Riemerella anatipestifer pre-bacterial ghost vaccine preparation method
A Riemerella anatipestifer and vaccine technology, applied in the field of poultry breeding disease prevention and control, can solve the problems of large immune stress, weakened immunogenicity, time-consuming and labor-intensive immunization operations, etc., achieve high immune protection rate and avoid stress , the effect of convenient application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Implementation Example 1: Preparation of Riemerella anatipestifer precursor shadows
[0020] 1.1 Toxicity verification of clinical isolates The virulence of a strain of Riemerella anatipestifer (coded as LY1) isolated from clinical laying ducks and identified as type 2 by serotype was verified. The strain does not produce gelatinase, and the inoculated ducklings have not shown pathogenicity for three consecutive generations, but they can produce more than 90% protection when challenged with the same type of strong virus, indicating that the strain is a non-pathogenic or relatively pathogenic strain. Strains with low and good immunogenicity can be used as vaccine candidate strains.
[0021] Preparation of protoplasts After the LY1 strain was activated, cultured to OD 600 When it is 0.7, place the bacterial solution in an ice-water bath for 30 minutes, take 10 mL of the bacterial solution, and centrifuge at 5000 r / min for 10 minutes at 4 °C, discard the supernatant, ...
Embodiment 2
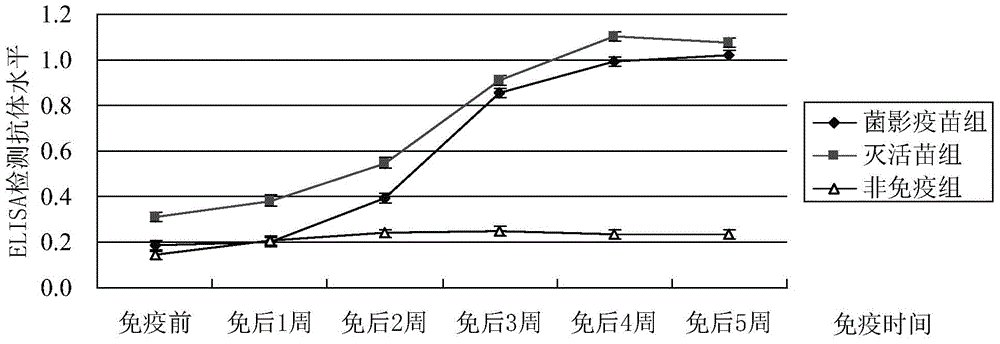
[0026] Implementation Example 2: Vaccine Immunization Protection Test
[0027] In order to verify the clinical protective effect of the mycobacteria vaccine prepared by genetic engineering means on ducks, Cherry Valley commercial meat ducks were selected as experimental animals, and the homologous strain oil adjuvant vaccine prepared by the method of formaldehyde inactivation was used to carry out the duckling under the same conditions. Duck immune protection test.
[0028] 1. Materials and methods
[0029] 1.1 Test material
[0030] Proghost vaccine: prepared according to the method described in Example 1.5.
[0031] Inactivated vaccine with oil adjuvant: formaldehyde-inactivated LY1 strain and mineral oil adjuvant were mixed at a ratio of 1:1 to make the bacterial content reach 10 10 cfu / mL.
[0032] Experimental animals: 1-day-old Cherry Valley commercial meat duck.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com