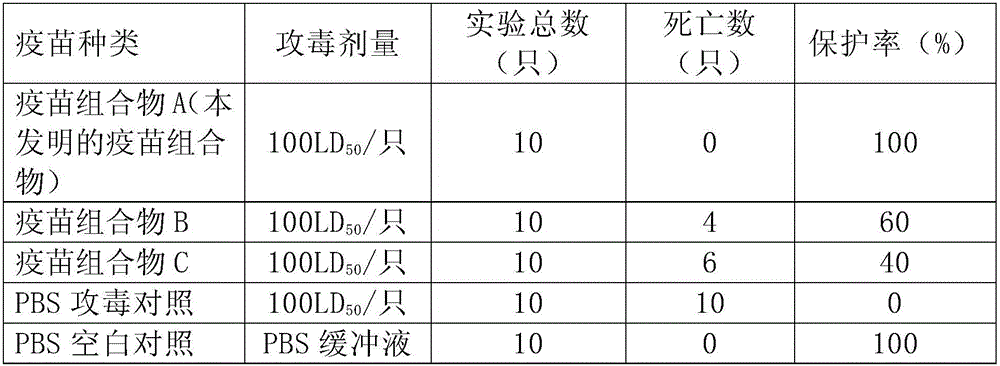
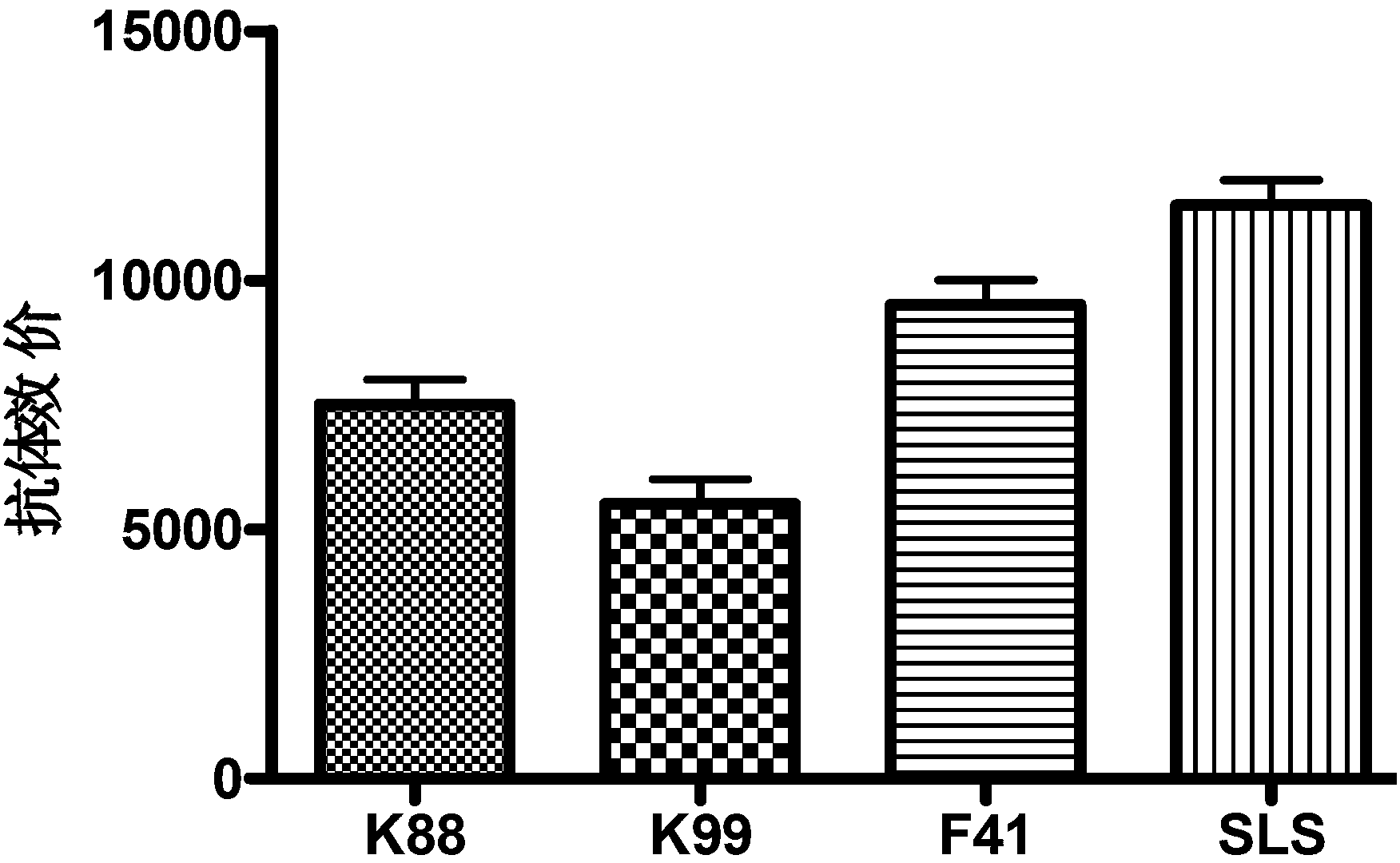Patents
Literature
68results about How to "High immune protection rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Fish compound traditional Chinese medicinal preparation and preparation method thereof
InactiveCN101829211ASolve the situation that there is no cure for intractable diseasesSolve the situation of no cureMetabolism disorderAntiinfectivesDiseaseHerbal preparations
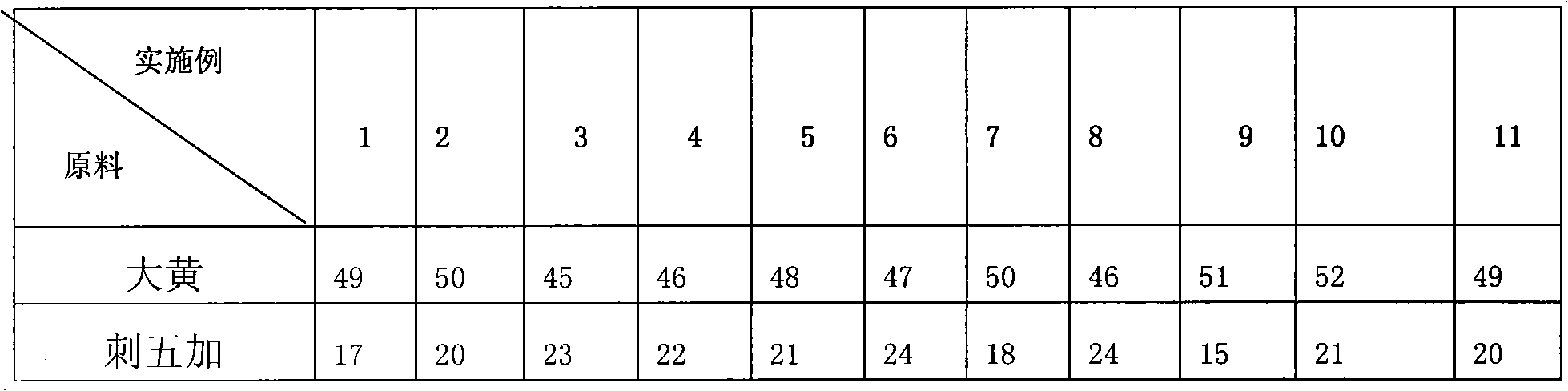
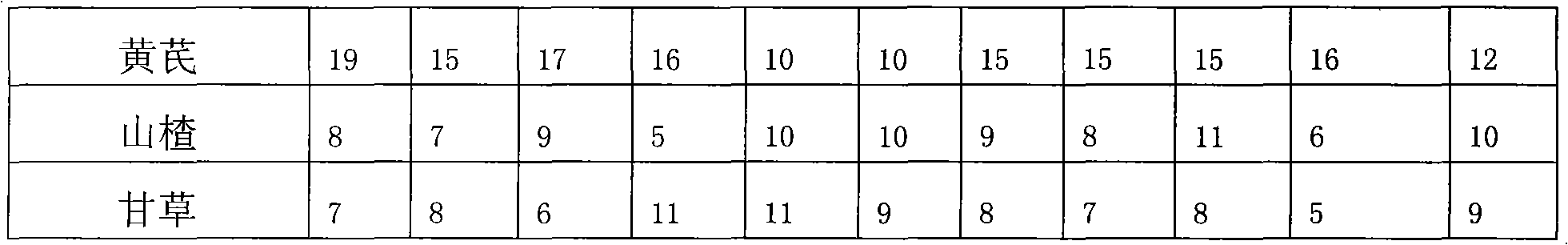
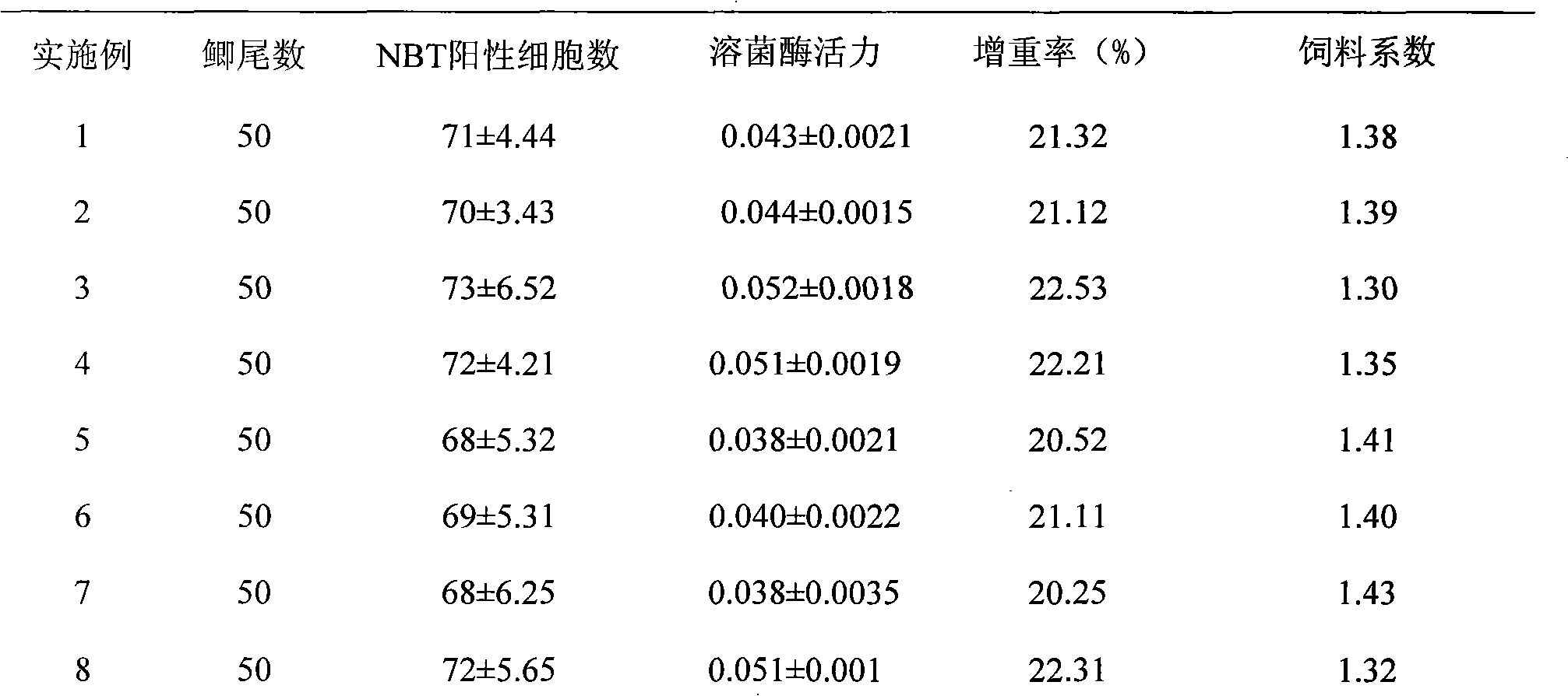
The invention discloses a fish compound traditional Chinese medicinal preparation prepared from raw materials including rhubarb, acanthopanax, radix astragali, hawthorn and liquorice according a certain weight ratio. A method for preparing fish compound traditional Chinese medicinal preparation comprises the following steps of: A. washing and drying the rhubarb, the acanthopanax, the radix astragali, the hawthorn and the liquorice in the sun; B. respectively crushing and sieving the rhubarb, the acanthopanax, the radix astragali, the hawthorn and the liquorice to ensure the granularity reaches 140-200 mu; and C. weighing up the rhubarb, the acanthopanax, the radix astragali, the hawthorn and the liquorice according to the ratio and mixing and subpackaging after uniformly mixed to obtain the fish compound traditional Chinese medicinal preparation. The method has the advantages of feasibility, convenient operation, reasonable formulation and convenient use; traditional Chinese medicines have wide sources, low price, remarkable effects and no toxic or side effects; various components mutually act; and the invention simultaneously has two properties of nutrition and medicament, not only contains nutrient substances, but also has components with antibacterial activity and other bioactivities, can improve the metabolism of cultivated fishes, promote the growth and the development, improve the immunologic function, prevent and treat diseases and the like.
Owner:武汉中博水产生物技术有限公司
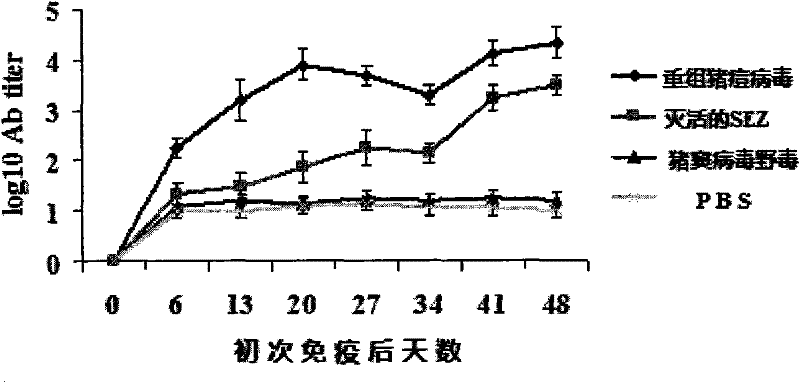
Recombinant Swine pox virus (SPV) vector vaccine for the expression of Streptococcus equi subsp zooepidemicus (SEZ) M-like protein (SzP)
InactiveCN102198268ANot pathogenicEasy Security EvaluationAntibacterial agentsBacterial antigen ingredientsProtein targetAdjuvant
The invention belongs to the field of biological pharmacy. The invention provides a recombinant vaccine, comprising a SPV and one pharmaceutically acceptable vector and / or adjuvant or a plurality of such vectors and / or adjuvants. The SPV comprises an SPV vector and the encoding genes of SzP. The recombinant SPV vaccine provided in the invention can proliferate in large quantities in immune animalbodies, express target protein SzP and induce the generation of high titer antibodies in animal bodies, and exerts a good protective effect on immune animals.
Owner:NANJING AGRICULTURAL UNIVERSITY
PRRSV (porcine reproductive and respiratory syndrome virus) virus-like particles with immunogenicity as well as preparation and application thereof
ActiveCN103555680AGood spatial configurationImproving immunogenicityViral antigen ingredientsAntiviralsVirus-like particleRespiratory syndrome virus
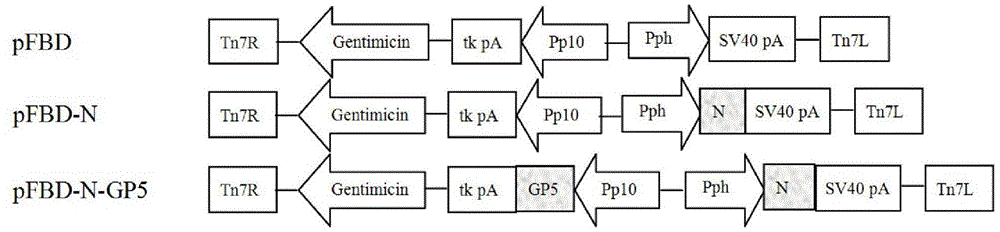
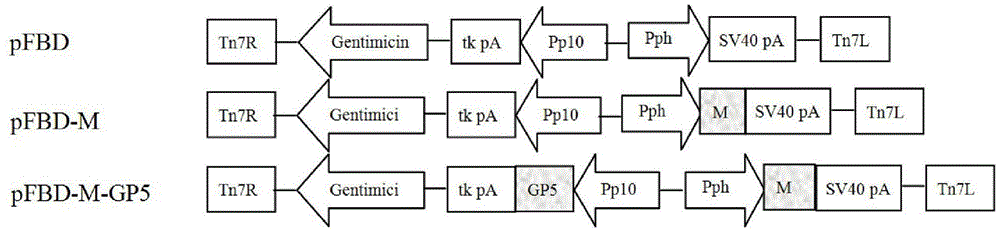
The invention discloses PRRSV (porcine reproductive and respiratory syndrome virus) virus-like particles with immunogenicity as well as preparation and application thereof, and belongs to the technical field of agricultural veterinary biology. The preparation comprises the following steps: cloning coding sequences of N protein and GP5 protein of PRRSV into rod granules to obtain recombinant rod granules; infecting the recombined rod granules on insect cells, culturing the transfected insect cells to obtain recombinant baculovirus. The N protein and GP5 protein are expressed by adopting the recombinant baculovirus infected insect cells, protein with better steric configuration can be obtained, and PRRSV virus-like particles can be automatically assembled. The virus-like particles with high immunogenicity can be used for preparing vaccine for preventing infection of PRRSV. The PRRSV virus-like particles have high immune protective rate, can produce attacking protection on PRRSV virulent strain, can be safer and more effective, and do not have the risk of virulence return.
Owner:WUHAN CHOPPER BIOLOGY
Recombinant PRRSV virus-like particles having immunogenicity and preparation thereof
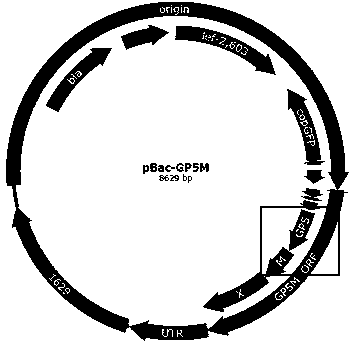
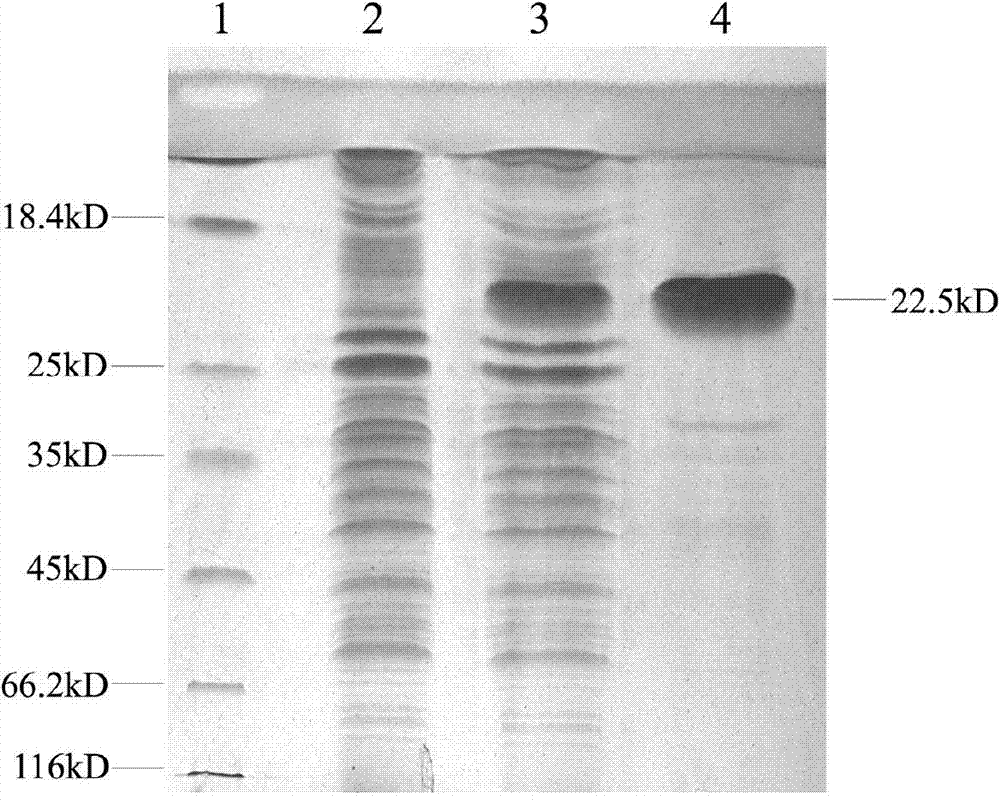
ActiveCN109385435ABroad-spectrum cross-immunogenicityImproving immunogenicitySsRNA viruses positive-senseViral antigen ingredientsSpecific immunityTransfer vector
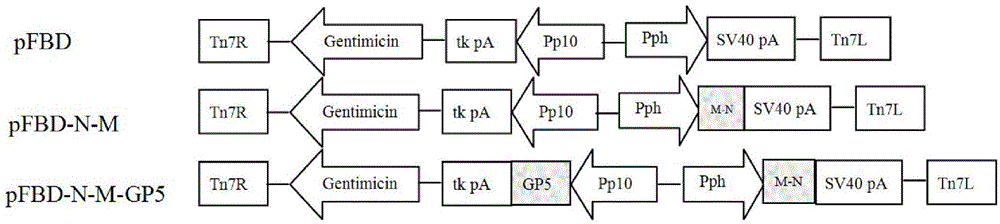
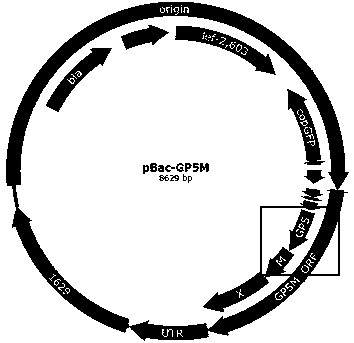
The invention discloses recombinant porcine reproductive and respiratory syndrome virus (PRRSV) virus-like particles (VLP) and a preparation method and an application thereof. Based on comparative analysis of GP5 of a PRRSV epidemic strain and an M gene sequence, a GP5 and M tandem sequence GP5M is synthesized artificially, the synthesized GP5M gene sequence is cloned into a vector with a pBAC5 plasmid as a skeleton, the baculovirus transfer vector pBAC-PRRSVGP5M is obtained, the recombinant bacmid rBacmid-GP5M is obtained, sf9 cells are transfected with the bacmid, and the recombinant baculovirus Ac-PRRSVGP5M is obtained. The PRRSV GP5 and M protein are expressed efficiently by the recombinant baculovirus, and the virus-like particles are formed. A subunit vaccine prepared by the proteinexpressed by the recombinant baculovirus can induce a body to produce a specific immune response after immunizing animals and can protect the pig body against the strong poison attacking of porcine reproductive and respiratory syndrome virus.
Owner:陕西诺威利华生物科技有限公司
Process for preparing subunit vaccine of reovirus antigen for grass carp
InactiveCN1616094AEasy to prepareEasy to operateViral antigen ingredientsAntiviralsInfected cellReovirus (antigen)
The preparation process of reovirus antigen subunit vaccine for grass carp includes the following steps: proliferation of GCRV873, which is the Hunan Shaoyang strain of reovirus, in grass carp kidney cell line; centrifugal purification and concentration of GCRV873 infected cell virus suspension; processing purified virus with surfactant to disintegrate complete virus structure to form viral nucleic acid and capsid protein subunit component; digesting with nuclease to degrade free virus genome dsRNA; dialysis to obtain reovirus protein subunit antigen preparation for grass carp containing no nuclease; and diluting with physiological saline to obtain the said vaccine. The vaccine of the present invention has the advantages of containing complete virus protein antigen component, containing no genetic virus matter RNA, simple preparation process, high product purity, etc.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Method for screening candidate bacterial strain from fish streptococcus agalactiae vaccine
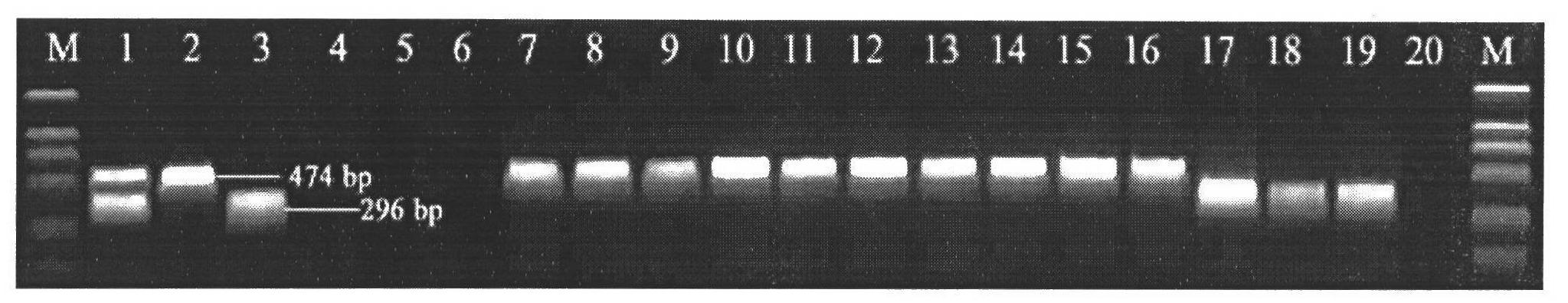
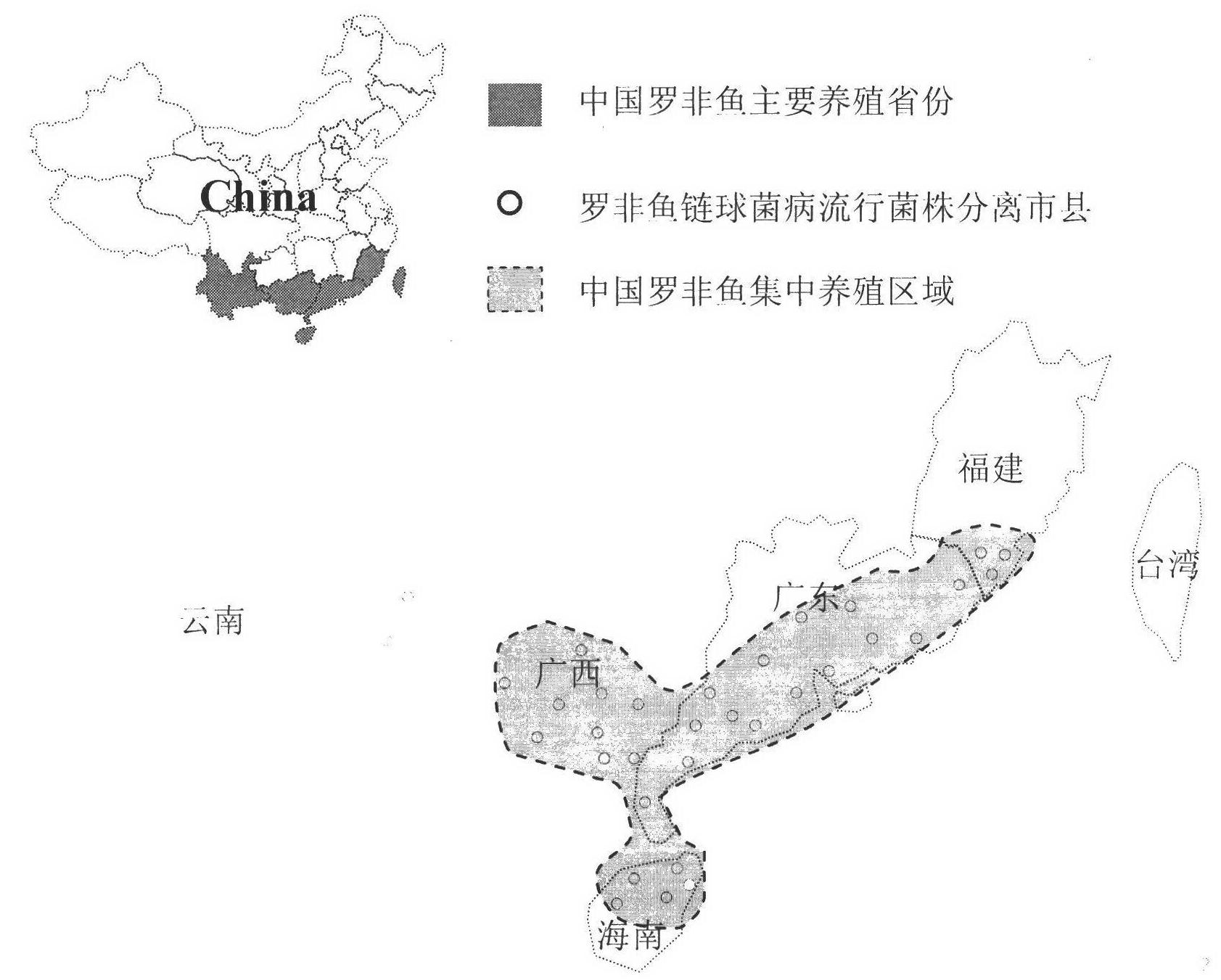
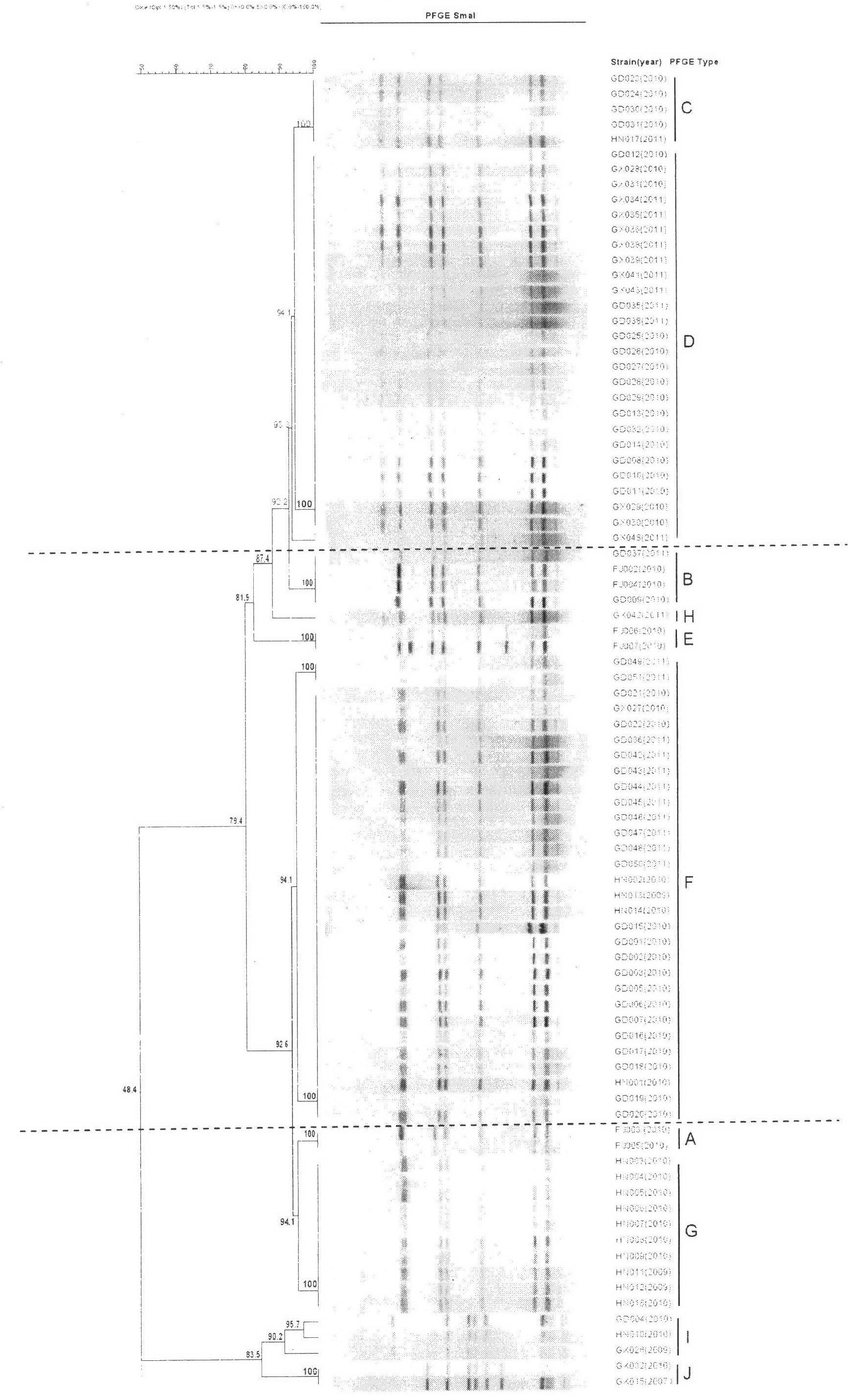
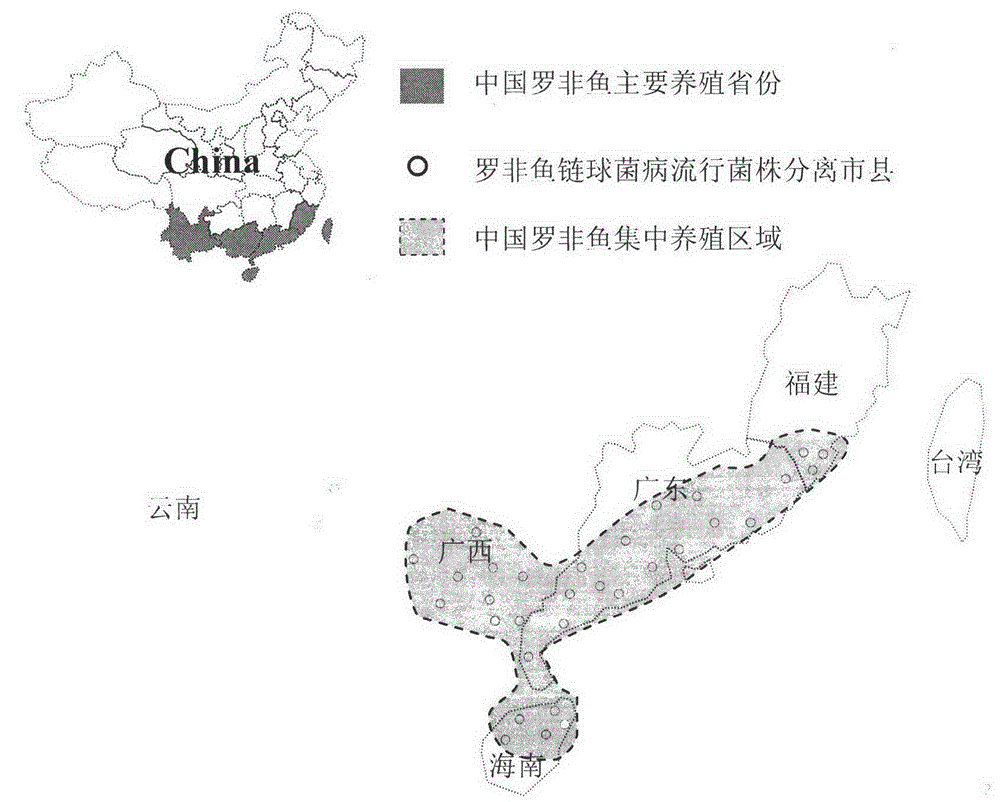
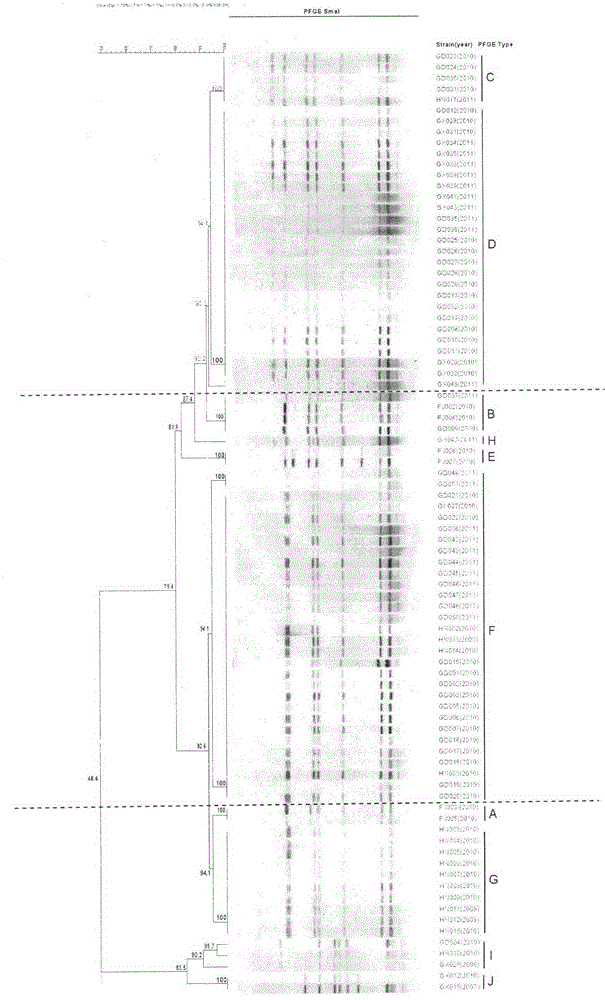
ActiveCN102676683AHigh immune protection rateLarge range of protectionMicrobiological testing/measurementMicroorganism based processesGenotype AnalysisBacterial strain
The invention discloses a method for screening candidate bacterial strains from fish streptococcus agalactiae vaccines. The method comprises the steps: separation and breed conservation of tilapia streptococcicosis agalactiae epidemic strains, identification of the epidemic strains and the establishing of a pathogenic library, PFGE genotype analysis of epidemic strains, toxicity determination of PFGE genotype representative strains, and immunogenicity and protection domain test of the PFGE genotype representative strains so as to obtain a candidate bacterial strains combination of a tilapia streptococcicosis agalactiae immunoprophylaxis vaccine in China. The technology has the characterizes of strong pertinence, high screening efficiency, remarkable effect and the like, and the screen candidate bacterial strains combination of the tilapia streptococcosis agalactiae immunoprophylaxis vaccine in China can protect 90% of genotypes and 96.47% of epidemic strains in the pathogenic library. The technology provides a new method for screening the candidate bacterial strains from the fish streptococcus agalactiae vaccines, is suitable for screening the candidate bacterial strains from the fish streptococcus agalactiae vaccines, and has significance and using value in immune prevention and control on fish streptococcicosis.
Owner:GUANGXI INST OF FISHERIES
Japanese encephalitis (JE) vaccine composition and preparing method thereof
InactiveCN105688202AImproving immunogenicityImprove protectionSsRNA viruses positive-senseViral antigen ingredientsWater basedJapanese encephalitis
The invention provides Japanese encephalitis (JE) vaccine composition. The JE vaccine composition comprises a JE virus antigen of immunization effective quantity and water based vaccine adjuvant. The invention further provides a preparing method and application of the JE vaccine composition. The vaccine composition has good immunogenicity and immunizing protection. The immune effect of the JE vaccine composition is remarkably superior to that of single pig JE live vaccine and other live vaccine diluted by immunologic adjuvant. The clinical application prospects are good.
Owner:SICHUAN AGRI UNIV
Attenuated live vaccine of eel vibrio
InactiveCN1600368AProtection against infestationImprove immunityAntibacterial agentsBacterial antigen ingredientsFish speciesAnguilliformes
An attenuated live vaccine of eel vibrio for preventing the sensitive fish from being infected by wild vibrio and its preparing process are disclosed.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method of immune-enhanced recombinant PRRSV virus-like particle subunit vaccine
ActiveCN109402145ABroad-spectrum cross-immunogenicityImproving immunogenicityViral antigen ingredientsVirus peptidesBaculovirus expressionVirus-like particle
The invention discloses a preparation method of an immune-enhanced recombinant PRRSV virus-like particle subunit vaccine. The subunit vaccine is prepared from PRRSV virus-like particles and compound immunological adjuvants. By a genetic engineering means, a PRRSV GP5-M gene is modified, and PRRSV virus-like particles with high immunogenicity are prepared by constructing a rhabdovirus expression vector. In addition, by improving the immunological adjuvants, the immuno-enhanced recombinant PRRSV virus-like particle subunit vaccine is obtained, and the subunit vaccine has better immunization effects.
Owner:陕西诺威利华生物科技有限公司
Edwardsiella tarda subunit vaccine, and preparation and application thereof
InactiveCN103690942AHigh immune protection rateEasy to applyAntibacterial agentsBacterial antigen ingredientsEdwardsiella tardaProtein coding
The invention relates to the field of immunology, and specifically relates to an Edwardsiella tarda subunit vaccine and a preparation method thereof. The protein gene of the vaccine has a base sequence shown in SEQ ID No.1 in a sequence table, and the vaccine protein coded by the protein gene has an amino acid sequence as shown in SEQ ID No.2 in the sequence table. The subunit vaccine provided by the invention is mixed with an oil emulsion adjuvant, and then capable of effectively exciting the generation of specific antibodies after immunizing the fish through intraperitoneal injection; the Edwardsiella tarda subunit vaccine is capable of efficiently improving the ability of resisting the infection of the Edwardsiella tarda of the immune fish and thus can be applied to controlling the Edwardsiella tarda of the fish.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Newcastle disease virus strain and application thereof in preparation of Newcastle disease vaccine
ActiveCN104164410AMake up for the shortcoming of too longProduce quicklyViral antigen ingredientsMicroorganism based processesMaternal antibodyAdjuvant
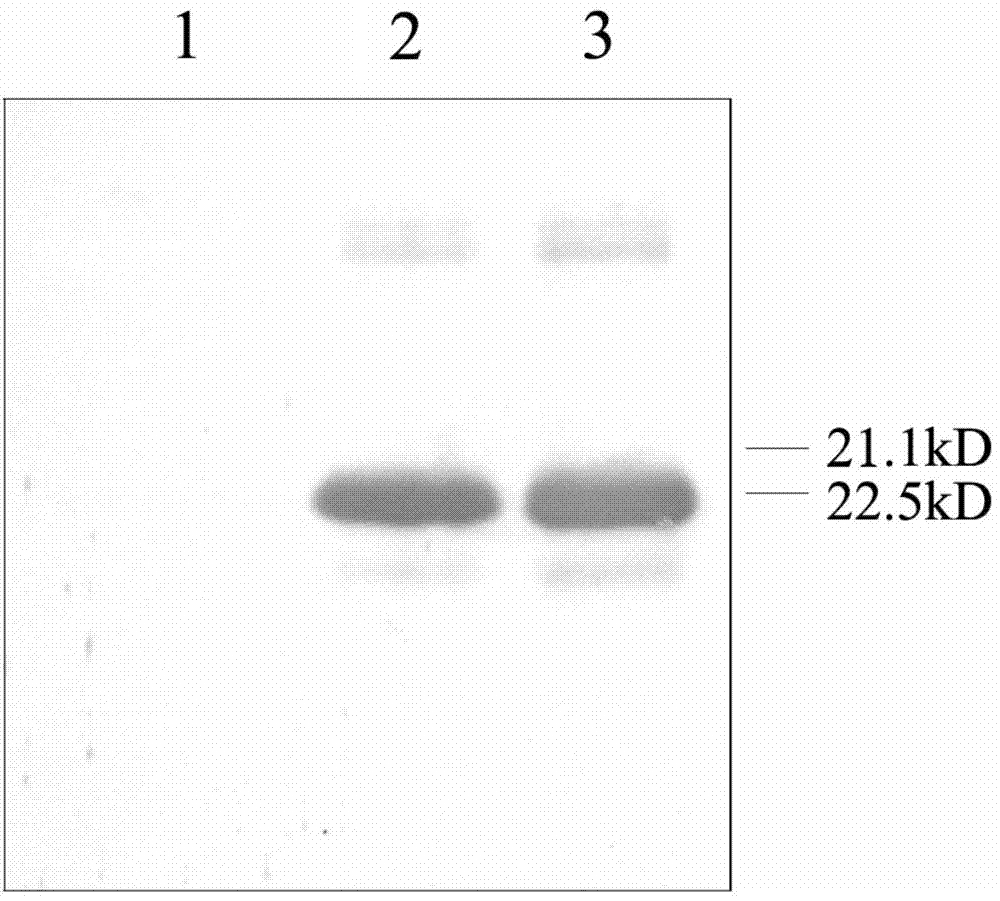
The invention discloses a Newcastle disease virus strain and an application thereof in preparation of a Newcastle disease vaccine. A preparation method of the Newcastle disease virus strain comprises the following step: culturing recombinant plasmids pBrClone30-GM-CSF, pBL-N plasmids, pBL-P plasmids and pBL-L plasmid cotransfection mammalian cells together so as to obtain the Newcastle disease virus strain, wherein the recombinant plasmids pBrClone30-GM-CSF are of the plasmids of DNA molecules shown in No.2703 to No.18408 nucleotides from the 5' tail end of a sequence 3 with a sequence table. According to the Newcastle disease virus strain, molecular adjuvants GM-CSF are led into the genomes of the existing Newcastle disease vaccine, so that the immune effect is effectively improved and the immune blank period is greatly shortened. Meanwhile, the Newcastle disease virus strain can be used for resisting a maternal antibody so as to improve the immune protective rate. Thus, the Newcastle disease virus strain has an important value for preventing and treating Newcastle diseases.
Owner:JIANGSU KANIONREAL BIOMEDICAL TECH CO LTD
Perch rhabdovirus subunit vaccine and preparation method thereof
ActiveCN113521265AHigh immune protection rateImproving immunogenicitySsRNA viruses negative-senseViral antigen ingredientsRhabdovirus carpioPerch rhabdovirus
Part of protein fragment G2 of MSRV glycoprotein is selected, G2 protein is obtained through prokaryotic recombinant expression, and mannosylation modification is carried out on the G2 protein to prepare a perch rhabdovirus nano-carrier targeting vaccine, and research shows that for perch after immunized by the subunit vaccine for 21 days is attacked by an MSRV FJ985 strain, the perch immune protection rate is 94%-96%, and the vaccine is safe and effective. After the perch is immunized, a better protection effect can be achieved, and the perch can effectively resist the attack of MSRV virulent virus.
Owner:深圳万可森生物科技有限公司
Immune-enhanced recombinant PRRSV (Porcine Reproductive and Respiratory Syndrome Virus)-like particle subunit vaccine
ActiveCN109395073ABroad-spectrum cross-immunogenicityHigh immune protection rateViral antigen ingredientsVirus peptidesImmunogenicitySubunit vaccines
The invention discloses an immune-enhanced recombinant PRRSV (Porcine Reproductive and Respiratory Syndrome Virus)-like particle subunit vaccine. The immune-enhanced recombinant PRRSV virus-like particle subunit vaccine consists of PRRSV-like particles and a compound immunologic adjuvant. The immune-enhanced recombinant PRRSV-like particle subunit vaccine disclosed by the invention has the beneficial effects that by the means of gene engineering, PRRSV GP5-M gene is modified, and the PRRSV-like particles with high immunogenicity are prepared by constructing rhabdovirus expression vectors. In addition, by improvement on the immunologic adjuvant, the immune-enhanced recombinant PRRSV-like particle subunit vaccine is obtained and the subunit vaccine has a better immune effect.
Owner:陕西诺威利华生物科技有限公司
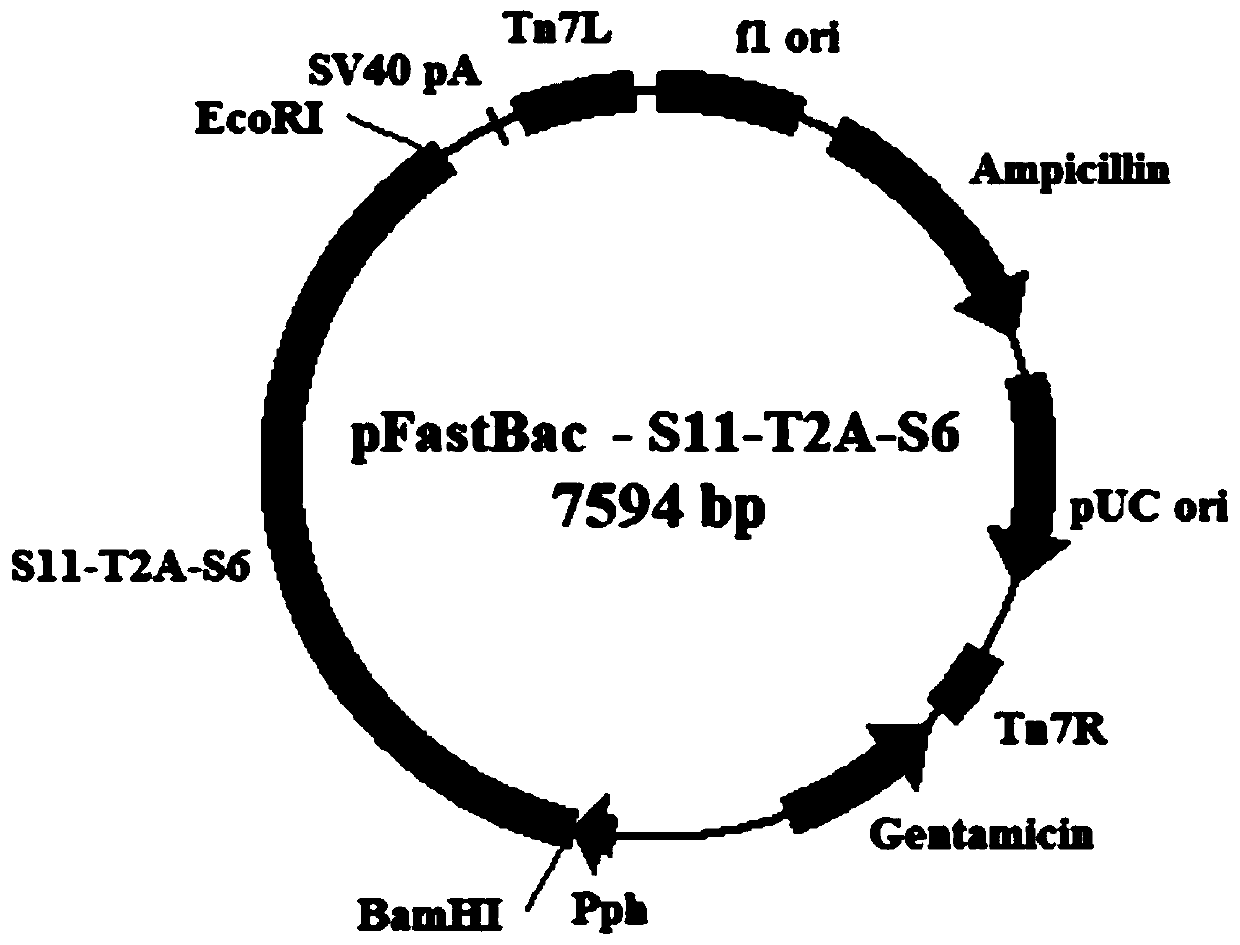
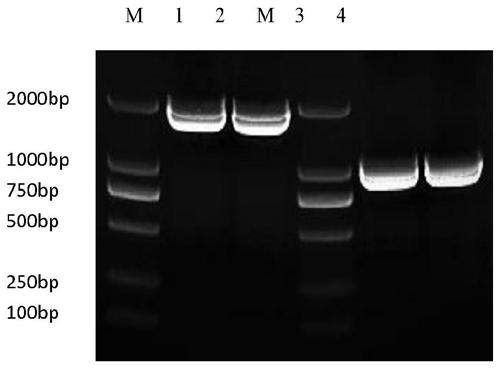
Preparation method and application of recombinant baculovirus co-expressing grass carp reovirus capsid proteins VP4 and VP35
ActiveCN110144334AEfficient expressionHigh immune protection rateViral antigen ingredientsVirus peptidesRestriction Enzyme Cut SiteCapsid
The invention belongs to the technical field of biology, and particularly discloses a preparation method and application of a recombinant baculovirus co-expressing grass carp reovirus capsid proteinsVP4 and VP35. Sequences as shown in SEQ ID NO.1, SEQ ID NO.3 and SEQ ID NO.2 are sequentially inserted among restriction enzyme cutting sites of BamHIand EcoRI pFastBacTMIto obtain recombinant plasmids pFastBac-S11-T2A-S6, and finally obtain the recombinant baculovirus co-expressing grass carp reovirus capsid proteins VP4 and VP35. The recombinant virus has high expression quantity, can be used for preparing proteins applicable to production of grass carp reovirus vaccines and have a wide application prospect.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Preparation method and application of Vibro harveyi and Vibrio parahaemolyticus bigeminy DNA vaccine
InactiveCN101879317AEasy to prepareStrong experimental operabilityAntibacterial agentsGenetic material ingredientsSide effectNucleotide
The invention relates to a preparation method and application of a Vibro harveyi and Vibrio parahaemolyticus bigeminy DNA vaccine. The Vibro harveyi and Vibrio parahaemolyticus bigeminy DNA vaccine is characterized by being a tdh2-oppA fusion gene protein eukaryotic expression engineering vector vaccine formed by connecting a Vibro harveyi oppA gene with a Vibrio parahaemolyticus tdh2 gene through 6 nucleotides GAATTC. The preparation method comprises the steps of: cloning the tdh2 gene and the oppA gene into a linear vector Pmd19-T Simple; inserting the fusion gene into a eukaryotic expression vector pEGFP-N1 by adopting a double-enzyme cleavage technology to construct a pEGFP-N1-tdh2-oppA fusion gene protein eukaryotic expression engineering vector vaccine; and finally preparing a Vibro harveyi and Vibrio parahaemolyticus bigeminy DNA vaccine suspension by using a conventional method, and immunizing fishes by the dosage of 100 microlitres per fish. The invention has strong operability, high repetition rate, safety, no toxic or side effect, double immune protection for fishes and higher immune effect than an inactivated vaccine, a recombinant protein vaccine and a common DNA vaccine.
Owner:OCEAN UNIV OF CHINA
Preparation method of grass carp reovirus genetic engineering vaccine
InactiveCN102266555AHigh expressionHigh expression yieldViral antigen ingredientsGenetic material ingredientsSaccharumSucrose
The invention provides a method for preparing grass carp reovirus (GCRV) gene engineering vaccines. The method comprises the following steps of: selecting recombinant baculoviruses (vAcGCRV-VP5 / VP7) of sf9 insect cell proliferated recombinant GCRV outer capsid proteins VP5 and VP7; separating recombinant protein components by freezing, thawing and cracking recombinant virus infected sf9 cells and adopting sucrose lining centrifuging method, and thus obtaining a purified in vitro expression VP5 and VP7 protein complex; and performing fish protection experiments on the purified VP5 and VP7 proteins to prove that the VP5 and VP7 proteins have good immune protection effect. The GCRV gene engineering vaccines prepared by the method not only have good stability and immunogenicity, but also have good immune protection effect on grass carp fries; the immune protection rate of the GCRV gene engineering vaccines reaches 90 percent; and the method is suitable for large-scale production and application of the vaccines.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Recombined enterotoxin (namely, fimbriae composite polyvalent vaccine) for resisting diarrhea of piglets and preparation method for recombined enterotoxin (namely, fimbriae composite polyvalent vaccine)
InactiveCN103007267AHigh immune protection rateAntibacterial agentsBacterial antigen ingredientsEnterotoxinPathogenic factor
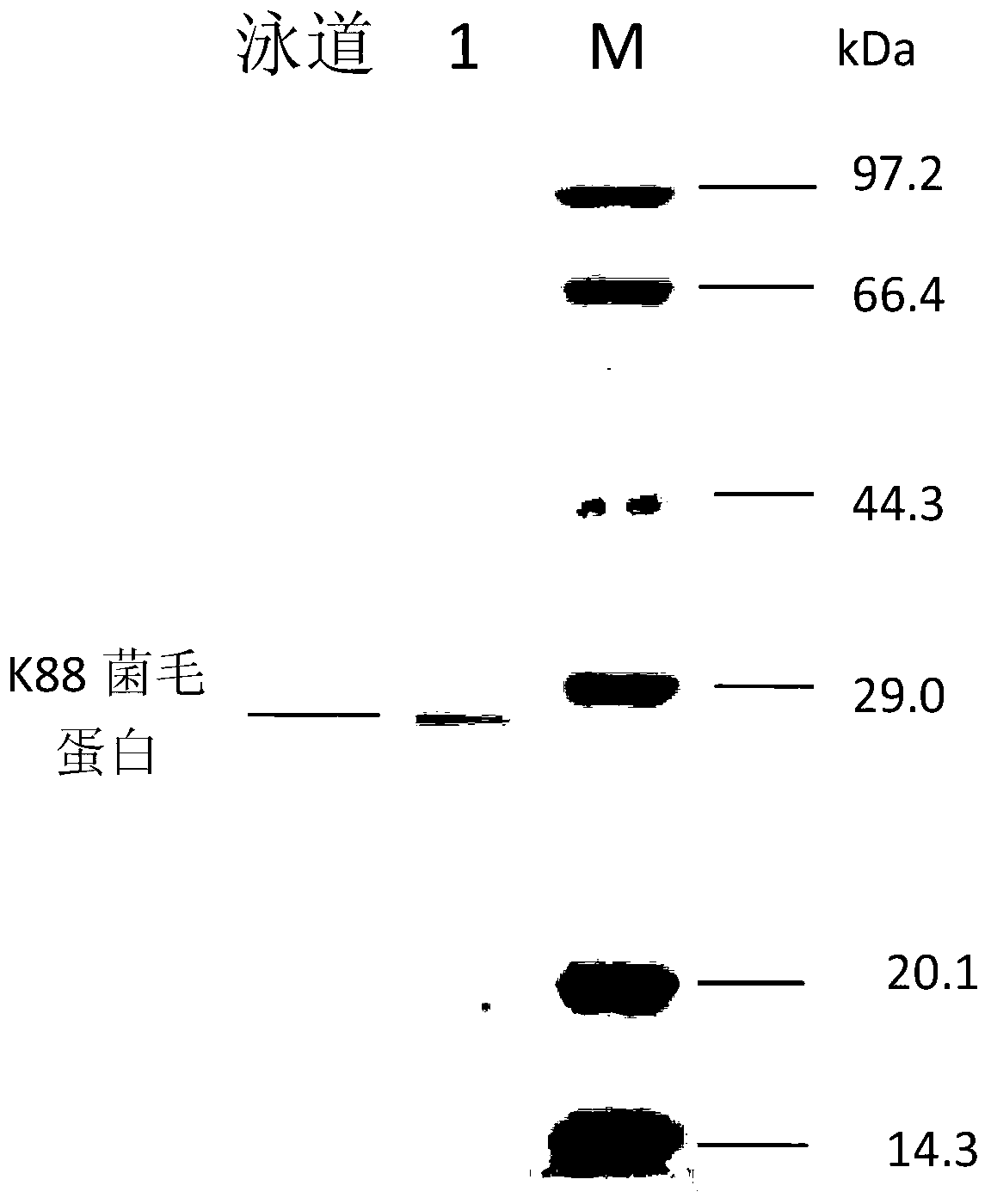
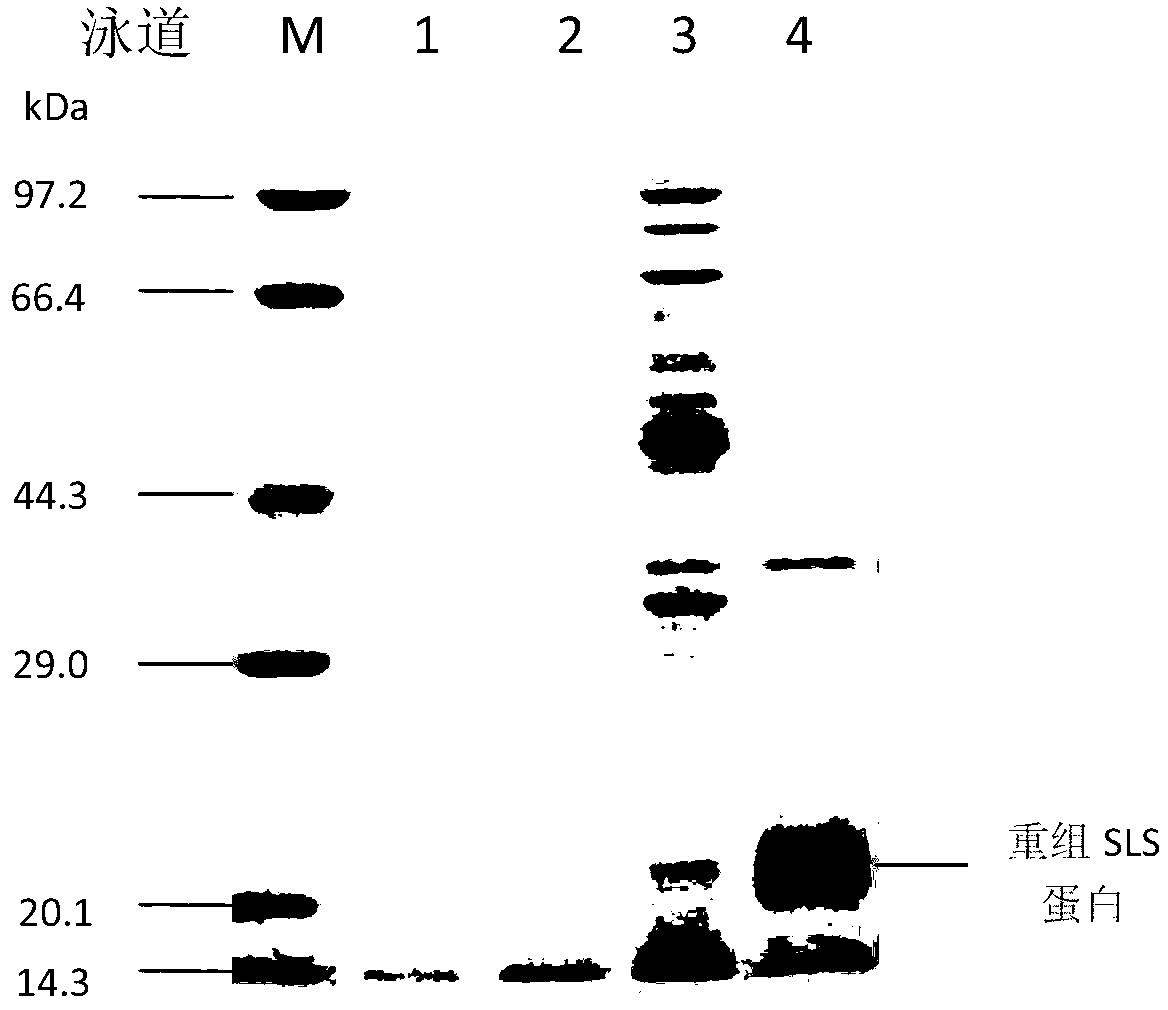
The invention discloses recombined enterotoxin (namely, fimbriae composite polyvalent vaccine) for resisting diarrhea of piglets and a preparation method for the recombined enterotoxin (namely, the fimbriae composite polyvalent vaccine), and belongs to the technical field of animal husbandry and veterinary. The composite vaccine comprising an enterotoxin protein antigen and a various fimbriae antigen protein is prepared by the following steps of: uniformly emulsifying recombined trivalent enterotoxin SLS proteins expressed by gene engineering recombination and fimbriae antigen proteins of the conventional pathogenic bacteria K88, K99 and F41 in China with an adjuvant. The recombined enterotoxin (namely, fimbriae composite polyvalent vaccine) comprises two important pathogenic factors of an ETEC (enterotoxigenic e.coli) pathopoiesia loop, namely, enterotoxin and fimbriae; each process of diarrhea generated by the ETEC is protected; in the first step, the growth of the ETEC is stopped; in the second step, the enterotoxin generated by a small amount of growing ETEC is neutralized, so that the generation of the diarrhea is controlled from respective layers; the vaccine can not be limited by domains; the use range is relatively wide; and the protection rate is relatively high. After the vaccine is used, the protection rate is up to 95 percent; the immune efficiency of the vaccine is 5 times higher than that of the vaccine used in the market; and the recombined enterotoxin (namely, the fimbriae composite polyvalent vaccine) is safe and reliable.
Owner:DALIAN UNIV OF TECH
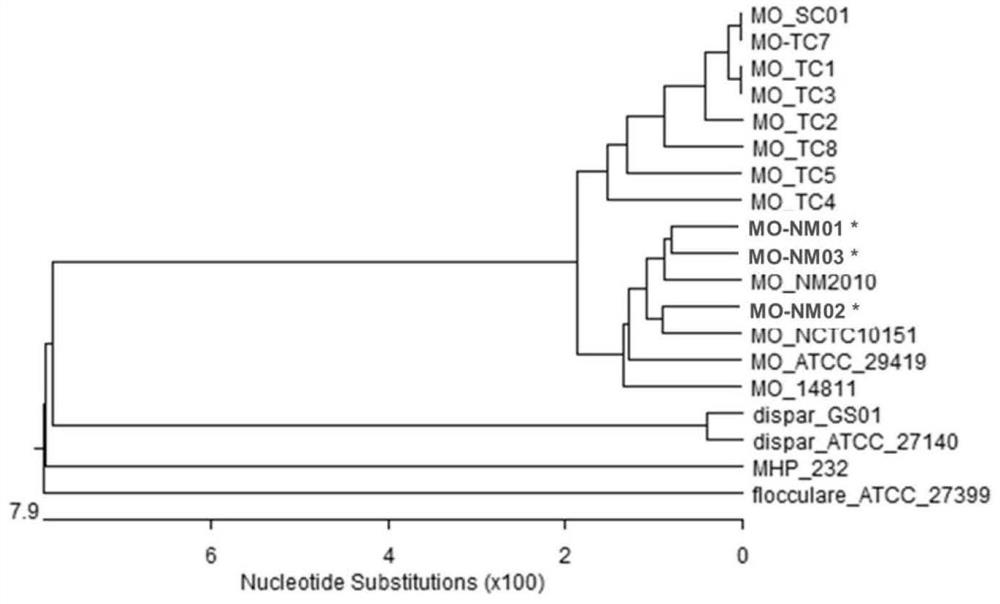
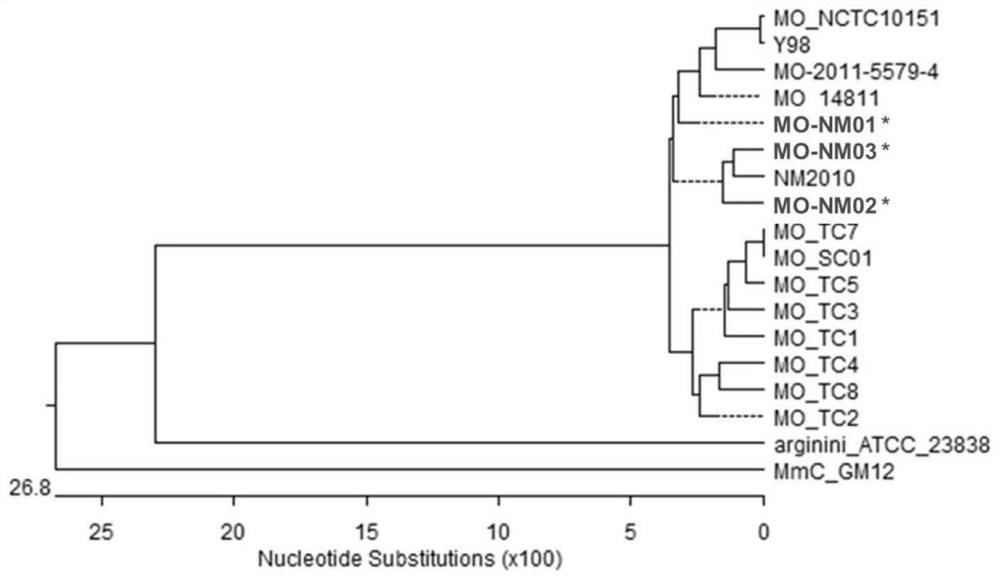
Combined strain for preparing mycoplasma ovipneumoniae vaccine, mycoplasma ovipneumoniae trivalent inactivated vaccine and preparation method of inactivated vaccine
ActiveCN111690554AHigh immune protection rateImprove protectionAntibacterial agentsBacterial antigen ingredientsBiotechnologyStructural protein
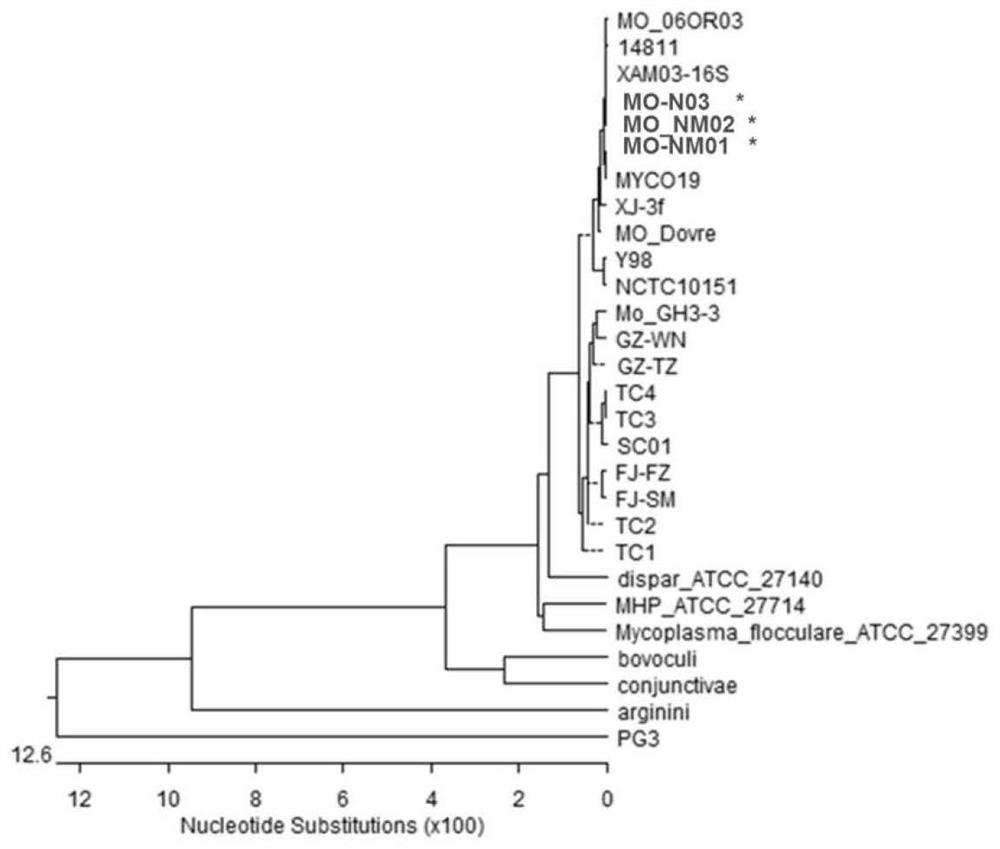
The invention provides a combined strain for preparing a mycoplasma ovipneumoniae vaccine, a mycoplasma ovipneumoniae trivalent inactivated vaccine and a preparation method of the inactivated vaccine,and belongs to the technical field of veterinary biological products. The combined strain comprises mycoplasma ovipneumoniae strains MO_NM01, MO_NM02 and MO_NM03, the preservation number of the mycoplasma ovipneumoniae strain MO_NM01 is CGMCC No.19698, the preservation number of the mycoplasma ovipneumoniae strain MO_NM02 is CGMCC No.19699, and the preservation number of the mycoplasma ovipneumoniae strain MO_NM02 is CGMCC No.19700. Housekeeping genes, structural proteins and polyclonal antibodies of the strains in the combined strain are different, the trivalent inactivated vaccine preparedfrom the combined strain can play a good role in protecting different strains, and has high immune protection rate.
Owner:INNER MONGOLIA AUTONOMOUS REGION ACAD OF AGRI & ANIMAL HUSBANDRY SCI
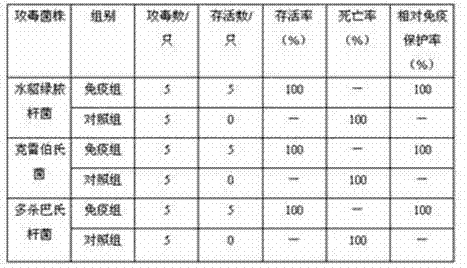
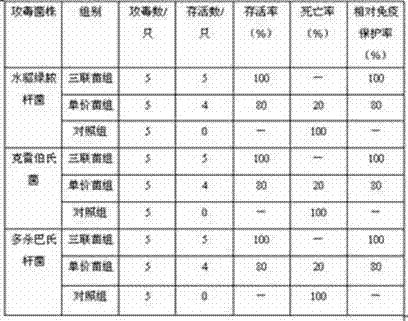
Pseudomonas aeruginosa, klebsiella and pasteurella triple-inactivated vaccine for mink
ActiveCN104740622APrevention of mixed infectionHigh immune protection rateAntibacterial agentsAntibody medical ingredientsDiseaseMicrobiology
The invention discloses a pseudomonas aeruginosa, klebsiella and pasteurella triple-inactivated vaccine for a mink. The vaccine is composed of inactivated pseudomonas aeruginosa, klebsiella and pasteurella, and is capable of simultaneously preventing a pseudomonas aeruginosa disease, a klebsiella disease and a pasteurella disease, and mixed infection with pseudomonas aeruginosa, klebsiella disease and pasteurella disease. The vaccine is small in side effect, has synergistic effect, has relatively good immunogenicity and safety, and can reach multiple prevention effects; the immune procedure for vaccination is reduced; and the protection efficiency is improved.
Owner:JILIN HEYUAN BIOENG LIMITED
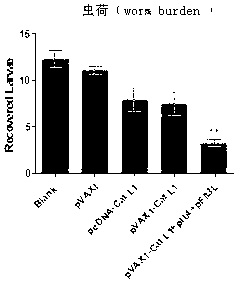
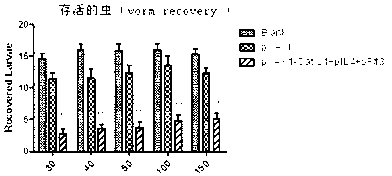
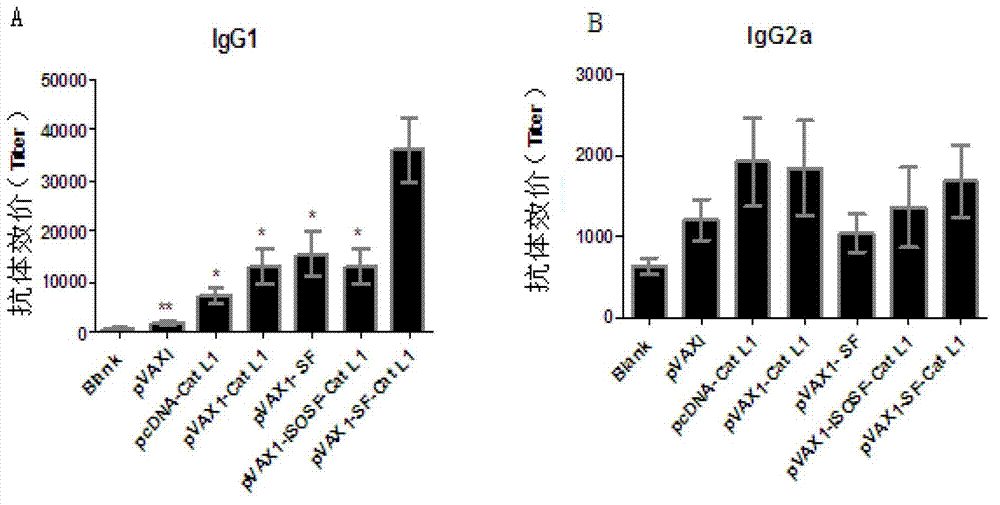
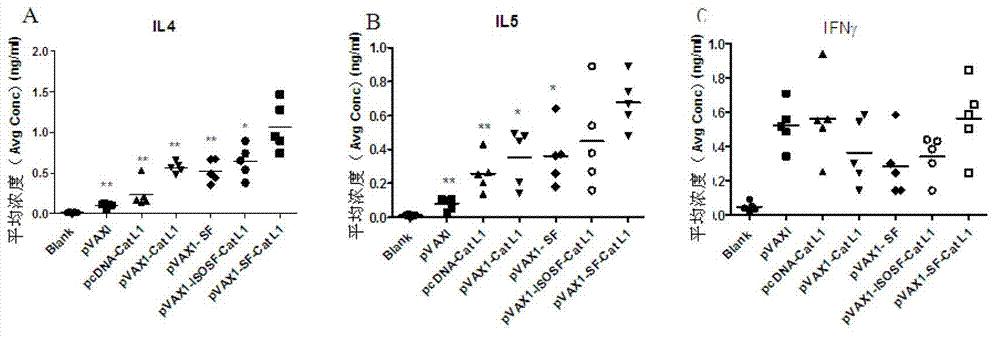
Vaccine composition for improving immune protective rate of anti-Fasciola hepatica Cat L1 DNA, and preparation method thereof
InactiveCN103203028AHigh protection rateHigh frequencyGenetic material ingredientsAntiparasitic agentsBALB/cDendritic cell
The invention relates to a vaccine composition for improving the immune protective rate of anti-Fasciola hepatica Cat L1 (FhCat L1) DNA. The vaccine composition is pVAX1-Cat L1+pIL4+pFlt3L. An FhCat L1 DNA plasmid is constructed and a host body Th2 immune response is utilized to well kill or inhibit worm infection, and Flt3L which can induce the maturity of dendritic cells (DCs) and increase the number and the frequency of the DCs and a cell factor IL4 which can induce the host immune response trending to Th2 are selected as a molecular adjuvant to improve the immunogenicity and the immune protective rate of FhCat L1 DNA after the immunization of BALB / c mice, so a new idea is provided for the research and development of an anti-Fasciola hepatica DNA vaccine.
Owner:SOUTHWEST UNIVERSITY
Method for screening candidate bacterial strain from fish streptococcus agalactiae vaccine
ActiveCN102676683BHigh immune protection rateLarge range of protectionMicrobiological testing/measurementMicroorganism based processesStreptococcus agalactiaeGenotype Analysis
The invention discloses a method for screening candidate bacterial strains from fish streptococcus agalactiae vaccines. The method comprises the steps: separation and breed conservation of tilapia streptococcicosis agalactiae epidemic strains, identification of the epidemic strains and the establishing of a pathogenic library, PFGE genotype analysis of epidemic strains, toxicity determination of PFGE genotype representative strains, and immunogenicity and protection domain test of the PFGE genotype representative strains so as to obtain a candidate bacterial strains combination of a tilapia streptococcicosis agalactiae immunoprophylaxis vaccine in China. The technology has the characterizes of strong pertinence, high screening efficiency, remarkable effect and the like, and the screen candidate bacterial strains combination of the tilapia streptococcosis agalactiae immunoprophylaxis vaccine in China can protect 90% of genotypes and 96.47% of epidemic strains in the pathogenic library. The technology provides a new method for screening the candidate bacterial strains from the fish streptococcus agalactiae vaccines, is suitable for screening the candidate bacterial strains from the fish streptococcus agalactiae vaccines, and has significance and using value in immune prevention and control on fish streptococcicosis.
Owner:GUANGXI INST OF FISHERIES
Vibrio paraheamolyticus bivalent DNA vaccine as well as preparation method and application thereof
InactiveCN101947325AImprove the efficiency of eukaryotic expressionSimple methodAntibacterial agentsGenetic material ingredientsSide effectDigestion
The invention provides a vibrio paraheamolyticus bivalent DNA vaccine as well as a preparation method and application thereof. The vibrio paraheamolyticus bivalent DNA vaccine is characterized in that a vibrio paraheamolyticus tdh2 gene and a vps gene are connected through 6 nucleotides to form a tdh2-vps fusion gene protein eukaryotic expression engineering vector vaccine. The preparation method of the vibrio paraheamolyticus bivalent DNA vaccine comprises the following steps of connecting the gene tdh2 and the gene vps by utilizing a double digestion technology after a cohesive end is obtained, and cloning into a prokaryotic expression vector pET28a to construct a fusion gene protein prokaryotic expression engineering vector; through the double digestion technology, inserting the fusion gene into a eukaryotic vector to construct a fusion gene protein eukaryotic expression engineering vector; finally, using a conventional method to prepare a vibrio paraheamolyticus bivalent DNA vaccine suspension. The invention has the advantages of strong operability, high repetition rate, safety without toxic and side effects, double immune protection on fish and higher immune effect than that of an inactivated vaccine, a recombinant protein vaccine and a monovalent DNA vaccine.
Owner:OCEAN UNIV OF CHINA
Fish feed additive, application method, application and feed containing additive
The invention discloses a fish feed additive, an application method, application and a feed containing the additive. The fish feed additive comprises the following components in parts by weight: 0.057-0.075 part of copper, 0.88-1.14 parts of zinc, 0.22-0.36 part of manganese and 0.008-0.013 part of xylanase. According to the invention, by adding the feed additive in the range, the specific and non-specific immune functions of fish are improved, the capacity against aeromonas hydrophila infection is enhanced, the immune protection rate is improved, and the death rate under infection is reduced; and moreover, without the substances causing toxic and side effects on fish body as well as antibiotic substances, the additive avoids pollution and drug resistance.
Owner:GUANGDONG LINKOCEAN GRP CO LTD +1
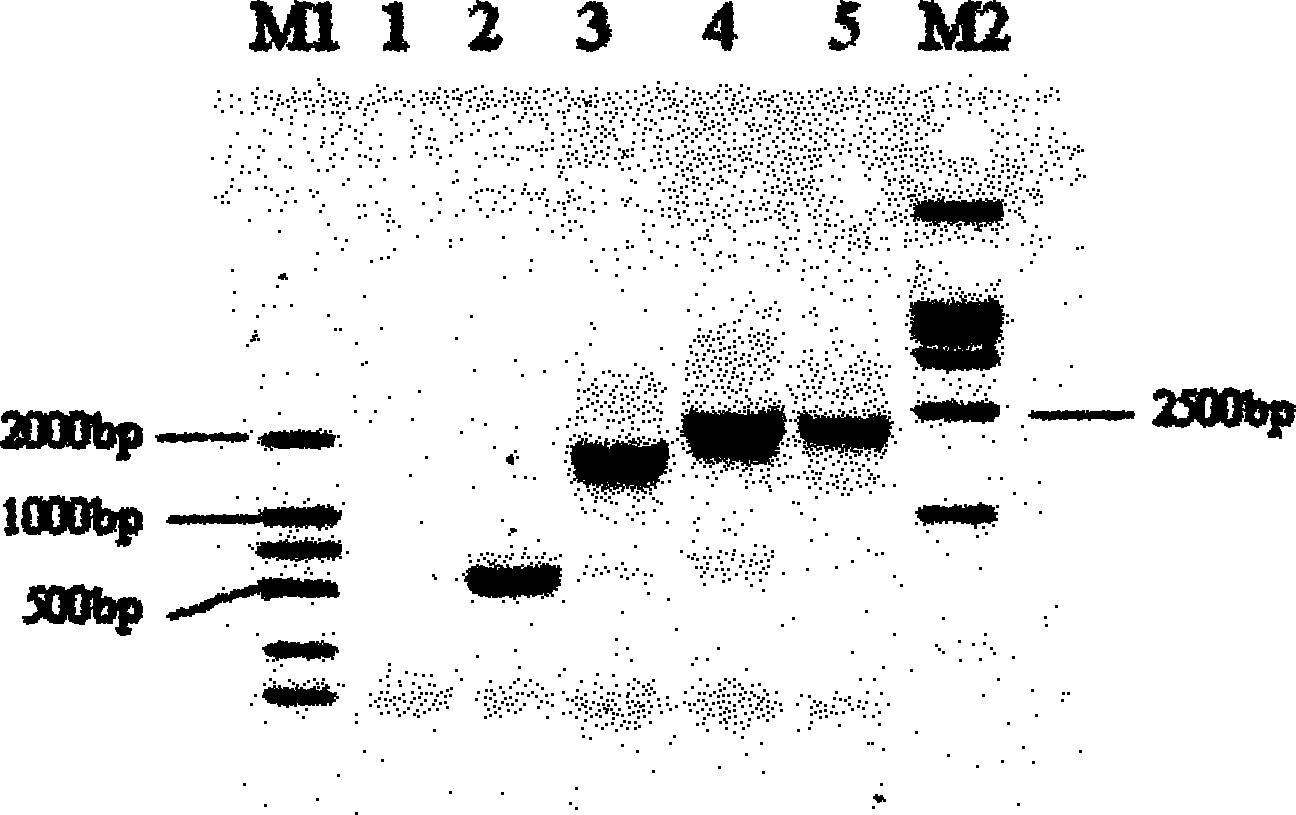
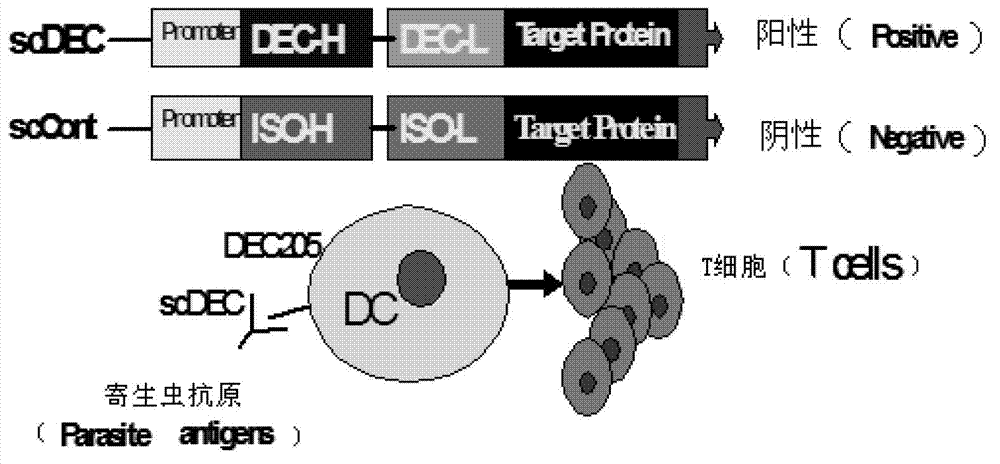
Method for improving immune protective rate of fasciola hepatica Cat L1 (FhCat L1) DNA (Deoxyribose Nucleic Acid) vaccine
InactiveCN103083684AImprove delivery efficiencyHigh immune protection rateGenetic material ingredientsAntiparasitic agentsDendritic cell3-deoxyribose
The invention provides a method for improving immune protective rate of a fasciola hepatica Cat L1 (FhCat L1) DNA (Deoxyribose Nucleic Acid) vaccine. The method is carried out based on the presenting effect of the antigen in dendritic cells, the influential role mechanism that the single-chain antibody scFvNLDC-145 of the DEC-205 of surface molecules of dendritic cells (DC) can target the DC cells is utilized, so that the resistance of a Spragae-Dawley rat to fasciola hepatica infection subjected to the immune encoding of the DNA vaccine of the FhCat L1 is improved, and as a result, the immune protective rate of the DNA vaccine of the FhCat L1 is improved.
Owner:SOUTHWEST UNIVERSITY
Method for preparing grass carp reovirus (GCRV) gene engineering vaccines
InactiveCN102266555BHigh expressionHigh expression yieldViral antigen ingredientsGenetic material ingredientsSaccharumSucrose
The invention provides a method for preparing grass carp reovirus (GCRV) gene engineering vaccines. The method comprises the following steps of: selecting recombinant baculoviruses (vAcGCRV-VP5 / VP7) of sf9 insect cell proliferated recombinant GCRV outer capsid proteins VP5 and VP7; separating recombinant protein components by freezing, thawing and cracking recombinant virus infected sf9 cells and adopting sucrose lining centrifuging method, and thus obtaining a purified in vitro expression VP5 and VP7 protein complex; and performing fish protection experiments on the purified VP5 and VP7 proteins to prove that the VP5 and VP7 proteins have good immune protection effect. The GCRV gene engineering vaccines prepared by the method not only have good stability and immunogenicity, but also have good immune protection effect on grass carp fries; the immune protection rate of the GCRV gene engineering vaccines reaches 90 percent; and the method is suitable for large-scale production and application of the vaccines.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Riemerella anatipestifer pre-bacterial ghost vaccine preparation method
ActiveCN104975038AIntact surface structureAntigenicity is not affectedAntibacterial agentsBacterial antigen ingredientsLocal immunityFreeze-drying
The present invention relates to a method for conversion preparation of a riemerella anatipestifer pre-bacterial ghost vaccine by using phage PhiX174 E gene and staphylococcal nuclease A gene, and an application method. According to the technical scheme, the phage PhiX174 E gene and the staphylococcal nuclease A gene are coupled to form the series coupling gene through the flexible coupling arm (Gly4Ser), a temperature control expression vector pBV-E-SN is further constructed, the pBV-E-SN is transformed into riemerella anatipestifer protoplast through electric shock conversion, the positive clones are screened and are subjected to bacterial amplification culture at a temperature of 28 DEG C, the bacteria are separated, a protection agent is added, and sub-packaging and freeze-drying are performed. According to the present invention, the prepared live bacterial vaccine is immunized through drinking water, such that the local immunity on the respiratory tract mucous membrane and the digestive tract mucous membrane can be irritated, and the humoral immunity can be stimulated.
Owner:QINGDAO AGRI UNIV
Trachinotus ovatus-derived streptococcus agalactiae DNA vaccine as well as preparation method and application thereof
ActiveCN112773891AImprove protectionHigh immune protection rateAntibacterial agentsBacterial antigen ingredientsEnzyme digestionNucleotide
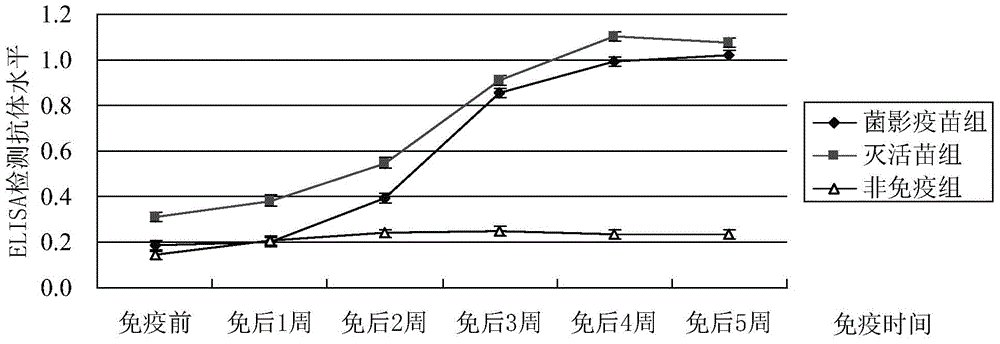
The invention discloses a trachinotus ovatus-derived streptococcus agalactiae DNA vaccine as well as a preparation method and application thereof. The nucleotide sequence of the vaccine is shown as SEQ ID NO: 1, and the preparation method of the vaccine comprises the following steps: designing primers according to an internalin gene sequence, carrying out PCR (Polymerase Chain Reaction) amplification, connecting the obtained product with pMD-18T, transforming DH5alpha, selecting positive bacterium clones, carrying out amplification culture, extracting plasmids, and verifying to obtain pMD-internalin recombinant plasmids; performing enzyme digestion with restriction enzymes EcoR I and Xho I on the obtained plasmids and pcDNA3.1 (+) respectively, ligation of enzyme digestion products with T4DNA ligase and transformation of DH5alpha cells, selecting positive bacterium clones, extracting plasmids and obtaining the trachinotus ovatus-derived streptococcus agalactiae DNA vaccine through verification. The vaccine disclosed by the invention is relatively high in immune protection rate and relatively long in immune protection period.
Owner:BEIBU GULF UNIV
Application of bacillus pumilus and immunopotentiator powdery preparation
InactiveCN104397358AImprove infection abilityImprove the body's immunityAntibacterial agentsOrganic active ingredientsBacterial diseaseWeight gain
The invention relates to application of a bacillus pumilus and immunopotentiator powdery preparation to preparation of biological agents for preventing and treating aquatic bacterial diseases. The bacillus pumilus and immunopotentiator powdery preparation comprises effective components of bacillus pumilus and an immunopotentiator, wherein the bacillus pumilus is a powdery bacterial agent prepared through solid fermentation or liquid fermentation of bacillus pumilus with the collection number of CCTCC M 2013240; the viable count of the bacillus pumilus is 109-1011cfu / g; the immunopotentiator comprises vitamin C, astragalus membranaceus, eucommia, codonopsis pilosula, Chinese angelica, honeysuckle, radix bupleuri, black plum, pomegranate peel and fructus schisandrae. The bacillus pumilus and immunopotentiator powdery preparation has the advantages as follows: the bacillus pumilus and immunopotentiator powdery preparation can significantly improve the resistance of fishes to infection of aeromonas hydrophila, enhance the body immunity of cultured fishes, significantly inhibit the growth of intestinal pathogenic bacteria of the fishes, promote the rapid growth of the fishes and improve the weight gain rate and the disease resistance of the cultured fishes.
Owner:SUZHOU IRIVET BIOTECH
Bacillus pumilus and immunopotentiator powdery preparation
InactiveCN104399071AHigh immune protection rateIncrease weight gainAntibacterial agentsPowder deliveryWeight gainingDisease
The invention relates to a bacillus pumilus and immunopotentiator powdery preparation. The bacillus pumilus and immunopotentiator powdery preparation comprises effective components of bacillus pumilus and an immunopotentiator, wherein the bacillus pumilus is a powdery bacterial agent prepared through solid fermentation or liquid fermentation of bacillus pumilus with the collection number of CCTCC M 2013240; the viable count of the bacillus pumilus is 109-1011cfu / g; the immunopotentiator comprises vitamin C, astragalus membranaceus, eucommia, codonopsis pilosula, Chinese angelica, honeysuckle, radix bupleuri, black plum, pomegranate peel and fructus schisandrae. The bacillus pumilus and immunopotentiator powdery preparation has the advantages as follows: the bacillus pumilus and immunopotentiator powdery preparation can significantly improve the resistance of fishes to infection of aeromonas hydrophila, enhance the body immunity of cultured fishes, significantly inhibit the growth of intestinal pathogenic bacteria of the fishes, promote the rapid growth of the fishes and improve the weight gain rate and the disease resistance of the cultured fishes.
Owner:SUZHOU IRIVET BIOTECH
A kind of Edwardsiella piscida derived from turbot and its application
ActiveCN112029696BHigh immune protection rateAntibacterial agentsBacterial antigen ingredientsBiotechnologyEdwardsiella sp.
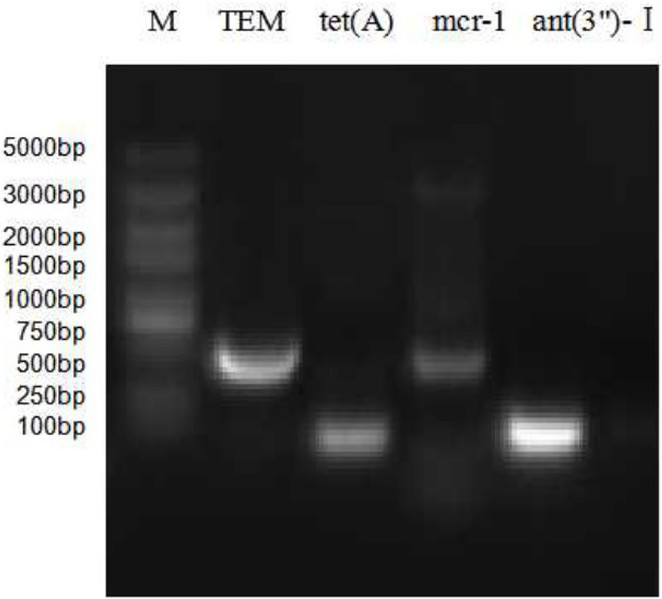
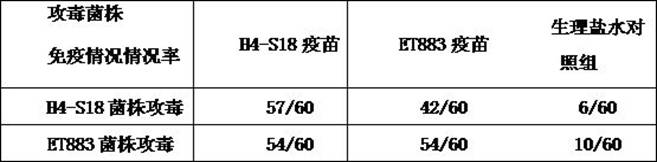
The present invention provides a turbot-derived Edwardsiella piscicida and its application; the Edwardsiella piscida derived from turbot is Edwardsiella piscida H4-S18 strain, and its gene sequence is as shown in SEQ ID No. .1. Shown in SEQ ID No.2 and SEQ ID No.3, the Edwardsiella piscicidae H4‑S18 strain is preserved with the preservation number CCTCC NO: M 2020450. The present invention also relates to the application of the Edwardsiella piscicida derived from turbot in the preparation of vaccines. The piscicidal Edwardsiella provided by the present invention is screened from the diseased turbot, and it is the first screening of the turbot-derived Edwardsiella piscicida carrying the drug-resistant gene in China, and the first application of the piscicidal Edwardsiella carrying the drug-resistant gene. Vaccines prepared from turbot-derived Edwardsiella piscicida can effectively improve the immune protection rate of turbot against drug-resistant Edwardsiella piscicida.
Owner:烟台市海洋经济研究院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com