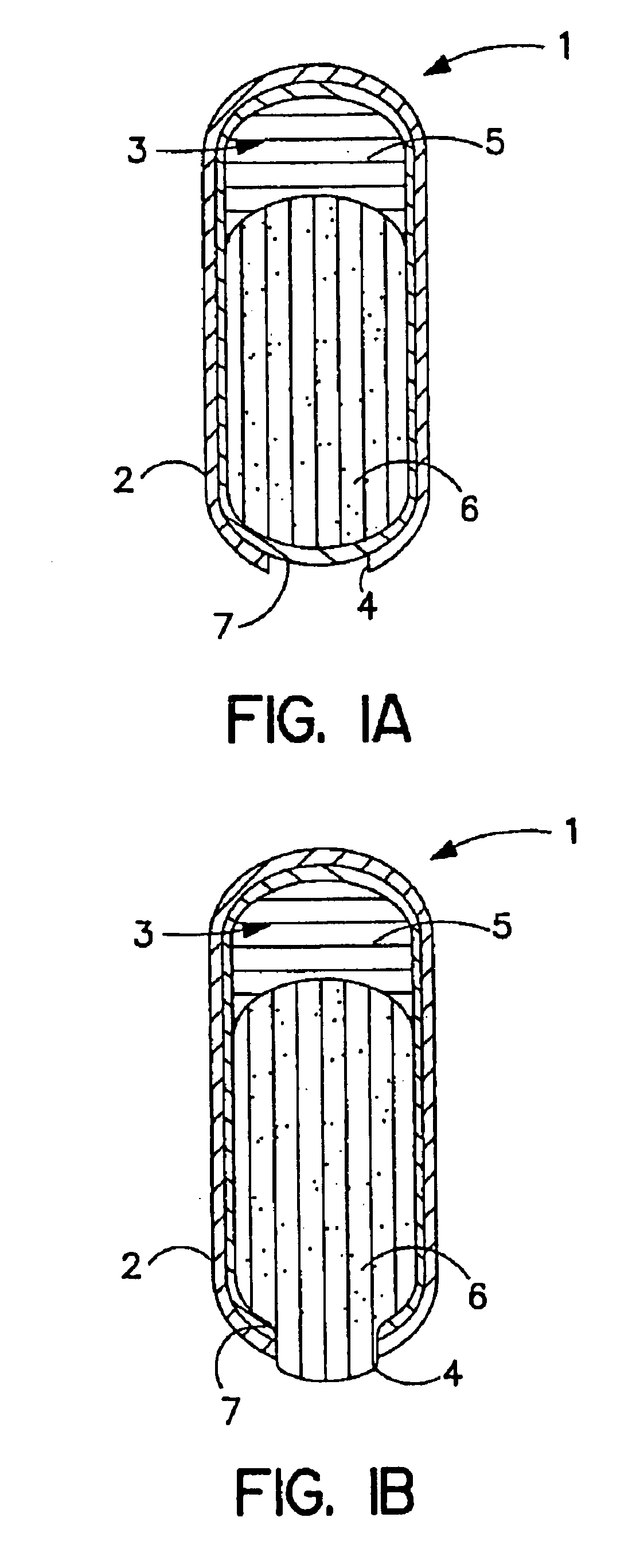
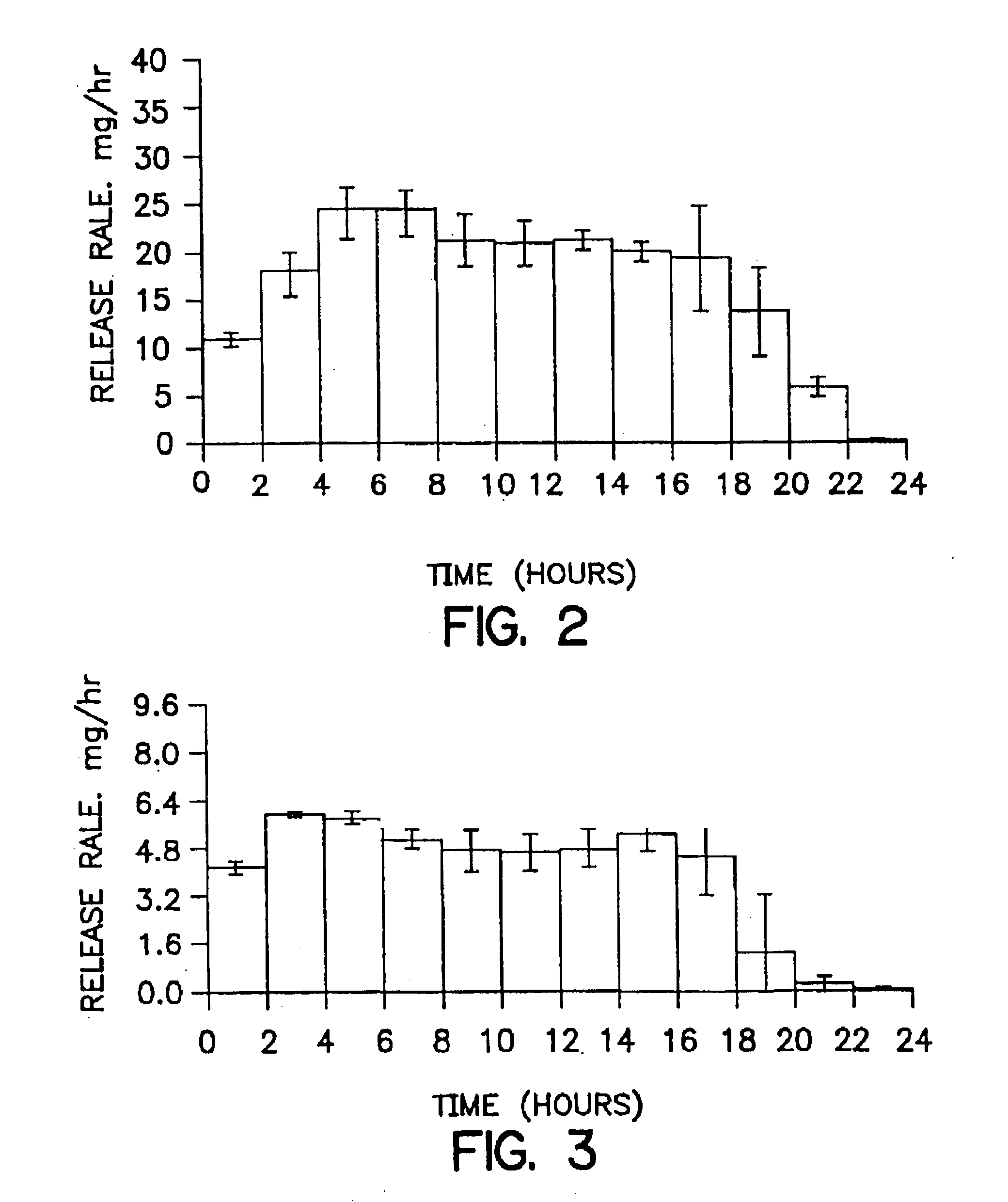
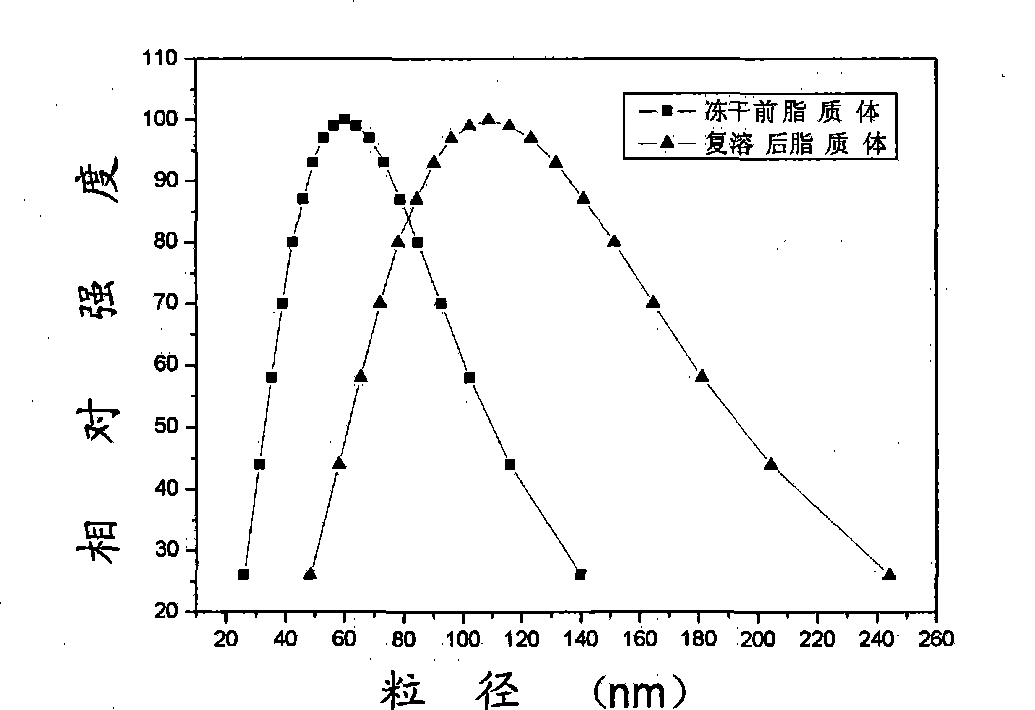
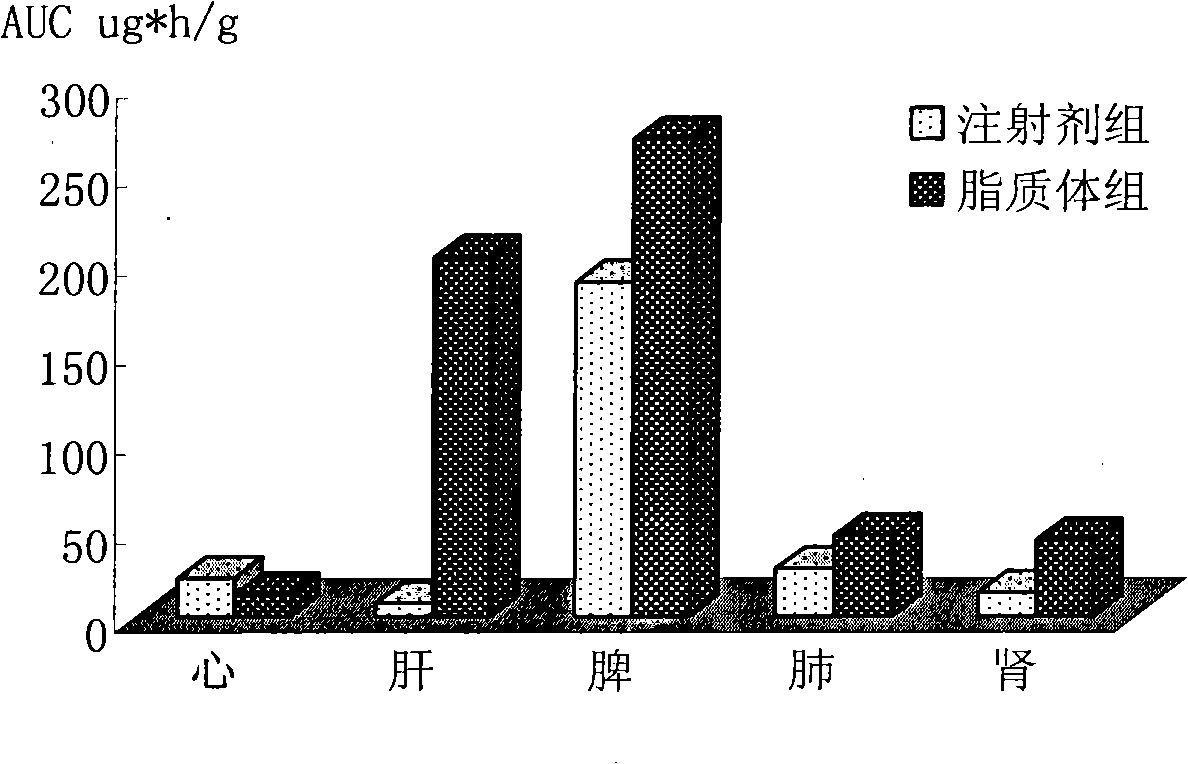
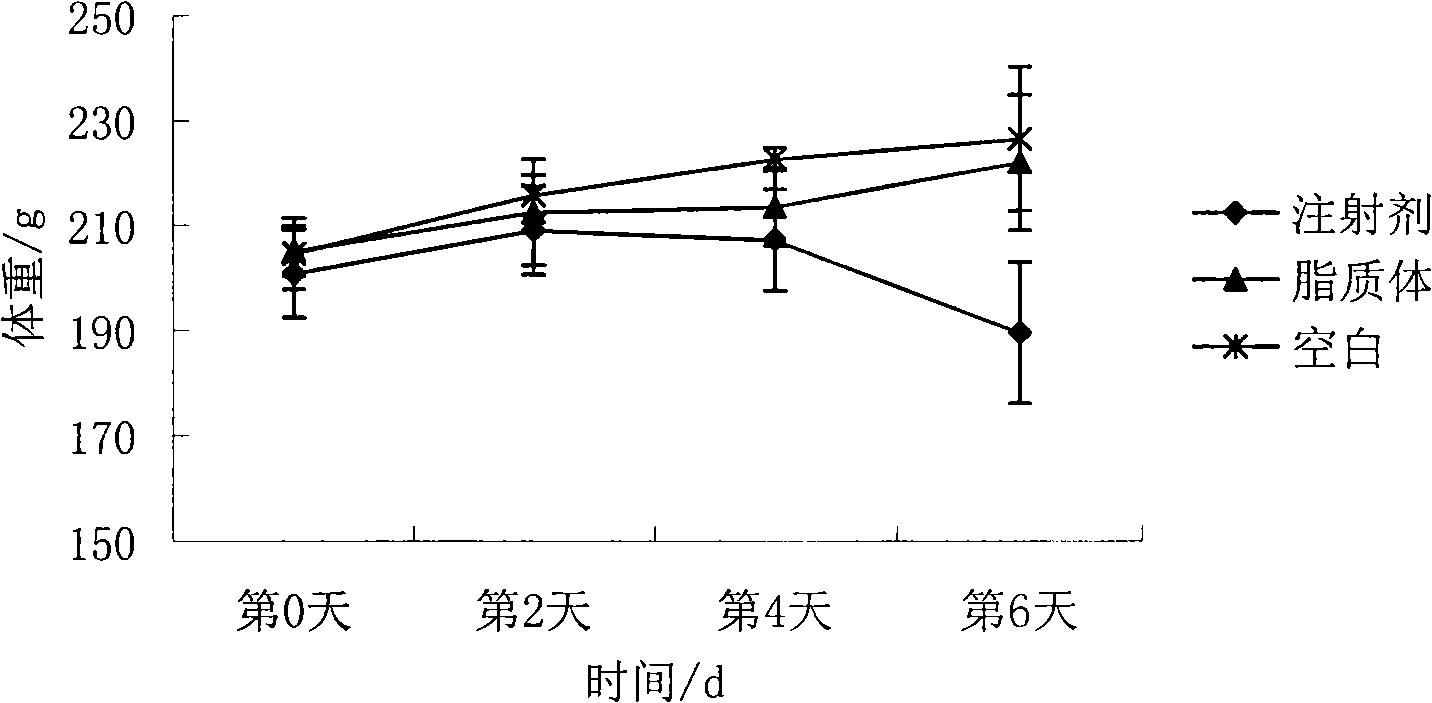
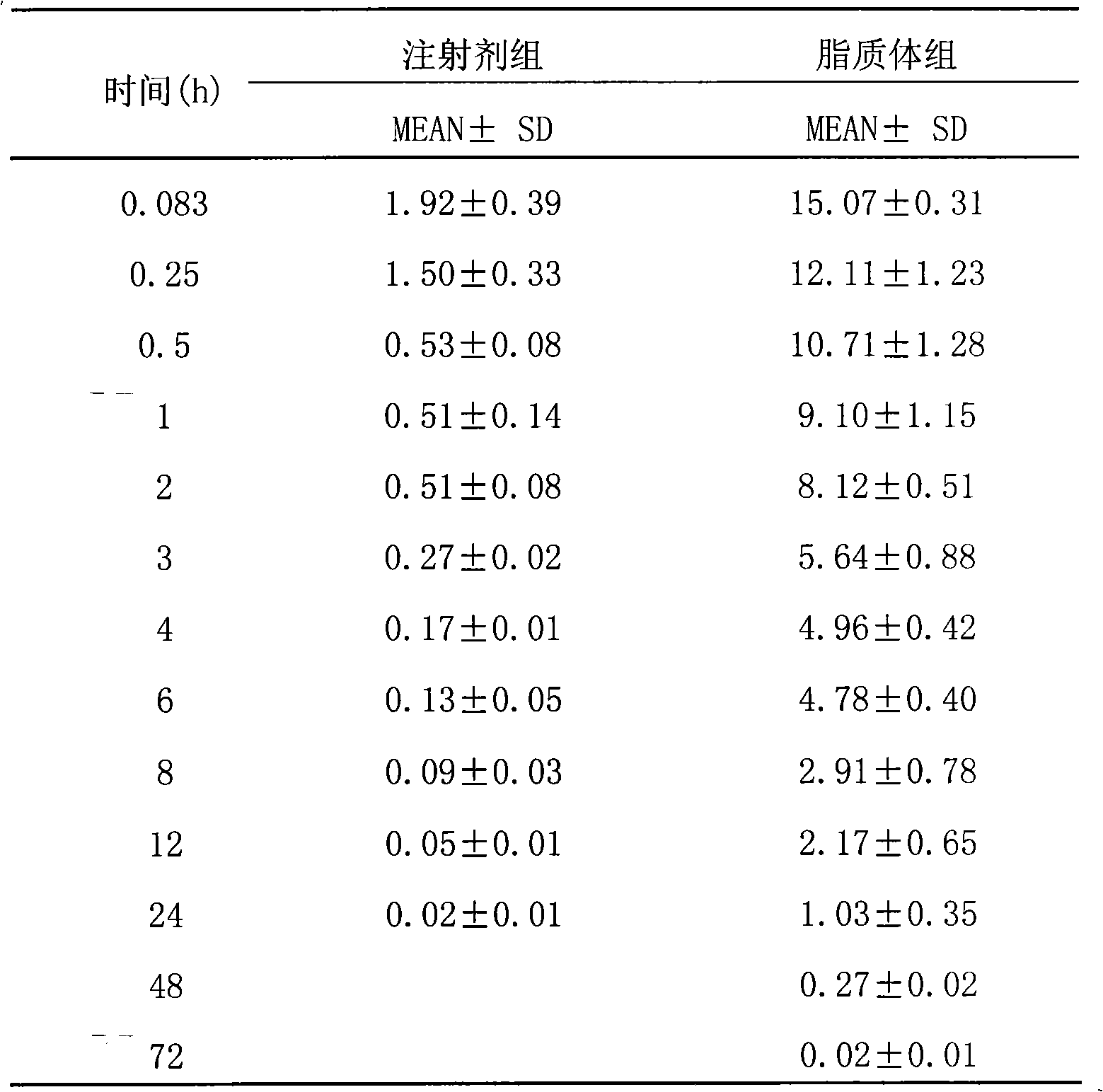
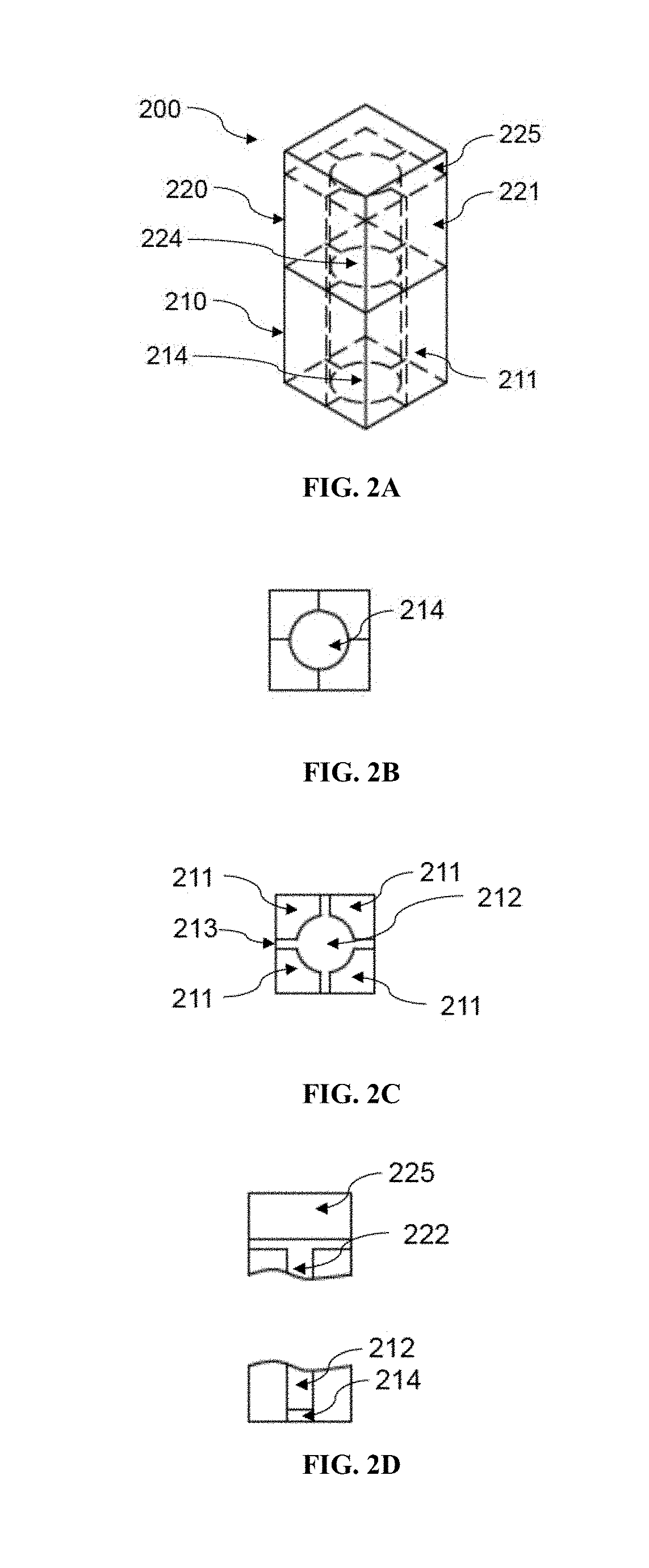
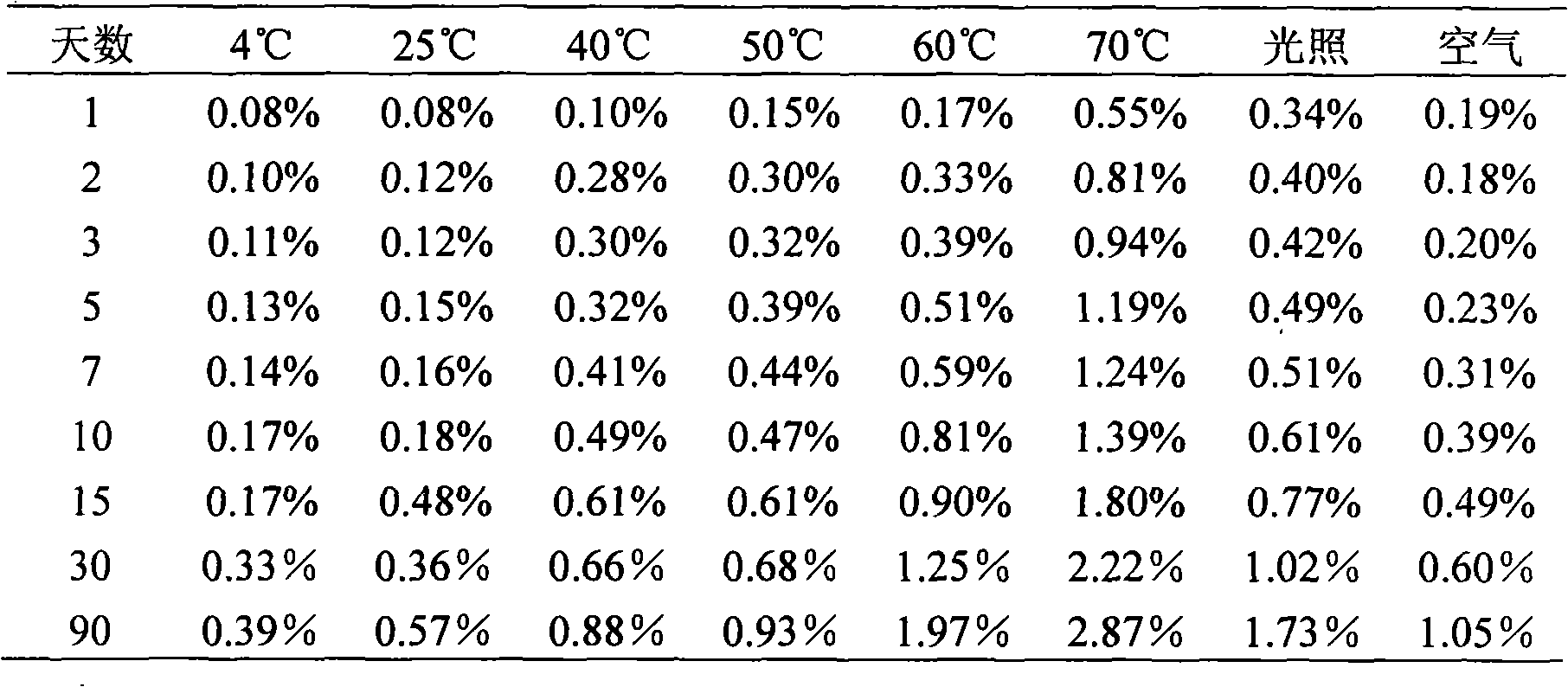
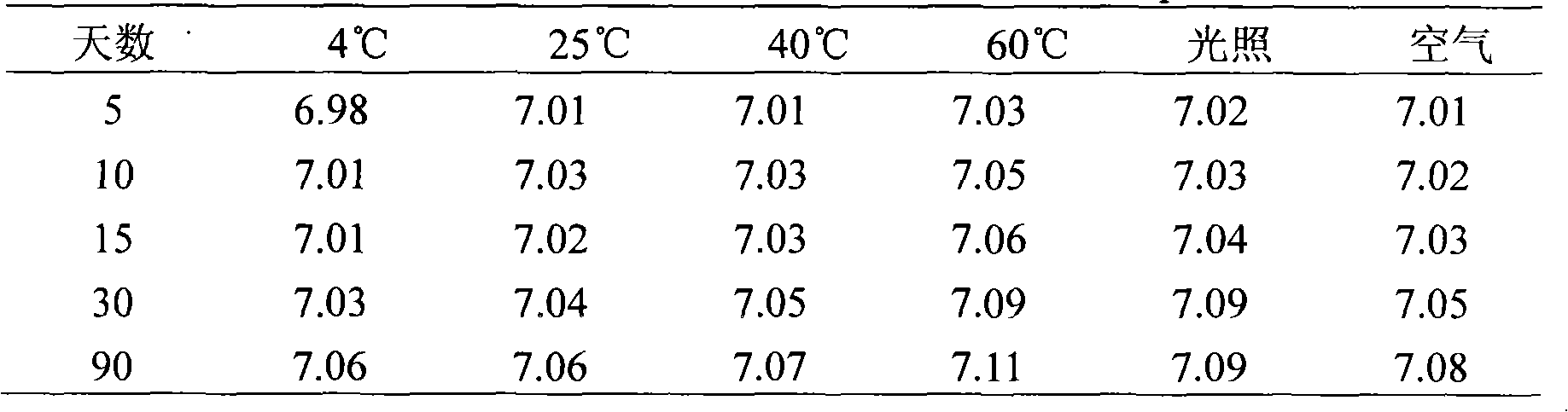
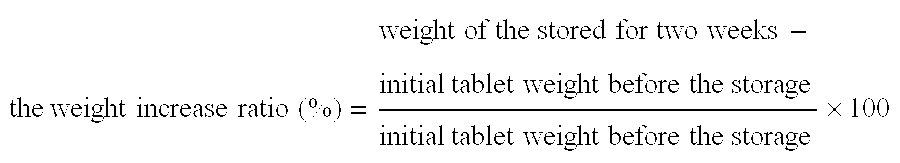
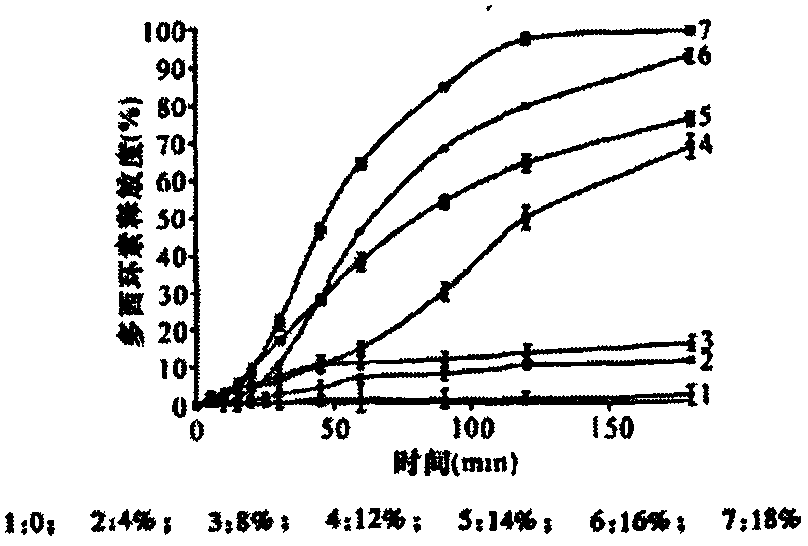
Patents
Literature
626 results about "Drug content" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
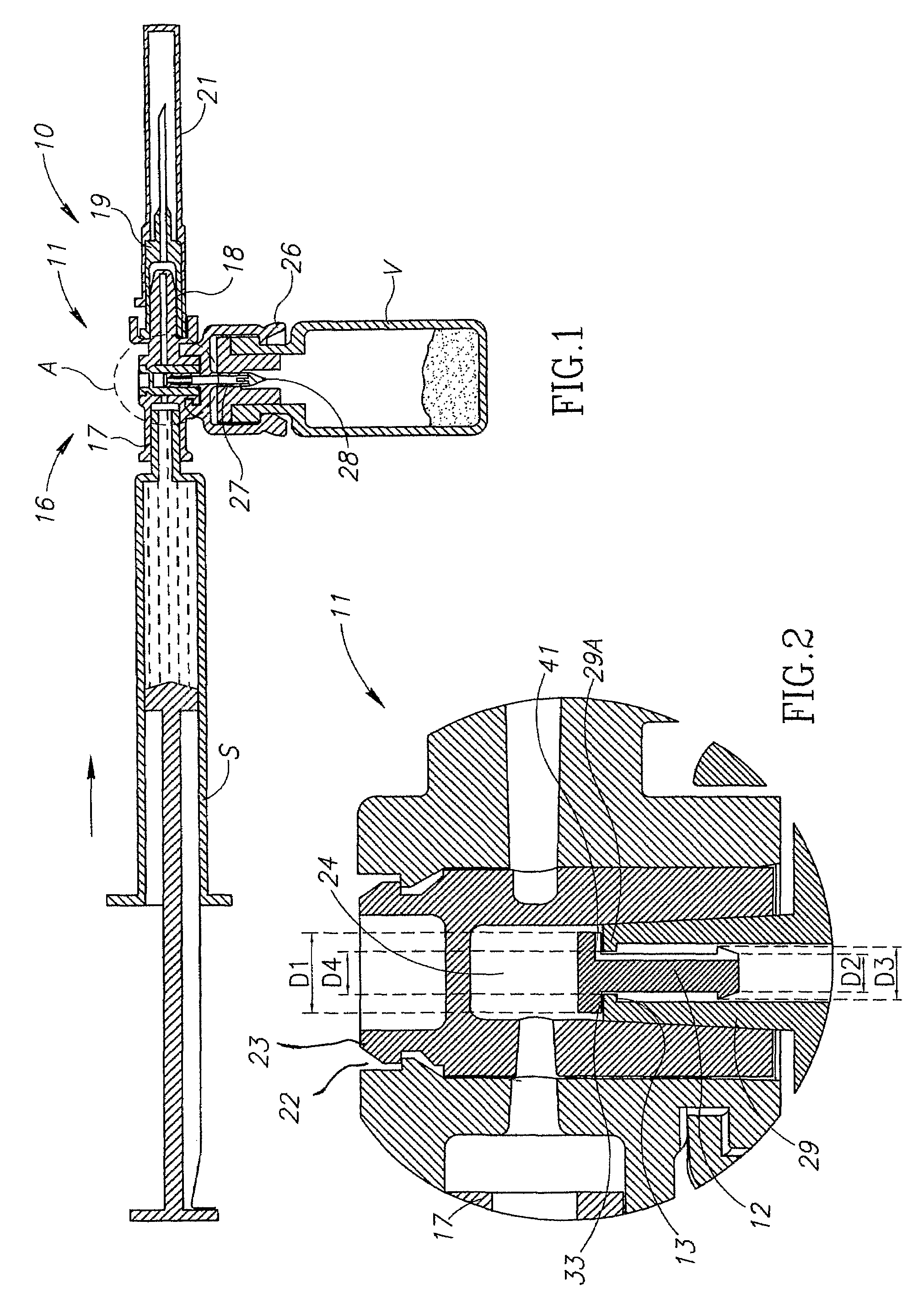
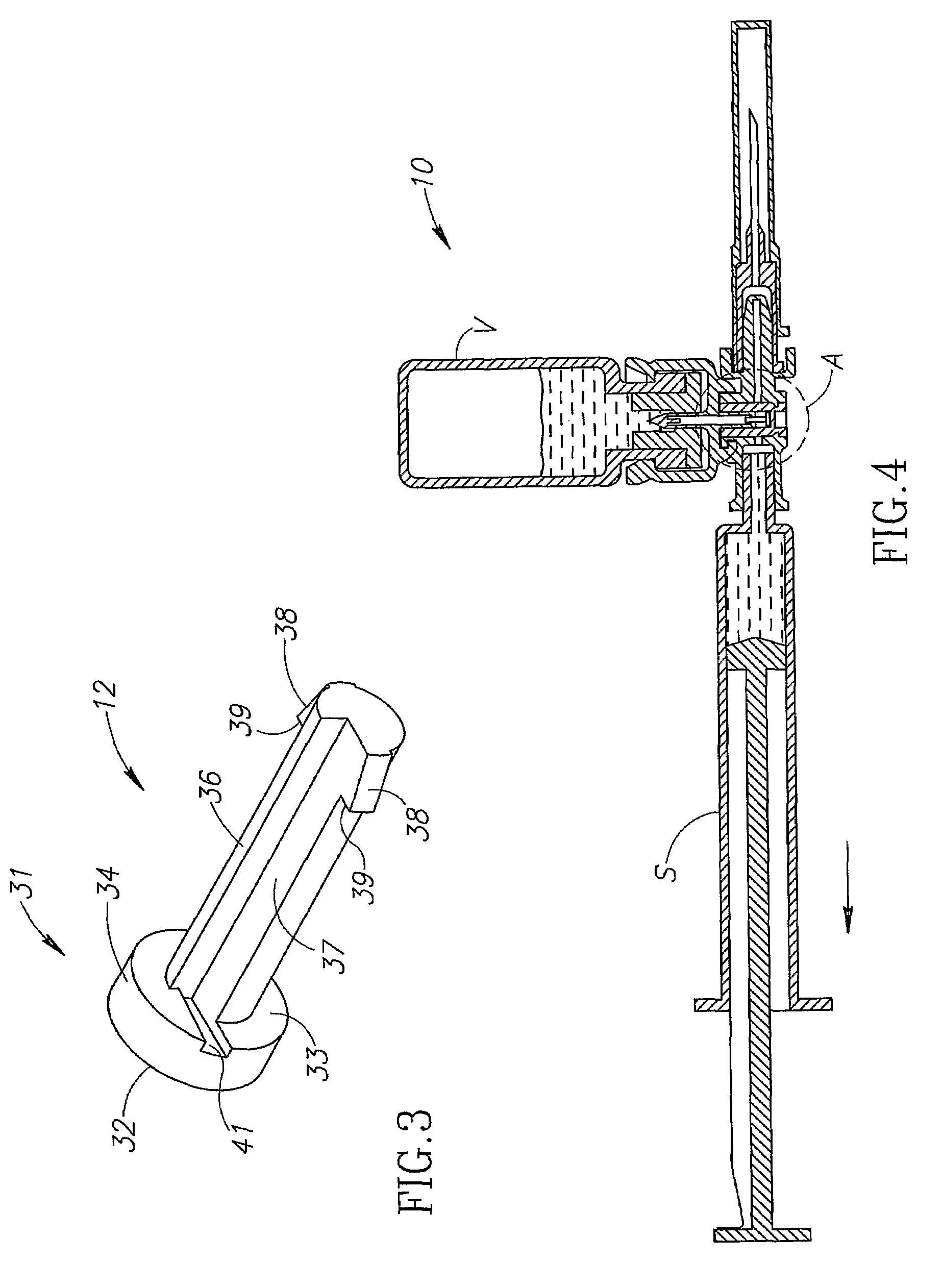
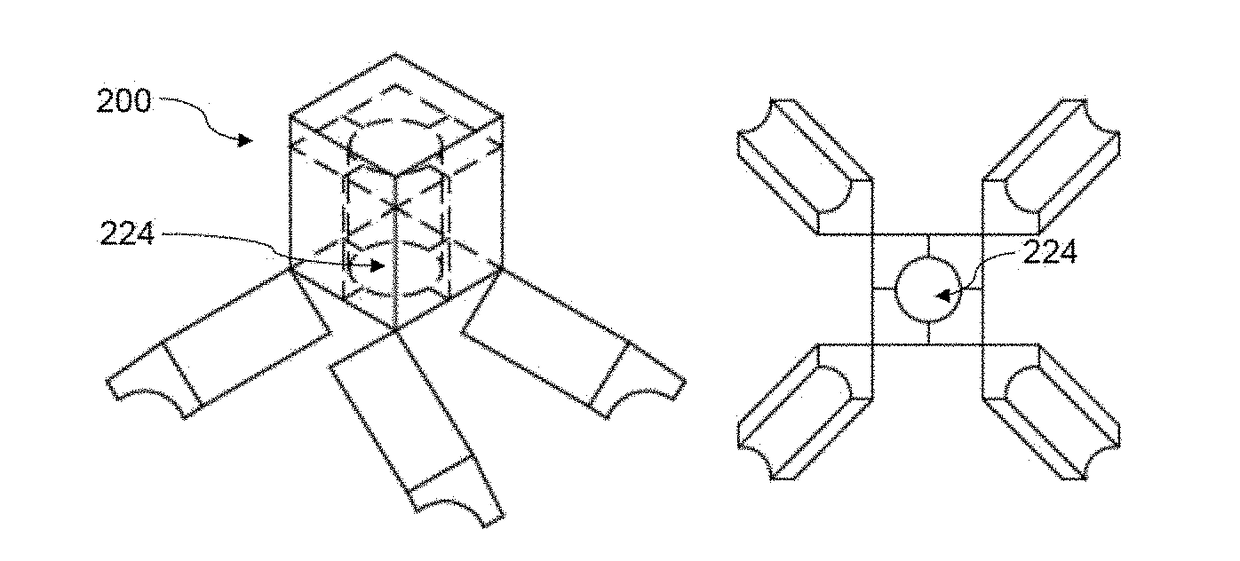
Fluid transfer assembly with venting arrangement
Owner:WEST PHARM SERVICES IL LTD
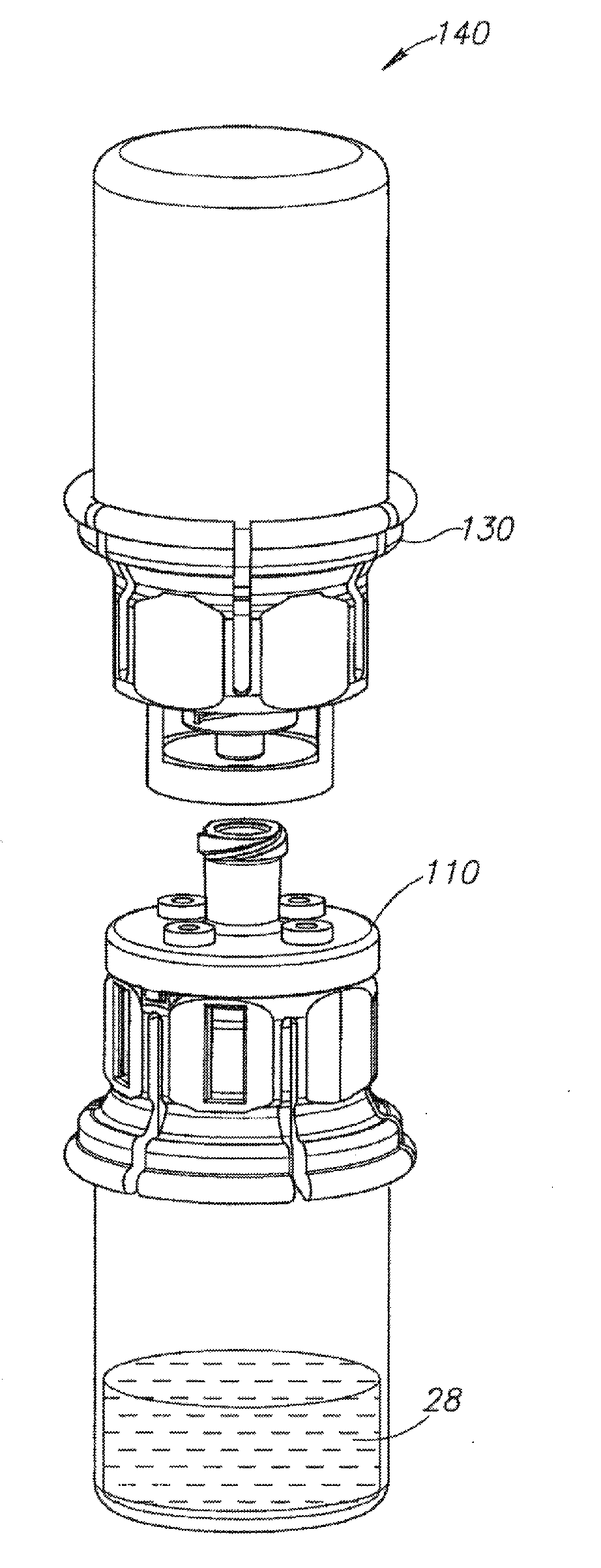
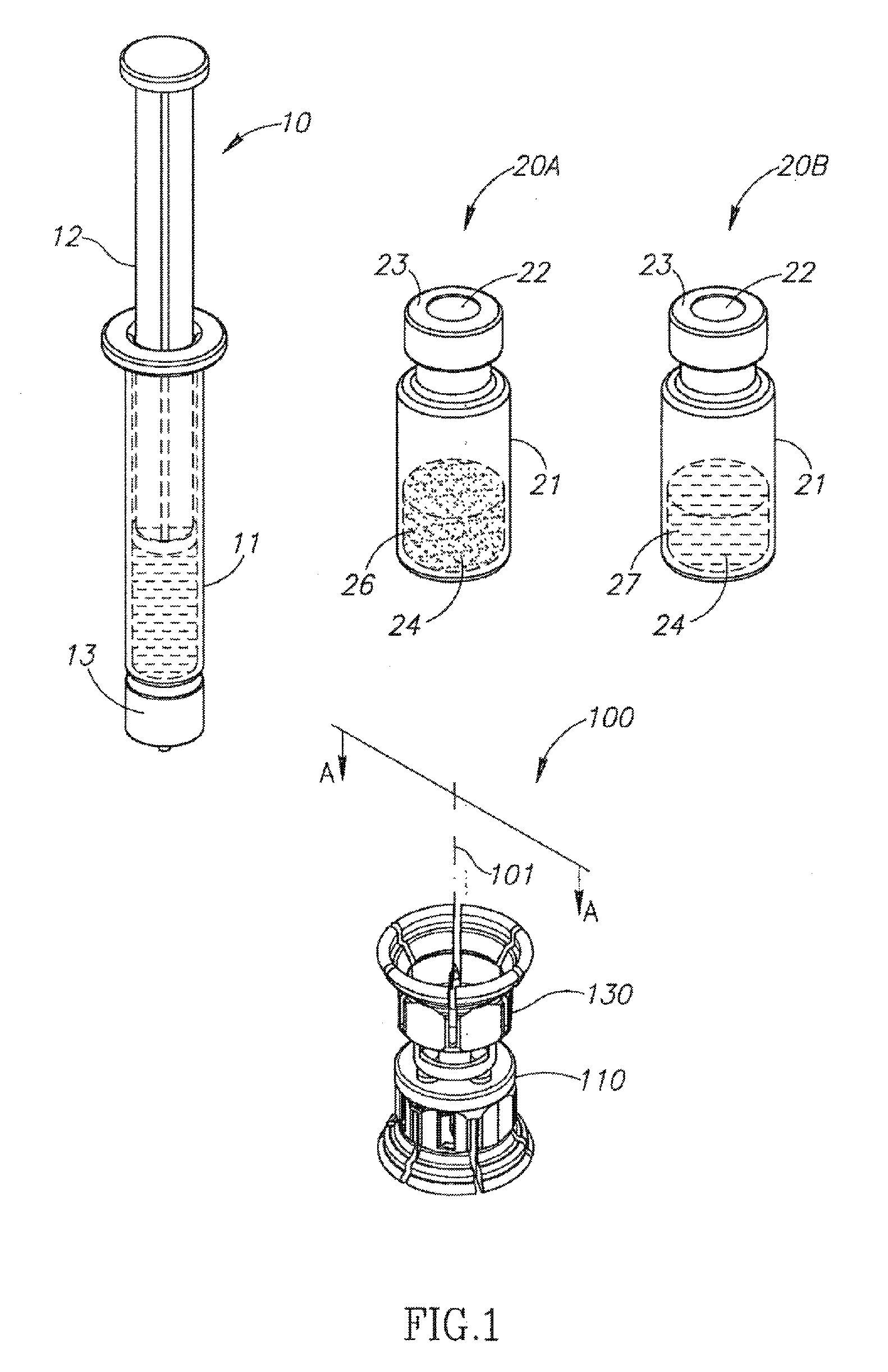
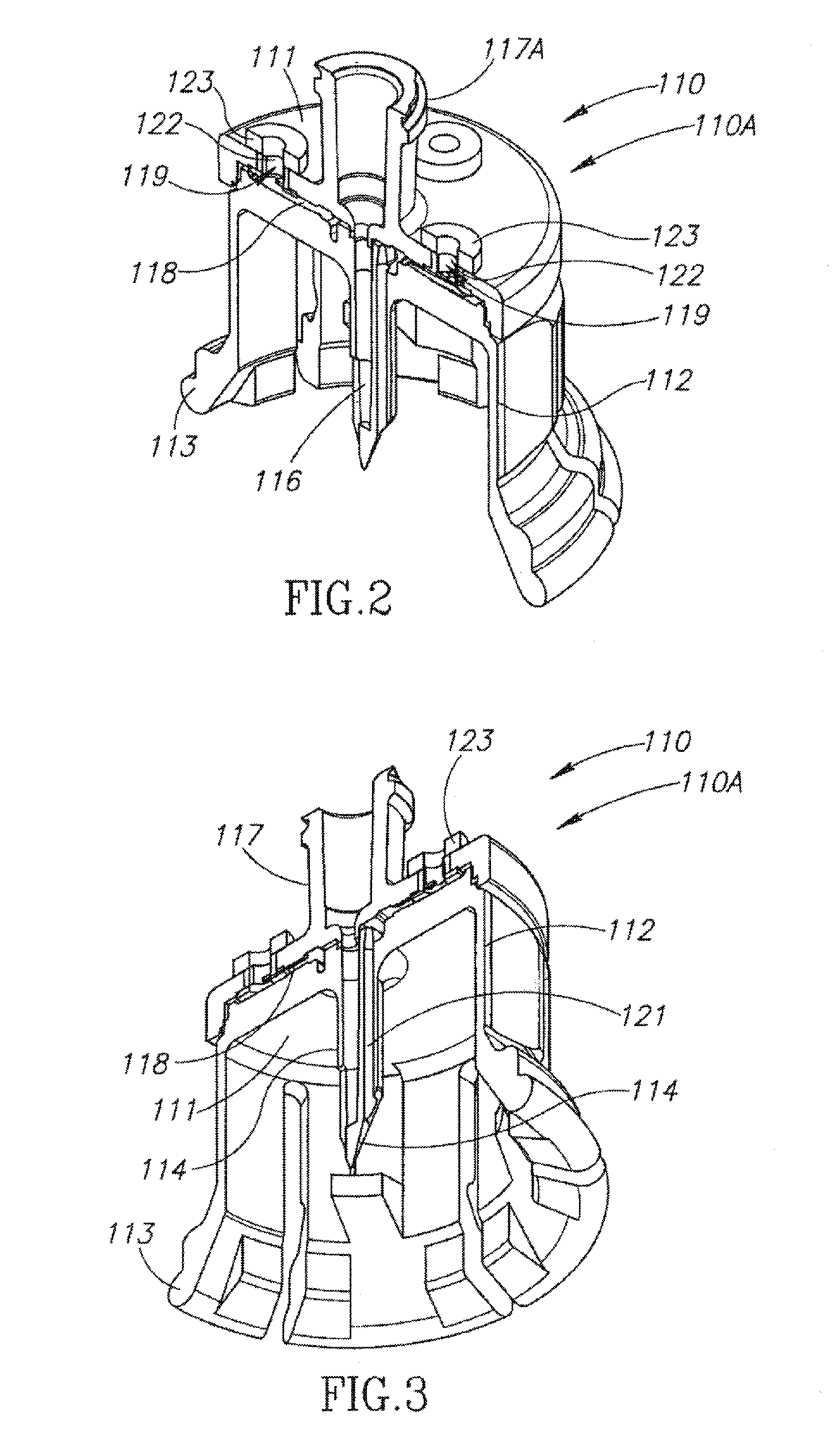
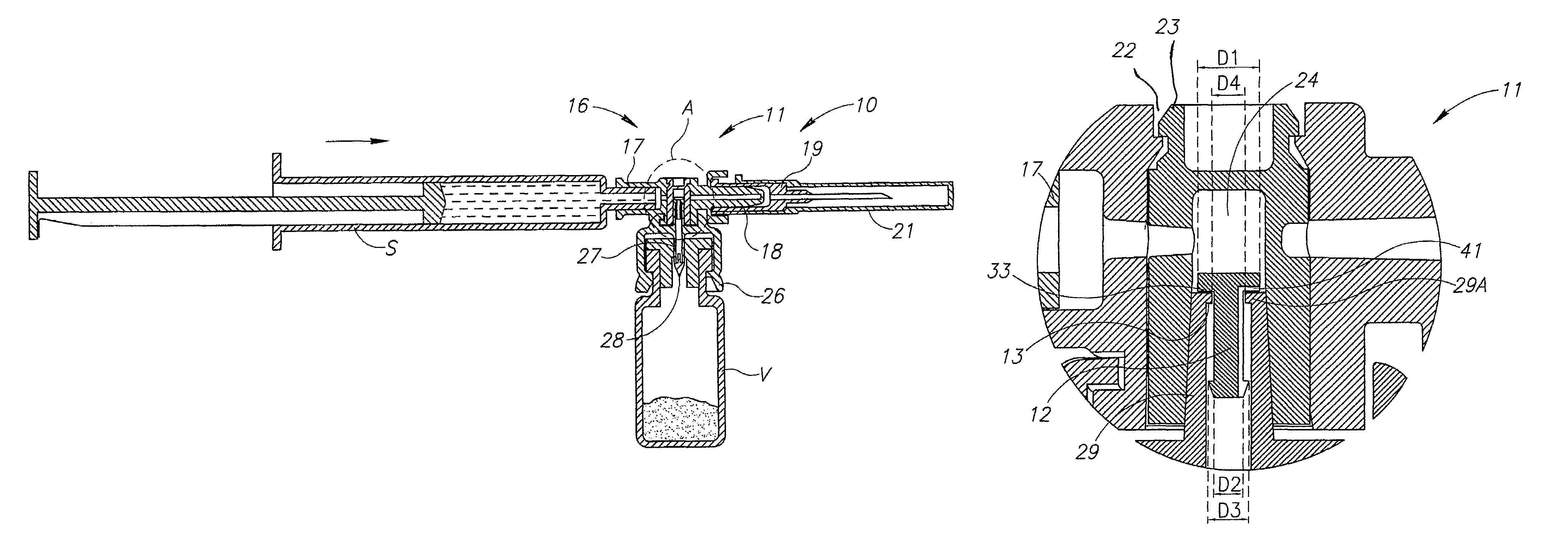
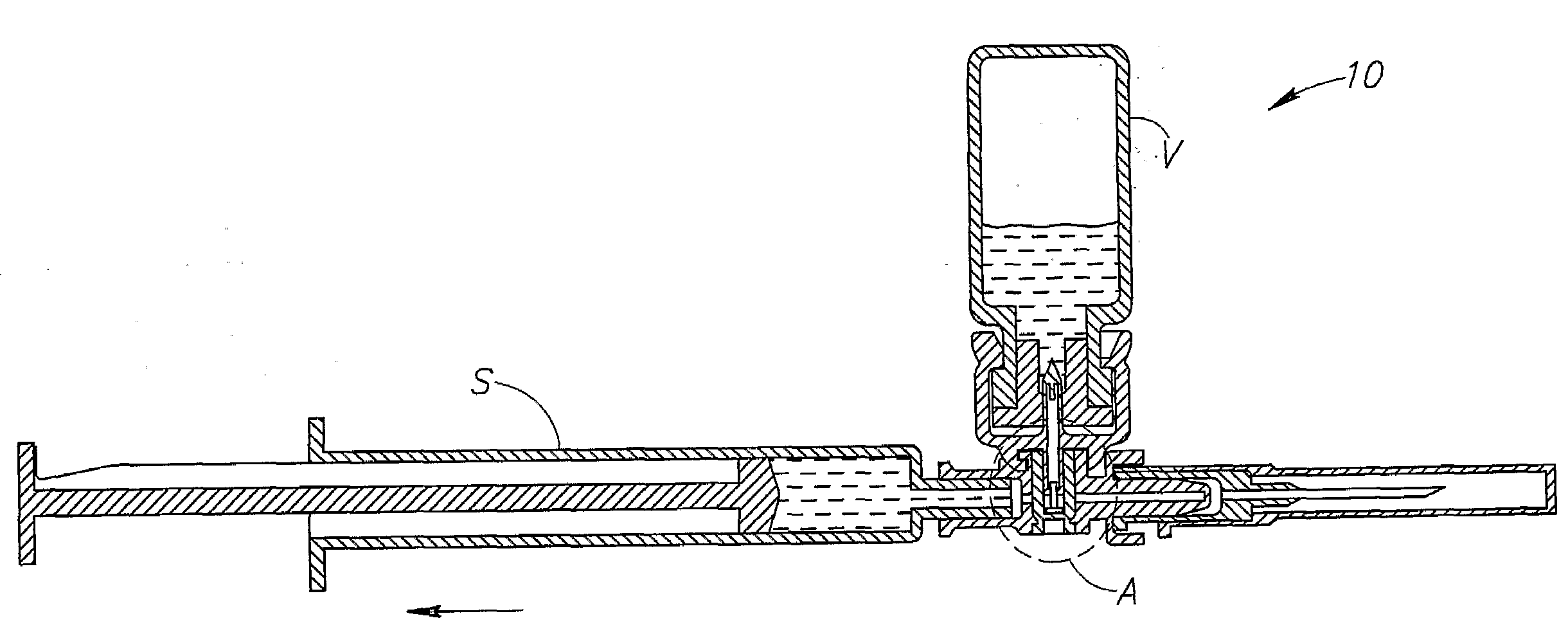
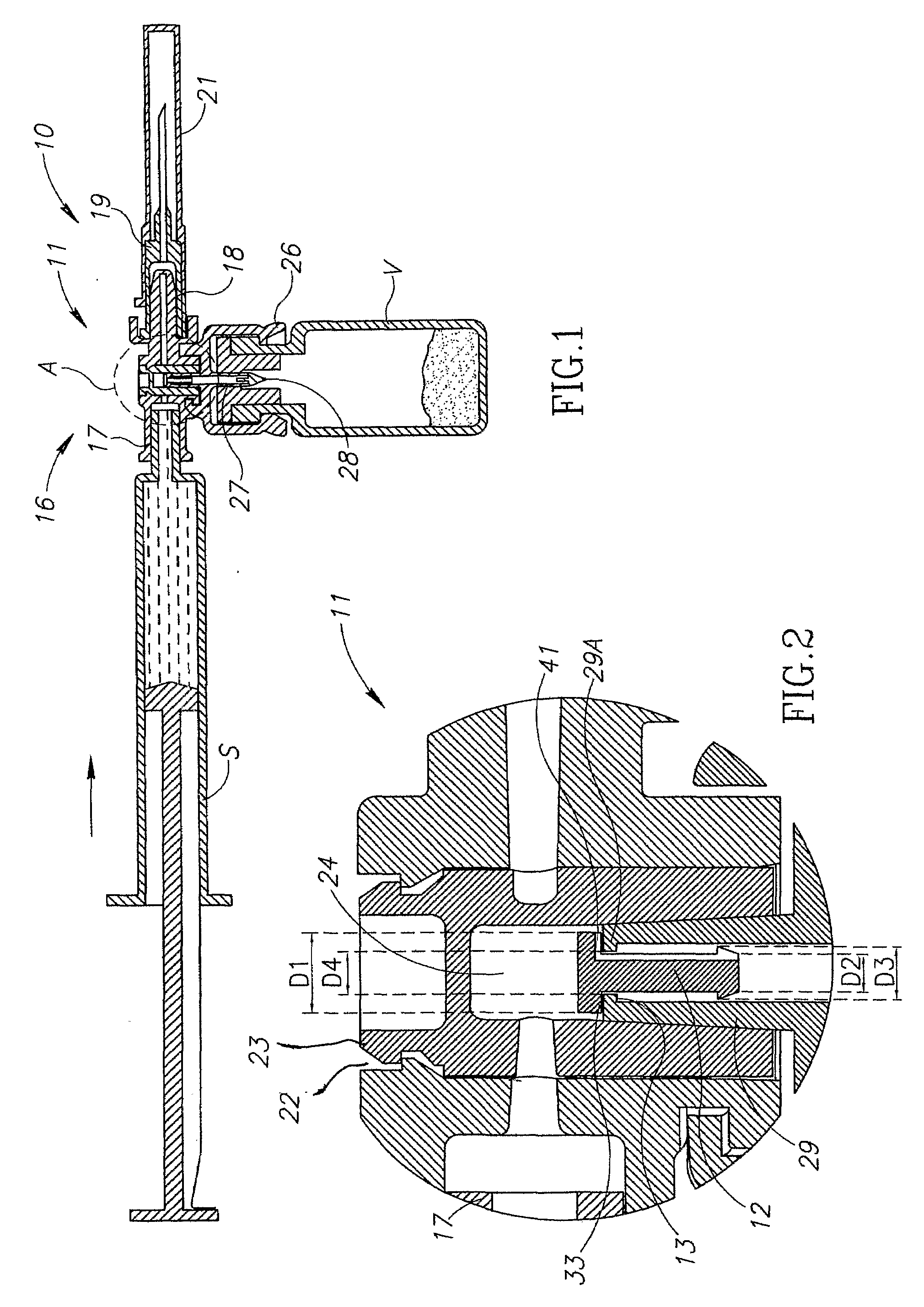
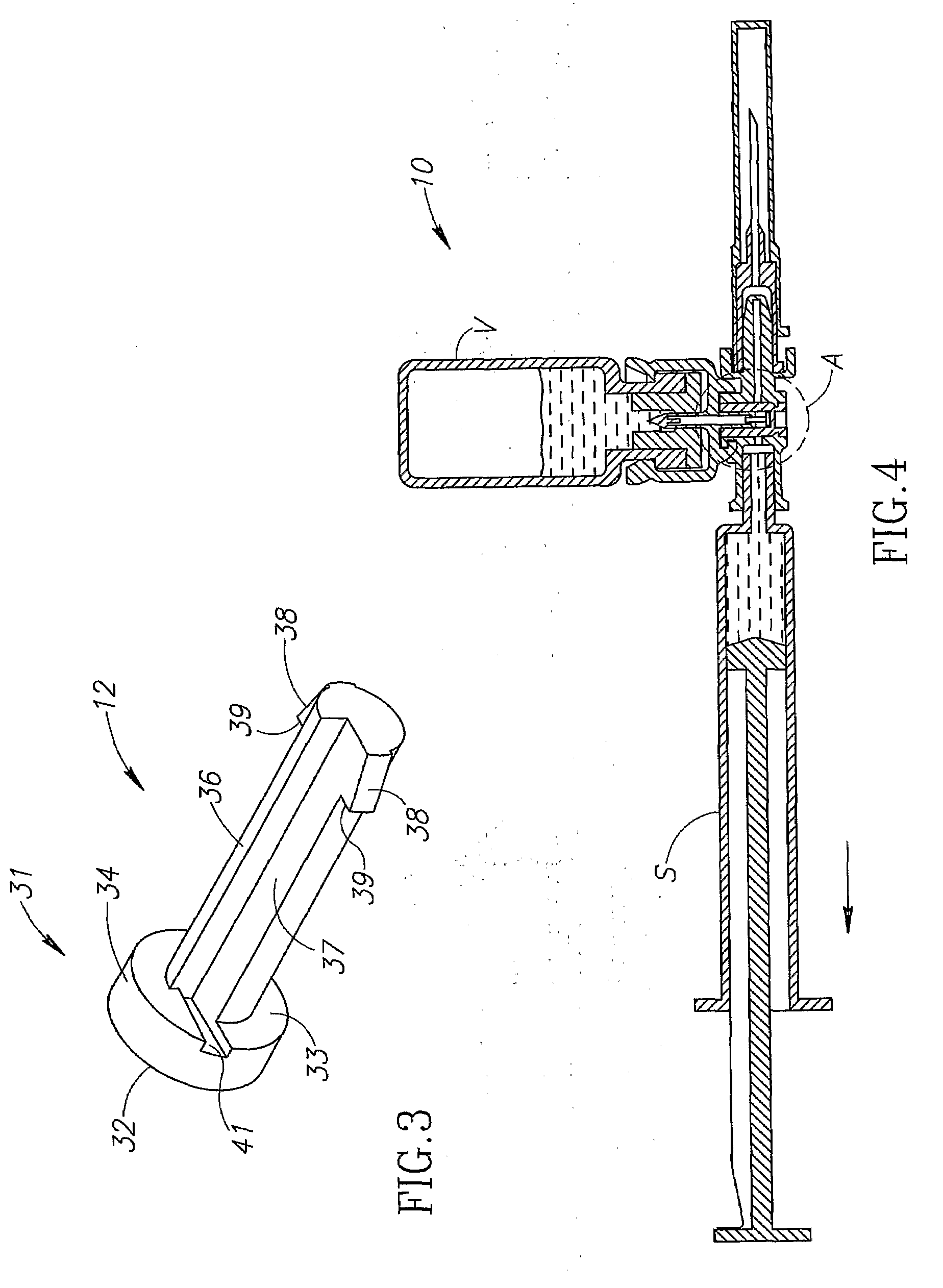
Medical device for in situ liquid drug reconstitution in medicinal vessels
ActiveUS7862537B2Little restrictionEasy to usePharmaceutical containersMedical devicesDrug contentDiluent
Medical device for in situ liquid drug reconstitution in medicinal vessels containing drug contents. The medical device includes a body member having a vessel port for insertion into a medicinal vessel containing drug contents, and a syringe port for receiving a syringe containing a diluent for reconstituting the drug contents into a reconstituted liquid drug. The medical device includes a one-way flow restriction mechanism between the two ports for positively restricting injection of diluent into a medicinal vessel and only slightly restricting aspiration of reconstituted liquid drug therefrom, if at all.
Owner:WEST PHARM SERVICES IL LTD
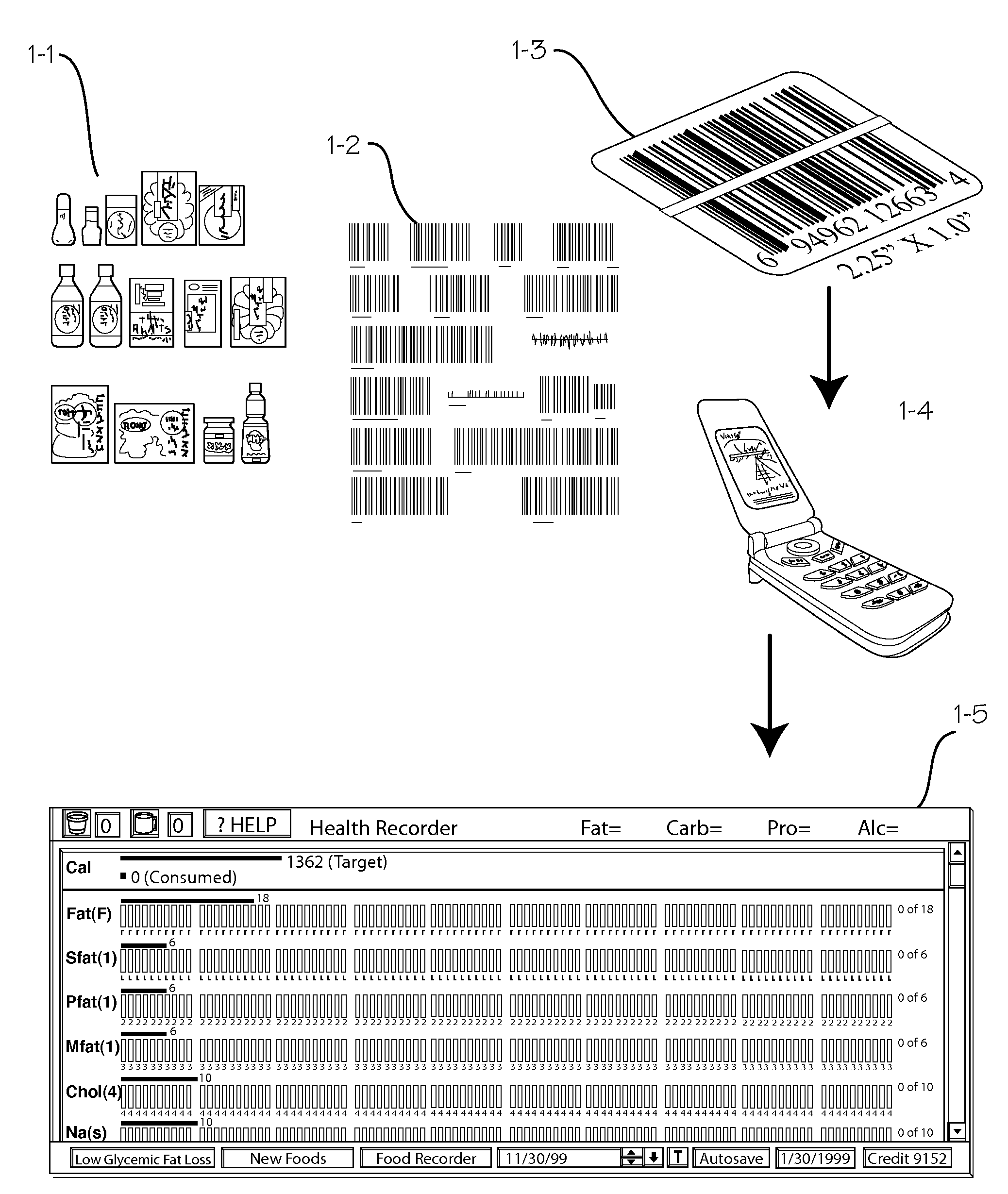
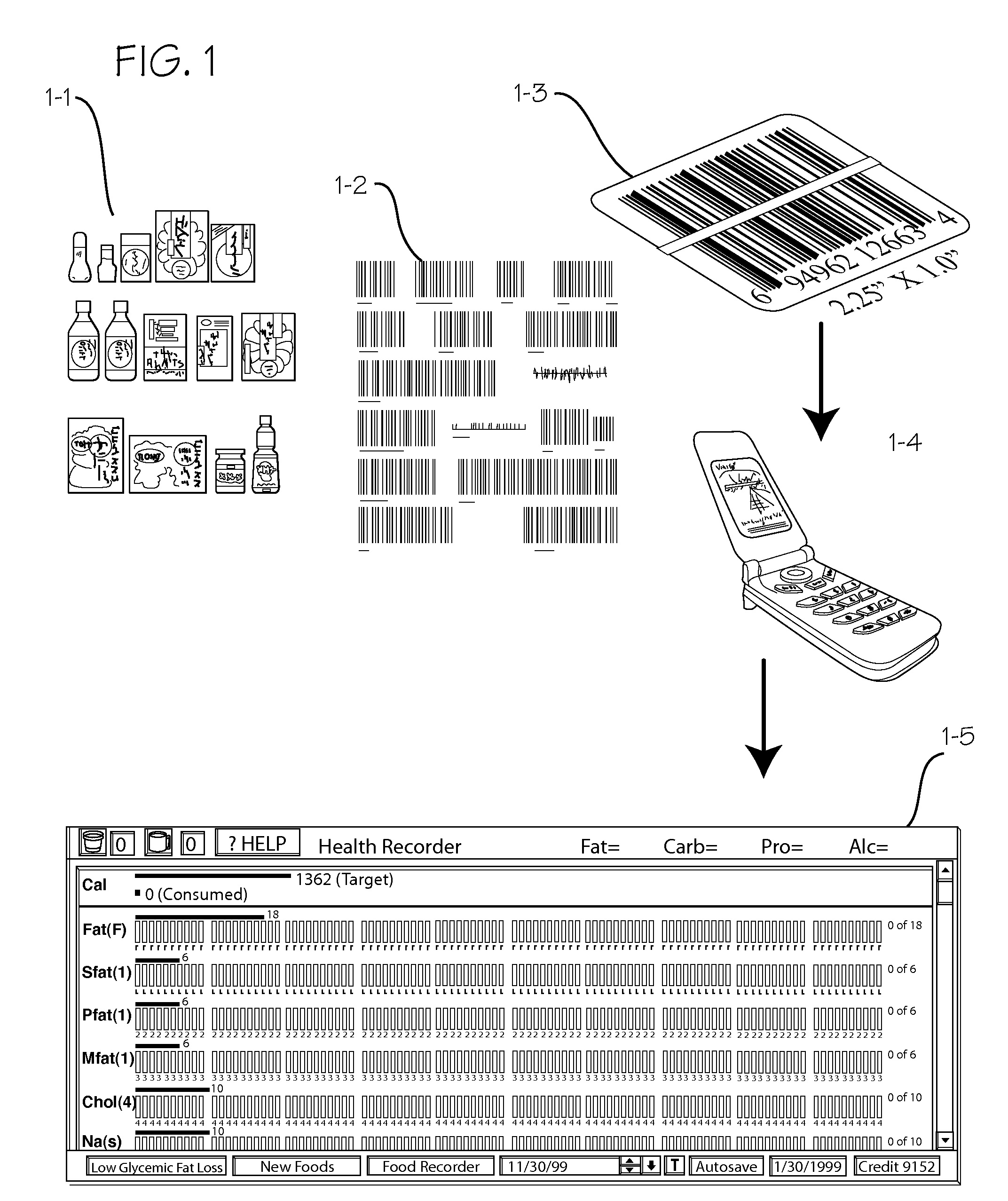
Longitudinal Personal Health Management System Using Mobile Data Capture
InactiveUS20090176526A1Enhanced couplingDrug and medicationsCharacter and pattern recognitionDrug contentCalorie intake
A system and method for tracking longitudinal data for the maintenance and management of health of an individual. The method entails marking numerous groceries, drugs, beverages, etc., with labels that may be read by a scanner or reader embedded in a PDA or cell phone or similar small electronic device, and providing users with a suitable scanner or reader. Users scan the labels of the products they consume during the course of a day, and the system keeps track of calories, fat content, carbohydrate content, etc. of the food consumed, drug content of the drugs consumed, etc. The system can also be operated to track exercise levels and energy expenditure during the day. The system is operable to provide a comparative display of various databased information, such as calorie intake versus calorie expenditure during exercise.
Owner:ALTMAN PETER A
Fluid transfer assembly with venting arrangement
A fluid transfer assembly including a vented female vial adapter and male vial adapter for use with a pair of vials including a vial with contents under negative pressure for liquid drug reconstitution and administration purposes. The vented female vial adapter includes a venting arrangement and the male vial adapter includes a sealing arrangement for selectively sealing the venting arrangement. The fluid transfer assembly is designed such that only filtered air is drawn into the vial under negative pressure subsequent to reconstitution of liquid drug contents to ensure sterile conditions.
Owner:WEST PHARM SERVICES IL LTD
Medical device for in situ liquid drug reconstitution in medicinal vessels
ActiveUS20090054834A1Little restrictionEasy to useInfusion syringesPharmaceutical containersDrug contentDiluent
Medical device for in situ liquid drug reconstitution in medicinal vessels containing drug contents. The medical device includes a body member having a vessel port for insertion into a medicinal vessel containing drug contents, and a syringe port for receiving a syringe containing a diluent for reconstituting the drug contents into a reconstituted liquid drug. The medical device includes a one-way flow restriction mechanism between the two ports for positively restricting injection of diluent into a medicinal vessel and only slightly restricting aspiration of reconstituted liquid drug therefrom, if at all.
Owner:WEST PHARM SERVICES IL LTD
Biodegradable fluorourcacil polyester medicine-carried nanospheres and its preparation method
InactiveCN101053553AHigh drug loadingSmall particle sizeOrganic active ingredientsPowder deliveryPolyesterPolymer science
The invention relates to biodegradable fluorouracil(Fu) polyester drug-bearing manoparticles with a coating material of polylactic acid, polylactic acid-glycolic acid, polylactic acid-polyethylene glycol block copolymer or polylactic acid-glycolic acid-polyethylene glycol block copolymer and the producing method including: firstly, fully dissolving the copolymer in the dichloromethane, under the ultrasonic shock, injecting the fluorouracil NaOH solution in the dichloromethane solution, dispersing uniformly, forming W / O primary latex, and beating up the primary latex and injecting into the fluorouracil saturated water solution containing 5 wt% of polyvinylalcohol (PVA), and storing in the refrigeratory after freeze-dry. The drug-bearing manoparticle has a drug content which is 10-25% of the microparticle mass, and has a smooth surface, an even diameter distribution, a remarkable slow release function and not adhesive. The micropartical size is 100-1000nm.
Owner:JILIN UNIV +1
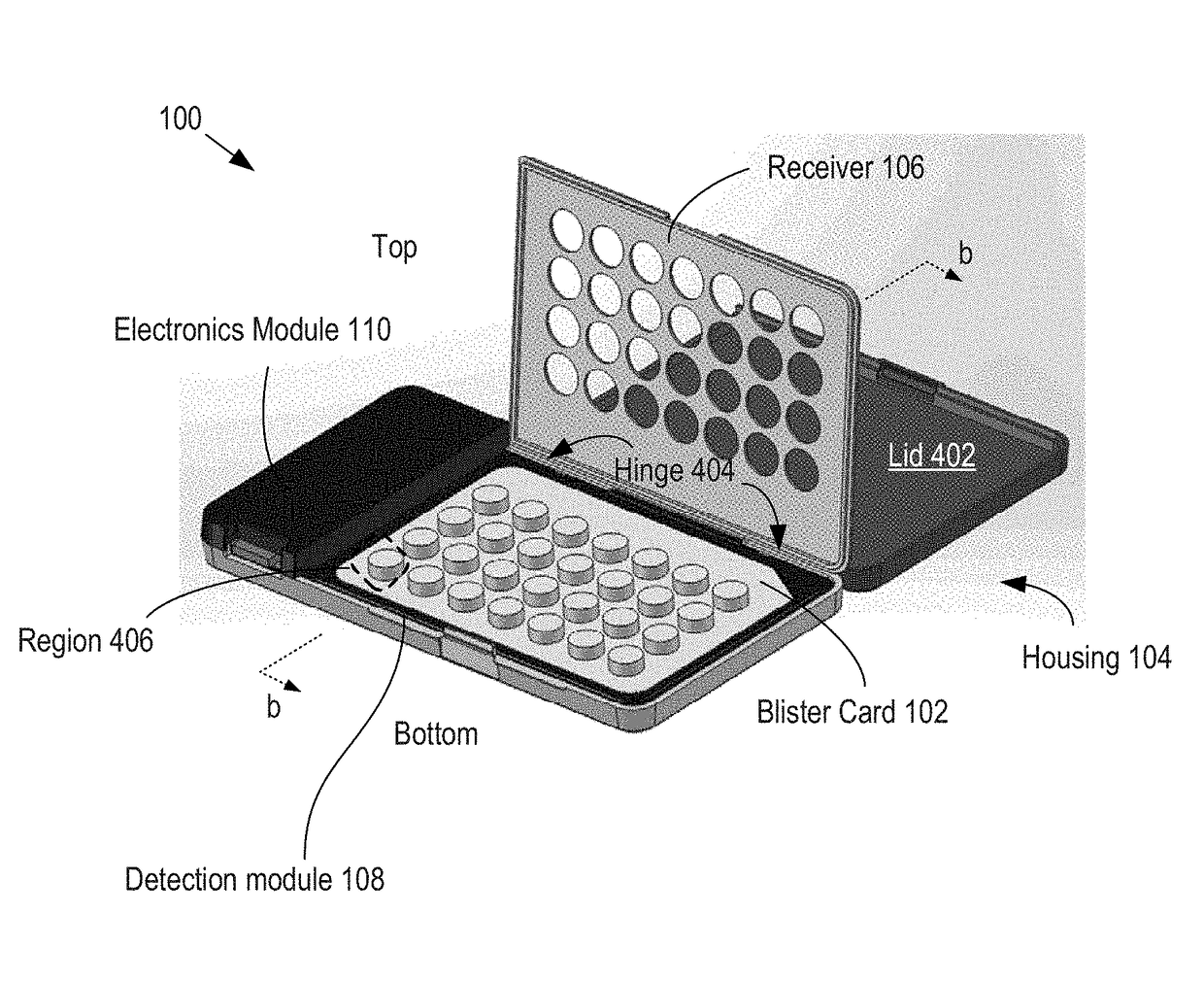
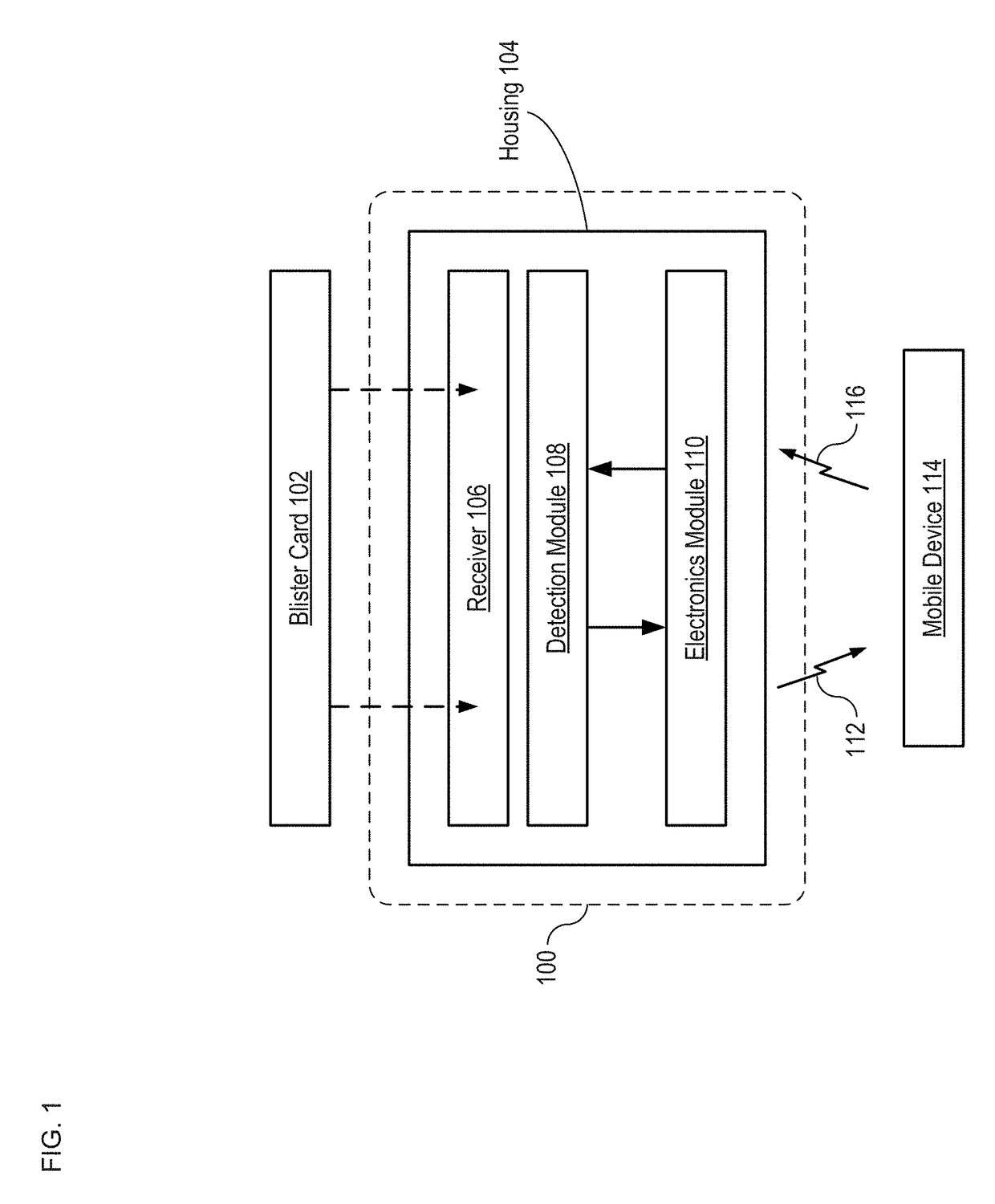
Apparatus and Method for Improved Drug Regimen Compliance
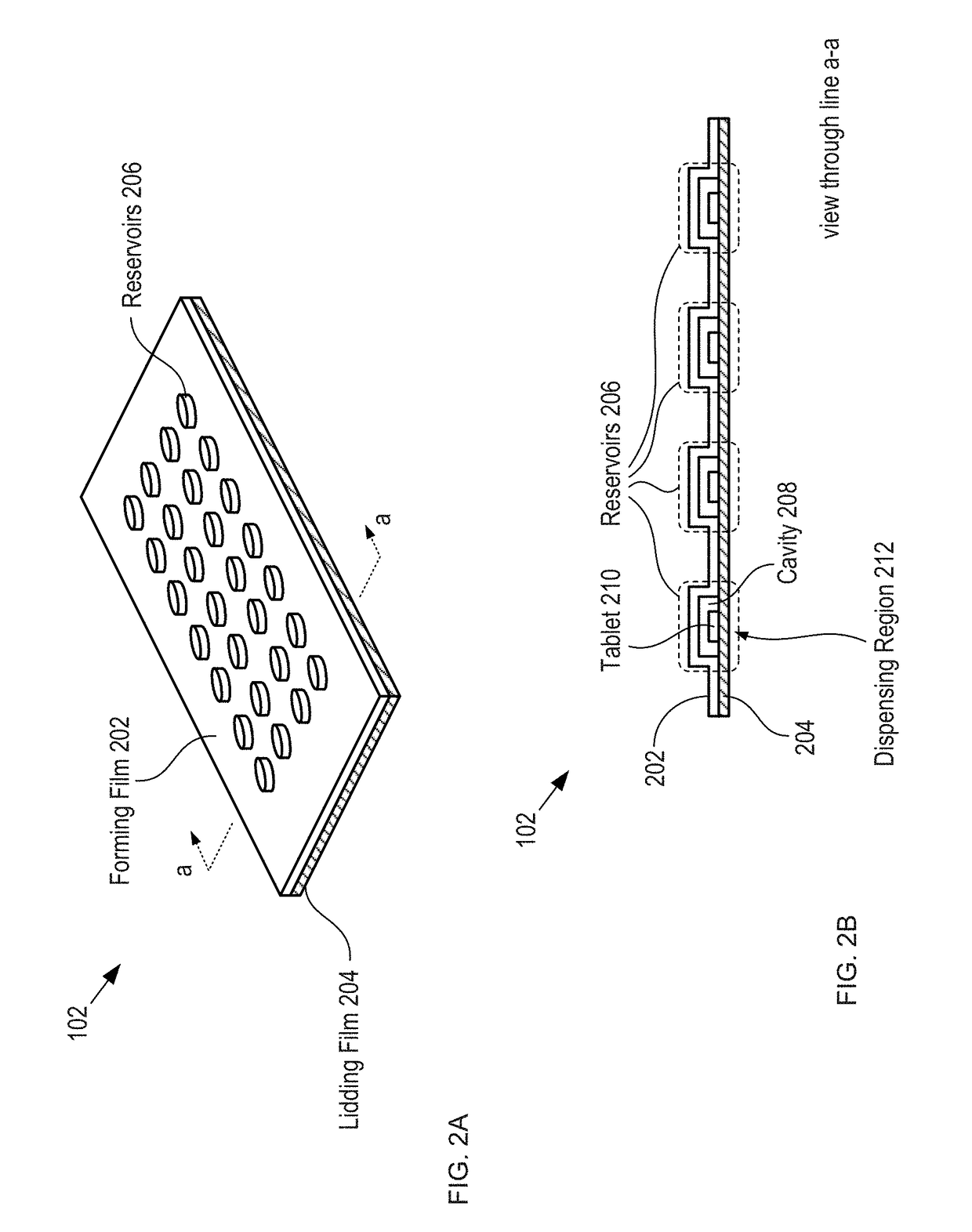
ActiveUS20170294105A1Improve adhesionEasy to managePharmaceutical containersMedical packagingCapacitanceMobile apps
A method and apparatus for monitoring drug-regimen compliance is disclosed. Systems in accordance with the present invention enable automatic monitoring of the state of medicine content of a blister card. Each tablet location on the blister card is operatively coupled with a different sensor that detects whether the tablet location is occupied and / or a dispensing event at a tablet location. In some embodiments, capacitive sensing is employed, where the capacitance of each sensor is based on the physical state of a dispensing region of the lidding film of the blister pack, which is located at the tablet location being monitored. Alternative sensing approaches are based on optical, acoustic, and tactile sensors that interrogate either the dispensing region at each tablet location or the tablets themselves to determine whether tablets have been dispensed. The sensors interface with a mobile app that provides the user instructions to help improve drug-regimen compliance.
Owner:QUANTAED +1
Controlled released compositions
ActiveUS20070092574A1Reduce degradationEasy loadingPowder deliveryOrganic active ingredientsDiseaseControlled release
The compositions disclosed herein are for use as controlled release therapeutics for the treatment of a wide variety of diseases. In particular, the compositions provide water soluble bioactive agents, organic ions and polymers where the bioactive agent is efficiently released over time with minimal degradation products. The resulting controlled release composition is capable of administration in a decreased dose volume due to the high drug content and predominance of non-degraded bioactive agent after release. Additionally, the compositions, of the present invention are capable of long term, sustained releases.
Owner:PR PHARMA +1
Dual-emission ratio-type quantum dot fluorescence probe, preparation method and application thereof
InactiveCN104198447AGood optical performanceReduce the impactFluorescence/phosphorescenceDrug contentPhotochemistry
The invention relates to a dual-emission ratio-type quantum dot fluorescence probe for visual detection of aspirin, a preparation method and an application thereof and belongs to the technical field of preparation of material and detection of content of medicines. The preparation method of the probe includes following steps: preparing a precursor NaHTe solution from sodium borohydride, tellurium powder and water under an ultrasonic environment; adding the precursor to an aqueous solution of CdCl2.2.5H2O in the presence of thioglycollic acid; carrying out a reflux reaction with a nitrogen protecting condition to obtain required green fluorescence quantum dot and red fluorescence quantum dot; by means of a sol-gel method, wrapping the red fluorescence quantum dot with silicon spheres with amino group being connected; dispersing a green fluorescence quantum dot solution and the red fluorescence quantum dot wrapped with the silicon spheres in an MES buffer solution with addition of an EDC / NHS solution; and carrying out a reaction at room temperature in a dark place to obtain the dual-emission ratio-type quantum dot fluorescence probe. The dual-emission ratio-type quantum dot fluorescence probe is used in detection of the content of the aspirin through fluorescence quantitation and visualized analysis. The quantum dot fluorescence probe is quite good in optical performance and stability and has a capability of visualizedly detecting the aspirin.
Owner:JIANGSU UNIV
Controlled delivery of active agents
Controlled release of active agents from sustained release push delivery devices having high drug loading are described wherein residual drug content in the device is minimized by the utilization of a flow-promoting layer between a semi-permeable wall and drug layer comprising the device.
Owner:ALTA
Chinese medicine health product preparation for preventing and treating alcoholic liver disease and preparation method thereof
InactiveCN101502627AMedicinal Flavor SimplifiedSignificant effectDigestive systemPlant ingredientsDrug contentSemen
The invention discloses a traditional Chinese medicine health product preparation for preventing and treating alcohol liver disease and a preparation method. According to the parts by weight, the traditional Chinese medicine health product preparation is prepared by adopting the following raw materials: 250 to 328 parts of pueraria flower, 250 to 328 parts of semen hoveniae, 250 to 328 parts ofradix puerariae, 125 to 164 parts of fructus amomi, 250 to 328 parts of dark plum fruit, 250 to 328 parts of date and 125 to 164 parts of liquoric root. The traditional Chinese medicine health product preparation can effectively prevent the alcohol from causing GSH exhaustion and MDA rising of the liver, reduce fatty degeneration of liver cells and has the function of preventing alcoholic liver damage; the health product preparation has condense medicinal odour, stable and controllable preparation technique and product quality, obvious curative effect and fast effect; the provided formulation has convenient in use, reduces the oral dosage; and in the formulation, all the drug contents are obtained from natural resources, has lower cost and is conveniently to promote and broad masses of the people are willing to adopt the traditional Chinese medicine health product preparation.
Owner:贵阳青青生物科技有限公司
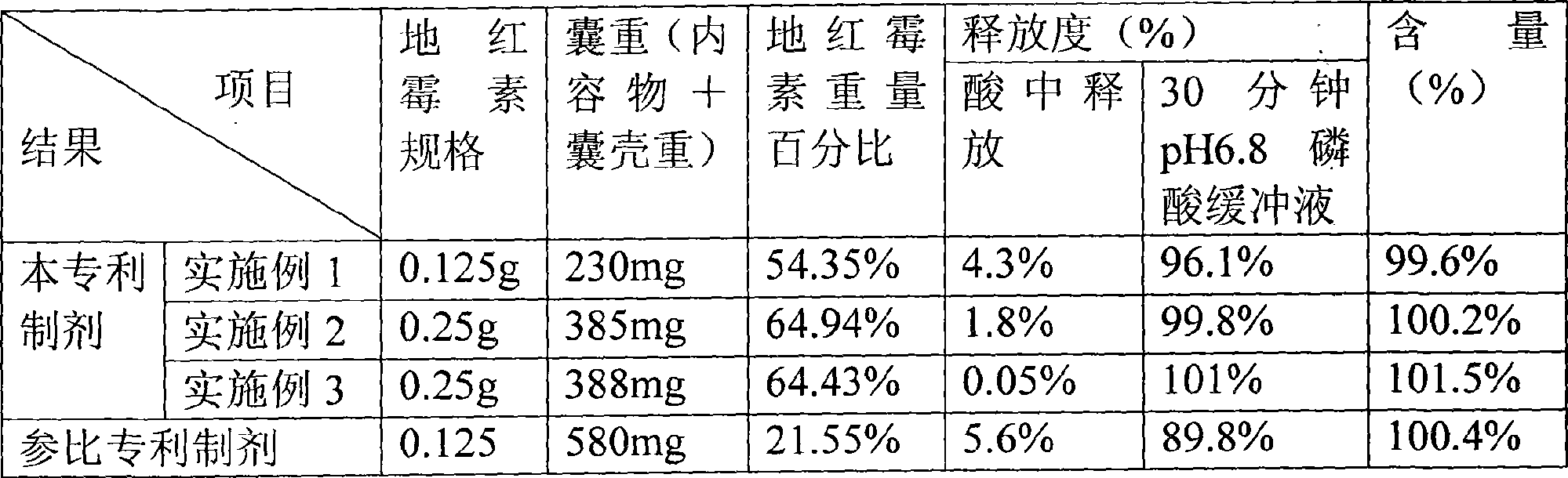
Dirithromycin enteric-coated formulation
The invention relates to a dirithromycin (DRM) enteric preparation with hydroxypropyl methylcellulose phthalate (HPMCP) being the framework material of drug-containing core and the coating material of an enteric coating layer. The enteric preparation has the advantages that the drug content is higher, the drug dissolution rate is less affected by the coating material, the preparation cost is low and the preparation process is simple.
Owner:SHANDONG INST OF PHARMA IND
High-safety ropivacaine hydrochloride injection and preparation method thereof
ActiveCN102552126AImprove adsorption capacityGuaranteed physical stabilityPharmaceutical delivery mechanismAnaestheticsDrug contentInjection solution
The invention relates to a high-safety ropivacaine hydrochloride injection and a preparation method of the high-safety ropivacaine hydrochloride injection. The formula of the high-safety ropivacaine hydrochloride injection comprises 20-200g of ropivacaine hydrochloride, 70-100g of sodium chloride, appropriate amount of sodium hydroxide or hydrochloric acid and 10000ml of water for injection. The formula is prepared into 1000 injections, and the pH value of the injection is 4.0-6.0. The injection has good stability, high drug content and safe and reliable effect.
Owner:GUANGDONG JIABO PHARM CO LTD
Quantum dot fluorescent aspirin imprinted sensor and its preparation method and use
InactiveCN104165874AGood optical stabilityAvoid slow recognitionFluorescence/phosphorescenceFunctional monomerDrug content
The invention provides a quantum dot fluorescent aspirin imprinted sensor and its preparation method and use and belongs to the technical field of material preparation and drug content detection. The preparation method comprises the following steps of preparing a precursor NaHTe solution from sodium borohydride, tellurium powder and water in an ultrasonic environment, injecting the precursor NaHTe solution into a CdCl2.2.5H2O solution fed with nitrogen for oxygen removal, having a pH value of 10.5-11.5 and containing thioglycollic acid (TGA), carrying out backflow reaction processes in a nitrogen protective atmosphere at a temperature of 100-110 DEG C to obtain quantum dots having different sizes according to different backflow time periods, and synthesizing a fluorescent molecule imprinted polymer from the CdTe quantum dots as fluorescent carriers, aspirin as a template molecule, (3-aminopropyl)triethoxysilane (APTES) as a functional monomer and tetraethyl orthosilicate (TEOS) as a cross-linking agent by a sol-gel method, wherein the fluorescent molecule imprinted polymer can be used for optical detection of aspirin. The fluorescent molecule imprinted polymer has good optical and pH stability and has an aspirin selective-identification function.
Owner:JIANGSU UNIV
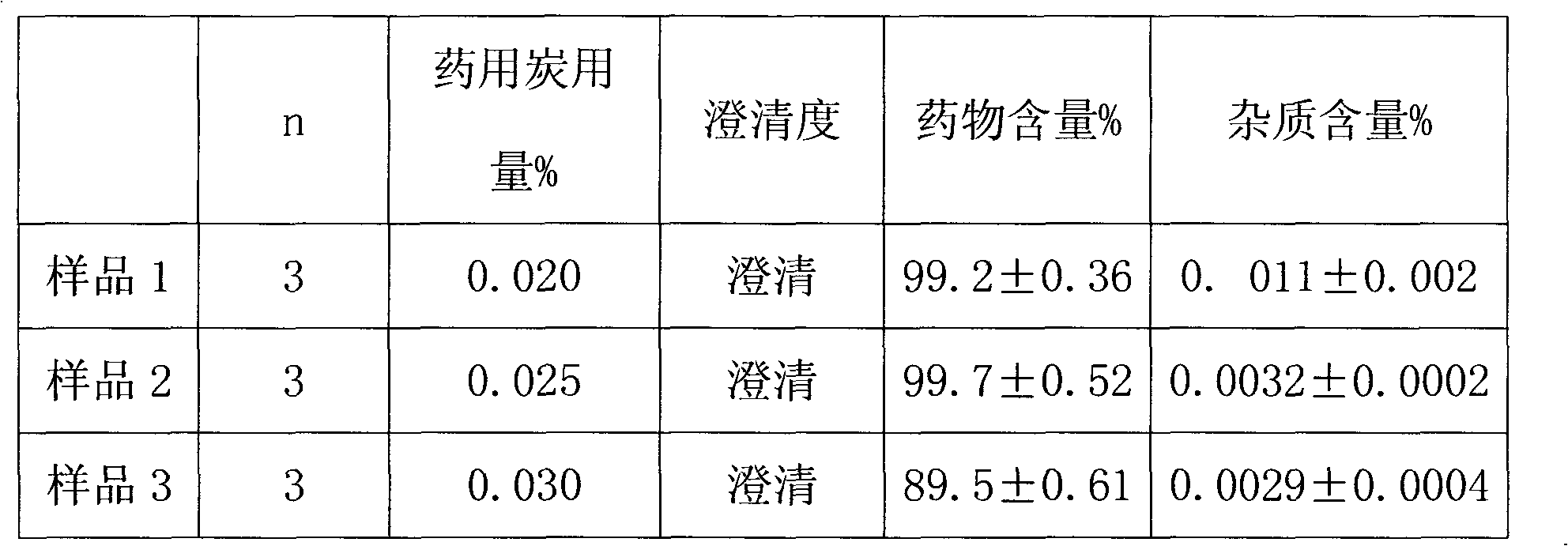
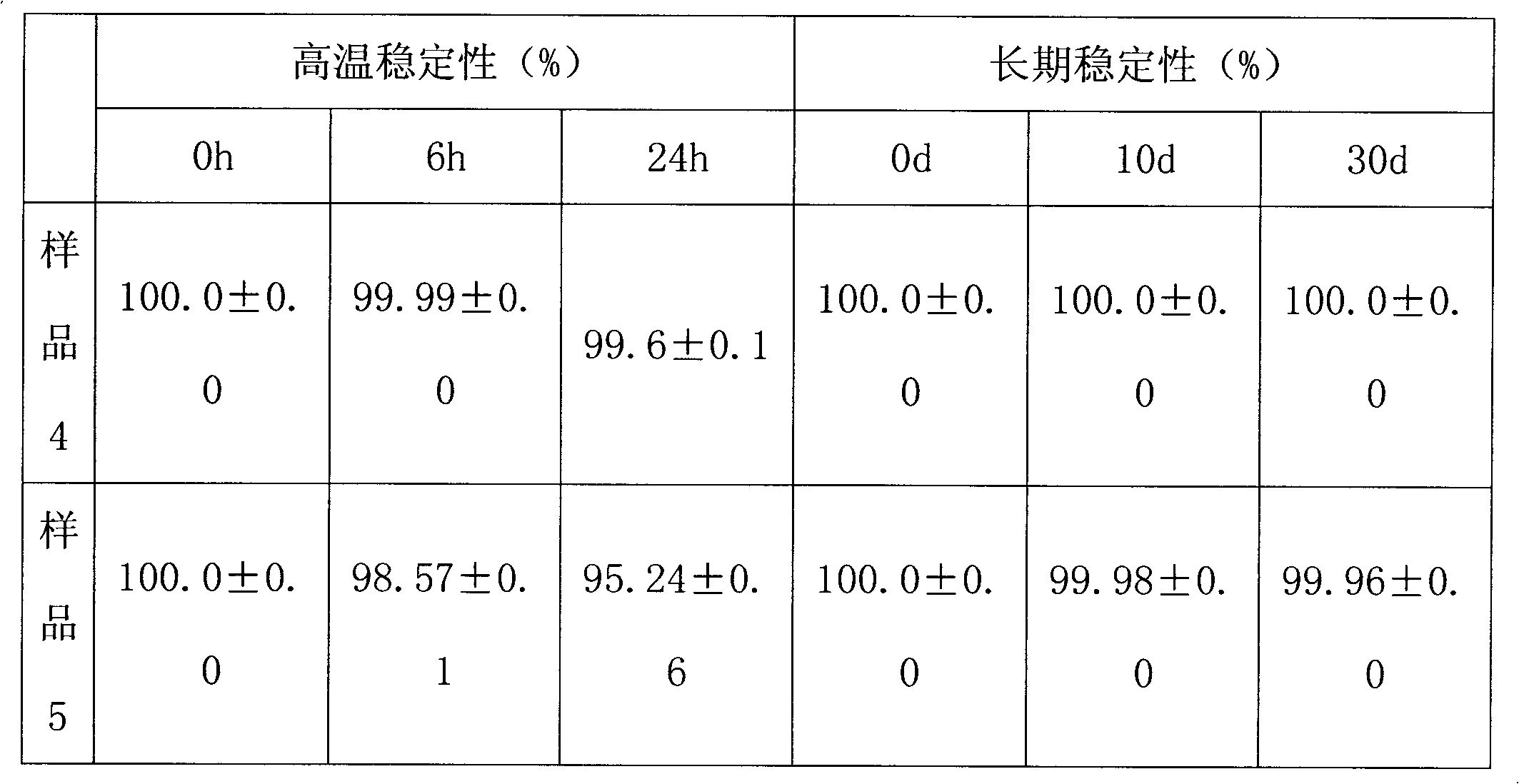
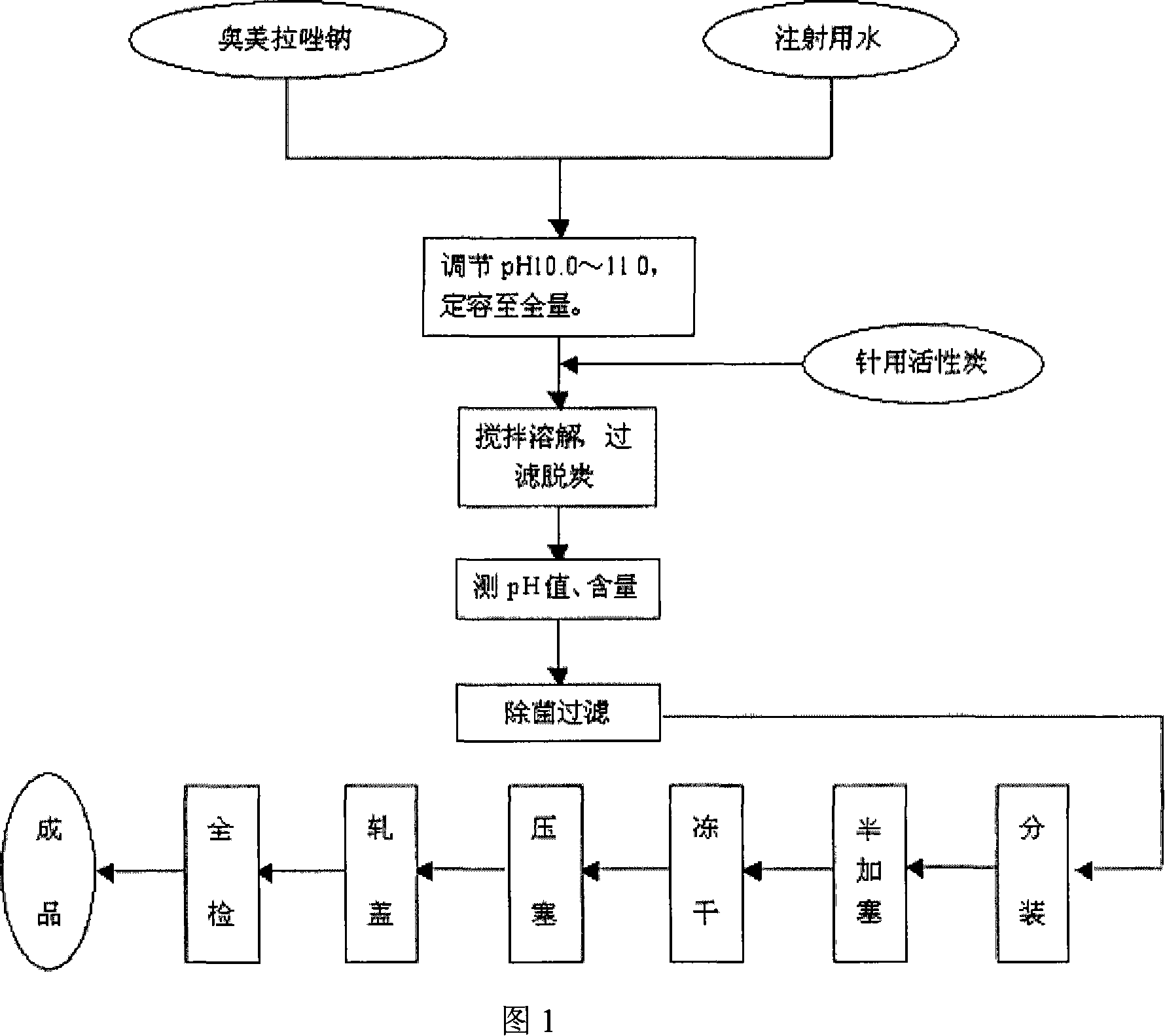
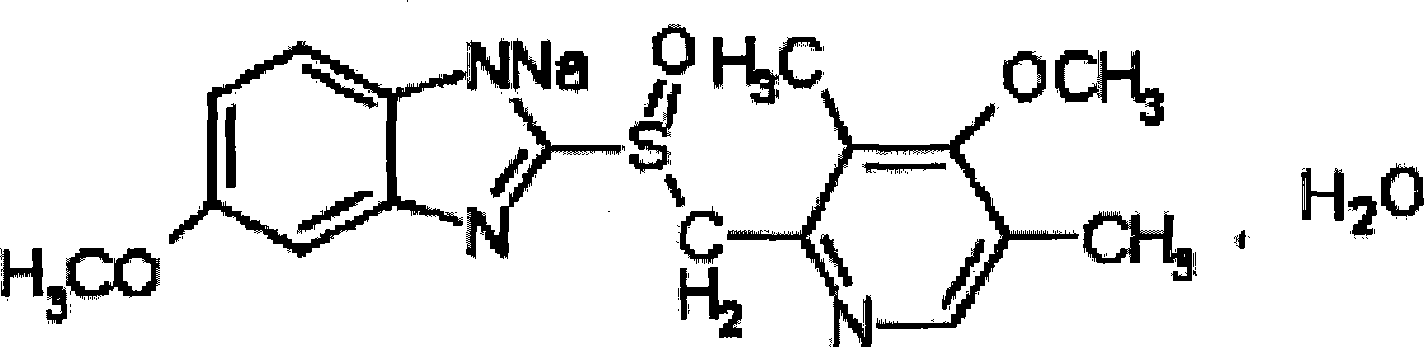
Omeprazole sodium freeze-dried powder injection and preparing method thereof
ActiveCN101229133ACatalysis against auto-oxidationHigh content of the main drugPowder deliveryOrganic active ingredientsOmeprazole SodiumDrug content
The invention provides an omeprazole sodium freeze-dried injection and a preparation method which is simple and feasible and the prepared freeze-dried injection is high in drug contents and low in related substances content. The method comprises the following steps: (1) the raw materials of the recipe quantity are stirred with injection water until the omeprazole sodium is completely dissolved to get omeprazole sodium solutions; sodium citrate solutions are put into the solutions got from step (1) and the pH value of the solutions is adjusted to 10.0 to 11.0; the injection water is put into the prepared products got from step (2) to the recipe quantity and then pin activated carbon is put into the prepared products, and filter decarburization is carried out after stirring to get filtrates; the filtrates got from step (3) are fine filling filtered by 0.22Mum removal bacteria microporous membrane, and the filtrates after fine filling filtering are put into a bottle which is partially stoppered, and then the filtrates are freeze-dried to get the freeze-dried injection.
Owner:SHANDONG YUXIN PHARMA CO LTD
Astragalus planting method
InactiveCN105917907AImprove physical and chemical propertiesReduce pests and diseasesSuperphosphatesAgriculture tools and machinesDiseaseDrug content
The invention discloses an astragalus planting method. The steps comprise: preparing field; treating seeds; sowing; performing field management, and controlling pests. In each step, a scientific treatment method is used. A soil conditioner in the step 1 can improve soil physical and chemical properties, reduce pest and disease damage in growth, and improve stress resistance. In step, on the premise of ensuring yield and quality of the astragalus, hard seed property of astragalus seeds is overcome through treatment before sowing, so as to improve rooting and developing efficiency of the astragalus seeds. In step 3 and step 4, fertilizer makes survival seedling rate of the astragalus high, and large amount of organic fertilizer generated in a yield increasing fertilizer can satisfy rapid growth requirements of the astragalus, so growth speed of the astragalus is fast, flowers are flourishing and fruits are rich, seeds are full, and leaf surface drug content is high. The method increases yield and solves a problem of repeated fertilization, and effectively controls incidence of astragalus root rot disease, improves yield and quality, and generates effective economical value.
Owner:ANHUI DACHUAN ECOLOGICAL AGRI DEV CO LTD
Controlled release dosage form
The present invention generally relates to a pharmaceutical dosage form and controlled release of biologically active agents, diagnostic agents, reagents, cosmetic agents, and agricultural / insecticide agents. In one embodiment, the dosage form has a substrate that forms a compartment, wherein the substrate includes at least a first piece and a second piece, wherein the first piece operably links to the second piece. The dosage form contains a drug content that is loaded into the compartment. The dosage form also has a releaser operably linked to the substrate which upon contact with water or body fluid is capable of separating the first and second piece to open the compartment and release the drug content.
Owner:TRIASTEK INC
Hypocrellin liposome preparation and preparation method thereof
ActiveCN101371828AHigh drug contentSatisfy productivityOrganic active ingredientsSenses disorderDrug contentFreeze-drying
The invention discloses a hypocrellin liposome preparation and a preparation method thereof. The hypocrellin liposome preparation comprises the following components according to parts by weight: 1-5 parts of hypocrellin, 40-200 parts of phospholipids, 2.4-12 parts of cholesterol and 50-100 parts of protective agent for freeze drying. The hypocrellin liposome preparation prepared by the high pressure homogenization is characterized in that: the preparation contains no surfactant of Tween; the drug content of the preparation can be increased to more than 1.2 percent from 0.6 percent; the drug concentration of redissolution reaches 1mg / ml, an increase of 100 percent. The invention substitutes the high pressure homogenization for the ultrasonic method so that the quality of the hypocrellin liposome preparation can be assured and the yield greatly increased which meets dual requirements of the industrial production and clinical application of drugs of hypocrellin liposome; meanwhile, the invention substitutes rapid pre-freezing for steady pre-freezing, which can ensure that solid powder of hypocrellin liposome with good monodispersity and more stable morphology after the redissolution is obtained.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Cinnamic aldehyde series preparation and its preparation process
InactiveCN1634000ALow priceReduce manufacturing costAntibacterial agentsPharmaceutical delivery mechanismDiseaseDrug content
The invention provides a preparation for treating viral myocarditis, wherein the medicinal reactivity of cinnamic aldehyde for antiviral, immunity adjustment, cardiac rhythm stabilization and blood vessel amplificatus, the cinnamic aldehyde can be prepared into cinnamic aldehyde slow release tablet, cinnamic aldehyde slow release capsule and cinnamic aldehyde injection for treating viral myocarditis. The invention has the advantages of stabilized medicinal content, high biological availability, small amount of administration, high curative effect, and low toxicity.
Owner:中国人民解放军第四军医大学药物研究所
Epirubicin hydrochloride liposome and preparation thereof
InactiveCN101264056ALow toxicityReduce manufacturing costOrganic active ingredientsPharmaceutical non-active ingredientsDrug contentSide effect
The invention belongs to the medicine technical field, relates to epirubicin hydrochloride liposome and a preparation method. The liposome comprises epirubicin hydrochloride and neutral phospholipid and cholesterol and / or negative charge phospholipids and / or long-circulating phospholipids and buffer solution, and actively carries drug with a PH gradient method or an ammonium sulphate gradient method; the entrapment rate of the achieved liposome is more than 90%, to make smaller volume contain more main drug component of epirubicin. Compared with the exiting techniques, the epirubicin hydrochloride liposome and the preparation method has the advantages of high drug content, high bioavailability, long internal circulation time, low toxic and side effect and other advantages; besides, the production cost can be reduced obviously, the epirubicin hydrochloride liposome is more suitable for industrial production after being made into lyophilized preparation. The liposome is clinically suitable for the tumor therapy, in particular to the tumor therapy for children.
Owner:FUDAN UNIV
Production process for polymeric micelle charged therein with drug and polymeric micelle composition
InactiveUS7223419B2Increase drug concentrationReadily and stablyAntibacterial agentsPowder deliveryEmulsionOrganic solvent
Provided are a production process for a polymeric micelle which is stable and has a high drug content and a composition containing such polymeric micelle. Disclosed are a production process for a polymeric micelle, comprising the steps of dissolving a drug and a specific copolymer in a water non-miscible organic solvent to prepare a solution, mixing the resulting solution with water to form an O / W type emulsion and then slowly volatilizing the organic solvent from the solution, and a polymeric micelle composition charged therein with a water-scarcely soluble drug, which can be obtained by the above production process.
Owner:NANOCARRIER
Preparation of solid dispersions and oral preparations of paclitaxel and homologous compounds thereof
InactiveCN103083240AImprove bioavailabilityPowder deliveryOrganic active ingredientsCelluloseDrug content
The invention relates to preparation of solid dispersions and oral preparations of paclitaxel and homologous compounds thereof, wherein solid dispersions and solid dispersion microspheres are prepared to increase rapid release and oversaturation maintenance of paclitaxel and homologous compounds thereof so as to increase bioavailability. According to the present invention, a carrier is hydroxypropylmethylcellulose acetate succinate or hydroxypropylmethylcellulose tetrabutyl titanate, or / and a mixture of hydroxypropylmethylcellulose acetate succinate and micro-powder silica gel or a mixture of hydroxypropylmethylcellulose tetrabutyl titanate and micro-powder silica gel; the drug and macromolecule are dissolved into a mixing solvent of a good solvent and a liquid bridging agent, and the obtained drug-containing solution is slowly added to a poor solvent under a stirring effect; under a shearing force effect of stirring, the drug-containing solution is emulsified and dispersed in the poor agent to form temporary translucent emulsion droplets, the emulsion droplets continuously diffuse to the poor solvent along with the good solvent and the bridging agent, and the drug and the macromolecule in the emulsion droplet are oversaturated so as to gradually solidify to form the solid dispersion microspheres; and the prepared solid dispersion microspheres have characteristics of a size particle of 100-600 mum, a yield of more than 80%, and a drug content of 5-35%.
Owner:SHENYANG PHARMA UNIVERSITY
Controlled release dosage form
The present invention generally relates to a pharmaceutical dosage form and controlled release of biologically active agents, diagnostic agents, reagents, cosmetic agents, and agricultural / insecticide agents. In one embodiment, the dosage form has a substrate that forms a compartment, wherein the substrate includes at least a first piece and a second piece, wherein the first piece operably links to the second piece. The dosage form contains a drug content that is loaded into the compartment. The dosage form also has a releaser operably linked to the substrate which upon contact with water or body fluid is capable of separating the first and second piece to open the compartment and release the drug content.
Owner:TRIASTEK INC
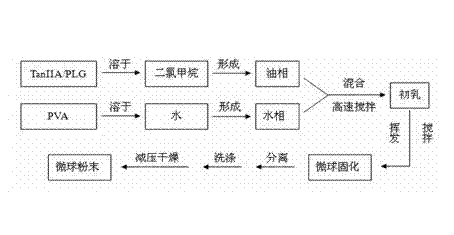
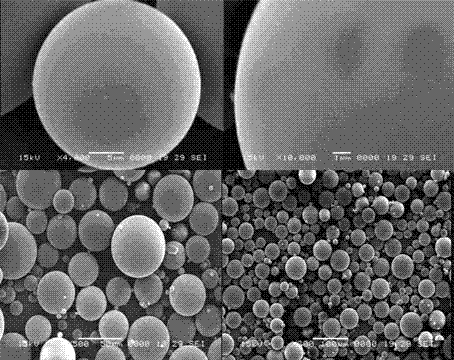
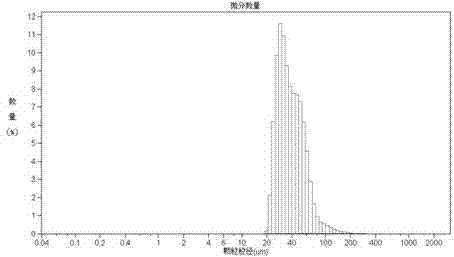
Tanshinone IIA-polyactic acid/hydroxyacetic acid microsphere and preparation method thereof
InactiveCN103083250AHigh specific activityHigh encapsulation efficiencyOrganic active ingredientsPharmaceutical non-active ingredientsDrug contentPolyvinyl alcohol
The invention discloses a tanshinone IIA-polyactic acid / hydroxyacetic acid microsphere and a preparation method thereof. The microsphere is prepared by drying oil-in-water type emulsion, wherein the oil phase is dichloromethane solution of a polyactic acid / hydroxyacetic acid copolymer and the water phase is the water solution of polyvinyl alcohol. The drug content of tanshinone IIA in the microsphere is 1-10% and the entrapment efficiency is 60-90%. The particle size range of the microsphere is 30-200mm. The tanshinone IIA-polyactic acid / hydroxyacetic acid microsphere provided by the invention is suitable for interventional therapy of liver cancers, has a good liver tumor peripheral vascular thrombosis function, has an effective thrombosis time of 7-60 days, can be distributed in tumor tissues in a targeted manner, slowly release drugs, increase the local concentrations of the drugs, prolong the drug metabolism time, obviously inhibit animal liver tumor growth and prolong the animal lifetime and can inhibit expressions of a human hypoxia inducible factor 1alpha and a vascular endothelial growth factor and reduce the tumor tissue microvessel density after thrombosis.
Owner:SHUGUANG HOSPITAL AFFILIATED WITH SHANGHAI UNIV OF T C M +1
Matrine type alkaloid microemulsion and preparation method thereof
InactiveCN101548679APlay a preventive roleIncrease contactBiocideArthropodicidesDispersitySolubility
The invention relates to matrine microemulsion and a preparation method thereof, which overcomes the defects of low solubility and low bioavailability of matrine and improves the stability of matrine microemulsion. The invention adopts microemulsion as a pharmaceutical carrier to prepare matrine O / W type microemulsion with liquid drop of 1 to 100nm particle size so as to improve the dispersity of enveloped medicaments, promote the percutaneous absorption of plant surface cuticle and strengthen the effect of eliminiting insect injury, thereby providing scientific basis for theory and application study. The invention also relates to a method of forming stable O / W emulsion by doping matrine into oil. The compound comprises matrine, oil phase, water and surfactant. In a preferred embodiment, the oil is tween 80, an emulgator is ethyl oleate, and an emulgator assistant is absolute ethyl alcohol. The medicament content of soluble water aqua of matrine type alkaloid microemulsion is improved,the medicament effect is strengthened, the cost is saved and the medicament is more effectively to be absorbed by crops by forming matrine type alkaloid O / W microemulsion.
Owner:SHENYANG PHARMA UNIVERSITY
Rapidly disintegrable tablet containing polyvinyl alcohol
InactiveUS20030086967A1Little changeImprove drug stabilityOrganic active ingredientsNervous disorderSolubilityDrug content
The present invention provides a quickly disintegrating tablet which has quick disintegrability and solubility in an oral cavity, and does not have uncomfortable tastes such as bitterness, has a small variation of a tablet physical property even in storage under a humidifying condition, and has substantially no change in a medicine content in the tablet and tablet appearance and which is superior in stability; and a manufacturing method of the tablet. That is, it provides: a quickly disintegrating tablet which is prepared by blending a medicine with a saccharide and polyvinyl alcohol, which has small variations of tablet weight, tablet hardness, tablet diameter and tablet thickness, and which is superior in medicine stability in the tablet; and a manufacturing method of the tablet.
Owner:EISIA R&D MANAGEMENT CO LTD
Preparation method for doxycycline modified release pellet and preparation
The invention discloses a preparation method for a doxycycline modified release pellet and preparation. The method includes the steps of: preparing a doxycycline hydrochloride drug loaded pellet, then mixing the pellet with an appropriate amount of a coating material, applying a fluidized bed to perform coating by bottom spraying, and carrying out drying to obtain the doxycycline hydrochloride modified release pellet; mixing auxiliary materials evenly, and mixing an adhesive with the auxiliary materials uniformly according to a weight ratio of 0.01-1:1; and under a molten state of the adhesive, sticking the auxiliary materials to the surface of the coated pellet prepared form doxycycline by the adhesive so as to obtain a pellet particle; and subjecting the pellet particle to direct tabletting, or mixing the pellet particle with a buffer particle prepared by a conventional method according to a weight ratio of 1:9-9:1 and then conducting tabletting, thus obtaining a pellet tablet. The preparation method provided by the invention is free of layering phenomenon in the tabletting process, and the prepared pellet tablet has uniform quality and drug content. Thus, the method is suitable for industrial production.
Owner:天津新济复兴药业科技有限公司
Fexofenadine Microcapsules and Compositions Containing Them
ActiveUS20110250281A1Suitable drug contentFast dissolutionBiocideAntipyreticFexofenadineDrug content
The present invention provides a pharmaceutical composition comprising taste-masked immediate release microcapsules which comprise fexofenadine and a water-insoluble polymer coating. These microcapsules and the pharmaceutical compositions comprising them have suitable drug content and desirable pharmaceutical properties, including a quick dissolution rate of fexofenadine combined with a taste masking effect.
Owner:ADARE PHARM SRL
Saussurea involucrata drop pill and its preparation method
InactiveCN1634508AIncrease surface areaHas a wetting effectAntipyreticAnalgesicsDiseaseEnteral administration
The invention relates to a medicinal oral preparation, i.e., a saussurea involucrata drop pill for treating rheumatic arthritis, chronic infectious arthritis and dysmenorrheal, which has the advantages of high biological availability, quick-speed medicine release, quick-speed effect, less toxic and side effects, higher medicinal content, smaller amount of administration, accurate administration dosage, easy administration, low price, and facilitated carrying. The medicine is prepared through the conventional drop pill preparing process.
Owner:北京博智绿洲医药科技有限公司
Asenapine maleate oral instant membrane and making method thereof
InactiveCN104000800ALow costRestrict or completely prevent extractionOrganic active ingredientsNervous disorderDrug contentSchizophrenia
The invention relates to an Asenapine maleate oral instant membrane used for treating adult schizophrenia, chronic severe brain dysfunction and type I bipolar disorder, and a making method thereof. The above dosage form has the advantages of convenient administration, administration without water, rapid dissolving in the oral cavity, restriction or complete prevention of the removal of a medicine by a schizophrenia patient, and improvement of the compliance f the patient. The main drug content of the dosage form made through the making method can reach the unit dosage of Asenapine maleate, and the dosage form has the characteristics of drug loading uniformity, good appearance and good stability. In the production process, the technology is simple, the cost is low, and almost no dust is generated.
Owner:AVENTIS PHARMA HAINAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com