Patents
Literature
66 results about "Murine cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
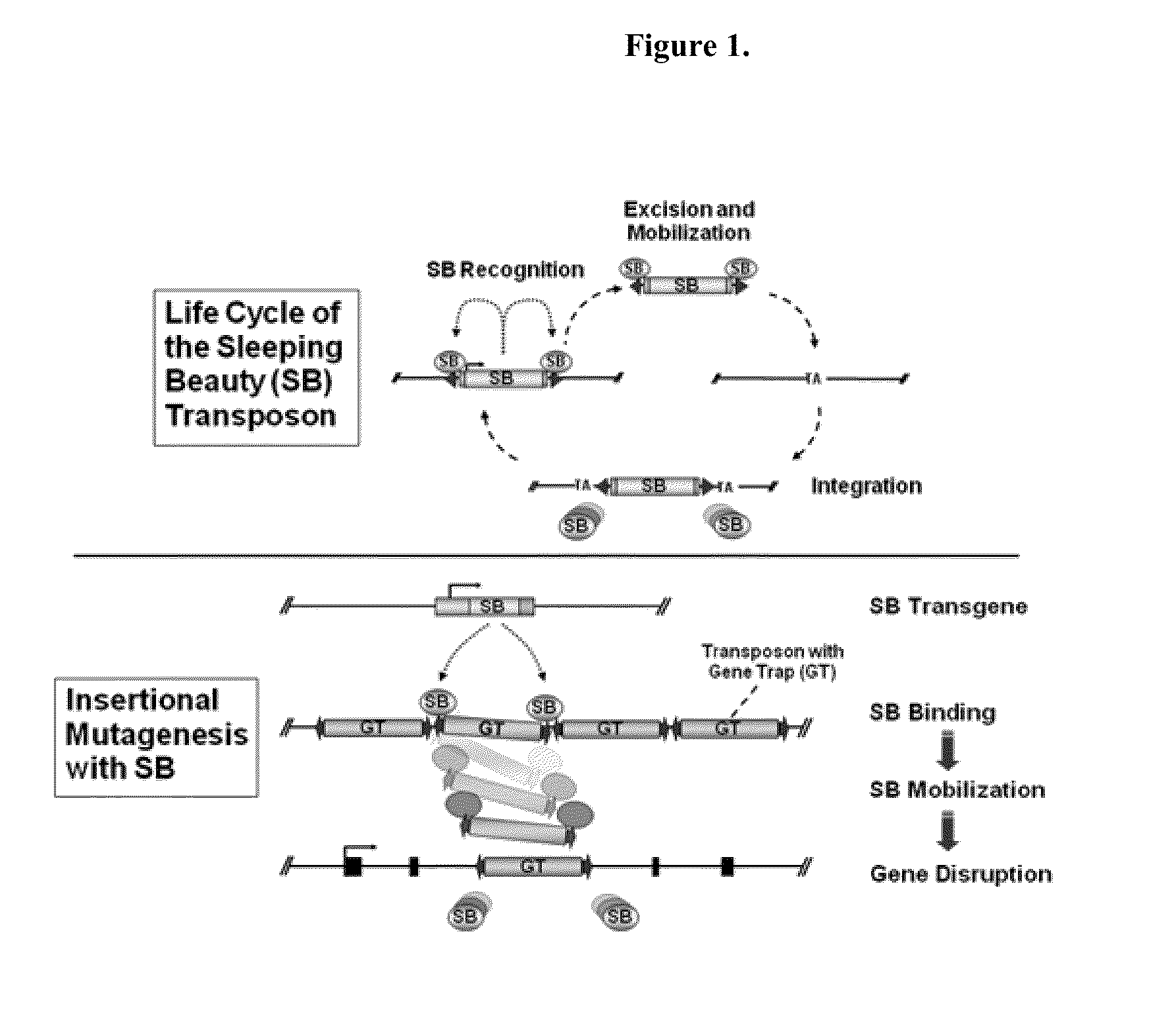
Murine cells are refractory to human immunodeficiency virus type 1 (HIV-1) replication at multiple stages of the viral life cycle. While this has allowed a finer inspection of the role of various host factors in HIV-1 replication, it has been an impediment to the development of a genetically modified mouse permissive to HIV-1 infection.
Process for producing polypeptide
InactiveUS7504256B1Efficient productionImprove metabolic efficiencyFused cellsImmunoglobulinsSerum igeSerum free media
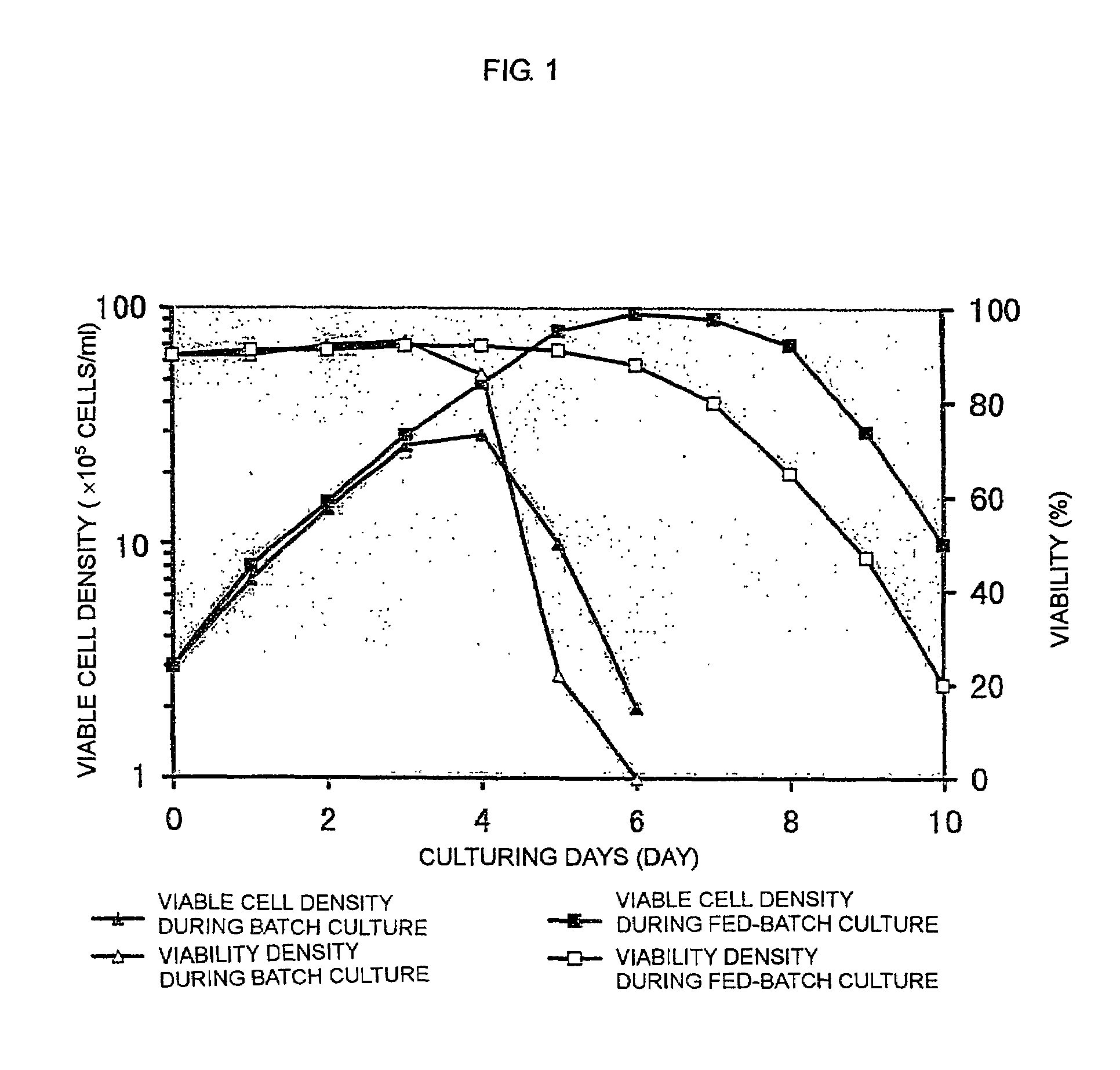
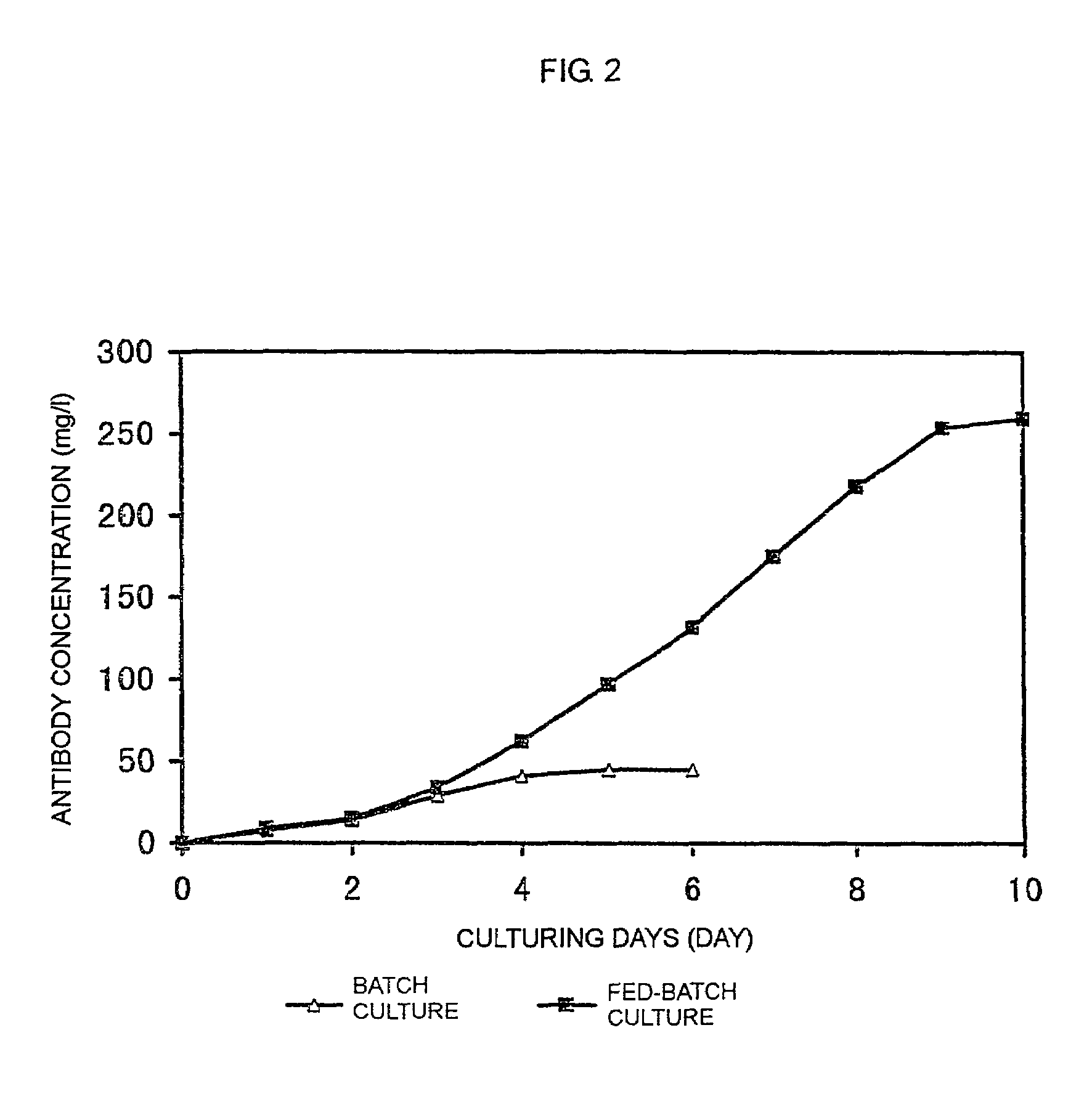
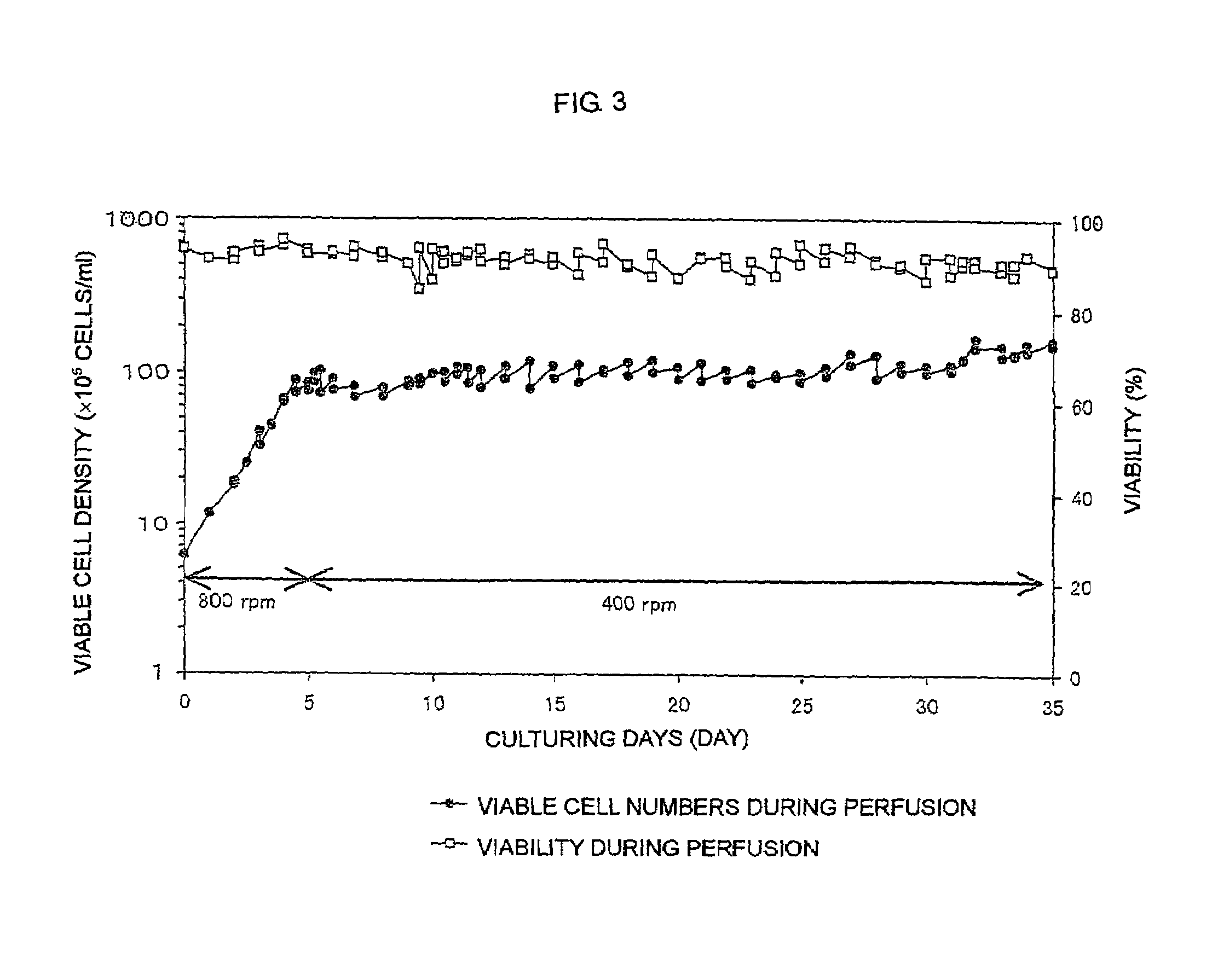
The present invention relates to a process for producing a desired polypeptide using rat cells. Specifically, the present invention relates to a process for producing the polypeptide which comprises culturing rat cells such as YB2 / 3HL.P2.G11.16Ag.20 (hereinafter referred to as YB2 / 0), preferably rat cells to which a recombinant DNA comprising DNA encoding a desired polypeptide such as an immunologically functional molecule is introduced, in a medium which does not contain serum (hereinafter referred to as a serum-free medium). Among the desired polypeptides obtained by the process of the present invention, an antibody obtained by using a transformant of YB2 / 0 has a high antibody-dependent cell-mediated cytotoxic activity (hereinafter sometimes referred to as ADCC activity) and is useful as a pharmaceutical agent.
Owner:KYOWA HAKKO KIRIN CO LTD
Mammalian common lymphoid progenitor cell
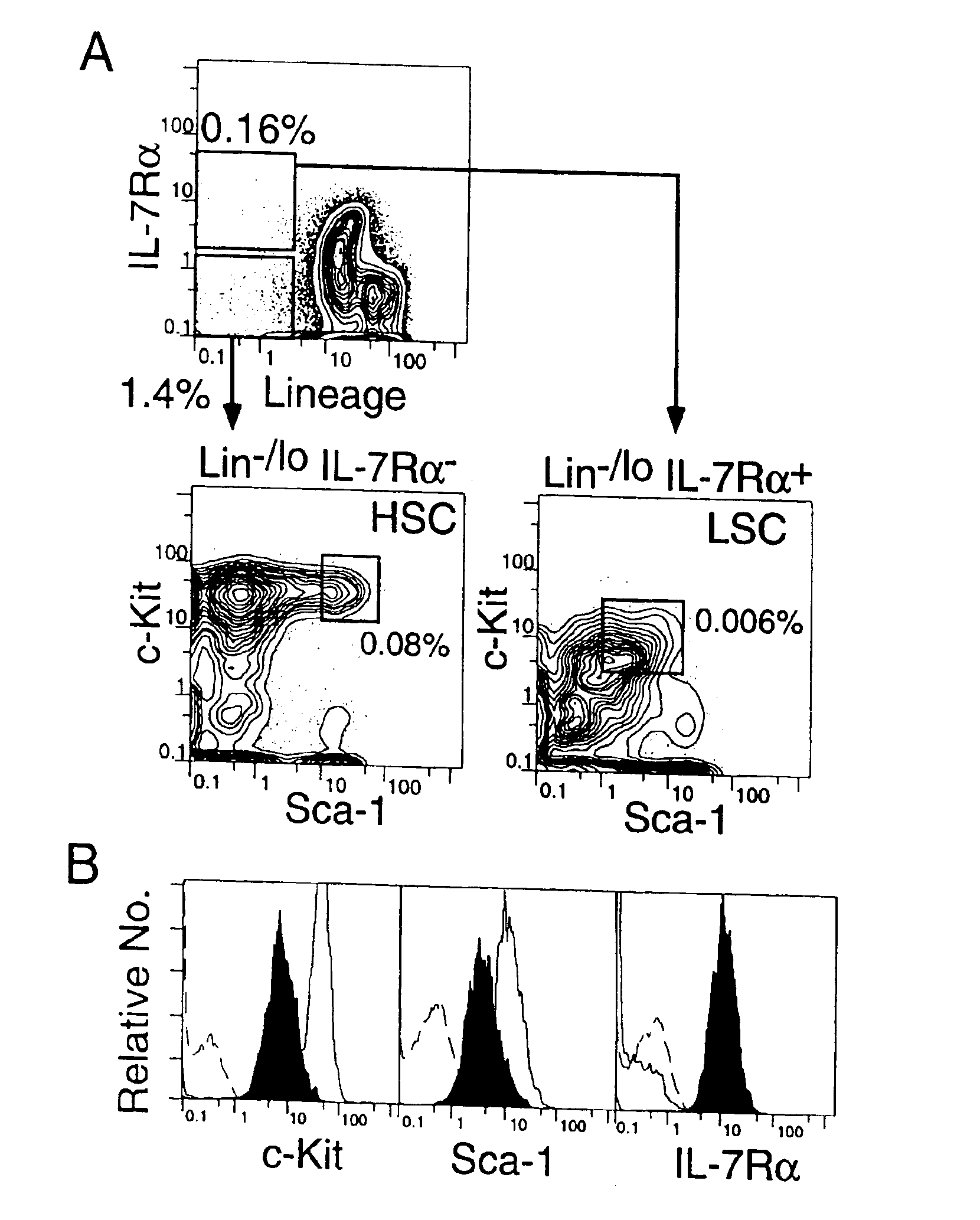
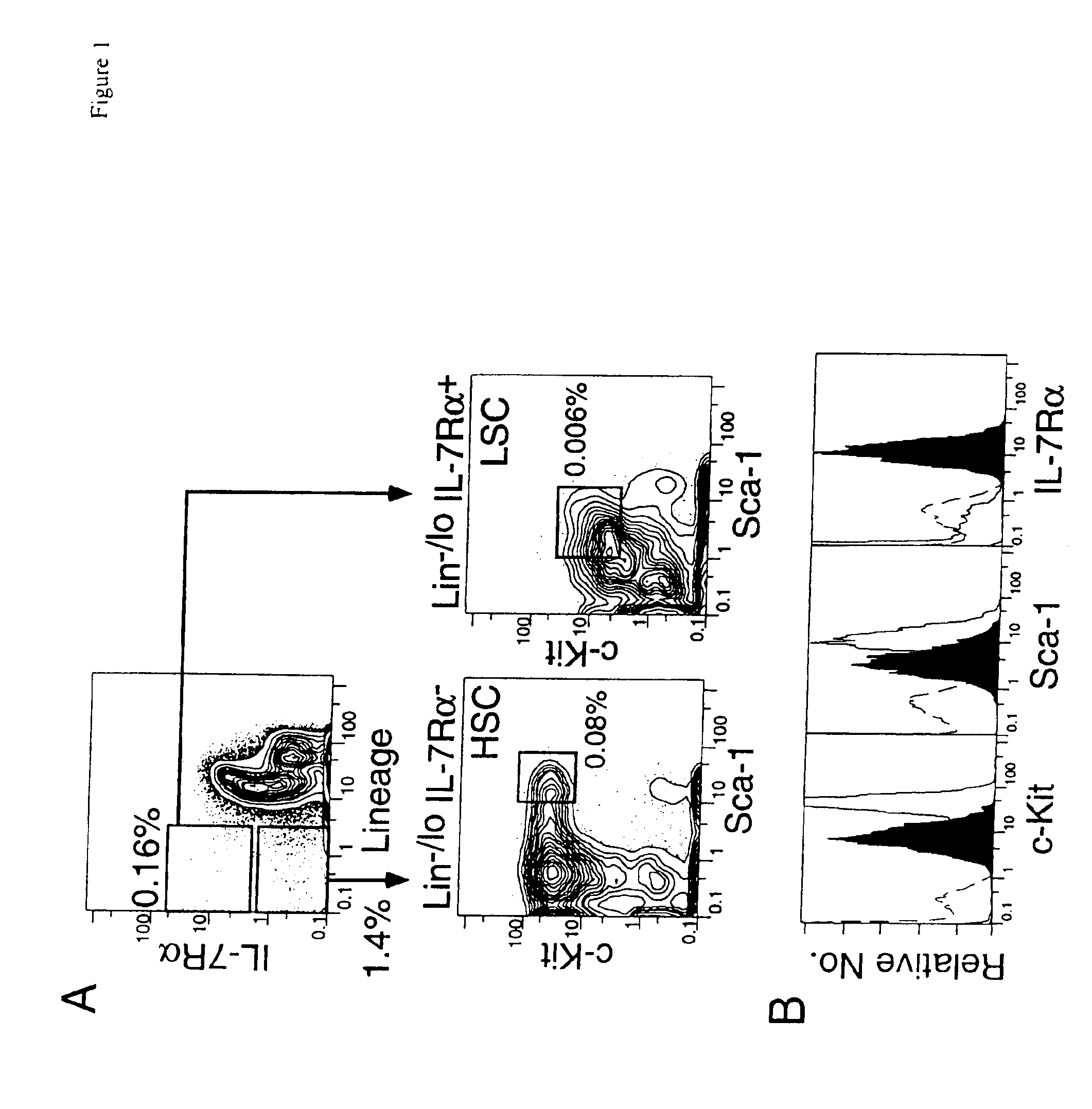
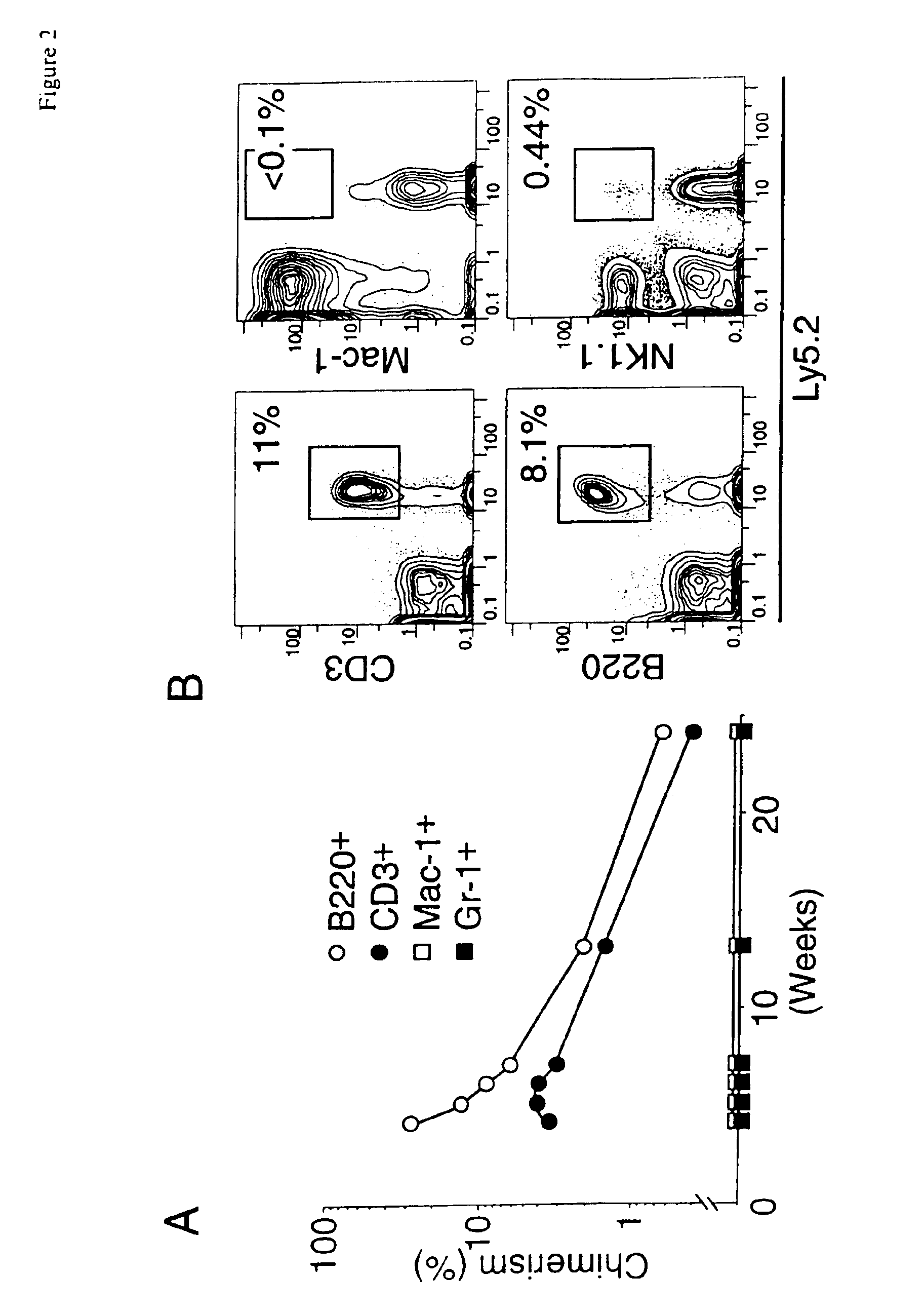
A substantially enriched mammalian hematopoietic cell subpopulation is provided, which is characterized by progenitor cell activity for lymphoid lineages, but lacking the potential to differentiate into myeloid and erythroid lineages. Methods are provided for the isolation and culture of this common lymphoid progenitor cell (CLP). The cell enrichment methods employ reagents that specifically recognize CDw127 (IL-7 receptor α); CD117 (c-kit) protein, in conjunction with other markers expressed on lineage committed cells. The murine cells are also characterized as expressing low levels of sca-1 (Ly-6E and Ly-6A). The CLPs are predominantly cycling, blast cells. These cells give rise to B cells, T cells and natural killer cells, as evidenced by their growth and differentiation in vitro and in vivo.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Method for tumor treatment using infusion of xenogeneic cells to induce hyperacute rejection and innocent bystander effect
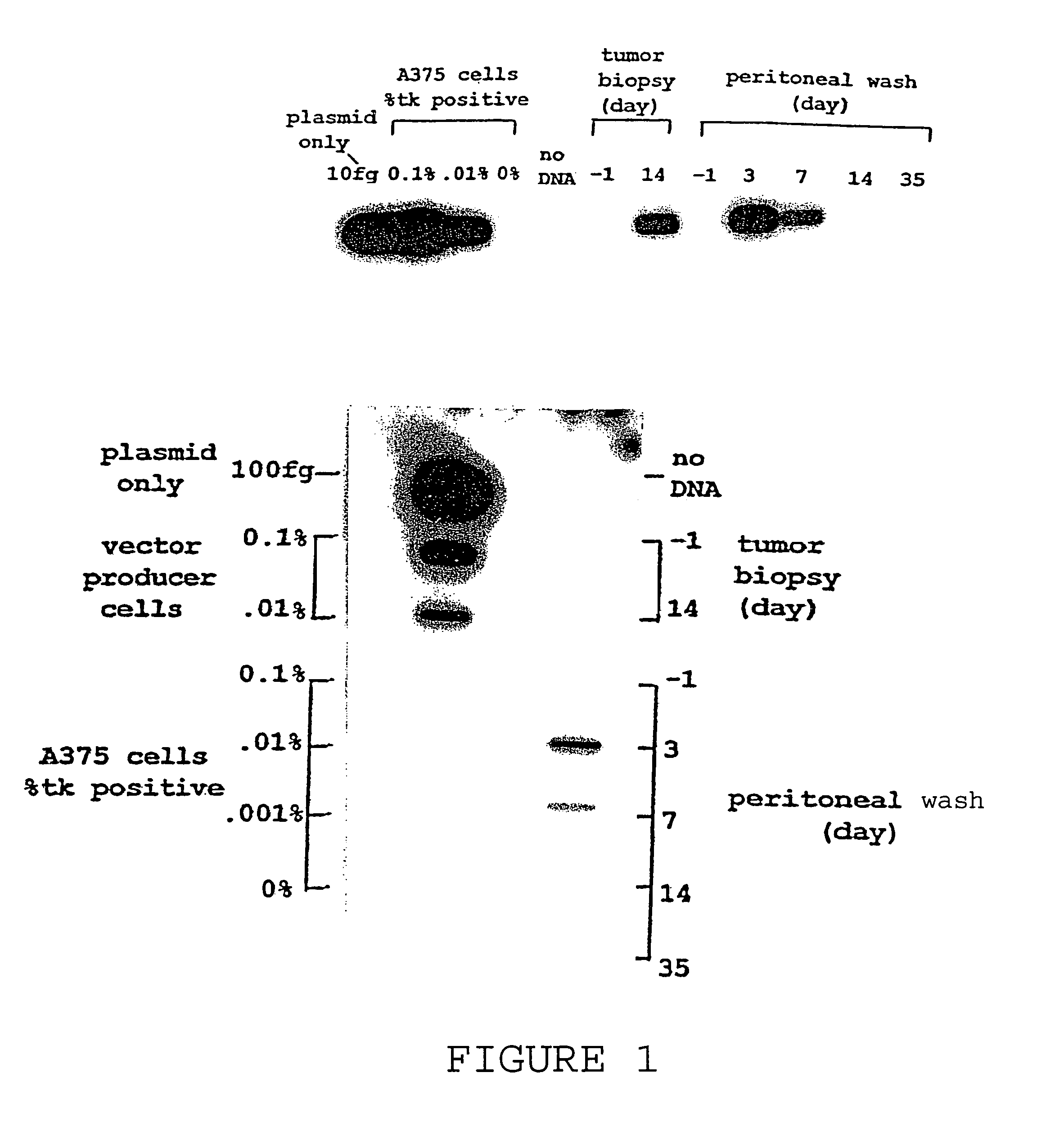
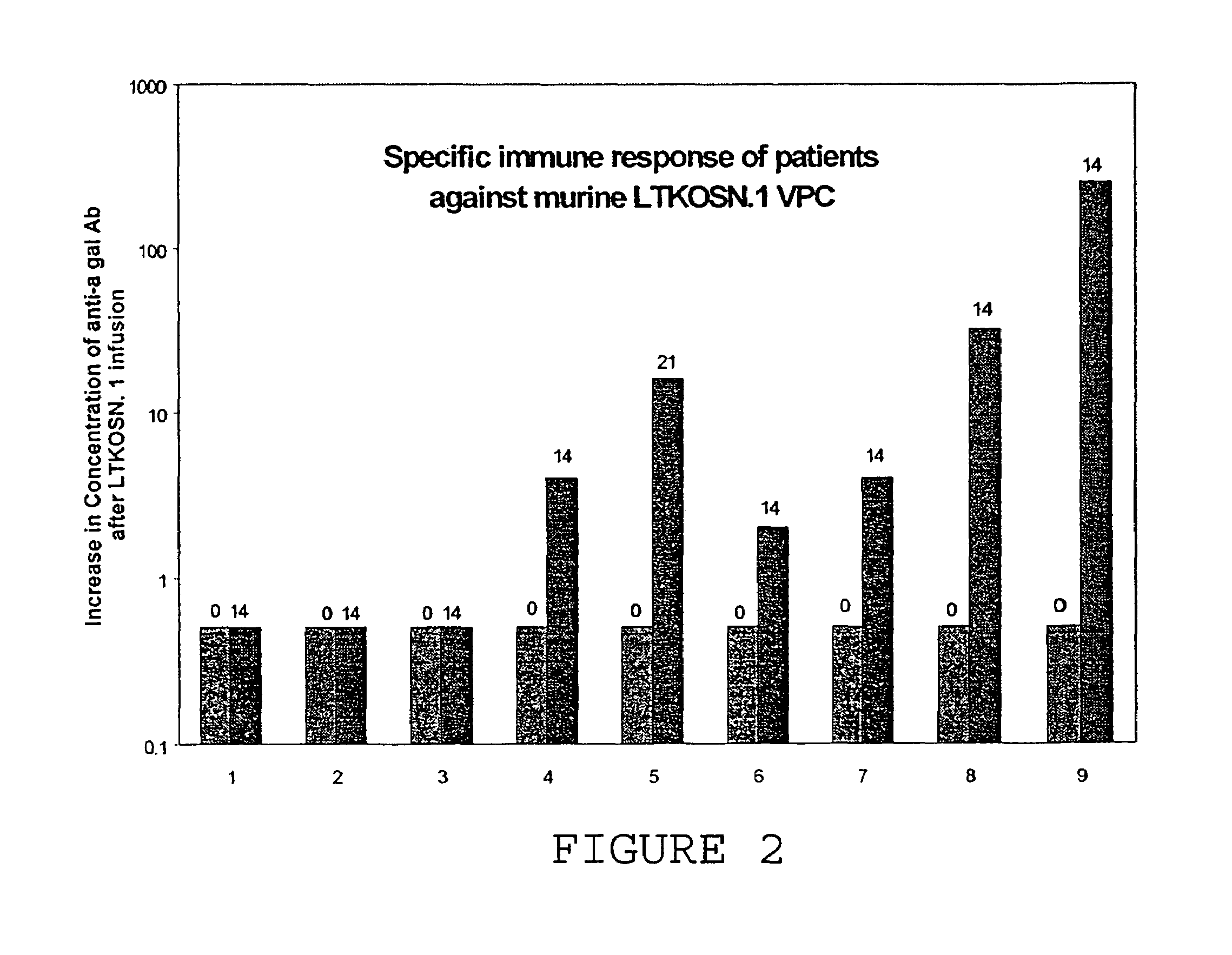
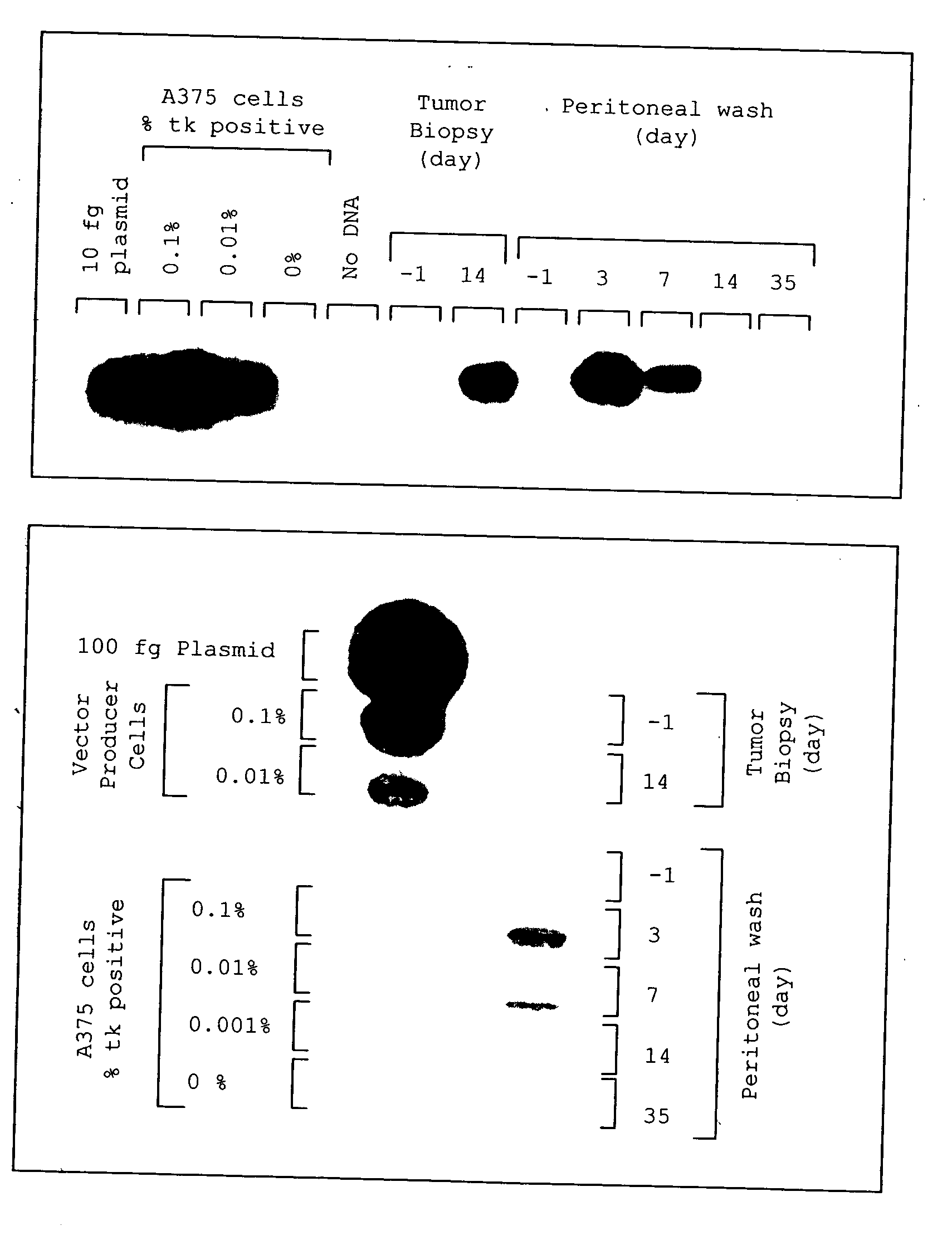
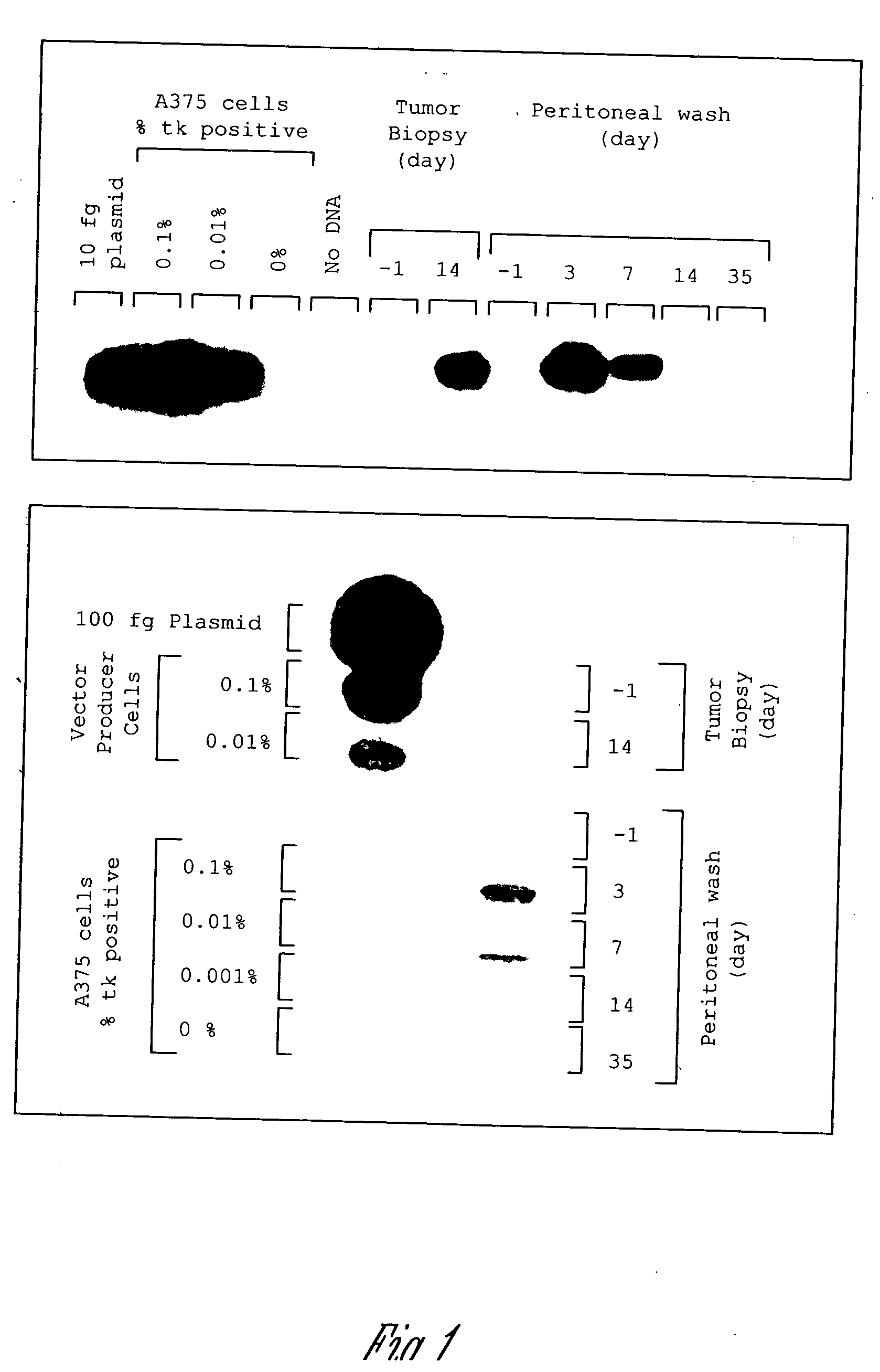
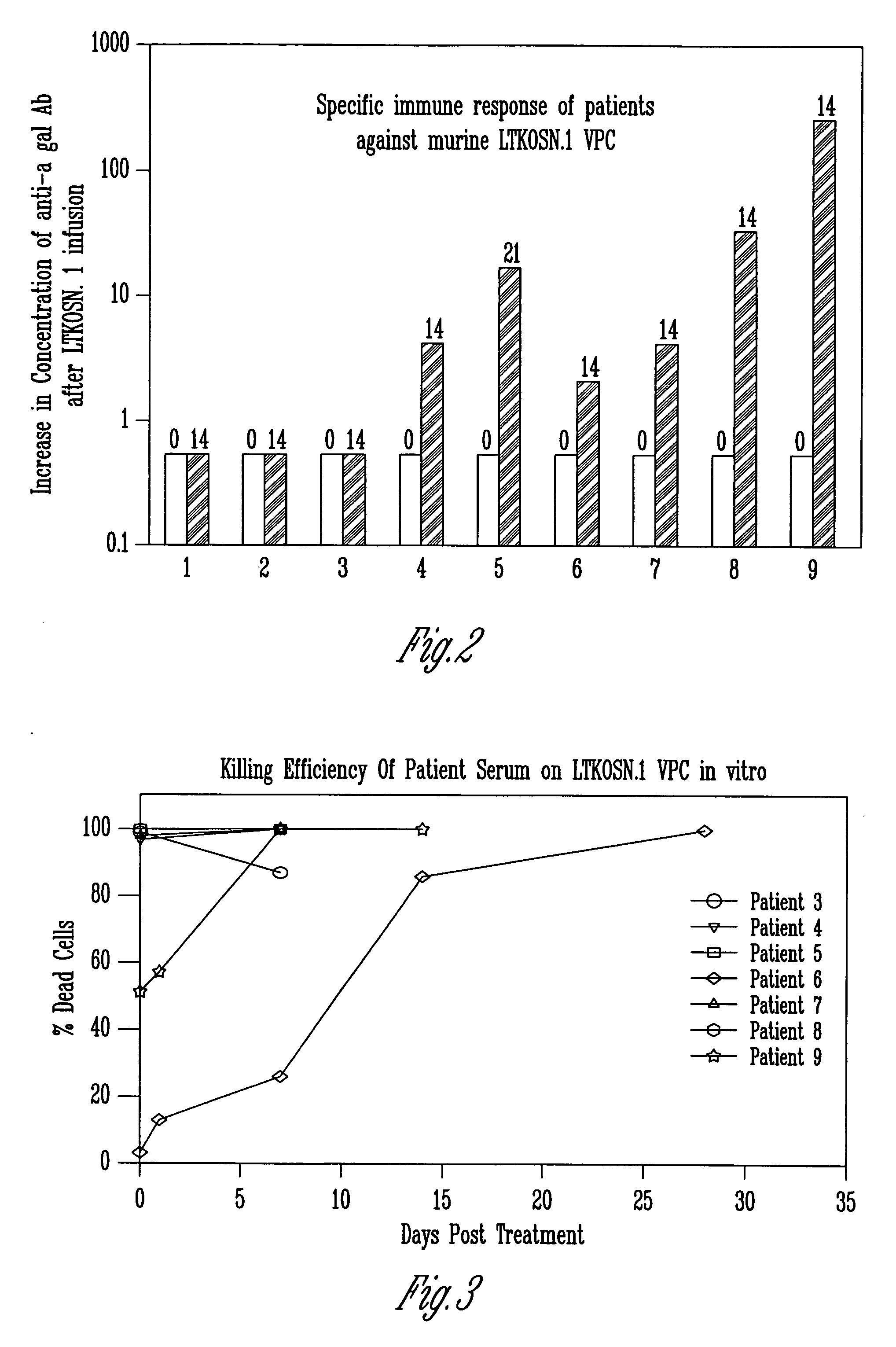
A method for treating tumors. Through infusion or xenotransplantation of xenogeneic cells, such as infusion of murine cells into the peritoneal cavity of humans, a hyperacute rejection response to the cells is induced. This in turn creates a bystander effect to the tumor. This effect creates tumor regression. This treatment can be used alone or in conjunction with gene therapy or chemotherapy treatments.
Owner:HUMAN GENE THERAPY RES INST
Covalently attached collagen VI for cell attachment and proliferation
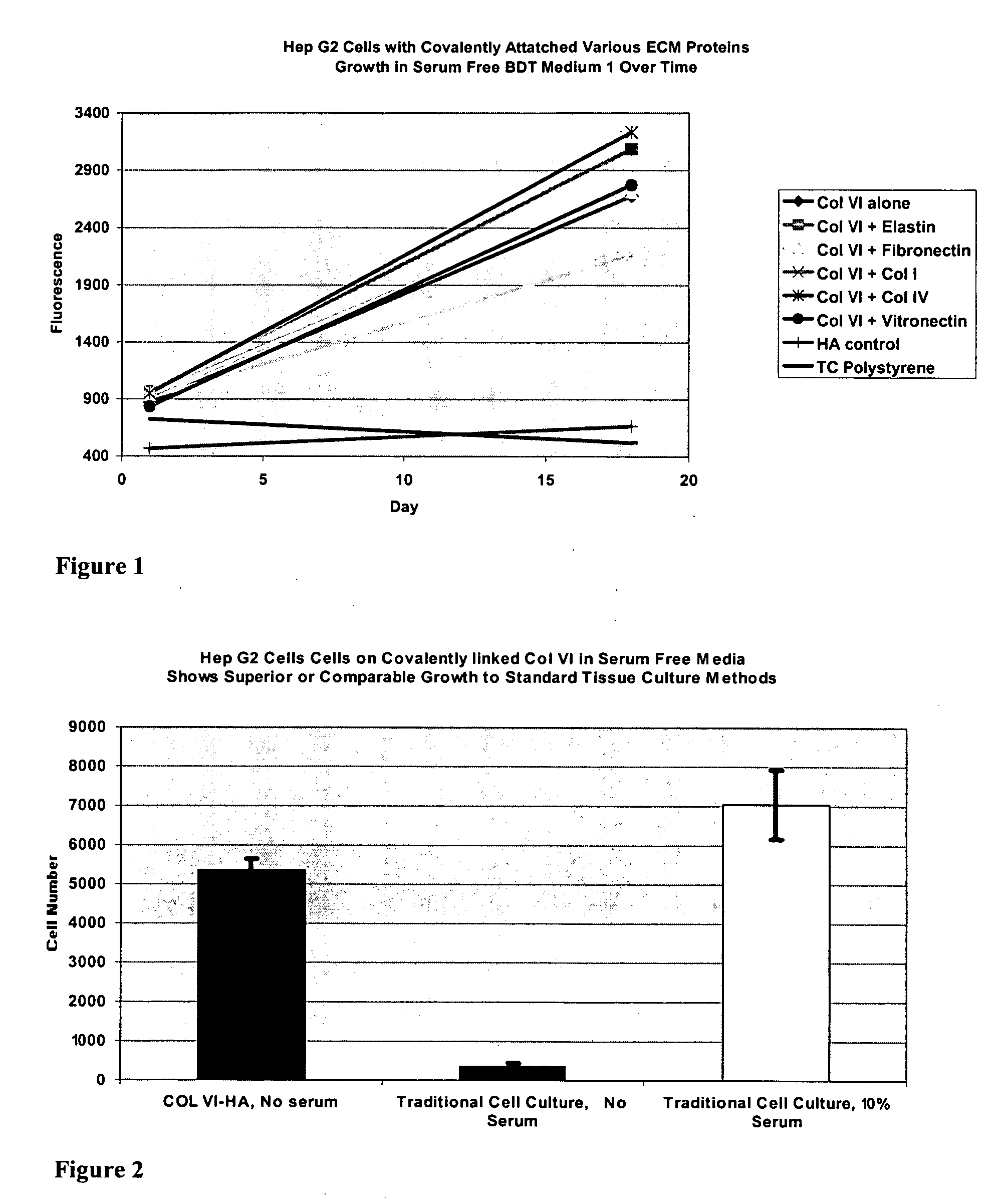
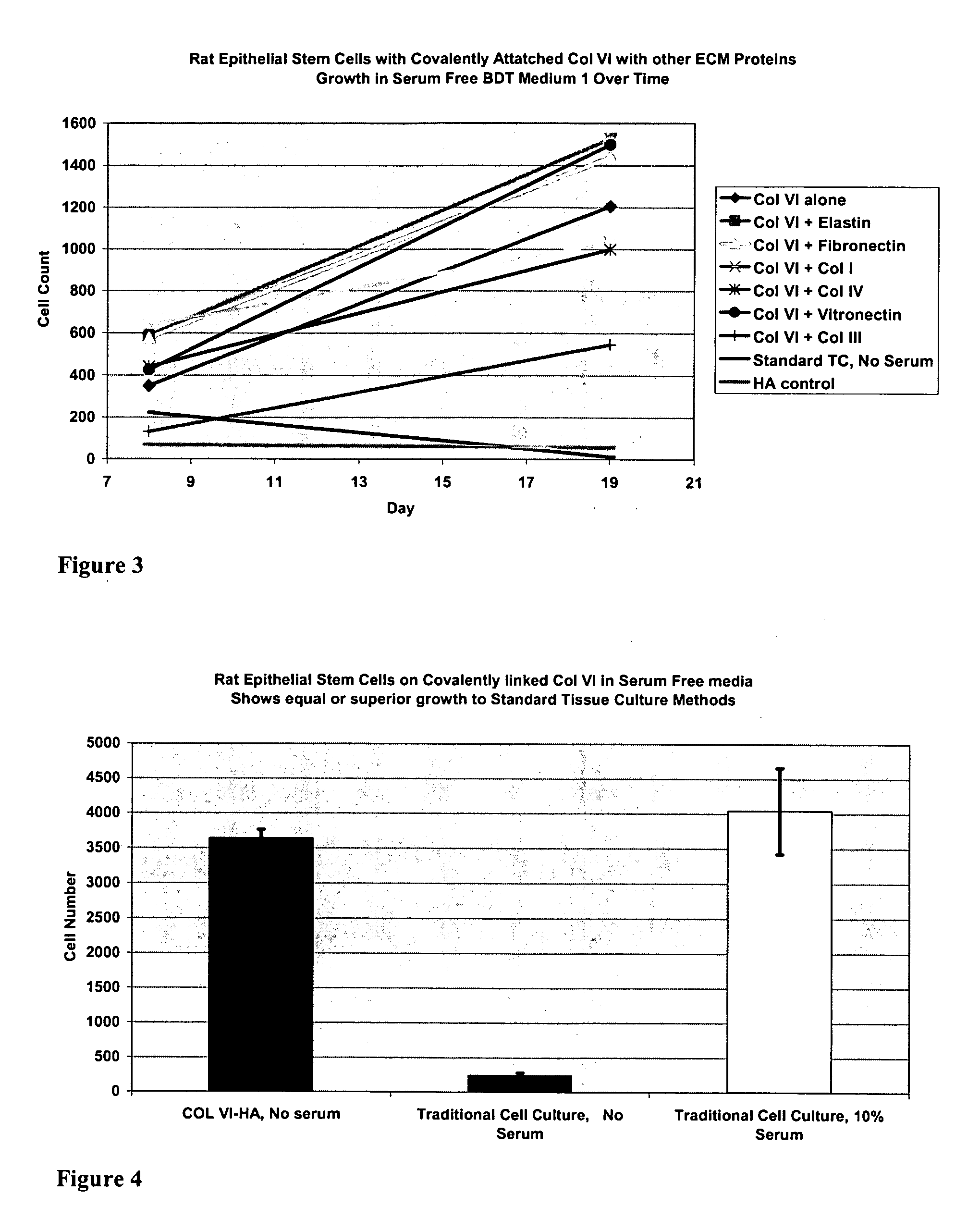
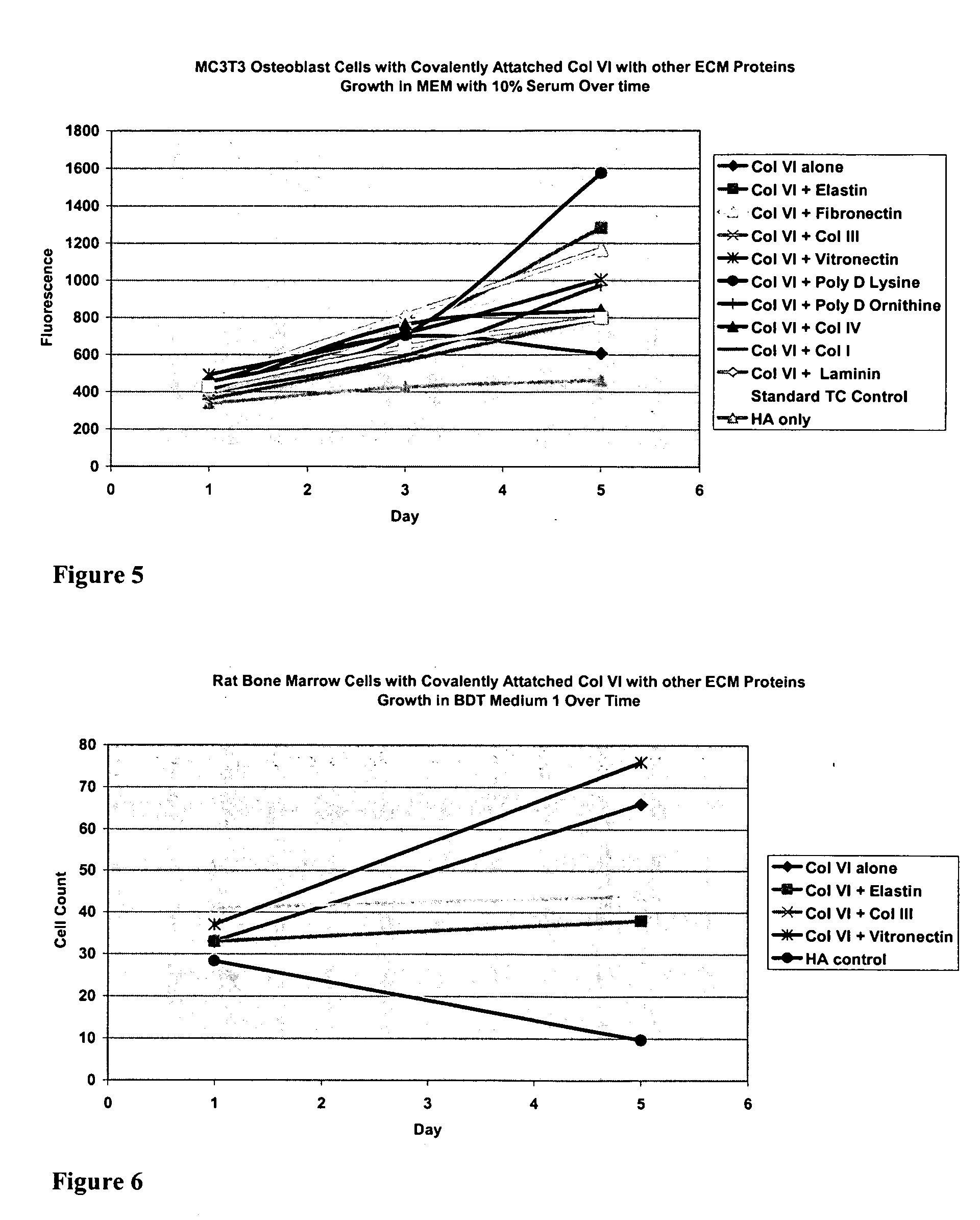
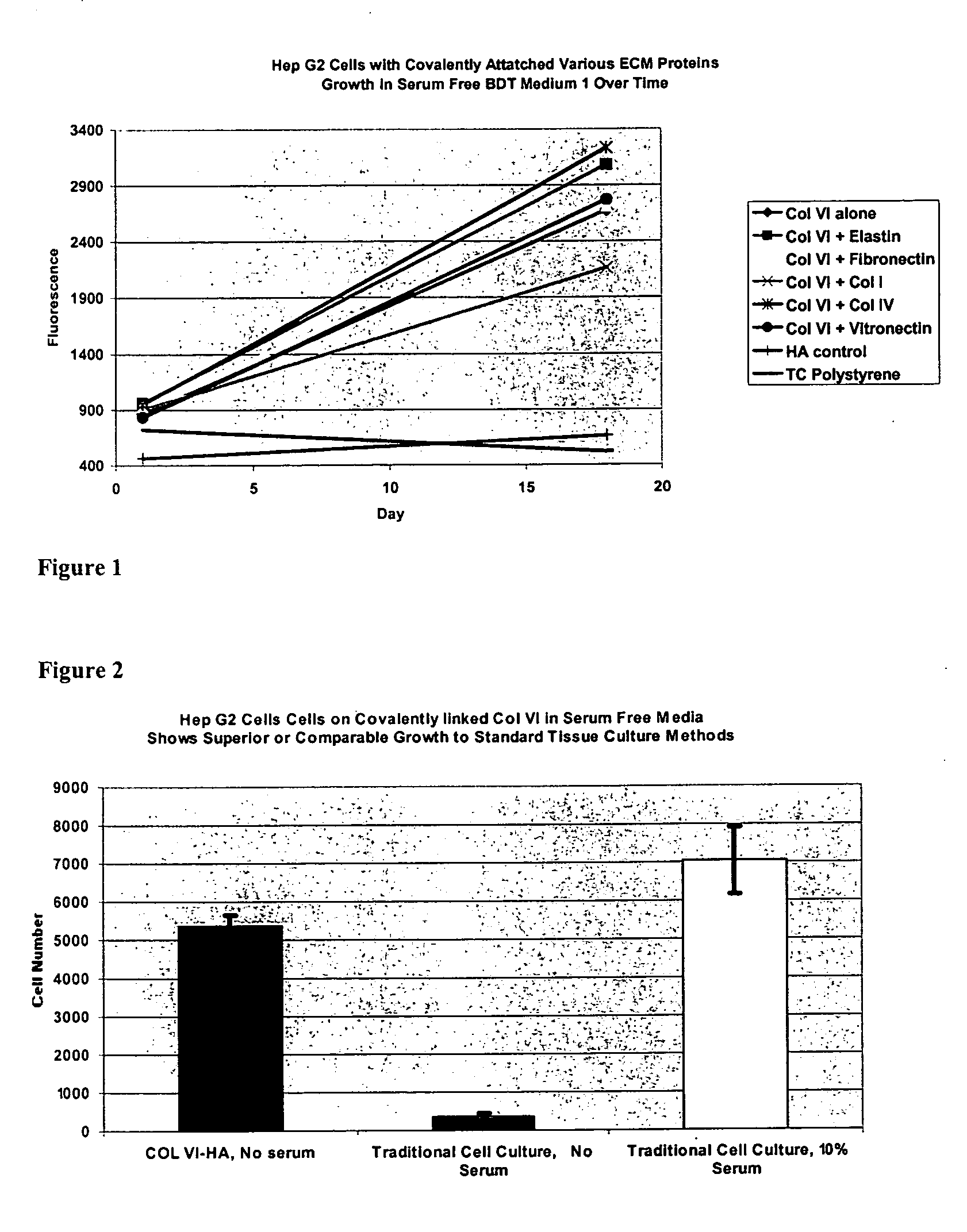
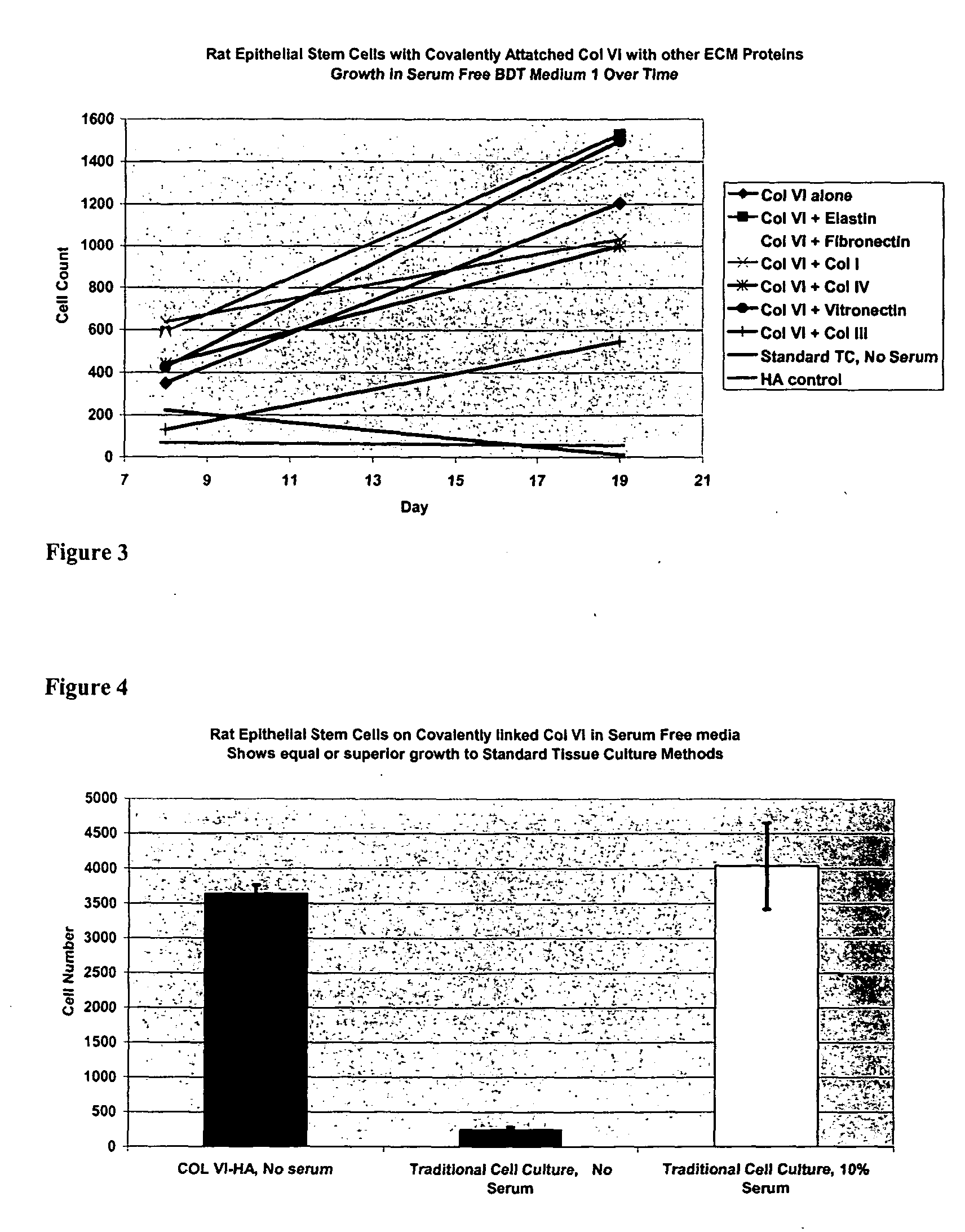
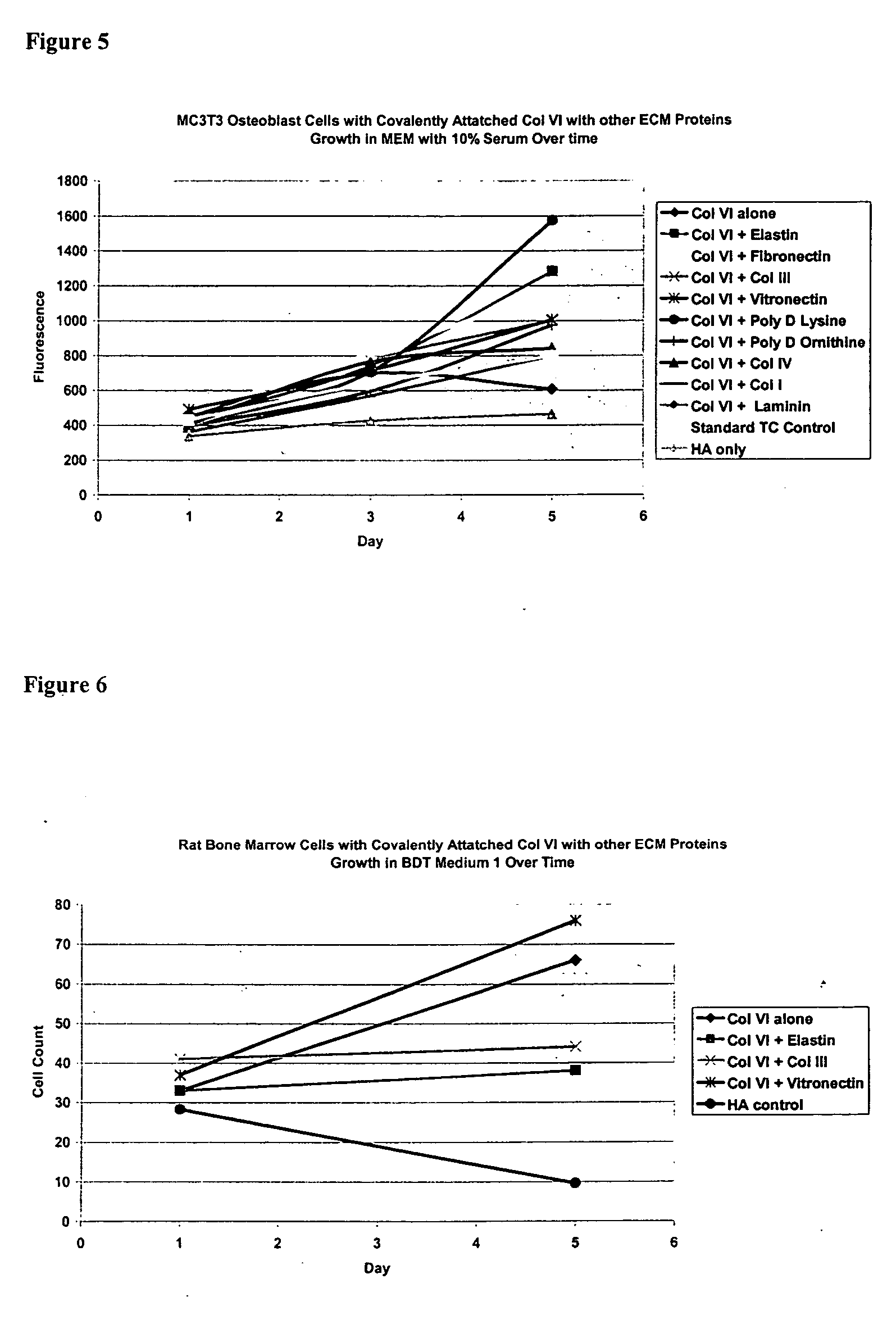
Surfaces useful for cell culture comprise a support to which is bound a CAR material, and, bound to the CAR material, collagen VI or a biologically active fragment or variant thereof and, optionally, one or more other ECM proteins (or fragments or variants thereof) such as elastin, fibronectin, vitronectin, tenascin, laminin, entactin, aggrecan, decorin, collagen I, collagen III, and collagen IV. Also, optionally present on the surface is one or more polycationic polymers, such as poly-D-lysine or poly-D-ornithine. This surface is used in cell culture to promote cell attachment, survival, and / or proliferation of a number of different cell types such as (a) liver cells (e.g., HepG2 tumor cells, and a newly discovered line of rat liver epithelial stem cells) (b) osteoblasts, such as the murine cell line MC3T3 cell line and (c) primary bone marrow cells. Kits comprising the surfaces and additional reagents are also disclosed.
Owner:BECTON DICKINSON & CO
Covalently attached collagen VI for cell attachment and proliferation
InactiveUS20050058687A1Promote proliferationEasy SurvivalBioreactor/fermenter combinationsBiological substance pretreatmentsCollagen VIPolymer
Surfaces useful for cell culture comprise a support to which is bound a CAR material, and, bound to the CAR material, collagen VI or a biologically active fragment or variant thereof and, optionally, one or more other ECM proteins (or fragments or variants thereof) such as elastin, fibronectin, vitronectin, tenascin, laminin, entactin, aggrecan, decorin, collagen I, collagen III, and collagen IV. Also, optionally present on the surface is one or more polycationic polymers, such as poly-D-lysine or poly-D-ornithine. This surface is used in cell culture to promote cell attachment, survival, and / or proliferation of a number of different cell types such as (a) liver cells (e.g., HepG2 tumor cells, and a newly discovered line of rat liver epithelial stem cells) (b) osteoblasts, such as the murine cell line MC3T3 cell line and (c) primary bone marrow cells. Kits comprising the surfaces and additional reagents are also disclosed.
Owner:BECTON DICKINSON & CO
Conditioned cell culture medium, method to obtain the same and use of it for maintenance, proliferation and differentiation of mammalian cells
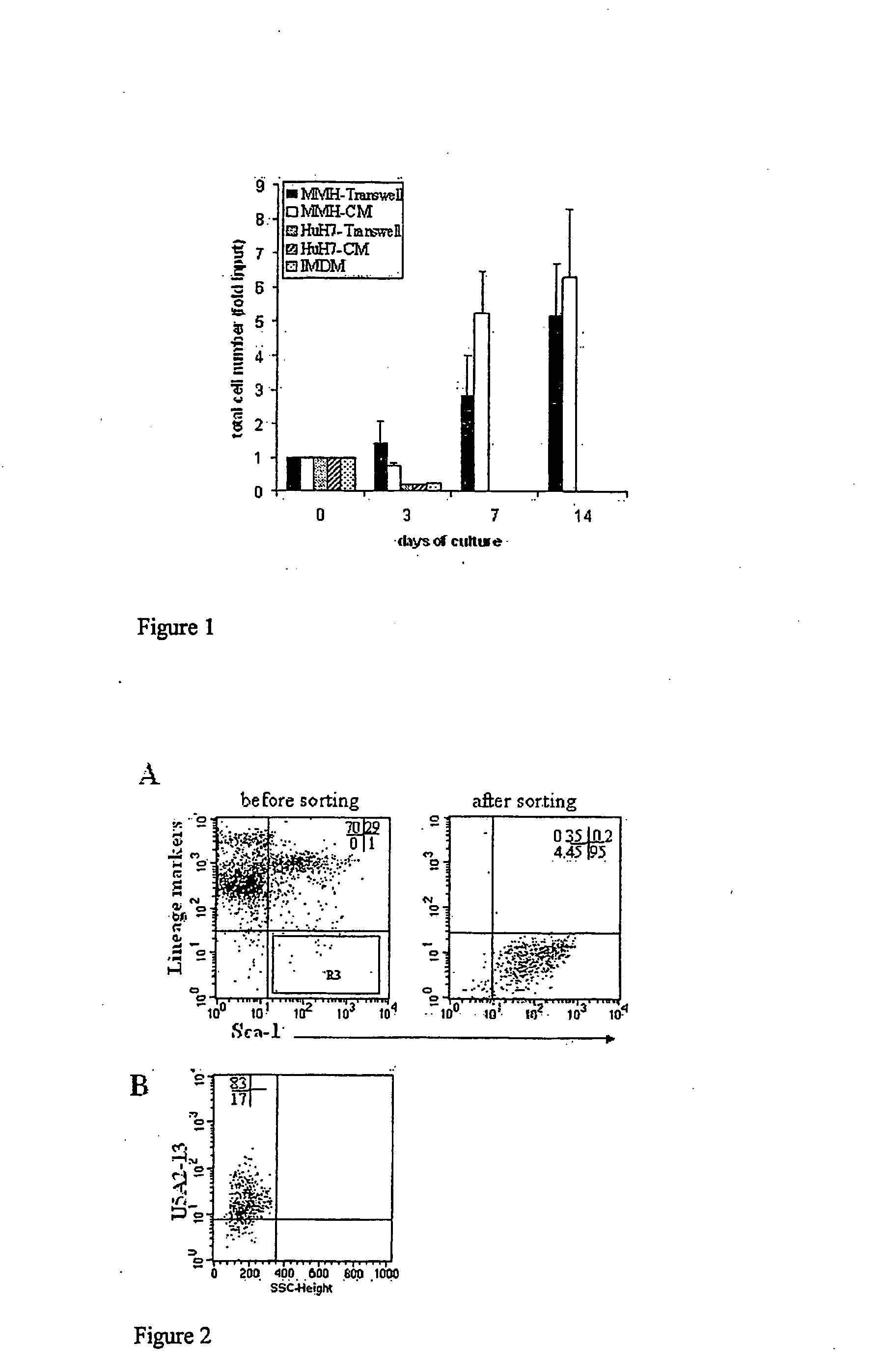
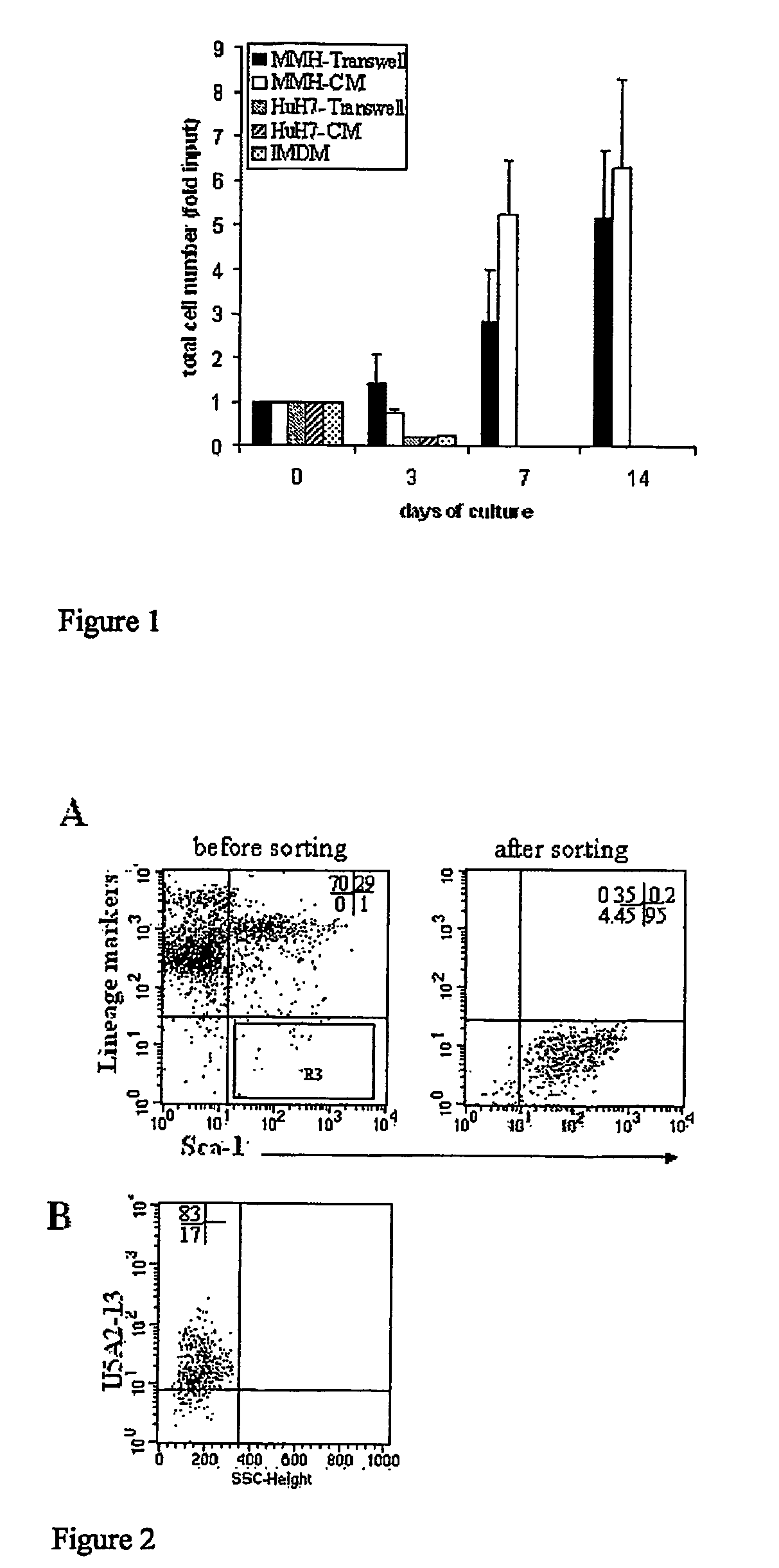
The invention relates to a conditioned cell culture medium and a corresponding method to obtain it. The invention also refers to methods of using this cellconditioned medium for the maintenance, proliferation and differentiation of mammalian cells. The culture medium produced in accordance with the present invention is conditioned by the cell secretion activity of murine cells, in particular, those differentiated and immortalized transgenic hepatocytes, named MMH (Met Murine Hepatocyte). These media are employed in in vitro cell culture systems to induce maintenance, proliferation and differentiation of mammalian cells. The cells named MMH are differentiated non transformed murine hepatocytes that produce important biological molecules (e.g cytokines and growth factors) and, in accordance with the present invention, they are used in in vitro cell culture systems for the maintenance, proliferation and differentiation of mammalian cells.
Owner:INST NAT PER LE MALATTIE INFETTIVE LAZZARO SPALLANZANI IRCCS
Nucleic acids, polypeptides, compositions, and methods for modulating apoptosis
InactiveUS20030064952A1Convenient amountEfficient screeningAntibacterial agentsOrganic active ingredientsInitiation factorDeoxyhypusine synthase
The present invention relates to isolated and / or purified rat apoptosis-specific eucaryotic initiation Factor-5A (eIF-5A) and deoxyhypusine synthase (DHS) nucleic acids and polypeptides. The present invention also relates to methods of modulating apoptosis using apoptosis-specific eIF-5A and DHS, and antisense oligonucleotides and expression vectors of apoptosis-specific eIF-5A and DHS useful in such methods.
Owner:SENESCO TECHNOLOGIES INC
Genetically Modified Rat Models for Drug Metabolism
The present invention provides a desired rat or a rat cell which contains a predefined, specific and desired alteration rendering the rat or rat cell predisposed to alterations in drug and chemical metabolism by modification of its structure or mechanism. Specifically, the invention pertains to a genetically altered rat, or a rat cell in culture, that is defective in at least one of two alleles of a drug metabolism gene such as the Cyp7b1 gene, the Cyp3a4 gene, etc. In another embodiment, the rat cell is a somatic cell. The inactivation of at least one drug metabolism allele results in an animal with a higher susceptibility to altered drug and chemical metabolism. In one embodiment, the genetically altered animal is a rat of this type and is able to serve as a useful model for altered drug and chemical metabolism or toxicology and as a test animal for autoimmune and other studies. The invention additionally pertains to the use of such rats or rat cells, and their progeny in research and medicine. In one embodiment, the invention provides a genetically modified or chimeric rat cell whose genome comprises two chromosomal alleles of a drug metabolism gene wherein at least one of the two alleles contains a mutation, or the progeny of the cell.
Owner:TRANSPOSAGEN BIOPHARM
Endothelial cell growth factor methods of isolation and expression
InactiveUS20060025577A1Peptide/protein ingredientsPeptide preparation methodsGranular leucocyteGranulosa cell
A novel growth factor specific for vascular endothelial cells has been identified in conditioned medium of bovine pituitary derived folliculo stellate cells. This factor, named folliculo stellate derived growth facto (FSdGF) or vascular endothelial growth factor (VEGF), was purified to homogeneity by a combination of heparin sepharose affinity chromatography, Bio Gel P-60 exclusion chromatography, Mono S ion exchange chromatography and hydrophobic chromatography on a C4 reverse phase HPLC column. The factor is also found in the murine AtT-20 cell line. Alternatively, the growth factor is purified by a first reverse phase HPLC using acetonitrile gradient followed by a second reverse phase HPLC using an isopropanol gradient. FSdGF, having a molecular weight of about 43,000 da, was characterized as a glycoprotein composed of two homologous sub units with MW of about 23 kDa. FSdGF was a potent mitogen for vascular endothelial cells with activity detectable at 10 pg / ml and saturation at 500 pg / ml. It did not stimulate the proliferation of other cell types such as bovine corneal endothelial cells, adrenal cortex cells, granulosa cells, BALB / MK cells or BHK-21 cells. Microsequencing revealed an amino terminal sequence containing no significant homology to any known protein. The release of FSdGF by pituitary cells and its unique target cell specificity indicate that FSdGF is useful in angiogenesis.
Owner:FERRARA NAPOLEONE +2
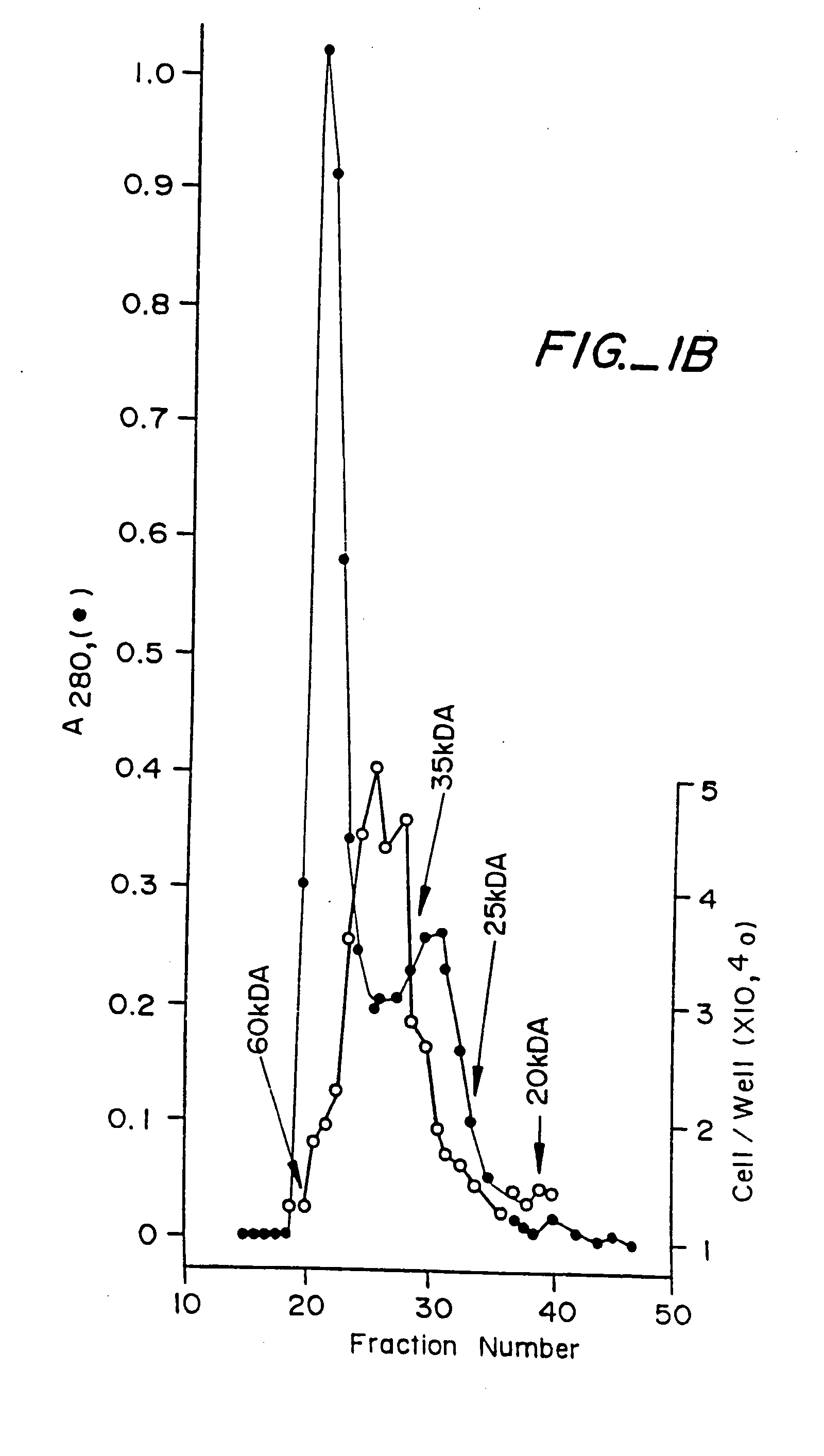
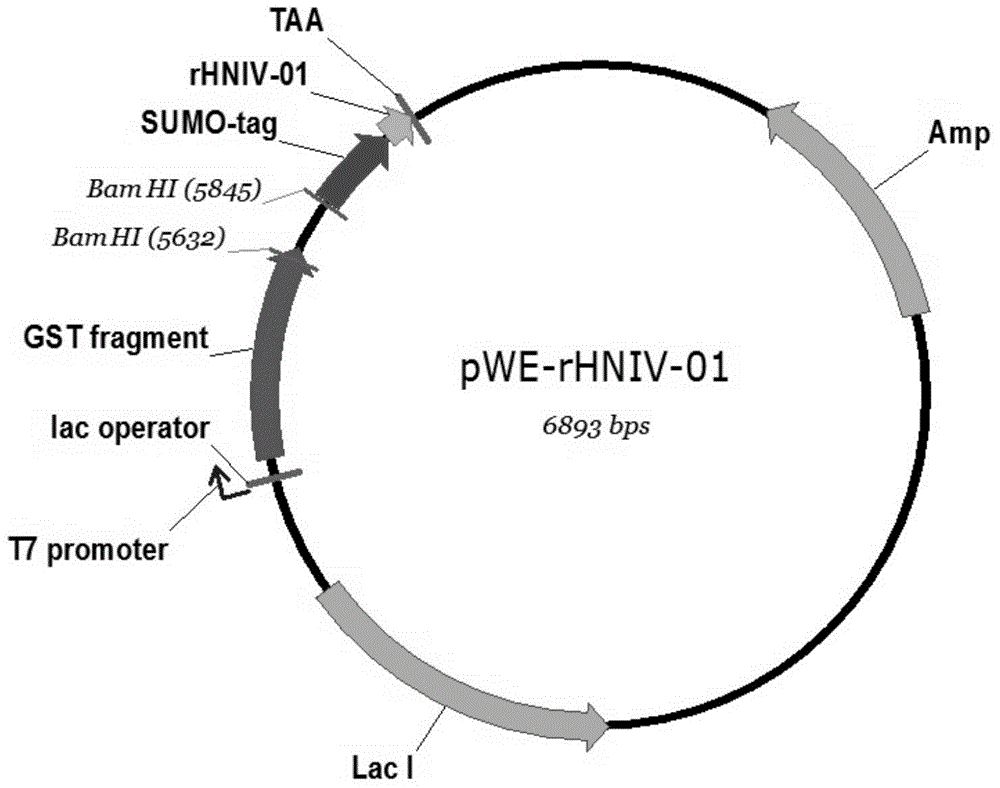
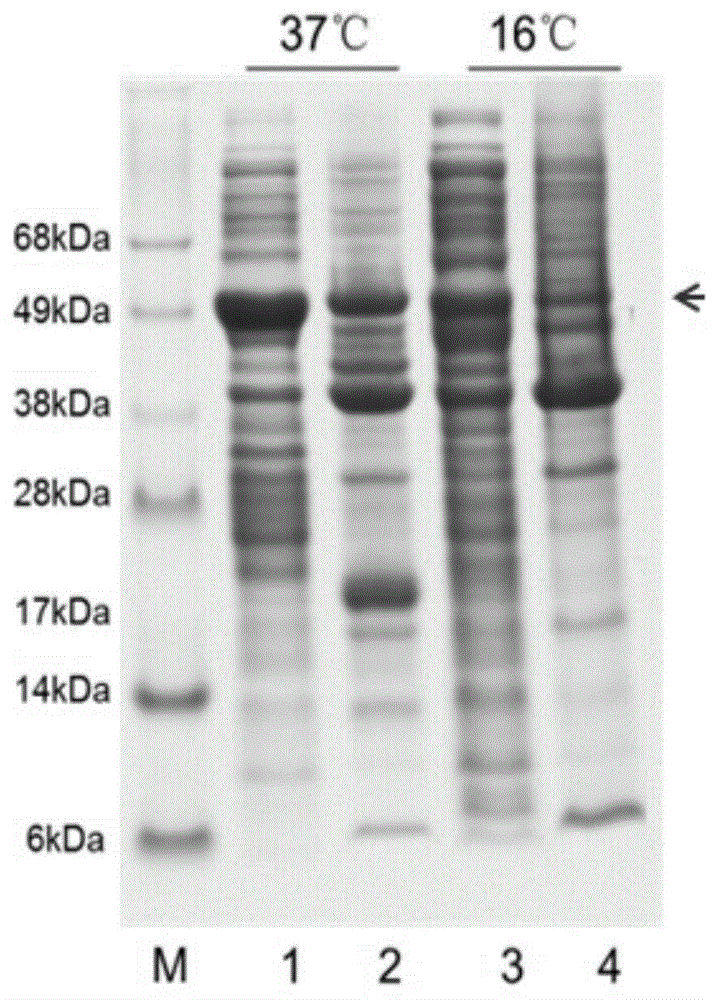
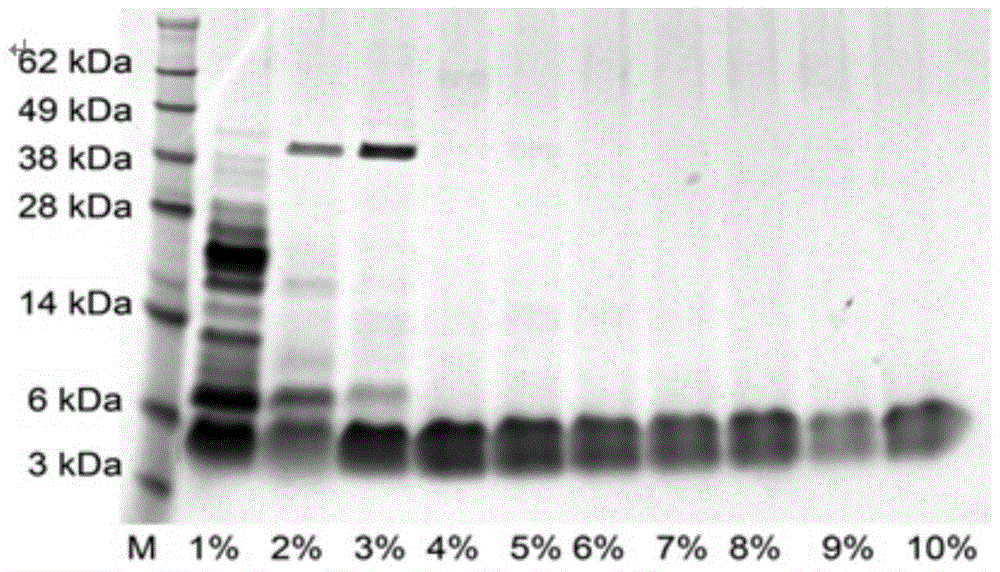
Expression vector and preparation method of HNTX (Hainantoxin)-IV analogue rHNIV-01
InactiveCN104099362ACheap purification methodEfficient separationFermentationVector-based foreign material introductionChemistryHigh-performance liquid chromatography
The invention provides an expression vector and a preparation method thereof, and high-purity recombinant HNTX (Hainantoxin)-IV analogue rHNIV-01 with biological activity can be rapidly prepared by the expression vector. Compared with natural HNTX-IV, an amino acid sequence of rHNIV-01 is almost the same as that of natural HNTX-IV, and only the C terminal of rHNIV-01 is not subjected to amidation modification. The method does not adopt conventional affinity chromatography for purifying fusion protein, protein kinase is directly added in a cell lysis product for cutting, then a cutting product rHNIV-01 is directly subjected to selective active extraction with a TCA (trichloroacetic acid) extraction method, and finally, RP-HPLC (reversed phase high-performance liquid chromatography) is adopted for purification. With the adoption of the method, 3 mg / L high-purity rHNIV-01 can be obtained stably; and purified rHNIV-01 can selectively inhibit TTX-S (tetrodotoxin-sensitive) sodium current on DRG (dorsal root ganglia) cells of a rat.
Owner:NAT UNIV OF DEFENSE TECH
Genetically modified rat models for pharmacokinetics
InactiveUS20150052623A1Compound screeningCell receptors/surface-antigens/surface-determinantsRat modelDrug transport
Owner:OSTERTAG ERIC M +1
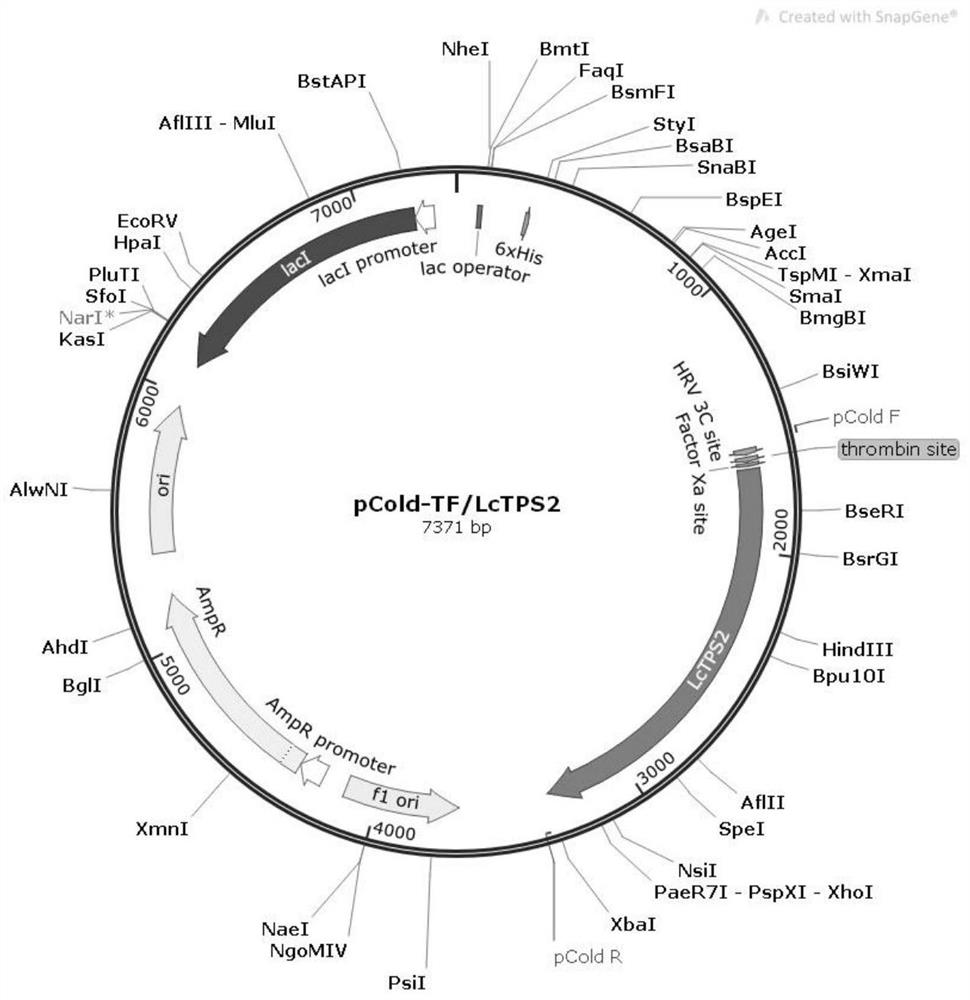
Difunctional sesterterpene/diterpene synthase LcTPS2, coding gene, product thereof and application of difunctional sesterterpene/diterpene synthase LcTPS2
ActiveCN113186183AShorten the production cycleHydroxy compound active ingredientsAntipyreticNucleotideEngineered genetic
The invention provides a difunctional sesterterpene / diterpene synthase LcTPS2, a coding gene and application thereof, and relates to the technical field of synthetic biology and natural pharmaceutical chemistry. According to the invention, a terpene synthase LcTPS2 coding gene for synthesizing a 14- / 18-membered ring terpene compound is cloned and functionally identified from labiatae plant Ipomoea cairica, and the nucleotide sequence of the terpene synthase LcTPS2 coding gene is as shown in SEQ ID NO.2. The genetically engineered cell constructed by the invention is safe and stable and short in production cycle, and the synthesized macrocyclic sesterterpene product has anti-inflammatory and immunosuppressive activity, and has an obvious inhibiting effect on inducing a Jurkat cell line to secrete IL-2 by combining phytohemagglutinin with 12-phorboester-13-acetate and stimulating mouse T cells to generate cell factors IFN-gamma by CD3 and CD28 monoclonal antibodies. However, the compound has no obvious cytotoxicity to Jurkat and splenocytes.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Humanized monoclonal antibody against hIL-33 and application of monoclonal antibody
ActiveCN111378037AAffinity ensuresLow heterogeneityNervous disorderAntipyreticAntiendomysial antibodiesDrug biological activity
In order to provide a antibody against hIL-33 with clinical application prospect, the invention provides a high-affinity mouse anti-human IL-33 antibody by adopting a panning technology of immunizingmouse B cells, based on the action mechanism of IL-33 in inflammation and tumors in the prior art, the function of IL-33 in regulating cytokine secretion, and cytokines involved in inflammation and tumors. After recombinant expression of mouse-derived antibodies, preliminary screening of biological activity, humanization transformation, affinity maturation and biological activity rescreening, a humanized monoclonal antibody against hIL-33 is obtained, and the monoclonal antibody has the characteristics of high stability, high affinity, good biological activity, broad clinical application prospects, etc.
Owner:NANJING NOVOACINE BIO-TECH CO LTD
Method of target-integrating foreign DNA (Deoxyribonucleic Acid) sequence to Rosa26 sites of rat and mouse as well as application thereof
ActiveCN103540587AClear genetic backgroundStable exogenous expressionMicrobiological testing/measurementVector-based foreign material introductionCell strainStable cell line
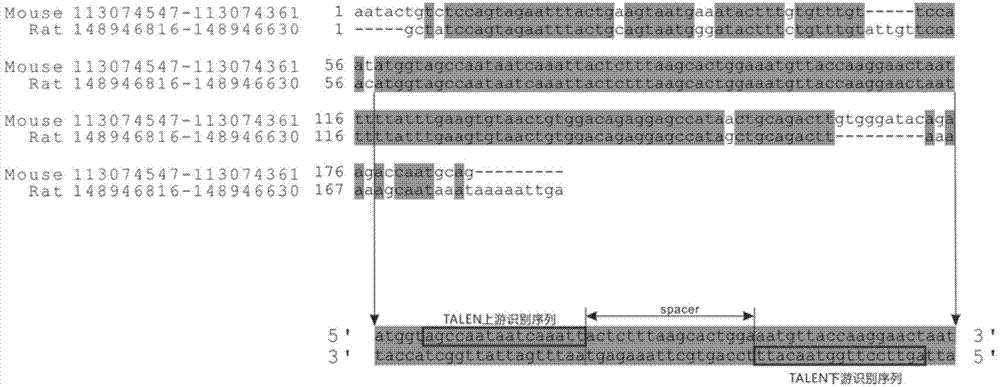
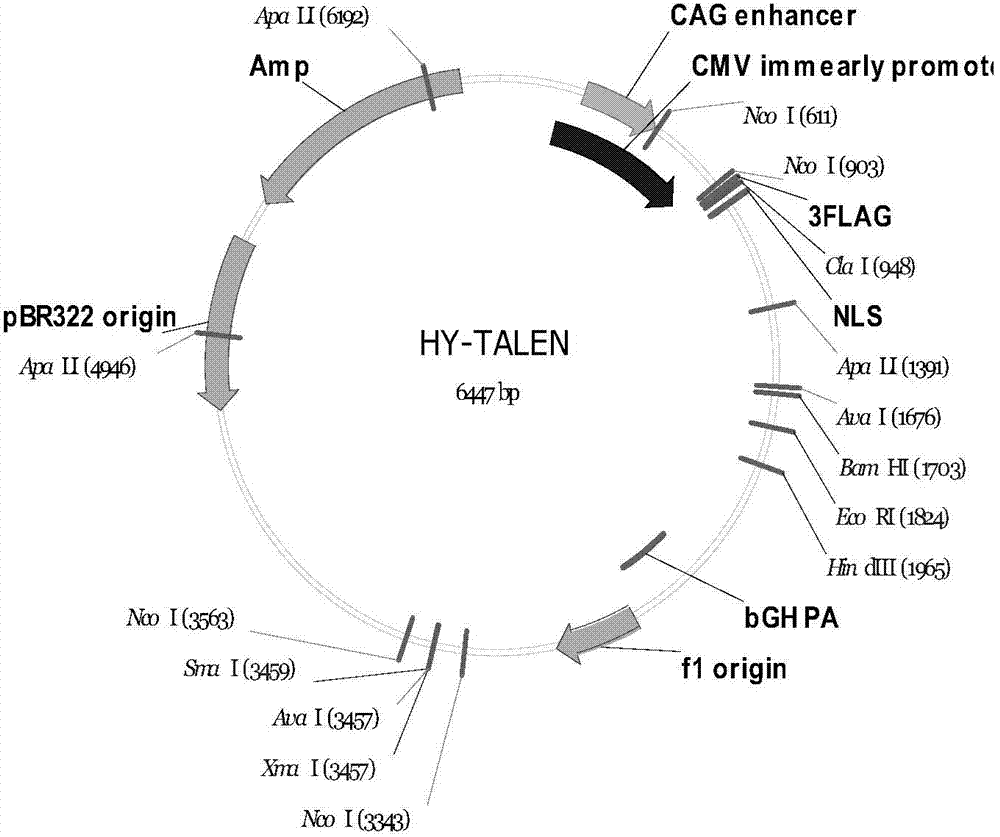
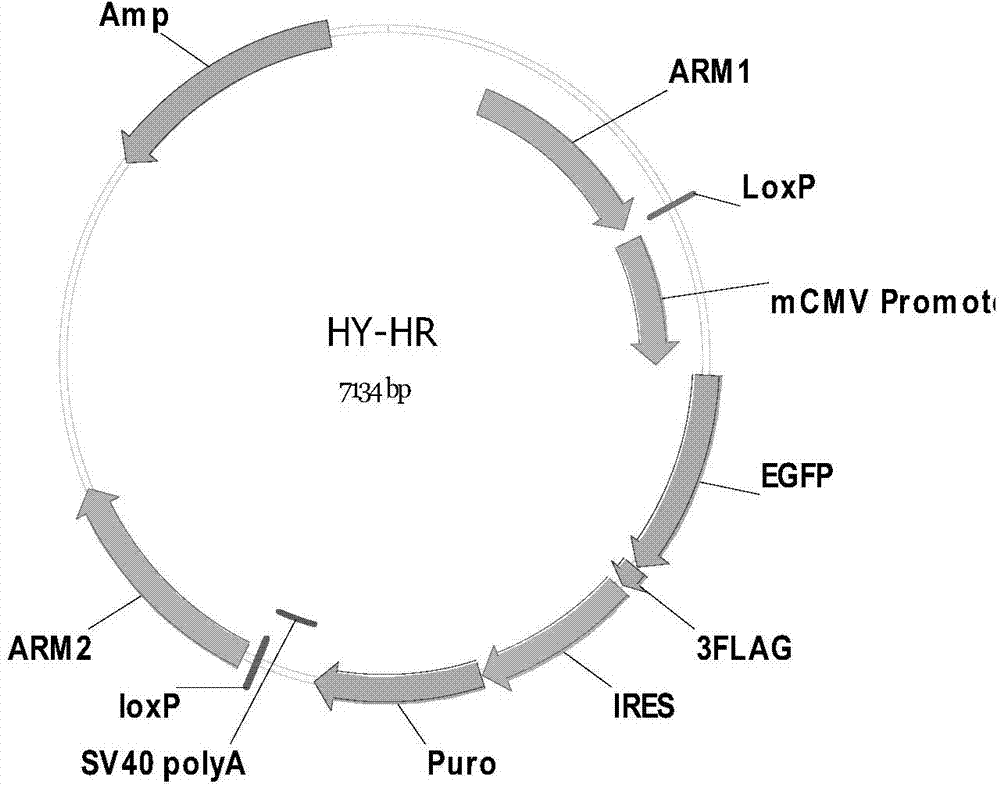
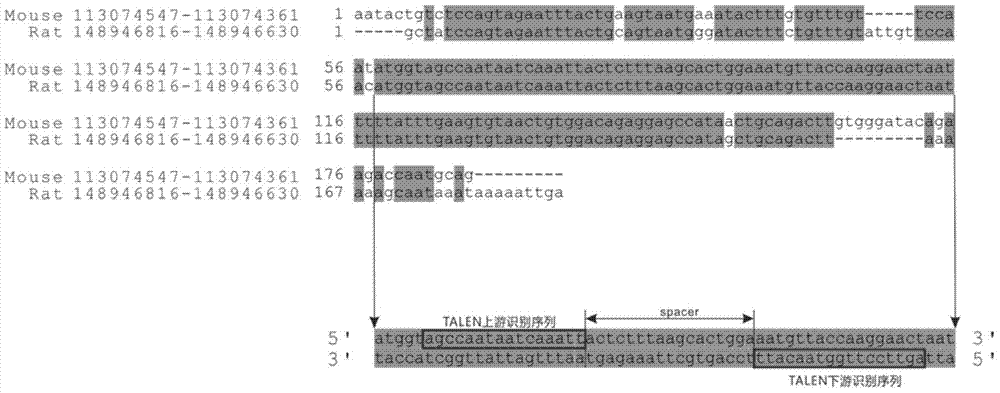
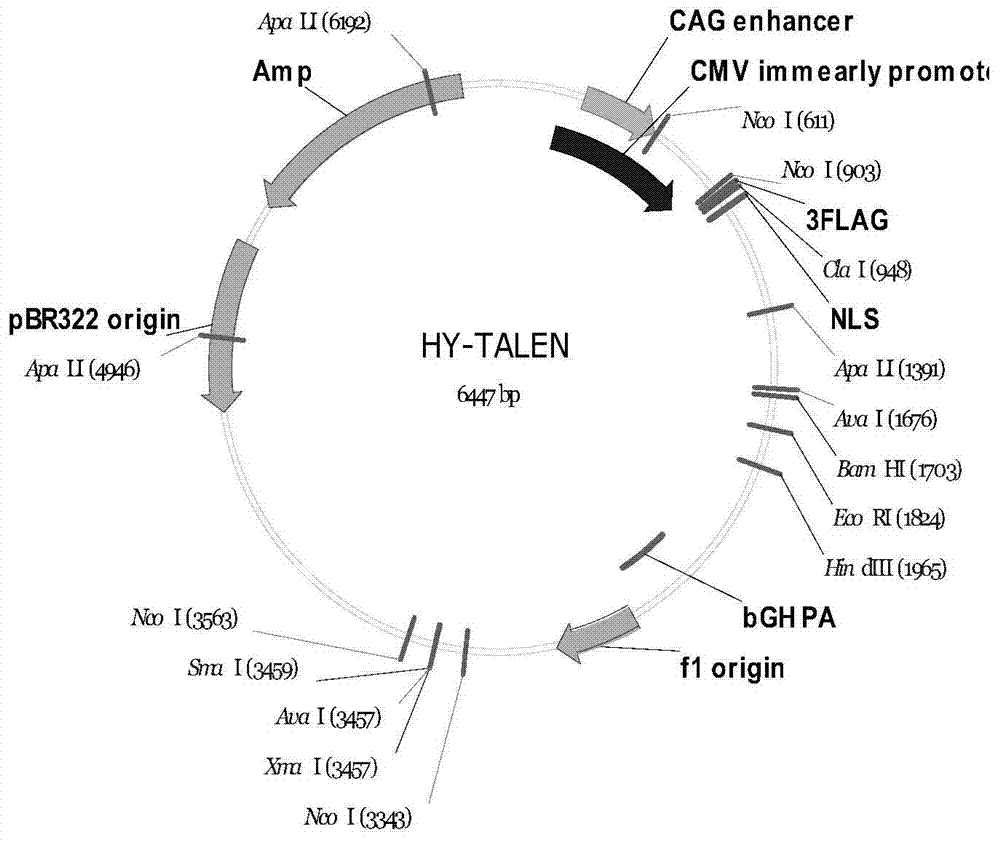
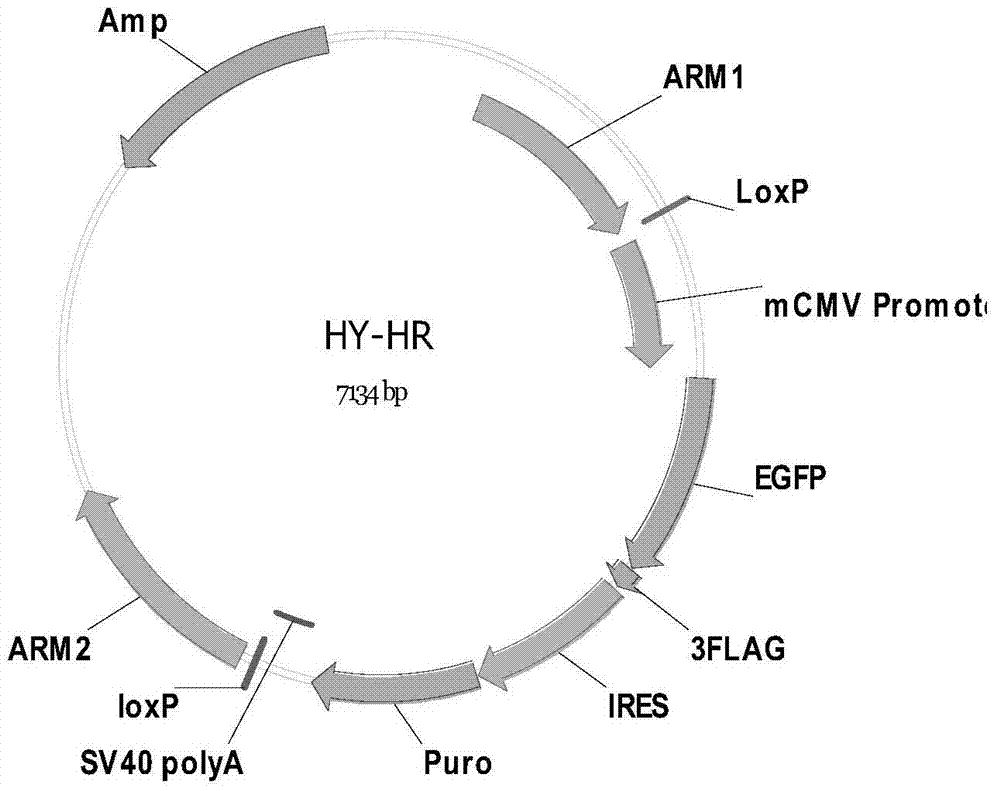
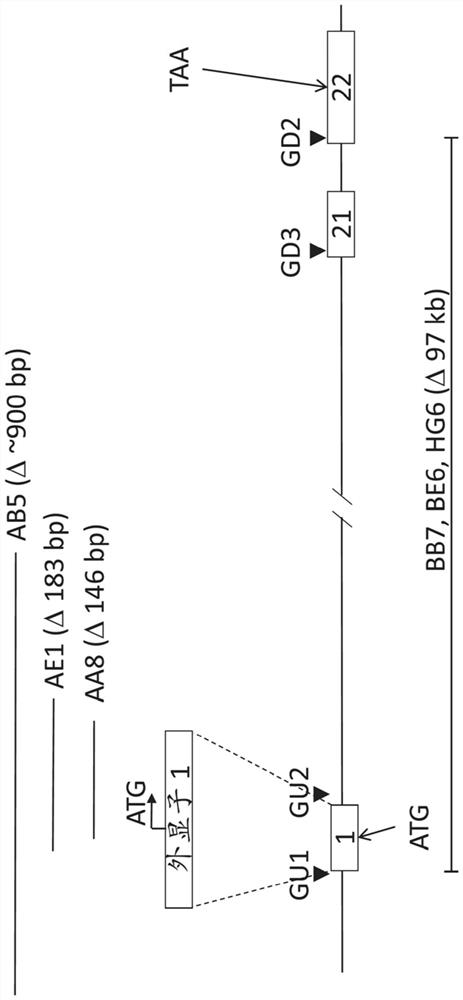
The invention discloses a method of target-integrating a foreign DNA (Deoxyribonucleic Acid) sequence to Rosa26 sites of rats and mice as well as application thereof. According to the invention, a stable cell line can be obtained for expressing various foreign genes through constructed TALEN plasmid, rat homologous recombination plasmid and mouse homologous recombination plasmid by means of respectively transferring corresponding plasmids to cells of rats and mice. The stable cell line obtained can be stably handed from generation to generation with clear genetic background, so that the biological function of the foreign gene is accurately represented.
Owner:OBIO TECH SHANGHAI CORP LTD
Genetically Modified Rat Models for Cytokine-Cytokine Signaling Pathways
The present invention relates to the engineering of animal cells, preferably mammalian, more preferably rat, that are deficient due to the disruption of gene(s) or gene product(s) resulting in cytokine-cytokine mediated autoimmune and inflammatory disease. In another aspect, the invention relates to genetically modified rats, as well as the descendants and ancestors of such animals, which are animal models of human autoimmune and inflammatory disease and methods of their use. Specifically, the invention pertains to a genetically altered rat, or a rat cell in culture, that is defective in at least one of two alleles of a cytokine gene such as the Faslg gene, the Fas gene, etc. In one embodiment, the cytokine gene is the Faslg gene. In another embodiment, the cytokine gene is one of several known cytokine genes, such as Fas, IFNγ, TNF-α, IL-2, IL-10, and IL-12. The inactivation of at least one of these cytokine alleles results in an animal with a higher susceptibility to cytokine-cytokine mediated autoimmune and inflammatory disease induction. In one embodiment, the genetically altered animal is a rat of this type and is able to serve as a useful model for cytokine-cytokine mediated autoimmune and inflammatory disease and as a test animal for autoimmune and other studies.
Owner:TRANSPOSAGEN BIOPHARM
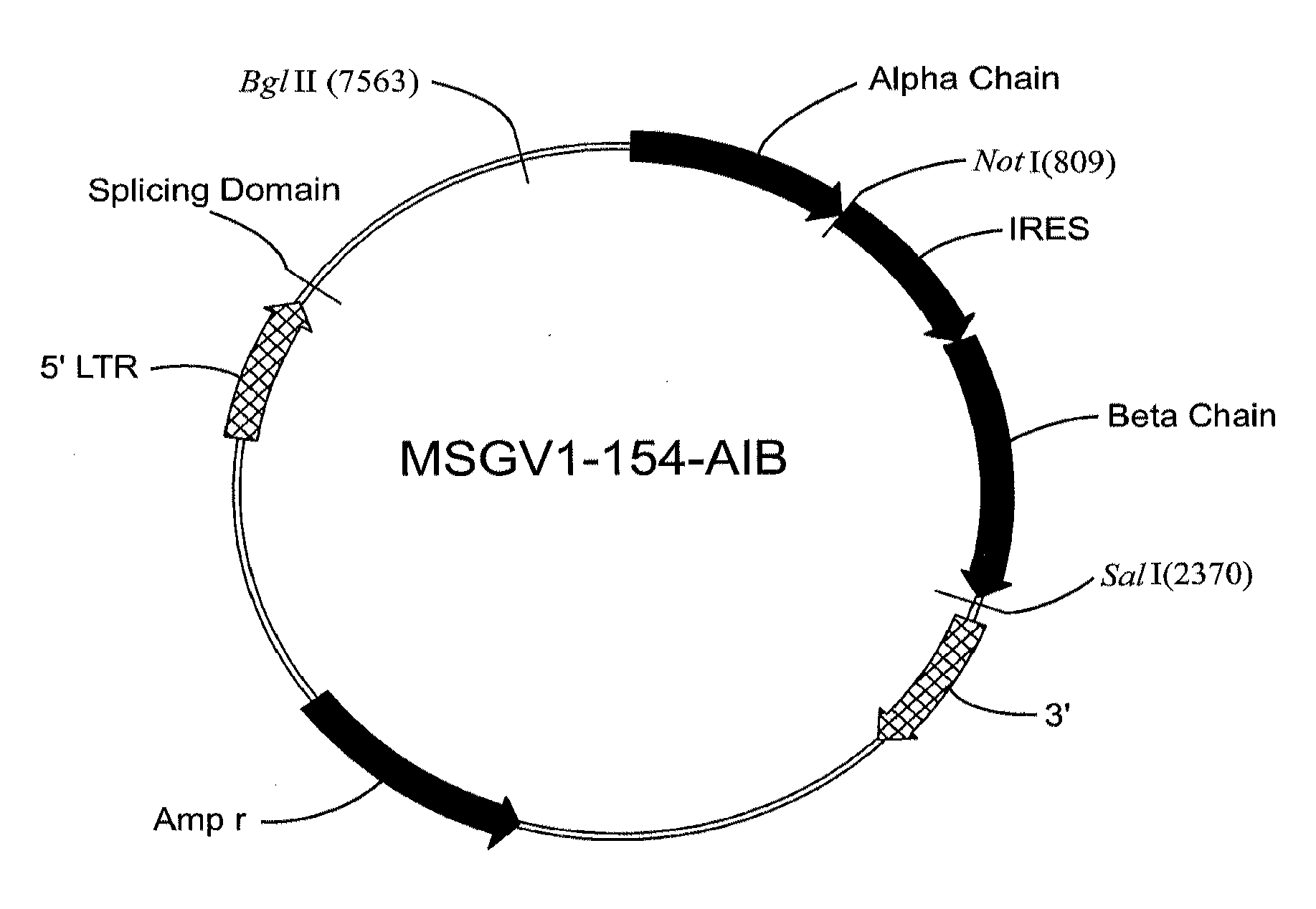
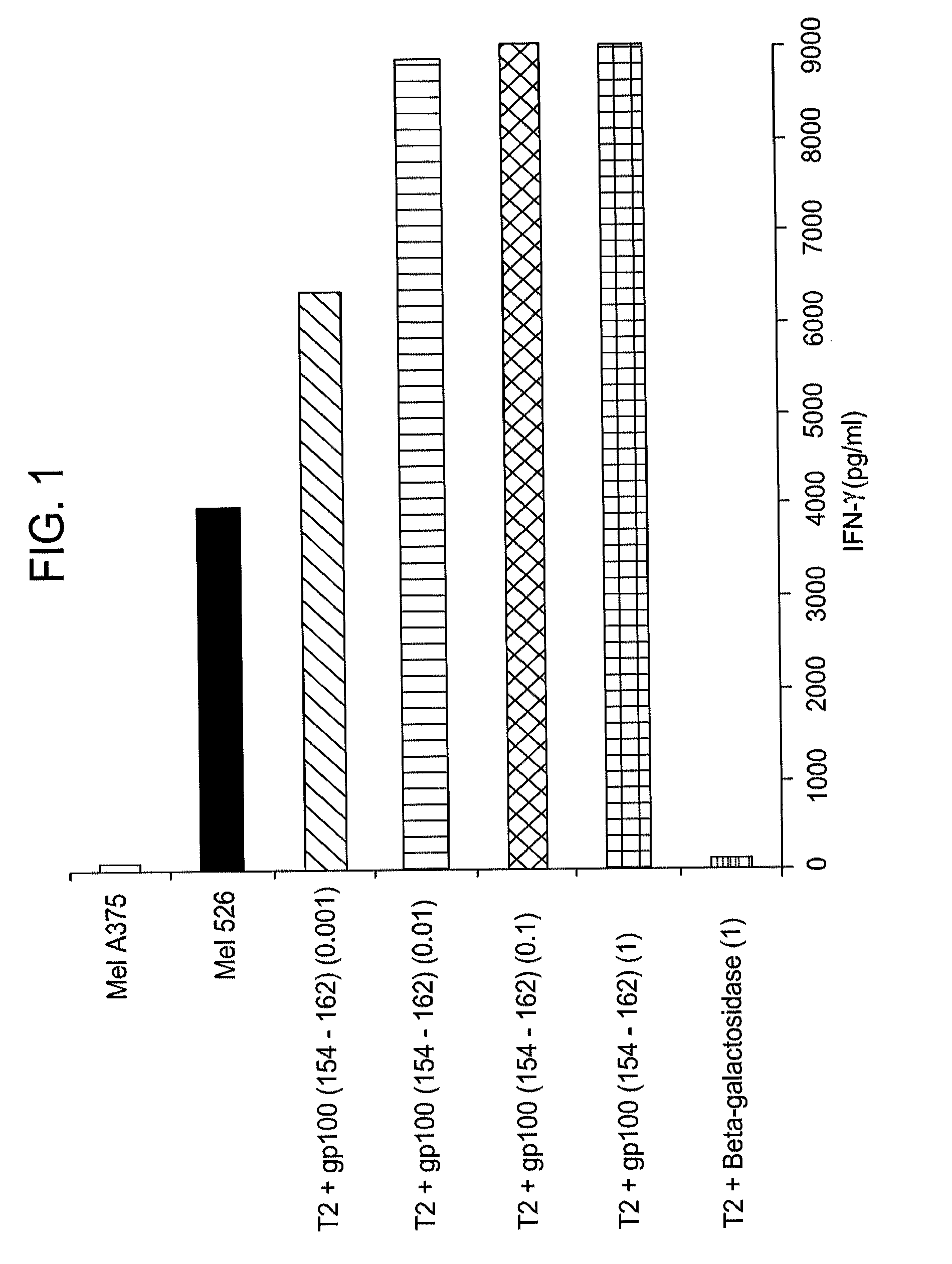
Gp100-specific t cell receptors and related materials and methods of use
Owner:UNITED STATES OF AMERICA
Polypeptide for inducing CD4CD25 regulatory T cells and application
InactiveCN101580539AIncrease the number ofImprove inhibitory functionPeptide/protein ingredientsAntipyreticRegulatory T cellLymphocyte
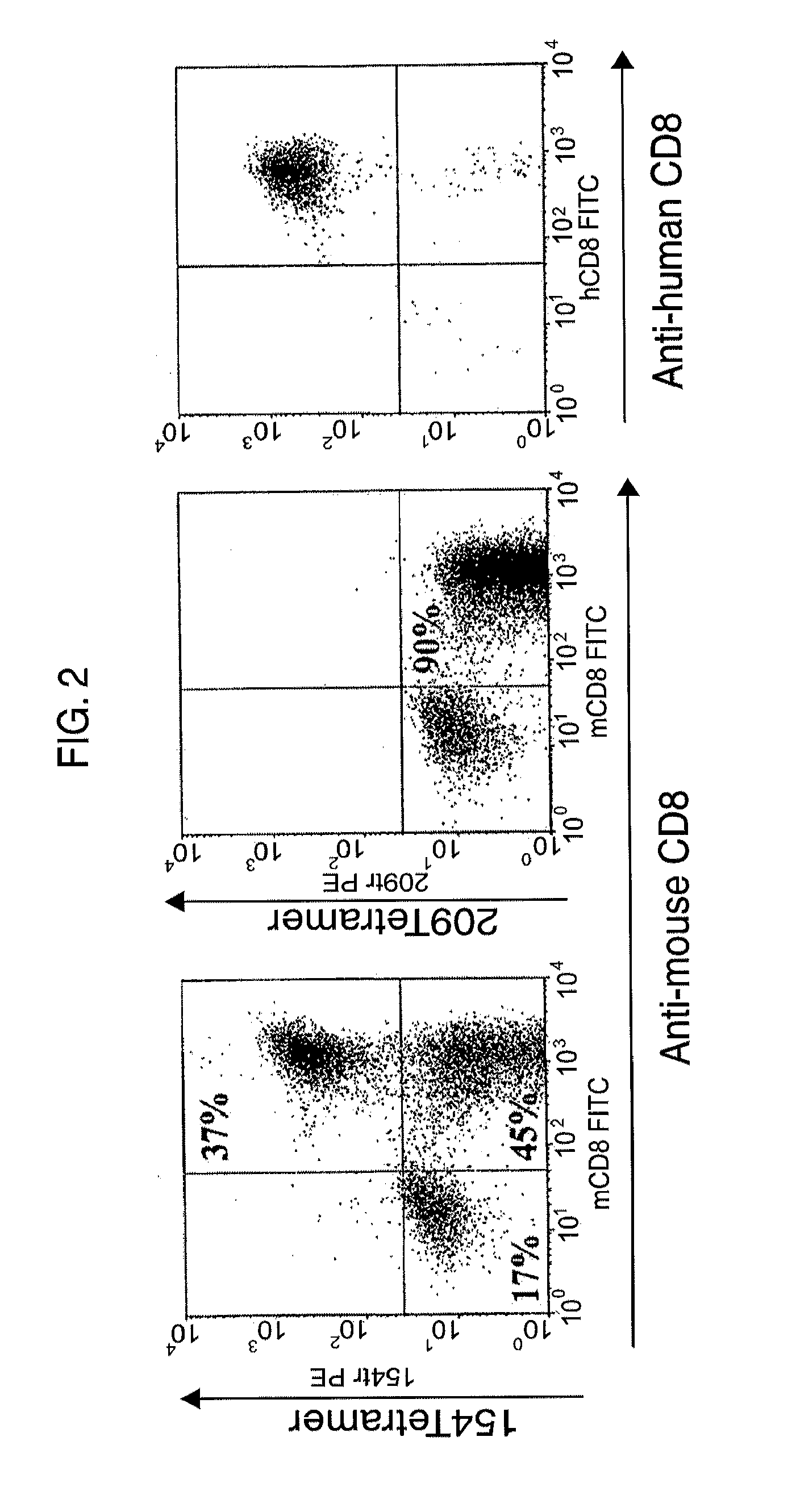
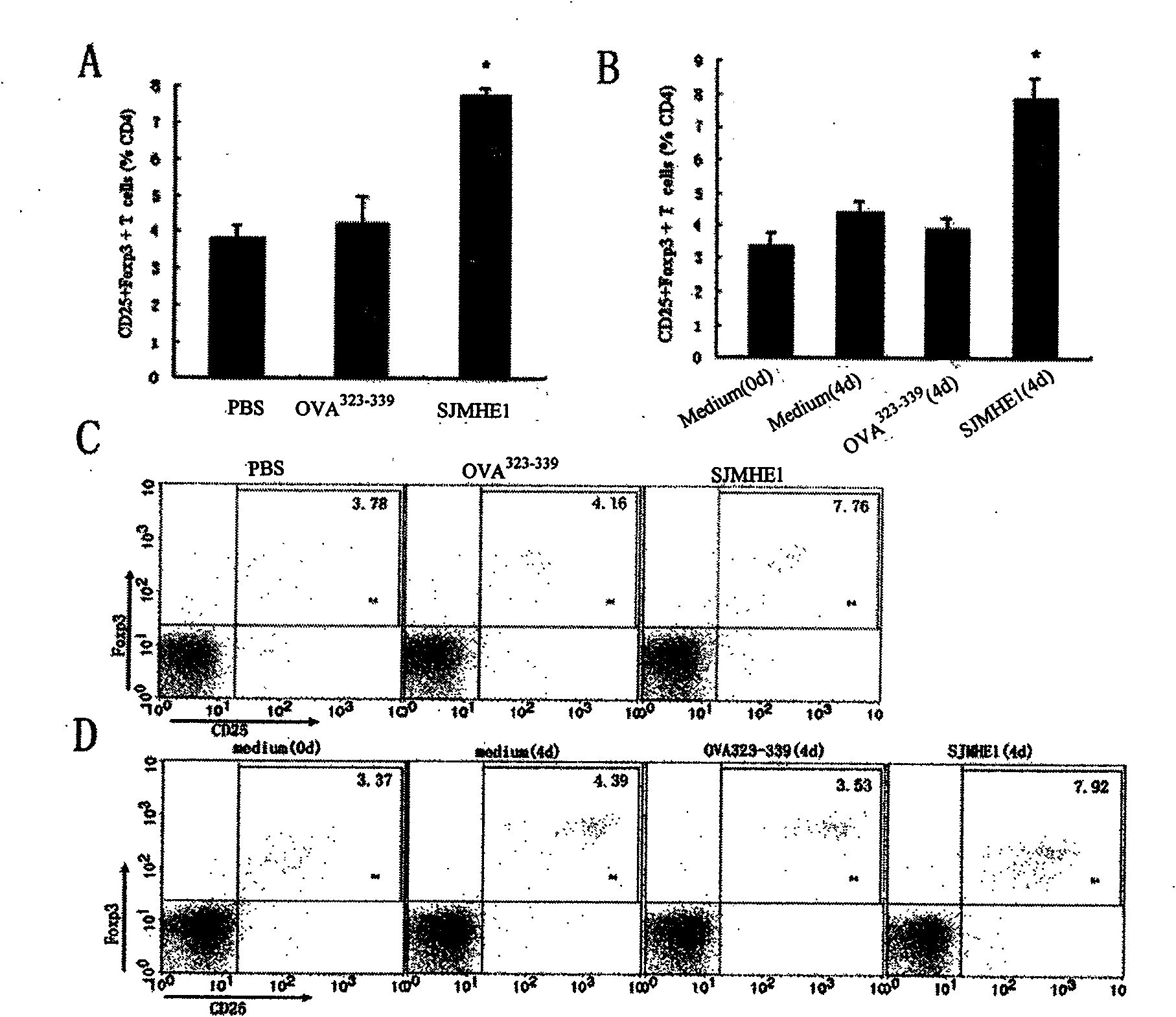
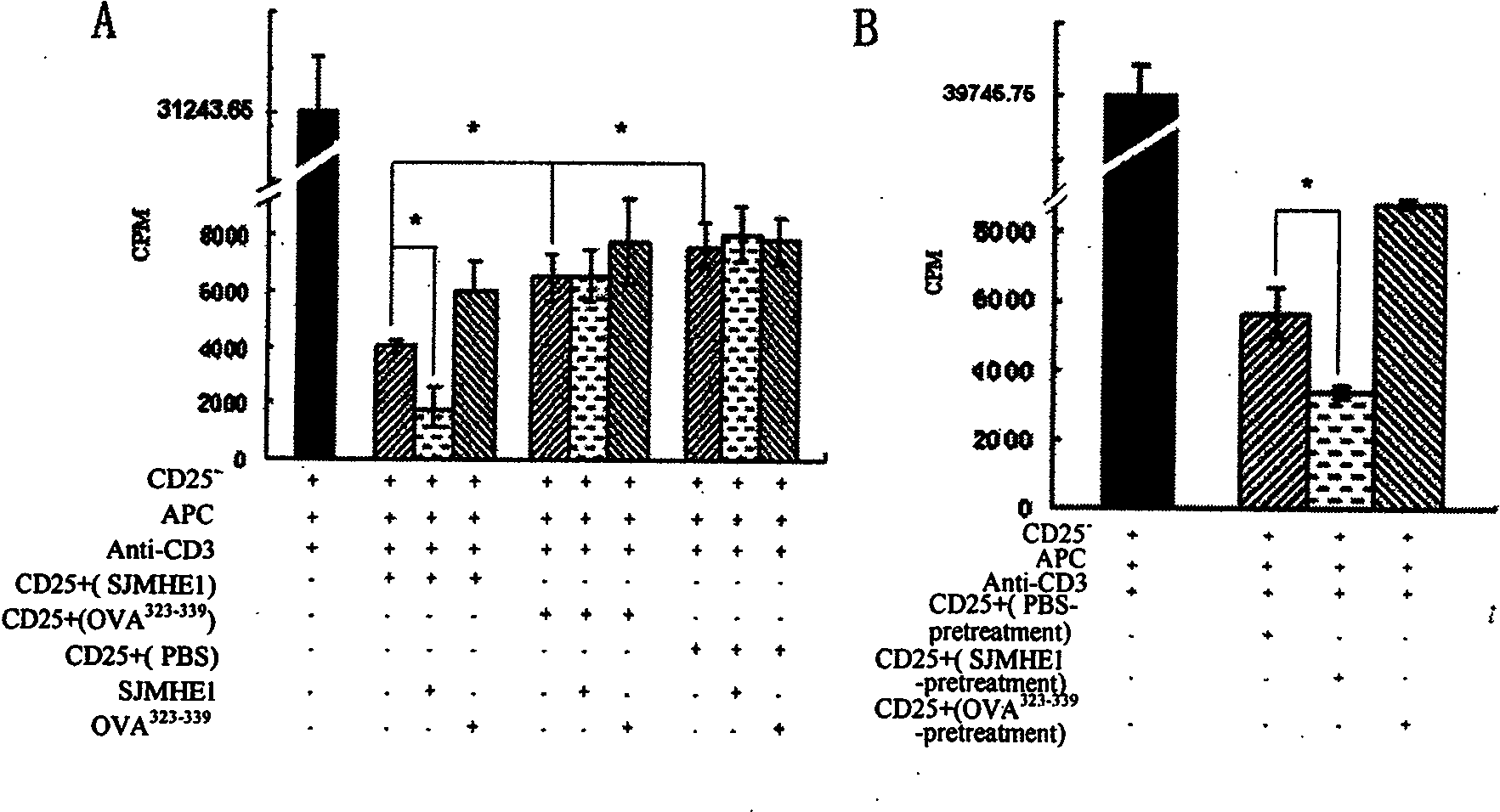
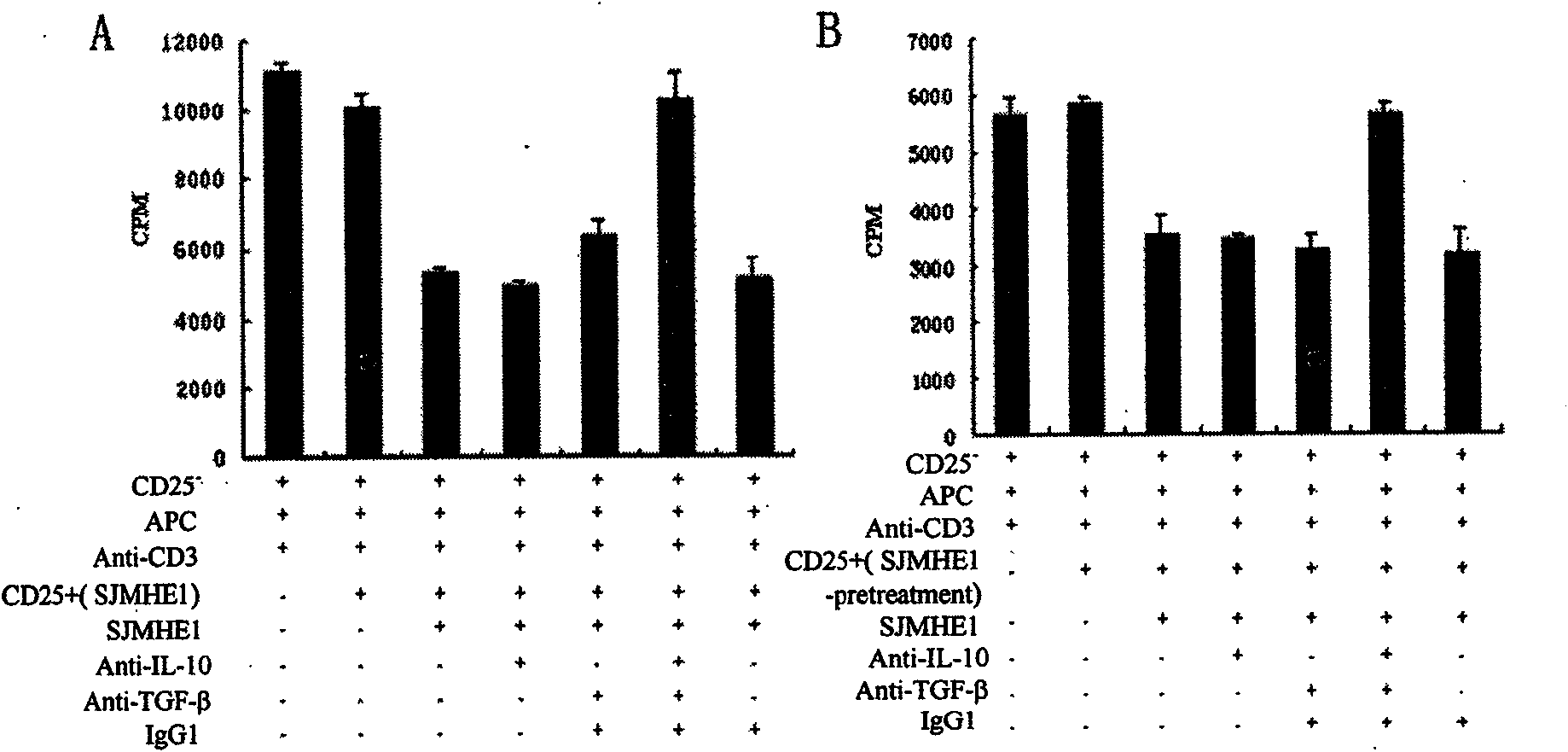
The invention belongs to the field of immunology, and in particular relates to a polypeptide for inducing CD4CD25 regulatory T cells (Tregs) and with immune-suppression function on schistosome source and application. The amino acid sequence of the polypeptide capable of inducing the CD4CD25 Tregs is VPGGGTALLRCIPVLDTLSTKNED. After in vivo immunization or in vitro pretreatment of the polypeptide on spleen and lymphocyte of a mouse, the number of CD4CD25Foxp3 T cells is remarkably increased; and after the in vivo immunization or the in vitro pretreatment of the polypeptide on the CD4CD25 Tregs cells of the mouse, the suppression function of the CD4CD25 Tregs is remarkably enhanced. The polypeptide provided by the invention can effectively suppress inflammation pathologic reaction, and has wide application prospect.
Owner:NANJING MEDICAL UNIV
Genetically Modified Rat Models for Obesity and Diabetes
InactiveUS20140041063A1Compounds screening/testingMicrobiological testing/measurementGene ModificationRat model
This invention relates to a genetically modified or chimeric rat cell whose genome comprises chromosomal alleles of an obesity-diabetes gene (especially, the Mc4r gene or Lep gene), wherein at least one of the two alleles contains a mutation, or the progeny of this cell. The obesity or diabetes gene may affect any of the pathways of obesity and diabetes. The obesity or diabetes gene may predispose the rat to a phenotype of obese and diabetic, lean and diabetic, obese and non-diabetic, non-obese and diabetic or any of the combinations thereof. In another aspect, the invention relates to a desired rat or a rat cell which contains a predefined, specific and desired alteration rendering the rat or rat cell predisposed to obesity or diabetes.
Owner:TRANSPOSAGEN BIOPHARM
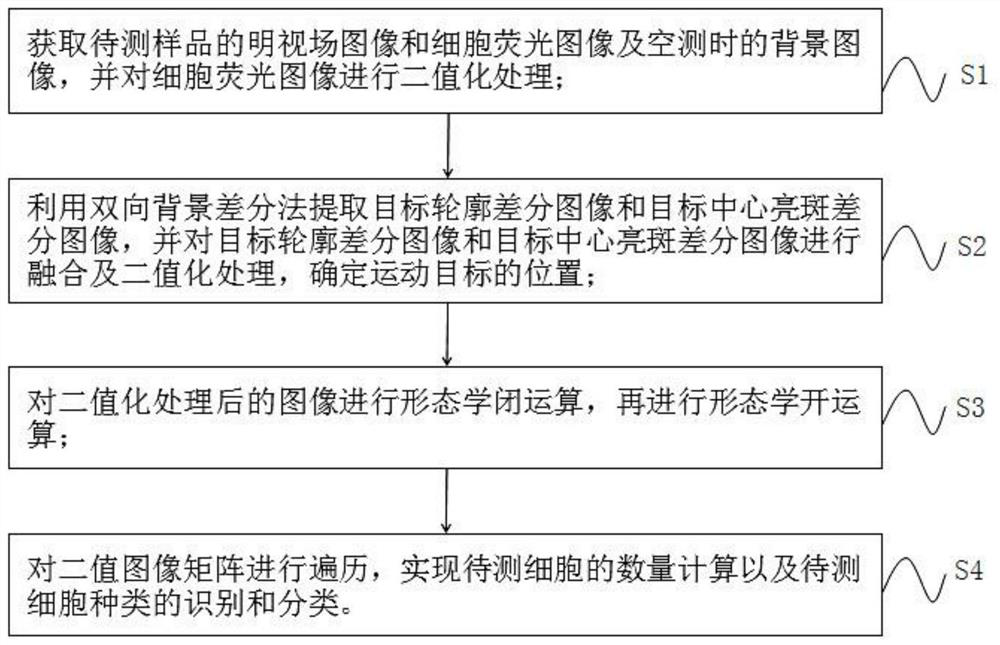
Imaging flow cytometry adhesion counting and activity detection method and device based on bidirectional background difference process
PendingCN113222969AIdentification indicators are stableAddressing the effects of countingImage enhancementImage analysisLymphocytic cellRadiology
The invention provides an imaging flow cytometry adhesion counting and activity detection method and device based on a bidirectional background difference process. The method comprises the following steps: acquiring a bright field image and a cell fluorescence image of a to-be-detected sample and a background image during empty detection, and carrying out binarization processing on the cell fluorescence image; extracting a target contour difference image and a target center bright spot difference image by using a bidirectional background difference method, and carrying out fusion and binarization processing on the two difference images to determine the position of a moving target; performing morphological closed operation on the binarized image, and then performing morphological open operation; and traversing the binary image matrix to realize quantity calculation of the cells to be detected and identification and classification of types of the cells to be detected. According to the technical scheme, the problems of time and labor consumption, incomplete functions, low flux, high price and the like in the prior art are solved. Counting and activity judgment of multiple cells such as microalgae cells, 293T lymphocytes and mouse cells can be achieved, the application range is wide, and identification indexes are stable.
Owner:DALIAN MARITIME UNIVERSITY
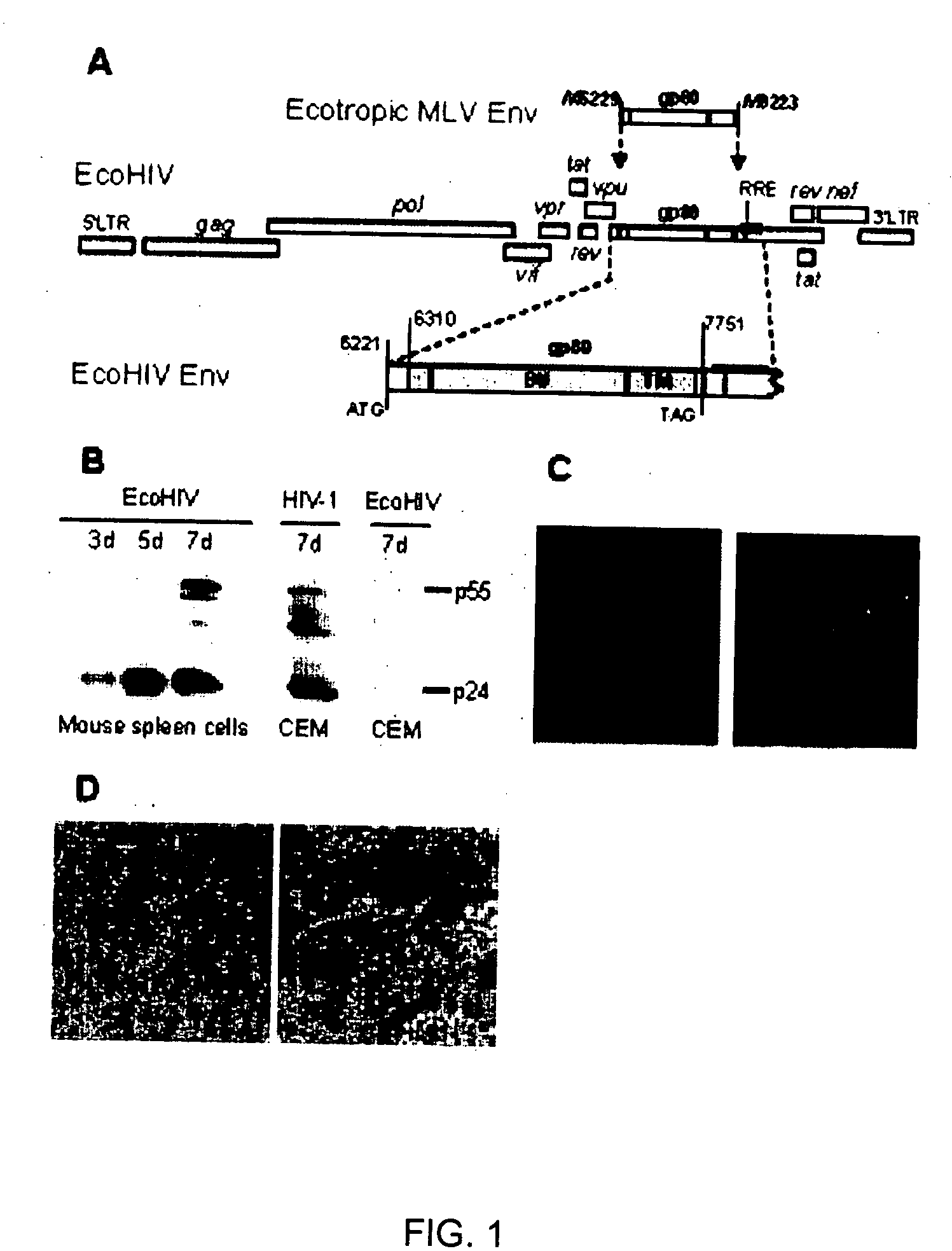
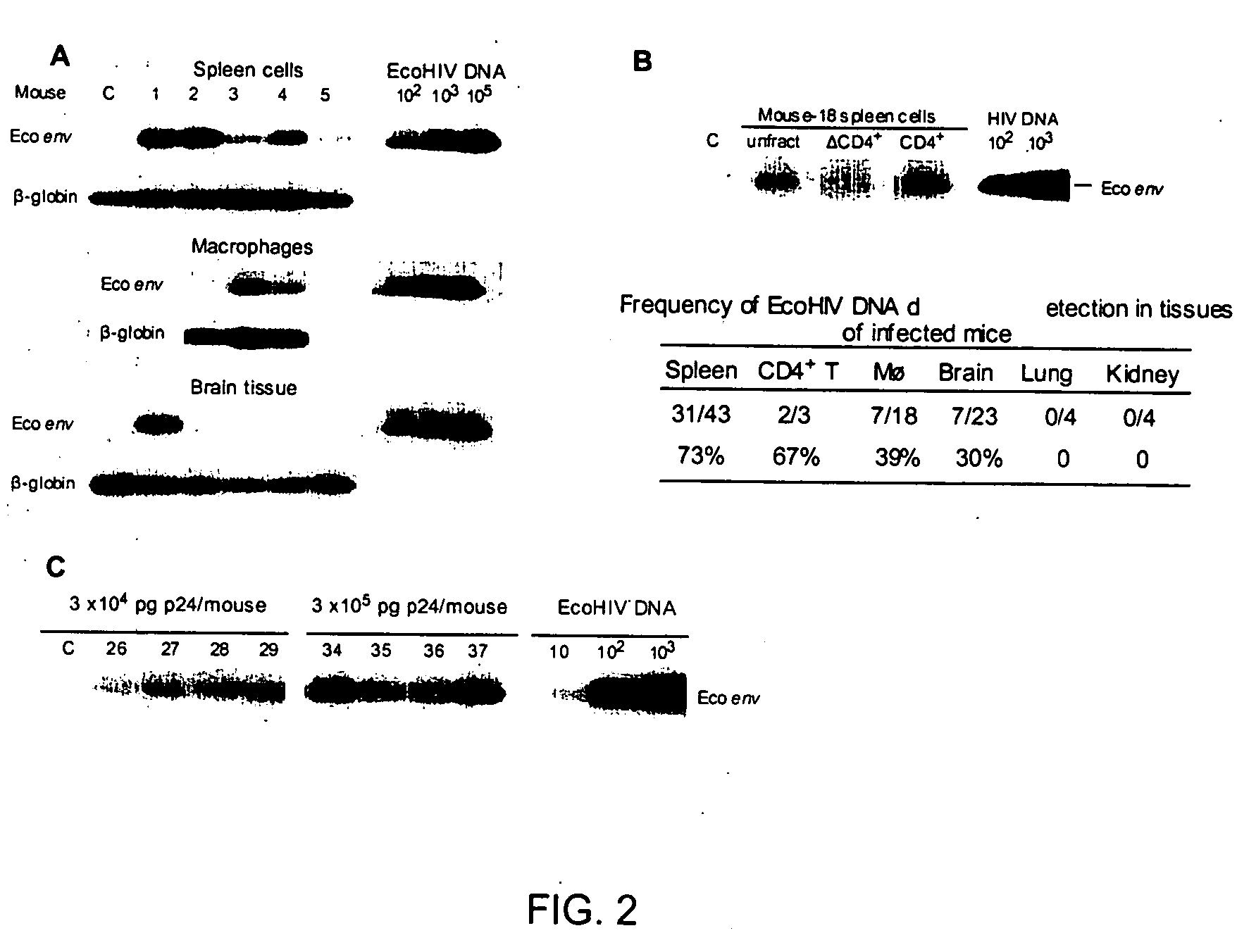
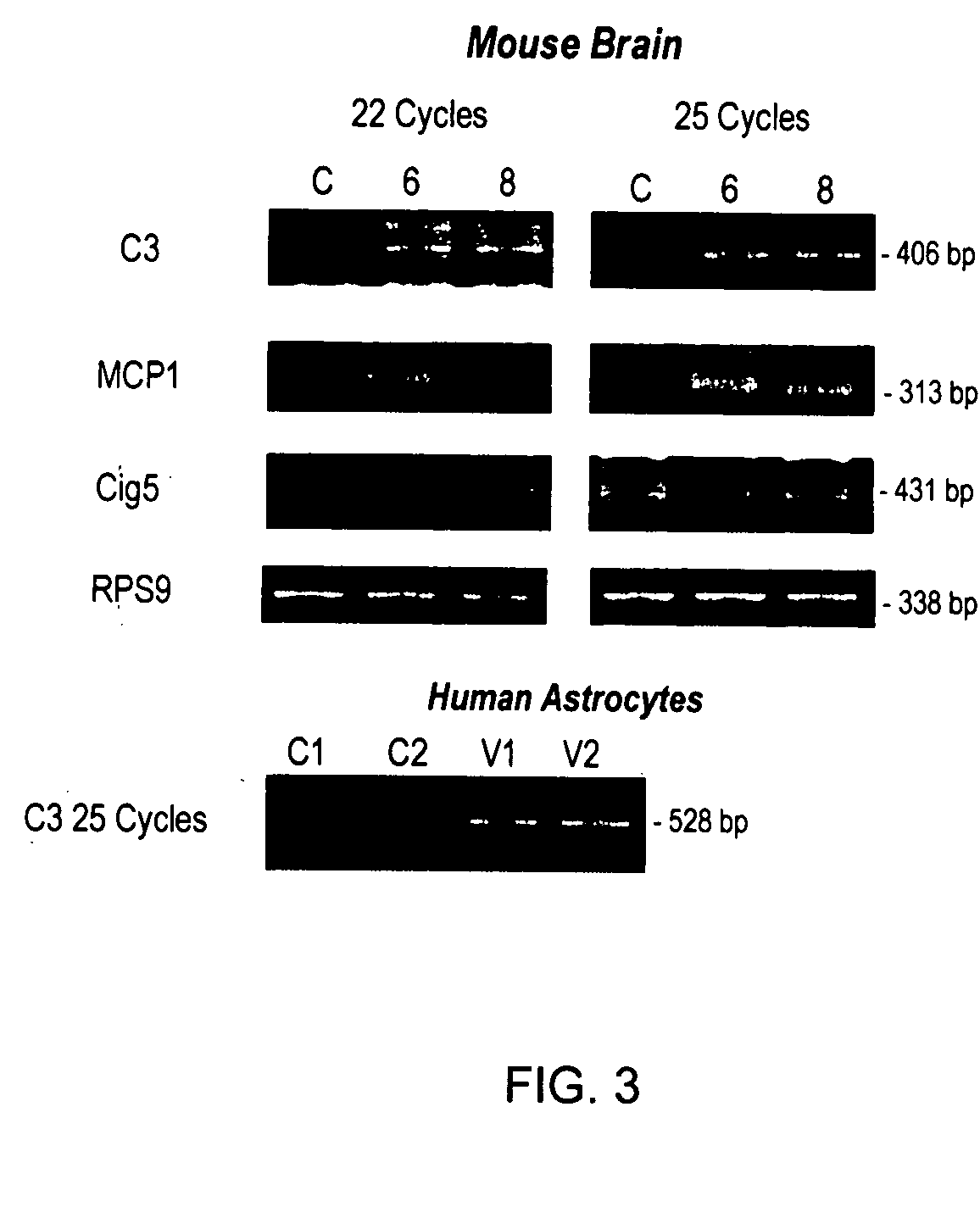
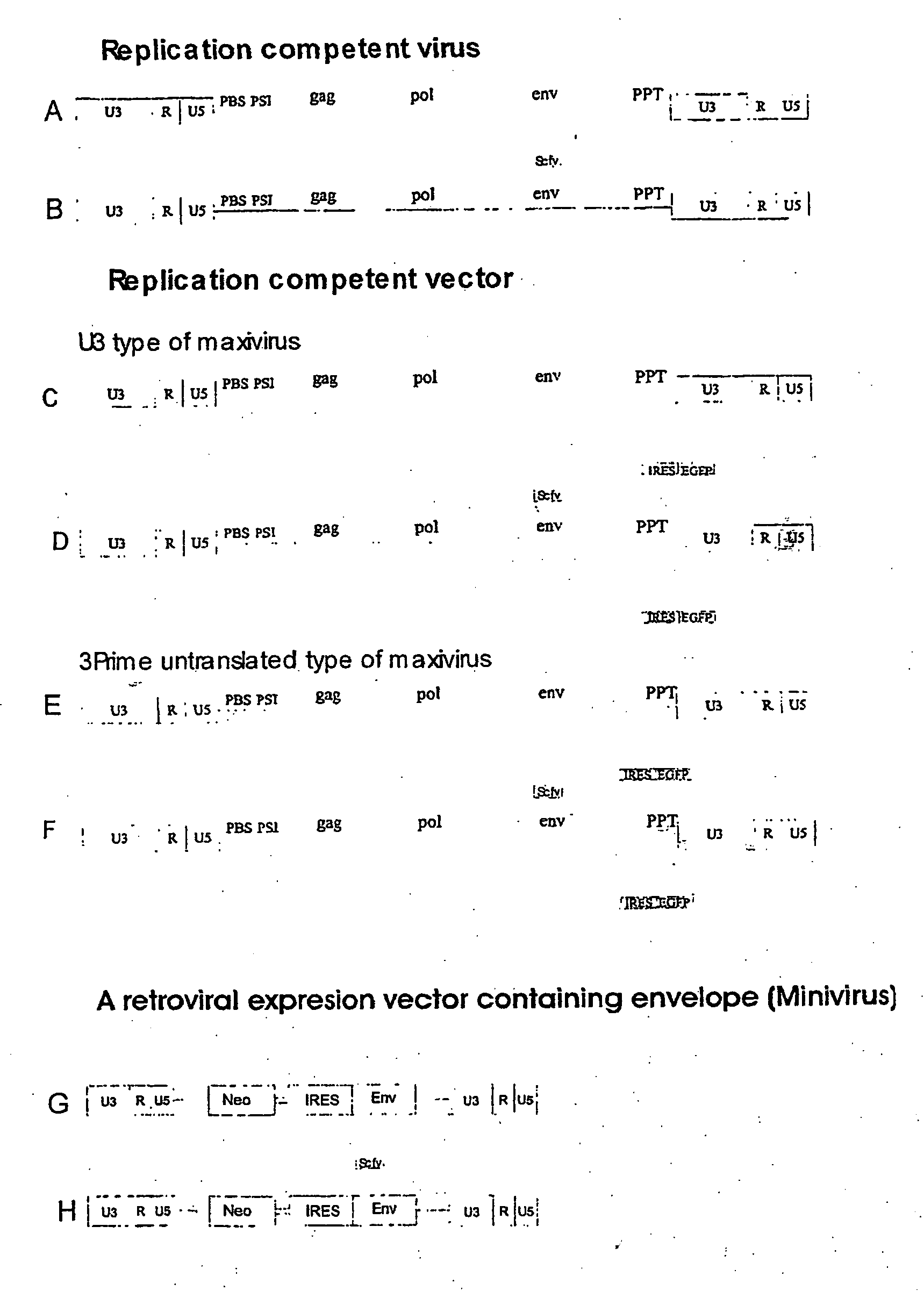
Development of a murine model of HIV-1 infection on the basis of construction of EcoHIV, a chimeric, molecular clone of human immunodeficiency virus type 1 and ecotropic moloney murine leukemia virus competent to infect murine cells and mice
The present invention provides a chimeric HIV-1 construct, EcoHIV, capable of replication in a rodent cell. The invention also provides a convenient and safe rodent model of HIV-1 infection and AIDS. Methods for producing a rodent model of HIV-1 infection are also provided. Additionally, the invention provides the means to test immunogenic compositions or pharmaceutical interventions effective in preventing infection, reducing viral load, or reducing disease symptoms in a subject.
Owner:POTASH MARY +1
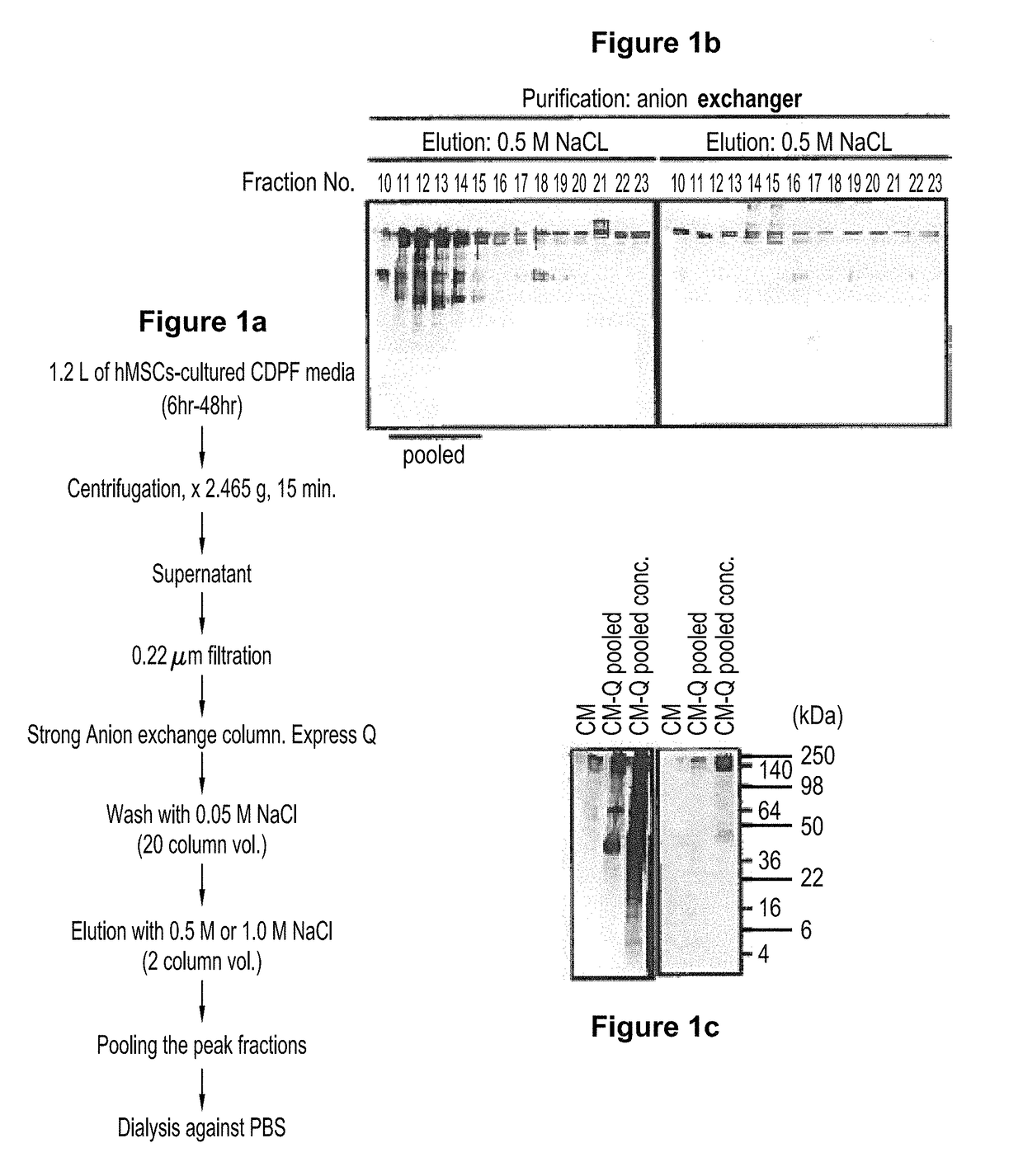
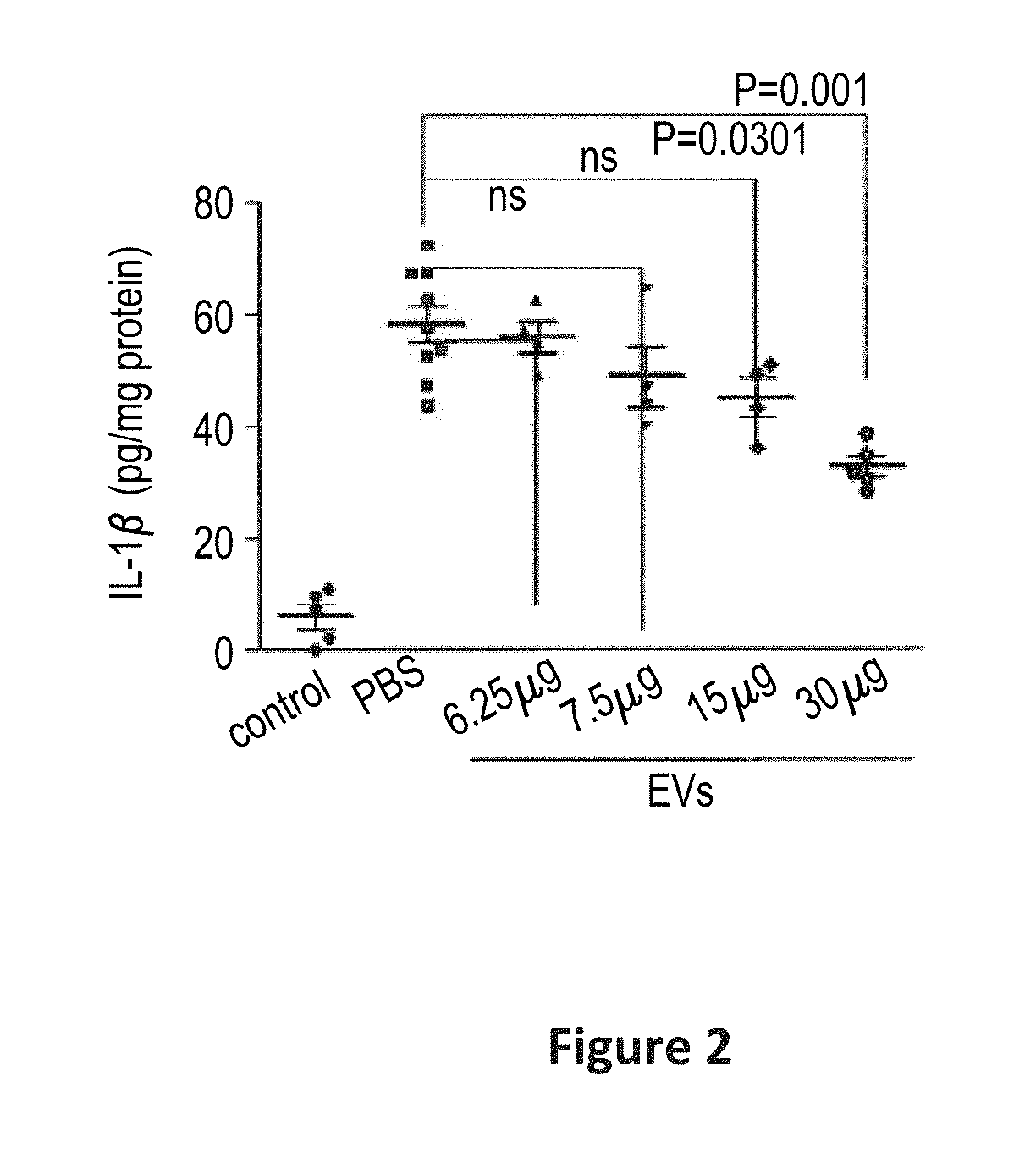
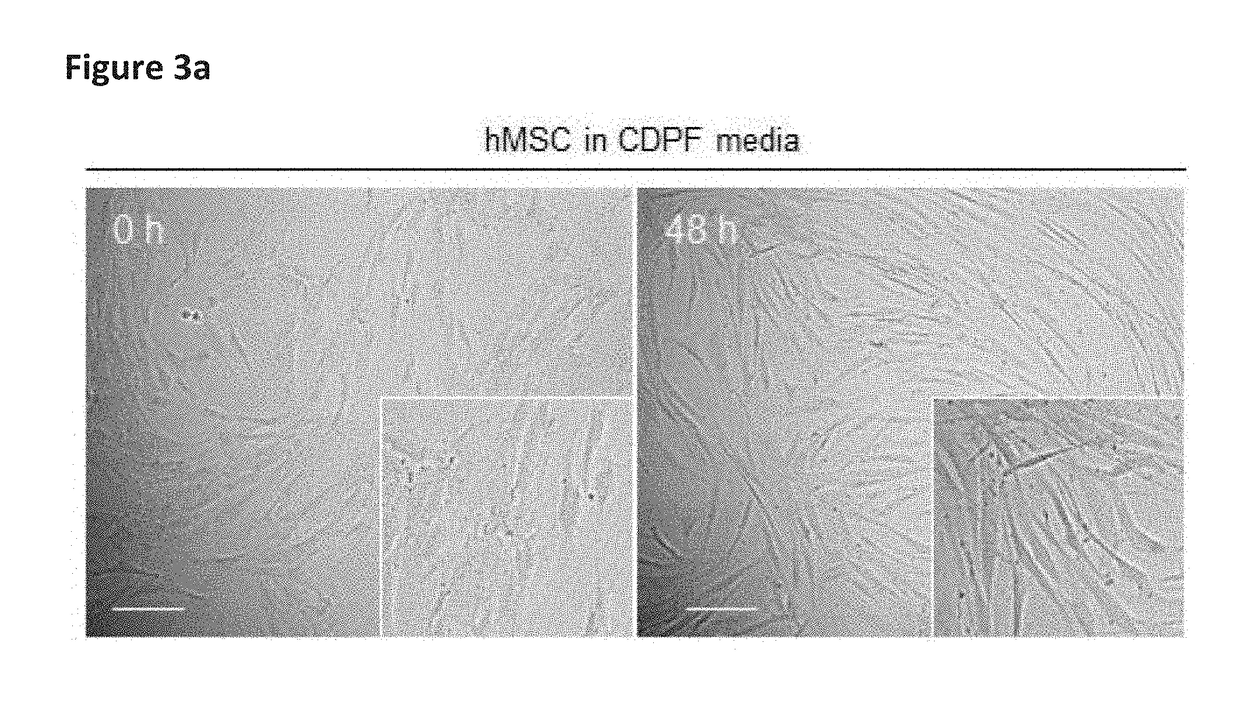
Scalable production of standardized extracellular vesicles, extracellular vesicle preparations and uses thereof
Preparations comprising an enriched population of extracellular vesicles (nEVs) having a negatively charged surface, and that are CD81+ and CD9−, are provided. Improved processes and methods for producing an enriched population of nEVs from non-murine cells, especially human origin cells and / or tissues, are disclosed. Therapeutic methods for using the preparations, including for reducing brain inflammation and treatment of various pathologies associated with brain inflammation, such as by intravenous or intranasal administration, are also described. Methods and preparations for reducing brain inflammation associated with traumatic brain injury (TBI) are also disclosed. A method for treating a patient having suffered a mild traumatic injury (mTMI), or concussion, such as a sports-related head injury, is also disclosed. The nEVs are also demonstrated to reduce the expression level of IL-Iβ in brain tissue of an animal having had traumatic brain injury. Methods for improving cognitive function and performance in animals after a traumatic brain injury is also demonstrated using the preparations of nEVs disclosed herein.
Owner:TEXAS A&M UNIVERSITY
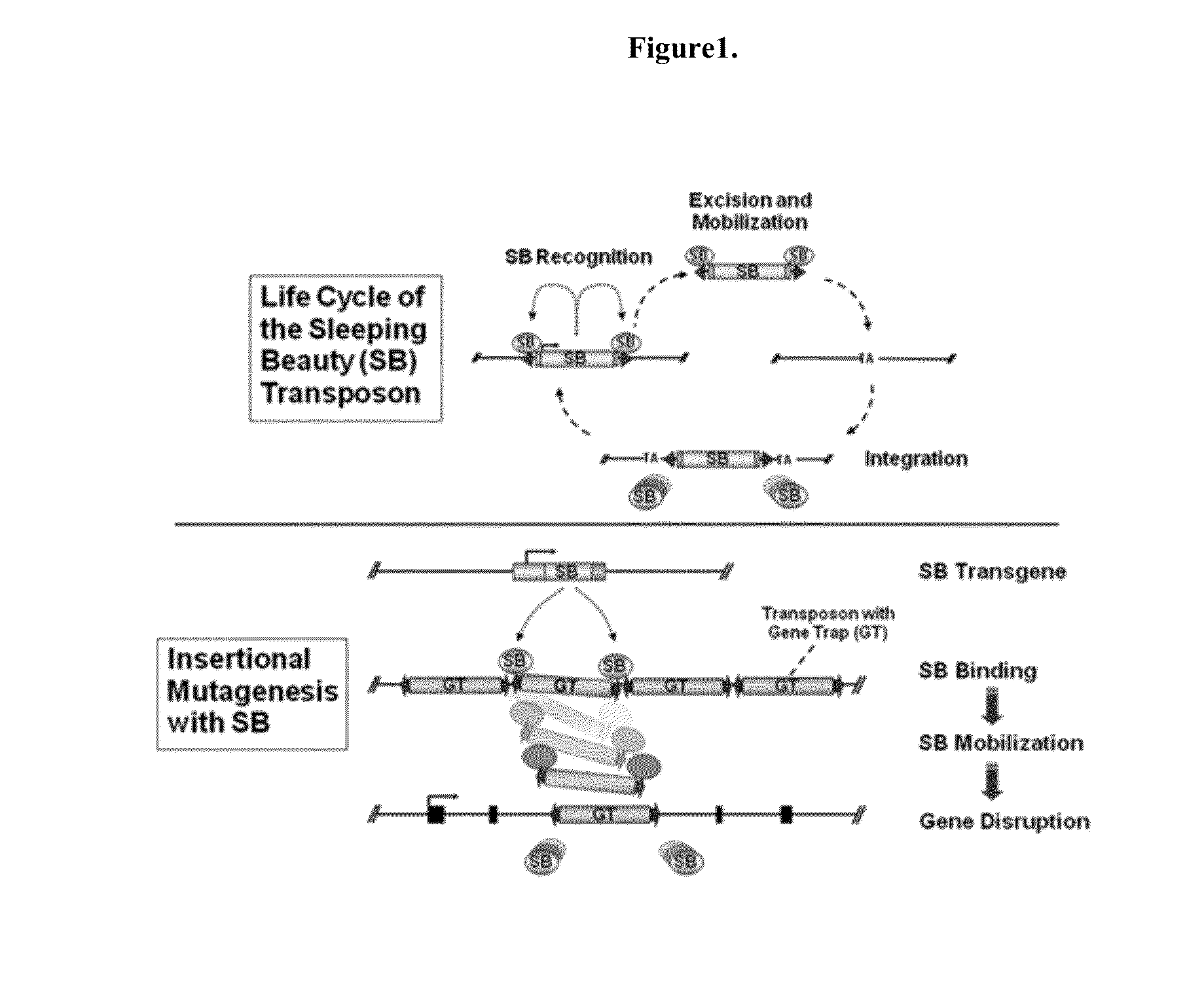
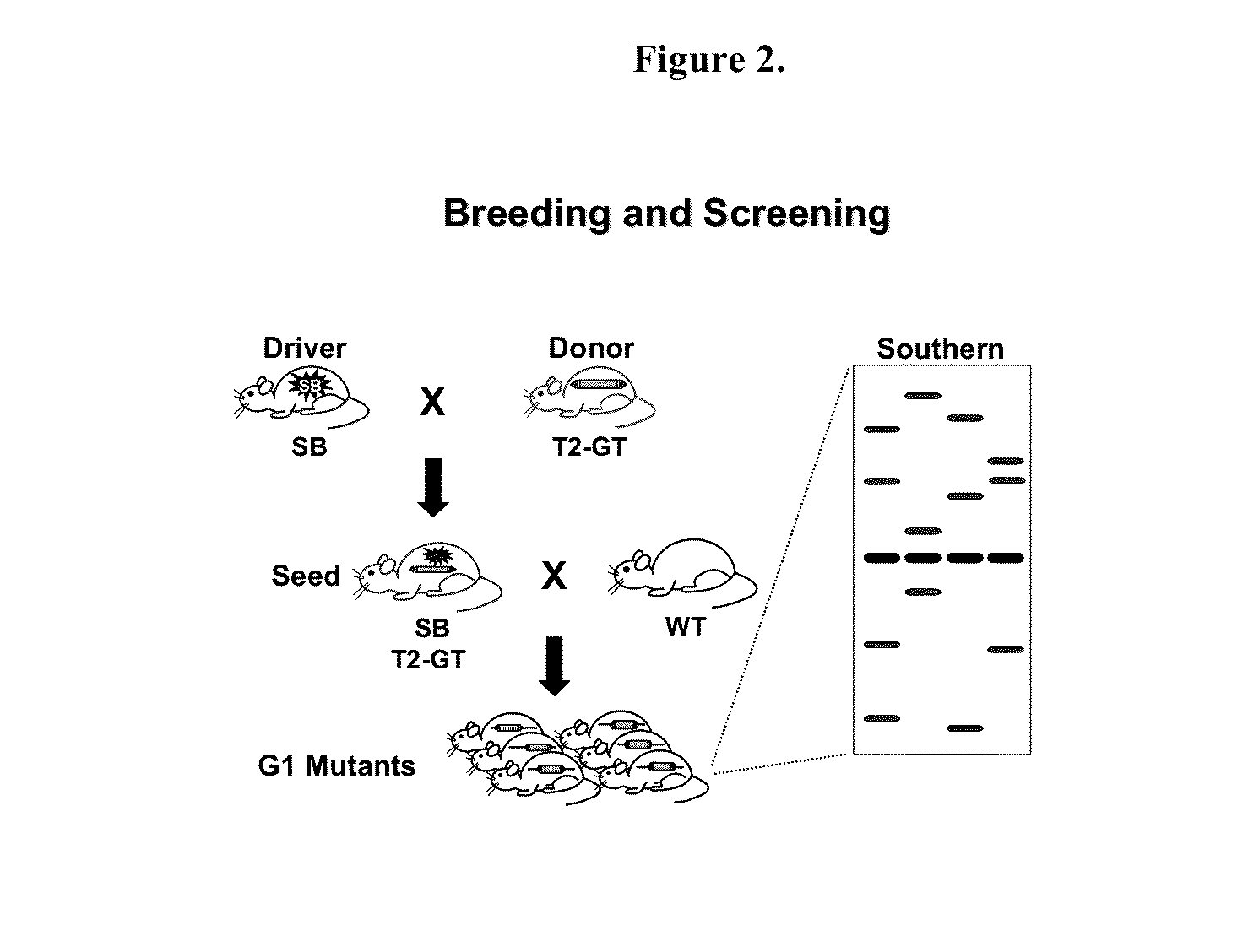
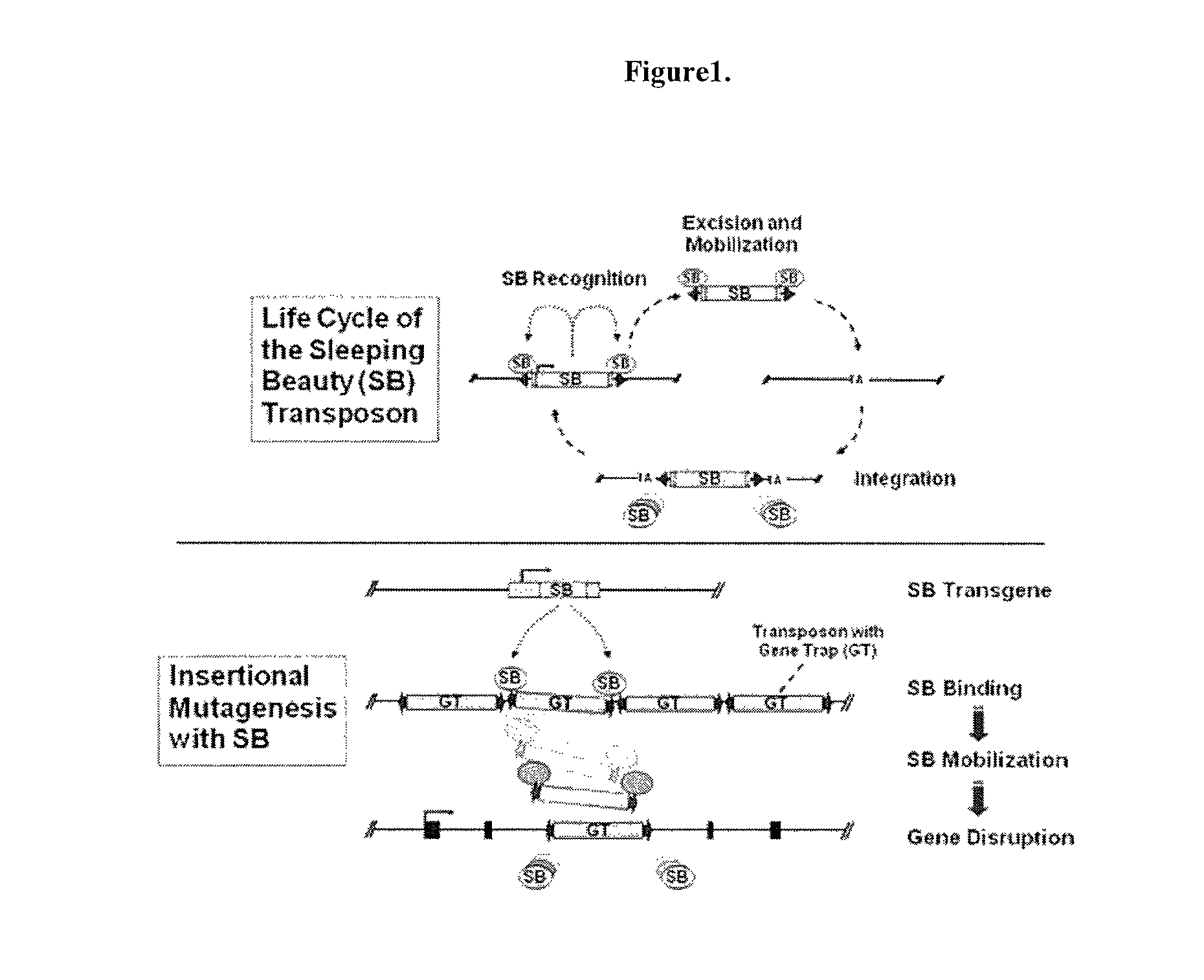
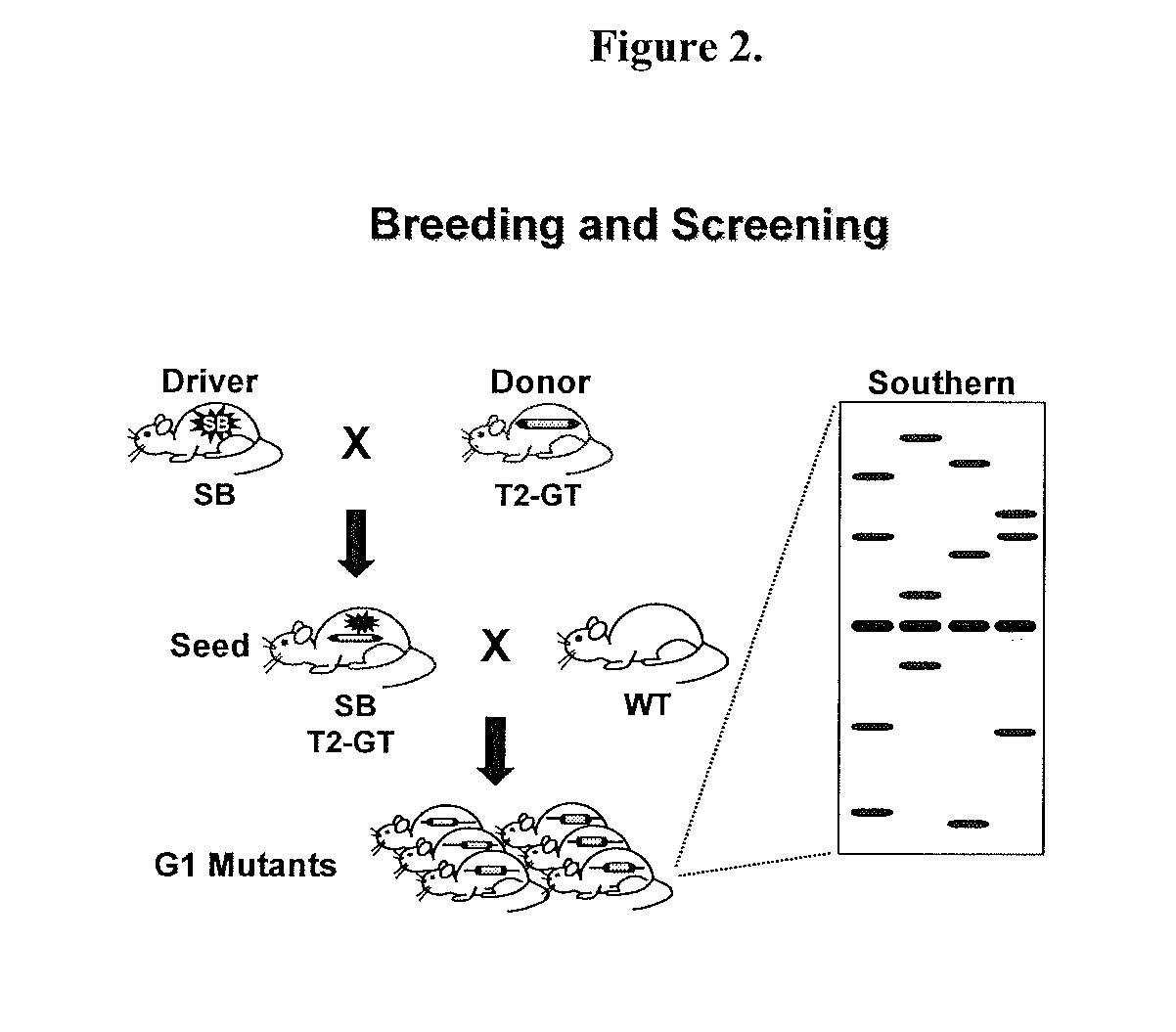
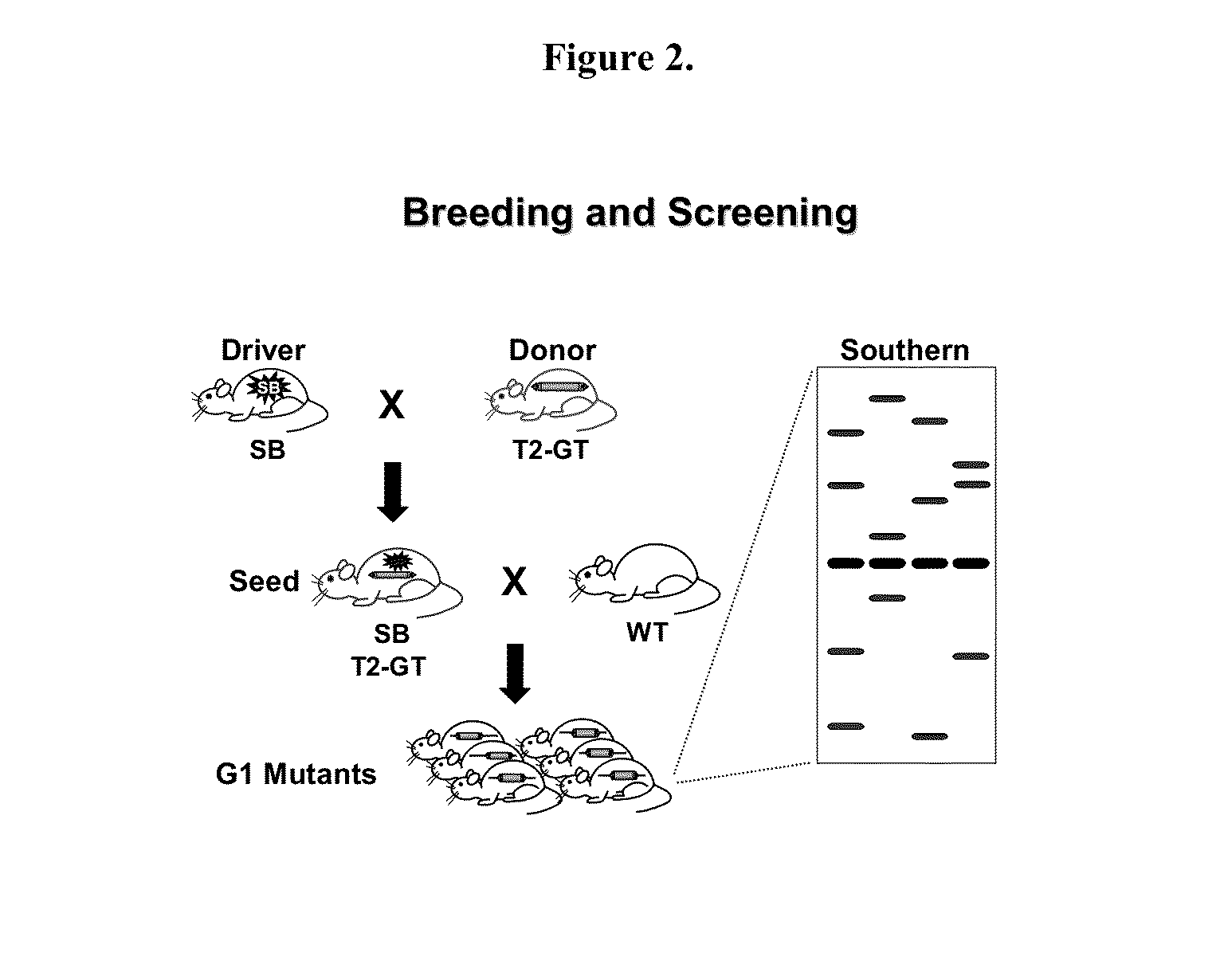
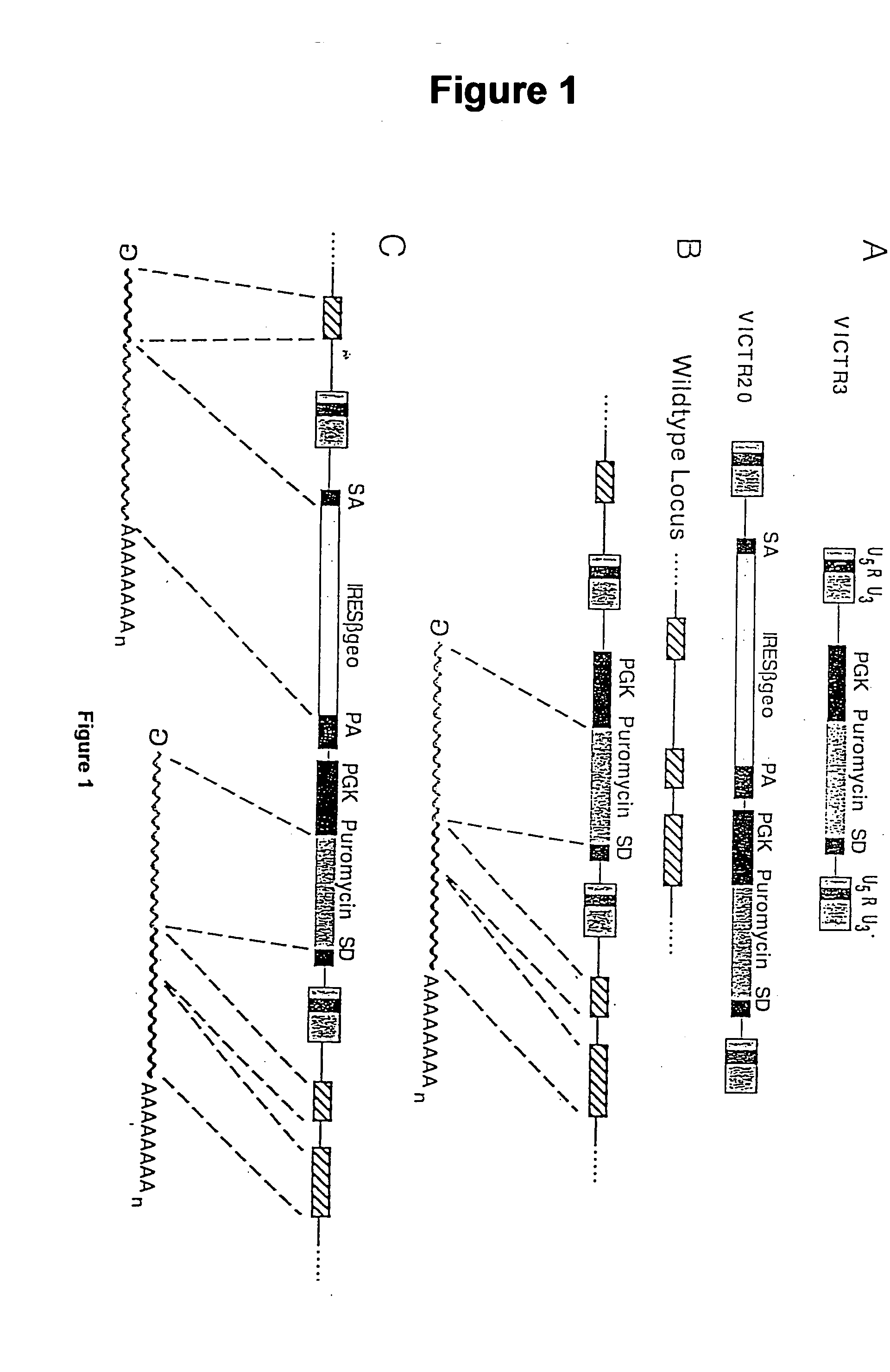
Novel mutated mammalian cells and animals
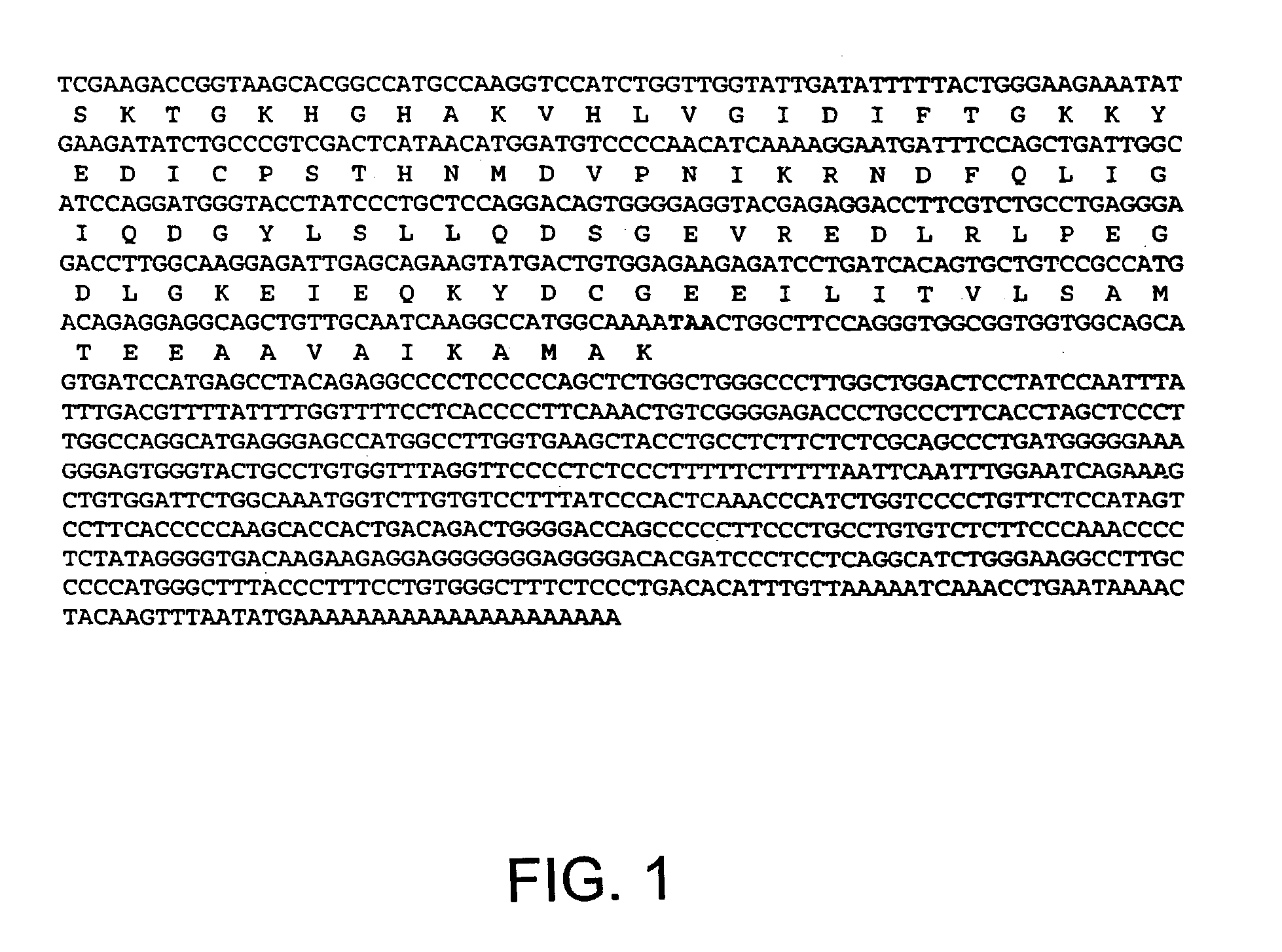
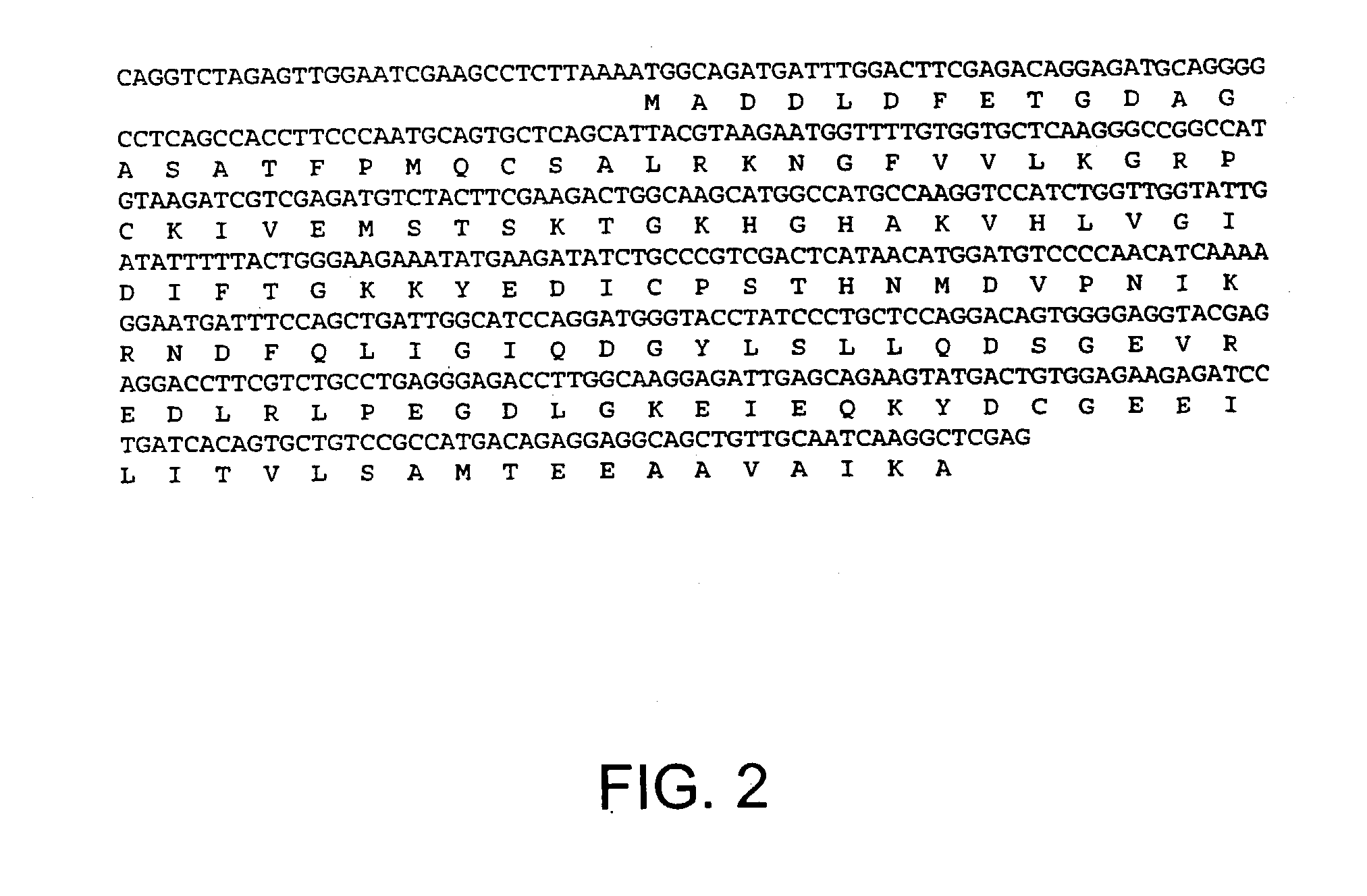
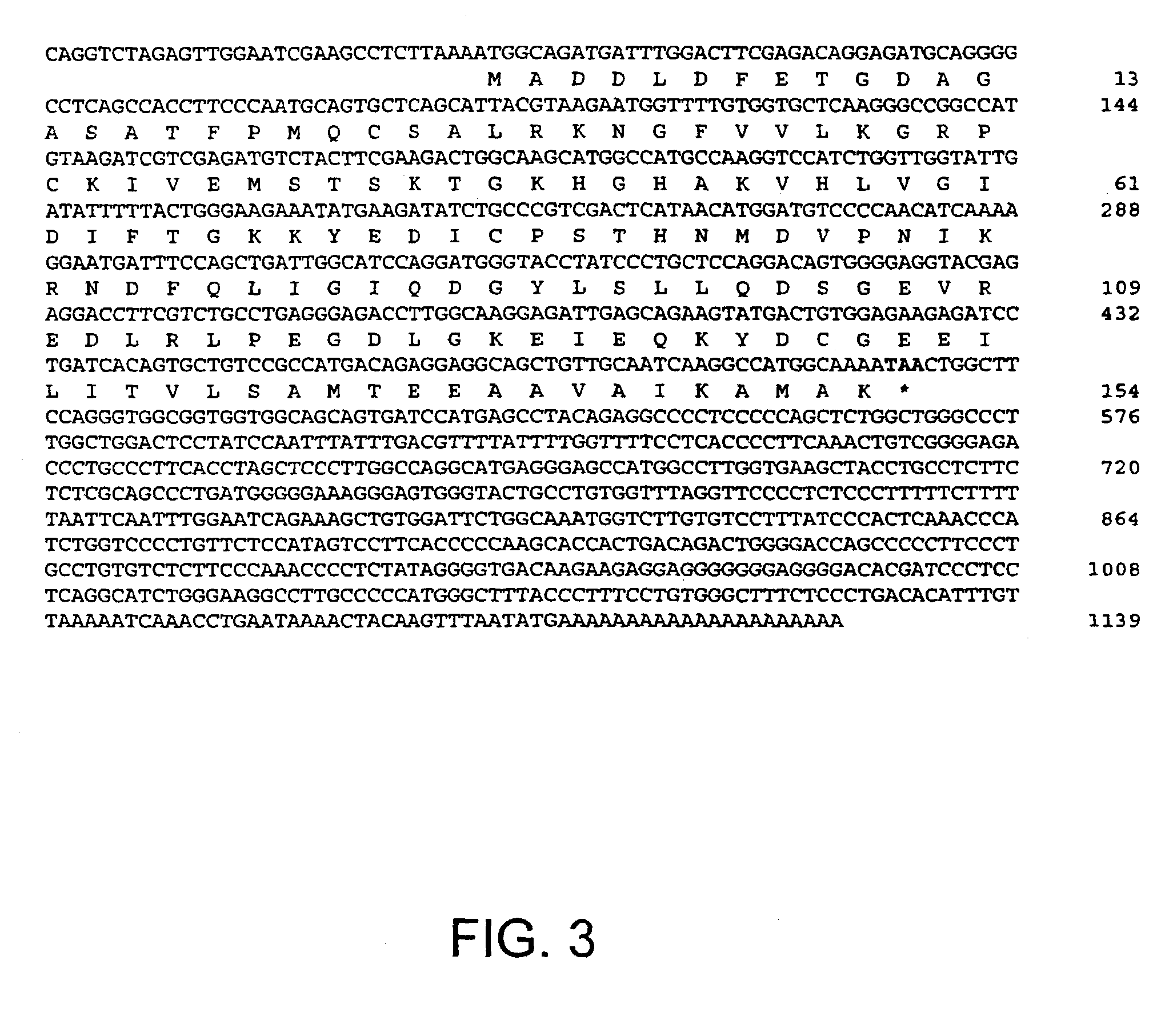
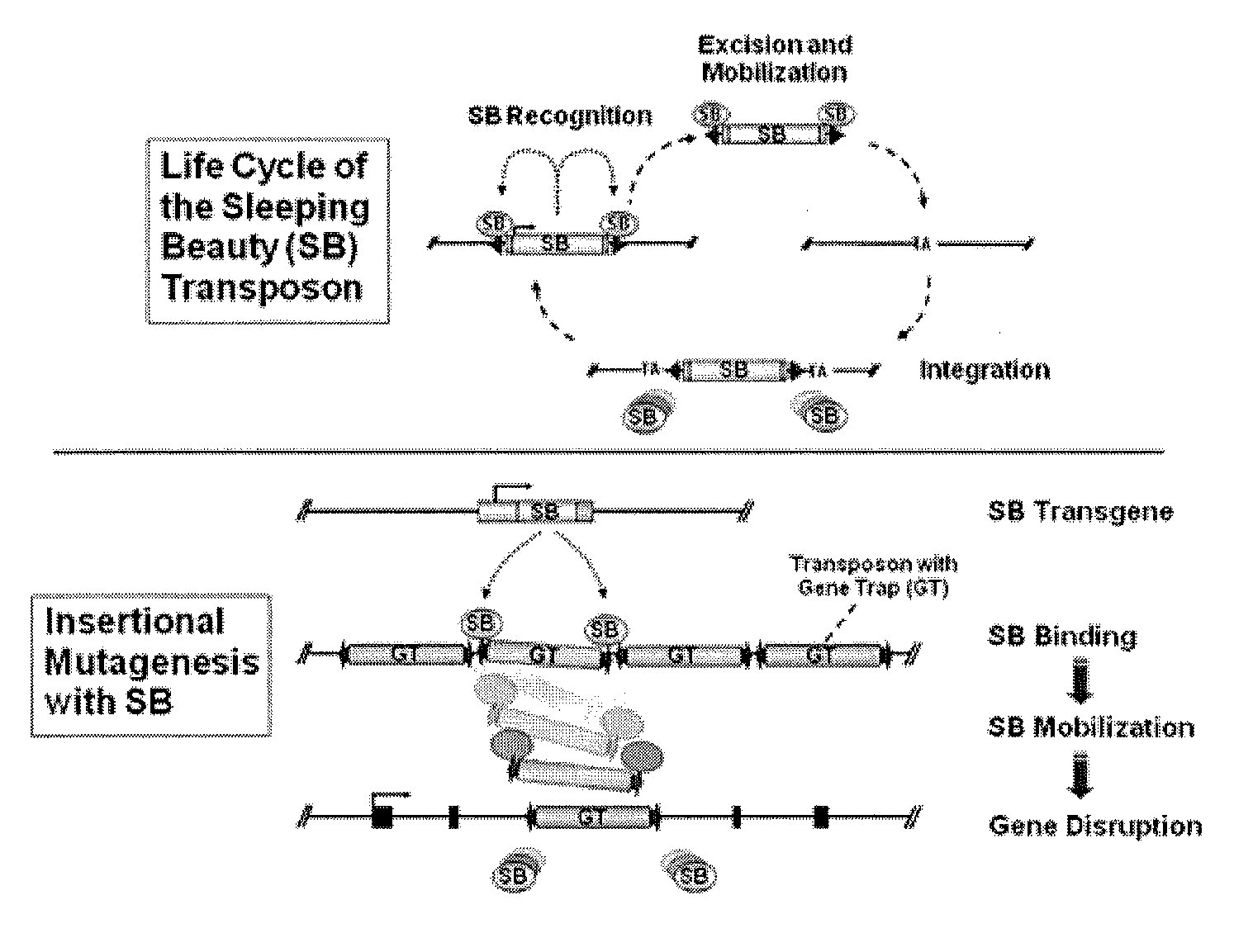
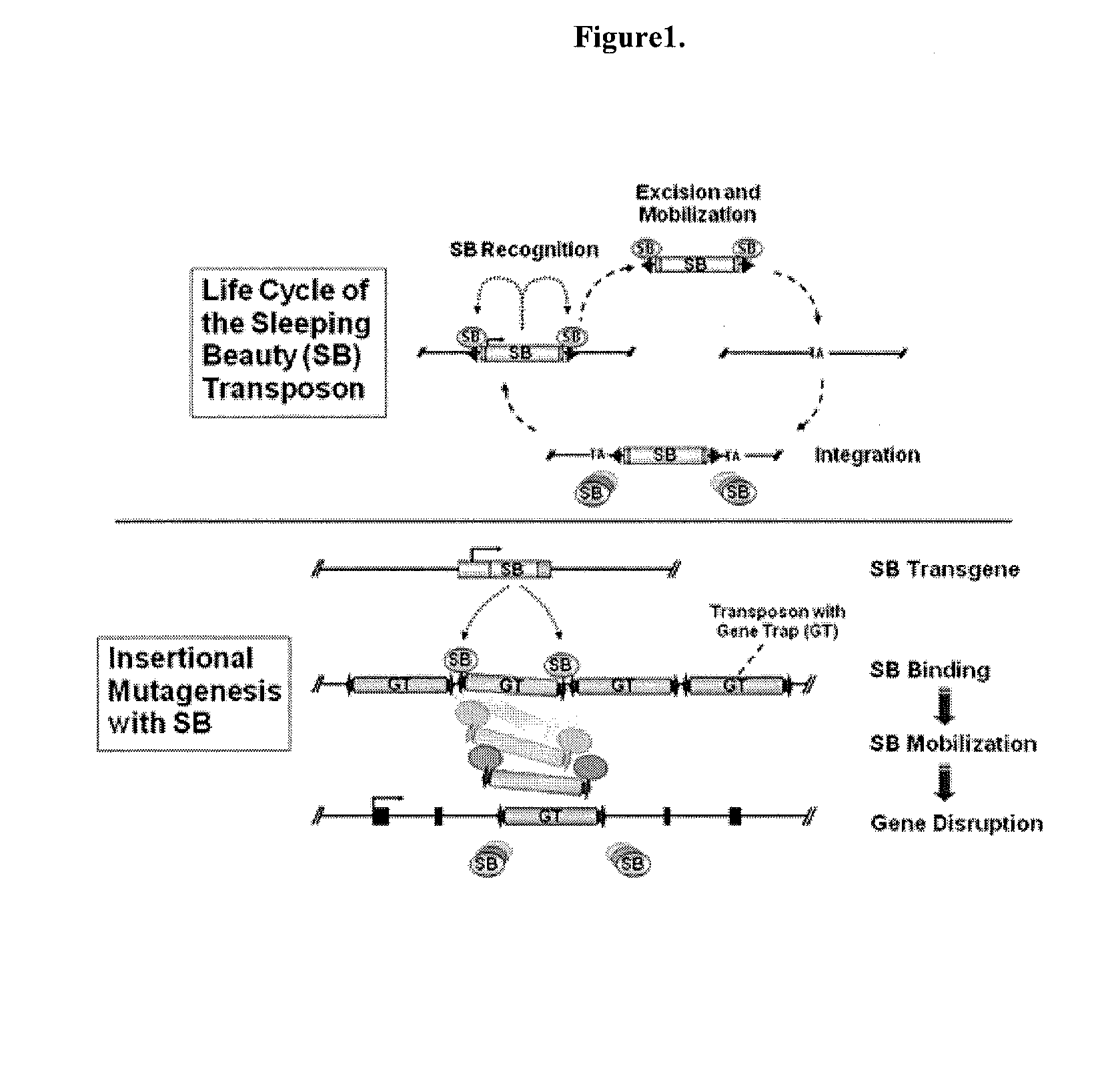
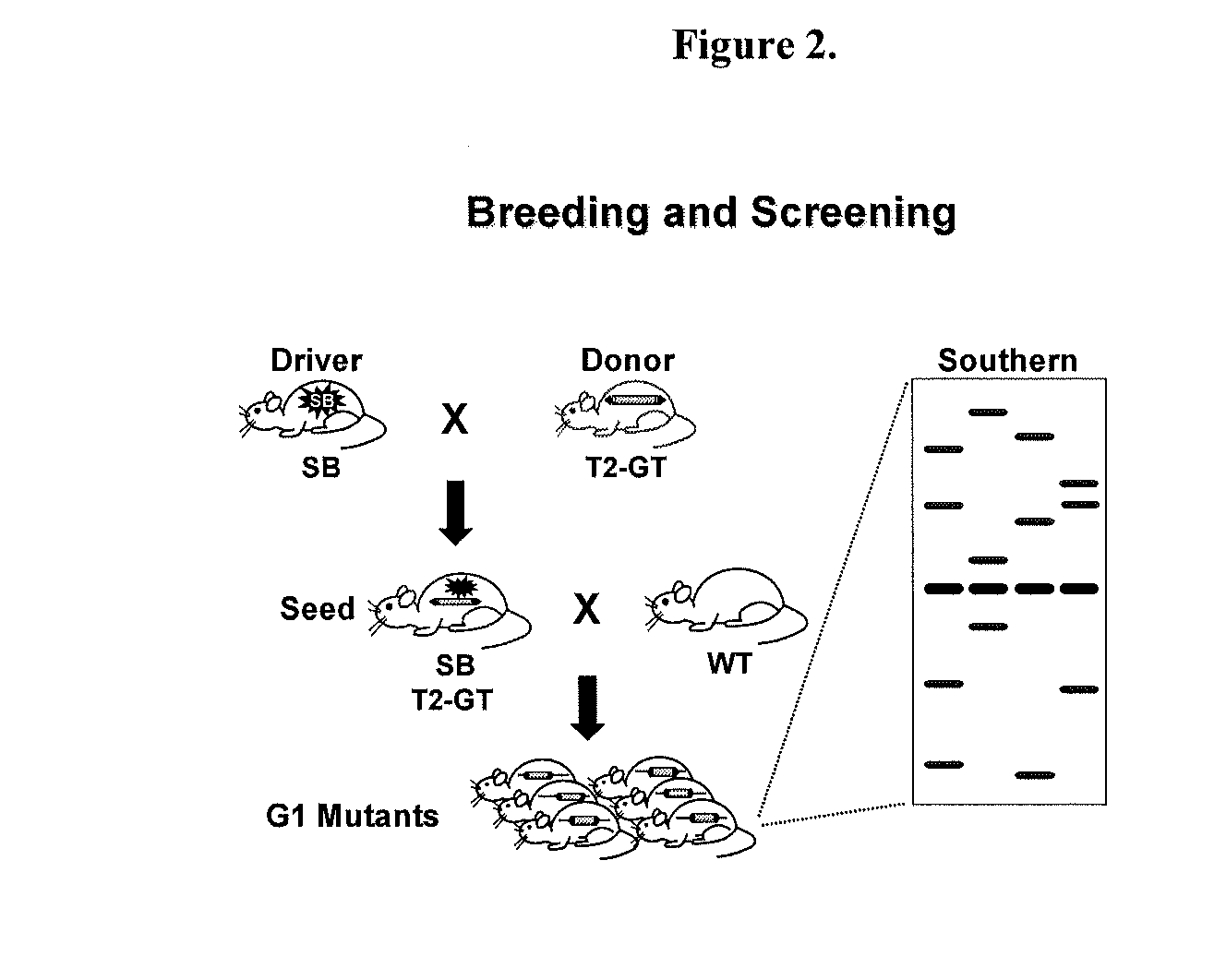
Novel mutated mammalian cells are provided that have been characterized by identifying the sequence of the genes that have been mutated. Preferably, novel mutated cells are murine ES cells that stably incorporate retroviral gene trap constructs in the specifically identified genes. The novel mutated cells and animals are useful in functional genomic analysis, and in the discovery and development of new therapeutic and diagnostics agents and methods.
Owner:FRIEDRICH GLENN +2
Sesquiterpene compound, preparation method thereof and application thereof in preparation of anti-inflammatory drugs and immunosuppressive drugs
ActiveCN111302924AEnhanced inhibitory effectOrganic active ingredientsAntipyreticImmunosuppressive drugAntiinflammatory drug
The invention provides a sesterterpene compound, a preparation method thereof and an application thereof in preparation of anti-inflammatory drugs and immunosuppressive drugs, and relates to the technical field of natural pharmaceutical chemistry. The sesterterpene compound provided by the invention has any one of structures shown in formulas 1 to 12. The sesterterpene compound provided by the invention is a sesterterpene compound with a very rare skeleton, has anti-inflammatory and immunosuppressive effects, and can be applied to preparation of anti-inflammatory drugs and immunosuppressive drugs. Results of embodiments show that the sesterterpene compounds provided by the invention have obvious inhibition effects on CD3 and CD28 monoclonal antibodies for stimulating mouse T cell proliferation and generating cytokine IFN-gamma. The invention further provides a pharmaceutical composition. The pharmaceutical composition comprises pharmaceutically acceptable pharmaceutical excipients andone or more of the sesterterpene compounds in the scheme.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Conditioned cell culture medium, method to obtain the same and use of it for maintenance, proliferation and differentiation of mammalian cells
The invention relates to a conditioned cell culture medium and a corresponding method to obtain it. The invention also refers to methods of using this cellconditioned medium for the maintenance, proliferation and differentiation of mammalian cells. The culture medium produced in accordance with the present invention is conditioned by the cell secretion activity of murine cells, in particular, those differentiated and immortalized transgenic hepatocytes, named MMH (Met Murine Hepatocyte). These media are employed in in vitro cell culture systems to induce maintenance, proliferation and differentiation of mammalian cells. The cells named MMH are differentiated non transformed murine hepatocytes that produce important biological molecules (e.g cytokines and growth factors) and, in accordance with the present invention, they are used in in vitro cell culture systems for the maintenance, proliferation and differentiation of mammalian cells.
Owner:INST NAT PER LE MALATTIE INFETTIVE LAZZARO SPALLANZANI IRCCS
Purified polypeptide, isolated nucleic acids encoding said polypeptide, vectors and use thereof
InactiveUS20060084791A1Improve bindingEfficient infectionVirus peptidesFermentationHuman cellMurine leukaemia virus
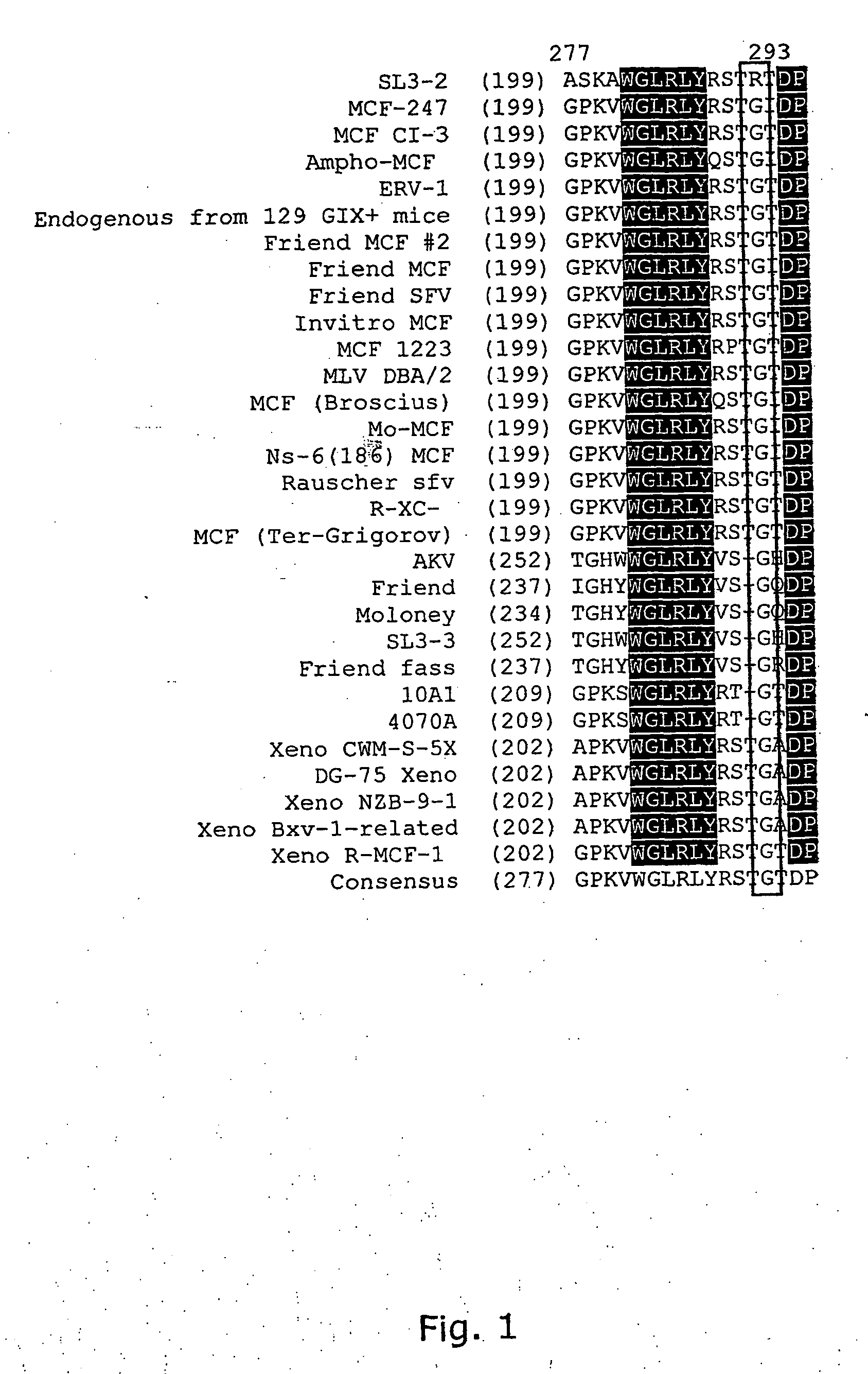
The present invention relates to a purified polypeptide, which is capable of mediating infection of a cell, by use of the polytropicixenotropic receptor encoded by the Rmc1 locus from a NIH Swiss inbred NFS / N mouse for entry, and unable of mediating infection of a cell by use of a human polytropic / xenotropic receptor encoded by the human RMC1 locus. The present invention especially relates to an envelope protein from the Murine leukaemia Virus (MLV) strain SL3-2, which is capable of infecting murine cells through usega of the polytropic receptor encoded by the Rruc1 locus, but lacks the ability of infecting human cells expressing the corresponding xenotropic receptor encoded by the RMC locus. The present invention furthermore demonstrate that replacements of at least one specified amino acid in the polypeptide can alter the tropism and enable the SL3-2 envelope to infect a human cell by use of the human polytropic / xenotropic receptor encoded by the RMC1 locus for entry.
Owner:RETROVEC +1
Method and application of targeted integration of exogenous dna sequence into rat and mouse rosa26 site
ActiveCN103540587BClear genetic backgroundStable exogenous expressionMicrobiological testing/measurementVector-based foreign material introductionExogenous DNACell strain
The present invention discloses a method and its application of targeted integration of exogenous DNA sequences into the Rosa26 site of rats and mice. Plasmids, and the corresponding plasmids are respectively transferred into mouse or rat cells, and the stable cell lines that can be obtained are used to express various foreign genes. The obtained stable cell line can be stably passaged, has a clear genetic background, and accurately expresses the biological function of the exogenous gene.
Owner:OBIO TECH SHANGHAI CORP LTD
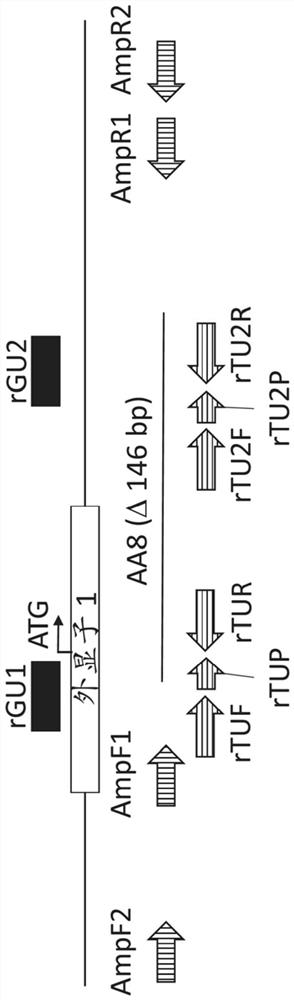
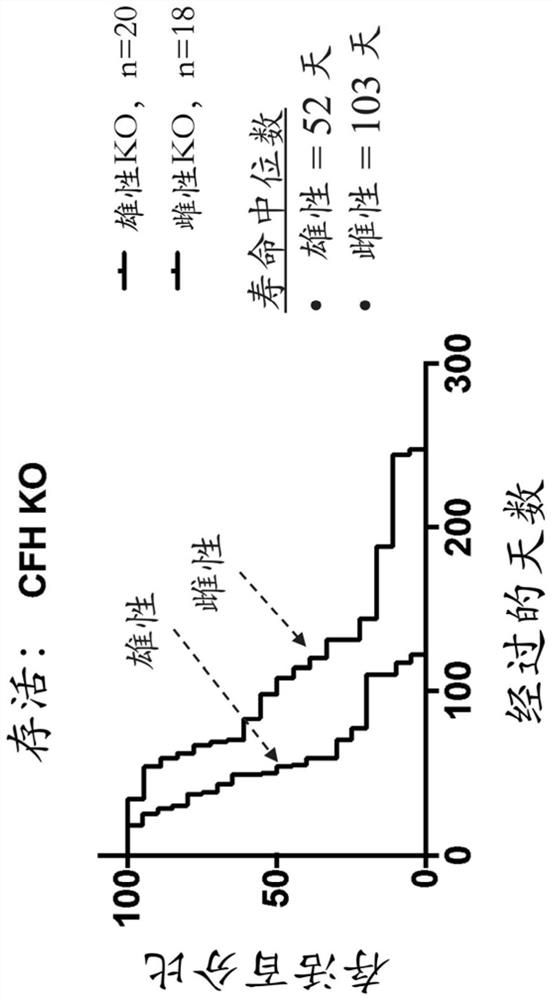
Complement factor h gene knockout rat as a model of c3 glomerulopathy
Rat cells and rats comprising an inactivated Cfh locus and methods of making and using such rat cells and rats are provided. The rats comprising an inactivated Cfh locus model C3 glomerulopathy (C3G). Methods are provided for using such rats comprising an inactivated Cfh locus to assess in vivo efficacy of putative C3G therapeutic agents.
Owner:REGENERON PHARM INC
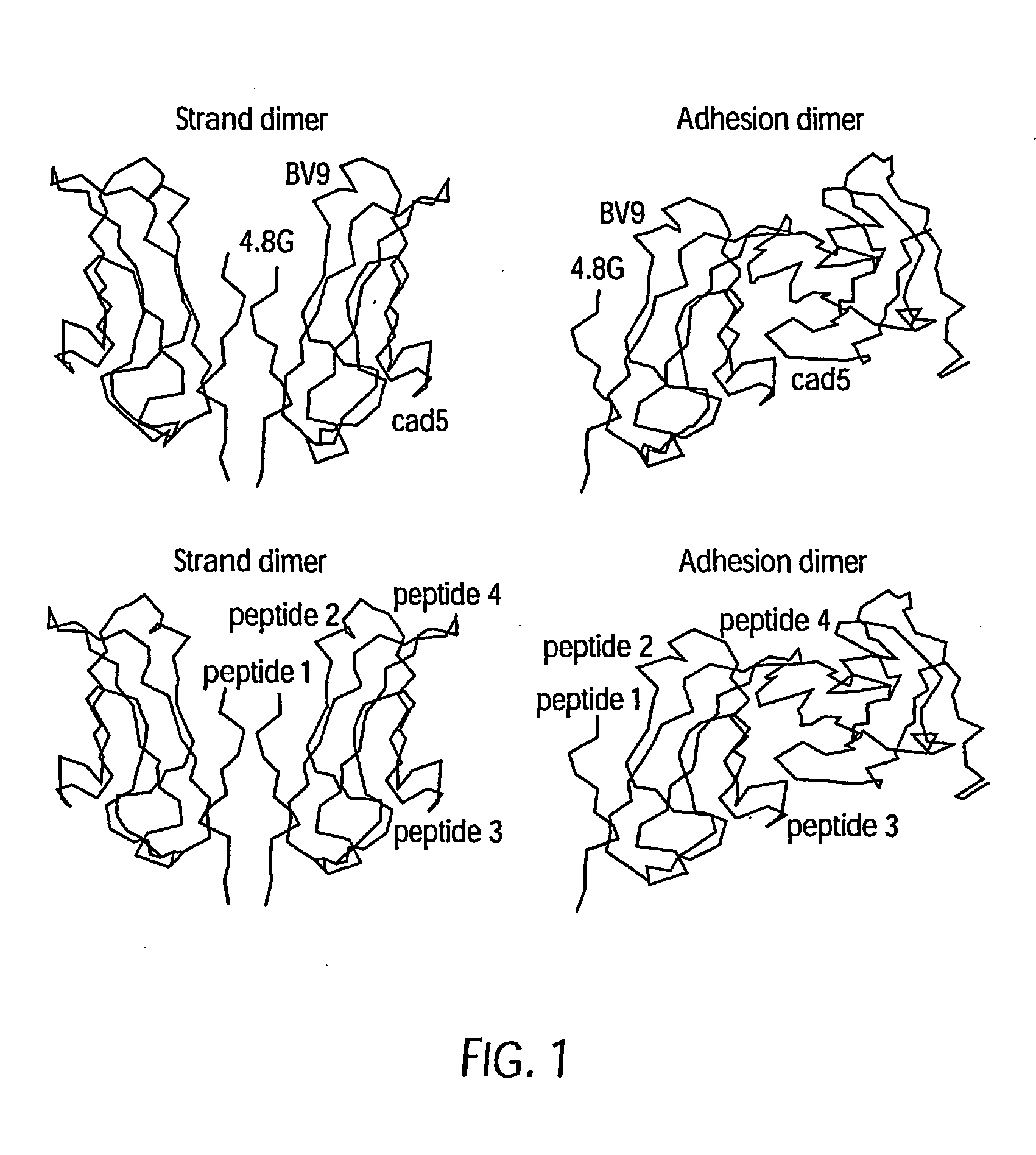
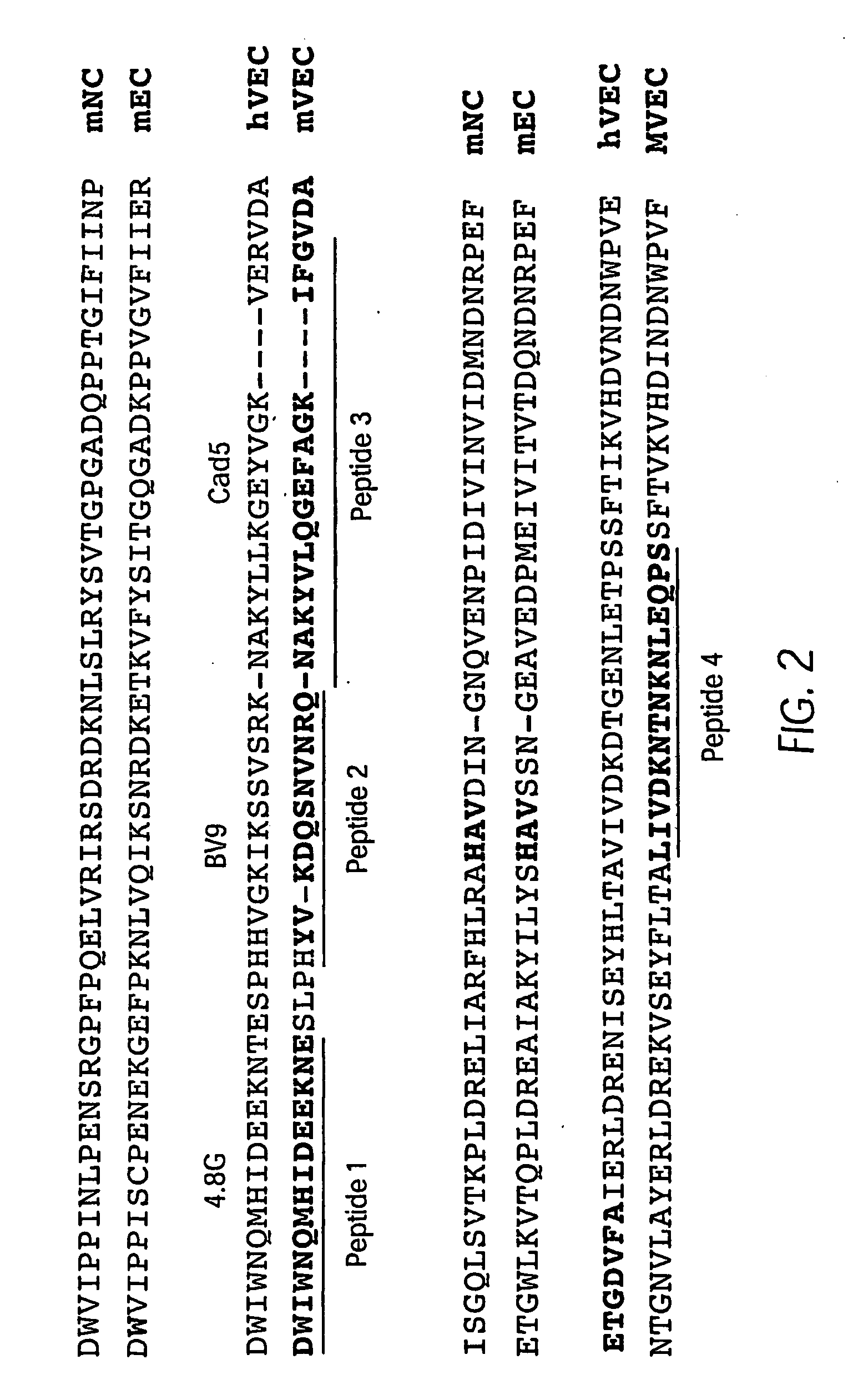
Antibody antagonists of ve-cadherin without adverse effects on vascular permeability
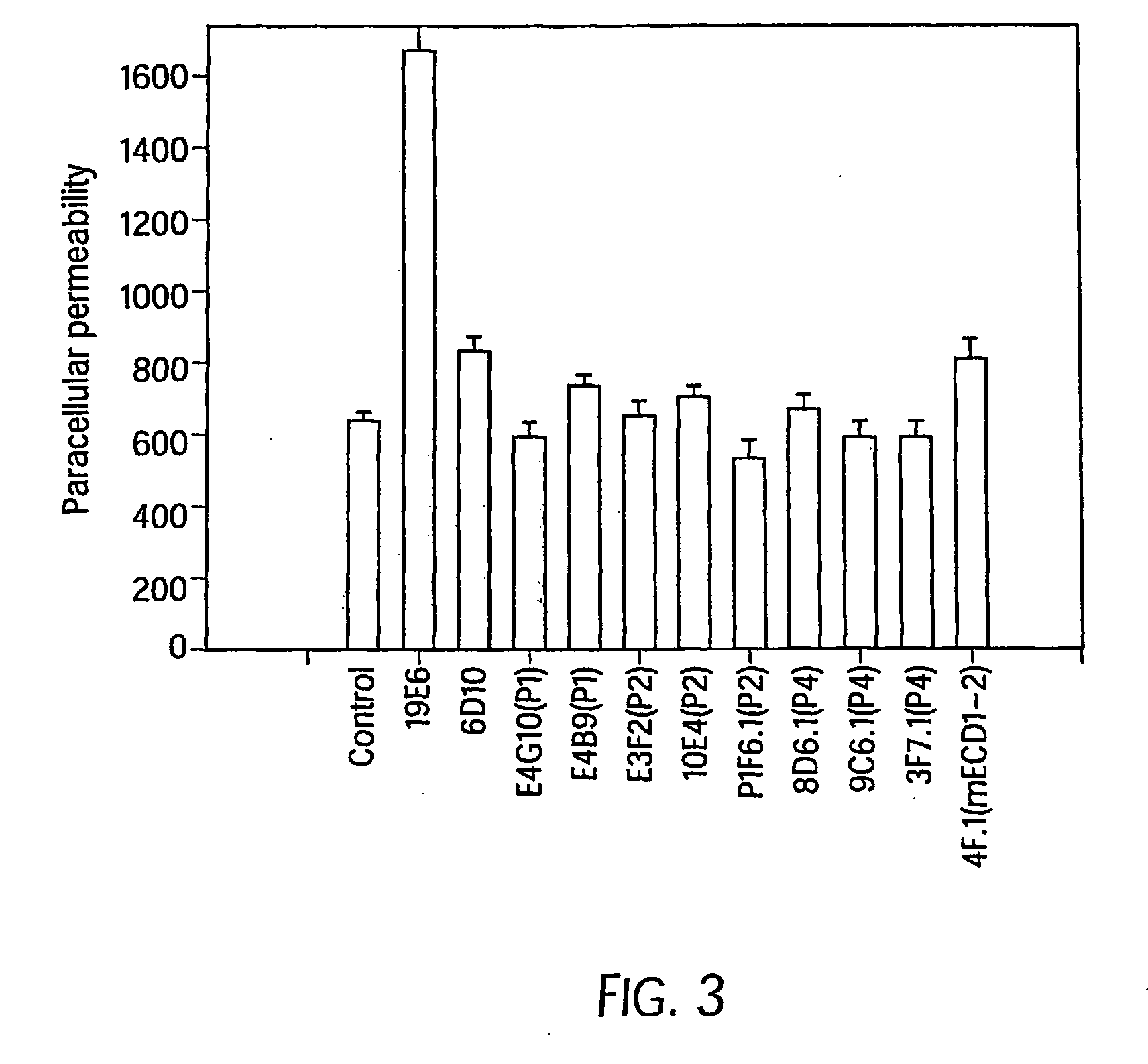
InactiveUS20080311121A1Reducing and inhibiting tumor vasculatureInhibition formationSenses disorderGenetic material ingredientsEpitopeWestern blot
The murine epitope sequence recognized by antibody E4B9 shares 100% homology with human VE-cadherin, so this antibody was examined to determine if it cross-reacts with human VE-cadherin. Western-blot analysis of several VE-cadherin expressing human and murine cells indicated that E4B9 indeed cross-reacts with human VE-cadherin. (FIG. 6). This finding facilitates development of a “humanized” E4B9 antibody and its success in the preclinical development since its anti-tumor activity can be tested extensively in several mouse models.
Owner:IMCLONE SYSTEMS
Method for tumor treatment using infusion of xenogeneic cells to induce hyperacute rejection and innocent bystander effect
InactiveUS20060034812A1Quick killAltered physiologyBiocidePeptide/protein ingredientsAbnormal tissue growthTumor therapy
A method for treating tumors. Through infusion or xenotransplantation of xenogeneic cells, such as infusion of murine cells into the peritoneal cavity of humans, a hyperacute rejection response to the cells is induced. This in turn creates a bystander effect to the tumor. This effect creates tumor regression. This treatment can be used alone or in conjunction with gene therapy or chemotherapy treatments.
Owner:HUMAN GENE THERAPY RES INST
Variable region sequence of specific anti-chlorothalonil antibody and recombinant full-length IgG antibody thereof
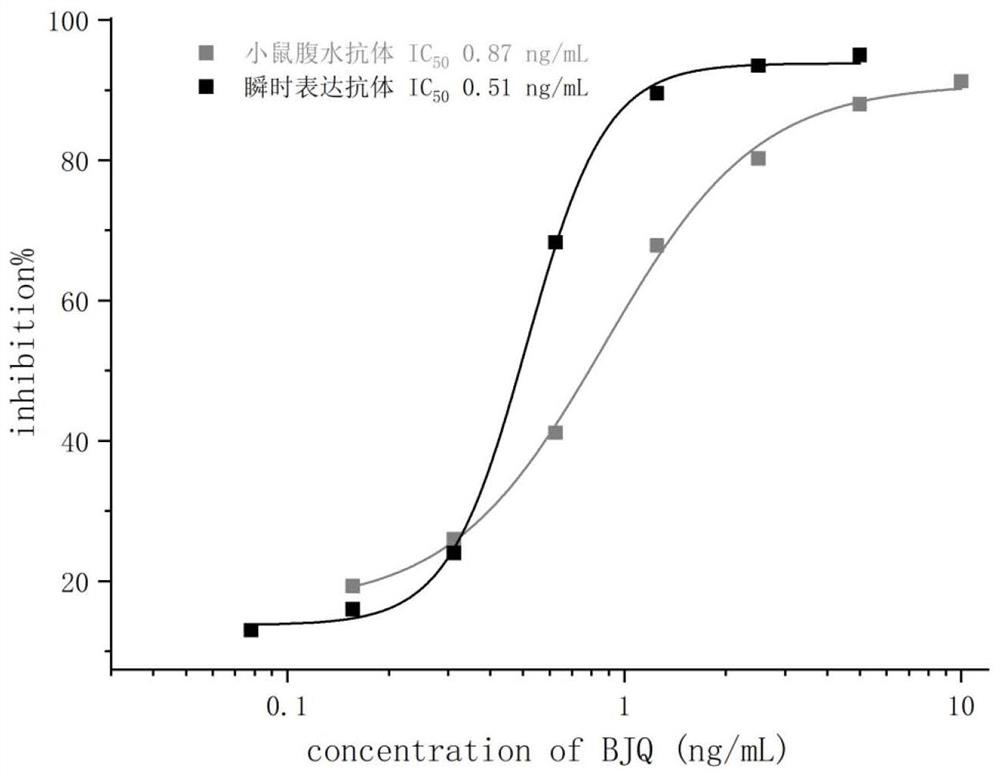
ActiveCN113563472AHigh sensitivityHigh selectivityImmunoglobulinsVector-based foreign material introductionImmune profilingAntiendomysial antibodies
The invention discloses a variable region sequence of a specific anti-chlorothalonil antibody. The amino acid sequence of a heavy chain variable region coding gene is shown in SEQ ID NO: 2. The invention also discloses an anti-chlorothalonil recombinant full-length IgG antibody. The invention also discloses a recombinant antibody expression plasmid. The heavy chain variable region and light chain variable region sequences of the anti-chlorothalonil recombinant full-length IgG antibody are derived from a monoclonal cell strain which secretes a high-affinity and high-specificity anti-chlorothalonil antibody and is obtained by immunizing mice for multiple times, performing cell fusion and screening. The sequence genes are respectively connected to expression vectors containing a mouse IgG1 heavy chain constant region gene and a mouse kappa light chain constant region gene, the recombinant full-length IgG antibody is obtained by expression of mammalian cells of a double-plasmid system, the recognition activity of the recombinant full-length IgG antibody is very close to that of a mouse-derived parent antibody, large-scale standardized production of the anti-chlorothalonil antibody can be realized, and reliable core raw materials are provided for various immunoassay methods for rapid detection of chlorothalonil residues.
Owner:ZHEJIANG UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com