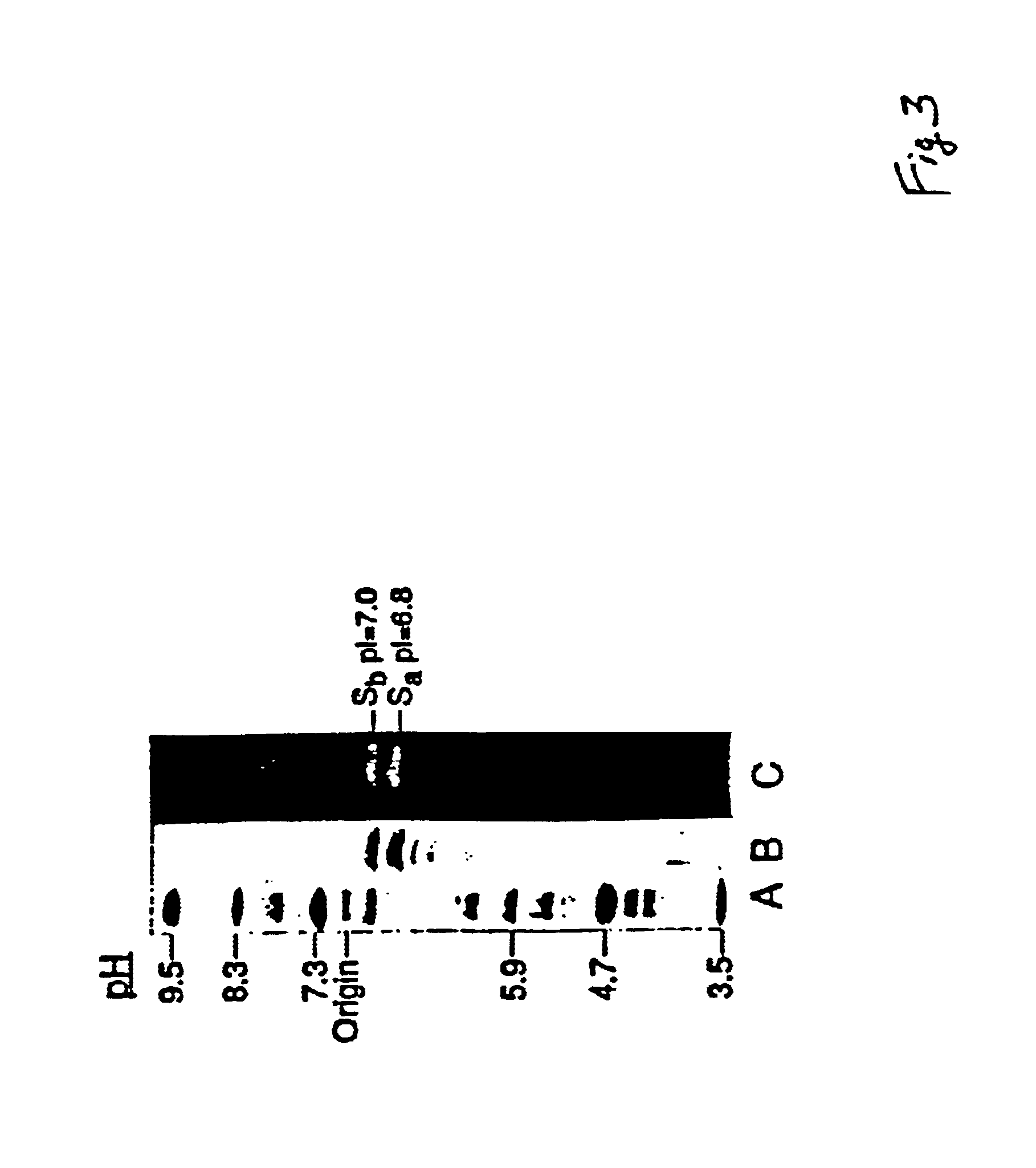
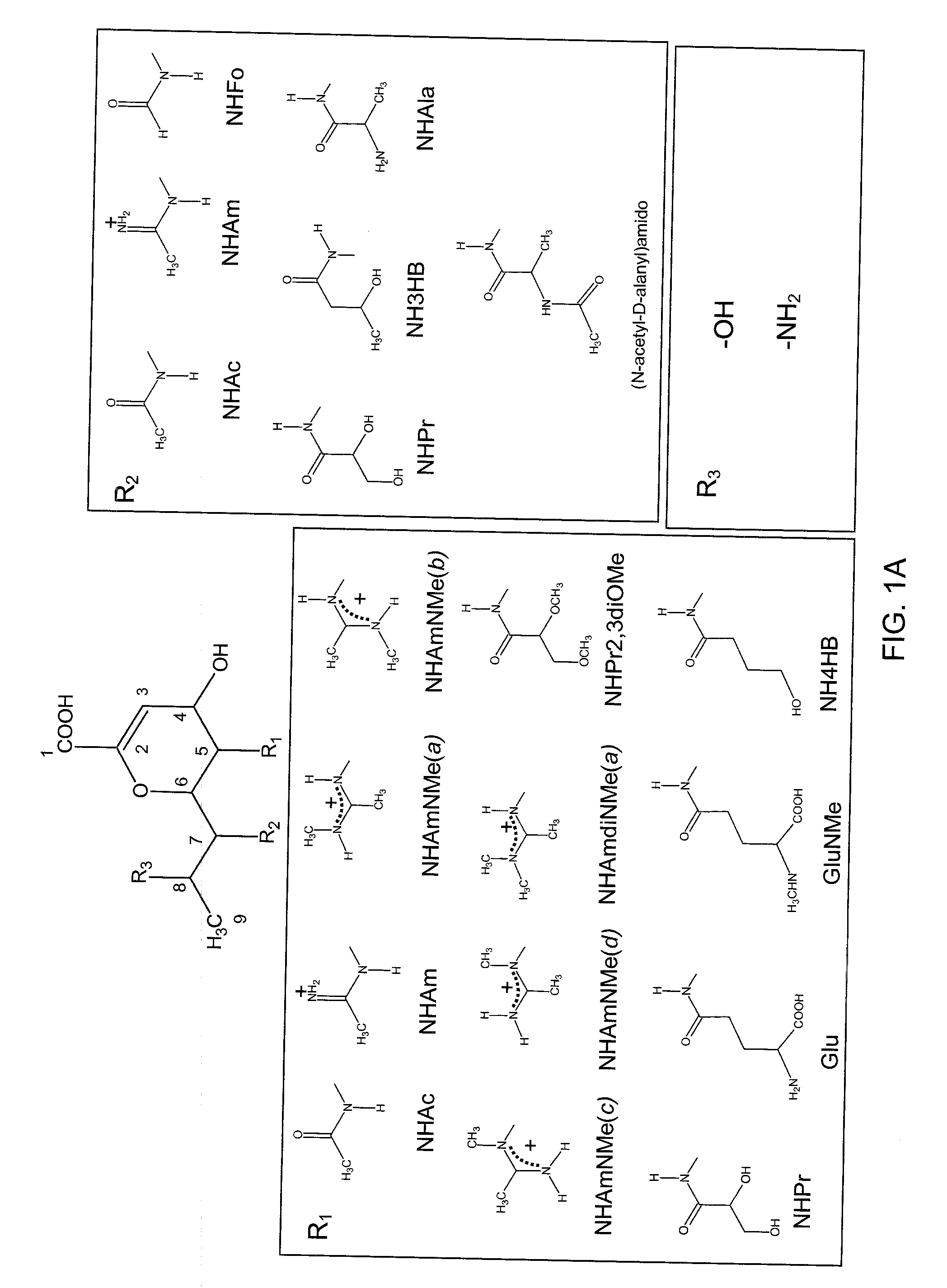
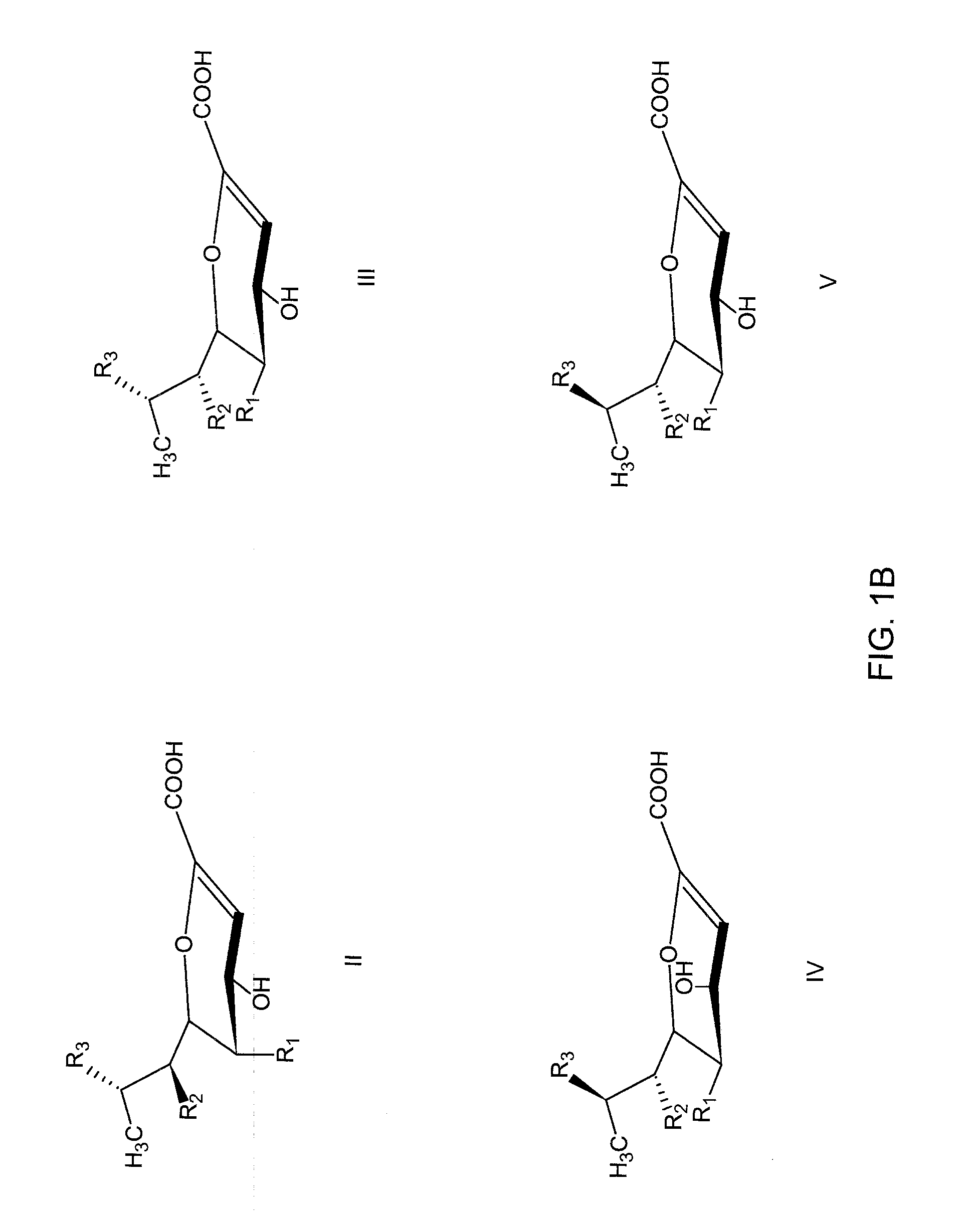
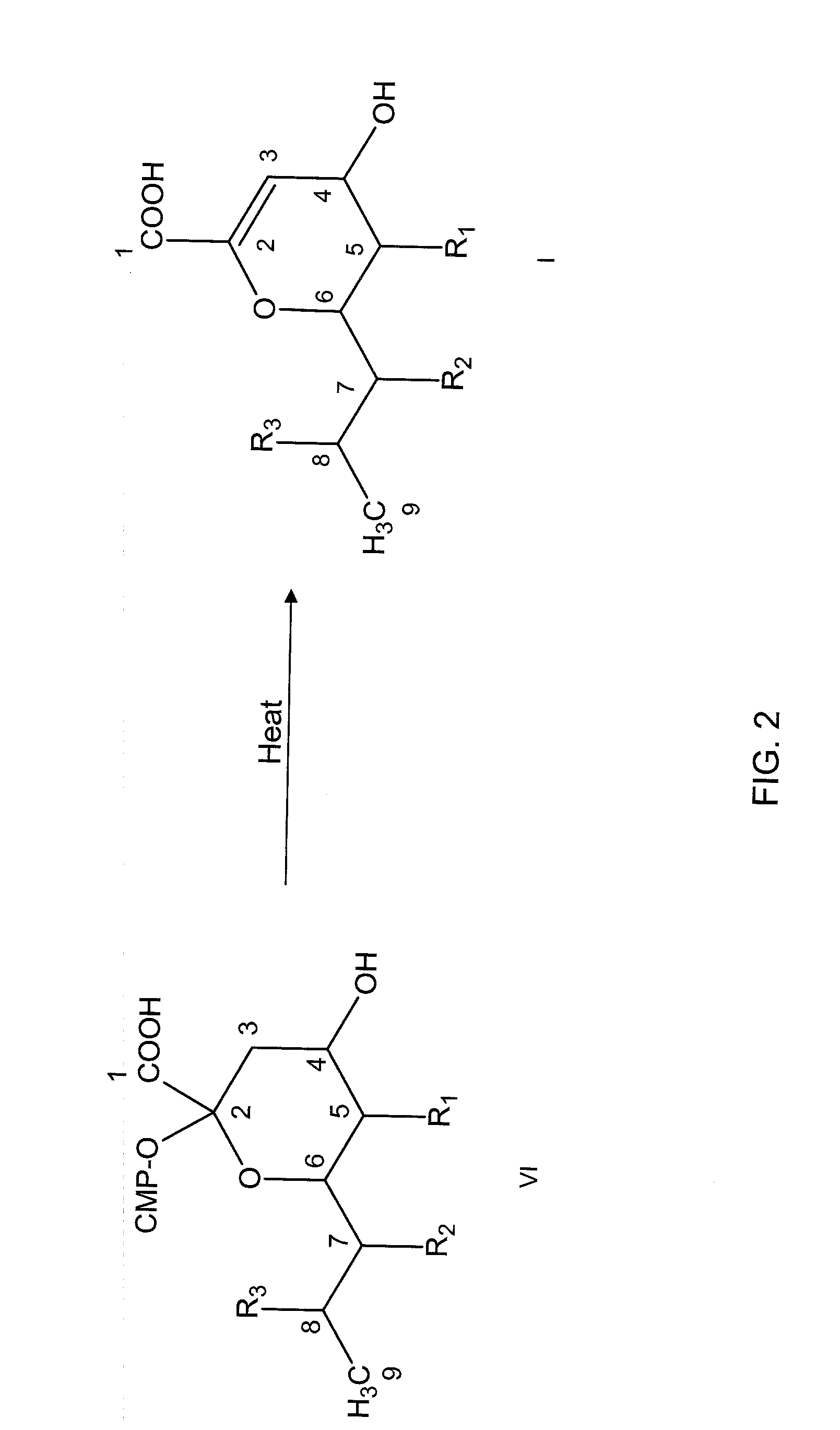
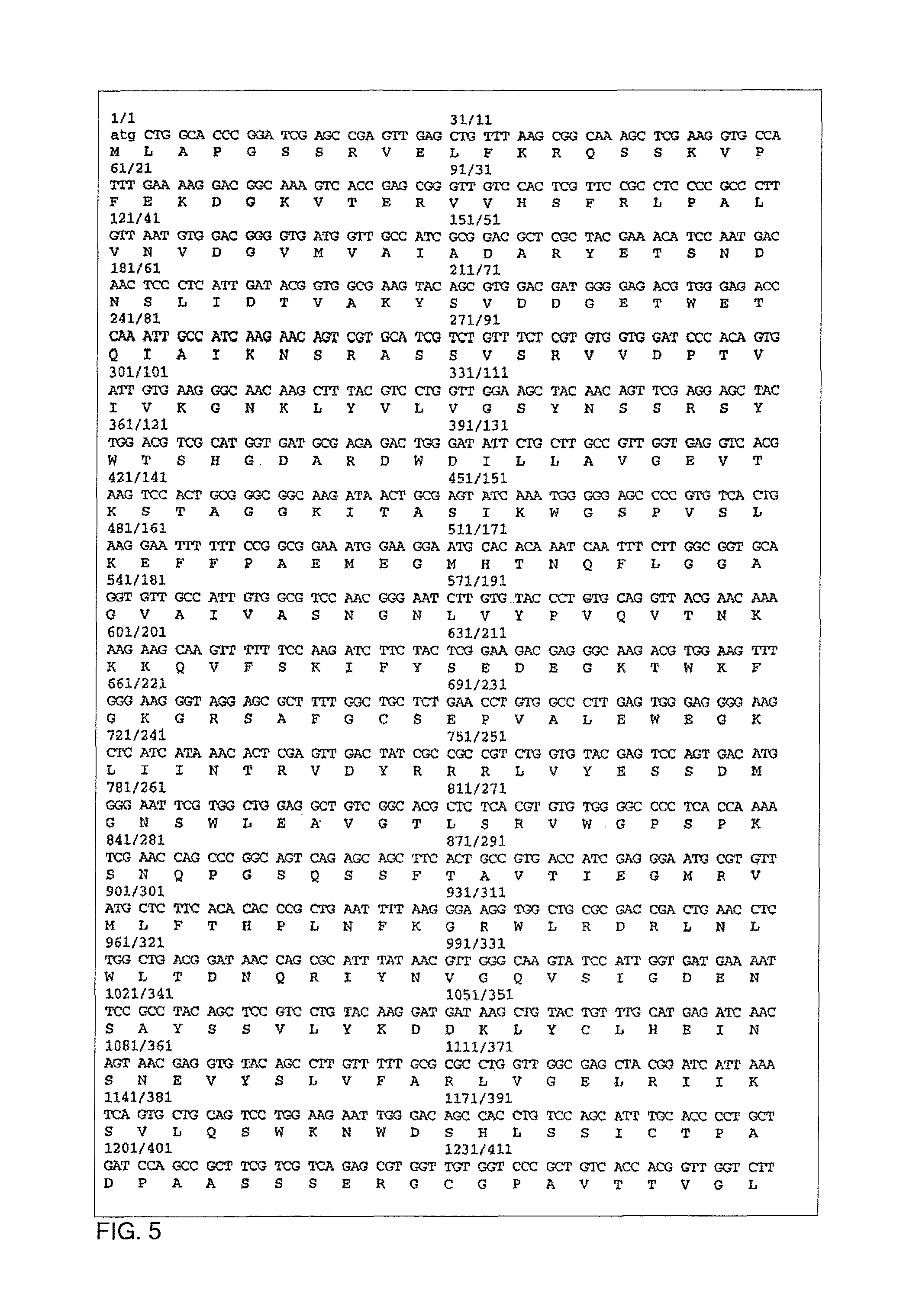
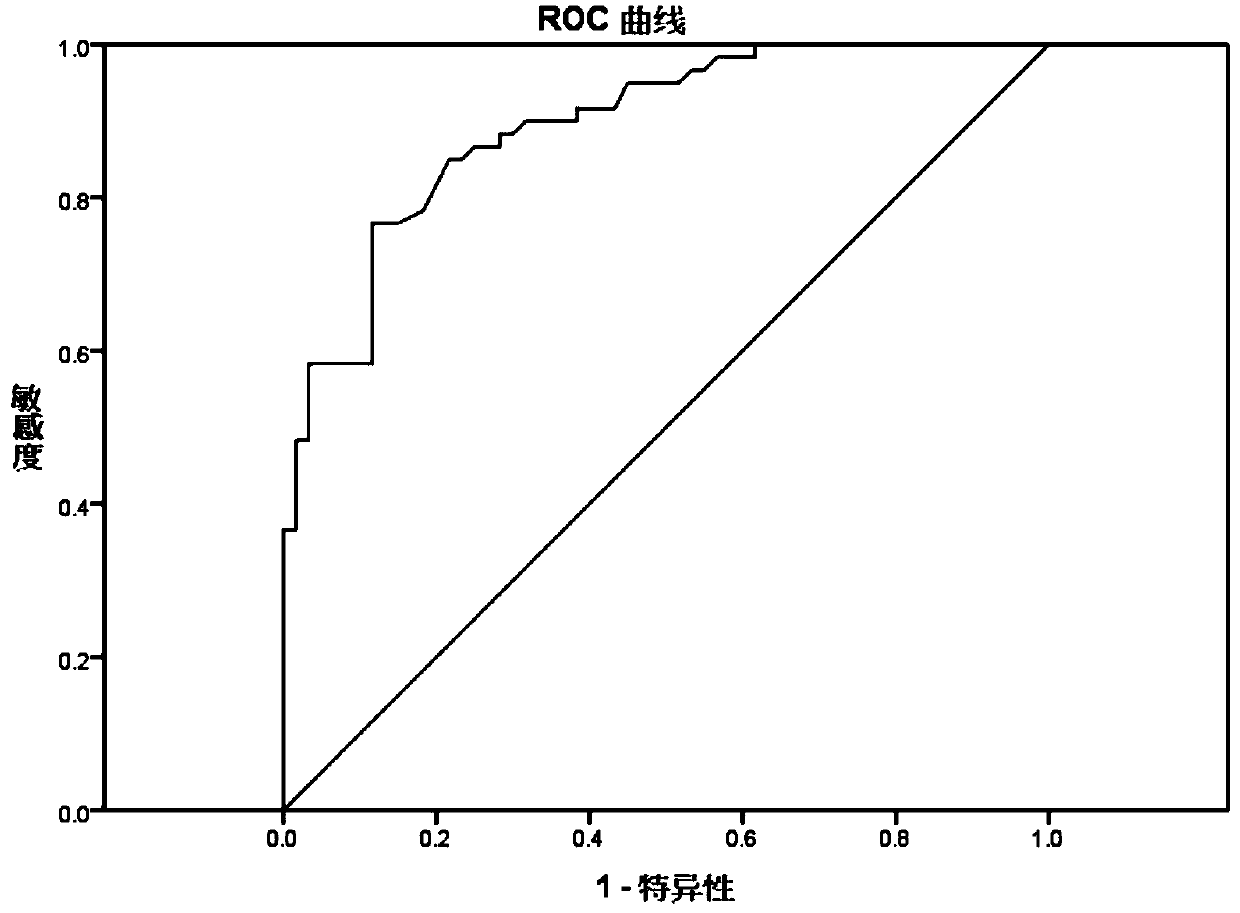
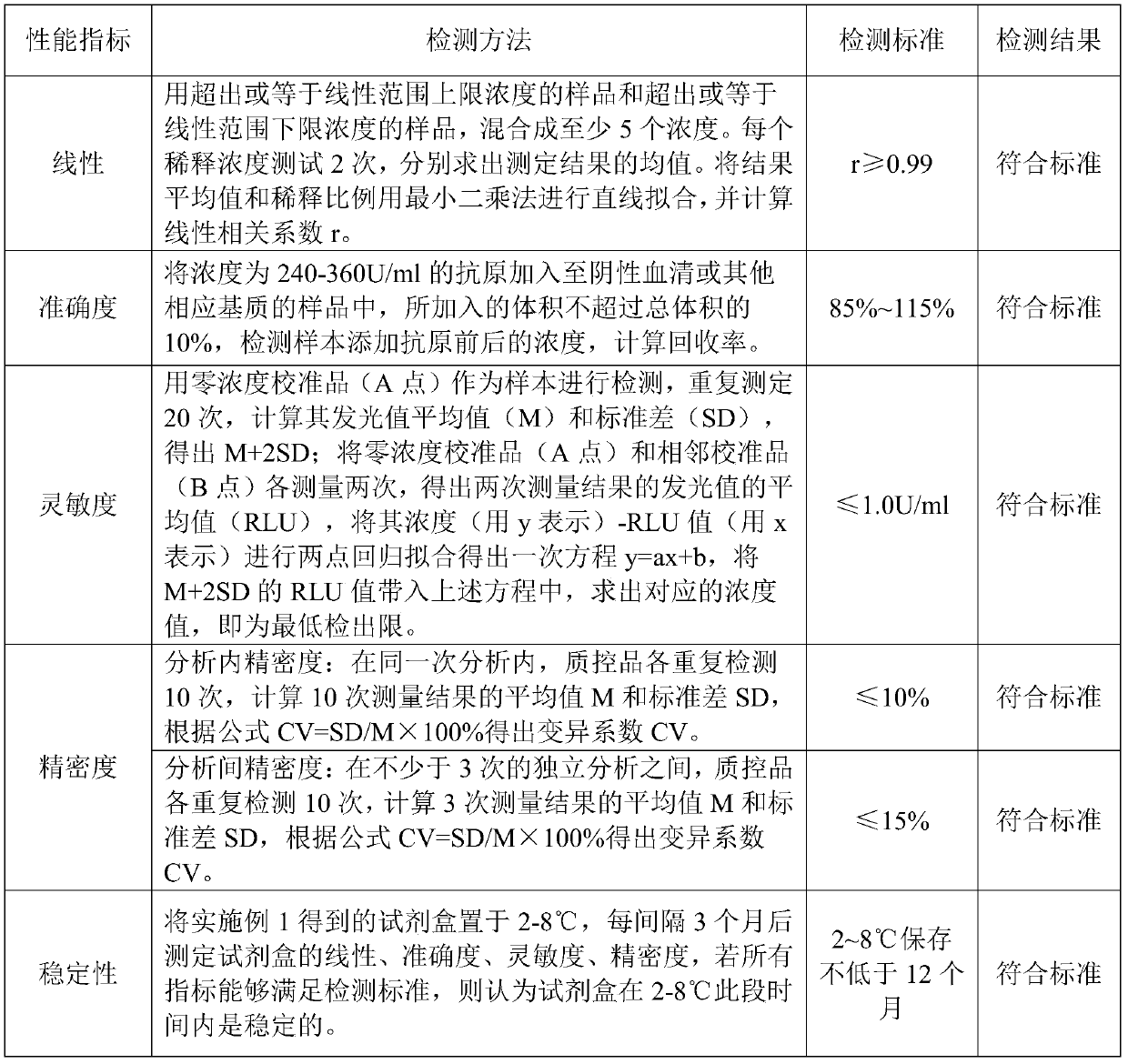
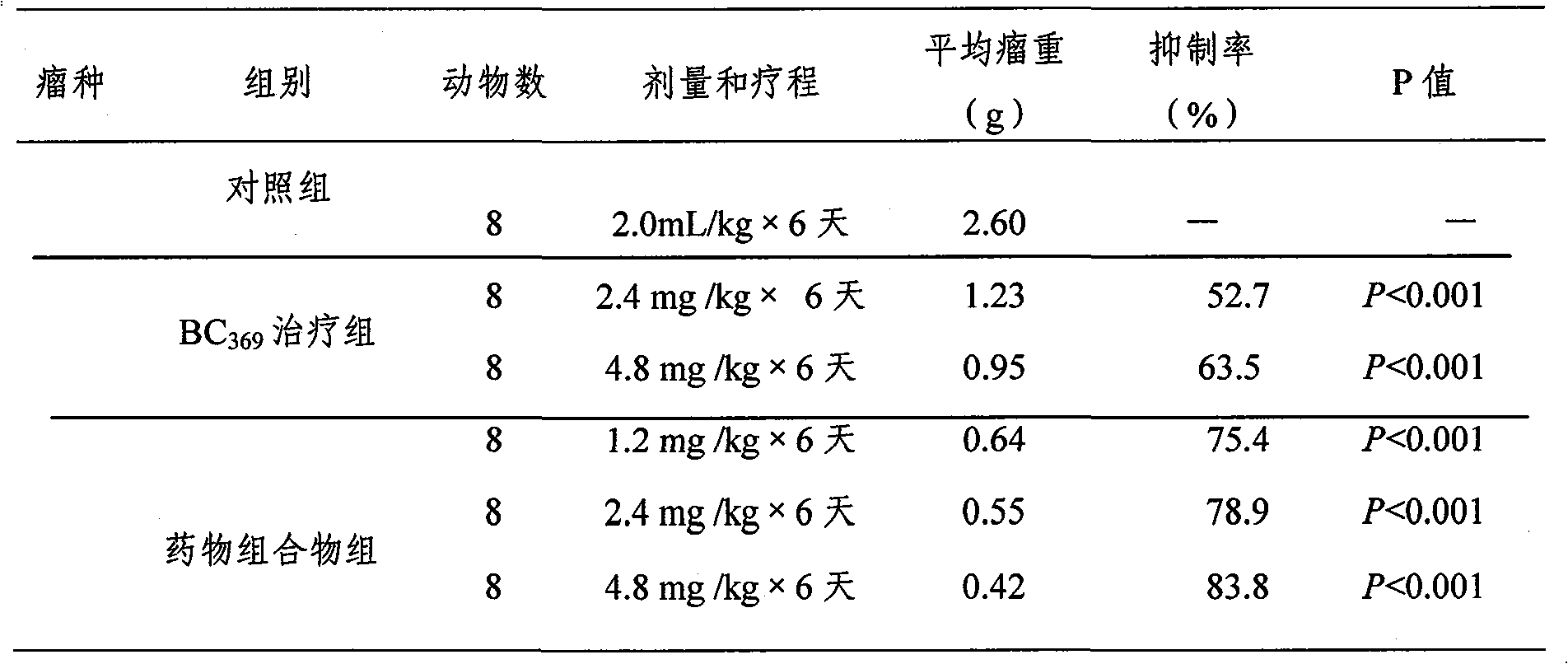
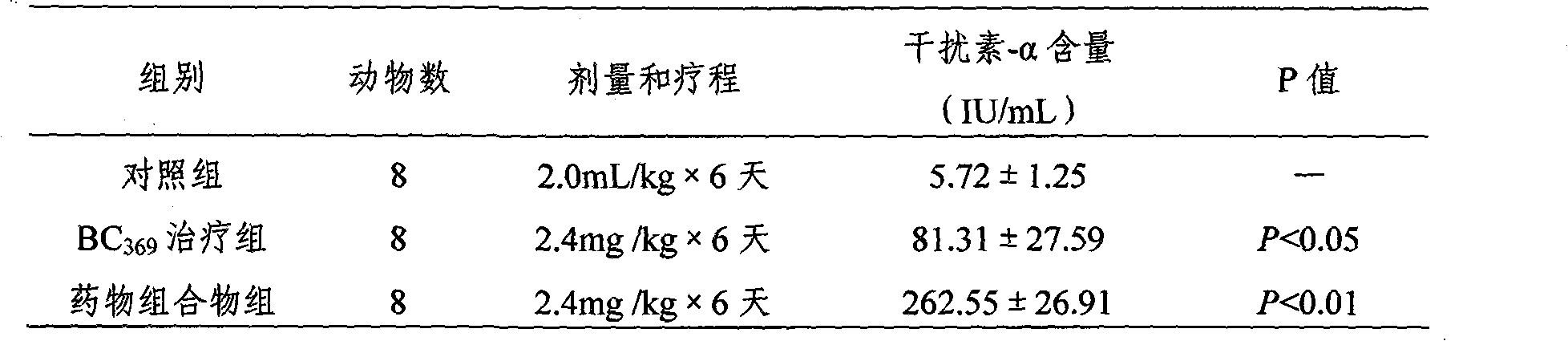
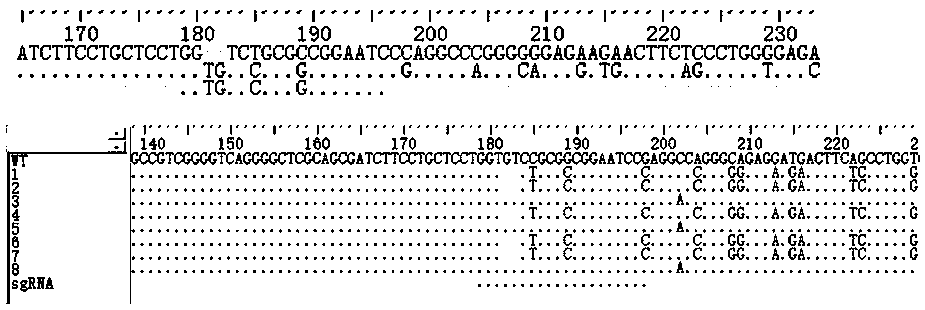Patents
Literature
111 results about "Sialidase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
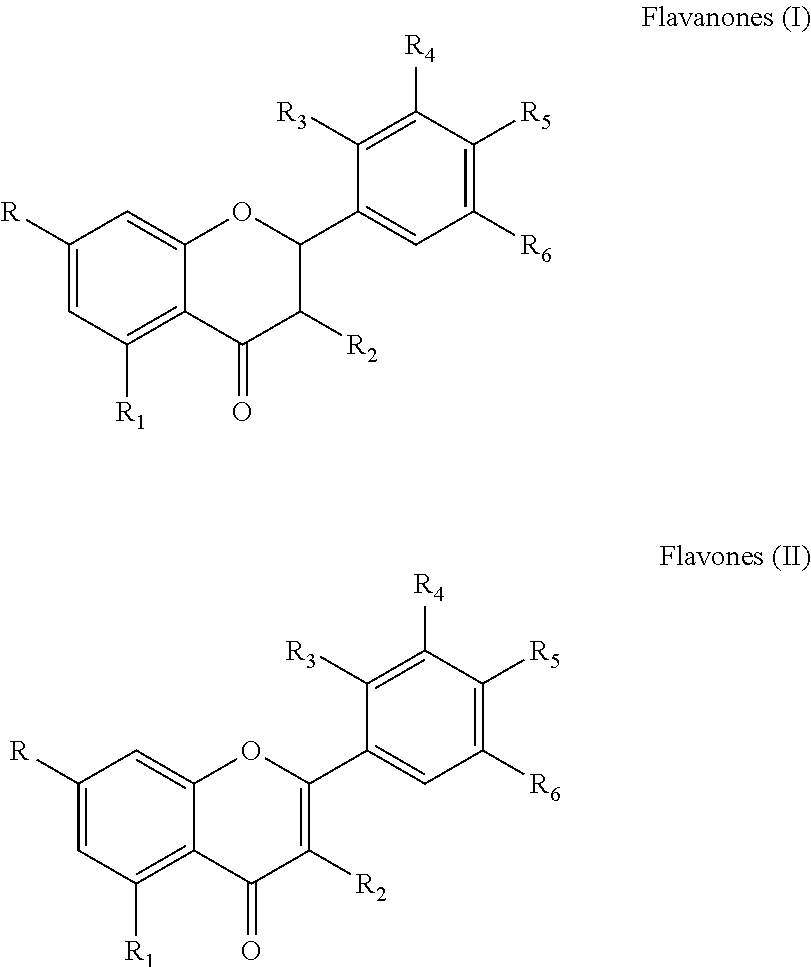
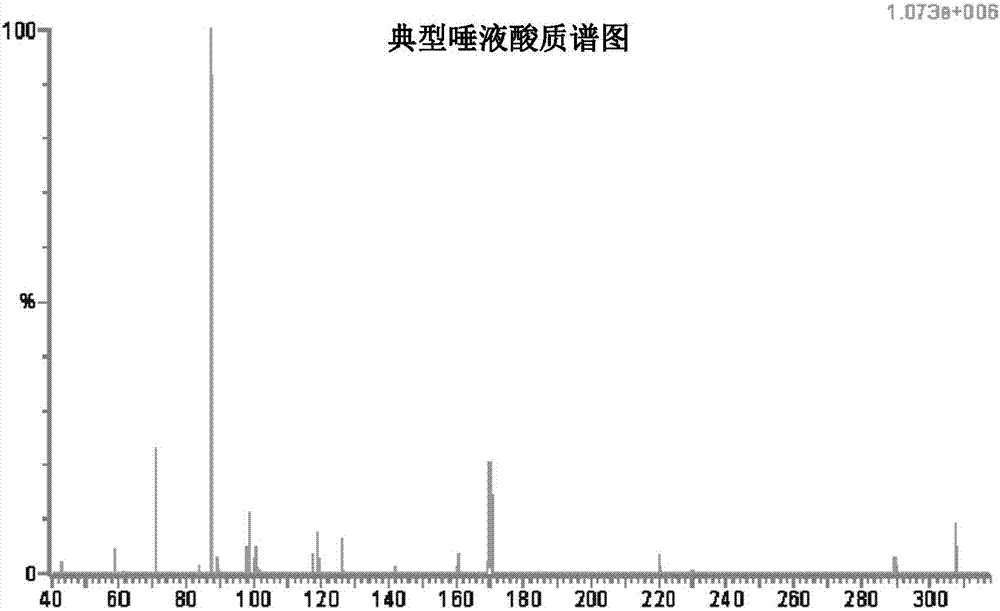
Sialidases hydrolyse alpha-(2->3)-, alpha-(2->6)-, alpha-(2->8)-glycosidic linkages of terminal sialic residues in oligosaccharides, glycoproteins, glycolipids, colominic acid and synthetic substrates. Sialidases may act as pathogenic factors in microbial infections.
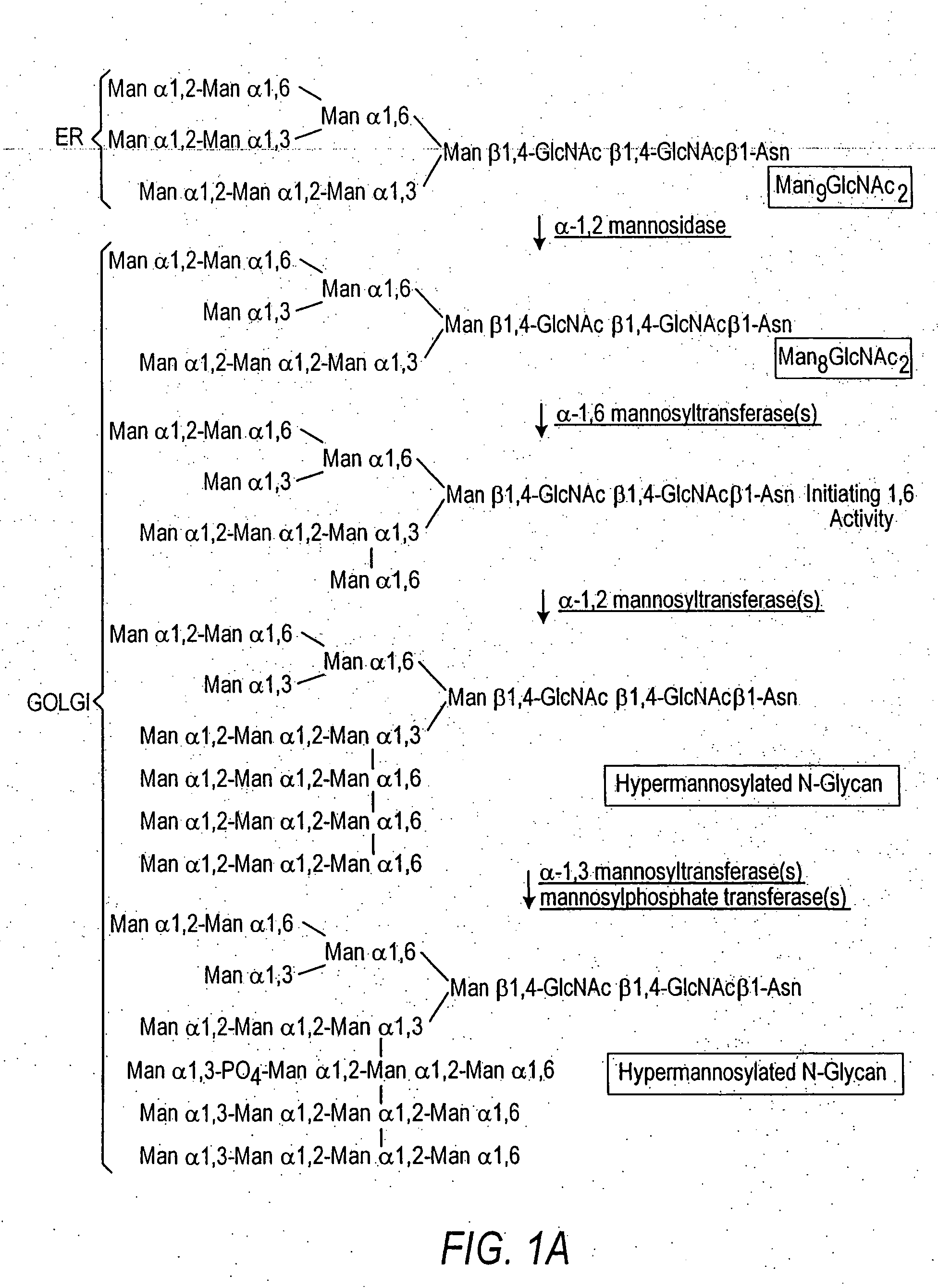
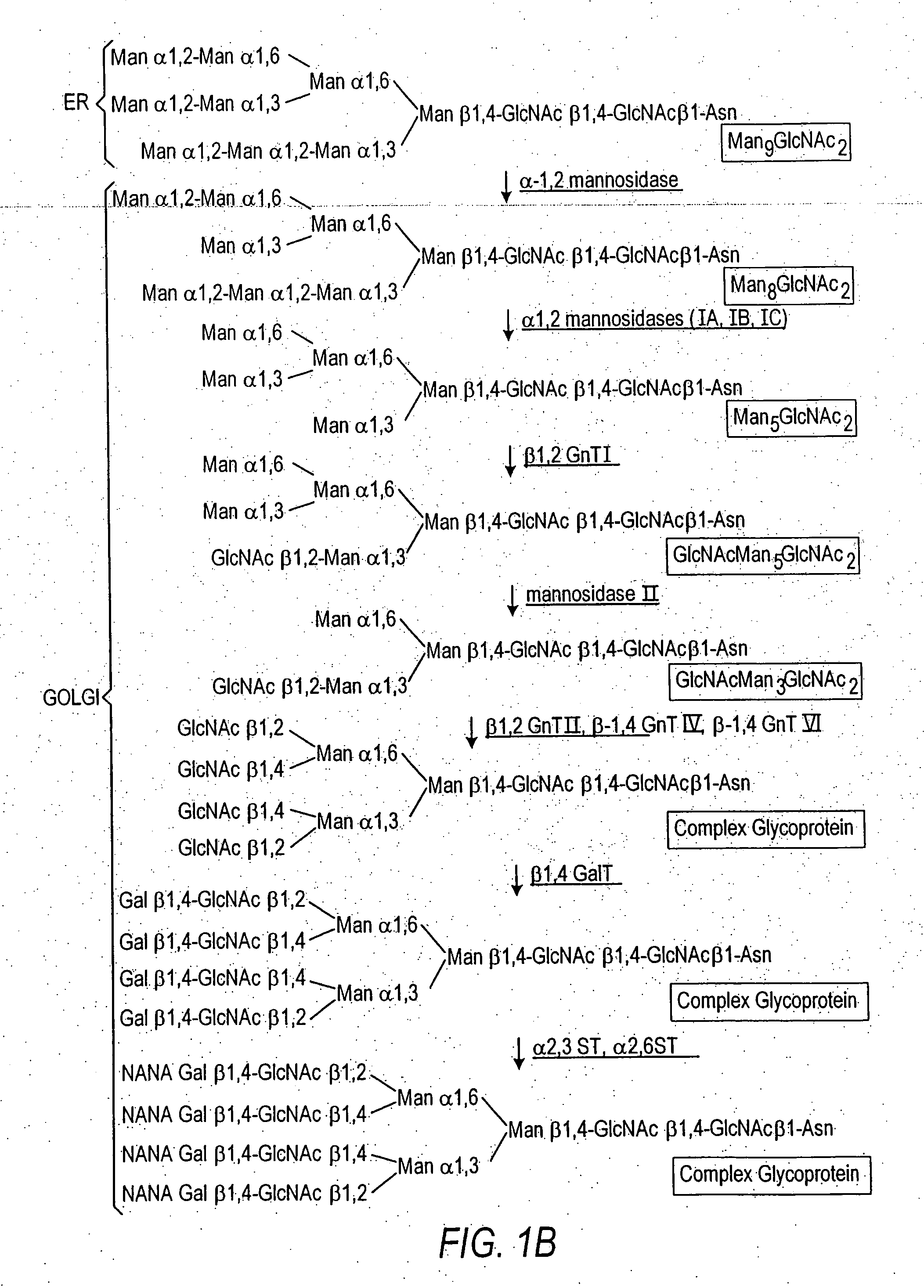
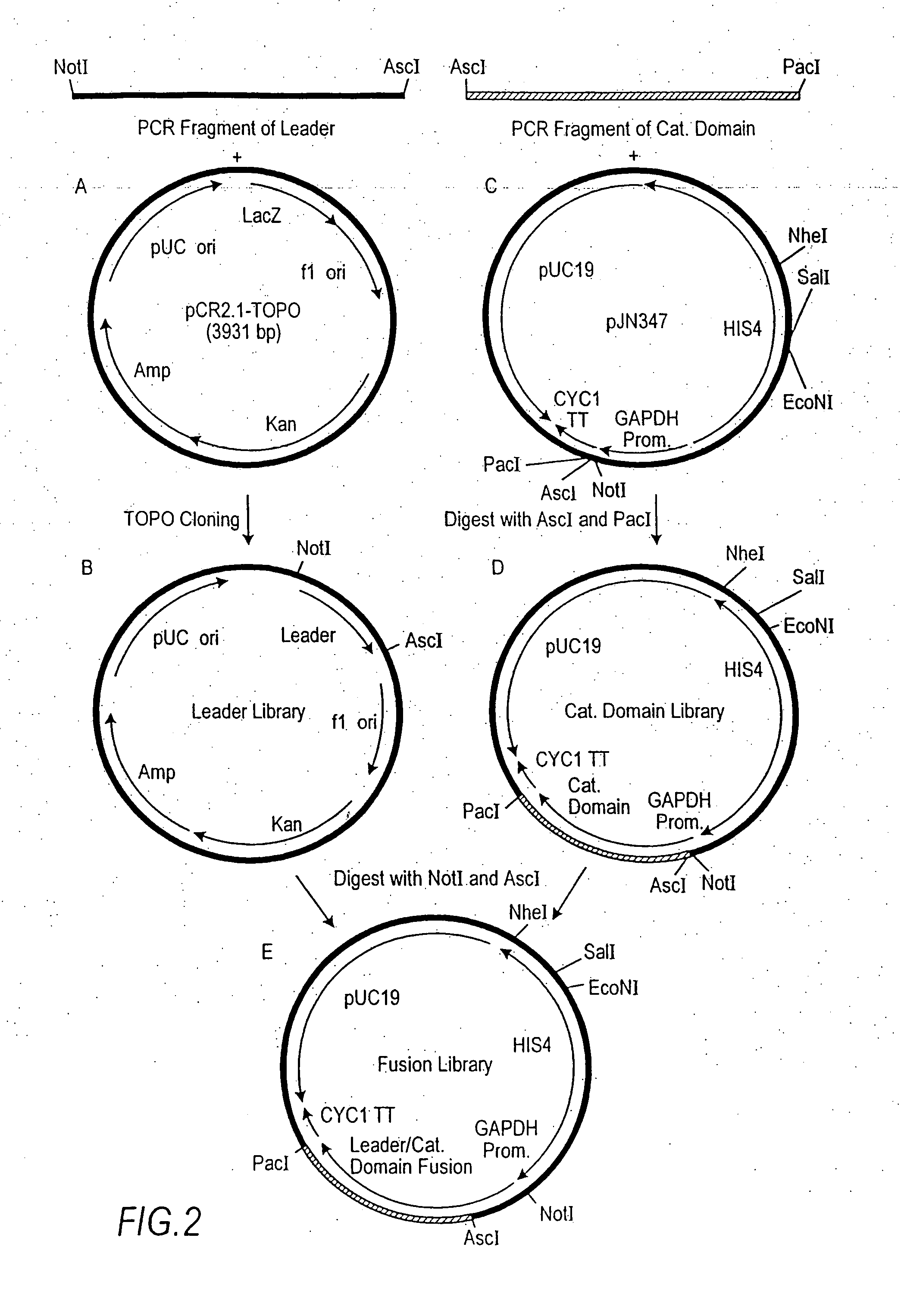
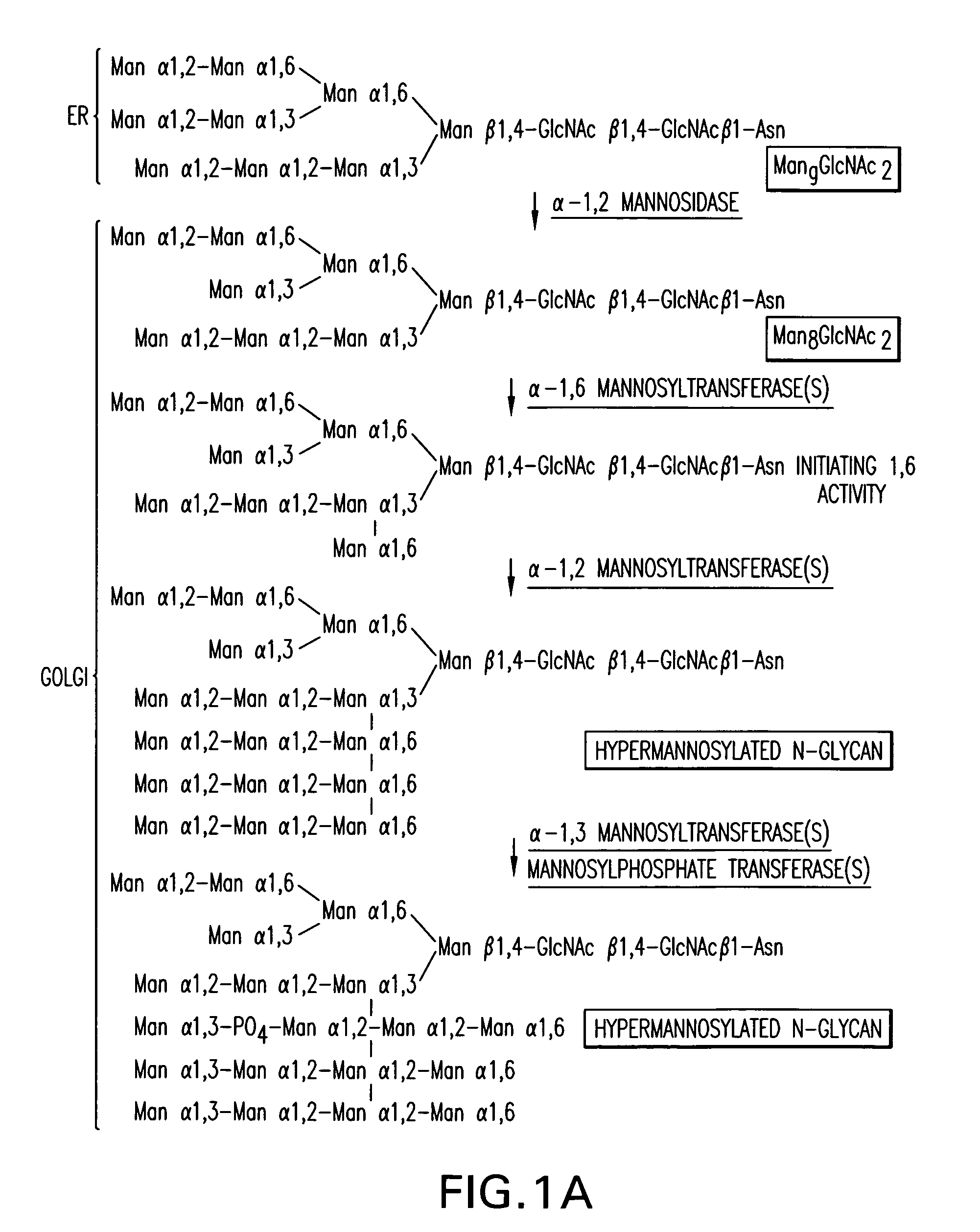
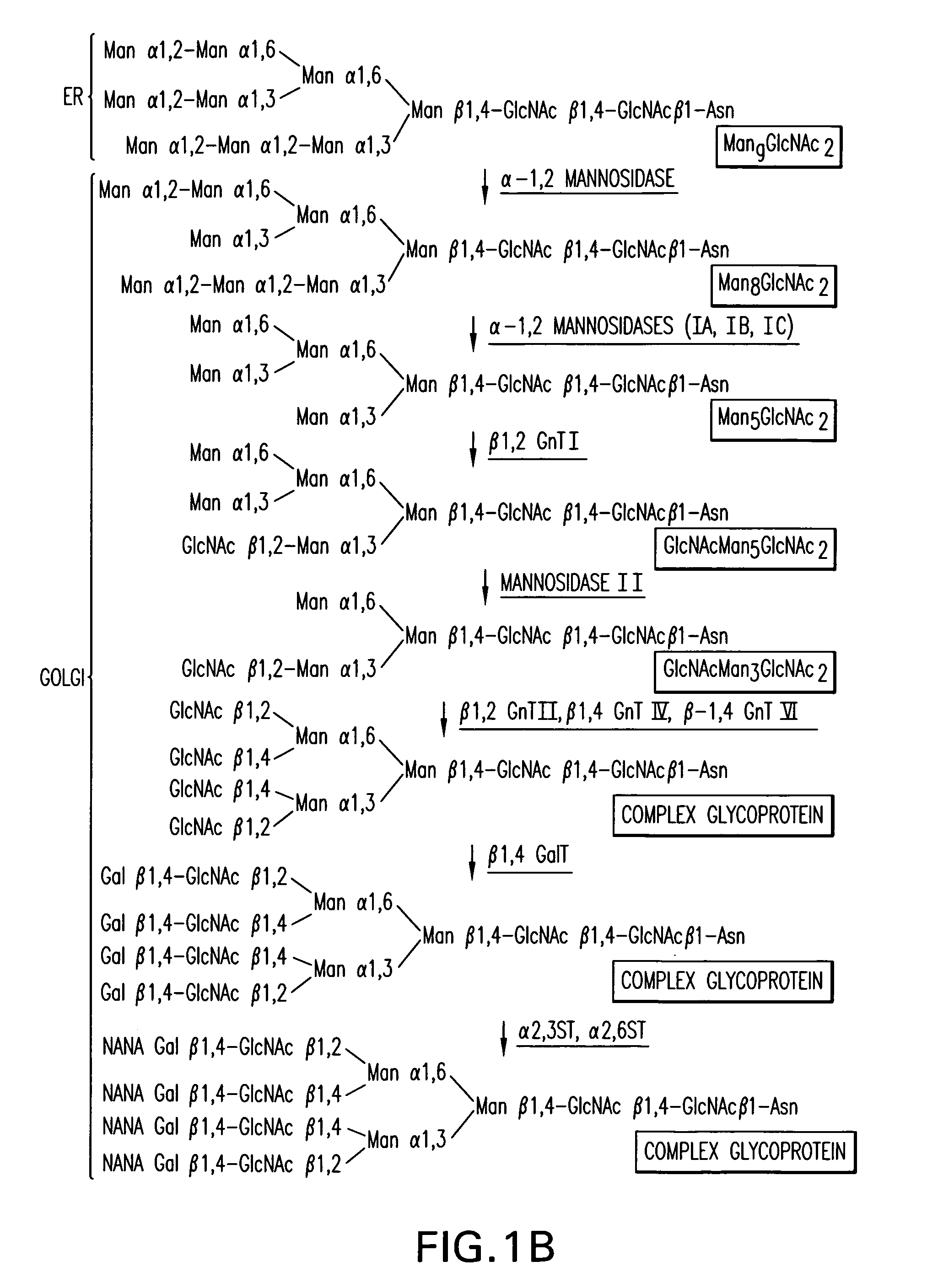
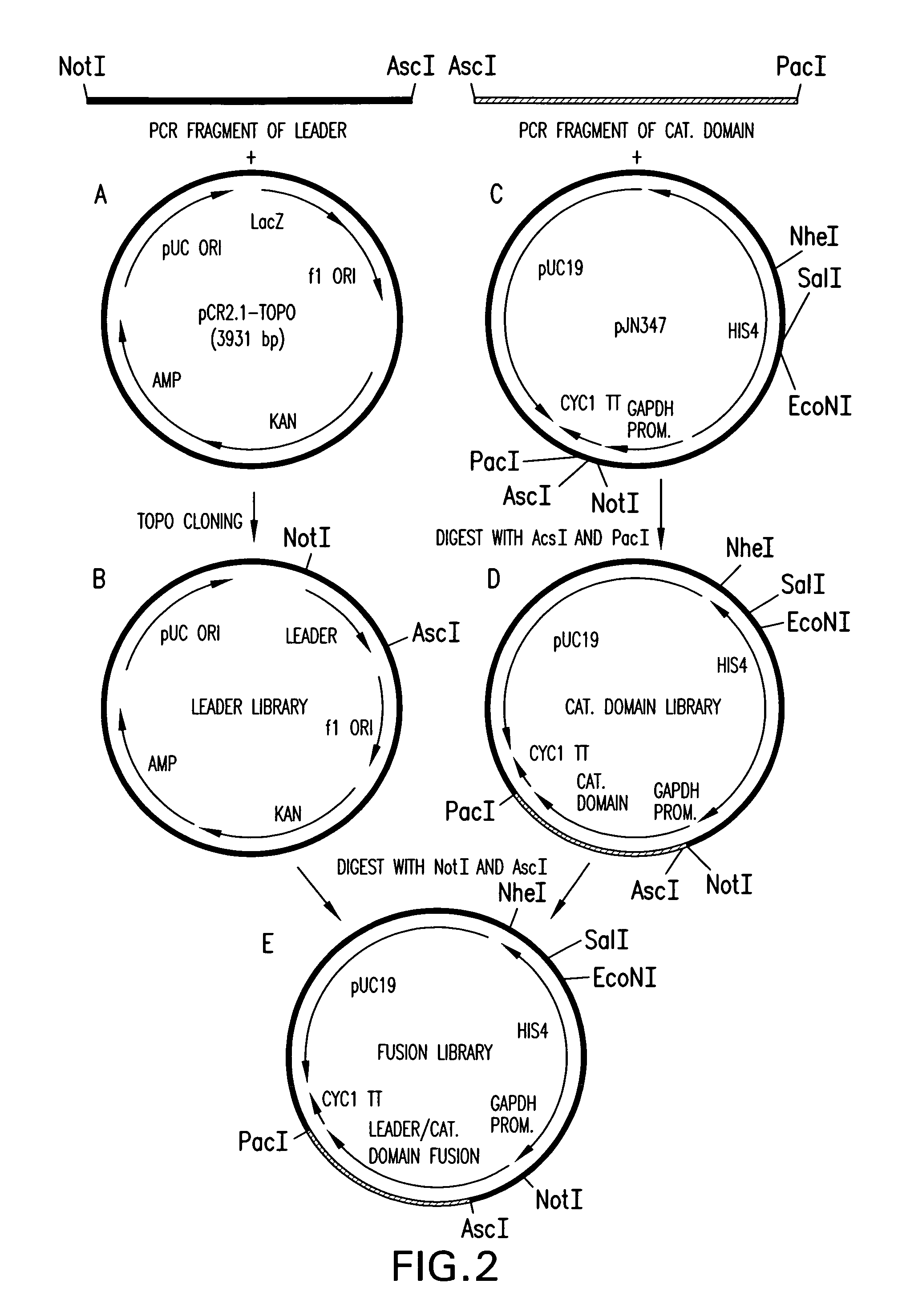
Production of sialylated N-glycans in lower eukaryotes
InactiveUS20060286637A1Improve efficiencyReducing competitive product inhibitionAntibody mimetics/scaffoldsTransferasesHeterologousSialic acid aldolase
The present invention relates to eukaryotic host cells which have been modified to produce sialylated glycoproteins by the heterologous expression of a set of glycosyltransferases, including sialyltransferase and / or trans-sialidase, to become host-strains for the production of mammalian, e.g., human therapeutic glycoproteins. Novel eukaryotic host cells expressing a CMP-sialic acid biosynthetic pathway for the production of sialylated glycoproteins are also provided. The invention provides nucleic acid molecules and combinatorial libraries which can be used to successfully target and express mammalian enzymatic activities (such as those involved in sialylation) to intracellular compartments in a eukaryotic host cell. The process provides an engineered host cell which can be used to express and target any desirable gene(s) involved in glycosylation.
Owner:GLYCOFI
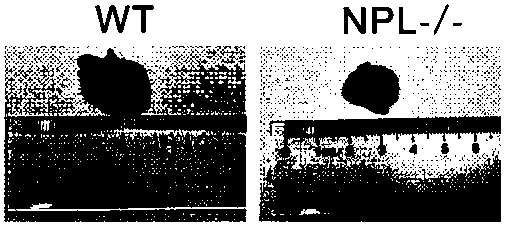
Construction method for sialidase gene knockout mouse model and application thereof
InactiveCN108486154AShort experiment cycleReduce experiment costVector-based foreign material introductionGlycosylasesCancer cellWilms' tumor
The invention relates to the field of biotechnologies, and provides a construction method for a sialidase gene knockout mouse model and application thereof. An in vitro and vivo targeted mouse genomeNPL is used for genetically recombining a CRISPR knockout plasmid vector, and a diploid double-knockout NPL mouse knockout model is successfully constructed through an oosperm microinjection technology. The NPL has the important function in a pathophysiological process of a body. Vicious transformation of a cancer cell is always along with overexpression of sialic acid. Now, the overexpression ofthe sialic acid has become one of important markers of the malignant transformation and transferring of a tumor. The model is capable of constructing a new research platform for researching a processof cell canceration, beneficial to a key step of researching generation and transferring of the tumor, and providing a foundation for the research and development of a novel anti-tumor drug.
Owner:FUZHOU UNIV
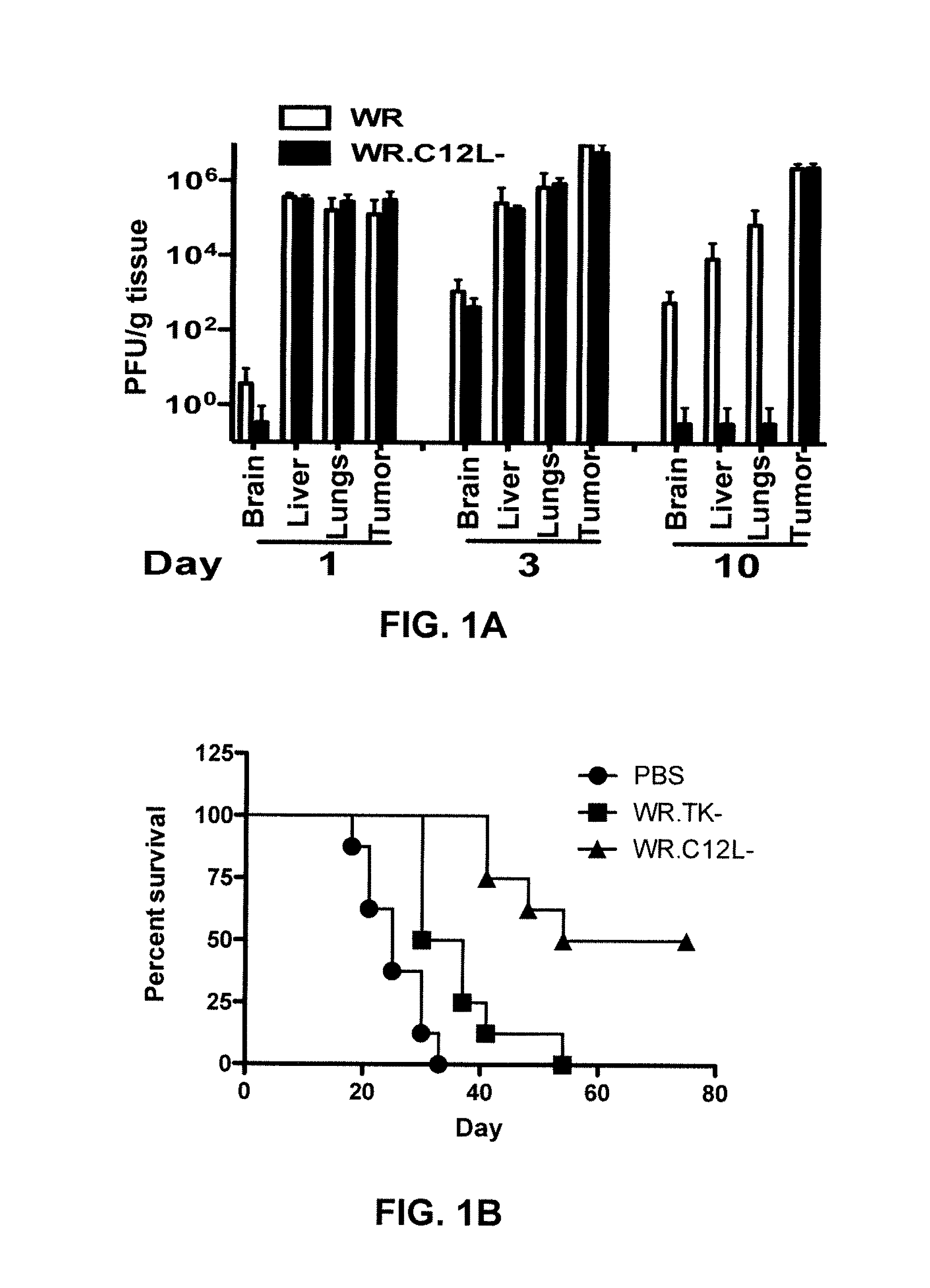
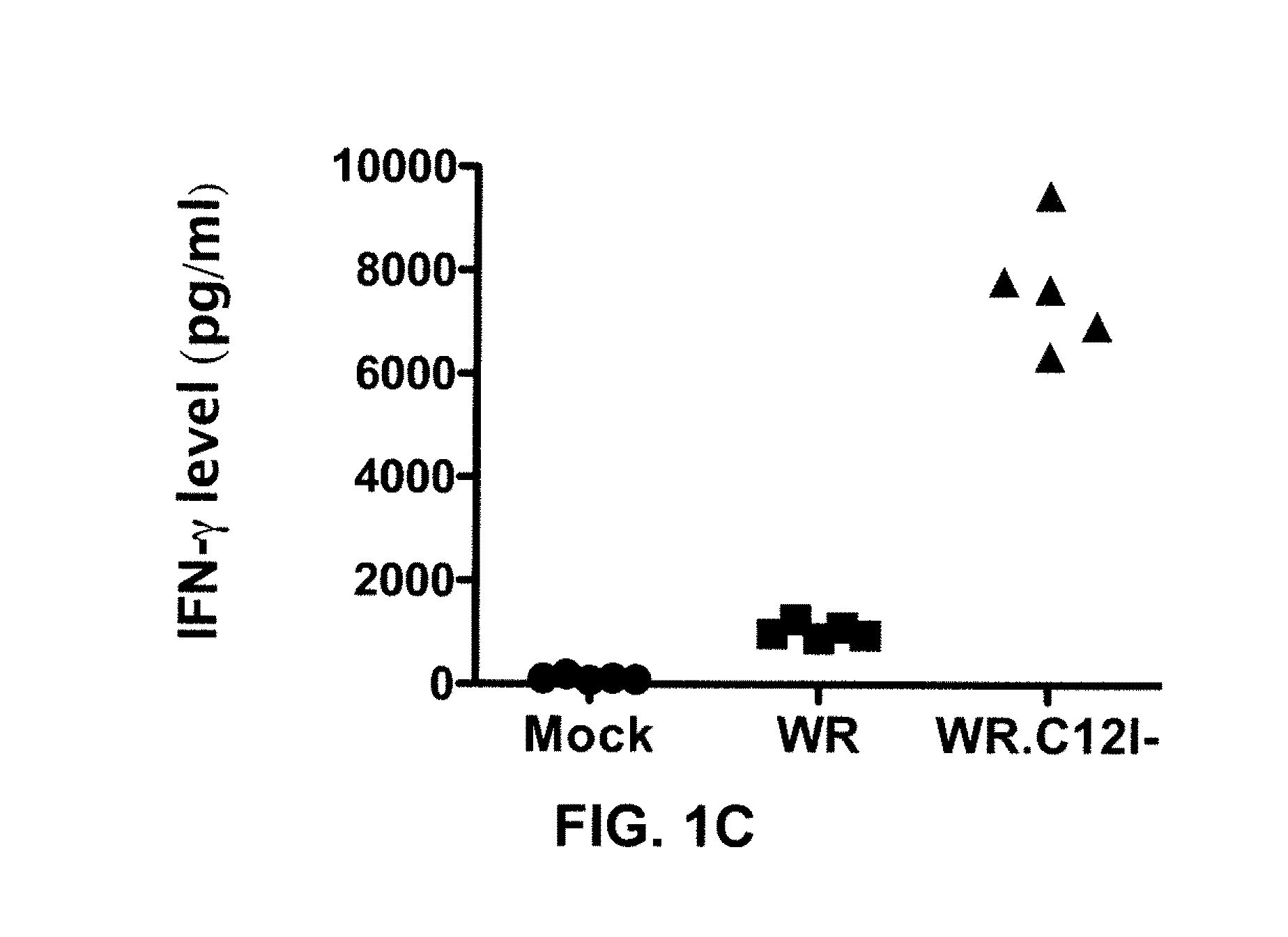
Immuno-Oncolytic Therapies
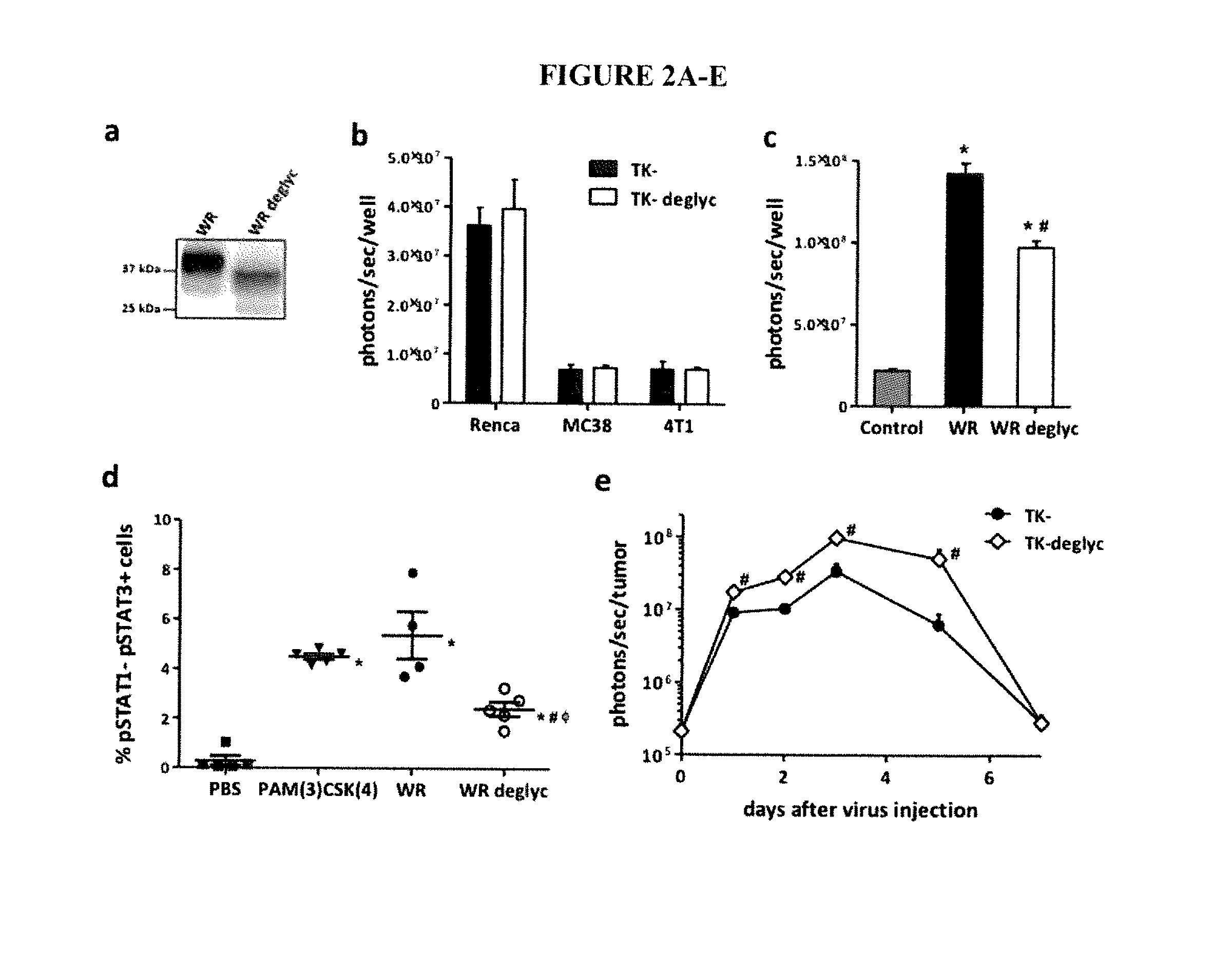
ActiveUS20160235793A1Reduced responseImprove immunityViral antigen ingredientsUnknown materialsAbnormal tissue growthInterleukin-18 binding protein
The present invention relates to oncolytic vaccinia viruses which have been modified to promote anti-tumor immunity and / or reduce host immunity and / or antibody response against the virus. It is based, at least in part, on the discovery that oncolytic vaccinia virus (i) bearing a genome deletion of a gene that reduces T cell immunity (interleukin-18 binding protein); (ii) treated with a sialidase enzyme which is believed to reduce TLR2 activation and therefore the antibody response; (iii) carrying a gene that enhances cytotoxic T lymphocyte induction (e.g., TRIF) and / or (iv) reduces tumor myeloid-derived suppressor cells by reducing prostaglandin E2 reduces tumor growth. Accordingly, the present invention provides for immunooncolytic vaccinia viruses and methods of using them in the treatment of cancers.
Owner:UNIVERSITY OF PITTSBURGH
Method and composition for preventing and treating viral infections
ActiveUS20160367517A1Reducing replication ratePreserving cellular integrityOrganic active ingredientsAntiviralsATPasePenetration enhancer
A method and composition for treating viral infections using a combination of naturally occurring compounds is provided. The method includes administering to a patient at risk of or diagnosed with a viral infection a composition including therapeutically effective amounts of a helicase ATPase inhibitor, a sialidase enzyme inhibitor, and ICAM-1 inhibitor which each also down regulate the immune response. The composition may further include a permeation enhancer.
Owner:GLOBAL BIOLIFE INC
Sialidase detection kit
InactiveCN102399851ASolve complexityFix stability issuesMicrobiological testing/measurementSialidasePreservative
The invention provides a sialidase detection kit. The kit comprises a reagent 1 and a reagent 2, wherein the reagent 1 comprises the components of buffer solution, neuraminidase, lactic dehydrogenase, surfactant, stabilizer and preservative; and the reagent 2 comprises the components of buffer solution, reducing coenzyme, neuraminic acid aldolase, stabilizer and preservative. The kit has accurate detection and low cost and is convenient, applicable, stable and reliable.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Production of sialylated N-glycans in lower eukaryotes
InactiveUS7863020B2High yieldImprove efficiencyPolypeptide with localisation/targeting motifSugar derivativesHeterologousNucleic acid molecule
The present invention relates to eukaryotic host cells which have been modified to produce sialylated glycoproteins by the heterologous expression of a set of glycosyltransferases, including sialyltransferase and / or trans-sialidase, to become host-strains for the production of mammalian, e.g., human therapeutic glycoproteins. Novel eukaryotic host cells expressing a CMP-sialic acid biosynthetic pathway for the production of sialylated glycoproteins are also provided. The invention provides nucleic acid molecules and combinatorial libraries which can be used to successfully target and express mammalian enzymatic activities (such as those involved in sialylation) to intracellular compartments in a eukaryotic host cell. The process provides an engineered host cell which can be used to express and target any desirable gene(s) involved in glycosylation.
Owner:GLYCOFI
Method of treating inflammation with inhibitors of sialyl transferases
Provided herein are a methods of reducing a level of activity of a sialyl transferase enzyme, a sialidase enzyme or a combination thereof using inhibitors of these enzymes, for example specific inhibitory compound or antibodies directed against the enzymes. The methods are effective to treat an inflammatory disease or disorder or a primary or metastatic cancer. Also provided is a method for screening potential inhibitors of sialyl transferase enzymes.
Owner:MARYLAND UNIV OF THE BALTIMORE
Sialidase detection reagent
InactiveCN1405562AImprove stabilityImprove accuracyMicrobiological testing/measurementBiological testingNeuraminateSialidase
This invention discloses a sialic acid enzyme test reagent for testing sialic acid enzyme activity in vagina secreta outside the body, containing substrate on the carrier named N-acetyl neuraminate and its salt which can be its derivant or thymolphthaleic N-acetyl neuraminate and its salt, 5-Br-4-Cl-3-indolyl-alpha-D-N-acytylneuraminate and its salt. We can diagnose the bacteriogenic vagina deseases quickly and simple by testing sialic acid enzyme to vagina searate with this sialic acid ester reagent which can be used as an independent diagnosis target with good stability, extremely excellent accuracy and simple operation (one step test).
Owner:肖洪武
Method for detecting neuraminidase influencing sperm functions
ActiveCN103454416AMore detectionMany clinical explanationsMaterial analysisMale infertilitySialidase
The invention provides a method for detecting neuraminidase influencing sperm functions. The method is a novel laboratory detecting method for assessing quality and functions of a sperm and can provide detection and clinical consultations for male infertility patients without clear clinical reasons for infertility. The method is characterized in that expressions of neuraminidase in the sperm are visually quantized through single sperm fluorescence strength analysis or are visually observed by means of fluorescence imaging under a microscope, and if the neuraminidase is highly expressed in the sperm, capacitation of the sperm is achieved through external capacitation liquid, and neuraminidase activity is detected in a fluorescence method.
Owner:成都思瑞多医疗科技有限公司
Methods and Reagents to Treat Tumor and Cancer
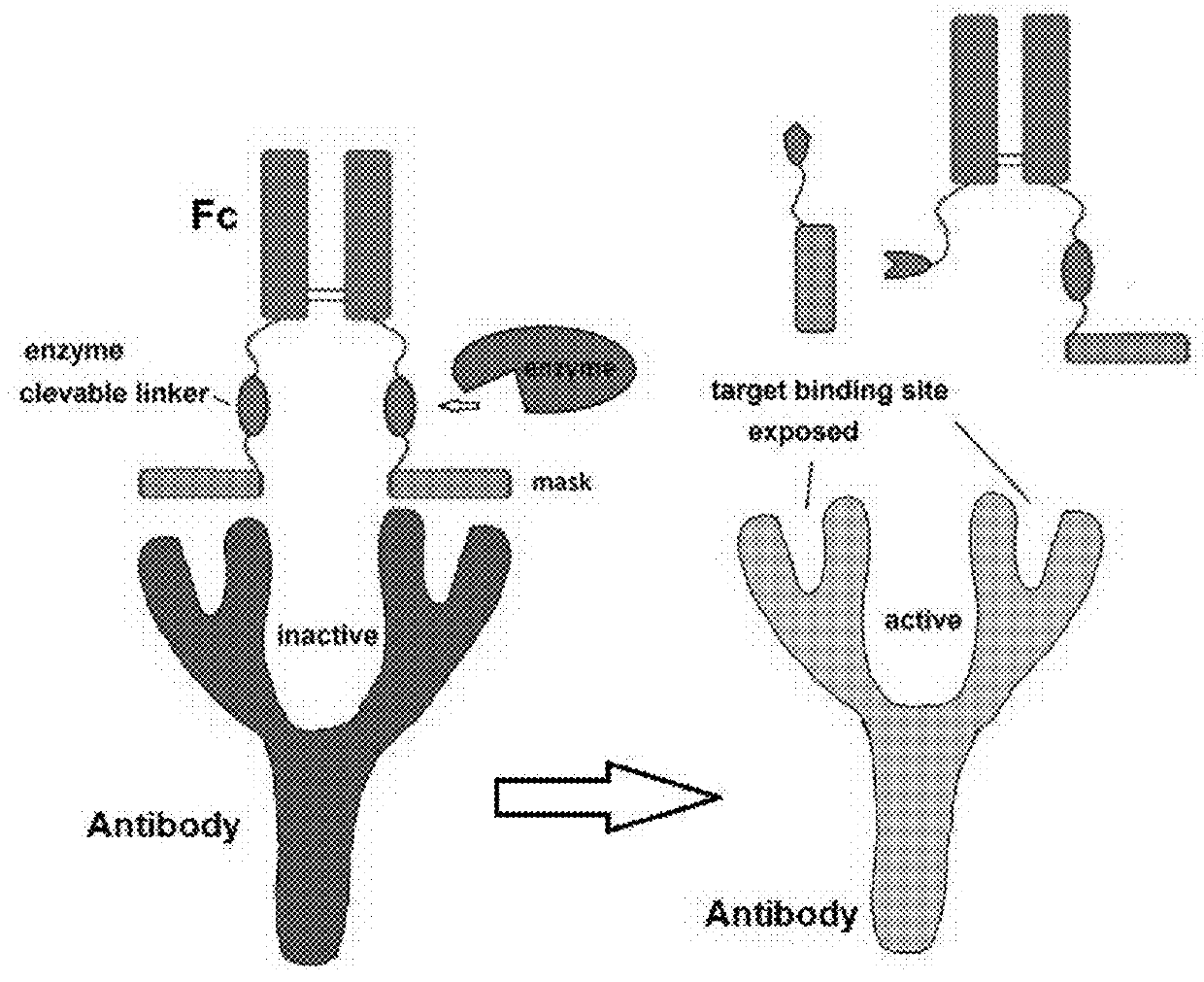
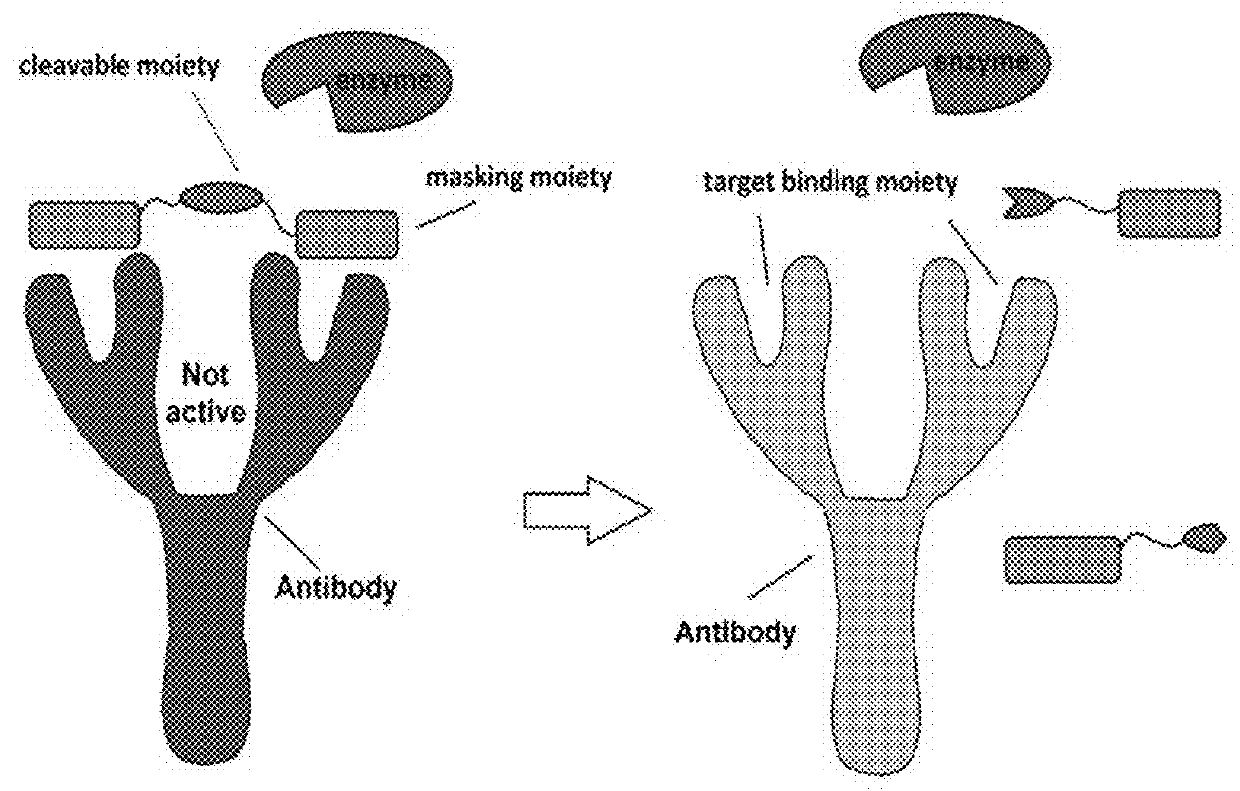
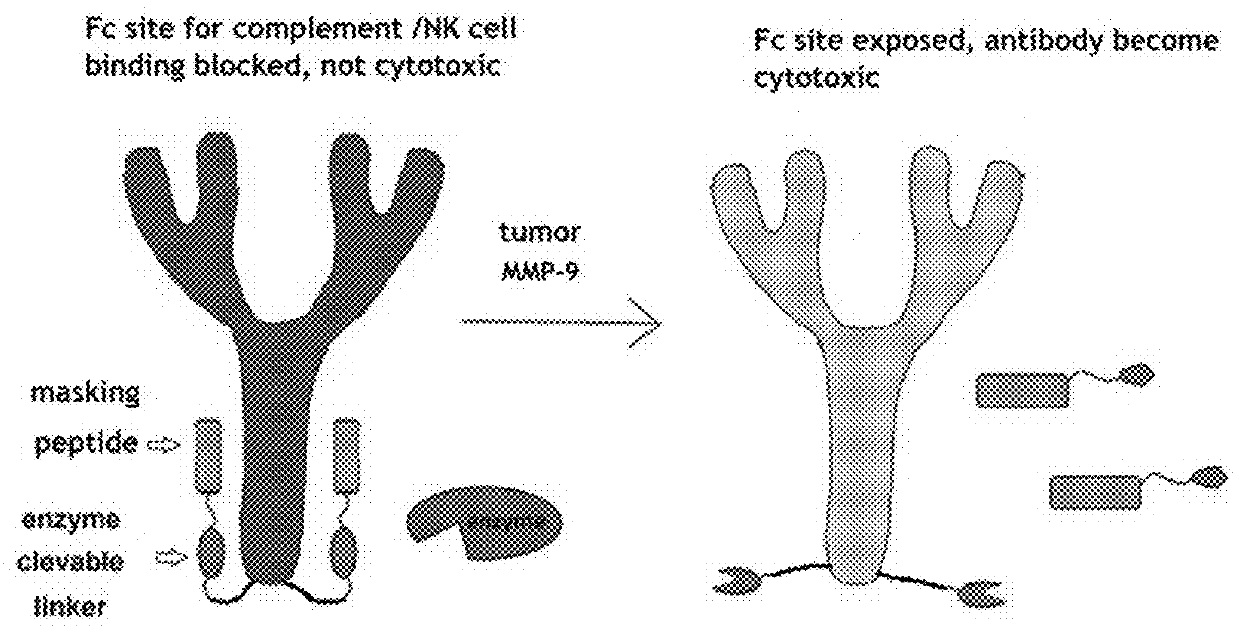
This disclosure provides reagent to treat tumor and cancer, and methods of using the same for treating tumor and cancer. One type of the reagent is pro-antibody, which is antibody that can be activated in tumor. Another type of the reagent is a conjugate of sialidase with affinity ligand that can bind to immuno cell surface or a conjugate of sialidase with affinity ligand that can bind to another antibody, therefore provide a sialidase based cancer immuno therapy strategy.
Owner:WANG TIANXIN
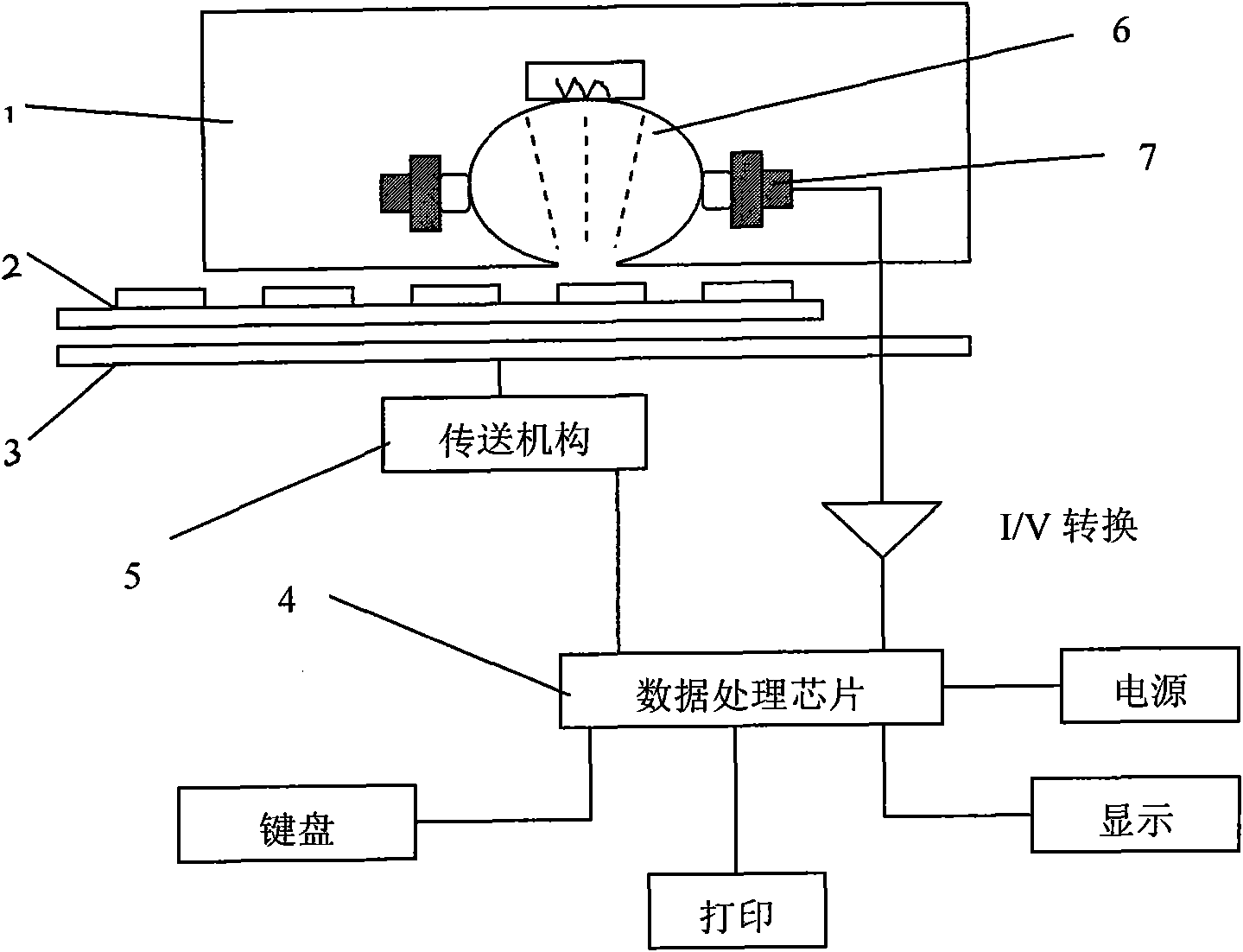
Rapid detection device, detection test strip and detection method for bacterial vaginosis
InactiveCN101975852APut time to concentrateSave operating timeBiological testingSialidaseBacterial vaginosis
The invention discloses a rapid detection device, a detection test strip and a detection method for bacterial vaginosis. The rapid detection device for bacterial vaginosis comprises an engine cover, a data processing chip, a delivery mechanism, an optical detection system and a control device, wherein a movable tray with a groove is arranged below the engine cover; and the optical detection system and the control device are arranged in the engine cover. The assorted test strip takes an oblong-shaped nontransparent white thin piece as a film base to which a blank block and detection reaction modules for pH, hydrogen peroxide, leucocyte esterase and sialidase are attached uniformly. The detection device and the test strip of the invention can quantitatively analyze four indexes of bacterial vaginosis at the same time, greatly shorten the test time of the four indexes, make operation more convenient, reduce errors caused by the conventional manual reading and make results more reliable by automated analysis and can be widely applied to the diagnosis and treatment observation of bacterial vaginosis by different levels of clinical institutions.
Owner:高翔 +2
Production of sialylated n-glycans in lower eukaryotes
InactiveUS20100279356A1High yieldImprove efficiencyPolypeptide with localisation/targeting motifFungiHeterologousBiology
The present invention relates to eukaryotic host cells which have been modified to produce sialylated glycoproteins by the heterologous expression of a set of glycosyltransferases, including sialyltransferase and / or trans-sialidase, to become host-strains for the production of mammalian, e.g., human therapeutic glycoproteins. Novel eukaryotic host cells expressing a CMP-sialic acid biosynthetic pathway for the production of sialylated glycoproteins are also provided. The invention provides nucleic acid molecules and combinatorial libraries which can be used to successfully target and express mammalian enzymatic activities (such as those involved in sialylation) to intracellular compartments in a eukaryotic host cell. The process provides an engineered host cell which can be used to express and target any desirable gene(s) involved in glycosylation.
Owner:GLYCOFI
Methods, Compounds, and Compositions for Treatment and Prophylaxis in the Respiratory Tract
ActiveUS20110171132A1Reduce in quantityCompounds screening/testingAntibacterial agentsSialidaseAnesthesia
The present invention provides a method of reducing the quanitity of mucus in the respiratory tract of a subject with elevated levels of mucus in said respiratory tract. The method includes administering to the subject a compound or composition containing a therapeutically effective amount of a fusion protein comprising a sialidase or an active portion thereof and an anchoring domain. The therapeutically effective amount comprises an amount of the fusion protein that results in a reduction of the quanitity of mucus in the respiratory tract after administration of the compound or composition when compared to the quantity of mucus present prior to administration of the compound or composition.
Owner:ANSUN BIOPHARMA
Lung cancer monitoring kit and use method thereof
ActiveCN106950379ATimely monitoring of disease progressionBiological testingNon invasiveWilms' tumor
The invention provides a lung cancer monitoring kit and a use method thereof. The kit includes: a reagent A, which is prepared by adding 5% of SDS to a 10 mM ammonium bicarbonate solution; a reagent B, which is prepared by adding not less than 2.2 unit / [mu]L of exoglycosidase to NP40 being 3.3% in concentration; a reagent C, which is a mixture liquid composed of, in equal volume, a solution of 20 mM of APTS dissolved in 1.2 M of citric acid and a solution of 1M of an organic reducing agent dissolved in DMSO; a reagent D, which includes 0.25 [mu]L of NH4Ac with concentration being 100 mM and pH value being 5, 0.2 [mu]L of sialidase, and 2.55 [mu]L of H2O2. The kit can evaluate lung cancer patients with detection on a fingerprint spectrum of an oligosaccharide chain in blood glycoprotein as a diagnosis index, thereby diagnosing the tumor stage of the lung cancer. The kit allows numerous lung cancer patients to take regular and non-invasive detection, and helps a medicinal worker to detect the lung cancer and monitor progression of the lung cancer timely.
Owner:XIANSIDA NANJING BIOTECH CO LTD +1
T. Cruzi-derived neurotrophic agents and methods of use therefor
InactiveUS20090117593A1Provide supportCompound screeningApoptosis detectionNeurophysinsNeuronal degeneration
The invention relates to T. cruzi trans-sialidase (TS) and to the neurotrophic and IL-6 secretion-inducing activities of the protein. TS, neurotrophic variants and / or neurotrophic peptides based upon the sequence of TS can be administered to a mammal to directly or indirectly provide neurotrophic support for neurons. A mammalian neurotrophic factor (e.g., CNTF, LIF) can be co-administered with the TS, neurotrophic variant and / or neurotrophic peptide. TS, IL-6 secretion-inducing variants and / or IL-6 secretion-inducing peptides based upon the sequence of TS can be administered to a mammal to induce the secretion of IL-6. TS, active variants and / or active peptides can be administered to a mammal having an acquired or congenital condition characterized by neuronal degeneration or to a mammal that has experienced trauma to the brain, spinal cord or peripheral nerves. The invention also relates to neurotrophic and IL-6 secretion-inducing variants of TS and to neurotrophic and IL-6 secretion-inducing peptides. The invention also relates to compositions comprising TS, active variants thereof and / or active peptides and a physiologically acceptable carrier.
Owner:TUFTS UNIV
Sialidase gene recombinant expression vector and construction method thereof and sialidase and preparation method thereof
ActiveCN106906236AGlycosylasesVector-based foreign material introductionProteinase activitySialidase
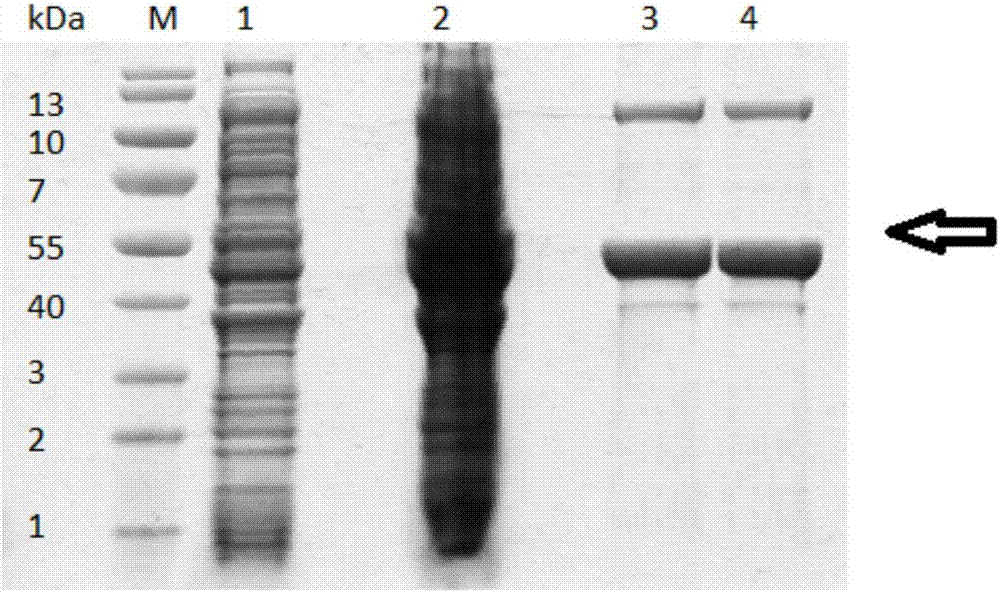
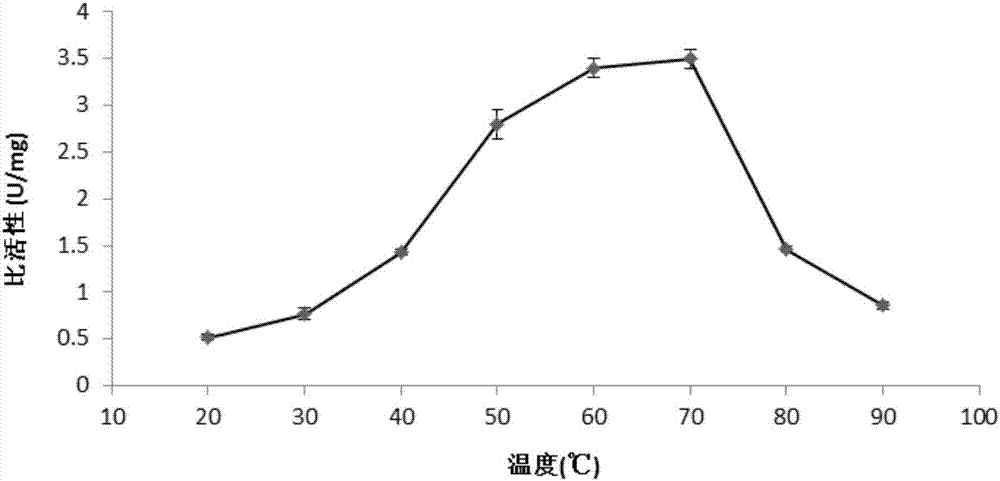
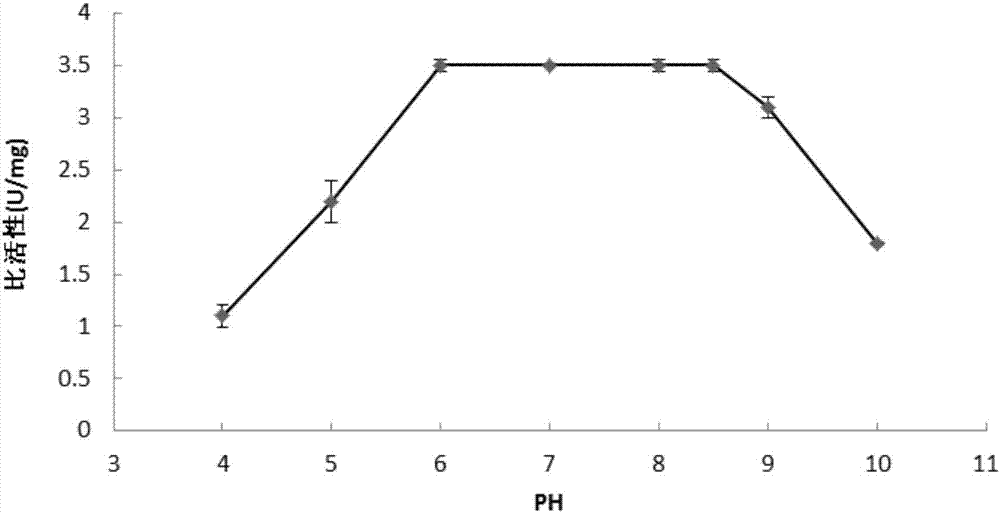
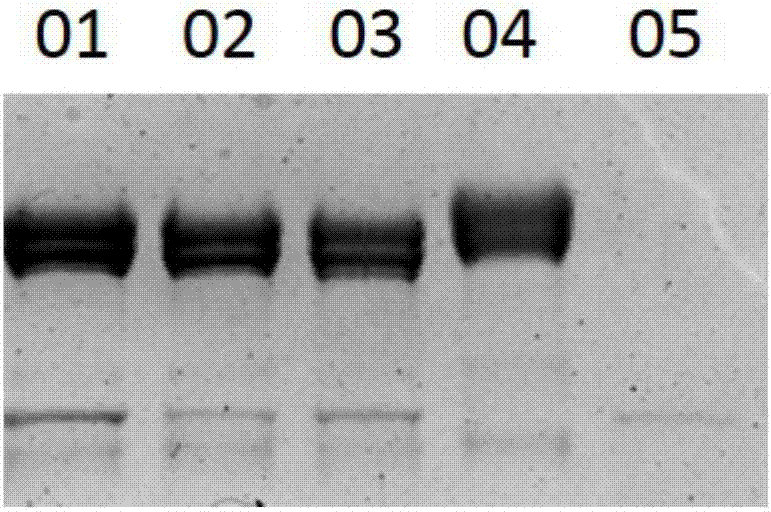
A preparation method of sialidase comprises the following steps: preparation of a sialidase gene fragment; cloning of the sialidase gene fragment into pMD-18T to obtain a connection product, conversion of the connection product into E.coliJM109, and selection of a positive clone for sequencing to obtain a gene sequence as shown in SEQIDNO:1; connection of the positive clone and a prokaryotic expression vector to obtain a recombinant expression vector; conversion of the recombinant expression vector to obtain recombinant engineering bacteria; fermentation, induced expression and purification of the recombinant engineering bacteria to obtain the sialidase. The preparation method of the sialidase comprises bacterial genome DNA extraction, PCR amplification, construction of the recombinant expression vector, conversion of the recombinant expression vector into host cells for construction of recombinant expression cells, culture and induced expression of the recombinant expression cells, isolation and purification of protease from the cultured host cells, and analysis of protease enzymatic properties. The sialidase prepared by the preparation method has high temperature resistance and Ph resistance, and provides more basic materials and scientific bases for industrial and social application.
Owner:INST OF DEEP SEA SCI & ENG CHINESE ACADEMY OF SCI
Diagnostic kit for early liver cancer and use method thereof
ActiveCN104807998AReduce missed diagnosis rateSave time and costDisease diagnosisSialidaseTherapeutic effect
The invention relates to a diagnostic kit for early liver cancer. The diagnostic kit comprises the following components of G1: 5% of SDS (sodium dodecyl sulfate); G2: 2.2 microgram / muL glycanase; G3A: 100mM trisulphonate fluorescent marker; G3B: 1M organic matter reducing agent; G4: sialidase; GW: deionized water. The invention also discloses a use method of the diagnostic kit. The diagnostic kit has the advantages that the problem of high missed diagnosing rate of alpha fetoprotein on the liver cancer is solved; the liver cancer can be quickly, simply, conveniently and accurately detected, and the therapy effect of the liver cancer can be tracked.
Owner:JIANGSU XIANSIDA BIOTECH CO LTD
Method for measuring sialic acid content of protein
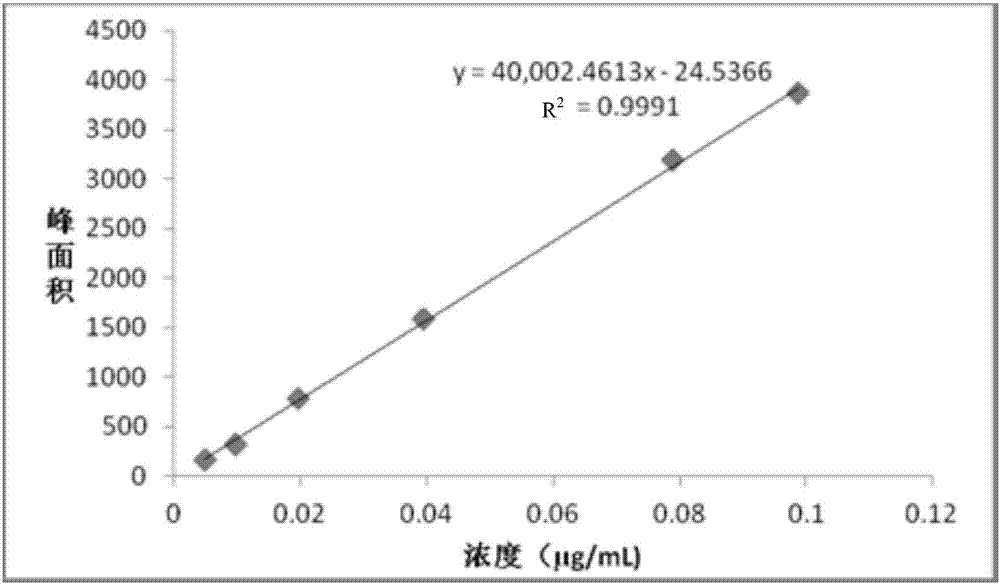
ActiveCN107091885AGood peak shapeGood peak separationComponent separationBiological macromoleculeDerivatization
The invention provides a method for measuring sialic acid content of protein. The method comprises the steps of 1, using sialidase to conduct digestive treatment on a protein sample, so that digestive juice containing sialic acid is obtained; 2, conducting sedimentation treatment on the digestive juice, and separating supernatant containing sialic acid; 3, using an ultra-high performance liquid chromatography-mass spectrometry method to analyze the supernatant, so that a mass spectrogram is obtained; 4, based on the mass spectrogram, determining the sialic acid content of protein. In the method for measuring the sialic acid content of protein, on the condition that there is no need to conduct derivatization on sialic acid, the total sialic acid content in the protein sample is subjected to rapid quantitative detection, the method is easy to operate, the sensitivity is high, the accuracy is high, the elapsed time is short, and the method can be used for intermediate quality control over biomacromolecule production or quality inspection of final products.
Owner:HUBEI BIO PHARMA IND TECHCAL INST +1
Sialidase and recombinant cell lines
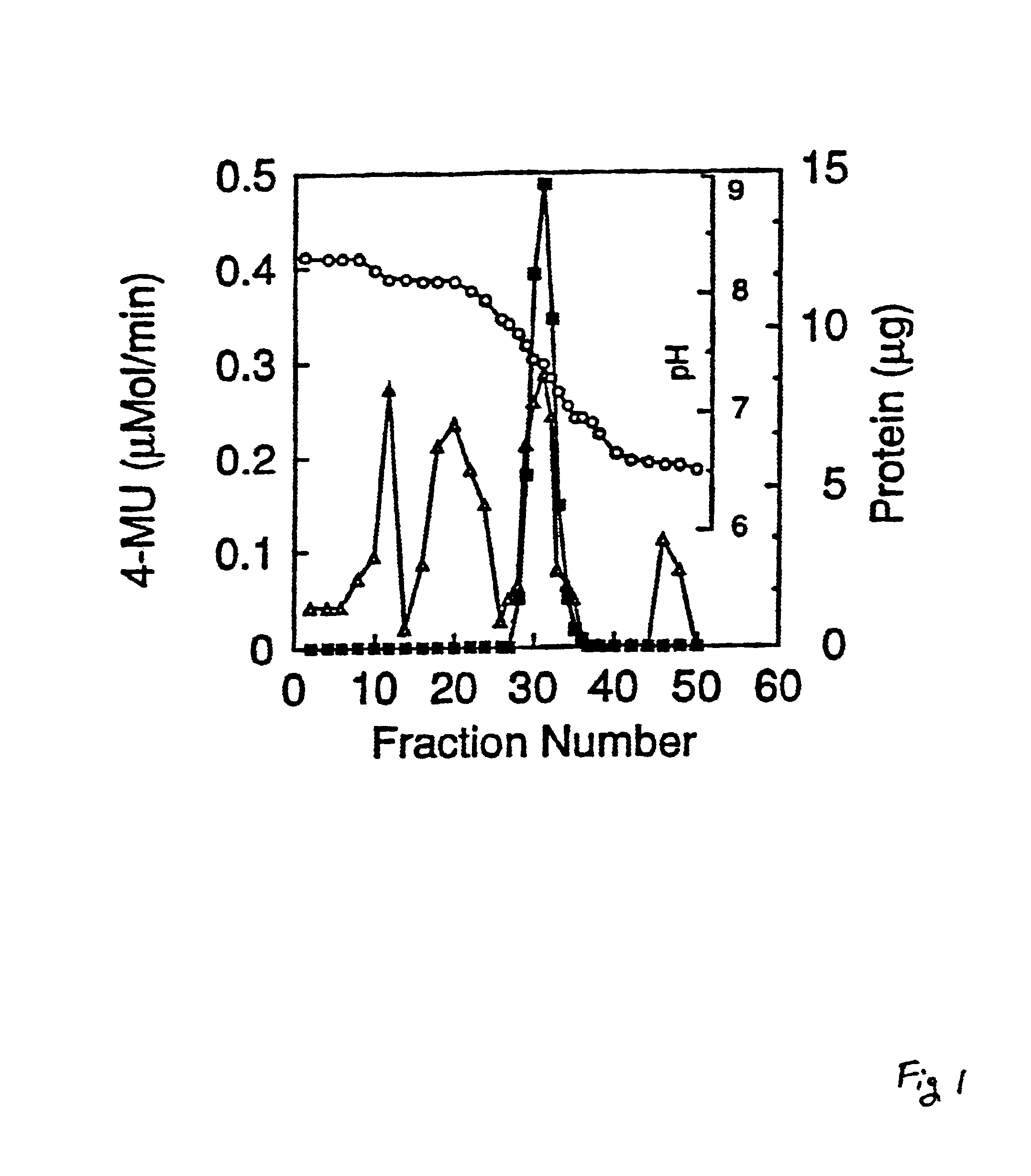
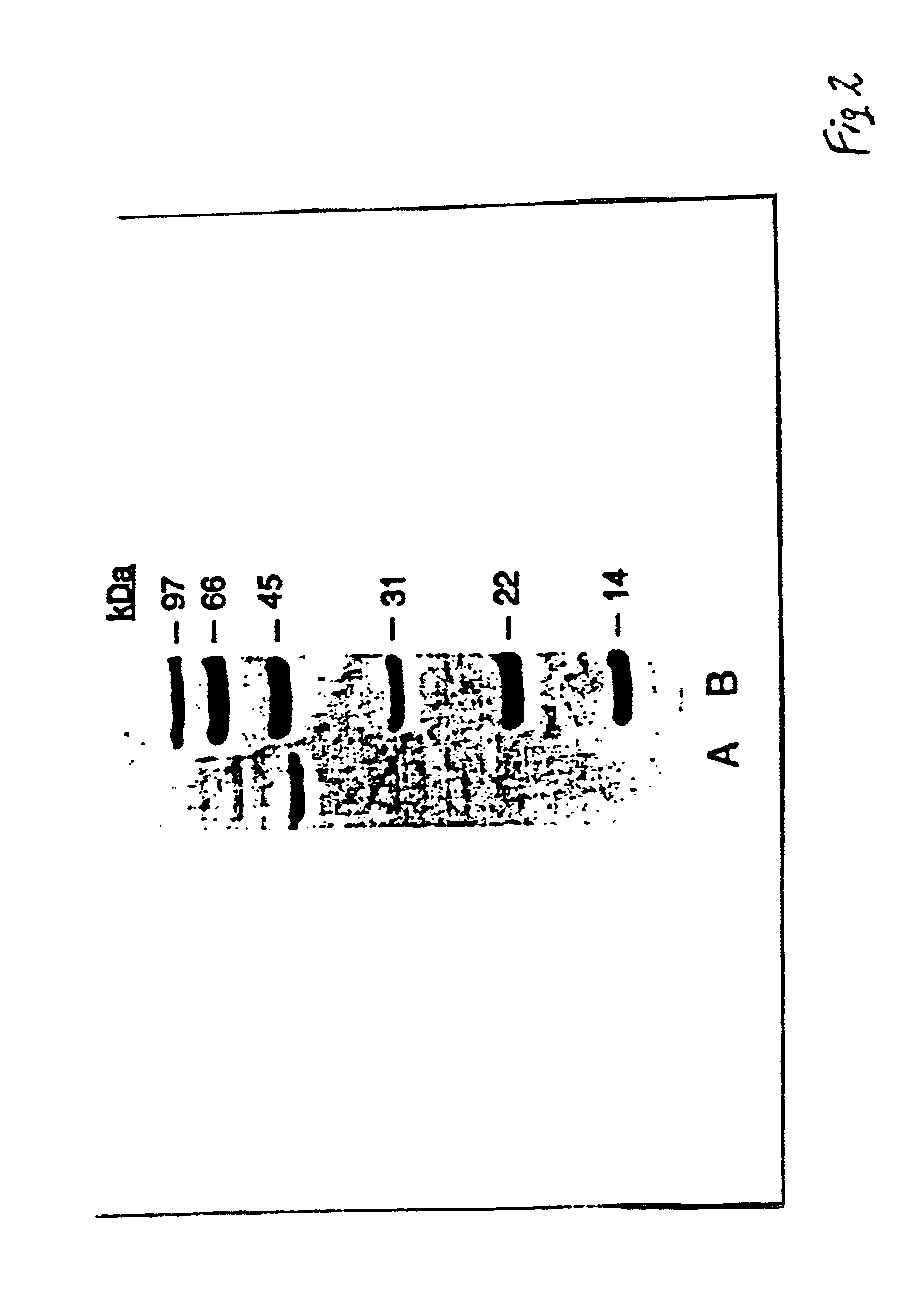
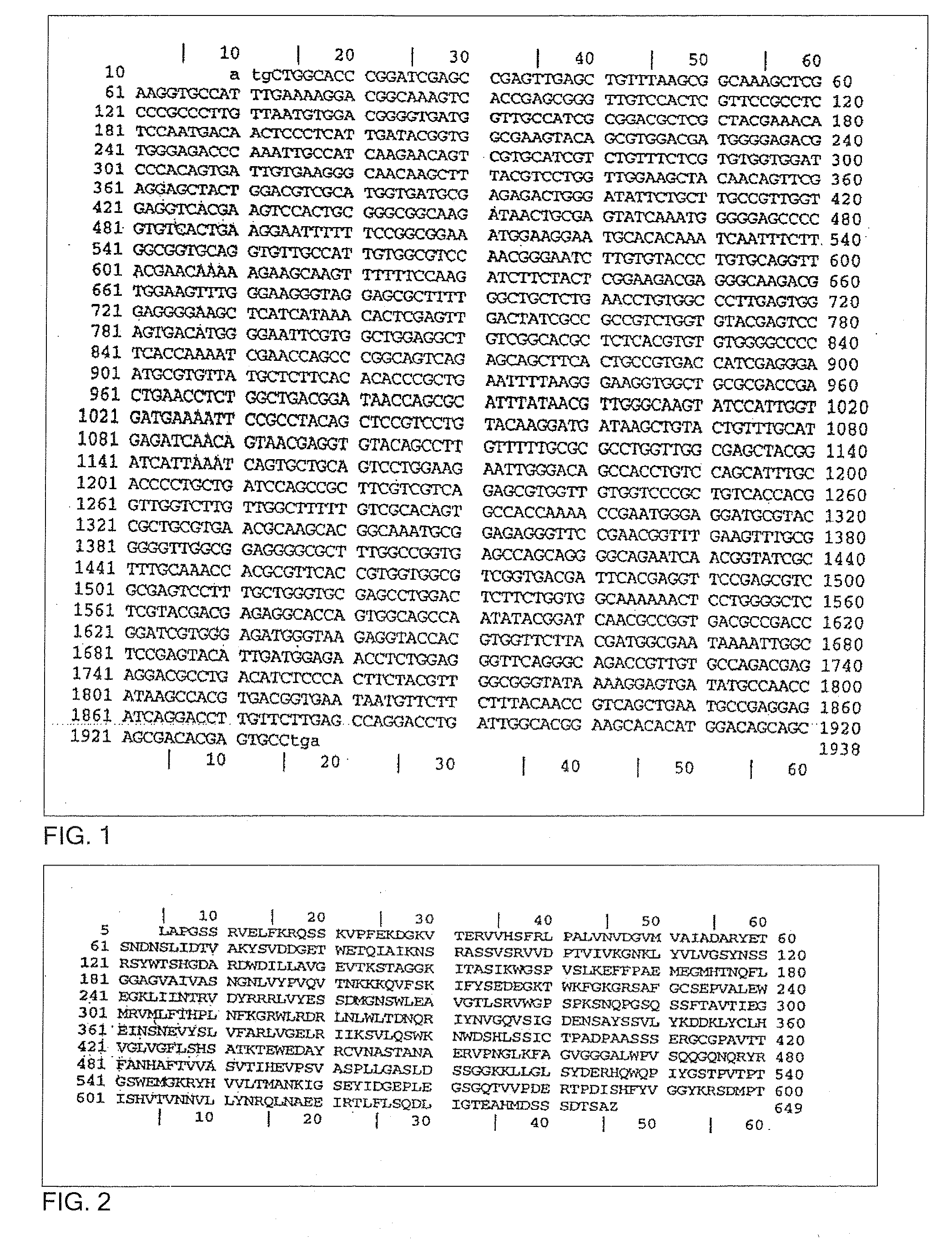
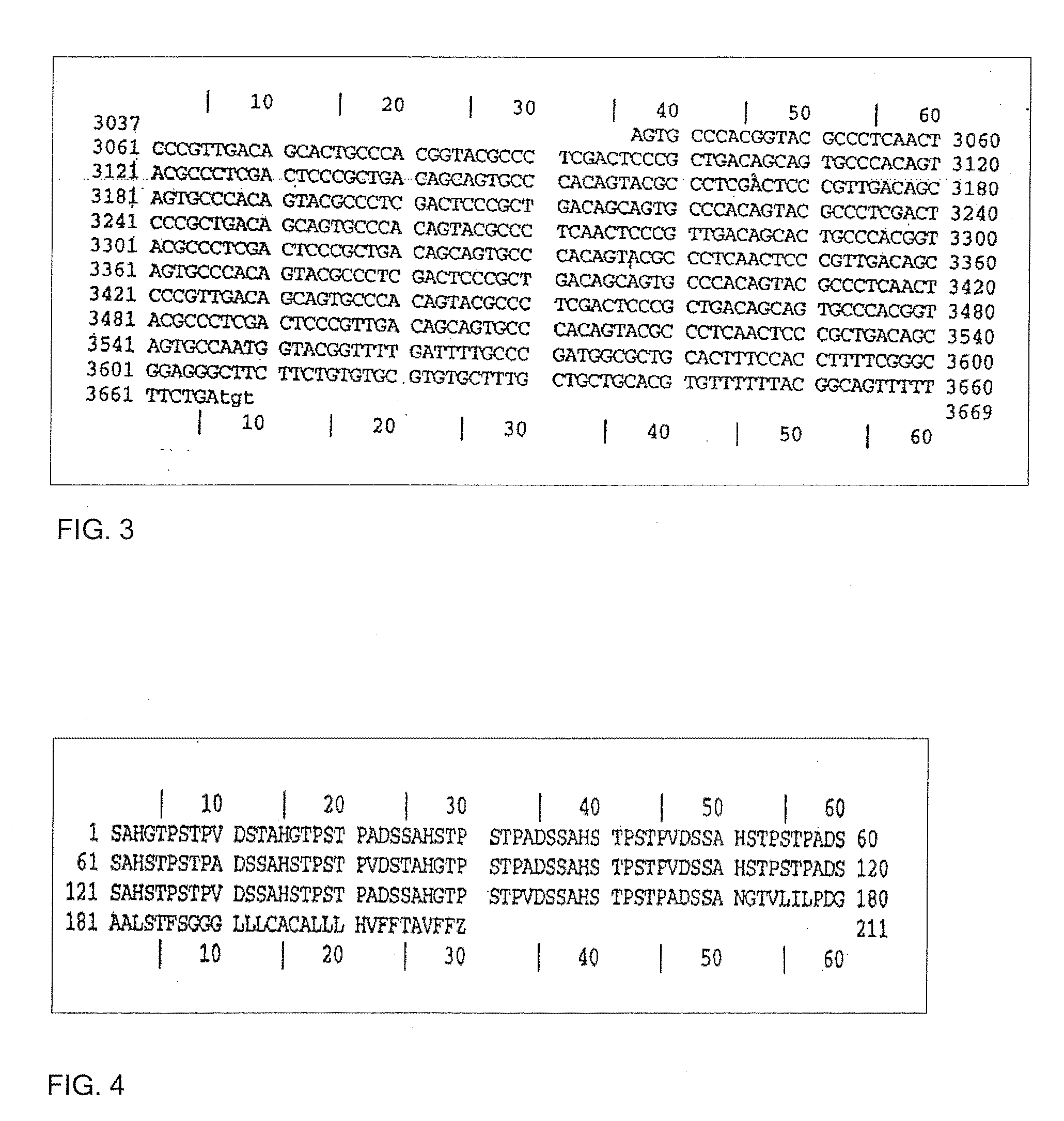
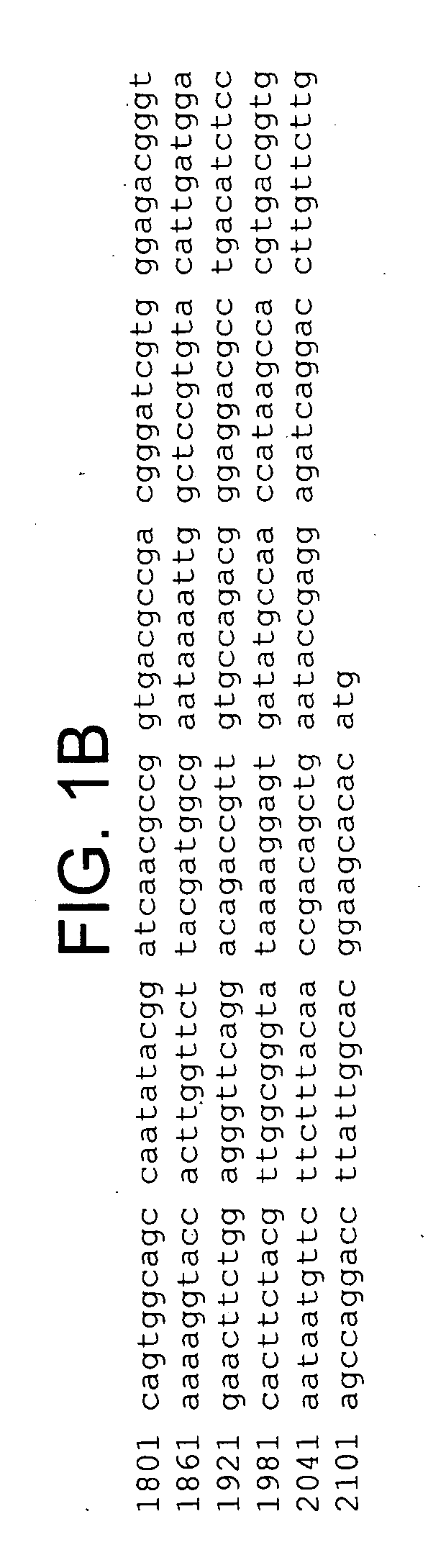
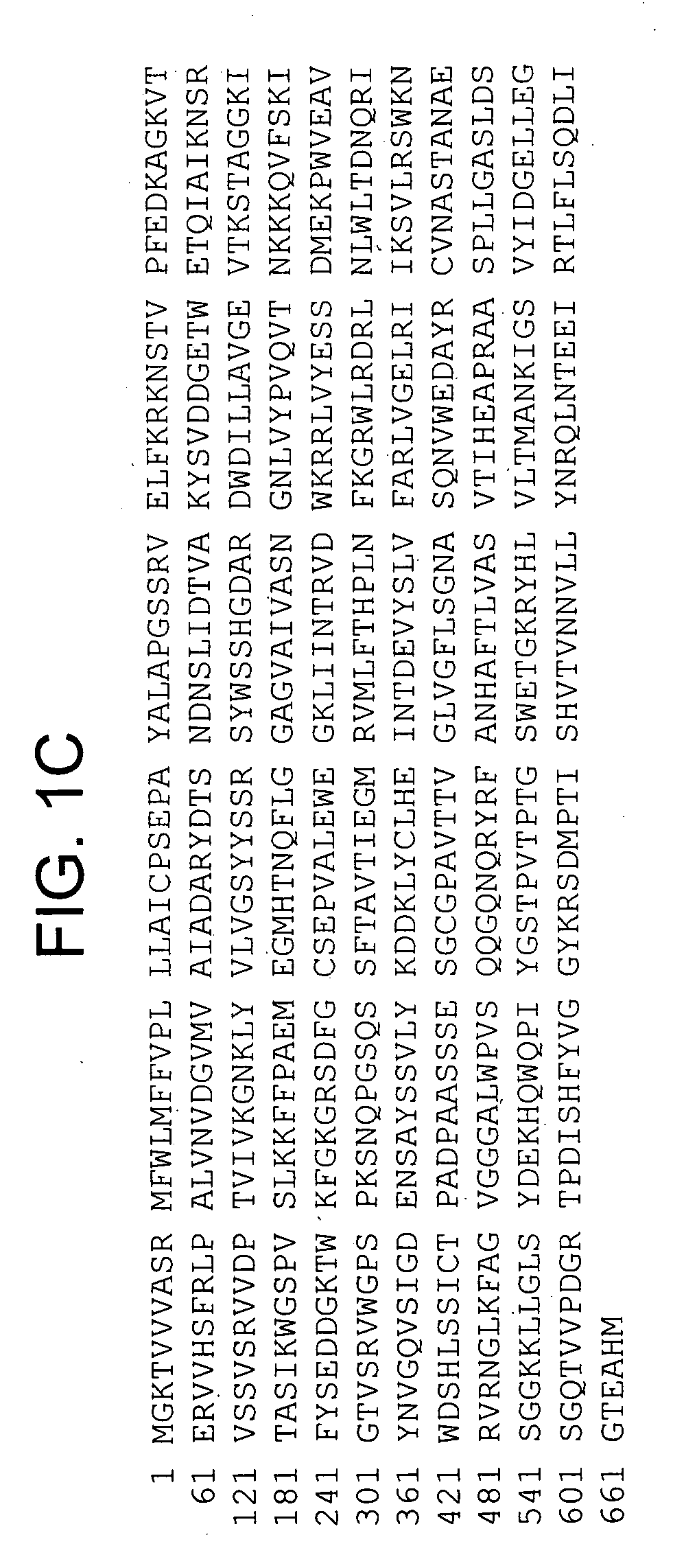
A recombinant cell line has a constitutive sialidase whose functional expression is disrupted, for example by homologous recombination or using antisense RNA. Sialidase is purified from cell culture fluid of Chinese hamster ovary cells. Nucleic acid encoding sialidase is obtained using an oligonucleotide probe designed using amino acid sequence data on the sialidase.
Owner:GENENTECH INC
Inhibitors of sialidase or sialidase-like enzymes
ActiveUS20120046355A1Improve purification effectEfficient processingAntibacterial agentsBiocideSialidaseCombinatorial chemistry
Owner:NAT RES COUNCIL OF CANADA
Pharmaceutical composition for treating cancer or diabetes
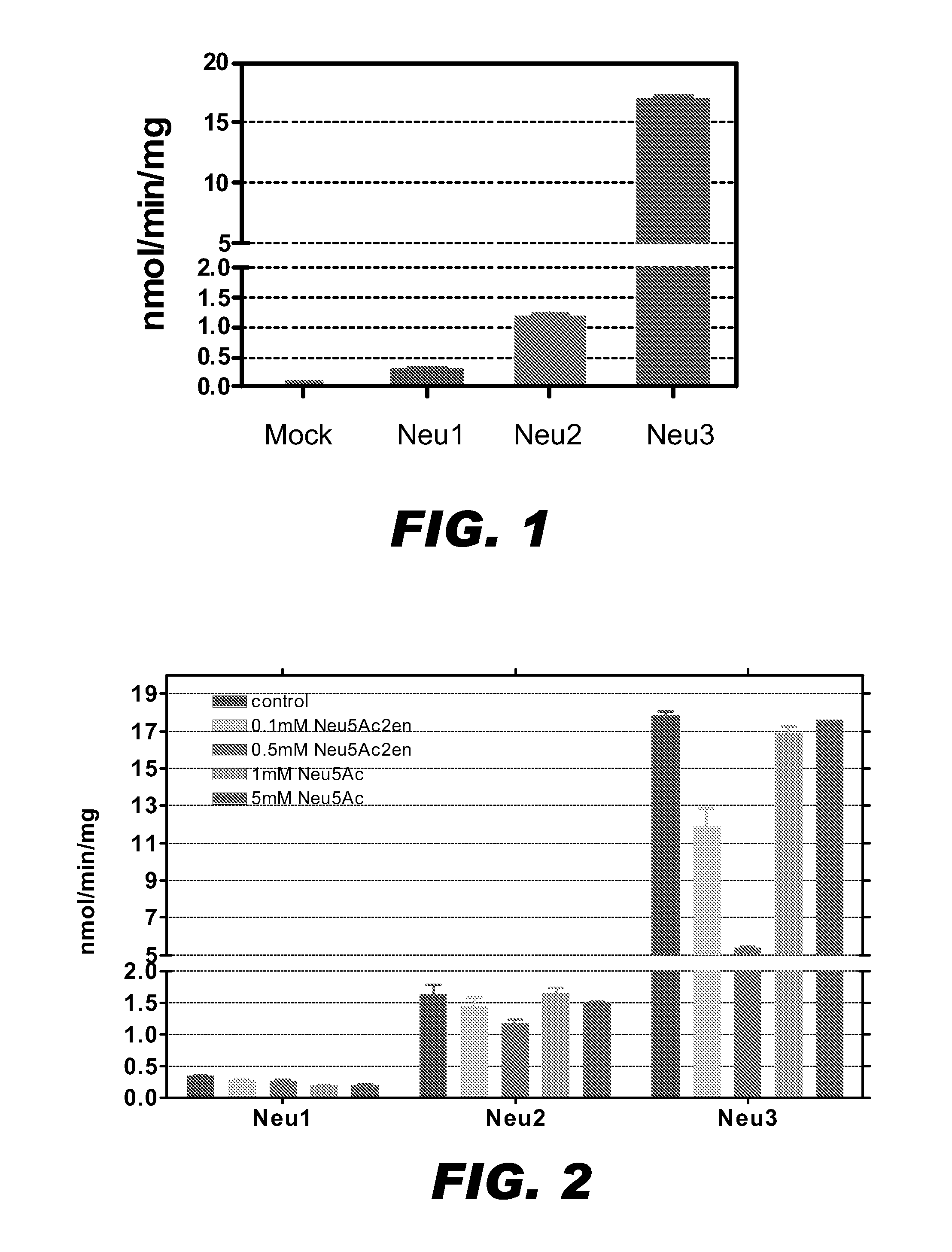
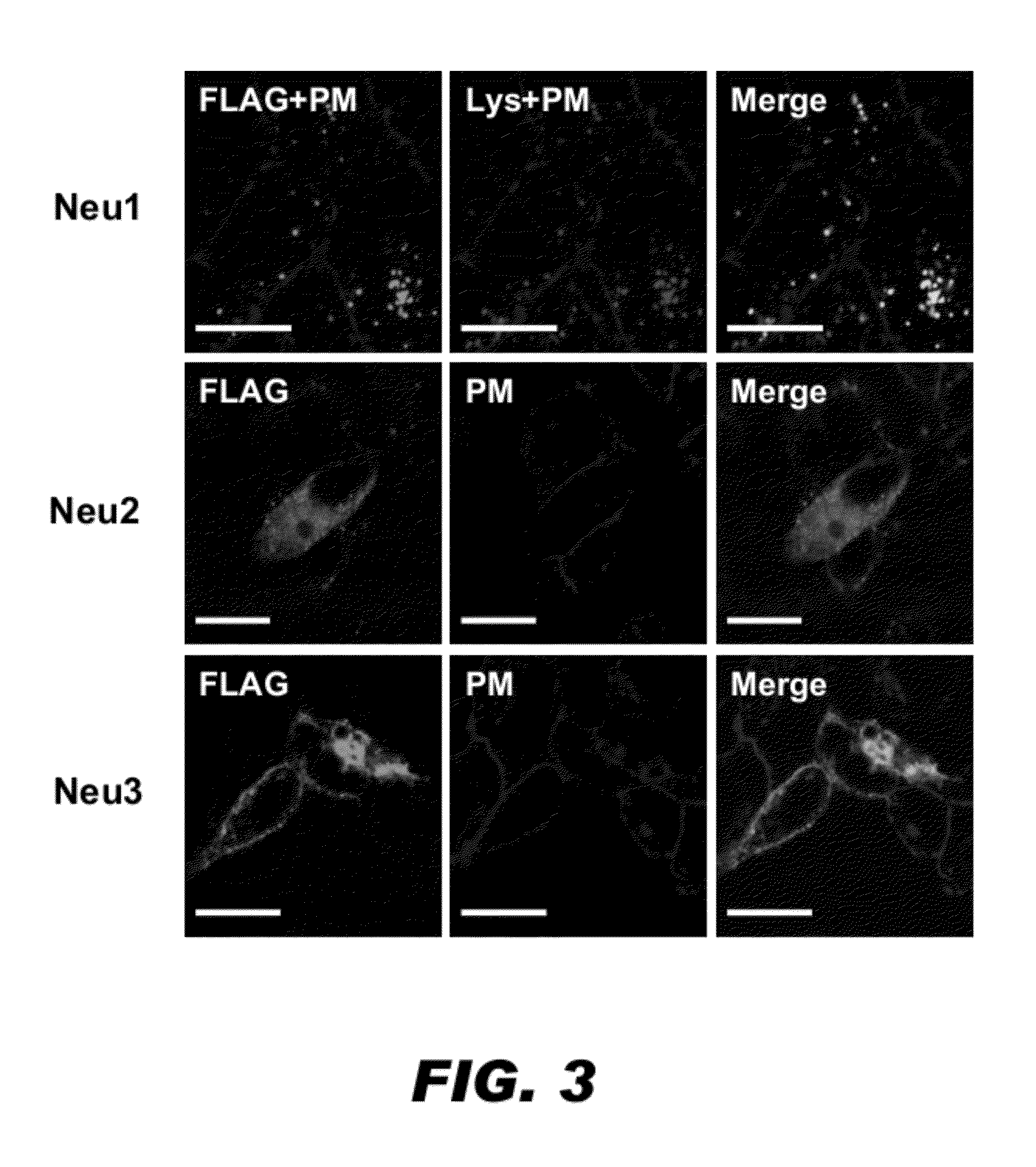
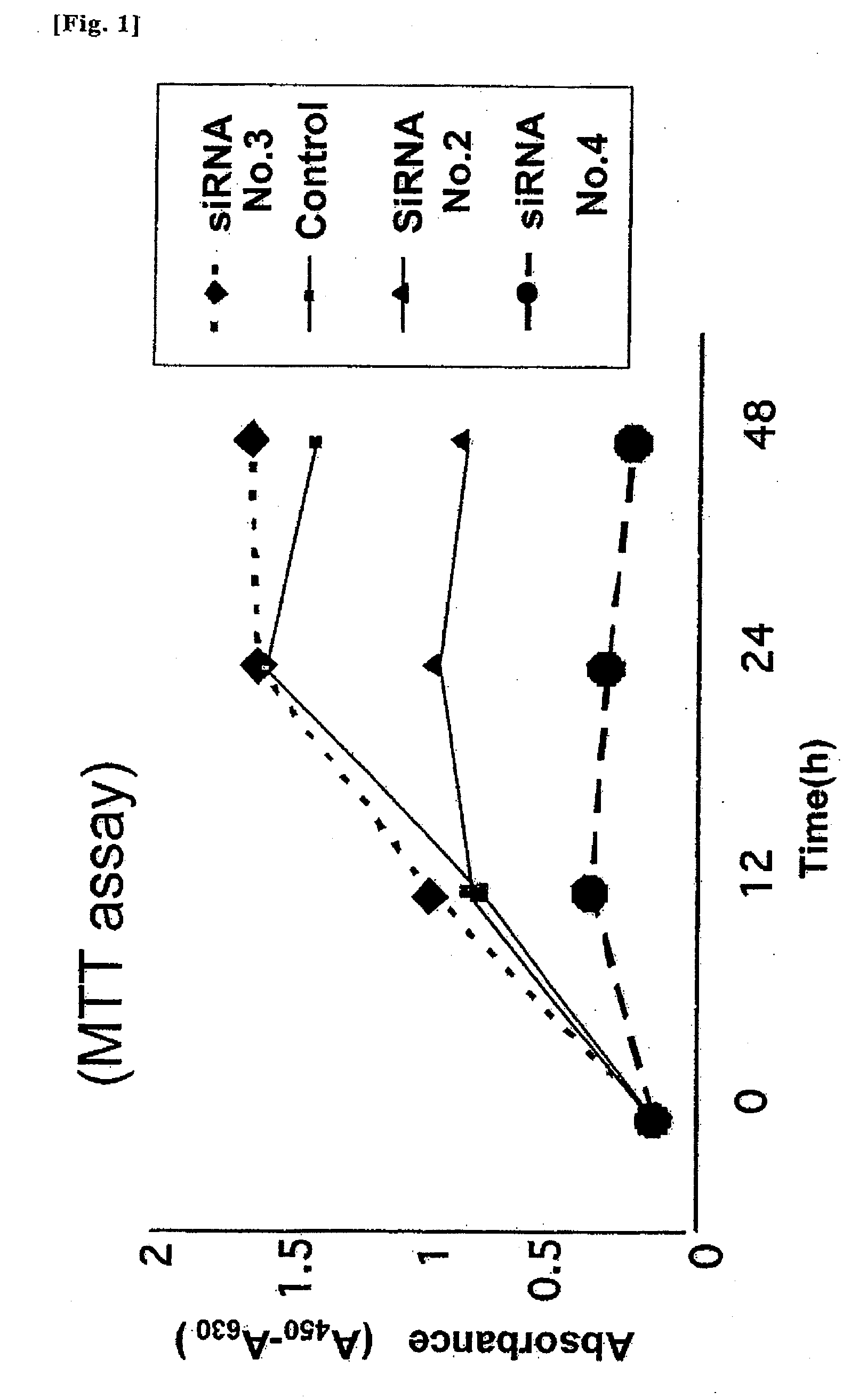
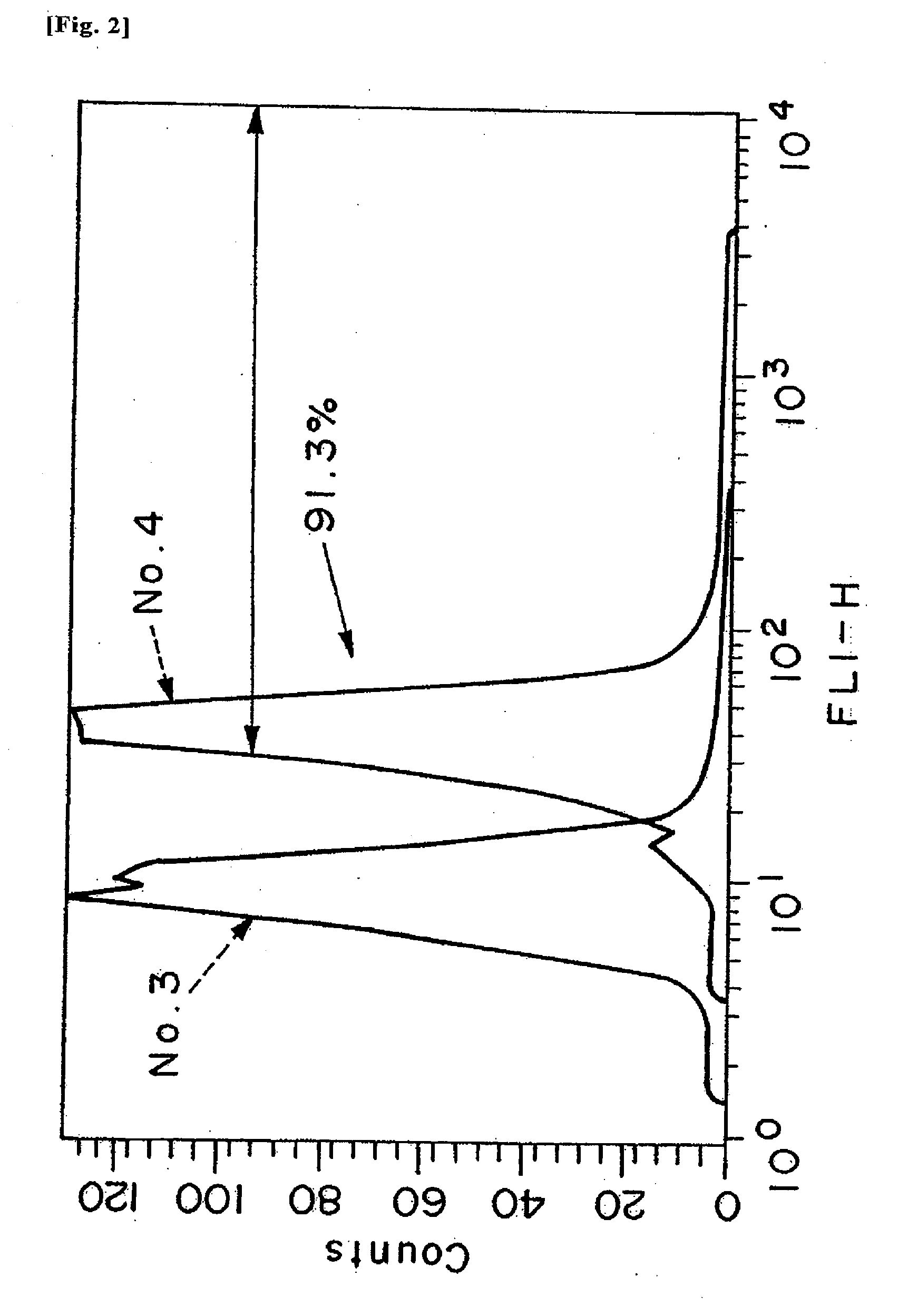
InactiveUS20090149402A1Inhibit expressionOrganic active ingredientsSugar derivativesDiabetes mellitusSialidase
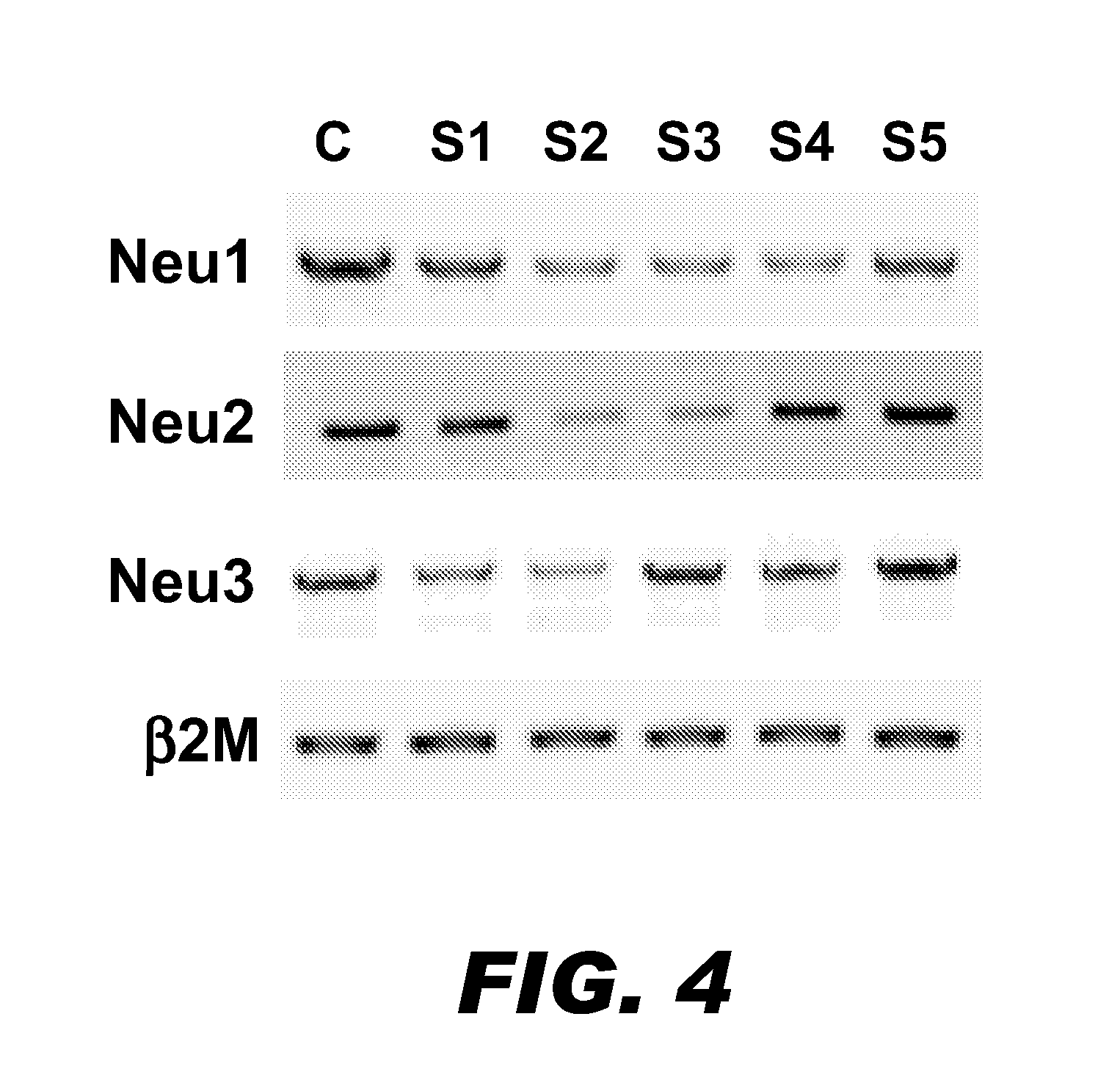
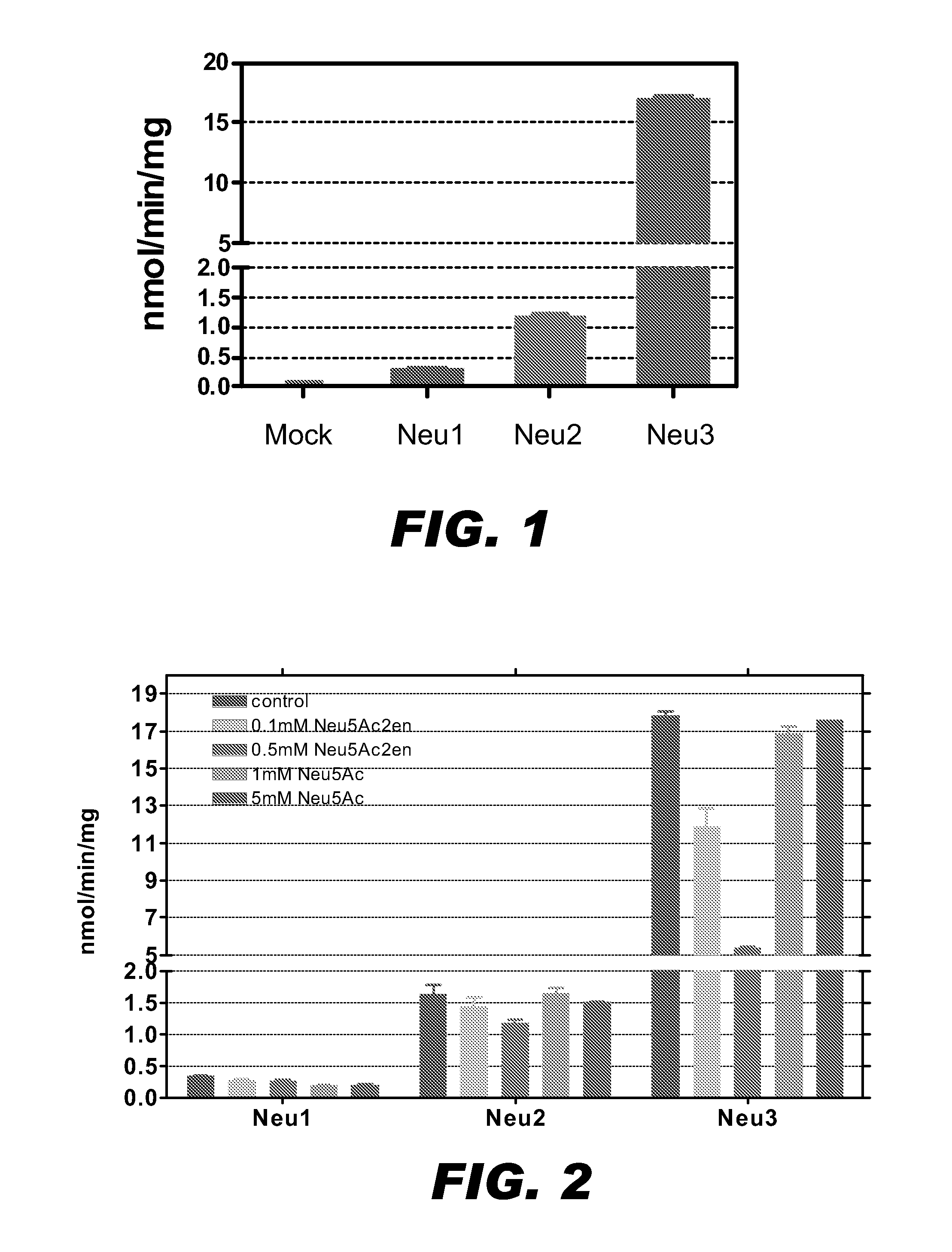
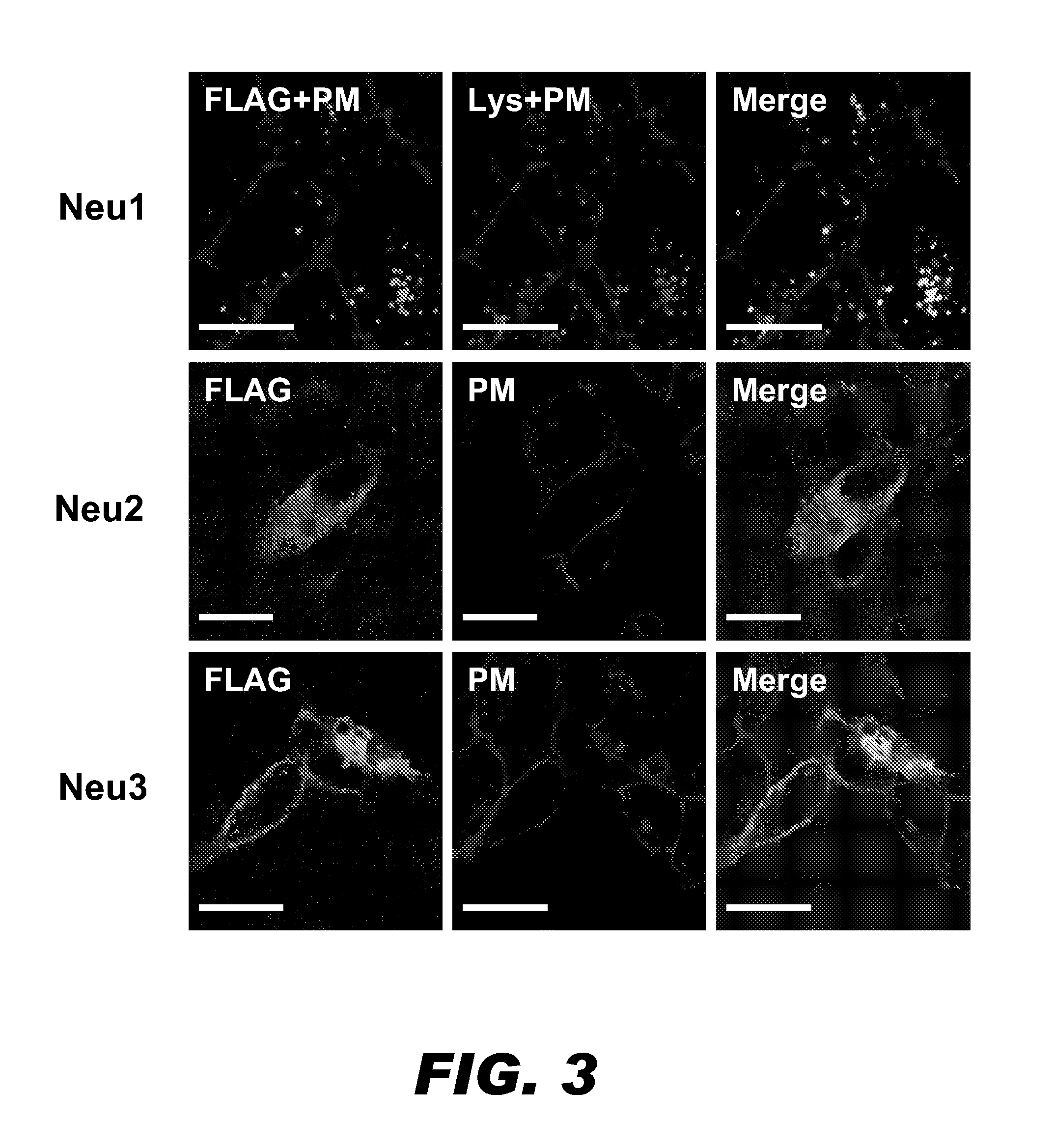
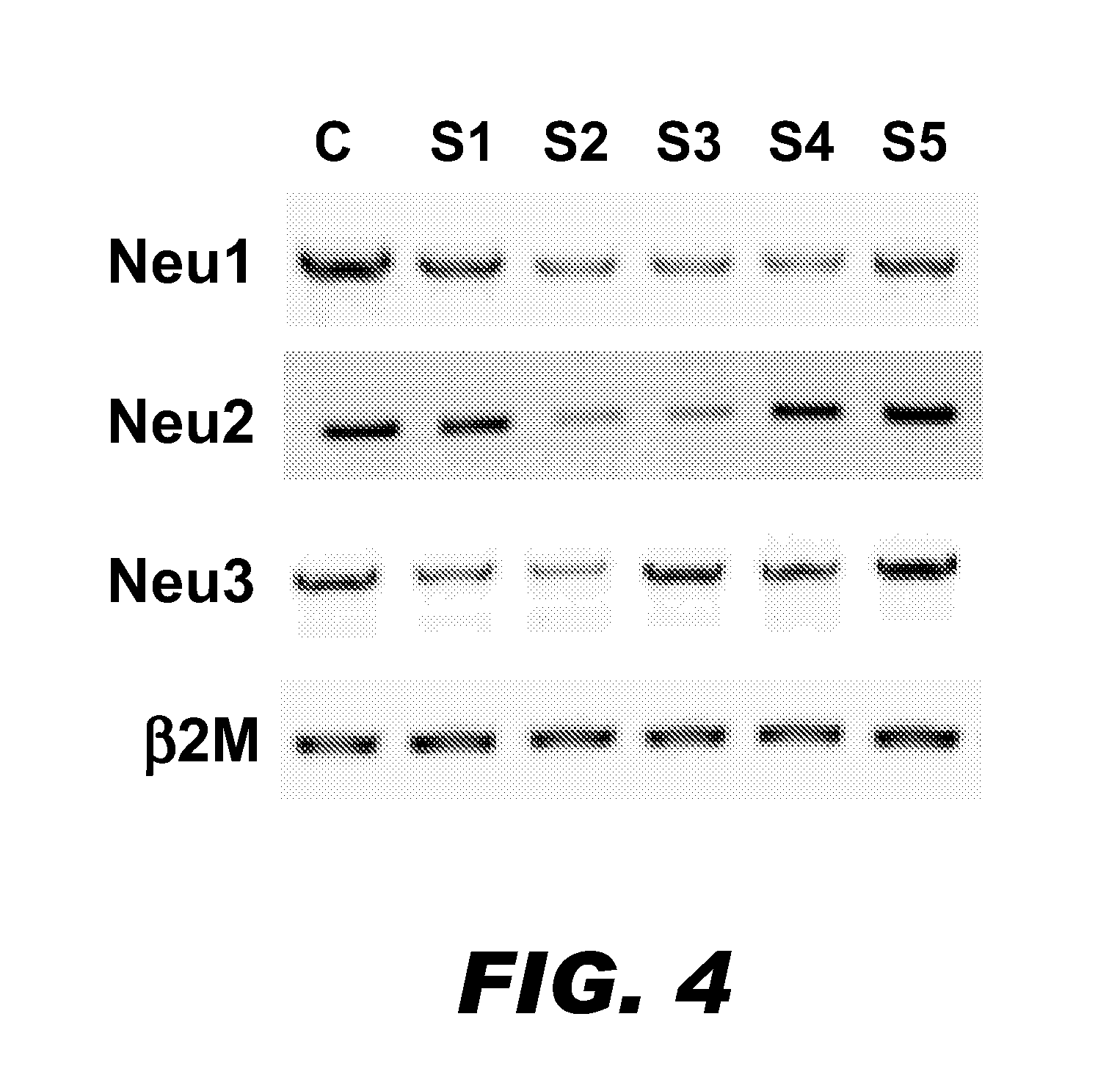
A pharmaceutical composition for treating cancer or diabetes which contains the following double-stranded RNA (A) or (B):(A) a double-stranded RNA having a sequence represented by SEQ ID NO:2, SEQ ID NO:4 or SEQ ID NO:8.(B) a double-stranded RNA which has a sequence of 20 to 30 nucleotides that is identical to a partial sequence of a gene encoding human plasma membrane-associated sialidase (NEU3) and contains the sequence represented by SEQ ID NO: 2, SEQ ID NO: 4, or SEQ ID NO: 8, and the double-stranded RNA inhibits the expression of a gene encoding human plasma membrane-associated sialidase (NEU3).
Owner:MIYAGI KEN
Multicomponent or monocomponent vaccine to be used against Chagas disease, pharmaceutical compositions containing them, procedure for the obtention of immunogen of said vaccines, and nucleic acid used in said procedure
InactiveUS8900598B2Stimulate immune responseEliminates itAntibacterial agentsPeptide/protein ingredientsChagas diseaseTrypanosomiasis
A vaccine against the Chagas disease, capable of stimulating the immune response against the trans-sialidase virulence factor of the Trypanosoma cruzi parasite, which is a multicomponent vaccine comprising: (a) an immunogenic portion formed by one or more recombinant or synthetic polypeptides or fractions of thereof and (b) one or more polynucleotides including the regions codifying one or more immunogenic polypeptides, or a monocomponent vaccine comprising at least one component selected among an immunogenic portion formed by one or more recombinant or synthetic polypeptides or fractions of them and a group of polynucleotides including the regions codifying one or more immunogenic polypeptides derived from Trypanosoma cruzi and pharmaceutical compositions containing said multicomponent and monocomponent vaccines, the procedures for obtaining the immunogen portion of said vaccines and the nucleic acid used in the procedure.
Owner:DE BAEREMAECKER BARROS CARLOS
Anti-trypanosomiasis vaccines and diagnostics
The present invention has as an object a novel genetic material coding for trans-sialidase-like proteins of African trypanosomes, and relates to the use of said genes and proteins in vaccines, therapeutics and diagnostics. The present invention also relates to the immunization of human and / or nonhuman animals against trypanosomosis.
Owner:UNIV BORDEAUX SEGALEN +1
Detection kit for bacterial vaginosis (BV) and sialidase detection method
InactiveCN102251021AShorten experiment timeFast reading stepsMicrobiological testing/measurementBacterial vaginosisSialidase
The purpose of the invention is to provide a detection kit for BV, and the detection kit has the advantages of a simple testing process, strong pertinency, high accuracy and fast and convenient operation. The technical scheme for the invention is that a detection kit for BV is provided and comprises a reaction solution and a colored solution, wherein the reaction solution comprises specific zymolytes, carbohydrates and a potassium acetate buffer, and the zymolytes are n-acetylneuraminic acid and salts thereof. The beneficial effect of the invention is simplification of testing methods and result interpretation of BV diagnosis. Discrimination with the sialidase detection method has the following advantages: 1, testing time is substantially saved, all the testing operation is finished with ten minutes or more, no other complex equipment is needed, and only a baking box or a culture case at a temperature of 37 DEG C is needed to provide; 2, the interpretation step is rapid and visual, and the rate of misoperation and misdiagnosis are greatly reduced; 3, the detection card is sensitive, accurate and convenient, and has a high accordance rate with the detection of BV by golden standard the Amsel method.
Owner:泰普生物科学(中国)有限公司
Multicomponent or monocomponent vaccine to be used against chagas disease, pharmaceutical compositions containing them, procedure for the obtention of immunogen of said vaccines, and nucleic acid used in said procedure
InactiveUS20100297186A1Stimulate immune responseReduces clinical consequenceAntibacterial agentsProtozoa antigen ingredientsChagas diseaseTrypanosomiasis
A vaccine against the Chagas disease, capable of stimulating the immune response against the trans-sialidase virulence factor of the Trypanosoma Cruzi parasite, which is a multicomponent vaccine comprising: (a) an immunogenic portion formed by one or more recombinant or synthetic polypeptides or fractions of thereof and (b) one or more polynucleotides including the regions that codifying one or more immunogenic polypeptides, or a monocomponent comprising at least one component selected among an immunogenic portion formed by one or more recombinant or synthetic polypeptides or fractions of them and a group of polynucleotides including the regions codifying one or more immunogenic polypeptides derived from Trypanosoma Cruzi and pharmaceutical compositions containing said multicomponent and monocomponent vaccines, the procedures for obtaining the immunogen portion of said vaccines and the nucleic acid used in the procedure.
Owner:DE BAEREMAECKER BARROS CARLOS
T. cruzi-derived neurotrophic agents and methods of use therefor
The invention relates to T. cruzi trans-sialidase (TS) and to the neurotrophic and IL-6 secretion-inducing activities of the protein. TS, neurotrophic variants and / or neurotrophic peptides based upon the sequence of TS can be administered to a mammal to directly or indirectly provide neurotrophic support for neurons. A mammalian neurotrophic factor (e.g., CNTF, LIF) can be co-administered with the TS, neurotrophic variant and / or neurotrophic peptide. TS, IL-6 secretion-inducing variants and / or IL-6 secretion-inducing peptides based upon the sequence of TS can be administered to a mammal to induce the secretion of IL-6. TS, active variants and / or active peptides can be administered to a mammal having an acquired or congenital condition characterized by neuronal degeneration or to a mammal that has experienced trauma to the brain, spinal cord or peripheral nerves. The invention also relates to neurotrophic and IL-6 secretion-inducing variants of TS and to neurotrophic and IL-6 secretion-inducing peptides. The invention also relates to compositions comprising TS, active variants thereof and / or active peptides and a physiologically acceptable carrier.
Owner:TRUSTEES OF TUFTS COLLEGE
Kit for preparing CA125 surface Tn antigen by magnetic particle chemiluminescence immunoassay and preparation method thereof
ActiveCN109781986ARemarkable substrate specificityEasy to identifyChemiluminescene/bioluminescenceFermentationBiotin-streptavidin complexAntigen
The invention relates to the technical field of immunoassay, in particular to a kit for preparing a CA125 surface Tn antigen by magnetic particle chemiluminescence immunoassay and a preparation methodthereof. The kit comprises a magnetic separation reagent coupled with a Fab fragment of a CA125 antibody, a biotin-lectin reagent, and a streptavidin-biotin-alkaline phosphatase coupling reagent, wherein the biotin-lectin reagent containing sialidase is capable of identifying the Tn antigen. The magnetic separation reagent is specifically bound to the CA125 antigen; the biotin-lectin specificallyrecognizes the Tn antigen of the CA125 antigen surface; the streptavidin-biotin-alkaline phosphatase coupling reagent is specifically bound to the biotin-lectin reagent to realize amplification of the detection signal; the sialidase hydrolyzes a glucosidic bond connected with the sialic acid on the Tn antigen surface and the sialic acid is released to expose the Tn antigen. Therefore, the detection accuracy, sensitivity and linear range are improved. The kit has advantages of high specificity and high sensitivity and is capable of realizing fully automated testing.
Owner:THE OBSTETRICS & GYNECOLOGY HOSPITAL OF FUDAN UNIV
Novel medicinal composition and preparation method
InactiveCN101850110AIncreased anti-lung cancer effectSmall side effectsPeptide/protein ingredientsViral/bacteriophage medical ingredientsCholesterolGlycoprotein G
The invention relates to a novel medicinal composition for improving organism immune function and resisting tumor to address both the symptoms and root cause, and a preparation method. The medicinal composition comprises the following components: 1,500 milliliters of Newcastle disease virus allantoic fluid with a titer of 1:640, 2 milliliters of neuraminidase, 2 milliliters of nuclease, 1.08 grams of glycoprotein G, 10 grams of galactose, 1.0 milligrams of biotin, 5 grams of fucose, 15 grams of astragalus polysaccharide, 2.0 milligrams of sialidase, 2.0 milligrams of phospholipid, 10 milligrams of uracil, 5 milligrams of Vit C, 4.0 milligrams of cholesterol, 200 milligrams of ATP, 22 milliliters of 10 percent KCl, 3.6 grams of MgSO4.7H2O, and 10 milligrams of prothrombin. The experiments for pharmacodynamics, pharmacology and the like, show that the medicinal composition has good inhibiting and killing effects on mouse transplantable tumor such as Lewis lung carcinoma. Compared with the medicament of the conventional invention, the medicinal composition has more remarkable diffidence in the growth inhibiting effect on the mouse Lewis lung carcinoma. The medicinal composition has obvious effect, and is a novel biological anticancer preparation with good social and economic benefits.
Owner:天津泽世德生物医药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com