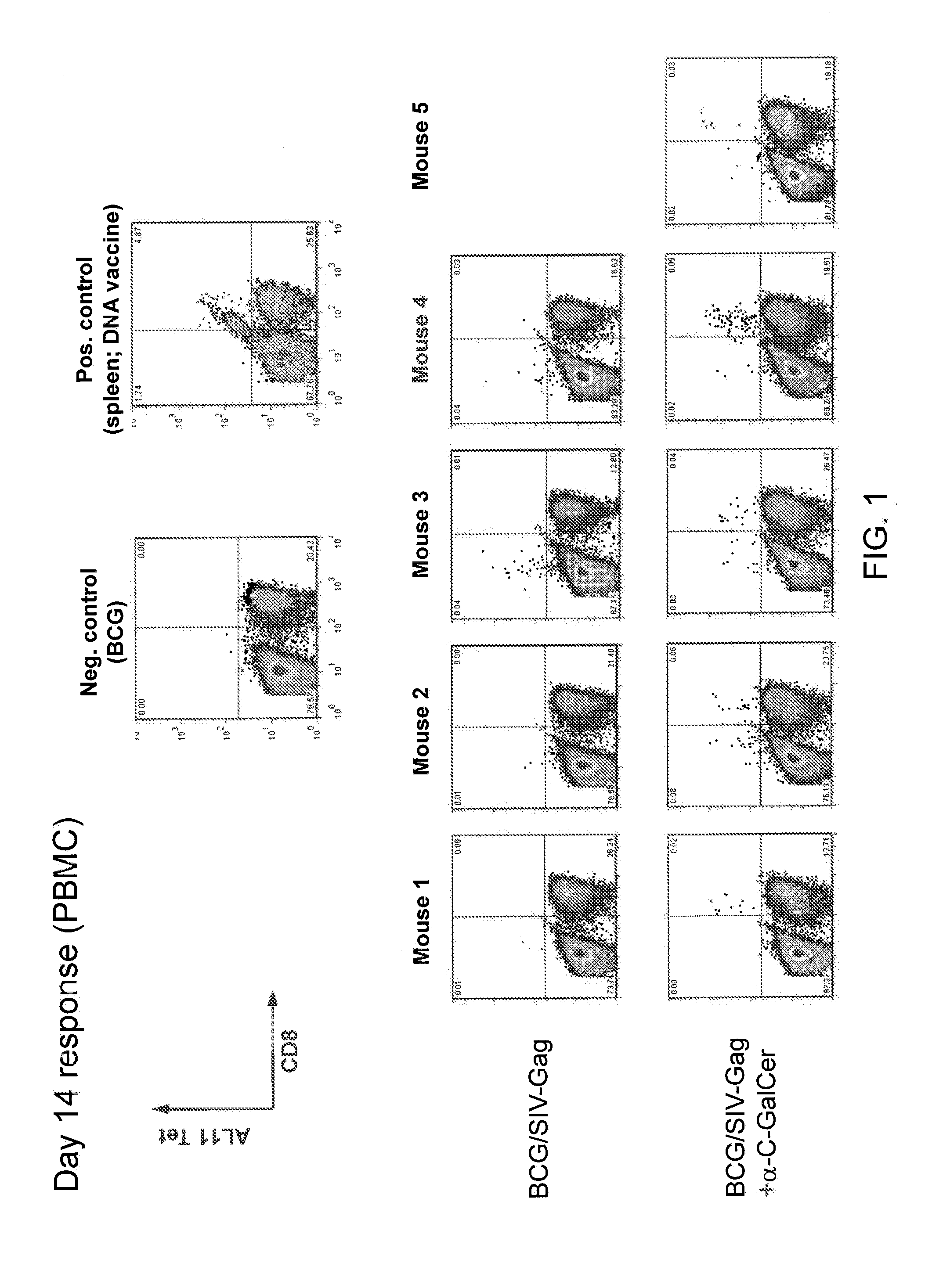
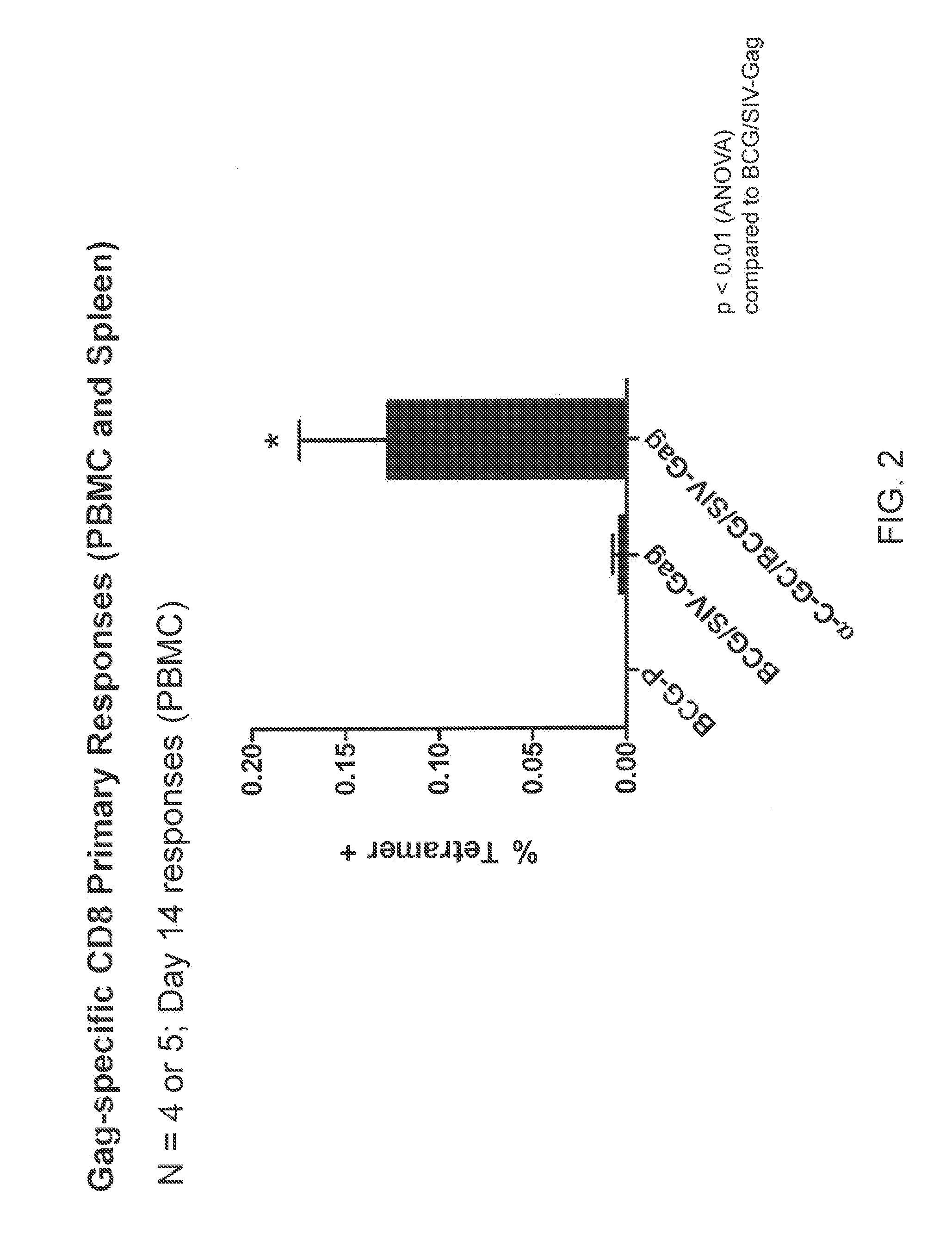
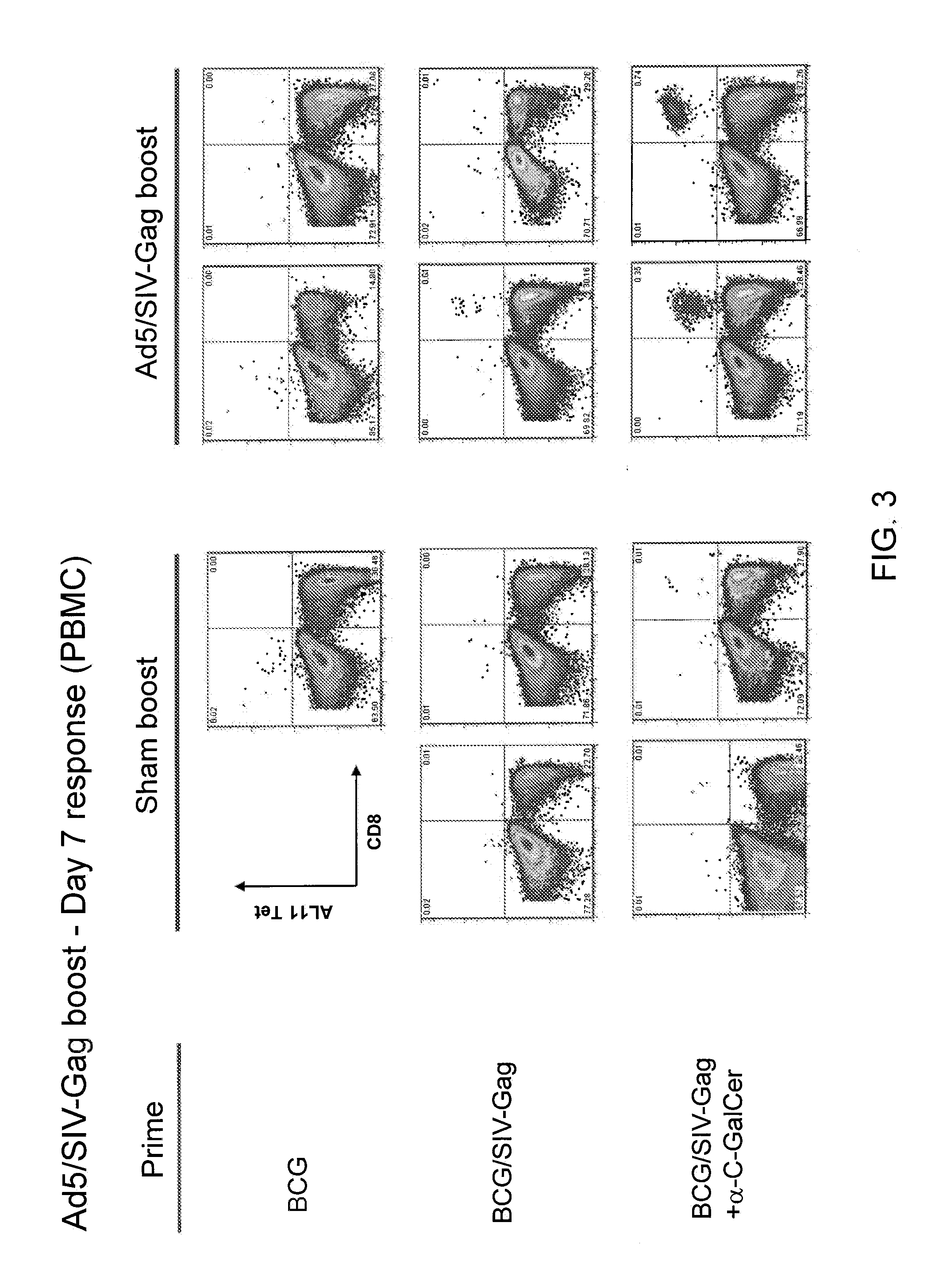
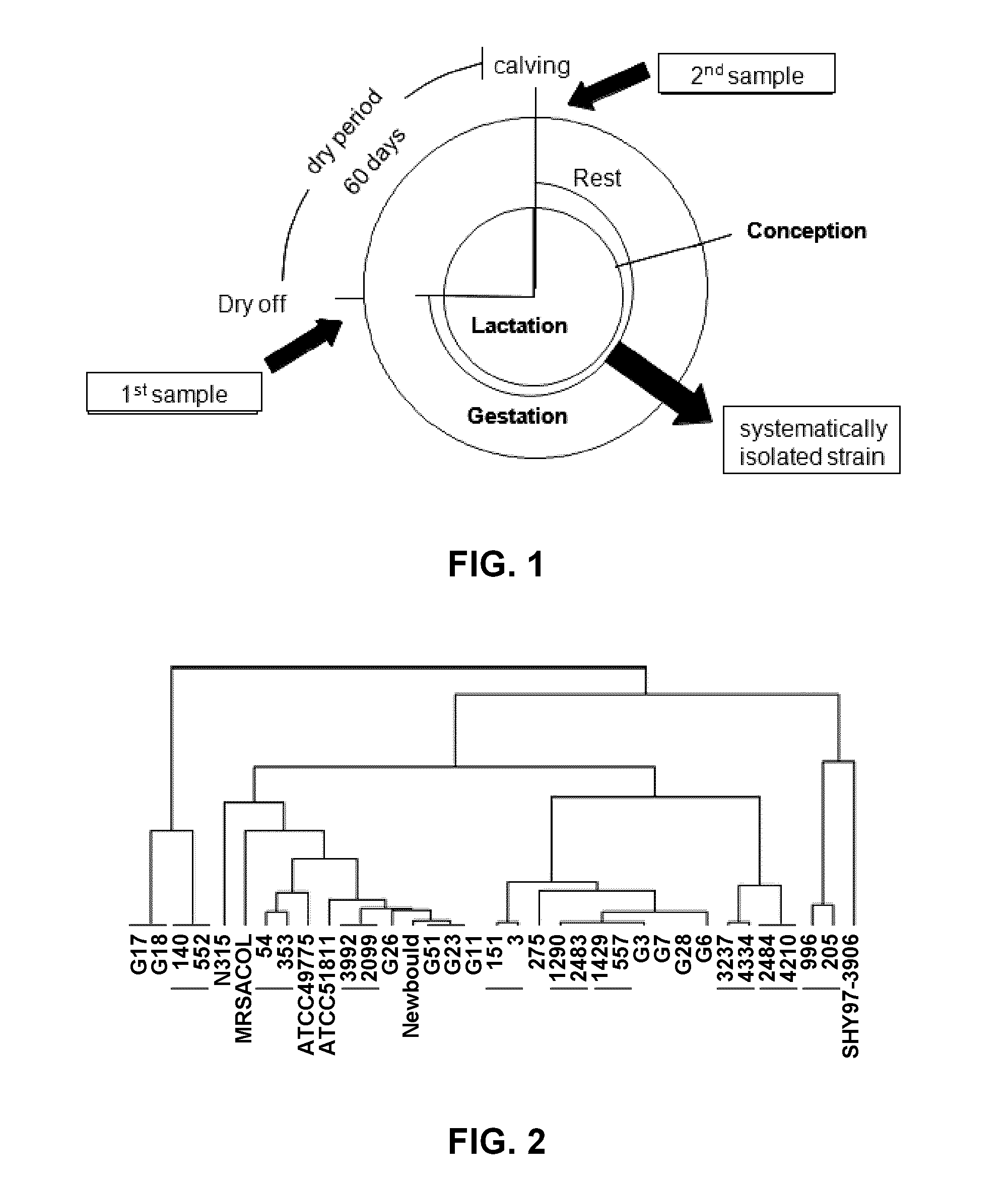
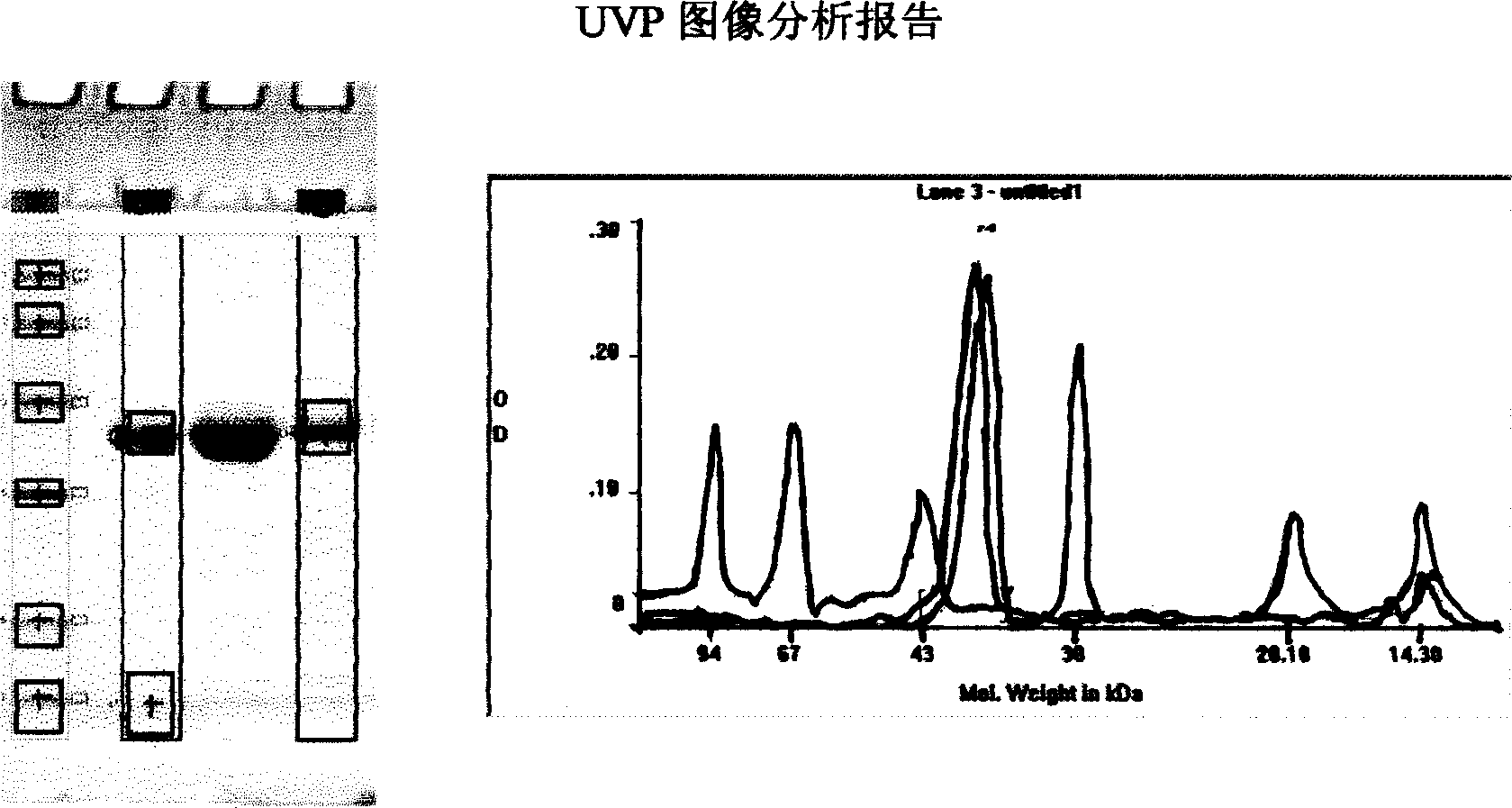
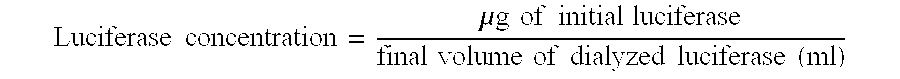Patents
Literature
94 results about "Bacterial vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Suspensions of bacteria, attenuated or killed bacteria, or their antigenic derivatives administered to induce an immune response for the prevention or treatment of bacterial disease.
Compositions, methods and kits for enhancing the immunogenicity of a bacterial vaccine vector
ActiveUS20060233835A1Improving immunogenicityOrganic active ingredientsBacterial antigen ingredientsImmunogenicityTGE VACCINE
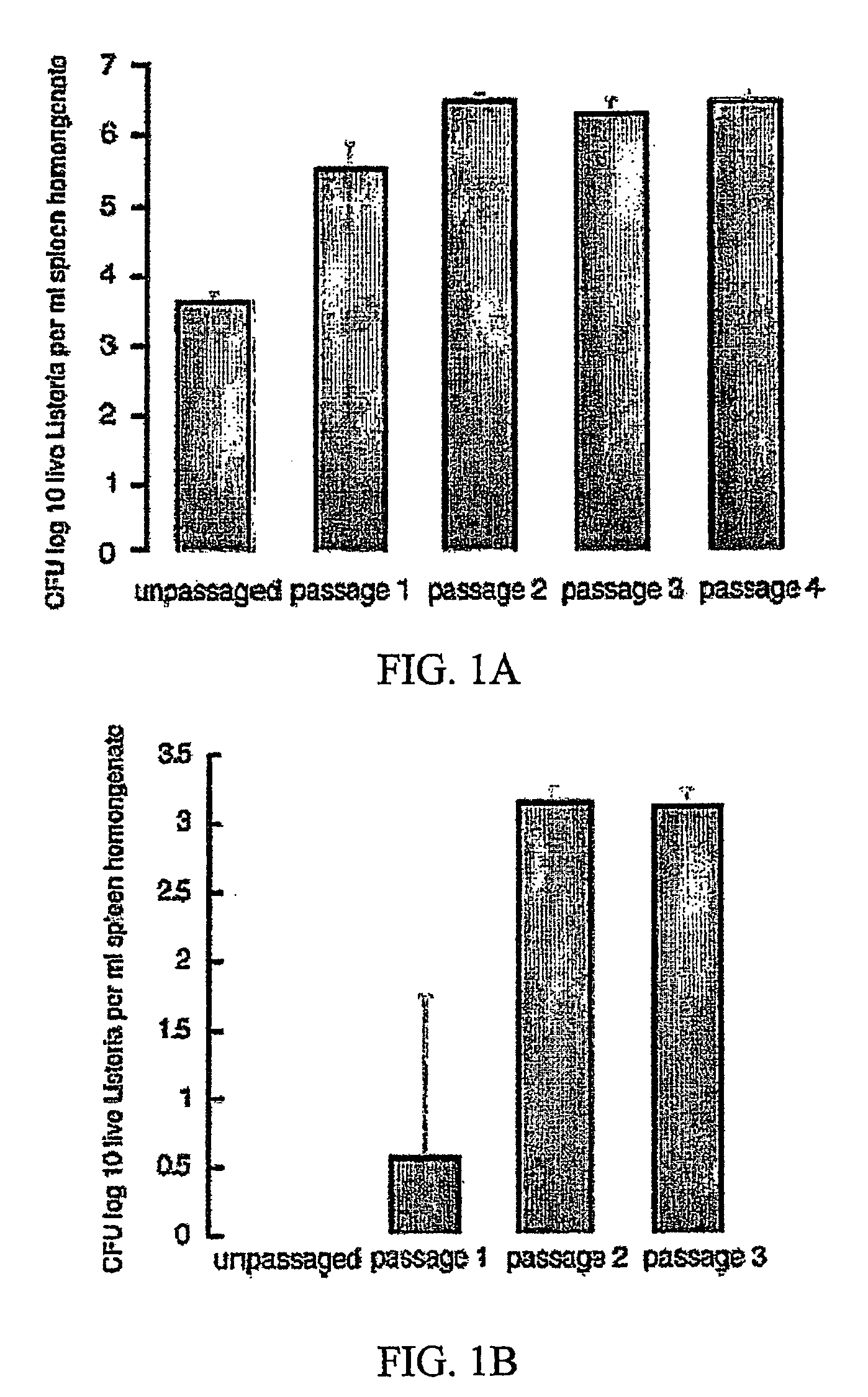
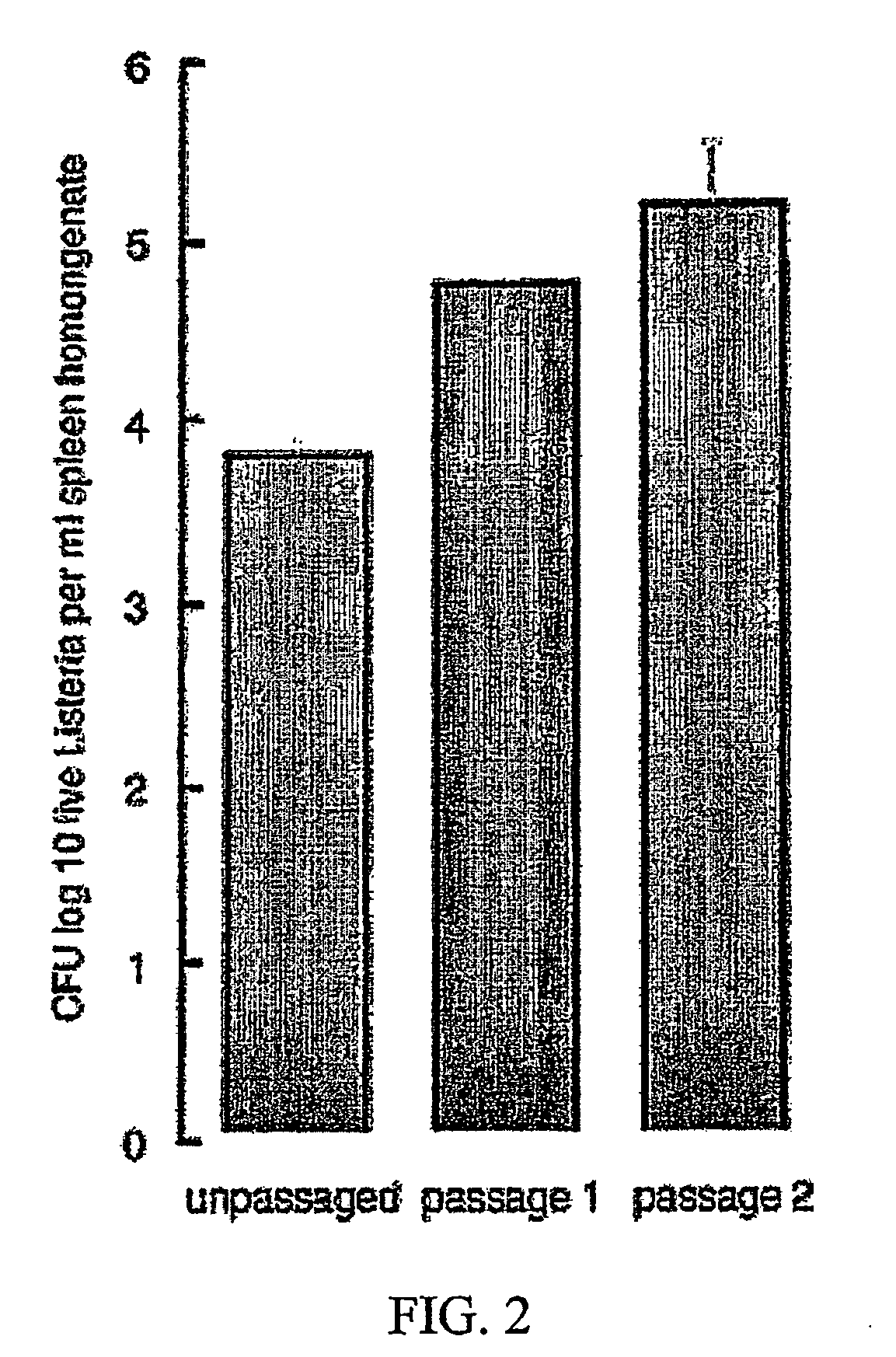
The present invention comprises methods for enhancing the immunogenicity of a bacterial vaccine vector and an antigen by passaging the bacterial vaccine vector through an animal.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Preservation and formulation of bioactive materials for storage and delivery in hydrophobic carriers
InactiveUS7153472B1Prevent backdiffusionReduce moistureMicroorganismsAerosol deliveryBacterial vaccineBiological activity
Owner:ELAN DRUG DELIVERY LTD
Live bacterial vaccines for viral infection prophylaxis or treatment
InactiveUS20080124355A1Enhance immune responseSsRNA viruses negative-senseBacterial antigen ingredientsHemagglutininBacteroides
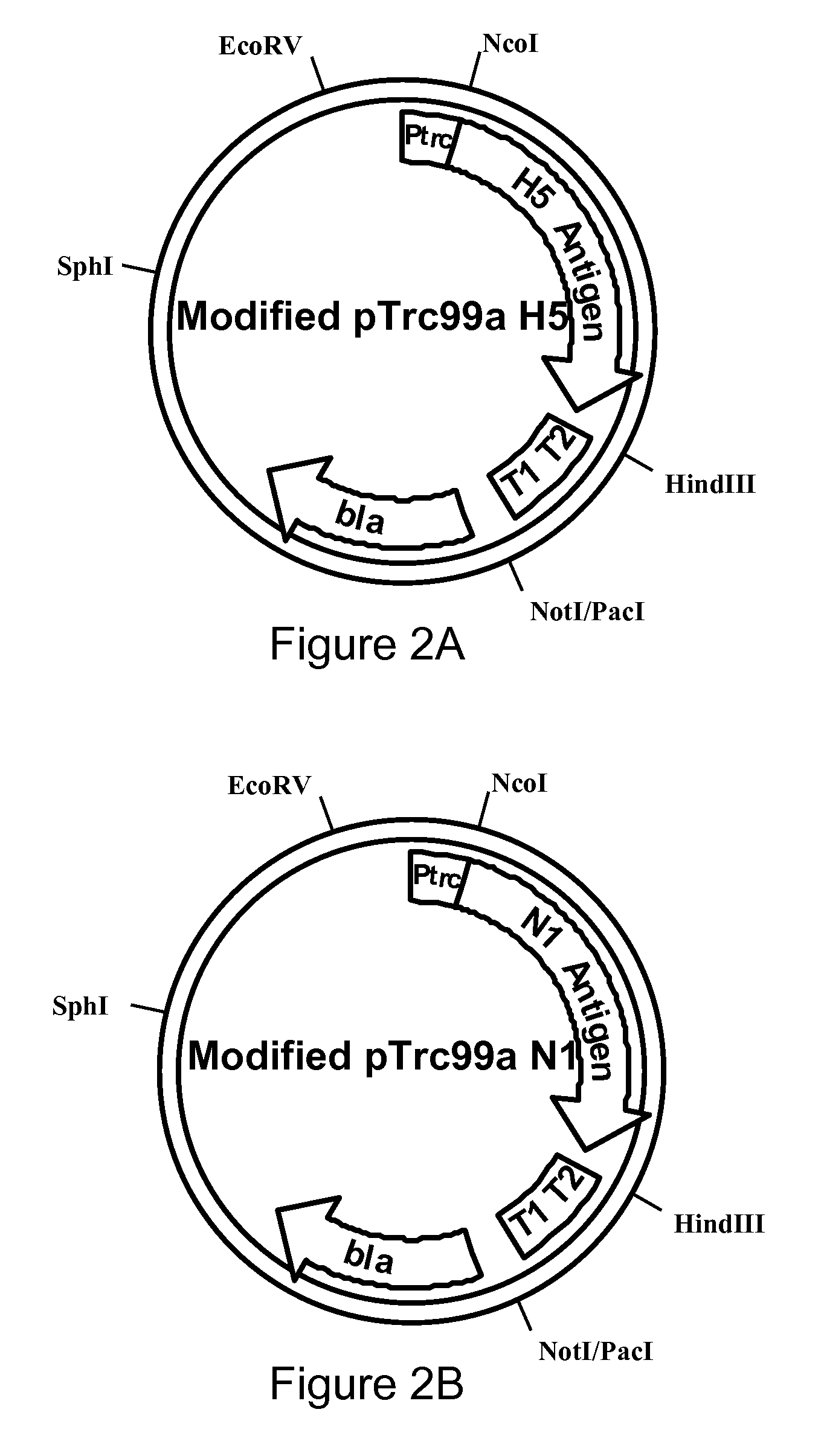
The present invention provides a vaccine, method of use, and kit employing genetically isolated and stabilized, live attenuated bacterial strains including Salmonella that express one or more avian influenza antigens for use in live vaccine compositions that can be orally administered to an individual to protect against avian influenza. Genetic stabilization may be achieved through deletion of IS200 elements and bacteria phage and prophage elements. The bacterial strains may be genetically isolated from external phage infection by constitutive expression of a P22 phage repressor. Nucleic acid sequences encoding antigenic hemagglutinin and neuraminidase avian influenza proteins, having at least one modified codon for optimum expression when transferred into a prokaryotic microorganism for improved immunogenicity
Owner:AVIEX TECH
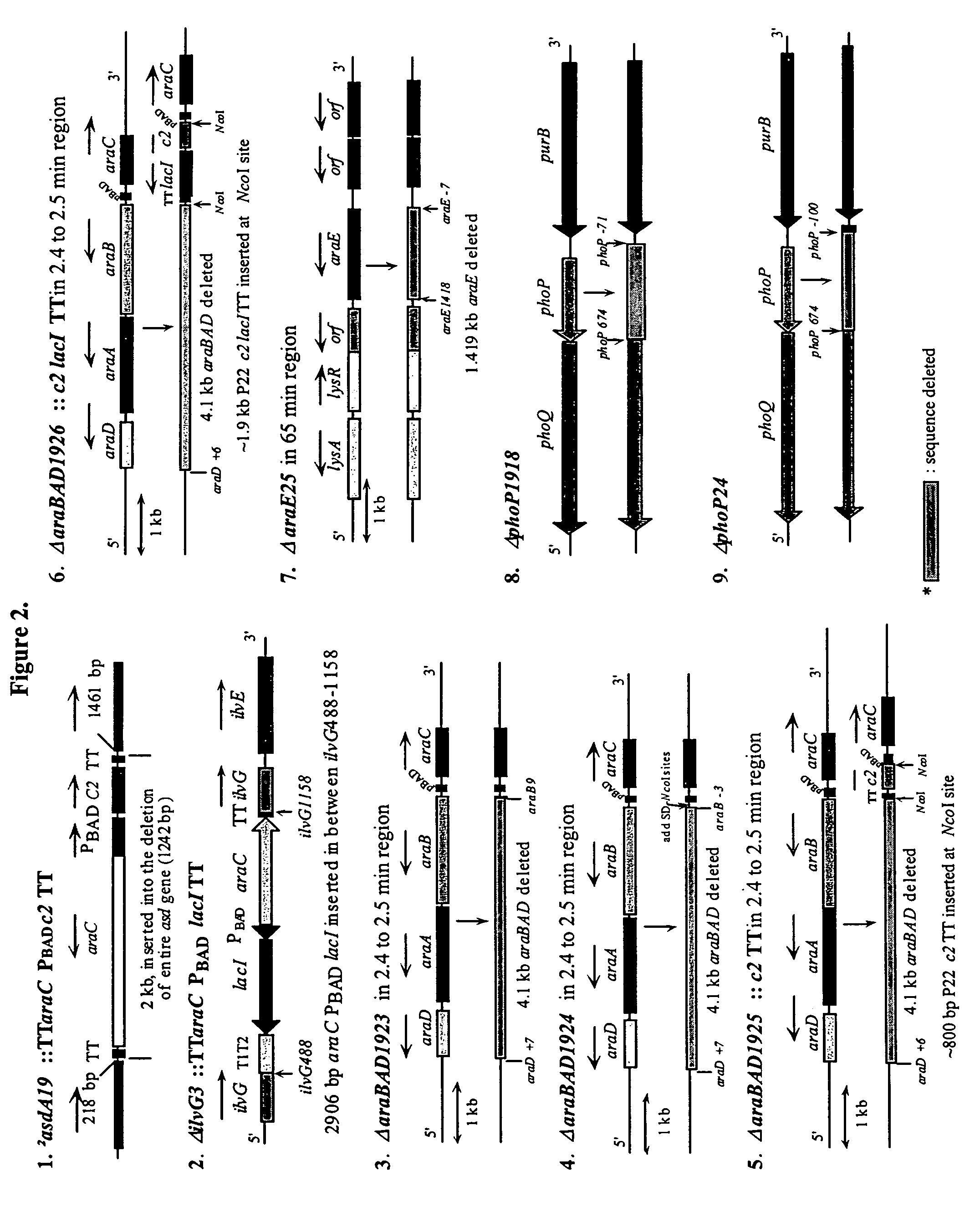
Recombinant bacterial vaccine system with environmentally limited viability
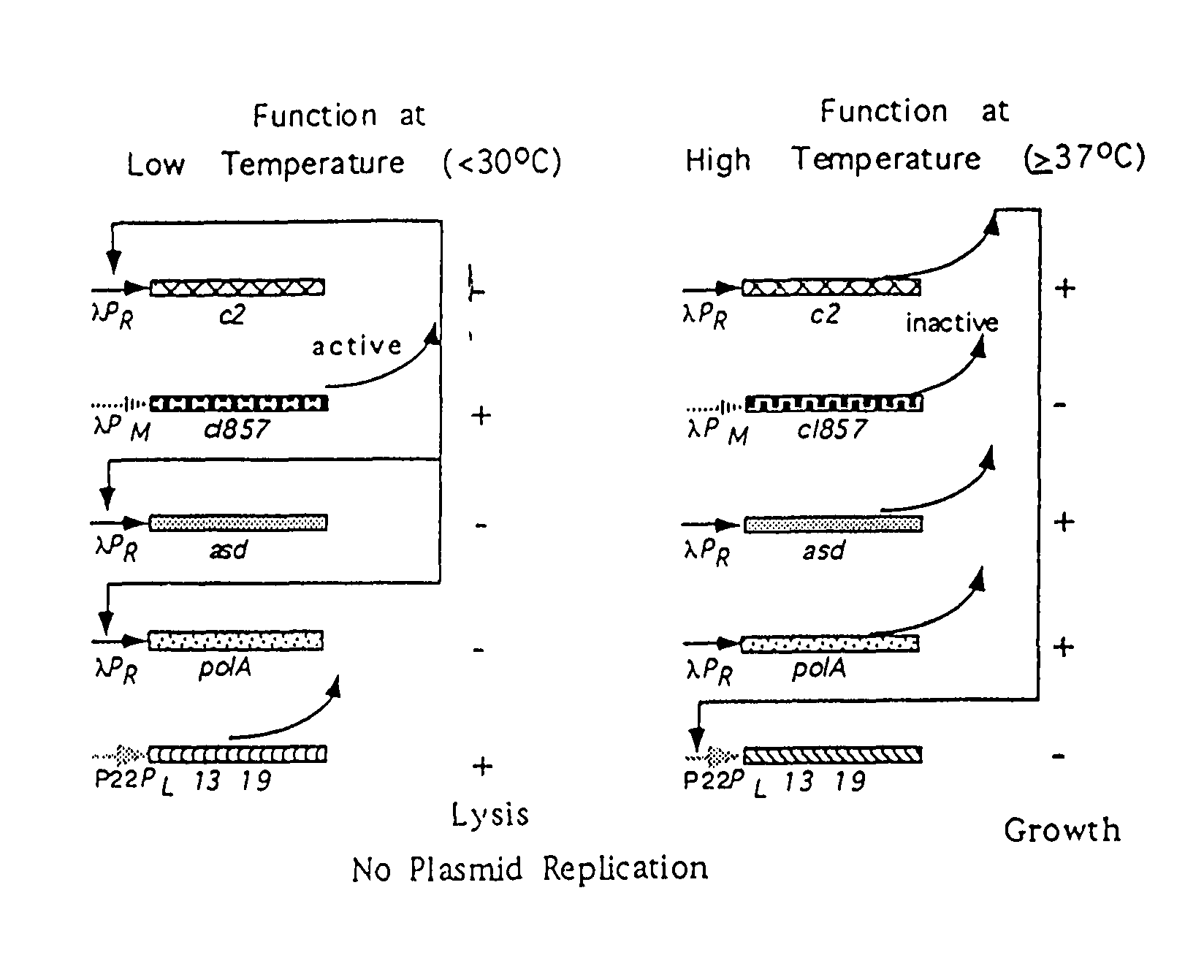
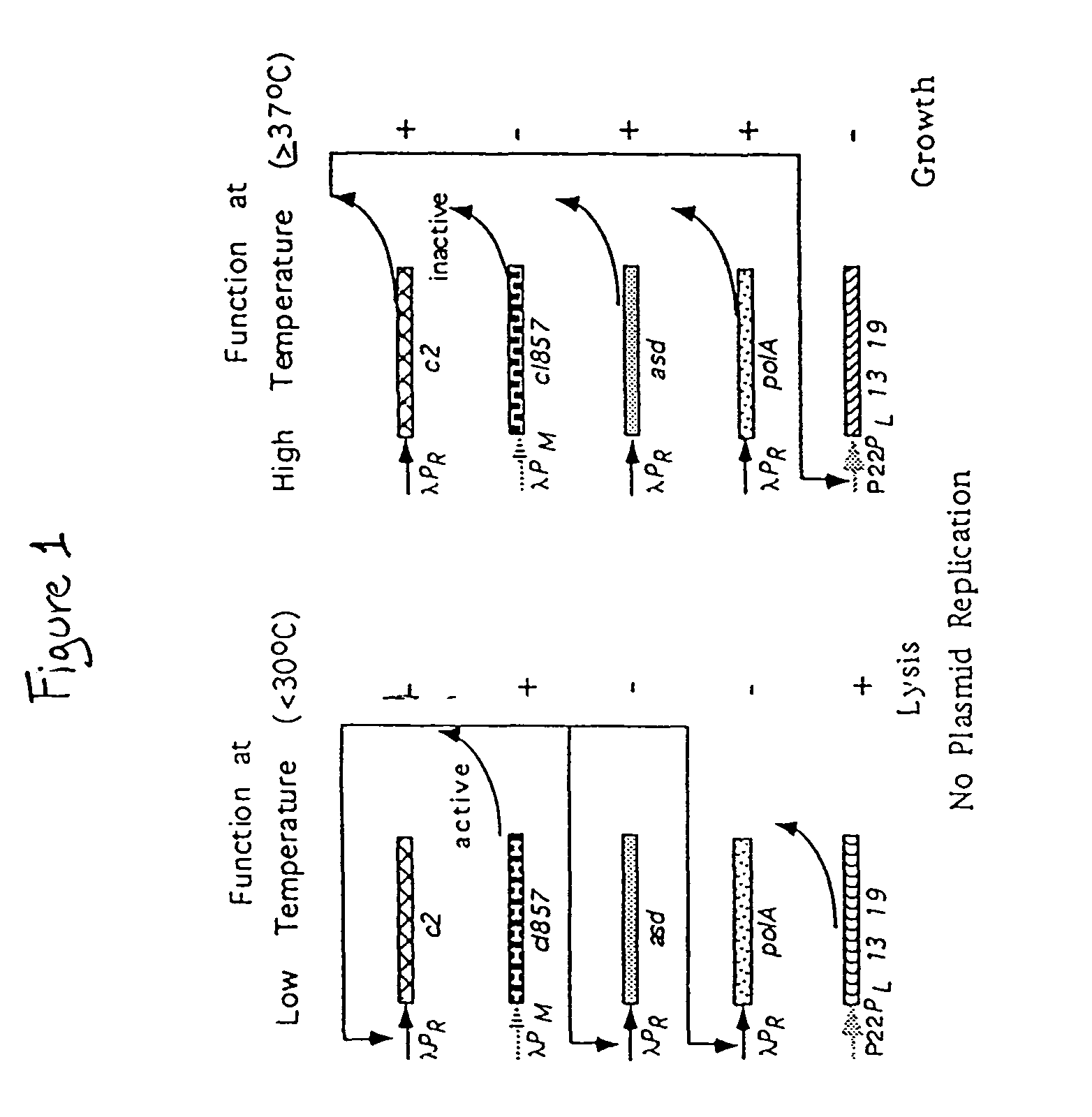
Disclosed is an Environmentally Limited Viability System (ELVS) for microorganisms based on temperature differences between permissive and non-permissive environments. Viability of the microorganisms are limited to the permissive environment by specifically expressing one or more essential genes only in the permissive environment, or expressing one or more lethal genes only in the non-permissive environment. Environmentally Limited Viability Systems are also disclosed involving coordinate expression of a combination of required genes and lethal genes. Microorganisms containing an Environmentally Limited Viability System are useful for release into a permissive environment. Temperature regulated Environmentally Limited Viability Systems are particularly suited for use with recombinant avirulent Salmonella vaccines by limiting their growth to the warmer environment inside the host. Such vaccines can be administered to protect humans or warm-blooded animals against bacterial, viral, mycotic and parasitic pathogens, especially those that colonize on or invade through mucosal surfaces. This antigen delivery system can also be used for expression of gamete-specific antigens to induce immune responses to block fertilization, or to induce immune responses to tumor antigens. In the event that an individual sheds live vaccine into the environment, the presence of the ELVS prevents survival of the vaccine. When environmentally regulated lethal genes are present on an extrachromosomal element and are regulated by chromosomal genes, transfer of the extrachromosomal element to other microorganisms will be limited by unregulated expression of the lethal genes in the recipient microorganism.
Owner:WASHINGTON UNIV IN SAINT LOUIS
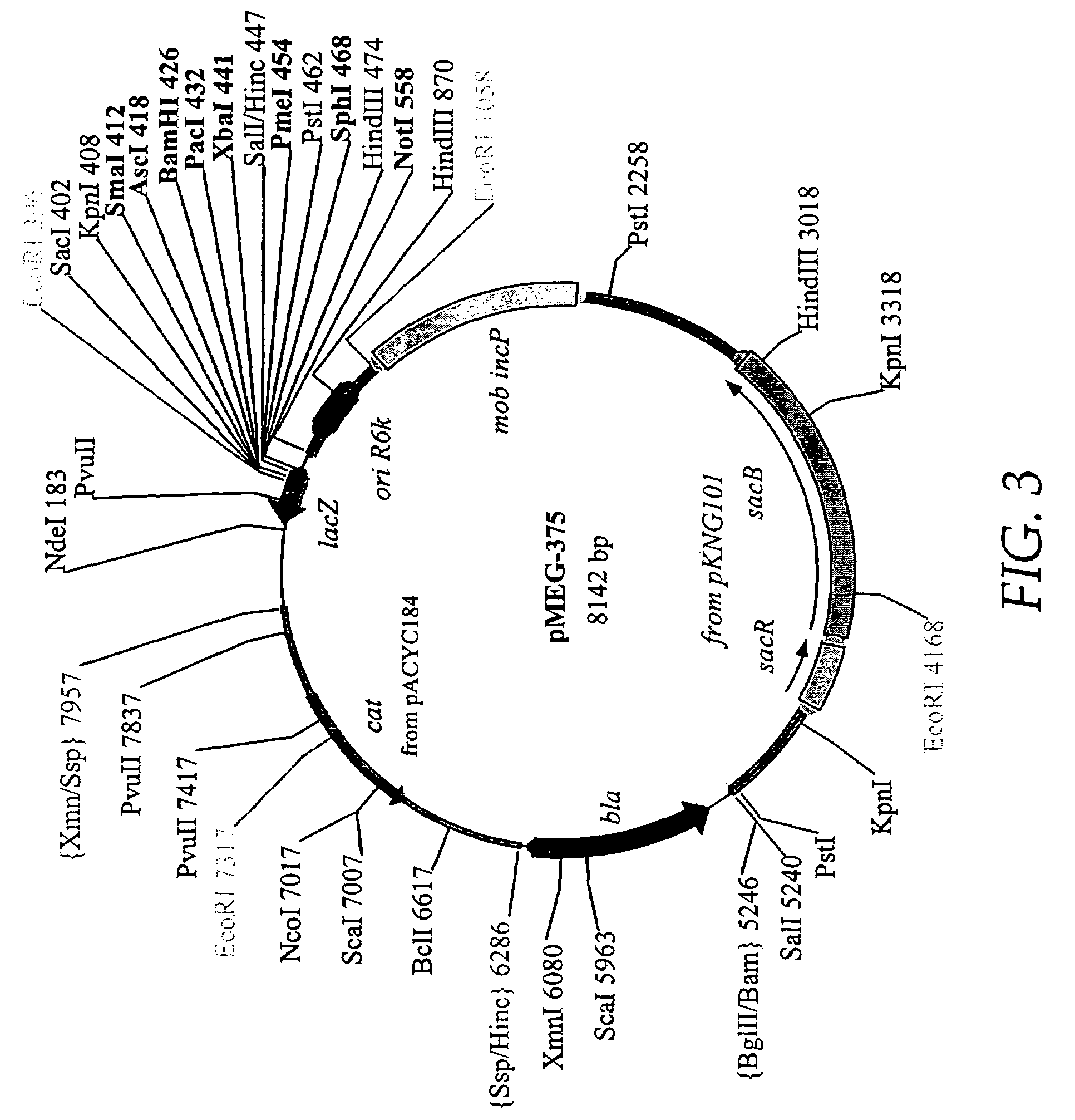
Regulated antigen delivery system (RADS)
InactiveUS7341860B2Increase heightImprove the level ofBacteriaMicroorganism based processesOrigin of replicationGene delivery
Owner:WASHINGTON UNIV IN SAINT LOUIS
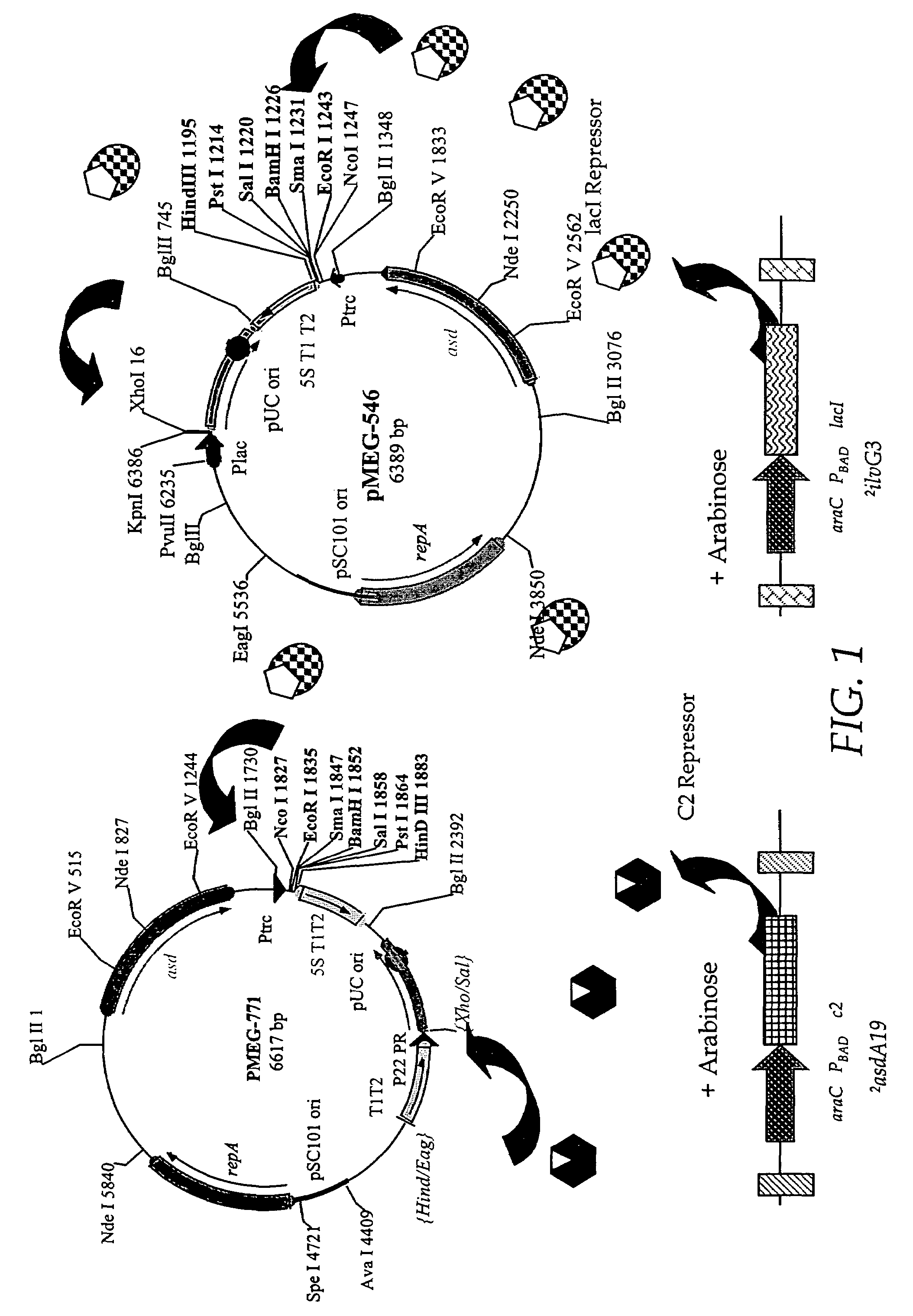
Live bacterial vaccines resistant to carbon dioxide (CO2), acidic PH and/or osmolarity for viral infection prophylaxis or treatment
InactiveUS8647642B2Enhance immune responseAntibacterial agentsSsRNA viruses negative-senseBacteroidesDisease
Owner:AVIEX TECH
Compositions and methods of enhancing immune responses
ActiveUS8604178B2Enhance immune responseReduce morbiditySsRNA viruses negative-senseBacteriaSalmonella enteritidisBacilli
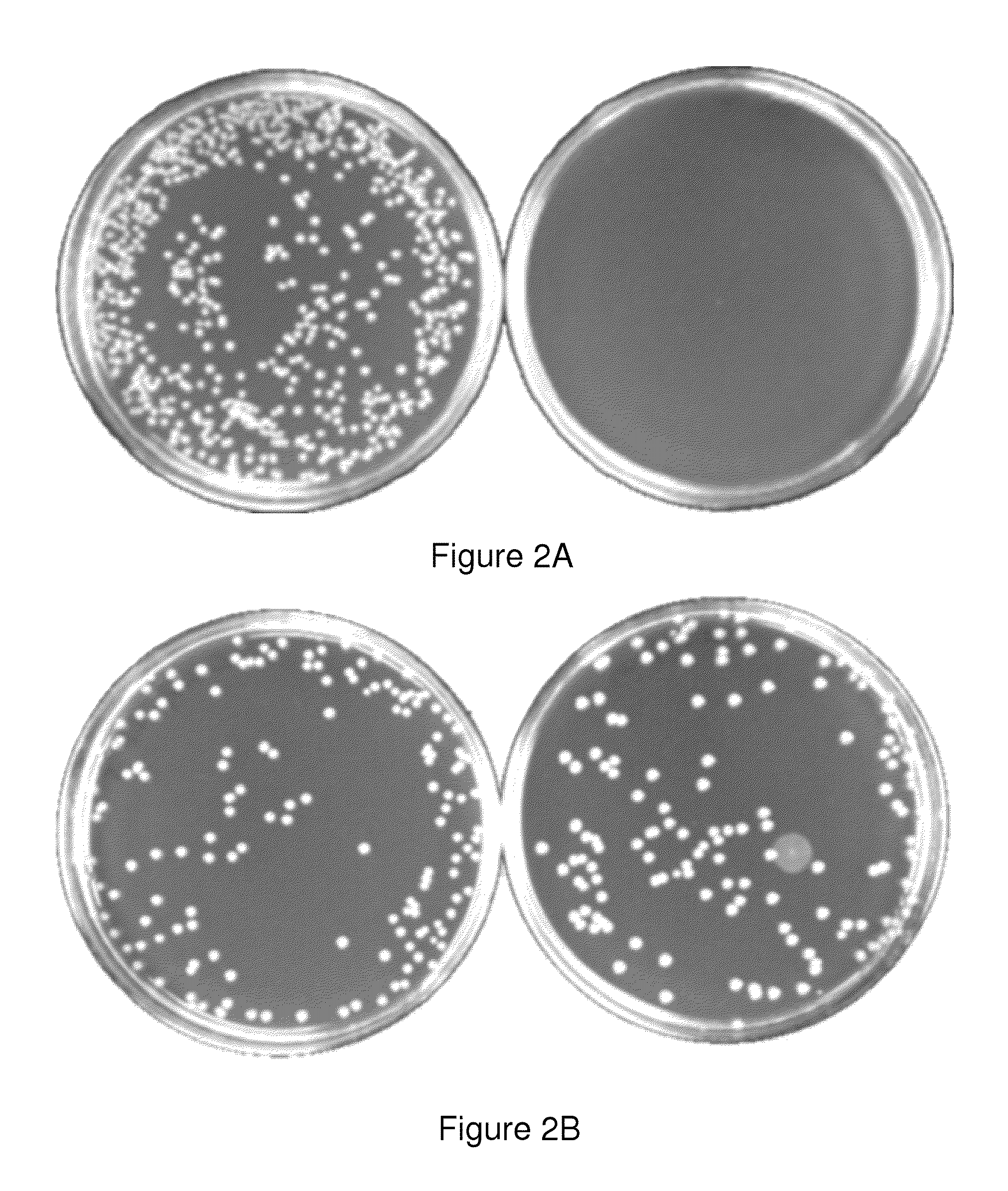
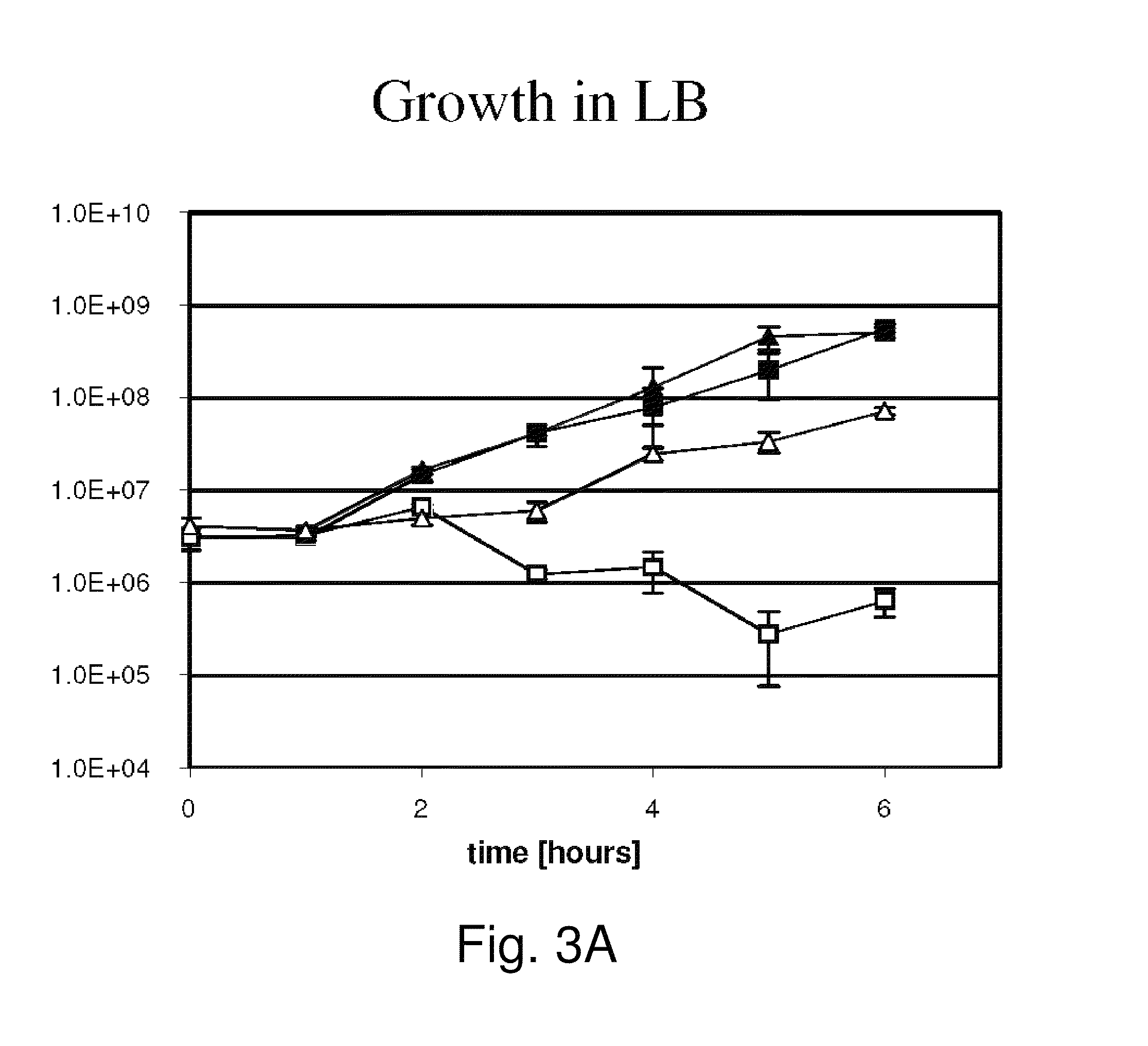
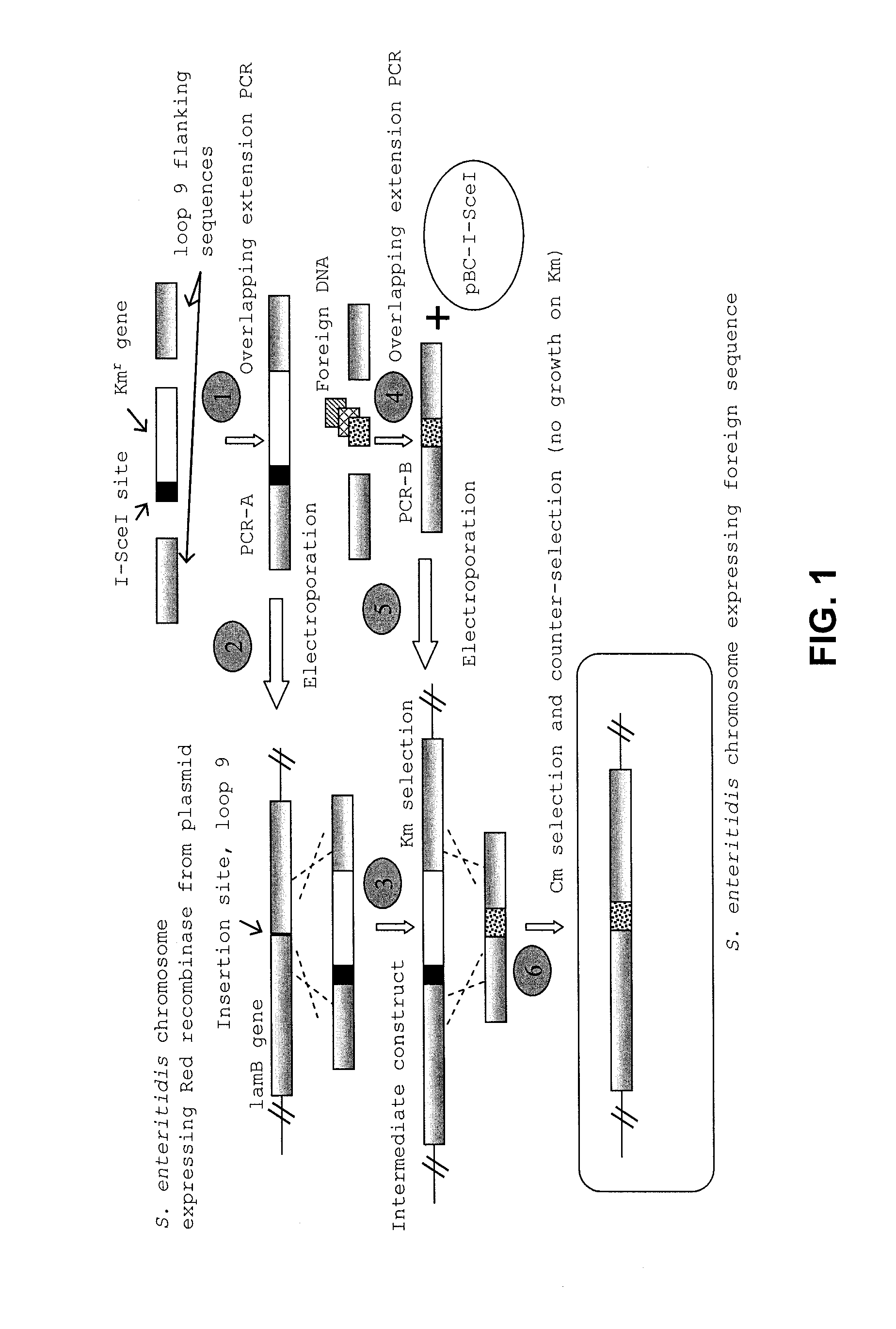
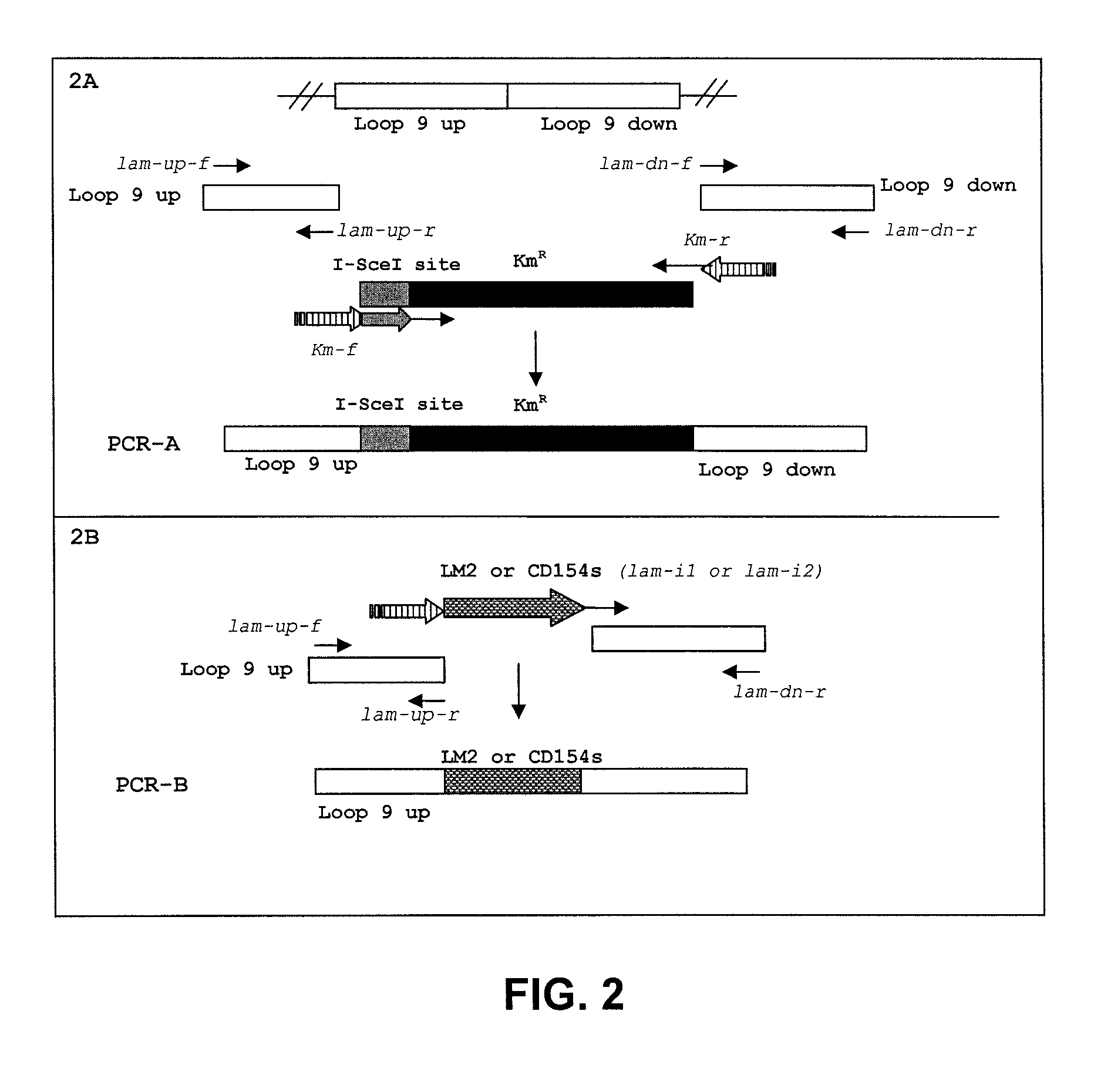
Provided herein are Salmonella enteritidis 13A strains and compositions comprising these strains. Also provided are methods of enhancing an immune response against Influenza A and methods of reducing morbidity associated with an Influenza A infection. Methods of enhancing an immune response to a vaccine vector by expressing a polypeptide of CD 154 capable of binding CD40 are also disclosed. Methods of developing a bacterial vaccine vector are disclosed. Methods of generating scarless site-specific mutations in a bacterium are also disclosed.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS +1
Live bacterial vaccines for viral infection prophylaxis or treatment
ActiveUS20120142080A1Enhance immune responseAntibacterial agentsSsRNA viruses negative-senseBacteroidesDNA construct
A live bacterium, having a DNA construct stabilized against transduction of other bacteria, having a promoter sequence and encoding a fusion peptide, comprising a bacterial secretion peptide portion and a non-bacterial immunogenic polypeptide portion, having a nucleotide sequence coding for the non-bacterial immunogenic polypeptide portion which has at least one codon optimized for bacterial expression. The bacterium has a secretion mechanism which interacts with at least the bacterial secretion peptide portion to cause a secretion of the fusion peptide from the bacterium, and a genetic virulence attenuating mutation. The bacterium is adapted to act as an animal vaccine, to transiently infect a tissue of the animal, and cause an immunity response to the non-bacterial immunogenic polypeptide portion in the animal to a non-bacterial organism associated with the non-bacterial immunogenic polypeptide portion.
Owner:AVIEX TECH
Regulated antigen delivery system (RADS)
InactiveUS20050106176A1Effective exposureIncrease productionBacterial antigen ingredientsBacteriaGene deliveryOrigin of replication
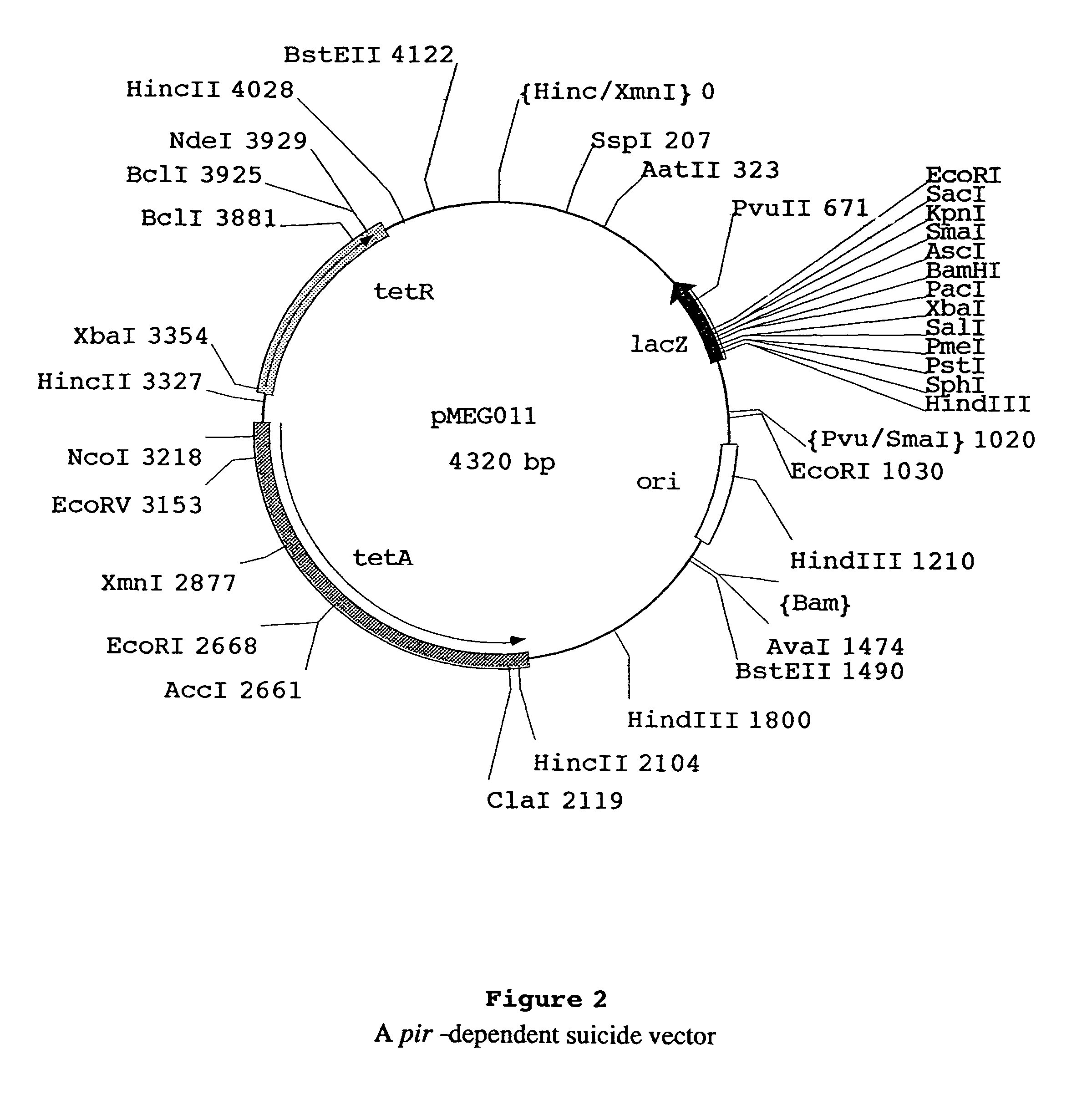
We describe a regulated antigen delivery system (RADS) that has (a) a vector that includes (1) a gene encoding a desired gene product operably linked to a control sequence, (2) an origin of replication conferring vector replication using DNA polymerase III, and (3) an origin of replication conferring vector replication using DNA polymerase I, where the second origin of replication is operably linked to a control sequence that is repressible by a repressor. The RADS microorganism also has a gene encoding a repressor, operably linked to an activatible control sequence. The RADS described provide high levels of the desired gene product after repression of the high copy number origin of replication is lifted. The RADS are particularly useful as live bacterial vaccines. Also described is a delayed RADS system, in which there is a delay before the high copy number origin is expressed after the repression is lifted. The delayed RADS is also particularly useful for live bacterial vaccines. Also described are several control elements useful for these systems, as well as methods for providing immunity to a pathogen in a vertebrate immunized with the RADS microorganisms.
Owner:WASHINGTON UNIV IN SAINT LOUIS
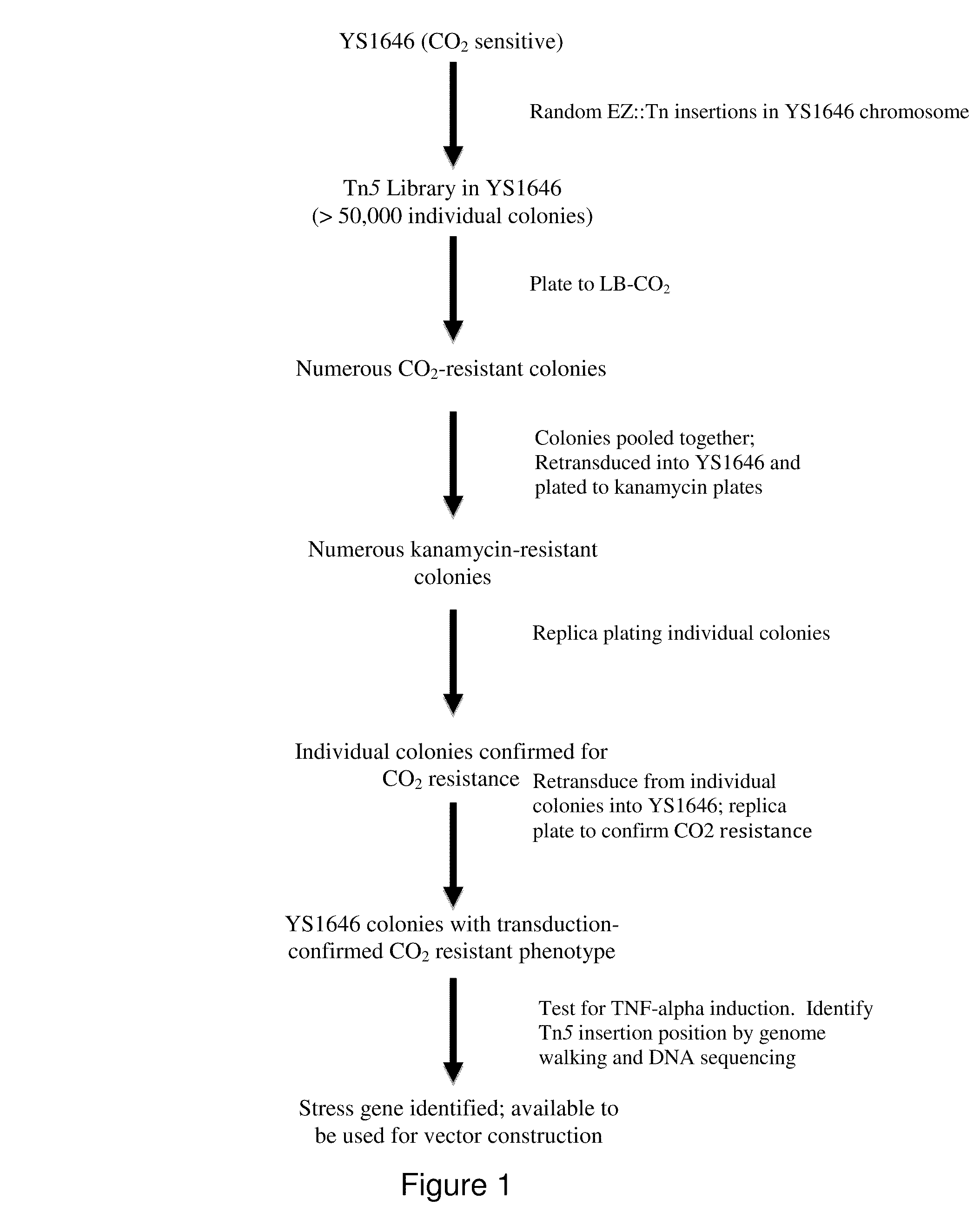
Live bacterial vaccines resistant to carbon dioxide (CO2), acidic ph and/or osmolarity for viral infection prophylaxis or treatment
InactiveUS20100136048A1Enhance immune responseSsRNA viruses negative-senseBacterial antigen ingredientsDiseaseBacteroides
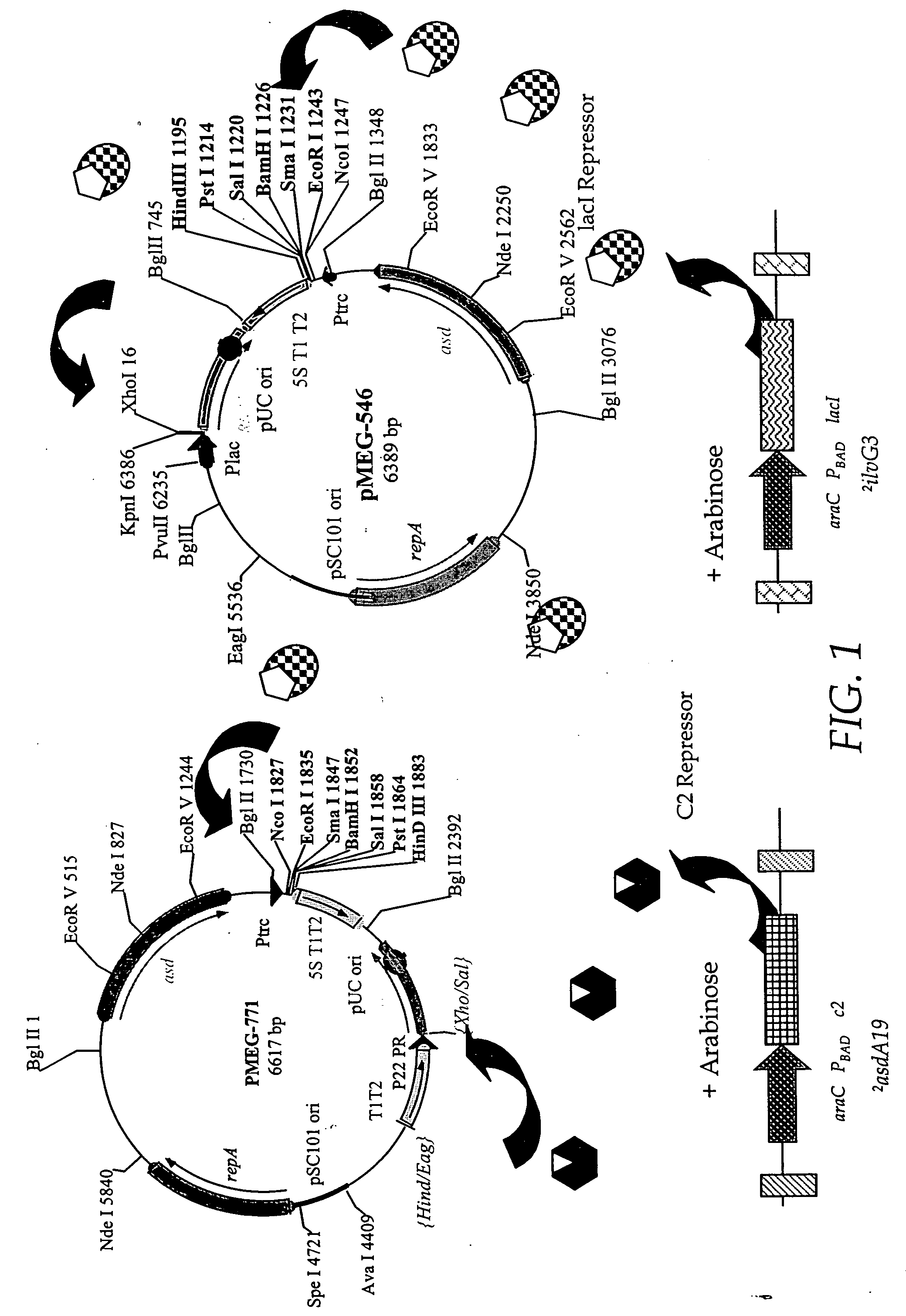
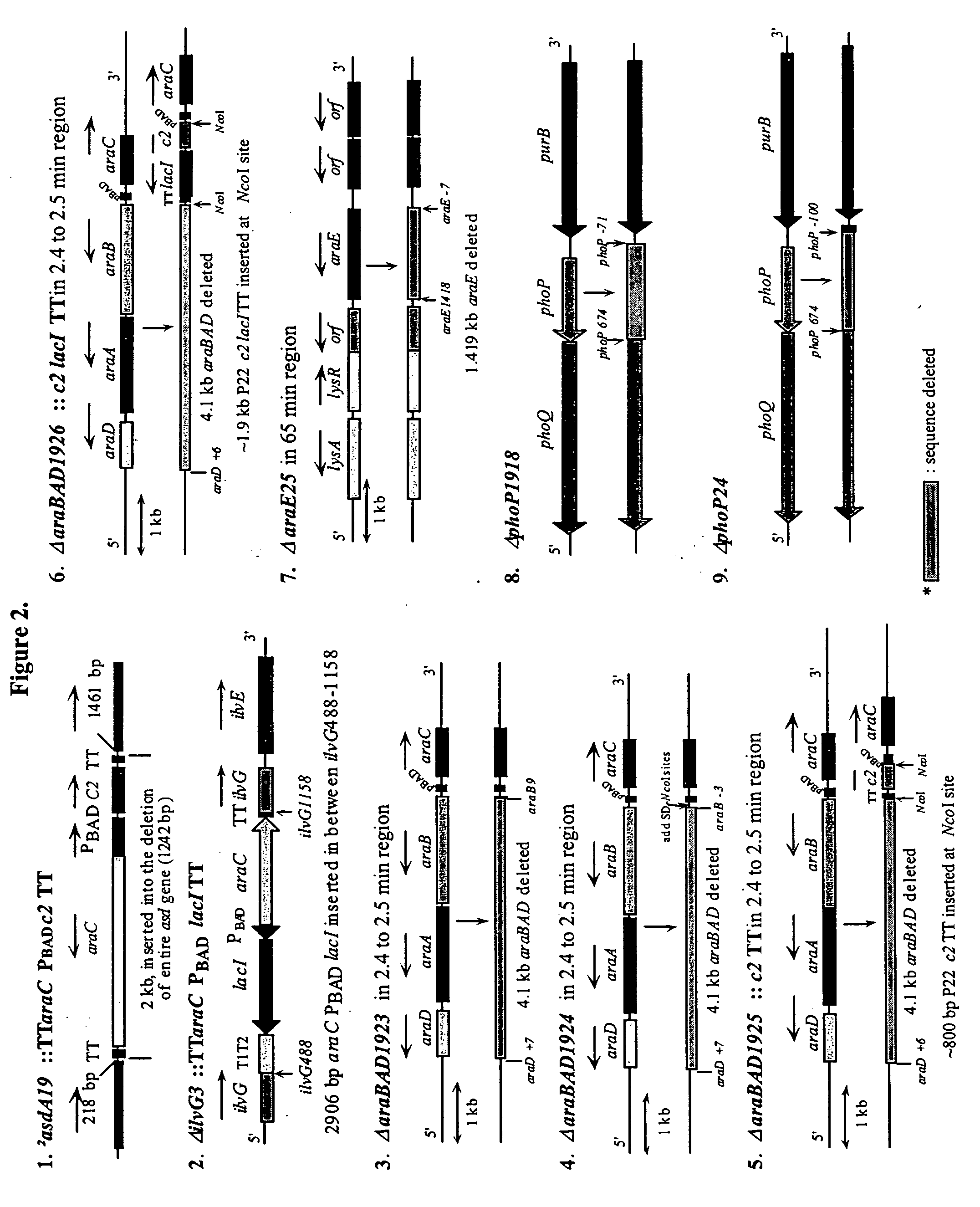
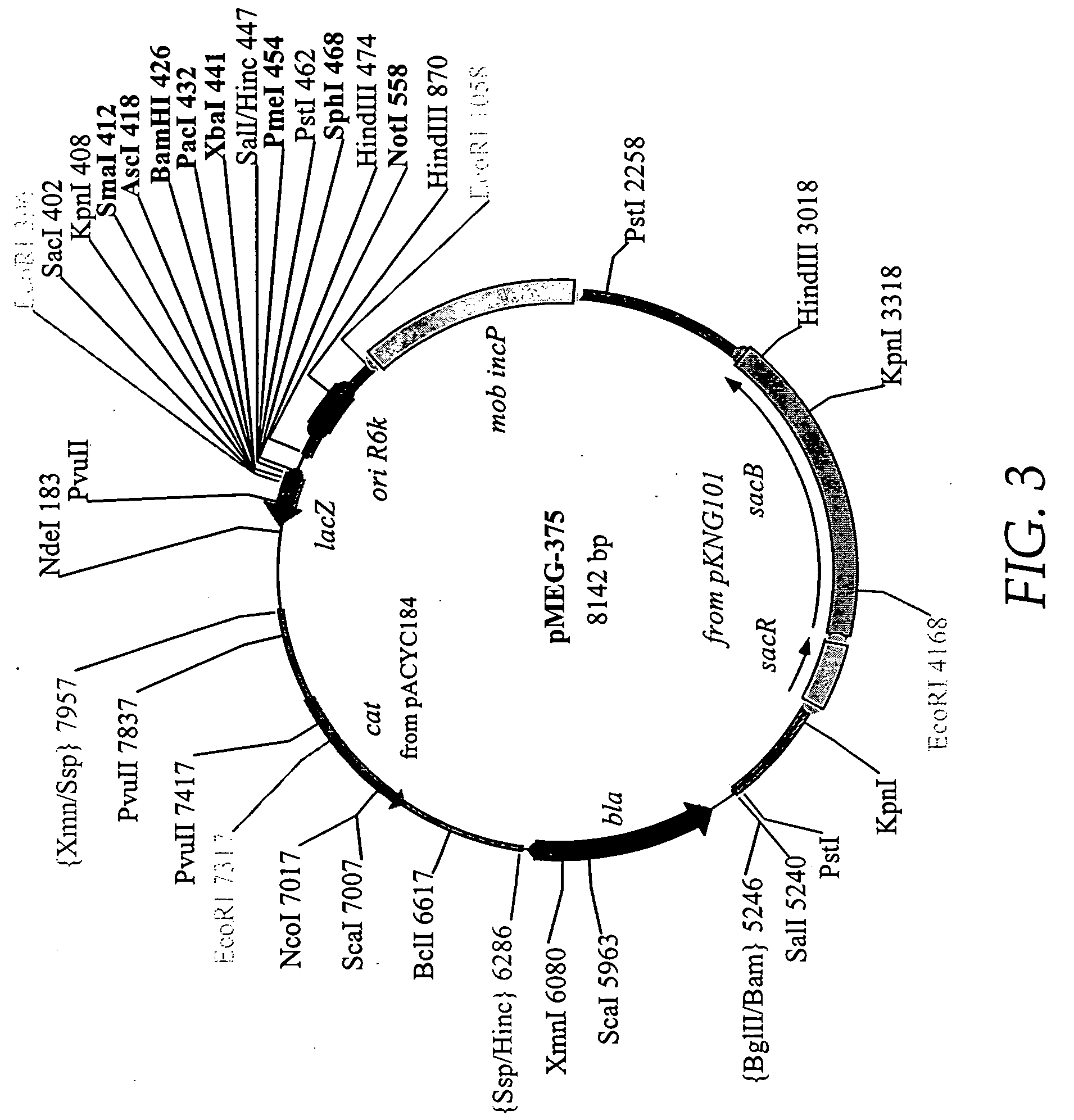
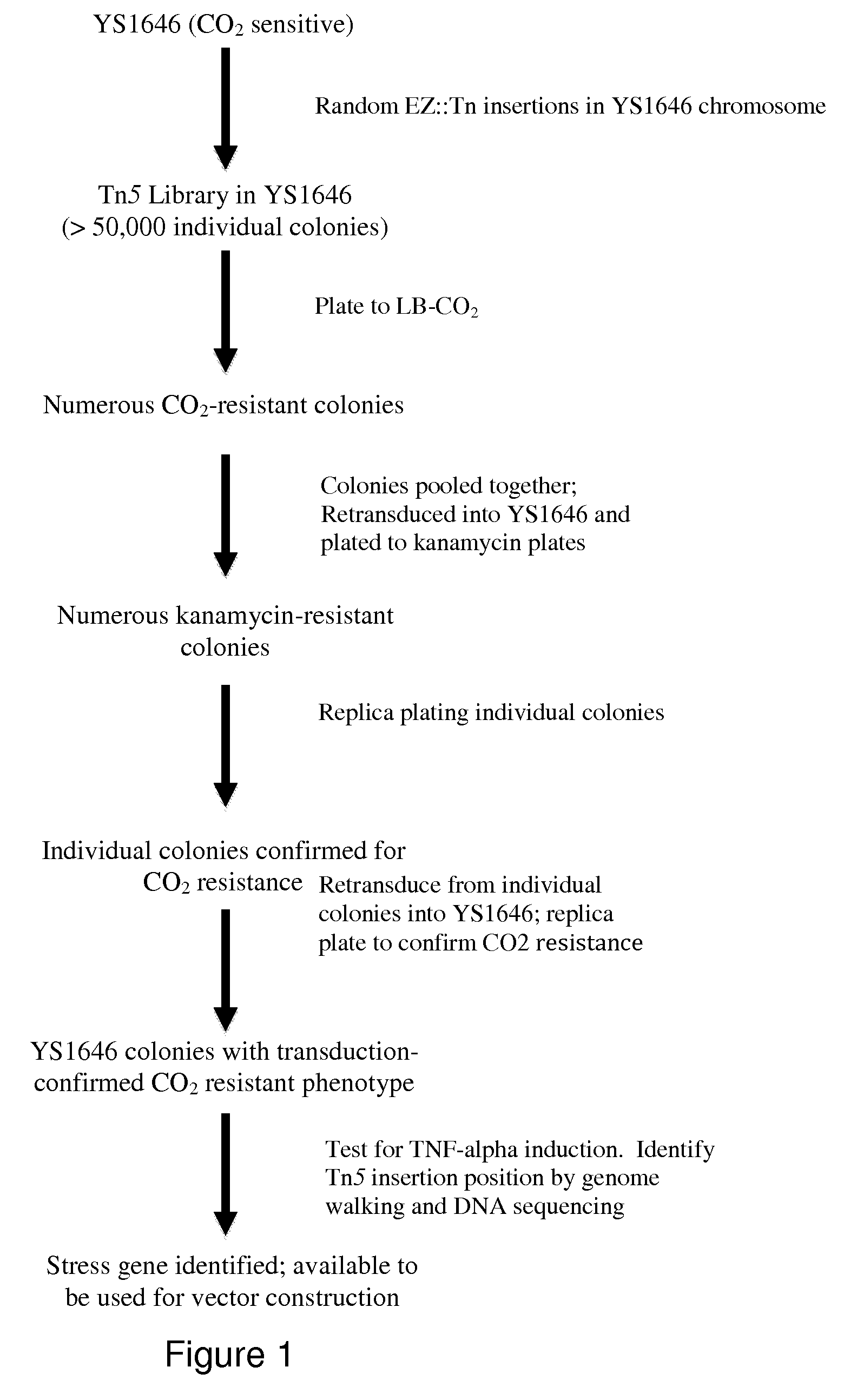
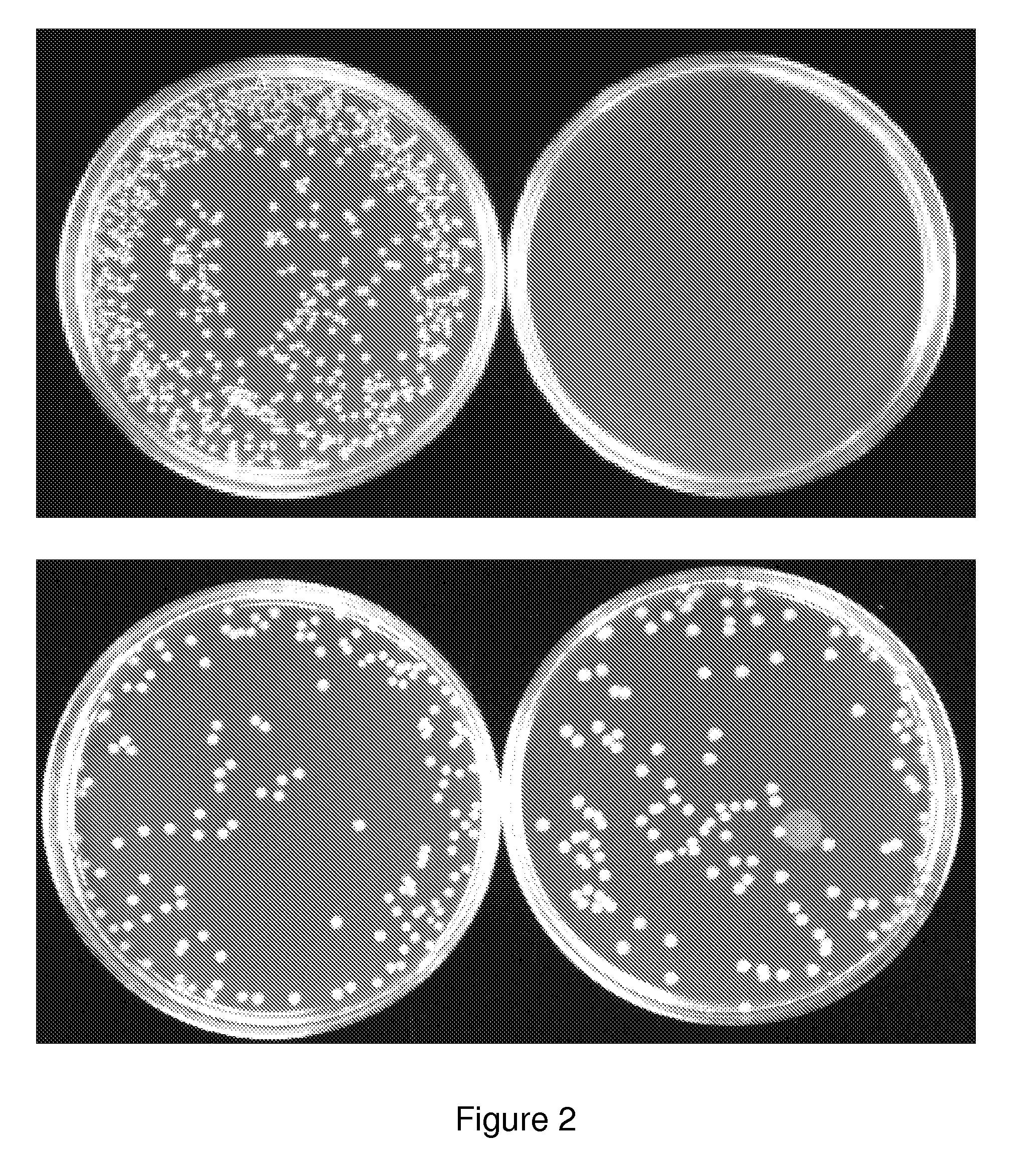
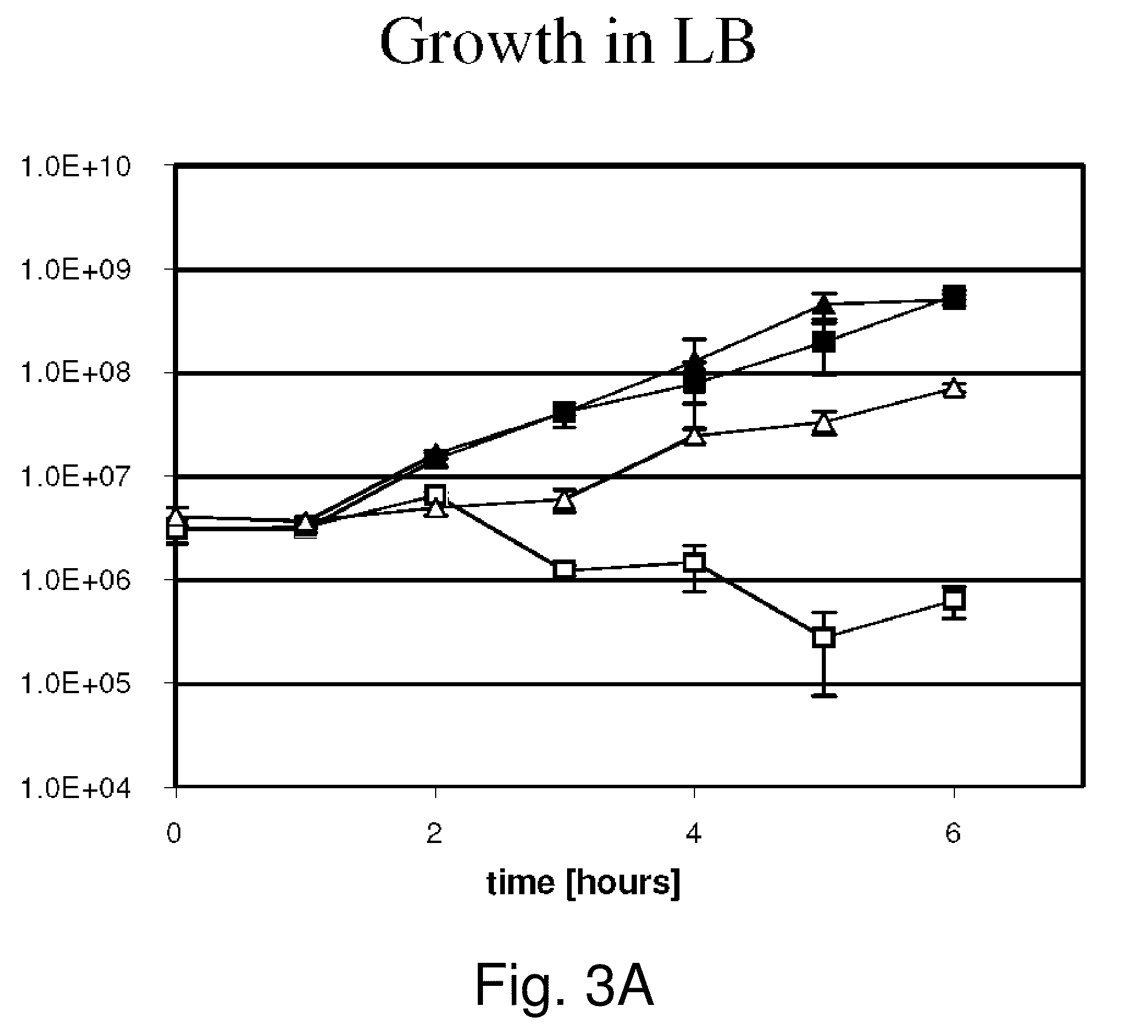
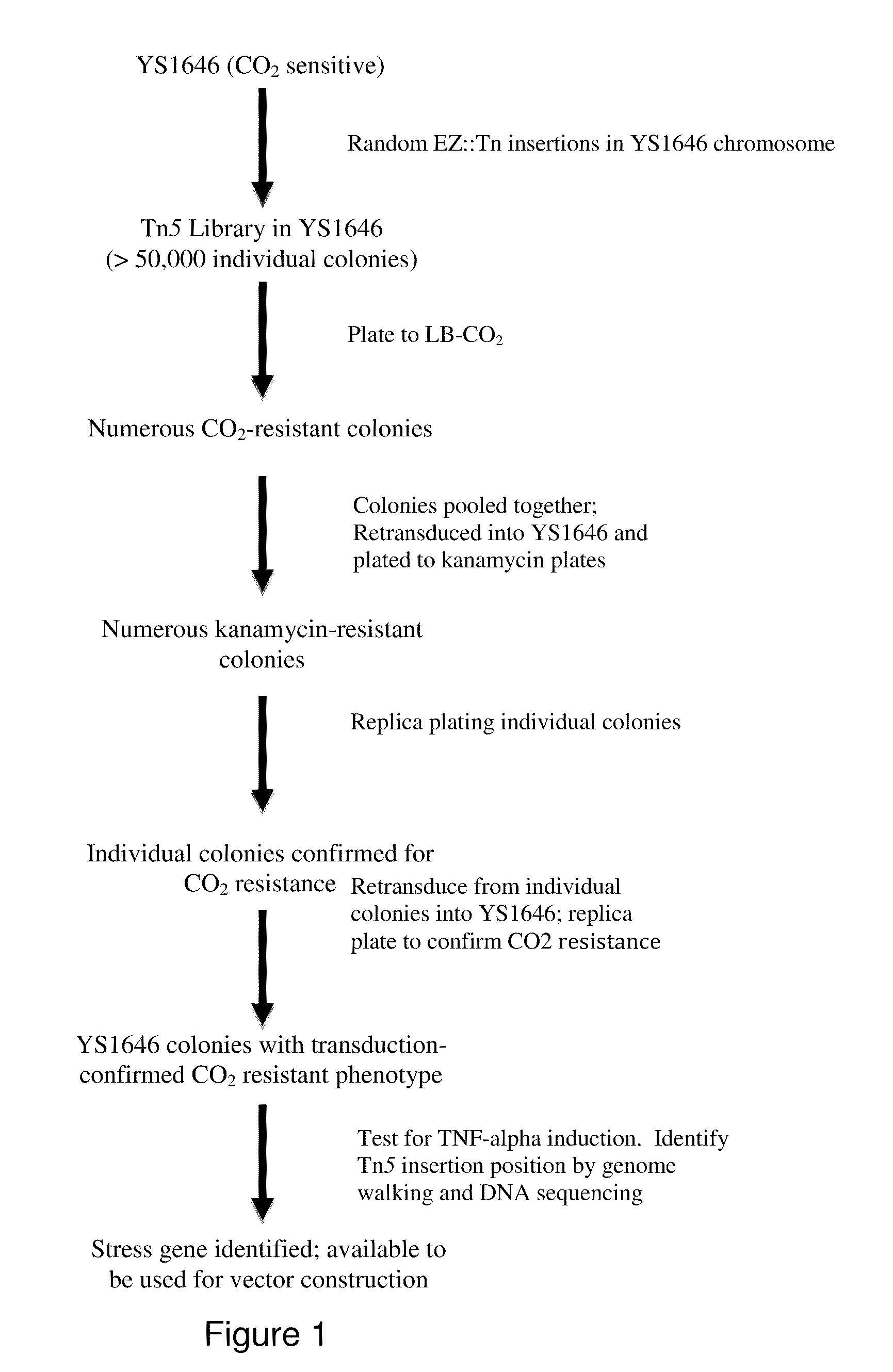
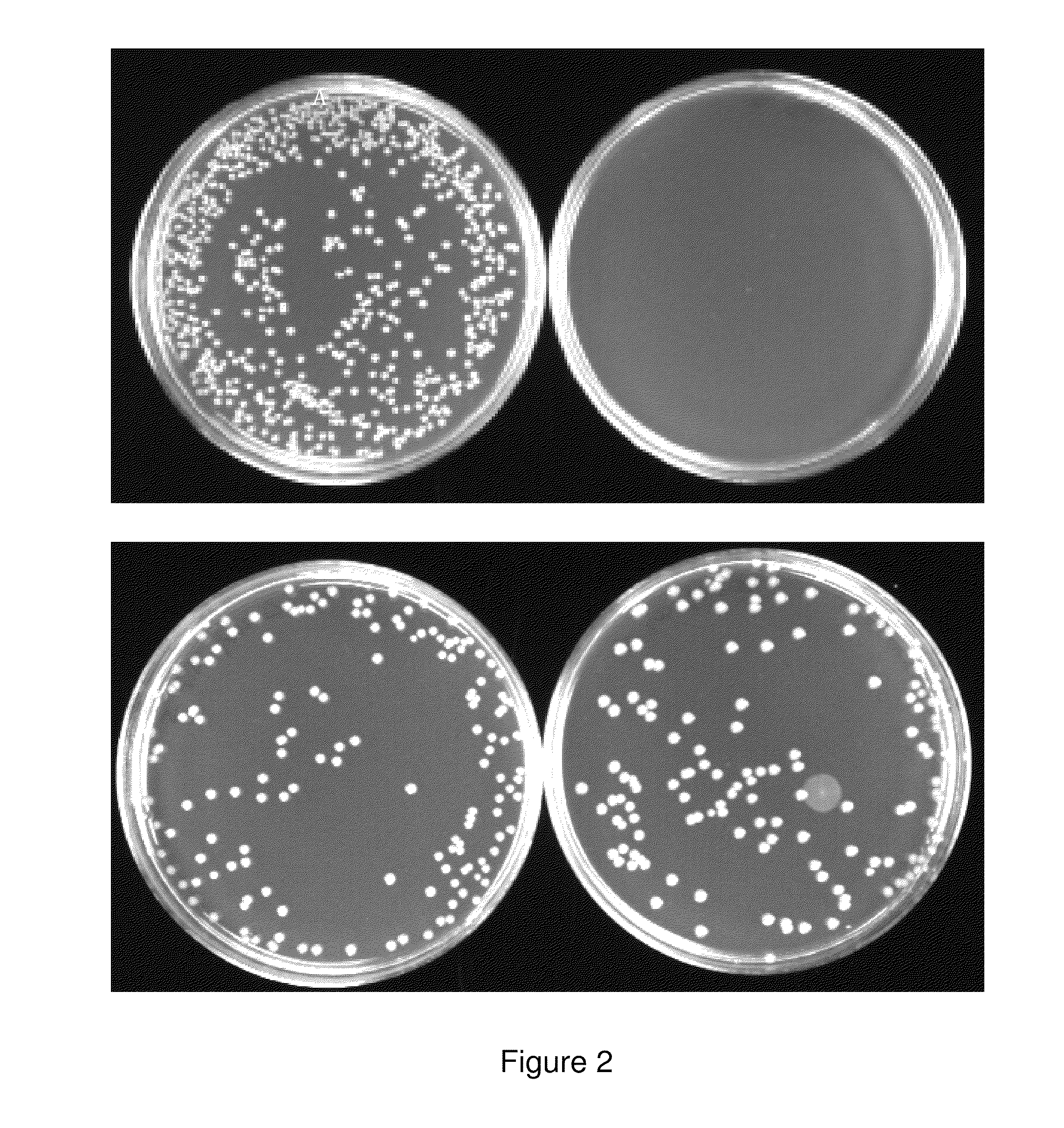
The present invention relates to gram-negative bacterial mutants resistant to one or more stress conditions, including, but not limited to, CO2, acid pH, and high osmolarity. The present invention also relates more particularly to gram-negative bacterial mutants with reduced TNF-α induction having a mutation in one or more lipid biosynthesis genes, including, but not limited to msbB, that are rendered stress-resistant by a mutation in the zwf gene. The present invention provides compositions comprising one or more stress-resistant gram-negative bacterial mutants, preferably attenuated stress-resistant gram-negative bacterial mutants. In particular, the present invention relates to methods for prophylaxis or treatment of a virally induced disease in a subject comprising administering to said subject one or more stress-resistant gram-negative bacterial mutants, preferably attenuated stress-resistant gram-negative bacterial mutants. The present invention further relates to methods for prophylaxis or treatment of a virally induced disease in a subject comprising administering to said subject one or more stress-resistant gram-negative bacterial mutants as vectors for the delivery of one or more therapeutic molecules. The methods of the invention provide more efficient delivery of therapeutic molecules by stress-resistant gram-negative bacterial mutants engineered to express said therapeutic molecules.
Owner:AVIEX TECH
Live bacterial vaccines resistant to carbon dioxide (CO2), acidic ph and/or osmolarity for viral infection prophylaxis or treatment
The present invention relates to gram-negative bacterial mutants resistant to one or more stress conditions, including, but not limited to, CO2, acid pH, and high osmolarity. The present invention also relates more particularly to gram-negative bacterial mutants with reduced TNF-α induction having a mutation in one or more lipid biosynthesis genes, including, but not limited to msbB, that are rendered stress-resistant by a mutation in the zwf gene. The present invention provides compositions comprising one or more stress-resistant gram-negative bacterial mutants, preferably attenuated stress-resistant gram-negative bacterial mutants. In particular, the present invention relates to methods for prophylaxis or treatment of a virally induced disease in a subject comprising administering to said subject one or more stress-resistant gram-negative bacterial mutants, preferably attenuated stress-resistant gram-negative bacterial mutants. The present invention further relates to methods for prophylaxis or treatment of a virally induced disease in a subject comprising administering to said subject one or more stress-resistant gram-negative bacterial mutants as vectors for the delivery of one or more therapeutic molecules. The methods of the invention provide more efficient delivery of therapeutic molecules by stress-resistant gram-negative bacterial mutants engineered to express said therapeutic molecules.
Owner:AVIEX TECH
Vaccine composition
The present invention relates to the field of Gram-negative bacterial vaccine compositions, their manufacture, and the use of such compositions in medicine. More particularly it relates to the field of useful Gram-negative bacterial outer membrane vesicle (or bleb) compositions comprising heterologously expressed Chlamydia antigens, and advantageous methods of rendering these compositions more effective and safer as a vaccine.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Compositions and methods of enhancing immune responses
ActiveUS20110027309A1Reduce morbidityEnhance immune responseSsRNA viruses negative-senseBacteriaSalmonella enteritidisBacilli
Provided herein are Salmonella enteritidis 13A strains and compositions comprising these strains. Also provided are methods of enhancing an immune response against Influenza A and methods of reducing morbidity associated with an Influenza A infection. Methods of enhancing an immune response to a vaccine vector by expressing a polypeptide of CD 154 capable of binding CD40 are also disclosed. Methods of developing a bacterial vaccine vector are disclosed. Methods of generating scarless site-specific mutations in a bacterium are also disclosed.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS +1
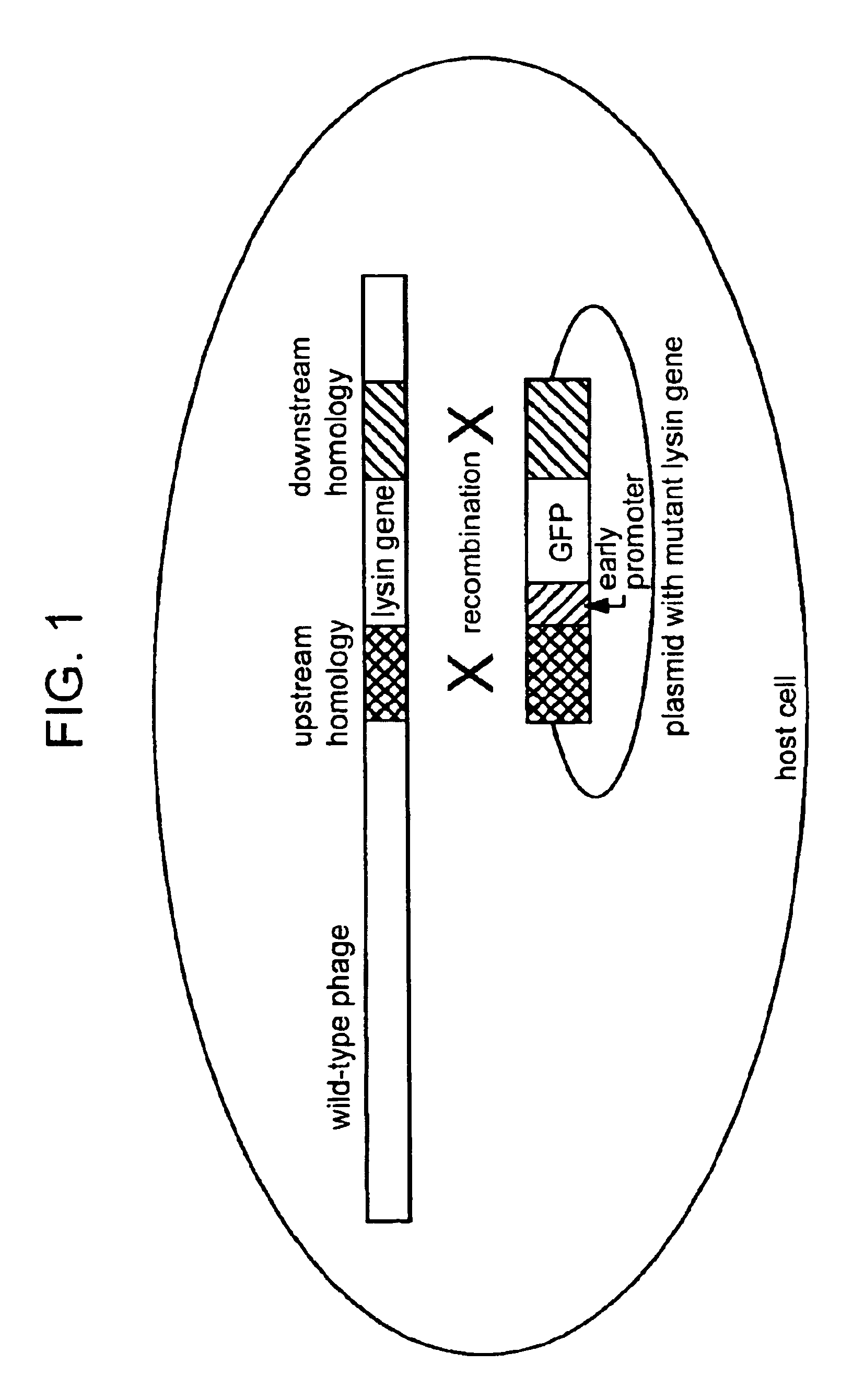
Incapacitated whole-cell immunogenic bacterial compositions
The invention features incapacitated whole cell bacterial immunogenic compositions produced by infecting a bacterium with Lys minus bacteriophage, which are deficient in the lysin protein. Lys minus bacteriophage retain activity in infection of its appropriate bacterial host, destruction of the bacterial genome, and replication, which are sufficient to inhibit bacterial growth and replication. The resulting, Lys minus-infected bacterium is provided in a state of bacteriostasis, and is not capable of replicating further (e.g., is “incapacitated”). The incapacitated bacterium can then be used as to elicit an immune response for prophylactic and / or therapeutic purposes. The invention thus also features incapacitated bacteria formulated appropriately for use in immunogenic compositions for eliciting an immune response, e.g., for production of antibodies in a non-human host or in a whole cell bacterial vaccine.
Owner:BACTOCLEAR HLDG PTE LTD
Compositions, methods and kits for enhancing the immunogenicity of a bacterial vaccine vector
ActiveUS8337861B2Improving immunogenicityOrganic active ingredientsBacterial antigen ingredientsBacteroidesImmunogenicity
The present invention comprises methods for enhancing the immunogenicity of a bacterial vaccine vector and an antigen by passaging the bacterial vaccine vector through an animal.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Live bacterial vaccine
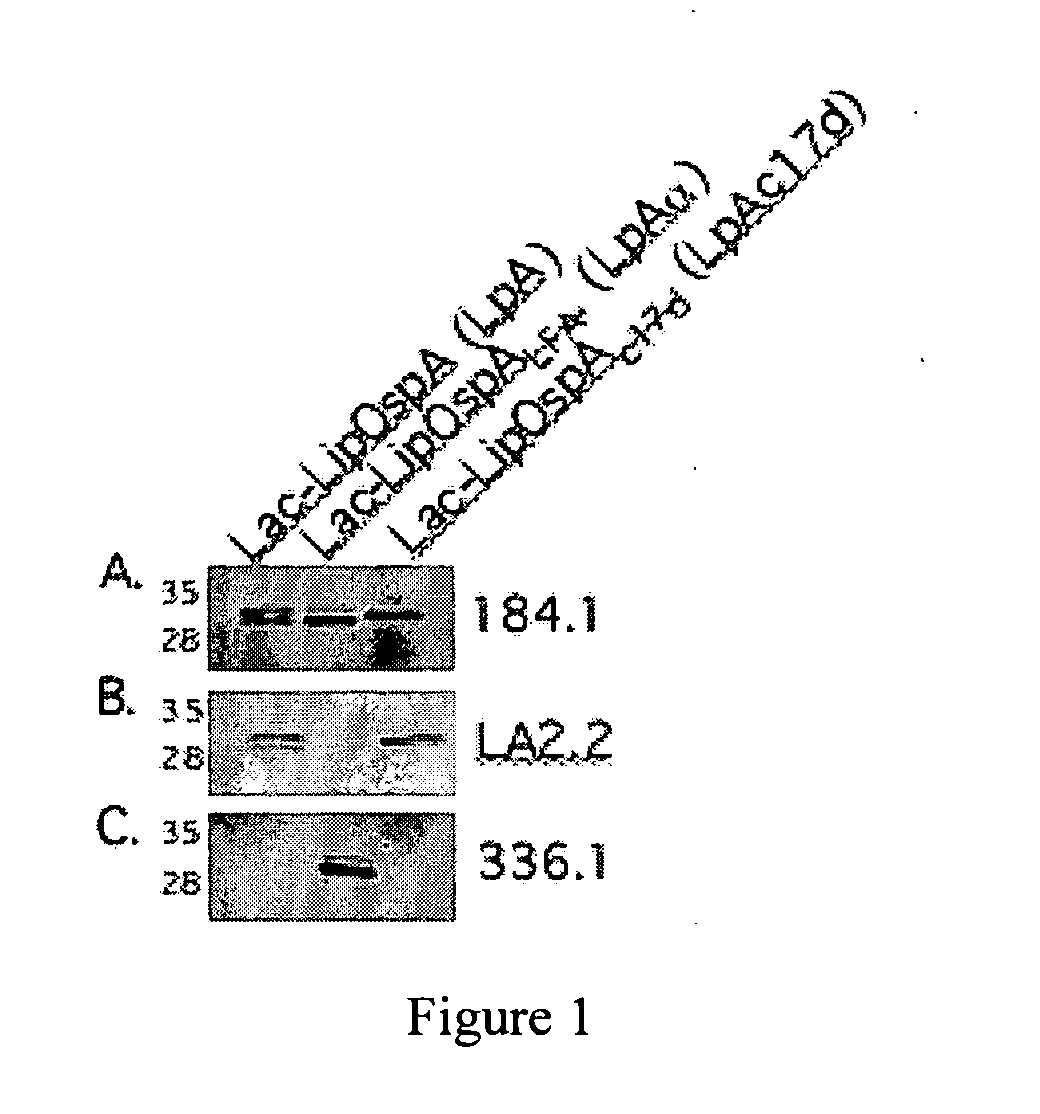
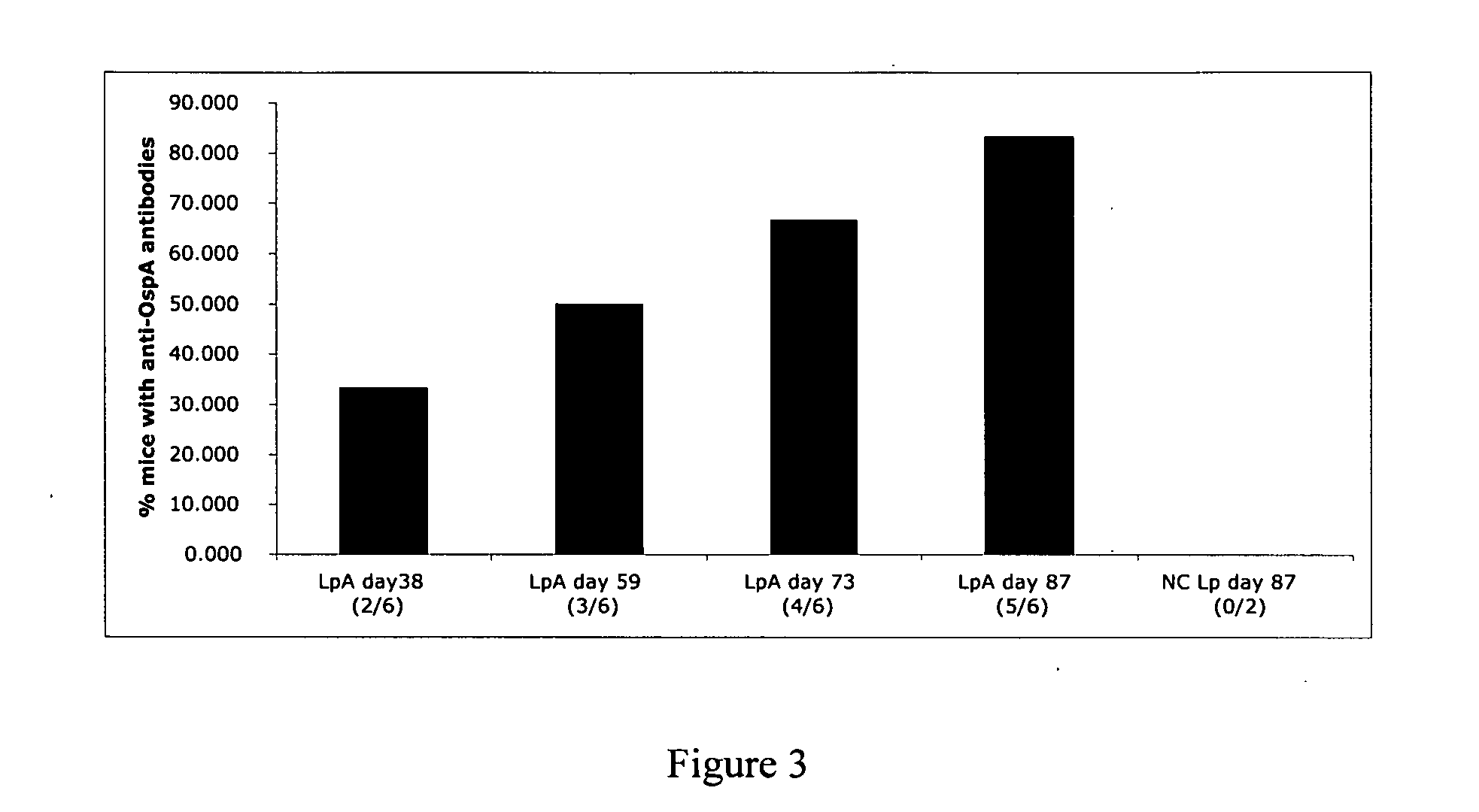
The present invention relates, e.g., to a Lactobacillus bacterium, which (1) expresses a recombinant polypeptide containing a lipoprotein signal sequence from the OspA protein of Borrelia burgdorferi, or an active variant of the leader sequence, operably linked to one or more heterologous polypeptide(s) of interest and / or (2) which comprises an expressible polynucleotide encoding a recombinant polypeptide, wherein the polynucleotide encodes a lipoprotein signal from the OspA protein of Borrelia burgdorferi, or an active variant thereof, which is operably linked to one or more heterologous polypeptide(s) of interest. In one embodiment, the heterologous polypeptide is from Yersinia pestis, the etiologic agent of plague. In another embodiment, the heterologous polypeptide is from Borrelia burgdorferi, the etiologic agent of Lyme disease. Also described are immunogenic compositions, such as live bacterial vaccines, comprising the bacterium; methods for eliciting an immune response against the polypeptide using the bacterium; and kits comprising the bacterium.
Owner:LACTRYS OCTROOI +1
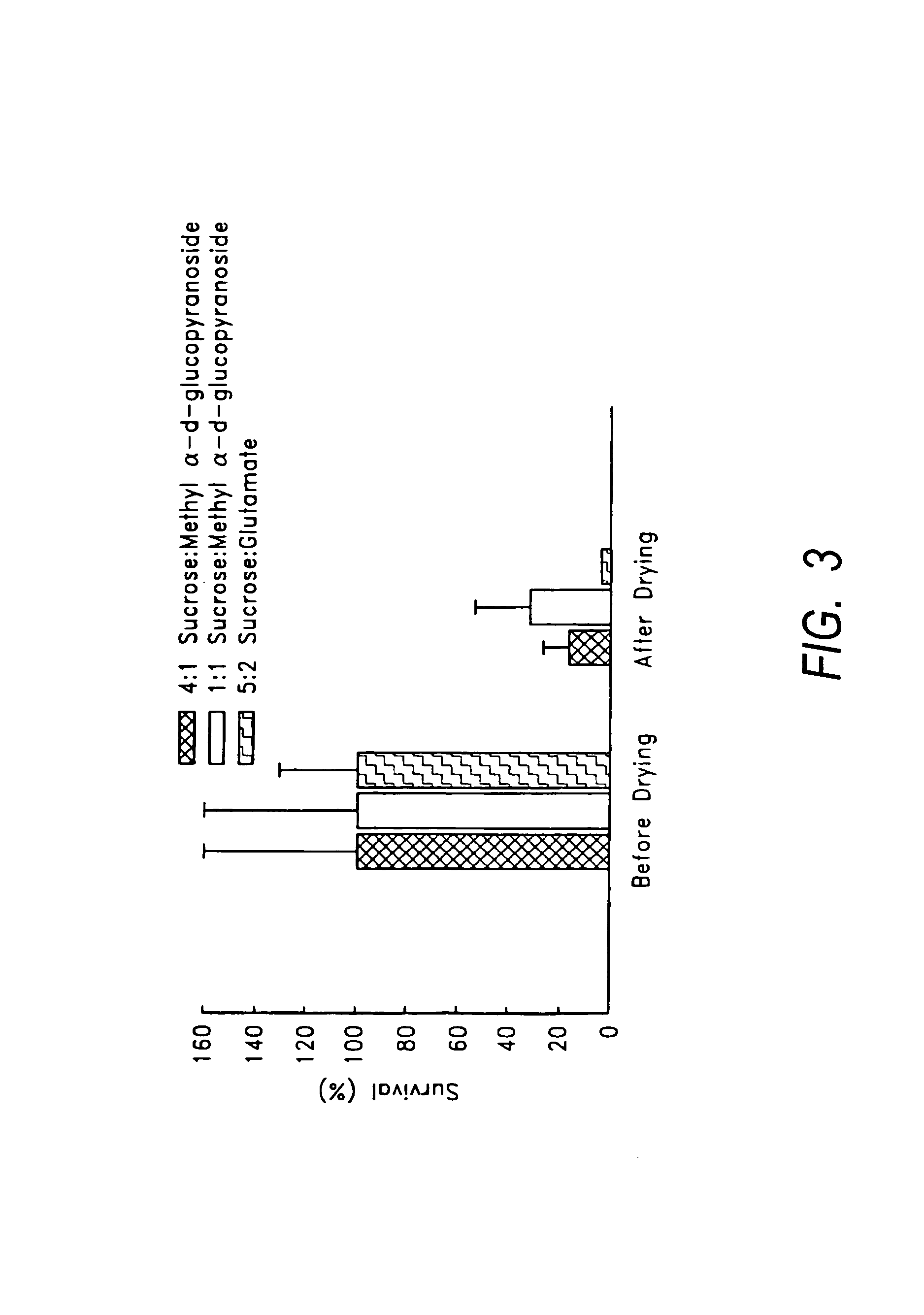
Formulation for room temperature stabilization of a live attenuated bacterial vaccine
InactiveUS20110064723A1Lower ratioGreat proportionSsRNA viruses negative-senseBacterial antigen ingredientsBacteroidesCavitation
This invention provides methods and compositions for stabilizing proteins and vaccines in dried formulations. In particular, a cavitation method and compositions of preparing a dried vaccine are provided that stabilize the viability of live bacteria and live virus vaccines at room temperature.
Owner:ARIDIS PHARMA INC
Ceramide-like glycolipid-associated bacterial vaccines and uses thereof
InactiveUS20130164325A1Improve responseAntibacterial agentsBacterial antigen ingredientsBacteroidesHeterologous Antigens
The invention is directed compositions and methods related to bacterial cells that are physically associated with ceramide-like glycolipids for use as antigen carriers for heterologous antigens. The invention further relates to methods of incorporating ceramide-like glycolipid to bacterial cell walls. The compositions and methods of the present invention are useful for the prevention and treatment of diseases.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE INC
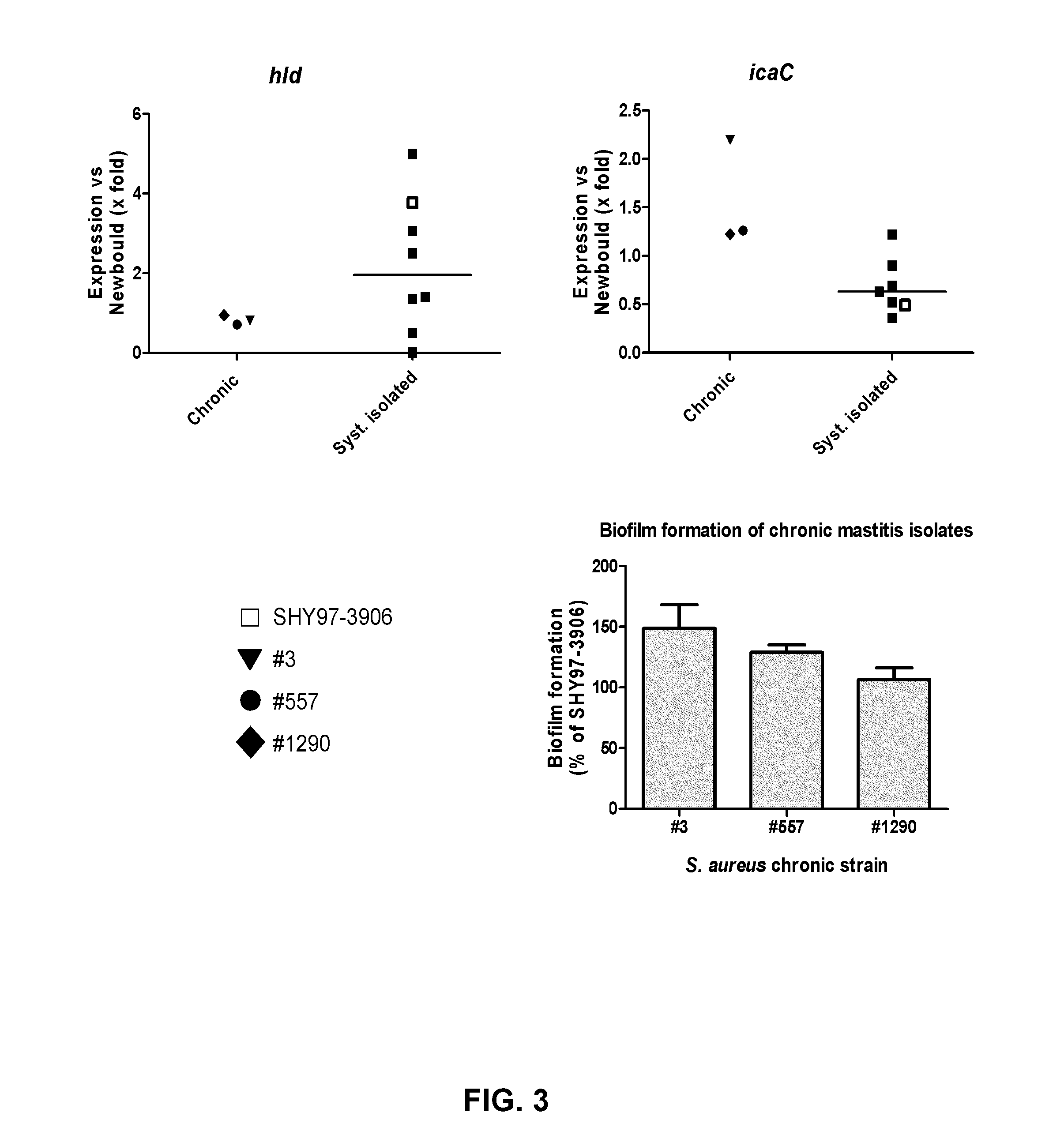
Bacterial vaccine components from Staphylococcus aureus and uses thereof
ActiveUS8889150B2Antibacterial agentsBacterial antigen ingredientsStaphylococcus aureusIntramammary infection
Agents, compositions, methods and kits useful for the treatment and diagnosis of Staphylococcal intramammary infection are disclosed. The agents, compositions, methods and kits are derived from genes expressed during Staphylococcal intramammary infection, and more particularly genes SACOL0029, SACOL0264, SACOL0442, SACOL0718, SACOL0720, SACOL1353, SACOL1416, SACOL1611, SACOL1944, SACOL2144, SACOL2365 or SACOL2599, based on the gene nomenclature from the Staphylococcus aureus COL (SACOL) genome.
Owner:SCOPRA SCI & GENIE SEC
Aquaculture creature disease controlling compound vaccine and its preparation method
InactiveCN1762387AExtensive controlSatisfied with the effectAntibacterial agentsBiocideDiseaseBacteroides
Disclosed is an aquaculture creature disease controlling compound vaccine which mainly comprises vibrio alginolyticus, bibrio parahemolyticus, vibrio alginolyticus, Aeromonas hydrophila, Aeromonas sobria, Pseudomonas aeruginosa, and Vibrio alginolyticus. The invention also discloses the preparing process for the composite bacterial vaccine.
Owner:DALIAN UNIV
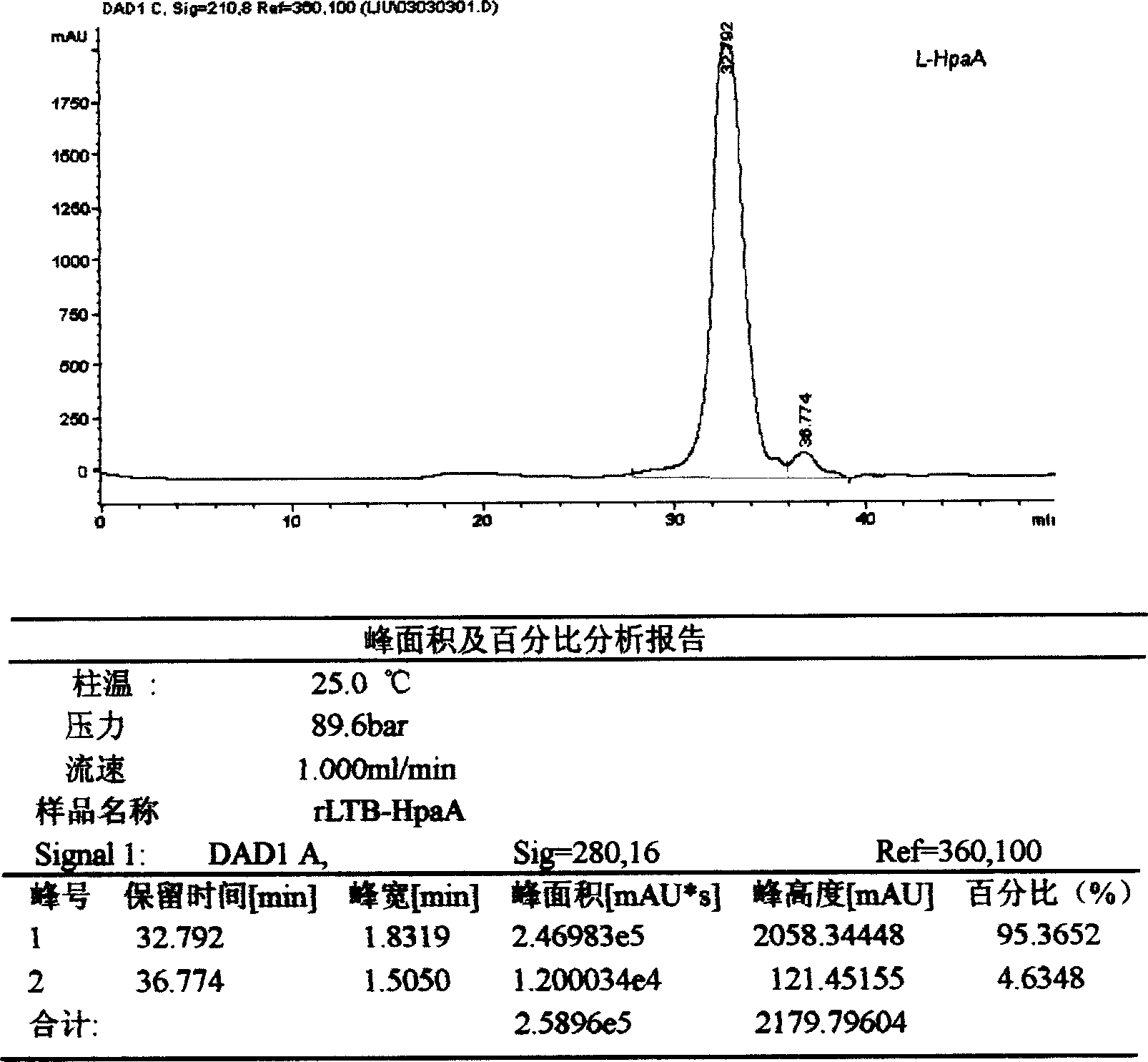
Constructing genetic engineering Vaccine of adhesin of confluent Helicobacter pylor and preparation method
InactiveCN1563388ANumber of consistent peaksGood attentionPeptide/protein ingredientsPeptide preparation methodsEscherichia coliAdjuvant
This invention discloses the construction of a fused poloric spirillum adhesion gene engineered bacterial vaccine characterizing that the adhesion HpaA is used to construct an adjuvant fused engineered vaccine of poloric spirillum adhesion and colibacillus heat-labile enterotoxin B sub unit or cholear toxin B sub unit intramolecular to get large quantity of necessary vaccine proteins by high density fermentation, occlusion body extraction, renaturation and purification to be proprared to peroral, injection and spray agents.
Owner:ARMY MEDICAL UNIV
Activated probiotic skin cream and preparation method thereof
ActiveCN103251547ARich varietyWith skin careCosmetic preparationsToilet preparationsWater bathsSludge
The invention discloses activated probiotic skin cream and a preparation method thereof. The method comprises the steps of carrying out high-intensity cultivation on probiotic, thereby obtaining bacterial sludge from culture solution by centrifugation; mixing glycerol with mycose; heating in a water bath until the mycose is completely dissolved; tightly cooling to 0-4 DEG C after sterilizing; mixing the obtained bacterial sludge with a mixing solution of glycerol and mycose, grinding and stirring evenly to form bacterial vaccine, filtering to obtain activated probiotic bacteria liquid; preparing a water-in-oil emulsion system, adding the activated probiotic bacteria liquid and a daily essence to the water-in-oil emulsion system, and evenly stirring to obtain the activated probiotic skin cream, wherein the obtained activated probiotic skin cream contains 1*10<6> to 1*10<8> CFU / g of activated probiotic. The skin cream disclosed by the invention can ensure the activity and the quality stability of the probiotic in the production and product storage processes; and effects of cleaning the skin by the probiotic, balancing the skin surface pH, restraining harmful bacteria, preventing damage of heavy metal ions on the cell, holding water and moisturizing are finally achieved.
Owner:广州集妍化妆品科技有限公司
Recombinant nucleic acid molecules, expression cassettes, and bacteria, and methods of use thereof
InactiveCN1921884APolypeptide with localisation/targeting motifBacterial antigen ingredientsHeterologousListerella paradoxa
The present invention provides recombinant nucleic acid molecules, expression cassettes and vectors for expressing polypeptides, including heterologous polypeptides such as antigens, in bacteria. Some recombinant nucleic acid molecules, expression cassettes and vectors include codon-optimized sequences encoding polypeptides and / or signal peptides. Some recombinant nucleic acid molecules, expression cassettes and expression vectors include sequences encoding non-Listerial and / or non-secAl signal peptides for secreted polypeptides. The present invention also provides bacteria comprising nucleic acid molecules, expression cassettes and expression vectors, and compositions such as vaccines comprising the bacteria. Methods of making and using the bacteria, recombinant nucleic acid molecules and expression cassettes are also provided.
Owner:ADURO BIOTECH
Porcine interferon gene family and application thereof in blue-ear porcine disease resistant medicament and vaccine adjuvant
InactiveCN101955941AGood immune protectionEasy gene insertionPeptide/protein ingredientsGenetic material ingredientsGenetic engineeringTGE VACCINE
The invention belongs to the technical field of genetic engineering, and particularly relates to a porcine interferon family gene and application thereof in a blue-ear porcine disease resistant medicament and a vaccine adjuvant. The porcine interferon gene is taken from a home pig genome and has anti-virus effect, and the amino acid sequence of the porcine interferon gene is SEQ ID No. 1, SEQ ID No. 3, SEQ ID No. 5, SEQ ID No. 7 or SEQ ID No. 9; and the medicament and the vaccine adjuvant are a recombinant plasmid obtained by cloning the home pig interferon gene to an eukaryotic expression vector through molecular biology technology and a live bacterial vaccine obtained by transferring the plasmid to a low virulent strain of Salmonella choleraesuls. Animal experiments prove that the medicament and the adjuvant have good safety and remarkable effect. After a home pig is directly injected, the toxicity is removed on the same day, and the protective rate can reach 80 percent. When the adjuvant is used with a blue-ear disease inactivated vaccine and after the home pig is subjected one-time immune injection, the toxicity is removed in 28 days, and the protective rate can reach 80 percent.
Owner:FUDAN UNIV
Multibacterial vaccines and uses thereof
InactiveUS20060292173A1Inhibiting and preventing diseaseBiocideOrganic active ingredientsDiseaseGram
The present invention provides methods for establishing standards for Gram-negative, Gram-positive, and mixed bacterial cultures. The present invention also provides methods for reproducing Gram-negative, Gram-positive, and mixed bacterial cultures. The present invention further provides methods for preparing multibacterial vaccines. Also provided are multibacterial vaccines prepared in accordance with these methods, and methods for treating and / or preventing disorders using these multibacterial vaccines. In addition, the present invention provides methods for predicting the efficacy of multibacterial vaccines, and methods for enhancing the efficacy of multibacterial vaccines.
Owner:MBVAX BIOSCI
Compound microbial agent solid particle product for environment protection and preparation method thereof
ActiveCN109321482AImprove microbial activityImprove survival rateBacteriaDispersed particle separationEnvironmental resistanceHigh density
The invention relates to the technical field of biological fermentation and in particular relates to a compound microbial agent solid particle product for environment protection and a preparation method thereof. The method comprises the following steps: (1) carrying out high-density fermentation culture on a compound microorganism for the environment protection, so as to obtain a high-density fermentation solution; adjusting the solid content of microorganisms in the high-density fermentation solution; (2) adding a drying protection agent into the adjusted high-density fermentation solution, wherein the adding amount of the drying protection agent is 0.1 to 10 percent of the weight of the adjusted high-density fermentation solution; uniformly mixing to obtain bacterial vaccine; (3) mixingthe bacterial vaccine and auxiliary materials and granulating to obtain solid particles; (4) drying the solid particles at 25 to 80 DEG C to obtain the compound microbial agent solid particle productfor the environment protection, wherein the drying time is 8 to 48h. According to the preparation method provided by the invention, raw materials are low in price, technological operation is simple and the production efficiency is high; the economical and efficient compound microbial agent solid particle product is provided for the environment protection industry.
Owner:GUANGDONG ZHONGWEI ENVIRONMENTAL PROTECTION BIOTECH CO LTD
Polyvalent Attenuated Live Vaccine For Preventing and Curing Vibriosis of Cultivated Fish
ActiveUS20080274136A1Eliminate environmentEliminate security concernsAntibacterial agentsBacterial antigen ingredientsBacteroidesVibrio anguillarum
A novel polyvalent attenuated live bacterial vaccine for preventing and curing vibriosis of cultivated fish is provided. The vaccine mainly comprises attenuated deletion strain of Vibrio anguillarum without marker gene, which has significant low toxicity, but remains immunogenicity against wild type strain of V. anguillarum, as compared with wild type strain MVM425. Moreover, the vaccine strain has excellent cross immunoprotection against Vibrio alginolyticus. The attenuated live vaccine made from the vaccine strain is effective to prevent and cure vibriosis of fish resulted from wild type strain of V. anguillarum and V. alginolyticus.
Owner:EAST CHINA UNIV OF SCI & TECH
Pro-Apoptotic Bacterial Vaccines To Enhance Cellular Immune Responses
InactiveUS20120276144A1Diminishing intracellular survivalReduced activityAntibacterial agentsBacterial antigen ingredientsVaccine PotencyVaccine efficacy
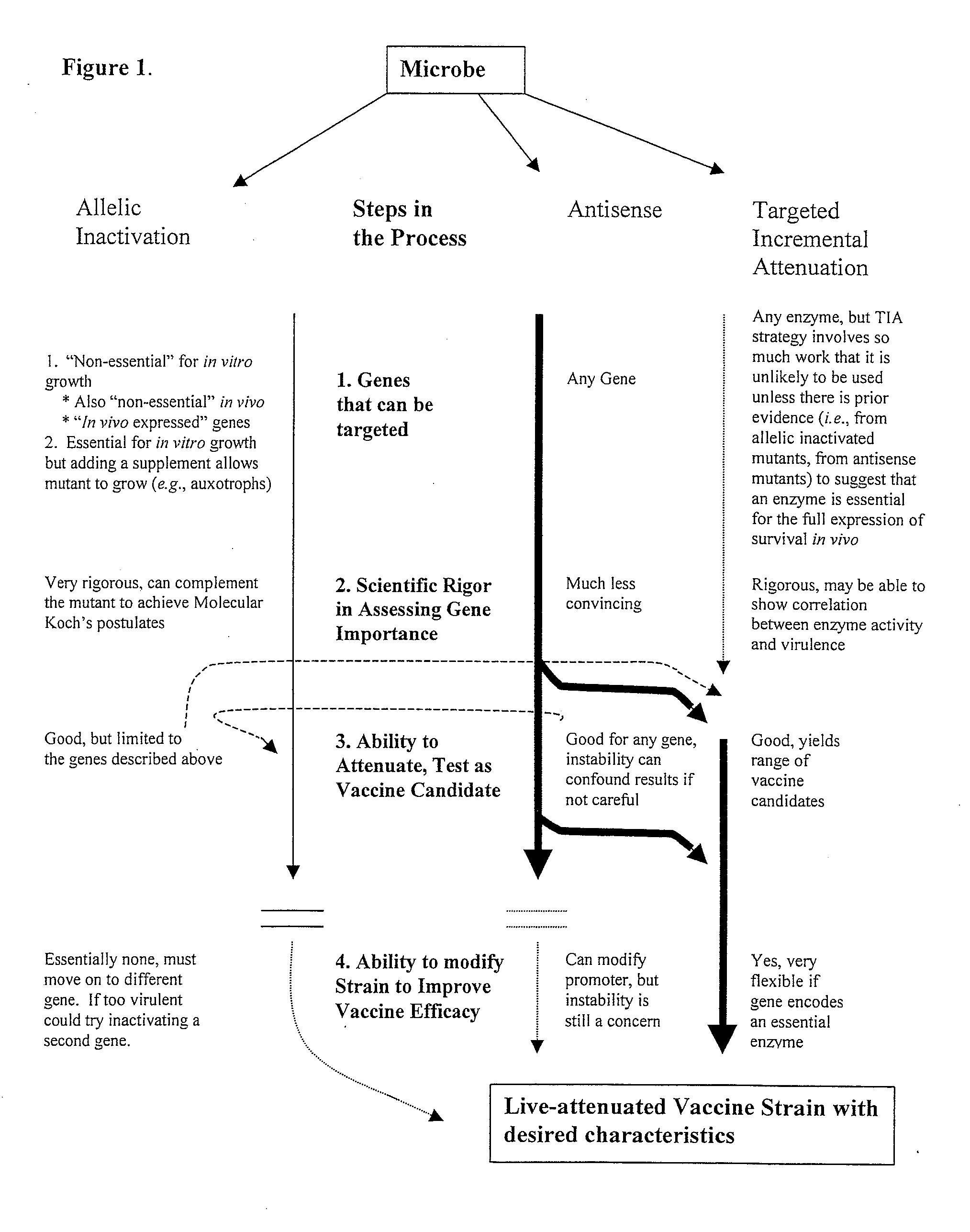
Whole-cell vaccines and methods for their use in producing protective immune responses in vertebrate hosts subsequently exposed to pathogenic bacteria. The present invention involves a method of enhancing antigen presentation by intracellular bacteria in a manner that improves vaccine efficacy. After identifying an enzyme that has an anti-apoptotic effect upon host cells infected by an intracellular microbe, the activity of the enzyme is reduced, thereby modifying the microbe so that it increases immunogenicity. Also, the present invention provides a method of incrementally modifying enzyme activity to produce incrementally attenuated mutants of the microbe from which an effective vaccine candidate can be selected.
Owner:VANDERBILT UNIV
Ultrasonic microvesicle as immuno adjuvant and vaccine carrier
ActiveCN100546649CImprove expression levelImprove immune activityPeptide/protein ingredientsCarrier-bound antigen/hapten ingredientsVaccine antigenPeptide vaccine
The invention belongs to the field of biomedical engineering, and more specifically, the invention relates to a new type of ultrasonic microbubble used as an immune adjuvant and vaccine carrier. Ultrasound microbubbles carrying vaccine antigens are synthesized by adhering the vaccine antigen material to the surface of the microbubbles and / or wrapping them in the microbubbles; when used, the microbubbles carrying the vaccine antigens are applied to the target tissue through the system and locally, and ultrasonic waves are used to destroy the target tissue. The microbubbles in the tissue area release vaccine antigen substances in a localized manner and enhance the immune activity of the antigens. It is expected to be developed into a new immune adjuvant and vaccine carrier, especially new adjuvants and carriers suitable for peptide vaccines, nucleic acid vaccines, viral and bacterial vaccines.
Owner:许川山 +2
Ultrasonic microvesicle as immuno adjuvant and vaccine carrier
ActiveCN1935259AImprove expression levelImprove immune activityPeptide/protein ingredientsCarrier-bound antigen/hapten ingredientsVaccine antigenTarget tissue
The present invention belongs to the field of biomedical engineering, in the more concrete, said invention relates to a new type ultrasonic microvesicle which can be used as immunological adjuvant and vaccine carrier. It is characterized by that the vaccine antigen substance is adhered on the microvesicle surface and / or covered in microvesicle interior so as to synthesize the vaccine antige carried ultrasonic microvesicle. When it is used, the vaccine antigen carried ultrasonic microvesicle can systematically or locally act on target tissue, the ultrasonic wave can be used to break the microvesicle of target tissue area, and orietationally release vaccine antigen substance so as to raise the immunological activity of antigen.
Owner:许川山 +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com