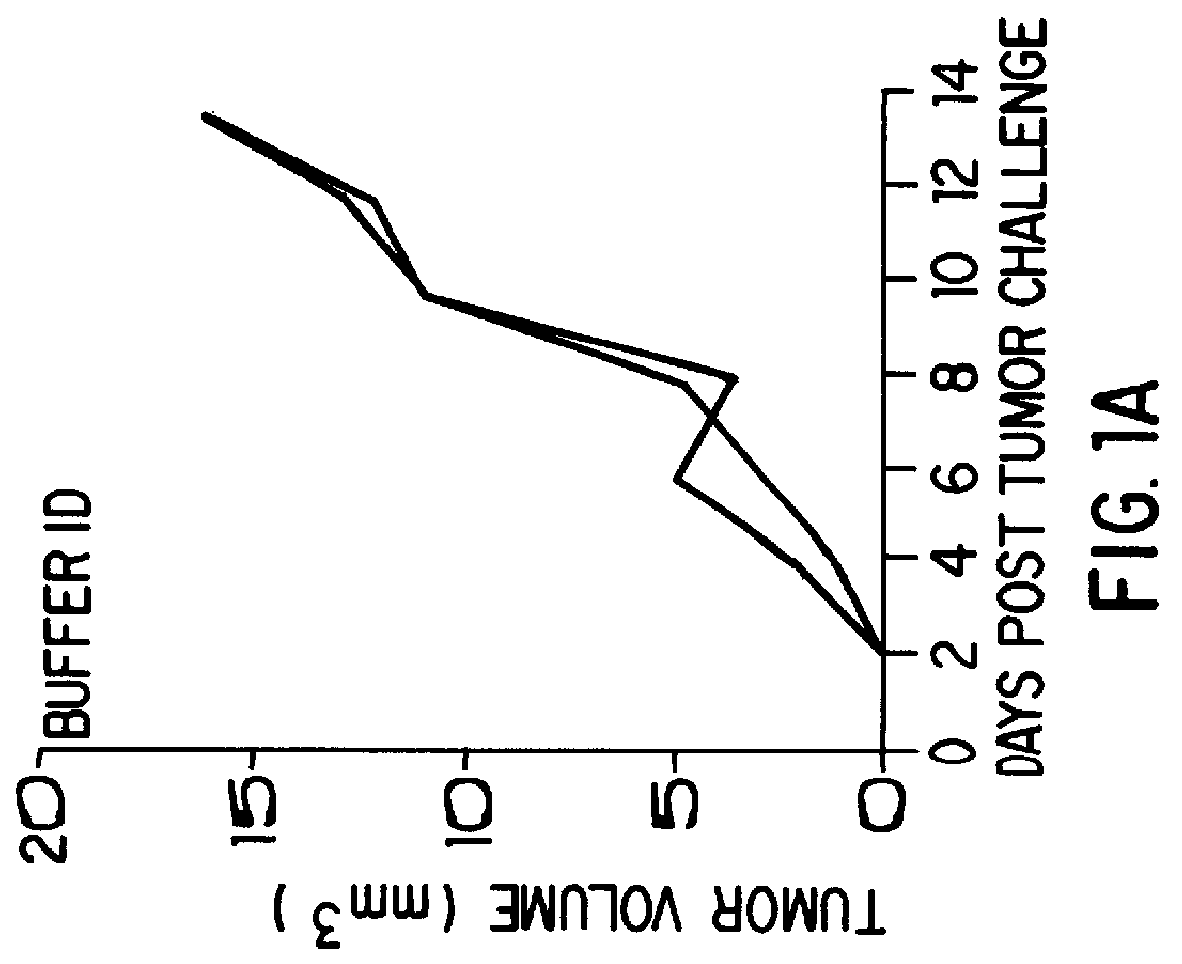
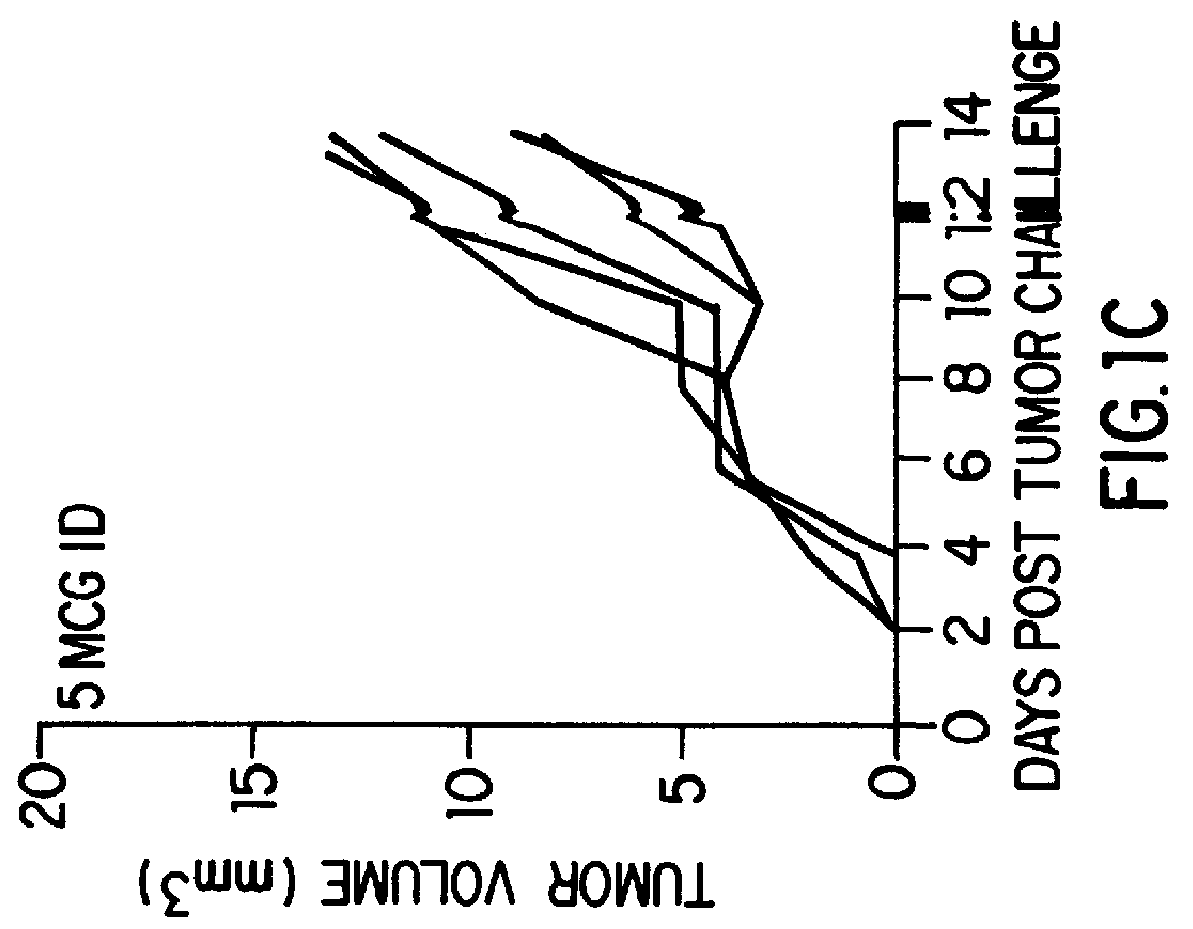
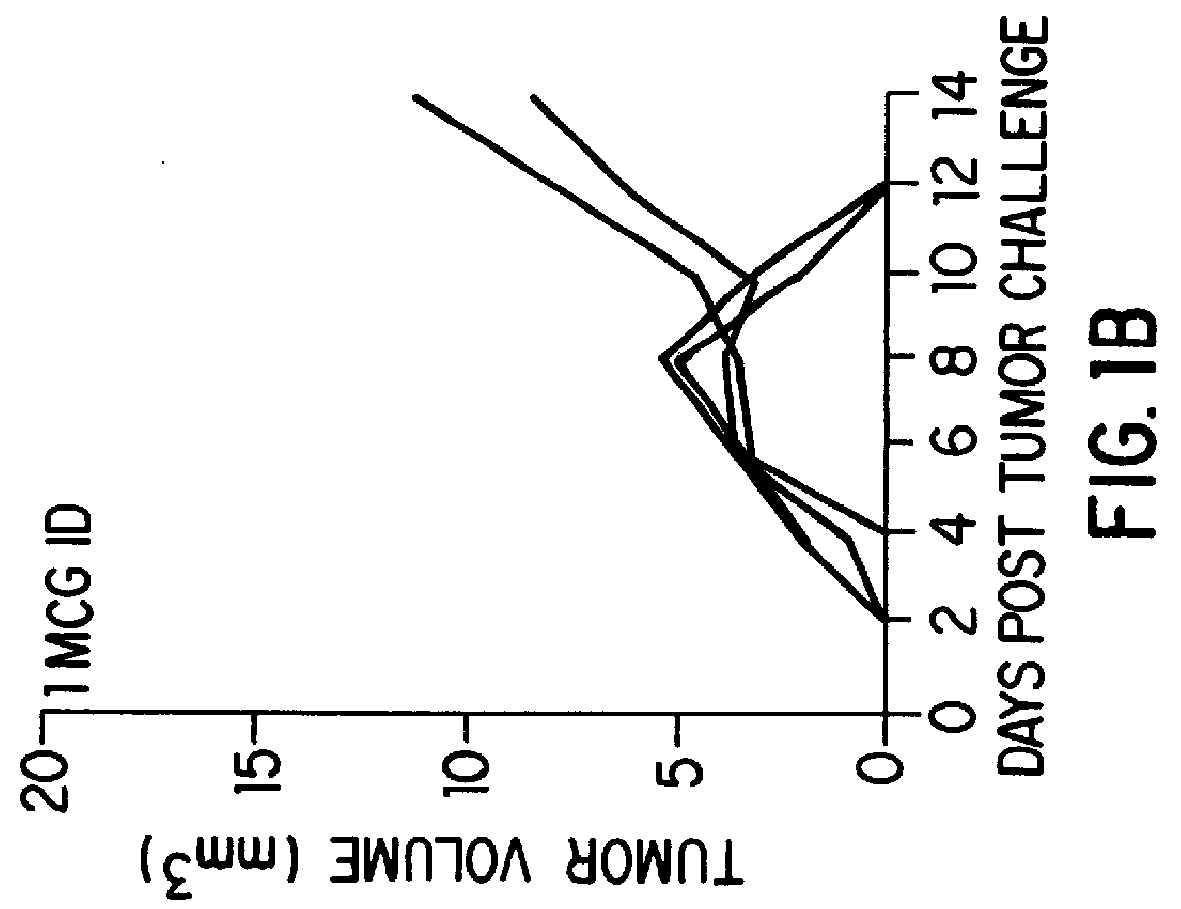
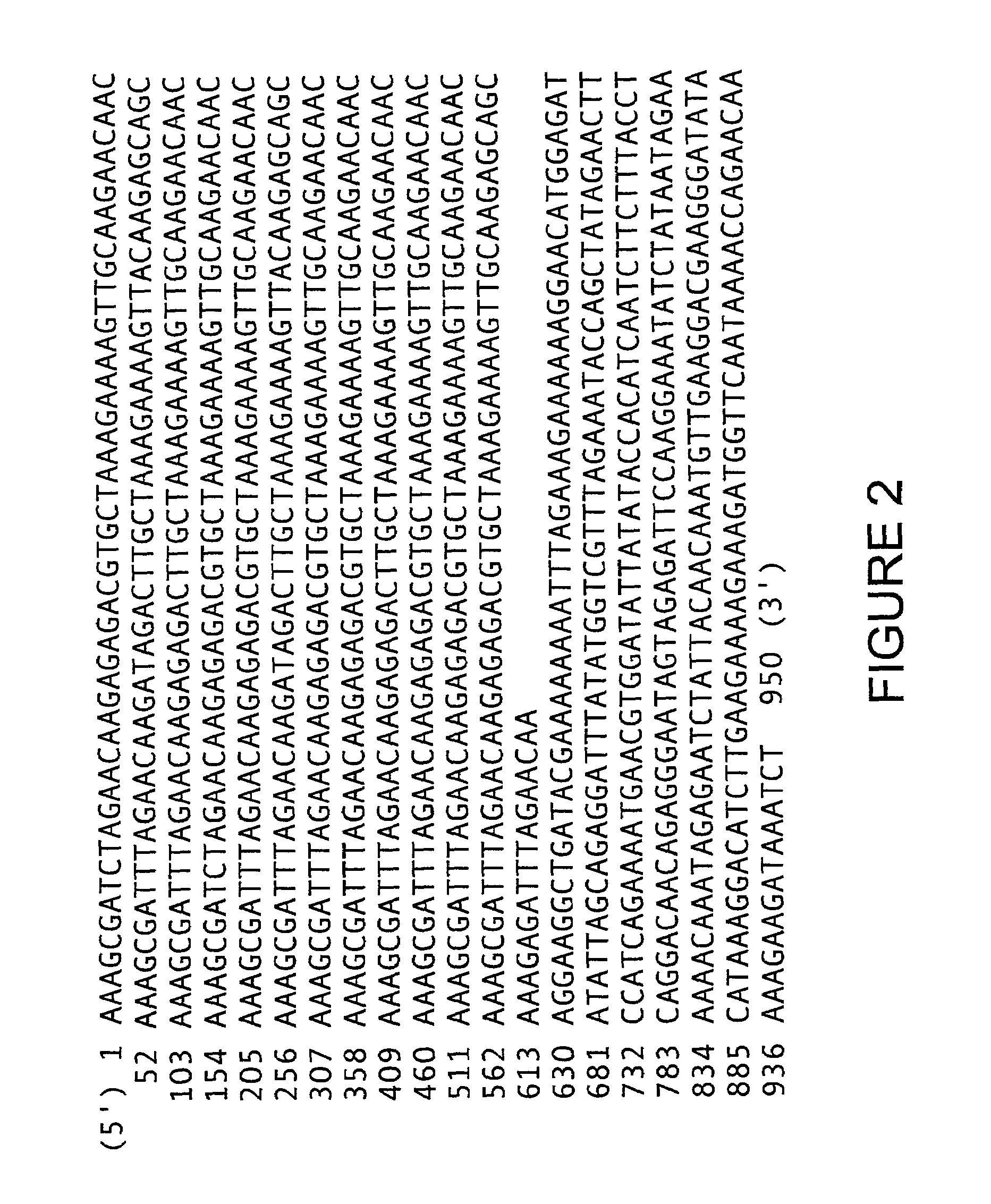
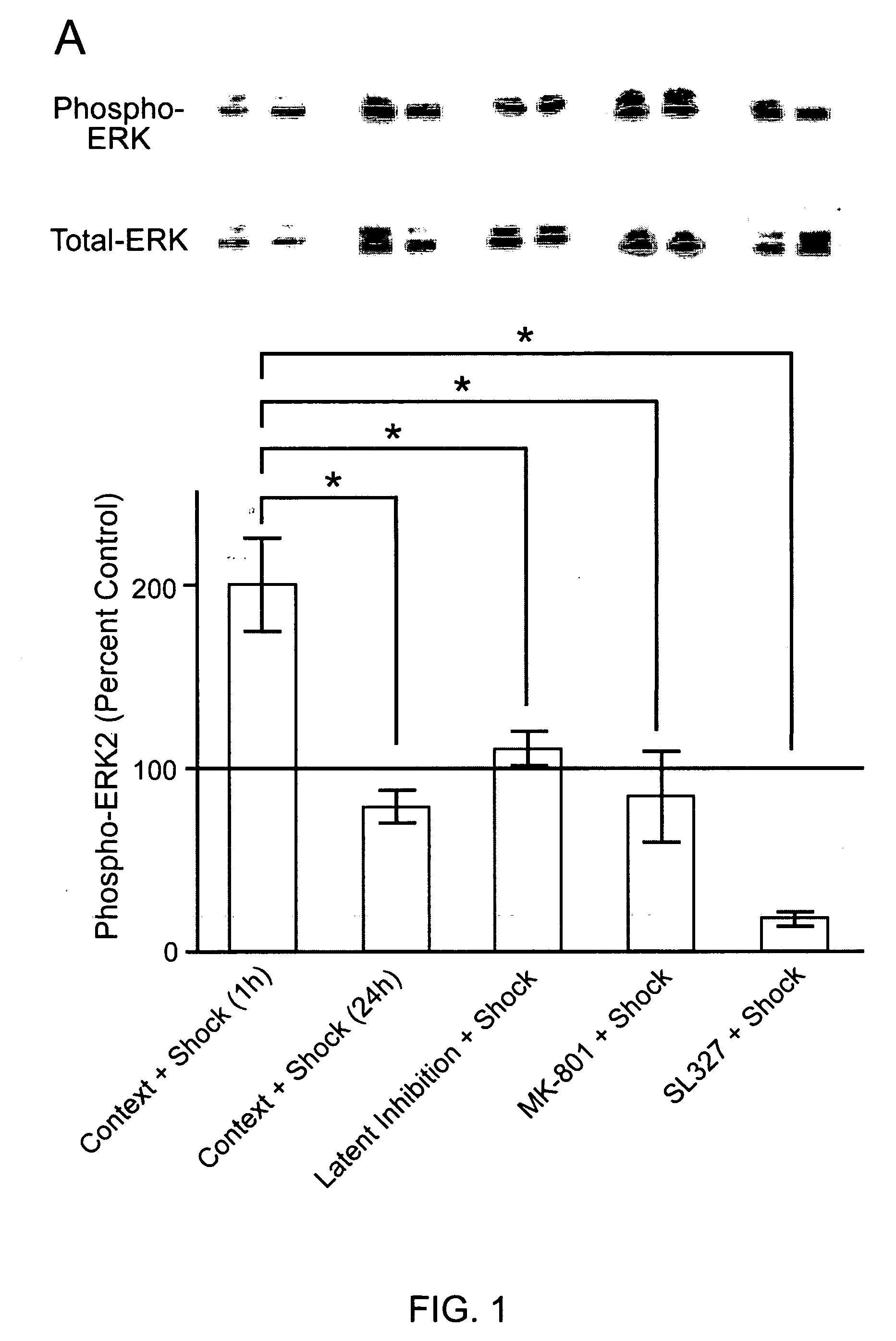
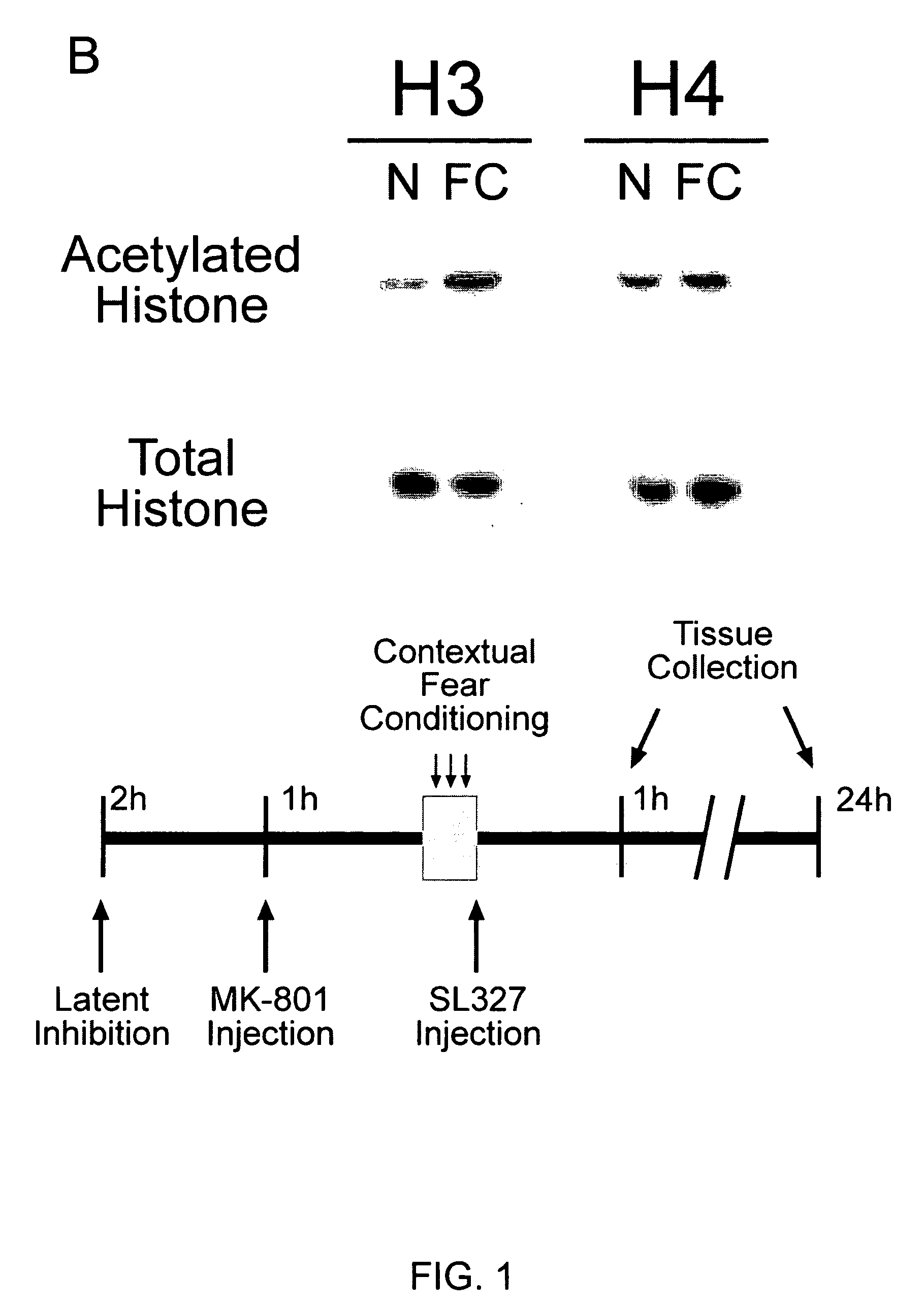
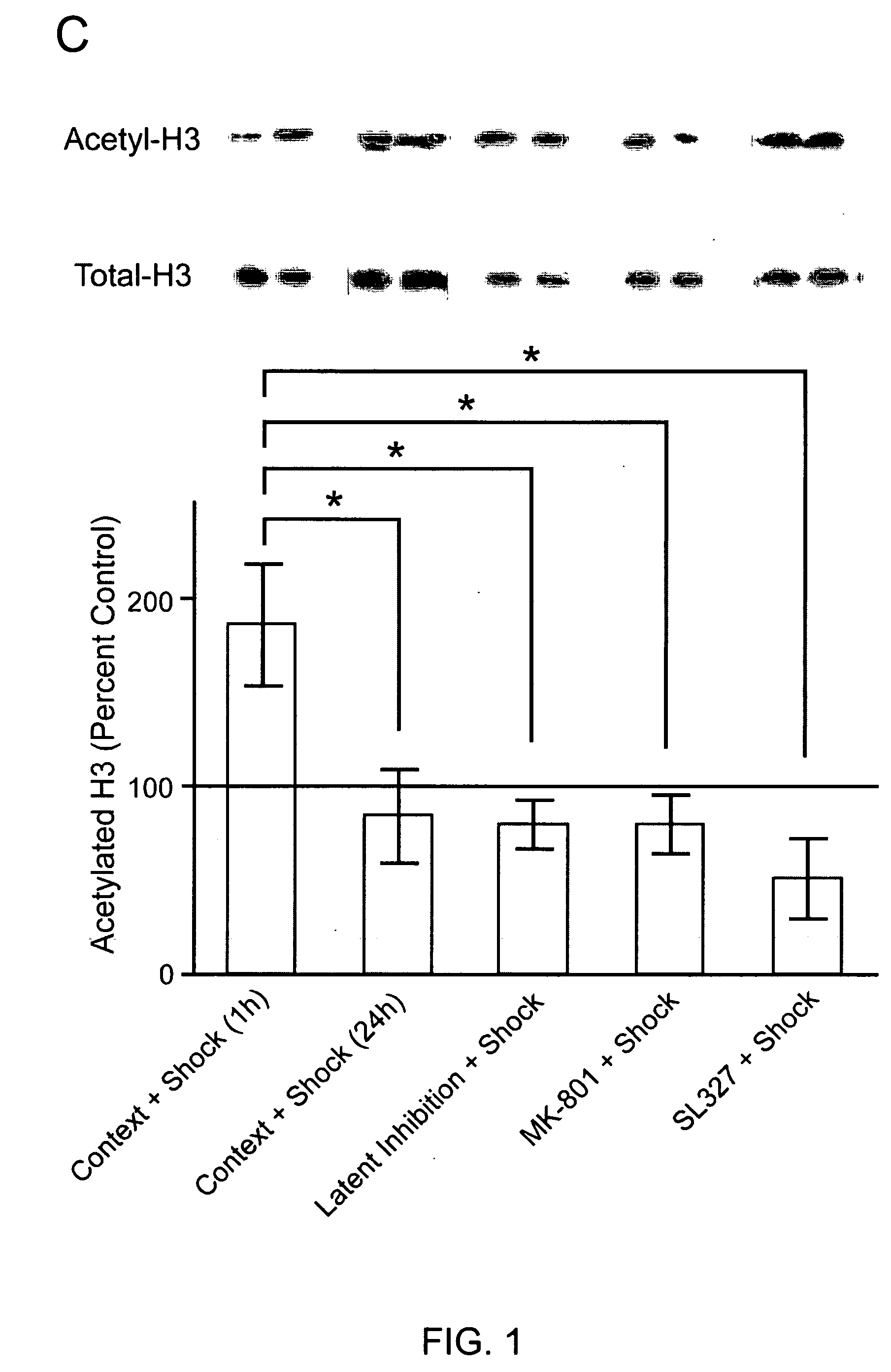
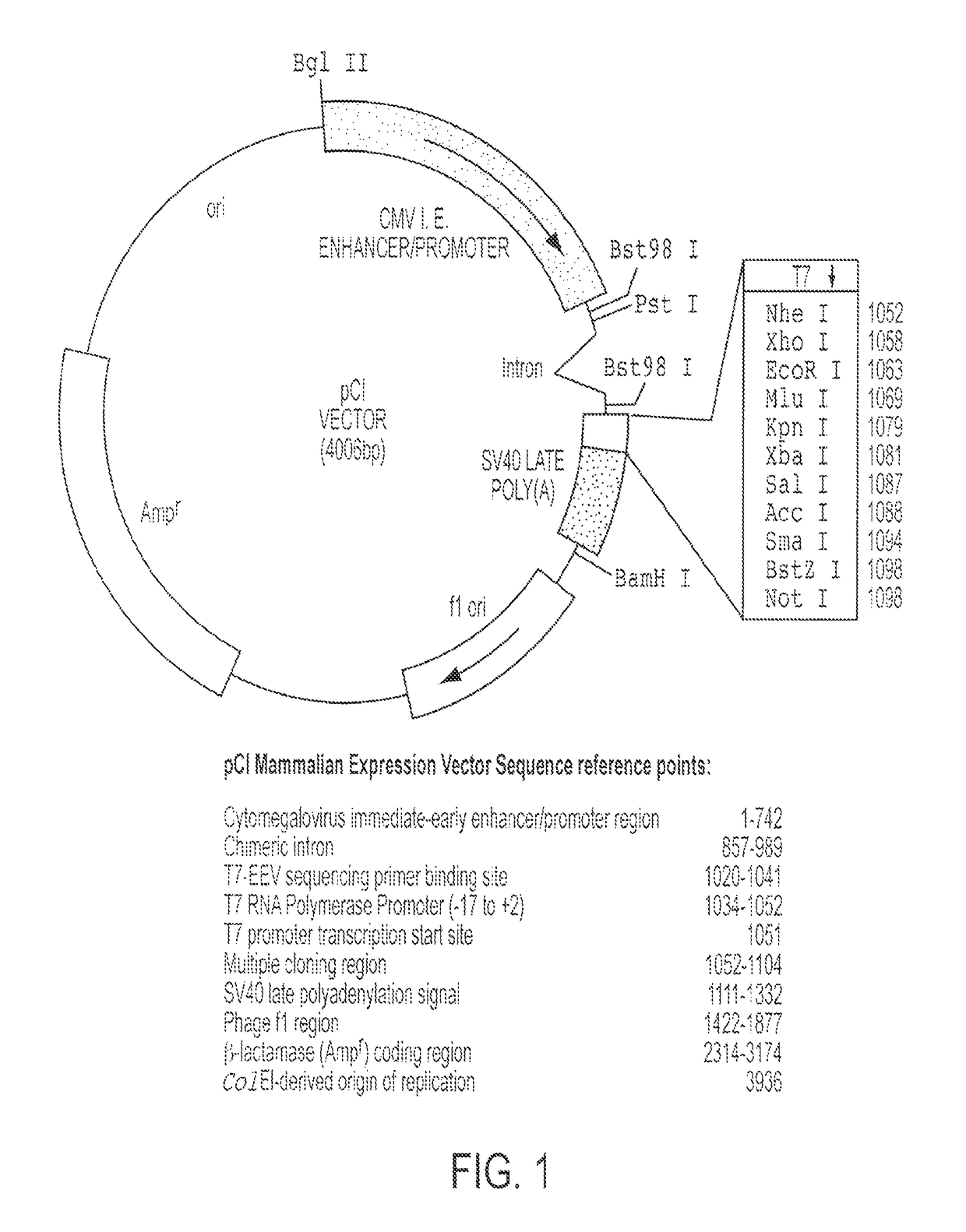
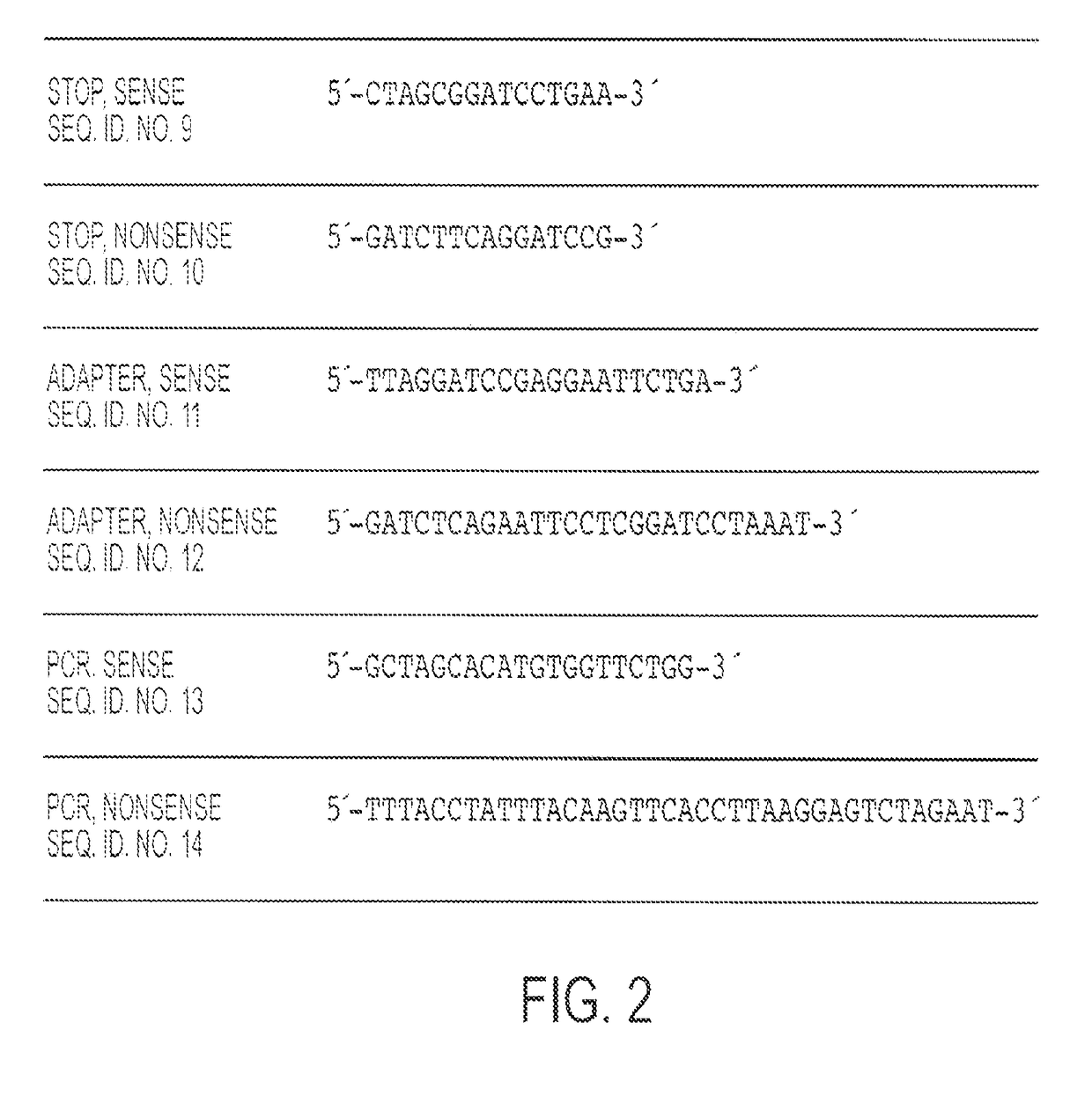
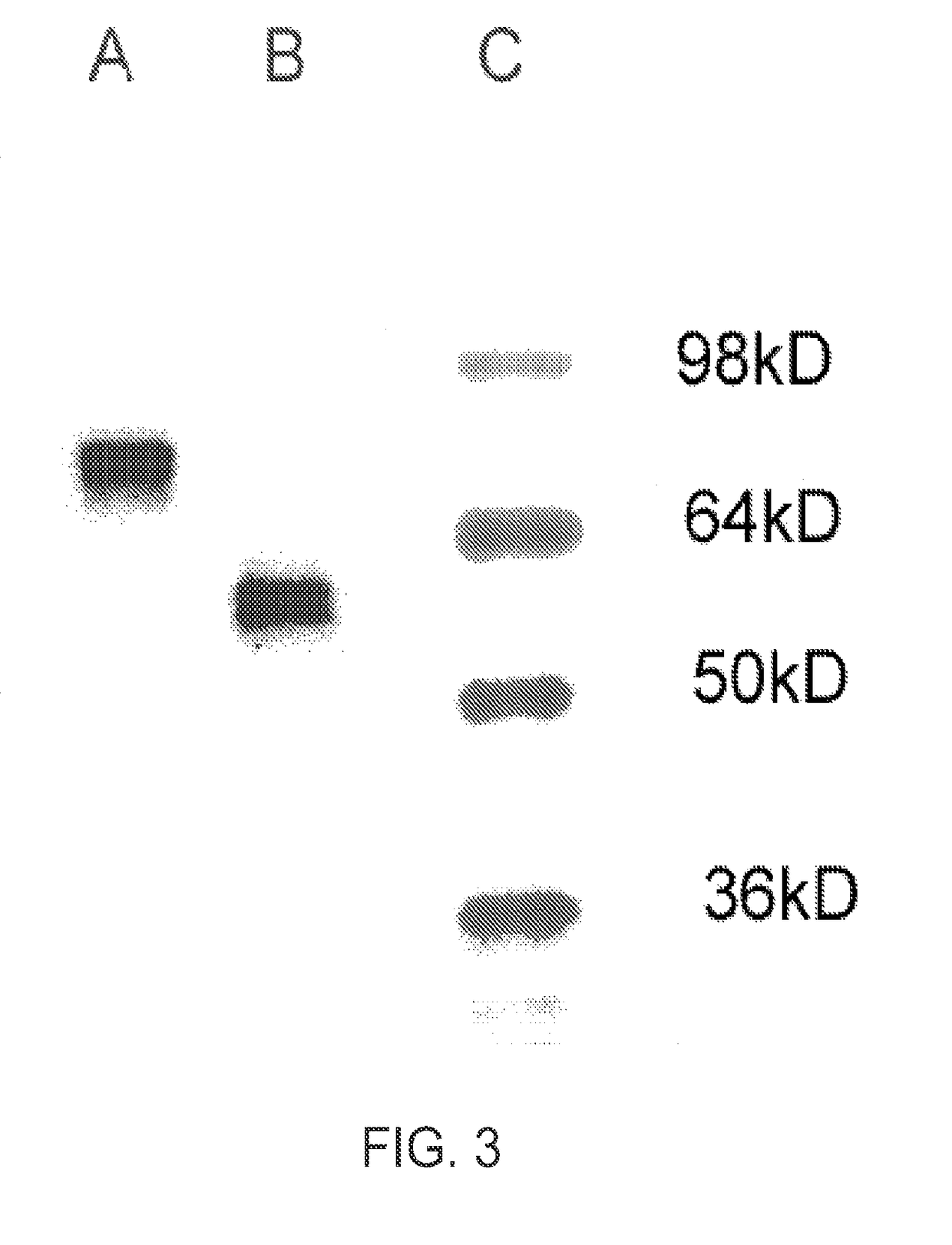
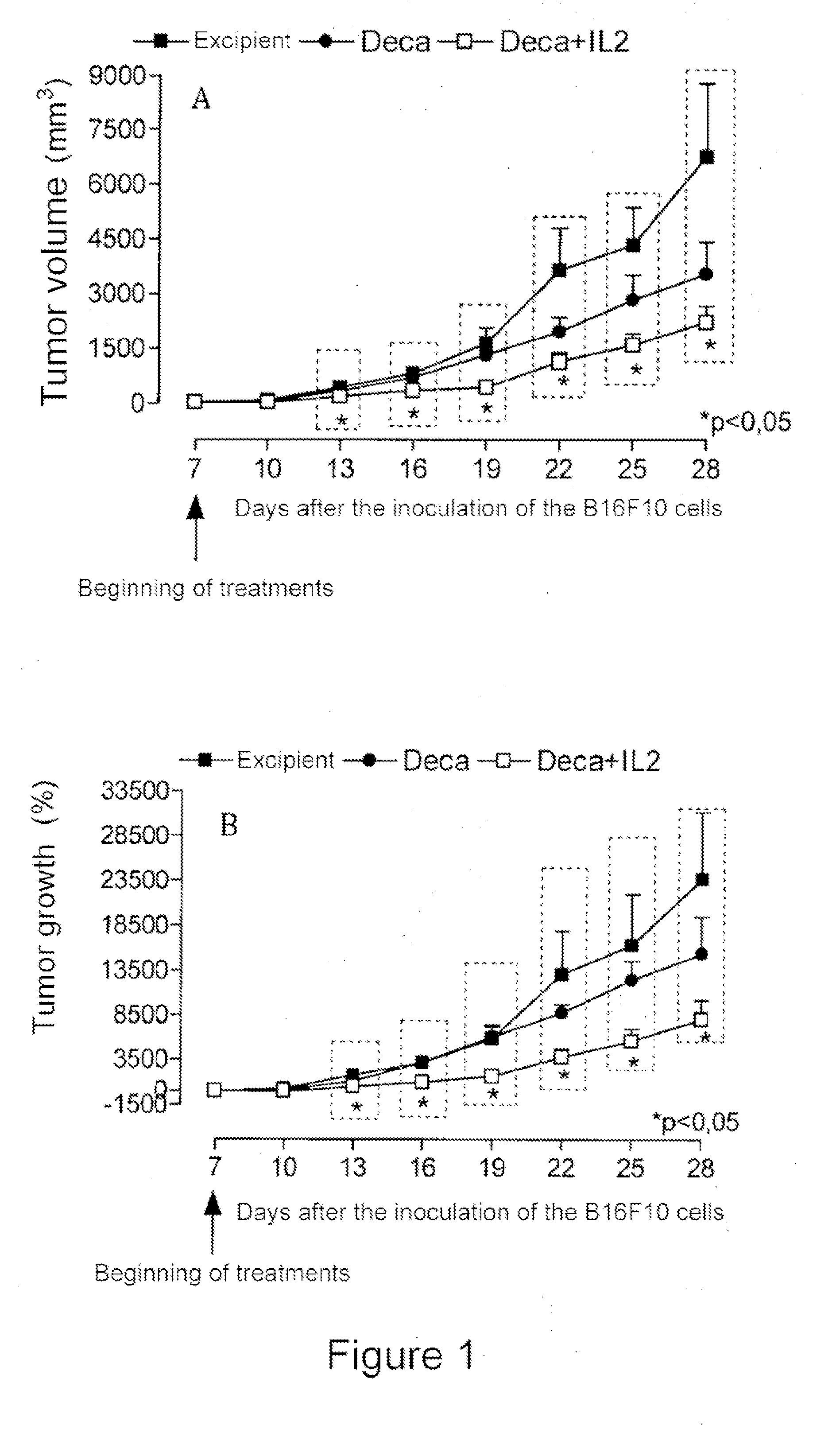
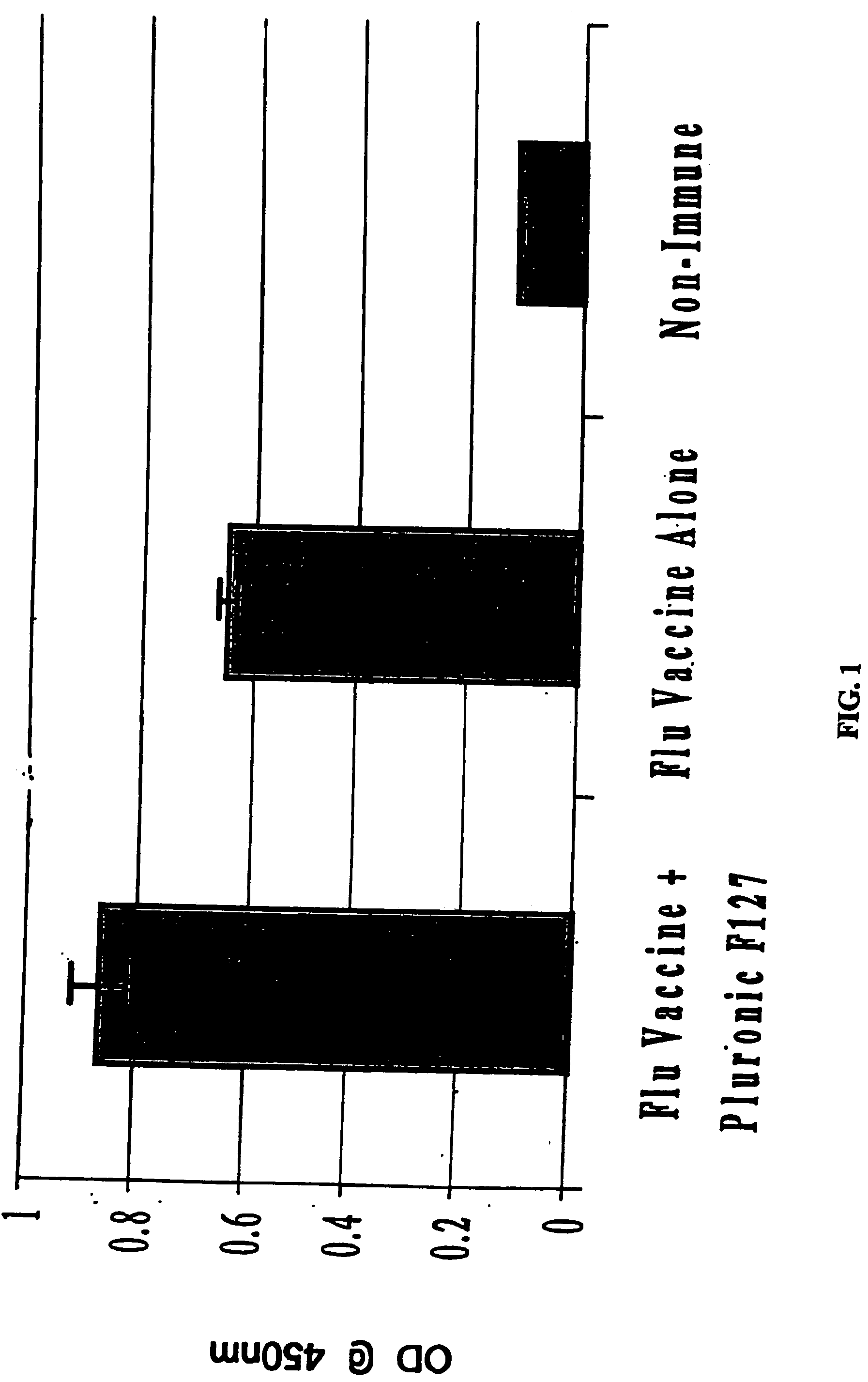
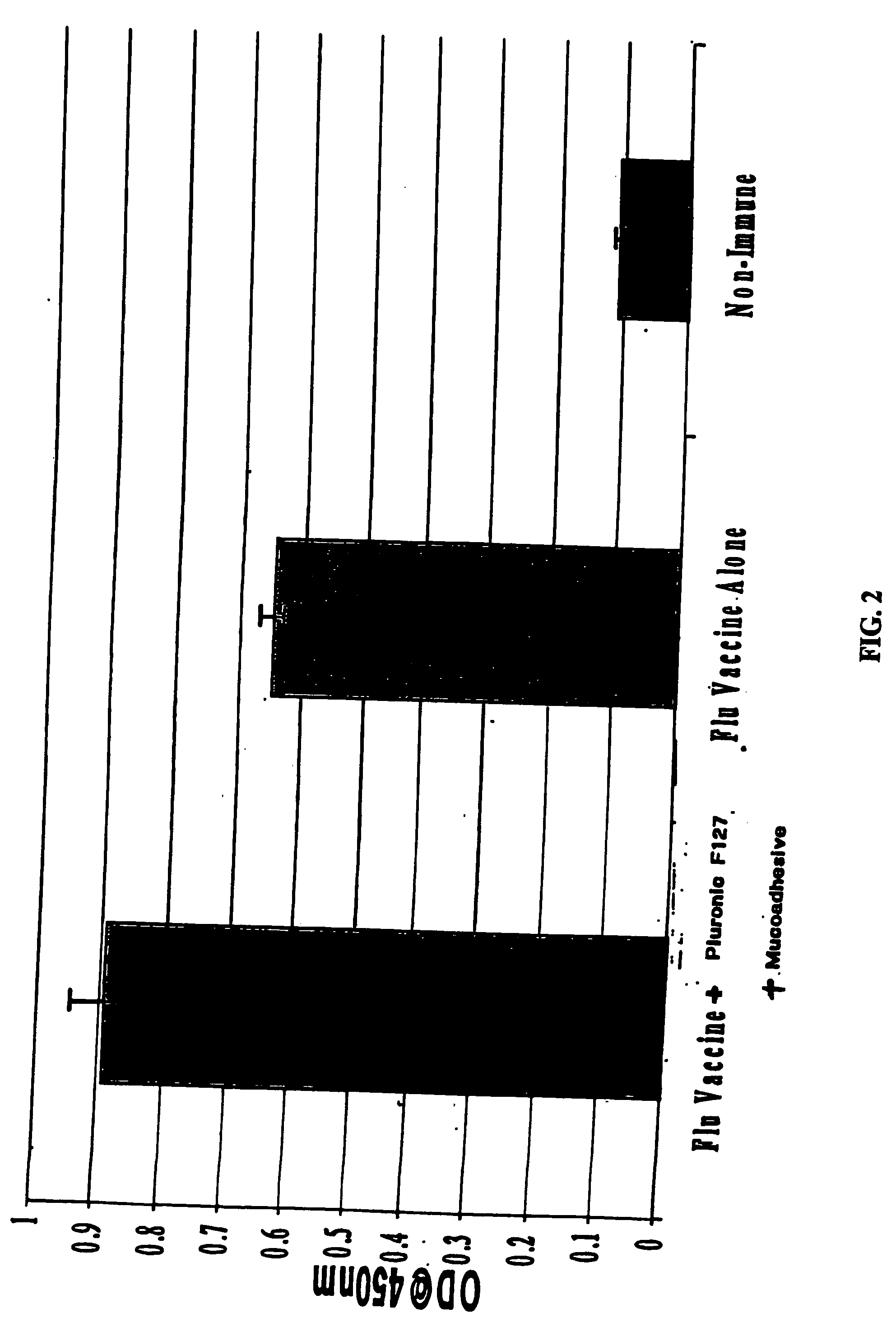
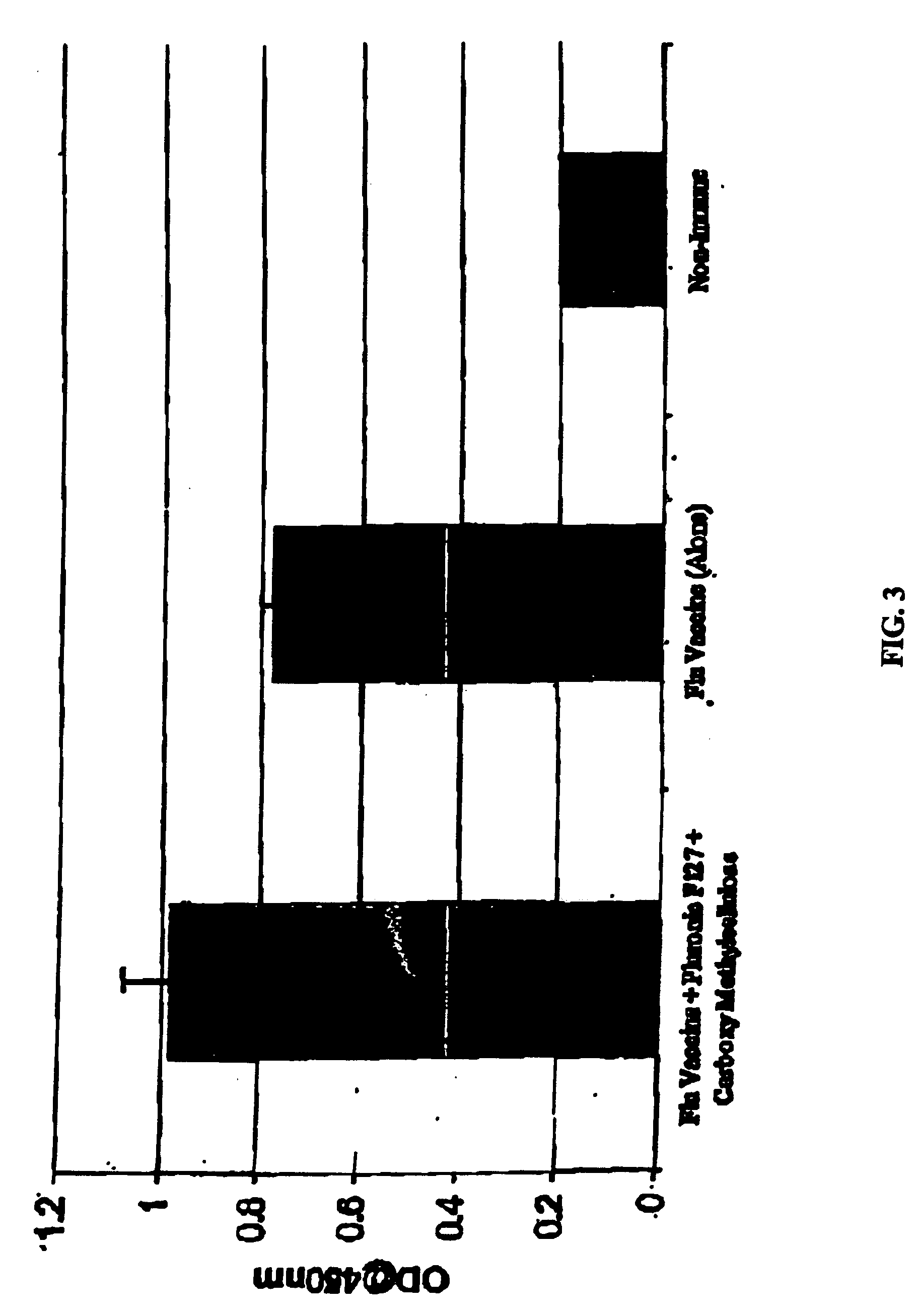
Patents
Literature
896results about "Protozoa antigen ingredients" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
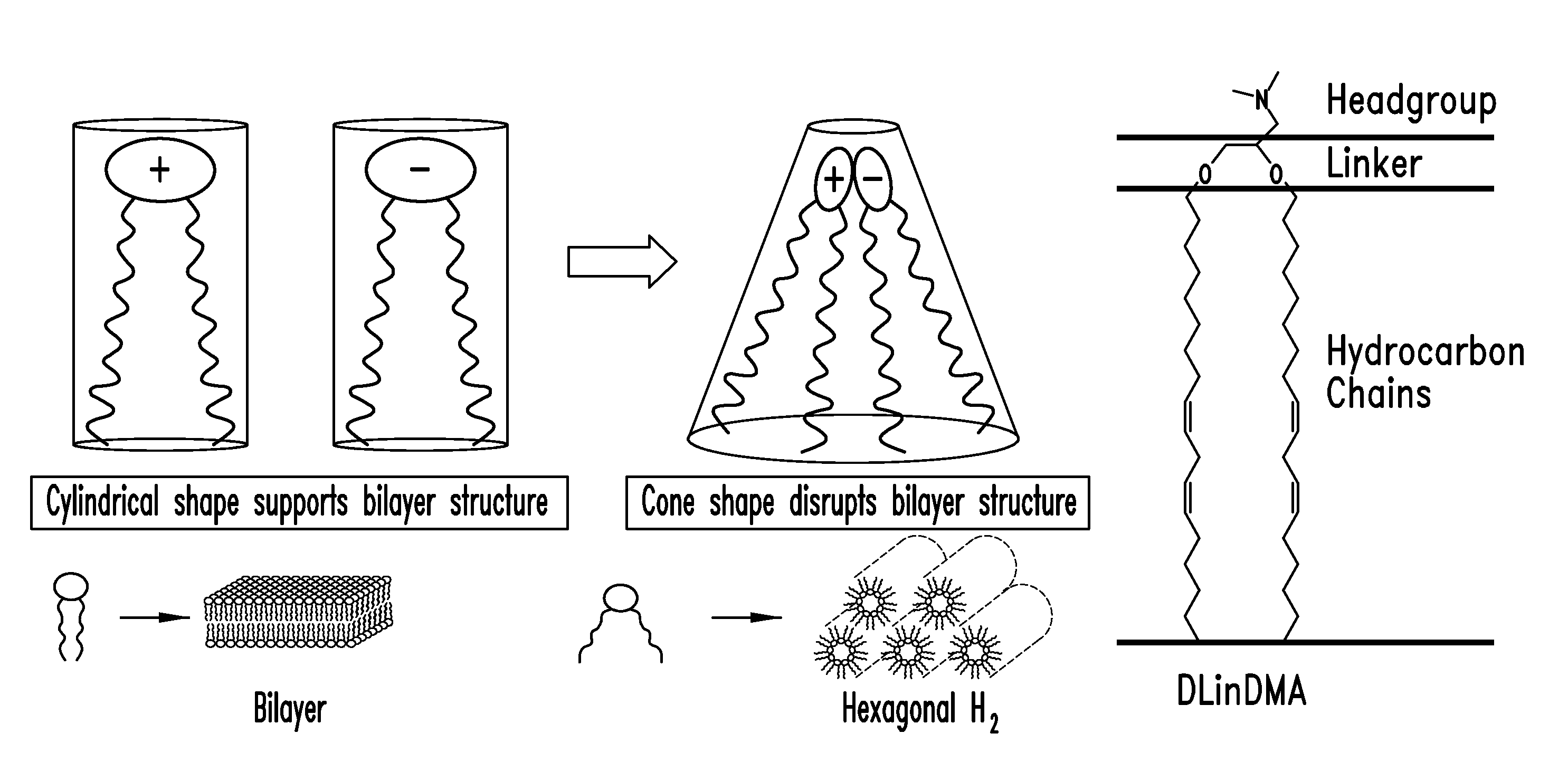
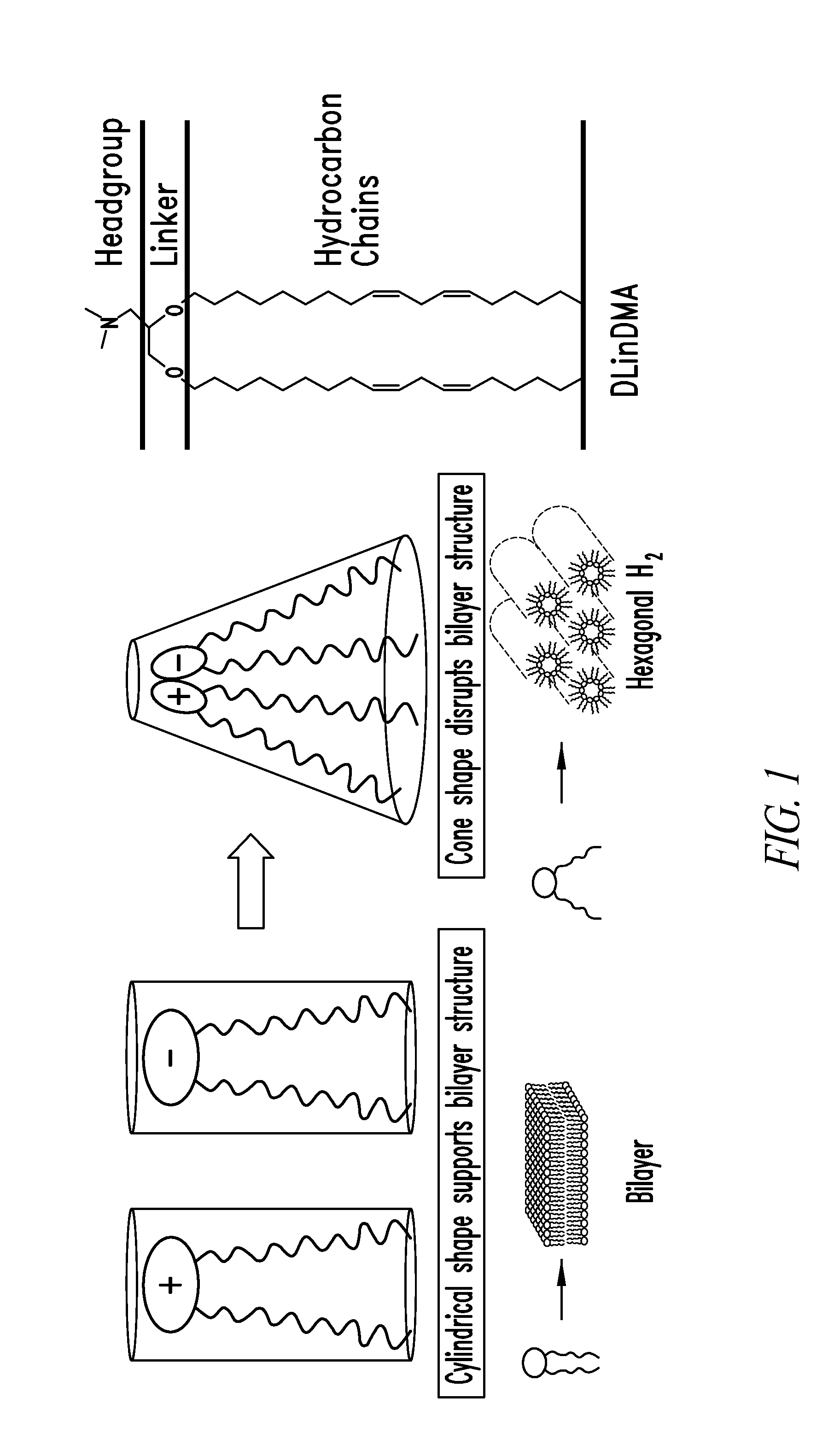
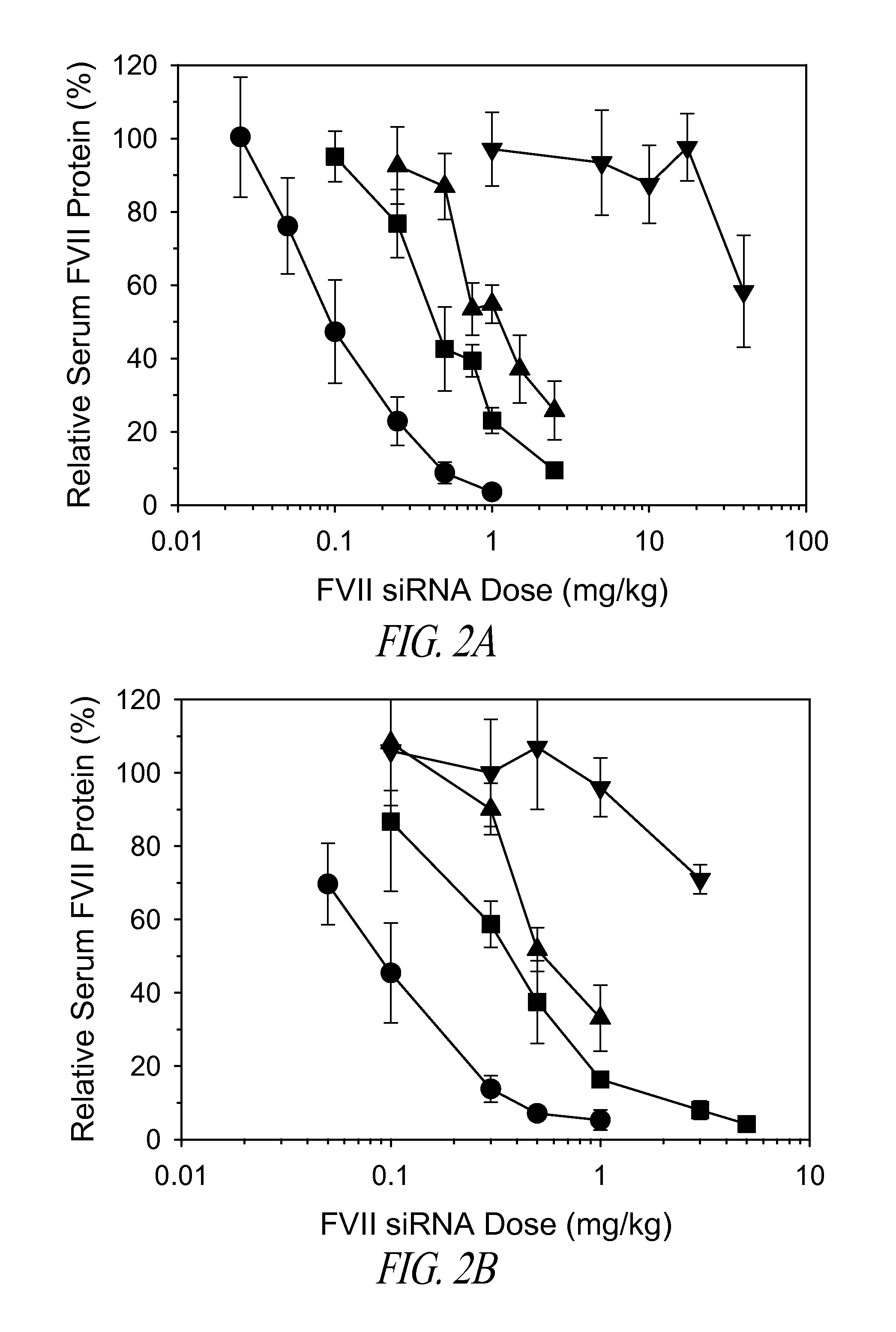
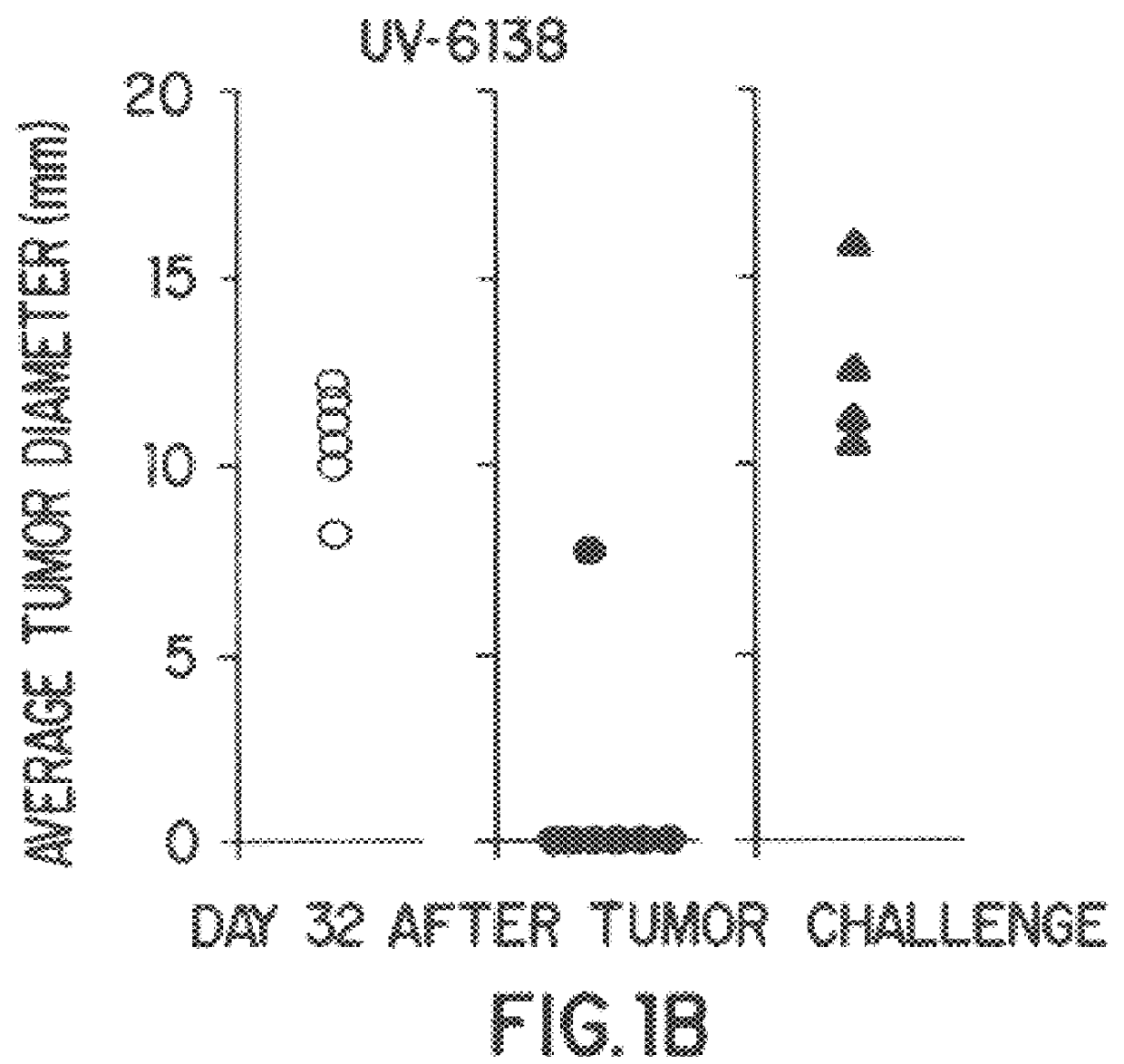
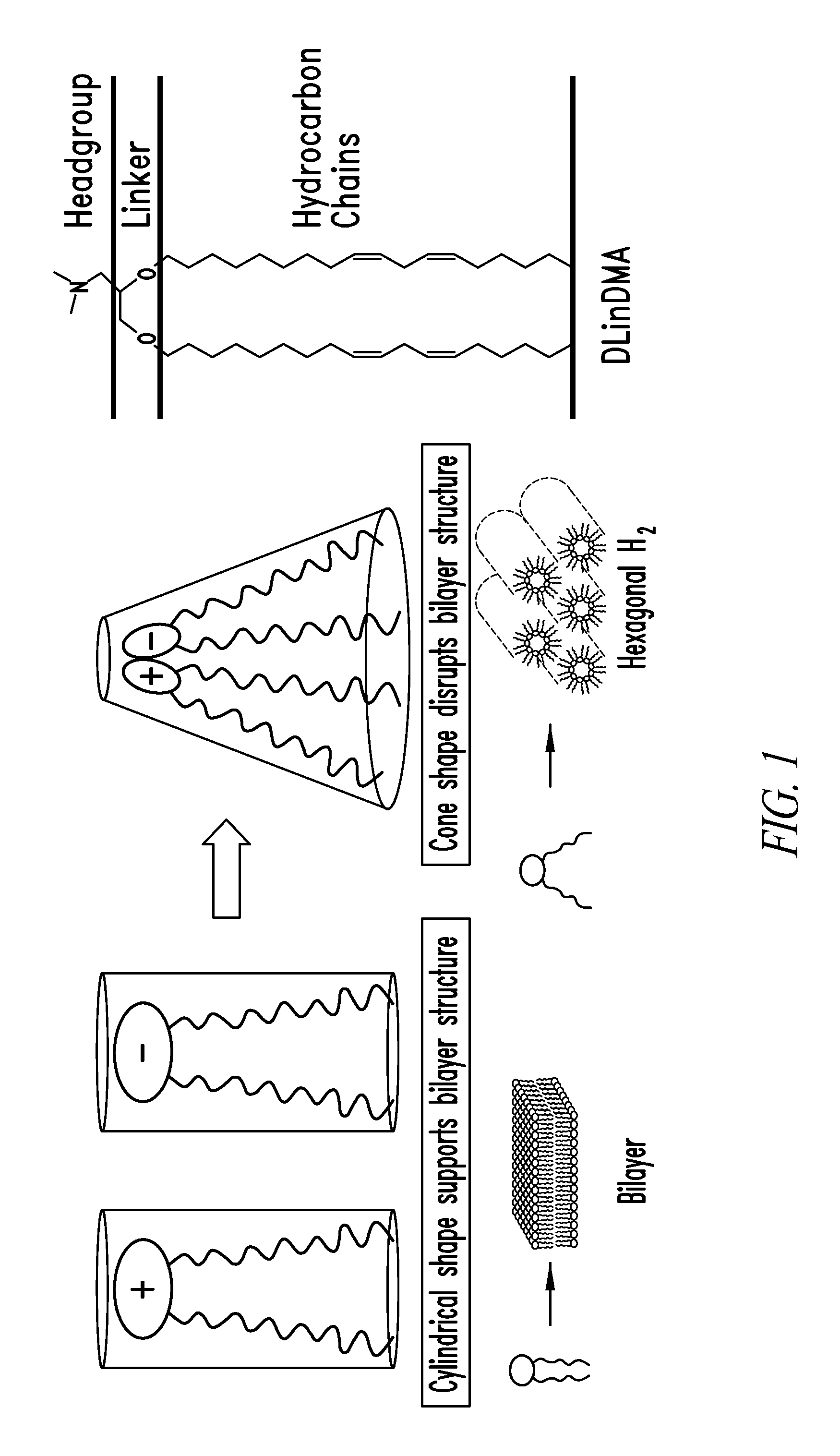
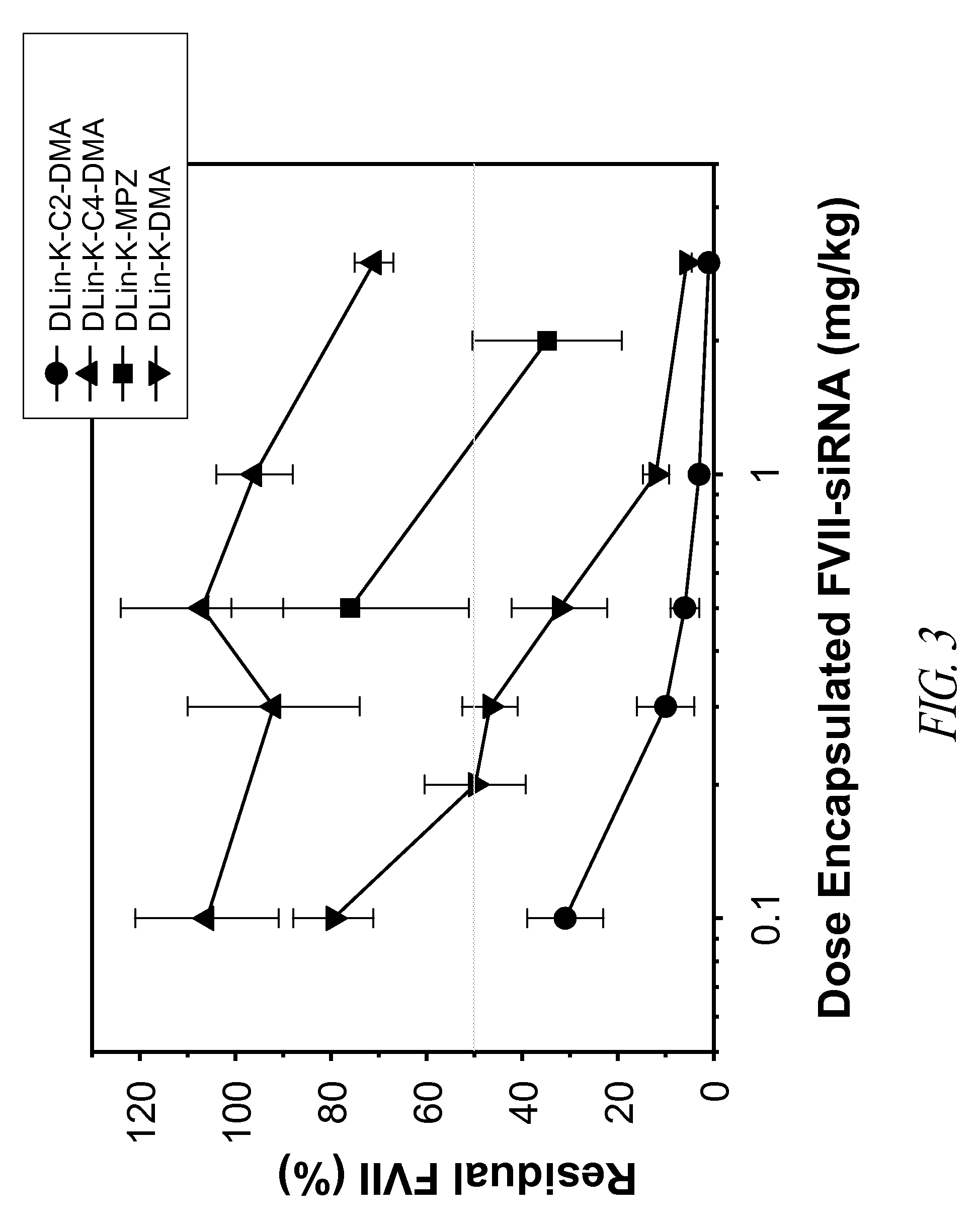
Amino lipids and methods for the delivery of nucleic acids
The present invention provides superior compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of specific target proteins at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:ARBUTUS BIOPHARMA CORPORAT ION +1
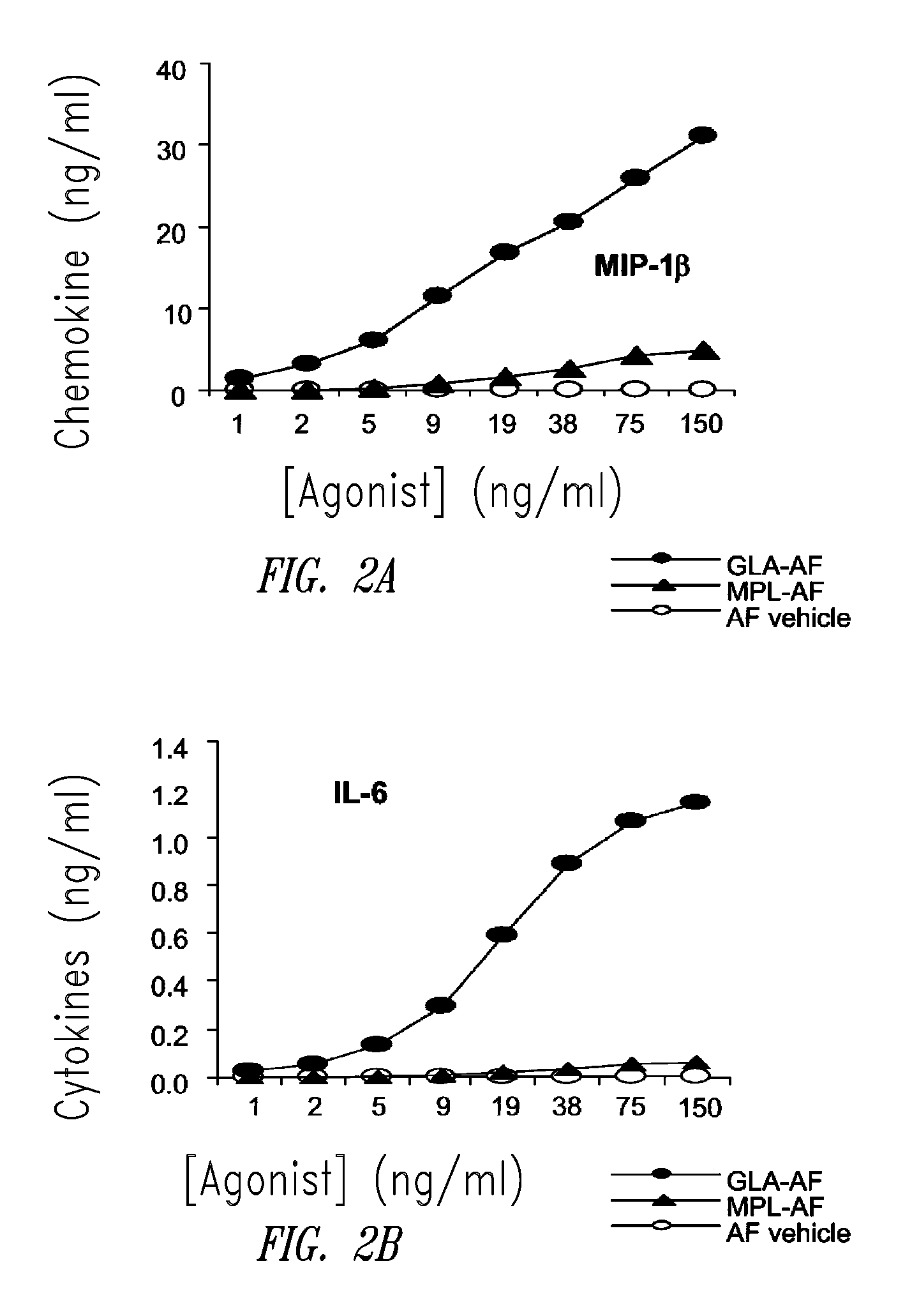
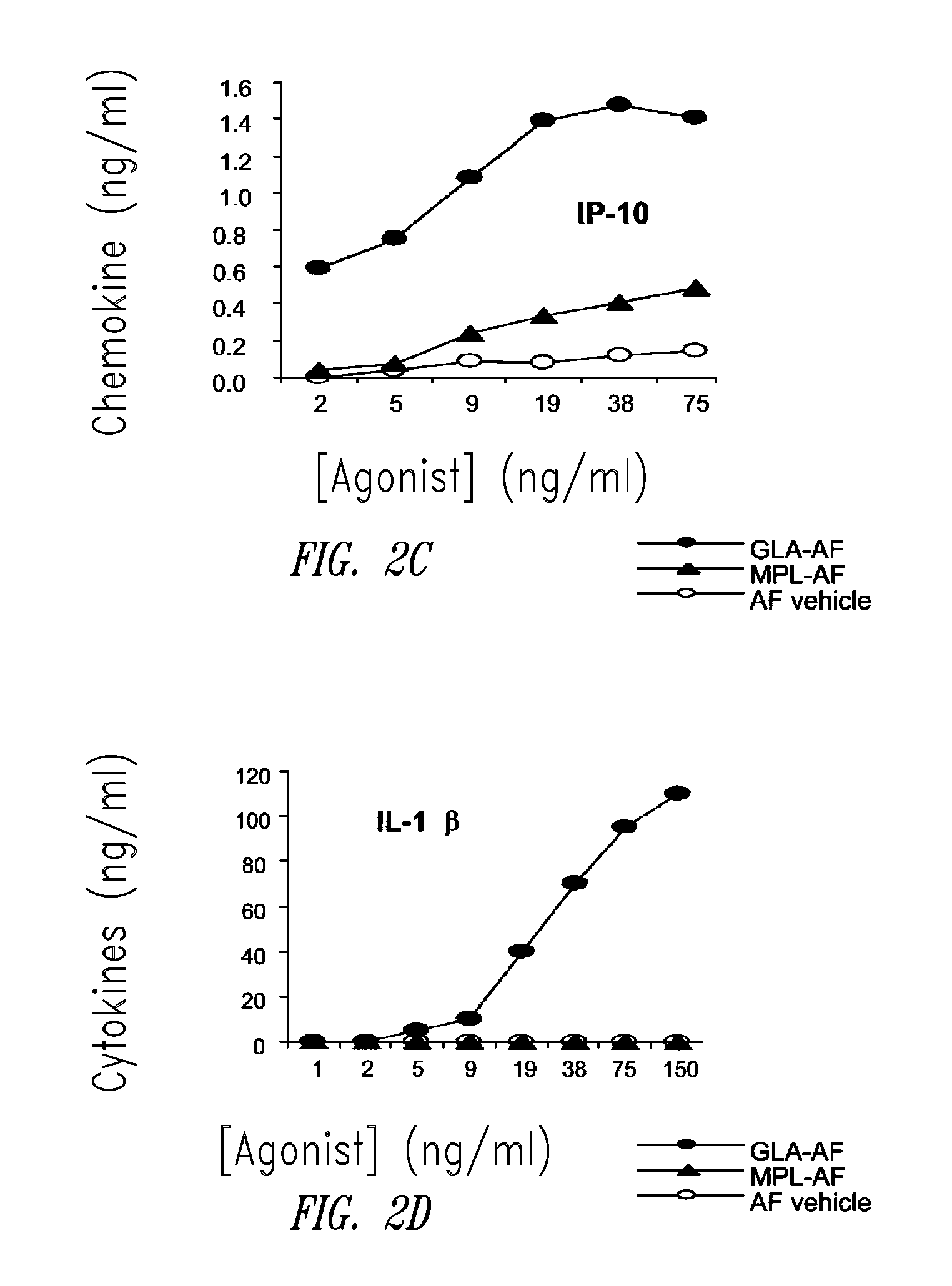
Vaccine composition containing synthetic adjuvant
ActiveUS20080131466A1Elicit immune responseAntibacterial agentsBacterial antigen ingredientsNatural productAdditive ingredient
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:ACCESS TO ADVANCED HEALTH INST
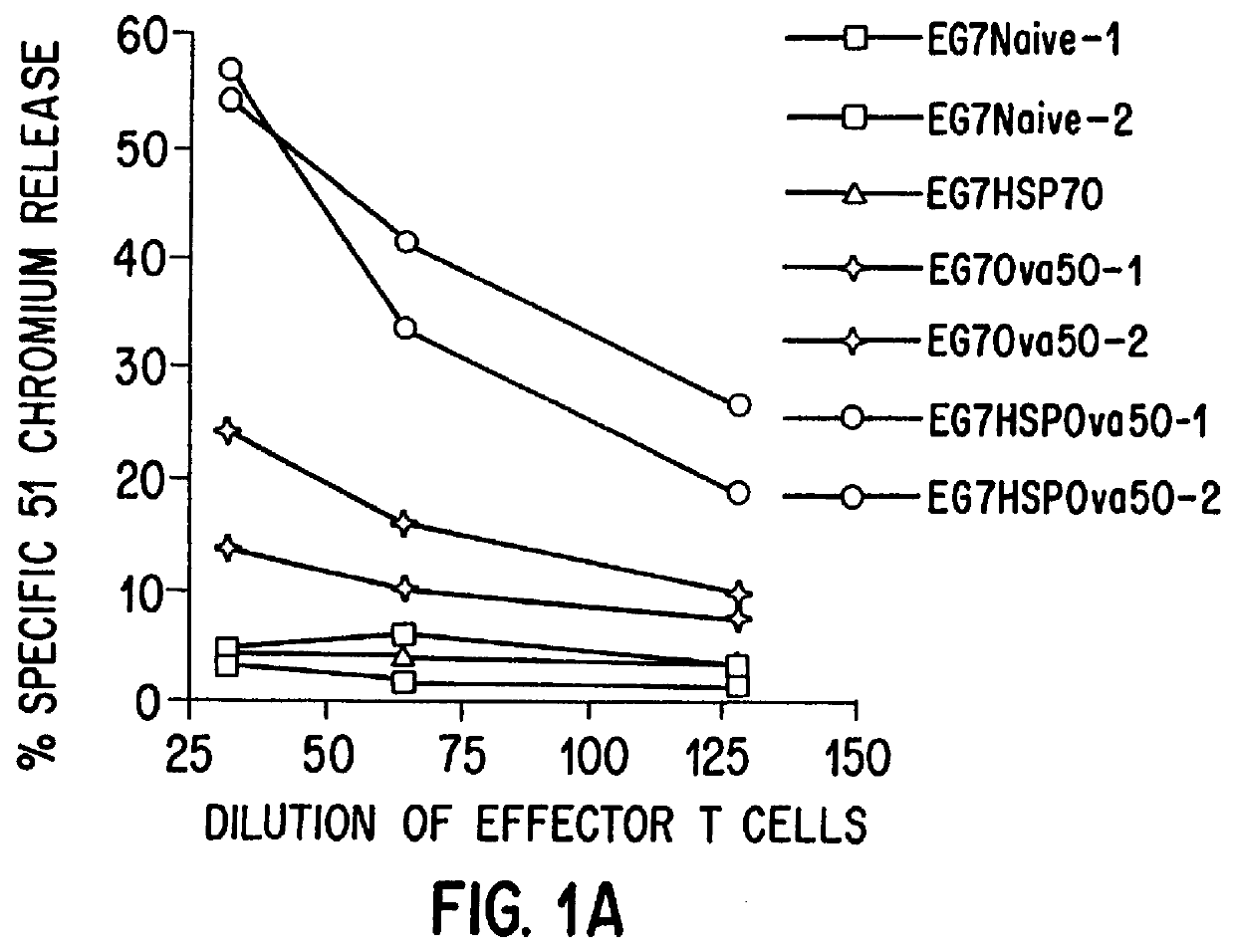
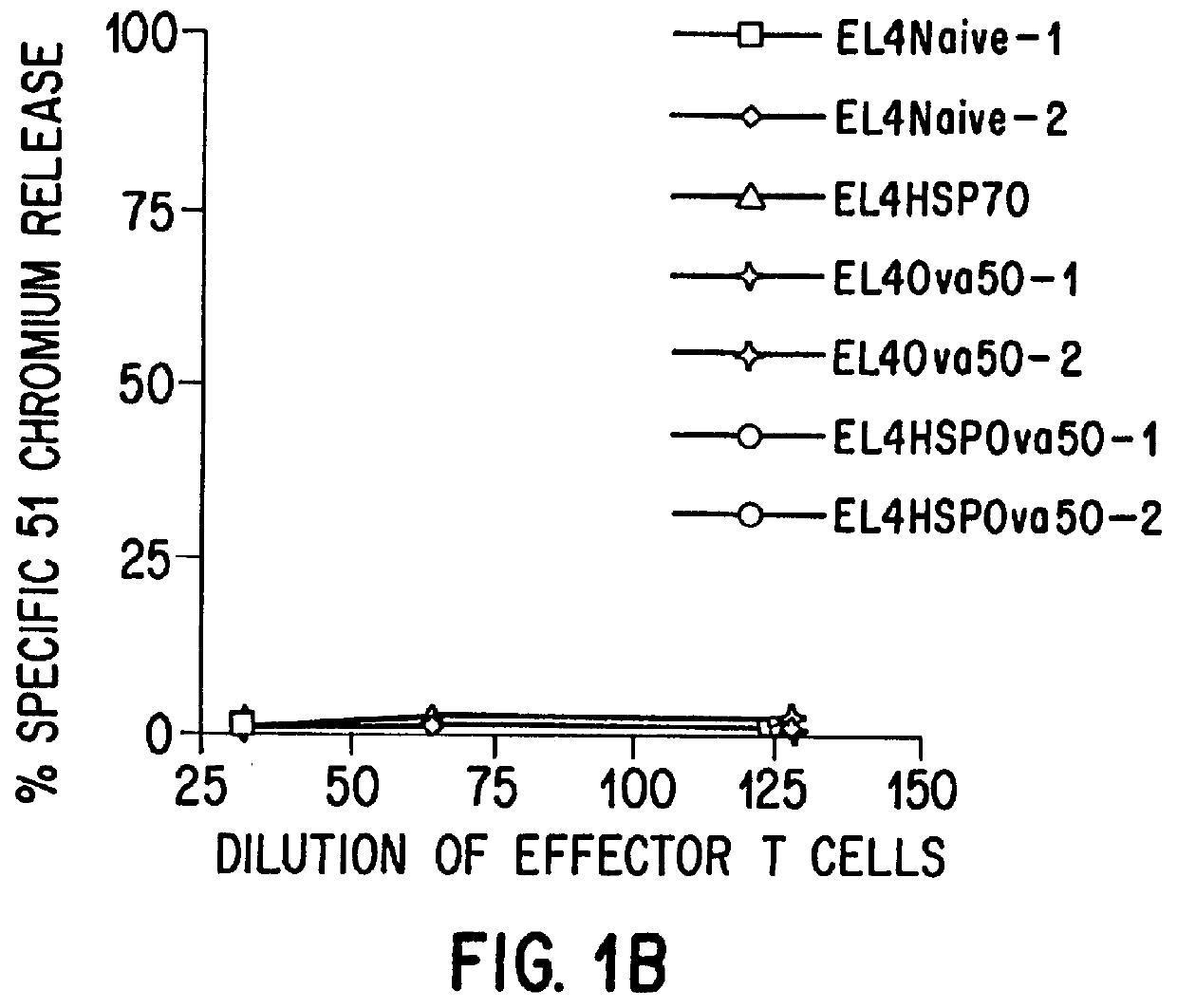
Therapeutic and prophylactic methods using heat shock proteins
The present invention relates to immunogenic complexes of heat shock proteins (hsp) noncovalently bound to exogenous antigenic molecules which when administered to an individual elicit specific immunological responses in the host. Methods of prevention and treatment of cancer and infectious disease are provided.
Owner:FORDHAM UNIVERSITY
Compositions and methods for the delivery of nucleic acids
InactiveUS20110117125A1Reduce particle aggregationReduce selection requirementsAntibacterial agentsOrganic active ingredientsLipid particleProtein target
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:THE UNIV OF BRITISH COLUMBIA +2
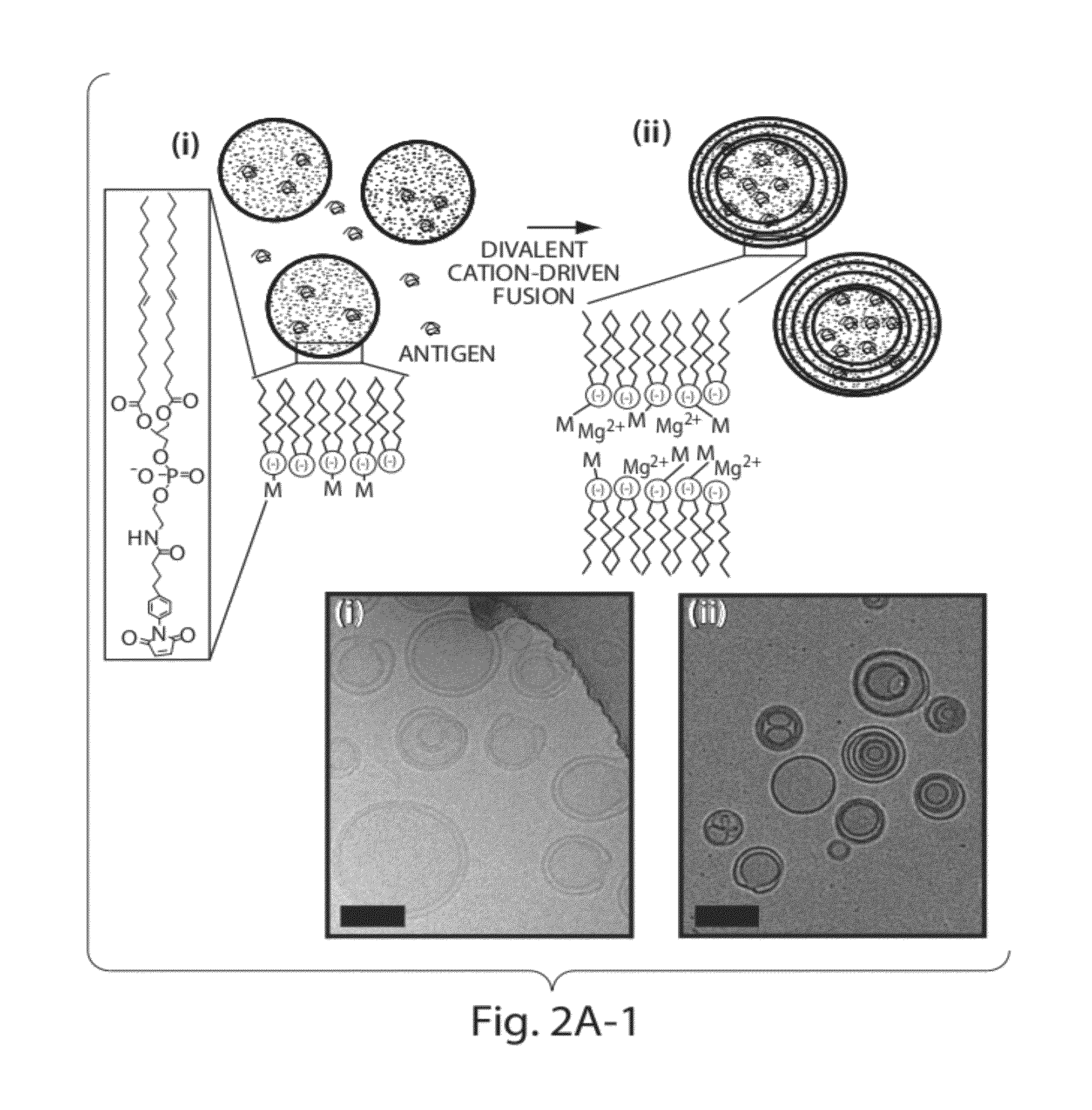
Lipid vesicle compositions and methods of use
ActiveUS20120177724A1Increase load capacityImprove the level ofPeptide/protein ingredientsMicroencapsulation basedAntigenVesicle/vacuole
The invention provides delivery systems comprised of stabilized multilamellar vesicles, as well as compositions, methods of synthesis, and methods of use thereof. The stabilized multilamellar vesicles comprise terminal-cysteine-bearing antigens or cysteine-modified antigens, at their surface and / or internally.
Owner:MASSACHUSETTS INST OF TECH +1
Use of synthetic glycolipids as universal adjuvants for vaccines against cancer and infectious diseases
InactiveUS20050192248A1Enhancement and extension of durationBiocideOrganic active ingredientsDiseaseAdjuvant
The present invention relates to methods and compositions for augmenting an immunogenicity of an antigen in a mammal, comprising administering said antigen together with an adjuvant composition that includes a synthetic glycolipid compound of Formula I, as described herein. According to the present invention, the use of a compound of Formula I as an adjuvant is attributed at least in part to the enhancement and / or extension of antigen-specific Th1-type responses, in particular, CD8+ T cell responses. The methods and compositions of the present invention can be useful for prophylaxis and treatment of various infectious and neoplastic diseases.
Owner:NEW YORK UNIV +1
Immunisation of large mammals with low doses of RNA
ActiveUS20130149375A1Conveniently preparedImprove stabilityAntibacterial agentsSsRNA viruses negative-senseMammalImmunity response
RNA encoding an immunogen is delivered to a large mammal at a dose of between 2 μg and 100 μg. Thus the invention provides a method of raising an immune response in a large mammal, comprising administering to the mammal a dose of between 2 μg and 100 μg of immunogen-encoding RNA. Similarly, RNA encoding an immunogen can be delivered to a large mammal at a dose of 3 ng / kg to 150 ng / kg. The delivered RNA can elicit an immune response in the large mammal
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases with heat shock/stress protein-peptide complexes
InactiveUS6017540AEnhancing host 's immunocompetenceHigh activityBiocidePeptide/protein ingredientsStress ProteinsIn vivo
The present invention relates to methods and compositions for eliciting an immune response and the prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases. The methods of the invention comprise administering a composition comprising an effective amount of a complex, in which the complex consists essentially of a heat shock protein (hsp) noncovalently bound to an antigenic molecule. Optionally, the methods further comprise administering antigen presenting cells sensitized with complexes of hsps noncovalently bound to an antigenic molecule. "Antigenic molecule" as used herein refers to the peptides with which the hsps are endogenously associated in vivo as well as exogenous antigens / immunogens (i.e., with which the hsps are not complexed in vivo) or antigenic / immunogenic fragments and derivatives thereof. In a preferred embodiment, the complex is autologous to the individual. In a specific embodiment, the effective amounts of the complex are in the range of 0.1 to 9.0 micrograms for complexes comprising hsp70, 5 to 49 micrograms for hsp90, and 0.1 to 9.0 micrograms for gp96.
Owner:FORDHAM UNIVERSITY
Mutant forms of cholera holotoxin as an adjuvant
InactiveUS7332174B2No loss in adjuvanting propertyLow toxicityAntibacterial agentsFungiAntigenAdjuvant
Mutant cholera holotoxins having single or double amino acid substitutions or insertions have reduced toxicity compared to the wild-type cholera holotoxin. The mutant cholera holotoxins are useful as adjuvants in antigenic compositions to enhance the immune response in a vertebrate host to a selected antigen from a pathogenic bacterium, virus, fungus, or parasite, a cancer cell, a tumor cell, an allergen, or a self-molecule.
Owner:WYETH HOLDINGS CORP +1
Mutant forms of cholera holotoxin as an adjuvant
InactiveUS7285281B2No loss in adjuvanting propertyLow toxicityAntibacterial agentsFungiAdjuvantCancer cell
Owner:UNIV OF COLORADO FOUND +1
Immunostimulatory Combinations for Vaccine Adjuvants
This invention discloses immunostimulatory combinations of Tumor Necrosis Factor Receptor Superfamily (TN-FRSF) agonists, Toll-Like Receptor (TLR) agonists, “domain present in NAIP, CIITA, HET-E, TP-I (NACHT)-Leucine Rich Repeat (LRR)” or “NLR” agonists, RIG-I-Like Helicase or “RLH” agonists, purinergic receptor agonists and cytokine / chemokine receptor agonists, together with delivery methods. The combinations, when used alone at the site of pathology, provide immunostimulation that induces host humoral and cellular immunologic responses to eliminate pathogens or neoplasms. Alternatively, when the combinations are used with a defined antigens, these combinations can induce focused humoral and cellular immunologic responses useful as prophylactic and / or ameliorative therapeutic modalities for infections and the treatment of neoplastic disorders.
Owner:RGT UNIV OF CALIFORNIA
Vaccine composition containing synthetic adjuvant
InactiveUS20090181078A1Promote maturitySsRNA viruses negative-senseVertebrate antigen ingredientsNatural productPharmaceutical drug
Compositions and methods, including vaccines and pharmaceutical compositions for inducing or enhancing an immune response are disclosed based on the discovery of useful immunological adjuvant properties in a synthetic, glucopyranosyl lipid adjuvant (GLA) that is provided in substantially homogeneous form. Chemically defined, synthetic GLA offers a consistent vaccine component from lot to lot without the fluctuations in contaminants or activity that compromise natural-product adjuvants. Also provided are vaccines and pharmaceutical compositions that include GLA and one or more of an antigen, a Toll-like receptor (TLR) agonist, a co-adjuvant and a carrier such as a pharmaceutical carrier.
Owner:INFECTIOUS DISEASE RES INST
Yeast-based vaccines as immunotherapy
InactiveUS7465454B2Enhance immune responseExtended half-lifeBiocideAntibody mimetics/scaffoldsYeastDisease
Compositions and methods for treating and / or preventing a variety of diseases and conditions that are amenable to immunotherapy and, in one particular embodiment, compositions and methods for treating and / or preventing cancer in an animal are described. Specifically improvements related to the use of a yeast-based vaccine comprising a yeast vehicle and an antigen that is selected to elicit an antigen-specific cellular and humoral immune response in an animal, for use in prophylactic and / or therapeutic vaccination and the prevention and / or treatment of a variety of diseases and conditions are disclosed.
Owner:GLOBE IMMUNE INC
Vaccine against streptococcus pneumoniae capsular polysaccharides
InactiveUS20030147922A1Antibacterial agentsSenses disorderAntigenStreptococcus pneumoniae capsular polysaccharide
The present invention relates to the field of bacterial polysaccharide antigen vaccines. In particular, the present invention relates to specific advantageous pnumococcal polysaccharide conjugates adjuvanted with 3D-MPL and substantially devoid aluminium-based adjuvant.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
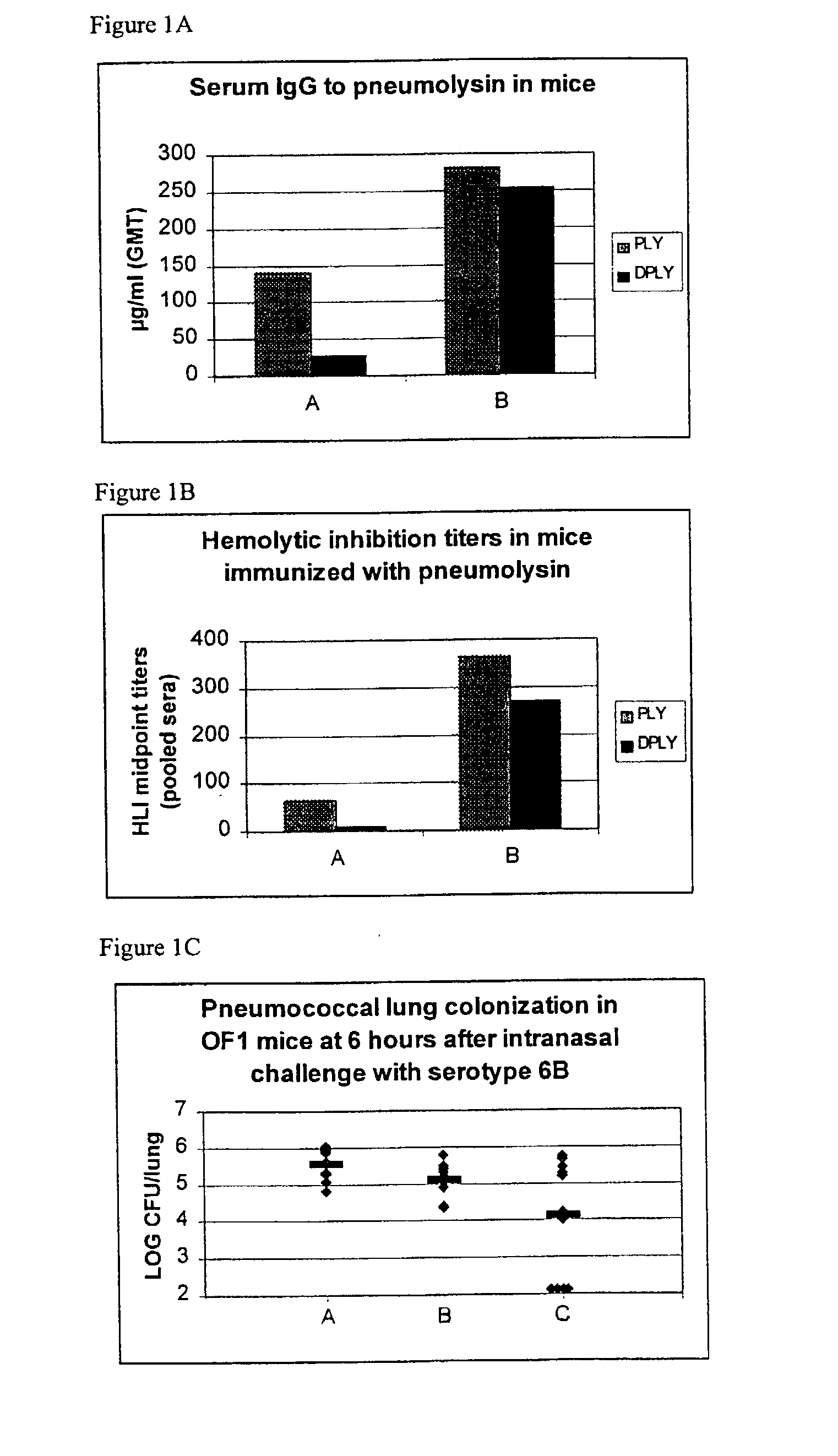
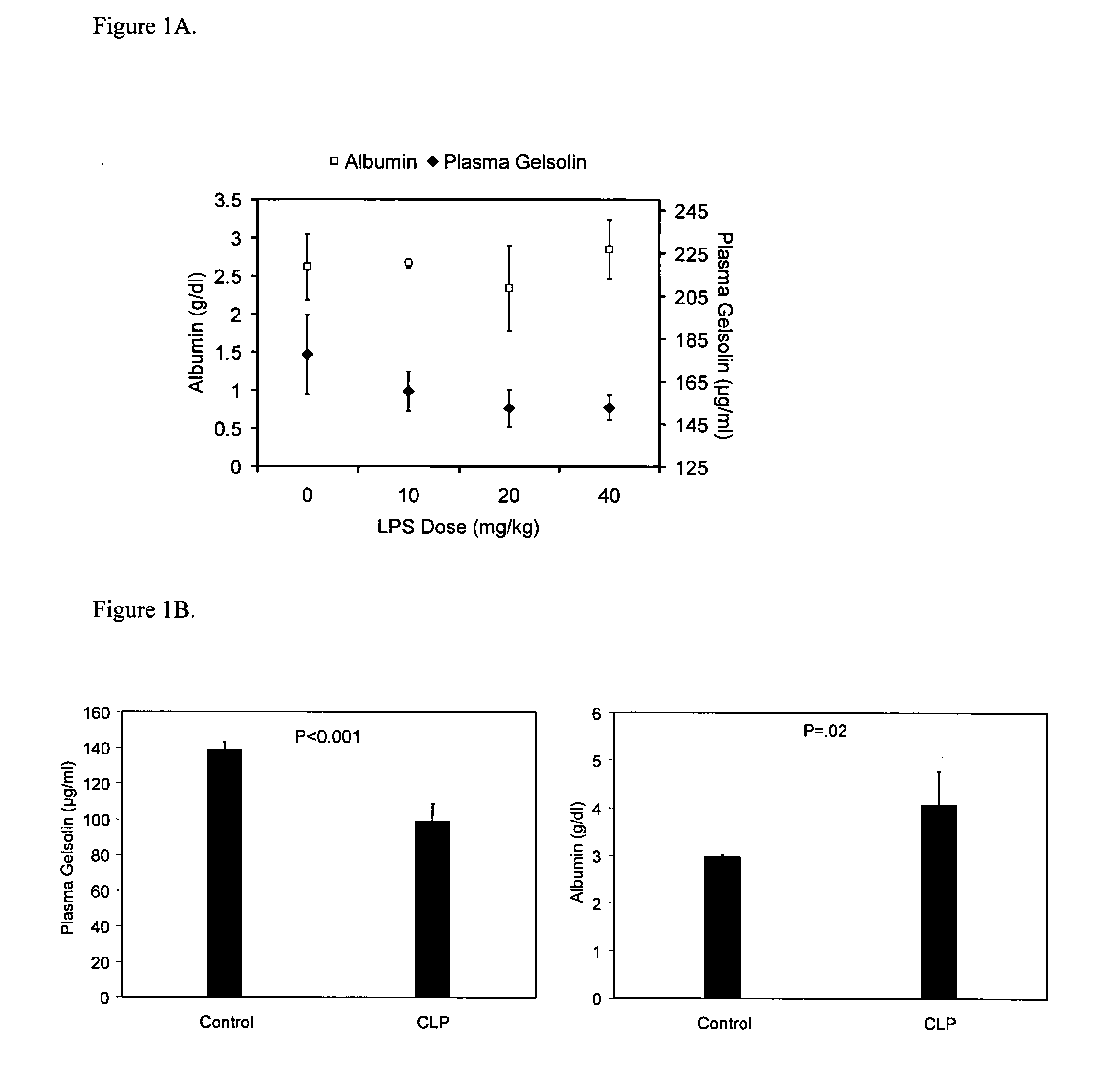
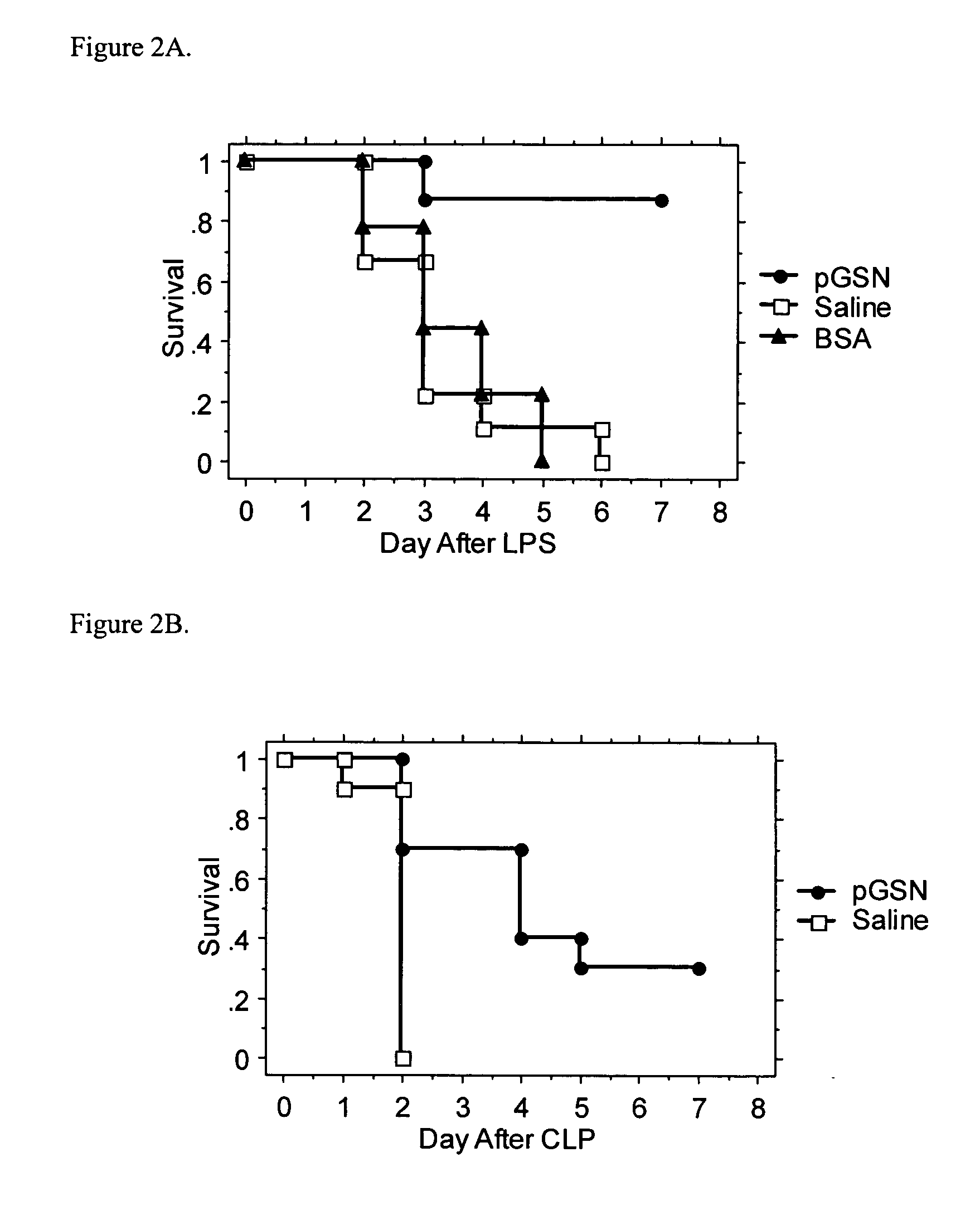
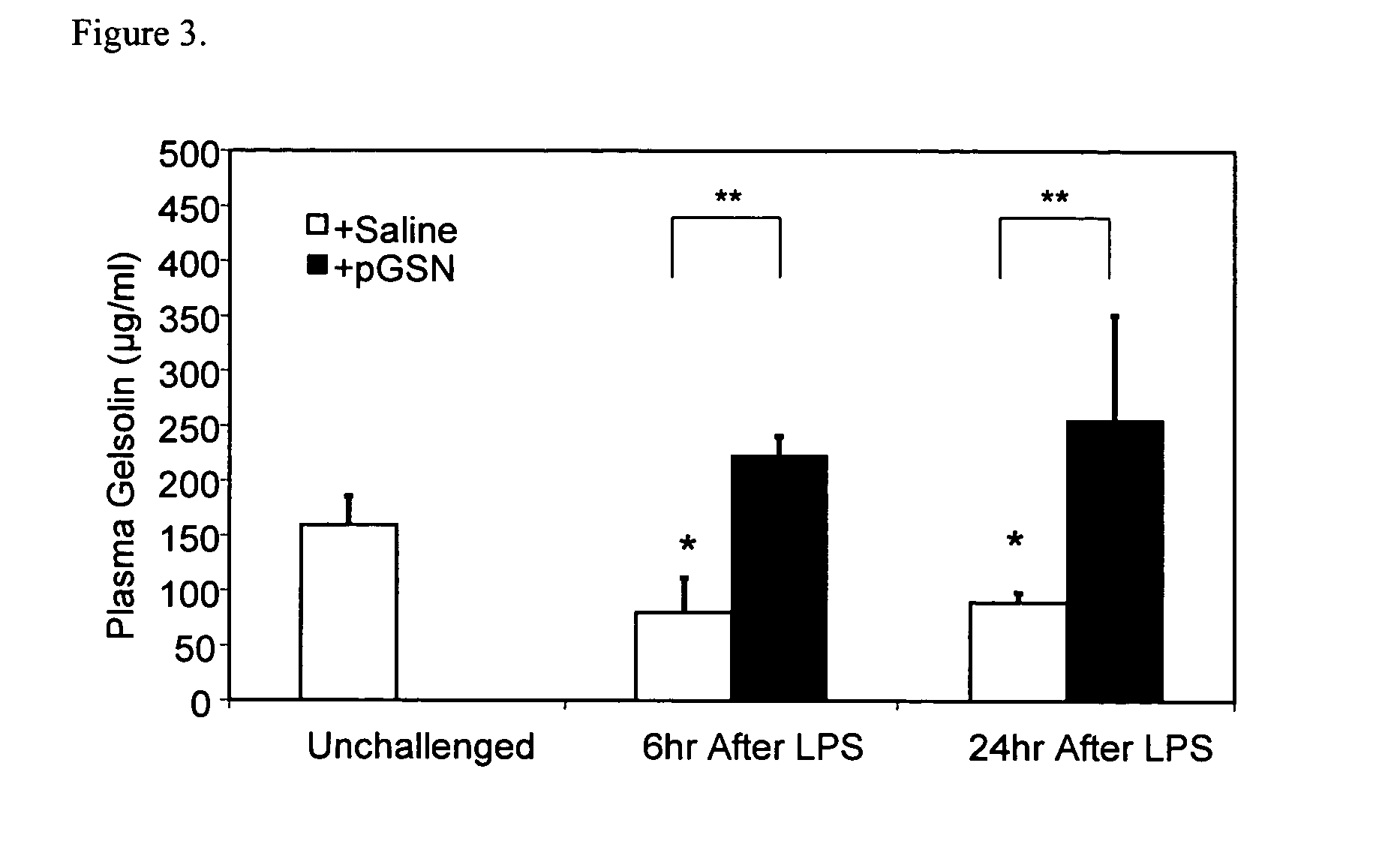
Use of gelsolin to treat infections
ActiveUS20060009386A1Reduce riskReduce the risk of infectionAntibacterial agentsBiocideWhite blood cellGelsolin
The invention relates to the use of gelsolin to treat infections and to monitor the treatment of infections. The invention also provides methods up-regulating interleukin expression and methods for down-regulating pro-inflammatory cytokine expression.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
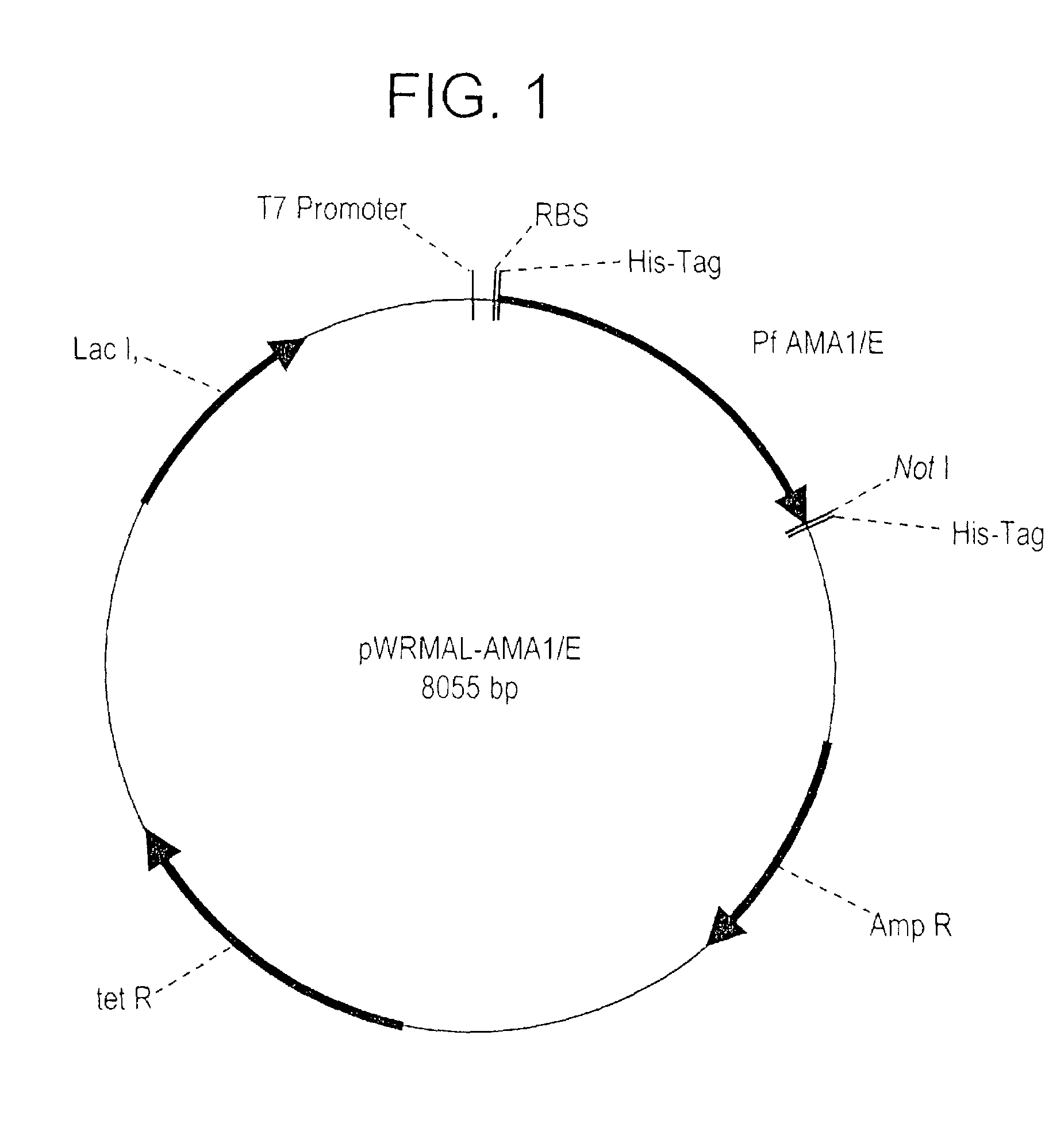
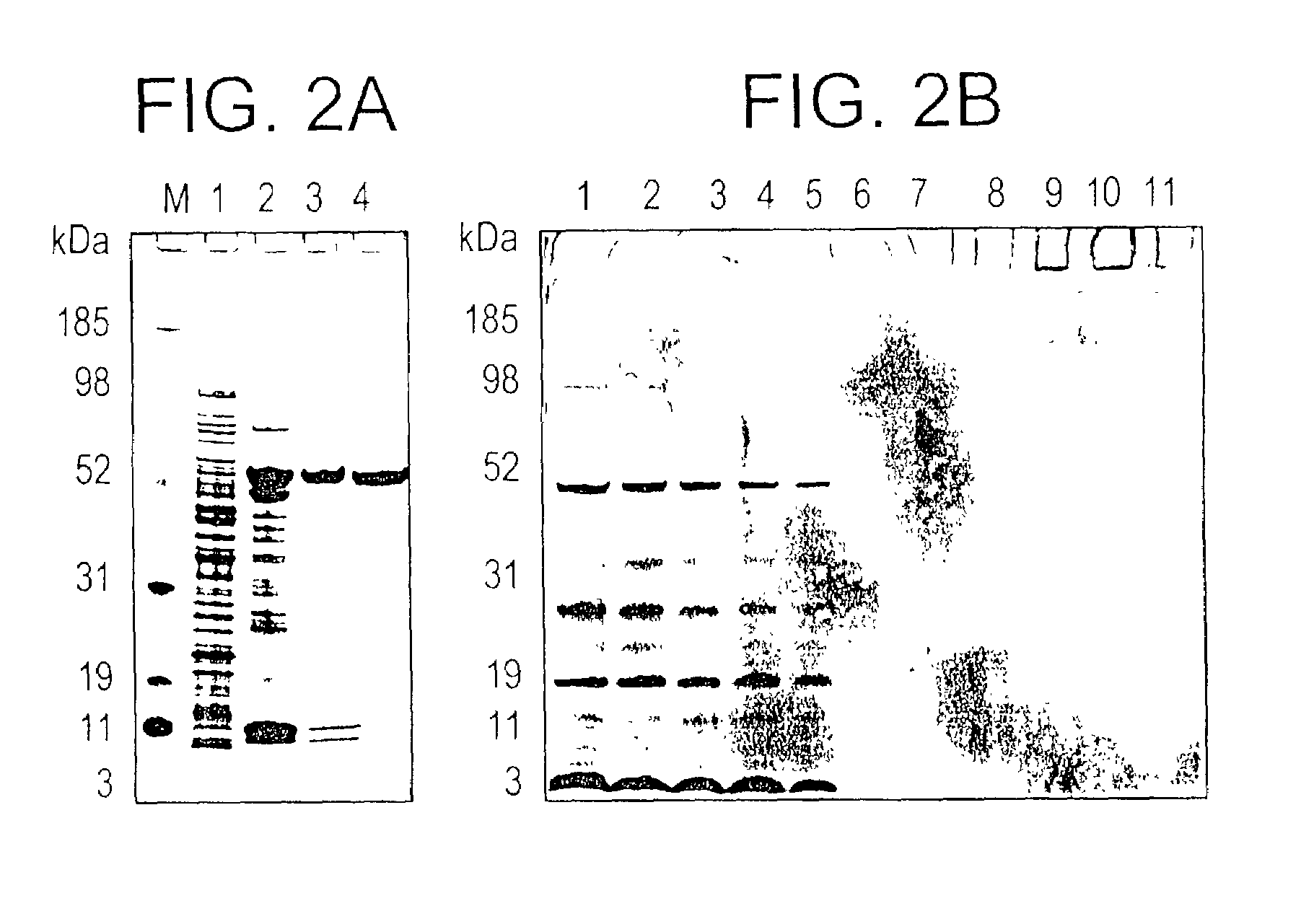
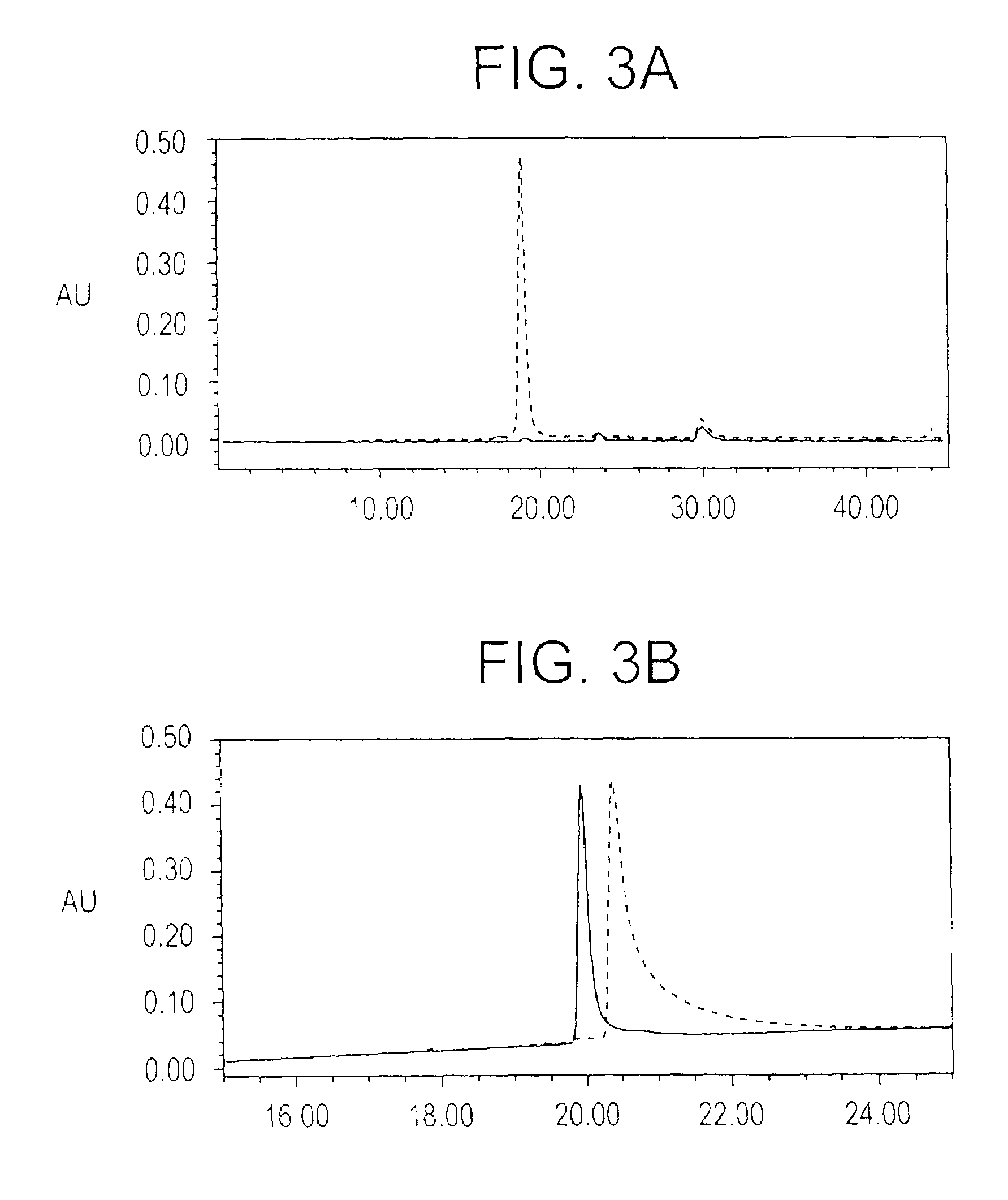
Plasmodium falciparum AMA-1 protein and uses thereof
InactiveUS7029685B2Eliminate the problemImprove responseProtozoaFermentationADAMTS ProteinsMalarial parasites
In this application is described the expression and purification of a recombinant Plasmodium falciparum (3D7) AMA-1 ectodomain. The method of the present invention produces a highly purified protein which retains folding and disulfide bridging of the native molecule. The recombinant AMA-1 is useful as a diagnostic reagent, for use in antibody production, and as a protein for use alone, or as part of, a vaccine to prevent malaria.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Monoclonal antibodies to Sarcocystis neurona and uses therefor
The present invention is directed to particular monoclonal antibodies that find use in the identification and purification of Sarcocystis neurona and related antigens. In particular, these antibodies permit the diagnosis of Sarcocystis related diseases such as equine protozoal myeloencephalitis (EPM).
Owner:UNIVERSITY OF MISSOURI
Compositions and methods using complexes of heat shock protein 90 and antigenic molecules for the treatment and prevention of neoplastic diseases
The present invention relates to methods and compositions for eliciting an immune response and the prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases. The methods of the invention comprise administering a composition comprising an effective amount of a complex, in which the complex consists essentially of a heat shock protein (hsp) noncovalently bound to an antigenic molecule. "Antigenic molecule" as used herein refers to the peptides with which the hsps are endogenously associated in vivo as well as exogenous antigens / immunogens (i.e., with which the hsps are not complexed in vivo) or antigenic / immunogenic fragments and derivatives thereof. In a preferred embodiment, the complex is autologous to the individual. The effective amounts of the complex are in the range of 10-600 micrograms for complexes comprising hsp7o, 50-1000 micrograms for hsp9o, and 10-600 micrograms for gp96. The invention also provides a method for measuring tumor rejection in viva in an individual, preferably a human, comprising measuring the generation by the individual of MHC Class I-restricted CD8+ cytotoxic T lymphocytes specific to the tumor. Methods of purifying hsp7o-peptide complexes are also provided.
Owner:FORDHAM UNIVERSITY
Amino lipids and methods for the delivery of nucleic acids
The present invention provides superior compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of specific target proteins at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:ARBUTUS BIOPHARMA CORPORAT ION +1
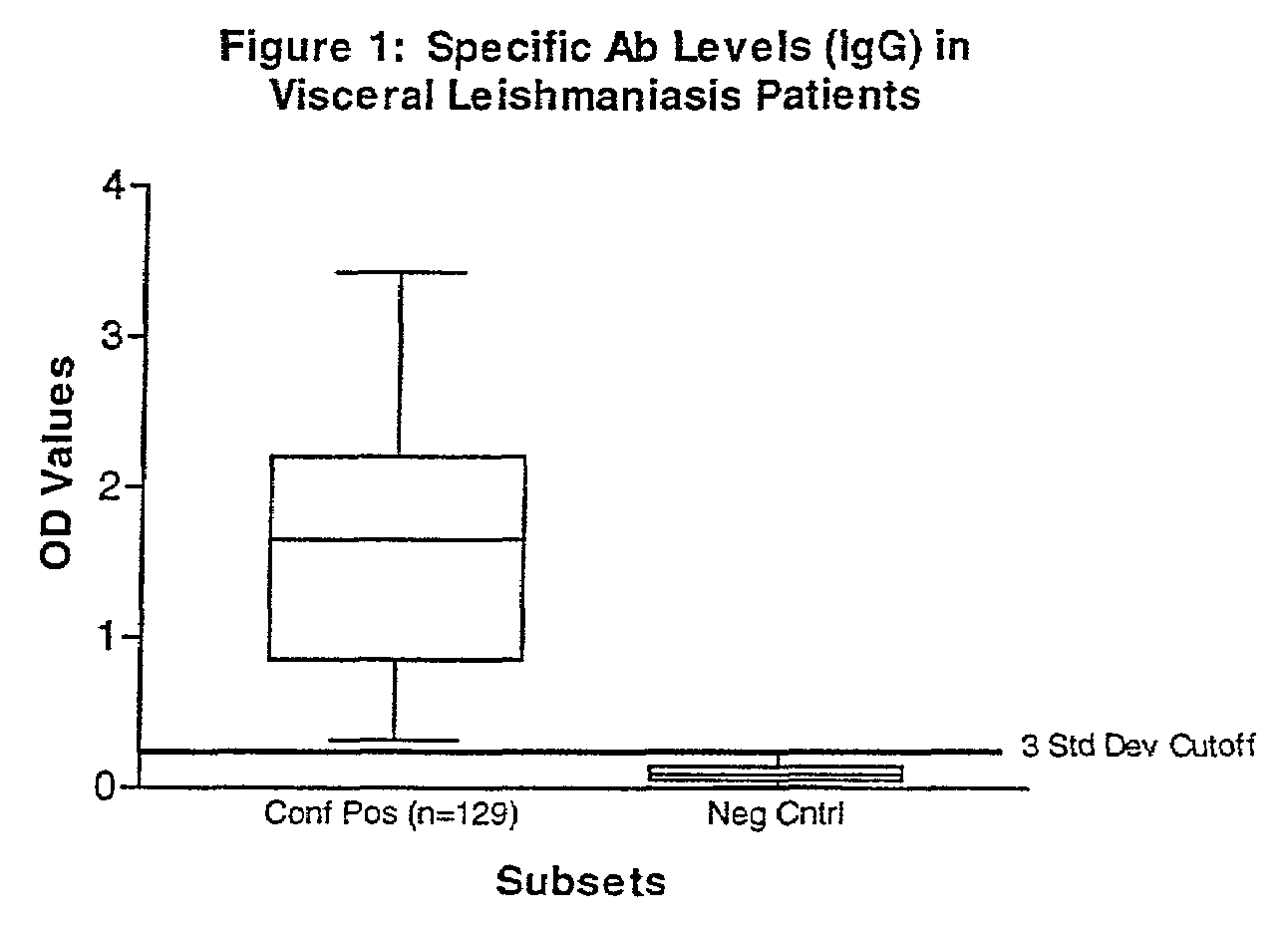
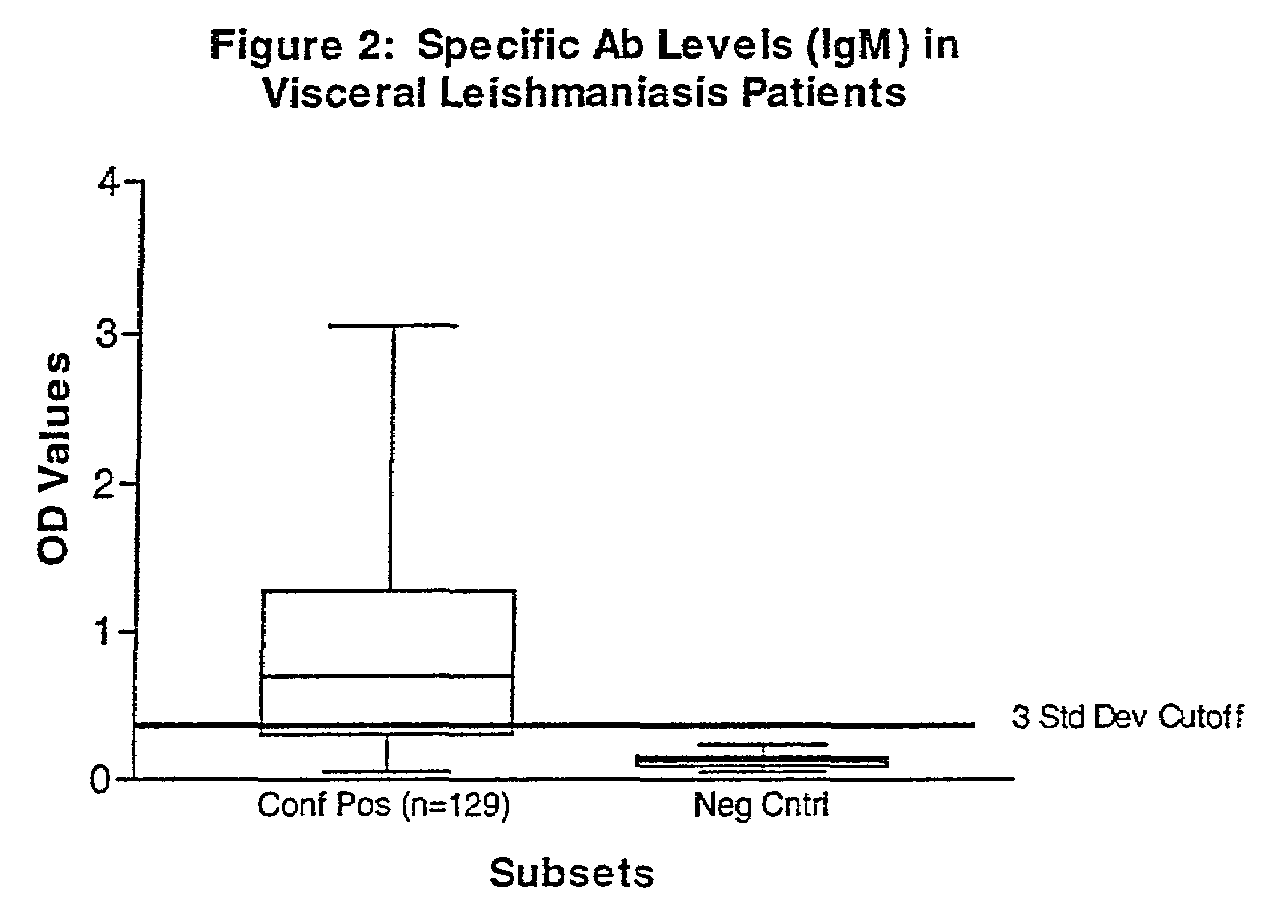
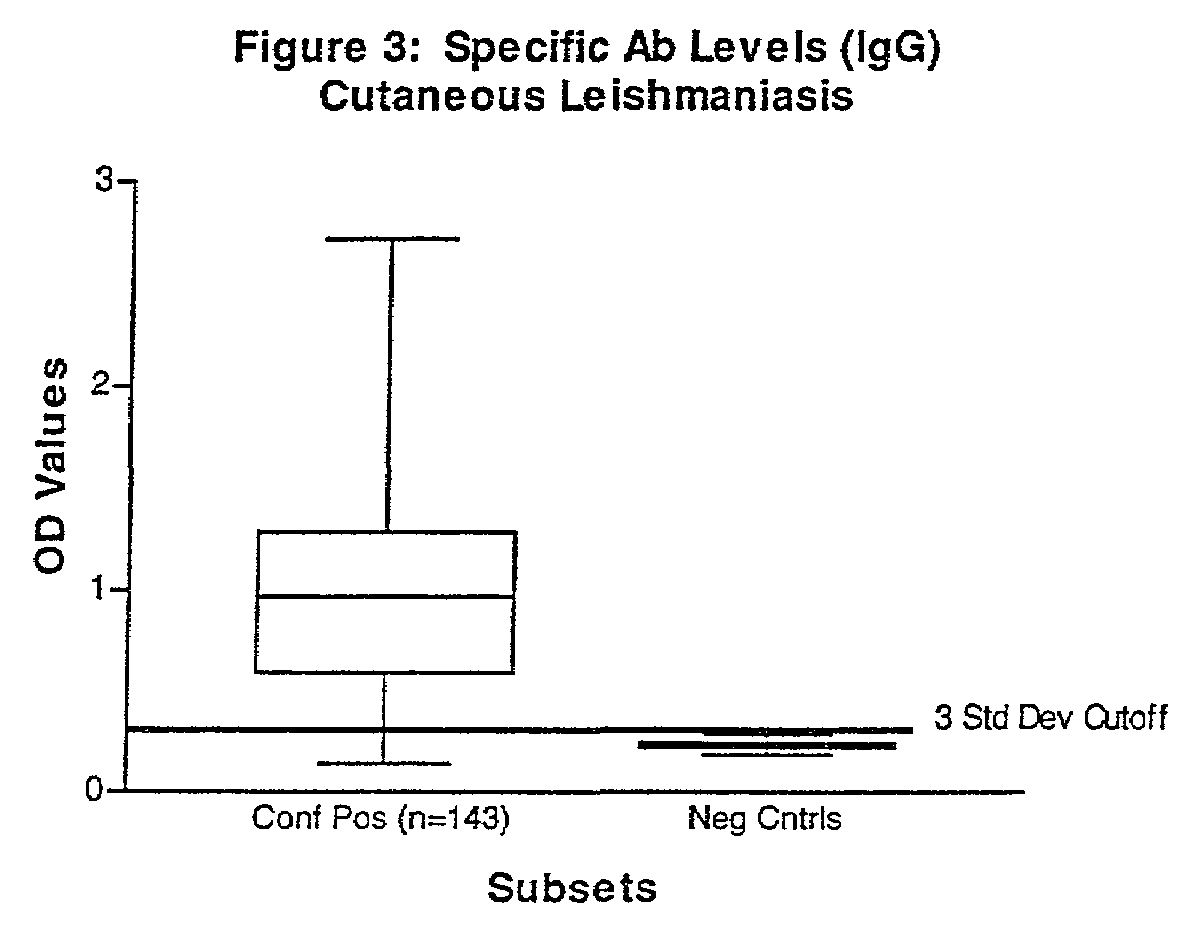
Practical serological assay for the clinical diagnosis of leishmaniasis
InactiveUS7008774B2Improve purification effectPurification is easy and lessBiocideProtozoa antigen ingredientsProtozoaAntigen capture
Methods for the diagnosis of visceral, cutaneous and canine leishmaniasis in a subject suspected of being infected with the parasitic protozoa Leishmania is disclosed. Disclosed are antibody-capture enzyme-linked immunosorbent assays (ELISAs) for the detection of antibodies to Leishmania parasite soluble antigens and antigen-capture ELISAs for the detection of Leishmania parasite soluble antigens in host samples. Also disclosed are immunodiagnostic kits for the detection of Leishmania parasite circulating antigens or IgM and IgG antibodies in a sample from subject having visceral, cutaneous or canine leishmaniasis. In these methods and kits, detection may be done photometrically or visually. The methods and kits also allow the visualization of Leishmania amastigotes or promastigotes in a sample.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Compositions containing bacteriophages and methods of using bacteriophages to treat infections
InactiveUS6942858B1Efficient and economically feasibleHigh frequencyBiocideOrganic active ingredientsBacteroidesPhage therapy
Purified, host-specific, non-toxic, wide host range and virulent bacteriophage preparations that are effective in killing bacterial organisms in vivo are disclosed. Also disclosed are compositions containing these bacteriophages, methods of making the bacteriophage preparations and methods of treating bacterial infections using the compositions. Methods of treating bacterial infections using the compositions containing the bacteriophages in combination with conventional antibiotics also are disclosed.
Owner:NYMOX CORP
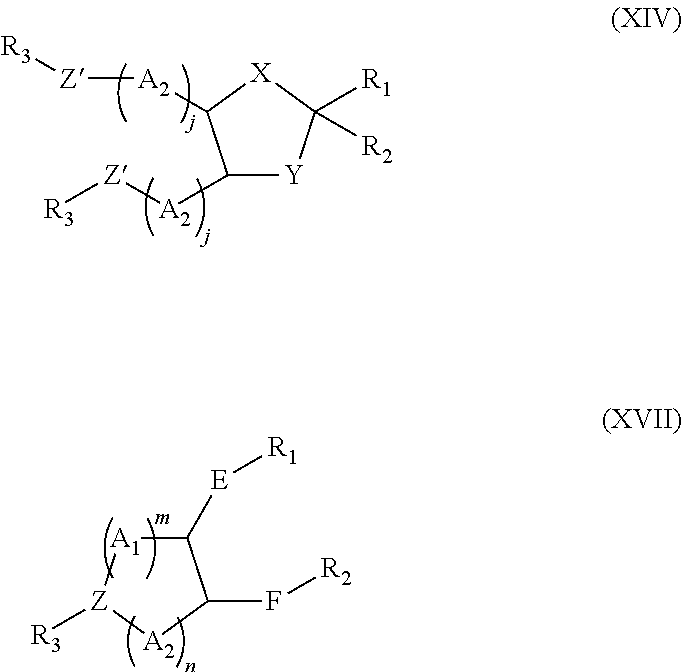
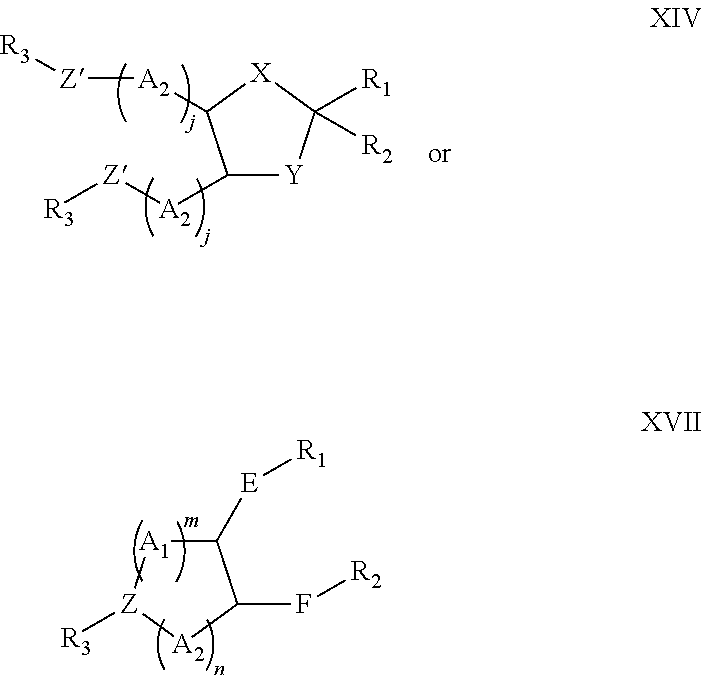
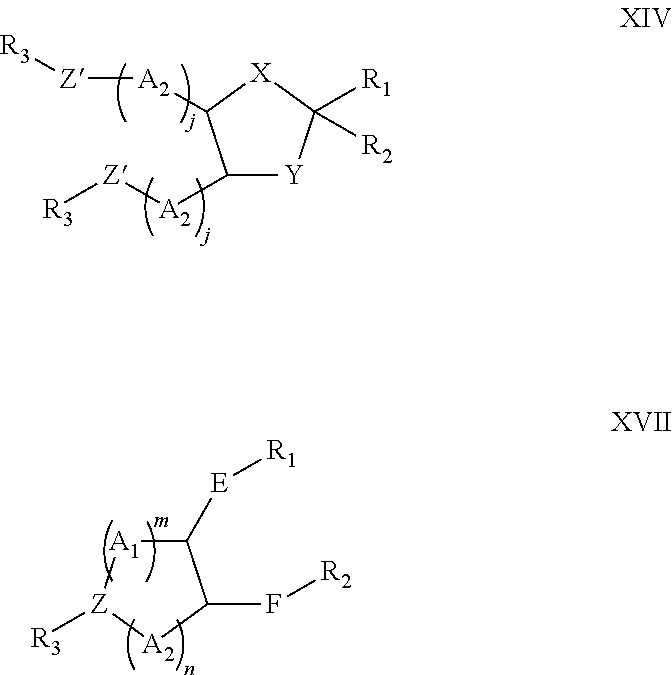
Lipids and compositions for the delivery of therapeutics
The present invention provides lipids that are advantageously used in lipid particles for the in vivo delivery of therapeutic agents to cells. In particular, the invention provides lipids having the following structures XIV or XVII.
Owner:TEKMIRA PHARMA CORP
Peptide sequences specific for the hepatic stages of P. falciparum bearing epitopes capable of stimulating the T lymphocytes
The present invention relates to an in vitro diagnostic method for malaria in an individual comprising placing a tissue or a biological fluid taken from an individual in contact with a molecule or polypeptide composition, wherein said molecule or polypeptide composition comprises one or more peptide sequences bearing all or part of one or more T epitopes of the proteins resulting from the infectious activity of P. falciparum, under conditions allowing an in vitro immunological reaction to occur between said composition and the antibodies that may be present in the tissue or biological fluid, and in vitro detection of the antigen-antibody complexes formed. The invention further relates to a polypeptide comprising at least one T epitope from a liver-stage specific protein produced by P. falciparum and a vaccine composition directed against malaria comprising a molecule having one or more peptide sequences bearing all or part of one or more T epitopes resulting from the infectious activity of P. falciparum in the hepatic cells.
Owner:INST PASTEUR
Histone deacetylase inhibitors and cognitive applications
InactiveUS20060018921A1Enhances long-term passive avoidance memoryImprove long-termBiocideNervous disorderAcetylationPoor memory
The present invention relates to the enhancement of cognition in an individual by delivery of a histone acetylation regulator, such as a histone deacetylase inhibitor. The individual may have normal or poor memory, and the poor memory may be the result of a pathogenic condition or a non-pathogenic condition, such as with normal aging-related impairment. In a specific embodiment, enhancement of cognition occurs in an individual having a mental retardation syndrome.
Owner:BAYLOR COLLEGE OF MEDICINE
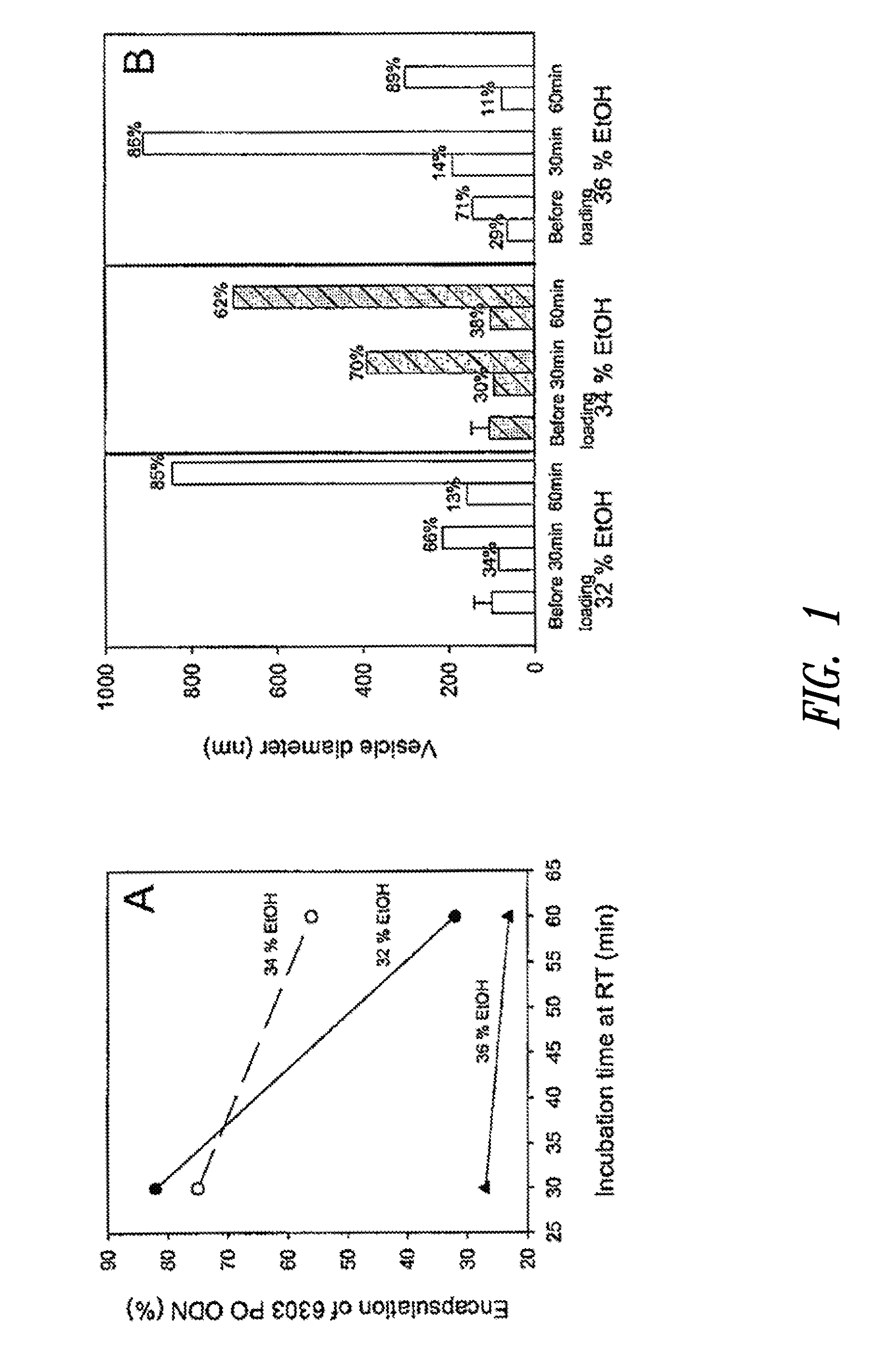
Oligodeoxynucleotide and its use to induce an immune response
Owner:UNIFORMED SERVICES UNIV OF THE HEALTH SCI AN INSTION OF HIGHER LEARNING WITHIN THE DEPT OF DEFENSE A GOVERNMENT AGENCY OF THE UNITED STATES OF AMERICA
CR-2 binding peptide P28 as molecular adjuvant for DNA vaccines
InactiveUS8470560B2Improve responseRobust productionBiocidePeptide/protein ingredientsAdjuvantBinding peptide
The invention is an DNA vaccine and method of use thereof for modulating the immune response against the circumsporozoite protein (CSP) of malaria parasites, using the CR2 binding motifs of C3d, especially p28.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Immunogenic composition for immune system modulation and use thereof, method for treating and preventing diseases, method for inducing cell regeneration and method for restoring immune response
ActiveUS20140037716A1Effective strikeIncrease opportunitiesSenses disorderNervous disorderBenign tumoursNervous system
The present invention relates to immunogenic compositions for modulating the immune system, comprising a therapeutically effective quantity of two or more immuno-active antigenic agents with pathogen-associated molecular patterns (PAMPs) and / or danger-associated molecular patterns (DAMPs) and one or more physiologically acceptable carriers, excipients, diluents or solvents. The immunogenic compositions according to the present invention are used for producing medicaments for preventing and / or treating, and for preventing and / or treating infectious diseases, auto-immune diseases, allergic diseases, inflammation, arthritis, inflammatory diseases, transplant rejection, affections caused by vascular disorders, diseases caused by haemorrhagic or ischaemic cardiovascular accidents, ischaemia, heart attack and haemorrhagia leading to tissue destruction, heart, kidney, respiratory or liver insufficiency, cancer, malign and benign tumours and neoplasia. The present invention further relates to methods for inducing the regeneration of cells, tissues, organs and organic systems such as the circulatory, nervous and endocrine systems. Finally, the present invention relates to methods for restoring immune response in an animal, in particular a human being.
Owner:NOWILL ALEXANDRE EDUARDO
Use of glycosylceramides as adjuvants for vaccines against infections and cancer
InactiveUS7488491B2Enhancement and extension of durationAntibacterial agentsOrganic active ingredientsAdjuvantMammal
The present invention relates to methods and compositions for augmenting an immunogenicity of an antigen in a mammal, comprising administering said antigen together with an adjuvant composition that includes glycosylceramide, preferably α-galactosylceramide (α-GalCer). According to the present invention, the use of glycosylceramide as an adjuvant is attributed at least in part to the enhancement and / or extension of antigen-specific Th1-type responses, in particular, CD8+ T cell responses. The methods and compositions of the present invention can be useful for prophylaxis and treatment of various infectious and neoplastic diseases.
Owner:NEW YORK UNIVERSITY
Transcutaneous immunization without heterologous adjuvant
InactiveUS20060002949A1Increase skin hydrationIncrease local concentrationSsRNA viruses negative-senseAntibacterial agentsDendritic cellSpecific immunity
Transcutaneous immunization can deliver antigen to the immune system through the stratum corneum without physical or chemical penetration to the dermis layer of the skin. This delivery system induces an antigen-specific immune response without the use of a heterologous adjuvant. Although perforation of intact skin is not required, superficial penetration or micropenetration of the skin can act as an enhancer; similarly, hydration may enhance the immune response. This system can induce antigen-specific immune effectors after epicutaneous application of a formulation containing one or more antigens. The formulation may initiate processes such as antigen uptake, processing, and presentation; Langerhans cell activation, migration from the skin to other immune organs, and differentiation to mature dendritic cells; contacting antigen with lymphocytes bearing cognate antigen receptors on the cell surface and their stimulation; and combinations thereof. Systemic and / or regional immunity may be induced. Immune responses that provide prophylactic and / or therapeutic treatments are preferred. Antigenic activities in the formulation may be found in the same molecule, two or more different molecules dissociated from each other, or multiple molecules in a complex formed by covalent or non-covalent bonds. For antigens which are proteinaceous, they may be provided in the formulation as a polynucleotide for transcutaneous genetic immunization. Besides simple application of a dry or liquid formulation to the skin, patches and other medical devices may be used to deliver antigen for immunization.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE OFFICE OF THE COMMAND JUDGE ADVOCATE
Molecules enhancing dermal delivery of influenza vaccines
InactiveUS20050255121A1Good antigenicityEnhance immune responseSsRNA viruses negative-senseBiocideInfluenza vaccineImmunogenicity
The present invention relates to dermal vaccine formulations, designed for targeted delivery of an immunogenic composition to a dermal compartment of skin including the intradermal and epidermal compartments. The dermal vaccine formulations of the invention comprise an antigenic or immunogenic agent, and at least one molecule, e.g., a chemical agent, which enhances the presentation and / or availability of the antigenic or immunogenic agent to the immune cells of the intradermal compartment or epidermal compartment resulting in an enhanced immune response. The dermal vaccine formulations of the invention have enhanced efficacy as the antigenic or immunogenic agent is delivered to the intradermal compartment or epidermal compartment with enhanced presentation and / or availability to the immune cells that reside therein. The enhanced efficacy of the dermal vaccine formulations results in a therapeutically effective immune response after a single intradermal or epidermal dose, with lower doses of antigenic or immunogenic agent than conventionally used, and without the need for booster immunizations.
Owner:BECTON DICKINSON & CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com