Synthesis method of parecoxib sodium impurity
A technology of parecoxib sodium and its synthesis method, which is applied in the field of chemical pharmacy, can solve problems affecting product quality, etc., and achieve the effects of improving accurate positioning and qualitative, easy-to-obtain raw materials, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Synthetic method of parecoxib sodium impurity D 4-[5-methyl-3-(3-phenylsulfonic acid)-1,2-oxazol-4-yl]benzenesulfonic acid
[0023] Add 3g of 5-methyl-3,4-diphenylisoxazole, 6g of dichloromethane, and 42g of chlorosulfonic acid into the reaction flask, reflux at 40°C for 14h, add the reaction solution dropwise to 50g of water, 12g of dichloro Extract with methane, concentrate to dryness, add 12g ethyl acetate to crystallize for 1.5h, filter to get 4-[5-methyl-3-(3-phenylsulfonyl chloride)-1,2-oxazol-4-yl]benzenesulfonate Acid chloride 4.1g, yield 74.4%.
[0024] Add 3 g of the above 4-[5-methyl-3-(3-phenylsulfonyl chloride)-1,2-oxazol-4-yl]benzenesulfonyl chloride to the reaction flask, add 10 g of water, 10 g of acetonitrile, Reflux at 85°C for 12h, depressurize to -0.09MPa and concentrate to obtain 2.6g of white solid , That is, 4-[5-methyl-3-(3-phenylsulfonic acid)-1,2-oxazol-4-yl]benzenesulfonic acid with a yield of 94.8%.
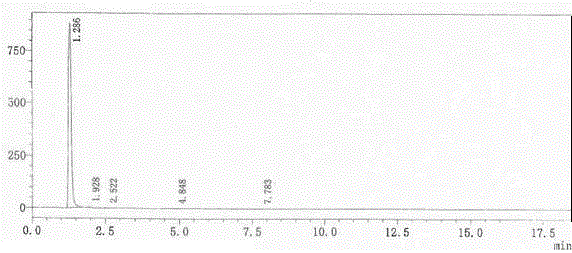
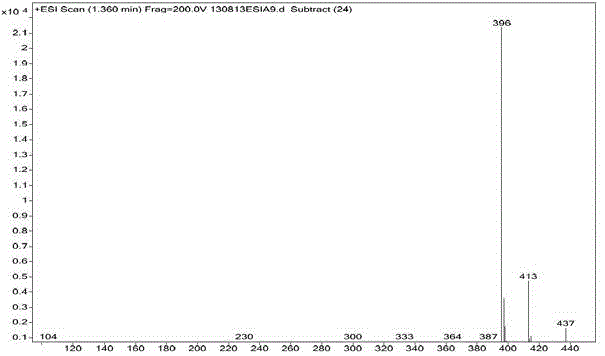
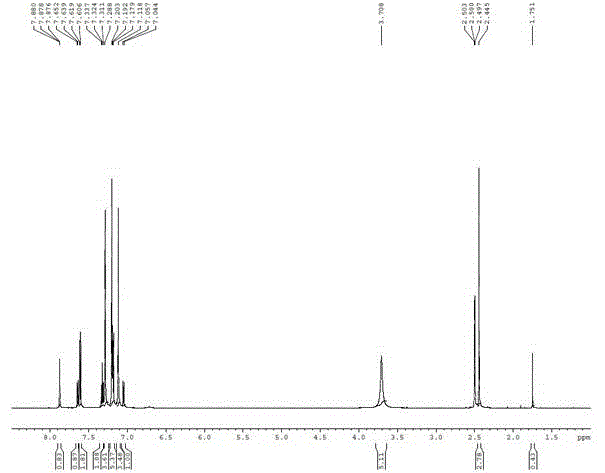
[0025] Parecoxib sodium imp...
Embodiment 2
[0028] Example 2: Synthetic method of parecoxib sodium impurity D 4-[5-methyl-3-(3-phenylsulfonic acid)-1,2-oxazol-4-yl]benzenesulfonic acid
[0029] Add 3g of 5-methyl-3,4-diphenylisoxazole, 18g of dichloromethane, and 60g of chlorosulfonic acid into the reaction flask, reflux at 50°C for 18 hours, add the reaction solution dropwise to 50g of water, 12g of dichloromethane Extract with methane, concentrate to dryness, add 12g ethyl acetate to crystallize for 2.5h, filter to get 4-[5-methyl-3-(3-phenylsulfonyl chloride)-1,2-oxazol-4-yl]benzenesulfonate Acid chloride 4.3g, yield 78.0%.
[0030] Add 4g of the above 4-[5-methyl-3-(3-phenylsulfonyl chloride)-1,2-oxazol-4-yl]benzenesulfonyl chloride to the reaction flask, add 20g of water and 20g of acetonitrile, Reflux at 95°C for 20 hours, depressurize to -0.08MPa and concentrate to obtain 3.5g of white solid, which is 4-[5-methyl-3-(3-phenylsulfonic acid)-1,2-oxazol-4-yl] Benzenesulfonic acid, yield 95.7%, purity 99.3%.
Embodiment 3
[0031] Embodiment 3: The synthetic method of parecoxib sodium impurity D 4-[5-methyl-3-(3-phenylsulfonic acid)-1,2-oxazol-4-yl]benzenesulfonic acid
[0032] Add 5g of 5-methyl-3,4-diphenylisoxazole, 20g of dichloromethane, and 85g of chlorosulfonic acid into the reaction flask, reflux at 43°C for 15 hours, add the reaction solution dropwise to 80g of water, 20g of dichloro Extract with methane, concentrate to dryness, add 20g ethyl acetate to crystallize for 1.8h, filter to get 4-[5-methyl-3-(3-phenylsulfonyl chloride)-1,2-oxazol-4-yl]benzenesulfonate Acid chloride 7.4g, yield 80.5%.
[0033] Add 5g of the above 4-[5-methyl-3-(3-phenylsulfonyl chloride)-1,2-oxazol-4-yl]benzenesulfonyl chloride to the reaction flask, add 15g of water and 15g of acetonitrile, Reflux at 88°C for 15 hours, reduce the pressure to -0.07MPa and concentrate to obtain 4.4g of white solid, which is 4-[5-methyl-3-(3-phenylsulfonic acid)-1,2-oxazol-4-yl] Benzenesulfonic acid, yield 96.2%, purity 99.5%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com