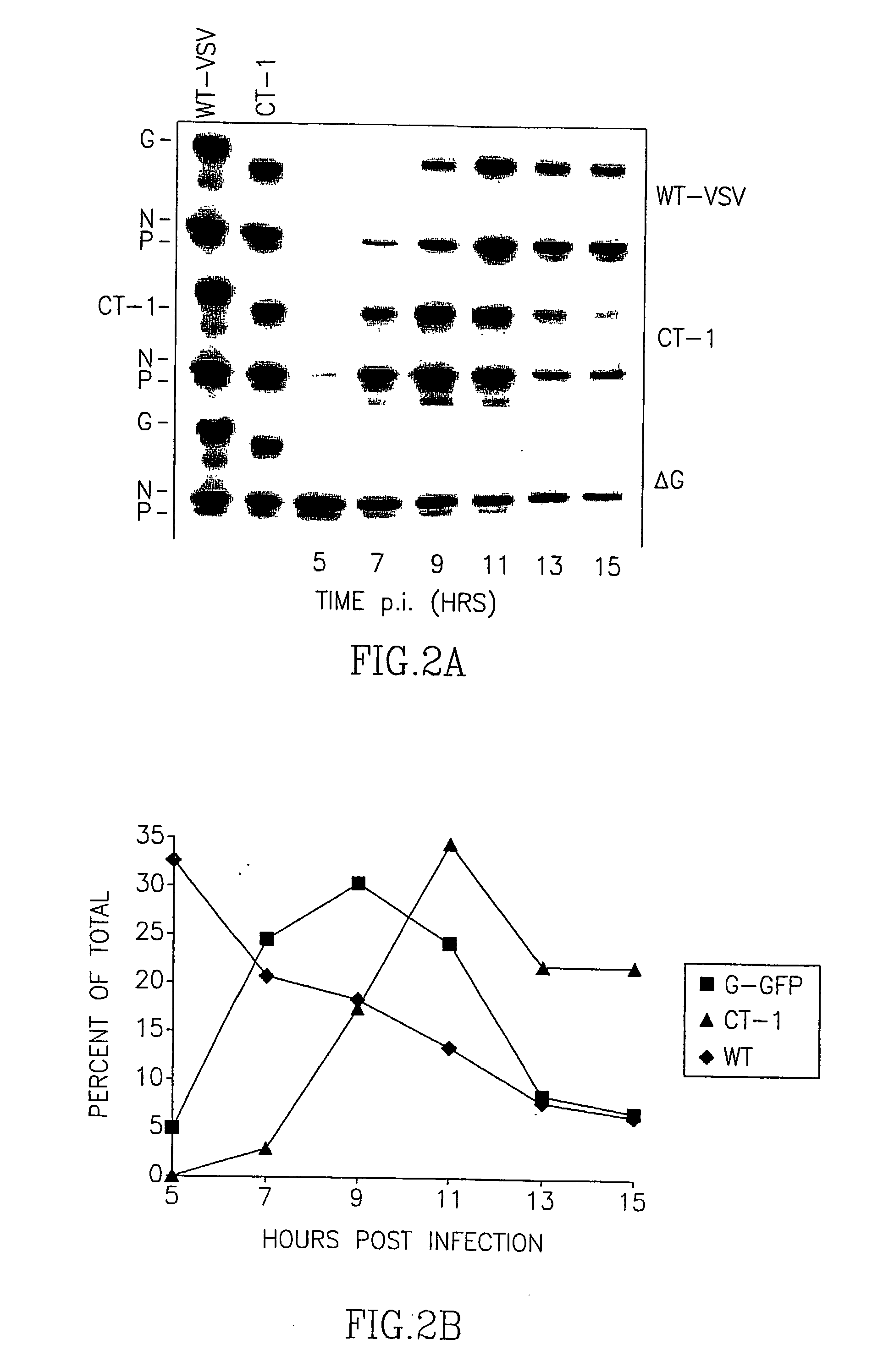
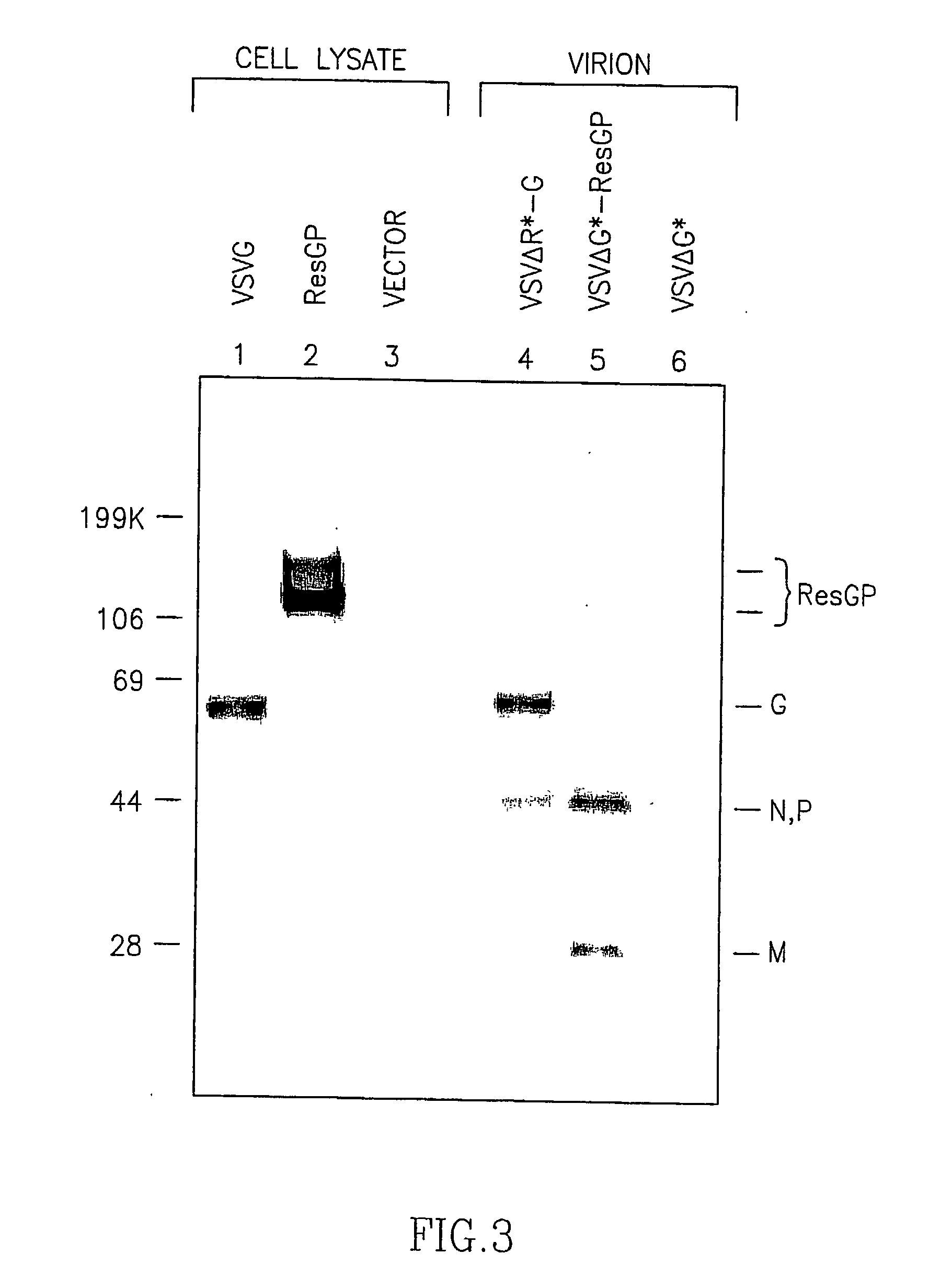
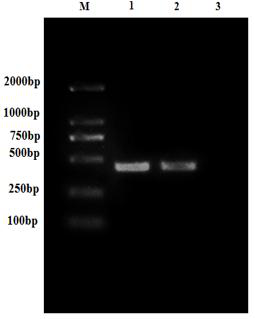
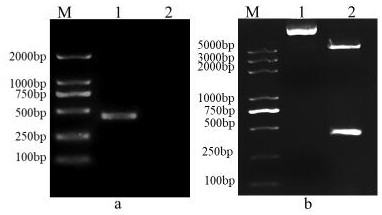
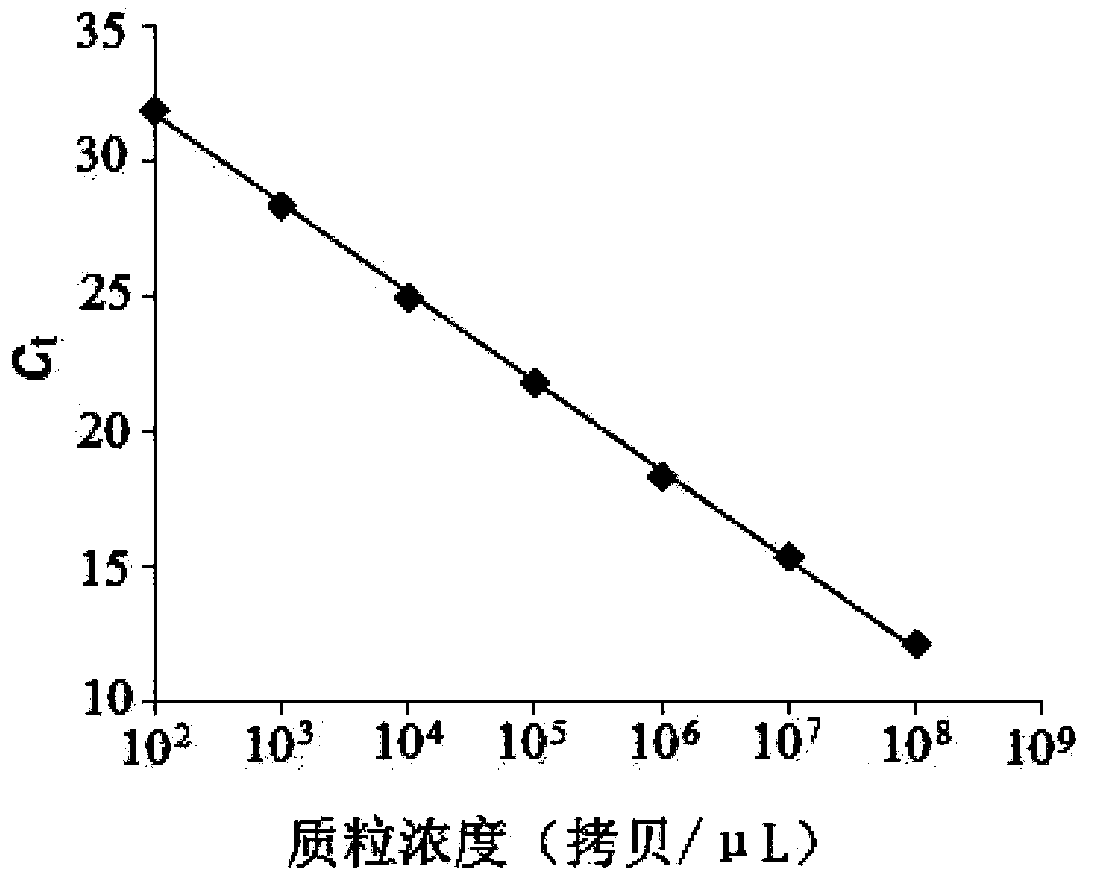
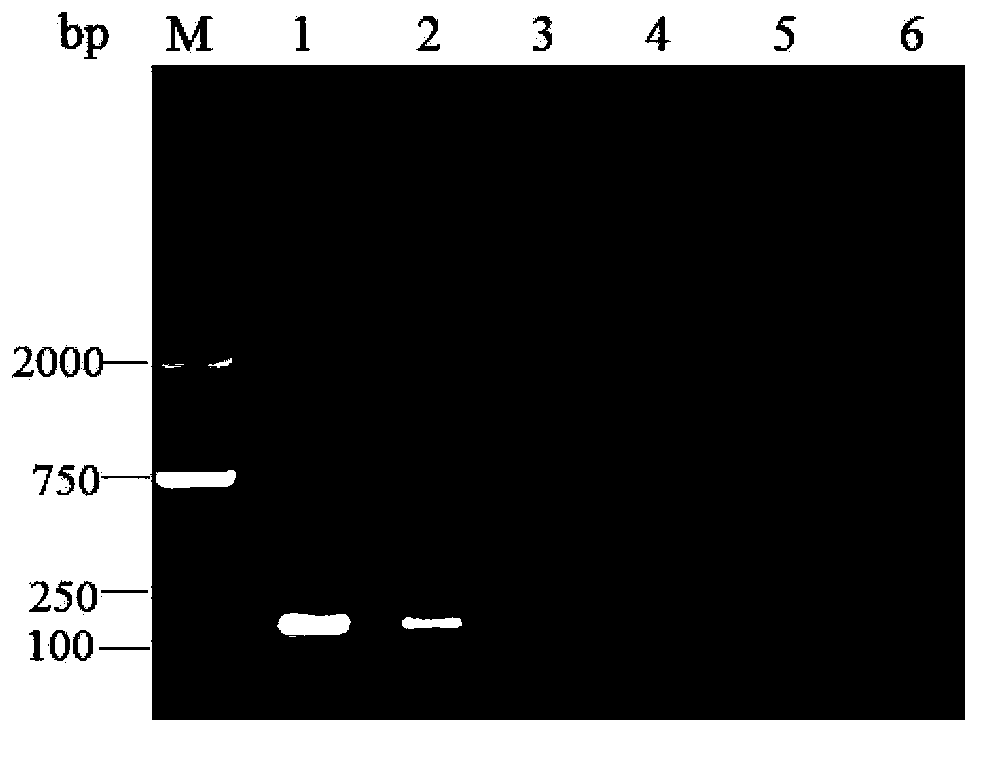
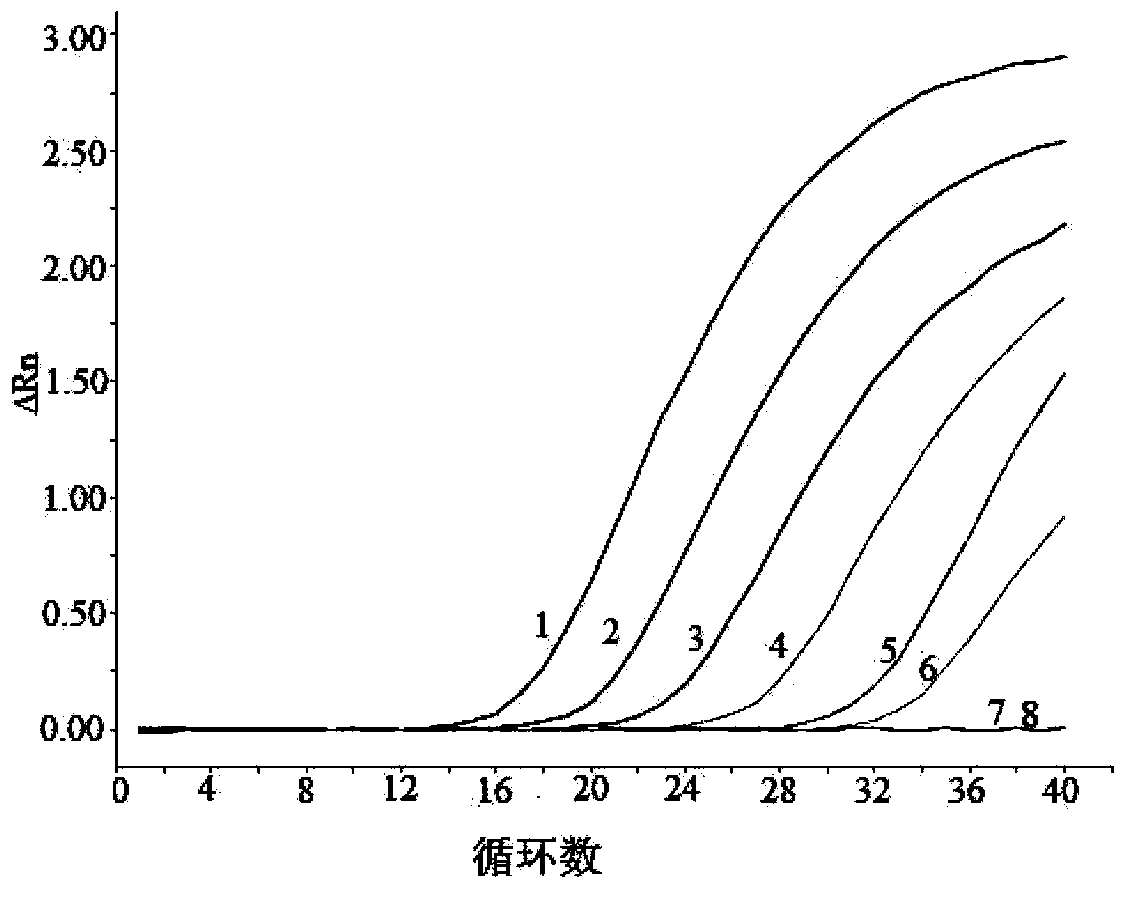
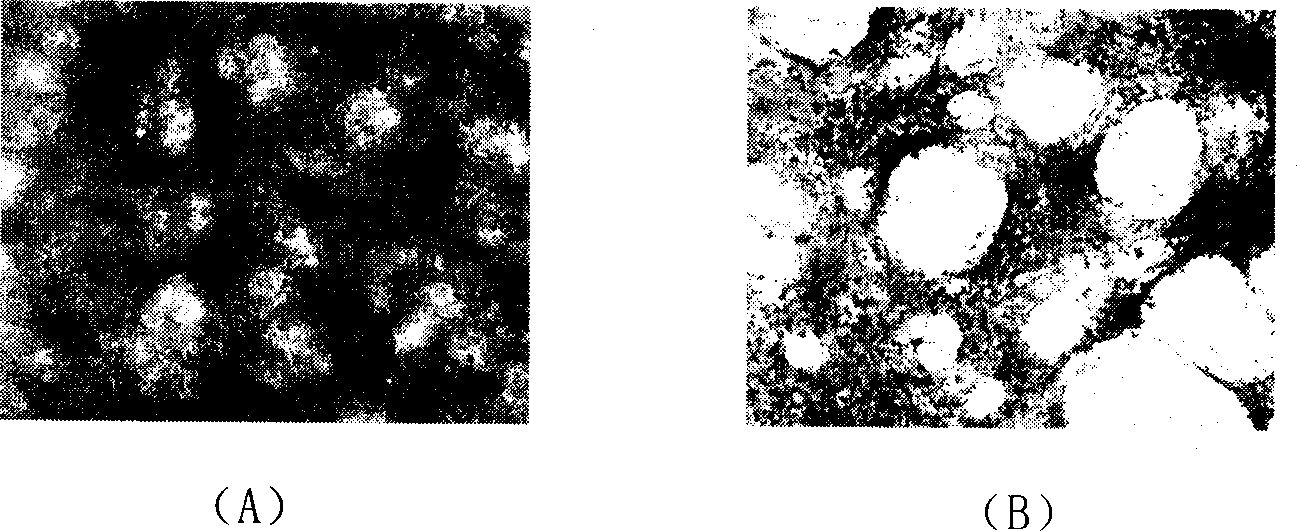
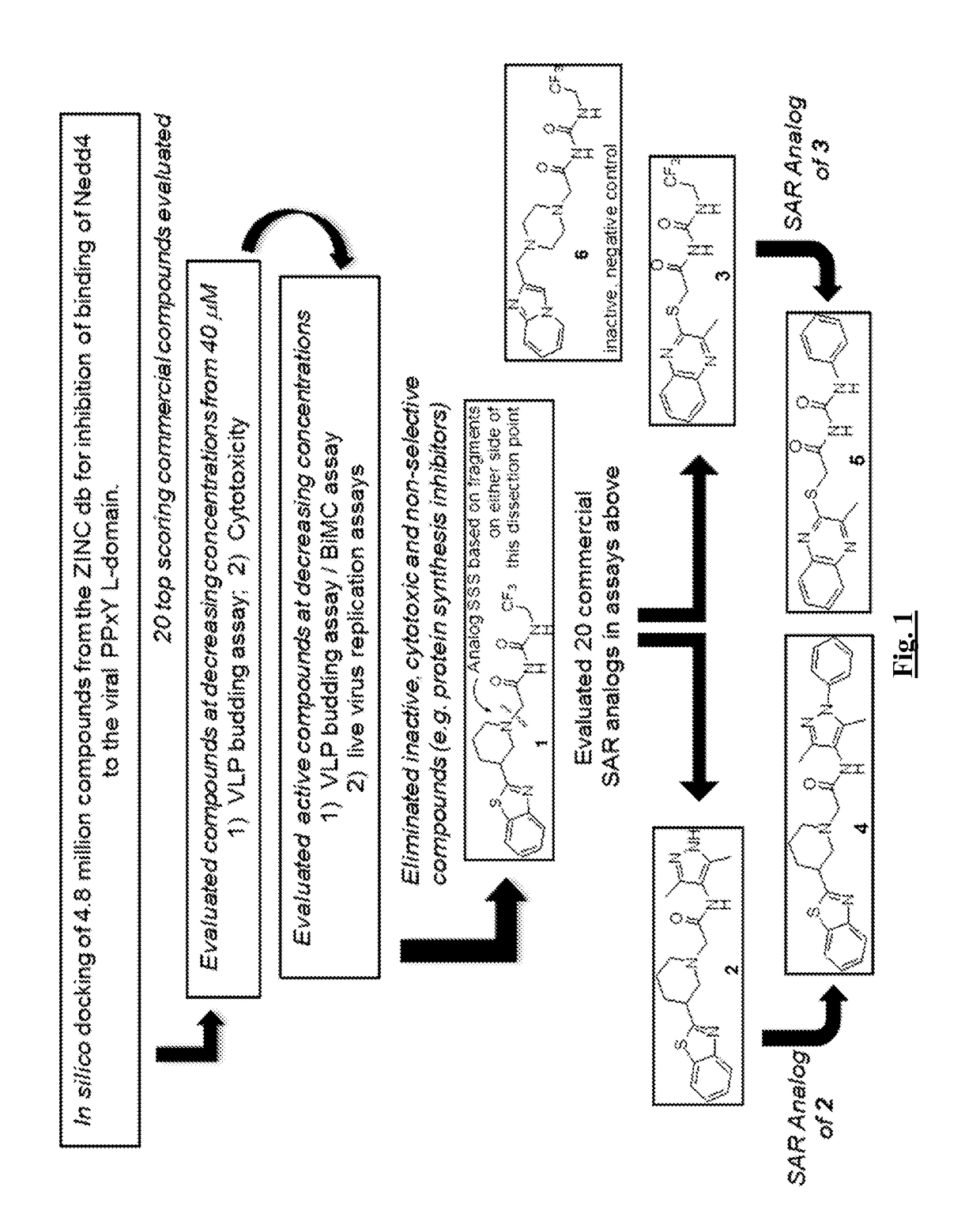
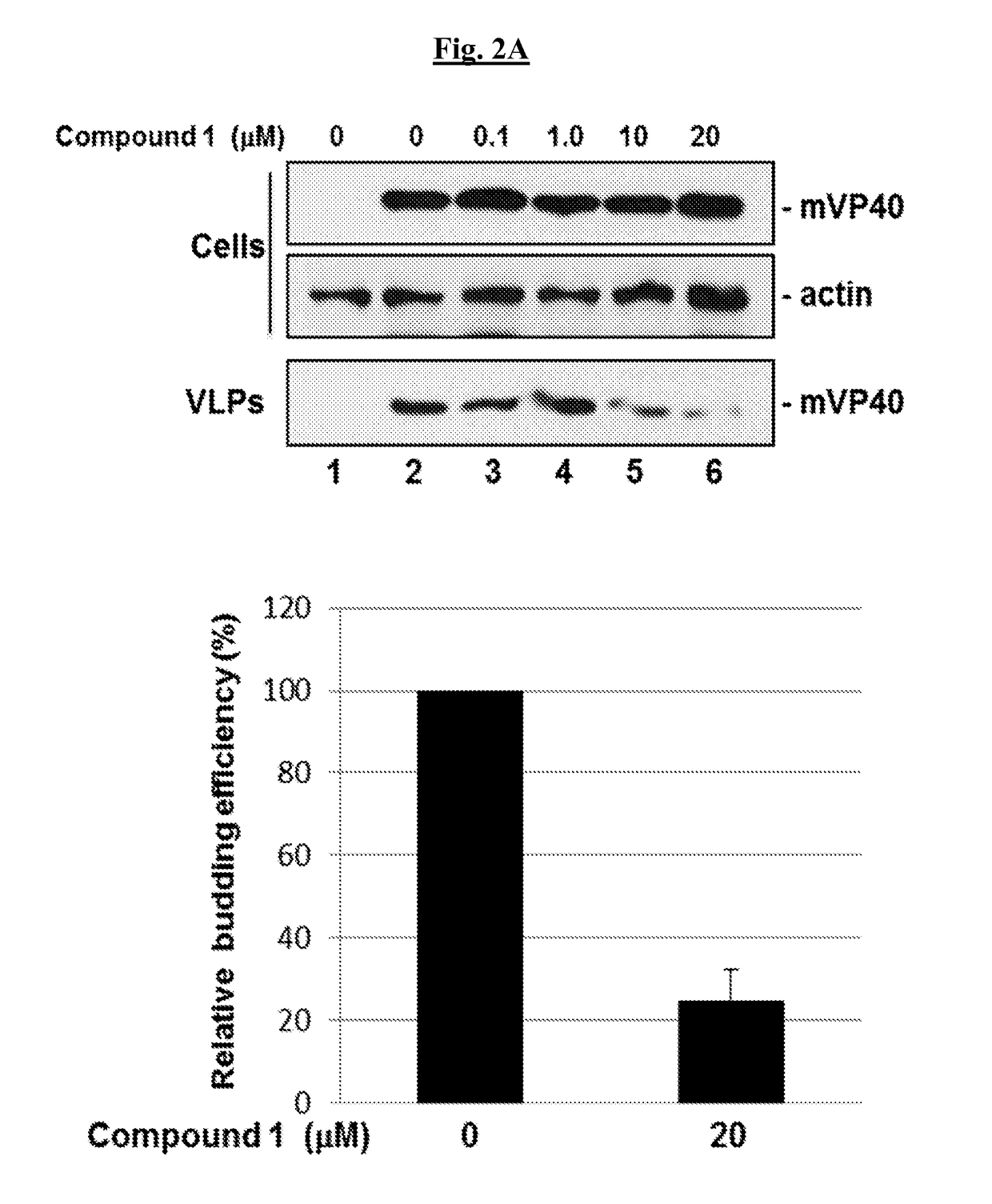
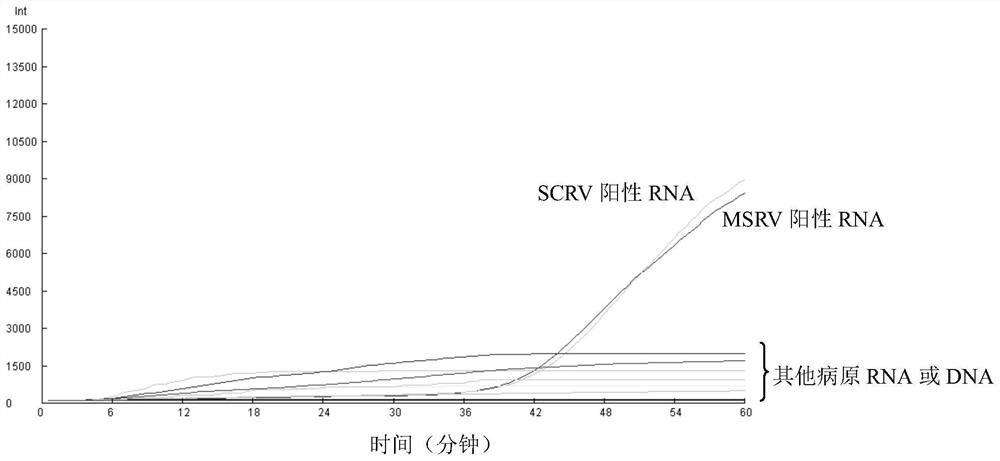
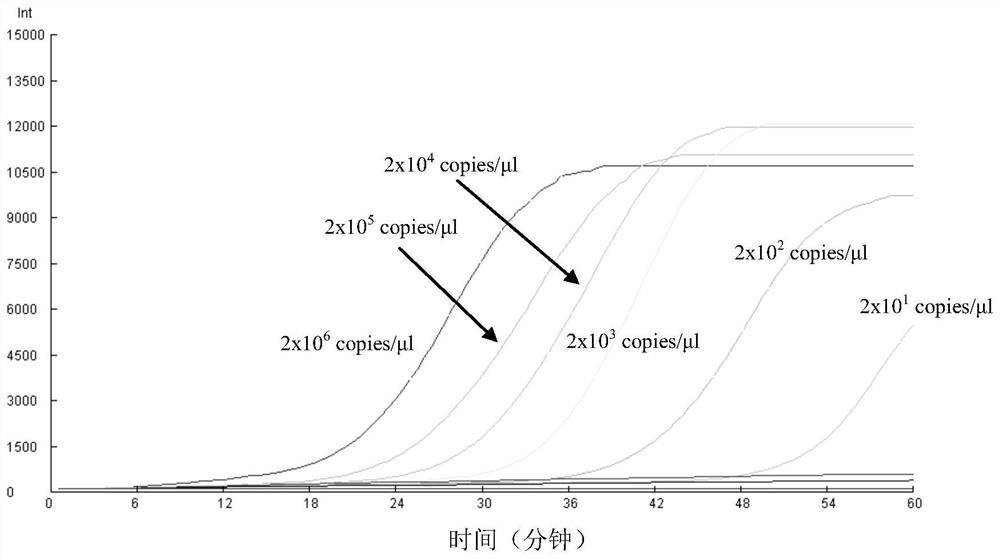
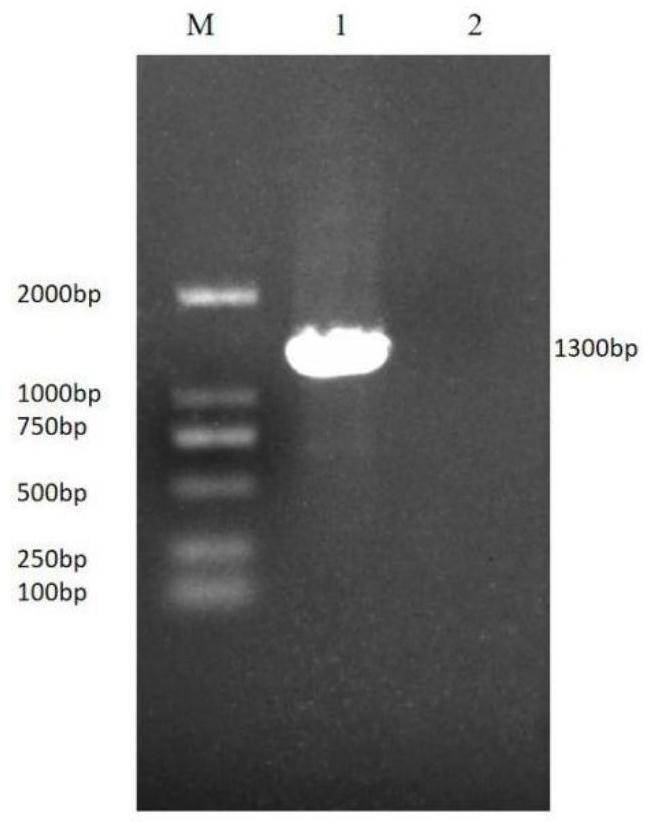
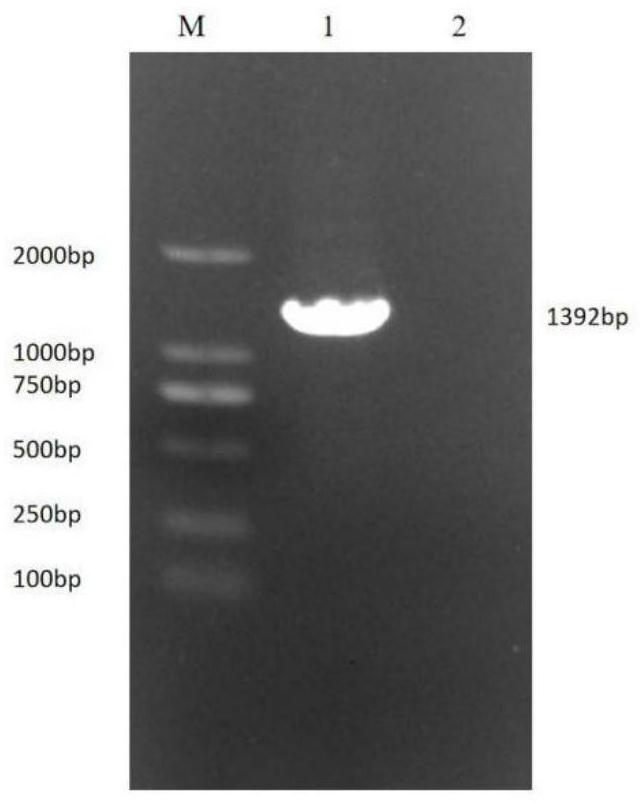
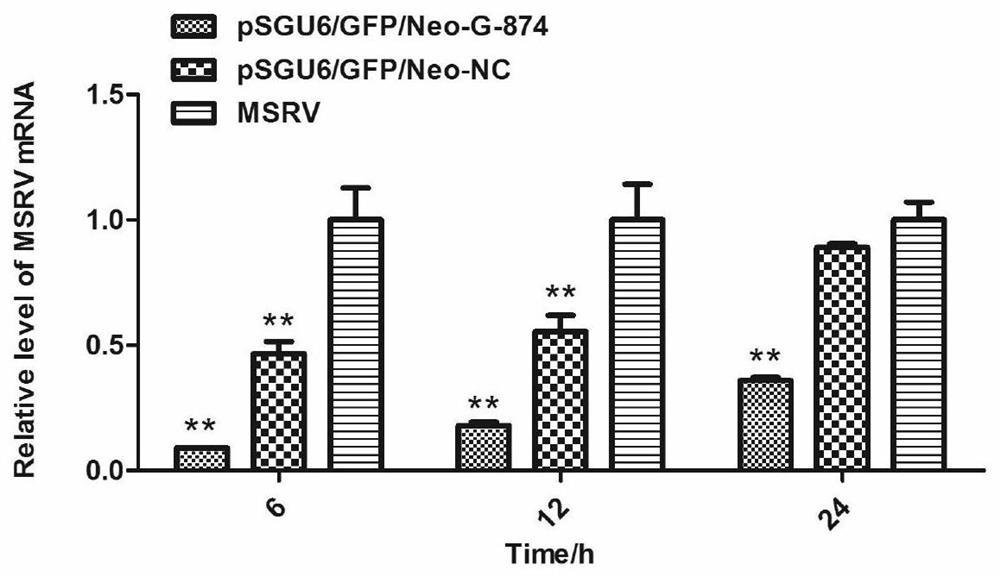
Patents
Literature
63 results about "Rhabdovirus carpio" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Dihydroorotate dehydrogenase inhibitors for the treatment of viral-mediated diseases
InactiveUS6841561B1Potent activityBiocideOrganic chemistryDiseaseDihydroorotate Dehydrogenase Inhibitor
Flavivirus, rhabdovirus and paramyxovirus infections may be treated by administering an inhibitor of the enzyme dihydroorotate dehydrogenase such as 6-fluoro-2-(2′-fluoro-1,1′-biphenyl-4-yl)-3-methyl-4-quinolinearcarboxylic acid sodium salt (Brequinar). A synergistic effect can be obtained if an interferon such as interferon α2, interferon α8 or interferon β, or an inhibitor of a second enzyme selected from inosine monophosphate dehydrogenase, guanosine monophosphate synthetase, cytidine triphosphate synthetase and S-adenosylhomocysteine hydrolase, is also administered.
Owner:INST OF MOLECULAR & CELL BIOLOGY
Recombinant Rhabdovirus containing a heterologous fusion protein
InactiveUS20030138457A1Easy to integrateOvercome limitationsSsRNA viruses negative-senseAntibody mimetics/scaffoldsHeterologousAttachment protein
This invention relates to a composition comprising a recombinant or genetically engineered Rhabdovirus that expresses a Fusion Protein, such as the F protein of the Paramyxovirus SV5 strain. This recombinant Rhabdovirus may express other non-Rhabdovirus attachment proteins and / or an enhancer protein. The invention also relates to methods of making recombinant Rhabdoviruses which express an F Protein. These recombinant compositions can be used for purposes of research, as well as for diagnostic and therapeutic compositions for treatment of diseases.
Owner:TENNESSEE RESEARCH CORPORATION
Weever rhabdovirus recombinant G2 protein and application thereof
ActiveCN113512096AGood immune ingredientsRaise antibody levelsSsRNA viruses negative-sensePeptide/protein ingredientsImmune profilingRhabdovirus carpio
G2 protein is obtained through prokaryotic recombinant expression, the protein fragment has good immunogenicity, can stimulate an organism to generate a high antibody level, and has better immunogenicity and immune protection performance compared with the whole G protein and other fragments; a carbon nano tube carrier sub-unit vaccine prepared on the basis of combination of a G2 protein and a functional carbon nano tube attacks an MSRV FJ985 strain 21 days after immunizing weever, the immune protection rate of the weever is 86% and is higher than the protection rate reported in the prior art, on the basis of selection of specific protein fragments, and compared with the original G protein and other G protein fragments obtained based on immunoassay, the G protein fragment has better protective efficacy, can generate a better protective effect after immunizing weever, and can enable the weever to effectively resist attack of MSRV virulent virus.
Owner:深圳万可森生物科技有限公司
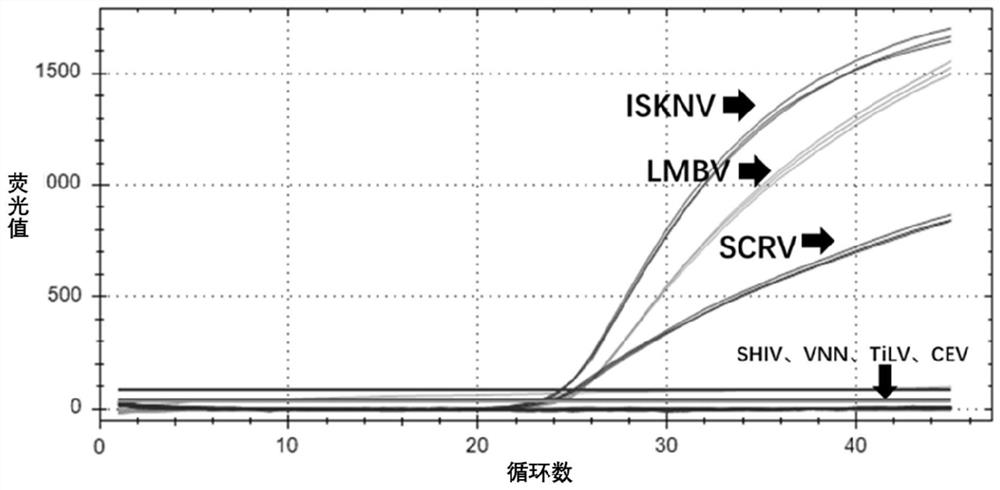
Triple fluorescent PCR detection kit for infectious spleen and kidney necrosis virus, largemouth bass ranavirus and siniperca chuatsi rhabdovirus
ActiveCN111733283AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationNecrovirusReverse transcriptase
The invention belongs to the technical field of virus detection, and specifically relates to a triple fluorescent PCR detection kit for the infectious spleen and kidney necrosis virus, largemouth bassranavirus and siniperca chuatsi rhabdovirus. The kit of the invention comprises virus-specific amplification primers and probes, a positive standard, a negative standard, Taq enzyme, reverse transcriptase, a RNA enzyme inhibitor, a reaction buffer, dNTP, nuclease-free water and a freeze-drying protective agent. The kit of the invention can perform multiple channel detection at the same time by using triple fluorescent PCR, uses the different fluorescently labeled probes, can detect the three viruses simultaneously in one reaction system, reduces the detection difficulty, shortens the detection time, and can help raisers accurately get detection results in time. The kit of the invention has high detection sensitivity, high stability and strong specificity, has no cross-reactions with otheraquatic viruses, and can be used for early monitoring and prevention and control of epidemic diseases.
Owner:GUANGDONG HAID ANIMAL HUSBANDRY & VETERINARY RES INST
Siniperca chuatsi ranairidovirus and rhabdovirus duplex PCR detection kit and detection method
PendingCN111254225AMultiple treatment timesQuick checkMicrobiological testing/measurementMicroorganism based processesDuplex pcrRhabdovirus carpio
The invention relates to a siniperca chuatsi ranairidovirus and rhabdovirus duplex PCR detection kit and detection method, and belongs to the technical field of PCR. The siniperca chuatsi ranairidovirus and rhabdovirus duplex PCR detection kit provided by the invention comprises primers, EasyTaq PCR SuperMix, a negative control solution and a positive control solution, wherein the primers comprisea primer designed by aiming at an MCP gene conserved region of the siniperca chuatsi ranairidovirus, and a primer designed by aiming at an N gene conserved region of the siniperca chuatsi rhabdovirus. The siniperca chuatsi ranairidovirus and rhabdovirus duplex PCR detection kit and detection method provided by the invention can be used for fast, efficiently and accurately detecting the sinipercachuatsi ranairidovirus and the siniperca chuatsi rhabdovirus at the same time; the time and the labor are saved; more treatment time is won for sick siniperca chuatsi; and important significance is realized on subsequent study, prevention and control.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
Perch rhabdovirus subunit vaccine and preparation method thereof
ActiveCN113521265AHigh immune protection rateImproving immunogenicitySsRNA viruses negative-senseViral antigen ingredientsRhabdovirus carpioPerch rhabdovirus
Part of protein fragment G2 of MSRV glycoprotein is selected, G2 protein is obtained through prokaryotic recombinant expression, and mannosylation modification is carried out on the G2 protein to prepare a perch rhabdovirus nano-carrier targeting vaccine, and research shows that for perch after immunized by the subunit vaccine for 21 days is attacked by an MSRV FJ985 strain, the perch immune protection rate is 94%-96%, and the vaccine is safe and effective. After the perch is immunized, a better protection effect can be achieved, and the perch can effectively resist the attack of MSRV virulent virus.
Owner:深圳万可森生物科技有限公司
Monopterus albus rhabdovirus Cr-ERV and RT-PCR detection primer and application thereof
ActiveCN109182277ASimple methodImprove detection efficiencySsRNA viruses negative-senseViral antigen ingredientsMonopterusAnguilliformes
The invention belongs to the virus detection field, in particular, discloses a Chinese rice virus-Field eel rhabdovirus Cr-ERV and RT-PCR detection primer and an application thereof. The Monopterus albus rhabdovirus is isolated from diseased Monopterus albus seedlings, and the virus is (Chinese rice virus-Field eel rhabdovirus) CrERV, deposited as CCTCC NO: V201819, is of pioneering significance for the study of Monopterus albus rhabdovirus. Primers are designed to detect rhabdovirus of Monopterus albus. The primers have good specificity and high sensitivity.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Novel effective antiviral compounds and methods using same
The present invention includes compounds that are useful in preventing or treating viral infections caused by an enveloped RNA virus, such as viral infections caused by a Filovirus, arenavirus, rhabdovirus, paramyxovirus, orthomyxovirus and / or retrovirus. The present invention further includes compositions comprising such compounds, and methods of treating a viral infection in a subject using such compounds.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Mandarin fish rhabdo virus toxic strain, its preparation method and application
InactiveCN1500864AAccurate Spot PurificationObtain Intuitive Results for ProliferationViral antigen ingredientsMicrobiological testing/measurementVirus antigenCell engineering
The Siniperca chuatsi rhabdovirus, SCRV0208, with preservation number of CCTCC No. V202008, is prepared through culture of GCF0208 cell in TC199 culture medium with ox serum to grow single layer of cell, inoculation with virus liquid, sampling in different stages, slicing, electronic microscope observation to determine the dot picking time, picking dot and inoculating to the single layer of cell for enlarged culture, and centrifugating to obtain pure virus. The said process is simple and practical, can separate rhabdovirus from accompanied virus to obtain high purity virus antigen for application in cell engineering vaccine and genetic engineering vaccine.
Owner:INST OF AQUATIC LIFE ACAD SINICA
Monoclonal antibody of anti-rhabdovirus glucoprotein of mandarin fish, preparation and use thereof
InactiveCN101503471AHigh activityPromote recoveryImmunoglobulins against virusesFermentationAntigenBALB/c
The invention discloses a monoclonal antibody of anti-mandarin fish rhabdovirus glycosidoprotein and a preparation method and the application thereof, and hybridoma cell strain SCRV-G-1B2,CCTCC No:C200918 for producing the monoclonal antibody. The preparation method comprises the following steps: taking purified mandarin fish rhabdovirus as antigen, immunizing a BALB / c mouse, producing fused hybridoma cells through cell fusion and obtaining hybridoma cell strain which stably secretes anti-mandarin fish rhabdovirus glycosidoprotein monoclonal antibody through indirection ELIS screening and limited dilution monocloning. The invention can obtain murine spleen lymphocytes with higher activity, stronger specification and more quantities so as to ensure that the obtained fused hybridoma cells have better effect. By using the murine spleen strengthening immunization technique, the fusion rate and the positive rate respective reach is 96.3 percent and 97 percent. The monoclonal antibody can be used for immunodetection of mandarin fish rhabdovirus infection and can also be used for quantitative and positioning analysis of mandarin fish rhabdovirus glycosidoprotein in host tissues or cells.
Owner:INST OF AQUATIC LIFE ACAD SINICA
Hybrid snakehead rhabdovirus fluorescent quantitative PCR (polymerase chain reaction) detection kit and detection method thereof
ActiveCN103397106AStrong specificityQuick QualificationMicrobiological testing/measurementFluorescence/phosphorescenceRhabdovirus carpioEpidemiologic study
The invention discloses a hybrid snakehead rhabdovirus fluorescent quantitative PCR (polymerase chain reaction) detection kit and a detection method thereof. The kit contains specific primers HSHRV-2F and HSHRV-2R and a probe HSHRV-2probe. When the kit is used for detecting hybrid snakehead rhabdovirus, the kit has the characteristics of high sensitivity, high repetitiveness and good specificity, can quickly and accurately realize qualitative and quantitative detection of hybrid snakehead rhabdovirus, and is of far reaching importance in early diagnosis of hybrid snakehead virus diseases, molecular epidemiology research, prevention and control technology research and the like.
Owner:湖南振业动物检验有限公司
Large brill rhabdo virus toxic strain and its preparation method and application
InactiveCN1410533AEfficient ProliferationSimple methodMicrobiological testing/measurementAntibody ingredientsPaleontologyScophthalmus
A Scophthalmus maximus rahbdovirus (SMRV0207, CCTCC No.202006) is prepared through sampling the liver, kidney, heart and spleen tissues from the scophthalmus maximus in ill, shearing, adding PBS, homogenizing, adding double antibody, freezing, thawing, centrifugal extracting, filtering, inoculating it to CLC 0207 cell, and constant-temp. continuous culture. Its advantages are high reproduction speed, and high reproduction valency up to 10 to the power 8 TCID 50 / ml. It can be used to prepare vaccine.
Owner:INST OF AQUATIC LIFE ACAD SINICA
Preparation method of mandarin fish rhabdovirus glycoprotein expressed by recombinant baculovirus
The invention discloses a preparation method of mandarin fish rhabdovirus glycoprotein expressed by recombinant baculovirus. The preparation method comprises the following steps: designing and synthesizing primers; amplifying and purifying a target gene; constructing a recombinant donor plasmid pFastBac-G; constructing a recombinant shuttle plasmid rBacmid-G; preparing a recombinant baculovirus; purifying the target protein; and carrying out Western blot detection. According to the invention, a large amount of soluble recombinant proteins with good antigenicity and immunogenicity and similar functions to natural proteins can be obtained, and the method is suitable for high-efficiency expression of mandarin fish rhabdovirus glycoproteins and preparation of DNA vaccines.
Owner:YANGZHOU UNIV
Novel antiviral compounds and methods using same
The present invention includes bicyclic compounds that are useful in preventing or treating viral infections, such as viral infections caused by a filovirus, arenavirus, rhabdovirus, paramyxovirus, and / or retrovirus. The present invention further includes compositions comprising such compounds, and methods of treating a viral infection in a subject using such compounds.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
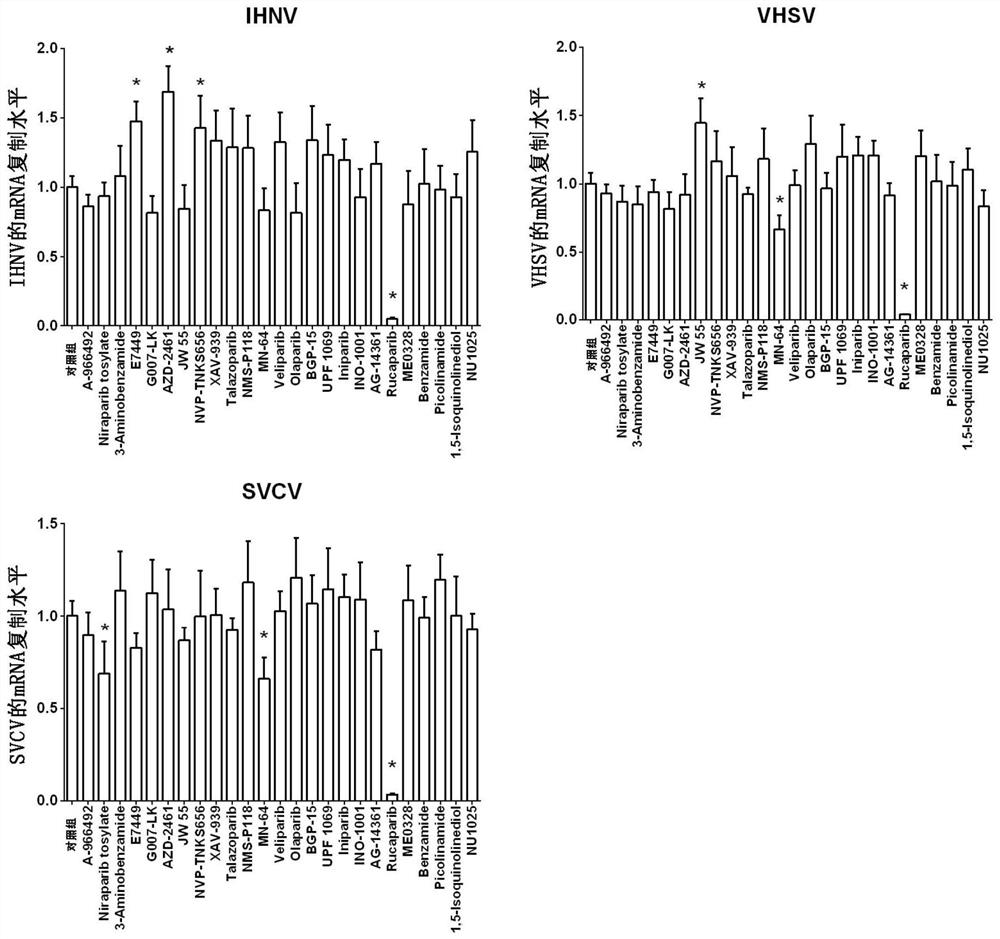
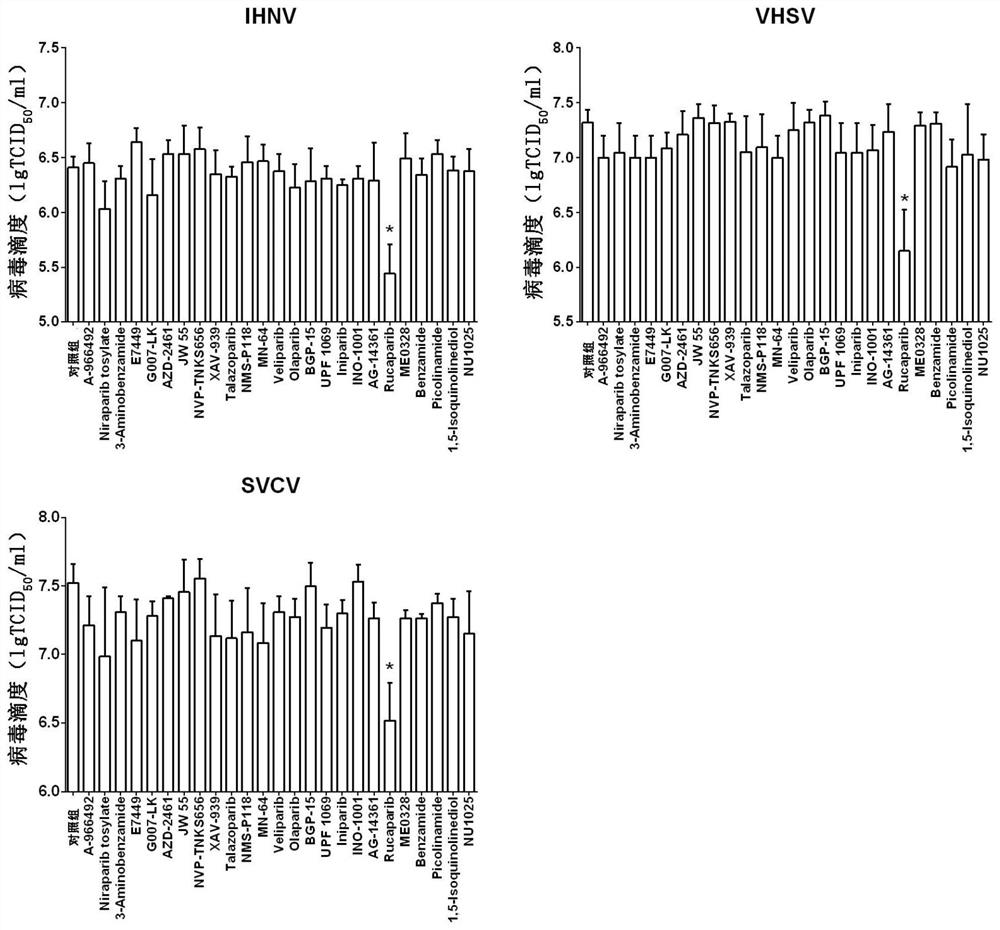
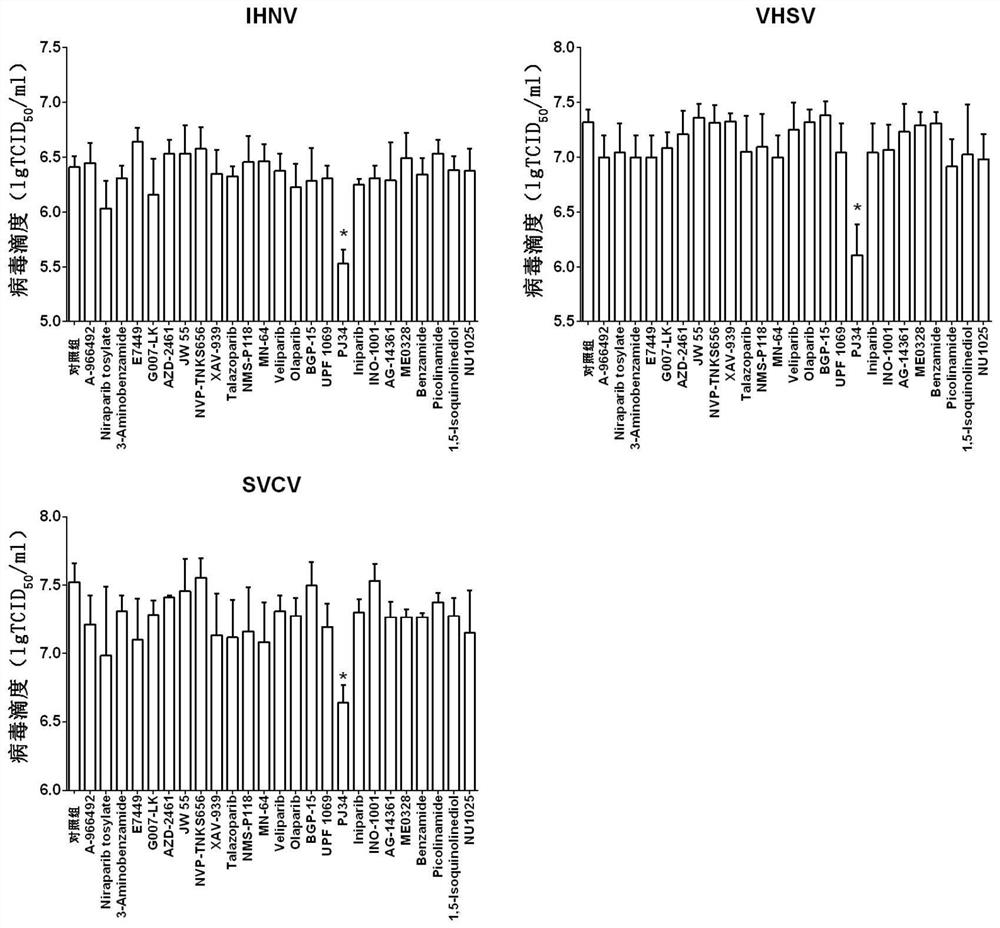
Application of PARP inhibitor Rucaparib in preparation of fish rhabdovirus resisting products
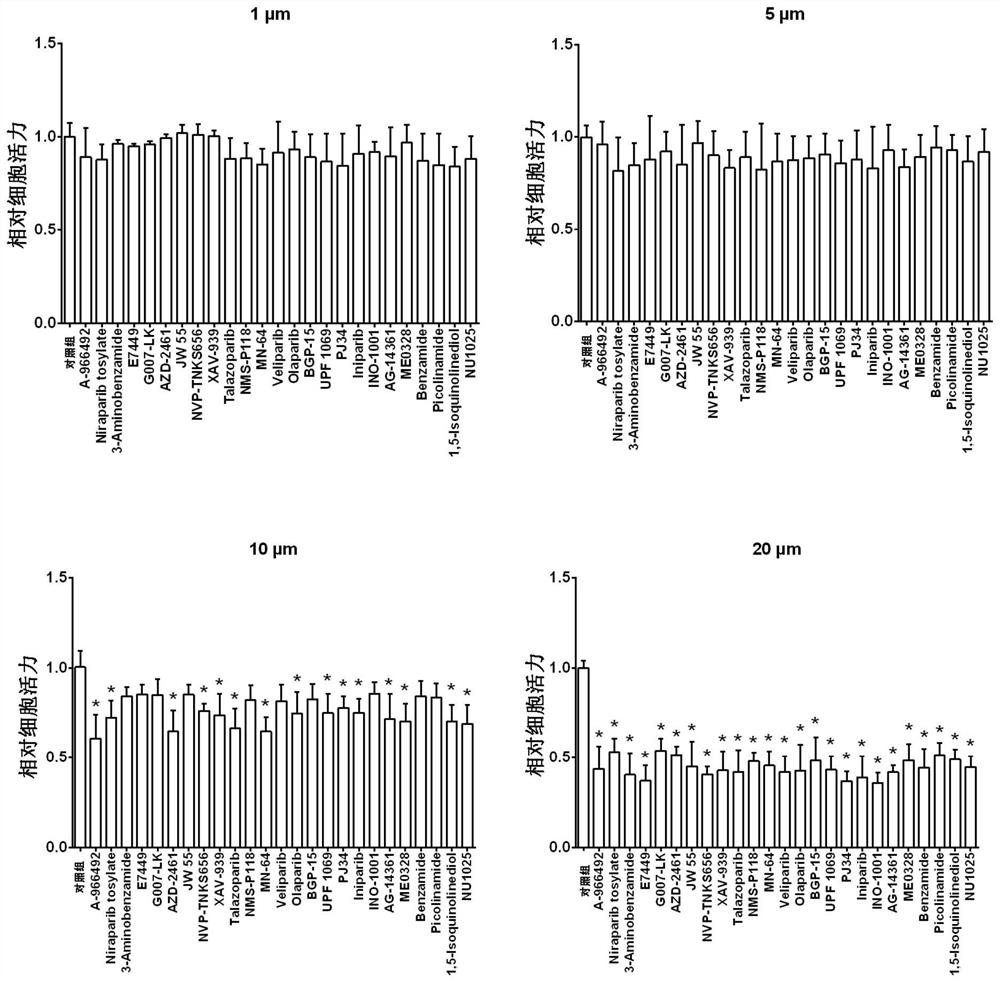
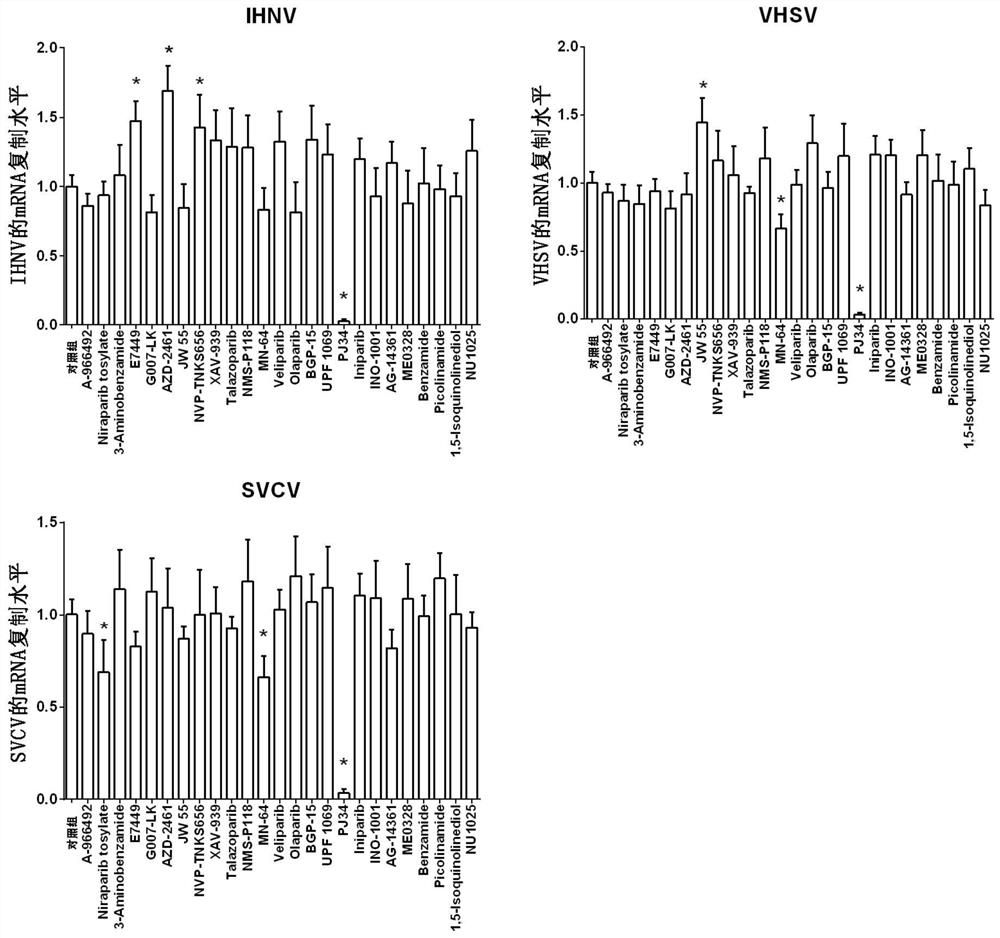
ActiveCN112587532AInhibition of replicationLower titerAntiviralsHeterocyclic compound active ingredientsRhabdovirus carpioPharmaceutical drug
The invention discloses application of a PARP inhibitor Rucaparib in preparation of fish rhabdovirus resisting products. According to the application, a plurality of PARP inhibitors are screened, andthe result shows that the PARP inhibitor Rucaparib can inhibit mRNA replication of viruses IHNV, VHSV and SVCV in cells and reduce the titer (TCID50) of the viruses IHNV, VHSV and SVCV; the inhibitioneffect of Rucaparib on IHNV, VHSV and SVCV is in dose-dependent relation. The PARP inhibitor Rucaparib is expected to become a novel drug for resisting fish rhabdovirus.
Owner:HEILONGJIANG RIVER FISHERY RES INST CHINESE ACADEMY OF FISHERIES SCI
Oncolytic rhabdovirus expressing il12
PendingCN110050062ASsRNA viruses negative-sensePeptide/protein ingredientsInfected cellWhite blood cell
Disclosed herein is an oncolytic recombinant Maraba virus whose genome comprises one or more nucleic acid sequences that, in combination, encode an interleukin-12 (IL12) protein or a functional portion thereof. A method for treating a cancer in a patient using the oncolytic recombinant Maraba virus is also disclosed. The present disclosure also provides a tumour cell infected with an oncolytic rhabdovirus whose genome comprises one or more nucleic acid sequences that, in combination, encode an interleukin-12 (IL12) protein or a functional portion thereof, for use as an infected cell vaccine (ICV) for the treatment of a cancer. A method for treating a cancer in a patient using the infected cell vaccine is also disclosed.
Owner:TURNSTONE LLP
Method and kit for detecting reverse transcription-loop mediated isothermal amplification (RT-LAMP) of pike fry rhabdovirus (RFRV)
InactiveCN102108415BHigh sensitivityShort detection timeMicrobiological testing/measurementReverse transcriptasePike fry rhabdovirus
The invention discloses a method and a kit for detecting reverse transcription-loop mediated isothermal amplification (RT-LAMP) of pike fry rhabdovirus (RFRV), wherein an adopted outer primer contains sequences indicated by Seq ID No.1 and Seq ID No.2 respectively. According to the method and the kit which are provided by the invention, PFRV can be detected specifically; and the sensitivity is high and particularly reaches 100.5TCID50 when the kit is used for detecting the extracted nucleic acid in a virus suspension.
Owner:SHENZHEN AUDAQUE DATA TECH
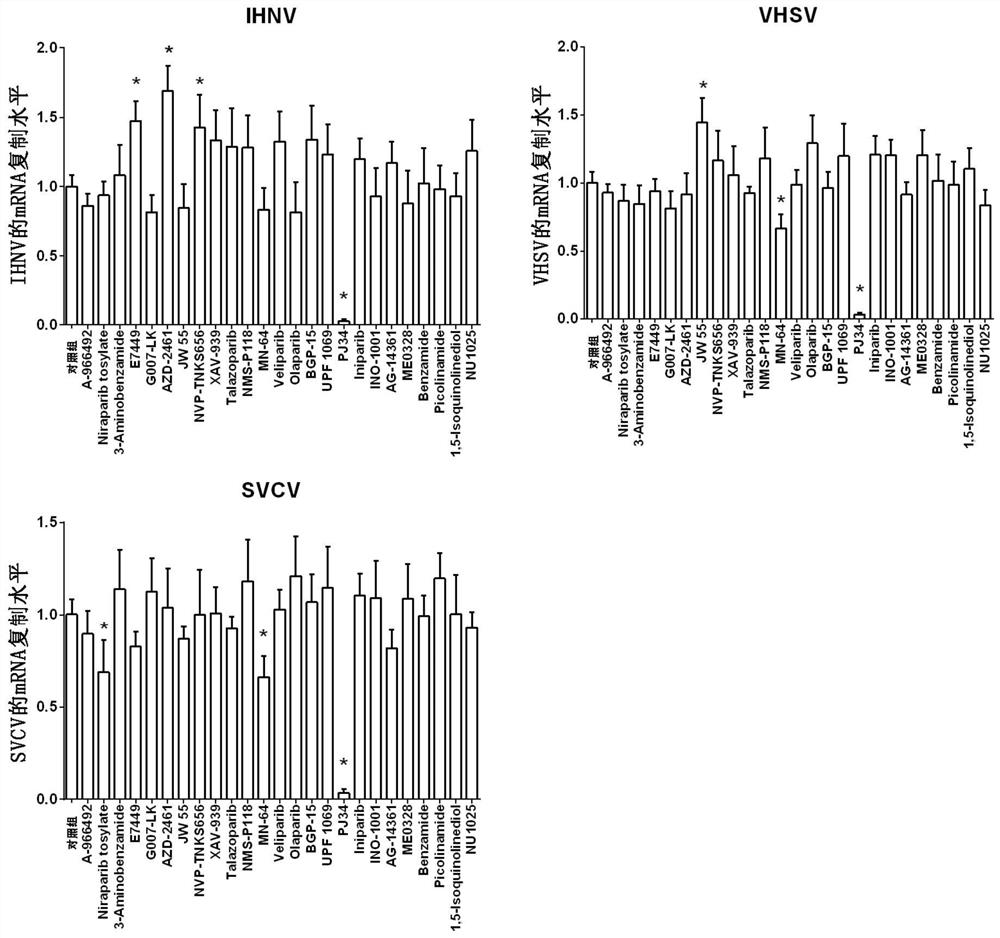
The invention also discloses application of PARP inhibitor PJ34 in preparation of anti-fish rhabdovirus products
ActiveCN112569233AInhibits mRNA replicationInhibition of replicationOrganic active ingredientsAntiviralsRhabdovirus carpioPharmaceutical drug
The invention discloses an application of a PARP inhibitor PJ34 in preparation of an anti-fish rhabdovirus product. The invention provides an application of a PARP inhibitor PJ34 or a derivative thereof or a pharmaceutically acceptable salt thereof or a substance taking the PARP inhibitor PJ34 or the derivative thereof or the pharmaceutically acceptable salt thereof as an active ingredient in preparation of an anti-fish rhabdovirus product. In the invention, a plurality of PARP inhibitors are screened, and results show that the PARP inhibitor PJ34 can inhibit mRNA replication of IHNV, VHSV andSVCV viruses in cells and reduce titer (TCID50) of the IHNV, the VHSV and the SVCV viruses at the same time; and the inhibition effect of the PJ34 on the IHNV, the VHSV and the SVCV shows a dose-dependent relationship. The PARP inhibitor PJ34 is expected to become a novel drug for resisting fish rhabdovirus.
Owner:HEILONGJIANG RIVER FISHERY RES INST CHINESE ACADEMY OF FISHERIES SCI
Rhabdovirus-sensitive monopterus albus kidney tissue cell line and application
ActiveCN112126619ALesion Effect (CPE) Stable and SignificantImprove biological activitySsRNA viruses negative-senseViral antigen ingredientsRhabdovirus carpioViral nucleic acid
The invention belongs to the field of aquatic organism cells and the technical field of aquaculture disease prevention and control, and discloses a rhabdovirus-sensitive monopterus albus kidney tissuecell line and application. The cell line in the invention is preserved in the China centre for type culture collection; and the preservation number is CCTCC NO:C2019286. The monopterus albus kidney tissue cell line (CrE-K) has a good growth state and is sensitive to CrERV newly discovered, separated and identified at present; after continuous passage of CrERV on CrE-K cells to the 16th generation, viral nucleic acid can still be detected; cytopathy is stable; the cells with the pathological change effect are subjected to ultrathin electron microscopy slicing; and a large number of CrERV mature virus particles and the replication process thereof can be observed in the CrE-K cells. The construction method of the monopterus albus kidney tissue cell line in the invention is high in repeatability, scientific and reasonable in condition and suitable for in-vitro culture of the rhabdovirus; and a technical platform is provided for separation, detection and culture of the rhabdovirus and complete biological characteristic research of the rhabdovirus.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Method for producing siniperca chuatsi infectious spleen and kidney necrosis virus and siniperca chuatsi rhabdovirus through microcarrier suspension culturing CPB cells
ActiveCN107326016AEasy to operateLow pollution rateSsRNA viruses negative-senseMicroorganism based processesBottleViral infection
The invention discloses a method for producing a siniperca chuatsi infectious spleen and kidney necrosis virus and a siniperca chuatsi rhabdovirus through microcarrier suspension culturing CPB cells. The method comprises the steps of selecting the CPB cells as a cell line for making vaccines; sequentially carrying out step-by-step magnifying microcarrier suspension culture on the CPB cells through stirring bottles and bioreactors, so that the CPB cells are used as hosts of virus infection; finally obtaining the siniperca chuatsi infectious spleen and kidney necrosis virus bulk and the siniperca chuatsi rhabdovirus bulk. The prepared ISKNV potency reaches up to 8.34LgTCID50 / mL, and the SCRV potency reaches up to 10.25LgTCID50 / mL. A microcarrier suspension culture process adopted by the invention is simple to operate, less in pollution probability, small in labor force compared with a roller bottle culture method, and lower in production cost.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
LAMP detection primer group for Siniperca chuatsi-perch rhabdovirus, application of LAMP detection primer group and detection kit
InactiveCN112063750AHigh sensitivityLow technical requirementsMicrobiological testing/measurementMicroorganism based processesRhabdovirus carpioMicrobiology
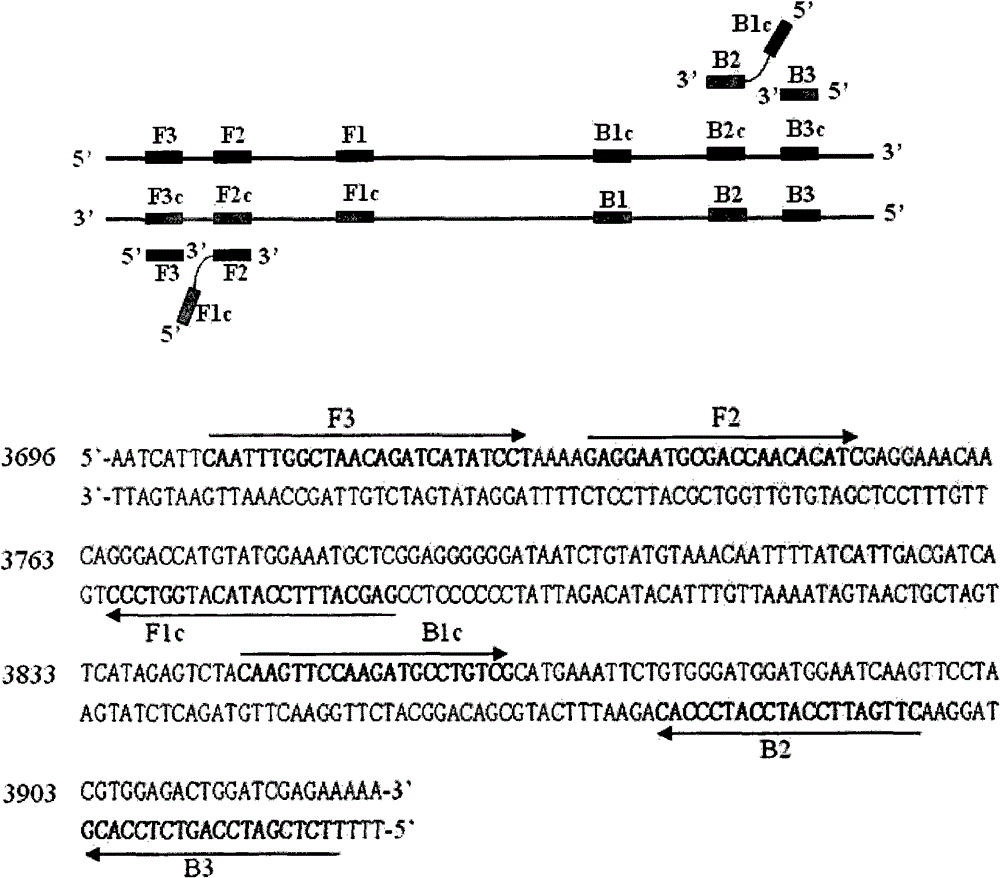
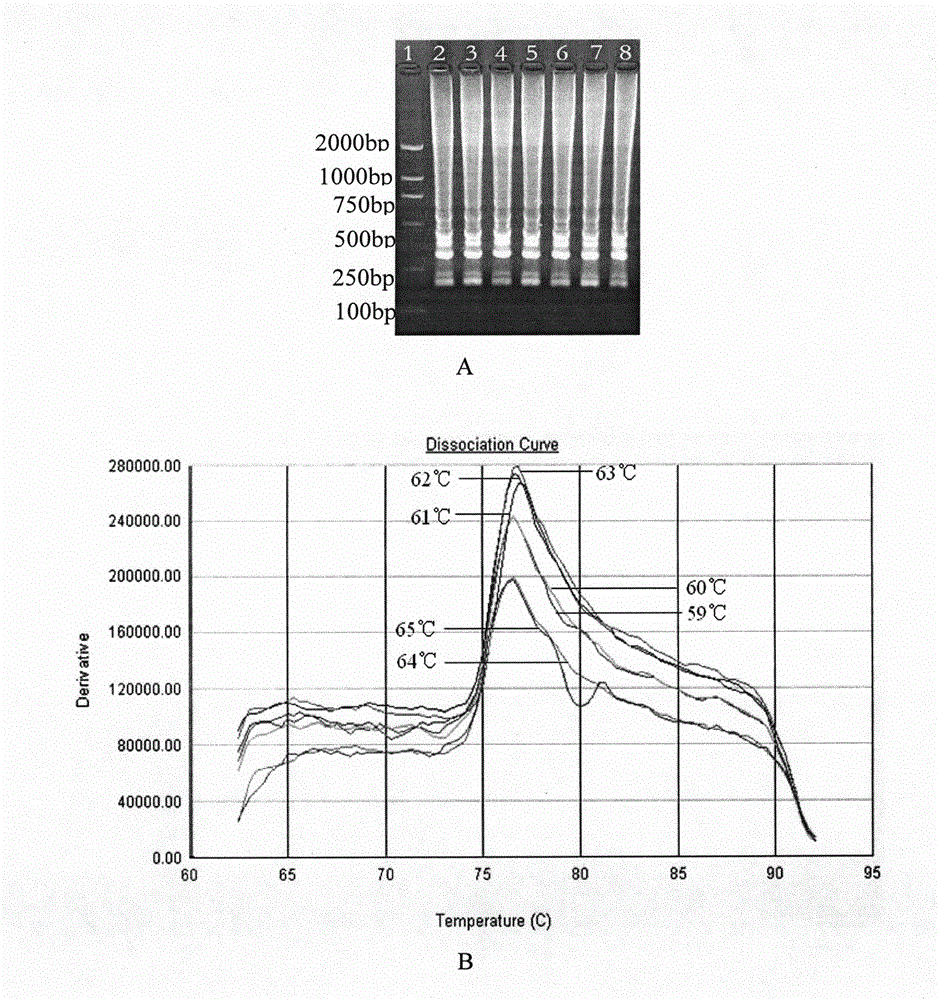
The invention discloses an LAMP detection primer group for Siniperca chuatsi-perch rhabdovirus, application of the LAMP detection primer group and a detection kit. The primer group comprises a pair ofouter primers, a pair of inner primers and a pair of loop primers; the outer primers comprise a sequence L-F3 and a sequence L-B3, the sequence L-F3 of the outer primers is shown in SEQ ID NO: 1, andthe sequence L-B3 of the outer primers is shown in SEQ ID NO: 2; the inner primers comprise a sequence L-FIP and a sequence L-BIP, the sequence L-FIP of the inner primers is shown in SEQ ID NO: 3, and the sequence L-BIP of the inner primers is shown in SEQ ID NO: 4; and the loop primers comprise a sequence L-LF and a sequence L-LB, the sequence L-LF of the loop primers is shown in SEQ ID NO: 5, and the sequence L-LB of the loop primers is shown in SEQ ID NO: 6. The invention further provides application of the primer group in preparation of a Siniperca chuatsi-perch rhabdovirus detection kitand the kit. The primer group and kit, provided by the invention, have the advantages of simplicity in operation, high detection speed, good specificity, high sensitivity, reliable result, and the like.
Owner:HOHAI UNIV +1
PCR primer group for rapidly detecting three fish viruses and application thereof
ActiveCN112359148AHigh sensitivityMeet detectionMicrobiological testing/measurementMicroorganism based processesNecrovirusRhabdovirus carpio
The invention discloses a triple PCR primer group for rapidly detecting three fish viruses and application thereof, and relates to the technical field of gene detection. The invention discloses a triple PCR primer group for rapidly detecting three fish viruses. The triple PCR primer group comprises an infectious spleen and kidney necrosis virus ISKNV primer, a mandarin fish frog virus SCRIV primerand a mandarin fish rhabdovirus SCRV primer, the triple PCR primer group has high sensitivity, the detection time is 2-3 hours earlier than that of conventional PCR, the efficiency of clinical diagnosis is greatly improved, richer reference bases can be provided for epidemiological investigation of ISKNV, SCRIV and SCRV infection, and the triple PCR primer group has important significance for subsequent research and prevention and treatment.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
Pharmaceutical composition for treating tumors or cancers and application thereof
ActiveCN111939262AEffective infiltrationPromote infiltrationSsRNA viruses negative-senseOrganic active ingredientsRhabdovirus carpioPharmaceutical drug
The invention relates to a pharmaceutical composition for treating tumors or cancers and application thereof. Particularly, the pharmaceutical composition comprises an oncolytic rhabdovirus deliveredthrough direct local injection or systemic administration or intratumoral delivery, and the oncolytic rhabdovirus can generate a synergistic inhibition effect on tumors and / or cancers in combination with administration of a CD38 small-molecule inhibitor. Besides, the oncolytic rhabdovirus has the characteristic of recognizing tumor cells and cannot damage normal cells, meanwhile, the CD38 small-molecule inhibitor has the specific T cell receptor molecule inhibiting activity, and the safety and the curative effect are remarkably excellent through combined use of the oncolytic rhabdovirus and the CD38 small-molecule inhibitor.
Owner:FANTASIA BIOPHARMA ZHEJIANG CO LTD
Fish pike fry rhabdovirus nested PCR detection primer group, kit and method and application
InactiveCN110607403AStrong specificityIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationTotal rnaBiology
The invention relates to a fish pike fry rhabdovirus nested PCR detection primer group, kit and method and application, and belongs to the technical field of molecular biology. The fish pike fry rhabdovirus nested PCR detection method comprises the steps that total RNA of a sample to be detected is used as a template, a reverse transcription reaction is carried out, and cDNA of the sample to be detected is obtained; the cDNA of the sample to be detected is used as a template to perform a first round PCR amplified reaction; then a first round PCR amplified product is used as a template to perform a second round PCR amplified reaction; and a second round PCR amplified product is subjected to agarose gel electrophoresis, gel is placed into an imaging system for observing whether a positive amplification stripe appears or not, and whether the sample to be detected contains fish pike fry rhabdovirus or not is judged. The fish pike fry rhabdovirus nested PCR detection method has the characteristics of high sensitivity and high specificity, and fish pike fry rhabdovirus-snakehead vesicular stomatitis virus can be rapidly and accurately detected.
Owner:YANGZHOU UNIV
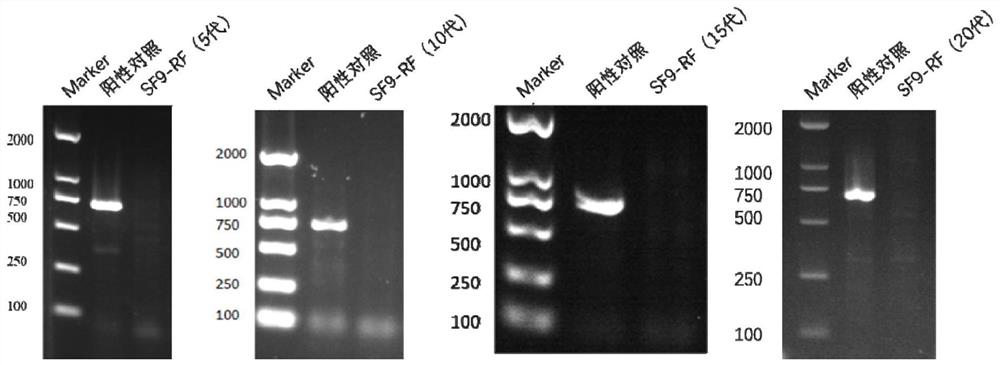
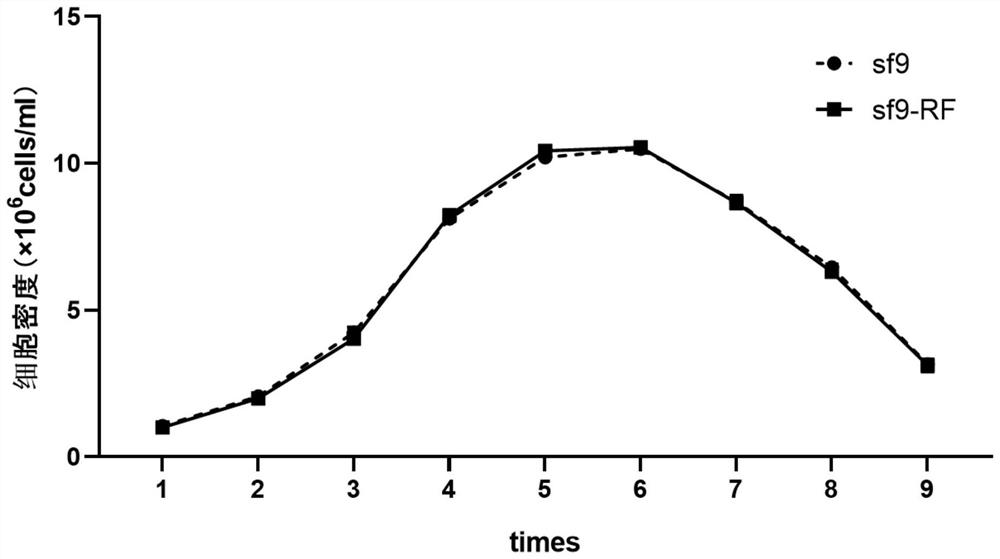
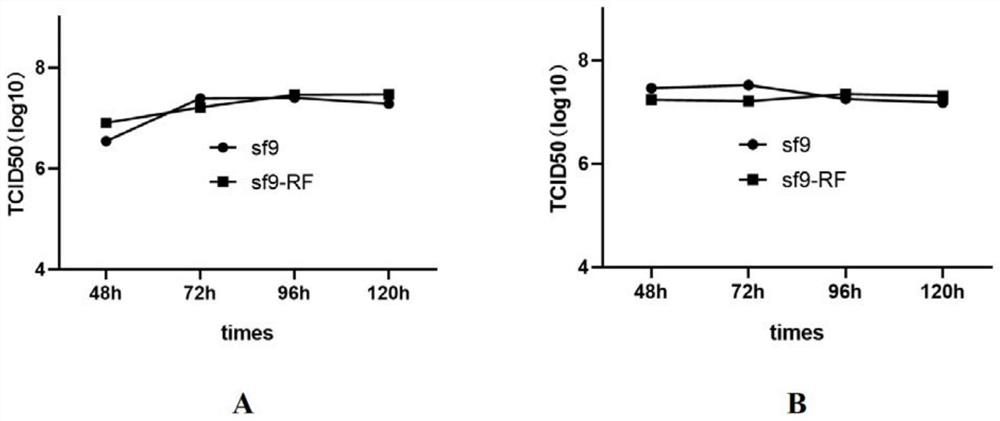
Serum-independent rhabdovirus-pollution-free sf9 cell strain, screening method and application
PendingCN114807009AEnsure safetyInvertebrate cellsMicrobiological testing/measurementEntire cellRhabdovirus carpio
The invention relates to cell screening, in order to solve rhabdovirus pollution in an sf9 cell strain, the invention provides a serum-independent rhabdovirus-pollution-free sf9 cell strain, the cell strain is named as an sf9-RF cell strain, and the biological preservation number of the cell strain is CGMCC No.45028. The invention also provides a method for preparing the sf9 cell strain. The invention further provides a screening method of the serum-free rhabdovirus-pollution-free sf9 cell strain, and screening is carried out under a serum-free condition. The invention also provides an application of the serum-independent rhabdovirus-pollution-free sf9 cell strain in foreign protein expression or AAV production based on a baculovirus expression system. According to the serum-free rhabdovirus-pollution-free sf9 cell strain disclosed by the invention, a serum-free insect cell culture medium is used in the whole cell culture and screening process, and any exogenous virus is not introduced, so that various indexes of the cell line meet the requirements of cell matrixes for production, and when the cell line is applied to an expression system of baculovirus, the cell line can be used for preparing the rhabdovirus-pollution-free sf9 cell strain. And the safety of biological products can be effectively ensured.
Owner:成都纳微金生物技术有限公司
ShRNA for inhibiting replication of micropterus salmoides rhabdovirus and application of shRNA
ActiveCN112574989ALower levelReduce cumulative mortalityOrganic active ingredientsGenetic material ingredientsNucleotideMedicine
The invention discloses shRNA for inhibiting the replication of micropterus salmoides rhabdovirus and an application of the shRNA, and belongs to the technical field of biological detection. The nucleotide sequence of the shRNA provided by the invention is as shown in SEQ ID No.35. The invention also provides a recombinant vector containing a DNA fragment for encoding the shRNA, a strain containing the recombinant vector, and applications of the recombinant vector and the strain in preparation of drugs for inhibiting the replication of micropterus salmoides rhabdovirus G glycoprotein. The shRNA recombinant vector provided by the invention is safe and non-toxic, can significantly inhibit the replication of the micropterus salmoides rhabdovirus G glycoprotein, and has a wide application prospect.
Owner:ZHEJIANG INST OF FRESH WATER FISHERIES +1
Fluorescent quantitation PCR detection primer and kit for Sf-rhabdovirus
PendingCN112063754AStrong specificityQuick QualificationMicrobiological testing/measurementDNA/RNA fragmentationNucleotideRhabdovirus carpio
The invention discloses a fluorescent quantitation PCR detection primer and kit for a Sf-rhabdovirus. The nucleotide sequence of the fluorescent quantitation PCR primer is disclosed by SEQ ID NO.1-SEQID NO.2. The fluorescent quantitation PCR primer disclosed by the invention can specifically detect the Sf-rhabdovirus, is high in detection sensitivity, has an extremely good linear relationship ina copy range of 8E*10-10, efficiently detects the Sf-rhabdovirus and improves detection efficiency.
Owner:SOUTH CHINA UNITED VACCINE INST
A kind of perch rhabdovirus recombinant g2 protein and its application
ActiveCN113512096BGood immune ingredientsRaise antibody levelsSsRNA viruses negative-sensePeptide/protein ingredientsImmune profilingRhabdovirus carpio
The present invention obtains G2 protein through prokaryotic recombinant expression. The protein fragment has good immunogenicity and can stimulate the body to produce a higher antibody level. Compared with the whole G protein and other fragments, this fragment has better immunogenicity and immune protection Performance, based on the combination of G2 protein and functional carbon nanotubes, the carbon nanotube carrier subunit vaccine prepared by immunizing perch with MSRV FJ985 strain 21 days later, the immune protection rate of perch was 86%, which was higher than the protection rate of existing reports. And based on the selection of specific protein fragments, it has a better protective effect than the original G protein and other G protein fragments based on immune analysis, and can produce better protection after immunization of sea bass, and can make sea bass effectively resist MSRV Poisonous attack.
Owner:深圳万可森生物科技有限公司
Application of parp inhibitor pj34 in the preparation of anti-fish rhabdovirus products
ActiveCN112569233BInhibits mRNA replicationInhibition of replicationOrganic active ingredientsAntiviralsRhabdovirus carpioPharmaceutical medicine
The invention discloses the application of PARP inhibitor PJ34 in the preparation of anti-fish rhabdovirus products. The present invention provides PARP inhibitor PJ34 or derivatives thereof or pharmaceutically acceptable salts thereof or substances with PARP inhibitors PJ34 or derivatives thereof or pharmaceutically acceptable salts thereof as active ingredients in the preparation of anti-fish bomb Applications in virus products. The present invention has screened multiple PARP inhibitors, and found that PARP inhibitor PJ34 can suppress the mRNA replication of IHNV, VHSV and SVCV virus in cells, and reduce the titer of IHNV, VHSV and SVCV virus simultaneously (TCID 50 ); and the inhibitory effect of PJ34 on IHNV, VHSV and SVCV showed a dose-dependent relationship. PARP inhibitor PJ34 is expected to be a new drug against rhabdovirus in fish.
Owner:HEILONGJIANG RIVER FISHERY RES INST CHINESE ACADEMY OF FISHERIES SCI
A kind of flounder rhabdovirus detection test paper and preparation method thereof
The invention discloses a flounder rhabdovirus detection test paper and a preparation method thereof. The detection test paper includes a carrier plate, a sample pad, a gold standard pad, a nitrocellulose membrane detection layer, and an absorbent pad. The sample pad and the absorbent pad are respectively at the two ends of the carrier plate, and one end of the gold standard pad is located below the sample pad. The gold label pad is loaded with colloidal gold-labeled anti-flounder rhabdovirus G protein monoclonal antibody, which is secreted by the hybridoma cell line HIRRV‑G‑4D10 (CCTCC NO: C201869), and the detection line and Quality control line, wherein the detection line is close to the side of the sample pad, and the quality control line is close to the side of the absorbent pad. The control line was coated with goat anti-mouse IgG. The invention can specifically detect the flounder rhabdovirus, has the advantages of rapidity, sensitivity, accuracy, little influence, low cost and the like, and is not restricted by professionals.
Owner:OCEAN UNIV OF CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com