Patents
Literature
237 results about "Benzenesulfonyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Benzenesulfonyl chloride is an organosulfur compound with the formula C₆H₅SO₂Cl. It is a colourless viscous oil that dissolves in organic solvents, but reacts with compounds containing reactive N-H and O-H bonds. It is mainly used to prepare sulfonamides and sulfonate esters by reactions with amines and alcohols, respectively. The closely related compound toluenesulfonyl chloride is often preferred analogue because it is a solid at room temperature and easier to handle.
Water-soluble high molecule intercalating agent containing thiourea group and preparation method
ActiveCN101456939AImprove responseReduce investmentSludge treatmentOther chemical processesSolubilityLiquid waste
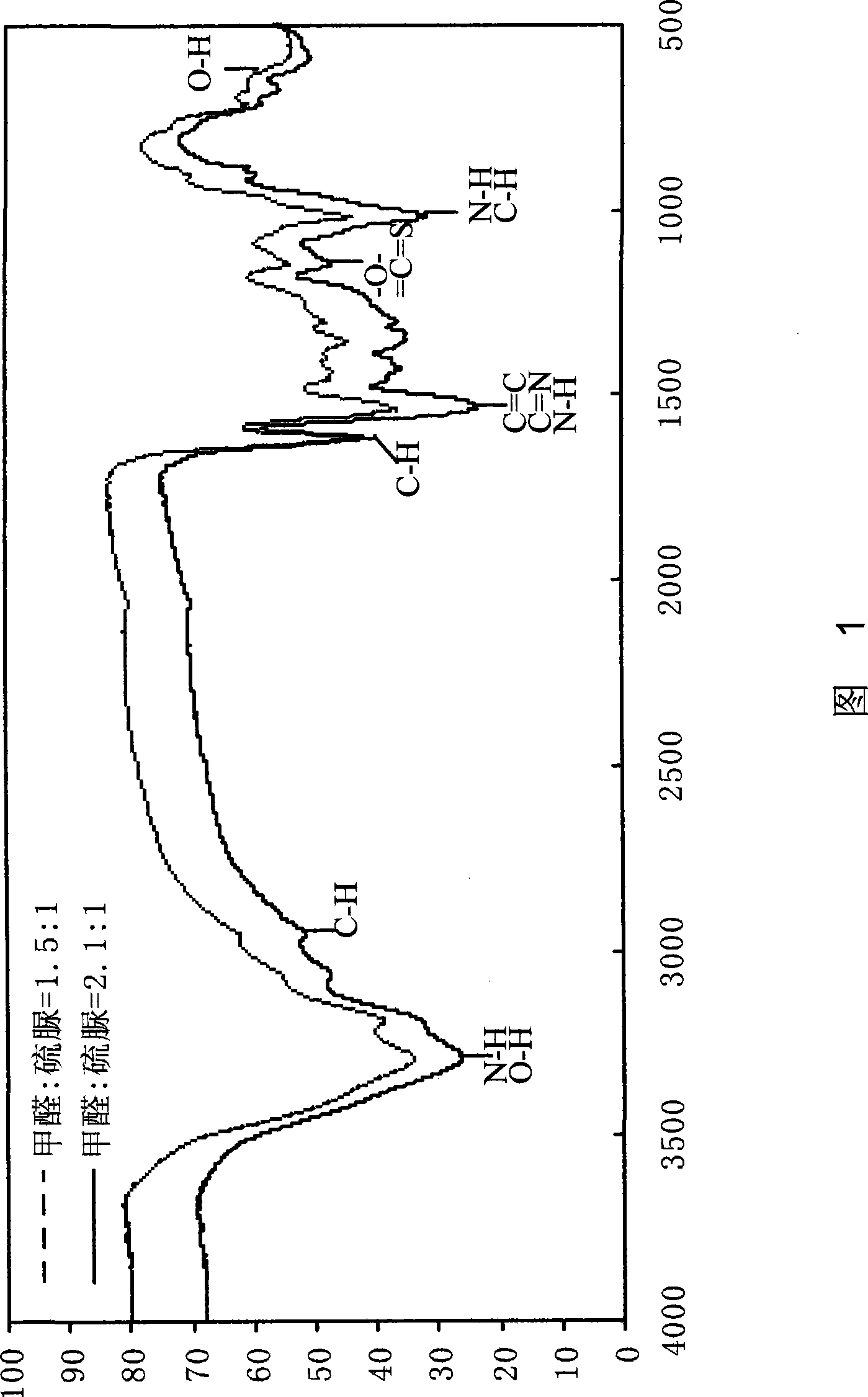
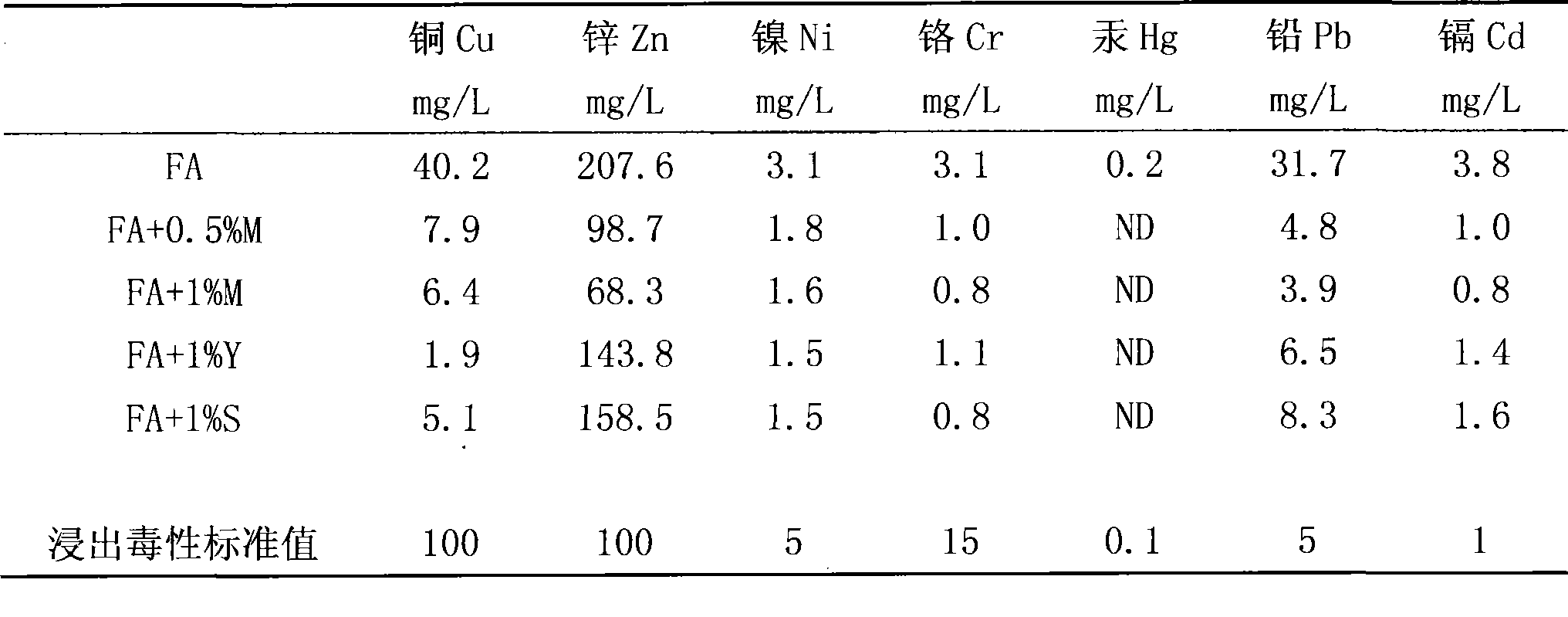
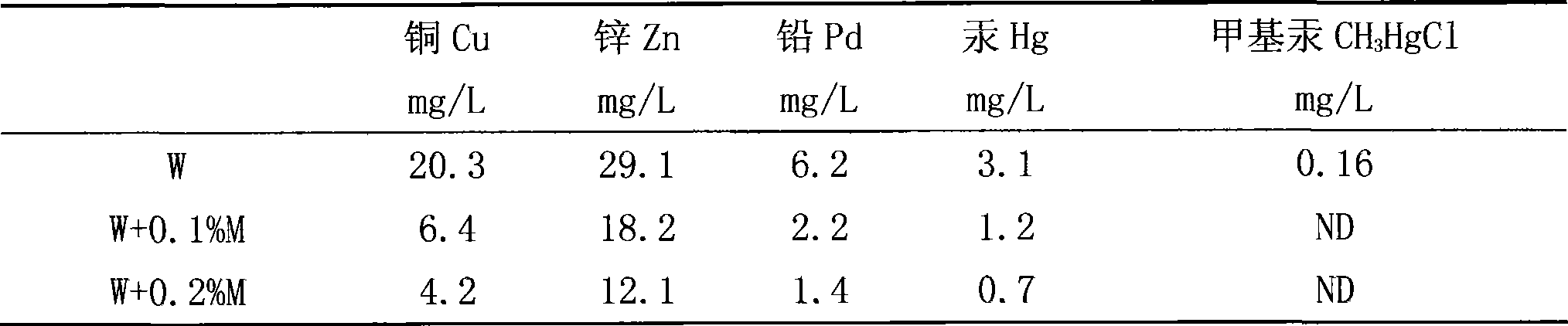
The invention discloses a water-solubility macromolecular chelant containing sulfourea group and a preparation method thereof. The chelant of the invention is prepared by the following method: (a) adjusting the pH value of formaldehyde with acid liquor to activate the formaldehyde; (b) adding the sulfourea, adjusting the pH value with alkali, and reacting at the temperature of 75-90 DEG C; (c) adjusting the pH value with the acid, adding the alcoholic solution of benzene sulfonyl chloride to react at 70-90 DEG C; (d) reducing temperature to 50-60 DEG C, adjusting the pH value with the alkali, and reducing the temperature to below 40 DEG C to prepare the chelant. The chelant of the invention has chelation sedimentation function to almost all of the transition stated heavy metals, which can not only be used in the treatment of the heavy metal sewage containing copper, zinc, nickel, mercury, cadmium, and the like, but also can be used in the stabilization treatment of the heavy metal in the solid wastes such as mud, waste incineration flying ashes, and the like. The preparation method of the invention has the advantages of no need of high temperature and high voltage, low one-time investment, easy availability of raw materials, and zero waste gas, waste liquid and waste residue in the production process.
Owner:TIANJIN YIMING ENVIRONMENTAL TECH CO LTD
Production technology of phenyl sulfuryl chloride
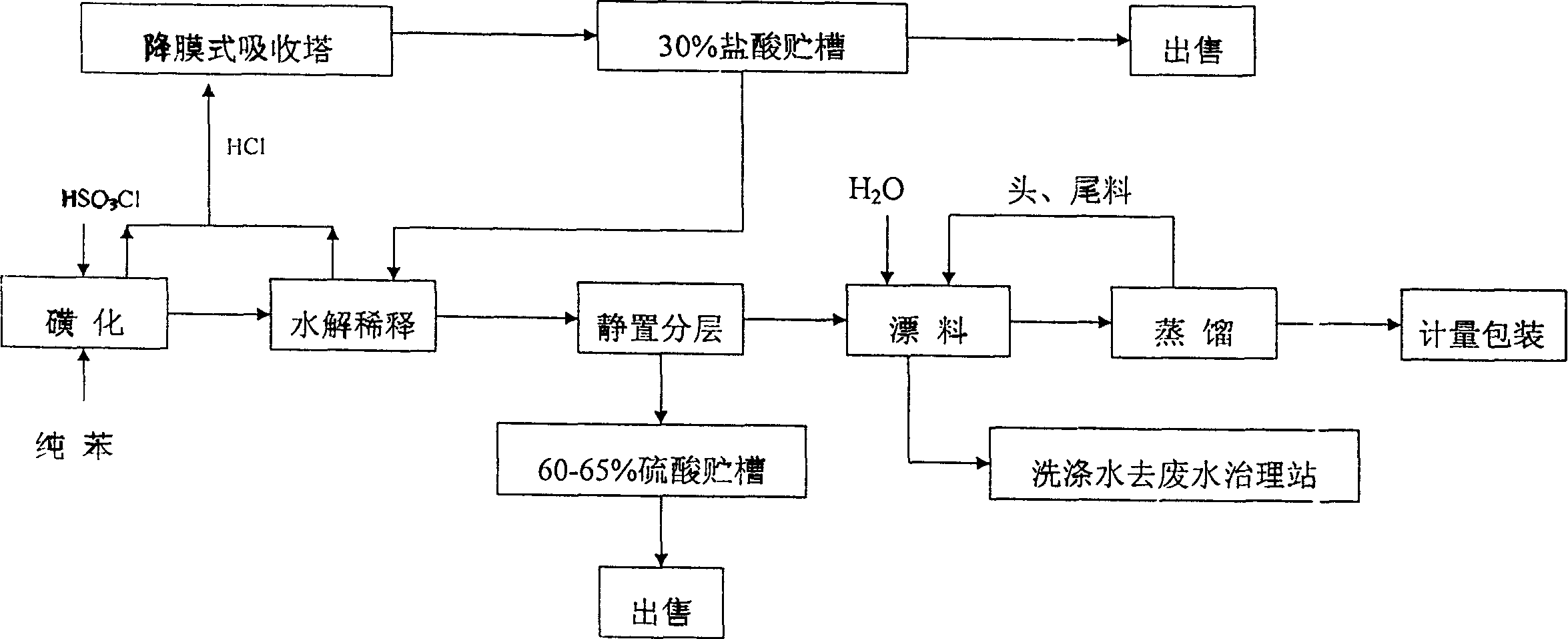
The present invention provides a production method of benzenesulfonyl chloride. It is characterized by that said invention uses benzene and chlorosulfonic acid as raw material, and makes them undergo the processes of sulfonation reaction, hydrolytic dilution, standing still and layer separation and reduced pressure distillation so as to obtain the invented product. Said invention also provides the concrete steps of every process and its concrete requirements.
Owner:江苏康祥实业集团有限公司
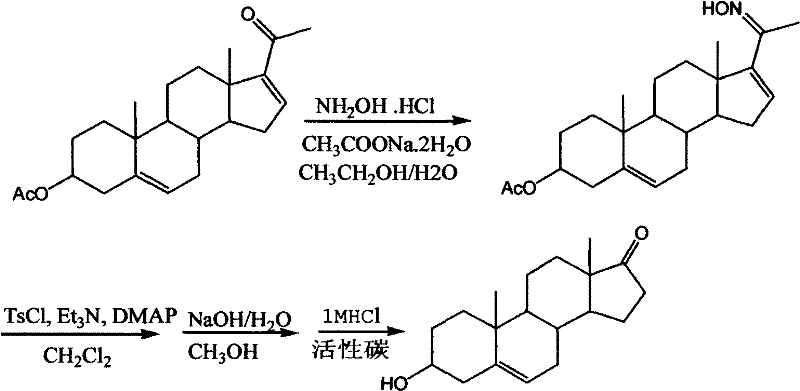
Synthesis method for dehydroepiandrosterone
InactiveCN102212099AOvercoming reactivityOvercoming processingSteroidsBeckmann rearrangementSodium acetate
The invention belongs to a novel synthesis method for dehydroepiandrosterone, which comprises the following steps: oximation of 16-dehydropregnenolone acetate, Beckmann rearrangement, hydrolysis and refining to obtain a product. The synthesis method is characterized by carrying out oximation of ketone by using sodium acetate as a base and water and ethanol as solvent, carrying out Beckmann rearrangement, hydrolysis and one-pot refining reaction, reacting 16-dehydropregnenolone acetate oxime with p-toluenesulfonamide chloride, benzenesulfonyl chloride, triethylamine or N,N-dimethyl-pyridine (DMAP) in a dichloromethane solution, concentrating the solvent after reaction, adding methanol and a sodium hydroxide solution for refluxing hydrolysis, cooling and regulating the pH value to 7-8, adding activated carbon, then refluxing for 30-60 minutes, filtering, concentrating, crystallizing, and centrifuging to obtain the product. The synthesis method provided by the invention is simple to operate, and has characteristics of mild reaction conditions, high yield, low environmental pollution and the like.
Owner:HUNAN KEREY BIOTECH
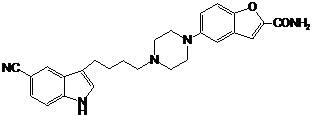
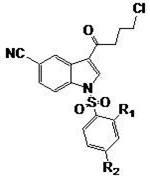
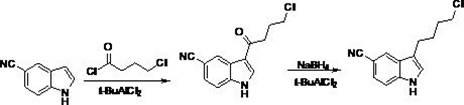
Preparation method of vilazodone
The invention provides a preparation method of vilazodone, which comprises the following steps: reacting 5-cyanoindole, which is used as the initial raw material, with substituted phenylsulfonyl chloride under alkaline conditions, carrying out Friedel-Crafts reaction under the catalytic action of Lewis acid, reducing the product, and carrying out substitution reaction with 5-(1-piperazino)-benzofuryl-2-formamide to obtain the vilazodone. The invention also provides three intermediate compounds related to the vilazodone preparation method. The preparation method provided by the invention has the advantages of low cost, high yield and simple after-treatment, and is easy to operate and convenient for industrial production; and all the reagents are conventional reagents.
Owner:上海泛凯生物医药科技有限公司 +1
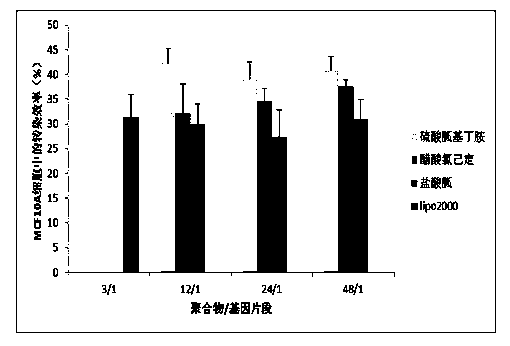
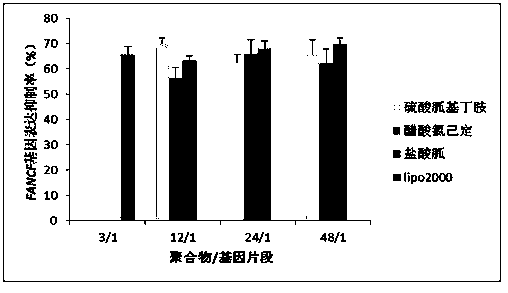
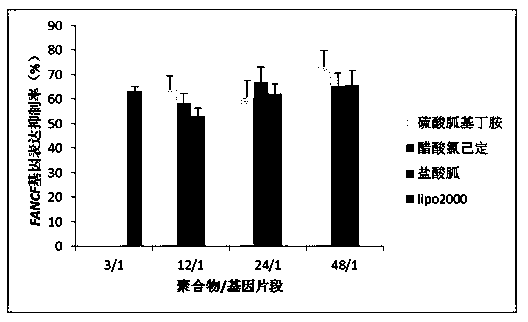
Guanidinylation SS-PAAs polymer as well as preparation and application thereof
InactiveCN103626996ALow toxicityGood membrane permeabilityGenetic material ingredientsOther foreign material introduction processesSulfonyl chlorideCross-link
The invention belongs to the technical field of medicine, and relates to a guanidinylation SS-PAAs gene vector polymer as well as preparation and application thereof. The preparation method comprises the following steps: the guanidinylation SS-PAAs gene vector polymer is finally prepared from a cross-linking agent with a dual-acroloyl structure and a guanidinylation reagent through the processes of protection reaction performed on sulfonyl chlorides by sulfonyl chlorides, Michael addition polymerization and removal of protection of a benzene sulfonyl chloride guanidyl protecting agent in sequence. The polymer gene vector can be self-assembled with different kinds of gene segments to form a compound, so that the excellent biological membrane permeability is achieved, and the responsive degradation is realized in the reducible environment of cells, and the nuclear localization effect is achieved. The capability of loading the gene segments of the vector and the transport process can be regulated through controlling the guanidinylation reagent species, the proportion of the guanidinylation reagent in the polymer, and the molecular mass. The guanidinylation SS-PAAs gene vector can improve the transfection efficiency of genes, and reduce cytotoxicity, and is a novel gene vector adopted in the process of gene therapy.
Owner:SHENYANG PHARMA UNIVERSITY
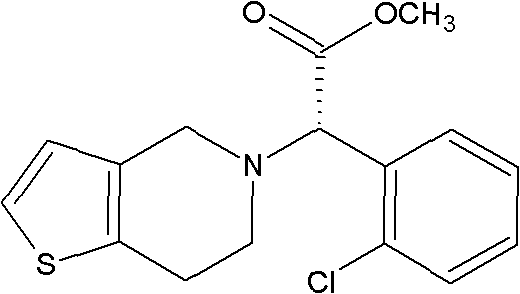
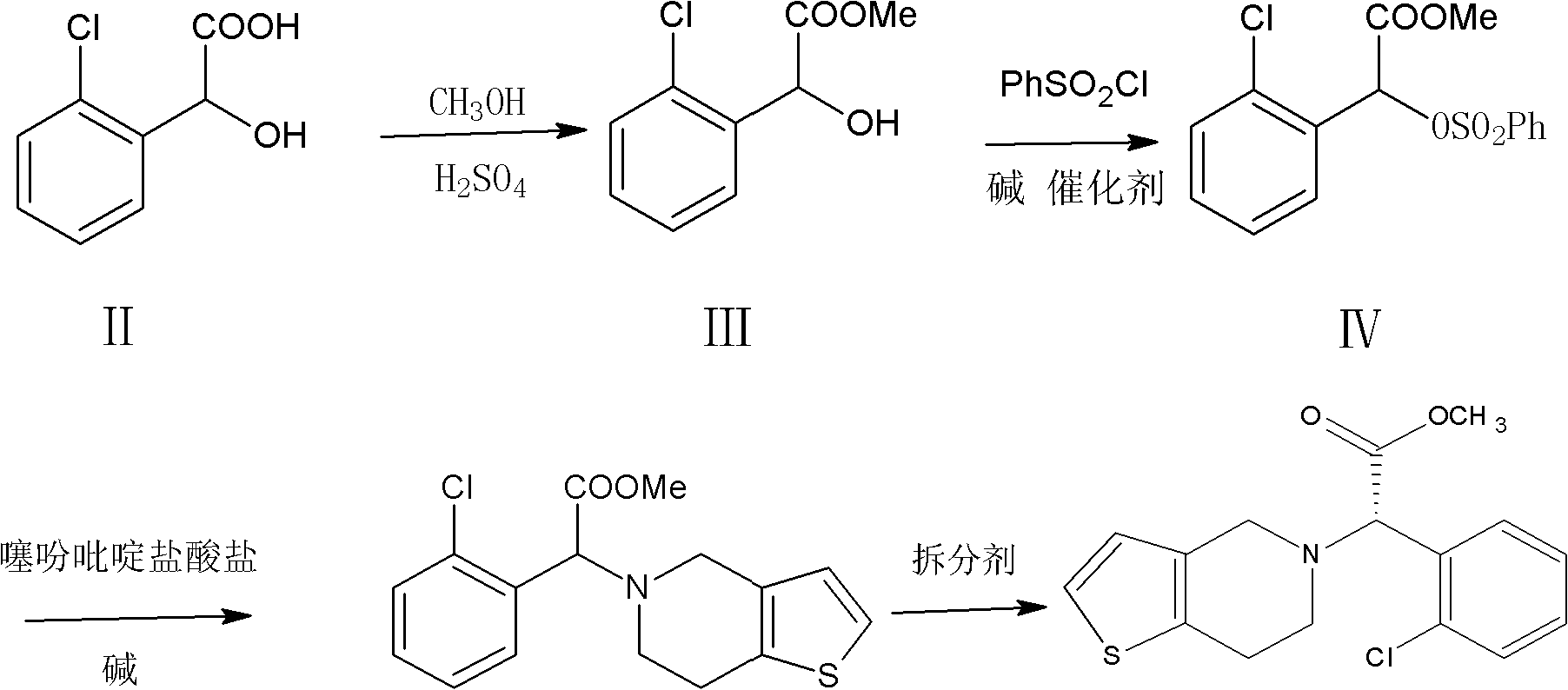
Method for preparing clopidogrel
InactiveCN101845050APromote environmental protectionEliminate the splitting stepOrganic chemistrySulfonyl chlorideMethyl o-chloromandelate
The invention relates to a method for preparing clopidogrel. The conventional synthetic methods have the disadvantages of poor environmental protection, disadvantageous industrial production, low optical purity of final products and high cost. The technical scheme adopted by the invention comprises the following steps of: performing a reaction on a compound, namely, R,S-o-chloromandelic acid and methanol to produce R,S-chloromandelic acid methyl ester; performing the reaction on the R,S-chloromandelic acid methyl ester and benzene sulfonyl chloride under the action of an alkaline catalyst to produce 2-benzenesulfonic acyloxy-2(2-chlorphenyl) methyl acetate; performing an SN2 substitution reaction on the 2-benzenesulfonic acyloxy-2(2-chlorphenyl) methyl acetate and 4,5,6,7-tetrahydro-thiophene pyridine hydrochloride under an alkaline condition to produce R,S-clopidogrel free alkali; resolving the R,S-clopidogrel free alkali in resolving solvent by using a resolving agent; and dissociating the resolved R,S-clopidogrel free alkali to prepare the clopidogrel. In a synthetic route of the invention, reaction conditions are temperate, used reaction substrates are environmentally friendly, reaction yield in each step is high, the optical purity of a final product is up to over 99.5 percent, and pollution-free production can be realized.
Owner:SHANGYU JINGXIN PHARMA
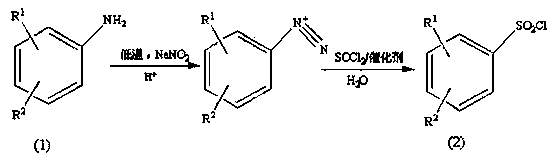
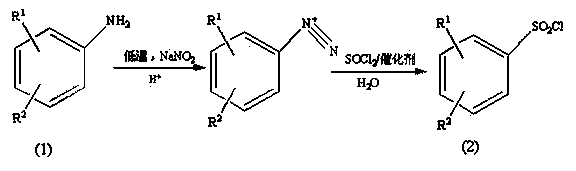
Preparation method of substituted benzene sulfonyl chloride
ActiveCN103739525AImprove solubilityThe synthesis steps are simpleSulfonic acid preparationSulfonyl chloridePtru catalyst
The invention provides a preparation method of a pesticide intermediate, and in particular relates to a preparation method of substituted benzene sulfonyl chloride. The preparation method comprises the following steps: by taking substituted aniline as a raw material, first, carrying out diazo-reaction with sodium nitrite; then, carrying out chlorosulfonation with thionyl chloride aqueous liquor containing a catalyst to prepare substituted benzene sulfonyl chloride, wherein the yield ranges from 78% to 91%. The preparation method provided by the invention simplifies the reaction step, is safe to operate, mild in reaction conditions which are easy to control and low in equipment demand, the yield is improved, the production cost is lowered, emission of the three wastes is reduced, and the environment-friendly load is reduced, so that the preparation method is suitable for industrialized production.
Owner:JINGBO AGROCHEM TECH CO LTD
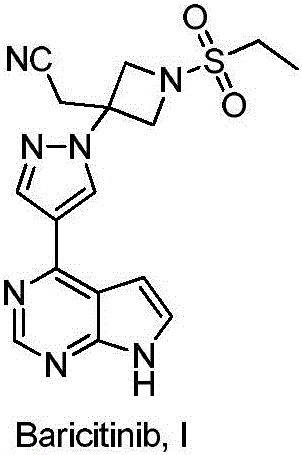
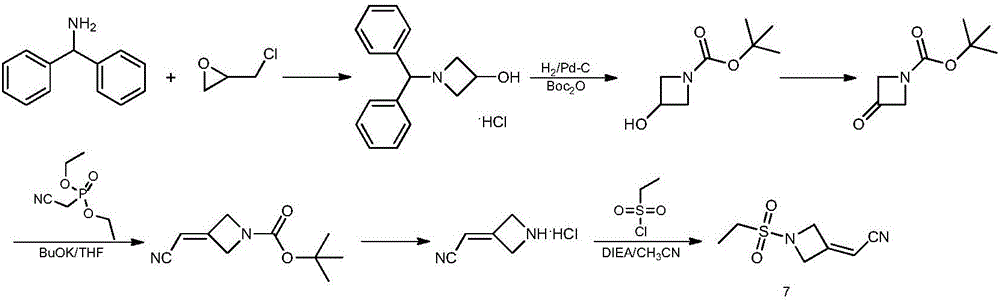
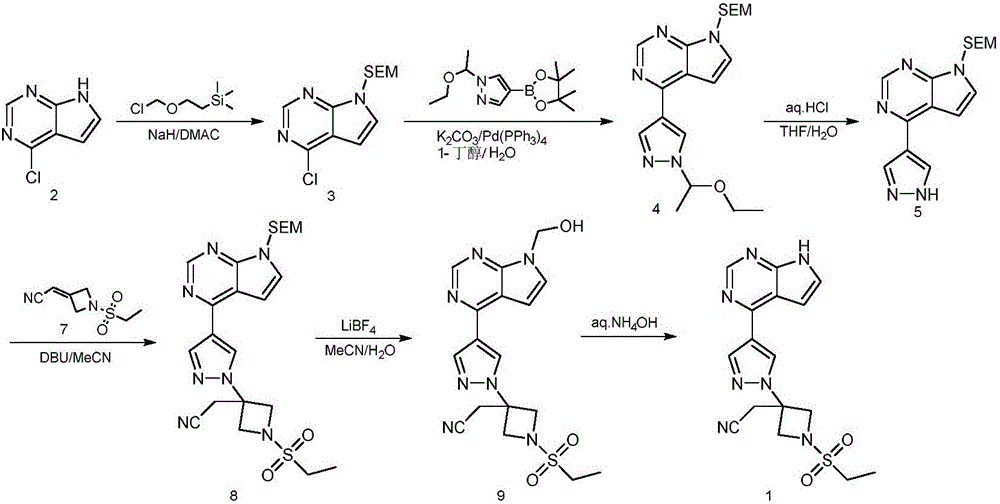
Preparation method of baricitinib
ActiveCN107176955ARaw materials are easy to getSimple processOrganic chemistrySulfonyl chlorideBoronic acid
The invention discloses a preparation method of baricitinib. The method comprises the following steps: performing a substitution reaction on 4-chloro-7H-pyrrolo[2,3-d]pyrimidine (II) serving as a raw material and benzene sulfonyl chloride in the presence of an alkali to obtain an intermediate III; then, performing a Suzuki coupling reaction on the intermediate III and 4-pyrazole-4-boronic acid pinacol ester in the presence of a palladium catalytic system and an alkali to obtain an intermediate V; then performing a Michael addition reaction on the intermediate V and 3-(cyanomethylene)azetidine-1-tert-butyl formate in the presence of a catalyst to obtain an intermediate VII; then removing Boc protection from the intermediate VII under the action of hydrochloric acid to obtain an intermediate VIII; then performing a sulfoamidate reaction on the intermediate VIII and ethyl sulfonyl chloride in an organic solvent in the presence of an alkali to obtain an intermediate IX; lastly, removing benzenesulfonyl protection from the intermediate IX under the action of tetramethylammonium fluoride or tetrabutylammonium fluoride or a trihydrate of the tetramethylammonium fluoride or the tetrabutylammonium fluoride to obtain baricitinib (I). Compared with the prior art, the method has the advantages of adoption of readily-available raw materials, low cost, high product yield and easiness for industrial production.
Owner:NANJING YOKO PHARMA +2
Preparation and refining method of 4-Cl-(trifluoromethyl) benzene sulfonyl chloride
InactiveCN102336689ABiologically activeMultipurposeOrganic compound preparationSulfonic acid preparationSulfonyl chlorideEthyl Chloride
The invention provides a preparation and refining method of 4-Cl-(trifluoromethyl) benzene sulfonyl chloride, which comprises the following steps of: reacting o-chloro benzo trifluoride and mixed acid to generate 2-Cl-5-nitro trifluoromethyl benzene, reducing to obtain 2-Cl-5-amino trifluoromethyl benzene, generating diazo salt with sodium nitride and hydrochloric acid in a polar solvent at a low temperature, reacting with sulfur dioxide and cuprous chloride in the polar solvent at a low temperature to generate rough 4-Cl-(trifluoromethyl) benzene sulfonyl chloride, distilling and recrystallizing to obtain a pure product. The invention has the characteristics that the reaction yield is improved, production danger is lowered, environment pollution is reduced, and the quality of the refined fine product is improved on the basis of improving the yield.
Owner:天津均凯农业科技有限公司
Preparation method of BBI
ActiveCN101671285AHigh purityShort melting rangeSulfonic acid amide preparationSulfonyl chlorideBenzene
The invention provides a preparation method of BBI, which includes the following steps: pouring sodium hydroxide and water into a reaction kettle which is provided with an electric mixer, a thermometer and a reflux condenser; after the sodium hydroxide is fully dissolved and the temperature thereof rises to 50 to 57 DEG C, adding benzsulfamide, and controlling the temperature between 53 to 57 DEGC; after the benzsulfamide is fully dissolved, starting to dropwise add benzene sulfonyl chloride; after dropwise adding for 30 minutes, refilling sodium hydroxide solution, and maintaining the PH value between 8 and 9; after finishing dropwise adding the benzene sulfonyl and carrying out heat insulation reaction for 2 to 4 hours, again rising the temperature to 100 to 105 DEG C; and back flowing for 1 hour, reducing the temperature to 26 to 30 DEG C, using hydrochloric acid to adjust the pH value between 4.0 to 7.5, and separating the solid-liquid mixtures by a centrifugal machine. The invention adopts the method of adding alkali to separate out the sodium salt of BBI directly from the filter liquor, while the benzsulfamide has better solubility in alkali liquor so that the benzsulfamide can not be separated out together with the sodium salt of BBI, and thereby the product with high purity and good dissolubility can be obtained.
Owner:武汉松石科技股份有限公司
Synthesis process of tolylsulfonyl chloride
The present invention is the new synthesis process of tolylsulfonyl chloride with toluene as material, chlorosulfonic acid and phosphorus oxychlorides as sulfonating agent and inorganic ammonium slat as sulfonating assistant. Under the action of sulfonating agent chlorosulfonic acid and phosphorus oxychloride and sulfonating assistant, the material toluene is sulfochlorinated to produce the reacted liquid; and the reacted liquid is made to pass through low temperature hydrolysis, solvent extraction, water washing, eliminating water layer, crystallization of the oil phase at temperature lower than 5 deg.c, filtering and drying to obtain solid tolylsulfonyl chloride. The filtrate is decompression distilled to eliminate solvent to obtain sulfonated oil with o-tolylsulfonyl chloride as main component with total yield as high as 98.8%.
Owner:ZHEJIANG UNIV
Method for producing sulfuric acid fluorinated surfactants
InactiveCN101406816AConvenient sourceThe synthesis method is simpleTransportation and packagingMixingOrganic baseSolvent
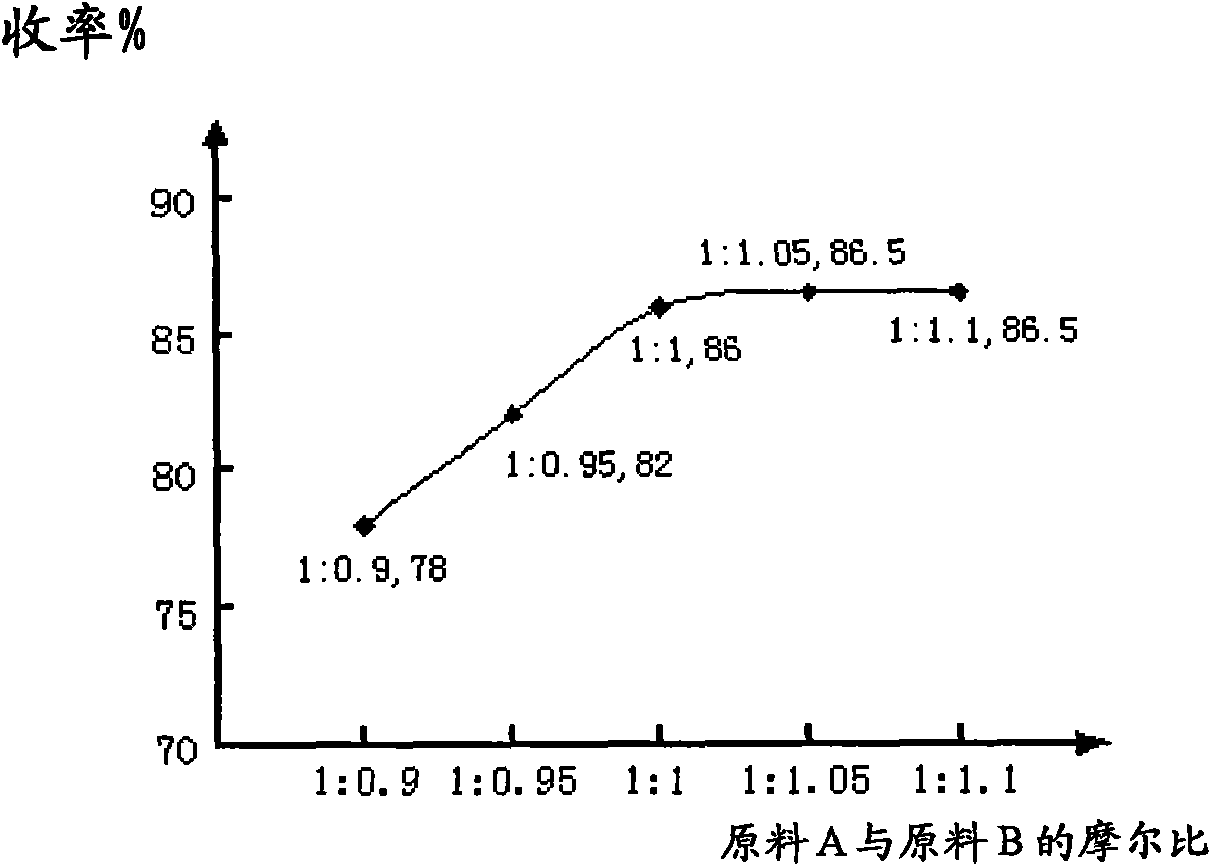
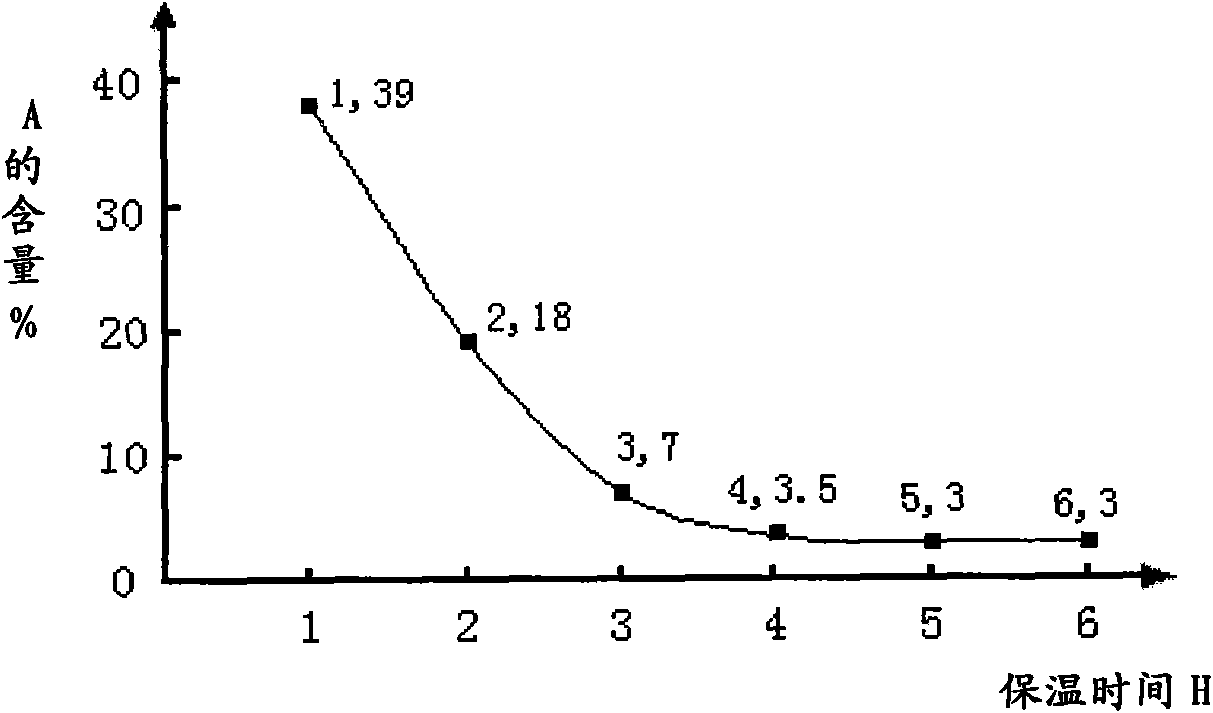
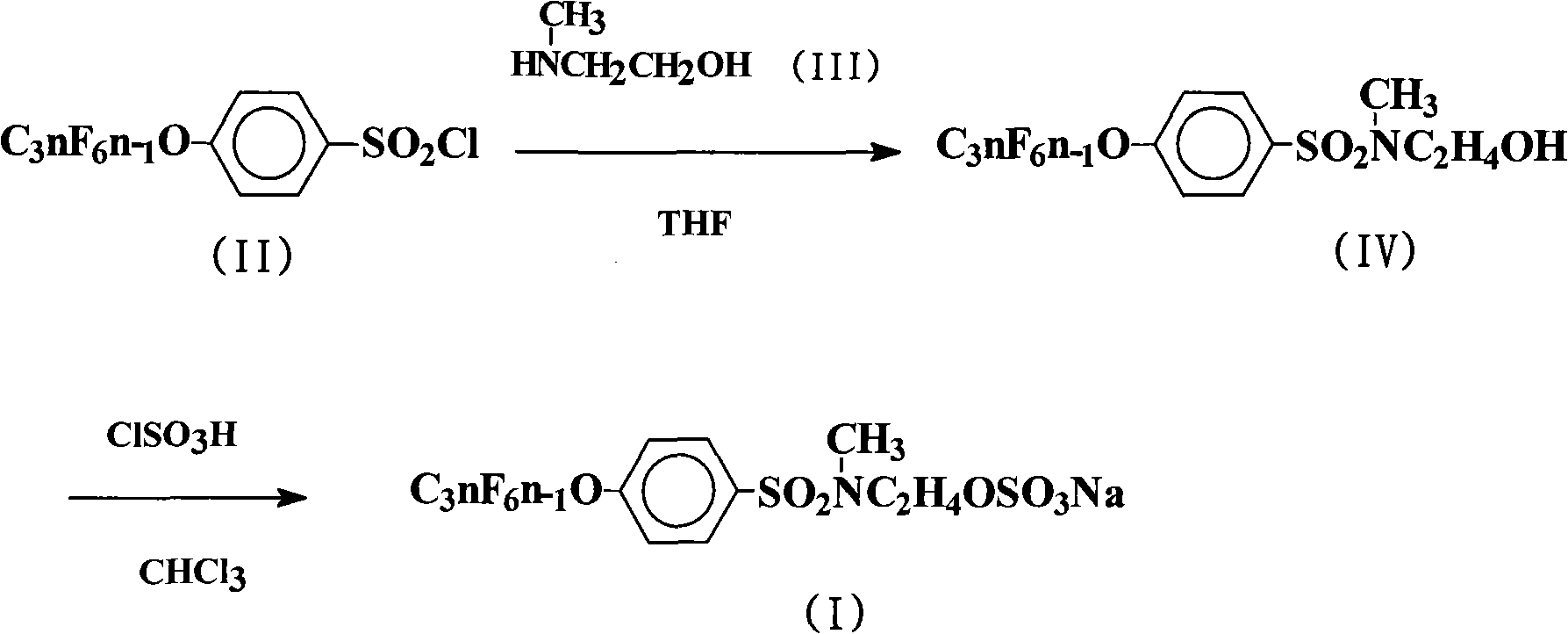
The invention discloses a preparation method for a sulfuric acid ester salt fluorine surfactant. The method comprises the following steps: taking 4-perfluoro alkene oxygen group benzene sulfonyl chloride and 2-methylamino alcohol as raw materials, taking inorganic base or organic base as an acid-binding agent, and carrying out an amidation reaction in an organic solvent 1 to obtain N- ethoxyl-N-methyl-4-perfluoro alkene oxygen group benzene sulfonyl chloride; and carrying out the esterification reaction of N-ethoxyl-N-methyl-4-perfluoro alkene oxygen group benzene sulfonyl chloride and sulfur trioxide in an organic solvent 2 for 1.5 to 15 hours, carrying out distillation to remove the solvent when the esterification reaction is finished, adding water for dissolution, then adding inorganic base aqueous solution with the mass content of between 10 and 50 percent into the solution till the reaction system is neutral, stirring the reaction system for reaction, and finally obtaining sulphuric acid 2-( N-methyl-4- perfluoro alkene oxygen group benzene sulfonyl chloride)ethyl ester sodium. The preparation method has a convenient raw material source, a simple synthesis method and few wastes. In particular, sulfur trioxide (SO3) and N- ethoxyl-N-methyl-4- perfluoro alkene oxygen group benzene sulfonyl chloride are adopted to carry out the esterification reaction, thereby bringing about high reaction yield.
Owner:ZHEJIANG UNIV OF TECH
Process for the manufacture of agomelatine and its intermediate
ActiveUS20110130571A1Available and inexpensive materialEasy to optimizeNervous disorderOrganic compound preparationEthyl groupAlkaline hydrolysis
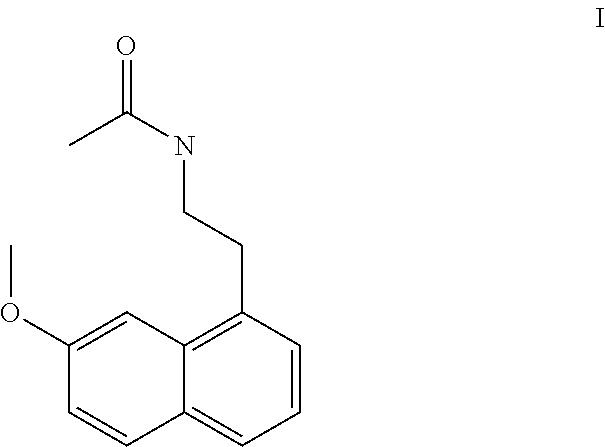
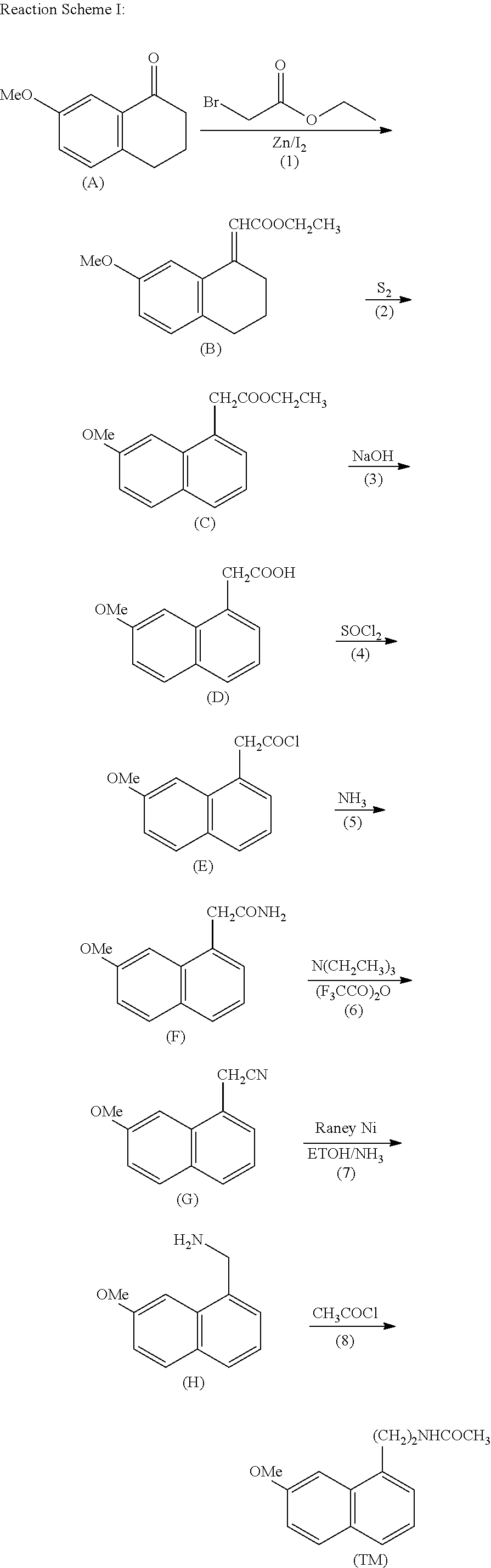
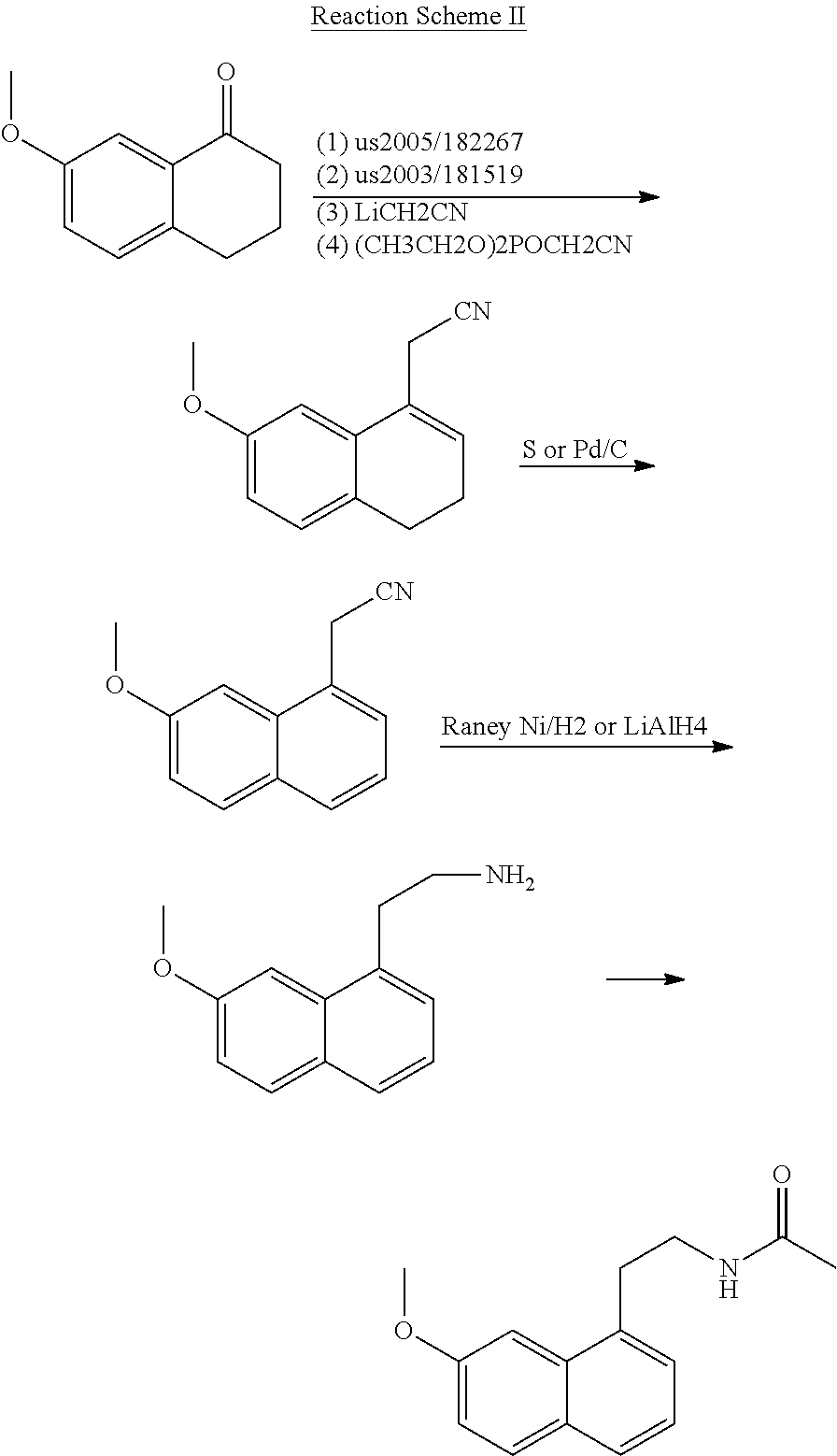
A process for the manufacture of agomelatine and its intermediate N-[2-(7-methoxy-1-naphthyl)ethyl]phthalimide is provided and includes reacting 7-methoxy-1-naphthyl ethanol (III) with benzenesulfonyl chloride to obtain 7-methoxy-1-naphthylethyl benzene sulfonate (IV), which is reacted with potassium phthalimide to produce N-[2-(7-methoxy-1-naphthyl)ethyl]phthalimide (II); and subjecting N-[2-(7-methoxy-1-naphthyl)ethyl]phthalimide (II) to alkaline hydrolysis and acetylation, to obtain agomelatine.
Owner:NHWA PHARMA CORPORATION
Sulfur-substituted podophyllotoxin derivative as well as synthetic method and application thereof
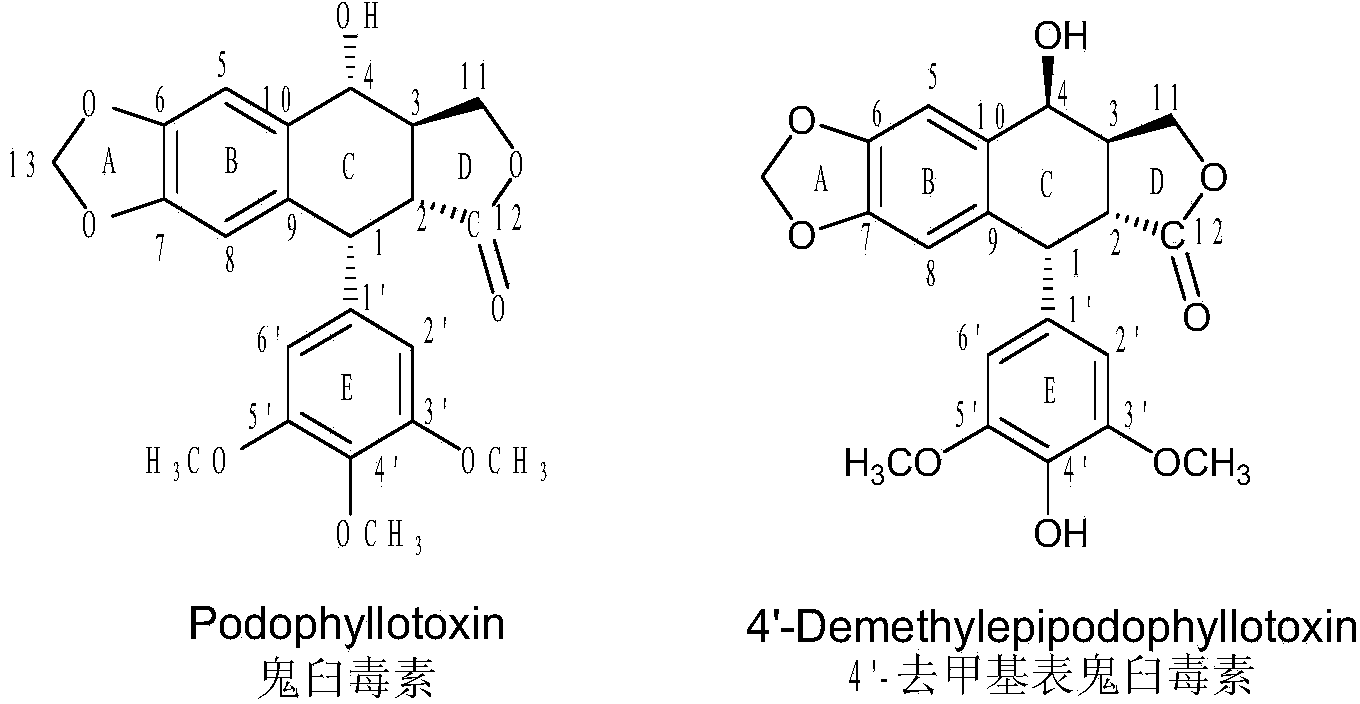
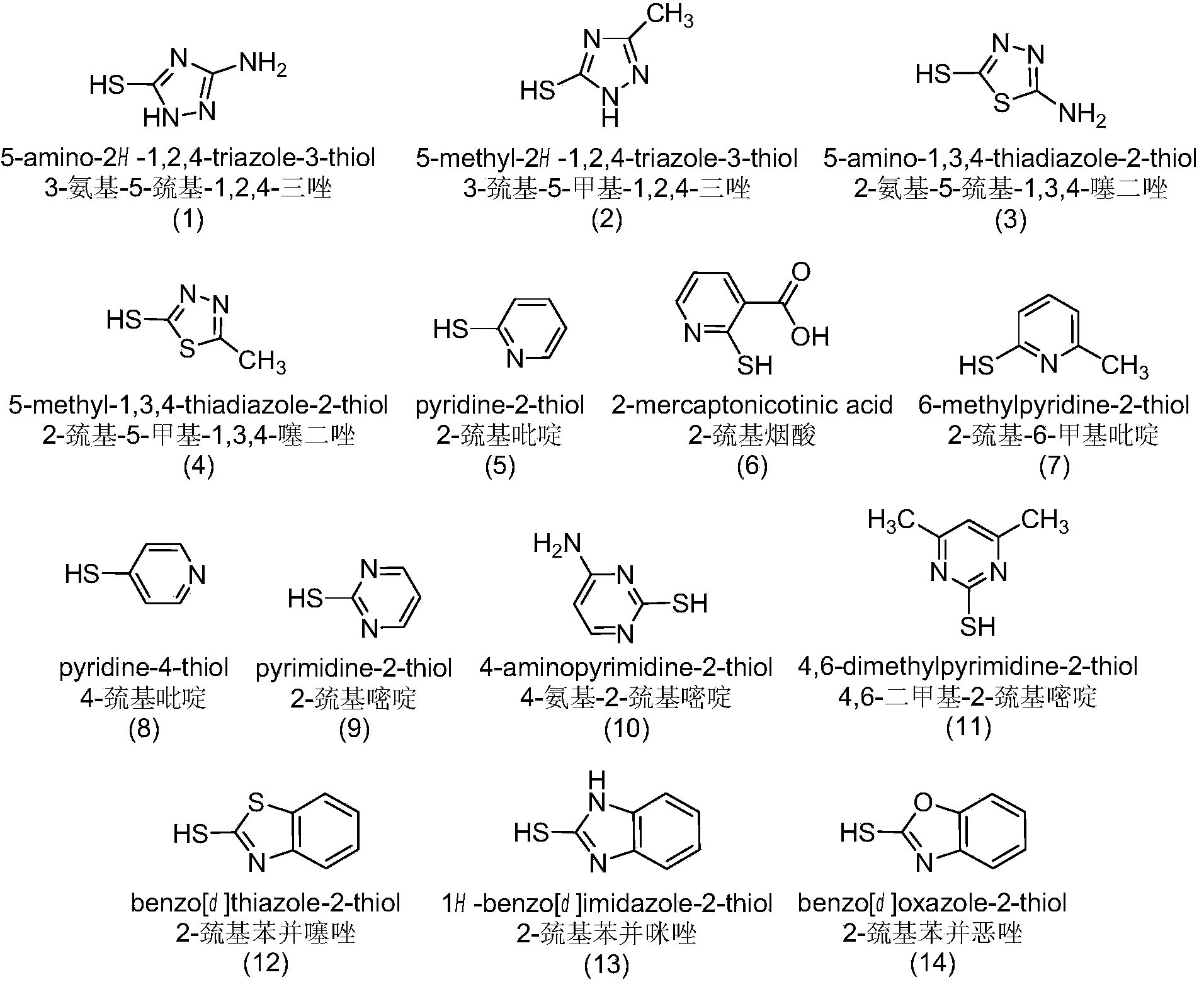
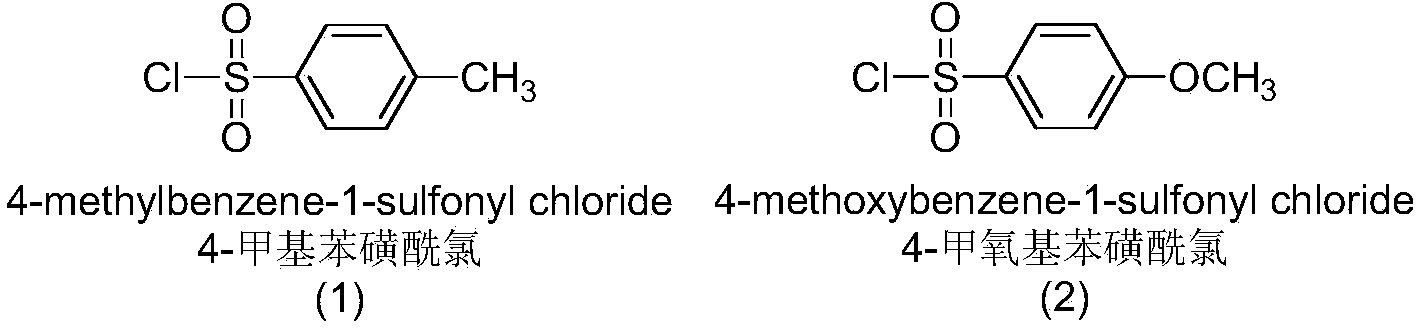
The invention discloses a sulfur-substituted podophyllotoxin derivative as well as a synthetic method and application thereof. The podophyllotoxin derivative, shown by a formula (V), with significantly improved anti-tumor activity and reduced toxic or side effect is obtained by introducing an aromatic heterocyclic compound with rigidity and a further sulfonamide product of 3-amino-5-mercapto-1,2,4-triazole, 2-amino-5-mercapto-1,3,4-thiadiazole, and 4-methyl benzenesulfonyl chloride or 4-methoxy benzenesulfonyl chloride, serving as substituent groups, into the fourth position of a C ring of podophyllotoxin or 4'-demethylepipodophyllotoxin respectively. An activity inhibition experiment of tumor cells in vitro shows that the anti-tumor activity of the compound shown by the formula (V) is significantly improved as compared with that of podophyllotoxin or 4'-demethylepipodophyllotoxin.
Owner:HUBEI UNIV OF TECH
Preparation method of mild diazomethane derivative
ActiveCN106608788AHigh puritySynthetic asymmetrySilicon organic compoundsCatalystsSulfonyl chlorideOrganic solvent
The invention discloses a preparation method of a mild diazomethane derivative. The preparation method comprises that EWG-substituted benzene sulfonyl chloride and hydrazine hydrate undergo a reaction to produce EWG-substituted benzene sulfonyl chloride, the EWG-substituted benzene sulfonyl chloride and aldehyde or ketone undergo a reaction to produce EWG-substituted benzenesulfonylhydrazone, and the EWG-substituted benzenesulfonylhydrazone, a base and an organic solvent are mixed and undergo a replacement reaction to produce a diazomethane derivative. The diazomethane derivative is not separated and purified and is further used for a tension small ring synthesis reaction and an insertion reaction. The benzene ring of benzenesulfonylhydrazone is introduced with an electron-withdrawing group EWG, and through electron effects and steric hindrance effects, the benzenesulfonyl group on the benzenesulfonylhydrazone is easily separated so that a diazomethane derivative is produced under very mild conditions and especially at the room temperature.
Owner:NORTHEAST NORMAL UNIVERSITY
Chlorinated glaucocalyxin A derivative and preparation method and application thereof
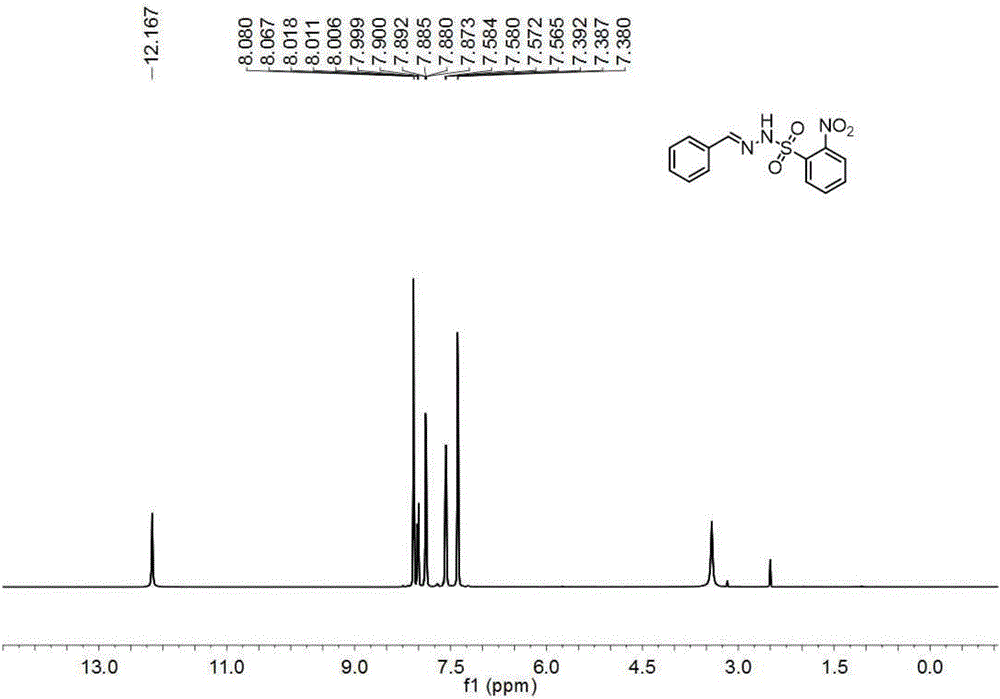
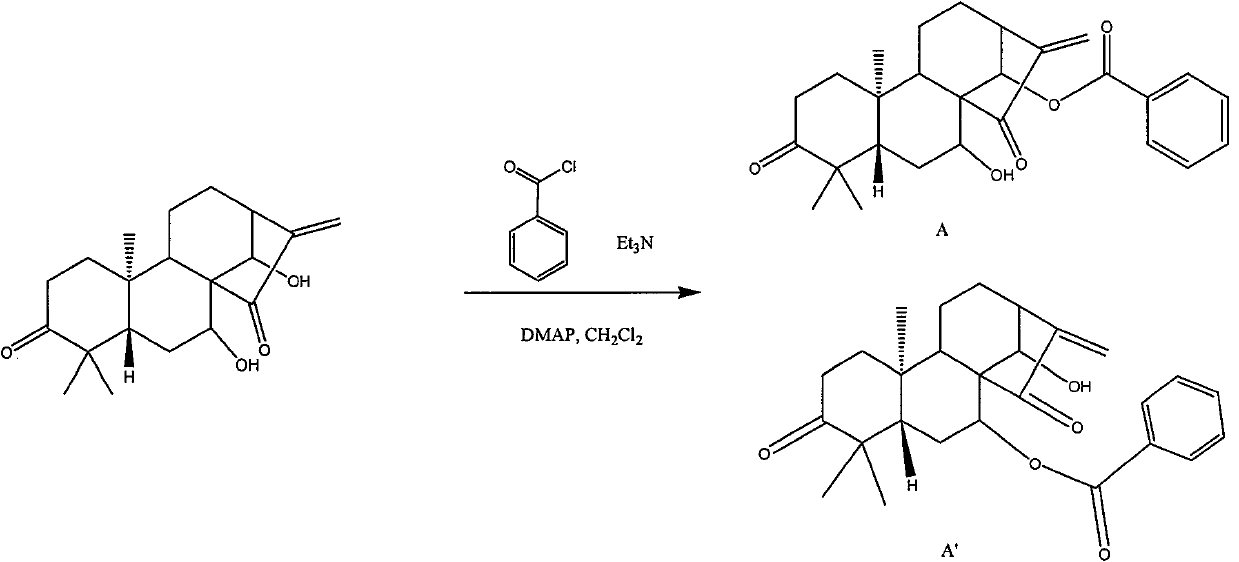
InactiveCN101993373AGood inhibitory effectSolve the polarity is smallOrganic active ingredientsPreparation from carboxylic acid halidesBenzeneSulfonyl chloride
The invention discloses a structure and a preparation method of a chlorinated glaucocalyxin A derivative, and application of the chlorinated glaucocalyxin A derivative to the treatment of cancer thereof. The general molecular structural formula of the chlorinated glaucocalyxin A derivative is shown below, wherein R1 is benzoyl chloride substituent(-C7H6O2), benzene sulfonyl chloride substituent(-C6H6O3S2) or -OH; and R2 is benzoyl chloride substituent(-C7H6O2), benzene sulfonyl chloride substituent(-C6H6O3S2) or -OH. The preparation method is to prepare the disclosed chlorinated glaucocalyxin A derivative by reacting the extracted glaucocalyxin A with benzoyl chloride or the benzene sulfonyl chloride in the presence of triethylamine and 4-dimethylamino pyridine.
Owner:SHANGHAI JINHAO PHARMA DEV
Preparation method of 2-(2',2'-difluoroethoxy)-6-(trifluoromethyl)benzene-1-sulfonyl chloride
InactiveCN105294515AHigh synthesis efficiencyEasy to synthesizeOrganic chemistryOrganic compound preparationSulfonyl chlorideBenzene
The invention discloses a preparation method of 2-(2',2'-difluoroethoxy)-6-(trifluoromethyl)benzene-1-sulfonyl chloride. The preparation method comprises following steps: (1) a sulfonyl chloride or a sulfonic anhydride is added into an organic solvent containing 2,2-difluoroethanol and an alkali, and a compound I is obtained via complete reaction, wherein the sulfonyl chloride is selected from alkyl sulfonyl chloride or benzene sulfonyl chloride, and the sulfonic anhydride is selected from alkyl sulfonic anhydride or benzene sulfonic anhydride; (2) m-trifluoromethylphenol, the compound I, and an alkali are added into an organic solvent, an obtained mixture is heated and stirred, and a compound II is obtained via complete reaction; and (3) an accelerant and the compound II are added into an organic solvent, a strong alkali is added, the sulfonyl chloride is added into an obtained reaction solution, and 2-(2',2'-difluoroethoxy)-6-(trifluoromethyl)benzene-1-sulfonyl chloride is obtained via complete reaction and purifying. The preparation method is simple, is high in efficiency, is safe and reliable, and is capable of increasing synthesis efficiency of penoxsulam greatly; and operation is simple and convenient.
Owner:TIANJIN MODERN VOCATIONAL TECH COLLEGE
Preparation method of ceftaroline fosamil intermediate parent nucleus
ActiveCN104910185AOrganic chemistryBulk chemical productionTrifluoromethanesulfonic anhydrideSulfonyl chloride
The invention provides a preparation method of ceftaroline fosamil intermediate parent nucleus, which comprises the following steps: 3-hydroxycephem and an activation reagent are reacted under existence condition of an acid binding agent and an organic solvent to obtain an activated intermediate; the activation reagent comprises p-toluene sulfonyl chloride, benzenesulfonyl choride, 4-nitrobenzene sulfonyl chloride, trifluoroactic anhydride or trifluoromethanesulfonic anhydride; the activated intermediate and 4-(4-pyridyl)-2-mercaptothiazole are reacted, then is reacted to a quaternized reagent to obtain pyridinium salt; and then deprotection is carried out to obtain the ceftaroline fosamil intermediate parent nucleus. Compared with the prior art, The p-toluene sulfonyl chloride, benzenesulfonyl choride, 4-nitrobenzene sulfonyl chloride, trifluoroactic anhydride or trifluoromethanesulfonic anhydride are used for substituting the usage of a severe toxicity material, reaction security is increased, due to increasing steric hindrance of an activating group, rate for generating a delta-3 isomer is reduced, and purity and yield of the reaction products are increased.
Owner:国药集团致君(苏州)制药有限公司
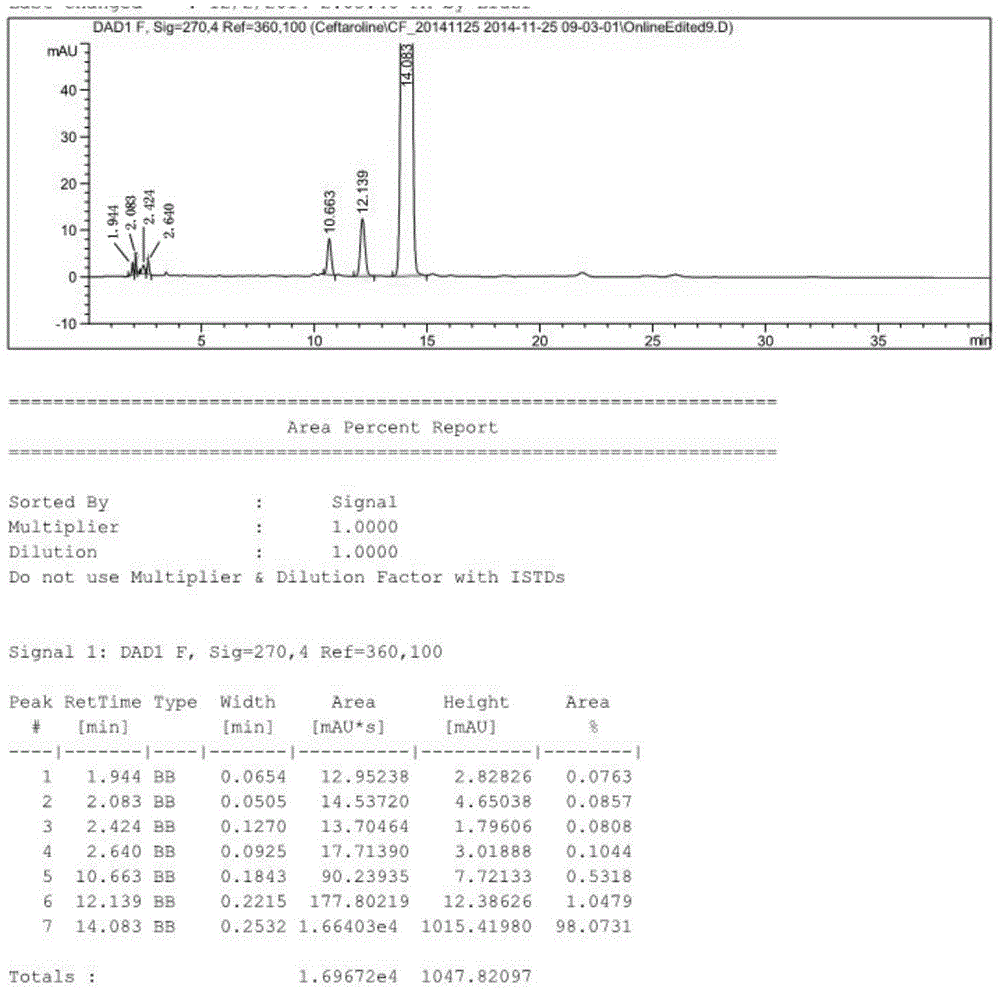
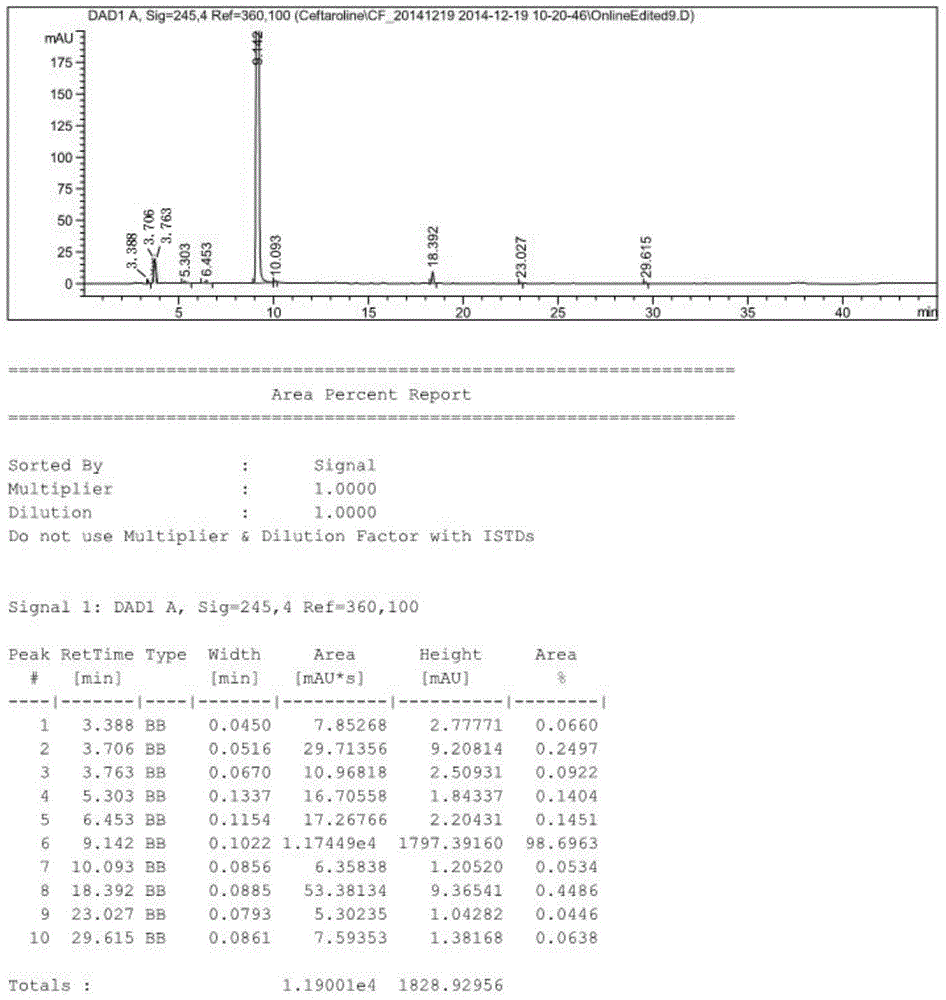
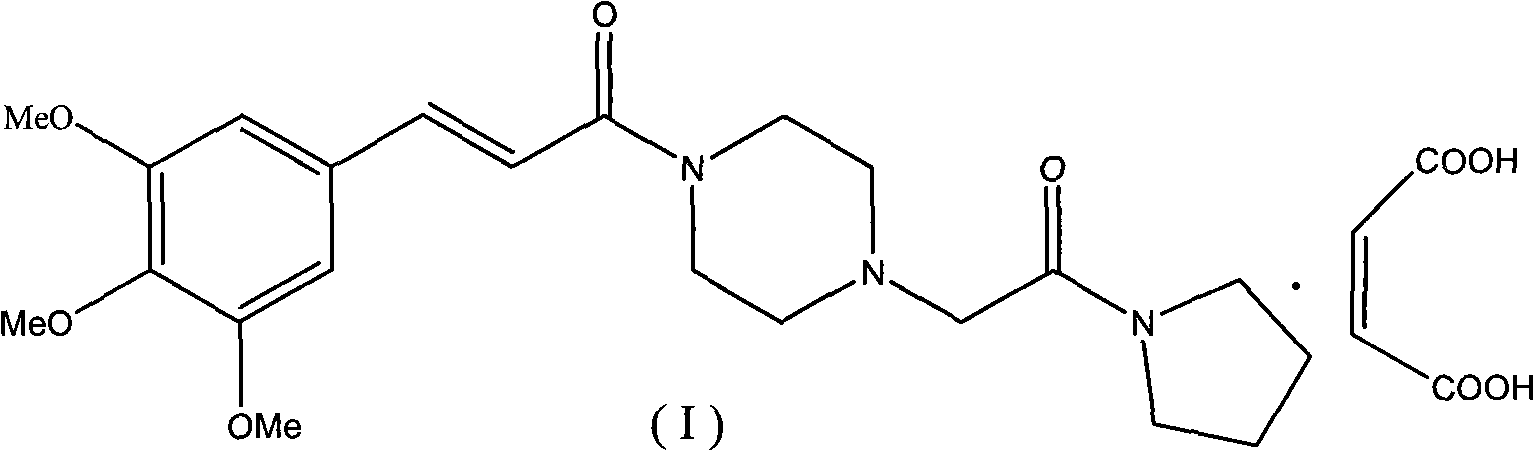
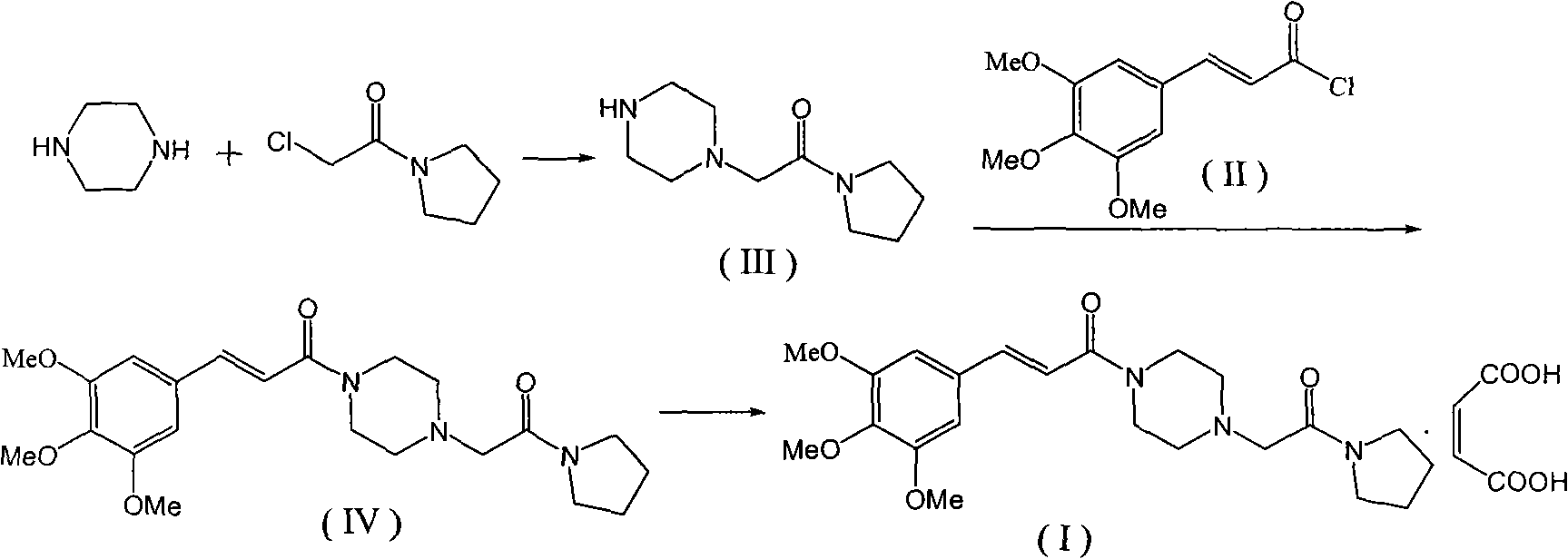
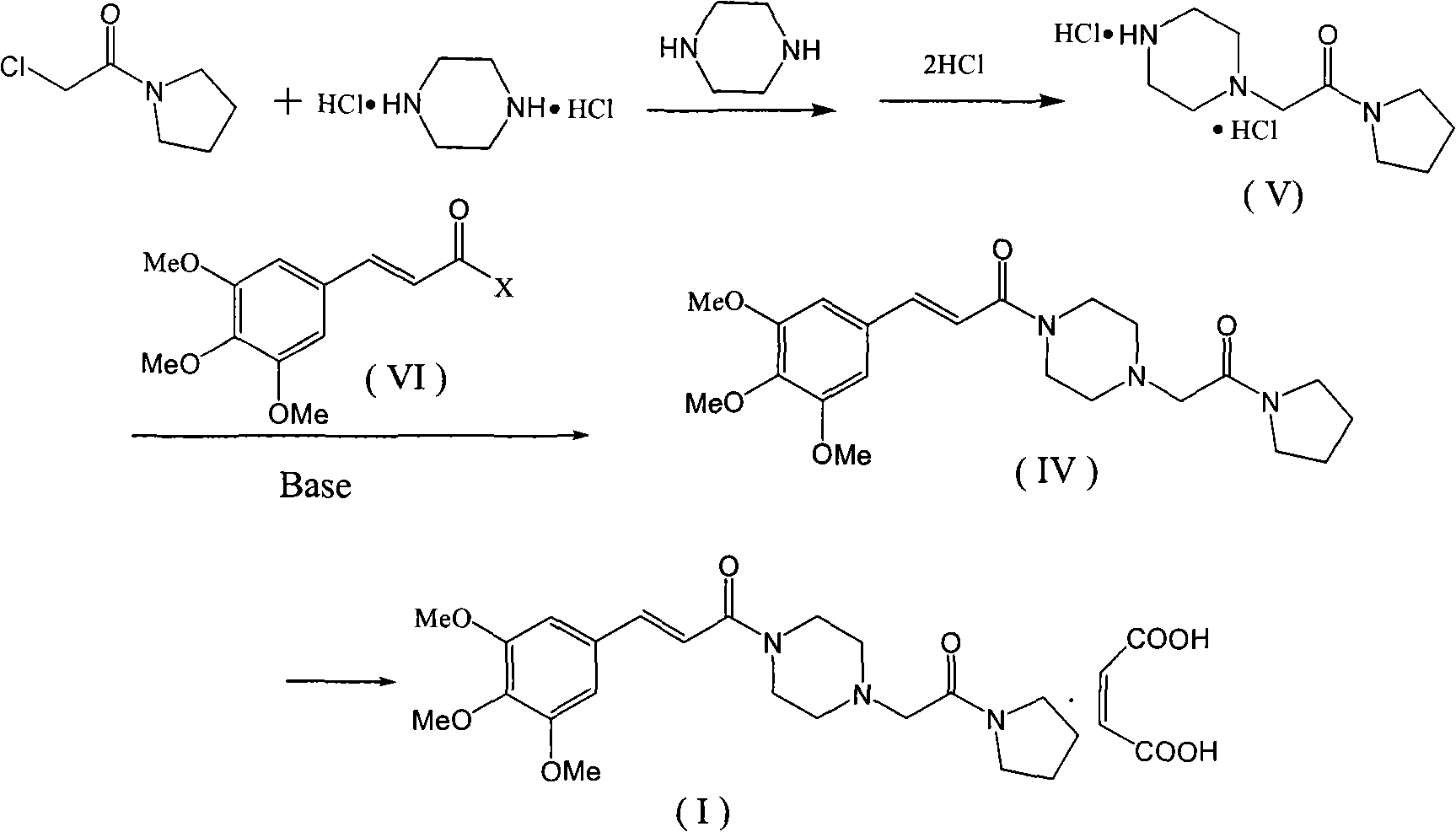
Method for preparing cinepazide maleate
InactiveCN101492431AGroup 5/15 element organic compoundsSulfonic acid preparationDiethyl phosphateMethyl group
The invention relates to a preparation method of cinepazide maleate, comprising the following steps: 3, 4, 5-trimethoxycinnamylic acid reacts with chlorinating agent, chloro diethyl phosphate and benzenesulfonyl chloride for preparing corresponding acyl active matters of the 3, 4, 5-trimethoxycinnamylic acid. The acyl active matters react with 1-[(1-pyrrolidine carbonyl) methyl] piperazine double hydrochloride for preparing 1-[(1-pyrrolidine carbonyl) methyl]-4-(3, 4, 5-trimethoxycinnamylic acid acyl) piperazine which is separated and forms salt with maleic acid for preparing cinepazide maleate or can be directly used in ethanol or acetone solution for forming salt with maleic acid. And after the crystallization, the cinepazide maleate with high melting point and stable crystal form is prepared. Chloroacetyl pyrrole perimidine reacts with mixture of piperazine and double hydrochloride of the piperazine with the ratio of 1 to 1 in lower alcohol, and then the chlorine hydride is pumped in to obtain the 1-[(1-pyrrolidine carbonyl) methyl] piperazine double hydrochloride. The process has the advantages of simple operation and high yield.
Owner:SHIJIANGZHUANG ZHIHENG PHARMACY TECH CO LTD
Surface modification method of magnetic iron oxide nano-particles
InactiveCN102552945AGood biocompatibilityUniform sizeEmulsion deliveryIn-vivo testing preparationsNano compositesBiocompatibility Testing
The invention relates to a surface modification method of magnetic iron oxide nano-particles. The method comprises the following steps: leading mixed solution consisting of iron acetylacetonate and triethylene glycol to react for a period at low and constant temperature, quickly heating to boil, and cooling to room temperature after the mixed solution reacts for a period to obtain a reaction solution; precipitating, magnetically separating and cleaning the reaction solution to obtain magnetic Fe3O4 nano-particles carrying hydroxyl on the surface; dispersing to dried toluene by ultrasonic, adding 2-(4-benzene sulfonyl chloride) trichlorosilane to obtain Fe3O4 nano-particles carrying a specific initiation group; and dispersing to mixed solution of water and ethanol by ultrasonic, and adding2,2'-bipyridyl, copper chloride, cuprous chloride and 2-(meth)acryloyloxyethyl phosphorylcholine to obtain phosphorylcholine polymer modified magnetic Fe3O4 nano-particles. The method is mild in reaction conditions, simple and feasible and strong in controllability; and the prepared magnetic Fe3O4-phosphorylcholine polymer nano-composite material has excellent stability and biocompatibility in aqueous solution.
Owner:QINGDAO UNIV OF SCI & TECH
Preparation method of chloromethyl styrene resin
InactiveCN103709278AReduce manufacturing costMaintain personal safetySulfonyl chloridePolymer science
The invention discloses a preparation method of chloromethyl styrene resin. The preparation method is as follows: first reacting a long chain diol with trioxymethylene in the presence of dry hydrogen chloride, thionyl chloride or benzene sulfonyl chloride for preparing long chain chloromethyl ether; performing chloromethylation reaction of the obtained long chain chloromethyl ether and styrene resin in conditions of aluminum chloride, stannous chloride, concentrated sulfuric acid or titanium tetrachloride catalysis, filtering an obtained reaction liquid to obtain resin balls, and washing with a mixed solution to remove a surface catalyst to obtain the chloromethyl polystyrene resin. The preparation method of the chloromethyl styrene resin has the advantages of environmental protection and no pollution, at the same time, the used long chain diol also can be recycled, so that the preparation method has the characteristic of low production cost.
Owner:SHANGHAI INST OF TECH
Preparation method of 2,4-disubstituted benzenesulfonyl chloride
InactiveCN108084062ASuppress generationAvoid overdoseSulfonic acid preparationChlorosulfuric acidSolvent free
Owner:TIANJIN RUILING CHEM CO LTD
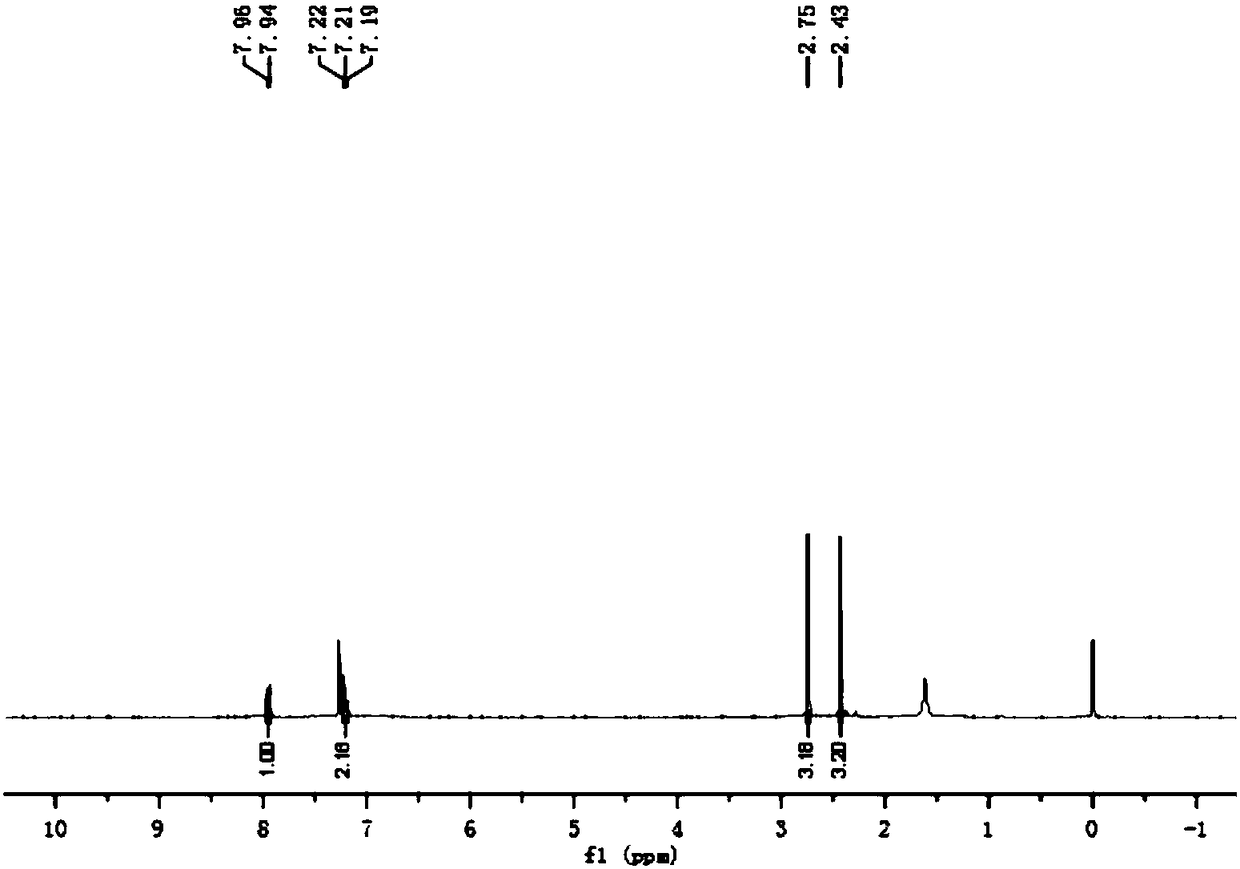
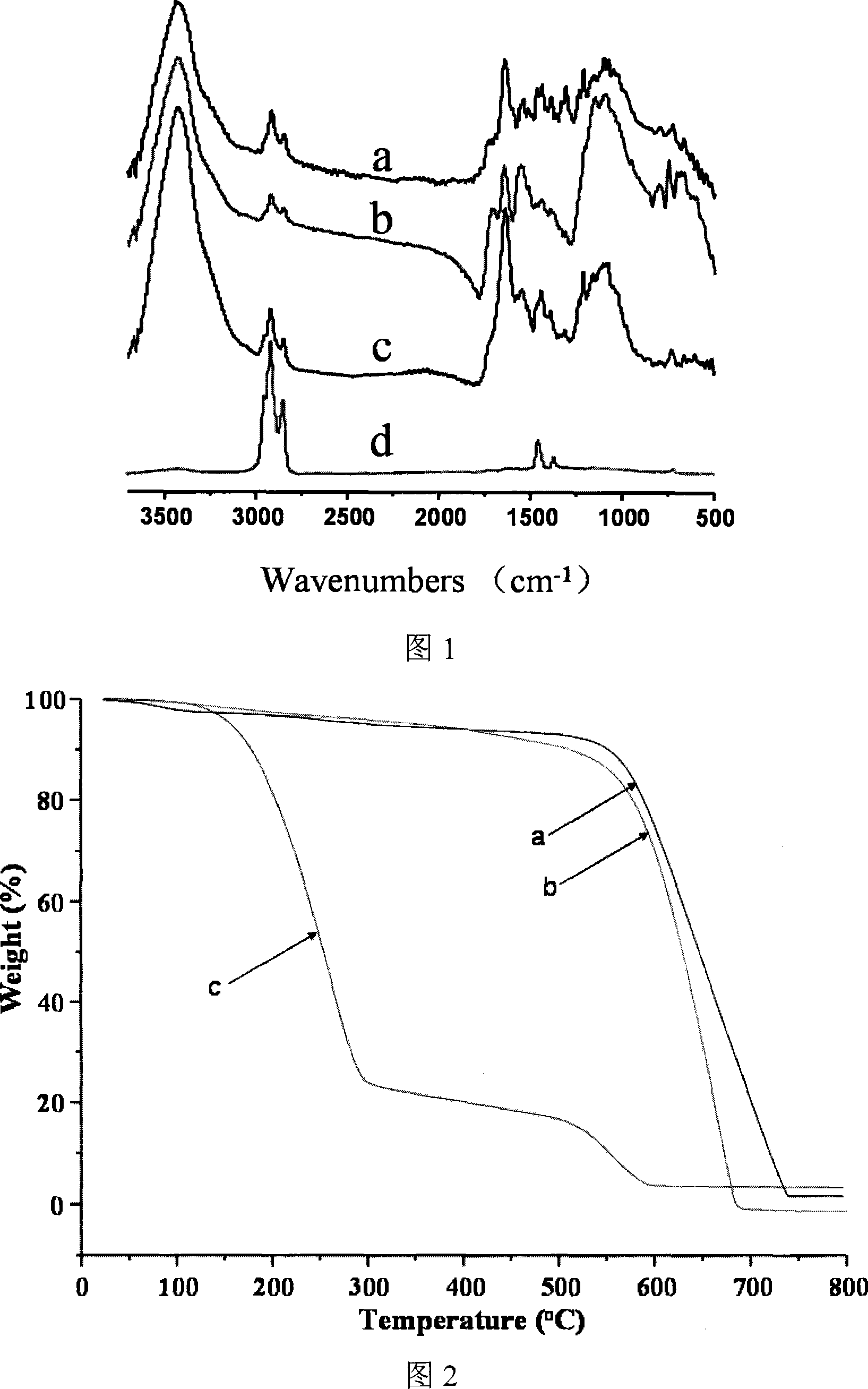
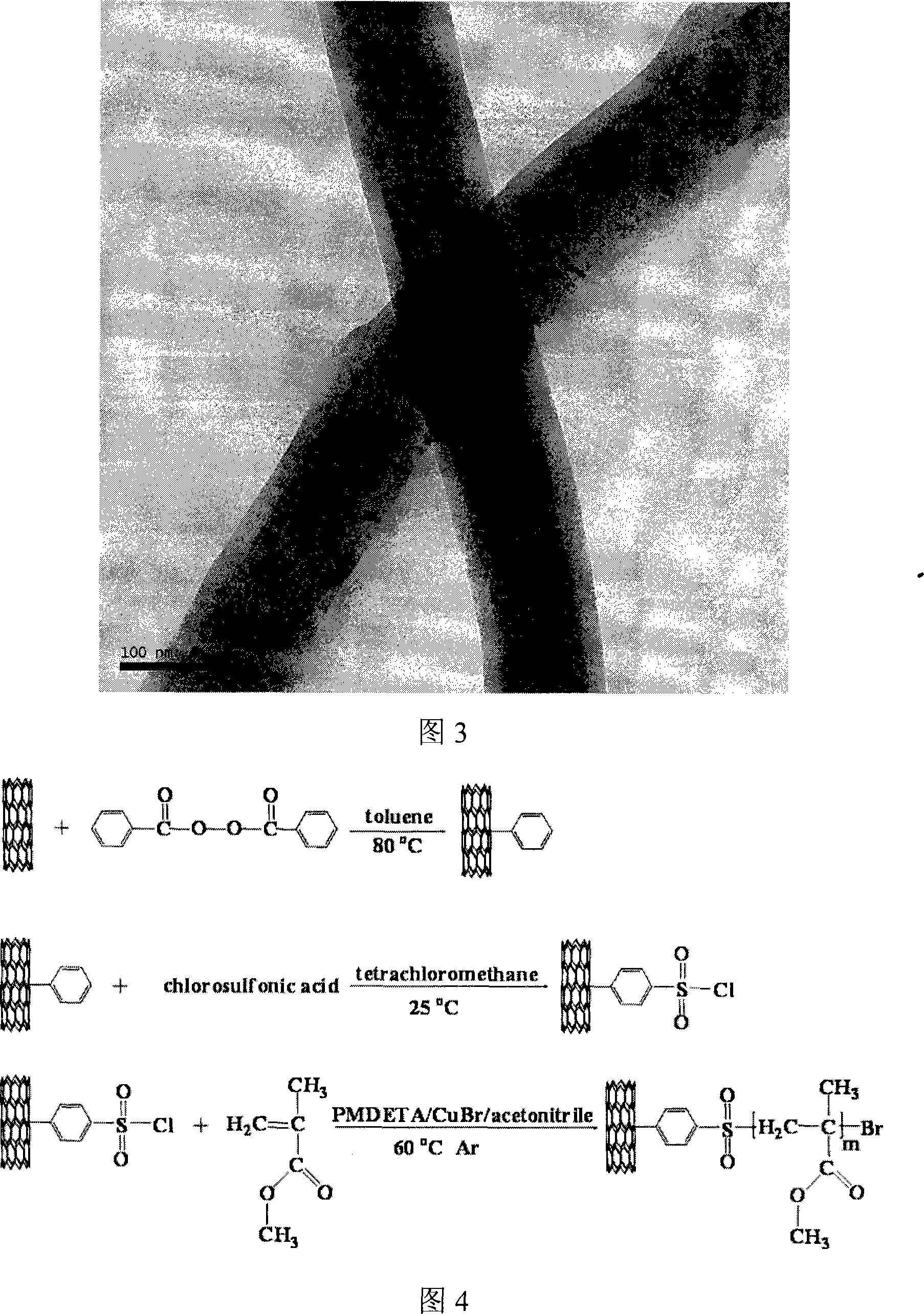
Preparation method of benzene sulfochloride substituted carbon nano-tube and grafting modification method initiated thereby
The invention relates to a method for processing a carbon nanometer tube so that a special initiation functional group can be formed on the surface, which is utilized to initiate and form graft decoration on the tube surface. The method provided by this invention is that purification treatment is carried out on the carbon tube, the attached catalyst and other impurities on the carbon tube surface, then the purified carbon tube is prepared into a benzene-substituted carbon tube, then substitution reaction for a benzene ring of the benzene-substituted carbon tube is carried out, thus producing benzene sulfonyl chloride-substituted carbon tube. In the invention, the carbon tube used for preparing the benzene sulfonyl chloride substitution can adopt free radical addition reaction.
Owner:LANZHOU UNIVERSITY
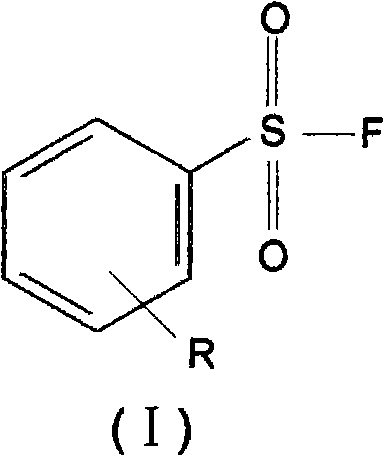
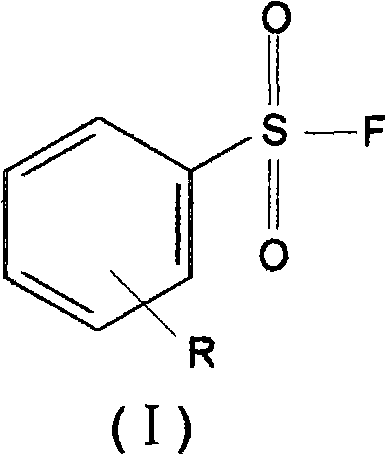
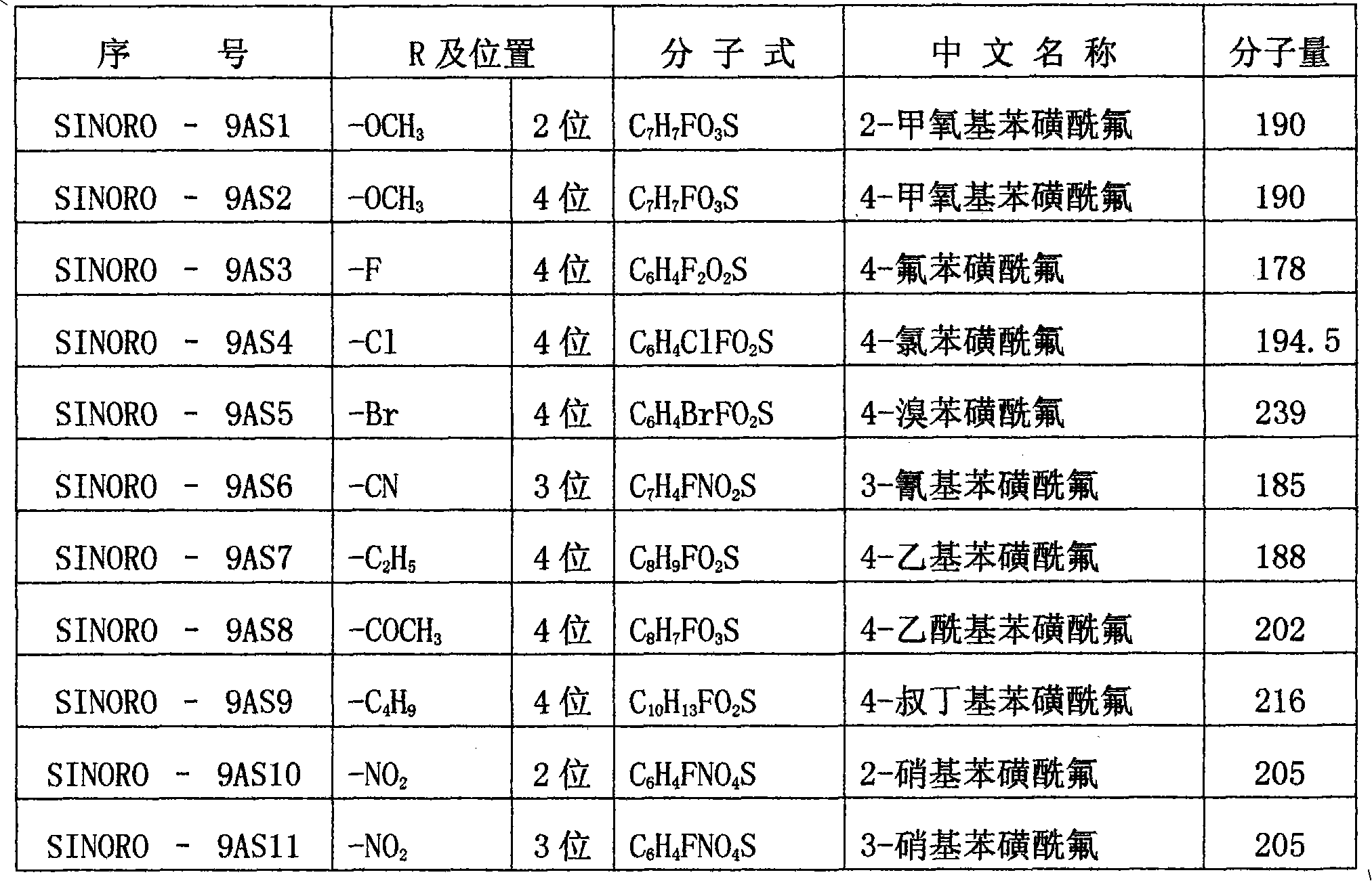
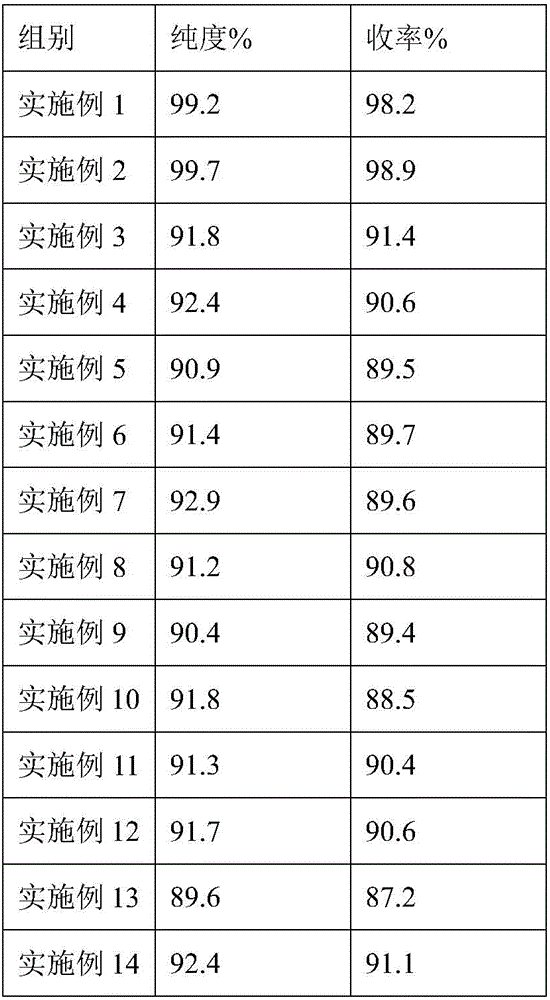
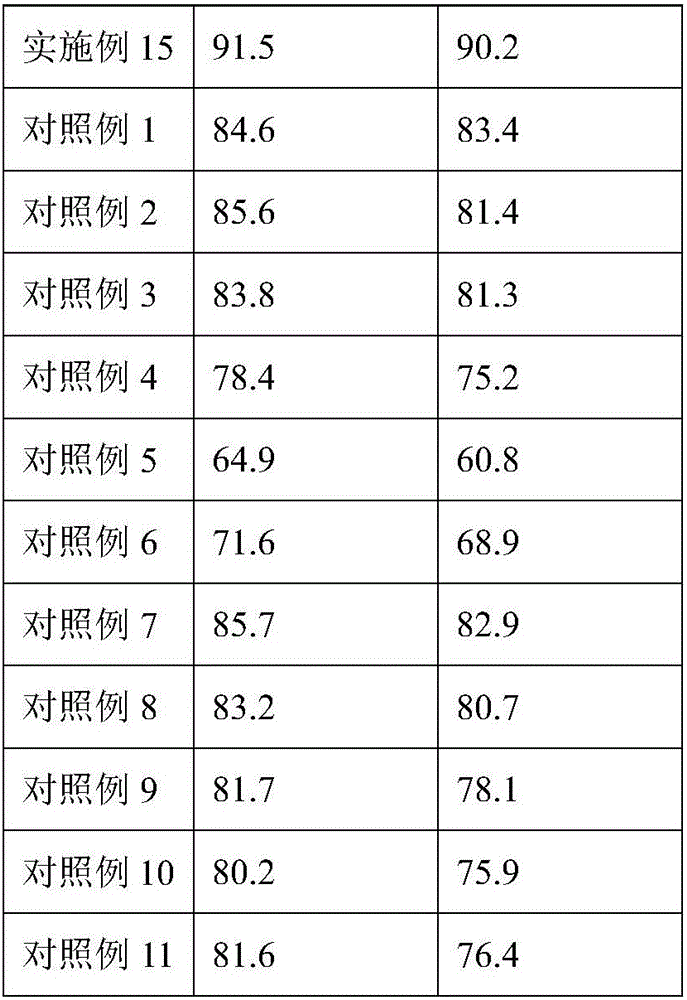
Benzenesulphonyl fluoride, preparing method and application thereof
InactiveCN101585787ASmall molecular weightImprove permeabilityPeptide preparation methodsBiological testingDiseaseBenzenesulfonyl chloride
The present invention relates to benzenesulphonyl fluoride, a preparing method and an application thereof. The invention relates to the compound with the general formula of (I), wherein R is alkyl, alkoxyl, acyl, carboxyl, cyano-group, nitryl, halogen or the combination of the groups. The compound is prepared through executing fluorination in the ethane nitrile while the precursor compound of benzene sulfochloride and the metal fluoride are used as raw materials. The new kind of benzenesulphonyl fluoride which is prepared thorugh using the sulfuryl fluoride (-SO2F) group as the reacting group, the parent benzene ring as a chromosphere and the R as the auxiliary substituent group not only can be used for measuring the protein, the peptide structure and the amino acid and researching the protein or the protemics, but also can be used for developing the new medicine or used for diagnosing the difficult and complicated disease in the medical health agency. Furthermore the benzenesulphonyl fluoride of the invention can also be used as a marking agent for quality monitoring by the national medicine checking agency and the quality supervision department.
Owner:李寿椿
Production technology of sulfanilamide intermediate acylamino benzenesulfonyl chloride
InactiveCN109796376AQuick touch responseReduce consumptionSulfonic acid preparationChemical synthesisSulfanilamide
The invention belongs to the technical field of chemical synthesis and particularly relates to a production technology of a sulfanilamide intermediate acylamino benzenesulfonyl chloride. According tothe production technology, a new solvent is introduced for dissolving a raw material A, the application amount of a raw material B chlorosulfonic acid is reduced, so that the raw material B and the raw material A are subjected to a complete reaction, and the acylamino benzenesulfonyl chloride is prepared. According to the production technology, after chlorosulfonation, water does not need to be used for decomposing chlorosulfonic acid, so that the acylamino benzenesulfonyl chloride is separated out just through a small amount of water, the environment protection problem caused by waste acid inthe sulfanilamide product production process is reduced, waste of the raw material B is also reduced, the production process and equipment input are reduced, and the aim of improving the economic benefit is achieved; a micro-reactor or a pipe reactor or a high-rotation-speed stirring-type reaction kettle is used as a reactor, so that the raw material A and the raw material B are in sufficient contact, a sufficient reaction is achieved, contact of the chlorosulfonic acid and water generated through a reaction is reduced, the application amount of the chlorosulfonic acid is controlled, the waste acid amount is greatly reduced, and the environment-friendly and economical technical effect is achieved.
Owner:顾一涛 +2
Technology for synthesizing glycol dibenzenesulfonate from nano-solid alkali catalyst
InactiveCN106588705AGood purity yieldEasy accessSulfonic acid esters preparationChemical recyclingSulfonyl chlorideIce water
The invention discloses a technology for synthesizing glycol dibenzenesulfonate from a nano-solid alkali catalyst. Glycol dibenzenesulfonate is prepared from raw materials including glycol, pyridine, benzene sulfonyl chloride, the home-made caesium modified nano-scale Ti / Al composite solid alkali catalyst and the like through operating means including ice-water bath, reduced-pressure distillation, magnetic stirring and the like.
Owner:盐城市胜达化工有限公司
2-[(4-dodecyloxy phenyl) sulfuryl] butyric acid synthetic method
ActiveCN104402782AHigh synthesis efficiencyReduce manufacturing costOrganic chemistryOrganic compound preparationSodium hydroxideSulfinic acid
The present invention discloses a 2-[(4-dodecyloxy phenyl) sulfuryl] butyric acid synthetic method which comprises the following steps: 1), reflux reaction of phenol, halogenated dodecane, an inorganic acid binding agent and an inorganic salt in organic solvent I to obtain dodecyl benzene ether; 2), reaction of the dodecyl benzene ether, sodium chloride and chlorosulfonic acid in dichloromethane to obtain 4-dodecyloxy benzene sulfonyl chloride; 3), reduction reaction of the 4-dodecyloxy benzene sulfonyl chloride, a reducing agent and a pH regulator in a mixture of water and organic solvent II to obtain 4-dodecyloxy benzene sulfinic acid sodium salt; 4), reflux reaction of the 4-dodecyloxy benzene sulfinic acid sodium salt, halogenated butyric acid methyl ester and a catalyst in methanol, then filtration of reaction liquid to obtain filtrate; 5), mixing of the filtrate and an aqueous sodium hydroxide solution for reaction to finally obtain 2-[(4-dodecyloxy phenyl) sulfuryl] butyric acid.
Owner:江西扬帆新材料有限公司
Pentaerythrityl tetramizole and preparation method thereof
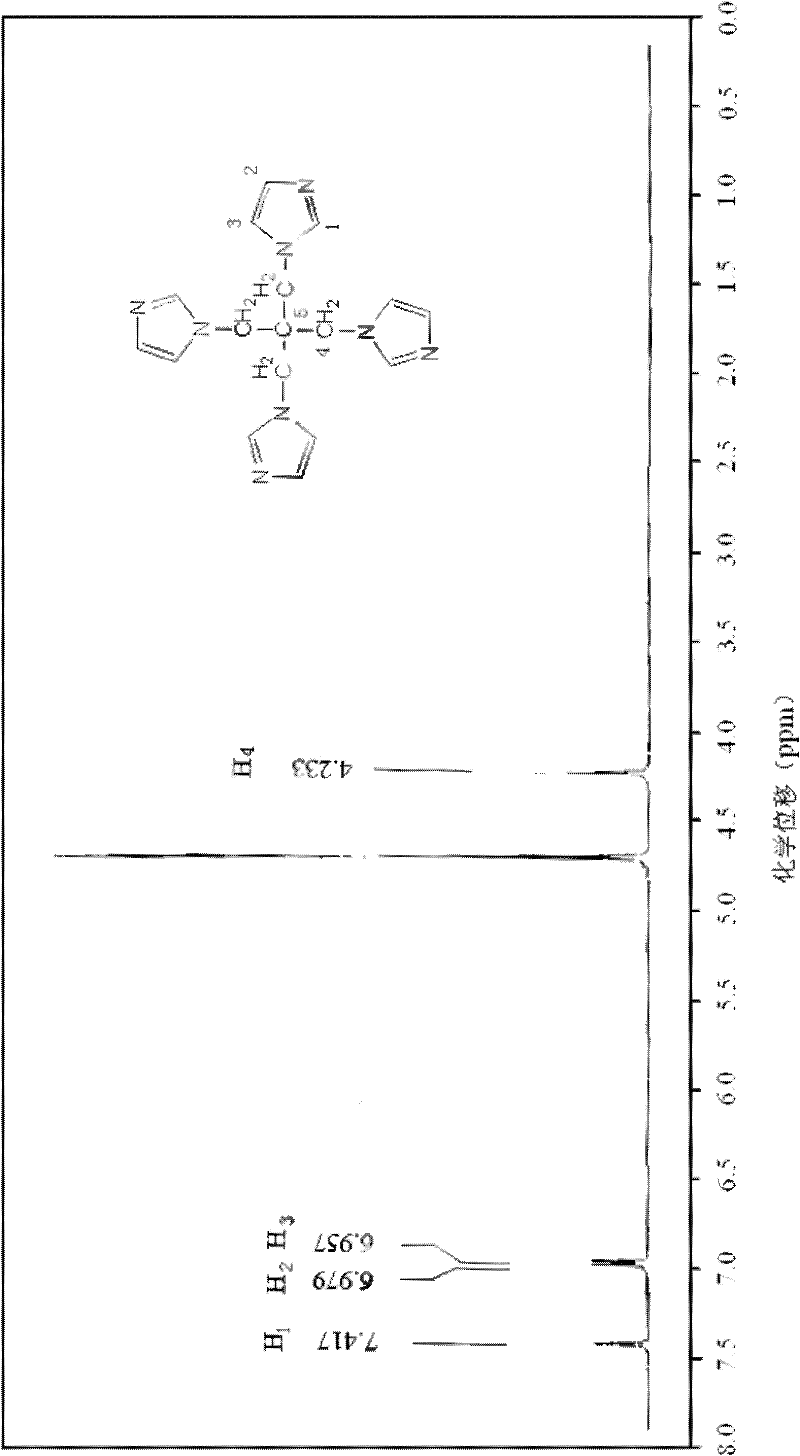
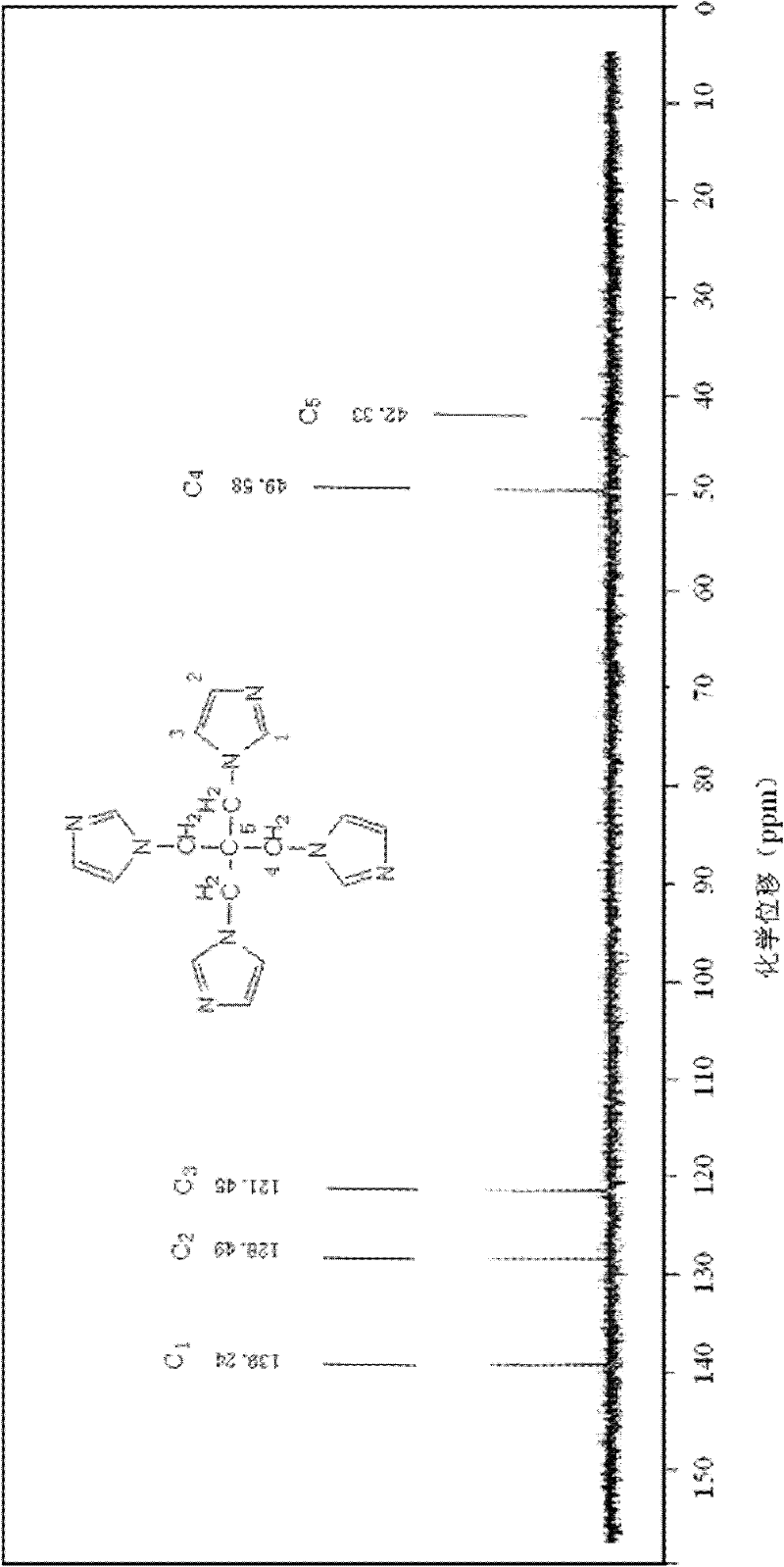
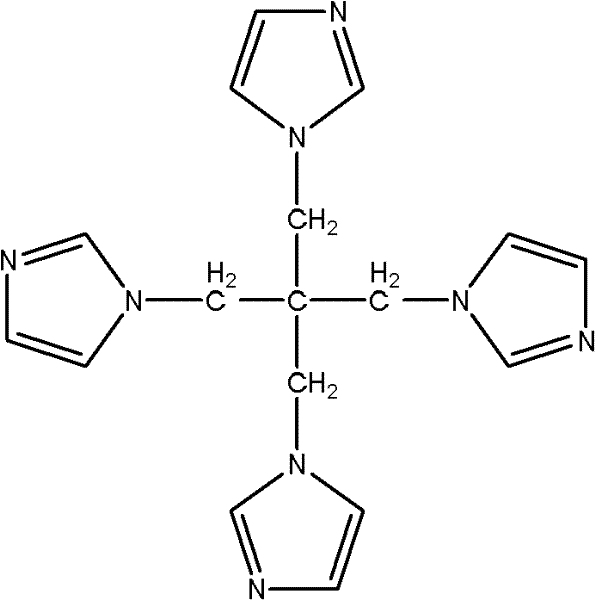
The invention provides a preparation method of pentaerythrityl tetramizole, relating to a multi-imidazolyl compound and a preparation method thereof. The invention solves the technical problem that the existing multi-imidazolyl compounds are difficult to be synthesized into tetrahedral compounds or dendritic macromolecules. The structural formula of the pentaerythrityl tetramizole is shown in the figure. The preparation method comprises the following steps: carrying out esterification reaction on pentaerythritol and benzene sulfonyl chloride to obtain pentaerythritol tetrabenzene sulfonate; carrying out reaction on the pentaerythritol tetrabenzene sulfonate and sodium bromide to obtain pentaerythrityl tetrabromide; and carrying out reaction on the pentaerythrityl tetrabromide and imidazole to obtain pentaerythrityl tetramizole. The pentaerythrityl tetramizole is a derivative of pentaerythritol. Because of the spatial tetrahedron of the pentaerythritol and the ring structure of the imidazole, the pentaerythrityl tetramizole can be used as an important intermediate for synthesizing multi-arm polymer initiators and dendritic macromolecules, and can be also used as a carrier for synthesizing multi-acid catalysts.
Owner:HARBIN INST OF TECH
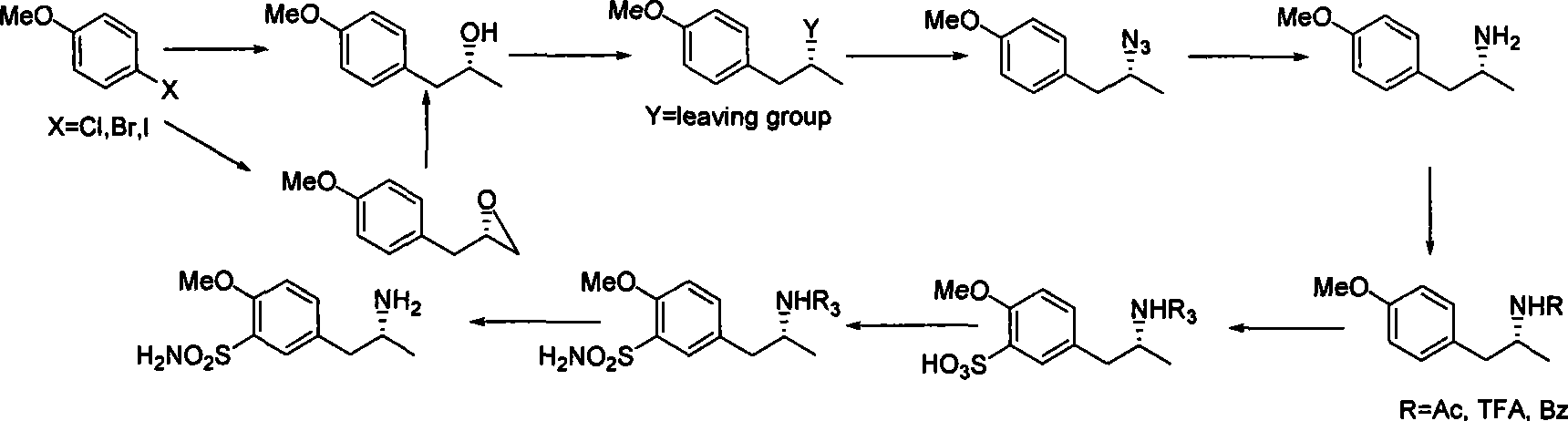
Method for synthesizing (R)-5-(2-amino propyl)-2-methoxybenzenesulfonamide
InactiveCN101462986ALow priceEasy to crystallize and purifySulfonic acid amide preparationEpoxyLeaving group
The invention relates to a method for synthesizing (R)-5-(2-aminopropyl)-2-methoxyl benzsulfamide. In the method, dihalogen anisole is used as initial raw material to react with metal. The reaction for opening ring of chiral propylene oxide or chiral epoxy chloropropane is the key reaction. Newly generated hydroxide radical reacts with substituted benzene sulfone chloride or methylsulfonyl chloride to generate leaving group to be ammonolyzed or azided and hydrolyzed or reduced into amine. (R)-5-(2-aminopropyl)-2-methoxyl benzsulfamide is prepared by protecting amido, benzene ring sulfonation, acylatation, aminolysis and deamination protection.
Owner:SHANGHAI BAILING PHARMA TECH CO LTD +1
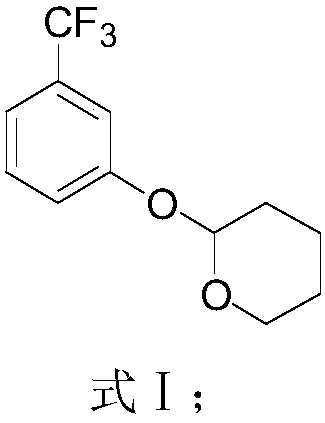
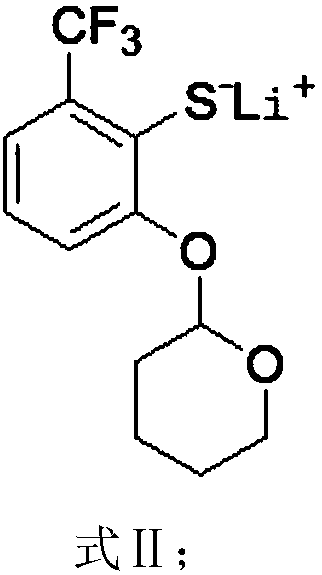
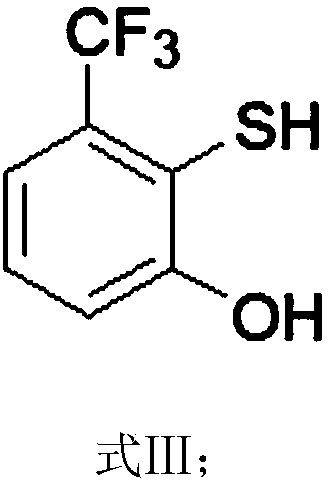
Preparation method of 2-(2,2-difluoroethyoxyl)-6-trifluoromethyl benzenesulfonyl chloride
ActiveCN108530323AEliminates foul odorHigh purityThiol preparationOrganic compound preparationIodideButyl lithium
The invention relates to the technical field of synthesis of pesticides and particularly relates to a preparation method of 2-(2,2-difluoroethyoxyl)-6-trifluoromethyl benzenesulfonyl chloride. The preparation method comprises the following steps: reacting m-trifluoromethylphenol with 3,4-dihydropyran to obtain a compound expressed as a formula (I), reacting the compound expressed as the formula (I) with n-butyl lithium and sulphur to obtain a compound expressed as a formula (II), reacting the compound expressed as the formula (II) under the action of an inorganic acid catalyst to obtain a compound expressed as a formula (III), reacting an alcoholic solution of elementary iodine or an alcoholic solution of iodide with the compound expressed as the formula (III) to obtain a compound expressed as a formula (IV), then reacting the compound expressed as the formula (IV) with carbonate and p-toluenesulfonate difluoroethanol sulfonate to obtain a compound expressed as a formula (V), and reacting the compound expressed as the formula (V) with a chlorination reagent and an oxidation reagent to obtain a final product. Through the preparation method, the generation of foul odor in the conventional synthesis process is avoided; chlorine gas with high risk is not used as the chlorination reagent; the safety in production is greatly improved.
Owner:CHANGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

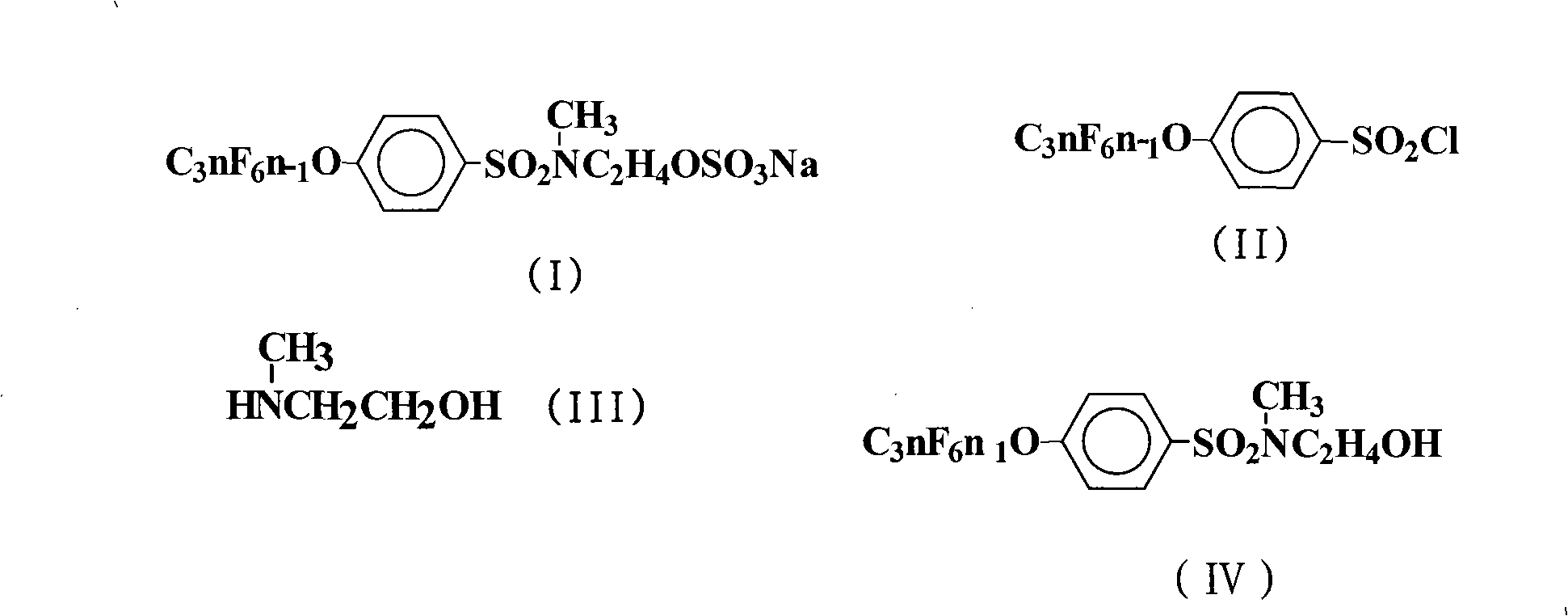
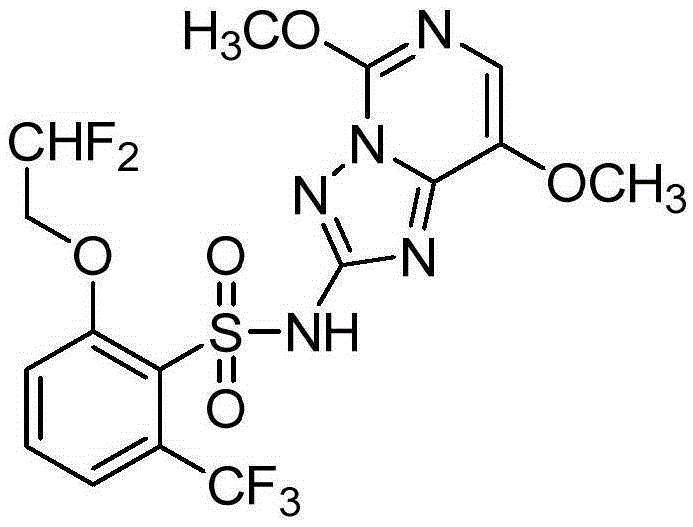
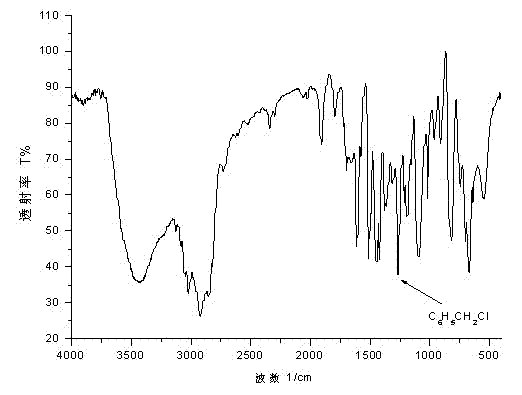
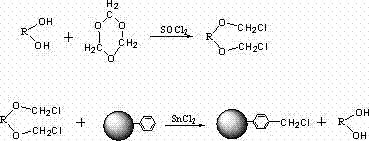
![2-[(4-dodecyloxy phenyl) sulfuryl] butyric acid synthetic method 2-[(4-dodecyloxy phenyl) sulfuryl] butyric acid synthetic method](https://images-eureka.patsnap.com/patent_img/7e4a6206-92f4-462a-bd04-f56898fd8e4f/BDA0000607409820000011.PNG)
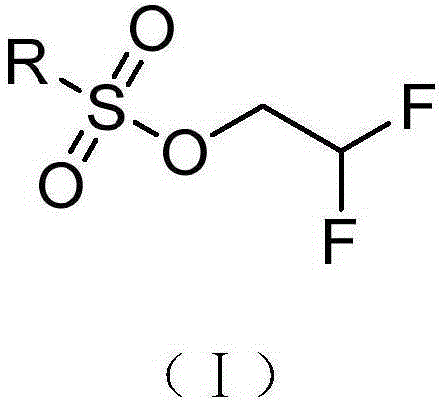
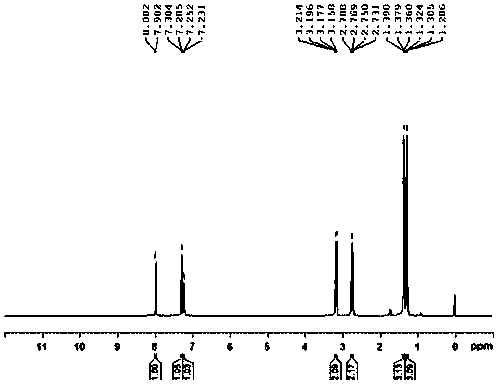
![2-[(4-dodecyloxy phenyl) sulfuryl] butyric acid synthetic method 2-[(4-dodecyloxy phenyl) sulfuryl] butyric acid synthetic method](https://images-eureka.patsnap.com/patent_img/7e4a6206-92f4-462a-bd04-f56898fd8e4f/BDA0000607409820000041.PNG)
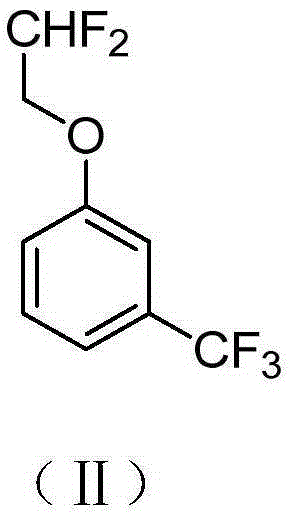
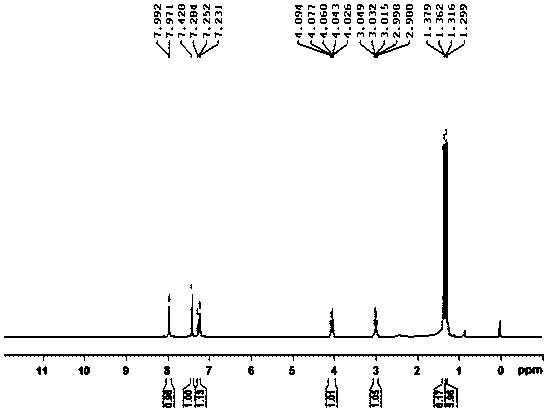
![2-[(4-dodecyloxy phenyl) sulfuryl] butyric acid synthetic method 2-[(4-dodecyloxy phenyl) sulfuryl] butyric acid synthetic method](https://images-eureka.patsnap.com/patent_img/7e4a6206-92f4-462a-bd04-f56898fd8e4f/BDA0000607409820000042.PNG)