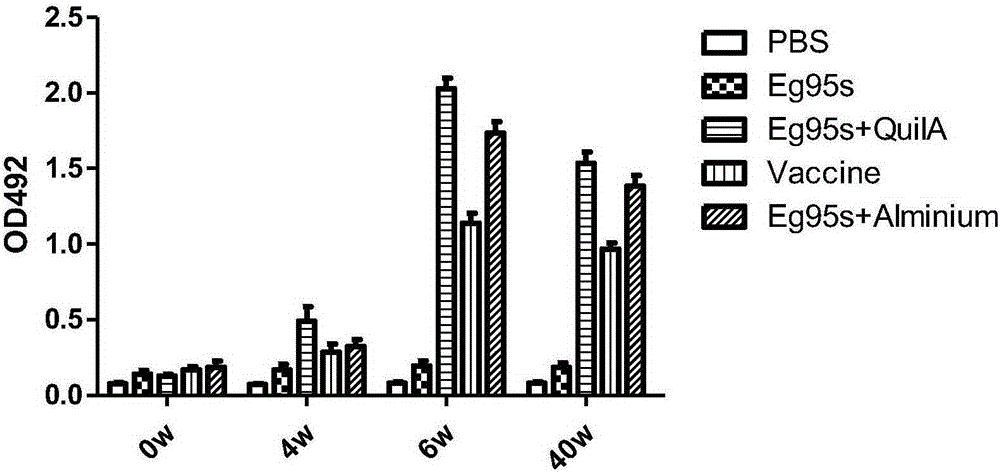
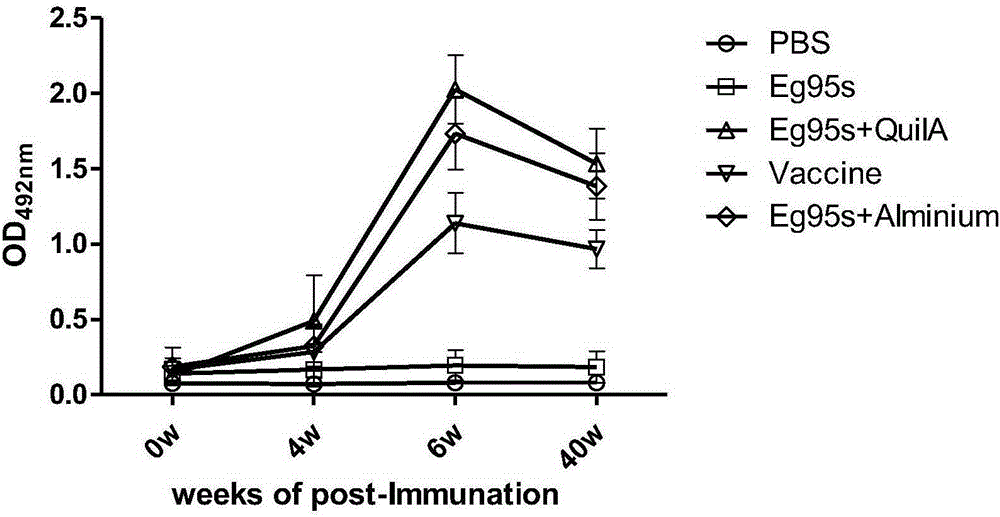
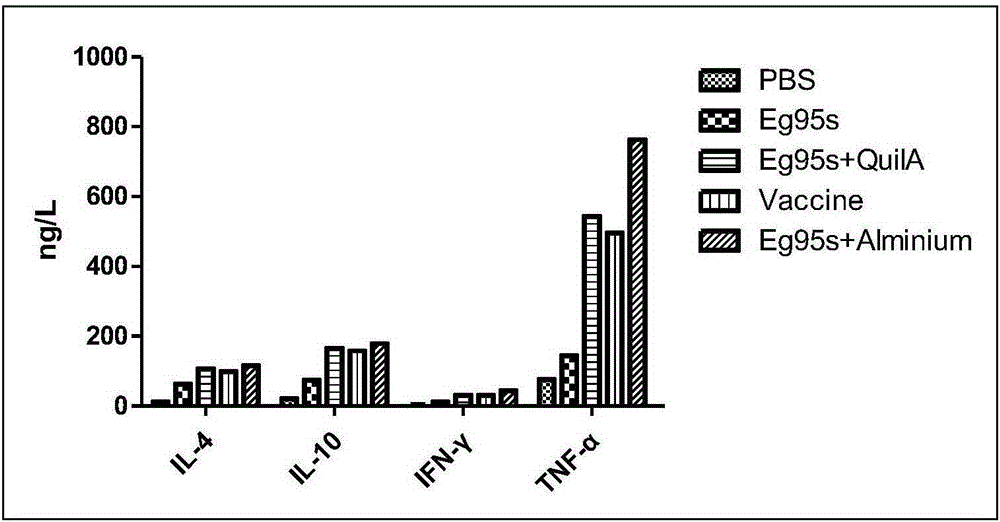
Novel water-soluble echinococosis granulosis vaccine with immune adjuvants
An immune adjuvant and echinococcosis technology, applied in the field of vaccine development, can solve the problems of limiting the popularization and application of vaccines, hemolysis and other side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The construction of embodiment 1 recombinant plasmid PET-EG95S
[0033] 1.1 Activation and emulsification of hydatid eggs
[0034] The aseptically treated hydatid eggs were washed and incubated and activated according to the method of Heath and Lawrence. Four million activated hydatid cells were incubated for 14 days in 40 ml of NCTC (Gibco) medium containing 260 mg / l L-cysteine and 20% lamb serum. During this period, the medium was changed every two days, and all culture media were filtered through a 0.22 μm filter membrane and stored at 4°C. After the cultivation, the culture solution was concentrated to 20 ml by dialysis and stored at -20°C.
[0035] 1.2 Preparation of total RNA
[0036] About 4×10 6 After the mature hydatid eggs were activated in vitro, the total RNA was extracted according to the instructions of the total RNA extraction kit (Total RNA Isolation System).
[0037] (1) When extracting tissue RNA, use 1ml Trizol reagent for every 50-100mg tissue...
Embodiment 2
[0086] Expression, purification and identification of embodiment 2 recombinant protein
[0087] 2.1 Induced expression and purification of recombinant protein
[0088] The recombinant plasmid PET-EG95s prepared in Example 1 was respectively transformed into E.coliBL21 (DE3) competent cells, and a single colony was picked and inoculated into 10 mL of LB medium containing ampicillin at a final concentration of 25 μg / ml, at 37°C 200r / Shake culture overnight, inoculate 1ml of the culture into 100ml LB medium containing ampicillin at a final concentration of 25μg / ml, and culture with shaking at 200r / min at 37°C until OD 600When nm=0.6, add IPTG with a final concentration of 1 mM, and culture at 22° C. with shaking at 160 rpm for 10 h. Collect the bacteria by centrifugation at 6000r / min for 10min, wash twice with 40mL PBS (pH7.4), resuspend in 10mlPBS (pH7.4), break the bacteria by ultrasonication in an ice bath, centrifuge the broken mixture at 12000rpm, 4℃ for 30min, and take it...
Embodiment 3
[0094] The preparation of embodiment 3 novel echinococcus vaccines
[0095] 3.1 Quantification of recombinant protein EG95s protein
[0096] After the endotoxin-free EG95s recombinant protein prepared in 2.2 was quantified by the BCA protein concentration detection kit, SDS-PAGE electrophoresis was performed, and the percentage of the target protein EG95s in the total protein was analyzed by Bandscan5.0 software, and the target protein content was finally calculated.
[0097] 3.2 Preparation of the vaccine
[0098] Eg95s: directly filter the quantified EG95s antigen through a 0.22μm filter, dilute it with PBS to contain 50μg / ml EG95s antigen per head, 1.0ml per head, pack in 100ml bottles, 50 heads per bottle, use sterile gel Plug it in and make a record.
[0099] Eg95s+QuilA: Mix 1.0ml per head, containing 1mgQuilA and 50μg EG95s antigen, filter through a 0.22μm filter, and vortex to mix. The prepared vaccine is divided into 100 ml bottles, 50 doses per bottle, covered wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com