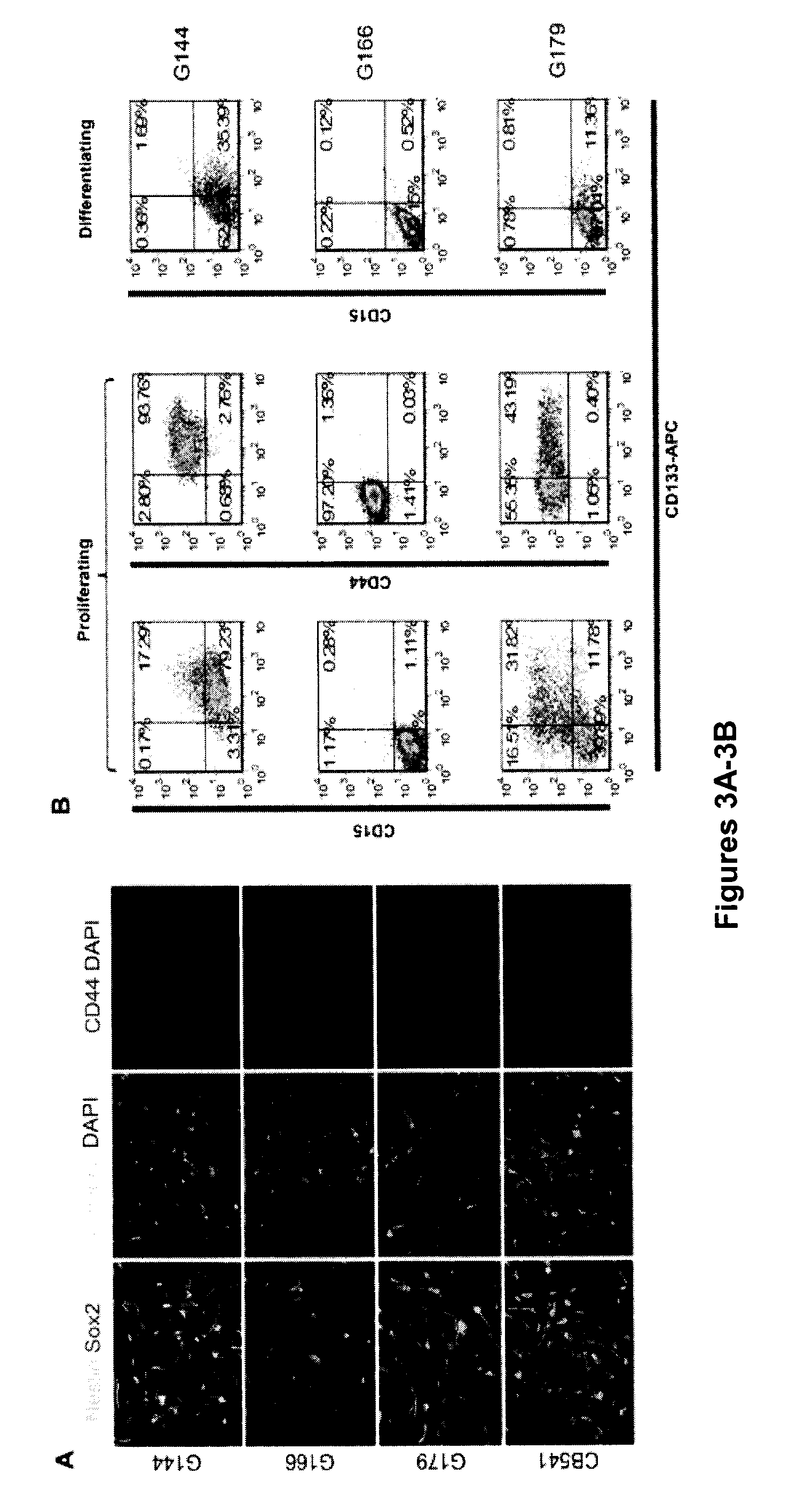
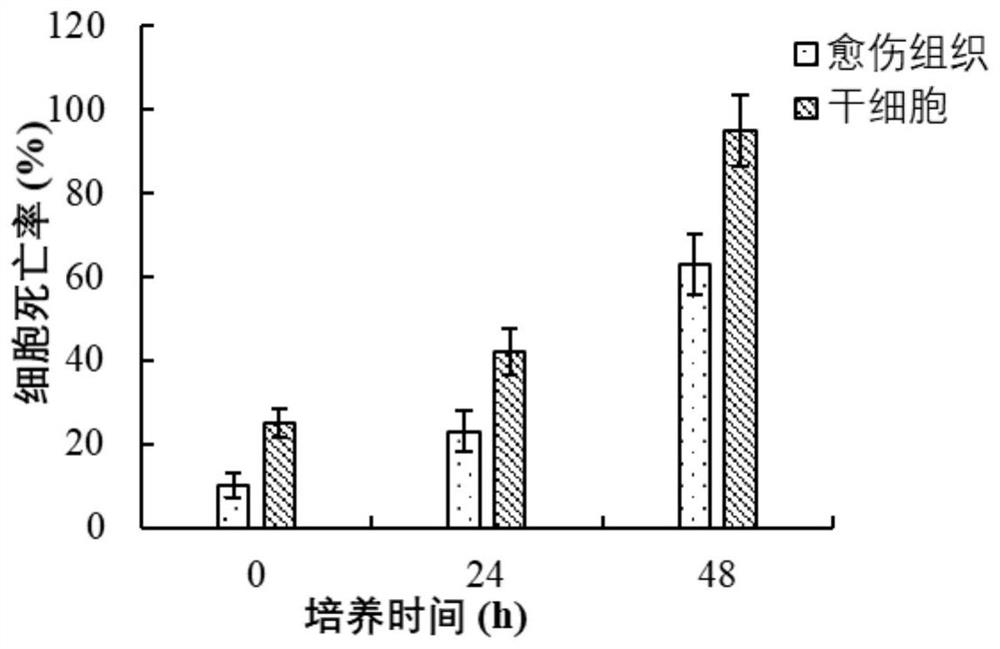
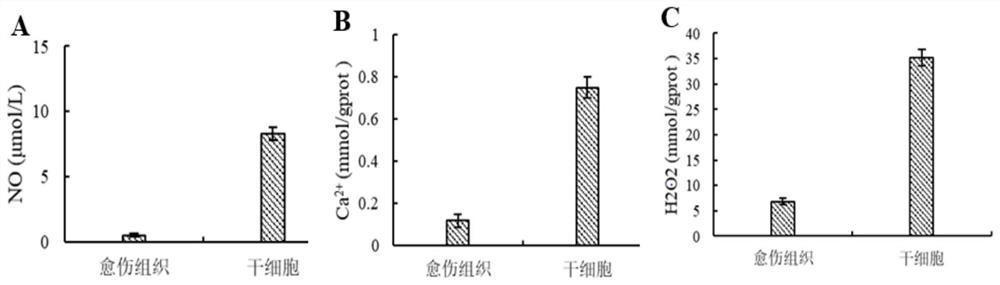
Patents
Literature
141 results about "Stem cell line" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A stem cell line is a group of stem cells that is cultured in vitro and can be propagated indefinitely. Stem cell lines are derived from either animal or human tissues and come from one of three sources: embryonic stem cells, adult stem cells, or induced stem cells. They are commonly used in research and regenerative medicine.
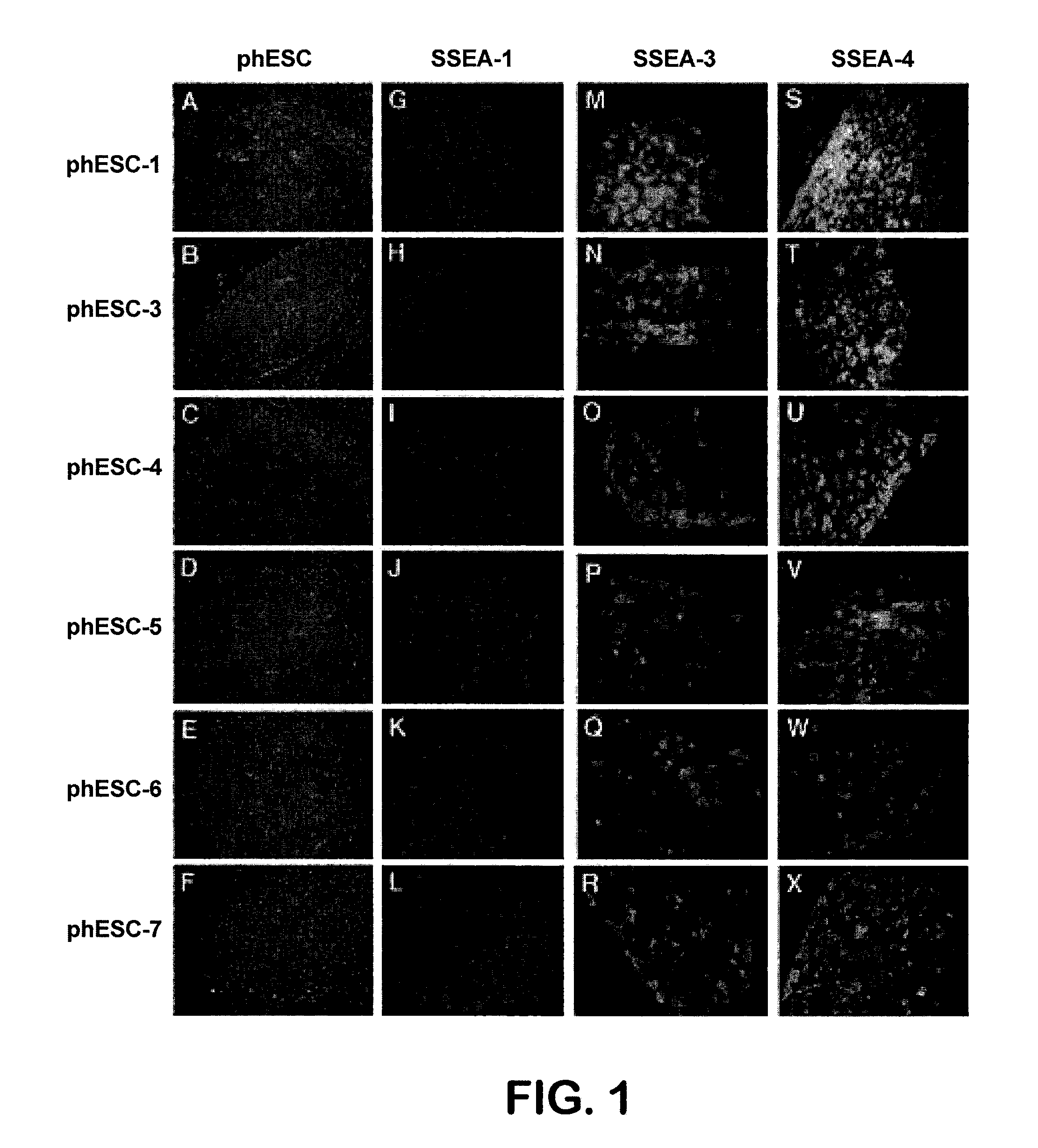
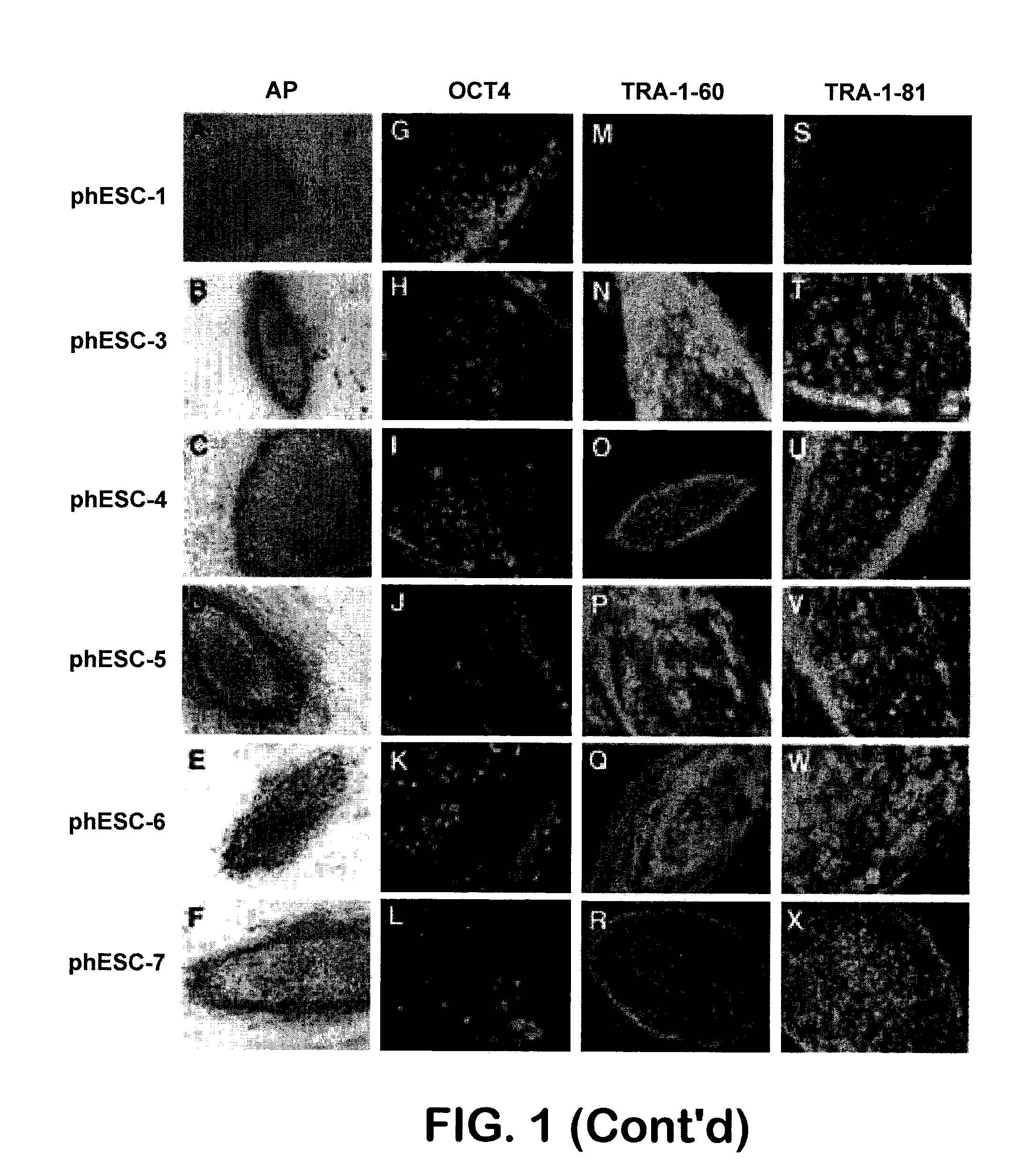
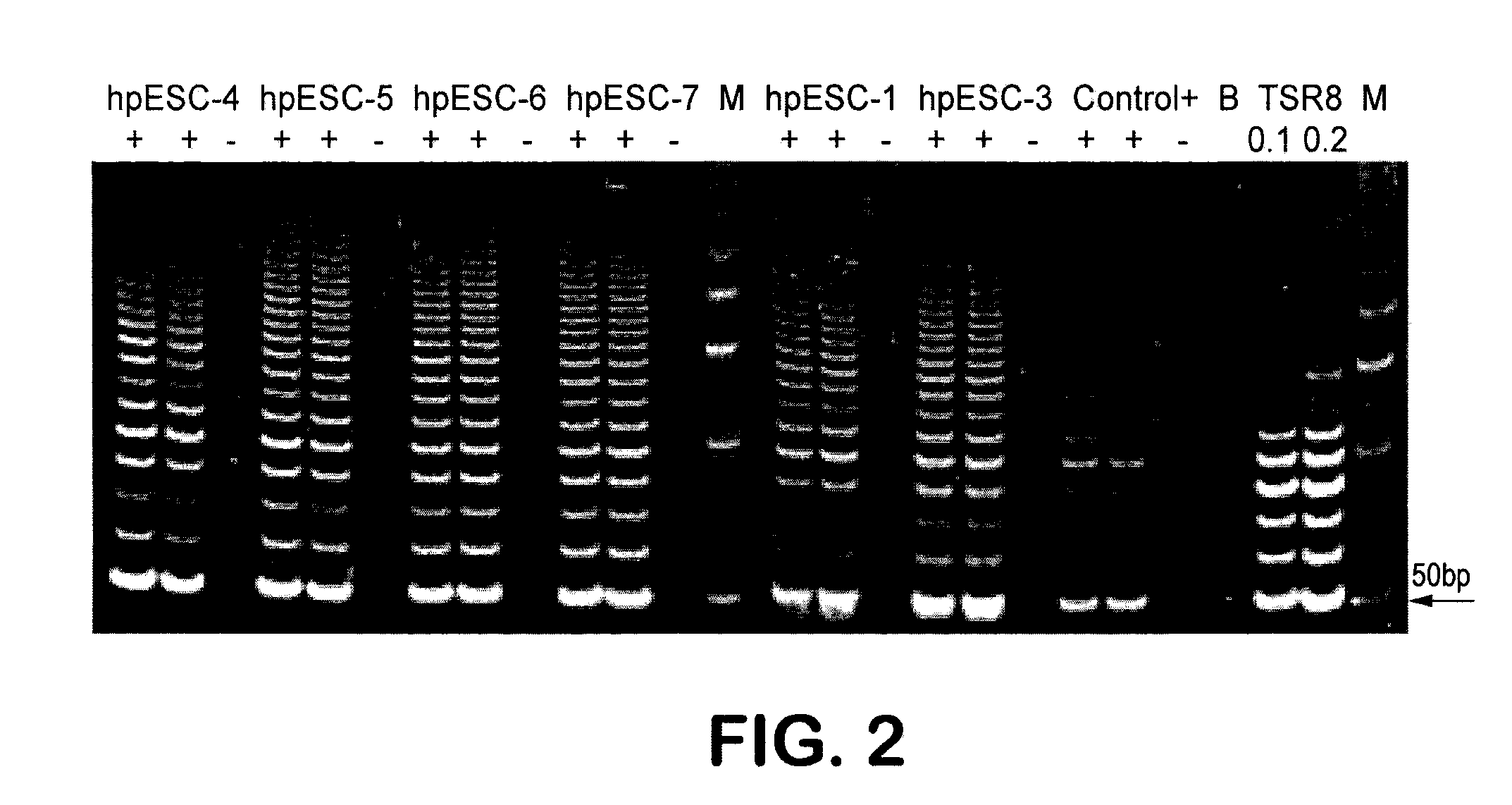
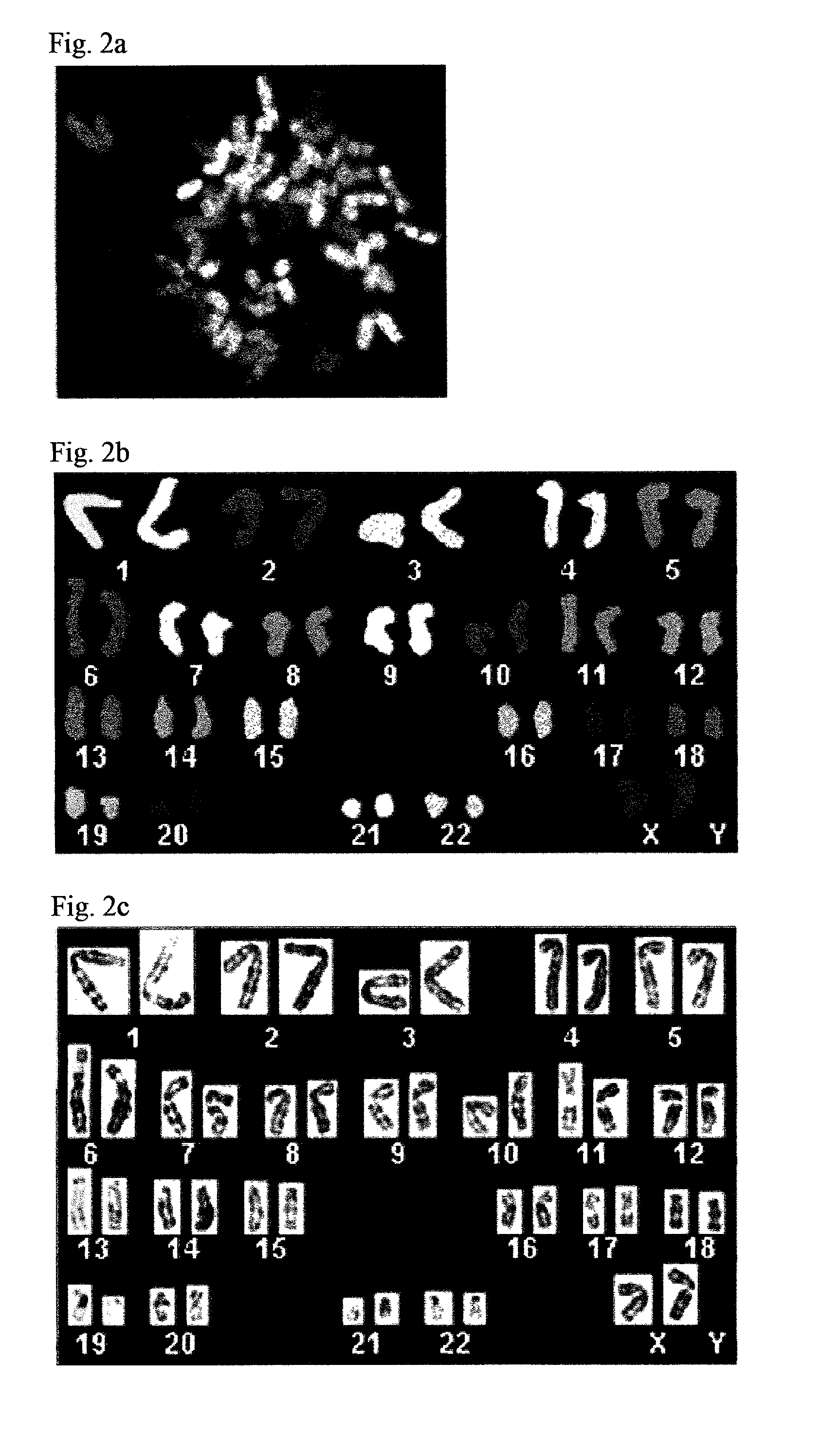
Patient-specific stem cell lines derived from human parthenogenetic blastocysts
InactiveUS20080299091A1Increase alkaline phosphatase activityHigh levelBiocideSenses disorderTelomeraseOocyte donor
Methods are disclosed for generating HLA homozygous parthenogenetic human stem cell (hpSC-Hhom) lines from both HLA homozygous and HLA heterozygous donors. These hpSC-Hhom lines demonstrate typical human embryonic stem cell morphology, expressing appropriate stem cell markers and possessing high levels of alkaline phosphatase and telomerase activity. Additionally, injection of these cell lines into immunodeficient animals leads to teratoma formation. Furthermore, in the case of HLA heterozygous donors, the hpSC-Hhom lines inherit the haplotype from only one of the donor's parents. SNP data analysis suggests that hpSC-Hhom lines derived from HLA heterozygous oocyte donors are homozygous throughout the genome as assessed by single-nucleotide polymorphism (SNP) analysis. The protocol as disclosed minimizes the use of animal-derived components, which makes the stem cells more practical for clinical application.
Owner:INT STEM CELL CORP
Clonal human embryonic stem cell lines and methods of generating same
InactiveUS20030073234A1Microbiological testing/measurementArtificial cell constructsSerum free mediaStem cell line
A method of establishing a clonal embryonic stem cell line capable of sustaining a phenotype of normal embryonic stem cells following at least eight months of in vitro culture is disclosed. The method is effected by culturing an individual embryonic stem cell for at least eight months in a serum-free medium, thereby establishing the clonal embryonic stem cell line capable of sustaining said phenotype of normal embryonic stem cells following at least eight months of in vitro culture.
Owner:WISCONSIN ALUMNI RES FOUND
Derivation of embryonic stem cells
ActiveUS20060206953A1Useful in treatmentBiocideSenses disorderStem cell lineEmbryonic Stem Cell Line
This present invention provides novel methods for deriving embryonic stem cells, those cells and cell lines, and the use of the cells for therapeutic and research purposes without the destruction of the embryo. It also relates to novel methods of establishing and storing an autologous stem cell line prior to implantation of an embryo, e.g., in conjunction with reproductive therapies such as IVF.
Owner:ADVANCED CELL TECH INC
Isolation of inner cell mass for the establishment of human embryonic stem cell (hESC) lines
InactiveUS20030104616A1Ensure purityMinimize contaminationMammal material medical ingredientsArtificial cell constructsMidblastulaStem cell line
A method for isolating an inner cell mass comprising the steps of immobilizing a blastocyst stage embryo having a zona pellucida, trophectoderm, and inner cell mass, creating an aperture in the blastocyst stage embryo by laser ablation, and removing the inner cell mass from the blastocyst stage embryo through the aperture. The aperture is through the zona pellucida and the trophectoderm. The laser ablation is acheived using a non-contact diode laser. The inner cell mass removed from the blastocyst stage embryo is used to establish human Embryonic Stem Cell lines.
Owner:RELIANCE LIFE SCI PVT
Derivation of embryonic stem cells and embryo-derived cells
This present invention provides novel methods for deriving embryonic stem cells and embryo-derived cells from an embryo without requiring destruction of the embryo. The invention further provides cells and cell lines derived without embryo destruction, and the use of the cells for therapeutic and research purposes. It also relates to novel methods of establishing and storing an autologous stem cell line prior to implantation of an embryo, e.g., in conjunction with reproductive therapies such as IVF.
Owner:ADVANCED CELL TECH INC
Dendrobium officinale stem cell and isolated culture method thereof
InactiveCN104195098AProvide stableDoes not cause environmental problemsCosmetic preparationsHair cosmeticsBiotechnologyStem cell line
The invention discloses a dendrobium officinale stem cell and an isolated culture method thereof. The method comprises the following steps: culturing plant root tissue containing a quiescent centre, performing subcultring and scale-up culture on quiescent centre stem cell, and then preparing stem cell dry powder or extracting solution according to requirements. The dendrobium officinale stem cell overcomes the problem that dendrobium officinale in vitro material is easy to brown, the cell of a stem cell line is not differentiated, the cell increment is large in a nutritional condition, the culture time is short, and the cell activity is high. The dendrobium officinale stem cell and the method have the advantages that the technical process is easy to operate, the quantity of technological processes is less, the output is high, and the dendrobium officinale stem cell obtained is anti-aging and oxidation resistant, promotes hair growth and high baldness-resistant activity, and can be manufactured into functional health care products and cosmetics for preventing and curing various senescence and baldness and relevant Chinese drugs for preventing senescence and baldness.
Owner:STEMCELL ESSENTIALS
Human embryonic stem cell clones
InactiveUS20070160974A1Microbiological testing/measurementArtificial cell constructsDiseaseStem cell line
The present invention provides methods for isolating individual viable stem cells from a stem cell line, methods for deriving one or more clones from a stem cell line, individual viable cells and clones derived from stem cell lines by the methods disclosed, methods for producing differentiated cells from the individual viable cells and clones so derived, differentiated cells so produced, methods for treating diseases using the cells and clones described herein and methods for proliferating cells and clones in undifferentiated form.
Owner:SOUTH EASTERN SYDNEY AND ILLAWARRA AREA HEALTH SERVICE
Derivation of embryonic stem cells
This present invention provides novel methods for deriving embryonic stem cells, those cells and cell lines, and the use of the cells for therapeutic and research purposes without the destruction of the embryo. It also relates to novel methods of establishing and storing an autologous stem cell line prior to implantation of an embryo, e.g., in conjunction with reproductive therapies such as IVF.
Owner:ADVANCED CELL TECH INC
Neural tumor stem cells and methods of use thereof
InactiveUS20100287638A1Microbiological testing/measurementLibrary screeningAbnormal tissue growthScreening method
Owner:HOSPITAL FOR SICK CHILDREN +1
Induction and in-vitro culture method of plant stem cells derived from apical meristem of catharanthus roseus roots
InactiveCN111876368AStable subcultureNo epigenetic variationCell culture mediaPlant cellsBiotechnologyStem cell line
The invention discloses an induction and in-vitro culture method of plant stem cells derived from apical meristem of catharanthus roseus roots. The induction method comprises the following steps: cutting off a root cap from the root tip of the catharanthus roseus, performing inoculating to an induction culture medium, performing culturing in dark, and proliferating and growing a stem cell layer inan explant to form a cell cluster; transferring the layered stem cell layer to a subculture medium for culturing for 10-15 days, and performing complete culturing in dark; and performing transferringto a new subculture medium, and performing culturing for 10-15 days under the same condition to obtain a catharanthus roseus stem cell line with stable cell morphology. A catharanthus roseus stem cell culture system is established by inducing apical meristem of the catharanthus roseus roots, stable subculture can be performed under certain conditions, and epigenetic variation is avoided. The catharanthus roseus stem cell culture system established by the invention has the characteristics of being high in growth speed, high in genetic stability and the like, and culture with a 10L bioreactor is preliminarily realized.
Owner:LINGNAN INST OF TECH
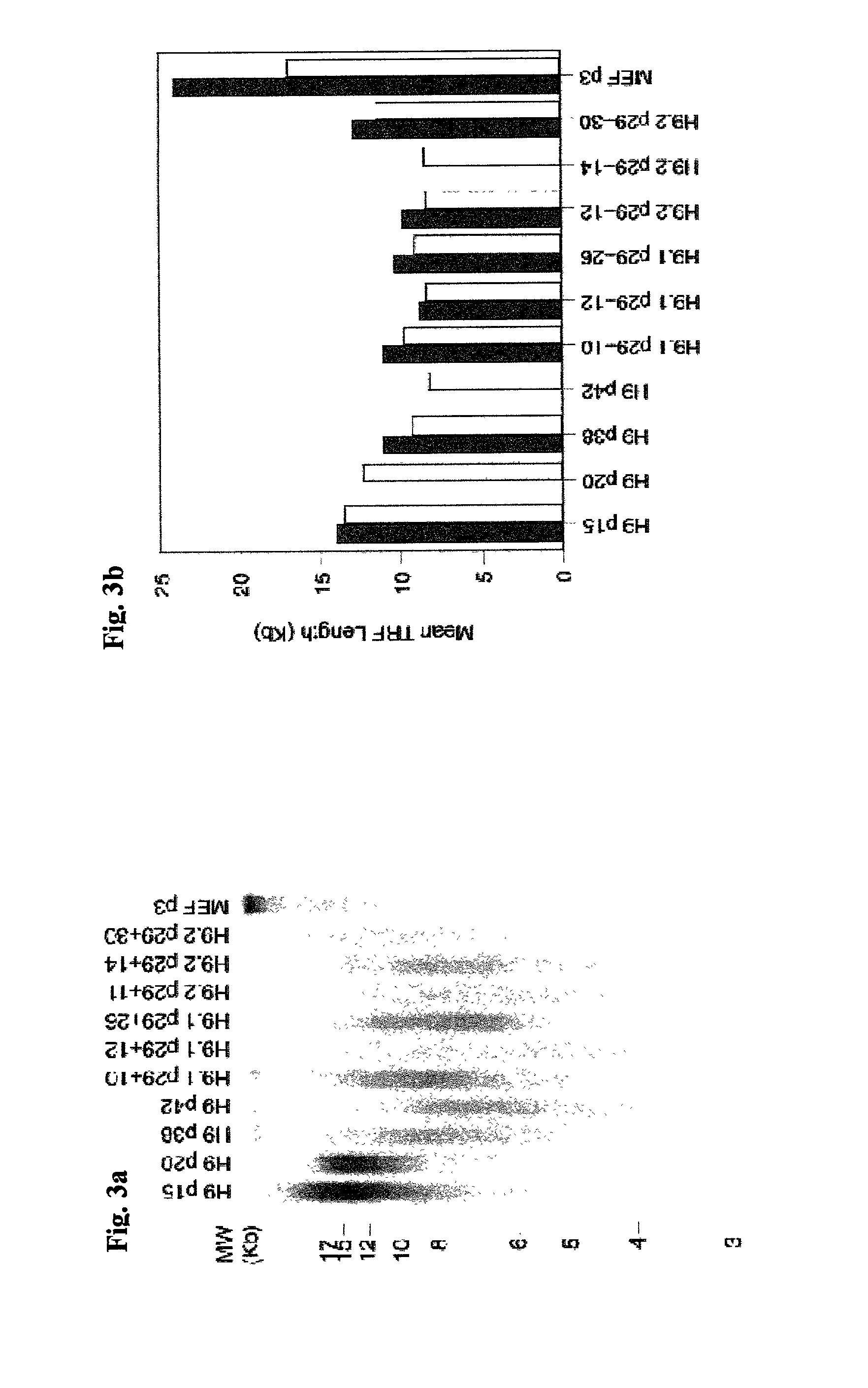
Methods for telomere length and genomic DNA quality control and analysis in pluripotent stem cells
InactiveUS20130011918A1Reduce riskDesired phenotypeMicrobiological testing/measurementLibrary screeningPluripotential stem cellClinical grade
The generation of clinical-grade cell-based therapies from human embryonic stem cells or cells reprogrammed to pluripotency from somatic cells, requires stringent quality controls to insure that the cells have long enough telomeres and resulting cellular lifespan to be clinically useful, and normal gene expression and genomic integrity so as to insure cells with a desired and reproducible phenotype and to reduce the risk of the malignant transformation of cells. Assays useful in identifying human embryonic stem cell lines and pluripotent cells resulting from the transcriptional reprogramming of somatic cells that have embryonic telomere length are described as well as quality control assays for screening genomic integrity in cells expanded and banked for therapeutic use, as well as assays to identify cells capable of abnormal immortalization,
Owner:LINEAGE CELL THERAPEUTICS INC
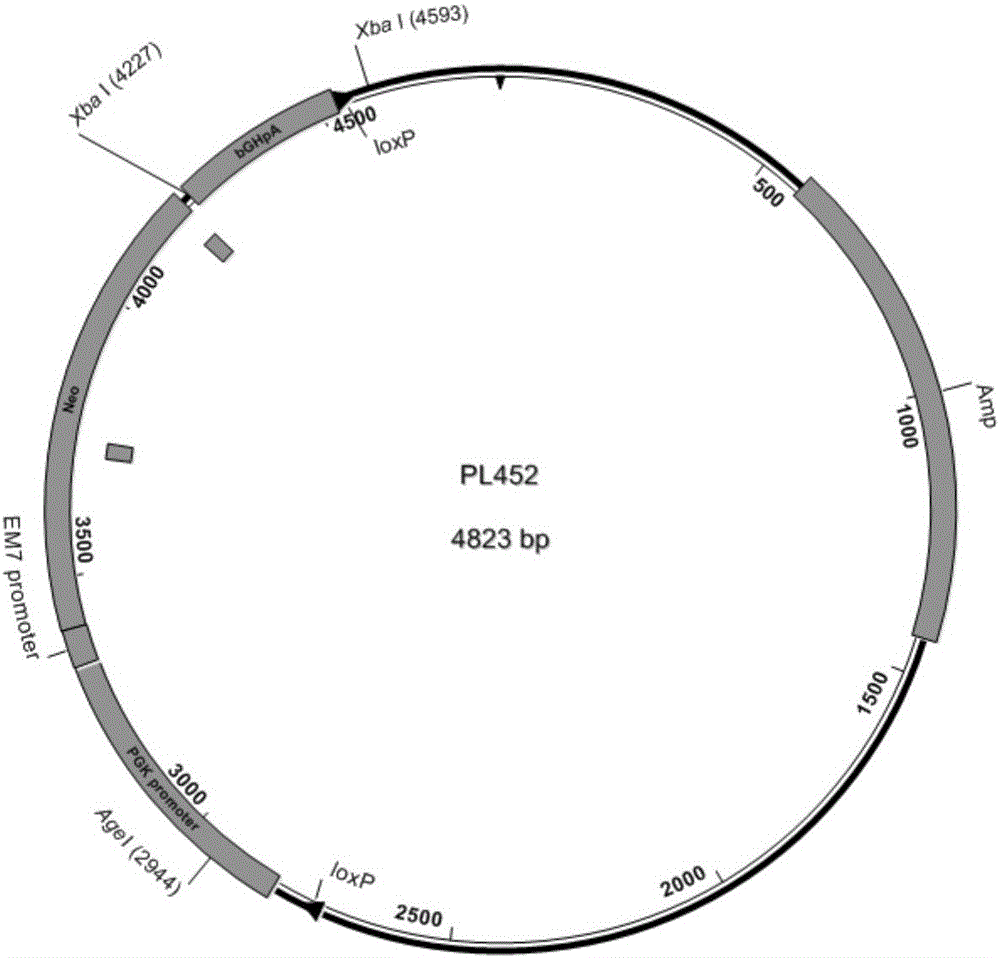
Method for high-efficiency establishment of genetically modified animal model through haploid stem cells
The invention discloses a method for high-efficiency establishment of a genetically modified animal model through haploid stem cells. Specifically, the method for high-efficiency establishment of the genetically modified animal model through haploid stem cells comprises the following steps: a) knocking out the H19 gene of male haploid embryonic stem cells through homologous recombination; b) screening and identifying the male haploid embryonic stem cells in which the H19 gene is correctly knocked out; and c) injecting the male haploid embryonic stem cells in which the H19 gene is correctly knocked out into cytoplasm of oocyte so as to produce a semi-cloned genetically modified animal. The method provided by the invention simulates the functions of sperms by establishing an H19 gene-knocked-out male haploid embryonic stem cell system so as to highly efficiently generate fertile progeny, which can then be used for implementation of novel genetic manipulation and establishment of corresponding genetically modified animal models.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Methods of Generating Stem Cells and Embryonic Bodies Carrying Disease-Causing Mutations and Methods of Using same for Studying Genetic Disorders
InactiveUS20070269790A1Microbiological testing/measurementGenetically modified cellsStem cell lineWaardenburg syndrome
Stem cells, stem cell lines and differentiated cells, tissues and organs which carry disease-causing mutations are provided. There is also provided a method of identifying agents suitable for treating disorders associated with at least one disease-causing mutations such as myotonic dystrophy and van Waardenburg syndrome.
Owner:TECHNION RES & DEV FOUND LTD
Immortalized porcine pancreatic stem cell line and construction and differentiation methods thereof
ActiveCN101974488ASolving activityPromote growthMicroorganism based processesVector-based foreign material introductionNormal blood glucoseReverse transcriptase
The invention discloses an immortalized porcine pancreatic stem cell line and construction and differentiation methods thereof. In the immortalized porcine pancreatic stem cell line, porcine pancreatic stem cells are taken as host cells and are transfected with a pCI-neo-hTERT eukaryotic expression vector, and the human telomerase reverse transcriptase screened by G418 is expressed and has a transformant with multi-directional differentiation potentiality. The cell line also has higher activity after transferring for over 50 generations in vitro, keeps split proliferation, does not generate aging or apoptosis, and is an immortalized cell line. The cell line can be differentiated to form functional islet cell mass after induction, can reduce the blood glucose concentration after being transplanted into a mouse model suffering from diabetes and can maintain a normal blood glucose level within two weeks.
Owner:陕西九州细胞基因工程有限公司
Immortalized canine adipic mesenchymal stem cell line and constructing method thereof
InactiveCN105779395APromote growthMultidirectional differentiation potential performanceMicroorganism based processesSkeletal/connective tissue cellsStem cell lineMesenchymal stem cell
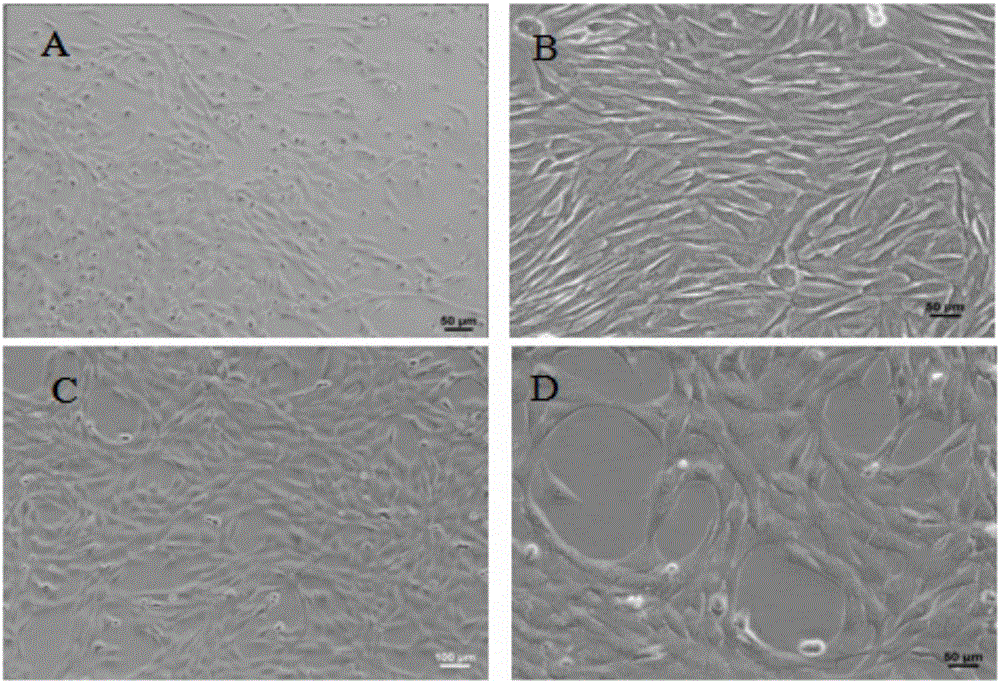
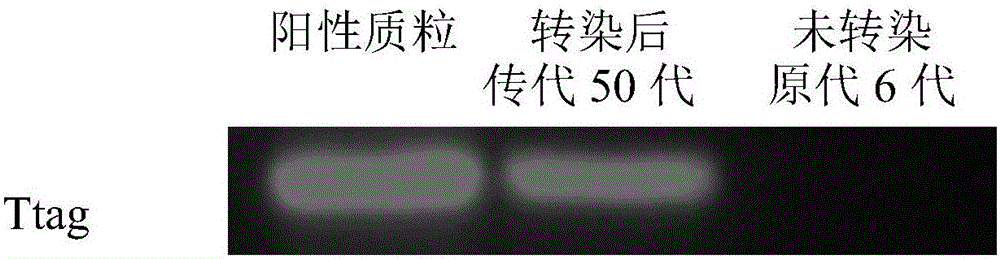
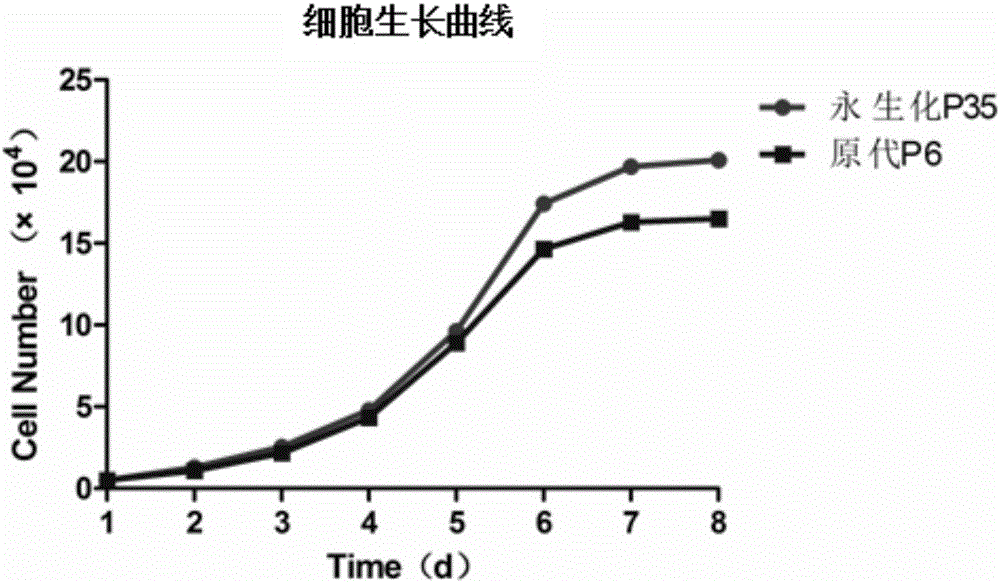
The invention discloses an immortalized canine adipic mesenchymal stem cell line and a constructing method thereof. The immortalized canine adipic mesenchymal stem cell line capable of expressing SV40 large T antigen genes and having multidirectional differentiation is obtained by: transfecting a lentiviral vector carrying SV40 large T antigen (Ttag) by using canine adipic mesenchymal stem cells as host cells, integrating the Tag genes into an adipic mesenchymal stem cell genome and carrying out continuous passage and screening. This cell line is still active in case of 50 in-vitro passages, is high in differentiation and proliferation performance, never ages or dies, can also save medium cost and is an immortalized cell line.
Owner:NORTHWEST A & F UNIV
Functional genomics assay for characterizing pluripotent stem cell utility and safety
ActiveCN103459611AAddress research questions of interestNervous disorderMetabolism disorderGenomicsEpigenetic Analysis
The present invention generally relates set of reference data or "scorecard" for a pluripotent stem cell, and methods, systems and kits to generate a scorecard for predicting the functionality and suitability of a pluripotent stem cell line for a desired use. In some aspects, a method for generating a scorecard comprises using at least 2 stem cell assays selected from: epigenetic profiling, differentiation assay and gene expression assay to predict the functionality and suitability of a pluripotent stem cell line for a desired use. In some embodiments, the scorecard reference data can be compared with the pluripotent stem cells data to effectively and accurately predict the utility of the pluripotent stem cell for a given application, as well as any to identify specific characteristics of the pluripotent stem cell line to determine their suitability for downstream applications, such as for example, their suitability for therapeutic use, drug screening and toxicity assays, differentiation into a desired cell lineage, and the like.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Functional genomics assay for characterizing pluripotent stem cell utility and safety
InactiveUS20130296183A1Rapidly and relatively inexpensively screenImprove throughputNervous disorderNucleotide librariesPluripotential stem cellCell lineage
The present invention generally relates set of reference data or “scorecard” for a pluripotent stem cell, and methods, systems and kits to generate a scorecard for predicting the functionality and suitability of a pluripotent stem cell line for a desired use. In some aspects, a method for generating a scorecard comprises using at least 2 stem cell assays selected from: epigenetic profiling, differentiation assay and gene expression assay to predict the functionality and suitability of a pluripotent stem cell line for a desired use. In some embodiments, the scorecard reference data can be compared with the pluripotent stem cells data to effectively and accurately predict the utility of the pluripotent stem cell for a given application, as well as any to identify specific characteristics of the pluripotent stem cell line to determine their suitability for downstream applications, such as for example, their suitability for therapeutic use, drug screening and toxicity assays, differentiation into a desired cell lineage, and the like.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Human tumor stem cell line and application thereof
ActiveCN101659940AImprove transfer abilitySelf-renewingMicrobiological testing/measurementMicroorganism based processesAbnormal tissue growthHuman tumor
The invention provides a human tumor stem cell line T3A-A3, which is obtained by separation of liver cancer tissue excised in an operation of a primary hepatic carcinoma patient. The separated cells can be in long-term passage culture in vitro, can grow rapidly and retain stem cell property after more than 100 times of passage. The cell can express markers of multiple stem cells, has self-updatingcapability of stem cells and directional differentiation potentials for different tumor cells, and also has tumor property, tumorigenicity ability and metastatic ability. The invention is a powerfultool for preparing stem cell drugs for targeting tumor, due to the facts that the cells can be in long-term passage culture in vitro and retain unchanged stem cell property, and the tumorigenicity ability and metastatic ability thereof are strong in immunodeficient mice.
Owner:SUZHOU BOJUHUA BIOMEDICAL TECH CO LTD
Method for efficient transfer of human blastocyst-derived stem cells (hBS cells) from a feeder-supported to a feeder-free culture system, long-term propagation of hBS cells under feeder-free conditions and use of cultured hBS cells for applications in myocardial regeneration
InactiveUS7638328B2Efficiently attachedPromote rapid proliferationNervous disorderNervous system cellsStem cell lineCell culture media
A method for the transfer of human blastocyst-derived stem cells (hBS cells) to feeder-free culture system and propagation of the cells in such a feeder-free culture system, the method comprising the following steps of (a) transferring the balstocyst-derived stem cells from feeder to feeder free culture by mechanical treatment, (b) optionally, culturing the blastocyst-derived stem cells under feeder cell free growth conditions in a suitable growth medium and / or on a suitable support substrate, and (c) optionally passaging the blastocyst derived stem cell line every 3-10 days by enzymatic and / or mechanical treatment. The invention also relates to the application of hBS cells cultured under feeder free condition in medicine (e.g., myocardial regeneration) and screening and toxicity tests.
Owner:TAKARA BIO EURO
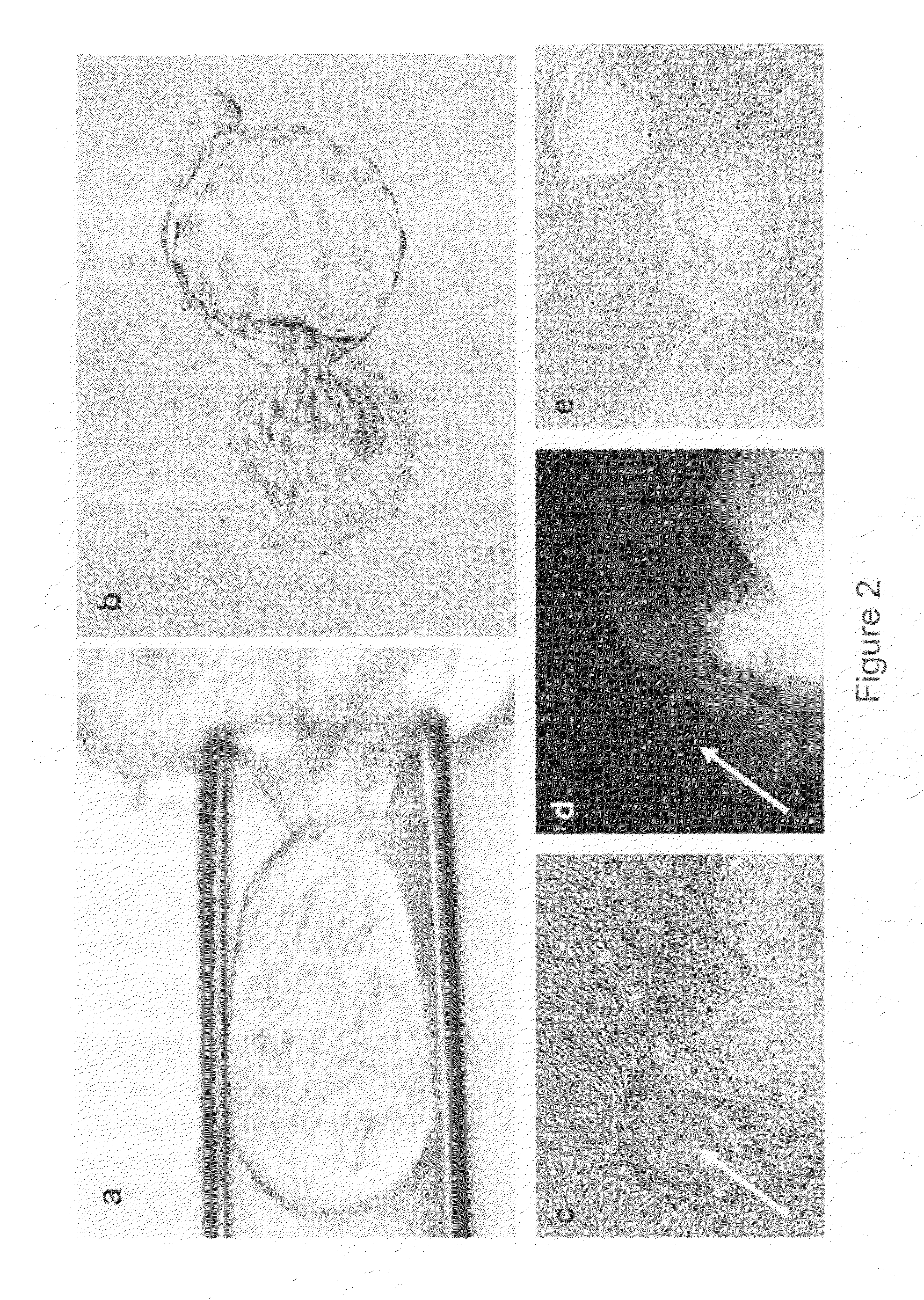
In-vitro maturation culture method for oocyte of mouse and method for establishing parthenogenetic embryonic stem cell line
InactiveCN101735978ASimple ingredientsLow costMicroorganism based processesEmbryonic cellsStem cell lineBiological activation
The invention discloses an in-vitro maturation culture method for an oocyte mouse and a method for establishing a parthenogenetic embryonic stem cell line. In the in-vitro maturation culture method, an immature oocyte is cultured in a basic culture solution in which the HCG (human chorionic gonadotropin) and PMSG (pregnant mare serum gonadotropin) are added, and the maturation rate of the oocyte can reach more than 83 percent. In the method for establishing a parthenogenetic embryonic stem cell line, the parthenogenetic activation culture development is carried out on the mouse oocyte after in-vitro maturation culture to obtain the embryonic stem cell line; a hepatocyte obtained by the method has no immunogenicity, is simultaneously equivalent to a stem cell derived from a fertilized embryo on totipotency and has guiding significance for utilizing in-vitro maturation of the immature oocyte of a human and separating parthenogenetic embryonic stem cells. Aiming at the current conditionsof human ovum donation shortage, great discarding of clinical immature oocytes by an assisted reproductive technology, and the like, the two methods are combined to lay the foundation for developing and utilizing the in-vitro maturation culture of the immature oocyte of people and further researching the parthenogenetic embryo by utilizing an in-vitro mature ovum and separating the embryonic stemcells.
Owner:SUN YAT SEN UNIV
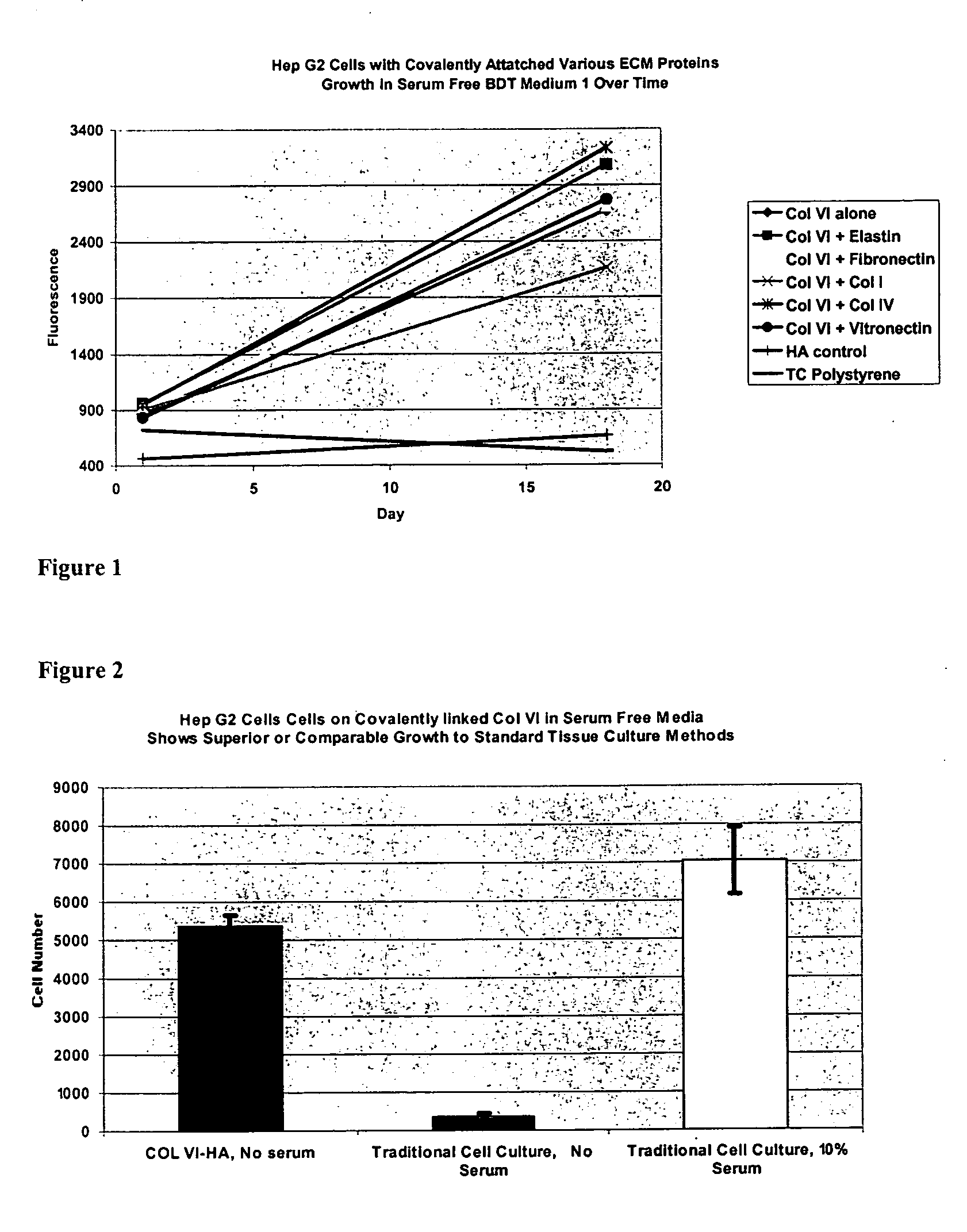
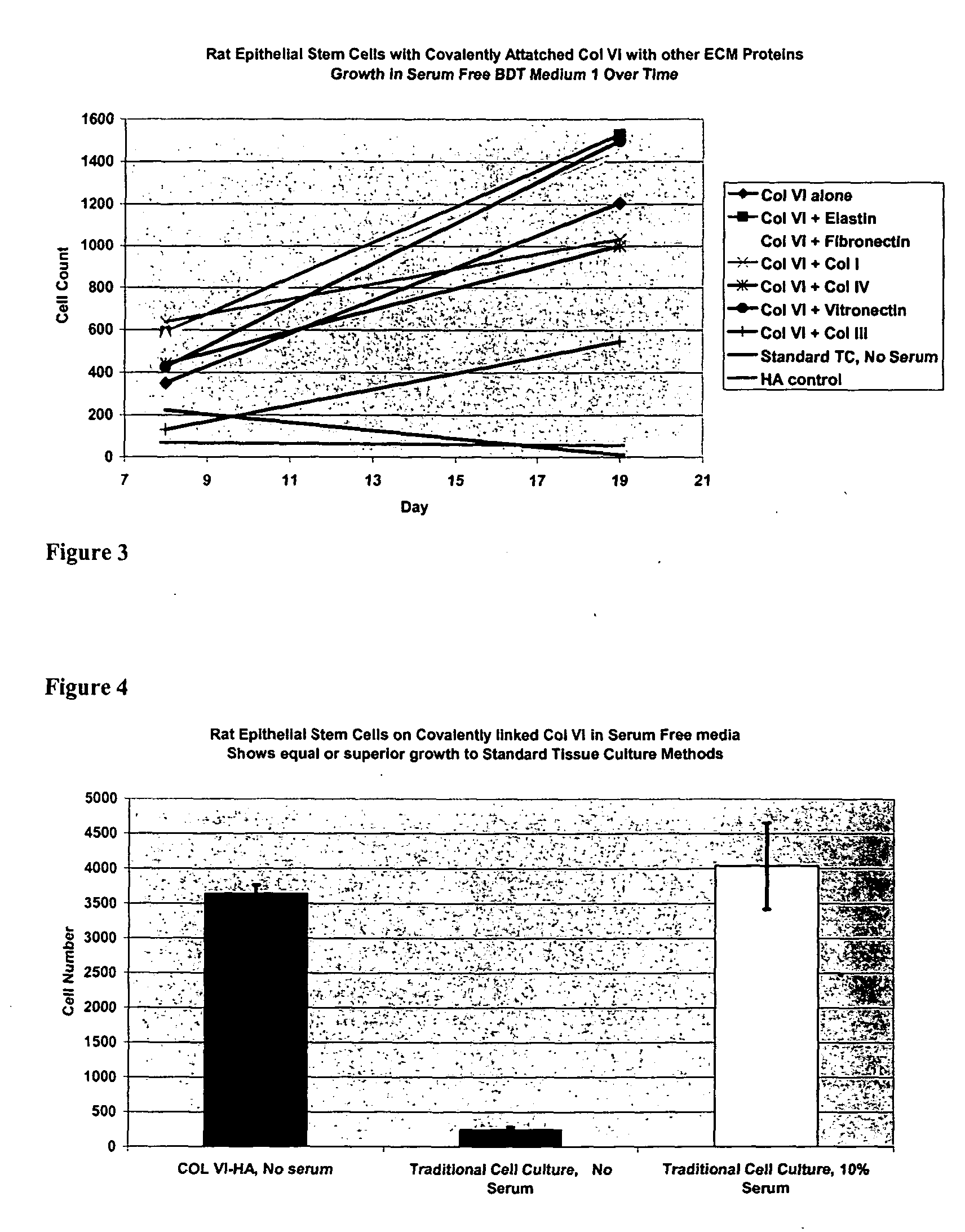
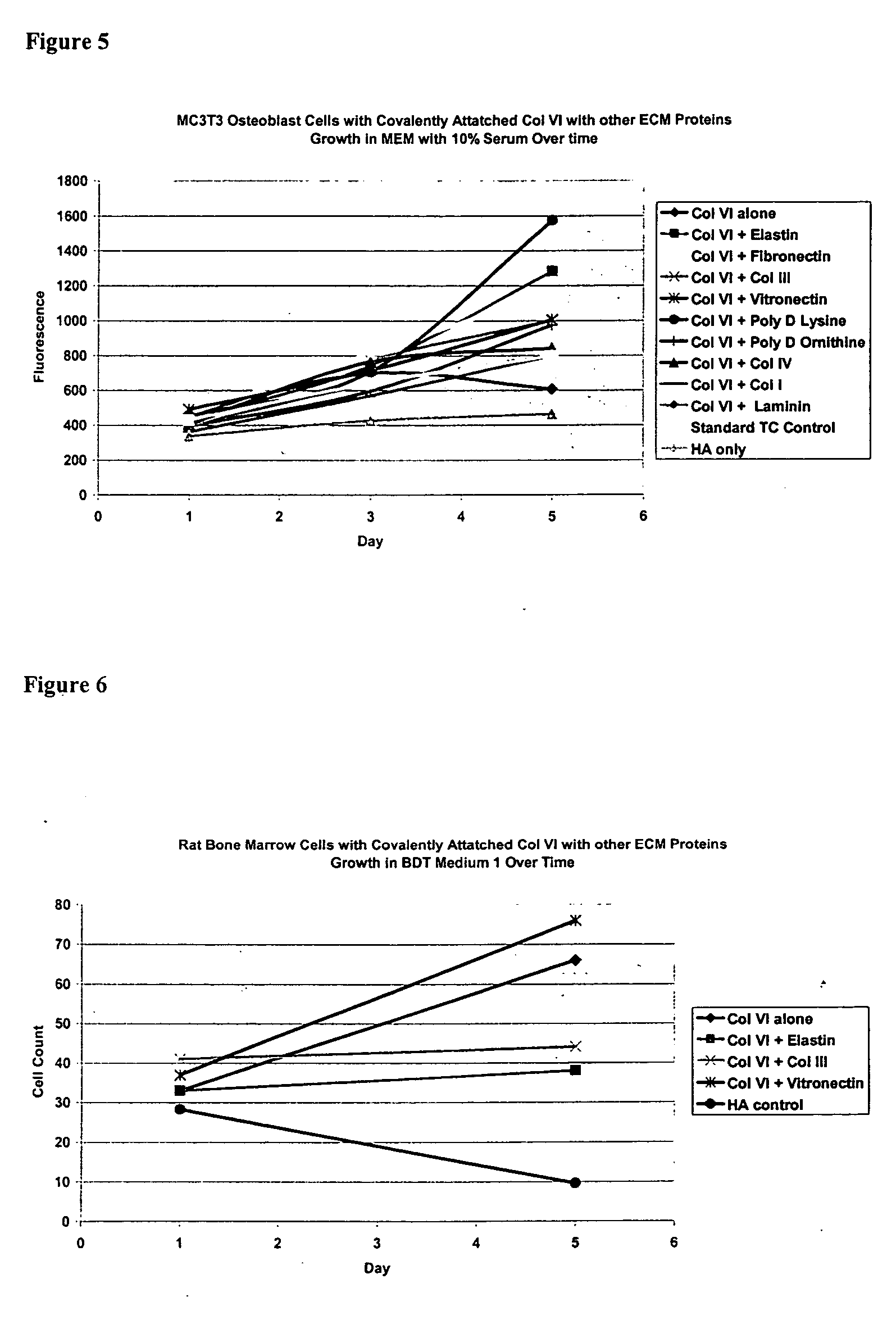
Covalently attached collagen VI for cell attachment and proliferation
InactiveUS20050058687A1Promote proliferationEasy SurvivalBioreactor/fermenter combinationsBiological substance pretreatmentsCollagen VIPolymer
Surfaces useful for cell culture comprise a support to which is bound a CAR material, and, bound to the CAR material, collagen VI or a biologically active fragment or variant thereof and, optionally, one or more other ECM proteins (or fragments or variants thereof) such as elastin, fibronectin, vitronectin, tenascin, laminin, entactin, aggrecan, decorin, collagen I, collagen III, and collagen IV. Also, optionally present on the surface is one or more polycationic polymers, such as poly-D-lysine or poly-D-ornithine. This surface is used in cell culture to promote cell attachment, survival, and / or proliferation of a number of different cell types such as (a) liver cells (e.g., HepG2 tumor cells, and a newly discovered line of rat liver epithelial stem cells) (b) osteoblasts, such as the murine cell line MC3T3 cell line and (c) primary bone marrow cells. Kits comprising the surfaces and additional reagents are also disclosed.
Owner:BECTON DICKINSON & CO
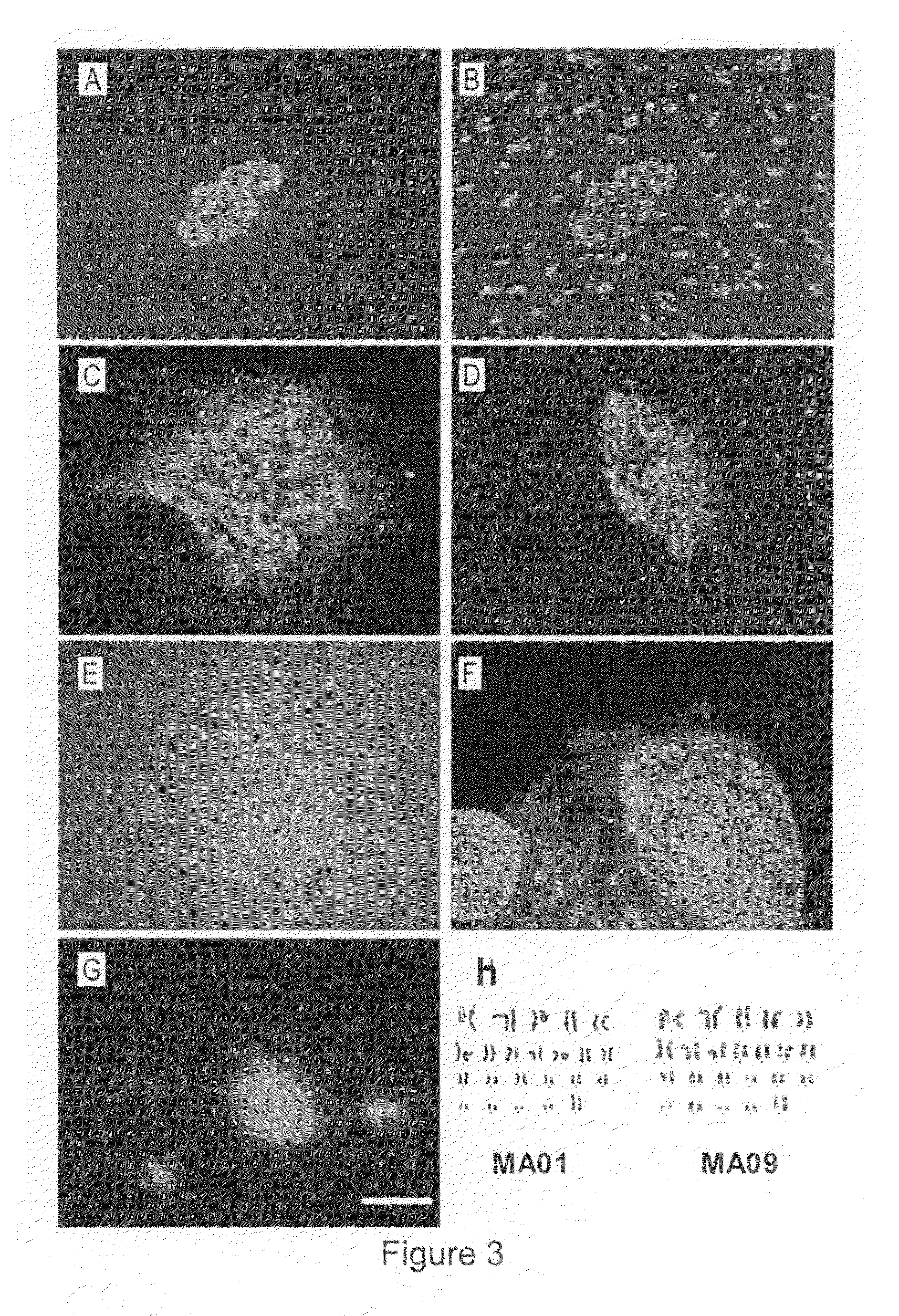
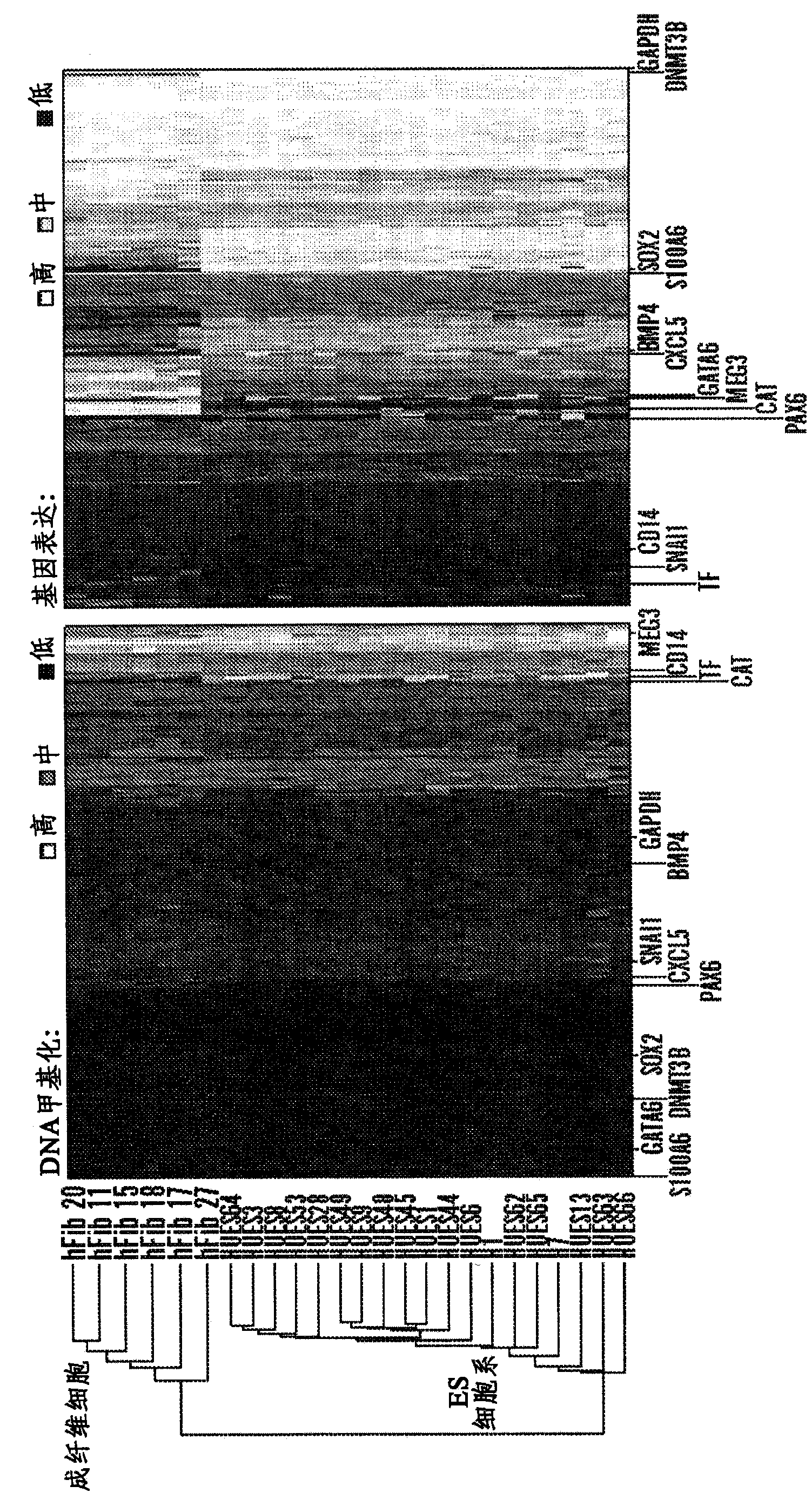
Human parthenogenetic embryo stem cell line with two active X chromosomes and derivatives thereof
The invention relates to a human parthenogenetic embryo stem cell line with two active X chromosomes and derivatives thereof. A karyotype of the human parthenogenetic embryo stem cell line is 46, XX before 10 generations; the karyotype of the cell line becomes a chimera from the 20th generation; all cells of the cell line lose an active X chromosome at the 35th generation; and in the inactivation detection of the X chromosome, only the state of the active X chromosome is maintained all along, but the presence of the inactivated X chromosome is not discovered. The parthenogenetic activation can be achieved by adopting an artificial activation condition causing a second polar body to be discharged; therefore, the human parthenogenetic embryo stem cell line and the derivatives thereof can be obtained. The cell line and the derivatives thereof have important significance on researching the characteristics of the cell on genetics and epigenetics and further applying the cell to clinically treating certain diseases; and a cell source for cell replacement therapy can be obtained by transforming genes of the cell or modifying the genes on the epigenetics.
Owner:广州医学院
Androgenesis haploid stem cell line as well as preparation method and application thereof
Provided are an androgenetic haploid stem cell line, preparation method and use thereof. Specifically, provided are an androgenetic haploid cell and androgenetic blastula. The nucleus of the cell or the blastula only comprises a haploid autosome and a sex chromosome, the sex chromosome being the X chromosome and containing no Y chromosome. The androgenetic haploid cell of the present application can replace a spermatid as a ligand to generate a fertile animal individual, thus facilitating gene manipulation and being capable of transferring genetic information to the offspring.
Owner:CENT FOR EXCELLENCE IN MOLECULAR CELL SCI CHINESE ACAD OF SCI
Composition for enhancing immunity containing plant stem cell line derived from cambium of panax ginseng including wild ginseng or ginseng as active ingredient
ActiveCN102325538AMinimize side effectsImprove immune activityImmunological disordersPlant tissue cultureAntigenSide effect
The present invention relates to a composition for enhancing immunity containing a cell line derived from the cambium of Panax ginseng including wild ginseng or ginseng, or the grounded substance, extract, or culture medium of the cell line as an active ingredient. The cell line and ground substance, extract, and culture medium of the cell line according to the present invention compose a natural product-derived composition. The cell line, ground substance, extract, and culture medium are safe to a human body by minimizing side effects of a conventional immunity booster, and also effectively increase activity of NK cells charging innate immunity. As well, the cell line, ground substance, extract, and culture medium appear to enhance acquired immunity by increasing proliferation rate of lymph node cells when re-exposing the cells to an antigen in a specific immune reaction, thus are useful as an immunity booster. Especially, the cell line, ground substance, extract, and culture medium also effectively increase the number of bone marrow cells, thus are not only used as an immune reaction adjuvant, but also used in preventing and treating anemia caused by hematopoiesis.
Owner:SHENZHEN LUQUAN IND CO LTD
Compositions Comprising Human Embryonic Stem Cells and Their Derivatives, Methods of Use, and Methods of Preparation
The present invention relates to a pharmaceutical composition comprising of preparations of human embryonic stem (hES) cells and their derivatives and methods for their transplantation into the human body, wherein transplantation results in the clinical reversal of symptoms, cure, stabilization or arrest of degeneration of a wide variety of presently incurable and terminal medical conditions, diseases and disorders. The invention further relates to novel processes of preparing novel stem cell lines which are free of animal products, feeder cells, growth factors, leukaemia inhibitory factor, supplementary mineral combinations, amino acid supplements, vitamin supplements, fibroblast growth factor, membrane associated steel factor, soluble steel factor and conditioned media. This invention further relates to the isolation, culture, maintenance, expansion, differentiation, storage, and preservation of such stem cells.
Owner:SHROFF GEETA
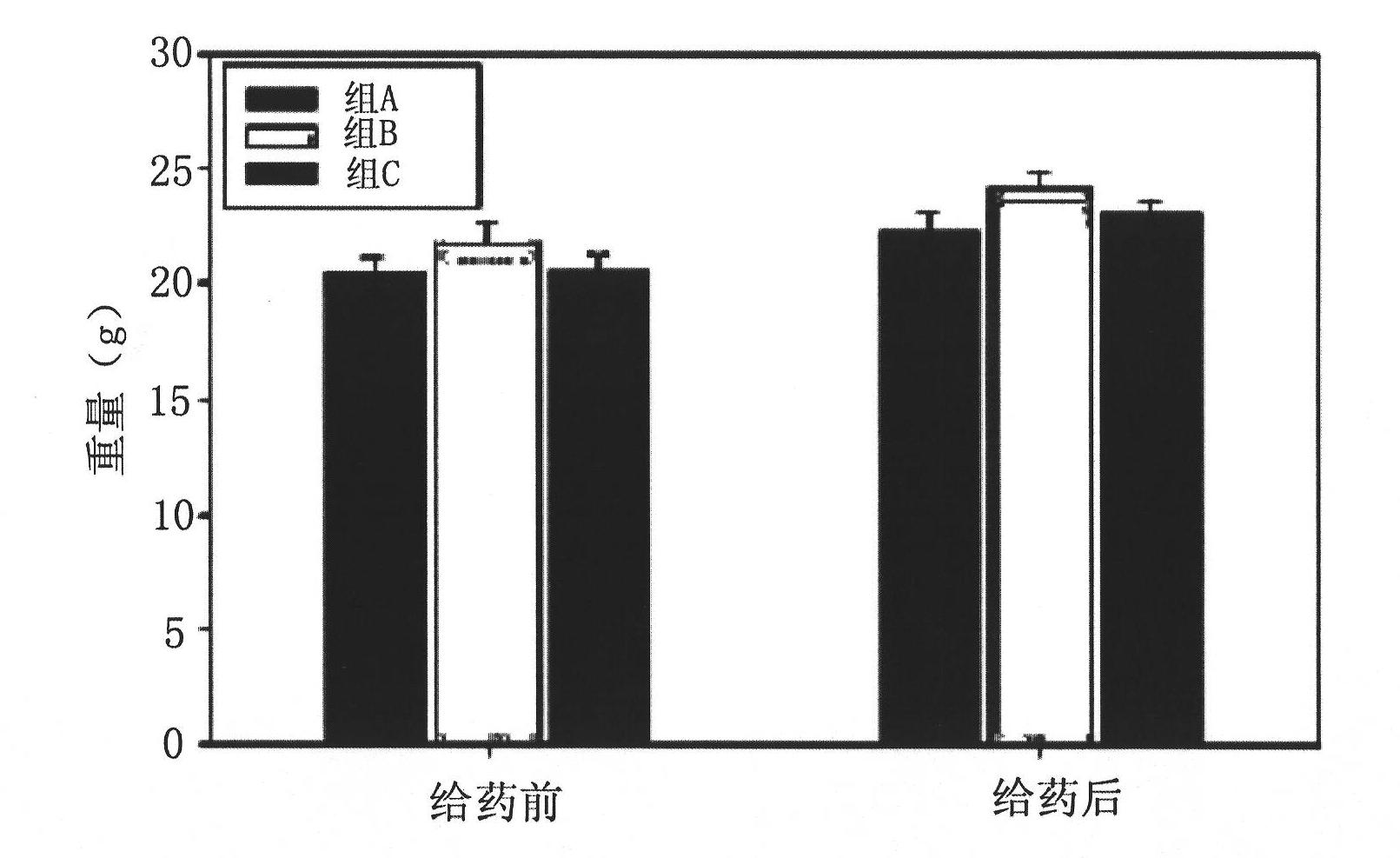
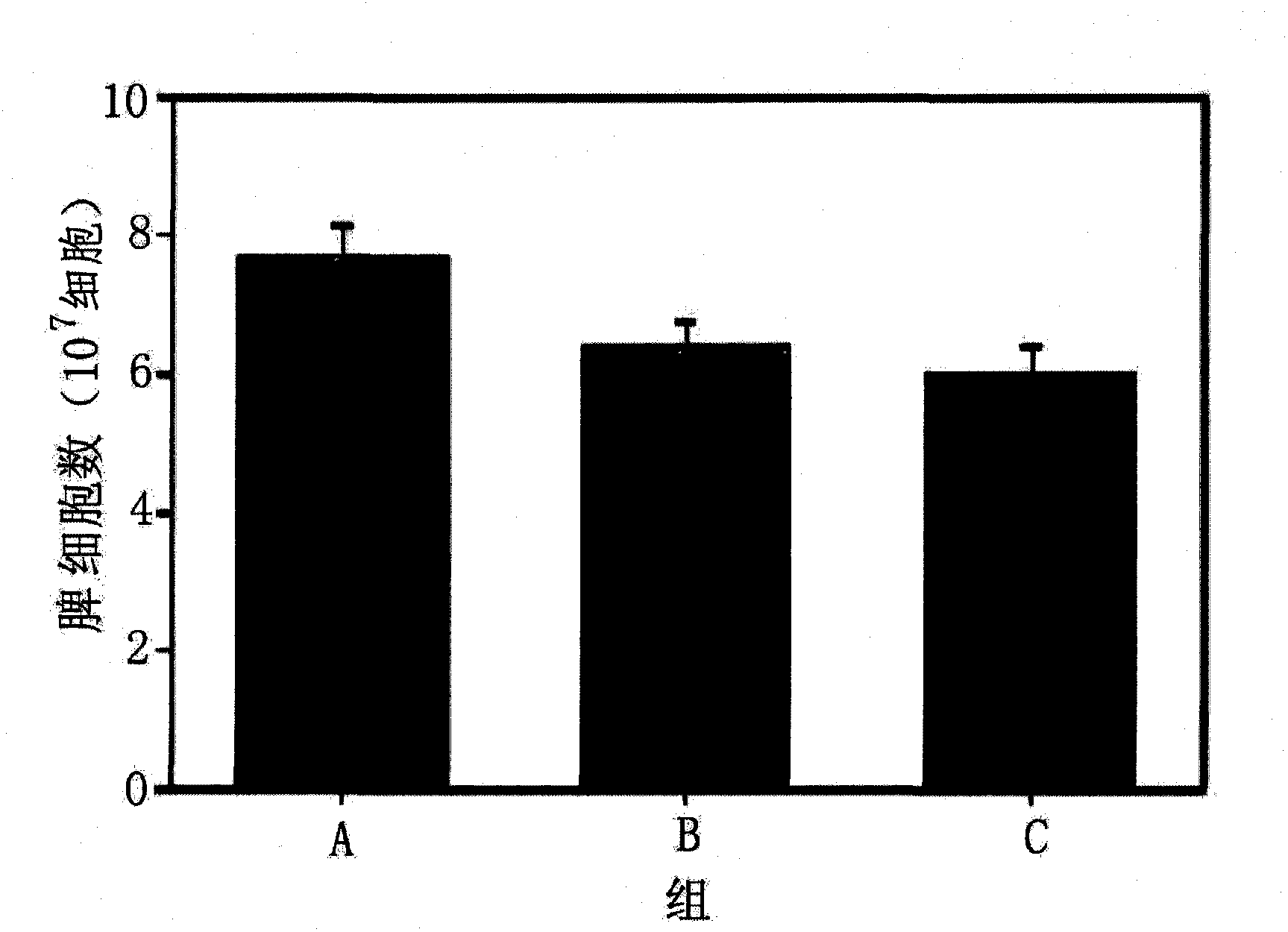
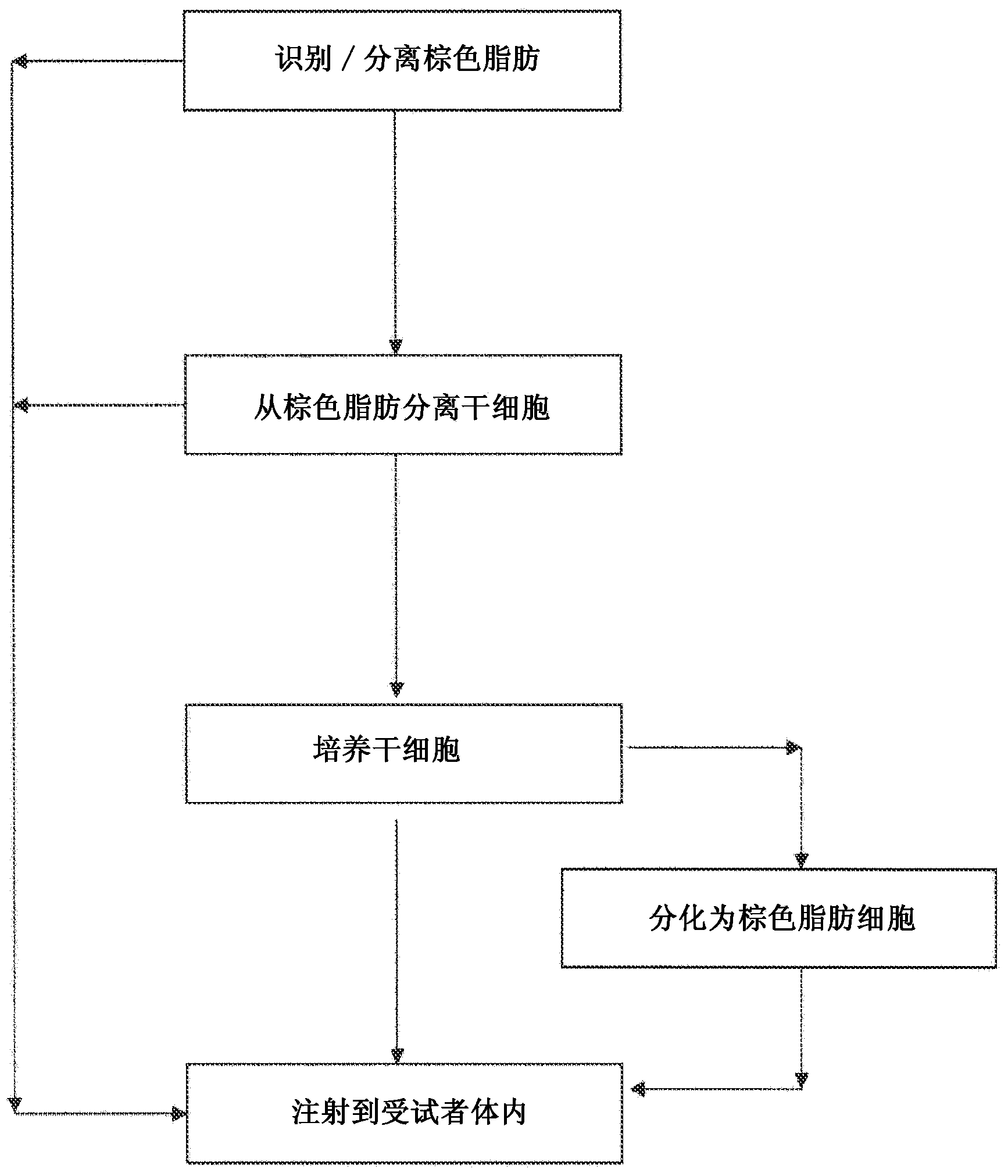
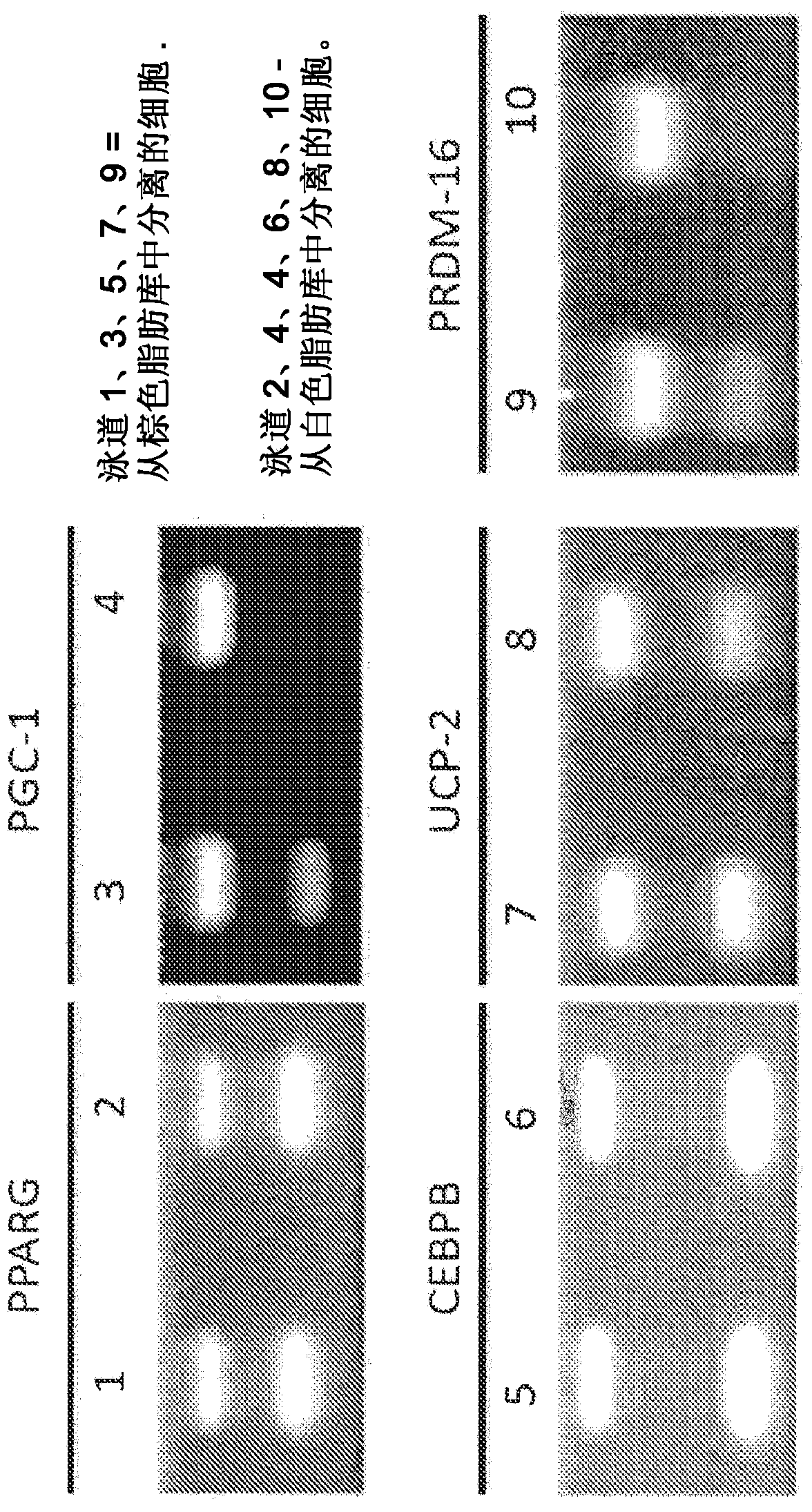
Brown fat cell compositions and methods
Methods of developing and using cell lines, such as stem cell lines, for therapeutic or cosmetic use. In one embodiment the cell lines are used to treat a wide range of degenerative and metabolic disorders including, but not limited to, obesity, diabetes, hypertension, and cardiac deficiency. Also described are methods of using such cell lines to screen for compounds that play a role in regulating a variety of processes.
Owner:BIORESTORATIVE THERAPIES
Compositions Comprising Human Embryonic Stem Cells and Their Derivatives, Methods of Use, and Methods of Preparation
Owner:SHROFF GEETA
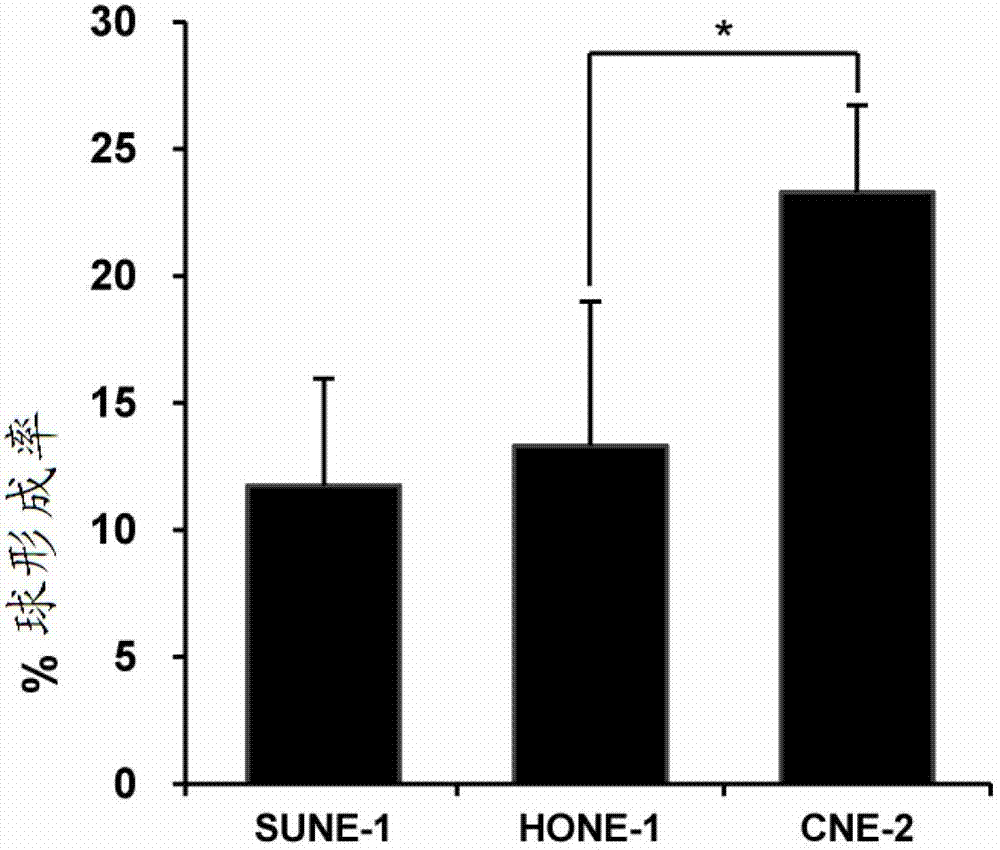
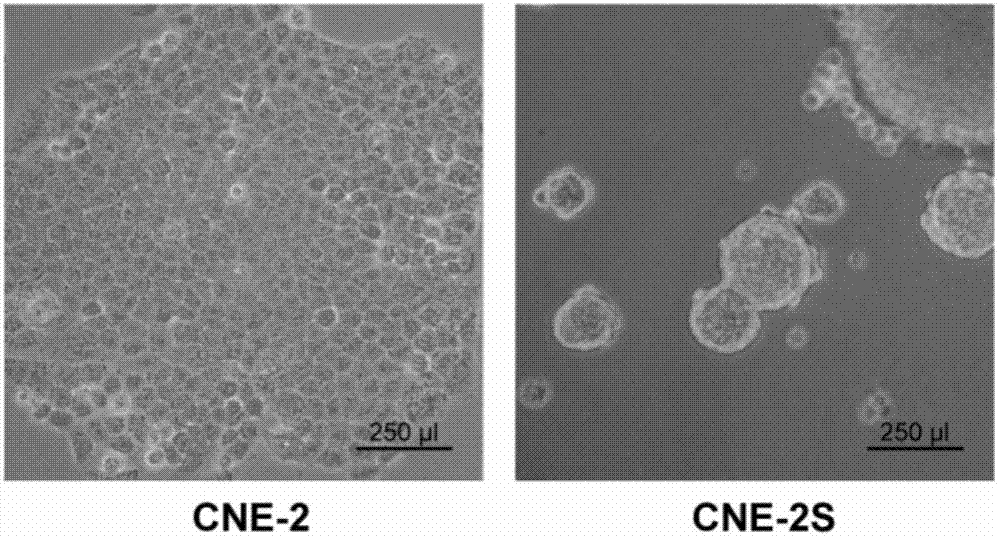
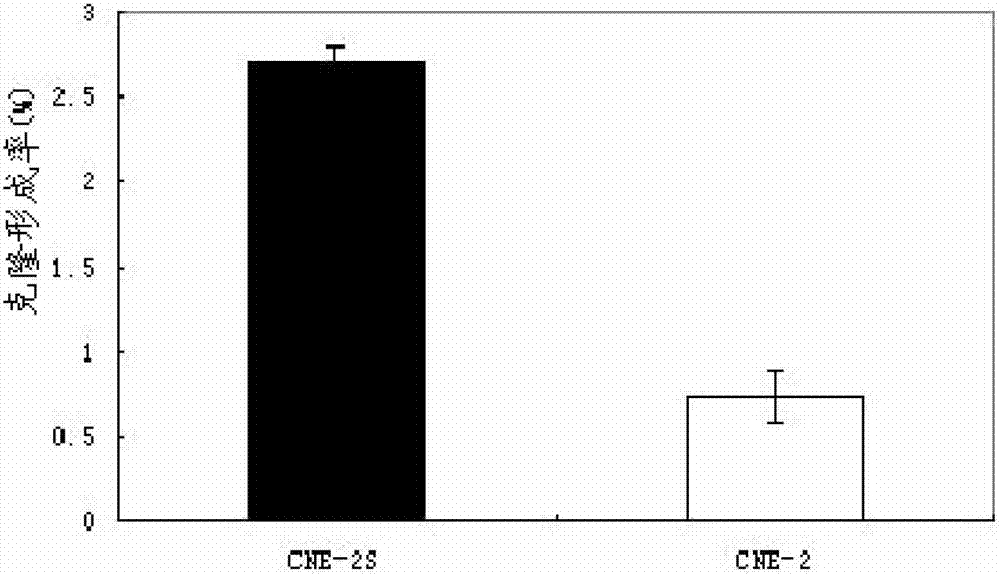
Method for establishing human nasopharyngeal carcinoma tumor stem cell line
InactiveCN102732484AStrong clonogenicityStrong drug toleranceTumor/cancer cellsCarcinoma cell lineStem cell line
The invention provides a method for establishing a human nasopharyngeal carcinoma tumor stem cell line. The method comprises the following steps: (1) obtaining human nasopharyngeal carcinoma cells in the single cell state; (2) adding the human nasopharyngeal carcinoma cells in the single cell state, obtained in the step (1), to a serum-free stem cell culture medium containing epidermal growth factors and basic fibroblast growth factors to obtain single cell suspension; (3) adjusting the concentration of the single cell suspension in the step (2) to 900-1100 single cells / ml; (4) inoculating 1ml of the single cell suspension obtained in the step (3) into single pores on a cell culture six-pore plate and then adding the equivoluminal stem cell culture medium for culture for 72-96 hours; and (5) collecting the cells obtained in the step (4), carrying out trypsinization till the single cell and carrying out subculture. The method provided by the invention is simple and convenient to operate; and the obtained nasopharyngeal carcinoma stem cell line has obvious stem cell characteristics.
Owner:GUANGDONG MEDICAL UNIV
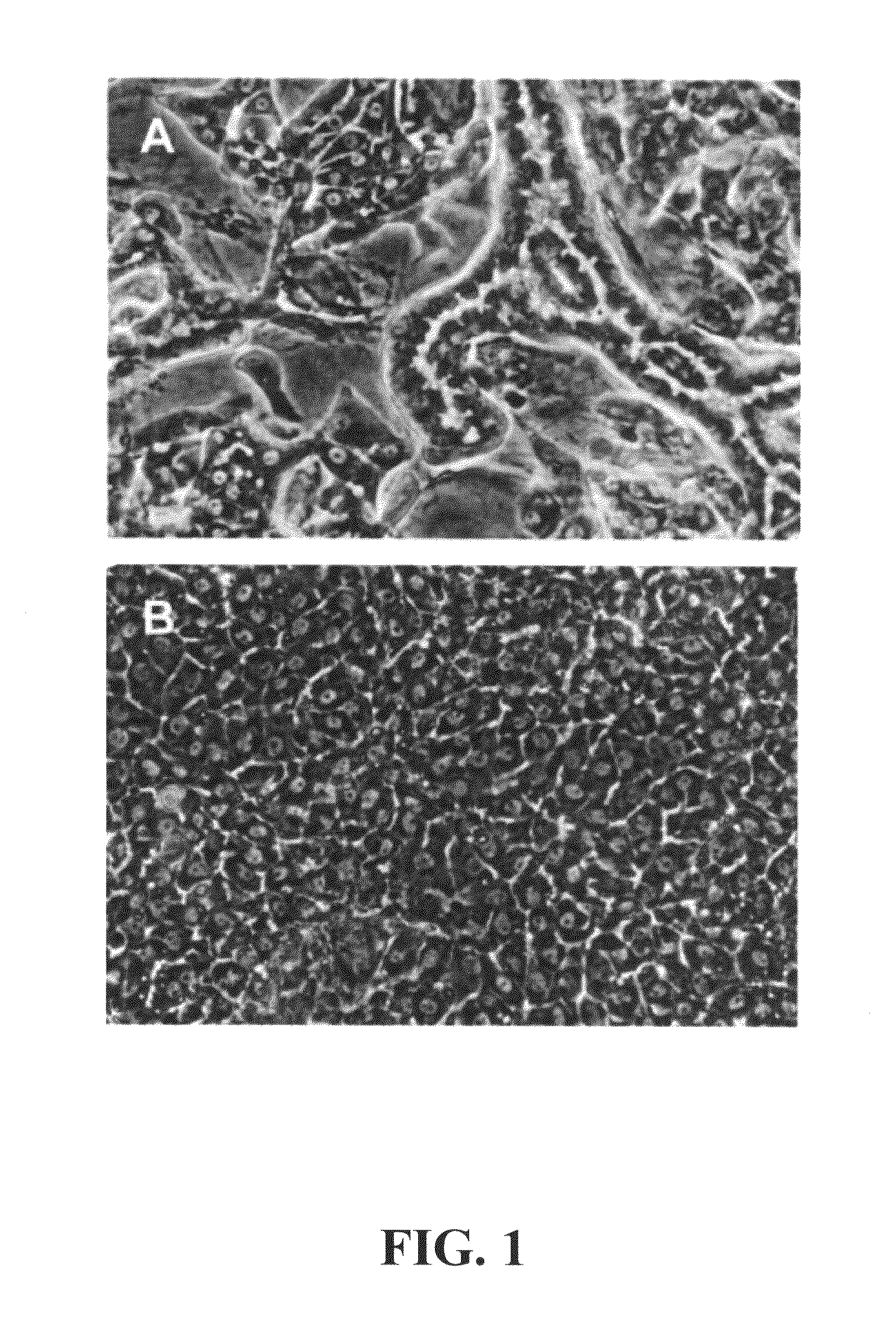
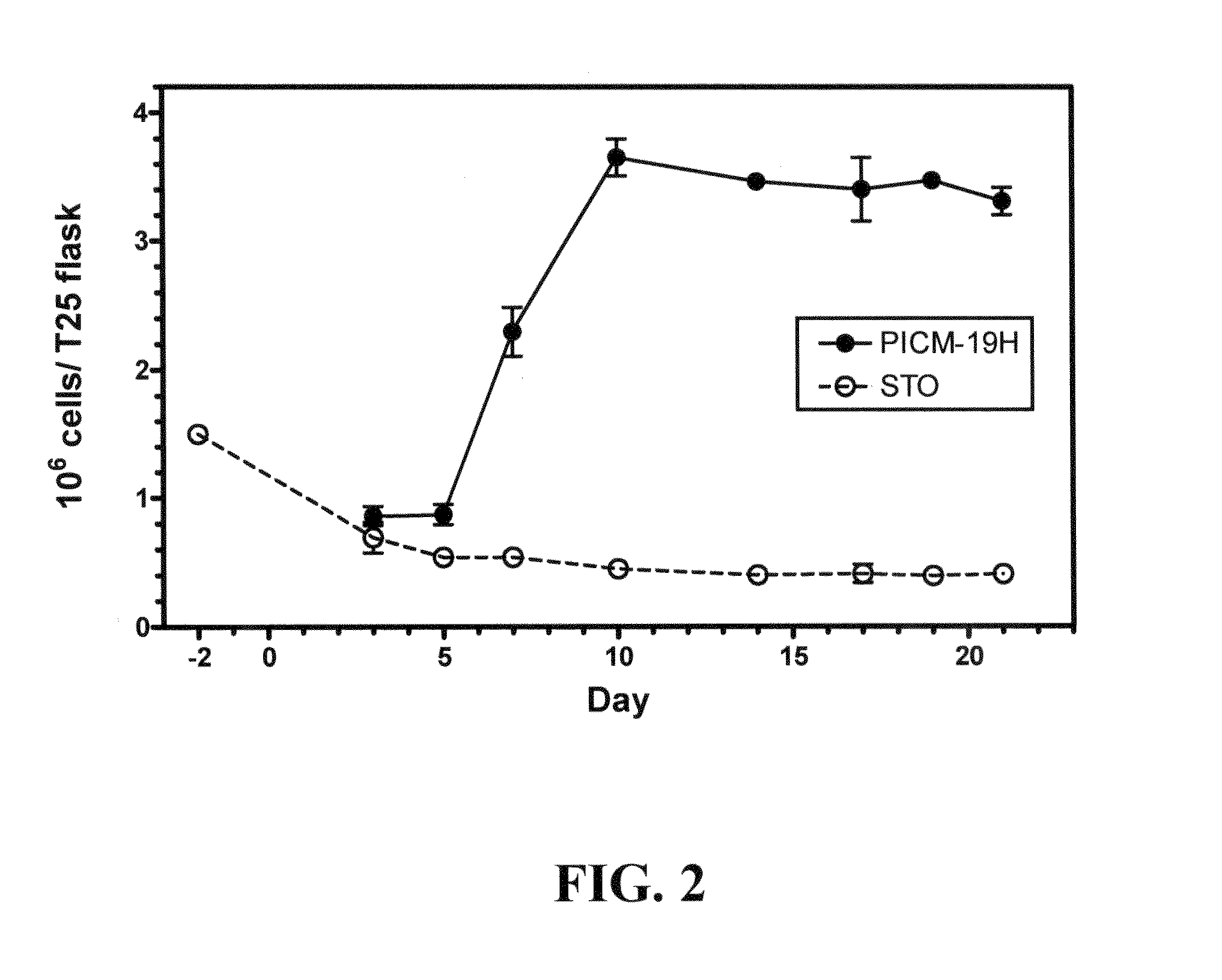
Immortal unipotent porcine PICM-19H and PICM-19B stem cell lines
InactiveUS8486699B2Confirmed its differentiationLow levelBiocideHepatocytesArtificial liverReticulum cell
Two cell lines, PICM-19H and PICM-19B, were derived from the bipotent ARS-PICM-19 pig liver stem cell line. The unipotent porcine stem cell line PICM-19H differentiates exclusively into hepatocytes and can be induced to express CYP450 enzymes. The growth rate and cell density in culture, morphological features, and hepatocyte detoxification functions, i.e., inducible CYP450 activity, ammonia clearance, and urea production of the PICM-19H cells were evaluated for their application in artificial liver devices. PICM-19H cells contain numerous mitochondria, Golgi apparatus, smooth and rough endoplasmic reticulum, vesicular bodies and occasional lipid vacuoles and display inducible CYP450 activity, clear ammonia, and produce urea in a glutamine-free medium. The data indicate that both cell lines, either together or alone, may be useful as the cellular substrate for an artificial liver device. The results demonstrate the potential for the use of PICM-19H cells in drug biotransformation and toxicity testing.
Owner:US SEC AGRI
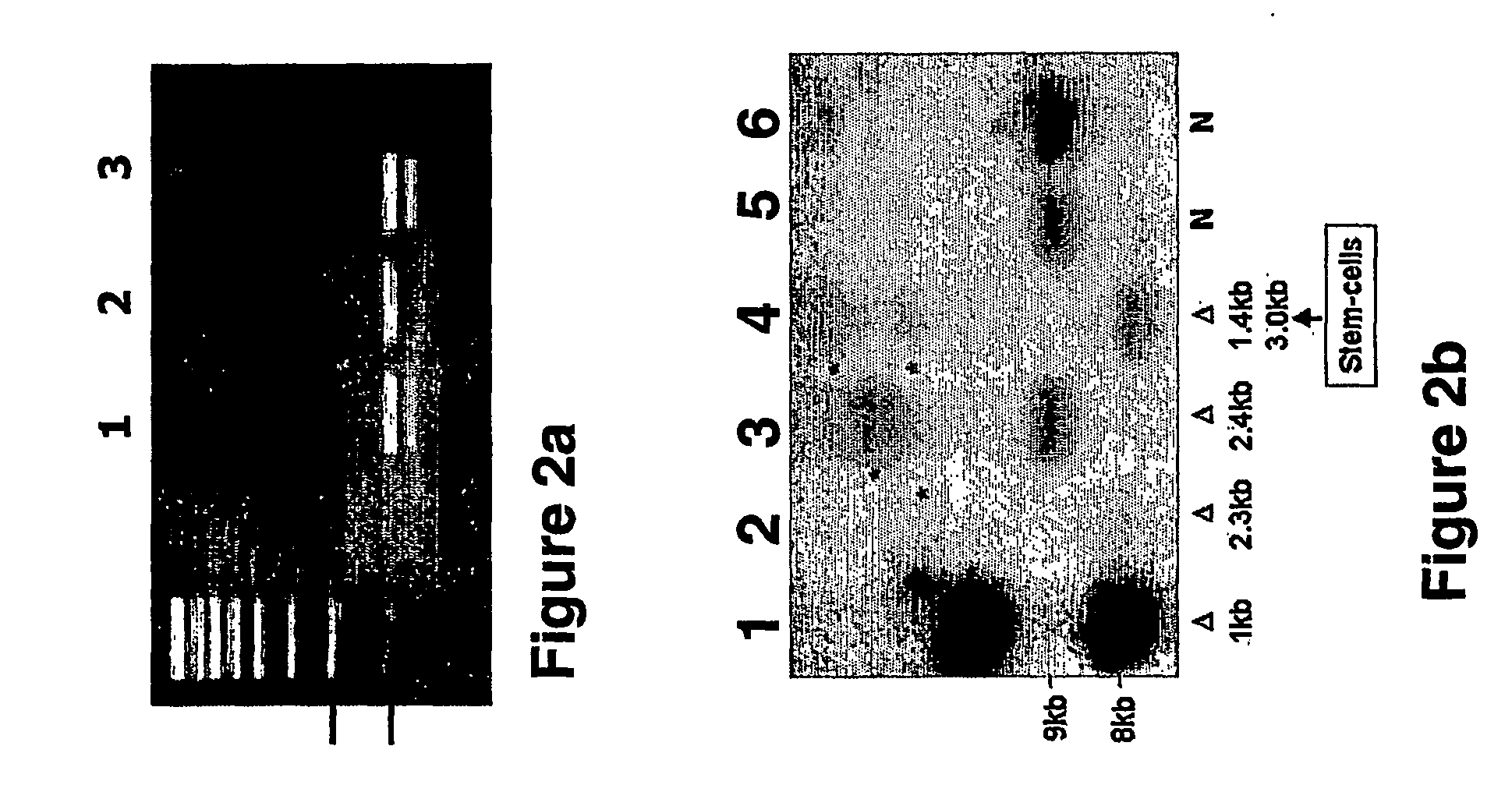
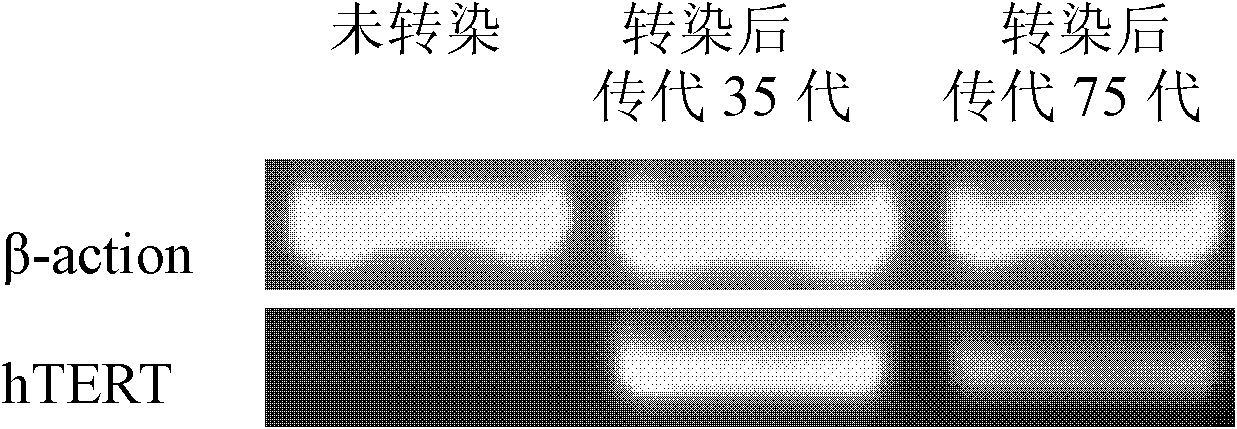
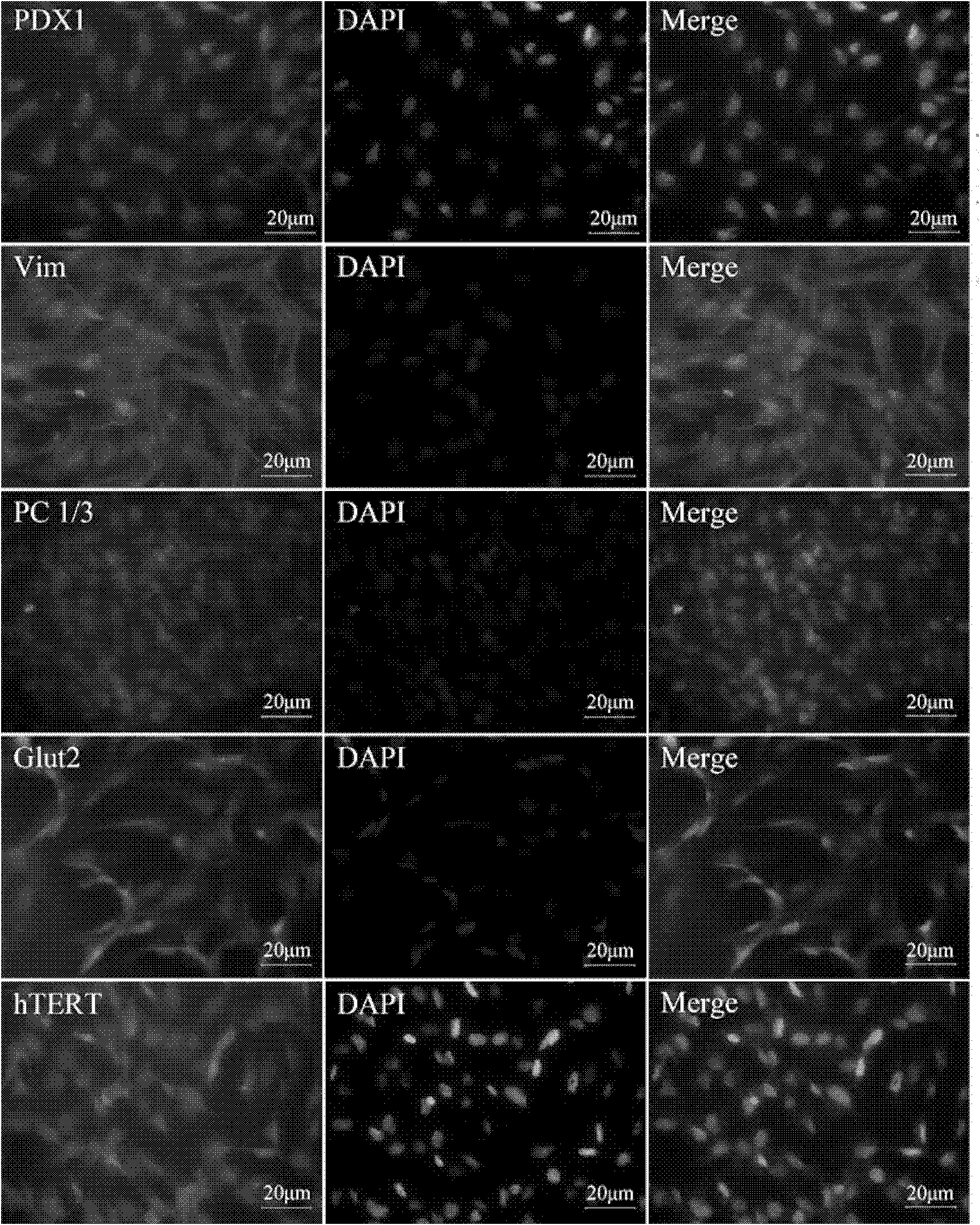
Method of preparing stem cell line for excreting insulin by using slow virus vectors of coding multiple exogenous gene
InactiveCN101100677AEasy to buildImprove practicalityVector-based foreign material introductionForeign genetic material cellsStem cell lineCell strain
Production of secretory insulin dry cell system by encoded various exogenous gene slow virus carrier is carried out by copying pWPST, cloning various insulin regulating genes to the carrier, transfecting 293T cell, packing, collecting virus grains, concentrating and transfecting human embryo pancreas dry cell. It's simple, efficient and practical, it can obtain one, two or three kinds of exogenous gene cells, which are in different expression and meet different needs.
Owner:CHINA JAPAN FRIENDSHIP HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com