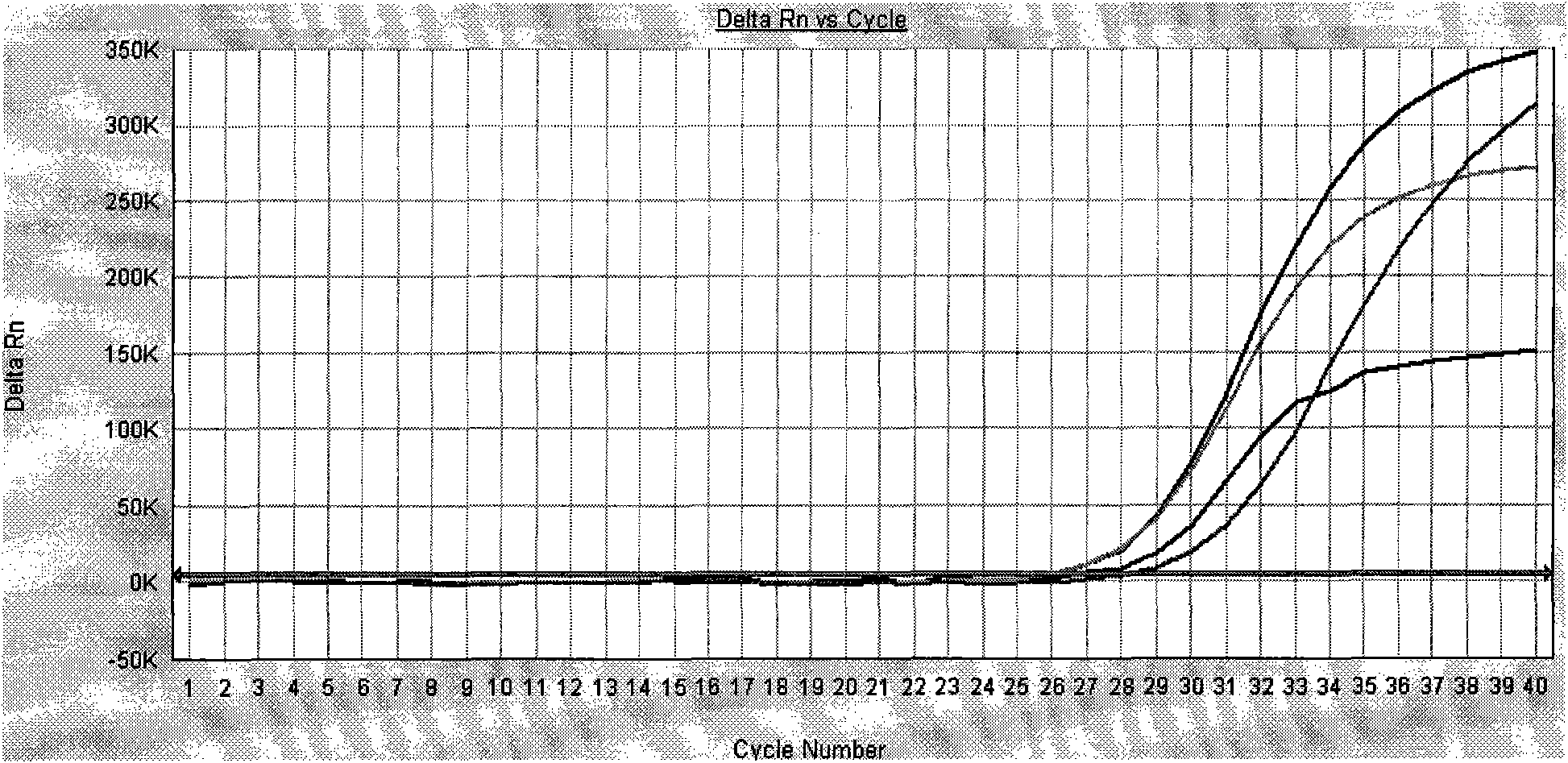
Patents
Literature
643 results about "Antigen Gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
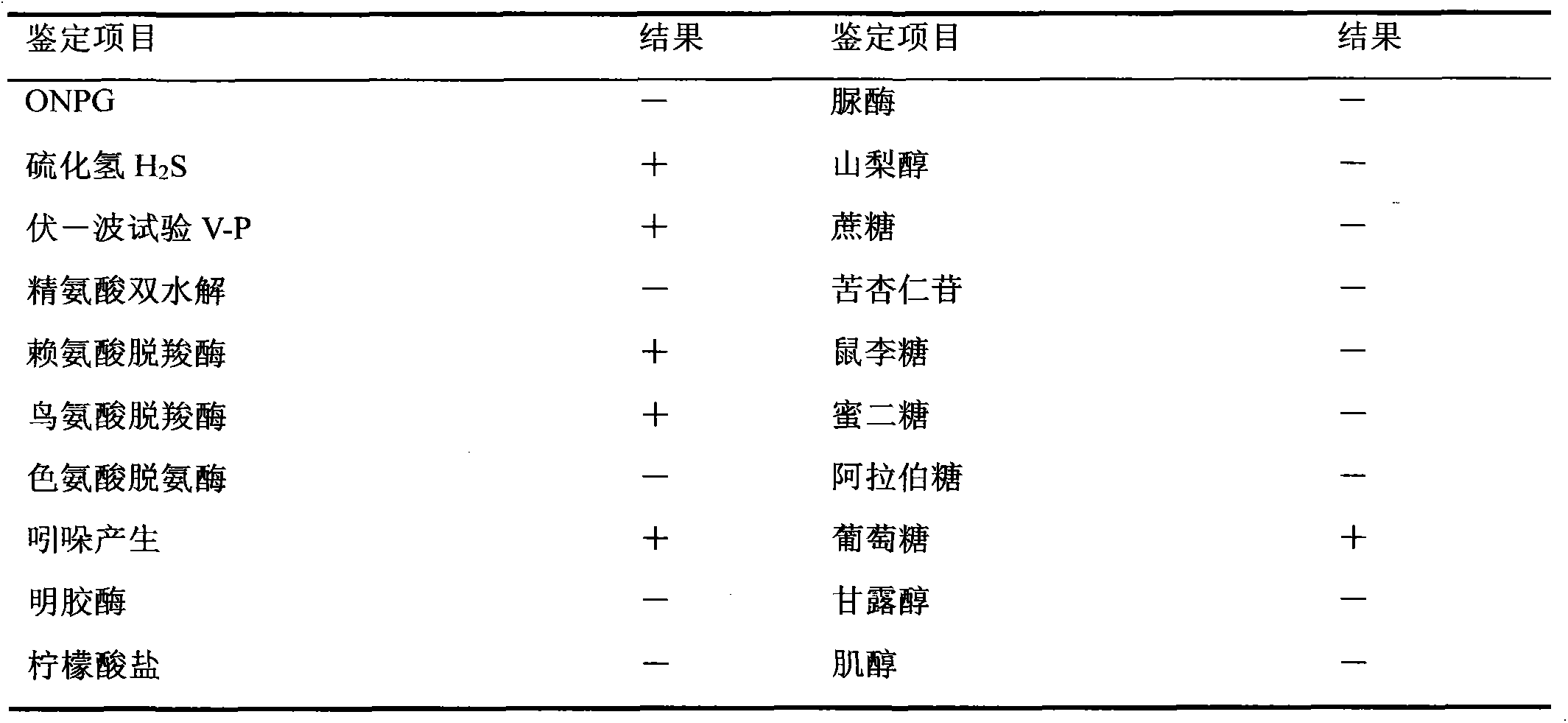
A gene that encodes a product, termed an antigen, which stimulates the immune system and results in the production of antibodies.
mVSV virus vector and virus vector vaccine, and COVID-19 vaccine based on mVSV mediation
ActiveCN111088283AEnhance immune responseStrong immune responseSsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousReceptor
The invention provides an mVSV virus vector, i.e., attenuated mVSV obtained after multiple modification mutations occur to an M protein amino acid site of a wild Indiana strain VSV, and an optimized heterologous antigen gene is preferentially integrated to a double cloning site area of an mVSV packaging core plasmid pmVSV-Core at the same time. The mVSV virus vector vaccine comprises a heterologous antigen gene which fuses or embeds a target virus between G and L genes of an mVSV vector envelope, wherein the antigen gene comprises an enveloped and embedded antigen gene encoding the target virus, an embedded combination antigen gene or a fused antigen gene; the mVSV virus vector is embedded or fused with a dominant antigen of spike protein S of an SARS-CoV-2 pathogen; the dominant antigen is preferably selected from a receptor binding domain of spike protein S, namely RBD; and a COVID-19 vaccine based on mVSV mediation is formed. The vaccine has good prevention or treatment effect on COVID-19 infected people.
Owner:FANTASIA BIOPHARMA ZHEJIANG CO LTD
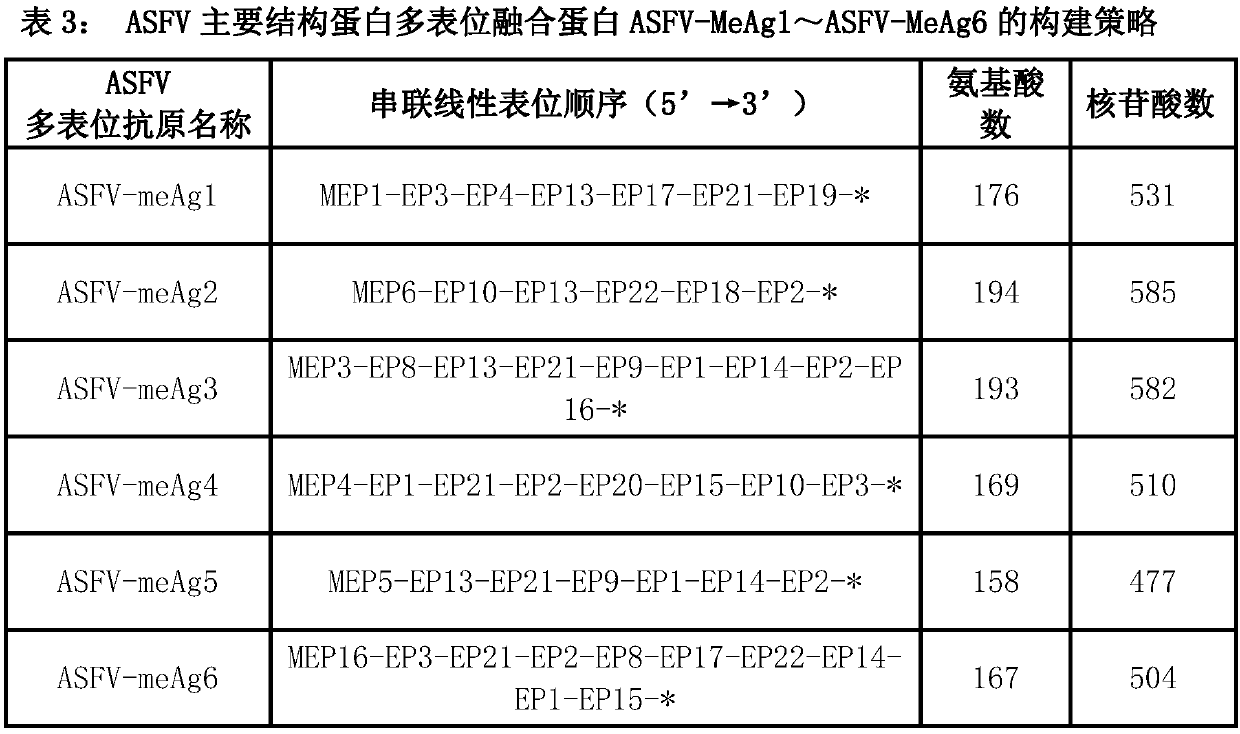
Multi-epitope fusion diagnosis antigen for African swine fever virus as well as preparation method and application thereof
InactiveCN108148138AImprove featuresIncreased sensitivityAntibody mimetics/scaffoldsVirus peptidesAntigenBacillus coli
The invention discloses a multi-epitope fusion diagnosis antigen for African swine fever virus as well as a preparation method and application thereof. An ASFV (African swine fever virus) important structural protein gene encoding amino acid sequence is analyzed, screened and recombined through bioinformatics software, a multi-epitope fusion antigen gene is built and synthesized and is expressed in bacillus coli; through screening, the recombinant multi-epitope fusion antigen ASFV-meAg6 is obtained, so that diagnosis antigen protein with strong specificity and high sensitivity is provided foran ASFV serological diagnosis method.
Owner:SHIHEZI UNIVERSITY
Antineoplastic dibasic polypeptide and application and preparation method thereof
ActiveCN101139613ASpecific targetingWon't attackPeptide/protein ingredientsHybrid peptidesNucleotideDouble chain
The invention provides a gene, recombinant plasmid and polypeptide for an anti-tumor binary polypeptide. The gene of the recombinant anti-tumor binary polypeptide is obtained by connecting in an operable way the gene of a coding antibody simulator with a recombinant bacillus anthraci protein antigen gene. The recombinant plasmid of the invention is formed by inserting the gene of the coding antibody simulator by double-chain oligomeric nucleotide directed mutagenesis method into the recombinant bacillus anthraci protein antigen gene. The obtained recombinant plasmid is infected into engineering bacillus coli BL-21 to get engineering bacillus coli cell of anti-tumor binary polypeptide; the anti-tumor binary polypeptide can be obtained by expanding the bacillus coli, settling in centrifugal way the bacillus coli body, crushing in altrasonic way, settling and crushing bacillus coli body by hi-speed centrifuging and treating the upper clean solution. The anti-tumor binary polypeptide is of special targeting characteristic, higher efficiency in killing special physical tumor than prior anti-tumor medicine, and will not attack normal cells, and has much lower toxicity and poor-reaction than prior anti-tumor medicine.
Owner:姜荣锡
Breeding method of polyclonal antibody cold-resistant two-line sterile line
ActiveCN103229712ALow costReduce pollutionPlant genotype modificationAnimal scienceTryporyza incertulas
The invention discloses a breeding method of a polyclonal antibody cold-resistant two-line sterile line, belonging to the technical field of breeding for disease resistance. According to the breeding method, by using a two-line sterile line as a recurrent parent, and using a tryporyza incertulas-resistant variety, a rice blast-resistant variety, a stunt-resistant variety and a cold-resistant variety as antigen parents, through a hybrid polymerization process, antigen genes of four rice diseases, insect and cold damages are polymerized in the same rice material, and then subjected to multi-generation backcross and screening together with the recurrent parent of the two-line sterile line, so that the insect and cold-resistant two-line sterile line, with economical characters, sterility period translative temperature and sterility highly consistent with those of the recurrent parent of the two-line sterile line, is bred. The two-line sterile line is used for preparing two-line hybrid rice, and thus the level of resisting insects and low-temperature coldness of the hybrid rice can be improved.
Owner:福建亚丰种业有限公司
Method for efficiently preparing porcine circovirus type 2 empty capsid particles
The invention provides a method for efficiently preparing porcine circovirus 2 type empty capsid, which comprises the following steps: cloning different viral strain lines of antigen genes cap required by forming circovirus empty capsid and artificially reconstructed cap mutant into a domestic silkworm baculovirus carrying vector to obtain a homologous recombinant vector, recombining or transpositioning the homologous recombinant vector and parent virus DNA (deoxyribonucleic acid) in an insect cell or bacterium to obtain recombinant baculovirus, and infecting the insect with the recombinant baculovirus containing the antigen gene; and culturing the infected insect host to express the corresponding circovirus 2 type empty capsid, determining the information required by efficiently assembling the virus empty capsid by comparison and experimentation, and obtaining a recombinant viral strain line capable of efficiently expressing and assembling virus empty capsid, thereby carrying out mass production on the circovirus 2 type empty capsid. The porcine circovirus 2 type empty capsid produced by the method can be used for preparing vaccines for preventing and treating porcine circovirus disease after being subjected to primary purification.
Owner:CHINA INST OF VETERINARY DRUG CONTROL +1
Liquid phase chip reagent kit detecting various anti EB viral antigen antibody
ActiveCN101063683AHigh throughputSmall amount of sampleMaterial analysisViral antigensNasopharyngeal carcinoma
This invention discloses one liquid phase chip test agent case and its process method for multiple kinds of anti-EB virus antibody, which comprises the following parts: coupling affinity element multi-color microball covering on polypeptide; at least one kind of coupling hydroxyl group multi-color microball covering on natural or gene antigen, wherein the antigen is EB virus crack object or shell antigen VCA; gene antigen is of one amino acid in list of No. 33-No. 37; the said integration polypeptide is one from No. 1-No. 32.
Owner:SUN YAT SEN UNIV CANCER CENT
Detection type gene chip for detecting various infectious desease and use thereof
InactiveCN1450171AStrong specificityHigh sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesEpidemic hemorrhagic fever virusMalaria
The present invention relates to a gene chip for detecting several infections lideases and its application. It can be used for detecting, typing and strain-identifying 7 main infections diseases of epidemic hemorrhagic fever, tsutsugamushi disease, leptospirosis, schistosomiasis, malaria, cholera and hemorrhagic enteritis due to 0157:H7. Said invention can select specific gene probe and PCR primer respectively from S gene of epidemic hemorrhagic fever virus, 56KD protein gene of oriental rickettsia, 23 SRNA gene of leptospirosis, 5D antigen gene of schistosomiasis, SSrRNA gene of malarial parasite, 0157 antigen gene of colibacillus 0157:H7, H7 antigen gene and toxic gene and outer membrane OWP protein gene of cholera vibrio and toxic gene.
Owner:陶开华 +1
Anthrax resisting polypeptide and its application and preparation method
ActiveCN101215568AFatal infection preventionAntibacterial agentsBacterial antigen ingredientsHuman cellEukaryotic plasmids
The invention provides an anti-anthrax polypeptide gene, a recombinant plasmid, a polypeptide, the application and a process for preparation which comprises following steps: operationally connecting a gene of an encoding antibody simulacrum with the gene of an encoding recombinant mutation bacillus anthracis proteantigen to obtain the gene which expresses a recombinant anti-anthrax polypeptide, inserting the gene of the encoding antibody simulacrum into the gene of the recombinant mutation bacillus anthracis proteantigen through the technique of double chain oligonucleotide point mutation to form the recombinant plasmid of the invention, transfecting the recombinant plasmid which is obtained into coli bacteria BL-21 project engineering bacteria to obtain engineering bacteria cells of the anti-anthrax polypeptide, obtaining the anti-anthrax polypeptide through extracting supernate which contains polypeptide from a great amount of increasing bacteria, centrifugal precipitation cells and saccharose with an addex-magnesiumand method and purifying the supernate with florisil column. The anti-anthrax polypeptide specifically can damage the biological activity of bacillus anthracis toxin and does not attack normal human cells.
Owner:PROTEIN DESIGN LAB LTD
Method for preparing rabies virus antigen
ActiveCN101307317AReduce consumptionImprove securityAntiviralsDepsipeptidesAntigenBaculovirus expression
The invention provides a method for making rabies virus antigen. The method comprises the following steps that: the antigen gene of rabies virus or the combined expression combination of the antigen gene is respectively cloned in a baculovirus carrier so as to obtain a transfer expression carrier; the transfer expression carrier and baculovirus undergo cotransfection so as to carry out homologous recombination or transposition, thereby obtaining recombined baculovirus; the recombined baculovirus is used to infect insect host and cell; the infected insect host is cultured to express corresponding rabies antigen; and the expressed antigen is ingathered and purified so as to obtain rabies virus antigen. The method adopts a baculovirus expression system to make safe and efficient rabies virus antigen in a domestic silkworm bioreactor; moreover, due to having extremely high safety, the made antigen can be directly used to make injection vaccine and oral vaccine used for animal immunization. The method can substantially reduce the production cost of rabies virus antigen, and has the advantages of safety, high efficiency, less energy consumption and low cost, etc.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
HLA high-resolution gene sequencing kit
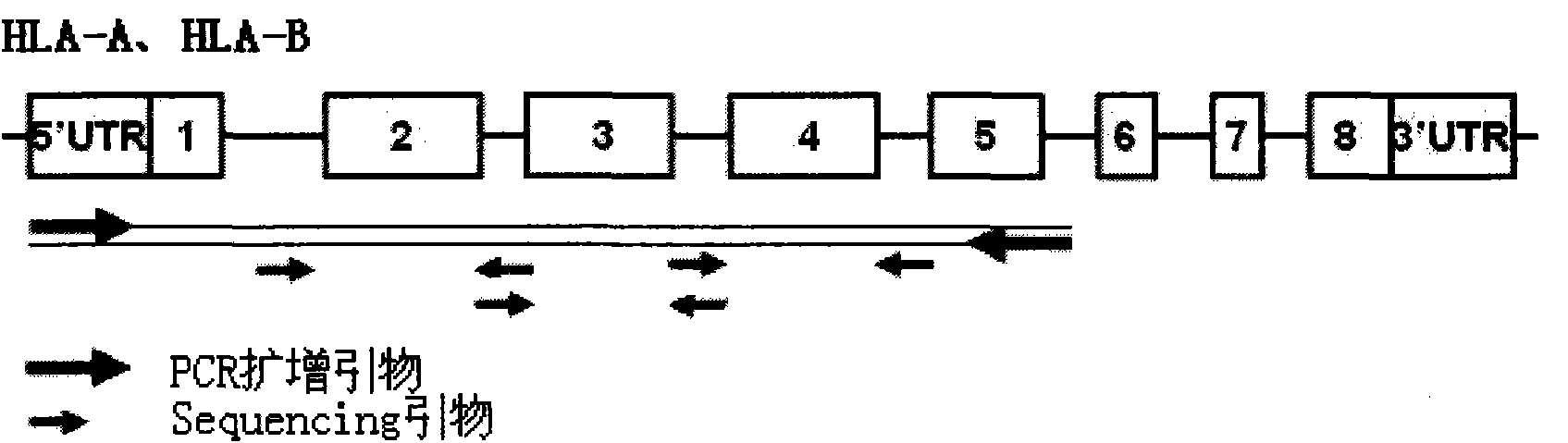
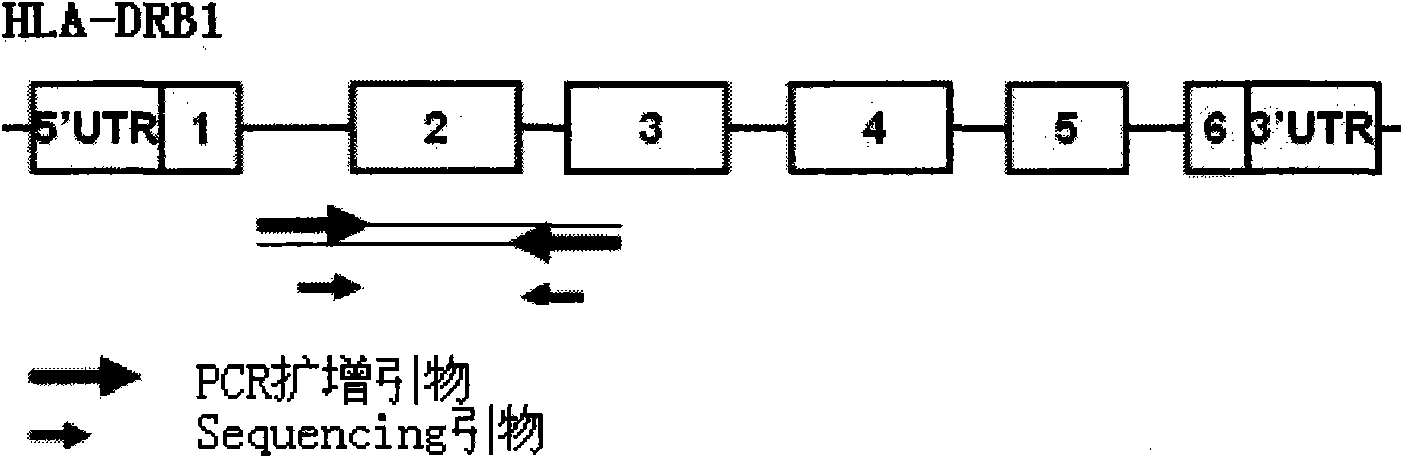
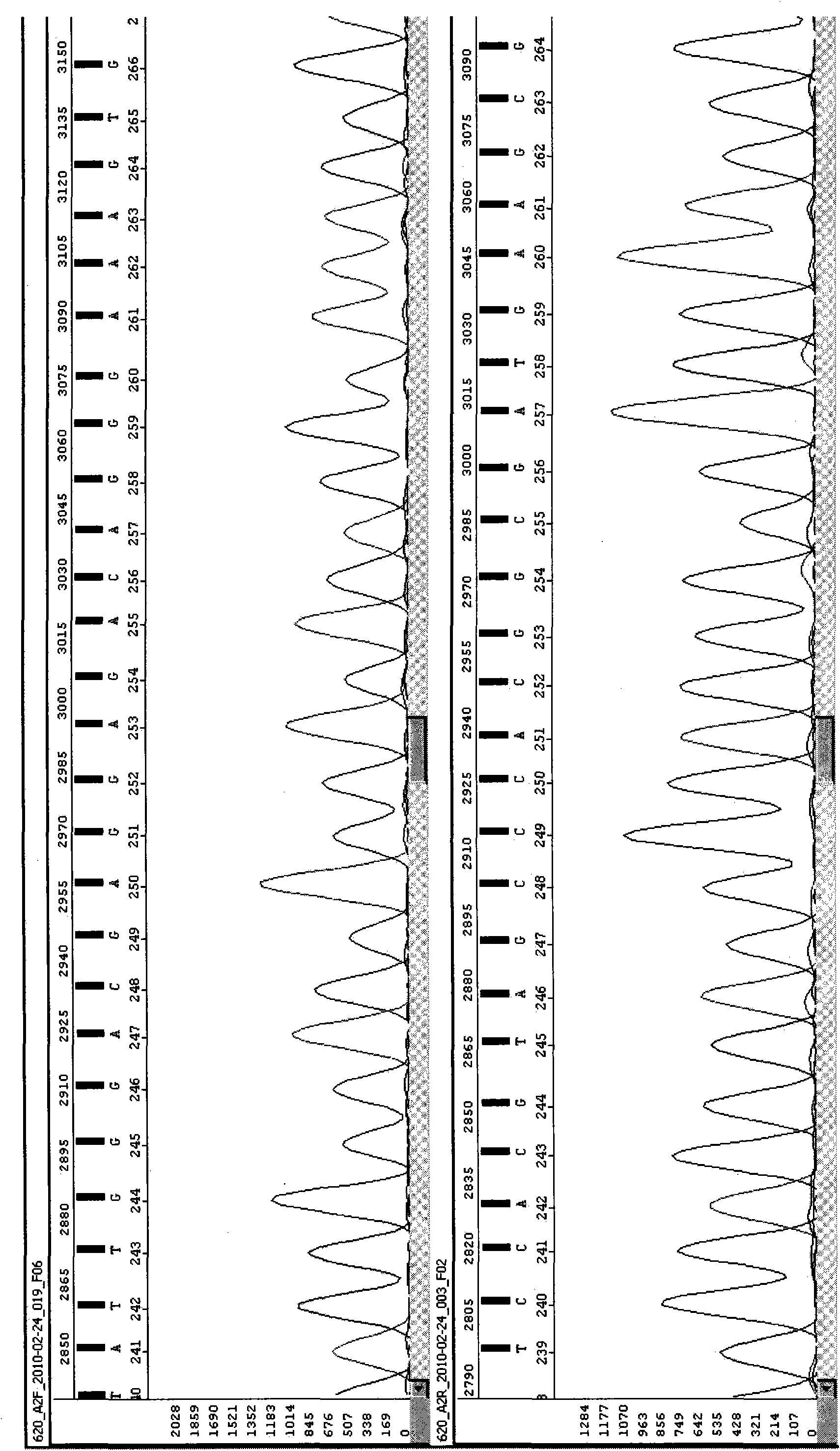
InactiveCN101892317AAvoid problems that cannot be effectively typedHigh resolutionMicrobiological testing/measurementDNA/RNA fragmentationHLA-BExon
The invention discloses a parting method of leucocyte antigen gene of human being, comprising the following steps of: (1) extracting genome DNA to be tested by a regular technology, and amplifying a destination gene fragment to be analyzed by using PCR amplification primer: 2,3,4 exon of HLA-A, 2,3,4 exon of HLA-B and exon on the locus 2 of HLA-DRB; and (2) amplifying the PCR output obtained in the step (1) by using sequencing primer, amplifying the exon, sequencing the amplified exon and comparing the sequencing result with the standard sequence in a database to determine the gene parting result. As the 2,3,4 exon of HLA-A, 2,3,4 exon of HLA-B and exon on the locus 2 of HLA-DRB are effectively amplified as a result of optimized combination of the HLA gene sequencing kit and the test condition, and the corresponding exon is sequenced, the invention solves the problem that effective parting can not be performed when certain allelic gene nucleotide is located outside an amplification area during further parting, thereby improving the parting resolution and accuracy of the HLA gene.
Owner:SUZHOU UNIV +1
Echinococcosis antigen gene (egG1Y162 antigen gene), and recombinant protein and use thereof
InactiveCN101475938AMicrobiological testing/measurementGenetic material ingredientsAntigenNucleotide
The present invention relates to the field of antigen gene technology, specifically to an echinococcosis antigen gene, namely egG1Y162 antigen gene and its recombinant protein and applications, wherein, the gene has a nucleotide sequence with a sequence 1. The invention obtains the echinococcosis antigen gene, namely egG1Y162 antigen gene and its recombinant protein from the granule echinococci, performs cloning, transforming and induce expression to the egg1y162 gene, the animal experiment further indicates that the egg1y162 recombinant protein vaccine has a protective effect on the infection of secondary granule echinococci to mice, becomes a candidate gene vaccine for preventing and controlling echinococcosis, and provides a new way for the development of practical vaccine.
Owner:XINJIANG MEDICAL UNIV
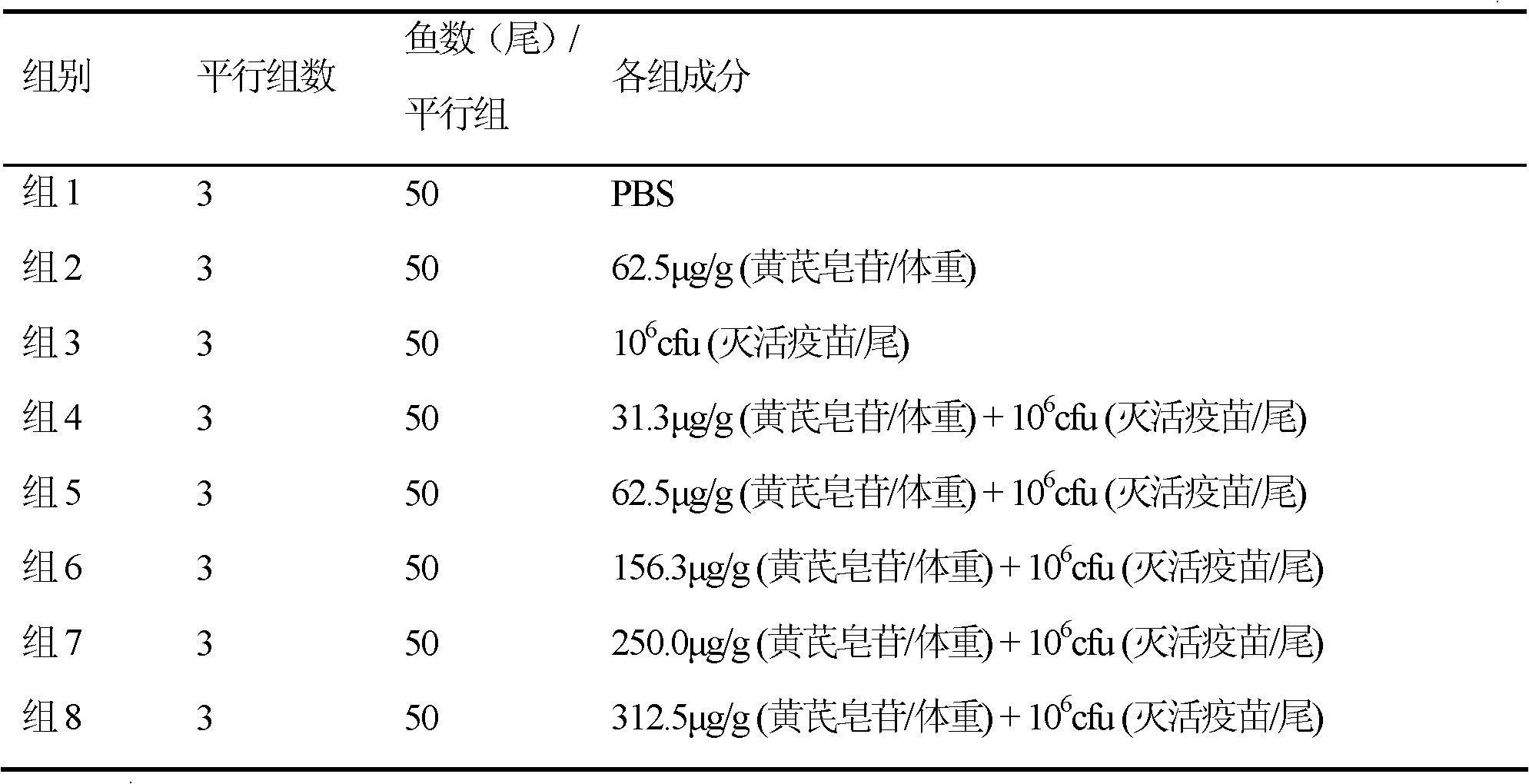
Adjuvant for enhancing fish vaccine immunization effect and application thereof
ActiveCN102430120AImprove featuresImprove protectionImmunological disordersAntibody medical ingredientsProtective antigenAstragaloside
The invention relates to an adjuvant for enhancing a fish vaccine immunization effect and an application thereof. The adjuvant for enhancing the fish vaccine immunization effect is characterized in that an immune potentiator is extracted from Astragalus mongholicus as a leguminous plant or effective components of the Astragalus mongholicus, comprising Astragaloside and Astragalus polysacharin, and natural products or manually modified products or manually synthetic products comprising the Astragaloside and the Astragalus polysacharin can be adopted to serve as the effective components. The adjuvant is capable of increasing the specific immune protection rate after vaccine component immunization is finished. When the adjuvant is applied, a vaccine can comprise the following components: any one or more than one of expression products of inactivated pathogens, bacterial ghost components, hypotoxic pathogens, attenuated pathogens, protective antigens, antigen subunits, antigen determinants or antigen gene expression vectors of bacteria, viruses and parasites. When in use, the adjuvant can be mixed with the components of the vaccine for application, also cannot be mixed with the components of the vaccine for application and also can be applied with the vaccine at different time.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
MRNA vaccine and synthesis method thereof and kit
ActiveCN111821433AIncreased immunogenicityFlexible designSsRNA viruses positive-senseViral antigen ingredientsReceptorGenetic engineering
The invention discloses an mRNA vaccine and a synthesis method and a kit. The mRNA vaccine is prepared from an epitope antigen gene sequence of trimerical spike glycoprotein S, and / or an epitope antigen gene sequence of a transmembrane protein-envelope E, and / or, an epitope antigen gene sequence of membrane glycoprotein M, and / or, an epitope antigen gene sequence of nucleocapsid N, and / or, an epitope antigen gene sequence of a receptor binding domain (RBD) in the trimerical spike glycoprotein. According to the technical scheme of the invention, the mRNA vaccine is designed through a genetic engineering method so as to achieve immunity to the novel coronavirus.
Owner:深圳瑞吉生物科技有限公司
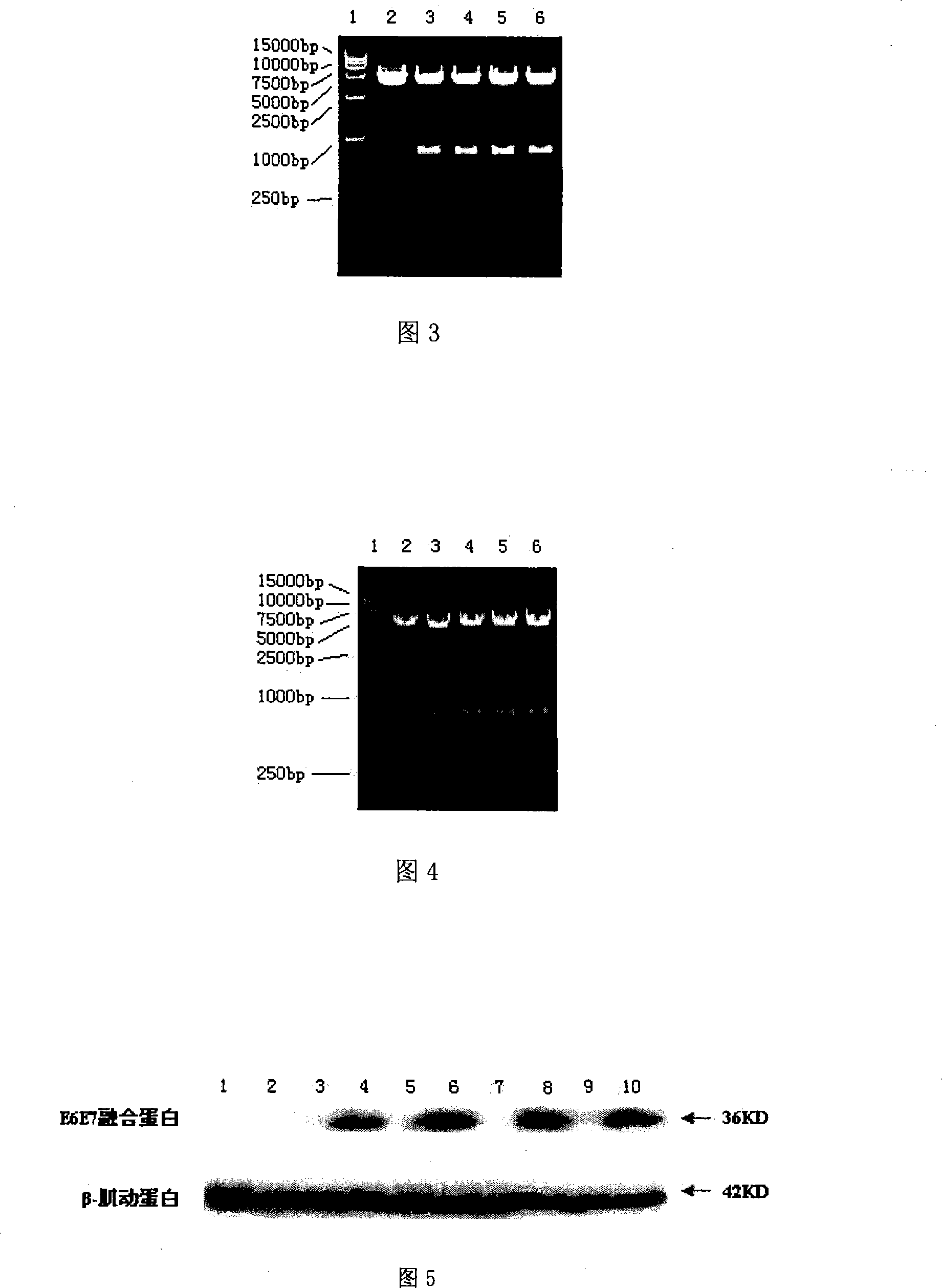
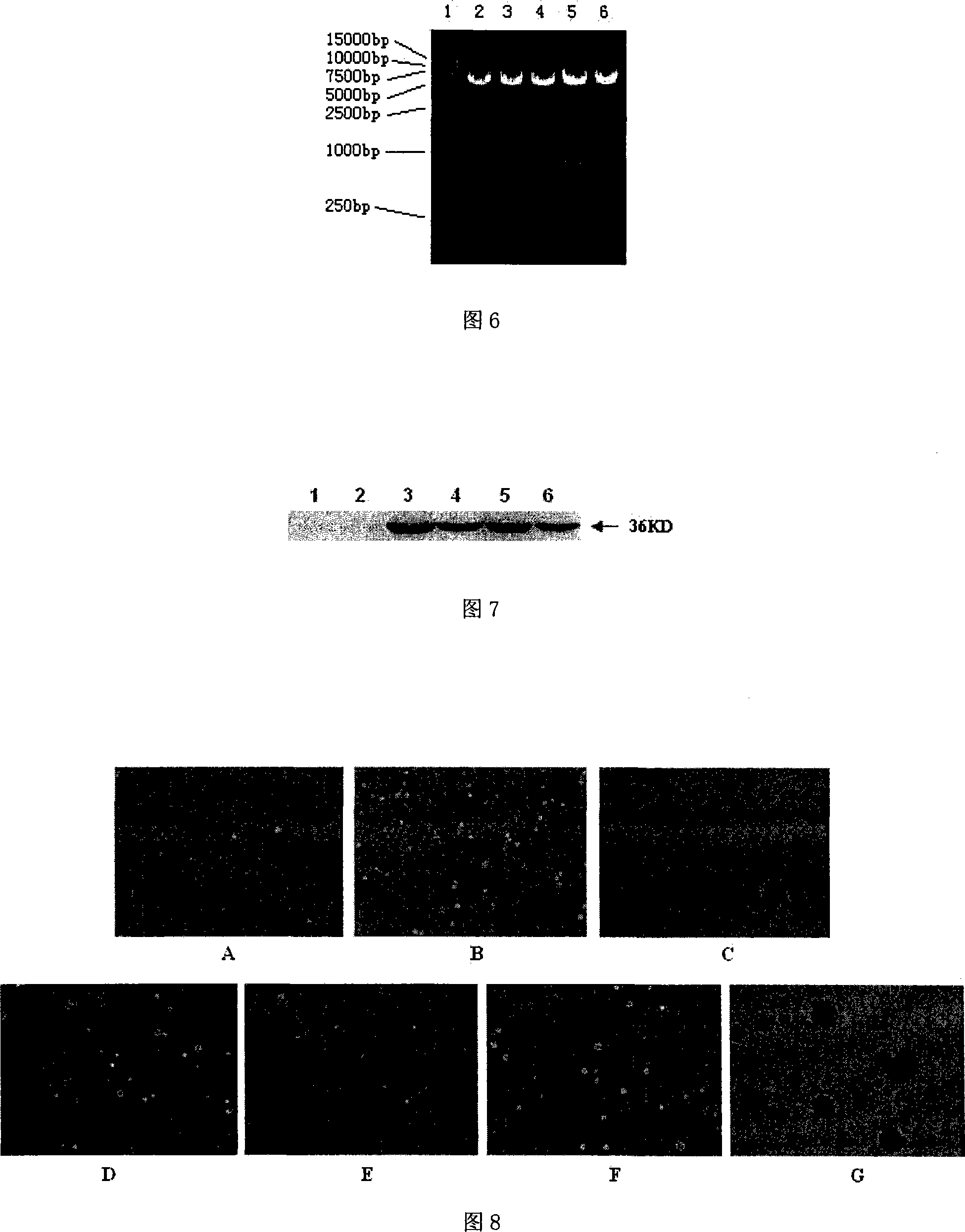
Modified HPV E6-E7 fusion gene and coding protein thereof
A code optimizing HPV16 E6-E7 fused gene, fused gene with carcinogenicity elimination by fixed-point mutagen and it encoded fused protein are disclosed. The modified fused genes are increased in mammal cell expression. It has excellent cell conversion activity and biological safety. It can be used to offer excellent provisional antigen gene for constructing related DNA vaccine.
Owner:曾毅
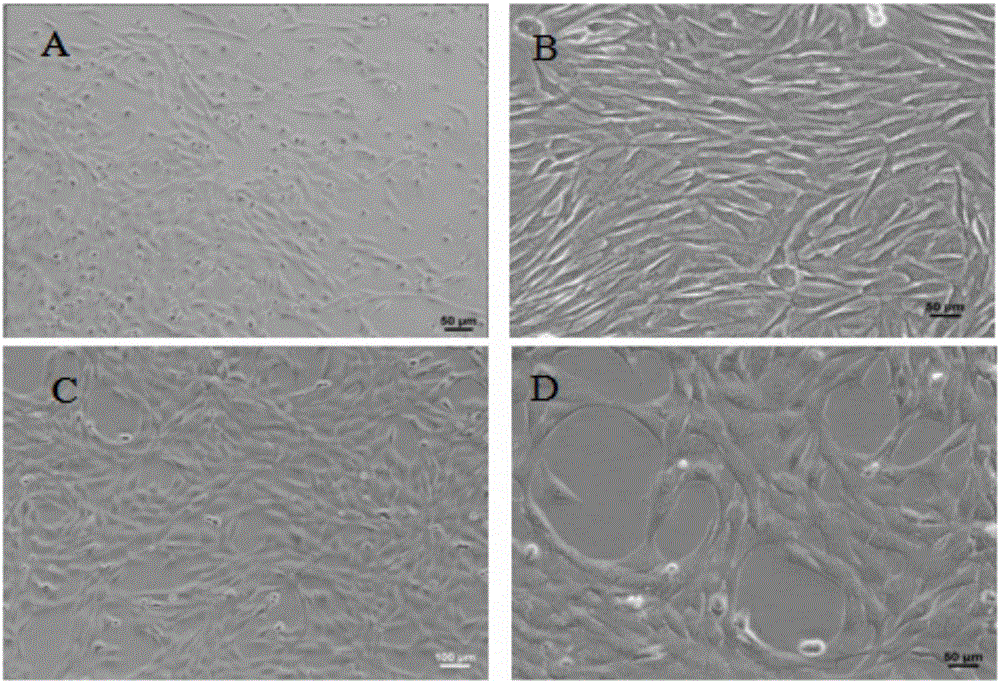
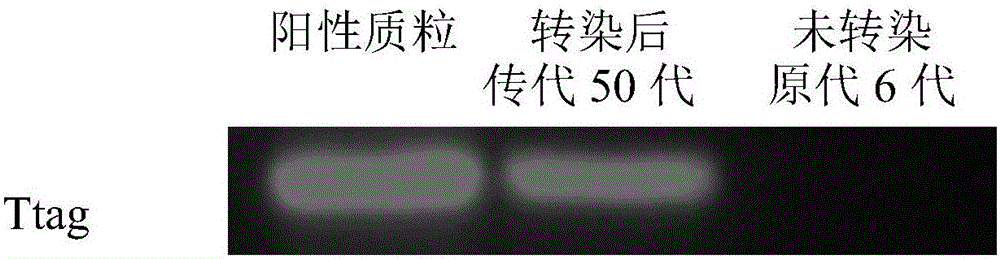
Immortalized canine adipic mesenchymal stem cell line and constructing method thereof
InactiveCN105779395APromote growthMultidirectional differentiation potential performanceMicroorganism based processesSkeletal/connective tissue cellsStem cell lineMesenchymal stem cell
The invention discloses an immortalized canine adipic mesenchymal stem cell line and a constructing method thereof. The immortalized canine adipic mesenchymal stem cell line capable of expressing SV40 large T antigen genes and having multidirectional differentiation is obtained by: transfecting a lentiviral vector carrying SV40 large T antigen (Ttag) by using canine adipic mesenchymal stem cells as host cells, integrating the Tag genes into an adipic mesenchymal stem cell genome and carrying out continuous passage and screening. This cell line is still active in case of 50 in-vitro passages, is high in differentiation and proliferation performance, never ages or dies, can also save medium cost and is an immortalized cell line.
Owner:NORTHWEST A & F UNIV
Establishment of immortal human ovary carcinoma cell strain
InactiveCN1336431AHigh degree of malignancyFused cellsVector-based foreign material introductionMicroorganismBiological property
The present invention relates to the method of establishing human ovary cancer immortal cell strian, the immortal gene-SV40T antigen gene is introduced into human ovary cancer original generation cell, then screened, resistance clone enlarge culture to obtain immortal cell strain. The established ovary cancer cell strain is named as BVPH:OVSC-2 and is kept by CGMCC with keeping No.0606, its biological characteristics includes strong invading powder, cell is spindle sarcoma call shape, apparent heteromorphism, retains biological characters of original cell.
Owner:PEOPLES HOSPITAL PEKING UNIV
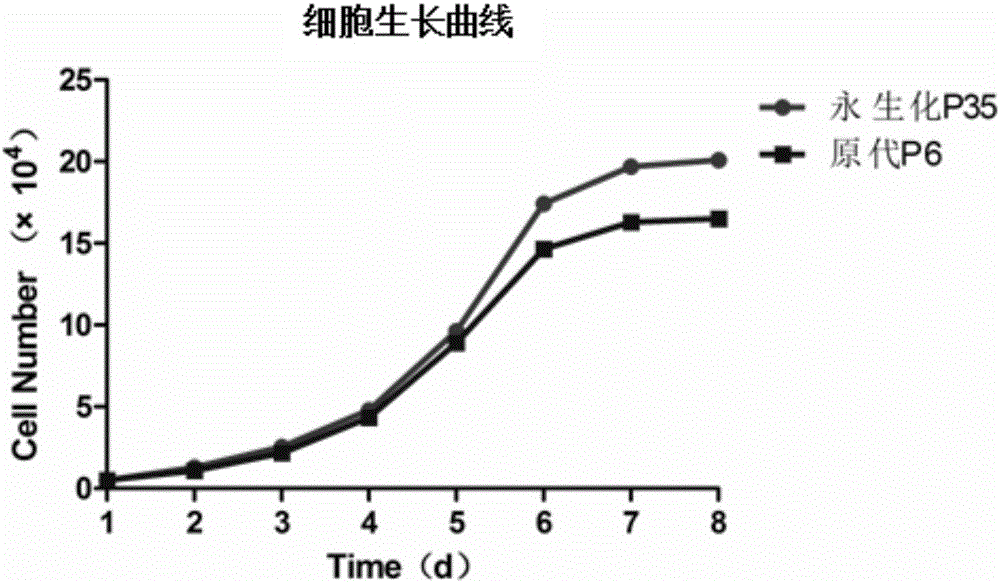
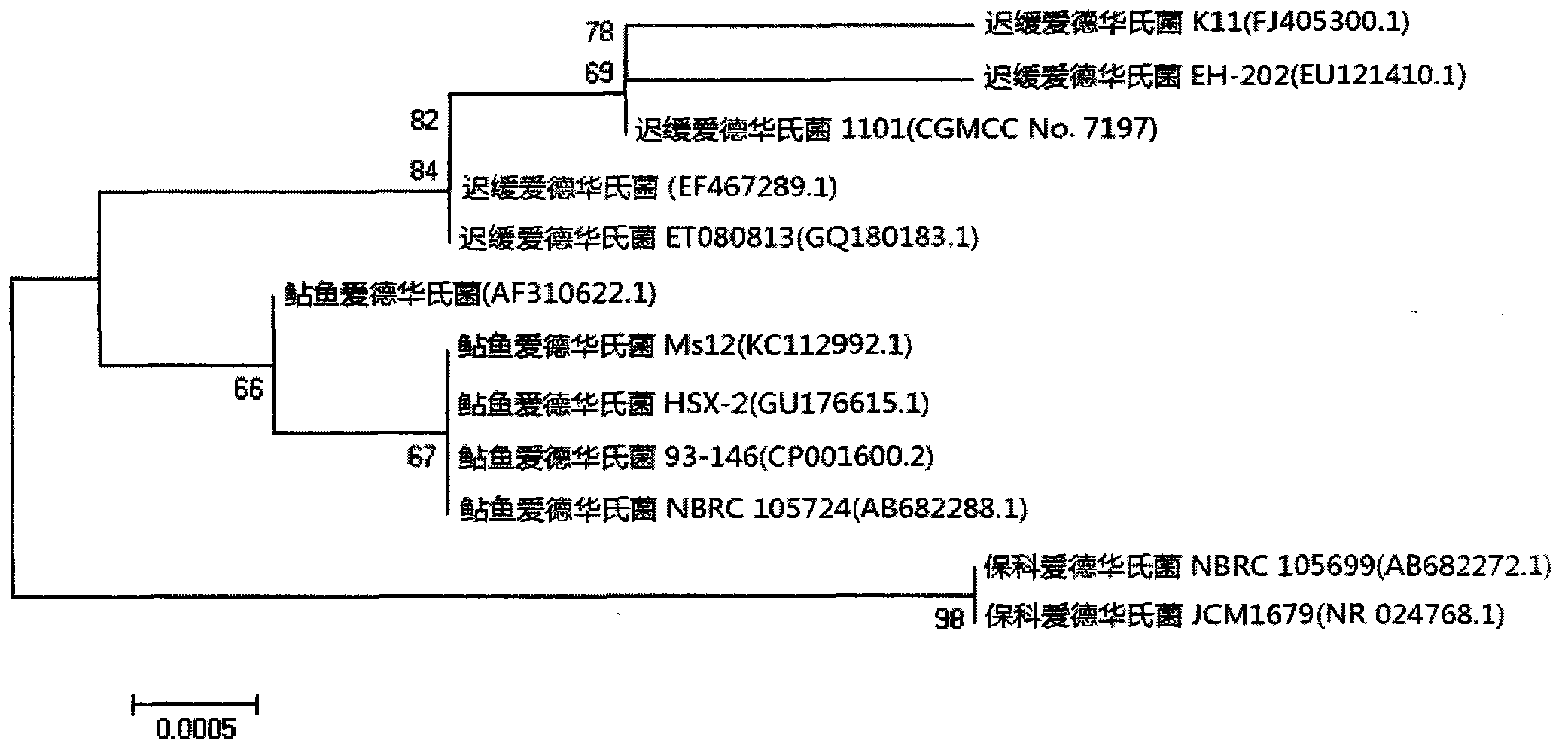
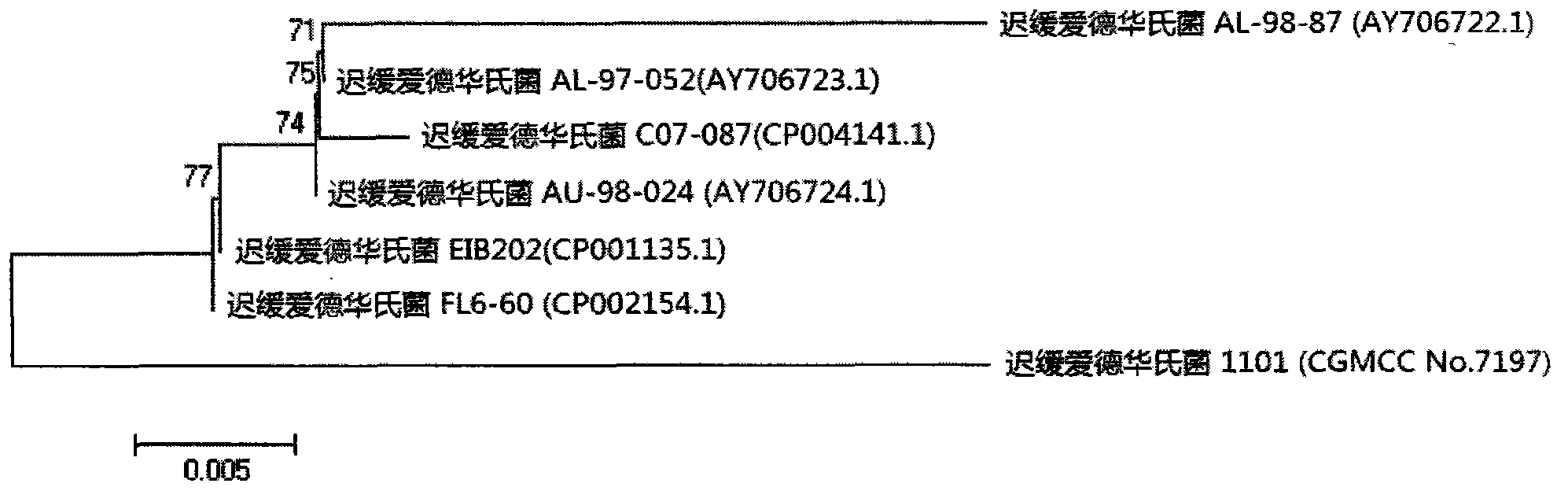
Velogenic Edwardsiella tarda vaccine strain and application thereof
ActiveCN103255089AStrong drug resistanceReduce lossesAntibacterial agentsBacterial antigen ingredientsBacteroidesProtective antigen
The invention relates to an Edwardsiella tarda strain and an application method thereof. The Edwardsiella tarda strain is separated from a turbot adult fish body and is a wild strain with strong virulence, and the preservation number of the Edwardsiella tarda strain is CGMCC No.7197. Preparation modes of an antigen of the Edwardsiella tarda strain comprise any one or more than one of an inactivated thallus, a bacteruak ghost ingredient, an attenuated strain, a protective antigen, an antigen subunit and an expression product of an antigen determinant or an antigen gene expression carrier; the produced vaccine can be a single ingredient of the antigen prepared by utilizing the Edwardsiella tarda strain and can also be a combined vaccine produced by mixing the antigen prepared by utilizing the Edwardsiella tarda strain with antigens of other bacteria, and the prepared single or combined vaccine antigen is added with an adjuvant to produce the vaccine; and an inoculation mode of the vaccine in immunization application can adopt injection immunization, wound immunization, immersion bath immunization or oral administration immunization.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Enzyme-linked immunologic diagnosis kit for core antigen of C type hepatitis virus and method for preparing same
Disclosed are a hepatitis C virus antigen enzyme-linked immunoassay reagent box and a method for making the same. The invention obtains the cell strain of excretive anti HCV core antigen by analyzing the core antigen array of the hepatitis C virus different type and cloning the core antigen gene of HCV, purifying out the high activity monoclonal antibody with four core aa expression sites of HCV, wherein the Cab1 and Cabs are used as coating antibodies, the Cab3 and Cab4 are used as enzyme labeled antibodies; employing double antibodies sandwich technology to prepare HCV-cAg ELISA diagnosing reagent box.
Owner:湖南景达基因有限公司
Recombinant baculovirus with surface displaying porcine epidemic diarrhea virus S protein
InactiveCN106085969AImprove Surface DisplayHigh expressionSsRNA viruses positive-senseViral antigen ingredientsSurface displayViral Vaccine
The invention provides a recombinant baculovirus with the surface displaying a porcine epidemic diarrhea virus S protein, and a preparation method thereof. The virus adopts PEDV spike protein S1 gene as antigen gene, the recombinant virus is constructed by using an insect baculovirus vector expression system, and an S1 protein is successfully expressed and displayed on the surface of the virus. The recombinant virus is used to immunize and inoculate mice as a PEDV pseudo-virus vaccine, and serum neutralization test and lymphocyte propagation experiment analysis shows that the recombinant virus can arouse an effective immune protection effect.
Owner:杭州洪晟生物技术股份有限公司
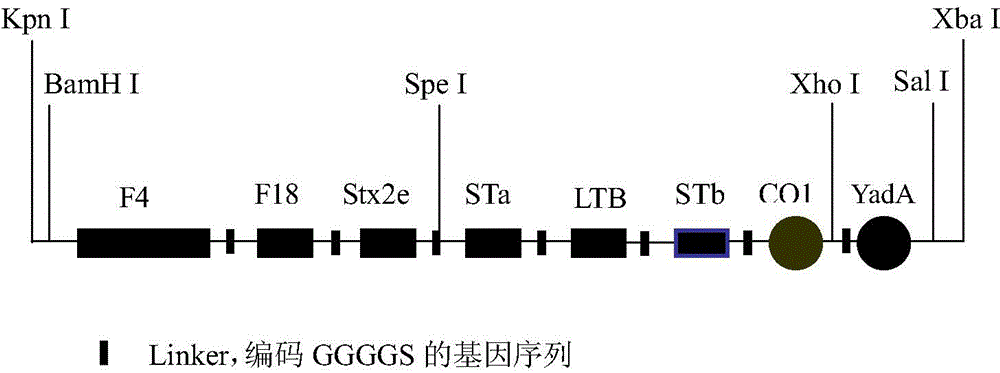
Optimized enterotoxigenic escherichia coli-producing polyvalent antigen gene sequence and application thereof in preventing weaned piglet diarrhea
InactiveCN104593397AFull protective responseHigh expressionAntibacterial agentsBacterial antigen ingredientsEscherichia coliInclusion bodies
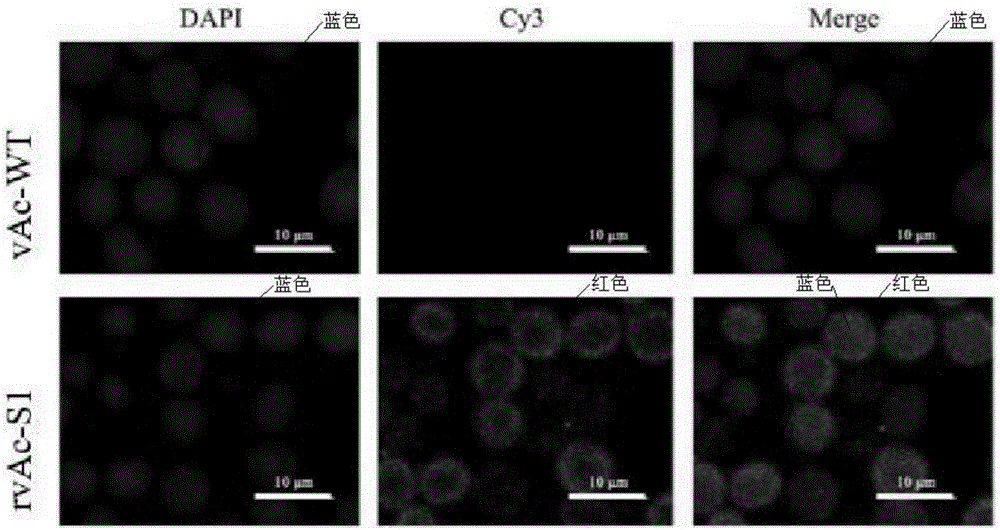
The invention discloses an optimized enterotoxigenic escherichia coli (ETEC)-producing polyvalent antigen gene sequence and the application thereof in preventing weaned piglet diarrhea, and belongs to the technical field of biology. The polyvalent antigen gene sequence is synthesized by a large fragment according to the preference of the lactococcus lactis codon, and the synthesized polyvalent antigen gene sequence contains six antigen genes, namely common ETEC dominant serotype F4<+> primary structure protein FaeG causing the weaned piglet diarrhea, the receptor binding domain RBD of F18<+>, and toxins Stx2e and STa mutant, LTB subunit and STb, and molecular peptides CO1 and YadA31 genes targeting the M cells and the intestinal cells, respectively; the genes are connected by GGGGS. The genes are applicable to constructing a lactic acid bacterium living-vector vaccine and remarkably improving the secretory expression quantity of the target protein, and have excellent immunogenicity and protection effect; the genes also are suitable for high-efficiency expression in the escherichia coli; experiments prove that the inclusion body of the genes has excellent immunocompetence and can be taken as the vaccine for preventing the weaned piglets from F4<+> and F18<+> ETEC infection.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
Vibrio cholerae typing and virulence gene detection kit and detection method
ActiveCN101967516ADetection helpsStrong specificityMicrobiological testing/measurementFluorescence/phosphorescenceVirulent characteristicsEnzyme system
The invention relates to a vibrio cholerae typing and virulence gene detection kit and a vibrio cholerae typing and virulence gene detection method. The kit comprises DNA extracting solution, PCR reaction solution, a Taq enzyme system, a positive quality control product and a negative quality control product, wherein the PCR reaction solution consists of PCR reaction buffer, four pairs of forward and reverse primers for the specificity of vibrio cholerae and four probes for the specificity of the vibrio cholerae; the primers and probes are designed according to the specificity conservative areas of nucleic acid sequences of a hemolysin gene, an O-antigen gene of O139 type vibrio cholerae, the O-antigen gene of O1 type vibrio cholerae and the virulence gene of cholera toxin (CTX). The detection kit and the detection method ensure a reliable and stable result, are easy and fast to operate and can realize the typing of the vibrio cholerae and fast judgment about whether the vibrio cholerae has a virulence gene or not, thereby facilitating the tracing and detection of the public health-related events caused by the vibrio cholerae and facilitating the routine monitoring of the vibrio cholerae.
Owner:DAAN GENE CO LTD +1
Raccoon Poxvirus Expressing Genes of Feline Antigens
InactiveUS20080299149A1Avoiding adjuvant-related sarcoma side effectBroad protectionSsRNA viruses positive-senseViral antigen ingredientsHemagglutininAnimals vaccines
The present invention relates to new recombinant raccoon poxvirus vectors comprising two or more exogenous nucleic acid molecules, each encoding at least one feline protein, wherein at least two of the nucleic acid molecules are inserted into the hemagglutinin (ha) locus or the thymidine kinase (tk) locus, or at least one of the nucleic acid molecules is inserted into each of the hemagglutinin and thymidine kinase loci. Described herein are monovalent and polyvalent recombinant feline vaccines that encompass an immunologically effective amount of the recombinant raccoon poxvirus vectors and, optionally, a suitable carrier or diluent. The vaccine of this invention optionally includes additional feline antigens to provide broad spectrum protection to cats against a variety of feline pathogens. The invention further concerns the method for inducing a protective immune response to the feline pathogens in a cat by administering the recombinant vaccines.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
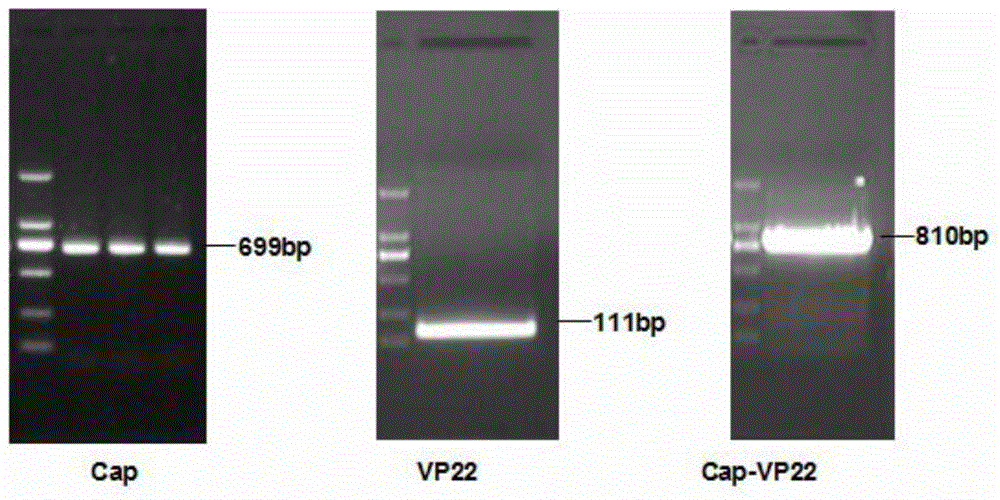
Recombinant porcine circovirus type 2 virus-like particle, and preparation method and application thereof
InactiveCN103555746AImproving immunogenicityStimulationViral antigen ingredientsGenetic material ingredientsAdjuvantPorcine circovirus
The invention discloses a recombinant porcine circovirus type 2 (PCV2) virus-like particle (VLP). The VLP is prepared by connecting a protein transduction domain originated from HSV-1VP22 with an antigen Cap gene needed for formation of PCV2 hollow capsid protein by using an SOE-PCR method, then carrying out cloning to a baculovirus transporter so as to obtain a homologous recombinant vector, carrying out encapsulation so as to produce recombinant baculovirus containing PCV2Cap protein and the protein transduction domain of HSV-1VP22, infecting an insect cell and expressing recombinant Cap-VP22 protein in which PCV2Cap protein and the protein transduction domain of HSV-1VP22 are fused. According to results of research, the VLP prepared in the invention can be individually used or used with adjuvant for inoculation to an animal and effectively stimulates generation of a specific antibody to PCV2. Thus, the VLP provided by the invention can be applied to development of a novel high-efficiency PCV2 subunit vaccine with a good immune effect.
Owner:CHANGCHUN SR BIOLOGICAL TECH +1
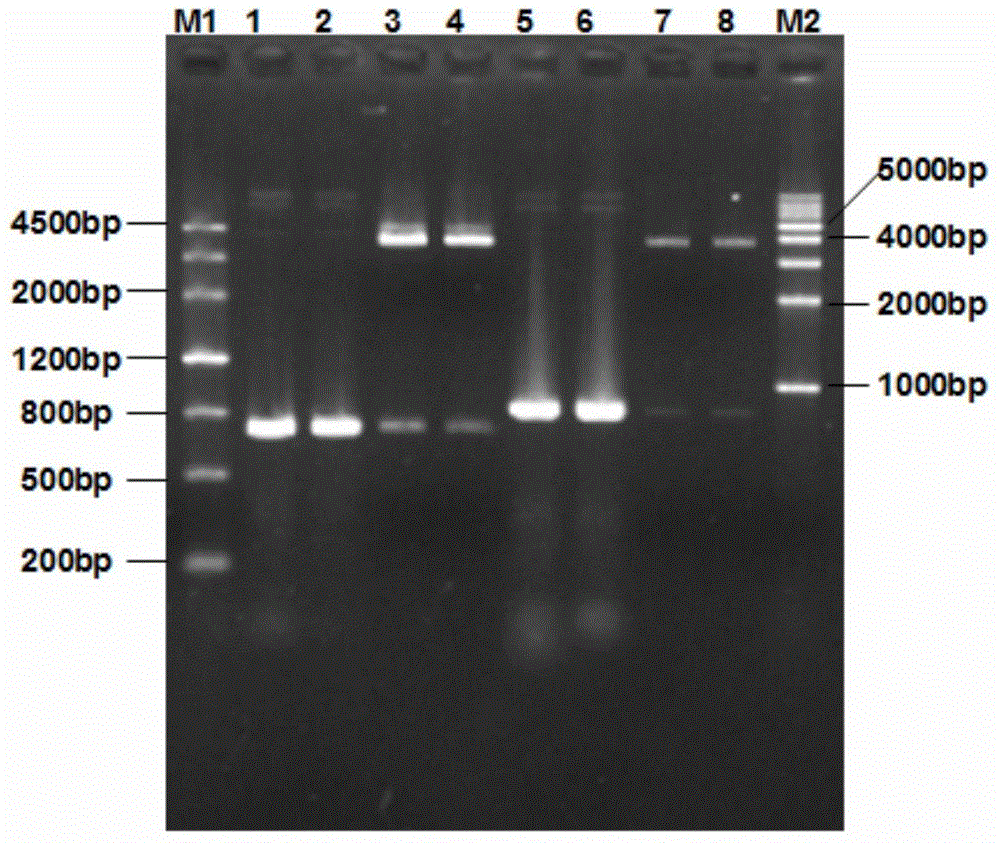
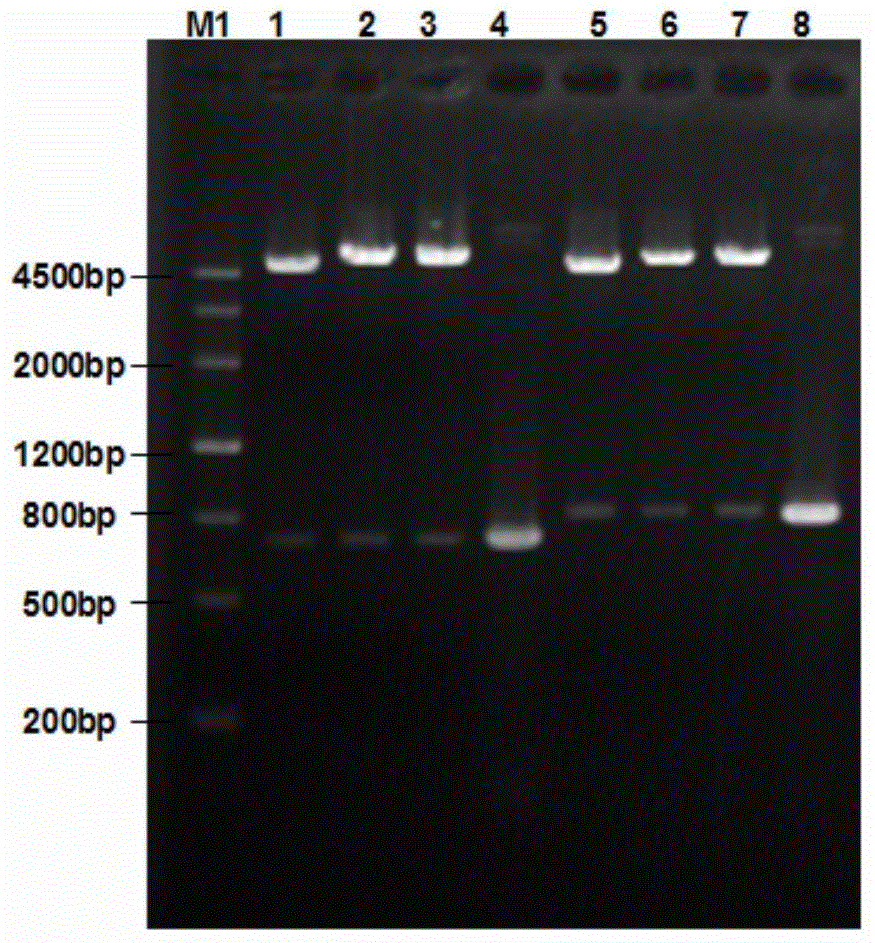
Recombinant Salmonella choleraesuis for expressing surface antigen gene sao of streptococcus suis type 2, vaccine and application
ActiveCN101979501AGood immune protectionPreserve immune efficiencyAntibacterial agentsBacterial antigen ingredientsBacteroidesAntigen
The invention belongs to the field of animal bacterium gene engineering, and in particular relates to construction of resistance marker-free recombinant Salmonella choleraesuis for expressing surface antigen sao gene segment of streptococcus suis type 2, preparation of a vaccine and application. The resistance marker-free recombinant Salmonella choleraesuis for expressing the surface antigen sao gene segment of the streptococcus suis type 2, namely asd-C500 / Pya-saoA is obtained, and the collection number is CCTCC NO: M2010156. The asd gene of the Salmonella choleraesuis is deleted in the recombinant strain, and the recombinant strain contains plasmid pYA-saoA capable of expressing the asd and the sao gene segment of the streptococcus suis type 2. The invention also discloses a method for preparing the recombinant strain and the vaccine, and application in preparing Salmonella choleraesuis-streptococcus suis type 2 vaccines.
Owner:HUAZHONG AGRI UNIV +1
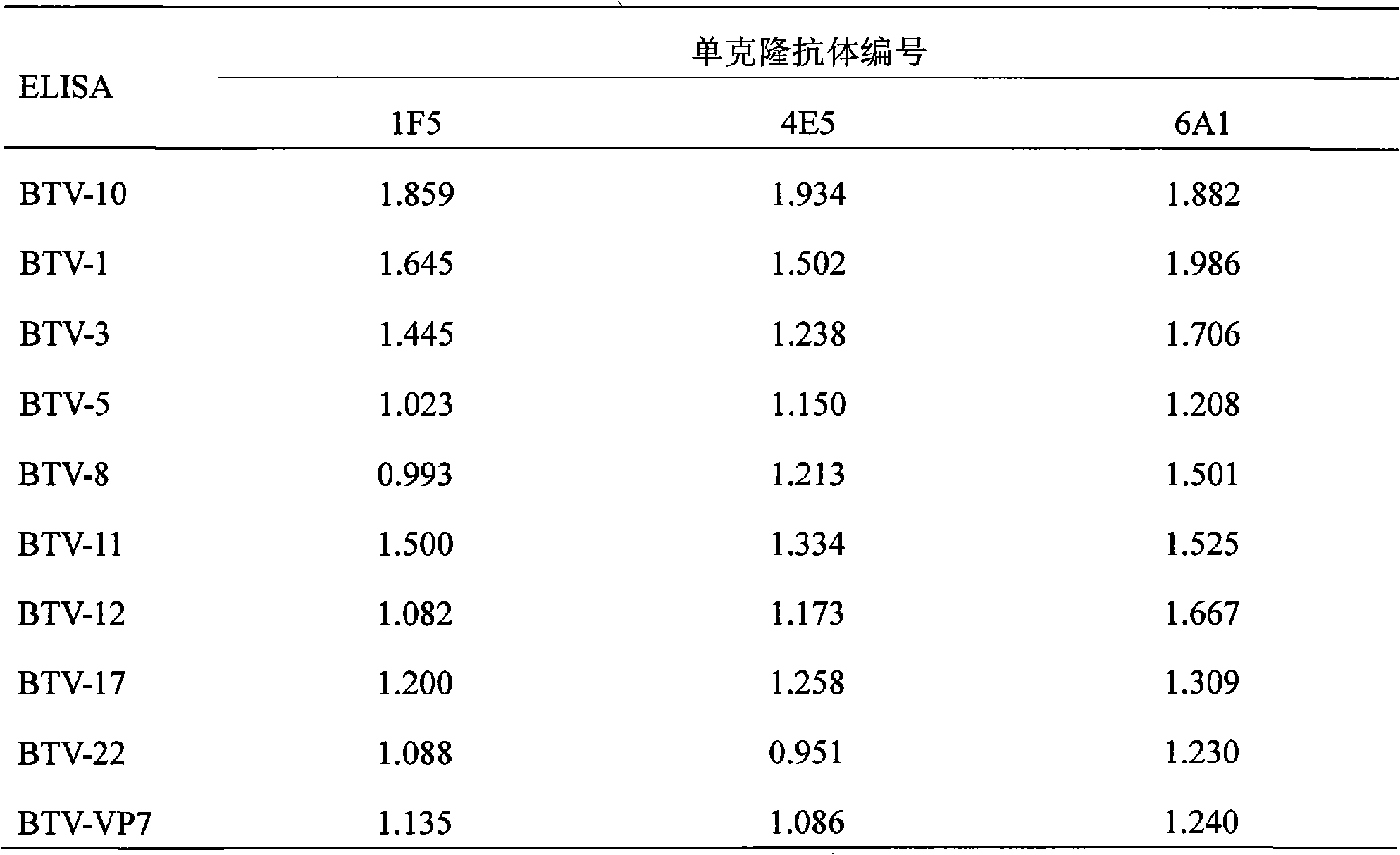
Monoclonal antibody of bluetongue virus (BTV) and preparation method and application thereof
InactiveCN101597334ASame structureUniform compositionImmunoglobulins against virusesFermentationBALB/cHamster
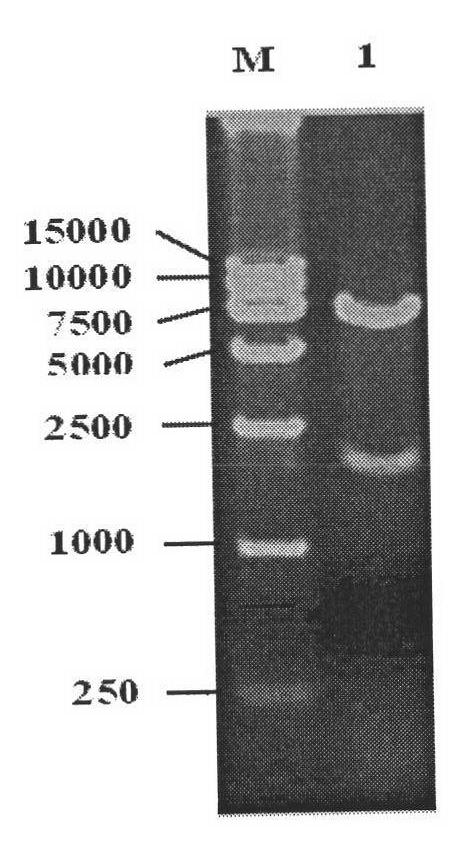
The invention relates to the biotechnology field. A monoclonal antibody against a BTV VP7 protein of a bluetongue virus (BTV) of the invention is prepared by the following steps: adopting the hybridoma cell technology, taking splenocytes of BALB / c mice immunized with purified BTV for fusion with a mouse myeloma cell (SP2 / 0), after culturing the cells with an HAT selective medium, carrying out screening with indirect ELISA coated by a purified BTV antigen, a BTV VP7 protein antigen expressed by a gene engineering and a control antigen of a normal hamster kidney continuous cell (BHK21), carrying out screening and cloning by limiting dilution to obtain a hybridoma cell line which has the capacities of stable continuous culture and secretion of the monoclonal antibody (McAb) against the specific BTV VP7 protein and preparing McAb mouse ascites of the BTV VP7 protein. The monoclonal antibody against the BTV VP7 protein features strong specificity, high ascites titer, high affinity and simple preparation method, can be used in the detection method of the BTV antibody and antigen and provides an important technical means for prevention and control of bluetongue in China.
Owner:花群义
Toxophasma gondii detecting kit based on recombined antigen
InactiveCN1861633AImprove immune activityExcellent repeatabilityBiological testingAnimals/human peptidesSephadexEscherichia coli
A reagent kit based on recombinant antigen for detecting Toxoplasma is prepared through taking 542-1218 fragment (t SAG1) from the primary surface antigen gene SAG1 of Toxoplasma, subcloning it to soluble expression carrier pET32a(+), transferring it to colibacillus, configuring engineering bacterium pET32a-tSAG1 / BL21, IPTG induced efficient expression, ultrasonic splitting to obtain supernatant, purifying by Ni-NTA and Sephadex-G75, and coating the microholes on ELISA plate. Its test paper can also be prepared by same way.
Owner:深圳市绿诗源生物技术有限公司
Immortalized dairy cow rumen endothelial cell line and construction method thereof
InactiveCN107881197ALow costProliferate fastGastrointestinal cellsGenetically modified cellsRumenSV40 T-antigen
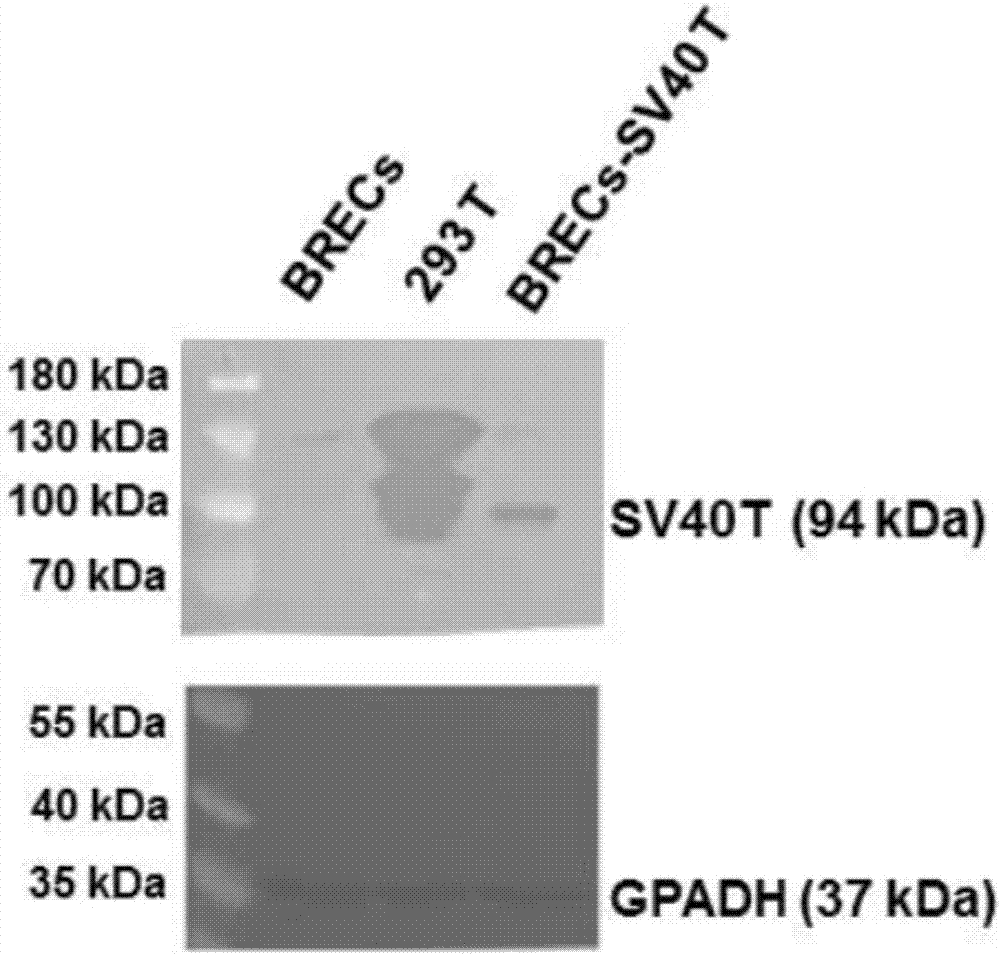
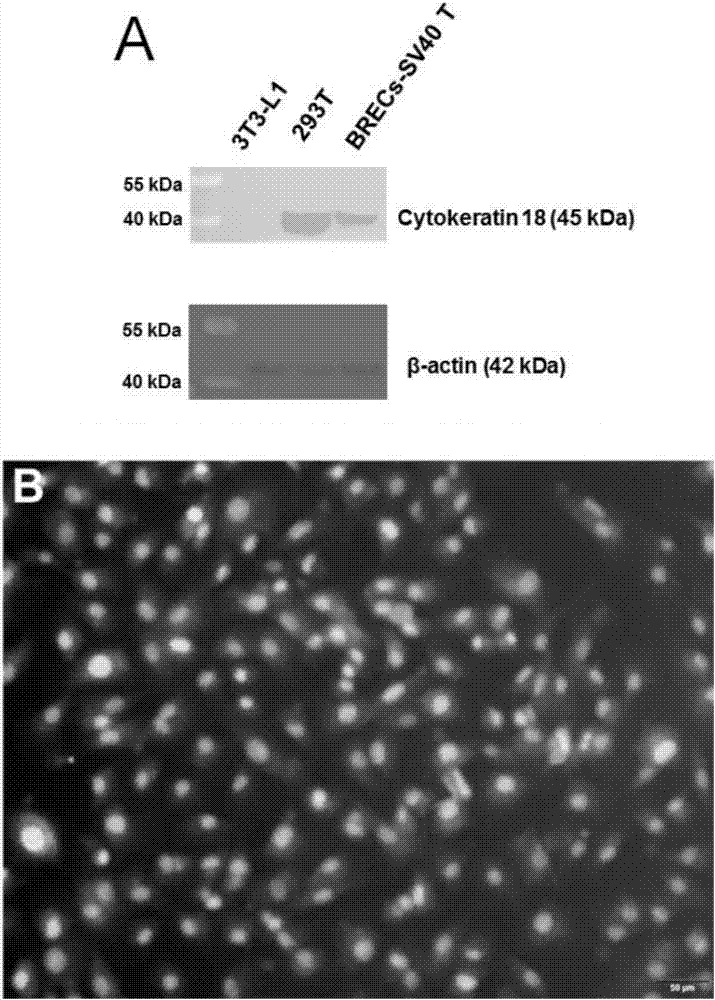
The invention provides an immortalized dairy cow rumen endothelial cell line and a construction method thereof. The construction method comprises the following steps: (a) cutting, washing, and digesting collected dairy cow rumen endothelial tissues in a culture medium, and carrying out primary culture to obtain rumen endothelial cells; step (b) incubating rumen endothelial cells obtained in the step (a) with a viral liquid containing an SV40T antigen gene to obtain infected rumen endothelial cells; and step (c) culturing the infected rumen endothelial cells obtained in the step (b) to obtain the immortalized dairy cow rumen endothelial cell line. The provided immortalized dairy cow rumen endothelial cell line can provide a test cell model for the research on physiological regulation and nutrient absorption mechanism of dairy cow rumen. The culture method is simple, the growth speed is quick, and the construction method can obtain BRECs, which have physiological functions and can carryout continuous passage.
Owner:YANGZHOU UNIV
Hepatitis B nucleic acid vaccine with optimized codon
InactiveCN101502650AHigh protein expressionImprove responseDigestive systemAntiviralsMammalDigestion
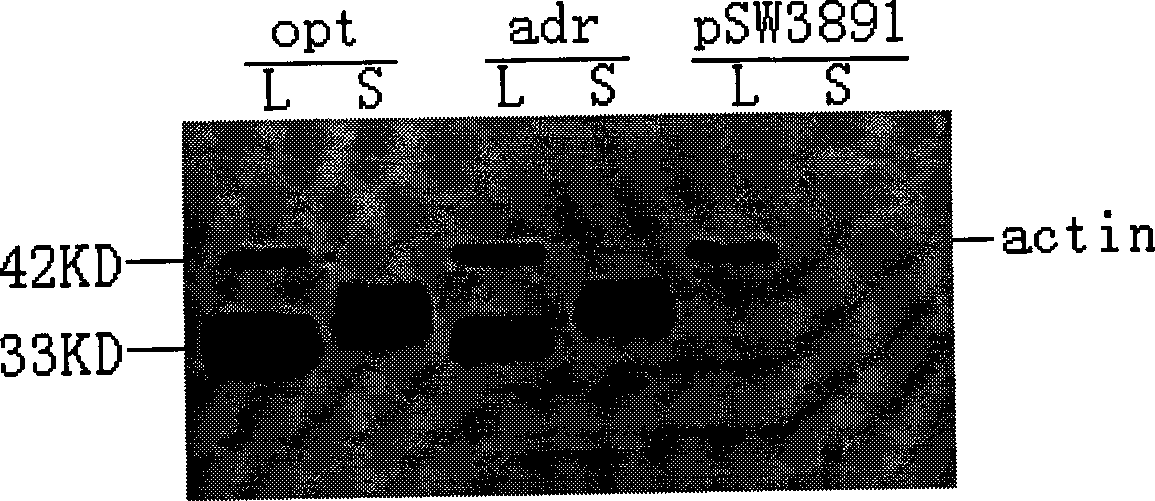
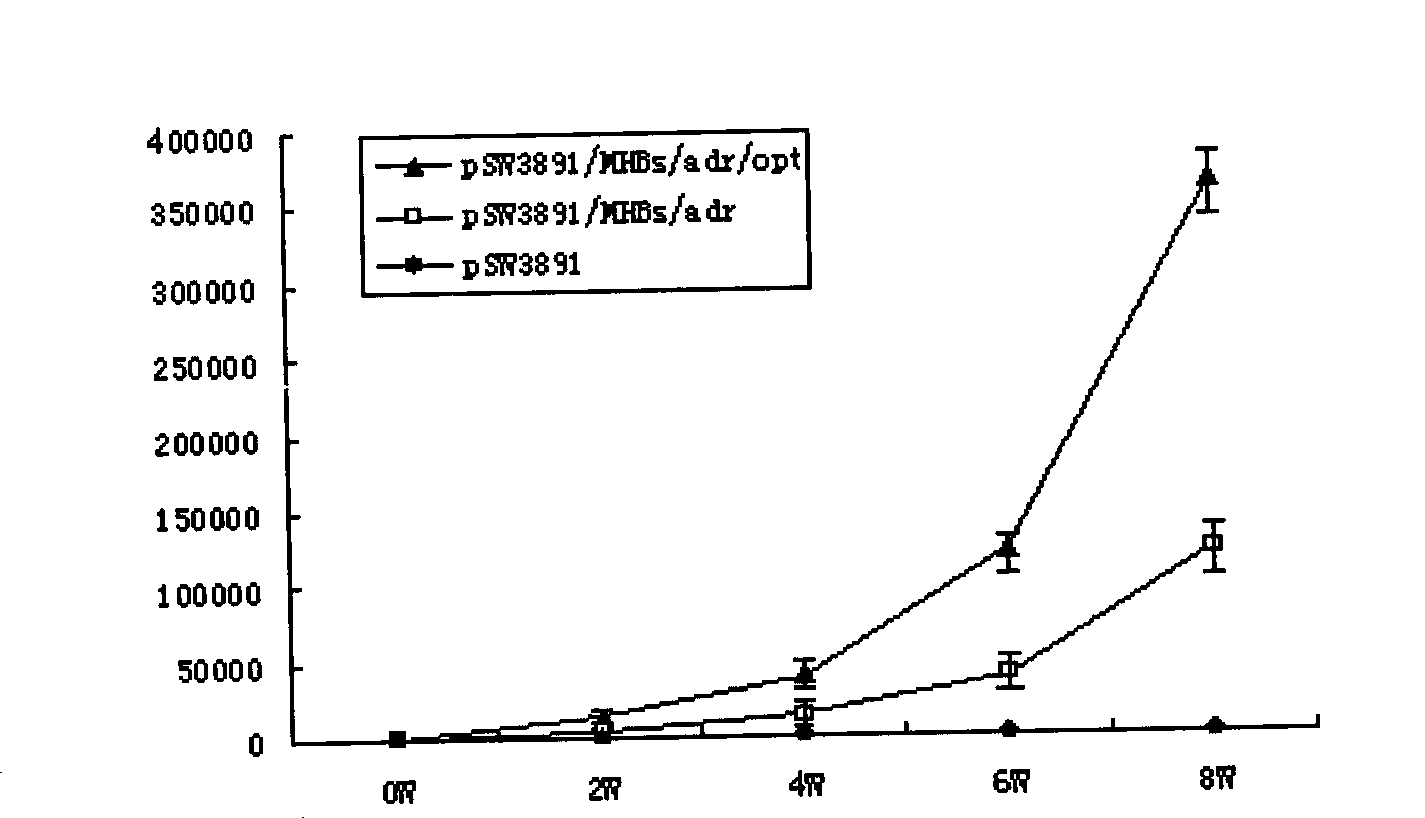
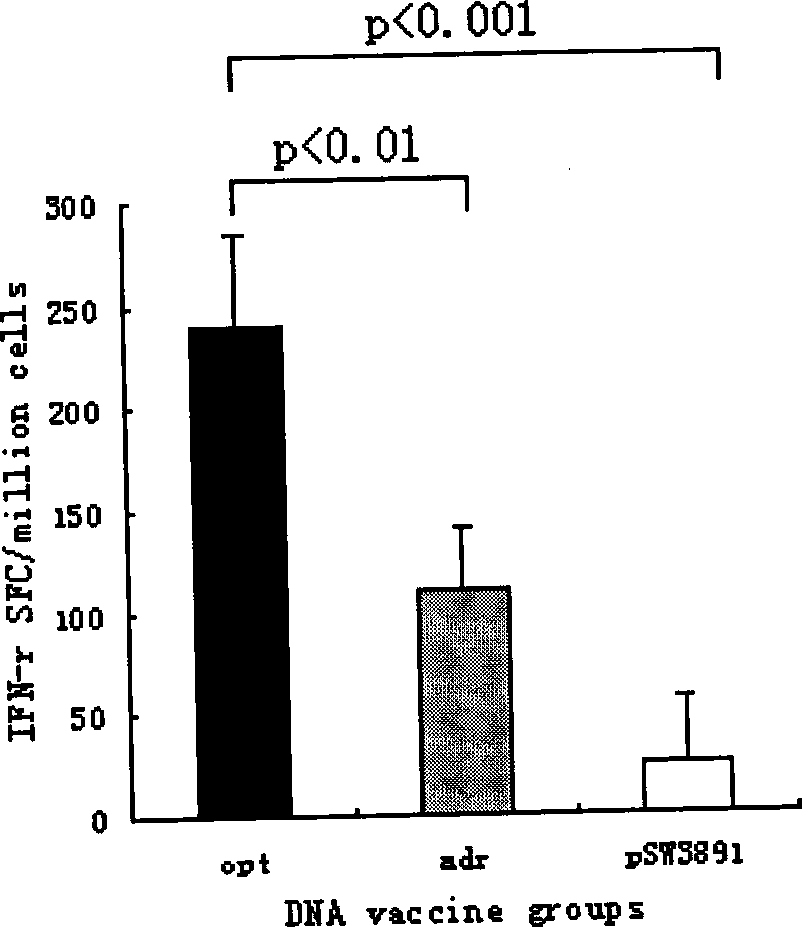
The invention relates to a hepatitis B virus nucleic acid vaccine optimized by codon. In the invention, hepatitis B surface antigen (HBs) gene order (adr subtype) is analyzed to find codon locus which tells the differences between the codon preferences of the gene order and the codon preferences of the mammal; the codon of the HBs gene order is replaced to obtain the surface antigen gene; the gene order is combined and expanded to obtain MHBs, Pst I, BamH I, double digestion MHBs gene and carrier pSW3891 plasmid optimized by the codon; 10ul connection system is configured to obtain a middle protein gene. The nucleic acid vaccine of the invention overcomes the defects that the differences between prokaryote and eukaryote in terms of codon preferences cause that the foreign gene can not be expressed effectively in mammal reservoir and can not generate relatively good immune sheltering effect; in addition, the invention remarkably improve protein expression of the foreign gene in the mammal reservoir, effectively stimulates immune system of the reservoir to generate relatively good immunological reaction of human body fluids and cellular immune response.
Owner:邢益平 +1
Cryptosporidium parvum divalent protein vaccine and preparation method thereof
InactiveCN101658667AGood immune protectionStrong immune responseAntiparasitic agentsAntibody medical ingredientsProtective antigenDisease
The invention provides a cryptosporidium parvum divalent protein vaccine, which is characterized in that prokaryotic expression vector pET28a is taken as a carrier, and two cryptosporidium parvum protective antigen genes are inserted in series to the multiple cloning sites of the prokaryotic expression pET28a, thus obtaining the cryptosporidium parvum divalent protein vaccine. The vaccine selectstwo advantageous protective antigens, combines the advantages of the two antigens, and can induce stronger functions of cellular immunity and humoral immunity, thus achieving the purposes of preventing cryptosporidium parvum disease of animal better.
Owner:JILIN UNIV
Recombinant porcine pseudorabies virus for expressing GP protein of porcine reproductive and respiratory syndrome virus, and application
ActiveCN110628730AInviolableStrong targetingSsRNA viruses positive-senseViral antigen ingredientsAntigenVirulent characteristics
The invention provides a recombinant porcine pseudorabies virus for expressing GP protein of a porcine reproductive and respiratory syndrome virus, and an application. A PRV virus strain genome is quickly edited through a Crispr / Cas9 gene editing technique and a Cre / lox recombination system, virulence genes gE, gI and TK of the PRV virus strain genome are subjected to fixedpoint deletion, and an antigenic gene of a NADC30-like strain is subjected to fixedpoint insertion at a gG position. According to the recombinant porcine pseudorabies virus for expressing GP protein of a porcine reproductiveand respiratory syndrome virus disclosed by the invention, non-transmembrane regional coding sequences of GP3 protein, GP4 protein, GP5 protein and GP6 protein of an epidemic PRRSV strain PRRSV NADC30-like are selected as antigenic genes for the first time, the kinds of antigens are more comprehensive, and the antigenic genes have higher applicability on current PRRSV epidemic situations, and arebetter in immunoprotection effects. A live vaccine provided by the invention can protect target animals from being invaded by PRV and PRRSV in a more pointed manner, and a powerful tool is provided for preventing and controlling epidemic situations of porcine pseudorabies and porcine reproductive and respiratory syndromes in China.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com