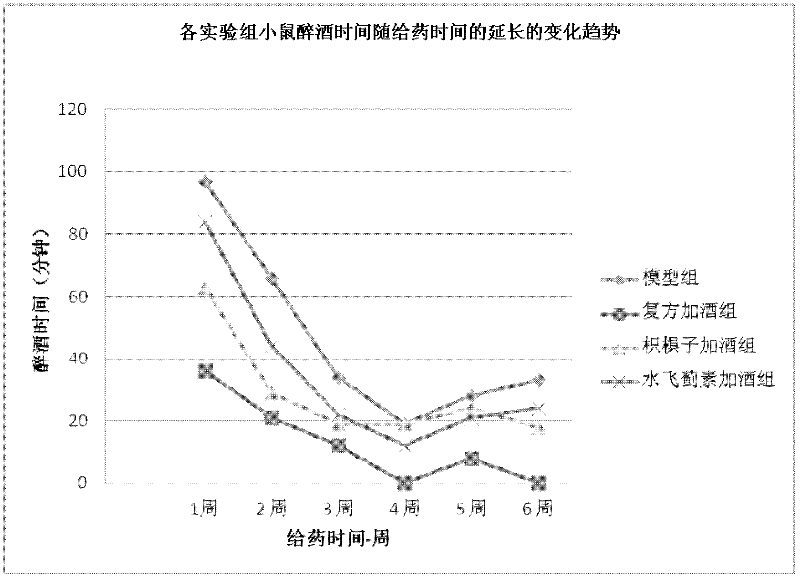
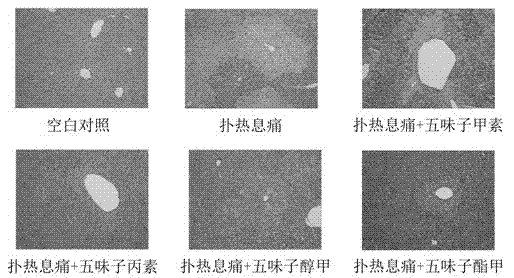
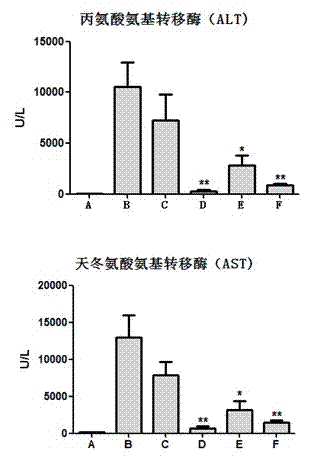
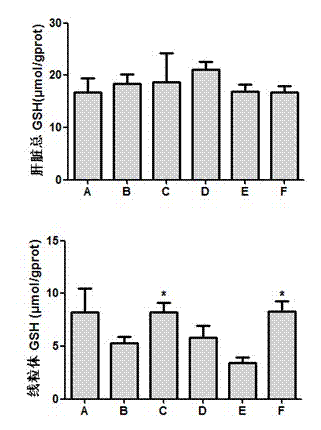
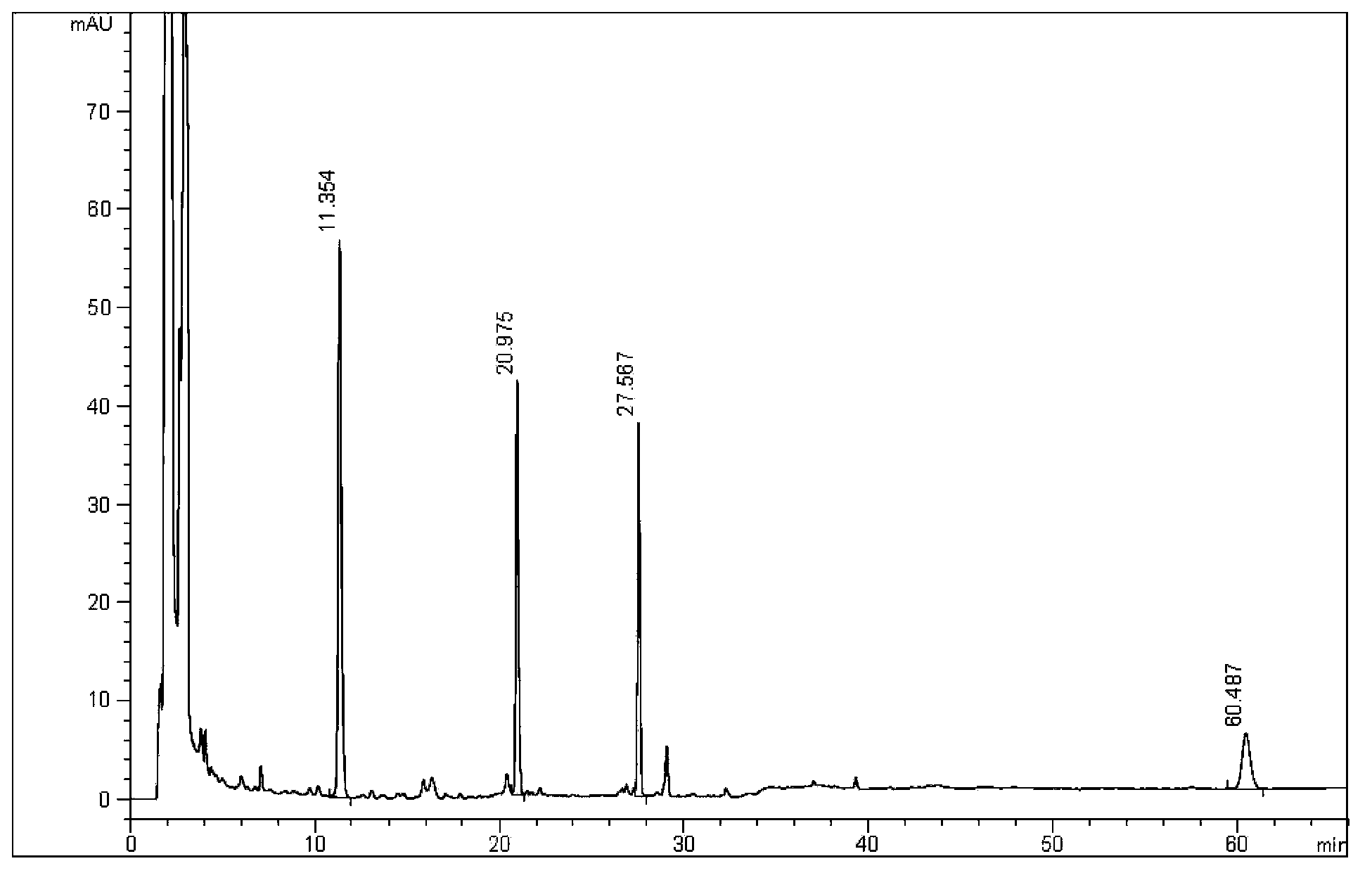
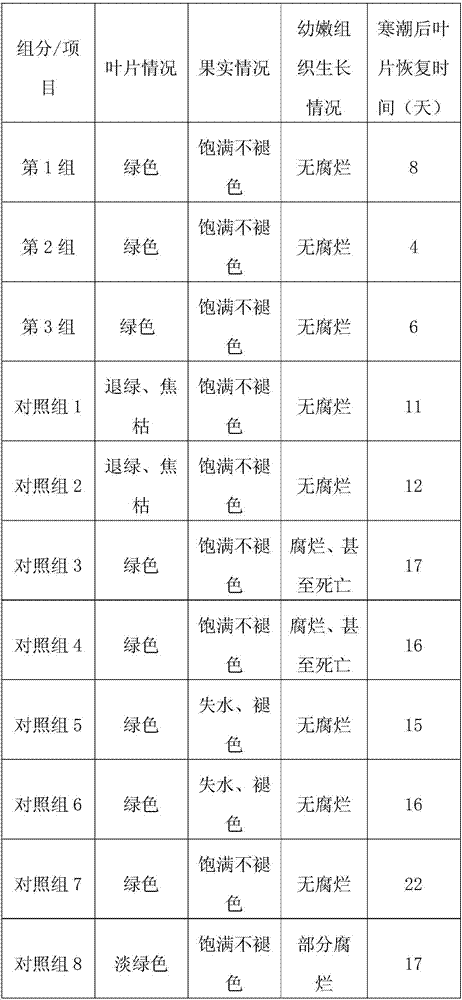
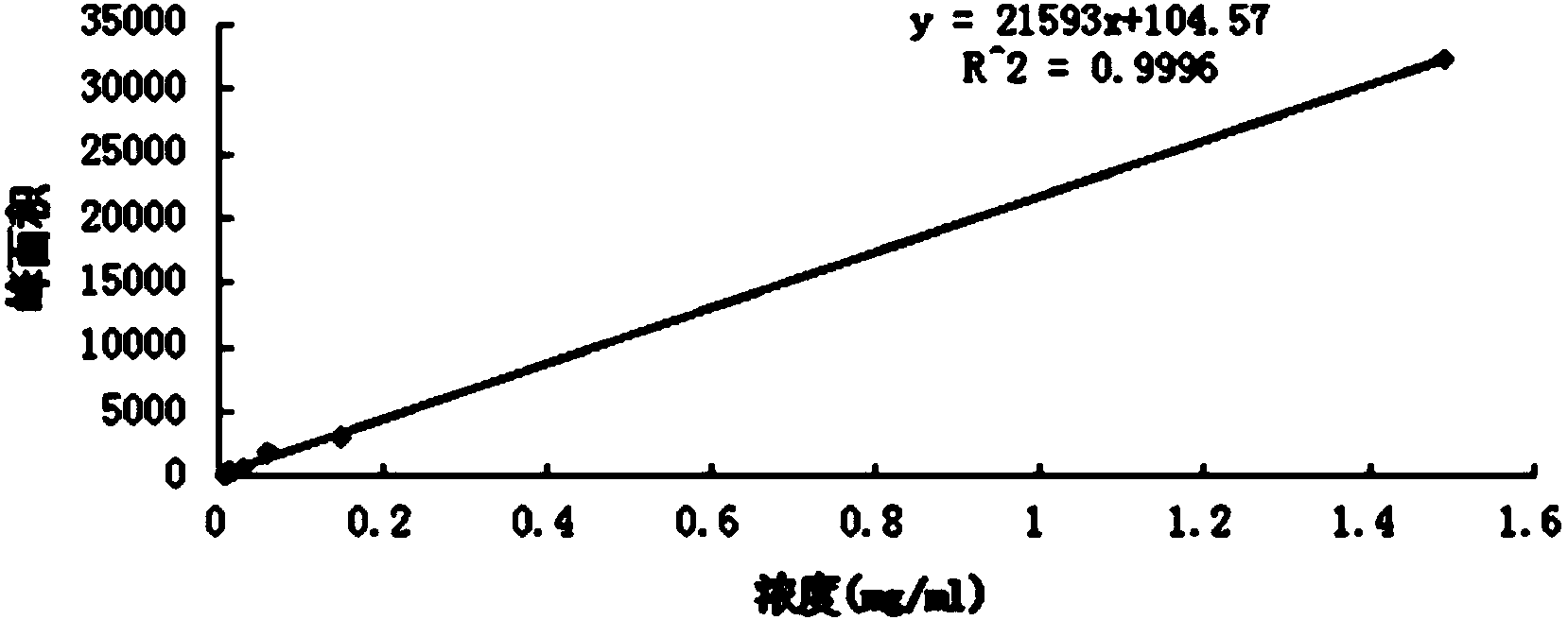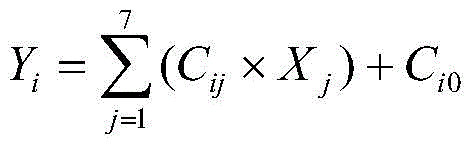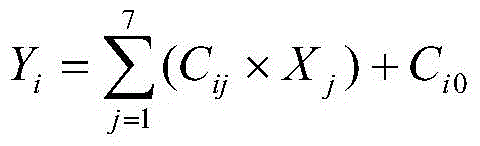Patents
Literature
50 results about "Schisandrin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
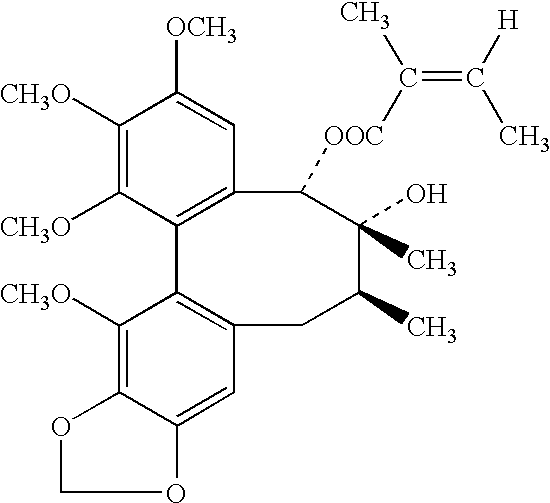
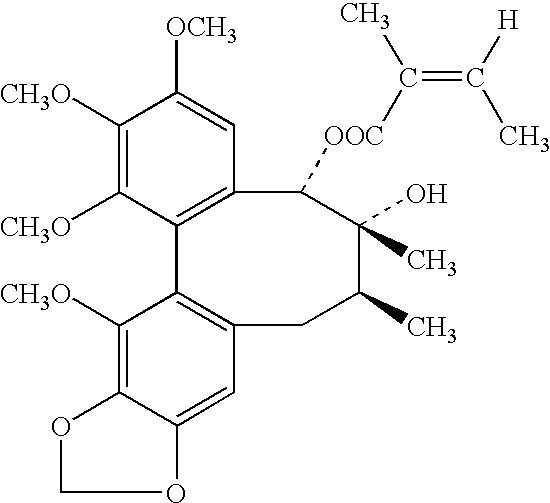
Schisandrins (schizandrins) are a group of bioactive chemical compounds found in Schisandra chinensis.
Pharmaceutical formulations with the raw material containing panax, ophiopogon root and schisandra fruit, processes for their preparation, the raw material and the quality control method for the prepa
InactiveCN101116722AImprove quality controlGood clinical efficacyPowder deliveryComponent separationMedicineOphiopogon japonicus
The present invention relates to a medicine preparation with raw material comprising gen-seng, RADIX OPHIOPOGONIS and schizan drin and relates to the methods of preparing and quality control of the invention as well as relates to the quality control method of the raw material comprising gen-seng, radix ophiopogonis and schizan drin. The present invention comprises saponins content of gen-seng, amylase content of gen-seng, saponins content of radix ophiopogonis and neolignan content of schizan drin. Each unit dosage of preparation comprises Rg1 and Re for 0.08 mg-12 mg, polysaccharide of gen-seng for 1mg-460mg, general saponins for 5 mg-800 mg and Schisandrin for 0.036mg-6mg. a medicine functioning as cooperation can be or can not be added in the present invention which can be any acceptable medicine preparation type comprising oral taking solid preparation, oral taking semi-solid preparation, oral taking liquid preparation and injection. The present invention also provides the quality control method of gen-seng, RADIX OPHIOPOGONIS and schizan drin as well as the quality control method of the present invention.
Owner:天津市轩宏医药技术有限公司 +1
Plant drug for treatment of liver disease
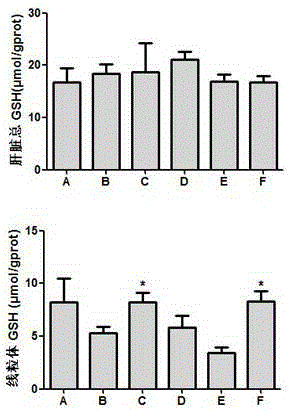
The present invention related to safe plant drug for treatment of liver disease, specifically, this invention proves a safe plant drug Schisandrin and its preparation. Schisandrin has the following pharmaceutical functions: increasing tumor suppresson genes express activity, decreasing activity of oncogenes, increasing immune function, increasing liver DNA synthesis, decreasing serum alamine aminotransferase activity, increasing glutathione level, increasing glutathione reductase activity, decreasing lipid peroxidation of liver, increasing hepatic microsomal monooxygenases activity, increasing ATP content in liver, increasing energy metabolism activity, decreasing density lipoprotein oxidation, protecting gastrointestinal function, increasing killer cell activity, increasing complement activity, decreasing induced liver cancer activity and decreasing grown of cancer cells.
Owner:ZHAO XINXIAN
Plant drug for treatment of liver disease
The present invention related to safe plant drug for treatment of liver disease, specifically, this invention proves a safe plant drug Schisandrin and its preparation. Schisandrin has the following pharmaceutical functions: increasing tumor suppresson genes express activity, decreasing activity of oncogenes, increasing immune function, increasing liver DNA synthesis, decreasing serum alamine aminotransferase activity, increasing glutathione level, increasing glutathione reductase activity, decreasing lipid peroxidation of liver, increasing hepatic microsomal monooxygenases activity, increasing ATP content in liver, increasing energy metabolism activity, decreasing density lipoprotein oxidation, protecting gastrointestinal function, increasing killer cell activity, increasing complement activity, decreasing induced liver cancer activity and decreasing grown of cancer cells.
Owner:ZHAO XINXIAN
Weight management composition
A method for promoting weight management in a mammal is provided, comprising administering to the mammal a composition comprising at least one weight loss agent and at least one mitochondria enhancingagent. The weight loss agent may be selected from the group consisting of psyllium, guar gum, capsaicin, chitosan, caffeine, garcinia cambogia, Pinus densiflora, capsaicin, yohimbre, hoodia, glucomannan, African mango, guarana, pyruvate, carnitine, beta-glucan, fucoxanthin, raspberry ketone, white kidney bean, kola nut, chromium, ginseng, psyllium, St. John's wort, dandelion, hydroxycitric acid,conjugated linoleic acid, green tea, black tea, green coffee beans extract, forskolin and bitter orange. The mitochondria enhancing agent may be selected from the group consisting of beetroot extract,nitrate, idebenone, nicotinamide riboside, elamepratide, vitamin C, vitamin D, vitamin E, thiamine, riboflavin, magnesium, calcium, phosphate, phospholipid, creatine, pyruvate, coenzyme Q10, NADH, nicotinic acid, L-camitine, dicholoracetate, curcumin, schisandrin and resveratrol. Compositions for weight management are also provided.
Owner:ASERKIN CO LTD
Liver-protecting product and method for preparing the same
InactiveCN101468103APrevent intrusionRecovery functionDigestive systemSolution deliveryDiseaseSchisandrin
The purpose of the present invention is to provide a Hugan products and their preparation methods, as described including chrysanthemum, dandelion, wolfberry, cassia seed, Angelica dahurica and schisandrin, carried out by a special combination of natural herbs, effective prevention, and treatment to improve liver dysfunction, such as the role of disease, and its preparation method is simple, stable process conditions suitable for the scale of chemical production.
Owner:沃德(天津)营养保健品有限公司
New method for purifying schisandrin
The invention belongs to the field of natural organic chemistry, and relates to a purifying method for schisandrin, and particularly to a method for purifying schisandrin in schisandra chinensis by using microwave countercurrent extraction, macroporous resin adsorption, and column chromatography purification. The method has the following advantages that: 1, the microwave extraction is adopted, and ethanol is adopted as a solvent to remove part of fatty oil so as to greatly reduce the extraction time, and improve production efficiency, in addition, the method is simple; 2, the macroporous resin can be conveniently and rapidly regenerated, and is suitable for large-scale production, water elution can remove most of pigments and impurities, and the macroporous resin is easy to operate; 3, the macroporous resin and the silica gel column chromatography are combined so as to provide a good separation and purification effect, at the late stage, recrystallization is adopted so as to greatly improve the product purity. With the present invention, the microwave countercurrent extraction, the macroporous resin separation and purification and the column chromatography are adopted to carry out further purification, such that the product purity is greatly improved, and the semi-finished product schisandrin with the content more than 65% and the final product schisandrin with the content more than 98% are obtained.
Owner:DAXINGANLING LINGOBERRY BOREAL BIOTECH CO LTD
Rehmannia decoction pharmaceutical composition and preparation method thereof, and detection method
ActiveCN110464825AConcentration efficiency is lowSolve technical problemsNervous disorderComponent separationMonkshoodsFreeze-drying
The invention relates to a rehmannia decoction pharmaceutical composition and a preparation method thereof, and a detection method. The pharmaceutical composition consists of the following formula: 0.5-1.5 parts of prepared rehmannia roots, 0.5-1.5 parts of Morinda pulp, 0.5-1.5 parts of cornus officinalis pulp, 0.5-1.5 parts of dendrobium, 0.5-1.5 parts of wine-processed cistanche, 0.5-1.5 partsof prepared common monkshood branched roots, 0.5-1.5 parts of Chinese magnoliavine fruits, 0.5-1.5 parts of cinnamon, 0.5-1.5 parts of poria cocos, 0.5-1.5 parts of dwarf lilyturf tuber, 0.5-1.5 partsof rhizoma acori graminei, 0.5-1.5 parts of milkwort root, 4-6 parts of raw ginger, 3-6 parts of Chinese dates and 0.5-1.5 parts of mint. The composition is prepared by a method including water extraction, freeze drying and the like. The process solves the technical problems that in the prior art, morroniside, loganin and schisandrin are lost due to reduced-pressure drying, and the dry paste powder is granular and hard. The invention determines a specific detection method for qualitative identification, fingerprint spectrum and content determination. The good detection method effectively reflects the advantages and disadvantages of the material reference mass and solves the technical problem that quality detection methods in the prior art are difficult to reflect the advantages and disadvantages of the material reference mass.
Owner:GUIZHOU JINGCHENG PHARMA
Method for simultaneously detecting contents of four effective ingredients in antitussive tablet
ActiveCN103048409AImprove quality controlQuality improvementComponent separationSilanesAdditive ingredient
The invention provides a method for simultaneously detecting contents of four effective ingredients (including schisandrin, praeruptorin A, praeruptorin and shionone) in an officinal antitussive tablet. The method comprises the steps of applying a modern high performance liquid chromatography technology, and taking octodecyl silane bonded silica gel as a filling agent; taking acetonitrile as a mobile phase A, and water as a mobile phase B; and calculating the number of theoretical plates to be not less than 5000 according to a schisandrin peak. In an elution process, gradient elution that allows a concentration proportion of the mobile phase A to the mobile phase B to change gradually is combined with isocratic elution that allows a proportion of the mobile phase A to the mobile phase B to be constant, so that a specific gradient elution procedure is formed; two or three gradient elutions are conducted; and the change detection is conducted on wavelength in a gradient elution process. The method can simultaneously detect the contents of schisandrin, praeruptorin A, praeruptorin and shionone in the antitussive tablet qualitatively and quantitatively, is simple, convenient, quick and high in specificity, and can raise the quality control level of the antitussive tablet effectively.
Owner:TEYI PHARMACEUTICAL GROUP CO LTD
Production method for extracting Chinese magnoliavine fruit volatile oil through CO2 supercritical extraction
InactiveCN104388185ASolve the problem of low extraction contentSettlement yieldEssential-oils/perfumesFatty-oils/fats productionPhysical chemistrySchisandrin
The invention relates to a production method for extracting Chinese magnoliavine fruit volatile oil through CO2 supercritical extraction. The method comprises the following steps: crushing Chinese magnoliavine fruits, putting the crushed fruits in an extraction kettle, controlling the temperature in a range of 30-45DEG C and the CO2 flow at 2L / min, extracting under a pressure of 200-320MPa for 8-15h, carrying out primary separation under 100-200MPa at 30-60DEG C, carrying out secondary separation under 40-70MPa at 30-60DEG C, discharging Chinese magnoliavine fruit oil to obtain the Chinese magnoliavine fruit oil with the yield of 6% and the Schisandrin Bcontent of 2.30-2.50%, preserving in a 5DEG C refrigerator, and recovering Chinese magnoliavine fruit residues to be used for making feeds. The production method has the advantages of simple process and low production cost, and solves the problems of low content and small yield of a Chinese magnoliavine fruit volatile oil pressing method.
Owner:辽宁大熊制药有限公司 +2
Preparation method of Fructus Schisandrae/indigo honeysuckle oral liquid containing crude polysaccharides
ActiveCN102908390AFast absorptionImprove absorption rateSenses disorderNervous disorderMedicineAdditive ingredient
The invention discloses a preparation method of fructus schisandrae / indigo honeysuckle oral liquid containing crude polysaccharides, relating to preparation method of nutritional health-promoting oral liquid, to solve the problem of incapability of rapid absorption of effective nutritional ingredients for existing fructus schisandrae oral liquid. The preparation method includes washing fresh fructus schisandrae and indigo honeysuckle; extracting fresh juice of fructus schisandrae; collecting natural juice of indigo honeysuckle; mixing the juices, performing enzymolysis and separation, fermenting at low temperature, coarse-filtering, and fine-filtering to obtain fermented juice; coarse-filtering and fine-filtering the fresh juice of fructus schisandrae to obtain clear fresh juice; mixing the fermented juice and clear fresh juice, and performing UHT sterilization to obtain the oral liquid. The inventive process is scientific and free of any biological auxiliary. Under action of the fermented juice, nutritional ingredients (such as crude polysaccharides and schisandrin) in the oral liquid can rapidly enter human micro-circulation system, to improve absorption speed and rate of nutritional ingredients.
Owner:黑龙江越橘庄园生物科技有限公司
Method for establishing Sishen tablet fingerprint spectrum
InactiveCN104198634AMonitor qualityHigh technical contentComponent separationGradient elutionSilica gel
The invention discloses a method for establishing a Sishen tablet preparation fingerprint spectrum. The method comprises the following steps: preparing a reference solution which contains 10-100mg of psoralen, isopsoralen and schisandrin per milliliter; and removing coatings from Sishen tablets, weighing out 0.1-10g of Sishen tablets without coating, performing ultrasonic extraction with methanol, performing filtration to obtain a test solution, precisely sucking the test solution, injecting the test solution into a liquid chromatograph, and determining in accordance with a liquid chromatography, thereby obtaining the fingerprint spectrum. The method is performed under the following chromatographic conditions: octadecyl silane-bonded silica gel serves as stuff of a chromatographic column, a mobile phase refers to a gradient eluent consisting of acetonitrile and water, the detection wavelength is 220-360nm, the column temperature is 20-50 DEG C, the flow velocity is 0.5-1.5ml / min and the time is 30-90 min. The method is simple and stable, has high precision and good reproducibility, and is capable of rapidly and correctly distinguishing the authenticity and the quality of products.
Owner:沈阳双鼎制药有限公司
Processing method of Chinese magnoliavine fruit green tea
InactiveCN108308346AReduce caffeine levelsReduce bitternessTea alkaloid content reductionSchisandrinSweetness
The invention relates to the technical field of green tea processing, in particular relates to a processing method of a Chinese magnoliavine fruit green tea. The processing method comprises the following 11 processing technologies: collection and storing of Chinese magnoliavine fruits, tea picking, enzyme deactivation, soaking, first stoving, rolling, tea strip tidying, secondary stoving, moistureregain, third stoving, and sterilization and packaging. A steam steaming technology and a soaking technology can blend schisandrin and schisantherin in tea, and a combination effect can reduce the content of tea polyphenols and caffeine in the tea so as to reduce the bitter and astringency of green tea; and meanwhile, with a spreading for cooling technology and a moisture regain technology, the components that the Chinese magnoliavine fruits contain and the green tea are perfectly fused, so that the components contained in the finished green tea are not easy to dissipate. The brewed green teasoup has no obvious difference from conventional green tea soup in color, and has a taste with faint sourness and sweetness in mouth feel, and the Chinese magnoliavine fruit green tea has the efficacy of nourishing yin and tonifying the kidney after long-term drinking.
Owner:晴隆县花贡镇巨鑫茶叶农民专业合作社
Agricultural product production place identification method
ActiveCN104764864AGuarantee the safety of medicine and foodRealize the protection of originComponent separationMaterial analysis by electric/magnetic meansAgricultural scienceFood safety
The present invention relates to a discrimination method, particularly to an agricultural product production place identification method, which is mainly used for schisandra chinensis production place identification. According to the agricultural product production place identification method, the independent variable factors in the crushed schisandra chinensis sample is qualitatively and / or quantitatively determined, and is used to carry out model regression, and the belonging production place of the sample is determined according to the dependent variable level, wherein the independent variable factors (Xj) are [delta]<15>N, the schisandrin content, the schisantherin A content, the schizandrin A content, the schisandrin B content, the B content and the Sr content. According to the present invention, the schisandra chinensis is adopted as the demonstration to develop the production place traceability research to screen the geographic labels for effectively indicating the schisandra chinensis production place source, such that the drug food safety of the consumer can be easily ensured, the original production place protection of the authentic herbs can be achieved, and the theoretical basis is provided for the optimized planting of the schisandra chinensis. In addition, the technology can further be promoted and applied for the agricultural product quality safety and control, and has broad application prospects.
Owner:SHENYANG INST OF APPLIED ECOLOGY - CHINESE ACAD OF SCI
Method for carrying out simultaneous quantitative analysis on four lignan components in Chinese magnoliavine raw material and Chinese magnoliavine extract
InactiveCN102419350ARealize simultaneous measurementSolve the problem that the raw materials of Schisandra chinensis cannot be measured at the same timeComponent separationLignanColumn temperature
The invention discloses a method for carrying out simultaneous determination on contents of schisandrin, schisantherin, deoxyschizandrin and schisandrin B in a Chinese magnoliavine raw material or a Chinese magnoliavine extract. The method employs means of crushing, sieving, methanol ultrasonic extraction, calibrating volume and filtering to treat schisandra chinensis or a Chinese magnoliavine extract to obtain a sample solution for testing; a high performance liquid chromatography is utilized for simultaneous determination on contents of four lignan components. The method utilizes a reverse direction distribution chromatographic theory to carry out analysis on the sample by gradient elution, under conditions of a column temperature of 25-45 DEG C, mobile phases of methanol and 0.05-0.2% trifluoroacetic acid aqueous solution (methanol and trifluoroacetic acid aqueous solution in a volume ratio of 50:50-100:0), an elution time of 30-60 min, a flow velocity of 0.7-1.5 ml / min and a detection wavelength of 210-280 nm. The quality detection method is simple, rapid, sensitive and accurate.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
Novel natural herbal compound composition with sobering and liver protection effects and application thereof
InactiveCN102416086AGood synergySignificantly sober upDigestive systemAntinoxious agentsVitamin E AcetateVitamin B12
The invention discloses a novel natural herbal compound composition with sobering and liver protecting effects and preparation as well as application thereof. The composition is prepared from active ingredients which mainly have the sobering and liver protecting effects and auxiliary materials, wherein the composition of the active ingredients comprise the following components in percentage by weight based on total weight of the active ingredients: 3-10 percent of sesamin, 0.05-5 percent of schisandrin, 10-45 percent of hovenia dulcis thumb extract, 10-45 percent of kudzu vine flower extract, 10-45 percent of nutgrass galingale rhizome extract, 0-0.02 percent of vitamin B12 and 1-10 percent of vitamin E acetate. The invention also relates to application of the composition to preparation of health care foods for preventing and / or treating acute alcoholism, health care foods for treating chronic alcoholic liver damage and health care foods for treating nonalcoholic fatty liver. The composition has the advantages of good synergistic reaction among the components, obvious curative effect, readily available raw materials, easiness in preparation and relatively low cost.
Owner:中哈福生物医药科技(上海)有限公司
Application of schisandra chinensis monomer compound in preparation of drugs for prevention and treatment of hepatotoxicity caused by acetaminophen
ActiveCN103751174ACurb consumptionImprove necrosisDigestive systemEther/acetal active ingredientsLiver necrosisAlanine aminotransferase
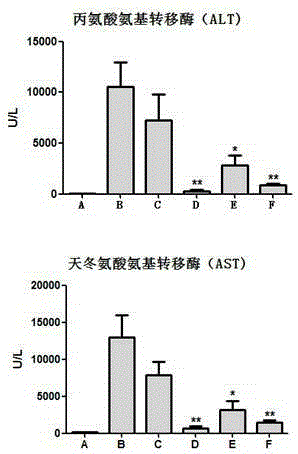
The invention discloses an application of a schisandra chinensis monomer compound in preparation of drugs for prevention and treatment of hepatotoxicity caused by acetaminophen (APAP), wherein the schisandra chinensis monomer compound is deoxyschizandrin, schisandrin C, schisandrin or schisantherin a. Experiment results show that: with the schisandra chinensis monomer compound, the ALT content and the AST content in the APAP-induced liver injury animal model can be significantly reduced, consumption of glutathione in liver cells can be inhibited, the liver cell necrosis degree can be effectively improved, and the schisandra chinensis monomer compound provides significant treatment and protection effects for hepatotoxicity symptoms caused by APAP.
Owner:SUN YAT SEN UNIV
Treating agent for removing drug residues in wastewater of amoxicillin production workshop
InactiveCN105084496AStrong complexing abilityFast precipitationWater contaminantsWater/sewage treatment by flocculation/precipitationCyclohexeneBeta-boswellic acid
The invention relates to a treating agent for removing drug residues in wastewater of an amoxicillin production workshop. The treating agent is compounded from components as follows: 2, 6-dimethyl-2,6-octadiene-8-ol, actein, methyl 2-aminobenzoate, ferrous gluconate, psoralen, 4-methyl-1-(1-methyl ethyl)-3-cyclohexene-1-ol, 2,4-dimethylthiazole, salicin, schisandrin, polyoxyethylene lauryl ether, cyclopentadecanolide, imperatorin, polyacrylamide, acetyl-11-carbonyl-beta-boswellic acid and isolicoflavone. The treating agent has high complexing capability with a target substance, and the removal rate can reach 99.9%.
Owner:HANGZHOU QICHENG TECH CO LTD
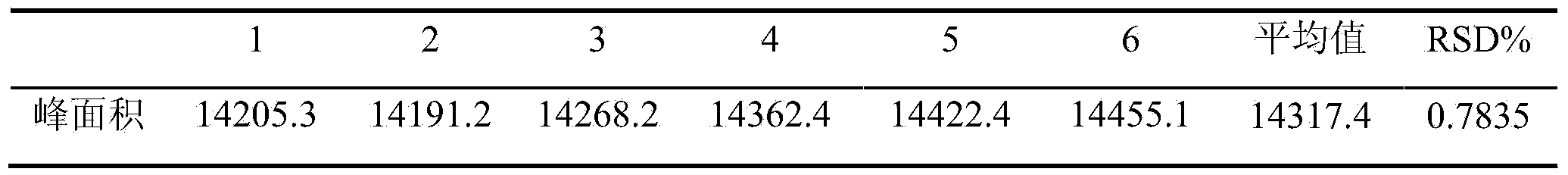
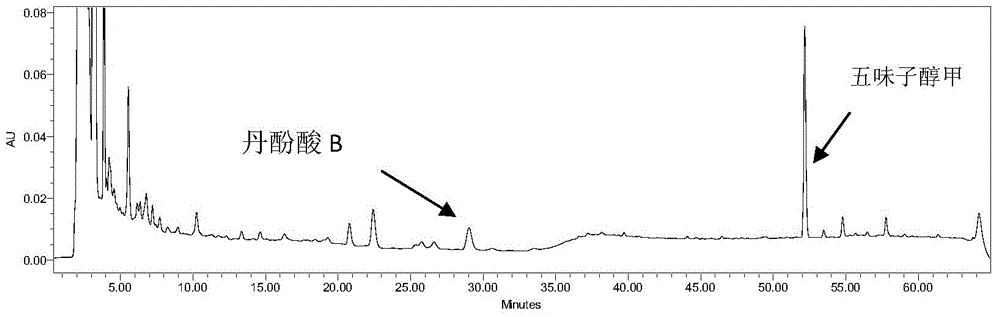
Method for determining content of schisandrin in Yixinshu traditional Chinese medicine preparation
InactiveCN103592387AEffective quality controlHigh precisionComponent separationSchisandrinTraditional medicine
The invention discloses a method for determining the content of schisandrin in a Yixinshu traditional Chinese medicine preparation. A schisandrin reference substance is taken as a contrast, methanol and water in a ratio of (65-75):(35-25) are taken as a mobile phase, and the content of the schisandrin in the Yixinshu traditional Chinese medicine preparation is determined with a high performance liquid chromatography. The method for determining the content of the schisandrin in the Yixinshu traditional Chinese medicine preparation with the high performance liquid chromatography is high in specificity, high in precision, good in repeatability, high in recovery rate, high in stability and accurate in measurement result, so that the medicine quality is effectively controlled, the stability of the product quality as well as the safety and effectiveness of clinical medication are guaranteed.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Healthy tea beneficial for protection of liver and relieving of alcohol effects
The invention relates to a healthy tea beneficial for protection of liver and relieving of alcohol effects, more specifically relates to a healthy tea which is prepared from lucid ganoderma, schisandra chinensis, and radix puerariae and is capable of protecting liver and relieving alcohol effects effectively, and belongs to the field of food processing. According to a preparation method, purple lucid ganoderma, schisandra chinensis, and wild radix puerariae are taken as raw materials, the healthy tea is obtained via scientific combination and processing, and the preparation method comprises steps of material preparation, processing, uniform mixing, sterilizing, and packaging. The healthy tea is rich in a plurality of functional ingredients which are capable of protecting liver and relieving alcohol effects effectively, such as ganoderan, schizandrin, and pueraria flavone, after mixing with water; is capable of protecting liver, relieving alcohol effects, and adjusting immunity; and is suitable for people that drink wine for long terms, and people with liver protection requirements. The healthy tea comprises 50% of purple lucid ganoderma, 20% of schisandra chinensis, and 30% of radix puerariae.
Owner:镇江市丹徒区南山溪园茶叶专业合作社
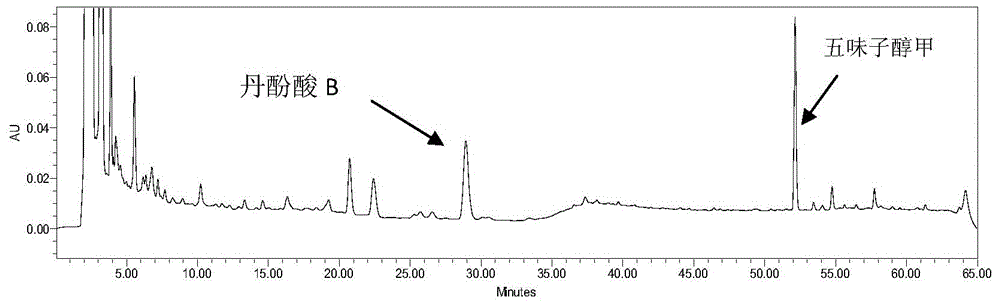
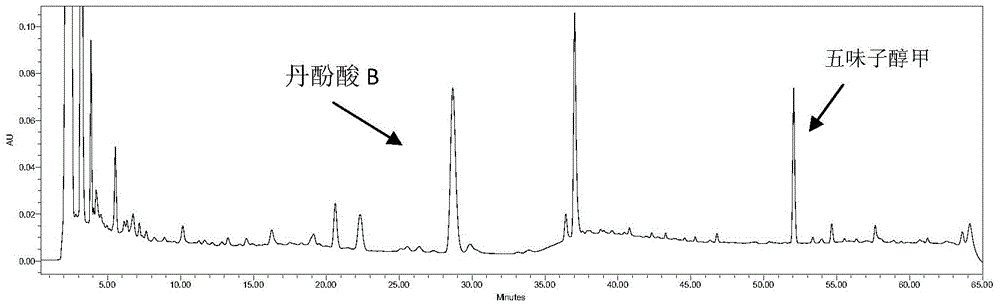
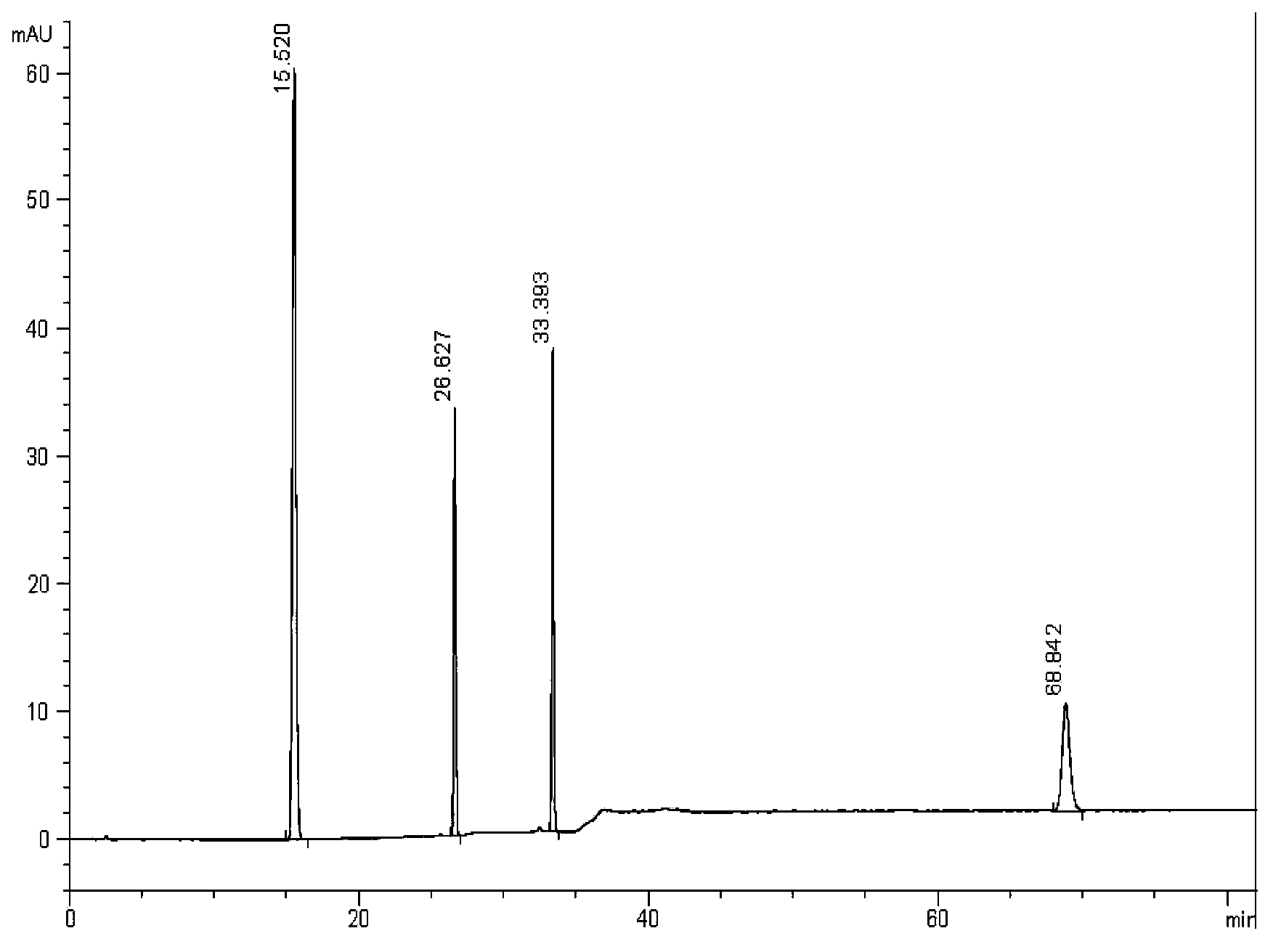
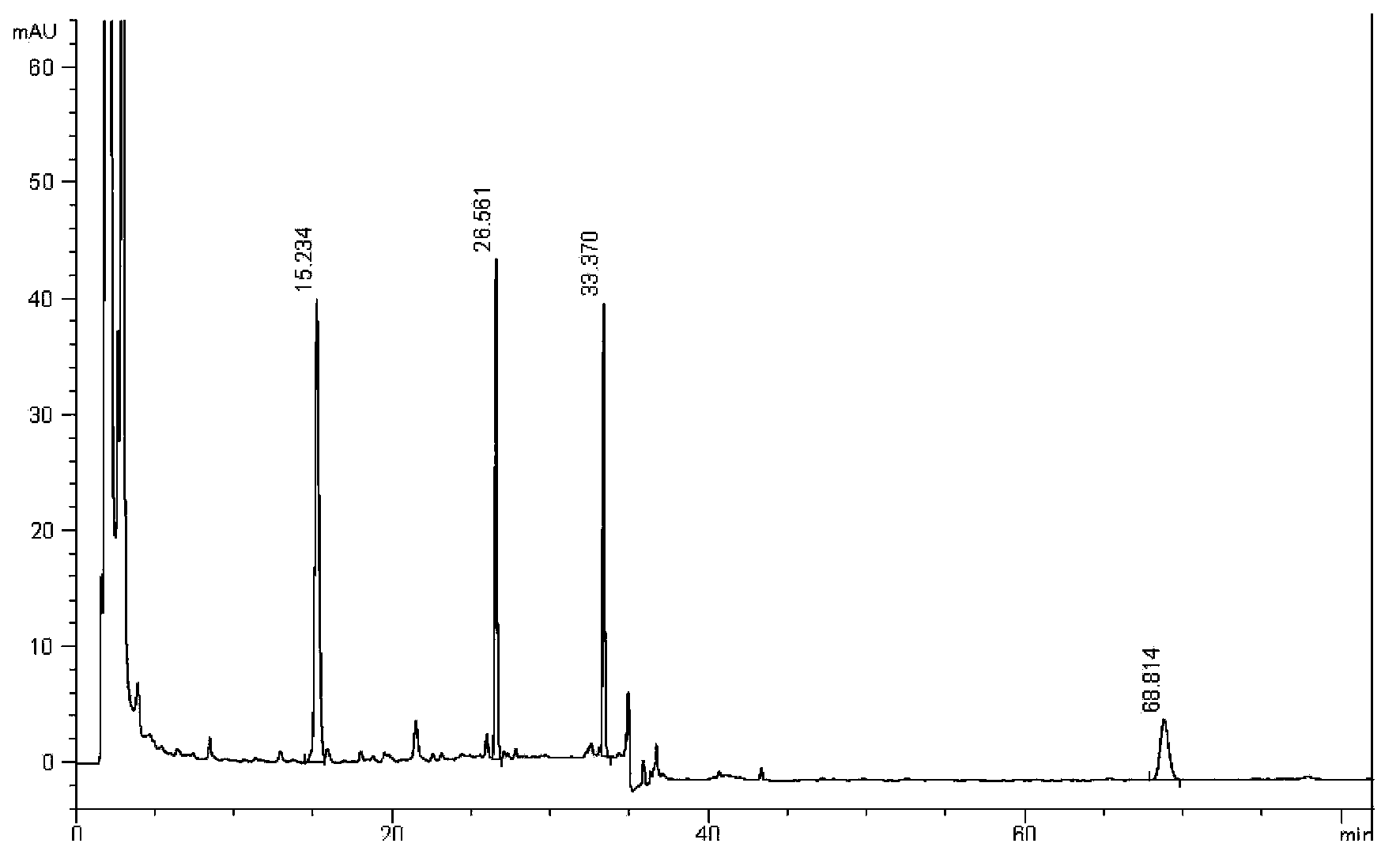
Method for measuring content of salvianolic acid B and schisandrin in heart-benefiting pulse-invigorating granule by quantitative analysis of multi-components by single marker(QAMS)
ActiveCN105823830AMeet the requirements of method validationSimple and fast operationComponent separationSalvianolic acid BQuality control
The invention relates to a method for measuring content of salvianolic acid B and schisandrin in heart-benefiting pulse-invigorating granule by a quantitative analysis of multi-components by single marker(QAMS). The method comprises the following steps: a tested object solution of heart-benefiting pulse-invigorating granule and a reference solution of schisandrin are prepared, then the materials are respectively injected into a high-performance liquid chromatography, gradient elution is carried out under specific chromatogram condition, the content of the schisandrin is determined, through the quantitative analysis of multi-components by single marker, and the content of the salvianolic acid B in heart-benefiting pulse-invigorating granule is calculated by using relative correction factors between the salvianolic acid B and the schisandrin. The established quantitative analysis of multi-components by single marker can increase a quality control level of the product, and at the same time, the detection cost is saved.
Owner:TIANJIN TASLY PHARMA CO LTD +1
Method for simultaneously detecting contents of four effective ingredients in antitussive tablet
ActiveCN103048409BImprove quality controlQuality improvementComponent separationSilanesAdditive ingredient
The invention provides a method for simultaneously detecting contents of four effective ingredients (including schisandrin, praeruptorin A, praeruptorin and shionone) in an officinal antitussive tablet. The method comprises the steps of applying a modern high performance liquid chromatography technology, and taking octodecyl silane bonded silica gel as a filling agent; taking acetonitrile as a mobile phase A, and water as a mobile phase B; and calculating the number of theoretical plates to be not less than 5000 according to a schisandrin peak. In an elution process, gradient elution that allows a concentration proportion of the mobile phase A to the mobile phase B to change gradually is combined with isocratic elution that allows a proportion of the mobile phase A to the mobile phase B to be constant, so that a specific gradient elution procedure is formed; two or three gradient elutions are conducted; and the change detection is conducted on wavelength in a gradient elution process. The method can simultaneously detect the contents of schisandrin, praeruptorin A, praeruptorin and shionone in the antitussive tablet qualitatively and quantitatively, is simple, convenient, quick and high in specificity, and can raise the quality control level of the antitussive tablet effectively.
Owner:TEYI PHARMACEUTICAL GROUP CO LTD
Quality control method of drug for nourishing blood and quieting spirit
InactiveCN104833661AGuaranteed production effectGuarantee clinical applicationPreparing sample for investigationFluorescence/phosphorescenceMedicineSchisandrin
A quality control method of a drug for nourishing blood and quieting spirit is used for controlling quality during production of the drug and comprising the steps of: identifications of spina date seeds, radix glycyrrhizae, rhizoma chuanxiong, rhizoma anemarrhenae and poria cocos, and content measurements of the spina date seeds, the radix glycyrrhizae, the rhizoma chuanxiong, the rhizoma anemarrhenae and the poria cocos. The quality control method is advantaged in that by means of thin layer chromatography, the spina date seeds, the radix glycyrrhizae, the rhizoma chuanxiong, the rhizoma anemarrhenae and the poria cocos are subjected to qualitative identification, thereby achieving excellent specificity, repeatability and durability; by means of high performance liquid chromatography, the schisandrin is subjected to quantitative analysis, so that the method is high in separation degree, is short in analysis time and is simple in operation, thereby effectively improving production efficiency and saving energy. In addition, by means of a DAD detector, the specificity of the method is inspected, and the durability of the method is inspected by means of different instruments and chromatographic columns. The quality control method is high in specificity, is good in repeatability, is accurate in result and is a safe and effective quality control method of the drug for nourishing blood and quieting spirit.
Owner:吉林修正药业新药开发有限公司
Chinese magnoliavine active extract
InactiveCN107513008AHigh purityHigh adsorption rateEther separation/purificationReflux extractionDeoxyschizandrin
The invention discloses a Chinese magnoliavine active extract, which comprises schisandrin, deoxyschizandrin, and schisandrin B. The preparation method comprises reflux extraction, concentration and water precipitation, adsorption, elution, and drying. Ethanol (85-95%) is used to extract the Chinese magnoliavine active substances through reflux extraction; then the active substances are purified by water precipitation, adsorption, macroporous resin elution, and reversed-phase high-performance liquid chromatography to obtain schisandrin, deoxyschizandrin, and schisandrin B, and the product quality is high. The production technology is simple, each operation has accurate data, the repeatability is good, the product quality is stable, ethanol is low in toxicity, and the extracted Chinese magnoliavine active substances are very safe and green.
Owner:浦江县美泽生物科技有限公司
Heart-nourishing nerve-calming traditional Chinese medicine composition
InactiveCN111544529AIt has a good effect of nourishing the mind and calming the nervesPromote absorptionNervous disorderConiferophyta medical ingredientsMedicinal herbsVitamin C
The invention discloses the technical field of traditional Chinese medicines for tranquilizing mind by nourishing heart. The invention relates to a traditional Chinese medicine composition for tranquilizing mind by nourishing heart. The traditional Chinese medicine composition is prepared from fried spina date seeds, platycladi seeds, glossy privet fruits, schisandra chinensis, tuber fleeceflowerstems, cortex albiziae, poria cocos, lotus seeds, lily, wheat, polygala tenuifolia, radix pseudostellariae, panax quinquefolius, dendrobe and prepared rhizome of rehmannia, wherein fried spina date seeds, platycladi seeds, tuber fleeceflower stems and cortex albiziae have the sedative effect, are mainly used for calming people with qi deficiency, preventing upset and irritability, and keeping thebody state of people with qi deficiency in a state of intensive nourishment; schisandra chinensis contains schisandrin, vitamin C, resin, tannin and a small amount of sugar, and has the efficacy of astringing the lung to stop cough, nourishing and arresting seminal emission and relieving diarrhea and sweat; glossy privet fruits and schisandra chinensis can overflow grease in the cooking process, and the grease is easily attached to the surfaces of medicinal materials with gaps such as poria cocos and lotus seeds, so that effective components in the raw medicinal materials are easy to fuse, andthe overall absorbability of the traditional Chinese medicine composition is improved.
Owner:张嘉炜
Special cold-resistant foliage fertilizer for bananas and preparation method thereof
InactiveCN107082678AImprove cold resistancePromote growthMagnesium fertilisersAlkali orthophosphate fertiliserChlorogenic acidPolydimethyl siloxane
The invention discloses a special cold-resistant foliage fertilizer for bananas and a preparation method thereof, and relates to the technical field of fertilizers. The foliage fertilizer comprises the following raw materials: potassium nitrate, magnesium sulfate, ammonium phosphate, zinc sulfate, manganese sulfate, cysteine, vitamins, ferrous chloride, copper sulfate, salicylic acid, methyl jasmonic acid, fucoidan in laminaria japonica, soy isoflavone, chlorogenic acid, schisandrin, polydimethyl siloxane and soybean lecithin. The foliar fertilizer of the invention can improve cold resistance of bananas, prevent banana leaves from being withered or plants from dying under low-temperature stress, effectively reduces cold damage and improves the economic benefit. After the cold wave, damaged banana tissues and various physiological functions after the cold wave are restored quickly, and yield is raised.
Owner:广西浩昌敏再生资源利用有限公司
Application of schisandrin B and schisandrin C in the preparation of anti-hepatic fibrosis drugs
ActiveCN104873491BHas anti-hepatic fibrosis effectDigestive systemEther/acetal active ingredientsLignanSchizandrin B
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Application of schisandrin B in preparation of drugs for preventing colitis or colon cancer
ActiveCN104825442BInhibit the activity of inflammatory cytokinesLow toxicityDigestive systemAntineoplastic agentsSide effectSchisandrin
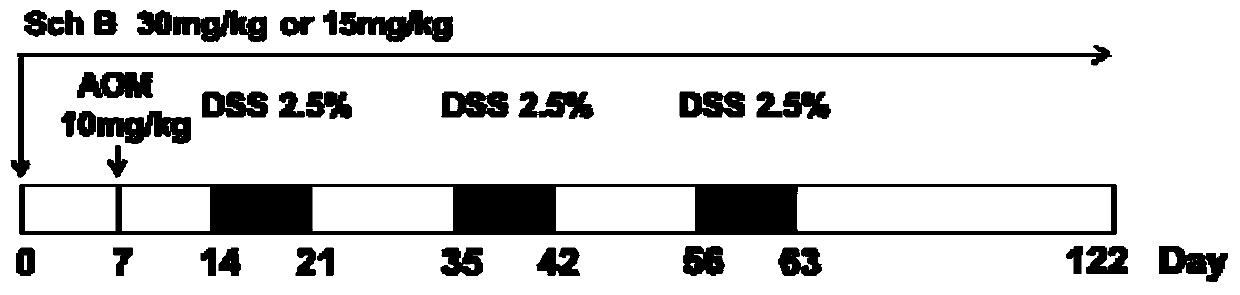
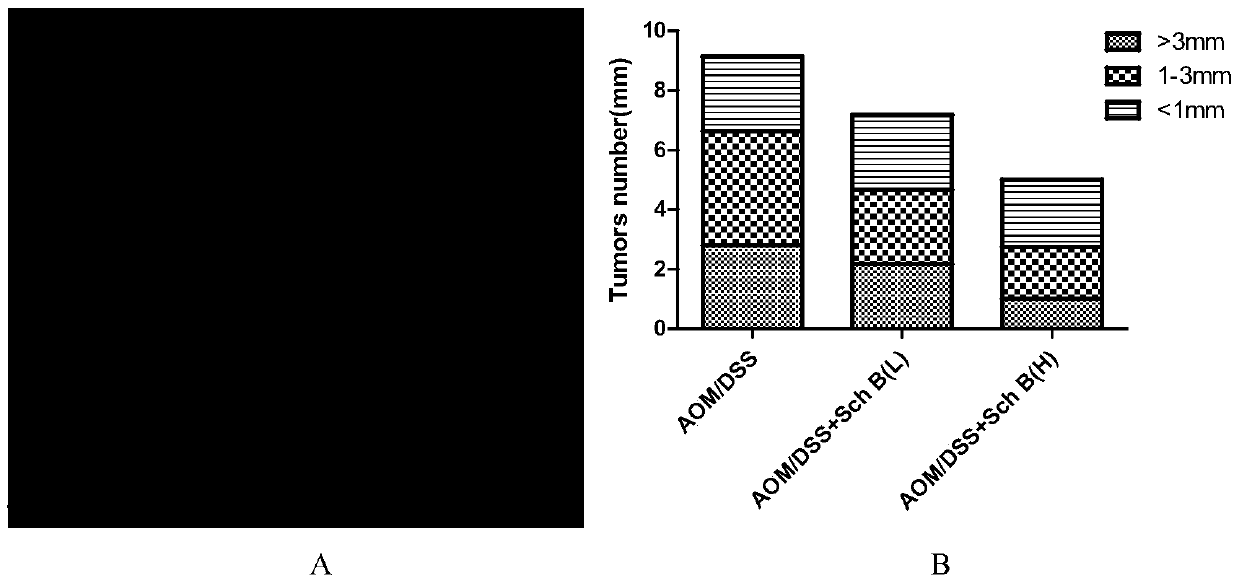
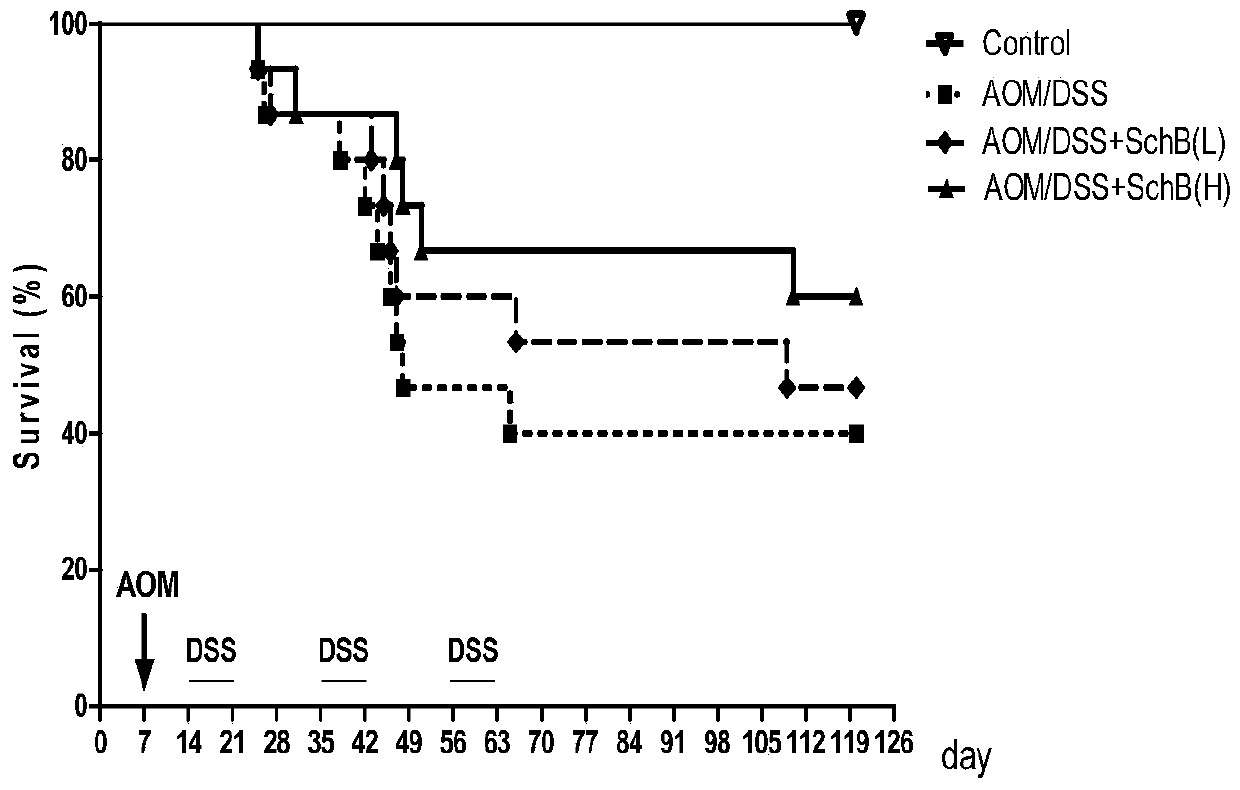
The invention discloses an application of schisandrin B in the preparation of a drug for preventing colitis or colon cancer; the dosage of the schisandrin B is 15-30 mg / kg body weight; the schisandrin B can effectively prevent colitis and colitis-related colon cancer, has clinical application prospects; Schizandrin B has low toxicity and few side effects, which can reduce the production of inflammatory factors; Schizandrin B has good drug-making properties; therefore, Schizandrin B has good clinical application prospects for preventive treatment.
Owner:ZHEJIANG DINGHUI PHARM
A method for identifying the origin of agricultural products
ActiveCN104764864BGuarantee the safety of medicine and foodRealize the protection of originComponent separationMaterial analysis by electric/magnetic meansAgricultural scienceFood safety
The invention relates to a discrimination method, in particular to a method for identifying the origin of agricultural products, which is mainly applicable to the origin identification of schisandra chinensis. Through qualitative and / or quantitative determination of the independent variable factor in the pulverized Schisandra chinensis sample, after regression using the independent variable factor model, determine the origin of the sample according to the level of the dependent variable; the independent variable factor (Xj) is δ15N, schisandra alcohol A content, schisandrin A content, schisandrin A content, schisandrin B content, B content, Sr content. Taking Schisandra chinensis as an example to carry out research on origin traceability technology, and to screen out "geo-tags" that effectively indicate the origin of Schisandra chinensis, it will not only help to ensure the safety of consumers' medicine and food, realize the protection of the origin of authentic medicinal materials, but also provide a theory for the optimal planting of Schisandra chinensis in accordance with. This technology can also be popularized and applied to the quality safety and control of agricultural products in our country, and has broad application prospects.
Owner:SHENYANG INST OF APPL ECOLOGY CHINESE ACAD OF SCI
Application of schisandra monomer compound in the preparation of drugs for the prevention and treatment of hepatotoxicity caused by paracetamol
ActiveCN103751174BCurb consumptionImprove necrosisDigestive systemEther/acetal active ingredientsGlucocorticoidLiver necrosis
The invention discloses the application of a schisandra monomer compound in the preparation of drugs for preventing and treating liver toxicity caused by APAP. The schisandra monomer compound is schisandrin A, schisandrin C, schisandrin alcohol A or schisandrin ester A. It was found through experiments that the Schisandra-related monomer compounds can significantly reduce the contents of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in APAP-induced liver injury model animals, and inhibit the reduction of glucocorticoids in liver cells. The depletion of glutathione, and effectively improve the degree of liver cell necrosis, the experimental results show that Schizandra monomer compounds have significant therapeutic and protective effects on the symptoms of liver toxicity caused by APAP.
Owner:SUN YAT SEN UNIV
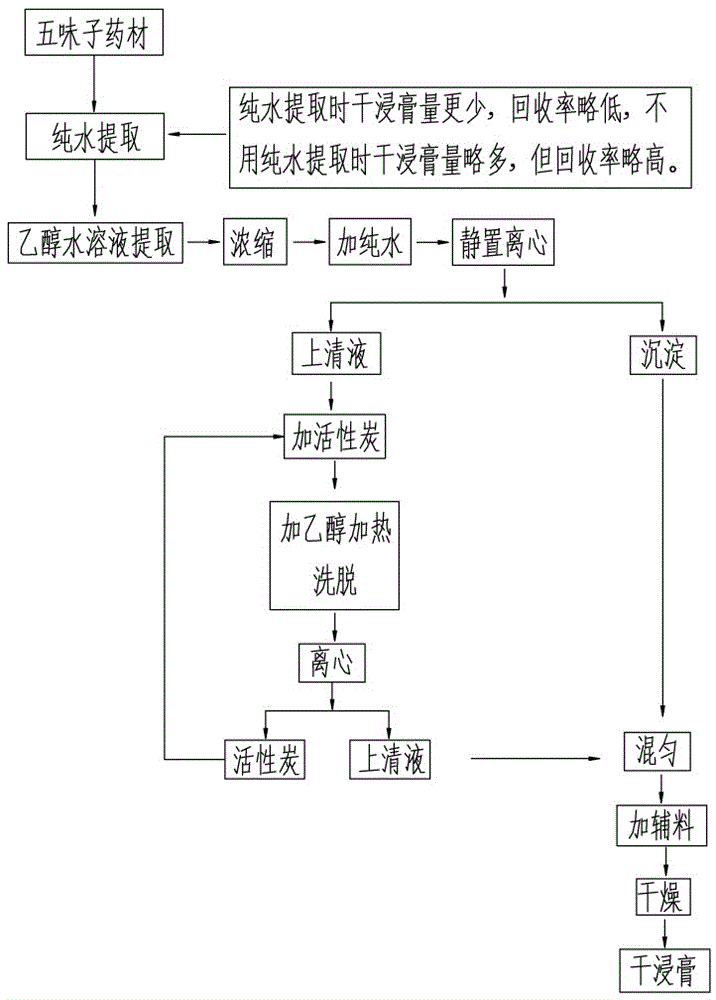
A method for purifying schisandrin A and schisandrin B from schisandra extract
ActiveCN104926624BEasy to operateReduce consumptionEther separation/purificationSchisandrin BChemistry
A method for purifying Schisandrin A and Schisandrin B from the Schisandra chinensis extract, which comprises the following steps: after the crude drug of Schisandra chinensis is extracted, collecting the extract, concentrating the extract to 0.3-0.8 times the weight of the crude drug, and adding a volume of Pure water 2-4 times the weight of the crude drug, cool and stand for 4-6 hours at 0-4°C for water sedimentation, perform the first high-speed centrifugation, and precipitate for treatment; add 10-20% of the crude drug weight to the supernatant After the activated carbon was stirred and adsorbed for 0.5-3 hours, the second high-speed centrifugation was performed, and the centrifugation supernatant was discarded; 4-20 times the weight of the crude drug was added to the activated carbon with 60-95% (v / v) ethanol solution, and heated to 50 Elute at -80°C for 1-3 hours, and collect the supernatant after the third high-speed centrifugation; add the supernatant to the precipitate after the first centrifugation, stir, and add 3-8% of the crude drug weight after homogenization. After drying, the dry extract containing schisandrin A and B can be obtained; the method has the advantages of simple operation, safety and non-toxicity, low cost and high recovery rate, can simultaneously recover schisandrin A and schisandrin B, and has good medicinal properties. and health food development prospects.
Owner:劲牌持正堂药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com