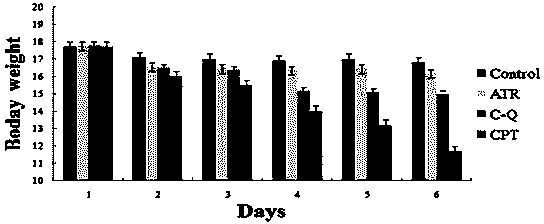Patents
Literature
364 results about "4-Dimethylaminopyridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
4-Dimethylaminopyridine (DMAP) is a derivative of pyridine with the chemical formula (CH₃)₂NC₅H₄N. This colourless solid is of interest because it is more basic than pyridine, owing to the resonance stabilisation from the NMe₂ substituent.
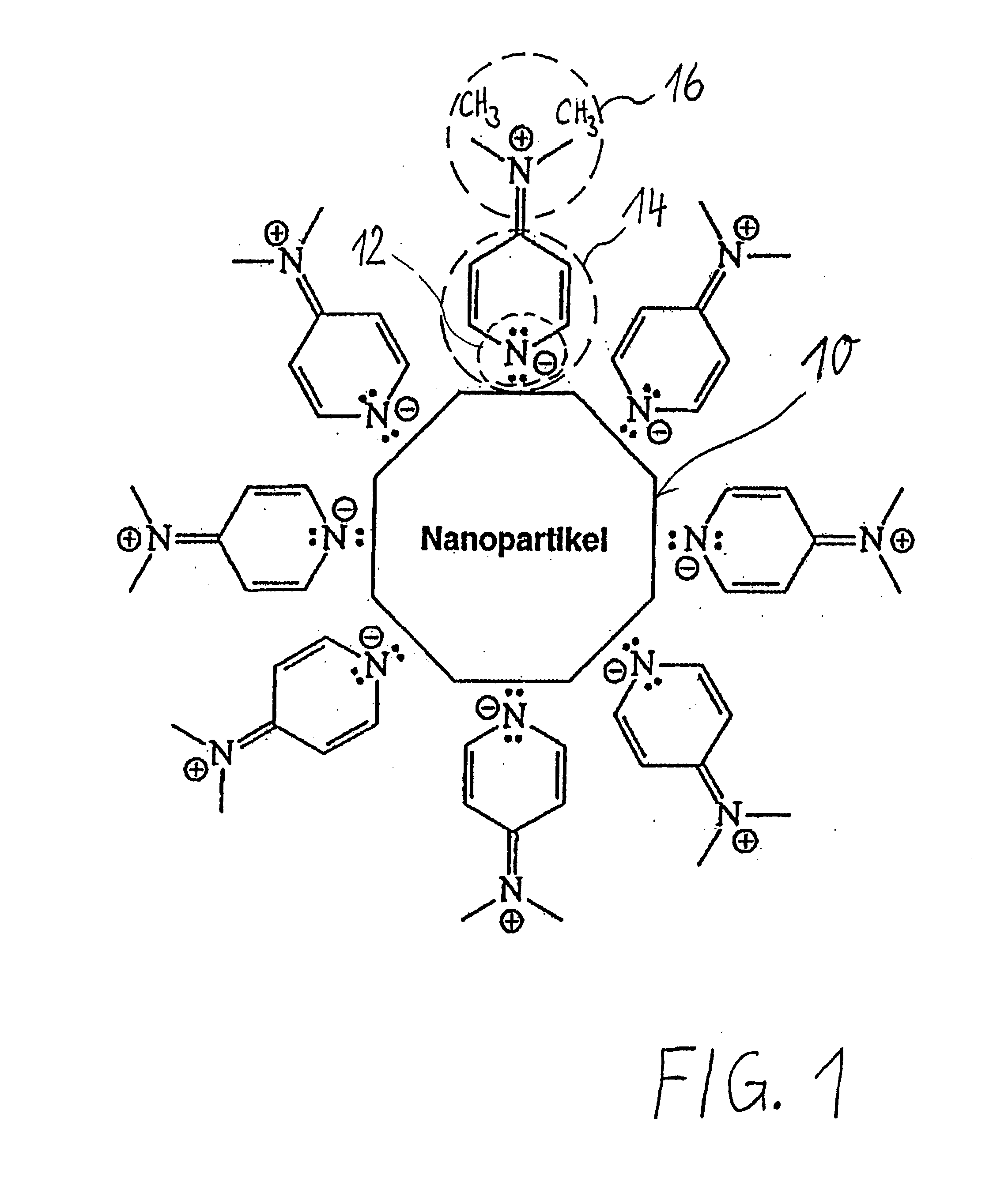
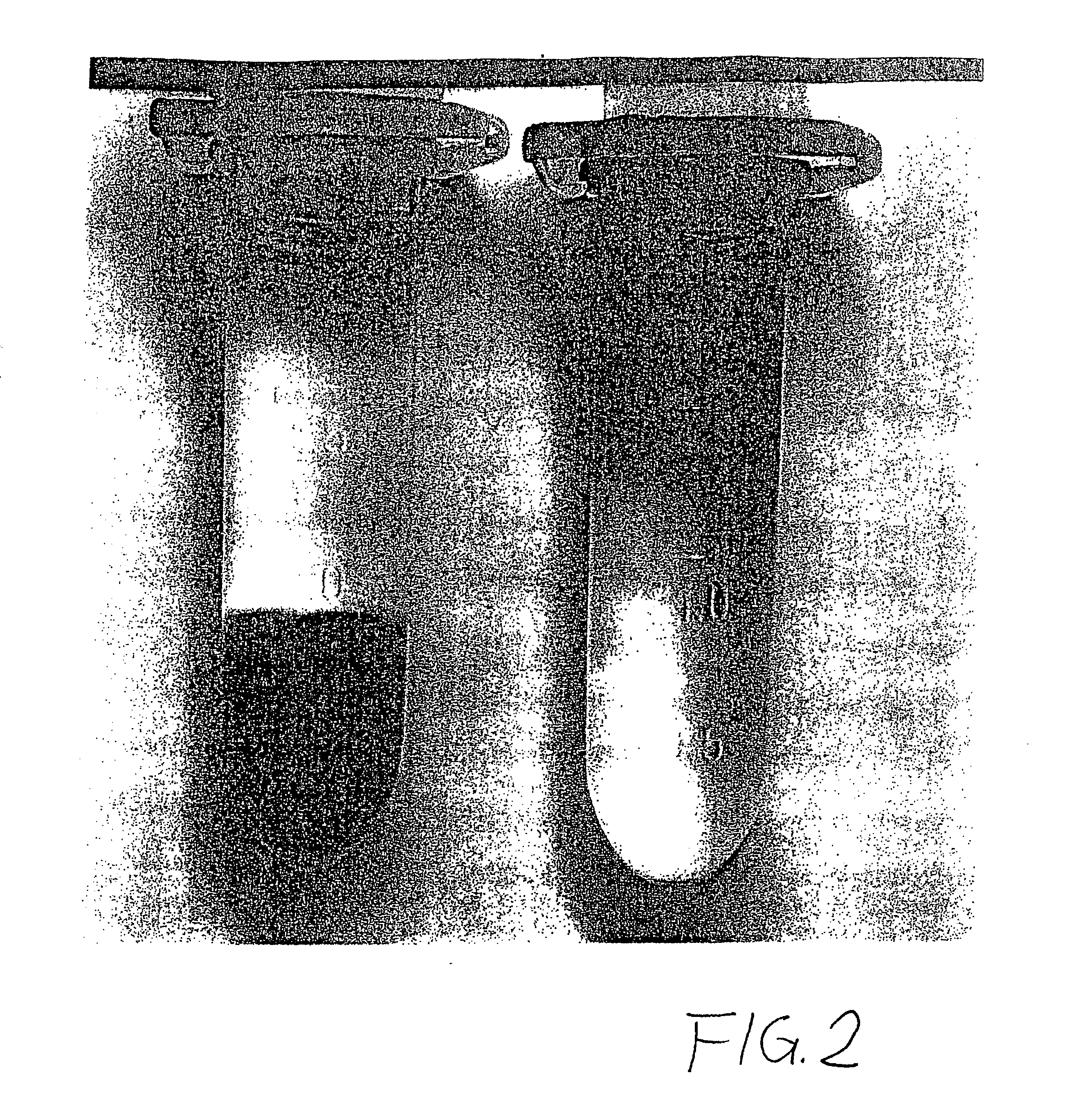
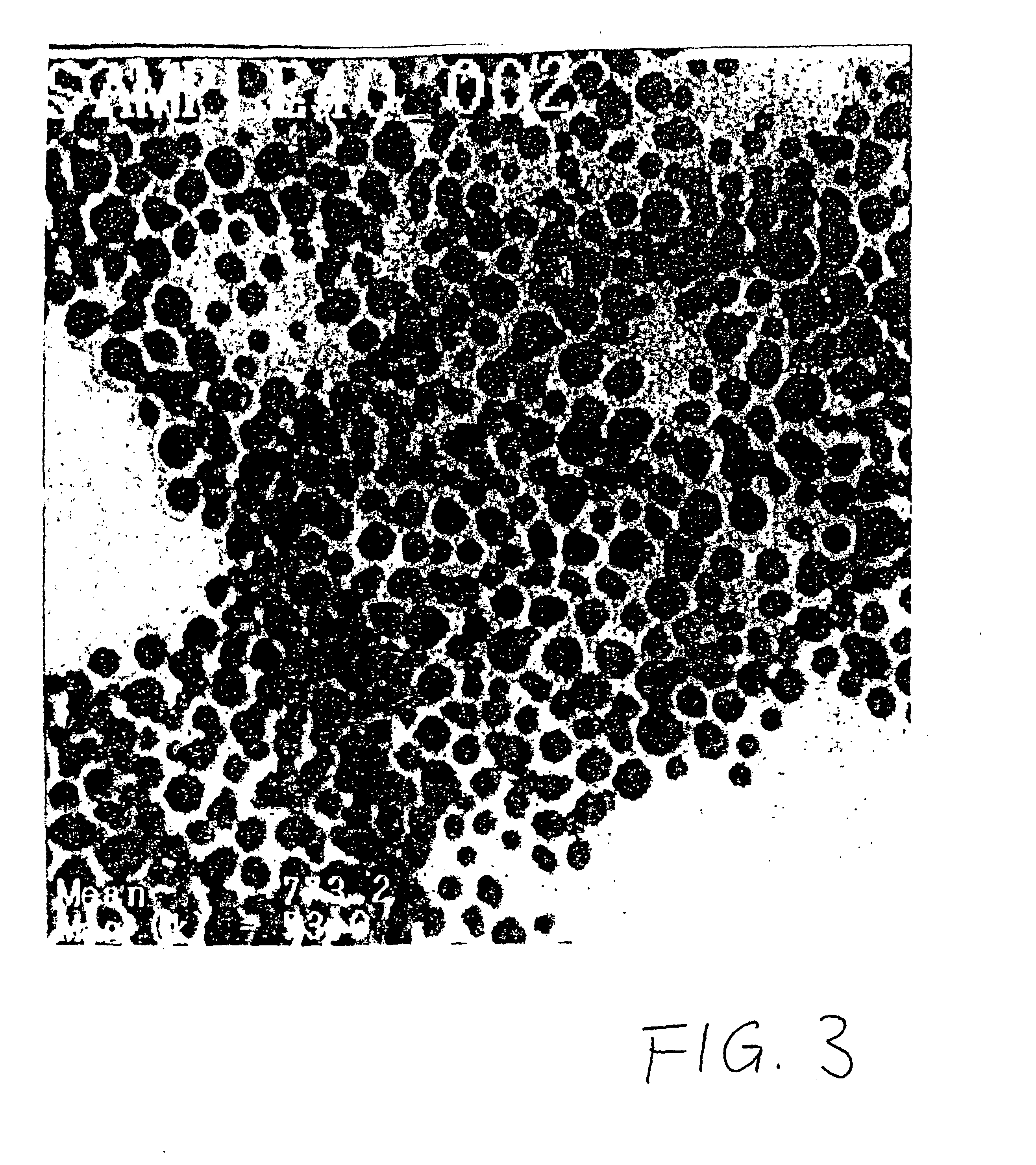
Phase transfer of nanoparticles
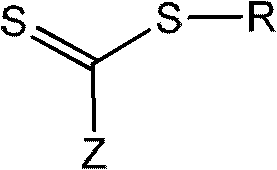
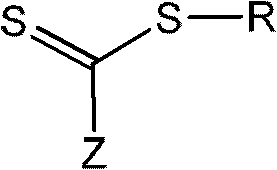
The invention relates to phase transfers of nanoparticles and to a catalysis using said nanoparticles. The aim of the invention is to facilitate a transfer of nanoparticles from an organic solution to an inorganic, especially, aqueous solution. To this end, a generically describable substance class, for example the commercially available 4-dimethylaminopyridine (DMAP), which is for example dissolved in water, is added to the organic solution in sufficient amounts. This measure has the effect that the nanoparticles are readily transferred in a one-step process from the organic phase (in each case in the top section) to the inorganic phase (in each case in the lower section) in the sample container.
Owner:CARUSO FRANK +1
Preparation method of segmented copolymer from vinylidene chloride copolymer and polyethylene glycol
The invention discloses a preparation method of a segmented copolymer from a vinylidene chloride copolymer and polyethylene glycol which comprises the steps of: reacting polyethylene glycol (PEG) and a reversible addition fragmentation transfer radical polymerization (RAFT) chain transfer reagent in dichloromethane under the action of a catalyst 4-dimethylamino pyridine to obtain a PEG macromolecular RAFT reagent; and performing RAFT polymerization on vinylidene chloride or vinylidene chloride and a comonomer in the PEG macromolecular RAFT reagent under the action of azo-bis-iso-butyrynitrile initiator and with 1,4-dioxane as a solvent to obtain a segmented copolymer from a vinylidene chloride copolymer and polyglycol with large molecular weight and narrow molecular weight distribution width. The invention provides a novel method for preparing a vinylidene chloride segmented copolymer, and the segmented copolymer composed of a vinylidene chloride copolymer and polyglycol prepared by the invention has a potential application preparation of hydrophilic modified vinylidene chloride films and vinylidene chloride polymer based mesoporous carbon.
Owner:ZHEJIANG UNIV
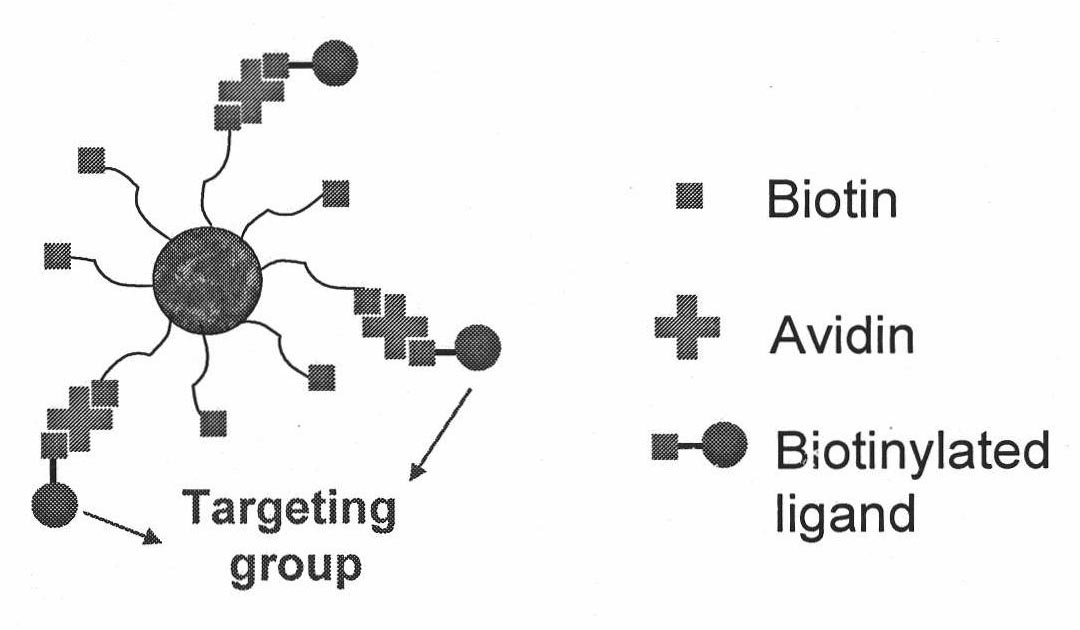
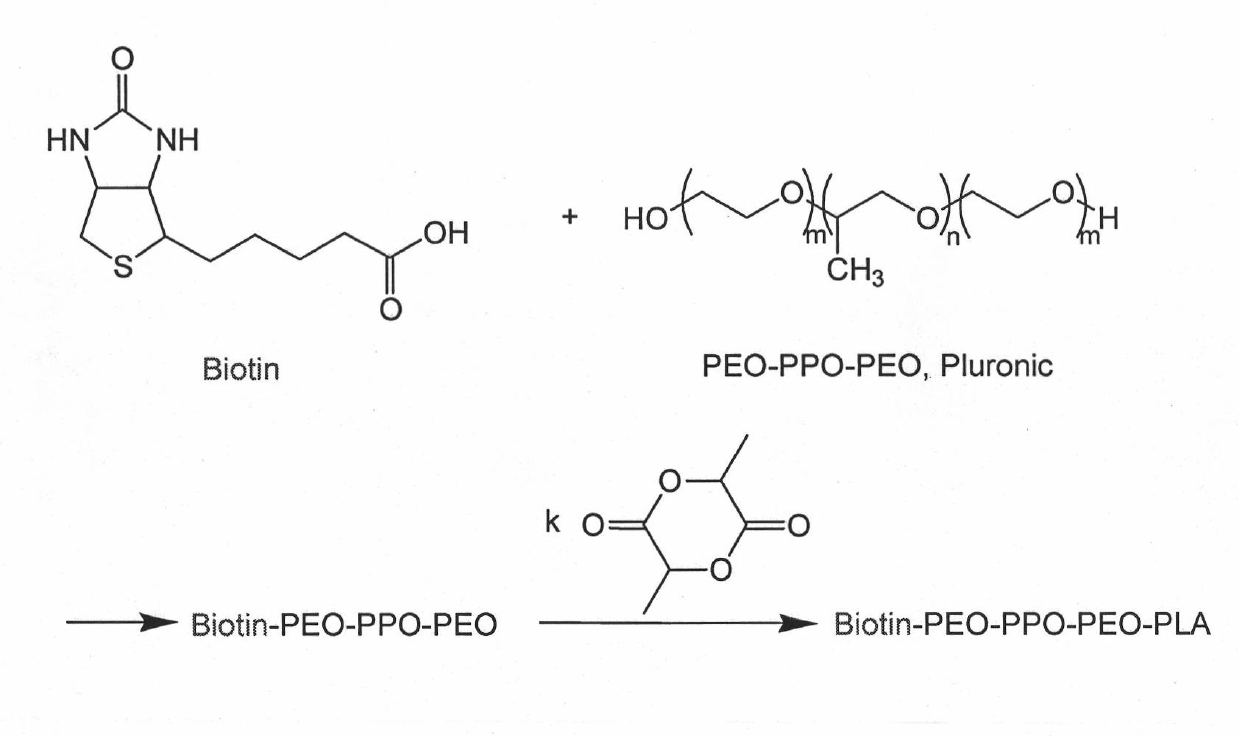
Targeted polymer medicament carrier and preparation method and application thereof
InactiveCN102000340AOvercome expensiveMultiple choicePharmaceutical non-active ingredientsIn-vivo testing preparationsWater bathsIce water
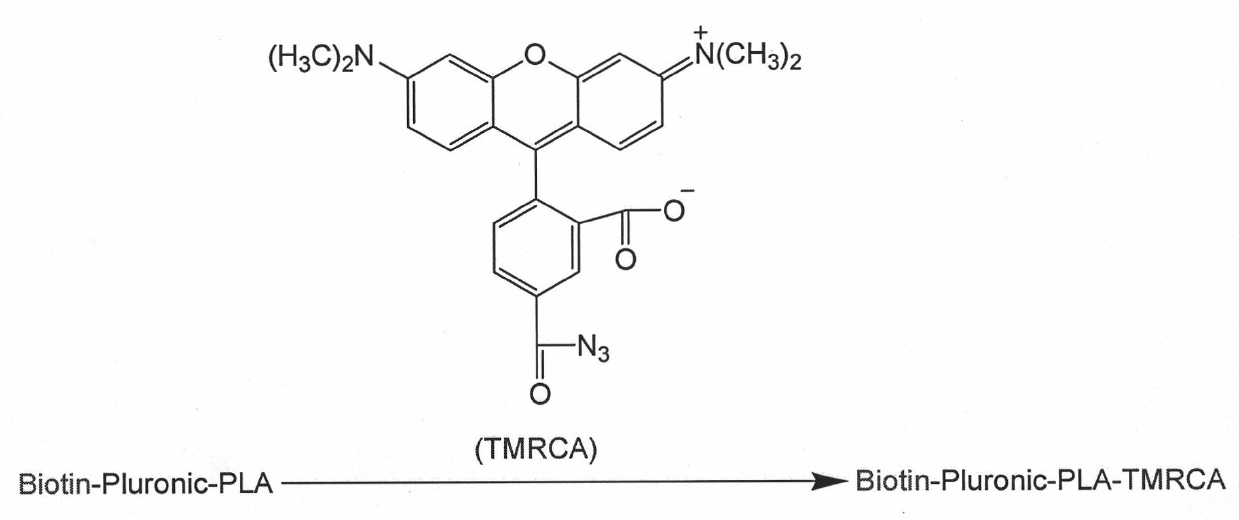
The invention relates to a targeted polymer medicament carrier and a preparation method and application thereof. The targeted polymer medicament carrier has a molecular structural formula shown in the graph. The preparation method comprises the following steps of: a) feeding Pluronic and Biotin in a molar ratio of 1:1.1-1.5; dissolving the mixture in dichloromethane; adding 4-dimethylamino pyridine; dropwise adding 1,3-dicyclohexyl carbodiimide in an ice water bath; reacting at room temperature for 24 to 48 hours; extracting reactive fluid with 10 to 15 percent NaHCO3; freezing over night; filtering to remove undissolved substances; concentrating the reactive fluid; dropping the reactive fluid into cold absolute ethyl ether; filtering, and drying in vacuum; and b) dissolving a product with dry toluene, and distilling the product in the presence of argon gas; dehydrating by an azeotropy method; cooling to the room temperature; adding lactide according to 50 to 90 percent of the weight of the product in the presence of the argon gas, and adding stannous octoate according to 0.1 to 0.15 percent of the weight of the lactide; heating to the temperature of between 120 and 140 DEG C; reacting for 6 to 8 hours under stirring; immersing a reactant into the cold ethyl ether, and filtering; and dissolving polymer by using dichloromethane, immersing into methanol, and filtering and drying. The carrier can be used as carriers of medicaments for treating and diagnosing cancers.
Owner:JIANGXI SCI & TECH NORMAL UNIV
Polyene ether compounds and preparation method thereof
The invention discloses a preparation method of polyene ether compounds. The method includes: organic small-molecule 4-dimethylamino pyridine (DMAP) is used as the catalyst, binary alkynyl compounds and binary hydroxy compounds are mixed with organic solvent, and the polyene ether compounds are obtained by using the click polymerization reaction of alkynyl-hydroxy. The polyene ether compounds comprise an inner unit indicated by formula (I). The polymerization step is showed in the formula (V), wherein n is larger than 1, and R1 and R2 are selected from organic groups. The method has the advantages that water and oxygen do not need to be removed during reaction, polymerization temperature is low, polymerization efficiency is high, and no metal residues exist in products; the prepared polyene ether compounds are high in steric regularity, good in machinability, and high in heat stability, degradability and aggregation-induced emission performance.
Owner:ZHEJIANG UNIV
Method for preparing hydrophilic or hydrophobic fiber
The invention relates to a manufacturing method for fiber with hydrophilicity or hydrophobicity. In the invention fiber macromolecular initiator is manufactured by the reaction between fiber stock and 2-bromoisobutyryl bromide with the 4-dimethylaminopyridine catalyst, then methyl methacrylate or dimethylamino ethyl methacrylate is initiated by fiber macromolecular initiator. The fiber in the invention is provided with hydrophilicity or hydrophobicity. By the method monomer can be controlled to polymerize on the fiber surface effectively so as to improve the usage of monomer and by the inputting quantity of monomer the hydrophilic or hydrophobic performance of manufactured fiber can be controlled.
Owner:SHAANXI NORMAL UNIV
Method for synthesizing clindamycin phosphate
InactiveCN101830946AImprove conversion rateIncrease contentSugar derivativesSugar derivatives preparationSolubilityHydrolysis
The invention discloses a method for synthesizing clindamycin phosphate, which comprises the following steps of: performing ketal protection reaction on clindamycin hydrochloride alcoholate at the temperature of between 2.0 below zero and 2.0 DEG C under the action of acetone and phosphorus oxychloride to form propylidene clindamycin; and performing esterification, hydrolysis, adsorption, washing, deabsorption, concentration, coarse crystallization, decoloration, refining and drying to obtain the finished product of clindamycin phosphate. Because a new catalyst 4-dimethylaminopyridine participates in the esterification in the rection system, the phosphorylating reaction is performed completely, and the conversion rate of raw materials is improved. Meanwhile, due to the secondary crystallization method, the problems of poor color grade and poor powder solubility are solved, and the operating conditions are mild and simple. By adopting triethylamine to replace partial pyridine, the esterification is pushed forwards; the reaction period is shortened; and importantly, related impurities in the finished product and the production cost are reduced, and the content is improved.
Owner:南阳普康药业有限公司 +1
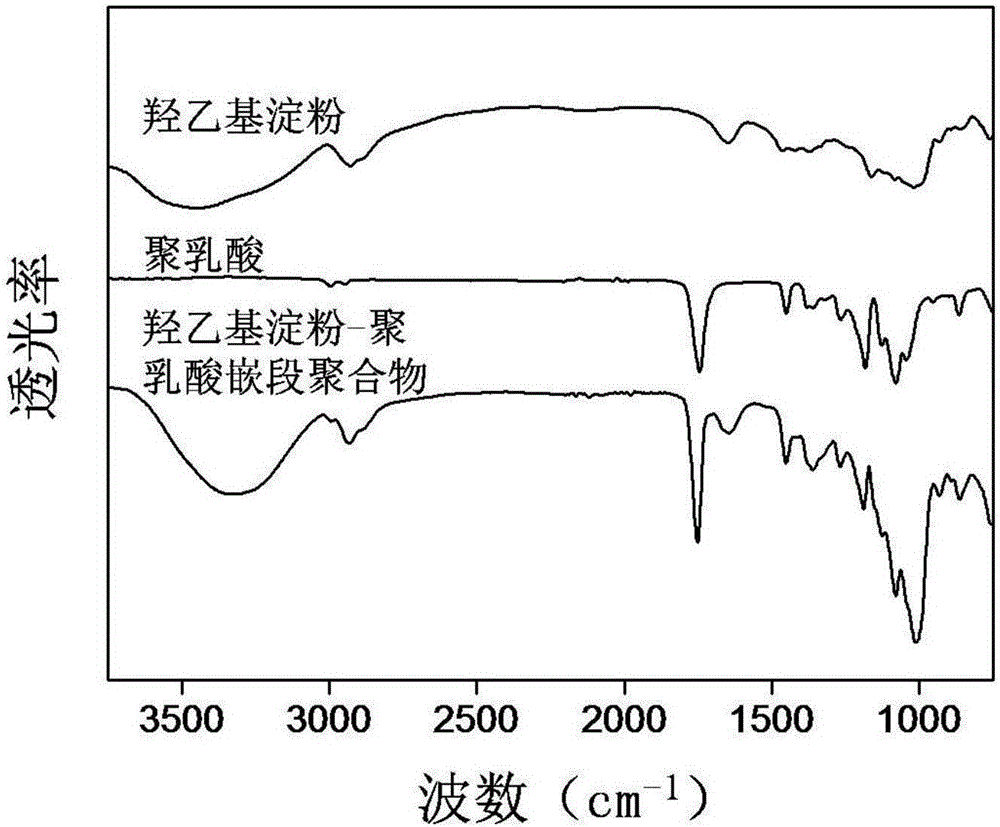
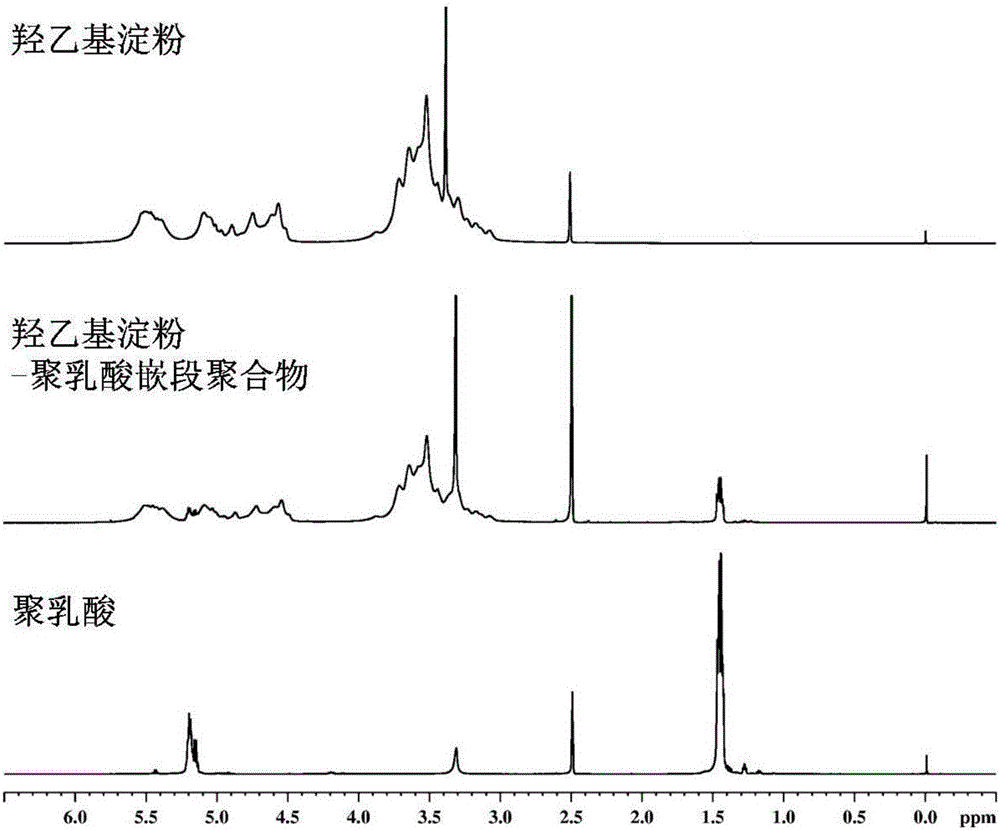
Amphipathy hydroxyethyl-starch-coupled-polylactic-acid copolymer and preparing method and application thereof
ActiveCN106432746AUniform chain lengthHigh degree of polymerizationOrganic active ingredientsPharmaceutical non-active ingredientsHydroxyethyl starchSide effect
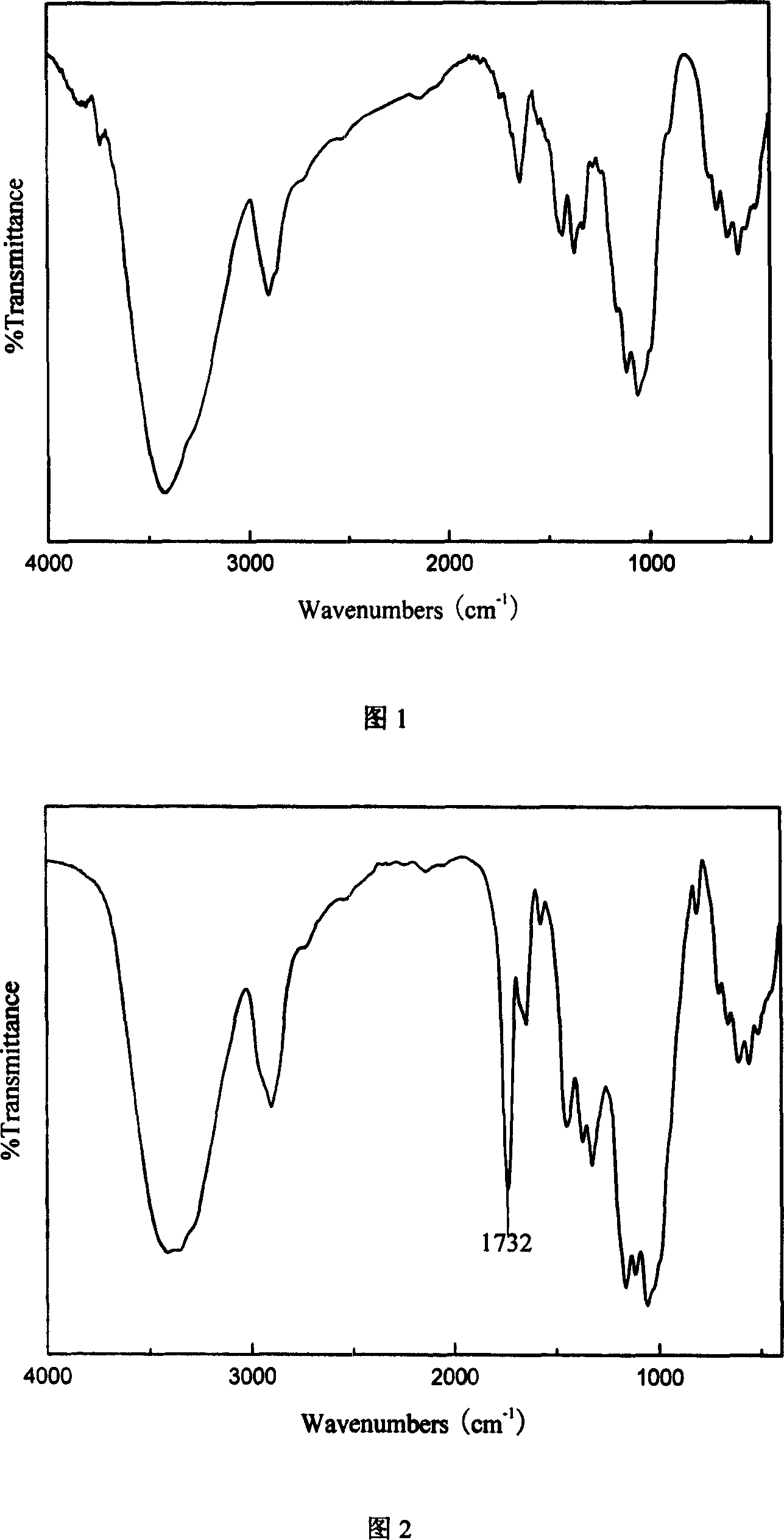
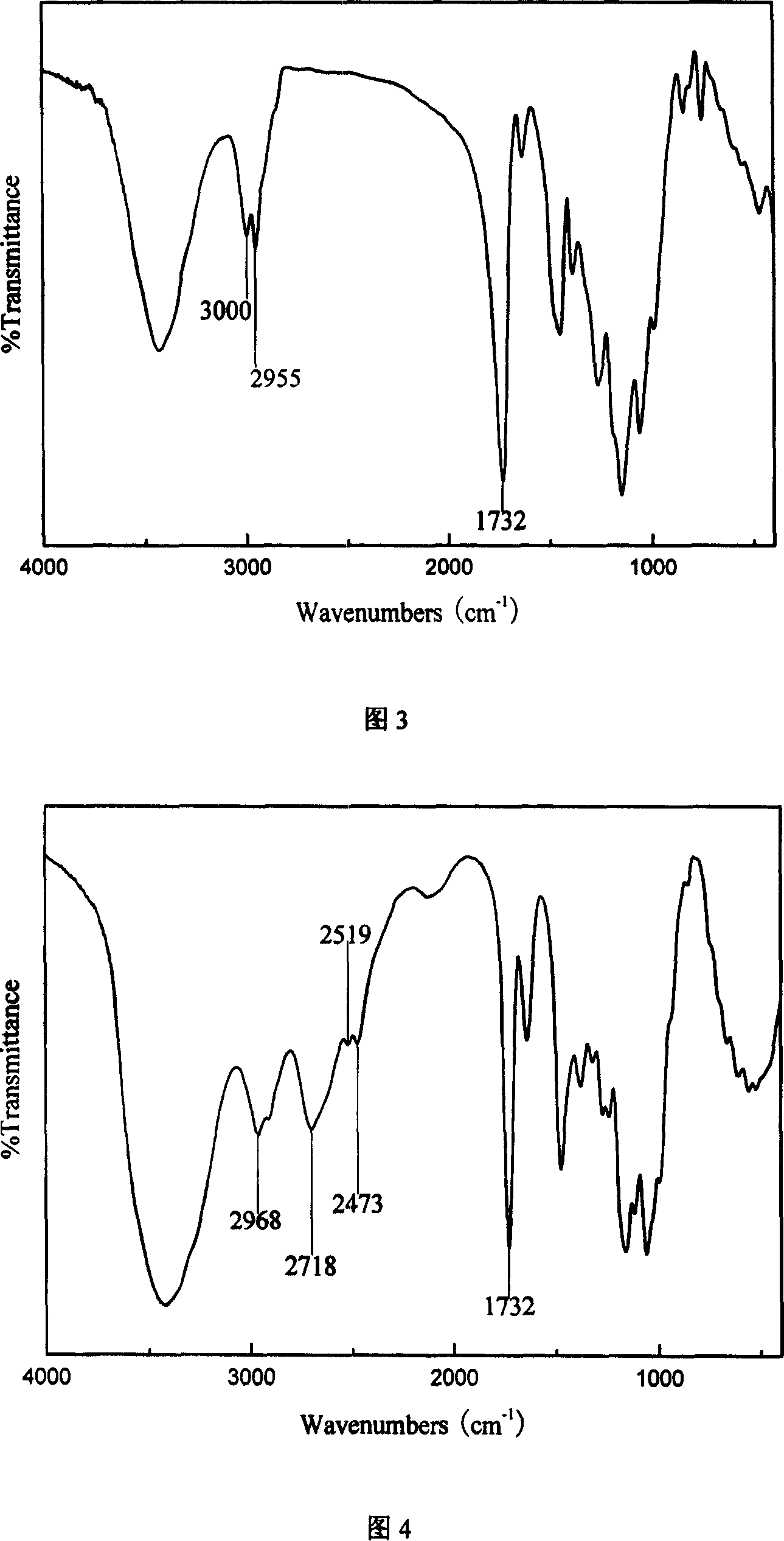
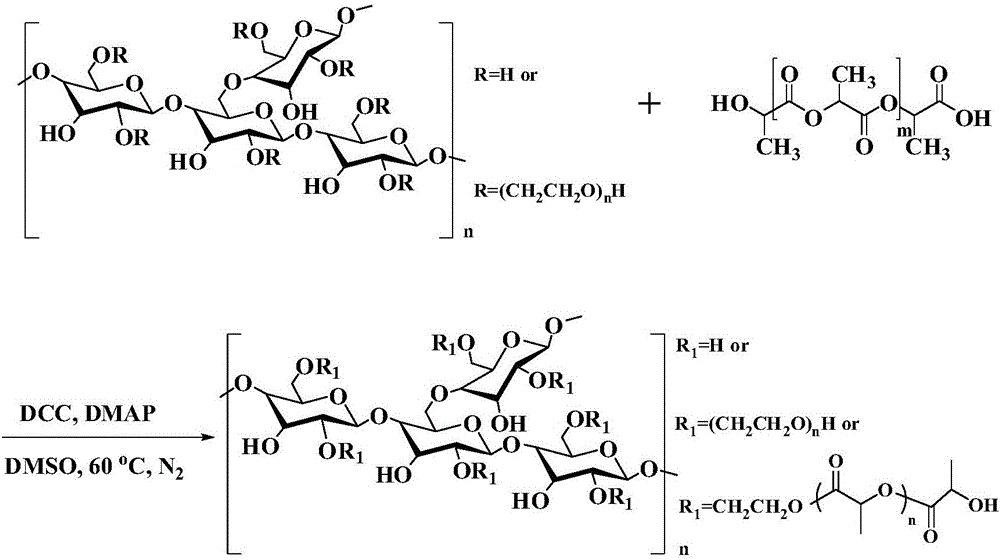
The invention discloses an amphipathy block-polymer hydroxyethyl-starch-olylactic-acid copolymer and a preparing method and nanometer medicine loading system thereof. The method includes the steps that 4-dimethylaminopyridine serves as a catalyst, N-N'-dicyclohexylcarbodiimide serves as a dehydrating agent, carboxyl at the tail end of polylactic acid and hydroxyl on hydroxyethyl-starch sugar ring are subjected o an esterification reaction, hydrophobic polylactic acid is coupled on hydrophilic hydroxyethyl starch accordingly, and the amphipathy hydroxyethyl-starch-polylactic-acid block polymer is synthesized. Debydrochlorination adriamycin amycin is loaded to a hydrophobic core with the emulsified solvent evaporation method and the high pressure homogenization technology, evenly-distributed medicine loading polymer nanometer particles with the particle-size of about 140 nm are formed, and are applied in preparing antitumor medicine, the medicine in-vivo cycling time is prolonged, the toxic and side effects of the medicine are reduced, and the good antitumor effect is achieved.
Owner:HUAZHONG UNIV OF SCI & TECH
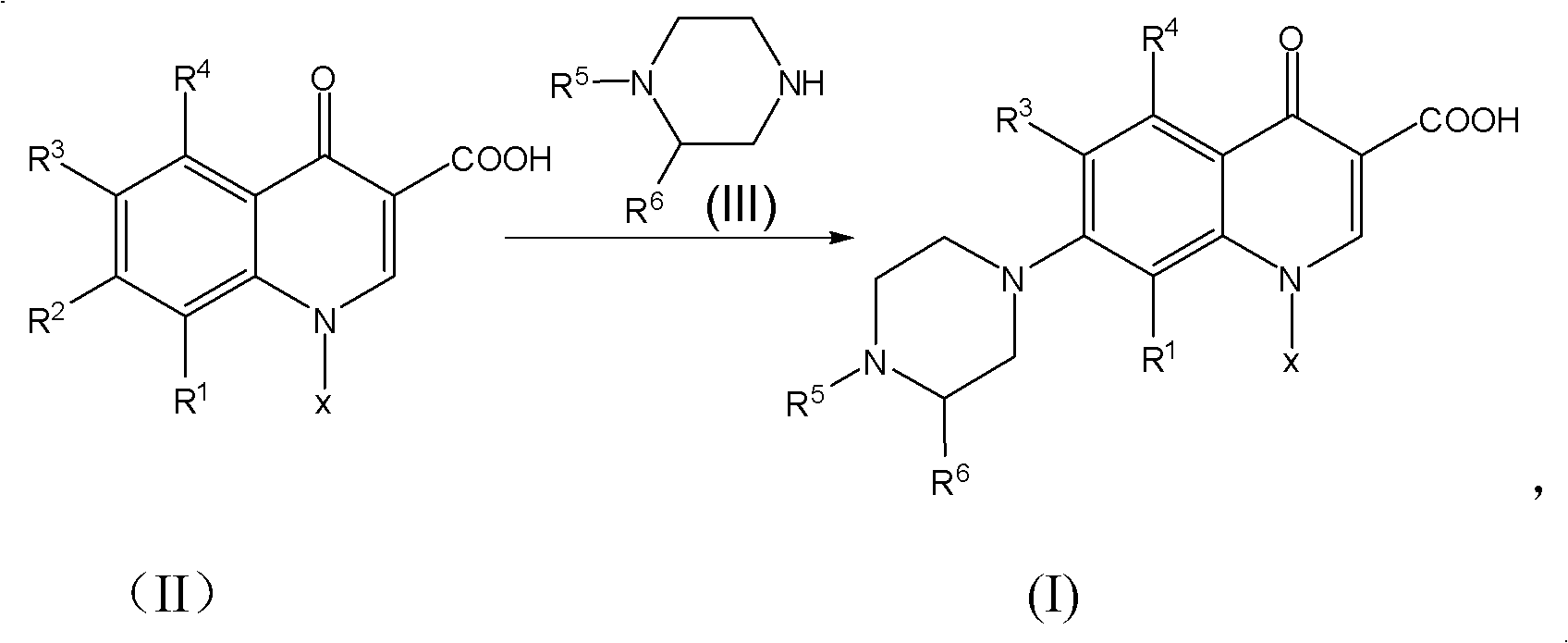
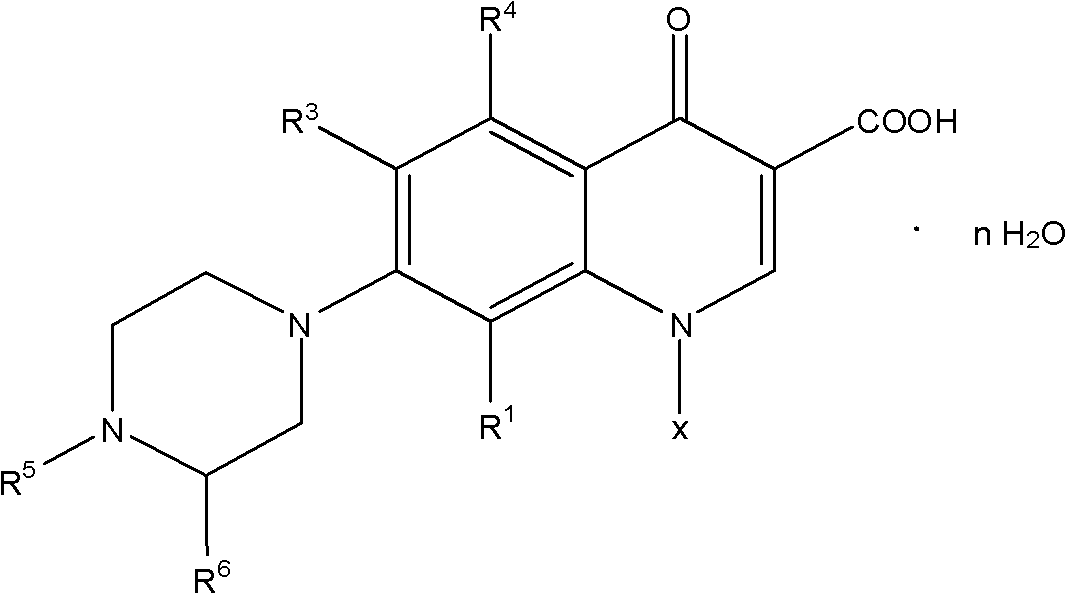
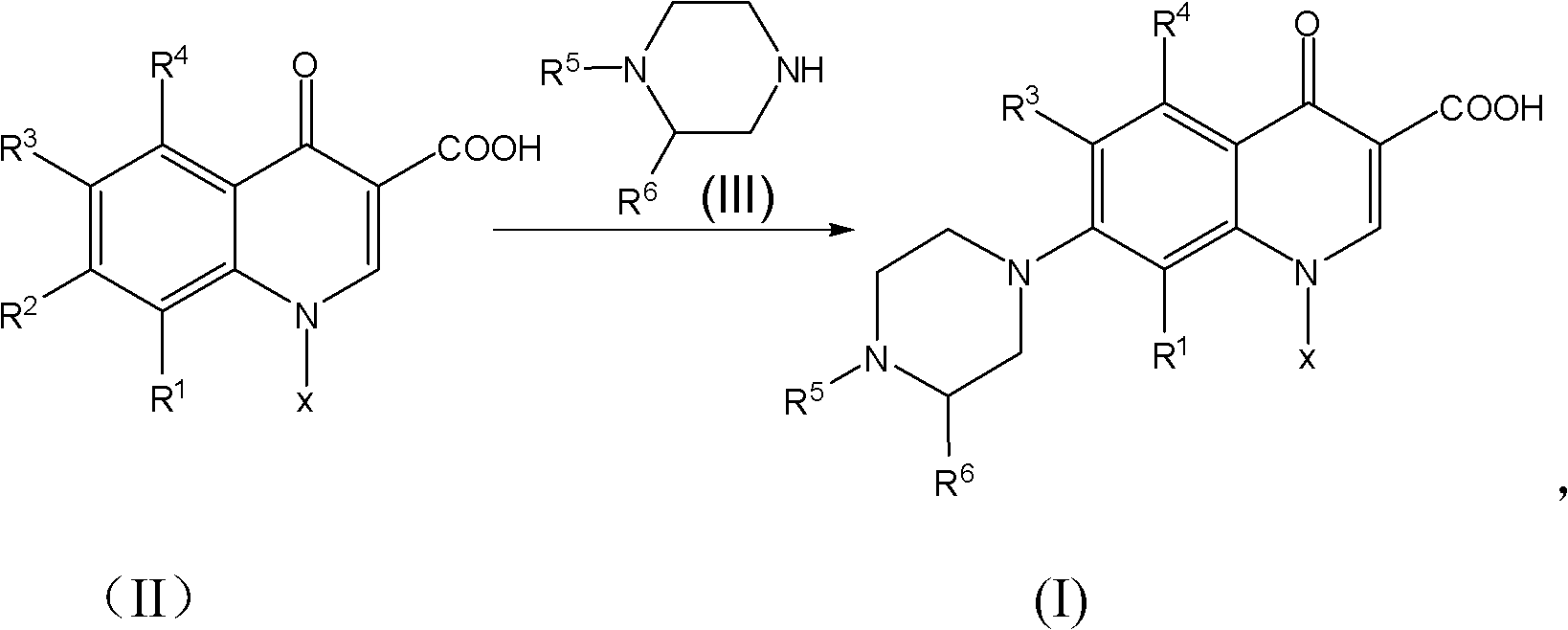
Method for synthesizing quinolone medicaments
InactiveCN101781313ASolve the environmental odor problemRealize cleaner productionOrganic chemistryCarbonyldiimidazoleCarboxylate
The invention discloses a method for preparing quinolone medicaments. The conventional methods using organic solvents with a high boiling pint and a large polarity, adopting other solid alkali materials except reaction materials or using other liquid alkali materials except the reaction materials have obvious defects. The method of the invention adopts a technical scheme that: quinolone compounds are prepared by a piperazidine reduction of quinolone carboxylate nuclear parent and piperazidine derivates in water; the piperazidine reduction is completed in the presence of a catalyst which may be one or a mixture of more than two of cerous chloride heptahydrate, N,N-carbonyldiimidazole, 4-dimethylamino pyridine, tetrabutylammonium bromide, benzyl triethylammonium chloride and tetrabutyl ammonium hydroxide. The method for preparing the quinolone medicaments radically solves the problems of terrible smell, realizes clean production, avoids using the other alkali substances except the reaction materials as an acid-binding agent and overcomes the defects of the prior art.
Owner:ZHEJIANG JINGXIN PHARMA +1
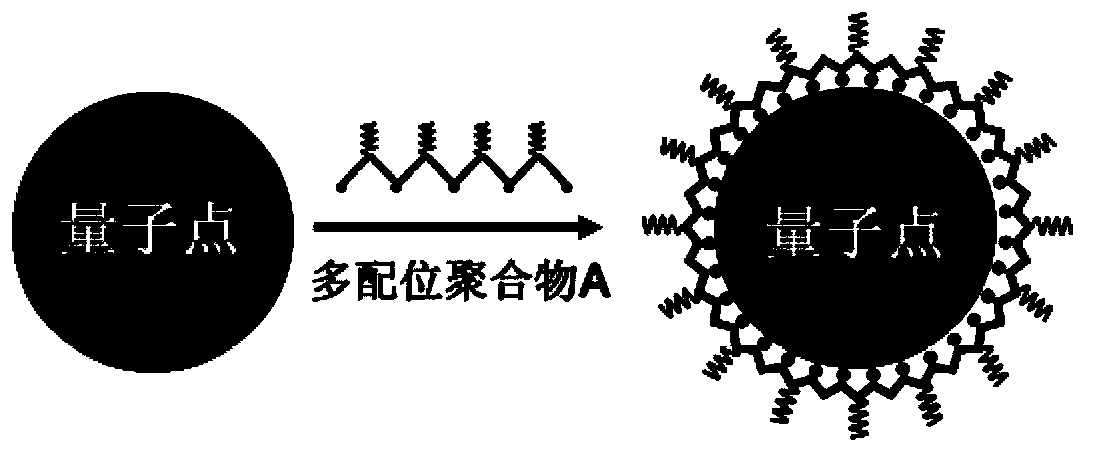
Method for conversion of oil soluble quantum dots into water soluble quantum dots
The invention provides a method for conversion of oil soluble quantum dots into water soluble quantum dots. The method comprises the steps of: 1. dissolving polymaleic anhydride, adenine or histamine, and 4-dimethylaminopyridine in dimethyl sulfoxide to let the materials react, thus obtaining a coordination polymer solution; 2. dissolving the oil soluble quantum dots in chloroform to obtain a chloroform solution of the oil soluble quantum dots; and 3. adding the coordination polymer solution obtained in step 1 into the chloroform solution of the oil soluble quantum dots obtained in step 2 slowly to form a transparent solution, then adding a sodium hydroxide aqueous solution, conducting centrifugation, then taking the upper water solution, removing the excess polymer by an ultrafiltration pipe to obtain filter residue, and dissolving the filter residue in water so as to obtain the water soluble quantum dot solution. The method is simple and practicable, has good reproducibility and universality.
Owner:SHENZHEN INST OF ADVANCED TECH
9-fluorenylmethyl chloroformate preparation method
InactiveCN103408427ASimple processMild conditionsOrganic compound preparationCarbonic/haloformic acid esters preparationChloroformateSolvent
The present invention relates to a 9-fluorenylmethyl chloroformate preparation method, particularly to an improved method for preparing 9-fluorenylmethyl chloroformate from 9-fluorenylmethanol. The method comprises the following steps: 1) adding chloroform, 9-fluorenylmethanol and triphosgene to a reactor, and stirring for 30 min, wherein raw materials comprise 9-fluorenylmethanol, triphosgene, chloroform and 4-dimethylaminopyridine, and a ratio of the 9-fluorenylmethanol to the triphosgene to the chloroform to the 4-dimethylaminopyridine is 20 g:46 g:200 ml:18.5 g; 2) in an ice bath, adding a chloroform solution of 4-dimethylaminopyridine in a dropwise manner, and carrying out a reaction for 2-4 h; and 3) filtering the reactant in the reactor to obtain a white solid and the filtrate, and sequentially carrying out pressure reduction solvent removing, cryogenic crystallization, organic solvent washing and drying on the filtrate to obtain the white solid 9-fluorenylmethyl chloroformate. The preparation method has characteristics of mild reaction conditions, low cost, simple process, and easy industrialization.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
Preparation method of 4-methyl-5-ethoxy oxazole acid ethyl
ActiveCN103435568AEasy to operateMild reaction conditionsOrganic chemistryState of artRoom temperature
The invention discloses a preparation method of 4-methyl-5-ethoxy oxazole acid ethyl. The preparation method comprises the following steps of (a) adding catalytic amount of 4-dimethylamino-pyridine to a cyclization system of phosphorus oxychloride and triethylamine to carry out cyclization reaction, wherein N-ethoxy oxalyl-alpha-ethyl aminopropionic acid is taken as a raw material; (b) cooling the reaction mixture obtained in the step (a) to the room temperature and then dropwise adding the reaction mixture into deionized water for quenching reaction; and (c) carrying out separation treatment, thus obtaining the 4-methyl-5-ethoxy oxazole acid ethyl. Not only does the preparation method have the advantages of simplicity in process operation, mild reaction conditions, short reaction time, economy, environment friendliness and the like but also the purity and the molar yield of the 4-methyl-5-ethoxy oxazole acid ethyl are higher than the levels of the prior art and the 4-methyl-5-ethoxy oxazole acid ethyl meets the requirements of industrial production.
Owner:DAFENG HEGNO PHARMA +2
Composite catalyst for preparing polyether-polylactide-aliphatic polycarbonate ternary block copolymer and application of composite catalyst
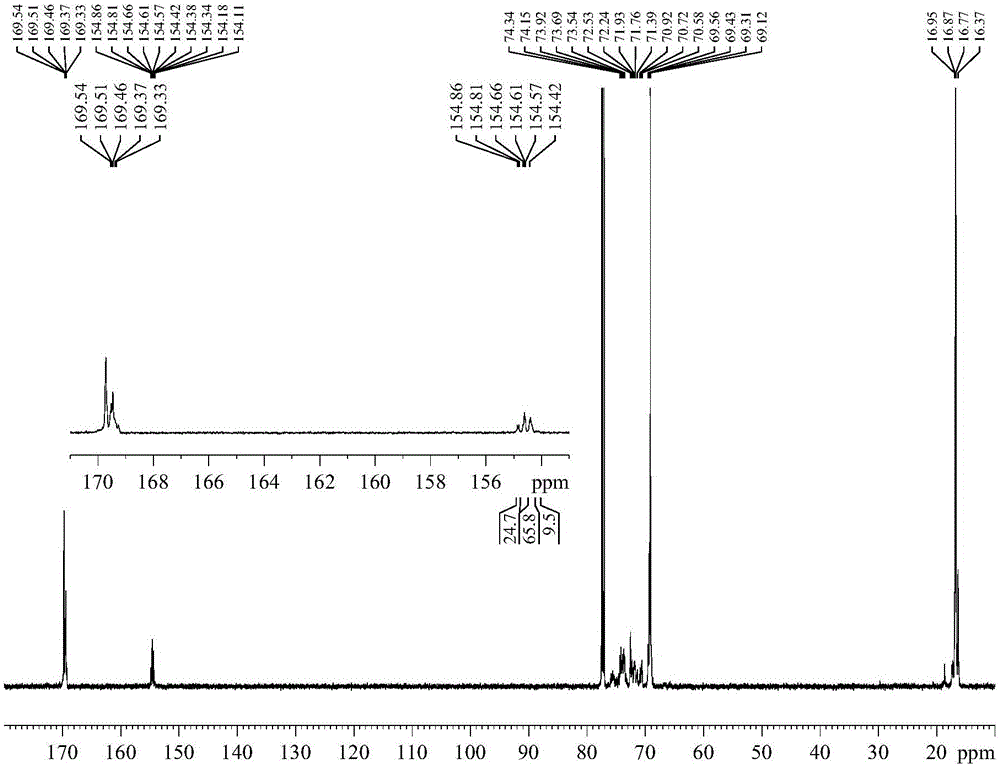
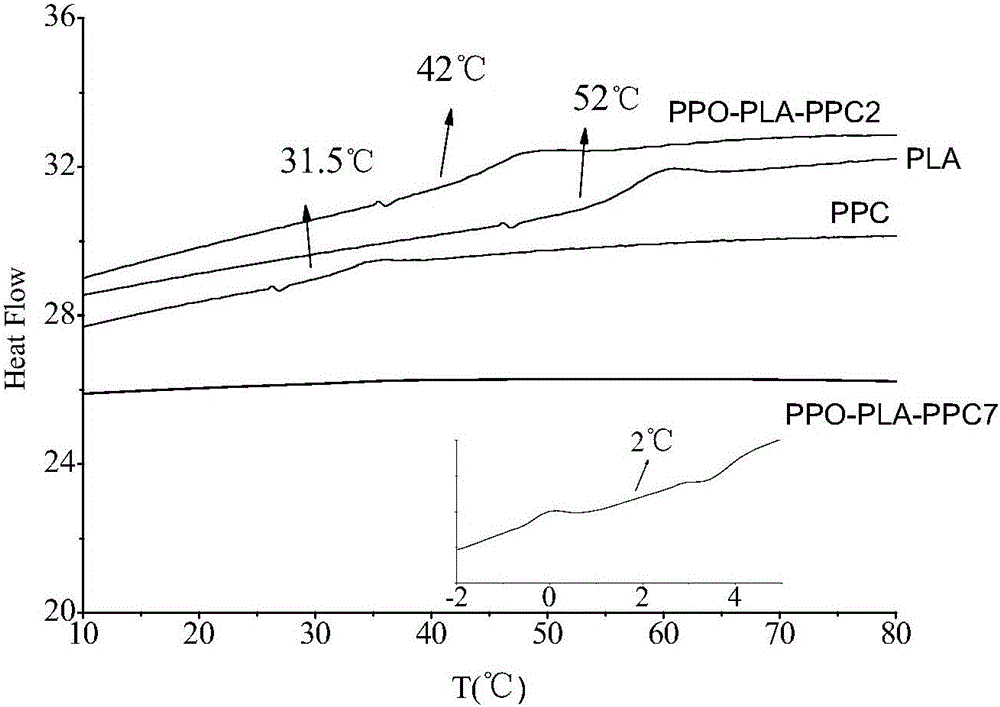
The invention relates to a composite catalyst for preparing a polyether-polylactide-aliphatic polycarbonate ternary block copolymer and an application of the composite catalyst. The composite catalyst comprises a metal Salen catalyst and a cocatalyst in a molar ratio of 1: (0.5-2), wherein the cocatalyst is bis-(triphenylphosphine)iminium chloride, 4-dimethylaminopyridine or 2,6-lutidine. The composite catalyst has the following beneficial effects: 1, The catalysis effect of the composite catalyst is good, the catalysis efficiency is high, the obtained ternary copolymer has larger number-average molecular weight and narrow molecular weight distribution; 2, composition of a polyether segment, a polylactide segment and a PPC (poly(propylene carbonate)) segment in the copolymer can be effectively controlled through regulation of step reaction time and monomer ratio, the glass transition temperature of the copolymer is 2-42 DEG C, the elongation at break is 55%-331%, and the tensile strength is 6-39 MPa.
Owner:WUHAN UNIV OF TECH
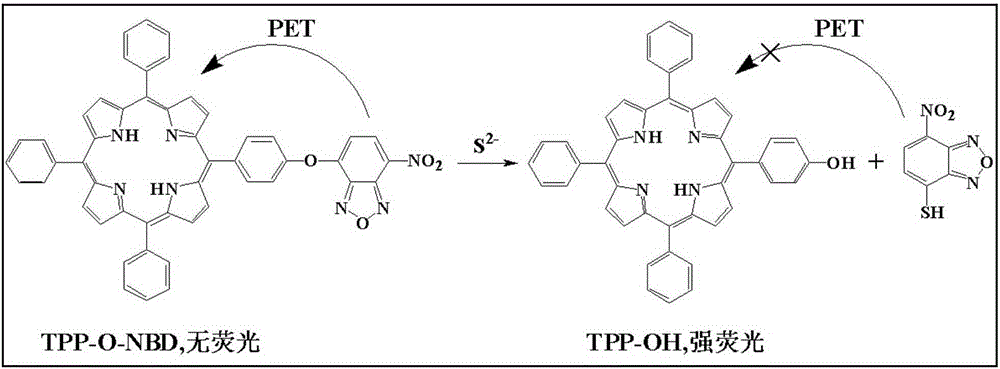
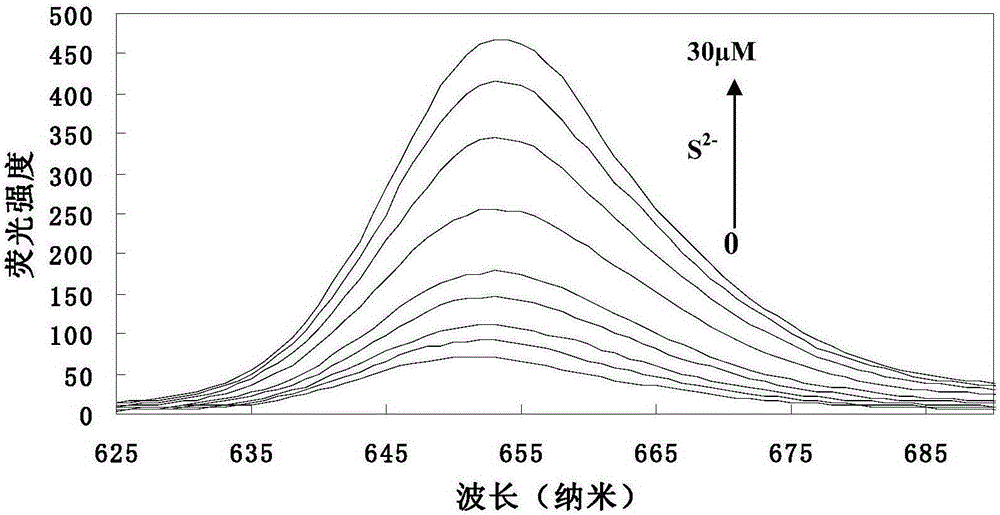
Preparation method and application of hydroxyl porphyrin-based high-selectivity near-infrared fluorescence sulfur ion probe
ActiveCN105295900AFast fluorescent responseHigh sensitivityOrganic chemistryFluorescence/phosphorescencePorphyrinHigh selectivity
The invention discloses a preparation method and application of a hydroxyl porphyrin-based high-selectivity near-infrared fluorescence sulfur ion probe. The fluorescence probe is prepared by taking 5-hydroxyphenyl-11,15,20-triphenylporphyrin (TPP-OH) and 4-chlorine-7-nitro-1,2,3-benzoxadiazole (NBD-Cl) as materials, taking 4-dimethylamino pyridine (DMAP) as a catalyst, taking dichloromethane as a solvent and performing one step reaction under room temperature. The preparation method of the probe is simple, and a product is easy to separate and purify. Compared with an existing small organic molecule fluorescence probe, the fluorescence probe obtained by the preparation method has higher fluorescence emission wavelength (lumbda em is greater than 650nm), high-sensitivity and high-selectivity quick recognition and detection on a trace of hydrogen sulphide can be realized in a near-infrared region, and the application prospect is great in the field of environmental and biological detection.
Owner:HUNAN UNIV OF SCI & TECH
Preparation method of nitrogen- and phosphorus-doped graphene sheet
The invention discloses a preparation method of a nitrogen- and phosphorus-doped graphene sheet. The preparation method particularly includes following steps: adding bentonite to a proper amount of a tetramethyl ammonium bromide solution, stirring the mixture, aging the mixture, adding 4-dimethylamino pyridine and n-butyl phosphate, stirring the mixture in a constant-temperature water bath at 60-70 DEG C for 6-8 h, separating a precipitation from liquid, washing the precipitation with deionized water for 2-3 times, drying and grinding the precipitation, sieving the precipitation through a sieve being 20-40 in meshes to obtain modified bentonite powder, heating the modified bentonite powder in a vacuum tubular furnace to 400-600 DEG C under a vacuum condition for calcining the powder for 2-4 h, adding the calcined powder in a hydrofluoric acid solution, separating the precipitation and finally heating the precipitation to 2000-2500 DEG C under the vacuum condition to perform heat treatment for 3-6 h, and cooling a product to obtain the nitrogen- and phosphorus-doped graphene sheet. The preparation method is simple in raw materials and is mild in conditions.
Owner:JINING XINRUIDA INFORMATION TECH CO LTD
High-yield synthesis method of trenbolone acetate
The invention relates to a synthesis method of trenbolone acetate, which comprises the following steps: 1. etherification reaction: adding methanol, acetyl chloride and oestrogenic steroid-4,9-diene-3,17-dione into a reaction tank, stirring, regulating the pH value, carrying out vacuum filtration, washing with water, and centrifuging; 2. reduction reaction: adding potassium borohydride, stirring, regulating the pH value, washing with water, and concentrating; 3. hydrolysis reaction: adding methanol and hydrochloric acid, dotting the plate, regulating the pH value, concentrating, adding dichloromethane to be dissolved, washing with water, and dehydrating; 4. dehydrogenation reaction: rinsing with dichloromethane, adding DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone), dotting the plate, carrying out vacuum filtration, washing with water, concentrating, cooling, and drying to obtain trenbolone; and 5. acylation reaction: sucking in benzene and pyridine, adding the trenbolone and 4-DMAP (4-dimethylaminopyridine), dropwisely adding acetyl chloride, keeping the temperature, dotting the plate, washing with water, and concentrating. The method provided by the invention enhances the yield of the trenbolone acetate from 88-90% to 92-93.5%, and can lower the cost by 200 yuan or so per kilogram.
Owner:YICHENG GOTO PHARMA
Dynamic kinetic resolution method of 1-(1-naphthyl) ethylamine
InactiveCN102392065AIncrease reaction rateReduce the temperatureFermentationPhenethyl alcoholAcetophenone
The invention discloses a dynamic kinetic resolution method for 1-(1-naphthyl) ethylamine, which comprises the following steps of: firstly, adding parachlorophenol, organic acid, dicyclohexylcarbodiimide and 4-dimethylaminopyridine, carrying out agitating reaction and filtering, drying, concentrating and carrying out column chromatography to obtain an acyl donor; or adding acetophenone and sodium borohydride, carrying out agitating reaction, depressurizing and distilling, washing, extracting, drying and concentrating to obtain phenethyl alcohol, adding the phenethyl alcohol, acetyl chloride and triethylamine, carrying out agitating reaction and washing, drying, concentrating and carrying out column chromatography to obtain an acyl donor; secondly, adding palladium salt and a carrier, heating, adding formaldehyde and sodium hydroxide, heating and centrifuging, washing and vacuum-drying to obtain a racemic catalyst for standby; and thirdly, adding the 1-(1-naphthyl) ethylamine in methyl benzene, the acyl donor, lipase and the racemic catalyst and carrying out hydrogenation reaction to obtain amide. The dynamic kinetic resolution method has high reaction speed, lower temperature, high conversion rate and product optical purity and great application value.
Owner:ZHEJIANG UNIV
Metronidazole modified graphene oxide composite material and preparation method thereof
The invention discloses a metronidazole modified graphene oxide composite material. A preparation method of the metronidazole modified graphene oxide composite material comprises the following steps of (1) preparing graphene oxide; (2) stirring and dissolving maleic anhydride into an oil bath pan at a temperature of 75 DEG C, adding the graphene oxide into the dissolved maleic anhydride to reach for 3 h, then adding distilled water and heating up to 86 DEG C to react for 16 h, and finally performing suction filtration, washing, drying and grinding treatments to obtain maleic anhydride modified graphene oxide; (3) dispersing the maleic anhydride modified graphene oxide in dimethyl formamide, then adding metronidazole, dicyclohexyl diimine and 4-dimethylamino-pyridine, and reacting at a temperature of 80 DEG C under the protection of nitrogen gas for 48 h so as to obtain the metronidazole modified graphene oxide composite material. The metronidazole modified graphene oxide composite material has the advantages that the composite material is prepared from conventional materials under mild conditions, and is added into a coating, so that the excellent properties of the composite material and the coating are fully exerted, and the pollution to environment is reduced.
Owner:成都恒固新材料科技有限公司
Camptothecin and artesunate conjugate, preparation method and application thereof
ActiveCN104163823AHas antitumor activityReduce harmOrganic active ingredientsOrganic chemistryOrganic solventMedicinal chemistry
The present invention discloses a camptothecin and artesunate conjugate represented by a formula (I). The preparation method comprises the steps of dissolving camptothecin represented by a formula (II) and artesunate represented by a formula (III) in an organic solvent, carrying out a stirring reaction at a temperature of 15-50 DEG C under the effects of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride and 4-dimethylaminopyridine, performing TLC tracking monitoring until the reaction is over, and post-treating the reaction solution to obtain the camptothecin and artesunate conjugate represented by the formula (I). The camptothecin and artesunate conjugate of the present invention can be used for preparing various anti-tumor drugs. The formulas (I), (II) and (III) are shown as follows.
Owner:ZHEJIANG UNIV OF TECH
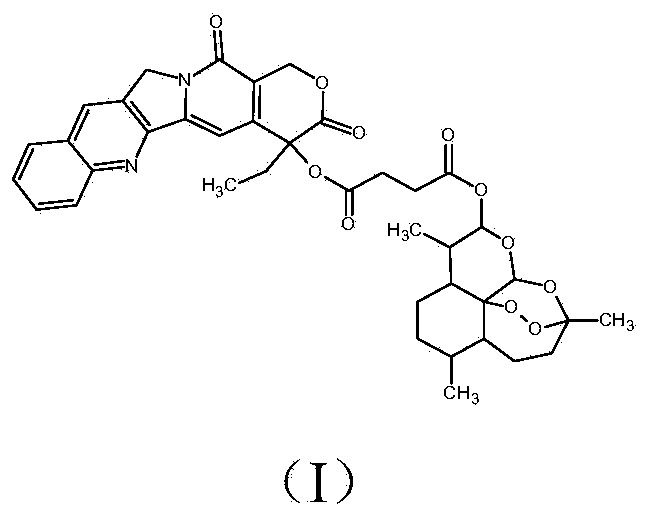
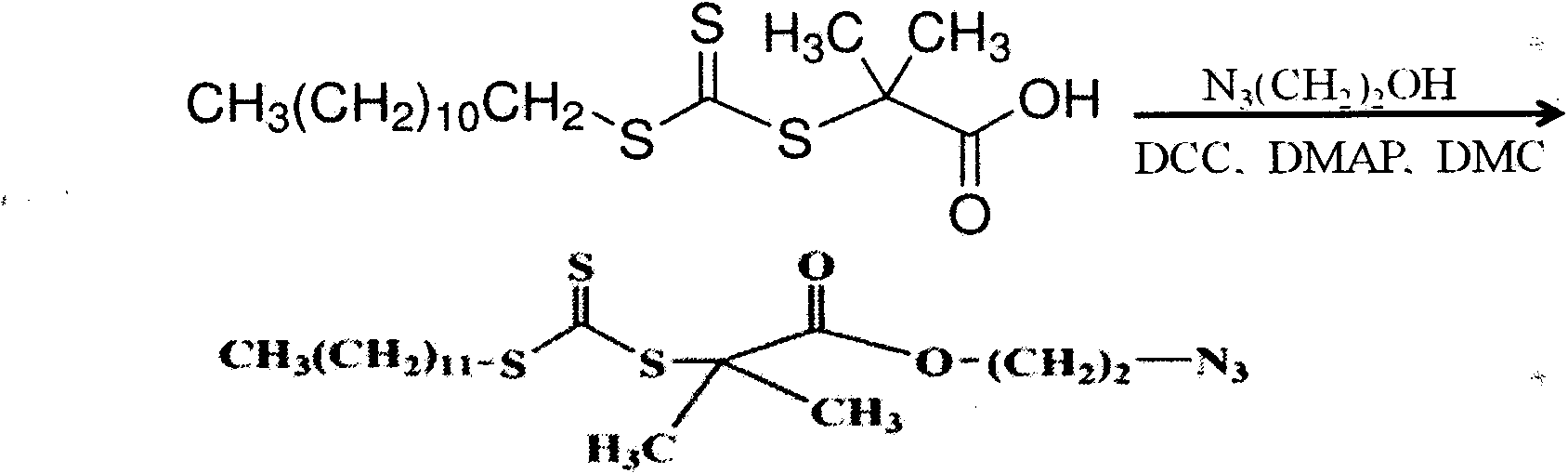
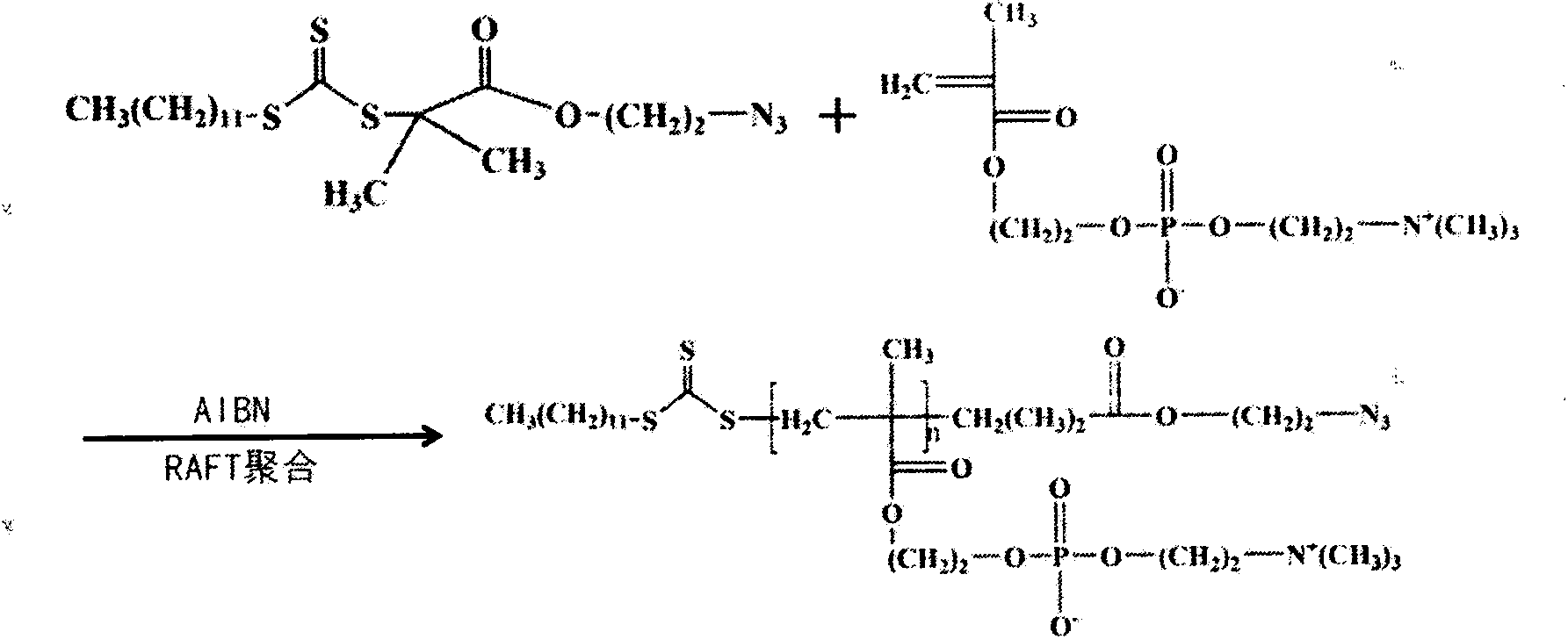
Artificial cell membrane materials applied to photoinduction stem grafting and synthesis method thereof
InactiveCN103483480ADoes not affect mechanical propertiesDoes not affect structural propertiesCoatingsPropanoic acidBiocompatibility Testing
The invention discloses artificial cell membrane materials applied to photoinduction stem grafting and a synthesis method of the artificial cell membrane materials. The synthesis method of the artificial cell membrane materials comprises the following steps that 2-(dodecyl trithiocarbonate)-2- methyl propionic acid, dicyclohexyl carbodiimide, 4- dimethylamino pyridine and dichloromethane are added into a flask, all the components are stirred and dissolved under the protection of nitrogen, azide ethanol is dropwise added and stirred, washing is carried out through diluted hydrochloric acid and distilled water, solvents are removed through a reduced pressure distillation method, and a yellow oily liquid a is obtained; MPC, a midbody a, azo different nitrile and absolute ethyl alcohol are added into a boiling tube and are evenly dissolved, then after the nitrogen is removed through three times of 'vacuumizing-induction of highly pure nitorgen' operations on the boiling tube, and reaction away from light is carried out on the boiling tube in a sealed mode under the protection of highly pure nitrogen; the reaction liquid is precipitated through trichloromethane / diethyl ether mixed solvents, and solid products are collected. By means of the technical scheme, the artificial cell membrane materials have good biocompatibility, achieve the various effects of restraining of protein adsorption, anticoagulation, surface lubrication and the like, and have good medical prospects.
Owner:西安维萃禾生物科技有限公司 +1
Kinetic resolution method of secondary alcohol
InactiveCN102154431AImprove performanceMeet various requirements of purityFermentationOrganic acidAlcohol
The invention discloses a secondary alcohol kinetic resolution method which comprises the following steps of: (1) adding parachlorophenol, organic acid, dicyclohexylcarbodiimide and 4- dimethylamino pyridine, the molar ratio of which is 1:1-2:1-2:0.03-0.05, stirring and reacting for 3 to 7 hours, filtrating, drying filtrate, concentrating and carrying out column chromatography so as to obtain pure ester being used as acyl donor for standby application; (2) adding secondary alcohol, acyl donor and 5 to20mg / mL lipase into 2-6mL of organic solvent, wherein the molar ratio between the secondary alcohol and the acyl donor is 1:0.5 to 3, reacting for 0.5 to 3 hours at the reaction temp[rapture of 30 to 60 DEG C so as to obtain ester with 50% conversion rate and 99% of e.e value. In the method, the conversion rate of the secondary alcohol reaches 50% in a very short time; the e.e value of the obtained ester reaches nearly 100%, properties of products is greatly increased, various requirements on purity can be met and very great application value is realized.
Owner:ZHEJIANG UNIV
Purification method of acetyl pullulan polysaccharide folate conjugate and preparation method of nanometer particles thereof
InactiveCN101831000AStable structureNon-immunogenicPowder deliveryPharmaceutical non-active ingredientsPullulanPurification methods
The invention relates to a purification method of acetyl pullulan polysaccharide folate conjugate and a preparation method of nanometer particles thereof. Folate, acetyl pullulan polysaccharide solution, 4-dimethylaminopyridine (DMAP) and a carbodiimide dehydrating agent are mixed for reaction for 5 to 7 days at the temperature of 25 to 28 DEG C, excessive absolute ethyl alcohol is dropwise added into filter liquor for sedimentation, and centrifugation is carried out, the sediment is collected, is dialyzed for 24 to 48 hours by sodium carbonate buffer solution and is dialyzed for 24 to 48 hours in deionized water, and a material is obtained through freezing-drying or vacuum drying. The material is dissolved in dimethyl sulfoxide (DMSO) or other organic solvents, and a dialysis method is adopted for preparing the nanometer particles. The invention has less steps, simple operation and good repeatability and dose not need other stabilizing agents and emulsifiers, and the nanometer particles are in ball shapes, have uniform grain sizes, low absolute potential, and good stability, and do not have significant toxicity for single mainline to mice.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Method for synthesizing phenylalanine esterified bagasse xylan-g-CHMA (cyclohexyl methacrylate) with anti-tumor activity
InactiveCN109438622AImprove biological activityHigh grafting rateAntineoplastic agentsBulk chemical productionMethacrylateSide chain
The invention discloses a method for synthesizing phenylalanine esterified bagasse xylan-g-CHMA with anti-tumor activity. The method comprises taking bagasse xylan as the raw material, and in an ionicliquid of [Hemim]Cl, taking ammonium persulfate as an initiator and cyclohexyl methacrylate as grafting monomers to synthesize a bagasse xylan-g-CHMA derivative; taking Boc-L-phenylalanine as esterification agent and 4-dimethylaminopyridine as catalysts for catalytic esterification in ionic liquid solvent to synthesize a bagasse xylan esterified grafted derivative phenylalanine esterified bagassexylan-g-CHMA; performing deprotection through trifluoroacetic acid to obtain the product of the phenylalanine esterified bagasse xylan-g-CHMA. Under the reaction environment of the ionic liquid, themethod for synthesizing the phenylalanine esterified bagasse xylan-g-CHMA with the anti-tumor activity introduces active groups to the main chain and the side chain of bagasse xylan through grafting and esterification reaction, thereby enhancing the bioactivity of raw bagasse xylan; the prepared phenylalanine esterified bagasse xylan-g-CHMA has a broad application prospect in the fields of biologyand medicine.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Reactions between ethylene oxide and derivatives of ethylene oxide and CO2 for generating cyclic carbonate from
InactiveCN104418832AGood reproducibilityShort reaction timeOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsReaction temperaturePotassium iodine
The invention discloses reactions between ethylene oxide and derivatives of ethylene oxide and CO2 for generating cyclic carbonate. According to the reactions, metal complexed conjugated micropore high-molecular polymers (CMP-M) of one type are taken as main catalysts and quaternary ammonium salts (such as tetrabutylammonium bromide, tetrabutyl ammonium chloride and tetrabutyl ammonium acetate), triethylamine and 4-dimethylamino or potassium iodide are taken as catalysis promoters. The reactions disclosed by the invention are characterized in that the molar ratio of the ethylene oxide and the derivatives of the ethylene oxide to the catalyst promoters and the catalysts is (500-2000):(1-50):1, the reaction temperature is 0-120 DEG C, the reaction time is 1-24 hours, and the reaction pressure is 0.1-3MPa. The limit of high temperature and high-pressure (6-9MPa and 160-200 DEG C) to similar reactions in prior art is broken through, and the yield of cyclic carbonate can be up to 29-99% under gentle conditions. The reaction has the advantages of short reaction time, gentle reaction condition, high reaction yield, good reaction repeatability and the like, and the possibility of industrialization is improved.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
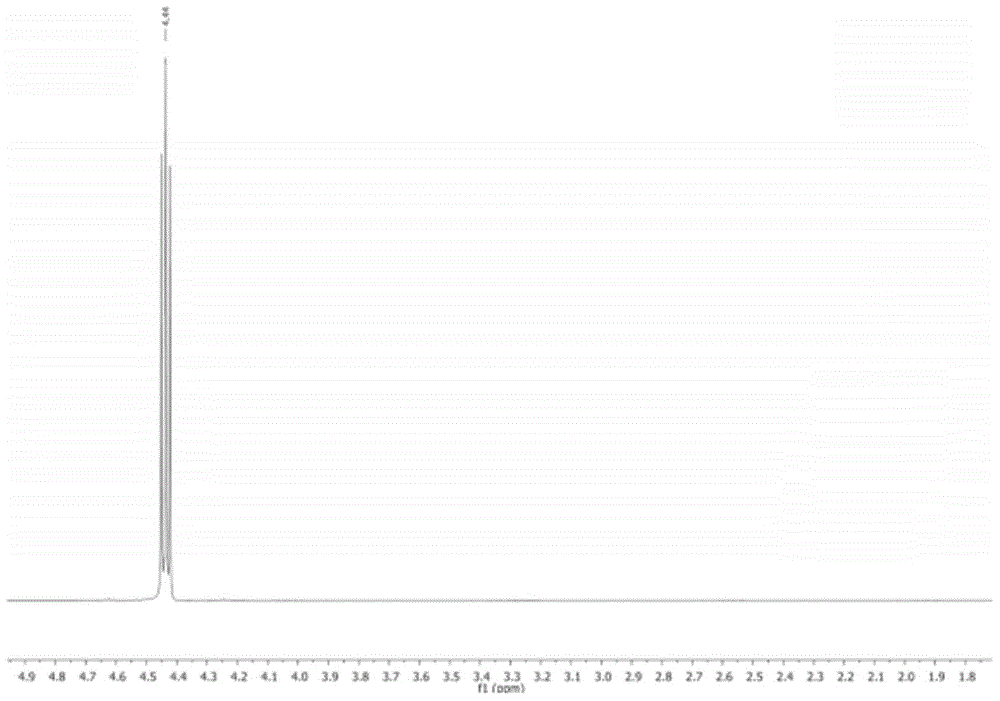
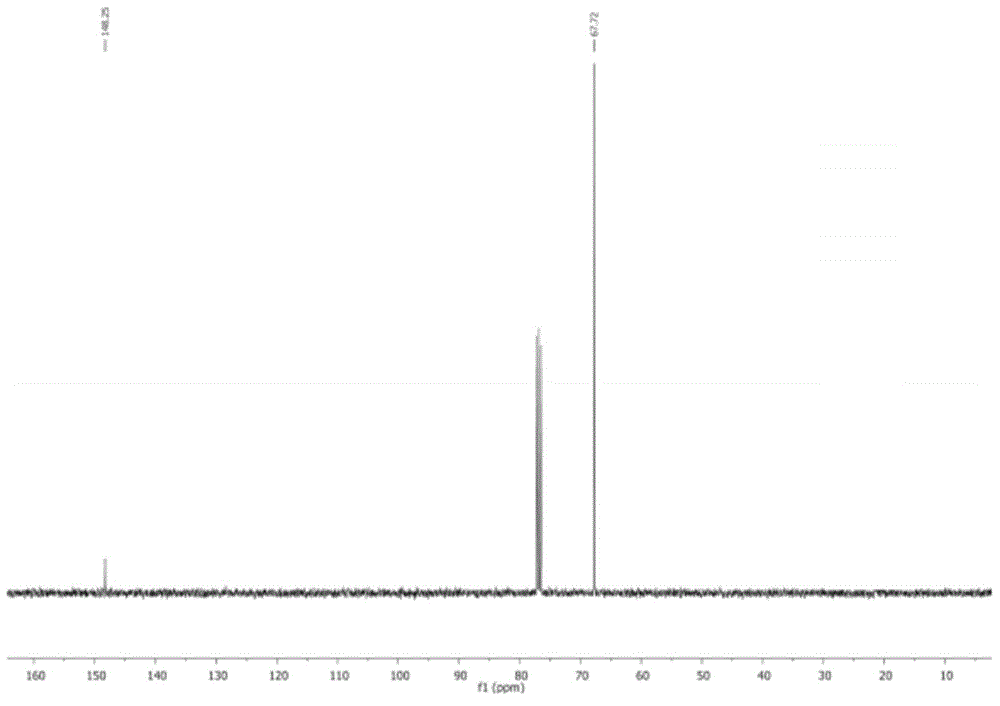
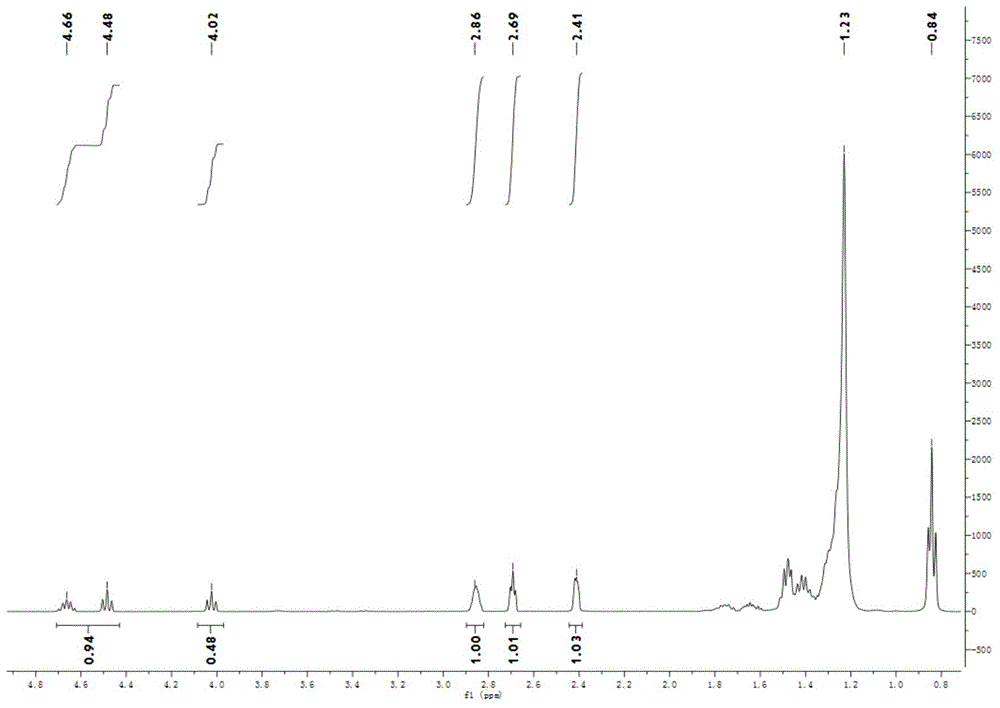
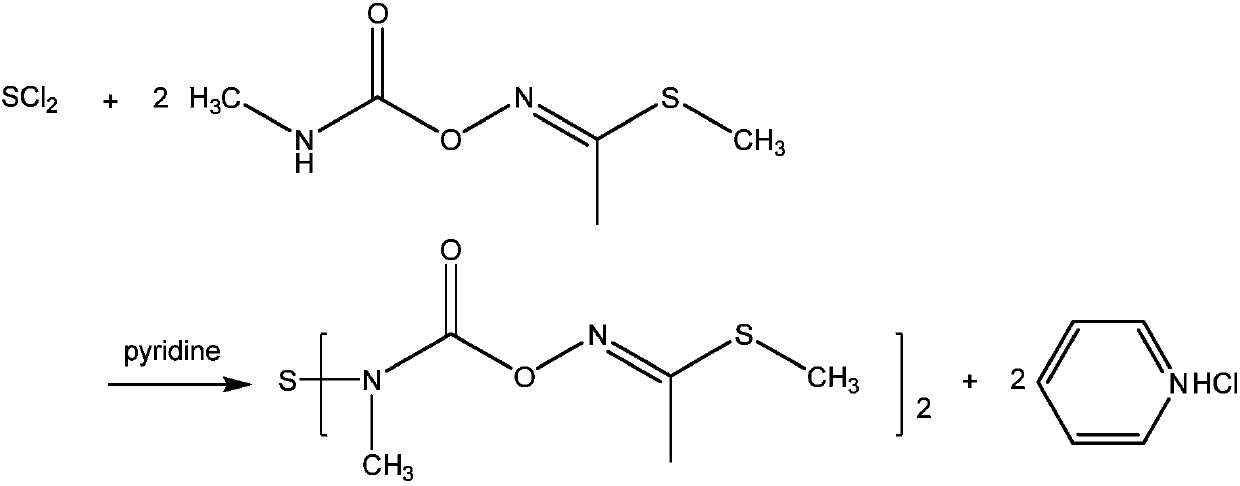
Preparation method of thiodicarb
The invention discloses a preparation method of thiodicarb. The preparation method comprises the following steps: (1) dividing a solvent into two portions, dissolving methomyl in one of the two portions to obtain a methomyl solution, and dissolving 4-dimethylaminopyridine in the other portion to obtain a solvent which contains a catalyst; and (2) dividing sulfur dichloride into two portions, dropwise adding one portion of the sulfur dichloride in the solvent which contains the catalyst under a closed condition, meanwhile, dropwise adding the other portion of sulfur dichloride and carrying outdropwise adding reaction on the other portion of sulfur dichloride and the methomyl solution obtained in step (1), and carrying out insulating reaction after dropwise adding is finished to obtain thethiodicarb. The preparation method of the thiodicarb has the advantages that the reaction selectivity is high, the purity of the obtained product is high, the content of thiodicarb isomer is smaller than 0.4%, the yield is high and the like.
Owner:湖南海利常德农药化工有限公司
Efficient preparation of 4-dimethylaminopyridine
The invention discloses a new method for preparing 4-dimethylaminopyridine (DMAP) with a one-kettle process by taking 4-cyanopyridine and acrylic acid as main raw materials. The method mainly comprises the following steps: performing quaternization of 4-cyanopyridine with acrylic acid to obtain an intermediate; reacting with an amination reagent; neutralizing acid in the system in the presence of a pH regulator to enable the product to be free, wherein the reaction period is greatly shortened; and performing simple separation and purification to obtain a target product DMAP. In the method disclosed by the invention, the reaction conditions are mild, the synthesis method is simple, the DMAP synthesis can be efficiently performed through simple technological operation, and the obtained product has high purity and high yield; and the method is suitable for large-scale industrial production.
Owner:ANHUI COSTAR BIOCHEM CO LTD
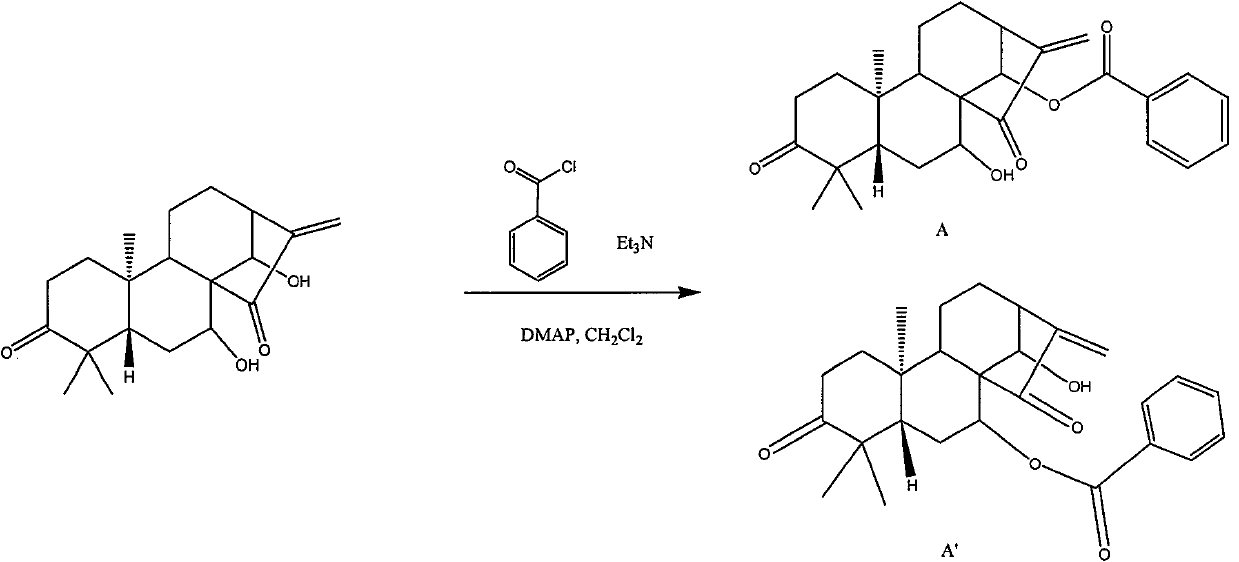
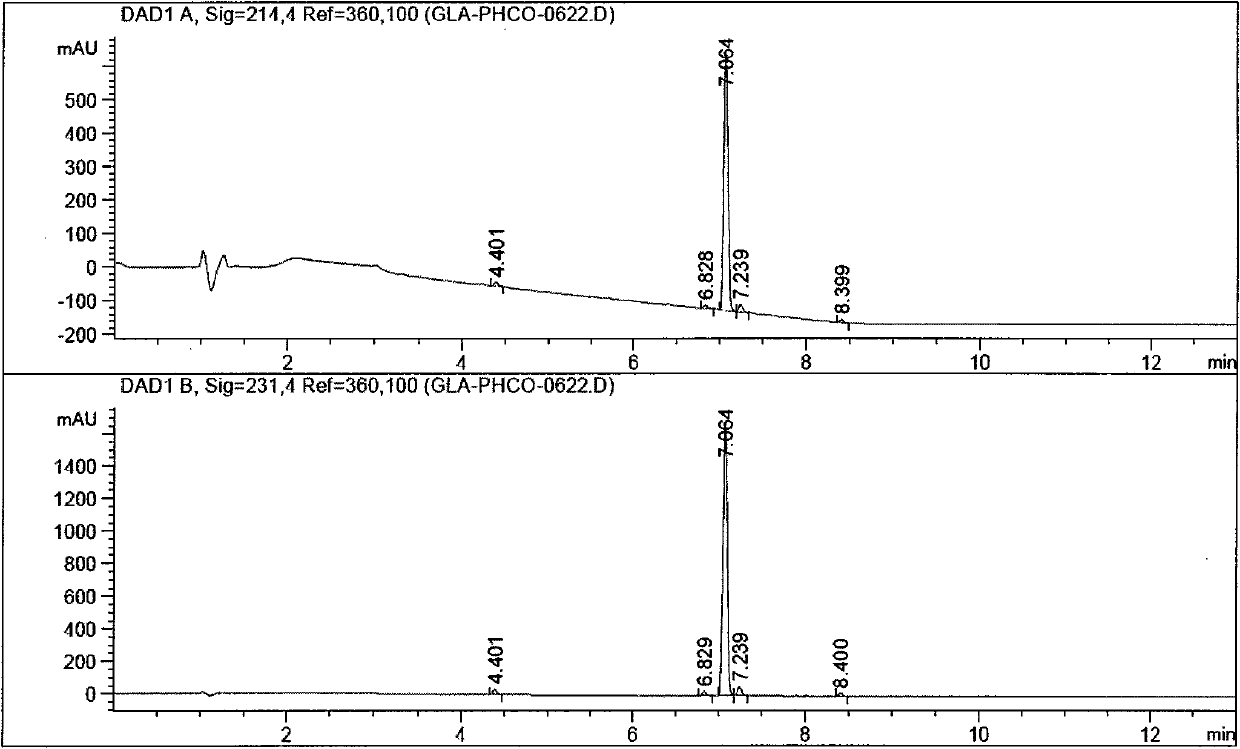
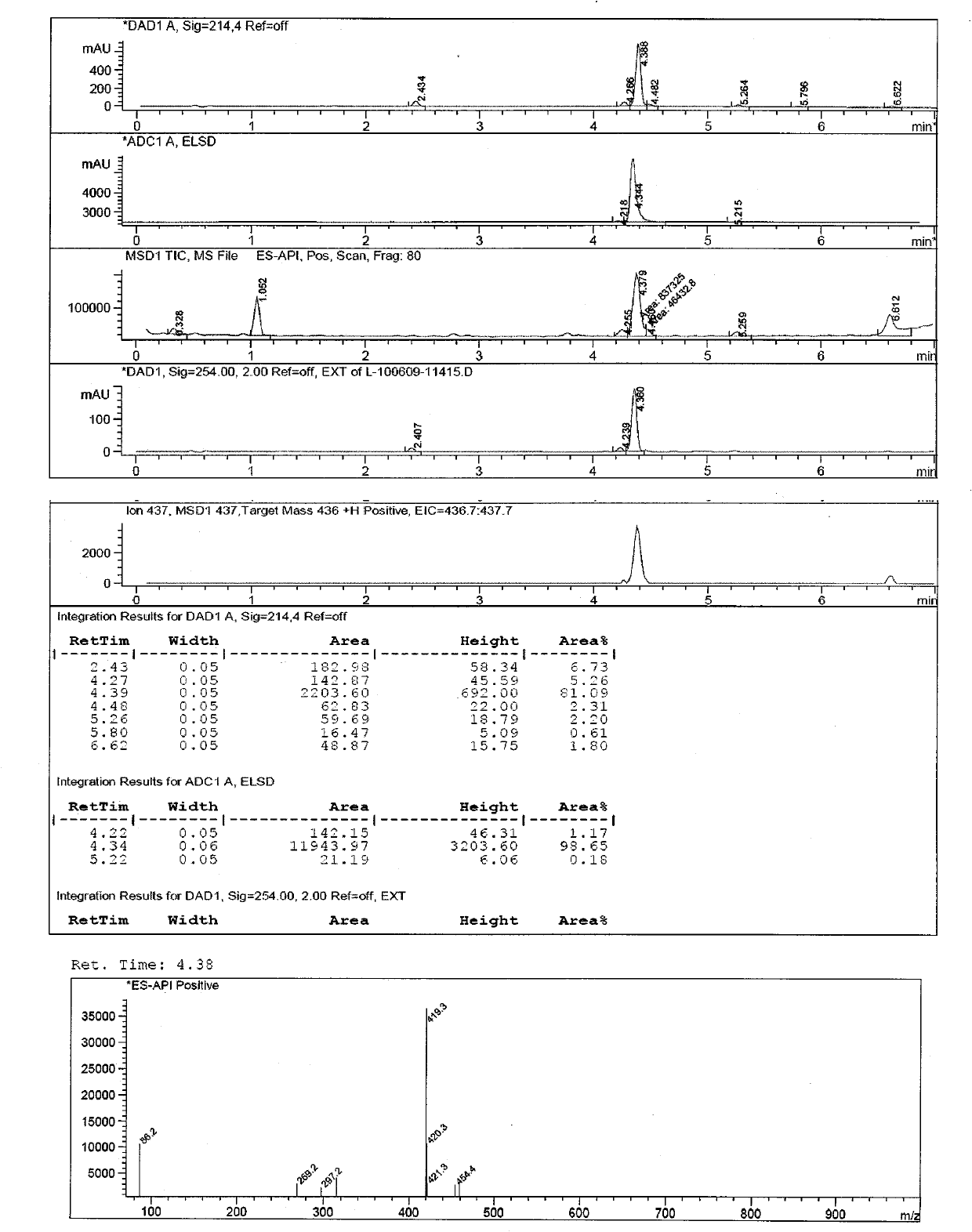
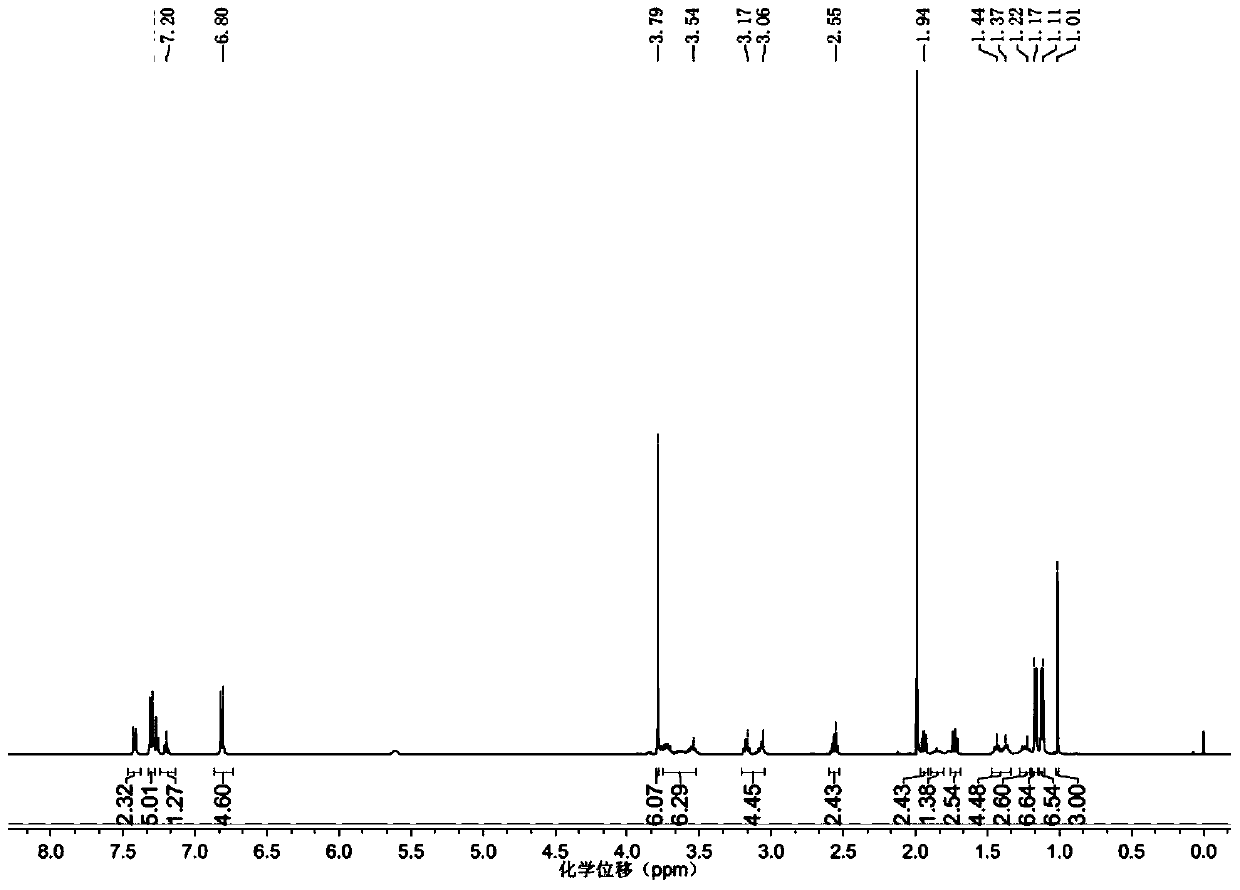
Chlorinated glaucocalyxin A derivative and preparation method and application thereof
InactiveCN101993373AGood inhibitory effectSolve the polarity is smallOrganic active ingredientsPreparation from carboxylic acid halidesBenzeneSulfonyl chloride
The invention discloses a structure and a preparation method of a chlorinated glaucocalyxin A derivative, and application of the chlorinated glaucocalyxin A derivative to the treatment of cancer thereof. The general molecular structural formula of the chlorinated glaucocalyxin A derivative is shown below, wherein R1 is benzoyl chloride substituent(-C7H6O2), benzene sulfonyl chloride substituent(-C6H6O3S2) or -OH; and R2 is benzoyl chloride substituent(-C7H6O2), benzene sulfonyl chloride substituent(-C6H6O3S2) or -OH. The preparation method is to prepare the disclosed chlorinated glaucocalyxin A derivative by reacting the extracted glaucocalyxin A with benzoyl chloride or the benzene sulfonyl chloride in the presence of triethylamine and 4-dimethylamino pyridine.
Owner:SHANGHAI JINHAO PHARMA DEV
Deoxyribonucleic acid (DNA) base analogue with photo-crosslinking group biaziridine and synthetic method thereof
ActiveCN103435648ASimple stepsRoutine operation is stableGroup 5/15 element organic compoundsMonomerN,N-Diisopropylethylamine
The invention discloses a deoxyribonucleic acid (DNA) base analogue with a photo-crosslinking group biaziridine and a synthetic method thereof, and relates to the DNA base analogue and the synthetic method thereof. The DNA base analogue with the photo-crosslinking group biaziridine is a biaziridine phosphoramidite monomer; an intermediate product with two hydroxyl groups is connected on the basis of a compound 1; a material 1-amino-hexylene glycol is led; an amido bond is formed under condensation of dicyclohexylcarbodiimide and 1-hydroxybenzotriazole, so as to obtain an immediate product 2; the material 4-4'-dimethoxy triphenyl methyl chloride is led; pyridine and dichloromethane are taken as solvents; an immediate product 3 is obtained under catalytic action of 4-dimethylamino-pyridine; the material 2-cyanoethyl N, N-diisopropyl chlorinated phosphoramide is led; N,N-diisopropylethylamine is taken as alkali and a catalyst; dichloromethane is taken as a solvent, so as to obtain the final product under the condition of anaerobic reaction without water.
Owner:XIAMEN UNIV
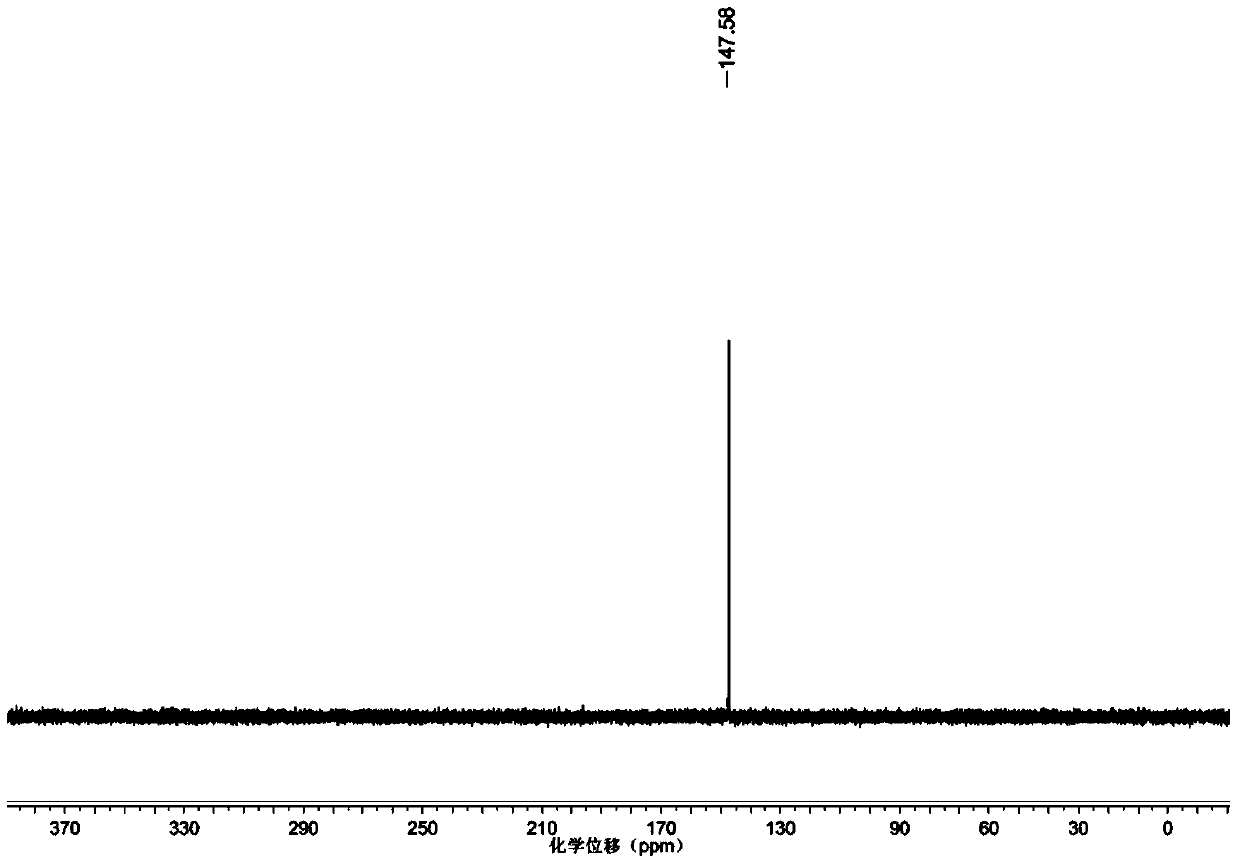
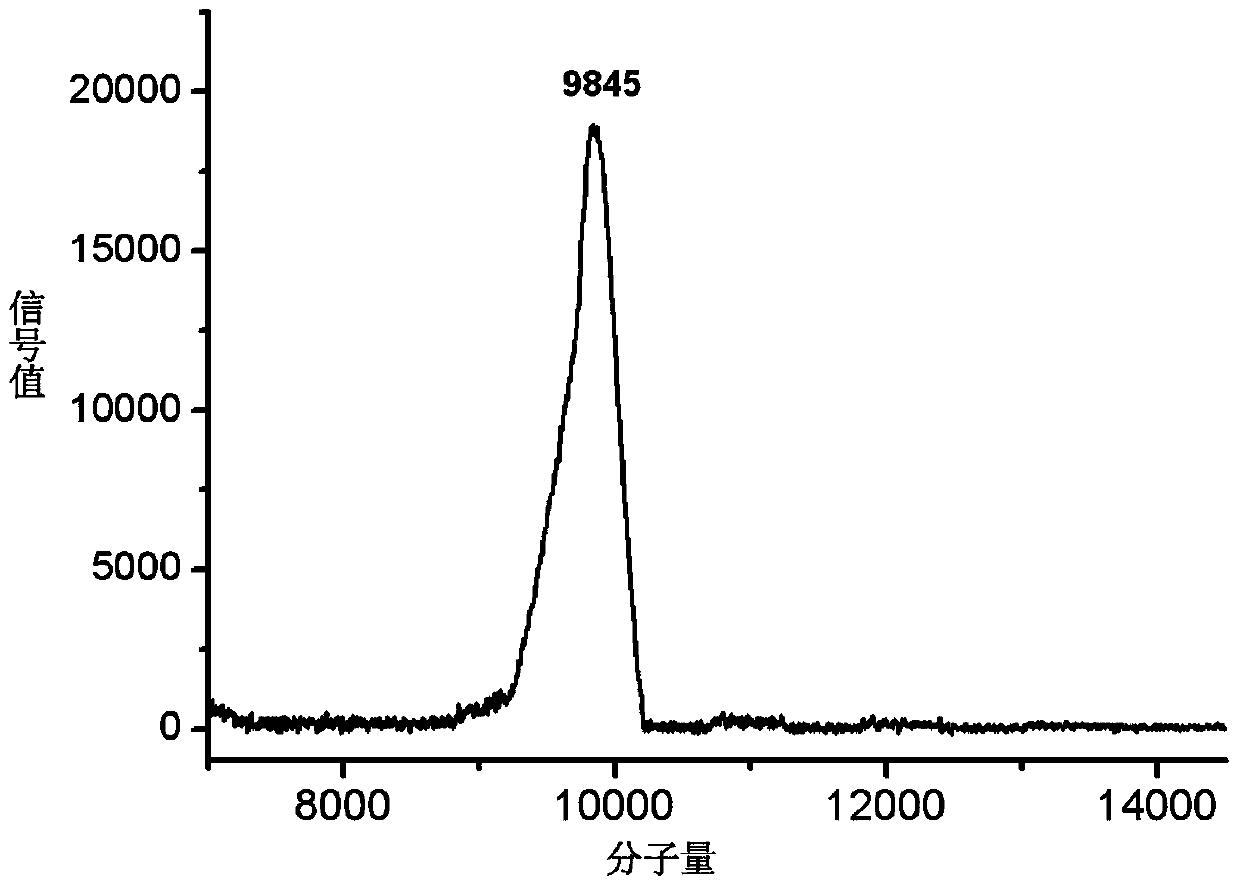
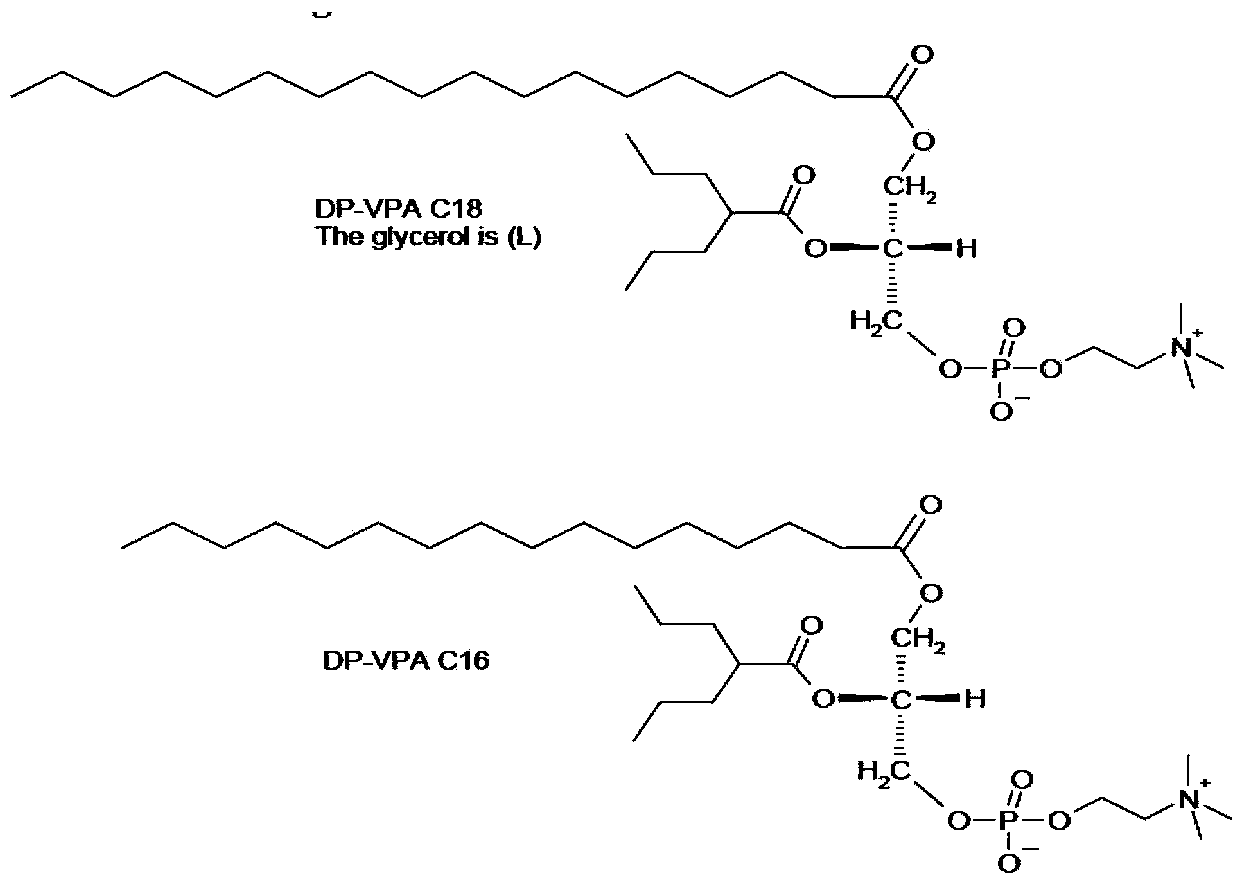
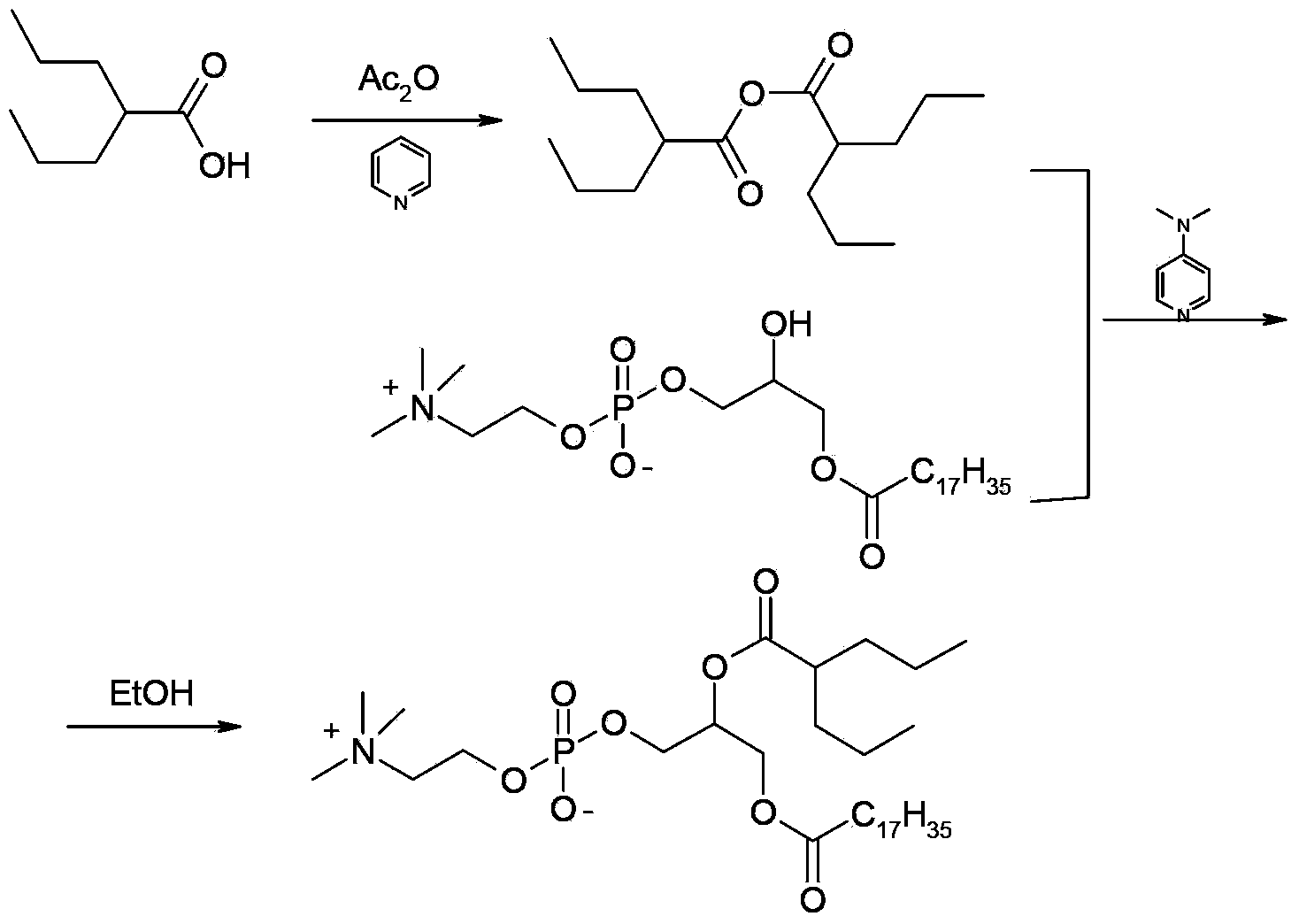
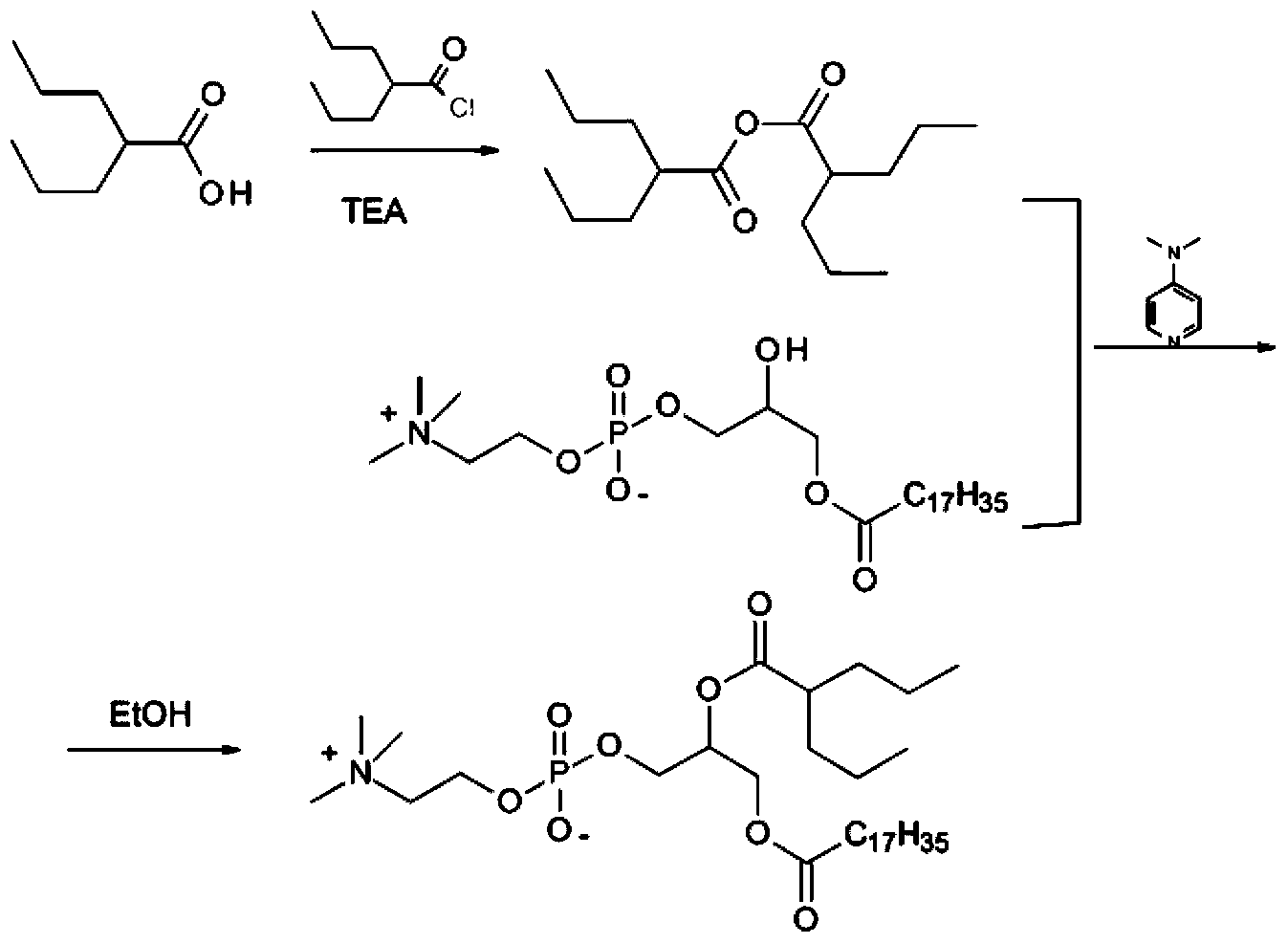
Preparation method of valproic acid phospholipid derivative
ActiveCN104230981AEasy to purifyHigh purityPhosphatide foodstuff compositionsAcetic anhydrideValproic Acid
The invention belongs to the field of organic compound preparation, and discloses a preparation method of a valproic acid phospholipid derivative. The method comprises the following steps: by using tetrahydrofuran as a solvent and triethylamine as an acid-binding agent, reacting propyl valeric chloride and equal mole of valproic acid at 10-50 DEG C, filtering, distilling to obtain valproic acid anhydride, adding lysophosphatidyl choline into the valproic acid anhydride solution, reacting at 60-85 DEG C under the catalytic action of 4-dimethylaminopyridine for 2-6 hours, adding acetone to precipitate the product, and refining with ethanol / acetone to obtain the valproic acid phospholipid derivative. By reacting the valproic acid and propyl valeric chloride to prepare the valproic acid anhydride, no influence of the acetic anhydride exists, and the valproic acid anhydride can be easily purified to obtain the high-purity valproic acid anhydride, thereby preparing the high-purity valproic acid phospholipid derivative.
Owner:NHWA PHARMA CORPORATION
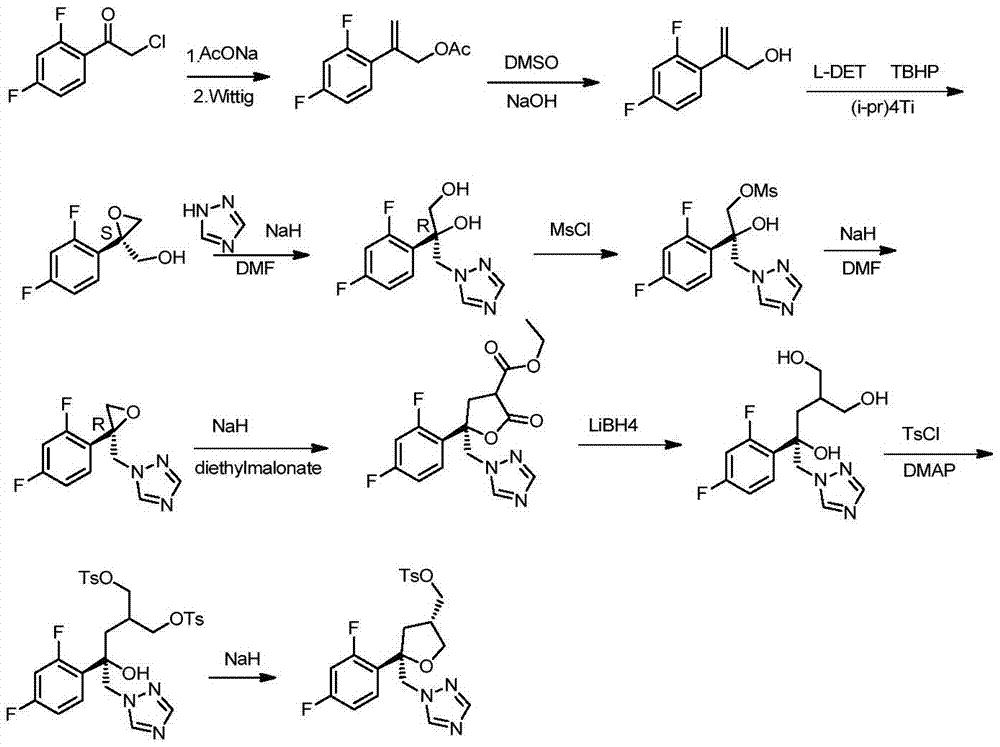
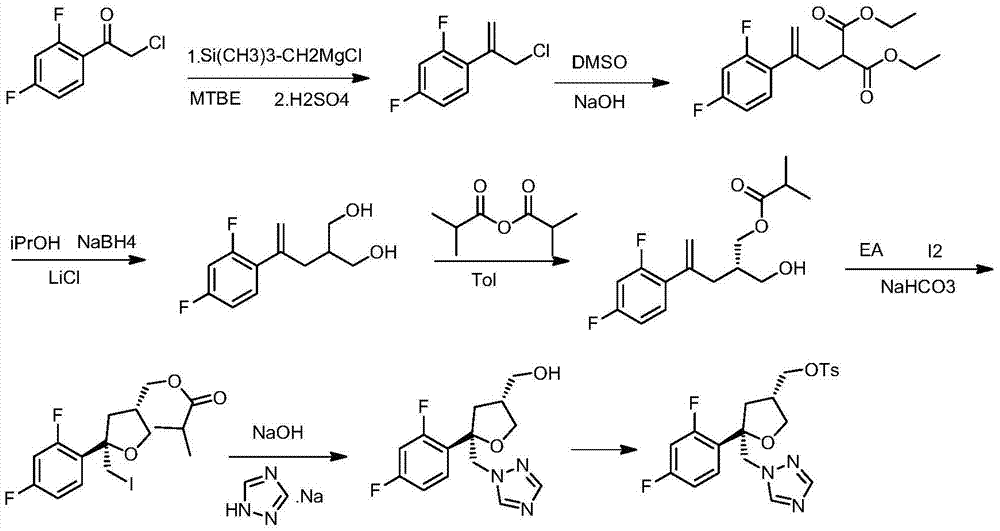
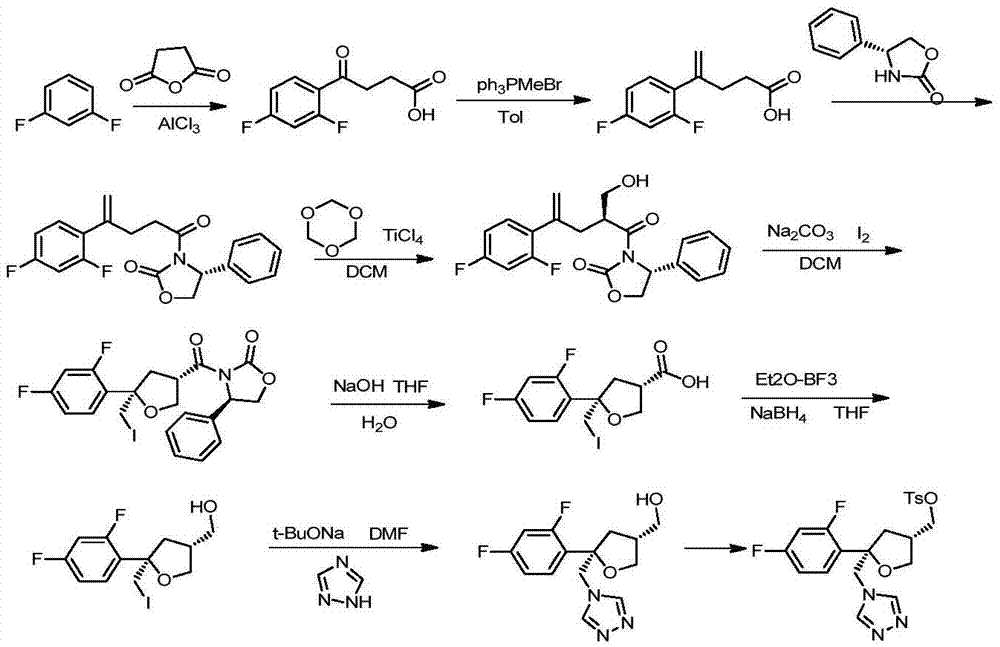
Preparation method of posaconazole main ring
ActiveCN105440022AGood choiceOperational securityOrganic chemistrySulfonyl chloridePotassium borohydride
The invention discloses a preparation method of a posaconazole main ring. The preparation method comprises steps as follows: 1), a compound F is dissolved in an organic solvent I, then thionyl chloride is added for reaction, and a compound G is obtained; 2), the compound G is added to an organic solvent II, triazole sodium and a dissolving promoter are added for reaction, and a compound H is generated; 3), the compound H is added to an organic solvent III, zinc chloride and sodium borohydride or potassium borohydride are added for reaction, and a compound I is obtained; 4), the compound I is added to an organic solvent IV, alkali, 4-dimethylaminopyridine and 4-methyl-benzene sulfonyl chloride are added for reaction, and a target compound J is obtained. The method has the advantages of good selectivity, safety in operation, stable reaction and high yield, and the problems of high probability of generation of intramolecular ring-closure impurities and low yield of an original synthetic route are solved.
Owner:CHONGQING WEIPENG PHARMA
Resin-based graphene heat conduction composite material and method for preparing same
InactiveCN106633366AImprove thermal conductivityImprove cooling effectHeat-exchange elementsEthylenediamineResin matrix
Owner:YAHAM OPTOELECTRONICS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com