Recombinant Rhabdovirus containing a heterologous fusion protein
a fusion protein and rhabdovirus technology, applied in the field of rhabdovirus recombinant, can solve the problems of virus not being able to replicate, gene therapy vectors that have encountered problems such as overcoming wild-type tropisms, effective incorporation of non-rvs, etc., and achieve the effect of facilitating the fusion of the lipid envelop
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Creation of a Modified Rhabdovirus
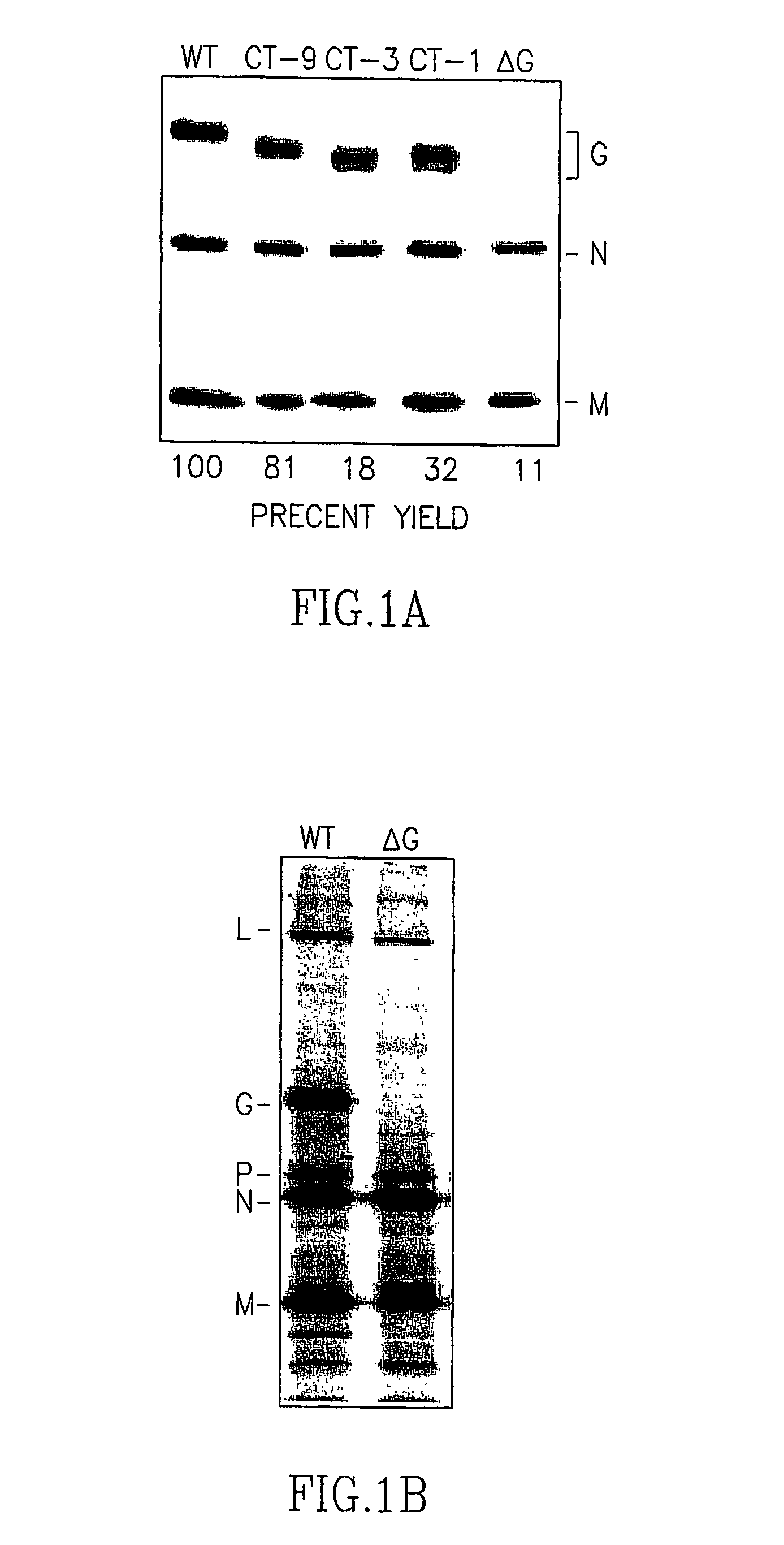
[0107] Construction of recombinant VSV cDNAs. A diagram of the method used, in this example, to construct a recombinant VSV that expresses the F protein of paramyxovirus SV5 is shown in FIG. 5. Full-length VSV cDNAs encoding G proteins with truncated cytoplasmic domains were generated by replacing the gene encoding the wild-type G protein in pVSVFL(+).sub.I / NIG (N. Lawson et al., Proc. Nat'l Acad. Sci. USA (1995) 92: 4477-4481). Another version of CT-1 was also constructed, which contained three consecutive stop codons, and which had the remaining portion of the cytoplasmic tail coding region to the NheI site deleted. This mutant is called CT-1TS (CT-1 Triple Stop) and was generated using standard PCR-mediated mutagenesis methods with a sense-strand primer (MW-36) complementary to nucleotides 975-997 (see the sequence for the complete genome of VSV-Indiana, GenBank # J02428), which are 5' to the KpnI site in the G protein cDNA, and an antisense olig...
example 2
In vitro Administration of Recombinant VSV Containing a Fusion Protein to Cells
[0113] The filtrate was then applied to cells in 10 cm dishes. After 18 to 24 hr, the cells were examined for cytopathic effects (CPE). Supernatants from cultures exhibiting CPE were titered, and individual plaques were purified. Plaque purified isolates were used to infect BHK cells at a m.o.i. of one for the production of stocks of the tail mutant viruses. Direct sequencing of viral RNA isolated from each of the mutants was performed to ascertain whether the recovered virus contained the appropriate mutations.
[0114] One-half of each filtered supernatant from the VSV-HAGS / minivirus technique was used to infect fresh cells. Successful recoveries were indicated when cultures showed the typical cytopathic effects indicative of a normal VSV infection 18 to 24 hr post infection. The supernatants from those cultures were then titered and stocks of the co-complementing viruses were produced by infecting fresh c...
example 3
Administration of Modified VSV to Cells in vivo
[0115] Recombinant VSV particles prepared by either of the methods described above are inoculated into a subject infected with HIV-1. The concentration of the virus to be administered to a subject may vary, but the titer of the virus to be administered preferably should be in the order of about 10.sup.6 to 10.sup.12 IU / ml. The amount of the virus used may be greater if the patient has a relatively high number of infected cells. The viruses harvested as described in Example 1 would e resuspended in an appropriate carrier or excipient for purposes of injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| Fluorescent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com