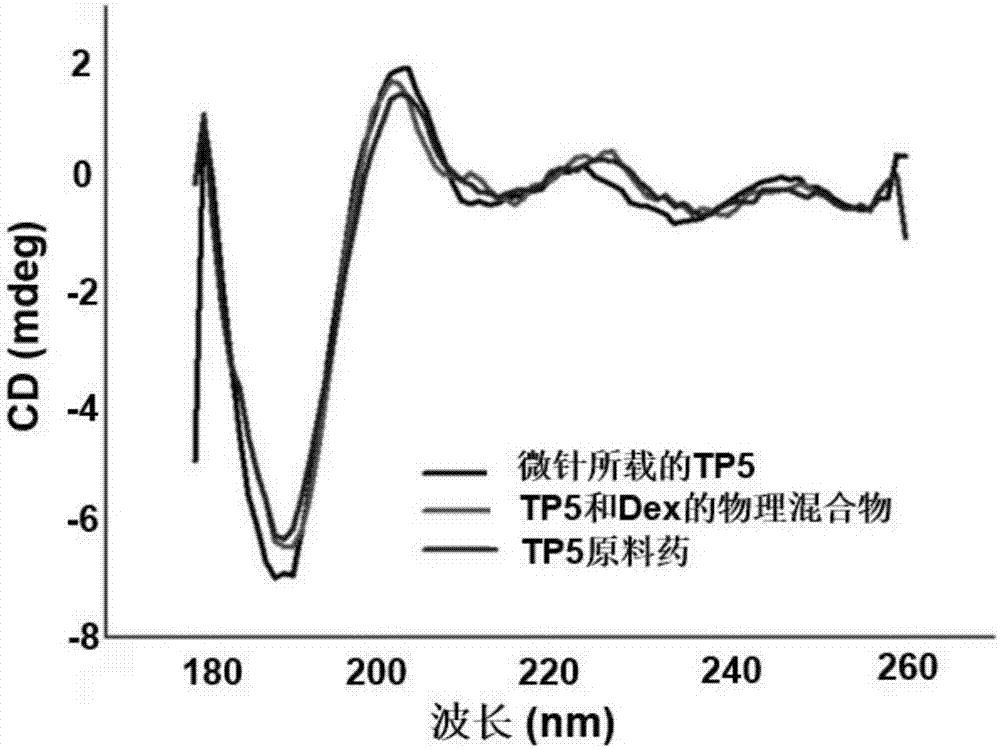
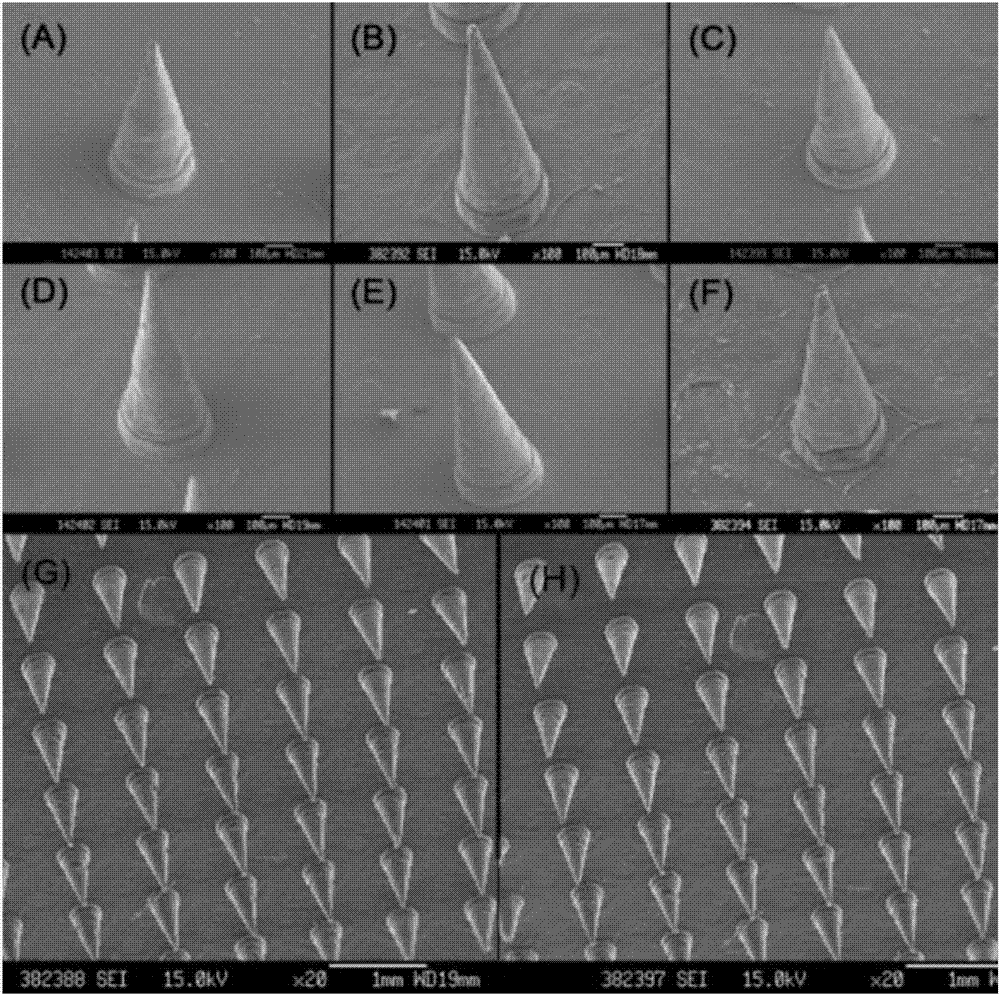
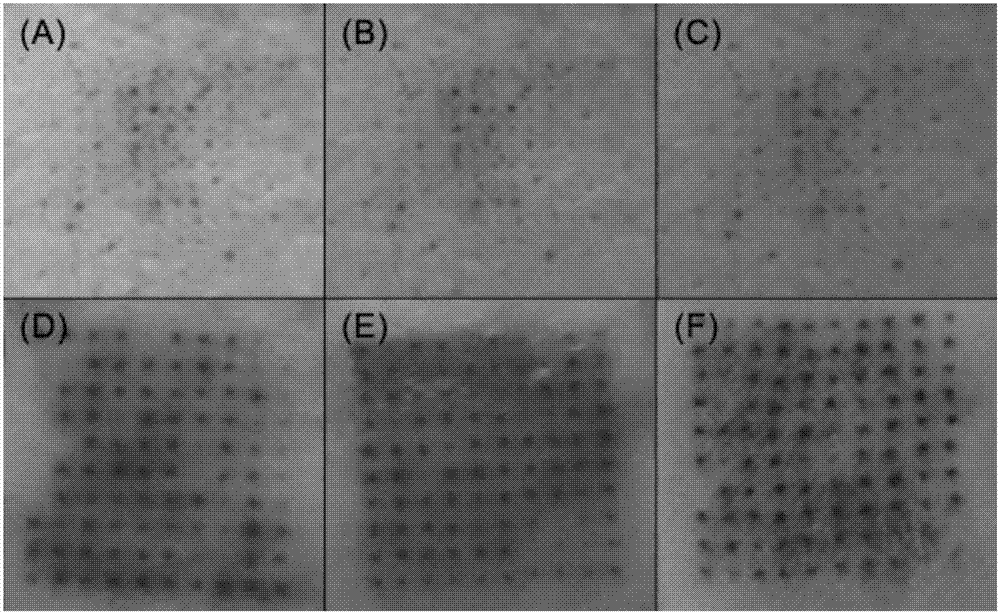
Soluble thymopentin microneedle and preparation method thereof
A soluble, thymic technology, used in microneedles, needles, pharmaceutical formulations, etc., to achieve the effects of small diameter, extended shelf life, and appropriate penetration depth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation of embodiment 1 Thymopentin soluble microneedles
[0050] This embodiment carries out the preparation of high-dose Thymopentin soluble microneedle (TP5-BSA-DMNA-H), its tip shape is conical, the height of the tip is 800 μm, the bottom of the tip is a circle with a diameter of 300 μm, adjacent The distance between the tops of the cones of the two needle tips is 900 μm, and the base is a square with a side length of 1 cm. The preparation of the soluble microneedle comprises the following steps:
[0051] 1. Preparation of needle tip solution
[0052] Precisely weigh 0.2501g of biodegradable material (Dex 40), 0.1000g of BSA, and 0.1000g of thymopentin raw drug, add 1mL of water, stir and dissolve to obtain a uniform viscous aqueous solution, namely the needle tip solution.
[0053] 2. Preparation of base solution
[0054] Accurately weigh 1.0002 g of high molecular weight polymer (PVPK90), add 2.5 mL of ethanol, stir evenly and let it swell overnight to ...
Embodiment 2
[0060] The preparation of embodiment 2 thymopentin soluble microneedles
[0061] In this example, a medium-dose Thymopentin soluble microneedle (TP5-BSA-DMNA-M) was prepared. The shape of the tip is conical, the height of the tip is 800 μm, and the bottom of the tip is a circle with a diameter of 300 μm. The distance between the tops of the cones of the two needle tips is 900 μm, and the base is a square with a side length of 1 cm. The preparation of the soluble microneedle comprises the following steps:
[0062] 1. Preparation of needle tip solution
[0063] Precisely weigh 0.2501g of biodegradable material (Dex 40), 0.1000g of BSA, and 0.0500g of thymopentin raw drug, add 1mL of water, stir and dissolve to obtain a uniform viscous aqueous solution, namely the needle tip solution.
[0064] 2. Preparation of base solution
[0065] Accurately weigh 1.0000 g of high molecular weight polymer (PVPK90), add 2.5 mL of ethanol, stir evenly and let it swell overnight to obtain the ...
Embodiment 3
[0071] Example 3 Preparation of Thymopentin Soluble Microneedles
[0072] This embodiment carries out the preparation of low-dose thymopentin soluble microneedles (TP5-BSA-DMNA-L). The distance between the tops of the cones of the two needle tips is 900 μm, and the base is a square with a side length of 1 cm. The preparation of the soluble microneedle comprises the following steps:
[0073] 1. Preparation of needle tip solution
[0074] Precisely weigh 0.2501g of biodegradable material (Dex 40), 0.1000g of BSA, and 0.0100g of thymopentin raw drug, add 1mL of water, stir and dissolve to obtain a uniform viscous aqueous solution, namely the needle tip solution.
[0075] 2. Preparation of base solution
[0076] Accurately weigh 1.0001 g of high molecular weight polymer (PVPK90), add 2.5 mL of ethanol, stir evenly and let it swell overnight to obtain the base solution.
[0077] 3. Preparation of soluble microneedle drug-containing needle tip
[0078] Take an appropriate amoun...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com