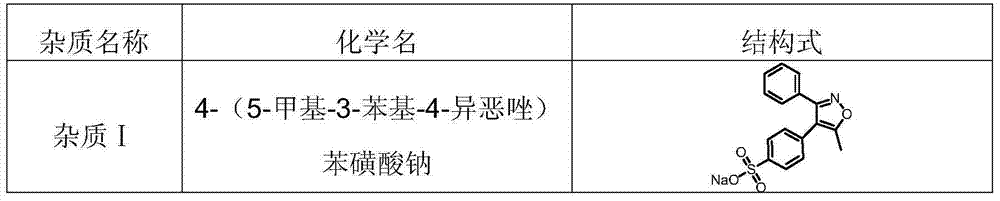
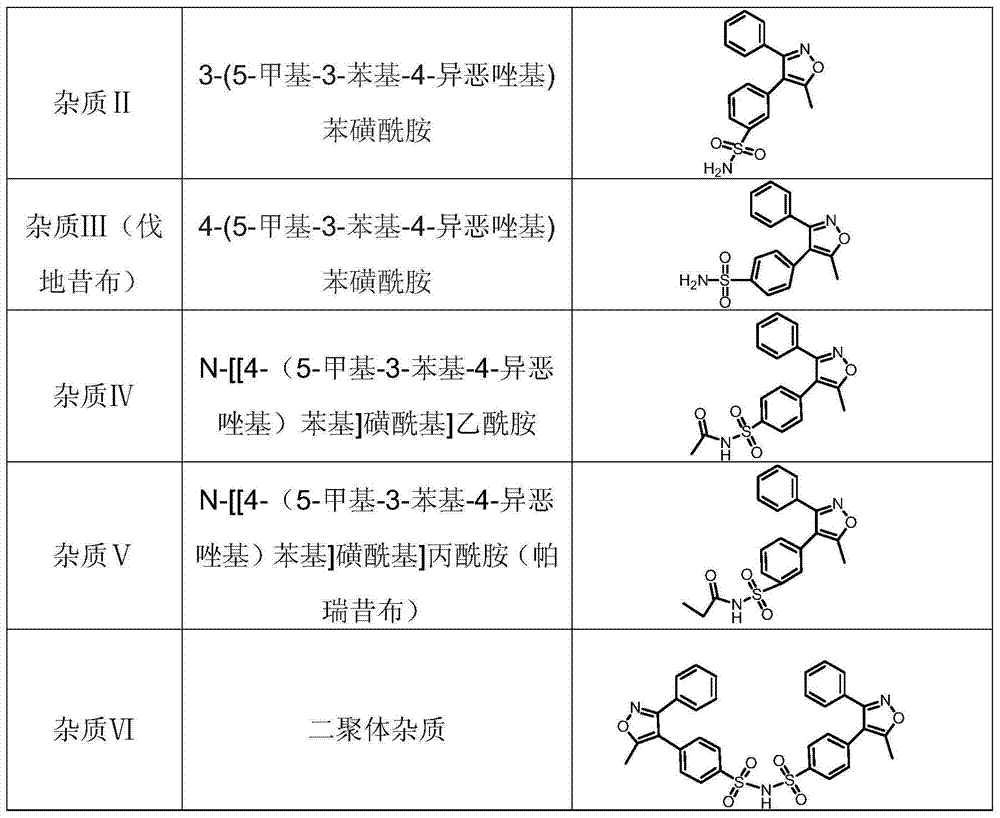
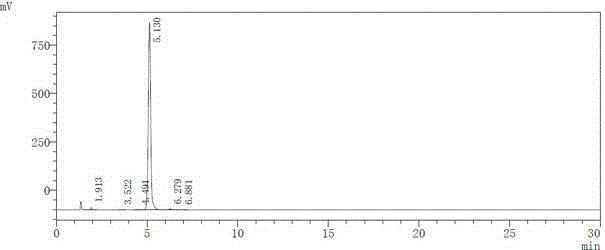
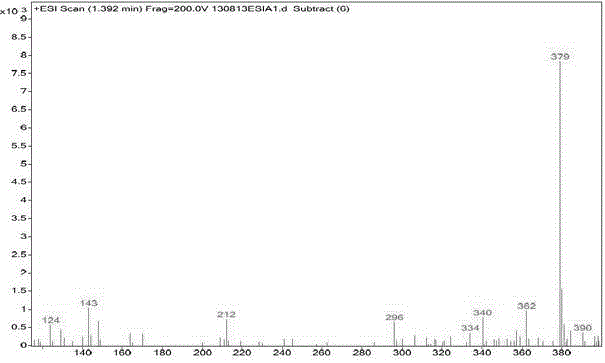
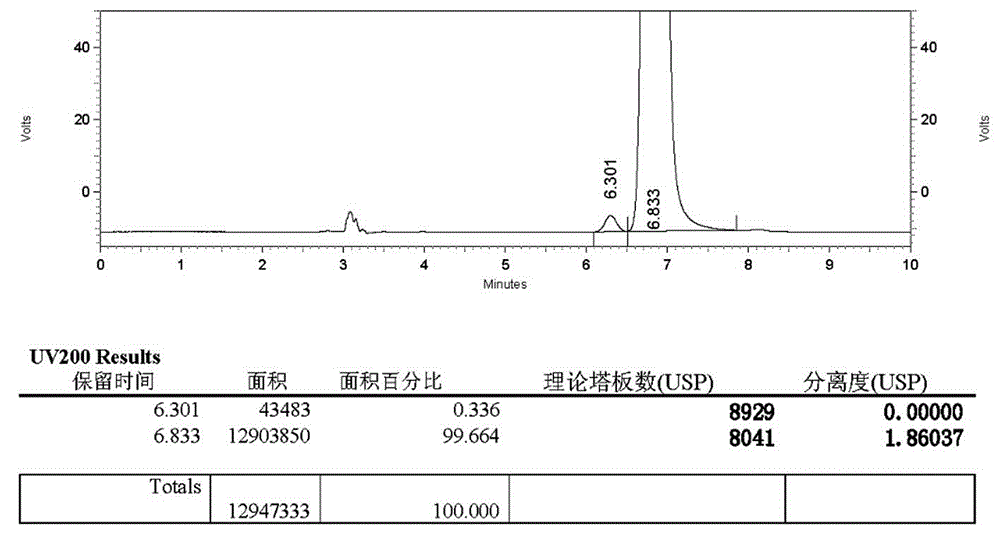
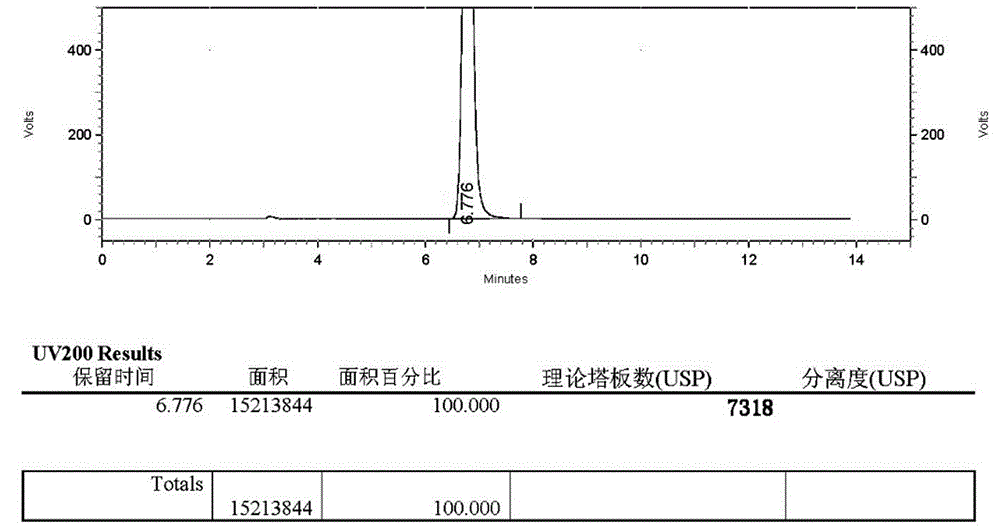
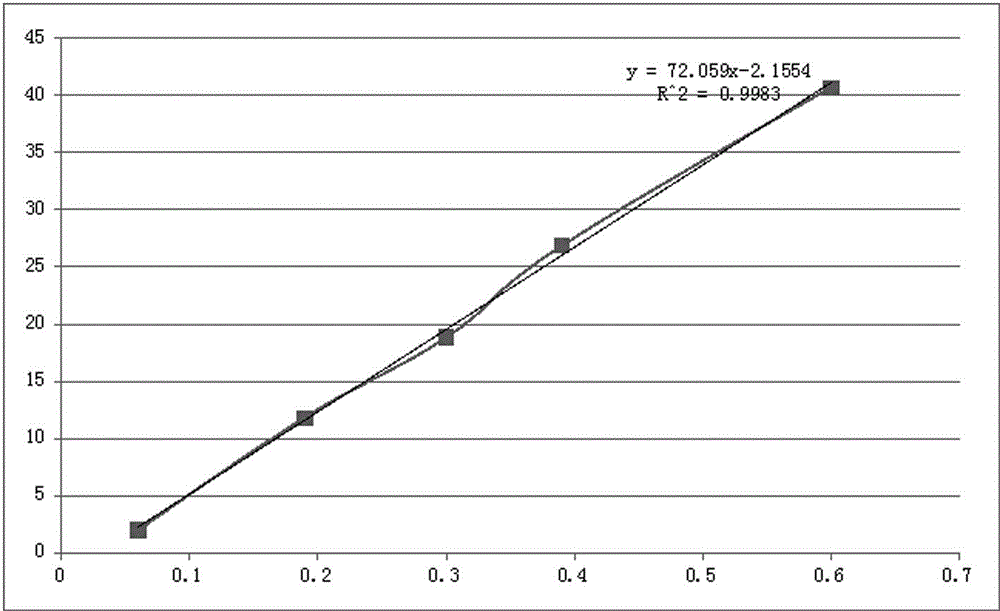
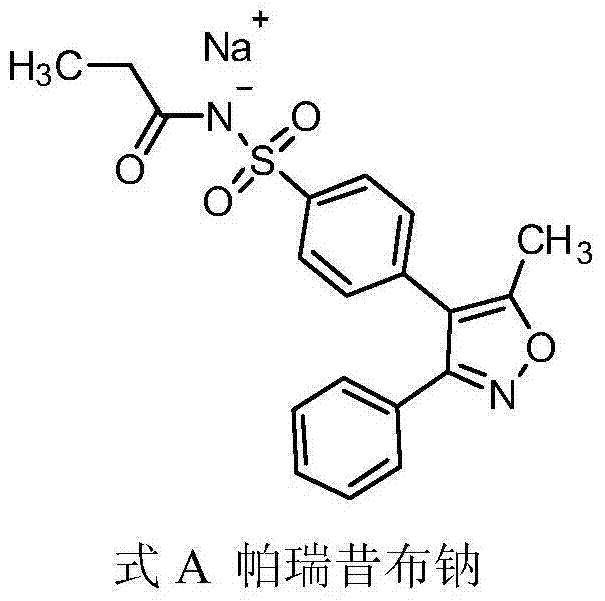
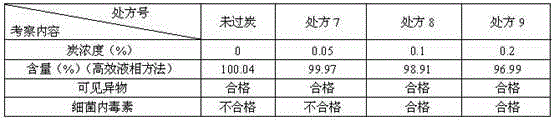
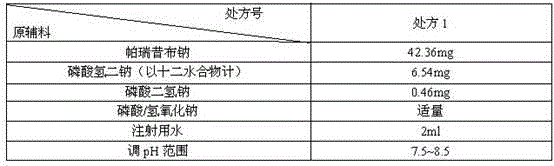
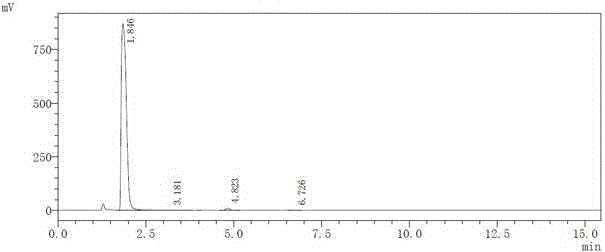
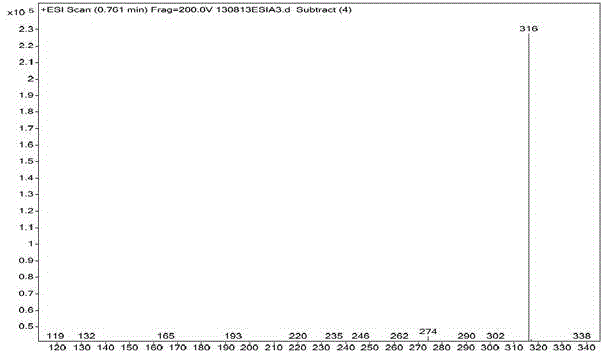
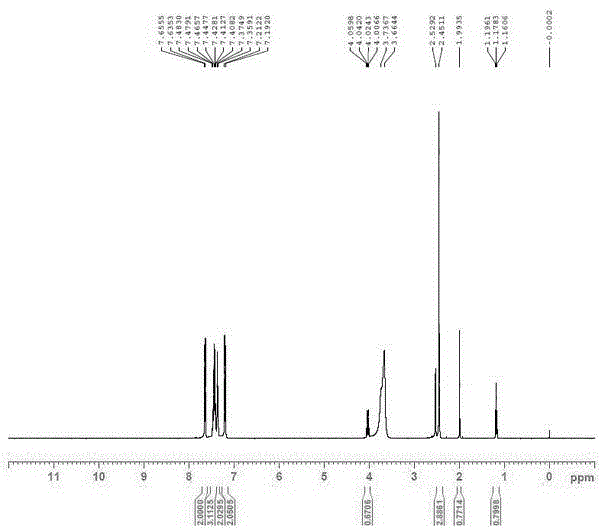
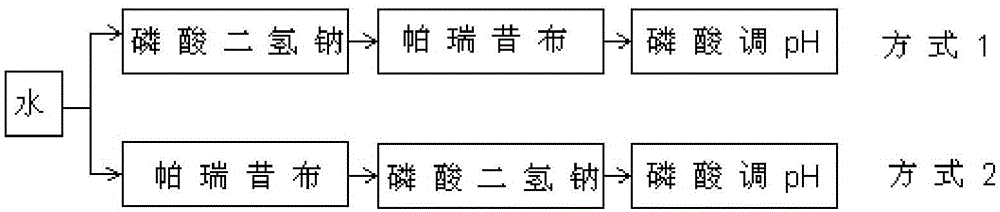
Patents
Literature
102 results about "Parecoxib sodium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
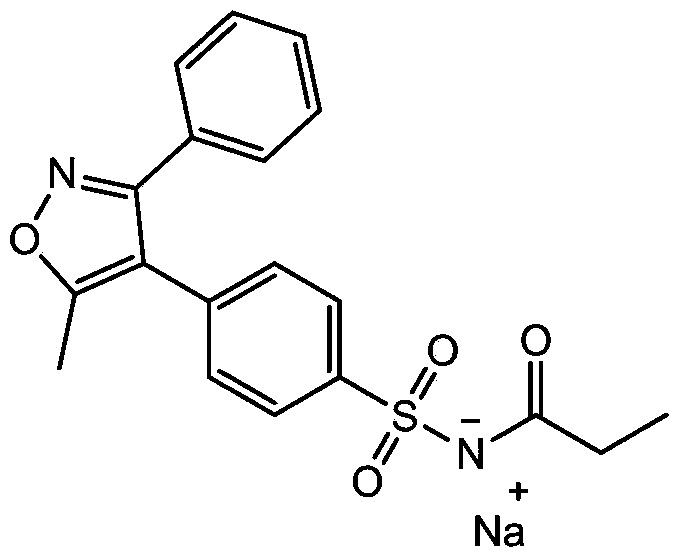
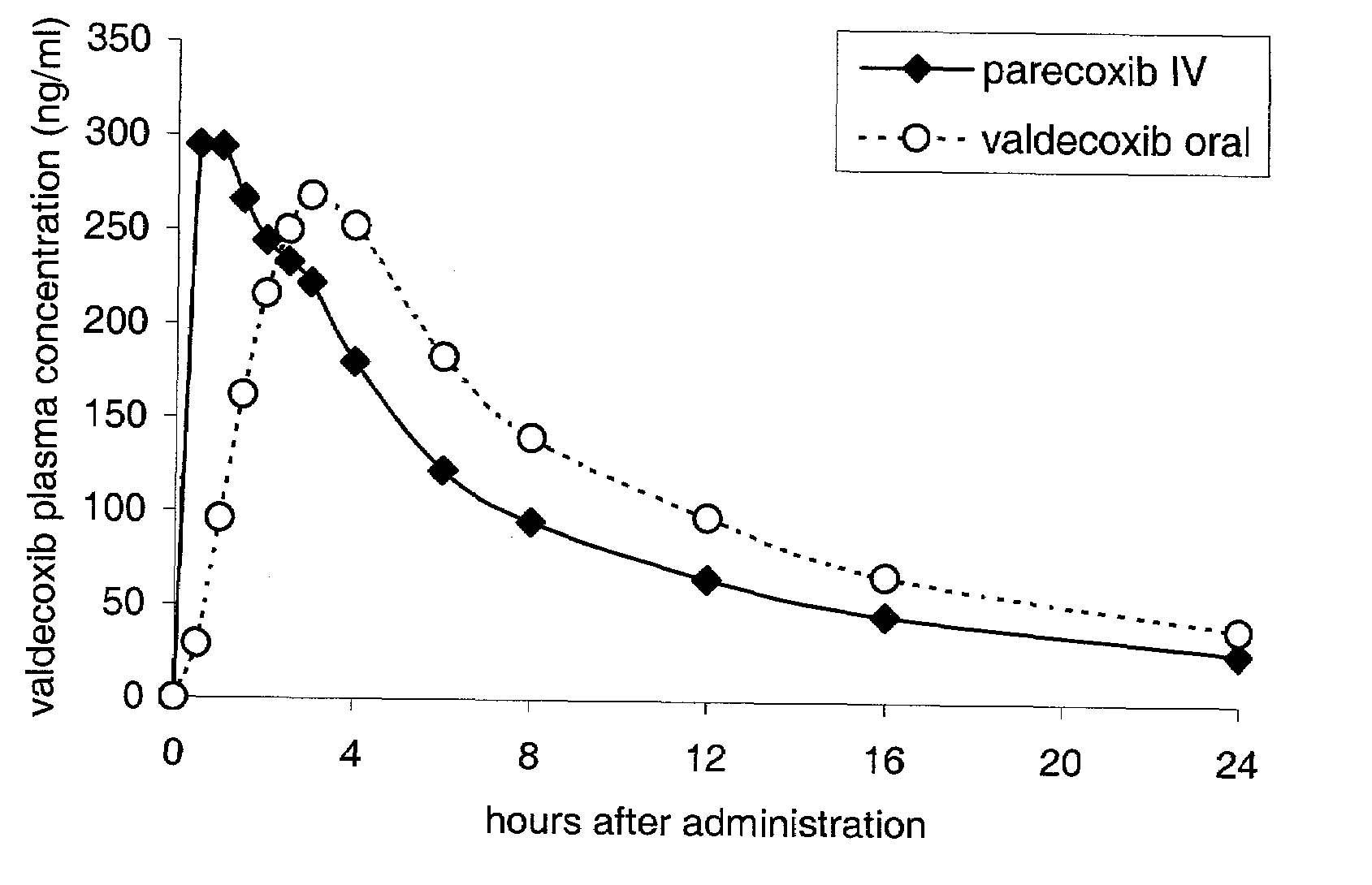
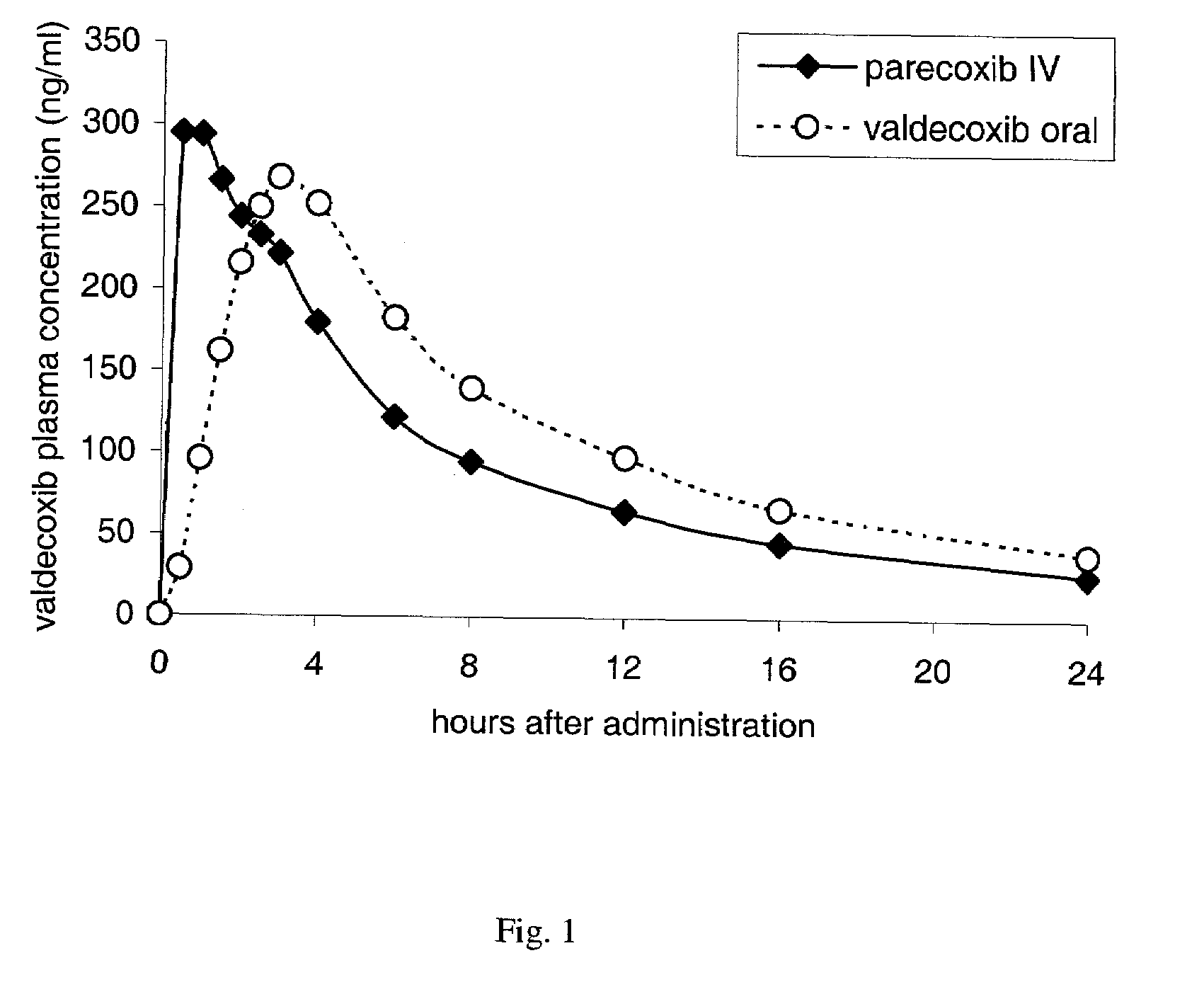
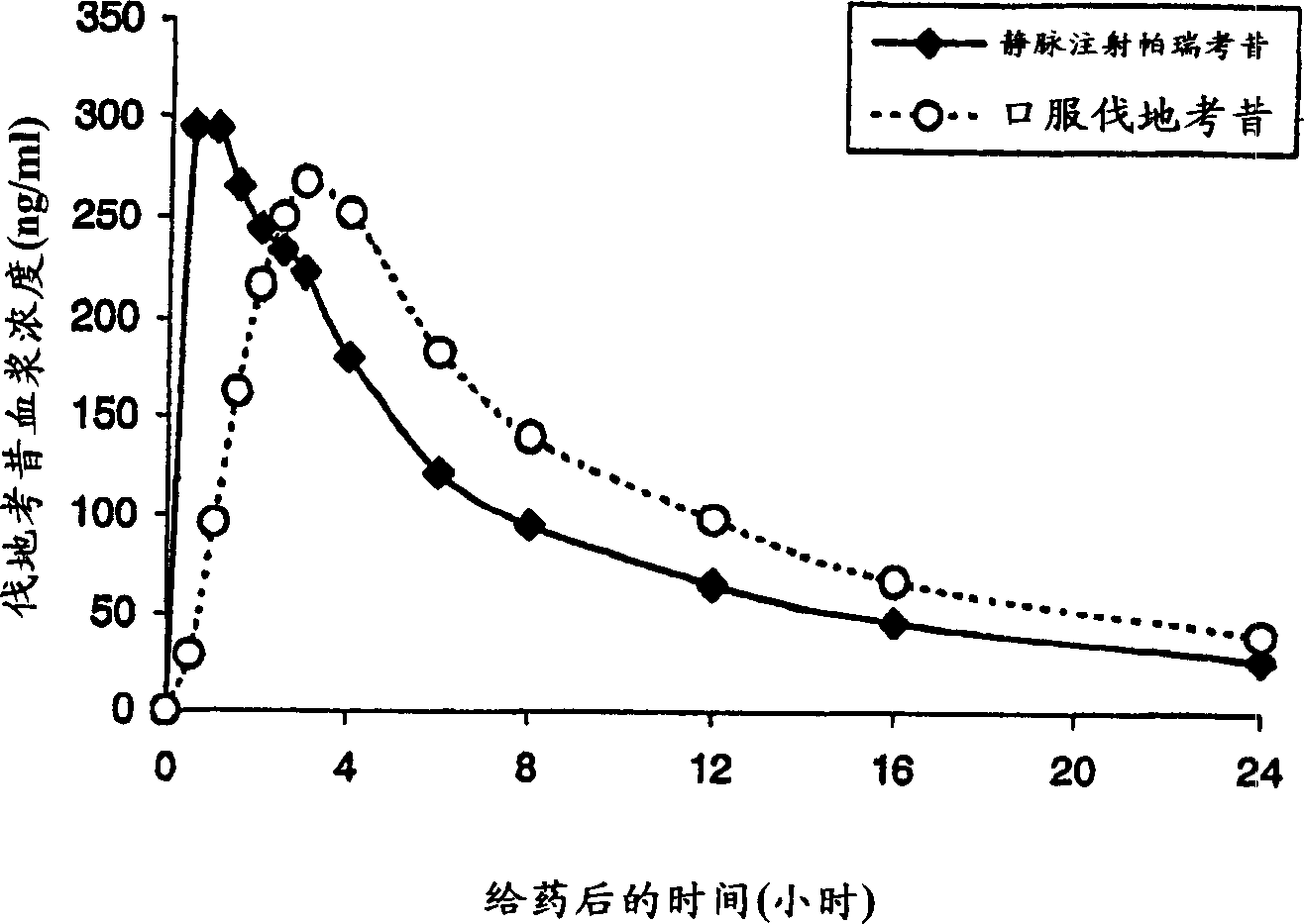
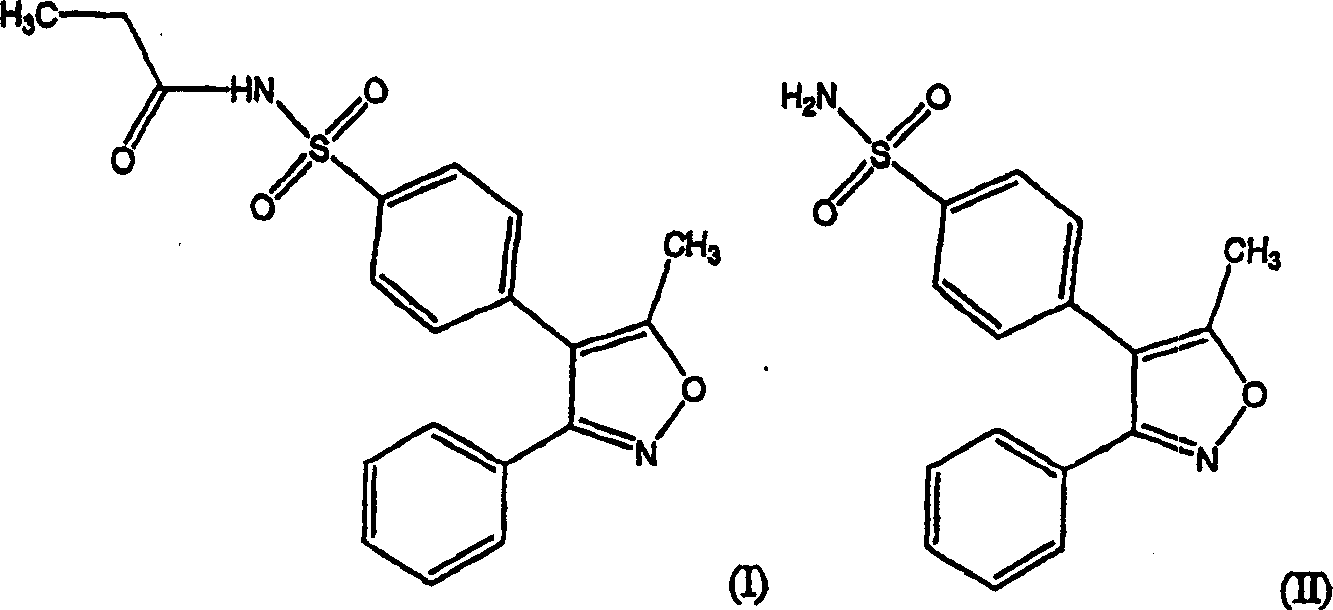
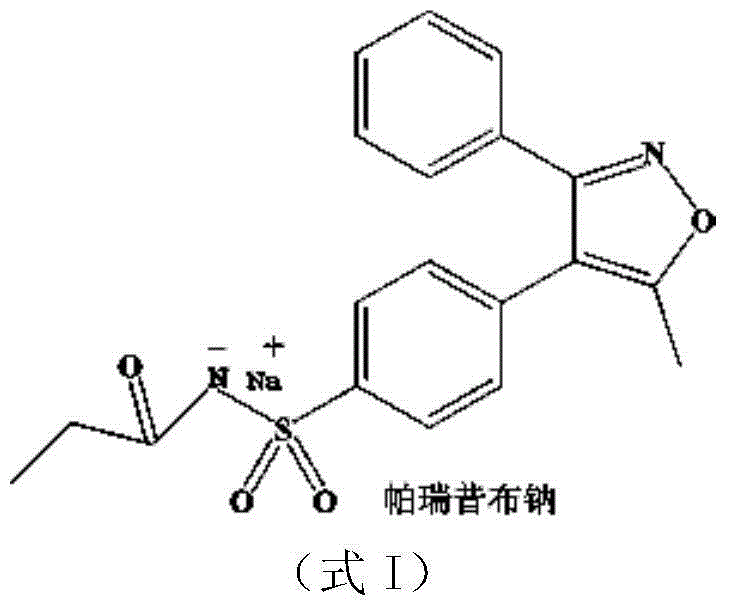
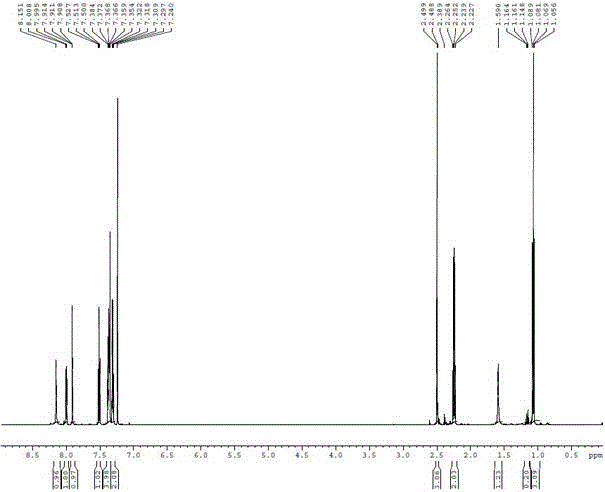
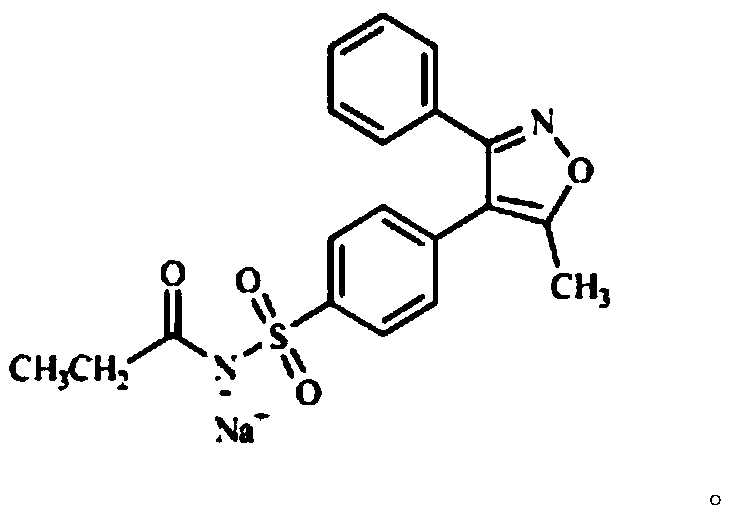
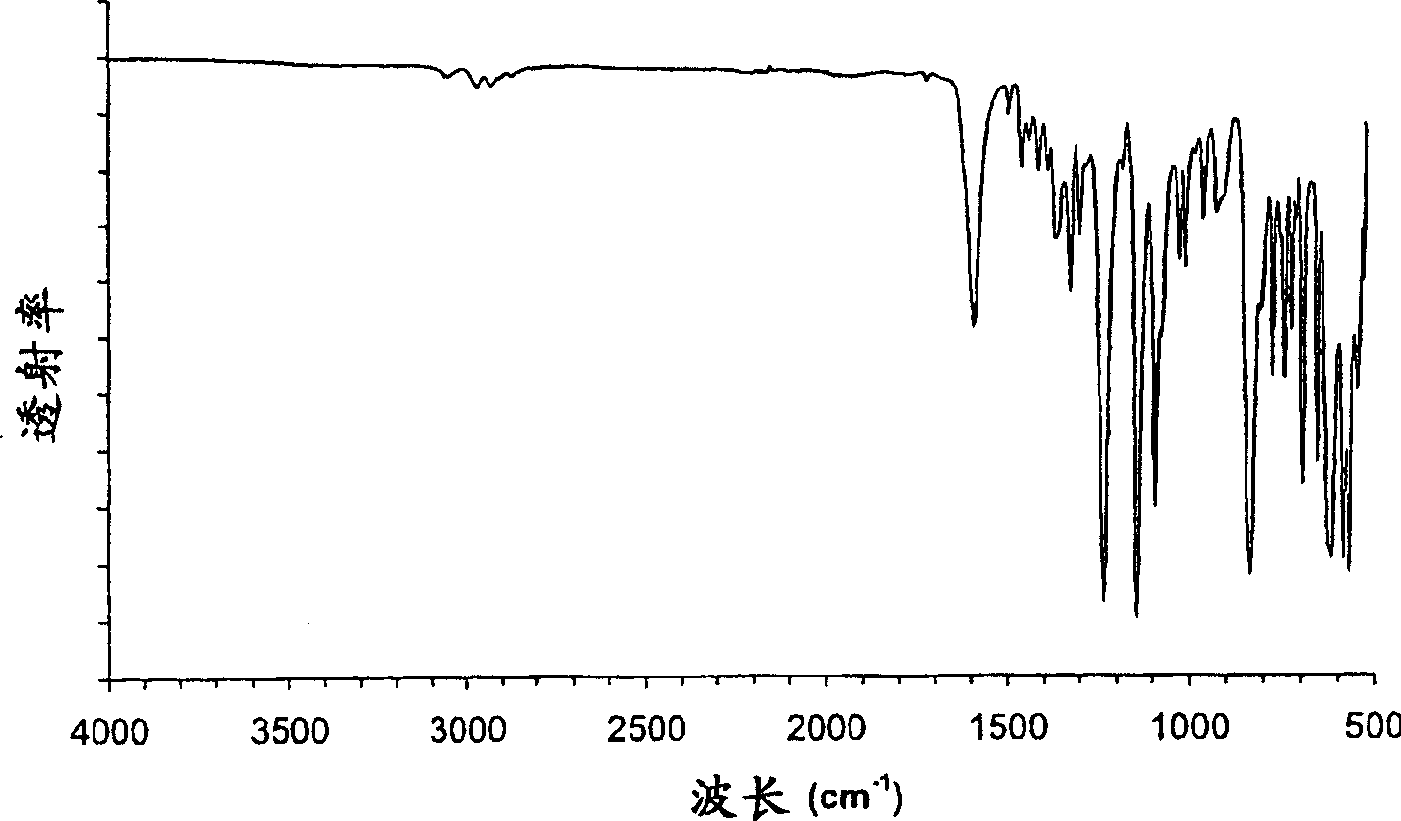
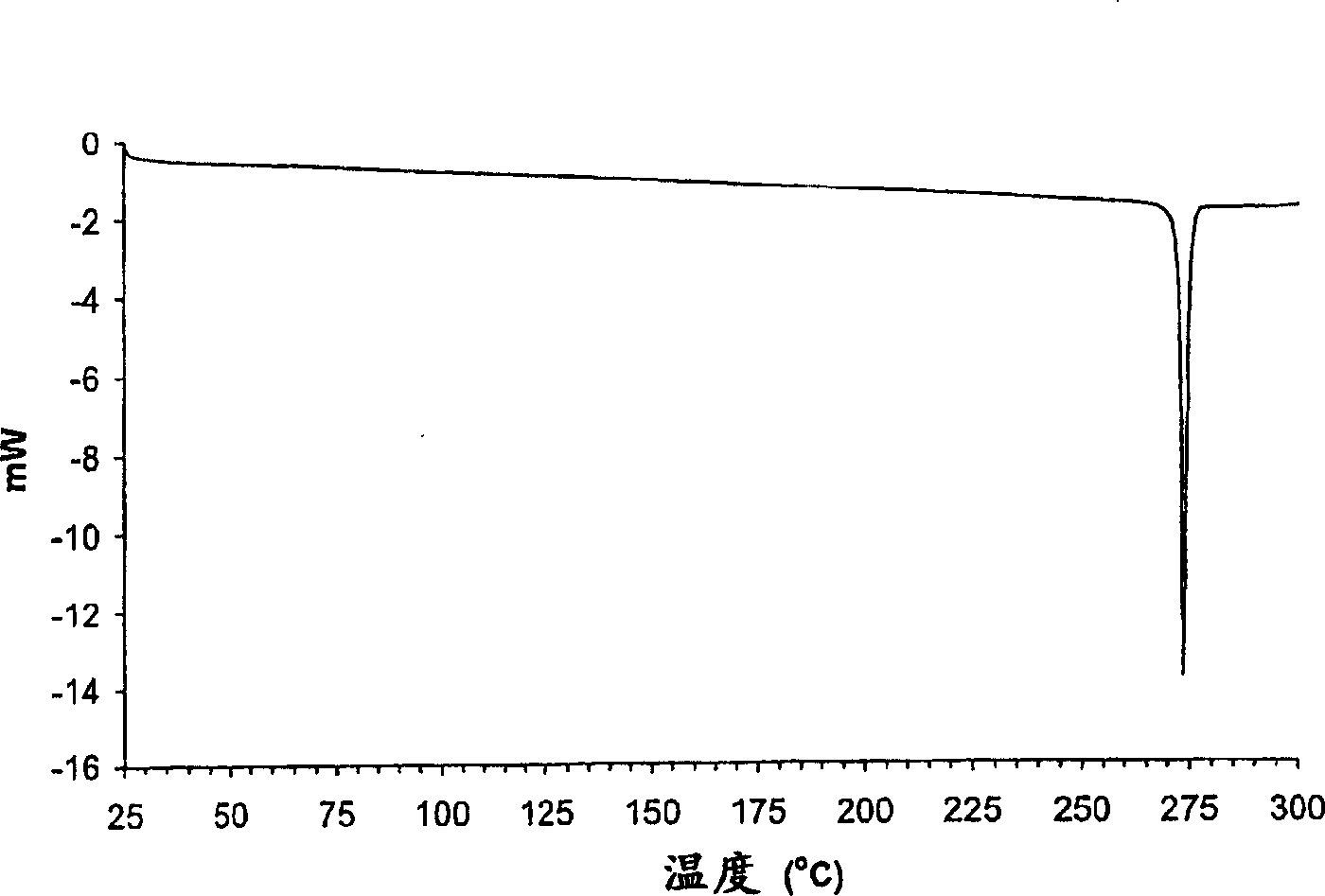
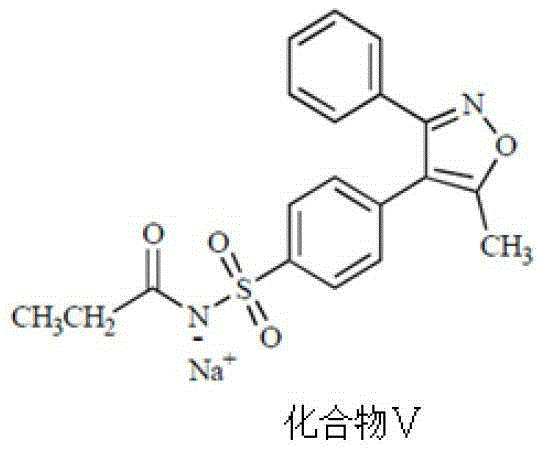
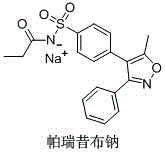
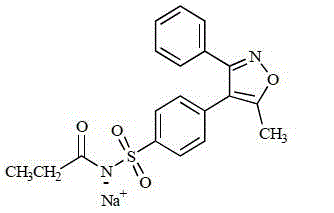
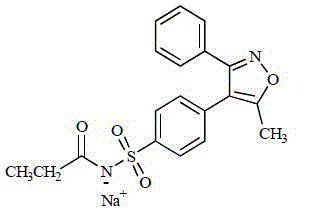
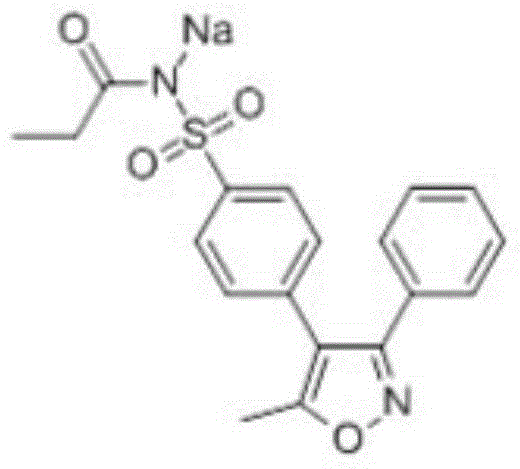
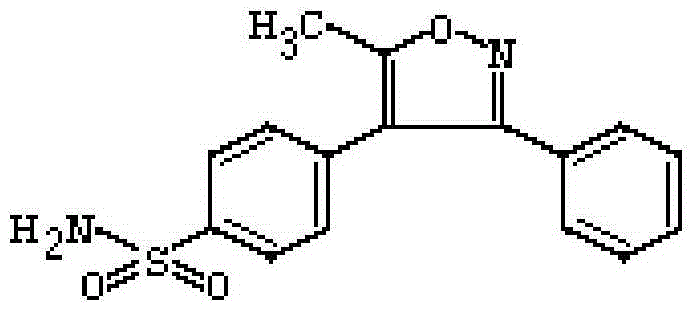
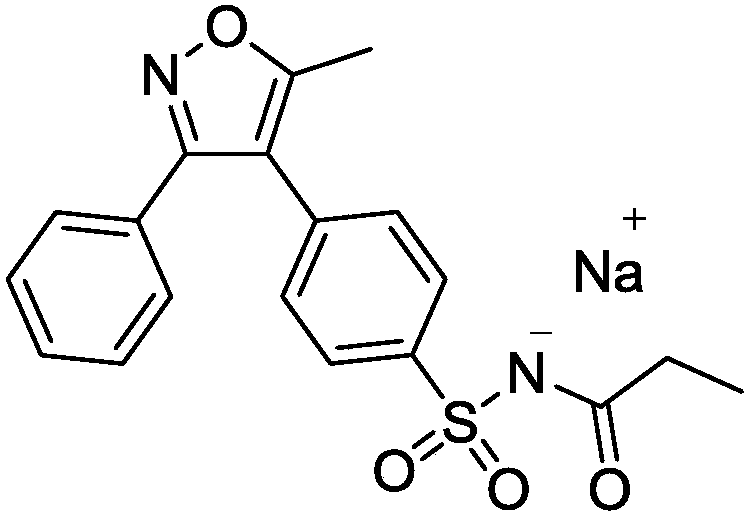
Parecoxib Sodium is a water-soluble, injectable sodium salt form of parecoxib, an amide prodrug of the cyclooxygenase II (COX-2) selective, non-steroidal anti-inflammatory drug (NSAID) valdecoxib, with anti-inflammatory, analgesic, and antipyretic activities.Upon intravenous or intramuscular administration, parecoxib is hydrolyzed by hepatic carboxyesterases to its active form, valdecoxib.
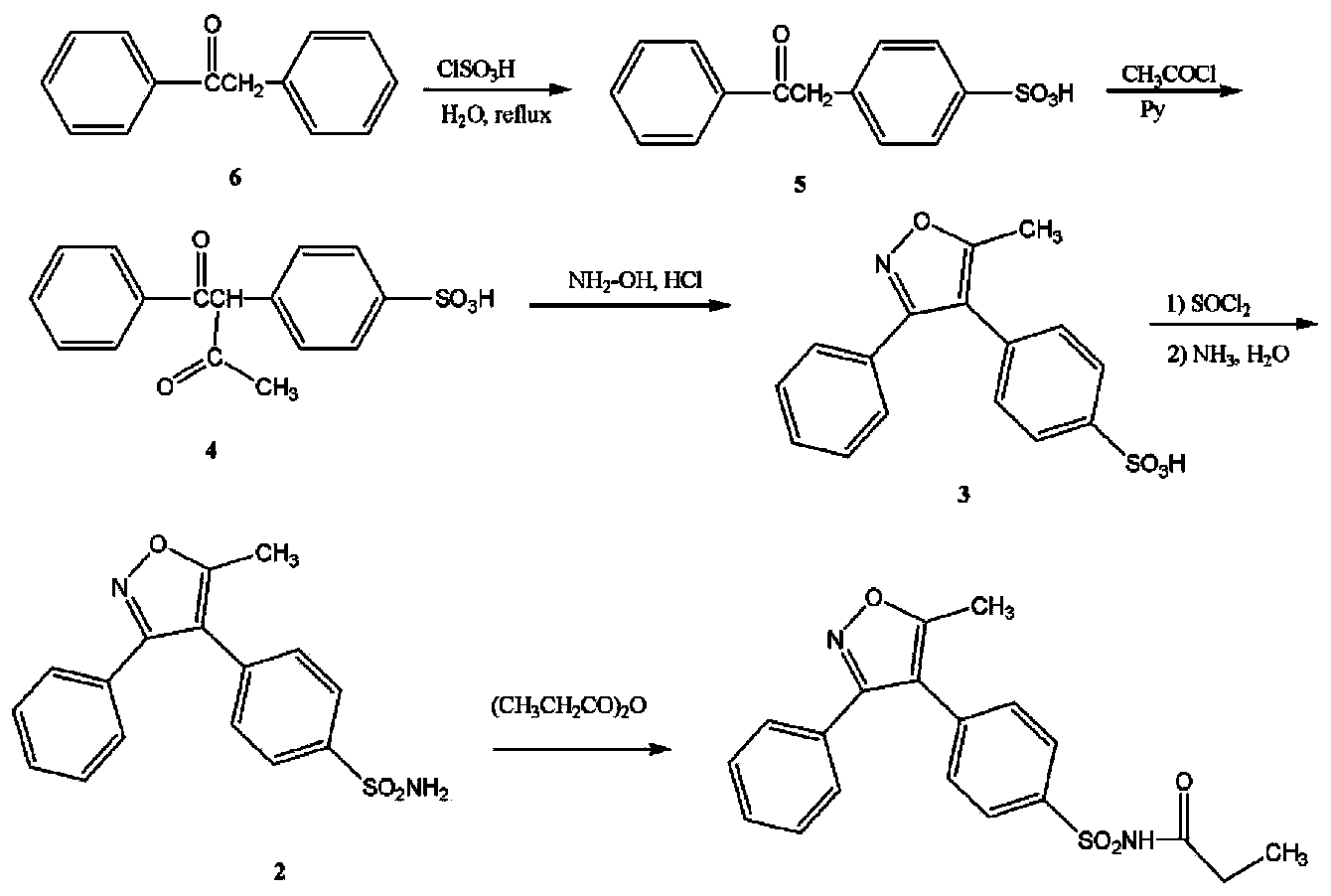
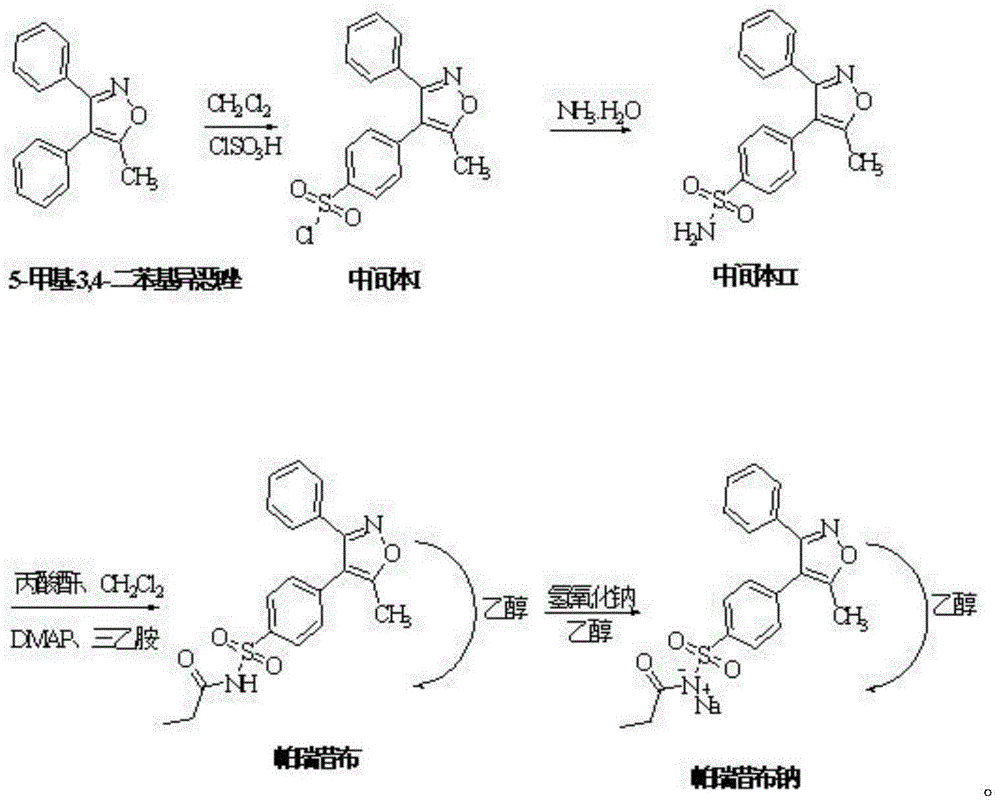
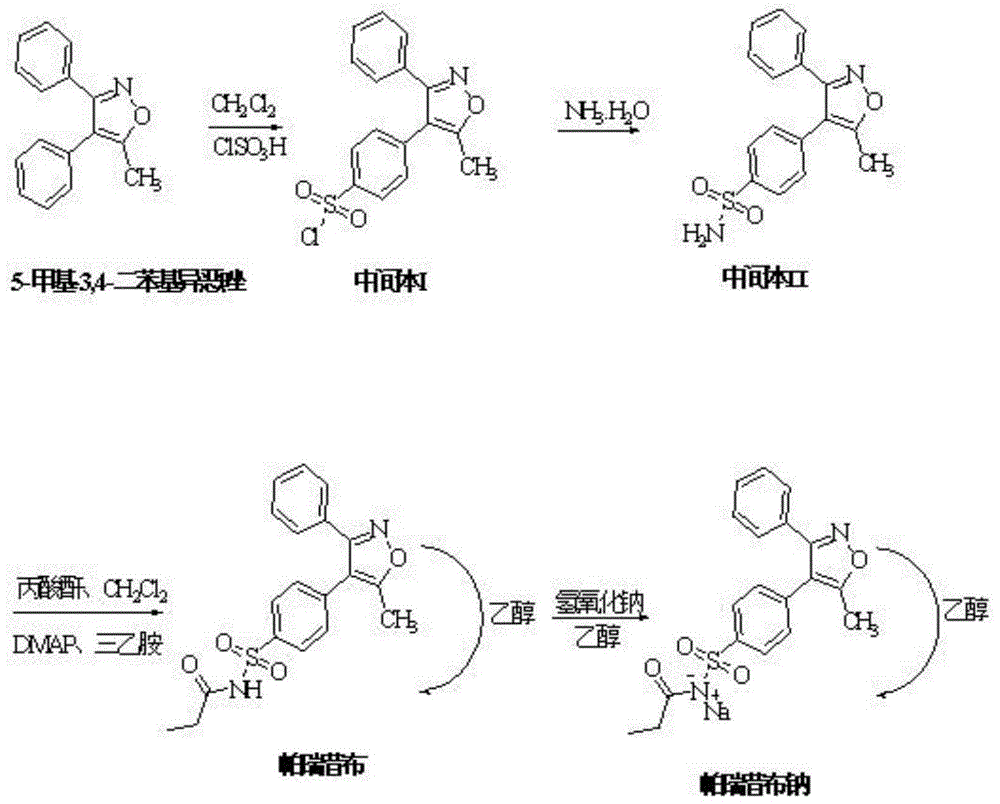
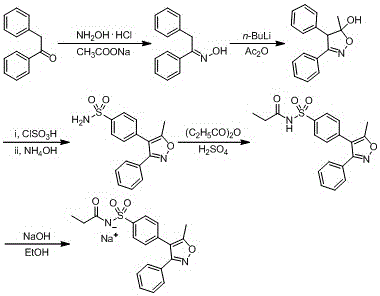
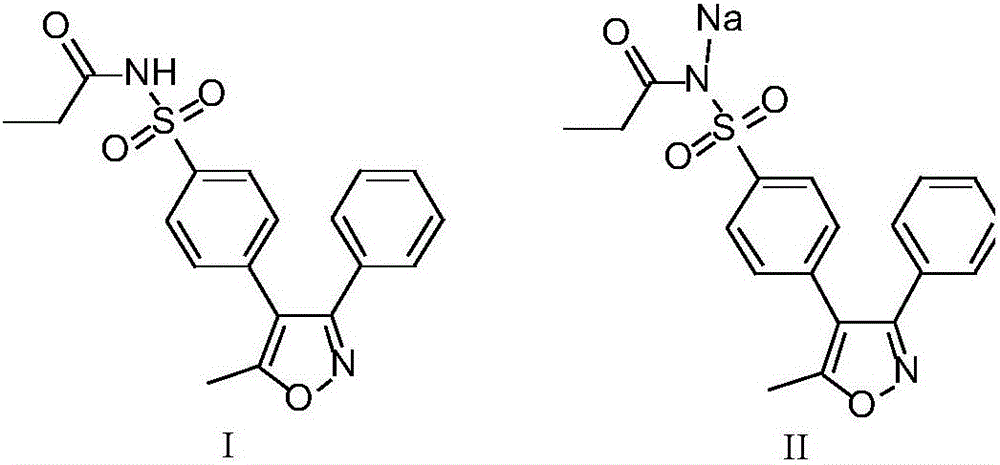
Preparation method of parecoxib sodium
InactiveCN104250232ASimple methodEasy to operateOrganic chemistryAntipyreticParecoxib sodiumImpurity
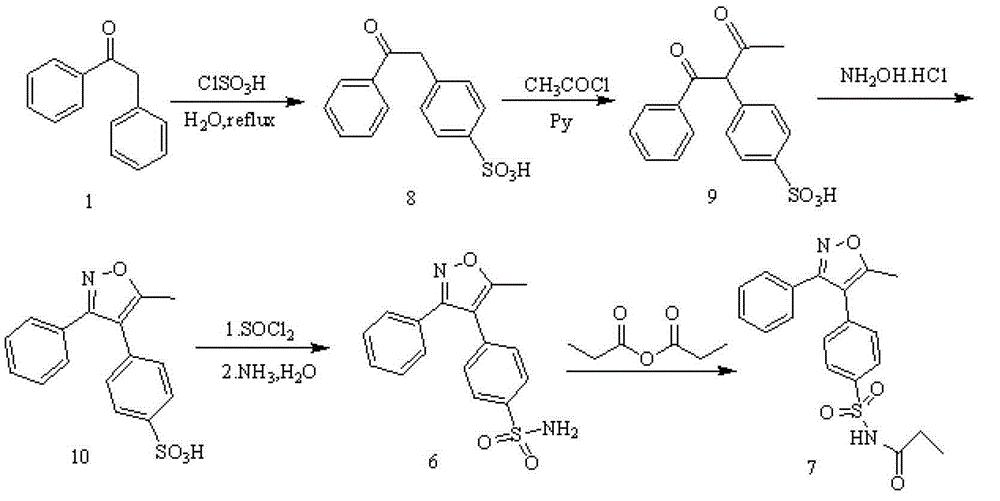
The invention discloses a preparation method of parecoxib sodium. The method comprises the following steps: sulfonating 3,4-diphenyl-5-methylisoxazole as a raw material, ammonolyzing, acylating, salifying, and re-crystallizing to obtain parecoxib sodium. The method has the advantages of simple operation, good repeatability, high yield and low cost, and is suitable for industrialized production, and the parecoxib sodium finished product obtained in the invention has a content of a separate impurity of below 0.1%, and is suitable for preparing medicinal parecoxib sodium preparations.
Owner:SICHUAN WEITUO BIOLOGICAL PHARMA
Detection method of parecoxib sodium genotoxicity impurity and application thereof
ActiveCN105372376AAchieve separationHigh sensitivityComponent separationPhosphateReversed-Phase Liquid Chromatography
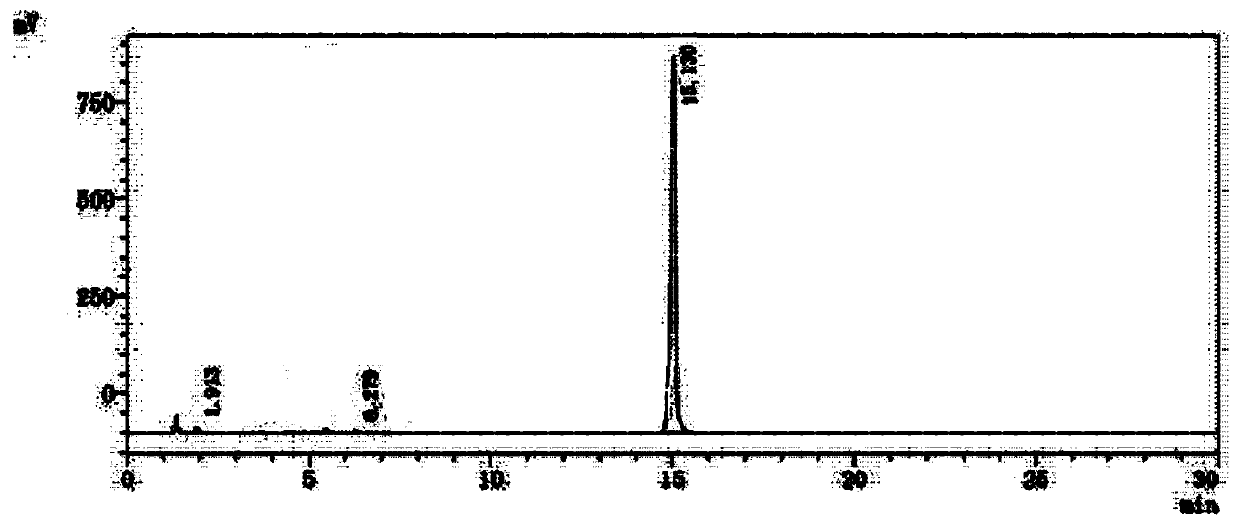
The present invention provides a detection method of parecoxib sodium genotoxicity impurity and application thereof. The method uses a reversed-phase liquid chromatography method. The chromatography conditions are as follows: the chromatographic column comprise a C18 column, a C8 column, a phenyl column and a Hilic column; a mobile phase consists of water-acetonitrile, dilute phosphoric acid-acetonitrile, and phosphate-acetonitrile; a flow phase comprises an aqueous phase and an organic phase in the ratio of 90:10-10:90; the column temperature is 25-40 DEG C; the flow rate of the mobile phase is 0.2-2ml / min; detection wavelength is 205-290nm; a detector is a UV detector or a photodiode array (PDA) detector; and the sample size is 0.1-40 mul. The detection method of parecoxib sodium genotoxicity impurity achieves the separation of parecoxib sodium and three genotoxicity impurities in a short period of time, has high sensitivity and specificity, and simple operation, reaches the separation rate of the main component and genotoxicity impurities, and the separation rate of genotoxicity impurities both greater than 1.5; and the method can be used for quality control of parecoxib sodium, and has practical value.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Preparation method of parecoxib sodium compound as well as intermediate impurity and application of parecoxib sodium compound
ActiveCN104447600AImprove quality controllabilityOrganic chemistryComponent separationAlcoholParecoxib sodium
The invention provides parecoxib sodium which is prepared by controlling an intermediate impurity and in particular provides a preparation method of a parecoxib sodium compound as well as the intermediate impurity and an application of the parecoxib sodium compound. According to the preparation method provided by the invention, 3-methyl-4,5-diphenyl-4,5-dihydro-isoxazole-5-alcohol is used as an isomer impurity for preparing 5-methyl-3,4-diphenyl-4,5-dihydro-isoxazole-5-alcohol as an intermediate of the parecoxib sodium, the quality of the 3-methyl-4,5-diphenyl-4,5-dihydro-isoxazole-5-alcohol is controlled in the preparation of the parecoxib sodium, specifically, the impurity content is required not to be higher than 0.5 percent, and an important significance is provided for the product quality of the parecoxib sodium; by obtaining the 3-methyl-4,5-diphenyl-4,5-dihydro-isoxazole-5-alcohol as an isomer impurity of the important 5-methyl-3,4-diphenyl-4,5-dihydro-isoxazole-5-alcohol and further studying 3-methyl-4,5-diphenyl-4,5-dihydro-isoxazole-5-alcohol in the aspects of preparation process, detection process and purification process, important quality monitoring significance is provided for the process with the 5-methyl-3,4-diphenyl-4,5-dihydro-isoxazole-5-alcohol as an industrial production raw material.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Reconstitutable parenteral composition
InactiveUS7695736B2Equal analgesic effectEqual anti-inflammatory effectPowder deliveryBiocideParecoxib sodiumAdditive ingredient
A pharmaceutical composition comprises, in powder form, (a) at least one water-soluble therapeutic agent selected from selective COX-2 inhibitory drugs and prodrugs and salts thereof, for example parecoxib sodium, in a therapeutically effective total amount constituting about 30% to about 90% by weight, (b) a parenterally acceptable buffering agent in an amount of about 5% to about 60% by weight, and optionally (c) other parenterally acceptable excipient ingredients in a total amount not greater than about 10% by weight, of the composition. The composition is reconstitutable in a parenterally acceptable solvent liquid to form an injectable solution. A lyophilization process is provided for preparation of such a composition.
Owner:PHARMACIA CORP
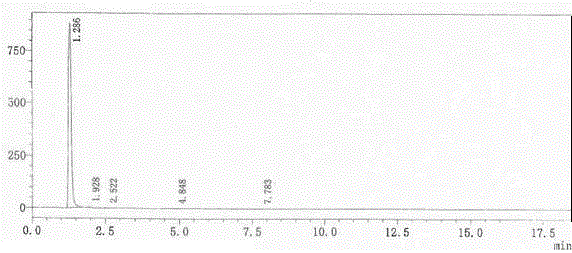
Liquid chromatographic analysis method for parecoxib-sodium related substances
ActiveCN104749288AEffective and accurate quantificationEffective and accurate quantitative detectionComponent separationParecoxib sodiumTest article
The invention provides a liquid chromatographic analysis method for parecoxib-sodium related substances. The liquid chromatographic analysis method comprises the following contents: 1. system-applicability experiment: (1) respectively taking contrast products of all impurities and parecoxib sodium, and diluting, wherein the obtained diluent is taken as system-applicability solution; (2) setting a liquid chromatograph, injecting the system-applicability solution into the liquid chromatograph, and recording a chromatogram till the separation degree among all peaks conforms to the specification; 2. determination experiment: (1) taking the parecoxib sodium to be tested and placing in a container, adding a solvent to dissolve and dilute, wherein the obtained diluent is taken as a test-article solution; (2) taking the test-article solution, and diluting, wherein the obtained diluent is taken as a contrast solution; (3) respectively taking and injecting the test-article solution and the contrast solution into the liquid chromatograph, and recording a chromatogram; and (4) calculating by a self-contrast method according to the peaks in the chromatogram of the test-article solution. The liquid chromatographic analysis method provided by the invention has the advantages that the trace potential impurities in the parecoxib sodium can be accurately detected in a high-efficiency and low-cost manner, and good quality guarantee can be provided; and the liquid chromatographic analysis method also can be used for detecting and analyzing other substances.
Owner:SHANGHAI CHENPON PHARM TECH CO LTD
Synthesis method for parecoxib sodium impurity
InactiveCN104557754APrecise positioningQualitative highOrganic chemistryParecoxib sodiumSynthesis methods
The invention disclose a synthesis method for a parecoxib sodium impurity namely N-[[4-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonyl] acetamide and belongs to the technical field of chemical pharmacy. According to the synthesis method, 5-methyl-3,4-diphenyl isoxazole is used as raw material, sulfonation reaction, aminating reaction and propionylation reaction are performed so as to obtain the parecoxib sodium impurity, and the synthesized high-purity parecoxib sodium impurity can be used as a standard impurity for detection analysis of a parecoxib sodium finished product, so that accurate positioning and quantification of the parecoxib sodium finished product detection analysis to the impurity are improved, control over the impurity is strengthened, and the quality of the parecoxib sodium finished product is improved. The method provided by the invention has the advantages that the raw material is cheap and easy to obtain, the operation is simple, the product yield is 65%+ / -5%, and the HPLC purity is larger than or equal to 98%.
Owner:CHENGDU CLIMB PHARMA TECH
Synthesis method for parecoxib sodium impurity
InactiveCN104557755APrecise positioningQualitative highOrganic chemistryParecoxib sodiumSynthesis methods
The invention disclose a synthesis method for a parecoxib sodium impurity namely 4-N-propionyl[5-methyl-3(3-N-propionyl phenyl sulfonamide)-1,2-oxazole-4-group] benzenesulfonamide and belongs to the technical field of chemical pharmacy. According to the synthesis method, 5-methyl-3,4-diphenyl isoxazole is used as raw material, sulfonation reaction, aminating reaction and propionylation reaction are performed so as to obtain the parecoxib sodium impurity, and the synthesized high-purity parecoxib sodium impurity can be used as a standard impurity for detection analysis of a parecoxib sodium finished product, so that accurate positioning and quantification of the parecoxib sodium finished product detection analysis to the impurity are improved, control over the impurity is strengthened, and the quality of the parecoxib sodium finished product is improved. The method provided by the invention has the advantages that the raw material is cheap and easy to obtain, the operation is simple, the product yield is 85%+ / -5%, and the HPLC purity is larger than or equal to 98%.
Owner:CHENGDU CLIMB PHARMA TECH
High performance liquid chromatography detection method for parecoxib sodium isomer
ActiveCN104965041AAccurate separation detectionAnalytical detection method is fast and simpleComponent separationSilanesSilica gel
The invention discloses a high performance liquid chromatography detection method for a parecoxib sodium isomer. The method uses a chromatographic column with silane bonded silica gel as the filler, and adopts n-hexane-isopropanol as the mobile phase to conduct separation and detection on parecoxib sodium, the volume ratio of the mobile phase n-hexane-isopropanol is 70-95:5-30, and the chromatographic conditions include: a mobile phase flow rate of 0.6-1.0ml / min, a chromatographic column temperature of 30DEG-40DEG C, and a detection wavelength of 215nm. The method specifically includes the steps of: S1. preparation of a test solution; S2. preparation of a reference solution; and S3. detection. The detection method provided by the invention has the advantages of simplicity and rapidity, accurate quantification and good repeatability, can accurately separate and detect a parecoxib sodium structure isomer, and plays a good promoting role in research and synthesis, and production quality control of parecoxib sodium.
Owner:CHENGDU CLIMB PHARMA TECH
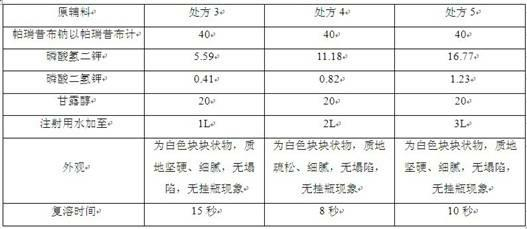
Parecoxib sodium freeze-dried powder injection and preparation method thereof
ActiveCN104771370AGood effectLow conversion levelPowder deliveryAntipyreticParecoxib sodiumFreeze-drying
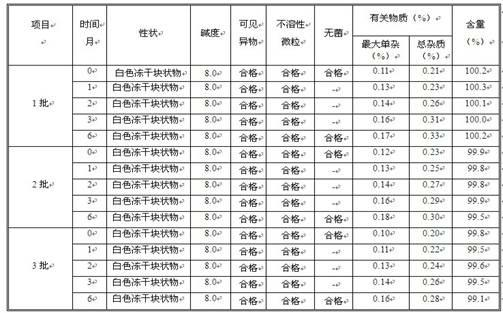
The invention belongs to the field of pharmaceutical preparation, and in particular, relates to a parecoxib sodium freeze-dried powder injection containing sodium chloride and a preparation method thereof. The parecoxib sodium freeze-dried powder injection for injection has the advantages of uniform color and luster, smooth surface, no dusting and wall sticking phenomena, no collapse and no crimping, has the most significant effects in good stability, re-dissolution after being placed for a long period of time and quite little valdecoxib conversion amount, and ensures the product injection safety.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
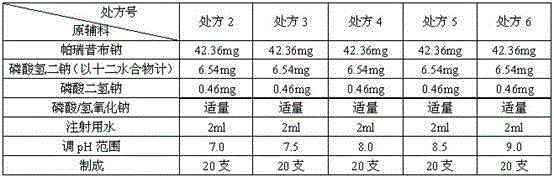
Parecoxib sodium pharmaceutical composition for injection
InactiveCN102512383ASolve the problem of unstable pH value when exposed to lightHigh yieldPowder deliveryAntipyreticDrug utilisationParecoxib sodium
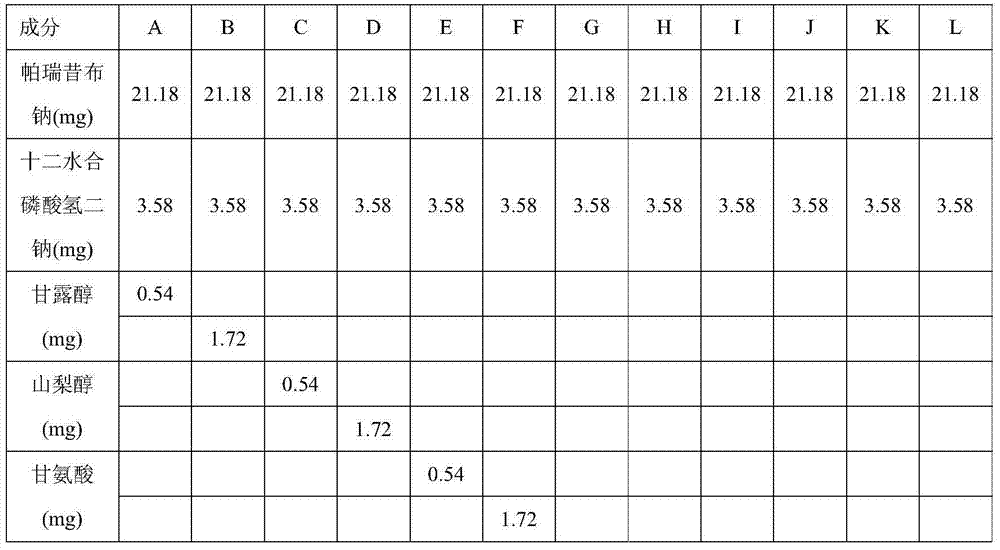
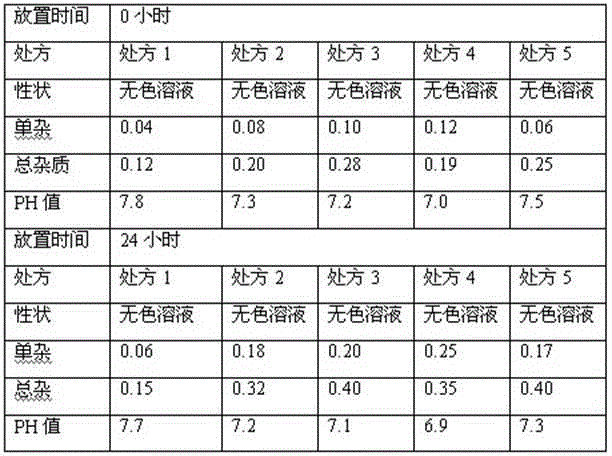
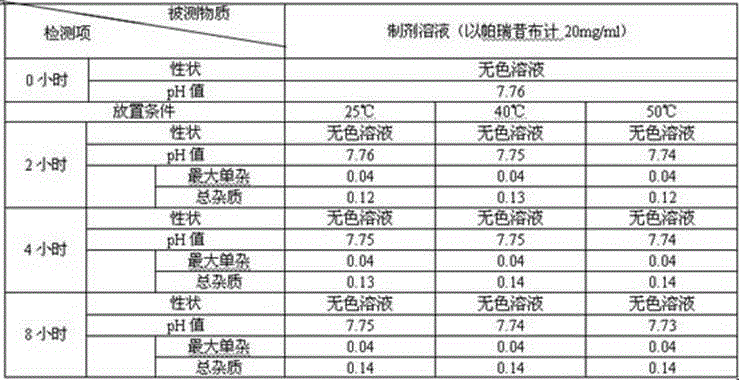
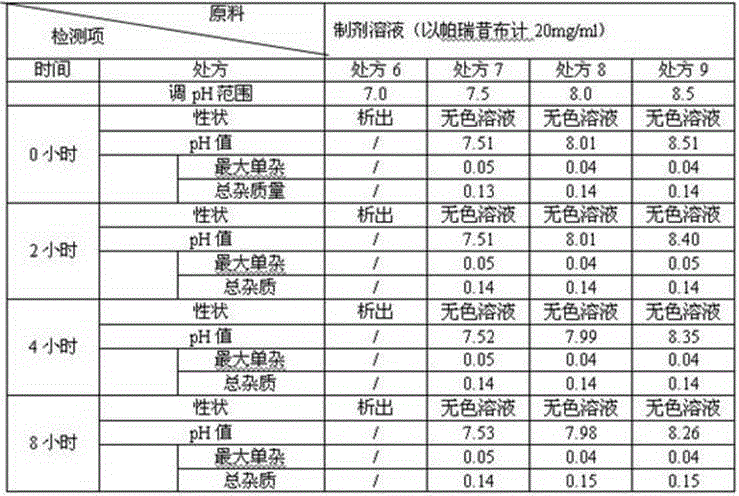
The invention relates to a stable parecoxib sodium pharmaceutical composition for injection. The pharmaceutical composition specifically comprises parecoxib sodium and additive for injection, wherein the additive at least contains a phosphatic buffer with a pH of 7.8-8.0, and other additive is selected from mannitol and sodium hydroxide. The parecoxib sodium pharmaceutical composition for injection provided by the invention is filled in a colorless neutral borosilicate glass tube injection bottle, has good stability, stable pH and little stimulation on skin, and is convenient for transportation and storage. The parecoxib sodium pharmaceutical composition for injection provides reasonable preparation prescription and preparation technology for clinical medicament usage and is easy for realization of industrialization.
Owner:TIANJIN SONGRUI MEDICAL TECH
Reconstitutable parenteral composition containing COX-2 inhibitor
A pharmaceutical composition comprises, in powder form, (a) at least one water-soluble therapeutic agent selected from selective COX-2 inhibitory drugs and prodrugs and salts thereof, for example parecoxib sodium, in a therapeutically effective total amount constituting about 30% to about 90% by weight, (b) a parenterally acceptable buffering agent in an amount of about 5% to about 60% by weight, and optionally (c) other parenterally acceptable excipient ingredients in a total amount not greater than about 10% by weight, of the composition. The composition is reconstitutable in a parenterally acceptable solvent liquid to form an injectable solution. A lyophilization process is provided for preparation of such a composition.
Owner:PHARMACIA CORP
Synthesis method of parecoxib sodium
InactiveCN104592141AReduce pollutionThe synthetic route is simpleOrganic chemistryParecoxib sodiumSynthesis methods
The invention discloses a synthesis method of parecoxib sodium, and belongs to the technical field of medicines. The target product, namely parecoxib sodium, is synthesized from 5-methyl-3,4-diphenyl isoxazole which is taken as an initial raw material through sulfonation, amination, propionylation and salt forming reaction. The invention has the following beneficial effects: the method disclosed by the invention has the advantages of simple synthesis route, mild reaction conditions, convenient operation, low cost, low environmental pollution and high yield, and is applicable to large-scale factory production.
Owner:CHENGDU CLIMB PHARMA TECH
Parecoxib sodium pharmaceutical composition for injection
InactiveCN104414966AHigh yieldReduce market riskNervous disorderAntipyreticUse medicationDisodium phosphate
The invention relates to a stable parecoxib sodium pharmaceutical composition for injection. The pharmaceutical composition specifically comprises parecoxib sodium and an additive for injection, wherein disodium hydrogen phosphate and sodium dihydrogen phosphate are used as buffer agents, and phosphoric acid / sodium hydroxide is used as a pH regulator. The medicine composition is feasible in production process, stable and controllable in quality, low in irritation on the skin, convenient to transport and store and capable of providing reasonable preparation prescription and preparation process for clinical medication, and easily realizes industrialization.
Owner:TIANJIN HANRUI PHARMA
Synthetic method of parecoxib sodium impurity
ActiveCN104557756APrecise positioningQualitative highOrganic chemistryParecoxib sodiumStandard samples
The invention discloses a synthetic method of a parecoxib sodium impurity, namely N-[[3-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonyl]propanamide, belonging to the technical field of chemical pharmacy; the parecoxib sodium impurity is obtained by carrying out sulfonation reaction, amination reaction and propionylation reaction by taking 5-methyl-3,4-diphenyl isoxazole as the raw material; the synthesized high-purity parecoxib sodium impurity can be used as an impurity standard sample in detection and analysis of parecoxib sodium finished products; therefore, the accurate positioning property and the qualitative diagnosis to impurities in detection and analysis of parecoxib sodium finished products are increased; control of the impurity is strengthened easily, so that the quality of the parecoxib sodium finished products is increased; the synthetic method disclosed by the invention is cheap and available in raw materials and simple to operate; the yield of the prepared parecoxib sodium impurity is 85+ / -5%; and the HPLC (High Performance Liquid Chromatography) purity is more than or equal to 98%.
Owner:CHENGDU CLIMB PHARMA TECH
Liquid chromatography method for detecting parecoxib sodium and related substances in synthesis intermediate
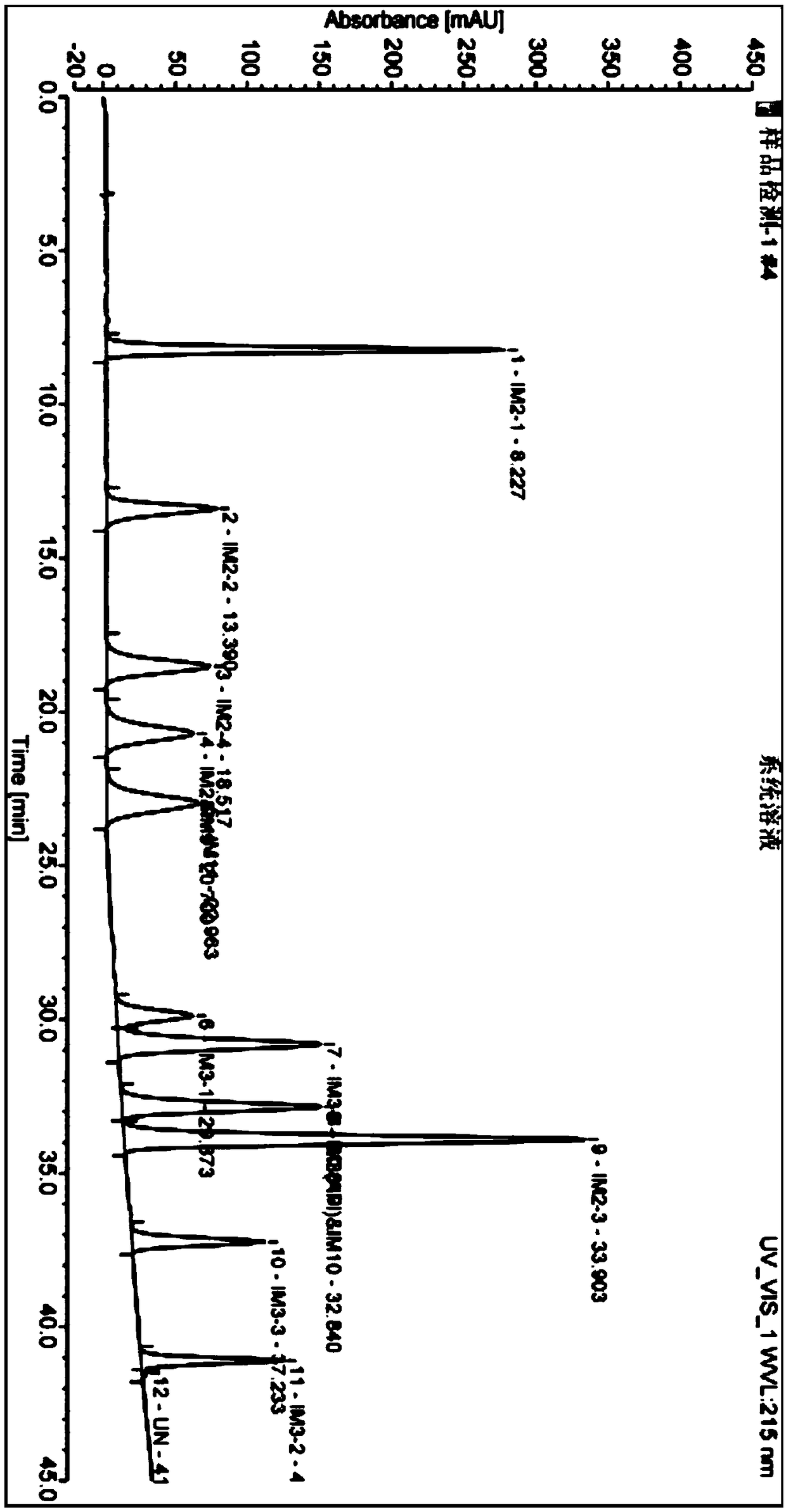
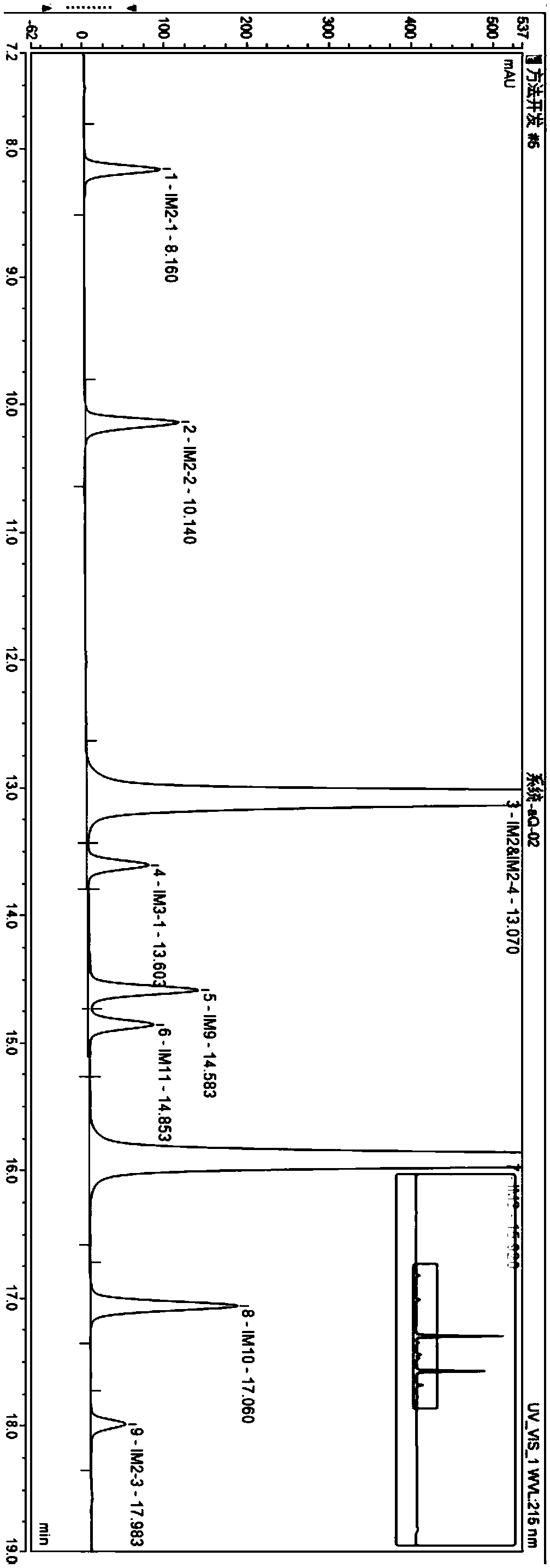
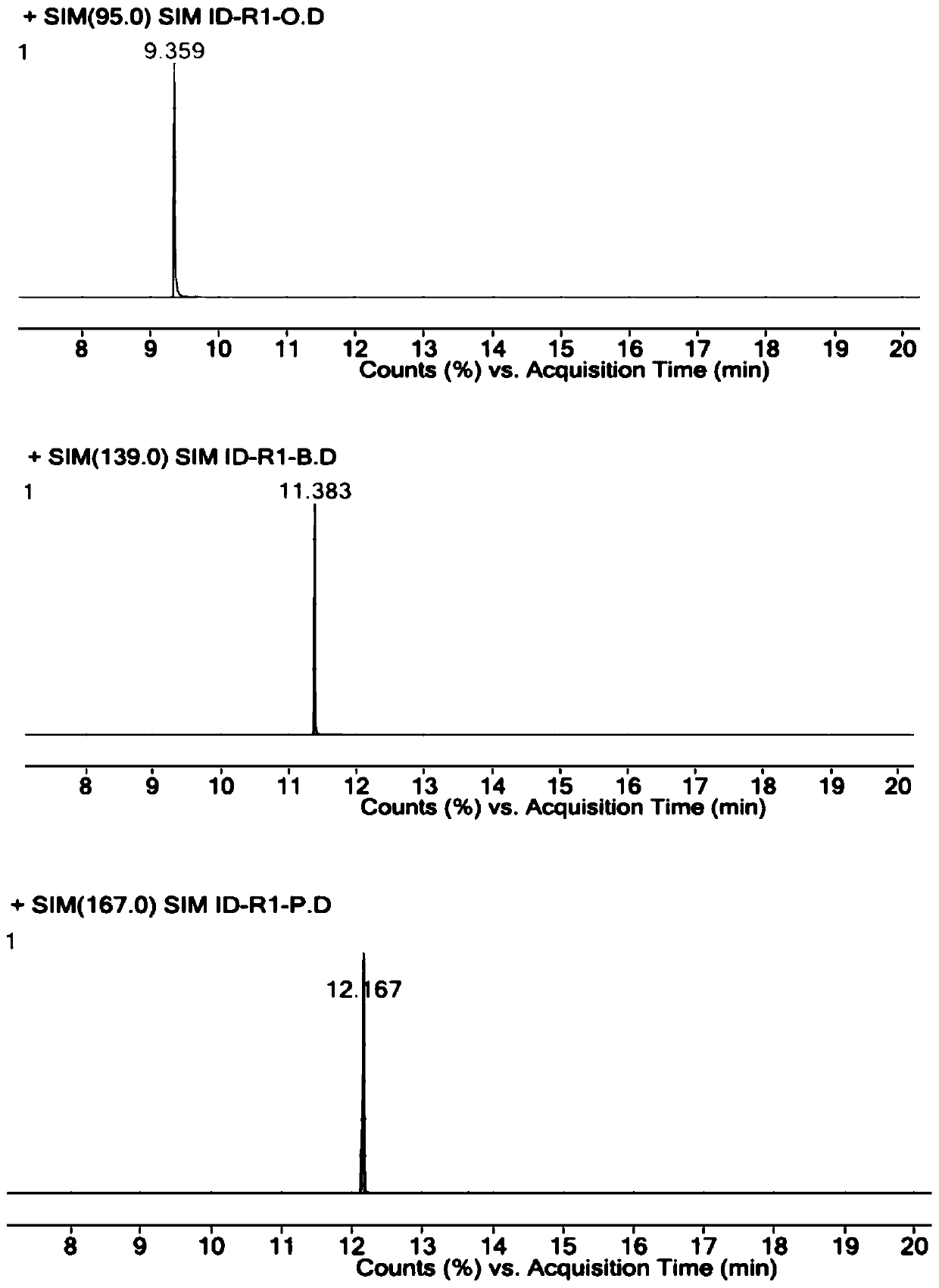
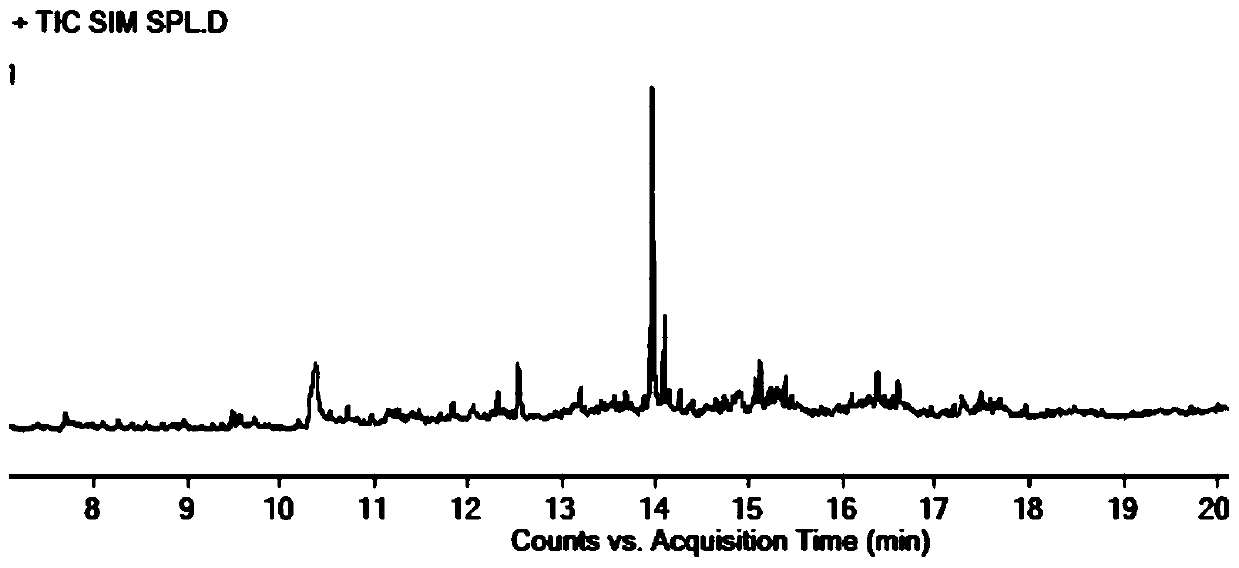
The invention discloses a liquid chromatography method for detecting parecoxib sodium and related substances in a synthesis intermediate. A reverse phase efficient liquid chromatography method is adopted, pentafluorophenyl bonded silica gel and octadecyl silane bonded silica gel are taken as chromatographic column fillers, an ultraviolet detector is adopted, a mobile phase containing potassium dihydrogen phosphate is selected to carry out elution, the related substances, i.e., first related substances and second related substances, in parecoxib sodium intermediate valdecoxib and parecoxib aremeasured in two times; the mobile phases of the first related substances and the second related substances are both a mixed solution of methyl alcohol and a monopotassium phosphate aqueous solution. Two gradient methods of the liquid chromatography method are combined to finish simultaneously detecting 11 types of parecoxib sodium and the related substances of the intermediate of the parecoxib sodium, wherein fives types of position isomer impurities which are difficult to separate are contained. The liquid chromatography method has the advantages of high sensitivity, good repeatability and high accuracy.
Owner:SHANGHAI PHARMA DONGYING JIANGSU PHARMA CO LTD
Stable parecoxib sodium pharmaceutical composition for injection
InactiveCN104434815AHigh yieldReduce market riskPowder deliveryAntipyreticParecoxib sodiumPharmaceutical drug
The invention relates to a stable parecoxib sodium pharmaceutical composition for injection. The pharmaceutical composition specifically includes parecoxib sodium and injection additives. Disodium hydrogen phosphate and sodium calcium edentate are adopted as the buffering agent, and sodium hydroxide is taken as the pH regulator. The production process is feasible, the quality is stable and controllable, the skin irritation is small, and at the same time the composition is convenient for transportation and storage. The invention provides the reasonable preparation prescription and preparation technology for clinical medication, and industrialization is easy to realize.
Owner:TIANJIN HANKANG PHARMA BIOTECH
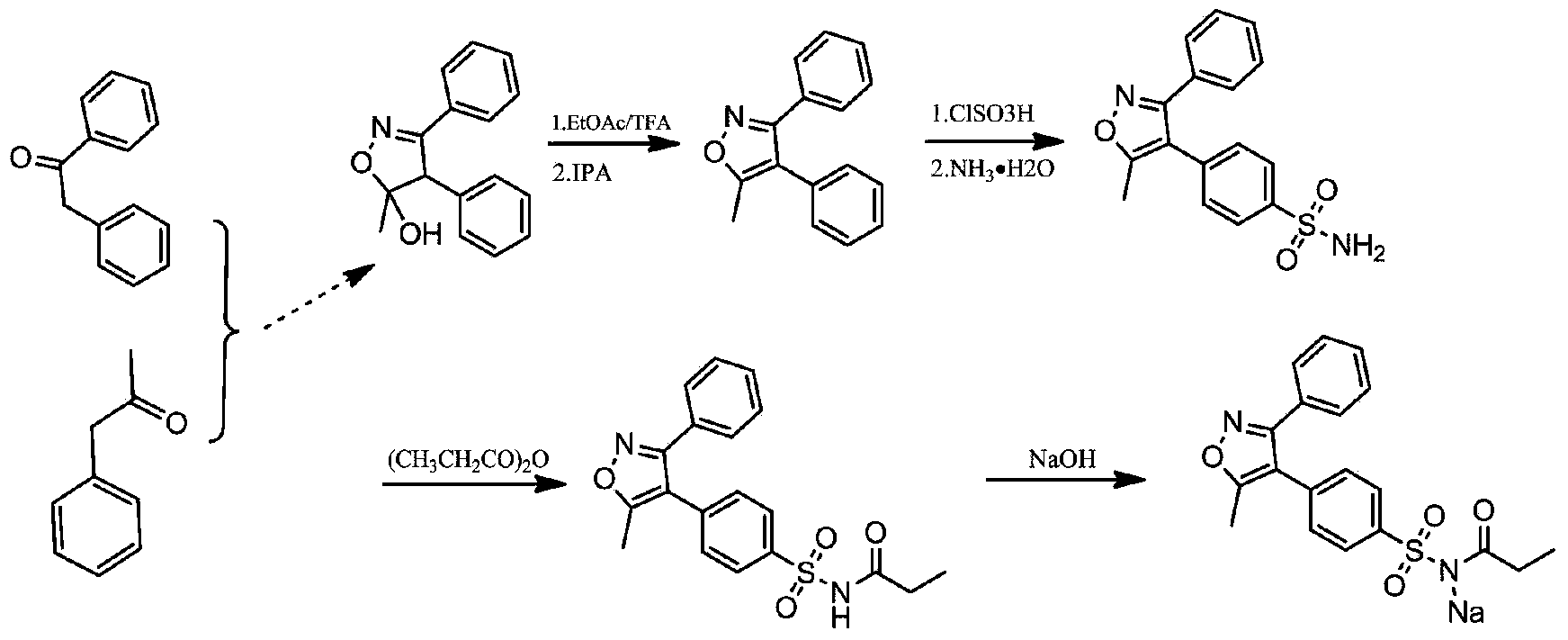
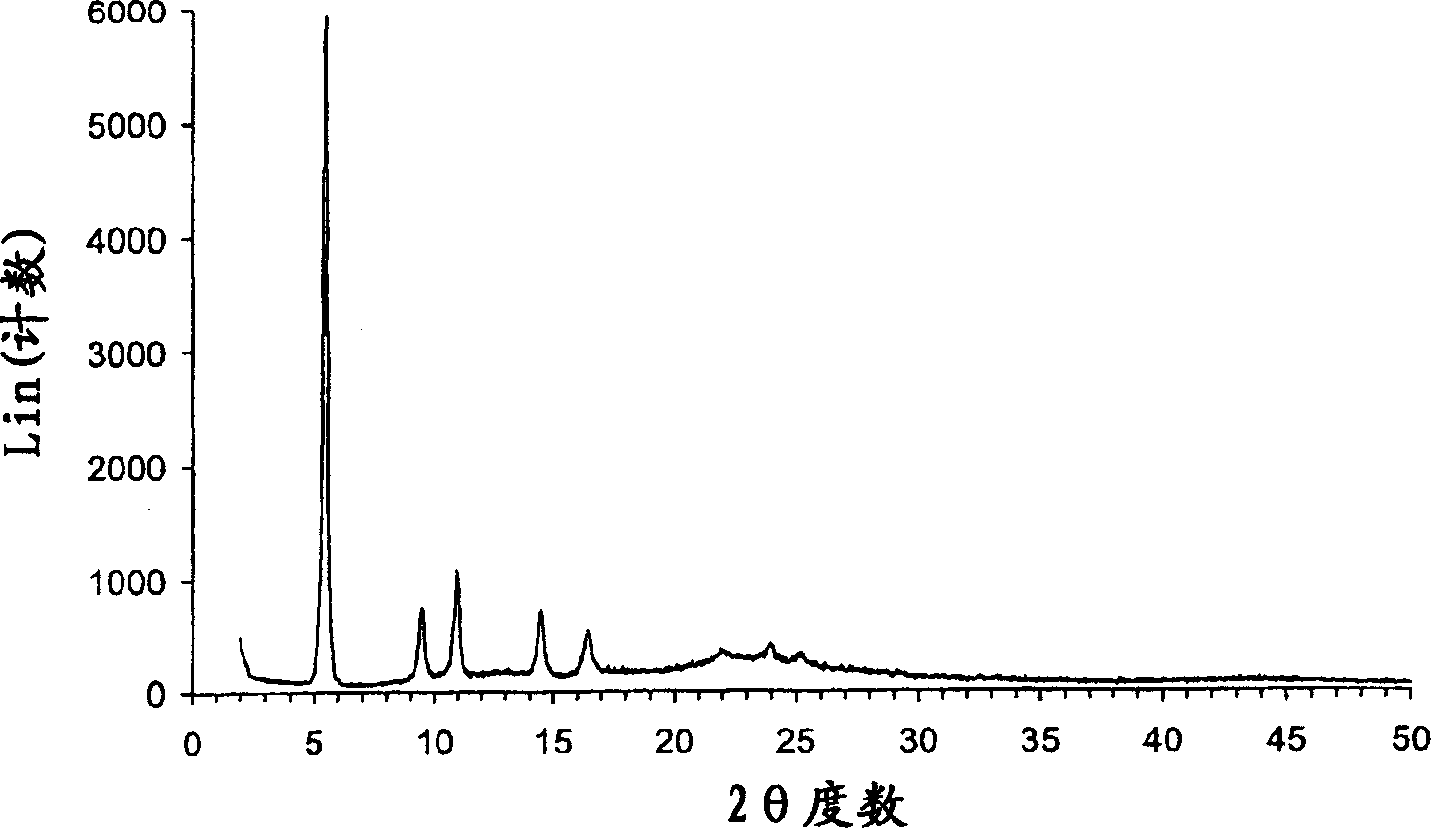
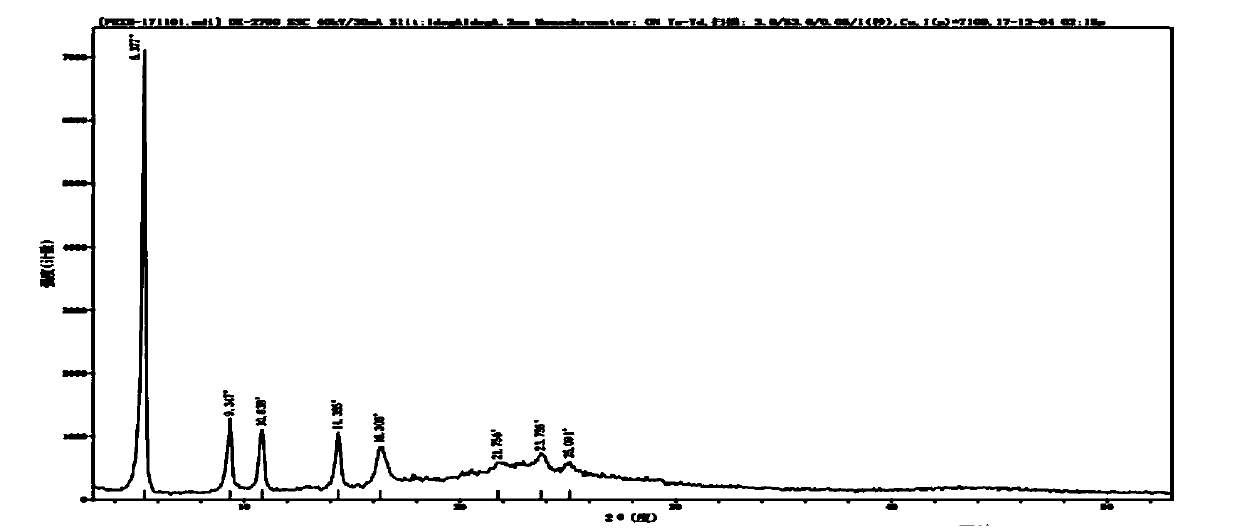
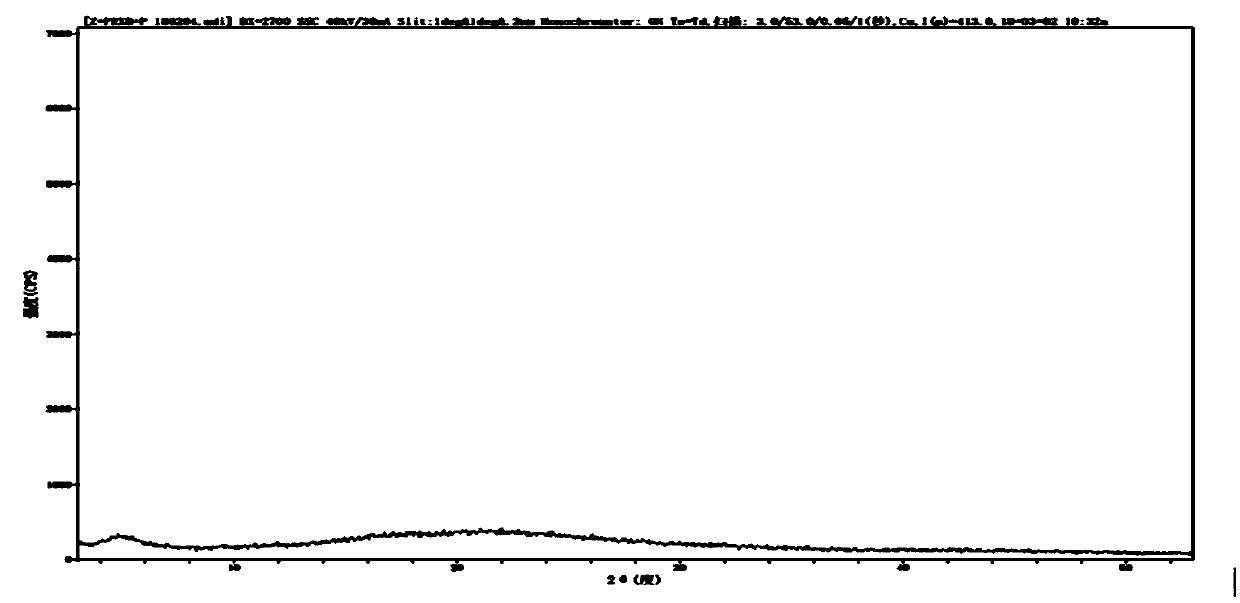
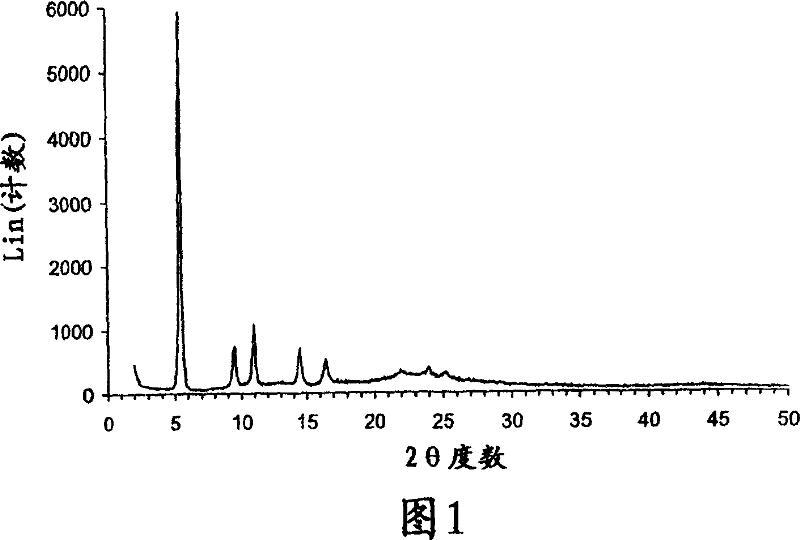
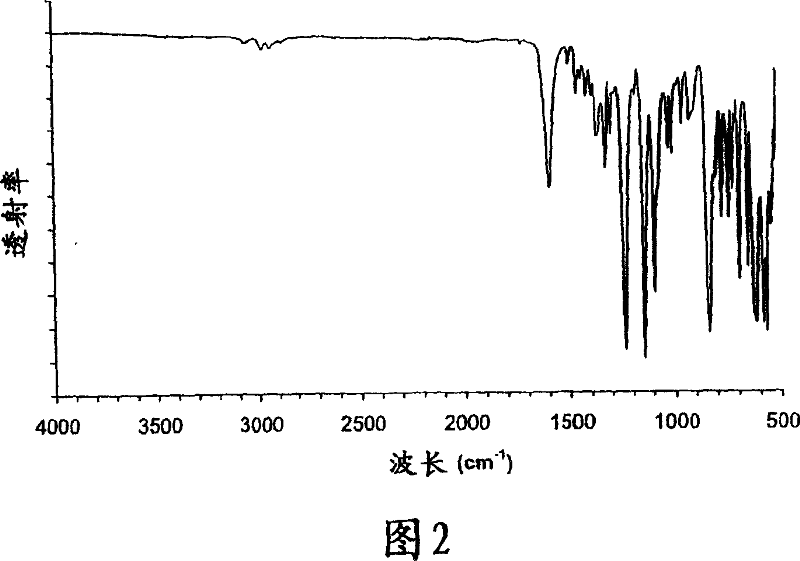
Crystalline parecoxib sodium
Parecoxib sodium is provided in crystalline form that is substantially anhydrous and substantially unsolvated. A number of such anhydrous, unsolvated crystalline forms have been identified, including Forms A, B and E described herein. Also provided is a pharmaceutical product of parecoxib sodium, wherein at least about 90% of the parecoxib sodium is in one or more anhydrous, unsolvated crystalline forms. Such drug products are storage-stable intermediates that can be further processed, for example, by dissolving or slurrying in an aqueous medium with one or more parenterally acceptable excipients, followed by lyophilization of the resulting solution or slurries to provide reconstitutable injectable compositions suitable for therapeutic use.
Owner:PHARMACIA CORP
Preparing method for parecoxib sodium
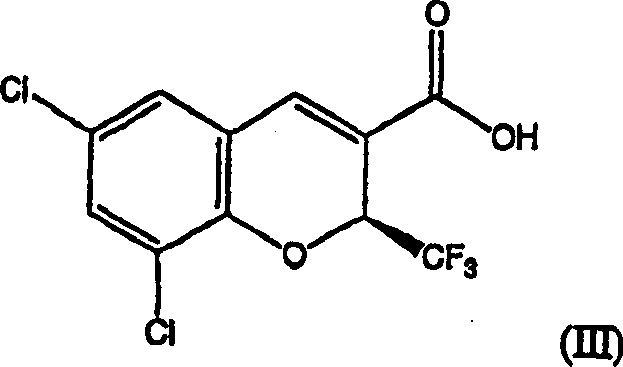
ActiveCN105418528AShort synthetic routeReaction raw materials are stable and easy to obtainOrganic chemistryChlorosulfuric acidPtru catalyst
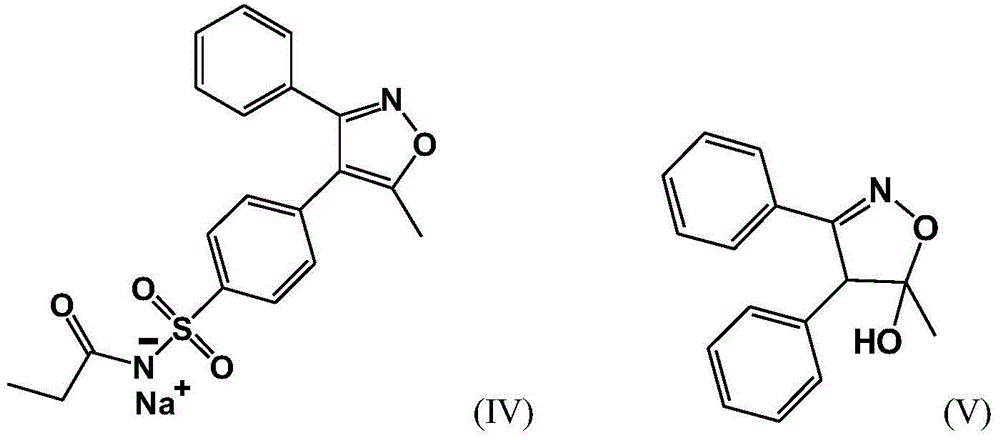
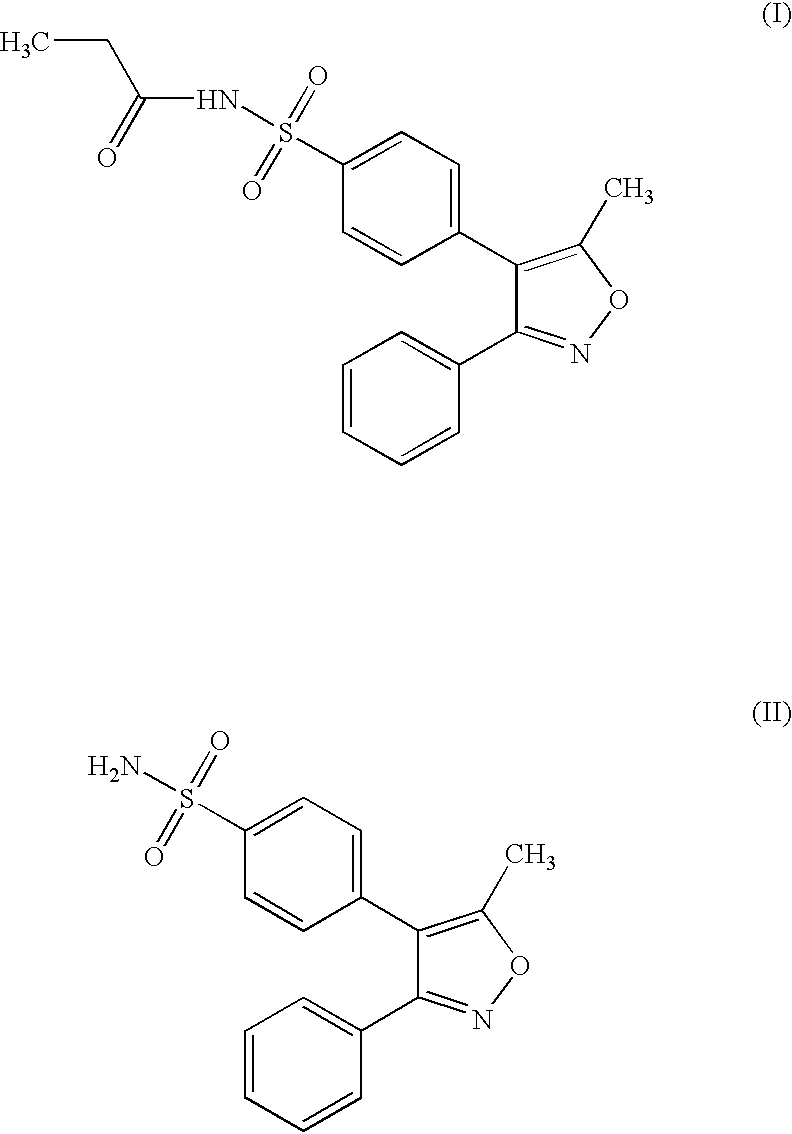
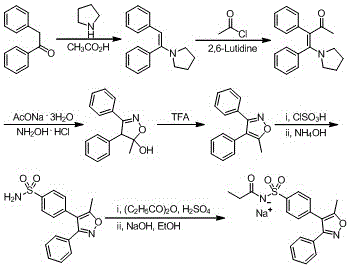
The invention belongs to the field of medicine chemical industry and particularly relates to a preparing method for parecoxib sodium. According to the method, benzaldoxime (compound I) and 1-phenyl-1-propyne (compound II) are subjected to an addition reaction under existence of a catalyst and an acid-binding agent to construct an isoxazole ring to obtain a parecoxib sodium intermediate (compound III); the compound III is subjected to a sulfonation and sulfonylation reaction to obtain a compound IV, and the compound IV and propionic anhydride react to form salt to obtain parecoxib sodium (compound V). According to the method, the dipole ring addition reaction is creatively adopted for preparing the compound III, common safe and low-toxicity reagents chlorosulfuric chlorosulfonic acid and ammonia water with relative stable nature are used to be subjected to the sulfonylation reaction, and the method has the advantages that the reaction condition is mild, operation is reasonable, selectivity is high, and product quality is high; raw and auxiliary materials in the reaction are low in price, and the production cost is reduced.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
Parecoxib sodium lyophilized preparation and preparation method thereof
The invention relates to a stable parecoxib sodium pharmaceutical composition for injection. The stable parecoxib sodium pharmaceutical composition for injection concretely comprises a main drug parecoxib sodium, a lyophilized protective agent glycine, a lyophilized additive tertiary butanol and pH regulators disodium hydrogen phosphate and phosphoric acid. The stable parecoxib sodium pharmaceutical composition for injection has the advantages that a production technology is feasible, quality is stable and controllable, skin irritation is less, multiple key parameters of time, temperature, vacuum degree, heating rate, cooling rate and the like are smartly controlled in a lyophilization process, a reasonable and effective preparation prescription and preparation technology scheme is provided for clinical medication, and industrialization can be easily realized.
Owner:NANJING HERON PHARMA SCI & TECH CO LTD
Synthesis method of parecoxib sodium impurity
InactiveCN104592142AQuality improvementPrecise positioningOrganic chemistryParecoxib sodiumSynthesis methods
The invention discloses a synthesis method of a parecoxib sodium impurity, namely N-[4-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonic acid, belonging to the technical field of chemical pharmacy. The synthesis method comprises the step of carrying out sulfonation reaction and hydrolysis reaction by taking 5-methyl-3, 4-diphenylisoxazole as a raw material to obtain the parecoxib sodium impurity. The synthesized high-purity parecoxib sodium impurity can be used as a standard impurity in parecoxib sodium finished product detection and analysis, so that the accurate impurity location and qualification realized through the parecoxib sodium finished product detection and analysis are improved, the impurity can be better controlled, and furthermore the quality of a parecoxib sodium finished product is improved. The synthesis method disclosed by the invention is simple in operation; the raw material is cheap and available; and the yield of the obtained product is up to 95+ / -5%, and the HPLC purity is larger than or equal to 98%.
Owner:CHENGDU CLIMB PHARMA TECH
Parecoxib sodium for injection and preparing method of parecoxib sodium for injection
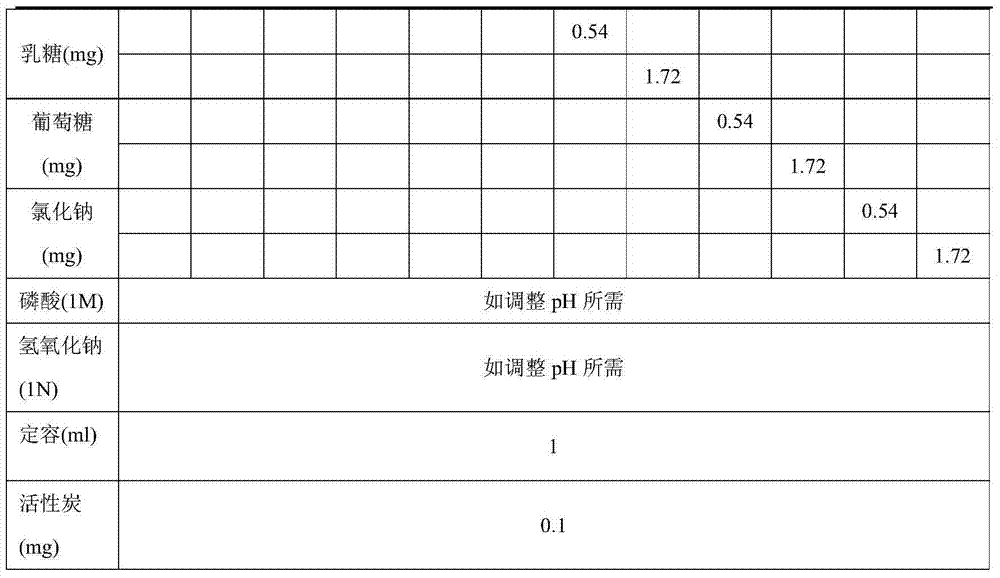
The invention provides a preparing method of parecoxib sodium for injection. The method comprises the following steps of: (1) adding disodium hydrogen phosphate into water for injection; (2) adding parecoxib sodium; (3) adding phosphoric acid or sodium hydroxide to regulate the pH value of the solution to be 8.0 to 9.0; (4) performing processing, detection and split charging; (5) performing freeze-drying processing: uniformly lowering the temperature to -20 DEG C from the room temperature in 30min; uniformly lowering the temperature to -30 DEG C in 90 to 150min; uniformly lowering the temperature to -45 DEG C in 20min and maintaining the temperature for 120min; then, uniformly raising the temperature to 0 DEG C in 120min and maintaining the temperature for 300 to 540min; uniformly raising the temperature to 40 DEG C in 120min and maintaining the temperature for 180 to 300min; and meanwhile, controlling the vacuum degree to be 0.3 to 0.8mbar; and (6) performing plug pressing, box discharging and cover milling. Finally, the parecoxib sodium for injection with the advantages of high stability and few problems in the preparing process is obtained.
Owner:SHANGHAI CHENPON PHARM TECH CO LTD
Synthesis method of parecoxib sodium
ActiveCN106008385AShort route stepsMild conditionsOrganic chemistryParecoxib sodiumSynthesis methods
The invention belongs to the technical field of medical production, and particularly relates to a preparation method of parecoxib sodium. The invention aims to provide a synthesis method of parecoxib sodium, which has the advantages of short synthesis route, mild and controllable conditions and low cost and is simple to operate. By using 5-methyl-3,4-diphenylisooxazole as the initial raw material, chlorosulfonation, acylation and salification are carried out to synthesize the target product parecoxib sodium. The synthesis method provided by the invention has the characteristics of short route steps, mild and controllable conditions, small environmental pollution, higher yield and the like, and is simple to operate and suitable for industrial production.
Owner:HONGGUAN BIO PHARMA CO LTD
Preparation methods of parecoxib sodium and intermediate thereof
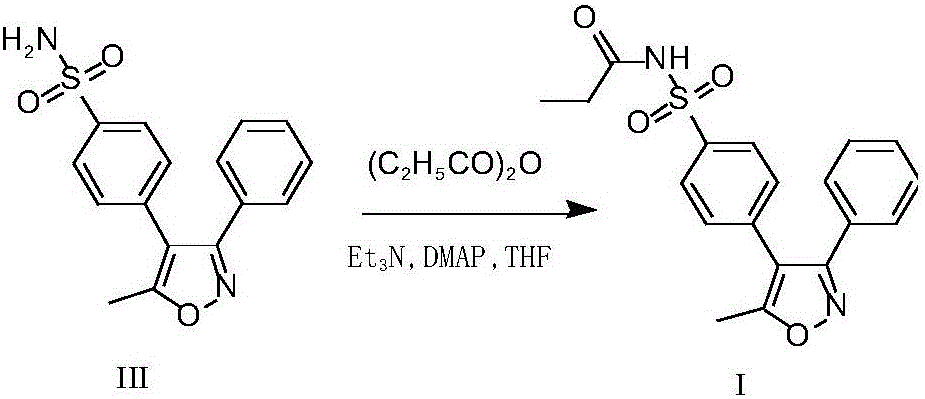
InactiveCN106674142AHigh puritySimple reactivityOrganic chemistryParecoxib sodiumPropionic anhydride
The invention discloses preparation methods of parecoxib sodium and intermediate thereof. The invention provides a preparation method of a parecoxib sodium intermediate I. The preparation method of the parecoxib sodium intermediate I comprises the following step: in the presence of a catalyst, performing condensation reaction on valdecoxib III and propionic anhydride to obtain the parecoxib sodium intermediate I, wherein the catalyst is one or more of benzenesulfonic acid, p-toluenesulfonic acid, methanesulfonic acid and sulfamic acid. The preparation method is simple in reaction and post-treating operation, high in yield and low in cost; the purity of the prepared intermediate product is high and can reach 99.80% or above, and the content of specific impurity valdecoxib reaches 0.02% or below and can meet the standard of an original researching manufacturer; therefore, the preparation method is suitable for industrial production. The purity of parecoxib sodium prepared from the parecoxib sodium intermediate I prepared by the preparation method provided by the invention can reach 99.80% or above and the content of the specific impurity valdecoxib reaches 0.01% or below and is higher than the standard of the original researching manufacturer.
Owner:SHANGHAI BOCIMED PHARMA CO LTD
Parecoxib sodium anhydrous compound
The invention belongs to the technical field of medicines and in particular relates to a parecoxib sodium anhydrous compound and a preparation method thereof. The parecoxib sodium anhydrous compound is basically anhydrous and not solvated and has the advantages that the chemical purity is 99.9 percent, the maximum impurity is smaller than one thousandth, the optical purity reaches up to 99.96 percent ee, and the stability is good. The invention also relates to application of a composition of parecoxib sodium in preparing a medicament for treating cyclooxygenase-2(COX-2) mediated diseases.
Owner:TIANJIN HANRUI PHARMA
Preparation method of anhydrous and non-solvation A crystallization parecoxib sodium
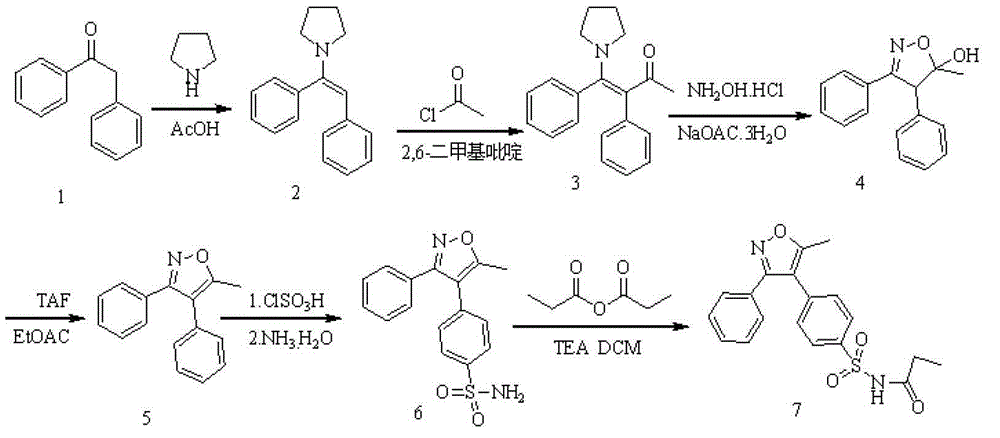
InactiveCN104910091AHigh crystallinityImprove stabilityOrganic chemistrySodium acetateChlorosulfuric acid
The invention relates to a preparation method of anhydrous and non-solvation A crystallization parecoxib sodium. The method comprises the following steps: 1,2-phenylacetophenone and pyrrolidine are condensed to generate 1-(1,2-diphenylvinyl)pyrrolidine, then acetylation is carried out, and 4,5-dihydro-5-methyl-3,4-dibenzyl-5-isoxzzole alcohol are subjected to cyclization under sodium acetate and hydroxylamine hydrochloride. The compound is directly reacted to chlorosulfonic acid, dehydration and chlorine sulfonation reaction are carried out, and then valdecoxib can be obtained through ammonification, valdecoxib is refined, then is subjected to acetylation to obtain parecoxib, and salt forming is performed to obtain the target compound parecoxib sodium.
Owner:北京华睿鼎信科技有限公司
Directional preparation method and application of diaryl substituted isoxazole compound
InactiveCN108047155AProcess conditions are easy to controlEasy to routeOrganic chemistryComponent separationSulfonyl chloridePhenyl group
The present invention discloses a parecoxib sodium impurity S, namely, N-{3-[(5-methyl-4-phenylisoxazol-3-yl)phenyl]sulfonyl}propionamide, and an preparation method thereof, and belongs to the technical field of chemical pharmacy. The method comprises the following steps: 5-methyl-3,4-diphenylisoxazole is used as a starting raw material, and the reaction conditions and an auxiliary reagent are controlled to increase the ratio of a sulfonyl chloride group connected to the meta-position of a phenyl ring at a 3-position of an isoxazole ring; and an amination reaction is performed, a crystallization mother liquid is concentrated to dryness, the obtained product is subjected to propionylation, and finally through preparative liquid chromatography separation, the parecoxib sodium impurity S is obtained. The high-purity parecoxib sodium impurity S provided by the present invention can be used as an impurity standard product in the detection and analysis of a finished product of parecoxib sodium, so that the accurate positioning and chemical composition determination of impurities in the detection and analysis of the finished product of the parecoxib sodium are promoted, reinforcement of control of the impurities is facilitated, and the quality of the finished product of the parecoxib sodium is further improved. The method provided by the invention has the advantages of cheap and easyraw materials, simple operation, good reproducibility, and an HPLC purity of 99.5% or more.
Owner:ZHEJIANG ZHENYUAN PHARMA CO LTD +1
Method for detecting parecoxib sodium sulfate genotoxic impurities
PendingCN111413440AHigh detection sensitivityHigh precisionComponent separationGas liquid chromatographicDiethylsulfate
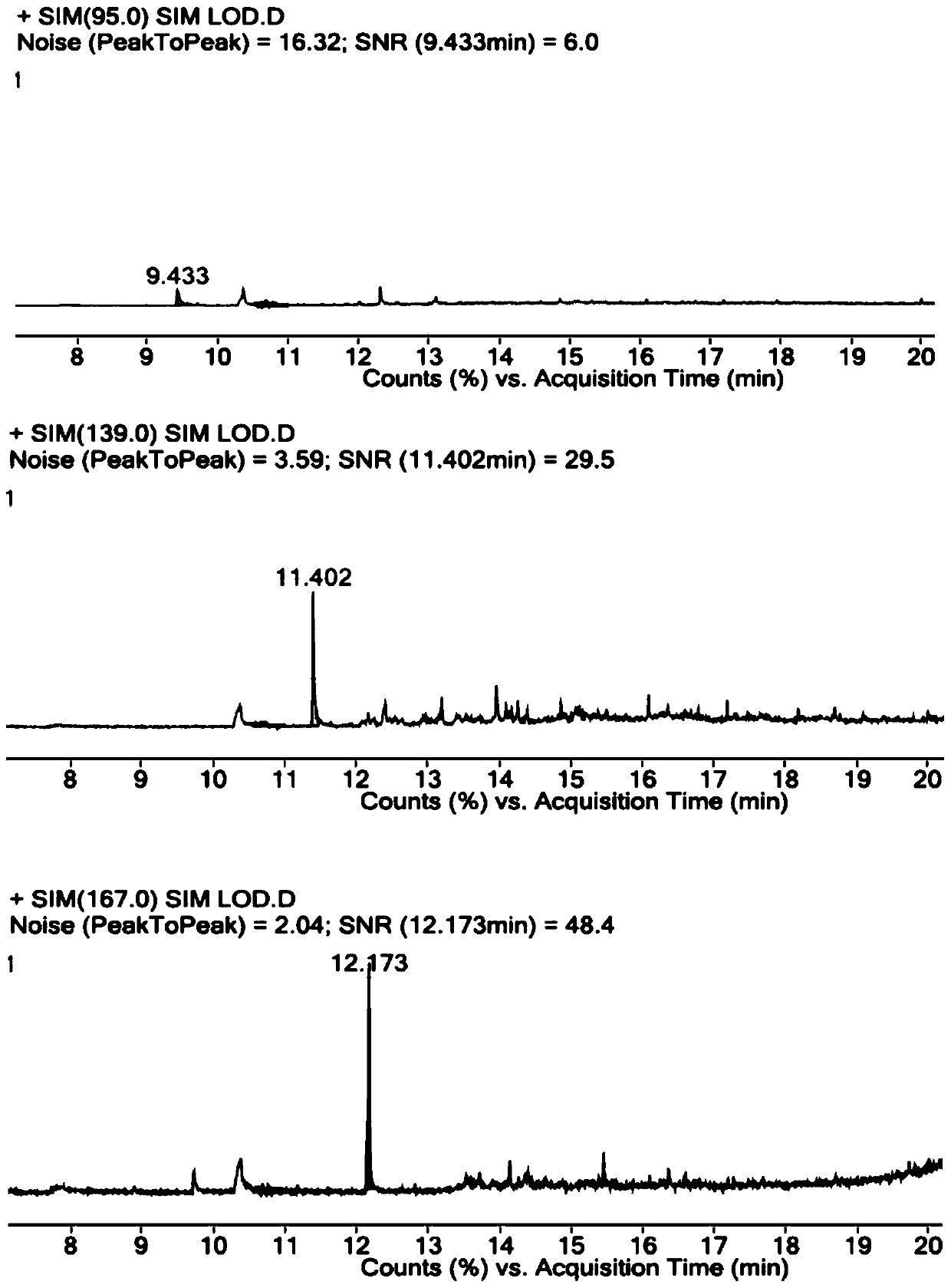
The invention relates to a method for detecting parecoxib sodium sulfate genotoxic impurities. The method comprises the following steps of dissolving a dimethyl sulfate reference substance, a diethylsulfate reference substance and a diisopropyl sulfate reference substance to obtain a sulfate impurity reference substance solution, dissolving parecoxib sodium to be detected to obtain a sample solution to be detected, and carrying out gas chromatography-mass spectrometry determination on the sulfate impurity reference substance solution and the sample solution to be detected. The chromatographicconditions are as follows: a filler of the gas chromatographic column is selected from one of a non-polar filler, a weak polar filler and a medium polar filler; and mass spectrum conditions comprisethat an electrospray ion source and a positive ion scanning mode are selected. The method is high in detection precision, has very high specificity and durability, and is simple and convenient to operate. The separation degree between genotoxic impurities is greater than 2.0, and the method can be used for quality control of parecoxib sodium bulk drugs.
Owner:SHANGHAI CHENPON PHARMA TECH
Application of citric acid in preparing parecoxib sodium freeze-drying preparation compound, compound and preparation method thereof
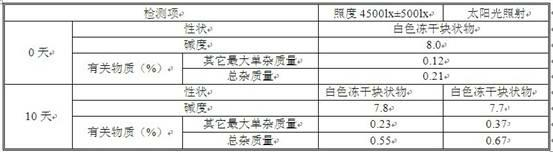
ActiveCN109568277APrevent or delay the production ofPowder deliveryAntipyreticParecoxib sodiumFreeze-drying
The invention discloses application of citric acid in preparing a parecoxib sodium freeze-drying preparation compound, a compound and a preparation method thereof. A photo-stable parecoxib sodium freeze-drying preparation compound comprises a pharmaceutically acceptable amount of parecoxib sodium, a pharmaceutically acceptable amount of pH regulator, a pharmaceutically acceptable amount of sodiumphosphate and a pharmaceutically acceptable amount of photo-stabilizer, wherein citric acid serves as the photo-stabilizer. The citric acid disclosed by the invention can be used as the photo-stabilizer for preparing the parecoxib sodium freeze-drying preparation compound, and meanwhile, a more stable parecoxib sodium freeze-drying preparation compound is supplied. The compound is capable of preventing or delaying generation of impurities U and T when the parecoxib sodium freeze-drying preparation compound is exposed to light source during the processes of production, storage, transportation and clinic use, and the compound is capable of obviously promoting product quality and clinic use safety.
Owner:CHENGDU XINJIE HIGH TECH DEV CO LTD
Synthesis method of parecoxib sodium impurity
InactiveCN104945343APrecise positioningQualitative highOrganic chemistryParecoxib sodiumSynthesis methods
The invention discloses a synthesis method of a parecoxib sodium impurity 4-[5-methyl-3-(3-phenylsulfonic acid)-1,2-oxazole-4-yl] benzenesulfonic acid, belonging to the technical field of chemical pharmacy. The synthesis method comprises the following step of performing sulfonation reaction and hydrolysis reaction on starting raw materials including 5-methyl-3,4-diphenyl isoxazole, dichloromethane and chlorosulfonic acid to produce the parecoxib sodium impurity. The synthesized high-purity parecoxib sodium impurity can be used as an impurity standard sample in the detection analysis of a finished product of parecoxib sodium, so that accurate positioning and qualifying effects of the detection analysis of the finished product of parecoxib sodium for the impurity can be improved, which is beneficial to enhancing the control over the impurity and further improving the quality of the finished product of parecoxib sodium. The synthesis method disclosed by the invention has the advantages that the raw materials are cheap and easily available, the operation is simple, the product yield is 95%+ / -5%, and HPLC purity is 99% or above.
Owner:CHENGDU CLIMB PHARMA TECH
Crystalline parecoxib sodium
A process for producing crystalline form I of cabergoline, which process comprises the preparation of Form V using heptane as precipitation solvent, and its exclusive conversion into crystalline Form I of cabergoline. The present crystallization process from toluene-heptane solvent system for form V involves ''reverse addition'' of toluene-cabergoline concentrate to cold heptane.
Owner:PHARMACIA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com