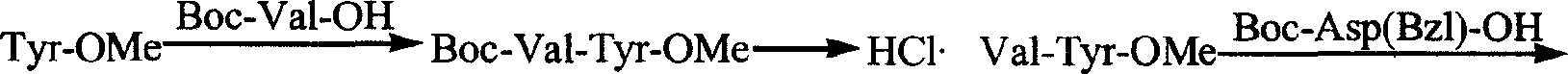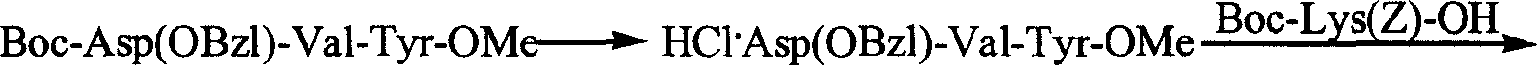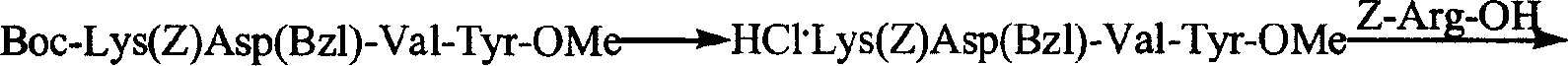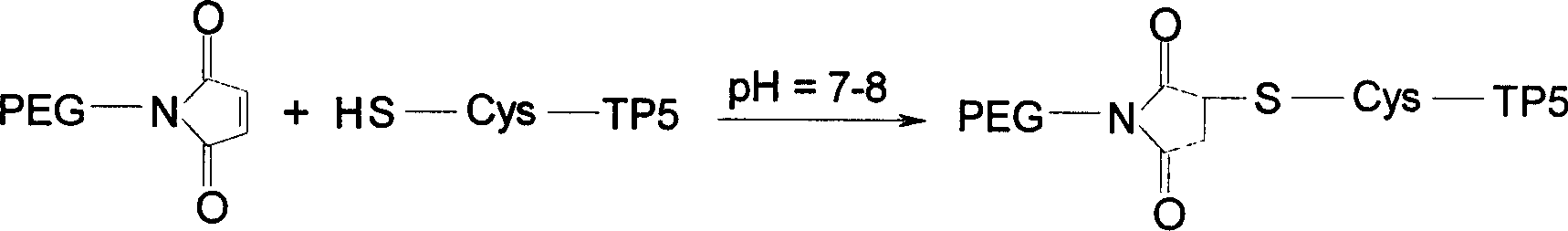Patents
Literature
122 results about "Thymopentin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thymopentin is an immunostimulant. As such, it has been used in several clinical studies in the early years of the AIDS pandemic (from 1983 to 1985). Thymopentin helped to improve immunological condition in several patients for a brief time under specific treatments.
Thymopentin dry powder formulation and preparation method thereof
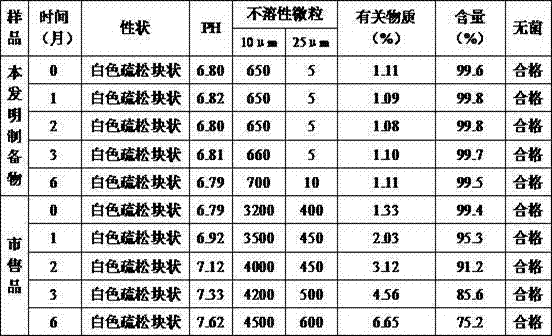
ActiveCN101411864AEasy to degradeImprove solubilityPowder deliveryPeptide/protein ingredientsSolubilityCurative effect
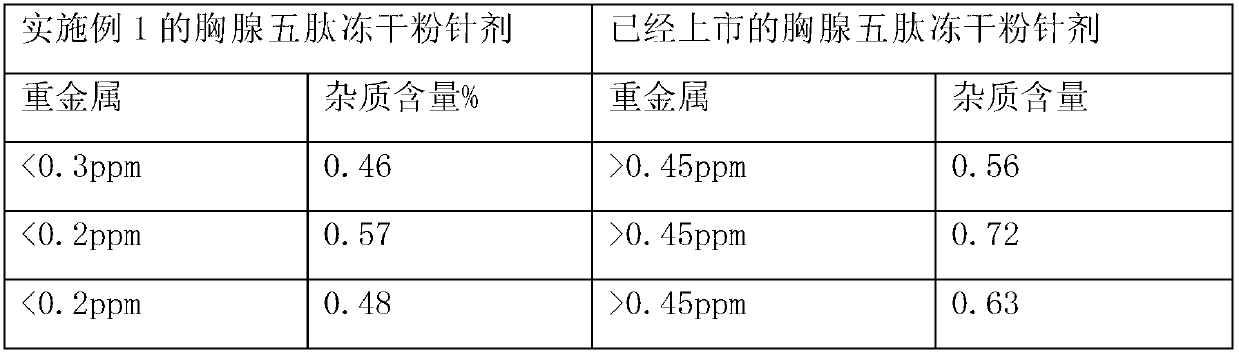
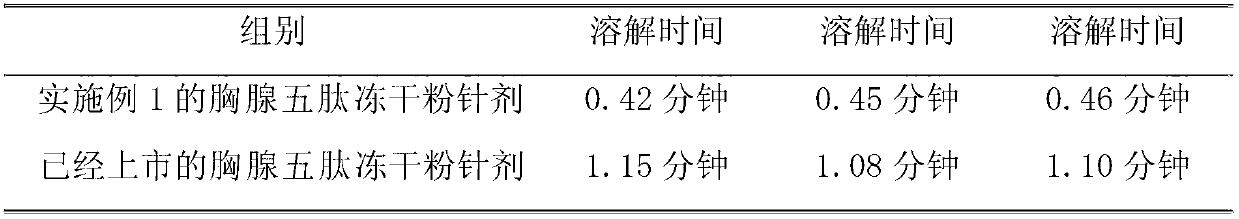
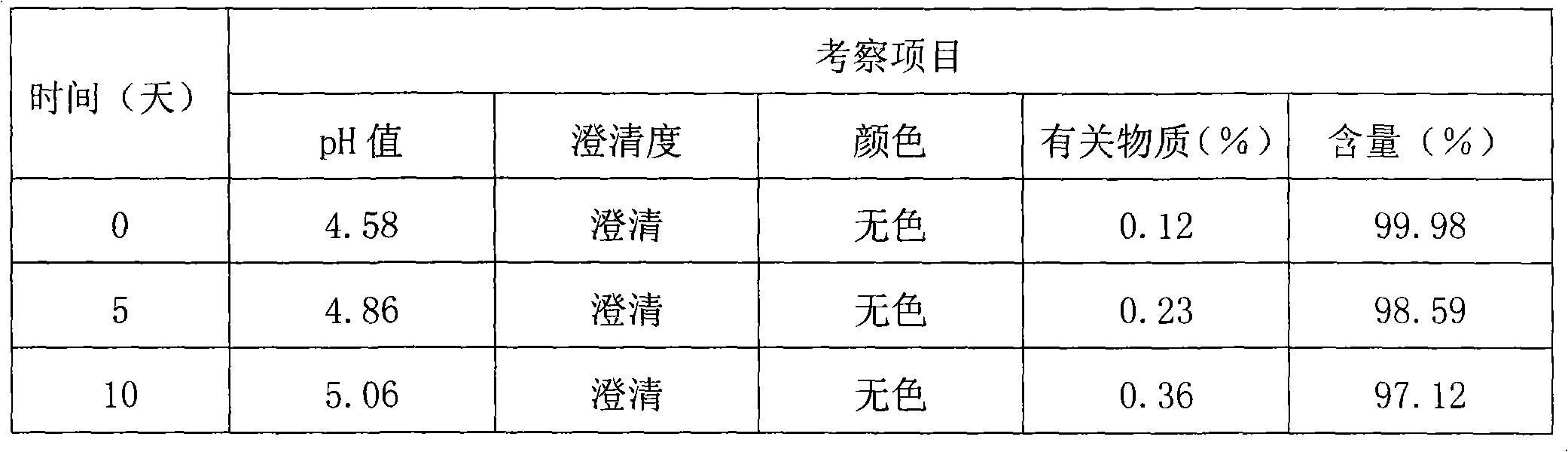
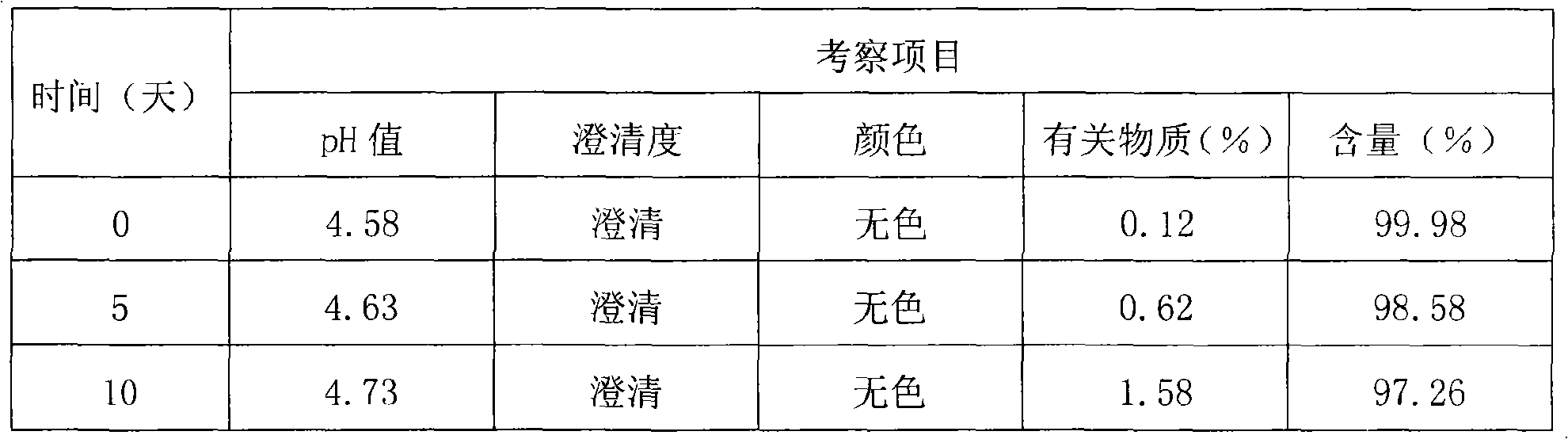
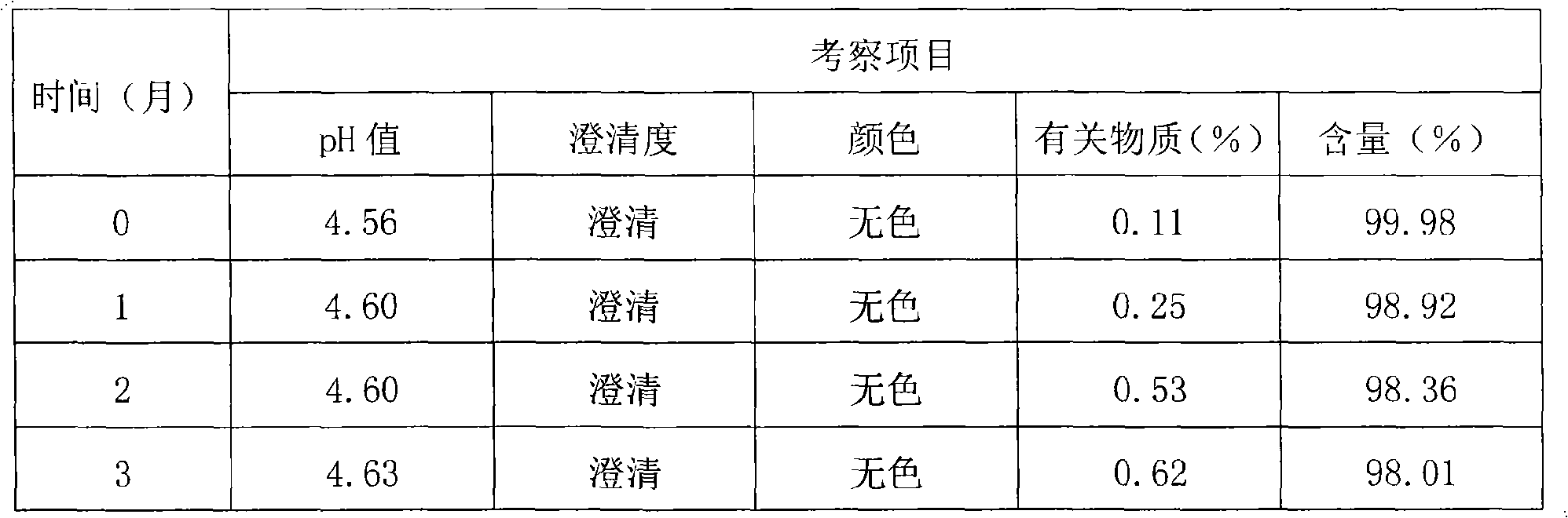
The invention discloses a thymopeptide-5 powder preparation and a preparation method thereof. The method comprises the following steps: dissolving excipient in water to produce solution I, and filtering the solution I to collect filtrate I; adding the thymopeptide-5 to the filtrate I to obtain solution II, and filtering solution II to collect filtrate II; and freezing and drying the filtrate II to obtain the thymopeptide-5 powder preparation. The excipient can be any one of mannite, lactose and glycin. Experiments prove that, in the thymopeptide-5 powder preparation, thymopeptide-5 is not easy to be degraded, and has good solubility; low content of 'related substances' which is below 1.0 percent, so that healing efficacy and safety of the thymopeptide-5 powder preparation can be greatly improved.
Owner:上海华源药业(宁夏)沙赛制药有限公司
Process for preparing thymopentin
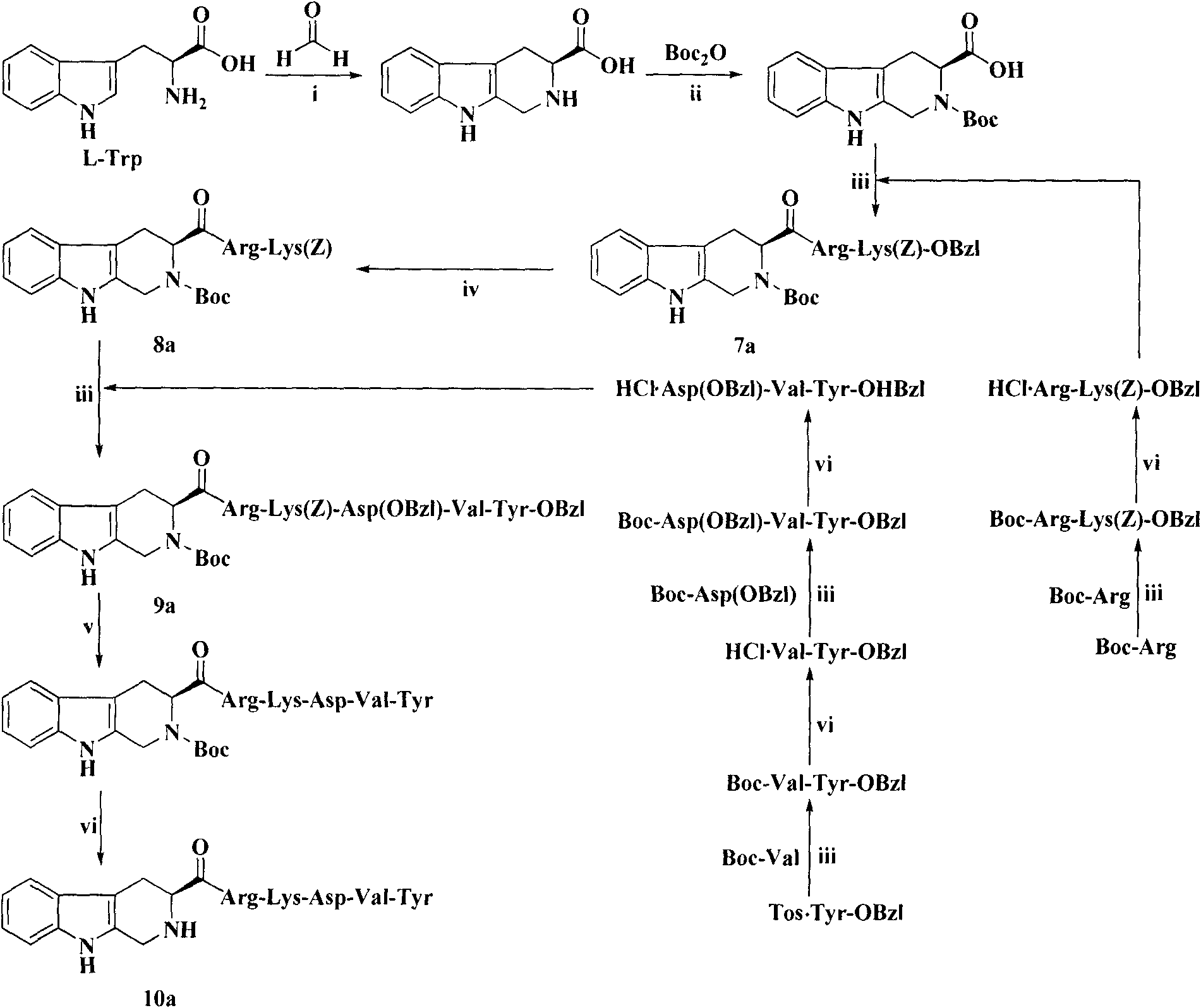
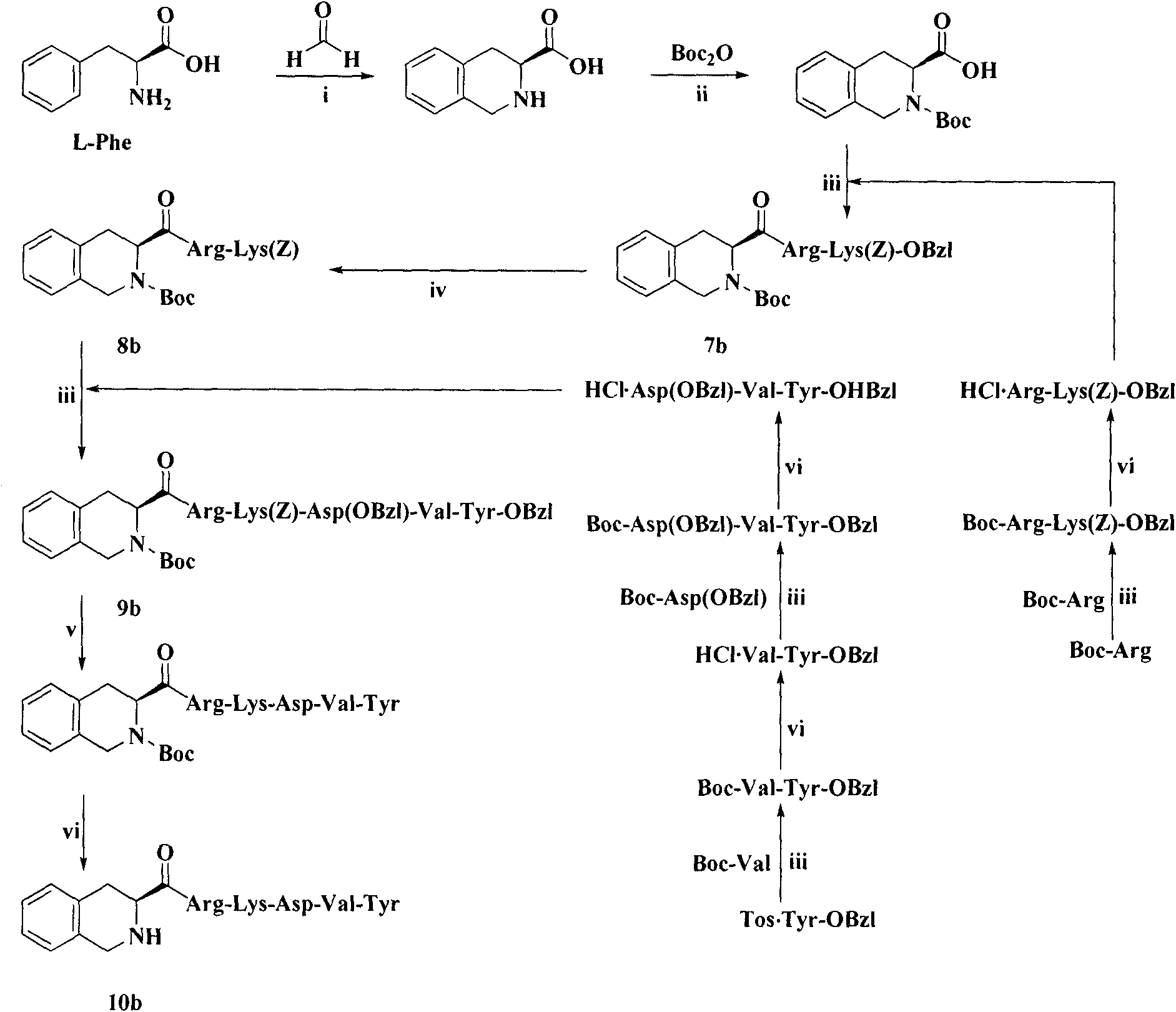
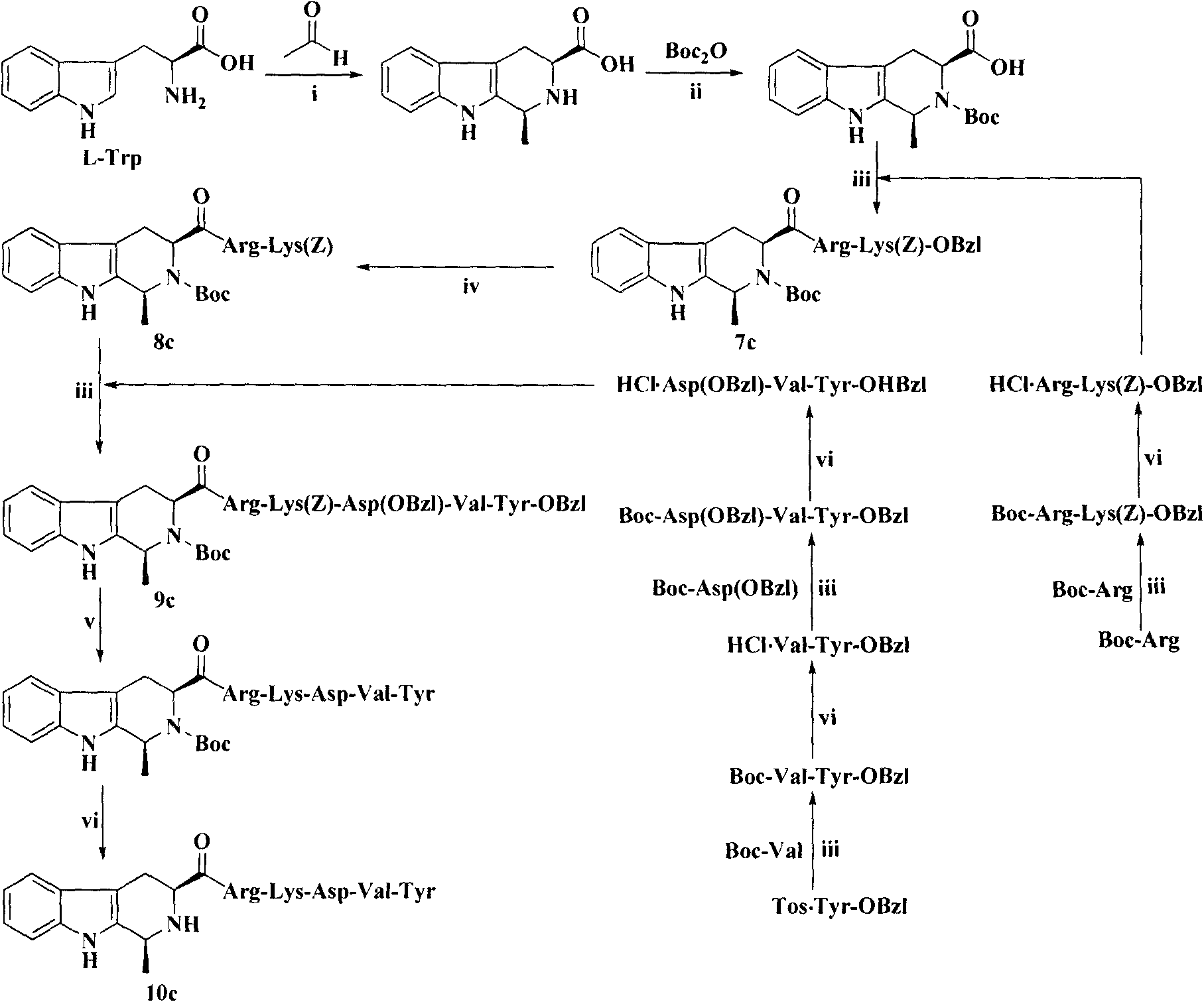
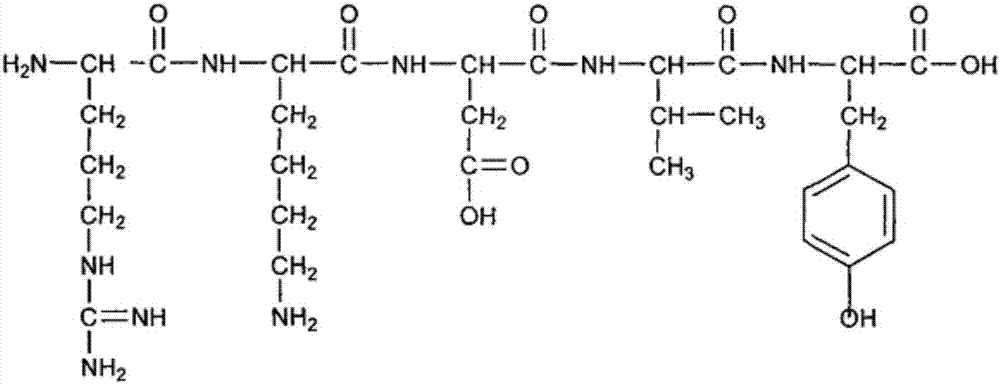
The invention provides a process for preparing thymopentin Arg-Lys-Asp-Val-Tyr-OH by employing minimum protection strategy, wherein Arg and Tyr whose lateral chain functional groups are not protected are used as raw material. The invention has the advantages of simple process, low cost and easy accessibility of raw material.
Owner:康哲(湖南)制药有限公司
Carbowax alcoholized ramification of TimopEntin, combination of medication and application
InactiveCN1626549ANovel chemical structureBiologically active and stablePeptide/protein ingredientsAntipyreticDiseaseSide chain
A polyglycolized derivative of thymopeptide-5, which can be used to prepaer the medicines for preventing and treating rheumatoid arthritis, allergic dermatisis, tumor, viral infection, and the diseases associated with immune deficiency and hypoimmunofunction, is disclosed. Said derivative features that the polyglycol, polyglycol modified amino acid, cysteine, maleamido glycol, vinyl glycol, or iodoacetylglycol is introduced or coupled to the N or C terminal of thymopeptide-5.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA
Thymopentin injecta liquid formulation
InactiveCN101024071AFix stability issuesImprove stabilityPeptide/protein ingredientsPharmaceutical delivery mechanismPreservative freePreservative
The present invention relates to a prescription of thymus pentapetide injection. Said prescription includes 1-100 mg of thymus pentapeptide, 0-100 mg of anhydrous sodium acetate, 0-50 mg of citric acid, 300-1000 mg of sodium chloride and 0.2-5 ml of injection water. Said thymus pentapeptide injection can be made into ampule package form.
Owner:HAINAN ZHONGHE PHARM CO LTD
Composition of thymic peptide alpha 1 and thymopeptide-5 and preparation method thereof
ActiveCN101683519AGood effectImprove economyPeptide/protein ingredientsDigestive systemDiseaseMedicine
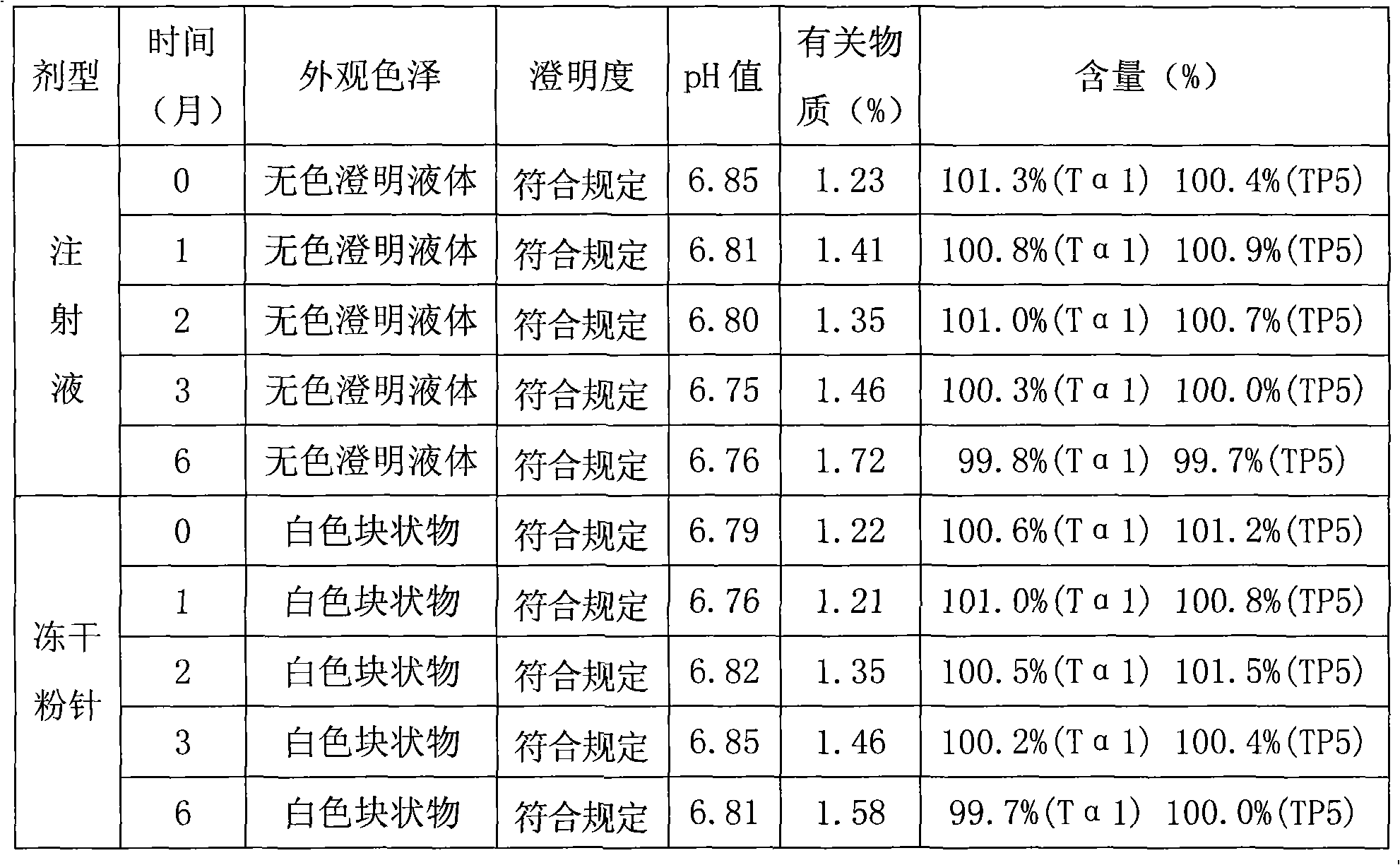
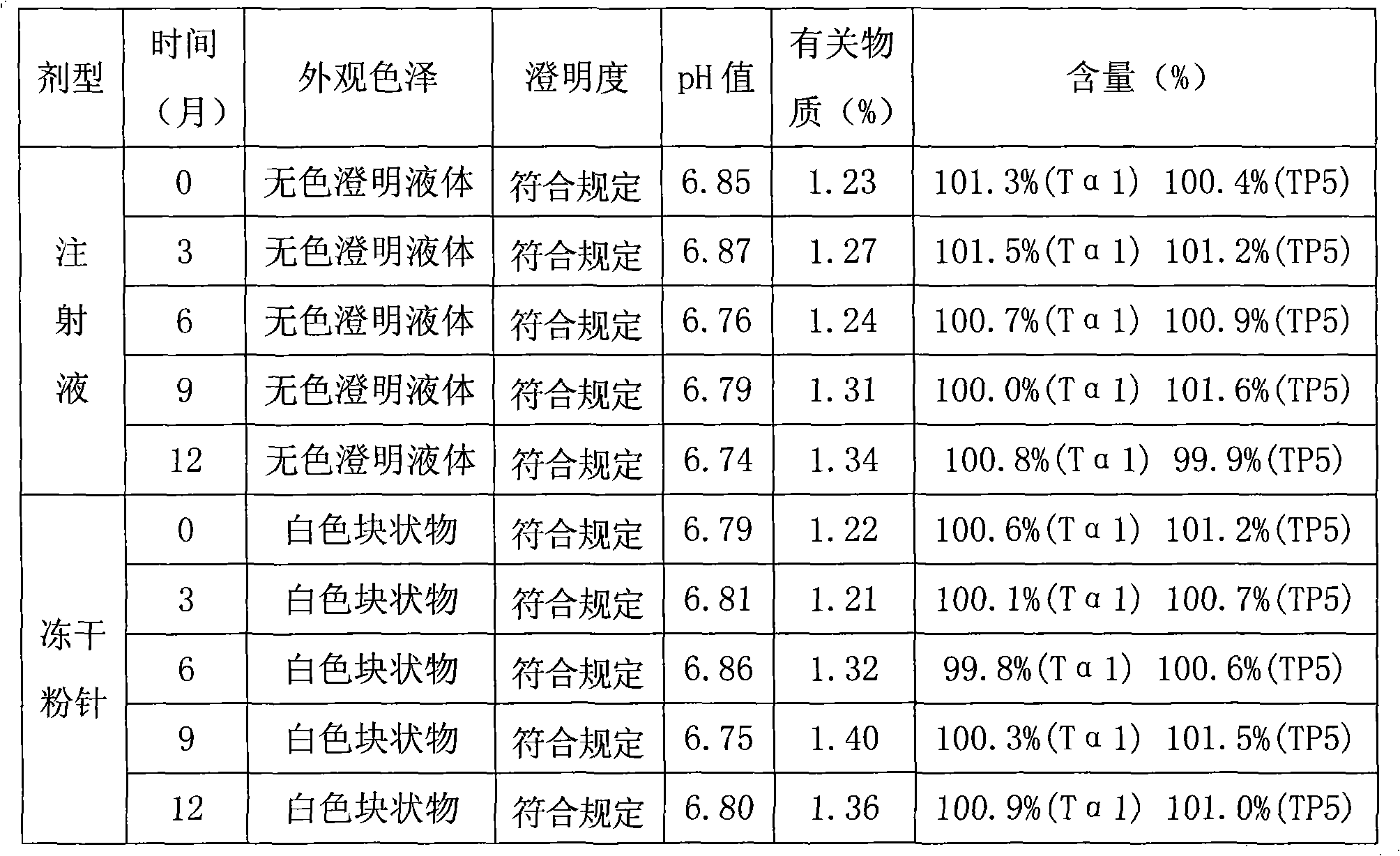
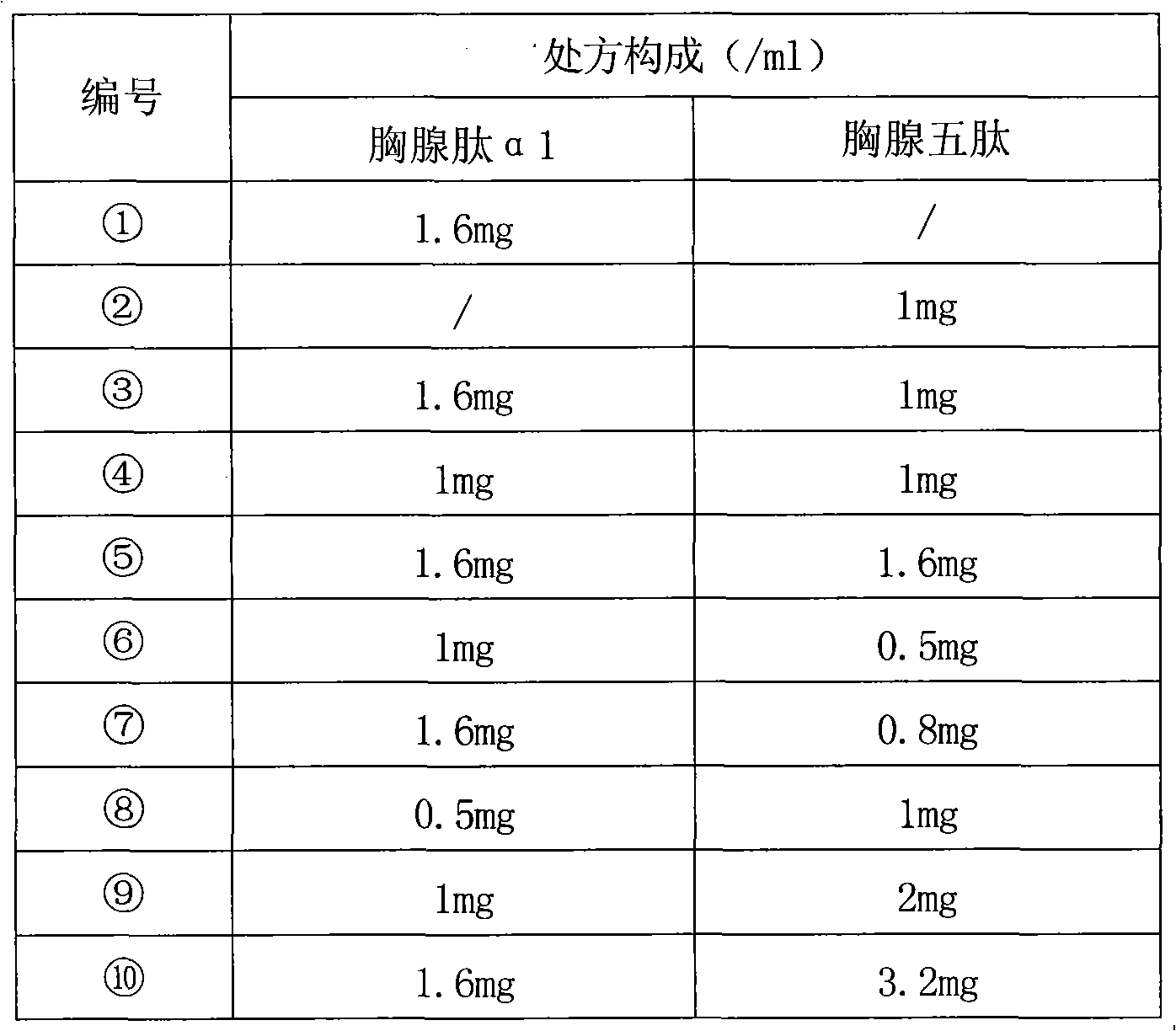
The invention relates to a composition of thymic peptide alpha 1 and thymopeptide-5 and a preparation method thereof. The compound preparation can be used for the treatment of chronic hepatitis B andvarious diseases with damaged immunological functions and the auxiliary treatment of tumours. The composition mainly comprises 0.1-10mg of thymic peptide alpha 1 and 0.1-100mg of thymopeptide-5. Simultaneously, proper auxiliary materials are added into the composition to prepare injection liquid or a freeze-drying medicament form. The composition prepared according to the proportion has obvious synergy action and can reach a better curative effect dose ratio for the treatment of the chronic hepatitis B and various diseases with damaged immunological functions and the auxiliary treatment of thetumours.
Owner:HAINAN ZHONGHE PHARM CO LTD
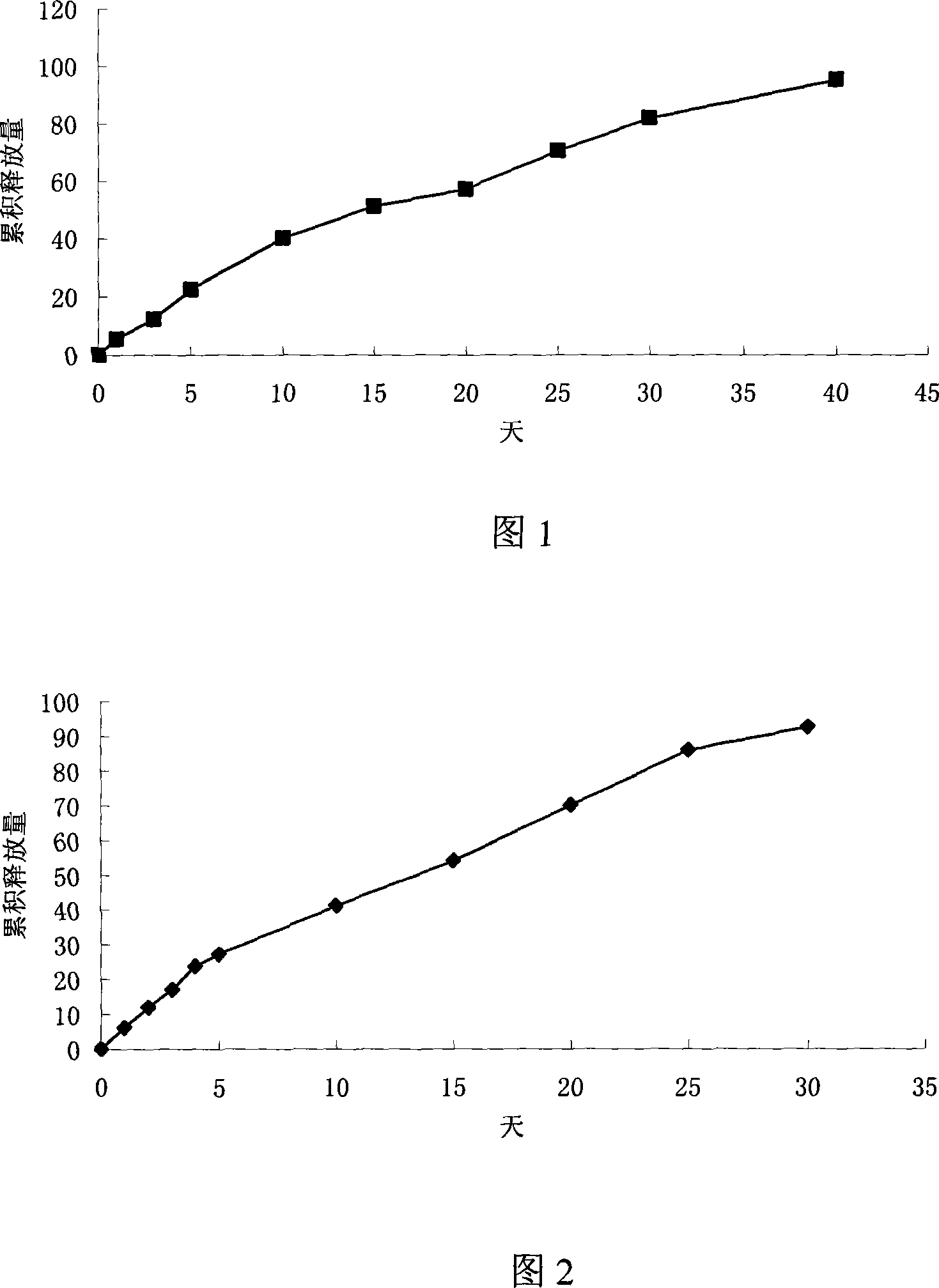
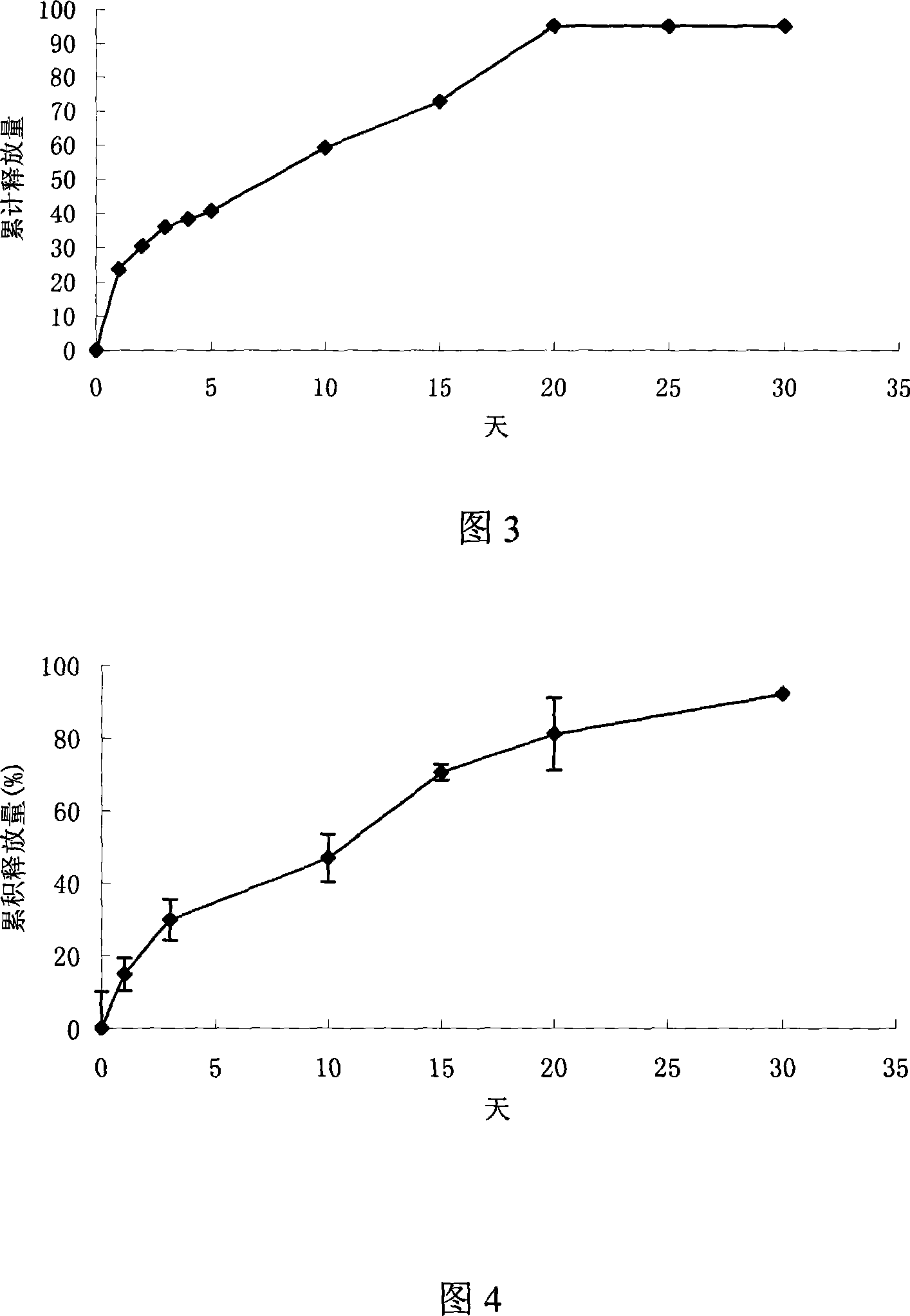
Thymus pentapeptide slow-release microshpere formulation for injection and its preparation method
The invention relates the thymus five-peptide slow release microsphere and preparing method. The slow release microsphere comprises 0.5-30wt% thymus five-peptide, 70-99.5wt% degradable high polymer findings. The molecular weight of high polymer findings is 5,000-500,000Dalton. The method can use W / O / W method, S / O / O method and spray-drying method. The particle mean size of the slow release microsphere is 5-40 mum, the encapsulated rate is more than 75%. The slow release microsphere has the advantages of long medicinal crop, less frequency, improving bioavailability and reducing side effects.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Medicinal composition containing thymopentin compound
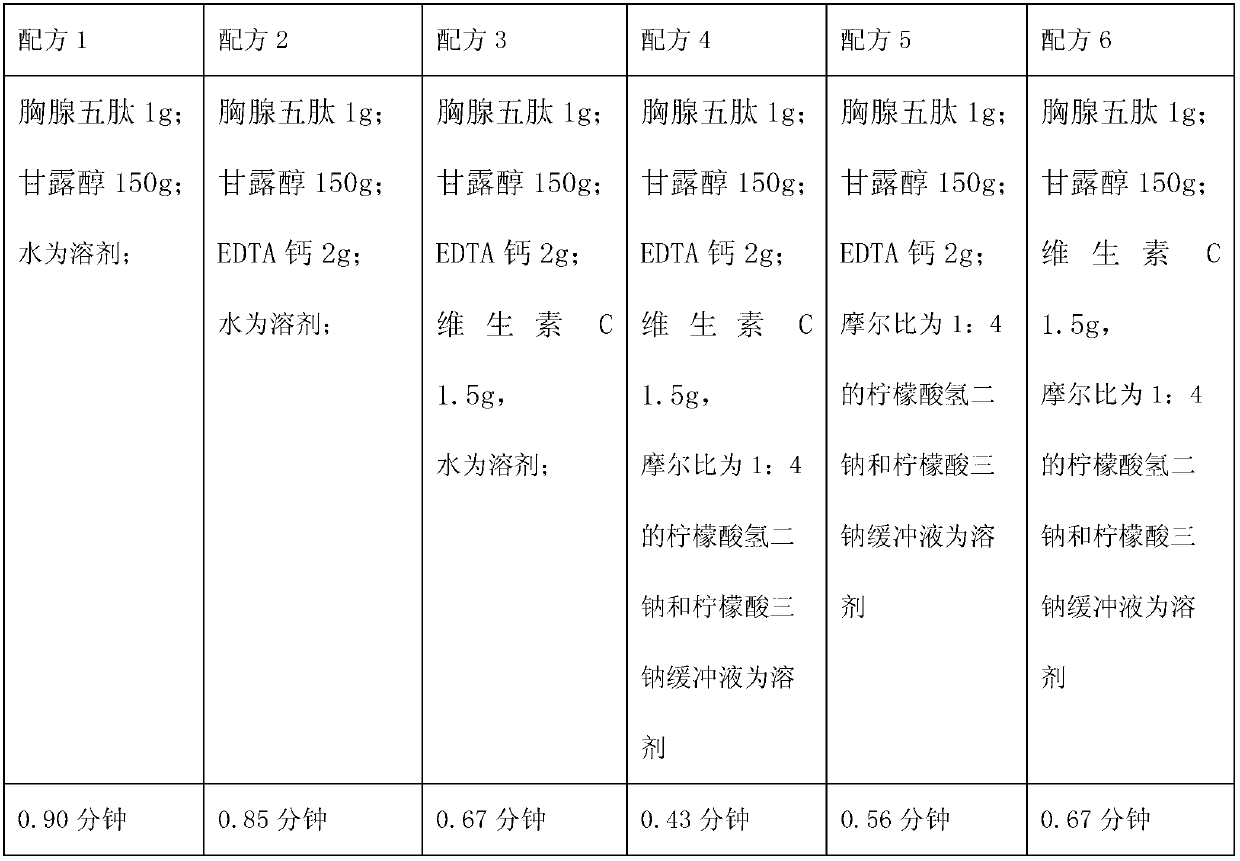
ActiveCN102988954AImprove stabilityImprove tolerancePowder deliveryPeptide/protein ingredientsEthylene diamineVitamin C
The invention relates to a medicinal composition containing a thymopentin compound, and in particular relates to an injection as well as a formula, application and a preparation method thereof. Each 1,000 injections are prepared from the following components: 0.5 to 2g of thymopentin, 50 to 200g of mannitol, 1 to 3g of ethylene diamine tetraacetic acid (EDTA) calcium, 1 to 2g of vitamin C, 1,000ml of buffer solution of disodium hydrogen citrate and trisodium citrate at a mole ratio of 1: 4, and the balance of injection water to the volume of 2,000ml.
Owner:姚云
Oral nanoparticle polypeptide composition tablets and preparation method thereof
The invention relates to a medicinal preparation and a preparation method thereof, and in particular relates to a series of polypeptide thymopentin, thymalfasin and thymosin beta4 combined enzyme inhibitors capable of improving the immunity. An oral preparation is prepared by adopting a nanoparticle technology. A pharmaceutically-acceptable biodegradable high polymer material is coated with a polypeptide medicament for improving the immunity to prepare nanoparticles, and tablets are made by tableting. The medicament-loading nanoparticles prepared by using the preparation method have high bioavailability.
Owner:深圳市健翔生物制药有限公司
Thymus penta peptide slow releasing micro ball and its preparing method
The present invention discloses a kind of slowly releasing thymus penta peptide microballoon and its preparation process. The thymus penta peptide microballoon consists of thymus penta peptide, biodegradable medicinal polymer material and emulsifying stabilizer. It may be prepared through emulsifying dispersion process, solvent volatilizing process, spray drying process or lower temperature spray extracting process. The thymus penta peptide microballoon with biodegradable medicinal polymer material may be released slowly for several days or even several months, resulting in obviously medicine taking times and being favorable to clinical treatment.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Thymus gland pentapeptide oral intestine-dissolved formulated product and method of preparing the same and use thereof
ActiveCN101108246ASolve the problem of oral drug deliveryImprove complianceOrganic active ingredientsPeptide/protein ingredientsIntestinal structureAdditive ingredient
The invention provides a Thymopentin Oral Enteric-coated Agent, which takes thymopentin of effective dosage as the active ingredient and enteric-coated agent as its accessories. Each dosage contains 5mg to 150 mg thymopentin. The invention also provides the preparation method and usage of the enteric-coated agent. The medicine effect tests prove that the medicine has the same indication and efficacy as the injections and can overcome that the gastrointestinal enzyme will easily degrade the thumopentin into amino acid and small peptide so as to lose the activity when orally taking the Thymopentin; the thumopentin can not easily penetrate the gastrointestinal mucosa, resulting in low bioavailability; and the liver has the First-pass effect on the thymopentin. The invention opens a new way to apply thumopentin and increases the patients' compliance.
Owner:CHENGDU DIAO JIUHONG PHARMA FAB
Immunomodulator polypeptide slow-release microsphere preparation and preparation method thereof
InactiveCN103961320AImprove adaptabilityEasy to acceptPeptide/protein ingredientsPharmaceutical non-active ingredientsSide effectMicrosphere
The invention belongs to the field of medical preparations, and relates to an immunomodulator polypeptide slow-release microsphere preparation and a preparation method thereof. Particularly, the immunomodulator polypeptide comprises thymopentin, thymalfasin and thymosin beta4. The slow-release microsphere comprises 0.1-30% (w / w) of immunomodulator polypeptide, 60-80% of biodegradable high polymer material with biocompatibility, of which the molecular weight is 5,000-200,000 Dalton, and 0.1-20% of other pharmaceutical acceptable auxiliary materials on the basis of the total weight of the microsphere. According to the slow-release microsphere disclosed by the invention, the mean grain size is 5-60mu m; the encapsulation efficiency is greater than 90%; the slow release period of the slow-release microsphere can be up to a few days and months, the medication times is obviously reduced, the bioavailability is improved, the toxic and side effects of the medicine are reduced, and clinical treatment is facilitated. The product is good in production process repeatability and good in feasibility.
Owner:SHENZHEN JYMED TECH
Drug composition of thymopentin composed of five kinds of amino acids and preparation method thereof
ActiveCN102764424AQuality improvementImprove stabilityPowder deliveryPeptide/protein ingredientsDiseaseFreeze-drying
The invention relates to a drug composition of thymopentin composed of five kinds of amino acids. The drug composition can improve immunity and cure diseases such as hepatitis, deficiency of T lymphocytes and autoimmune diseases, and contains, by weight, 1-10 parts of thymopentin composed of five kinds of amino acids, 0.4-0.6 part of citric acid, 1.4-1.6 parts of dipotassium phosphate and 48-52 parts of glucose. The preparation method includes adding the citric acid, the dipotassium phosphate and the glucose into water for injection to dissolve; adding thymopentin to dissolve with stirring and then stirring evenly, adding active carbon for injection and stirring, filtering for decarburization, and obtaining a filter solution through filter membrane filtration sterilization; and obtaining the drug composition of the thymopentin through filter solution subpackaging or freeze drying. Prepared thymopentin for injection and thymopentin injection have good quality and stability, and can maintain a high stability even at a room temperature and be stored for more than 36 months.
Owner:湖北美林药业有限公司
Heterocyclic carboxylic acid-modified thymopentins and their preparation method, anti-tumor effect and use
InactiveCN103450338APeptide/protein ingredientsPeptide preparation methodsThymopentinCarboxylic acid
The invention discloses heterocyclic carboxylic acid-modified thymopentins. The heterocyclic carboxylic acid-modified thymopentins have a general formula I. In the general formula I, RCO represents 1,2,3,4-tetrahydro-beta-carboline-3-formyl, beta-carboline-3-formyl, 1,2,3,4-tetrahydroisoquinol-3-formyl, isoquinol-3-formyl, 1-methyl-1,2,3,4-tetrahydro-beta-carboline-3-formyl, 1-methyl-beta-carboline-3-formyl or 4,5,6,7-tetrahydro-3H-imidazo[4,5-C]pyridyl-6-formyl. The invention also discloses a preparation method of the heterocyclic carboxylic acid-modified thymopentins, and in-vitro tumor cell proliferation-resistant activity of the heterocyclic carboxylic acid-modified thymopentins. The invention further discloses heterocyclic carboxylic acid-modified thymopentin activity of inhibiting weight gain of a tumor in a S180 tumor-loading mice, and use of the heterocyclic carboxylic acid-modified thymopentins in preparation of anti-tumor drugs. The general formula I is RCO-Arg-Lys-Asp-Val-Tyr.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Pentapeptide nose spraying agent for thymus gland, preparation method and application thereof
ActiveCN101318011AFormulation stabilityExtended storage timePeptide/protein ingredientsAntipyreticAutoimmune conditionAutoimmune disease
The invention relates to a nasal spraying agent of thymopentin, and a preparation method and applications thereof. The preparation is used in over18-year-old sufferers of chronic hepatitis B, various primary or secondary defect diseases of T cellular, certain autoimmune diseases, various diseases of low cellular immune function and adjutant therapy to tumours; the preparation consists of thymopentin, pharmaceutic adjuvant and water; the preparation method of the agent includes the steps of liquid preparation, degerming, split charging, stoppering, capping, package, etc. The medicament form overcomes the difficulty that polypeptide drugs are unsteady in water, thus keeping the activity of thymopentin well and having good stability; meanwhile, pain is not produced when the medicament form is used, thus being accepted by the general consumers.
Owner:HAINAN ZHONGHE PHARM CO LTD
Immunomodulator thymopentin powder injection pharmaceutical composition and quality control method
The invention relates to an immunomodulator thymopentin powder injection pharmaceutical composition and a quality control method. The immunomodulator thymopentin powder injection pharmaceutical composition comprises thymopentin, mannitol and an optional acid or alkali modulator, wherein the mannitol is 20-100 parts by weight on the basis of each 100 parts by weight of thymopentin. The thymopentin pharmaceutical composition can be used for treating chronic viral hepatitis, such as chronic hepatitis B and can be used for treating various primary or secondary T cell defects such as congenital immunodeficiency in children. In addition, the immunomodulator thymopentin powder injection pharmaceutical composition can be used for treating a few of autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus, can be used for treating various diseases caused by a low cellular immune function, and can also be used for tumor treatment, especially adjuvant treatment of tumors. The immunomodulator thymopentin powder injection pharmaceutical composition has an excellent technical effect.
Owner:北京元延医药科技股份有限公司
Prescription and preparation method of thymopeptide-5 injection
InactiveCN102380088AEasy to manufactureImprove stabilityPeptide/protein ingredientsDigestive systemDiseaseImmunologic disorders
The invention provides a prescription and a preparation method of a thymopeptide-5 injection for treating chronic disease toxic hepatitis, tumor, and other immunity diseases. The injection is prepared through taking thymopeptide-5 as an active ingredient, and adding injection-use auxiliary materials and injection-use water. The invention aims to overcome the shortages of pharmaceutical preparations taking thymopeptide-5 as the active ingredient, and provides the prescription and the preparation method of the thymopeptide-5 injection. The thymopeptide-5 injection provided by the invention has stable stability and convenience in preparation, reduces contamination rate during the use of medication, provides great convenience to the use of the medication, and improves the safety of the medication and adaptability of patients.
Owner:双鹤药业(海南)有限责任公司
Fmoc-strategy solid-phase synthesis method of thymopentin
InactiveCN103709233AChemically stableEasy to transportPeptide preparation methodsBulk chemical productionSide chainThymopentin
The invention discloses an Fmoc-strategy solid-phase synthesis method of thymopentin. The method comprises the following steps: taking Fmoc protected amino acids as the monomers and sequentially connecting the amino acids to a resin, wherein while synthesizing pentapeptide, the solvent of the added amido acid Fmoc-Arg(Pbf)-OH solution is the mixed solution of DMF (Dimethyl Formamide) and THF (Tetrahydrofuran); depriving all Fmoc protecting groups by using a piperazine solution, and finally, removing the resin and the side chain protecting groups to obtain the thymopentin. According to the invention, the piperazine is taken as the deprotection agent for depriving Fmoc in stead of the traditional pyridine, and the piperazine is a substance unregulated as the dangerous chemical and the poison-making chemical, and is cheap and easy to get, and also stable in chemical properties; the piperazine is in the form of white acicular crystals at the normal temperature, while piperidine is a liquid at the normal temperature, and therefore, the piperazine is more convenient to transport and store; under the same concentration, the Fmoc depriving efficiency of the piperazine is higher than that of piperidine; and obviously, the piperazine has many advantageous in the synthesis of the thymopentin, and also has great stimulative significance in production and study of polypeptides.
Owner:HAINAN UNIVERSITY
Compsns. of thymopentin and interferon
ActiveCN1833721AEnhanced inhibitory effectSame synergistic effectPeptide/protein ingredientsAntiviralsMedicineThymopentin
A composition used to prepare the medicine for treating viral disease, especially the viral hepatitis B, and tumor is prepared from themopeptide-5 (TP-5) and an interferon. Its preparing process is also disclosed.
Owner:SINOPHARM A THINK PHARMA
Compound biological insecticide
InactiveCN105123801AGood prevention and treatment effectAvoid drug resistanceBiocideMolluscicidesGround beetleAdditive ingredient
The invention discloses a compound biological insecticide. The compound biological insecticide is prepared from, by mass, 2-6% of chenopodium oil, 6-45% of graceful jessamine herb, 10-35% of taro rhizomes, 5-40% of skunk bugbane, 10-30% of taro stalks, 4-6% of solanine, 1-5% of thymopentin and 2-6% of emulsifying agent. Compared with the prior art, the compound biological insecticide has the following advantages that effective ingredients of multiple plants are compounded, supplement each other, have synergistic effect, have obvious prevention and control effect on aphids, beetles and other worms, the effect can be improved according to the insect pest situation, and the problem that the same insecticide is used for a long period of time and accordingly insecticide resistance is produced is avoided. Due to the fact that all the ingredients exist naturally, the compound biological insecticide can be naturally degraded after a long period of time, no residue exists on crops or no health problem is directly caused.
Owner:界首市保华种植专业合作社
Thymopentin oral microsphere preparation and preparation method thereof
ActiveCN101721677AImprove oral bioavailabilityDoes not reduce clinical efficacyPeptide/protein ingredientsDigestive systemClinical efficacyMicrosphere
The invention discloses a thymopentin oral microsphere preparation and a preparation method thereof. The oral microsphere preparation comprises the following raw materials: thymopentin, gelatin, a lactide-glycolide copolymer, dichloromethane, polyvinylalcohol-124, an aprotinin or trypsin inhibitor, and sodium glycyl-cholate or deoxysodium cholate or sodium caprate. The thymopentin oral microsphere preparation has high bioavailability, can replace injection administration without reducing clinical curative effects and overcomes a lot of pains and inconvenience of patients caused by frequent injections.
Owner:北京博恩特药业有限公司
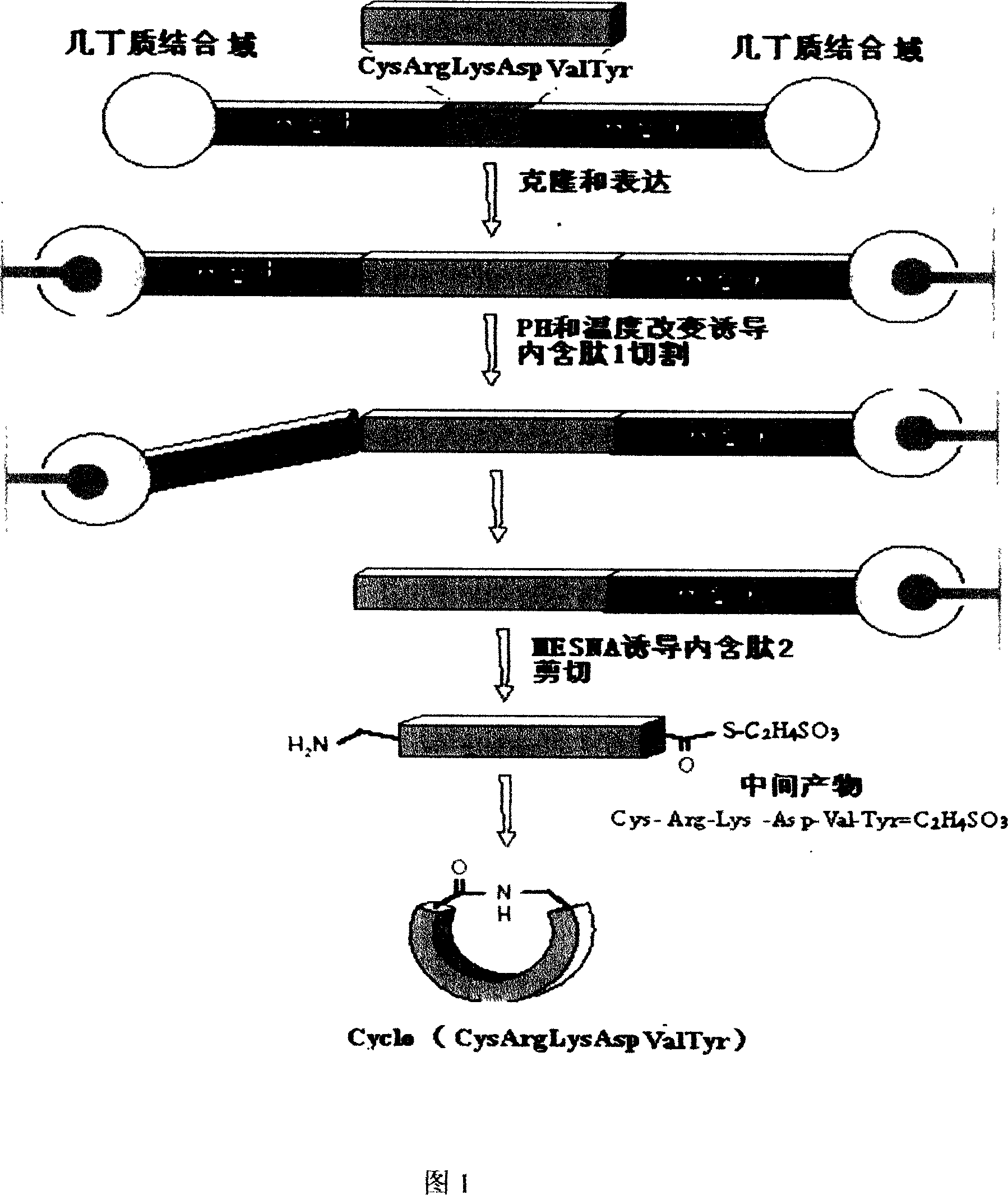
Recombinant thymus pentapeptide structural analogs, its production and use
Recombinant thymus pentapeptide structural analogs, its production, expression and use for preparing related immune diseases medicines are disclosed. The structural formula is cyclo-(Cys-Arg-Lys-Asp-Val-Tyr-), and it consists of Cys-guided circular thymic peptide and 6 amino acids. It has better biological activity and stability.
Owner:广州达信生物技术有限公司
Frontal polymerization preparation method for thymopentin molecular imprinted hydrogel
The invention relates to a frontal polymerization preparation method for a thymopentin molecular imprinted hydrogel which is prepared from the raw materials in percentage by mass: 4.33%-11.94% of thymopentin, 10.00%-36.44% of N-isopropylacrylamide, 5.88%-21.86% of acrylic acid, 0.36%-0.74% of N, N'-methylenebisacrylamide, 9.92%-33.58% of choline chloride and 30.12%-53.68% of ethylene glycol. By using the frontal polymerization preparation method, a molecular imprinted hydrogel sustained-release material is prepared by taking bioactive molecule thymopentin as a template and a green solvent DESsystem as a pore-foaming agent, and the molecular imprinted hydrogel sustained-release material serving as a drug carrier is stable in physical and chemical properties and obvious in imprinting effect; the molecular imprinted hydrogel sustained-release material is large in drug loading capacity and has an obvious sustained-release effect for the template molecule thymopentin; and compared with a thermal polymerization imprinted hydrogel, the molecular imprinted hydrogel sustained-release material is capable of prolonging the in-vivo metabolism time of a drug and increasing the biological availability of the drug.
Owner:TIANJIN MEDICAL UNIV
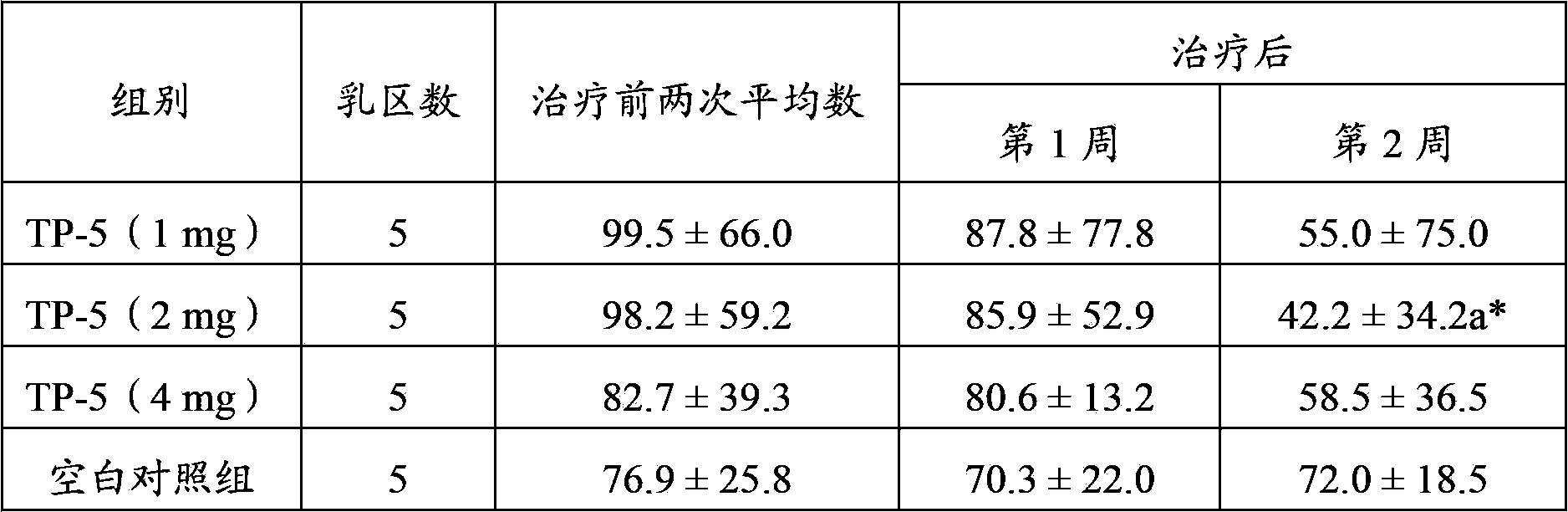
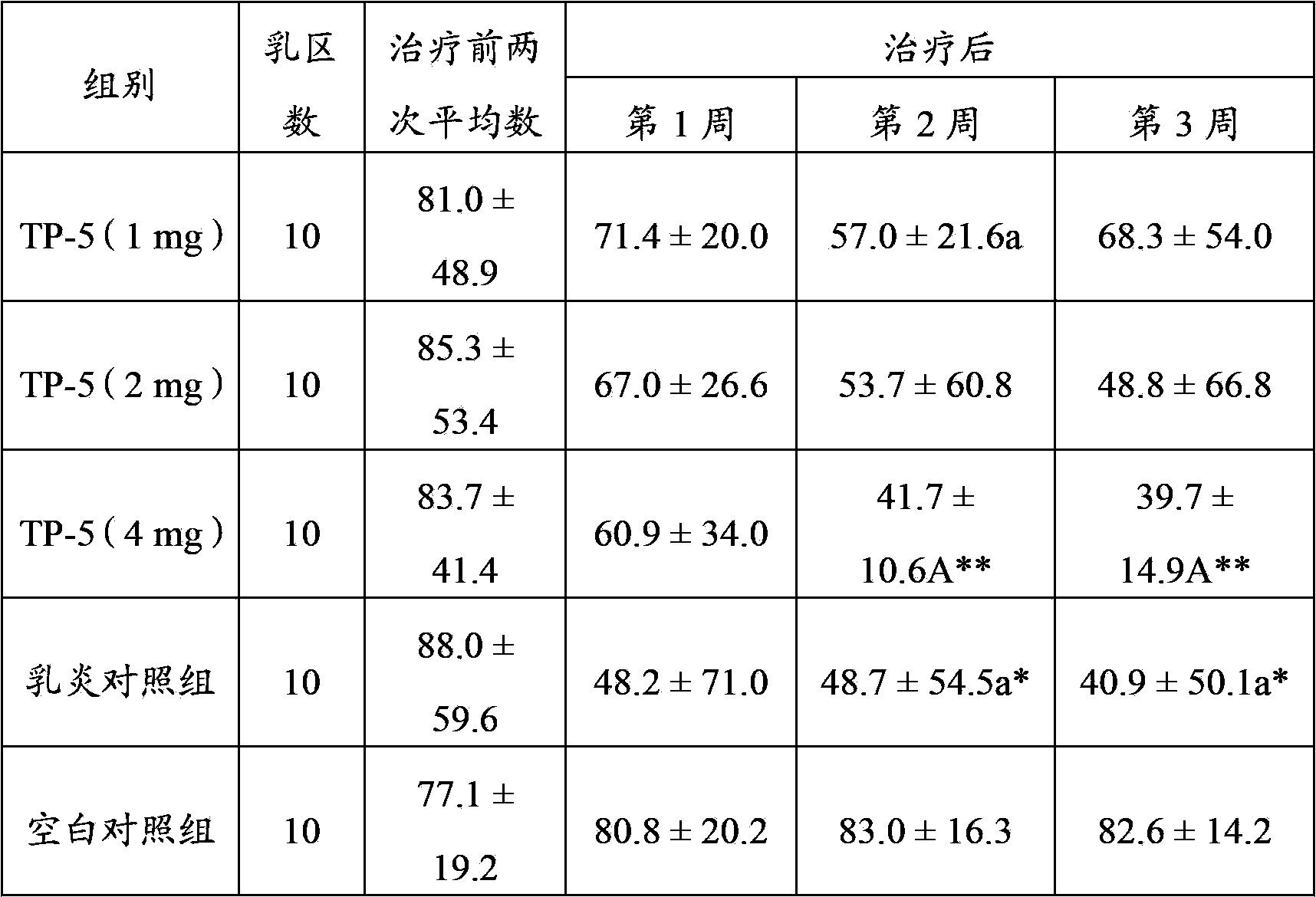
Application of thymopentin in preparing medicine used for treating mastitis
ActiveCN103386115AImproved ability to remove pathogenic bacteriaSwelling improvedPeptide/protein ingredientsSexual disorderAfter treatmentThymopentin
The invention relates to the field of medicine, and especially relates to an application of thymopentin in preparing medicine used for treating mastitis. As a result of subclinical mastitis efficacy test, infection mammary area numbers of thymopentin treatment groups, especially a high dose (4mg) group, have a reduction tendency after treatment. As a result of clinical mastitis efficacy test, through injection of thymopentin (TP-5) in breast lymph node for continuously 3 days, milk SCC of two TP-5 dose groups (10mg and 20mg) both have reduction tendencies, and cow breast swellings are substantially ameliorated in appearance. Therefore, medicines prepared with high-dose thymopentin (higher than 10mg) have significant effects against cow clinical mastitis. Therefore, the medicines prepared by using thymopentin have significant effects against subclinical mastitis and / or clinical mastitis.
Owner:ZHEJIANG HUAERCHENG PHARMA
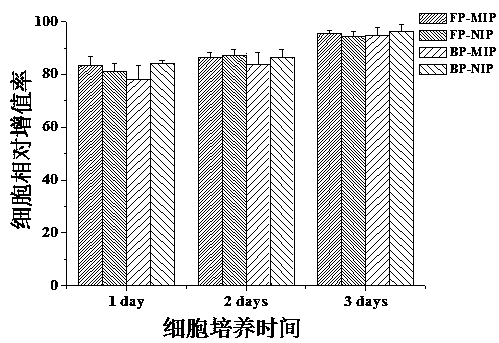
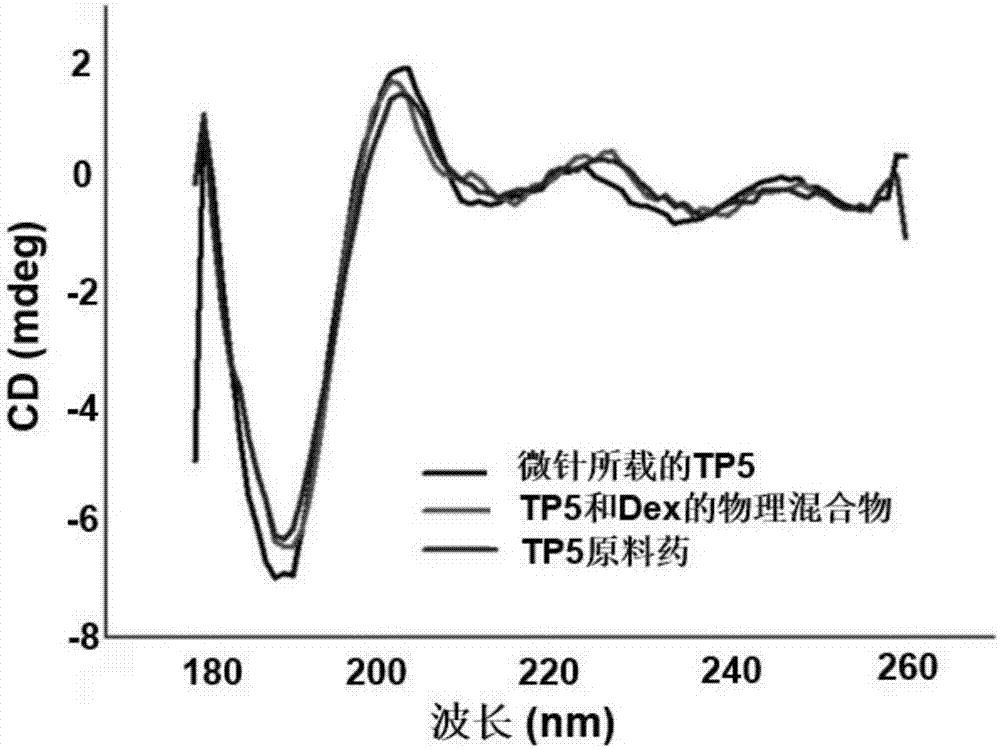
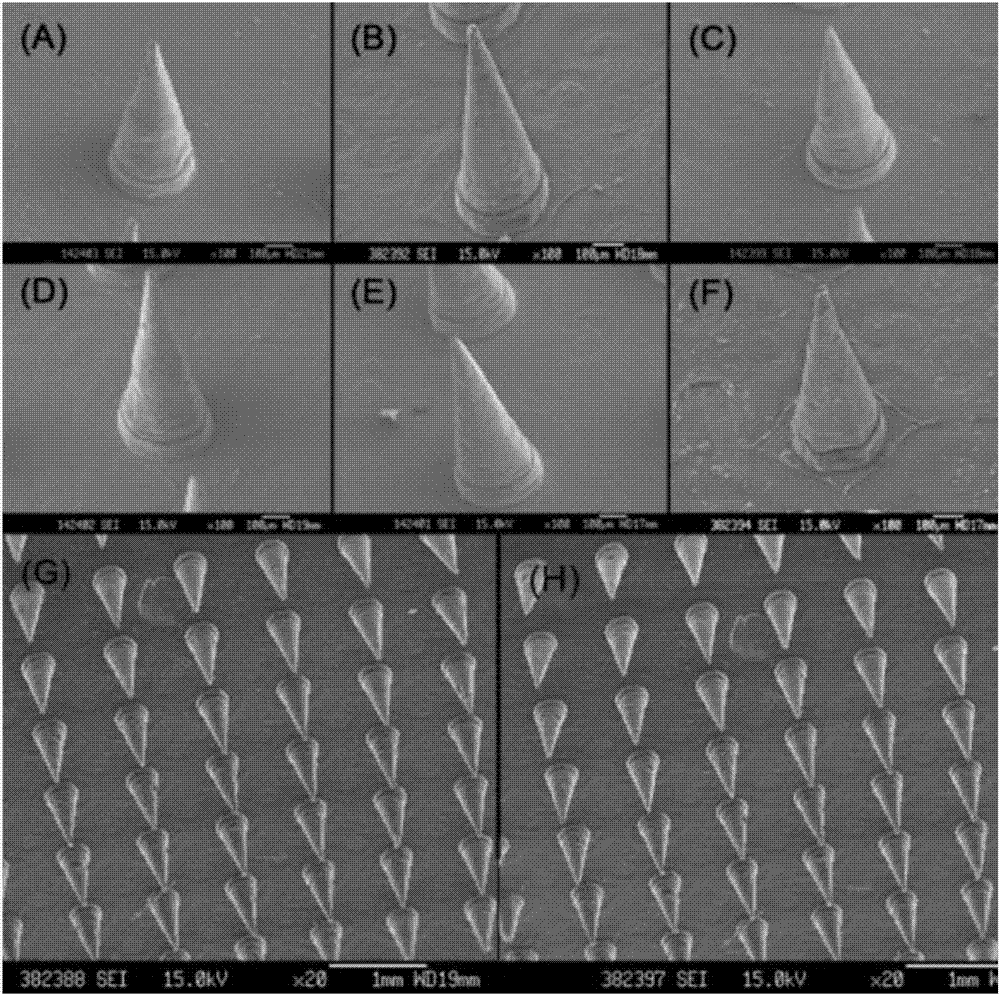
Soluble thymopentin microneedle and preparation method thereof
ActiveCN107233296AHigh drug loadingHigh drug contentPeptide/protein ingredientsMicroneedlesDrug contentPolyvinyl alcohol
The invention relates to a soluble thymopentin microneedle and a preparation method thereof. The soluble thymopentin microneedle comprises a needle tip and a substrate, wherein the needle tip is prepared from a water solution of a biodegradable material, bovine serum albumin and thymopentin in a mass ratio of 25 to (3-15) to (0.5-15); and the biodegradable material is selected from at least one of dextran, polyvinylpyrrolidone, polyvinyl alcohol, hyaluronic acid, carboxymethylcellulose and dihydrate-D-trehalose. By adding the bovine serum albumin into the needle tip material to prepare the soluble thymopentin microneedle, the mechanical strength of the needle tip can be improved, so that the problems that the needle tip of an existing soluble thymopentin microneedle is low in mechanical strength and a percutaneous drug delivery pore cannot be formed are effectively solved; and by adding the bovine serum albumin, the drug carrying quantity of a drug in the needle tip can be further increased, and the drug content of the needle tip part of the microneedle can be effectively increased.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Long-acting implantation agent of pentapeptide for thymus gland and method of producing the same
InactiveCN101130057AHigh encapsulation efficiencyLow burst dosePeptide/protein ingredientsPharmaceutical delivery mechanismThymopentinThymus Glands
The invention discloses a long-effective implantation agent of thymic pentapeptide in the drug agent domain, which is characterized by the following: adopting thymic pentapeptide as treating active material and biologically decomposed material as base; making the appearance as fine rob shape or cylinder shape; injecting or surgerying to transplant into body; releasing drug completely without surgery and fetching; obtaining high-packing rate and drug-carrying quantity to approach constant speed to release one to several months; reducing the medical cost; improving the compliance for patients. The invention has simple preparing technique and high safety, which projects he clinical treatment superiority and good applying prospect.
Owner:FUDAN UNIV
Thymopentin (TP-5) drug composition
The invention relates to a thymopentin (TP-5) drug composition and in particular relates to a drug composition. The drug composition comprises 1 part of TP-5, 10-200 parts of sugar and 0-2000 parts of optional water for injection by weight. The TP-5 drug composition can be used for treating chronic viral hepatitis such as chronic hepatitis B, various primary or secondary T cell deficiency diseases such as congenital immunodeficiency diseases in children, some autoimmune diseases such as rheumatoid arthritis and systematic lupus erythematosus, various diseases caused by cellular immune hypofunction and tumors, and can be especially used for adjuvant treatment of tumors.
Owner:CHENGDU TIANTAISHAN PHARMA
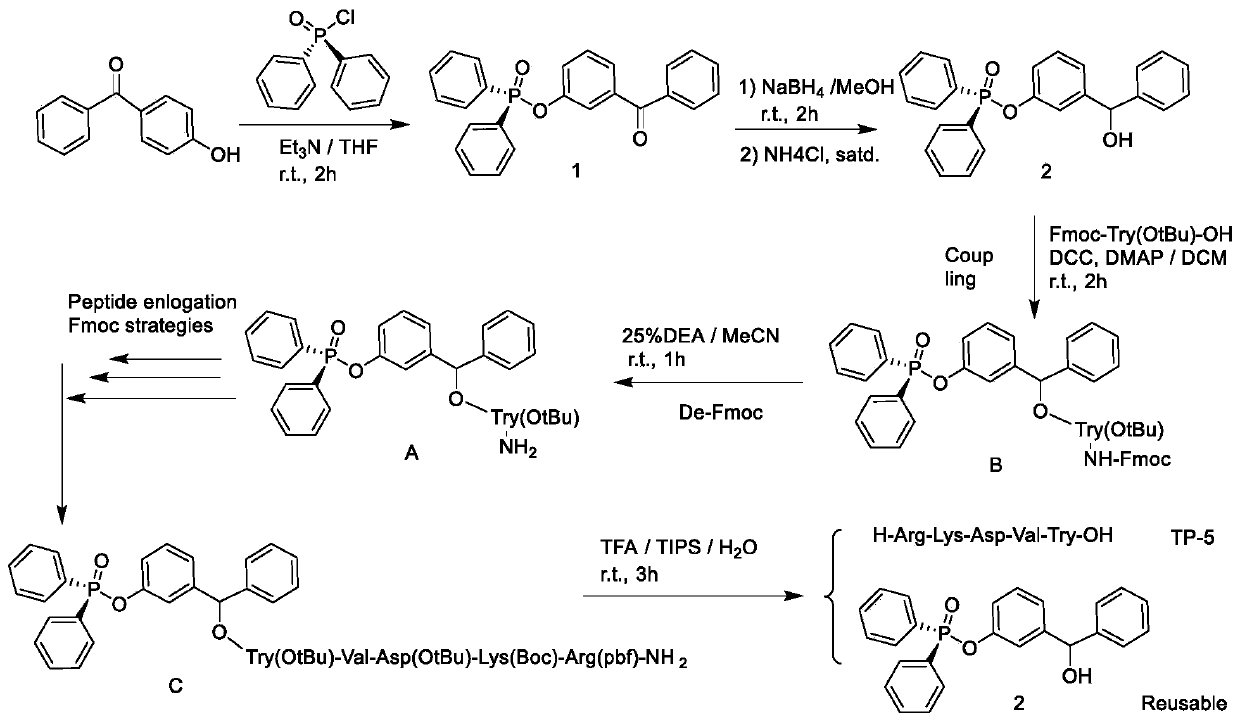
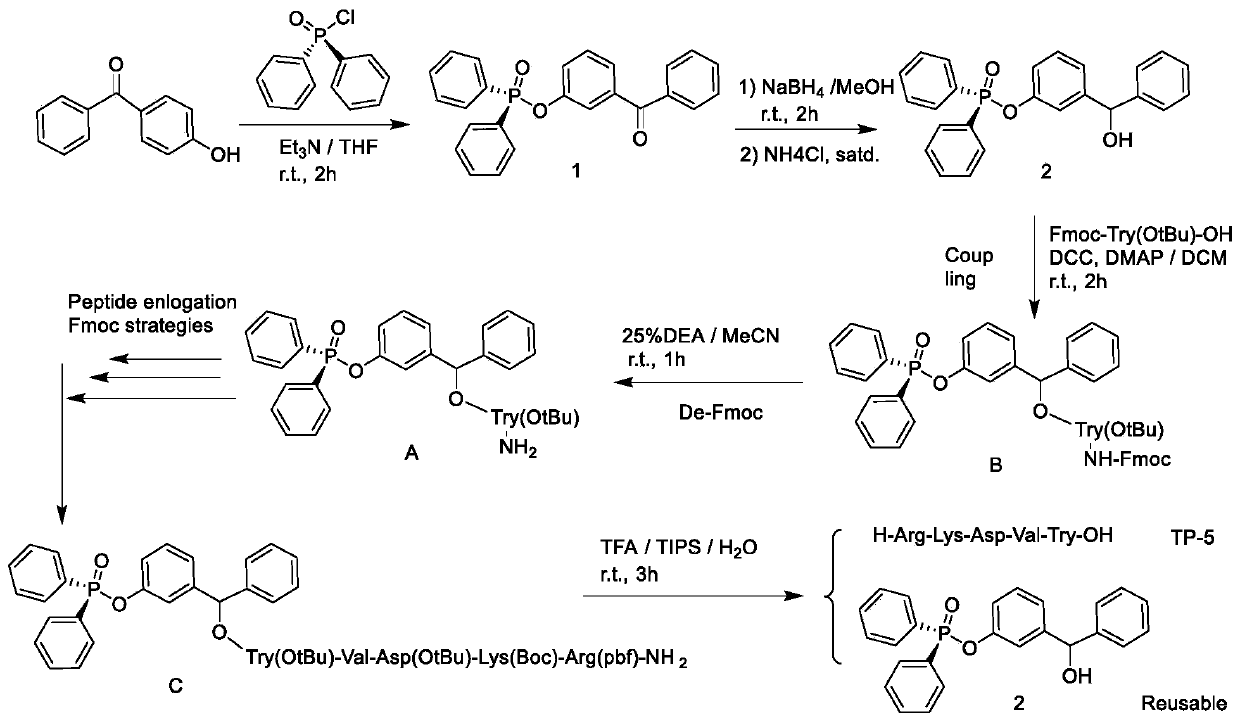
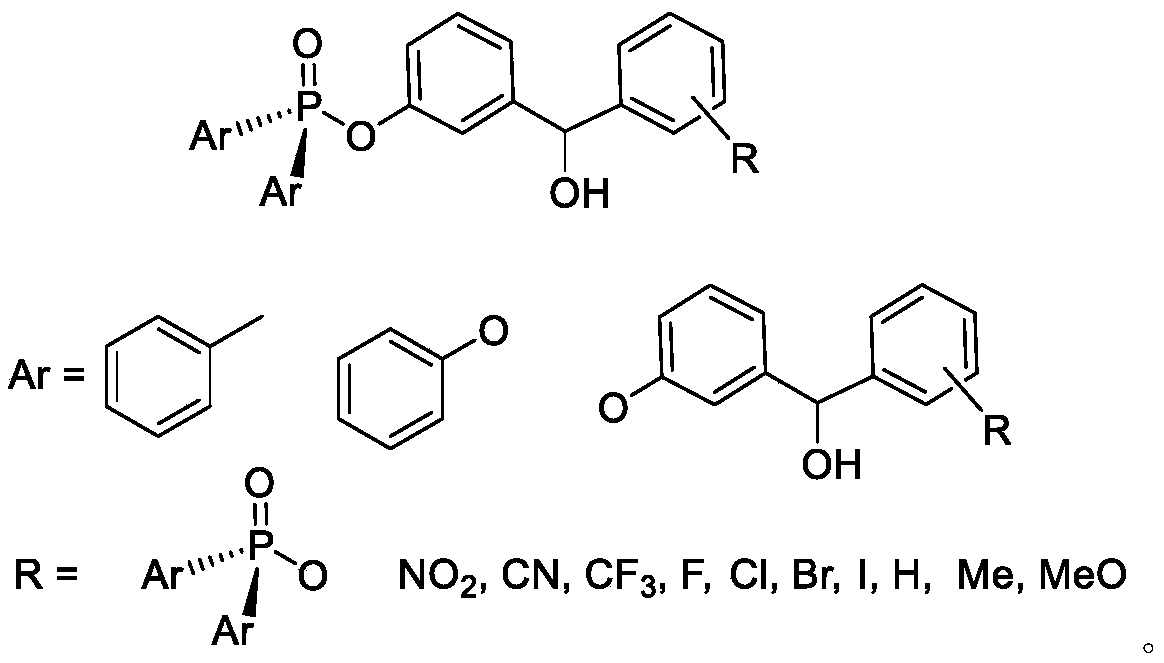
Liquid-phase synthesis method of thymopentin based on phosphorus or phosphorousoxy diphenylmethanol and derivatives and auxiliary groups
ActiveCN109836455AEfficient synthesisReduce wasteGroup 5/15 element organic compoundsPeptide preparation methodsDiphenylmethanolArginine
The invention relates to a liquid-phase synthesis method of thymopentin (TP-5) based on polyarylphosphorus or phosphoroxyphenylcarbinol (POB) compounds and their derivatives and auxiliary groups, comprising the following steps: a phosphorus or phosphite carrier replaces solid phase resin to be connected with the C- end of Fmoc-protected tyrosine under the action of a coupling dehydrant; N-end Fmocis removed after separation and purification; coupling with Fmoc, valine with side chain protection, asparaginic acid, lysine and arginine and Fmoc removal reaction are carried out to prepare a precursor C of thymopentin; and side chain is deprotected and the carrier is cleaved to obtain a solid of thymopentin. Compared with existing synthetic methods, the synthesis method of the invention has the advantages of liquid-phase and solid-phase synthesis, and can be adopted for simpler, faster, economical, efficient and large-scale synthesis of thymopentin. The POB auxiliary groups can be recovered and directly reused, raw material waste is reduced, waste pollution is lowered, costs are saved, and the method is good for environmental protection.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Process for synthesis of thymopentin
InactiveCN1844142AEasy to separate and purifyReduce usagePeptidesTert-Butyloxycarbonyl protecting groupArginine
This invention is about the synthesis of thymic-pentapeptide. Using tyrosine and its derivative as raw material, after four condensation reactions, we add valine with tertiary-butyl or tertiary-butyl ketonic oxygen group protection, aspartate, lysine, and arginine accordingly to obtain protected pentapeptide. With the method we reported here, the all protection groups could be removed once to obtain targeted products, due to our adoption of tertiary-butyl ketonic oxygen group protection strategy. The method is advantageous in simplified procedure, high yield, cost cutting and high purity.
Owner:CHONGQING HUAPONT PHARMA
Dilution for live vaccine of porcine reproductive and respiratory syndrome
InactiveCN106620689ALittle batch-to-batch variabilityImprove immunitySsRNA viruses positive-senseViral antigen ingredientsImmune effectsThymopentin
The invention belongs to the technical field of veterinary biological products, and in particular to a dilution for a live vaccine of porcine reproductive and respiratory syndrome. Every 1000ml of phosphate buffer with the pH being 7 contains the following Chinese medicine polysaccharide components: 1-8g of folium mori polysaccharide, 0.1-5g of coriolus versicolor polysaccharide, 1-5g of rchmannia glutnosa polysaccharide and 0.1-5g of thymopentin. The dilution disclosed by the invention has the following beneficial effects: on one hand, the difference between the vaccine batches can be reduced and the overall immune level can be improved; and on the other hand, the protection period of vaccine can be prolonged, and the immune effect of the live vaccine for porcine reproductive and respiratory syndrome can be greatly improved.
Owner:浙江美保龙生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com