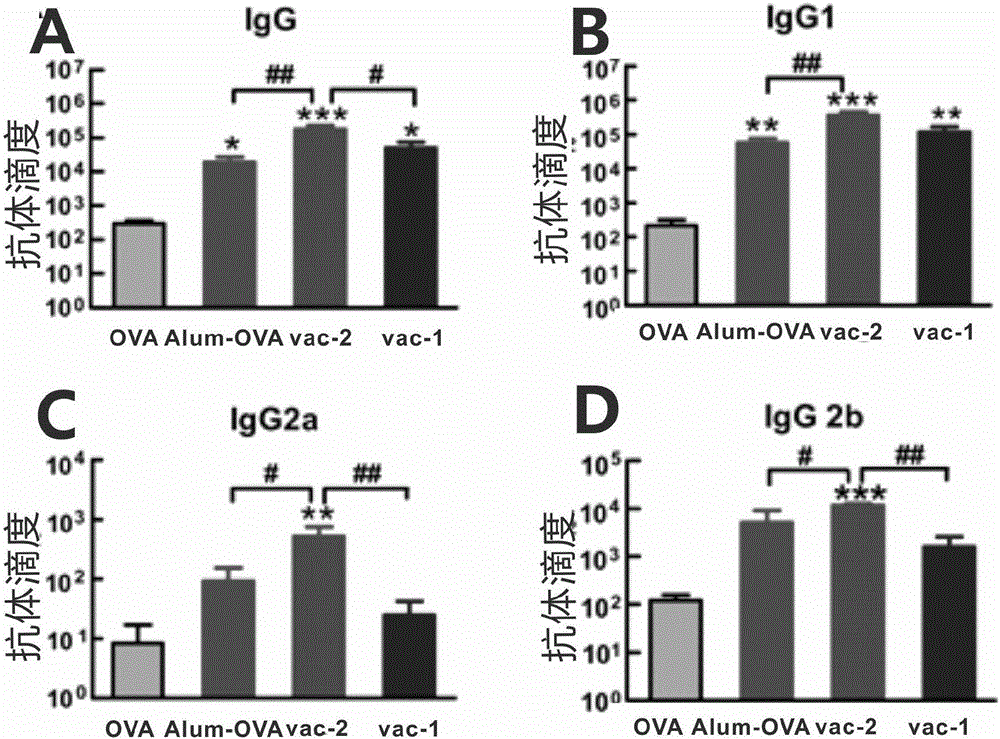
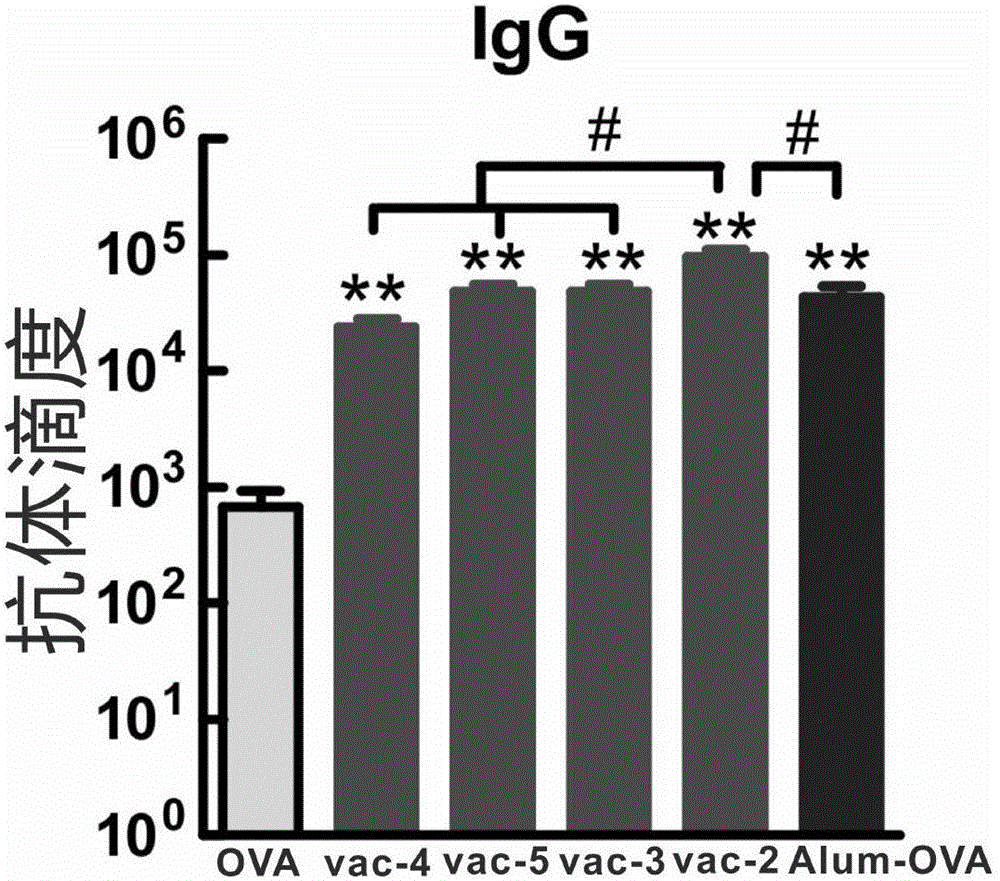
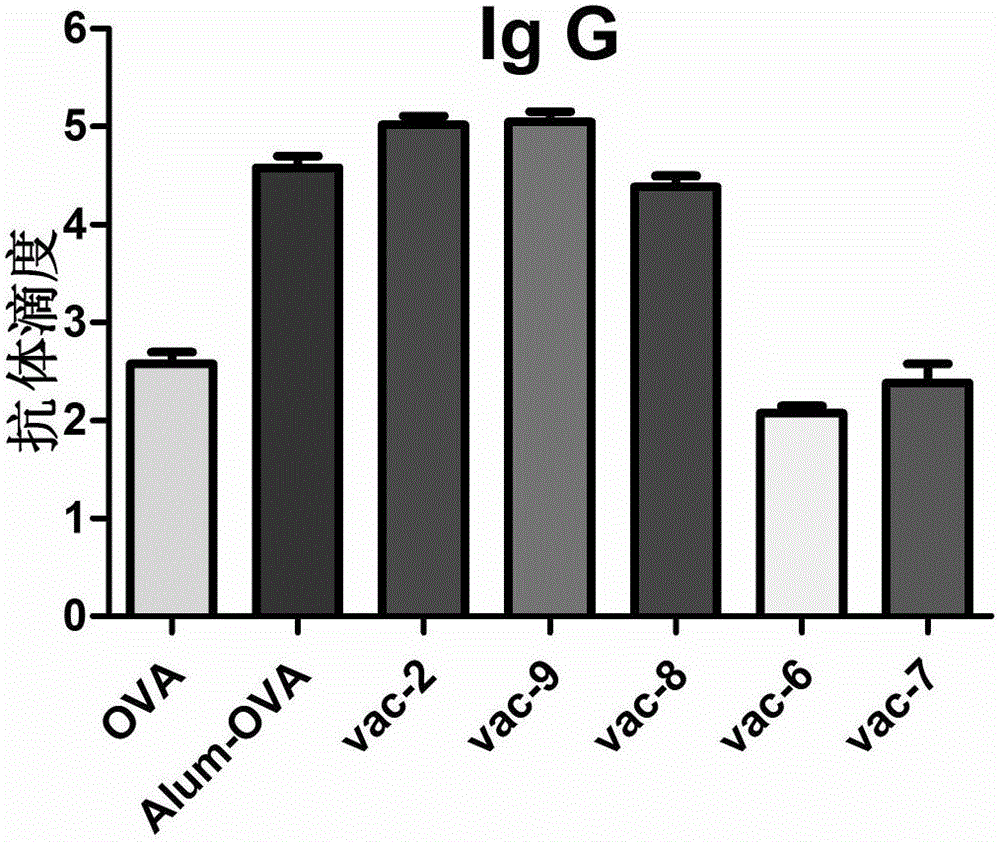
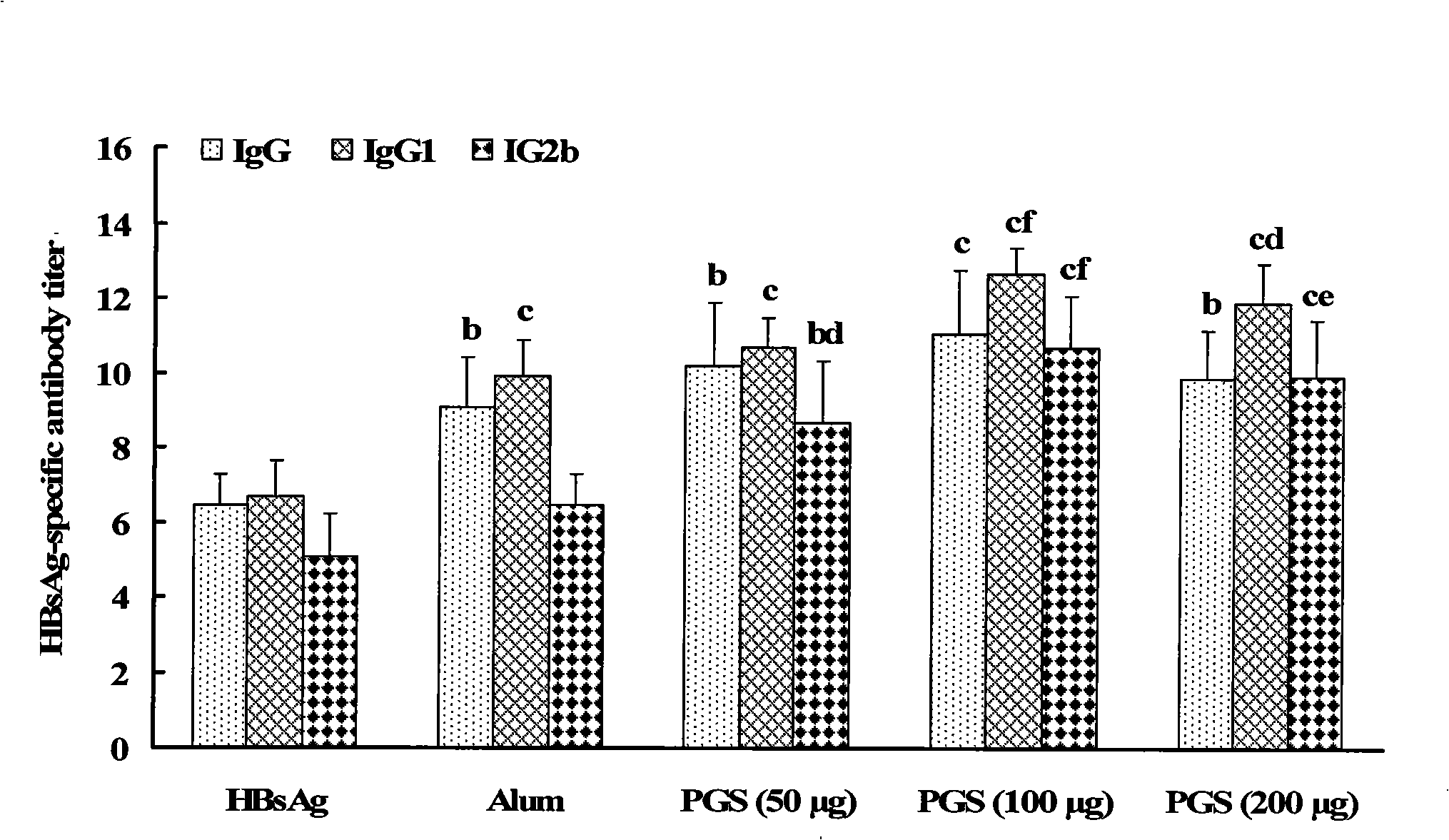
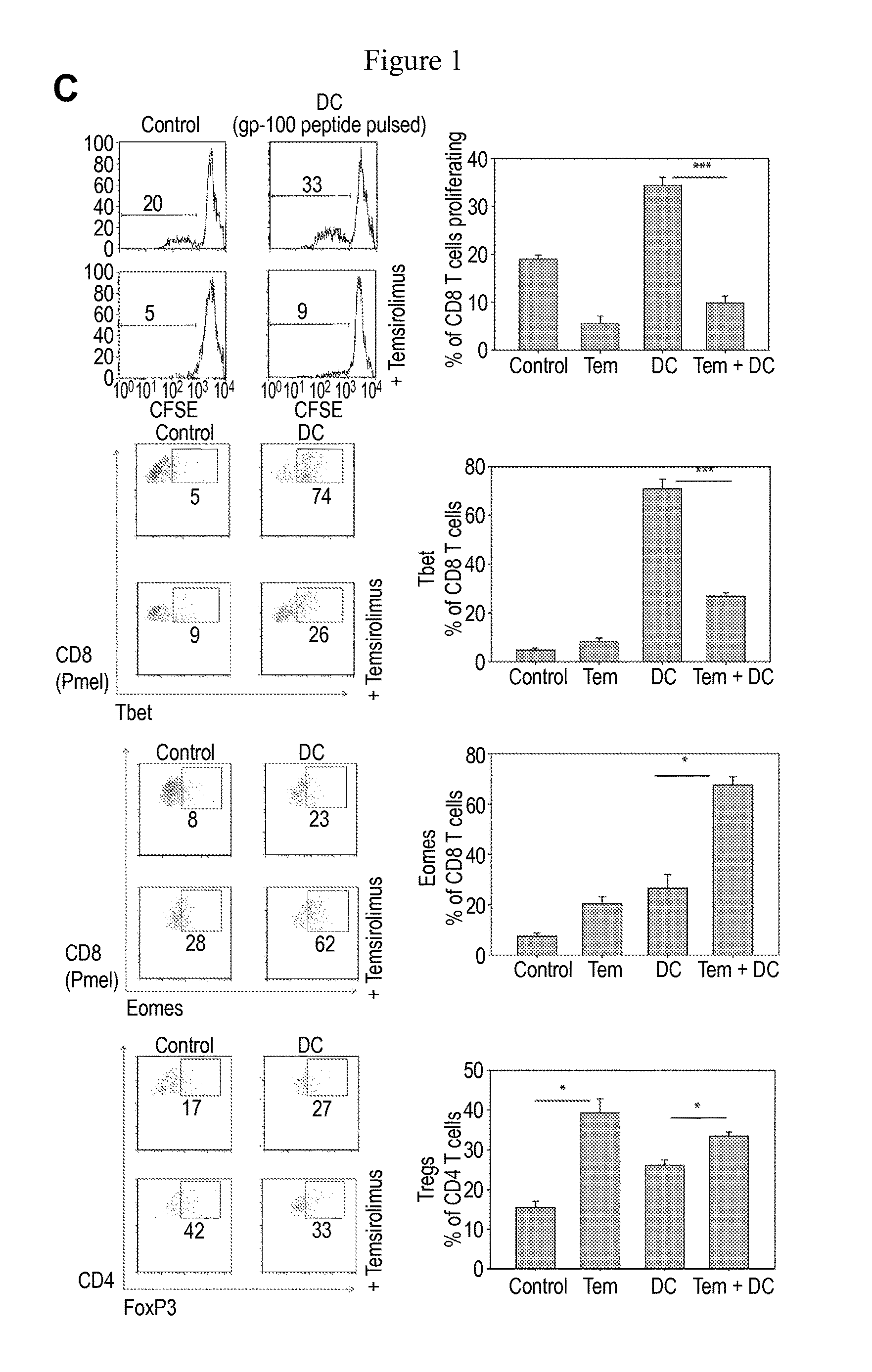
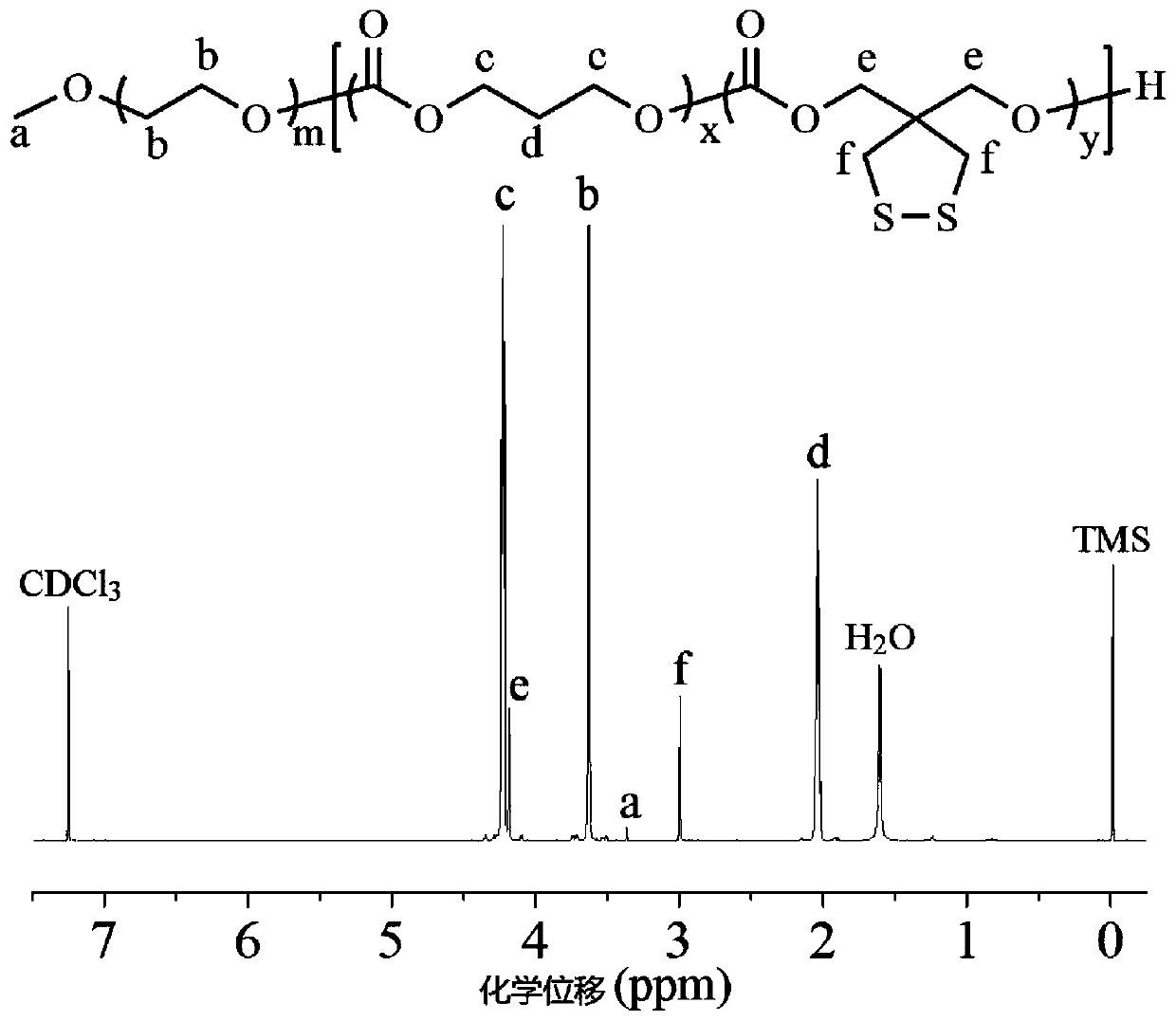
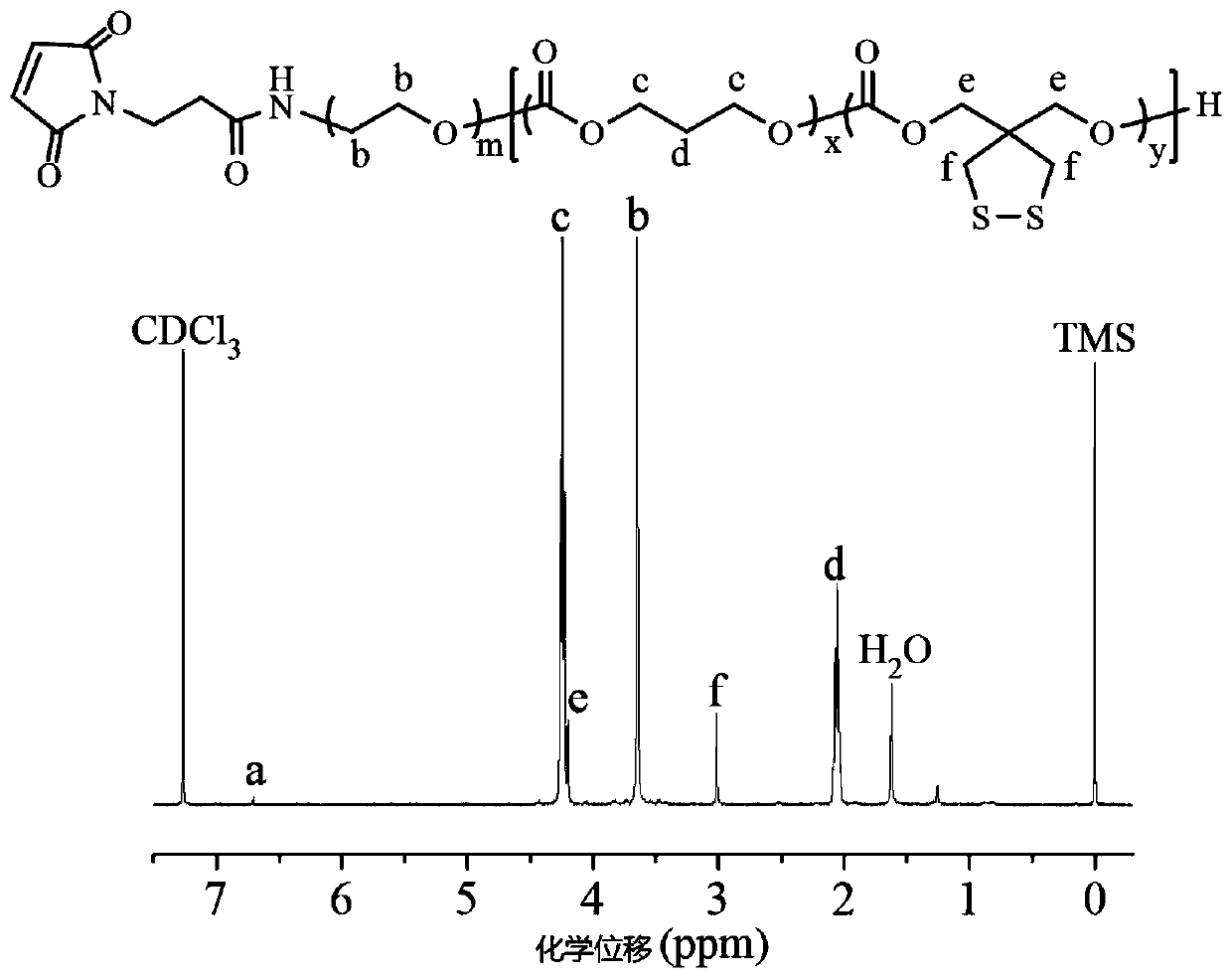
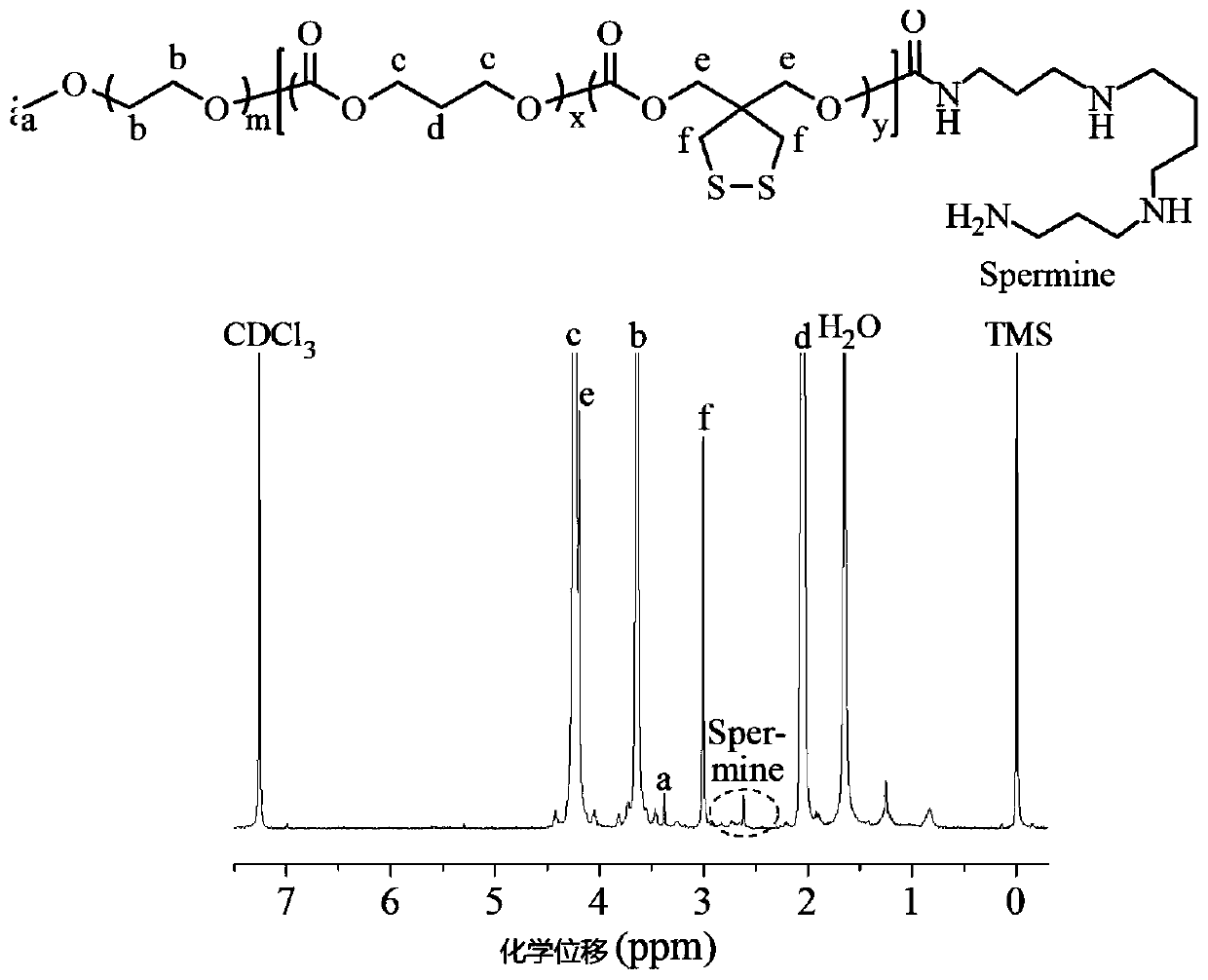
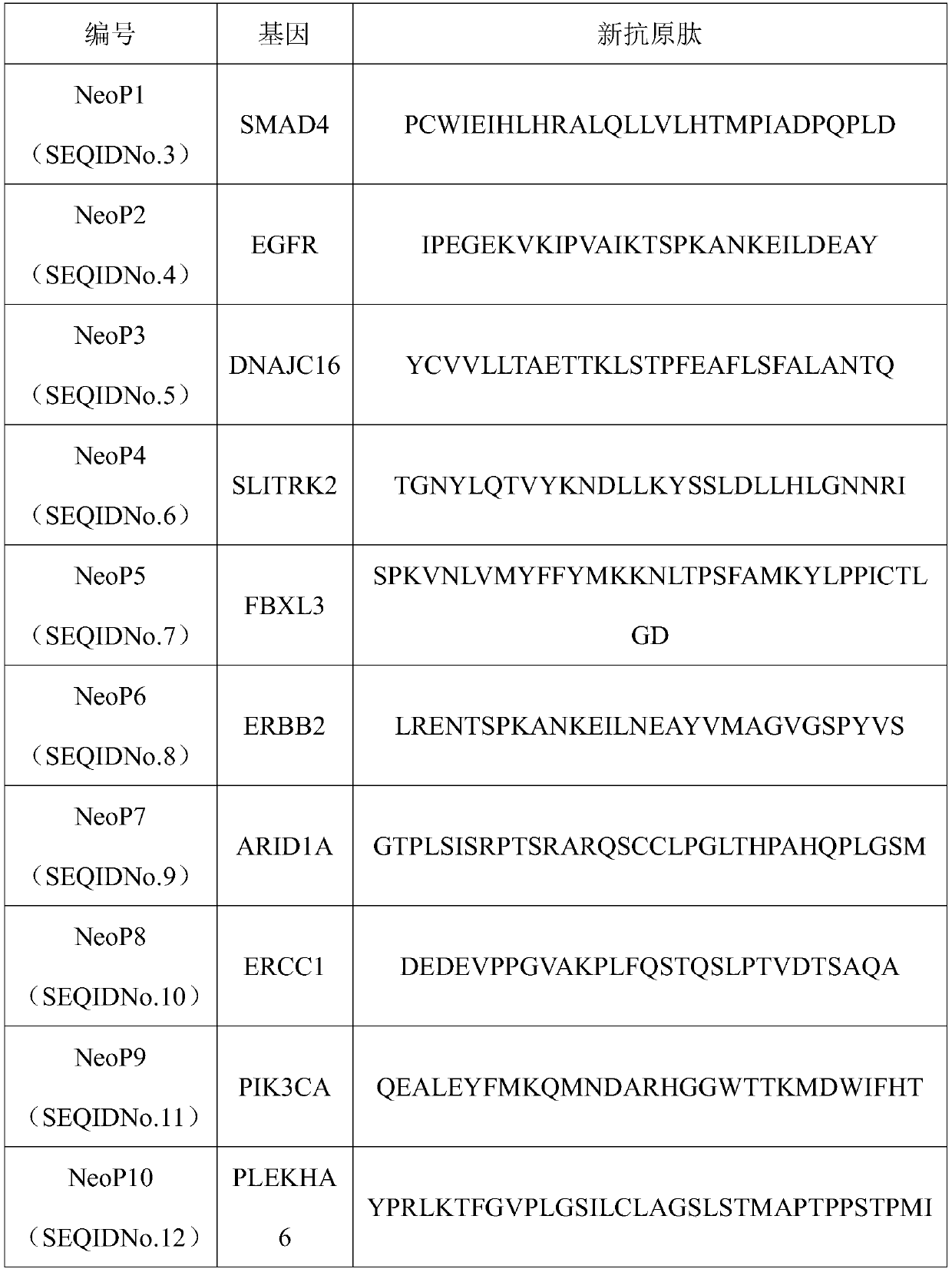
Patents
Literature
193 results about "Immune adjuvant" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A drug that stimulates the immune system
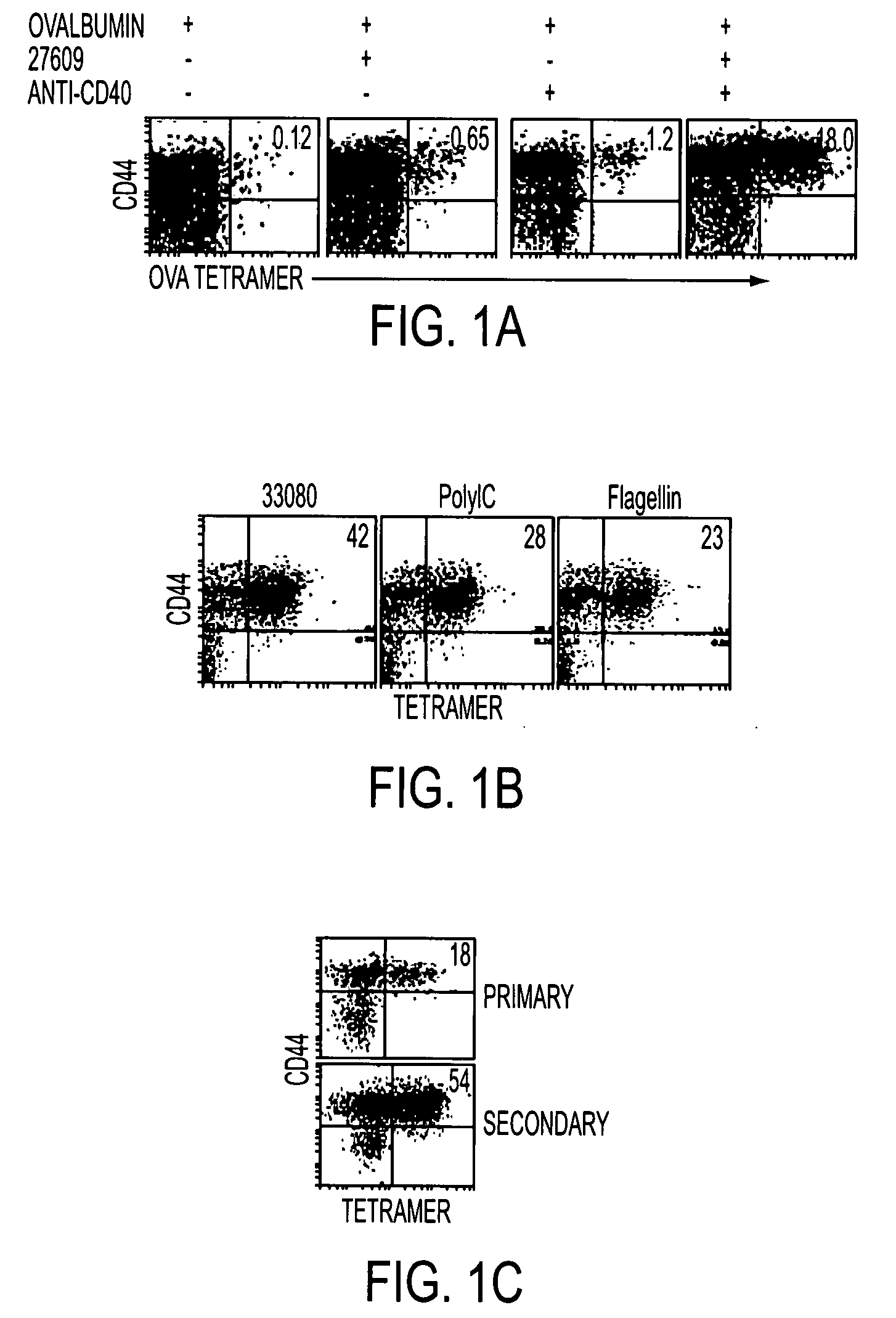
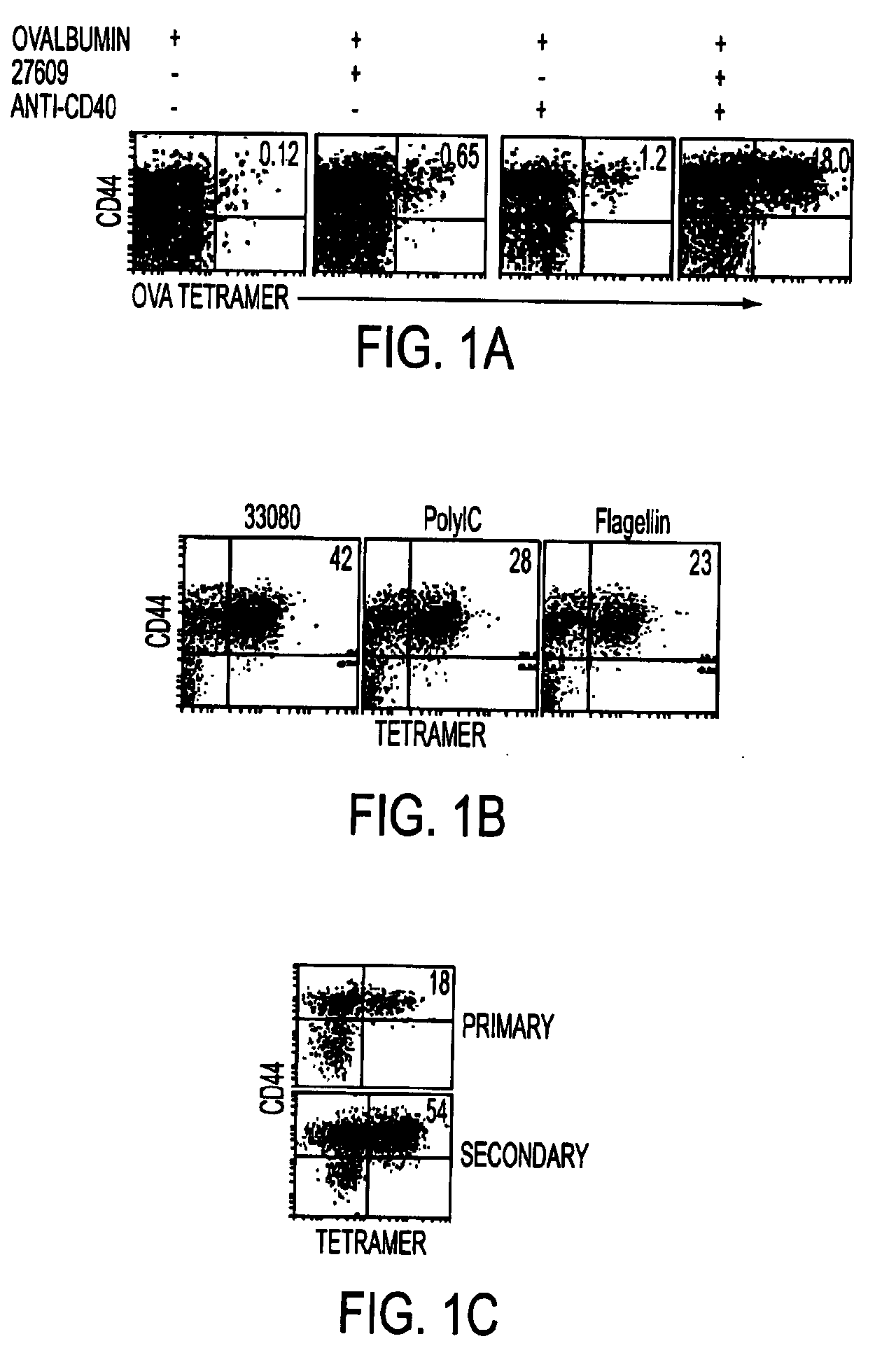
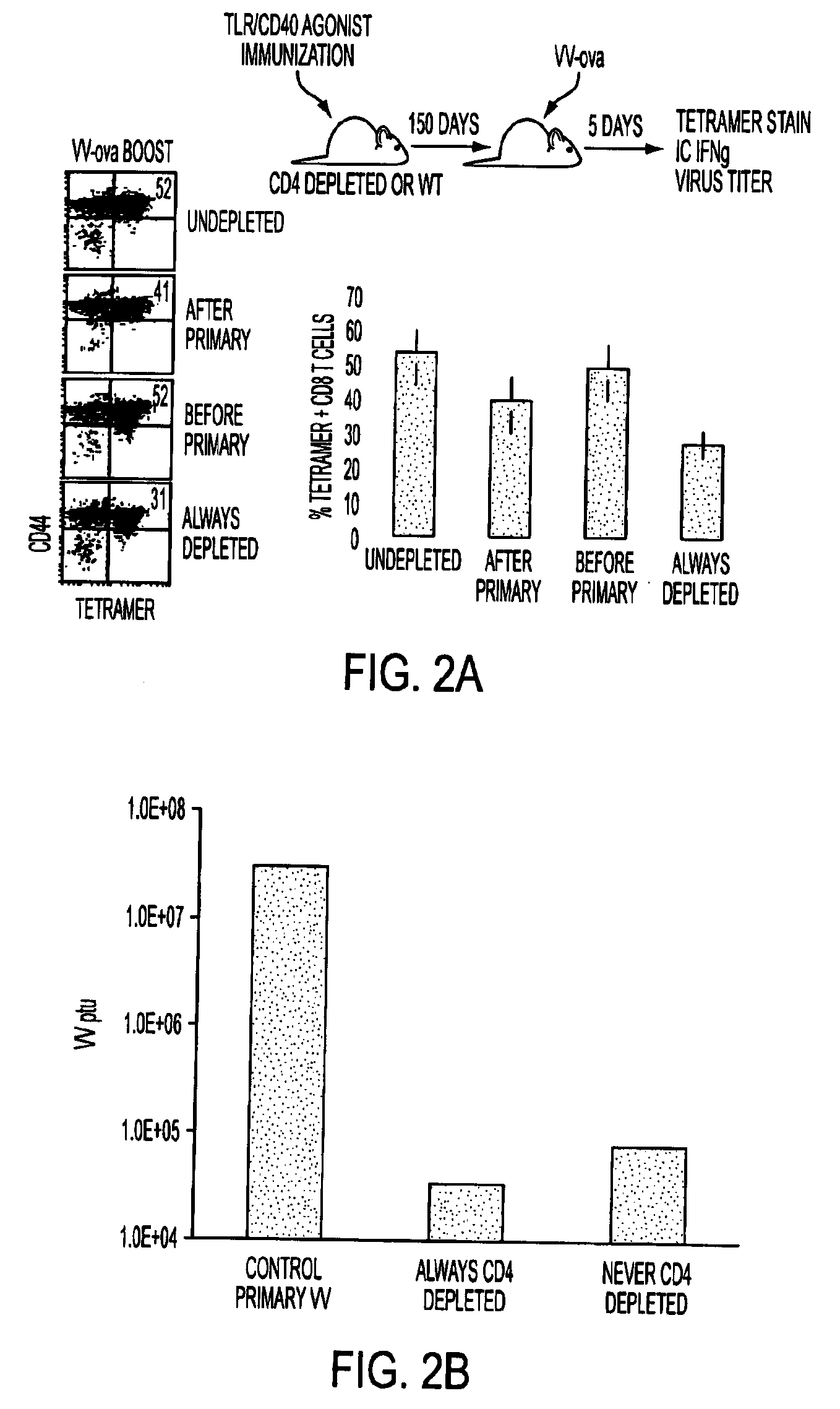
Adjuvant combinations comprising a microbial tlr agonist, a cd40 or 4-1bb agonist, and optionally an antigen and the use thereof for inducing a synergistic enhancement in cellular immunity
InactiveUS20080241139A1Enhanced T cell responseImprove responseAntibacterial agentsAntimycoticsDiseaseYeast
Adjuvant combinations comprising at least one microbial TLR agonist such as a whole virus, bacterium or yeast or portion thereof such a membrane, spheroplast, cytoplast, or ghost, a CD40 or 4-1BB agonist and optionally an antigen wherein all 3 moieties may be separate or comprise the same recombinant microorganism or virus are disclosed. The use of these immune adjuvants for treatment of various chronic diseases such as cancers and HIV infection is also provided.
Owner:UNIV OF COLORADO THE REGENTS OF
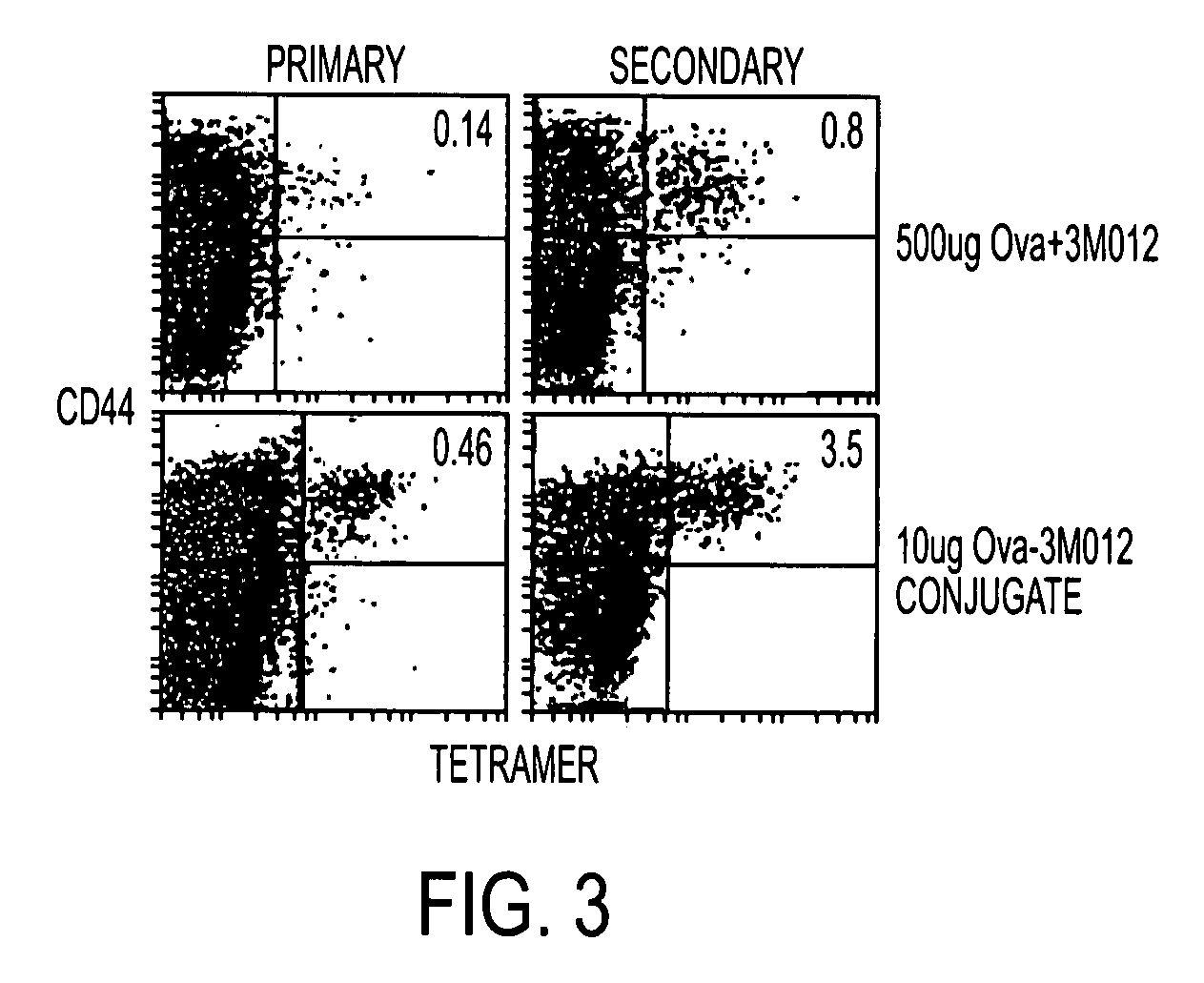
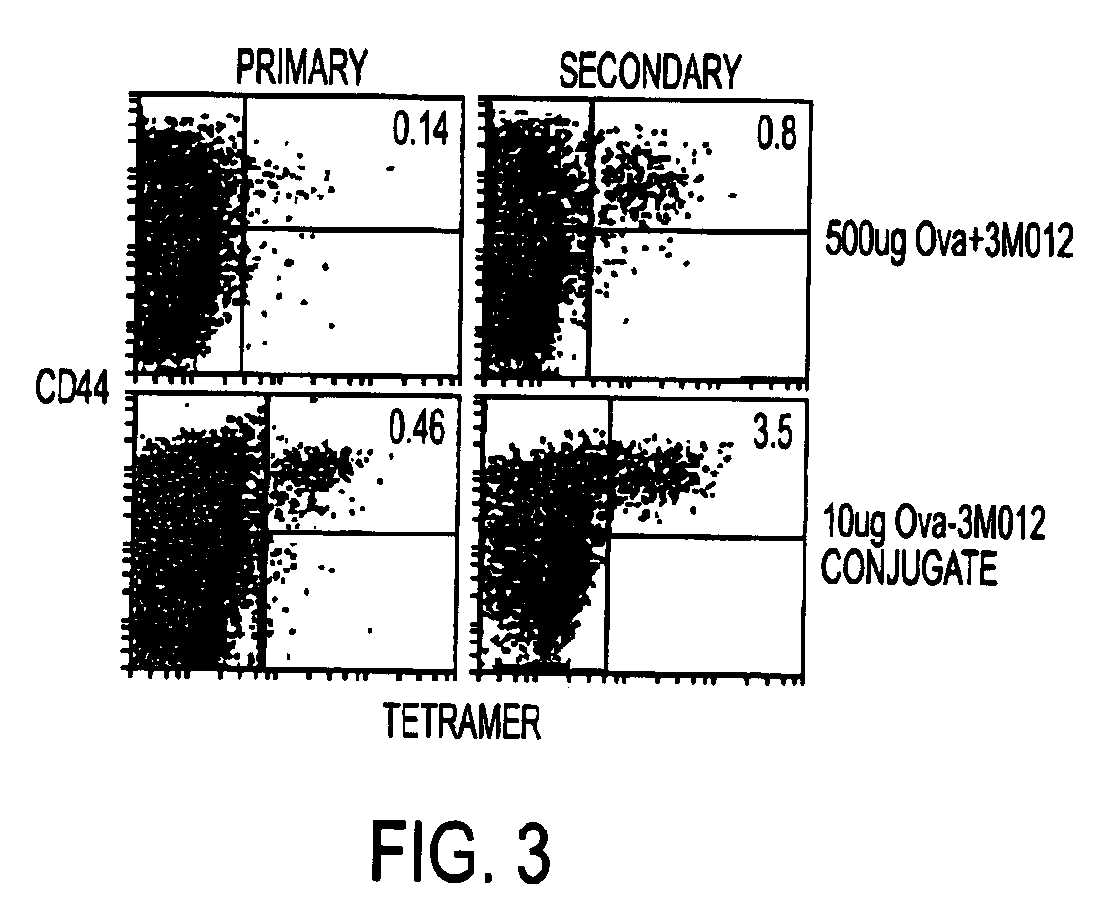
Tlr agonist (flagellin)/cd40 agonist/antigen protein and DNA conjugates and use thereof for inducing synergistic enhancement in immunity
InactiveUS20090004194A1Improve immunityEnhance cellular immune responseAntibacterial agentsFungiDiseaseTlr agonists
Owner:UNIV OF COLORADO THE REGENTS OF
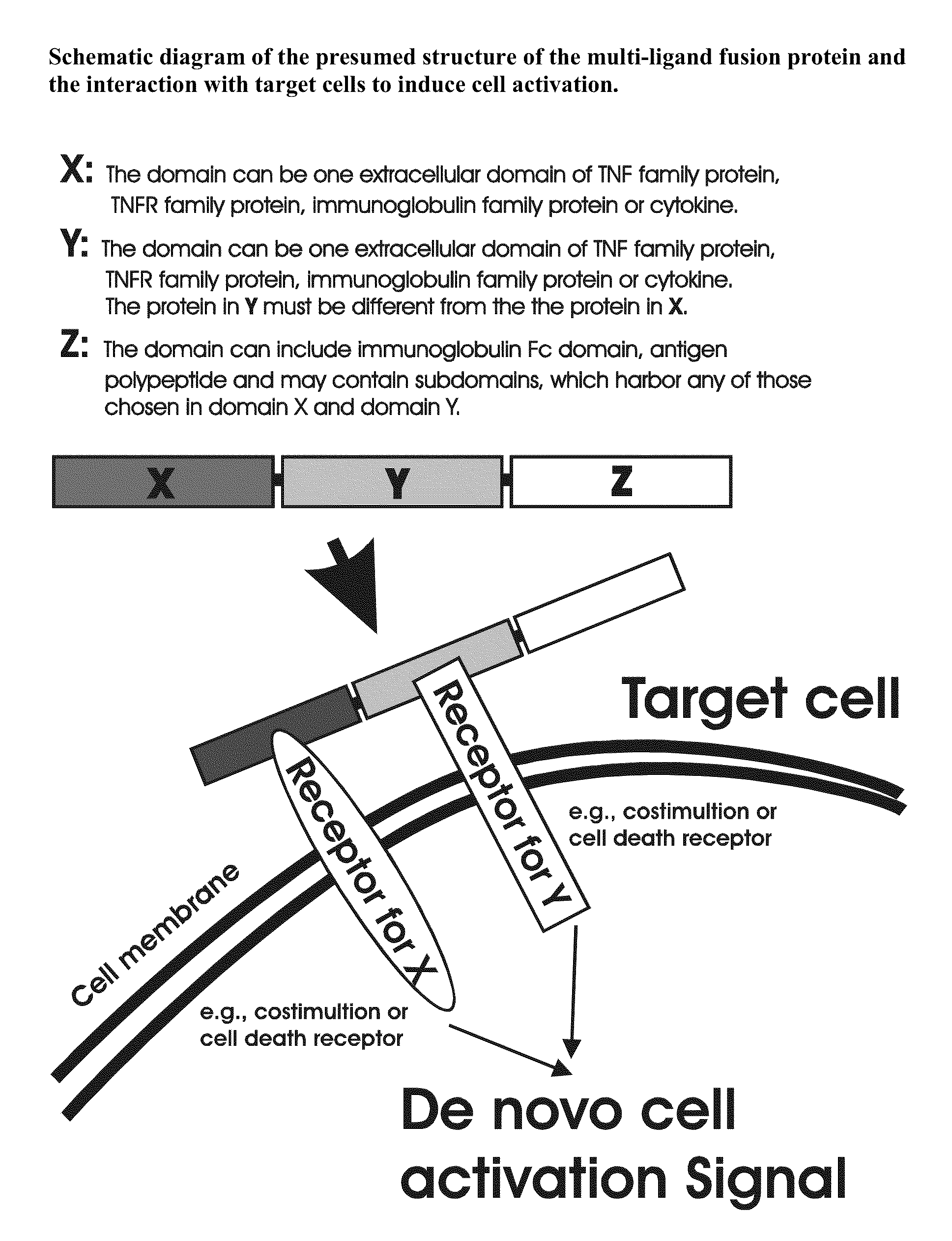
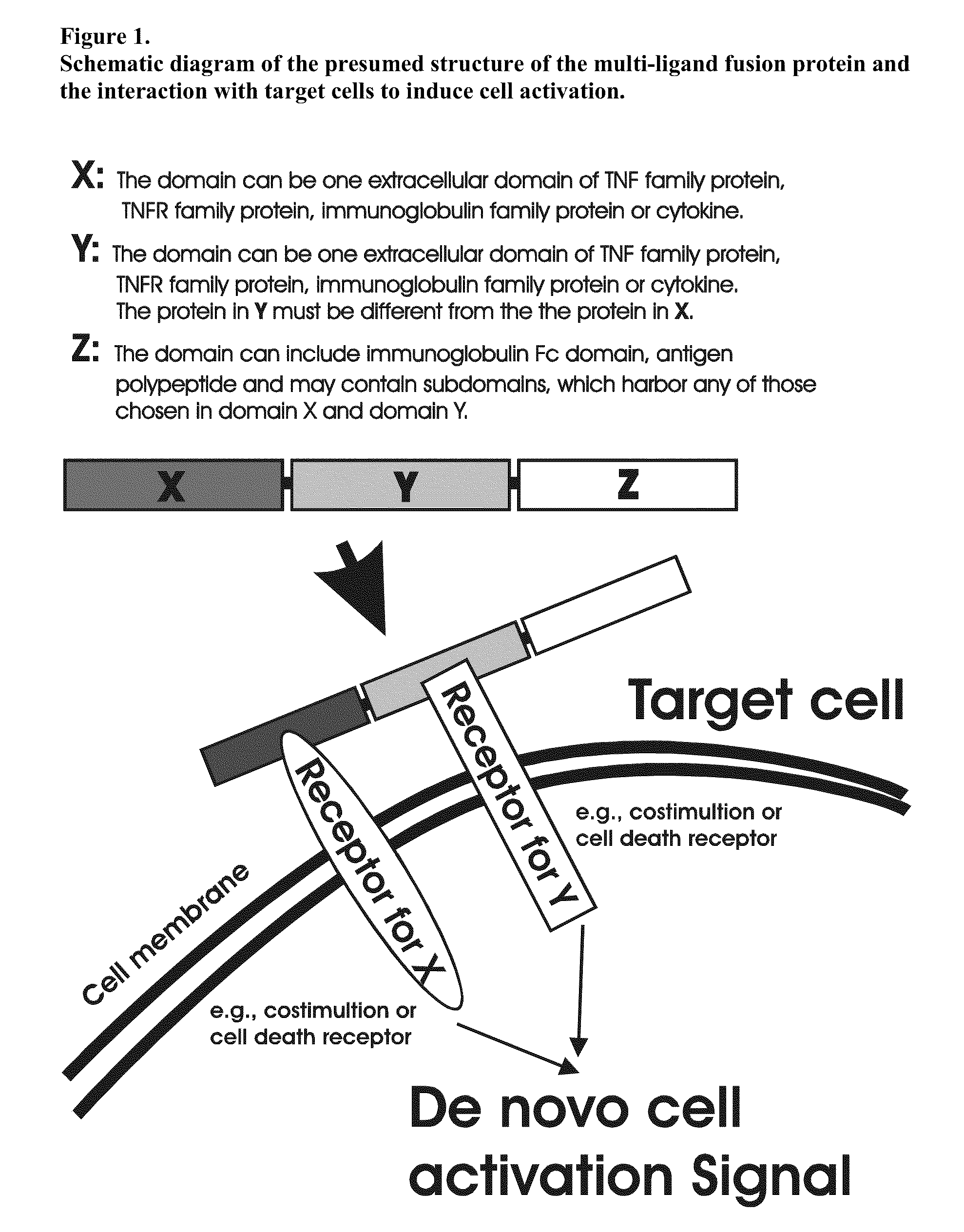
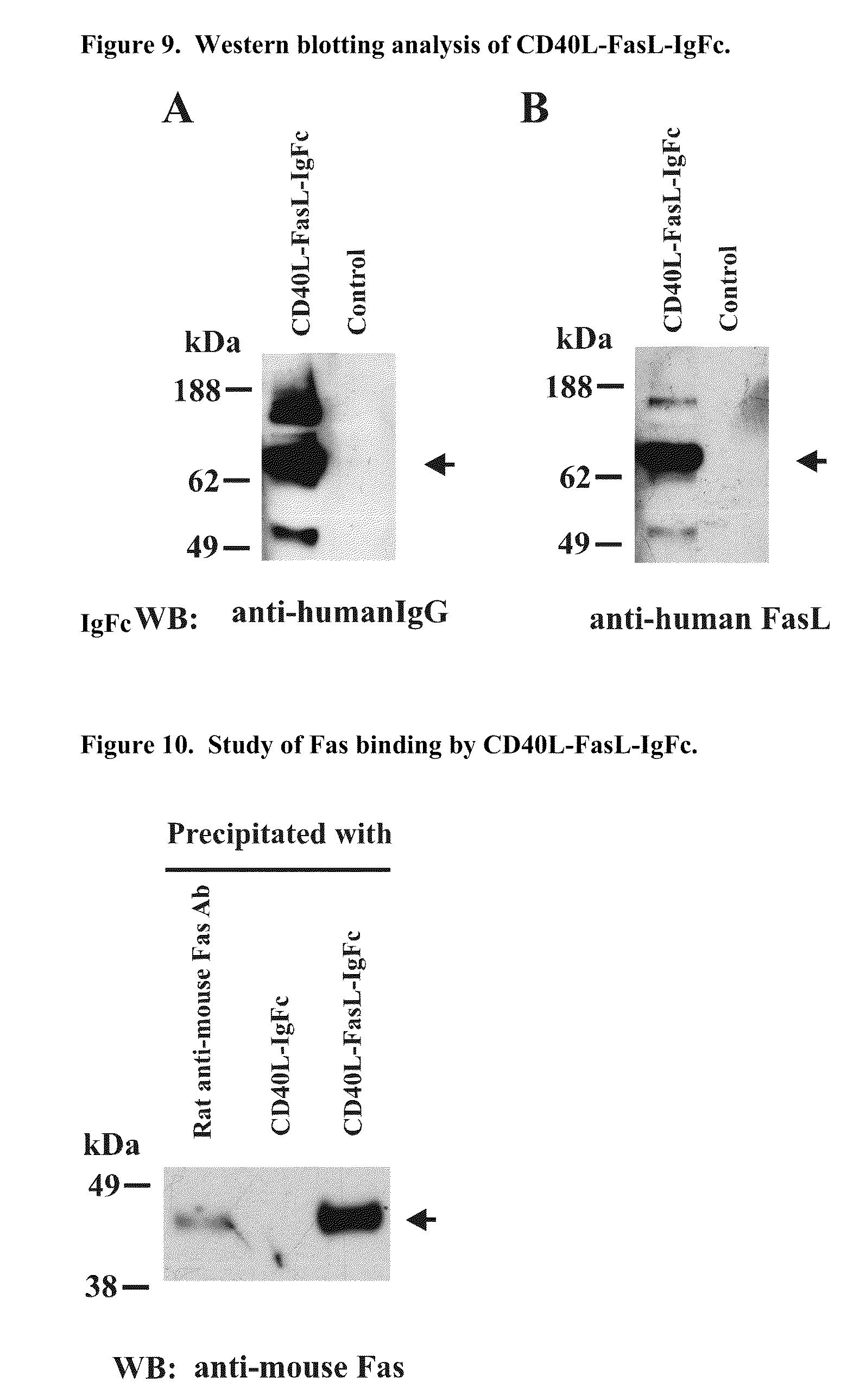
Recombinant multiple domain fusion protein mitogens and use thereof for inducing enhancement or repression of antigen-specific immunity.
ActiveUS20100303811A1Increase heightVirusesPeptide/protein ingredientsIMMUNE SUPPRESSANTSAutoimmune responses
The invention relates to cell stimulatory fusion proteins and DNA sequences, vectors comprising at least two agonists of TNF / TNFR super family, immunoglobulin super family, cytokine family proteins and optional antigen combination. Instructions for use of these proteins and DNA constructs as immune adjuvants and vaccines for treatment of various chronic diseases such as viral infection are also provided. Additionally, the use of these protein and DNA constructs as immune suppressant for treatment of various chronic diseases, such as autoimmunity and organ transplant rejection, is also illustrated.
Owner:OCHI ATSUO
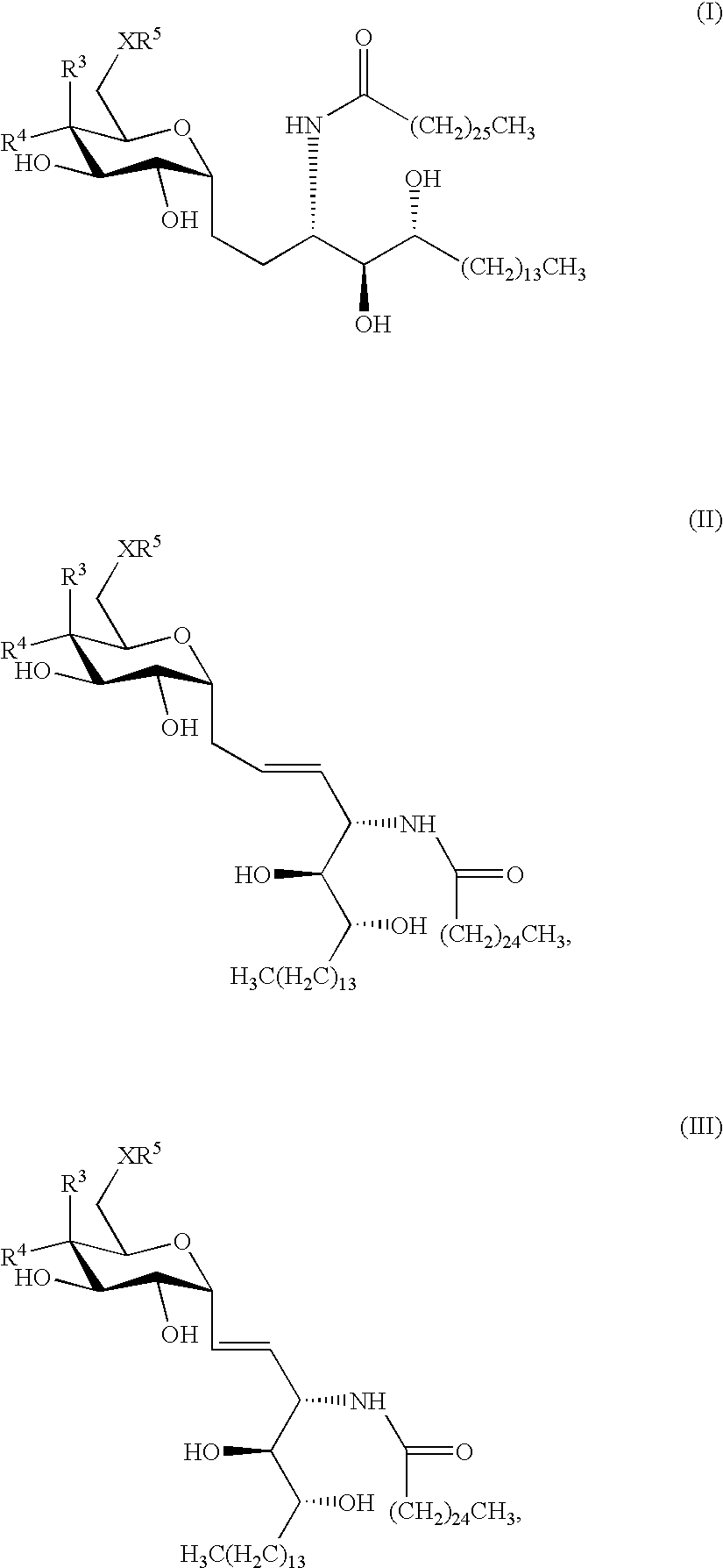
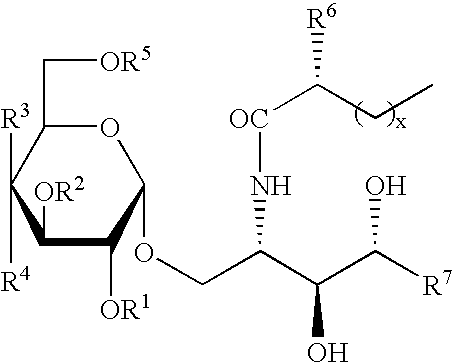
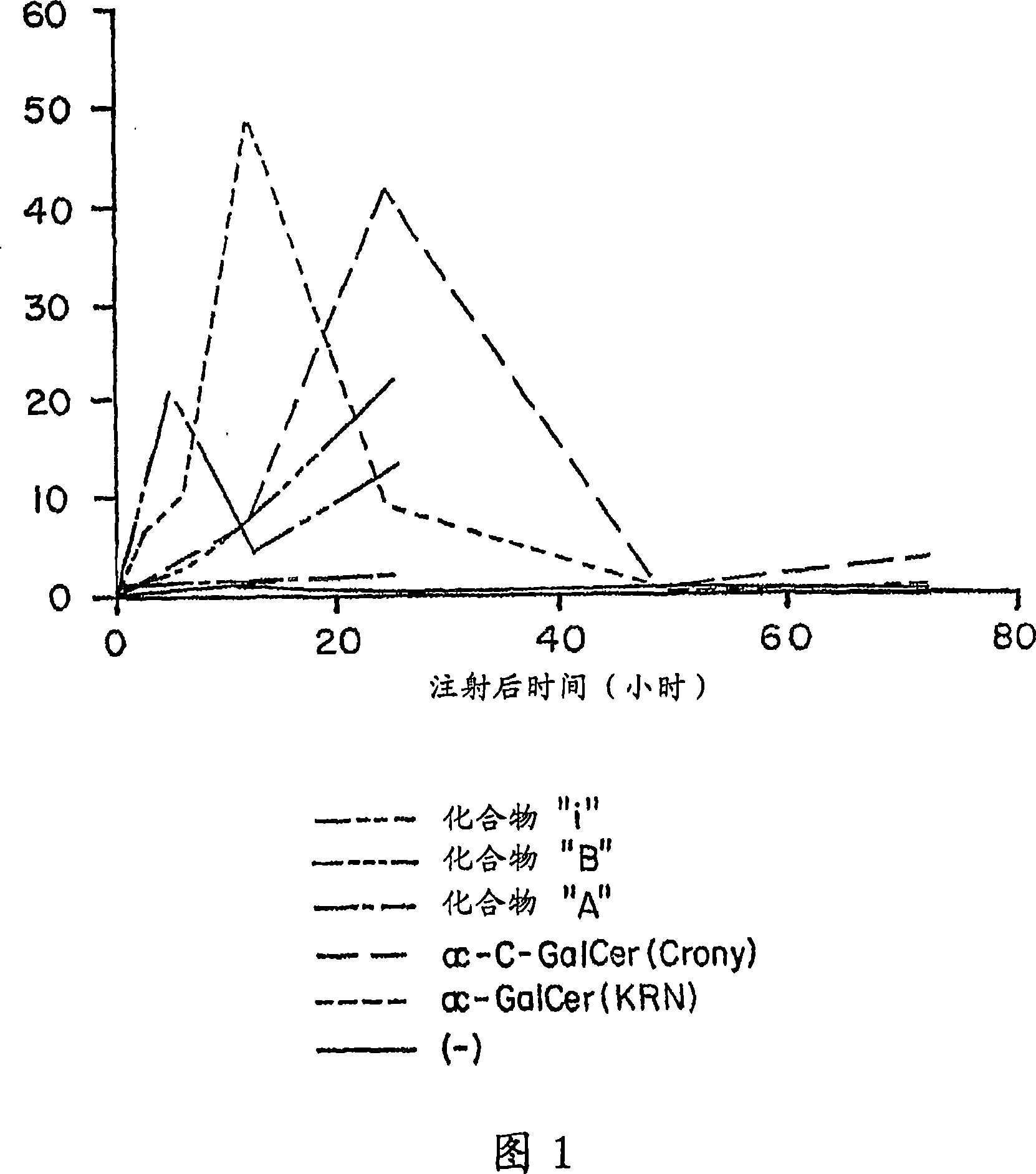
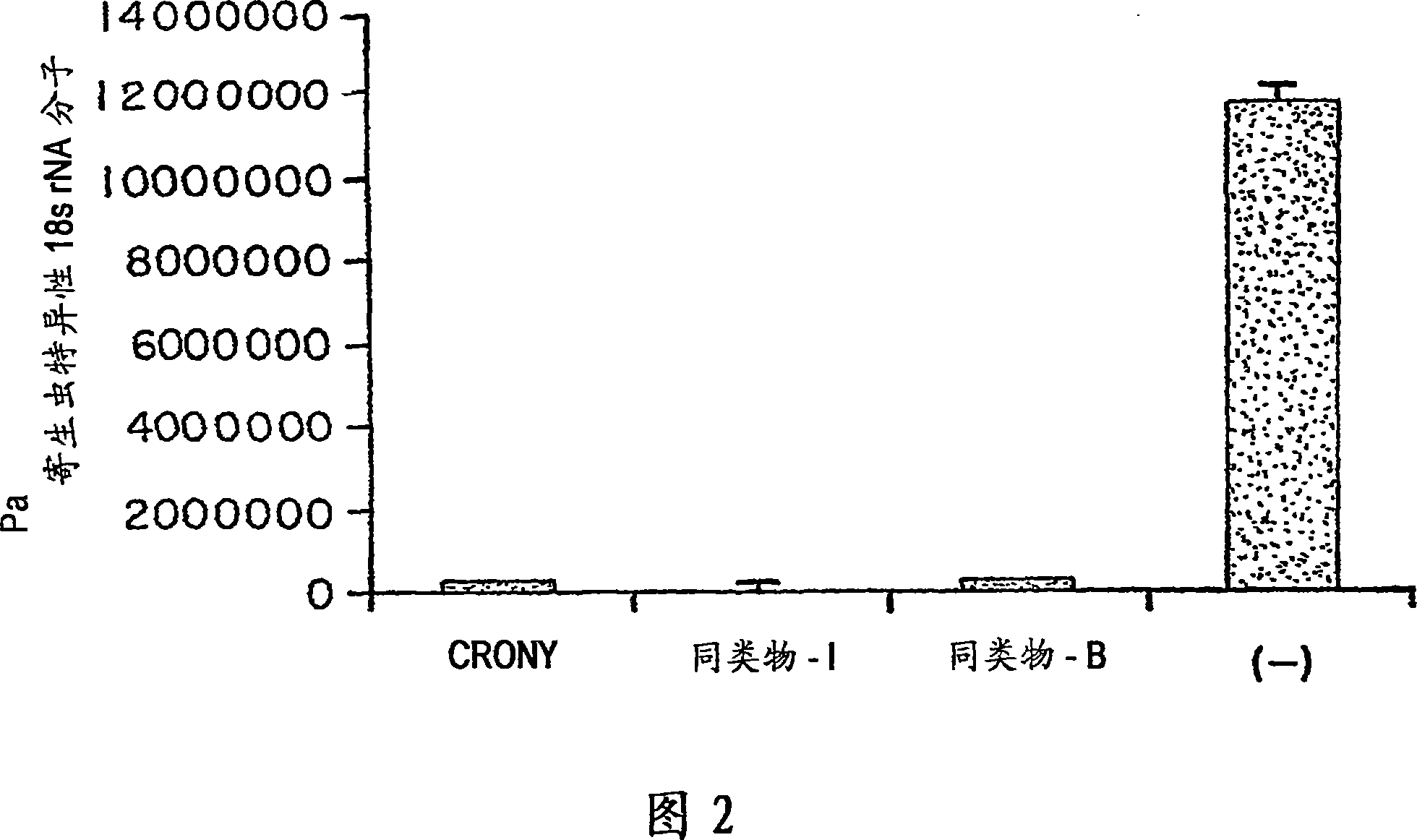
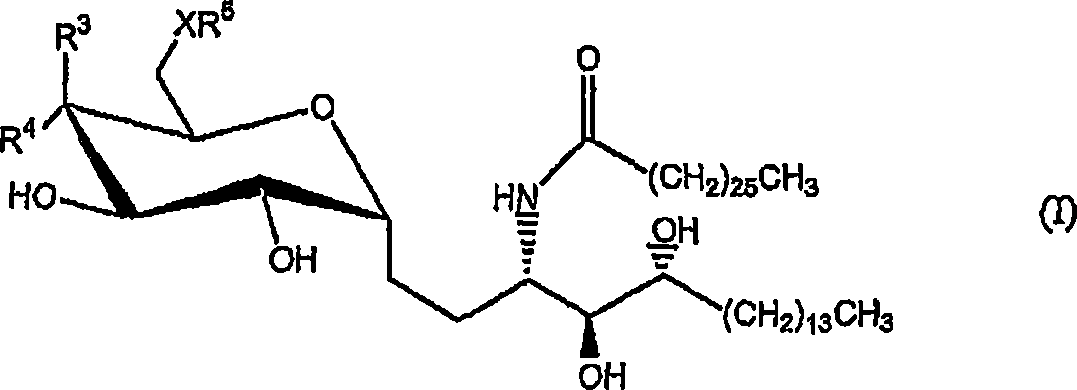
Novel synthetic C-glycolipids, their synthesis and use to treat infections, cancer and autoimmune diseases
InactiveUS20050222048A1Esterified saccharide compoundsAntibacterial agentsAutoimmune diseaseGlycolipid
The invention is directed to novel compounds of formulae (I), (II) and (III): wherein X is O or NH; R3 is OH or a monosaccharide and R4 is hydrogen, or R3 is hydrogen and R4 is OH or a monosaccharide; R5 is hydrogen or a monosaccharide; and pharmaceutically acceptable salts or esters thereof. The invention is also directed to the use of the compounds both directly and as immune adjuvants for treating cancer, infectious diseases and autoimmune diseases. The invention is also directed to syntheses of the intermediates which can be used to make these novel compounds.
Owner:RES FOUND THE CITY UNIV OF NEW YORK +1
Tlr agonist (flagellin)/cd40 agonist/antigen protein and DNA conjugates and use thereof for inducing synergistic enhancement in immunity
Owner:KEDL ROSS
Adjuvant combinations comprising a microbial tlr agonist, a cd40 or 4-1bb agonist, and optionally an antigen and the use thereof for inducing a synergistic enhancement in cellular immunity
Adjuvant combinations comprising at least one microbial TLR agonist such as a whole virus, bacterium or yeast or portion thereof such a membrane, spheroplast, cytoplast, or ghost, a CD40 or 4-1BB agonist and optionally an antigen wherein all 3 moieties may be separate or comprise the same recombinant microorganism or virus are disclosed. The use of these immune adjuvants for treatment of various chronic diseases such as cancers and HIV infection is also provided.
Owner:DELUCIA DAVE
Application of short-peptide serving as vaccine adjuvant and vaccine
The invention provides application of short-peptide serving as vaccine adjuvant and a vaccine taking the short-peptide as the vaccine adjuvant. The short-peptide is easy to prepare, and after the short-peptide is easily, conveniently and physically mixed with an antigen, the immune response capacity of the antigen can be effectively enhanced. In the application of the short-peptide in serving as the vaccine adjuvant, the short-peptide is subjected to end-capping through a group X, the short-peptide sequence is X-GFF, or the short-peptide sequence contains a sequence FFY. Preferably, the short-peptide sequence containing the sequence FFY is X-FFY or X-GFFY or X-GFFYK or X-GFFYE or X-GFFYG; preferably, the short-peptide has a D configuration. The short-peptide can serve as immune adjuvant to enhance immunogenicity of the antigen, a high cellular immunity and humoral immunity response of antigenic specificity of a main body occurs, and the short-peptide can be applicable to various antigens; the short-peptide is easy to prepare, and the component is single and controllable.
Owner:NANKAI UNIV
Saponin with immunoadjuvant function, preparation method, vaccine preparation containing the saponin as adjuvant and uses thereof
InactiveCN101402666ASimple preparation processSimple methodAntiinfectivesSteroidsDiseaseCell immune response
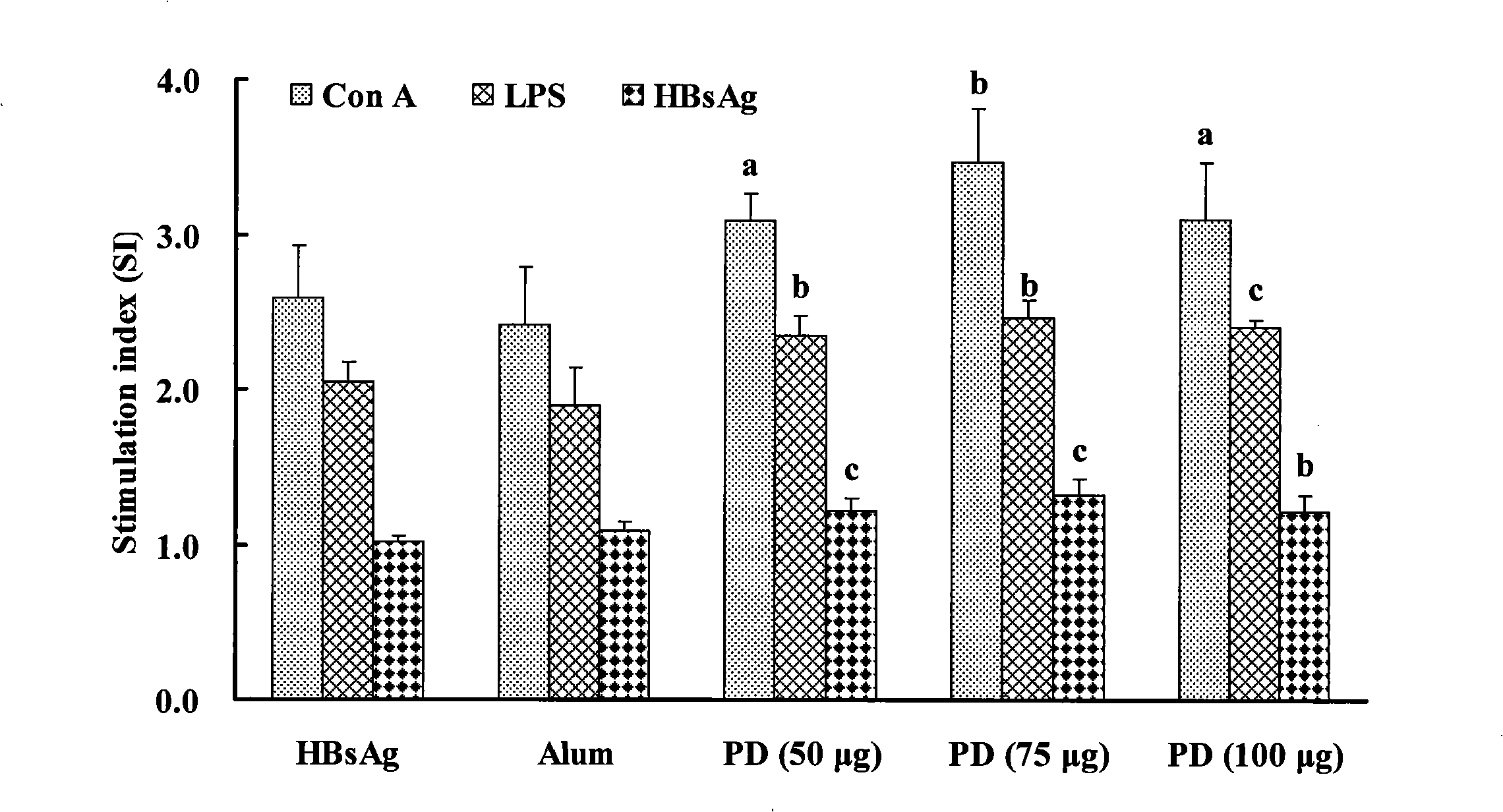
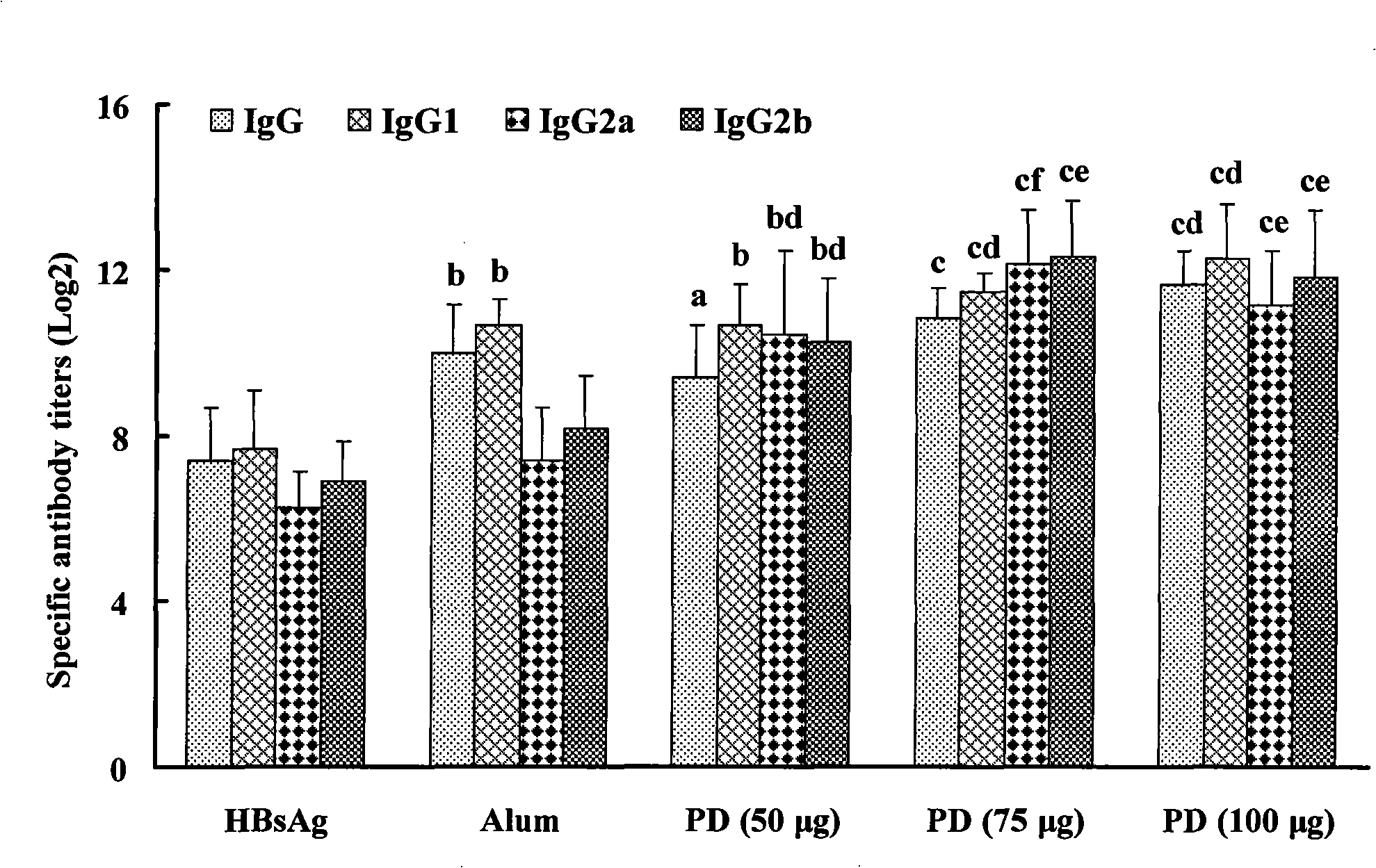
The invention relates to a saponin with immune adjuvant function and a preparation method thereof, a vaccine preparation containing the saponin as an adjuvant, and applications of the saponin and the vaccine preparation in the prevention and treatment of infectious diseases and cancers of human and animals. The saponin is platycodin D, platycodin D2, or a total-saponin containing the two saponin compounds. The platycodin D and platycodin D2 are both extracted and separated from balloonflower, a Chinese medicine. The saponin can induce an organism to generate Th1-type and Th2-type immune responses, show the capability of inducing the organism to generate stronger cell immune response and humoral immune response to a vaccine than the alhydrogel adjuvant known in the prior art, and can be taken as the immune adjuvant for a plurality of vaccines and achieve an ideal immunity effect. The vaccine which takes the saponin as the adjuvant has simple preparation technology and simple and convenient method, and the quality is easy to control and the saponin can be reserved by freezing.
Owner:ZHEJIANG UNIV
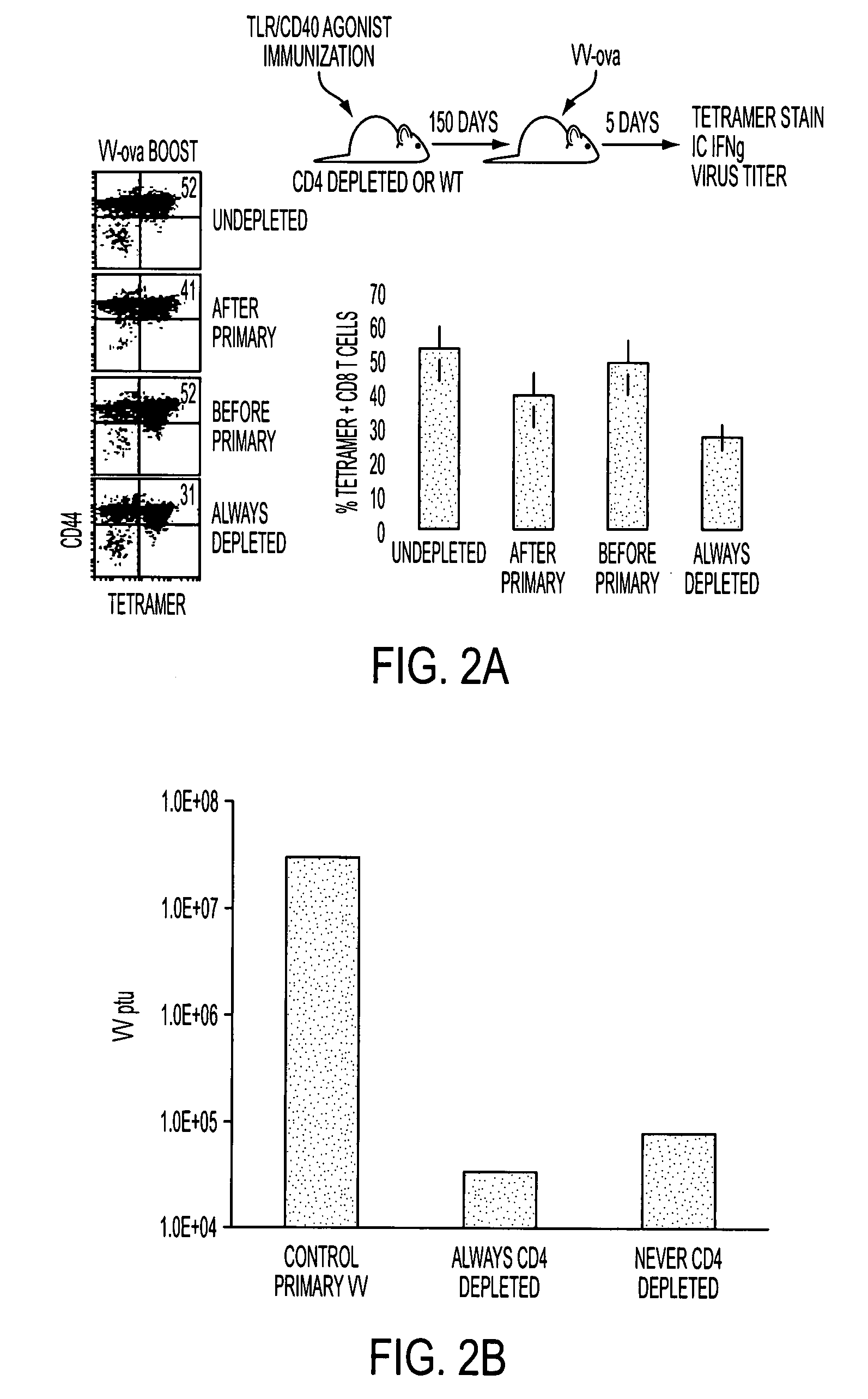
Methods and compositions for treating cancer and infectious diseases
ActiveUS20170007698A1Prevention and reduction of severityShorten the progressOrganic active ingredientsAntibody ingredientsInfectious DisorderImmune modulator
The invention relates to compositions comprising a CD4 lymphocyte depleting agent; and methods of using the compositions to treat, prevent, reduce the severity of and / or slow the progression of a condition in a subject. The invention also relates to use of combinations of a CD4 lymphocyte depleting agent and at least one additional agent to treat, prevent, reduce the severity of and / or slow the progression of a condition in a subject. The additional agent may be an immune check point inhibitor, an adoptive immune therapeutic, an immune adjuvant, or an immune modulating agent, or their combinations.
Owner:CEDARS SINAI MEDICAL CENT
Polyelectrolyte hybrid hollow silica nano particles and preparation method and application thereof
ActiveCN108434122AHigh drug loadingEfficient loadingAntiviralsPharmaceutical non-active ingredientsPolyelectrolyteSilica nanoparticles
The invention provides polyelectrolyte hybrid hollow silica nano particles and a preparation method and application thereof. The insides of polyelectrolyte hybrid hollow silica nanoparticles are of hollow structures, and outer shell layers are hybrid structures of polyelectrolyte and silica; mesopores are formed in the shell layers. The preparation method comprises the steps of firstly synthesizing the silica nano particles with core-shell structures; then removing surfactant in the core-shell structures of the silica nano particles; finally etching the obtained mesoporous silica nano particles with the polyelectrolyte, thereby obtaining the polyelectrolyte hybrid hollow mesoporous silica nano particles. The preparation method is simple, and the obtained polyelectrolyte hybrid hollow mesoporous silica nano particles have good monodispersity, good biocompatibility and large specific surface area and pore volume, and can be applied to drug carriers and immunity adjuvant.
Owner:HUAZHONG UNIV OF SCI & TECH +1
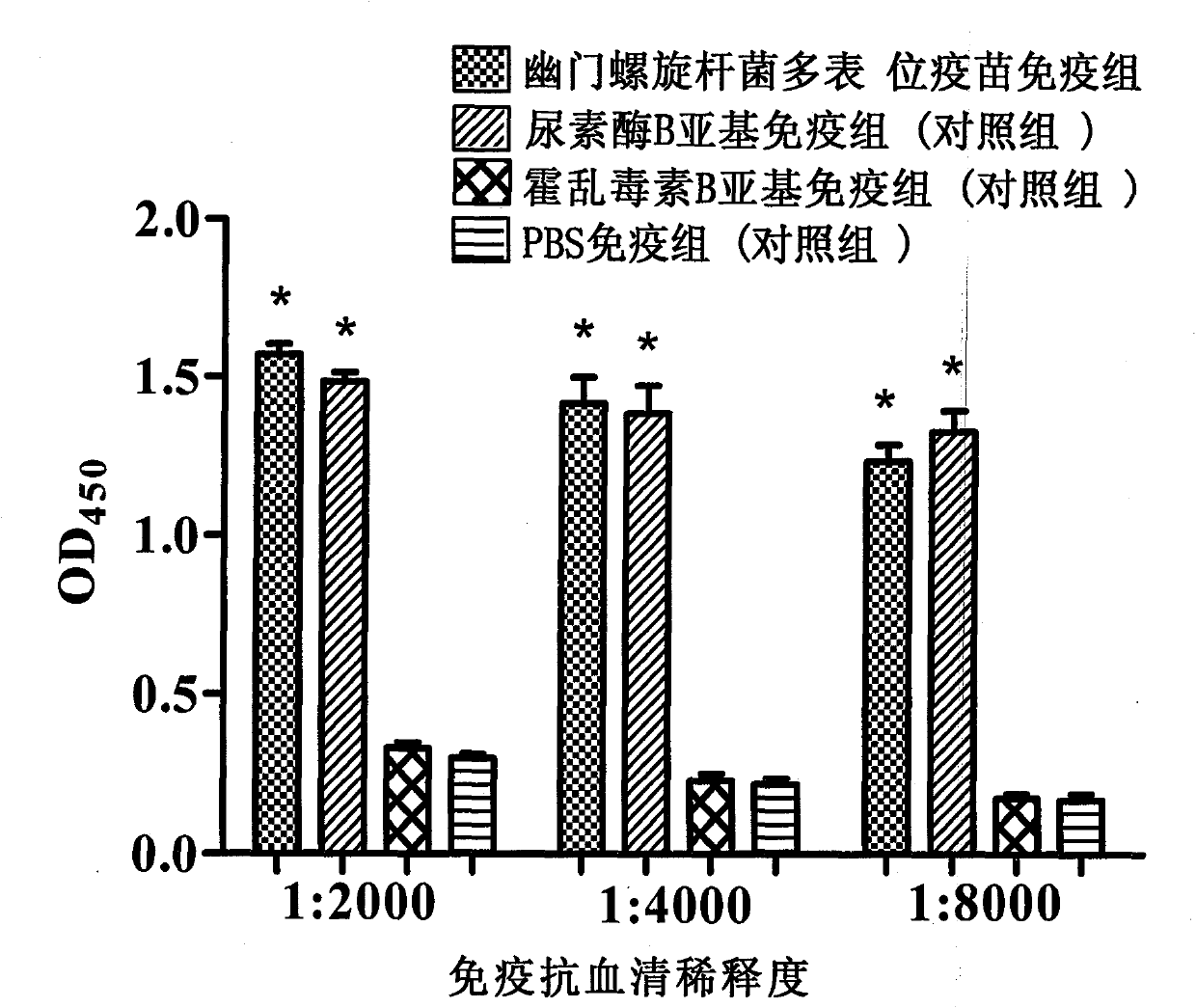
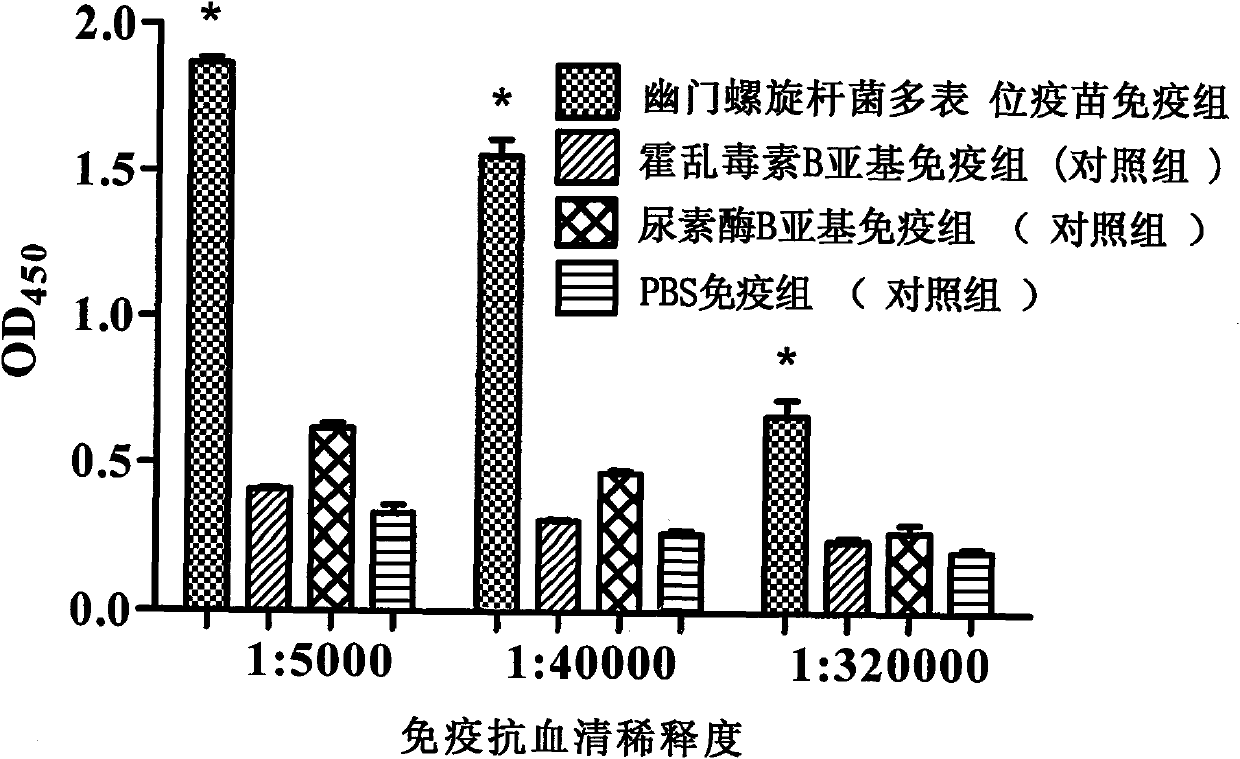
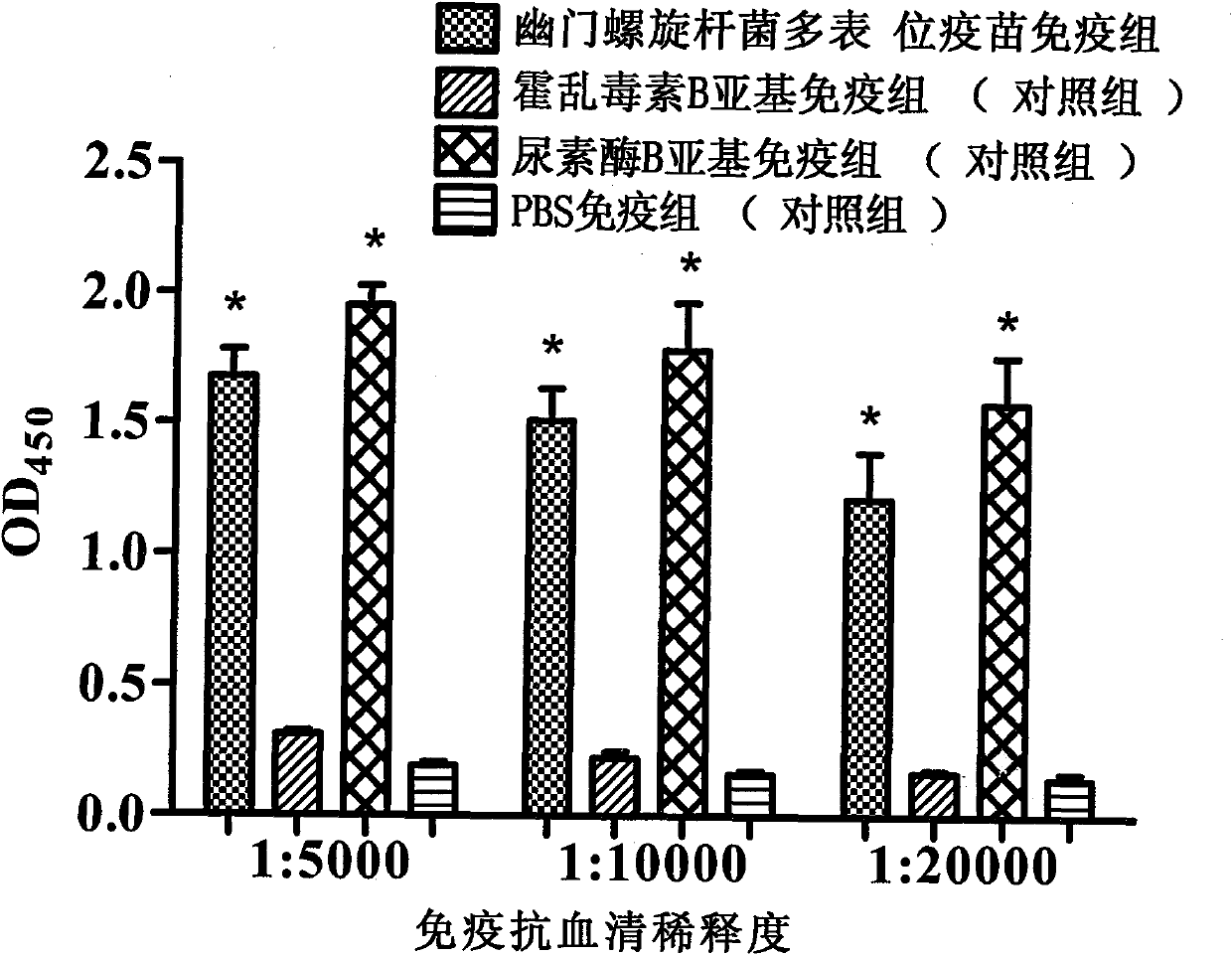
Novel helicobacter pylori multiepitope vaccine and preparation method thereof
InactiveCN102178941ANovel structureImproving immunogenicityAntibacterial agentsBacterial antigen ingredientsDiseaseEscherichia coli
The invention provides a helicobacter pylori multiepitope vaccine. The active ingredient of the helicobacter pylori multiepitope vaccine is a piece of polypeptide; and the helicobacter pylori multiepitope vaccine mainly comprises a multi-copy body of Th and B cell antigen epitopes of helicobacter pylori urease A and B bi-subunit and a mucosal immune adjuvant of a cholera toxin B subunit. The preparation method mainly comprises the following steps of: synthesizing an artificial gene by using a gene synthesis technology, wherein the artificial gene comprises a gene sequence of the multi-copy body of the Th and B cell antigen epitopes of the helicobacter pylori urease A and B bi-subunit; coupling the artificial gene with the gene sequence of the cholera toxin B subunit to form a fusion gene;and expressing the fusion gene by using an escherichia coli prokaryotic expression system, and performing protein purification to obtain the helicobacter pylori multiepitope vaccine. The helicobacterpylori multiepitope vaccine can induce an organism to generate T cell immune response and high-titred specific antibody humoral immune response aiming at the urease A and B bi-subunit, and can be used for preventing and treating helicobacter pylori infection related diseases.
Owner:CHINA PHARM UNIV
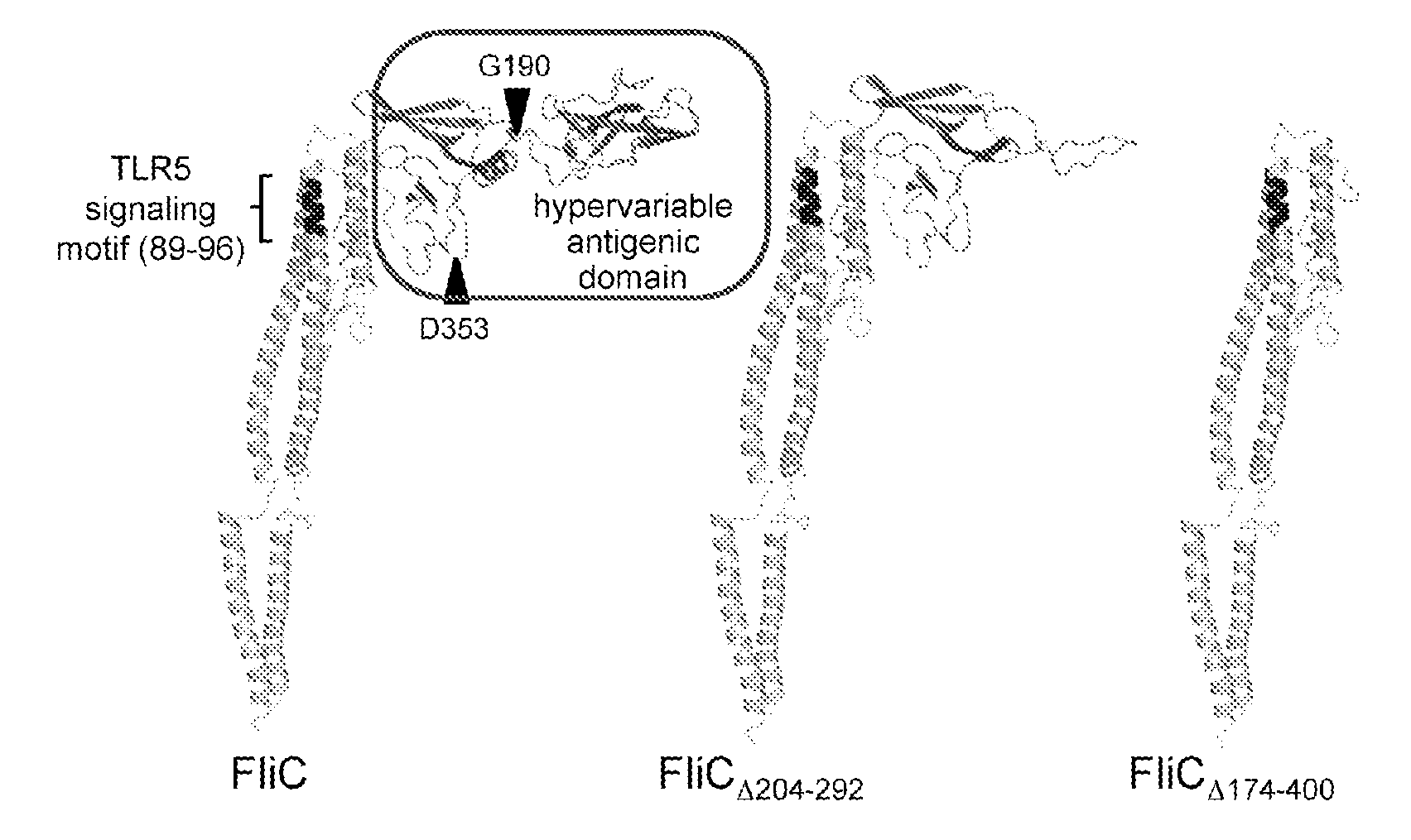
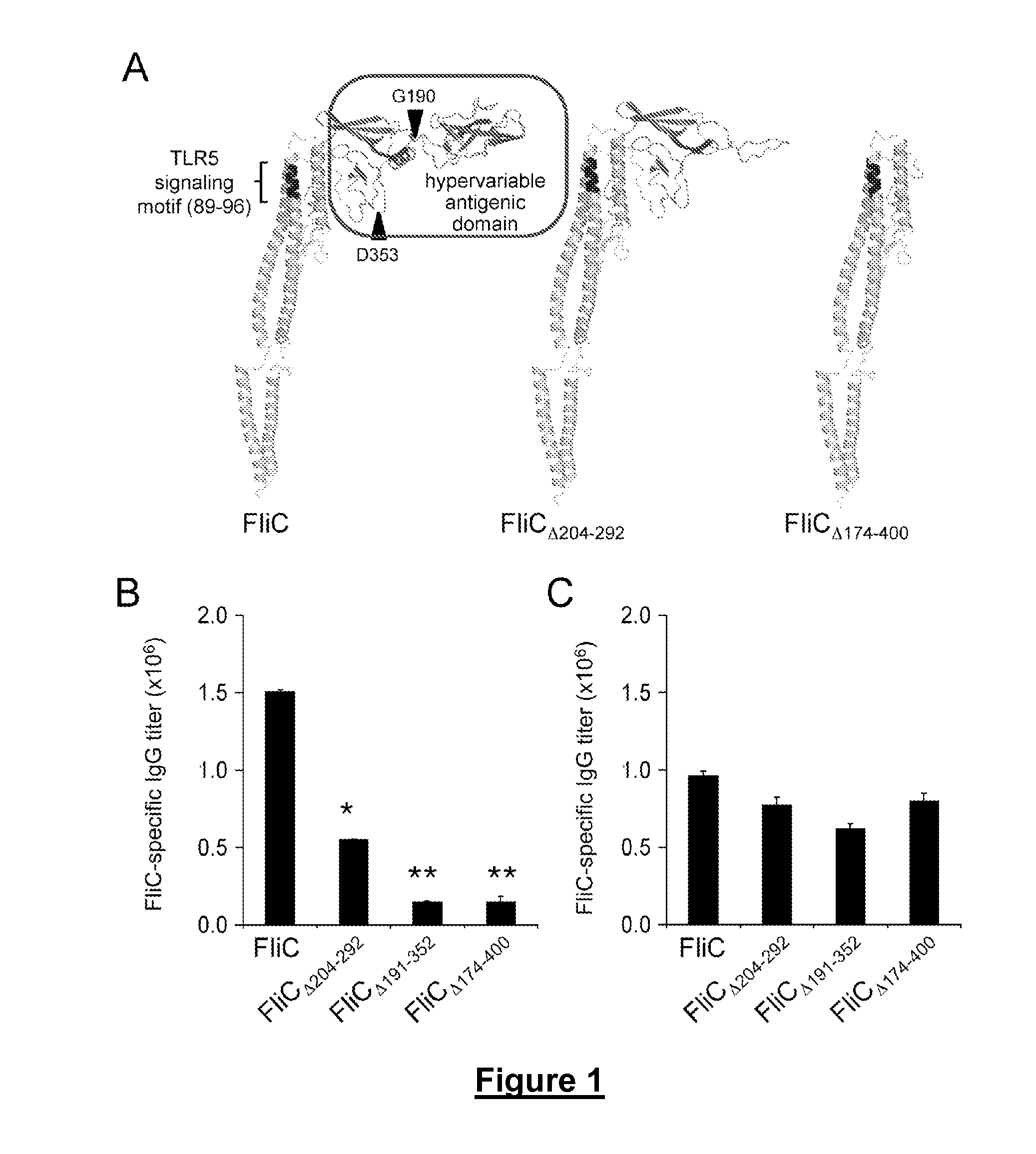
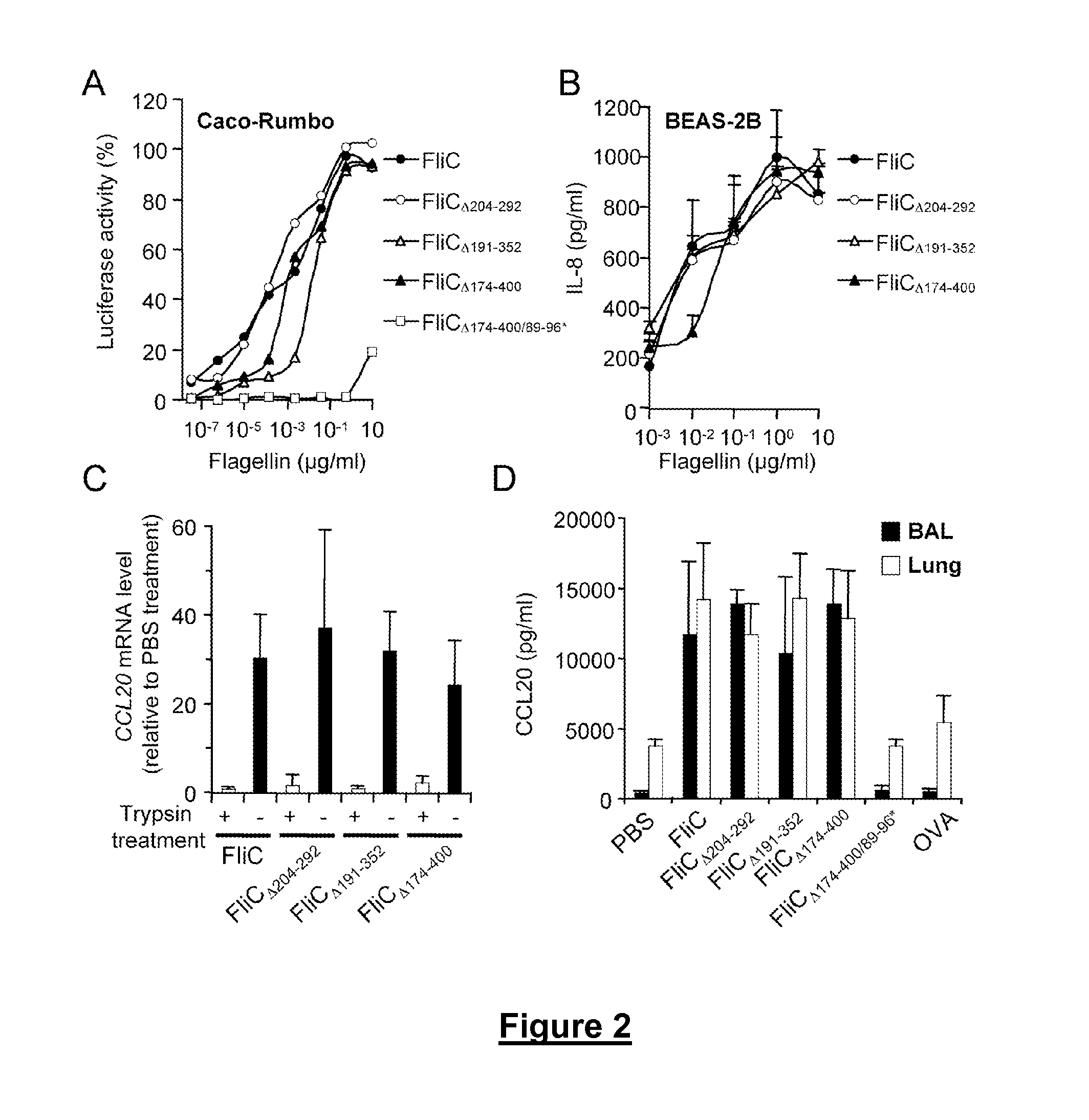
Novel Immunoadjuvant Flagellin-Based Compounds and Use Thereof
The present invention relates to novel peptide compounds derived from flagellin originating from Salmonella enterica that exhibit an in vivo immune adjuvant activity.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +1
Immunoglobulin for anti virus of poultry influenza, preparation method and preparation
InactiveCN1796416AStrong specificityImprove infection abilityPowder deliverySerum immunoglobulinsAnti virusSerum ige
This invention publishes an anti-avian influenza virus immunoglobulin and its preparation method. Such an immunoglobulin refers to anti-avian influenza virus immunoglobulin G and / or its effective fragment ([fab]2) which is extracted from the mixture of inactivated avian influenza virus and immune adjuvants and / or the serum and / or plasma of avian influenza vaccine immunized animals. It is strongly specific and applicable in diagnosis and therapy of avian influenza in animals. It has significant effects in treating avian influenza infected patients and promoting immunity of both high-risk and common population but shows no side effects to human. Besides, this invention also publishes the medicament forms of the anti-avian influenza virus immunoglobulin: injection, spray or lyophilized powder for injection, in which there is anti-avian influenza virus immunoglobulin mentioned in this invention and their pharmacologically acceptable adjuvants.
Owner:SUN YAT SEN UNIV +1
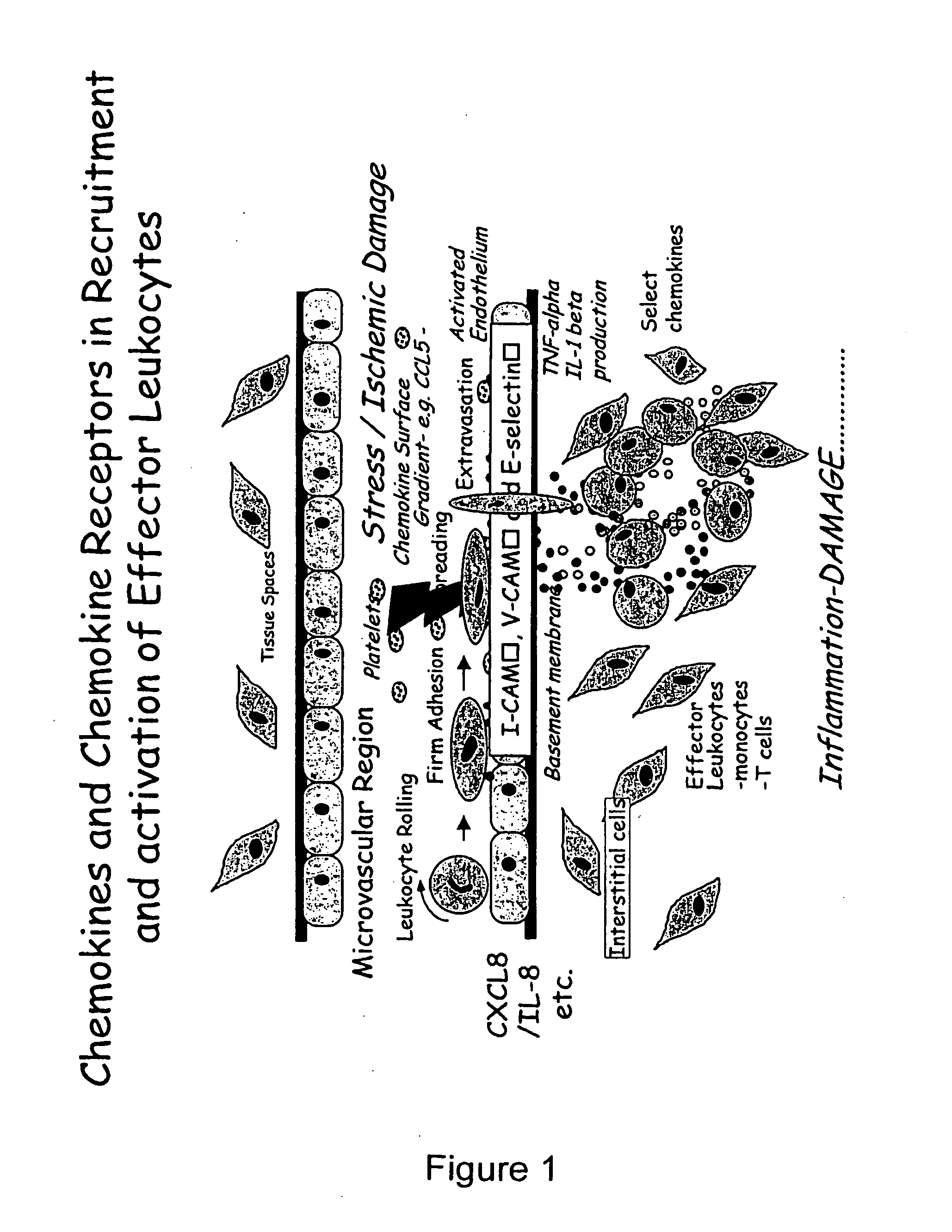
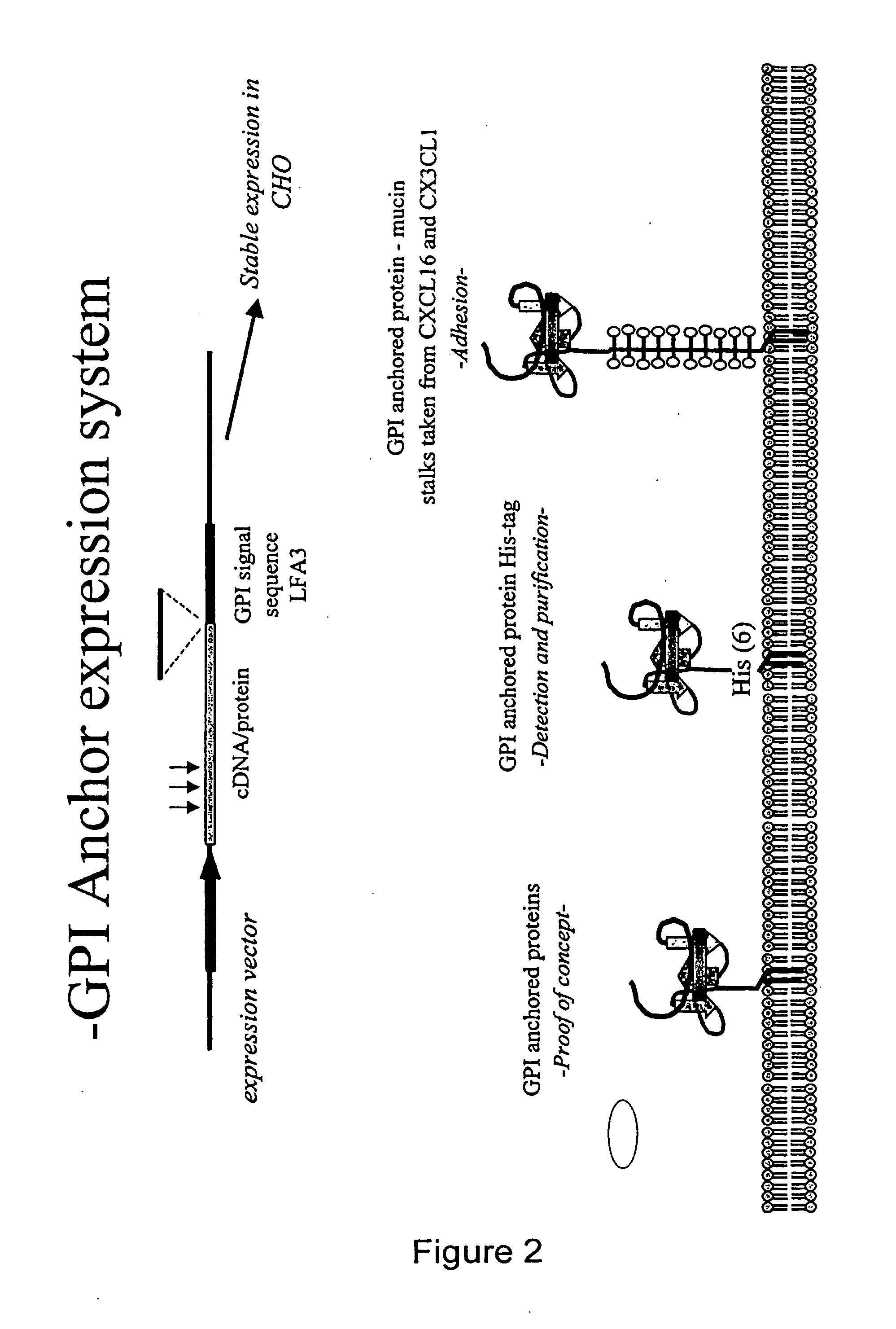
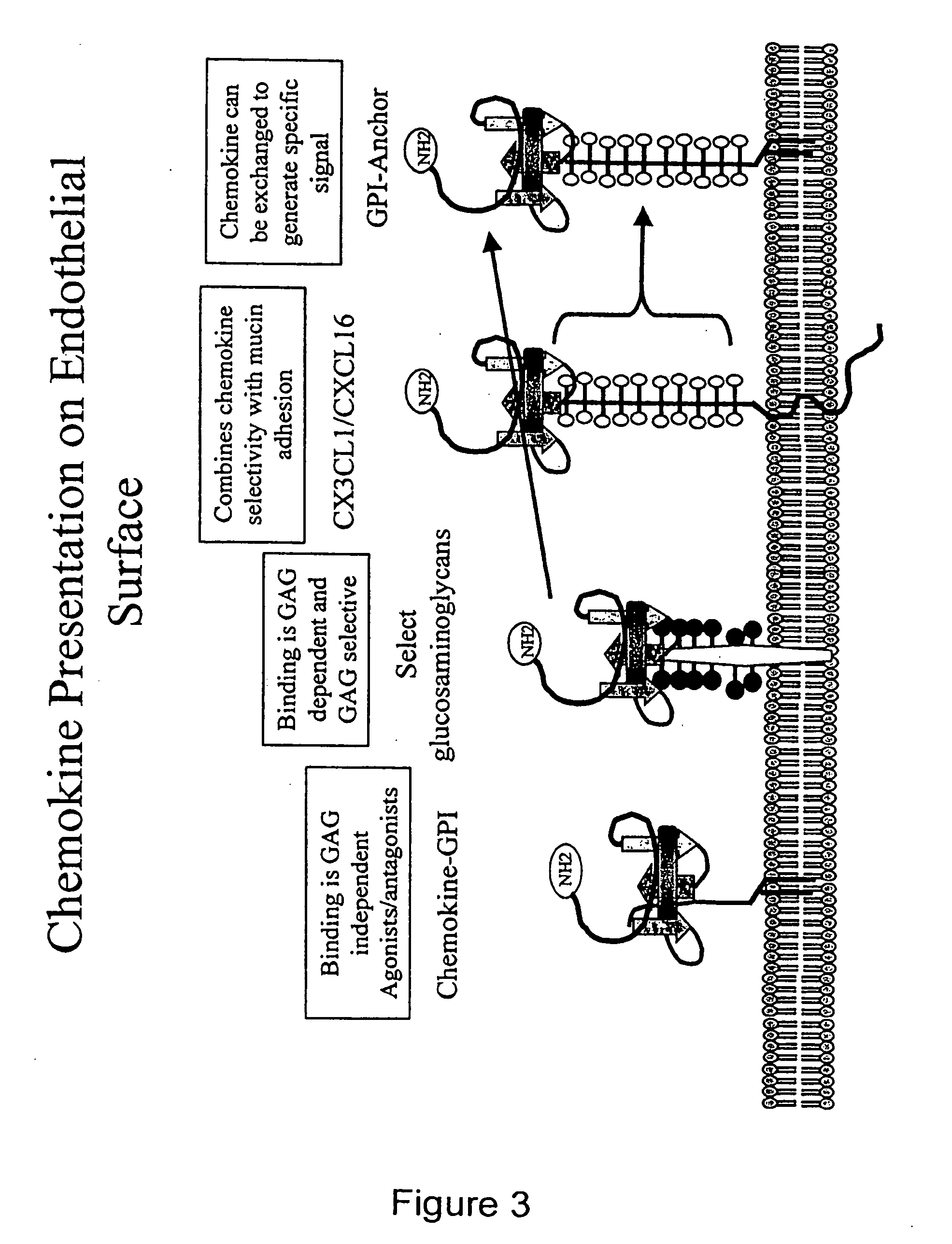
Chemokine-Mucin Fusions Linked to Glycosylphosphatidylinositol (GPI)-Anchors in Tissue Regeneration and as Tumour Immune Adjuvants
InactiveUS20120208769A1Reduction of immune-induced vascular damageEnhance immune responseOrganic active ingredientsBacteriaNatural Killer Cell Inhibitory ReceptorsCytotoxicity
The present invention relates to chemokine-mucin fusions linked to glycosylphosphatidylinositol (GPI)-anchors and their use as anti-cancer adjuvants and as agents in tissue regeneration and suppression of vascular damage. GPI-linked chemokines are incorporated in the surface membrane of tumour cells and effect a recruitment of cytotoxic immune cells to the tumour site following injection in vivo. Leukocytes, cytotoxic T-cells and NK cells target the chemokine-GPI-anchored tumour cells and modulate cell-mediated lysis of the tumour cells. The efficiency of GPI-anchoring and modulation of immune cells can be further enhanced by linking the chemokine to a mucin domain followed by the GPI-anchor. The GPI-anchored chemokines, with or without mucin domain, are remarkably useful for the inhibition of tumour growth, tissue regeneration, and suppression of acute vascular damage to allografts.
Owner:APCETH GMBH & CO KG
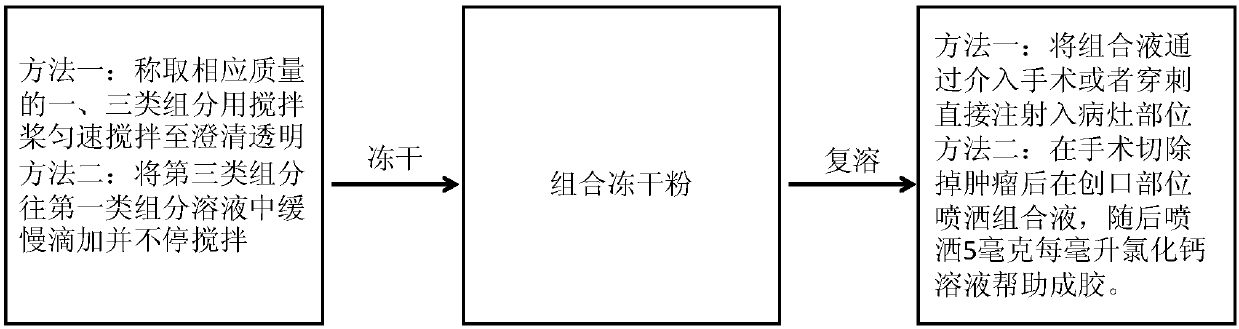
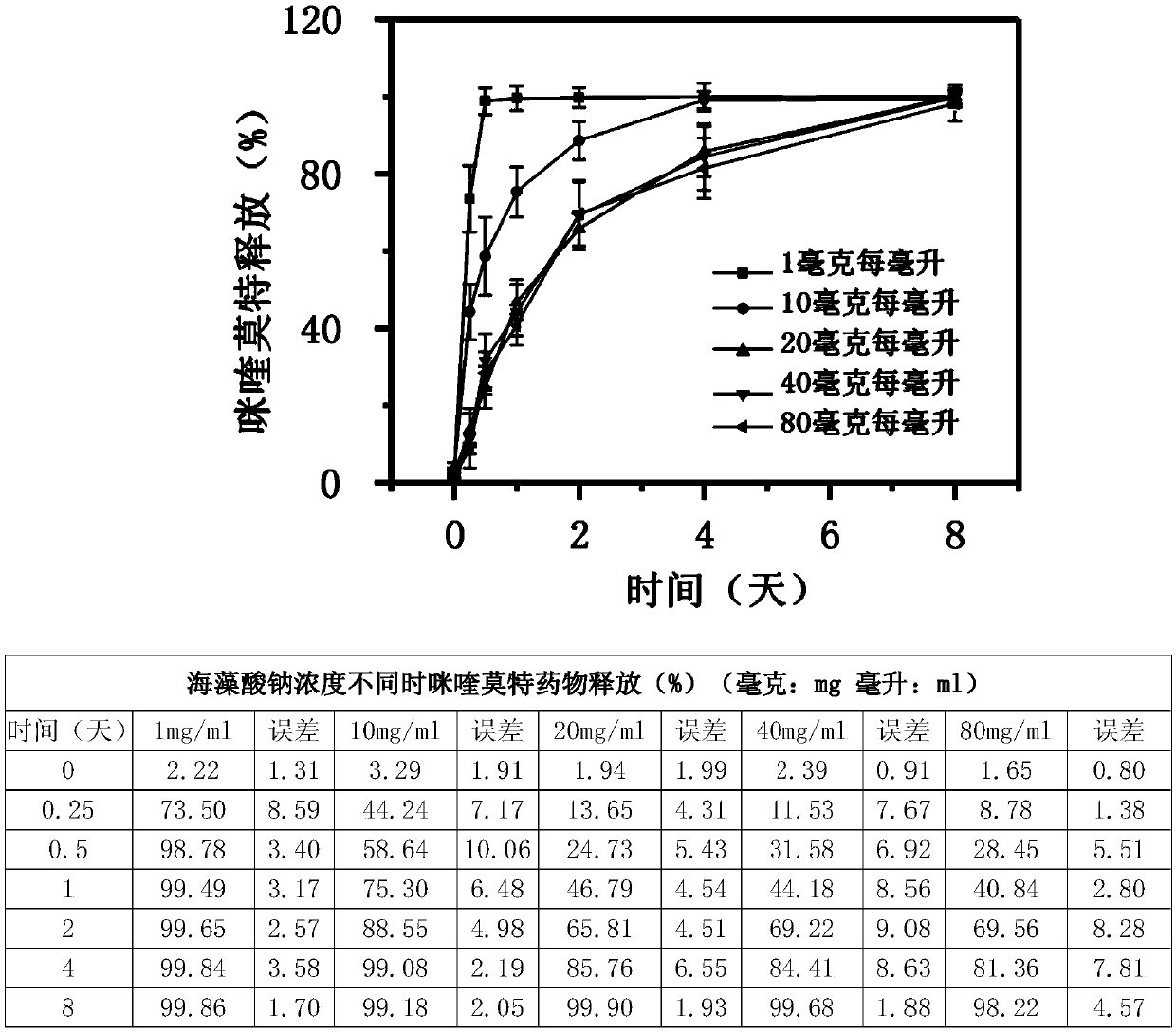
Biopolymer pharmaceutical composition used for tumor therapy
PendingCN111375065AGood biocompatibilityGood sustained release effectOrganic active ingredientsPharmaceutical delivery mechanismAMMONIUM ALGINATEBiopolymer
The invention discloses a biopolymer pharmaceutical composition for tumor therapy. The biopolymer pharmaceutical composition comprises a first component and a third component, wherein the first component is alginate; the alginate can form porous gel together with calcium ions in vivo, and the alginate is one or more of sodium alginate, potassium alginate and ammonium alginate; and the third component is an immune adjuvant. The biopolymer pharmaceutical composition can generate a synergistic anti-cancer effect, and reduces side effects, the cancer metastasis probability, and the cancer recurrence probability.
Owner:SUZHOU INNOVATIVE BIOMATERIALS & PHARM CO LTD
Anti-tumor nano-adjuvant based on cross-linked biodegradable polymer vesicles and preparation method and application thereof
ActiveCN111437258ALow toxicityImprove loading performanceImmunological disordersMacromolecular non-active ingredientsPolyesterCancer cell
The invention discloses an anti-tumor nano-adjuvant based on cross-linked biodegradable polymer vesicles and a preparation method and application thereof. The nano-adjuvant is obtained by loading drugs with reversibly cross-linked biodegradable polymer vesicles with asymmetric membrane structure. The drug is an oligonucleotide capable of activating the immune response. Degradable polymer vesiclesare obtained by cross-linking after self-assembly of polymers. The molecular chain of the polymer includes successively connected hydrophilic segments, hydrophobic segments and positively charged molecules. The hydrophobic segments are polycarbonate segments and / or polyester segments that are compounded and loaded with drugs through electrostatic interaction. The film is a polycarbonate and / or polyester segment that is reversibly cross-linked, biodegradable and biocompatible. The side chain of dithiolane is similar to natural antioxidant lipoic acid of the human body. The outer shell is basedon PEG, and can target cancer cells. The adjuvant is expected to become a nano-vaccine or nano-immune adjuvant that integrates the advantages of simplicity, stability, and multi-function, and can be used for efficient tumor immunotherapy.
Owner:SUZHOU UNIV
Compound I and compound II as well as preparation methods and application thereof
ActiveCN105315281AHas the effect of immune adjuvantImprove anti-tumor effectOrganic active ingredientsImmunoglobulin superfamilyMelanomaChemical compound
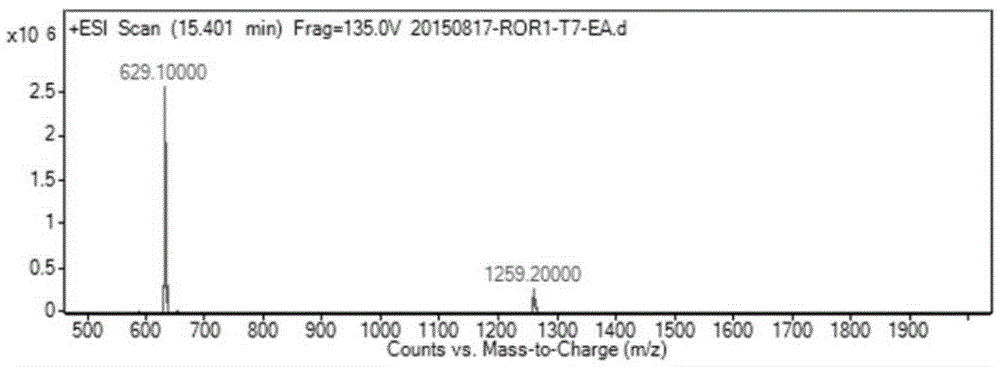
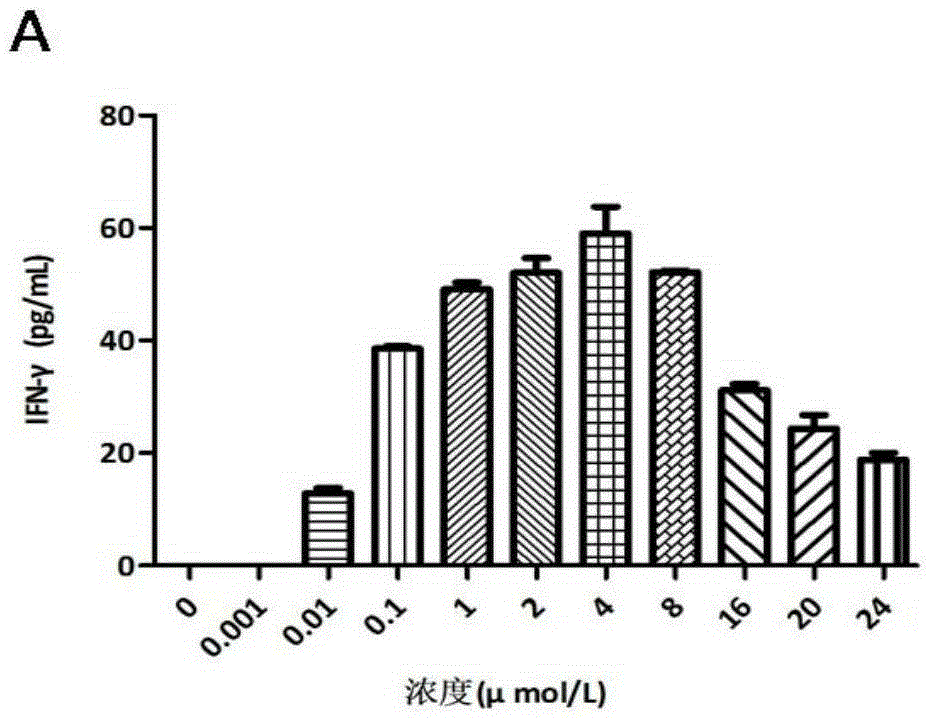
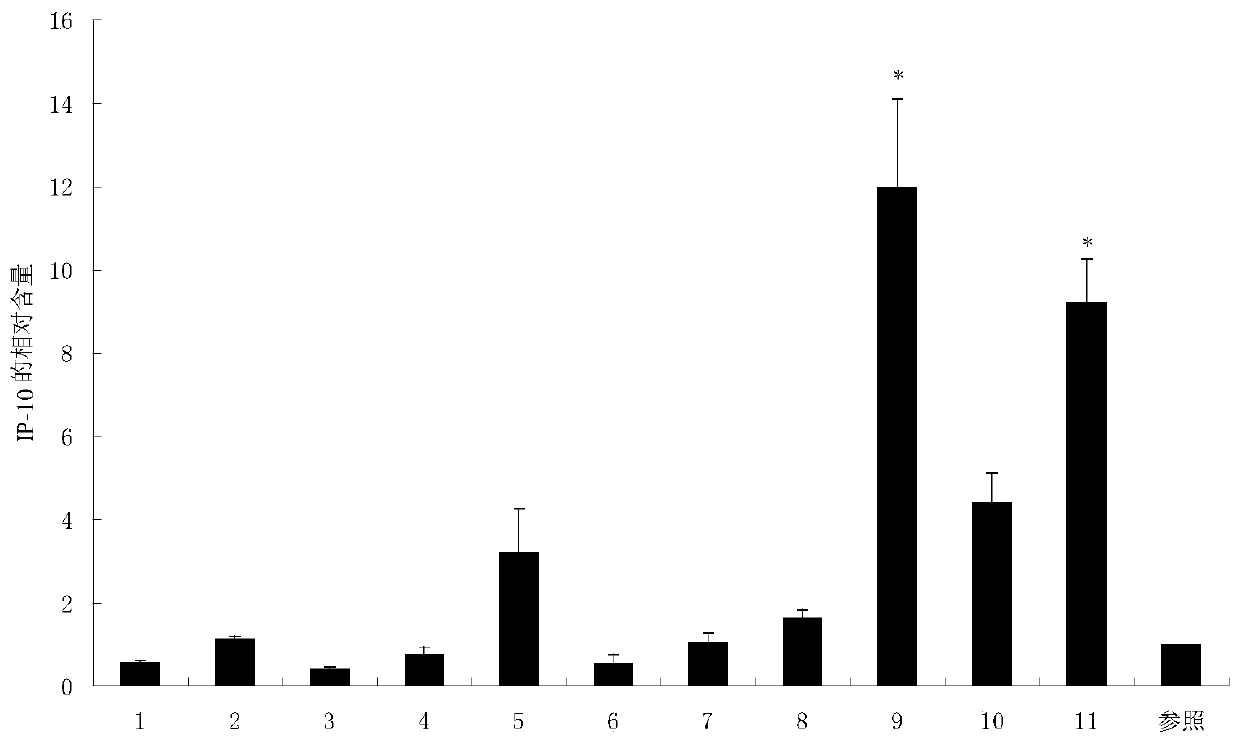
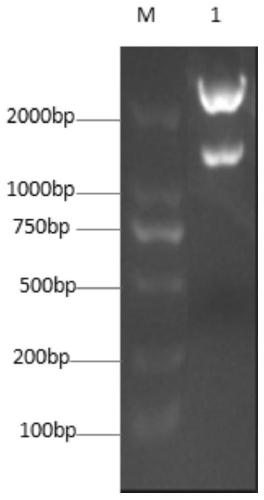
The invention relates to a compound I and a compound II as well as preparation methods and application thereof. The newly synthesized compound shown in the formula I can stimulate anti-tumor congenital immunity and cellular immunity while greatly improving the anti-tumor effect of ethacrynic acid (EA) and drug design of anti-tumor immunity synergetic integration is explored; the anti-melanoma immune response mechanism of the compound shown in the formula I is proved; the compound shown in the formula II and prepared through covalence of the compound shown in the formula I and ROR1 obviously reduces growth of breast cancer subcutaneously transplanted tumors, the breast cancer treatment immune response mechanism of the compound shown in the formula II is proved, and meanwhile, the fact that the compound shown in the formula I not only has the functions of stimulating congenital immunity and adaptive immunity, but also has the effect of immune adjuvant is proved.
Owner:SHENZHEN UNIV +1
CpG-ODN having specific immuno-stimulatory effect on pigs and application thereof
ActiveCN110042103AEnhanced induction of immune responseReduce manufacturing costAntibacterial agentsOrganic active ingredientsNucleotidePig breeding
The present invention discloses a CpG-ODN having a specific immuno-stimulatory effect on pigs and an application thereof. A nucleotide sequence of the CpG-ODN is shown in SEQ ID NO:1. The CpG-ODN canimprove induction of innate immunity and specific humoral and cell-mediated immune responses of the pigs, and can be transferred into a plasmid vector pFAR4 to construct a recombinant plasmid pFAR4-CpG to prepare a preparation containing the recombinant plasmid pFAR4-CpG. The preparation can effectively activate natural immune functions of the pigs, also successfully replaces or partially replaces antibiotics as an immune activator, can also effectively activate acquired immune functions of the pigs, especially cellular immunity, can be used as an immune adjuvant to improve a protective effect of vaccines, also has little toxic and side effects on body, has great potential, can be widely used in live pig breeding industry, and is conducive to reducing breeding cost, reducing incidence ofthe pigs, and improving survival rate and breeding efficiency.
Owner:SOUTH CHINA AGRI UNIV
Antitumor nanoparticles and preparation method and preparation thereof
ActiveCN109481418AEasy to operateEasy to masterPowder deliveryLuminescence/biological staining preparationSide effectTherapeutic effect
The invention provides antitumor nanoparticles and a preparation method and preparation thereof and relates to the technical field of nano-medicine. Each antitumor nanoparticle comprises a core, a wrapping layer coating the core and an immune layer coating the wrapping layer. The antitumor nanoparticles obtained by wrapping nanoparticles with an immune adjuvant can realize therapeutic effect combining photodynamics therapy and immune therapy, have selectivity to target tissue and injury degree and can reduce injury to normal tissue; the antitumor nanoparticles treat tumor by enhancing own immunity of a patient, is quick in response, free of side effect and lasting in therapeutic effect and can play an important role in preventing postoperative recurrence. The preparation method includes: providing high polymer organic compound-photosensitizer nanoparticles; coating the immune adjuvant outside the nanoparticles to obtain the antitumor nanoparticles. The method is simple in operation, easy to master and high in universality.
Owner:SHENZHEN INST OF ADVANCED TECH
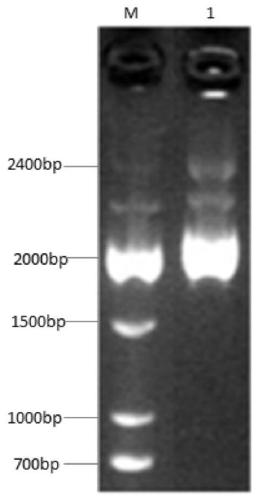
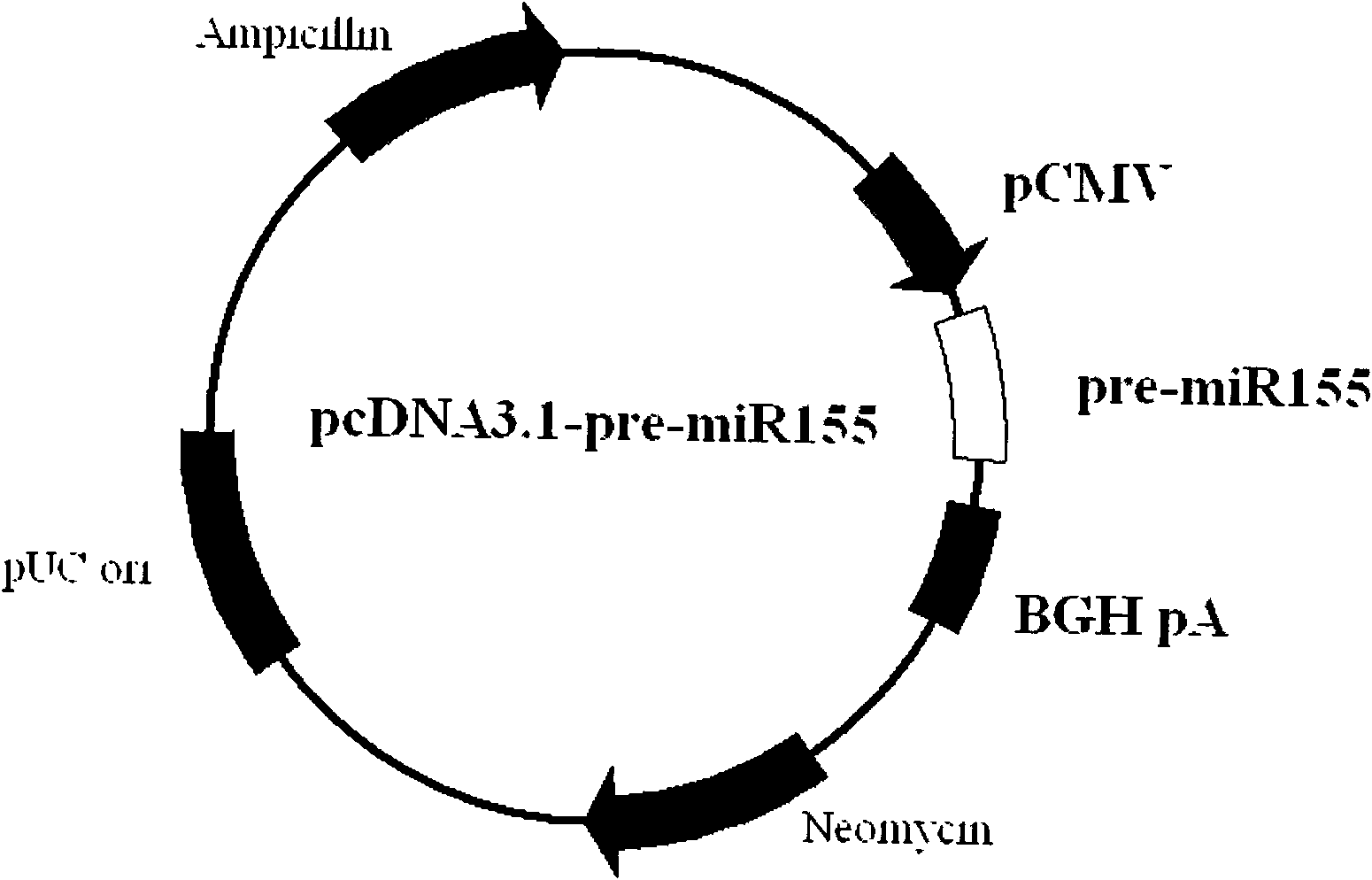
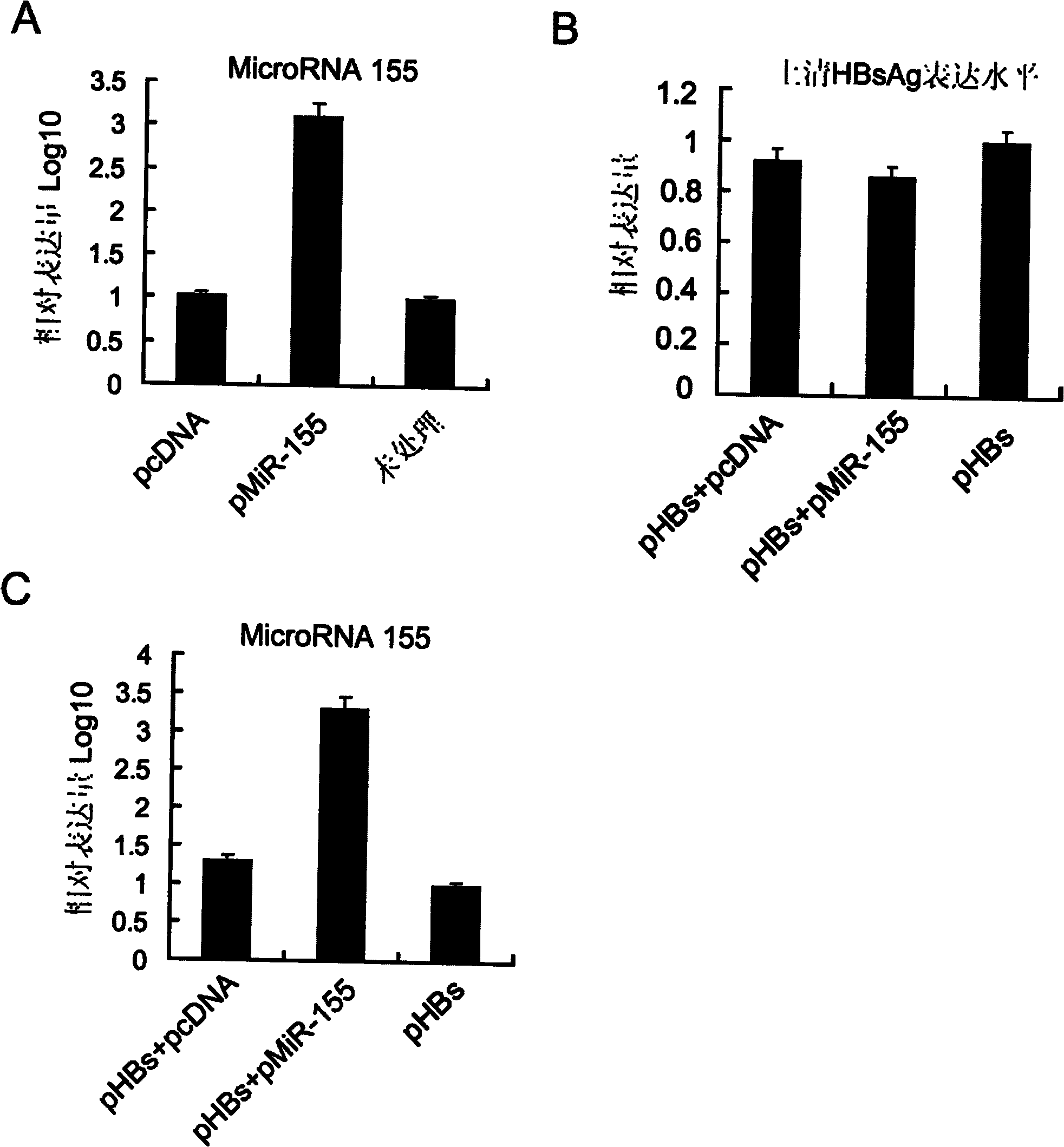
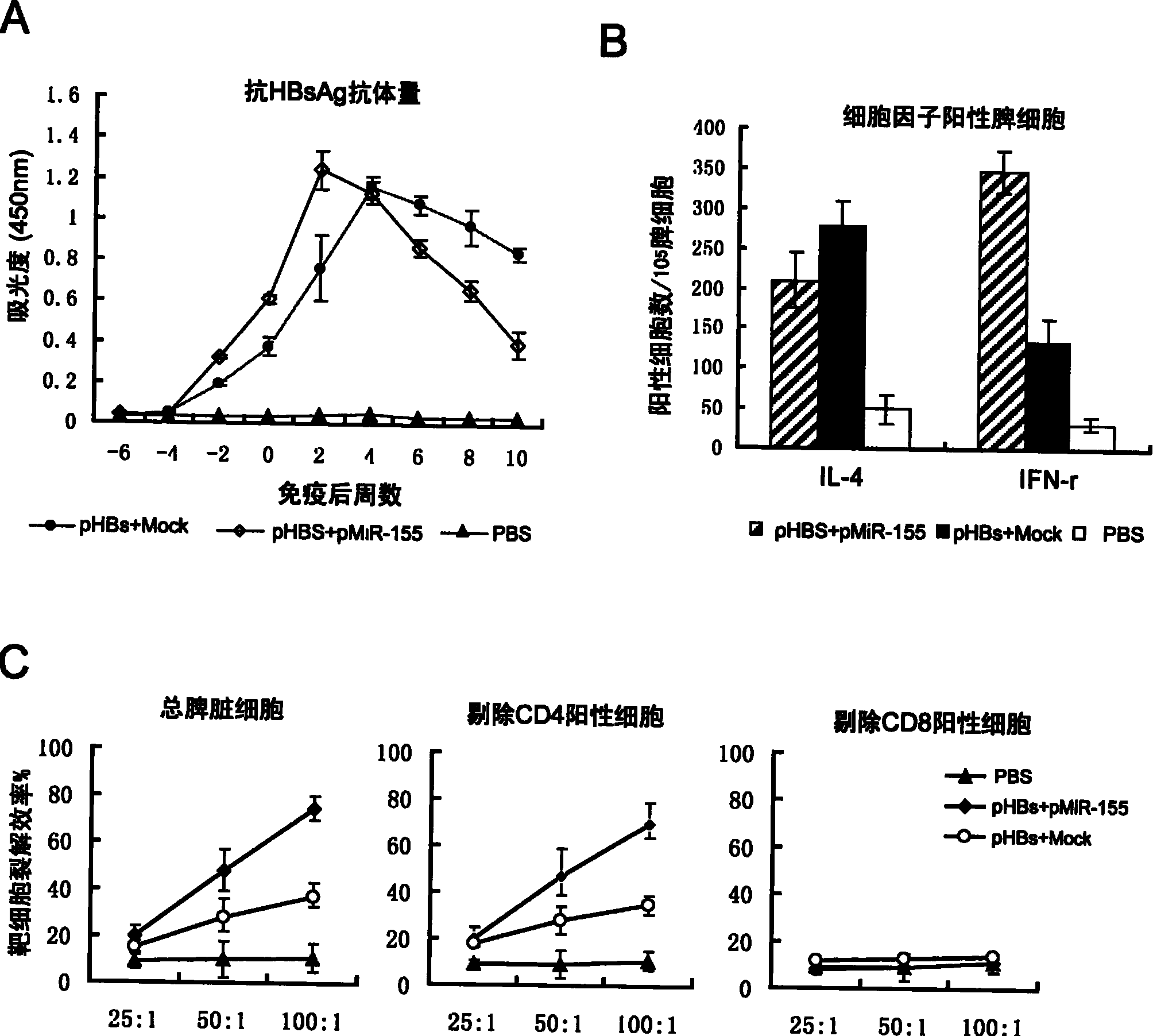
DNA vaccine adjuvant using Micro RNA-155 and construction method thereof
InactiveCN101972476AImprove immune efficiencyIncreased mature body concentrationGenetic material ingredientsAntibody medical ingredientsRegulatory T cellCD8
The invention relates to the technical field of medical biology. Micro RNA-155 is micro ribonucleic acid (RNA) for regulating the maturation and natural immunoreaction of T cells and B cells. The invention provides a micro RNA-155-based plasmid for enhancing the immune effect of deoxyribonucleic acid (DNA) vaccine cells. A plasmid pcDNA 3.1-pre-miR-155 is obtained by inserting an expression sequence of a micro RNA-155 precursor molecule into a pcDNA-3.1 plasmid, and the micro RNA-155 precursor molecules can be expressed efficiently without influencing the expression capability of the co-transfected DNA vaccine. In an in-vivo test, the plasmid and the DNA vaccine are injected at the same time and have the capability of effectively introducing cellular immunoreaction, and such capability isrepresented by the increase of antigenically specific spleen IFN-gamma positive cells and enhancement of target killing capability of spleen CD8 positive cells. The plasmid of the invention can be added into the conventional DNA vaccine preparation and injected together so as to obviously promote the cellular immunoreaction induced by the DNA vaccine, and serves as a novel cell immune adjuvant.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Novel synthetic c-glycolipids, their synthesis and use to treat infections, cancer and autoimmune diseases
InactiveCN1964626AAntibacterial agentsEsterified saccharide compoundsHydrogenAutoimmune thyroid disease
The invention is directed to novel compounds of formulae (I), (II) and (III): wherein X is O or NH; R<3> is OH or a monosaccharide and R<4> is hydrogen, or R<3> is hydrogen and R<4> is OH or a monosaccharide; R<5> is hydrogen or a monosaccharide; and pharmaceutically acceptable salts or esters thereof. The invention is also directed to the use of the compounds both directly and as immune adjuvants for treating cancer, infectious diseases and autoimmune diseases. The invention is also directed to syntheses of the intermediates which can be used to make these novel compounds.
Owner:NEW YORK UNIV +1
Preparation method of recombinant panda IL-2 immune adjuvant
InactiveCN103509815AAntibody medical ingredientsVector-based foreign material introductionPANDASDisease
The invention discloses a preparation method of a recombinant panda IL-2 immune adjuvant. The recombinant panda IL-2 immune adjuvant is a recombinant panda IL-2 (gpIL-2) immune adjuvant, which has a prominent effect and a convenient extraction process, can be directly applied to production and clinical application, improves the immune effect of canine distemper vaccine strain and other conventional vaccines, controls the occurrence and epidemic of diseases, and protects the health of panda. Canine distemper is usually known as "the fatal infectious disease", has become the first fulminating infectious disease that threatens the species number and life safety of pandas, and badly affects the healthy development of pandas. The recombinant panda IL-2 (gpIL-2) immune adjuvant can prominently strengthen the immune effect of canine distemper vaccine strain, and has an important meaning on preventing and treating of canine distemper.
Owner:四川卧龙国家级自然保护区管理局 +1
Swine trichinella spiralis vaccine as well as preparation method and application thereof
InactiveCN107551265AImproving immunogenicityGood immune protectionHydrolasesImmunoglobulins against animals/humansEscherichia coliTrichinella spiralis infection
The invention discloses a swine trichinella spiralis vaccine as well as a preparation method and an application thereof. Firstly, a mature region of a gene TsCPB2 is amplified and cloned into an expression vector of a His tag, and then after transformation of escherichia coli and IPTG induced expression, TsCPB2 recombinant protein is purified by Ni-affinity chromatography, and a trichinella spiralis excretion-secretion recombinant antigen is obtained; TsCPB2 recombinant protein and an immune adjuvant are subjected to mixed emulsification, and the TsCPB2 recombinant antigen vaccine is obtained.With adoption of the vaccine for immunizing a mouse or swine, the host can be induced to produce an immune reaction, a good immunoprotective effect on trichinella spiralis infection is realized, andgreater application prospect is achieved.
Owner:SUN YAT SEN UNIV
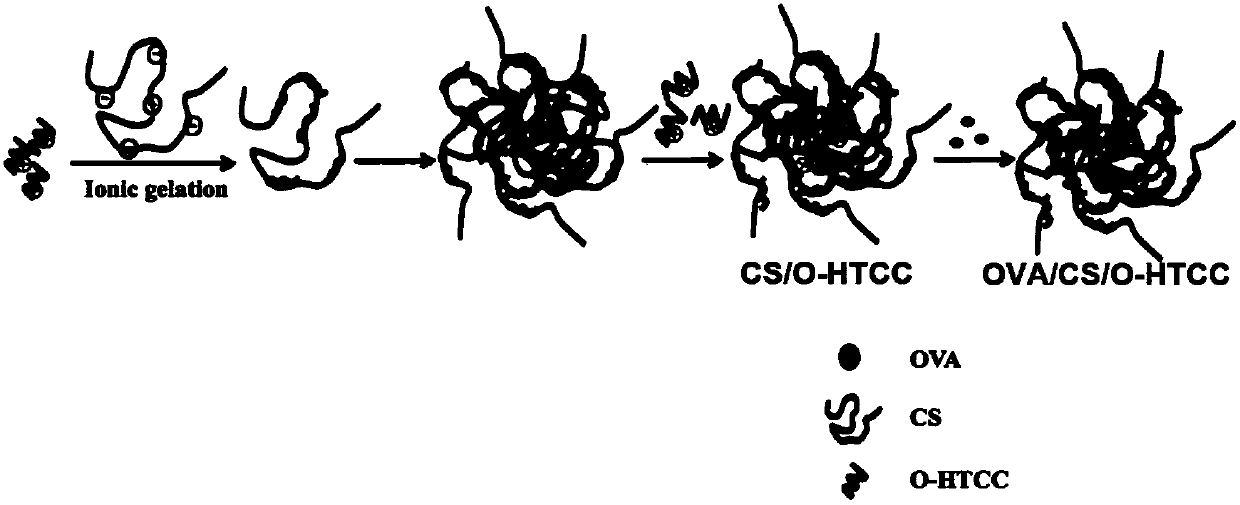
Curdlan sulfate/6-O-quaternized chitosan nanoparticles and application thereof in mucosal vaccines
InactiveCN107625961APromote proliferationPromote activationAntiinfectivesRespiratory disorderEpitheliumMucosal adjuvant
Nanoparticles are prepared from curdlan sulfate and 6-O-quaternized chitosan by an ion gelation method for the first time; the method is simple and convenient, green, safe and pollution-free, and through controlling experimental steps and parameters, the prepared curdlan sulfate / 6-O-quaternized chitosan nanoparticles have uniform size and good stability. Evaluation of the membrane penetrating ability, the immune regulation ability and the mucosal adjuvant effect of the curdlan sulfate / 6-O-quaternized chitosan nanoparticles shows that the positively charged characteristic of the nanoparticles make the nanoparticles more easily act on effective parts through epithelium mucosae, so as to play a role of immune enhancers and help an antigen reach the effective parts to play relatively good immune adjuvant effect; the nanoparticles have great industrial application value.
Owner:SHANDONG UNIV
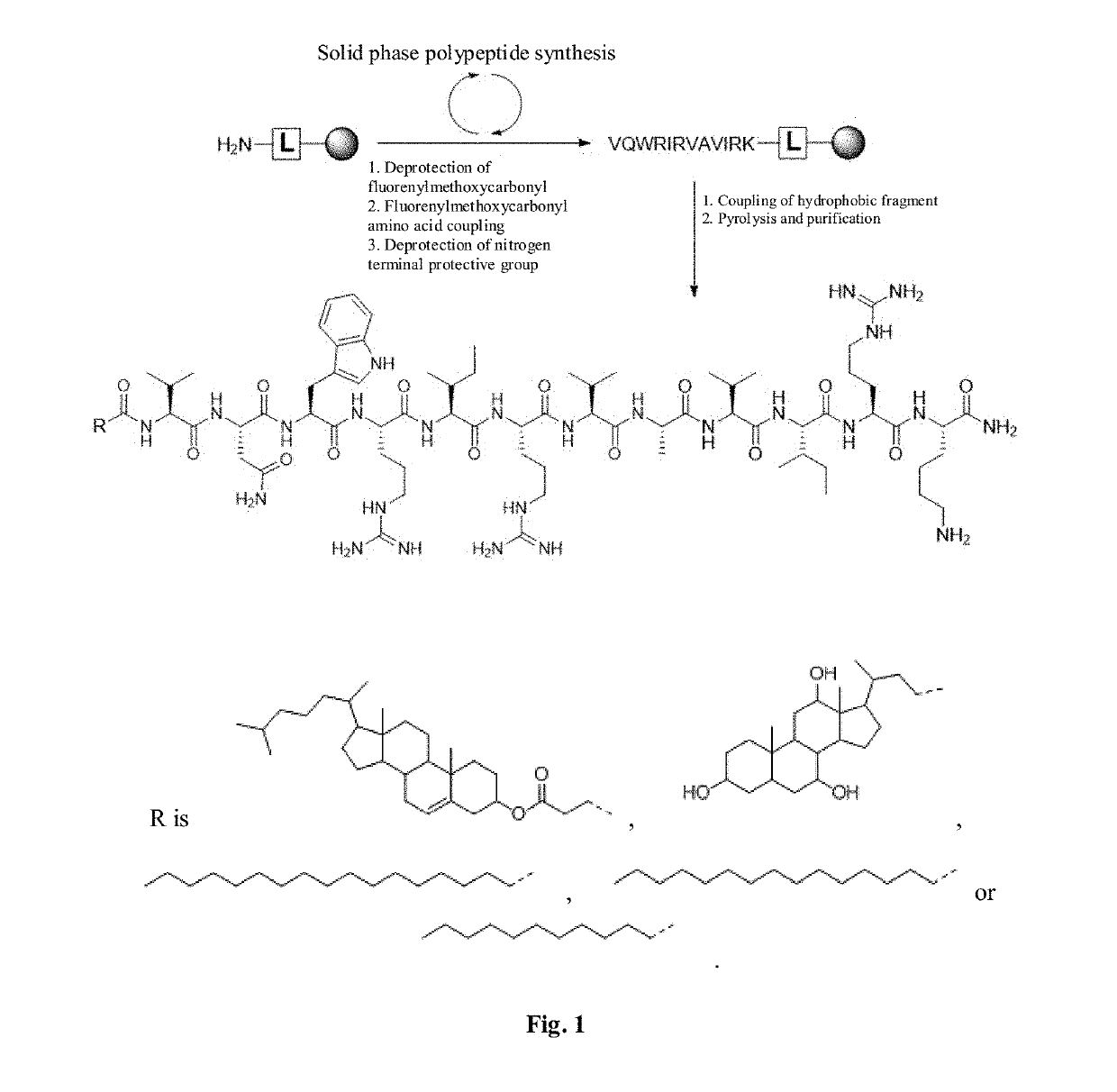
Antimicrobial peptide derivative and use thereof
PendingUS20190216939A1Low toxicitySimple methodAntibacterial agentsOrganic active ingredientsChemical synthesisCrystallography
The invention relates to the field of biomedicine and particularly to a hydrophobically modified antimicrobial peptide and a use thereof. The technical problem to be solved by the invention is to provide a hydrophobically modified antimicrobial peptide, the hydrophobic modification is to couple a hydrophobic fragment at the nitrogen terminal of the antimicrobial peptide. The invention further provides a micelle prepared from the hydrophobically modified antimicrobial peptide, and use of the hydrophobically modified antimicrobial peptide and the micelle in preparing antimicrobial drugs, nucleic acid transporter, immune adjuvant and the like. Due to small molecular weight, the antimicrobial peptide of the invention can be conveniently synthesized by Fmoc solid phase polypeptide, and coupled to a hydrophobic fragment by the chemical synthesis method in a simple and feasible way.
Owner:SICHUAN UNIV
Preparation method of tumor vaccine and tumor vaccine prepared by using the method
PendingCN110464840AHigh precisionImprove efficiencyTumor rejection antigen precursorsVaccinesAgricultural scienceTumor antigen
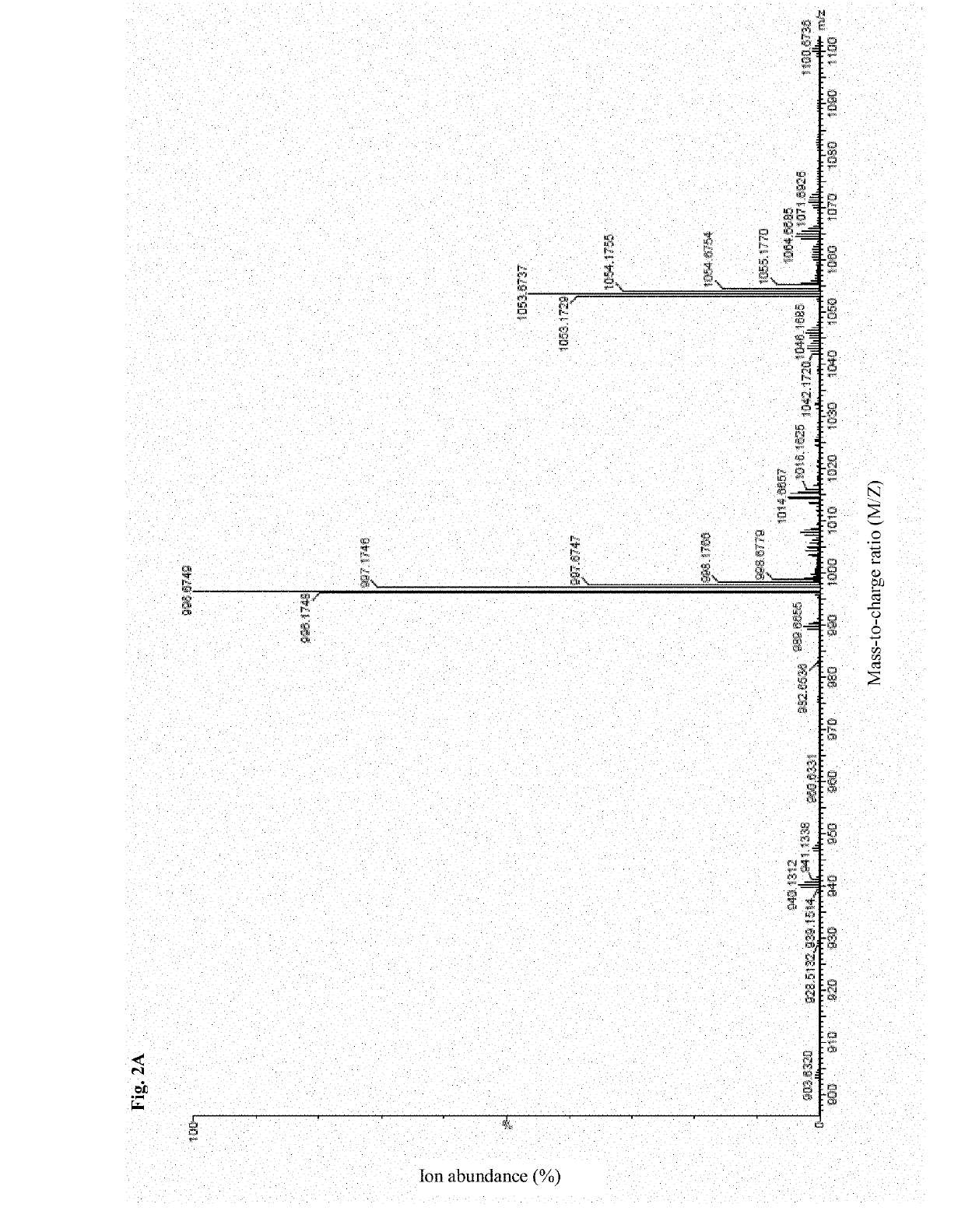
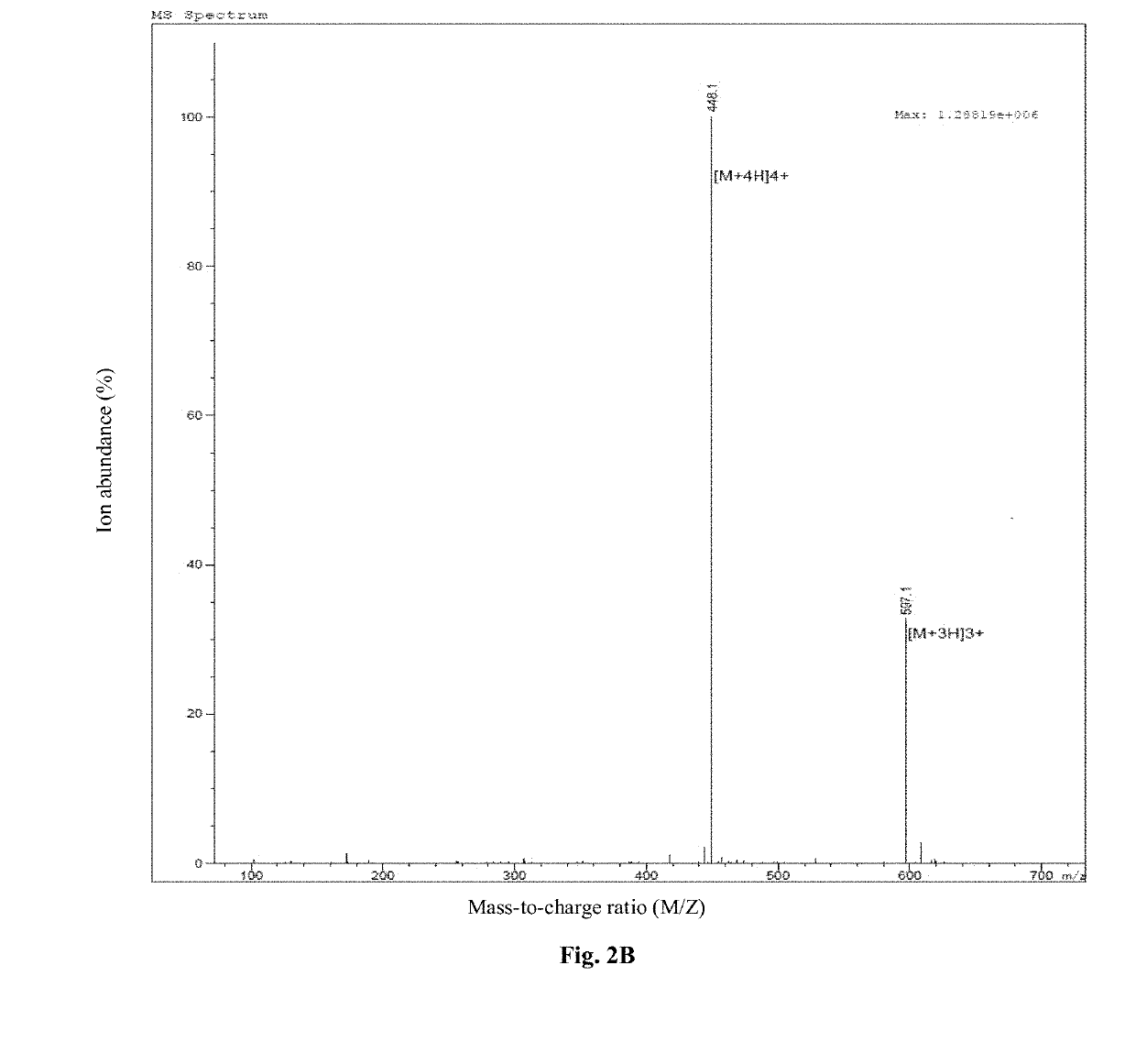
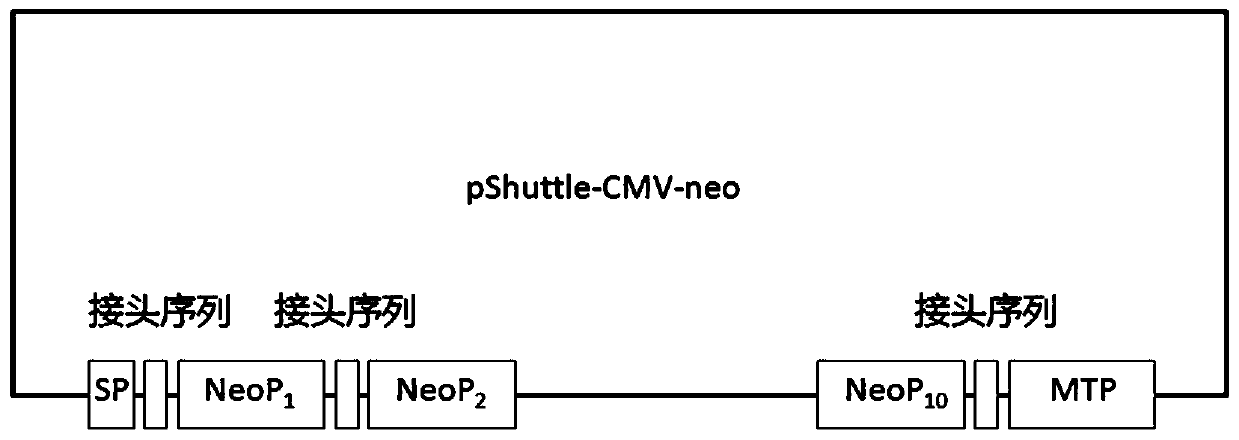
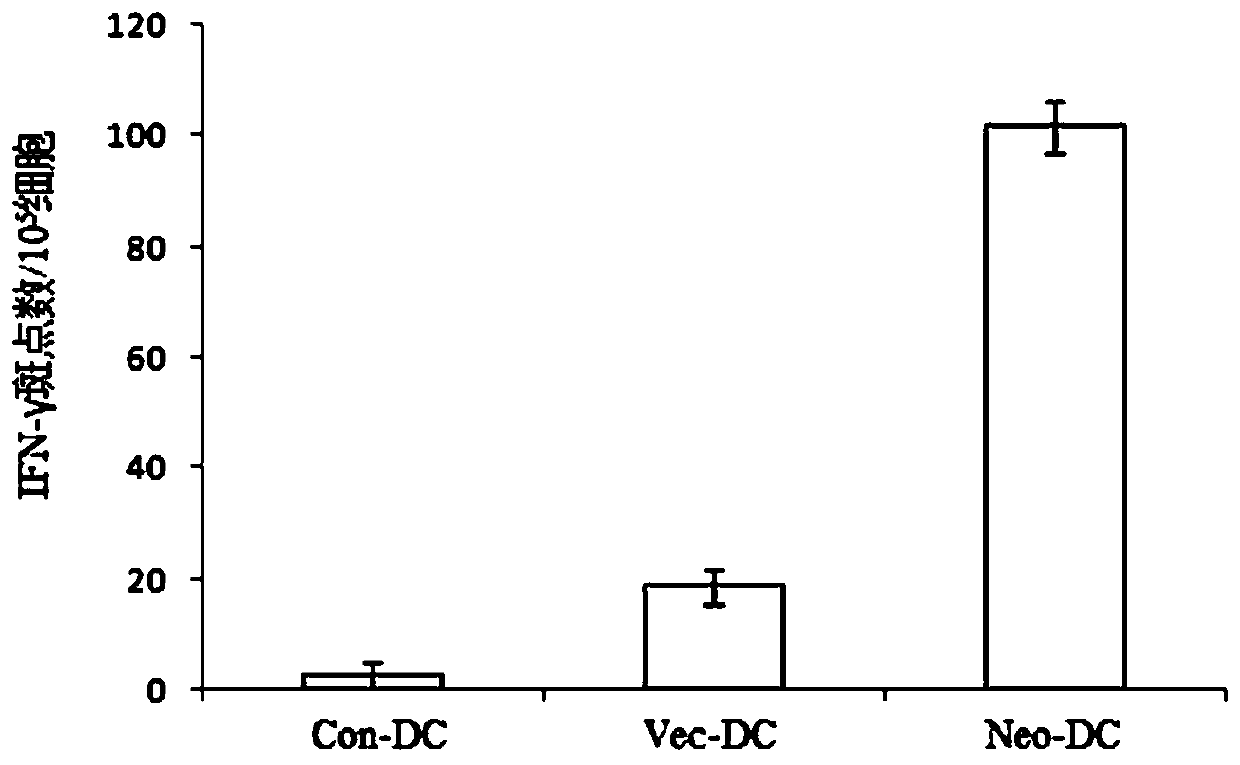
The invention discloses a preparation method of a tumor vaccine. The method includes: cloning a gene sequence of a tumor antigen obtained by screening into a virus expression vector to prepare a recombinant virus to obtain the tumor vaccine; inserting a signal peptide sequence shown in SEQ ID No. 1 or a sequence having a homology of more than 80% with the signal peptide sequence to 5 'end of the gene sequence of the first tumor antigen, and inserting an MHC transport signal sequence shown in SEQ ID No. 2 or a sequence having more than 80% homology with the MHC transport signal sequence to 3 'end of the gene sequence of the last tumor antigen; wherein the virus expression vector is an adenovirus, a retrovirus or a lentivirus, preferably the adenovirus. According to the method, the tumor antigen is screened by a specific method, the virus vector, particularly the adenovirus vector, is used for expressing and preparing the vaccine for the tumor antigen. The vaccine has strong immune stimulation activity and can reduce the requirement on immune adjuvants.
Owner:北京微九九科技有限公司
Novel echinococosis granulosis vaccine with CPG DNA (deoxyribonucleic acid) immune adjuvants
InactiveCN105267988AImprove immunityGood humoral immunityGenetic material ingredientsAntiparasitic agentsDna immunizationIntermediate host
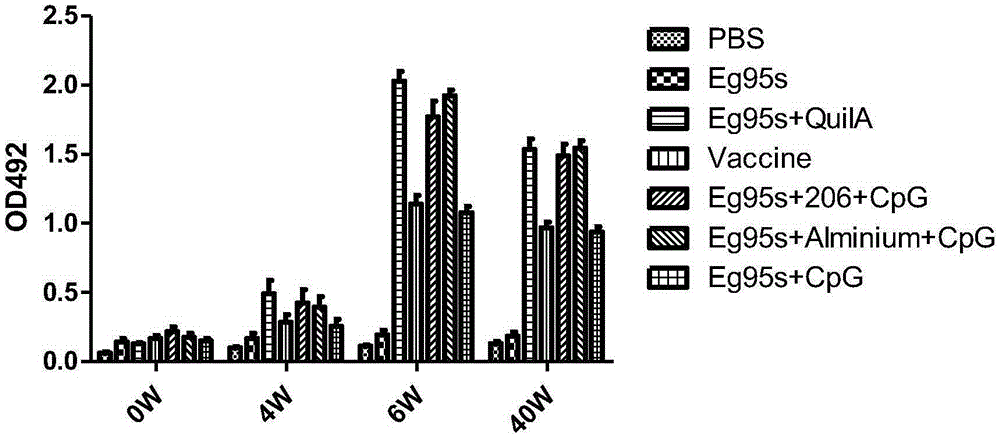
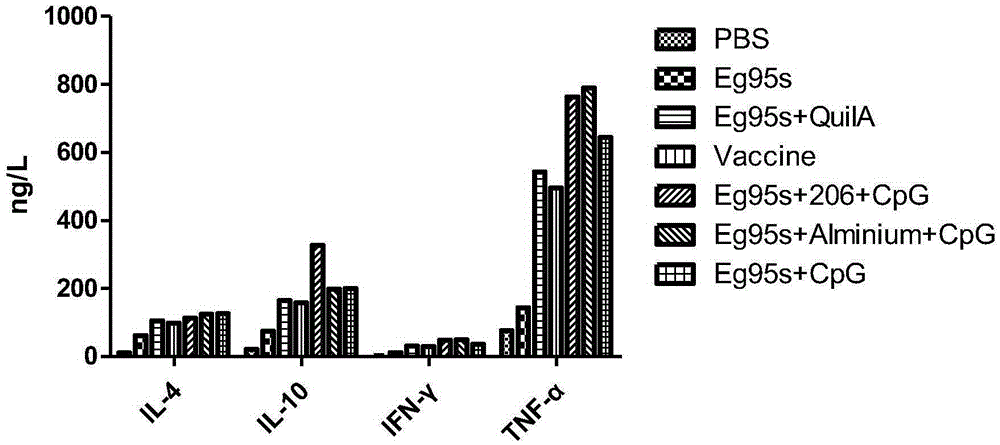
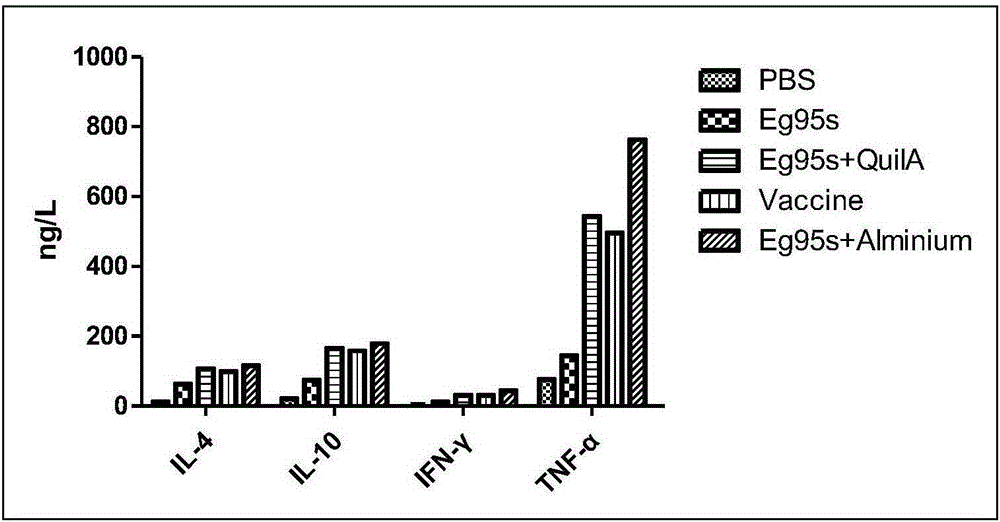
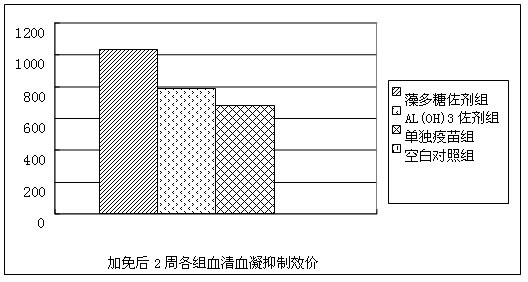
The invention belongs to the technical field of vaccine development, and provides novel echinococosis granulosis vaccine for preventing intermediate hosts of echinococosis for human, livestock and the like. The novel echinococosis granulosis vaccine comprises soluble expression mixtures of Eg95 recombinant proteins, aluminum hydroxide and CpG immune adjuvants. The novel echinococosis granulosis vaccine has the advantage that excellent cellular immunity and humoral immunity of bodies can be effectively stimulated by the novel echinococosis granulosis vaccine.
Owner:BEIJING ZHONGNONG BIOLOGICAL ENG CO LTD
Panax japonicus saponin as well as preparation method and application thereof
ActiveCN103611157AEasy to prepareEasy to operateImmunological disordersAntibody medical ingredientsPANAX JAPONICUS ROOTImmunity
The invention relates to panax japonicus saponin used as a vaccine adjuvant as well as a preparation method and application thereof. The method comprises the following steps: extraction of the panax japonicus, extraction separation, resin adsorption and the like. The panax japonicus saponin is further separated and purified, the weaknesses of the traditional method that only the total saponins component is used and the main components of the samponin is not studied can be overcome, so that the problem that the drug ingredients are not known by people can be solved; an active part of the panax japonicus saponin has an obvious immunity adjuvant effect, and a way for developing the vaccine adjuvant is provided.
Owner:湖北森元药业有限公司
Novel water-soluble echinococosis granulosis vaccine with immune adjuvants
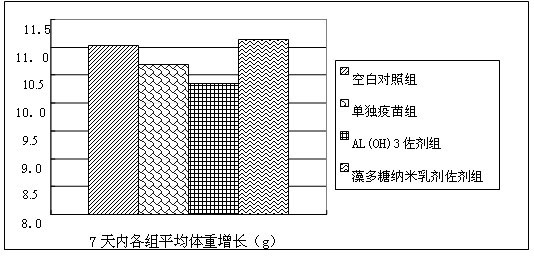
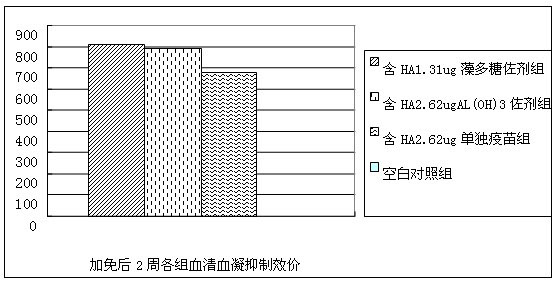
InactiveCN105267989AImprove immunityGood humoral immunityGenetic material ingredientsAntiparasitic agentsWater solubleHumoral immune reaction
The invention belongs to the technical field of vaccine development, and provides novel echinococosis granulosis vaccine for preventing intermediate hosts of echinococosis for human, livestock and the like. The novel echinococosis granulosis vaccine comprises soluble expression mixtures of Eg95 recombinant proteins, aluminum hydroxide and immune adjuvants. The novel echinococosis granulosis vaccine has the advantage that excellent cellular immunity and humoral immunity of bodies can be effectively stimulated by the novel echinococosis granulosis vaccine.
Owner:BEIJING ZHONGNONG BIOLOGICAL ENG CO LTD
Spirulina polysaccharide immune adjuvant and influenza vaccine containing the same
ActiveCN102028945AEasy to prepareReduced activityAntiviralsAntibody medical ingredientsSide effectVaccine antigen
The invention provides a spirulina polysaccharide immune adjuvant and an influenza vaccine containing the same, wherein the spirulina polysaccharide immune adjuvant is prepared from the components in mass ratio as follows: 0.001-100% of spirulina polysaccharide and 0-99.999% of carrier, which is capable of preferably improving immunogenicity of the vaccine and greatly reducing dosage of antigen vaccine; the dosage of the antigen vaccine taking the spirulina polysaccharide as adjuvant is only a half of the dosage of the antigen vaccine taking the aluminum hydroxide as adjuvant or less, which can achieve ideal immune protection effect as well with high safety and small side effect; the influenza vaccine taking the spirulina polysaccharide as adjuvant is convenient for preparation and the quality thereof is easy to control.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com