Patents
Literature
135 results about "Epirubicin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Epirubicin is used to treat breast cancer.
Antineoplastic conjugates of transferrin, albumin and polyethylene glycol
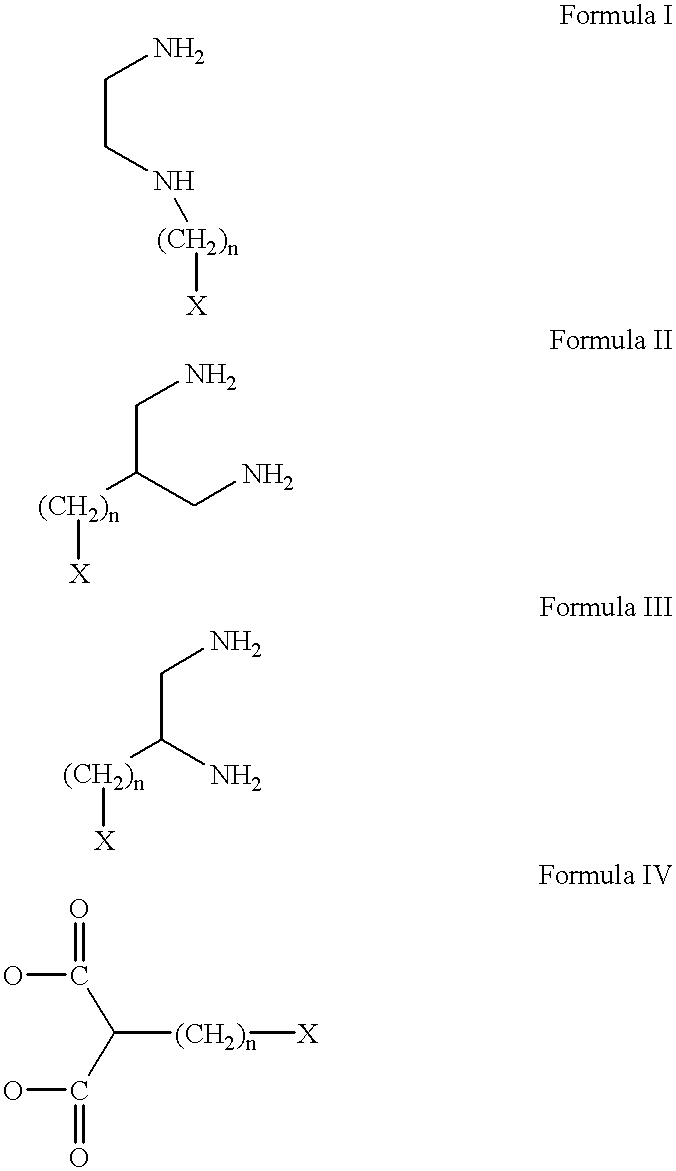
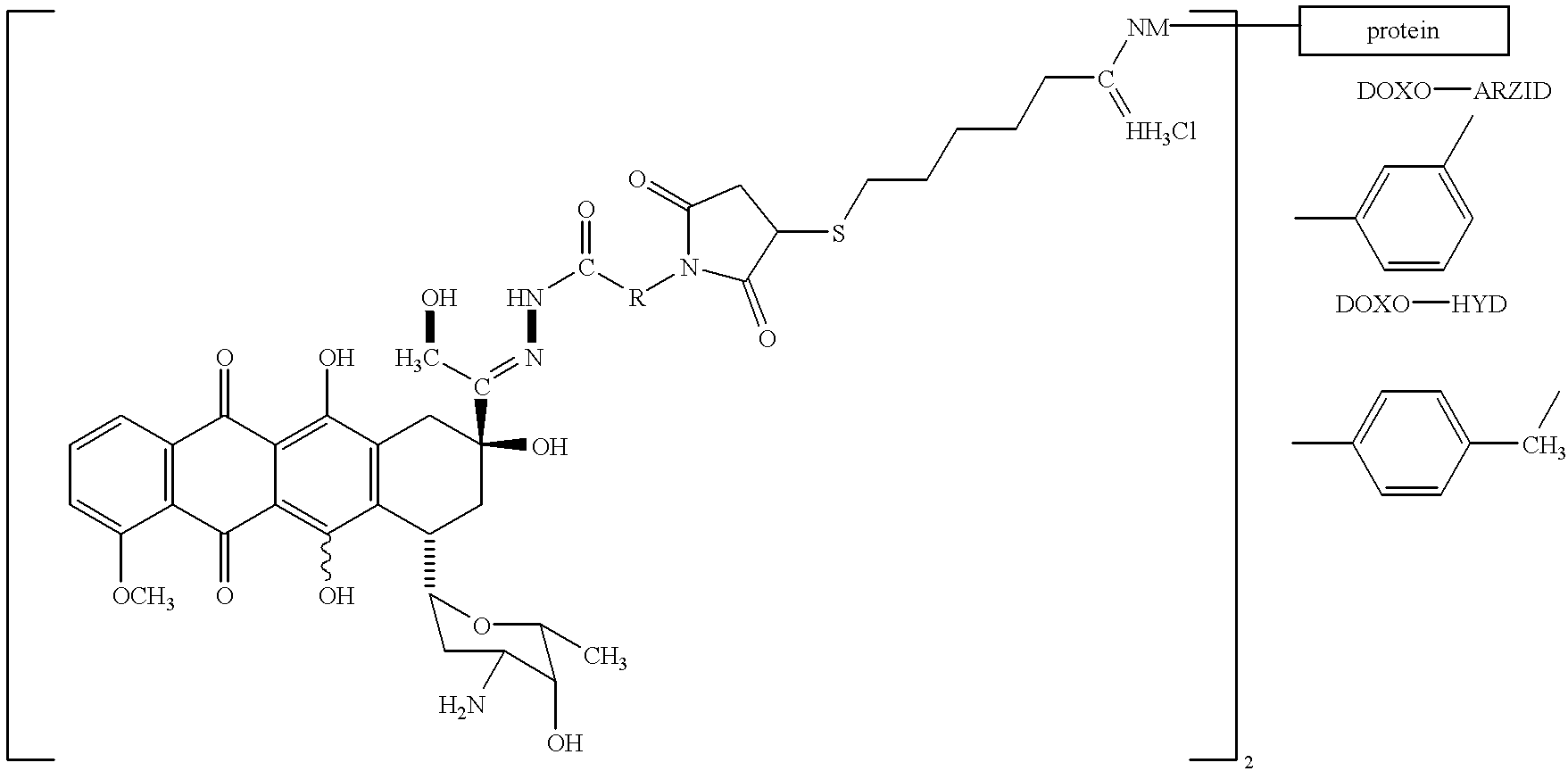
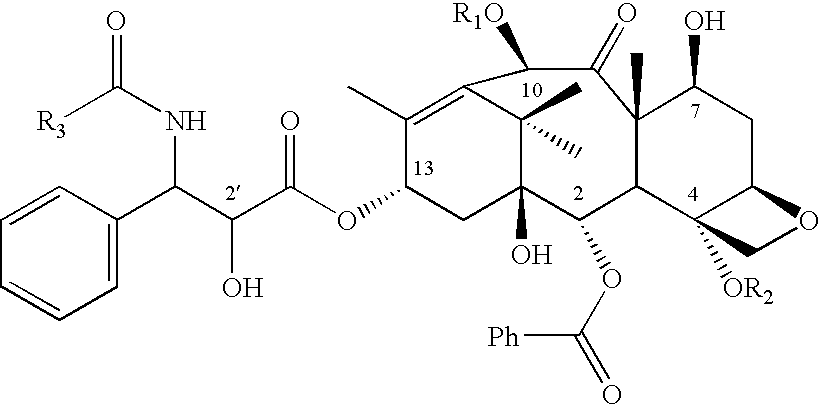
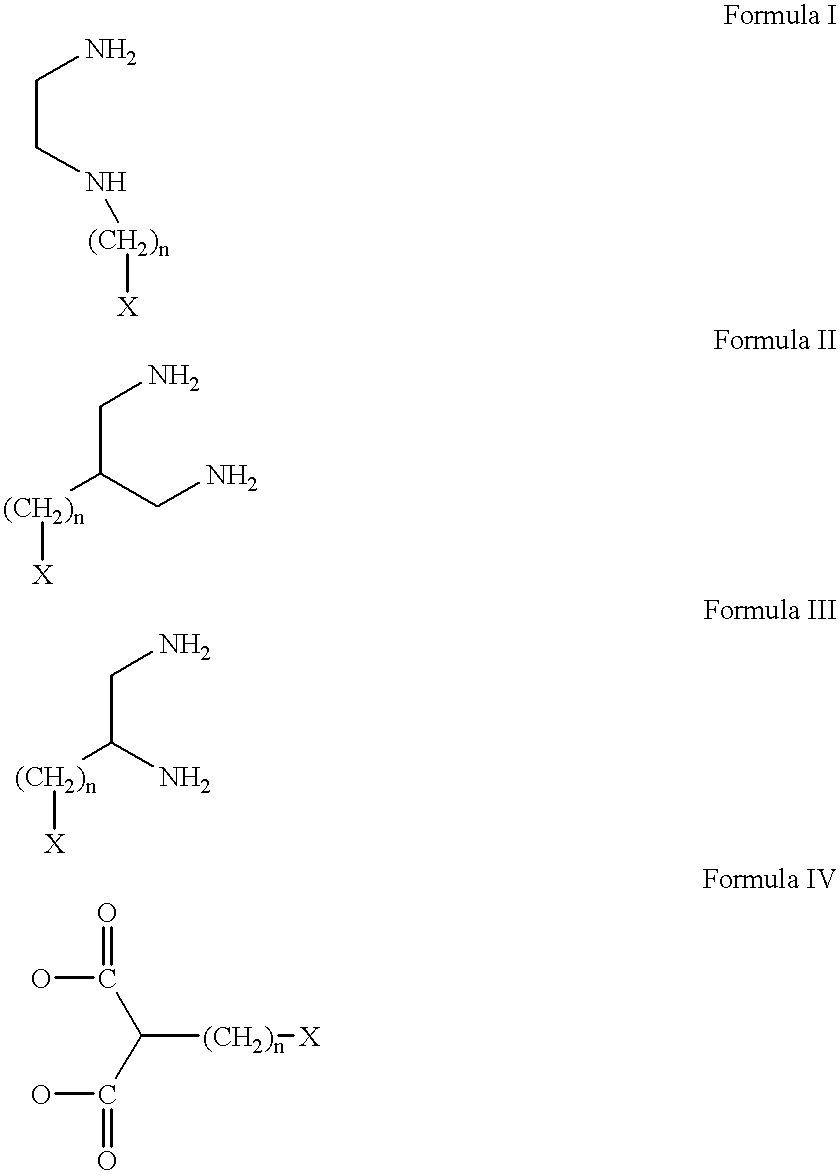
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R* H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
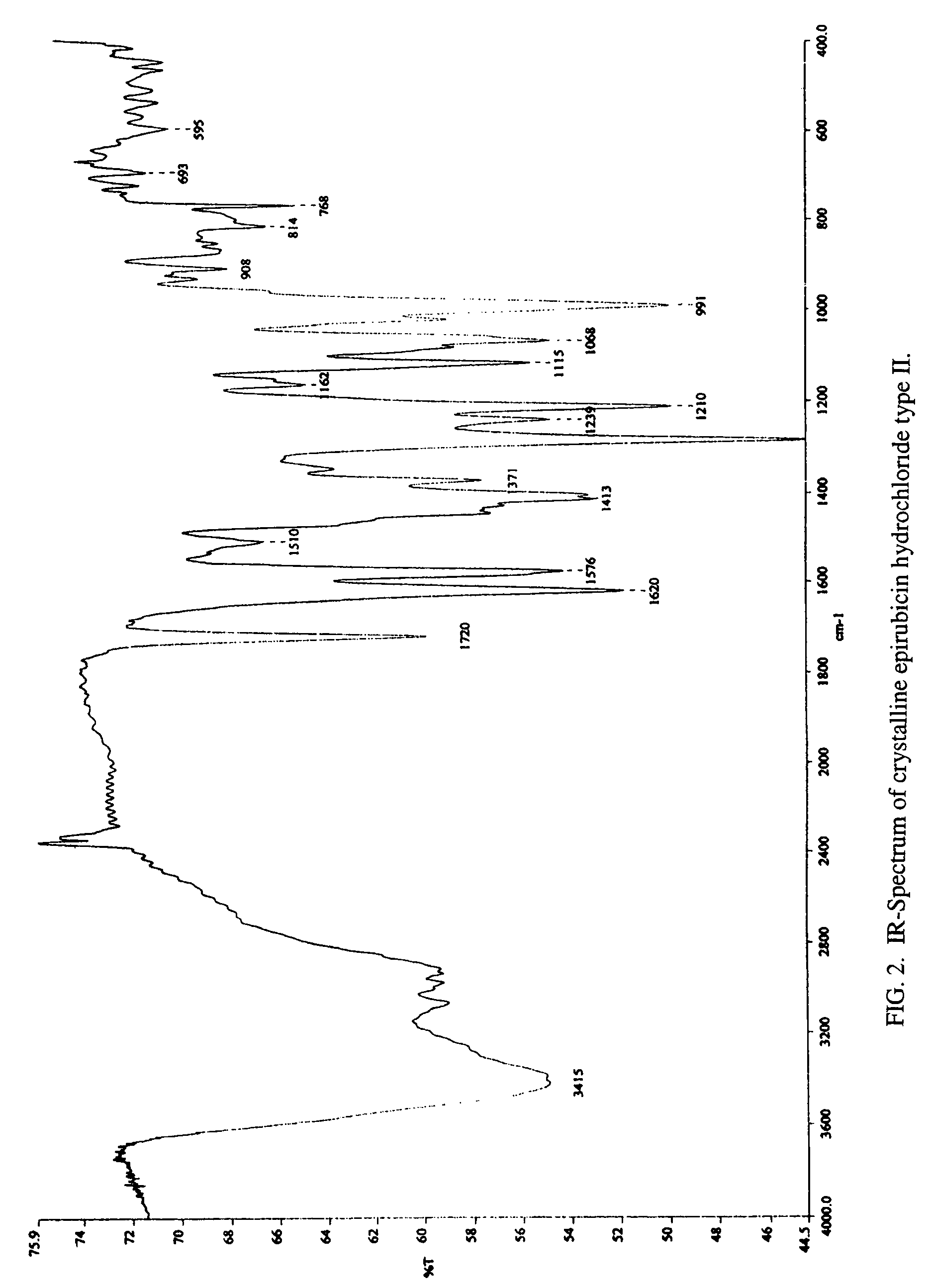
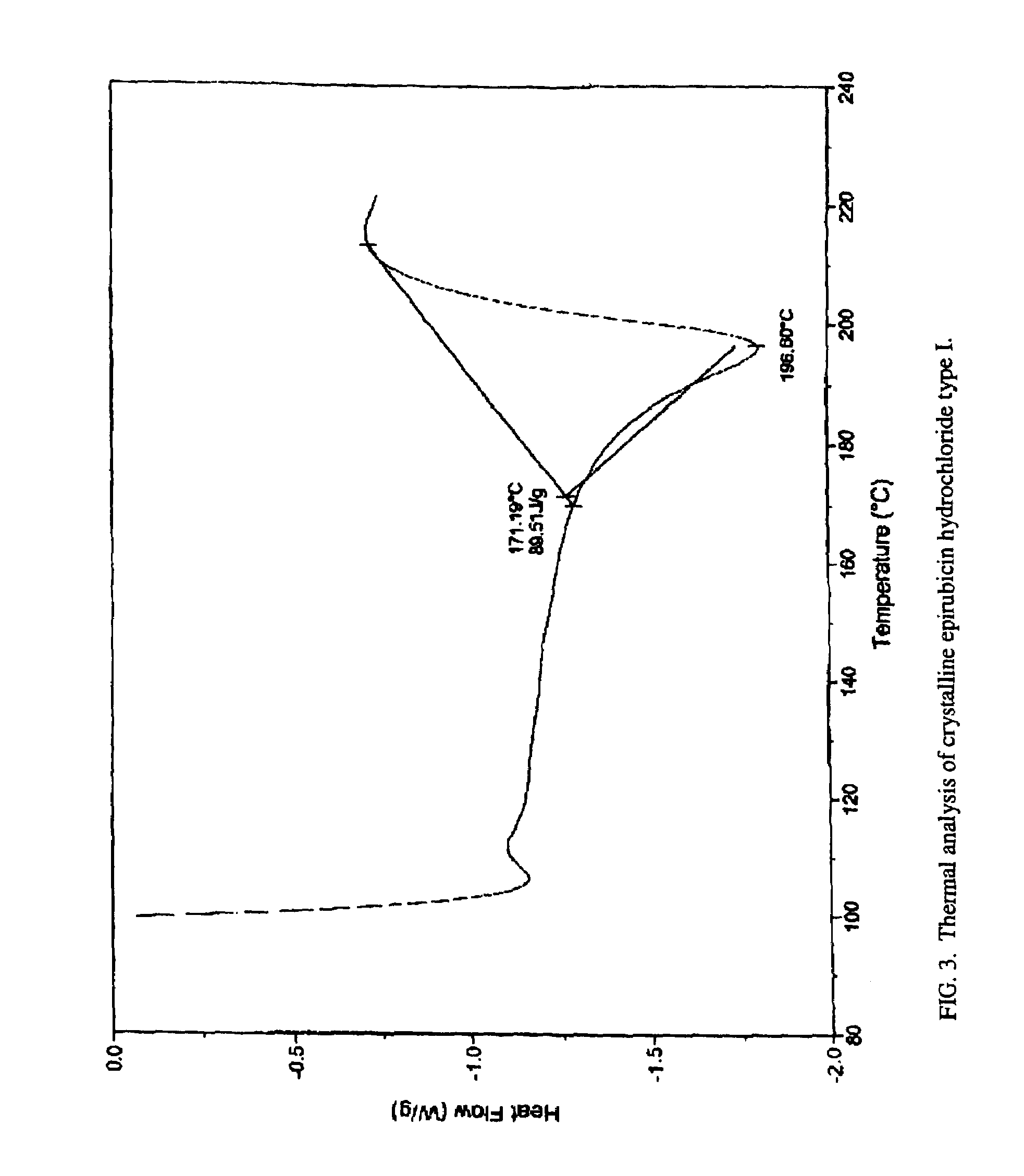
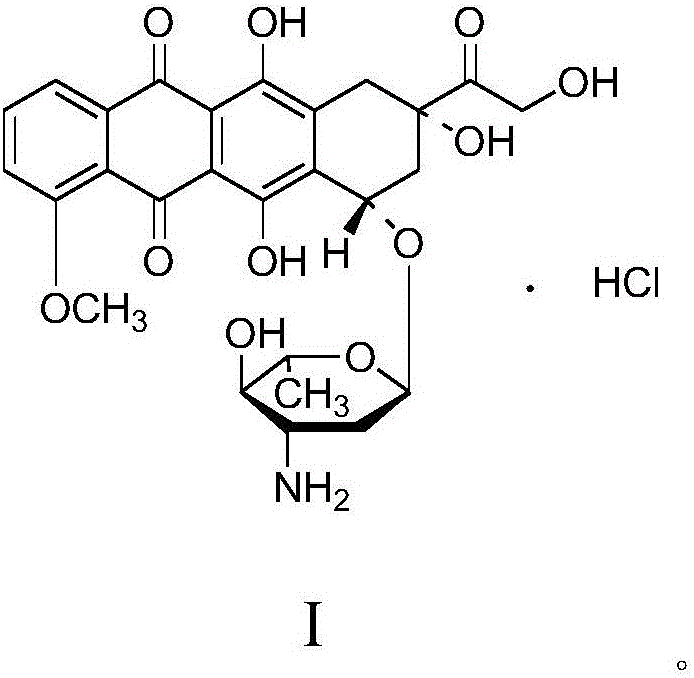
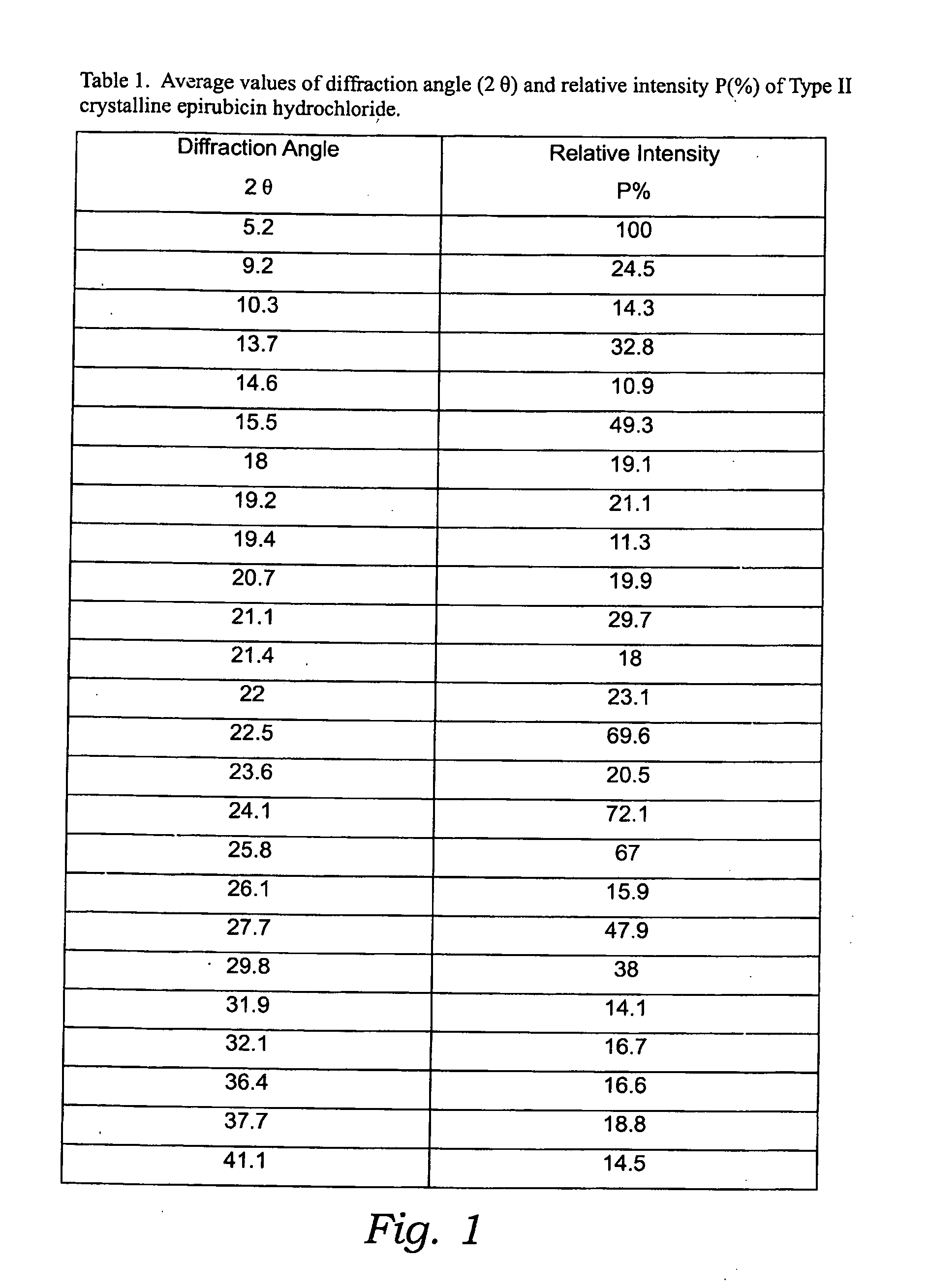
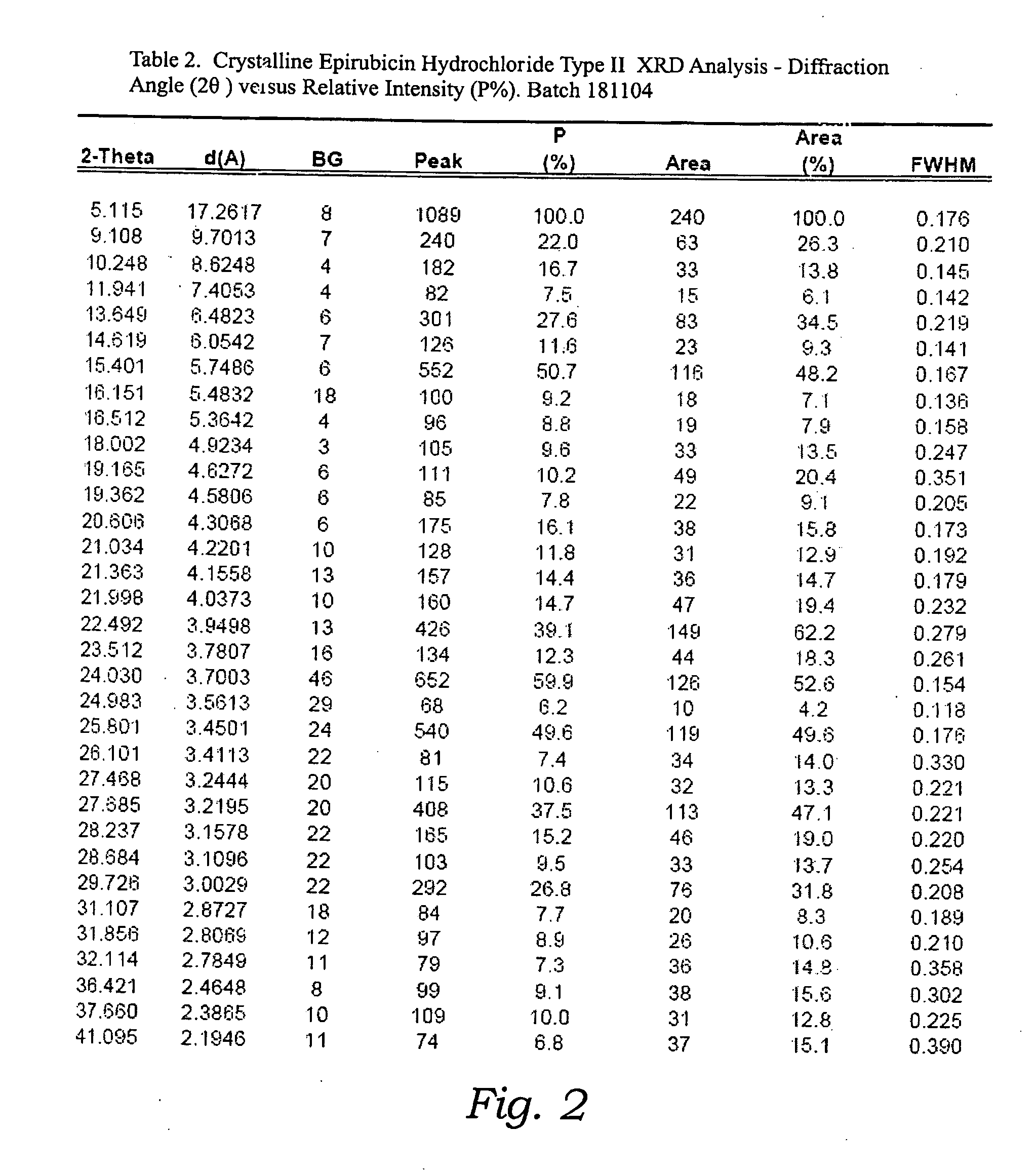
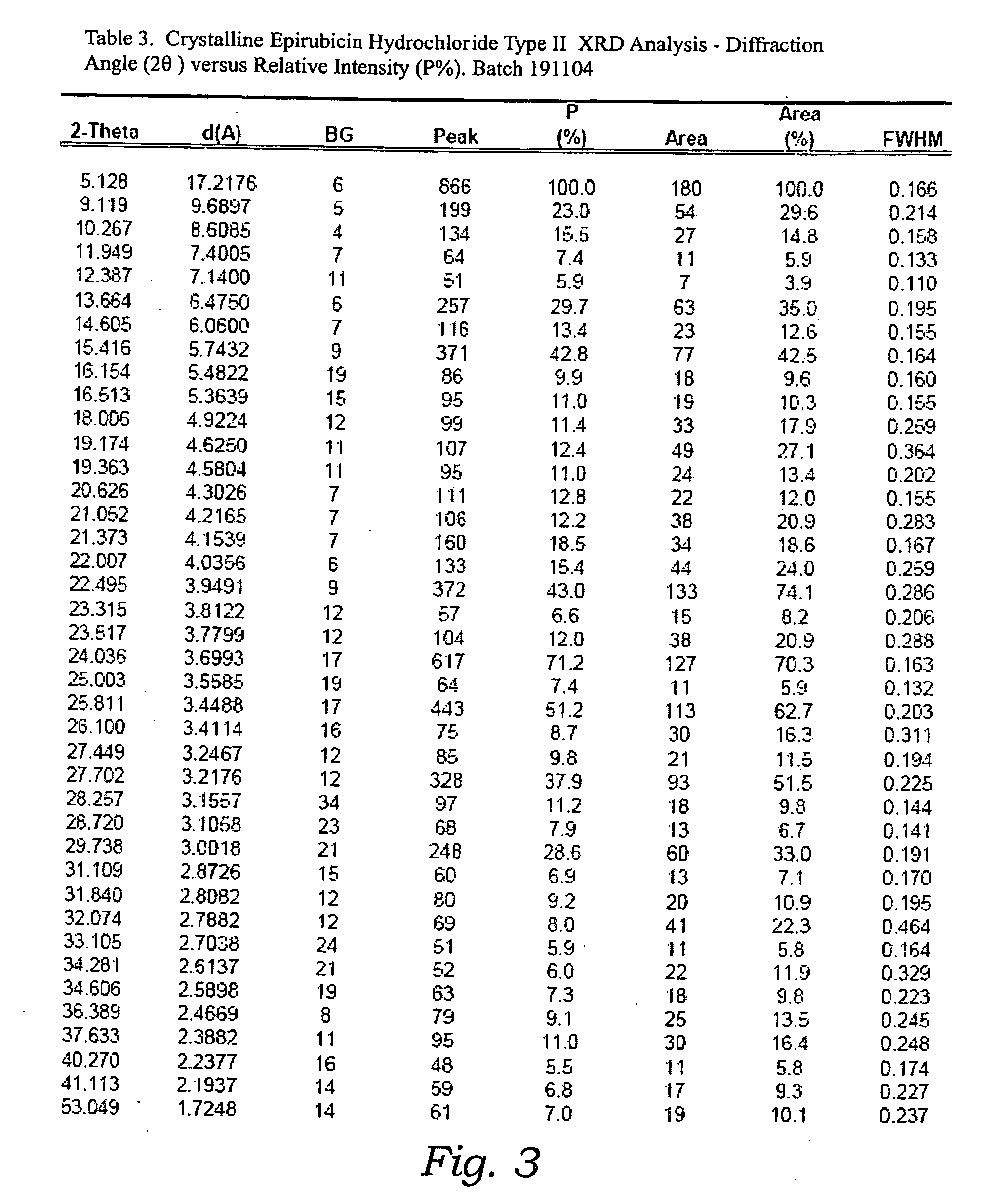
Thermally stable crystalline epirubicin hydrochloride and method of making the same
ActiveUS7485707B2Improve thermal stabilityImprove featuresSugar derivativesMedical preparationsCrystallographyThermal stability
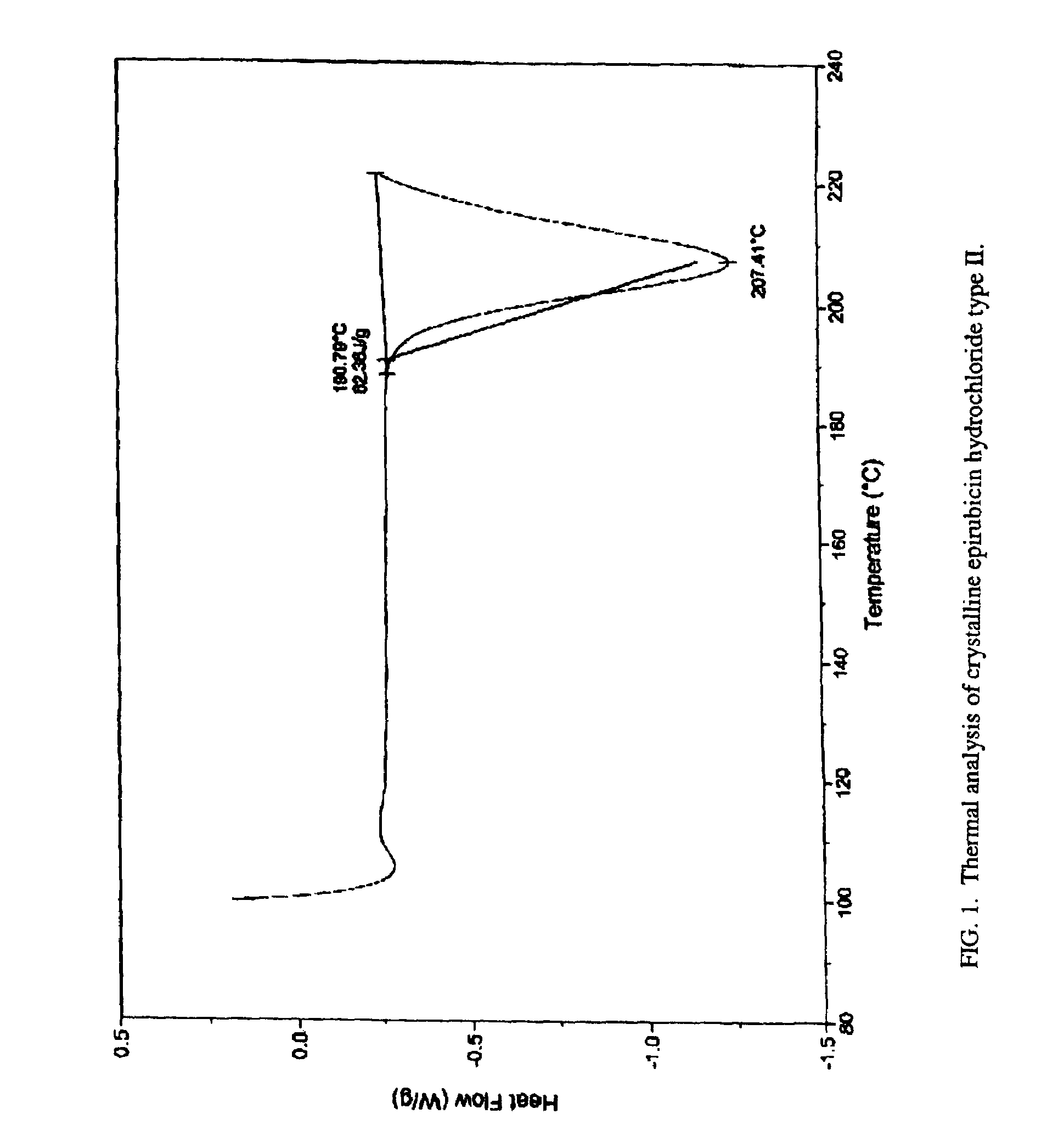
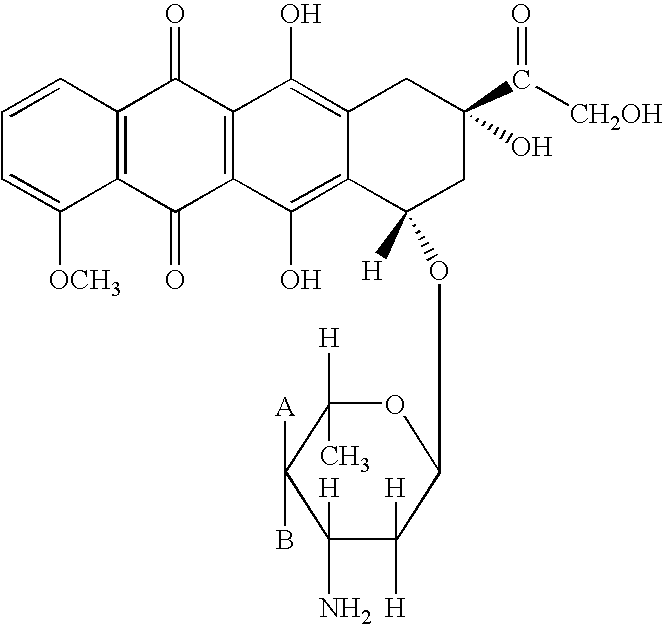
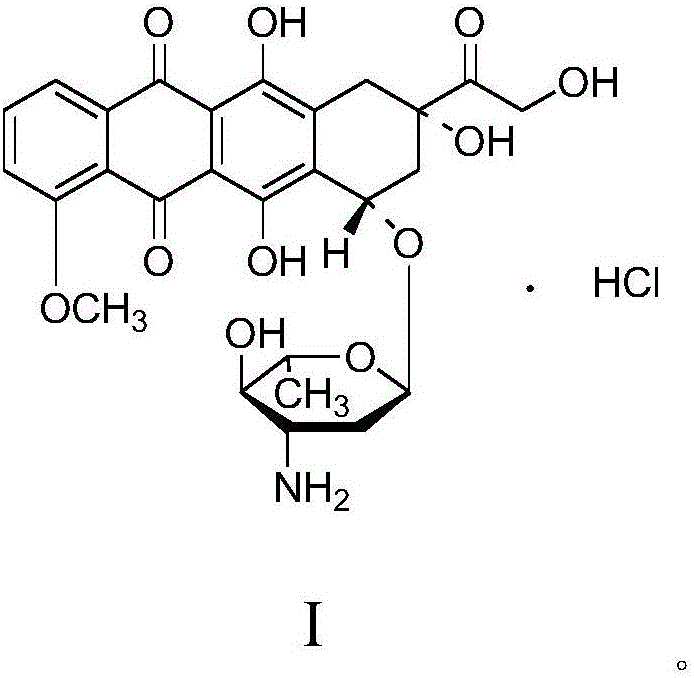
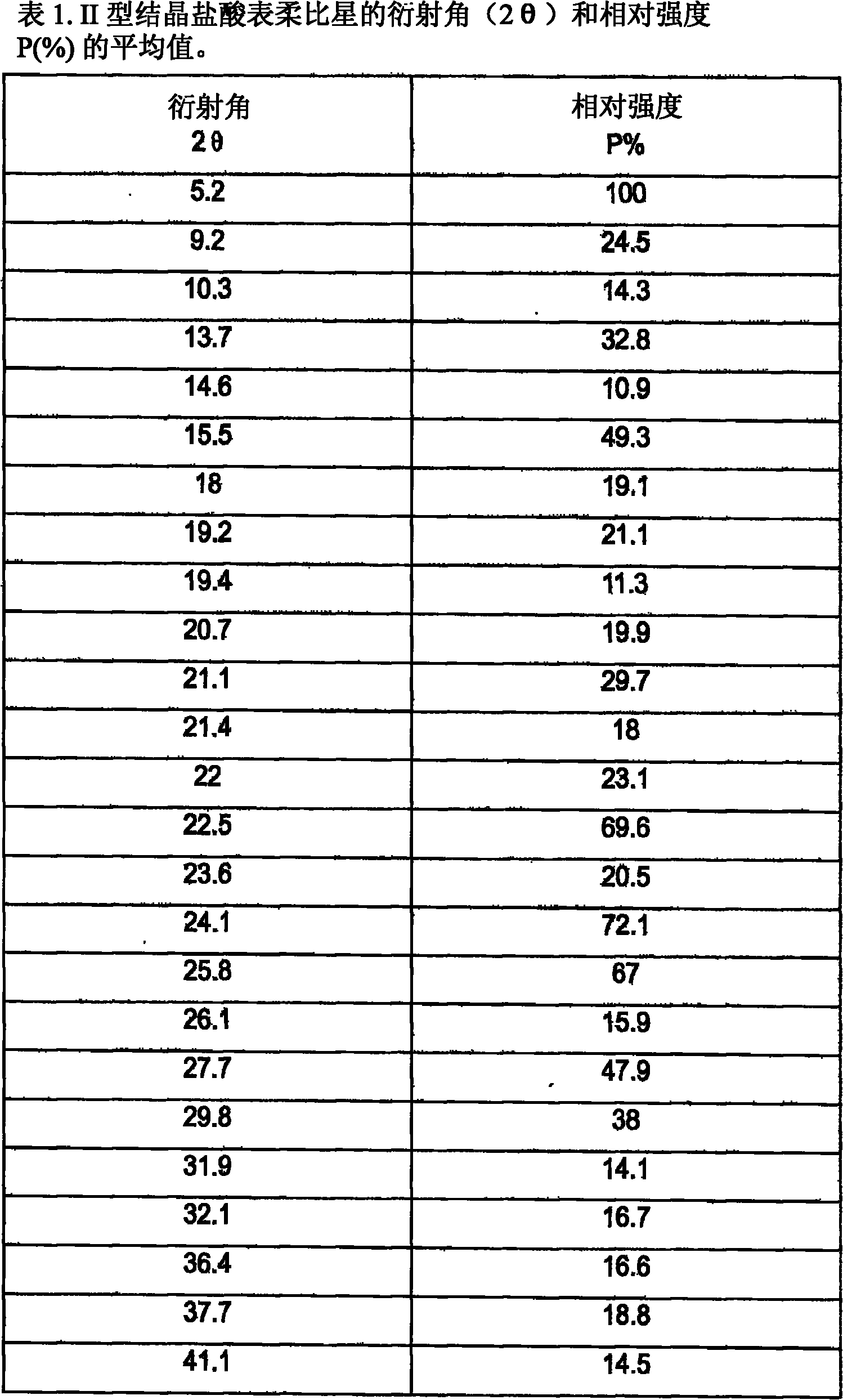
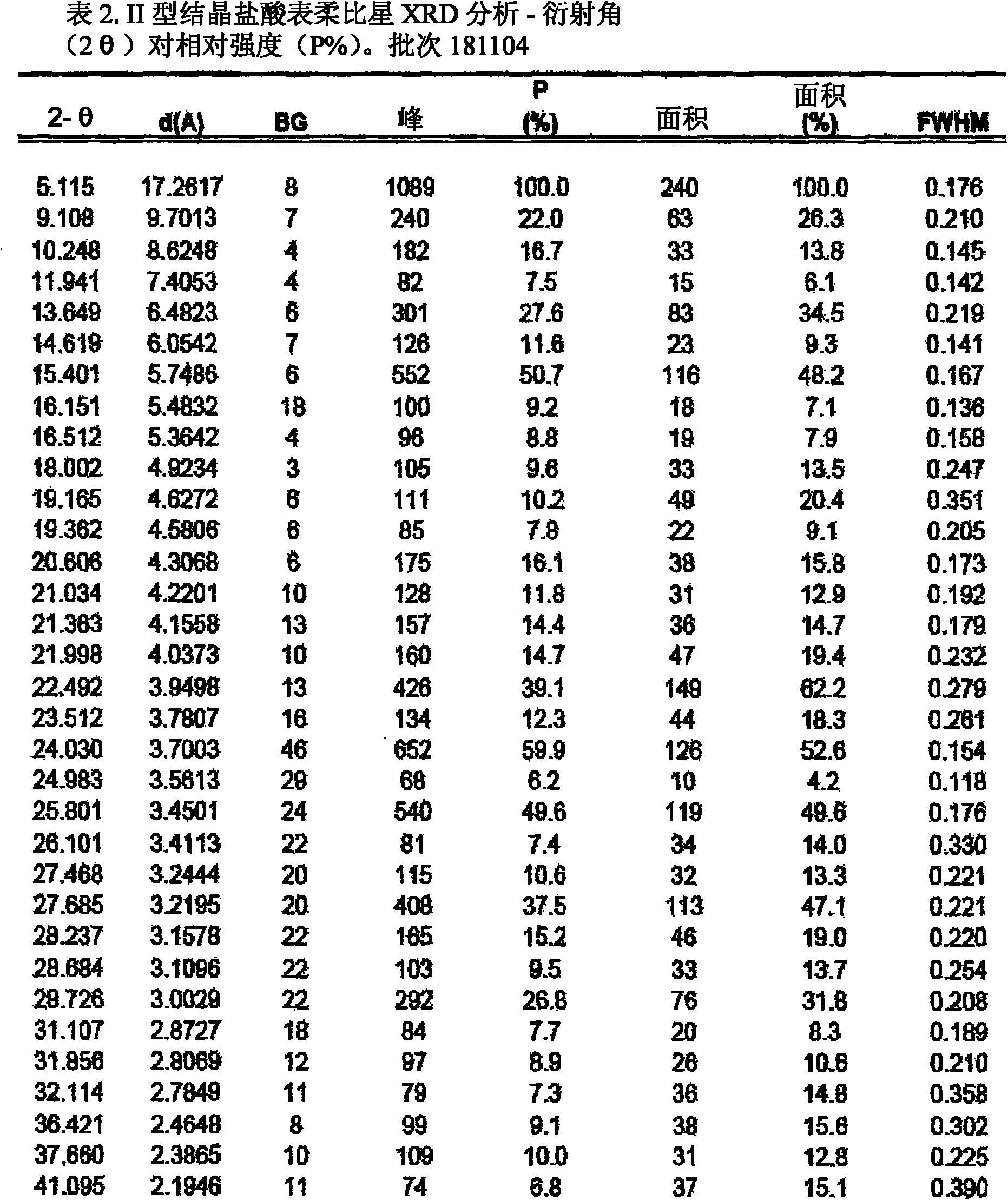
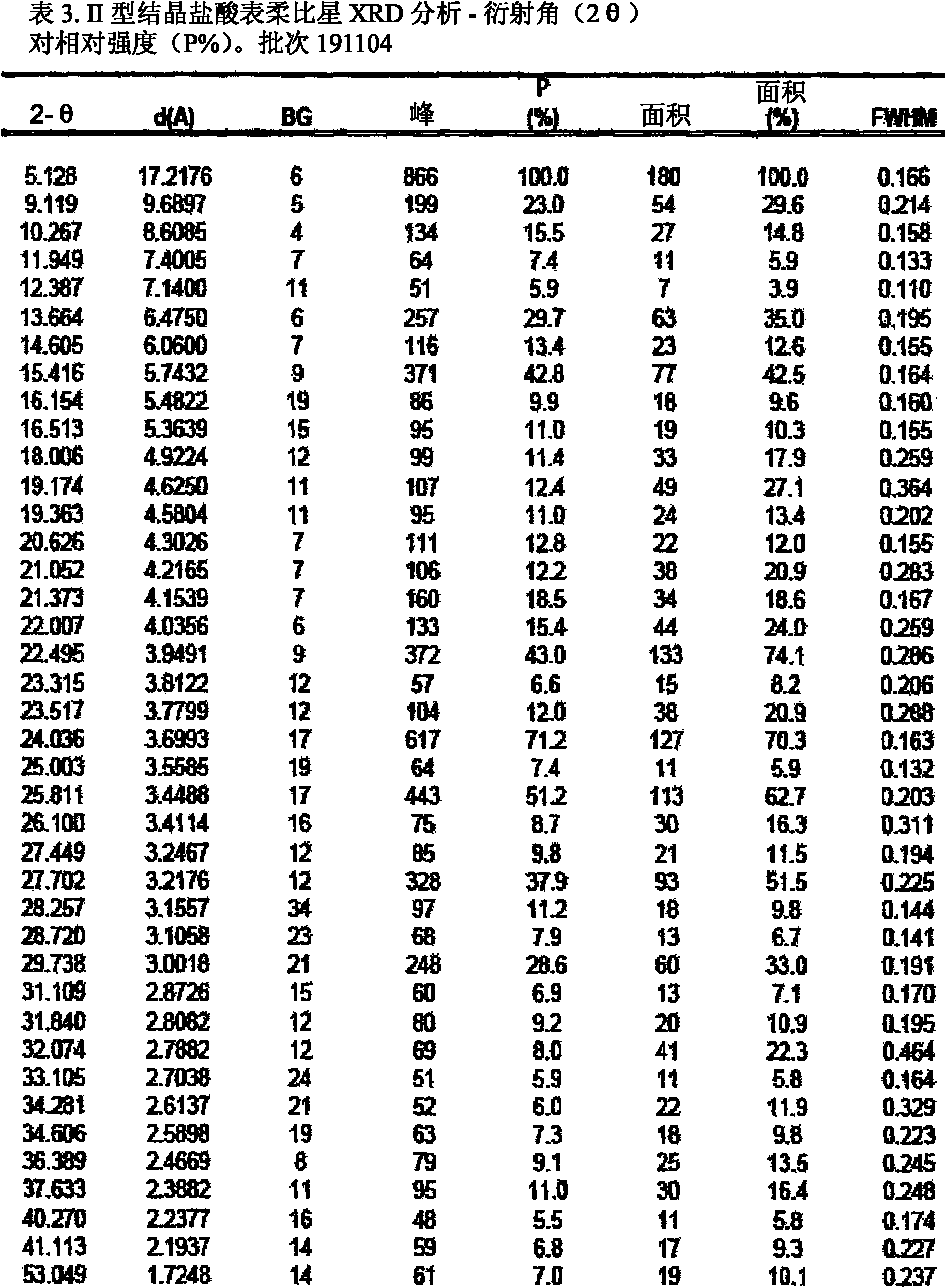
A crystalline form of epirubicin hydrochloride, named herein as “type II” crystalline epirubicin hydrochloride, has excellent thermal stability. Type II crystalline epirubicin hydrochloride has a powder X-ray diffraction pattern having average values of diffraction angle (2θ) and relative intensity P(%) as presented in the following table:DiffractionAngleRelative Intensity2ΘP (%)5.2369.89.21212.513.73215.516.4464.818.234521.1149.722.52925.524.07129.925.87918.427.76216.529.75710.134.3924.438.15713.144.2935.964.6997.777.815100.
Owner:SYNBIAS PHARMA
Folacin receptor mediated targeted acetyl pullulan polysaccharide nano granule and preparation thereof
InactiveCN101254309ASmall particle size distribution rangeImprove bioavailabilityPharmaceutical non-active ingredientsAntineoplastic agentsPullulanHydrophobic polymer
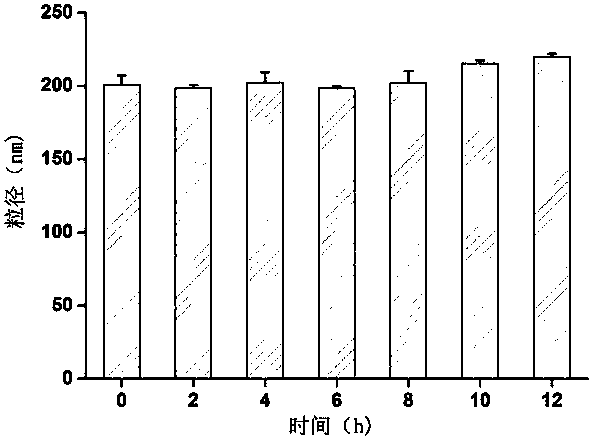
The invention discloses a method for preparing folic acid coupled acetyl pullulan polysaccharide and the nanoparticles thereof, a preparation of drug-loaded nanoparticles which take the compound as the carrier and the role of the drug-loaded nanoparticles on the tumor cells. Firstly, the water soluble pullulan polysaccharide is converted to hydrophobic polymers by acetylation, so as to be conductive to the preparation of the nanoparticles and the loading of a hydrophobic drug, and the tumor cells with the high expression of the folate receptor can be targeted after the coupling of the folic acid by esterification. The drug-loaded nanoparticles are prepared by taking epirubicin as a model drug and adopting the solvent dispersion method, and the role of the drug-loaded nanoparticles on the tumor cells are evaluated by the in vitro cell uptake test. The results show that the method for preparing the folic acid-acetyl pullulan polysaccharide nanoparticles by the solvent dispersion method is simple, the reproducibility is good, the expanded production is easy, the drug-loading ratio is high, and the drug-loaded nanoparticles can be taken into the tumor cells by the route of the folate receptor.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Medicinal composition for treating non-small cell lung cancer and application thereof
InactiveCN103948689AReduce dosageLow toxicityAntineoplastic agentsHeavy metal compound active ingredientsSalvia miltiorrhizaCarboplatin
The invention discloses a medicinal composition for treating non-small cell lung cancer. The medicinal composition comprises a target medicament, a chemotherapeutic medicament and a traditional Chinese medicament, wherein the target medicament is one or more of bortezomib, imatinib, gefitinib and sunitinib; the chemotherapeutic medicament is one or more of 5-fluorouracil, carboplatin, epirubicin, adriamycin and fludarabine; and the traditional Chinese medicament is one or more of salvia miltiorrhiza, astragalus membranaceus, sappanwood, Chinese pulsatilla root and portulaca oleracea. The medicinal composition has a remarkable synergetic treatment effect when being used for treating the non-small cell lung cancer, and can be used for remarkably strengthening the cancer-inhibition effect compared with treatment of a single medicament, so that the medicament dosage can be reduced, and the toxic and side effects of chemotherapeutic medicaments can be reduced.
Owner:NORTHWEST A & F UNIV
Epirubicin loaded graphene quantum dot drug carrying system and preparation method thereof
ActiveCN104984349ARealize the loadIncrease loading capacityOrganic active ingredientsPharmaceutical non-active ingredientsMedicineDrug carrier
The invention discloses an epirubicin loaded graphene quantum dot drug carrying system and a preparation method thereof. The preparation method includes the following steps: preparation of graphene quantum dots by a hydrothermal method; loading an anti-cancer drug with the graphene quantum dots; and performing a drug slow-release experiment, and performing a cytotoxicity test on the drug carrying system. The graphene quantum dots prepared by the hydrothermal method are used as a drug carrier for being loaded with epirubicin, greatly improve the drug loading rate, and show a quite good inhibitory effect on tumor HeLa cells. The preparation method of the drug carrying system is simple to operate, mild in reaction conditions and high in drug loading rate, and is expected to have a broad application prospect in the field of biological medicine.
Owner:南京美材科技有限公司
Chemotherapeutic drug-photosensitizer co-assembled nanoparticles and construction thereof
ActiveCN109718207APromote enrichmentExtend cycle timePowder deliveryOrganic active ingredientsChlorophyll derivativesPorphyrin
The invention belongs to the field of new auxiliary materials and new dosage forms of medicine preparations and relates to a chemotherapeutic drug-photosensitizer co-assembled nanoparticles and construction thereof. A chemotherapeutic drug is an anthracycline chemotherapeutic drug selected from mitoxantrone, doxorubicin or epirubicin; a photosensitizer is a porphyrin photosensitizer selected fromchlorine e6, hematoporphyrin monomethyl ether or a chlorophyll derivative, wherein the molar ratio of the chemotherapeutic drug to the photosensitizer is 3:1-1:3. A certain quantity of the chemotherapeutic drug and the photosensitizer or a mixture of the chemotherapeutic drug, the photosensitizer and PEG is dissolved in a proper quantity of organic solvent, and the solution is slowly dropwise added to water while stirring to form uniform nanoparticles spontaneously. The preparation process is simple, enlarged production is easy, particle size is small and uniform, and the nanoparticles can beenriched at tumor parts through a reinforced permeation retention effect; the nanoparticles have ultrahigh drug loading capacity and can reduce related toxicity of auxiliary materials; and surface modification is easy, and the circulation time of the nanoparticles in blood can be prolonged by PEG modification.
Owner:SHENYANG PHARMA UNIVERSITY
Multi drug response markers for breast cancer cells
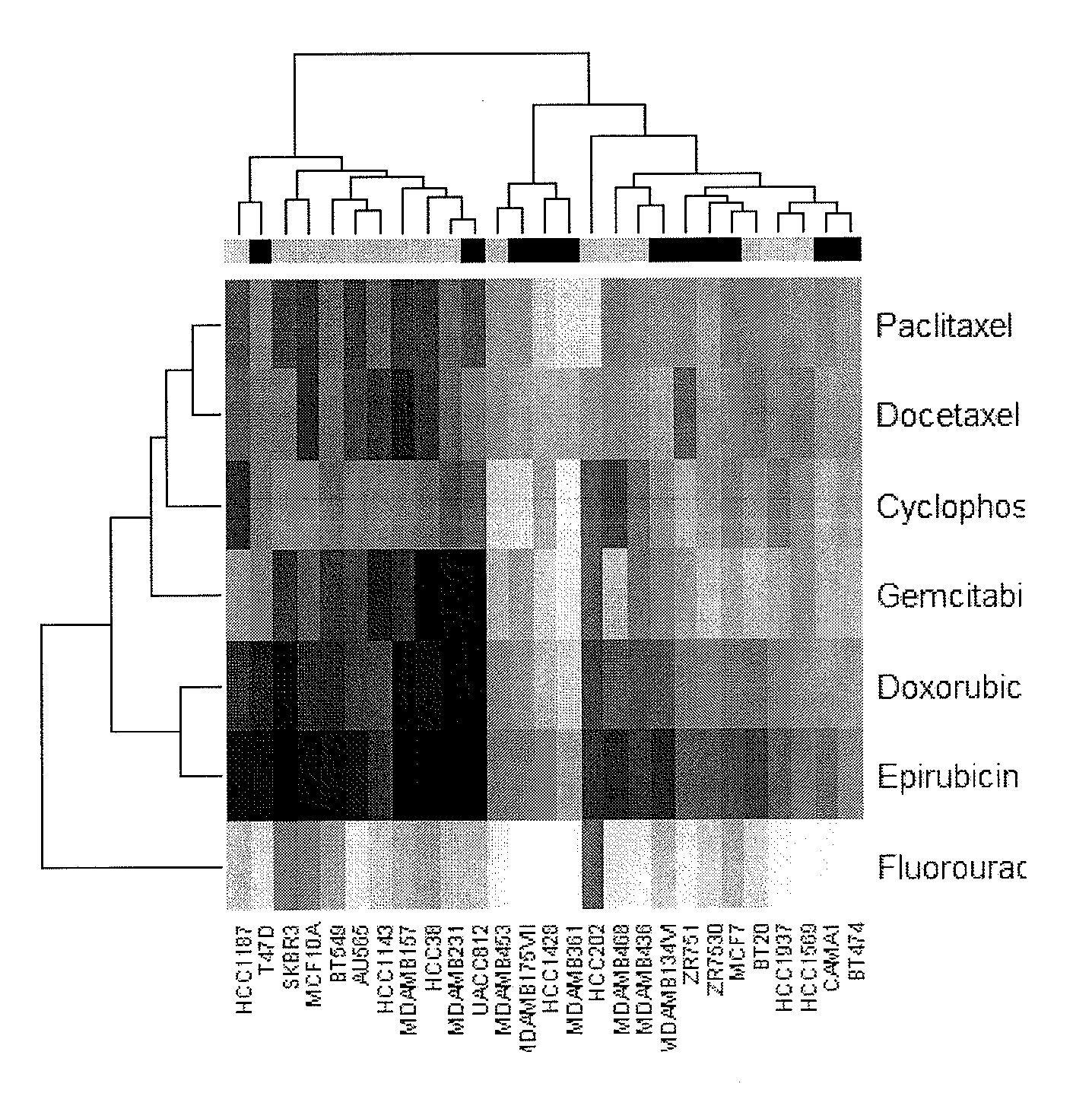
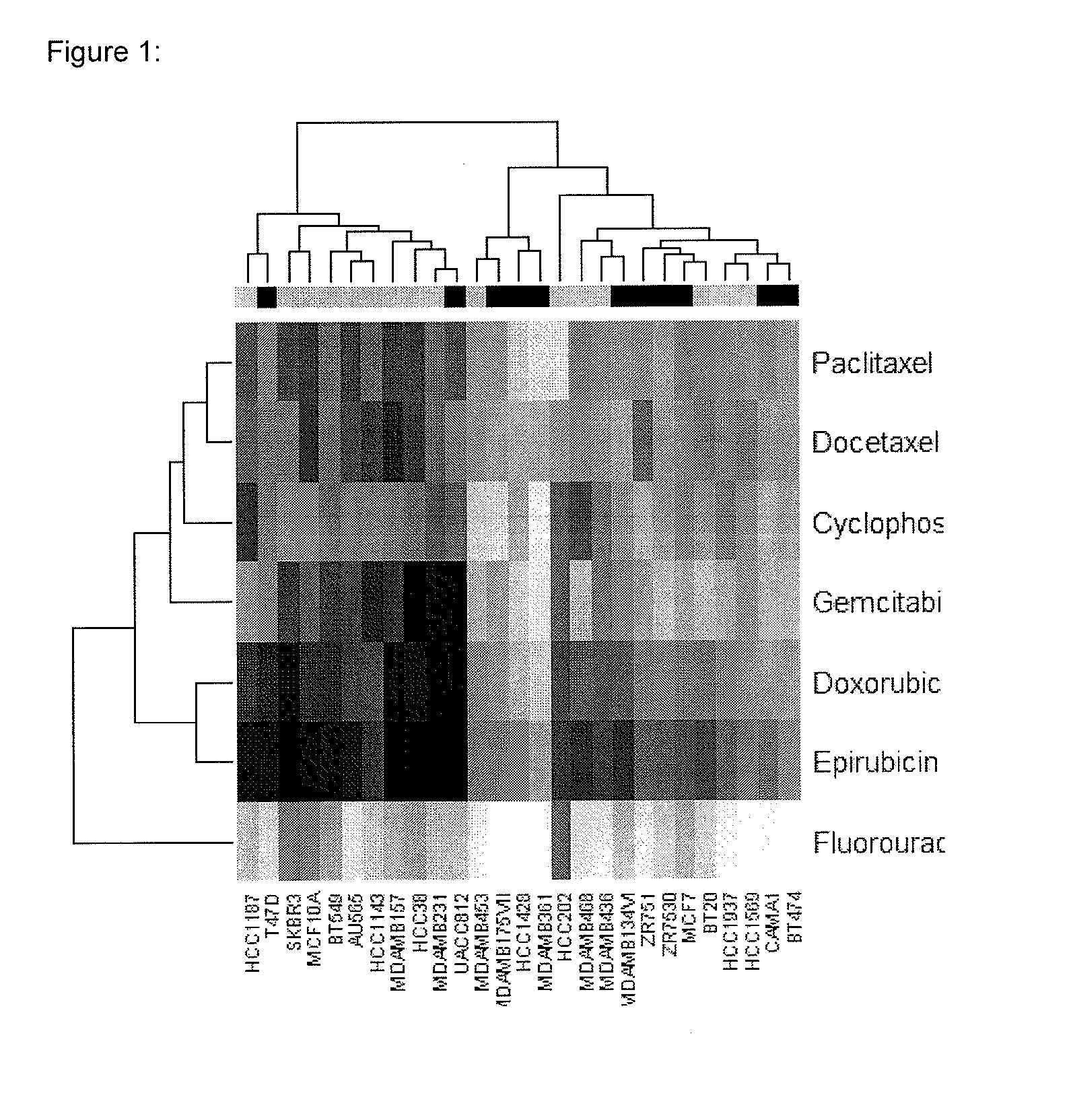
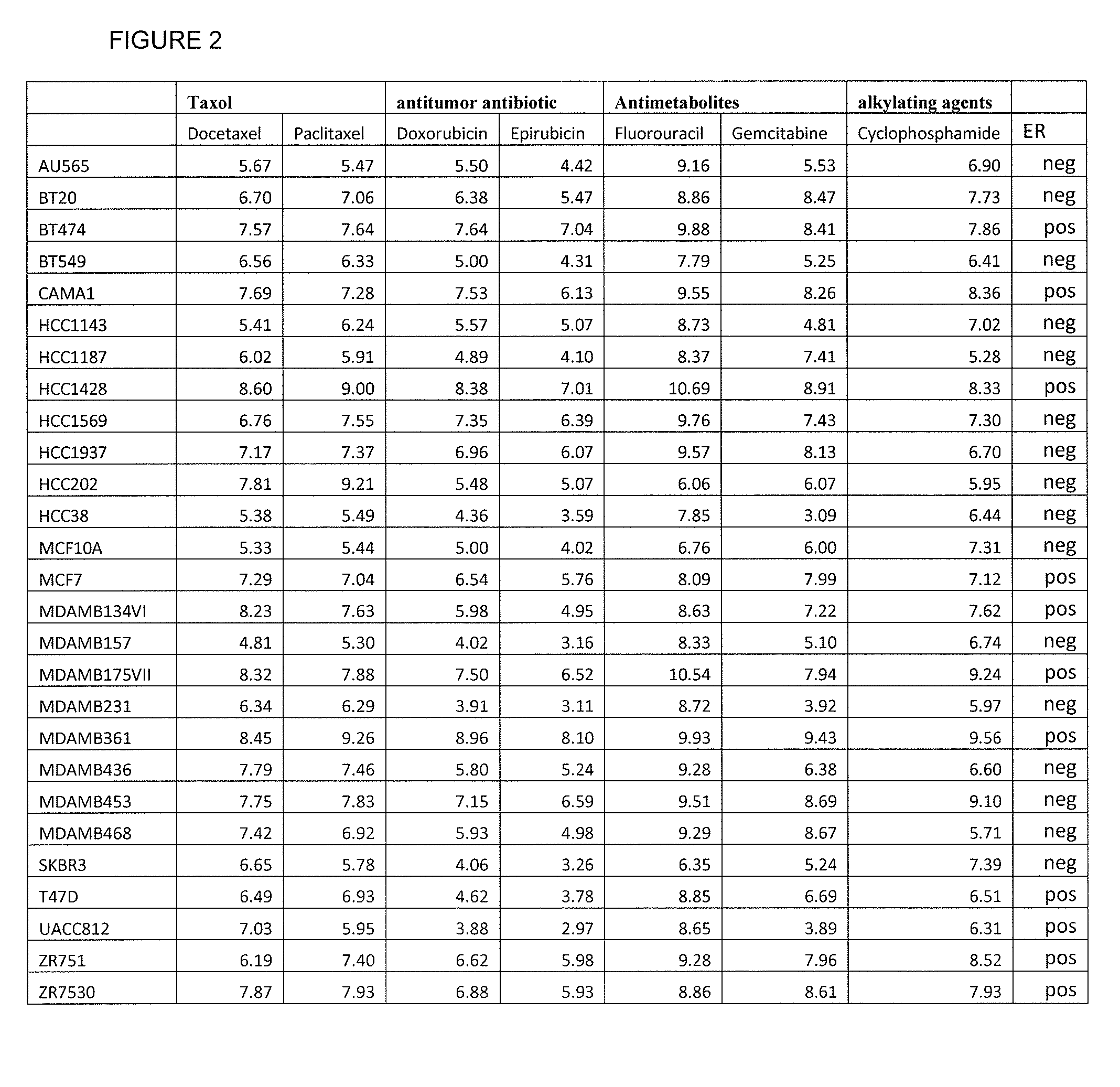
The present invention provides methods for preparing a gene expression profile of a breast cancer cell, tumor, or cell line, where the gene expression profile may be evaluated for one or more gene expression signatures indicative of multidrug resistance. The signature may be indicative of resistance to one or more chemotherapeutic agents selected from a Taxol (e.g., Docetaxel or Paclitaxel), an antibiotic (e.g., Doxorubicin or Epirubicin), an antimetabolite (e.g., Fluorouracil and / or Gemcitabine), and an alkylating agent (e.g., Cyclophosphamide). Generally, the gene expression profile contains the level of expression for a plurality of genes listed in FIGS. 3, 4, and / or 5. Gene expression profiles for evaluating multidrug resistance for ER positive and ER negative breast cancers are also provided.
Owner:PRECISION THERAPEUTICS
Epirubicin hydrochloride liposome and preparation thereof
InactiveCN101264056ALow toxicityReduce manufacturing costOrganic active ingredientsPharmaceutical non-active ingredientsDrug contentSide effect
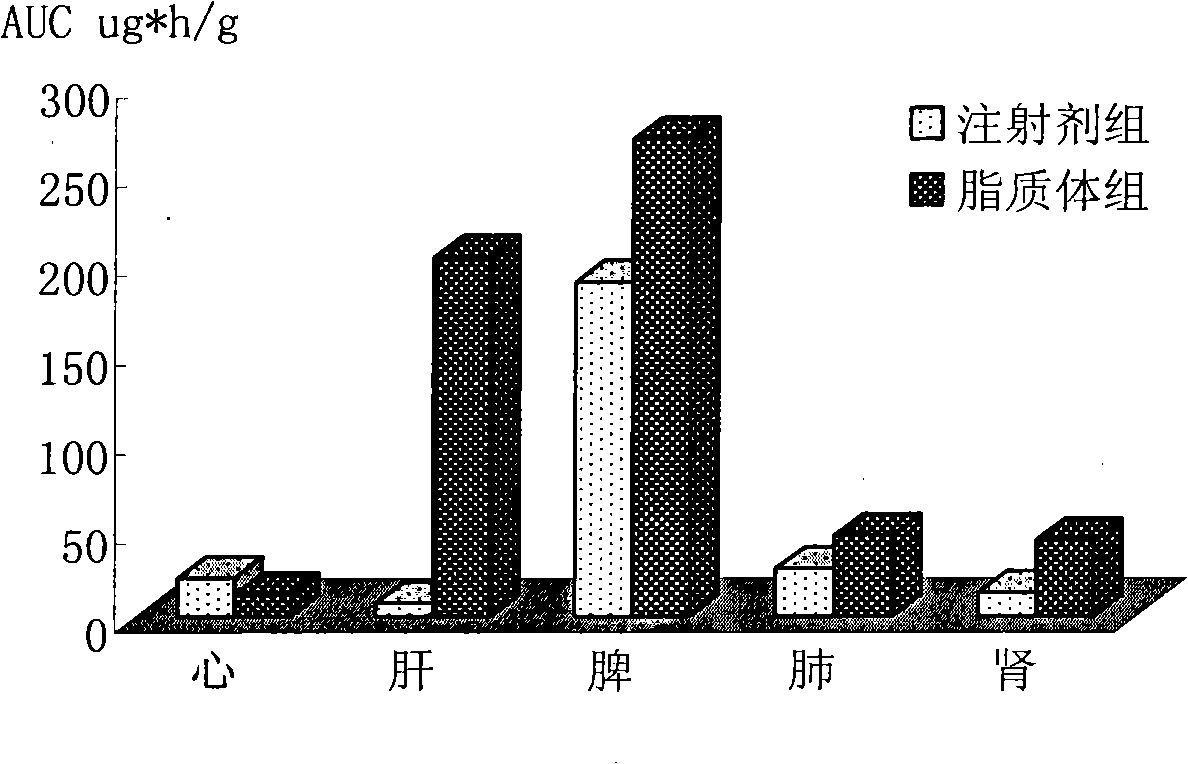
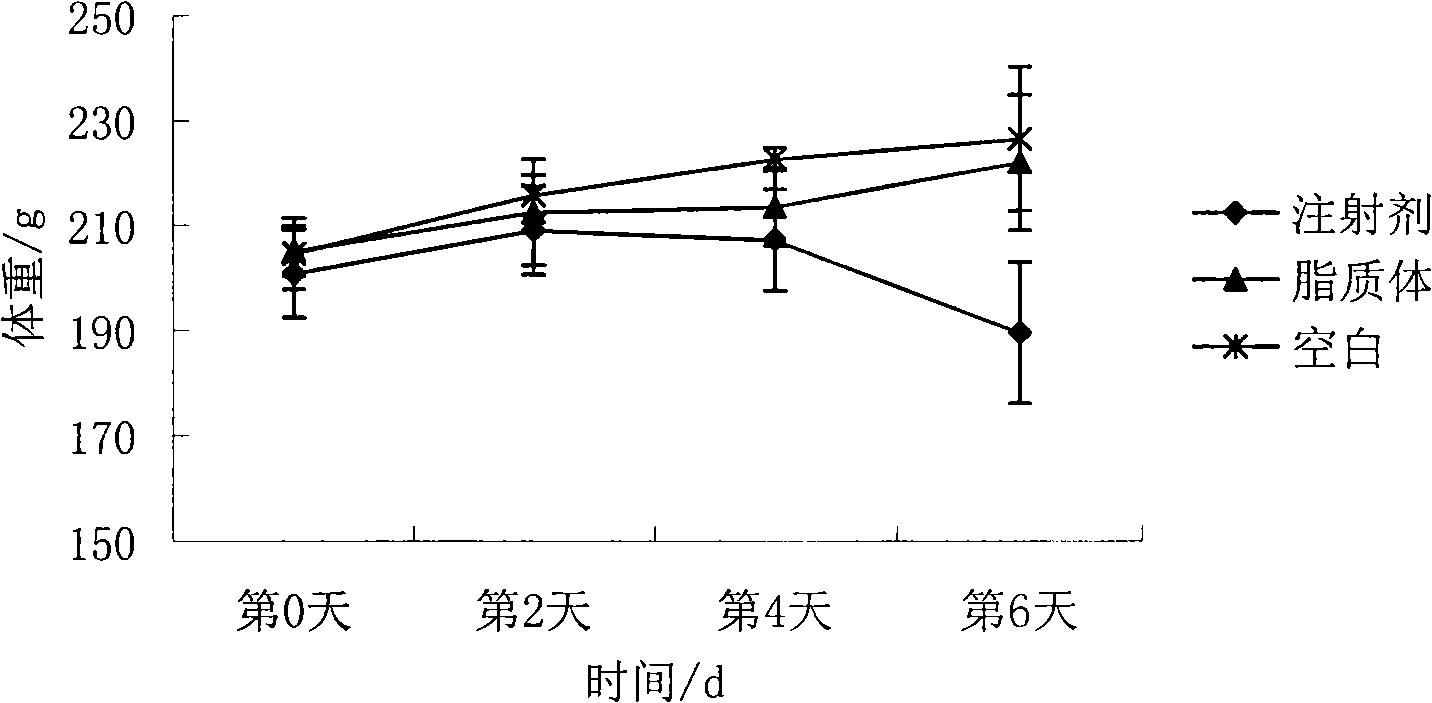
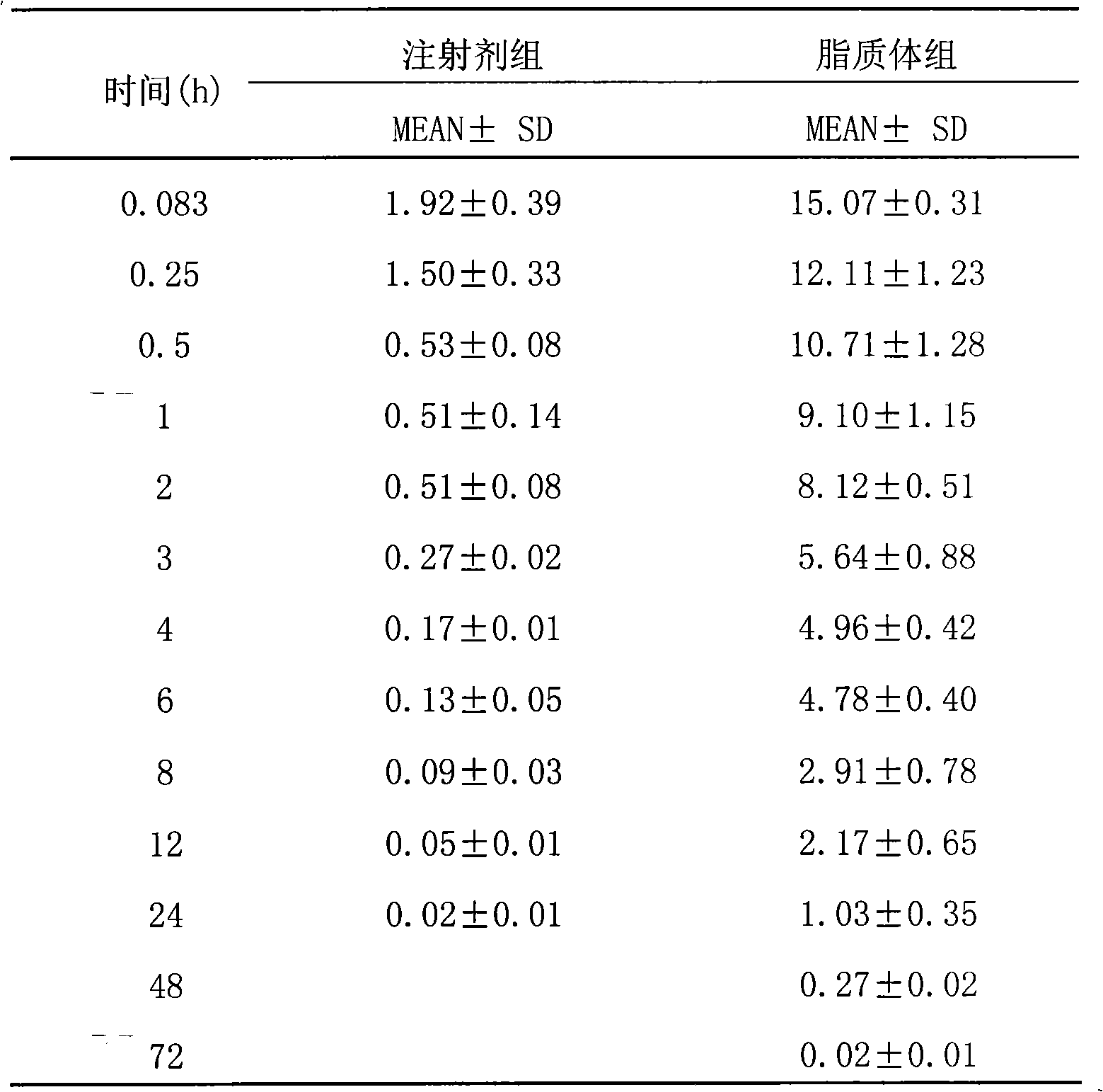
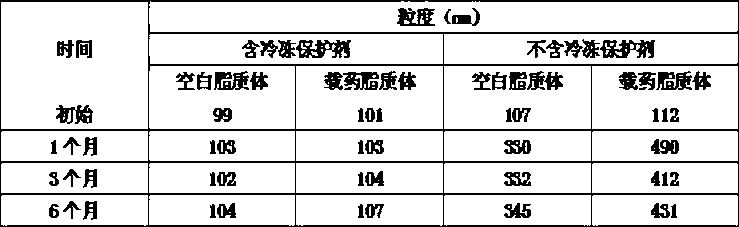
The invention belongs to the medicine technical field, relates to epirubicin hydrochloride liposome and a preparation method. The liposome comprises epirubicin hydrochloride and neutral phospholipid and cholesterol and / or negative charge phospholipids and / or long-circulating phospholipids and buffer solution, and actively carries drug with a PH gradient method or an ammonium sulphate gradient method; the entrapment rate of the achieved liposome is more than 90%, to make smaller volume contain more main drug component of epirubicin. Compared with the exiting techniques, the epirubicin hydrochloride liposome and the preparation method has the advantages of high drug content, high bioavailability, long internal circulation time, low toxic and side effect and other advantages; besides, the production cost can be reduced obviously, the epirubicin hydrochloride liposome is more suitable for industrial production after being made into lyophilized preparation. The liposome is clinically suitable for the tumor therapy, in particular to the tumor therapy for children.
Owner:FUDAN UNIV
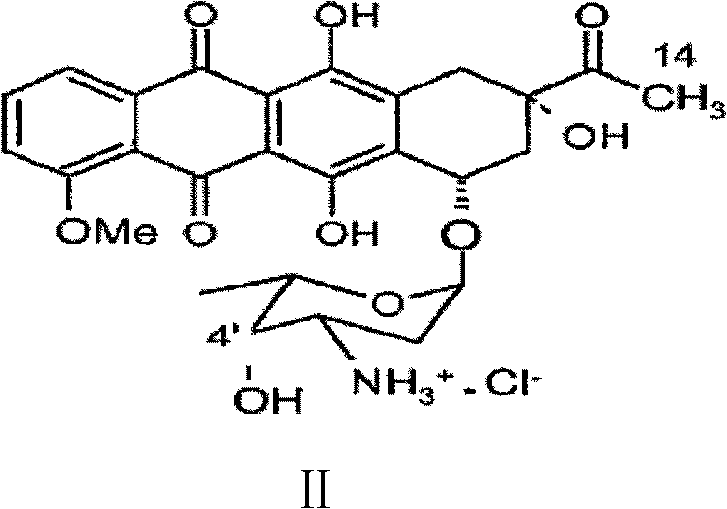
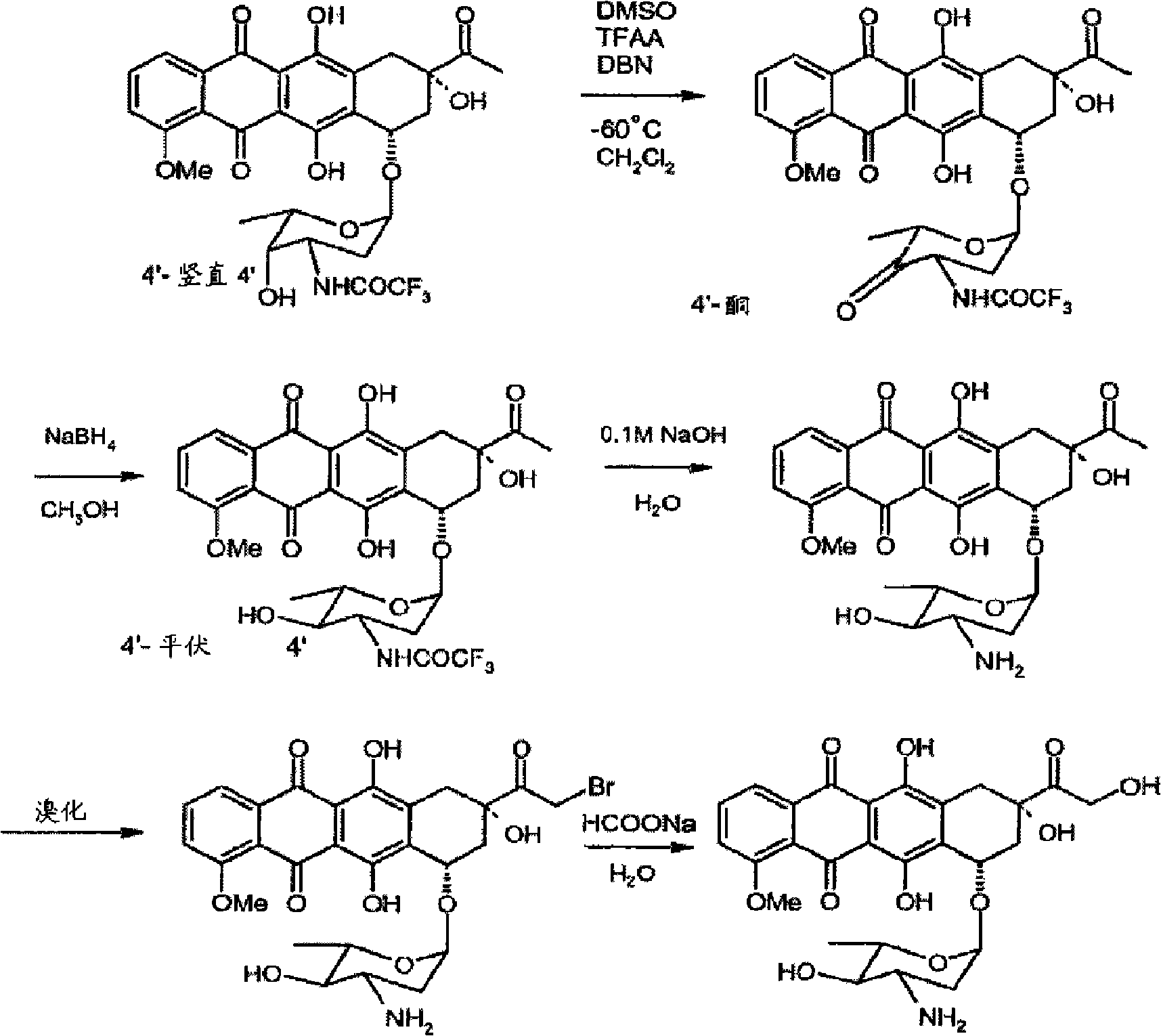
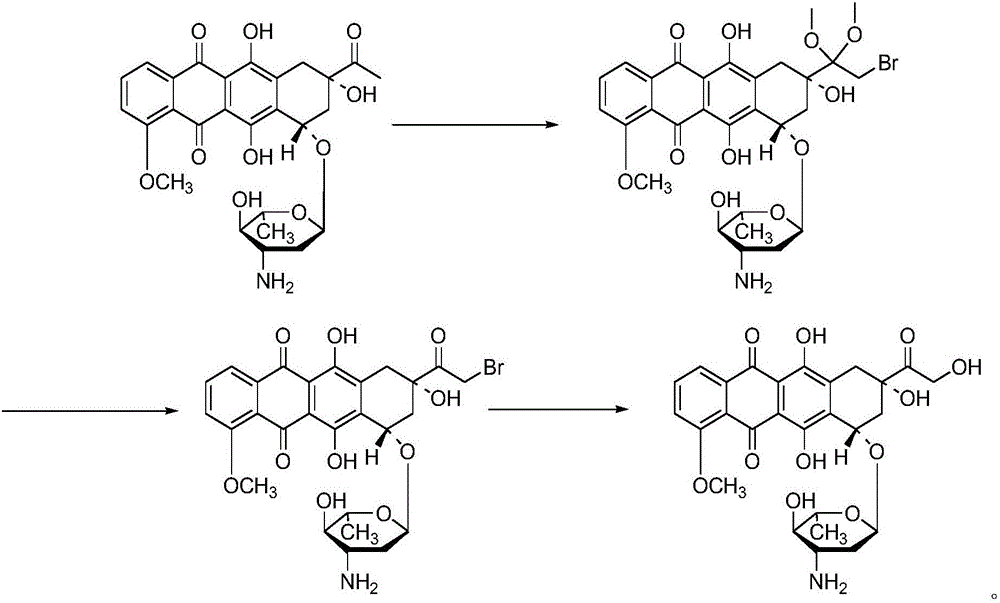
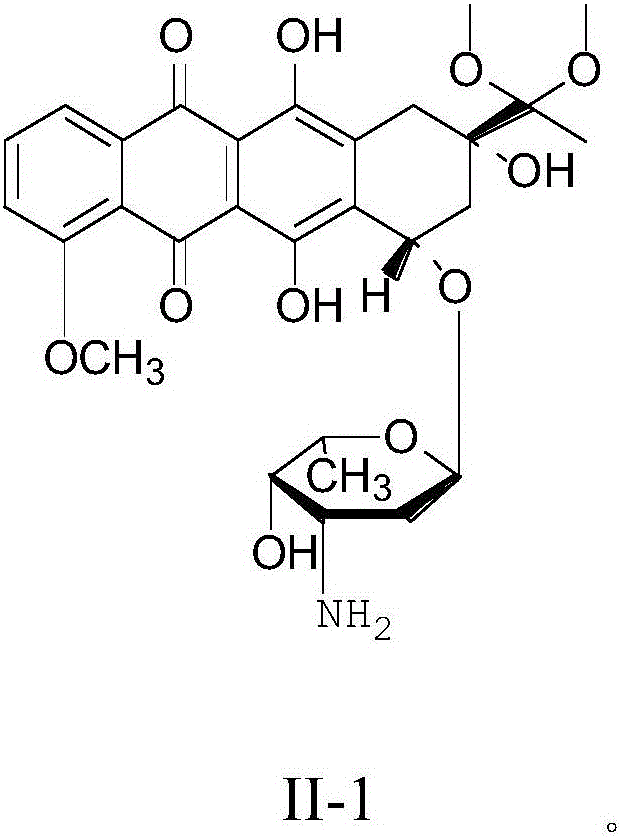
Preparation method for intermediate of epirubicin hydrochloride
ActiveCN103204888AReduce pollutionAvoid generatingSugar derivativesSugar derivatives preparationEtherSodium borohydride
The invention relates to a preparation method for an intermediate 4'-epidaunorubicin hydrochloride of epirubicin hydrochloride. The preparation method comprises the steps of trifluoroacetyl protection, oxidation, reduction, hydrolyzation, etc. The method effectively inhibit generation of (methylthio)methyl ether, increases selectivity for sodium borohydride during the reduction process and is mild in reaction conditions. The total yield of the reaction is over 40% (based on epidaunorubicin hydrochloride) and the purity reaches 99.5%.
Owner:SHANDONG NEWTIME PHARMA
Preparation process for Sappan Wood extract perfusate and application thereof in treating bladder cancer
InactiveCN101904892ANo adverse reactionSignificant clinical effectAntineoplastic agentsPlant ingredientsFreeze-dryingEthyl acetate
The invention relates to a preparation method for a Sappan Wood extract, in particular to a preparation process for Sappan Wood extract perfusate and application thereof in treating bladder cancer, which solves the problem of the application of the Sappan Wood extract in treating bladder cancer tumors. The preparation process comprises the following steps: taking and smashing Sappan Wood, carrying out water extracting for three times, combining the extracting solutions obtained by three-time water extracting, and carrying out decompression concentrating; standing overnight, and removing sediments; adding petroleum ether, extracting, and retaining aqueous phase; adding ethyl acetate, extracting, and removing the aqueous phase; carrying out decompression volatilizing to remove all the ethyl acetate; weighing dry substances, adding purified water to enable the dry substance content to be 2%, heating for dissolving, standing overnight at room temperature, and filtering out sediments; and freeze-drying, and carrying out split charging. The experiment research result shows that the extract has obvious killing effect on bladder neoplasm cells; and compared with mitomycin, epirubicin, Pirarubicin and other anticancer drugs, which are clinically and frequently used, the Sappan Wood extract perfusate has the advantages of high anticancer activity, less toxicity, safety, effectiveness and the like.
Owner:山西华尚汇商贸有限公司
Non-spherical drug-loaded particles and controlled release preparation of lactyl polymer and preparation methods thereof
InactiveCN101953776AStable structureNo hemolyticPowder deliveryHydroxy compound active ingredientsSolventSustained-Release Preparations
The invention relates to non-spherical drug-loaded particles and a controlled release preparation of a lactyl polymer and preparation methods thereof. The non-spherical particles of polylactic-co-glycolic acid (PLGA) are prepared by using an emulsion-solvent volatilization method assisted by small molecule materials. The PLGA is used as raw material coated with at least one of the following hydrophobic drugs: all-trans retinoic acid, paclitaxel, epirubicin, camptothecin or roxithromycin, wherein the mass ratio of the hydrophobic drug to a lactyl polymer high polymer material is 1:4-40. The drug-loaded particles of the all-trans retinoic acid are prepared and subjected to in vitro drug release evaluation. The results show that the preparation method has the advantages of simple preparation process, good reproducibility, significantly increased drug loading amount and encapsulation efficiency relative to spherical particles, very good controlled-release effect, no hemolysis initiation and safety use. The novel carrier and preparation have a potential industrial production value in the field of long-circulating controlled release of the hydrophobic drugs.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Imaging of drug accumulation as a guide to antitumor therapy
InactiveUS7175830B2Effective judgmentAvoid the needX-ray constrast preparationsRadioactive preparation carriersDocetaxel-PNPDocetaxel
The use of radio-labeled antitumor drugs in the treatment of solid tumors by the method of administering a radio-labeled anticancer drug to a patient and imaging at least a part of the patient using Positron Emission Tomography imaging is described. The method can be used to monitor delivery of antitumor drugs to tumors, to predict the effectiveness of therapy with a particular antitumor drug or combination of antitumor drugs, to assess the effectiveness of modulators of cellular accumulation, to individualize therapy and to evaluate the effectiveness of antitumor drugs with respect to particular cancers. Particularly preferred drugs are labeled taxanes, e.g., 11C-paclitaxel and 11C-docetaxel, labeled anthracyclines, e.g., 11C-doxorubicin and 11C-epirubicin, and other radiolabeled drugs, e.g. 11C-topotecan, 11C-SN-38, and 11C-imatinib. The invention further describes antitumor drugs labeled with the radioactive label 11C and methods of preparing radio-labeled drugs.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US REPRESENTED BY THE SEC
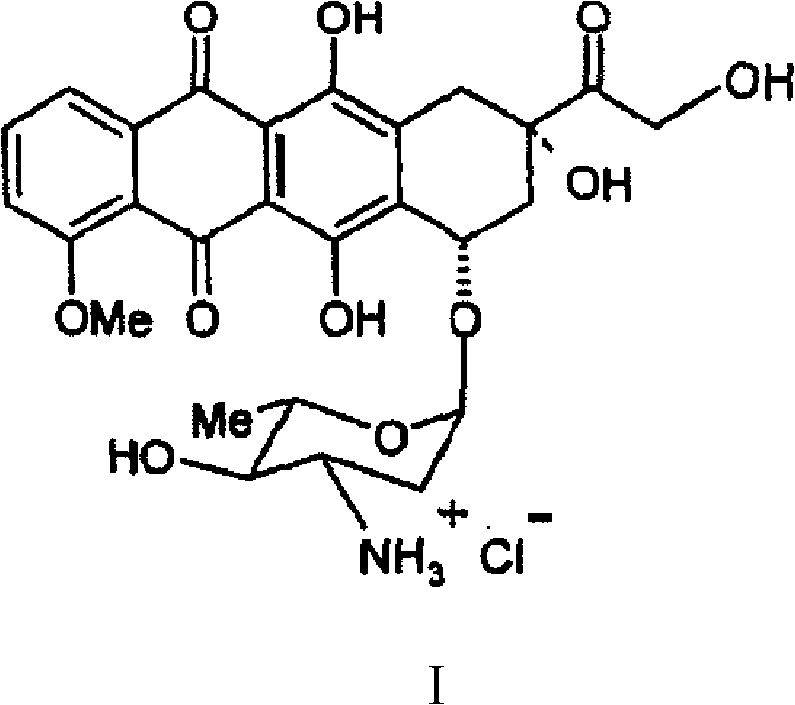
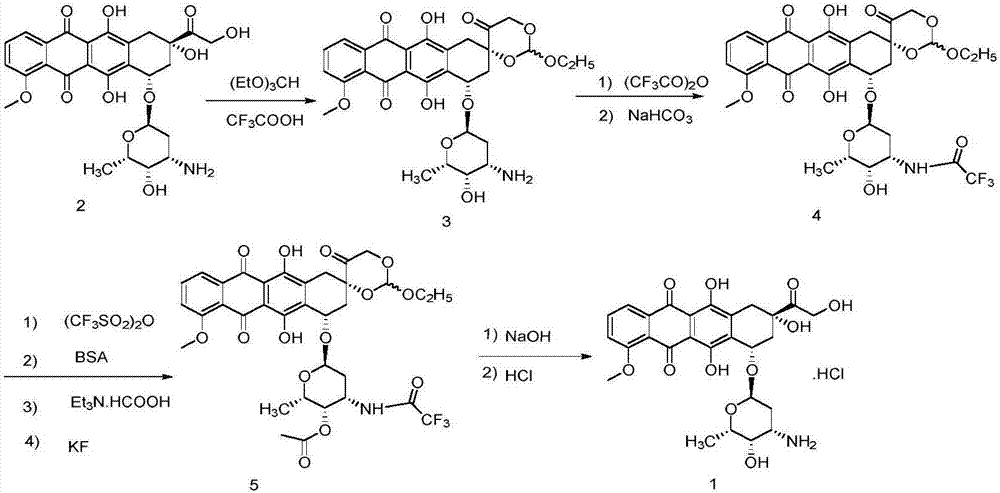
Epirubicin hydrochloride intermediate compound
ActiveCN106749447AReduce pollutionNot technically demandingSugar derivativesSugar derivatives preparationImpurityReagent
The invention provides a novel intermediate and a novel route for synthesizing epirubicin hydrochloride by utilizing the intermediate. The route is simple, cheap and efficient, achieves better energy conservation and emission reduction, and avoids the problems that an intermediate generated by the existing epirubicin hydrochloride synthesis route is water-instable and easy to decompose and a yield of the whole route is low. The route uses a low-cost and easy-to-obtain reagent and adopts a removable selective protection means; generated impurities are few; the purity is high; a yield is high.
Owner:LUNAN PHARMA GROUP CORPORATION
Antitumor agent and process for producing the same
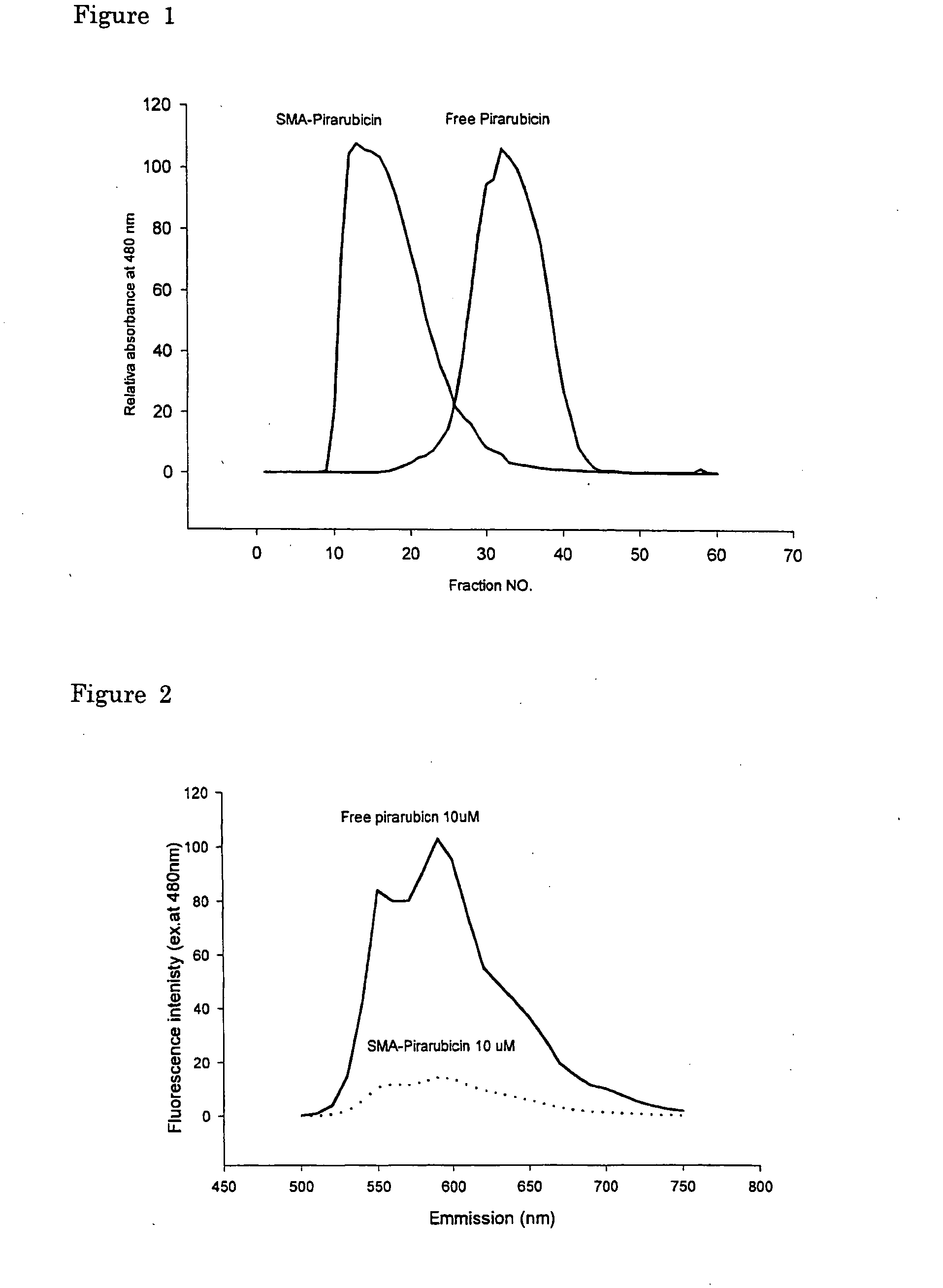
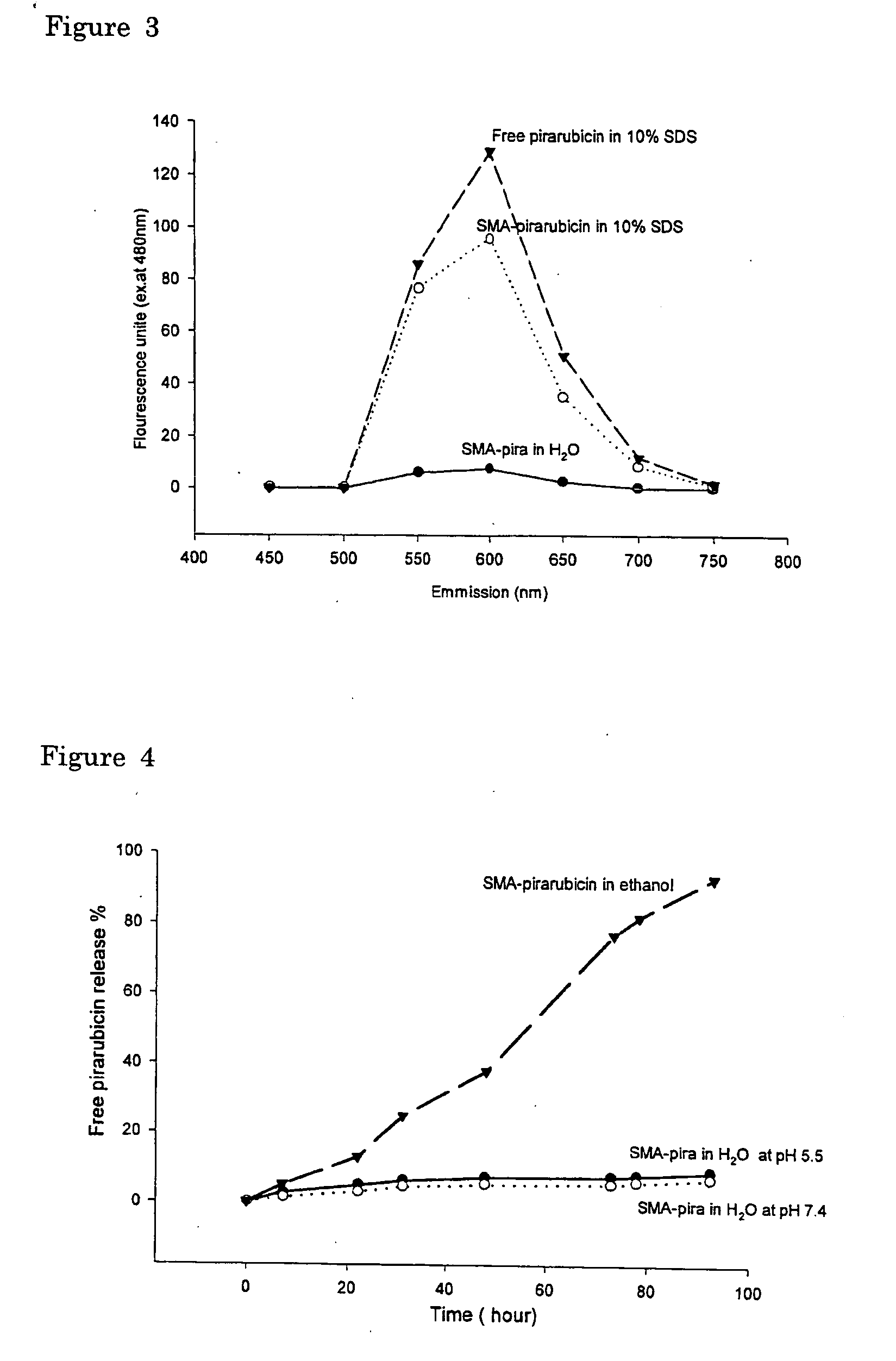
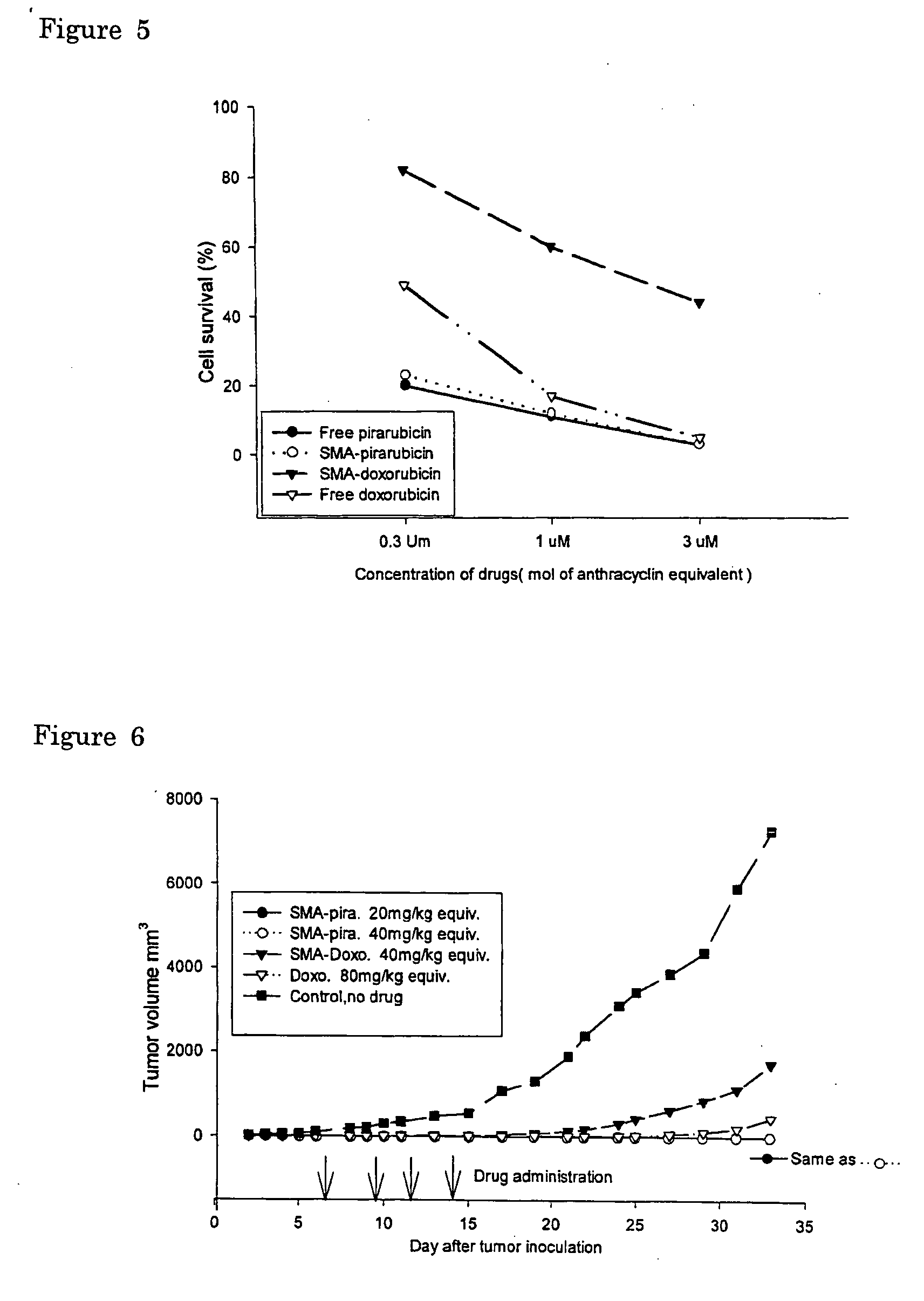
InactiveUS20050208136A1Improving selective solid targeting capacityEliminate side effectsHeavy metal active ingredientsBiocideCancer cellPolyamine
The present invention relates to polymeric antitumor agent which is formed in polymeric micelle complex by intermolecular bonding or mutual interaction between styrene maleic acid copolymer (SMA) and low molecule antitumor agent which is anthracyclins drug such as pirarubicin, doxorubicin, epirbicin, daunorbicin, acralbicin, or alkaloid antitumor agent such as cis-platinum, and taxol These polymeric antitumor agents may improve specificity to cancer cells so that it improves antitumor effect, while it may not be concentrated at normal organ or tissue, so that adverse effect may be diminished. These polymeric antitumor agents may be prepared by dissolving SMA and low molecule antitumor agent in aqueous solution, then in the presence of aqueous soluble carbodiimide, amino acids, or polyamine, adjusting pH to form micelle complex and recovering polymer fraction.
Owner:MAEDA HIROSHI
Epi-doxorubicine liposome and its preparing method
InactiveCN1554354AQuality improvementHigh encapsulation efficiencyOrganic active ingredientsAntineoplastic agentsSide effectCholesterol
The present invention relates to the field of pharmaceutical technology, and is especially epirubicin liposome and its preparation process. The epirubicin liposome of the present invention consists of epirubicin, phosphatide, cholesterol, sodium deoxycholate, etc.; has stable quality and less toxic side effect; and is suitable for clinical application.
Owner:CHINA PHARM UNIV
Pseudomonas engineering bacteria for producing epirubicin
InactiveCN102154192AIncrease productionTo achieve the purpose of large-scale industrial productionBacteriaMicroorganism based processesHeterologousStreptomyces peucetius
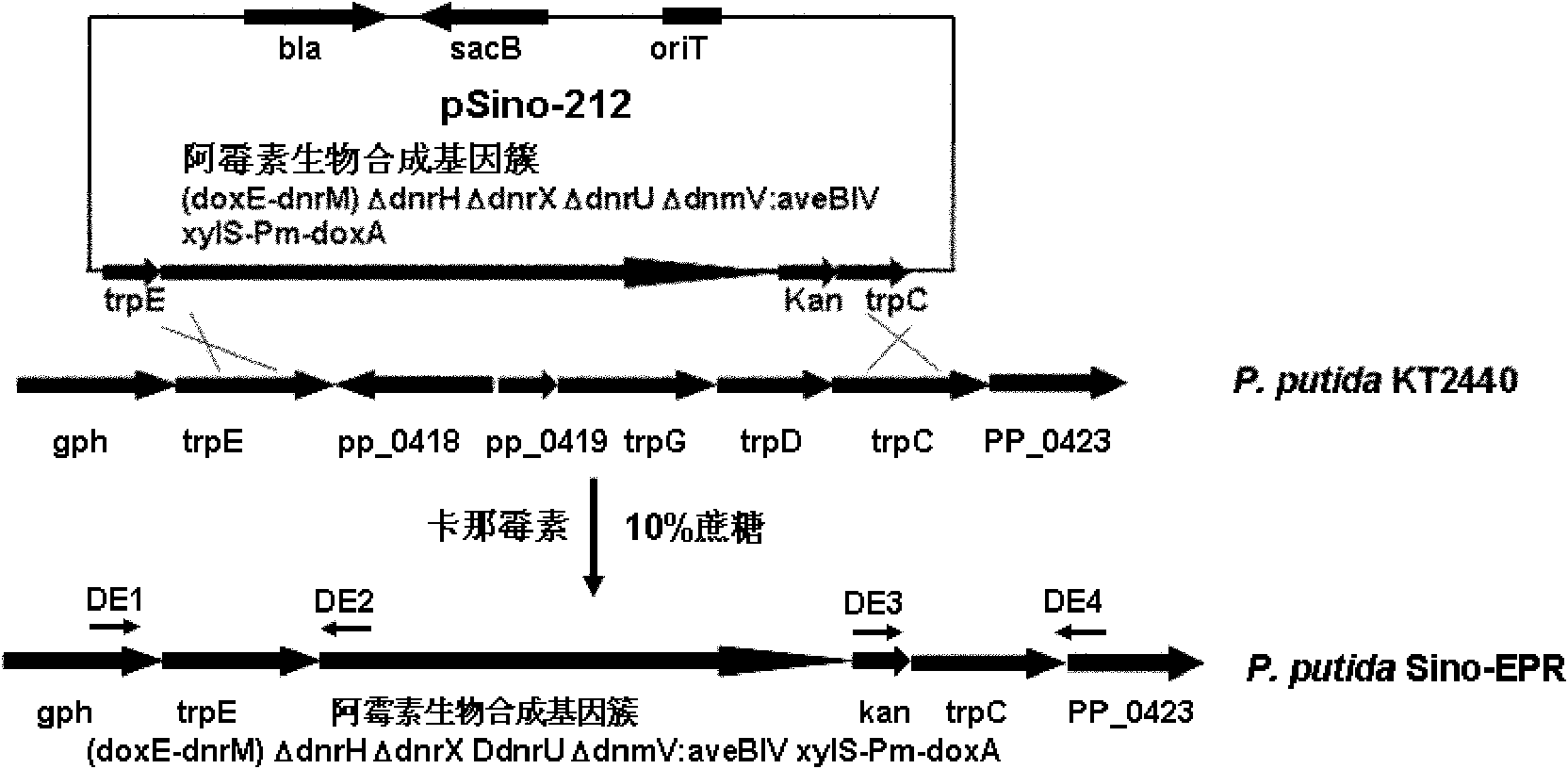
The invention provides a pseudomonas engineering bacteria for producing an epirubicin. The preparation method of the pseudomonas engineering bacteria comprises the following steps: eliminating dnrH gene, dnrX gene and dnrU gene in an adriamycin biosynthesis gene cluster from streptomyces peucetius ATCC 29050; permuting dnmV gene to be aveBIV gene from an abamectin biosynthesis gene cluster; inserting a Pm promoter from pseudomonas before coding doxA gene of oxidation-reduction enzyme; and integrating the modified gene cluster and the screening marker gene into the genome of the pseudomonas putida KT2440 to obtain the pseudomonas engineering bacteria. The pseudomonas engineering bacteria lays a foundation for the heterologous expression and the large-scale industrial production of the epirubicin, thereby being wide in application value.
Owner:北京赛诺百奥生物技术有限公司
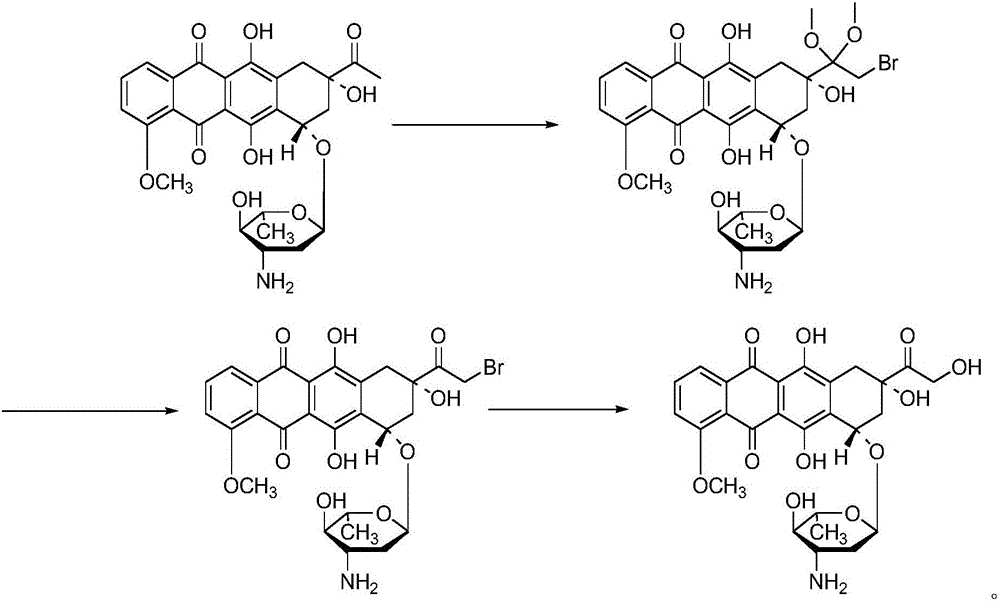
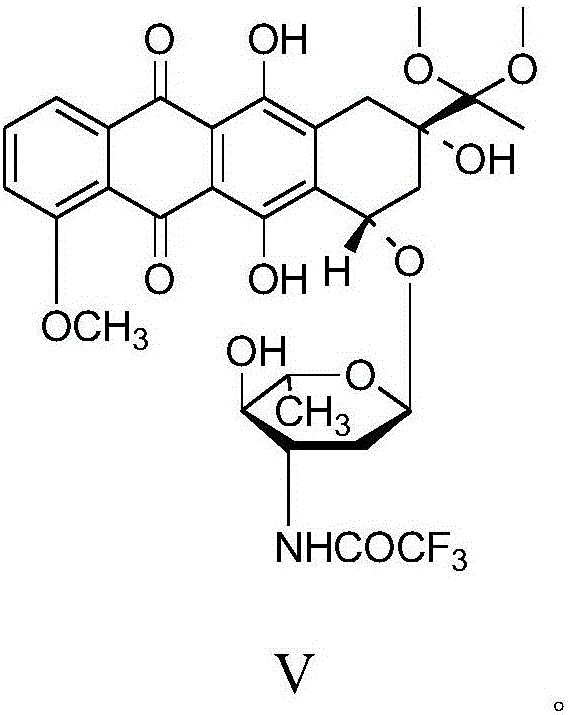
Epirubicin hydrochloride intermediate compound V
ActiveCN106749446AReduce pollutionNot technically demandingSugar derivativesSugar derivatives preparationImpurityEnergy conservation
The invention provides a novel intermediate and a novel route for synthesizing epirubicin hydrochloride by utilizing the intermediate. The route is simple, cheap and efficient, achieves better energy conservation and emission reduction, and avoids the problems that an intermediate generated by the existing epirubicin hydrochloride synthesis route is water-instable and easy to decompose and a yield of the whole route is low. The route uses a low-cost and easy-to-obtain reagent and adopts a removable selective protection means; generated impurities are few; the purity is high; a yield is high.
Owner:LUNAN PHARMA GROUP CORPORATION
Compound recipe anti-cancer drugs slow release agent comprising anticancer antibiotics and booster thereof
Disclosed is a compound anticancer slow release agent which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer effective ingredients include Aclarubicin, Idarubicin, Doxorubicin, Epirubicin, Valtaxin, Pirarubicin, Losaxantrone, Losoxantrone and / or anticancer antibiotic synergistic agents selected from phosphoinositide-3-kinase inhibitor, pyrimidine analogues and / or DNA restoration enzyme inhibitor, the slow release auxiliary materials are selected from polylactic acid copolymer EVAc, or sebacic acid copolymer, the viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C). The slow release microspheres can also be prepared into slow release implanting agent for lowering down the whole body toxicity reaction of the medicament when locally dispensing on the tumor, and for selectively increasing the tumor local medicinal concentration.
Owner:SHANDONG LANJIN PHARMA
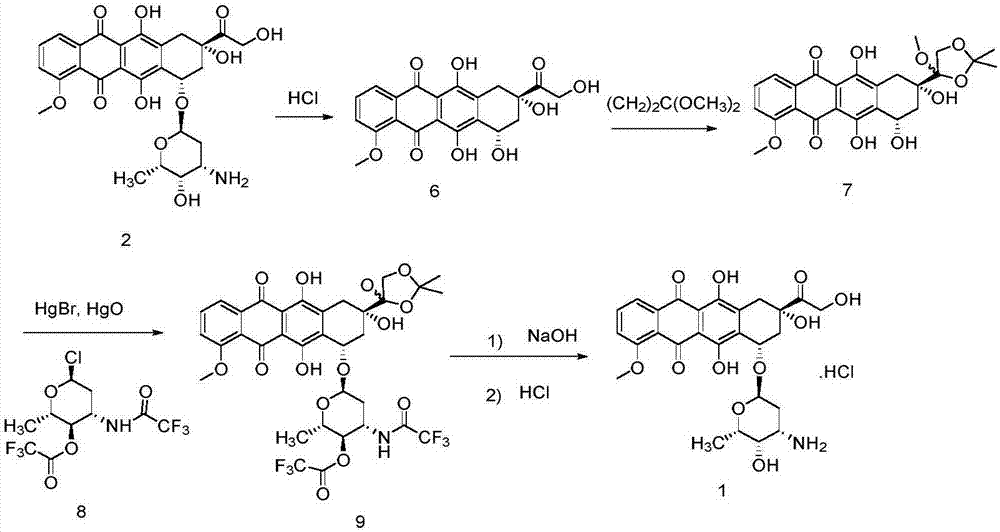
Method for preparing epirubicin hydrochloride and intermediate compound
InactiveCN106977564AReduce consumptionThe reaction steps are simpleSugar derivativesSugar derivatives preparationHydrochlorideRaw material
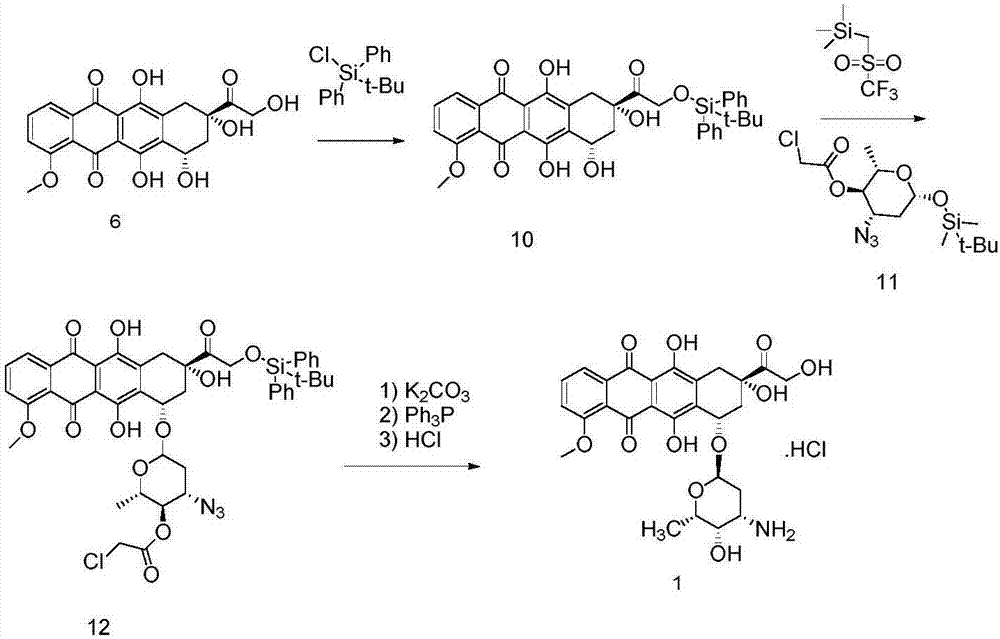
The invention discloses a novel method for synthesizing epirubicin hydrochloride (compound 1) from adriamycin. Epirubicin is directly obtained by selective oxidation and reduction. Compared with the existing method, the novel method has the advantages that the reaction route is more concise, the synthesis efficiency is improved, raw materials for reaction are easily obtained, conditions are mild, the energy consumption and the cost are reduced, and the total yield reaches 68 percent.
Owner:CHENGDU UNIV
Epirubicin liposome as well as preparation method and preserving method thereof
ActiveCN102552146BProperties are not affectedAvoid side effectsOrganic active ingredientsPharmaceutical non-active ingredientsCholesterolActive agent
Owner:JIANGXI HERBFINE HI TECH
Thermally stable crystalline epirubicin hydrochloride
A crystalline form of epirubicin hydrochloride, named herein as 'type II' crystalline epirubicin hydrochloride, has excellent thermal stability. Type II crystalline epirubicin hydrochloride has a powder X-ray diffraction pattern having average values of diffraction angle (2 theta) and relative intensity P(%) as presented in the Table of Fig. 1.
Owner:苏洛克股份有限公司
Method for synthesizing polyvinyl alcohol embolization microspheres capable of loading chemotherapeutic drug epirubicin
InactiveCN108236736AHigh drug loadingSimple methodOrganic active ingredientsSurgical adhesivesSulfonateMicrosphere
The invention relates to a method for synthesizing polyvinyl alcohol embolization microspheres capable of loading a chemotherapeutic drug epirubicin. According to the present invention, by researchingthe formula and the synthesis process of the synthesis reaction, the epirubicin loading of the polyvinyl alcohol embolization microspheres is increased; the technical scheme comprises that polyvinylalcohol is completely dissolved, the dissolved polyvinyl alcohol is cooled to a temperature of 10-15 DEG C, a ratio of Calli-B to 2-acrylamide-2-methyl propyl sodium sulfonate is 1.00:0.15, an axial flow type stirring paddle is selected, the stirring speed is controlled at 400 rpm, and the temperature of the reaction system is controlled at 40-50 DEG C when a polymer monomer solution is added; andby researching the formula, the stirring mode, the stirring speed and the reaction temperature of the synthesis reaction, the problem of the low epirubicin loading of the polyvinyl alcohol embolization microspheres is solved, the epirubicin loading of the polyvinyl alcohol embolization microspheres is increased, and the drug-loading microspheres can continuously release epirubicin to the targeting tumor area at the tumor site.
Owner:SUZHOU HENGRUI CALLISYN BIOLOGICAL MEDICINE TECH CO LTD
Method for treating bladder cancer through promoting pharmorubicin by bacillus calmette guerin vaccine
PendingCN109675023ASmall toxicityImprove treatment efficiencyCompound screeningOrganic active ingredientsSide effectRetention time
The invention discloses a method for treating bladder cancer through promoting pharmorubicin by a bacillus calmette guerin vaccine (BCG), and specifically relates to the field of combined treatment ofthe bladder cancer in clinical medicine. The method comprises a method for researching synergism of the BCG to the pharmorubicin in a cellular level and a method for synergism of the in-vivo BCG to the pharmorubicin. According to the invention, through using the method for researching the synergism of the BCG to the pharmorubicin in the cellular level and the method for the synergism of the in-vivo BCG to the pharmorubicin, a combined medication scheme is provided to improve treatment efficiency and reduce toxic and side effects during drug perfusion. The method disclosed by the invention isnot high in prefused drug concentration, but long in intra-bladder retention time, thereby being better in the anti-tumor effect without generating an adverse reaction.
Owner:GUANGDONG PHARMA UNIV
Chemotherapy pharmaceutical composition and application thereof
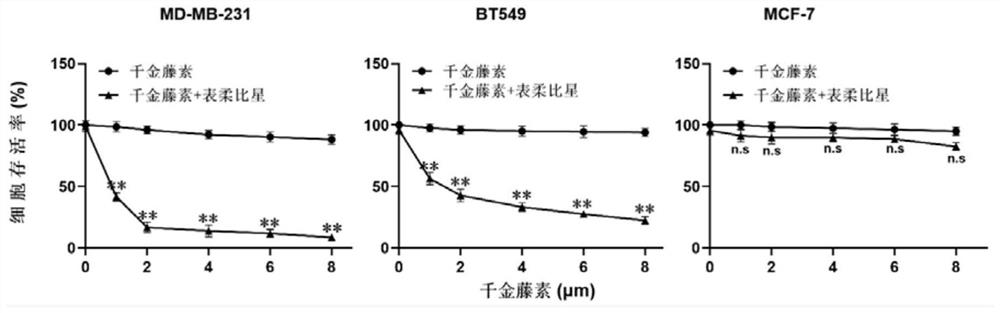
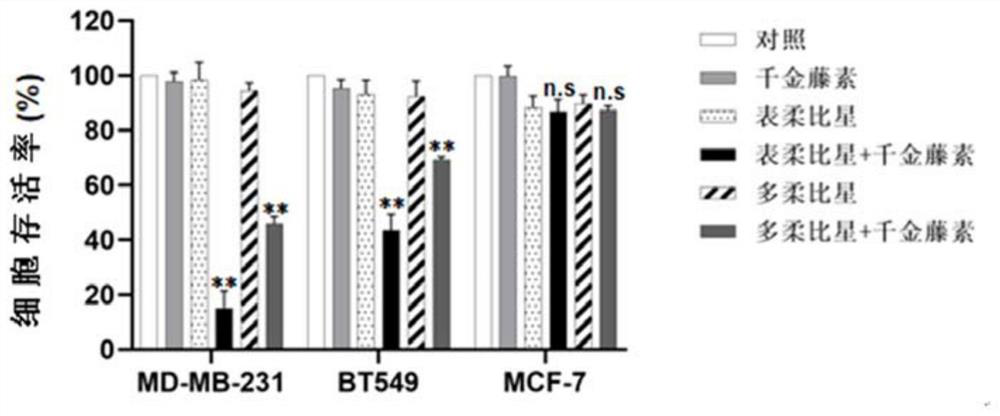
ActiveCN112274525AReduce dosing frequencyOrganic active ingredientsPharmaceutical delivery mechanismCepharanthinCancer cell
The invention relates to the technical field of biological medicine, in particular to a chemotherapy pharmaceutical composition and application thereof. The invention relates to a chemotherapy pharmaceutical composition, which comprises cepharanthine and epirubicin. Before epirubicin is administrated, cepharanthine is administrated firstly, and the cancer cell inhibiting effect of epirubicin can be synergistically enhanced. According to the scheme, the technical problems that the curative effect of epirubicin is limited, and adverse reactions and drug resistance are easily generated when a cancer patient uses epirubicin for a long time are solved. The composition can be applied to clinical practices of treatment of various cancers, reduces adverse reactions of epirubicin, and prevents drugresistance of epirubicin at the same time.
Owner:ZUNYI MEDICAL UNIVERSITY
Thermally stable crystalline epirubicin hydrochloride
InactiveUS20090099346A1Improve thermal stabilityHigh puritySugar derivativesX ray diffractogramEpirubicin
A crystalline form of epirubicin hydrochloride, named herein as “type II” crystalline epirubicin hydrochloride, has excellent thermal stability. Type II crystalline epirubicin hydrochloride has a powder X-ray diffraction pattern having average values of diffraction angle (2θ) and relative intensity P(%) as presented in the following table:Diffraction AngleRelative Intensity2ΘP %5.21009.224.510.314.313.732.814.610.915.549.31819.119.221.119.411.320.719.921.129.721.4182223.122.569.623.620.524.172.125.86726.115.927.747.929.83831.914.132.116.736.416.637.718.841.114.5
Owner:SYNBIAS PHARMA
Temperature controlled sustained-release injection containing anti-cancer medicine
InactiveCN101273965APharmaceutical delivery mechanismPharmaceutical non-active ingredientsTherapeutic effectVinorelbine
The invention relates to a temperature-controlled sustained-release injection containing an anti-cancer drug, which consists of the anti-cancer drug and an amphiphilic block copolymer hydrogel and has the temperature-sensitive gelatinization feature, the temperature-controlled sustained-release injection is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, thus allowing the drug to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months; the temperature-controlled sustained-release injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus significantly reducing the systemic reaction of the drug, strengthening the treatment effects of chemotherapy, radiotherapy and other non-surgical therapies, and being used for the treatment of the tumors in different stages. The anti-cancer drug can be vincristine, vinorelbine, navelbine, vindesine, vinleurosine, vinrosidine, cephalotaxine, bleomycin, daunomycin, aclarubicin, epirubicin, idarubicin, pirarubicin, valrubicin, mitomycin C, actinomycin D, losoxantrone, mitoxantrone, mitozolomide, temozolomide and so on.
Owner:SHANDONG LANJIN PHARMA +1
Antineoplastic conjugates of transferin, albumin and polyethylene glycol
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R*H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
Epirubicin hydrochloride lyophilized injectable powder and preparation method thereof
ActiveCN103006586AAvoid allergiesAvoid harmOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLHydrogen
The invention belongs to the technical field of pharmaceutical preparations, and provides epirubicin hydrochloride lyophilized injectable powder and a preparation method of the epirubicin hydrochloride lyophilized injectable powder. According to the preparation method, mannitol is adopted and used as a propping agent during preparing the epirubicin hydrochloride lyophilized injectable powder, the pH (Potential of Hydrogen) of the liquid medicine is adjusted to 4.5 to 6.0 by hydrochloric acid or sodium hydroxide solution, and therefore, the hydrolyzing of the main medicine is inhibited, and the stability of the main medicine is improved; the pre-freezing temperature is controlled to below -40 DEG C, and the sublimation temperature in lyophilization is controlled to -15 DEG C, and as a result, the stability of the medicine can be improved. The prepared epirubicin hydrochloride lyophilized injectable powder is stable, and can prevent the human body from damage due to the anaphylaxis of lactose in an original preparation and preservative methylparaben, thus the safety of the epirubicin hydrochloride lyophilized injectable powder can be improved.
Owner:SHANDONG NEWTIME PHARMA
Imaging of drug accumulation as a guide to antitumor therapy
InactiveUS7141234B1Avoid the needShorten the timeX-ray constrast preparationsRadioactive preparation carriersDocetaxel-PNPDocetaxel
The present invention describes the use of radio-labeled antitumor drugs in the treatment of solid tumors by the method of administering a radio-labelled anticancer drug to a patient and imaging at least a part of the patient using Positron Emission Tomography imaging. The method is used to monitor delivery of antitumor drugs to tumors and may be used to predict the effectiveness of therapy with a particular antitumor drug or combination of antitumor drugs, to assess the effectiveness of modulators of cellular accumulation, to individualize therapy and to evaluate the effectiveness of antitumor drugs with respect to particular cancers. Particularly preferred drugs are labeled taxanes, e.g., 11C-paclitaxel and 11C-docetaxel, labeled anthracyclines, e.g., 11C-doxorubicin and 11C-epirubicin, and other radio-labeled drug, e.g. 11C-topotecan and 11C-mitoxantrone. The invention further describes antitumor drugs labeled with the radioactive label 11C and methods of preparing radio-labeled drugs.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US REPRESENTED BY THE SEC
Anti-cancer composition loading both platinum compound and synergist
InactiveCN101011351APharmaceutical delivery mechanismPharmaceutical non-active ingredientsCarboplatinMitozolomide
Disclosed is a slow release injection agent of anticancer composition containing platinum-group compounds and synergistic agent, which comprises slow release microspheres and dissolvent, wherein the slow release microspheres comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being conventional dissolvent or specific dissolvent containing suspension adjuvant. The viscosity of the suspension adjuvant is 100-3000cp (at 20-30 deg C), and is selected from sodium carboxymethylcellulose, the platinum-group compounds are selected from cisplatin, Carboplatin, Nedaplatin or Oxaliplatin, the synergistic agent can be selected from tetrazine drugs such as Mitozolomide or Temozolomide, and / or anticancer antibiotics such as Adriamycin, Aclarubicin, Epirubicin, mitomycin or pidorubicin, the slow release auxiliary materials are selected from polyphosphate ester copolymers such as p(LAEG-EOP), p(DAPG-EOP), copolymer or blend of polyphosphate ester with polylactic acid, Polifeprosan, sebacylic acid and PLGA. The anticancer composition can also be prepared into slow release implanting agent for injection or placement in or around tumor with a period of effective concentration maintenance over 60 days, as well as the treatment effect of appreciably lowering general reaction of the drugs, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com