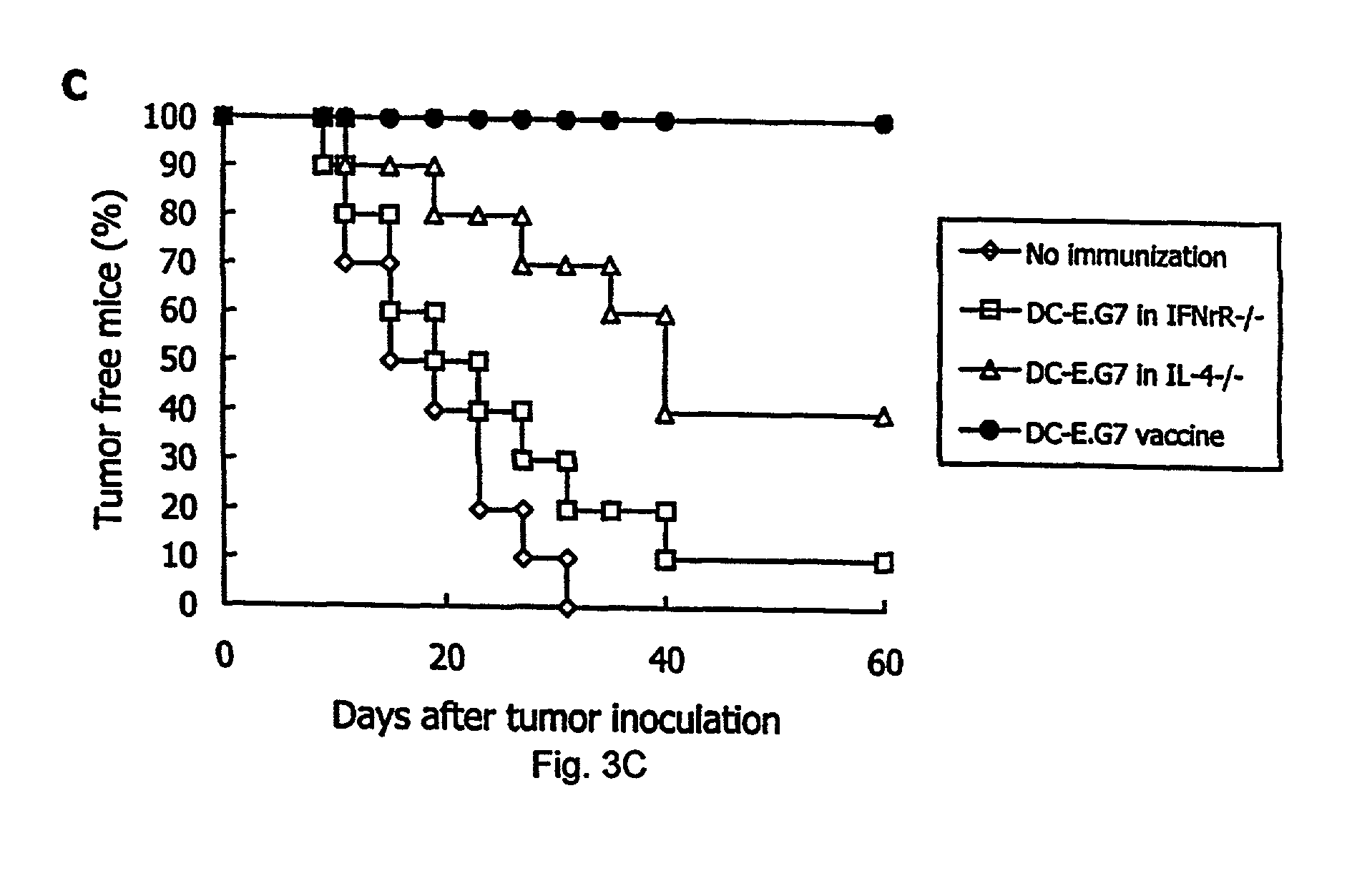
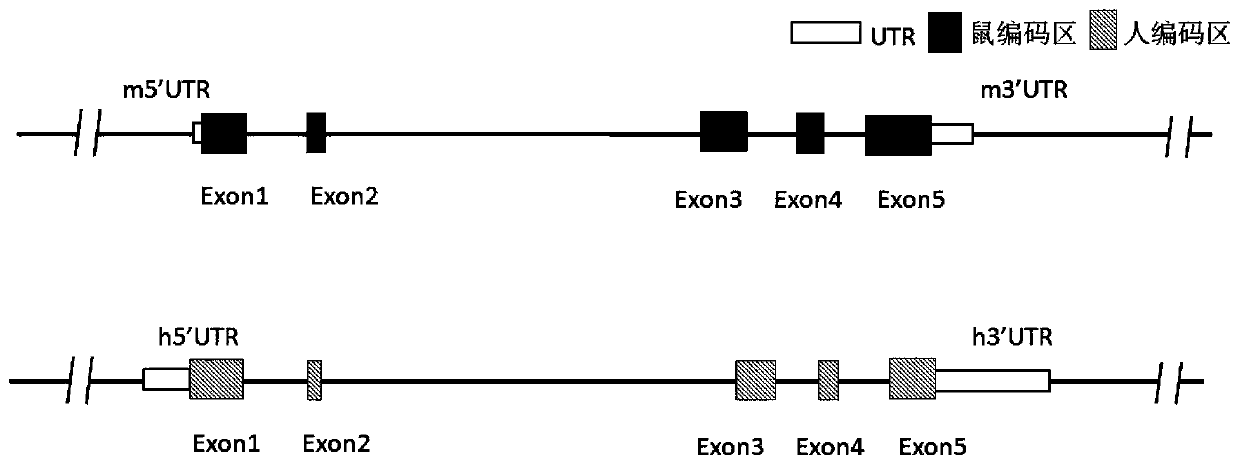
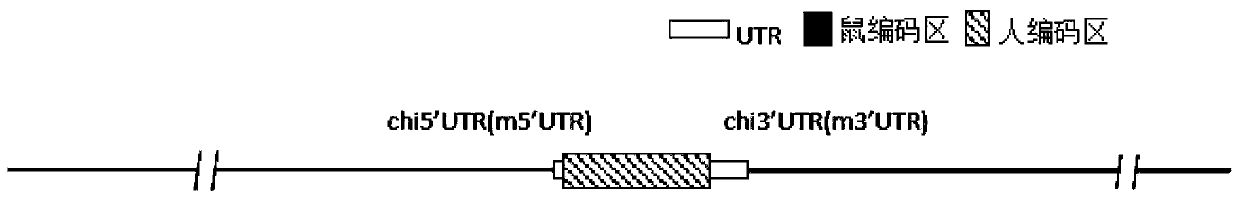
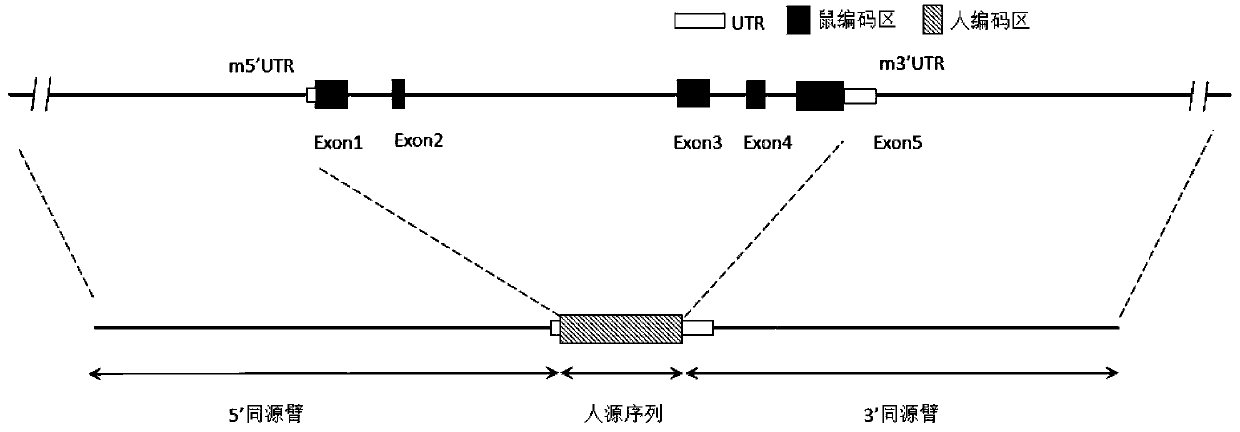
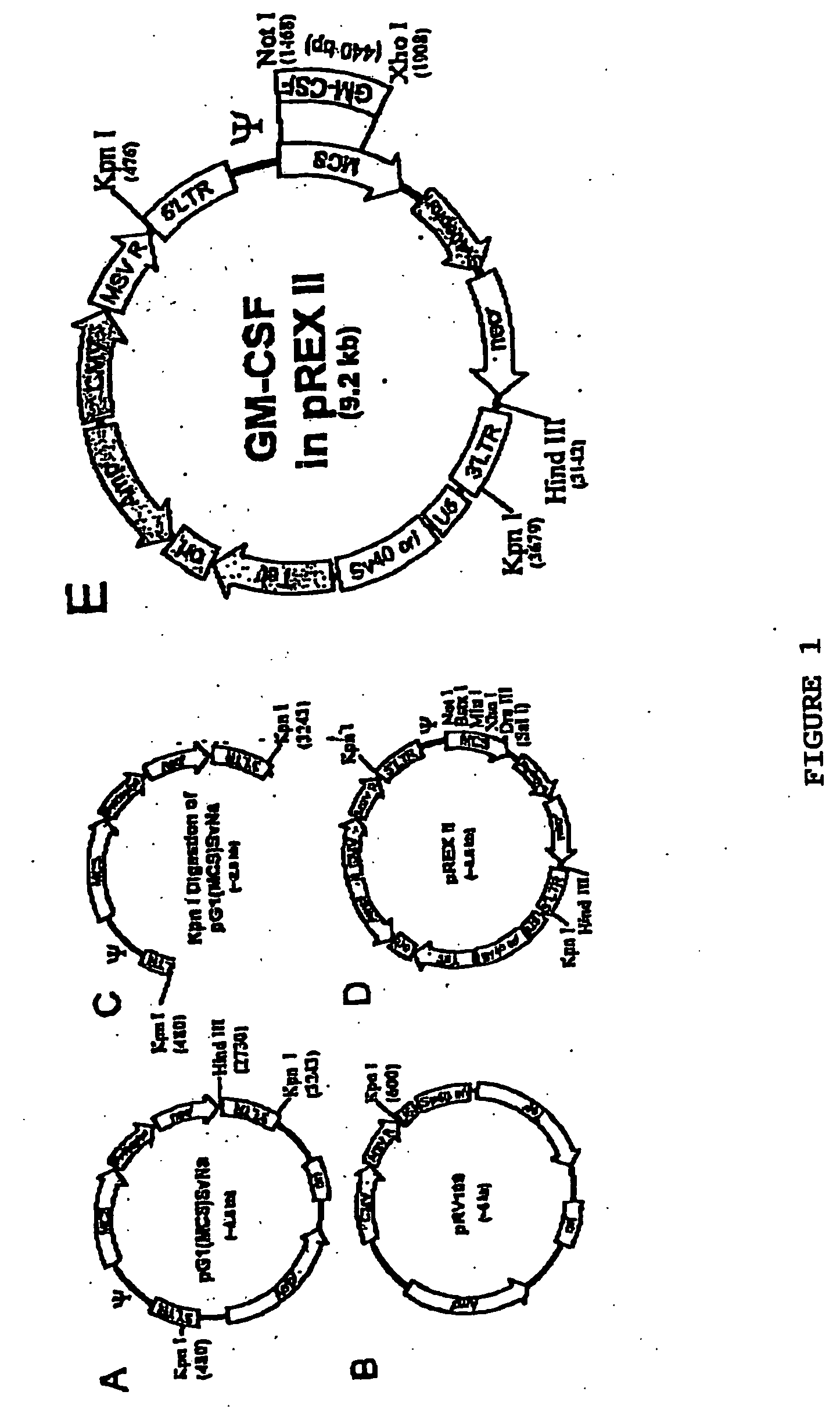
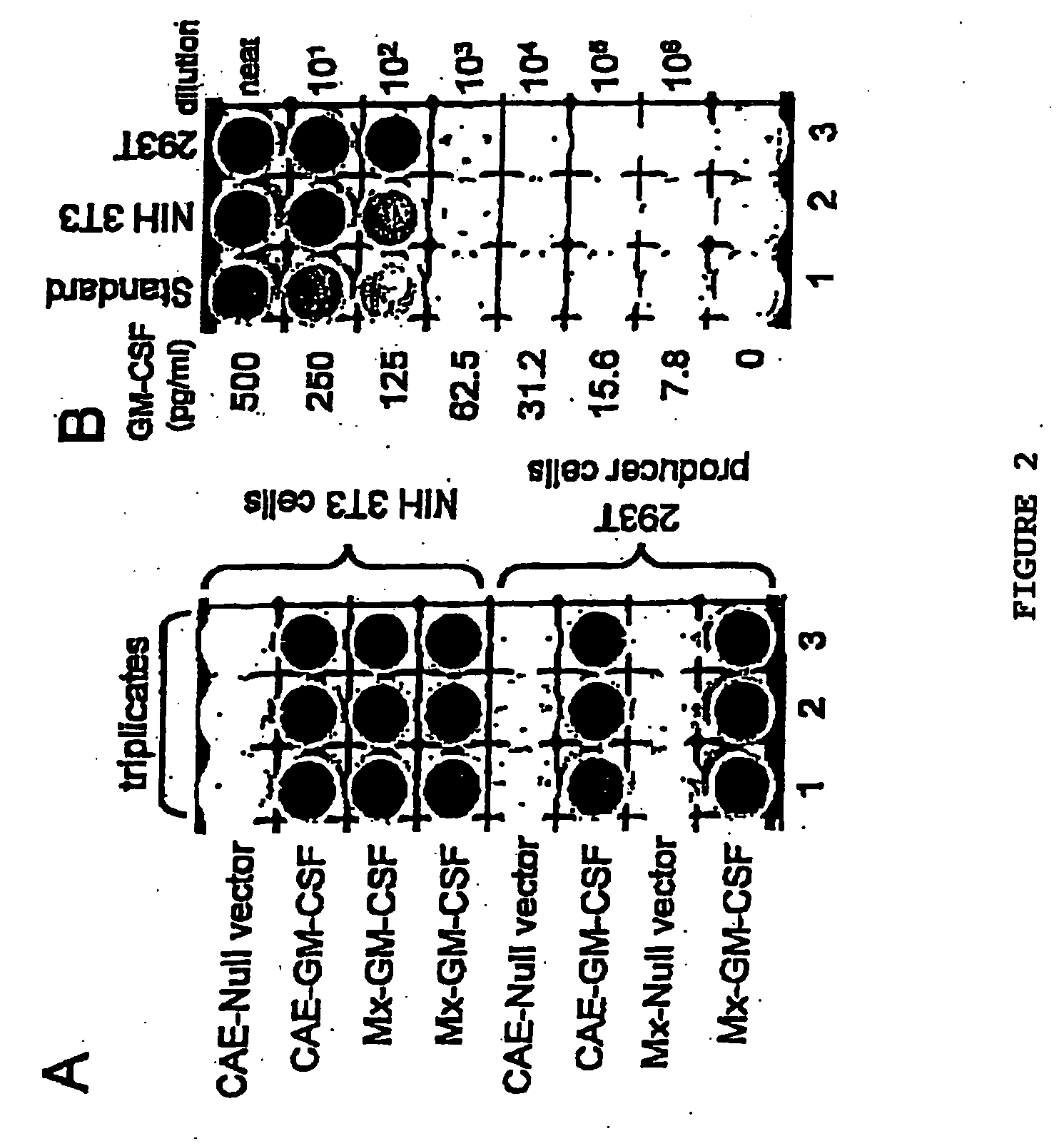
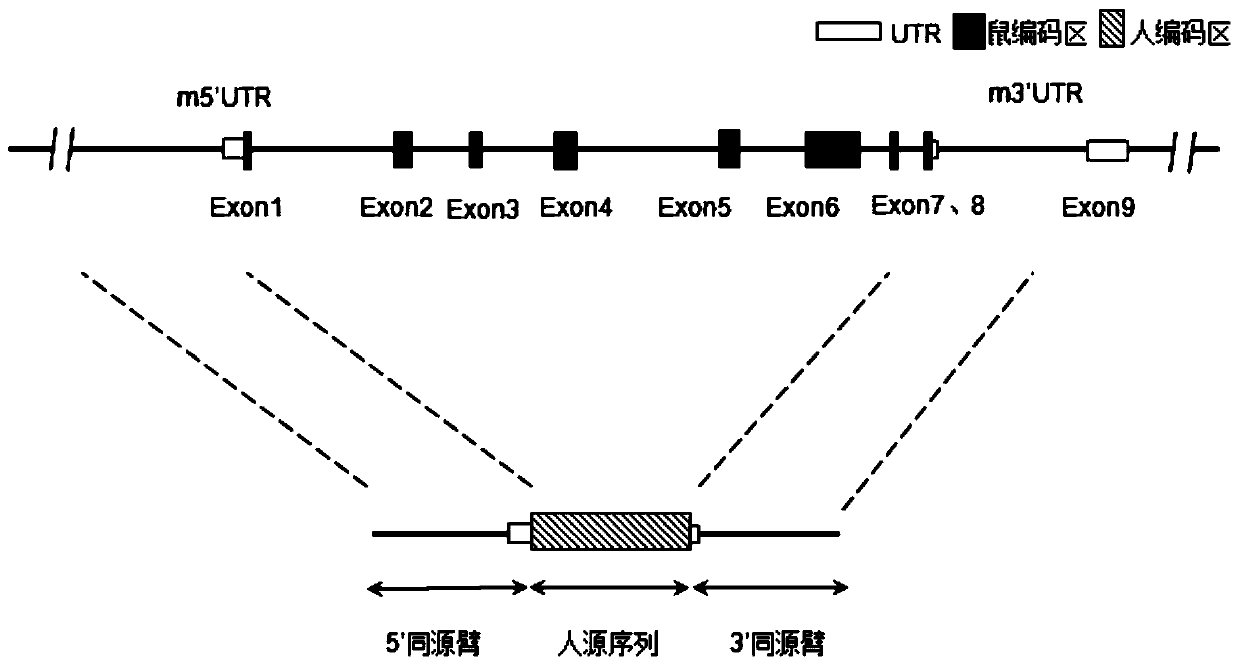
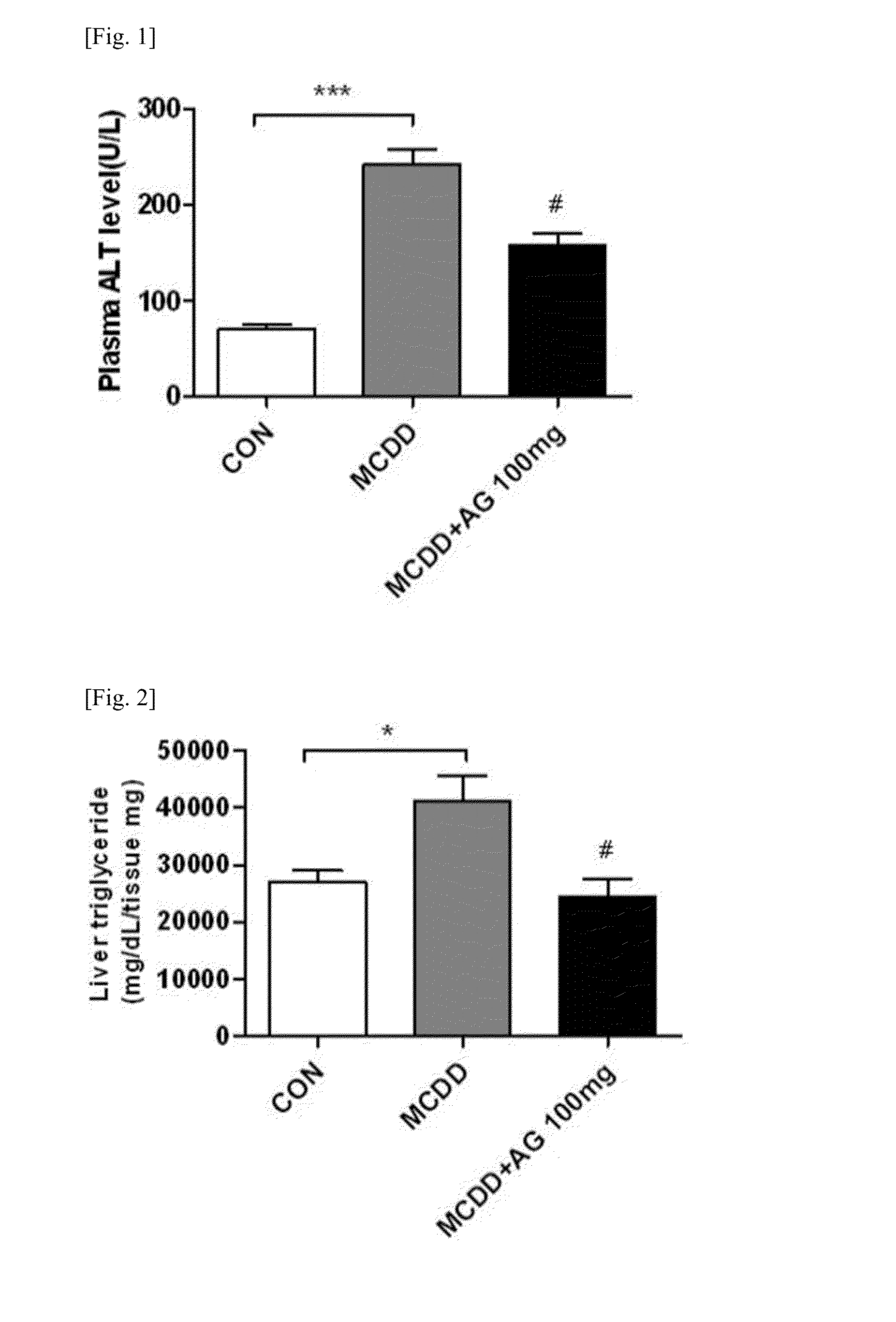
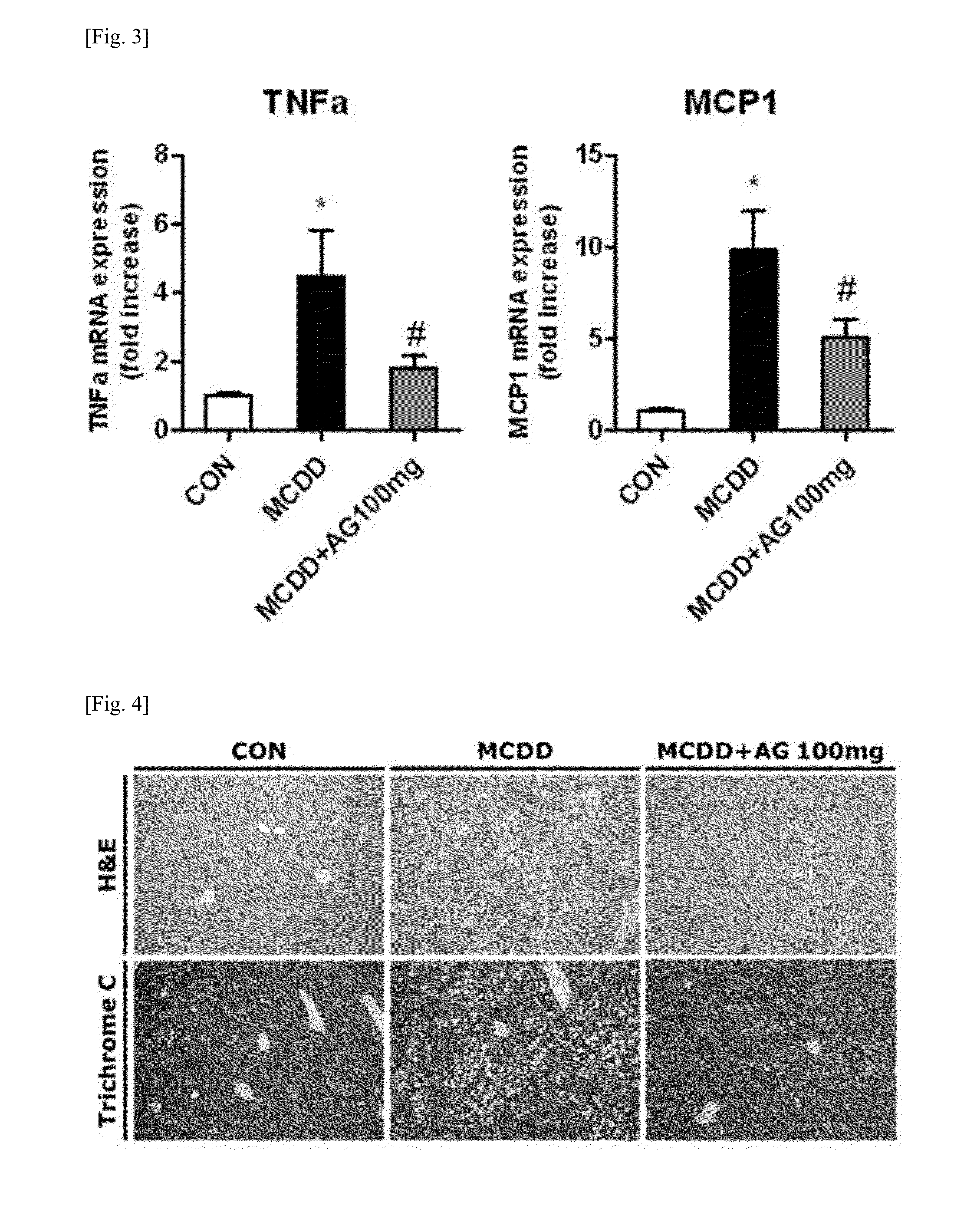
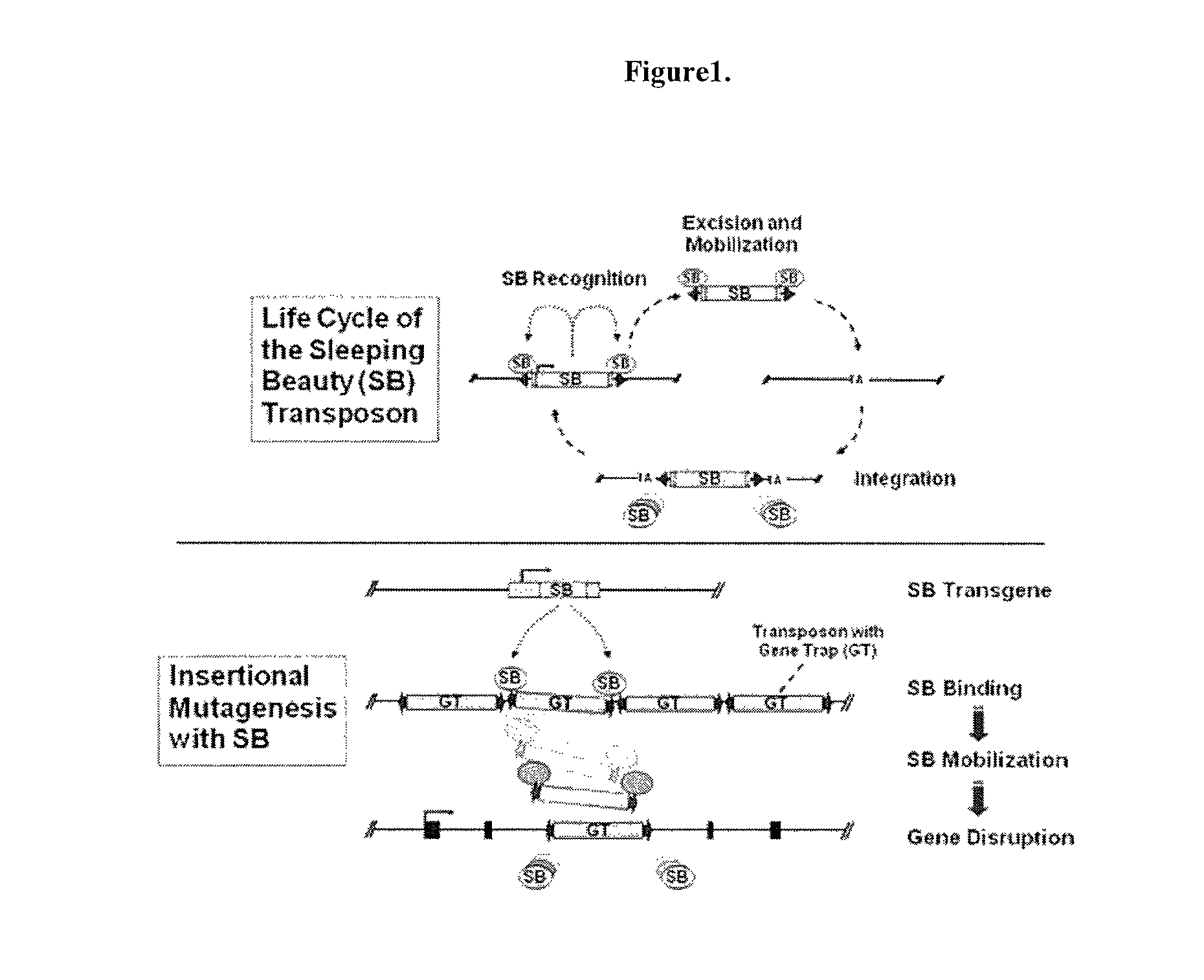
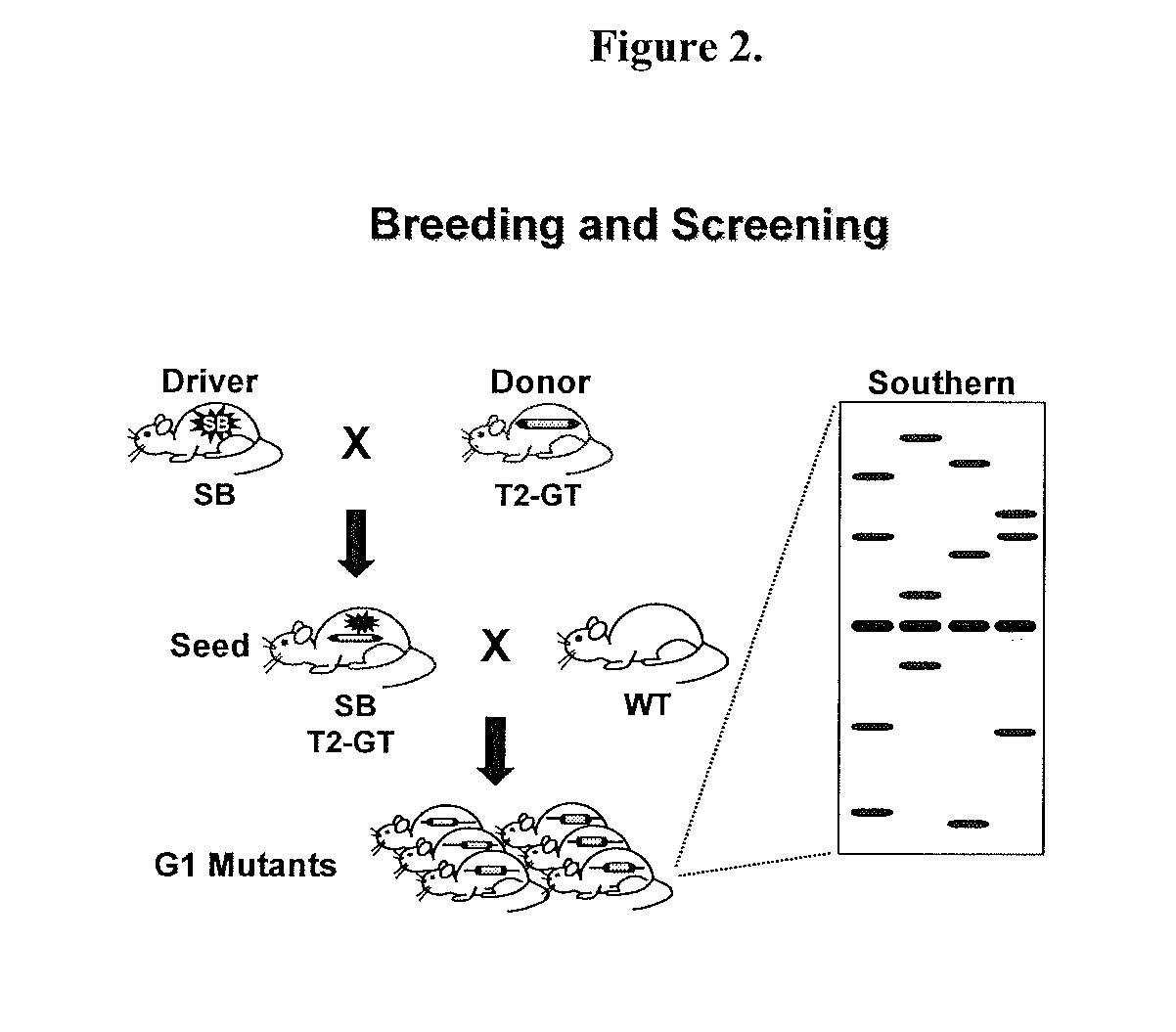
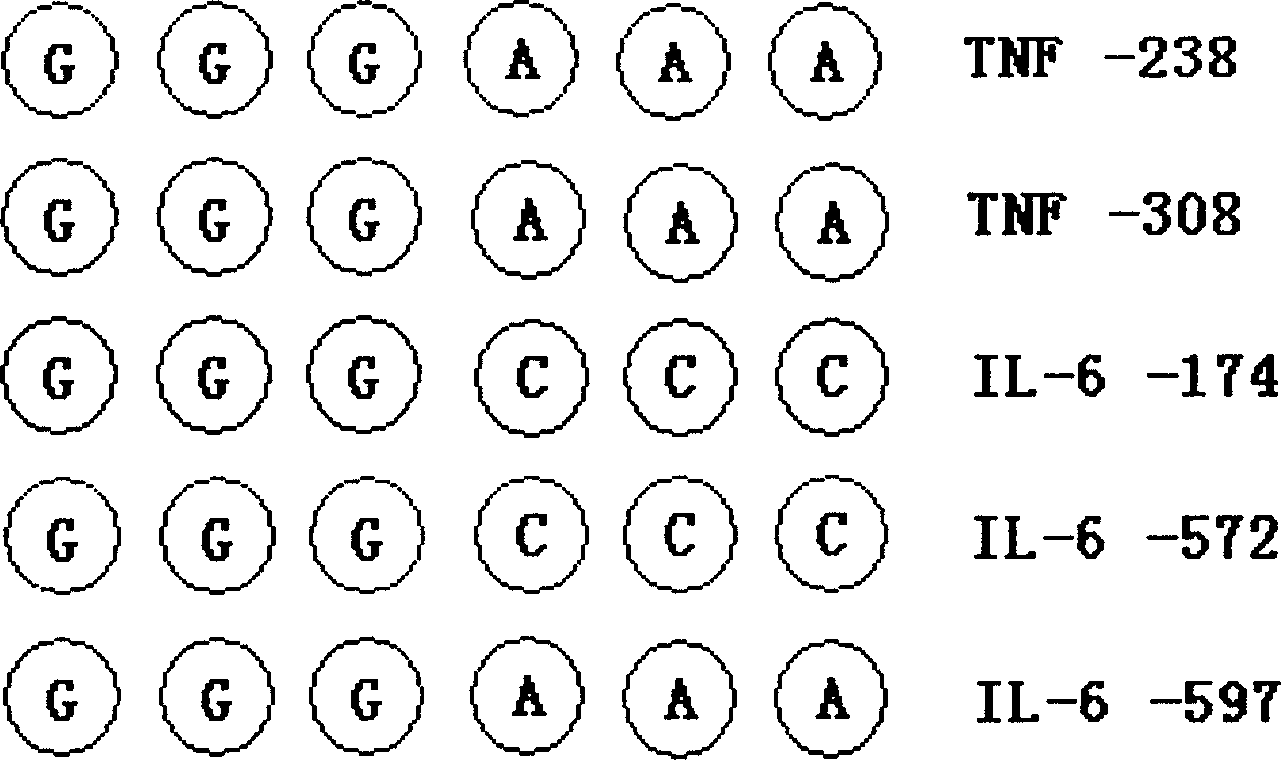
Patents
Literature
75 results about "Cytokine genes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cytokine genes instruct cells to produce proteins called cytokines that influence immune system response. As with many genes, the cytokine genes differ slightly from person to person.
Noninvasive genetic immunization, expression products therefrom and uses thereof
InactiveUS6348450B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideHemagglutininWhole body
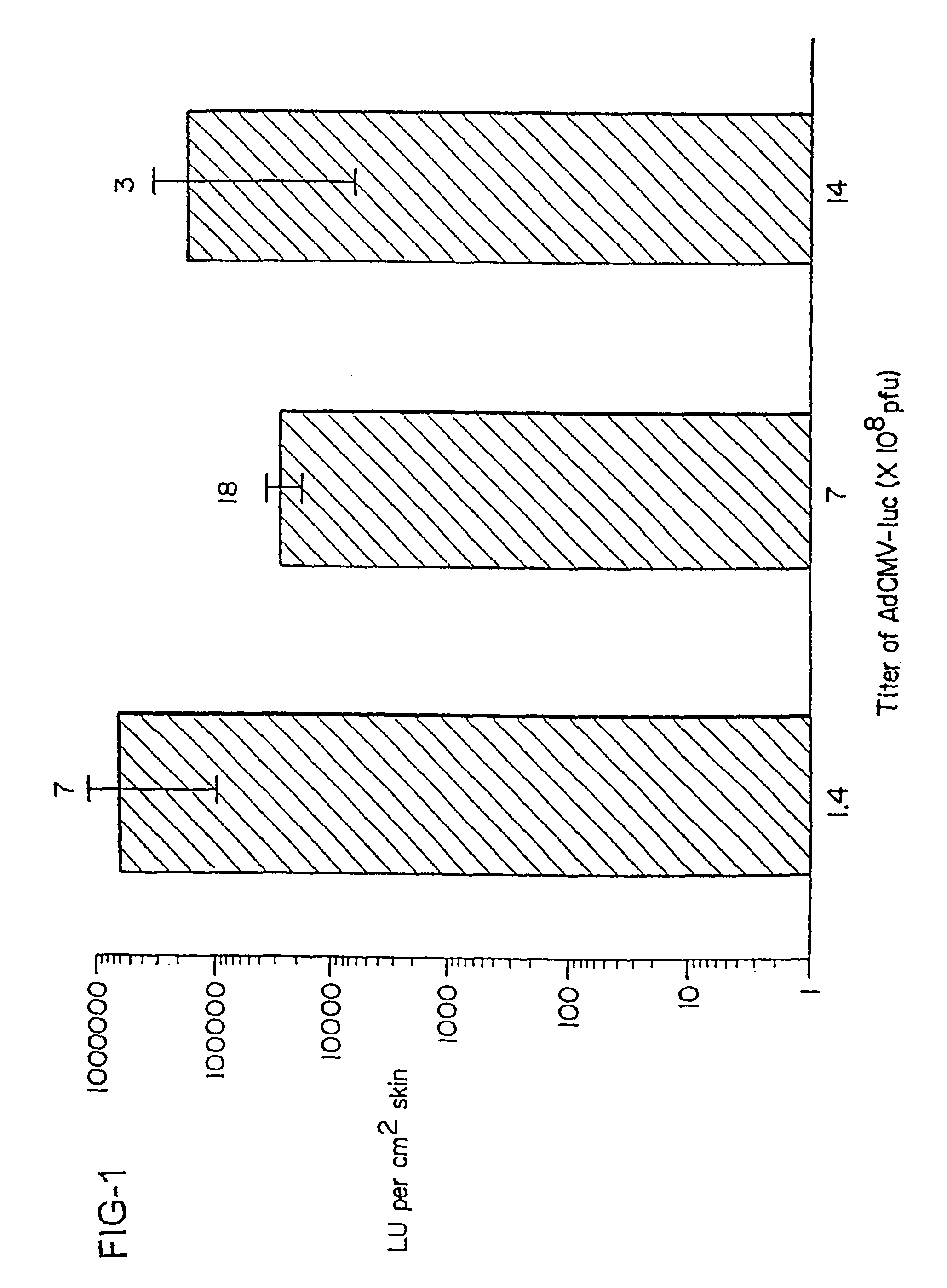
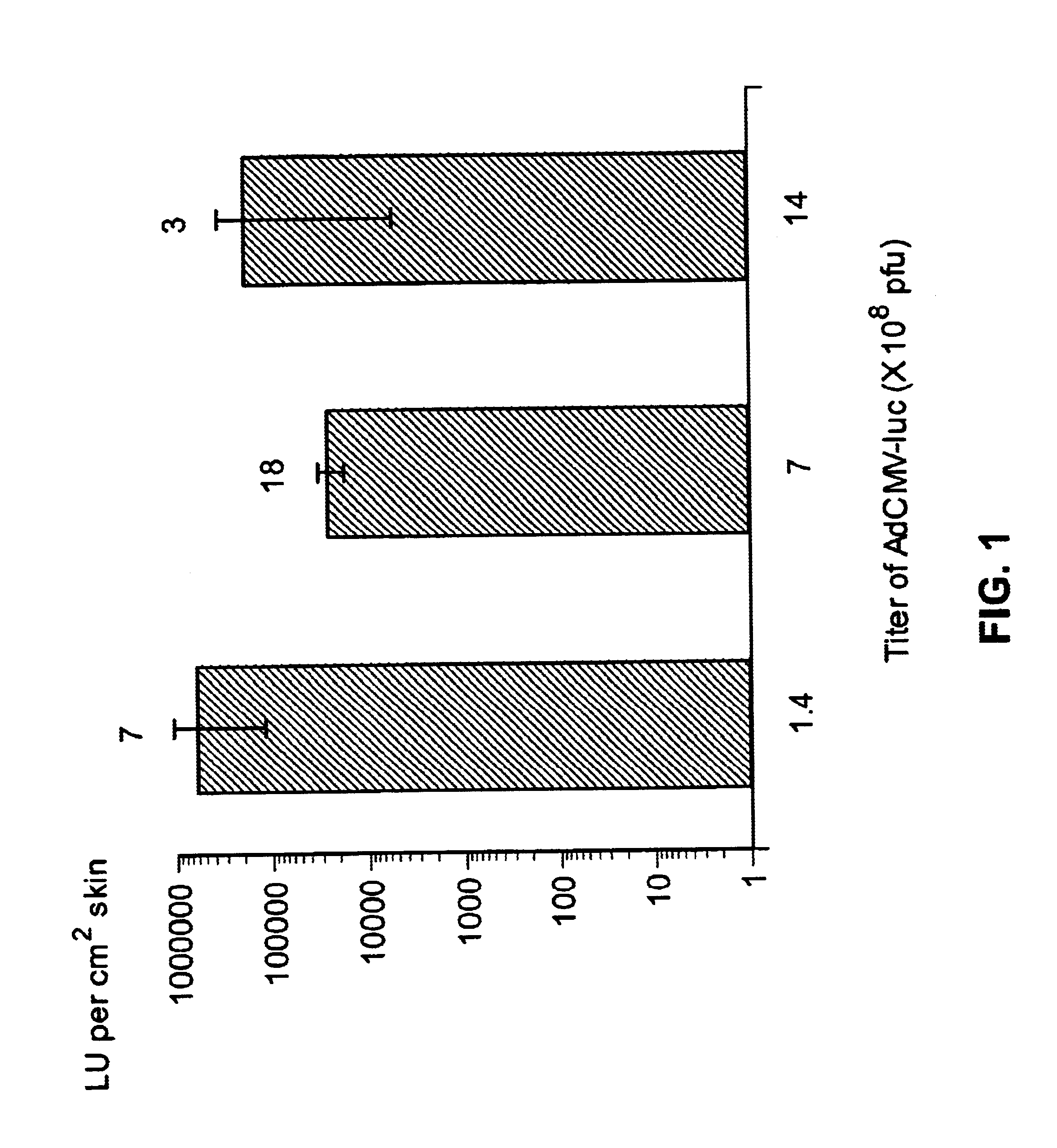
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, tetanus toxin C-fragment, anthrax protective antigen, HIV gp 120, human carcinoembryonic antigen, and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector.
Owner:UAB RES FOUND
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Process to study changes in gene expression in granulocytic cells
InactiveUS6365352B1Resistant to digestionDigestion of every cDNA is assuredMicrobiological testing/measurementBacteroidesNeutrophil granulocyte
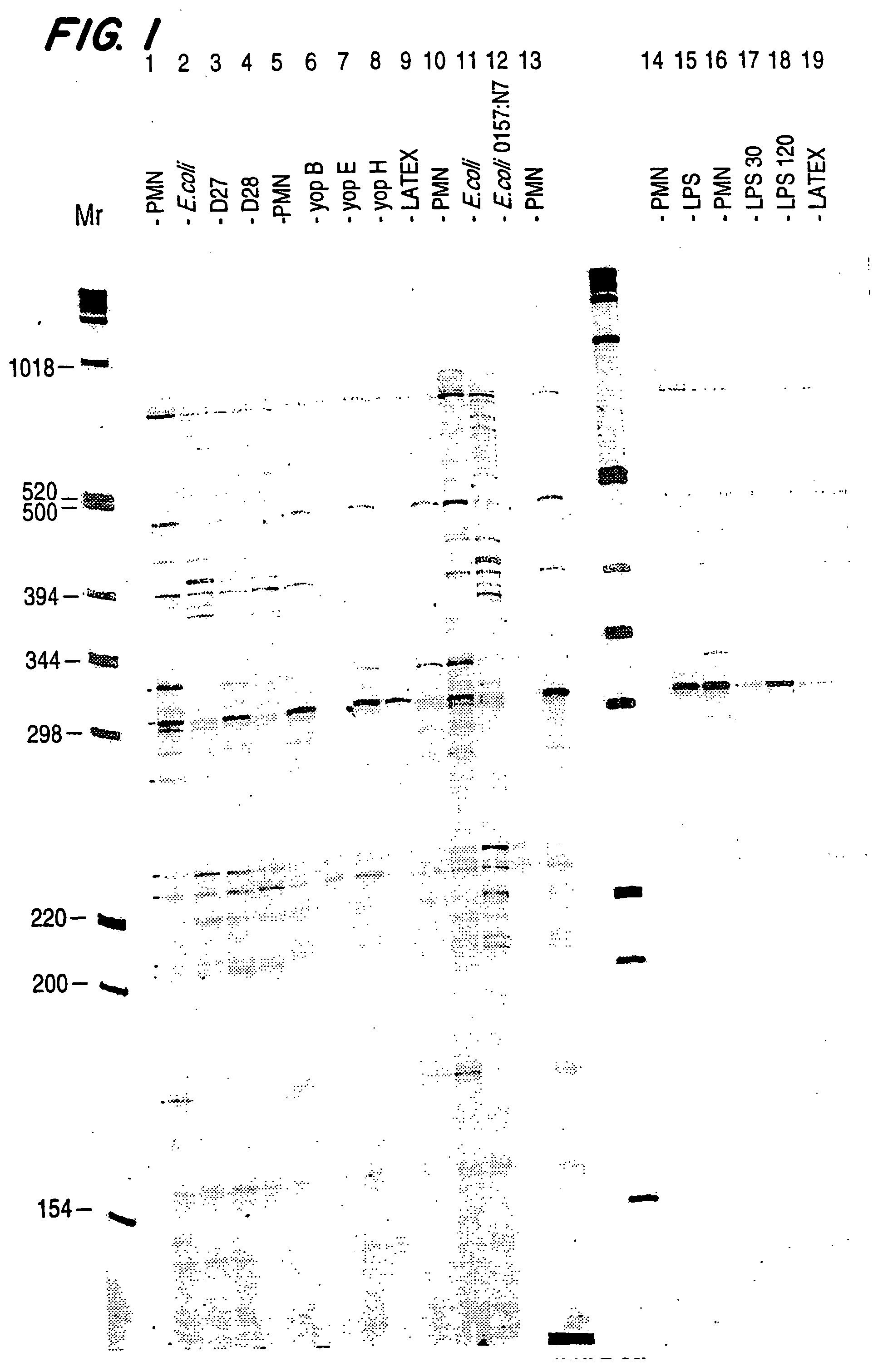
The present invention comprises a method to identify granulocytic cell genes that are differentially expressed upon exposure to a pathogen or in a sterile inflammatory disease by preparing a gene expression profile of a granulocytic cell population exposed to a pathogen or isolated from a subject having a sterile inflammatory disease and comparing that profile to a profile prepared from quiescent granulocytic cells. The present invention is particularly useful for identifying cytokine genes, genes encoding cell surface receptors and genes encoding intermediary signaling molecules. The invention also includes methods to identify a therapeutic agent that modulates the expression of at least one gene in a granulocytic population. Genes which are differentially expressed during neutrophil contact with a pathogen, such as a virulent bacteria, or that are differentially expressed in a subject having a sterile inflammatory disease are of particular importance.
Owner:YALE UNIV +2
Targeted vectors for cancer immunotherapy
InactiveUS20020177571A1Improve leveling efficiencyBiocidePeptide/protein ingredientsCytokine genesImmuno therapy
This invention provides compositions and methods for treating cancer. More specifically this invention is directed to a targeted retroviral vector comprising a cytokine gene that can be administered either alone or in combination with a targeted retroviral vector comprising a cytocidal gene for treating cancer in a subject. Also provided are a kit or drug delivery system comprising the compositions for use in the methods described.
Owner:UNIV OF SOUTHERN CALIFORNIA
Recombinant virus with coexpression of anthropogenic glutamate decarboxylase and cell factor
InactiveCN101586096ANervous disorderPeptide/protein ingredientsGlutamate decarboxylaseAdeno associate virus
The invention relates to a recombinant virus containing a gene expression box expressing an anthropogenic GAD gene and a cell factor, in particular to a method for constructing a recombinant adeno-associated virus with the coexpression of an anthropogenic GAD 65 gene and a GDNF gene, an application and a meaning thereof. Anthropogenic GAD 65 gene and the GDNF gene protein products generated by the expression of the recombinant virus have favorable synergistic effect on the treatment of diseases relative to Parkinson's disease and the central nervous system and have important values for treatment of relative diseases.
Owner:王尚武 +2
In vivo electroporation-mediated cytokine/immunocytokine-based antitumoral gene
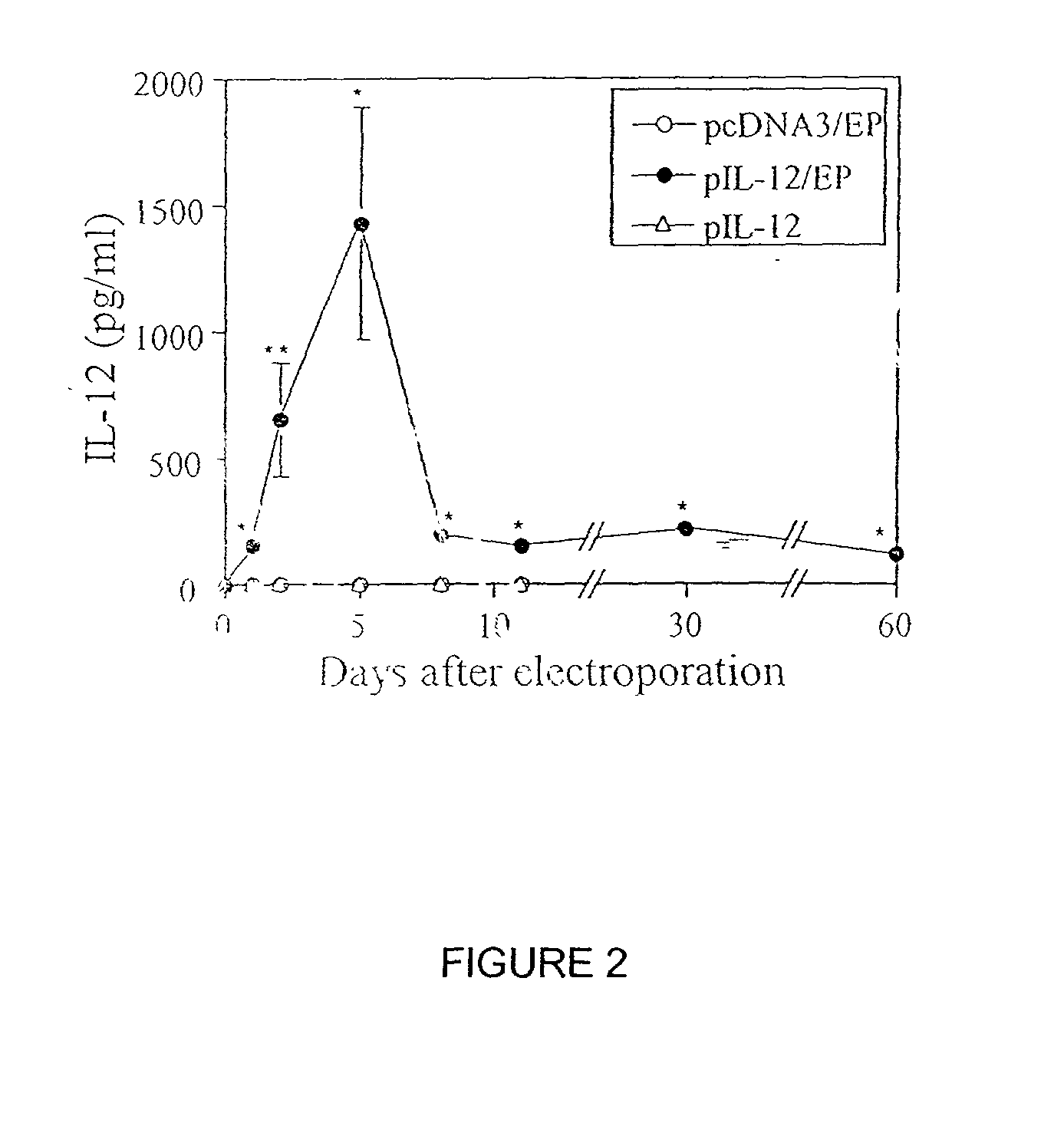
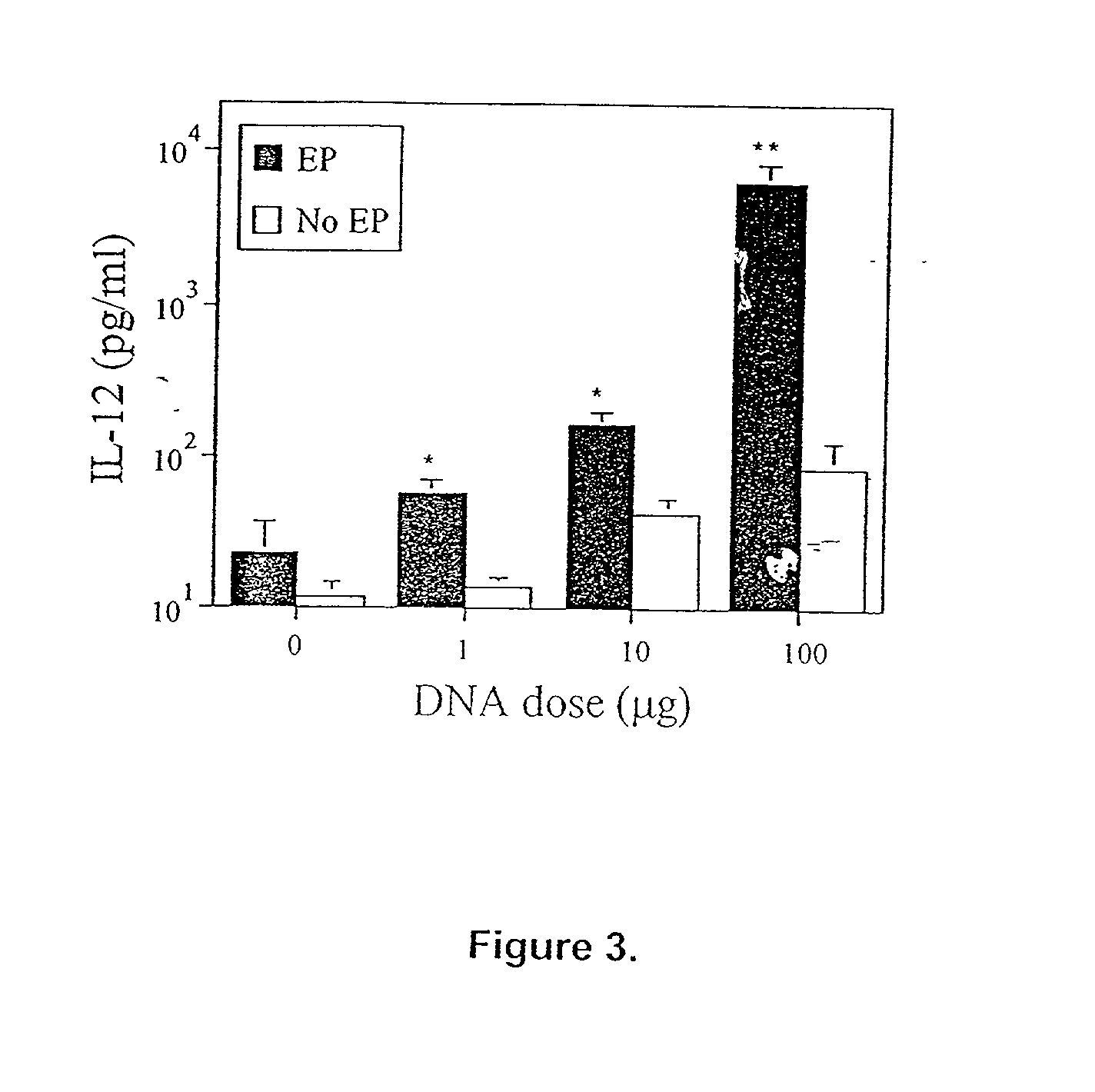
InactiveUS20030018006A1Polypeptide with localisation/targeting motifDepsipeptidesMammalElectroporation
Owner:ACAD SINIC
Cytokine gene modified antigen-presenting cell/tumor cell conjugate, its preparation and use
InactiveUS7067120B2Strong specificityLittle side effectsBiocideGenetic material ingredientsAntigenTumor vaccines
The present invention provides an antigen-presenting cell(APC) / tumor cell conjugate, wherein the antigen-presenting cell (APC) is modified by a cytokine gene selected from the group consisting of IL-2, IL-3, IL-4, IL-6, IL-12, IL-18, IFNα, IFNβ, IFNγ, TNF, TGF, GM-CSE, and the combination thereof. The conjugate is useful as a tumor vaccine to significantly induce an immunity specifically against the tumor cell The present invention also provides the method for preparing the conjugate and a pharmaceutical composition containing said conjugate.
Owner:SHANGHAI MEDIPHARM BIOTECH
Construction method and application of non-human animal modified by humanized cell factor IL3 (interleukin3) gene
ActiveCN111118019AAntibody mimetics/scaffoldsGenetically modified cellsBiotechnologyColony-stimulating factor
The invention relates to the modification of a non-human animal by a humanized gene, in particular to a construction method of a non-human animal modified by a humanized cell factor IL3 (interleukin3)gene, and application of the construction method in the biological medicine field, and in particular to an animal model which expresses a humanized IL3 protein. In certain examples, the genetic modification non-human animal which expresses the human IL3 protein also contains IL2rg deletion and / or contains the humanization of more cell factor, including CSF2 (colony stimulating factor 2) and thelike. The invention also provides a construction method for the above animal model and the application of the construction method in the biological medicine field.
Owner:BIOCYTOGEN JIANGSU CO LTD +1
Process for producing physiologically active protein using genetically modified silkworm
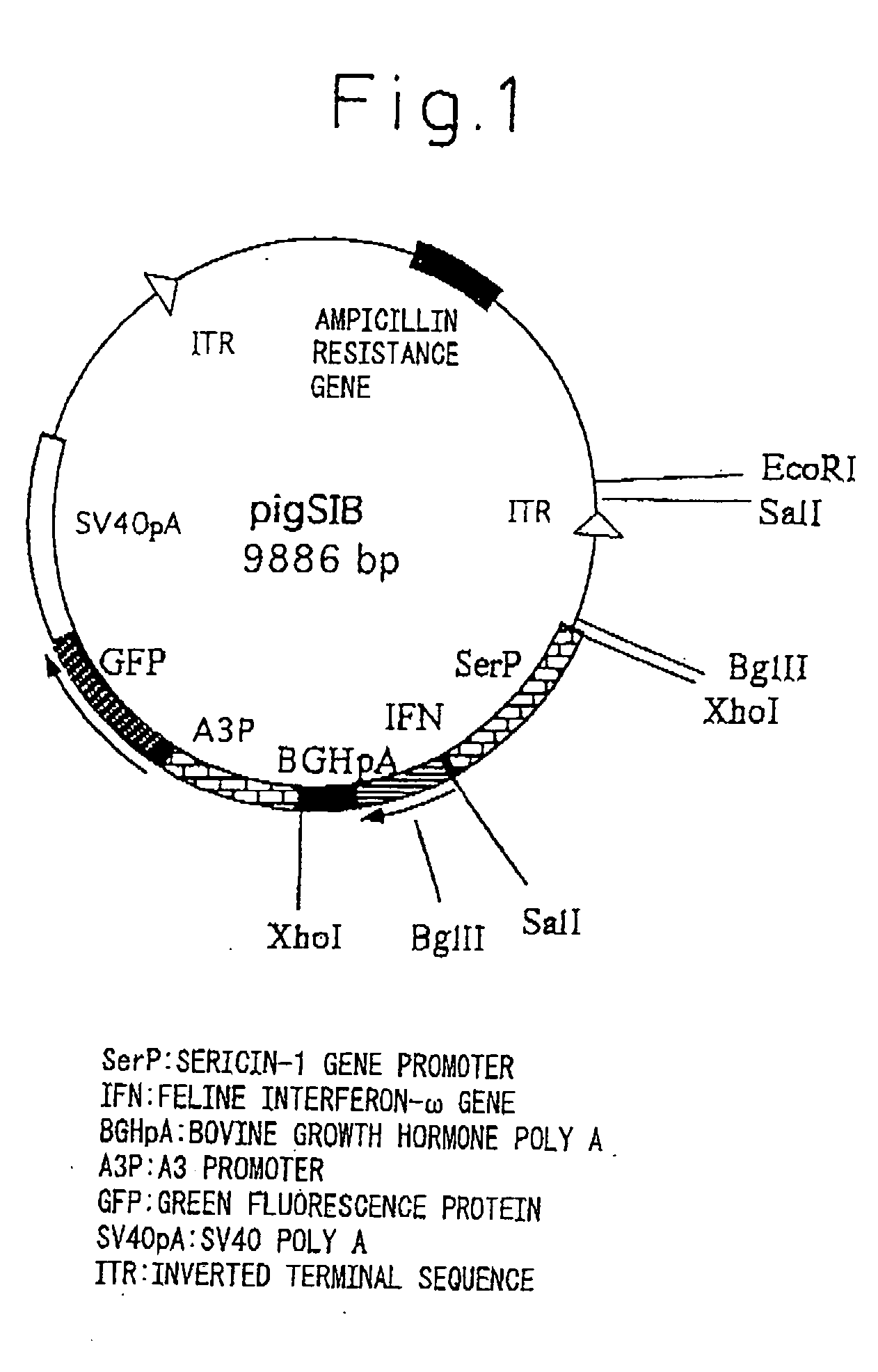
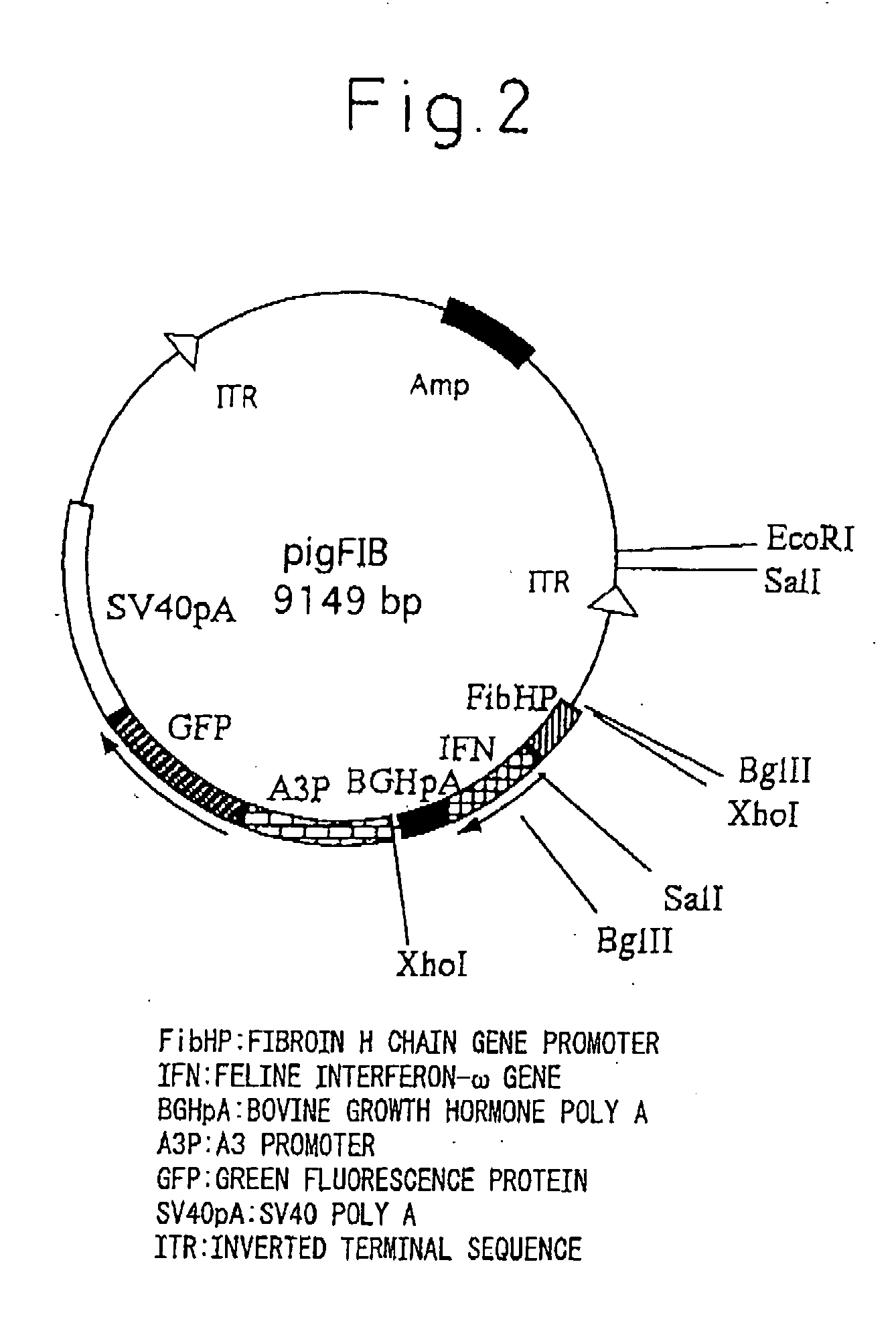
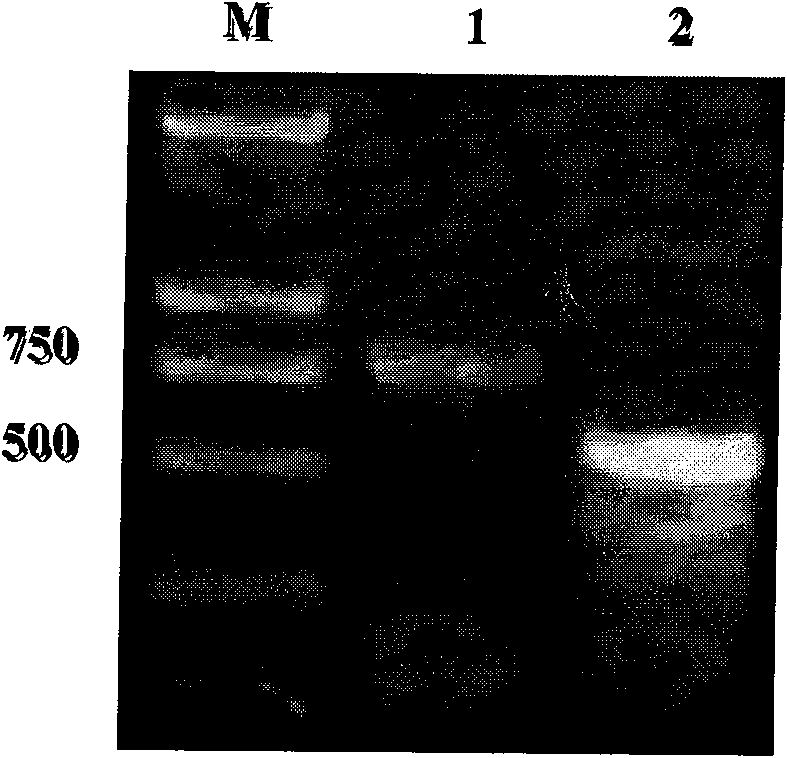
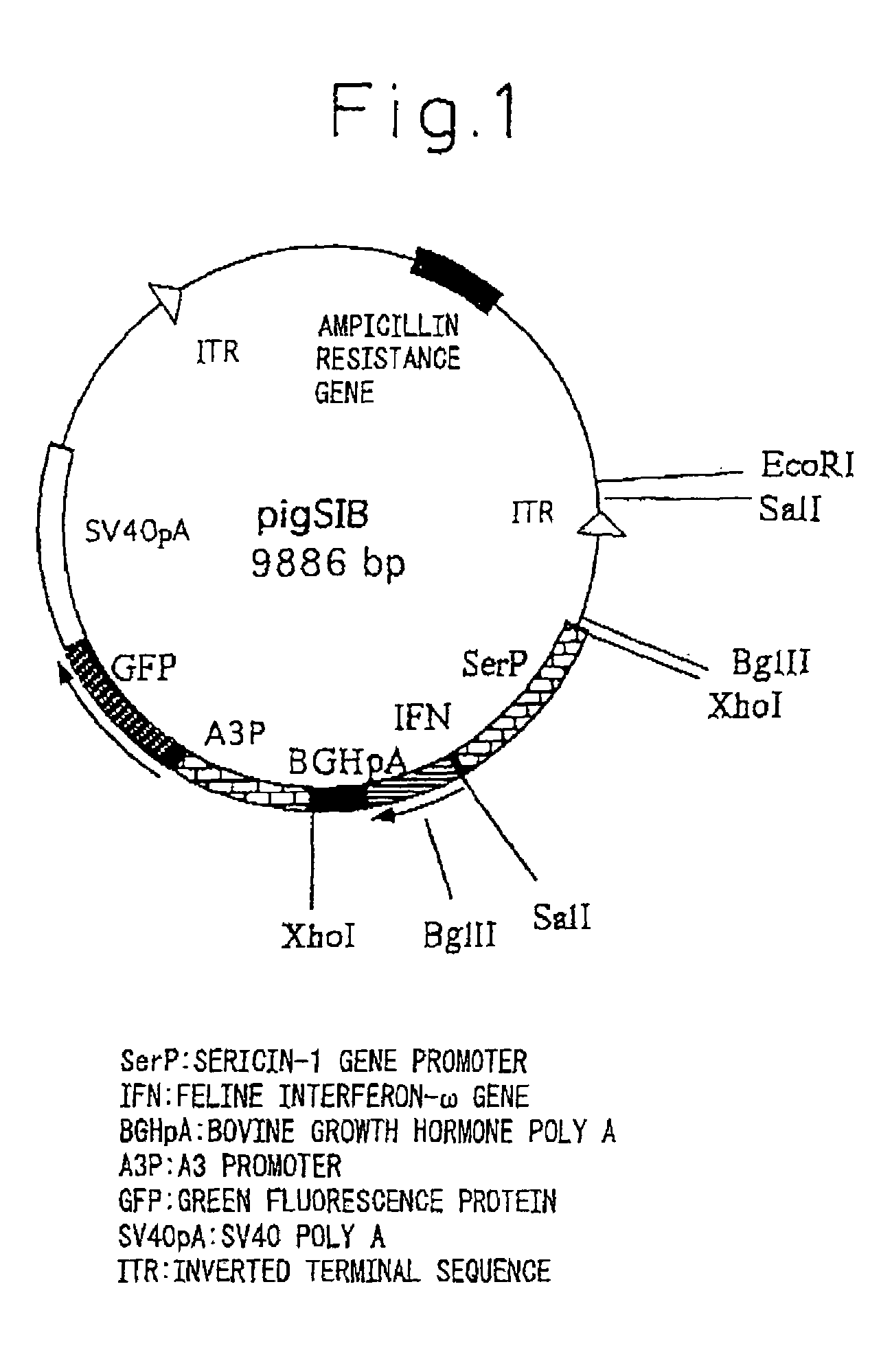
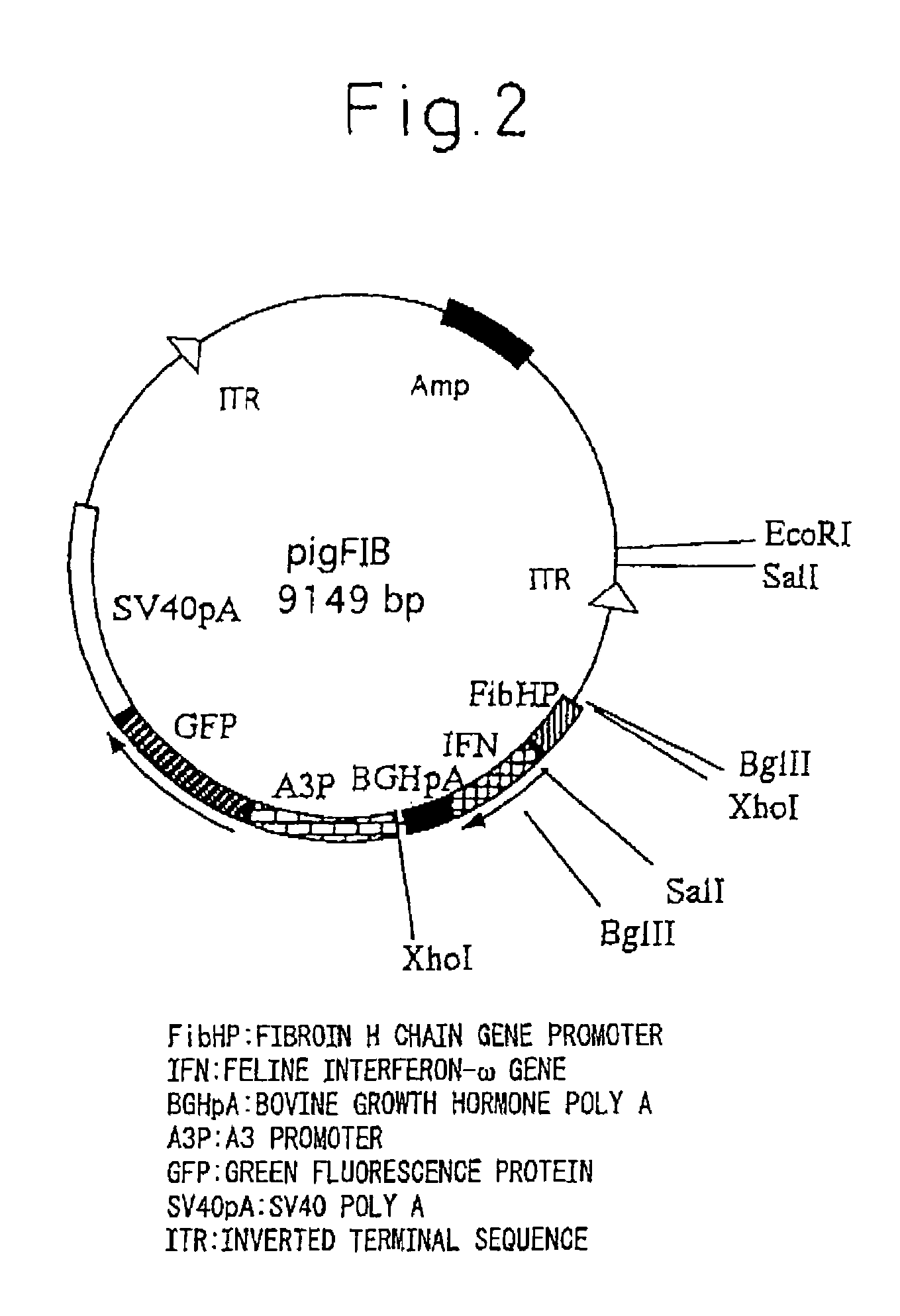
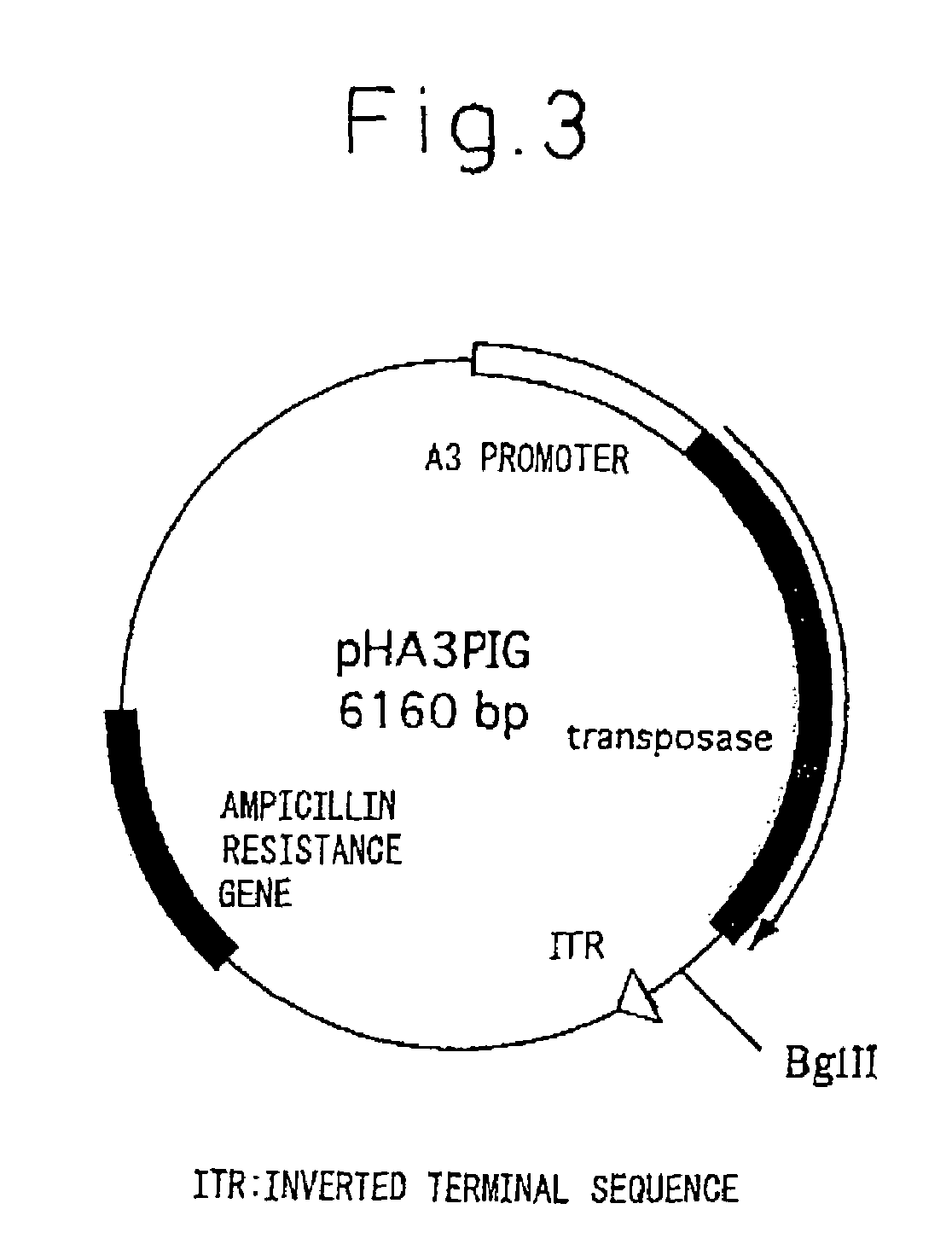
InactiveUS20050177877A1Improve purification effectEasy to aimAnimal cellsDepsipeptidesProtein targetActive protein
The present invention provides a genetic engineering material for insects that enables a target protein to be purified easily, without requiring the use of recombinant baculovirus, while simultaneously providing a process for producing exogenous protein using that genetic engineering material. A gene recombinant silkworm is obtained by inserting an exogenous protein gene such as a cytokine gene coupled to a promoter that functions in silk glands into a silkworm chromosome. An exogenous protein such as a cytokine is then extracted and purified from the silk glands or cocoon of that silkworm or its offspring. A large amount of exogenous protein can be produced within silk gland cells, outside silk gland cells or in silk thread or a cocoon by inserting an expression gene cassette, in which the DNA sequence of the 3′ terminal portion and the DNA sequence of the 5′ terminal portion of fibroin H chain gene are fused to the exogenous protein gene, into silk gland cells and so forth.
Owner:NAT INST OF AGROBIOLOGICAL SCI
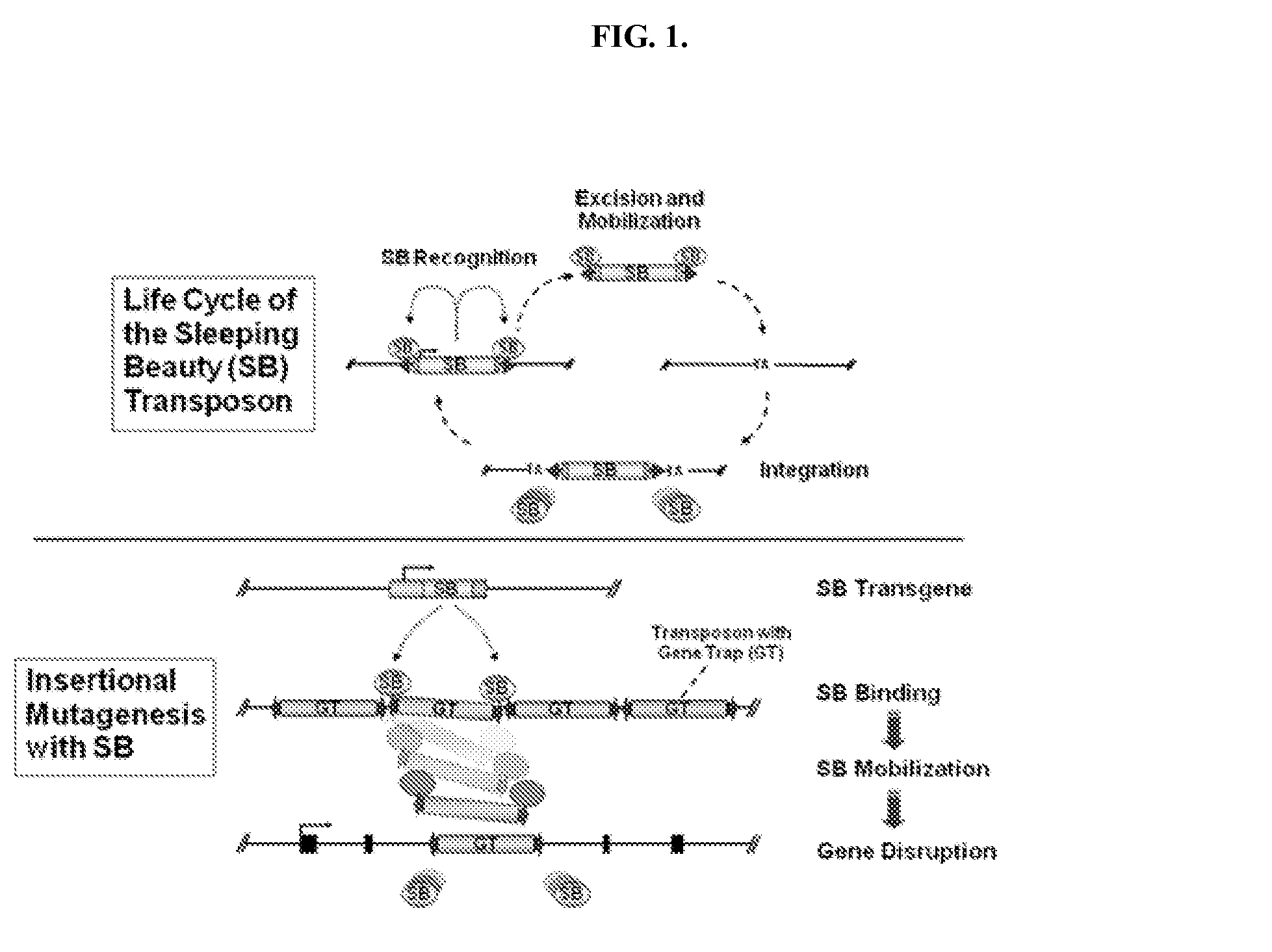
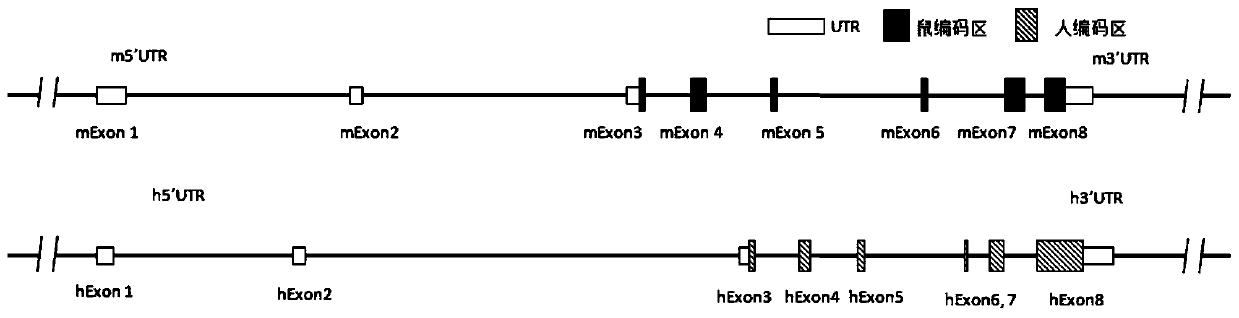
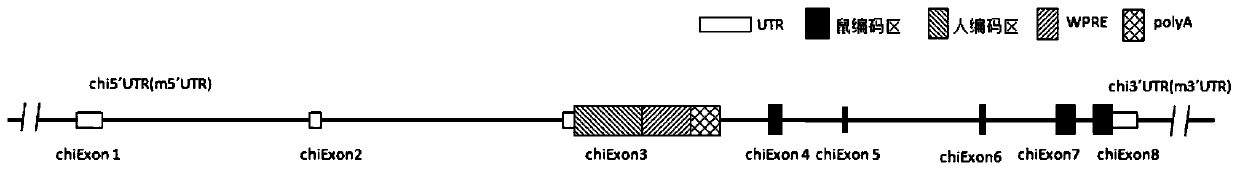
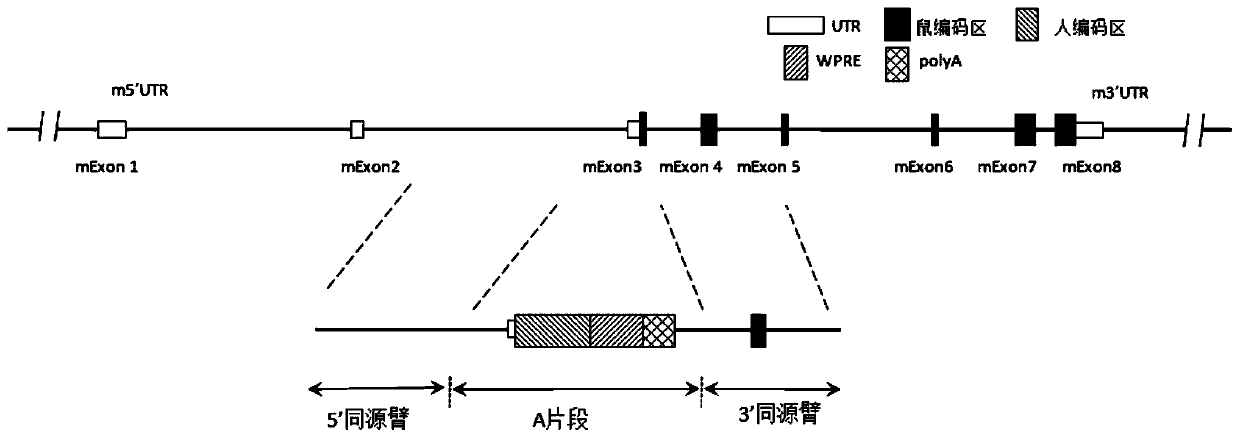
Genetically modified rat comprising a cytokine gene disruption and exhibiting a greater susceptibility to a cytokine-mediated autoimmune and/or inflammatory disease
The present invention relates to the engineering of animal cells, preferably mammalian, more preferably rat, that are deficient due to the disruption of gene(s) or gene product(s) resulting in cytokine-cytokine mediated autoimmune and inflammatory disease. In another aspect, the invention relates to genetically modified rats, as well as the descendants and ancestors of such animals, which are animal models of human autoimmune and inflammatory disease and methods of their use. Specifically, the invention pertains to a genetically altered rat, or a rat cell in culture, that is defective in at least one of two alleles of a cytokine gene such as the Faslg gene, the Fas gene, etc. In one embodiment, the cytokine gene is the Faslg gene. In another embodiment, the cytokine gene is one of several known cytokine genes, such as Fas, IFNγ, TNF-α, IL-2, IL-10, and IL-12. The inactivation of at least one of these cytokine alleles results in an animal with a higher susceptibility to cytokine-cytokine mediated autoimmune and inflammatory disease induction. In one embodiment, the genetically altered animal is a rat of this type and is able to serve as a useful model for cytokine-cytokine mediated autoimmune and inflammatory disease and as a test animal for autoimmune and other studies.
Owner:TRANSPOSAGEN BIOPHARM
Hyperthermia agent for malignant tumor comprising cytokine and magnetic fine particles
The present invention discloses a hyperthermia agent for malignant tumor which comprises cytokine or a vector in which a cytokine gene is integrated so that cytokine can express in malignant tumor cells, and magnetic fine particles, and a hyperthermia method using the same.
Owner:TTC +1
Construction method and application of humanized cell factor IL15 gene modified non-human animal
The invention provides a construction method of a humanized IL15 gene modified non-human animal. A nucleotide sequence encoding a human protein IL15 is introduced into a genome of an animal by using ahomologous recombination mode, so as to construct a gene modified humanized animal. A body of the animal can normally express the human or humanized protein IL15, promote development of human T cellsand NK cells and can serve as an animal model for human IL15 signal mechanism researching and screening of drugs for diseases such as tumors, and thus, the animal has an important application value in research and development of new drugs for immunization target points.
Owner:BIOCYTOGEN JIANGSU CO LTD +1
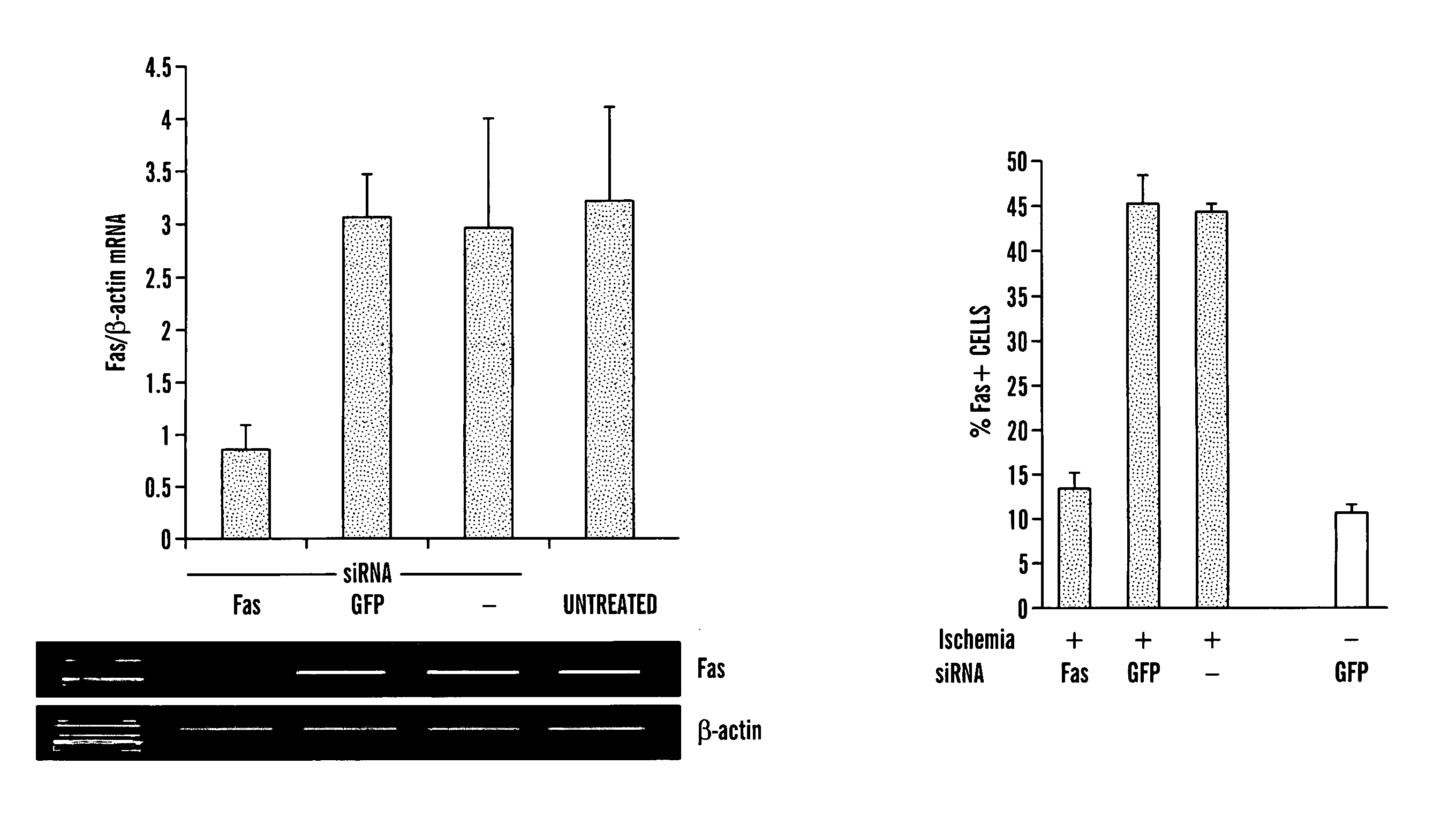
Method for Treating and Preventing Ischemia-Reperfusion Injury Using Rna Interfering Agent
InactiveUS20080227733A1Avoid ischemiaInhibition of translationOrganic active ingredientsDead animal preservationReperfusion injuryProtein activity
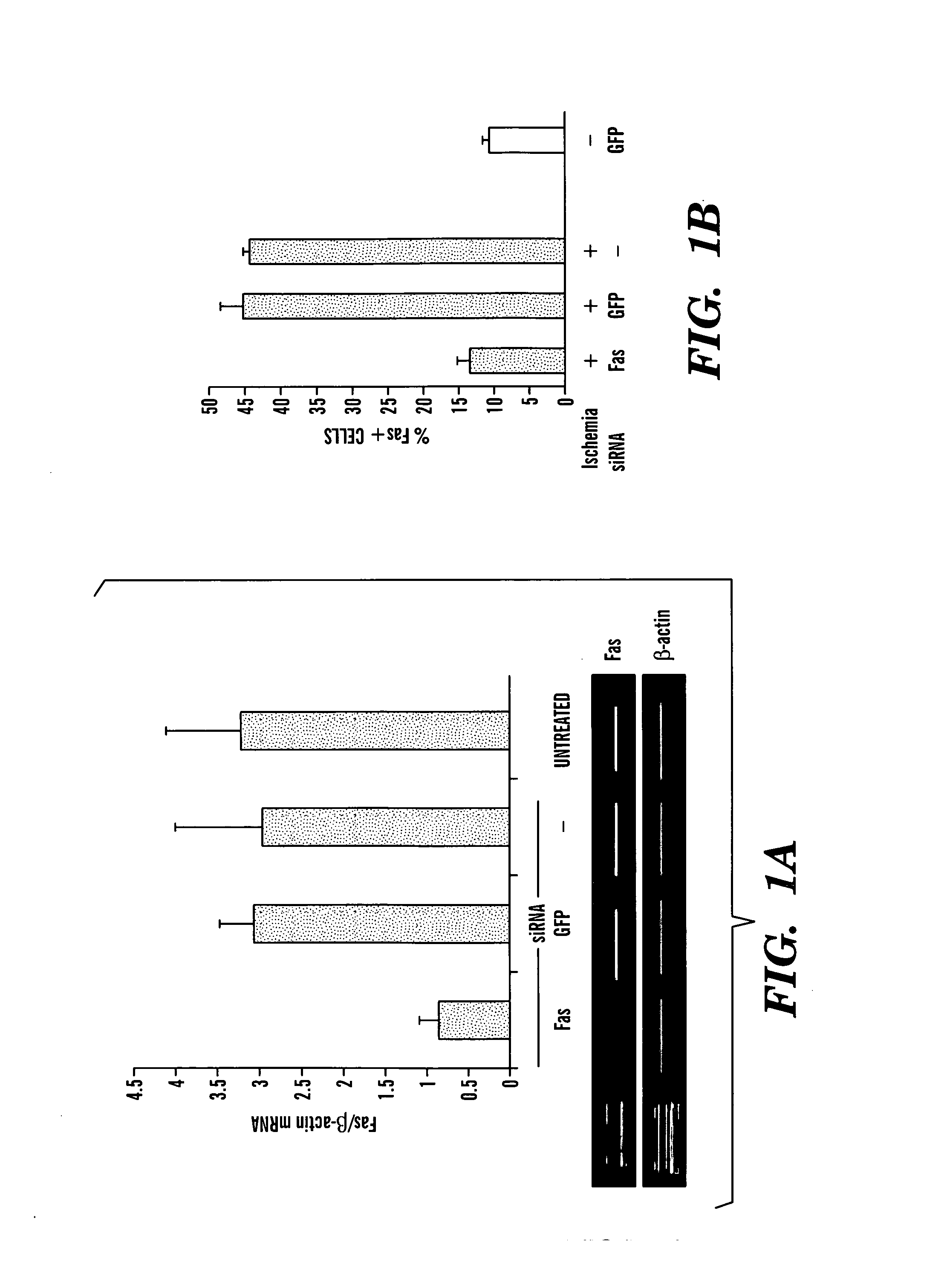
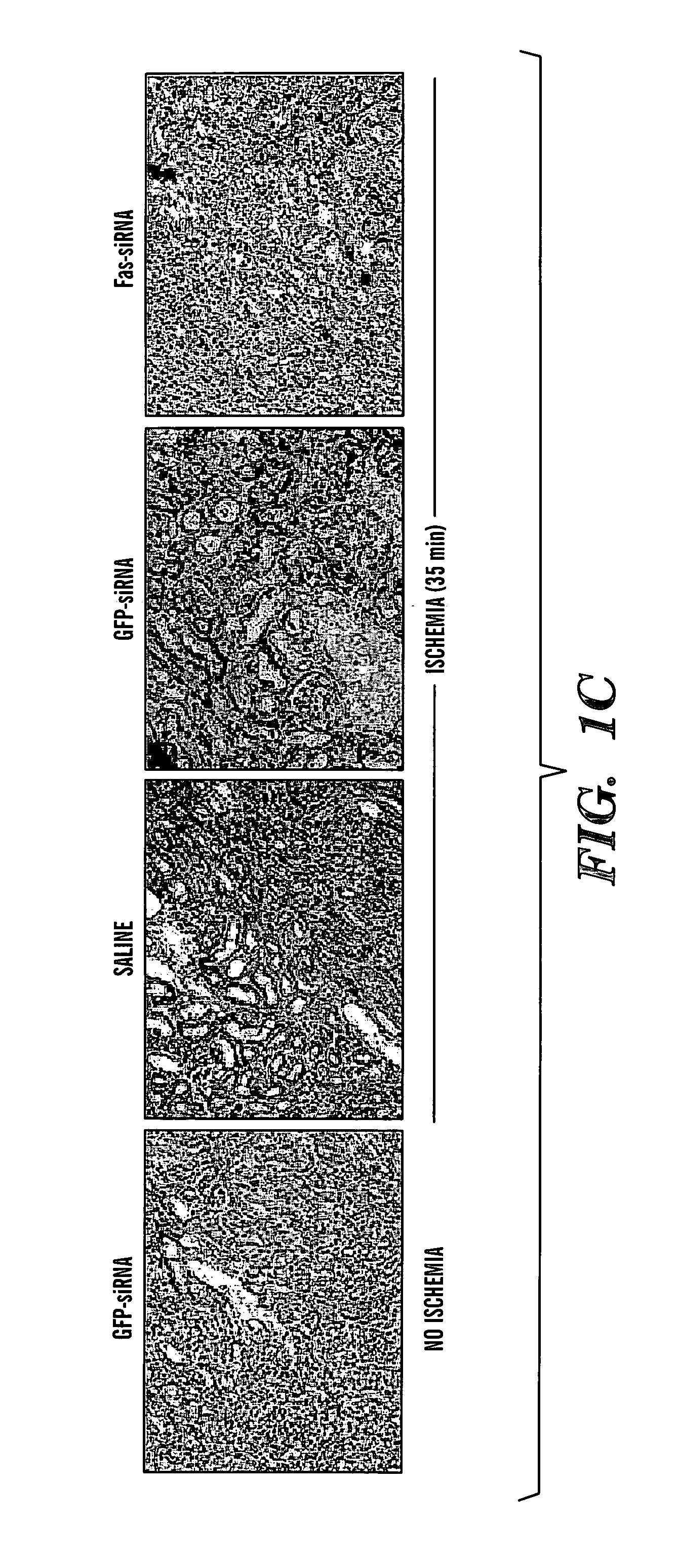
The present invention is based, at least in part, on the discovery of methods useful in the modulation, e.g., inhibition, of gene expression or protein activity, e.g., apoptosis-related gene expression, e.g., Fas gene expression or cytokine expression, e.g., proinflammatory cytokine expression. In particular, the present invention is based on novel RNA interfering agents, e.g., siRNA in reduction, e.g., prolonged reduction, of apoptosis-related gene expression or cytokine expression in cells. Inhibition of apoptosis-related gene expression or protein activity or cytokine gene expression or protein activity, e.g. by the siRNAs used in the methods of the invention, inhibits ischemia-reperfusion injury.
Owner:IMMUNE DISEASE INST INC
Human Bcl-2 and human VEGF165 double-gene co-expression recombinant vector and building method thereof
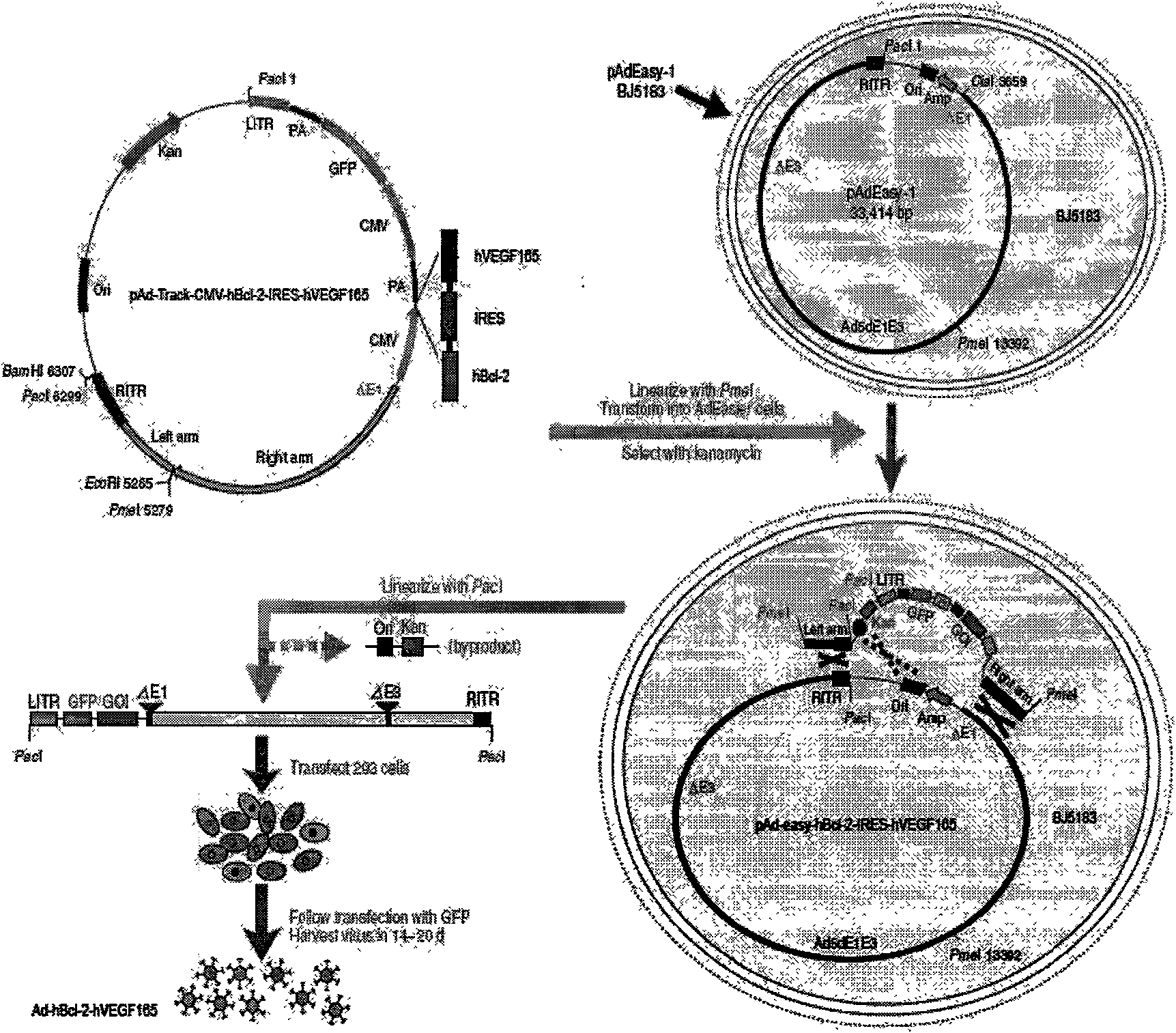
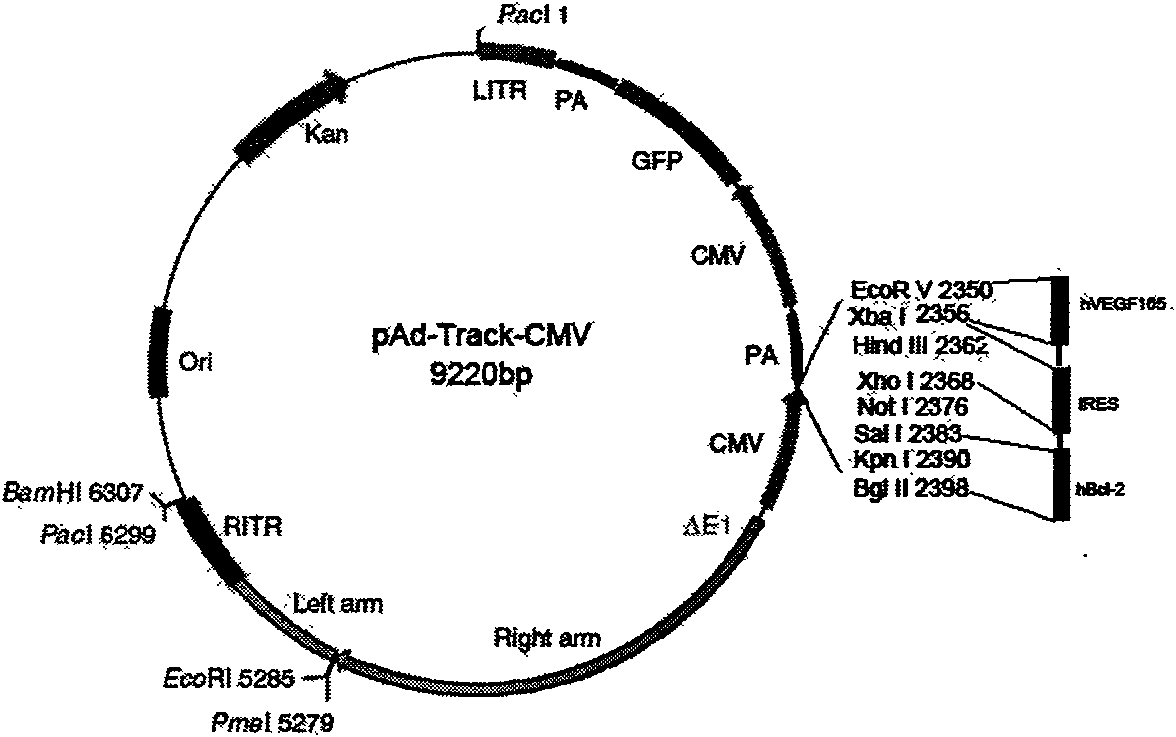
InactiveCN101654685APromote regenerationSolve the problem of low survival rateFermentationVector-based foreign material introductionAngiogenesis EffectAngiogenesis growth factor
The invention relates to a human Bcl-2 and human VEGF165 double-gene co-expression recombinant vector and a building method thereof, and is characterized in that IRES segment is utilized to connect an hBc1-2 gene and an hVEGF165 gene, and homologous recombination mechanisms in bacterium are utilized to recombine and build the human Bcl-2 and human VEGF165 double-gene co-expression recombinant vector according to an AdEasy system. The invention leads the Bcl-2 gene with anti-apoptosis capacity and the VEGF165 gene which currently promotes angiogenesis cytokine with strongest function to recombine on a same vector to be co-expressed, and solves the problem of low survival rate of transplanted cells under the condition of hypoxia and inflammation, simultaneously plays the function of promoting angiogenesis of VEGF165, plays an important part in the gene therapy of myocardial infarction and the cell transplantation research field, and has wide application prospect.
Owner:THE SECOND AFFILIATED HOSPITAL OF GUANGZHOU MEDICAL UNIV
Process for producing physiologically active protein using genetically modified silkworm
InactiveUS7659112B2Improve purification effectEasy to aimAnimal cellsDepsipeptidesProtein targetActive protein
The present invention provides a genetic engineering material for insects that enables a target protein to be purified easily, without requiring the use of recombinant baculovirus, while simultaneously providing a process for producing exogenous protein using that genetic engineering material. A gene recombinant silkworm is obtained by inserting an exogenous protein gene such as a cytokine gene coupled to a promoter that functions in silk glands into a silkworm chromosome. An exogenous protein such as a cytokine is then extracted and purified from the silk glands or cocoon of that silkworm or its offspring. A large amount of exogenous protein can be produced within silk gland cells, outside silk gland cells or in silk thread or a cocoon by inserting an expression gene cassette, in which the DNA sequence of the 3′ terminal portion and the DNA sequence of the 5′ terminal portion of fibroin H chain gene are fused to the exogenous protein gene, into silk gland cells and so forth.
Owner:NAT INST OF AGROBIOLOGICAL SCI
Targeted vectors for cancer immunotherapy
InactiveUS20060251627A1Improve leveling efficiencyBiocidePeptide/protein ingredientsCancer researchCytokine genes
This invention provides compositions and methods for treating cancer. More specifically this invention is directed to a targeted retroviral vector comprising a cytokine gene that can be administered either alone or in combination with a targeted retroviral vector comprising a cytocidal gene for treating cancer in a subject. Also provided are a kit or drug delivery system comprising the compositions for use in the methods described.
Owner:UNIV OF SOUTHERN CALIFORNIA
Process to study changes in gene expression in T lymphocytes
InactiveUS20070020618A1Value maximizationMicrobiological testing/measurementBiological testingAutoimmune conditionSignalling molecules
Methods are disclosed to identify T lymphocyte genes that are differentially expressed upon exposure to a pathogen (viral or bacterial), immunogen, antigen, or in a sterile inflammatory disease, autoimmune disease, immunodeficiency disease, lymphocytic cancers, or graft versus host rejection. The method involves the preparation of a gene expression profile of a T lymphocyte population exposed to a pathogen or isolated from a subject having one of the aforementioned pathologies and comparing that profile to a profile prepared from quiescent T lymphocytes. The present invention is useful for identifying cytokine genes, genes encoding cell surface receptors and genes encoding intermediary signalling molecules. Related methods for identifying therapeutic or prophylatic immunomodulatory agents are present. Articles of manufacture are disclosed that comprise selected grouping of nucleic acids, affixed to a solid support, that correspond to genes that are differentially expressed in various populations or subpopulations of T lymphocytes at variations stages of T cell differentiation, in quiescent versus activated T lymphocytes or normal versus diseased T lymphocytes.
Owner:GENE LOGIC
Process to study changes in gene expression in granulocytic cells
InactiveUS20050084896A1Microbiological testing/measurementFermentationNeutrophil granulocyteReceptor degradation
The present invention comprises a method to identify granulocytic cell genes that are differentially expressed upon exposure to a pathogen or in a sterile inflammatory disease by preparing a gene expression profile of a granulocytic cell population exposed to a pathogen or isolated from a subject having a sterile inflammatory disease and comparing that profile to a profile prepared from quiescent granulocytic cells. The present invention is particularly useful for identifying cytokine genes, genes encoding cell surface receptors and genes encoding intermediary signaling molecules. The invention also includes methods to identify a therapeutic agent that modulates the expression of at least one gene in a granulocyte population. Genes which are differentially expressed during neutrophil contact with a pathogen, such as a virulent bacteria, or that are differentially expressed in a subject having a sterile inflammatory disease are of particular importance.
Owner:GENE LOGIC +2
Pharmaceutical composition for promoting hematopoiesis and preparation method thereof
InactiveCN101530427AQuick effectImprove efficiencyHeavy metal active ingredientsMammal material medical ingredientsBlood-forming stem cellCancer research
The invention relates to a pharmaceutical composition for promoting hematopoiesis, which comprises hemopoietic stem cells and melanterite, wherein the preferred hemopoietic stem cells are cells which are imported with an expression vector with cell factor genes. The invention also relates to a method for preparing the pharmaceutical composition and application of the pharmaceutical composition in preparing a medicament for treating the damage state of hematopoietic function, wherein the hemopoietic stem cells and the melanterite have the function of synergistically promoting the hematopoiesis.
Owner:SOUTHWEST HOSPITAL OF CHONGQING
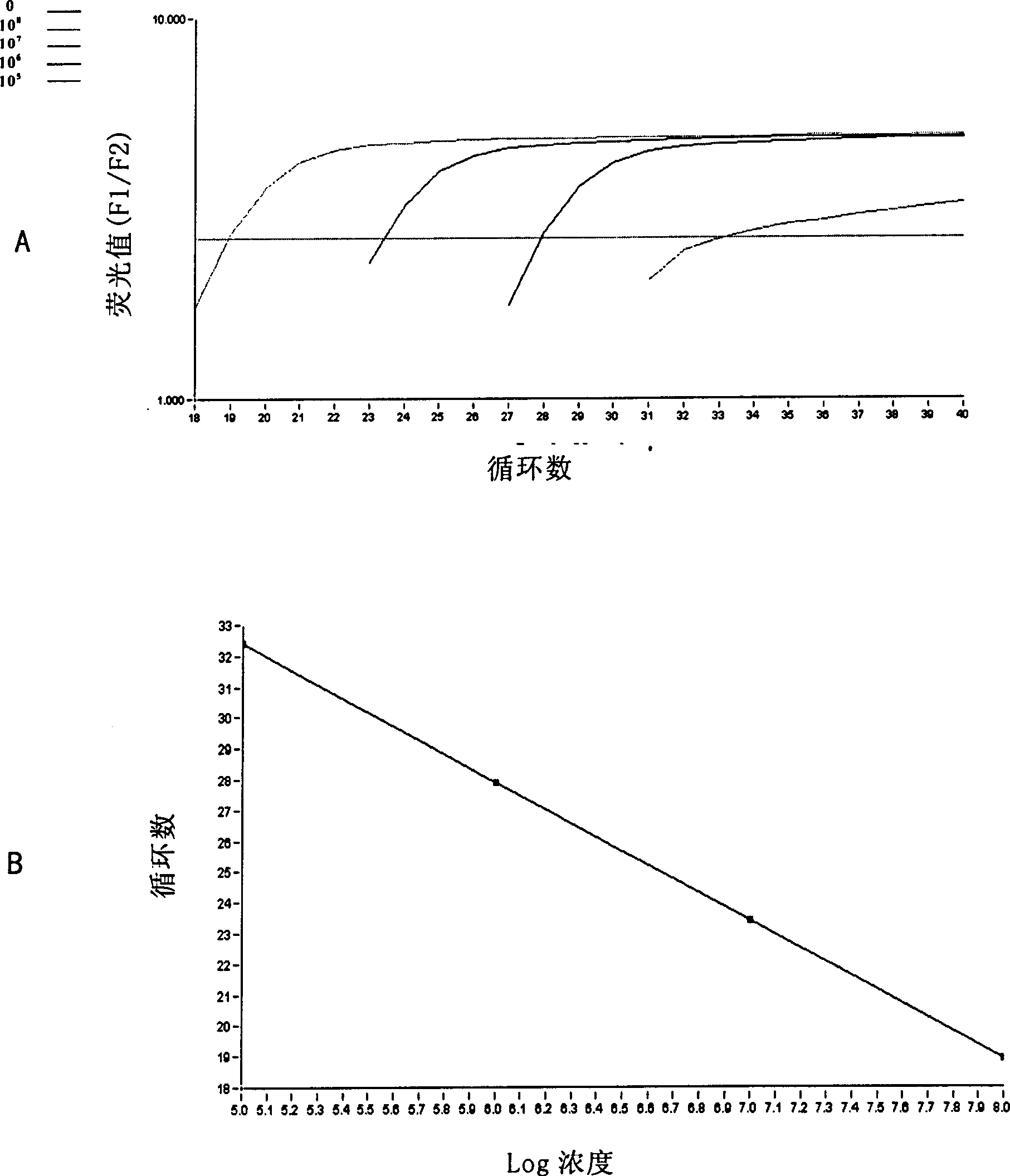
Fluorogenic quantitative PCR method for detecting interleukin 2 gene expression in peripheral-blood under physiological state
InactiveCN1629315AHigh detection sensitivityMicrobiological testing/measurementBiological testingWhite blood cellQuantitative determination
The invention provides a method for performing quantitative determination to immunity-relating cytokine in peripheral blood, the method can be applied for specifically detect the cytokine gene expression (IL-2) from 2ml of peripheral blood, and no any stimulation is needed before detection. The method can detect the cytokine gene expression under physiologic status, thus can be used for determining early stage fatigue, preventing decrease of body immunity function caused by exercises of excess sport load. The invention also provides a corresponding detection kit.
Owner:SHANGHAI UNIV OF SPORT
A tumor immune T cell detection kit and method
InactiveCN107099603AAccurately determine quantitative expression profilesAccurately identify personalized treatment targetsMicrobiological testing/measurementWilms' tumorReceptor for activated C kinase 1
A tumor immune T cell detection kit and method are provided. The kit includes specific primers for detecting specific quantitative expression of immune-related target genes in human peripheral blood T cells through subjecting the target genes to PCR amplification based on SYBR-Green quantitative PCR amplification. The immune-related target genes include receptor and ligand related target genes, NK cell related target genes and key cytokine genes. Expression profiles of the immune system related target genes in the human peripheral blood T cells can be accurately and quantitatively detected by the kit and the method successfully so that targets can be provided for accurate immune treatment of tumor patients. In a treatment process, cytokine storm response to immune treatment of a patient can be detected timely, thus providing guidance for avoiding adverse reactions. States of the immune system in the treatment process and after treatment are assessed, thus providing supports for adjusting the amount of an antibody medicine used for treatment or the feedback number of modified T cells and even target adjustments. The kit and the method are comprehensive in detection, rapid, quantitative, accurate, high in sensitivity and low in cost.
Owner:成都克里斯博生物科技有限公司
Mutant cytokine fusion protein for treating metabolic diseases
ActiveCN106397569ALower blood sugar levelsHigh activityPeptide/protein ingredientsMetabolism disorderDiseaseMammal
The invention discloses the effect of a novel mutant human cytokine and fusion protein thereof on treatment of metabolic diseases. The human cytokine with mutant expression in mammalian cells is protein as shown in SEQ ID No. 3 in a sequence table. According to the invention, a wild human cytokine gene is subjected to mutation and N-terminal reconstruction, and the fusion protein contains a human cytokine mutant and the Fc segment (CH2-CH3) of human IgG4. The mammalian cell-expressed recombinant cytokine fusion protein has good activity in reducing blood sugar; in particular, the fusion protein has the advantages of high stability, long half life, etc.; and the fusion protein can well control fluctuation of blood sugar and stably maintain blood sugar at a normal level within 24 h.
Owner:哈尔滨资治生物科技有限公司
Construction method of humanized cytokine CSF1 gene modified-non-human animal and application
The invention relates to a humanized gene modified non-human animal, in particular to a construction method of a humanized CSF1 gene modified non-human animal for expressing humanized cytokine proteinand application of the method in the field of biological medicine, and particularly relates to an animal model for expressing human or humanized CSF1 protein. In some examples, a genetically modifiednon-human animal expressing the human CSF1 protein also contains an IL2rg deletion, and / or contains humanization of more genes such as IL3 and CSF2.
Owner:BIOCYTOGEN JIANGSU CO LTD +1
Compound material for internal fixation of tendons carrying transgenic cells and preparation method thereof
InactiveCN101574542AStable internal environmentGood biocompatibilityProsthesisPorous substratePolyester
The invention discloses a compound material for the internal fixation of tendons carrying transgenic cells. The compound material is formed by compounding a non-degradable tendon surface internal fixation polyester material layer as the outer layer and a pharmacologically acceptable biodegradable porous substrate film material layer as the inner layer, wherein, the inner layer adopts the biodegradable substrate film as the base material obtained through the surface modification treatment of substances capable of improving the hydrophilicity and cell affinity thereof and the inoculation of tendon cells subjected to the transfection of genes carrying the related cell factors for promoting the healing of tendons. The preparation method of the compound material comprises the following steps: firstly, preparing the non-degradable tendon surface internal fixation polyester material compound and the surface-modified biodegradable substrate film; then, preparing the transgenic tendon cells, and inoculating the transgenic tendon cells onto the biodegradable substrate film; and finally compounding to obtain the compound material of the invention. The compound material prepared by the invention has the advantages of good biocompatibility and higher tensile strength and is capable of stabilizing the internal environment of the tendons and promoting the healing of the tendon injury.
Owner:张朝跃
Pharmaceutical composition for preventing or treating liver diseases, containing plasmalogen precursor, plasmalogen or plasmalogen analog as effective component
InactiveUS20160263130A1Reduce accumulationInhibit expressionDispersion deliveryDigestive systemDiseaseSteatosis
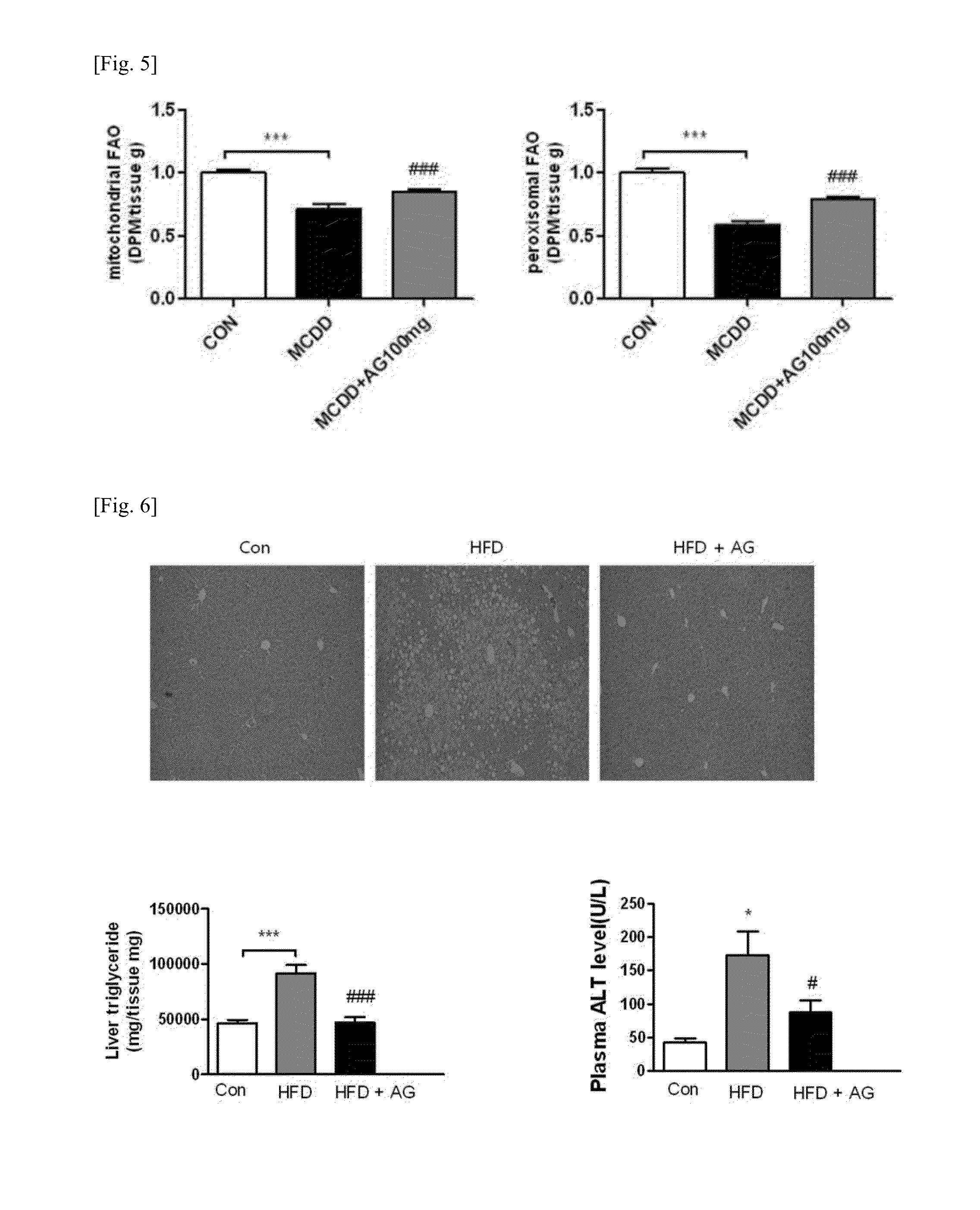
The present invention relates to a pharmaceutical composition for preventing or treating liver diseases, containing a plasmalogen precursor, plasmalogen, or a plasmalogen analog as an effective component. More specifically, a plasmalogen precursor, or plasmalogen or a plasmalogen analog produced by metabolizing the plasmalogen precursor, can prevent or treat liver diseases such as hepatic steatosis, hepatitis, or liver cirrhosis, etc. by decreasing accumulation of neutral fats in the liver, repressing expressions of inflammatory cytokine genes, and increasing fatty acid oxidation capacity of peroxisome.
Owner:UNIV OF ULSAN FOUND FOR IND COOPERATION
Hepatitis B nucleic acid vaccine and construction method thereof
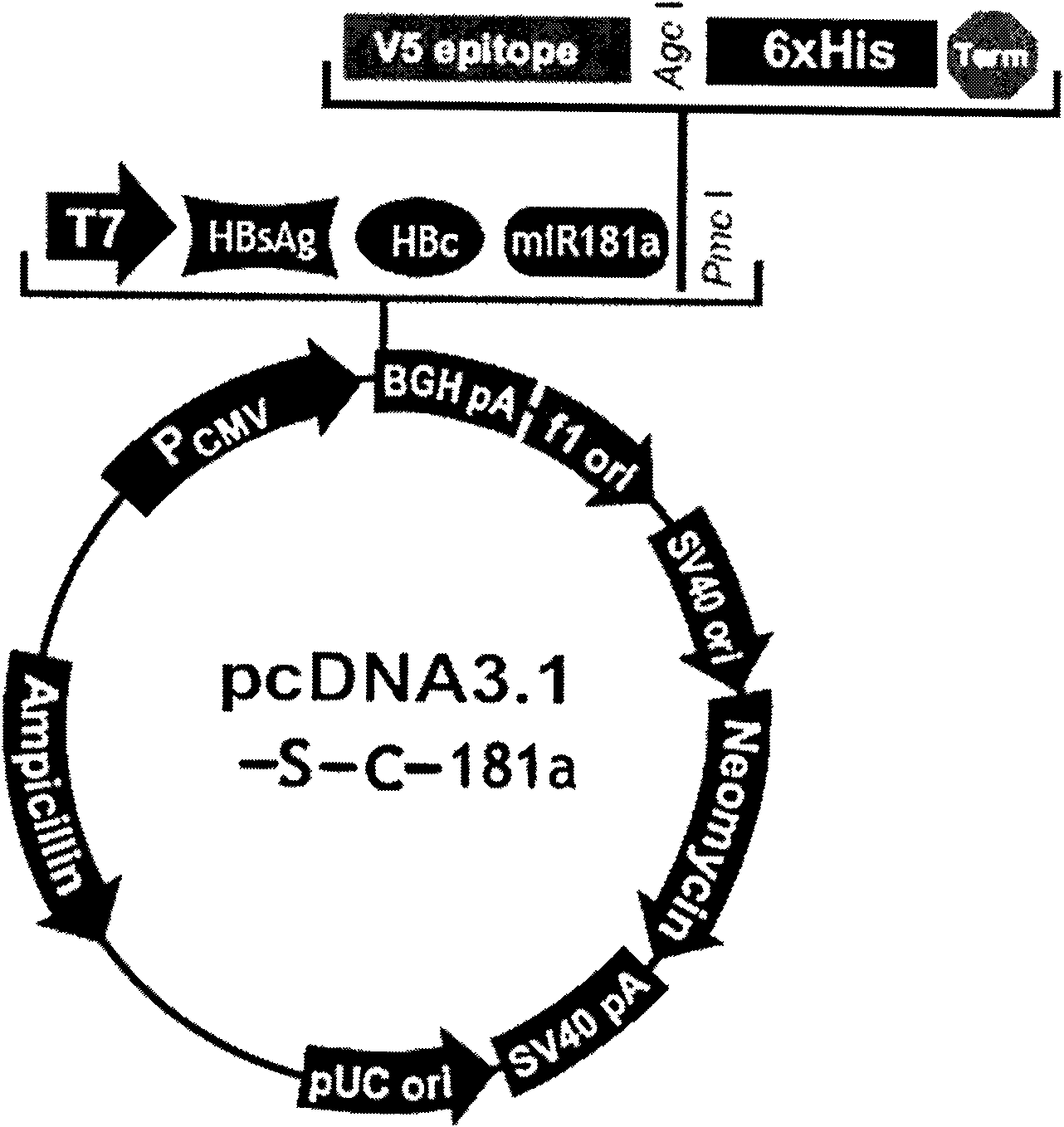
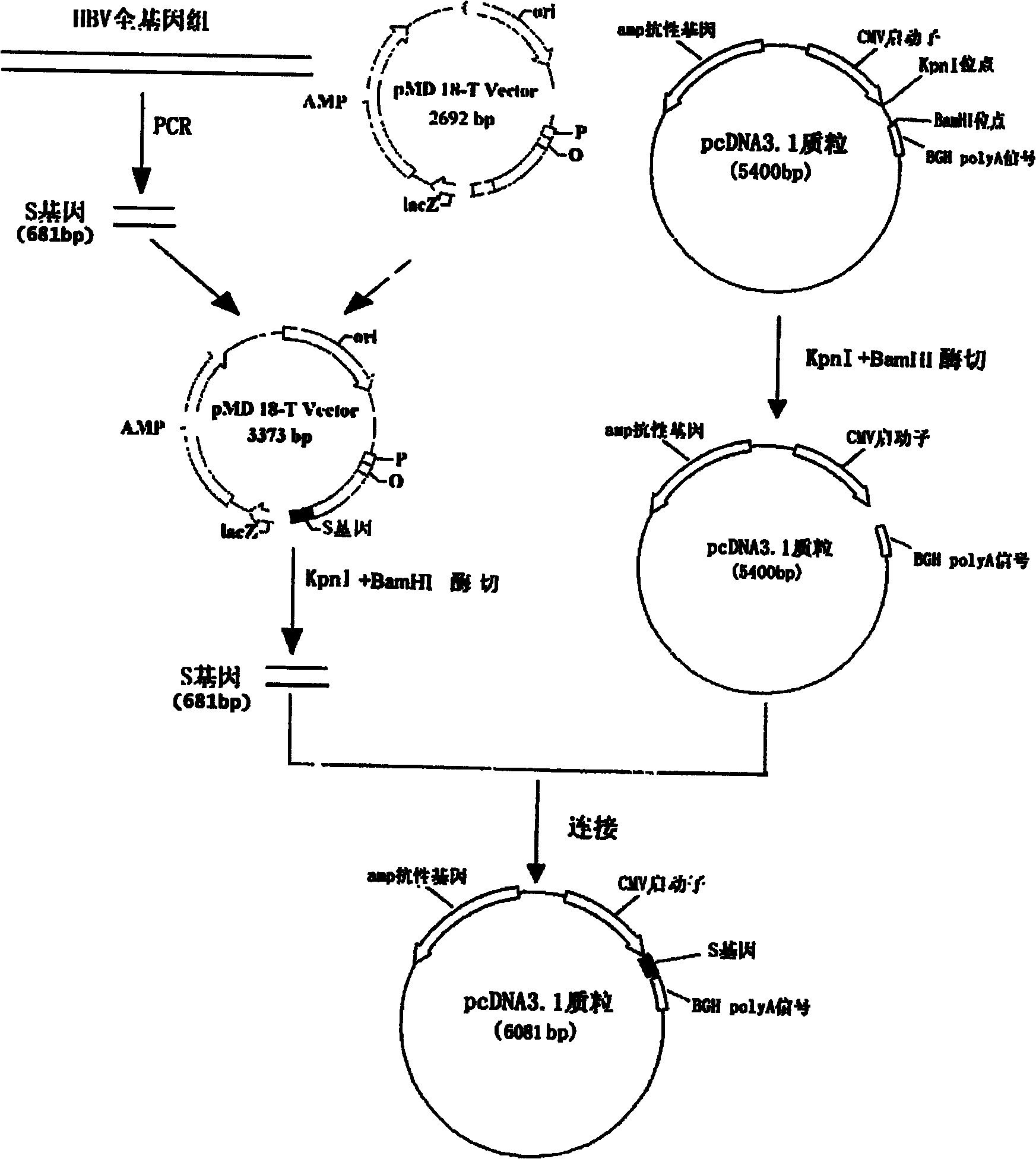
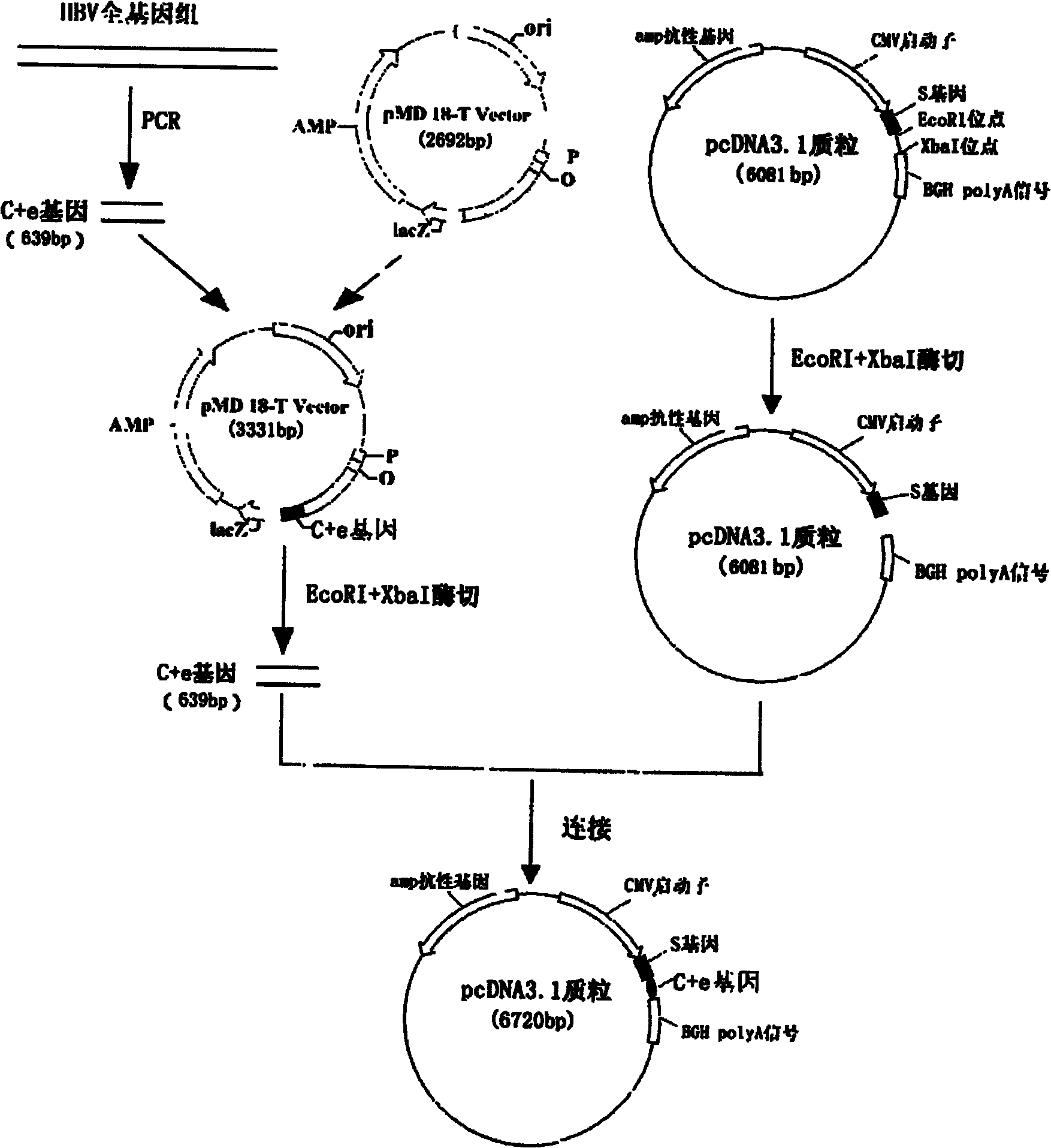
InactiveCN101954093AImprove protectionGood immune effectGenetic material ingredientsDigestive systemTreatment effectHepatitis B virus DNA
The invention relates to the technical field of biomedicines. The currently reported hepatitis B virus vaccine mainly comprises one or several of HBsAg, preS 1, preS2 and HBcAg, some cell factor genes with immunological enhancement function or lymphocyte epitope genes and the like, however, the immunoprophylaxis effect and treatment effect of the vaccine are always unsatisfactory. The invention provides a hepatitis B vaccine which comprises hepatitis B virus surface antigen gene HBsAg, core protein gene HBc and e-antigen gene, also comprises a human microRNA181a precursor gene sequence, and can assist stimulating the body immunity pathway. The construction process of the vaccine relates to PCR, enzyme cutting, connection, conversion and other molecularly biological operating means. The hepatitis B vaccine has the advantages of effectively activating the body to produce a specific antibody against the hepatitis B virus, stimulating body cell immunity and secreting various cell factors. Therefore, the aim of treating chronic hepatitis B is fulfilled.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Fluorogenic quantitative PCR method for detecting interleukin 4 gene expression in peripheral-blood under physiological state
InactiveCN1629316AHigh detection sensitivityMicrobiological testing/measurementBiological testingWhite blood cellQuantitative determination
The invention provides a method for performing quantitative determination to immunity-relating cytokine in peripheral blood, the method can be applied for specifically detect the cytokine gene expression (IL-4) from 2ml of peripheral blood, and no any stimulation is needed before detection. The method can detect the cytokine gene expression under physiologic status, thus can be used for determining early stage fatigue, preventing decrease of body immunity function caused by exercises of excess sport load. The invention also provides a corresponding detection kit.
Owner:SHANGHAI UNIV OF SPORT
Genetically Modified Rat Models for Cytokine-Cytokine Signaling Pathways
The present invention relates to the engineering of animal cells, preferably mammalian, more preferably rat, that are deficient due to the disruption of gene(s) or gene product(s) resulting in cytokine-cytokine mediated autoimmune and inflammatory disease. In another aspect, the invention relates to genetically modified rats, as well as the descendants and ancestors of such animals, which are animal models of human autoimmune and inflammatory disease and methods of their use. Specifically, the invention pertains to a genetically altered rat, or a rat cell in culture, that is defective in at least one of two alleles of a cytokine gene such as the Faslg gene, the Fas gene, etc. In one embodiment, the cytokine gene is the Faslg gene. In another embodiment, the cytokine gene is one of several known cytokine genes, such as Fas, IFNγ, TNF-α, IL-2, IL-10, and IL-12. The inactivation of at least one of these cytokine alleles results in an animal with a higher susceptibility to cytokine-cytokine mediated autoimmune and inflammatory disease induction. In one embodiment, the genetically altered animal is a rat of this type and is able to serve as a useful model for cytokine-cytokine mediated autoimmune and inflammatory disease and as a test animal for autoimmune and other studies.
Owner:TRANSPOSAGEN BIOPHARM
Testing chip in cytokine gene type and application
A gene chip for detecting INF and IL-6 genotypes, a reagent kit containing said gene chip for detecting INF and IL-6 genotypes, and its application for predicting the information about the danger of the diseases associated with said genes and providing the method to develop the relative medicines are disclosed.
Owner:JINAN CENTER HOSPITAL +1
Safer Attenuated Virus Vaccines With Missing Or Diminished Latency Of Infection
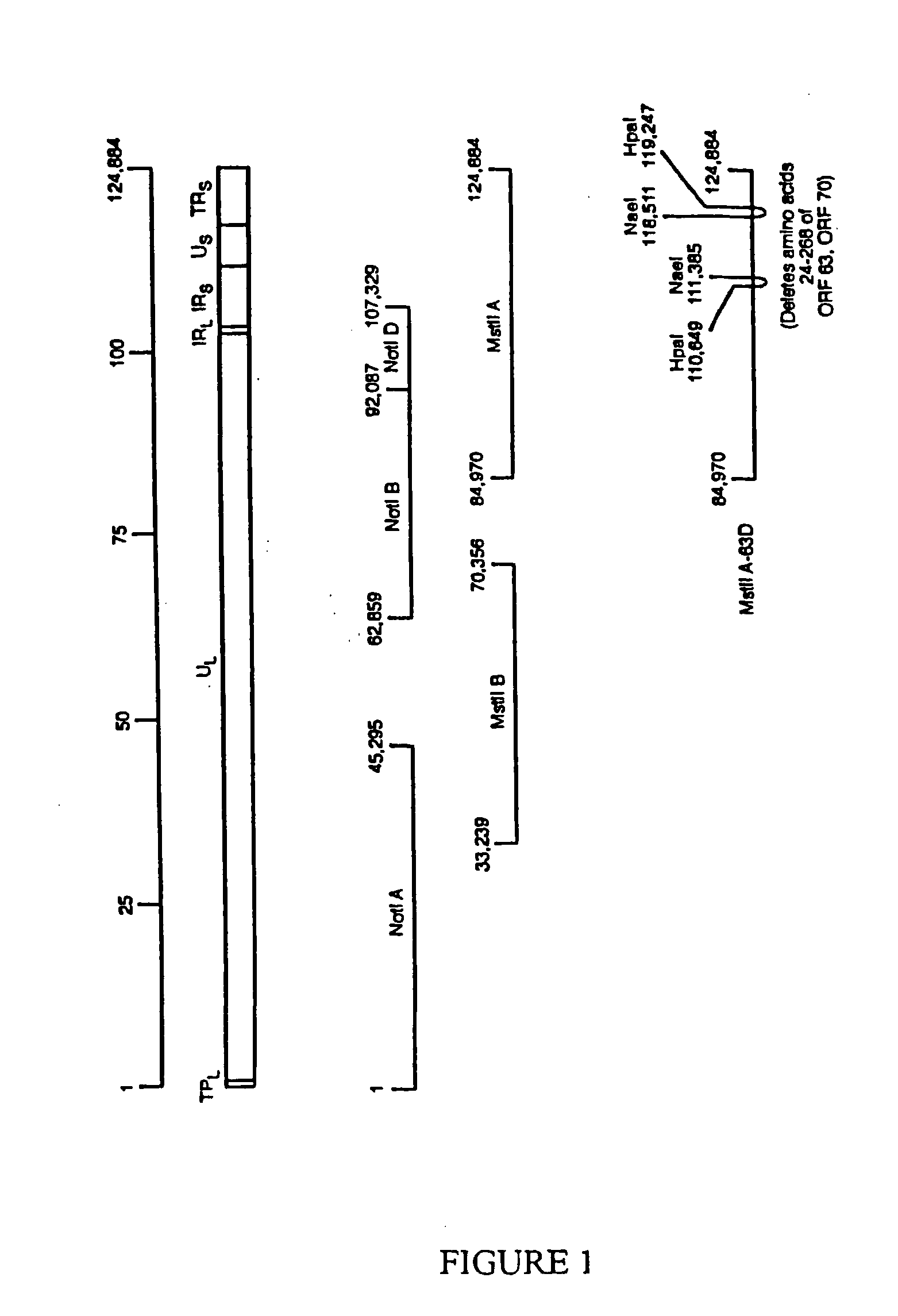
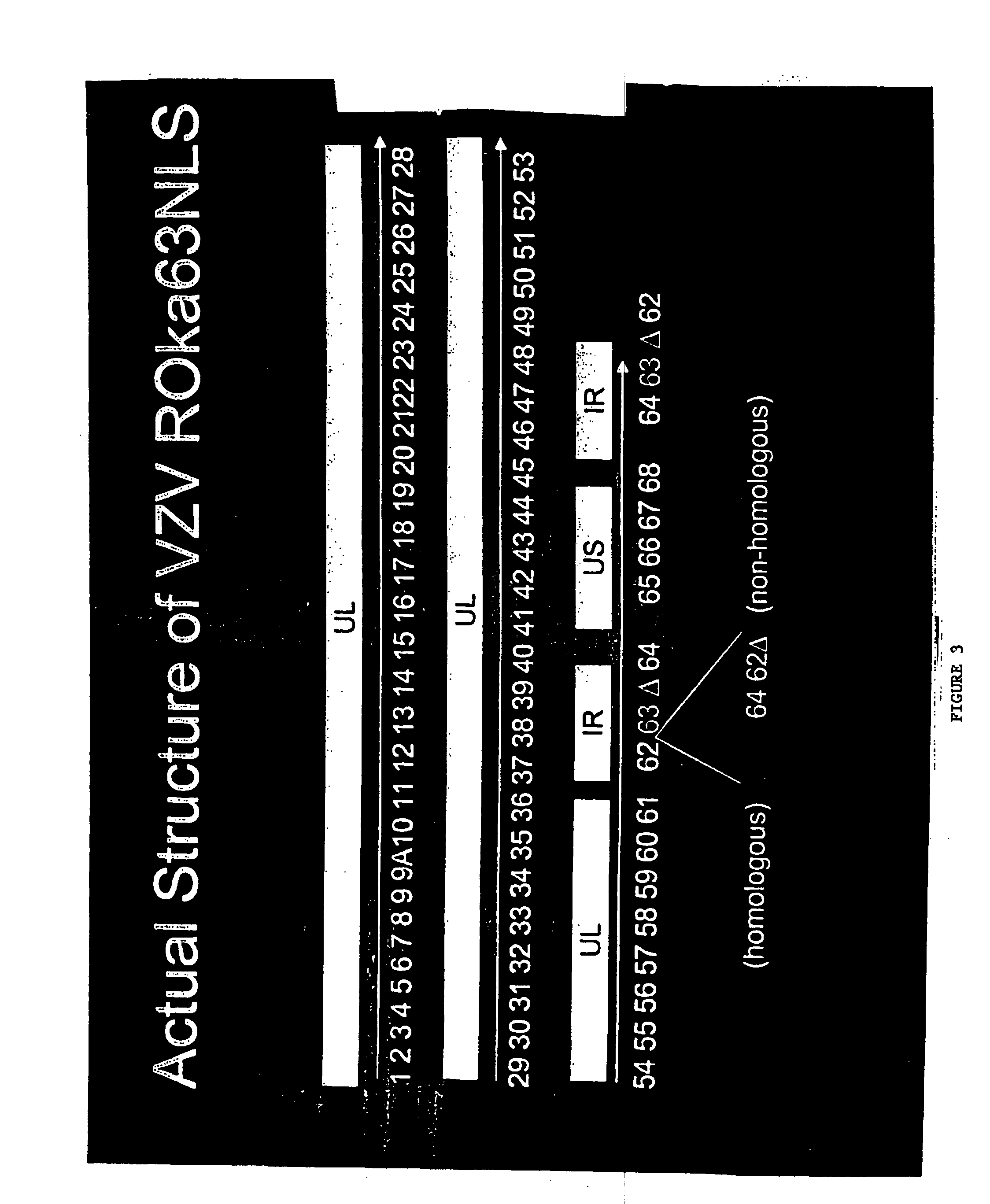
ActiveUS20080279886A1Increase delayEasy to copyViral antigen ingredientsAntiviralsGenetic MaterialsNormal growth
Viruses having weakened ability to establish and / or maintain latency and their use as live vaccines are described. The vaccines have one or more alterations in genes that provide continued virus replication but that inhibit latency. The vaccine materials and methods for their construction are exemplified with the varicella zoster virus. Deletion of a significant portion from both copies of the varicella zoster gene ORF63 was shown to inhibit establishment of a latent infection from a live vaccine form of the virus. Insertion of an additional ORF62 gene which is partially truncated with the ORF63 deletion inhibited establishment of latency and allowed normal growth of the virus. Other desirable viral antigen encoding sequence(s) and / or cytokine genes advantageously may replace deleted genetic material to enhance a desired immunological response. Aspects of the discovery pertain to live vaccines of other viruses, and can provide a variety of vaccines having greater safety.
Owner:UNITED STATES OF AMERICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com