Kit for quantitative detection on O type foot-and-mouth disease virus antibody through fluorescence immunoassay magnetic particles
A quantitative detection and fluorescence immunoassay technology, which is applied in the field of animal disease detection, can solve the problems of time-consuming operation, long detection time, cumbersome and time-consuming operation, etc., and achieve the effect of shortening the reaction time, simple and fast operation, and improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In this embodiment, the kit for quantitative detection of type O foot-and-mouth disease antibody by fluorescent immunomagnetic particles consists of a reagent strip and type O foot-and-mouth disease negative and positive serum.
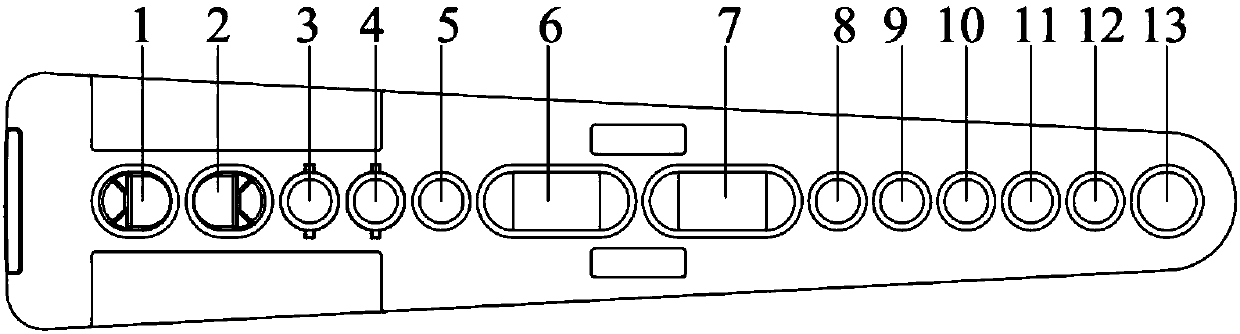
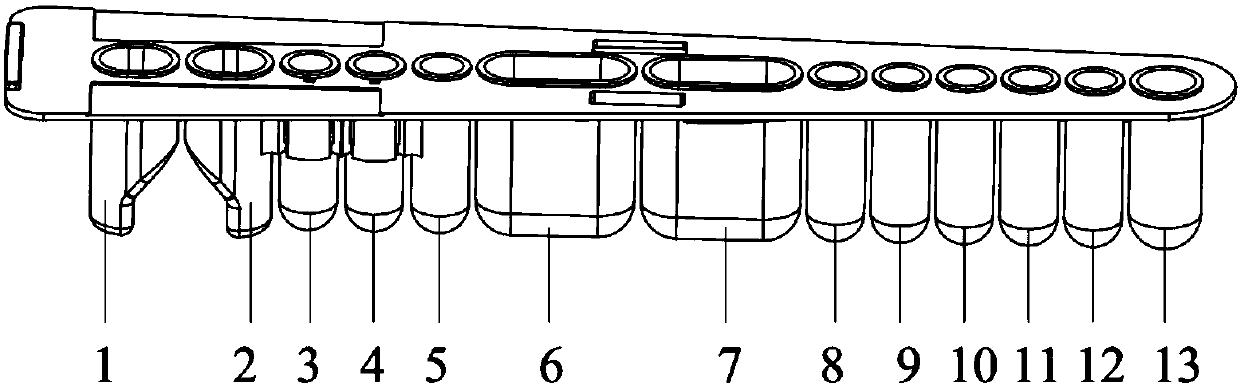
[0033] Among them, the reagent strip consists of test well 1 (VP1 protein coated carboxyl magnetic beads), well 3 (biotinylated goat anti-pig secondary antibody), well 4 (streptavidin-labeled europium), washing liquid well 6 and 7. Strengthen the composition of liquid hole 12, such as figure 1 with figure 2 Shown.
[0034] The preparation process of the fluorescent immunomagnetic particle quantitative detection kit for O-type foot-and-mouth disease antibody is as follows:
[0035] (1) Preparation method of immunomagnetic beads:
[0036] Washing magnetic beads
[0037] Pipette 1mg of carboxyl magnetic beads with a diameter of 1μm into a 1.5ml centrifuge tube, place the centrifuge tube in a magnetic stand, use the magnetic stand to separate the magneti...
Embodiment 2
[0072] Example 2 Preparation and detection of reagent strips
[0073] The reagent strips in this example are semi-finished products assembled through the following processes: Dispense 100 μL of immunomagnetic beads, 200 μL of biotinylated goat anti-pig antibody, SA-Eu into the first, third, fourth, sixth, seventh, and twelfth stage. 3+ , 900μL lotion, 900μL lotion, 300μL enhancement solution, and then sealed with a film sealing machine. The sealing film is coated with product information identification that can be scanned and identified by the automatic fluorescence detection analyzer, including corporate standard curves, batches , Production date, expiration date. The finished reagent strip, the pre-packed O-type foot-and-mouth disease negative serum, positive serum and other accessories are assembled into a kit.
[0074] Sample testing:
[0075] ①Add sample:
[0076] Put the sample to be tested or the O-type foot-and-mouth disease antibody standard into the loading system of the au...
Embodiment 3
[0090] Example 3 Sensitivity detection
[0091] The steps of this method are as follows:
[0092] (1) Dilute the porcine type O FMDV positive standard serum with a calibrated antibody titer of 1:512 from 1:32 to 1:32, 1:64, 1:128, 1 : 256, 1:512, 1:1024 six dilutions, put the diluted sample into the sample compartment.
[0093] (2) Add 100μl of biotinylated goat anti-pig antibody to the test well of the reagent strip, and incubate at 37°C for 10min; then discard the supernatant, wash with washing solution 5 times, adding 200μl washing solution each time;
[0094] (3) Add 100μl SA-Eu diluted 1:80000 to the test hole of the reagent strip 3+ , Incubate at 37°C for 10 minutes, then discard the supernatant and wash with lotion;
[0095] (4) Add 100μl of enhancement solution to the test hole of the reagent strip and incubate for 5 minutes;
[0096] (5) The detector automatically detects the fluorescence value.
[0097] If the fluorescence value of the serum to be tested is ≥ 37695, the serum t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com