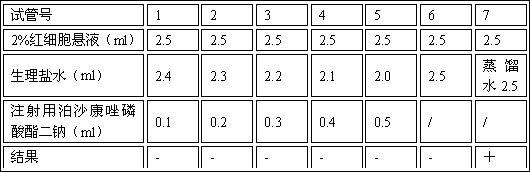
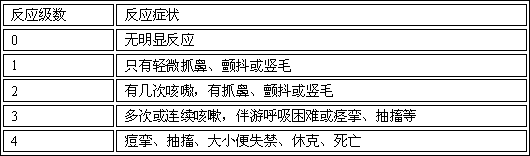
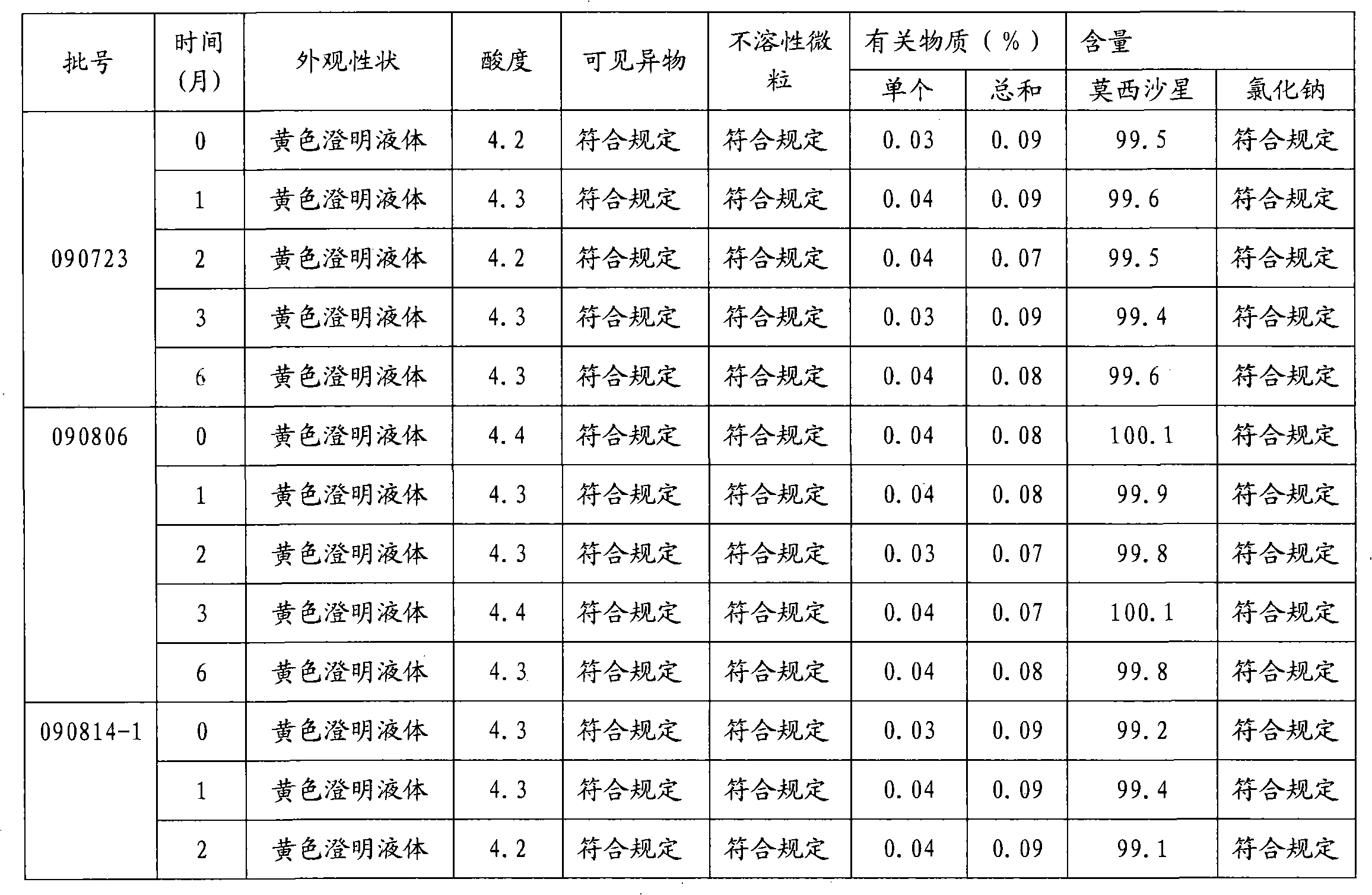
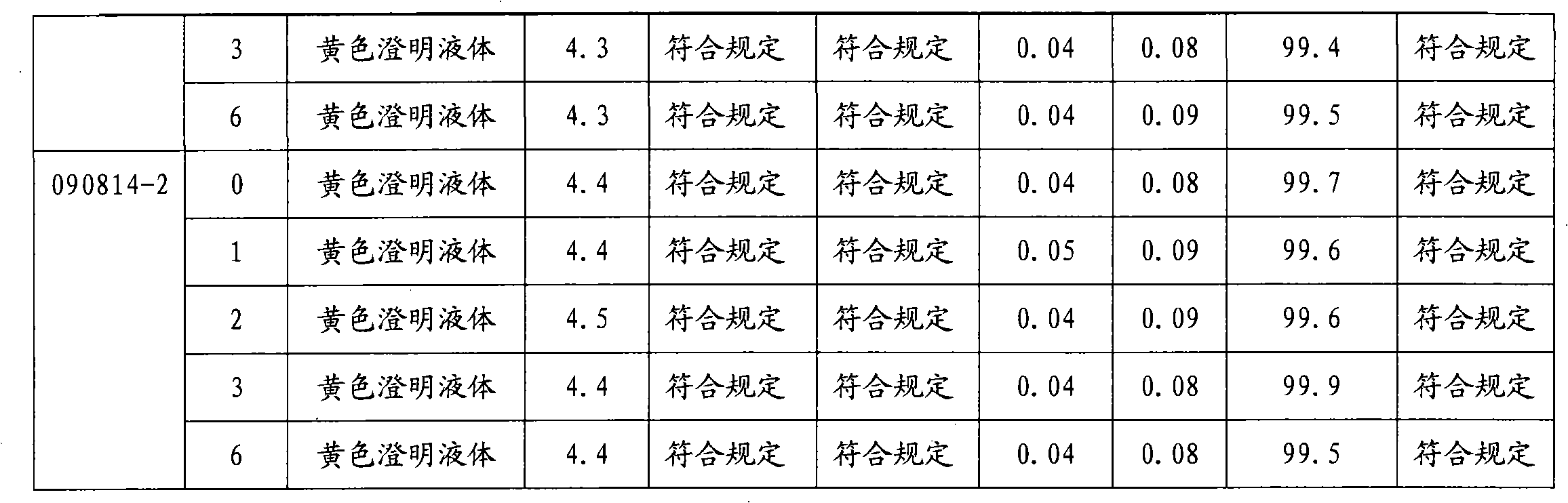
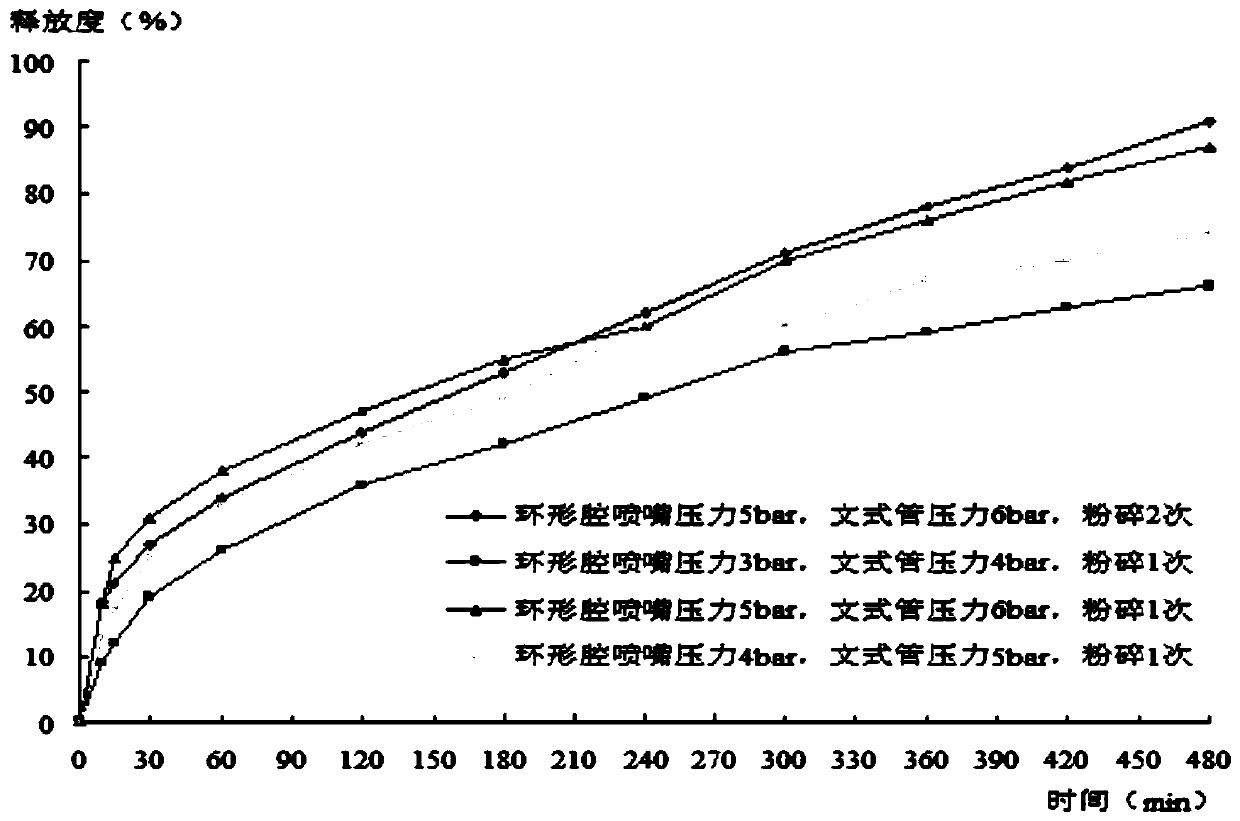
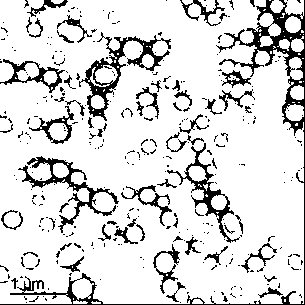
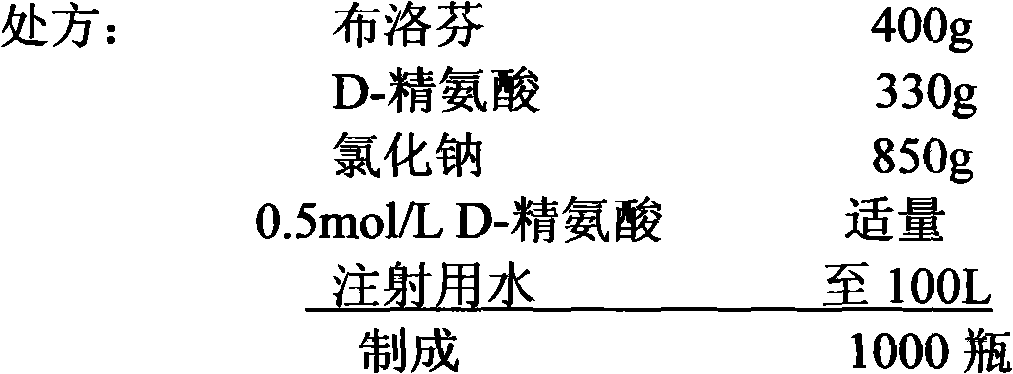
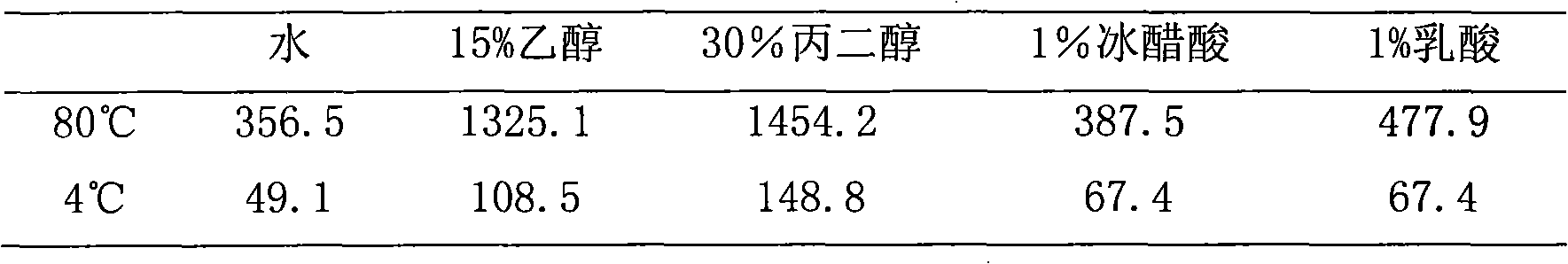
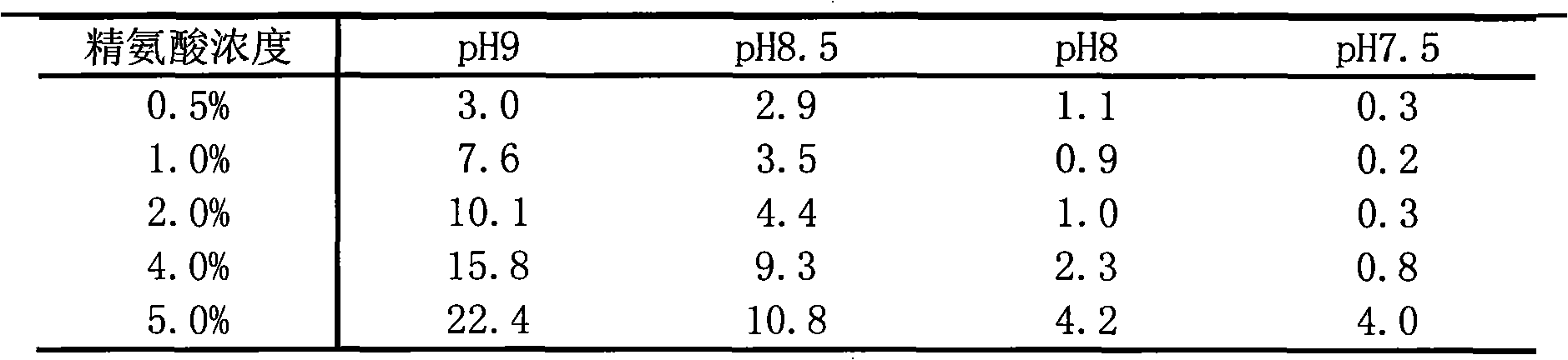
Patents
Literature
55results about How to "No hemolytic" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
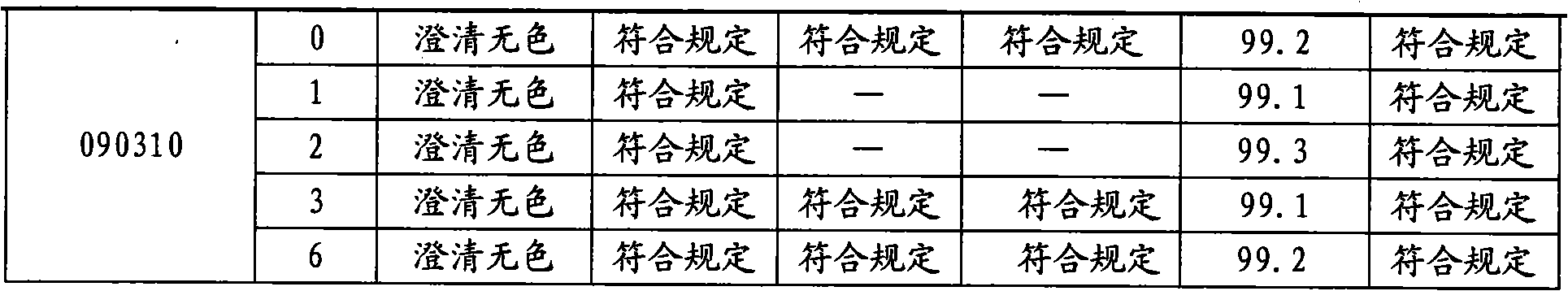
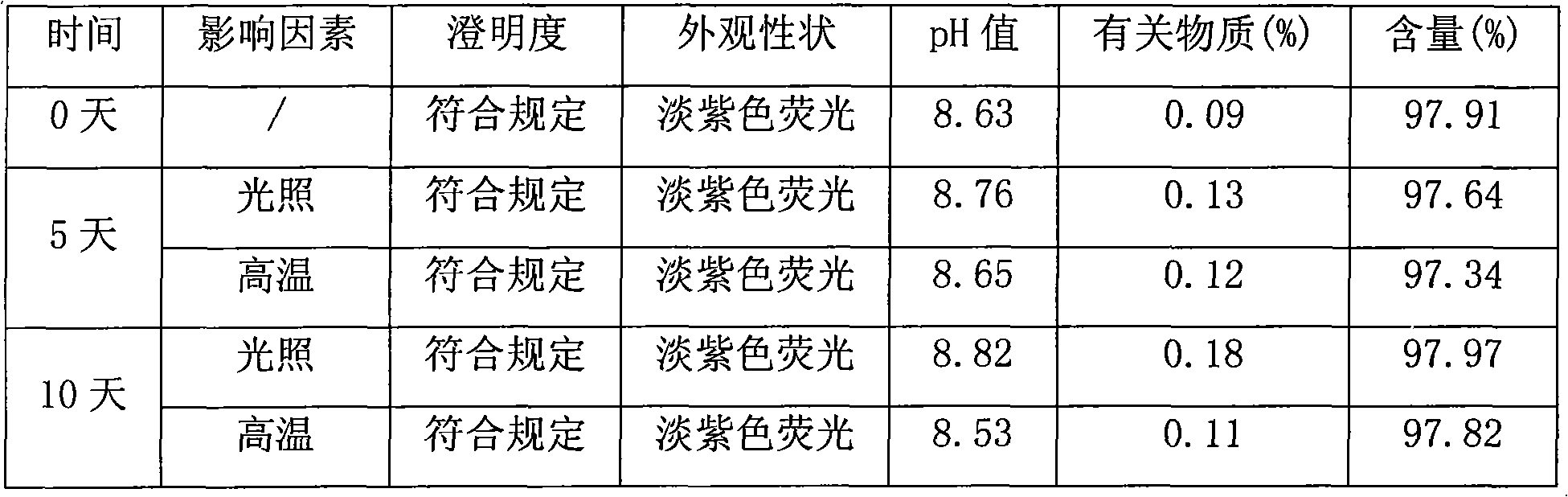
A stable torasemide injection and its preparation method
InactiveCN101007003AReduced dosing timeNo hemolyticPharmaceutical delivery mechanismPharmaceutical non-active ingredientsMaterial growthActivated carbon
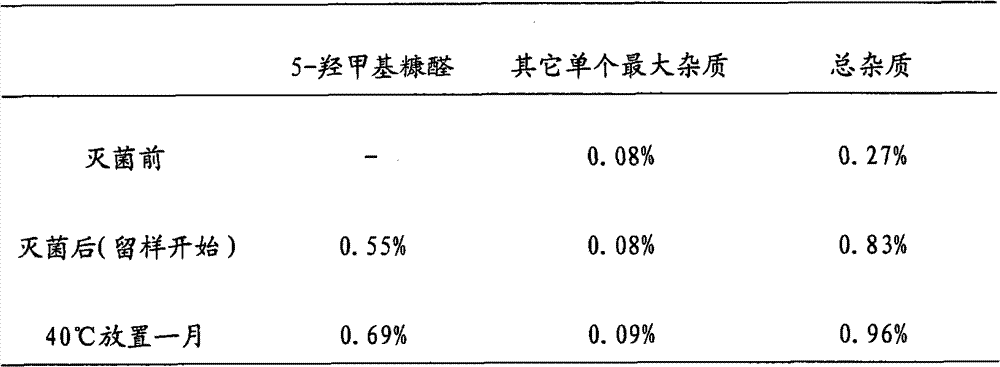
There is provided an injection with torasemide as the main active ingredient in the invention, the preparing method of the injection is also included in the invention. The stability of the injection in the invention has been greatly improved. There is solubilizing agent and stabilizer in the preparation, the pH value is 9.0 or larger. The preparing method includes the following steps: adding 1-50% (by weight) of solubilizing agent to certain amount of injecting water; adding torasemide to the water, after it is completely dissolved, adding 0.1-5% part of stabilizer; adding water to requested amount and stirring uniformly; adding activated char to adsorb for 30min at high temperature; decarbonizing and filtering; adding medically used alkali to regulate the ph value to 9.0 or larger; aseptically filtering; after the assay is approved, packaging, degerming and stocking. The advantages of the invention include: high stability, short preparing time, low cost, no haemolyticus, blood vessel stimulus and allerfic response, low growing speed of relative substances.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD +1
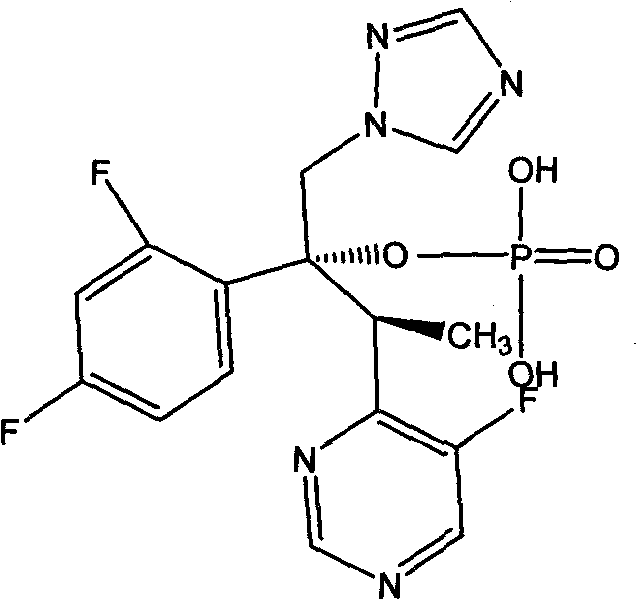
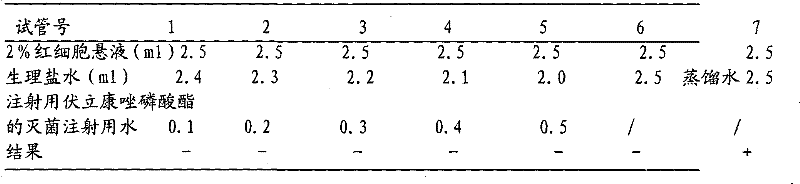
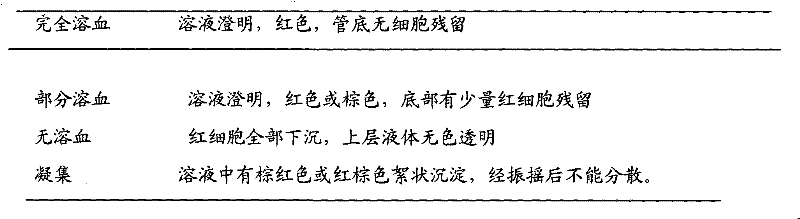
Voriconazole phosphate ester for injection and preparation method thereof
ActiveCN101744778AImprove performanceNo pollution in the processOrganic active ingredientsPowder deliveryPhosphateMedical prescription
The invention provides voriconazole phosphate ester for injection and a medicinal salt thereof and a preparation method for the voriconazole phosphate ester for injection and the medicinal salt thereof. The preparation method comprises the following steps: adding 5 to 98 percent water for injection in a liquid preparation container; adding 90 to 110 percent of the accurate formula dosage of voriconazole phosphate ester and the medicinal salt thereof in the container; stirring the mixture; slowly dropwise adding a pH value regulator; regulating pH to between 6.0 and 11; supplementing water to the full dosage and then adding 0.01 to 1.0 percent (weight in volume) medicinal carbon into the product; stirring the mixture for 15 to 60 minutes; using a sand filter stick to carry out rough filtration and decarburization on the obtained product, and using a 0.22mum millipore filter to carry out fine filtration on the product until the clarity is qualified; after determining that the content of the midbody is qualified, determining the filling quantity and subpackaging the finished product in the vial; adding the semi-plug; carrying out freezing and drying on the sample; controlling the moisture content between 1 and 8 percent; pressing the plug; and carrying out capping.
Owner:HC SYNTHETIC PHARMA CO LTD
Ibuprofen injection composite and preparation method thereof
ActiveCN101966147AImprove stabilityMeet the needs of clinical medicineOrganic active ingredientsAntipyreticIbuprofen InjectionMedicine
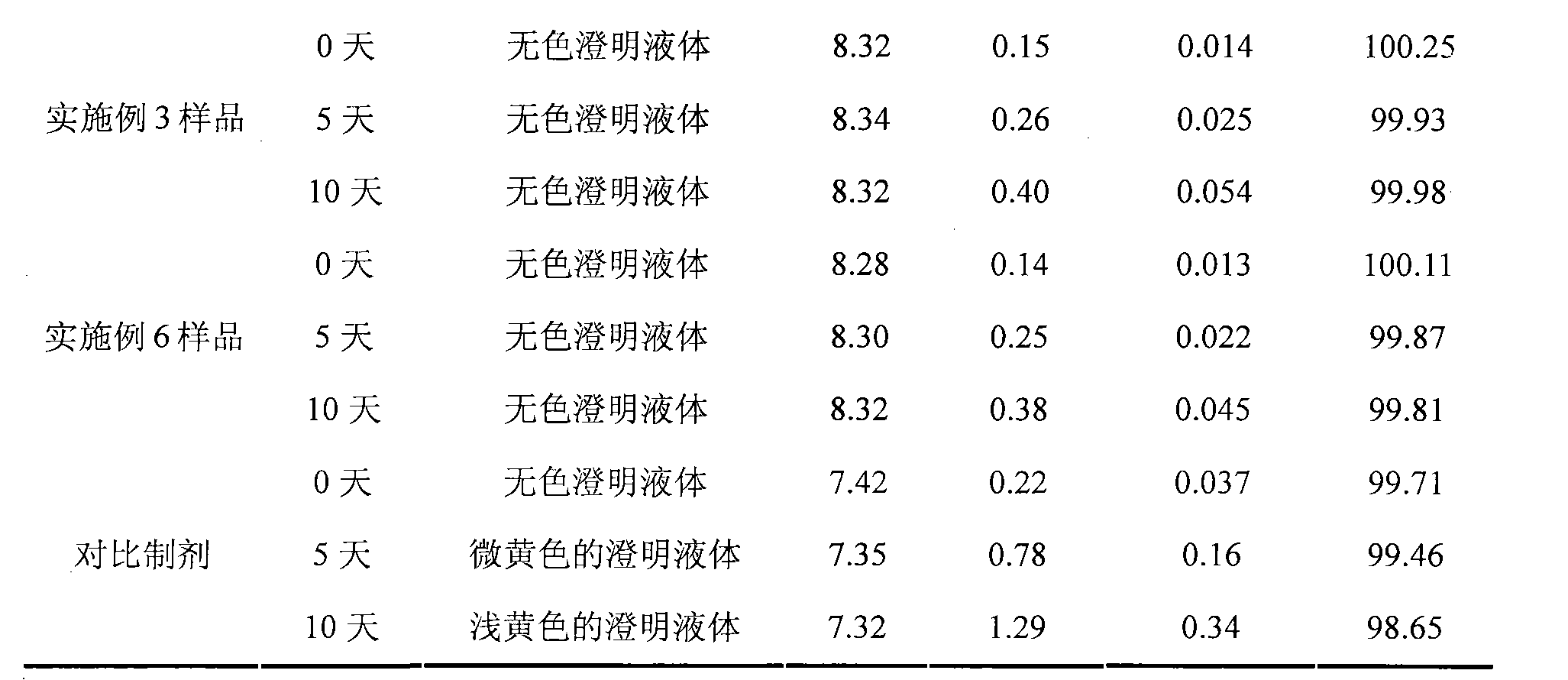
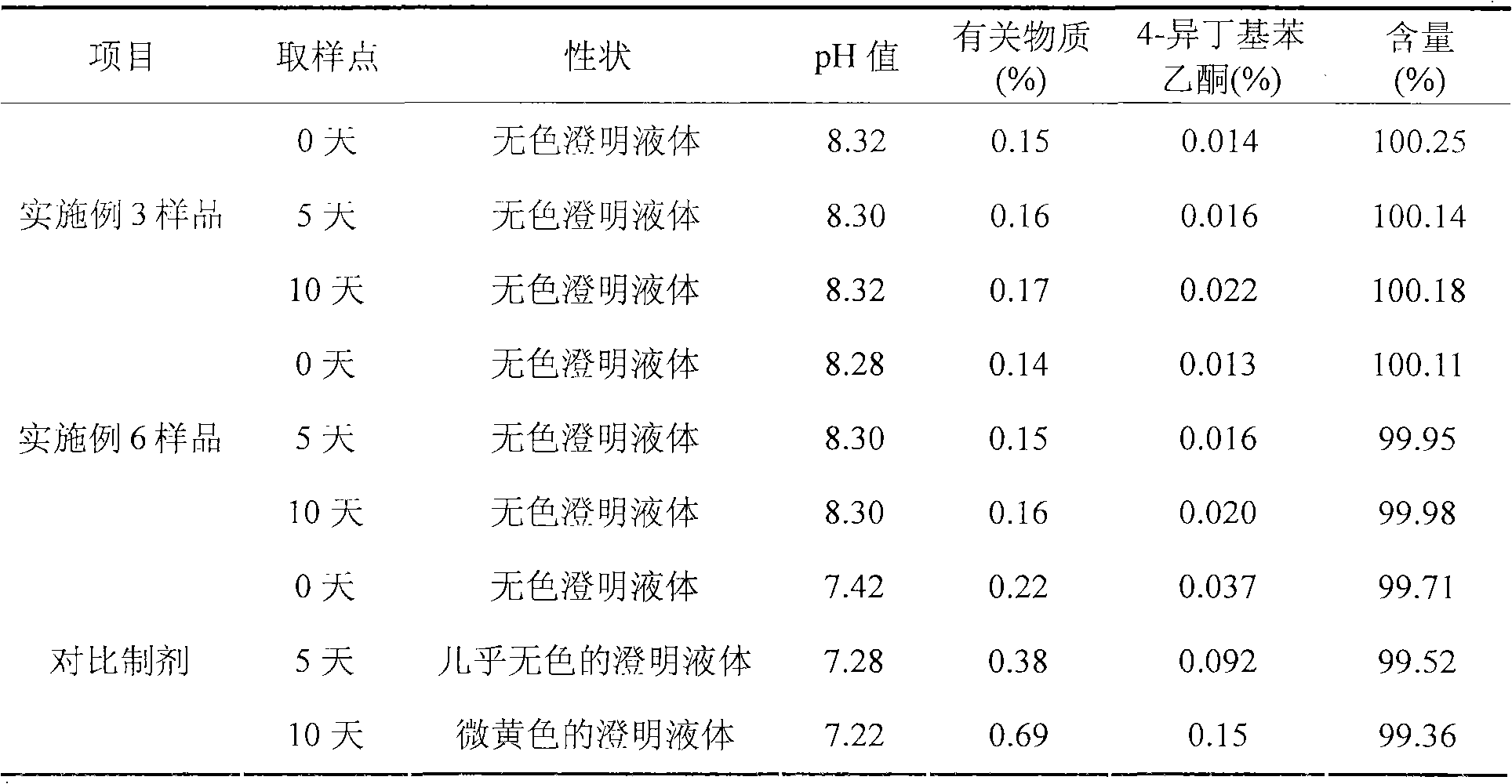
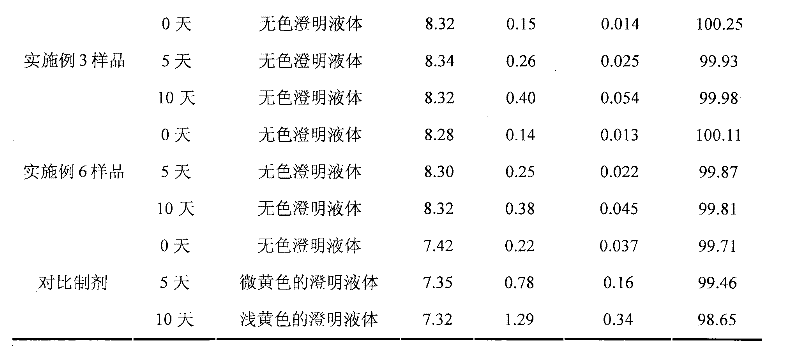
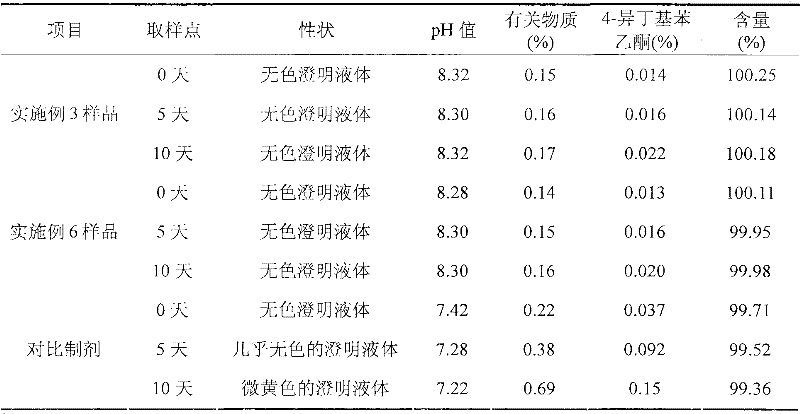
The invention discloses an ibuprofen injection composite which can obviously improve the quality stability of products and meet the clinical compatibility demand and a preparation method thereof to overcome the defect of the traditional ibuprofen injection. The injection is prepared by mixing ibuprofen and cosolvent according to a molar ratio of 1: 1.001-2, and the pH value of the injection is 7.5-9.0. The ibuprofen injection prepared through the method has obviously improved tolerance capability to high temperature and strong light and better stability and no irritant to vessels, and can effectively meet the requirement of the clinical intravenous drip. Moreover, the preparation method of the invention has the advantages of simple process and good stability of the prepared product.
Owner:四川阳光润禾药业有限公司
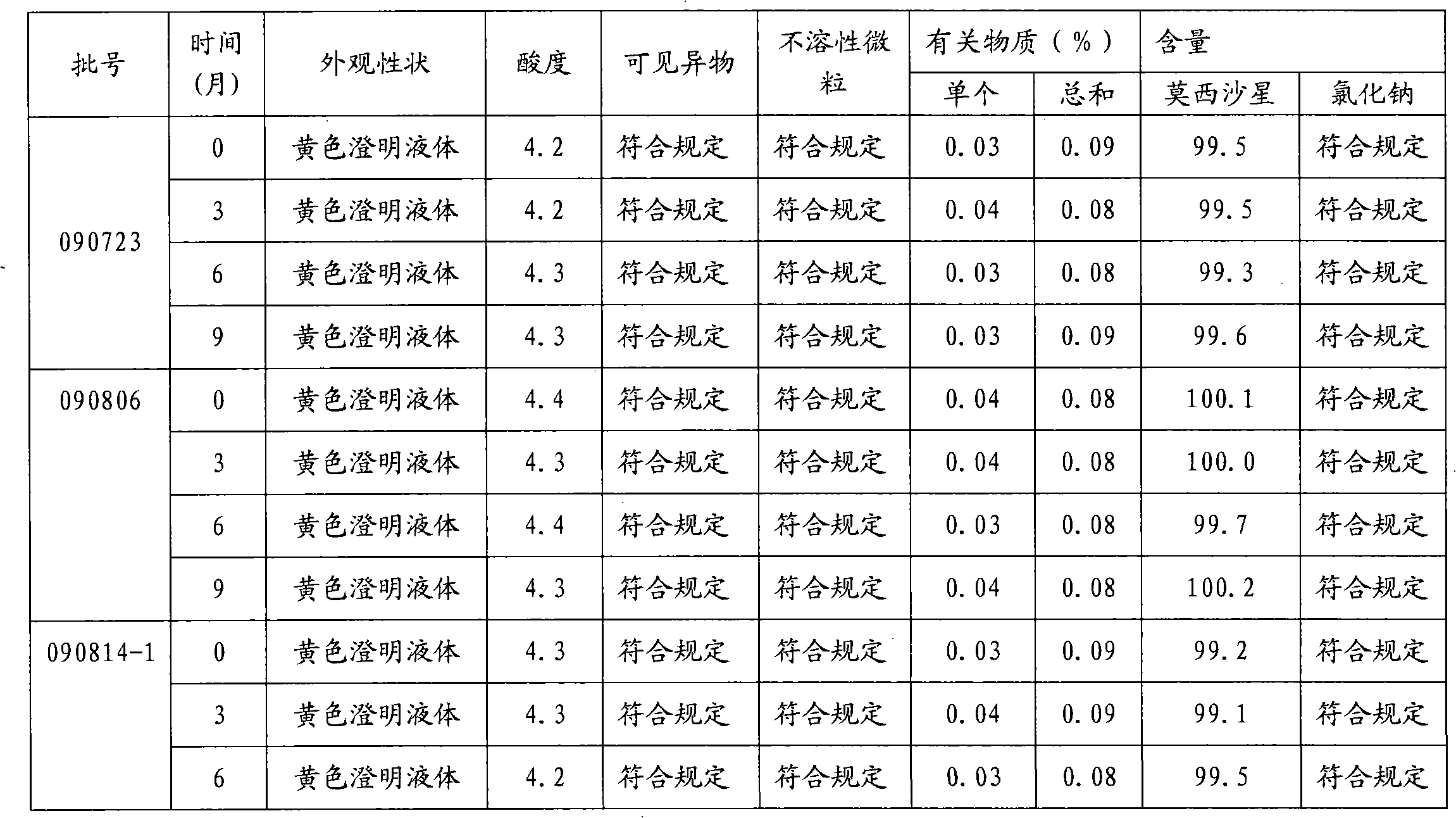
Moxifloxacin hydrochloride glucose injection and preparation method and use thereof
InactiveCN101836950APrecipitation does not occurReduce solubilityAntibacterial agentsOrganic active ingredientsAdditive ingredientMoxifloxacin hydrochloride
The invention provides moxifloxacin hydrochloride glucose injection and a preparation method and use thereof. The method for preparing the injection comprises the following steps of: adding water for injection accounting for 20 to 98 percent of the batch volume into an ingredient tank, and adding glucose, a metal complexing agent and the moxifloxacin hydrochloride in a ratio; after stirring to fully dissolve the components, regulating the pH value to between 4.0 and 4.5 by using 1mol / L hydrochloric acid solution or 1mol / L sodium hydroxide, adding medicinal carbon accounting for 0.05 percent (W / V) of the total volume, uniformly stirring, maintaining the temperature of between 70 and 80 DEG C for 20 minutes, and performing circular filtering for over 20 minutes; replenishing the water for injection to the batch scale, stirring for 5 to 10 minutes, and detecting the pH value of the prepared solution (controlling to between 4.0 and 4.5); after determining that no residual water is present in an elevated tank and a pipeline, opening a valve of the elevated tank, and sampling liquid medicament at a self-circulation pipeline sampling port after the liquid medicament circulates for 20 minutes through a filter element and the elevated tank; detecting according to the intermediate quality standard, requiring that the content of the moxifloxacin hydrochloride is between 1.52 and 1.68 mg / ml, the glucose content is between 47.5 and 52.5 mg / ml, and the pH value is between 4.0 and 4.5; after the intermediate is detected to be qualified, beginning to fill; and conveying the filled semi-finished products into a sterilizing cabinet for sterilization, wherein the sterilization condition is to sterilize for 8 to 30 minutes at 121 DEG C through thermal pressure steam.
Owner:HC SYNTHETIC PHARMA CO LTD
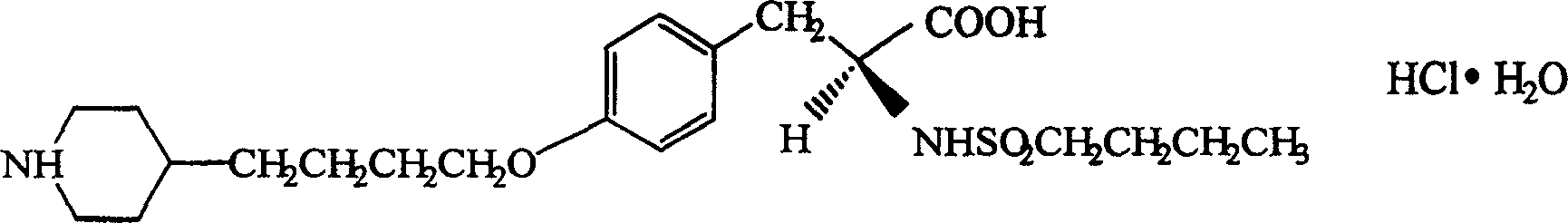
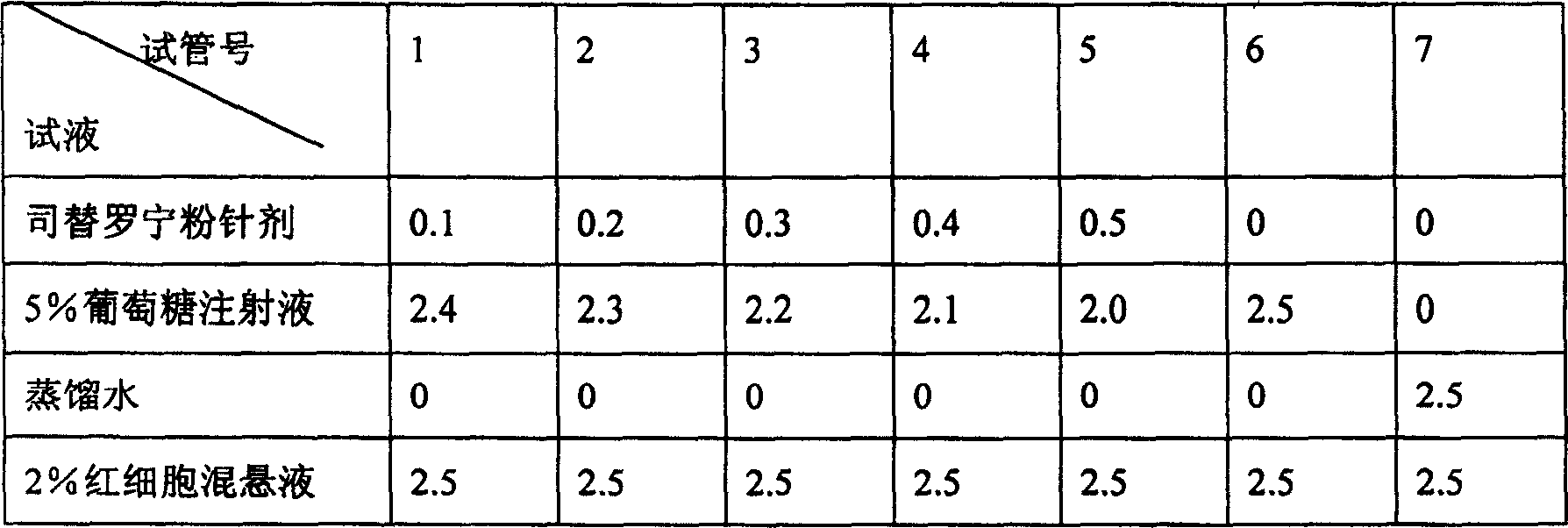
Tirofiban powder injection and its preparing method
InactiveCN1820751ANo vascular irritationNo hemolyticPowder deliveryOrganic active ingredientsPh bufferingActive component
The present invention relates to new preparation form of Tirofiban, especially Tirofiban powder for injection, as antiplatelet medicine and its preparation process. The powder for injection consists of Tirofiban as active component, freeze drying support agent and pH buffering agent. The present invention expands the administration range of Tirofiban and raises the clinical administration level of Tirofiban.
Owner:HUAZHONG UNIV OF SCI & TECH
Freeze-drying composition of posaconazole prodrug and preparation method and application of freeze-drying composition of posaconazole prodrug
InactiveCN105287403AImprove performanceNo pollution in the processOrganic active ingredientsPowder deliverySolubilityCase fatality rate
The invention relates to a freeze-drying composition of a posaconazole prodrug and a preparation method and application of the freeze-drying composition of the posaconazole prodrug. The freeze-drying composition has the advantages that the freeze-drying composition is high in water solubility, and safety of the freeze-drying composition is guaranteed due to the fact that cyclodextrins auxiliary materials need not to be added during the preparation of the freeze-drying composition; the freeze-drying composition is suitable for being used for treating various amphotericin-intolerant or refractory adult invasive fungal infections; the freeze-drying composition is used as a preventive drug for high-risk patients, the freeze-drying composition is applicable to patients above 13 years old and with impaired immunity and especially applicable to patients who have graft versus host disease (GVHD) after hematopoietic stem cell transplant, patients with leukemia and patients with long-term leukopenia due to chemotherapy; compared with control drugs such as fluconazole and itraconazole, the freeze-drying composition can effectively prevent invasive aspergillosis and can lower the mortality related to the invasive fungal infections.
Owner:HC SYNTHETIC PHARMA CO LTD
Moxifloxacinhydrochloride sodium chloride injection, preparation method thereof and use thereof
InactiveCN101884613AWill not crystallizePrecipitation does not occurAntibacterial agentsOrganic active ingredientsPhosphateSodium Chloride Injection
The invention relates to moxifloxacinhydrochloride sodium chloride injection. The injection comprises the following components in percentage by weight / volume: 0.03 to 1 percent of moxifloxacinhydrochloride, 0.01 to 3 percent of weak acid, 0.01 to 3 percent of salt of weak acid, 0.01 to 3 percent of phosphoric acid, 0.01 to 3 percent of amino acid or phosphate and 0.65 to 0.95 percent of sodium chloride.
Owner:HC SYNTHETIC PHARMA CO LTD
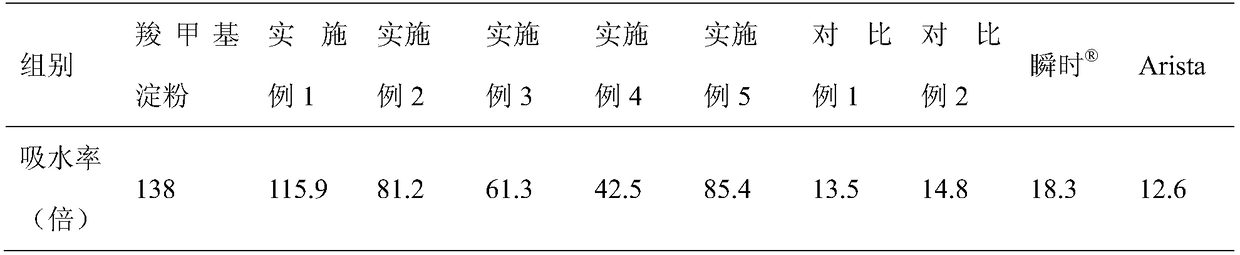
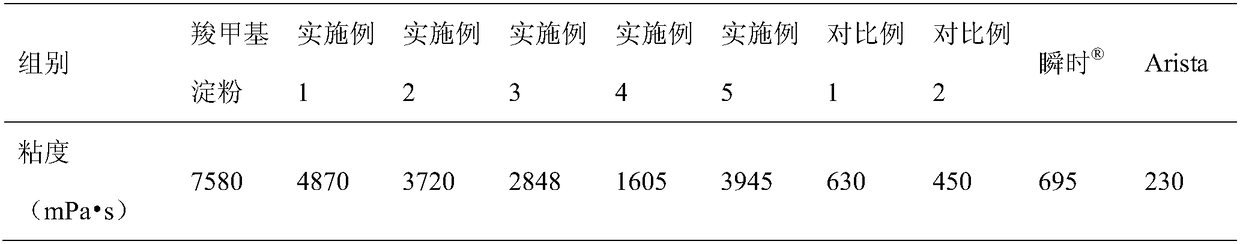
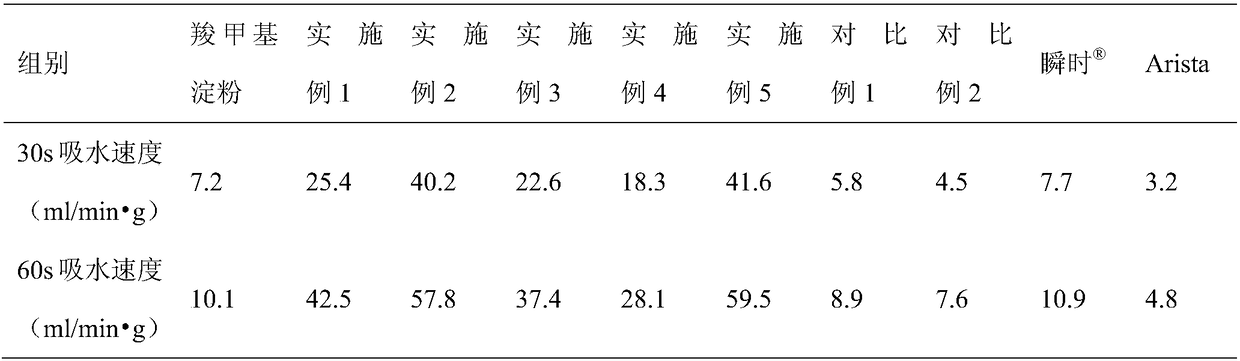
Medical absorbable polysaccharide composite material and application thereof
ActiveCN109498833AGood biocompatibilityNon-cytotoxicSurgical adhesivesPharmaceutical delivery mechanismFreeze-dryingBiocompatibility Testing
The invention discloses a medical absorbable polysaccharide composite material and application thereof. According to the medical absorbable polysaccharide composite material, starch with poor water absorbability is used as one material in the polysaccharide composite material; furthermore, a polysaccharide material with good water absorbability is added; the composite polysaccharide material is obtained through directly mixing, or through adding water, uniformly mixing and then carrying out freeze drying, without the need of cross-linking reaction. The material has the advantages of high waterabsorption rate, rapid water absorption speed, higher gel strength, good biocompatibility and the like, and can be directly used on wound surfaces with blood and for stopping bleeding of body surfaces, tissue organs in inner parts of bodies and in body cavities; the material can be used for rapidly stopping bleeding, can be absorbed by human bodies and has a sticky plugging effect. The material also can be further used as a post-operative anti-adhering material, a tissue healing promoting material, a surgical sealant, a wound suture-free tissue adhesive, a tissue filling material and a tissuedebridement material.
Owner:济南格莱威医疗科技有限公司
Antimicrobial peptide Pc-CATH1 and gene thereof, chemical synthesis method and application thereof
InactiveCN102115496ASmall molecular weightImprove the bactericidal effectMicrobiological testing/measurementDepsipeptidesChemical synthesisArginine
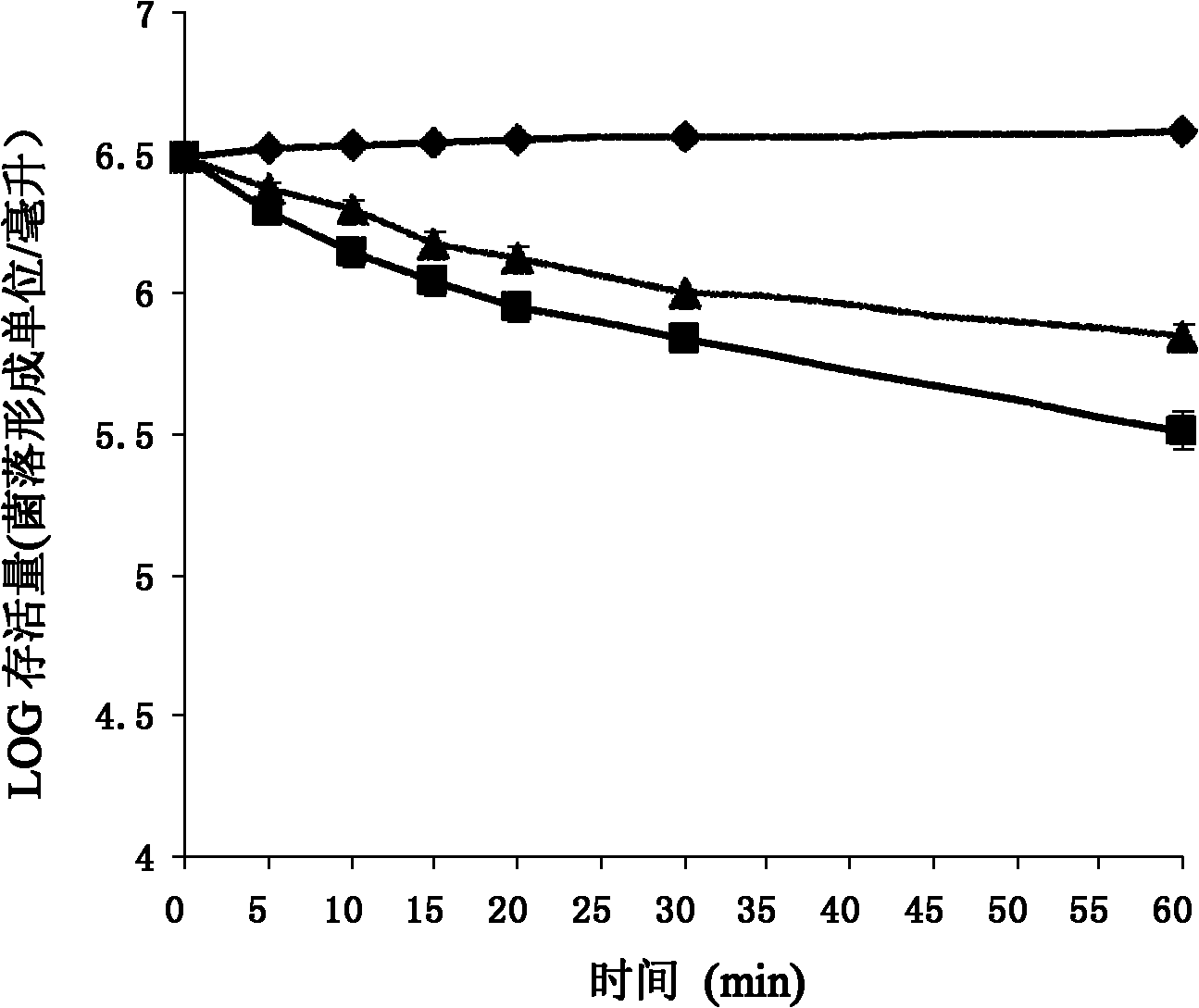
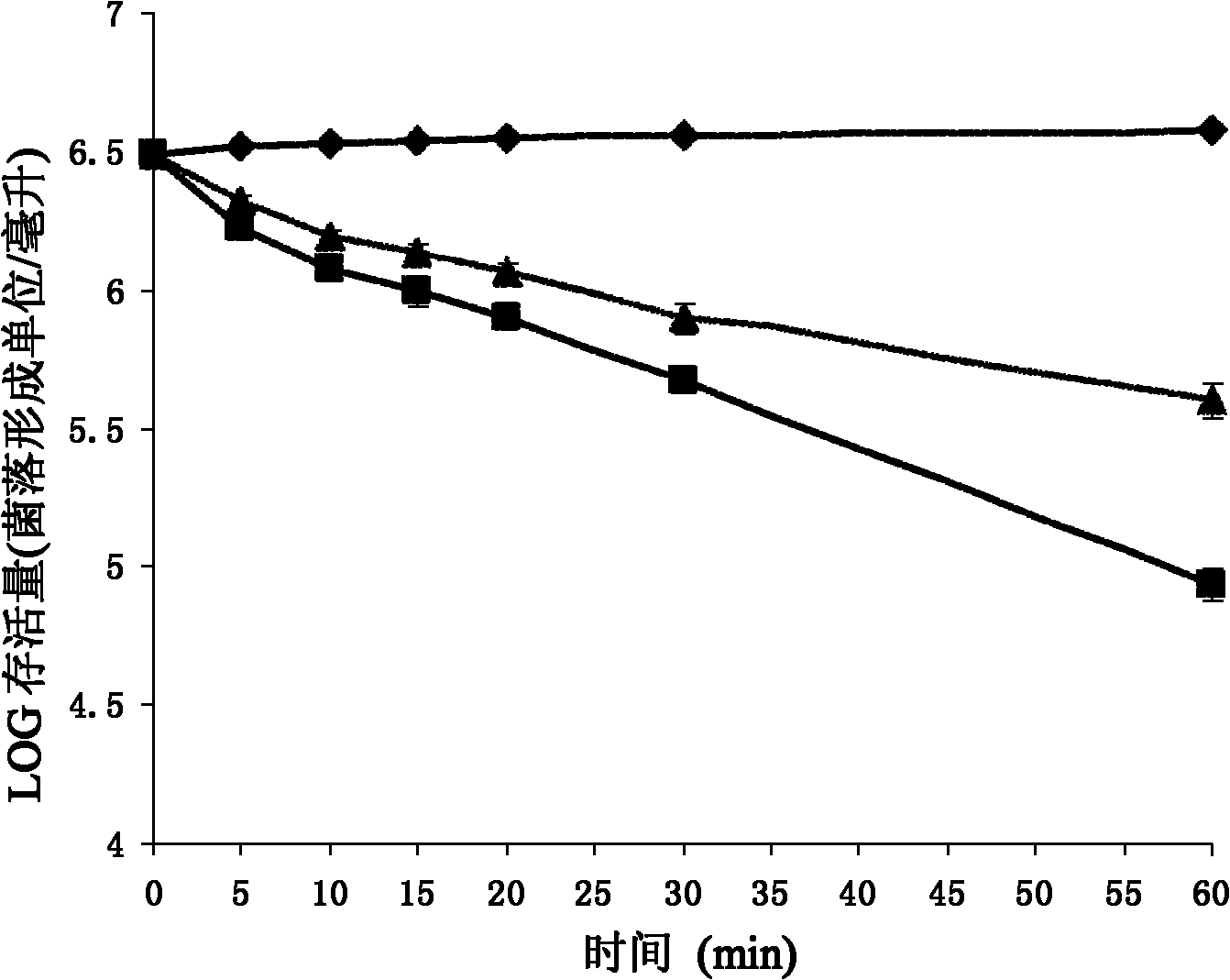
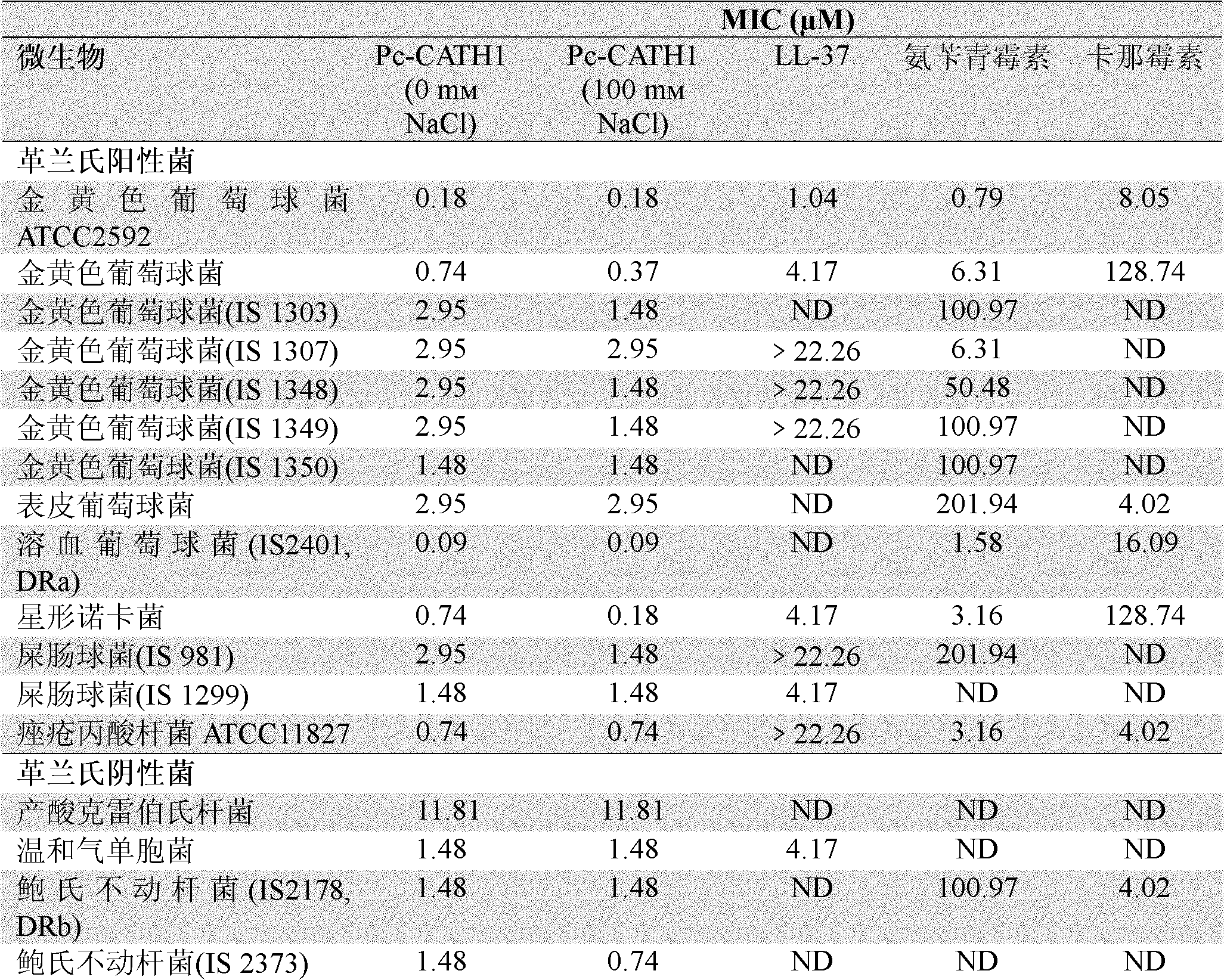
The invention relates to an antimicrobial peptide Pc-CATH1 from Phasianus colchicus and a gene thereof, a chemical synthesis method and application in the field of biopharmaceuticals and belongs to the technical field of biomedicine. The antimicrobial peptide Pc-CATH1 is a straight-chain polypeptide encoded by (Phasianus colchicus) Cathelicidin genes, and the complete sequence of the antimicrobial peptide Pc-CATH1 is as follows: arginine-isoleucine-lysine-arginine-phenylalanine-tryptophan-proline-valine-valine-isoleucine-arginine-threonine-valine-valine-alanine-glycine-tyrosine-asparaginate-leucine-tyrosine-arginine-alanine-isoleucine-lysine-lysine-lysine; and a gene for encoding a precursor of the antimicrobial peptide Pc-CATH1 consists of 607 nucleotides, wherein the 367th to 445th nucleotides are used for coding mature peptide part. The antimicrobial peptide Pc-CATH1 is smaller in molecular weight, stronger in bactericidal action and broader in spectrum and has strong effect of killing various clinical drug-resistant bacteria; the antimicrobial peptide Pc-CATH1 has a simple structure without a disulfide bond or a ring structure and is convenient for chemical synthesis and the preparation of gene engineering; and in addition, the antimicrobial peptide Pc-CATH1 has the beneficial characteristics of no hemolysis, no cytotoxicity, super-strong serum stability and the like.
Owner:DALIAN UNIV OF TECH
Brachymystax lenok Cathelicidin antimicrobial peptide CATH_BARLE, and gene, preparation and application thereof
InactiveCN102816223AGood killing effectImprove the bactericidal effectAntibacterial agentsAntimycoticsChemical synthesisEngineered genetic
The invention relates to a Brachymystax lenok Cathelicidin antimicrobial peptide CATH_BARLE, and a gene, a preparation and an application thereof, and belongs to the technical field of biomedicine. The gene of the CATH_BARLE is composed of 909 nucleotides, and a part coding the mature peptide is nucleotides from the 439th bit to the 597th bit. The mature peptide CATH_BARLE is rich in alkaline amino acids, and has a strong bactericidal effect on common pathogens in aquatic product culture. The CATH_BARLE has a simple structure, does not contain a disulfide bond or an annular structure, and is convenient for chemical synthesis and gene engineering preparation; and the CATH_BARLE also has a good killing effect on many clinical drug-resistant bacteria, especially fungi.
Owner:DALIAN UNIV OF TECH
Fat-soluble drug submicron capsule glucose injection and preparation method thereof
ActiveCN107693488AEvenly wrappedUniform particle sizeEmulsion deliveryMacromolecular non-active ingredientsPolyvinyl alcoholPolyethylene glycol
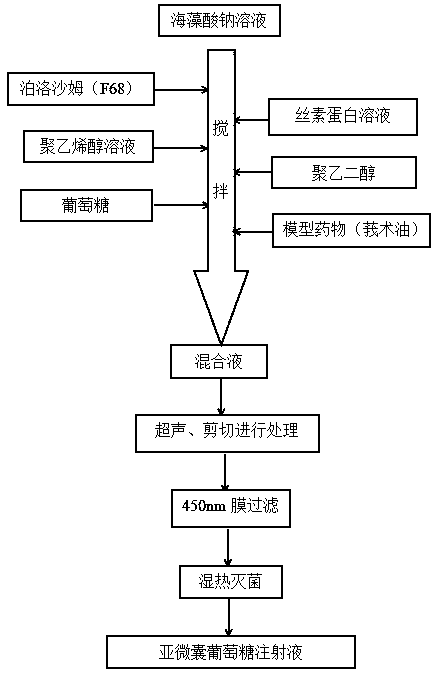
The invention discloses a fat-soluble drug submicron capsule glucose injection and a preparation method thereof. The invention is characterized in that the capsule material comprises the following components in parts by weight: 15-45 parts of sodium alginate, 2.5-9 parts of poloxamer 188, 1.5-5 parts of silk fibroin, 0.5-3 parts of polyvinyl alcohol and 0.2-3 parts of polyethylene glycol. The preparation method comprises the following steps of: sequentially adding the poloxamer, a silk fibroin aqueous solution, a polyvinyl alcohol aqueous solution, the polyethylene glycol and glucose into a sodium alginate aqueous solution at intervals under stirring, and finally dropwise adding a corresponding fat-soluble drug; adopting an ultrasonic and shearing treatment mode; and finally, carrying outfiltration with a 450nm membrane and damp-heat sterilization to obtain the submicron capsule glucose injection. The preparation process of the submicron capsule glucose injection provided by the invention is simple and economic, the particle size of submicron capsules is controllable and uniform, and the biological safety is high.
Owner:CHONGQING UNIV OF TECH
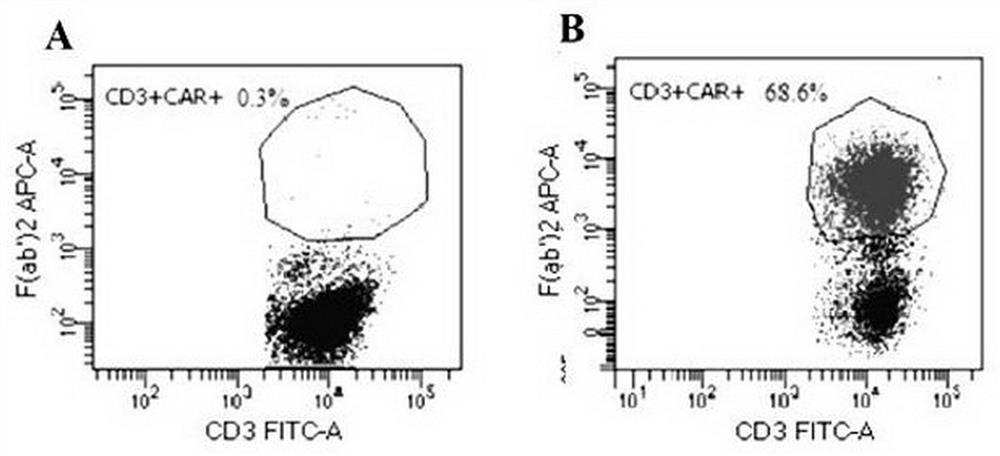
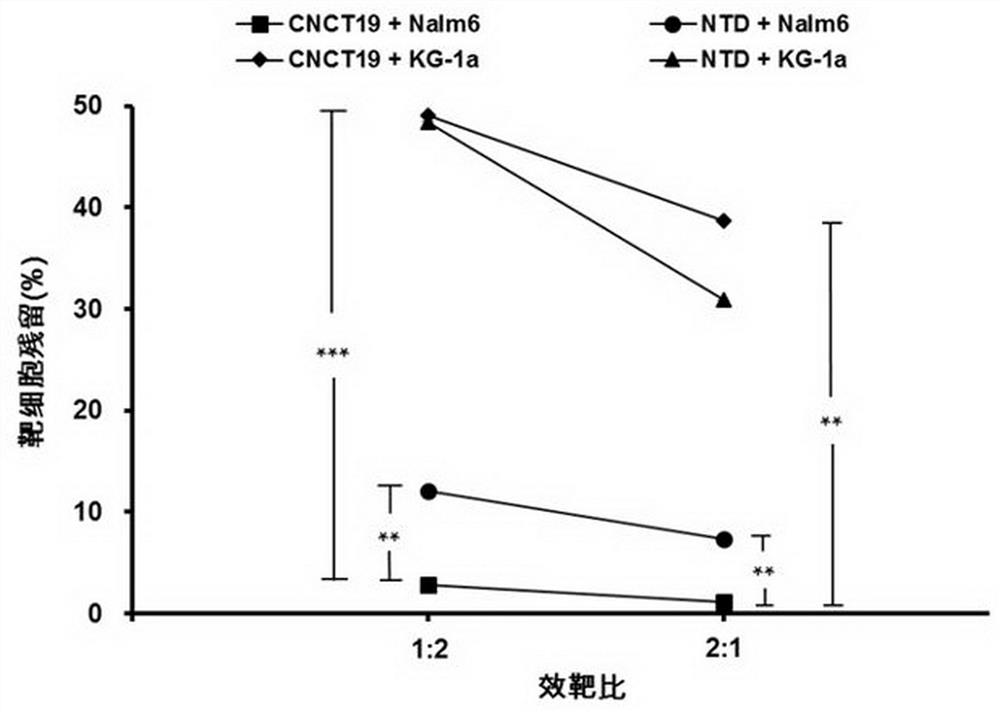
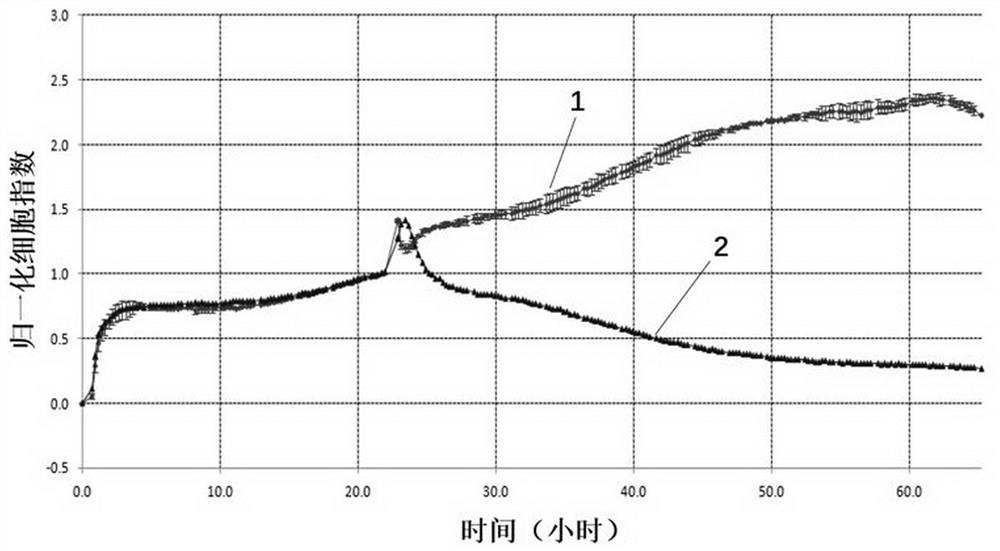
CD19 targeting chimeric antigen receptor and application thereof
ActiveCN112079934APromote secretionProlong survival timeNGF-receptor/TNF-receptor superfamilyImmunoglobulinsAntigen receptorImmune effector cell
The invention provides a chimeric antigen receptor. The chimeric antigen receptor comprises an amino acid sequence shown in SEQ ID NO. 1. The application also provides nucleic acids encoding the chimeric antigen receptor, vectors comprising the nucleic acids, immune effector cells comprising the chimeric antigen receptor, the nucleic acid molecules and / or the vectors, methods of preparing the immune effector cells, compositions comprising the immune effector cells, and uses of the chimeric antigen receptor.
Owner:JUVENTAS CELL THERAPY LTD
Lactobacillus plantarum with inhibition effect on ETEC, and fermentation product and application thereof
ActiveCN111484958AImprove conversion ratePromote growthAntibacterial agentsBacteriaBiotechnologyAntibacterial activity
The invention provides lactobacillus plantarum with an inhibiting effect on ETEC as well as a fermentation product and application of the lactobacillus plantarum, and belongs to the technical field ofpreparation of probiotics and feed additives. The lactobacillus plantarum (Lactobacillus plantarum) provided by the invention is named as DMS LP1210, and the preservation number is GDMCC No.60717. The bacterium has the function of inhibiting ETEC, the bacteriostatic activity of the produced bacteriocin is still kept at 80% or above after the bacteriocin is subjected to heat treatment at 121 DEG Cfor 30 min, and the bacteriocin has tolerance to high temperature; 80% or above of the antibacterial activity is reserved when the pH value is 2-10, 90% or above of the antibacterial activity is reserved after pepsin, trypsin and protease K are used for treating the bacteria, the tolerance to the intestinal tract environment is good, the diarrhea rate is reduced, the feed conversion efficiency isimproved, the effect is safe and reliable, and the probiotics additive is suitable for being developed into feed probiotics additives.
Owner:泰安肽普德蛋白有限公司
Application of Pluronic F-127 solution in chick embryo chorioallantoic membrane experiment
InactiveCN103134910ADoes not affect growthEasy to observeTesting medicinal preparationsMedicineLiquid state
The invention relates to application of Pluronic F-127 solution in chick embryo chorioallantoic membrane experiment as medicine carrying material. The application includes the steps of setting the Pluronic F-127 solution at 4 DEG C through still-setting method to dissolve the solution, configurating biological isotonic solution of certain concentration, and thus the medicine carrying material is obtained. The Pluronic F-127 solution is in liquid state in low temperature, and thus the solution can be mixed with tested medicine conveniently, and possibility of temperature-sensitive medicine degeneration is reduced to the greatest extent. The Pluronic F-127 solution is transparent when the solution is in solid state in high temperature, and thus chick embryo chorioallantoic membrane does not deform when the Pluronic F-127 solution is used and observation is convenient.
Owner:SHANXI UNIV
Three-dimensional printing material as well as preparation method and application thereof
InactiveCN111330073AImprove mechanical propertiesPromote proliferationAdditive manufacturing apparatusTomographyPoly ether ether ketoneHemolysis
The invention relates to a three-dimensional printing material as well as a preparation method and application thereof. The method uses polycaprolactone, hydroxyapatite, polylactic acid, polyether ether ketone and strontium doped mesoporous bioactive glass as raw materials to successfully prepare the composite material for 3D printing, and mechanical performance tests and biological experiments confirm that the obtained material has excellent mechanical properties, can significantly promote cell proliferation and osteogenic differentiation, has no hemolysis, has good biocompatibility, and canbe degraded in vivo, and therefore the composite material can be used to prepare a medical artificial bone to treat bone injury, such as orbital zygomatic jaw complex injury.
Owner:SHANGHAI TONGJI HOSPITAL
Medicinal composition and preparation method and application thereof
ActiveCN109984999ANon-irritatingHigh solubilizing abilityOrganic active ingredientsNervous disorderWater insolublePolyethylene glycol
The invention provides a medicinal composition. The composition consists of a water-insoluble antipsychotic, polyethylene glycol fatty acid ester, sorbitan ester and an aqueous intermedium; the medicinal composition forms an aqueous and injectable suspension. The water-insoluble antipsychotic drug is an aripiprazole prodrug, namely lauroyl aripiprazole, and the medicinal composition which is proper in particle size and is prepared according to a specific prescription proportion is used for preparing a clinical suspension injection which has high stability, meets the requirements for the release degree and particularly has high safety and an extremely high application value.
Owner:重庆仁泽医药科技有限公司
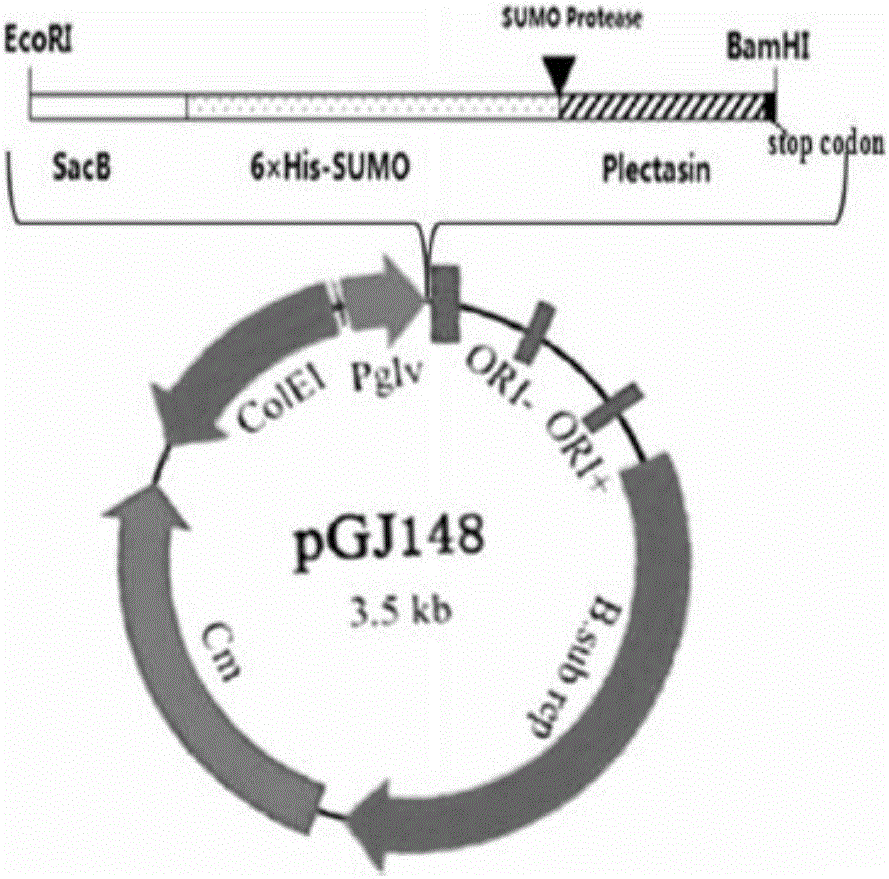
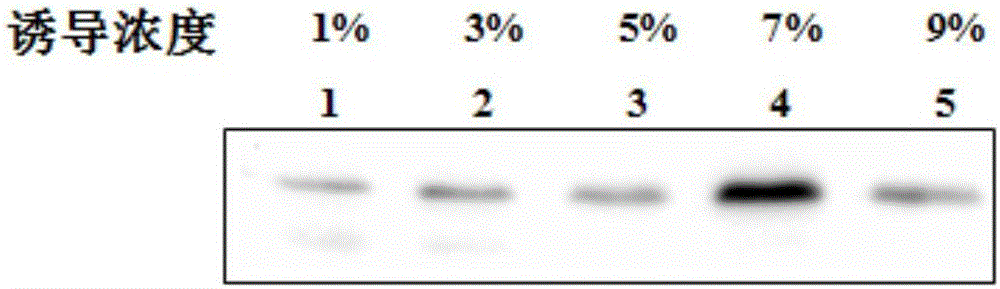
Plectasin expressed through bacillus subtilis and expressing method thereof
InactiveCN106554396AGood killing effectNo hemolyticAntibacterial agentsPeptide/protein ingredientsEscherichia coliHemolysis
The invention provides plectasin expressed through bacillus subtilis and an expressing method thereof. The sequence of the plectasin is shown as a sequence table SEQ ID No.1. The expressing method includes the steps that a plectasin gene with the signal peptide SacB and protein tag 6*His-SUMO is connected onto an expression vector pGJ148 and then the connected product is electrically transferred into competent escherichia coli cells DH5alpha; correctly-authenticated recombinant plasmid is converted into the bacillus subtilis WB800N; and under the action of an inductive agent maltose, fusion protein can be expressed, and plectasin protein can be obtained by means of purification. The protein has an efficient killing effect on gram positive bacteria and does not have hemolysis to red blood cells of human bodies, production cost is reduced, and application of the plectasin as an anti-infection drug is further promoted; and meanwhile a theoretical basis is provided for application of the plectasin.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
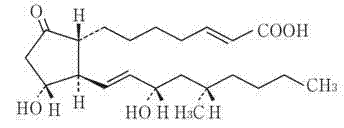
PGA1 ((13E,15S)-15-hydroxy-9-oxo-prosta-10,13-dien-1-oic acid)-containing lipid emulsion and preparation method thereof
ActiveCN102988290AImprove curative effectOpaque appearanceOrganic active ingredientsEmulsion deliveryChemistrySide effect
The invention provides a PGA1 ((13E,15S)-15-hydroxy-9-oxo-prosta-10,13-dien-1-oic acid)-containing lipid emulsion and a preparation method thereof, and in particular provides a PGA1 lipid emulsion preparation which is prepared by wrapping an oil component containing a main medicine PGA1 by a lipid agent which serves as a soft shell structure, wherein phosphatidylinositol or distearoyl phosphatidylglycerole sodium is added to the preparation, so that the stability of the main medicine PGA1 can be improved, and the main medicine PGA1 can be fused with the externally wrapped lipid agent structure better; and furthermore, the preparation prescription contains a stabilizer for improving the stability of the lipid agent structure. According to the PGA1-containing lipid emulsion, the phosphatidylinositol or distearoyl phosphatidylglycerole sodium is added and can be chemically combined with PGA1, so that the stability of PGA1 can be improved, PGA1 can be prevented from being damaged under the preparation conditions such as high temperature and high pressure, the useful life and the storage period of the medicine can be obviously prolonged, and adverse reaction such as vascular irritation and a plurality of defects such as toxic or side effect and inconvenience in use of conventional PGA1 preparation can be avoided.
Owner:BEIJING TIDE PHARMA
Ibuprofen sodium chloride injection preparation with pH of 6.0-6.5, and preparation method thereof
InactiveCN103565733AReduce dosageSolve the problem of opalescence opacityOrganic active ingredientsAntipyreticActivated carbon filtrationMicrofiltration membrane
The invention provides an ibuprofen sodium chloride injection preparation with pH of 6.0-6.5, and a preparation method thereof. The injection contains 1-10 mg / ml of ibuprofen, and injection water, wherein the pH is adjusted to 6.0-6.5 by an acid-base modifier; the dosage of the required acid-base modifier accounts for about 2-6% by weight / volume of the injection; the sodium chloride content of an isoosmotic adjusting agent is 0.70-0.90% by weight / volume. The preparation technology comprises the following steps: firstly, dissolving the acid-base modifier and the ibuprofen; adding the injection water to 20% of total mass; adding sodium chloride to stir and dissolve; adding activated carbon, filtering and decarburizing; adding water to 90% of total mass, adjusting the pH to 6.0-6.5, and adding water to total mass; filtering by 0.45 microns and 0.22 microns of microfiltration membranes; and filling and sealing and sterilizing at 121 DEG C for 8-20 minutes.
Owner:南京帝易医药科技有限公司
Medicinal composition solution and preparation method and use thereof
InactiveCN101966146AQuick effectConducive to clinical rescueOrganic active ingredientsNervous disorderArginineAntioxidant
The invention relates to medicinal composition solution. The medicinal composition solution comprises 2-methy-5-imido-benzo-[d][1,3] oxazine [5-b] pyrazole, arginine, an antioxidant and a solvent, wherein the weight ratio of the 2-methy-5-imido-benzo-[d][1,3] oxazine [5-b] pyrazole, the arginine, the antioxidant to the solvent is 1:0.5 to 15:0.1 to 1.5:10 to 400; and the solvent is pharmaceutical water. The medicinal composition has high water dissolubility, and is difficult to degrade under the protection of the antioxidant. The medicinal composition solution has stable injection exposure, has stable effect of inhibiting coloring change, is not hemolytic, does not separate crystal in serum out and is safe to use.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Houttuynia injection and its processing technology
InactiveCN1559522AAvoid harmGood water solubilityDigestive systemPharmaceutical delivery mechanismHouttuyniaSolvent
A houttuynia injection is prepared from houttuynia through distilling to obtain distilled liquid, adding polyethanediol, alcohol and sodium chloride, regulating isotonic pressure by physiological saline, thermal reflux, cooling, regulating pH=4-6 by citric acid, storage at 4-10 deg.C for 24-48 hr, millipore membrane filtering, and sterilizing.
Owner:姚仲青
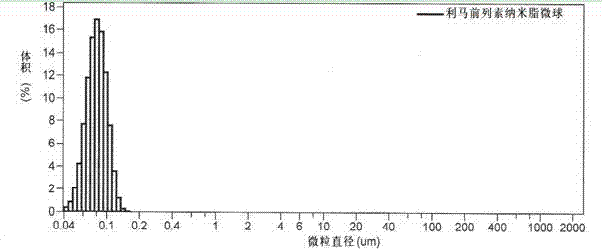
Limaprost nanoemulsion preparation for vertebral canal injection of antisternum
The invention provides a limaprost nanoemulsion preparation for vertebral canal injection of antisternum, particularly relates to a limaprost nanoemulsion which contains citric acid and sodium citrate emulsion stabilizer, wherein the weight proportion of the citric acid and the sodium citrate in the emulsion stabilizer is 1:1, so the problem of unstable limaprost liquid preparation is solved. Thepreparation can be used for vertebral canal injection of the antisternum, so the defect that only few medicines of the existing oral limaprost tablet reach the focus part due to a first pass effect is solved, the therapeutic effect is improved, and the operative treatment rate is lowered, so the clinical use is benefited.
Owner:BEIJING TIDE PHARMA
Active antibacterial dressing based on bamboo fungus embryo proper extract
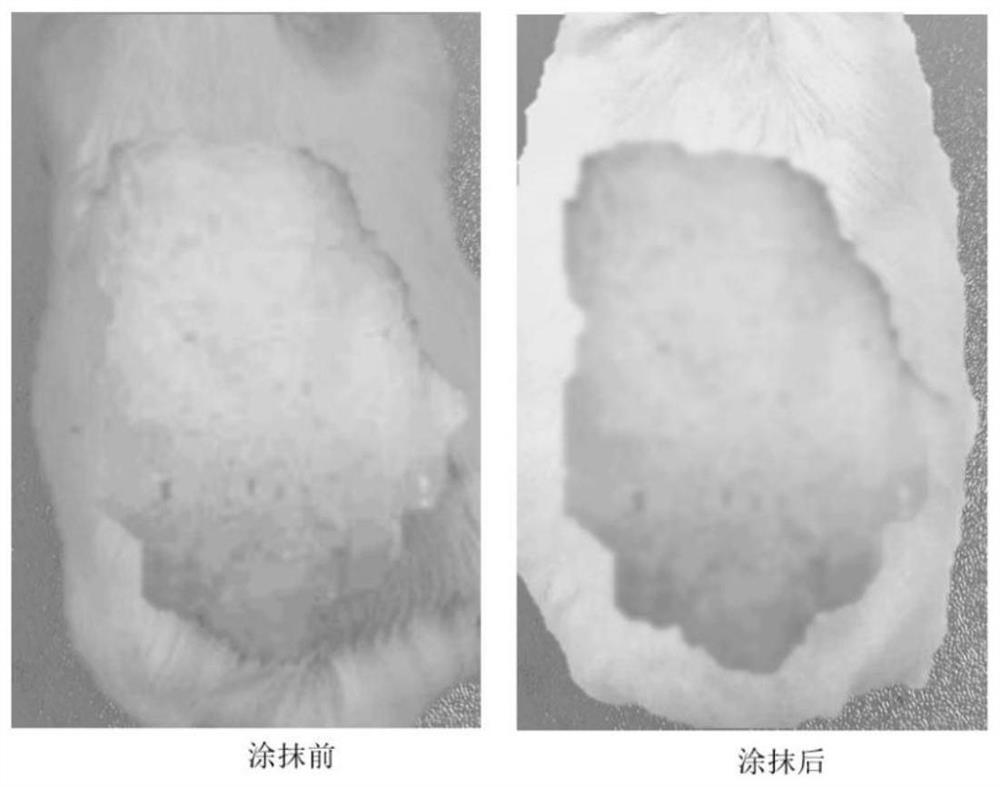
The invention discloses an active antibacterial dressing based on a bamboo fungus embryo proper extract, and belongs to the technical field of biological medicine products. By selecting chitosan as acarrier material to be compounded with the antibacterial bamboo fungus embryo proper extract, three novel medical antibacterial dressings are prepared. The antibacterial dressing is good in antibacterial effect and free of immunogenicity and hemolysis, a gel protective layer is formed at a wound, and wound exudate is effectively absorbed. The antibacterial dressing is moisture-preserving, breathable and non-sticky, and has the advantages of being high in biocompatibility and good in treatment effect on wound healing and meeting the requirements of ideal functional external dressings.
Owner:XI AN JIAOTONG UNIV
Ketoprofen intravenous administration preparation and preparation method thereof
InactiveCN102895220AAvoid secondary pollutionConvenient for clinical operationOrganic active ingredientsAntipyreticKetoprofenInjection solution
The invention provides a ketoprofen intravenous administration preparation and a preparation method thereof. The injection contains 0.4mg / ml-4mg / ml ibuprofen, an alkali modifier in the amount required for adjusting pH to 6.5-9.0, 0.70%-0.90% w / v sodium chloride and water for injection.
Owner:HC SYNTHETIC PHARMA CO LTD
Ibuprofen injection composite and preparation method thereof
ActiveCN101966147BImprove stabilityLow in 4-isobutylacetophenoneOrganic active ingredientsAntipyreticIbuprofen InjectionMedicine
The invention discloses an ibuprofen injection composite which can obviously improve the quality stability of products and meet the clinical compatibility demand and a preparation method thereof to overcome the defect of the traditional ibuprofen injection. The injection is prepared by mixing ibuprofen and cosolvent according to a molar ratio of 1: 1.001-2, and the pH value of the injection is 7.5-9.0. The ibuprofen injection prepared through the method has obviously improved tolerance capability to high temperature and strong light and better stability and no irritant to vessels, and can effectively meet the requirement of the clinical intravenous drip. Moreover, the preparation method of the invention has the advantages of simple process and good stability of the prepared product.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD +1
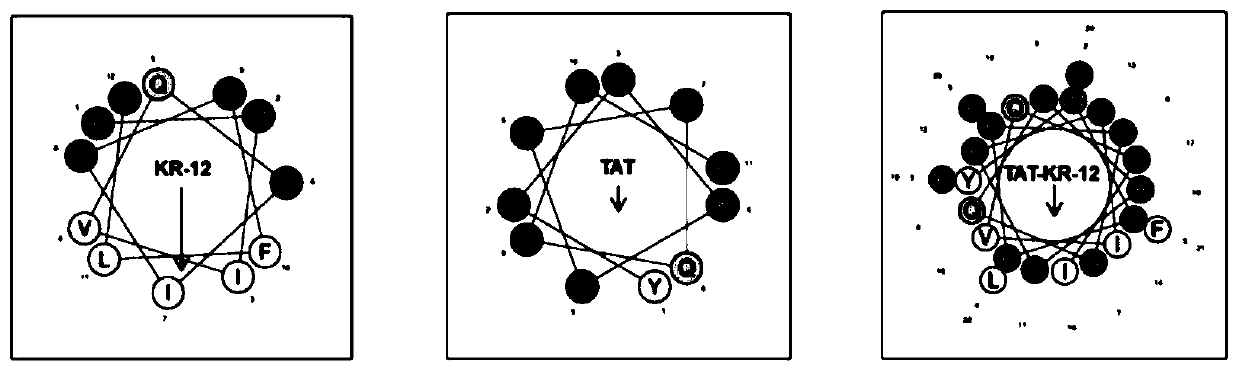
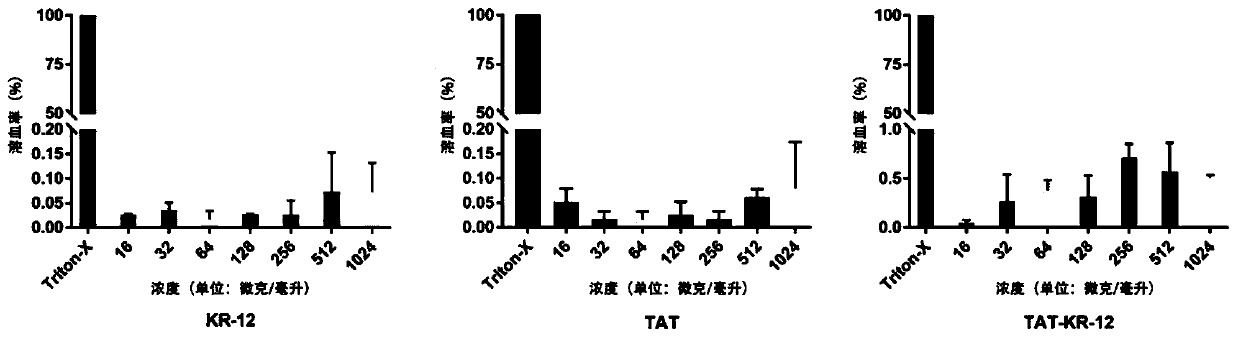
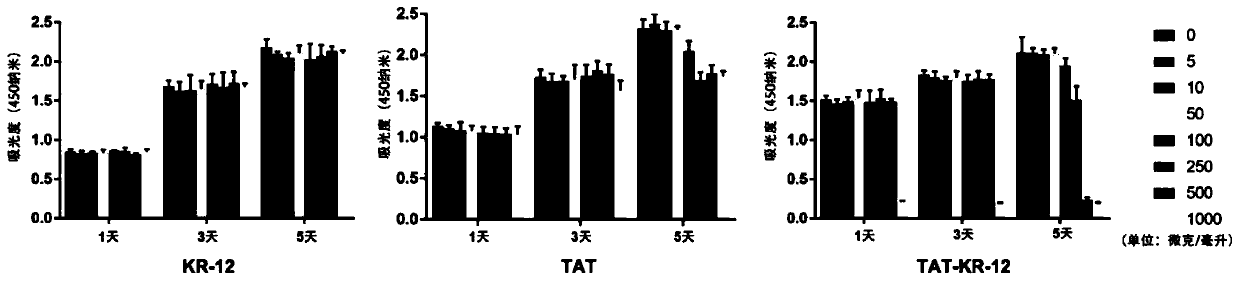
Antibacterial peptide TAT-KR-12 resisting infection of airborne bacteria and intracellular bacteria as well as preparation method and application of antibacterial peptide
InactiveCN111171159AHas anti-intracellular bacteria effectHas anti-inflammatory effectsPolypeptide with localisation/targeting motifAntibacterial agentsAntimicrobial drugPharmaceutical drug
The invention discloses a novel antibacterial peptide resisting infection of airborne bacteria and intracellular bacteria and an application of the antibacterial peptide. The novel antibacterial peptide TAT-KR-12 is formed by joining a biological active fragment KR-12 of anthropogenic antibacterial peptide LL-37 and a cell penetrating peptide TAT. The novel synthesized antibacterial peptide TAT-KR-12 has a function of resisting airborne bacteria and has the effect of resisting intracellular bacteria while having good biocompatibility. Besides, the antibacterial peptide has an anti-inflammatoryeffect. The antibacterial peptide is prepared with a simple preparation process, is high in efficiency and good in repeatability, has a good effect on the intracellular bacteria while effectively resisting the airborne bacteria, has certain anti-inflammatory property, makes up the defect of conventional antibacterial drugs and has great clinical significance.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
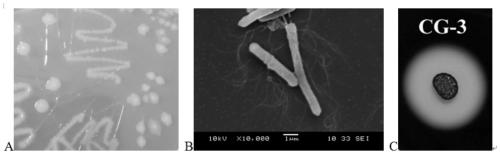
Clostridium butyricum CG3 with probiotic activity and culture method and application thereof
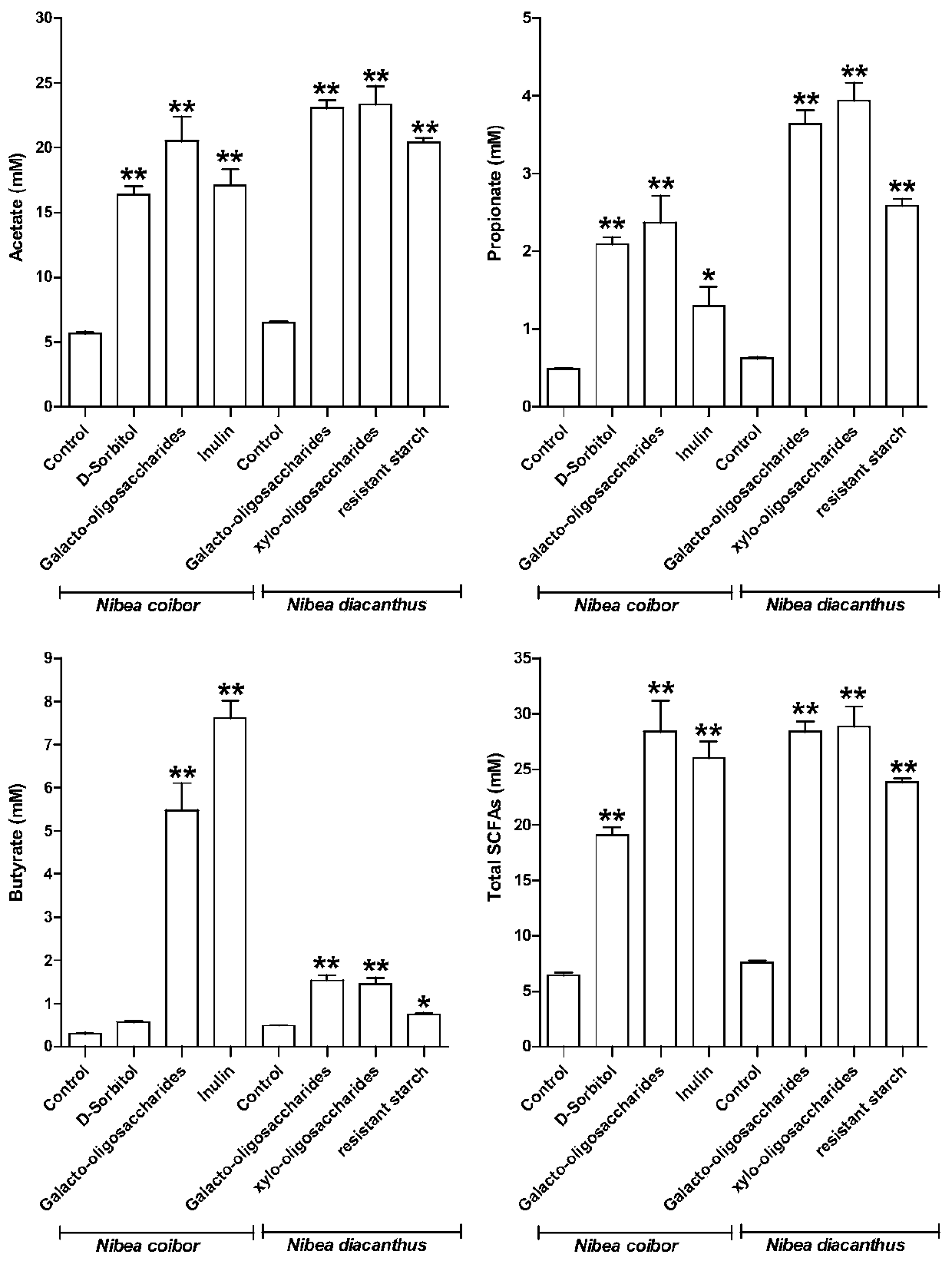
ActiveCN110004094AImprove growth performanceIncrease intestinal SCFAs contentBacteriaAnimal feeding stuffSynbioticsGut flora
The invention relates to clostridium butyricum CG3 with probiotic activity and a culture method and application thereof. The clostridium butyricum CG3 is preserved in the China Type Culture Collection, and the preservation number is CCTCC NO. M2019071. The clostridium butyricum CG3 and synbiotics thereof significantly improve the growth performance of fishes, improve intestinal digestive enzyme activity, change the composition and diversity index of intestinal flora, enrich many SCFA production bacteria, and increase the content of fish intestinal SCFAs. In addition, a light nibea albiflora immune system can be activated by regulating serum complement and cytokine levels, lysozyme activity and intestinal antioxidant capacity, and the clostridium butyricum CG3 is non-hemolytic, sensitive tomost antibiotics, non-drug resistant and highly safe, and can be used as a feed additive for the light nibea albiflora to promote growth and prevent diseases.
Owner:SHANTOU UNIV
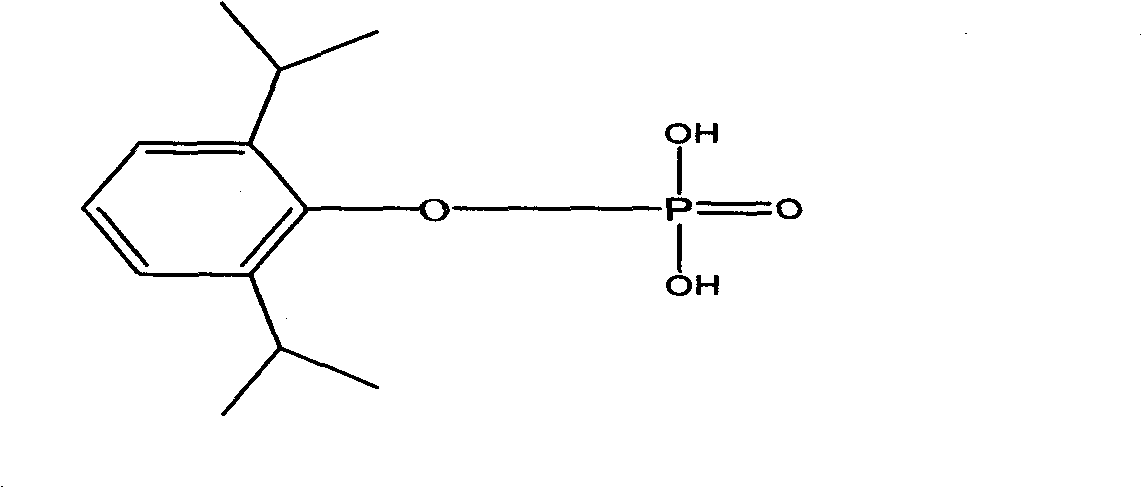
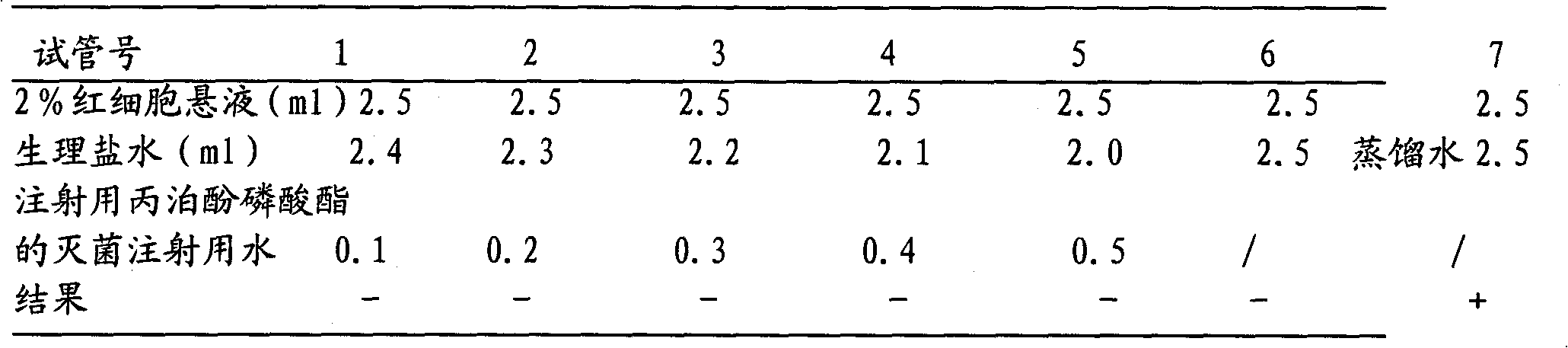
Propofol phosphate for injection and preparation method and application thereof
InactiveCN101780096AImprove performanceNo pollution in the processPowder deliveryOrganic active ingredientsPhosphateMedical prescription
The invention relates to propofol phosphate for injection, pharmaceutical salt thereof and a preparation method thereof. The preparation method comprising the following steps: adding 5-98 percent of water for injection into a preparation container, adding propofol phosphate and pharmaceutical salt thereof of 90-110 percent of accurate prescriptions, slowly dropping a pH value regulator while stirring so as to adjust the pH to be 6.0-11, adding water to the full volume, then adding medicinal carbon of 0.01-1.0 percent (W / V), stirring for 15-60 minutes, roughly filtering with a sand filter rod to remove the carbon, and finely filtering with a 0.22mum microporous membrane until the clarity is qualified; and measuring until the intermediate content is acceptable, fixing the filling amount, sub-packaging into cillin bottles, plugging in plugs by half, freezing and drying samples to control the moisture content being 0.1-8 percent, pressing the plugs, and capping.
Owner:HC SYNTHETIC PHARMA CO LTD
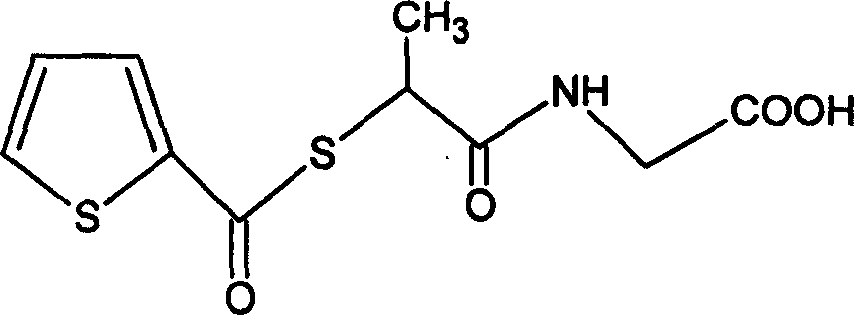
Stepronin powder injection and its preparing method
InactiveCN1820746AQuick effectExpansion of drug occasionsOrganic active ingredientsPowder deliveryFreeze-dryingActive component
The present invention is new preparation form of Stepronin, especially Stepronin powder for injection, and its preparation process. The powder for injection consists of Stepronin or its metal salt or amino salt as active component and freeze drying support agent. Compared with available orally taken Stepronin preparation, the present invention has the advantages of high bioavailability, good medicine absorption, fast medicine dispersion, fast acting, etc. The present invention expands the administration range of Stepronin and raises the clinical administration level of Stepronin.
Owner:HUAZHONG UNIV OF SCI & TECH
Medicinal composition
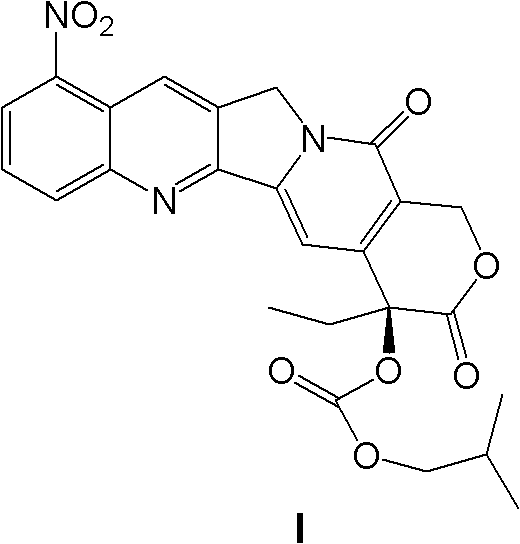
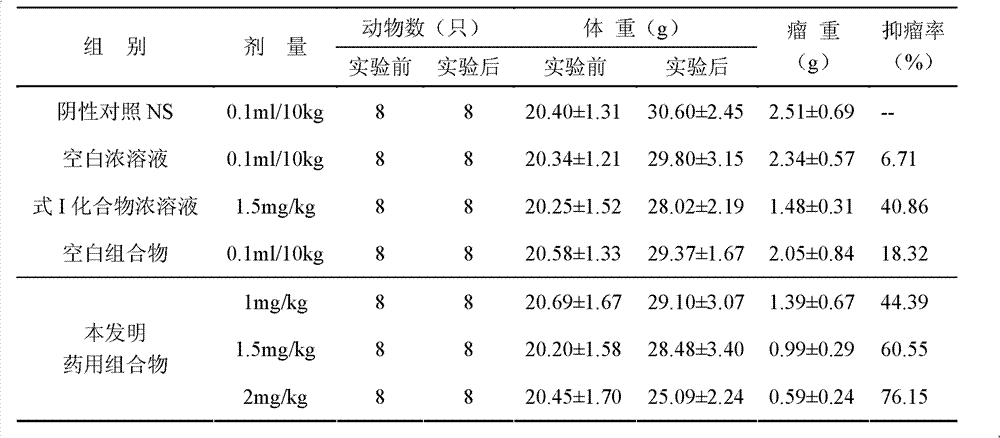
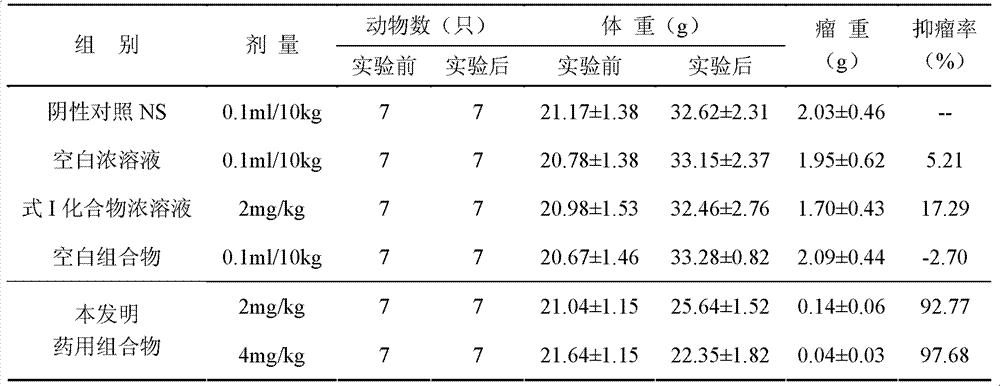
InactiveCN103356619AImprove tumor inhibition rateNo hemolyticPowder deliveryOrganic active ingredientsHemolysisMelanoma
Disclosed in the present invention is a pharmaceutical composition, comprising a weight ratio of 1 : 120 to 1 : 1000 of Camptothecin compounds of formula I and beta-cyclodextrin or derivatives thereof, and an acidic buffer to adjust the pH = 3.5-6.0. The composition can be used to treat solid tumours, such as melanoma, pancreatic cancer, liver cancer, etc. The pharmaceutical composition of the present invention is miscible with a water-miscible co-solvent system in any proportion, and can be used as an intravenous infusion solvent, and has no obvious hemolysis or vascular stimulation; the pharmaceutical composition has a better tumour inhibiting rate than solubilization of surfactants.
Owner:SHANGHAI HUATUO MEDICAL SCI CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com