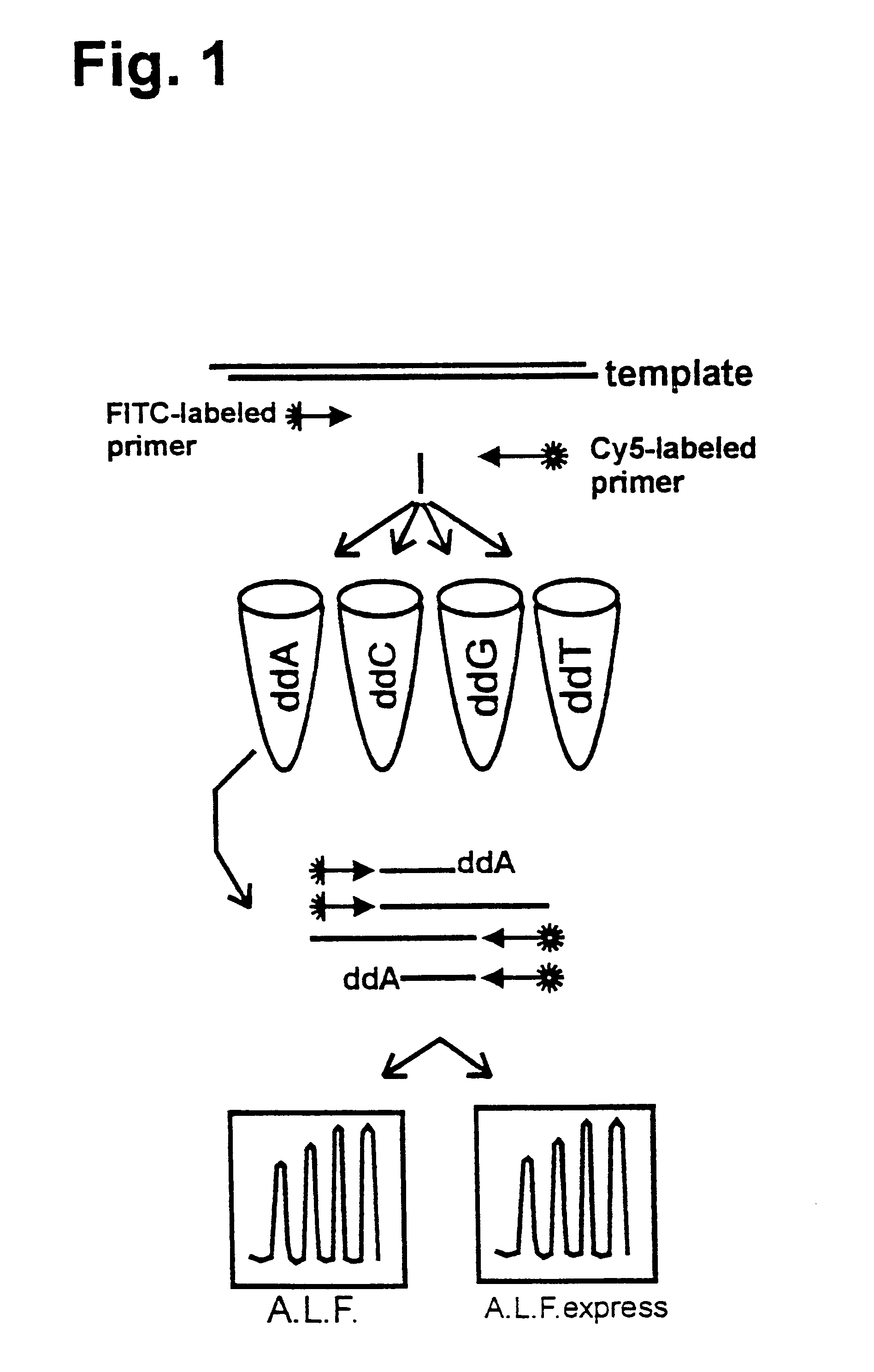
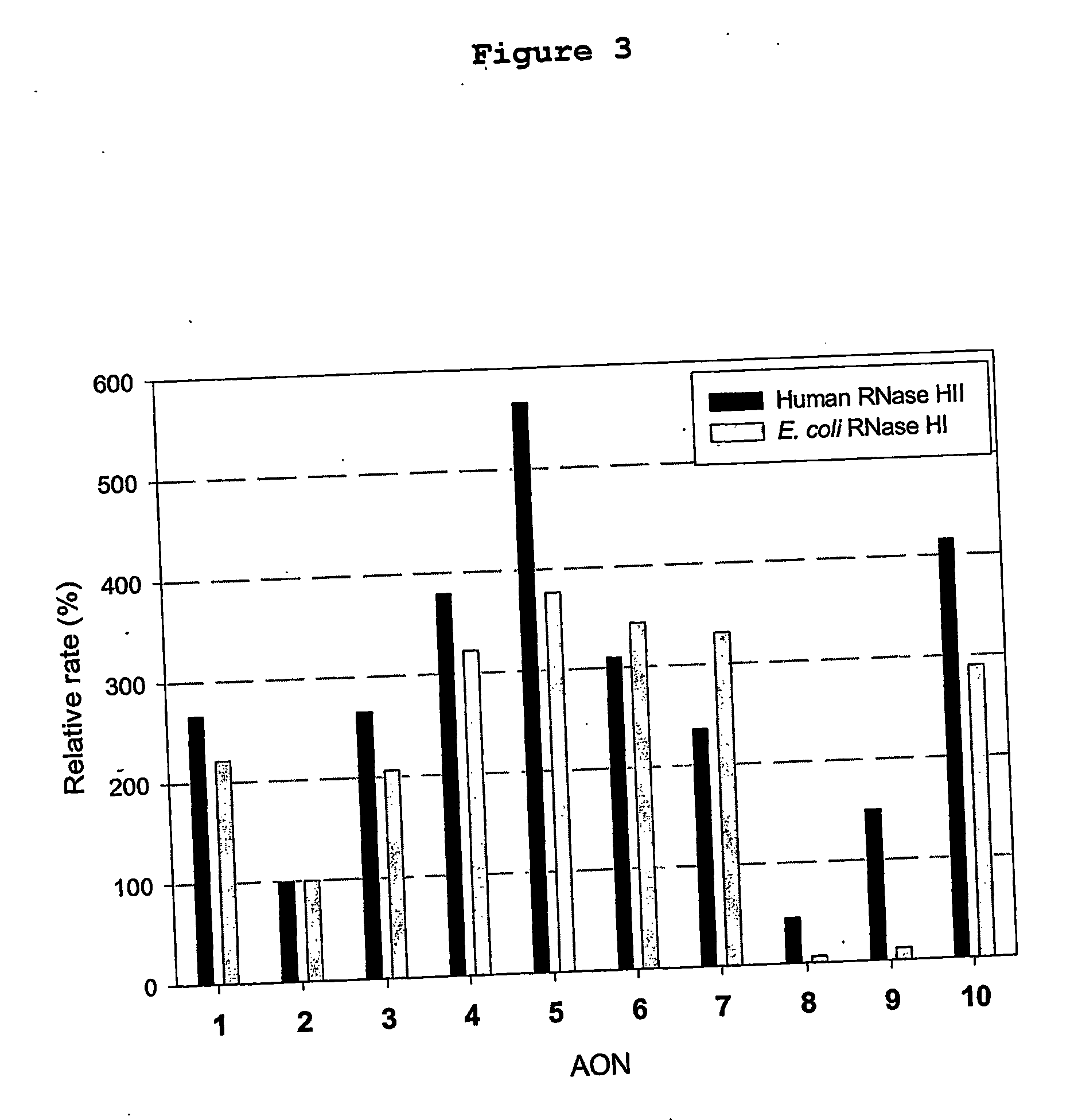
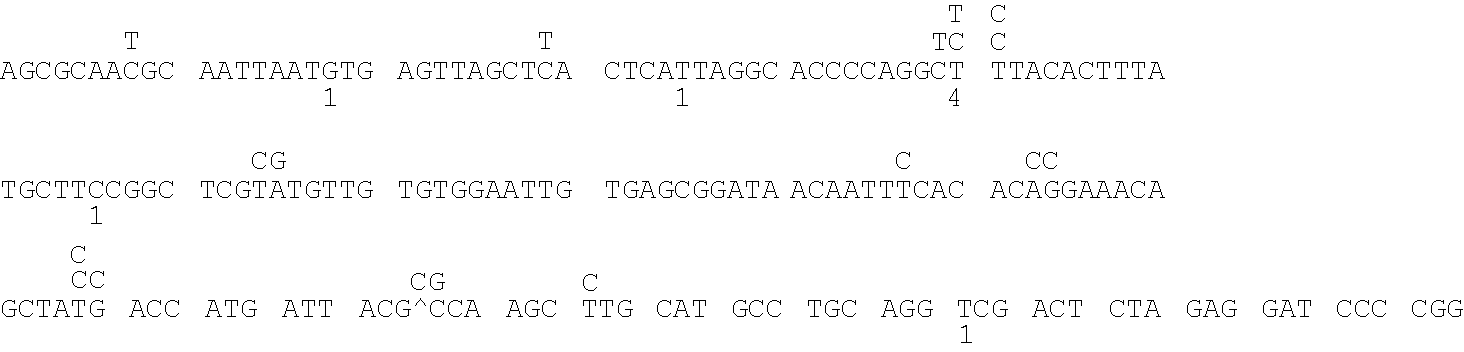
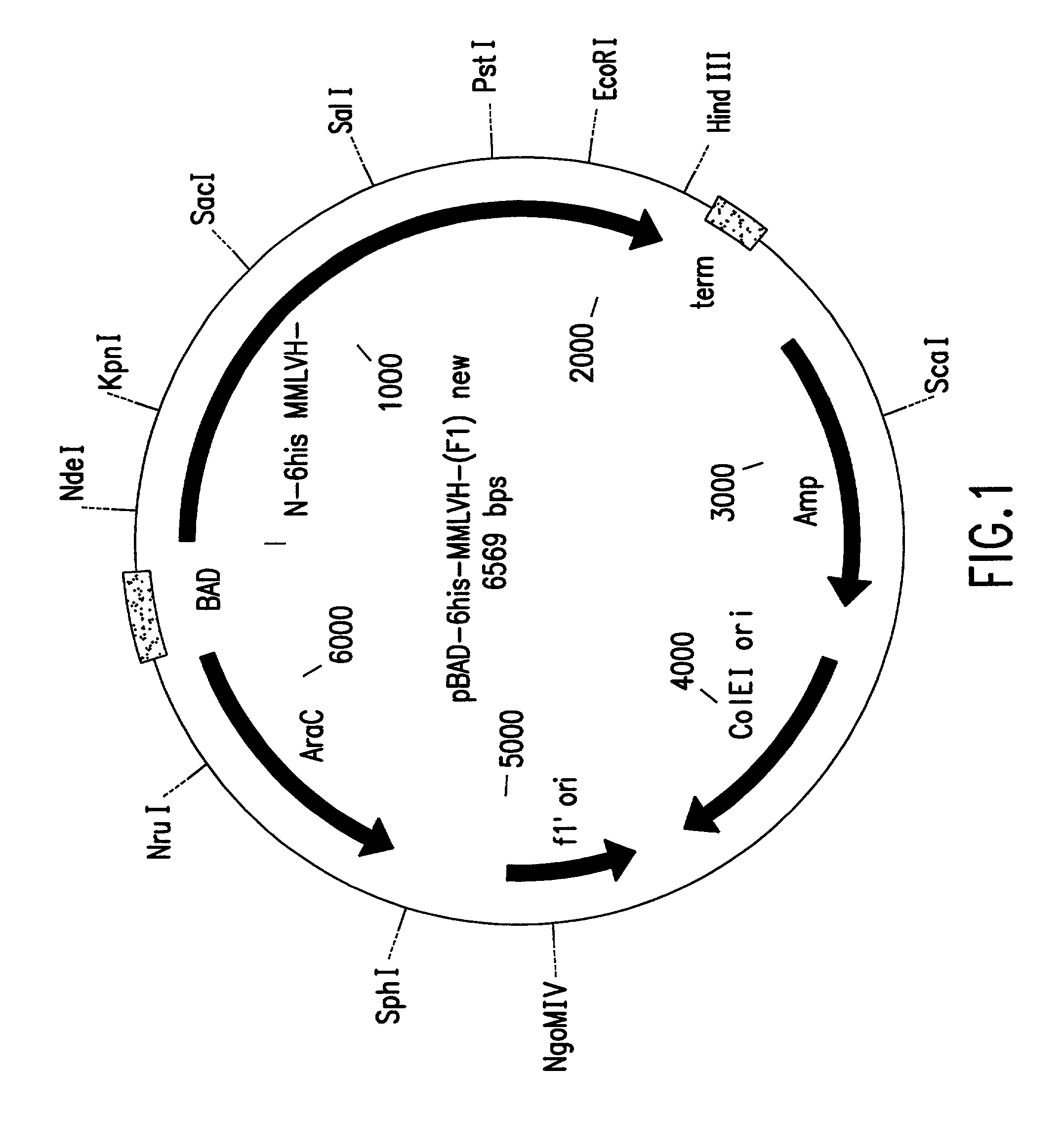
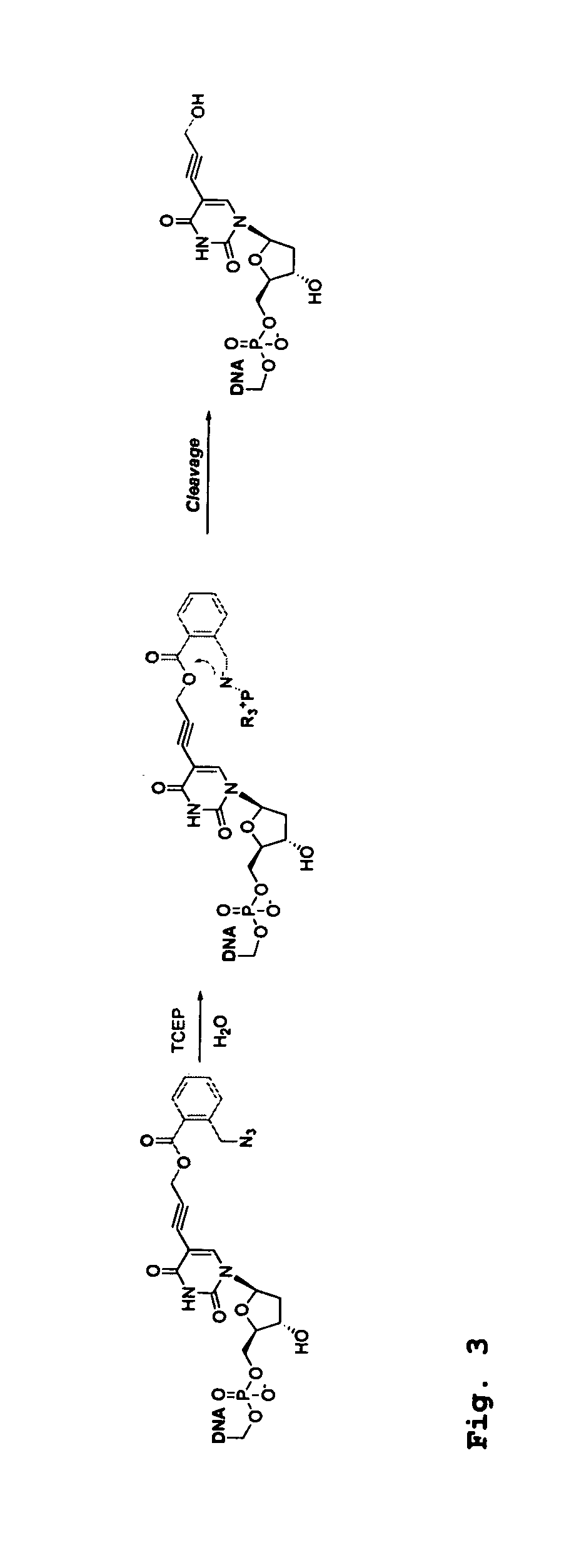
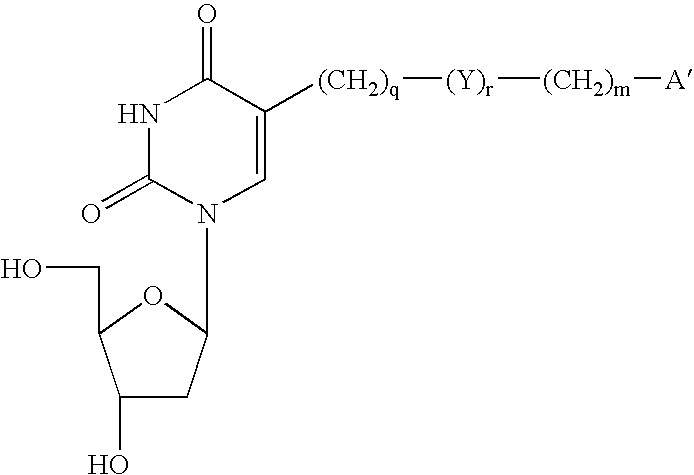
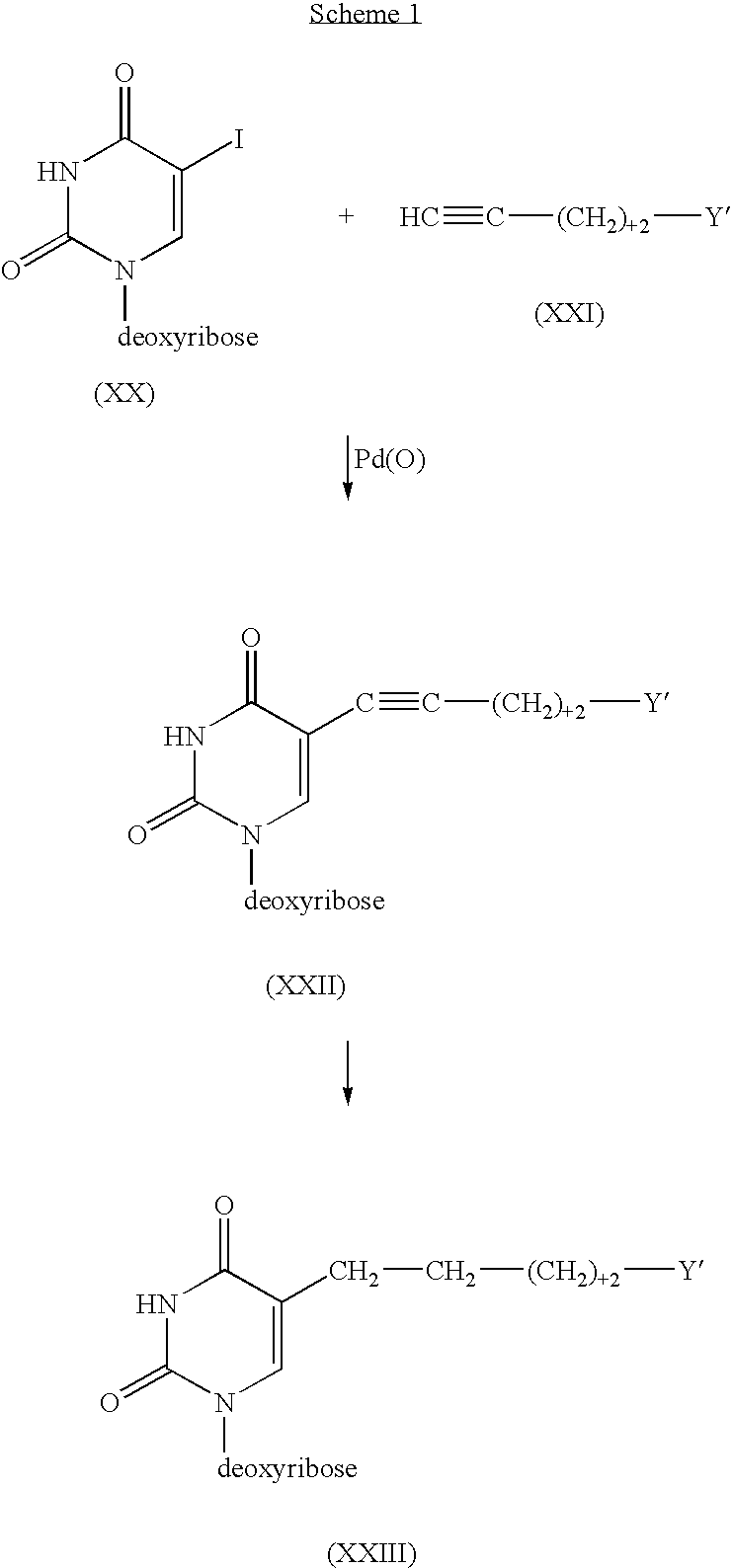
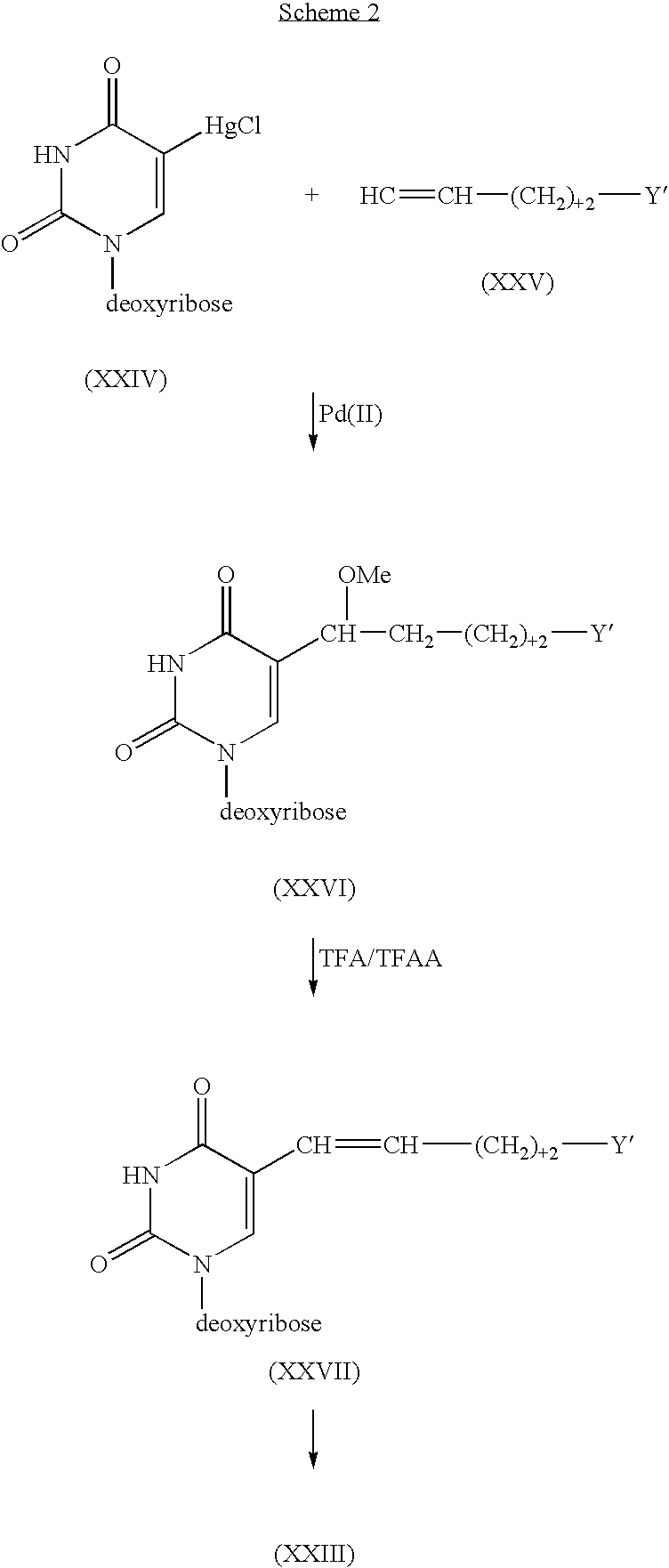
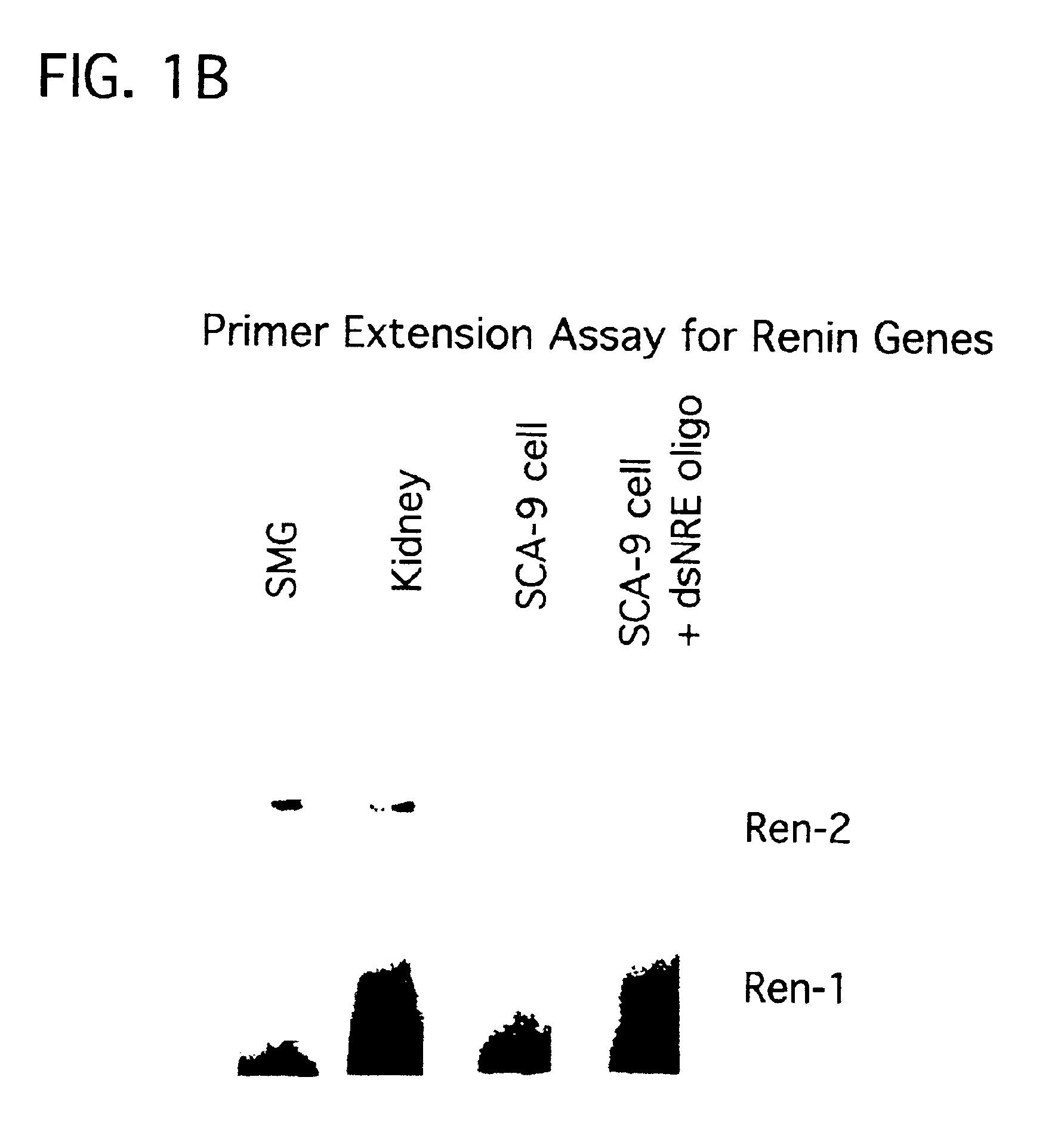
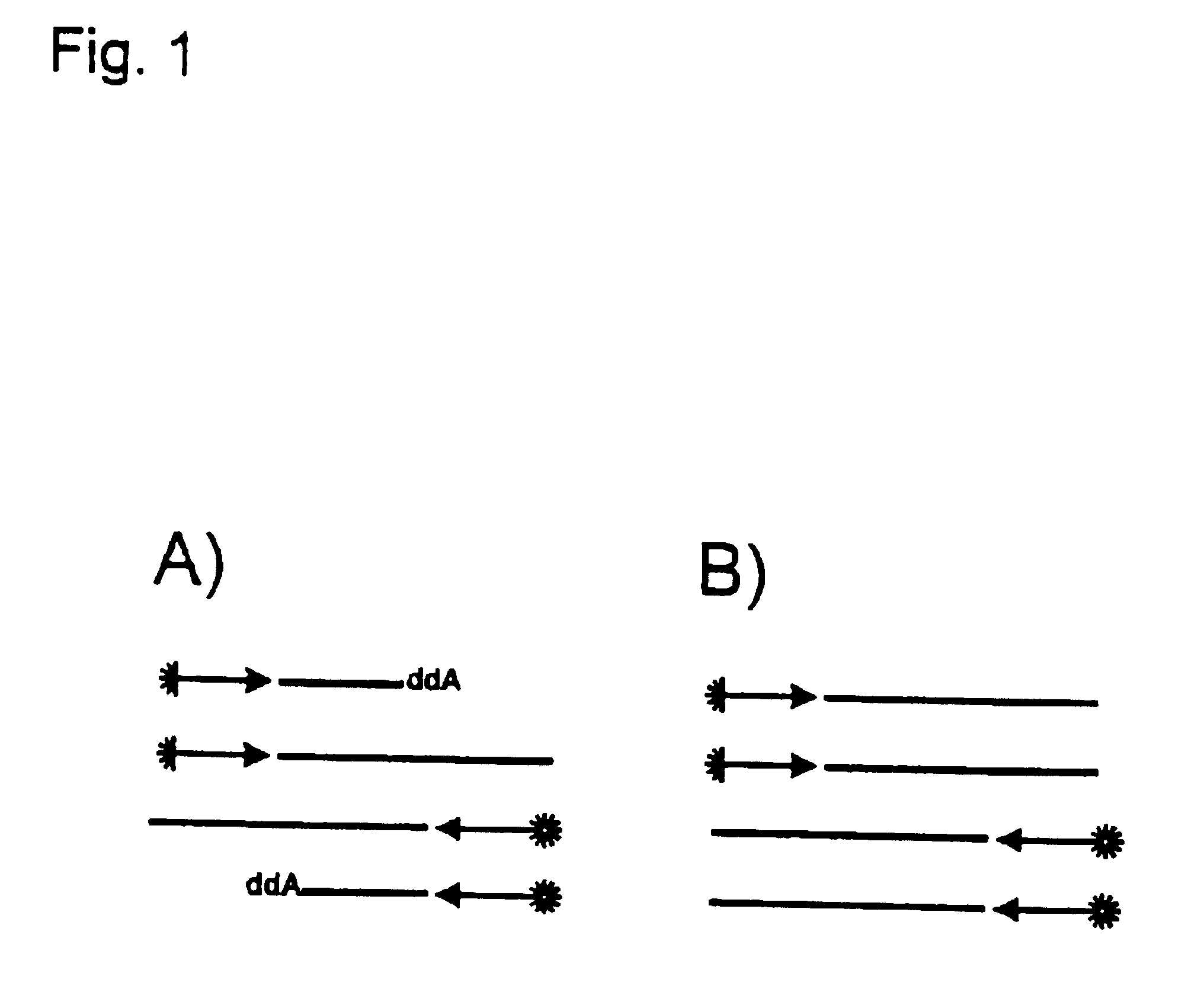
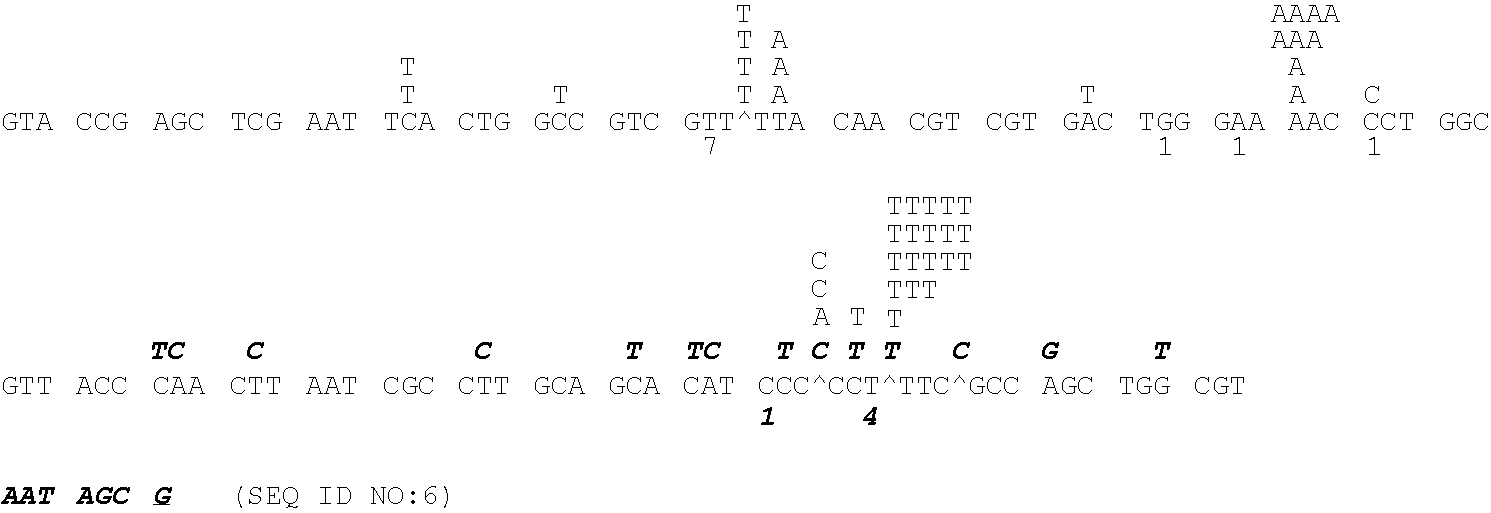Patents
Literature
364 results about "Deoxyribonucleotide synthesis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
When deoxyribonucleotides polymerize to form DNA, the phosphate group from one nucleotide will bond to the 3' carbon on another nucleotide, forming a phosphodiester bond via dehydration synthesis.
New sequencing method for sequencing rna molecules
InactiveUS20060166203A1Reduce decreaseMicrobiological testing/measurementReverse transcriptaseNucleotide
The present invention provides a method for determination of the identity of at least one nucleotide in a RNA-molecule comprising the steps of: (i) providing the RNA-molecule, an oligonucleotide primer binding to a predetermined position of the RNA molecule, a reverse transcriptase, deoxynucleotides and other necessary reagents, in a reaction vessel; (ii) performing a primer extension reaction, whereby the oligonucleotide primer is extended on the RNA-molecule through incorporation of at least one deoxynucleotide by the action of a reverse transcriptase, resulting in the release of a PPi molecule only upon incorporation of a deoxynucleotide; and (iii) detecting the presence or absence of incorporation, thereby indicating the nucleotide identity of the RNA molecule in the relevant position. In a preferred embodiment, the sequencing of the invention is coupled to the Pyrosequencing™ reaction. A variant of the method employs incorporation of modified nucleotides, with an optionally cleavable linker arm to which is attached a label.
Owner:TOOKE NIGEL
Method for the direct, exponential amplification and sequencing of DNA molecules and its application
InactiveUS6605428B2Improved and rapid and reliable methodReduction of initial amountSugar derivativesMicrobiological testing/measurementDideoxynucleotide TriphosphatesPolymerase L
A method is described for the direct, exponential amplification and sequencing ("DEXAS") of a DNA molecule from a complex mixture of nucleic acids, wherein truncated DNA molecules as well as DNA molecules of full length are synthesized simultaneously and exponentially between two positions on the said DNA molecule, which initially contains a DNA molecule in a thermocycling reaction, a first primer, a second primer, a reaction buffer, a thermostable DNA polymerase, a thermostable pyrophosphatase (optionally), deoxynucleotides or derivatives thereof and a dideoxynucleotide or derivatives thereof. In a preferred embodiment of the method of the invention, direct sequencing of RNA can be performed using one polymerase having a Tabor-Richardson mutation, or a functional derivative thereof, and reverse transcriptase activity. In a more preferred embodiment of the method of the invention, direct sequencing of RNA can be performed in one step, in one vessel.
Owner:ROCHE DIAGNOSTICS GMBH
Antisense oligonucleotides against VR1
InactiveUS7662948B2Reduce in quantityAvoid developmentOrganic active ingredientsFungiPain therapyNucleotide
Antisense oligodeoxynucleotides against VR1, corresponding nucleotide constructs, cells containing said nucleotide constructs, pharmaceutical and diagnostic substances, uses thereof in pain therapy, and methods for diagnosing symptoms related to VR1 and for identifying pain-modulating substances.
Owner:GRUNENTHAL GMBH
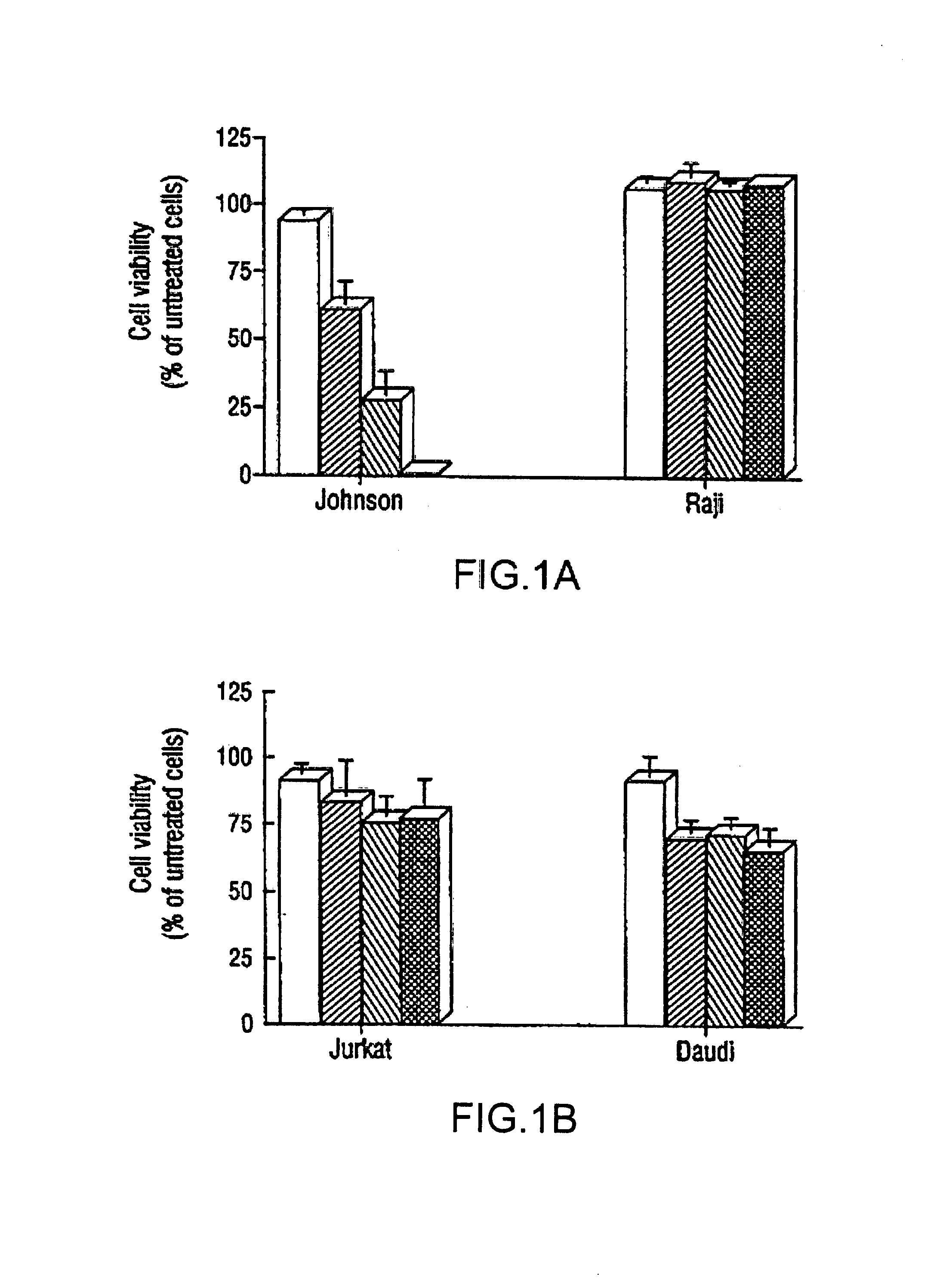
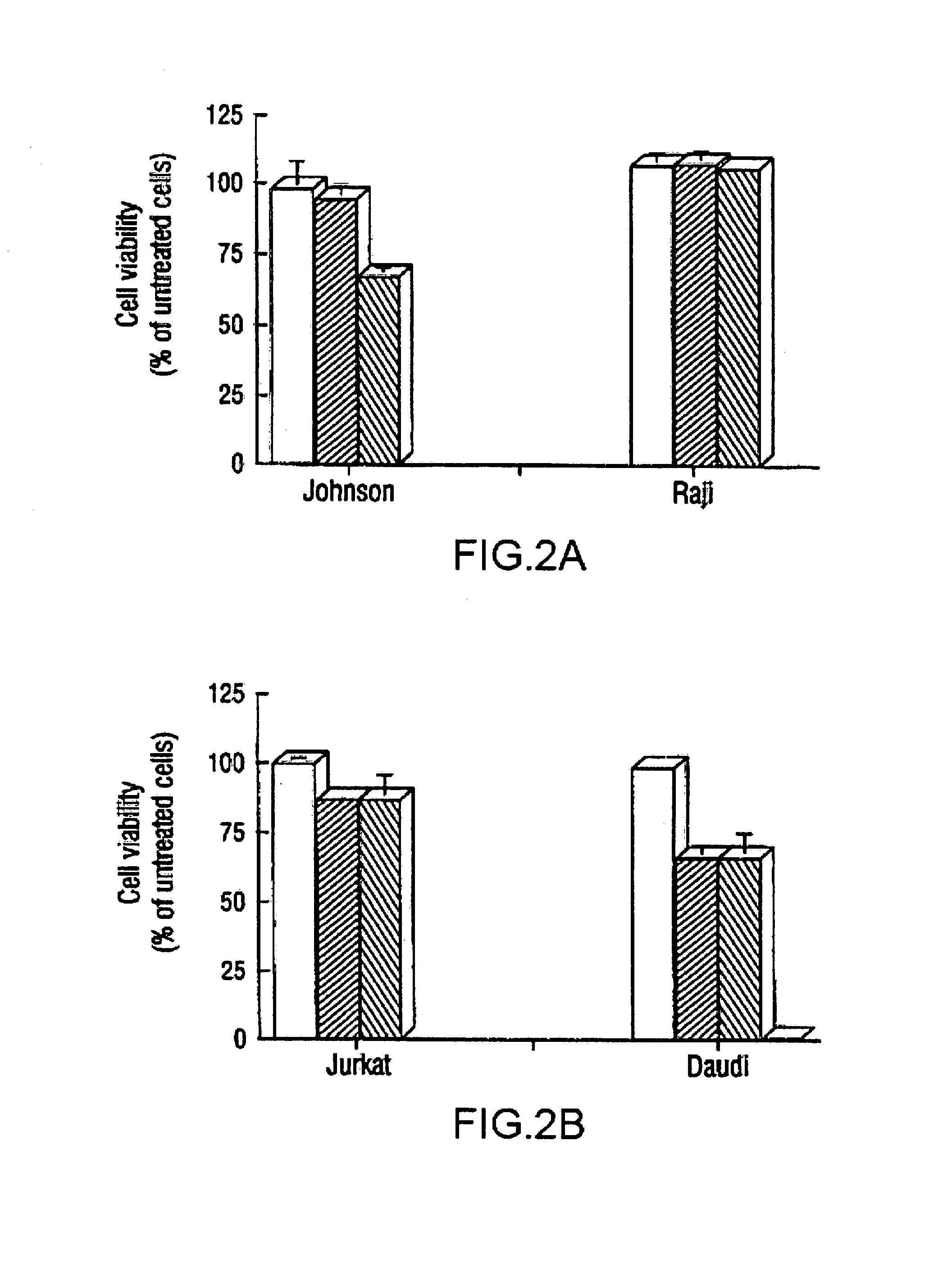
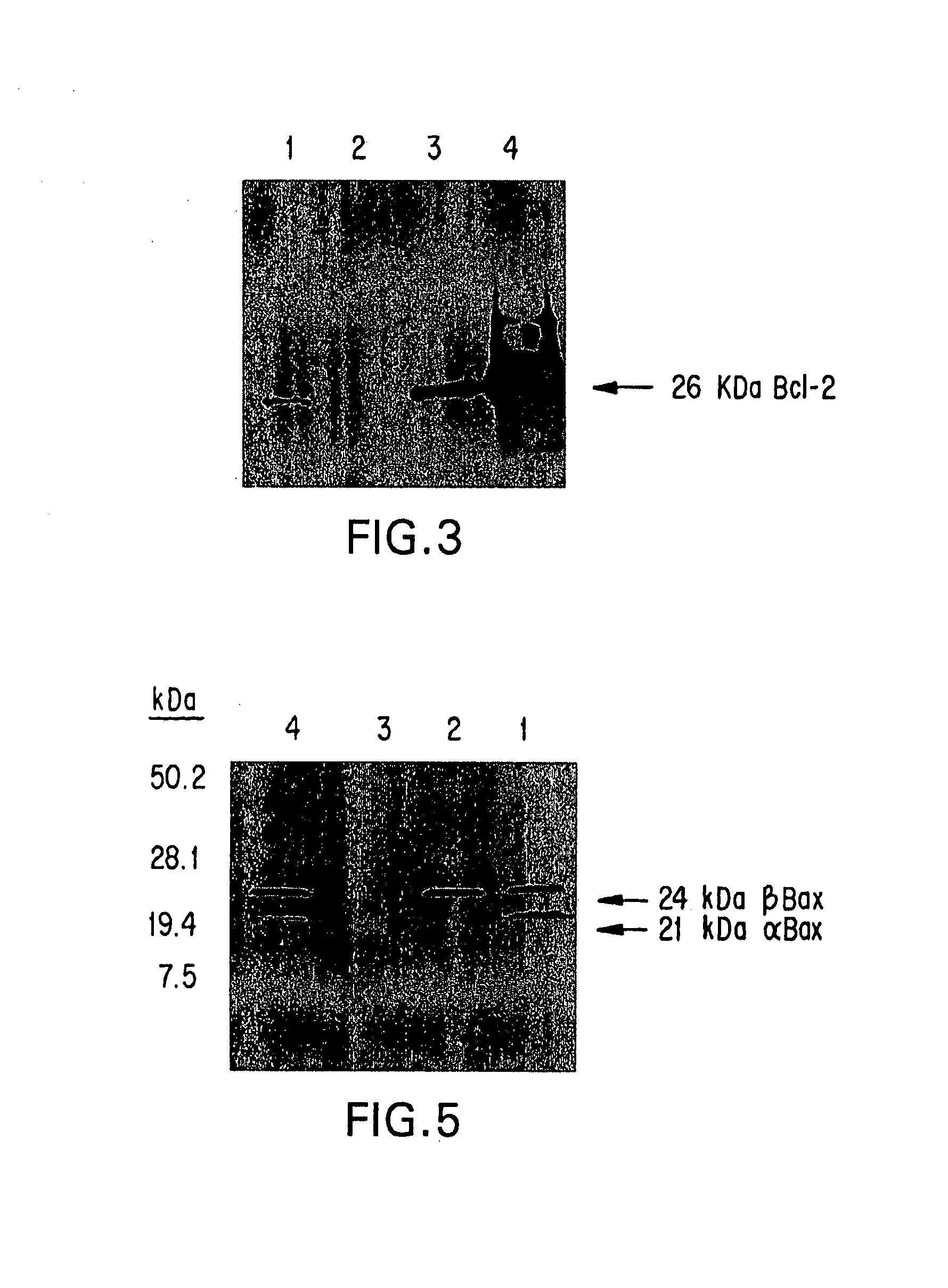
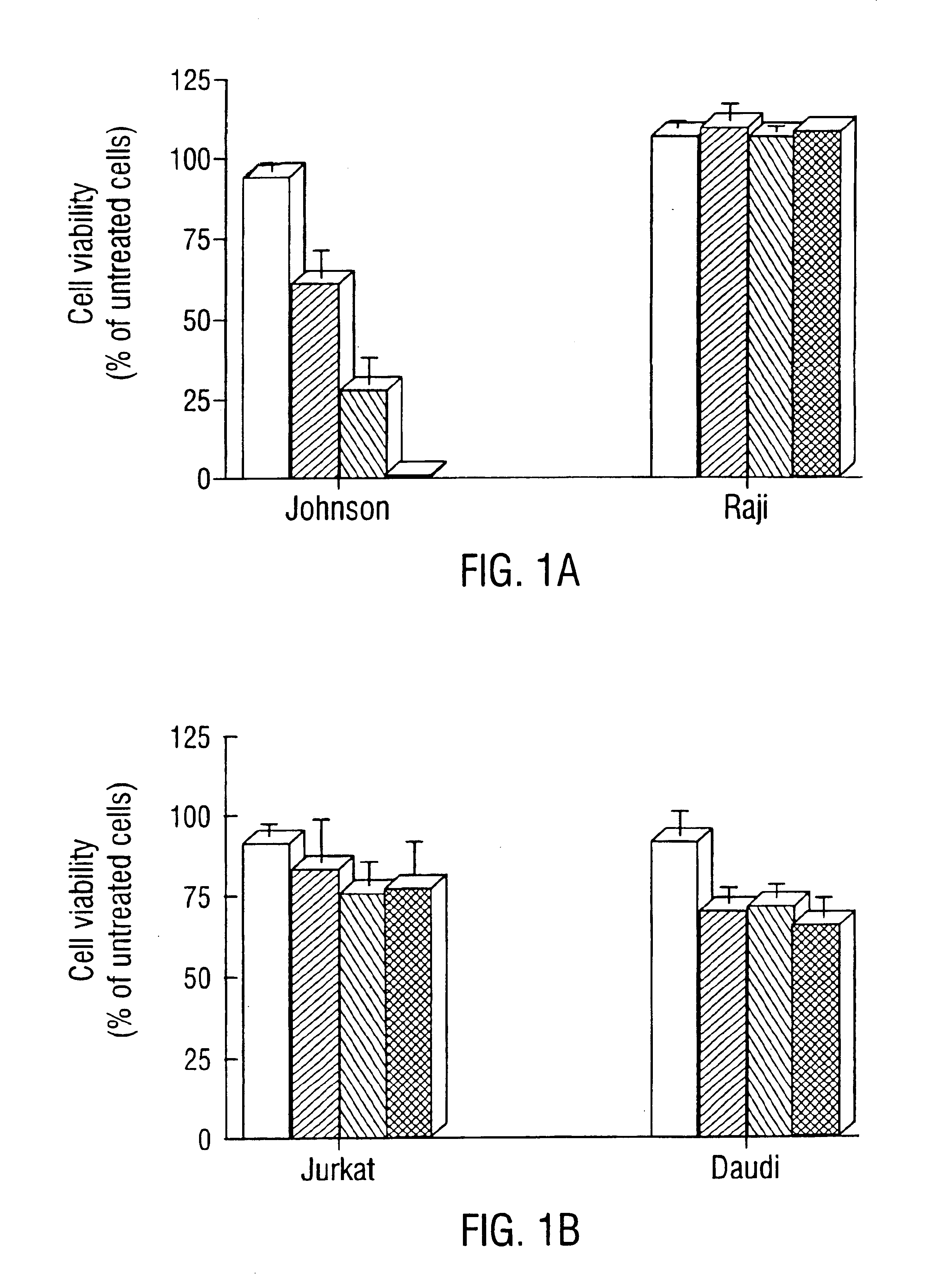
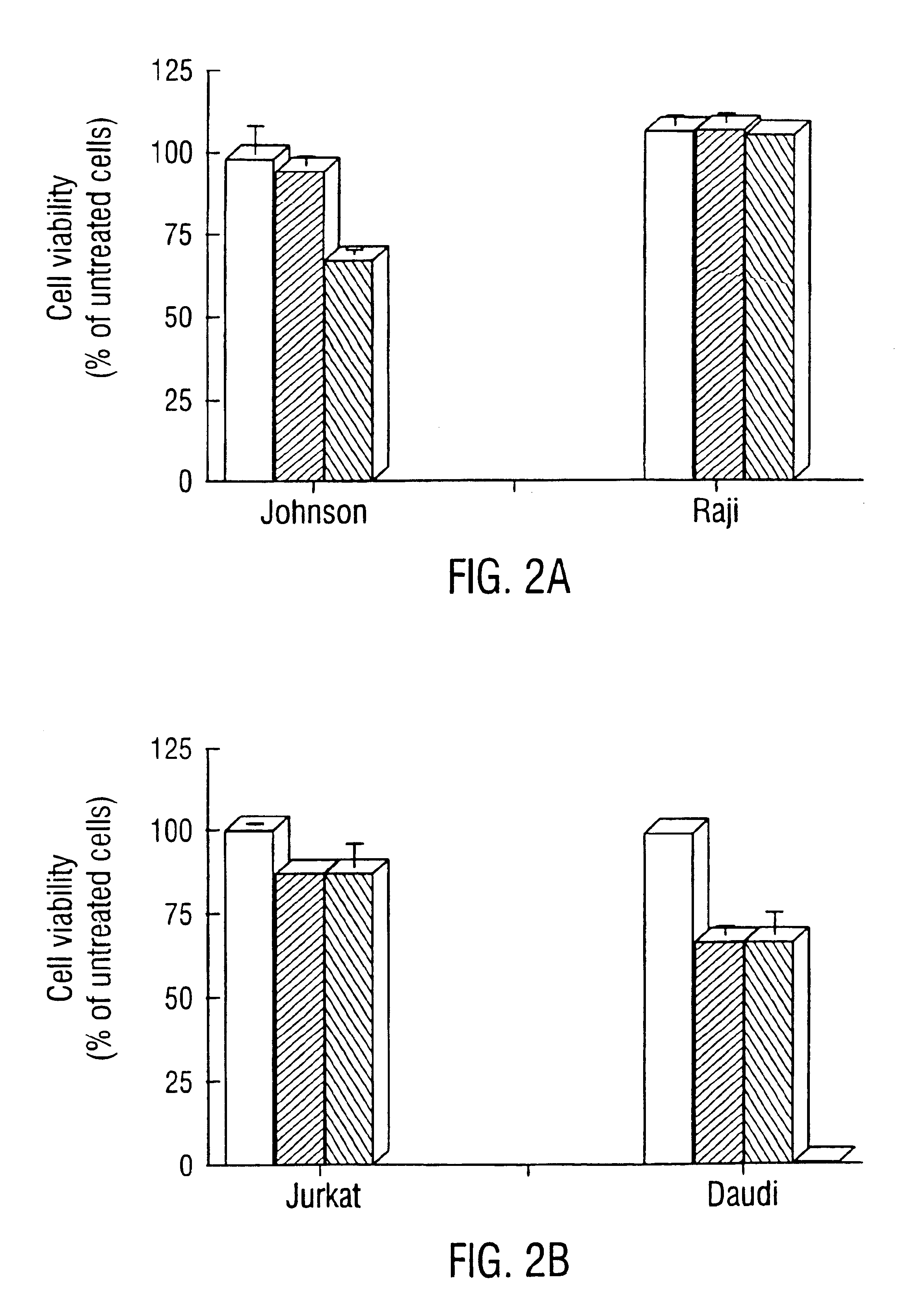
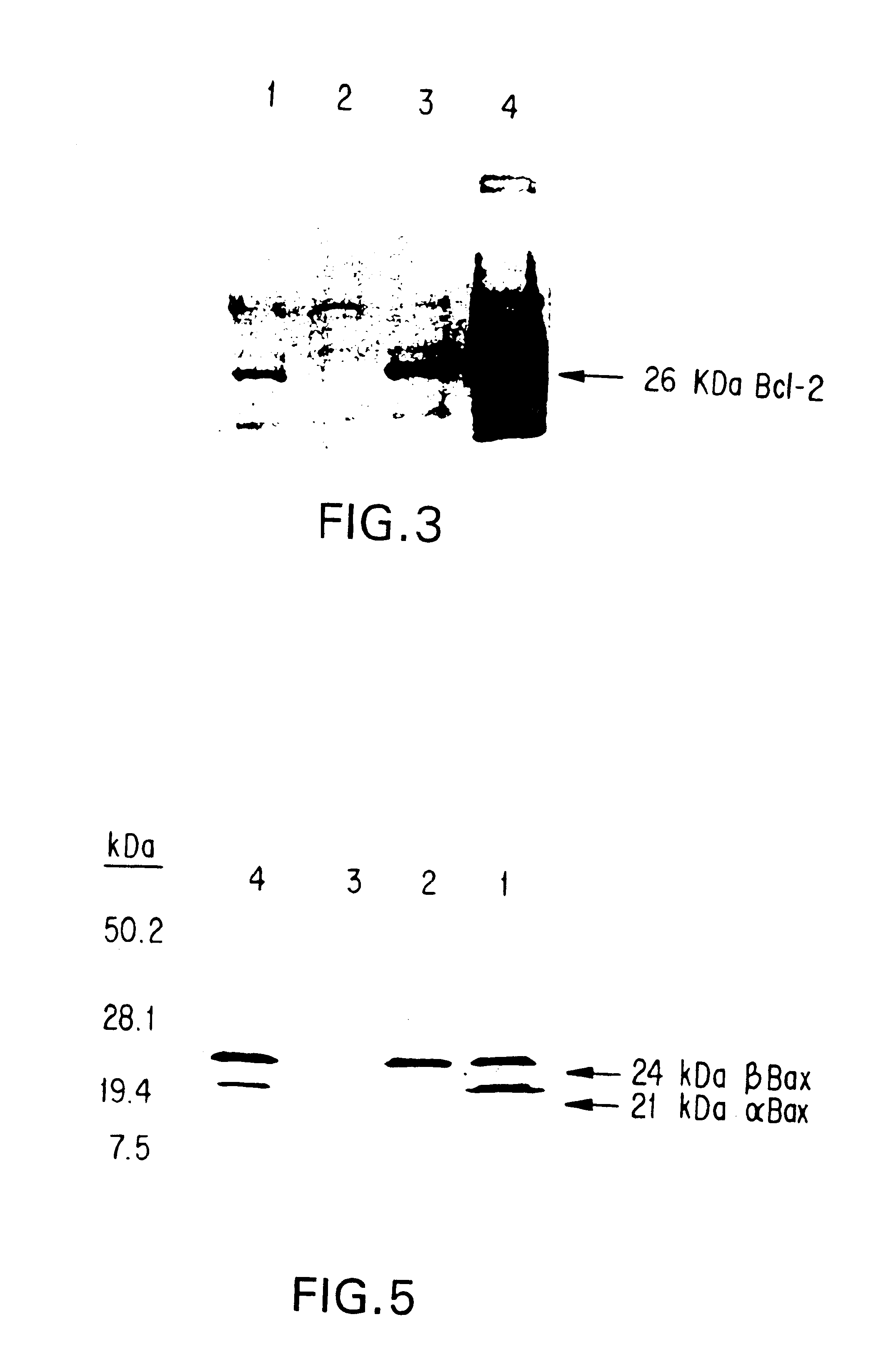
Inhibition of Bcl-2 protein expression by liposomal antisense oligodeoxynucleotides
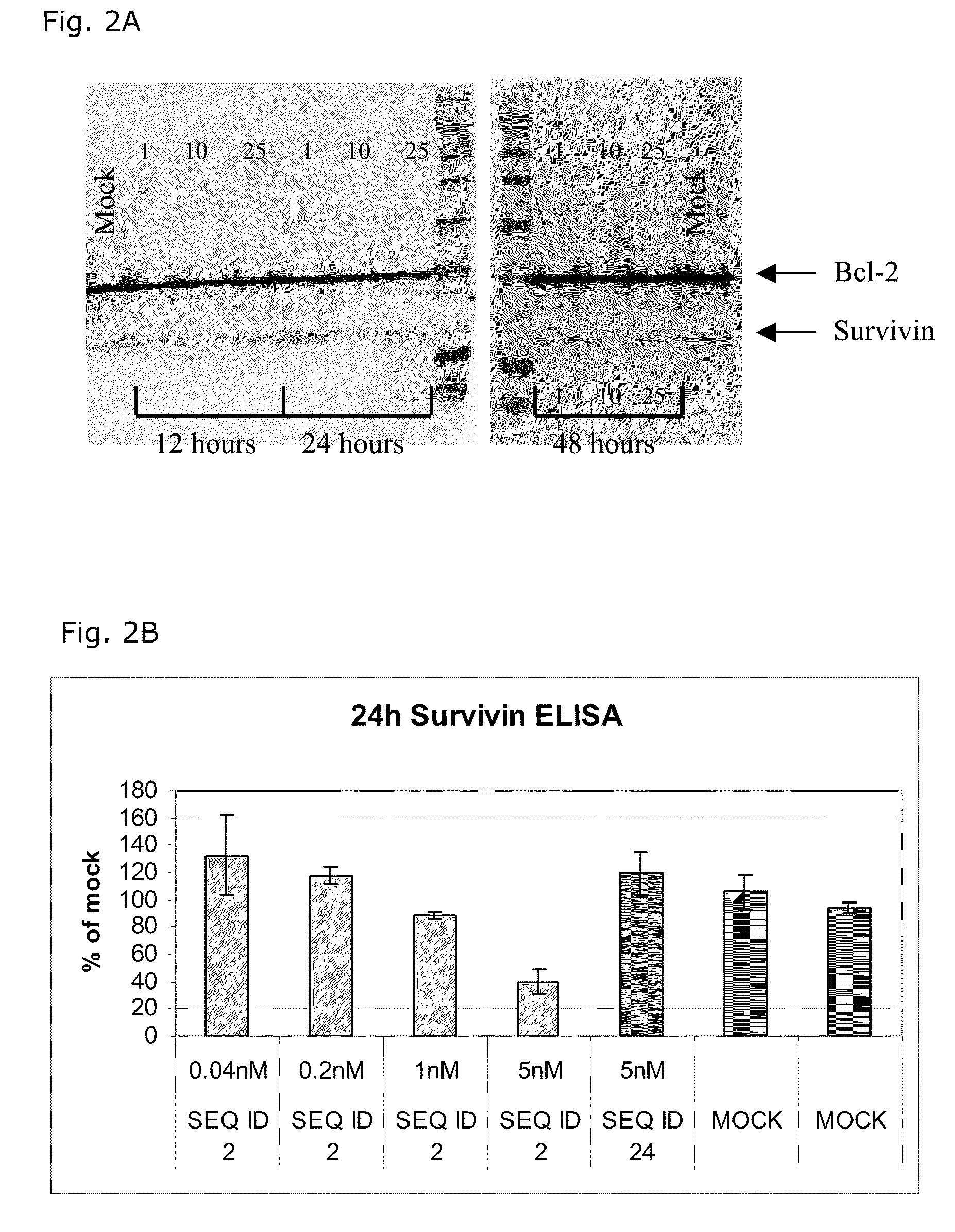
InactiveUS7285288B1Lower the volumeIncrease the effective concentrationSugar derivativesMicrobiological testing/measurementDiseaseCancer cell
The present invention provides novel compositions and methods for use in the treatment of Bcl-2-associated diseases like cancer, specifically, in the treatment of follicular lymphoma (FL). The compositions contain antisense oligonucleotides that hybridize to Bcl-2 nucleic acids, the gene products of which are known to interact with the tumorigenic protein Bcl-2. Used alone, or in conjunction with other antisense oligonucleotides, these compositions inhibit the proliferation of FL cancer cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
DNA polymorphism identity determination using flow cytometry
InactiveUS6287766B1Sensitive, homogenous, and flexibleSugar derivativesMicrobiological testing/measurementMicrosphereDideoxynucleotide Triphosphates
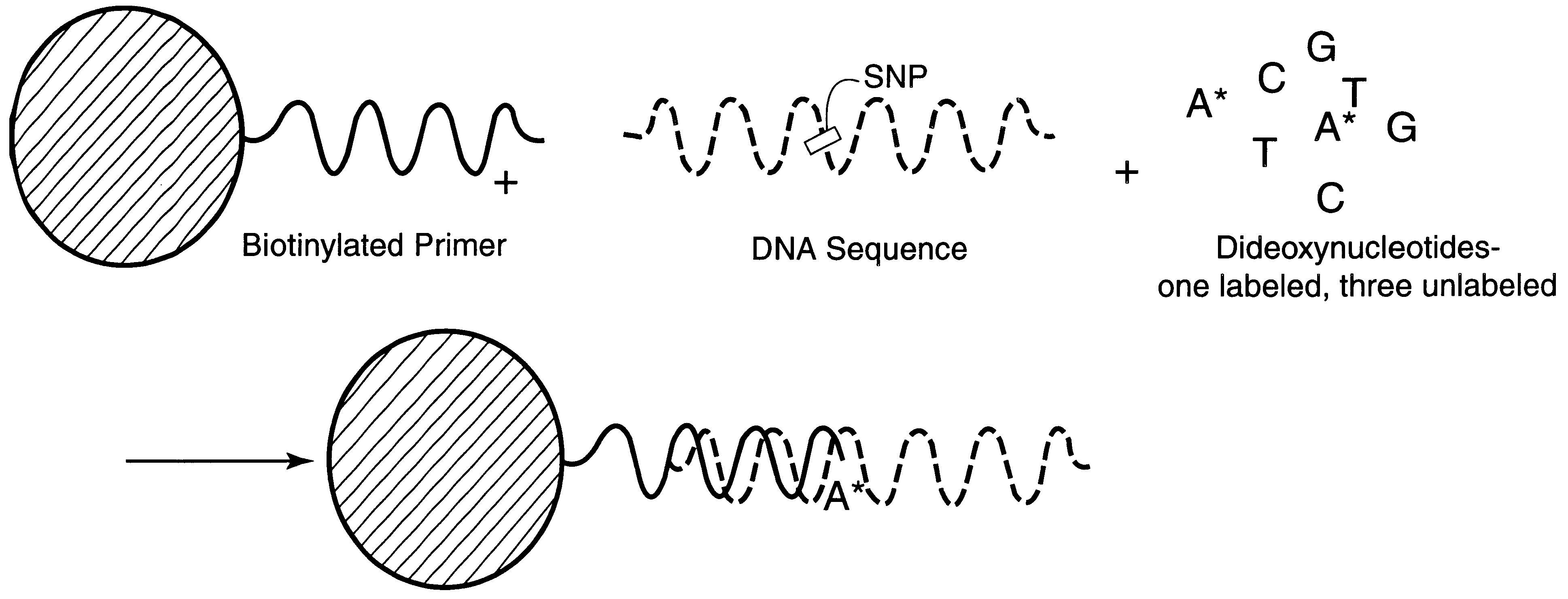
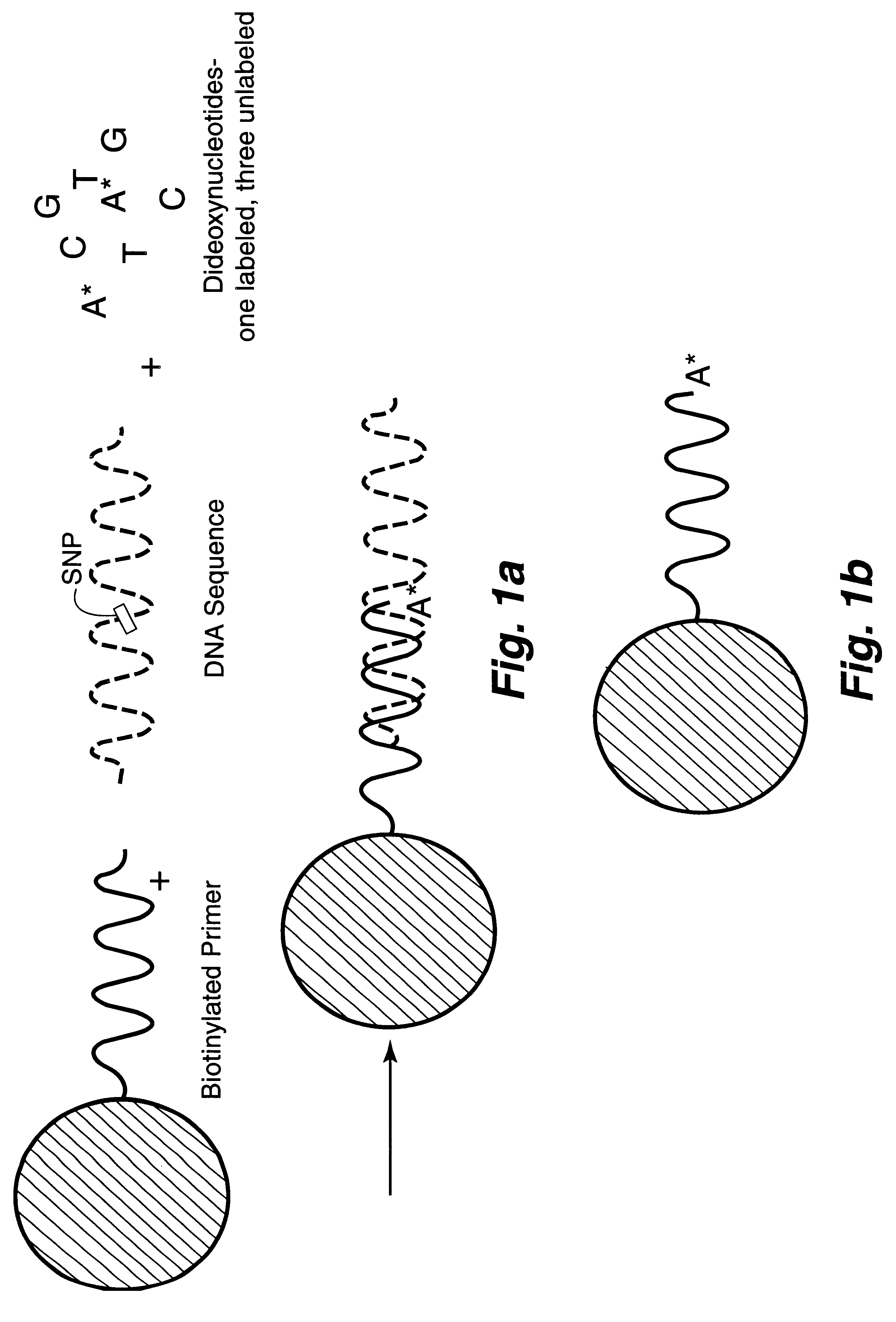
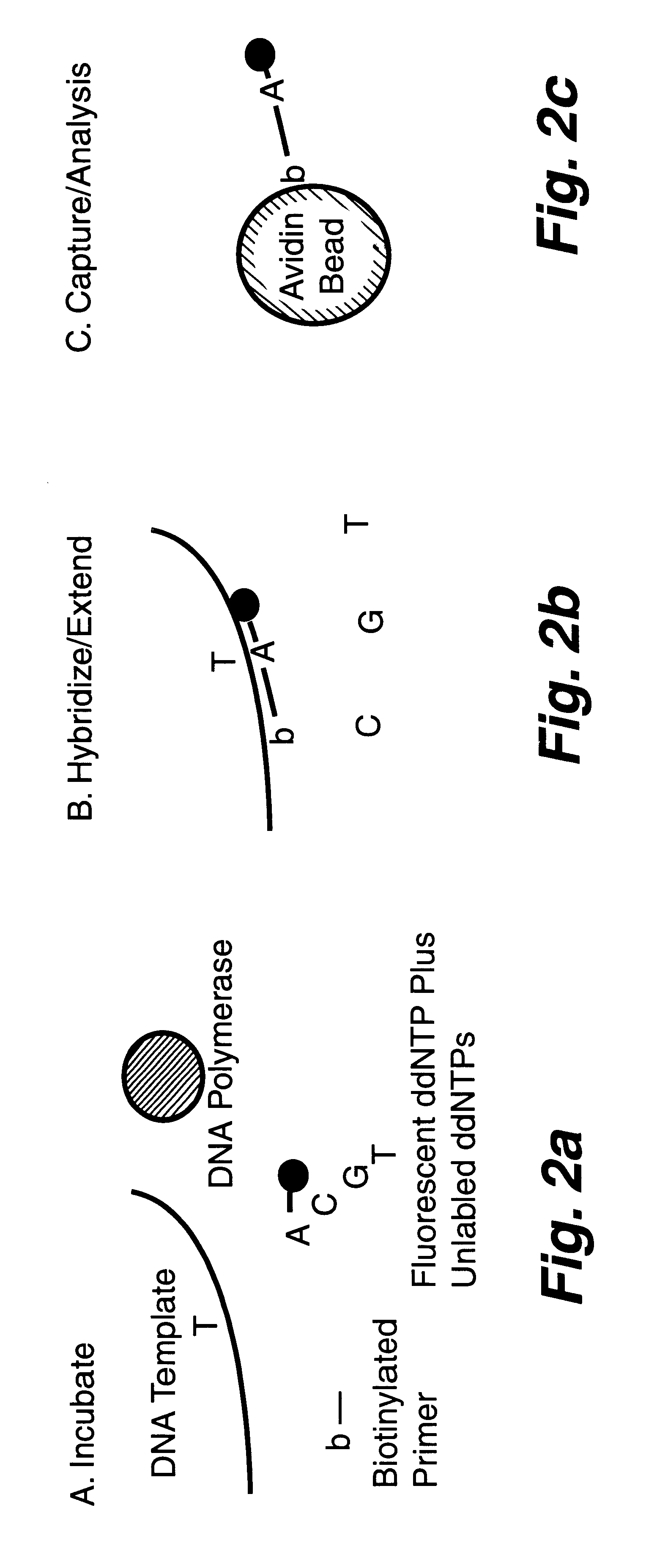
DNA polymorphism identity determination using flow cytometry. Primers designed to be immobilized on microspheres are allowed to anneal to the DNA strand under investigation, and are extended by either DNA polymerase using fluorescent dideoxynucleotides or ligated by DNA ligase to fluorescent reporter oligonucleotides. The fluorescence of either the dideoxynucleotide or the reporter oligonucleotide attached to the immobilized primer is measured by flow cytometry, thereby identifying the nucleotide polymorphism on the DNA strand.
Owner:TRIAD NAT SECURITY LLC
High fidelity reverse transcriptases and uses thereof
InactiveUS7056716B2FungiSugar derivativesReverse transcriptaseTerminal deoxynucleotidyltransferase activity
The invention relates to reverse transcriptases which have increased fidelity (or reduced misincorporation rate) and / or terminal deoxynucleotidyl transferase activity. In particular, the invention relates to a method of making such reverse transcriptases by modifying or mutating specified positions in the reverse transcriptases. The invention also relates to nucleic acid molecules containing the genes encoding the reverse trancriptases of the invention, to host cells containing such nucleic acid molecules and to methods to make the reverse trancriptases using the host cells. The reverse transcriptases of the invention are particularly suited for nucleic acid synthesis, sequencing, amplification and cDNA synthesis.
Owner:LIFE TECH CORP
Ogligonucleotides comprising alternating segments and uses thereof
ActiveUS20050142535A1Preventing and decreasing translationOrganic active ingredientsPeptide/protein ingredientsSugarBiology
The invention relates to oligonucleosides having alternating segments of sugar-modified nucleosides and 2′-deoxynucleosides, and uses thereof. The invention further relates to oligonucleotides having alternating segments of sugar-modified nucleotides and 2′-deoxynucleotides, and uses thereof. Such uses include the preparation of antisense oligonucleotides and their use for the prevention or depletion of function of a target nucleic acid of interest, such as an RNA, in a system. Accordingly, an oligonucleotide of the invention is useful for therapeutic, analytical and diagnostic methods and uses, as well as a component of compositions and commercial packages corresponding to such methods and uses.
Owner:MCGILL UNIV
Thermostable reverse transcriptases and uses thereof
InactiveUS7078208B2Reduced RNase H activityImprove fidelityFungiBacteriaMessenger RNAReverse transcriptase
The present invention is in the fields of molecular and cellular biology. The invention is generally related to reverse transcriptase enzymes and methods for the reverse transcription of nucleic acid molecules, especially messenger RNA molecules. Specifically, the invention relates to reverse transcriptase enzymes which have been mutated or modified to increase thermostability, decrease terminal deoxynucleotidyl transferase activity, and / or increase fidelity, and to methods of producing, amplifying or sequencing nucleic acid molecules (particularly cDNA molecules) using these reverse transcriptase enzymes or compositions. The invention also relates to nucleic acid molecules produced by these methods and to the use of such nucleic acid molecules to produce desired polypeptides. The invention also concerns kits comprising such enzymes or compositions.
Owner:LIFE TECH CORP
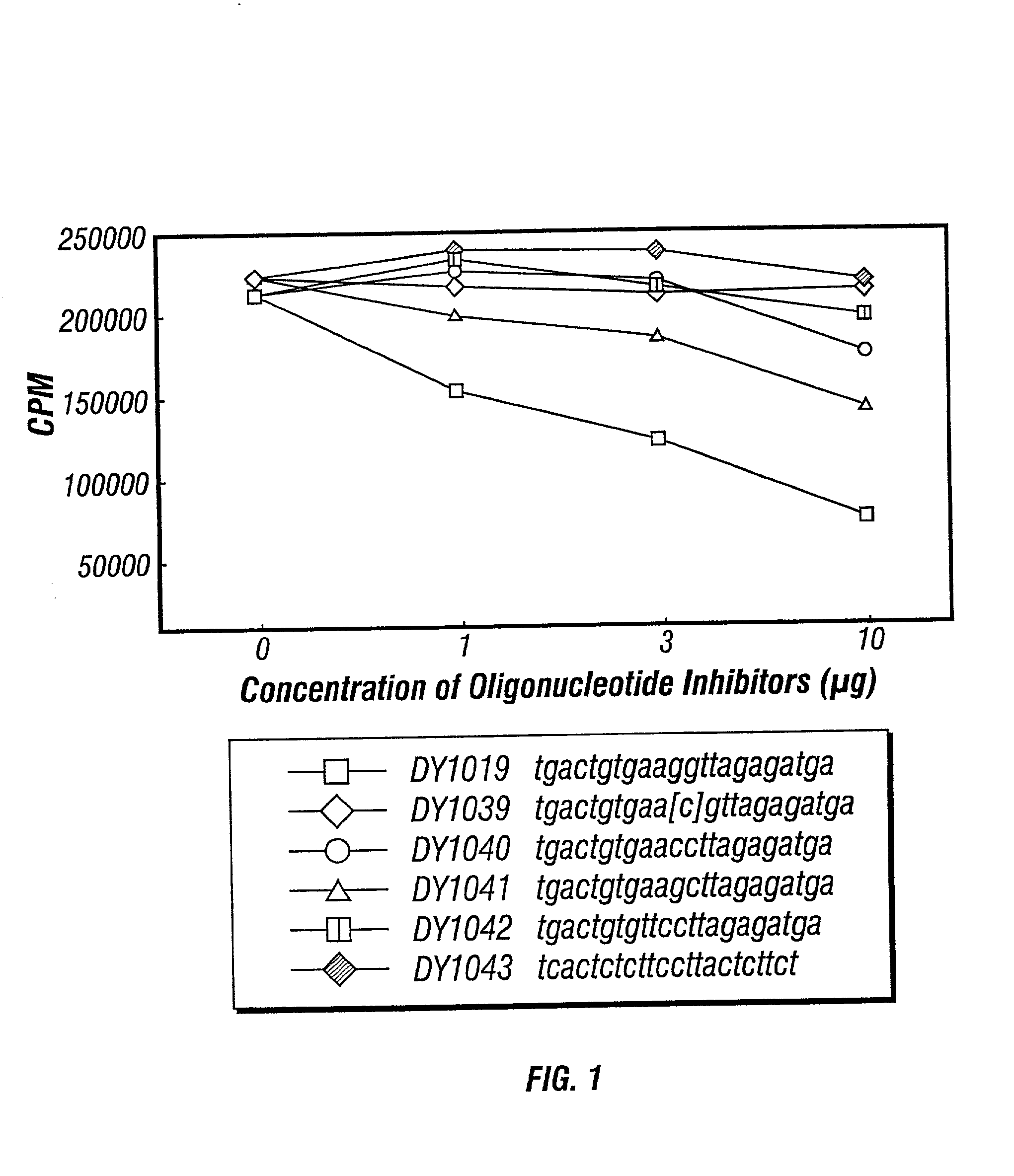
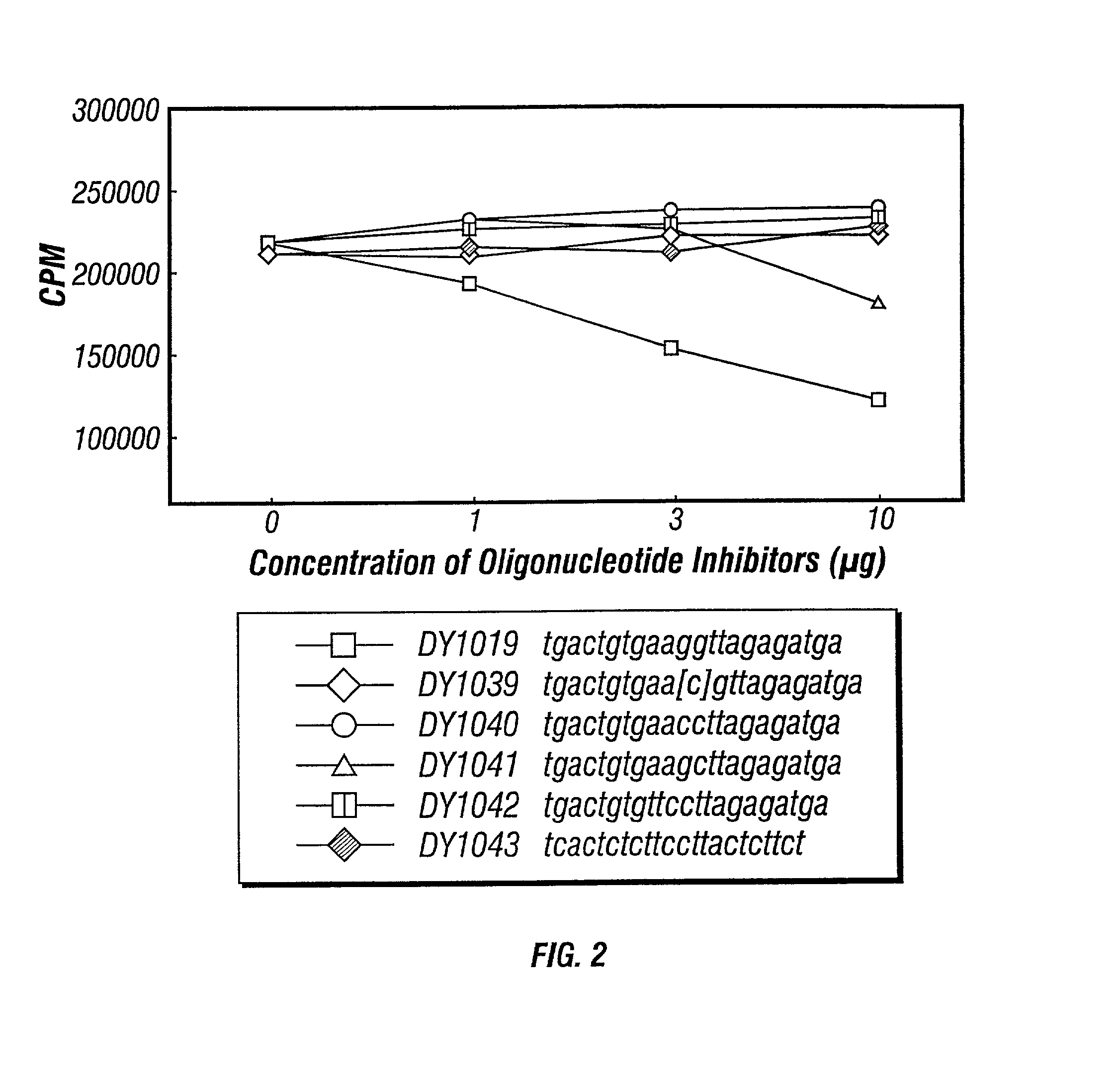
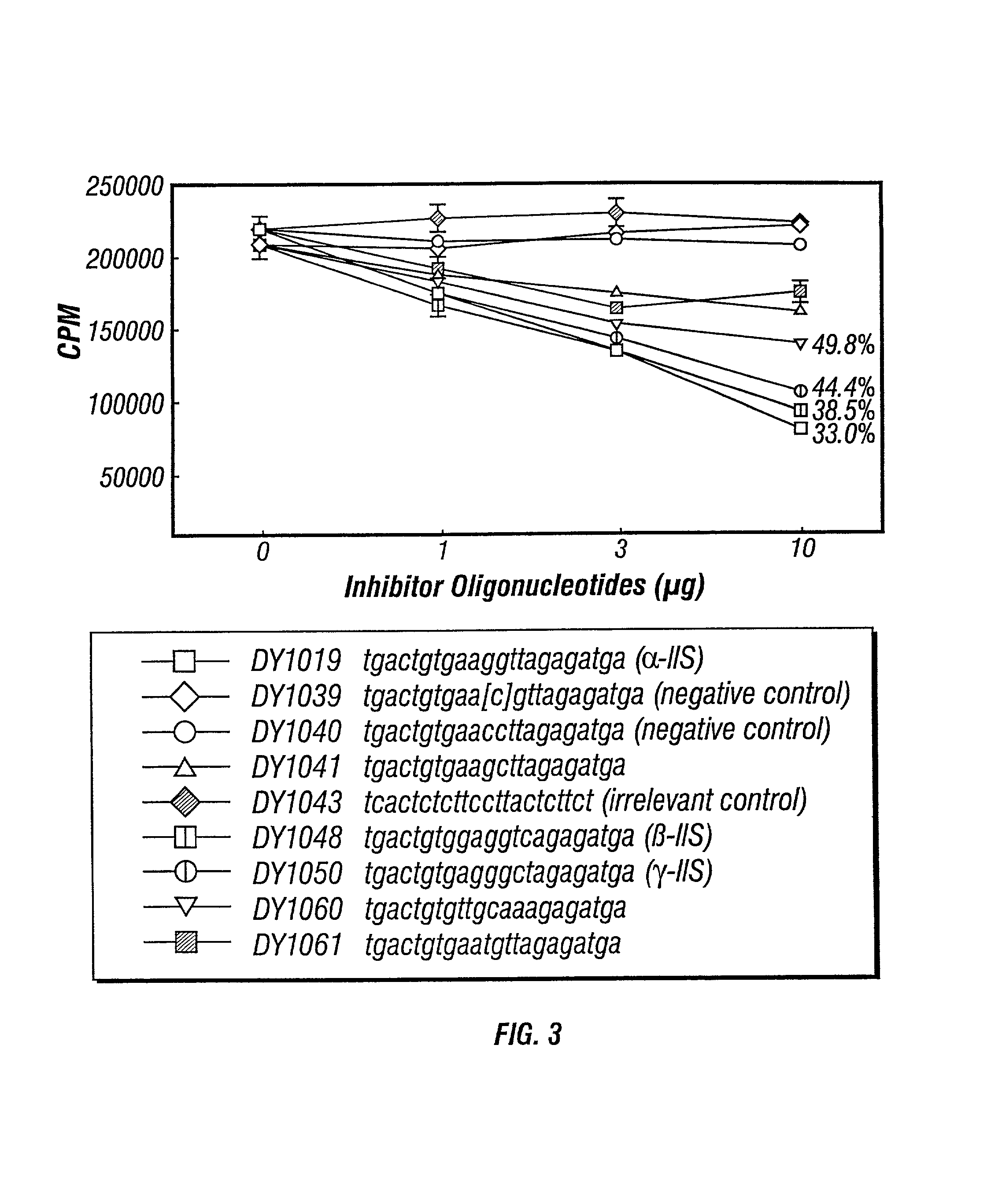
Inhibitors of DNA immunostimulatory sequence activity
InactiveUS20020086839A1Modulating immunostimulatory activityImprove responseOrganic active ingredientsPeptide/protein ingredientsAntigenDisease
The invention consists of oligonucleotides which inhibit the immunostimulatory activity of ISS-ODN (immunostimulatory sequence oligodeoxynucleotides) as well as methods for their identification and use. The oligonucleotides of the invention are useful in controlling therapeutically intended ISS-ODN adjuvant activity as well as undesired ISS-ODN activity exerted by recombinant expression vectors, such as those used for gene therapy and gene immunization. The oligonucleotides of the invention also have anti-inflammatory activity useful in reducing inflammation in response to infection of a host with ISS-ODN containing microbes, in controlling autoimmune disease and in boosting host Th2 type immune responses to an antigen. The invention also encompasses pharmaecutically useful conjugates of the oligonucleotides of the invention (including conjugate partners such as antigens and antibodies).
Owner:DYNAVAX TECH CORP +1
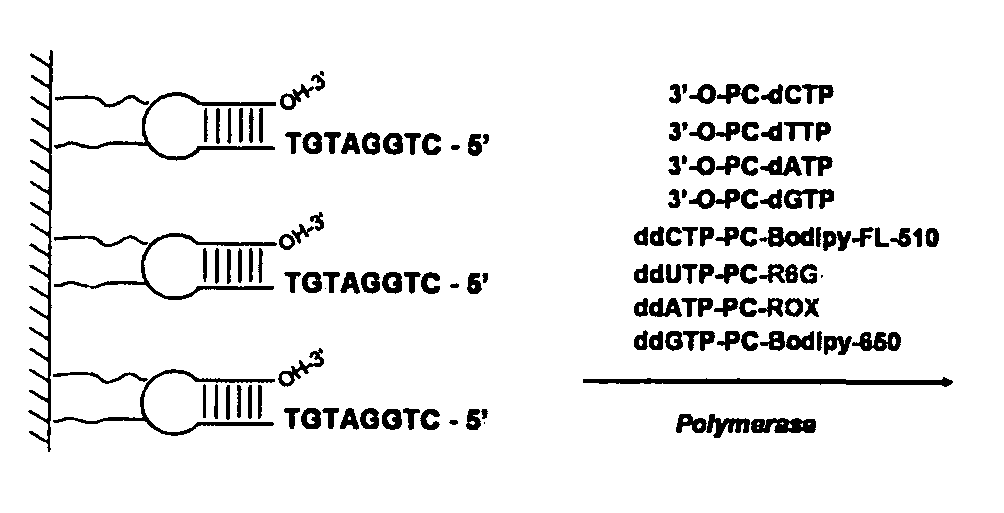
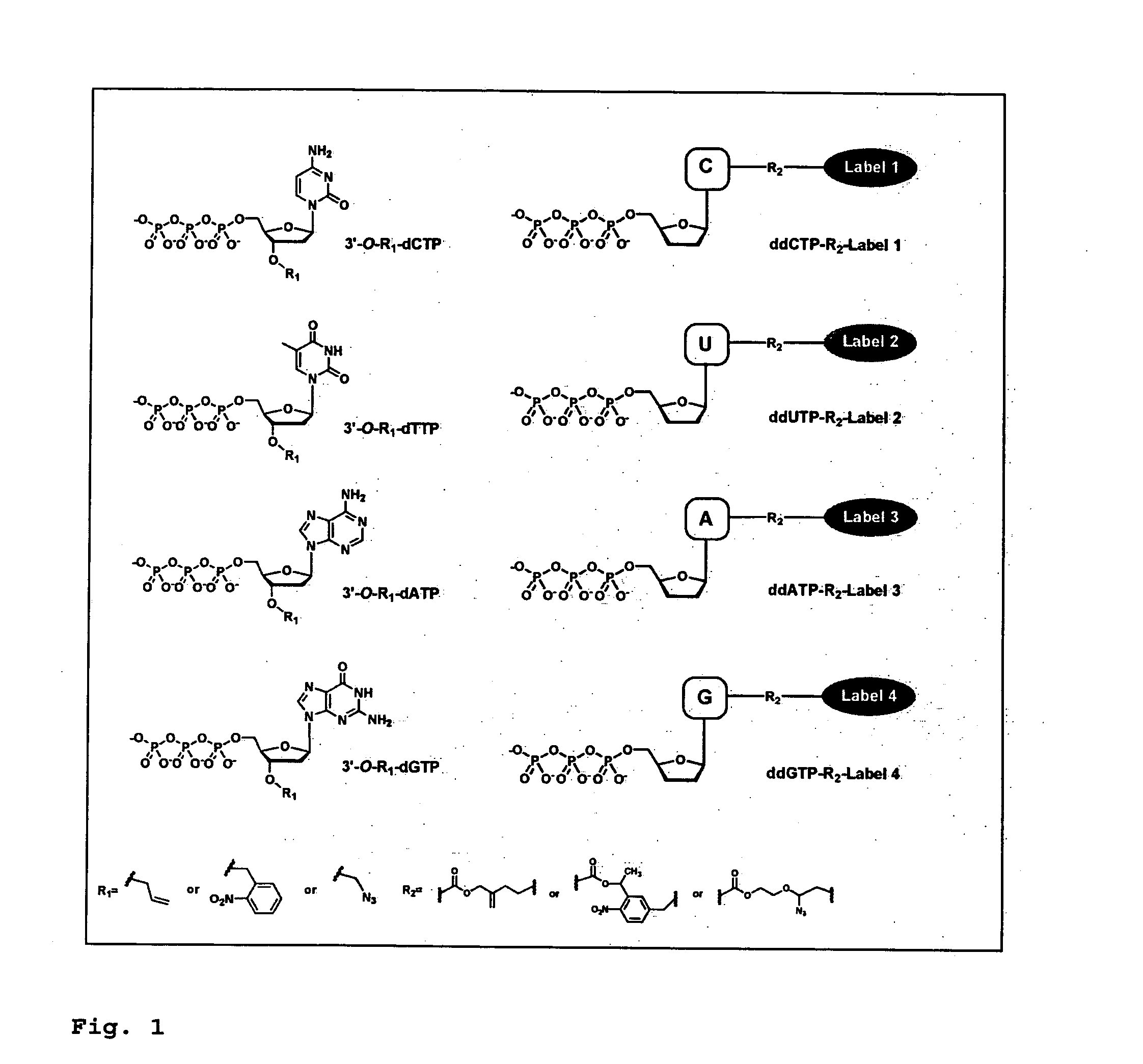
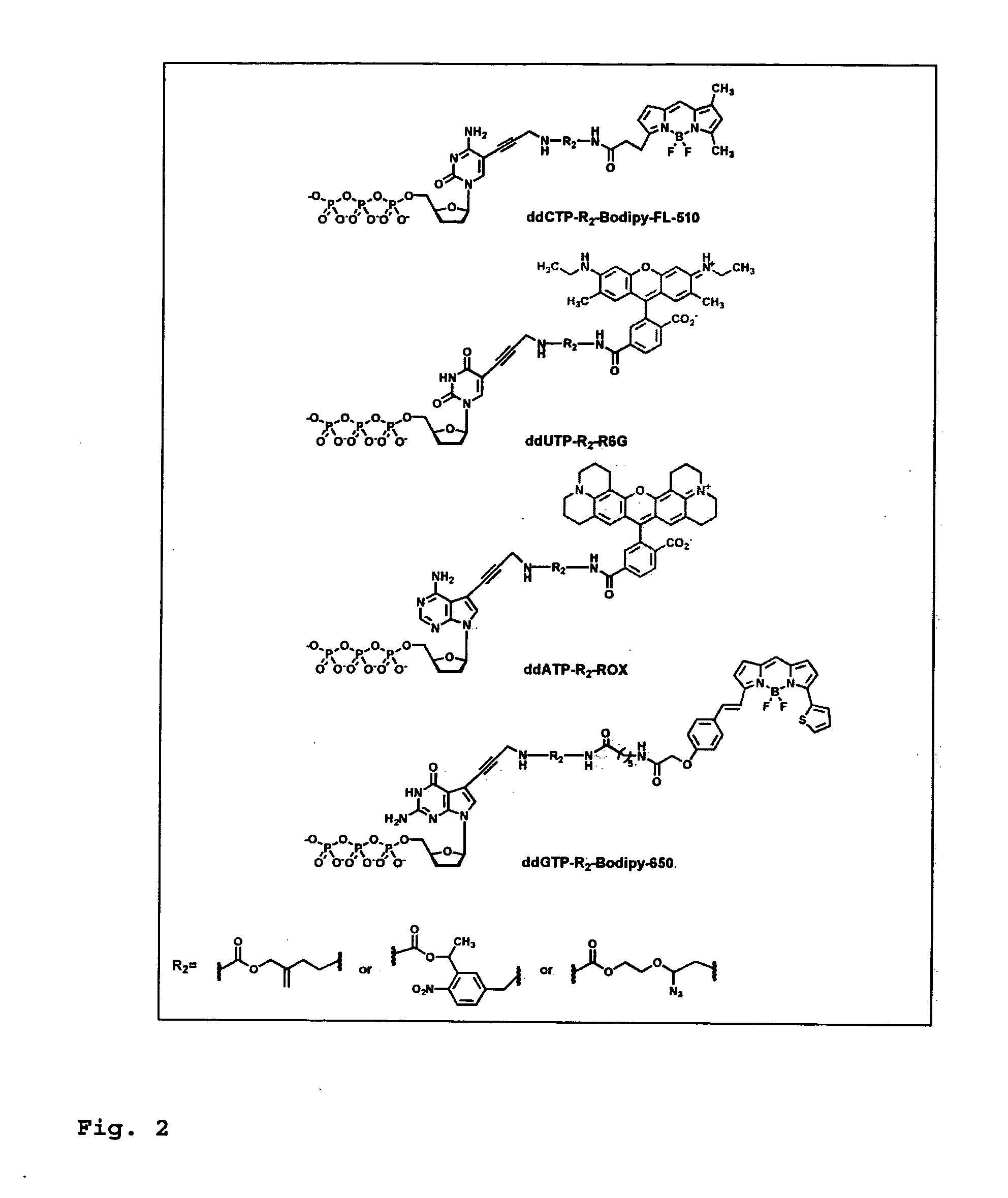
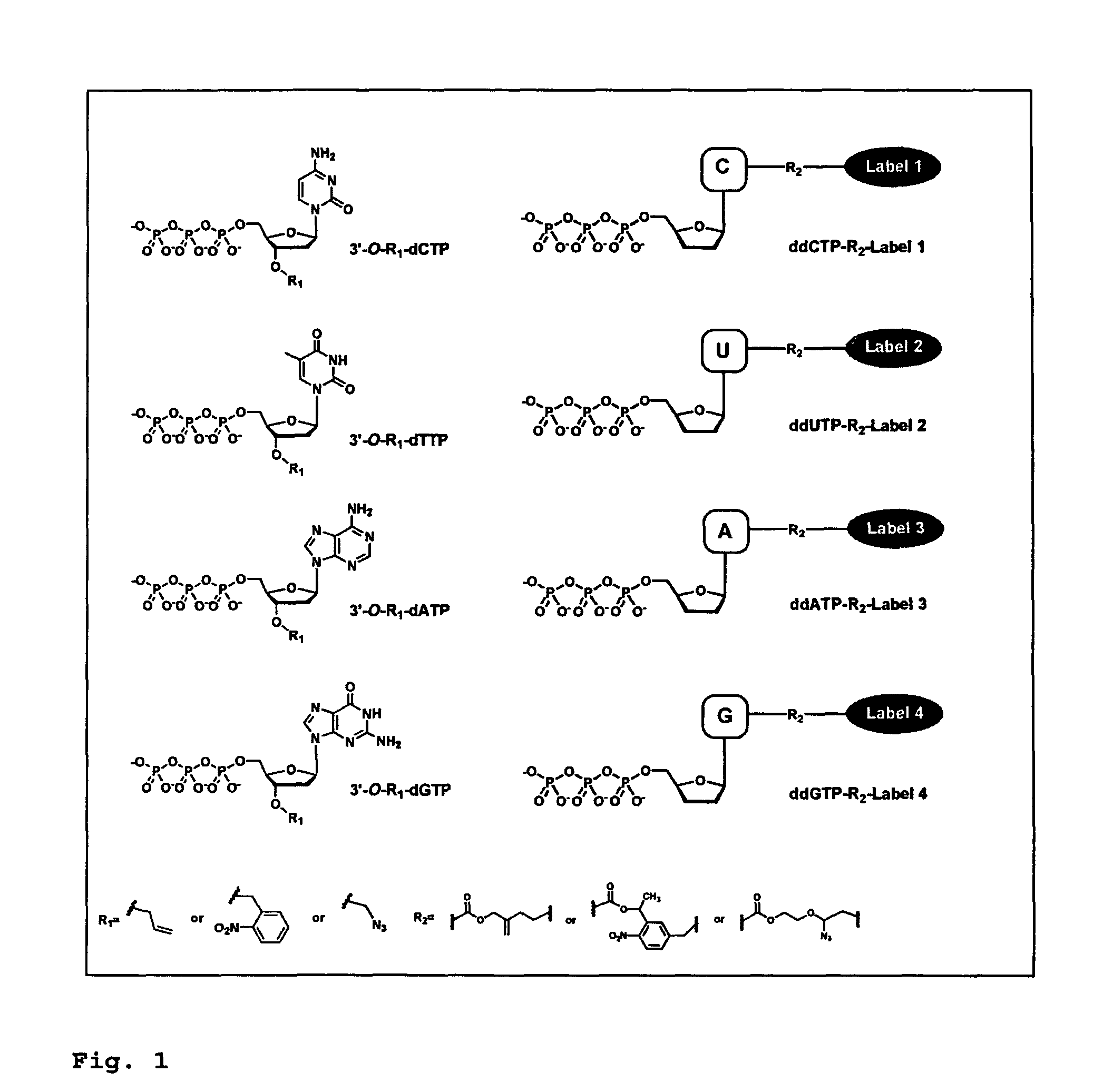
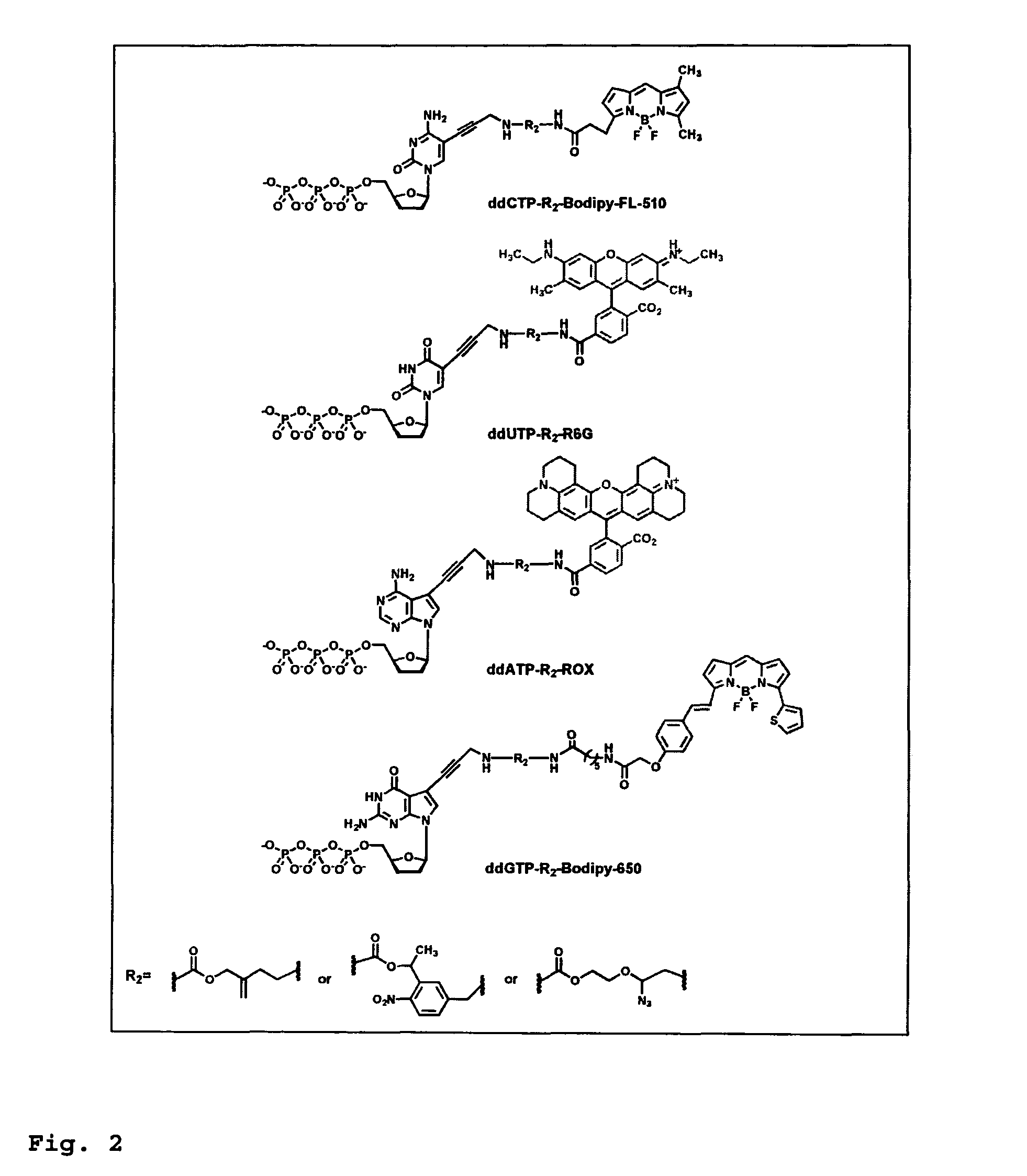
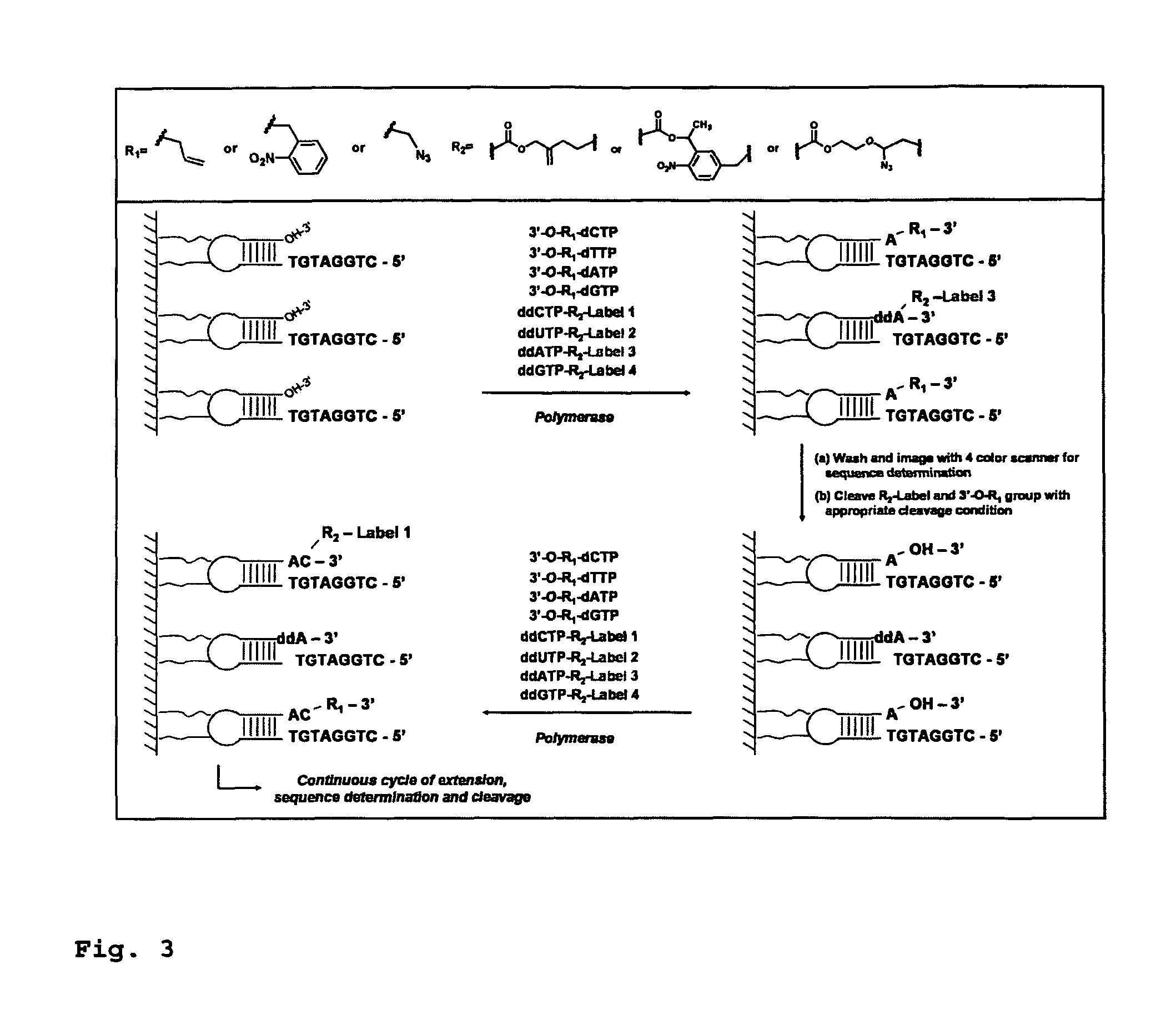
DNA sequence with non-fluorescent nucleotide reversible terminators and cleavable label modified nucleotide terminators
ActiveUS20110039259A1Sugar derivativesMicrobiological testing/measurementDideoxynucleotide TriphosphatesDNA
This invention provides a process for sequencing nucleic acids using 3′ modified deoxynucleotide analogues or 3′ modified deoxyinosine triphosphate analogues, and 3′ modified dideoxynucleotide analogues having a detectable marker attached to a base thereof.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Oligodeoxynucleotide and its use to induce an immune response
Owner:UNIFORMED SERVICES UNIV OF THE HEALTH SCI AN INSTION OF HIGHER LEARNING WITHIN THE DEPT OF DEFENSE A GOVERNMENT AGENCY OF THE UNITED STATES OF AMERICA
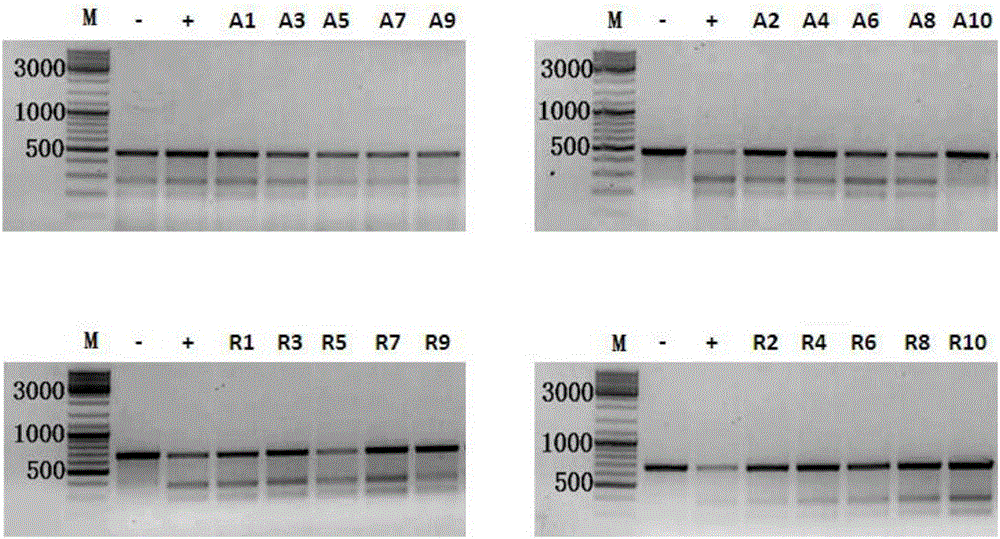
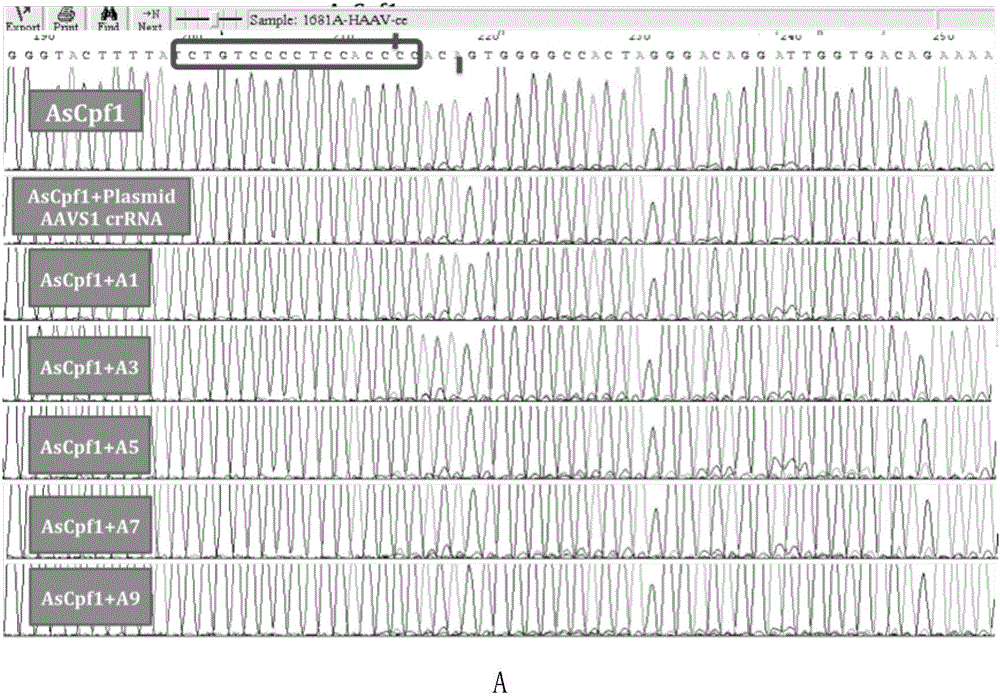
Applications of modified crRNA in CRISPR/Cpf1 gene editing system
InactiveCN106244591AStrong gene editing abilityNucleic acid vectorVector-based foreign material introductionChemical synthesisThymine
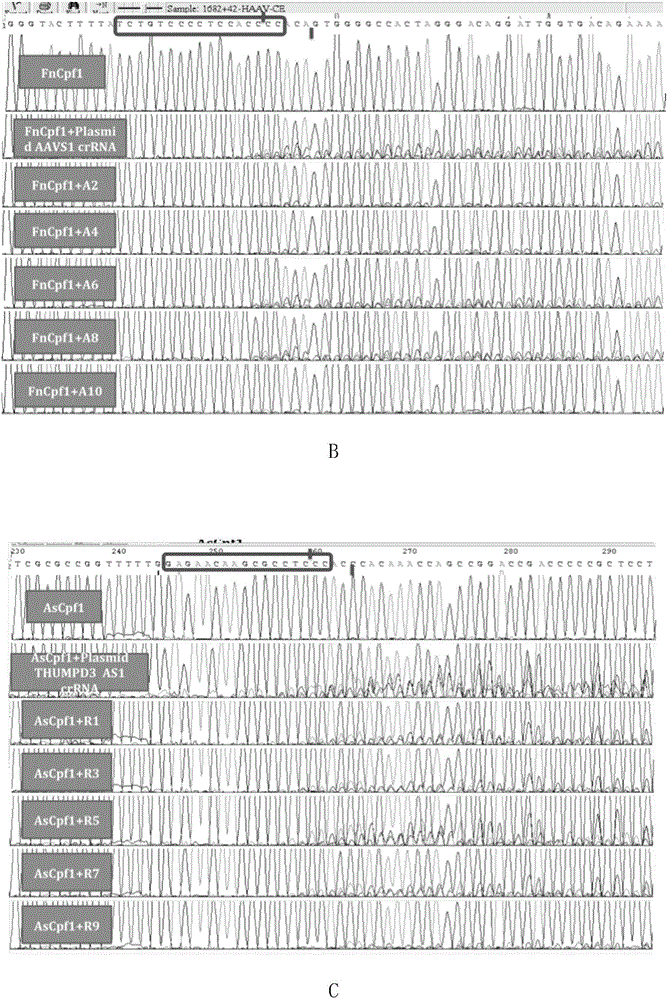
The invention discloses applications of a CRISPR / Cpf1 system in gene editing. CrRNA in the CRISPR / Cpf1 system is modified crRNA; the modification manner is desoxyribonucleic acid modification; the desoxyribonucleic acid modification method comprises the step of increasing 1-3 desoxyribonucleic acid at the 5' terminal and / or increasing 1-3 desoxyribonucleic acid at the 3' terminal of crRNA; and the desoxyribonucleic acid is deoxythymidine acid. The experiment proves that the crRNA formed through chemical synthesis and having the modification can cause the mutation of an hAAVS1 gene and a THUMPD3-AS1 gene when used for the AsCRISPR / Cpf1 system or the FnCRISPR / Cpf1 system, namely, the modified crRNA has certain gene editing capacity when used for the AsCRISPR / Cpf1 system or the FnCRISPR / Cpf1 system, and therefore, the modified crRNA has a significant application value in gene editing utilizing the CRISPR / Cpf1 system.
Owner:SUZHOU GENEPHARMA
Dideoxynucleotide-triphosphate utilization by the hyper-thermophilic DNA polymerase from the archaeon Pyrococcus furiosus
InactiveUS6333183B1High sensitivityImprove thermal stabilityBacteriaSugar derivativesDideoxynucleotide TriphosphatesBinding site
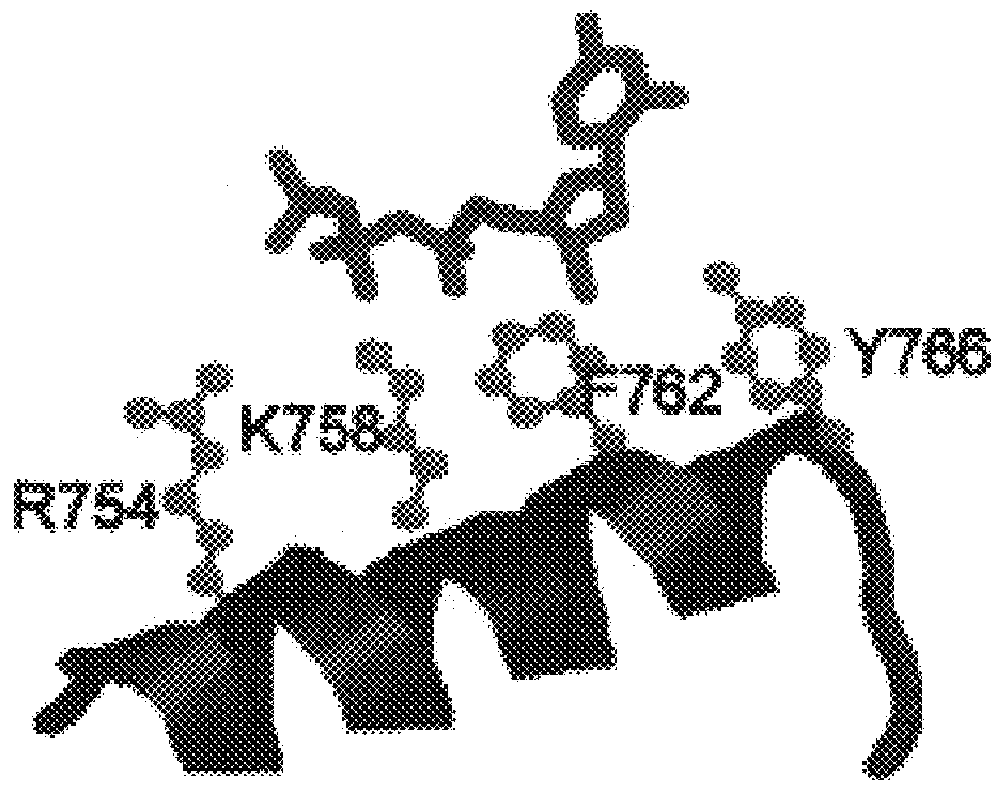
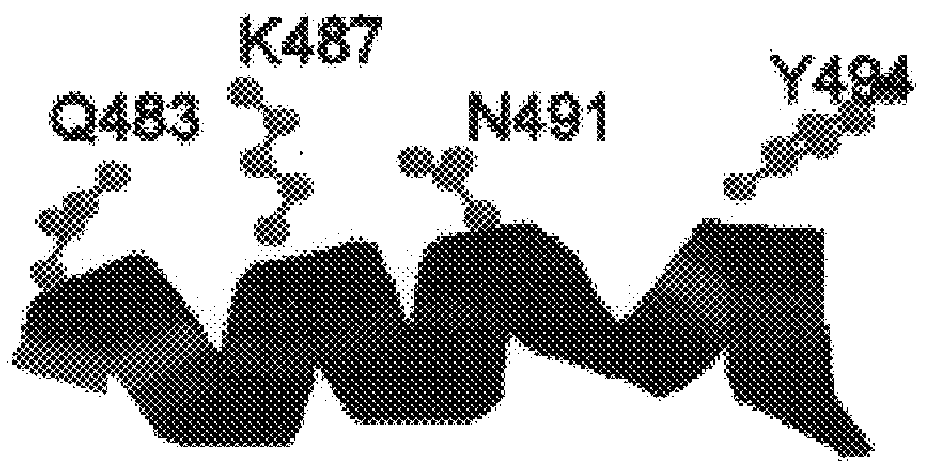
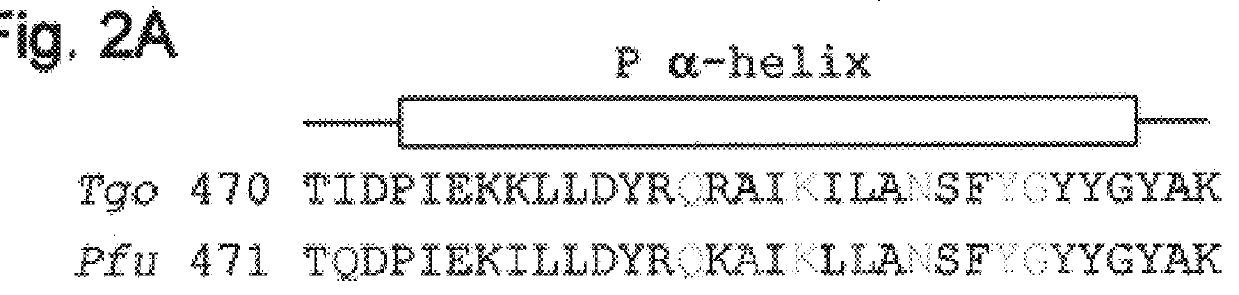
Polymerases from the Pol I family which are able to efficiently use ddNTPs have demonstrated a much improved performance when used to sequence DNA. A number of mutations have been made to the gene coding for the Pol II family DNA polymerase from the archaeon Pyrococcus furiosus with the aim of improving ddNTP utilisation. "Rational" alterations to amino acids likely to be near the dNTP binding site (based on sequence homologies and structural information) did not yield the desired level of selectivity for ddNTPs. However, alteration at four positions (Q472, A486, L490 and Y497) gave rise to variants which incorporated ddNTPs better than the wild type, allowing sequencing reactions to be carried out at lowered ddNTP:dNTP ratios. Wild type Pfu-Pol required a ddNTP:dNTP ratio of 30:1; values of 5:1 (Q472H), 1:3 (L490Y), 1:5 (A486Y) and 5:1 (Y497A) were found with the four mutants; A486Y representing a 150-fold improvement over the wild type. A486, L490 and Y407 are on an alpha-helix that lines the dNTP binding groove, but the side chains of the three amino acids point away from this groove; Q472 is in a loop that connects this alpha-helix to a second long helix. None of the four amino acids can contact the dNTP directly. Therefore, the increased selectivity for ddNTPs is likely to arise from two factors: 1) Small overall changes in conformation that subtly alter the nucleotide triphosphate binding site such that ddNTPs become favoured; 2) interference with a conformational change that may be critical both for the polymerisation step and discrimination between different nucleotide triphosphates.
Owner:GE HEALTHCARE BIO SCI CORP
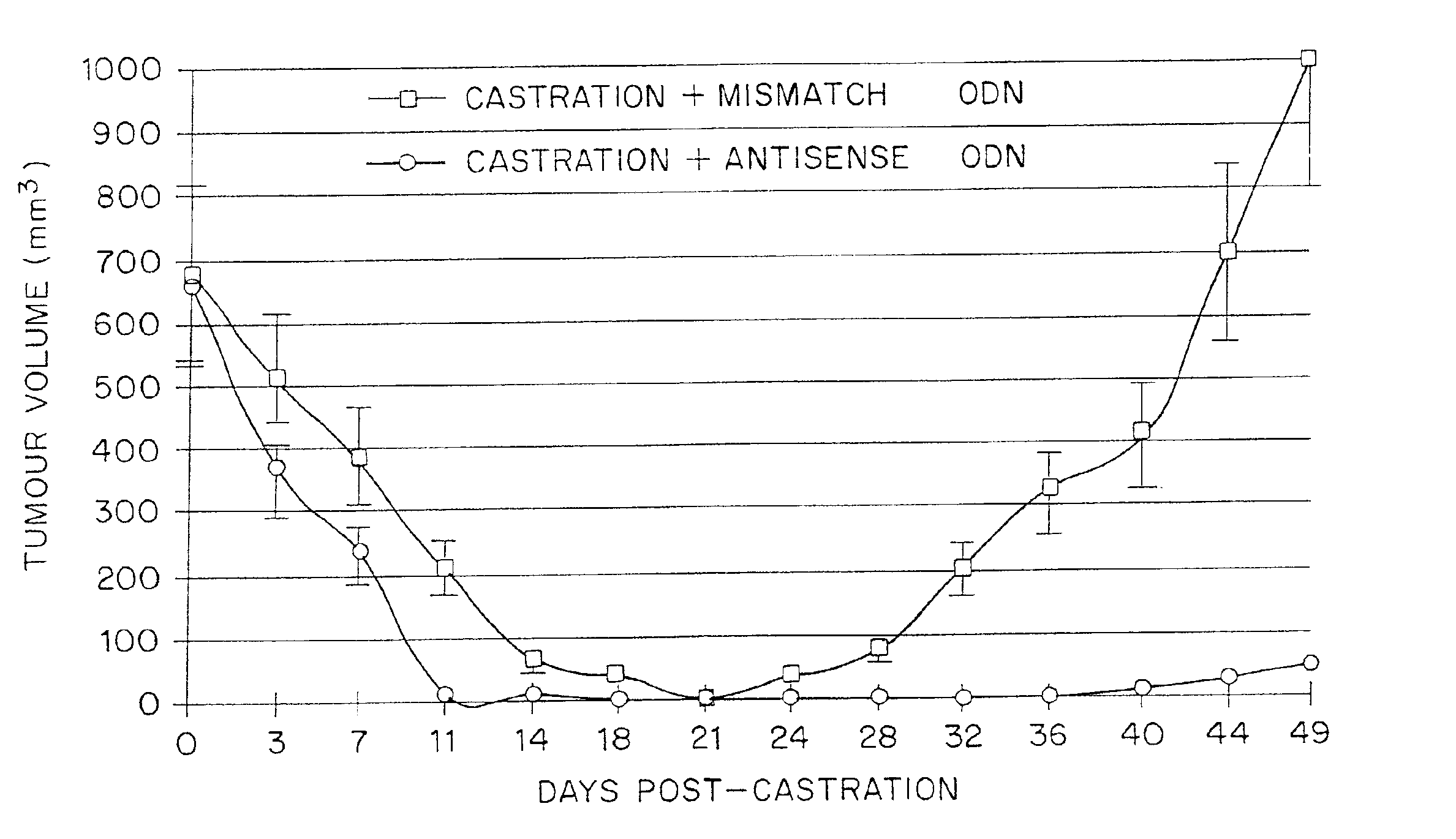
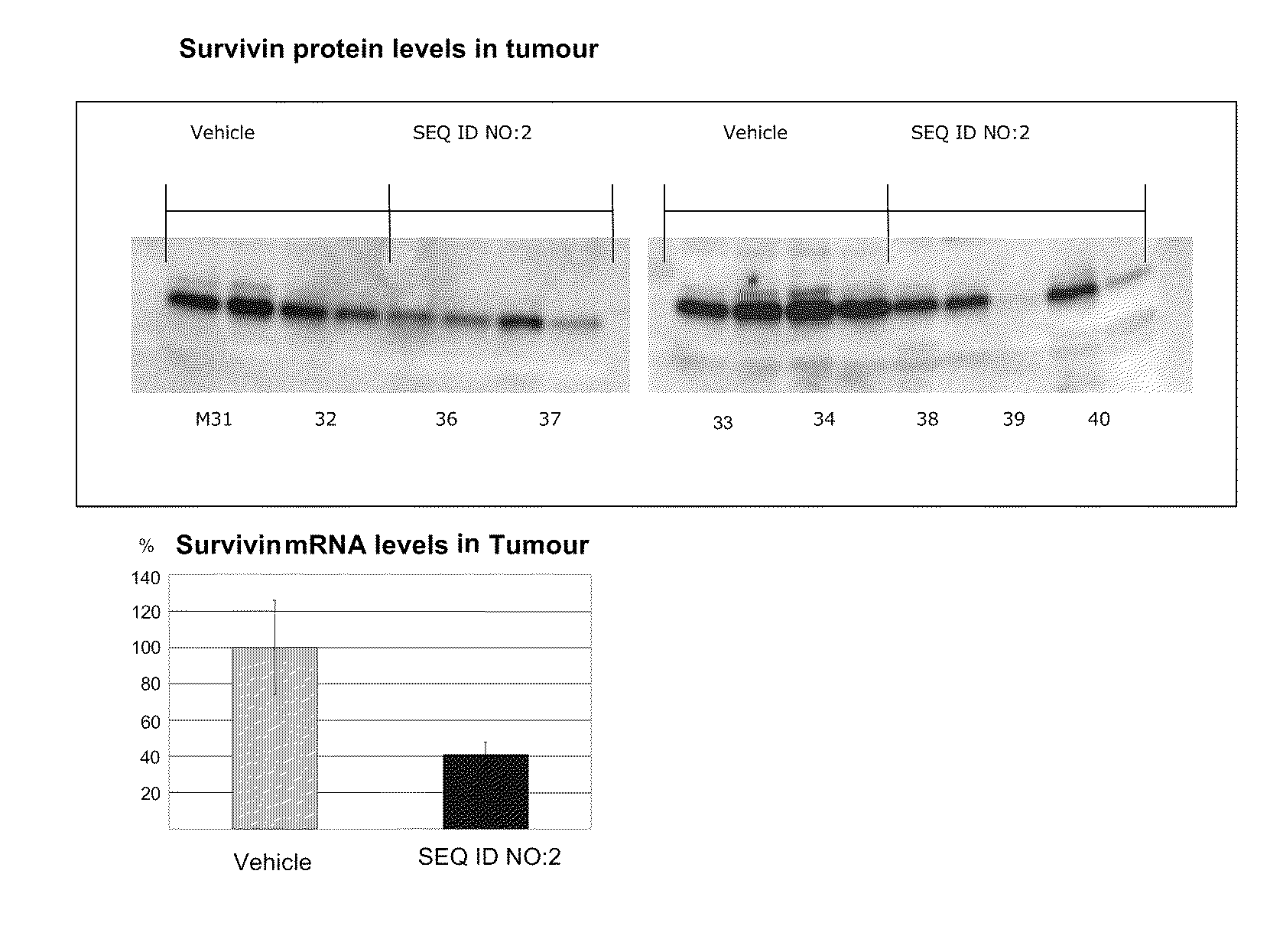
TRPM-2 antisense therapy using an oligonucleotide having 2′-O-(2-methoxy)ethyl modifications
InactiveUS6900187B2Improve in vivo stabilityImproved in vitroPeptide/protein ingredientsGenetic material ingredientsIn vivoOligonucleotide
A compound consisting of an oligonucleotide of sequence CAGCAGCAGAGTCTTCATCAT, where the oligonucleotide has a phosphorothioate backbone throughout, the sugar moieties of nucleotides 1-4 and 18-21 bear 2′-O-methoxyethyl modifications, and the remaining nucleotides (nucleotides 5-17) are 2′-deoxynucleotides, and where the cytosines of nucleotides 1, 4 and 19 are 5-methylcytosines. The compound has increased stability in vivo and improved in vitro and in vivo antitumor activity.
Owner:THE UNIV OF BRITISH COLUMBIA
Thermostable or thermoactive DNA polymerase molecules with attenuated 3′-5′ exonuclease activity
The present invention provides thermostable or thermoactive DNA polymerases with attenuated 3′-5′ exonuclease activity, methods for their synthesis, methods for their use, kits comprising the polymerases, nucleic acids encoding the polymerases and cells comprising such a nucleic acid. The DNA polymerases of the invention are useful in many recombinant DNA techniques, such as nucleic acid amplification by the polymerase chain reaction. The DNA polymerases of the invention allow higher fidelity replication and amplification of a template DNA sequence, allow less degradation of primers and / or more efficient use of deoxynucleotide triphosphates and are in general more efficient and less costly to make and use.
Owner:ROCHE MOLECULAR SYST INC
Lna oligonucleotides and the treatment of cancer
Owner:ENZON PHARM INC
DNA sequence with non-fluorescent nucleotide reversible terminators and cleavable label modified nucleotide terminators
ActiveUS9115163B2Sugar derivativesMicrobiological testing/measurementDideoxynucleotide TriphosphatesDNA
This invention provides a process for sequencing nucleic acids using 3′ modified deoxynucleotide analogues or 3′ modified deoxyinosine triphosphate analogues, and 3′ modified dideoxynucleotide analogues having a detectable marker attached to a base thereof.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Method for the uncoupled, direct, exponential amplification and sequencing of DNA molecules with the addition of a second thermostable DNA polymerase and its application
InactiveUS20020034792A1Increases exponential amplificationReduction of initial amountMicrobiological testing/measurementRecombinant DNA-technologyDideoxynucleotide TriphosphatesDNA
Method for sequencing a nucleic acid molecule in a thermocycling reaction which initially comprises a nucleic acid molecule, a first primer, a second primer, a reaction buffer, a first thermostable DNA polymerase, (optionally) a thermostable pyrophosphatase, deoxynucleotides or derivatives thereof and a dideoxynucleotide or a derivative thereof and which is characterized in that the thermocycling reaction additionally contains a second thermostable DNA polymerase which, in comparison to the said first thermostable DNA polymerase, has a reduced ability to incorporate dideoxynucleotides as well as the use of the said method.
Owner:ROCHE DIAGNOSTICS GMBH
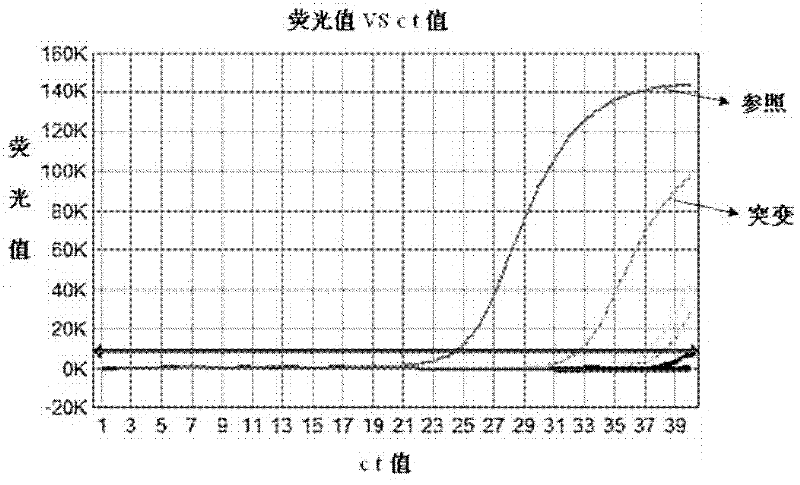
ARMS-qPCR (Allele Refractory Mutation System-quantitative Polymerase Chain Reaction) detection kit for KRAS (Kirsten Rat Sarcoma Viral Oncogene Homolog) gene mutation subtype and detection method
InactiveCN102367478AIncreased sensitivityQuick checkMicrobiological testing/measurementViral OncogenePositive control
The invention relates to the field of molecular biology and aims to provide an ARMS-qPCR (Allele Refractory Mutation System-quantitative Polymerase Chain Reaction) detection kit for KRAS (Kirsten Rat Sarcoma Viral Oncogene Homolog) gene mutation subtype and a detection method. The kit comprises a qPCR hybrid reaction solution, a locked nucleic acid retardant probe, a reference primer, an ARMS primer and a positive control sample, wherein the qPCR hybrid reaction solution comprises a PCR buffer solution, dNTPs (Deoxynucleotide Triphosphates), MgCl2, GoldStarbest Taq enzyme, a universal PCR reverse primer and a universal TaqMan probe. The kit provided by the invention can be used for rapidly and accurately detecting specific locus mutation of KRAS genes in various cancer tissues with high sensitivity, has high sensitivity, and can be used for detecting genome DNA with various tissue origins, specially free DNA segments adopting cell-free systems, such as blood serum and blood plasma, orother body fluid origins, wherein the genome DNA is derived from cell systems. Compared with direct sequencing and other mutation detection technologies, the kit and the detection method thereof havethe advantages of strong specificity, high sensitivity, simplicity and rapidness in operation, high throughput, safety, definiteness and objectivity in result identification and the like for detecting the KRAS gene mutation.
Owner:ZHEJIANG UNIV
Design and synthesis of cleavable fluorescent nucleotides as reversible terminators for DNA sequencing by synthesis
ActiveUS20140093869A1Increase read lengthSugar derivativesMicrobiological testing/measurementFluorescenceNucleotide
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Nutrient for enhancing plant growth and preparation method thereof
InactiveCN102079665BThe proportion of mutual cooperation is reasonableReduce manufacturing costFertilizer mixturesSide effectAmino acid composition
The invention relates to a nutrient for enhancing plant growth and a preparation method thereof. The nutrient comprises the following components in percent by weight: 20-80 percent of nucleotide salts and deoxynucleotide salts and 20-80 percent of sugar or amino acid. The preparation method comprises the following steps of: preparing nucleic acid from mycelium in side products of a fermentation plant, next, hydrolyzing the nucleic acid into the nucleotide salts and the deoxynucleotide salts, and then mixing the nucleotide salts and the deoxynucleotide salts with the sugar or amino acid. The invention has the advantages that (1) the nutrient has no toxicity, harm, residue and any adverse side effect to plants; (2) the nutrient is beneficial to increase of the yield, the income and the efficiency, and has good effects of enhancing the resistance and improving the quality for plants; and (3) the preparation method has a simple process and low cost and is easy for large-scale production and promotion. The nutrient is widely applied to plants such as grain crops, cotton crops, oil crops, tobaccos, tea trees, fruit trees, vegetables, mushrooms, Chinese medicinal plants, flowers, forests and the like.
Owner:BOZHOU HONGYU TRADITIONAL CHINESE MEDICINE
Cross-linking oligonucleotides
Owner:DRUG ROYALTY TRUST 9
Polypeptide with brain targeted medicine delivery characteristic and preparation method thereof
The invention belongs to the technical field of protein polypeptides, relates to a polypeptide with a brain targeted medicine delivery characteristic, and provides a molecular structure of the polypeptide, and application of the polypeptide to brain targeted medicine delivery. A phage monoclonal antibody which has the function of penetrating a blood brain barrier and can be enriched in a brain is obtained through repeated in-vivo Sprague Dawley (SD) outbred stock rat screening by a phage display technology; deoxyribonucleic acid (DNA) sequencing proves that the phase display polypeptides comprise a consensus sequence; meanwhile, an immunohistochemical method and in-vivo recovery rate comparison prove that the obtained polypeptide has the brain targeted medicine delivery characteristic. The polypeptide and derivative peptides thereof can be used for modifying medicine-carrying nanoparticles, liposomes, vesicles or micelles to construct a brain targeted medicine delivery system; and the delivery of medicines in the brain can be improved, the treatment effect on brain diseases is enhanced and systemic toxic and side effects are reduced.
Owner:FUDAN UNIV
Therapeutic use of cis-element decoys in vivo
The invention provides for the use of oligodeoxynucleotide decoys for the prophylactic or therapeutic treatment of diseases associated with the binding of endogenous transcription factors to genes involved in cell growth, differentiation and signalling or to viral genes. By inhibiting endogenous trans-activating factors from binding transcription regulatory regions, the decoys modulate gene expression and thereby regulating pathological processes including inflammation, intimal hyperplasia, angiogenesis, neoplasia, immune responses and viral infection. The decoys are administered in amounts and under conditions whereby binding of the endogenous transcription factor to the endogenous gene is effectively competitively inhibited without significant host toxicity. The subject compositions comprise the decoy molecules in a context which provides for pharmacokinetics sufficient for effective therapeutic use.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Medicine-carrying nanometer polymer particle and its prepn and use
InactiveCN1608675AGenetic material ingredientsPharmaceutical non-active ingredientsWater insolublePolyethylene glycol
The present invention discloses one medicinal polymer (PELGE / PELGA) nanometer particle carrier and its preparation process and use. The carrier material is PELGE material of different molecular weights, different LA / GA ratios and different PEG contents, and the nanometer PELGE / PELGA carrier particle is prepared through evaporation process. The said copolymer is self-assembled in water into nanometer particle or micelle, and its hydrophobic PLGA segment coagulates into the core while the hydrophilic polyglycol forms hydrophilic shell. The carrier may be used for the nanometer preparation of plasmid, nucleic acid vaccine, antisense oligodeoxynucleotide or ribozyme for genetic treatment; the nanometer preparation of various chemical medicines; and the nanometer preparation of polypeptide and protein medicines.
Owner:SICHUAN UNIV +1
Method for the uncoupled, direct, exponential amplification and sequencing of DNA molecules with the addition of a second thermostable DNA polymerase and its application
InactiveUS6828094B2Increases exponential amplificationReduction of initial amountMicrobiological testing/measurementRecombinant DNA-technologyDideoxynucleotide TriphosphatesDNA
Method for sequencing a nucleic acid molecule in a thermocycling reaction which initially comprises a nucleic acid molecule, a first primer, a second primer, a reaction buffer, a first thermostable DNA polymerase, (optionally) a thermostable pyrophosphatase, deoxynucleotides or derivatives thereof and a dideoxynucleotide or a derivative thereof and which is characterized in that the thermocycling reaction additionally contains a second thermostable DNA polymerase which, in comparison to the said first thermostable DNA polymerase, has a reduced ability to incorporate dideoxynucleotides as well as the use of the said method.
Owner:ROCHE DIAGNOSTICS GMBH
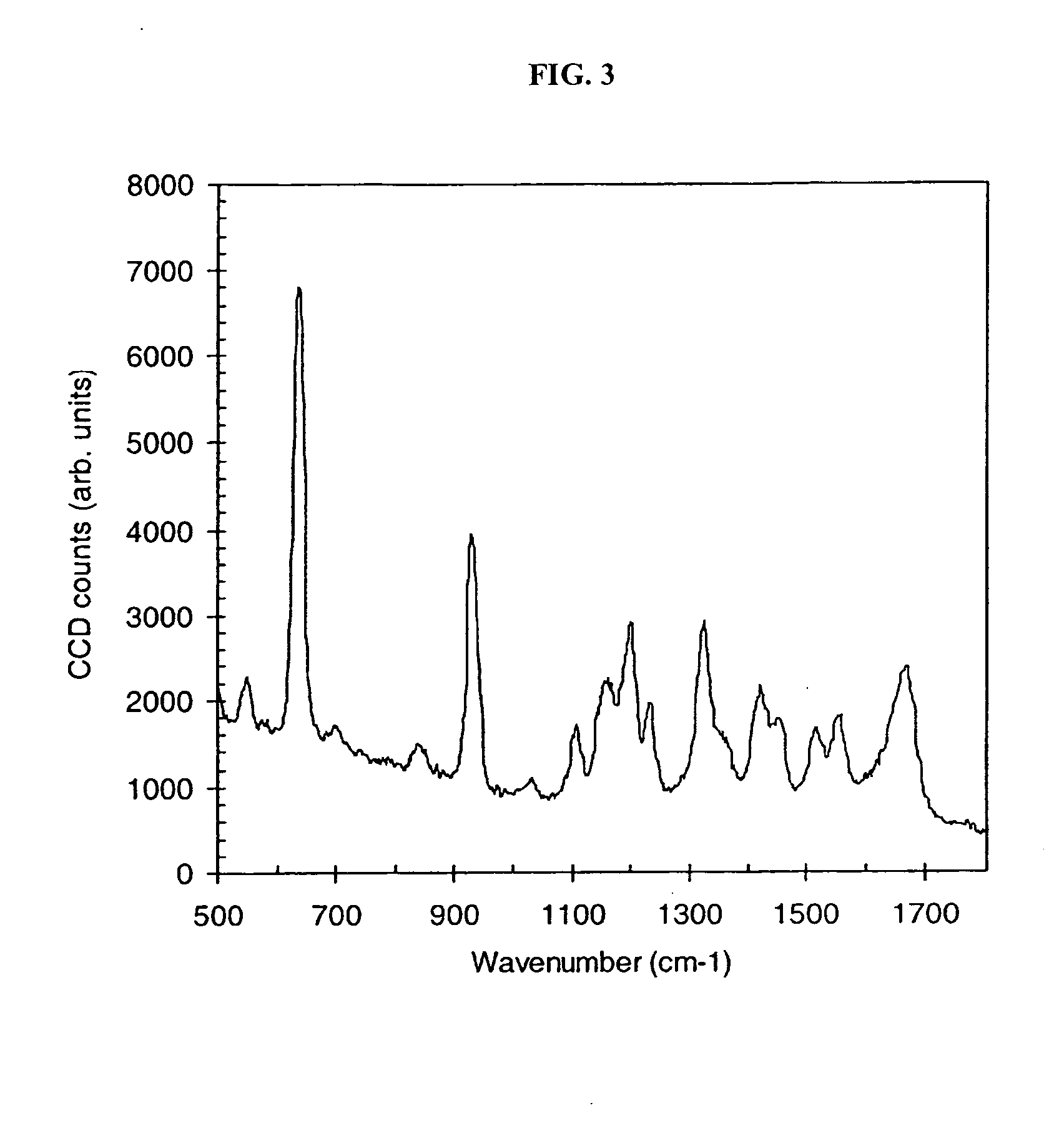
Nucleic acid sequencing by Raman monitoring of uptake of nucleotides during molecular replication
InactiveUS20050147979A1Microbiological testing/measurementNanomedicineSurface-enhanced Raman spectroscopyNucleotide
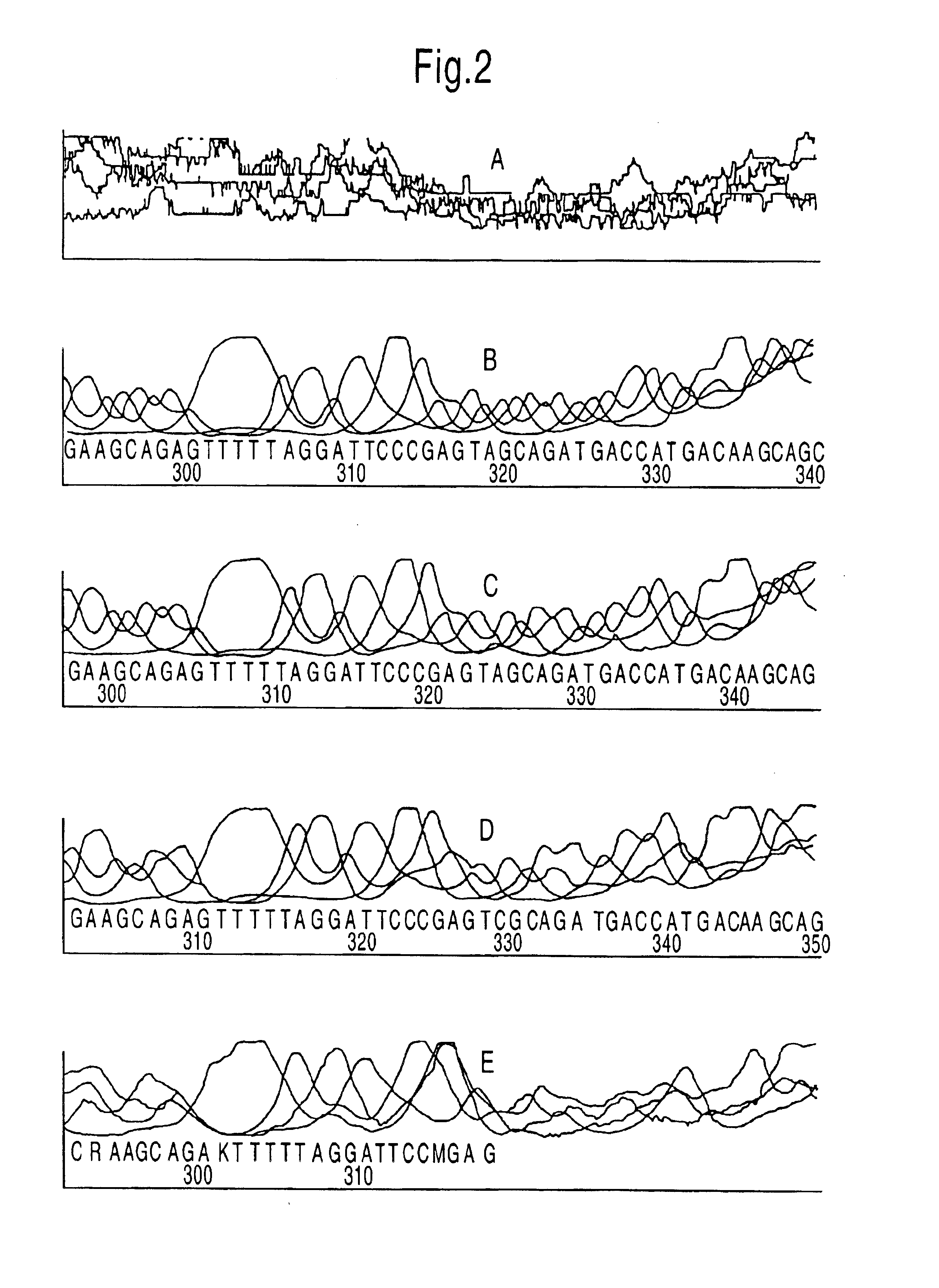
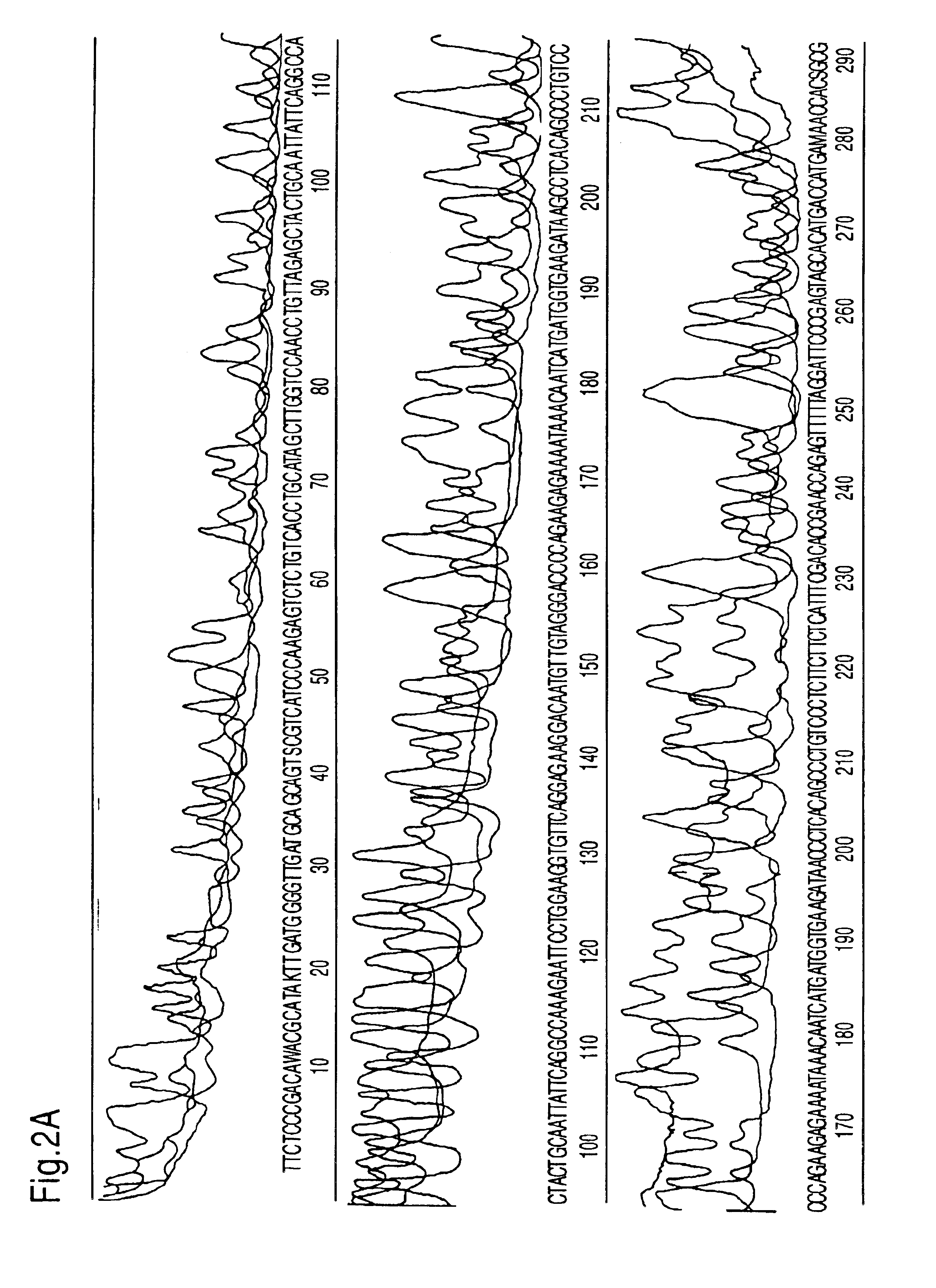
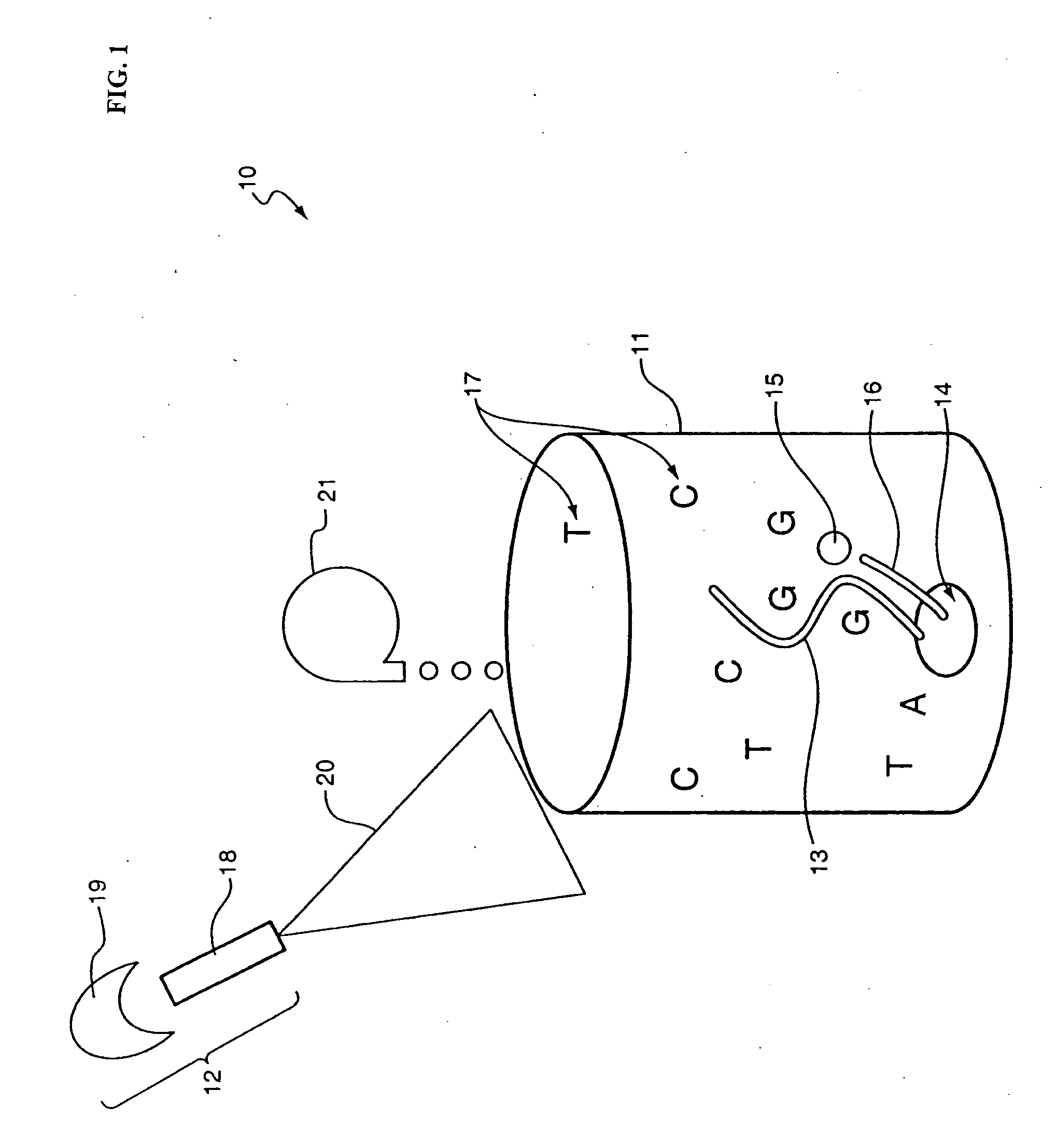
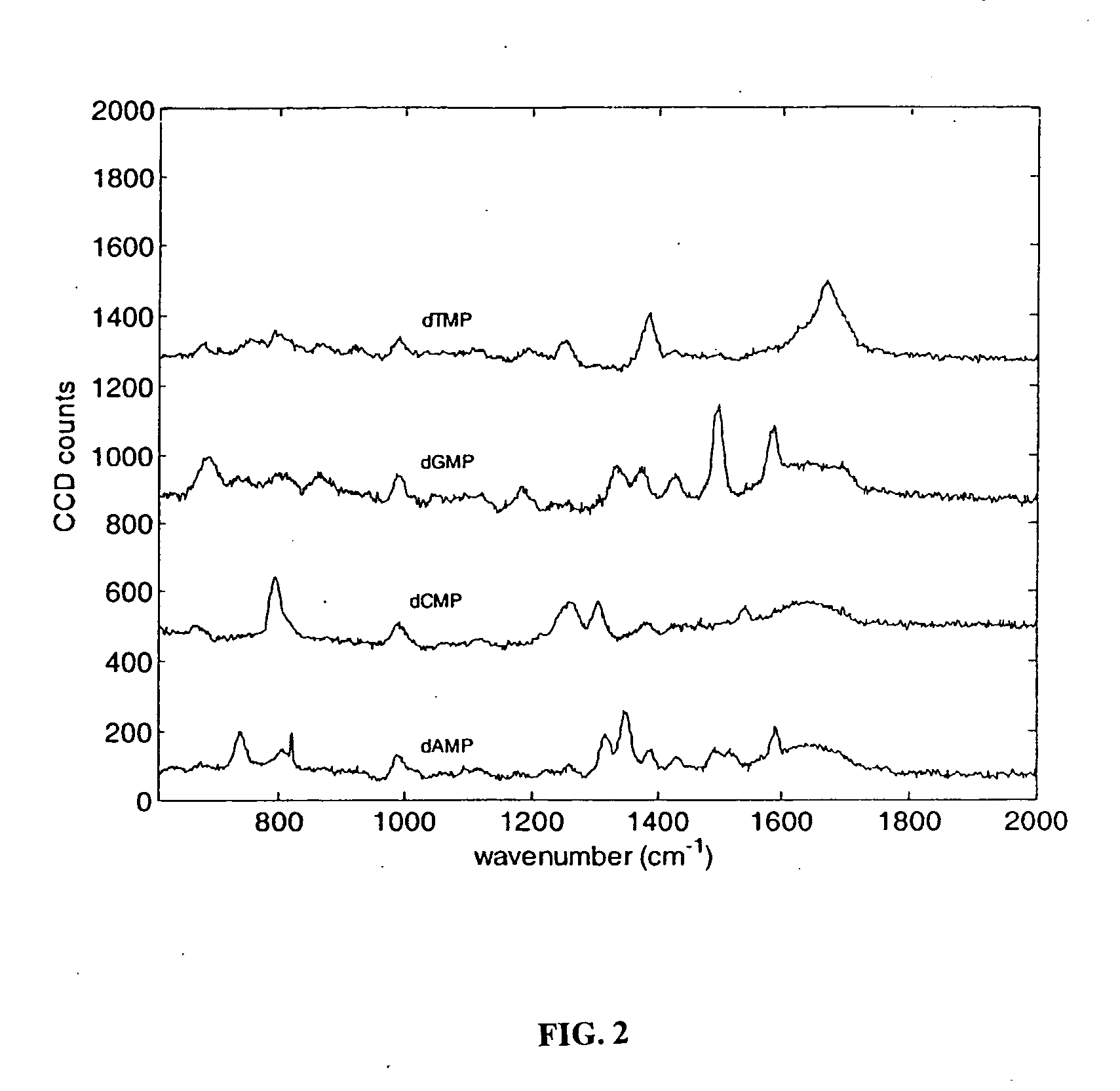
The methods and apparatus disclosed herein are useful for detecting nucleotides, nucleosides, and bases and for nucleic acid sequence determination. The methods involve detection of a nucleotide, nucleoside, or base using surface enhanced Raman spectroscopy (SERS) or surface enhanced coherent anti-Stokes Raman spectroscopy (SECARS). The detection can be part of a nucleic acid sequencing reaction to detect uptake of a deoxynucleotide triphosphate during a nucleic acid polymerization reaction, such as a nucleic acid sequencing reaction. The nucleic acid sequence of a synthesized nascent strand, and the complementary sequence of the template strand, can be determined by tracking the order of incorporation of nucleotides during the polymerization reaction. Methods for enhancing the SERS signal of a nucleotide or nucleoside by cleaving the base from a sugar moiety are provided. Furthermore, methods for detecting single base repeats are provided.
Owner:INTEL CORP
Inhibition of Bcl-2 protein expression by liposomal antisense oligodeoxynucleotides
The present invention provides novel compositions and methods for use in the treatment of Bcl-2-associated diseases like cancer, specifically, in the treatment of follicular lymphoma (FL). The compositions contain antisense oligonucleotides that hybridize to Bcl-2 nucleic acids, the gene products of which are known to interact with the tumorigenic protein Bcl-2. Used alone, or in conjunction with other antisense oligonucleotides, these compositions inhibit the proliferation of FL cancer cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Medicinal composition for treatment of chronic hepatitis c
InactiveUS20070110714A1Enhance immune functionPreventing sideration of hepatic cirrhosisBiocideOrganic active ingredientsChronic hepatitisBULK ACTIVE INGREDIENT
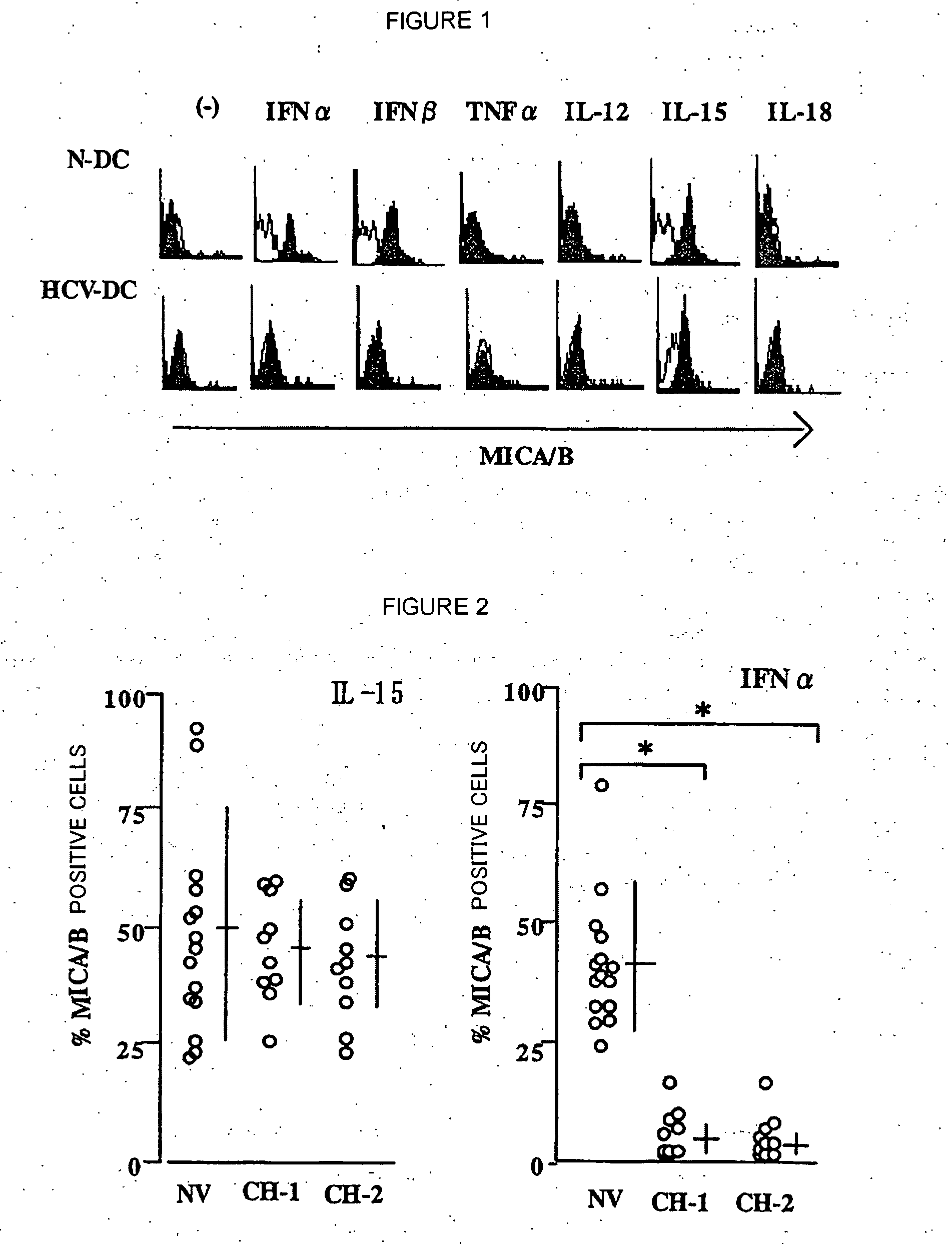
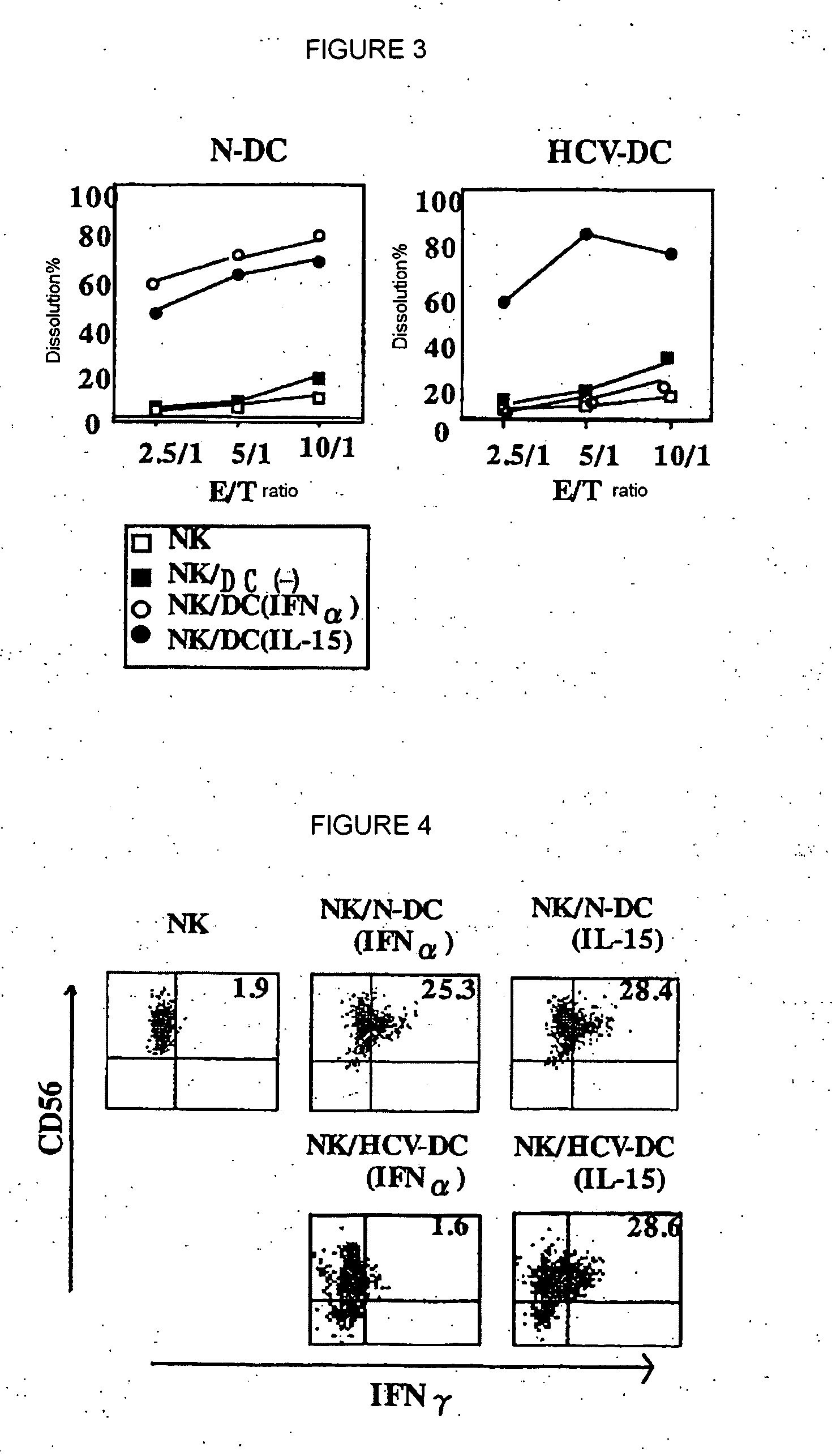
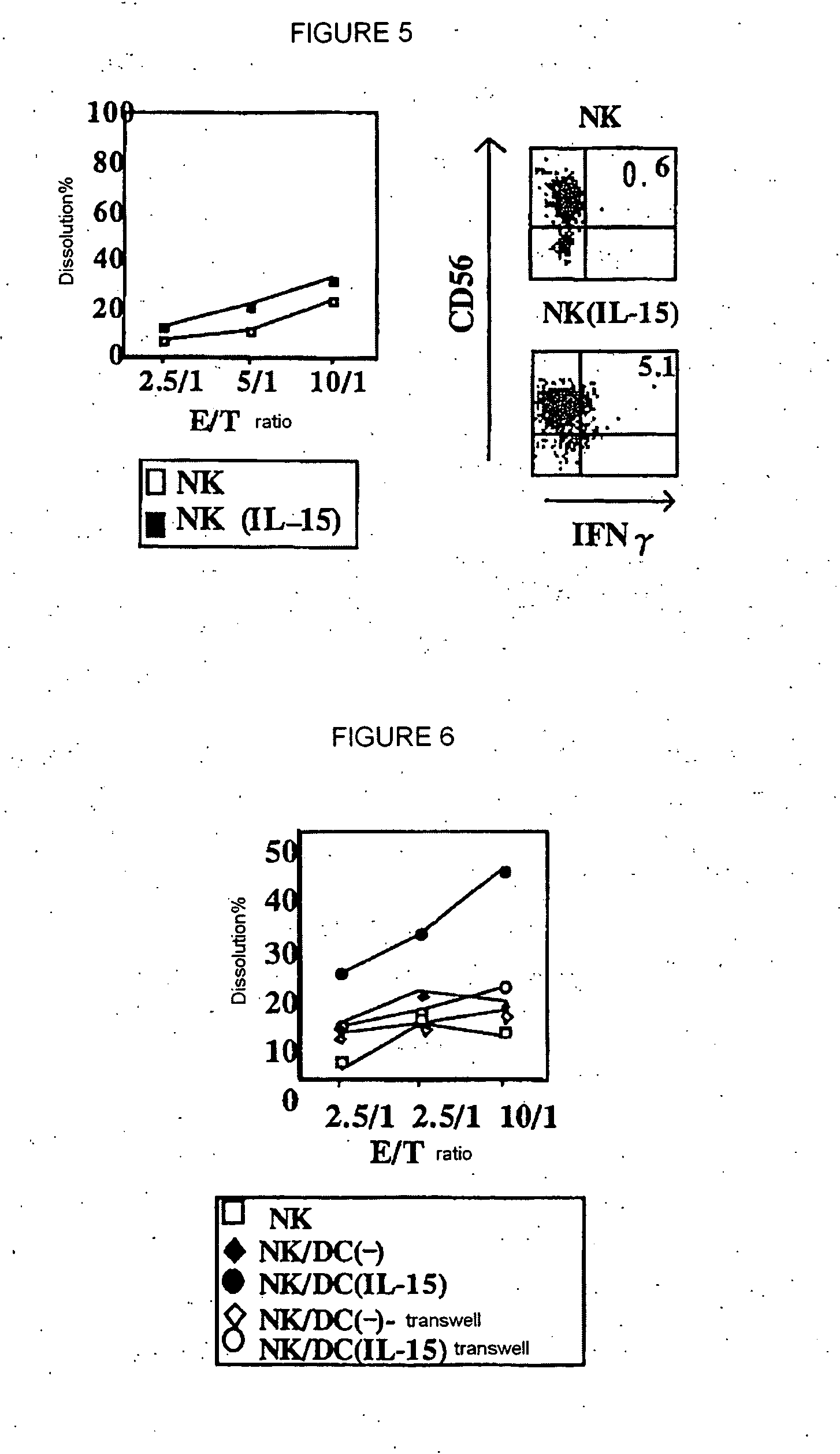
The present invention provides a pharmaceutical composition for treating chronic hepatitis C characterized by comprising at least one active ingredient selected from the group consisting of IL-15, myeloid dendritic cell maturation stimulators (for example, CpG oligo deoxynucleotide, GM-CSF, IL-4, LPS, CD40L, polyI:C, TNF-a and IFN-?) and lectin-binding substances (for example, mannose carbohydrate, fucose carbohydrate and anti-lectin antibody); and a method of treating chronic hepatitis C by using such active ingredient(s). In the above pharmaceutical composition and therapeutic method, it is possible to use INF-a together with these active ingredients.
Owner:INTPROP CONSULTING +1
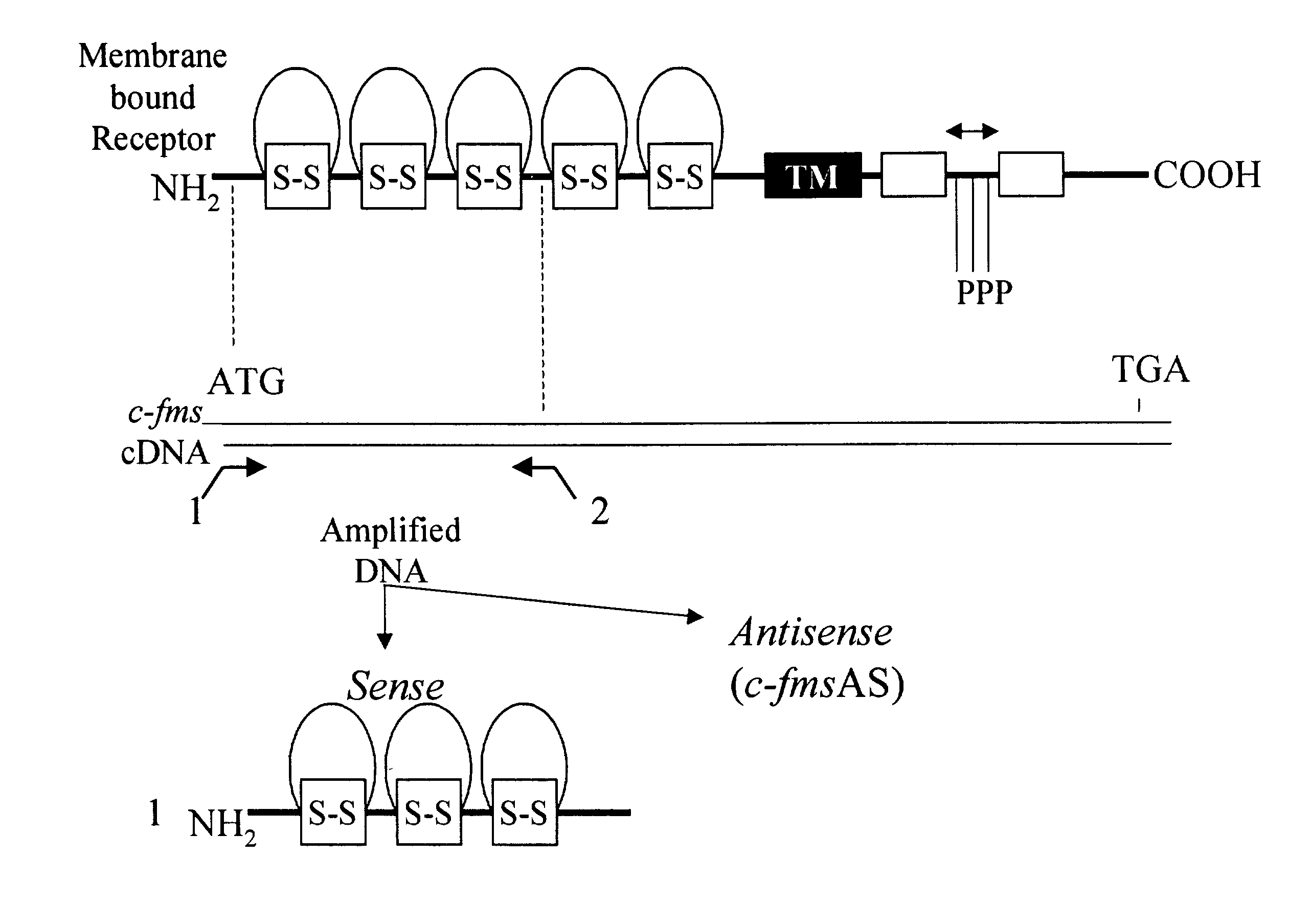
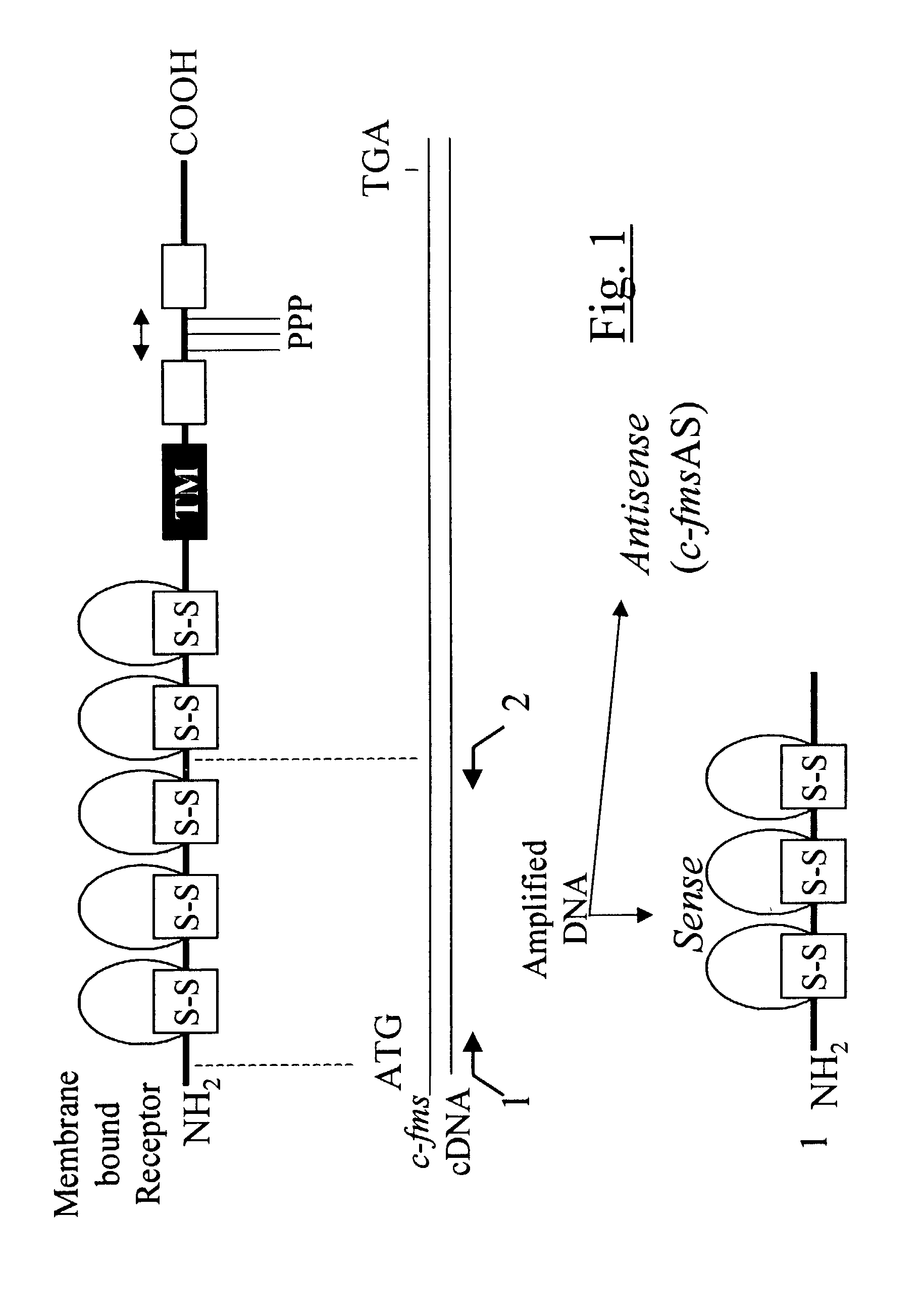
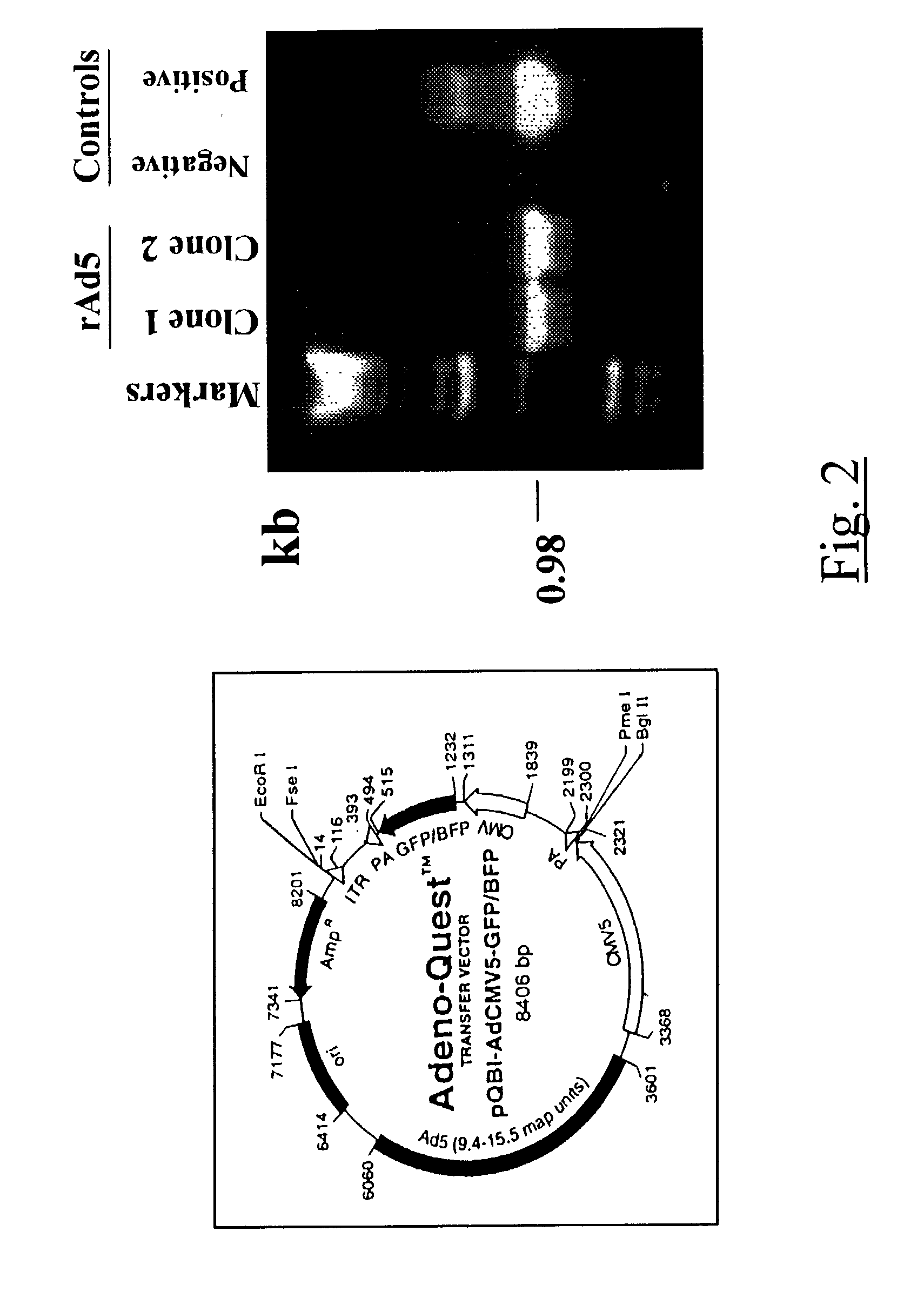
Methods for inhibiting macrophage colony stimulating factor and c-FMS-dependent cell signaling
Owner:RAJAVASHISTH TRIPATHI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com