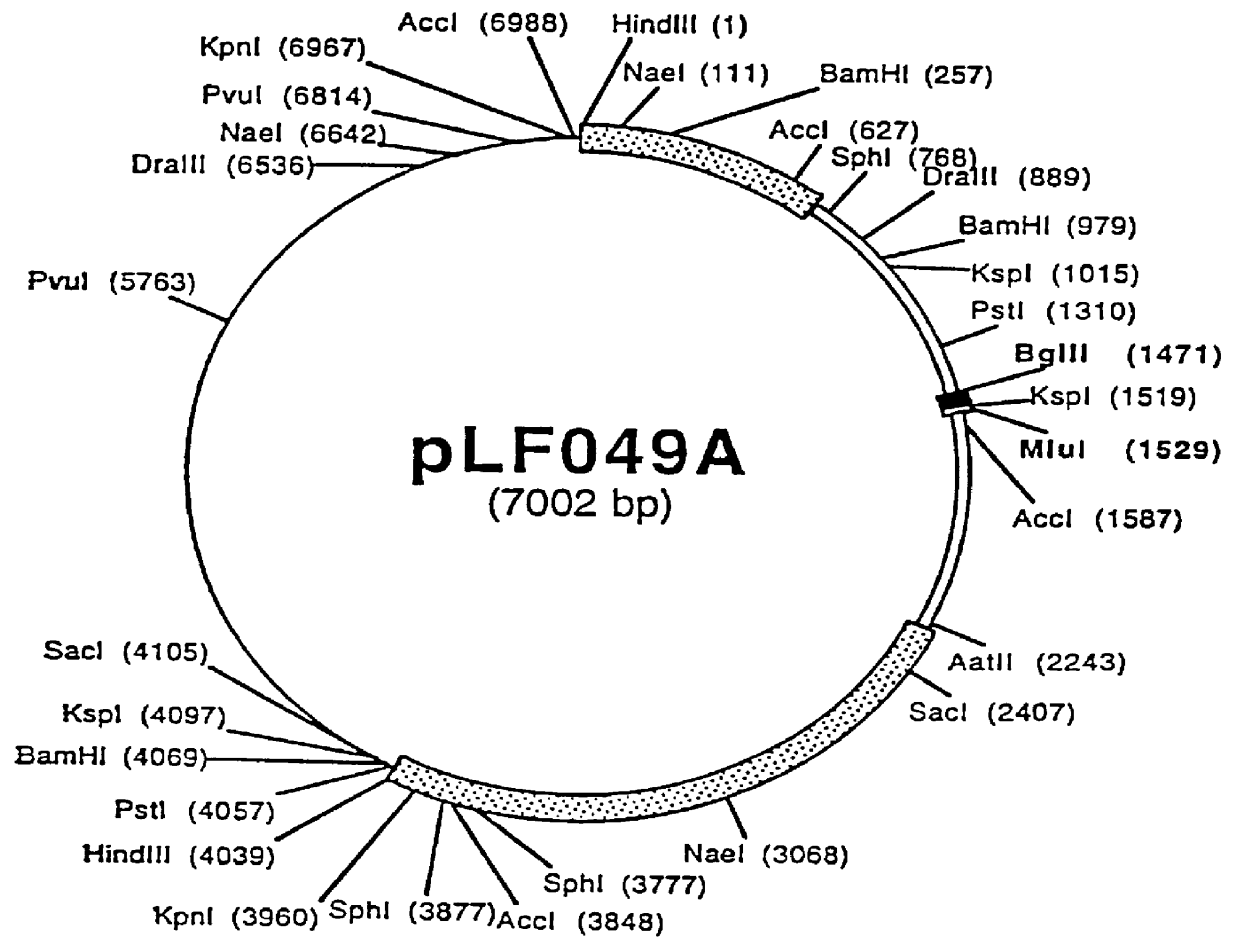
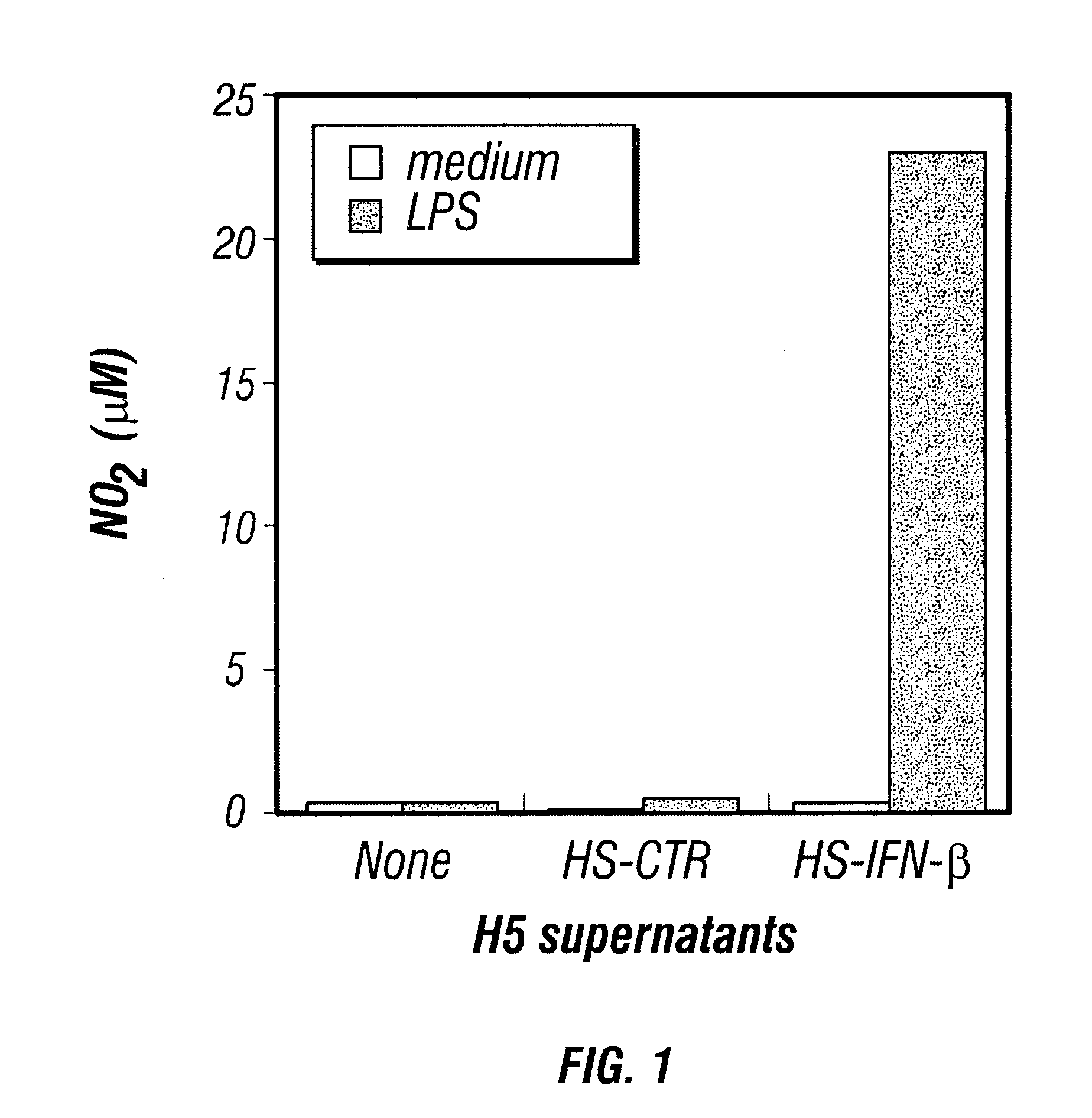
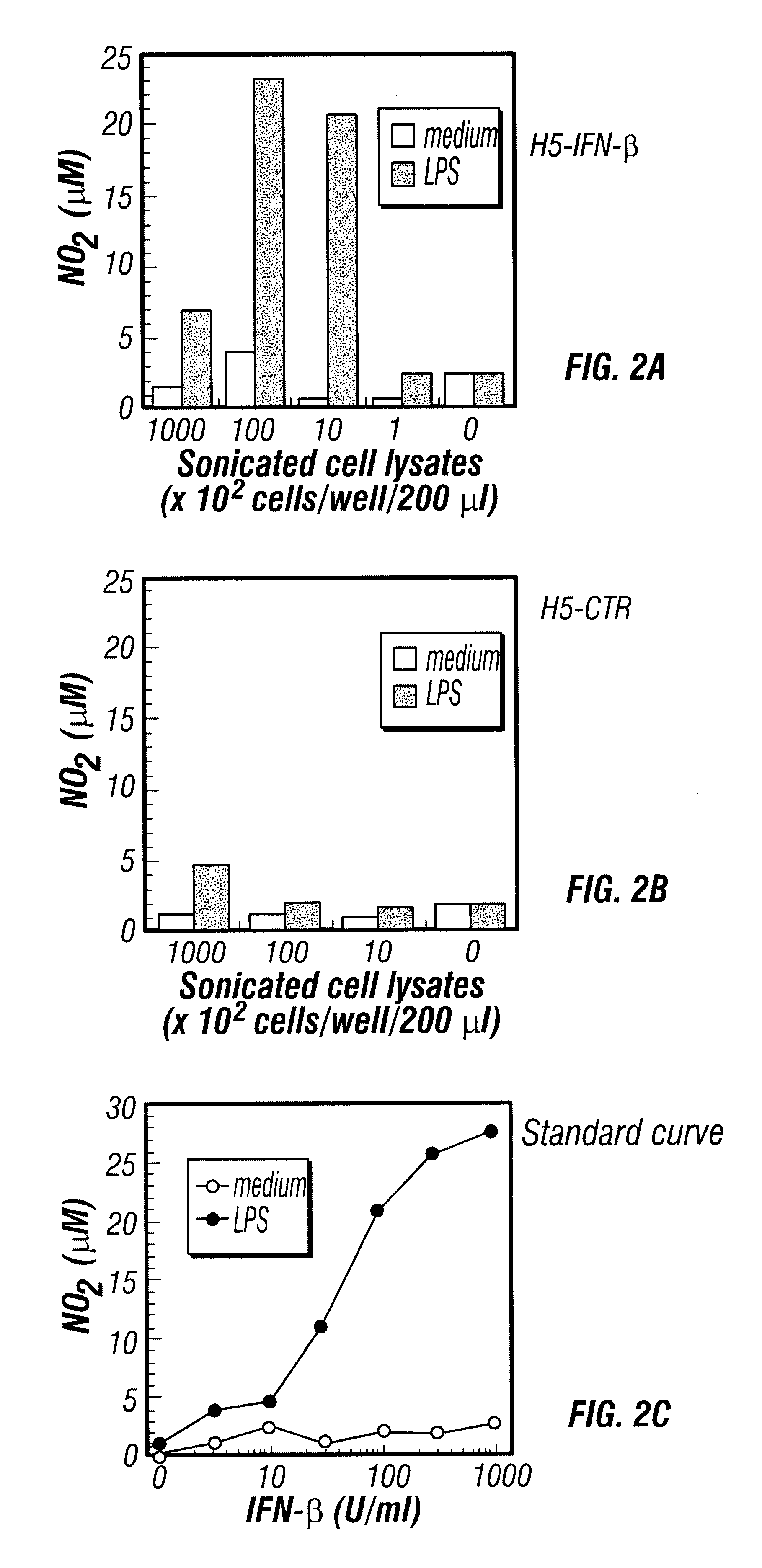
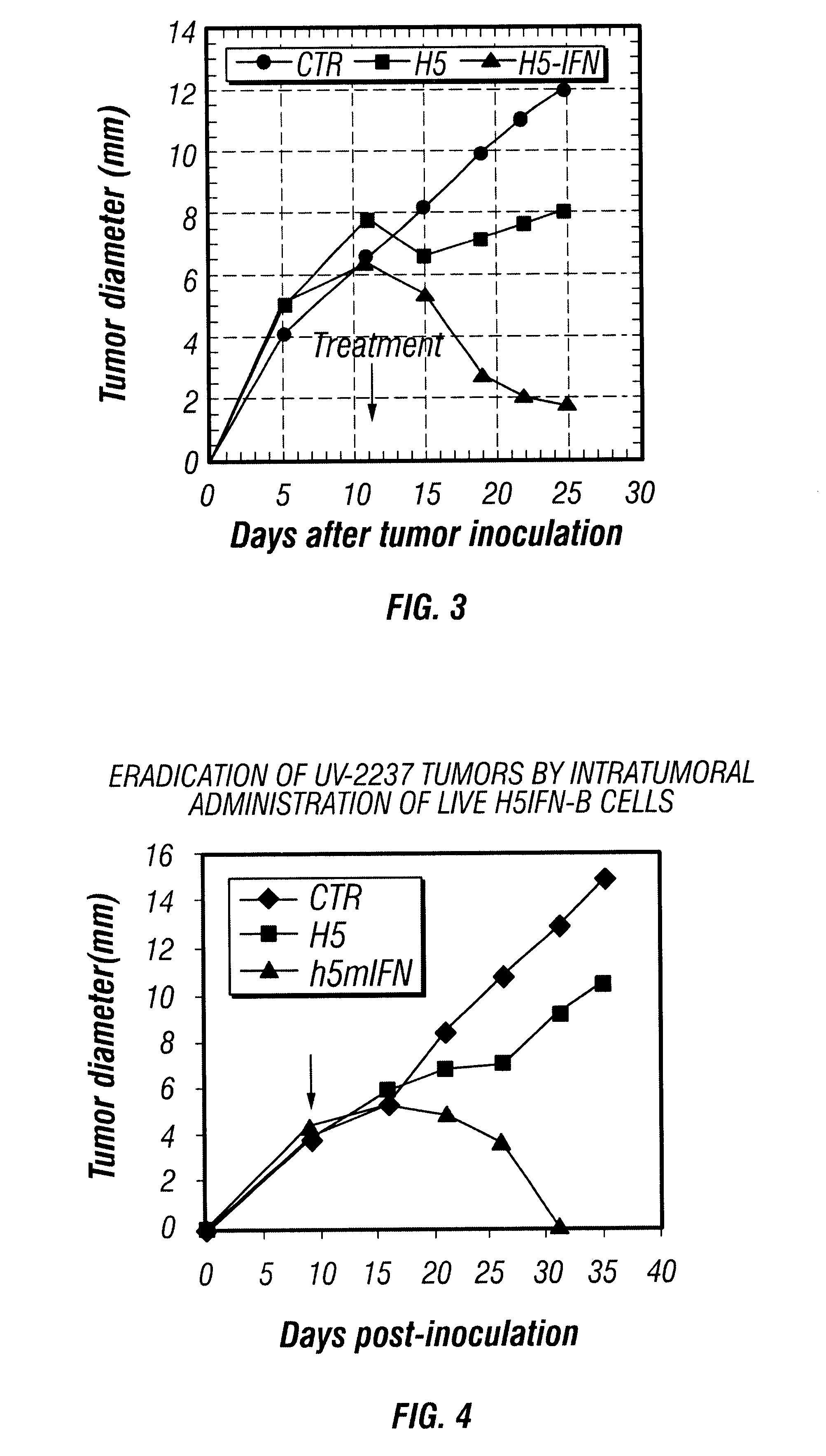
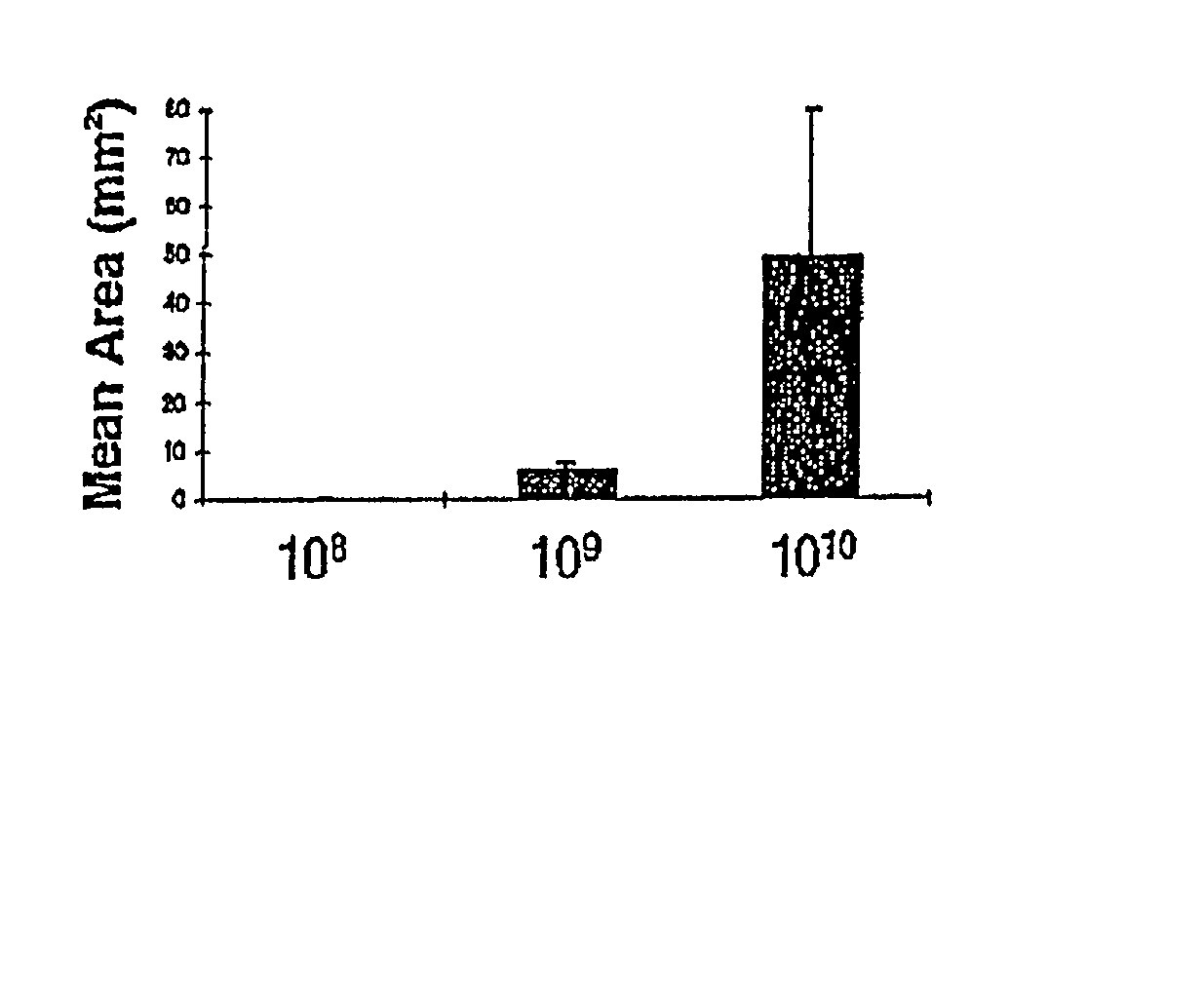
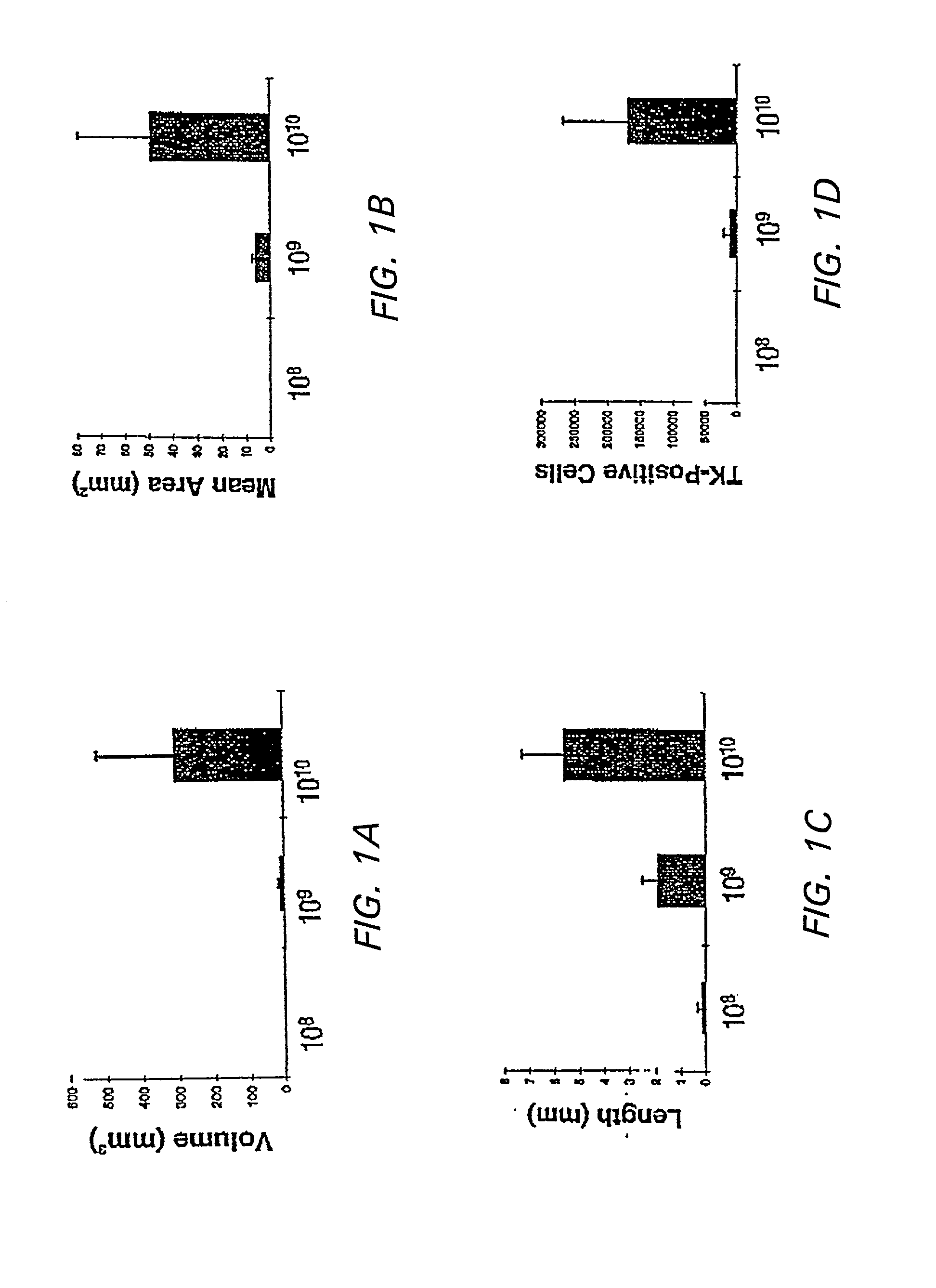
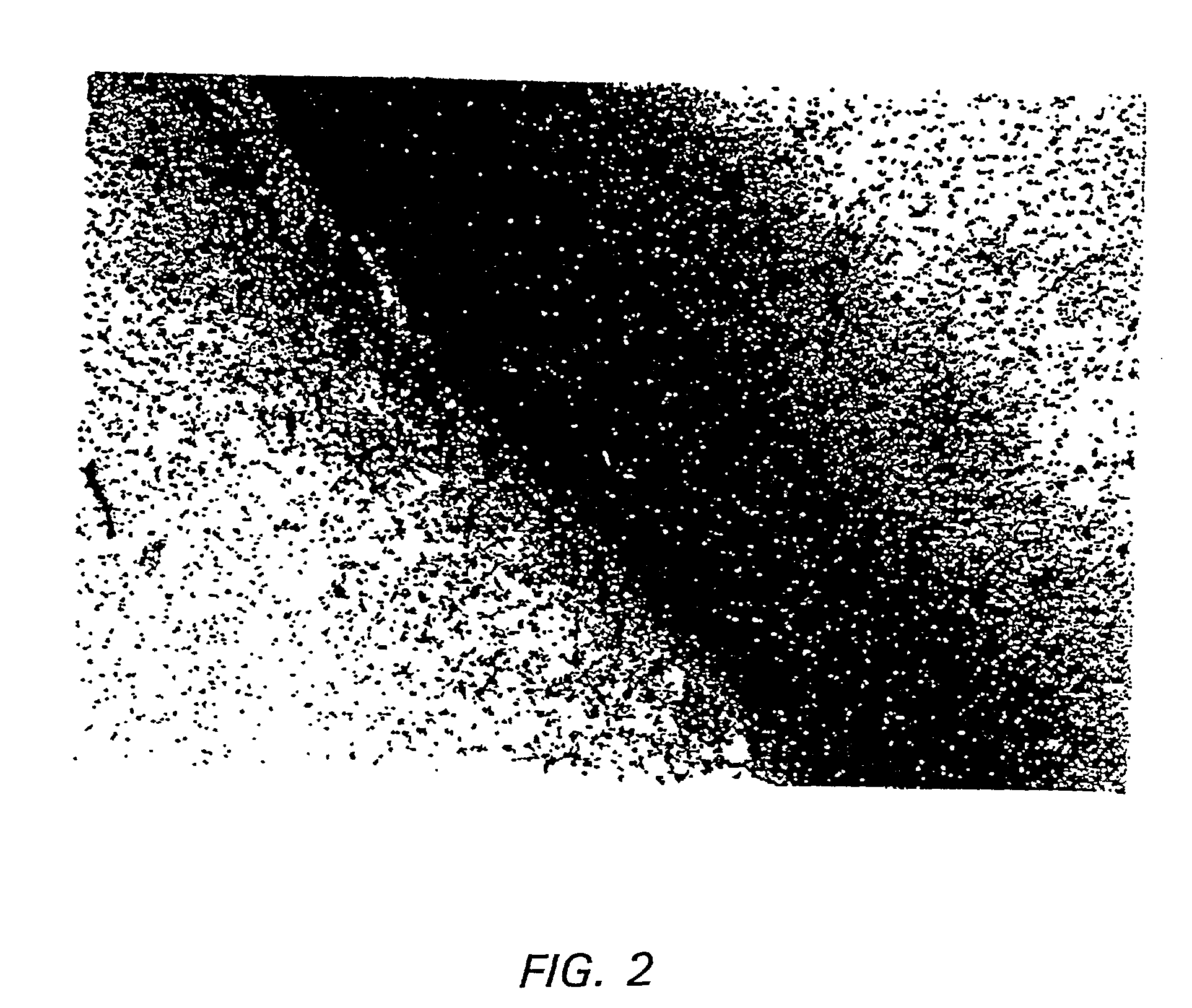
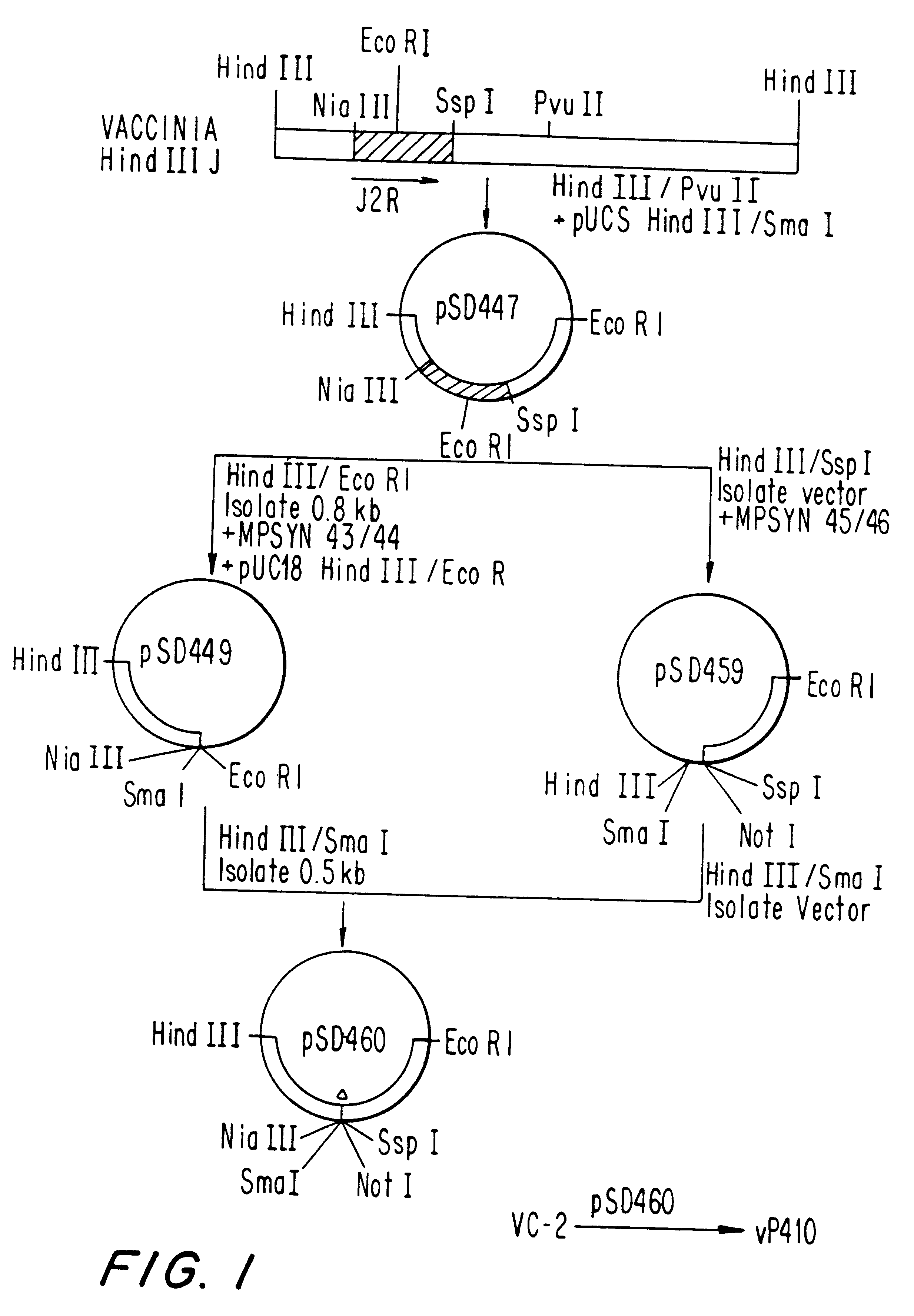
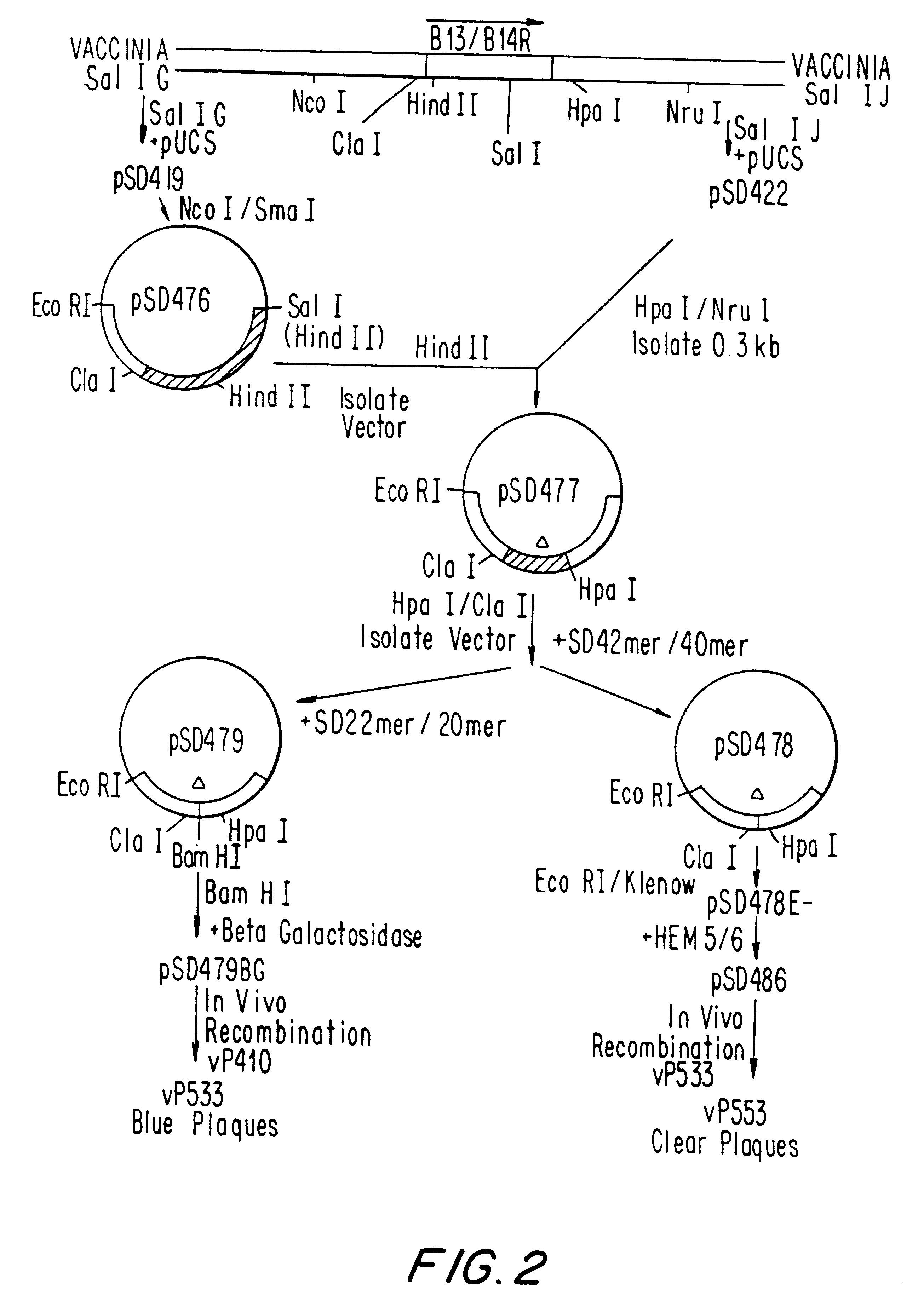
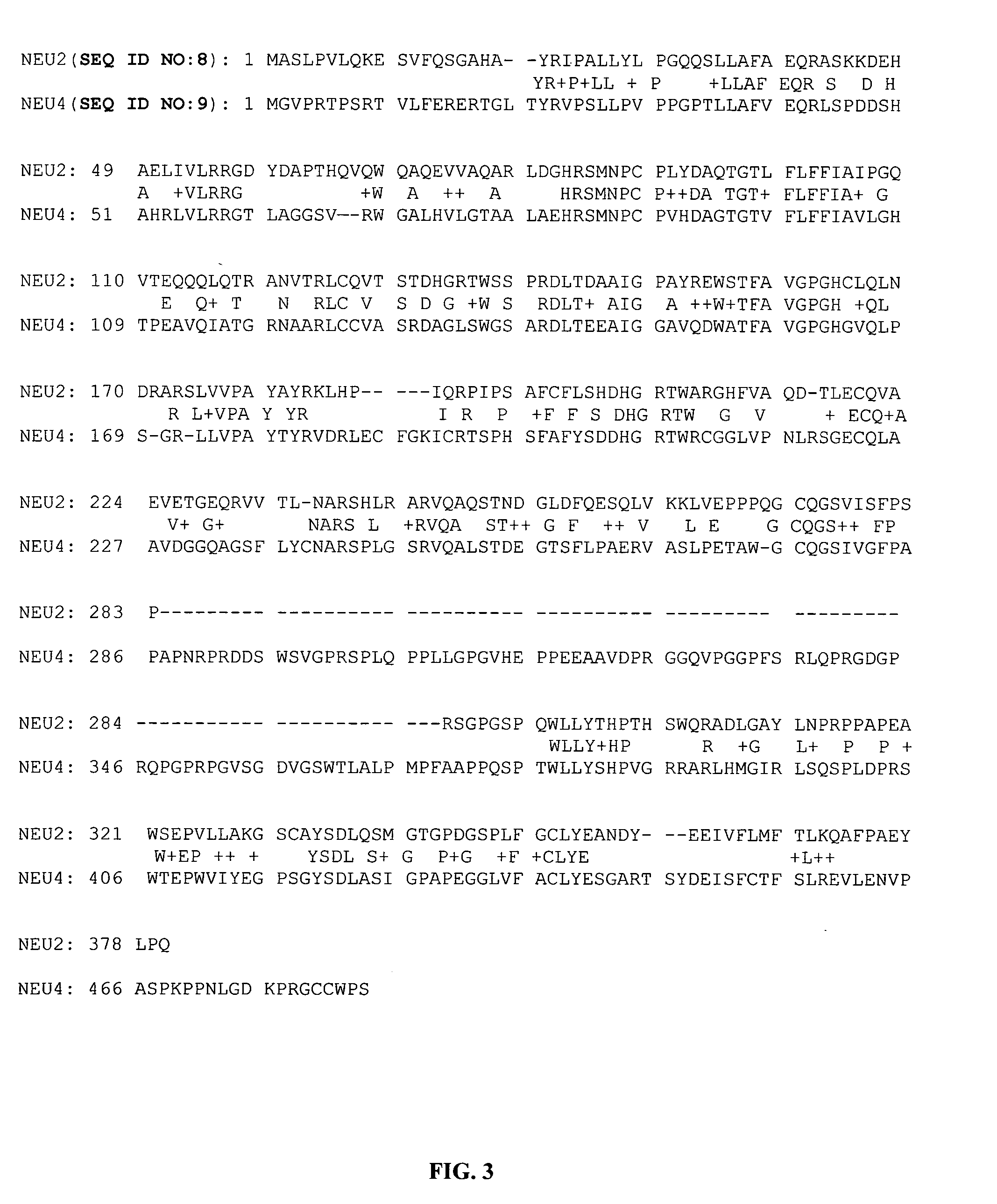
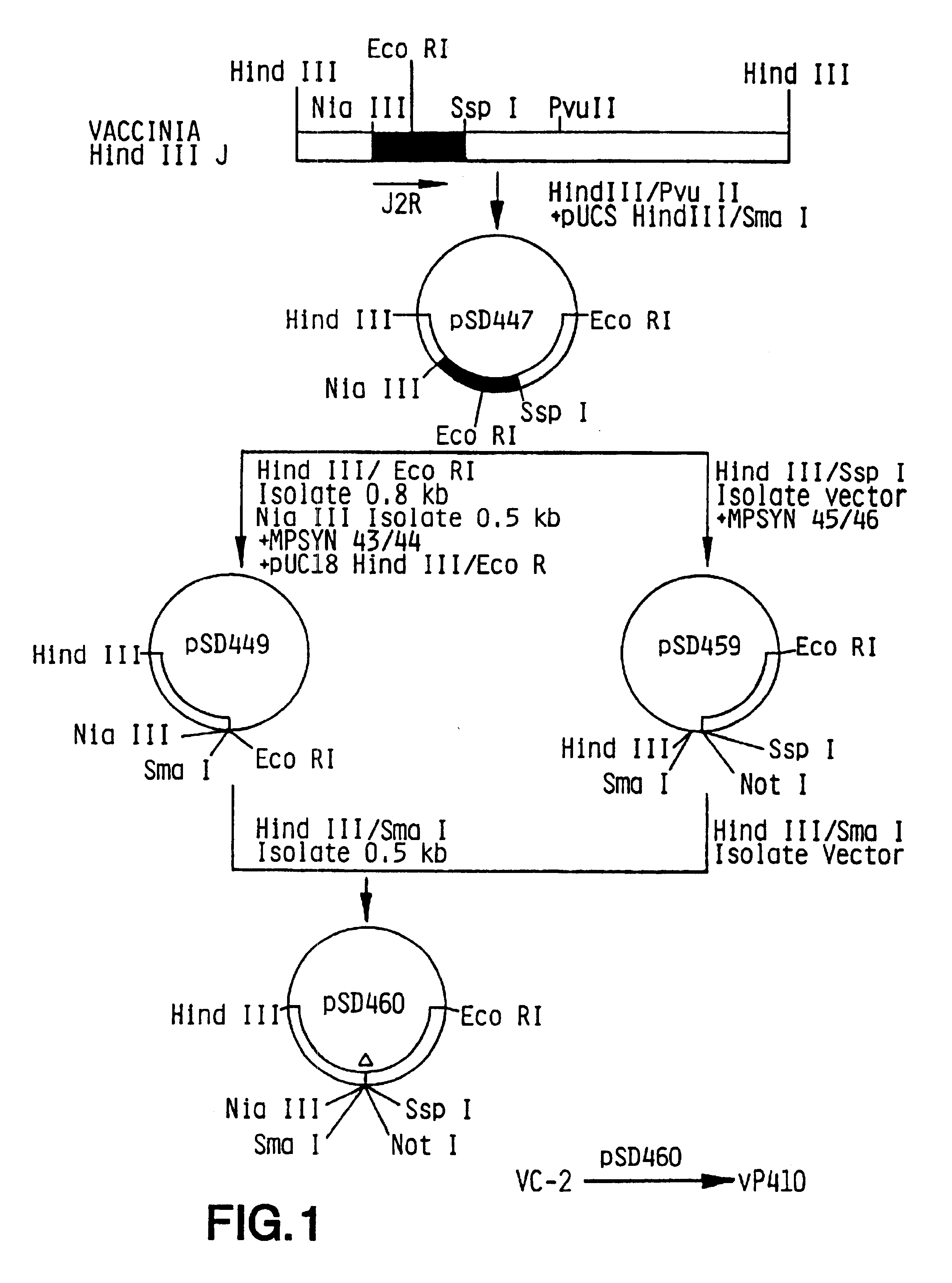
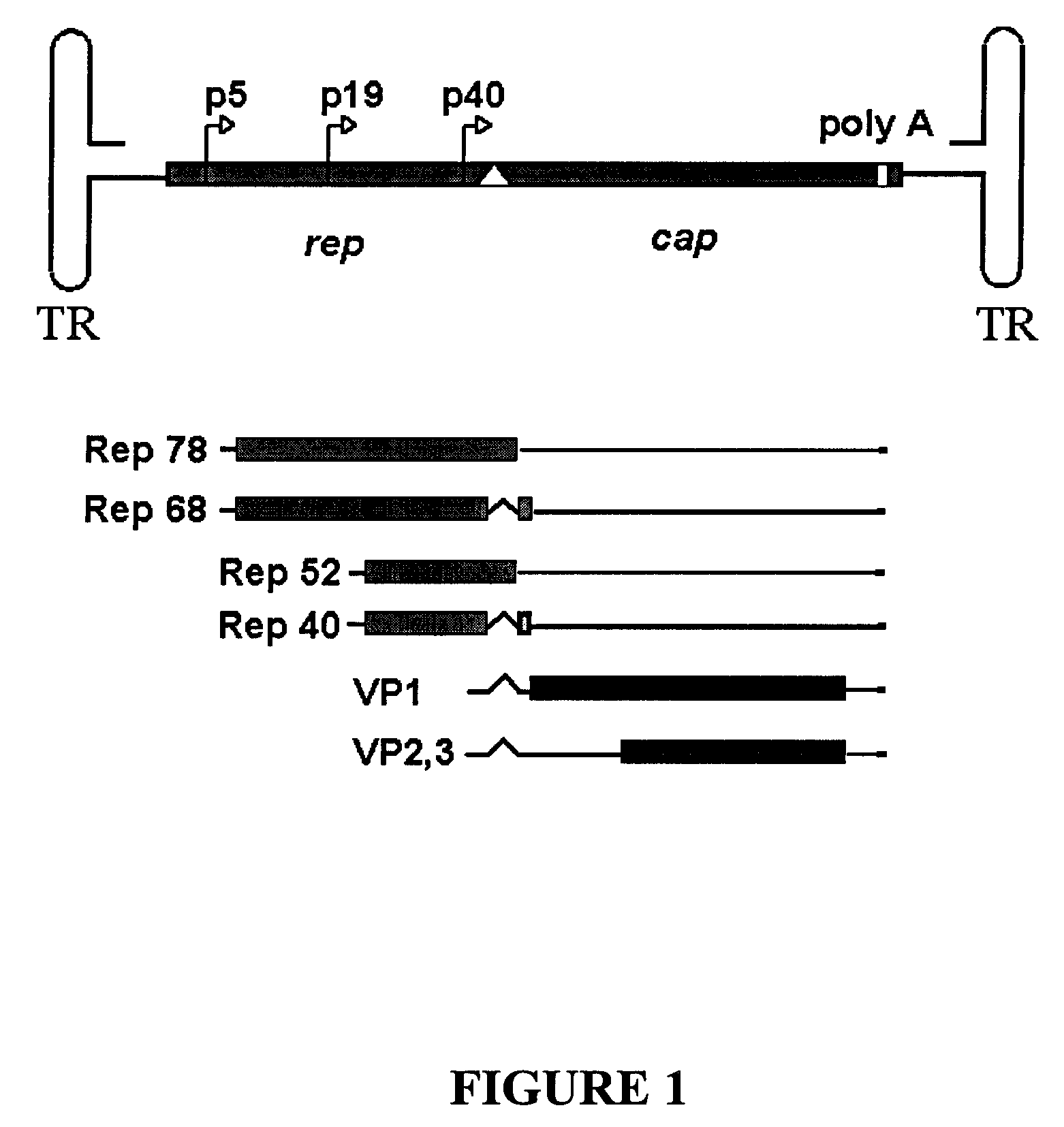
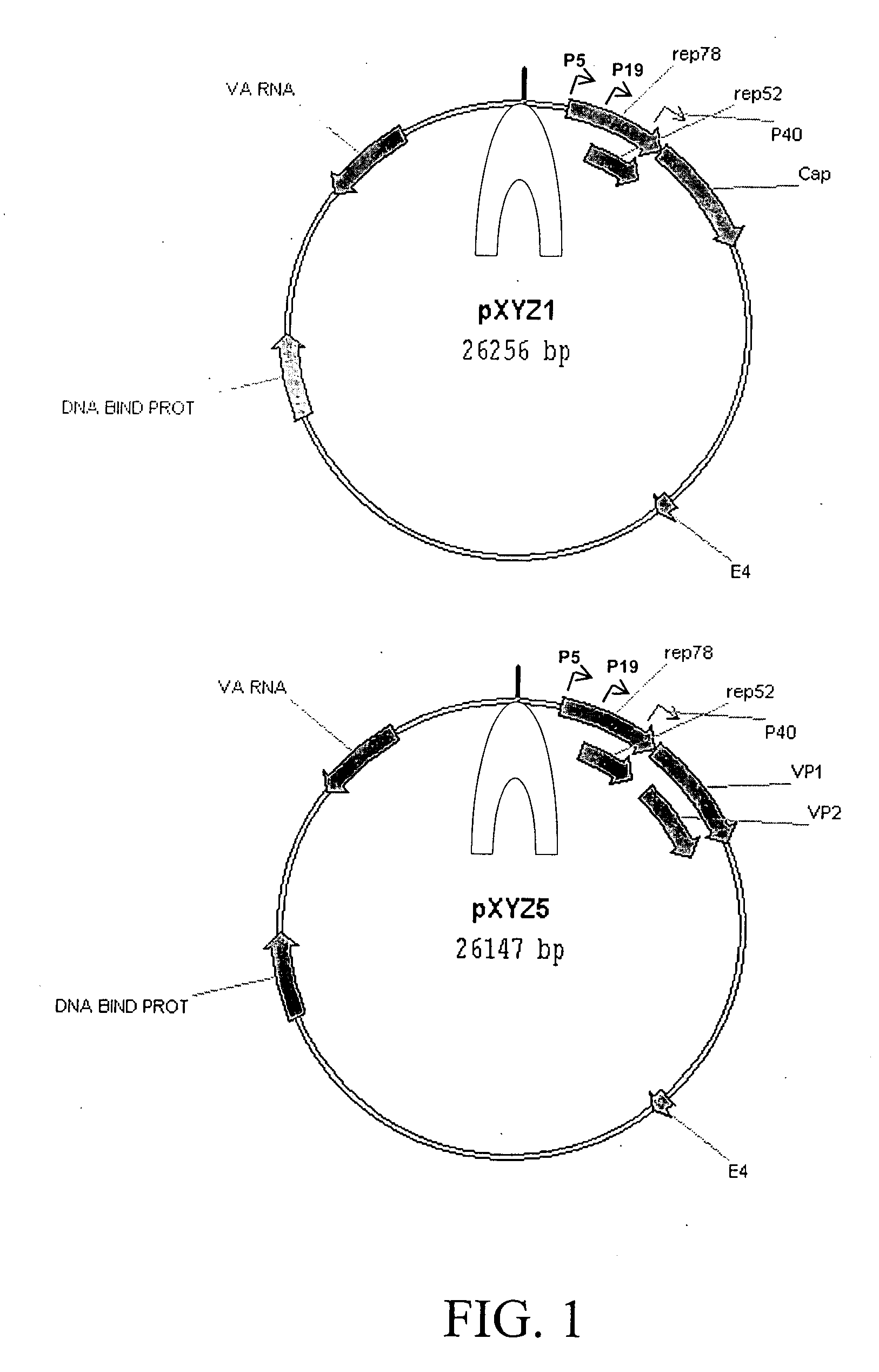
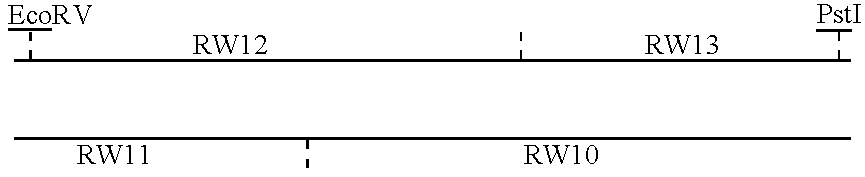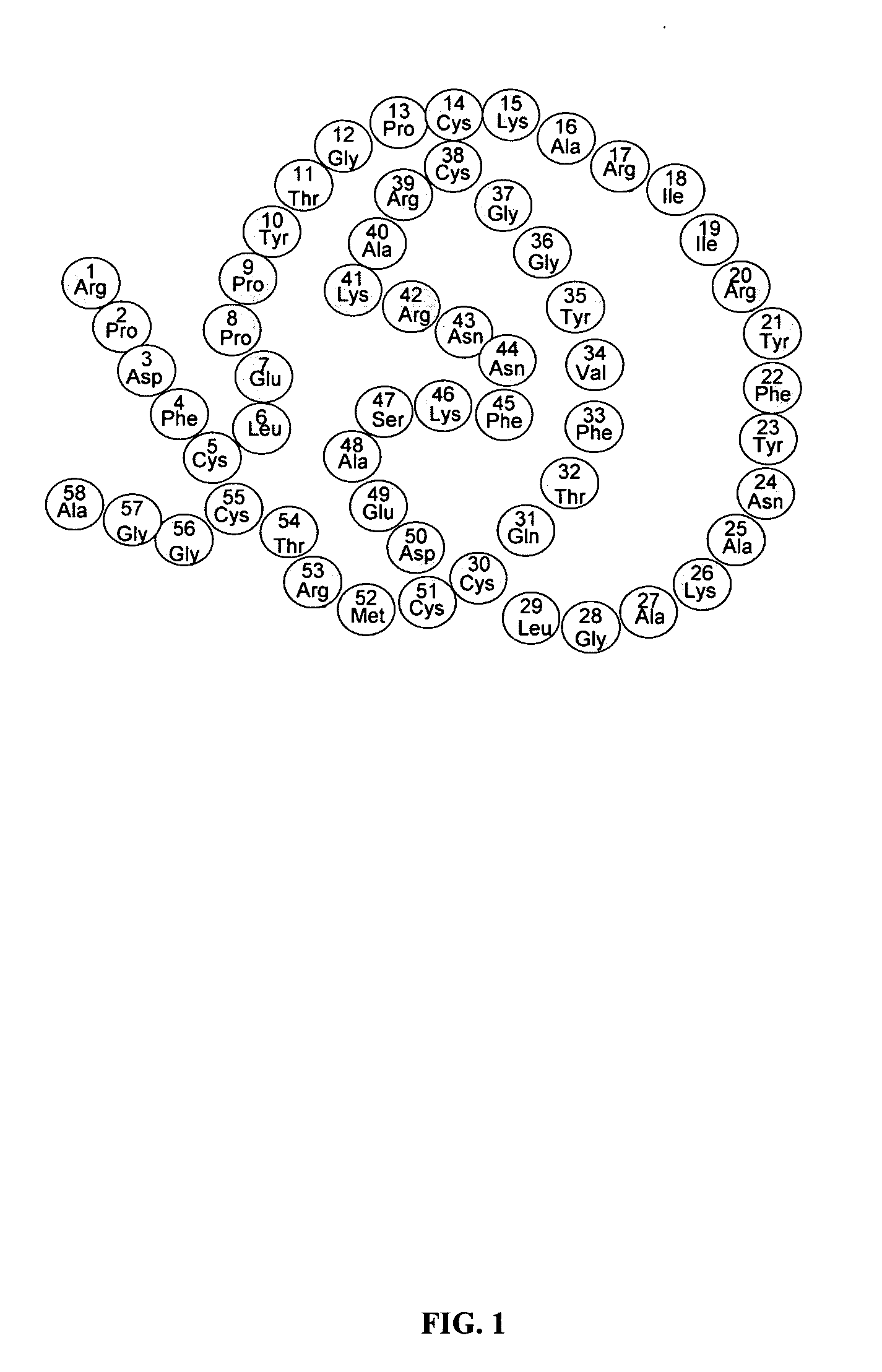Patents
Literature
1491 results about "Recombinant virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A recombinant virus is a virus produced by recombining pieces of DNA using recombinant DNA technology. This may be used to produce viral vaccines or gene therapy vectors. It is also used to refer to naturally occurring recombination between virus genomes in a cell infected by more than one virus strain. This occurs either by homologous crossing over of the nucleic acid strands or by reassortment of genomic segments. Both these and mutation within the virus have been suggested as ways in which influenza and other viruses evolve. An example of a recombinant virus is Western equine encephalitis virus (WEE), which is a recombinant virus between two other closely related yet distinct encephalitis viruses. In addition, reassortment is most important for pandemic influenza viruses.
Metabolically activated recombinant viral vectors and methods for their preparation and use
Recombinant viral vectors, especially parvovirus vectors such as adeno-associated virus (AAV) vectors, capable of enhanced expression of heterologous sequences, and methods for their construction and use, are provided. The vectors have a structure, or are capable of rapidly adopting a structure, which involves intrastrand base pairing of at least one region in a heterologous sequence.
Owner:GENZYME CORP
Production of pseudotyped recombinant AAV virions
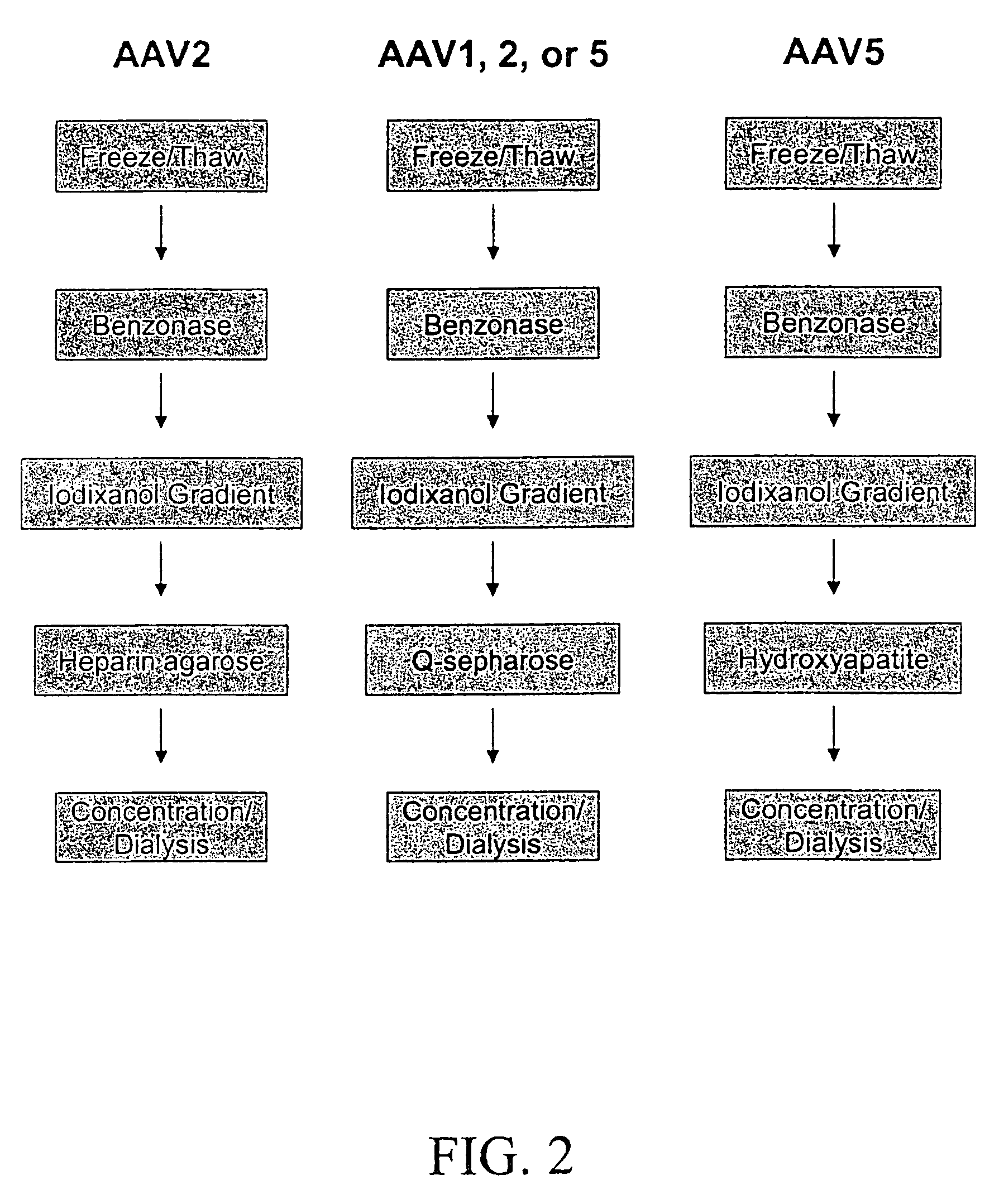
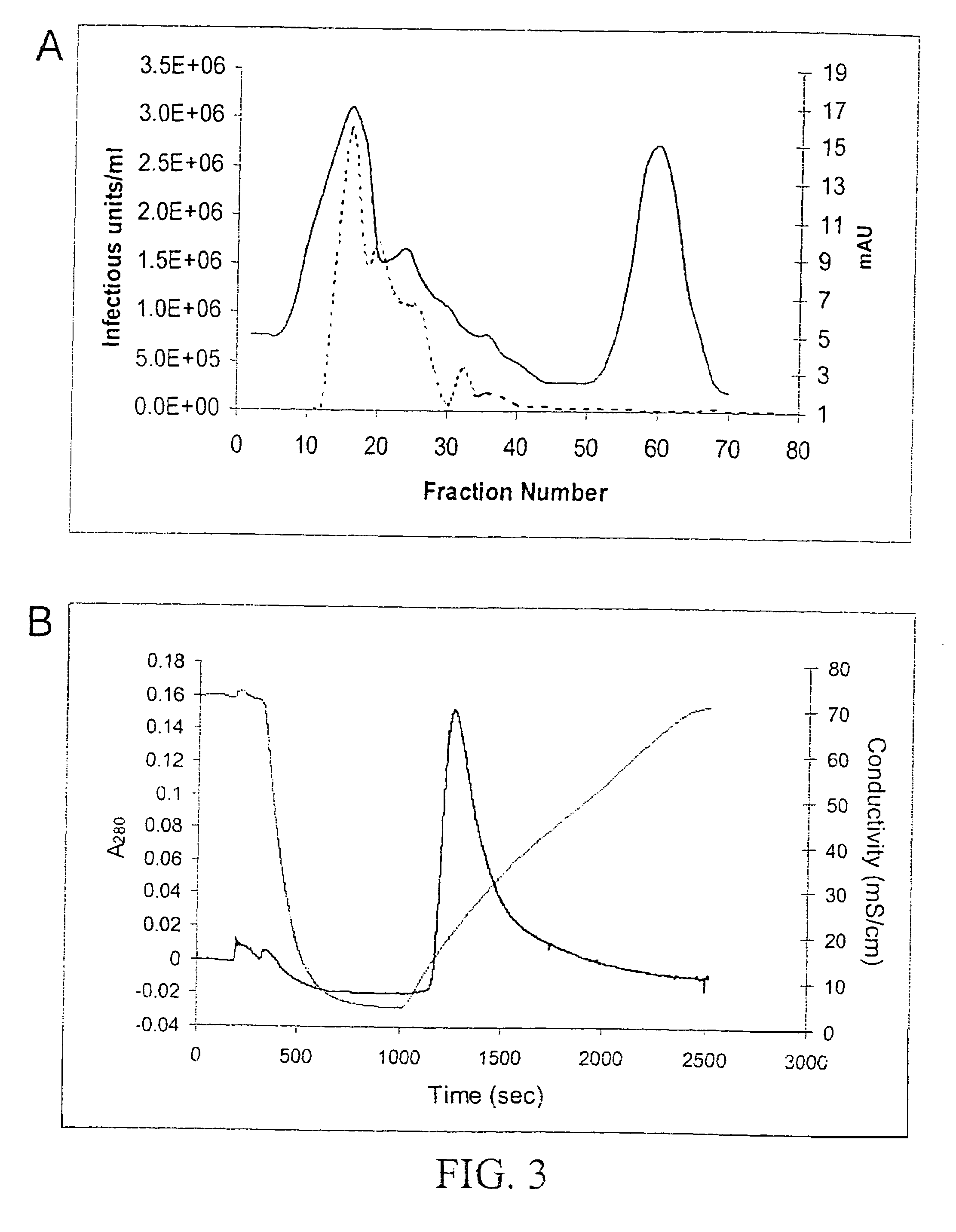
InactiveUS7094604B2Highly purified and concentratedEfficient and large-scale productionVectorsSugar derivativesPurification methodsSerotype
Vectors that encode Adeno-Associated Virus (AAV) Rep and Cap proteins of different serotypes and Adenovirus transcription products that provide helper functions were used to produce pseudotyped recombinant AAV (rAAV) virions. Purification methods generated pseudotyped rAAV virion stocks that were 99% pure with titers of 1×1012–1×1013 vector genomes / ml.
Owner:UNIVIRSITY OF FLORIDA RES FOUND INC
Recombinant canine adenoviruses, method for making and uses thereof
InactiveUS6090393AProvide securityIncrease capacitySsRNA viruses negative-senseVectorsBiotechnologyImmunogenicity
Disclosed and claimed are recombinant adenoviruses, methods of making them, uses for them (including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector), expression products from them, and uses for the expression products. More particularly, disclosed and claimed are recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses. Additionally, disclosed and claimed are truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes.
Owner:VIROGENETICS +1
Therapy of cancer by insect cells containing recombinant baculovirus encoding genes
Provided are compositions and methods of use for insect cells comprising baculovirus encoding non-surface expressed proteins and peptides. The claimed invention particularly relates to compositions comprising insect cells containing baculovirus that express cytokines. Such compositions may be administered by, for example, direct intratumoral injection into tumors in mammals, resulting in tumor reduction or recission. Another aspect of the claimed invention concerns methods of promoting resistance to the reoccurence of tumors in mammals who have undergone such tumor recission. In a specific aspect of the claimed invention, the mammals are human subjects presenting with various forms of cancer.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Pox virus containing DNA encoding a cytokine and/or a tumor associated antigen
InactiveUS6265189B1Improve securityImprove security levelVirusesPeptide/protein ingredientsHuman tumorWild type
Attenuated recombinant viruses containing DNA coding for a cytokine and / or a tumor associated antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least on of: human tumor necrosis factor; nuclear phosphoprotein p53, wildtype or mutant; human melanoma-associated antigen; IL-2; IFNgamma; IL-4; GNCSF; IL-12; B7; erb-B-2 and carcinoembryonic antigen. The recombinant viruses and gene products therefrom are useful for cancer therapy.
Owner:VIROGENETICS
Truncated transcriptionally active cytomegalovirus promoters
InactiveUS6156567AProvide securityIncrease capacitySsRNA viruses negative-senseGenetic material ingredientsBiotechnologyEukaryotic plasmids
Recombinant adenoviruses, methods of making them, uses for them, including in immunological, immunogenic, vaccine or therapeutic compositions, or, as a vector for cloning, replicating or expressing DNA and methods of using the compositions and vector, expression products from them, and uses for the expression products are provided. More particularly, recombinant canine adenoviruses (CAV) and methods of making them, uses for them, expression products from them, and uses for the expression products, including recombinant CAV2 viruses are provided. Additionally, truncated promoters, expression cassettes containing the promoters, and recombinant viruses and plasmids containing the promoters or expression cassettes are provided.
Owner:VIROGENETICS
Mutant adeno-associated virus virions and methods of use thereof
ActiveUS20050053922A1Reduce the binding forceAltered infectivityAntibacterial agentsVirusesReassortant VirusesNeutralizing antibody
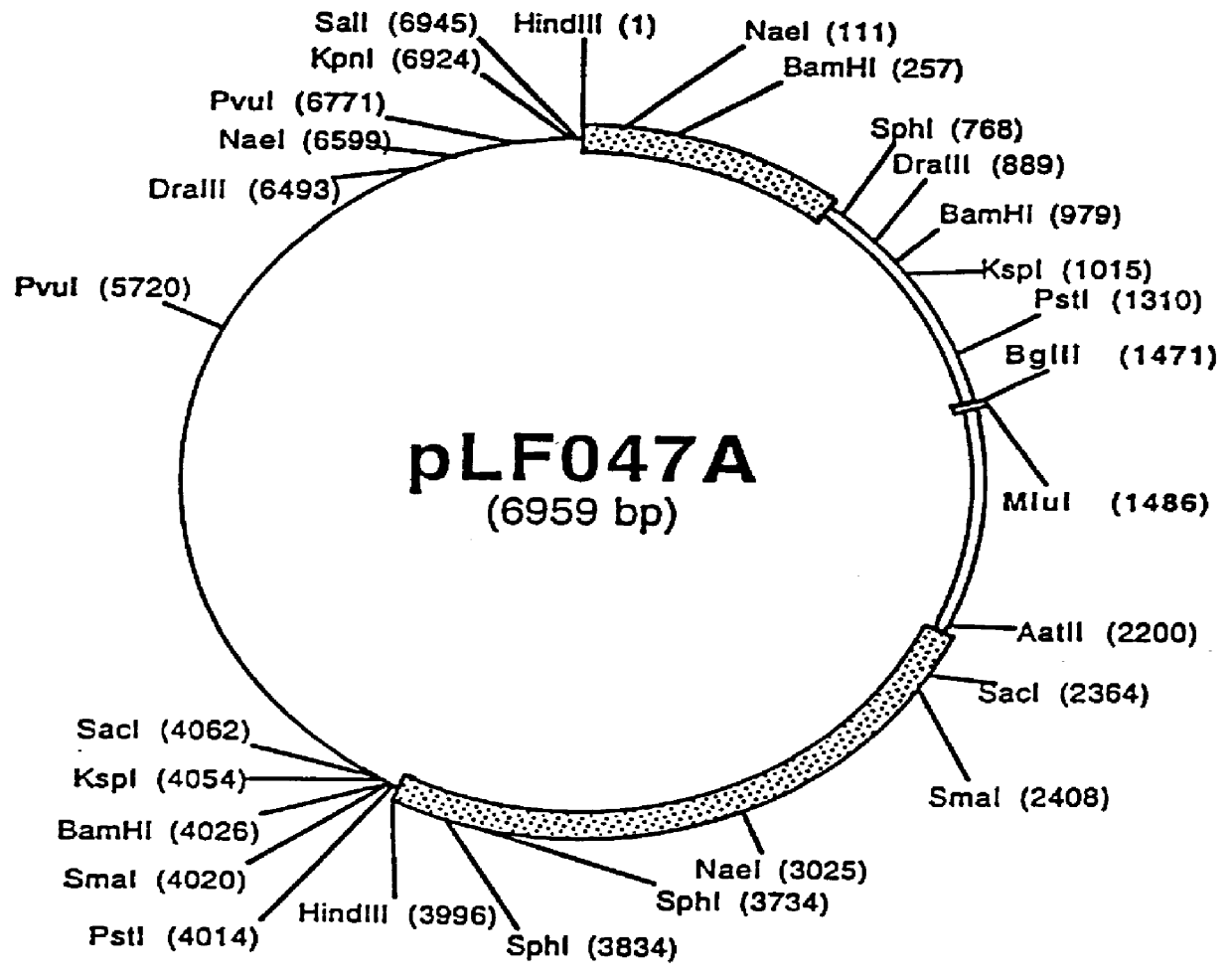
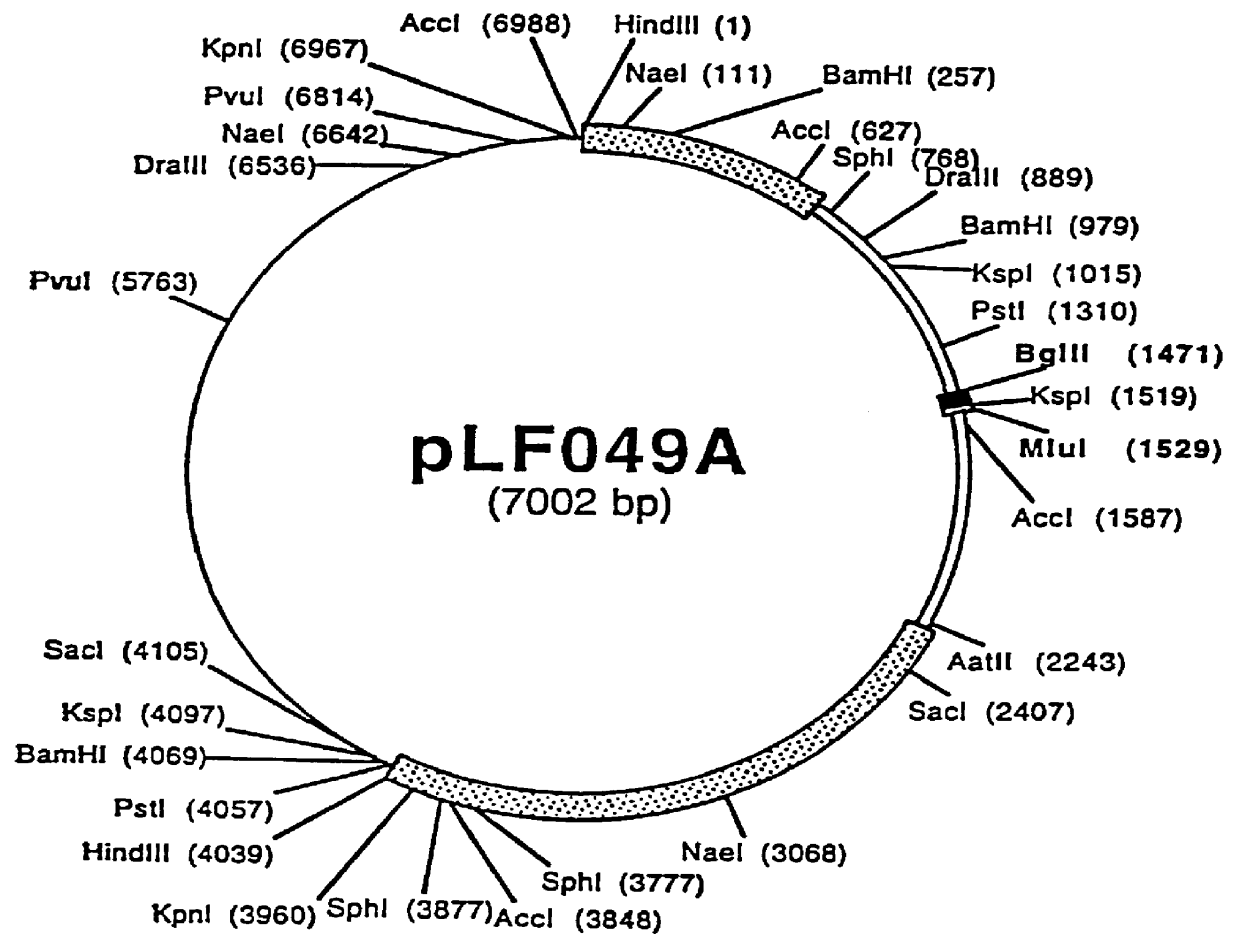
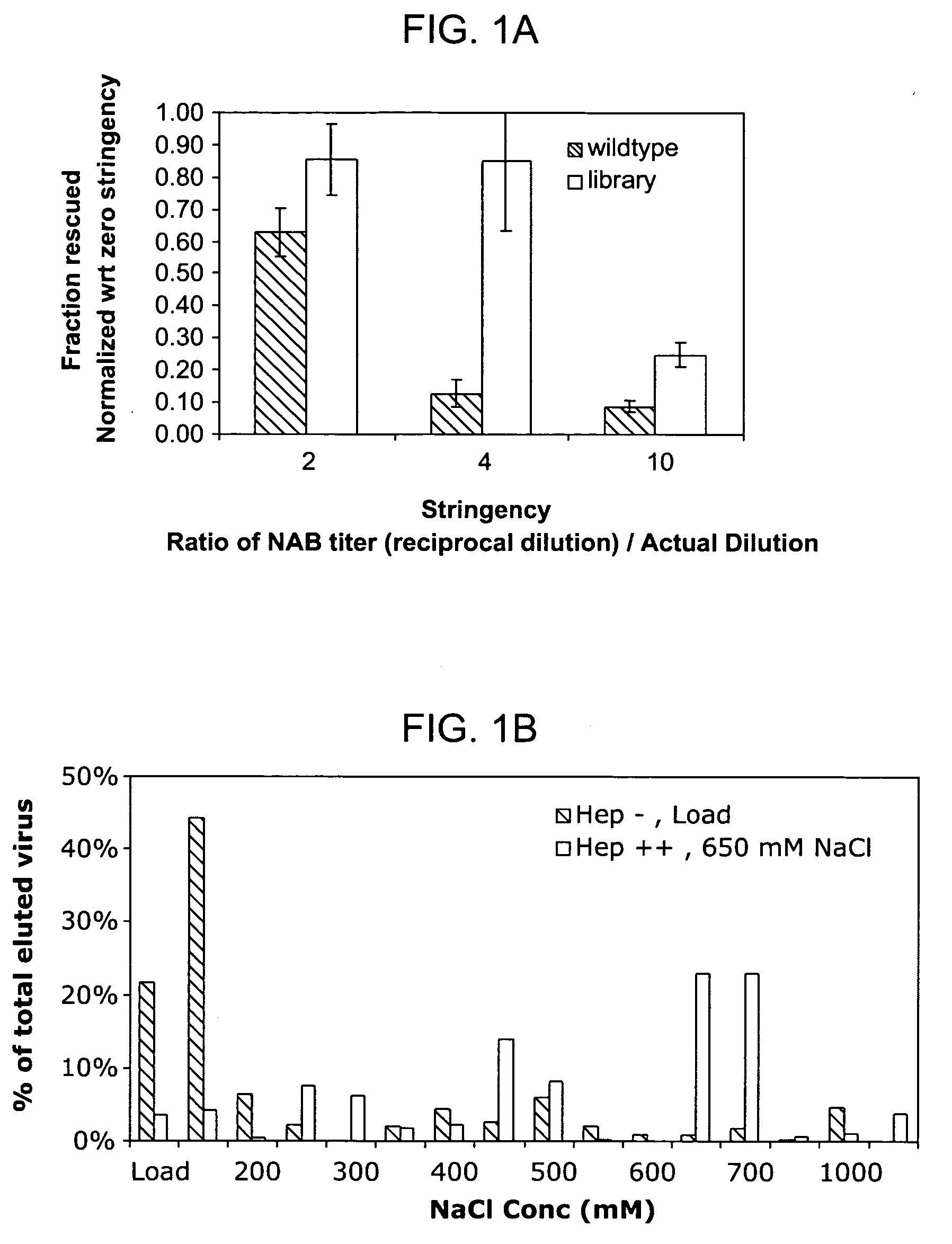
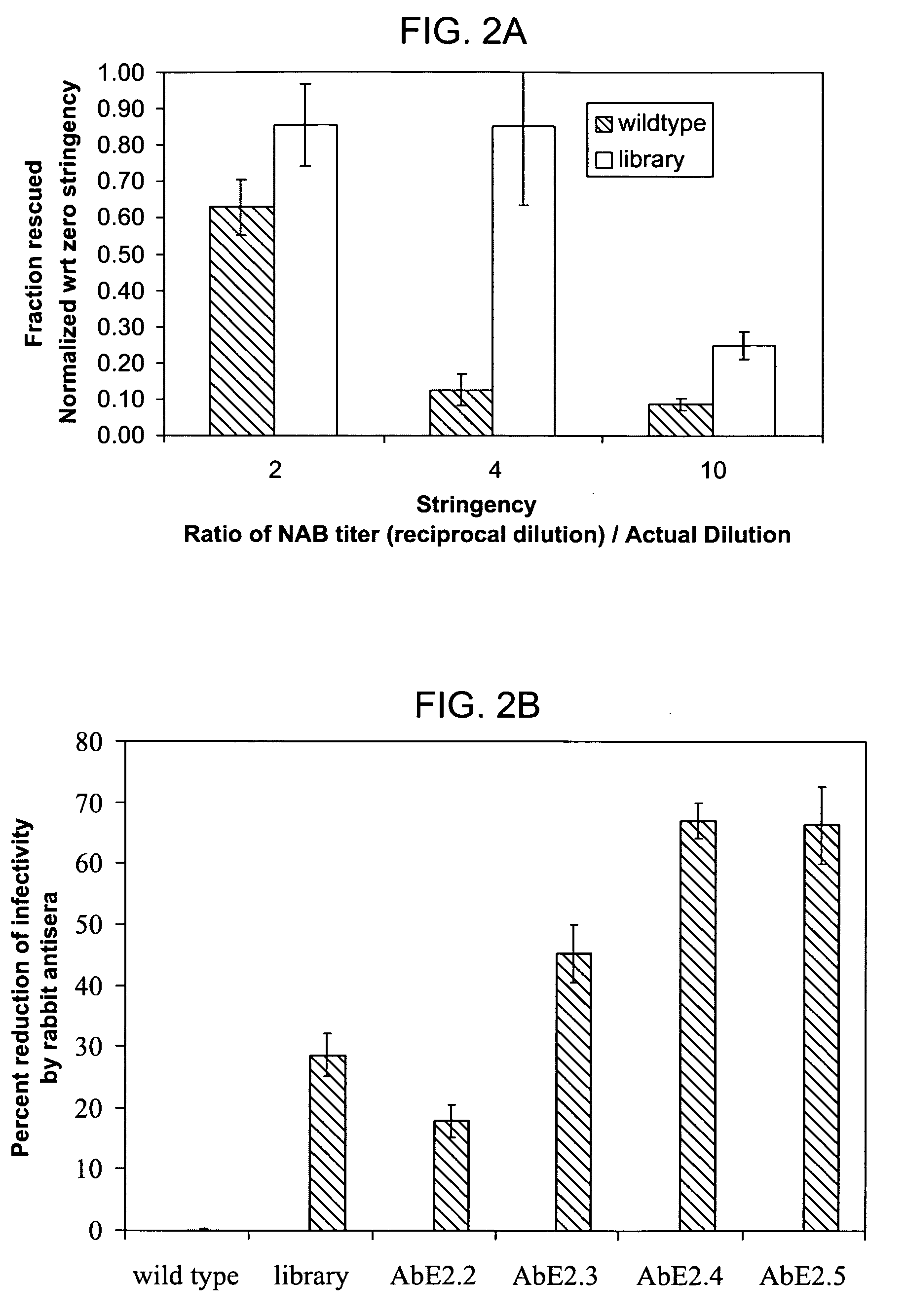
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:INTEGRATIVE GENE THERAPEUTICS +1
Retrovirus and viral vectors
InactiveUS6635472B1Prevent and attenuate diseaseEnhance immune responseGenetic material ingredientsVirus peptidesVirus-RetrovirusDna viral
This invention relates to the fields of genetic engineering, virus replication and gene transfer. More specifically, this invention relates to polynucleotide construct, recombinant virus, transposon, and their vectors, wherein an ori derived from a DNA virus capable of replicating in vertebrate cells is inserted into the retrovirus, allowing the retrovirus following the reverse transcription to efficiently replicate as extrachromosomal or episomal DNA without the necessity of integration into the host cell chromosome. Additionally, this invention relates to polynucleotide construct, recombinant virus, transposon, and their vectors replicating episomally without aid of an ori and related elements. Also, this invention encompasses preventive, therapeutic, and diagnostic applications employing said constructs, viruses and vectors.
Owner:RUBICON LABS
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Methods for increasing the efficiency of recombinant AAV product
InactiveUS6548286B1Altering the stability of REP mRNAShort lifeGenetic material ingredientsVirus peptidesPost translationalStable cell line
The present invention relates to methods and compositions for increasing the production of high titre stocks of recombinant AAV (rAAV) through regulation of expression of the AAV REP and CAP proteins. The methods and compositions of the invention are based on the observation that the low level expression of the AAV REP protein increases the production of AAV viral capsid protein and efficiency of packaging resulting in production of higher titre recombinant viral stocks. The invention encompasses recombinant AAV vectors that direct the expression of AAV REP and CAP proteins and the use of such vectors for the production of novel stable cell lines capable of generating high titre rAAV vectors. The invention provides methods for regulating the expression of the AAV REP gene at the transcriptional and post-translational level. The methods and compositions of the invention can be used to produce high titre stocks of rAAV which can be used in gene therapy for the purpose of transferring genetic information into appropriate host cells for the management and correction of human diseases including inherited and acquired disorders.
Owner:CELLS GENESYS INC +1
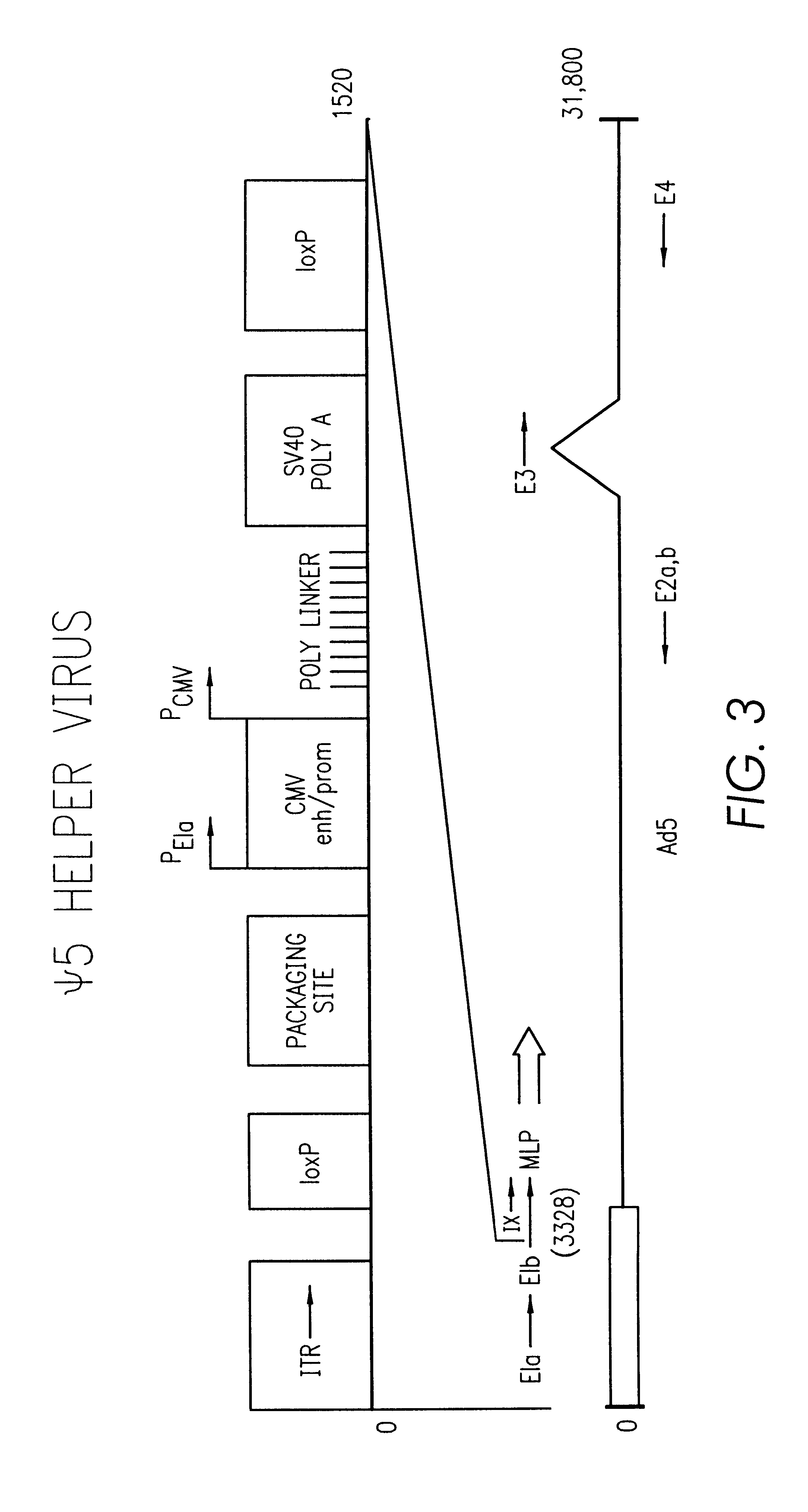
Helper-free, totally defective adenovirus for gene therapy
InactiveUS6228646B1Encapsidation efficiencyMaximal recombinationInactivation/attenuationNucleic acid vectorIn vivoHelper virus
A method for producing in vivo packaged recombinant adenovirus vectors is provided. The recombinant Ad vectors do not contain any Adenovirus genes and are therefore useful for gene therapy. The recombinant Adenovirus vectors are packaged in vivo using a helper virus which is itself very inefficiently packaged, providing a recombinant viral preparation with very little or no contamination with helper virus. In particular, the method makes use of a helper virus in which the packaging site can be easily excised in vivo by recombination mediated by a recombinase. The helper virus is also useful for the in vivo construction of new recombinant adenovirus vectors containing substitutions in the E1 or other adenoviral region.
Owner:RGT UNIV OF CALIFORNIA
Immunodeficiency recombinant poxvirus
InactiveUS6596279B1SsRNA viruses negative-senseSsRNA viruses positive-senseFeline immunodeficiency virusCtl epitope
Attenuated recombinant viruses containing DNA encoding an immunodeficiency virus and / or CTL antigen, as well as methods and compositions employing the viruses, expression products therefrom, and antibodies generated from the viruses or expression products, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: HIV1gag(+pro)(IIIB), gp120(MN)(+transmembrane), nef(BRU)CTL, pol(IIIB)CTL, ELDKWA or LDKW epitopes, preferably HIV1gag(+pro)(IIIB), gp120(MN) (+transmembrane), two (2) nef(BRU)CTL and three (3) pol(IIIB)CTL etpitopes; or two ELDKWA in gp120 V3 or another region or in gp160. The two (2) nef(BRU)CTL and three (3) pol(IIIB)CTL epitopes are preferably CTL1, CTL2, pol1, pol2 and pol3. The recombinant viruses and gene products therefrom and antibodies generated by the viruses and gene products have several preventive, therapeutic and diagnostic uses. DNA from the recombinant viruses are useful as probes or, for generating PCR primers or for immunization. Also disclosed and claimed are HIV immunogens and modified gp160 and gp120.
Owner:VIROGENETICS
Convection-enhanced delivery of AAV vectors
InactiveUS20020141980A1Efficient deliveryEfficient expressionBiocideOrganic active ingredientsDiseaseConvection-Enhanced Delivery
Methods of delivering viral vectors, particularly recombinant AAV virions, to the CNS are provided. Also provided are methods of treating Parkinson's Disease.
Owner:GENZYME CORP
Poxvirus-canine distemper virus (CDV) or measles virus recombinants and compositions and methods employing the recombinants
InactiveUS6309647B1Improve securityImprove security levelSsRNA viruses negative-senseSsRNA viruses positive-senseHemagglutininCanine distemper virus Antigen
Attenuated recombinant viruses containing DNA coding for a canine distemper virus antigen or measles M or N antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: canine distemper virus fusion protein, canine distemper virus hemagglutinin glycoprotein, canine distemper nucleocaspid protein, canine distemper matrix protein, measles virus nucleocaspid protein, and measles virus matrix protein. The recombinant viruses and gene products therefrom are useful for eliciting protection against canine distemper virus and / or measles virus, and, the gene products and antibodies elicited thereby are useful in assays. Additionally, DNA from the recombinants is used for probes or for generating PCR primers.
Owner:AVENTIS PASTEUR LTD
Novel class of therapeutic protein based molecules
ActiveUS20050112751A1Prevent and inhibit adhesion and functionInhibitory responseAntibacterial agentsSenses disorderTherapeutic proteinViral infection
The present invention provides new compositions and methods for preventing and treating pathogen infection. In particular, the present invention provides compounds having an anchoring domain that anchors the compound to the surface of a target cell, and a therapeutic domain that can act extracellularly to prevent infection of a target cell by a pathogen, such as a virus. The present invention also comprises therapeutic compositions having sialidase activity, including protein-based compounds having sialidase catalytic domains. Compounds of the invention can be used for treating or preventing pathogen infection, and for treating and reducing allergic and inflammatory responses. The invention also provides compositions and methods for enhancing transduction of target cells by recombinant viruses. Such compositions and methods can be used in gene therapy.
Owner:ANSUN BIOPHARMA
Methods for removal, purification, and concentration of viruses, and methods of therapy based thereupon
InactiveUS6861001B2Effect clotting timeEasy to zoom inBioreactor/fermenter combinationsBiological substance pretreatmentsSide chainBlood plasma
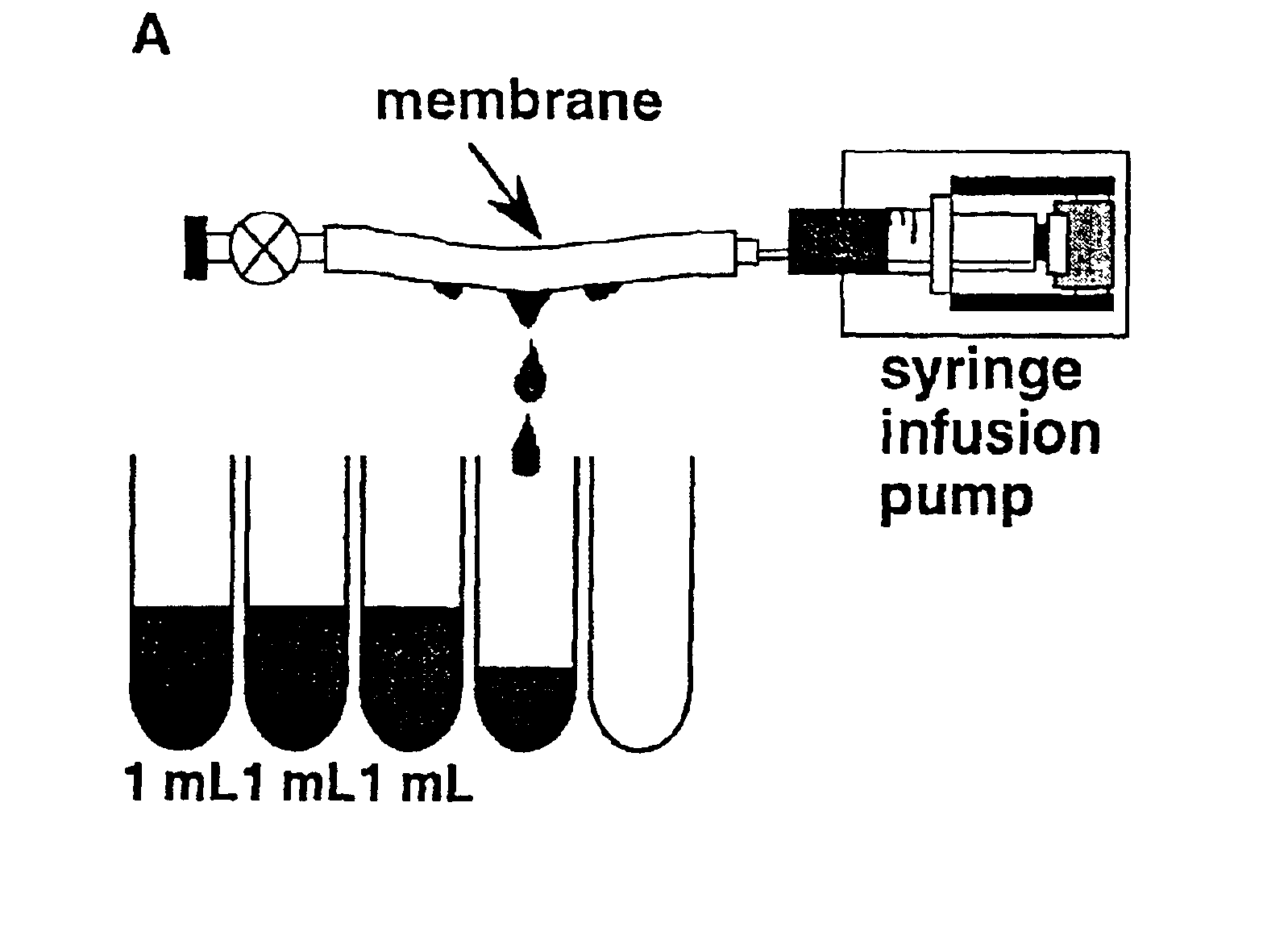
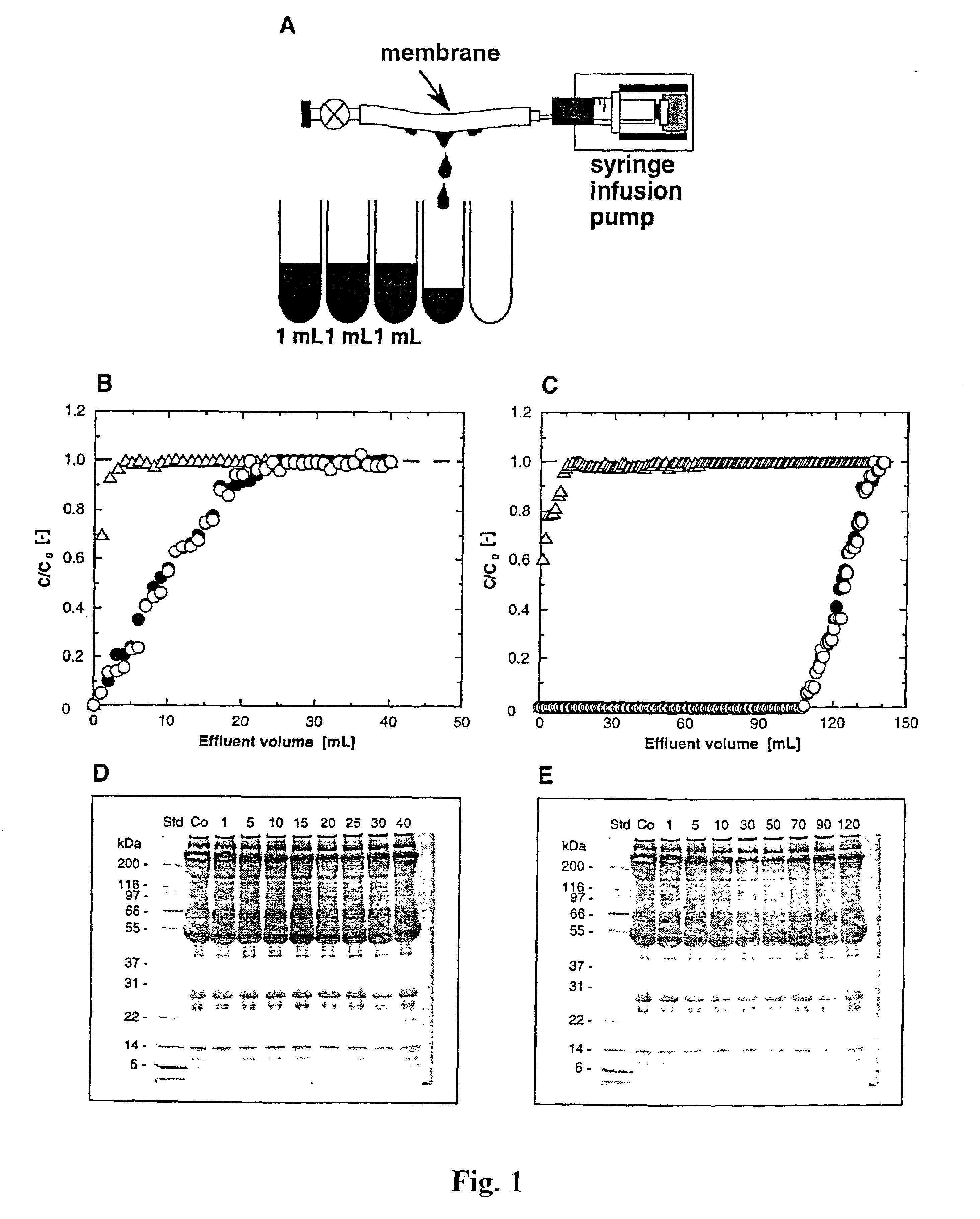
The invention is based on the discovery that certain membranes, which include side chains or molecular “brushes” having, for example, tertiary amino functional groups, can be used as highly effective filters to capture viruses / virus particles from liquids without removal of proteins. New methods based on this discovery include removing viruses from liquids such as blood or plasma, removing viruses from pharmaceuticals, concentrating and / or purifying viruses, e.g., for use in gene therapy, and producing recombinant viruses in new bioreactors. The invention also includes new methods of therapy or adjunct therapy for viral infections, in which a patient's blood or plasma is filtered through the membranes to remove viruses to reduce the viral load. The invention also includes new bioreactors and viral filters containing the membranes.
Owner:THE GENERAL HOSPITAL CORP
Vaccina virus comprising cytokine and/or tumor associated antigen genes
InactiveUS6537594B1Improve securityImprove security levelVirusesPeptide/protein ingredientsHuman tumorWild type
Owner:VIROGENETICS
Production of recombinant AAV virions
ActiveUS7927585B2Simple working processCost effective productionBiocideSugar derivativesYeastVirosome
Stocks of infectious rAAV are generated using yeast strains, bacterial strains, and bacteriophages engineered to express the required AAV proteins and harboring rAAV vector sequences. Stocks of rAAV virions of all serotypes and pseudotypes can be generated in prokaryotic and eukaryotic cells using the methods described herein.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Production of pseudotyped recombinant AAV virions
InactiveUS20070015238A1Highly purified and concentratedEfficient and large-scale productionVectorsTissue culturePurification methodsSerotype
Vectors that encode Adeno-Associated Virus (AAV) Rep and Cap proteins of different serotypes and Adenovirus transcription products that provide helper functions were used to produce pseudotyped recombinant AAV (rAAV) virions. Purification methods generated pseudotyped rAAV virion stocks that were 99% pure with titers of 1×1012−1×1013 vector genomes / ml.
Owner:SNYDER RICHARD O +12
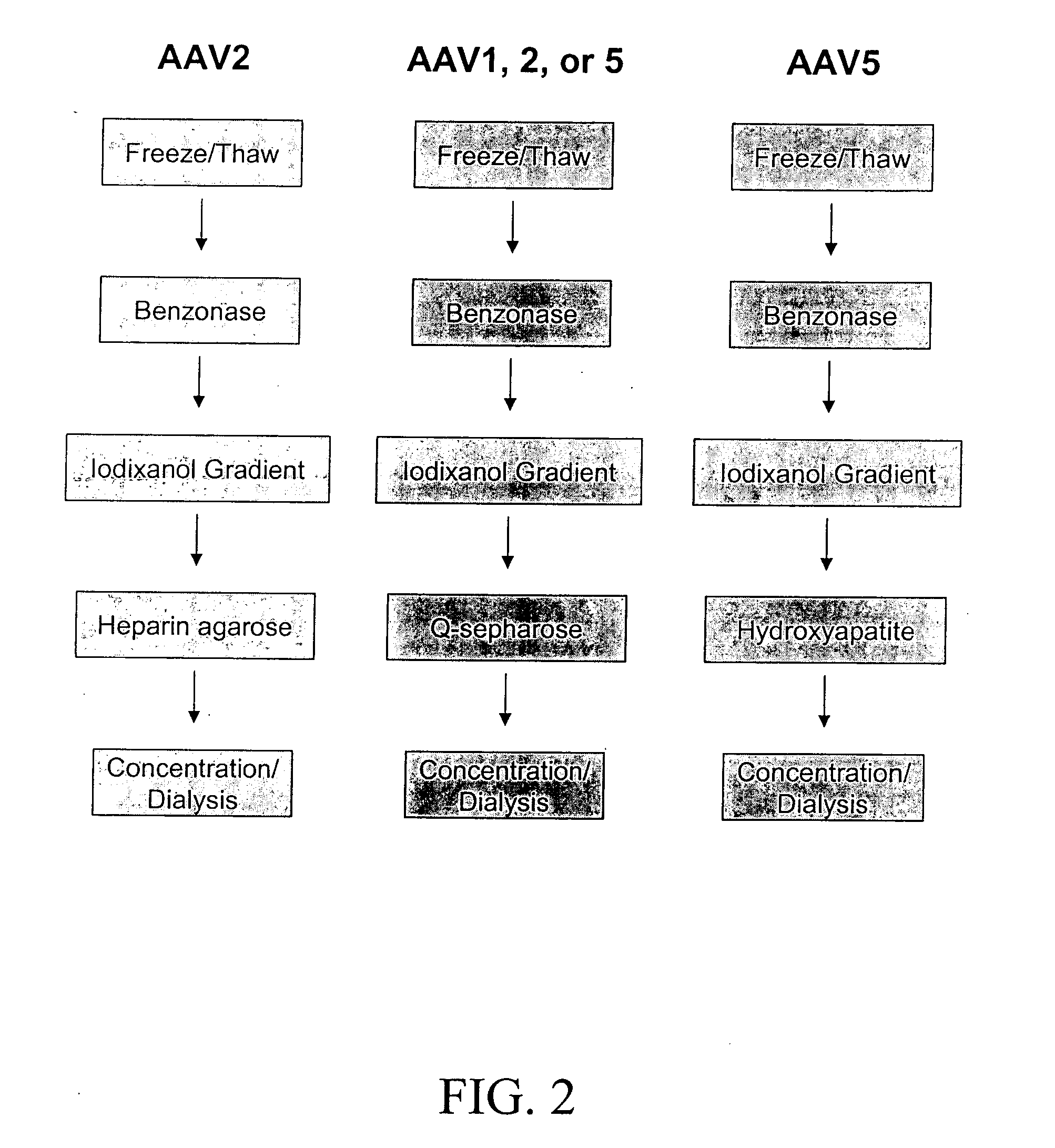
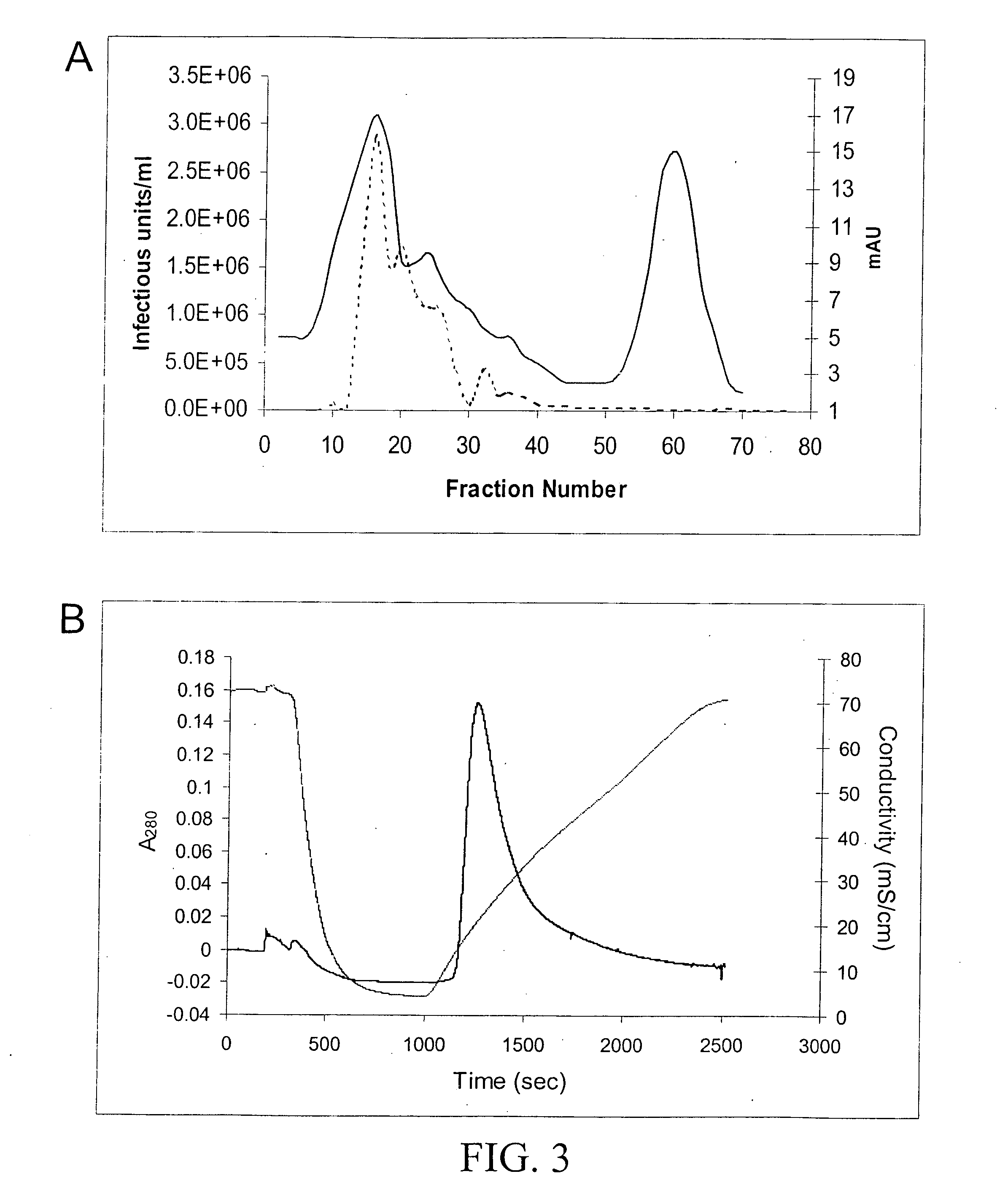
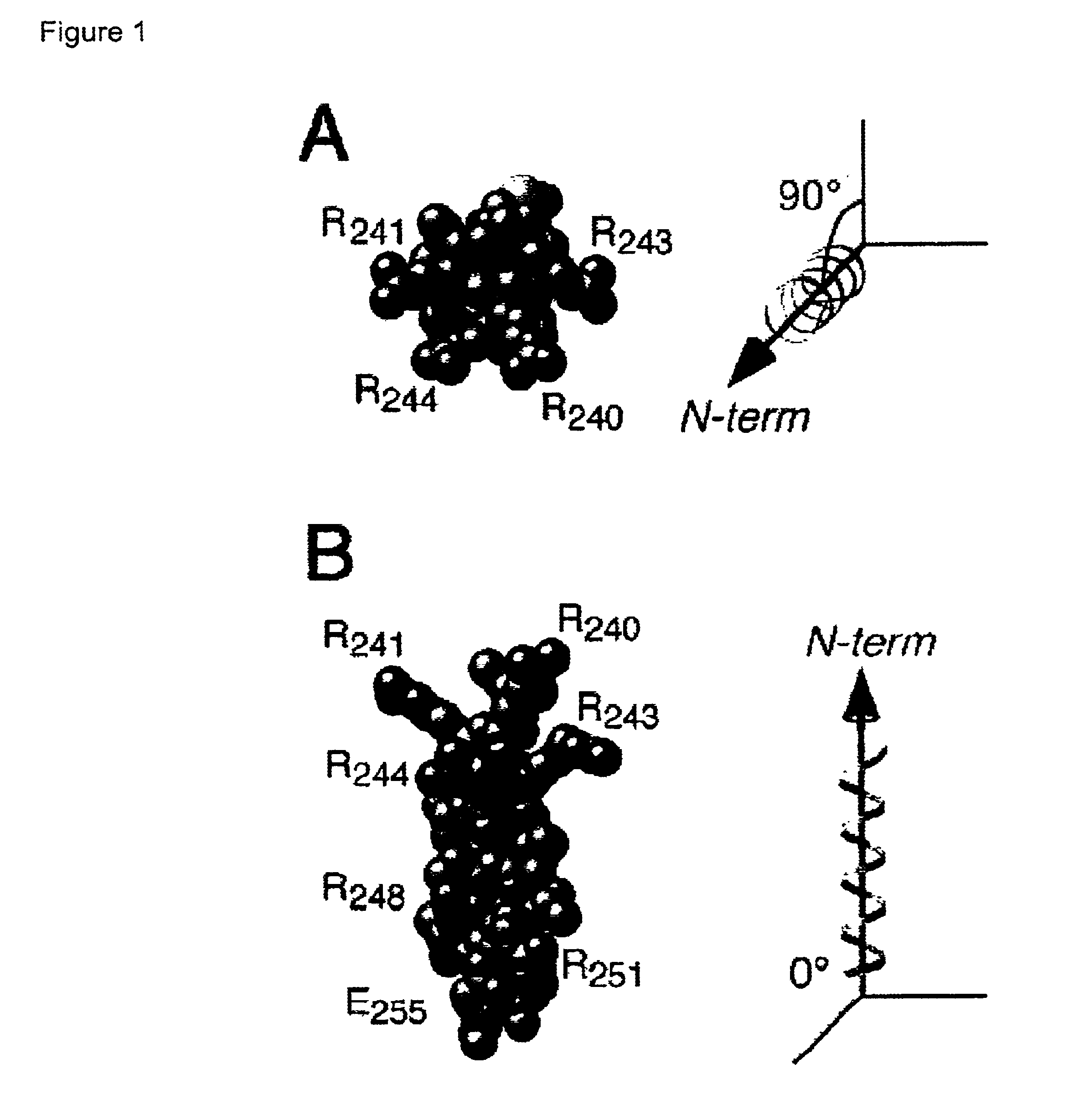
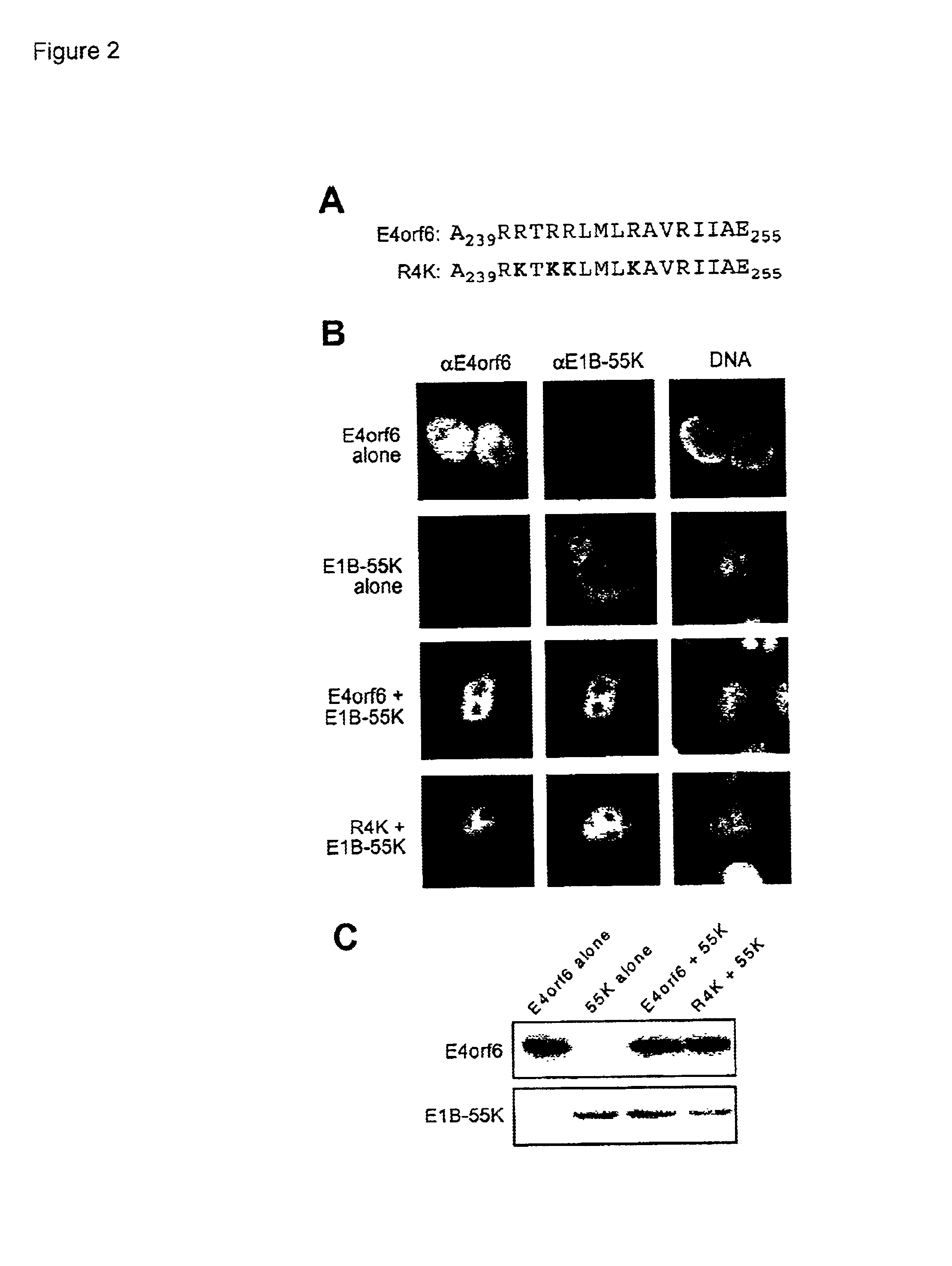
Adenovirus E4 protein variants for virus production
A method of packaging a recombinant viral vector is carried out by: (a) providing a packaging cell, the packaging cell containing and expressing a nucleic acid encoding a mutant adenovirus E4orf6 protein, the E4orf6 protein containing at least one mutation that renders the protein non-toxic to the host cell; (b) transfecting or infecting the packaging cell with a nucleic acid that encodes a recombinant viral vector (e.g., an adenovirus vector or an adeno-associated virus vector), where the vector lacks a functional gene encoding E4orf6 protein; (c) culturing the transfected cells; and then (d) collecting packaged recombinant viral vector from the cultured cells. Nucleic acids, vectors and packaging cells used for carrying out the methods, as well as proteins utilized in the methods, are also described.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Recombinant viral vectors
ActiveUS20130266611A1Escalate trimer abundanceImprove stabilitySsRNA viruses negative-senseViral antigen ingredientsViral vectorImmunogenicity
The present relation relates to recombinant vesicular stomatitis virus for use as prophylactic and therapeutic vaccines for infectious diseases of AIDS. The present invention encompasses the preparation and purification of immunogenic compositions which are formulated into the vaccines of the present invention.
Owner:INT AIDS VACCINE INITIATIVE
Enhanced immune response to an antigen by a composition of a recombinant virus expressing the antigen with a recombinant virus expressing an immunostimulatory molecule
The present invention is a composition of recombinant virus which has incorporated into its genome or portion thereof a gene encoding an antigen to a disease causing agent and a recombinant virus which has incorporated into its genome or portion thereof a gene encoding an immunostimulatory molecule(s) for the purpose of stimulating an immune response against the disease causing agent. Methods of treatment of diseases such as cancer and diseases caused by pathogenic microorganisms is provide using the composition.
Owner:UNITED STATES OF AMERICA
Modified Vaccinia Ankara virus variant and cultivation method
InactiveUS7445924B2Reduce riskGenetic material ingredientsVirus peptidesSerum free mediaModified vaccinia Ankara
The present invention provides an attenuated virus, which is derived from Modified Vaccinia Ankara virus and characterized by the loss of its capability to reproductively replicate in human cell lines. It further describes recombinant viruses derived from this virus and the use of the virus, or its recombinants, as a medicament or vaccine. A method is provided for inducing an immune response in individuals who may be immune-compromised, receiving antiviral therapy, or have a pre-existing immunity to the vaccine virus. In addition, a method is provided for the administration of a therapeutically effective amount of the virus, or its recombinants, in a vaccinia virus prime / vaccinia virus boost innoculation regimen. The present invention relates to a method of virus amplification in primary cells which are cultivated in a serum free medium. Viruses produced by this method are advantageously free of any infectious agents comprised in animal sera.
Owner:BAVARIAN NORDIC AS
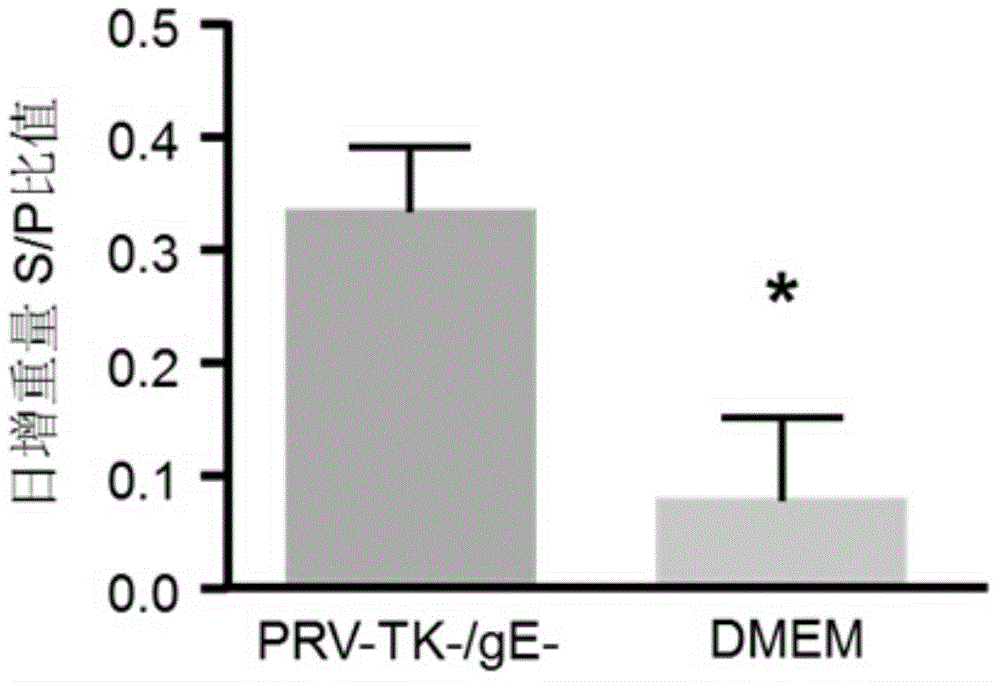
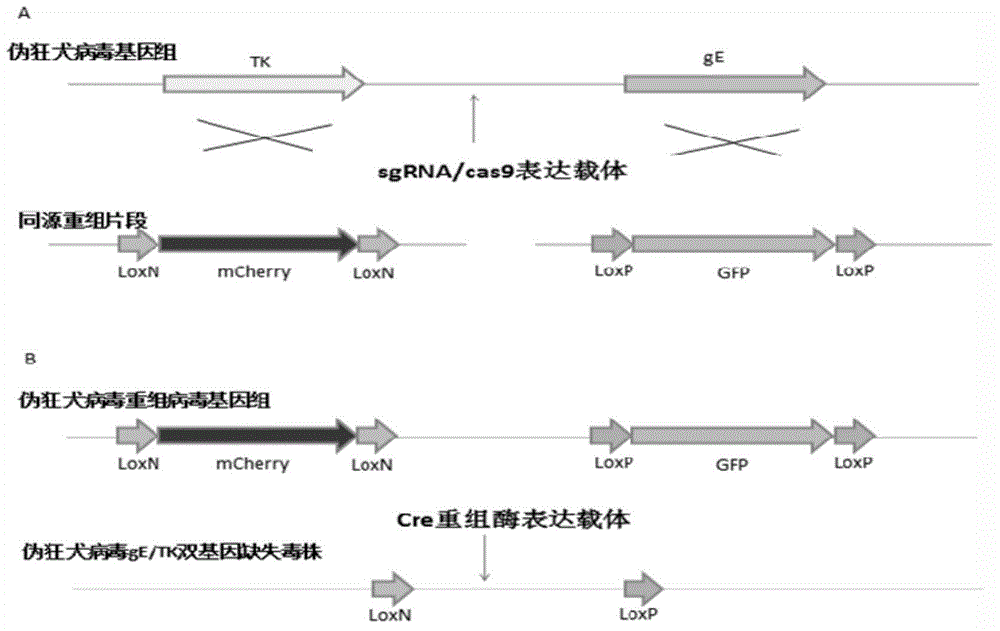
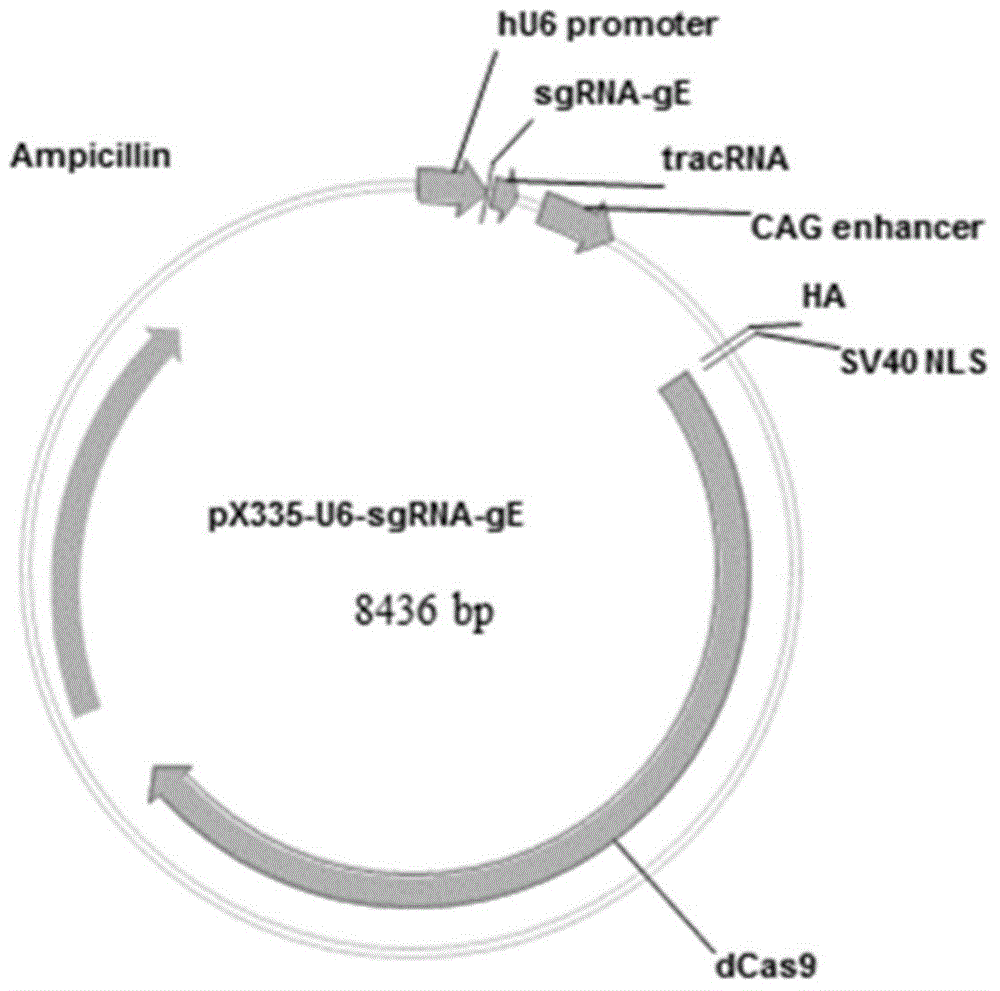
Method for preparing vaccine by editing pseudorabies virus genomes based on CRISPR/Cas9 and Cre/lox systems and application of method
ActiveCN104894075AReduce disease lossEasy to operateAntiviralsViruses/bacteriophagesMCherry fluorescent proteinBiology
The invention discloses a method for quickly preparing a vaccine by editing pseudorabies virus genomes based on a CRISPR / Cas9 gene editing system and a Cre / lox recombination system and an application of the method. According to the method, the CRISPR / Cas9 gene editing system is used for synchronously and efficiently recombining a GFP gene and a mCherry gene to a pseudorabies virus gE gene site and a TK gene site respectively to obtain conditional deletion strains of a gE gene and a TK gene; after purification, the Cre / lox system is used for cutting off extraneous GFP and mCherry genes in the pseudorabies virus recombinant virus genome so as to perform purification quickly to obtain a live pseudorabies virus vaccine lack of gE / TK genes; multiple genes are operated at the same time, so that multiple rounds of flows for knocking out multiple genes in the conventional method are reduced to one round; and meanwhile, the efficient edition of virus genes by using the CRISPR / Cas9 and Cre / lox systems simplifies about thirty generations of plaque purification processes into 3-4 generations, so that the preparation efficiency of the virus vaccine is greatly improved, and the method provides a strong guarantee for effectively preventing and controlling the larger-range popularization of variant pseudorabies viruses and reducing heavy economic losses.
Owner:武汉都为康生物科技有限公司
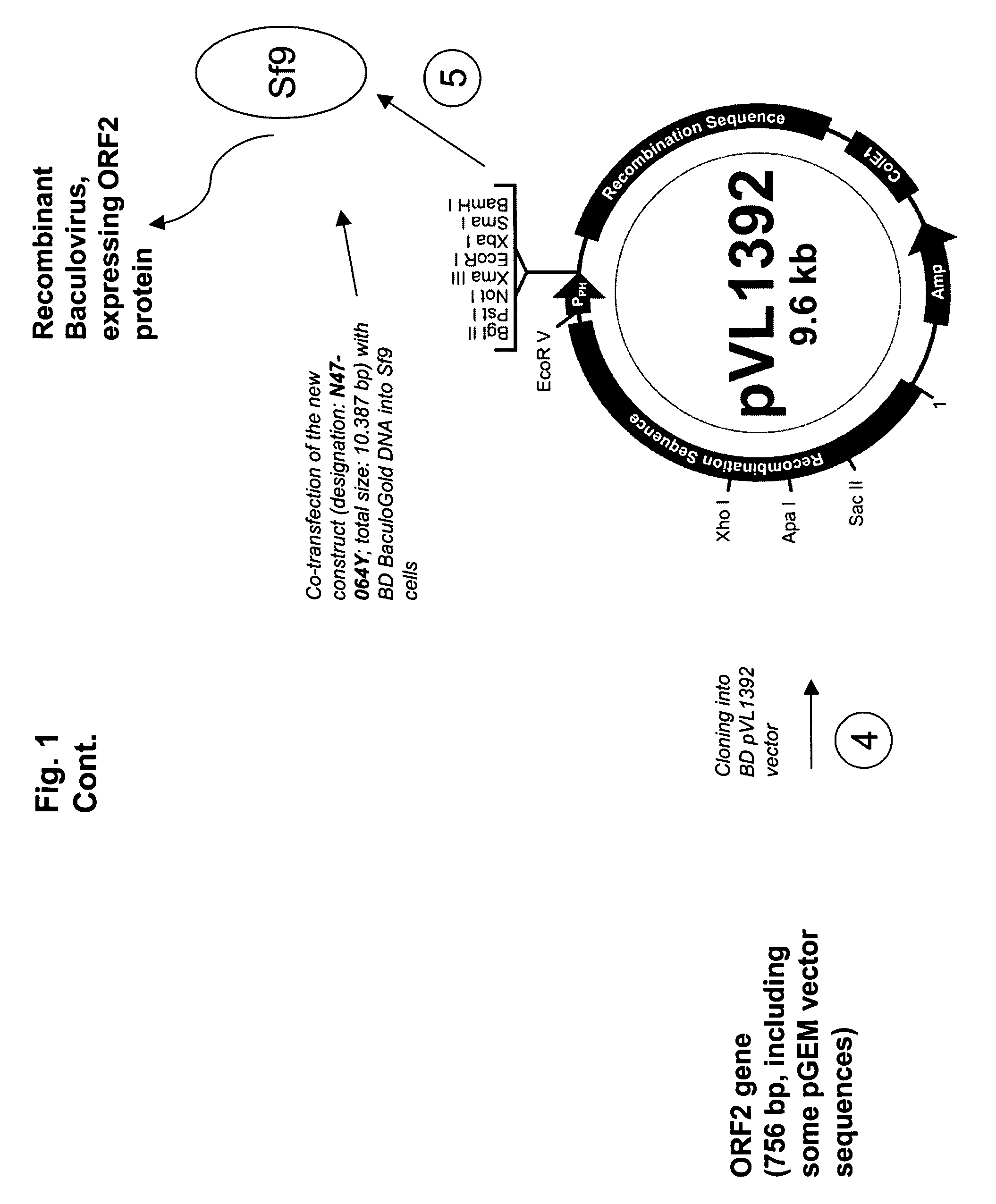
Methods of overexpression and recovery of porcine circovirus type 2 ORF2
ActiveUS20090042245A1SsRNA viruses positive-senseInorganic active ingredientsOpen reading frameIntracellular
An improved method for recovering the protein expressed by open reading frame 2 from PCV2 is provided. The method generally involves the steps of transfecting recombinant virus containing open reading frame 2 coding sequences into cells contained in growth media, causing the virus to express open reading frame 2, and recovering the expressed protein in the supernate. This recovery should take place beginning approximately 5 days after infection of the cells in order to permit sufficient quantities of recombinant protein to be expressed and secreted from the cell into the growth media. Such methods avoid costly and time consuming extraction procedures required to separate and recover the recombinant protein from within the cells.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
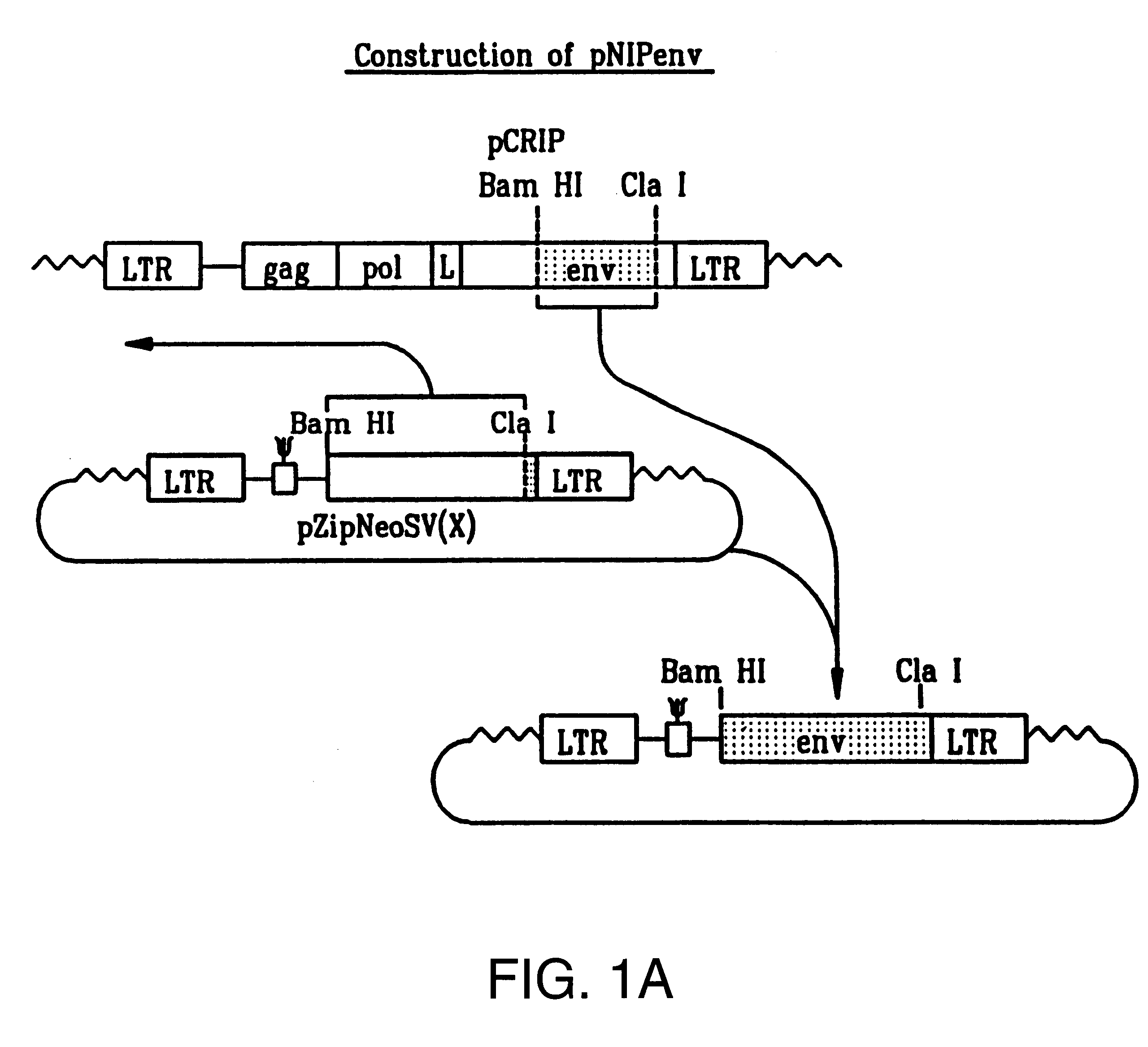
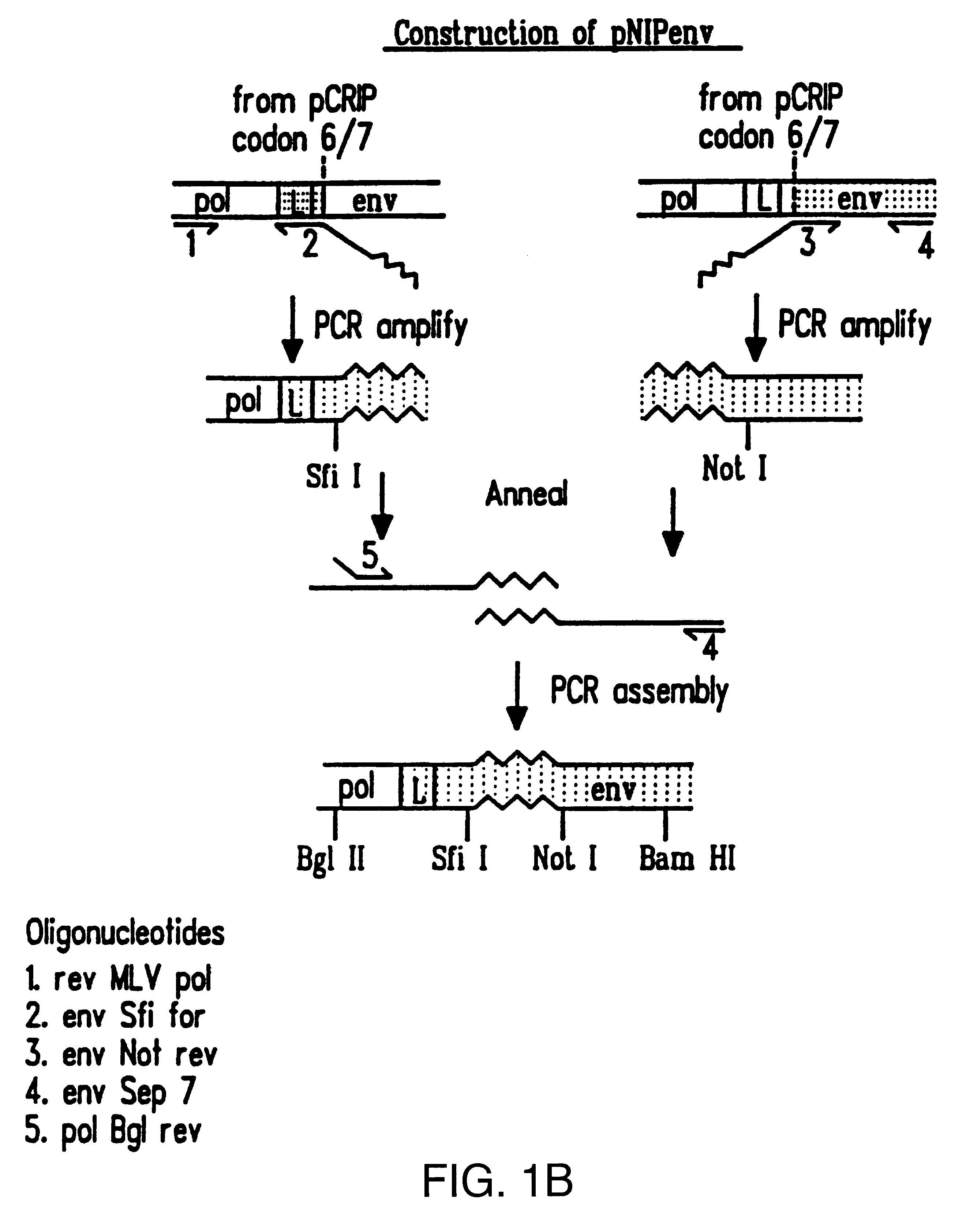
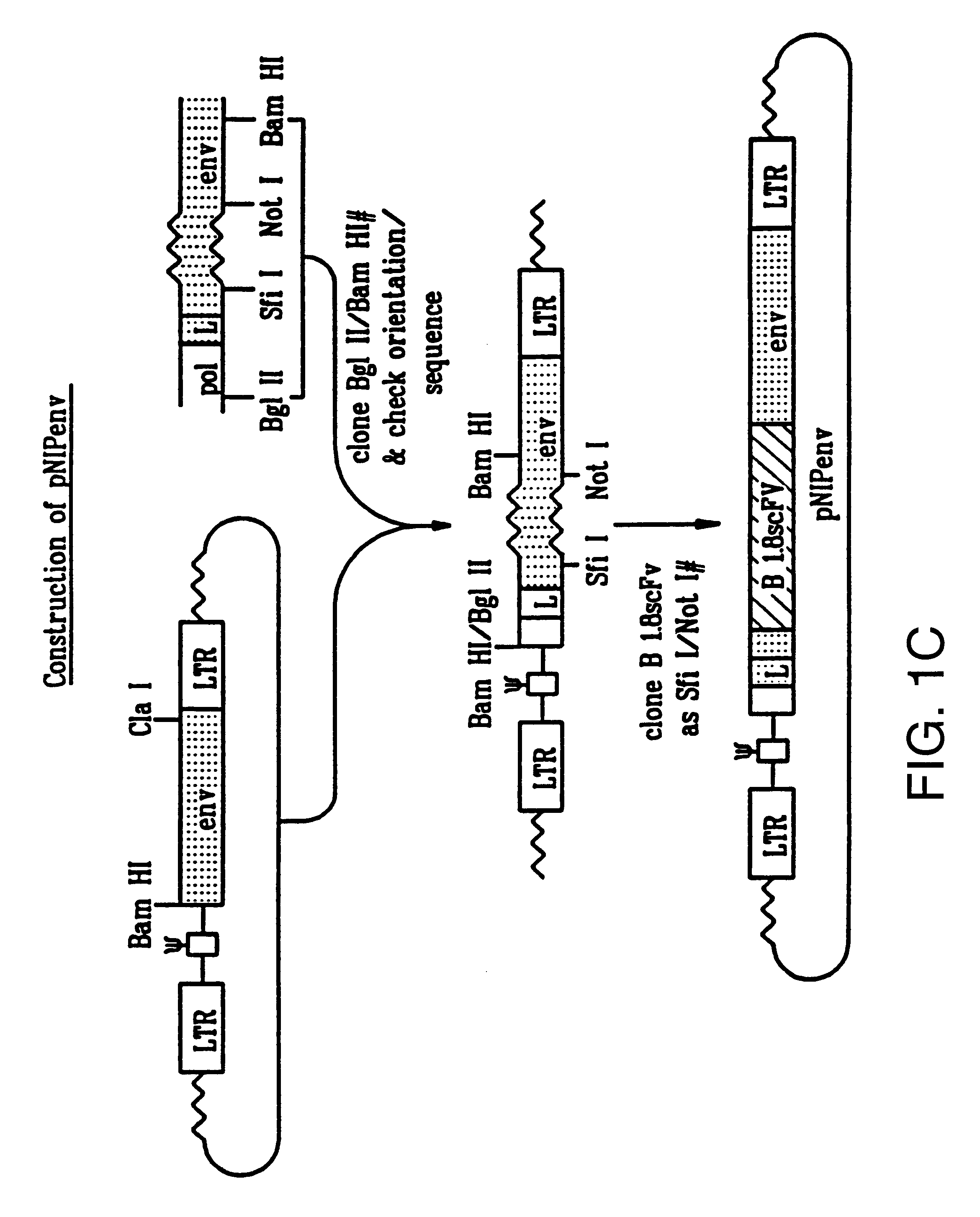
Recombinant viruses displaying a nonviral polypeptide on their external surface
InactiveUS6297004B1Genetic material ingredientsImmunological disordersGene deliveryAntibody fragments
We have made retrovirus particles displaying a functional antibody fragment. We fused the gene encoding an antibody fragment directed against a hapten with that encoding the viral envelope protein (Pr80env) of the ecotropic Moloney murine leukemia virus. The fusion gene was co-expressed in ecotropic retroviral packaging cells with a retroviral plasmid carrying the neomycin phosphotransferase gene (neo), and retroviral particles with specific hapten biding activities were recovered. Furthermore the hapten-binding particles were able to transfer the neo gene and the antibody-envelope fusion gene to mouse fibroblasts. In principle, the display of antibody fragments on the surface of recombinant retroviral particles could be used to target virus to cells for gene delivery, or to retain the virus in target tissues, or for the construction of libraries of viral display packages.
Owner:BIOFOCUS DICOVERY
Metapneumovirus strains and their use in vaccine formulations and as vectors for expression of antigenic sequences and methods for propagating virus
ActiveUS20050019891A1Narrow downSymptoms improvedSsRNA viruses negative-senseVirus peptidesNegative strandHeterologous
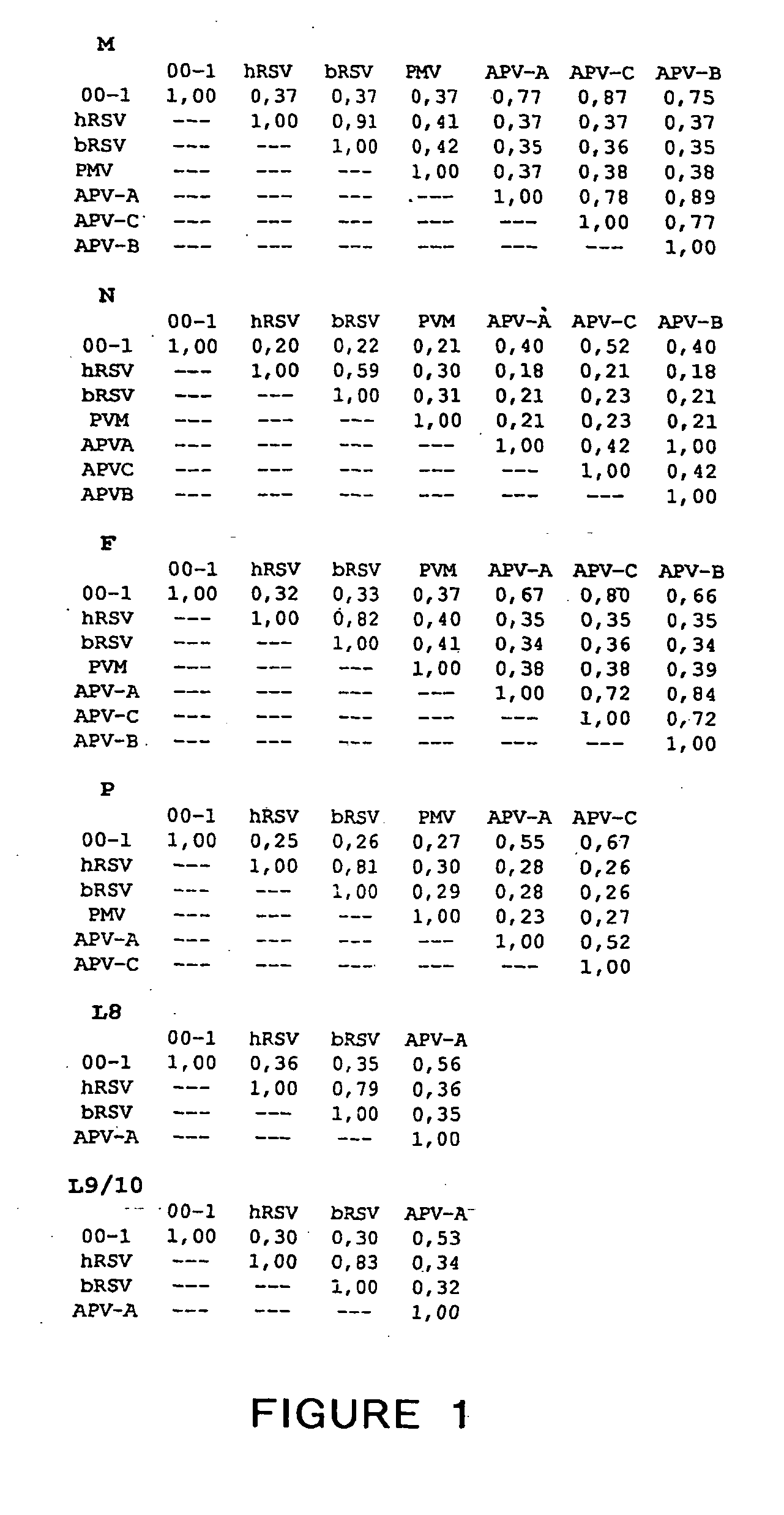
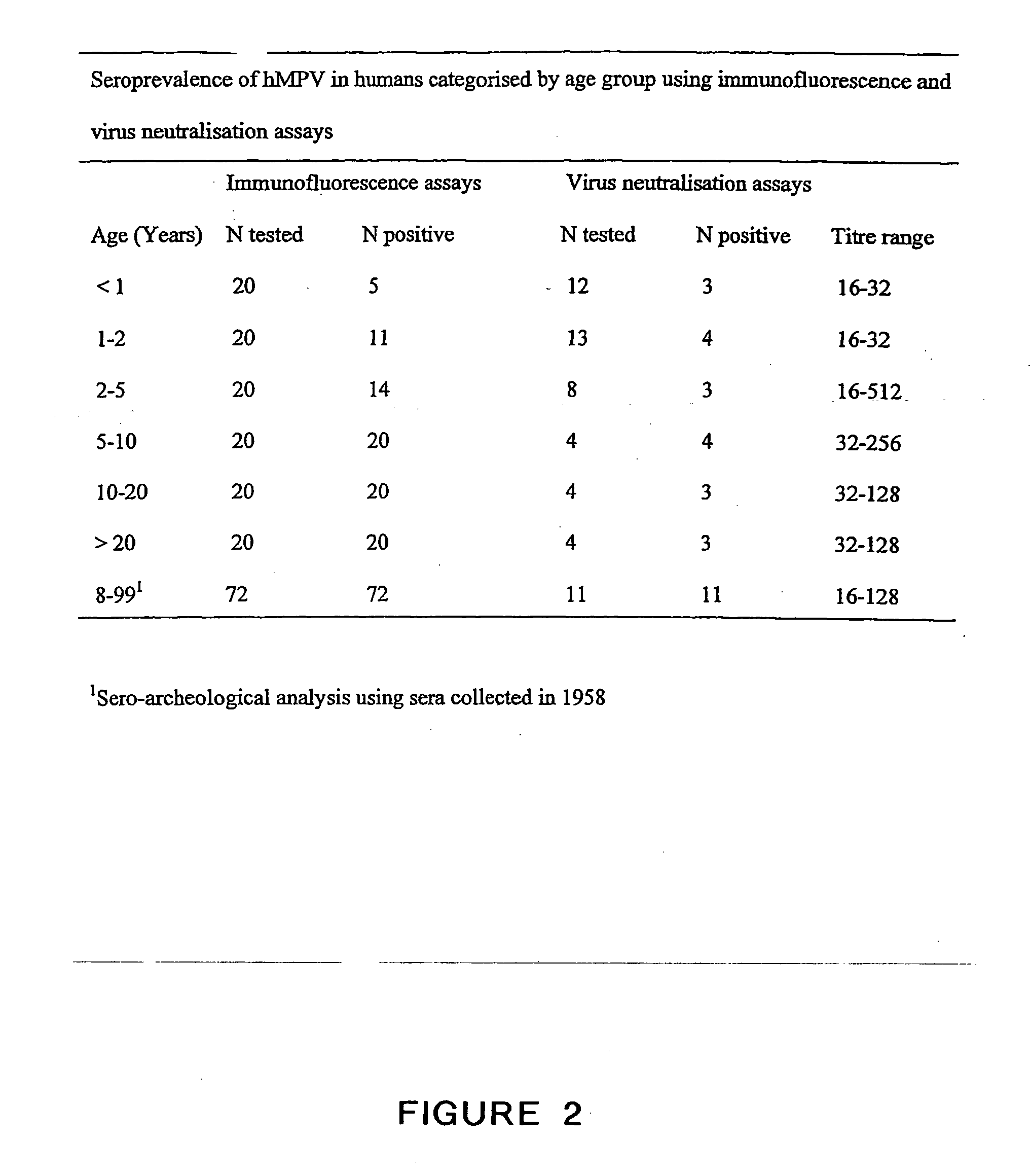
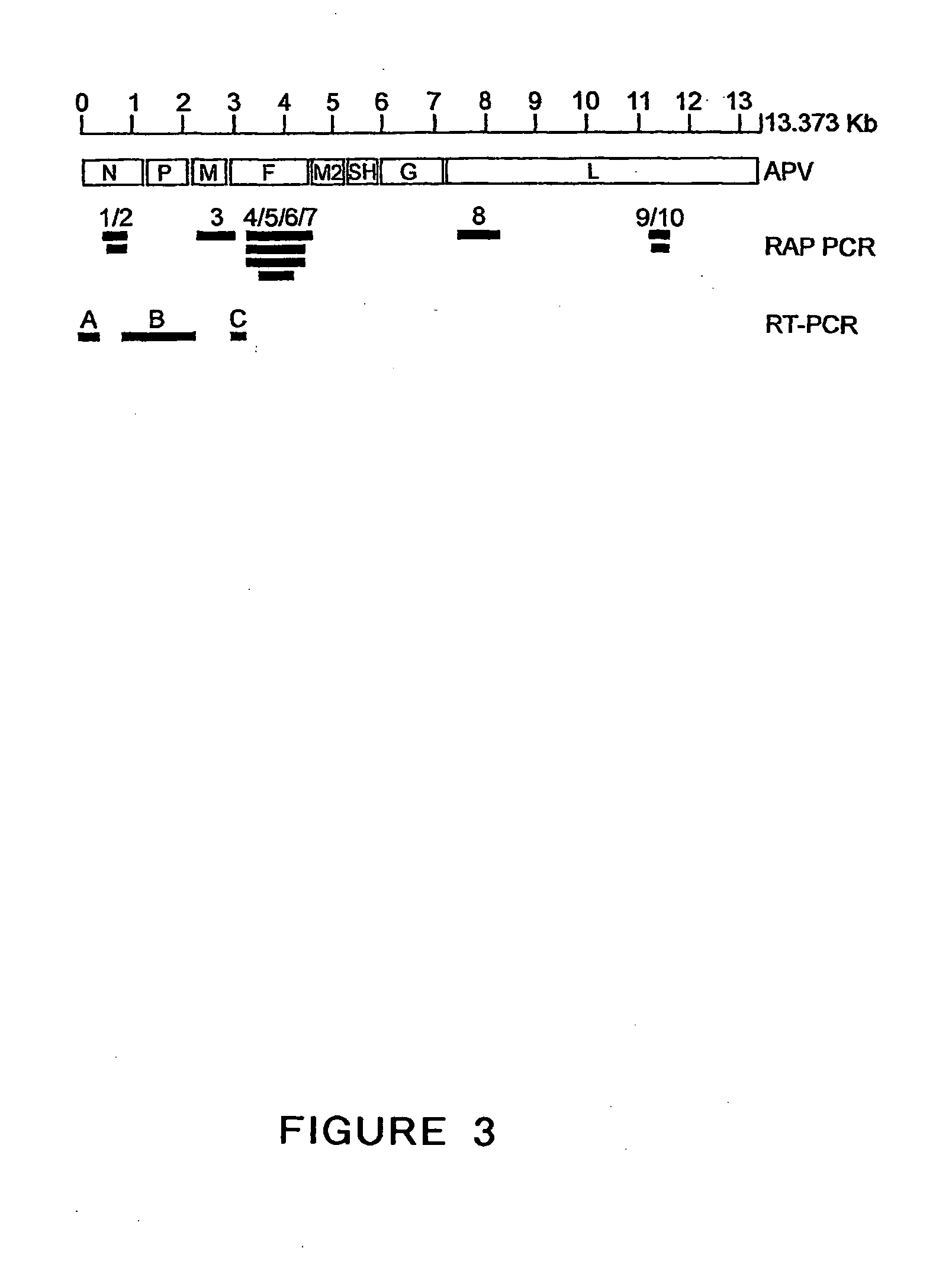
The present invention provides an isolated mammalian negative strand RNA virus, metapneumovirus (MPV), within the sub-family Pneumoviridae, of the family Paramyxoviridae. The invention also provides isolated mammalian negative strand RNA viruses identifiable as phylogenetically corresponding or relating to the genus Metapneumovirus and components thereof. In particular the invention provides a mammalian MPV, subgroups and variants thereof. The invention relates to genomic nucleotide sequences of different isolates of mammalian metapneumoviruses, in particular human metapneumoviruses. The invention relates to the use of the sequence information of different isolates of mammalian metapneumoviruses for diagnostic and therapeutic methods. The present invention relates to nucleotide sequences encoding the genome of a metapneumovirus or a portion thereof, including both mammalian and avian metapneumovirus. The invention further encompasses chimeric or recombinant viruses encoded by said nucleotide sequences. The invention also relates to chimeric and recombinant mammalian MPV that comprise one or more non-native or heterologous sequences. The invention further relates to vaccine formulations comprising mammalian or avian metapneumovirus, including recombinant and chimeric forms of said viruses. The vaccine preparations of the invention encompass multivalent vaccines, including bivalent and trivalent vaccine preparations. The invention also provide methods for propagating virus.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
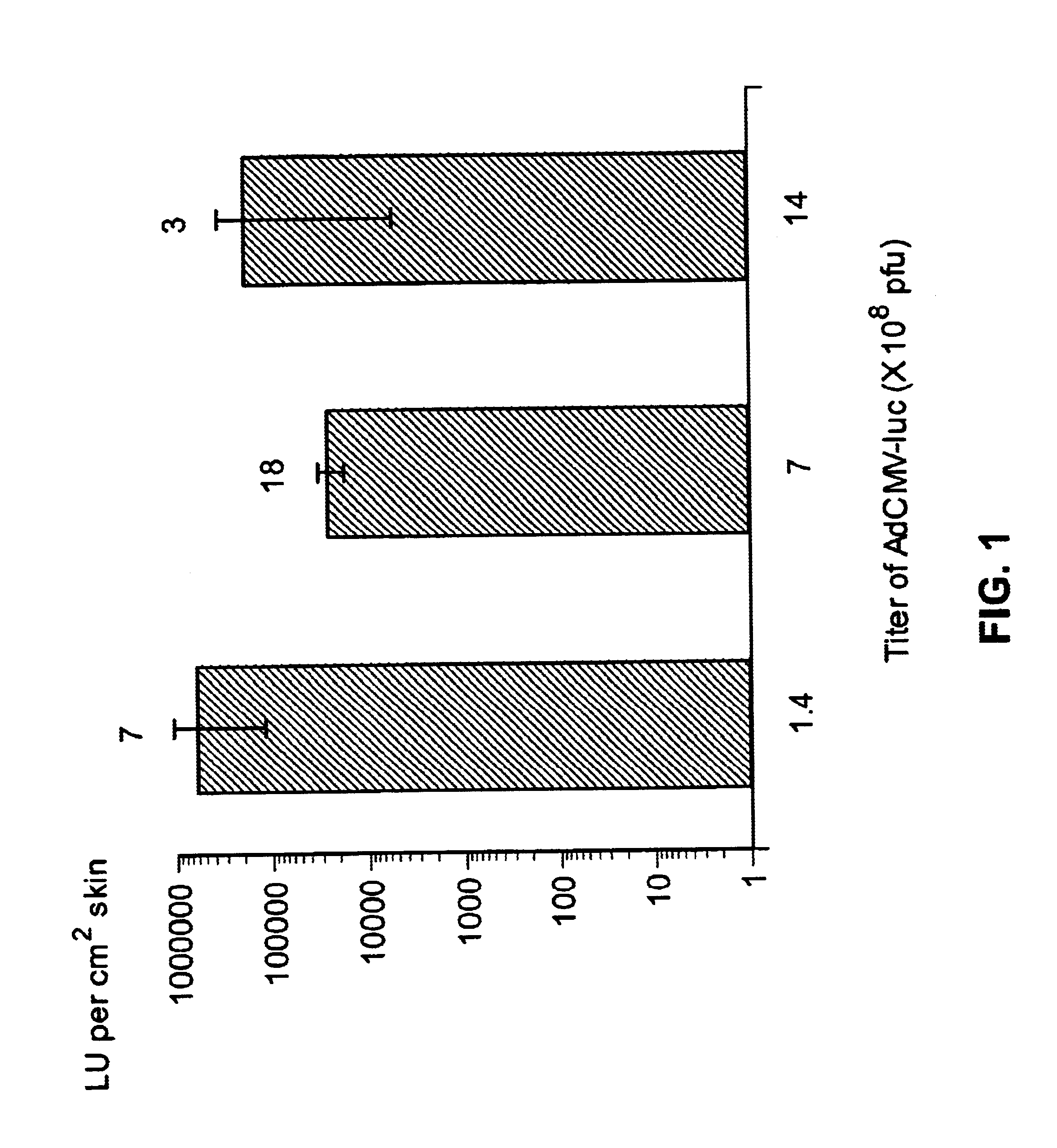
Vaccination by topical application of recombinant vectors
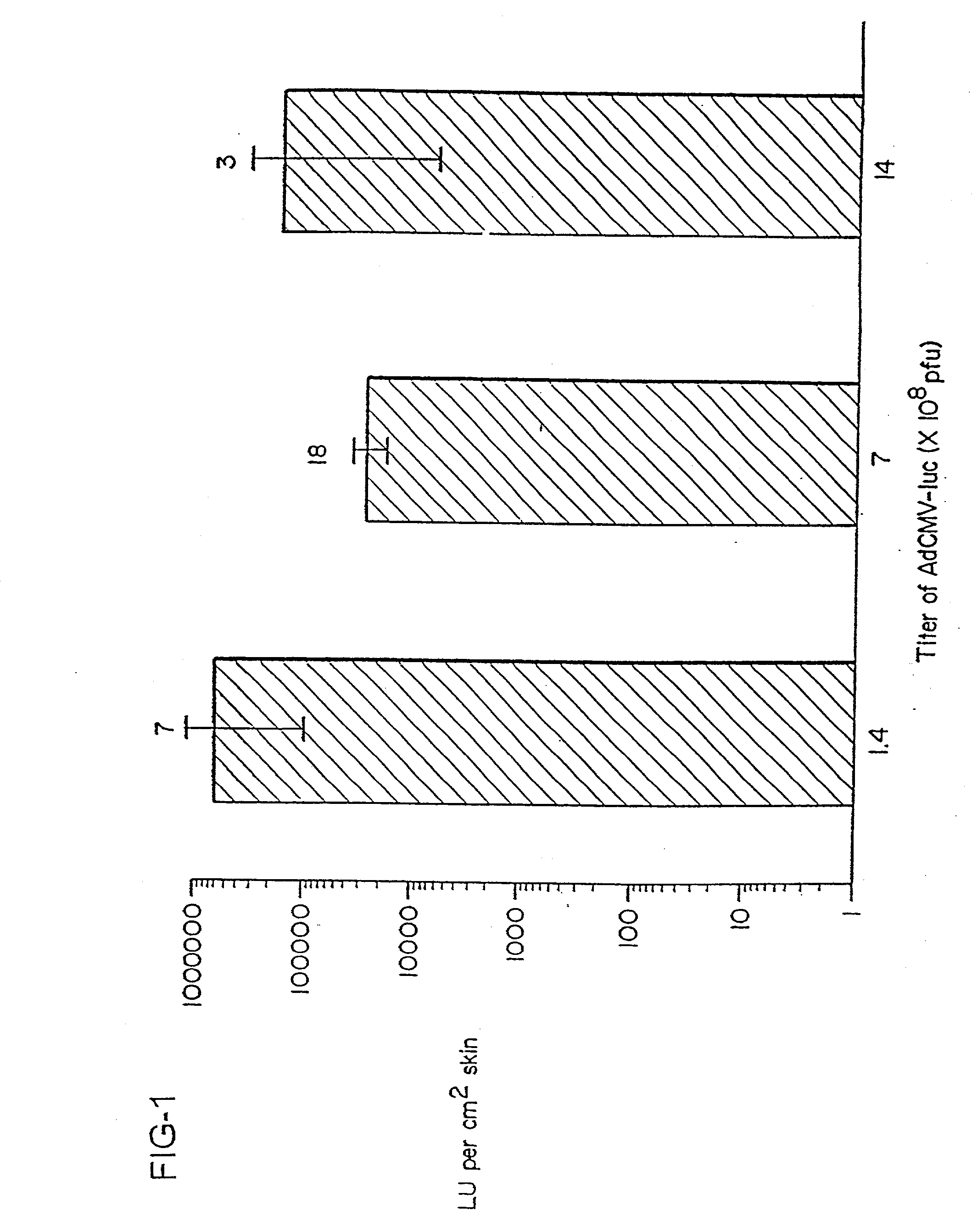
InactiveUS20030045492A1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseGenetic material ingredientsGene deliveryVaccination
The present invention relates to techniques of skin-targeted non-invasive gene delivery to elicit immune responses and uses thereof. The invention further relates to methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal following topical application of vectors, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal as well as such a method further including disposing the vector in and / or on the delivery device. The vector can be gram negative bacteria, preferably Salmonella and most preferably Salmonella typhimurium.
Owner:UAB RES FOUND
Optimization of gene sequences of chimeric virus-like particles for expression in insect cells
InactiveUS20050118191A1Minimize the numberSequence minimizedAnimal cellsViral antigen ingredientsDiagnostic testTGE VACCINE
Owner:NOVAVAX
Pox virus comprising DNA sequences encoding CEA and B7 antigen
Attenutated recombinant viruses containing DNA coding for a cytokine and / or a tumor associated antigen, as well as methods and compositions employing the viruses, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: human tumor necrosis factor; nuclear phosphoprotein p53, wiltype or mutant; human melanoma-associated antigen; IL-2; IFNgamma; IL-4; GMCSF; IL-12; B7; erb-B-2 and carcinoembryonic antigen. The recombinant viruses and gene products therefrom are useful for cancer therapy.
Owner:AVENTIS PASTEUR LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com