Preparation method of hydrolysis-resistant hyperbranched polycaprolactone
A technology of polycaprolactone and hyperbranched polyether, applied in the field of biodegradable materials, can solve the problems of complex preparation process of hyperbranched polycaprolactone, metal residues, fast hydrolysis speed and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
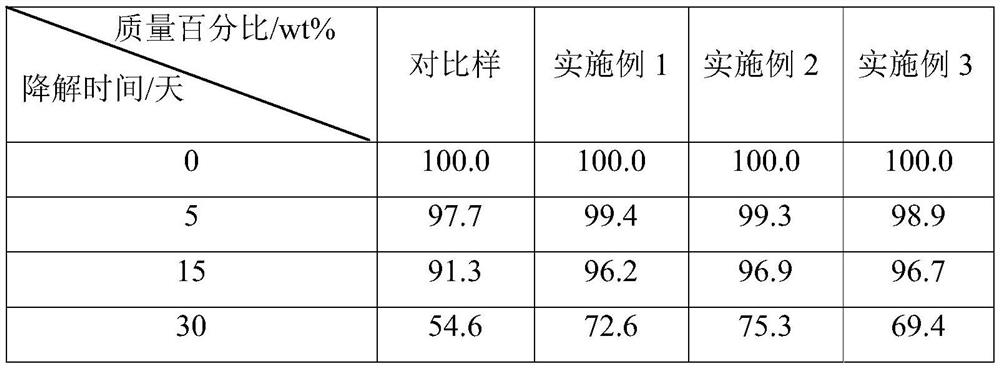
Embodiment 1
[0027] Ditrimethylolpropane 150.0g, phosphazene base (t-BuP 4 ) 3.0g, transferred to a high-pressure reactor, replaced with nitrogen three times, then heated up to 115°C for dehydration for 1 hour, added dropwise 1950g of 3-ethyl-3-hydroxymethylbutylene oxide, and controlled the pressure during the reaction to be less than 0.4MPa. The temperature is set at 115°C, the feeding time is 14 hours, the internal pressure is 4 hours after the feeding is completed, and the temperature is lowered after 1 hour of monomer removal, and the material is discharged to obtain a yellowish liquid. The hyperbranched polyether polyol 7g that reaction is made, phosphazene base (t-BuP 4 ) 0.35g and ε-caprolactone 63g were added to a 200mL eggplant-shaped reaction bottle, nitrogen was replaced three times and then sealed with a rubber stopper, 50mL of anhydrous toluene was added using a syringe, and the reaction bottle was placed in an oil bath at 120°C for 10 hours. After the reaction, the reaction...
Embodiment 2
[0030] Ditrimethylolpropane 75.0g, dipentaerythritol 82.4g, phosphazene base (t-BuP 2 ) 5.5g, transferred to a high-pressure reaction kettle, replaced with nitrogen three times, then heated up to 110°C for dehydration for 1h, added dropwise 1830g of 3-methyl-3-hydroxymethylbutene oxide, and controlled the pressure during the reaction to be less than 0.4MPa. The temperature is set at 120°C, the feeding time is 13 hours, the internal pressure is 4 hours after the feeding is completed, the temperature is lowered and the material is discharged after 1 hour of monomer removal, and a slightly yellow liquid is obtained. The hyperbranched polyether polyol 6.5g that reaction is made, phosphazene base (t-BuP 2 ) 0.33g and 62.5g of ε-caprolactone were added to a 200mL eggplant-shaped reaction bottle, nitrogen was replaced three times and then sealed with a rubber stopper, 55mL of anhydrous toluene was added with a syringe, and the reaction bottle was placed in an oil bath at 110°C for 11...
Embodiment 3
[0033] Mannitol 114.1g, phosphazene base (t-BuP 4 ) 5.0g, transferred to a high-pressure reactor, replaced with nitrogen three times, then raised the temperature to 110°C for dehydration for 1 hour, added 2336g of 3,3-dimethylolbutylene oxide dropwise, controlled the pressure during the reaction to be less than 0.4MPa, and set the reaction temperature to Set at 110°C, the feeding time is 16 hours, the internal pressure is 5 hours after the feeding is completed, the temperature is lowered and the material is discharged after 1 hour of monomer removal, and a light yellow liquid is obtained. The hyperbranched polyether polyol 8.0g that reaction is made, phosphazene base (t-BuP 2 ) 0.34g and ε-caprolactone 72.0g were added to a 200mL eggplant-shaped reaction bottle, nitrogen was replaced three times and then sealed with a rubber stopper, 70mL of anhydrous toluene was added using a syringe, and the reaction bottle was placed in an oil bath at 115°C for 14 hours. After the reaction...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Branching factor | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com