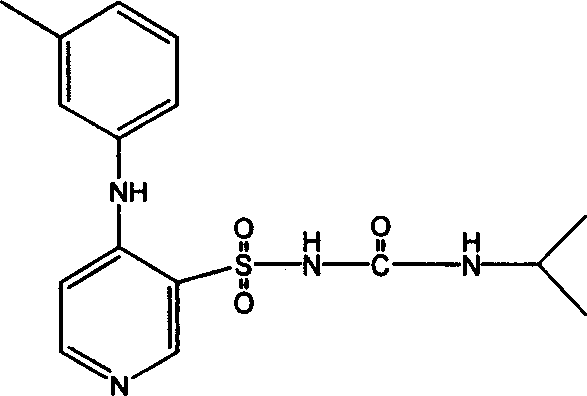
A stable torasemide injection and its preparation method
A technology of torasemide and injection, which is applied in blood diseases, pharmaceutical formulations, extracellular fluid diseases, etc., and can solve problems such as poor stability, slow dissolution rate, and large growth of substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] Prescription 1
[0020] Preparation process: Measure a certain amount of water for injection, add torasemide after adding a solubilizer, add a stabilizer after the dissolution is complete, add water to the full amount, stir evenly, add activated carbon for high-temperature adsorption for 30 minutes, decarbonize and filter, add medicine with Alkali (NaOH, sodium acetate buffer solution), adjust the pH to 9.5, sterilize and filter, fill after passing the intermediate inspection, sterilize, pack and store.
[0021] Stability test is carried out on the samples prepared by prescription 1, prescription 2 and prescription 3, and the results of related substances are shown in the table
[0022] 1. High temperature 60℃ influence factor test
[0023] relative substance%
[0024] 2. Accelerated 40°C test
[0025] relative substance%
[0026] The results showed that the stability of the injection after adding stabilizers and solubilizers was sig...
example 2
[0028] Prescription 4
[0029] Preparation process: Measure a certain amount of water for injection, add propylene glycol, add torasemide, add tromethamine after the dissolution is complete, replenish water to the full amount, stir evenly, add activated carbon for high temperature adsorption for 30 minutes, decarbonize and filter, add NaOH Gradually adjust the pH to 4.5, 7.0, and 9.0 for sterilizing and filtering, and after passing the intermediate inspection, fill, sterilize, and pack for storage.
[0030] Comparing the growth degree of related substances before and after sterilization of injections with different pH values
[0031]
[0032] The results show that when the pH value of the injection is greater than 9.0, there is almost no growth of related substances before and after sterilization, and the stability is significantly better than that of the injection with a pH value of less than 9.0.
example 3
[0034] Prescription 5
[0035] Preparation process: Measure a certain amount of water for injection, add propylene glycol, add torasemide, add tromethamine after the dissolution is complete, replenish water to the full amount, stir evenly, add activated carbon for high temperature adsorption for 30 minutes, decarbonize and filter, add NaOH Gradually adjust the pH to 4.5, 7.0, and 9.0 for sterilizing and filtering, and after passing the intermediate inspection, fill, sterilize, and pack for storage.
[0036] The samples of prescription 4-prescription 8 are tested for influencing factors, and the changes of related substances are shown in the table
[0037] relative substance%
[0038] As can be seen from the table, the stabilizer trometamol is added in the prescription, and the consumption is 0.1%-5%, and the indicated sample has better stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com