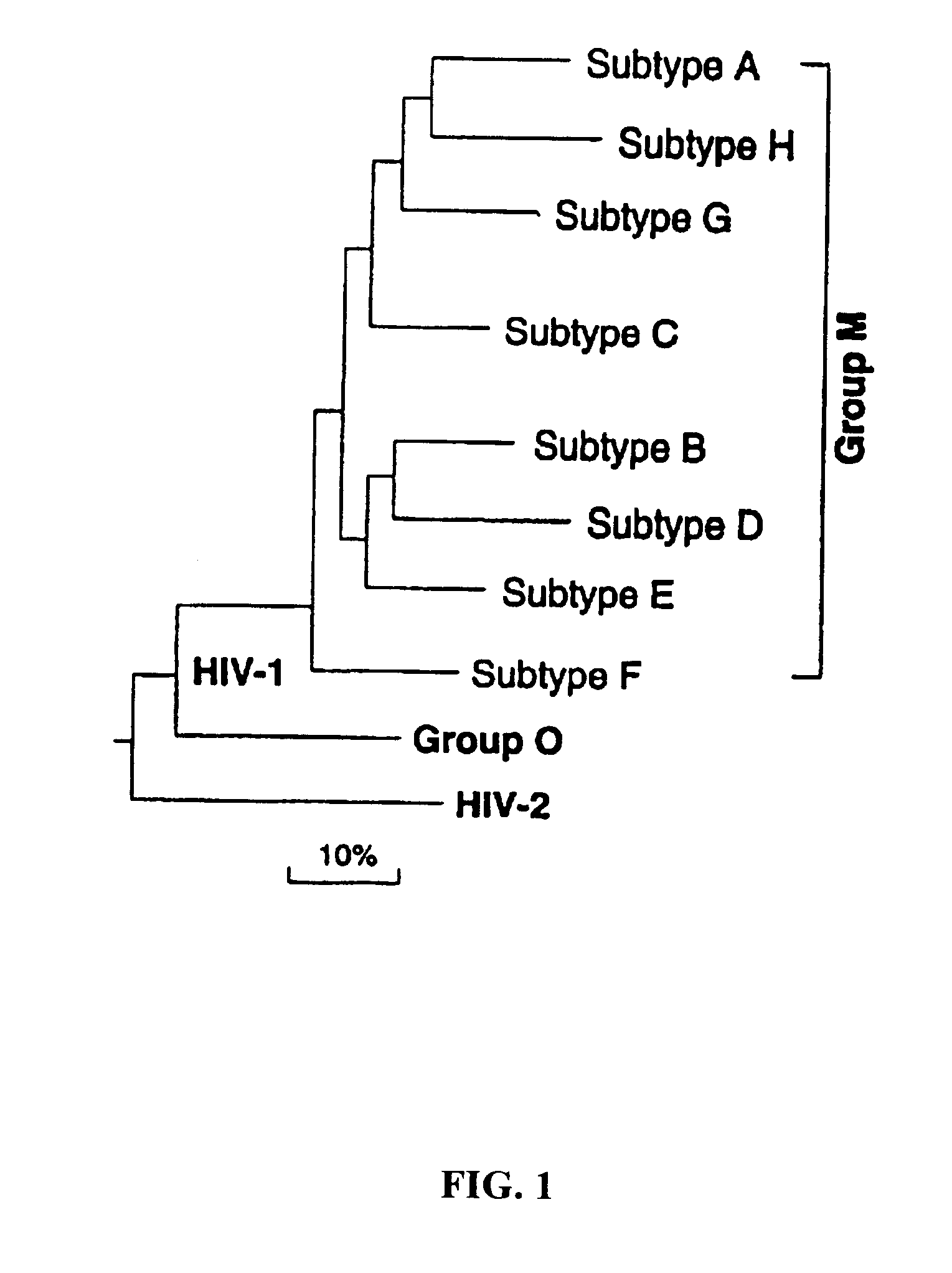
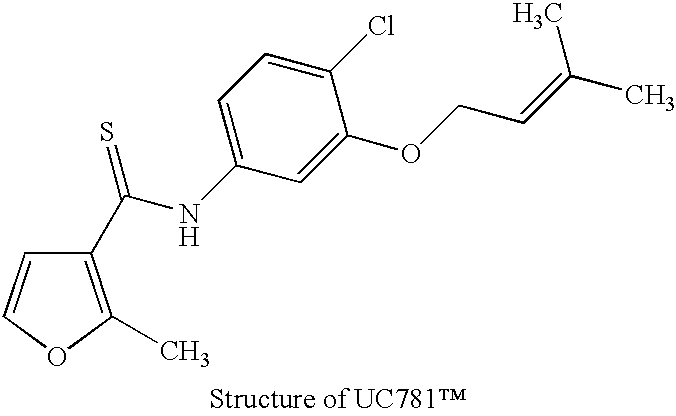
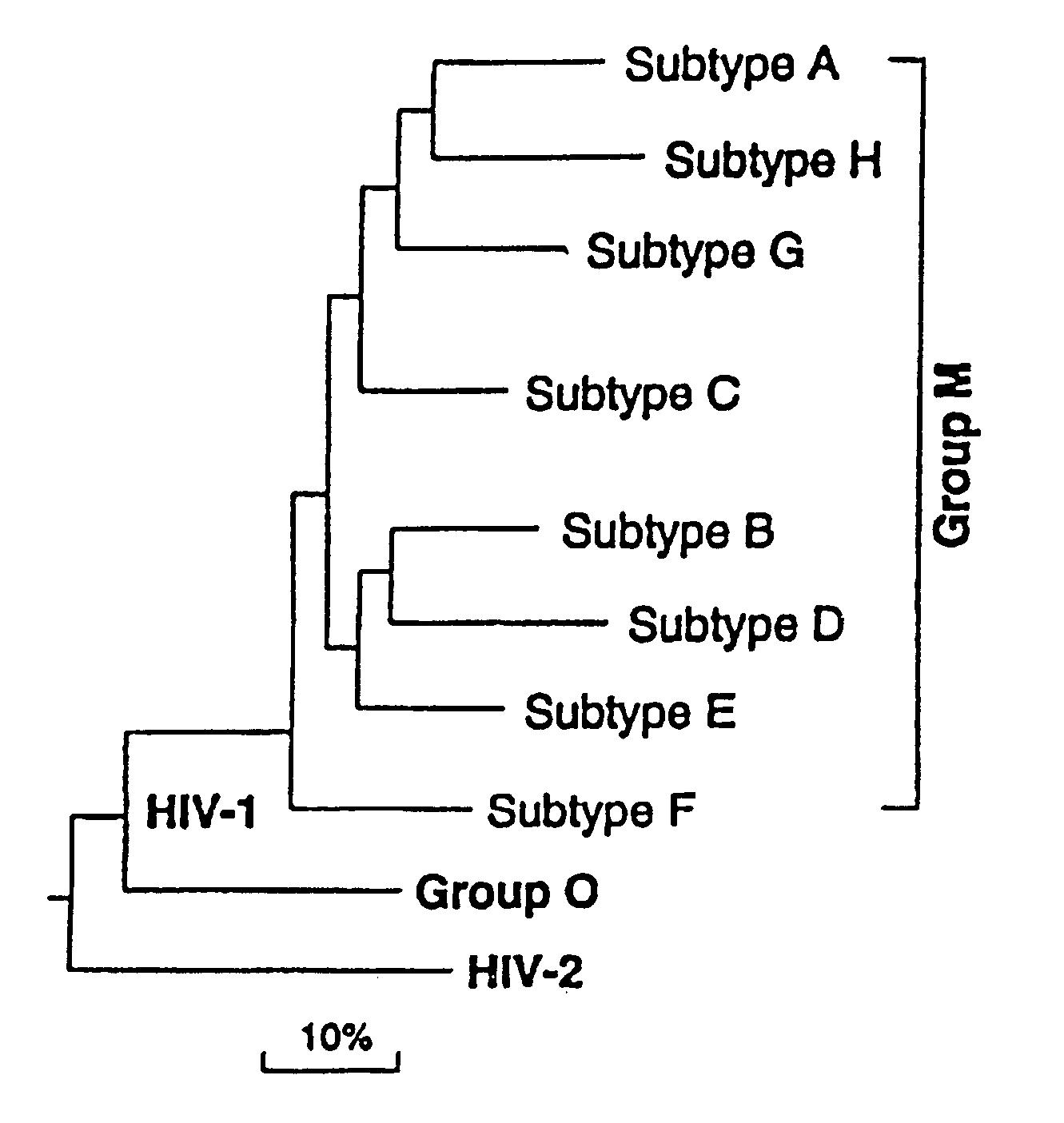
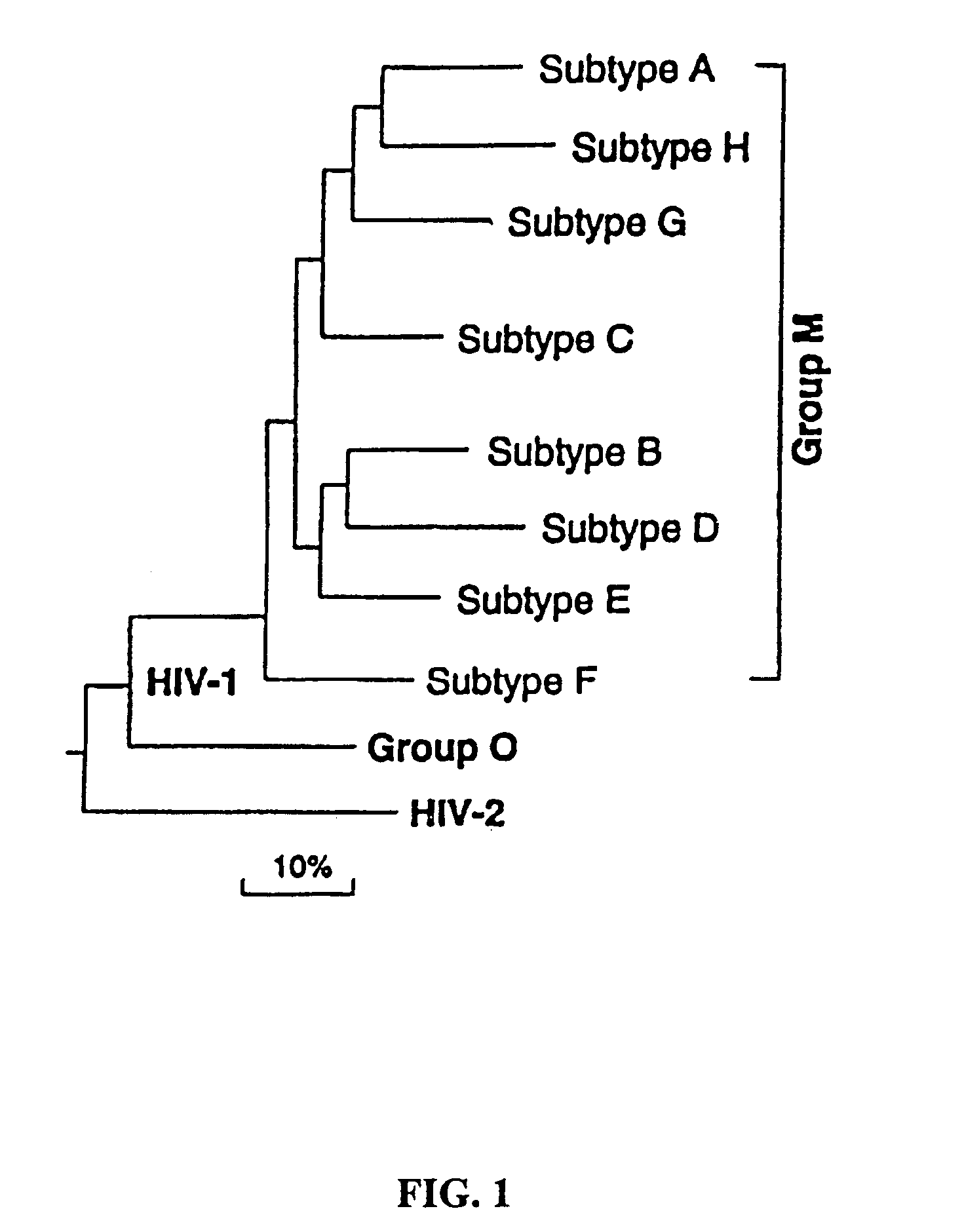
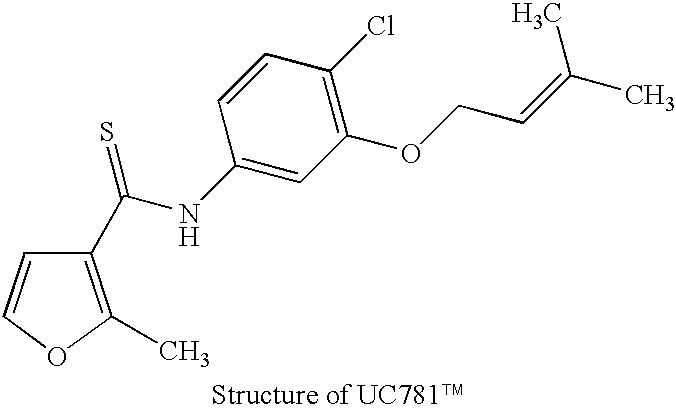
Patents
Literature
506 results about "Live virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A live virus vaccine contains a weakened, live virus that is designed to help your body develop an immune response without you developing symptoms of the disease it is intended to prevent. The virus is teaching your immune system what the virus looks like and allows the body to develop an immune response.
Interferon inducing genetically engineered attenuated viruses
InactiveUS6468544B1Reduce in quantityReduced characteristicsSsRNA viruses negative-senseVectorsGenetic engineeringRecombinant DNA
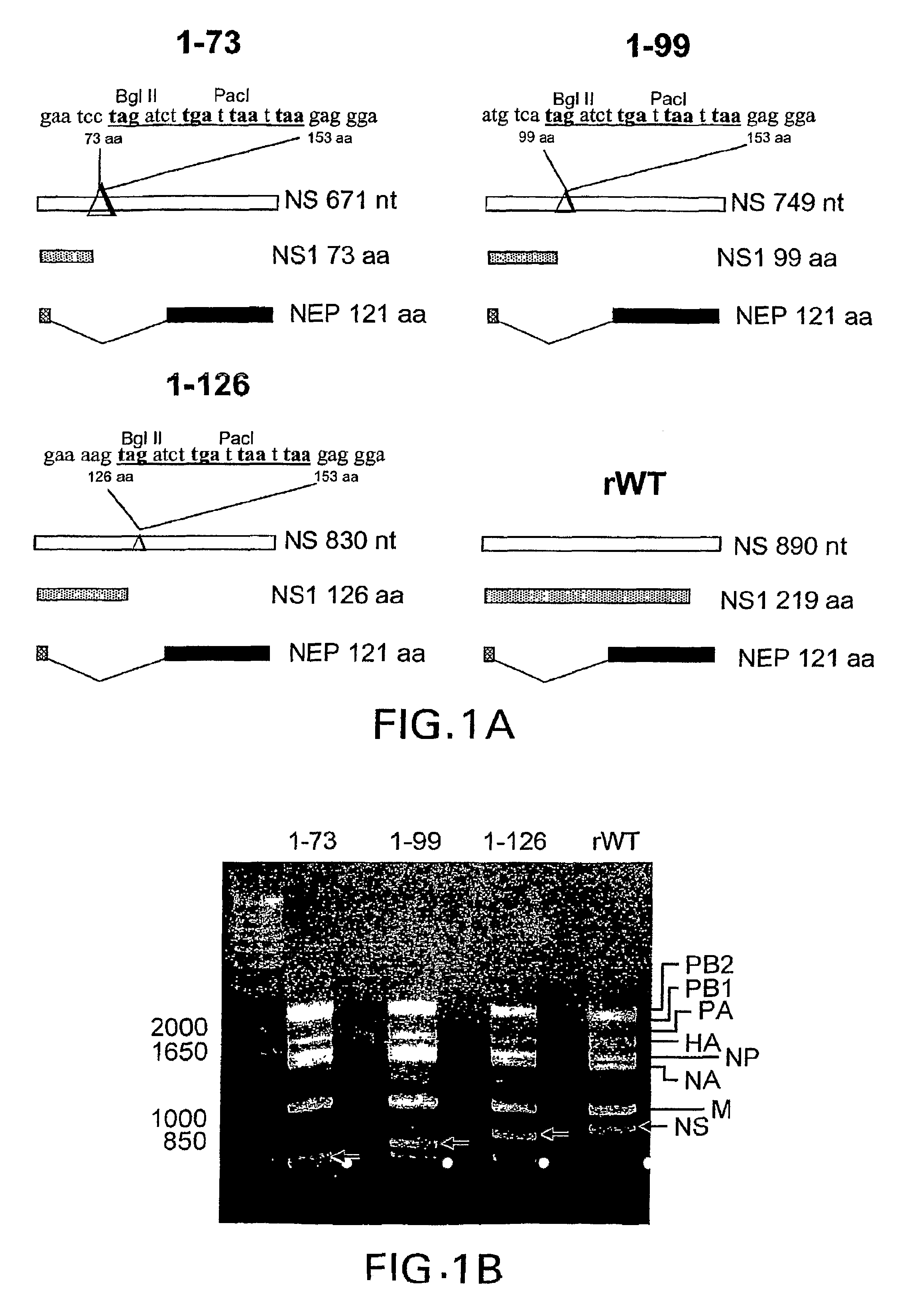
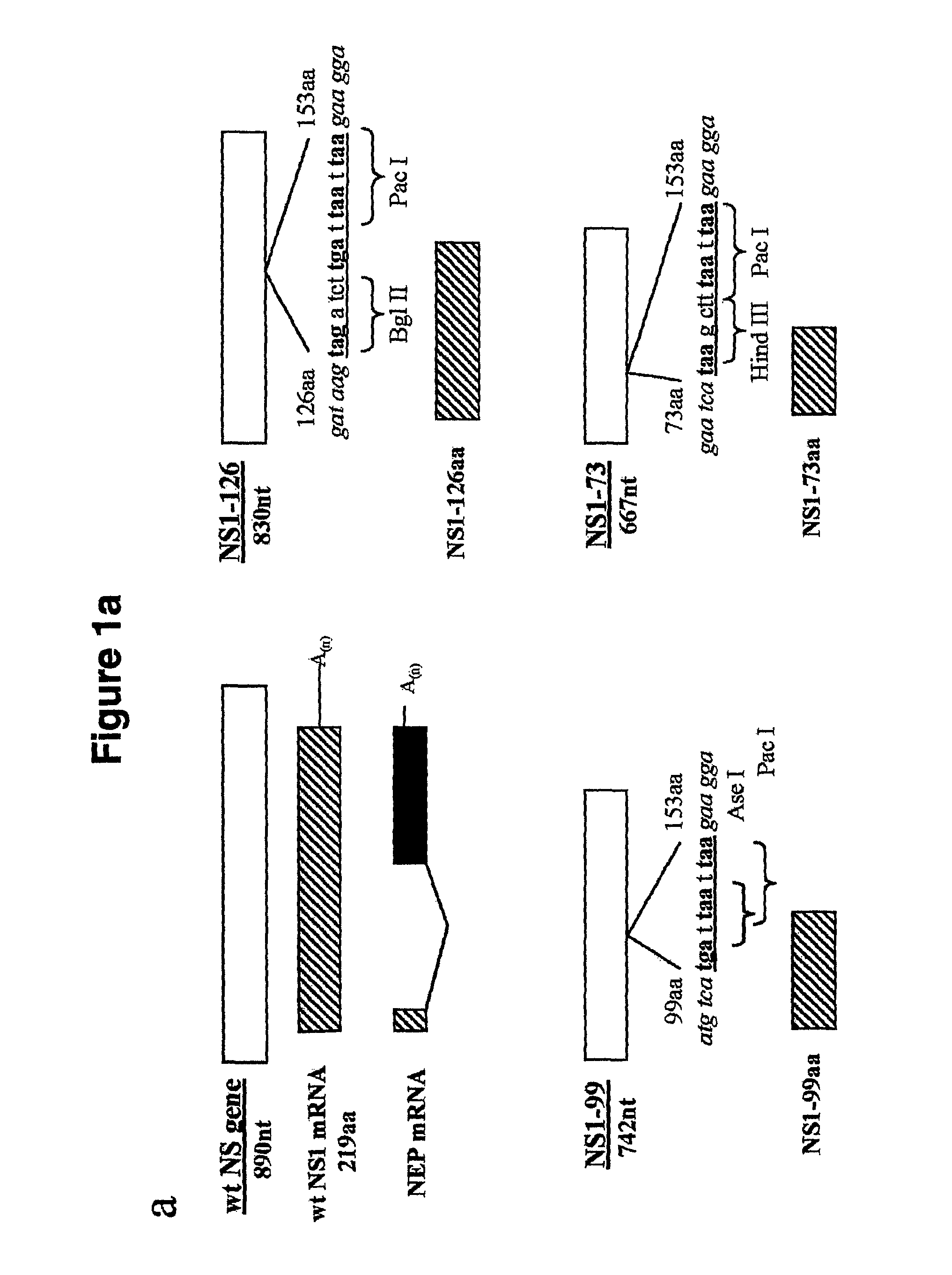
The present invention relates to genetically engineered attenuated viruses and methods for their production. In particular, the present invention relates to engineering live attenuated viruses which contain a modified NS gene segment. Recombinant DNA techniques can be utilized to engineer site specific mutations into one or more noncoding regions of the viral genome which result in the down-regulation of one or more viral genes. Alternatively, recombinant DNA techniques can be used to engineer a mutation, including but not limited to an insertion, deletion, or substitution of an amino acid residue(s) or an epitope(s) into a coding region of the viral genome so that altered or chimeric viral proteins are expressed by the engineered virus.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Attenuated negative strand viruses with altered interferon antagonist activity for use as vaccines and pharmaceuticals
InactiveUS6669943B1Impaired ability to antagonizeAvoiding and minimizing side effectSsRNA viruses negative-senseVirus peptidesNegative strandPharmaceutical drug
Owner:AVIR GREEN HILLS BIOTECH RES DEVMENT TRADE +1
Cellular and viral inactivation
The invention provides compositions of inactivated viruses, bacteria, fungi, parasites and tumor cells that can be used as vaccines. Methods for making such inactivated viruses, bacteria, fungi, parasites and tumor cells are also provided.
Owner:UNITED STATES OF AMERICA
Attenuated negative strand viruses with altered interferon antagonist activity for use as vaccines and pharmaceuticals
InactiveUS20040109877A1Inhibition of replicationReduce the numberSsRNA viruses negative-senseViral antigen ingredientsNegative strandPharmaceutical drug
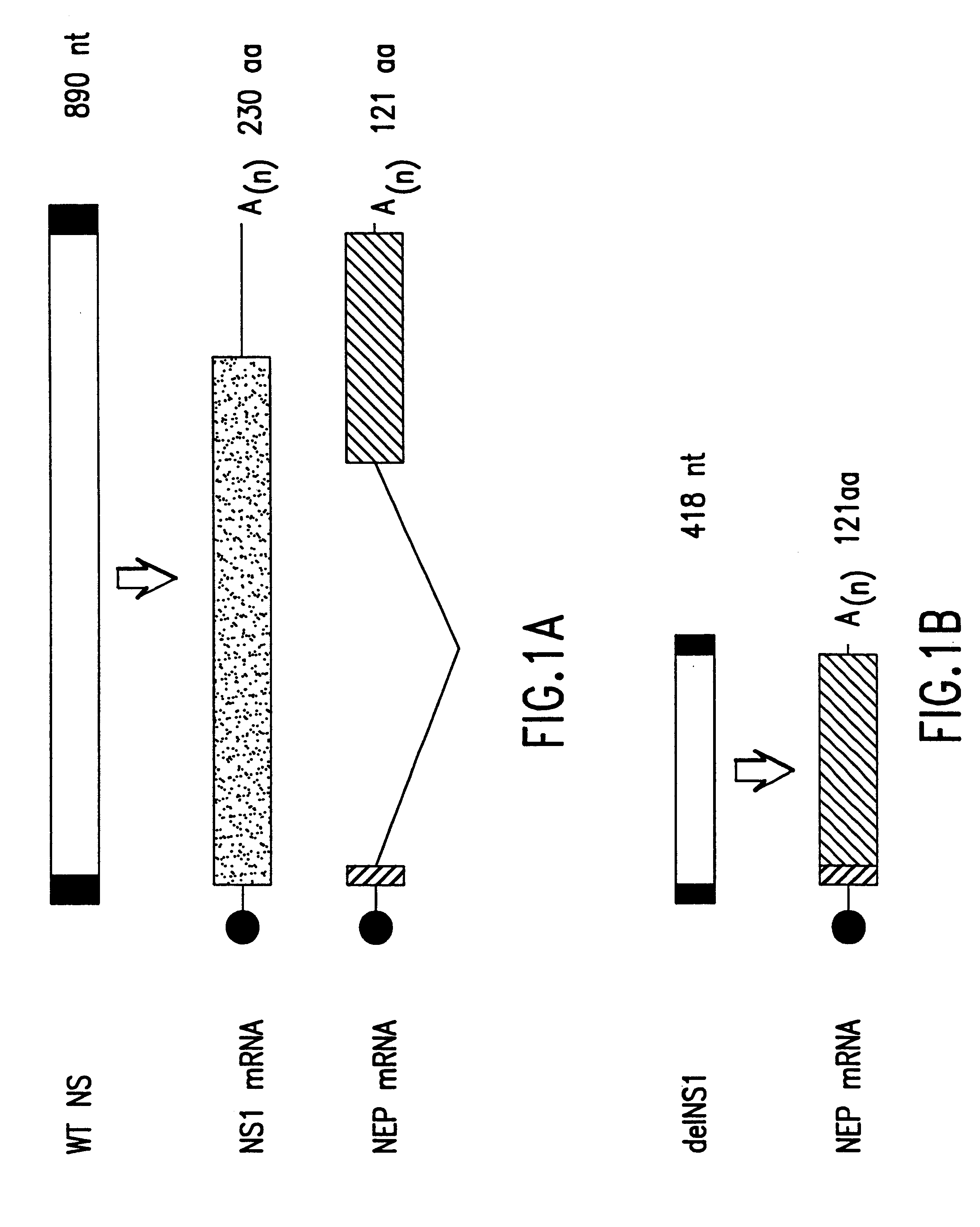
The present invention relates, in general, to attenuated negative-strand RNA viruses having an impaired ability to antagonize the cellular interferon (IFN) response, and the use of such attenuated viruses in vaccine and pharmaceutical formulations. The invention also relates to the development and use of IFN-deficient systems for selection of such attenuated viruses. In particular, the invention relates to attenuated influenza viruses having modifications to the NS1 gene that diminish or eliminate the ability of the NS1 gene product to antagonize the cellular IFN response. The mutant viruses replicate in vivo but demonstrate reduced pathogenicity, and therefore are well suited for live virus vaccines, and pharmaceutical formulations.
Owner:AVIR GREEN HILLS BIOTECH RES DEVMENT TRADE +1
Oral smallpox vaccine production and methods to evaluate safety, efficacy, and potency of orally delivered vaccine
InactiveUS20040175398A1Efficient responseSafety and efficacyViral antigen ingredientsMicrobiological testing/measurementHuman useDiagnostic test
This invention relates to methods and systems for generating a safe and effective oral smallpox vaccine for humans using a genetically defective strain of vaccinia virus to confer immunity following oral delivery of the vaccine. This invention is one that expands on current use of vaccinia virus propagation developed for gene therapy applications, and pharmaceuticals and nutraceuticals packaging and formualtion technologies. The vaccine invention can be delivered as a live virus with the ability to express viral proteins but unable to achieve complete, lytic virus replication, or it may be derived from such a virus, contain additional immunogens, or be delivered as viral antigens. Furthermore, the invention establishes innovative methods for formulation and packaging and for preclinical testing of the vaccine invention for safety, efficacy and potency with the use of human intestinal and other test cells and diagnostic test systems and kits.
Owner:INCELLS
Method for the development of an HIV vaccine
InactiveUS6383806B1Effective immune responseInhibiting infectivityAnimal cellsMicrobiological testing/measurementReverse transcriptaseImmunodeficiency virus
Human immunodeficiency virus (HIV) comprising reverse transcriptase inactivated by photoinactivation used to evoke an immune response. The immune response may protect an individual from challenges with live virus. Alternatively, the inactivated HIV particles may be used to augment the immune response to HIV in an infected individual.
Owner:PHOTOIMMUNE BIOTECH
Method for the development of an HIV vaccine
InactiveUS6503753B1Reduce riskSource securityAnimal cellsViral antigen ingredientsImmunodeficiency virusReverse transcriptase
Human immunodeficiency virus (HIV) comprising reverse transcriptase inactivated by photoinactivation. The inactivated virus may be more safely handled, stored, and analyzed, used in diagnostic procedures and kits, and may be used as an immunogen to evoke an immune response. The immune response may protect an individual from challenges with live virus. Alternatively, the inactivated HIV particles may be used to augment the immune response to HIV in an infected individual.
Owner:PHOTOIMMUNE BIOTECH
Methods and Compositions for Simultaneously Isolating Hemoglobin from Red Blood Cells and Inactivating Viruses
InactiveUS20080138790A1Facilitate viral inactivationFacilitating red blood cell lysisHaemoglobins/myoglobinsCentrifugal force sediment separationLysisRed blood cell
The present invention relates to methods arid compositions for isolating hemoglobin from red blood cells. Such methods and compositions also facilitate viral inactivation in a manner that allows recovery of biologically active hemoglobin. More particularly, this method relates to the use of solvents and detergents that are capable of facilitating red blood cell lysis to release hemoglobin (and solubilizing red blood cell membranes), while simultaneously inactivating viruses.
Owner:SANGART INC
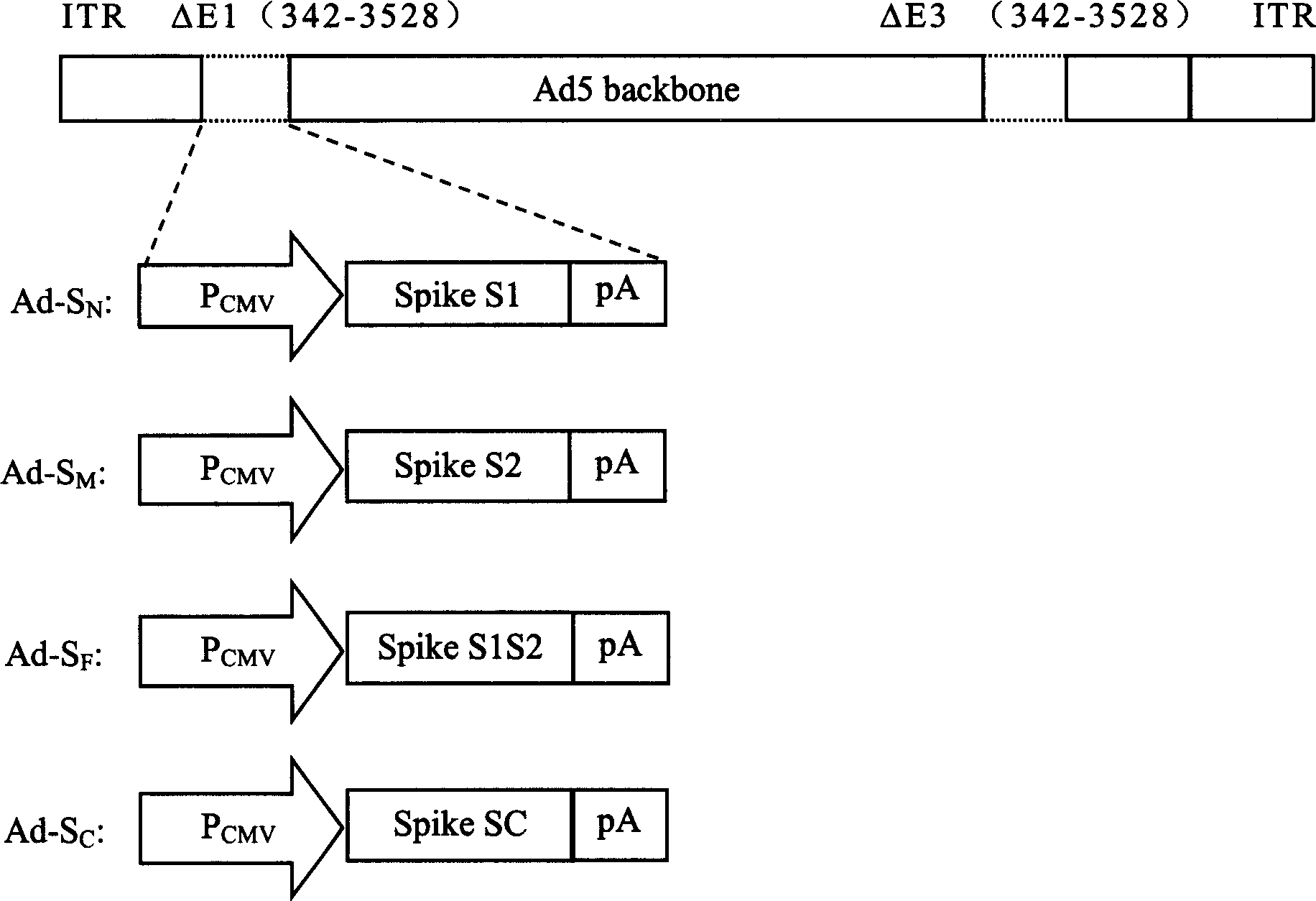
SARS vaccine of adenovirus carrier and preparation method, application of coronavirus S gene
InactiveCN1562365AEffective generationEffective induction ofGenetic material ingredientsAntiviralsGenetic engineeringOrganism
The invention pertains to biological genetic engineering field, specificly relating to SARS vaccine of adenovirus carrier and preparation method, application of related coronavirus S gene in preparation of the SARS vaccine for preventing SARS. Through a bioengineering means, the related coronary virus S gene and a defective adenovirus are recombined, which makes protective immunogen protein or polypeptide expressing in it. A genic vaccine that can arouse the mucosal immunogenicity is produced through amplifying training, purification, and preparation, which induces a immunity reaction in the respiratory mucosa, makes the organism produce the corresponding antibody, and prevents virus from infection. Compared with the traditional inactivated viral particles vaccine, the invention has a high safety; it is convenient to operate; it is not limited by the specific conditions such as intramuscular injection; and it has an extensive clinical application prospect.
Owner:SUN YAT SEN UNIV CANCER CENT
Immunogenic CEA
InactiveUS20050063952A1Effective immune responseBiocideGenetic material ingredientsVaccinationCarcinoembryonic antigen
The present invention provides for methods for immunizing actively against autologous carcinoembryonic antigen (CEA). The method encompasses that the immune system is engaged with variant CEA which is either administered as a protein vaccine, or is effected expressed by nucleic acid vaccination or live-viral vaccination. Preferred embodiments include immunization with variants that include at least one foreign T-helper epitope introduced in the CEA sequence. Also disclosed is variant proteins, DNA, vectors, and host cells useful for practising the method of the invention.
Owner:PHARMEXA
Compositions for the inactivation of virus replication and methods of making and using the same
ActiveUS20170049909A1Improve the level ofAccurate separationPolypeptide with localisation/targeting motifHydrolasesEpitopeNucleotide
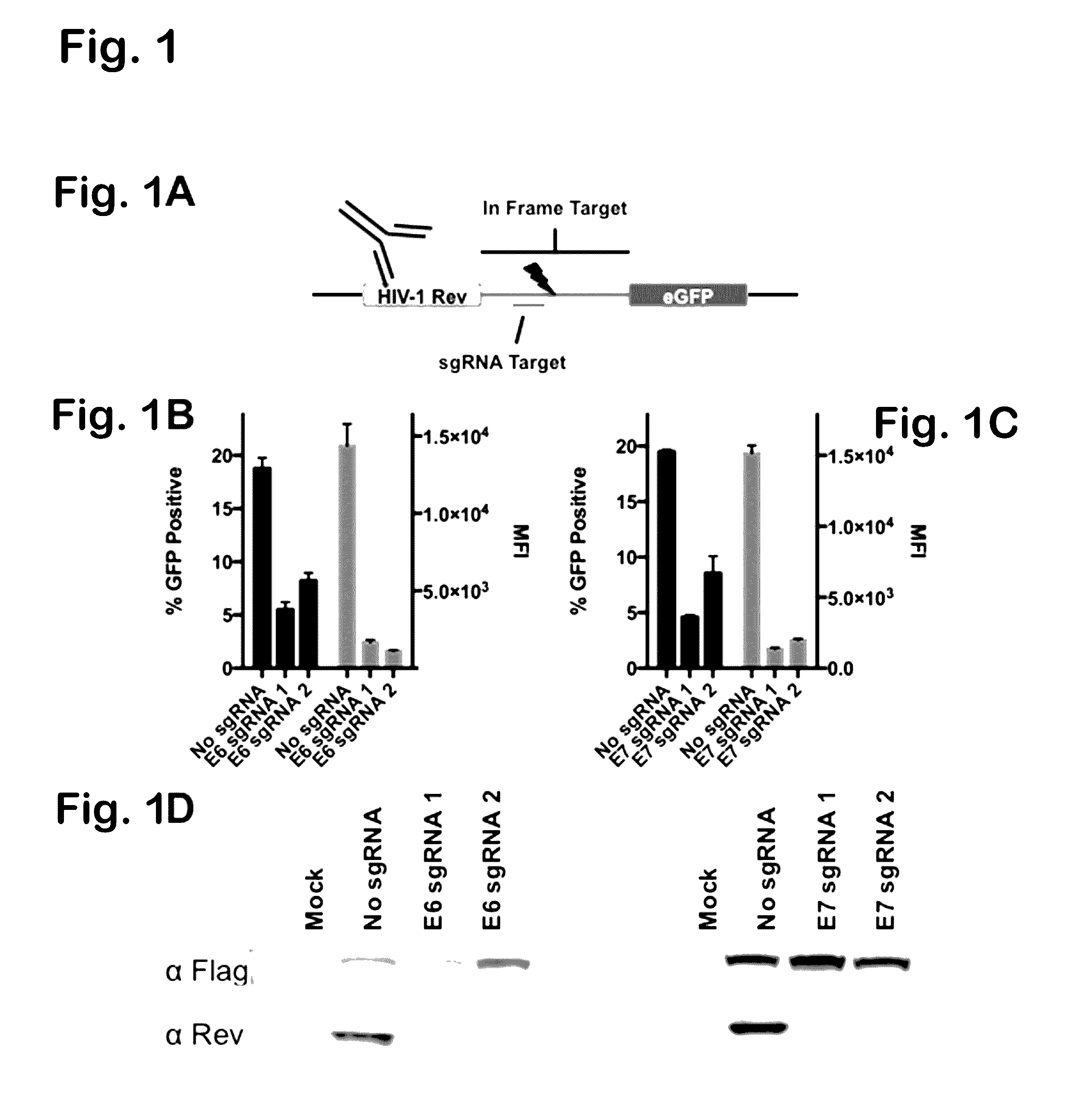
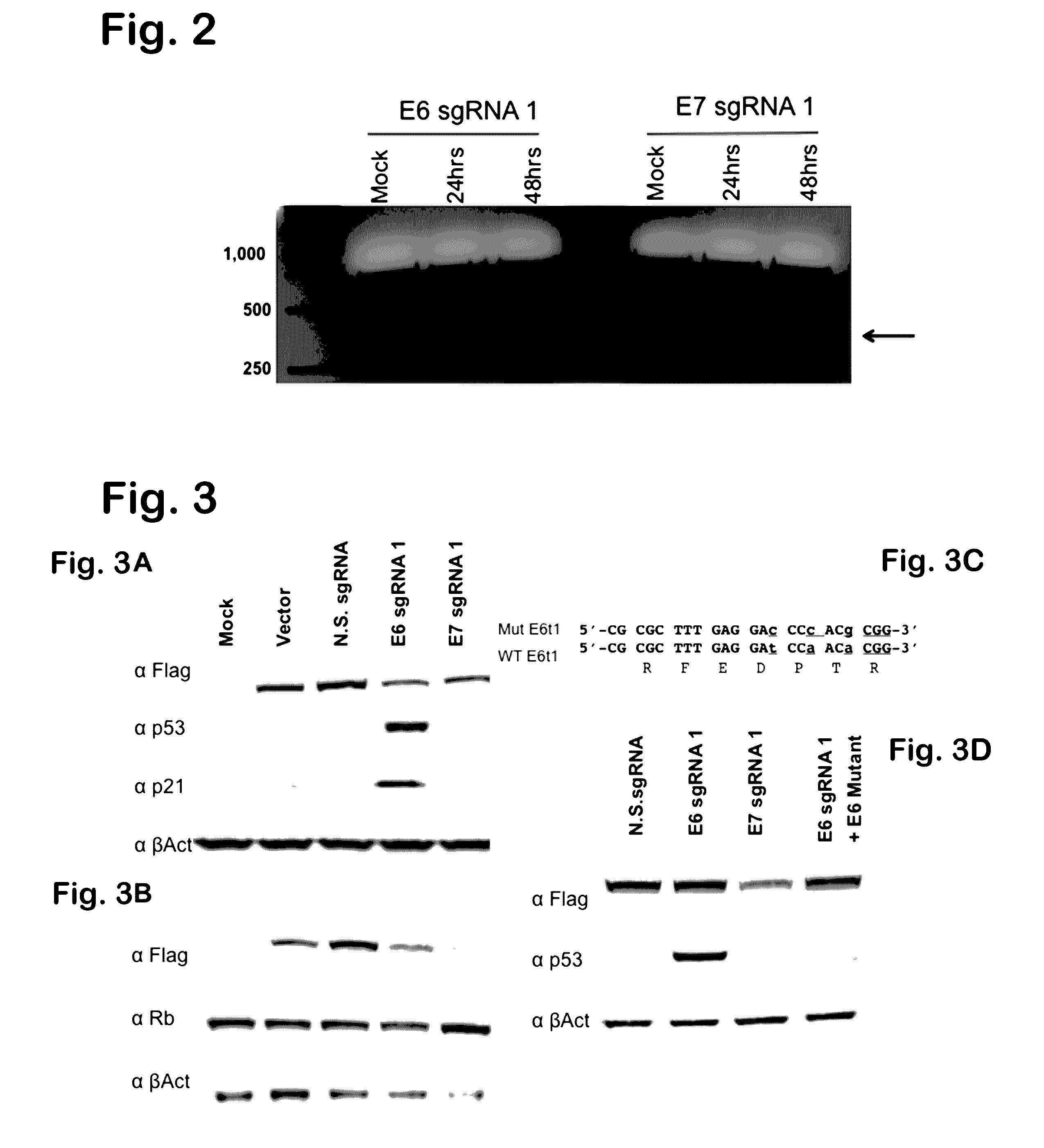
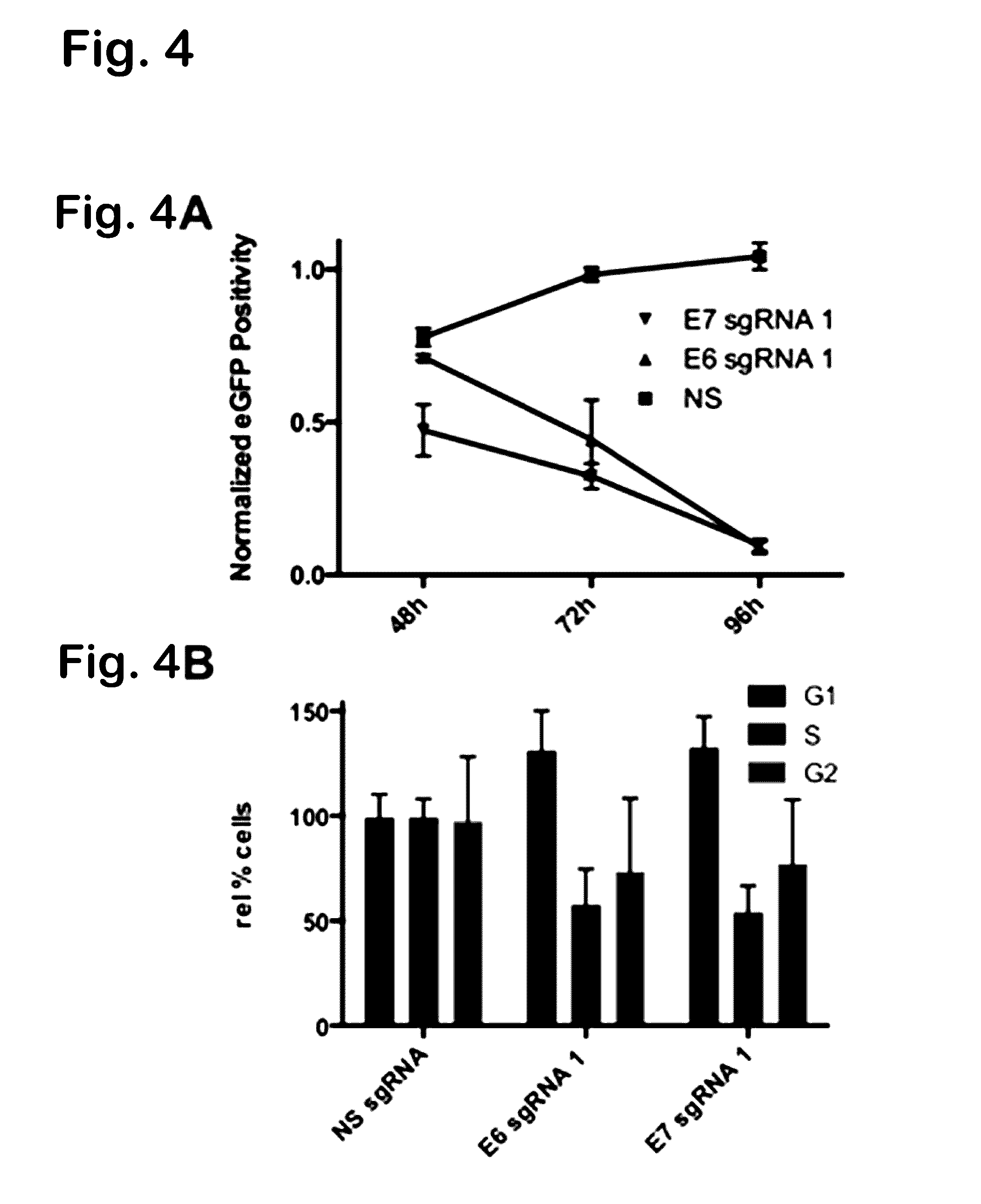
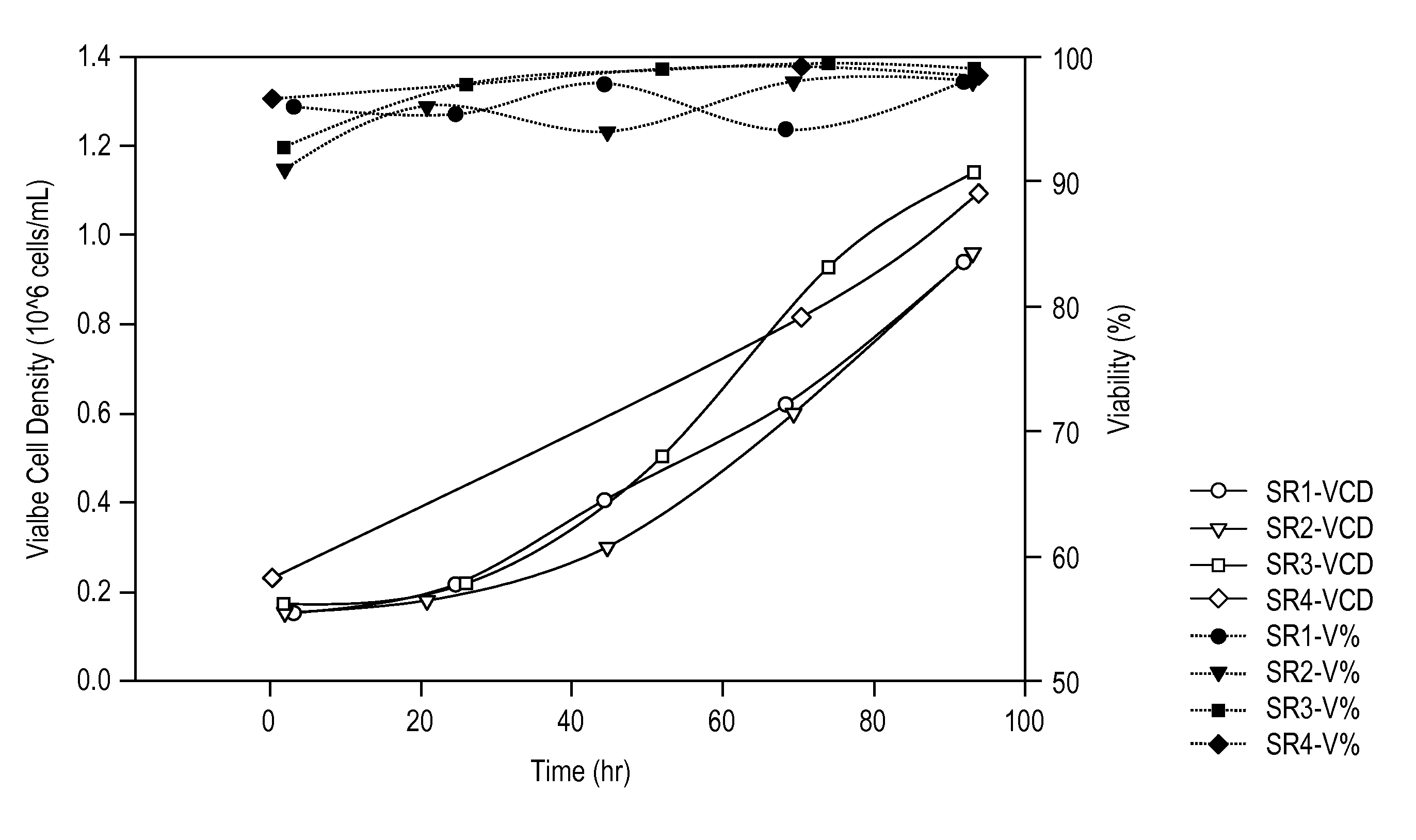
Provided herein are recombinant constructs, vectors and expression cassettes including a first promoter which is suitably a tRNA promoter operably connected to a first polynucleotide encoding a first single guide RNA and a second promoter operably connected to a second polynucleotide encoding a Cas9 polypeptide. The first single guide RNA includes a first portion complementary to a strand of a target sequence of a DNA virus and a second portion capable of interacting with the Cas9 polypeptide. Also provided are codon optimized Staphylococcus aureus derived Cas9 polynucleotides and polypeptides with nuclear localization signals and optionally an epitope tag. Also provided are constructs for production of sgRNAs including a tRNA. Methods of inhibiting viral replication, inhibiting expression of a target sequence from a virus or treating a viral infection or viral induced cancer using the compositions are also provided.
Owner:EMORY UNIVERSITY +2
Disease-suppression antibacterial beautiful sealant and preparation technology thereof
InactiveCN105969046AReduce pollutionReduce pollution sourcesAntifouling/underwater paintsPaints with biocidesDiseaseEpoxy
The present invention provides a disease-suppressing and antibacterial beautifying agent and a preparation process thereof, wherein the disease-suppressing and antibacterial beautifying agent includes components A and B; component A includes: 50-90 parts of epoxy resin, benzyl alcohol 1-5 parts, 0.2-1.2 parts of defoaming agent, 1-9 parts of fumed silica, 0.3-1.2 parts of thixotropic agent, 0.2-0.8 parts of silver antibacterial agent, 0.5-1.2 parts of ultraviolet absorber, and 1 part of water repellent ‑6 parts, 3‑40 parts of toner, 1‑20 parts of flexibility additive; component B includes: 10‑20 parts of polyetheramine, 9‑35 parts of alicyclic amine, 25‑45 parts of epoxy resin, benzyl alcohol 20-40 parts, 0.5-2 parts of defoamer, 0.5-4 parts of fumed silica, 1-5 parts of thixotropic agent, 0.2-0.9 parts of silver antibacterial agent. The invention can fill the gaps to bring aesthetics, and at the same time, suppress live viruses and bacteria existing in the gaps, and reduce indoor microbial pollution sources.
Owner:苏州久居环保科技有限公司
Methods for cultivating cells, propagating and purifying viruses
InactiveUS20100098725A1Increase cell densityHigh viral titersSsRNA viruses negative-senseViral antigen ingredientsCell culture mediaCultured cell
The present invention provides novel serum-free cell culture medium and methods for cultivating MDCK cells. In particular, non-tumorigenic MDCK cells. The present invention also provides methods for producing influenza viruses (e.g., particularly cold-adapted, and / or temperature sensitive, and / or attenuated influenza viruses) that eliminate the need for a cell culture medium exchange step. The novel medium and methods are useful to grow influenza viruses, in cell culture to high titer. The present invention further provides purification methods for purifying influenza viruses with high overall recovery of live virus and result in levels of host cell DNA (HCD), host cell protein (HCP) and non-specific endonuclease (e.g., Benzonase), which are below the specifications required by regulatory agencies. The immunogenic compositions can be used to actively immunize subjects or to generate antibodies for a variety of uses, including passive immunization and diagnostic immunoassays.
Owner:MEDIMMUNE LLC
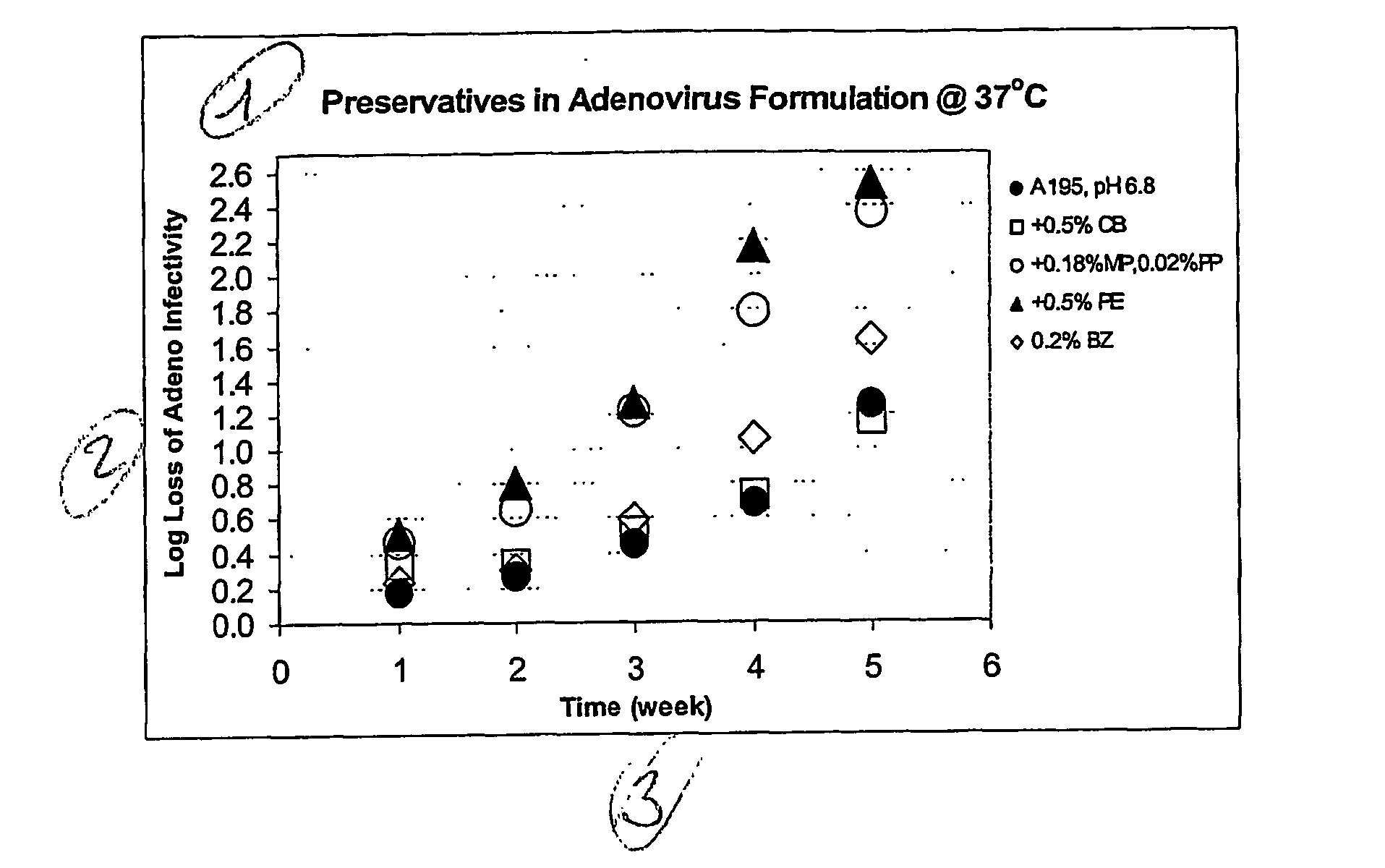
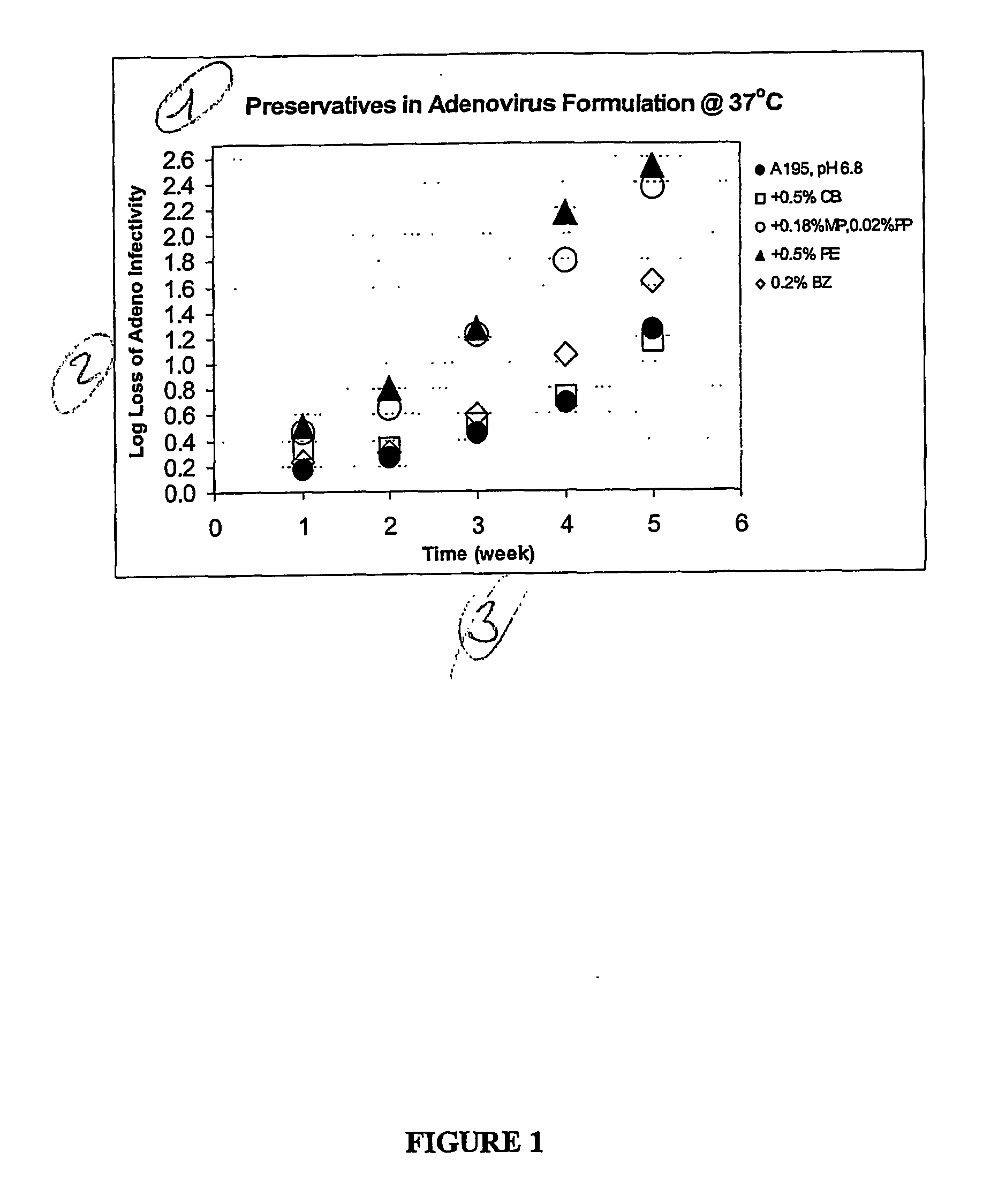
Preservative-containing virus formulations
InactiveUS20070148765A1Negligible lossHigh antibacterial activityMicrobiological testing/measurementPharmaceutical delivery mechanismViral VaccineGene
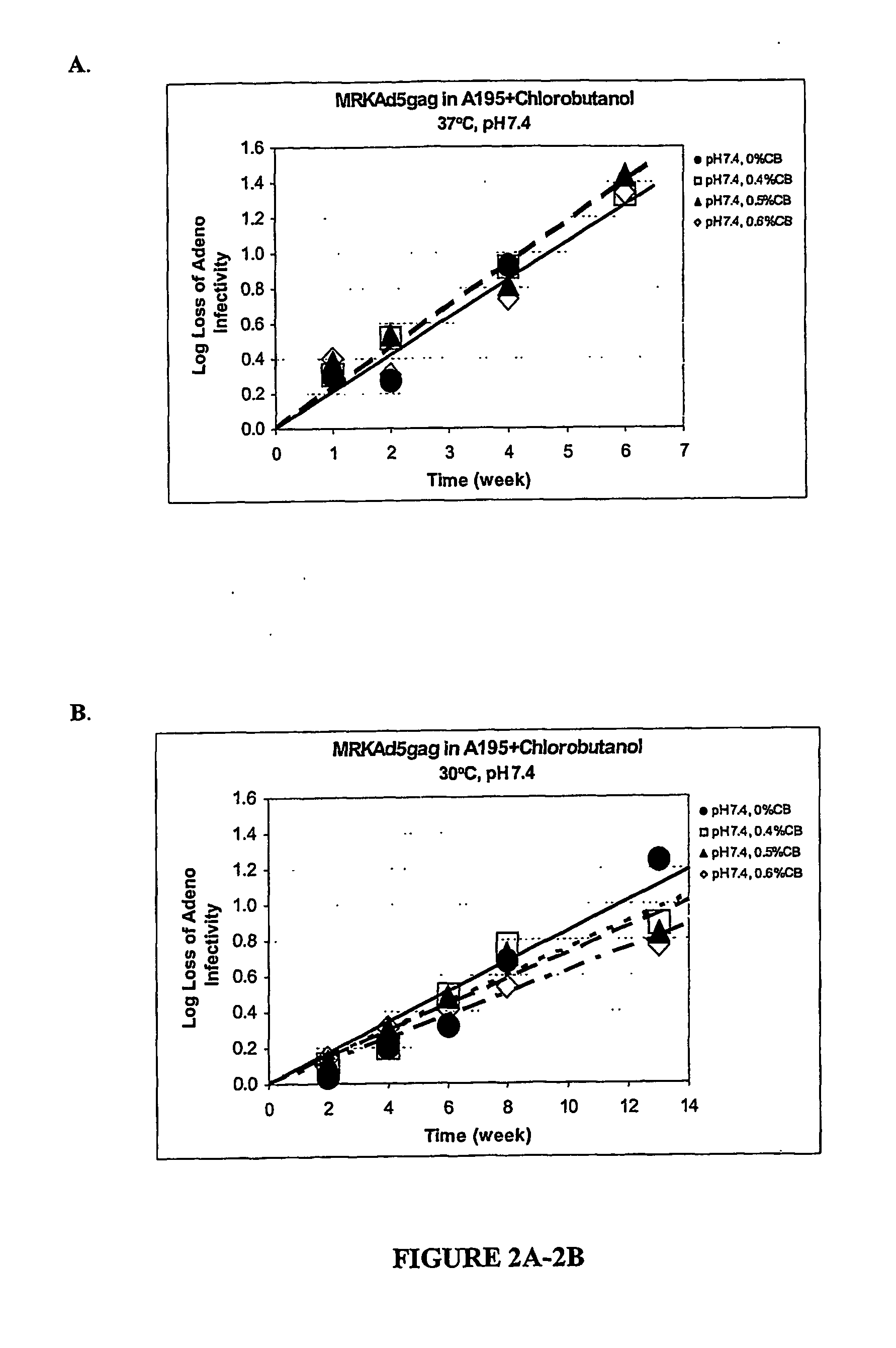
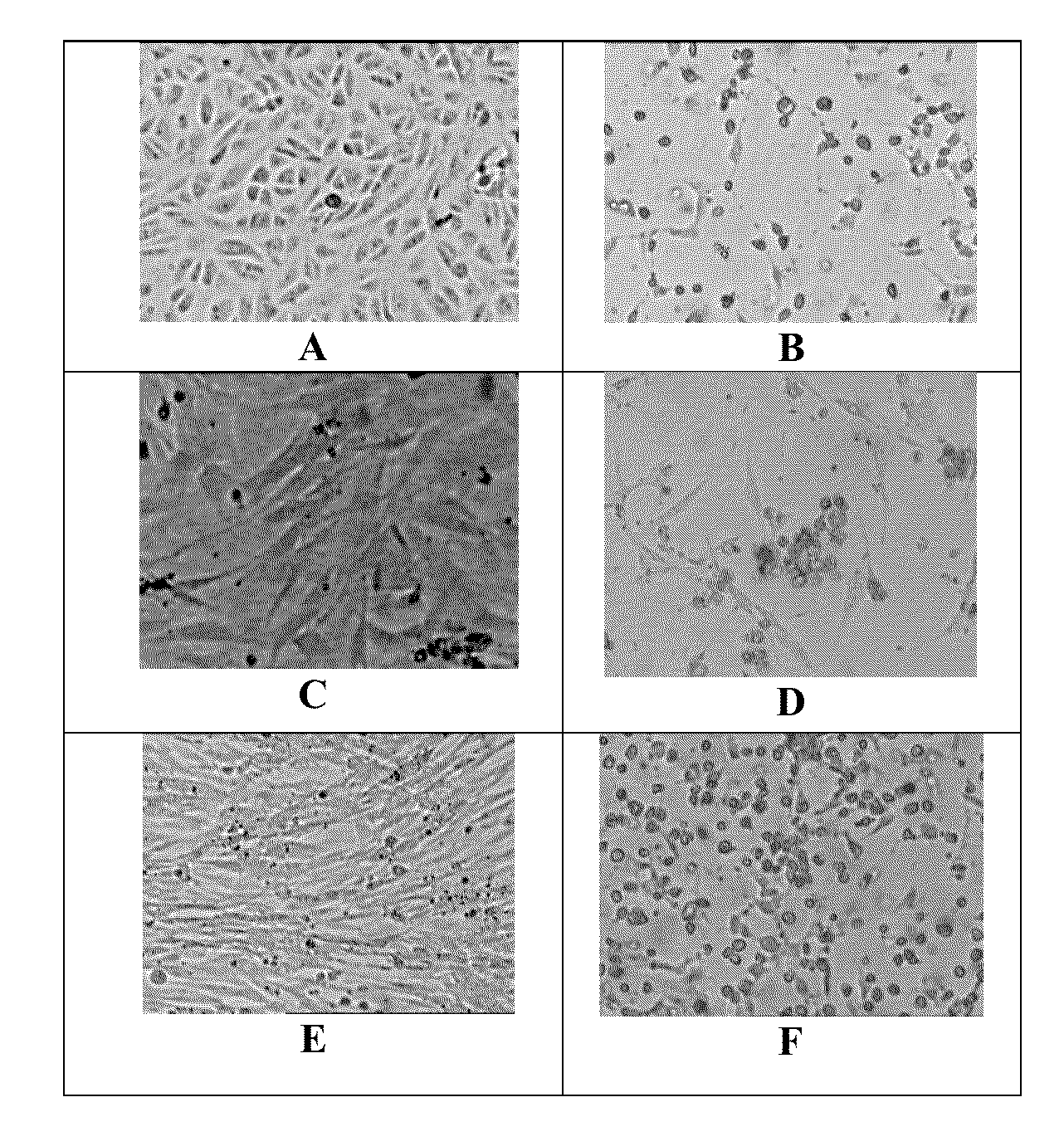
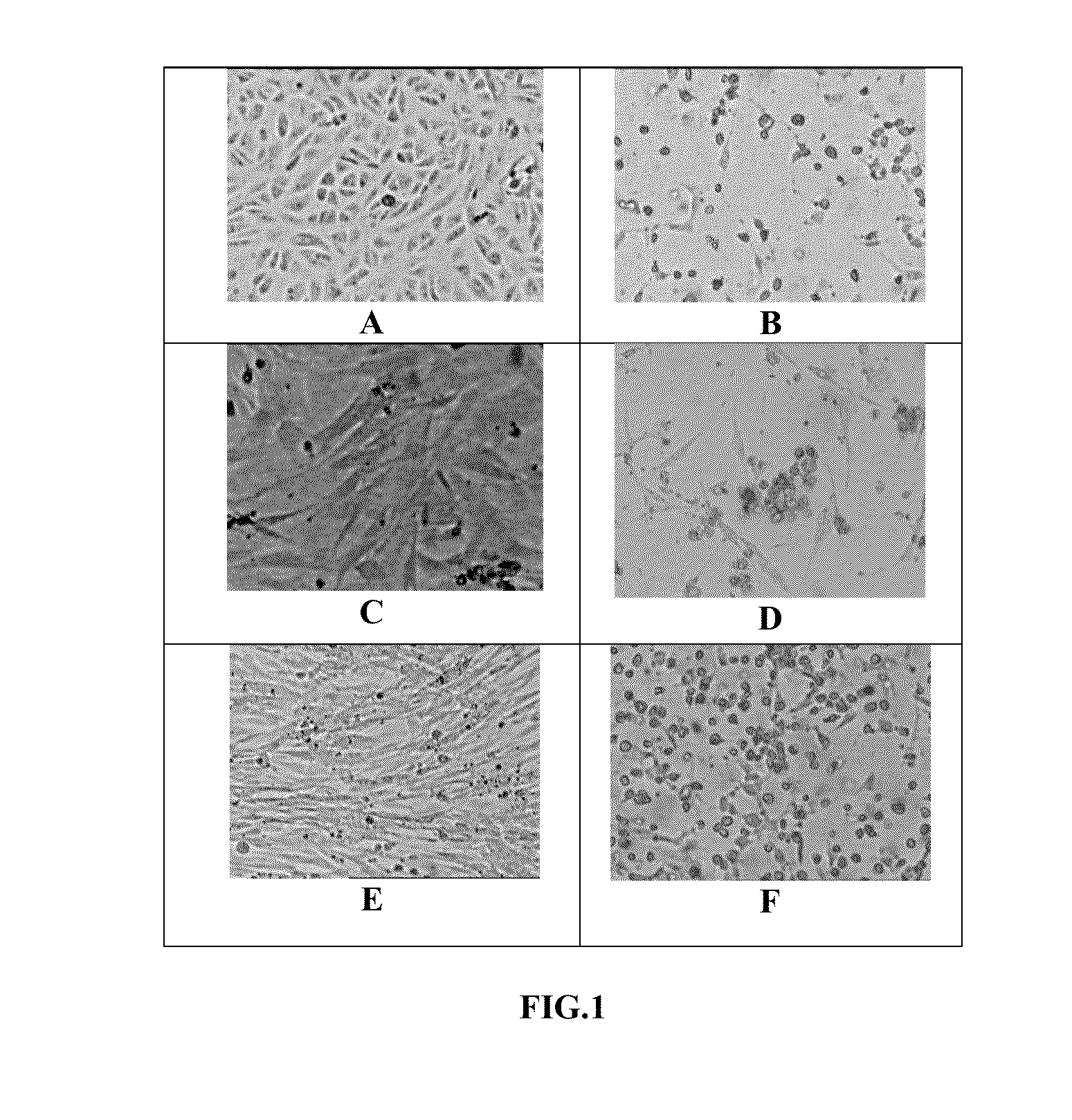
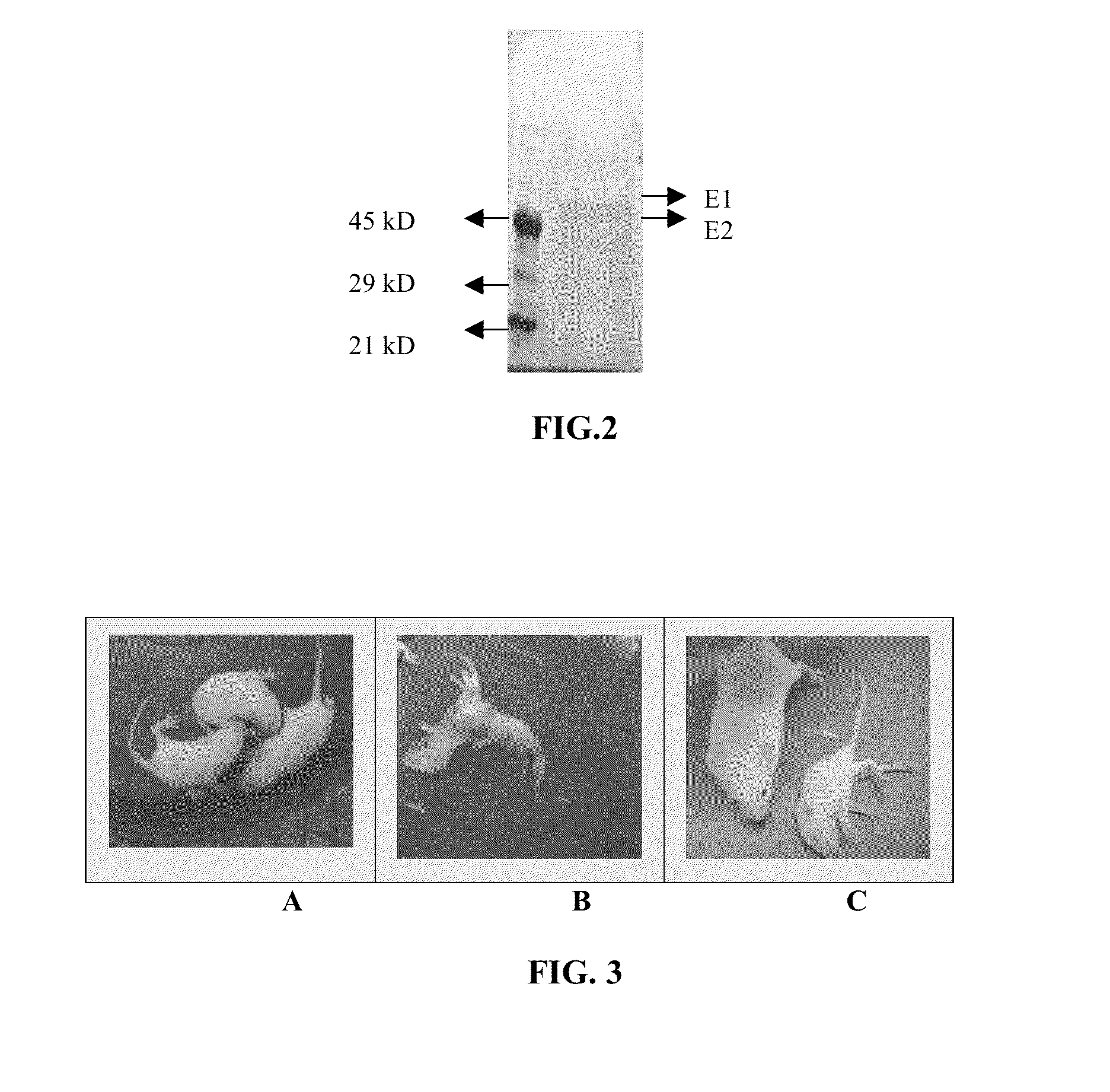
The preservation of live viral vaccines is disclosed. These liquid formulations comprise a live virus and a preservative, namely chlorobutanol. The preserved, live virus formulations of the present invention are (1) suitable for a vaccine or gene therapy product with a multi-dose image; (2) compatible with parenteral administration; and (3) are stable for extended periods of time with negligible loss of activity.
Owner:MERCK SHARP & DOHME CORP
Vaccine for chikungunya virus infection
ActiveUS20130022631A1Easy to be validatedSsRNA viruses positive-sensePeptide/protein ingredientsProtection sexIn vivo
The present invention relates to vaccine formulation capable of eliciting protective immune response against Chikun-gunya virus infection in humans and other mammalian hosts. The immunogenic formulation comprises purified inactivated Chikun-gunya virus in a stable formulation. Methods of propagation and purification of the virus are discussed. The inactivated virus formulation is non-infectious, immunogenic and elicits protective immune response in mammalian host. The immunogenic composition is formulated for in vivo administration to humans. The invention also discusses the strategy of developing a subunit vaccine using the recombinant viral proteins as antigens for immunization. The recombinant virus antigens that are potentially immunogenic can be used in diagnosing for the presence of the virus.
Owner:BHARAT BIOTECH INTERNATIONAL
Vaccine composition and preparation method and application thereof
The invention provides a method for preparing a vaccine composition. The method comprises the following steps: (1) preparing a liquid containing a porcine pseudorabies virus; (2) adding a nonionic surfactant or a solution containing the nonionic surfactant into the liquid in the step (1) and solubilizing envelope proteins in the liquid containing the porcine pseudorabies virus; (3) removing the nonionic surfactant in the step (2) to obtain a purified porcine pseudorabies virus antigen; and (4) preparing the vaccine composition by using the antigen prepared in the step (3). The invention further provides the vaccine composition containing porcine pseudorabies antigen prepared by the preparation method. Immunosuppression components are removed from the vaccine composition and meanwhile, immunity active ingredients are retained to the maximum extent. The vaccine composition has a relatively good protective effect on porcine lung diseases; when the vaccine composition and a live virus antigen of porcine reproductive and respiratory syndrome are jointly acted, the vaccine composition has no inhibiting effect to the live virus antigen, showing that the vaccine composition can be jointly used with various other antigen components.
Owner:PU LIKE BIO ENG
Infectious bursal virus and method for propagating bursal virus with chicken embryo cell line and bioreactor to prepare inactivated vaccine and combined vaccine
InactiveCN102260649AImprove adaptabilityImproving immunogenicityViral antigen ingredientsMicroorganism based processesTGE VACCINEEmbryo cell
The invention relates to an infectious bursal disease virus (IBDV) HQ strain CGMCC NO.4935 and a preparation method of inactivated vaccines and combined vaccines for infectious bursal disease (IBD). The preparation method mainly comprises the following steps of: (1) carrying out chain amplification on cell seeds; (2) adding cell growth-promoting liquid into a sterilized bioreactor, and inoculating cells for preparing vaccines for suspension culture; (3) replacing maintenance liquid containing an IBDV strain when cells are of the maximum density, and continuing to culture; (4) collecting viruses in time, and measuring virus titer; and (5) inactivating virus liquid, and preparing inactivated vaccines and combined vaccines thereof for the IBD according to different proportions. The method provided by the invention increases the cell density, improves the virus titer, improves the vaccine titer, reduces the side reaction, reduces the labor intensity, lowers the production cost, improves the controllability of production processes, and ensures the uniformity and stability of product quality. The produced inactivated vaccines and combined vaccines thereof for the IBD have the advantagesof good safety and high immune efficiency and have a complete protective effect on the IBDV attack.
Owner:POULTRY DISEASE RES INST OF HENAN AGRI UNIV
Canine distemper live vaccine and preparation method thereof
ActiveCN101612396AReduced risk of introducing other live virusesAntiviralsAntibody medical ingredientsDiseaseCanine distemper virus CDV
The invention relates to canine distemper live vaccine and a preparation method thereof. The preparation method takes a canine distemper virus natural attenuated strain CGMCC No.3201 with excellent immunogenicity and obtained by the inventor through field separation as a production strain to prepare the safe, effective and single-component canine distemper live vaccine. The canine distemper live vaccine effectively reduces the risk of importing other live viruses during canine distemper vaccine immunization and provides conditions for controlling the spread of fur-bearing animal diseases.
Owner:QILU ANIMAL HEALTH PROD
Heat-resisting protective agent and application thereof
ActiveCN103041399AReduced vital activityImprove heat resistancePowder deliveryViral antigen ingredientsBiotechnologyMicroorganism preservation
The invention provides a heat-resisting protective agent and an application thereof, and relates to the field of live microorganism deposit. The heat-resisting protective agent comprises the following substances: 40-80% of oligosaccharide, 3-12% of amino acid, 3-12% of gelatin, 3-12% of casein hydrolysate, 3-12% of polyvinyl pyrrolidone, 3-12% of glycerol, and 3-12% of poloxamer-68. The invention further provides the application of the heat-resisting protective agent in preparation of live vaccine freeze-dried powder. The heat-resisting protective agent is dissolved with a solvent; a protective agent solution is obtained; the protective agent solution is mixed with live virus liquid; a live vaccine is obtained; the live vaccine is freeze-dried; and then the live vaccine freeze-dried powder is obtained. The heat-resisting protective agent can improve heat resistance of the live vaccine, and the live vaccine freeze-dried powder prepared by the heat-resisting protective agent is stored for 2 months at 37 DEG C, and stored for 24 months at 15 DEG C.
Owner:南京国创生物技术研究院有限公司
Method for the development of an HIV vaccine
InactiveUS20030104011A1Reduce riskSeriousness of riskAnimal cellsViral antigen ingredientsReverse transcriptaseHIV vaccine
Owner:PHOTOIMMUNE BIOTECH
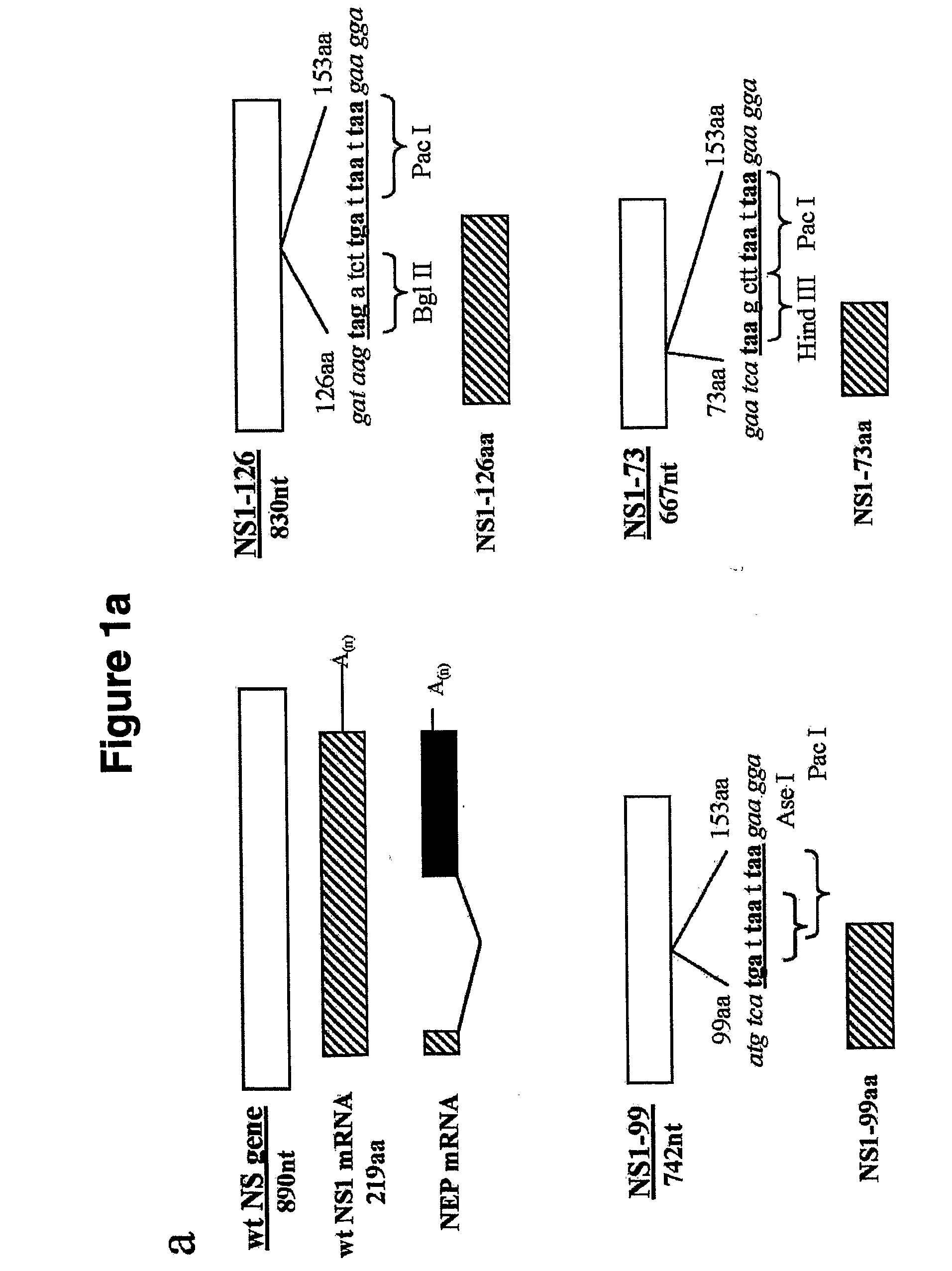
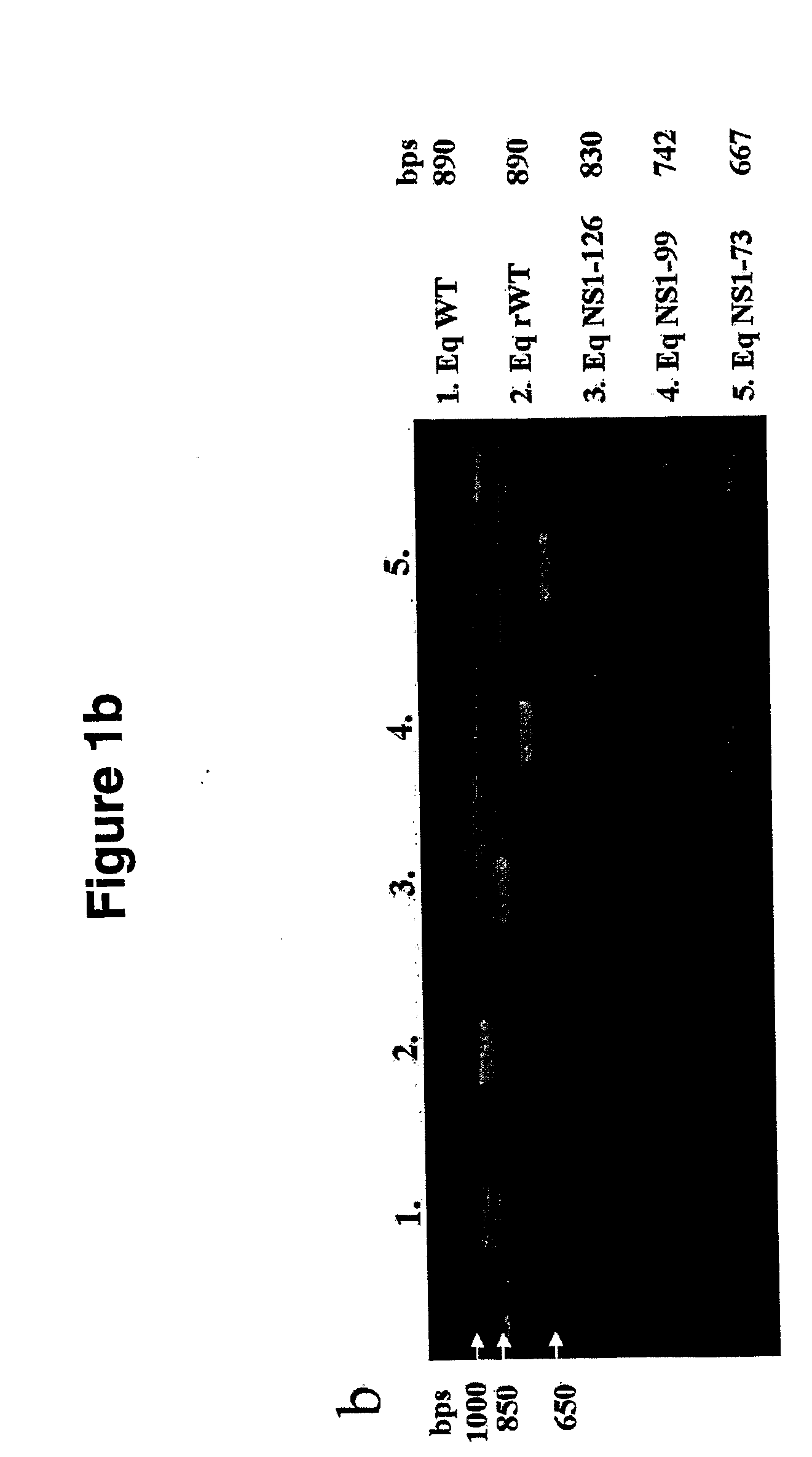
Genetically Engineered Equine Influenza Virus and Uses Thereof
ActiveUS20080254060A1Reduce capacityLow toxicitySsRNA viruses negative-senseVectorsUltrasound attenuationVirulent characteristics
The present invention relates, in general, to attenuated equine influenza viruses having an impaired ability to antagonize the cellular interferon (IFN) response, and the use of such attenuated viruses in vaccine and pharmaceutical formulations. In particular, the invention relates to attenuated equine influenza viruses having modifications to an equine NS1 gene that diminish or eliminate the ability of the NS1 gene product to antagonize the cellular IFN response. These viruses replicate in vivo, but demonstrate decreased replication, virulence and increased attenuation, and therefore are well suited for use in live virus vaccines, and pharmaceutical formulations.
Owner:UNIVERSITY OF KENTUCKY +1
Genetically engineered swine influenza virus and uses thereof
ActiveUS8124101B2Reduce capacityLow toxicitySsRNA viruses negative-senseViral antigen ingredientsUltrasound attenuationGene product
The present invention relates, in general, to attenuated swine influenza viruses having an impaired ability to antagonize the cellular interferon (IFN) response, and the use of such attenuated viruses in vaccine and pharmaceutical formulations. In particular, the invention relates to attenuated swine influenza viruses having modifications to a swine NS1 gene that diminish or eliminate the ability of the NS1 gene product to antagonize the cellular IFN response. These viruses replicate in vivo, but demonstrate decreased replication, virulence and increased attenuation, and therefore are well suited for use in live virus vaccines, and pharmaceutical formulations.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC +2
Methods and compositions for live attenuated viruses
ActiveUS8084039B2Improve stabilitySsRNA viruses negative-senseSsRNA viruses positive-senseAttenuated vaccineBiology
Embodiments herein relate to compositions of and methods for live viruses. In certain embodiments, a live, attenuated virus composition includes, but is not limited to, one or more live, attenuated viruses and compositions to reduce inactivation and / or degradation of the live, attenuated virus. In other embodiments, the live, attenuated virus composition may be a vaccine composition. In yet other compositions, a live, attenuated virus composition may include at least one carbohydrate, at least one protein and at least one high molecular weight surfactants for reducing inactivation and / or degradation of the live, attenuated virus.
Owner:TAKEDA VACCINES INC
Vaccine composition, preparation method and application thereof
The invention provides a vaccine composition, which comprises influenza virus-like particles and new castle disease virus-like particles. According to the invention, bird flu virus-like particles and new castle disease virus-like particles are prepared to obtain the vaccine composition, compared with chick embryo-produced vaccine compositions, The production of the vaccine composition can employ a large-volume bioreactor technology and large scale culture of insect cells to prepare antigen, when output is ensured, product quality stability and homogeneity are guaranteed, no live virus is related during the production process, and no hidden trouble on biological safety aspect is generated.
Owner:PU LIKE BIO ENG
Formulation for room temperature stabilization of a live attenuated bacterial vaccine
InactiveUS20110064723A1Lower ratioGreat proportionSsRNA viruses negative-senseBacterial antigen ingredientsBacteroidesCavitation
This invention provides methods and compositions for stabilizing proteins and vaccines in dried formulations. In particular, a cavitation method and compositions of preparing a dried vaccine are provided that stabilize the viability of live bacteria and live virus vaccines at room temperature.
Owner:ARIDIS PHARMA INC
Canine influenza virus and related compositions and methods of use
ActiveUS20070098742A1SsRNA viruses negative-sensePeptide/protein ingredientsImmunity responseHypotype
The present invention provides an isolated canine influenza virus of subtype H3N8 comprising an HA having SEQ ID NO: 4 or an amino acid sequence that is greater than 99% identical to SEQ ID NO: 4, with the proviso that the amino acids at positions 94 and 233 are identical to SEQ ID NO: 4; a composition comprising attenuated or inactivated virus; isolated or purified HA, NM, NP, M1, NS1, PA, PB1, and PB2 proteins and fragments thereof and compositions comprising same or nucleic acids, optionally as part of a vector, encoding same; and a method of inducing an immune response to canine influenza virus in an animal comprising administering to the animal an aforementioned composition.
Owner:IOWA STATE UNIV RES FOUND
Pathogenic microorganism waste liquor treatment system
InactiveCN101691247ATimely processingThe overall collection and processing of the processing station is timelyWater/sewage treatment by heatingLiquid wastePathogenic microorganism
The invention discloses a pathogenic microorganism waste liquor treatment system, which comprises a waste liquor collecting pipeline system (1), a waste liquor sterilizing tank (2) and a sewage treatment station (3). The invention adopts a technical scheme that waste liquor is integrally collected and treated through the waste liquor collecting pipeline system, the high-temperature steam bath waste liquor sterilizing tank and the sewage treatment station special for the waste liquor so as to overcome the defects that the liquor collecting vehicle and liquor collecting bucket mode in the prior art has low waste liquor treatment capacity, occupation of on-site space of a clean area for transit storage, indefinite sterilization effect and easy chemical pollution to environment. The pathogenic microorganism waste liquor treatment system provided by the invention directly conveys the waste liquor generated by various viable bacteria and live virus areas of buildings to the waste liquor sterilizing tank for high-temperature steam sterilization, and then conveys the waste liquor to the common sewage treatment station for physicochemical treatment through a pipeline so as to achieve the aims of timely, massively, highly efficiently and reliably treating the pathogenic microorganism waste liquor with low cost, and reducing the chemical pollution.
Owner:NINGBO RONGAN BIOLOGICAL PHARMA
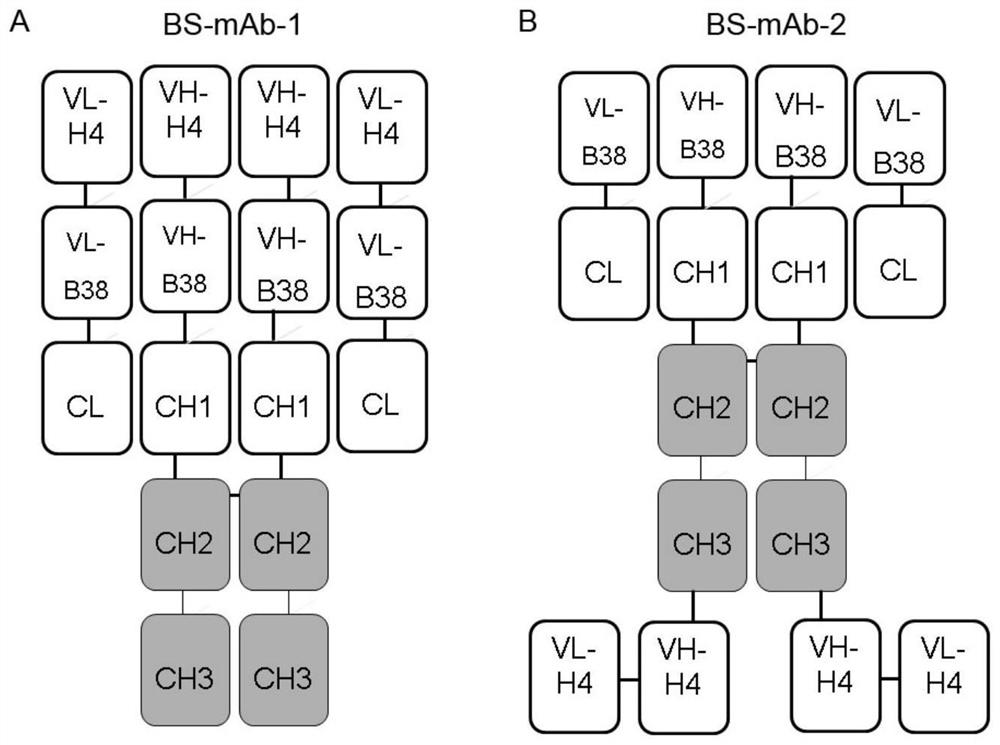
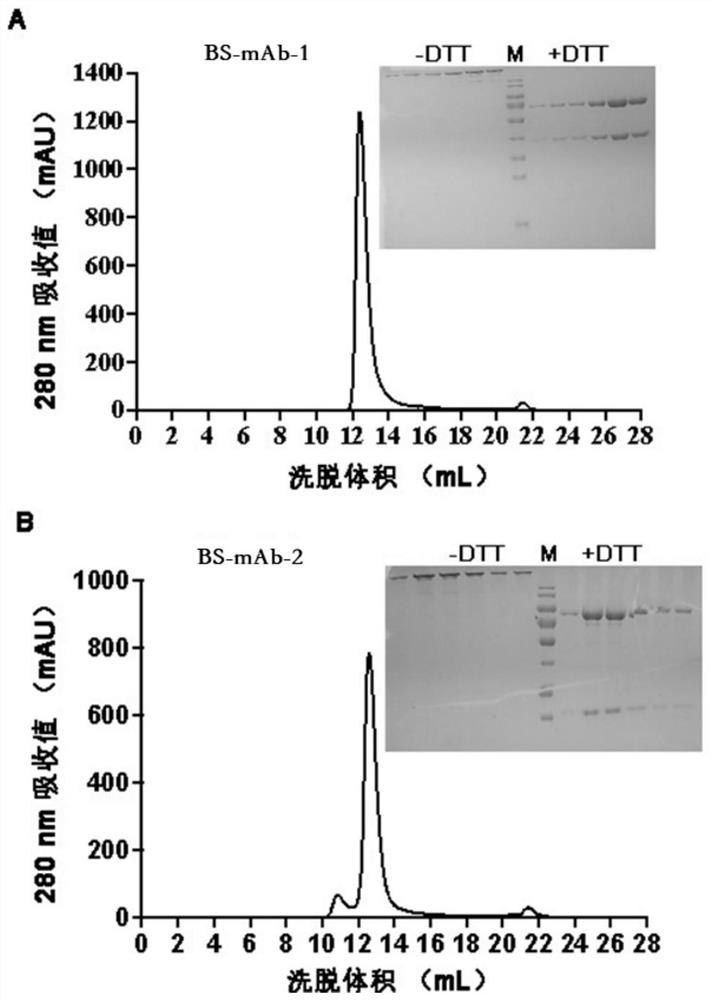
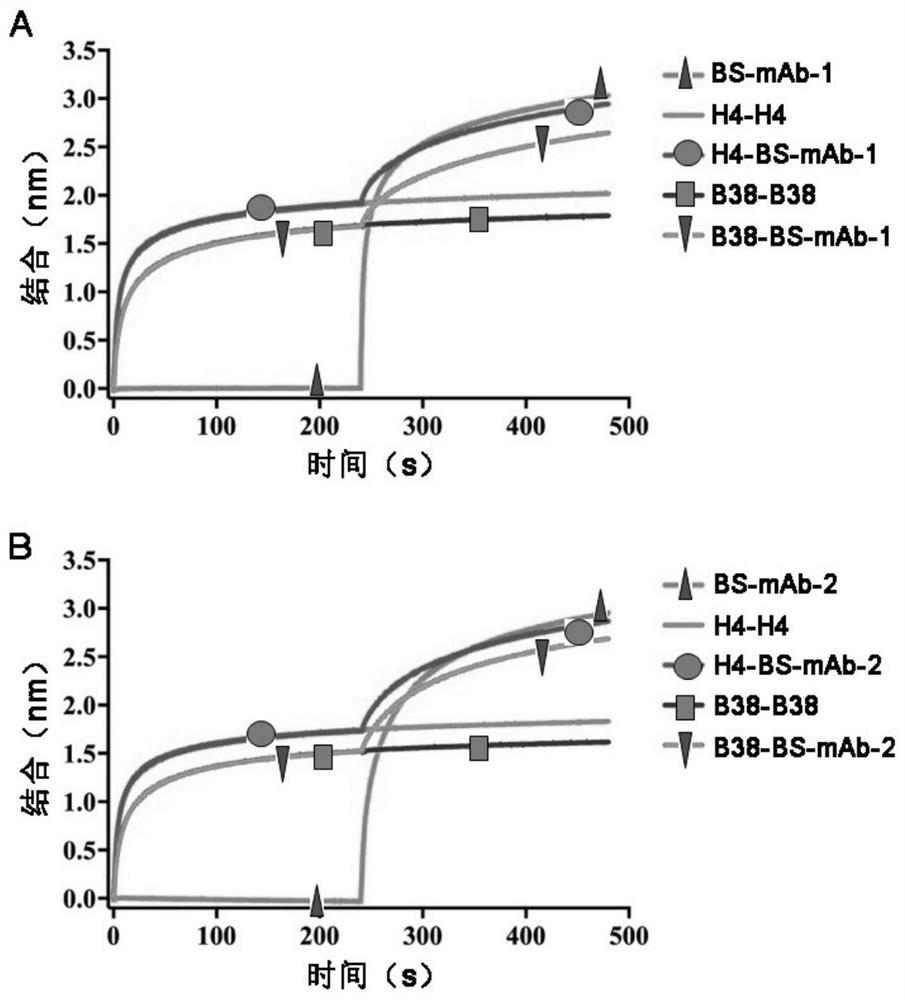
Bispecific antibody for resisting novel coronavirus and application of bispecific antibody
ActiveCN113563475AHigh neutralizing activityStrong inhibitory activityHybrid immunoglobulinsImmunoglobulins against virusesBispecific antibodyPharmaceutical drug
The invention relates to a bispecific antibody for resisting novel coronavirus and application of the bispecific antibody. The bispecific antibody is obtained by modifying new coronavirus monoclonal antibodies H4 and B38 through a genetic engineering method, different sites of novel coronavirus S protein RBD can be recognized at the same time, the neutralizing activity on new coronavirus pseudovirus is far higher than that of a maternal monoclonal antibody, and the inhibitory activity on new coronavirus live virus is further higher than that of the maternal monoclonal antibody. According to the bispecific antibody for resisting novel coronavirus, the selectivity and neutralizing activity of the maternal monoclonal antibody are improved, and the safety and effectiveness of monoclonal antibody drugs are improved; and the bispecific antibody can be used for preparing potential drugs for diagnosing, preventing and treating diseases caused by novel coronavirus and is huge in market value and good in application prospect.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Novel prrs virus inducing type i interferon in susceptible cells
ActiveUS20130028931A1SsRNA viruses positive-senseMaterial analysis by observing effect on chemical indicatorStructural proteinAttenuated vaccine
The present invention relates to the field of attenuated live viruses useful as vaccine or medicament for preventing or treating Porcine Reproductive and Respiratory Syndrome (PRRS) in swine, and is based on the surprising finding of a PRRS virus which is able to induce the interferon type I response of a cell infected by said virus. In one embodiment, the PRRS virus according to the invention is a PRRS virus mutant comprising, in comparison with the genome of a wild type strain, a mutation in the gene encoding the non structural protein 1 (nsp1) of said virus.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Genetically engineered equine influenza virus and uses thereof
ActiveUS8137676B2Reduce capacityLow toxicitySsRNA viruses negative-senseVectorsVirus influenzaIn vivo
The present invention relates, in general, to attenuated equine influenza viruses having an impaired ability to antagonize the cellular interferon (IFN) response, and the use of such attenuated viruses in vaccine and pharmaceutical formulations. In particular, the invention relates to attenuated equine influenza viruses having modifications to an equine NS1 gene that diminish or eliminate the ability of the NS1 gene product to antagonize the cellular IFN response. These viruses replicate in vivo, but demonstrate decreased replication, virulence and increased attenuation, and therefore are well suited for use in live virus vaccines, and pharmaceutical formulations.
Owner:UNIVERSITY OF KENTUCKY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com