Reactions between ethylene oxide and derivatives of ethylene oxide and CO2 for generating cyclic carbonate from
A technology of cyclic carbonate and ethylene oxide, which is applied in the chemical industry, can solve the problems of difficult industrial production and long reaction time, and achieve the effects of short reaction time, high reaction yield and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
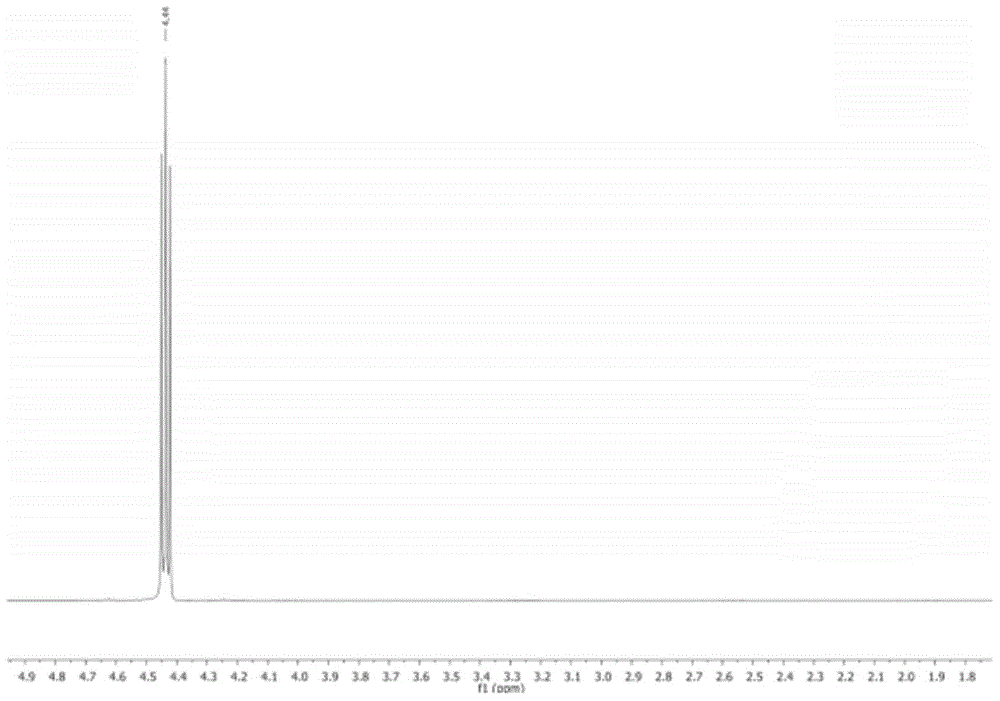
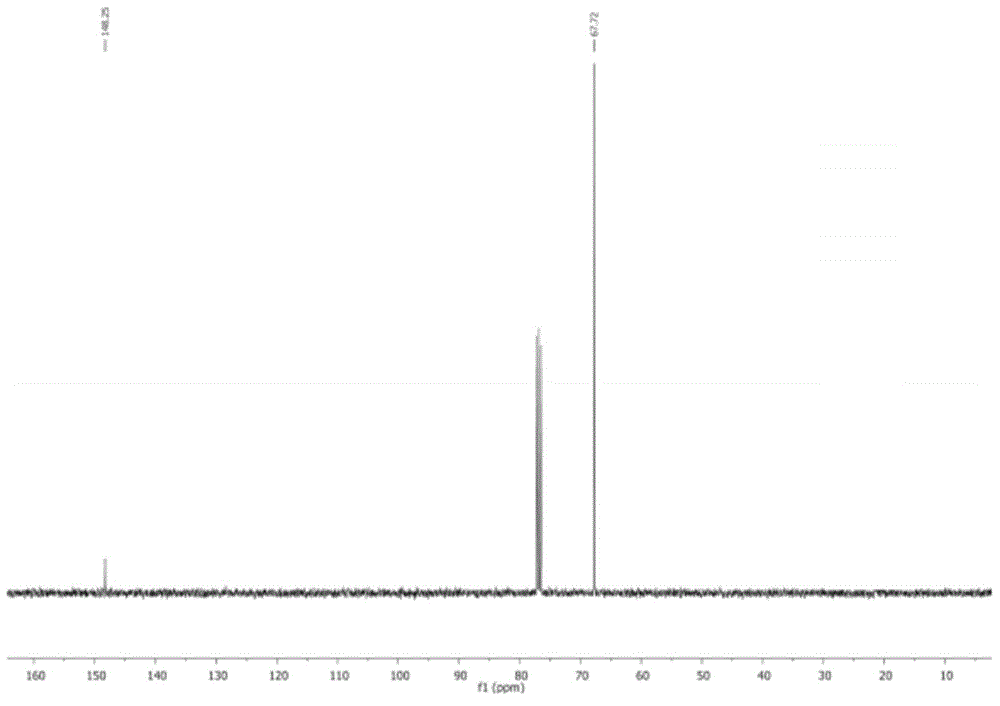
[0048] Taking CMP-Co as a catalyst to catalyze the reaction of ethylene oxide and carbon dioxide as an example to explore the influence of reaction conditions (temperature, pressure and time) on the reaction yield. The structural formula of the product was characterized by NMR. 1 H NMR (ethylene carbonate, 400 MHz, CDCl 3 ):δ=4.2(t,4H); 13 C NMR (400MHz, CDCl 3 ): δ=63.3,155. ( 1 H NMR and 13 C NMR spectrum see figure 1 and 2 ).
[0049] ① 100mg CMP-Co, 400mg tetrabutylammonium bromide; 1.1g ethylene oxide, carbon dioxide at room temperature for 48 hours, the yield of ethylene carbonate was 78.2%;
[0050] ② 100mg CMP-Co, 600mg tetrabutylammonium bromide; 1.1g ethylene oxide, carbon dioxide at room temperature for 48 hours, the yield of ethylene carbonate was 81.2%;
[0051] ③ 100mg CMP-Co, 600mg tetrabutylammonium bromide; 1.1g propylene oxide, carbon dioxide pressure 1.0MPa, 40°C for 12h, the yield of ethylene carbonate was 84.5%;
[0052] ④ 100mg CMP-Co, 600mg tet...
Embodiment 2
[0060] Taking CMP-Zn as a catalyst to catalyze the reaction of ethylene oxide derivatives and carbon dioxide to generate corresponding cyclic carbonates as an example. Table 2 shows the structures and reaction yields of oxirane derivatives and corresponding cyclic carbonates.
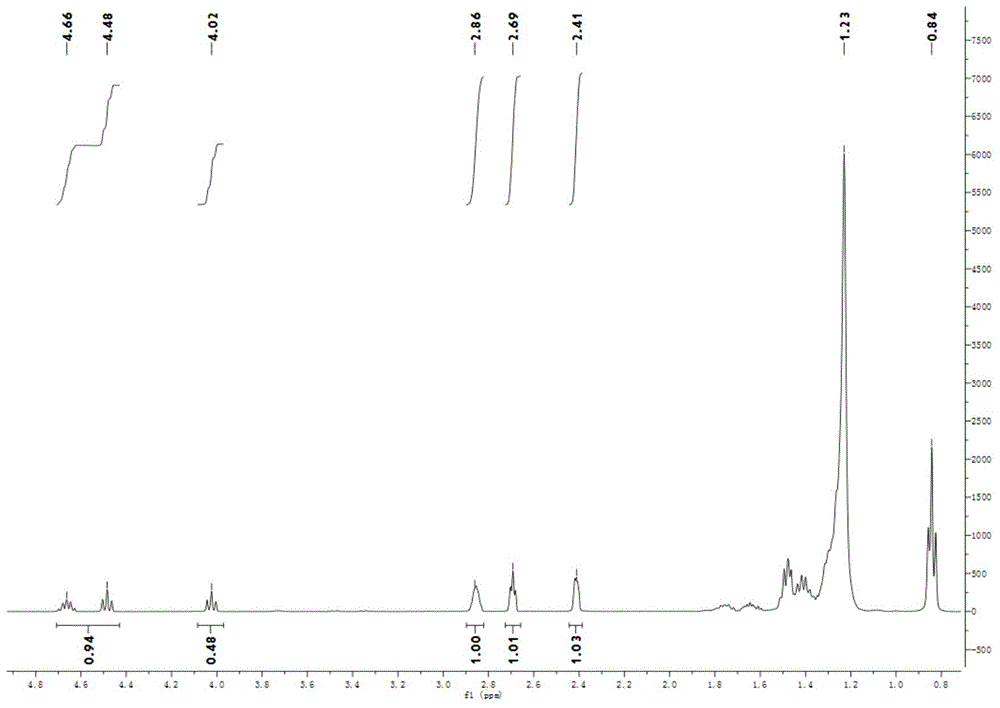
[0061] ① 50mg of CMP-Zn, 600mg of tetrabutylammonium bromide, 5.46ml of 1,2-epoxydodecane, and a carbon dioxide pressure of 3.0MPa were reacted at 120°C for 2h to obtain the product (a) with a yield of 91.6%. 1 H NMR (400MHz, CDCl 3 ),δ=4.43-4.70(m,1H),4.01-4.44(2,2H),2.82-2.89(m,2H),2.68-2.63(m,2H),2,35-2.42(m,2H) ,1.60-1.79(m,2H),1.30-1.54(m,8H),0.75-0.89(m,5H). 13 C NMR (400MHz, CDCl 3 ), δ=155.31 (C=O), 77.31, 71.26, 52.58, 47.28, 34.14, 32.76, 32.13, 29.55, 26.23, 24.64, 22.93, 14.33. Spectrum see image 3 and 4 .
[0062] ② 50mg CMP-Zn, 600mg tetrabutylammonium bromide, 3.32ml glycidol, carbon dioxide pressure 3.0MPa, react at 100°C for 1h, and the yield of product (b) is 91.2%. 1 H NMR (4...
Embodiment 3
[0073] A class of metal-complexed conjugated microporous polymers provided by the present invention catalyze the reaction of ethylene oxide and its derivatives with carbon dioxide to generate cyclic carbonates. The reaction conditions used are also applicable to 1,2-epoxycyclohexane Alkanes and the reaction of 1,2:7,8-dioxoctane with carbon dioxide.
[0074] The following uses CMP-Cr as an example to illustrate the reaction process of these two compounds with carbon dioxide. Table 3 shows the structures of these two compounds, the structures of the corresponding cyclic carbonates and the reaction yields.
[0075] ① 50mg CMP-Cr, 600mg tetrabutylammonium bromide, 5.06mL 1,2-epoxycyclohexane, carbon dioxide pressure 3.0MPa, reaction at 120°C for 12h, the yield of product (j) was 66.3%. 1 H NMR (400MHz, CDCl 3 ),δ=4.63-4.68(m,1H),1.75-1.95(m,2H),1.31-1.65(m,2H). 13 C NMR (400MHz, CDCl 3 ), δ=155.68 (C=O), 76.07, 26.98, 19.38. Spectrum see Figure 19 and 20 .
[0076] ② 50m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com