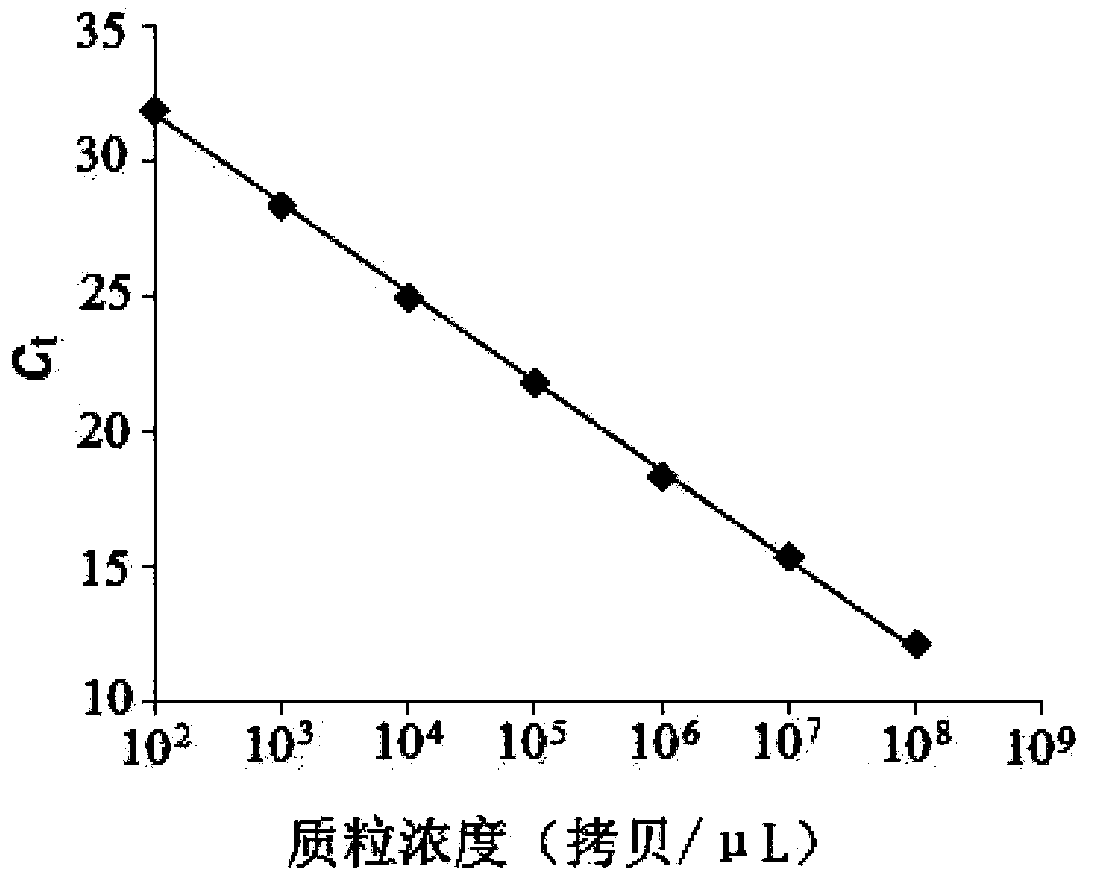
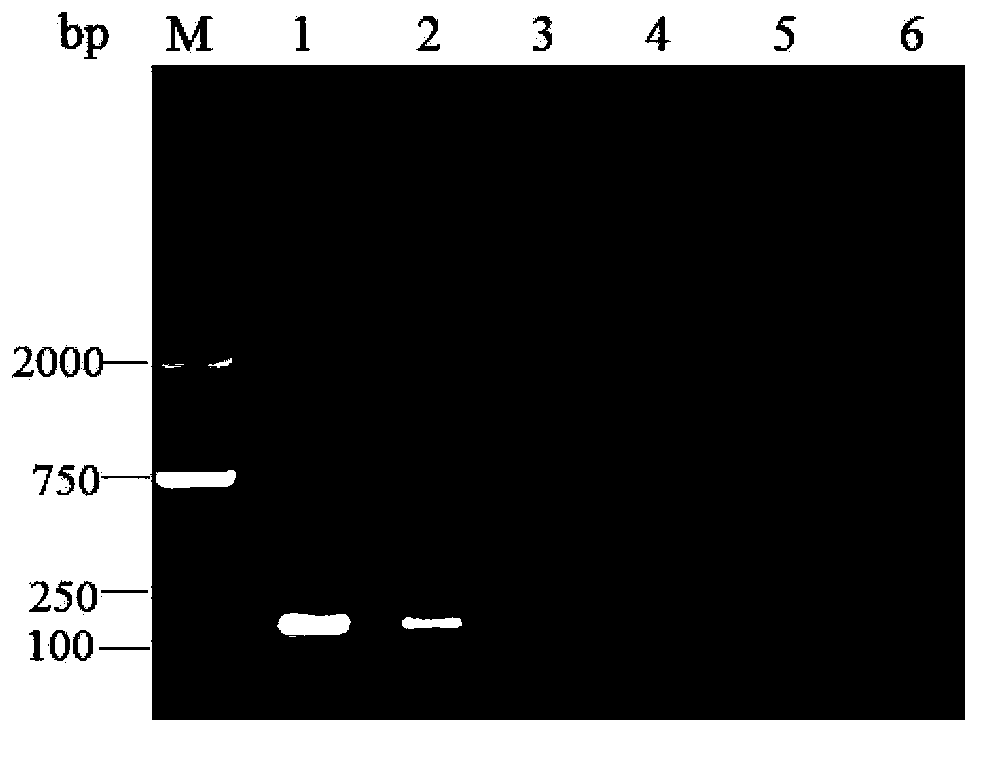
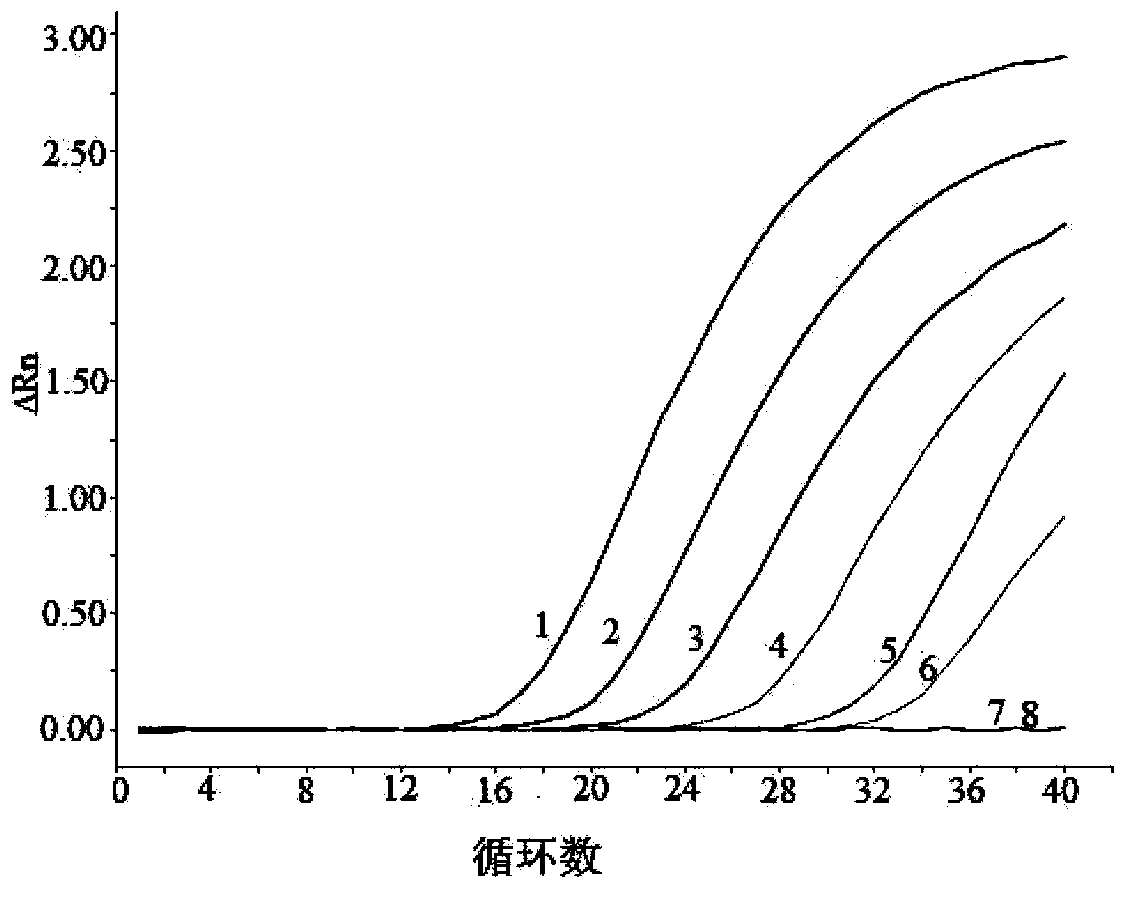
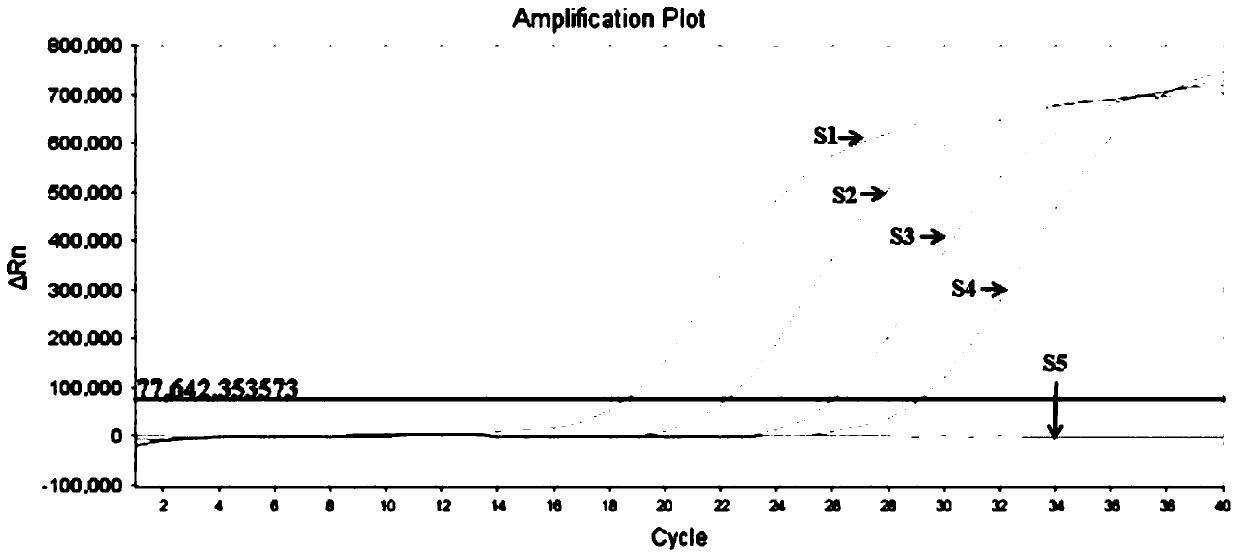
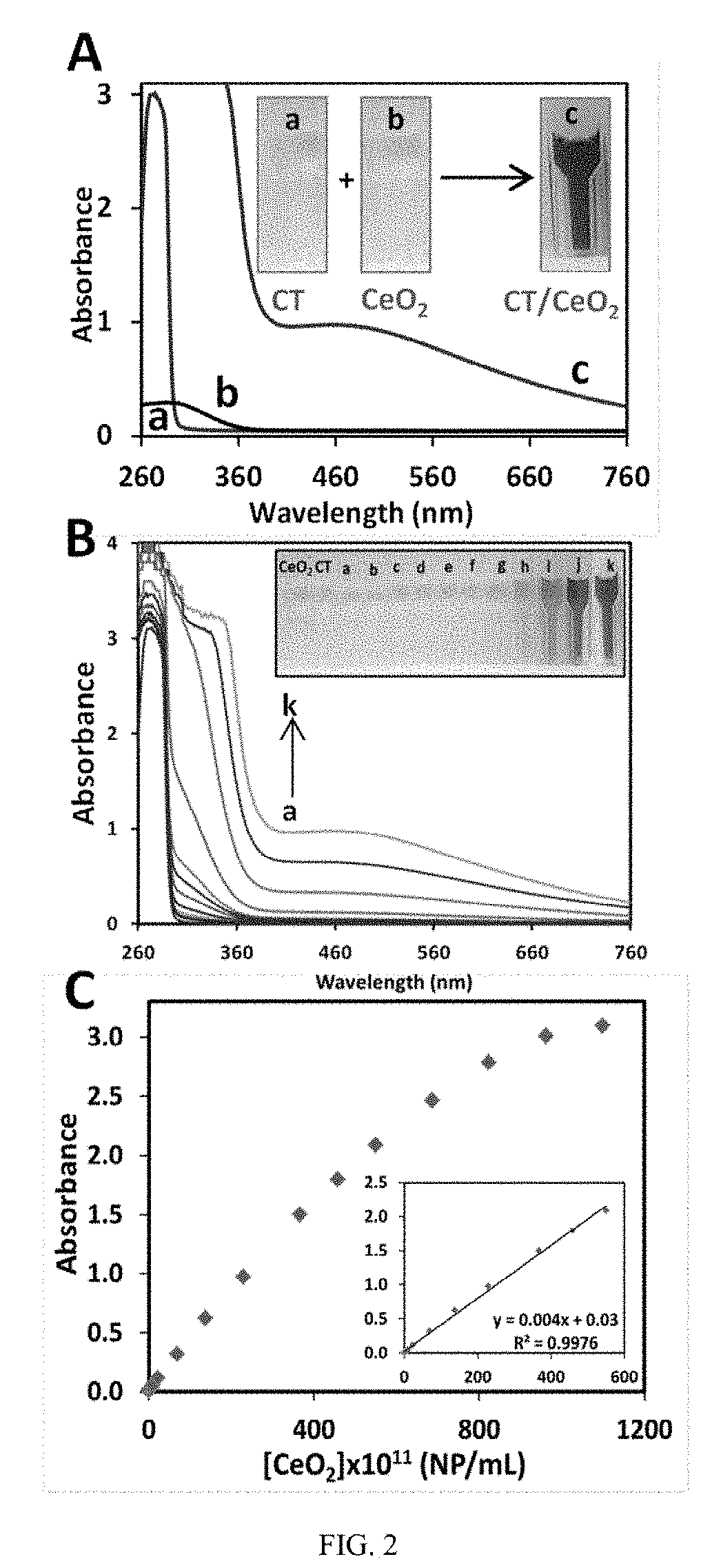
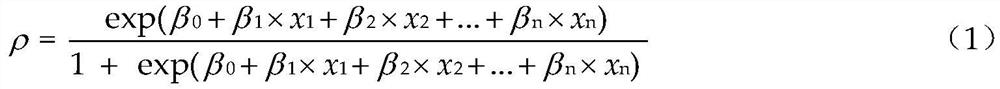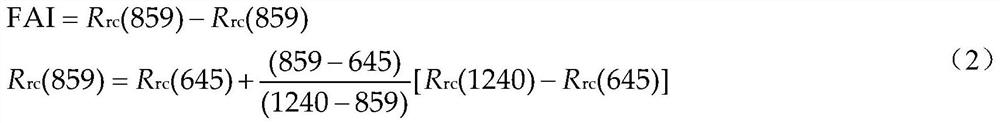Patents
Literature
39 results about "Epidemiologic study" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Epidemiologic study. A study that compares 2 groups of people who are alike except for one factor, such as exposure to a chemical or the presence of a health effect; the investigators try to determine if any factor is associated with the health effect.
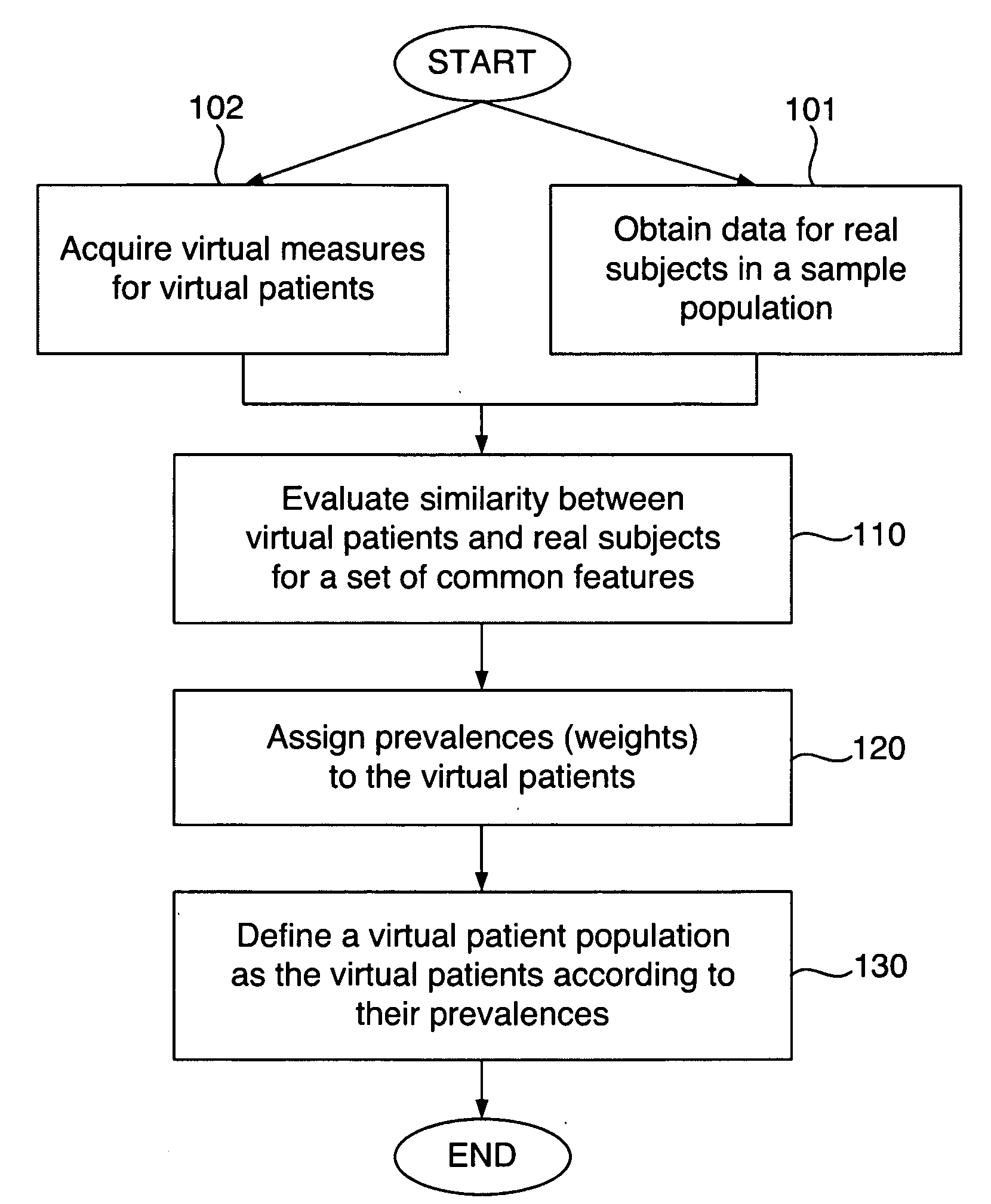
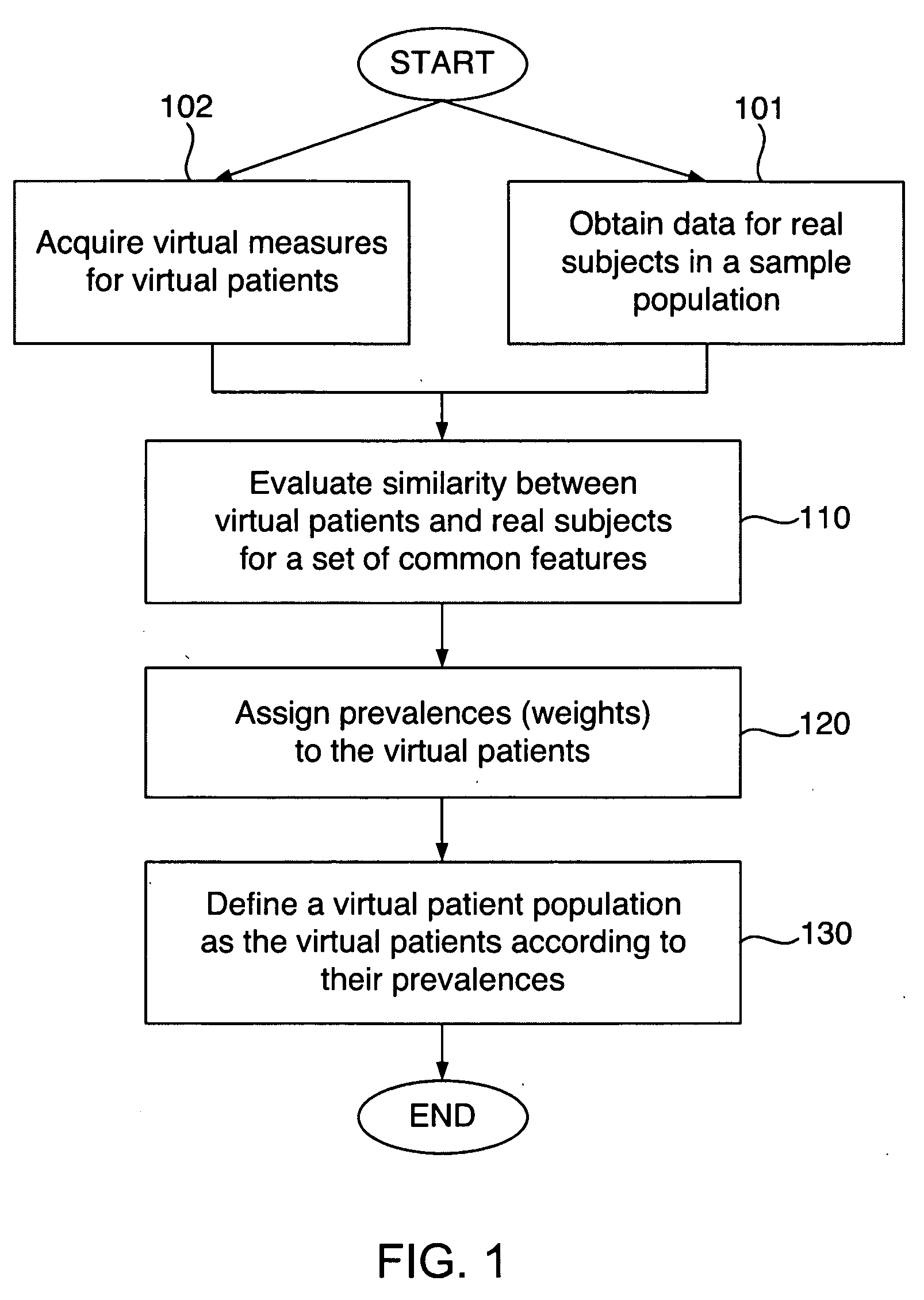
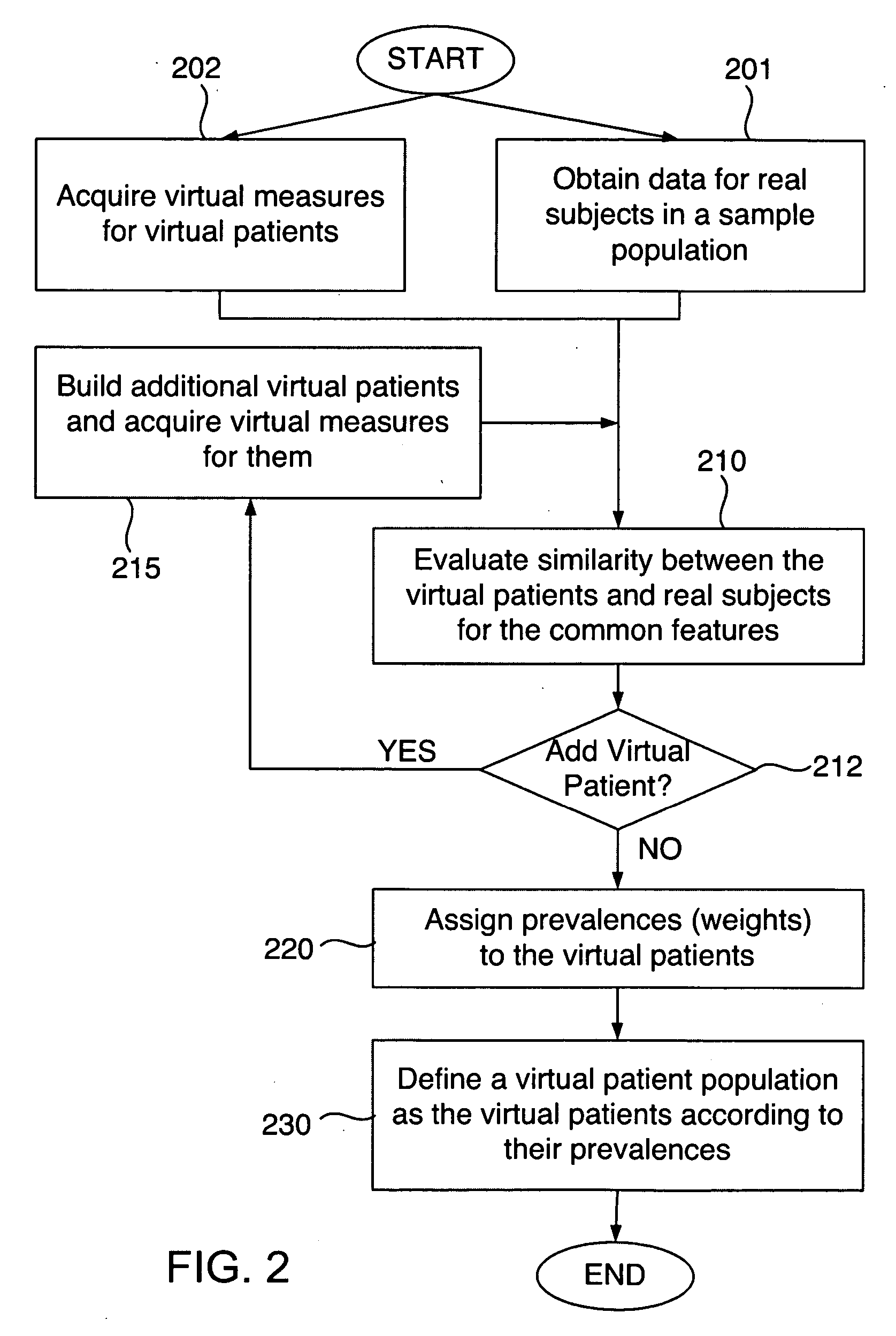
Defining virtual patient populations
InactiveUS20070026365A1Medical simulationData processing applicationsClinical psychologyClinical trial
The invention encompasses methods, including computer-implemented methods, of defining a virtual patient population and mapping the virtual patient population to a population of real patients. The invention utilizes virtual measures from one or more virtual patients, and data representative of multiple real subjects in a sample population, such as data collected from patients in a clinical trial or epidemiological study of a real population. The invention includes evaluating the similarity between the virtual patients and the real subjects, and assigning prevalences to the virtual patients based on the evaluation. The similarity can be assessed using some or all of the virtual measures of the virtual patients and some or all of the data obtained for the real subjects. Any of various goodness-of-fit measures can be used to evaluate the similarity or to help identify prevalences. The virtual patient population is defined as the virtual patients according to their respective prevalences.
Owner:ENTELOS INC
Cardiovascular health station methods and apparatus
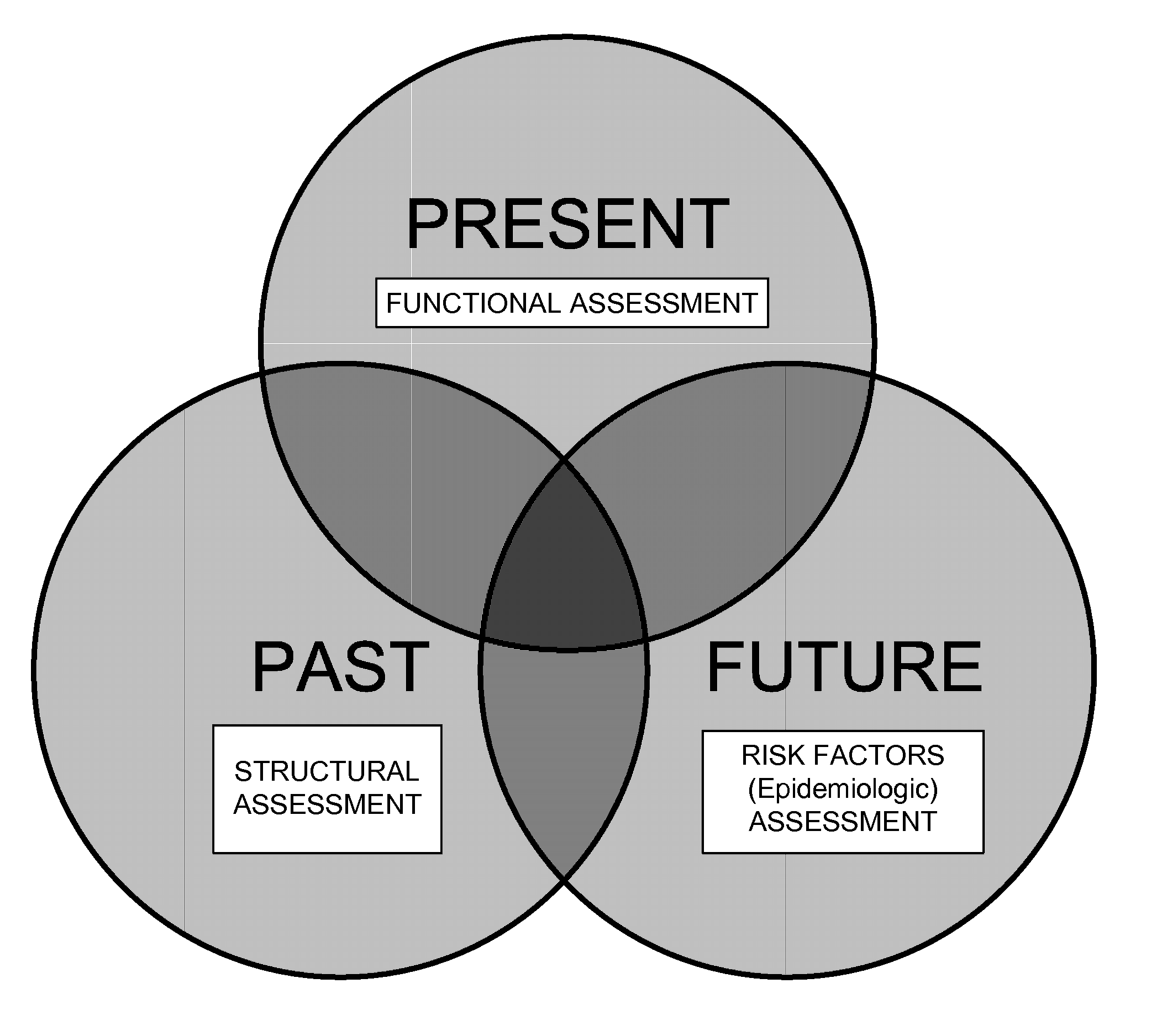
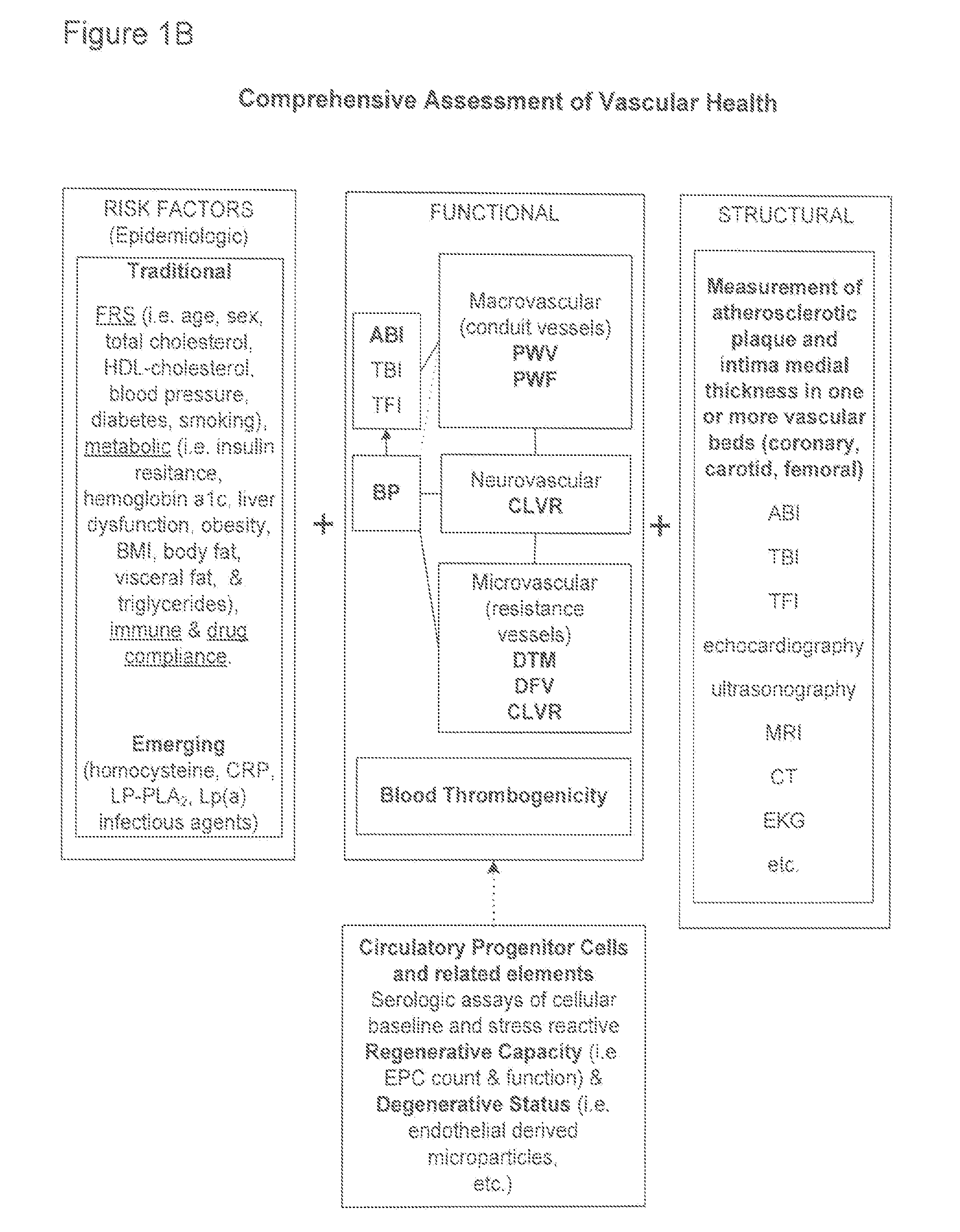
InactiveUS20100081941A1Easy to measureImprove administrationBlood flow measurement devicesEvaluation of blood vesselsEpidemiologic studyCardiovascular health
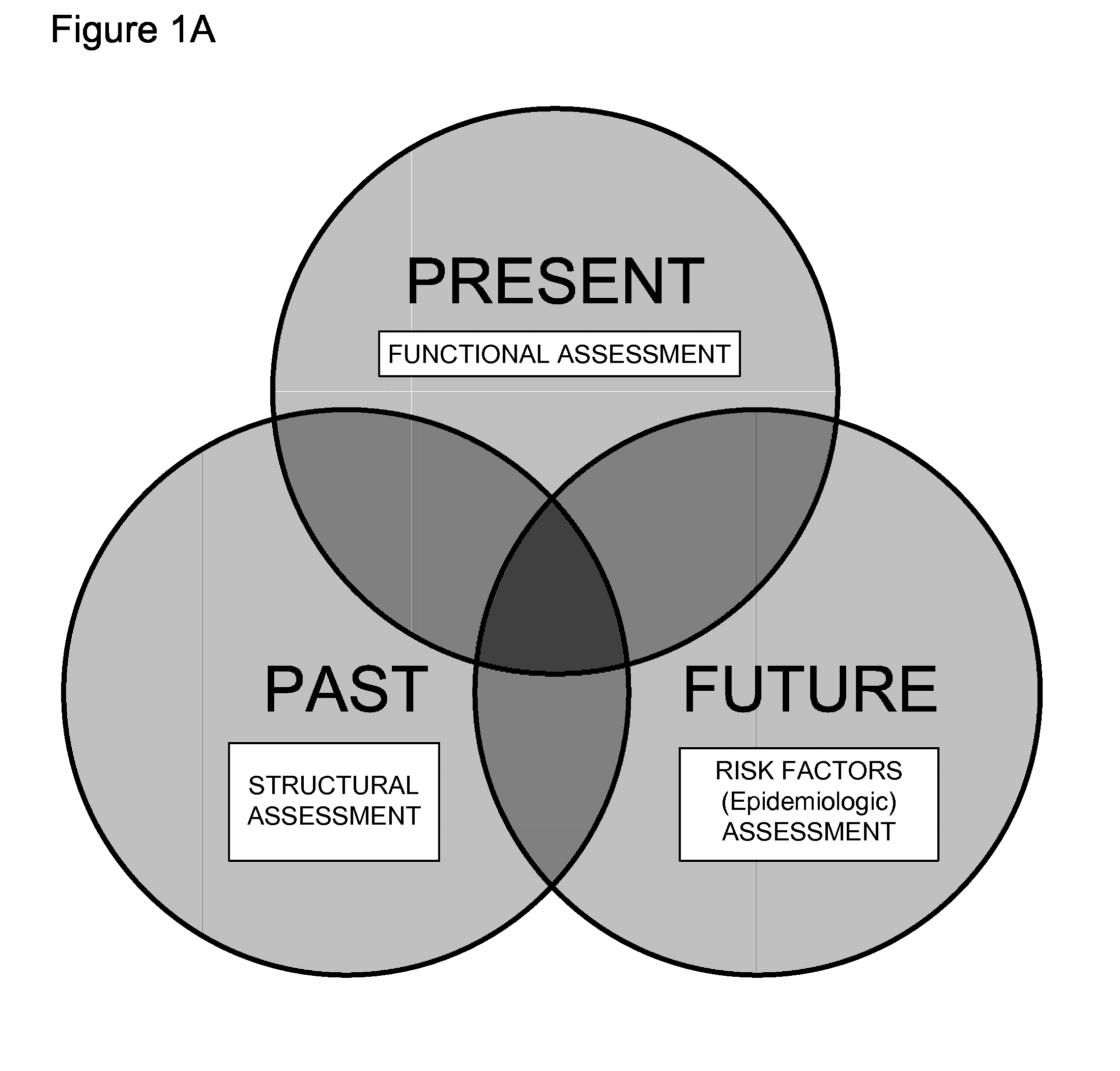
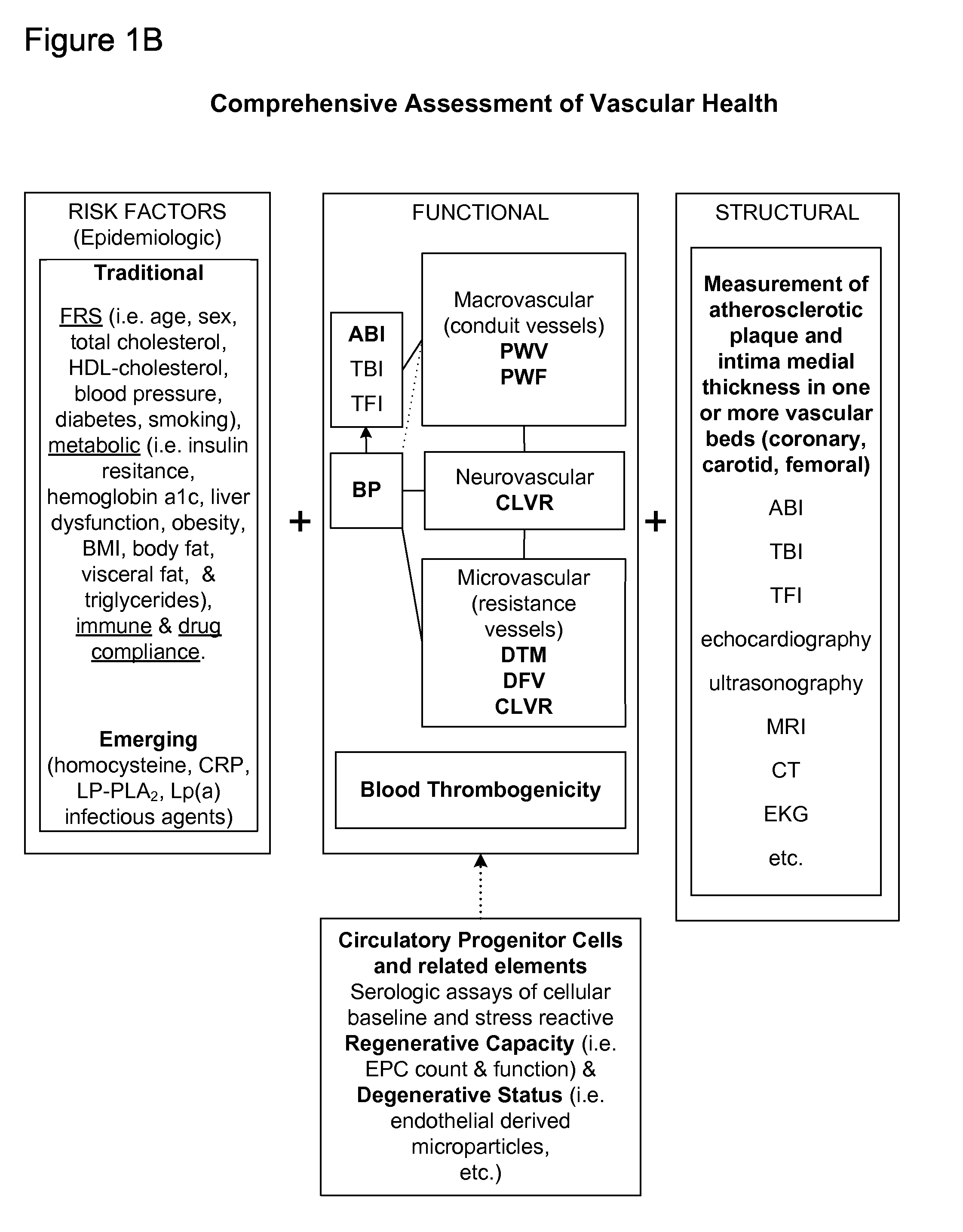
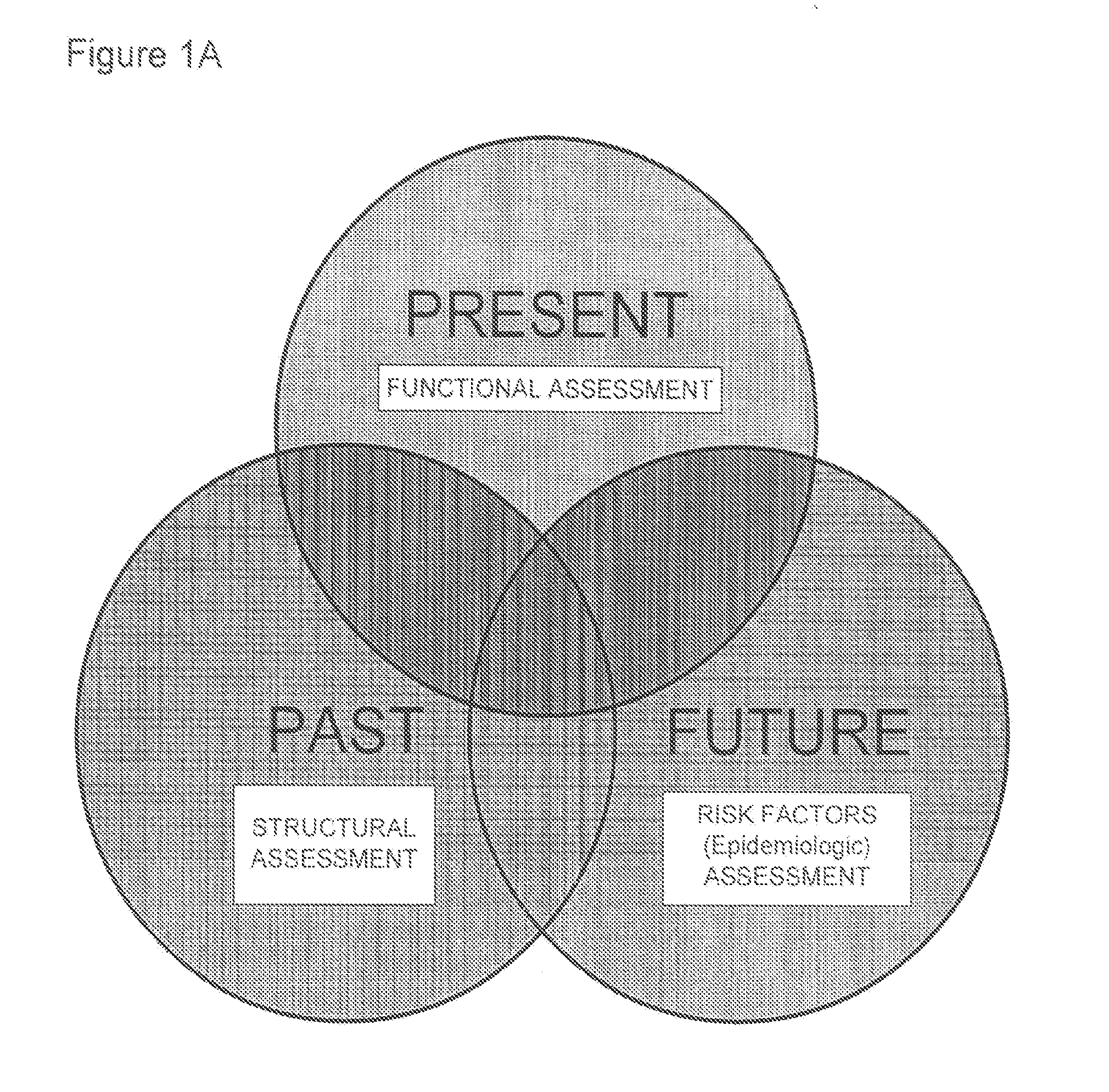
Methods and apparatus for improving measurements of cardiovascular health status in a given individual are provided. The comprehensive assessment of cardiovascular health includes at least two components: risk factor assessment based on epidemiologic studies and functional status of the individual. Structural studies of the individual can also be included in the comprehensive assessment of cardiovascular health. The invention aims to improve detection, treatment, devices, and administration of cardiovascular risk assessment.
Owner:AMERICAN HEART TECH LLC
Cardiohealth Methods and Apparatus
InactiveUS20120065514A1Reduce riskPowerful measureElectrocardiographyBlood flow measurement devicesCardiovascular healthEpidemiologic study
Methods and apparatus for improving measurements of cardiovascular health status in a given individual are provided. The comprehensive assessment of cardiovascular health includes at least two components: risk factor assessment based on epidemiologic studies and functional status of the individual. Structural studies of the individual can also be included in the comprehensive assessment of cardiovascular health. The invention aims to improve detection, treatment, devices, and administration of cardiovascular risk assessment.
Owner:ENDOTHELIX
Hybridoma cell strain, foot-and-mouth disease resistant O-type (O/GX/09-7) virus specificity monoclonal antibody secreted by hybridoma cell strain and application of hybridoma cell strain and antibody to detection of foot-and-mouth disease O-type viruses
ActiveCN105296435AHigh sensitivityAccurate detectionImmunoglobulins against virusesMicroorganism based processesVaccine ProductionEpidemiologic study
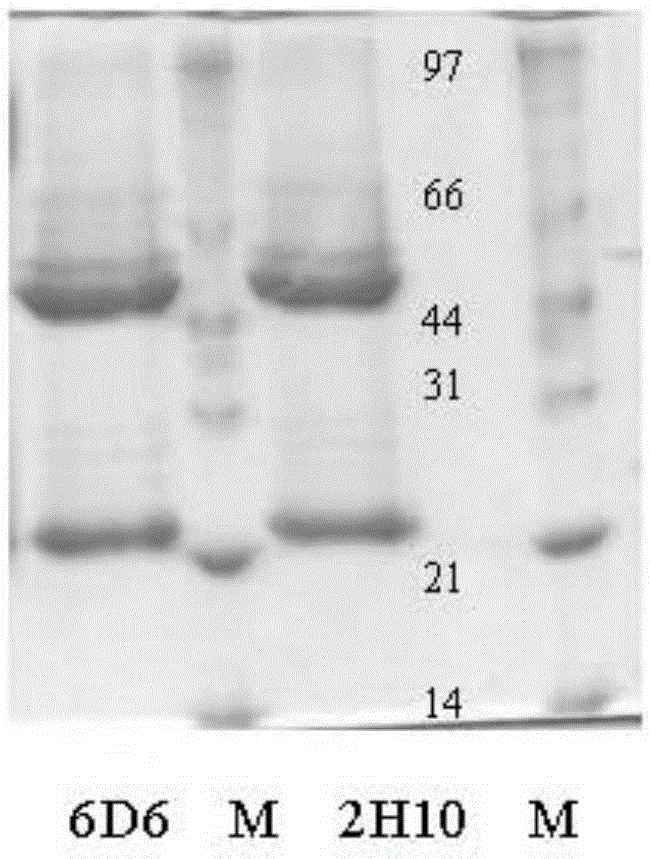
The invention discloses a hybridoma cell strain 6D6 of a specificity monoclonal antibody capable of continuously and stably secreting foot-and-mouth disease resistant O-type (O / GX / 09-7) viruses, and the specificity monoclonal antibody secreted by the hybridoma cell strain. The 6D6anti can recognize the foot-and-mouth disease O-type (O / GX / 09-7) viruses in a specificity mode, the 6D6anti is used as a coating and an enzyme labeled antibody in ELISA detection and can be used for detecting the foot-and-mouth disease O-type (O / GX / 09-7) viruses, and the advantages of high specificity and high sensitivity are achieved; the hybridoma cell strain, the antibody and the application can play an important role in detection of the foot-and-mouth disease O-type (O / GX / 09-7) viruses, vaccine production and epidemiologic study.
Owner:JINYUBAOLING BIO PHARMA CO LTD +1
Primer combination used for detecting SARS-CoV-2 whole genome, and application method
PendingCN111676325AHigh amplification efficiencyAmplification results are accurateMicrobiological testing/measurementMicroorganism based processesEpidemiologic studyMolecular diagnostics
The invention discloses a primer combination used for detecting SARS-CoV-2 whole genome. The primer combination comprises specific primers used for SARS-CoV-2RNA reverse transcription and a primer group for amplification of the whole genome; by adoption of the primer combination, after target genes are amplified by reverse transcription and nest type PCR, on the basis of a next-generation semiconductor sequencing platform, a next-generation semiconductor sequencing technology used for detecting the SARS-CoV-2 whole genome and mutation sites is constructed; the method has the advantages of being wide in applicability, high in flux and good in accuracy, and can detect multiple samples one time; the convenient method is provided for deep analysis of the SARS-CoV-2 genome; and the method has important meanings for epidemiologic study, clinical early molecular diagnosis and the like.
Owner:云南科耀生物科技有限公司
Malaria serum with anti-tumor function and preparation method and application of malaria serum with anti-tumor function
InactiveCN105748515AObvious apoptosisIncreased apoptotic rateMammal material medical ingredientsAntineoplastic agentsPlasmodium traguliMalaria
The invention discloses a malaria serum with an anti-tumor function and a preparation method and application of the malaria serum with the anti-tumor function.According to long-term arduous scientific experiments, the serum of patients with malaria has a certain anti-tumor function, and particularly the serum of patients with a syndrome of malaria and thrombocytopenia is effective in treatment or prevention of tumors, especially liver cancers, under specific concentration conditions.According to experiments, the serum has an evident apoptosis induction function on hepatoma cell strains (7721) cultured in vitro, and the hepatoma cell apoptosis induction ratio is increased along with increase of serum concentration.The malaria serum with the anti-tumor function and the preparation method and application of the malaria serum with the anti-tumor function provide theoretical and experimental bases for further researches on tumor cell apoptosis induction factors generated by plasmodia, breaks a new path for rediscovery pathogenesis of critical and cerebral malaria cases and epidemiologic researches of various plasmodium species and strains, and make new effort in application of the malaria serum or preparations thereof to preparation of medicines for treatment or prevention of the tumors, thereby having great scientific research values and significances.
Owner:GUANGXI ZHUANG AUTONOMOUS REGION CENT FOR DISEASE CONTROL & PREVENTION
Method for detecting canine distemper virus by using one-step RT-PCR method
InactiveCN101974653AHigh sensitivityQuick checkMicrobiological testing/measurementCanine distemper virus CDVEpidemiologic study
The invention discloses a method for detecting canine distemper virus by using a one-step reverse transcription-polymerase chain reaction (RT-PCR) method. The method comprises the following steps of: preprocessing a sample for the RT-PCR detection; extracting total ribonucleic acid (RNA); obtaining a specific amplification product by one-step RT-PCR reaction; and performing 1 percent agarose gel electrophoresis identification on the amplification product. The method of the invention has the characteristics of high flexibility, high specificity, simple and convenient operation procedure, low contamination rate and reliable detection result; the acquired sequence information is directly used for analyzing genetic derivation relation of an epidemic strain; and the method is suitable for etiological diagnosis and epidemiological research of canine distemper.
Owner:ZHENGZHOU HOUYI PHARMA
Kit for detecting ST251-type virulent aeromonas hydrophila and application
ActiveCN105969907AIncreased sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesDiseaseGenomic DNA
The invention discloses a PCR kit for detecting ST251-type aeromonas hydrophila and application. The kit comprises a PCR Premix, a primer pair, a standard positive DNA template, a standard negative DNA template and sterilization double distilled water. An upstream primer sequence is 5'-GCGGCGTAAGCGGATTTGTAGC-3', a downstream primer sequence is 5'-CCGGTCGGATTGGTCAGGTTCAT-3', the annealing temperature is 63 DEG C, a correct amplification product is 826bp in size, the standard positive DNA template is genomic DNA extract of an ST251-type aeromonas hydrophila XS91-4-1 strain, and the DNA concentration is 500 nanograms per microliter; the negative DNA template is genomic extractive of an aeromonas veronii IB340 strain, and the DNA concentration is 500 nanograms per microliter. The kit is applicable to all kinds of gene amplification instruments, high in detection specificity and sensitivity, quick, accurate and good in stability. The kit can be used for quickly diagnosing freshwater fish outbreak diseases and blood poisoning caused by aeromonas hydrophila of other fishes and quickly detecting and identifying bacteria and then is widely applied to disease prediction and forecast and epidemiologic studies.
Owner:INST OF AQUATIC LIFE ACAD SINICA
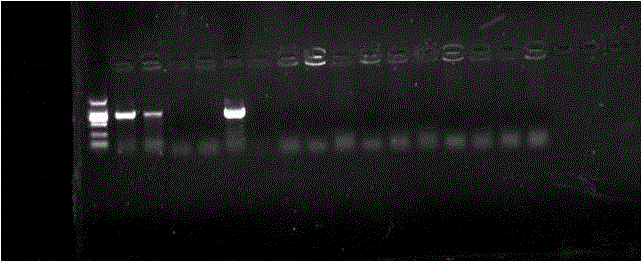
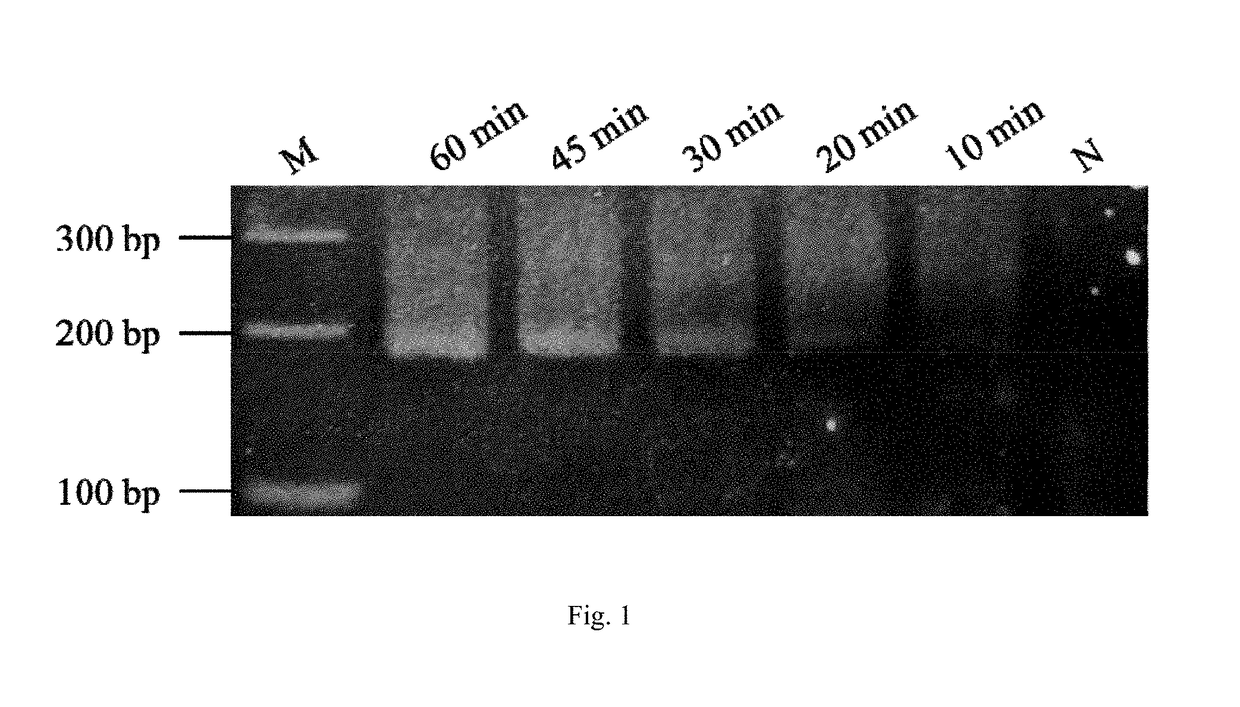
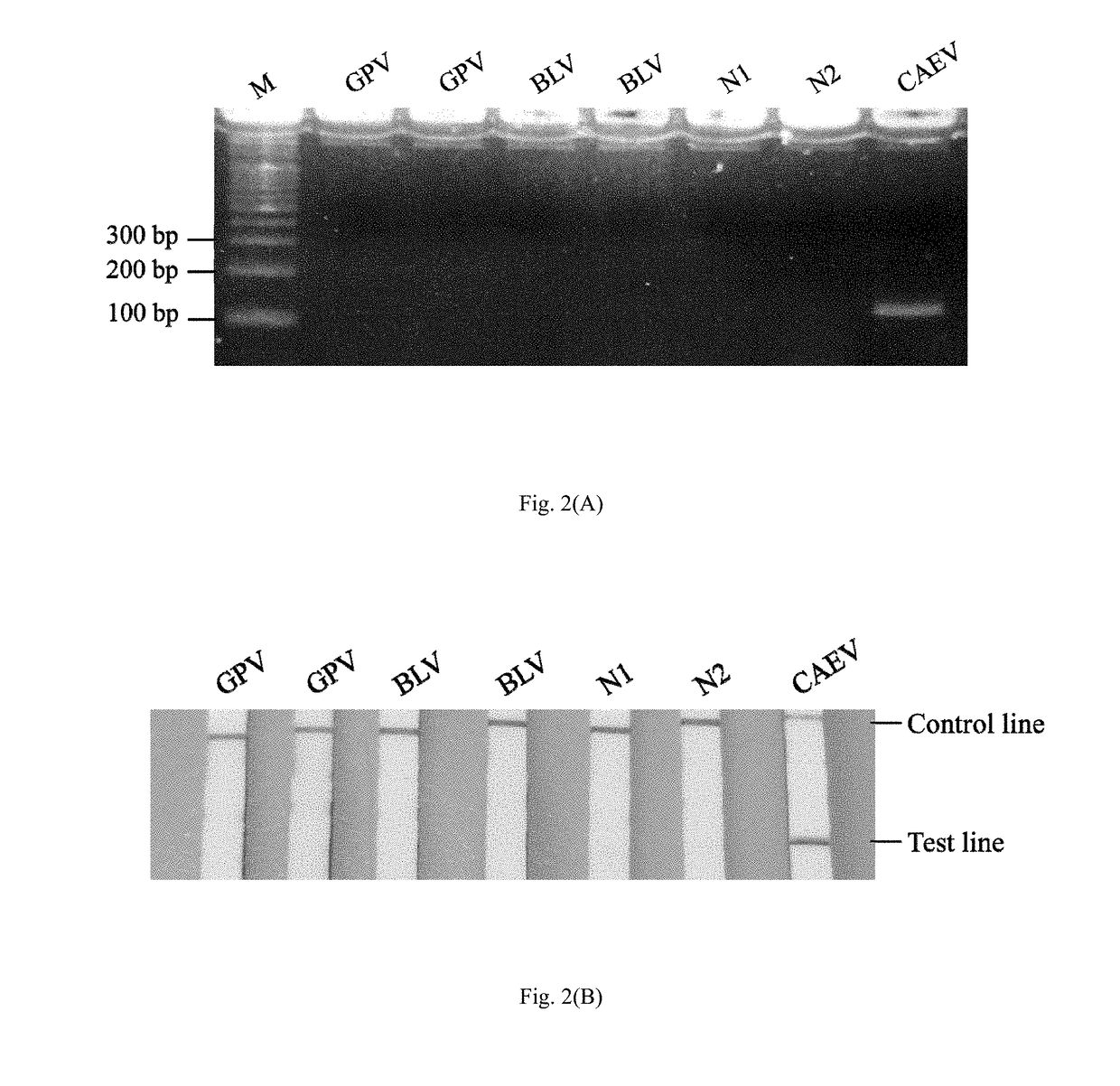
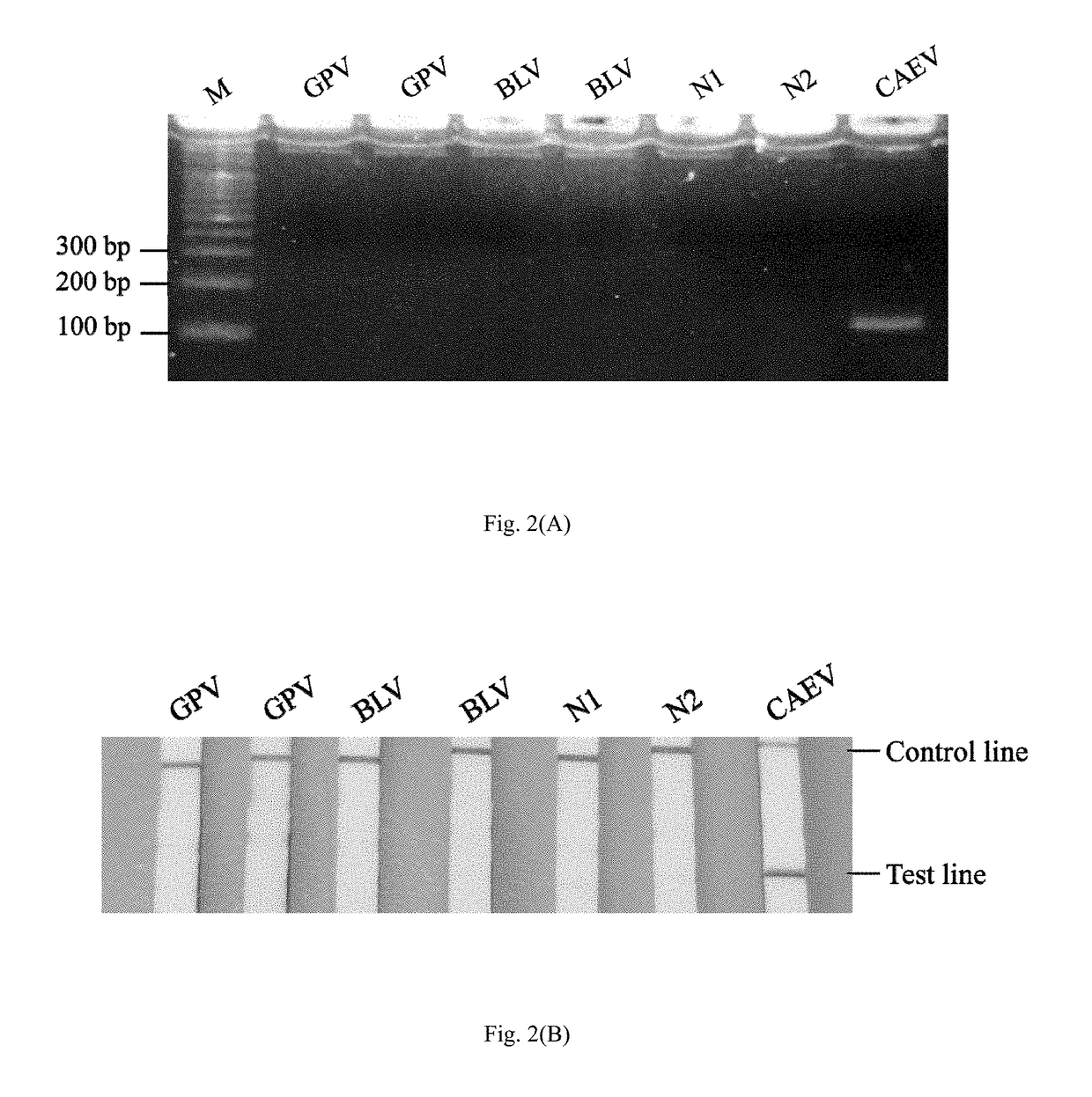
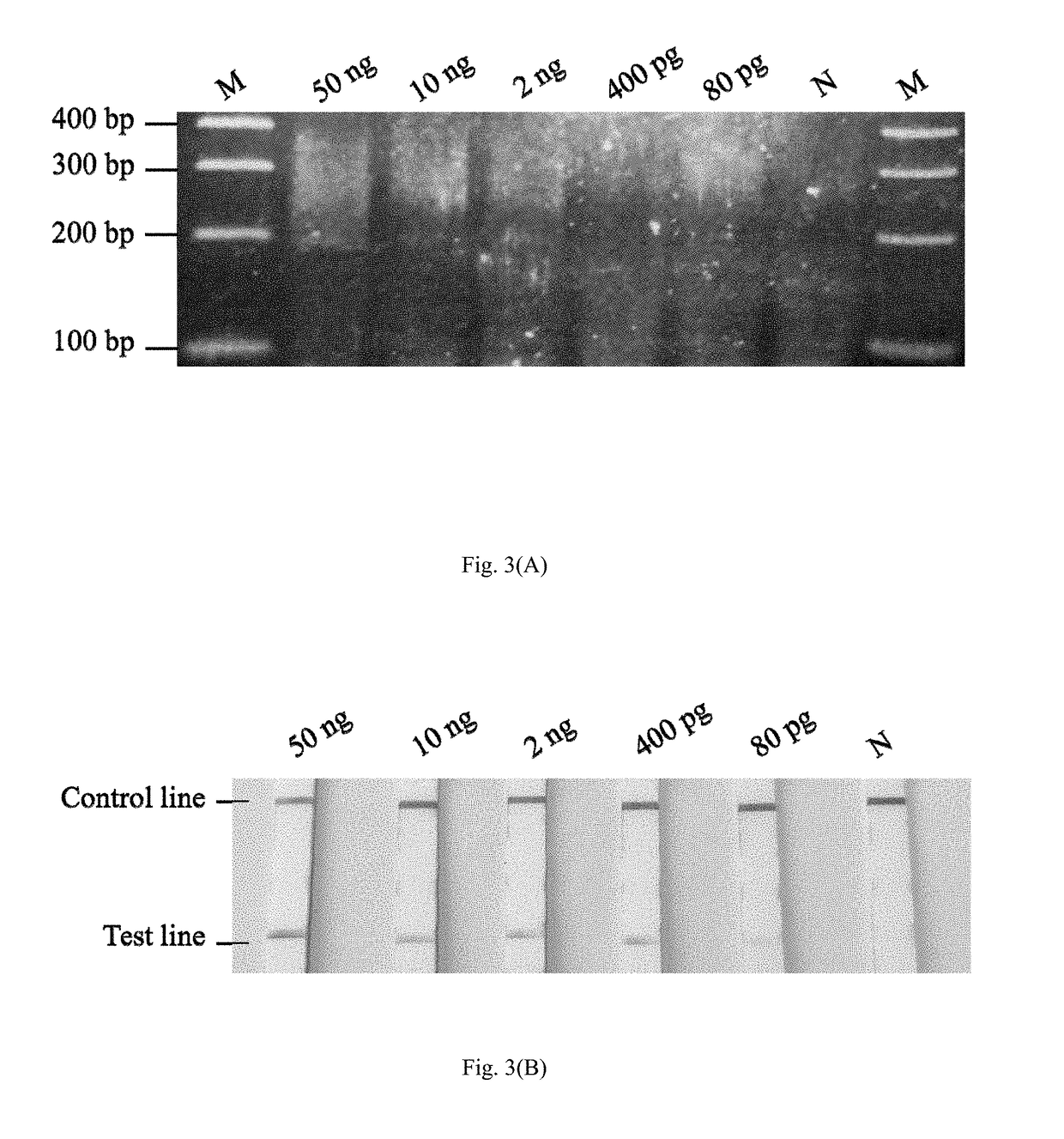
Method and kit for the field diagnosis of caprine arthritis-encephalitis virus (CAEV) infection
ActiveUS20180237868A1Avoid lateral flowMicrobiological testing/measurementMaterial analysisProviral dnaEncephalitis Viruses
The invention is to provide a method and kit based on recombinase polymerase amplification (RPA) and lateral flow dipstick (LFD) for detection of caprine arthritis-encephalitis virus (CAEV) infection. The method and kit are suitable for both laboratory and field application, and are specific and sensitive for detecting CAEV proviral DNA in goats in a fast manner. The method and lit of the invention are also applicable for on-site utilization at farms and should be useful in both eradication programs and epidemiological studies.
Owner:NAT TAIWAN UNIV
Hybrid snakehead rhabdovirus fluorescent quantitative PCR (polymerase chain reaction) detection kit and detection method thereof
ActiveCN103397106AStrong specificityQuick QualificationMicrobiological testing/measurementFluorescence/phosphorescenceRhabdovirus carpioEpidemiologic study
The invention discloses a hybrid snakehead rhabdovirus fluorescent quantitative PCR (polymerase chain reaction) detection kit and a detection method thereof. The kit contains specific primers HSHRV-2F and HSHRV-2R and a probe HSHRV-2probe. When the kit is used for detecting hybrid snakehead rhabdovirus, the kit has the characteristics of high sensitivity, high repetitiveness and good specificity, can quickly and accurately realize qualitative and quantitative detection of hybrid snakehead rhabdovirus, and is of far reaching importance in early diagnosis of hybrid snakehead virus diseases, molecular epidemiology research, prevention and control technology research and the like.
Owner:湖南振业动物检验有限公司
Method for simultaneously detecting three groups of rotaviruses A, B and C
InactiveCN110714097AImprove detection accuracyStrong specificityMicrobiological testing/measurementMicroorganism based processesEpidemiologic studyMicrobiology
The invention discloses a method for simultaneously detecting three groups of rotaviruses A, B and C, and belongs to the field of biological detection. A pair of specific primers and a specific probewith different fluorescent labels are designed for target genes of group A rotavirus, group B rotavirus and group C rotavirus, a pair of universal primers is further designed, and during PCR amplification, the specific primers with universal primer tags are used for enrichment and amplification; and then universal primer tags are used for exponential amplification, and fluorescence signals of exponential amplification products are collected for detection. The method can simultaneously detect the group A rotavirus, the group B rotavirus, and the group C rotavirus. The method is high in specificity, high in sensitivity and short in detection time, and can be used for early rapid diagnosis of rotavirus infection and epidemiological study of the rotavirus.
Owner:山东凯景生物技术有限公司
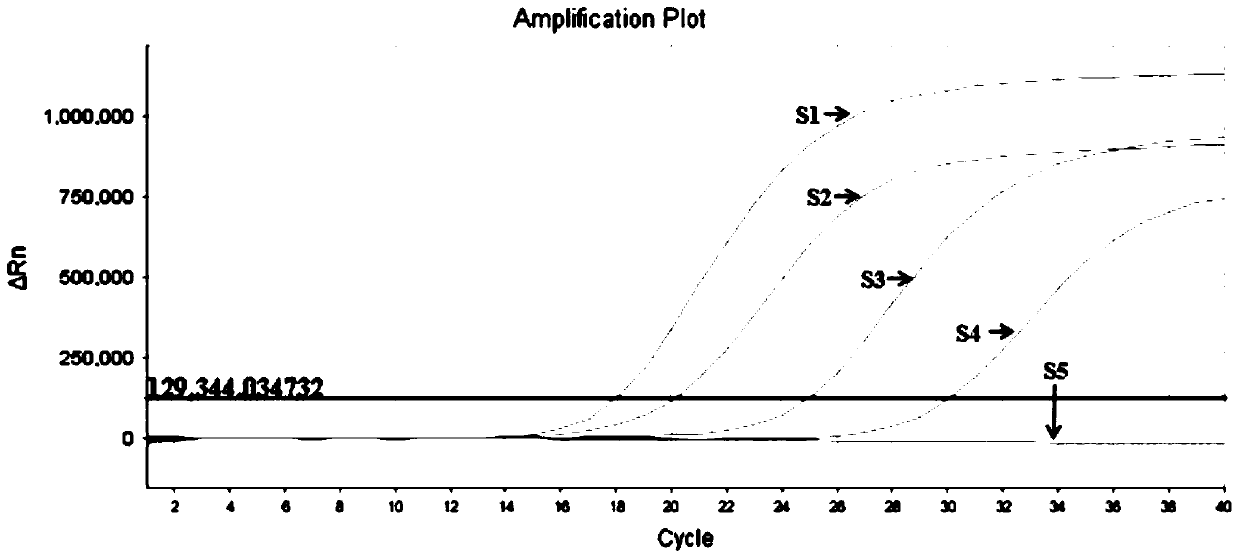
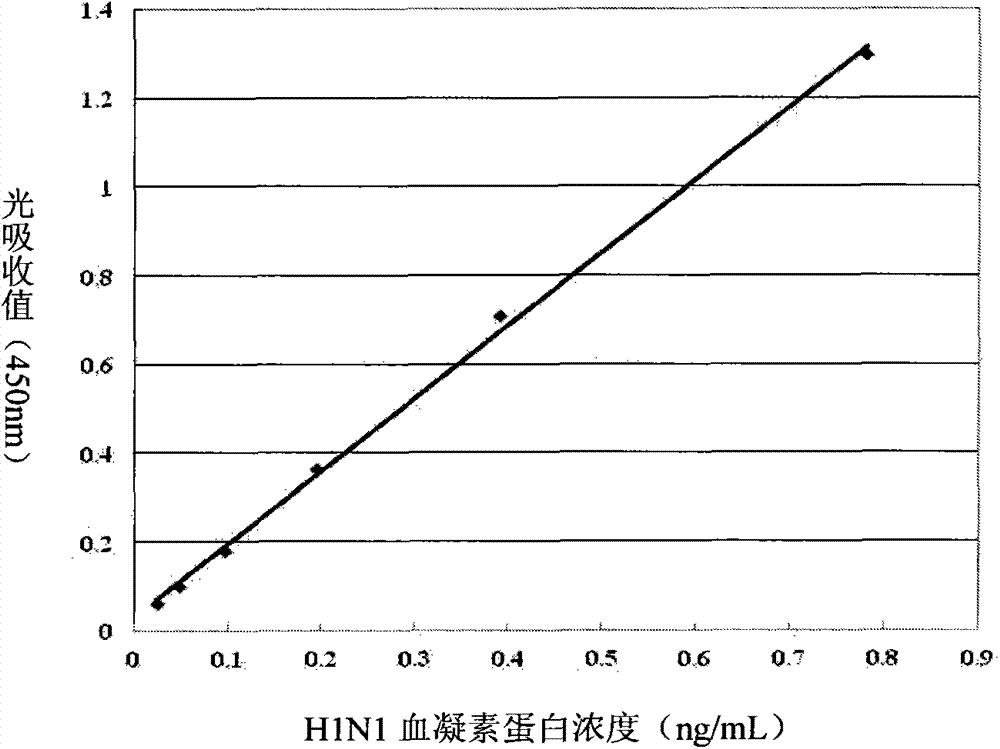
A monoclonal antibody for H1N1 swine influenza A virus hemagglutinin protein and a double-antibody sandwich ELISA kit
ActiveCN104744590AQuick checkThe inspection method is convenient and easyImmunoglobulins against virusesBiological testingElisa kitInfluenza virus A hemagglutinin
A monoclonal antibody for H1N1 swine influenza A virus hemagglutinin protein and a double-antibody sandwich ELISA kit are disclosed. The kit comprises a solid phase vector coated with the monoclonal antibody, a rabbit polyclonal antibody labeled by horseradish peroxidase, an H1N1 hemagglutinin protein standard substance, a sample diluting liquid, a washing liquid, a substrate coloring liquid and a reaction terminating liquid. The kit is good in sensitivity, capable of performing quantitative detection for the swine influenza virus hemagglutinin protein, and specifically recognizing main prevalent strains of A(H1N1) influenza outbreak in 2009, and free of cross reactions with other strains of the H1N1 influenza viruses and other influenza virus subtypes. The kit is simple to operate, can be used for supporting fundamental research of the H1N1 swine influenza virus, and has important significance for performing epidemiologic study on the H1N1 influenza viruses in different zones and different years.
Owner:SINO CELL TECH INC
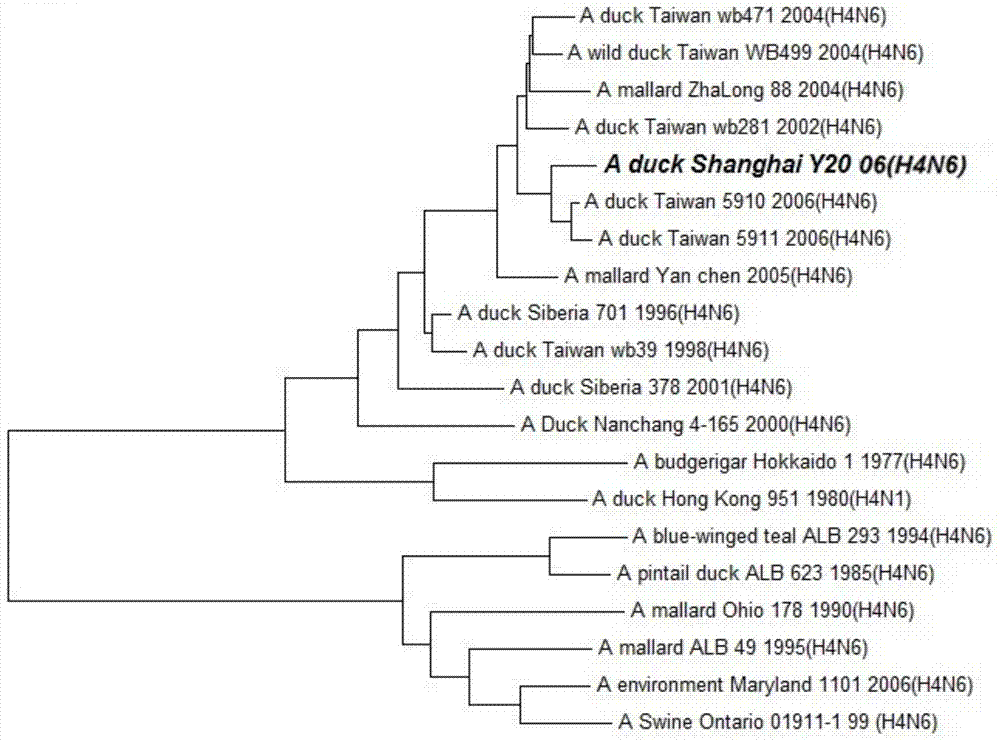
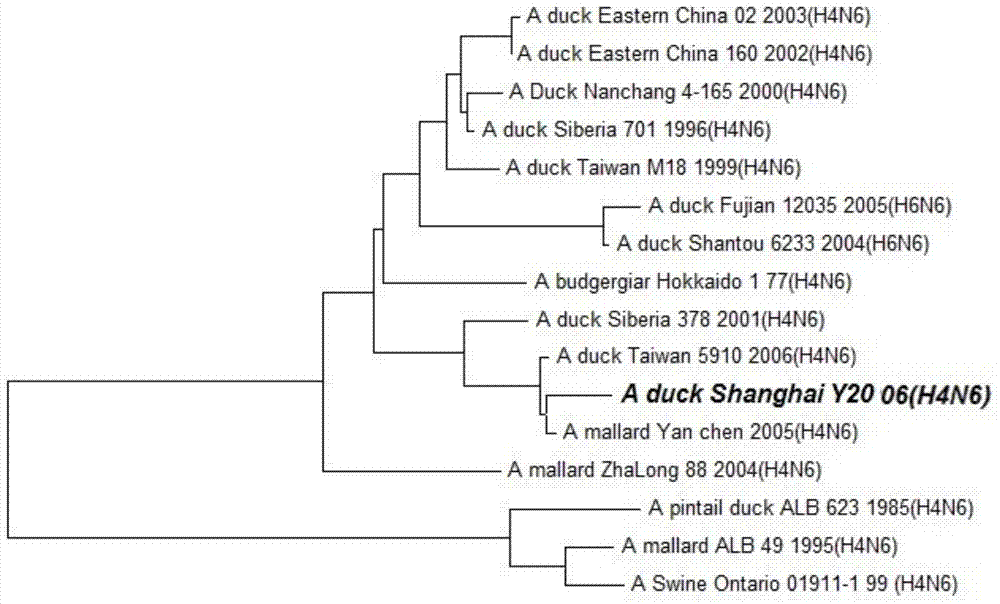
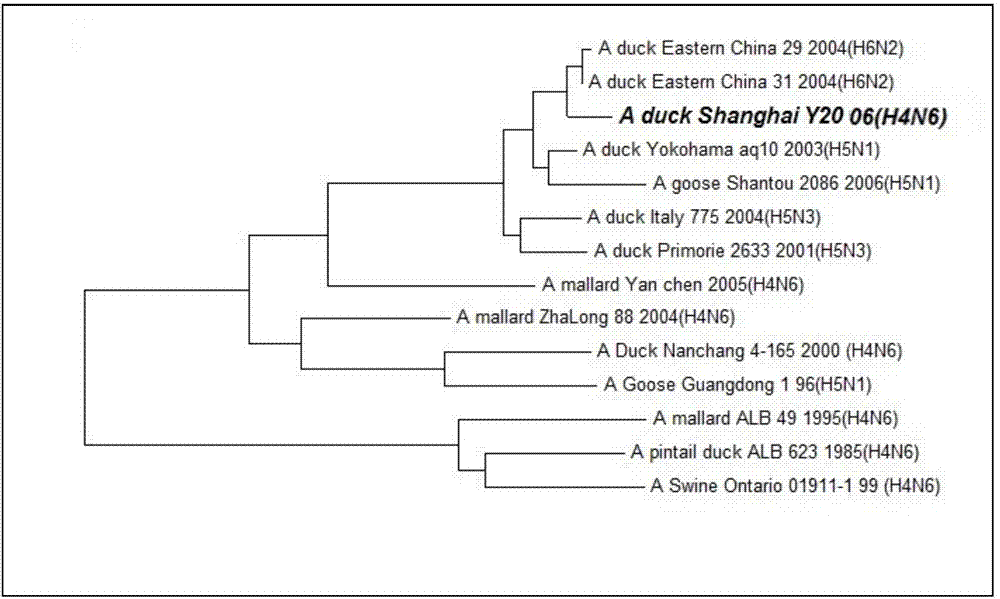
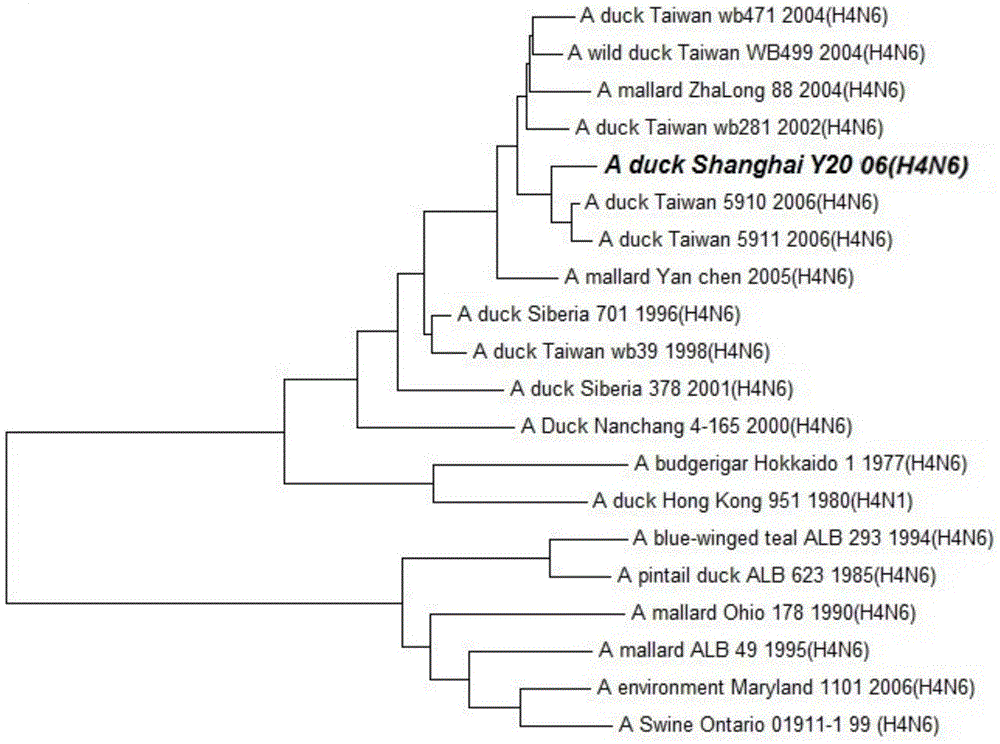
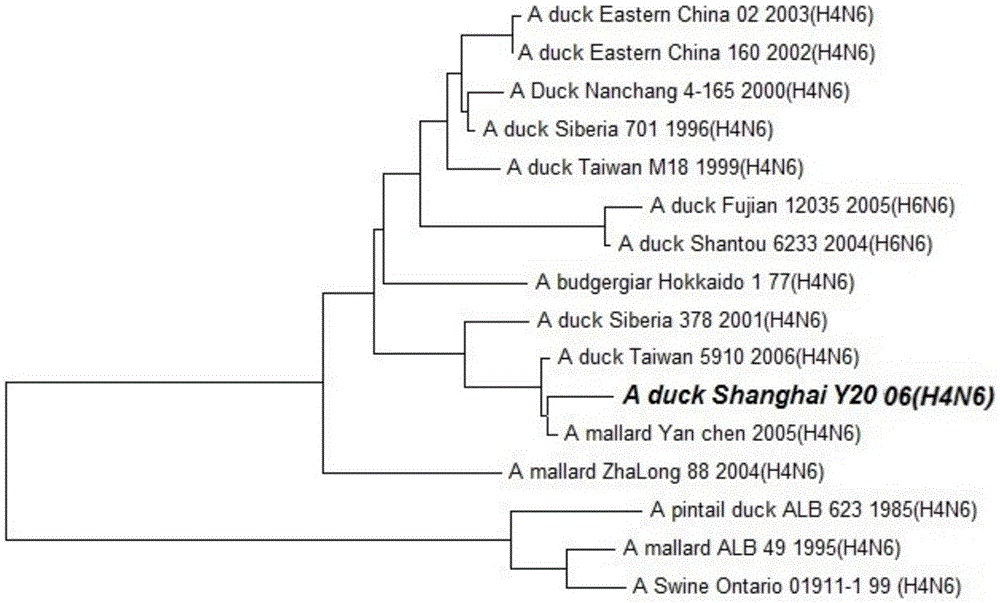
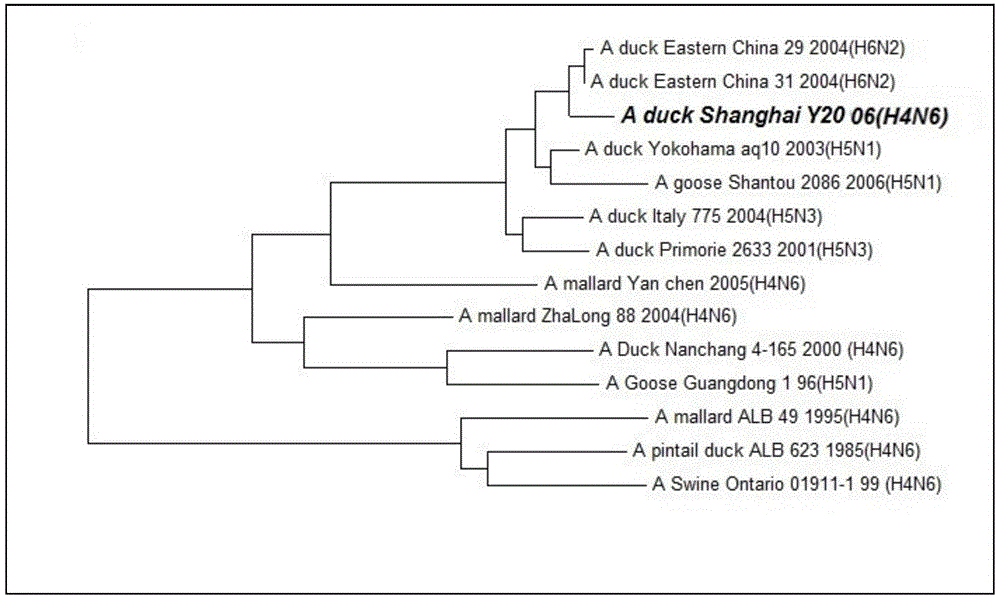
Duck-sourced H4N6 subtype avian influenza virus strain and application thereof
InactiveCN103571799AMicroorganism based processesAntiviralsAvian influenza virusWhole genome sequencing
The invention discloses a duck-sourced H4N6 subtype avian influenza virus strain and an application thereof. The preservation No. of the virus strain is CGMCC NO.7606. The virus strain is duck-sourced H4N6 subtype avian influenza virus containing 8 fragments of which the CDS sequence shown in SEQ ID NO.1-SEQ ID NO.8, wherein the 8 fragments respectively correspond to PB2, PB1, PA, HA, NP, NA, M and NS. Whole genome sequencing is carried out on the virus strain, and the information is used for research on the detection, distribution and epidemic rule of the avian influenza virus as well as control of prevalence and spreading of the avian influenza. The virus strain supplements valuable genetic information of the H4N6 subtype avian influenza virus in China, provides valuable information for molecular evolution and epidemiologic study of H4N6 subtype avian influenza virus and can be used for preparing reagents for rapidly diagnosing H4N6 subtype influenza virus infection and vaccine study.
Owner:SHANGHAI ANIMAL EPIDEMIC PREVENTION & CONTROL CENT
Related gene, method, primer group and kit for identifying mycobacterium tuberculosis complex flora and detecting drug resistance
InactiveCN113249502AOvercoming sensitivityOvercome the cycleMicrobiological testing/measurementFermentationEpidemiologic studyMycobacterium
The invention provides a related gene, method, primer group and kit for identifying mycobacterium tuberculosis complex flora and detecting drug resistance. According to the related gene, method, primer group and kit, not only can the mycobacterium tuberculosis complex flora be identified, but also 16 common anti-tuberculosis first-line and second-line drug resistance gene mutations of the mycobacterium tuberculosis complex flora can be distinguished and detected at high resolution, and rapid, simultaneous and accurate drug resistance detection is achieved. The related gene, method, primer group and kit can be used for clinical treatment reference and epidemiological research.
Owner:上海康黎诊断技术有限公司
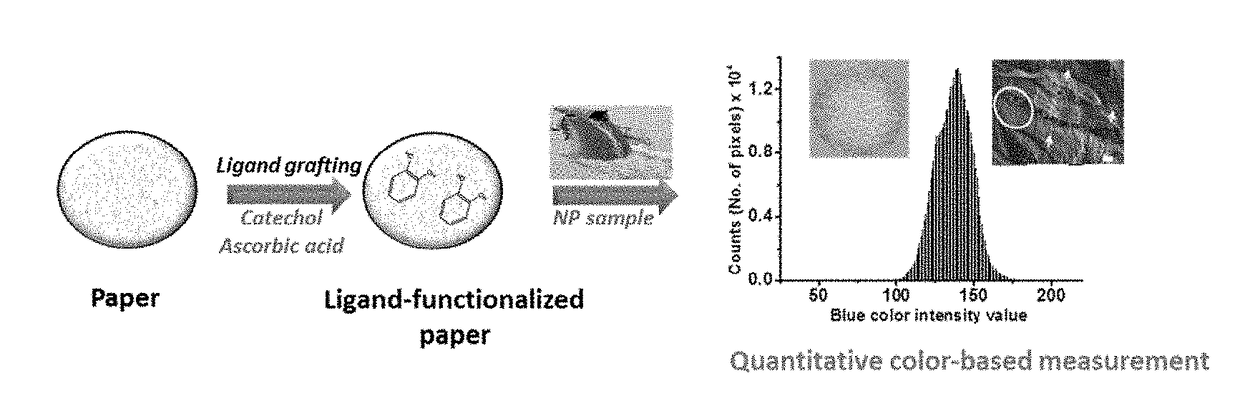
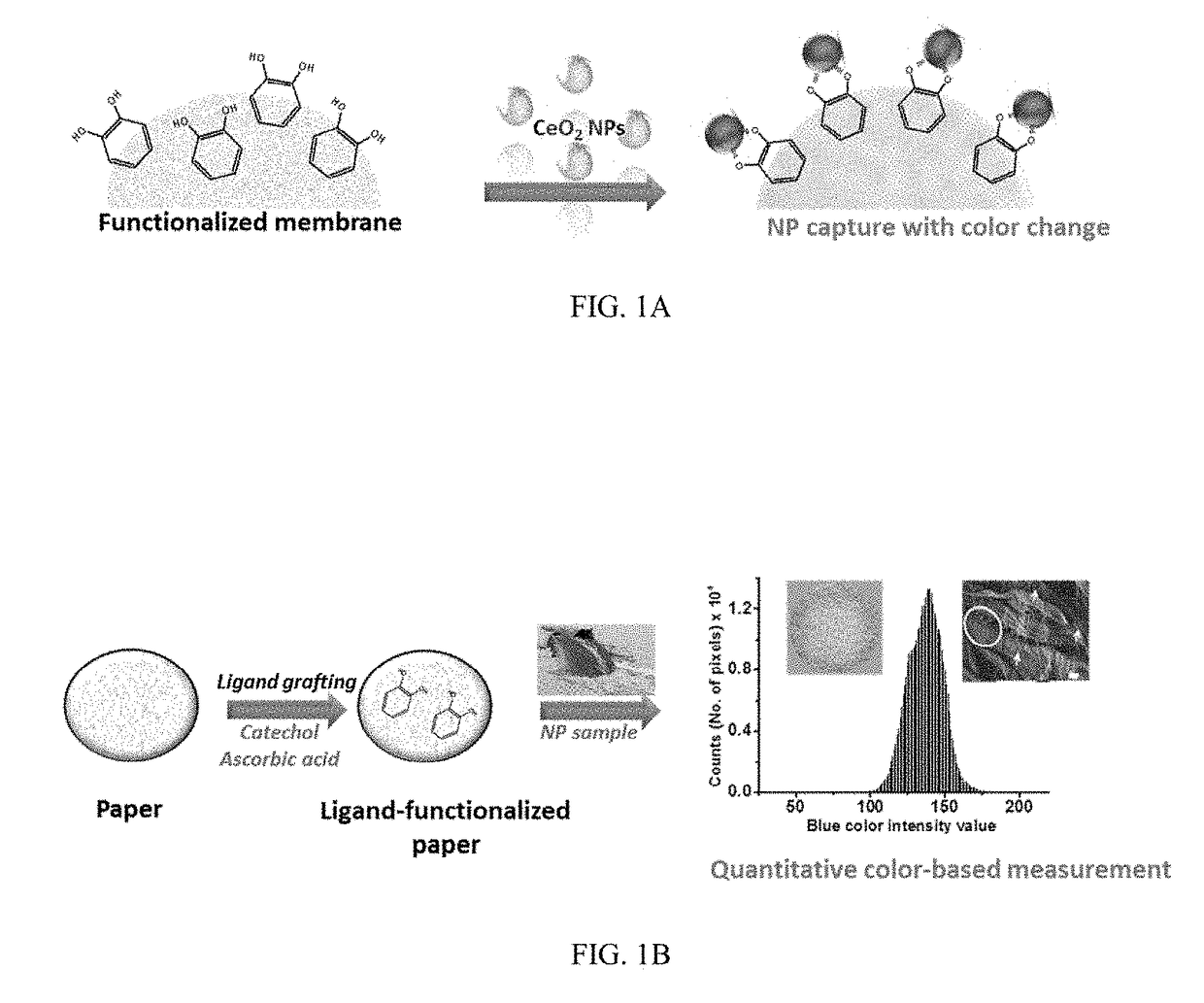
Functional Platform for Rapid Capture and Removal of Nanoparticles
ActiveUS20180022604A1Facilitate rapid assessmentEasy to measureWater treatment parameter controlWater treatment compoundsBinding siteBiochemical engineering
Device, method, and system for nanoparticle capture, tracking, and / or detection. A functional paper-based platform is modified with capture ligands to create binding sites for nanoparticles. According to an embodiment, nanoparticle binding produces visual images of the particle content and distribution on the modified sensing surface, which provides capabilities for both NP sequestration and real-time detection. According to an embodiment the system may be utilized for environmental decontamination, fabrication of personal protective equipment, field monitoring, and epidemiological studies. The availability of inexpensive and easy-to-use quantitative methods can facilitate rapid assessment and measurement of NPs concentration and the level of exposure for large scale toxicological and epidemiological testing
Owner:CLARKSON UNIVERSITY
Kit for detecting foot-and-mouth disease type-A virus as well as preparation and using methods of kit
ActiveCN104212916AMicrobiological testing/measurementMicroorganism based processesDiseaseElectrophoreses
The invention discloses a kit for detecting a foot-and-mouth disease type-A virus, a preparation method of the kit and a using method of the kit used for achieving aims except for disease diagnosis. Primers for amplifying the foot-and-mouth disease type-A virus have four gene sequences; and the kit for detecting the foot-and-mouth disease type-A virus internally comprises the four primers with the gene sequences. Relevant experiments prove that nucleic acid capable of specifically and rapidly amplifying the foot-and-mouth disease type-A virus is capable of detecting a specific band through nucleic acid electrophoresis and also has specificity. The relevant experiments hint that the sequences can be applied to preparation of GeXP diagnosis kits for rapidly identifying the foot-and-mouth disease type-A virus and epidemiologic study of the foot-and-mouth disease virus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
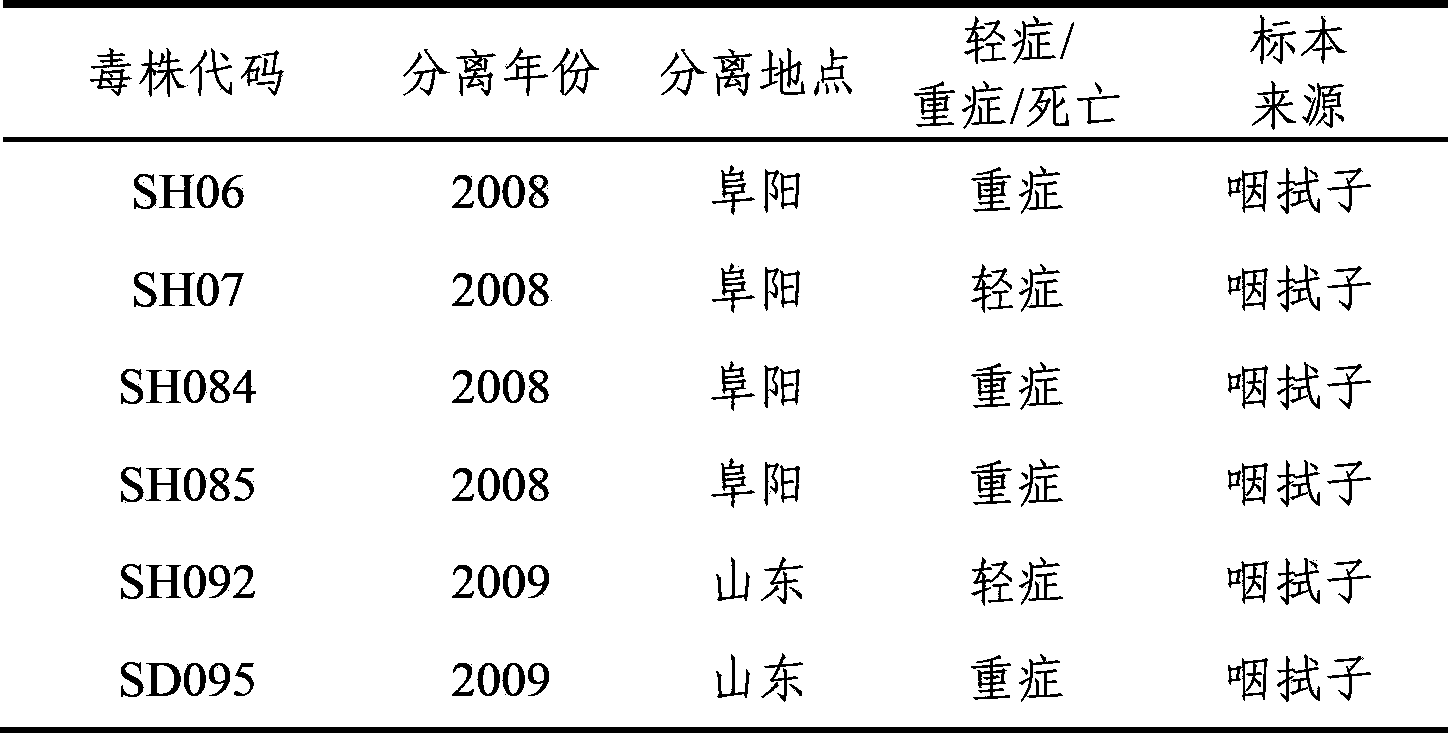
Human enterovirus 71-type C4-subtype lethal strain SD095 and its application
ActiveCN103834617AHigh titerHigh purityMicroorganism based processesImmunoglobulins against virusesDiseaseClinical study
The invention provides a human enterovirus71-type C4-subtype lethal strain SD095 with the accession number of CGMCC (China General Microbiological Culture Collection Center) NO.6601 and its application. A rat adaptive lethal strain is acquired by conventional virus isolation cultivation, animal infection and a series of technical means, and an animal lethal model based on the virus strain SD095 can be established. The acquisition of the lethal virus strain and the establishment of the animal lethal model have important significance for promoting clinical treatment and researches in China on diseases and death cases caused by EV71 (human enterovirus 71) virus, and have important values for further promotion of experimental and clinical studies and epidemiologic studies on the EV71, and new vaccine development and evaluation.
Owner:SINOVAC BIOTECH
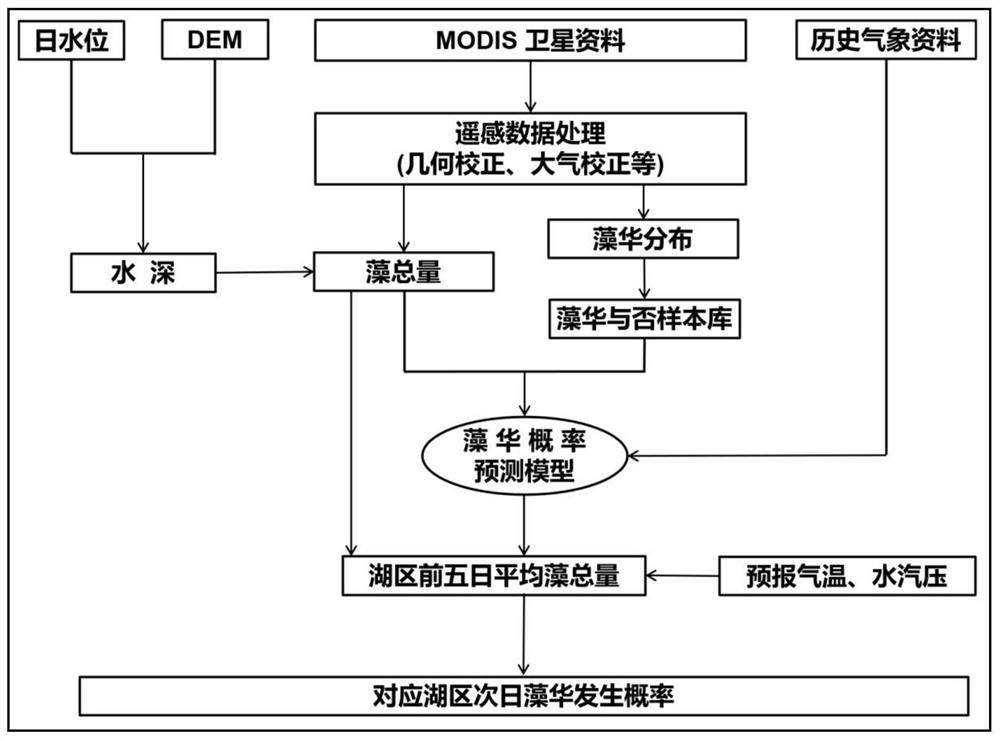
Algae bloom prediction method based on remote sensing total algae quantity
ActiveCN112989281AImplements probability of occurrence (continuous) predictionImprove scalabilityScattering properties measurementsComplex mathematical operationsSoil scienceEpidemiologic study
The invention relates to an algal bloom prediction method based on remote sensing total algae quantity, which comprises the following steps: performing big data analysis on algal bloom occurrence condition, remote sensing total algae quantity and different meteorological indexes monitored by remote sensing in historical periods, and then constructing a Logistic prediction model of algal bloom occurrence probabilities in different lake areas, thereby realizing algal bloom occurrence probability prediction based on the remote sensing total algae quantity. According to the method, Logistic regression analysis which is mostly used in epidemiological research is adopted, and the (continuous) prediction of the algal bloom occurrence probability is realized.
Owner:NANJING INST OF GEOGRAPHY & LIMNOLOGY
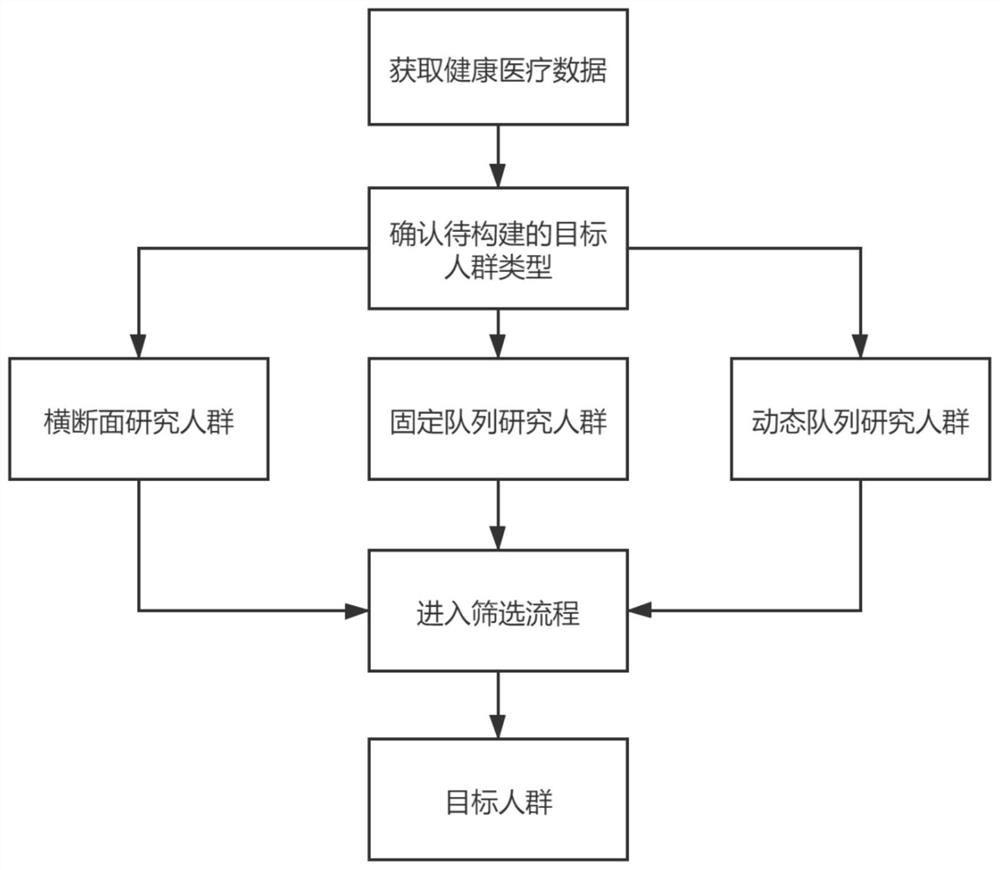
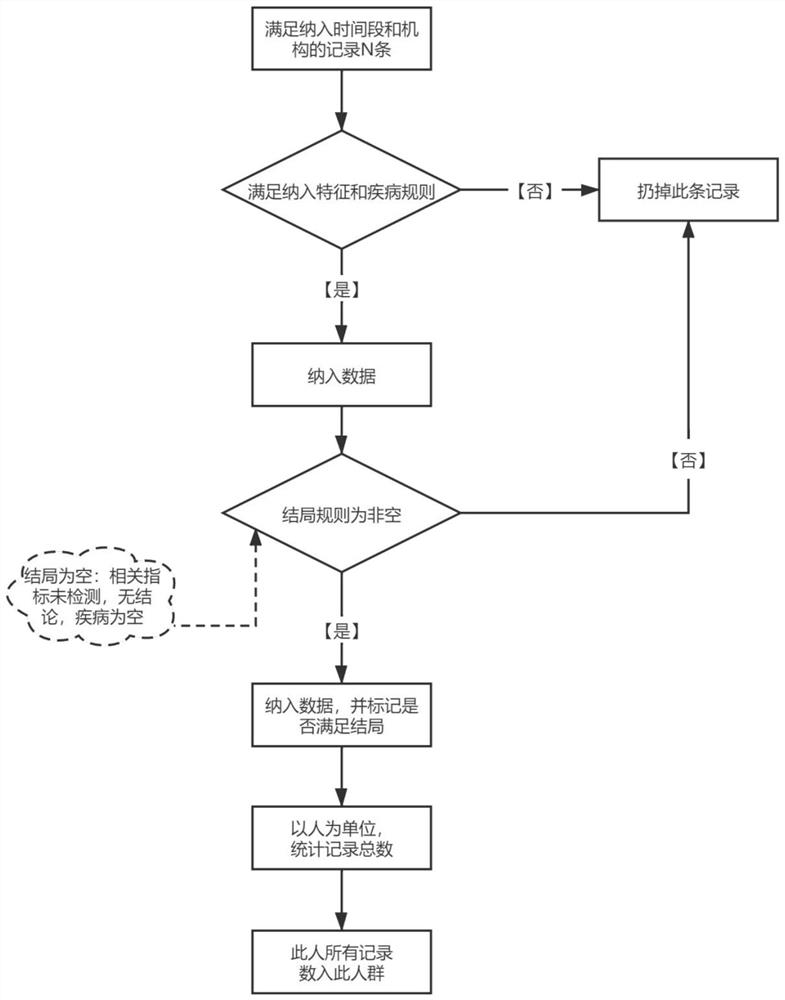
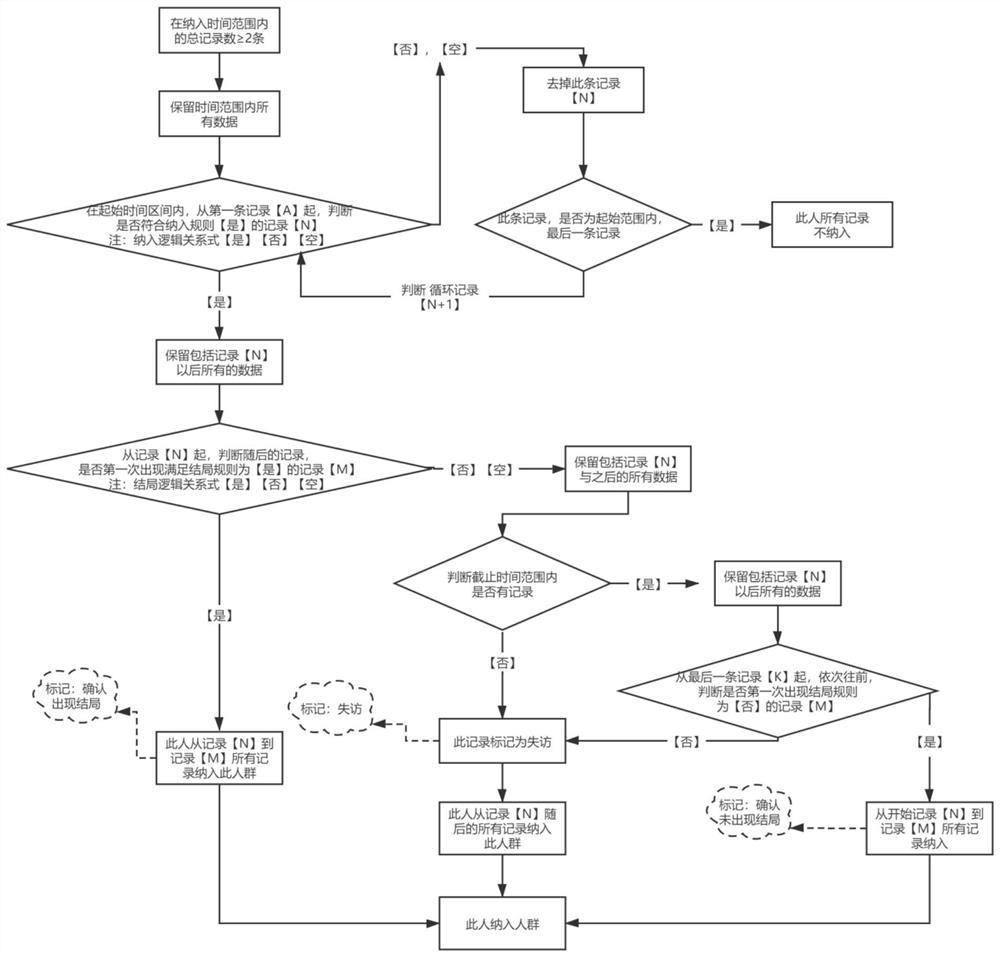
Medical big data-based epidemiological research population screening method and storage medium
ActiveCN112967817AImprove screening efficiencyImprove accuracyEpidemiological alert systemsEngineeringEpidemiologic study
The invention discloses an epidemiological research population screening method based on medical big data and a storage medium. The method comprises the following steps: acquiring health medical data; determining to-be-constructed target population types according to requirements of epidemiological research, wherein the to-be-constructed target population types comprise a cross section research population, a fixed queue research population and a dynamic queue research population; setting screening conditions including a research time range, follow-up visit time, a time window, an inclusion rule and an ending rule; and screening records meeting the requirements of the target crowd from the health medical data, and constructing the target population. According to the medical big data-based epidemiological research population screening method and the storage medium, the target population is directly screened from the existing massive medical data to be brought into epidemiological research, and the target population comprises a cross section research population, a fixed queue research population and a dynamic array research population; the efficiency and correctness of epidemiological research population screening based on medical big data are improved, and manpower is saved.
Owner:WUHAN UNIV
A kit for detecting st251-type pathogenic Aeromonas hydrophila and its application
ActiveCN105969907BIncreased sensitivityStrong specificityMicrobiological testing/measurementMicroorganism based processesBiotechnologyDisease
The invention discloses a PCR kit for detecting ST251-type aeromonas hydrophila and application. The kit comprises a PCR Premix, a primer pair, a standard positive DNA template, a standard negative DNA template and sterilization double distilled water. An upstream primer sequence is 5'-GCGGCGTAAGCGGATTTGTAGC-3', a downstream primer sequence is 5'-CCGGTCGGATTGGTCAGGTTCAT-3', the annealing temperature is 63 DEG C, a correct amplification product is 826bp in size, the standard positive DNA template is genomic DNA extract of an ST251-type aeromonas hydrophila XS91-4-1 strain, and the DNA concentration is 500 nanograms per microliter; the negative DNA template is genomic extractive of an aeromonas veronii IB340 strain, and the DNA concentration is 500 nanograms per microliter. The kit is applicable to all kinds of gene amplification instruments, high in detection specificity and sensitivity, quick, accurate and good in stability. The kit can be used for quickly diagnosing freshwater fish outbreak diseases and blood poisoning caused by aeromonas hydrophila of other fishes and quickly detecting and identifying bacteria and then is widely applied to disease prediction and forecast and epidemiologic studies.
Owner:INST OF AQUATIC LIFE ACAD SINICA
Bovine monocyte chemoattractant protein-1 hybridoma cell line, its secreted monoclonal antibody and its application
ActiveCN108048408BHigh potencyStrong specificityBiological material analysisImmunoglobulins against cytokines/lymphokines/interferonsEpitopeImmunofluorescence
The invention provides a hybridoma cell strain 4G9 secreting a bovine monocyte chemoattractant protein 1 (MCP-1) resisting monoclonal antibody as well as a monoclonal antibody MAb 4G9 secreted by same. The monoclonal antibody MAb 4G9 has the advantages of high titer, good specificity and high affinity with natural bovine MCP-1 antigen, and a Western-Blot detection kit, a direct immunofluorescence(DFA) detection kit and a flow cytometry (FCM) detection kit established on the monoclonal antibody MAb 4G9 have good sensitivity and specificity and can be used for detecting recombinant bovine MCP-1, detecting in vitro tissues, researching and identifying the epitope, performing the import and export inspection and quarantine, and performing the non-diagnosis research such as epidemiologic studyand the like.
Owner:YANGZHOU UNIV
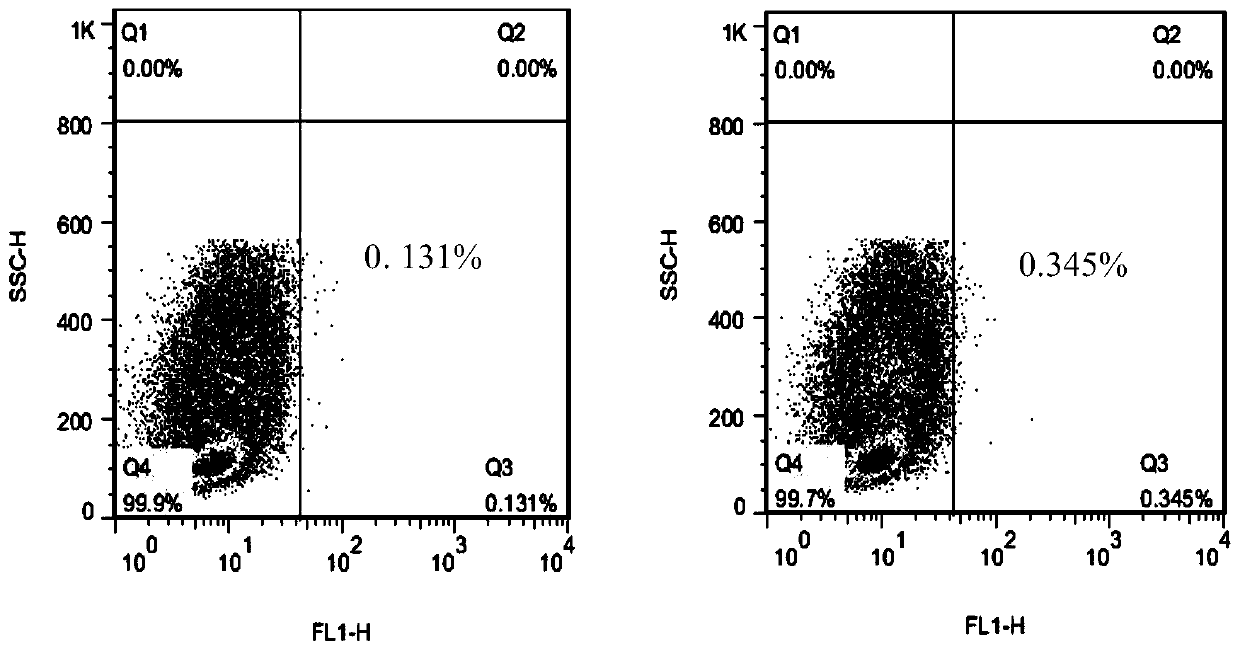
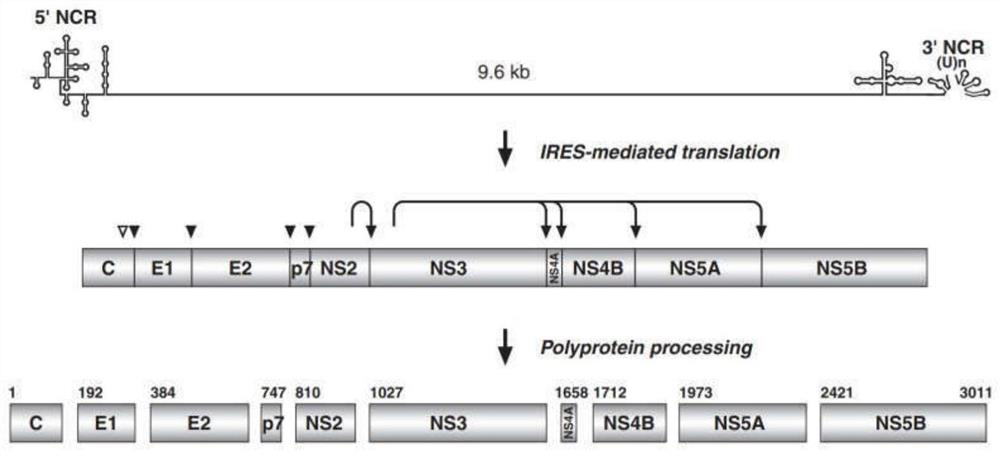
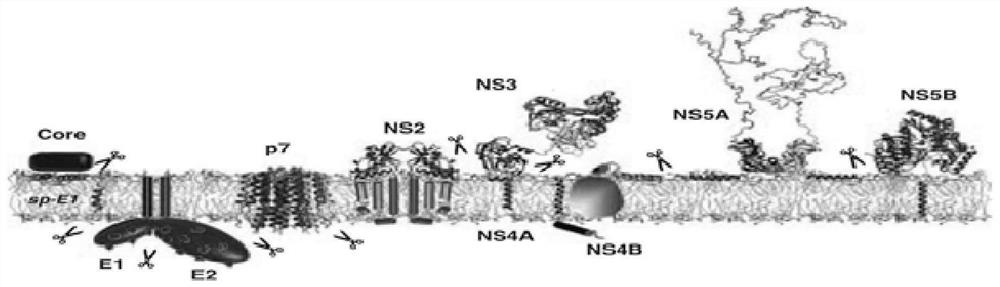
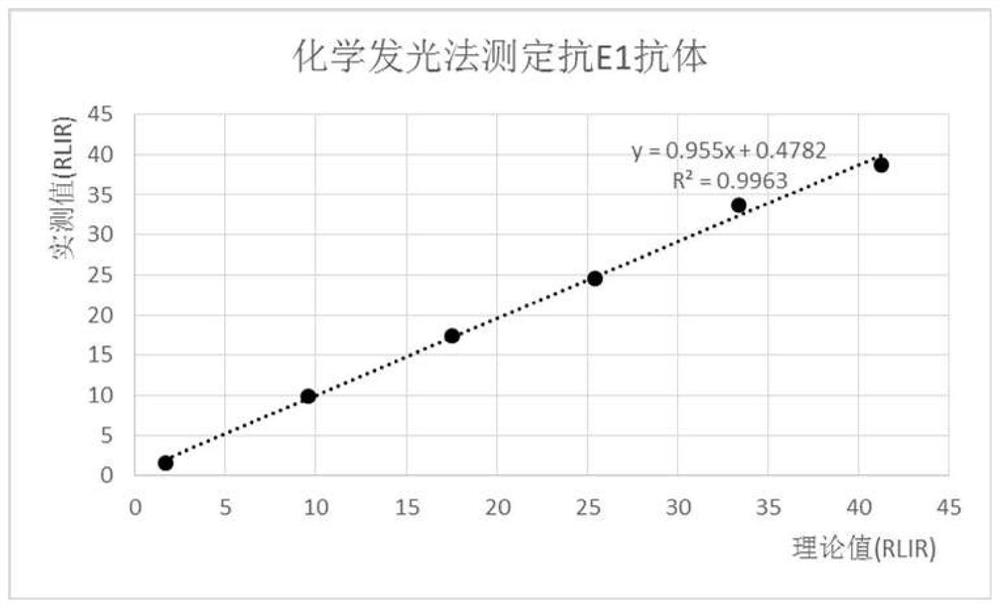
Chemiluminiscence kit for detecting anti-envelope glycoprotein antibody in serum of HCV infected person, and detection method
PendingCN112255412AHigh catalytic efficiencyIncreased sensitivityChemiluminescene/bioluminescenceBiological testingDisease monitoringAntiendomysial antibodies
The invention relates to the technical field of biology, and discloses a chemiluminiscence kit for detecting anti-envelope glycoprotein (anti-E1 and anti-E2) antibodies in serum of an HCV infected person. The kit comprises a microwell plate obtained by coating with a recombinant HCV envelope glycoprotein (E1, E2) antigen, and a sample diluent, a negative control, a positive control, an enzyme marker working solution, a substrate solution A, a substrate solution B and a washing solution which are independently packaged, wherein the substrate solution A comprises luminol, o-phenylphenol, 4-imidazole phenol and a carbonic acid buffer solution (CB), and the substrate solution B comprises urea peroxide and a phosphate buffer solution (PB). According to the invention, the kit can be used for carrying out in-vitro qualitative / quantitative determination on anti-E1 and anti-E2 antibodies in serum of an HCV infected person, establishing a detection technology of the anti-E1 and anti-E2 antibodies in the serum of the HCV infected person and carrying out methodological evaluation, and can be applied to screening diagnosis, disease monitoring, prognosis evaluation, disease mechanism and epidemiological research of the HCV infected person.
Owner:中国人民解放军联勤保障部队第九0三医院
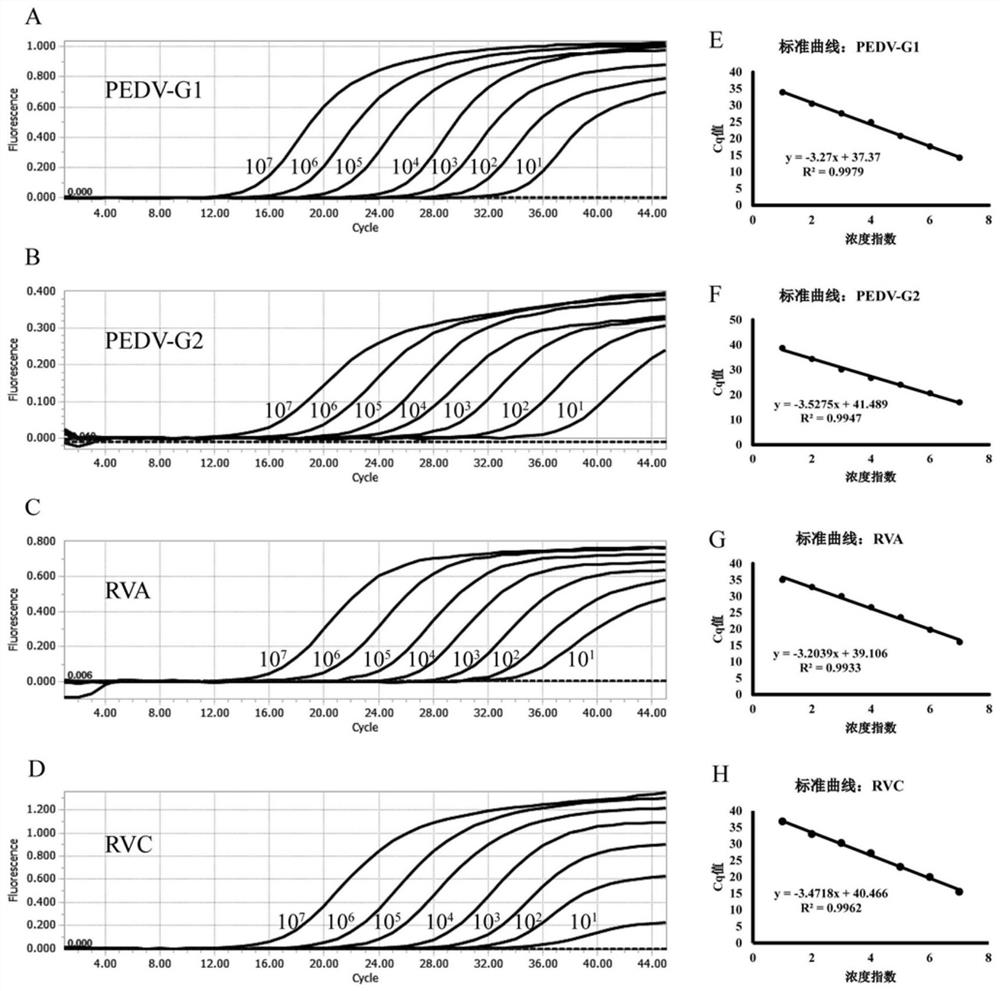
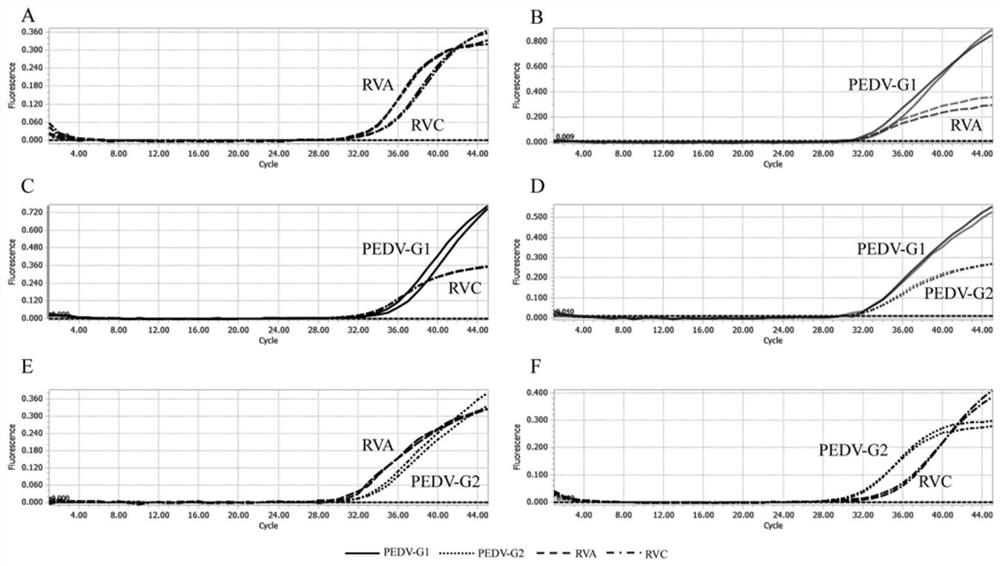
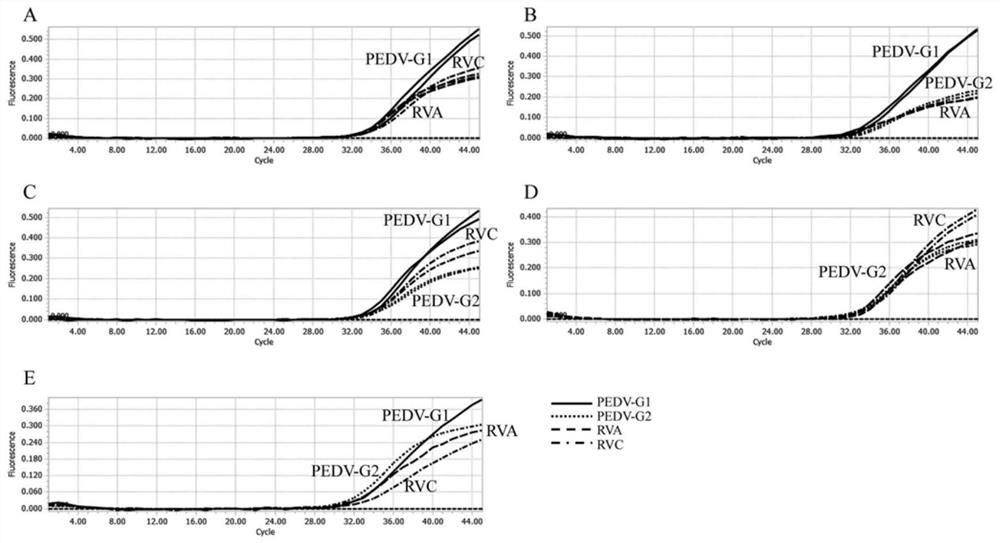
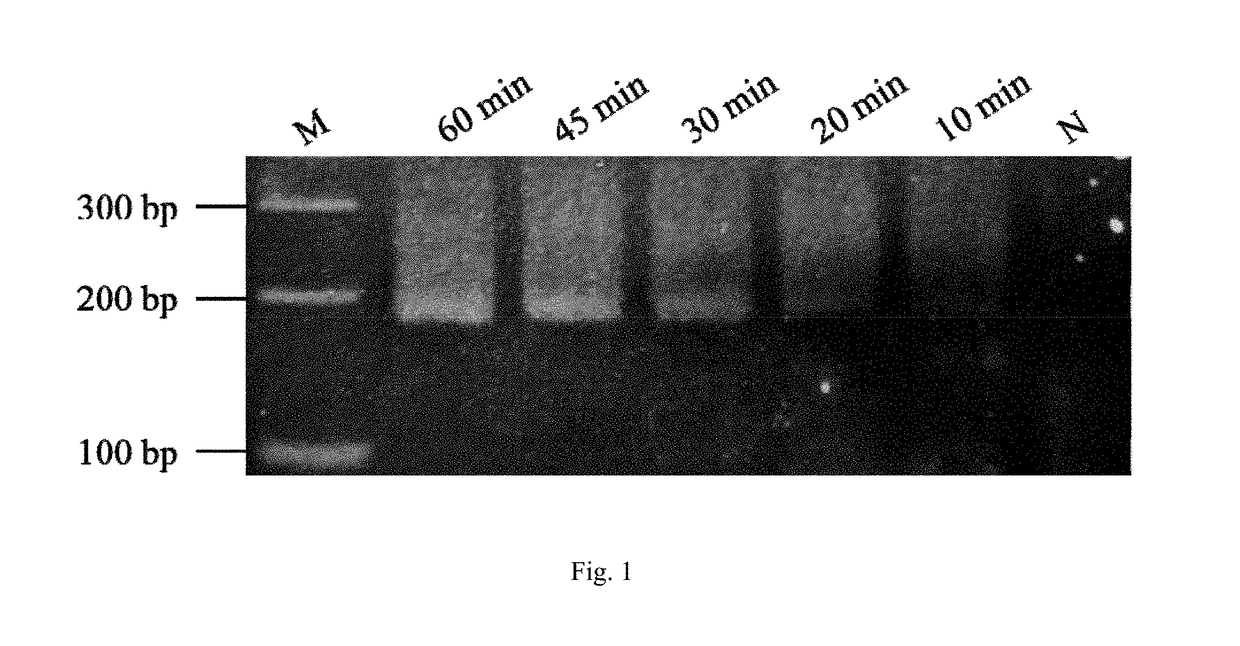
Quadruple fluorescent quantitative PCR (Polymerase Chain Reaction) detection kit for detecting porcine epidemic diarrhea virus and porcine rotavirus and application of quadruple fluorescent quantitative PCR detection kit
ActiveCN114807437ALow costProof of feasibilityMicrobiological testing/measurementAgainst vector-borne diseasesEpidemic diarrheaEpidemiologic study
The invention discloses a quadruple fluorescent quantitative PCR (Polymerase Chain Reaction) detection kit for detecting porcine epidemic diarrhea virus and porcine rotavirus and application of the quadruple fluorescent quantitative PCR detection kit. The kit comprises a hot start Taq DNA polymerase, enzyme-free water, a PCR reaction solution and a probe method fluorescent quantitative PCR matched Buffer, the four pairs of specific primers, the corresponding TaqMan probes and the reference substances are used for detecting the G1 genotype porcine epidemic diarrhea virus, the G2 genotype porcine epidemic diarrhea virus, the porcine group A rotavirus and the porcine group C rotavirus. The kit provided by the invention realizes simultaneous detection of four viruses capable of causing porcine diarrhea in one PCR reaction tube, has excellent specificity and repeatability, and can be directly applied to conventional laboratory diagnosis of porcine clinical diarrhea samples; and a rapid and accurate detection method is provided for epidemiological research of the porcine epidemic diarrhea virus and the porcine rotavirus.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method and kit for the field diagnosis of caprine arthritis-encephalitis virus (CAEV) infection
ActiveUS10161012B2Microbiological testing/measurementMaterial analysisProviral dnaEncephalitis Viruses
The invention is to provide a method and kit based on recombinase polymerase amplification (RPA) and lateral flow dipstick (LFD) for detection of caprine arthritis-encephalitis virus (CAEV) infection. The method and kit are suitable for both laboratory and field application, and are specific and sensitive for detecting CAEV proviral DNA in goats in a fast manner. The method and lit of the invention are also applicable for on-site utilization at farms and should be useful in both eradication programs and epidemiological studies.
Owner:NAT TAIWAN UNIV
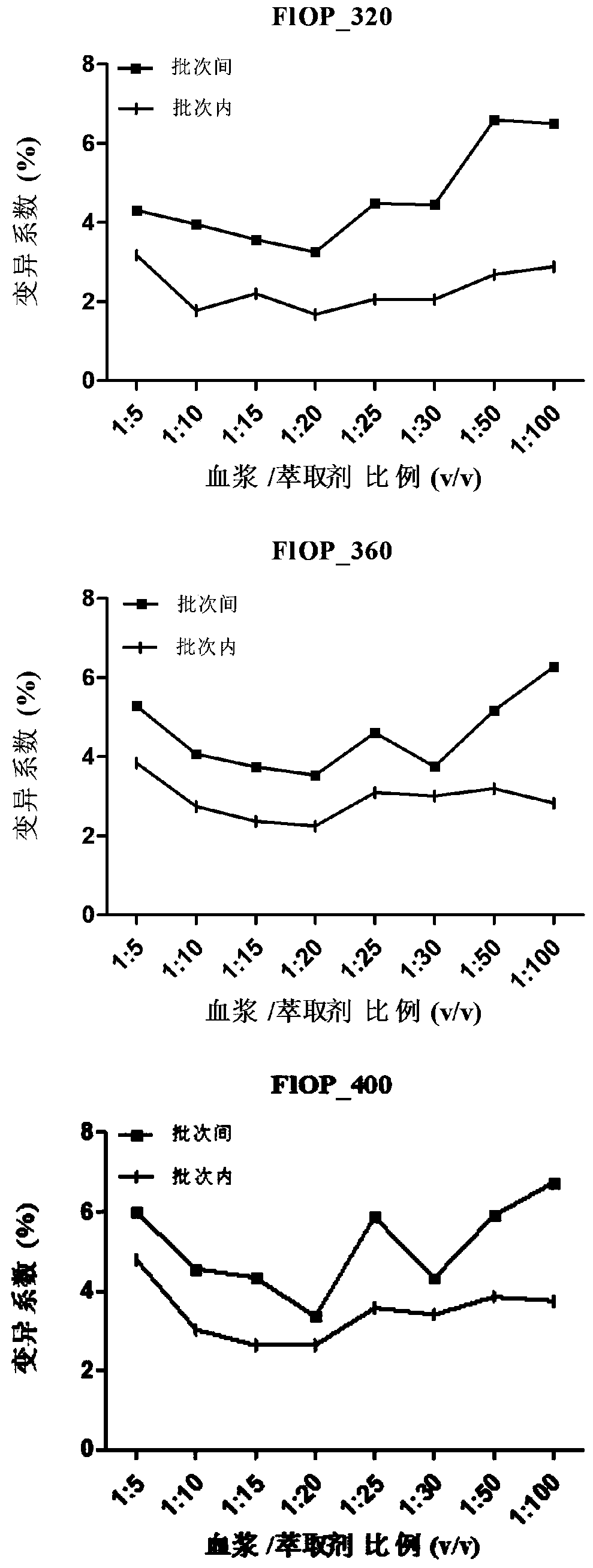
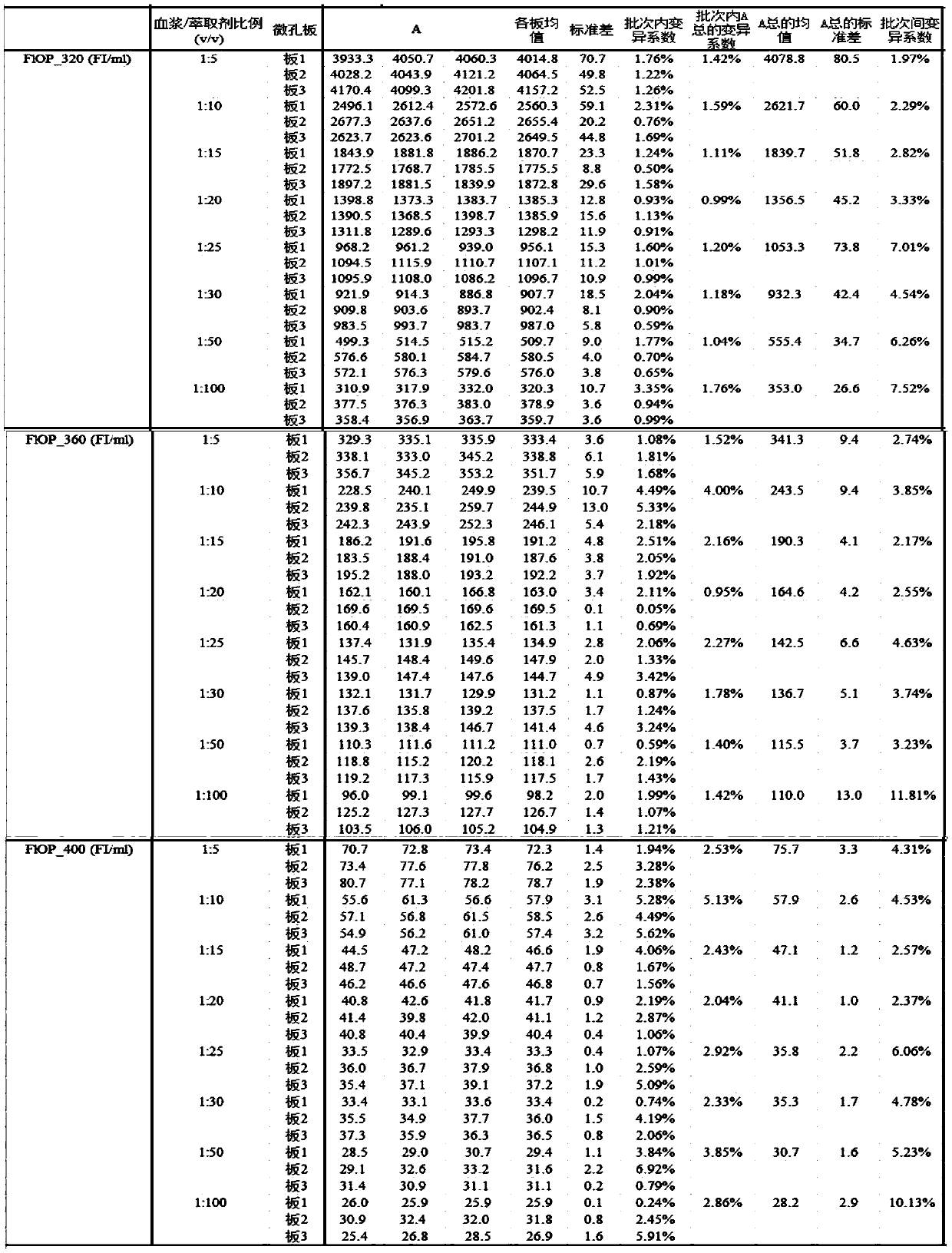
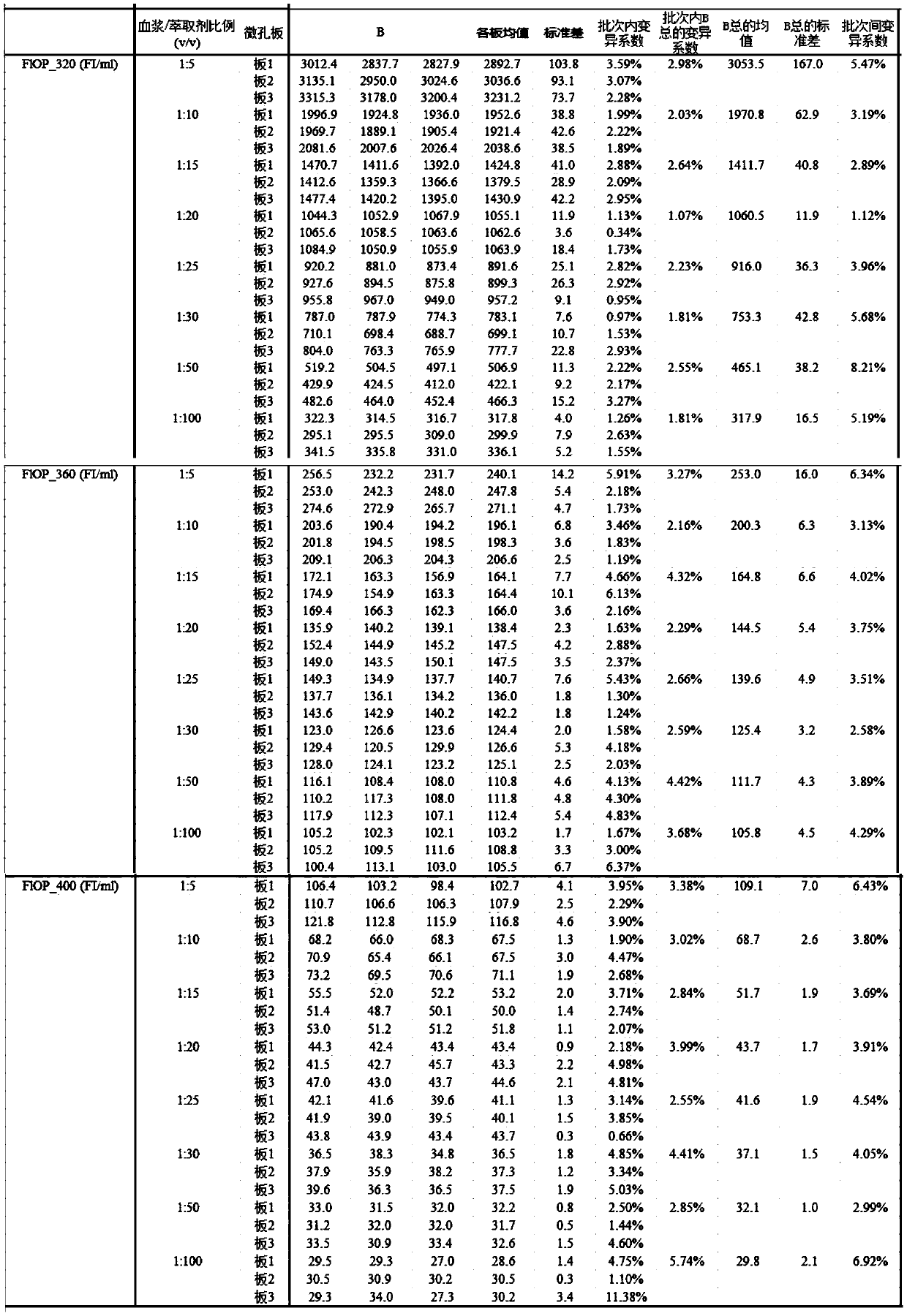
Method for testing oxidative stress biomarkers in blood plasma with low coefficient of variation
InactiveCN110987891AImprove the possibility of applicationRaise the possibilityFluorescence/phosphorescenceOxidative stressEpidemiologic study
The invention belongs to the technical field of oxidative stress biomarker detection, in particular to a method for testing oxidative stress biomarkers in blood plasma with a low coefficient of variation. The method comprises the following steps: taking a mixture of blood plasma and an extractant with the mixed volume ratio of 1: 20 as a to-be-detected substance solution, taking a supernatant of the to-be-detected substance solution and carrying out fluorescence determination by adopting a fluorescence microplate reader under the conditions of three excitation or emission wavelengths. According to the method, the coefficient of variation of oxidative stress biomarkers in blood plasma is remarkably reduced, the inter-batch coefficient of variation of FlOPs is reduced to be less than 3.6%, and the intra-batch coefficient of variation is reduced to be less than 2.7%, so that a more reliable FlOPs measurement method is provided for future epidemiological research and potential clinical practice.
Owner:JILIN UNIV
Primers, kit and preparation method for detecting vesicular stomatitis virus
The invention discloses a primer for detecting vesicular stomatitis viruses, a detection kit composed of the primer and a preparation method of the detection kit. The primer for detecting the vesicular stomatitis viruses has gene sequences, namely SEQ ID No.1, SEQ ID No.2, SEQ ID No.3 and SEQ ID No.4. A related experiment shows that a nucleic acid of the VSV can be specifically and rapidly amplified by the primer sequence and a specific band can be detected by nucleic acid electrophoresis; and meanwhile, the primer has the specificity. The primer can be applied to preparation of a GeXP diagnostic kit for rapidly identifying the VSV and applied to epidemiologic studies of vesicular stomatitis.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
A method for high-throughput detection of multiple different types of environmental pollutants in urine using LC-MS-MS
ActiveCN109507335BShort analysis timeSmall sample sizeComponent separationBiological testingEthylic acidEpidemiologic study
The invention discloses a method for using LC-MS / MS high-throughput detection of various types of environmental pollutants in urine, which includes the following steps: (1) sample pretreatment: the urine after enzymolysis is treated with formic acid Fully vortexed after the acetonitrile treatment, centrifuged to get the supernatant, the supernatant was concentrated by vacuum centrifugation to obtain the injection sample detected on the machine; (2) adopt LC-MS / MS injection to detect the injection sample: liquid phase The conditions are: the chromatographic column adopts a reversed-phase C18 chromatographic column; the mobile phase A is 4-6 mmol / L ammonium acetate aqueous solution; the mobile phase B is methanol; gradient elution is adopted; Multiple ion detection mode. The method of the invention has the characteristics of rapid detection, high efficiency, high throughput, strong versatility, high sensitivity, good stability and reproducibility, and is suitable for large-scale detection of biological samples in epidemiological research.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
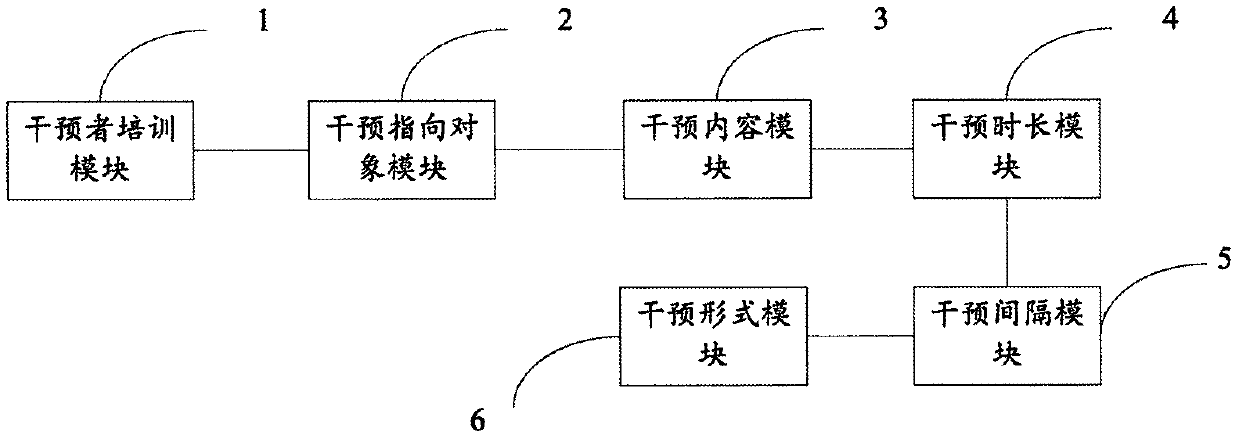
Cognition behavior intervention system for depression of senile dementia family caregiver
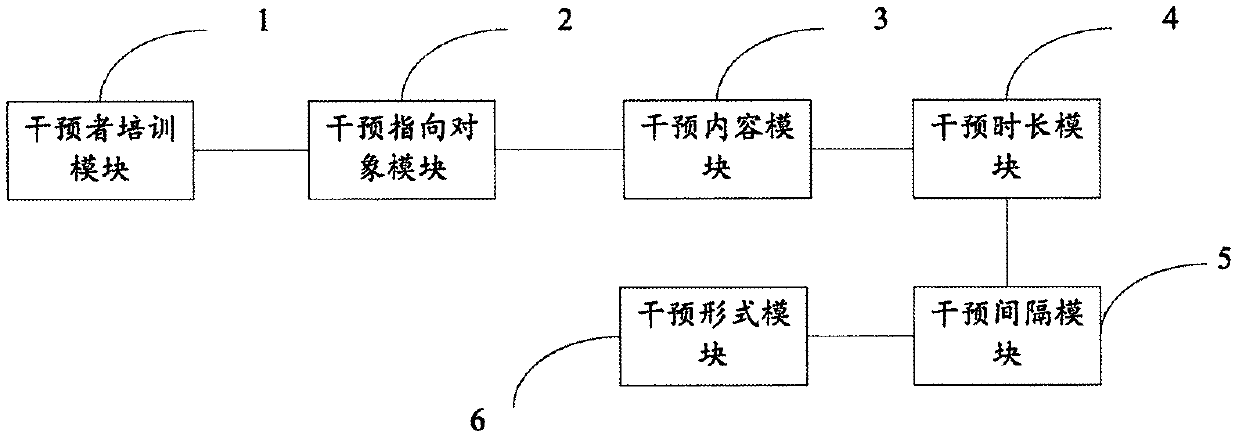
PendingCN109979567ADepression lessened or relievedForecastingMental therapiesCommunication skillsIntervention program
The invention discloses a cognition behavior intervention system for depression of a senile dementia family caregiver. The intervention comprises the components of a caregiver training module which isused for training professional nurses so that the professional nurses master an intervention program, an intervention method and a therapeutic communication skill; an intervention pointing object module which is used for screening the caregiver with a depression symptom; an intervention content module which is used for performing integrated cognition behavior intervention according to the characteristics of the Chinese local senile dementia family caregiver; an intervention time length module which is used for determining an intervention time length of five hours, a face-to-face interventiontime length of 1 hour in each month and a telephone follow-up intervention time length of 20-30 minutes; an intervention interval module which is used for face-to-face intervention in a frequency of one time in each month, telephone follow-up surveying intervention in a frequency of one time in each month, wherein the interval time between the face-to-face intervention and the telephone follow-upsurveying intervention is two weeks; and an intervention form module which is used for performing the face-to-face intervention and the telephone intervention. Ten centers for epidemiologic studies depression scale are used for testing and screening the caregiver who accords with a preset depression standard, thereby realizing a purpose of mitigating or alleviating depression of the caregiver.
Owner:JINHUA VOCATIONAL TECH COLLEGE
A duck-derived h4n6 subtype avian influenza virus strain and its application
InactiveCN103571799BResearch fastMicroorganism based processesAntiviralsWhole genome sequencingEpidemiologic study
Owner:SHANGHAI ANIMAL EPIDEMIC PREVENTION & CONTROL CENT
Isolation, identification and purification method of a genotype vii chicken Newcastle disease virus strain and its application
InactiveCN105543180BSsRNA viruses negative-senseViral antigen ingredientsAntigenPurification methods
Owner:INST OF ANIMAL SCI & VETERINARY MEDICINE SHANDONG ACADEMY OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com