preparing purified foot-and-mouth disease vaccine
InactiveCN103374547ARule out emergency responseReduced risk of contamination with exogenous agentsAntiviralsVertebrate cellsContinuous flow centrifugationSaccharum
The invention discloses a method for preparing a purified foot-and-mouth disease vaccine, a serum or animal-derived ingredient free culture medium and an application of the serum or animal-derived ingredient free culture medium to the preparation of the foot-and-mouth disease vaccine, belonging to the filed of biotechnology. The method for preparing the purified foot-and-mouth disease vaccine comprises the following steps of: culturing a foot-and-mouth disease virus by using the serum or animal-derived ingredient free culture medium, purifying an obtained virus solution to obtain a purified antigen, subjecting a cell strain BHK-21 or BSR to the multiple-generation acclimatization culture and the suspension culture by 300L of a microcarrier through the serum-free culture medium, inoculating the cell strain BHK-21 or BSR against the foot-and-mouth disease vaccine, stirring at the rotating speed of 30-50rpm, microfiltrating, ultrafiltrating, concentrating 50-200 times, carrying out chromatography with a Sephawse6FF molecular sieve or density gradient zonal centrifugation with a continuous flow, and inactivating with beta-propiolactone to obtain the serotype univalent or multivalent vaccine for cattle, sheep and pigs.
Owner:北京必威安泰科技有限公司 +1
LAMP kit for detecting hogcholera virus and preparation method thereof
InactiveCN101358246AEasy to operateJudging whether to expand or notMicrobiological testing/measurementFood safetyQuarantine
The invention belongs to the field of sanitary examination, and relates to a LAMP kit for testing classical swine fever virus and an establishing method and an application thereof. The kit contains a test system which is composed of the LAMP reaction liquid of six LAMP primers. The tests prove that the kit of the invention has good specificity and sensitivity, fast amplification speed, high efficiency and simple and convenient identification. The test system of the invention can rapidly and conveniently test the classical swine fever virus in high-efficiency, high-specificity and high-sensitivity under the isothermal condition of 65 DEG C without complicate instruments, can better satisfy the spot tests for the classical swine fever virus, provides a novel technical platform for food safety testing, can better meet the urgent requirements for the spot testing of foot and mouth diseases at present, is used for the spot testing of import and export quarantine, food sanitary departments, animal breeding farms, etc, and is easy to be popularized in a wide range.
Owner:SHANGHAI ENTRY EXIT INSPECTION & QUARANTINE BUREAU OF P R C
Method for detecting foot-and-mouth disease antigen 146S content with sucrose density gradient ultraviolet light quantitative method
ActiveCN101655452AStrong specificityGood repeatabilityPreparing sample for investigationColor/spectral properties measurementsAntigenSucrose
The invention provides a method for detecting foot-and-mouth disease antigen 146S content with a sucrose density gradient ultraviolet light quantitative method; the method comprises the following steps: sucrose density gradient ultra centrifugation is carried out to virus concentration liquid to be detected, and then OD259nm value of all fractions is continuously detected by an ultraviolet light spectrophotometer; next, peak area of OD value of a sample to be detected is calculated, and the 146S content in the virus liquid is calculated out according to the formula (FR*PA*FSD*1000) / (S*PL*E*W),wherein FR is flow rate of sucrose which passes through a sample cell; PA is peak area; S is speed, FSD is an absorbance unit, PL is optical path length of the flowing sample cell, W is original weight or volume of a sample which is added on the gradient, E is correction coefficient which is optional positive number. The method has better repetitiveness and applicability, and can be used for accurate and quantitative detection of 146S relative content of various foot-and-mouth viruses.
Owner:JINYUBAOLING BIO PHARM CO LTD
Application of baby hamster kidney(BHK)-21 cell serum-free suspension culture technology in foot-and-mouth disease vaccine production
ActiveCN102178946AShort cycleIncrease productionMicroorganism based processesAntiviralsSerum igeSerum free
The invention discloses application of a baby hamster kidney(BHK)-21 cell serum-free suspension culture technology in foot-and-mouth disease vaccine production, which comprises the following steps of: 1) performing cell recovery; 2) performing reactor culture and cell amplification culture; and 3) inoculating foot-and-mouth disease virus seed venom and collecting the venom. A process for producing foot-and-mouth disease inactivated vaccines by culturing the BHK-21 cells through serum-free suspension culture and a step-by-step cell amplification method make the production period f the foot-and-mouth disease vaccines shortened and yield increased, and ensure stable quality and obvious benefit. The production process reduces the using amount of a culture medium, and the amount of the collected virus liquid is the culture medium consumption amount, while the culture medium consumption amount in a roller bottle production process is 2 times higher than the amount of the collected virus liquid, and bovine serum which is about 5 percent of the culture medium amount is needed.
Owner:马忠仁 +5
Foot-and-mouth disease virus (FMDV) resistant monoclonal antibody and identified epitope and application thereof
ActiveCN101724605AGood passive immunityImprove immunityVirus peptidesImmunoglobulins against virusesIn vivoAmino acid
The invention discloses a foot-and-mouth disease virus (FMDV) resistant monoclonal antibody and an identified epitope and application thereof, and belongs to the field of prevention and control of the FMDV. The microbial collection number of a hybridoma cell line, which can secrete the neutralizing monoclonal antibody resisting to Asia-1 FMDV, is CGMCC No.2692; and the microbial collection number of the hybridoma cell line, which can secrete the neutralizing monoclonal antibody resisting to O-type FMDV, is CGMCC No.2691. The invention also discloses amino acid sequences of a conformational neutralizing epitope of the Asia-1 FMDV VP1 protein and a linear neutralizing epitope of the O-type FMDV VP1 protein which are identified by the two monoclonal antibodies respectively. In-vitro neutralization tests and in-vivo animal protection tests show that both the two monoclonal antibodies have excellent passive immunity effect, can be applied to emergency prevention of the FMDV and have excellent immunity effect on the passive immunity of the FMDV.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Antiviral serotype shared monoclonal antibody of foot-and-mouth disease and distinguished epitope thereof
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Asia1 type multi-epitope recombinant vaccine of bovine foot-and-mouth disease viruses and preparation method thereof
ActiveCN101775399AImproving immunogenicityFacilitate presentationGenetic material ingredientsVirus peptidesAdjuvantRecombinant vaccines
The invention discloses an Asia1 type multi-epitope recombinant vaccine of bovine foot-and-mouth disease viruses and a preparation method thereof. The recombinant vaccine comprises the following components: proteins coded by foot-and-mouth disease virus multi-epitope genes and the fusion genes of carrier proteins, and foot-and-mouth disease virus 3D proteins. The preparation method comprises the following steps: diluting the proteins expressed by the foot-and-mouth disease virus multi-epitope genes and the fusion genes of the carrier proteins and the foot-and-mouth disease virus 3D proteins, mixing the diluted proteins uniformly, adding an adjuvant into the mixture to emulsify the mixture. Animal models and animal immune effect experiments show that the bovine Asia1 epitope recombinant vaccine can make comprehensive immune protective response, can induce injected and immunized bovine and guinea pigs to generate high level neutralizing antibodies, and can also induce cell immune response, so the recombinant vaccine can effectively protect animals against the virulent attack of the foot-and-mouth disease viruses.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Reagent box for detecting aftosa A type, O type and Asia 1 type viruses and preparing method thereof
ActiveCN104195268AMicrobiological testing/measurementMicroorganism based processesSwine vesicular diseaseElectrophoresis
The invention discloses a primer capable of being used for detecting aftosa A type, O type and Asia 1 type viruses, a reagent box comprising the primer and a preparing method thereof. Six gene sequences are available in the primer for detecting the aftosa A type, O type and Asia 1 type viruses, and two universal primers are additionally arranged. Related experiments show that the nucleic acids of the aftosa A type, O type and Asia 1 type viruses can be specifically and rapidly amplified by the primer sequences, and a specific strip is detected by nucleic acid electrophoresis, but the nucleic acids of vesicular stomatitis viruses and swine vesicular disease viruses cannot be amplified under the same conditions. The primer can be used for rapidly identifying the aftosa A type, O type and Asia 1 type viruses and applied in the epidemiological research of aftosa viruses.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Type O foot-and-mouth disease virus mutant and preparation method and application thereof
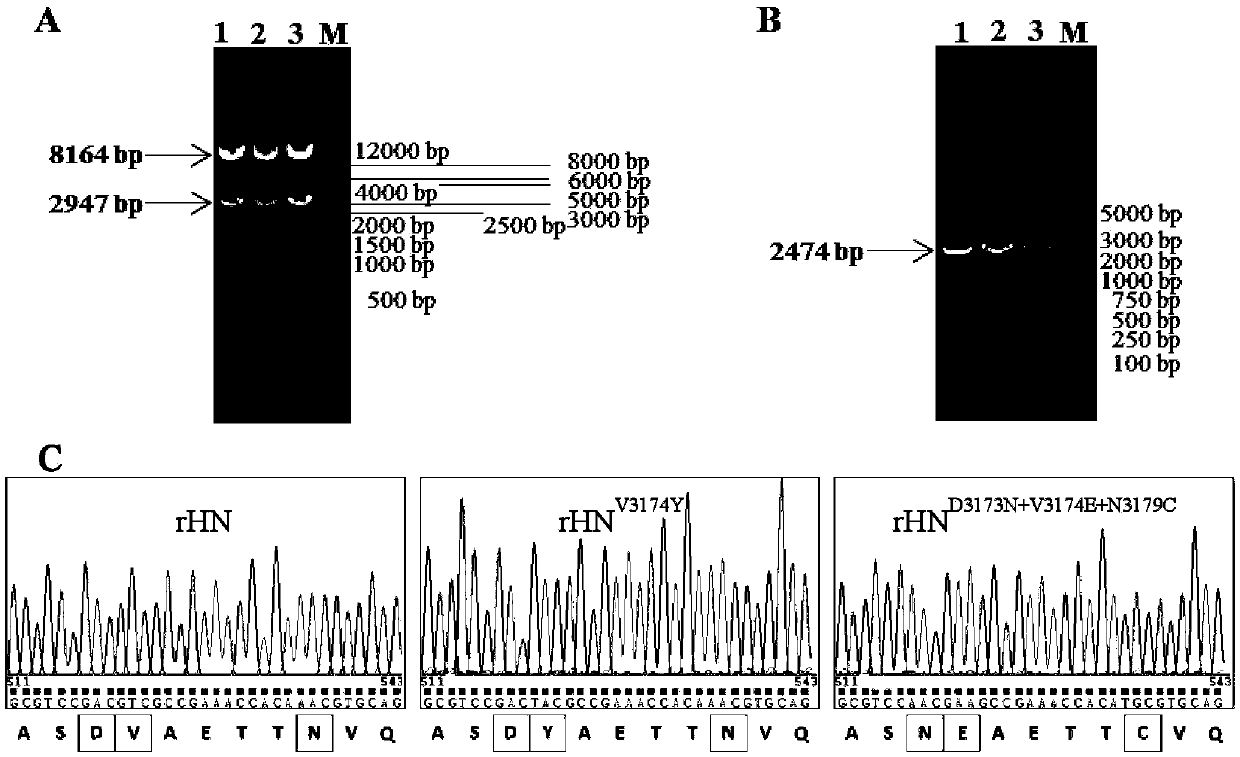
The invention provides a type O foot-and-mouth disease virus mutant and a preparation method and application thereof, and belongs to the technical field of vaccine candidate strains. According to thetype O foot-and-mouth disease virus mutant disclosed by the invention, an rHN virus strain is used as a female parent virus strain, and the following amino acids in a G-H ring of VP3 protein are mutated: the 173rd aspartic acid is mutated into asparagine, the 174th valine is mutated into glutamic acid and the 179th asparagine is mutated into cysteine. The virus mutant has heredity stability and obtains the capacity of caveolin for performing mediated infestation on CHO-K1cells. The result of detecting the cross neutralization capacity of immune positive serum indicates that compared with a female parent virus strain, the virus mutant has the advantages that the cross protection capacity of the virus mutant for inducing organisms to produce foot-and-mouth disease virus neutralizing antibodies is notably improved, and the virus mutant shows excellent antigen broad spectrum properties. Vaccines prepared through inactivation of the virus mutant can be used for preventing infection with type O foot-and-mouth disease viruses.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Primers, probes and kit for on-site detection of a variety of serotype foot and mouth disease viruses
ActiveCN104946795AHigh sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationForward primerRNA extraction
The invention discloses a universal primer and probe combination for detecting foot and mouth disease viruses through RPA (recombinase polymerase amplification)-lateral flow assay technology. The forward primer sequence is shown as SEQ ID No.1, the reverse primer sequence is shown as SEQ ID No.2, and the probe sequence is shown as SEQ ID No.3. The invention also discloses a kit for detecting foot and mouth disease viruses. Detection with the primers and probes involved in the invention only needs virus RNA extraction on clinical samples and isothermal amplification after one-step process reverse transcription into cDNA, thermal cycling reaction is not needed, amplification in a PCR instrument is unnecessary, and the result can be clearly displayed on a lateral flow chromatography test strip. The primer and probe combination and the kit provided by the invention have the advantages of high sensitivity, strong specificity, simple reaction procedure, short detection time and the like, and are suitable for rapid, accurate and simple detection of foot and mouth disease viruses on a cattle farm.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI
Chinese medicinal composition for preventing and treating hand, foot and mouth disease in children
The invention relates to a Chinese medicinal composition for preventing and treating hand, foot and mouth disease in children and belongs to the technical field of the traditional Chinese medical science and Chinese medicines. The Chinese medicinal composition is prepared from the following Chinese herbal medicines by weight: 10g of towel gourd vegetable sponge, 30g of rhubarb, 10g of Chinese gall, 35g of common cnidium fruit, 30g of calamine, 25g of salted jellyfish, 5g of wrinkled gianthyssop herb, 4g of gypsum rubrum, 15g of cassia twig, 20g of incised notopterygium rhizome, 18g of heracleum, 15g of clematis root, 10g of bark of oriental variegated coralbean, 8g of fructus amomi, 8g of katsumade galangal seed, 20g of coix seed, 15g of hypericum japonicum, 10g of abrus herb, 12g of borax and 10g of Spanish fly. The Chinese medicinal composition has quick response when used for treating the hand, foot and mouth disease, can take effect in two days, has a good therapeutic effect, and can effectively prevent herpes from ulcerating and relive the pain of the children, and the cure rate reaches over 90 percent; and the Chinese medicinal composition can prevent the hand, foot and mouth disease.
Owner:张成河
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com