Patents
Literature
137 results about "Virus influenza" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Influenza, or flu, is a contagious respiratory infection caused by several flu viruses that infect the nose, throat and lungs. People infected with the seasonal flu virus feel miserable with fever, chills, muscle aches, coughing, congestion, headache and fatigue for a week or so.
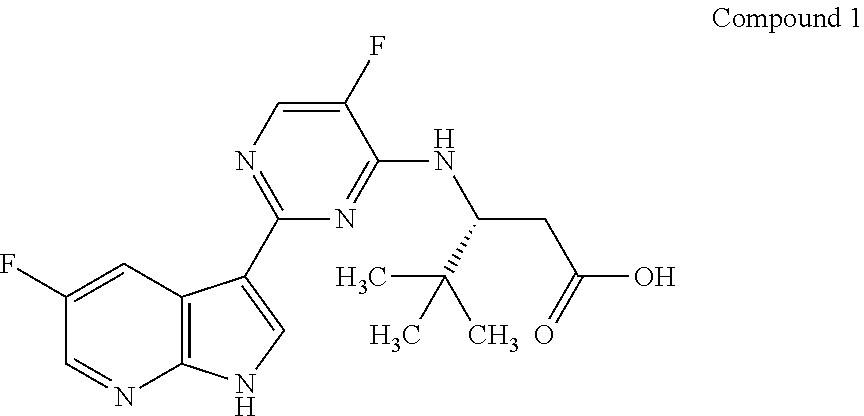
Inhibitors of influenza viruses replication
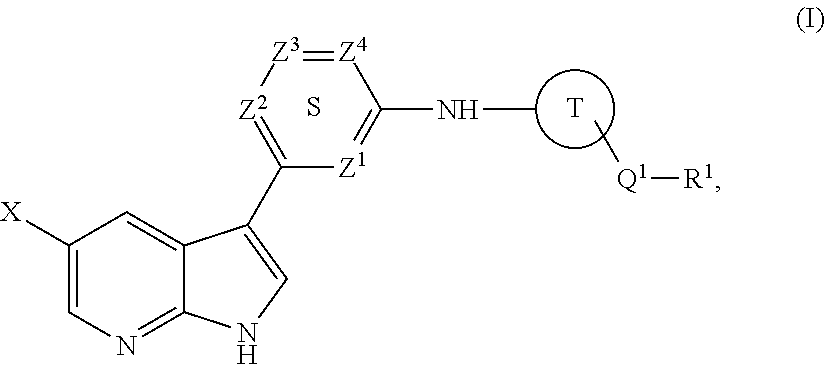
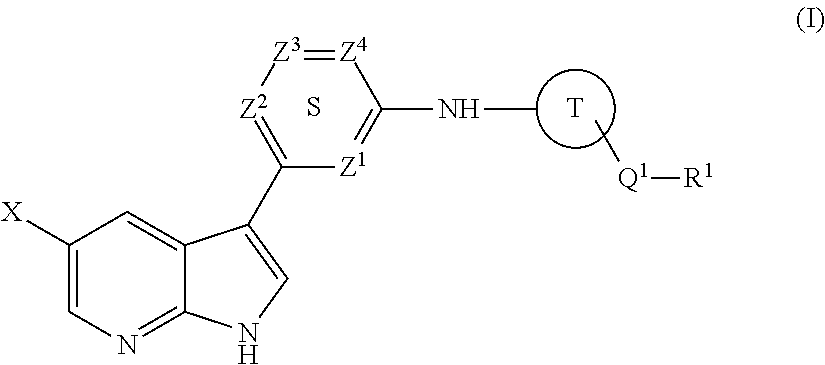
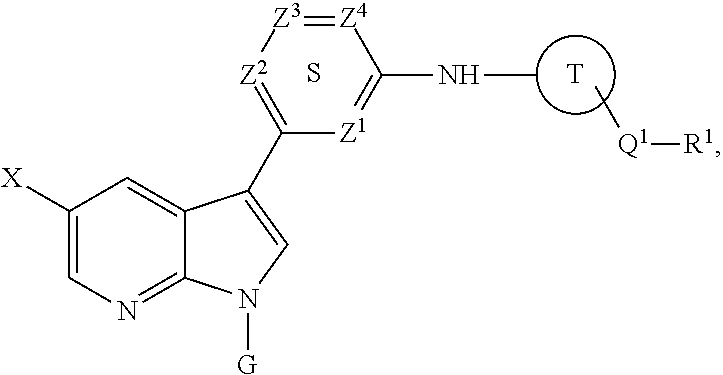
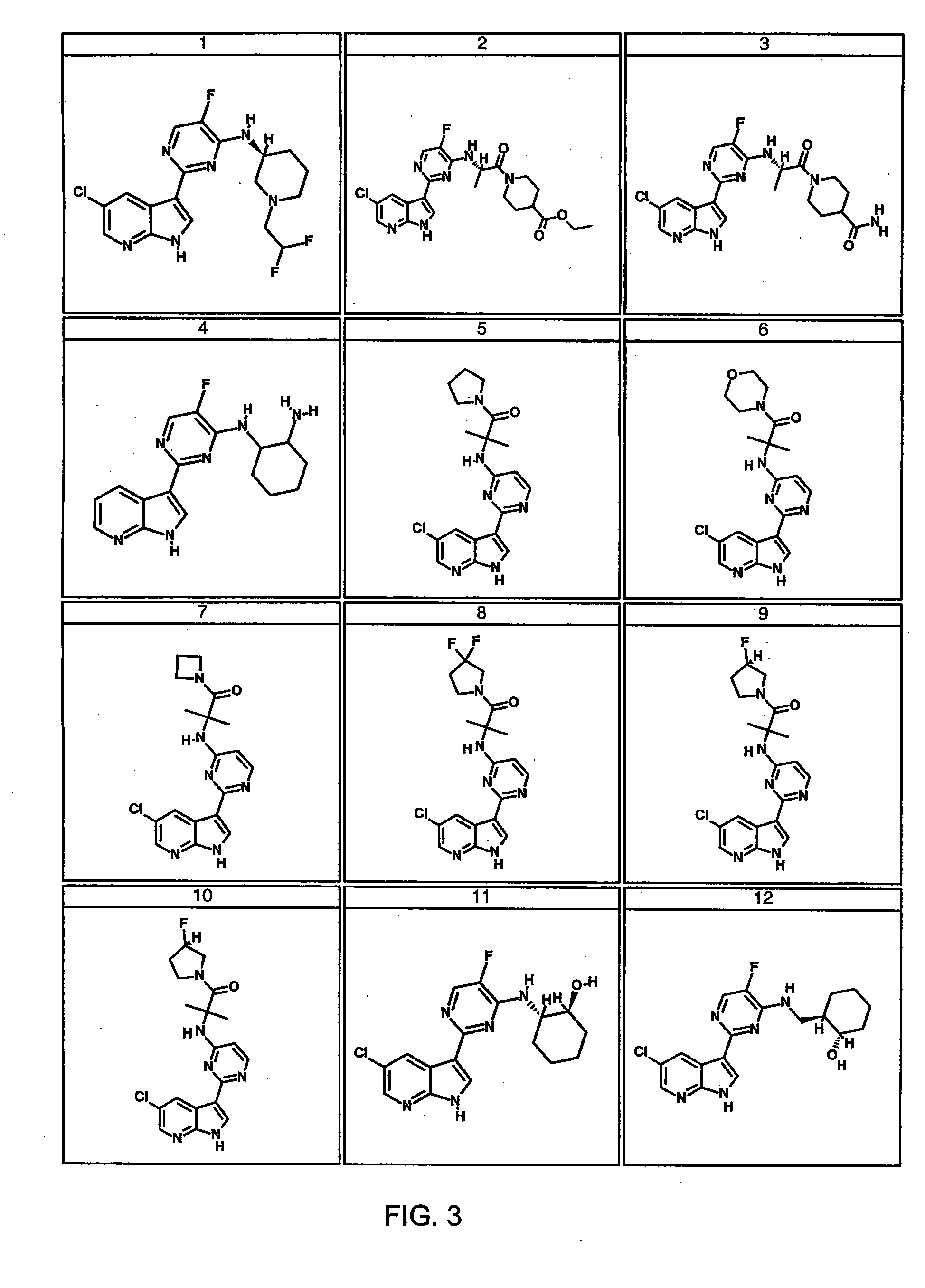
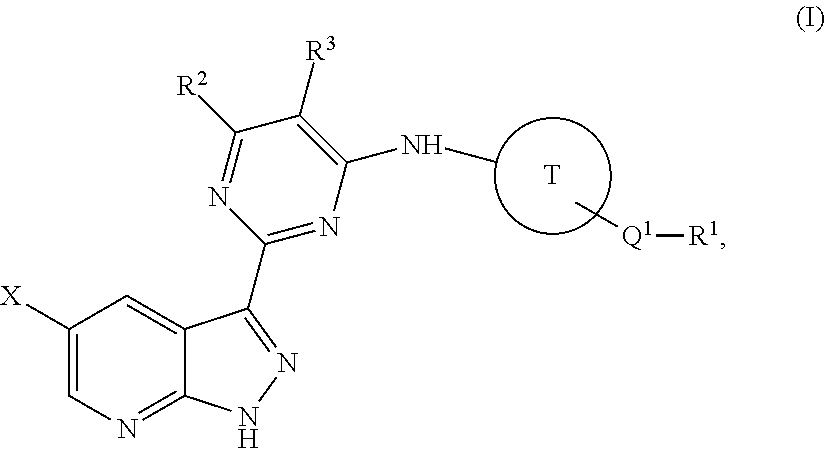
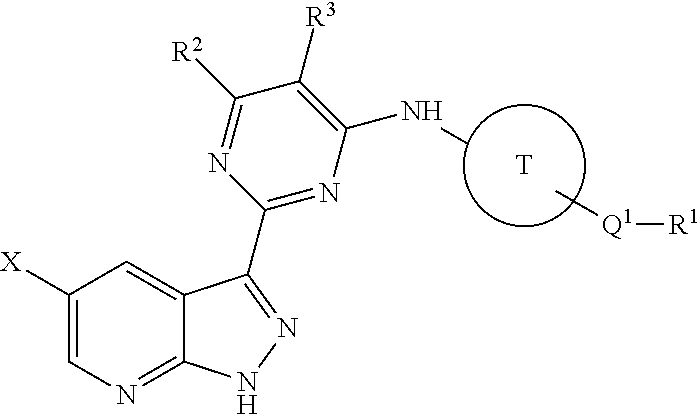
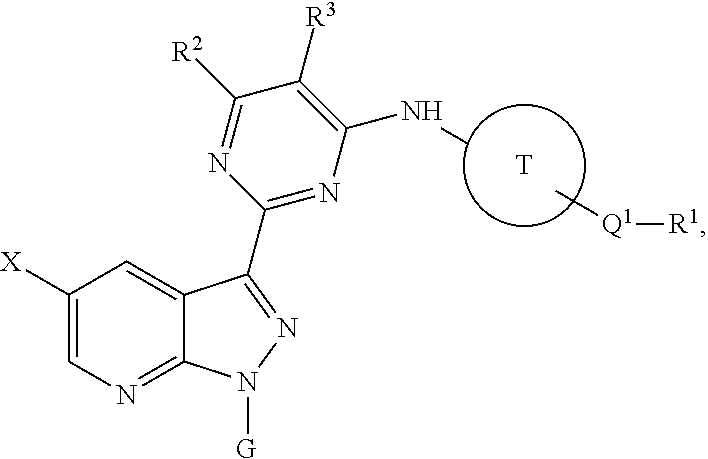
Methods of inhibiting the replication of influenza viruses in a biological sample or patient, of reducing the amount of influenza viruses in a biological sample or patient, and of treating influenza in a patient, comprises administering to said biological sample or patient an effective amount of a compound represented by Structural Formula (I):or a pharmaceutically acceptable salt thereof, wherein the values of Structural Formula (I) are as described herein. A compound is represented by Structural Formula (I) or a pharmaceutically acceptable salt thereof, wherein the values of Structural Formula (I) are as described herein. A pharmaceutical composition comprises an effective amount of such a compound or pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier, adjuvant or vehicle.
Owner:VERTEX PHARMA INC
Vaccines for the rapid response to pandemic avian influenza
InactiveUS20070003576A1Reduce riskImprove the level ofSsRNA viruses negative-senseViral antigen ingredientsAvian influenza virusVirus influenza
The present invention relates to adenovirus-based vaccines against avian influenza viruses with pandemic potential. The present invention provides replication-defective adenoviral vectors, each having a nucleic acid encoding an influenza A polypeptide. When introduced into a subject, the expressed influenza A polypeptide induces the production of antibodies that bind to influenza. The present invention also provides methods for inducing an immune response in a subject. Subjects are administered a replication-defective adenoviral vector, wherein the vector has a nucleic acid encoding an influenza A polypeptide. When the vector is expressed in the subject, the influenza A polypeptide induces the subject to produce antibodies to influenza.
Owner:UNIVERSITY OF PITTSBURGH
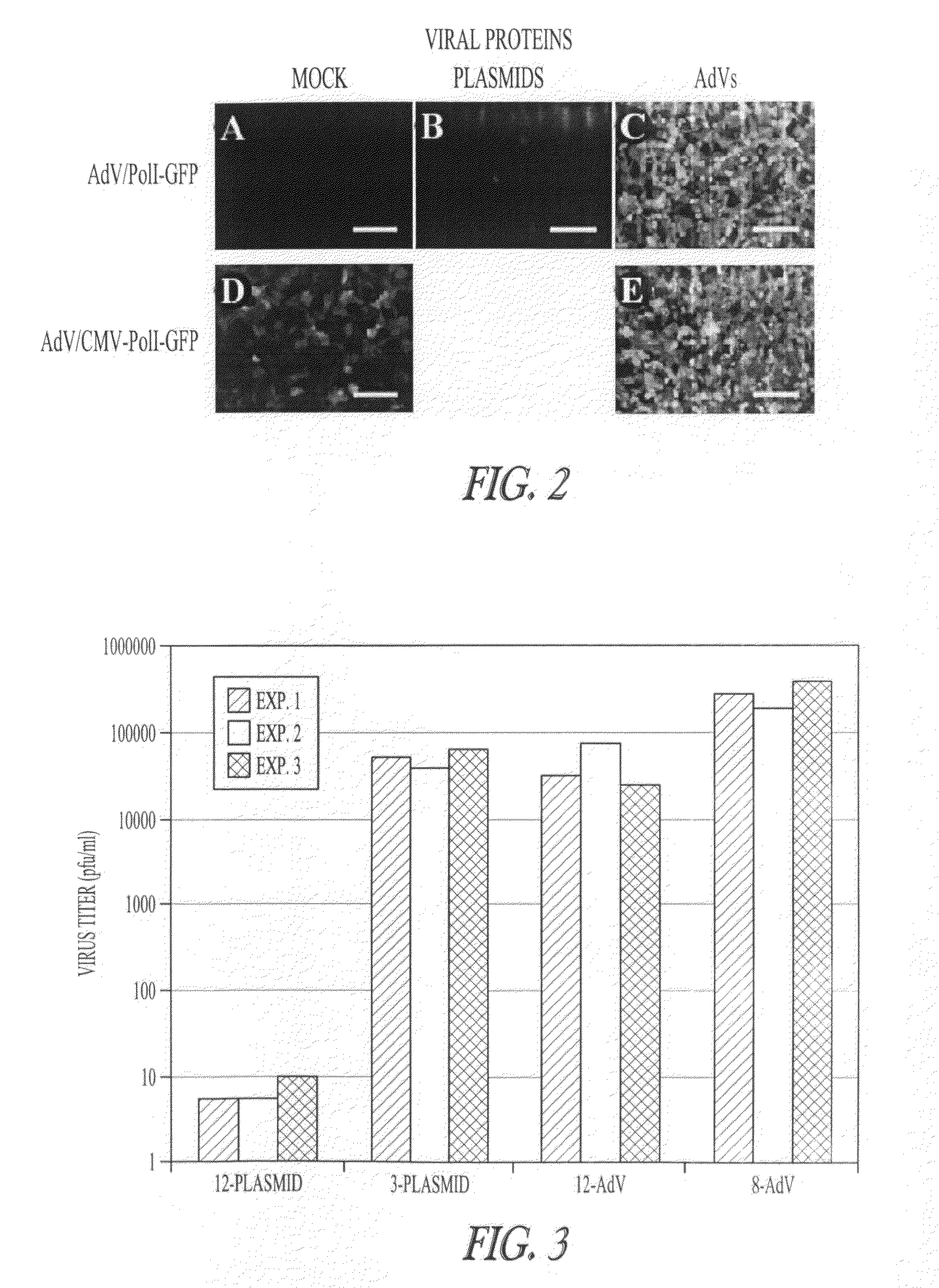
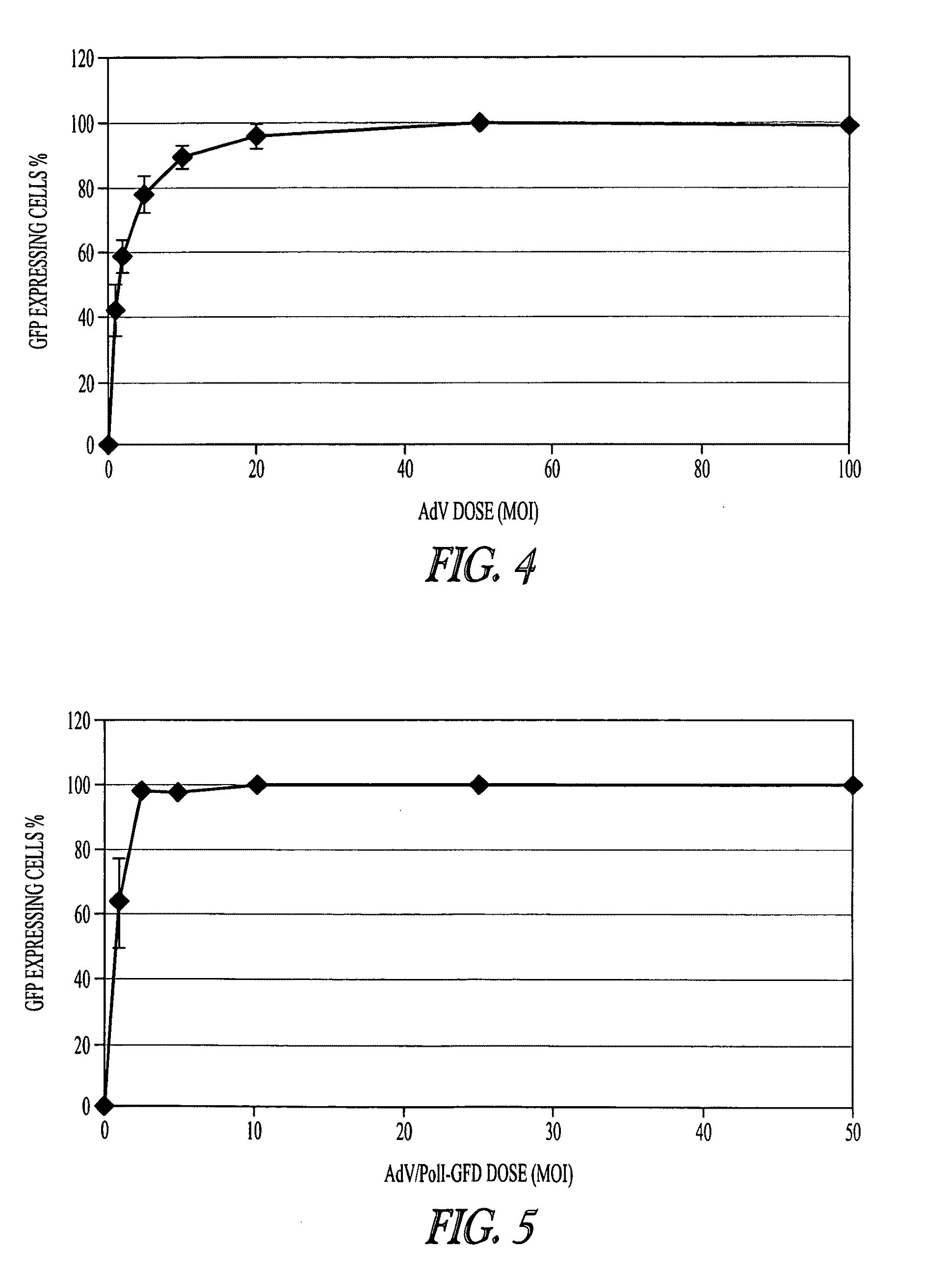
Multi plasmid system for the production of influenza virus
InactiveUS8354114B2Easy to copyEnhanced ability to replicateSsRNA viruses negative-sensePeptide/protein ingredientsEmbryonated chicken eggVirus influenza
Vectors and methods for the production of influenza viruses suitable as recombinant influenza vaccines in cell culture are provided. Bi-directional expression vectors for use in a multi-plasmid influenza virus expression system are provided. Additionally, the invention provides methods of producing influenza viruses with enhanced ability to replicate in embryonated chicken eggs and / or cells (e.g., Vero and / or MDCK) and further provides influenza viruses with enhanced replication characteristics.
Owner:MEDIMMUNE LLC
Novel vaccines against multiple subtypes of influenza virus
ActiveUS20090169505A1Elicit immune responseSsRNA viruses negative-sensePeptide/protein ingredientsHemagglutininMammal
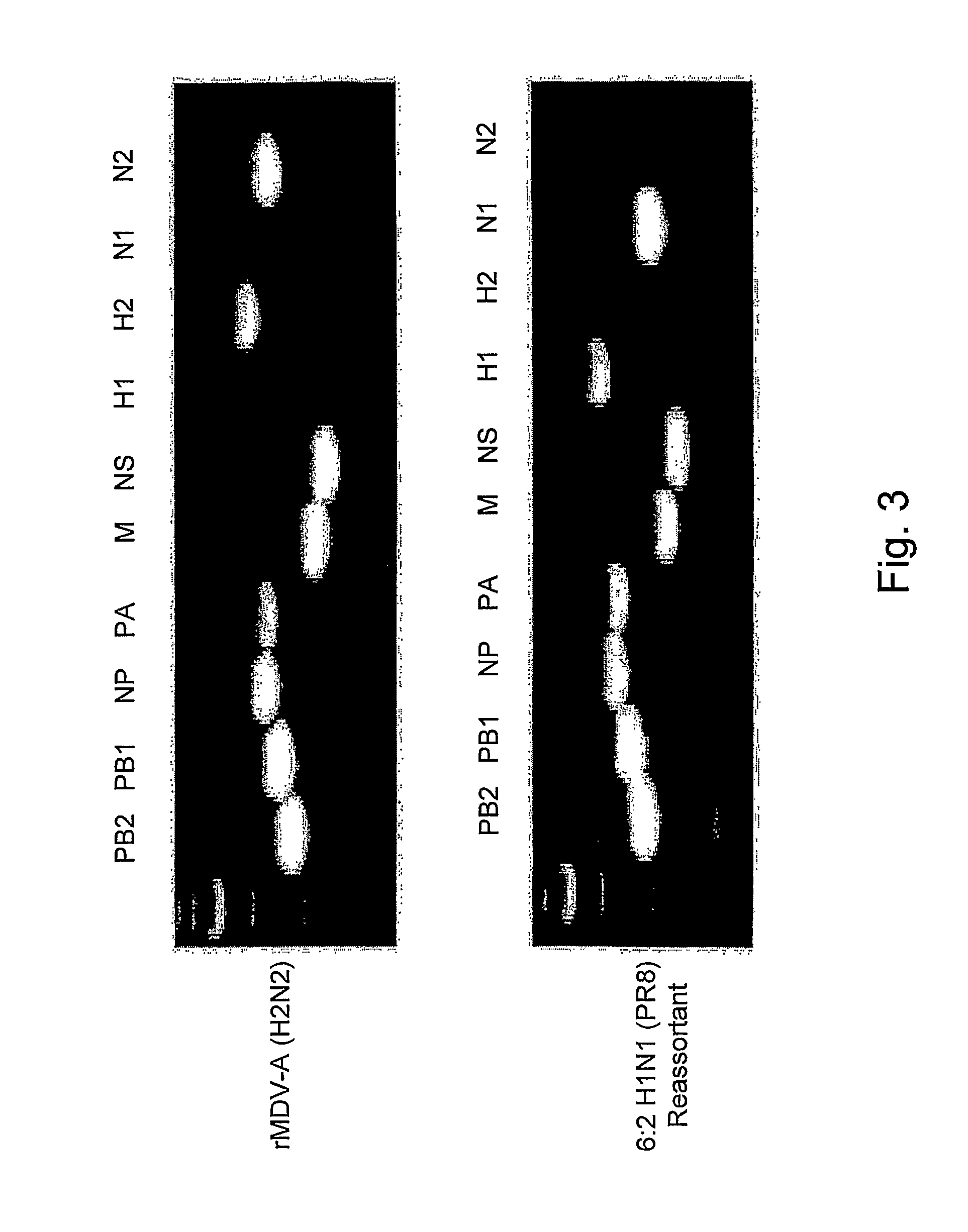
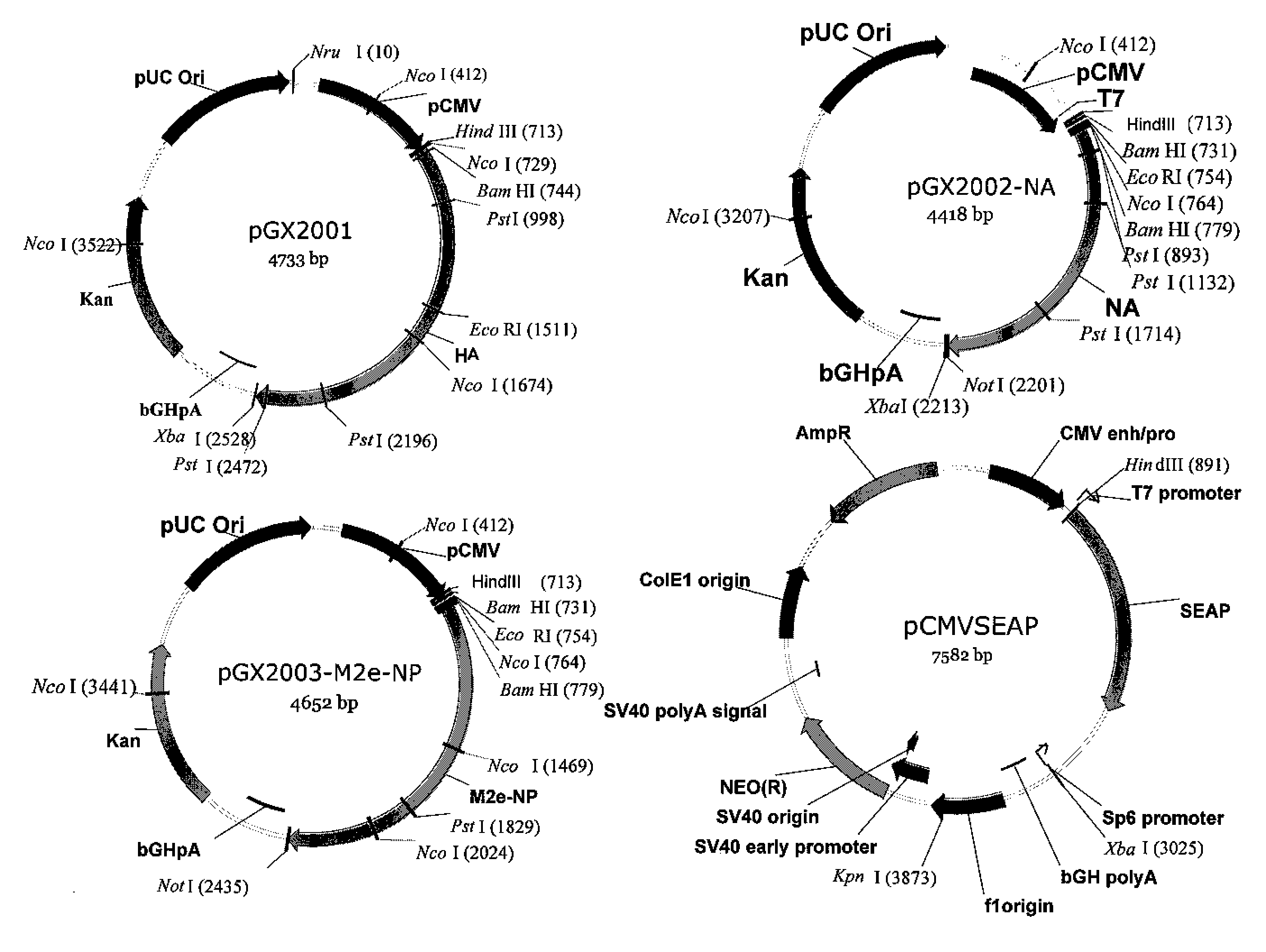
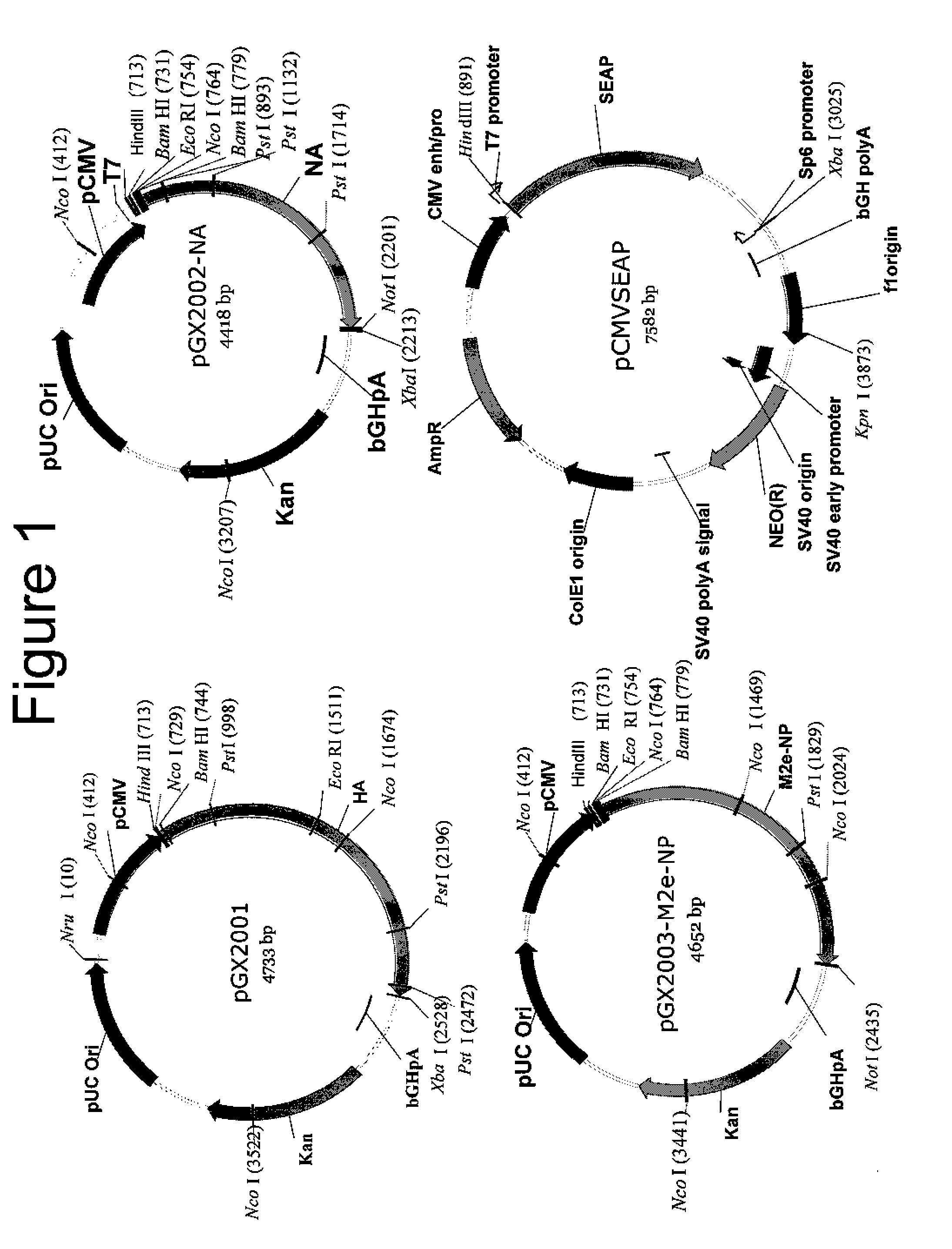
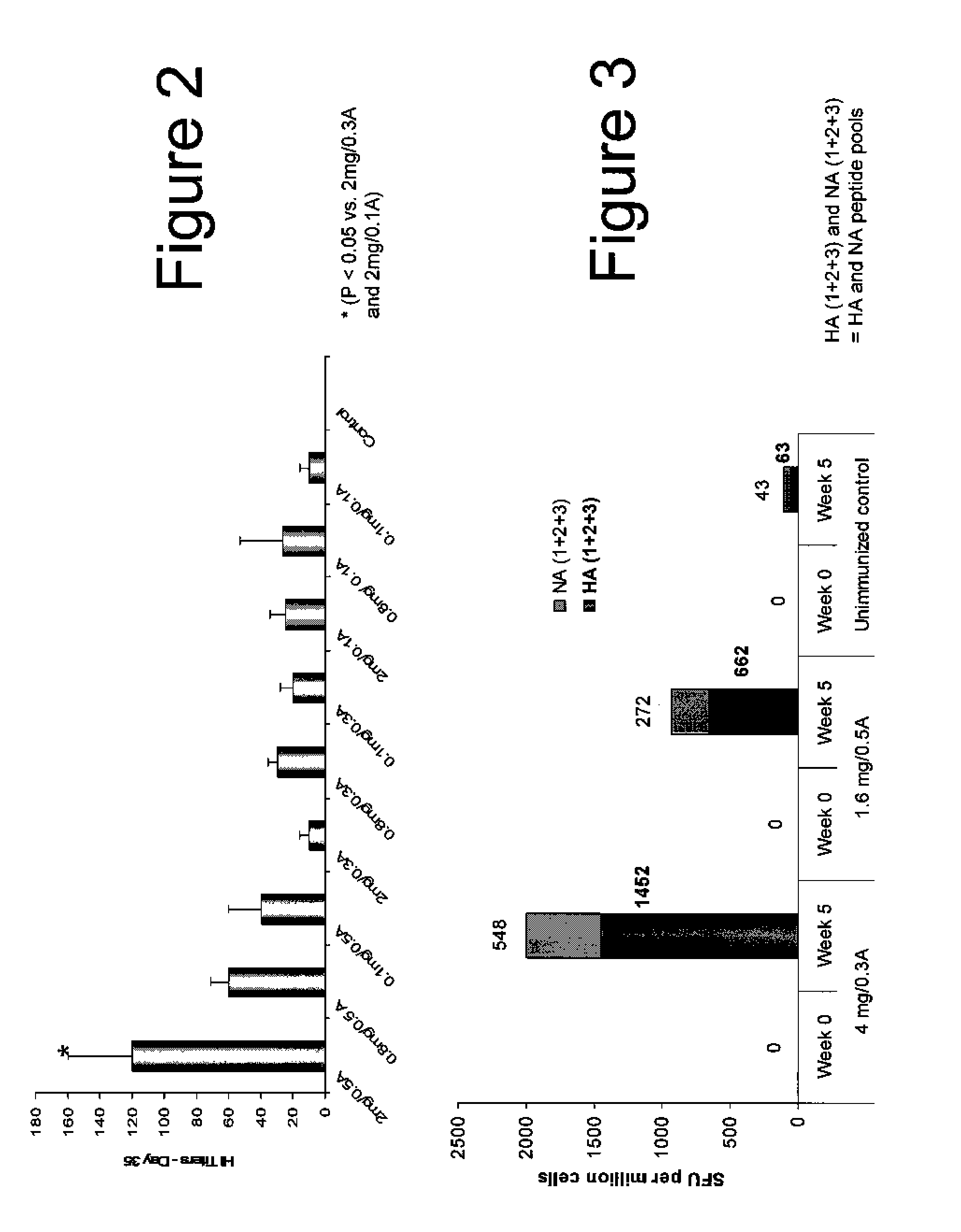
An aspect of the present invention is directed towards DNA plasmid vaccines capable of generating in a mammal an immune response against a plurality of influenza virus subtypes, comprising a DNA plasmid and a pharmaceutically acceptable excipient. The DNA plasmid is capable of expressing a consensus influenza antigen in a cell of the mammal in a quantity effective to elicit an immune response in the mammal, wherein the consensus influenza antigen comprises consensus hemagglutinin (HA), neuraminidase (NA), matrix protein, nucleoprotein, M2 ectodomain-nucleo-protein (M2e-NP), or a combination thereof. Preferably the consensus influenza antigen comprises HA, NA, M2e-NP, or a combination thereof. The DNA plasmid comprises a promoter operably linked to a coding sequence that encodes the consensus influenza antigen. Additionally, an aspect of the present invention includes methods of eliciting an immune response against a plurality of influenza virus subtypes in a mammal using the DNA plasmid vaccines provided.
Owner:VGX PHARMA +1
Technology for preparing anti influenza virus transfer factors
InactiveCN101057864AConvenient sourceNo pollution in the processPeptide/protein ingredientsAntiviralsFiberMedicine
The invention relates to a method for preparing anti-influenza virus transfer factor, comprising following steps: preparing raw material, pre-treating, disintegrating, freezing and thawing, extracting, ultra-filtering, disinfecting and packing. The invention employs process of first immune and second extraction. The preparation time is reduced because of low-temperature freezing and thawing and hollow fiber tube ultra-filtering, so the substance activity will not be destroyed. It can be made into oral liquid.
Owner:ZHAOQING UNIV
Herb tea for preventing virus influenza effectively and its preparing method
InactiveCN101011093AGood antiviral effectGreat tasteTea substituesAntiviralsVirus influenzaWindbreak
The invention relates to a method for producing herb tea which can prevent influenza virus. The invention is characterized in that the herb tea comprises windbreak, mother chrysanthemum, honeysuckle, or the like to improve the antiviral ability of human body; and it comprises licorice root, plantain herb or the like to improve the taste. The inventive herb tea can be prepared into variable states to support the application and transmission.
Owner:蔡向源 +1
Inactivated Influenza Virus Compositions
InactiveUS20090297558A1InactivatesMaintain activitySsRNA viruses negative-senseBacterial antigen ingredientsVirus influenzaInfluenza a
The invention provides compositions of inactivated influenza virus that can be used as vaccines and immunological compositions useful for inhibiting, preventing and treating influenza.
Owner:UNIV OF GEORGIA RES FOUND INC +1
Inhibitors of influenza viruses replication
ActiveUS20140142119A1Reduce the amount requiredInhibition of replicationBiocideOrganic chemistryAdjuvantReplication method
Methods of inhibiting the replication of influenza viruses in a biological sample or patient, of reducing the amount of influenza viruses in a biological sample or patient, and of treating influenza in a patient, comprises administering to said biological sample or patient an effective amount of a compound represented by Structural Formula (I):or a pharmaceutically acceptable salt thereof, wherein the values of Structural Formula (IA) are as described herein. A compound is represented by Structural Formula (IA) or a pharmaceutically acceptable salt thereof, wherein the values of Structural Formula (IA) are as described herein. A pharmaceutical composition comprises an effective amount of such a compound or pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier, adjuvant or vehicle.
Owner:VERTEX PHARMA INC
Artificially recombinant H9N2 hypotype influenza virus and its uses
The invention relates to an artificial recombination N9 hypotype influenza virus A / Harbin / Re-2 / 2003 (H9N2), artificial recombination H9 hypotype influenza virus A / Harbin / Re-2 / 2003(H9N2) characterized in that, it includes a HA of H9N2 hypotype fowl influenza virus A / chicken / Guangxi / 10 / 99(H9N2) and six internal genes of influenza virus A / PR / 8 / 34(H1N1), PB2, PB1, PA, NP, M and NS. its storage number is CCTCC-V200311.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Genetically Engineered Equine Influenza Virus and Uses Thereof
ActiveUS20080254060A1Reduce capacityLow toxicitySsRNA viruses negative-senseVectorsUltrasound attenuationVirulent characteristics
The present invention relates, in general, to attenuated equine influenza viruses having an impaired ability to antagonize the cellular interferon (IFN) response, and the use of such attenuated viruses in vaccine and pharmaceutical formulations. In particular, the invention relates to attenuated equine influenza viruses having modifications to an equine NS1 gene that diminish or eliminate the ability of the NS1 gene product to antagonize the cellular IFN response. These viruses replicate in vivo, but demonstrate decreased replication, virulence and increased attenuation, and therefore are well suited for use in live virus vaccines, and pharmaceutical formulations.
Owner:UNIVERSITY OF KENTUCKY +1
Influenza vaccine
ActiveUS20110243987A1Boosting antibodyBoosting cellular immune responseSsRNA viruses negative-senseViral antigen ingredientsAdjuvantSterol
The present invention relates to monovalent influenza vaccine formulations and vaccination regimes for immunising against influenza disease, their use in medicine, in particular their use in augmenting immune responses to various antigens, and to methods of preparation. In particular, the invention relates to monovalent influenza immunogenic compositions comprising an influenza antigen or antigenic preparation thereof from an influenza virus strain being associated with a pandemic outbreak or having the potential to be associated with a pandemic outbreak, in combination with an oil-in-water emulsion adjuvant comprising a metabolisable oil, a sterol and / or a tocopherol such as alpha tocopherol, and an emulsifying agent.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Genetically engineered equine influenza virus and uses thereof
ActiveUS8137676B2Reduce capacityLow toxicitySsRNA viruses negative-senseVectorsVirus influenzaIn vivo
The present invention relates, in general, to attenuated equine influenza viruses having an impaired ability to antagonize the cellular interferon (IFN) response, and the use of such attenuated viruses in vaccine and pharmaceutical formulations. In particular, the invention relates to attenuated equine influenza viruses having modifications to an equine NS1 gene that diminish or eliminate the ability of the NS1 gene product to antagonize the cellular IFN response. These viruses replicate in vivo, but demonstrate decreased replication, virulence and increased attenuation, and therefore are well suited for use in live virus vaccines, and pharmaceutical formulations.
Owner:UNIVERSITY OF KENTUCKY +1
Cold-adapted equine influenza viruses
InactiveUS20060121521A1Antibacterial agentsSsRNA viruses negative-senseVirus influenzaRespiratory disease
The present invention provides experimentally-generated cold-adapted equine influenza viruses, and reassortant influenza A viruses comprising at least one genome segment of such an equine influenza virus, wherein the equine influenza virus genome segment confers at least one identifying phenotype of the cold-adapted equine influenza virus, such as cold-adaptation, temperature sensitivity, dominant interference, or attenuation. Such viruses are formulated into therapeutic compositions to protect animals from diseases caused by influenza A viruses, and in particular, to protect horses from disease caused by equine influenza virus. The present invention also includes methods to protect animals from diseases caused by influenza A virus or other infectious agents utilizing the claimed therapeutic compositions. Such methods include using a therapeutic composition as a vaccine to generate a protective immune response in an animal prior to exposure to an infectious agent, as well as using a therapeutic composition as a treatment for an animal that has been recently infected with an infectious agent leading to respiratory disease, or is likely to be subsequently exposed to such an agent in a few days whereby the therapeutic composition reduces such respiratory disease, even in the absence of antibody-mediated immunity. The present invention also provides methods to produce cold-adapted equine influenza viruses, and reassortant influenza A viruses having at least one genome segment of an equine influenza virus generated by cold-adaptation.
Owner:DOWLING PATRICIA W +1
Mutations that confer genetic stability to additional genes in influenza viruses
ActiveUS10053671B2High genetic stabilityImprove stabilitySsRNA viruses negative-senseViral antigen ingredientsVirus influenzaValine
The disclosure provides for an isolated recombinant influenza virus having at least one of: a PA gene segment encoding PA with a residue at position 443 that is not arginine, a PB1 gene segment encoding PB1 with a residue at position 737 that is not lysine, a PB2 gene segment encoding PB2 with a residue at position 25 that is not valine or a residue at position 712 that is not glutamic acid, a NS gene segment encoding a NS1 with a residue at position 167 that is not proline, a HA gene segment encoding a HA with a residue at position 380 that is not threonine, or any combination thereof, and methods of making and using the virus.
Owner:WISCONSIN ALUMNI RES FOUND
Chicken-origin H9N2 avian influenza virus strain and application thereof
ActiveCN102443571AImprove prevention capabilitiesMicroorganism based processesAntiviralsMicroorganismAvian influenza virus
The present invention relates to a chicken-origin H9N2 avian influenza virus strain and an application thereof. The avian H9N2 influenza A virus AIV-SD strain is preserved in the China General Microbiological Culture Collection Center (CGMCC) on March 22, 2011. The preservation number of the strain is CGMCC No.4689. The AIV-SD strain of the present invention provides pathogenicity for the chicken, and provides low toxicity for mammalians. After the virus solution of the AIV-SD strain is inactivated, the resulting inactivated AIV-SD strain can be used as the vaccine. After vaccinating with thevaccine of the present invention, the prevention ability of the chickens to the avian influenza can be substantially increased so as to substantially improve the economic benefits of the cultivation industry. The chicken-origin H9N2 avian influenza virus strain of the present invention provides great values for prevention and control of the avian influenza virus.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
Vaccines for Pandemic Influenza
InactiveUS20110305748A1High potencyReduce doseSsRNA viruses negative-senseViral antigen ingredientsHemagglutininAdjuvant
Owner:IMMUNE DESIGN CORP
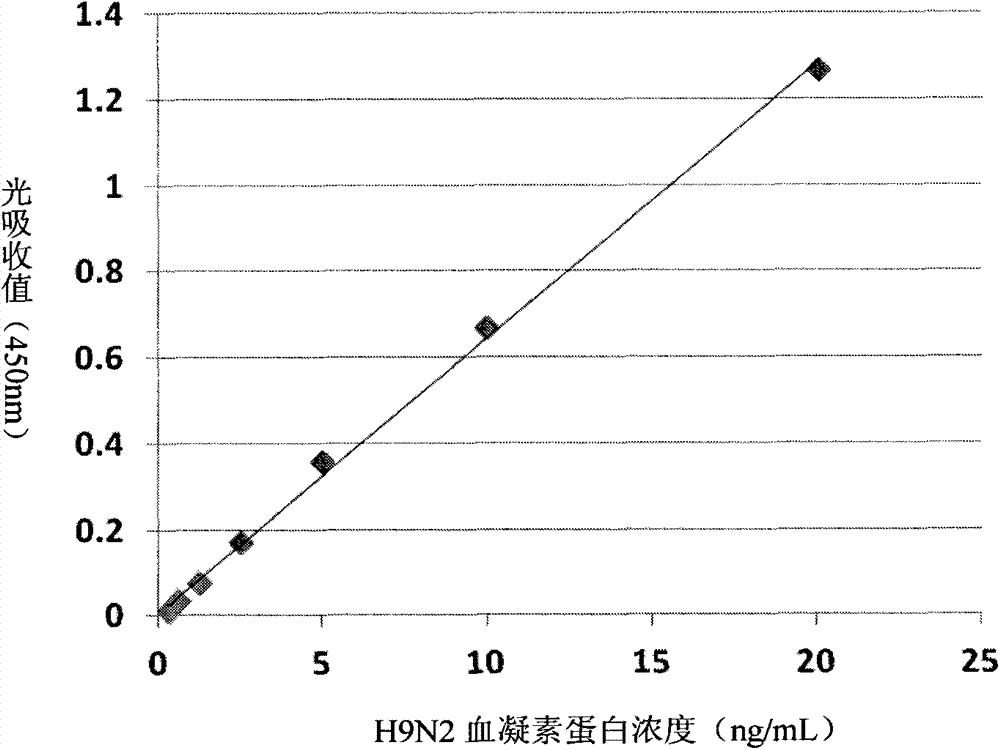
ELISA kit for H9N2 influenza virus hemagglutinin protein
ActiveCN104749372AQuick checkThe inspection method is convenient and easyBiological material analysisBiological testingElisa kitVirus influenza
The invention discloses a double-antibody sandwich ELISA kit for H9N2 influenza A virus hemagglutinin protein. The kit comprises a solid phase vector coated with a monoclonal antibody, a rabbit polyclonal antibody labeled by horseradish peroxidase, an H9N2 hemagglutinin protein standard substance, a sample diluting liquid, a washing liquid, a substrate coloring liquid and a reaction terminating liquid. The kit is good in sensitivity, capable of performing quantitative detection for the H9N2 influenza virus hemagglutinin protein and specifically recognizing H9N2 influenza A viruses, and free of cross reactions with hemagglutinin protein of other main subtypes comprising H1N1, H2N2, H3N2, H5N1 and H7N7 of the influenza A viruses and hemagglutinin protein of influenza B viruses. The kit is simple to operate and capable of rapidly detecting a large number of samples simultaneously, can be used for supporting fundamental research of the H9N2 influenza viruses, and has important significance for performing epidemiologic study on influenza viruses.
Owner:北京义翘神州科技股份有限公司
Primer pair for identifying newcastle disease virus and multi-subtype avian influenza virus and application thereof
InactiveCN102321769AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesNewcastle disease virus NDVVirus influenza
The invention discloses a primer pair for identifying newcastle disease virus and multi-subtype avian influenza virus and an application thereof. The PCR primer pair composition provided by the invention comprises primer pair NDV, primer pair H3V, primer pair H5V, and primer pair H9V; the PCR primer pair composition is applicable to the preparation of reagents for the diagnosis or auxiliary diagnosis of newcastle disease and avian influenza, or to the preparation of reagents for the identification or auxiliary identification of newcastle disease virus and multi-subtype avian influenza virus. When the PCR primer pair composition provided by the invention is used to simultaneously detect newcastle disease virus, H3, H5 and H9-subtype avian influenza virus, the specificity is strong; the sensitivity is 1EID50 / 100 microliters, 1EID50 / 100 microliters, 1*10-2EID50 / 100 microliters, and 1EID50 / 100 microliters respectively; compared with identification results of routine test methods such as virus isolation and hemagglutination inhibition tests, the results obtained by using the PCR primer pair composition of the invention has a coincidence rate of up to 100%; the PCR primer pair composition provided by the invention is applicable to the development of corresponding multiplex RT-PCR kits for the clinical diagnosis and epidemiological control of newcastle disease and avian influenza.
Owner:CHINA AGRI UNIV
Method for inhibiting infection and reproduction of influenza type A WSN virus
InactiveUS20090042801A1Maintain biological activityOrganic active ingredientsBiocideAdditive ingredientVirus influenza
The present invention relates to a method for inhibiting infection and reproduction of influenza type A WSN virus, which comprises providing an effective amount of a pharmaceutical composition; and contacting said composition with said influenza type A WSN virus, wherein said pharmaceutical composition contains C-phycocyanin (C-PC), allophycocyanin (APC), and spirulina growth factor (SGF). The present invention also provides a method for extracting said pharmaceutical composition, comprising the steps of: (a) adding hypotonic buffer solution to organic blue-green algae powder and mixing thoroughly; (b) incubating the mixture below room temperature overnight; (c) separating and purifying the mixture by a centrifuge; (d) collecting the suspending supernatant and detecting it by a spectrometer to determine ingredients and content; and (e) spray drying the supernatant; characterized in which low-temperature extraction is employed to maintain the bioactivity and nutrients of the pharmaceutical composition.
Owner:FAR EAST BIO TEC
Avian influenza virus infection or avian influenza virus vaccine immunophenotyping kit
The invention relates to an avian influenza virus infection or avian influenza virus vaccine immunophenotyping kit. The detection kit disclosed by the invention comprises one or a plurality of solid carriers and a specific polypeptide assembly which is independently connected to the one or the plurality of the solid carriers.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
Inhibitors of influenza viruses replication
Methods of inhibiting the replication of influenza viruses in a biological sample or patient, of reducing the amount of influenza viruses in a biological sample or patient, and of treating influenza in a patient, comprises administering to said biological sample or patient an effective amount of a compound represented by Structural Formula (I):or a pharmaceutically acceptable salt thereof, wherein the values of Structural Formula (I) are as described herein. A compound is represented by Structural Formula (I) or a pharmaceutically acceptable salt thereof, wherein the values of Structural Formula (I) are as described herein. A pharmaceutical composition comprises an effective amount of such a compound or pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier, adjuvant or vehicle.
Owner:VERTEX PHARMA INC
H1-subtype influenza A virus double-antibody sandwich ELISA kit and application
ActiveCN102633878AStrong specificityHigh sensitivityMicroorganism based processesImmunoglobulins against virusesHemagglutininElisa kit
The invention belongs to the fields of detection technology of animal virology and epizootiology. The invention particularly relates to an H1-subtype influenza A virus double-antibody sandwich ELISA kit and an application. The kit of the invention comprises an enzyme label plate which contains anti-H1-subtype influenza A virus hemagglutinin, has a preservation number of CCTCC NO: C201106, and is coated by a monoclonal antibody, and a horseradish peroxidase labeled H1-subtype influenza A virus hemagglutinin monoclonal antibody is used as a second antibody. The invention discloses separation, amplification, inactivation, and purification methods of the H1-subtype influenza A virus, and preparation and purification methods of the H1-subtype influenza A virus hemagglutinin monoclonal antibody. The invention also discloses an H1-subtype influenza A virus double-antibody sandwich ELISA detection method.
Owner:HUAZHONG AGRI UNIV
H7 avian influenza virus monoclonal antibody and application thereof
ActiveCN107475203ALevel results are accurateAccurate evaluationImmunoglobulins against virusesTissue cultureClinical immunologyAvian influenza virus
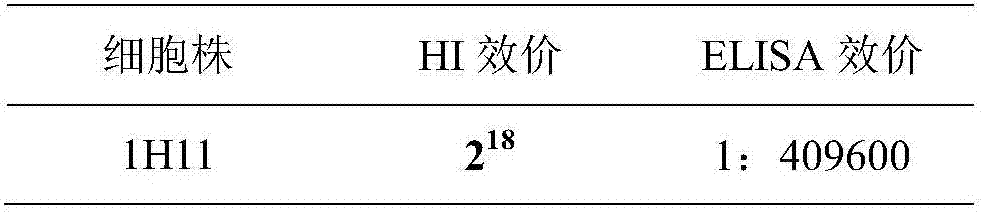
The invention belongs to the technical field of clinical immunology, and concretely discloses a H7 avian influenza virus monoclonal antibody and an application thereof. The monoclonal antibody is secreted by a hybridoma cell 1H11, and the hybridoma cell is preserved in CCTCC with the preservation number of CCTCC NO:C2017112. The monoclonal antibody has the advantages of high titer, good specificity and good blocking effect, and can be used for preparing an H7 subtype avian influenza blocking ELISA antibody detection kit. The kit is used for testing a sample to be tested, and has a coincidence rate of 98% or above with HI test; and compared with the HI, the kit has the advantages of shortening of the test time, and simple operating steps.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Live-Attenuated Vaccine Having Mutations in Viral Polymerase for the Treatment and Prevention of Canine Influenza Virus
ActiveUS20180243401A1SsRNA viruses negative-senseViral antigen ingredientsVirus influenzaPolymerase L
The present invention relates to compositions and methods for the treatment and prevention of canine influenza virus (CIV) and CIV-related pathology. The present invention is based in part upon the discovery that various mutations in segment 1 and segment 2 of the CIV genome, thereby encoding mutant PB2 and PB1 protein, render the virus to be temperature-sensitive.
Owner:CORNELL UNIVERSITY +1
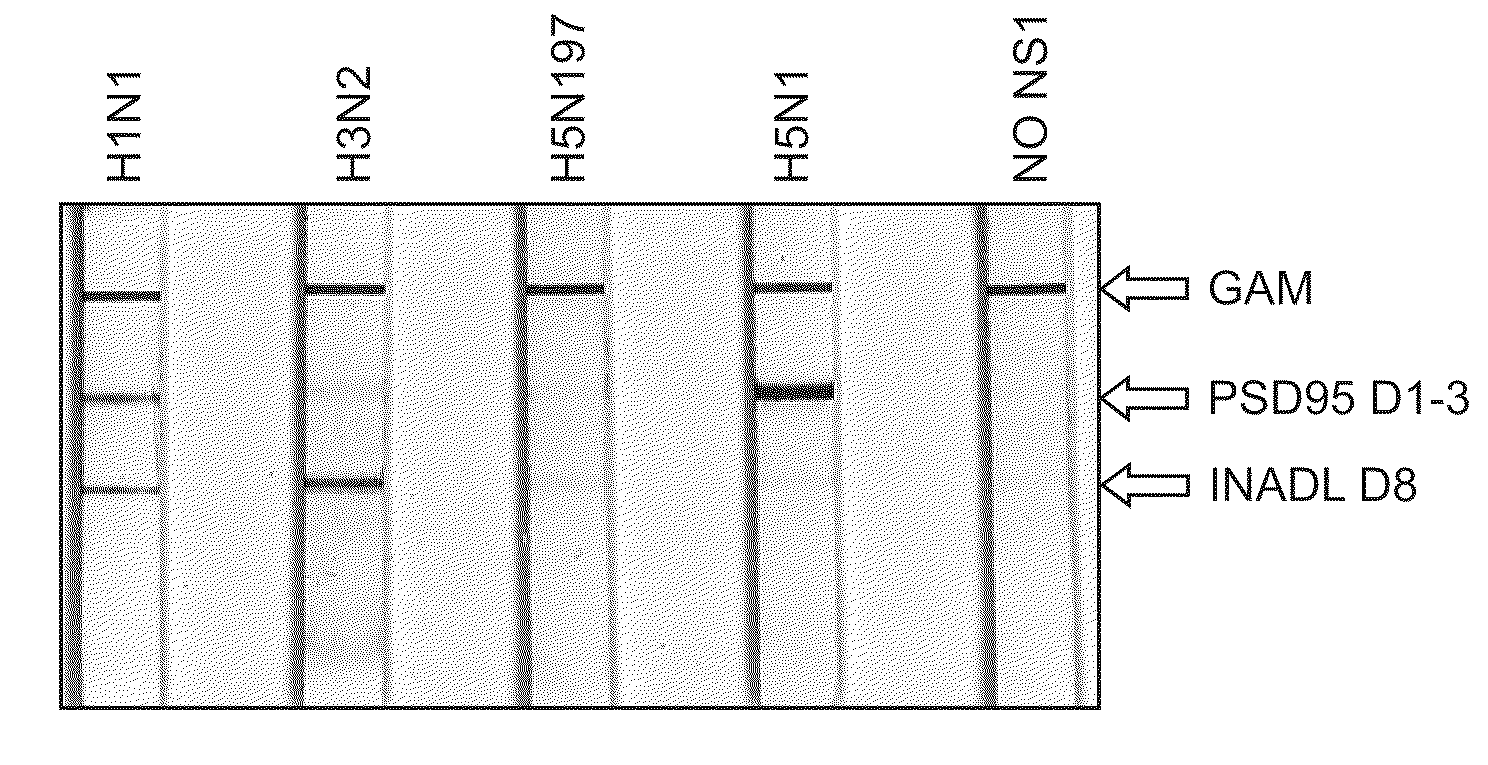
Detection of influenza virus
The present application describes methods for detecting influenza A and / or influenza B and / or distinguishing between pathogenic and seasonal influenza A subtypes. Many of these preferred formats employ pan-specific antibodies (i.e., that react with all or at least multiple strains within an influenza type) to detect presence of influenza A and / or influenza B and PDZ domains in combination with panspecific antibodies to influenza A to distinguish pathogenic and seasonal influenza A subtypes.
Owner:AVC ROYALTY FUND I
Reagent set and method for detecting avian influenza viruses and chicken parvoviruses
ActiveCN106636472AVery goodImprove accuracyMicrobiological testing/measurementMicroorganism based processesChicken parvovirusAvian influenza virus
The invention discloses a reagent set and a method for detecting avian influenza viruses and chicken parvoviruses. The reagent set for detecting or assisting in detecting the avian influenza viruses and the chicken parvoviruses comprises a primer pair named as M-P and a primer pair named as ChPV-P, the M-P comprises two single-chain DNA (deoxyribonucleic acid) shown as SEQ ID No.1 and SEQ ID No.2 in a sequence table, and the ChPV-P comprises two single-chain DNA shown as SEQ ID No.3 and SEQ ID No.4 in the sequence table. Experimental results show that the reagent set and the method for detecting or assisting in detecting the avian influenza viruses and the chicken parvoviruses are good in specificity and accuracy and high in sensibility and can be used for specifically detecting the avian influenza viruses and the chicken parvoviruses, and the sensibility of detecting the avian influenza viruses and the chicken parvoviruses is 100fg.
Owner:GUANGXI VETERINARY RES INST
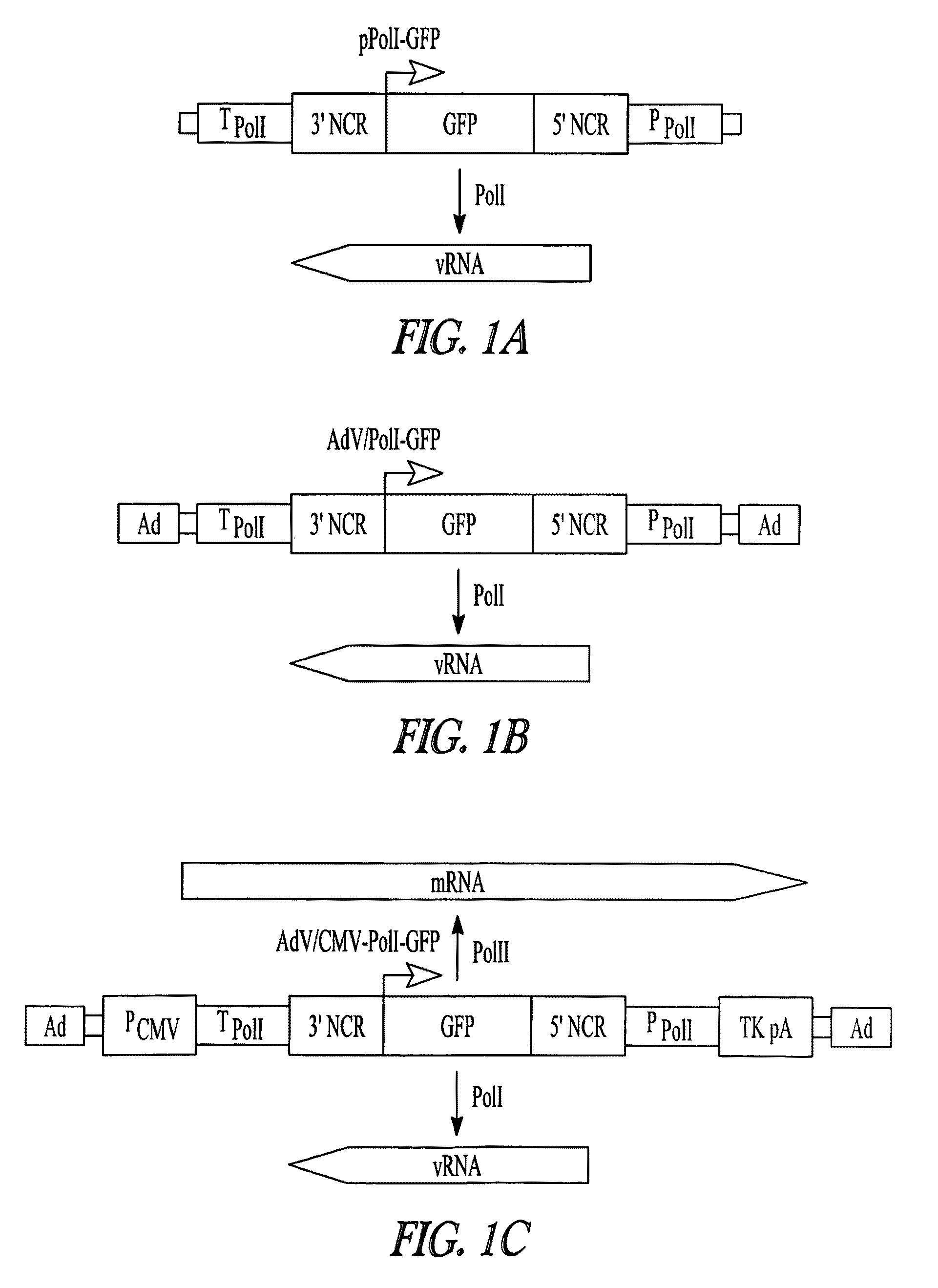
Adenoviral vectors for influenza virus production
ActiveUS8043856B2High expressionIncrease productionSsRNA viruses negative-senseAnimal cellsVirus-RetrovirusD'Aguilar virus
The invention provides adenovirus and retrovirus vectors useful to prepare influenza virus. Also provided is a canine RNA polymerase I promoter and vectors having that promoter.
Owner:WISCONSIN ALUMNI RES FOUND
Influenza a virus variants
The present invention relates to influenza A virus variants, particularly variants that are resistant to a polymerase inhibitors. Also provided are methods and compositions related to the influenza A virus variants. Further provided are methods of isolating, identifying, and characterizing multiple viral variants from a patient.
Owner:VERTEX PHARMA INC
Vaccination against influenza
InactiveUS20130236494A1Broad protectionSsRNA viruses negative-senseViral antigen ingredientsHemagglutininHeterologous
Described are methods for vaccinating a subject against influenza comprising administering a vaccine multiple times to a subject where the vaccine comprises influenza hemagglutinin (HA) and neuraminidase (NA) proteins from at least a first influenza strain, wherein the HA and NA proteins of the first influenza strain are administered to the subject at least three times within a period of less than one year. Such immunization schemes induce cross-protection against heterologous and heterosubtypic influenza strains.
Owner:JANSSEN VACCINES & PREVENTION BV
Shuttling intracellular antibody TAT-4F for H3N2-type canine influenza virus
ActiveCN108728461AInterfere with copyingPlay an antiviral rolePolypeptide with localisation/targeting motifAntiviralsHemagglutininVirus influenza
The invention discloses a shuttling intracellular antibody TAT-4F for H3N2-type canine influenza virus. The H3N2 virus is A-type influenza virus, the main neutralizing antibody is from hemagglutinin (HA), therefore, the HA becomes an original main research target; then the influenza virus has high variability, the immune cross reaction among different variable branches is weaker, M1 antibody can be combined with M1 protein to inhibit the activity and disturb multiplication, transcription and release of the influenza virus so as to play a role in resisting the virus; therefore, the M1 protein is selected for preparing the corresponding antibody to obtain stable titer, coupling the antibody with TAT protein PTD (Protein Transduction Domain) capable of transducing biomacromolecules into cellsand expressing fusion protein TAT PTD-M1 ScFv, and then a shuttling antibody for resisting the canine influenza virus is prepared to provide a new path for treating the canine influenza virus.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com