Medicine-carrying nanometer polymer particle and its prepn and use
A polymer material and drug-loaded nanotechnology, which is applied in the field of new polymer material drug-loaded nanoparticles and their preparation methods and uses, can solve the problems of ineffective reach of target tissues and poor stability of liposome carriers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
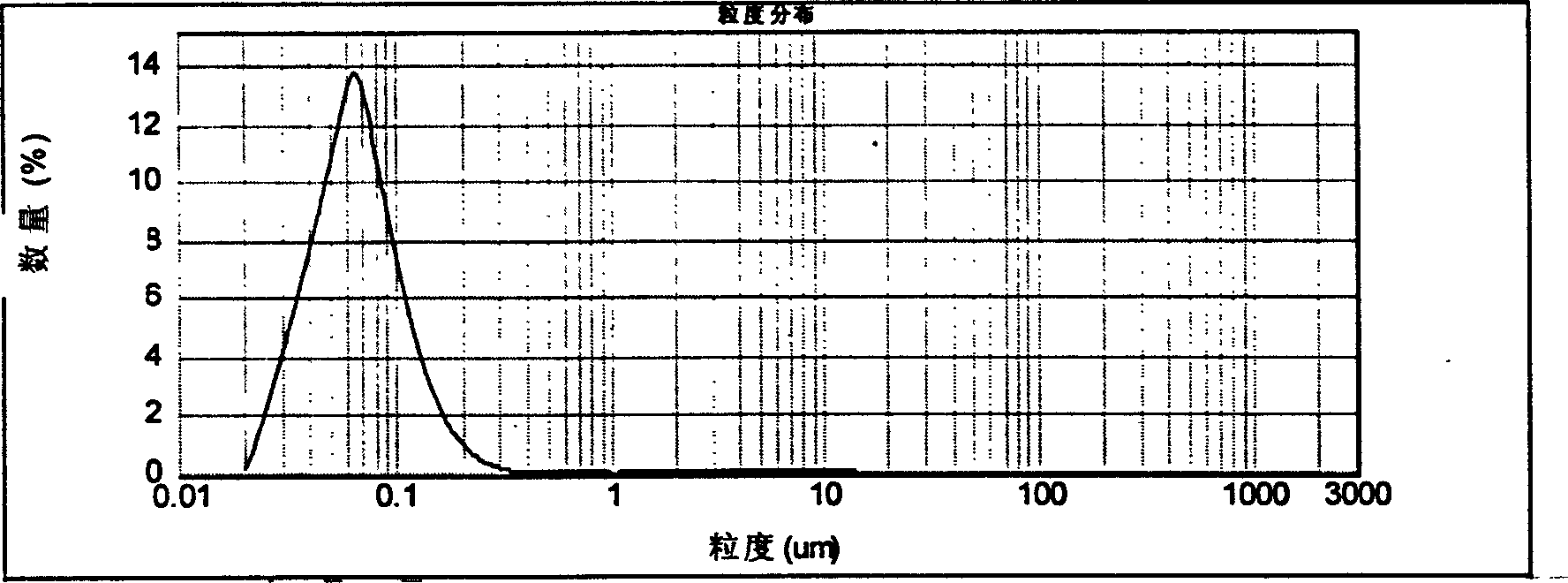
Image
Examples
Embodiment 1
[0045] Accurately weigh 10 mg of PELGE and add it to acetone to form the organic phase. Dissolve 100 μg of a therapeutic gene such as pORF IL-12 plasmid in double distilled water to form an aqueous phase. The plasmid solution was added to the organic phase and homogenized to form colostrum. Weigh Pluronic F68 and add it into double-distilled water to make it fully dissolved, adjust the concentration to 3%, and form the outer water phase. The volume ratio of the inner water phase to the outer water phase is 1:6-1:10. Add colostrum into the external water phase, emulsify with a high-pressure homogenizer, stir the obtained emulsion with magnetic force to evaporate the organic solvent for 4-5 hours, and completely volatilize the acetone to obtain the colloidal solution of PELGE nanoparticles. 3% mannose was added as a scaffold, which was routinely lyophilized for storage.
Embodiment 2
[0047] Accurately weigh 10 mg of PELGE and add it to a mixed organic solvent of dichloromethane (DCM) and acetone (the volume ratio of DCM and AC is 60-100 / 0-40) to form the organic phase. Dissolve 100 μg of a therapeutic gene such as pORF IL-12 plasmid in double distilled water to form an inner water phase. The plasmid solution was added to the organic phase and homogenized to form colostrum. Weigh polyvinyl alcohol (PVA) and add it into double-distilled water to make it fully dissolved, adjust the concentration to 2%, and form the external water phase. Add colostrum into the external water phase, ultrasonically emulsify for 30 seconds, put the obtained emulsion into a beaker, and magnetically stir to evaporate the organic solvent for 3 hours, so that dichloromethane and acetone are completely volatilized, and the colloidal solution of PELGE nanoparticles is obtained. 3% glucose was added as a scaffold, which was routinely lyophilized and stored.
Embodiment 3
[0049] Accurately weigh 20 mg of PELGA and add it into dichloromethane (DCM) to form the organic phase. Dissolve 300 μg of a therapeutic gene such as pORF IL-12 plasmid in double distilled water to form an inner water phase. The plasmid solution was added to the organic phase and homogenized to form colostrum. Weigh polyvinyl alcohol (PVA) and add it into double-distilled water to make it fully dissolved, and adjust its concentration to 1% to form the external water phase. Add colostrum into the external water phase, emulsify with a high-pressure homogenizer for 1 min, and magnetically stir the obtained emulsion to evaporate the organic solvent for 4-5 hours, so that the dichloromethane is completely volatilized, and the colloidal solution of PELGA nanoparticles is obtained. 3% lactose was added as a scaffolding agent and routinely lyophilized for storage.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com