Preparation method of 2,4-disubstituted benzenesulfonyl chloride
A technology of benzenesulfonyl chloride and disubstituted, applied in the field of compound preparation, can solve the problems of complex post-processing, many by-products, and a large amount of waste acid by-products, simplifies the difficulty of separation and purification, and solves serious side reactions and waste acid. The effect of spawn reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
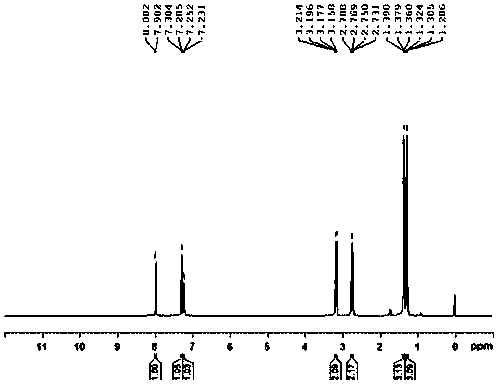
Embodiment 1
[0030] The preparation method of 2,4-diethylbenzenesulfonyl chloride, the steps are as follows:
[0031] (1) In the reactor of 50L, add m-diethylbenzene 3.35kg, add anhydrous sodium sulfate 0.05kg under stirring, ice-water bath control reaction temperature is 10 ℃, slowly drips chlorosulfonic acid 3.25kg from dropping funnel, After the addition was completed, the reaction was incubated at 10°C for 0.5 hours;
[0032] (2) Control the reaction temperature to be 10°C, slowly add 6kg of chlorosulfonic acid dropwise from the dropping funnel, after the addition is complete, add 0.75kg of thionyl chloride, and incubate at 10°C for 5.0 hours to obtain the sulfonylated material;
[0033] (3) In a 100L glass storage tank, add 25kg of ice-water mixture, start stirring, slowly add the above-mentioned sulfonylated material into the ice-water mixture, keep it under 15°C for 0.5 hours, put the material in a separatory funnel After standing for 0.5 hours, the lower organic layer was separate...
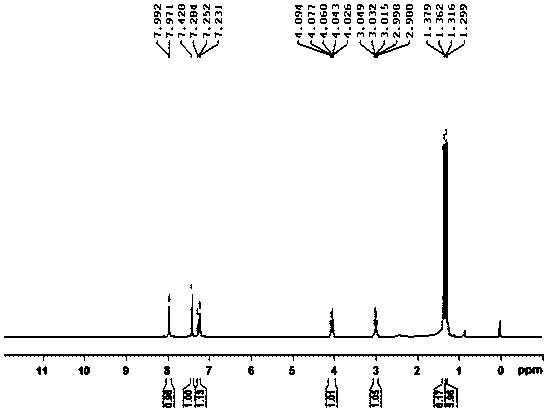
Embodiment 2
[0035] The preparation method of 2,4-diethylbenzenesulfonyl chloride, the steps are as follows:
[0036] (1) In the reactor of 50L, add m-diethylbenzene 3.35kg, add anhydrous ammonium sulfate 0.07kg under stirring, ice-water bath control reaction temperature is 5 ℃, slowly drips chlorosulfonic acid 3.35kg from dropping funnel, After the addition was completed, the reaction was incubated at 10°C for 0.5 hours;
[0037] (2) Control the reaction temperature to 15°C, slowly add 6.5kg of chlorosulfonic acid dropwise from the dropping funnel, after the addition is complete, add 0.75kg of oxalyl chloride, and incubate at 15°C for 5.0 hours to obtain the sulfonylated material;
[0038] (3) In a 100L glass storage tank, add 24 kg of ice-water mixture, start stirring, slowly add the above-mentioned sulfonylated material into the ice-water mixture, keep it under 15°C for 1.0 hour, put the material in a separatory funnel After standing for 0.5 hours, the lower organic layer was separated...
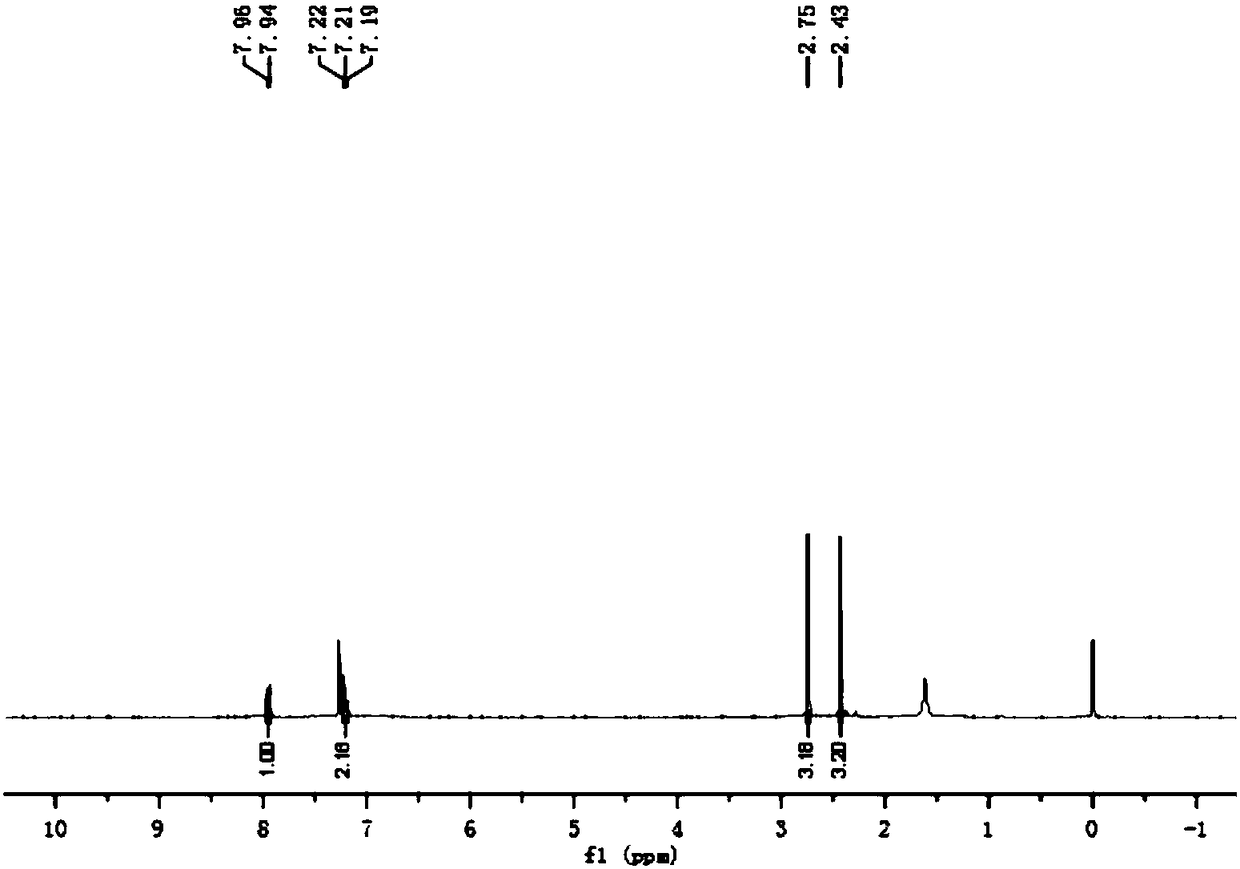
Embodiment 3
[0040] The preparation method of 2,4-dimethylbenzenesulfonyl chloride, the steps are as follows:
[0041] (1) In a 50L reaction kettle, add 2.65kg of m-xylene, add 0.05kg of anhydrous potassium sulfate under stirring, control the reaction temperature in an ice-water bath to be 20°C, slowly drop 3.25kg of chlorosulfonic acid from the dropping funnel, add After finishing, heat preservation reaction at 20°C for 0.5 hours;
[0042] (2) Control the reaction temperature to be 20°C, slowly add 6kg of chlorosulfonic acid dropwise from the dropping funnel, after the addition is complete, add 0.5kg of phosphorus trichloride, and keep the temperature at 20°C for 5.0 hours to obtain the sulfonylated material;
[0043] (3) In a 100L glass storage tank, add 25 kg of ice-water mixture, start stirring, slowly add the above-mentioned sulfonylated material into the ice-water mixture, keep it under 15°C for 1.0 hour, put the material in a separatory funnel After standing for 1.0 hour, the lower o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com