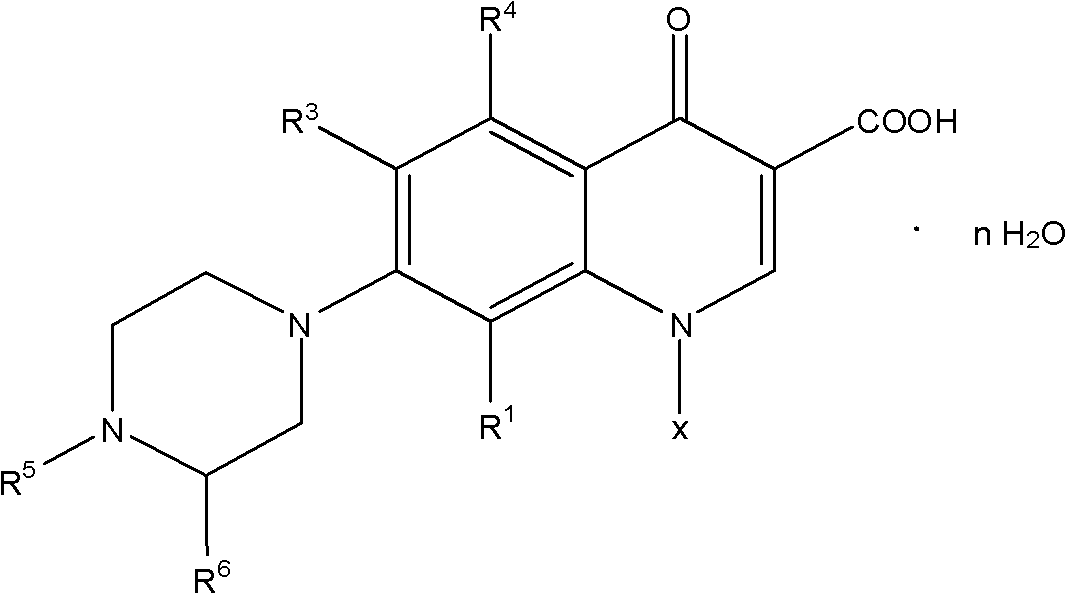
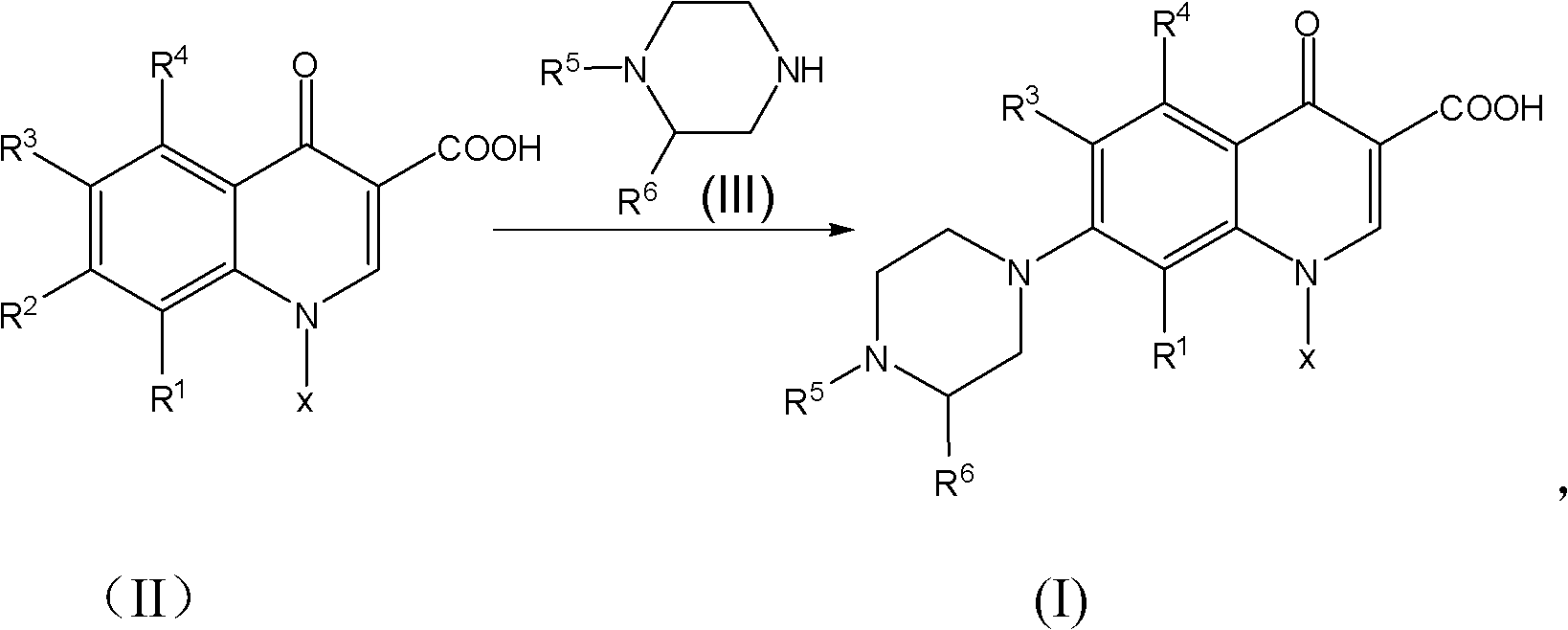
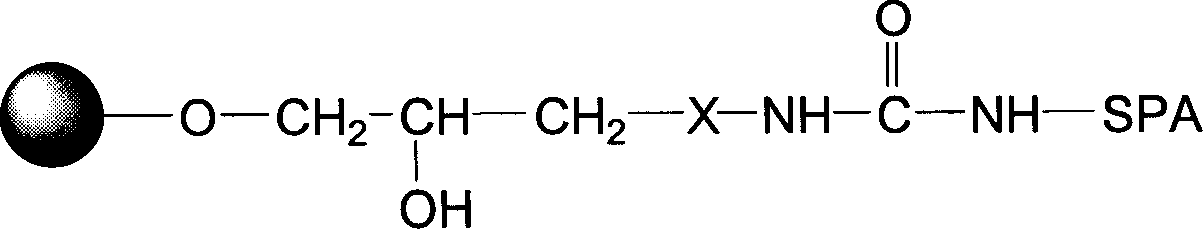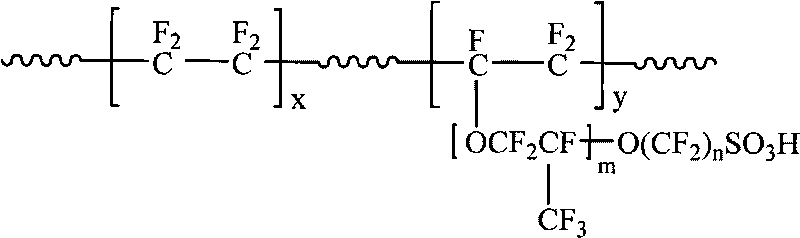Patents
Literature
399 results about "Carbonyldiimidazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
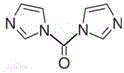
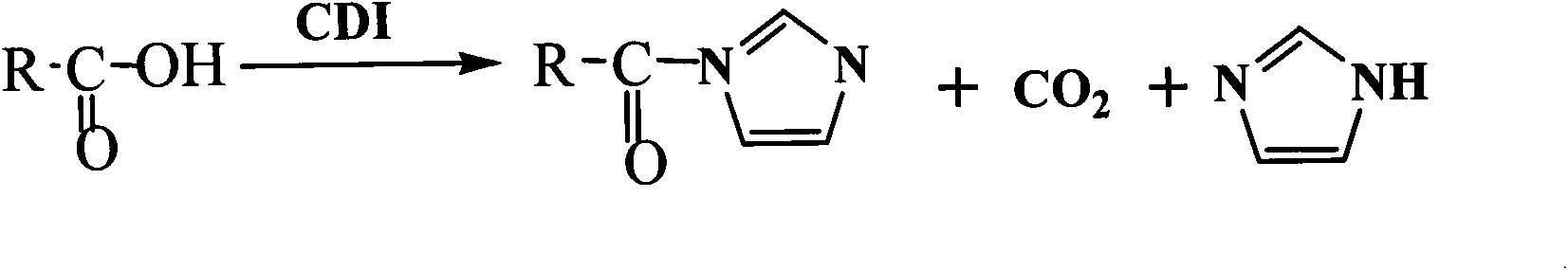
1,1'-Carbonyldiimidazole (CDI) is an organic compound with the molecular formula (C₃H₃N₂)₂CO. It is a white crystalline solid. It is often used for the coupling of amino acids for peptide synthesis and as a reagent in organic synthesis.
Magnetic nanometer ion liquid composite particles as well as preparation method and application thereof
InactiveCN103100358ASimple and fast operationLow costMicroballoon preparationWater/sewage treatment by extractionCarboxyl radicalSilicic acid
The invention discloses magnetic nanometer ion liquid composite particles as well as a preparation method and application of the magnetic nanometer ion liquid composite particles in removing pollutants in water bodies. The preparation method of the magnetic nanometer ion liquid composite particle comprises the following steps of: firstly synthesizing ferric oleate into magnetic Fe3O4 nanometer particles by taking ferric oleate as an iron source and adopting a chemical precipitation method; preparing nanometer nuclear shell type magnetic silicon dioxide with surface amino-functionalization by utilizing the nanometer magnetic particles as a kern, taking ethyl orthosilicate and a silane coupling agent as silicon sources, and utilizing a colloidal sol-gel method; and synthesizing a functionalized ion liquid by utilizing reaction between N, N-carbonyldimidazole (CDI) and an ion liquid containing carboxyl, and thus preparing the magnetic nanometer ion liquid composite particle by utilizing reaction between the functionalized ion liquid and amino on the surface of the magnetic nanometer silicon dioxide; and the method is used for removing pollutants in the water bodies. The method provided by the invention has the advantages that the operation is simple and convenient, the cost is low, the treatment process is simple, and the removal efficiency is high.
Owner:SOUTH CHINA UNIV OF TECH
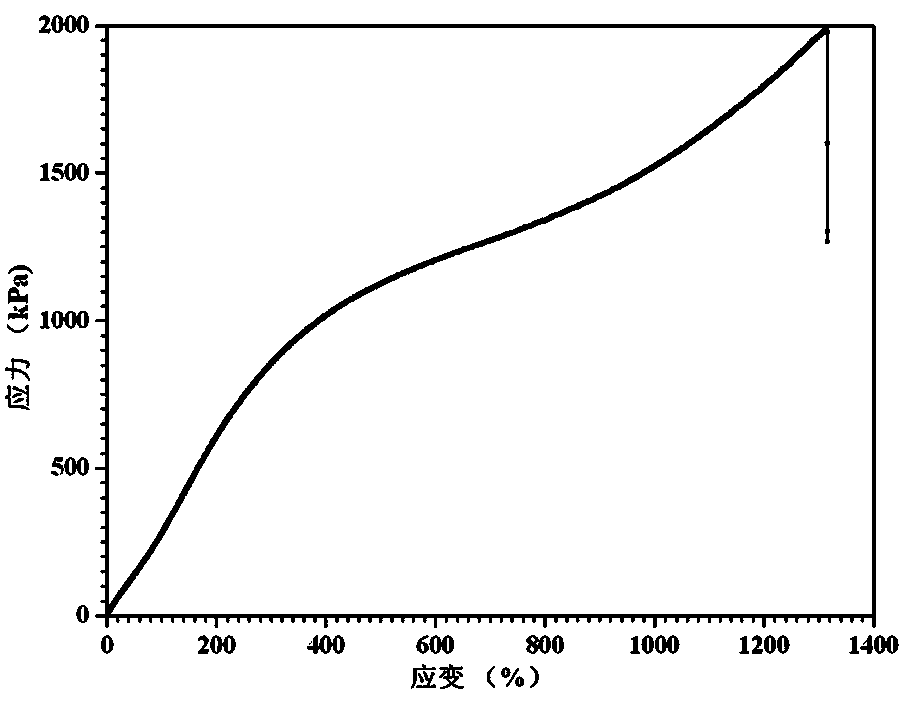
Polyurethaneurea hydrogel and preparation methods therefor
InactiveCN103524697AHigh strengthProvides effective mechanical propertiesOrganic solventPolyethylene glycol
The invention relates to polyurethaneurea hydrogel and preparation methods therefor. The preparation methods comprise two preparation methods. The first preparation methods is as follows: diisocyanate and catalysts are added in a polyethylene glycol (PEG) organic solution for reaction, then diamine chain extender is added, and products are obtained in the organic solvent after precipitation, wherein, the molar ratio of PEG, diisocyanate and diamine is 1:2:1. The second preparation method is as follows: N,N'-dicarbonyl diimidazole is added in a PEG organic solution for reaction, then excess diamine chain extender is added, macromolecules with amidogens at two ends are obtained, finally, diisocyanate is added, and products are obtained in the organic solvent after precipitation, wherein, the molar ratio of PEG and diisocyanate is 1:2. The polyurethaneurea hydrogel has advantages of large elongation and high tensile strength, and has shape memory functions. The polyurethaneurea hydrogel has application prospects at aspects of biomedical hydrogel, tissue engineering, hydrogel support materials, biomedical devices with shape memory functions and the like.
Owner:SUZHOU UNIV
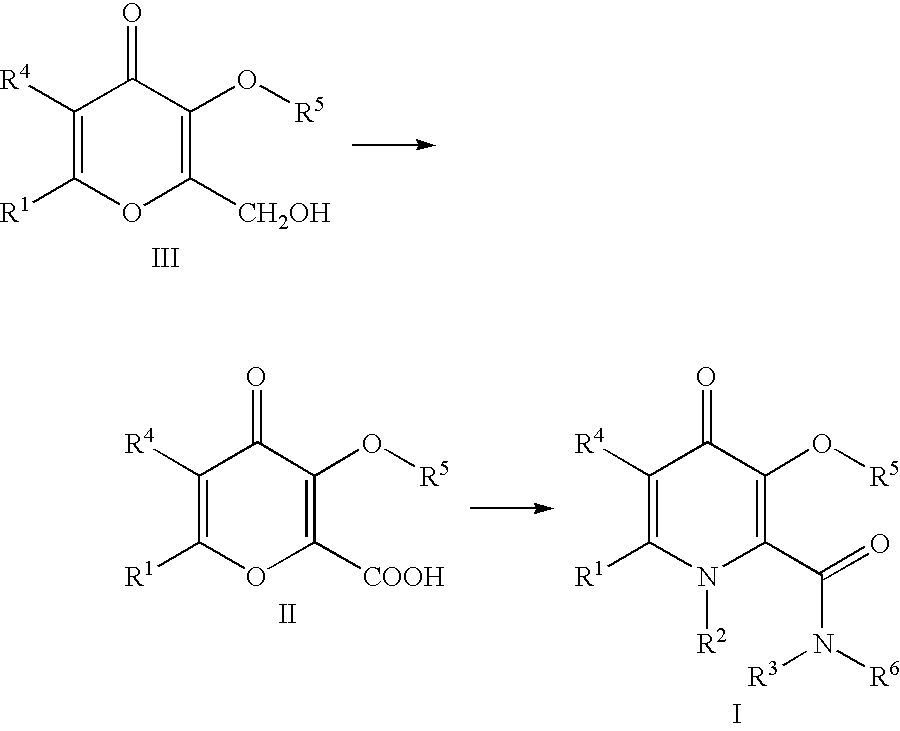
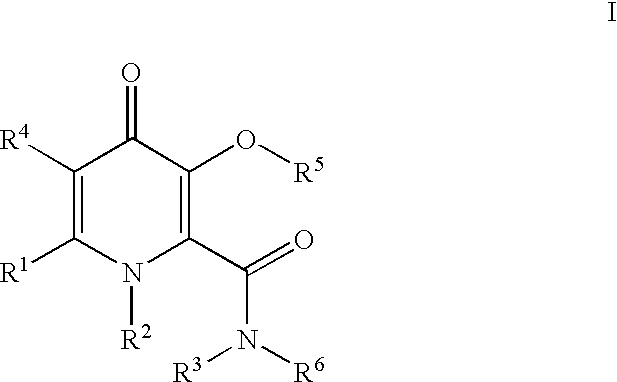
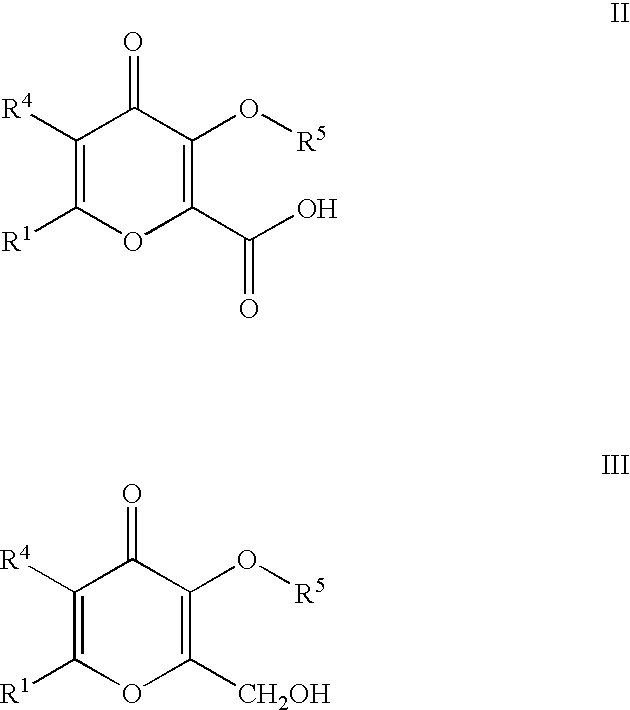
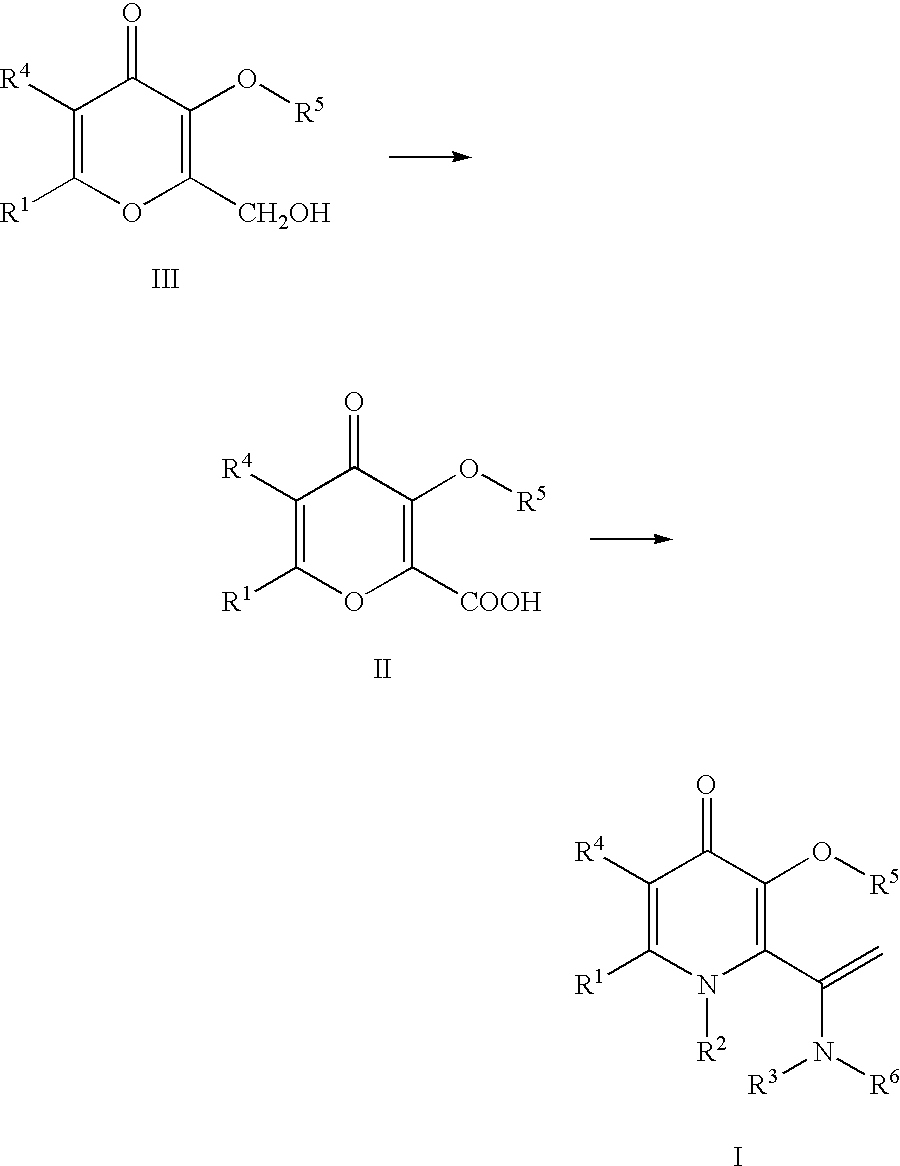
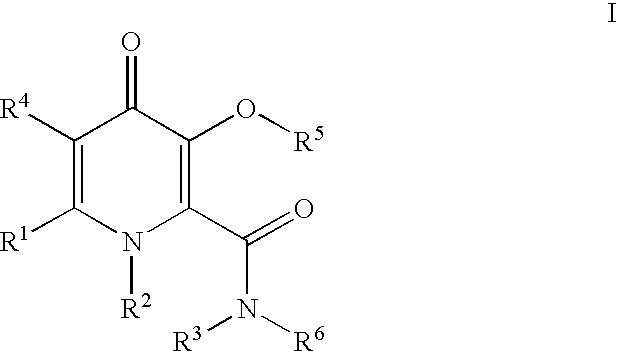
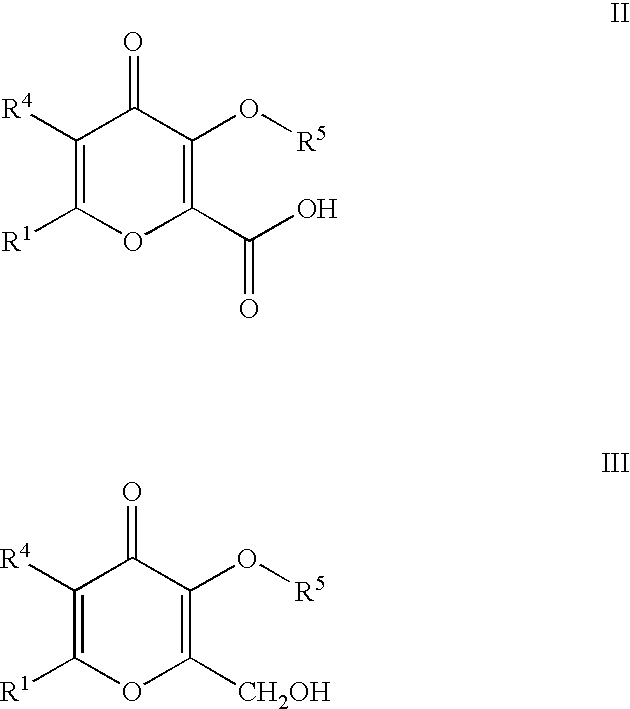
Processes for the manufacturing of 3-hydroxy-N,1,6-trialkyl-4-oxo-1,4-dihydropyridine-2-carboxamide
InactiveUS6426418B1High yieldAmenable for industrial scaleOrganic active ingredientsOrganic chemistrySolventCarbonyldiimidazole
The present invention relates to a novel process for the preparation of 3-hydroxy-N,1,6-trialkyl-4-oxo-1,4-dihydropyridine-2-carboxamide of formula I:The method comprises of the TEMPO oxidation of a primary alcohol of 3-O-protected-2-hydroxymethyl-6-alkyl-4H-pyran-4-one of formula III to 3-O-protected-6-alkyl-4-oxo-4H-pyran-2-carboxylic acid of formula II. Reaction of compound of formula II with methylamine and 1,1-carbonyldiimidazole in an inert solvent affords 3-O-protected-N,1,6-trialkyl-4-oxo-1,4-dihydropyridine-2-carboxamide, which is deprotected to give of 3-hydroxy-N,1,6-trialkyl-4-oxo-1,4-dihydropyridine-2-carboxamide of formula I.
Owner:APOTEX TECH INC
CGRP Antagonist Salt
InactiveUS20100286122A1Efficient synthesisEfficient preparationBiocideNervous disorderPotassiumFormamide
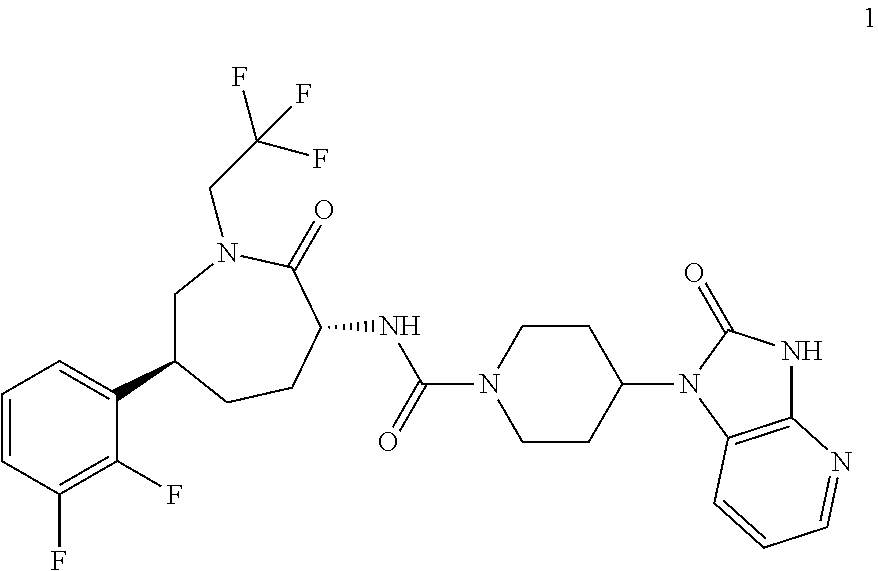
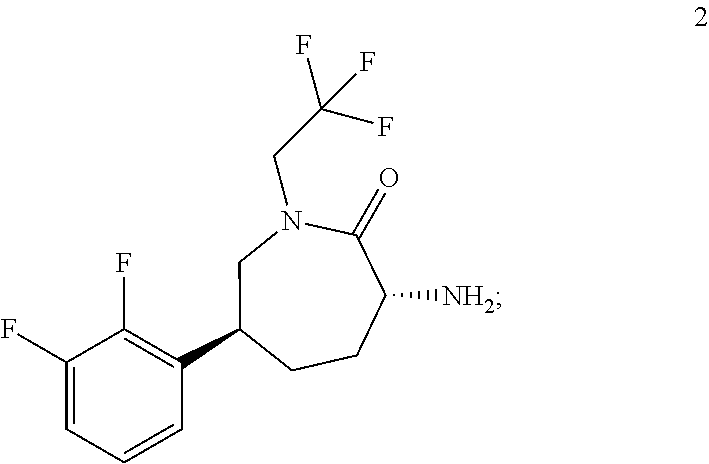
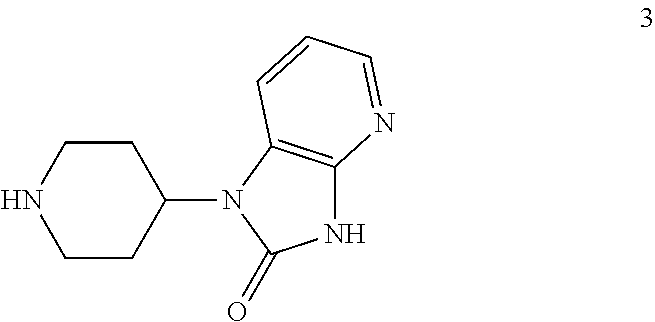
An efficient synthesis for the preparation of N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxamide, by coupling (3R,6S)-3-amino-6-(2,3-difluorophenyl)-1-(2,2,2-trifluoroethyl)azepan-2-one and 2-oxo-1-(4-piperidinyl)-2,3-dihydro-1H-imidazo[4,5-b]pyridine dihydrochloride with 1,1′-carbonyldiimidazole (“CDI”) as carbonyl source; an efficient preparation of the potassium salt of N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxamide; efficient syntheses for the preparation of intermediates (3R,6S)-3-amino-6-(2,3-difluorophenyl)-1-(2,2,2-trifluoroethyl)azepan-2-one and 2-oxo-1-(4-piperidinyl)-2,3-dihydro-1H-imidazo[4,5-b]pyridine dihydrochloride, and the potassium salt of N-[(3R,6S)-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl]-4-(2-oxo-2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxamide including the potassium salt ethanolate and potassium salt hydrate.
Owner:MERCK SHARP & DOHME CORP
Magnetic nanoparticle-based immobilized laccase and ionic liquid composite particle and application thereof
InactiveCN103007847AImprove stabilityHigh reuse rateOn/in inorganic carrierMicroballoon preparationSilicic acidSilanes
The invention discloses a magnetic nanoparticle-based immobilized laccase and an ionic liquid composite particle, a preparation method thereof and application in removal of contaminants in water. The preparation method comprises the steps that carbonyl iron is taken as an iron source and is synthesized to be gamma-Fe2O3 nano-particles through a chemical precipitation method; the magnetic nanoparticles are taken as cores, tetraethoxysilane and a silane coupling agent are taken as a silicon source, and by means of a sol-gel method, surface amino-functionalized nano core-shell type magnetic silicon dioxide is prepared; 1-ethyl-(3-dimethyllaminopropyl) carbodiie hydrochlide is taken as a coupling agent, and laccase is bonded on the surface of the nano core-shell type magnetic silicon dioxide in a covalent bond manner to obtain the immobilized laccase of the magnetic nanoparticle silicon dioxide particles; and then the immobilized laccase is reacted with a functionalized ionic liquid which is synthesized by reacting N,N-carbonyldiimidazole and an ionic carboxy contained liquid to prepare the magnetic nanoparticle immobilized laccase and ionic liquid composite particle, and the composite particle is applied to the removal of the contaminants in water.
Owner:SOUTH CHINA UNIV OF TECH
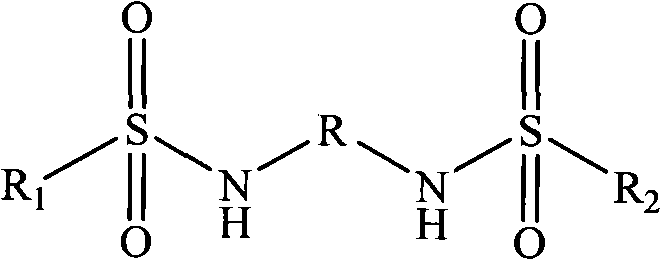
A proton exchange film for direct carbinol fuel battery and its making method
InactiveCN101188301AImprove proton conductivityImprove power densityFinal product manufactureSolid electrolyte fuel cellsElectrical conductorDiffusion resistance
The invention discloses a preparation method of a proton exchange membrane used for a direct methanol fuel cell. The method is realized by that polyether ether ketone is added into concentrated sulfuric acid to carry out sulfonation reaction, thereby obtaining sulfonated polyether ether ketone, then the sulfonated polyether ether ketone is dissolved in organic solvent, N, N (1)-Carbonyldiimidazole is added to stir for one to three hours, coupling agent is mixed for stirring the reaction for 1.5 to 4 hours, then inorganic crosslinking agent is mixed to react under the temperature of 50 to 80 DEG C. Proton conductors are mixed to continue getting the mixed solution of the sulfonated polyether ether ketone or the inorganic crosslinking agent or proton conductors under the temperature. Finally the proton exchange membrane for a direct methanol fuel cell is obtained by that the mixed solution of the sulfonated polyether ether ketone or the inorganic crosslinking agent or proton conductors is / are processed through membrane forming, drying and exuviation. The membrane has the advantages of good methanol diffusion resistance, low cost, high proton conductivity and good water-resistant swelling performance under high temperature. The preparation method is simple, the raw materials have low price, and the production cost is low.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method of pimavanserin
The invention discloses a preparation method of pimavanserin. The method comprises the following two steps: firstly, 4-isobutoxy benzene methylamine and carbonyl diimidazole are subjected to acylation reaction to obtain N-(4-isobutoxy phenyl)-1H-imidazole-1-formamide, and the N-(4-isobutoxy phenyl)-1H-imidazole-1-formamide and N-(4-fluorophenyl)-1-methylpiperidine-4-amine are subjected to urea reaction, so as to obtain the pimavanserin. The prepared pimavanserin is good in quality and high in yield, the reagent toxicity is relatively low, the operation is simple and easy to control, and the pimavanserin is suitable for industrial production.
Owner:NKD PHARMA CO LTD
Method for modifying nano metal oxide by coupling graft
InactiveCN101880482AHigh grafting rateImprove grafting efficiencyPigment treatment with organosilicon compoundsPigment treatment with non-polymer organic compoundsCarbonyldiimidazoleEnergy conservation
The invention discloses a method for modifying a nano metal oxide by coupling graft, and belongs to the technical field of nano granule surface modification. The nano metal oxide is used as a raw material, N,N'-carbonyl diimidazole (CDI) is used as an activating agent, and a finished product is obtained by simple processes of drying treatment, coupling reaction, separation and purification, coupling graft, re-separation and re-purification. The method has the advantages of simplicity, mild reaction conditions, high grafting efficiency which reaches 87 percent, energy conservation, emission reduction, low cost and suitability for large-scale industrial production. The nano metal oxide prepared by using the method can be widely applied in the fields of rubber, coating, ink, dye, glass, piezoelectric ceramics, photoelectrons, medicament and the like.
Owner:CHONGQING UNIV
Preparation method of endotoxin absorbent for blood perfusion
InactiveCN1864755AReduce non-specific adsorptionAvoid instabilityOther blood circulation devicesHaemofiltrationEndotoxin removalBiocompatibility Testing
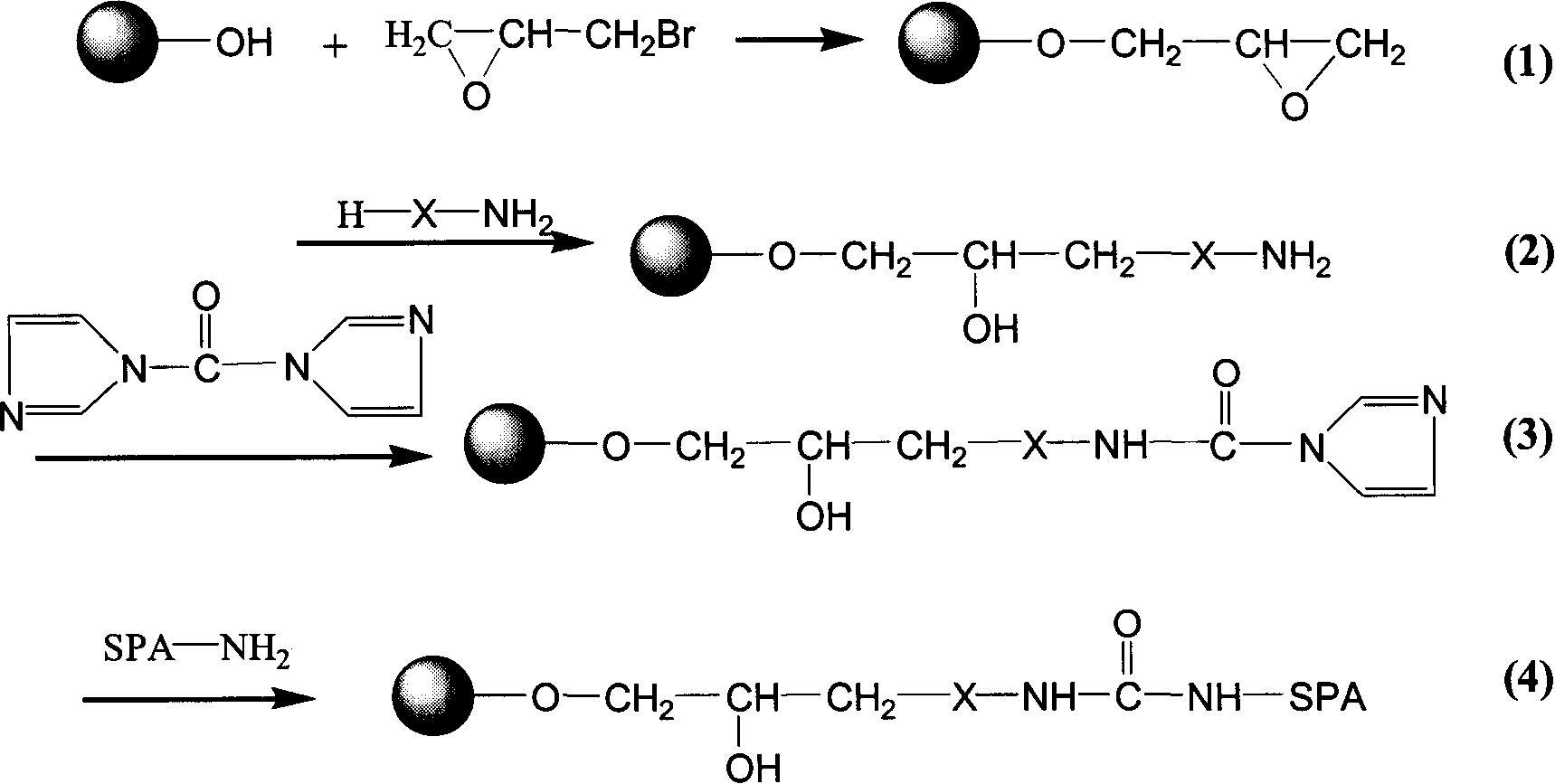
The invention relates to an endotoxin adsorbent, which in detail relates to a method of preparing endotoxin adsorbent for blood perfusion. The method employs spherical agarose gel with good biocompatibility as base material, employs epichlorohydrin, hexamethylene diamine, 1, 1'-carbonyldiimidazole as activating agent, and bonds polymyxin B for endotoxin removal. The invention is characterized in that it overcomes the toxicity of cyanogen bromide and instability of aglycone, improves the safty and reliability of operation, reducesnon-specific adsorption through bonding polymyxin B with 1, 1'-carbonyldiimidazole, and improves biocompatibility of adsorbent and suits for treating endotoxemia. The invention is also characterized by simple experimental procedure and high clearance for endotoxin.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of double-targeting cationic ultrasound microbubbles carrying cell-penetrating peptide iRGD
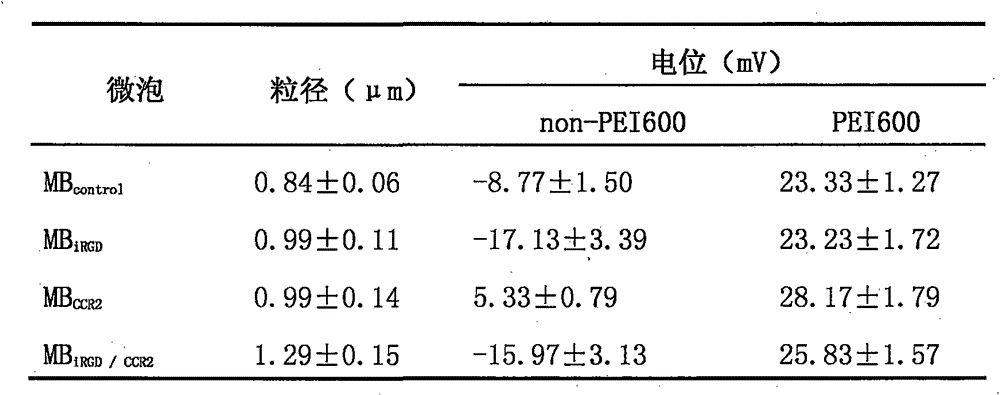
InactiveCN105106977AUniform particle size distributionSpeed up entryEnergy modified materialsGenetic material ingredientsPolyetherimidePlasmid dna
The invention discloses a preparation method of double-targeting cationic ultrasound microbubbles carrying cell-penetrating peptide iRGD and belongs to the field of fundamental research. The preparation method uses materials, including DSPC, DSPE-PEG2000-maleimide, DSPE-PEG2000-Biotin, iRGD peptide, CCR2 antibody, stearic acid, branched-chain polyetherimide-600, N, N'-carbonyldiimidazole, and avidin; by bonding integrin Alpha v Beta3 targeting cell-penetrating peptide iRGD and the CCR2 antibody to surfaces of the microbubbles, double-targeting cationic ultrasonic contrast agent is constructed and has the double functions of carrying plasmid DNA and targeting tumor cells; combining ultrasound-mediated biological effect and iRGD cell-penetrating action, the agent is capable of promoting entry of genes into the tumor cells and improving gene transfection efficiency and is expected to be developed and applied to visual ultrasound-controlled-release genetic treatment of tumors.
Owner:SHENZHEN PEOPLES HOSPITAL
Preparation method of high-purity olaparib
The invention discloses a preparation method of high-purity olaparib. The preparation method comprises: subjecting 2-fluoro-5-[(4-oxo-3,4-dihydrophthalazine-1-yl)methyl] benzoic acid as a starting material to activation and aminolysis crystallization to obtain high-purity olaparib, wherein the activation refers to adding carbonyldiimidazole activating agent into a solution containing 2-fluoro-5-[(4-oxo-3,4-dihydrophthalazine-1-yl)methyl] benzoic acid to obtain active amide intermediate; with separation and purification, subjecting the active amide intermediate to direct aminolysis crystallization with 1-(cyclopropanecarbonyl)piperazine to obtain the olaparib. The purity of the olaparib prepared by the method is greater than 99.8 %, and the process is simple, high in yield, low in cost and more suitable for industrial production.
Owner:合肥启旸生物科技有限公司
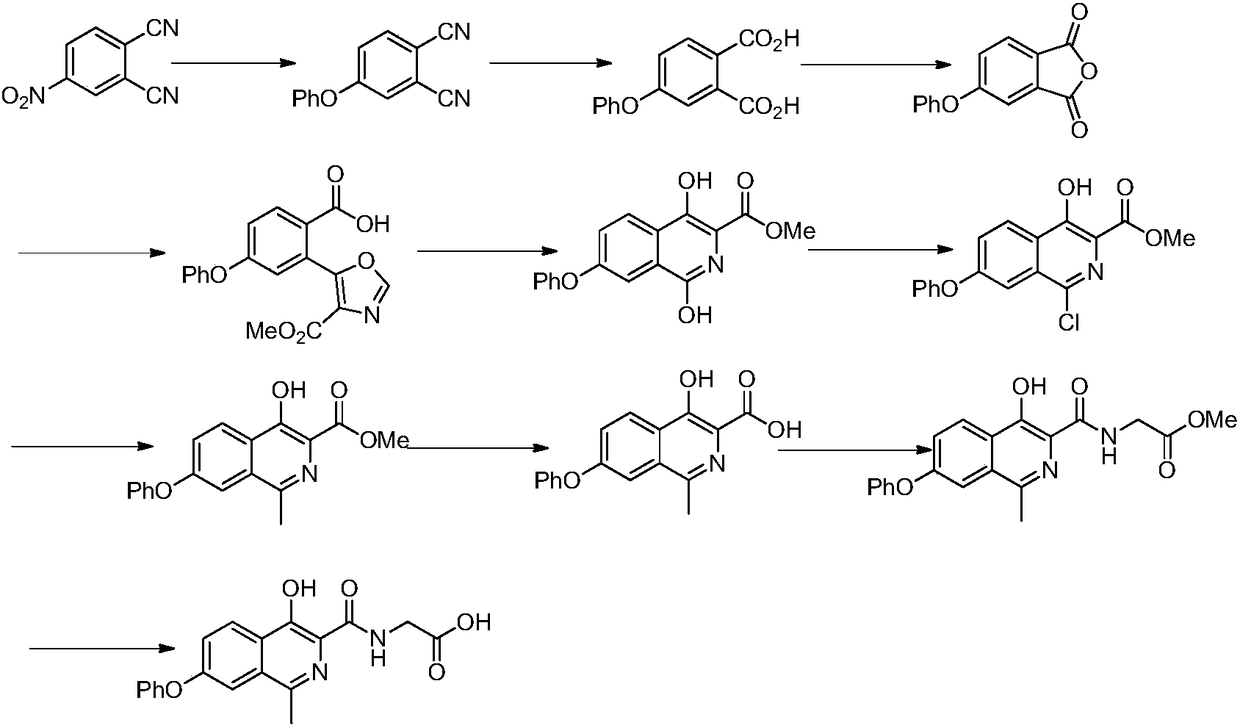
Preparation method of drug for chronic anemia
The invention discloses a synthesis method for a drug Roxadustat for chronic anemia. The synthesis method comprises the following steps: hydrolyzing and acidifying a compound shown as a formula 6 under the action of alkali to obtain a key intermediate compound shown as a formula 7; carrying out condensation reaction on the compound shown as the formula 7 and carbonyl diimidazole under suitable conditions to obtain an intermediate compound shown as a formula 8, and separating or not separating the compound shown as the formula 8 from a system to directly take part in subsequent reaction; and finally, reacting the product with glycine to obtain a final product Roxadustat shown as a formula 9. The preparation method has the advantages that the route efficiency is improved, the process cost isreduced, side products are reduced and the purity of a final product is favorably improved (The formula is shown in the description), wherein R2 in the compound shown as the formula 6 represents alkyl, and includes but not limited to methyl, ethyl, isopropyl, tertiary butyl or benzyl.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
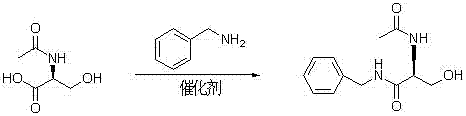
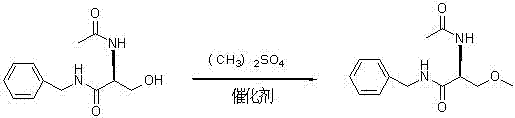
Synthetizing method of lacosamide
InactiveCN103113256AHighlight substantive featuresSignificant progressOrganic compound preparationCarboxylic acid amides preparationPtru catalystAmmonium chloride mixture
The invention provides a synthetizing method of lacosamide. The method comprises the steps of: based on D-serine as a raw material, performing an acylation reaction with acetic anhydride and then performing a condensation reaction with benzylamine; and finally, performing a methylation reaction with dimethyl sulfate, thereby obtaining lacosamide, wherein N,N' dicyclohexylcarbodiimide (DCC) or N,N' carbonyl diimidazole (CDI) is used as a catalyst in the condensation reaction; and a phase transfer catalyst including triethyl benzyl ammonium chloride (TEBA), tetrabutylammonium chloride (TBAC), tetrabutylammonium bromide (TBAB) or tetrabutylammonium hydrogen sulfate (TBAHS) is adopted in the methylation reaction. The method has the advantages of being simple in synthetizing process, moderate in reaction condition, simple in after-treatment, high in yield and high in product purity.
Owner:SUZHOU HONGRUI MEDICAL TECH
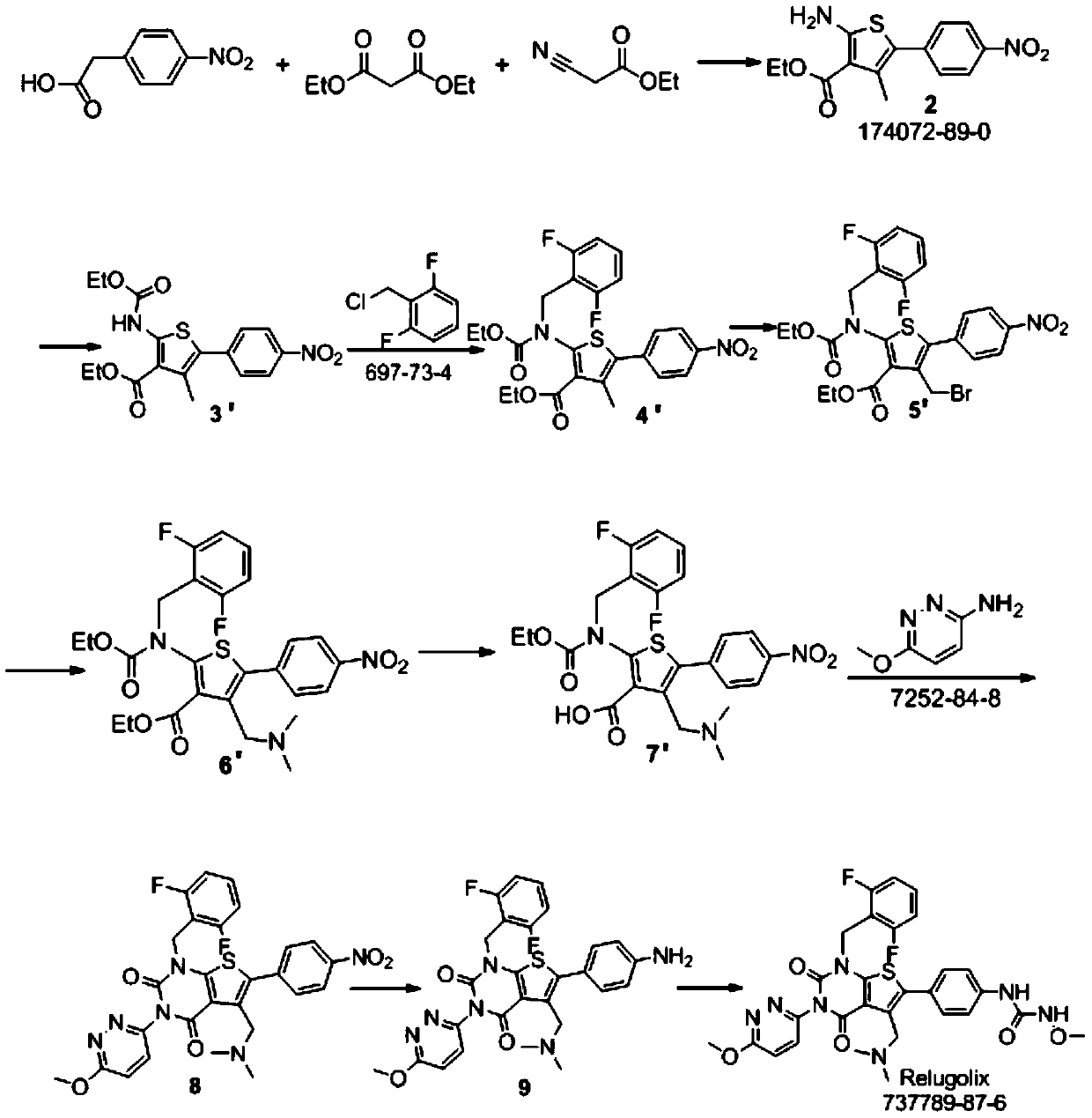
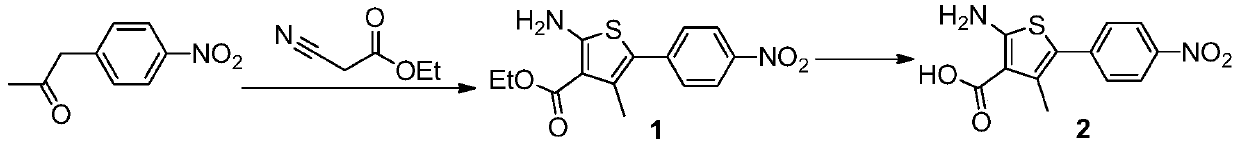
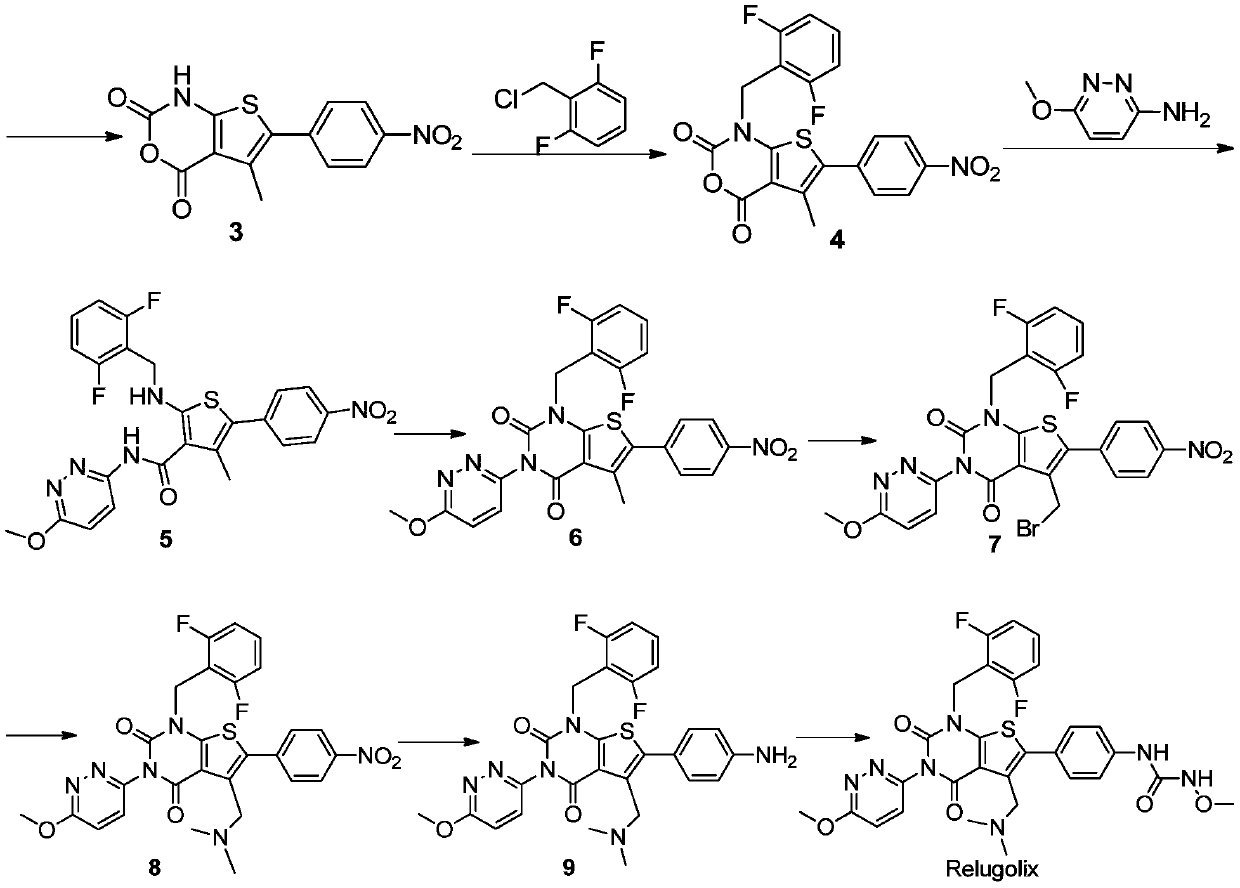
Relugolix synthesis method
The present invention provides a method for preparing a relugolix intermediate compound 8. The method comprises: (a) carrying out a reaction on a compound 2 and N,N'-carbonyldiimidazole to obtain a compound 3; (b) carrying out a reaction on the compound 3 and 2,6-difluorobenzyl chloride to obtain a compound 4; (c) carrying out a reaction on the compound 4 and 3-amino-6-methoxypyridazine to obtaina compound 5; (d) carrying out a reaction on the compound 5 and N,N'-carbonyldiimidazole to obtain a compound 6; (e) carrying out a reaction on the compound 6, N-bromosuccinimide and azobisisobutyronitrile to obtain a compound 7; and (f) carrying out a reaction on the compound 7 and dimethylamine hydrochloride to obtain a compound 8. The invention further provides a relugolix preparation method, which comprises: (g) carrying out a reaction on the compound 8 obtained by the method and hydrogen under a catalyst to obtain a compound 9; and (h) carrying out a reaction on the compound 9, N,N'-carbonyldiimidazole and methoxy amine hydrochloride to obtain relugolix. According to the present invention, the method adopts the route sequentially comprising loop closing and coupling, such that the method has characteristics of simple operation, less side-reaction, mild reaction condition, high yield, high product purity and easy product purification, and is suitable for commercial scale production.
Owner:四川伊诺达博医药科技有限公司
Preparation method of azilsartan intermediate
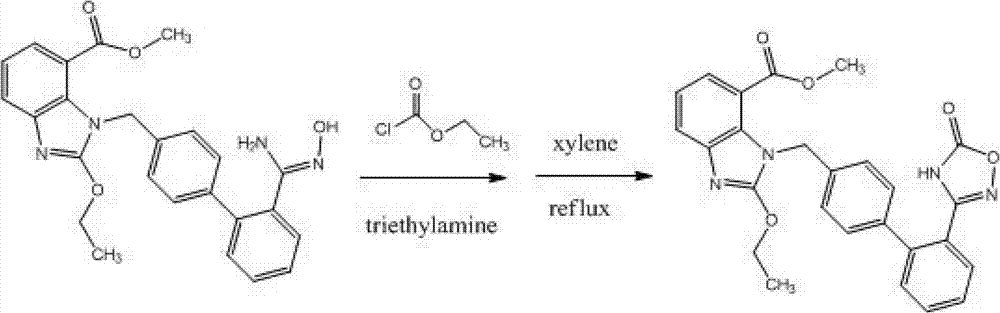
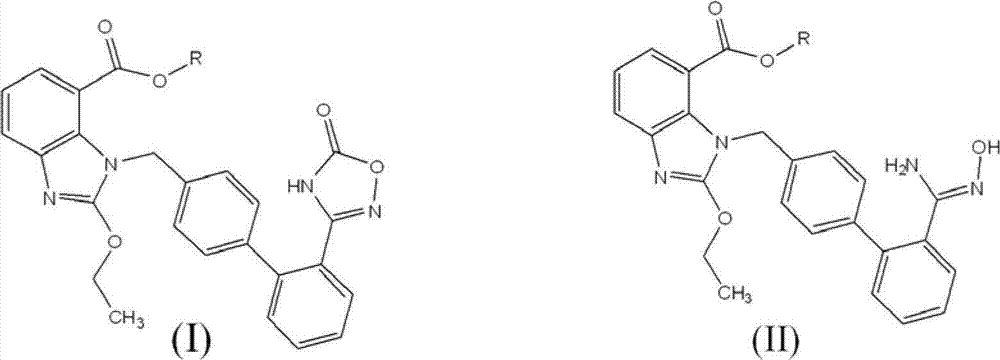
The invention discloses a preparation method of an azilsartan intermediate shown as in the formula I. According to the invention, the intermediate shown as in the formula I is obtained through a condensation cyclization reaction between a compound shown in the formula II and N, N'-carbonyl diimidazole or bis (trichloromethyl) carbonate in an aprotic solvent. Organic alkali can be added in the above reaction system to improve the reaction rate. The preparation method of the azilsartan intermediate shown as in the formula I has the advantages of high yield and low cost.
Owner:BEIJING COLLAB PHARMA
Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate
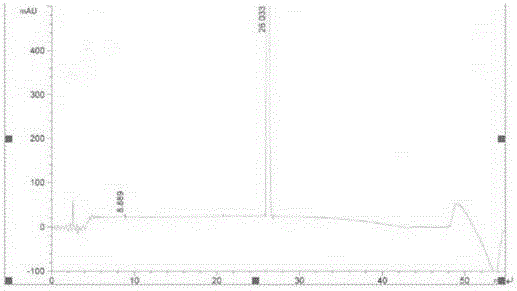
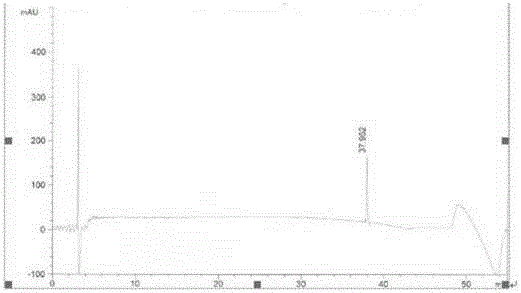
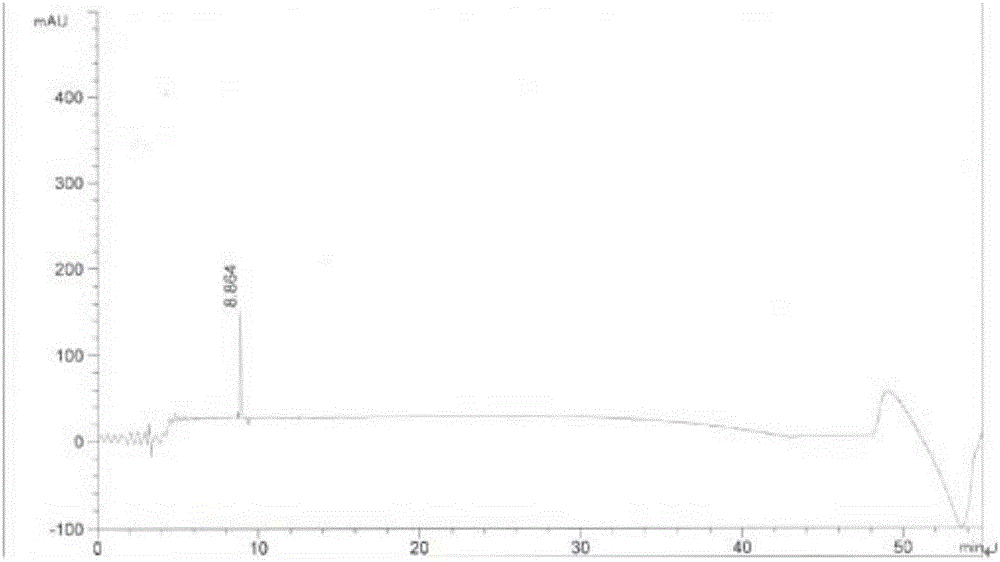
ActiveCN108689876AReduce usageLow toxicityOrganic compound preparationCarboxylic acid amide separation/purificationEthyl chloroformateCarbonyldiimidazole
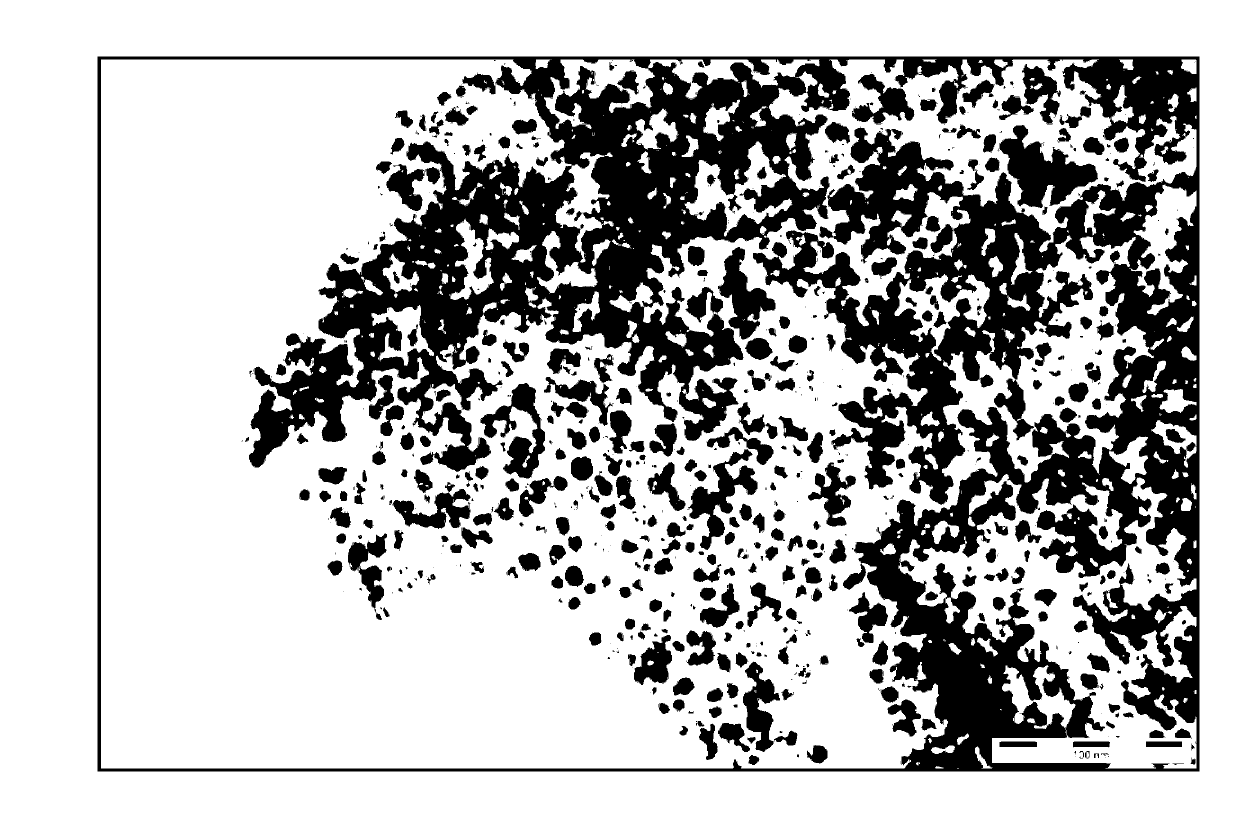
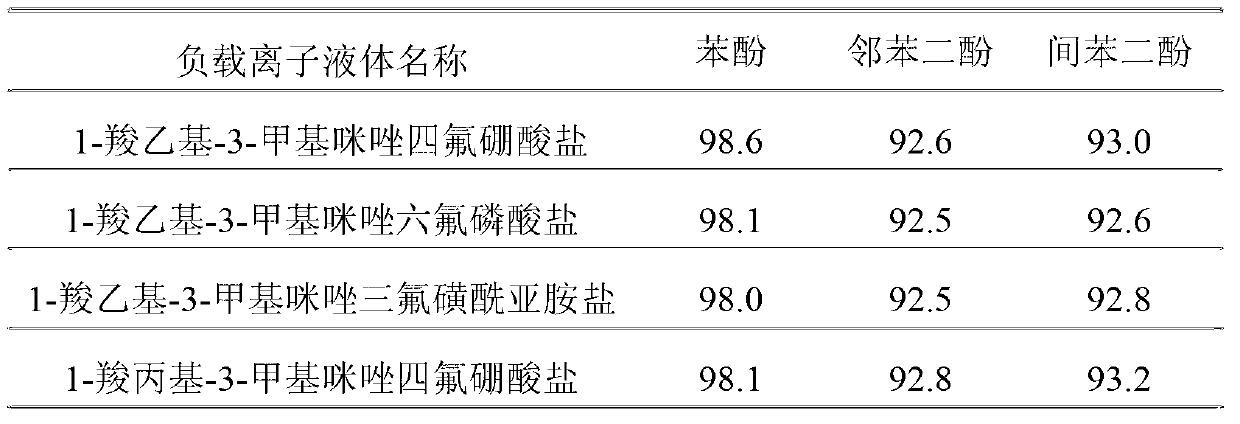
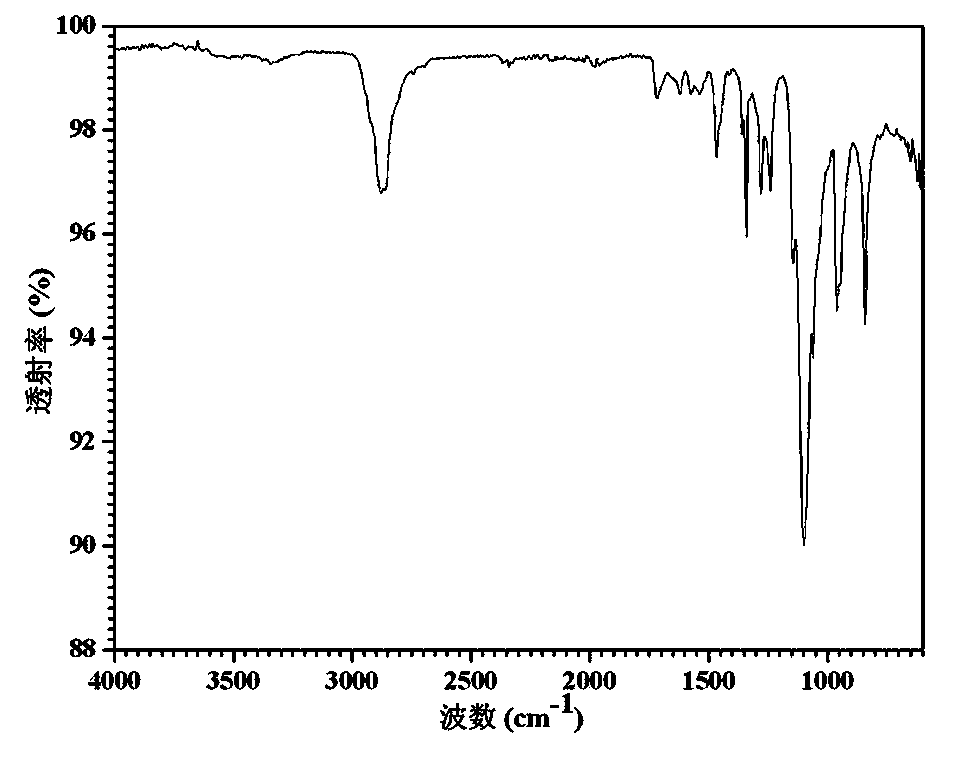
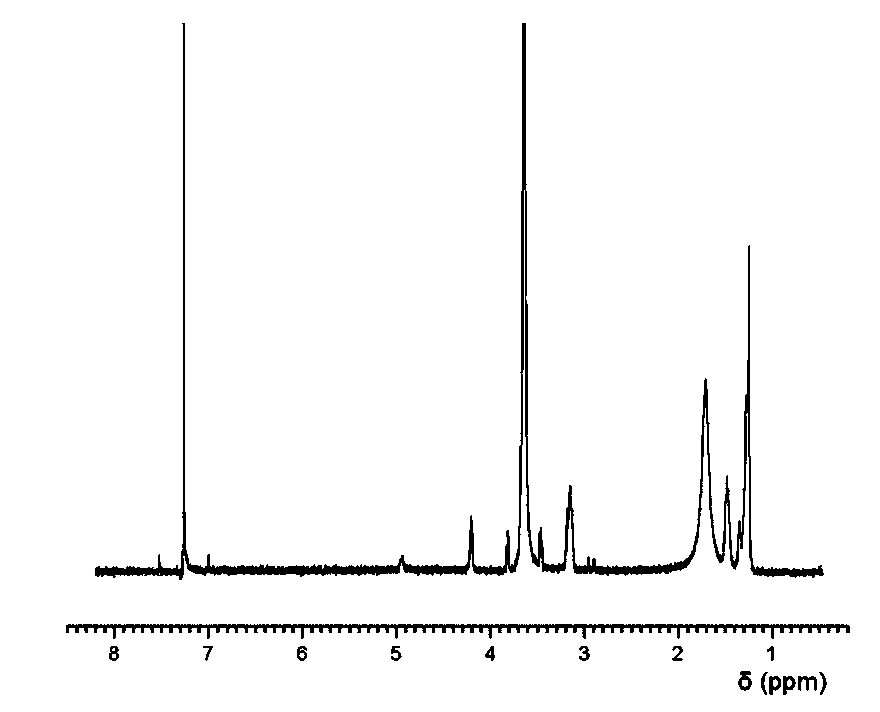
The invention discloses a preparation method of a pharmaceutical intermediate, i.e., sodium 8-[(2-hydroxybenzoyl) amino] octanoate. The preparation method comprises the steps of enabling salicylamide,used as a raw material, to react with N' N-carbonyl diimidazole so as to generate an intermediate, i.e., 2H-benzo[e][1,3]oxazine-2, 4 (3H)-dione; enabling the intermediate to react with 8-ethyl bromooctanoate to obtain 8-(2, 4-dicarbonyl-2H-benzo[e][1, 3]oxazine-3(4H)-yl) ethyl caprylate; hydrolyzing by using sodium hydroxide, and then acidizing to obtain 8-[(2-hydroxybenzoyl) amino] caprylic acid; then, enabling the 8-[(2-hydroxybenzoyl) amino] caprylic acid to react with sodium hydroxide to obtain the final product, i.e., the sodium 8-[(2-hydroxybenzoyl) amino] octanoate. The preparation method avoids the use of a genotoxic raw material-ethyl chloroformate, is low in reaction energy consumption, less in by-products and high in yield, greatly lowers the production cost, and is simple inprocess and suitable for industrial production.
Owner:江苏东南纳米材料有限公司
Preparation method of nylon-shell glycan compound film for affinity microfilter
InactiveCN1413760AImprove mechanical propertiesGood chemical stabilitySemi-permeable membranesBiocompatibility TestingCarbonyldiimidazole
A process for preparing the nylon-chitosan membrane used for affinity microfilter includes such steps as activating the nylon membrane with 0.4-3 microns of pore diameter by the solution of formaldehyde, biepoxyane, etc at 20-90 deg.C, coupling reaction acidic chitosan solution at 20-50 deg.C to obtain nylon-chitosan membrane, and then washing. The said membrane features high mechanical performance, chemical stability, hydrophilicity and biocompatibility, low non-specific adsorpativity, and rich active groups.
Owner:TIANJIN UNIV
Method of synthesizing landiolol hydrochloride
InactiveCN101012217AGuaranteed spatial conformationHigh reaction yieldOrganic chemistryPropanoic acidMorpholine
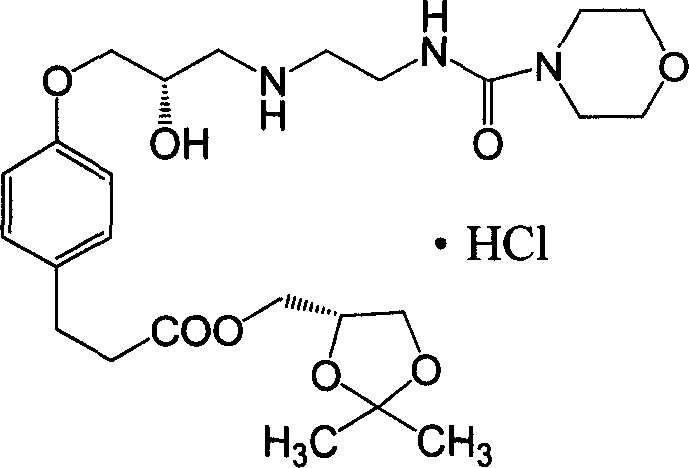
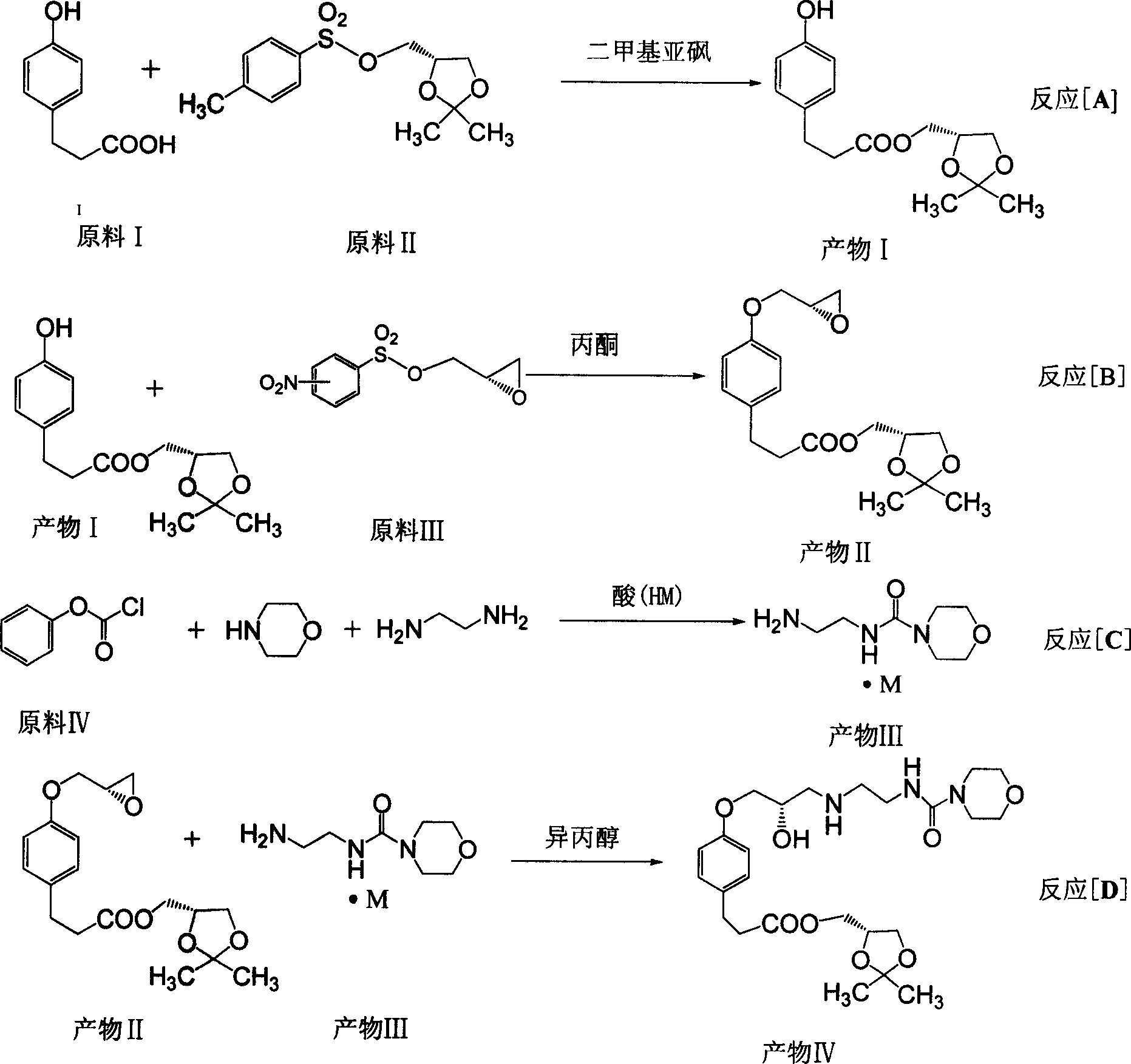
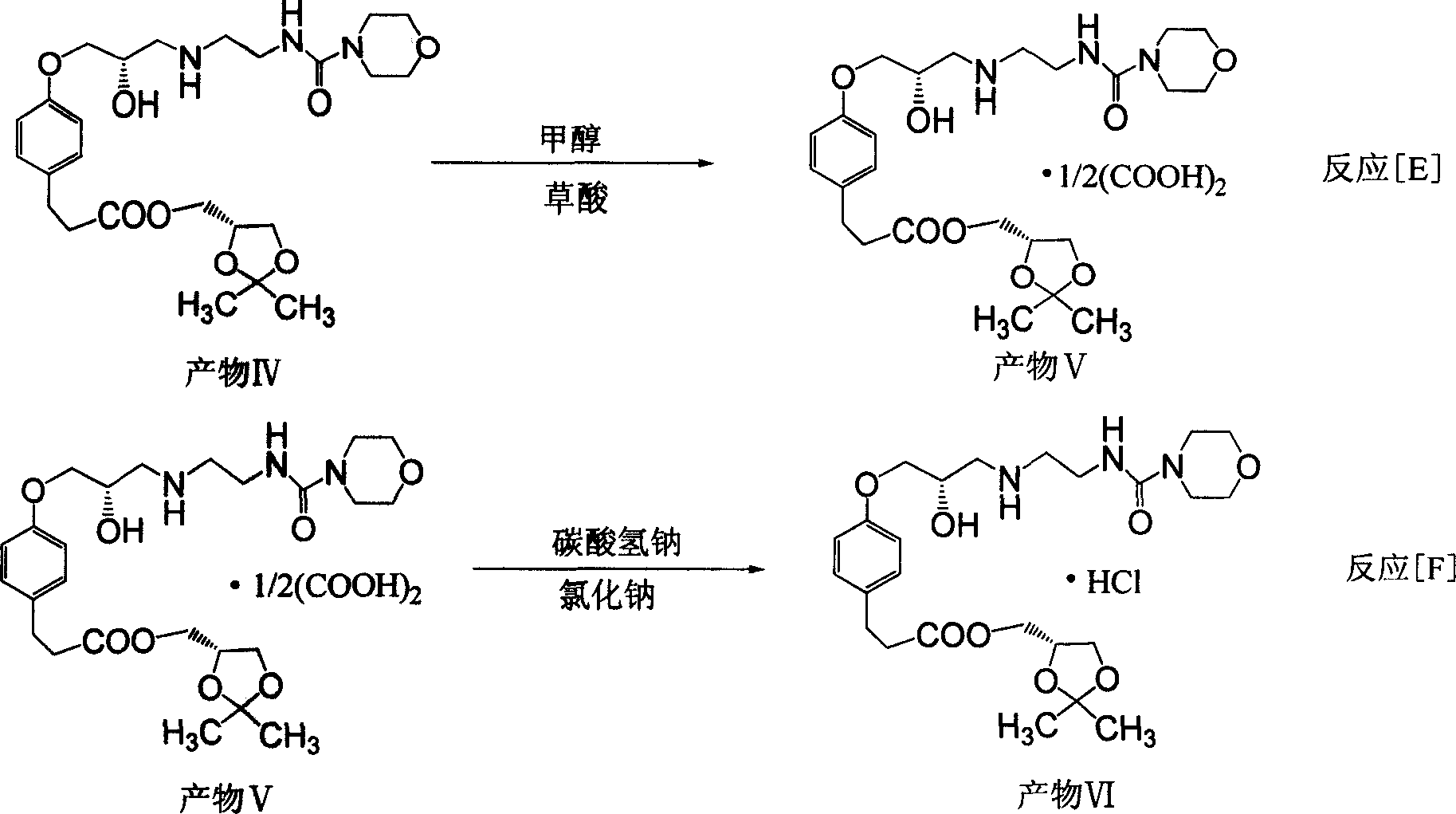
The invention discloses a synthesizing method of alcaine landol, which comprises the following steps: adopting S(+)-epichlorohydrin to replace 3-chlorine 1, 2-propanediol as raw material; optimizing; condensing; esterifying; etherifying; obtaining 3-[4-(2S, 3-epoxy propoxy) phenyl] propanoic acid (2, 2-dimethyl-1, 3-dioxolane-4S) methyl ester; opening loop with carbonyl diimidazole to make N-(2-aminoethyl) morpholine formamide oxalate in the isopropanol solution; proceeding salt precipitation directly to obtain the product.
Owner:BEIJING UNIV OF CHEM TECH
Method for synthesizing quinolone medicaments
InactiveCN101781313ASolve the environmental odor problemRealize cleaner productionOrganic chemistryCarbonyldiimidazoleCarboxylate
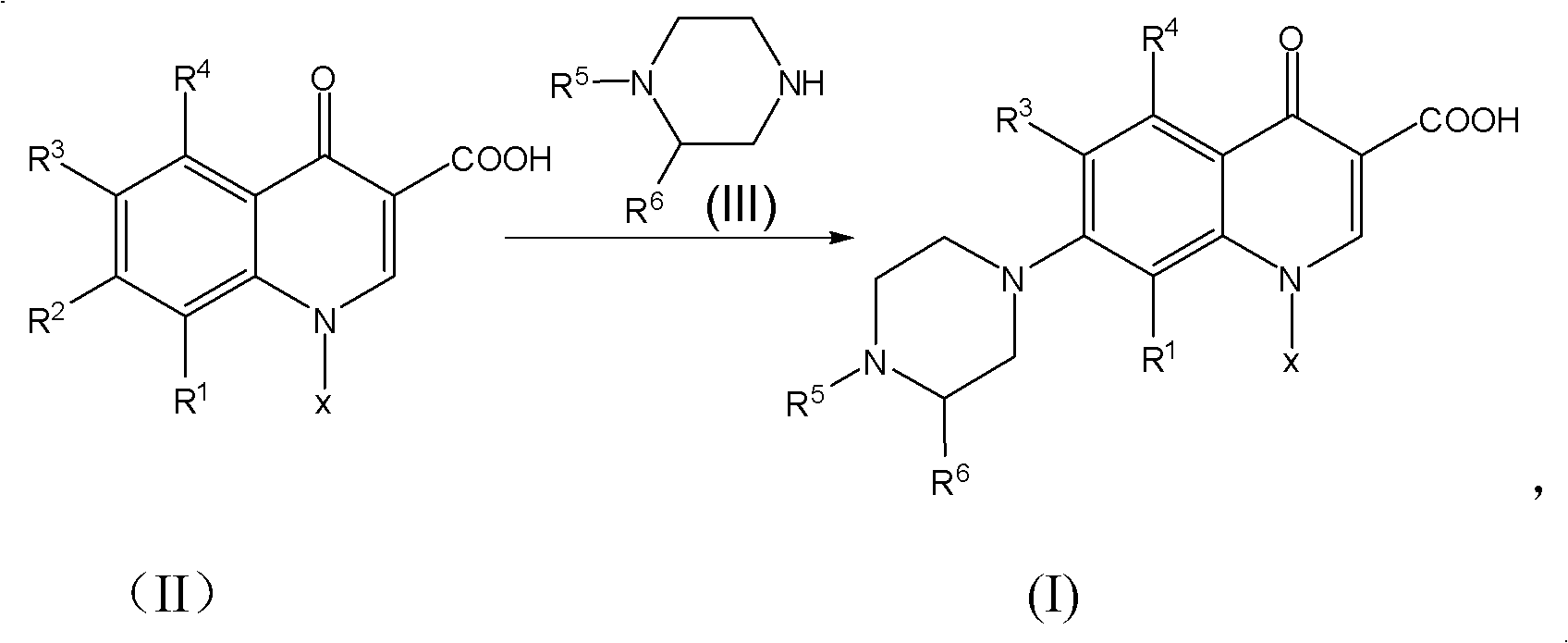
The invention discloses a method for preparing quinolone medicaments. The conventional methods using organic solvents with a high boiling pint and a large polarity, adopting other solid alkali materials except reaction materials or using other liquid alkali materials except the reaction materials have obvious defects. The method of the invention adopts a technical scheme that: quinolone compounds are prepared by a piperazidine reduction of quinolone carboxylate nuclear parent and piperazidine derivates in water; the piperazidine reduction is completed in the presence of a catalyst which may be one or a mixture of more than two of cerous chloride heptahydrate, N,N-carbonyldiimidazole, 4-dimethylamino pyridine, tetrabutylammonium bromide, benzyl triethylammonium chloride and tetrabutyl ammonium hydroxide. The method for preparing the quinolone medicaments radically solves the problems of terrible smell, realizes clean production, avoids using the other alkali substances except the reaction materials as an acid-binding agent and overcomes the defects of the prior art.
Owner:ZHEJIANG JINGXIN PHARMA +1
Bilirubin adsorption material for treating hyperbilirubinemia
InactiveCN1876226AImprove securityGood blood compatibilityOther blood circulation devicesOther chemical processesBILIRUBINAEMIAArginine
The related bilirubin adsorption material selects agarose gel as carrier activated by N, N'-carbonyldiimidazole, the dual-amido agent and dual-aldehydo agent as separation arm, and couples all of lycine, aethanolamina, arginine and n-butylamine on back end, wherein in 324mg / L blood, the adsorption capacity for former material with different coupled is: 0.71~1.21g / Lgel, 0.78~1.29g / Lgel, 0.91~1.44g / Lgel and 1.20~1.81g / Lgel, and the removal rate to bilirubin by material coupled n-butylamine is up to 50.0%. this invention has well adsorption rate with low cost.
Owner:DALIAN UNIV OF TECH
Blood purifying protein A immunoadsorption material and synthesizing method thereof
ActiveCN101190409AHigh chemical activityImprove adsorption capacityOther chemical processesEpoxySynthesis methods
The invention relates to a staphylococcal protein A (SPA) immunoadsorption material used for blood purification and a preparation method thereof. The invention discloses a macromolecule material which is coupled by agarose gel and SPA. The material is prepared by taking the agarose gel as carrier substrate which is reacted with epoxy bromopropane to obtain the epoxy-based active carrier; after that, polyamines reagent is taken as a space arm which is then reacted with carbonyl diimidazole and is then coupled with the SPA. The material has the advantages of short synthesis time, safe preparation, strong specificity of product, high adsorption efficiency and good regeneration performance to immunoglobulin and the compound thereof, and being able to be applied to clinic immunoadsorption cure.
Owner:GUANGZHOU KONCEN BIOSCI +1
Synthetic method for avibactam sodium salt
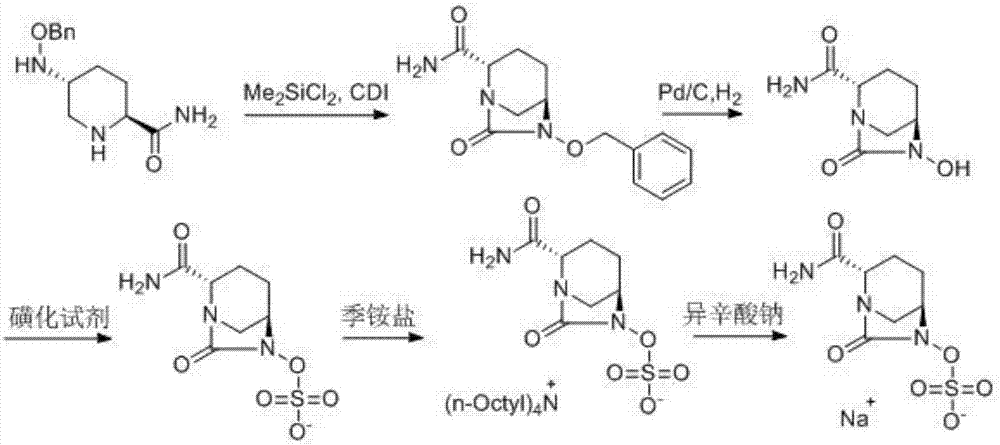
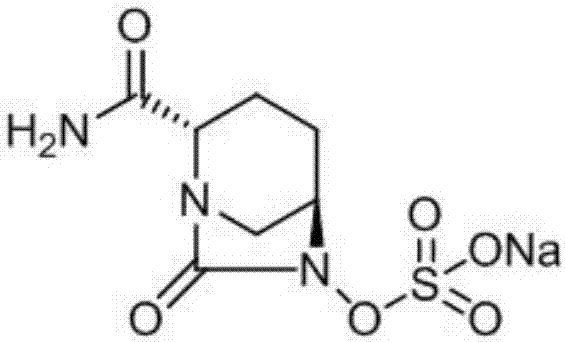
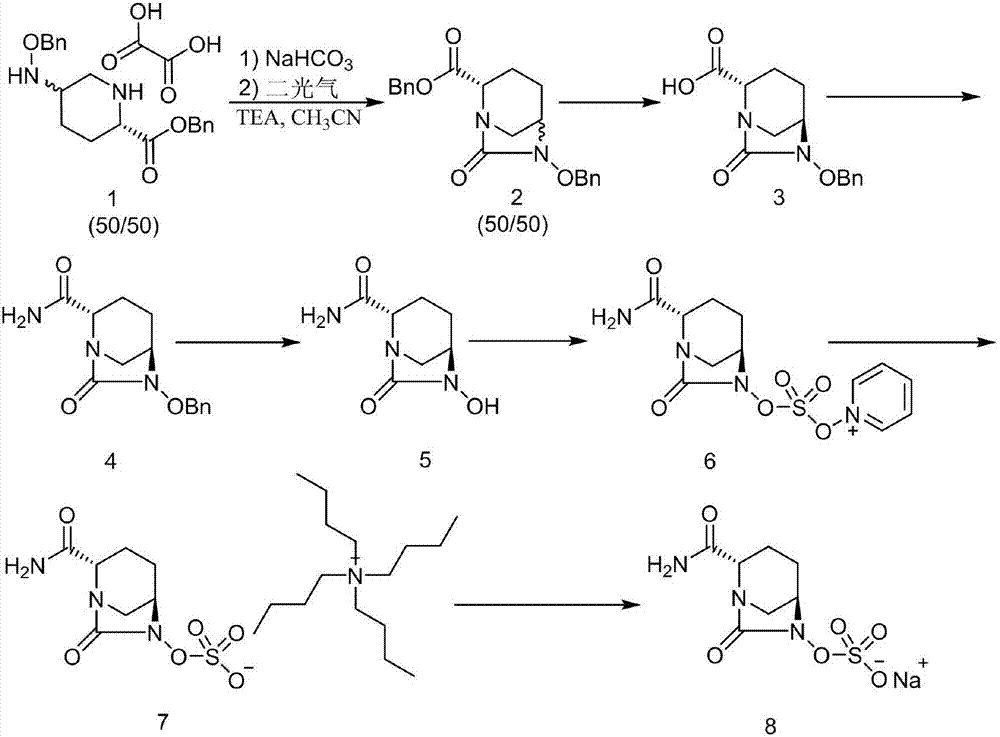
ActiveCN107417686ALow priceReduce manufacturing costOrganic chemistryIon exchangeCarbonyldiimidazole
The invention discloses a synthetic method for avibactam sodium salt. The method comprises the following steps: taking (2S, 5R)-5-[(benzyl oxyl) amino] piperidine-2-formamide as a starting material; constructing a urea ring by carbonyl diimidazole under the effect of dimethyldichlorosilance to obtain (2S, 5R)-6-(benzyl oxyl)-7-oxo-1,6-diazabicyclo [3.2.1] octane-2-formamide; then carrying out hydrogenation to remove benzyl; carrying out sulfonation reaction on the compound and a sulfonated reagent; synthesizing into a quaternary ammonium salt intermediate by using quaternary ammonium salt; and finally carrying out ion exchange to obtain the avibactam sodium salt. The improved process is low in cost, simple and convenient to operate, good in product quality and suitable for industrial production. In a process of synthesizing the intermediate (2S, 5R)-6-(benzyl oxyl)-7-oxo-1,6-diazabicyclo [3.2.1] octane-2-formamide, dimethyldichlorosilance which is low in price is used, and therefore, the production cost is greatly reduced.
Owner:JIANGXI FUSHINE PHARMA CO LTD
Cross-linking perfluorinated sulfonic acid ion exchange membrane and preparation method thereof
The invention belongs to the field of polymer functional membrane materials, and particularly relates to an ion exchange membrane for a proton exchange membrane fuel cell (PEMFC) and a vanadium redox battery (VRB), in particular to a cross-linking perfluorinated sulfonic acid ion exchange membrane and a preparation method thereof. The method comprises the following steps: dissolving carbonyldiimidazole and perfluorinated sulfonic acid ion exchange resin into an organic solvent to prepare perfluorinated sulfonic acid solution (perfluorinated membrane casting solution); adding a cross-linking agent into the membrane casting solution, mixing well, and pouring the membrane casting solution onto the surface of a horizontal glass plate or a Hastelloy alloy steel plate, thereby preparing a cross-linking ion exchange membrane. The cross-linking perfluorinated sulfonic acid ion exchange membrane prepared in the invention has oxidation resistance, good dynamic stability and good mechanical stability, and can effectively reduce the cost of the battery and promote the commercial popularization of the battery.
Owner:SHANDONG DONGYUE WEILAI HYDROGEN ENERGY MATERIAL CO LTD
Method for preparing high-purity torasemide and crystal form I thereof
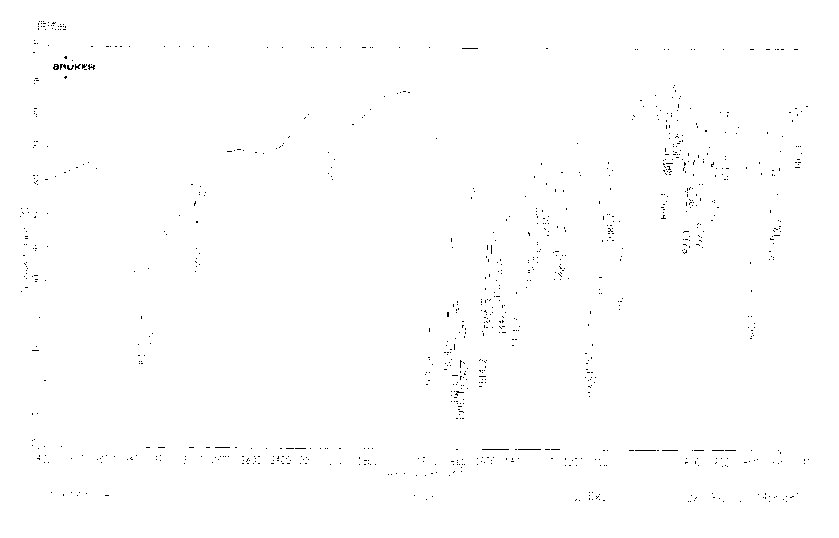
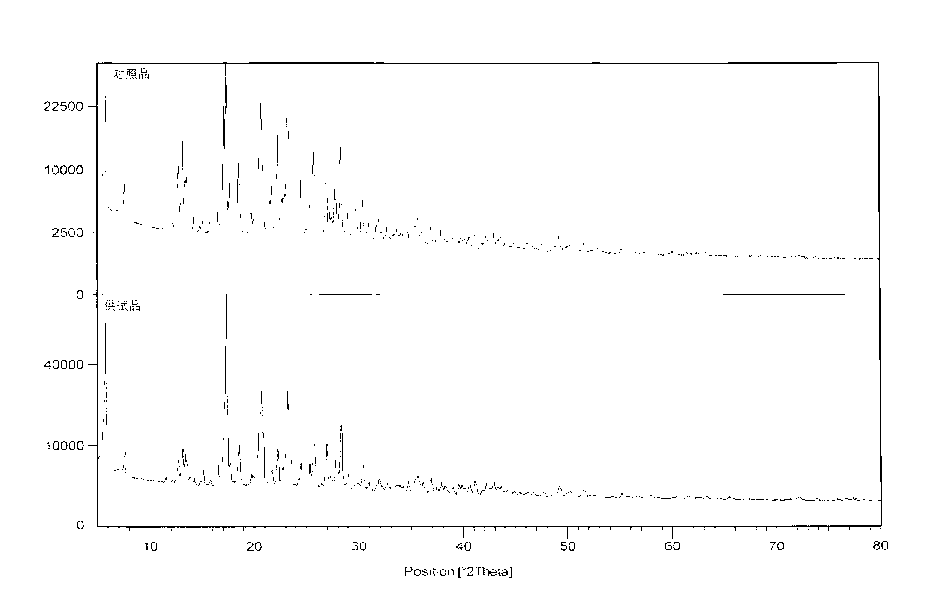
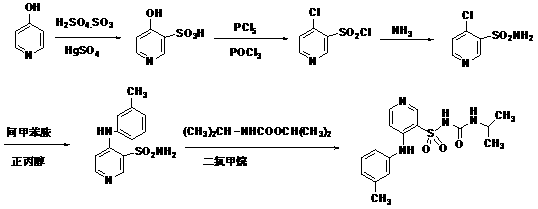
ActiveCN102702089AOvercome the disadvantages of greater toxicityHigh purityOrganic chemistryIsopropamideGranularity
The invention discloses a method for preparing high-purity torasemide and a crystal form I thereof. The method comprises the following steps of: reacting 4-chloro-3-pyridine sulfonamide and m-toluidine serving as raw materials to obtain 3-sulfamoyl-4-(3-methyl phenyl) aminopyridine, refining, and reacting with 1,1'-carbonyldimidazole and isopropamide, directly introducing isopropyl carbamyl, thus obtaining the high-purity torasemide, wherein the purity (high performance liquid chromatography (HPLC)) of the torasemide is more than 99.5 percent. The prepared torasemide has proper granularity, seed crystal is not required for crystal transformation, the torasemide can be directly transformed into the variant crystal form I, and a chemically pure torasemide variant I is obtained. The preparation method is strong in process controllability, easy and convenient to operate and high in reproducibility, and facilitates industrialized production.
Owner:连云港杰瑞药业有限公司
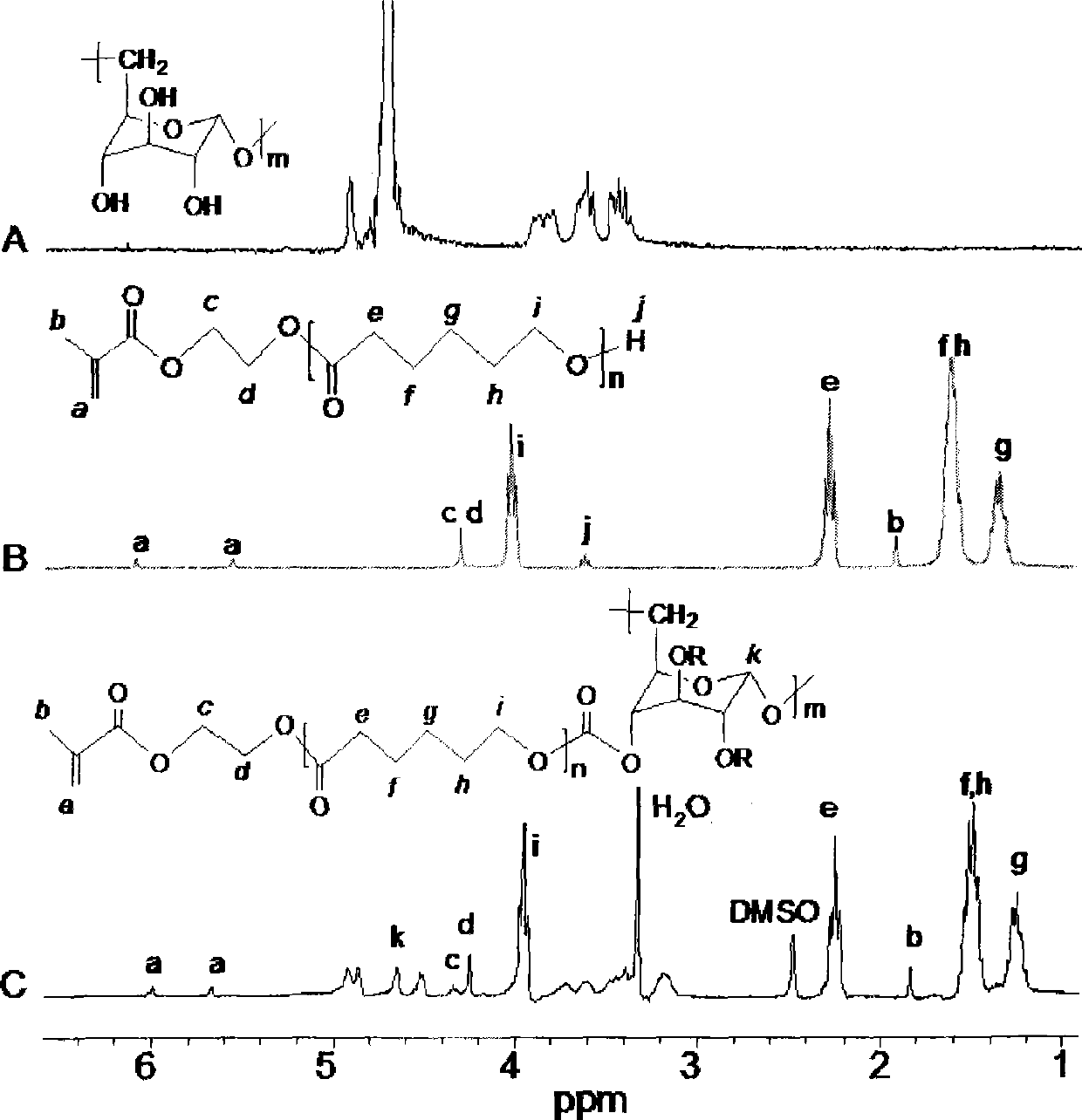
Biodegradable hydrogel with temperature sensitivity and production method and use thereof
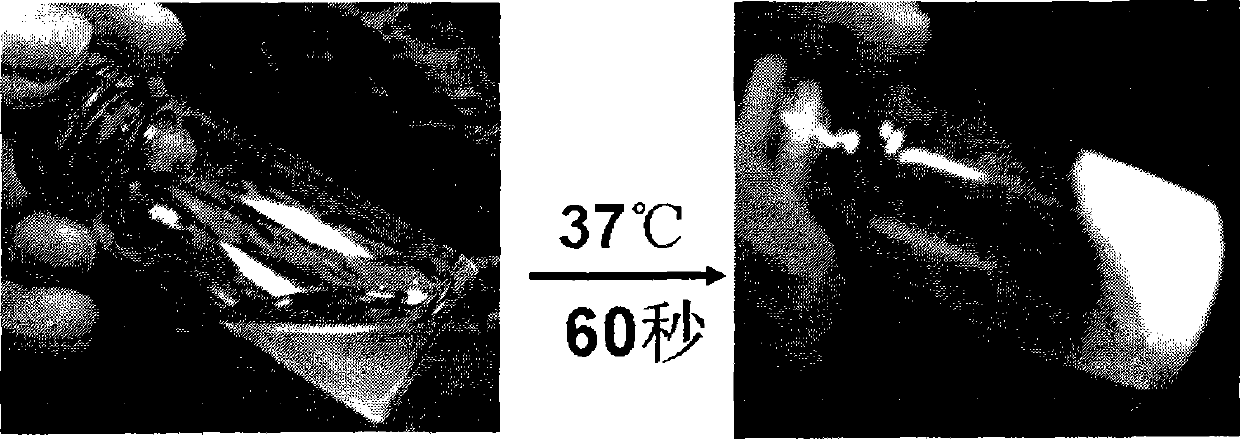
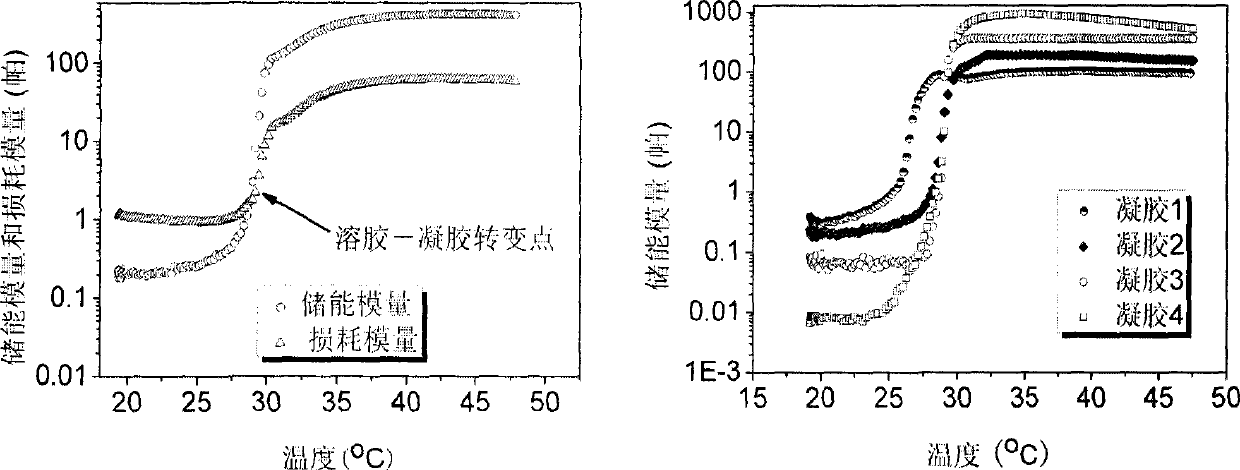
InactiveCN101371933ASensitive to temperatureBiodegradableSurgeryPharmaceutical non-active ingredientsPhosphorous acid(Hydroxyethyl)methacrylate
The invention relates to a hydrogel which is temperature-sensitive and biodegradable simultaneously, the hydrogel is prepared by the following method: hydroxyethyl methacrylate-polycaprolactone is obtained by the reaction of hydroxyethyl methacrylate and epsilon-caprolactone; the hydroxyethyl methacrylate-polycaprolactone reacts with carbonyldiimidazole, hydroxyethyl methacrylate-polycaprolactone-imidazole is obtained by rotary evaporation; the hydroxyethyl methacrylate-polycaprolactone-imidazole and Alpha-(1->6)-D-glucan are dissolved in dimethyl sulfoxide, p-dimethylaminopyridine is added, mixture which is obtained by the reaction at room temperature is settled in isopropyl alcohol, hydroxyethyl methacrylate-polycaprolactone-g-Alpha-(1->6)-D-glucan is obtained by vacuum drying; N-isopropylacrylamide and the hydroxyethyl methacrylate-polycaprolactone-g-Alpha-(1->6)-D-glucan are dissolved in phosphorous acid buffer solution with pH of 7.4, then ammonium persulfate and N,N,N',N'-tetramethylethylenediamine are added, the hydrogel is obtained by the reaction at room temperature; dialysis is carried out on the obtained hydrogel by phosphorous acid buffer solution, thereby obtaining the temperature-sensitive and biodegradable hydrogel.
Owner:WUHAN UNIV
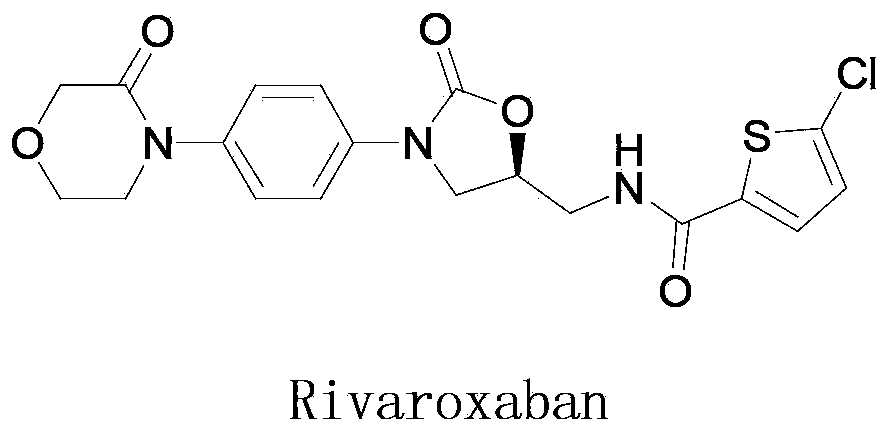
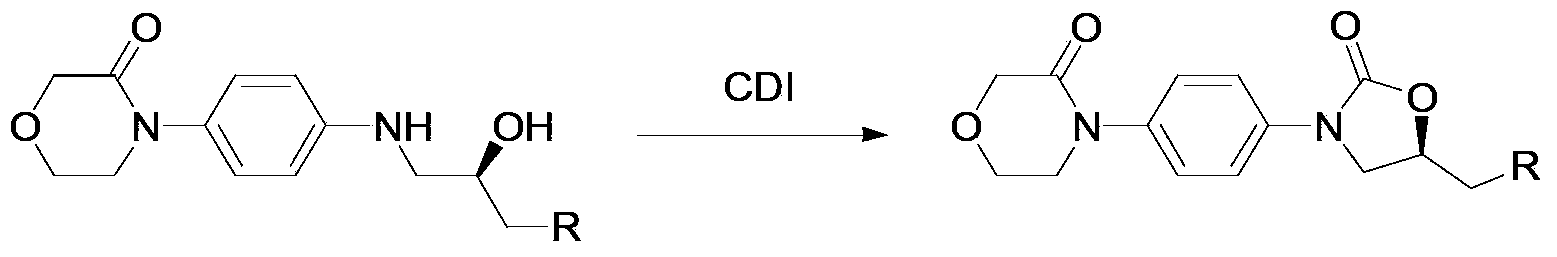
Preparation method for rivaroxaban intermediate
The invention provides a preparation method for a rivaroxaban intermediate. The preparation method is characterized in that a compound represented by formula 1 and triphosgene are used as raw materials and undergo a reaction in an organic solvent at a temperature of 40 to 100 DEG C for 1 to 10 h under the action of an organic amine catalyst, then pressure reduction is carried out to remove the solvent, and an obtained crude product is subjected to refining in the organic solvent so as to prepare a compound represented by formula 2, wherein the formula 1 and the formula 2 are described in the specification. According to the invention, the starting raw materials are easily available on the market and are cheap; triphosgene is used to replace expensive carbonyldiimidazole, CO2 and HCL gas are generated after the reaction, a product is easy to purify, and reaction yield and purity are effectively improved; triphosgene is used to replace expensive carbonyldiimidazole, HCL gas generated after the reaction can be utilized after recovery, so the purpose of green production is achieved; operation process is simple, and rigor conditions like low temperature, no water and no oxygen are not needed.
Owner:ZHEJIANG LIAOYUAN PHARM CO LTD
Preparation method of novel hydroxyapatite graft polylactic acid
The invention discloses a preparation method of a novel hydroxyapatite graft polylactic acid. The preparation method comprises the following steps: (1) adopting 3-aminopropyltriethoxysilane (APTES) to modify -NH2 to the surface of HA; and (2) using N,N'-carbonyl di-imidazole (CDI) to activate an end carboxyl group on a polylactic acid molecular chain so as to form a reaction intermediate, then reacting the modified HA with the polylactic acid to obtain the hydroxyapatite graft polylactic acid (HA-PLLA), and thus effectively solving the problem that hydroxyapatite is easily clustered in a polylactic acid basal body. The process is relatively simple in preparation process, toxic small molecular substances are not introduced, the preparation method has the advantages of being low in cost, short in preparation time, mild in reaction conditions, green, environment-friendly and the like, and the application prospect is wide.
Owner:HENAN INST OF ENG
Antibiotic sustained release system building method of medical titanium alloy implant surface
InactiveCN101224314AImprove adsorption capacityImprove bindingCoatingsProsthesisSodium bicarbonateMicrosphere
The invention relates to a construction method of medical titanium alloy implant surface antibiotics slow release system. At first, titanium beads or titanium is mixed with an additive, and then the mixture is coated on the surface of prosthesis and titanium beads are sintered together by sintering, and at the same time, the titanium beads are sintered with the prosthesis; after being washed by ultrasound, the matter is immerged into the hydrogen peroxide solution, and then after being washed by the acetone solution, the matter is put into the carbonyldiimidazole acetone solution, and then after being washed by acetone solution, the matter is put into the sodium bicarbonate solution with dissolved RGD, then is washed by PBS after being taken out. Drugs curing and preventing bone infection and gelatin are combined into drug-loaded microspheres, then the drug-loaded microspheres are combined with the micro-porous layer on the surface of the prosthesis by protein cross-linker, thus obtaining the medical titanium alloy implant surface antibiotics slow release system. The medical titanium alloy implant surface antibiotics slow release system of the invention improves the physical absorption on metal surface and the chemical bond binding capacity, and stable combination strength between the low release microspheres and the implant can be obtained along with the function of the protein cross-linker.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Processes for the manufacturing of 3-hydroxy-n,1,6-trialkyl-4-OXO-1,4-dihydropyridine-2-carboxamide
InactiveUS6472532B1High yieldAmenable for industrial scaleOrganic active ingredientsOrganic chemistryCarboxylic acidCarbonyldiimidazole
The present invention relates to a novel process for the preparation of 3-hydroxy-N,1,6-trialkyl-4-oxo-1,4-dihydropyridine-2-carboxamide of formula I:The method comprises of the TEMPO oxidation of a primary alcohol of 3-O-protected-2-hydroxymethyl-6-alkyl-4H-pyran-4-one of formula III to 3-O-protected-4-alkyl-4-oxo-4H-pyran-2-carboxylic acid of formula II. Reaction of compound of formula II with methylamine and 1,1-carbonyidiimidazole in an inert solvent affords 3-O-protected-N,1,6-trialkyl-4-oxo-1,4-dihydropyridine-2-carboxamide, which is deprotected to give of 3-hydroxy-N,1,6-trialkyl-4-oxo-1,4-dihydropyridine-2-carboxamide of formula I.
Owner:APOTEX TECH INC
Lithium ion battery containing additives
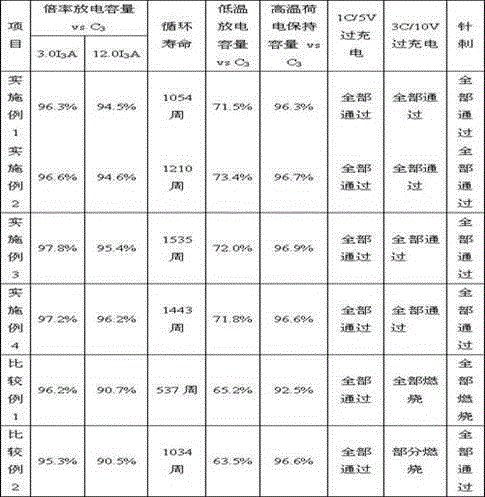
ActiveCN105428703AHigh magnificationGood low temperatureSecondary cellsPhysical chemistryLithium-ion battery
The invention provides a lithium ion battery containing additives. The lithium ion battery is characterized in that the lithium ion battery contains organic additives, and the additives mainly act on a lithium ion negative electrode or a negative electrode process; the organic additives are at least two selected from N,N-carbonyl diimidazole (CDI) or derivatives of N,N-carbonyl diimidazole, or at least two selected from N,N-carbonyl diimidazole (CDI) and derivatives of N,N-carbonyl diimidazole (CDI). The addition amount is 0.01%-5% of a battery adding electrolyte or a negative electrode material. The usage method is as follows: N,N-carbonyl diimidazole (CDI) and derivatives of N,N-carbonyl diimidazole (CDI) are dissolved in a lithium ion battery electrolyte solvent, then mixed dissolving in the lithium ion battery electrolyte or respective addition of the lithium ion battery electrolyte is carried out, or N,N-carbonyl diimidazole (CDI) or derivatives of N,N-carbonyl diimidazole (CDI) is dissolved in a solvent of the negative electrode process of the lithium ion battery, and then the additive is distributed in the battery negative electrode uniformly along with the negative electrode substance. The additive can raise the electric performances and safety performances of the lithium ion battery greatly.
Owner:ZHEJIANG NARADA POWER SOURCE CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

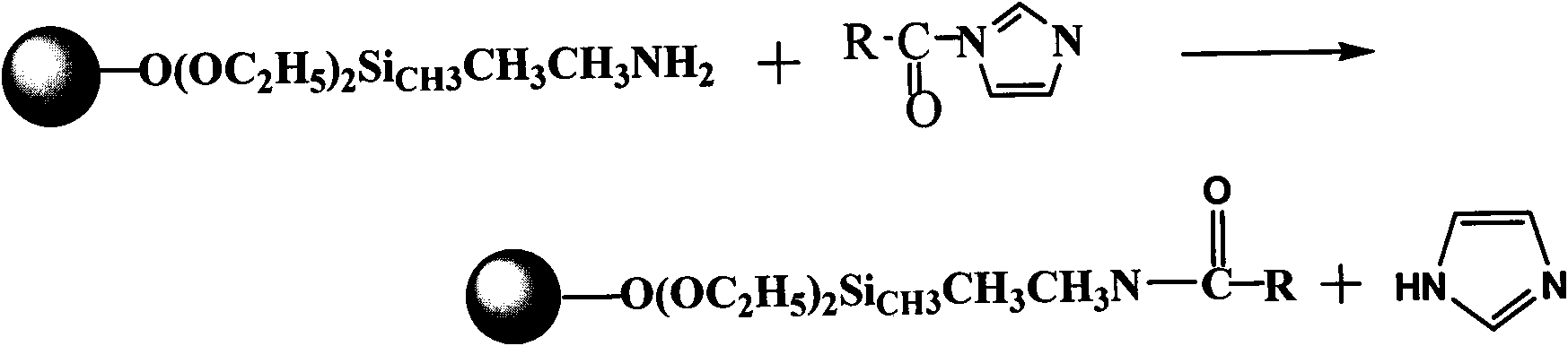
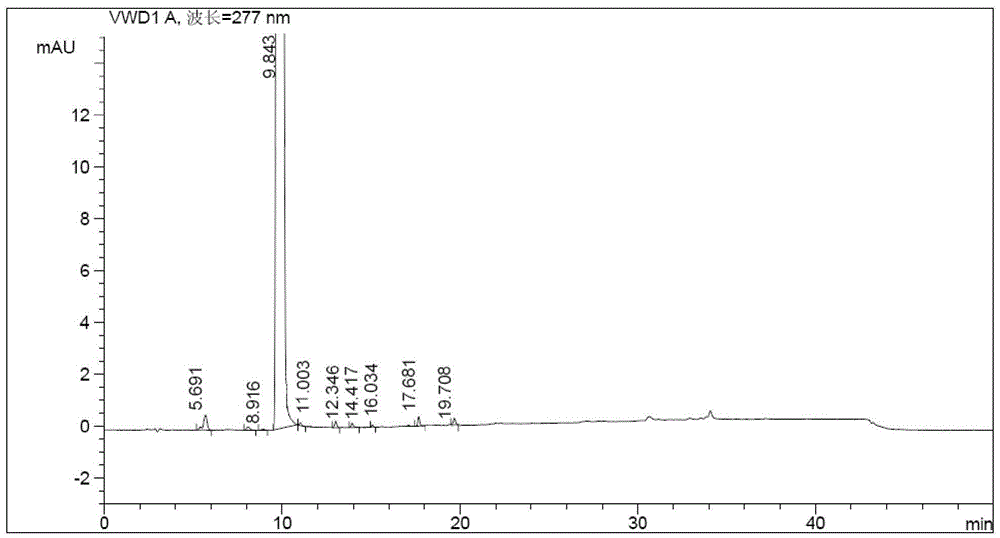

![Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate](https://images-eureka.patsnap.com/patent_img/dedce3e0-89a9-4c1f-8423-a087da736200/HDA0001711615060000011.png)
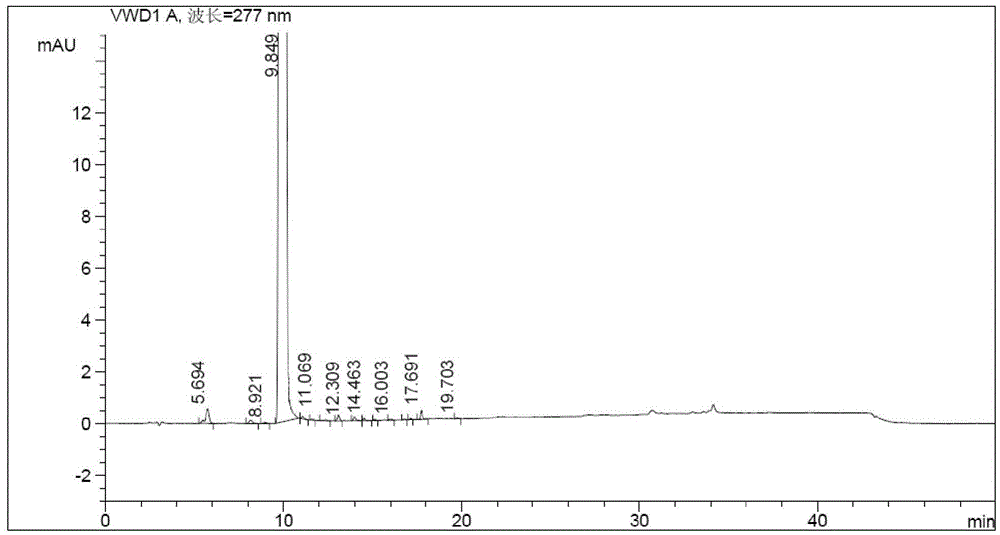
![Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate](https://images-eureka.patsnap.com/patent_img/dedce3e0-89a9-4c1f-8423-a087da736200/HDA0001711615060000021.png)
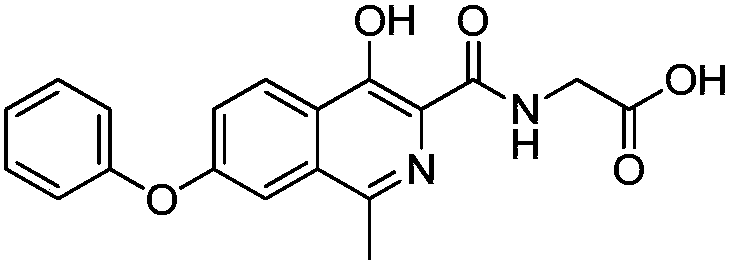
![Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate Preparation method of sodium 8-[(2-hydroxybenzoyl) amino] octanoate](https://images-eureka.patsnap.com/patent_img/dedce3e0-89a9-4c1f-8423-a087da736200/HDA0001711615060000022.png)