mVSV virus vector and virus vector vaccine, and COVID-19 vaccine based on mVSV mediation
A viral vector and virus technology, applied in the field of genetic engineering, can solve the problems of low response degree and inability to respond to mucosal immune response, and achieve the effects of high antigen loading capacity, enhancement of specific mucosal immune response, and stability of the viral genome
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 The toxicity of the three-site mutation in the Indiana strain of VSV is greatly reduced
[0049] It is known that the main pathogenic gene of VSV wild strain is M, and at the same time, M protein can induce the apoptosis of host cells, which is the main factor for the disease of artiodactyls infected by VSV wild strain. Based on this, synthetic biology technology can reduce The best way of VSV wild strain is to carry out genetic engineering mutation in the M gene. Existing studies have shown that after the non-synonymous mutation of the 51st amino acid of the M protein, the neurotoxicity of wild VSV will be reduced. Therefore, in the implementation of the technology of the present invention In , the mutation comparison of a single site was first carried out, and methionine was mutated into phenylalanine, alanine, leucine and arginine (control) at the 51st position, such as Figure 7As shown in A, some results show that mVSV-M51F has weaker cytotoxicity in norm...
Embodiment 2
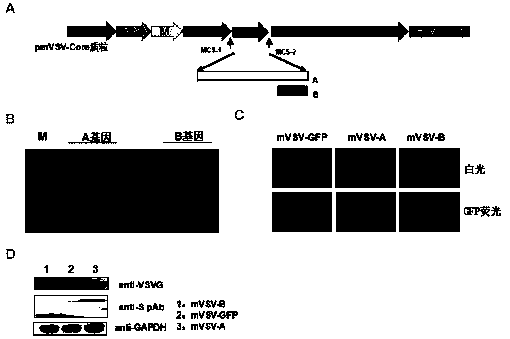
[0050] Example 2 Construction and identification of two chimeric vaccines based on VSV viral vectors
[0051] According to the S gene sequence of SARS-CoV-2 released by NCBI, the codon is optimized to facilitate its expression in cells (named as antigen gene A), and multiple potential antigenic epitopes predicted from the sequence of SARS-CoV-2 are synthesized The new antigen gene (named as antigen gene B), the antigen gene A and antigen gene B sequences were handed over to Nanjing GenScript to synthesize them on the pCDNA3.1 and pUC57 vectors respectively, after the target gene was amplified by PCR, it was purified by fragment purification reagents Recover and purify the target band from the cassette, digest the fragment and the pmVSV-GFP vector with restriction endonucleases, MCS1 (Xhol) and MCS2 (Nhel) at 37°C for 3 hours, recover the vector and the target fragment from the gel, and perform a ligation reaction. Then transfer to competent cells, positive clones were screened...
Embodiment 3
[0073] Example 3 Immune response effects of two chimeric vaccines based on VSV viral vectors under different immunization schemes
[0074] Detect the specific IgA and IgG antibody levels in mice after different immunization schemes by indirect ELISA: After coating the microtiter plate with the recombinant RBD protein of SARS-CoV-2S virus, it will be immunized by three immunization methods: intramuscular, intravenous and intranasal 21 days after the first time, the mouse serum was diluted 1:200 and added to the corresponding wells. After incubation for 2 hours, different types of (IgA, IgG) secondary antibodies were diluted 1:10000 to detect the specific antibody level ( figure 2 ), the specific steps are as follows:
[0075] 1) Dilute the coated antigen (S-RBD) with coating buffer to a final concentration of 5 μg / ml, take the microplate plate, add samples to the wells in sequence (100 μl / well), and then place it at 4 degrees Celsius for coating overnight;
[0076] 2) Pour o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com