Preparation method of high-purity olaparib
A high-purity, monohydric alcohol technology, applied in the field of medicine, can solve the problems of unfavorable industrial production, high cost, large dosage, etc., and achieve the effects of cost saving, pollution reduction and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Activation
[0042] Dissolve 2-fluoro-5-[(4-oxo-3,4-dihydronaphthalene-1-yl)methyl]benzoic acid (5.1g, 17mmol) in 50ml of dichloromethane, cool to 0-10°C, carbonyldiimidazole (4.1g, 25.5mmol) was added, stirred and reacted at 20-30°C for 10 hours, and the reaction solution was concentrated to dryness to obtain an active amide intermediate.
[0043] 2. Aminolytic crystallization
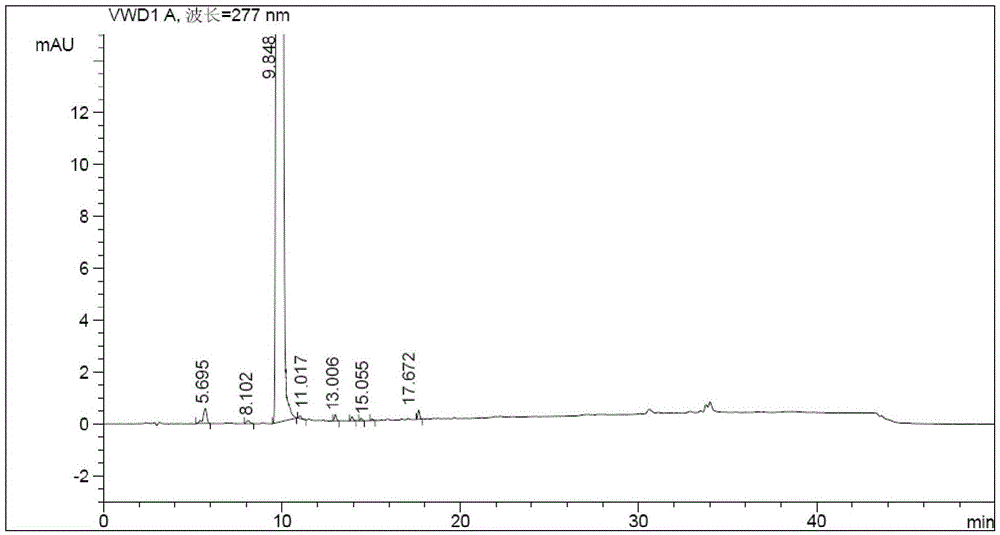
[0044] Dissolve the active amide intermediate prepared in step 1 in 10ml of dichloromethane, cool down to 0-10°C, and drop into ) in 20ml of dichloromethane solution, reacted at 0-10°C for 1.5 hours, washed 3 times with 30ml of water, concentrated the organic layer to dryness, added 50ml of ethanol-water mixed solution (1:2, v / v) to the concentrate, and refluxed Dissolve, add 0.5 g of activated carbon and reflux for 15 min, filter, stir and crystallize the filtrate at 0-5°C for 10 hours, filter and dry to obtain 6.8 g of olaparib, with a yield of 92.0% and a purity of 99.87%.
Embodiment 2
[0046] 1. Activation
[0047] Dissolve 2-fluoro-5-[(4-oxo-3,4-dihydronaphthalene-1-yl)methyl]benzoic acid (5.1g, 17mmol) in 50ml of chloroform, cool to 0-10°C, carbonyldiimidazole (4.1g, 25.5mmol) was added, stirred and reacted at 20-30°C for 9 hours, and the reaction solution was concentrated to dryness to obtain an active amide intermediate.
[0048] 2. Aminolytic crystallization
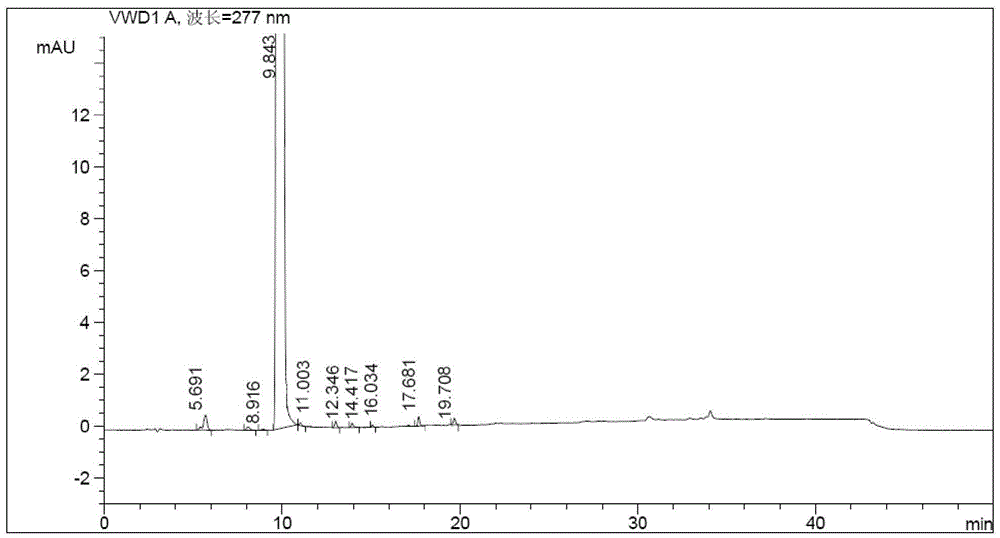
[0049]The active amide intermediate prepared in step 1 was dissolved in 10ml of chloroform, cooled to 0-10°C, and added dropwise to a solution containing 1-cyclopropanoylpiperazine (2.62g, 17mmol) and pyridine (4.1ml, 51mmol). In 20ml of chloroform solution, react at 0-10°C for 2 hours, wash with 30ml of water three times, concentrate the organic layer to dryness, add 70ml of methanol-water mixed solution (1:2, v / v) to the concentrate, and reflux to dissolve, Add 0.5 g of activated carbon and reflux for 15 min, filter, stir and crystallize the filtrate at 0-5°C for 8 hours, filter and dry to obt...
Embodiment 3
[0051] 1. Activation
[0052] Dissolve 2-fluoro-5-[(4-oxo-3,4-dihydronaphthalene-1-yl)methyl]benzoic acid (5.1g, 17mmol) in 50ml tetrahydrofuran, cool to 0- At 10°C, carbonyldiimidazole (4.1 g, 25.5 mmol) was added, stirred and reacted at 20-30°C for 12 hours, and the reaction solution was concentrated to dryness to obtain an active amide intermediate.
[0053] 2. Aminolytic crystallization
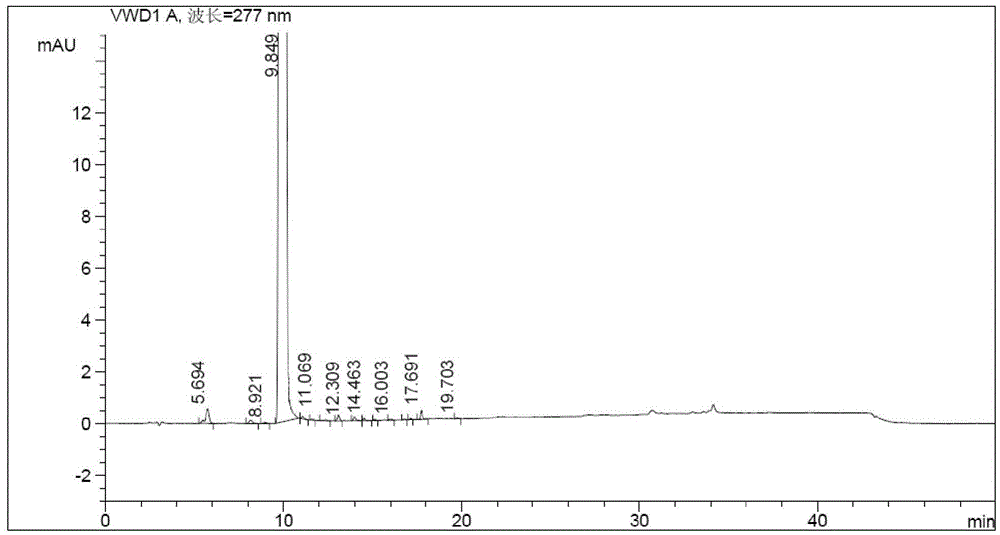
[0054] Dissolve the active amide intermediate prepared in step 1 in 10ml of toluene, cool down to 0-10°C, and add dropwise (8.4ml, 51mmol) in 20ml toluene solution, reacted at 0-10°C for 1.5 hours, washed 3 times with 30ml water, concentrated the organic layer to dryness, added isopropanol-water mixed solution (2:1, v / v) 80ml, reflux to dissolve, add 0.5g of activated carbon to reflux for 15min, filter, stir and crystallize the filtrate at 0-5°C for 10 hours, filter and dry to obtain olaparib 6.5g, yield 87.8%, purity 99.89%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com