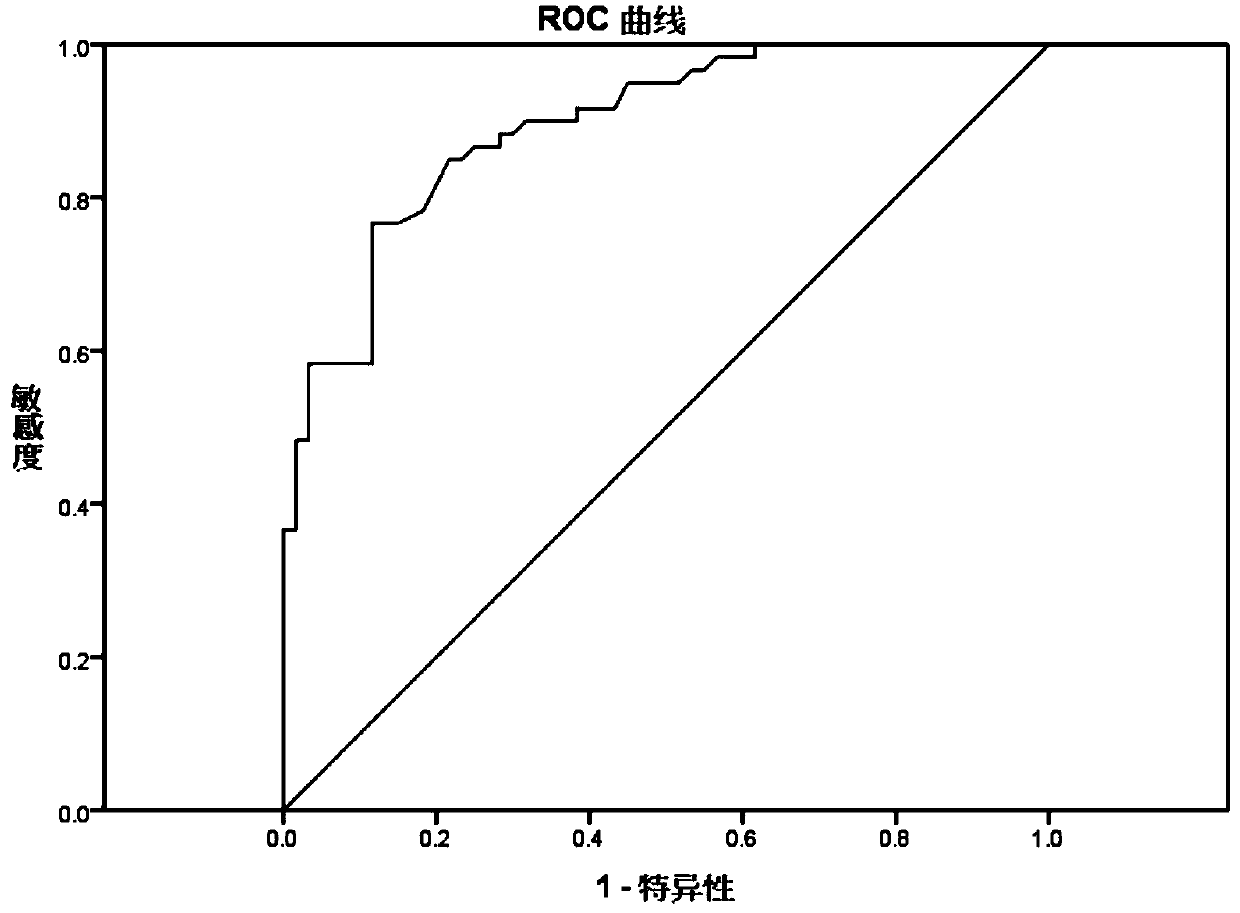
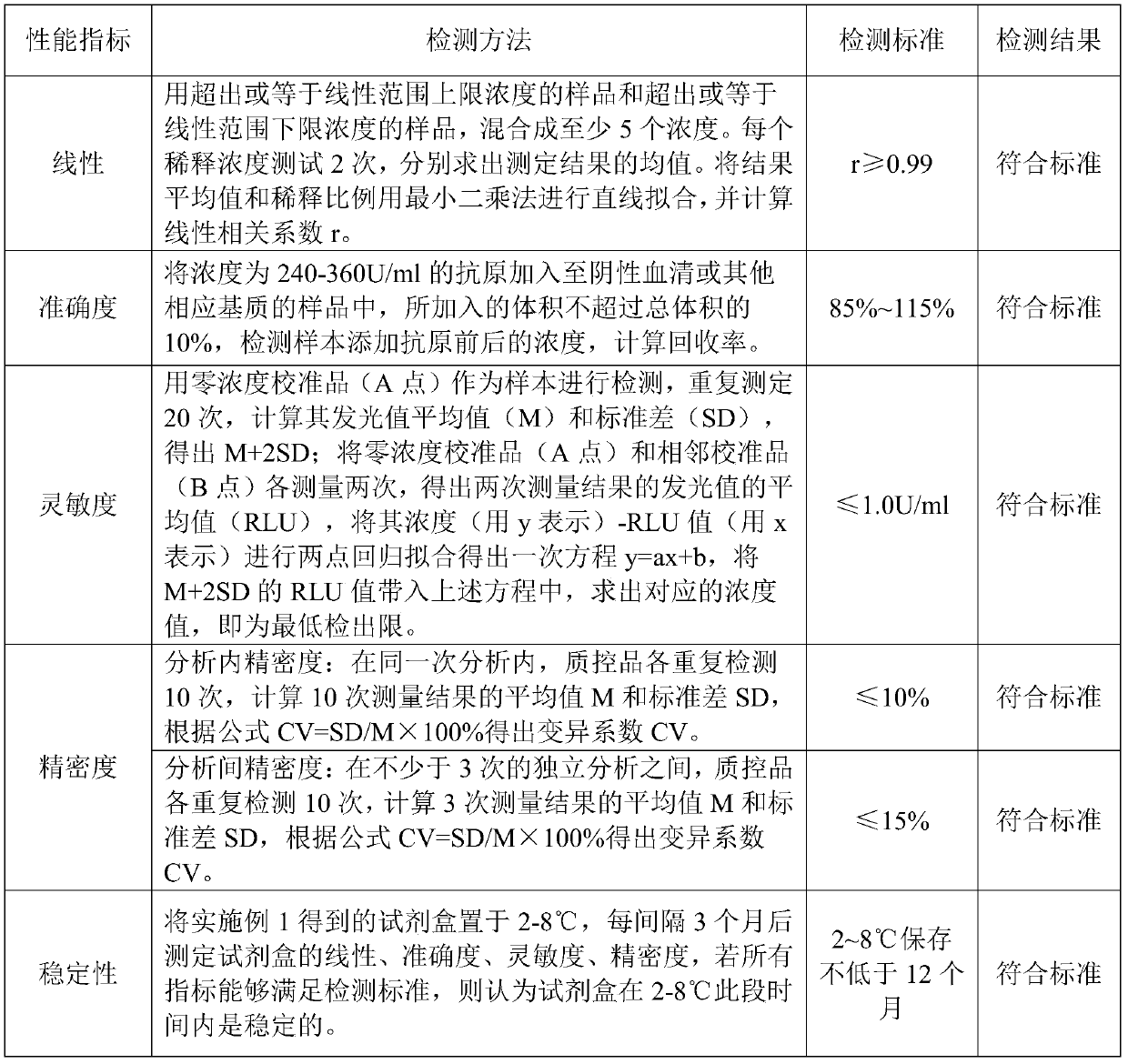
Kit for preparing CA125 surface Tn antigen by magnetic particle chemiluminescence immunoassay and preparation method thereof
A chemiluminescence immunoassay, CA125 technology, applied in chemiluminescence/bioluminescence, analysis, fermentation and other directions by making materials undergo chemical reactions, which can solve the problem of difficult integration and full automation, complicated operation steps, and large fluctuations in final results. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] The kit for the magnetic particle chemiluminescence immunoassay of CA125 surface Tn antigen in this embodiment includes a magnetic separation reagent coupled with the Fab fragment of the Ov185 monoclonal antibody, Biotin-VVA reagent containing sialidase, SA-Biotin-ALP reagent, chemical Luminescence substrate solution, washing solution and calibrator working solution.
[0077] The preparation method of the kit for the magnetic particle chemiluminescence immunodetection of CA125 surface Tn antigen in this embodiment comprises the following steps:
[0078] 1. Preparation of magnetic separation reagent coupled with Ov185 monoclonal antibody Fab fragment:
[0079] 1. Preparation of immobilized papain:
[0080] Ⅰ. Accurately measure 50mg Fe 3 o 4 Nano-magnetic particles were placed in a 10ml polystyrene plastic test tube, placed on a magnetic rack, removed from the supernatant after precipitation, washed twice with 5ml pure water, and added 5ml 0.01M PBS buffer (0.14M NaCl...
Embodiment 2
[0146] Different from Example 1,
[0147] Step Ⅰ is to accurately measure 50mg Fe 3 o 4 Nano-magnetic particles were placed in a 10ml polystyrene plastic test tube, placed on a magnetic rack, and the supernatant was removed after precipitation, washed twice with 10ml pure water, and 10ml 0.01M PBS buffer (0.14MNaCl, 3mM KCl, 10mM Na 2 HPO 4 , 2mM KH 2 PO 4, pH=7.2±0.05), then add papain with 0.05 times the mass of magnetic beads, and mix at room temperature for 1.5 hours;
[0148] Step II is to place the magnetic beads on the magnetic rack after mixing, remove the supernatant after precipitation, add 7.5ml 0.2mM hydrochloric acid buffer, and mix at room temperature for 10 minutes;
[0149] Step III is to add 0.3mg of 0.5M HEPES buffer solution (pH=7.4±0.1) after mixing, adjust the pH to 7.3±0.1, and mix at 37°C for 2.5 hours;
[0150] Step IV is to add 225 mg of glycine after mixing, and mix for 1 hour at 37°C;
[0151] Step Ⅴ is to place the magnetic bead solution on ...
Embodiment 3
[0167] Different from Example 1,
[0168] Step Ⅰ is to accurately measure 50mg Fe 3 o 4 Nano-magnetic particles were placed in a 10ml polystyrene plastic test tube, placed on a magnetic rack, removed from the supernatant after precipitation, washed twice with 25ml pure water, and added 25ml 0.01M PBS buffer (0.14MNaCl, 3mM KCl, 10mM Na 2 HPO 4 , 2mM KH 2 PO 4 , pH=7.2±0.05), then add papain with 0.04 times the mass of magnetic beads, and mix at room temperature for 1 hour;
[0169] Step II is to place the magnetic beads on the magnetic rack after mixing, remove the supernatant after precipitation, add 15ml 0.2mM hydrochloric acid buffer, and mix at room temperature for 10 minutes;
[0170] Step III is to add 0.125mg of 0.5M HEPES buffer solution (PH=7.4±0.1) after the mixing, adjust the pH to 7.3±0.1, and mix at 37°C for 2.5 hours;
[0171] Step IV is to add 75 mg of glycine after mixing, and mix for 1 hour at 37°C;
[0172] Step Ⅴ is to place the magnetic bead solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com