Polyene ether compounds and preparation method thereof
A technology of compound and polyalkene ether, which is applied in the fields of polymer chemistry and material science, can solve the problems of no preparation of polyalkene ether compounds, few and few synthesis of polyalkene ether compounds, etc., and achieve the elimination of cytotoxicity and damage to materials The effect of photoelectric performance, excellent area selection type and three-dimensional selection type, simple and controllable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
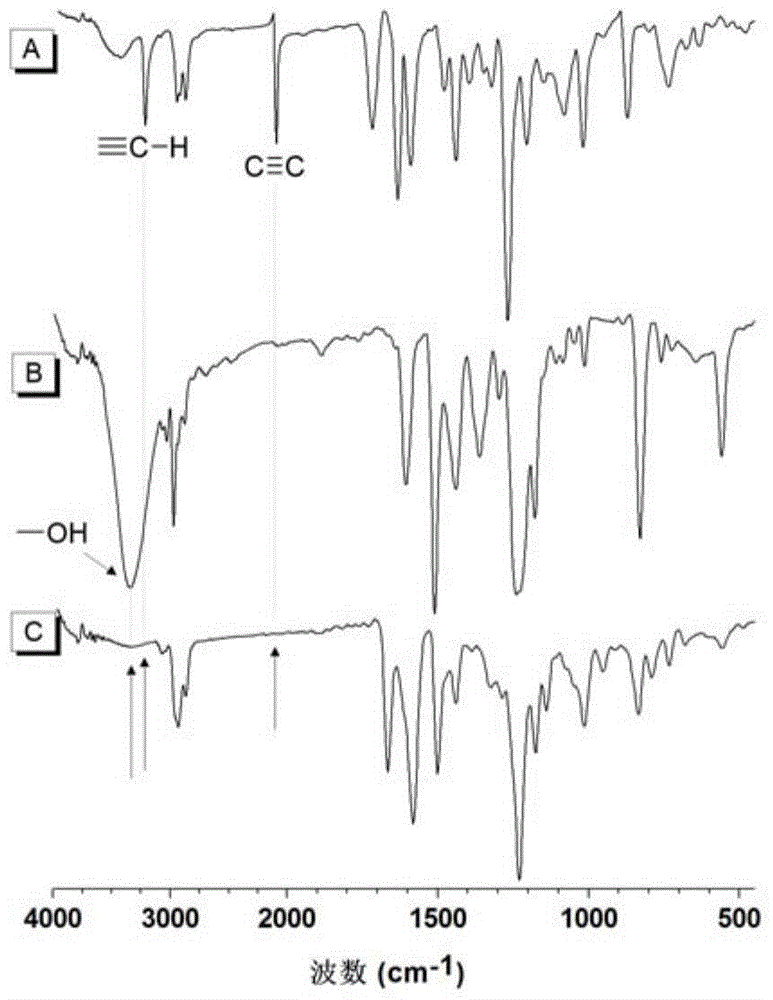
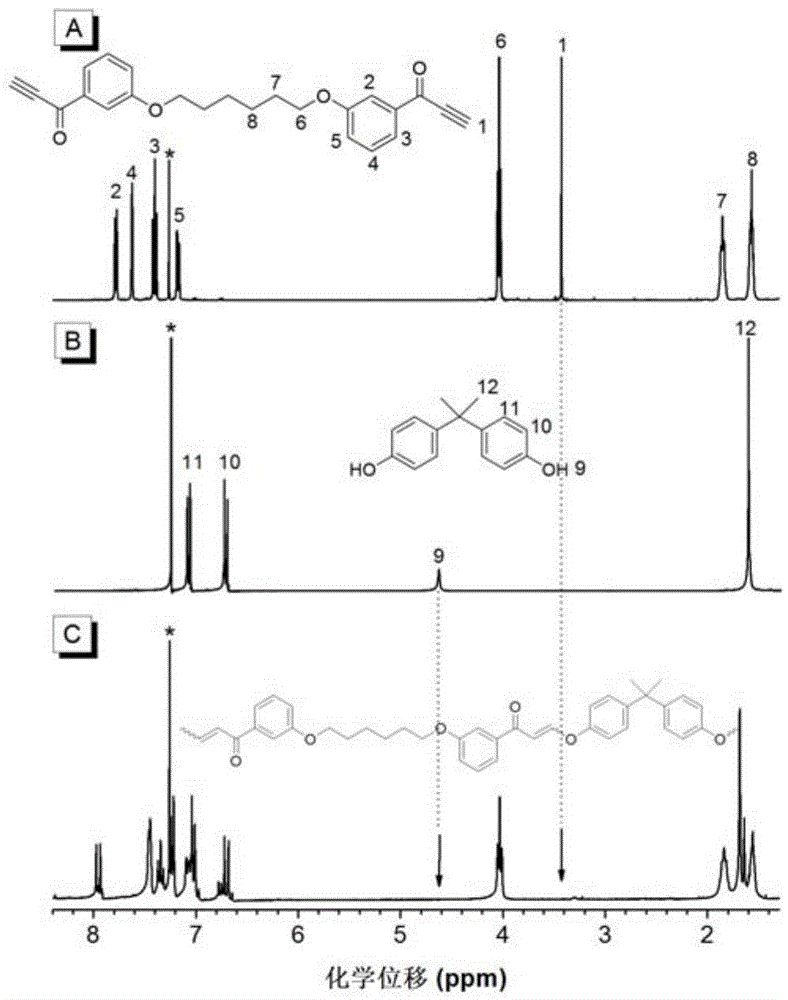
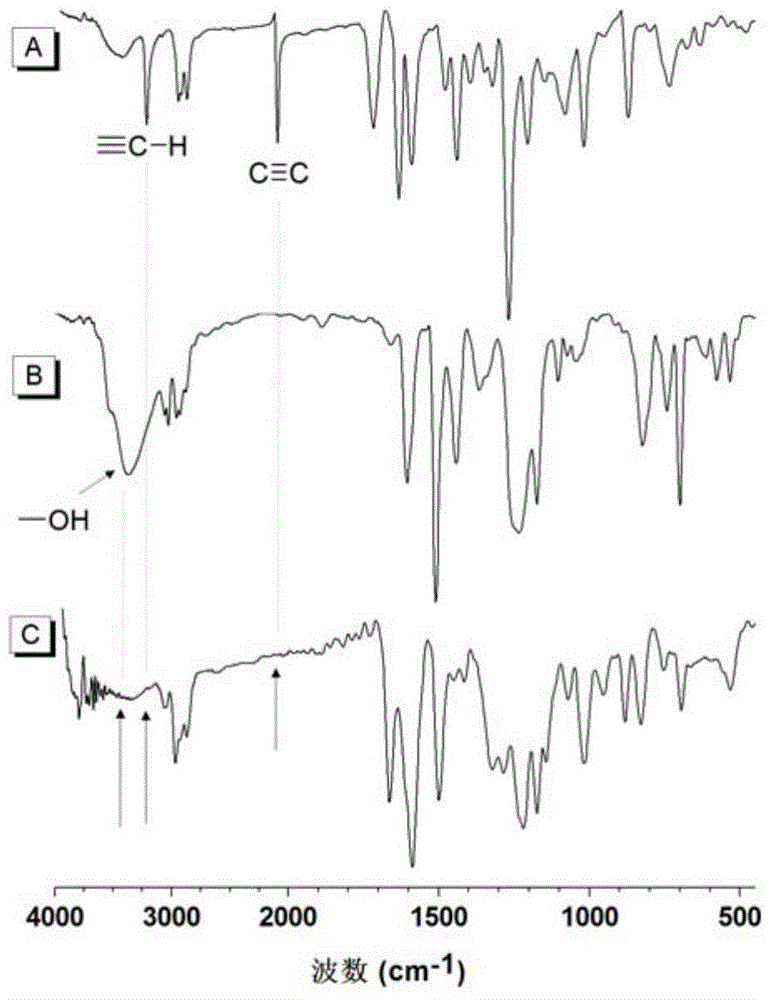
[0060] (1) The first monomer binary alkynyl compound The synthesis method of is synthesized according to the synthesis method of the applicant in the published literature (Macromolecules, 2005, 38, 6382).
[0061] (2) The second monomeric dihydroxy compound direct purchase
[0062] (3) Hydroxy-alkyne polymerization to prepare polyalkene ether compounds, the structural formula is as formula (Ⅰ-1)
[0063]
[0064] Add 37.4mg (0.10mmol) of the first monomer, 22.8mg (0.10mmol) of the second monomer and 0.9mL tetrahydrofuran into a 10mL polymerization tube. After the monomers are completely dissolved, raise the temperature to 25°C. Dissolve 0.976 mg (0.008 mmol) of DMAP in 0.1 mL of tetrahydrofuran, and after the system is kept at constant temperature, add DMAP and react for 4 hours. After the reaction solution was diluted with 2 mL of tetrahydrofuran, it was added dropwise into 150 mL of rapidly stirring n-hexane through a cotton filter to obtain a pink flocculent precipi...
Embodiment 2~6
[0069] Investigated the impact of different reaction times on the click polymerization reaction among the embodiment 2~6, and raw material kind and polymerization process are all identical with embodiment 1, influence a The results are shown in Table 1.
[0070] Table 1
[0071]
[0072] a Reaction in THF in air; T=25°C; [M] 0 = 100 mM; [DMAP] = 10 mM.
[0073] b t = reaction time.
[0074] c Determined by GPC with linear polystyrene as calibrator and THF as mobile phase.
[0075] It is completely soluble in common organic solvents such as THF, chloroform, dichloromethane and DMF.
Embodiment 7~11
[0077] Embodiment 7~11 has investigated the impact of different solvents on click polymerization reaction, and raw material kind and polymerization process are all identical with embodiment 1, influence a The results are shown in Table 2.
[0078] Table 2
[0079]
[0080] a Reaction under air for 4 hours; T=25°C; [M] 0 = 100 mM; [DMAP] = 10 mM.
[0081] b Solubility (S) tested in common organic solvents such as THF, dichloromethane, toluene, chloroform and DMF: √=completely soluble; Δ=partially soluble.
[0082] c Determined by GPC with linear polystyrene as calibrator and THF as mobile phase.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com