H7 avian influenza virus monoclonal antibody and application thereof
A monoclonal antibody and avian influenza virus technology, applied in the field of clinical immunology technology, to achieve the effects of shortening the test time, simple operation steps, and improving specificity and sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Obtaining of hybridoma cell line 1H11:
[0020] Establishment of monoclonal antibody hybridoma cell lines
[0021] animal immunity
[0022] A bottle of H7 standard antigen purchased from Harvey was resuspended in 500 μL of normal saline, fully emulsified with an equal volume of Freund’s complete adjuvant, and injected subcutaneously with 200 μL / mouse of 8-week-old BALB / c mice; 2 weeks, Four weeks later, the antigens emulsified with Freund's incomplete adjuvant were used to immunize again in the same way; six weeks later, the same dose of antigen was injected intraperitoneally without adjuvant; four days later, they were fused.
[0023] cell fusion
[0024] Preparation of myeloma cells Select SP2 / 0 cells in good growth state, discard the supernatant when the density grows to 75% of the bottom of the culture bottle, wash once with incomplete DMEM medium, blow the cells gently with 10mL incomplete DMEM medium Down.
[0025] Preparation of splenic lymphocytes Take mice ...
Embodiment 2
[0033] Preparation and potency determination of ascites:
[0034] Take 10-week-old BALB / c mice, intraperitoneally inject sterilized liquid paraffin, 0.5 mL / mouse; 1 week later, intraperitoneally inject hybridoma cells 1H11 in logarithmic growth phase diluted with PBS, 5×10 5 One per mouse; when the abdomen of the mouse was obviously raised, ascites was collected from the abdominal cavity with a 16# needle, centrifuged at 2500g for 10 minutes, the fat tissue was removed, the supernatant was absorbed, and stored at -70°C for later use.
[0035] Characterization of mAbs:
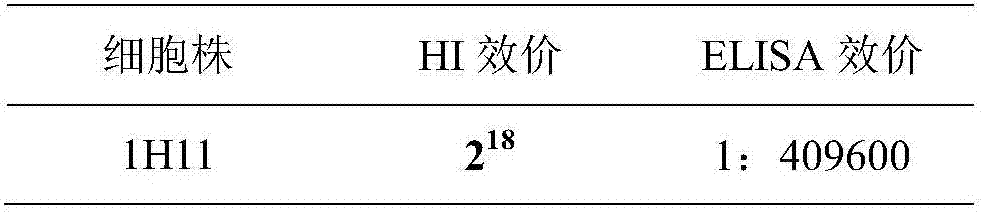
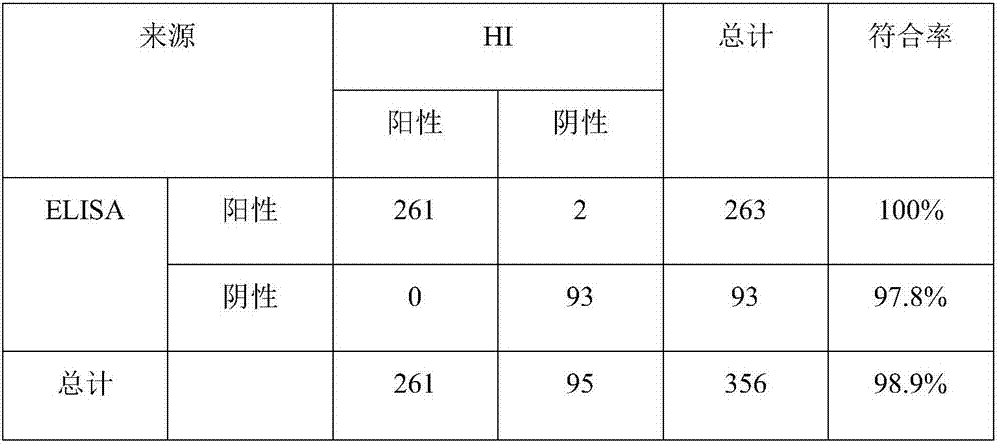
[0036] Determination of ascites titer Two methods of indirect ELISA and HI test were used to detect the titer of ascites, and the results are as follows:
[0037]
[0038] Subclass identification was carried out according to the method introduced in the instruction manual of the monoclonal antibody subclass kit, and the results showed that monoclonal antibody 1H11 was IgG 1 subclass.
Embodiment 3
[0040] Stability identification of hybridoma cell 1H11
[0041] The obtained hybridoma cell line 1H11 was continuously passaged for 15 generations, and the titer of the cell supernatant was determined by HI test. The results showed that the cell line can stably secrete antibodies, and the HI antibody titers of the cell culture supernatant were all above 2 7 above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com