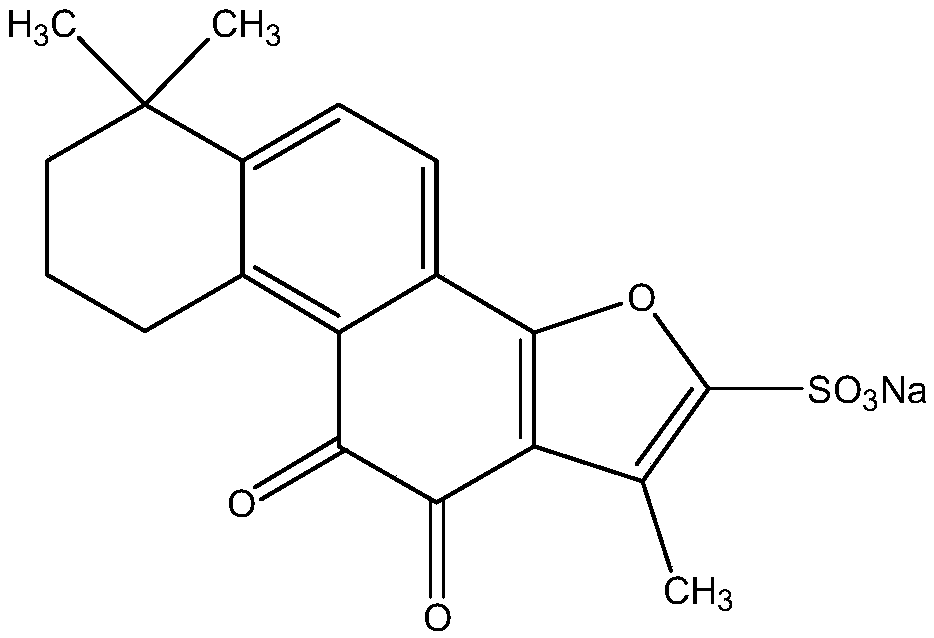
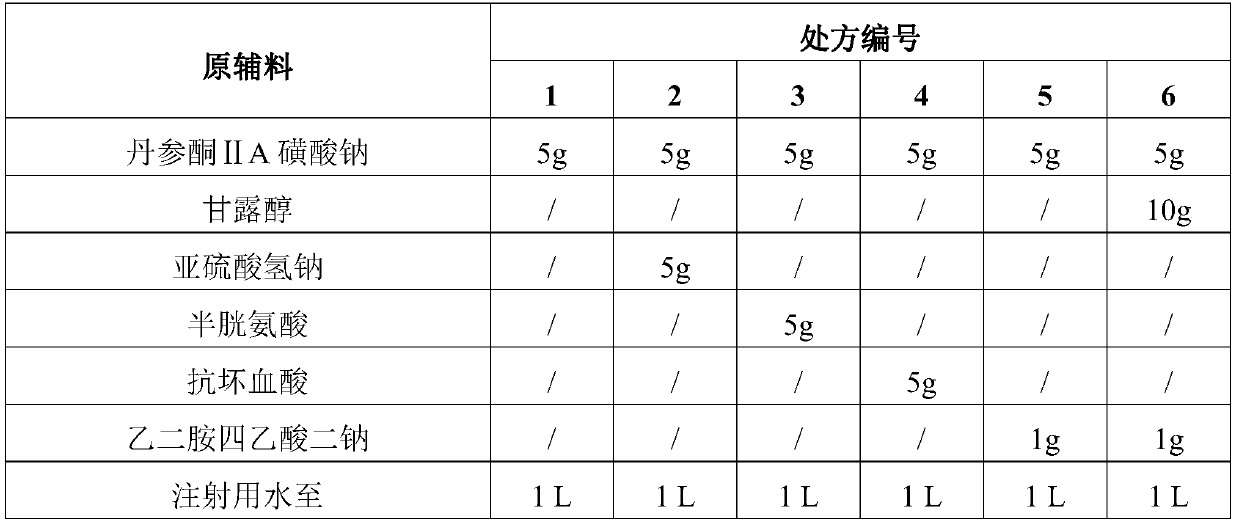
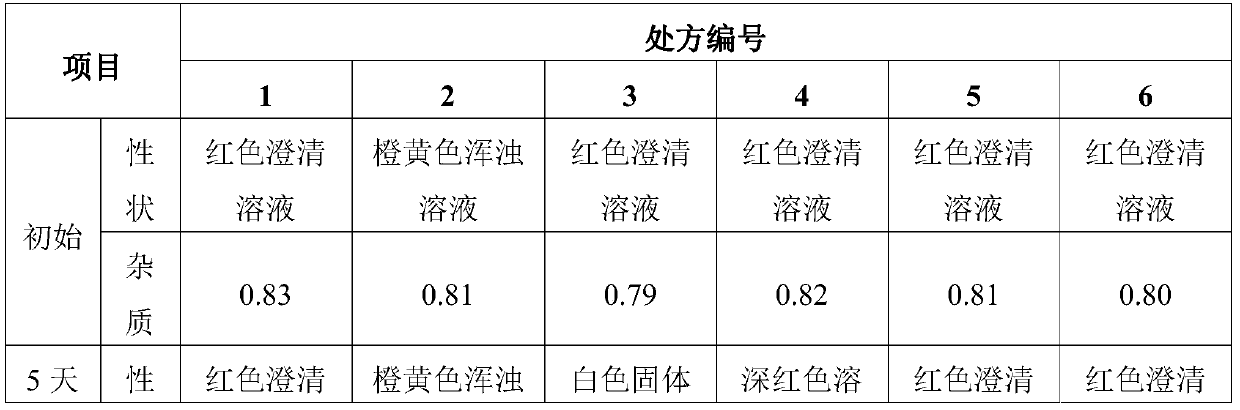
Sulfotanshinone IIA sodium lyophilized powder injection preparation and preparation method thereof
A technology of freeze-dried powder injection and sodium sulfonate, which is applied in freeze-dried delivery, pharmaceutical formulation, powder delivery, etc., can solve the problems of increasing insecurity, increasing production cost, poor product stability, etc., and reducing solid powder and bubbles. Mixture, safety for clinical use, effect of shortening dispensing time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Prescription: specification (10mg / bottle, 1000 bottles)
[0086] Sodium Tanshinone Ⅱ A Sulfonate
10g
60g
0.2g
Water for Injection
Add to 2000mL
[0087] Preparation:
[0088] Dosing: Take 1900mL water for injection, mix tanshinone IIA sodium sulfonate and mannitol, add it to the above-mentioned water for injection stirred in a vortex shape at a temperature of 25°C, stir to dissolve it; add the prescribed amount of sulfuric acid, stir evenly, Adjust the pH to 3.5-4.5; add water for injection to the full amount; filter with a 0.22 μm microporous membrane;
[0089] Freeze-drying: Fill in 7mL vials, 2mL per bottle, half-tightened, put into a freeze-dryer, and freeze-dry according to the designed freeze-drying curve; after freeze-drying, press the bottle stopper, deflate, and take out the sample , crimping and packaging to obtain tanshinone IIA sodium sulfonate freeze-dried powder.
[0090] The desi...
Embodiment 2
[0097] Prescription: specification (40mg / bottle, 1000 bottles)
[0098] Sodium Tanshinone Ⅱ A Sulfonate
10g
100g
sulfuric acid
0.5g
Water for Injection
Add to 2000mL
[0099] Preparation:
[0100] Dosing: with embodiment 1;
[0101] Freeze-drying: Fill in 20mL vials, 8mL per bottle, half-tightened, put into a freeze-dryer, and freeze-dry according to the designed freeze-drying curve; after freeze-drying, press the bottle stopper, deflate, and take out the sample , crimping and packaging to obtain tanshinone IIA sodium sulfonate freeze-dried powder.
[0102] The designed freeze-drying curve is as follows:
[0103] Pre-freezing stage: the plate layer is cooled at full speed, and the temperature of the product reaches about -30°C, and it is maintained for 2 hours to completely freeze the sample;
[0104] Pre-freezing to sublimation: Turn on the condenser, and when the temperature of the condenser drops below -40°C, t...
Embodiment 3
[0109] Prescription: specification (60mg / bottle, 1000 bottles)
[0110] Sodium Tanshinone Ⅱ A Sulfonate
10g
glucose
200g
0.5g
Water for Injection
Add to 2000mL
[0111] Preparation:
[0112] Dosing: with embodiment 1;
[0113] Freeze-drying: Fill in 30mL vials, 12mL per bottle, half-tightened, put into a freeze-dryer, and freeze-dry according to the designed freeze-drying curve; after freeze-drying, press the bottle stopper, deflate, and take out the sample , crimping and packaging to obtain tanshinone IIA sodium sulfonate freeze-dried powder.
[0114] The designed freeze-drying curve is as follows:
[0115] Pre-freezing stage: the plate layer is cooled at full speed, and the temperature of the product reaches about -40°C, and it is maintained for 3 hours to completely freeze the sample;
[0116] Pre-freezing to sublimation stage: Turn on the condenser, and when the temperature of the condenser drops below -...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com