Patents
Literature
456 results about "Oncogene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An oncogene is a gene that has the potential to cause cancer. In tumor cells, these genes are often mutated, or expressed at high levels. Most normal cells will undergo a programmed form of rapid cell death (apoptosis) when critical functions are altered and malfunctioning. Activated oncogenes can cause those cells designated for apoptosis to survive and proliferate instead. Most oncogenes began as proto-oncogenes, normal genes involved in cell growth and proliferation or inhibition of apoptosis. If normal genes promoting cellular growth, through mutation, are up-regulated (gain-of-function mutation), they will predispose the cell to cancer and are thus termed oncogenes. Usually multiple oncogenes, along with mutated apoptotic or tumor suppressor genes will all act in concert to cause cancer. Since the 1970s, dozens of oncogenes have been identified in human cancer. Many cancer drugs target the proteins encoded by oncogenes.
Noninvasive genetic immunization, expression products therefrom, and uses thereof
InactiveUS6716823B1Improve vaccination schemeEfficient methodSsRNA viruses negative-senseBiocideMalariaNon invasive
Disclosed and claimed are methods of non-invasive genetic immunization in an animal and / or methods of inducing a systemic immune or therapeutic response in an animal, products therefrom and uses for the methods and products therefrom. The methods can include contacting skin of the animal with a vector in an amount effective to induce the systemic immune or therapeutic response in the animal. The vector can include and express an exogenous nucleic acid molecule encoding an epitope or gene product of interest. The systemic immune response can be to or from the epitope or gene product. The nucleic acid molecule can encode an epitope of interest and / or an antigen of interest and / or a nucleic acid molecule that stimulates and / or modulates an immunological response and / or stimulates and / or modulates expression, e.g., transcription and / or translation, such as transcription and / or translation of an endogenous and / or exogenous nucleic acid molecule; e.g., one or more of influenza hemagglutinin, influenza nuclear protein, influenza M2, tetanus toxin C-fragment, anthrax protective antigen, anthrax lethal factor, rabies glycoprotein, HBV surface antigen, HIV gp 120, HIV gp 160, human carcinoembryonic antigen, malaria CSP, malaria SSP, malaria MSP, malaria pfg, and mycobacterium tuberculosis HSP; and / or a therapeutic, an immunomodulatory gene, such as co-stimulatory gene and / or a cytokine gene. The immune response can be induced by the vector expressing the nucleic acid molecule in the animal's cells. The animal's cells can be epidermal cells. The immune response can be against a pathogen or a neoplasm. A prophylactic vaccine or a therapeutic vaccine or an immunological composition can include the vector. The animal can be a vertebrate, e.g., a mammal, such as human, a cow, a horse, a dog, a cat, a goat, a sheep or a pig; or fowl such as turkey, chicken or duck. The vector can be one or more of a viral vector, including viral coat, e.g., with some or all viral genes deleted therefrom, bacterial, protozoan, transposon, retrotransposon, and DNA vector, e.g., a recombinant vector; for instance, an adenovirus, such as an adenovirus defective in its E1 and / or E3 and / or E4 region(s). The method can encompass applying a delivery device including the vector to the skin of the animal, as well as such a method further including disposing the vector in and / or on the delivery device. The vector can have all viral genes deleted therefrom. The vector can induce a therapeutic and / or an anti-tumor effect in the animal, e.g., by expressing an oncogene, a tumor-suppressor gene, or a tumor-associated gene. Immunological products generated by the expression, e.g., antibodies, cells from the methods, and the expression products, are likewise useful in in vitro and ex vivo applications, and such immunological and expression products and cells and applications are disclosed and claimed. Methods for expressing a gene product in vivo and products therefor and therefrom including mucosal and / or intranasal administration of an adenovirus, advantageously an E1 and / or E3 and / or E4 defective or deleted adenovirus, such as a human adenovirus or canine adenovirus, are also disclosed and claimed.
Owner:UAB RES FOUND
Adipose-derived stromal cells (ASC) as delivery tool for treatment of cancer
InactiveUS20110027239A1Efficient killingSystemic non-specific responses will be minimalBiocideSpecial deliveryAnticarcinogenStromal cell
The present invention generally relates to use of adult Adipose-derived stromal cells (ASC) and genetically engineered ASC for the treatment of cancer. In particular, the present invention generally relates, in part to a method for treating a subject with cancer comprising administering to the subject a composition comprising engineered ASCs which have been modified to express a gene encoding at least one anti-cancer agent. In some embodiments, an anti-cancer agent is a pro-apoptotic agent. In some embodiments an anti-cancer agent is an agent which inhibits the expression of an oncogene.
Owner:TISSUE GENESIS
Generation of tumor-free embryonic stem-like pluripotent cells using inducible recombinant RNA agents
InactiveUS20090203141A1Improve target specificityReduce the number of copiesVectorsFermentationCancer cellMammal
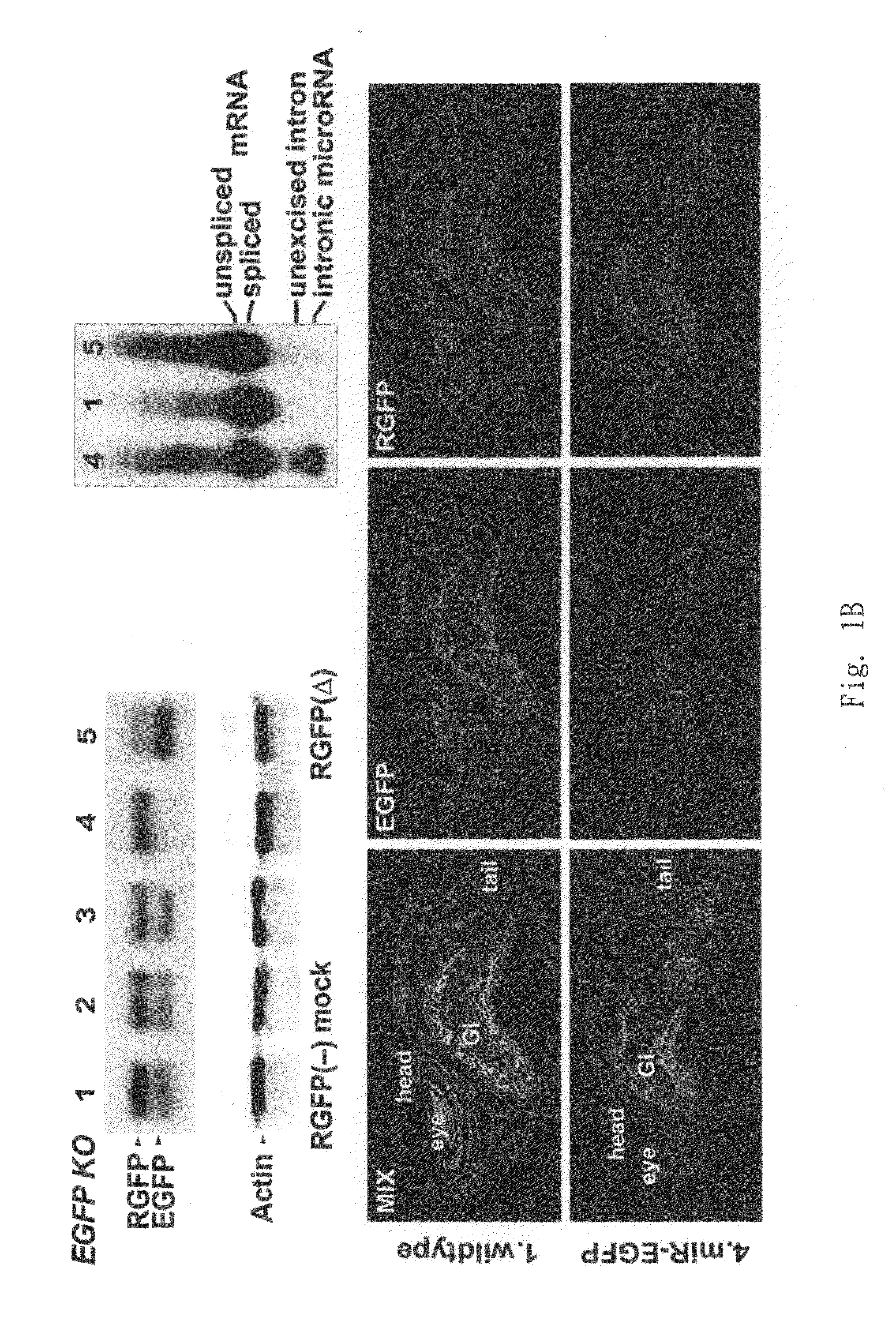
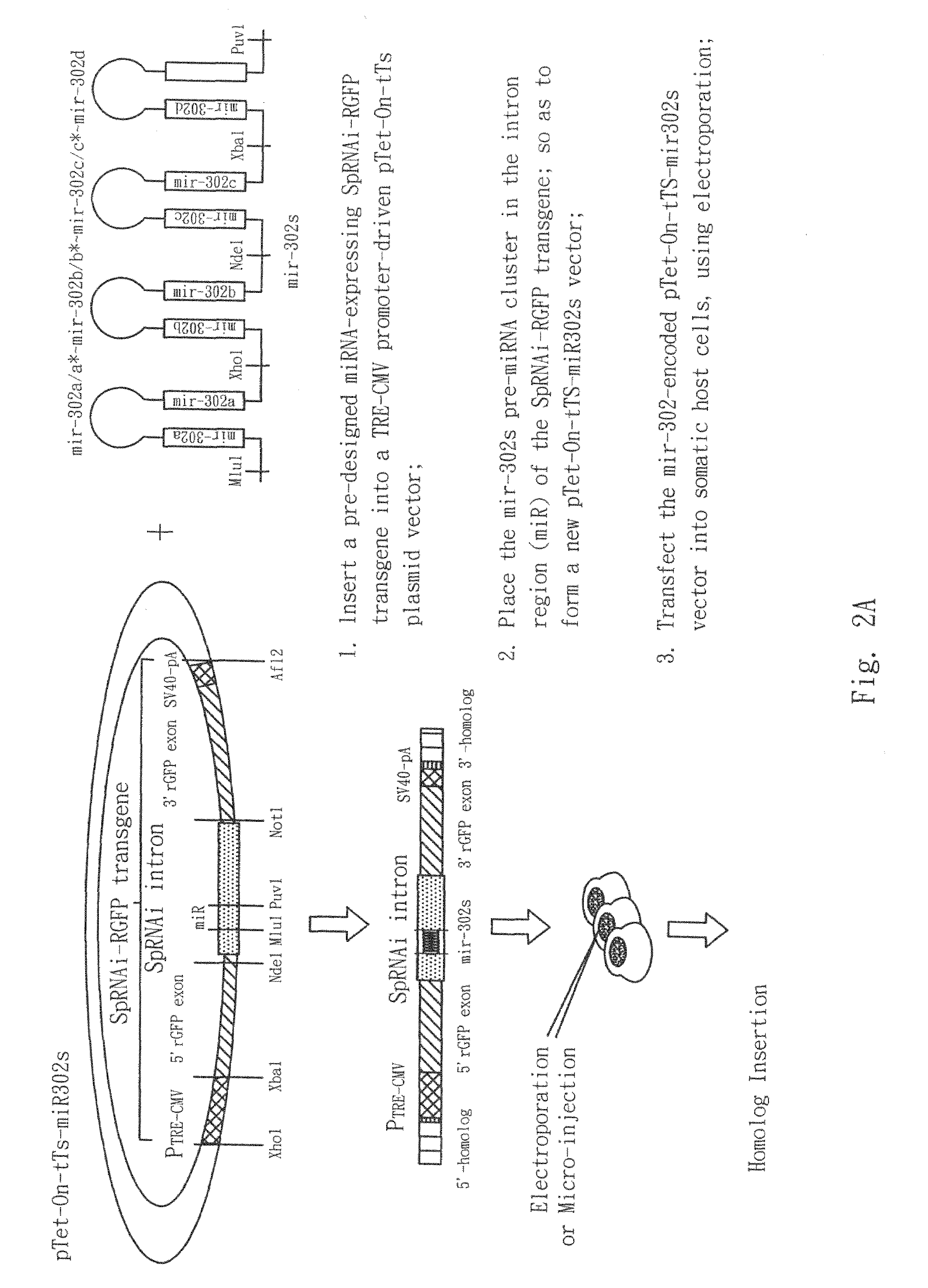
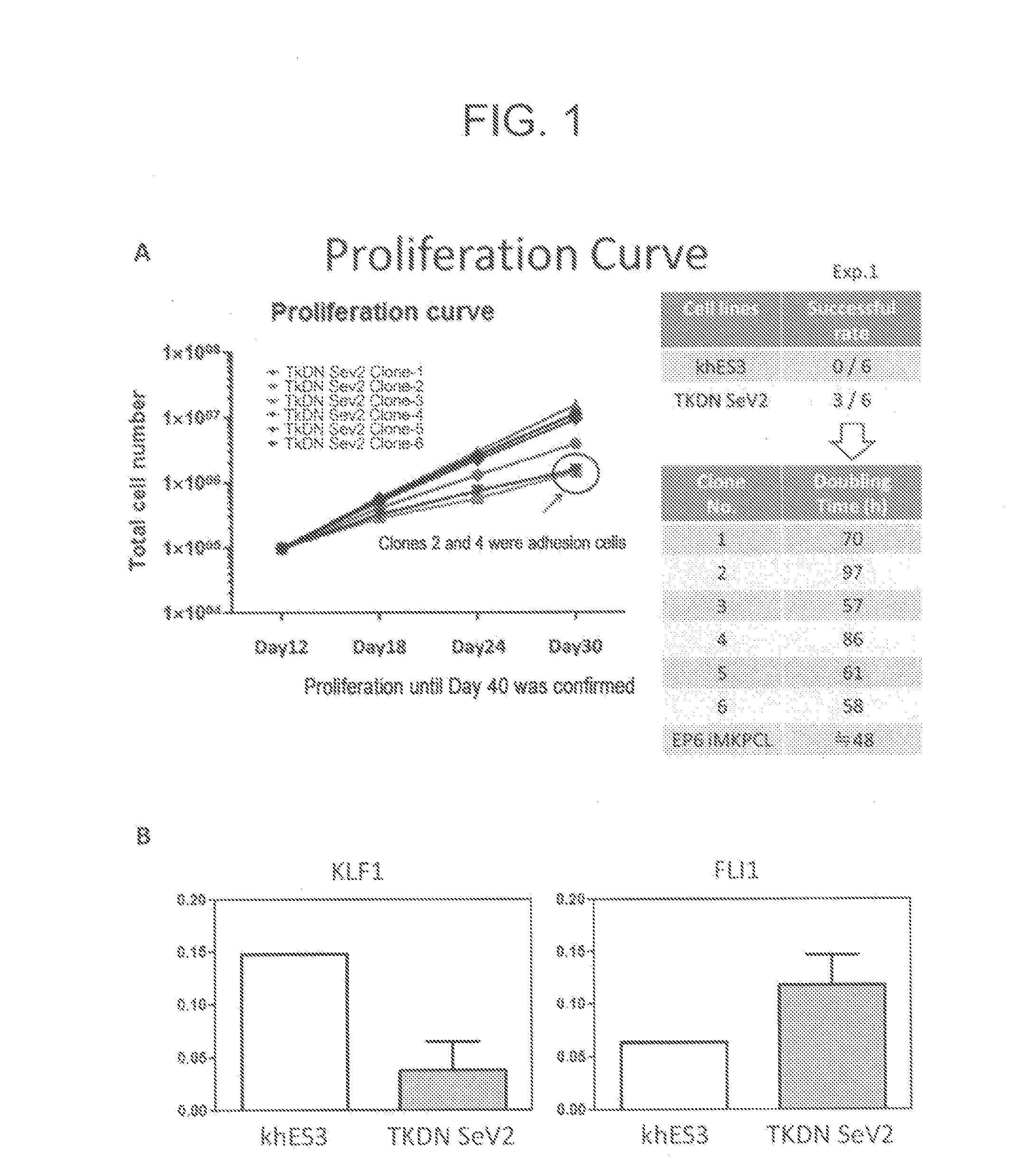
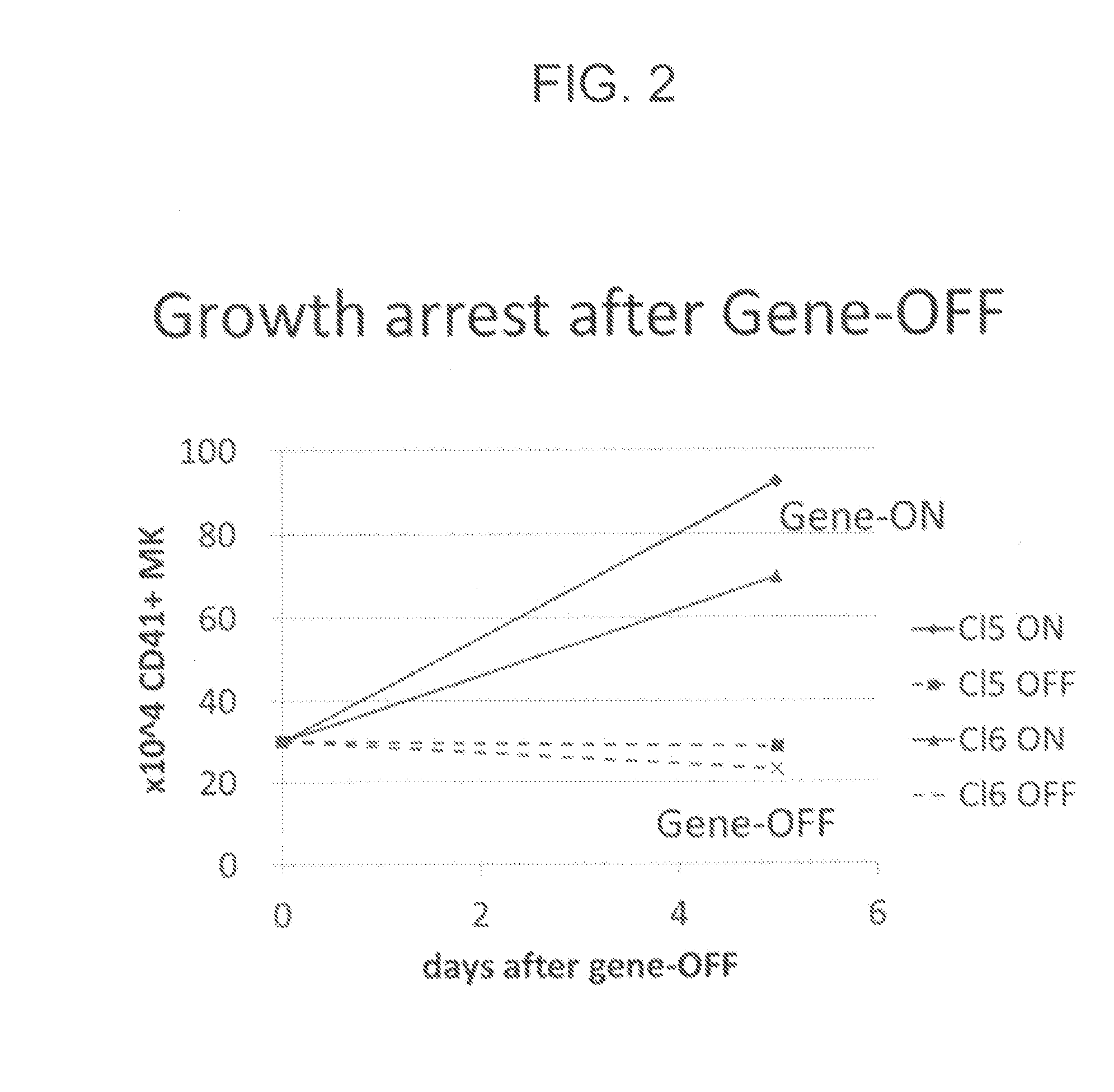
The present invention generally relates to a method for developing, generating and selecting tumor-free embryonic stem (ES)-like pluripotent cells using electroporation delivery of an inducible tumor suppressor mir-302 agent into mammalian cells. More particularly, the present invention relates to a method and composition for generating a Tet-On / Off recombinant transgene capable of expressing a manually re-designed mir-302 microRNA (miRNA) / shRNA agent under the control of doxycyclin (Dox) in human somatic / cancer cells and thus inducing certain specific gene silencing effects on the differentiation-associated genes and oncogenes of the cells, resulting in reprogramming the cells into an ES-like pluripotent state.
Owner:LIN SHI LUNG +1
Novel use of aim 3 acting as a tumor suppressor
InactiveUS20060046250A1Prevent proliferationPromote phosphorylationCompound screeningApoptosis detectionApoptosisInducer Cells
The present invention relates to novel uses of AIM3 acting as a tumor suppressor, and more particularly to methods for using an AIM3 protein or a nucleic acid encoding the protein to activate ATM or ATR and to treat ATM- or ATR-mediated diseases. The AIM3 protein according to the present invention interacts directly with ATM / ATR so as to activate ATM / ATR and proteins regulated by ATM / ATR. Also, the AIM3 protein upregulates tumor suppressor gene p53 and its target genes so as to not only inhibit the proliferation of cells but also to induce apoptosis.
Owner:SCHWARZ HERBERT +1
Induced malignant stem cells
InactiveUS20140137274A1High and low degree of methylationSugar derivativesPeptide/protein ingredientsMicrosatelliteSomatic cell
PROBLEMThere are provided induced malignant stem cells capable of in vitro proliferation that are useful in cancer research and drug discovery for cancer therapy, as well as processes for production thereof, cancer cells derived from these cells, and applications of these cells.MEANS FOR SOLVINGAn induced malignant stem cell capable of in vitro proliferation are characterized by satisfying the following two requirements:(1) having at least one aberration selected from among (a) an aberration of methylation (high or low degree of methylation) in a tumor suppressor gene or a cancer-related genetic region in endogenous genomic DNA, (b) a somatic mutation of a tumor suppressor gene or a somatic mutation of an endogenous cancer-related gene in endogenous genomic DNA, (c) abnormal expression (increased or reduced / lost expression) of an endogenous oncogene or an endogenous tumor suppressor gene, (d) abnormal expression (increased or reduced / lost expression) of a noncoding RNA such as an endogenous cancer-related microRNA, (e) abnormal expression of an endogenous cancer-related protein, (f) an aberration of endogenous cancer-related metabolism (hypermetabolism or hypometabolism), (g) an aberration of endogenous cancer-related sugar chain, (h) an aberration of copy number variations in endogenous genomic DNA, and (i) instability of microsatellites in endogenous genomic DNA in an induced malignant stem cell; and(2) expressing genes including POU5F1 gene, NANOG gene, SOX2 gene, and ZFP42 gene.
Owner:ISHIKAWA
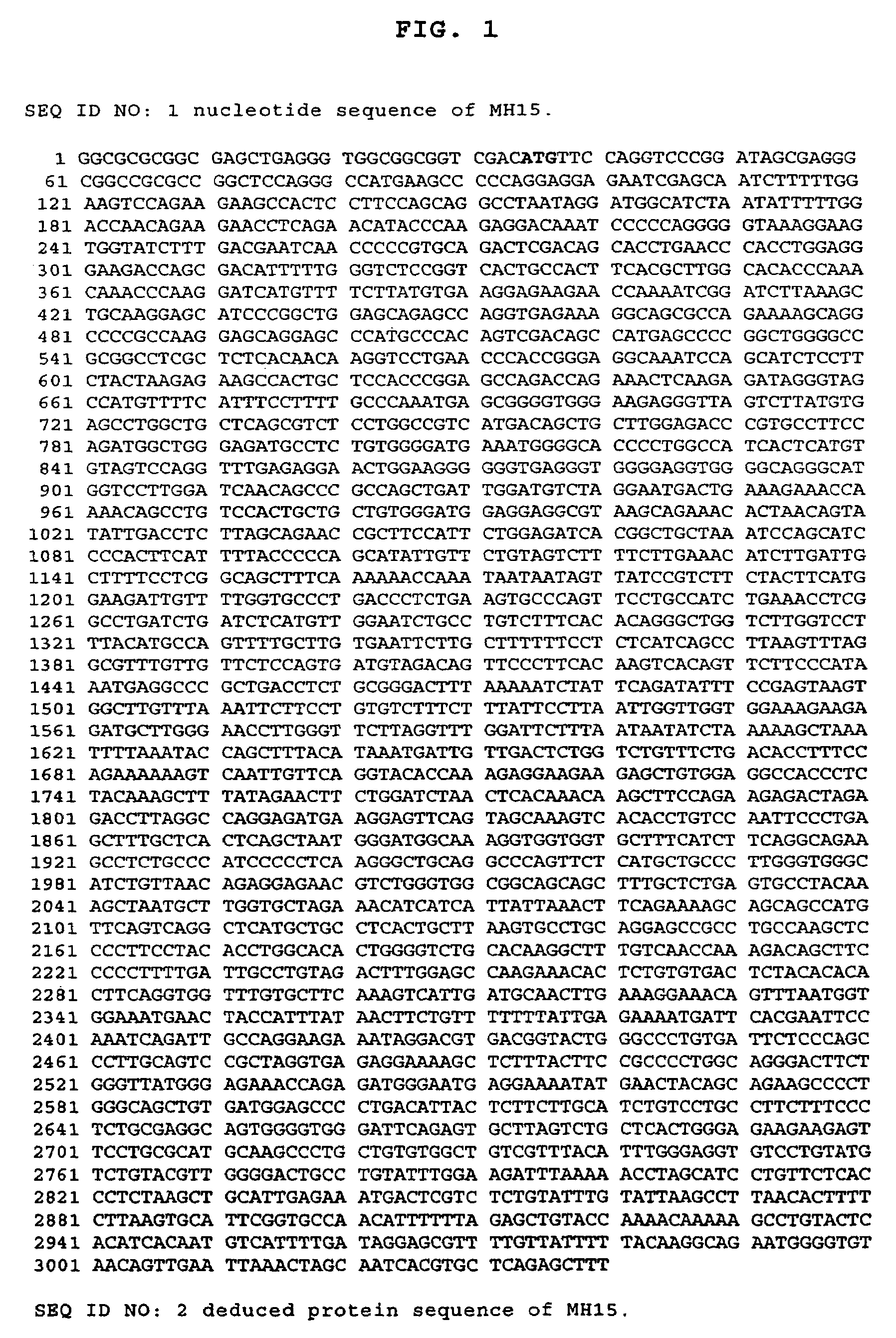
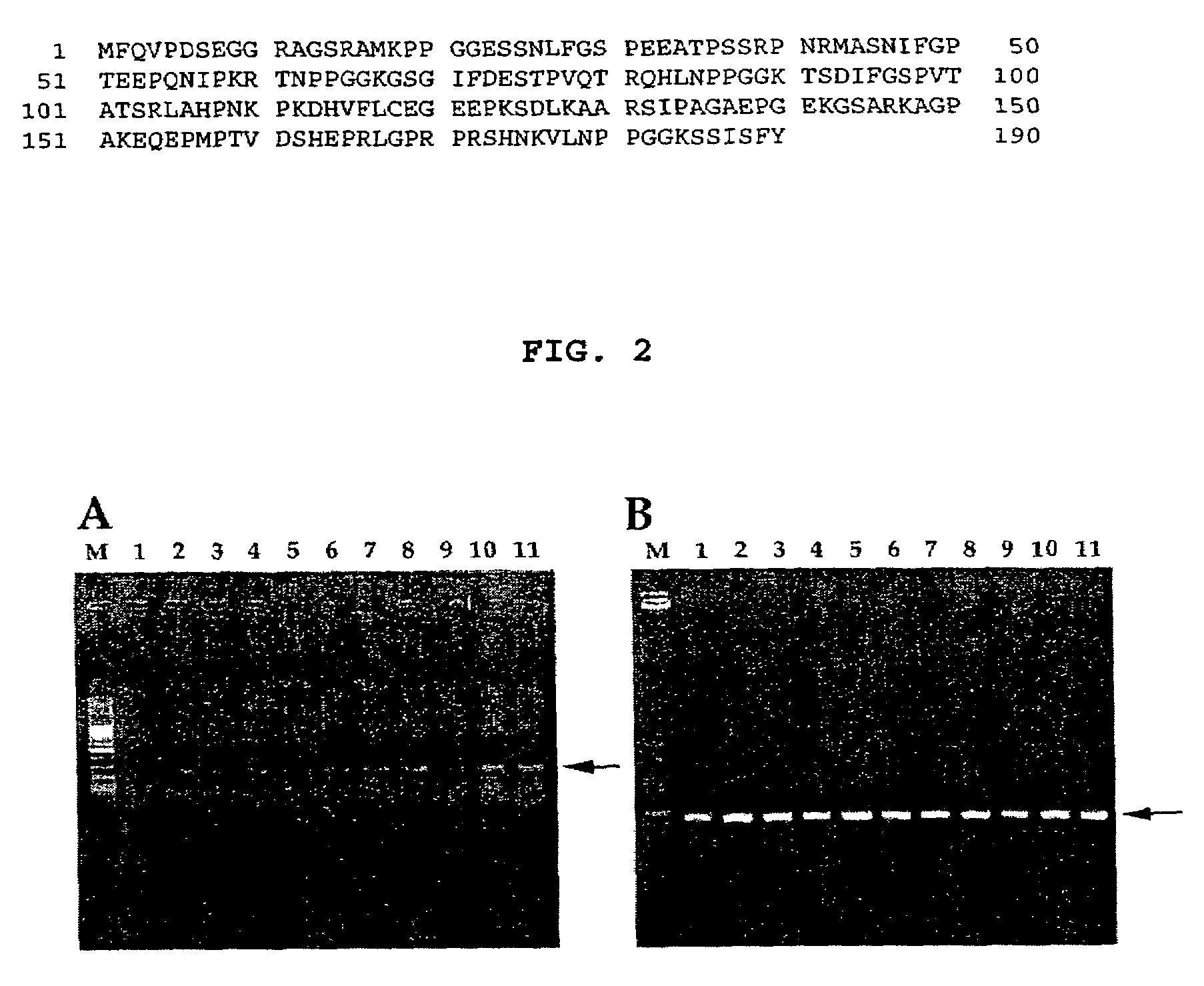
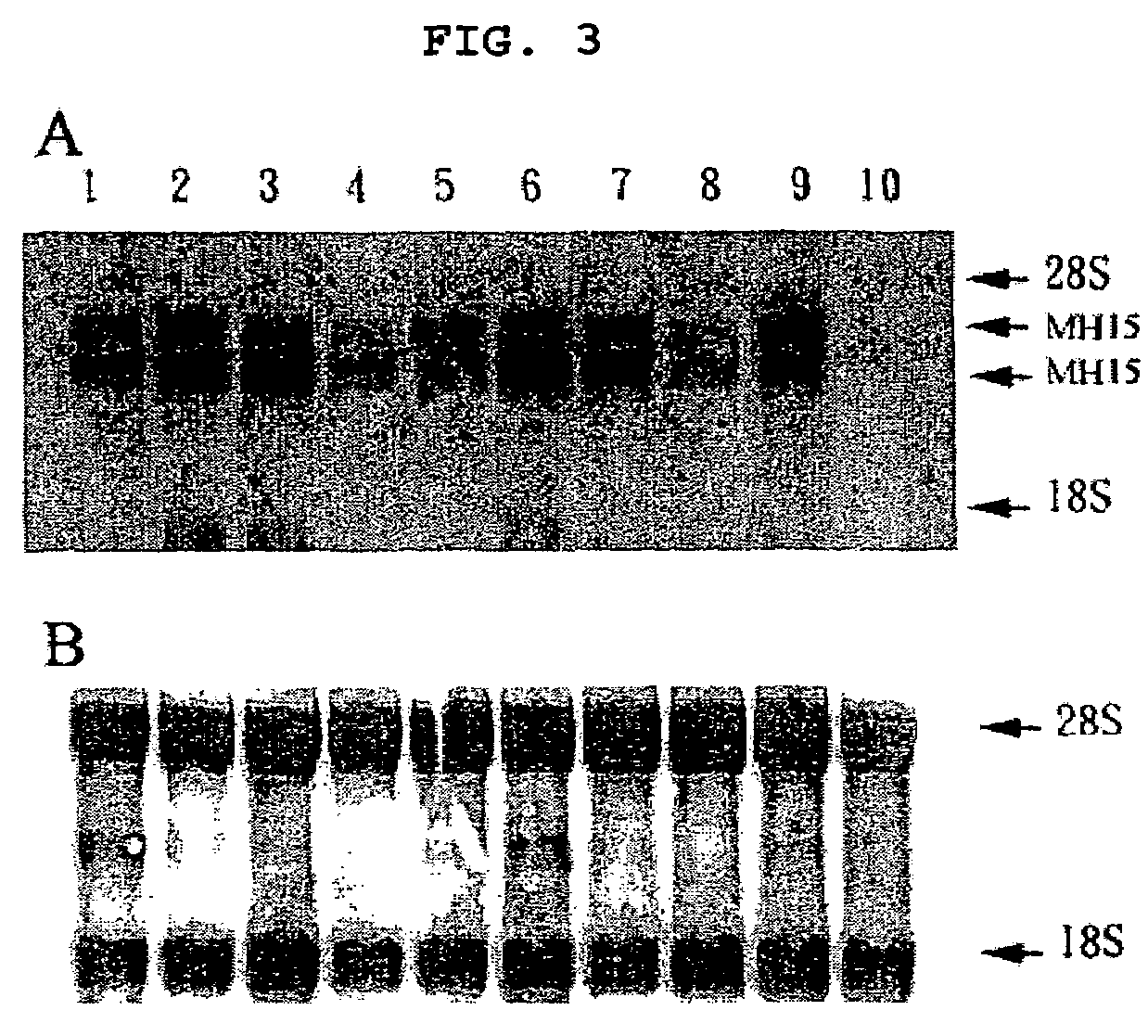
Cancer specific gene MH15
InactiveUS7507532B2Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsChemical compoundOncology
Owner:MEDIGEN BIOTECH
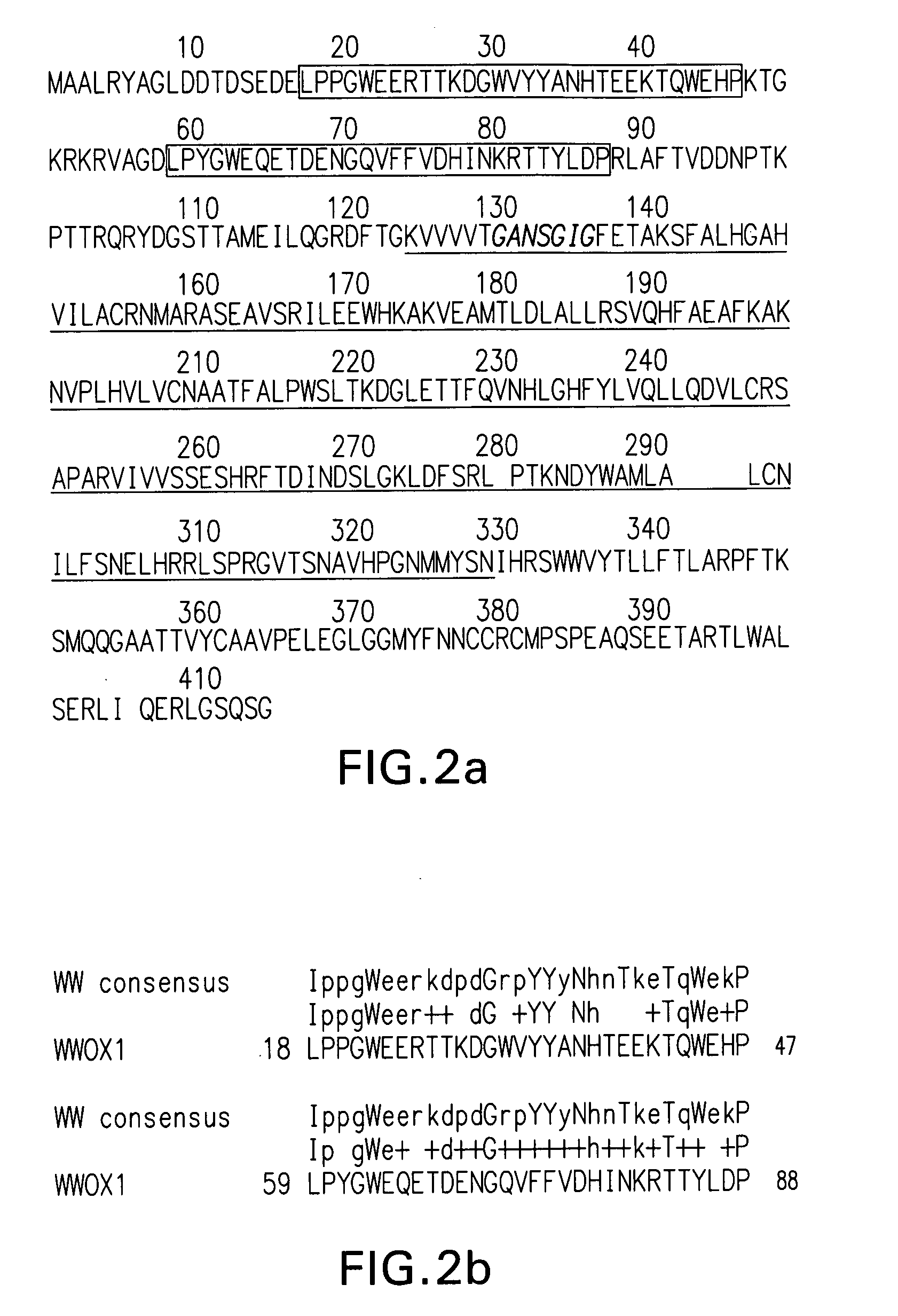
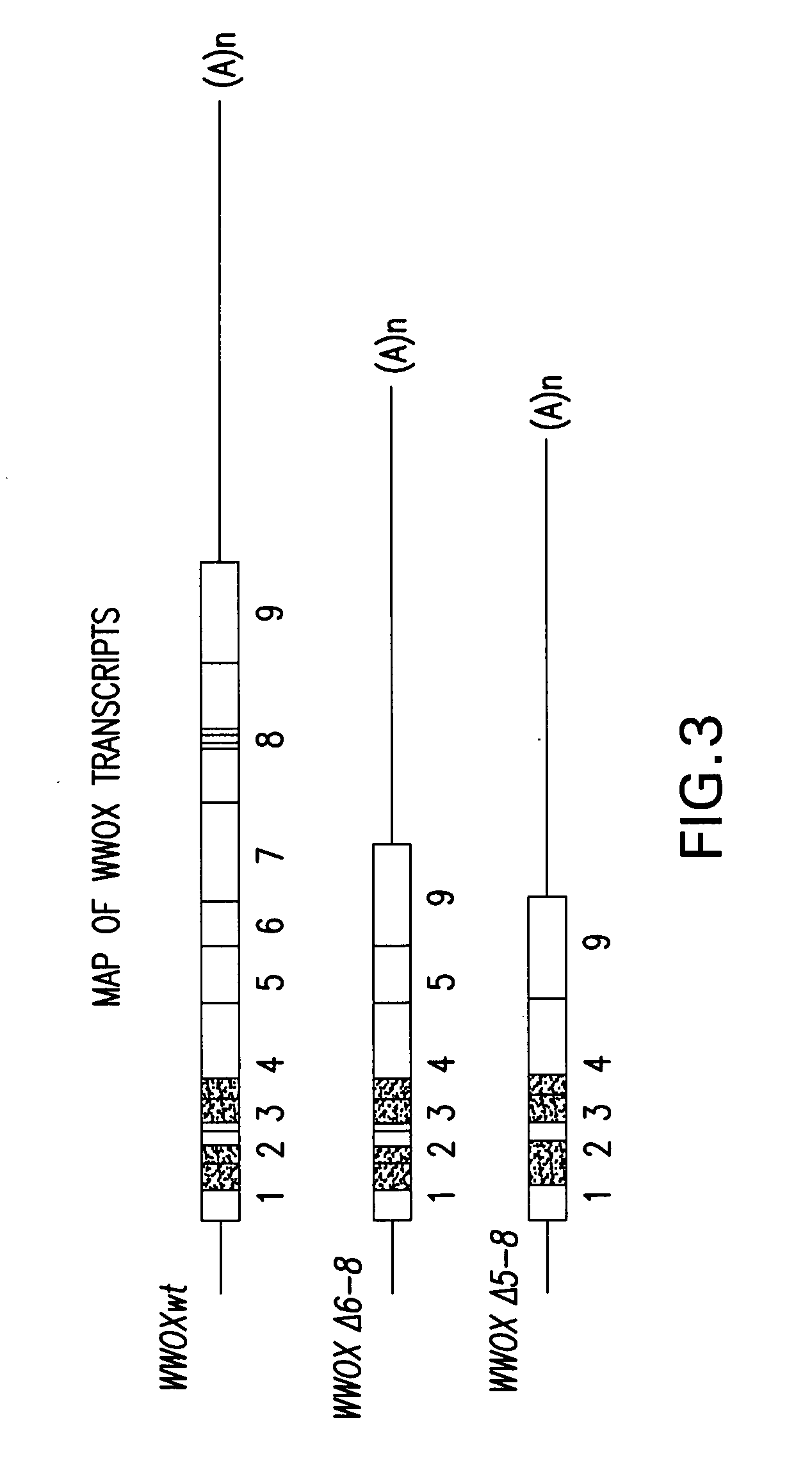
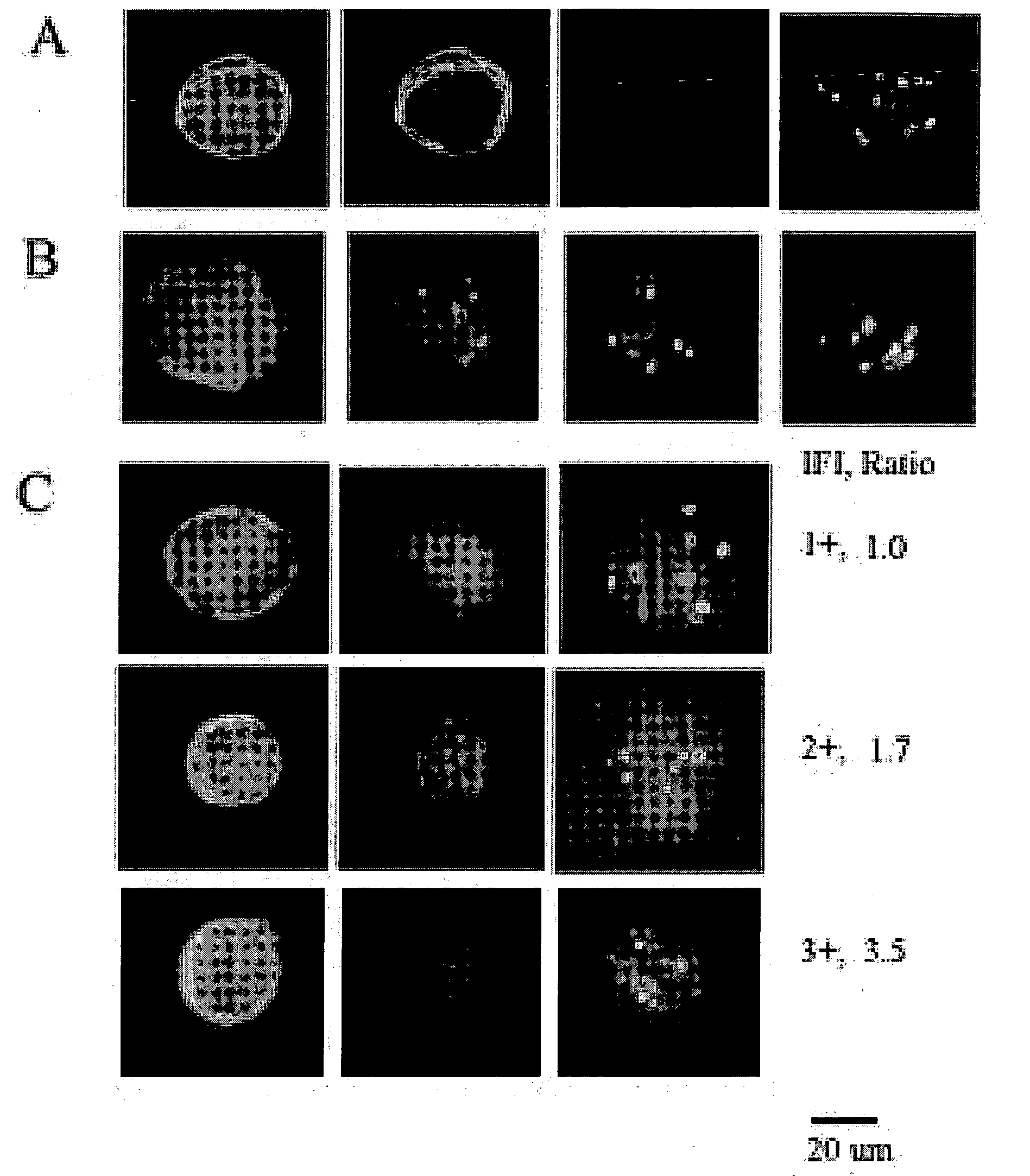
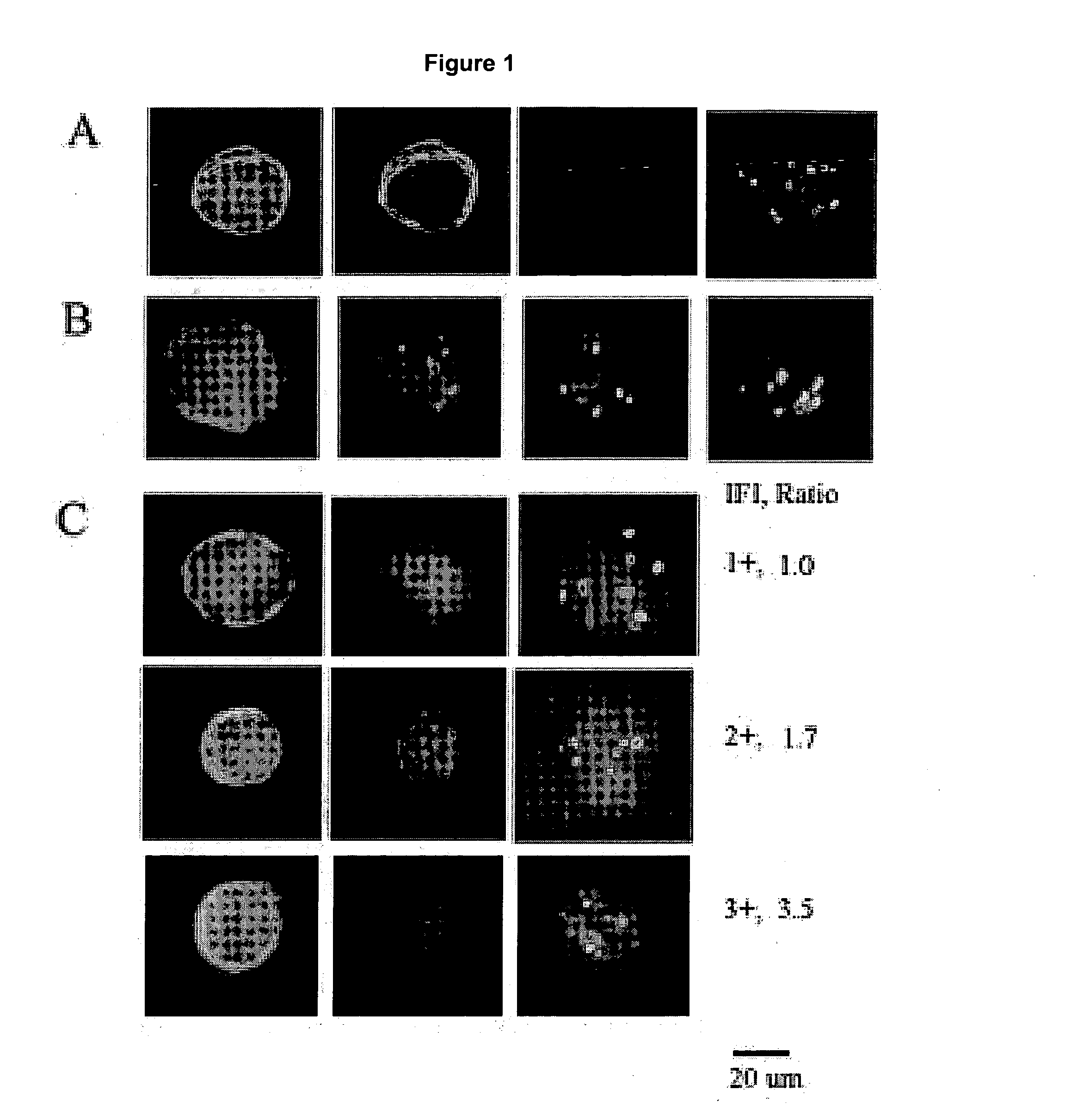
WWOX: a tumor suppressor gene mutated in multiple cancers
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Blood test to monitor the genetic changes of progressive cancer using immunomagnetic enrichment and fluorescence in situ hybridization (FISH)
InactiveUS20080113350A1Accurate measurementEasy accessMicrobiological testing/measurementLymphatic SpreadGenetic Change
Amplification and overexpression of theHER-2 oncogene in breast cancer is felt to be stable over the course of disease and concordant between the primary tumor and metastases. Therefore, patients with HER-2 negative primary tumors will rarely receive anti-HER-2 antibody therapy. A very sensitive blood test is used to capture circulating tumor cells (CTC's) and evaluate their HER-2 gene status by FISH evaluation. The HER-2 status of the primary tumor and corresponding CTC's is used to assess the ratio of CTC's as a reliable surrogate marker. HER-2 expression of 10 CTC's is sufficient to make a definitive diagnosis of the HER-2 gene status for the whole population of CTC's in patients with recurrent breast cancer.
Owner:JANSSEN DIAGNOSTICS LLC
MDM2-Containing Double Minute Chromosomes And Methods Therefore
InactiveUS20140350130A1Rapid identificationBiocideMicrobiological testing/measurementSequence analysisMdm2 Protein
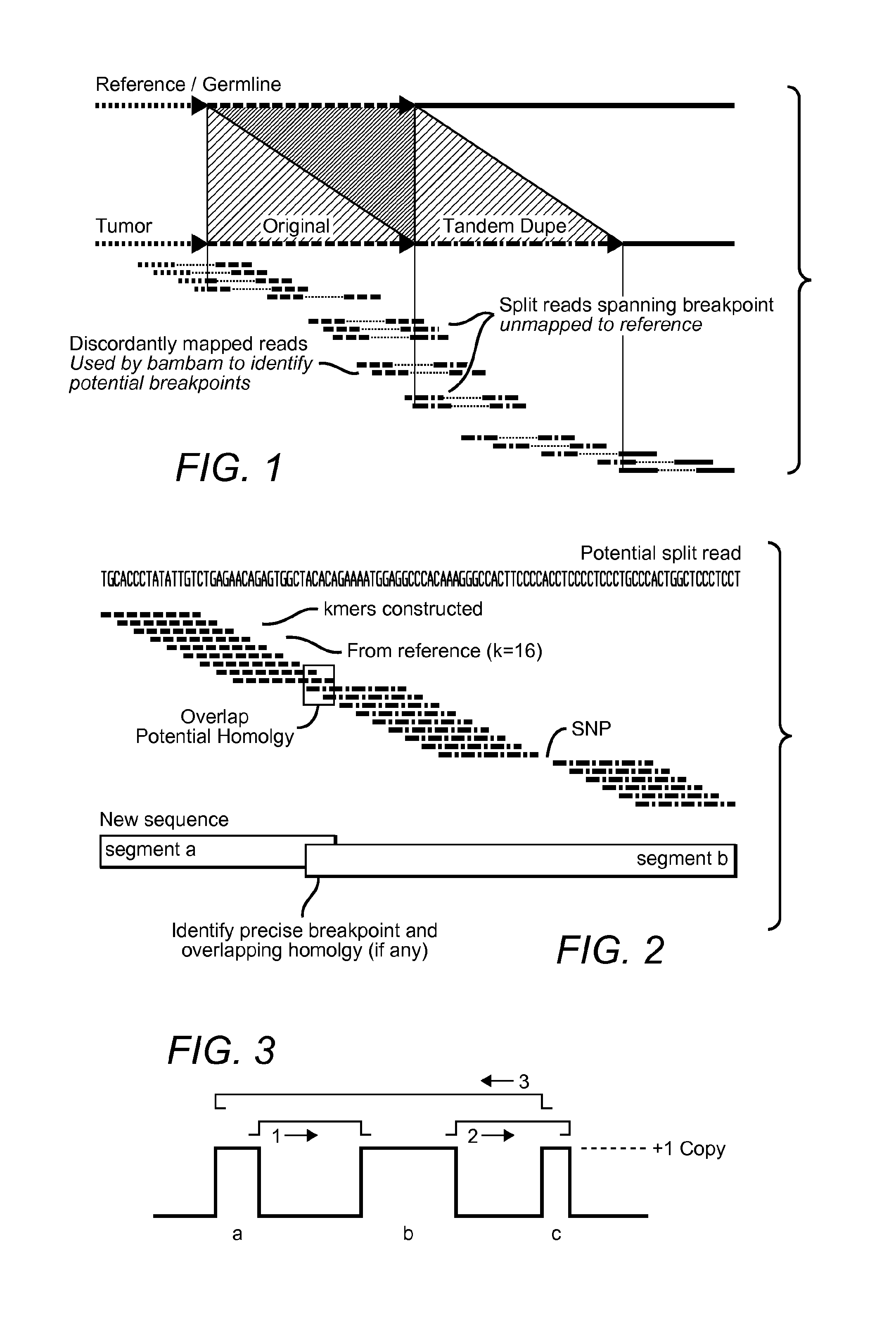
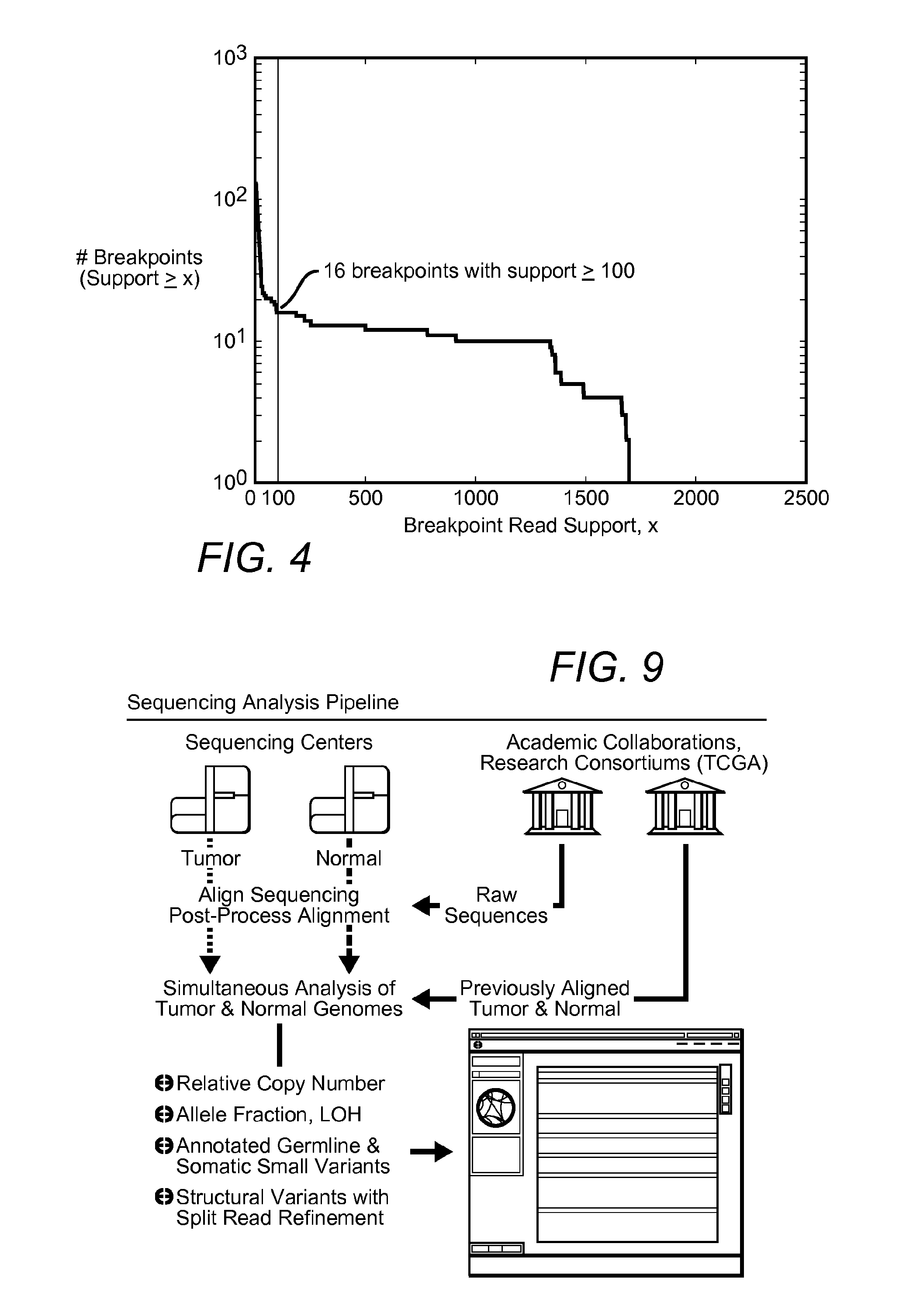
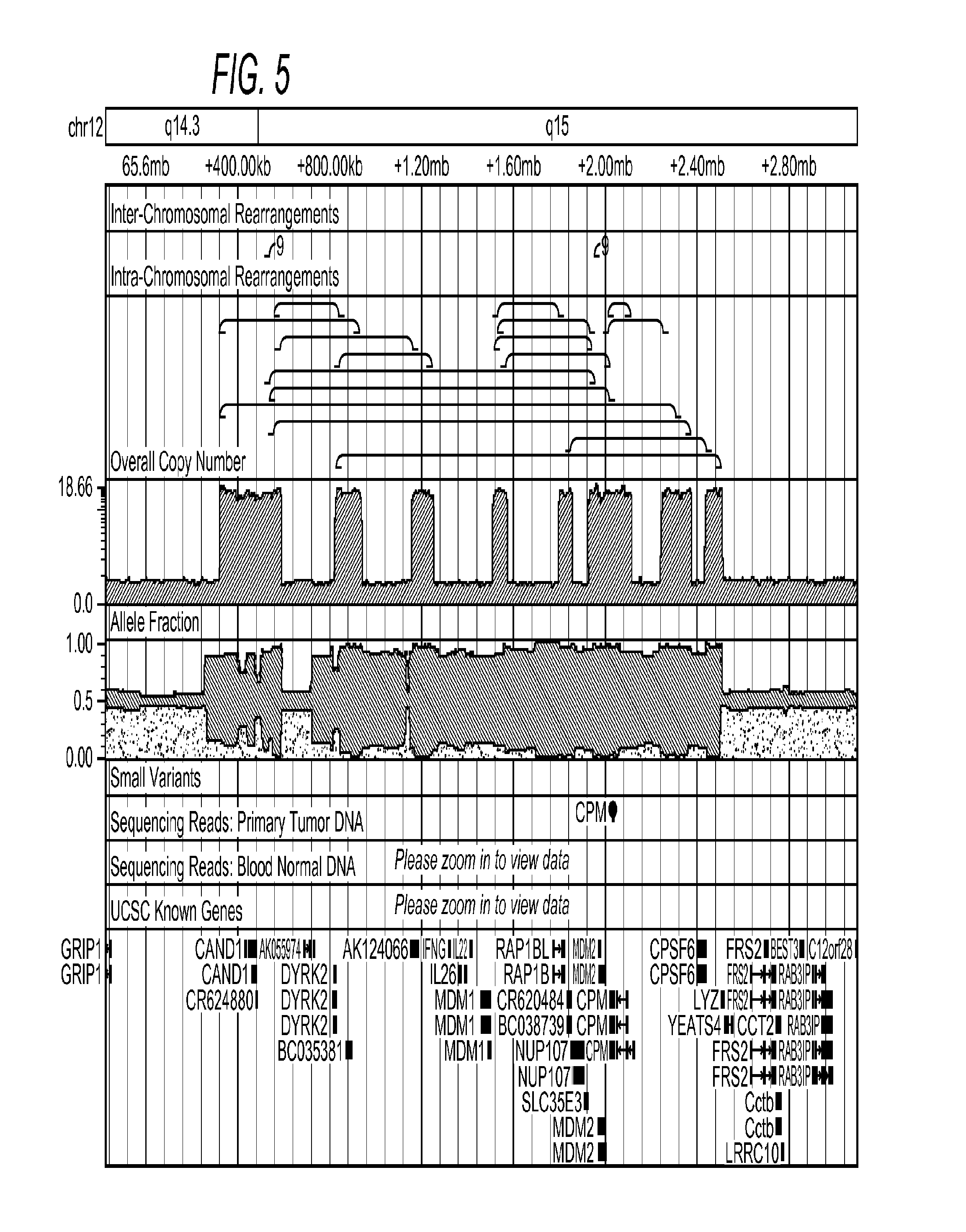
Contemplated systems and methods allow for computational genomic analysis using paired-end sequence analysis and split read refinement to thereby identify high-confidence breakpoints associated with high copy numbers and orientation of rearrangements, which is then the basis for full reconstruction of double minutes (DM). In especially preferred aspects, the DM will also include an oncogene or tumor suppressor gene, and / or may be found in blood or blood derived fluids.
Owner:FIVE3 GENOMICS
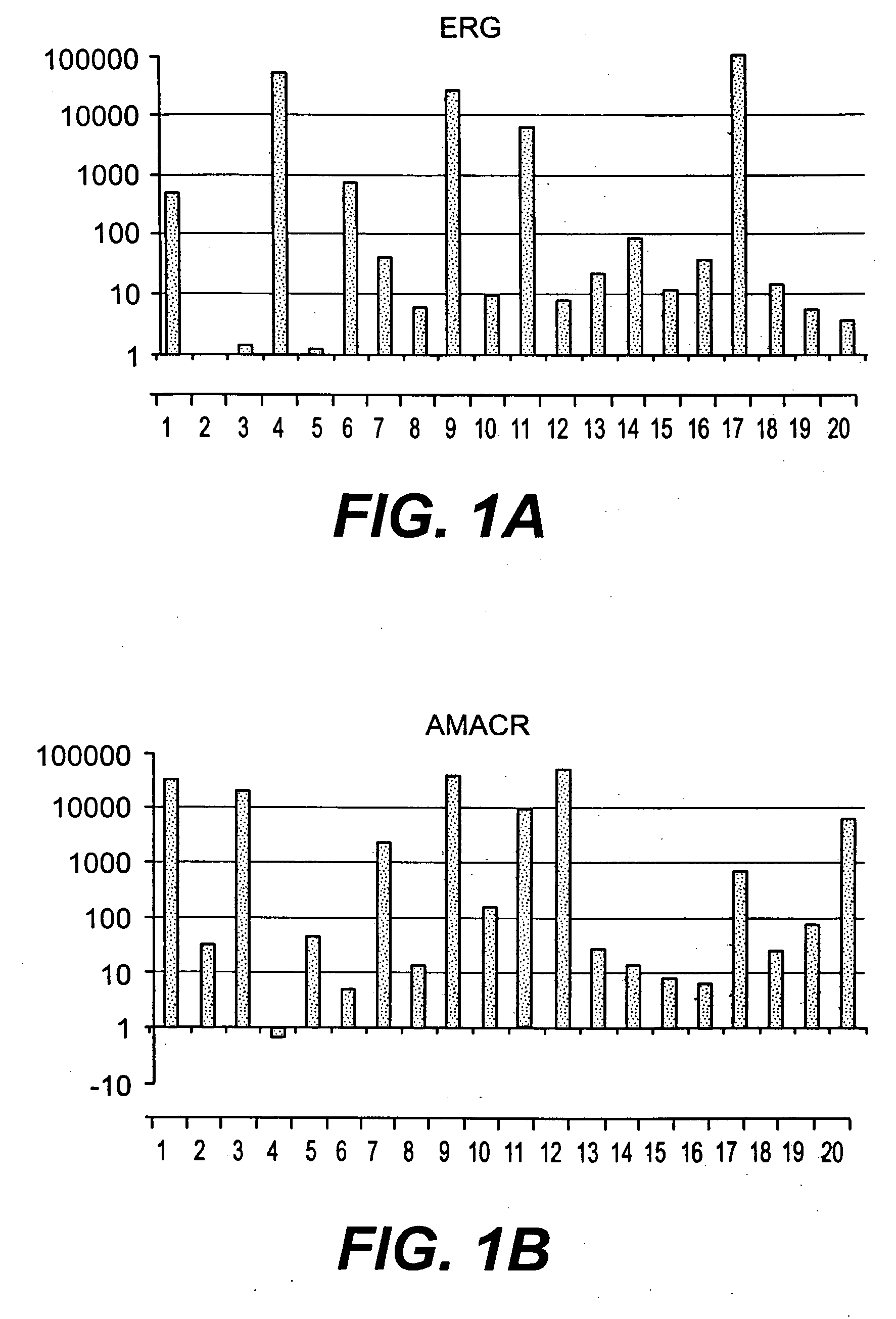
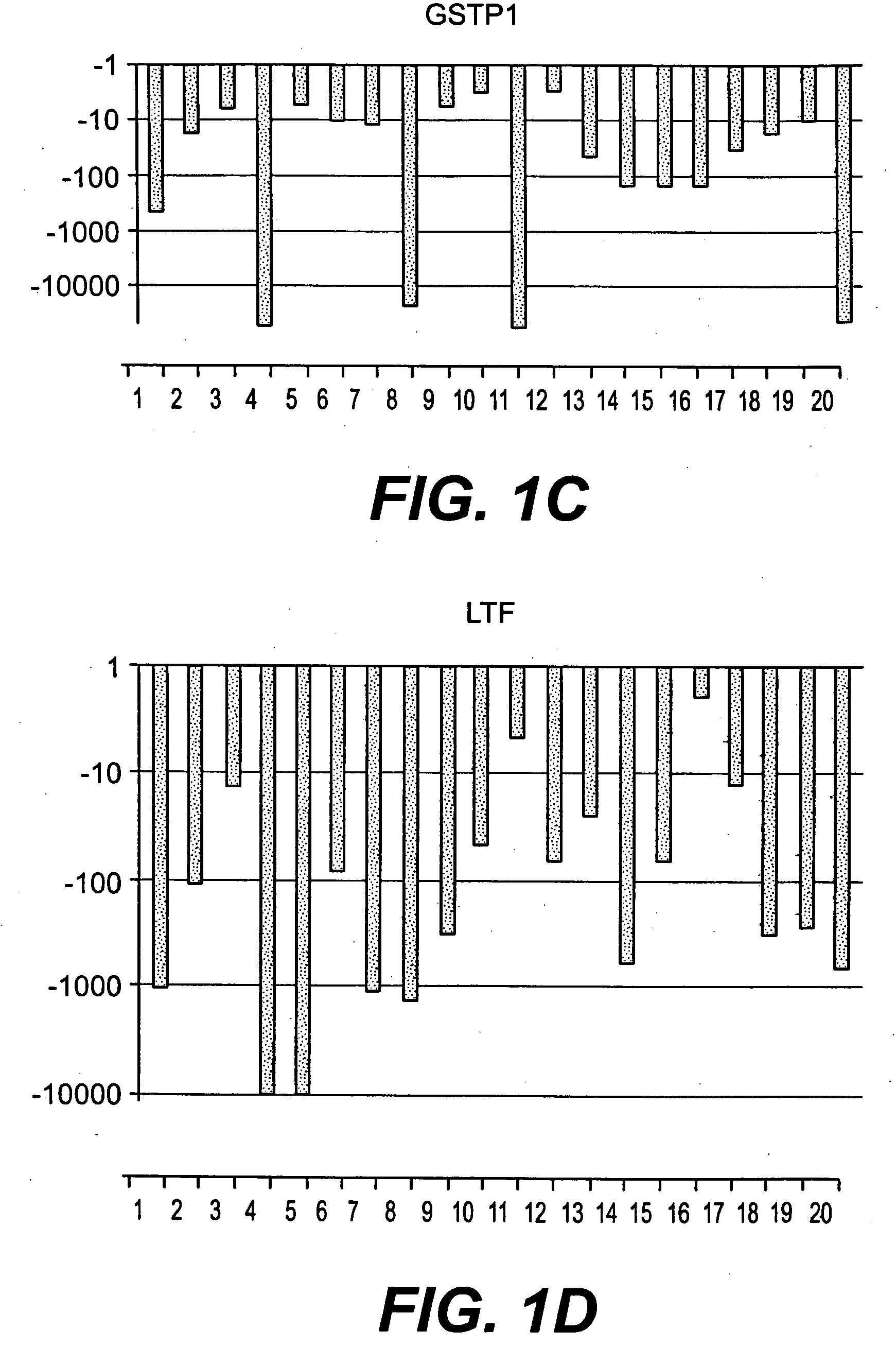
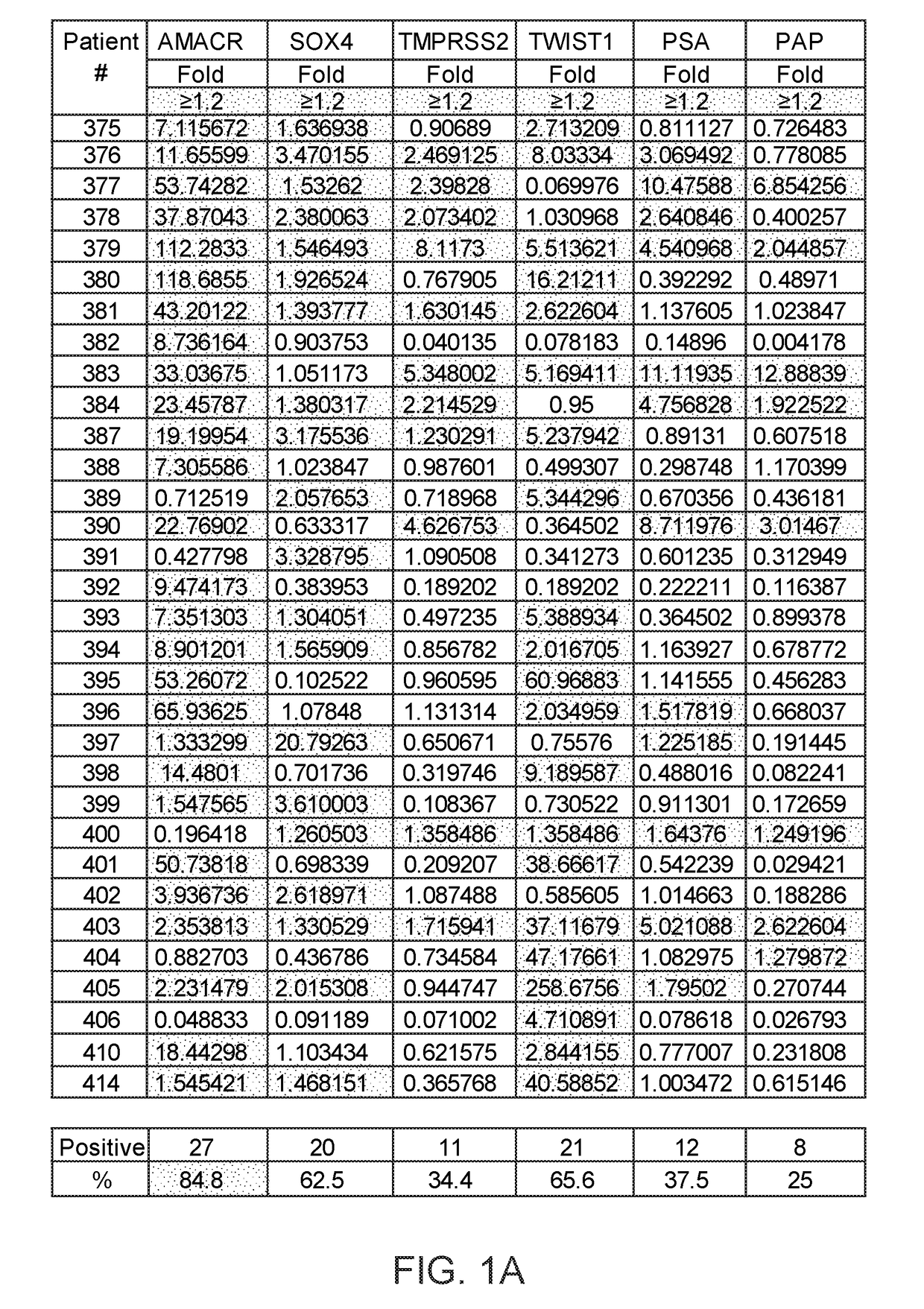
Methods of diagnosing or treating prostate cancer using the erg gene, alone or in combination with other over or under expressed genes in prostate cancer
ActiveUS20090170075A1Microbiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsProstate cancer cellOncology
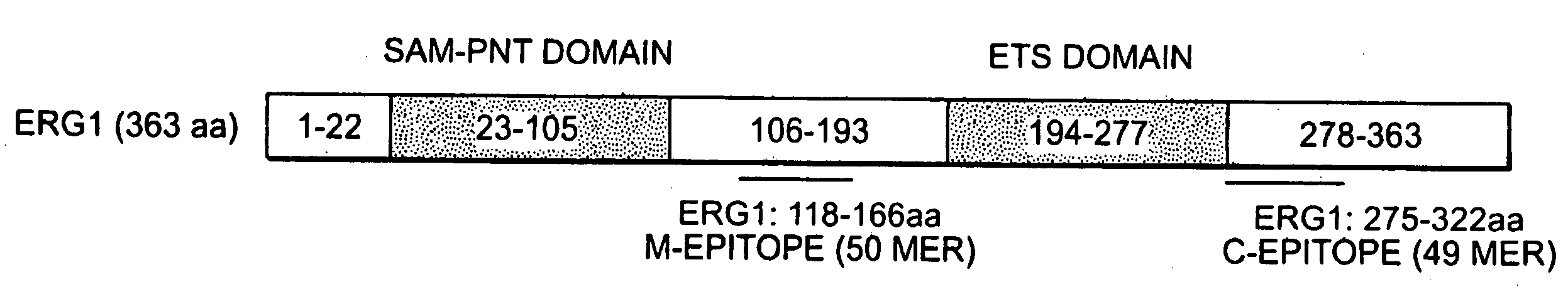
The present invention relates to oncogenes or tumor suppressor genes, as well as other genes, involved in prostate cancer and their expression products, as well as derivatives and analogs thereof. Provided are therapeutic compositions and methods of detecting and treating cancer, including prostate and other related cancers. Also provided are methods of diagnosing and / or prognosing prostate cancer by determining the expression level of at least one prostate cancer-cell-specific gene, including, for example, the ERG gene or the LTF gene alone, or in combination with at least one of the AMACR gene and the DD3 gene.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC
Mutant Viruses
An herpes simplex virus wherein the herpes simplex virus genome comprises nucleic acid encoding an antisense to the squamous cell carcinoma related oncogene (asSCCRO); and an herpes simplex virus wherein the herpes simplex virus genome comprises nucleic acid encoding a short interfering ribonucleic acid (siRNA) molecule that is capable of repressing or silencing expression of squamous cell carcinoma related oncogene (SCCRO) nucleic acid or polypeptide are disclosed together with methods for generation and applications of such viruses.
Owner:SLOAN KETTERING INST FOR CANCER RES +1
Conditional immortalization of cells
A recombinant, or genetically engineered, mammalian cell, comprises a conditionally-inducible oncogene and an exogenous polynucleotide encoding the catalytic sub-unit of the telomerase complex. The recombinant cells are useful in therapy, to replace lost or damaged cells.
Owner:RENEURON LTD +1
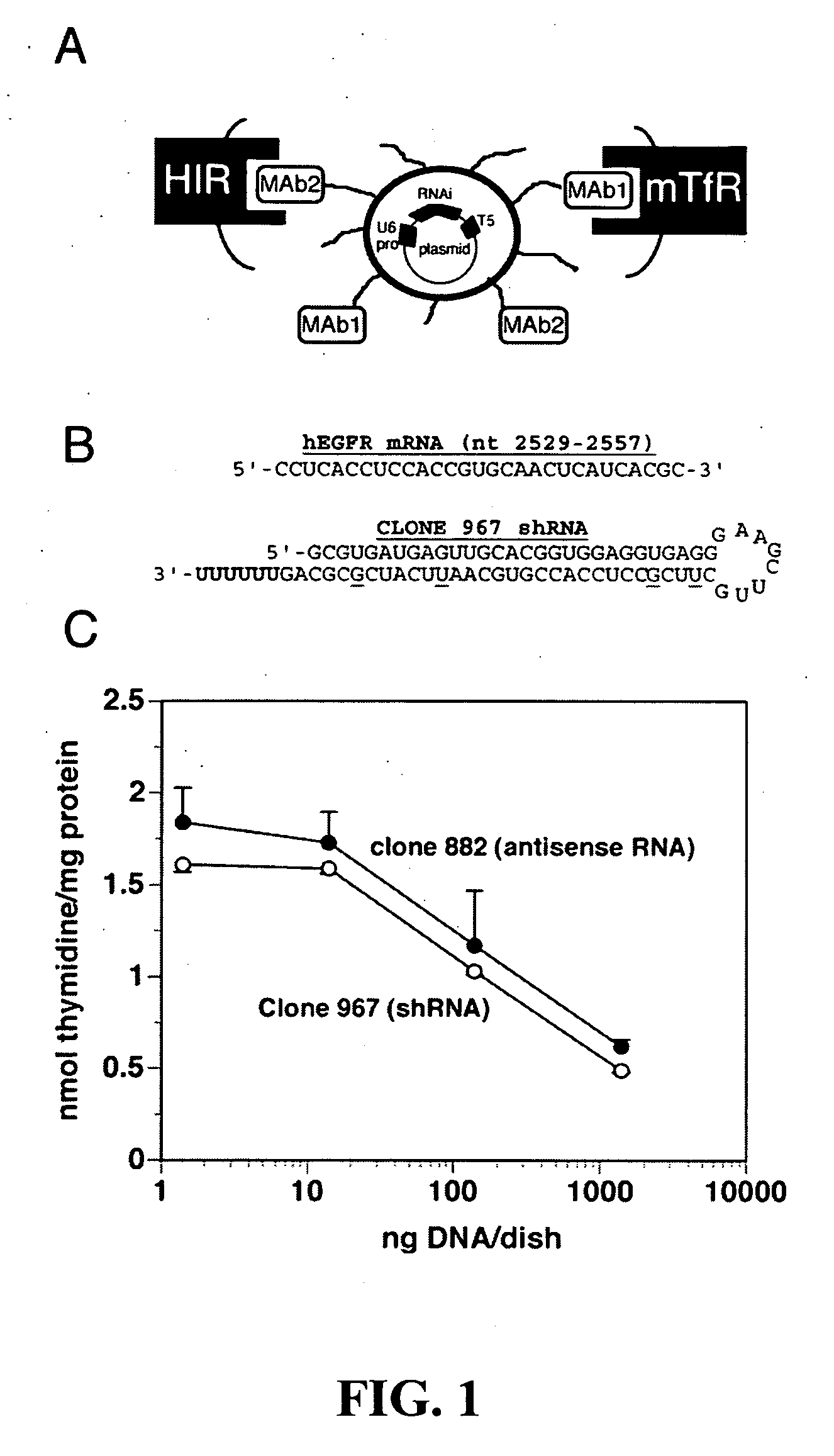
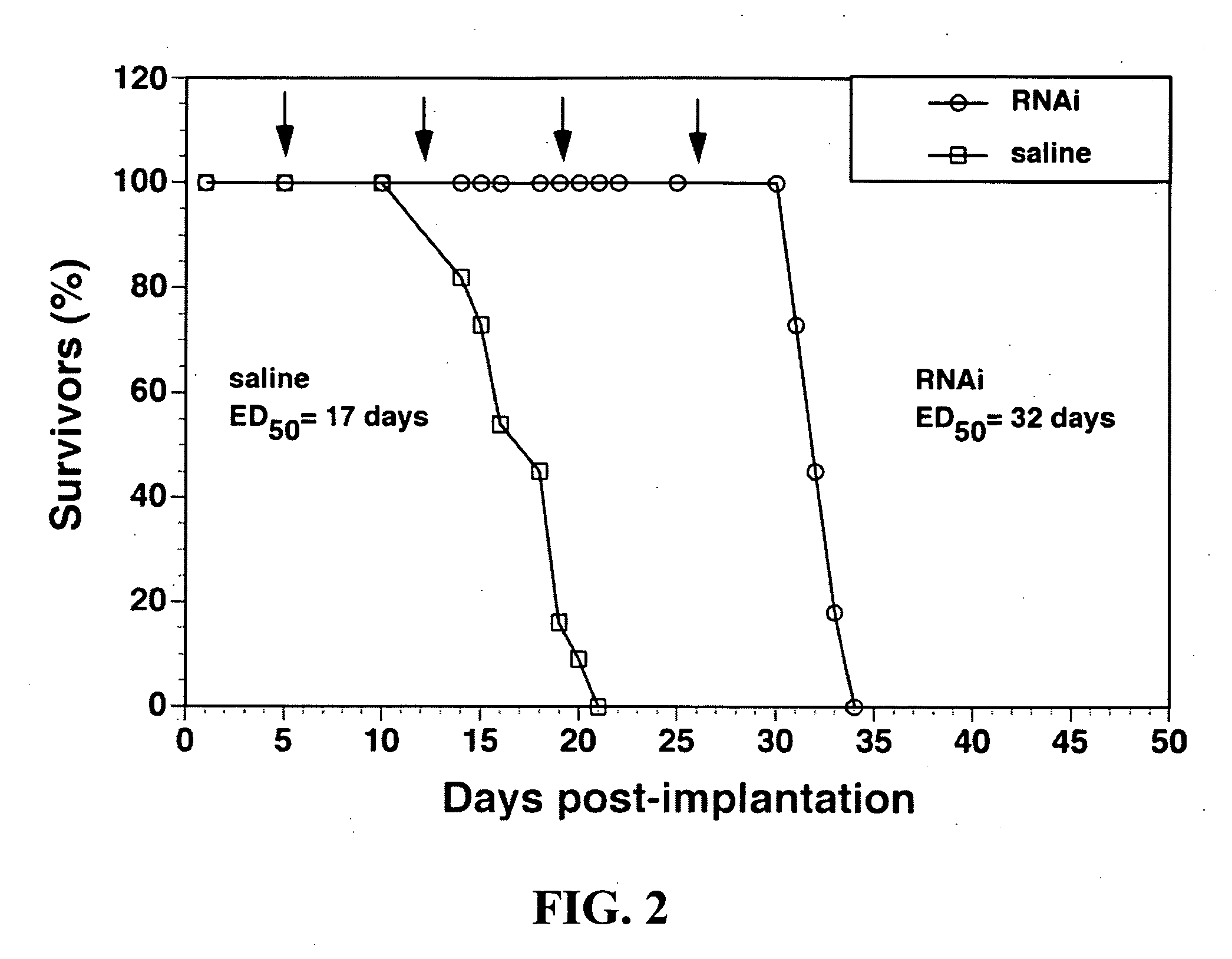
Delivery of genes encoding short hairpin RNA using receptor-specific nanocontainers
Receptor-specific nanocontainers are used to deliver a gene that encodes short hairpin RNA to cells having a given receptor. Once inside the cell, the gene expresses short hairpin RNA that includes a nucleotide sequence that is antisense to at least a portion of an oncogene, such as human epidermal growth factor receptor (EGFR) mRNA, or other disease causing nucleotide sequence. The short hairpin RNA is converted, in the cellular cytoplasm, into short RNA duplexes that are effective in deactivating (knocking down) the oncogenic or disease causing gene.
Owner:RGT UNIV OF CALIFORNIA
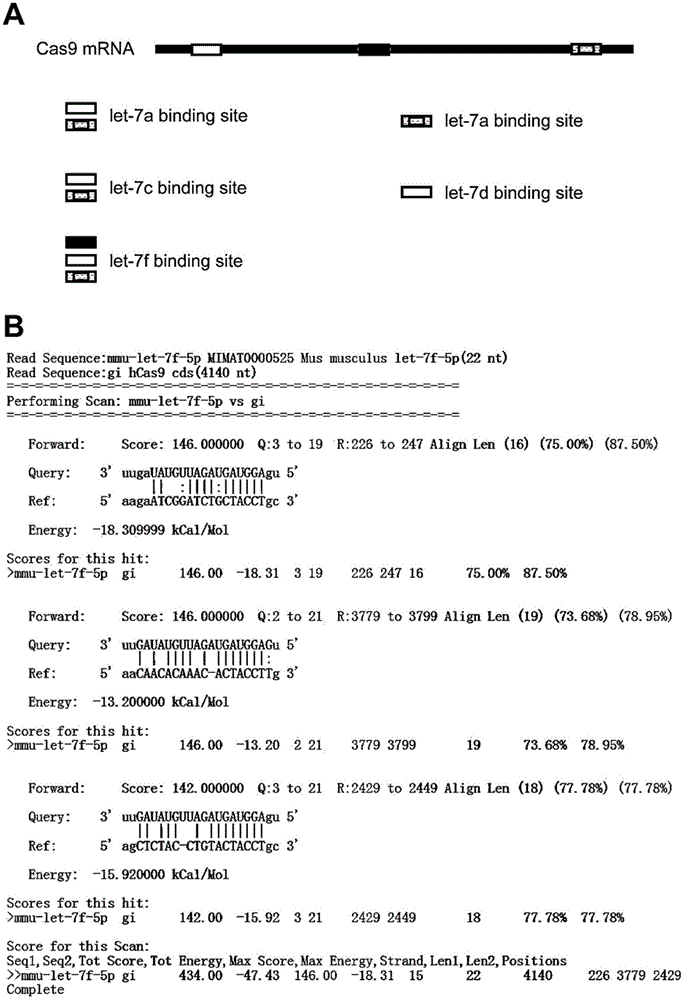
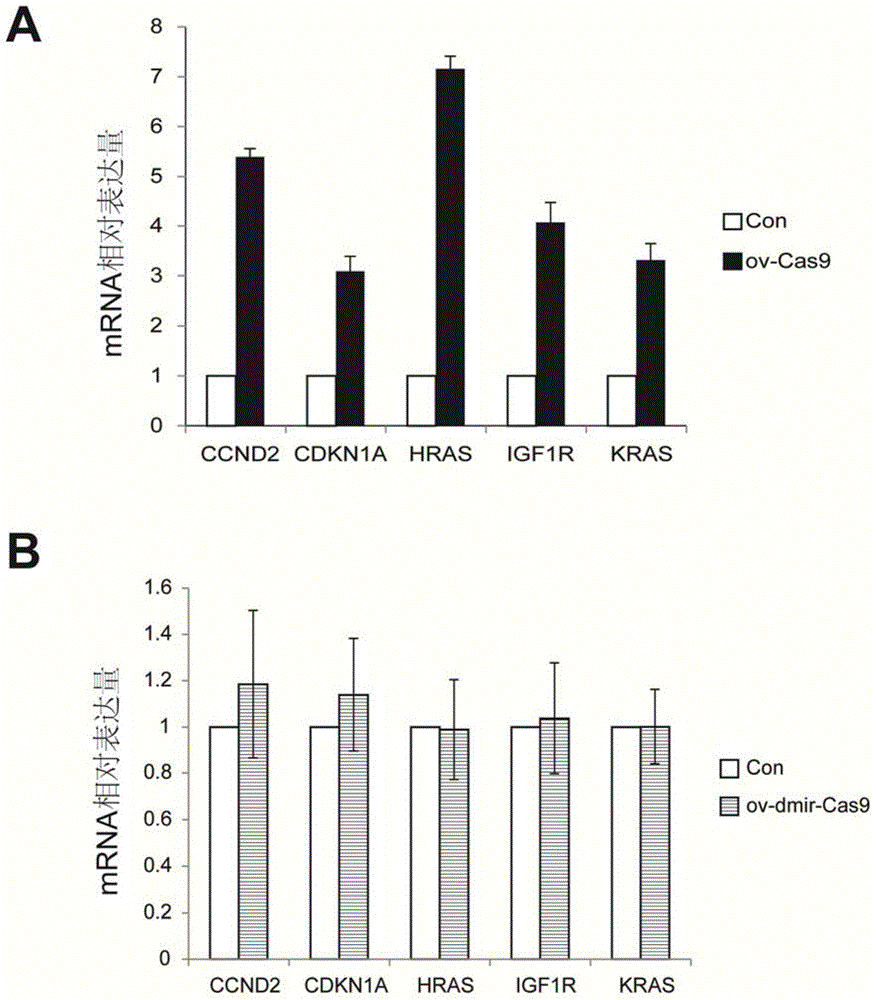
Nucleic acid molecule for encoding Cas9 (CRISPR (clustered regularly interspaced short palindromic repeat)-associated endonuclease 9) protein securely and expression vector of nucleic acid molecule
The invention relates to the technical field of biology, and provides a nucleic acid molecule for encoding Cas9 (clustered regularly interspaced short palindromic repeat-associated endonuclease 9) protein securely, a recombinant expression vector of the nucleic acid molecule, an application of the nucleic acid molecule in the CRISPR-Cas9 technology and a method for reducing combination of Cas9 nucleic acid molecule and microRNA (ribonucleic acid). Experiments find that although the extensively applied Cas9 expression vector can express the Cas9 protein successfully and play a role, an mRNA sequence transcribed by the vector can serve as an adsorber of the microRNA let-7 family to inhibit functions of let-7 which can inhibit expression of multiple oncogenes, accordingly, the Cas9 expression vector applied at present can increase expression of the oncogenes, and the risk of cancer is increased. According to the provided M-mir-Cas9 expression vector, the transcribed mRNA cannot adsorb let-7 and can be still translated into complete Cas9 protein to play a role, and the safety of CRISPR-Cas9 gene editing tool is improved.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
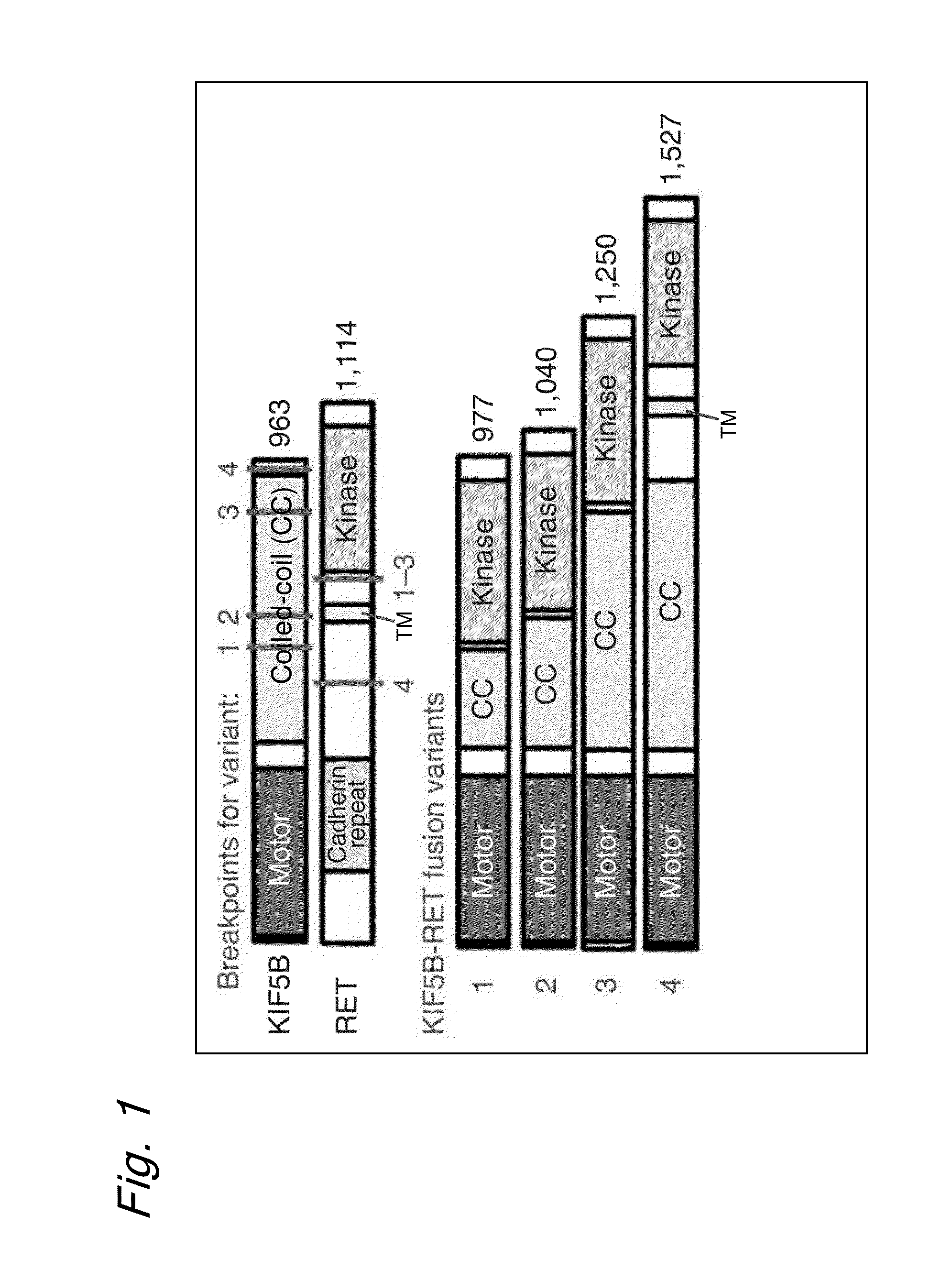
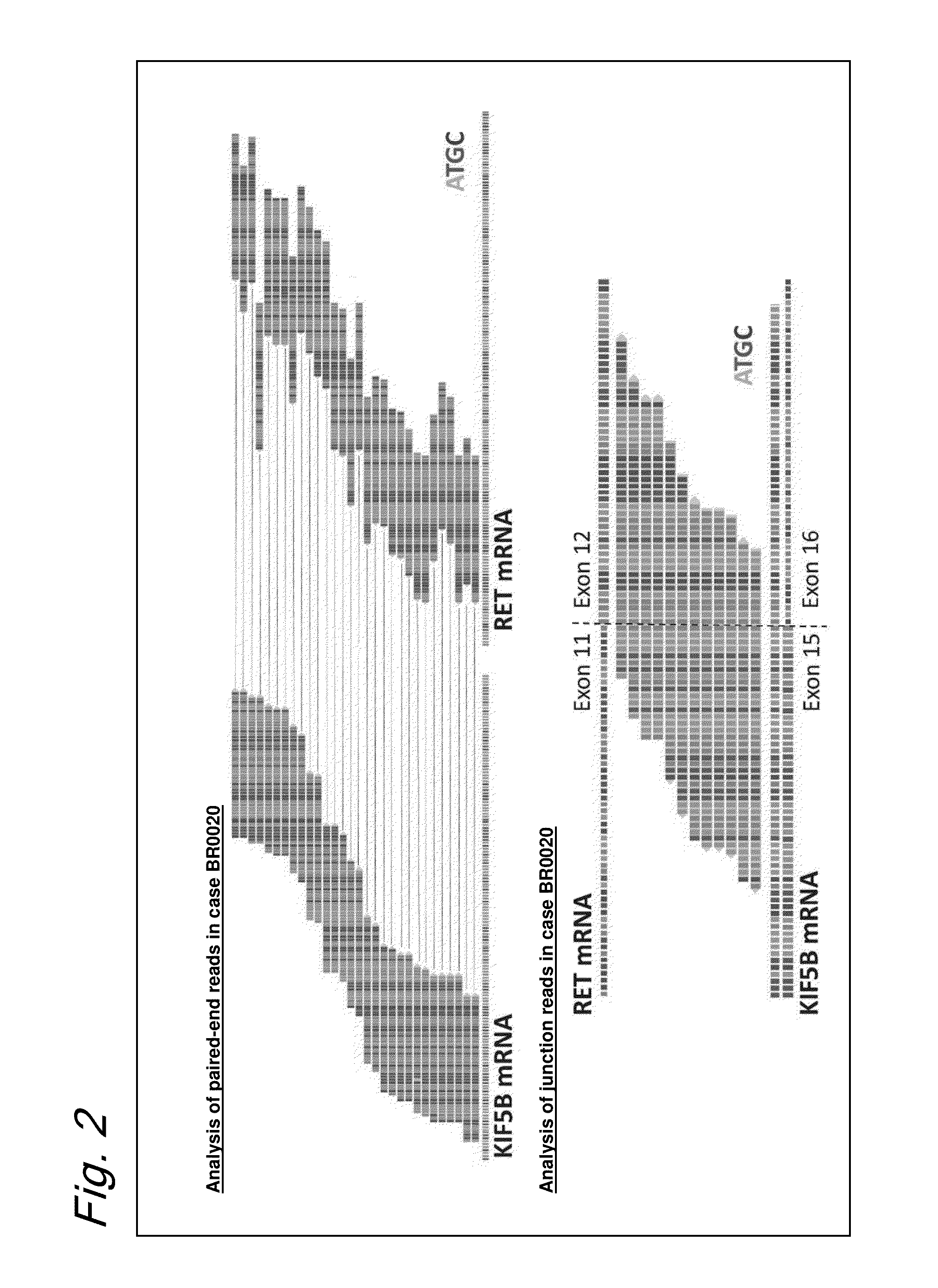
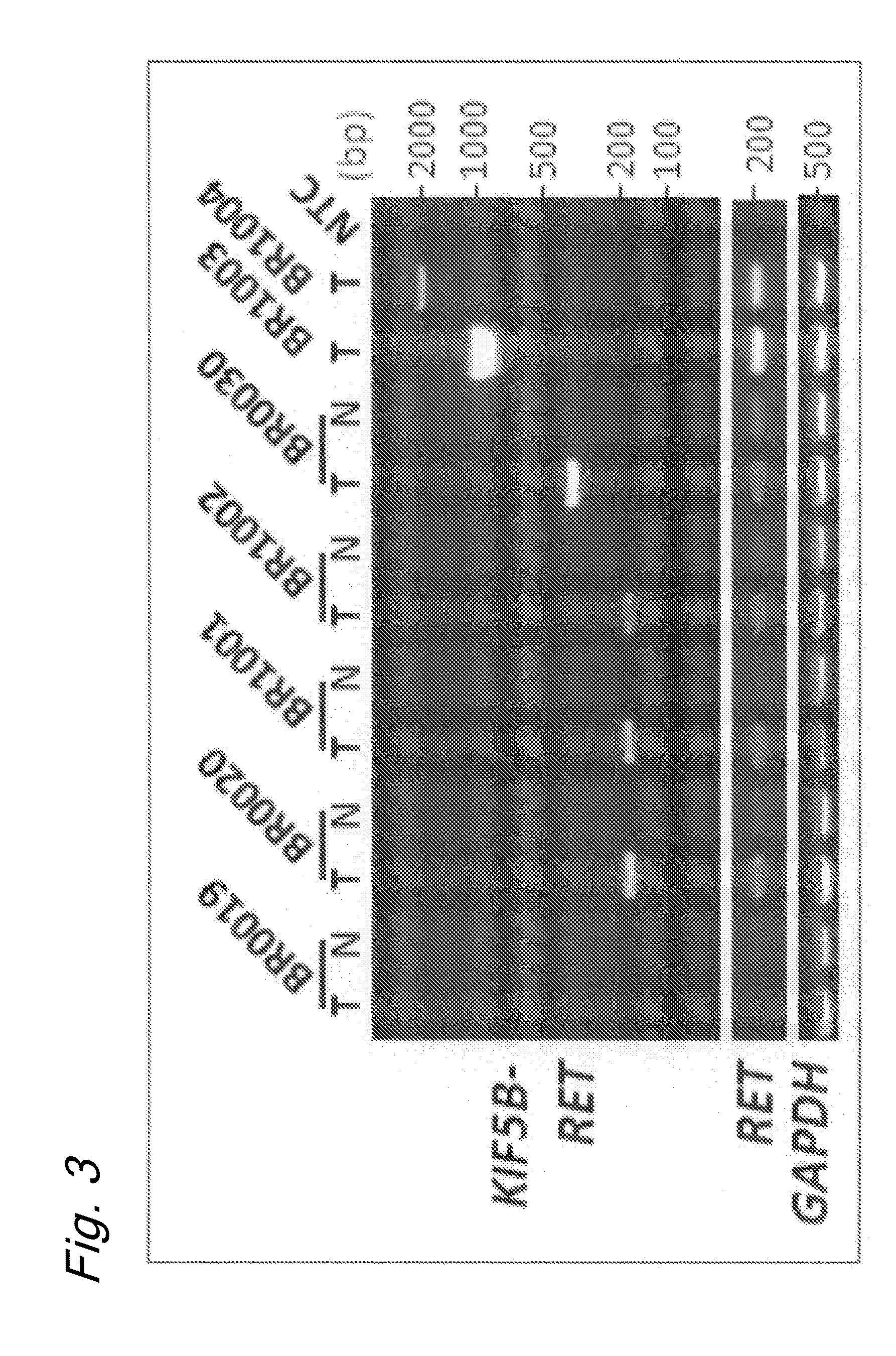
Method for determining effectiveness of cancer treatment by assessing the presence of a KIF5B-RET chimeric gene
ActiveUS9216172B2Prediction of effectivenessEffective treatmentHydrolasesPeptide/protein ingredientsActivating mutationTyrosine-kinase inhibitor
Owner:NAT CANCER CENT
Transcription regulatory gene and peptide
ActiveUS20050183169A1Simple structurePotent capacity for repressing transcription of genePeptide/protein ingredientsImmunoglobulinsTranscription Regulation GeneMetabolic enzymes
This invention relates to a peptide or protein capable of converting a transcription factor into a transcriptional repressor, a gene encoding such peptide or protein, a chimeric protein in which the aforementioned peptide or protein is fused to a transcription factor, a chimeric gene in which the gene encoding a peptide or protein is fused to a gene encoding a transcription factor, a recombinant vector having such chimeric gene, and a transformant comprising such recombinant vector. The peptide of the invention that is capable of converting a transcription factor into a transcriptional repressor is very short. Thus, it can be very easily synthesized, and it can effectively and selectively repress the transcription of a specific gene. Accordingly, such gene is applicable to and useful in a wide variety of fields, such as repression of the expression of cancerous genes and regulation of the expression of genes encoding pigment-metabolic enzymes.
Owner:GREENSOGNA +1
Tissue specific promoters and transgenic mouse for the screening of pharmaceuticals
InactiveUS6313373B1Restores promoter activityVectorsSugar derivativesHuman papillomavirusPromoter activity
The present invention provides human involucrin (hINV) sequences having tissue specific and cell type specific promoter activity. The sequences provided herein direct expression to suprabasal cells of stratifying epithelia. The invention further provides methods for the production of transgenic animals which contain a hINV promoter sequence which directs the expression of human papillomavirus 16 oncogenes (or other oncogenes). These animals display cervical and epidermal hyperplasias as well as cancer of the trachea, esophagus, colon, epidermis, anus / rectum, lymph nodes, spleen and lung. The animals of the invention provide a useful model for screening potential anti-neoplastic compounds, carcinogens, and co-carcinogens for a number of cancers.
Owner:CASE WESTERN RESERVE UNIV
Anti-Tumor Activity of an Oncolytic Adenovirus-Delivered Oncogene siRNA
InactiveUS20070258952A1Minimizing potential cytotoxicityIncrease infectivityBiocideSpecial deliveryOncolytic adenovirusOncogene MYC
The present invention includes compositions and methods for the knockdown of one or more genes to a target cell in need of gene therapy by using an siRNA transgene that is integrated into a replication-competent, oncolytic adenovirus.
Owner:GRADALIS +2
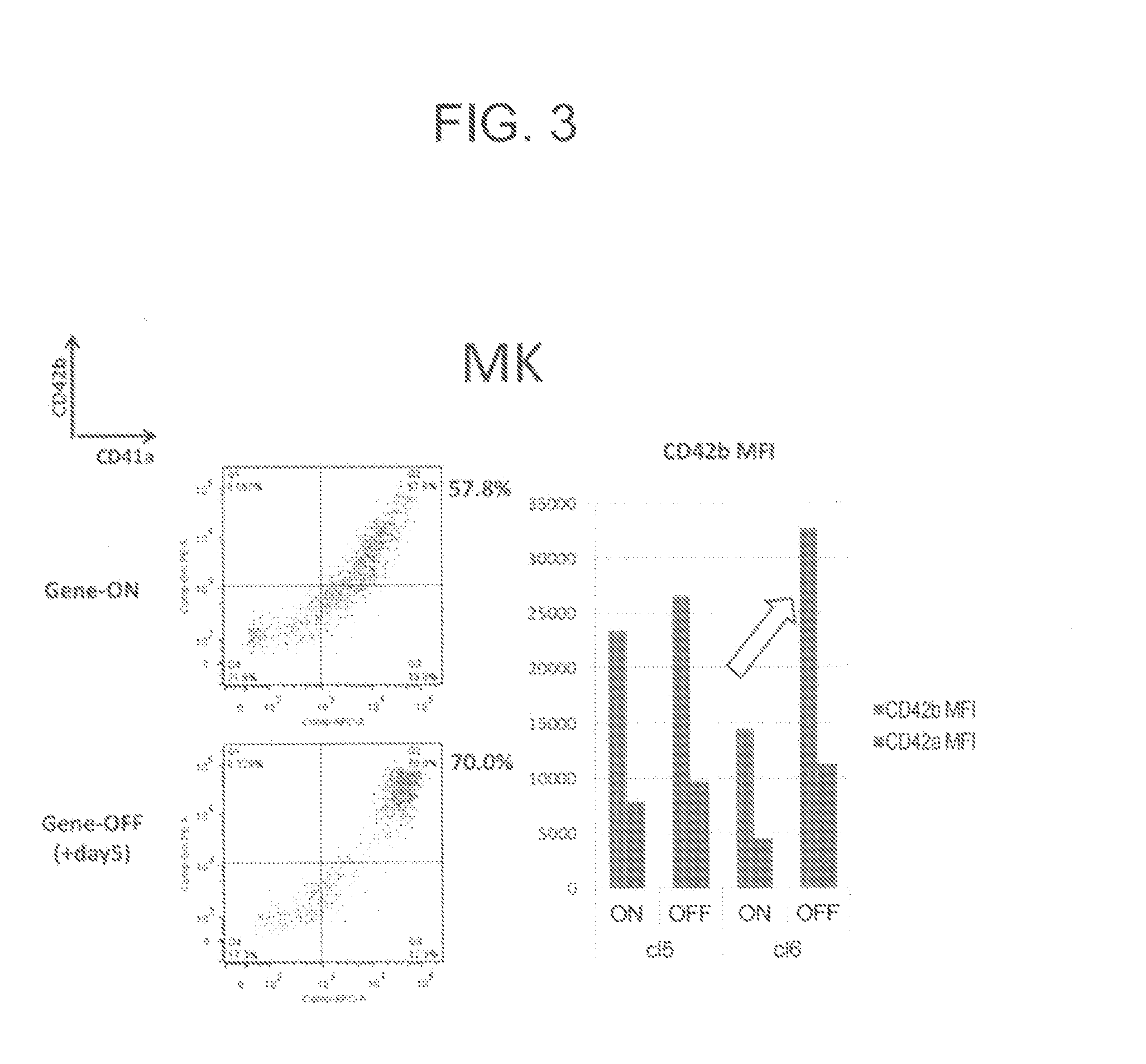
Production methods for megakaryocytes and platelets
InactiveUS20160002599A1Suitably producedMammal material medical ingredientsSkeletal/connective tissue cellsApoptosisPlatelet
An object of the present invention is to provide a method of efficiently producing a maturated megakaryocytic cell line from hematopoietic progenitor cells. The present invention provides a method for producing megakaryocytes from hematopoietic progenitor cells, comprising(i) forcibly expressing an apoptosis suppression gene and an oncogene in hematopoietic progenitor cells and culturing the cells, and(ii) arresting forced expression of the apoptosis suppression gene and the oncogene and culturing the hematopoietic progenitor cells.
Owner:KYOTO UNIV
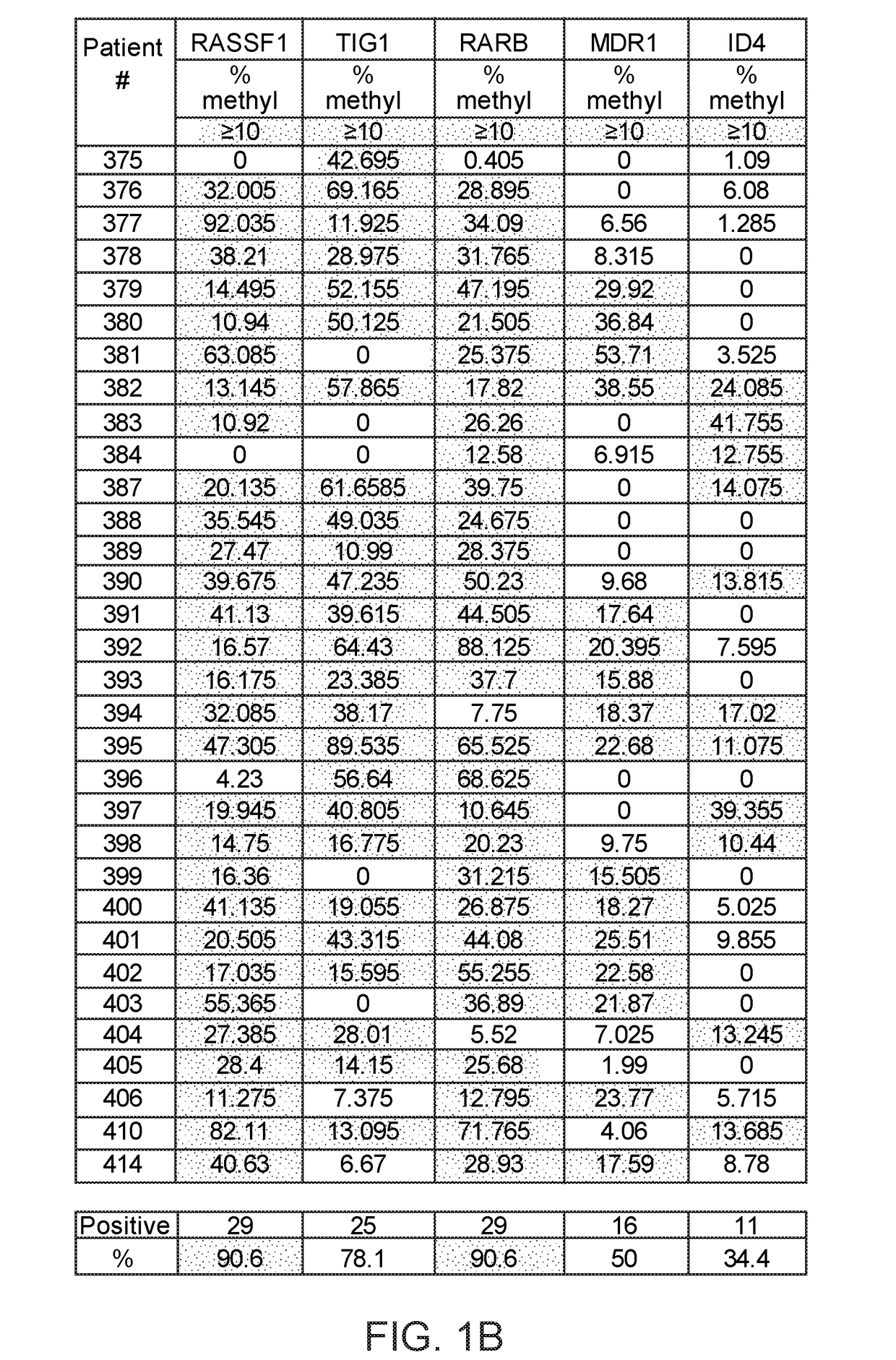
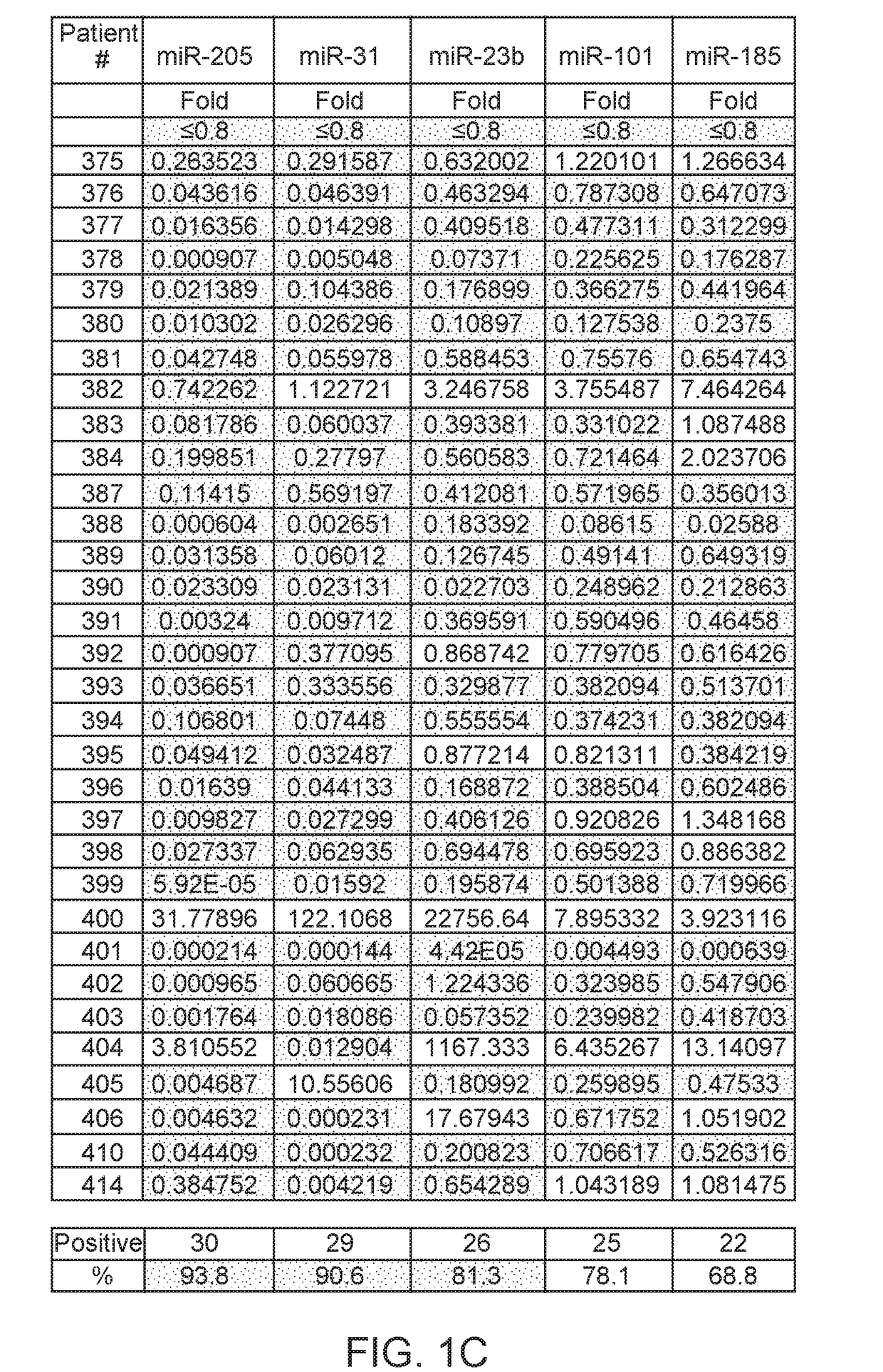
Method of analysis allowing avoidance of surgery
ActiveUS20170275702A1Prevent removalAvoid surgeryMicrobiological testing/measurementMedical automated diagnosisDNA methylationCervix
A multi-gene-based assay for analysis of the following: (a) oncogene expression; (b) DNA methylation of tumor suppressor genes; (c) non-coding RNA expression (microRNA profiling); and (d) long non-coding RNA expression in cancer samples is disclosed. The assay method and device for conducting the assay are applicable to diagnosis, prognosis, and treatment of various cancers such as lung, breast, colorectal, prostate, liver, bladder; kidney, cervix, pancreatic, gastric, brain, oral, endometrium, and ovary. The assay methods allow avoidance of surgery to remove cancerous tissue.
Owner:GENEVERIFY INC
Plant drug for treatment of liver disease
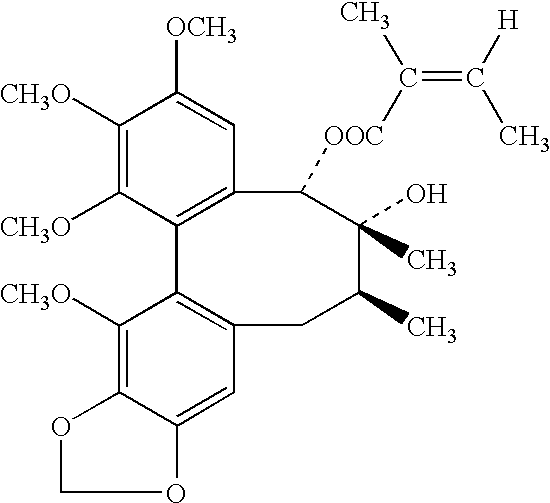
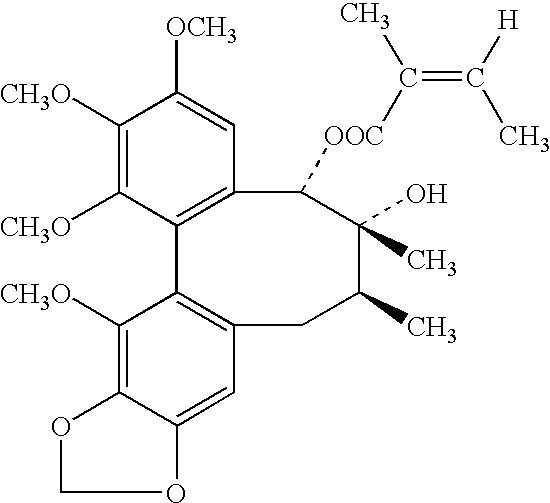
The present invention related to safe plant drug for treatment of liver disease, specifically, this invention proves a safe plant drug Schisandrin and its preparation. Schisandrin has the following pharmaceutical functions: increasing tumor suppresson genes express activity, decreasing activity of oncogenes, increasing immune function, increasing liver DNA synthesis, decreasing serum alamine aminotransferase activity, increasing glutathione level, increasing glutathione reductase activity, decreasing lipid peroxidation of liver, increasing hepatic microsomal monooxygenases activity, increasing ATP content in liver, increasing energy metabolism activity, decreasing density lipoprotein oxidation, protecting gastrointestinal function, increasing killer cell activity, increasing complement activity, decreasing induced liver cancer activity and decreasing grown of cancer cells.
Owner:ZHAO XINXIAN
Tumor suppressor and oncogene biomarkers predictive of Anti-immune checkpoint inhibitor response
ActiveUS20170130271A1Prevent cancerMicrobiological testing/measurementBiological material analysisAbnormal tissue growthSuppressor
The present invention is based on the identification of novel biomarkers predictive of responsiveness to anti-immune checkpoint inhibitor therapies.
Owner:DANA FARBER CANCER INST INC +1
Mutated ras peptides for generation of CD8+Cytotoxic T lymphocytes
InactiveUS7709002B1Growth inhibitionAvoid it happening againBacterial antigen ingredientsPeptide/protein ingredientsCtl epitopeMutated Ras Peptide
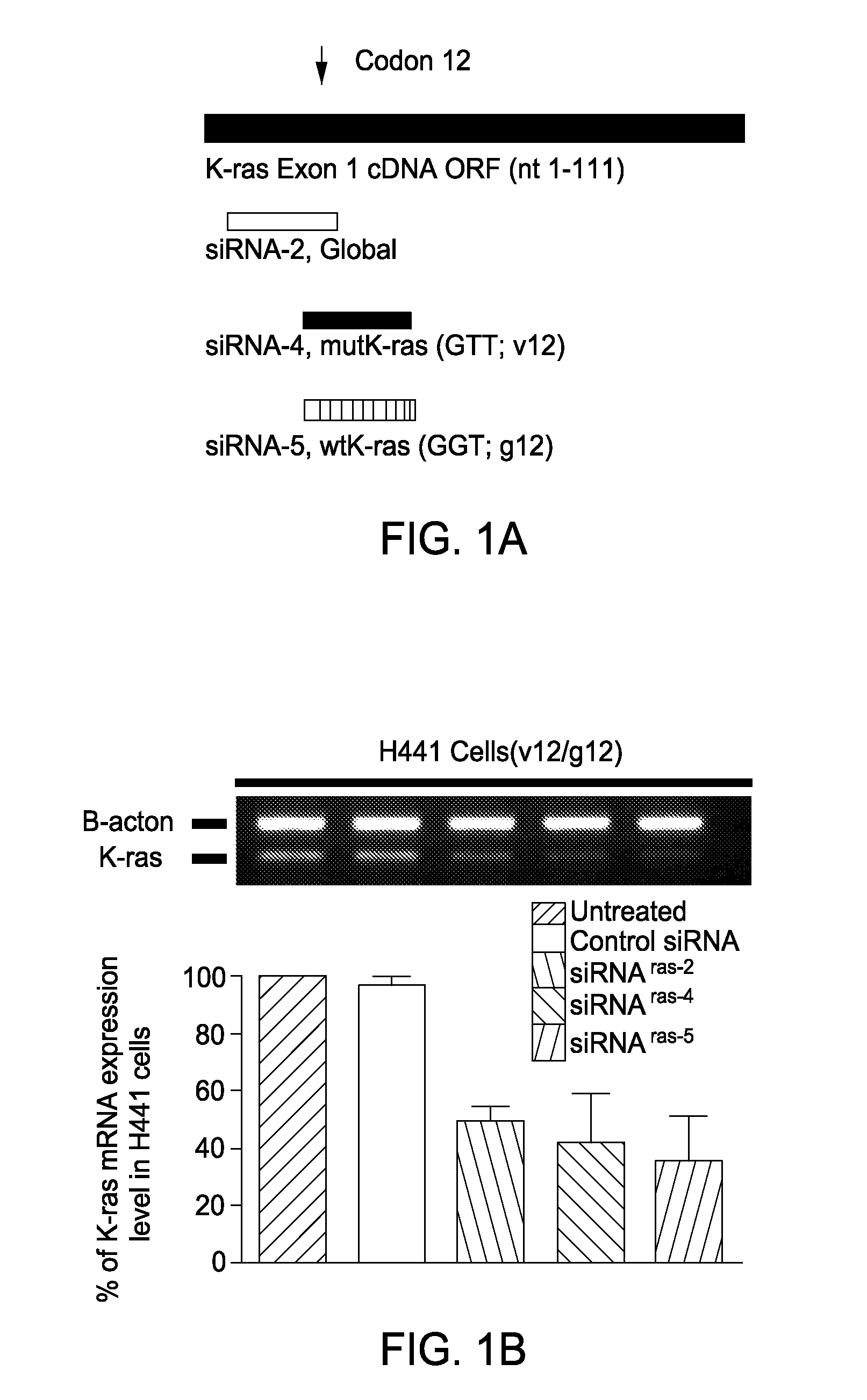
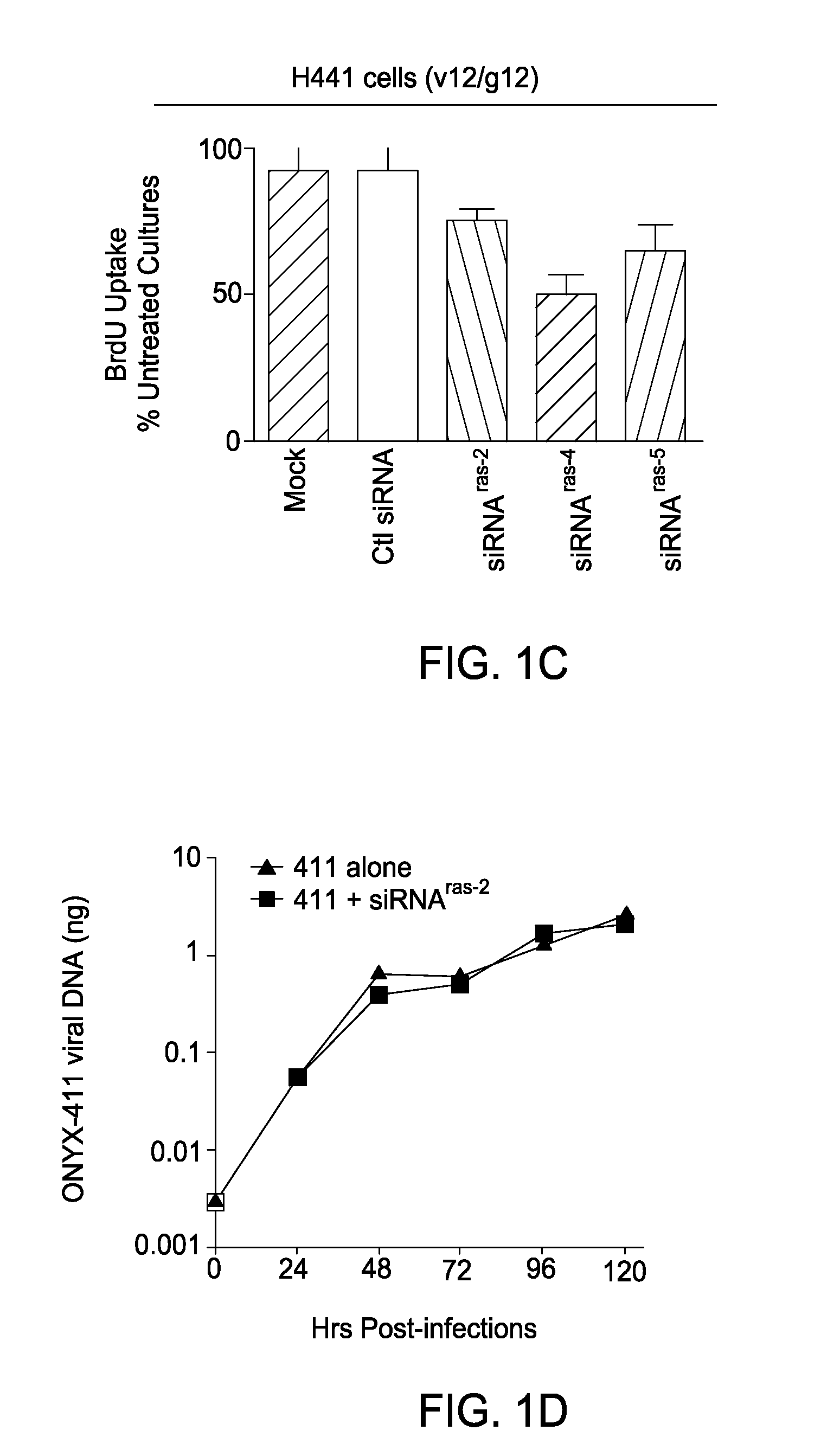
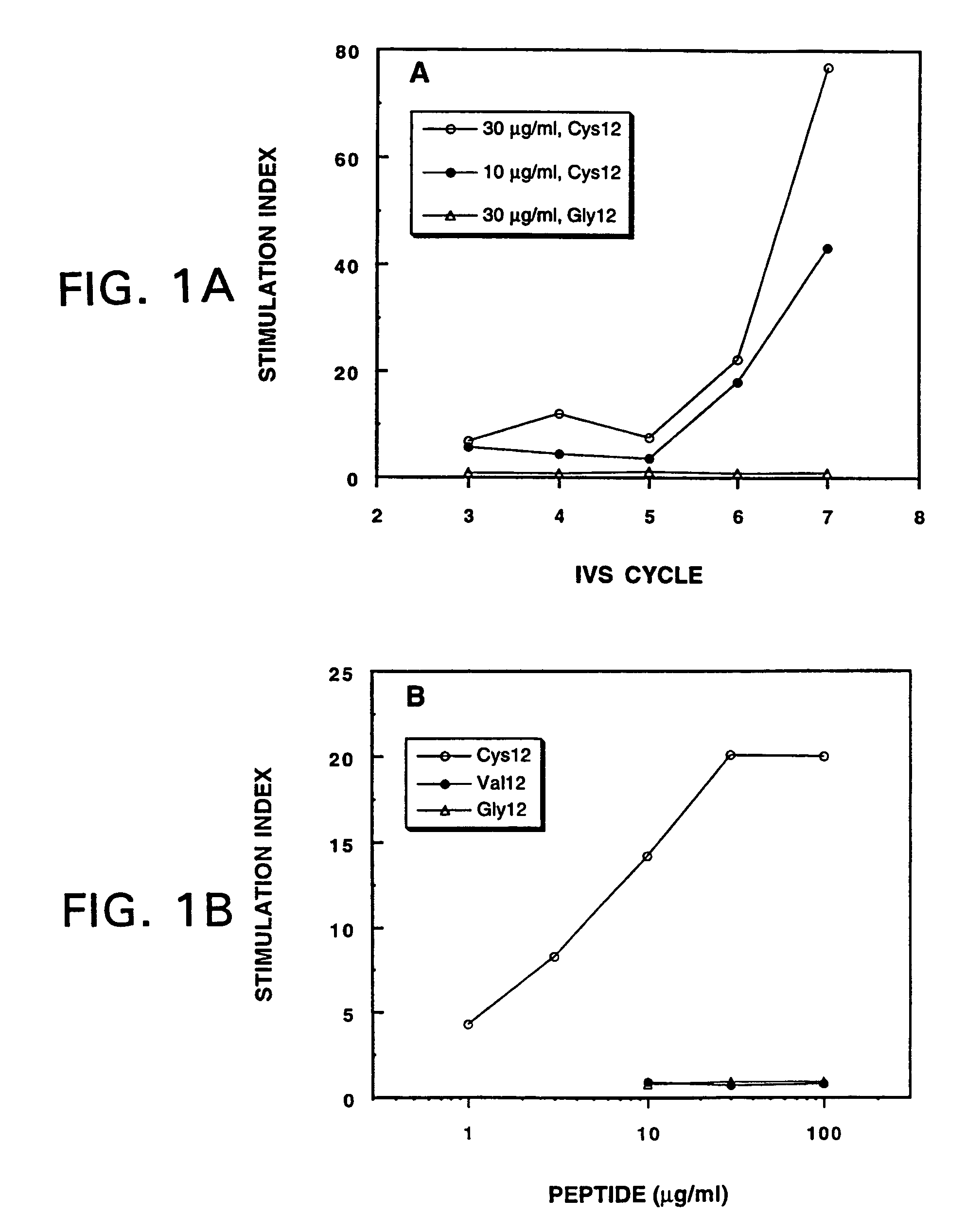
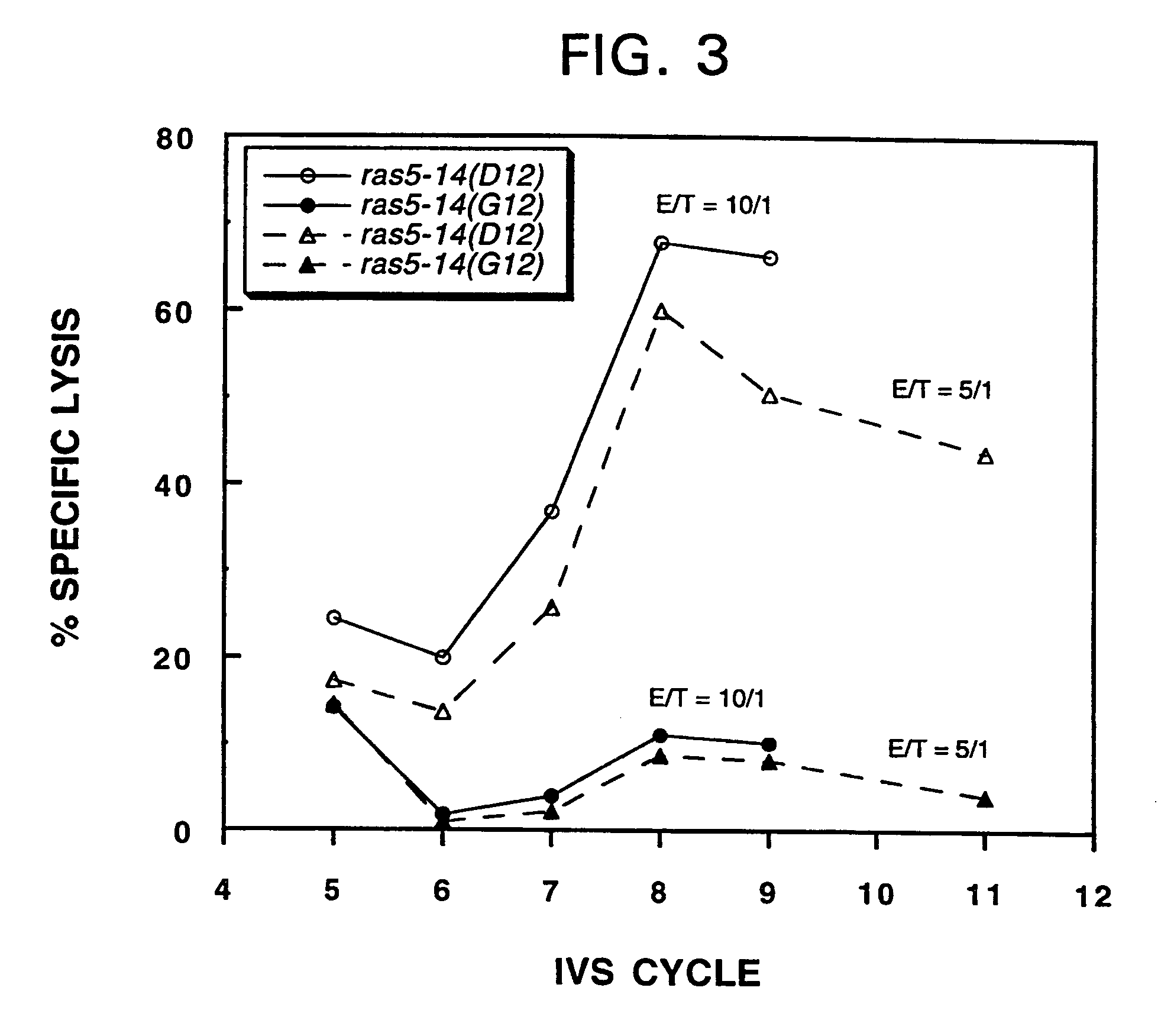
Mutant ras oncogene peptides may induce specific anti-ras cellular immune responses in vaccinated patients. Moreover, a human CD8+ CTL epitope(s) reflecting a specific point mutation in the K-ras oncogene at codon 12 was identified. The mutant ras peptide has implications for both active and passive immunotherapies in selected carcinoma patients. A nested 10-mer peptide was identified [i.e., ras5-14(Asp12)], which was shown to bind to HLA-A2 and display specific functional capacity for expansion of the in-vivo-primed CD8+ CTL precursors.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
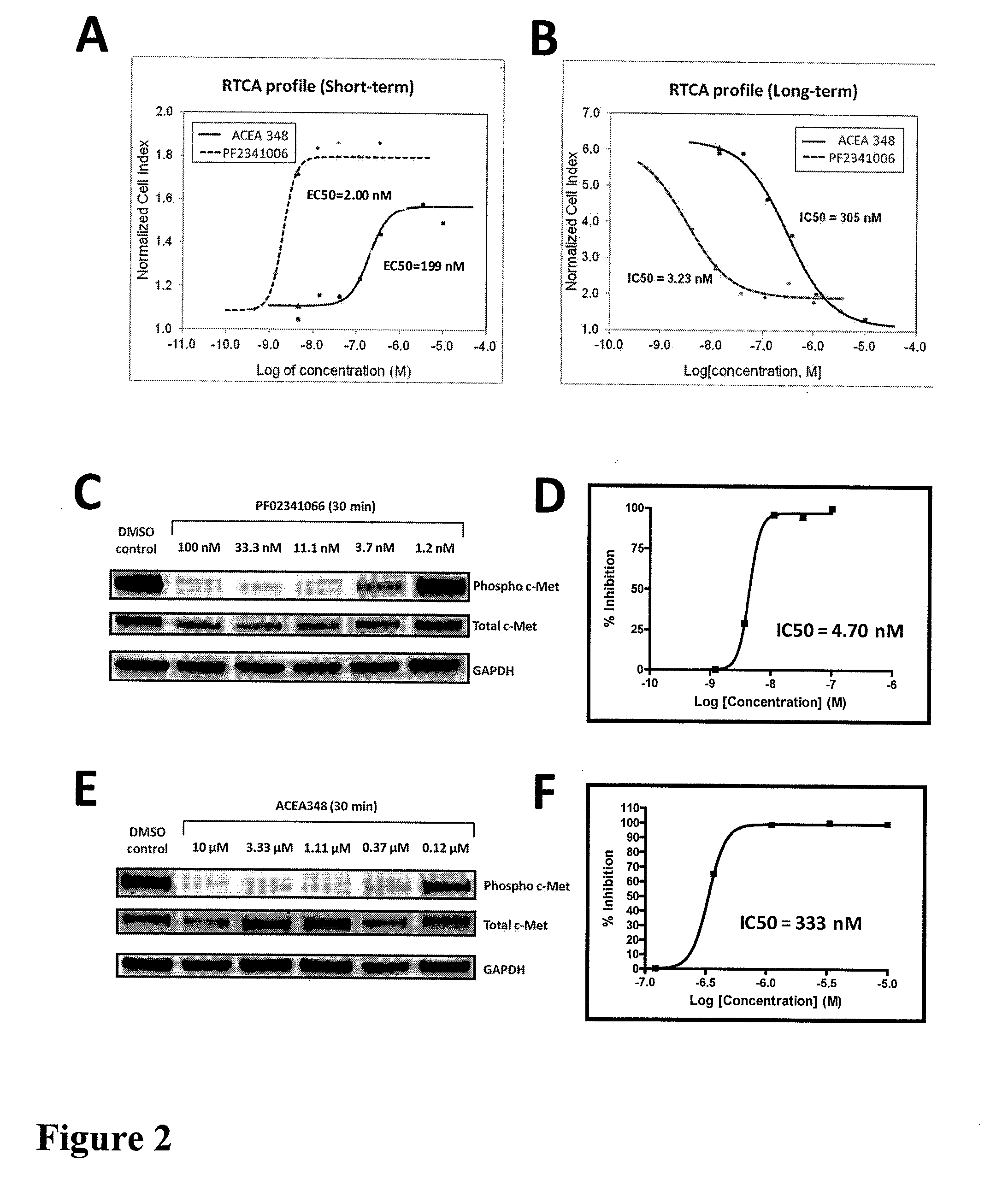
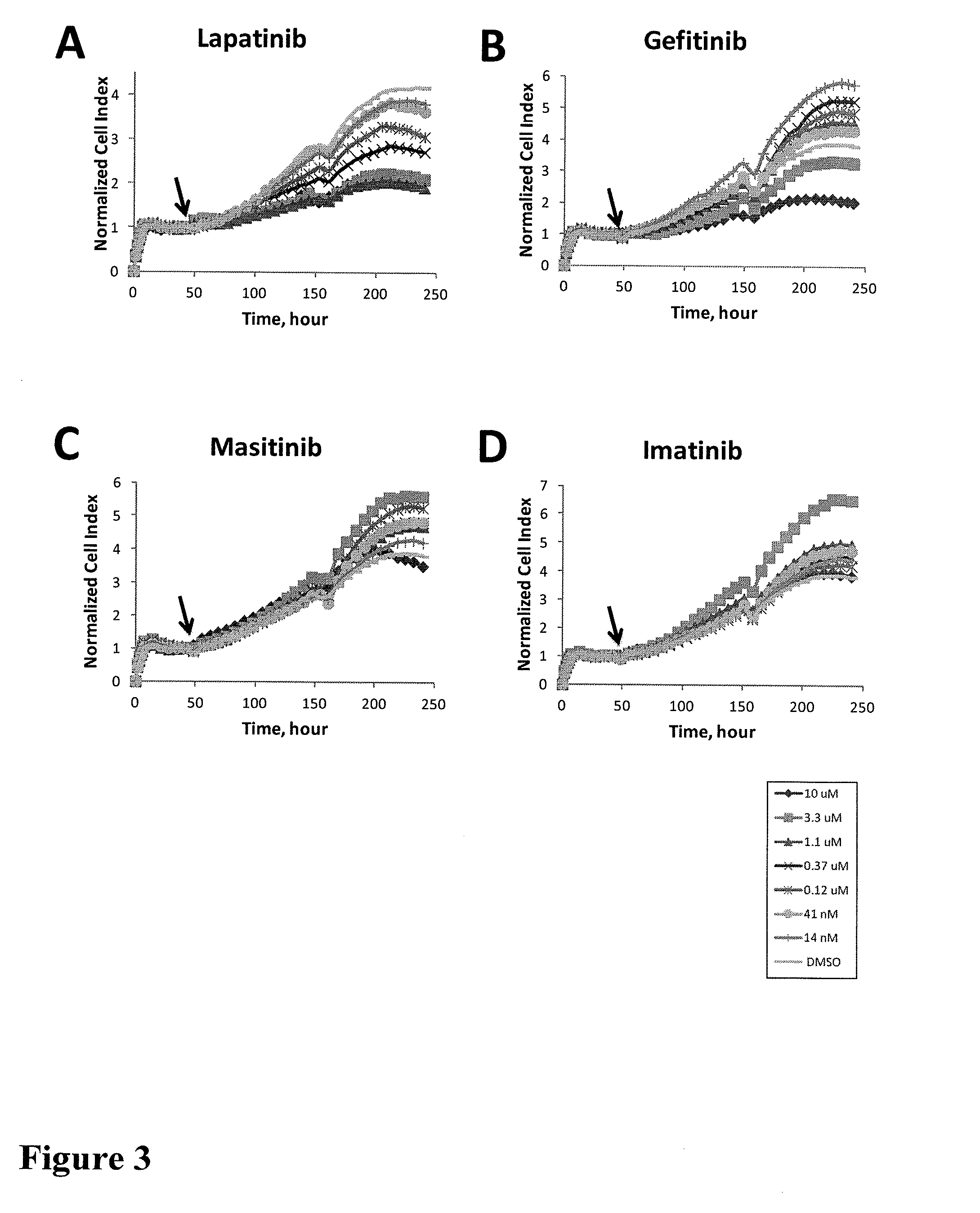
Using impedance-based cell response profiling to identify putative inhibitors for oncogene addicted targets or pathways
Use of impedance devices in methods of generating a time dependent cellular profiles (TCRP) for the modulation of oncogene addicted cells and comparing the impedance-based TCRP to controls or knowns to identify signature time dependent cellular profiles.
Owner:AGILENT TECH INC
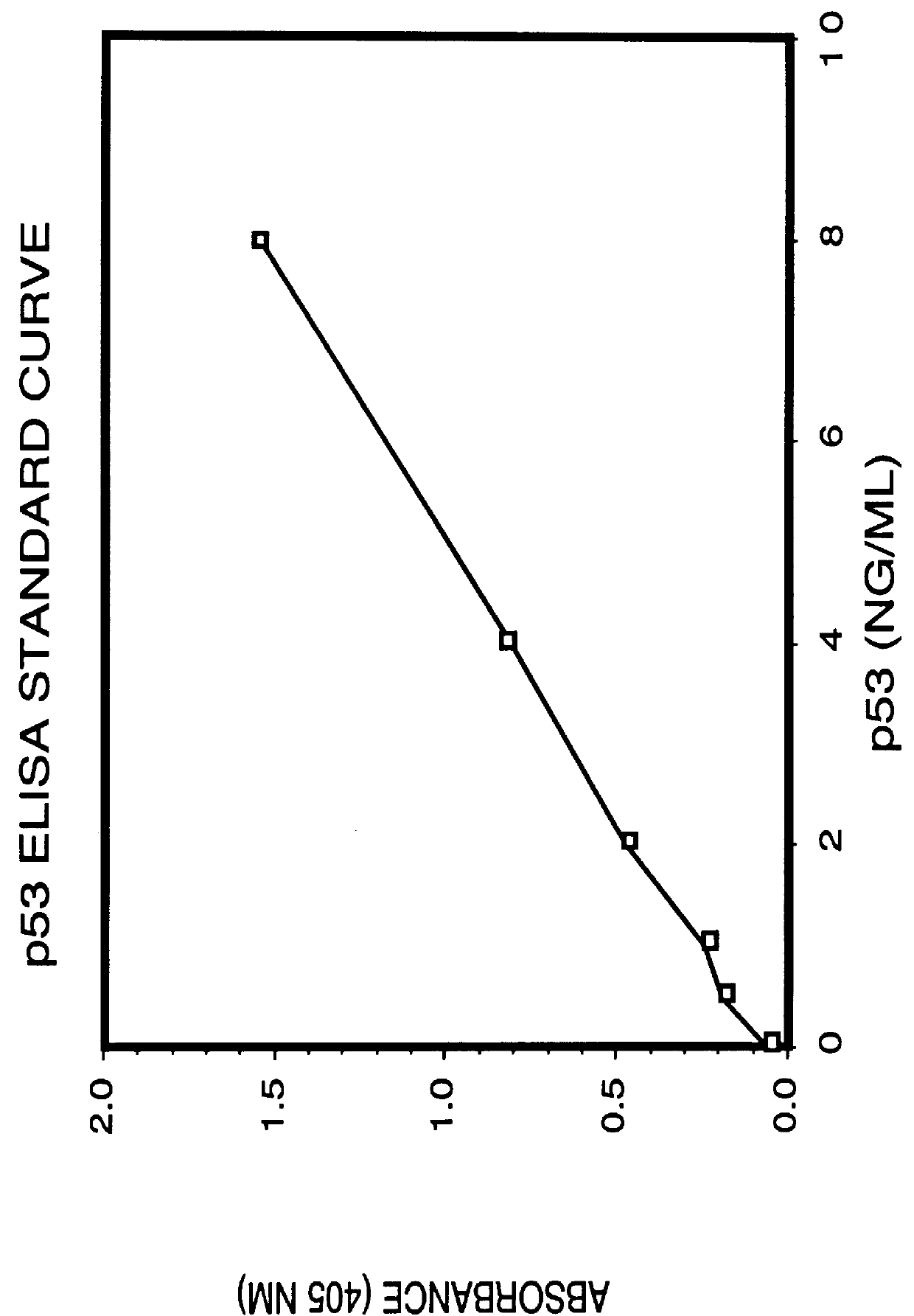
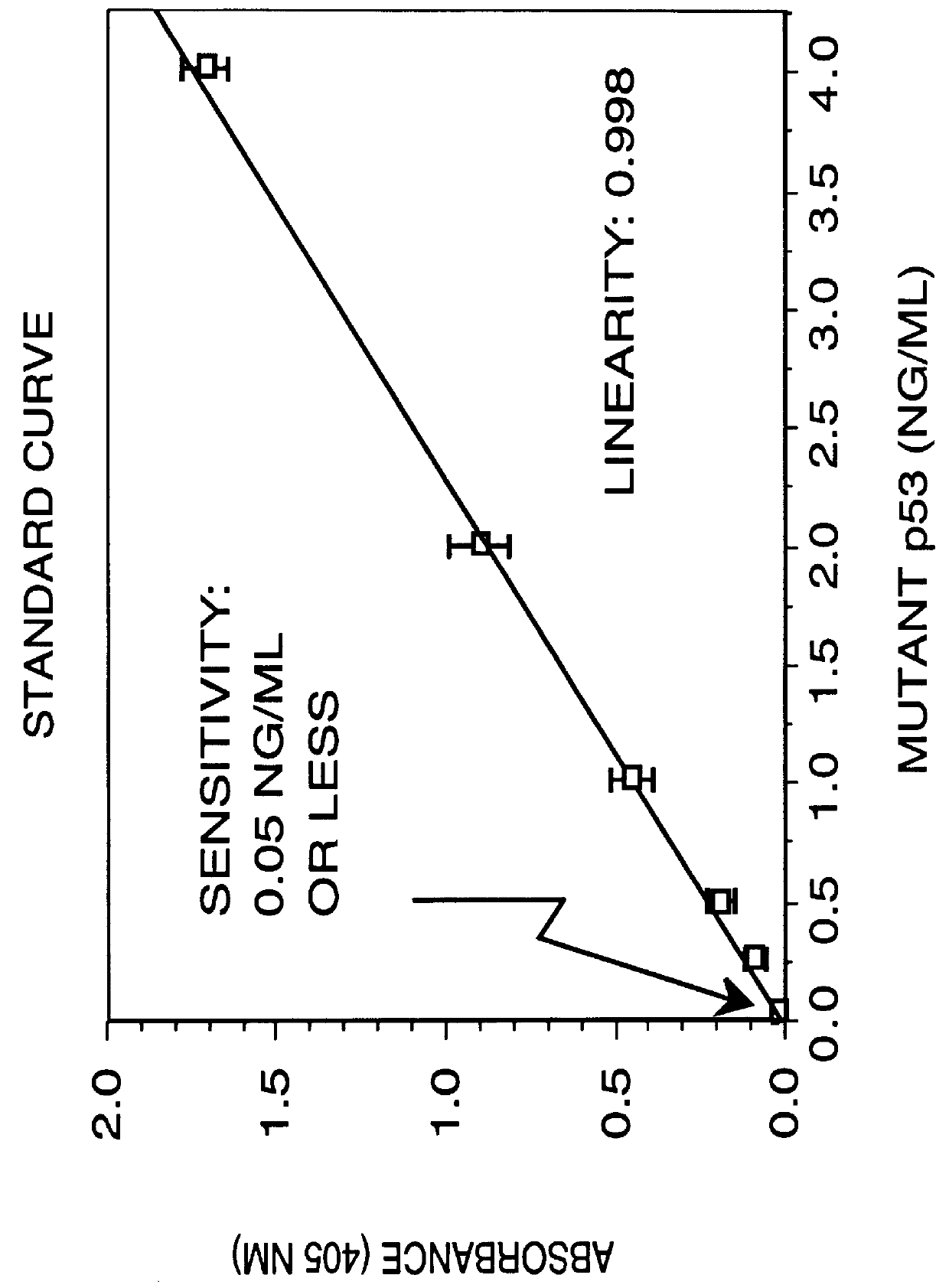
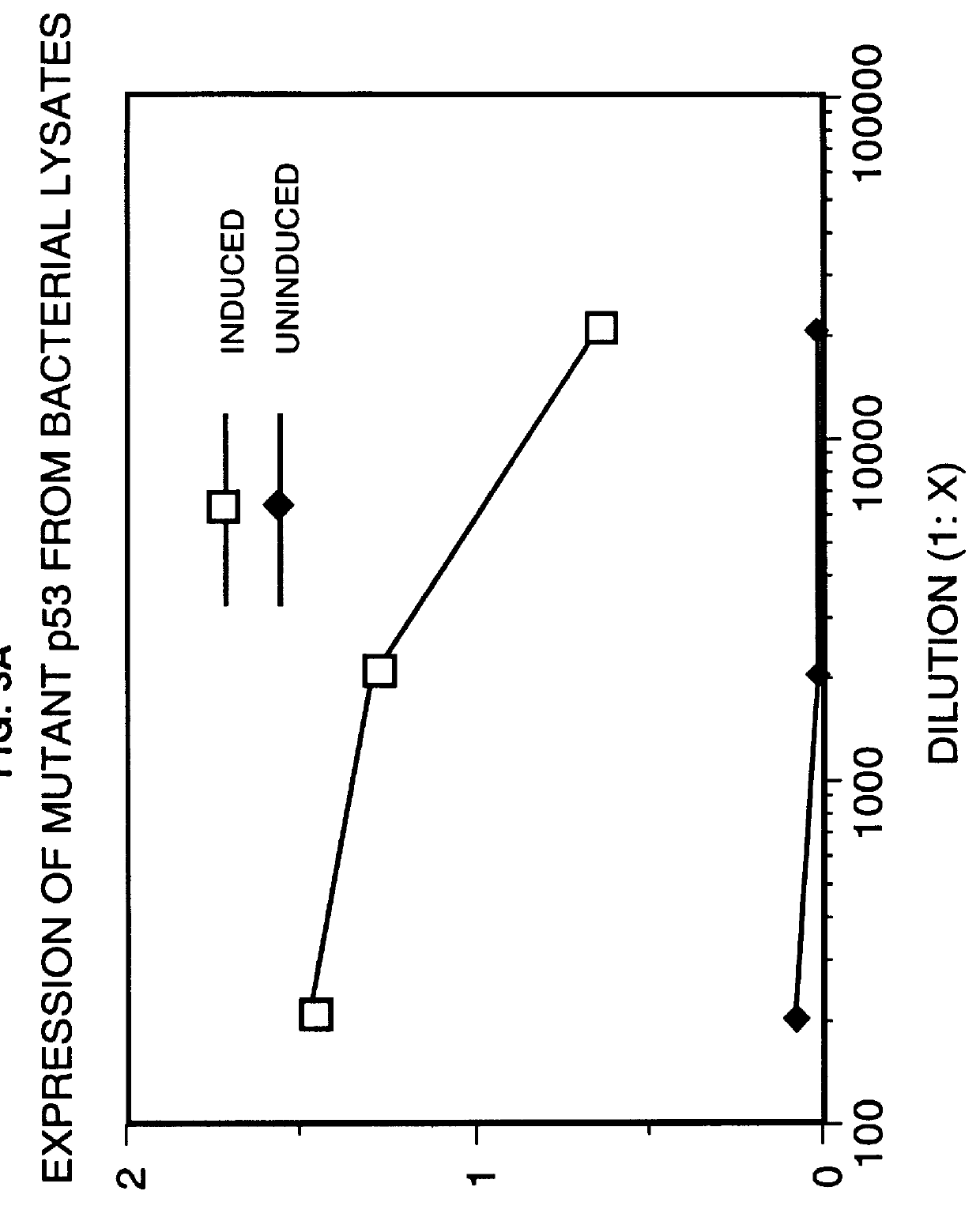
Immunoassay for detection of mutant P53 polypeptide in serum
The invention provides a method for diagnosing in a subject a neoplastic condition which comprises (a) obtaining from the subject a sample of a biological fluid; and (b) detecting the presence in the sample of a mutant p53 polypeptide encoded by an activated p53 oncogene, the presence of the mutant p53 polypeptide in the sample indicating that the subject has the neoplastic condition.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
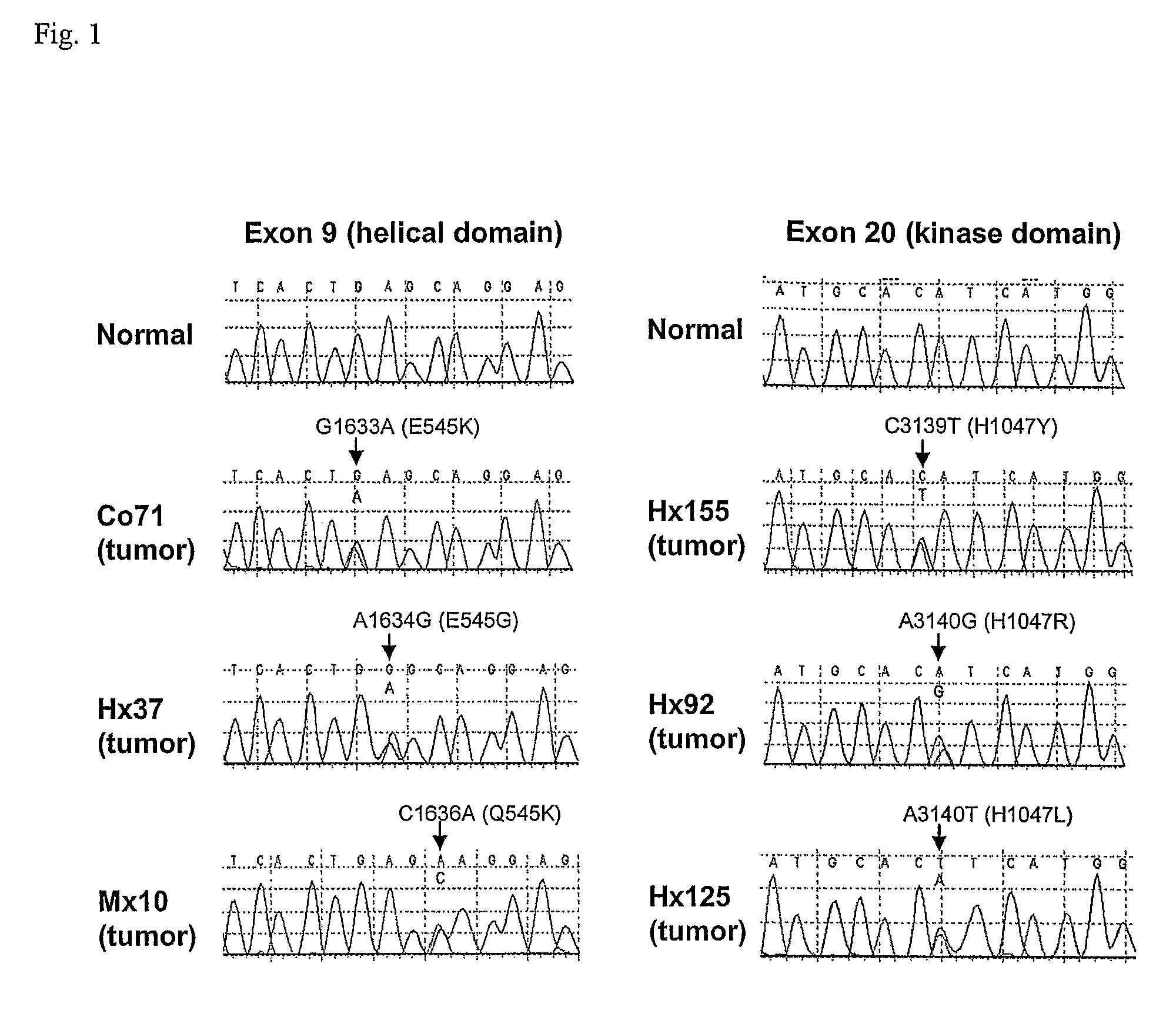
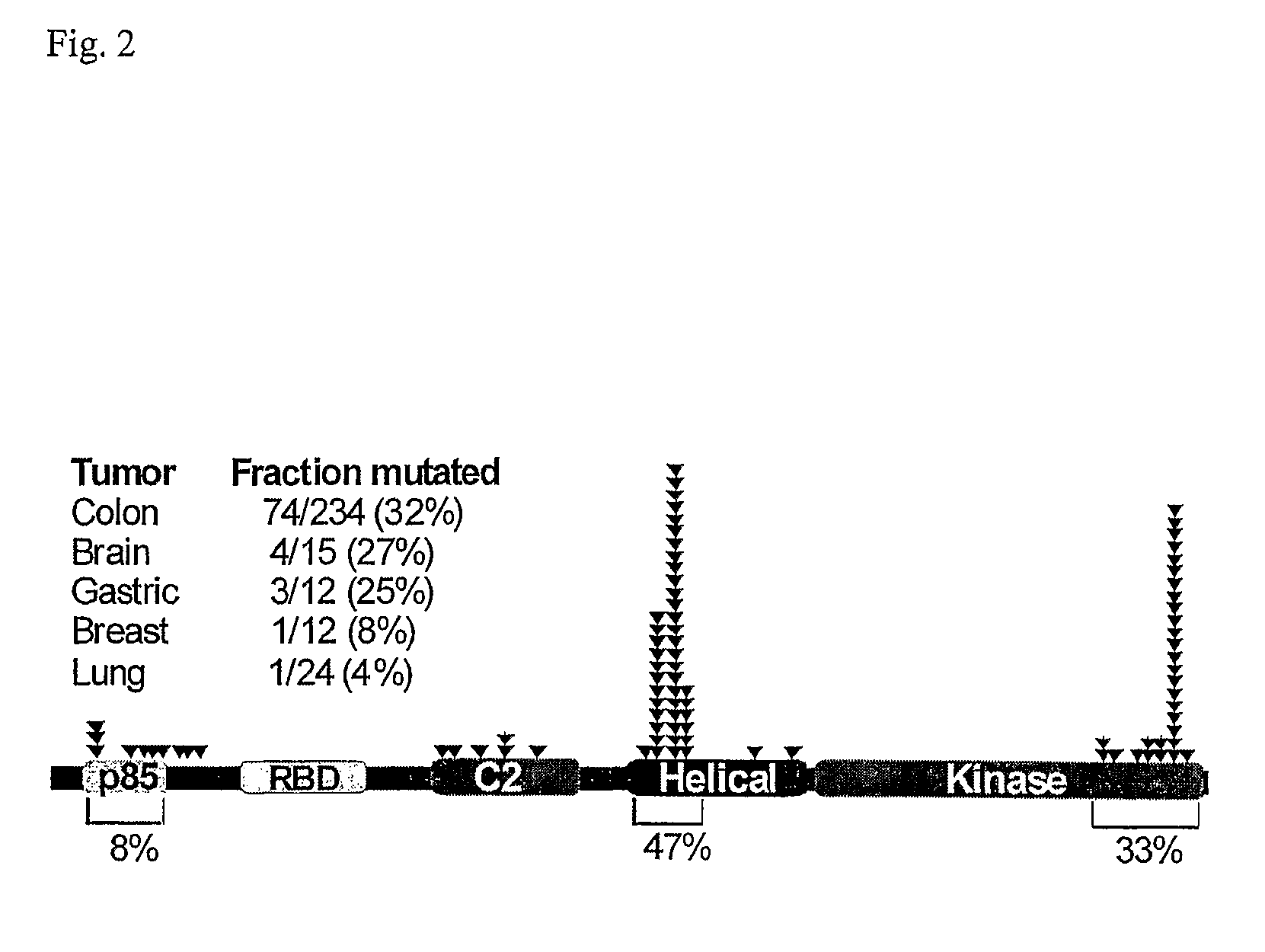
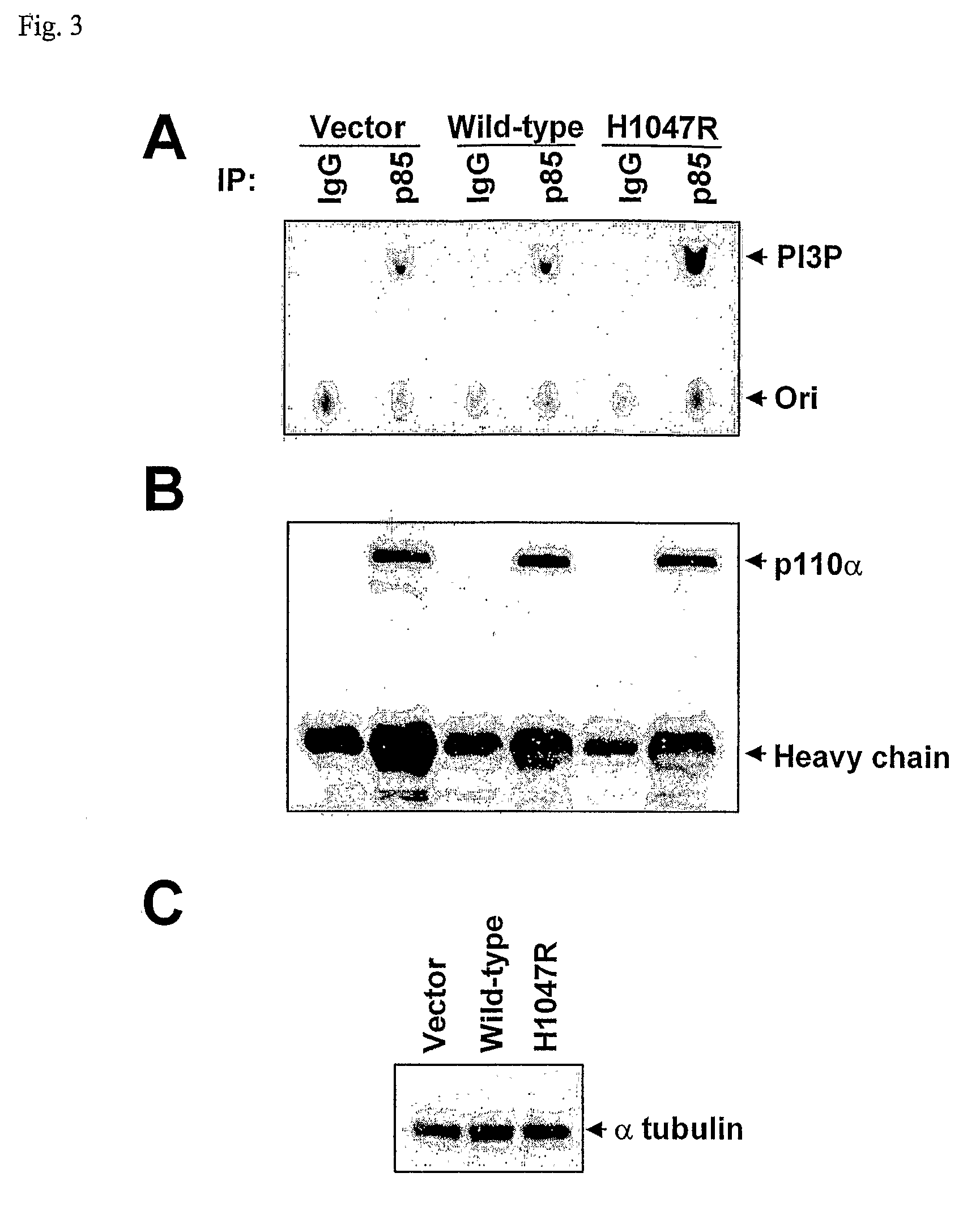
Mutations of the PIK3CA gene in human cancers
ActiveUS8026053B2Inhibit progressReduce the amount requiredMicrobiological testing/measurementAntineoplastic agentsHuman cancerKinase
Phosphatidylinositol 3-kinases (PI3Ks) are known to be important regulators of signaling pathways. To determine whether PI3Ks are genetically altered in cancers, we analyzed the sequences of the P13K gene family and discovered that one family member, PIK3CA, is frequently mutated in cancers of the colon and other organs. The majority of mutations clustered near two positions within the P13K helical or kinase domains. PIK3CA represents one of the most highly mutated oncogenes yet identified in human cancers and is useful as a diagnostic and therapeutic target.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methods and compositions for cancer therapy
The present invention relates to methods and compositions for the inhibition of gene expression. In particular, the present invention provides oligonucleotide-based therapeutics for the inhibition of oncogenes involved in cancers.
Owner:PRONAI THERAPEUTICS INC
siRNA targeting v-myc myelocytomatosis viral oncogene homolog (MYC)
InactiveUS7951935B2Improve efficiencyGood curative effectSugar derivativesActivity regulationViral OncogeneSilent gene
Efficient sequence specific gene silencing is possible through the use of siRNA technology. By selecting particular siRNAs by rational design, one can maximize the generation of an effective gene silencing reagent, as well as methods for silencing genes. Methods, compositions, and kits generated through rational design of siRNAs are disclosed including those directed to MYC.
Owner:THERMO FISHER SCIENTIFIC INC
Non-small cell lung cancer targeted therapy gene detection method
InactiveCN105969857AReduce mutual interferenceStrong specificityMicrobiological testing/measurementCell sensitivityOncogene
The invention discloses a non-small cell lung cancer targeted therapy gene detection method, and belongs to the field of gene detection. The method for detecting 466 mutations of 12 oncogenes is developed by multiplex PCR and high throughput sequencing technologies, wherein the oncogenes are AKT1, ALK, BRAF, EGFR, ERBB4, FGFR1, FGFR2, FGFR3 , KRAS, MET, PIK3CA, and PTEN, and the mutations may be substitutions, insertions and / or deletions of one or more bases. The detection method provided by the invention has the advantages of high detection sensitivity of up to 0.01%, and clear and objective detection results, can be directly used for reflecting specific mutation sites of the relevant gene, directly used for guiding clinical non-small cell lung cancer targeted dosage, and used for early diagnosis or auxiliary diagnosis and screening of cancers as well as post-cancer surveillance.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI
Plant drug for treatment of liver disease
The present invention related to safe plant drug for treatment of liver disease, specifically, this invention proves a safe plant drug Schisandrin and its preparation. Schisandrin has the following pharmaceutical functions: increasing tumor suppresson genes express activity, decreasing activity of oncogenes, increasing immune function, increasing liver DNA synthesis, decreasing serum alamine aminotransferase activity, increasing glutathione level, increasing glutathione reductase activity, decreasing lipid peroxidation of liver, increasing hepatic microsomal monooxygenases activity, increasing ATP content in liver, increasing energy metabolism activity, decreasing density lipoprotein oxidation, protecting gastrointestinal function, increasing killer cell activity, increasing complement activity, decreasing induced liver cancer activity and decreasing grown of cancer cells.
Owner:ZHAO XINXIAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com