Chlorinated glaucocalyxin A derivative and preparation method and application thereof
A technology for acyl chloride blue calyx A and derivatives is applied to the preparation of acyl chloride blue calyx A derivatives, the preparation of acyl chloride blue calyx A derivatives, and the application field in the treatment of cancer, and can solve the problem of excessive elimination, GLA Small polarity, not easy to dissolve in water, etc., to achieve a good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1: the preparation of cyanine A
[0066] Using fragrant tea herbs (aerial parts) as raw materials, through the steps of extraction, solvent treatment, resin treatment and recrystallization, to prepare cyanine (GLA), the specific process is as follows:
[0067] Step 1 is an extraction step, which further includes:
[0068] Step 1.1: Take fragrant tea and vegetable medicinal materials (aerial parts) and grind them to 20-50 mesh;
[0069] Step 1.2: Mix the obtained pulverized product with 95% ethanol (A.R.) at a volume ratio of 1:5 to 1:15, heat, reflux at a temperature of 80°C to 90°C for 1 to 2 hours, extract and filter to obtain the extract liquids and residues;
[0070] Step 1.3: Mix the residue obtained in step 1.2 with 95% ethanol (A.R.) at a volume ratio of 1:5 to 1:15, heat, reflux at 80°C to 90°C for 1 to 2 hours, extract and filter, To obtain the extract and the residue, repeat this step 1 to 3 times;
[0071] Step 1.4: Combine the extracts obtaine...
Embodiment 2
[0089] Example 2: Derivatization
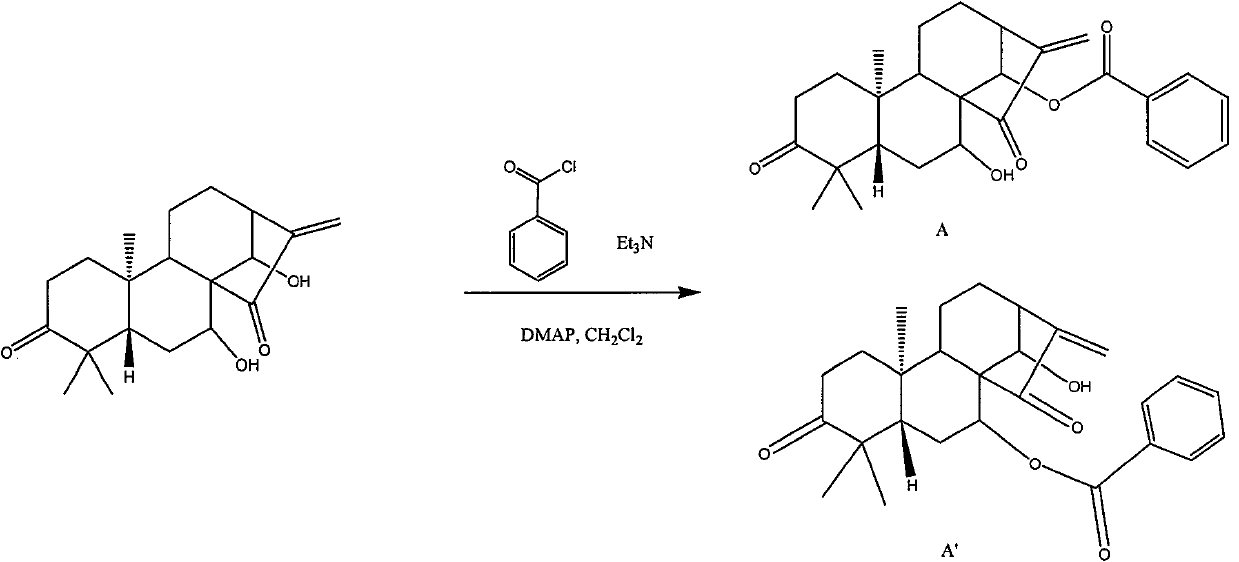
[0090] according to figure 1 In the reaction equation shown, the cyanine prepared in Example 1 is derivatized with benzoyl chloride, thereby introducing a modified substituent into the cyanine to obtain a benzoyl chloride derivative of cyanine , the derivatization step 5 further comprises:
[0091] Step 5.1: Dissolve 90-110 mg of cyanine obtained in Step 4 in 3-7 ml of dichloromethane, and slowly add 41.5-51.5 mg of benzoyl chloride, 40.6- 50.6 mg of triethylamine and 3.1-4.1 mg of DMAP were stirred and reacted at room temperature for 12 hours;
[0092] Step 5.2: add 5-15ml of water to the reaction solution in step 5.1 to dilute, then extract 2-4 times with 5-15ml of dichloromethane, combine the organic phases, wash with saturated brine, and dry over anhydrous sodium sulfate;
[0093]Step 5.3: The organic phase washed and dried in step 5.2 was heated to 35°C-40°C and concentrated under reduced pressure to evaporate the solvent, and dried a...
Embodiment 3
[0095] Example 3: Derivatization
[0096] according to image 3 As shown in the reaction equation, the cyanine A prepared in Example 1 is derivatized with benzenesulfonyl chloride, thereby introducing a modified substituent into the cyanine A to obtain the benzenesulfonyl chloride derivative of cyanine A , the derivatization step 5 further comprises:
[0097] Step 5.1: Dissolve 90-110 mg of cyanine obtained in step 4 in 3-7 ml of dichloromethane, and slowly add 106.8-126.8 mg of benzenesulfonyl chloride, 86.3- 96.3mg of triethylamine and 6.2-8.2mg of DMAP were stirred and reacted at room temperature for 12 hours;
[0098] Step 5.2: add 5-15ml of water to the reaction solution in step 5.1 to dilute, then extract 2-4 times with 5-15ml of dichloromethane, combine the organic phases, wash with saturated brine, and dry over anhydrous sodium sulfate;
[0099] Step 5.3: The organic phase washed and dried in step 5.2 was heated to 35°C-40°C and concentrated under reduced pressure t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com